Method for separating and detection palonosetron hydrochloride and impurity
A palonosetron hydrochloride impurity, separation method technology, applied in the field of analytical chemistry, to achieve the effect of shortening the separation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
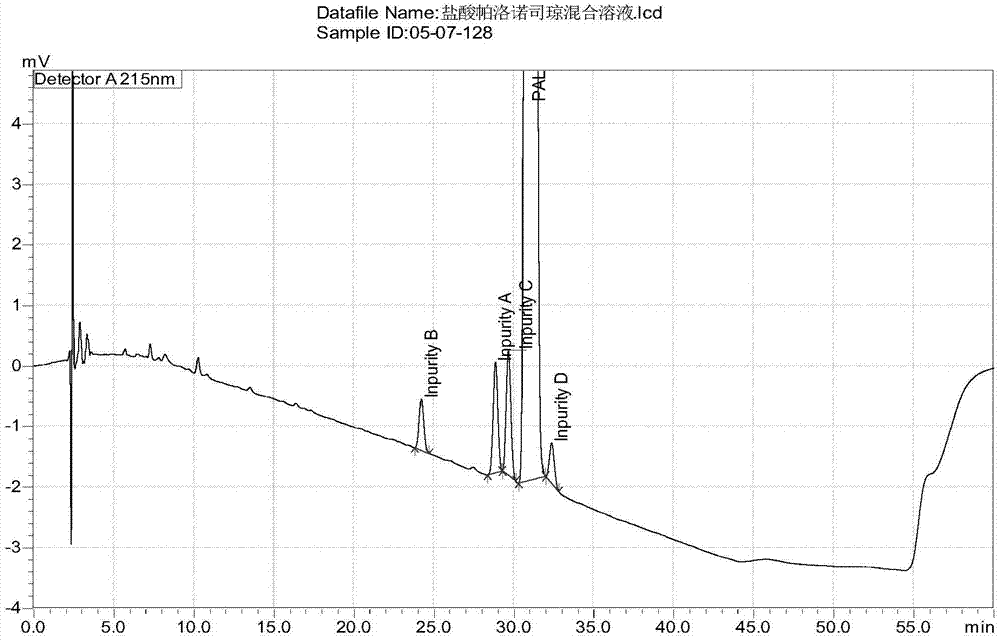
[0063] The chromatogram of embodiment 1 palonosetron hydrochloride and 4 kinds of process impurities
[0064] Impurity A is shown in molecular formula I; impurity B is shown in molecular formula II; impurity C is shown in molecular formula III; impurity D is shown in molecular formula IV.
[0065] Preparation of the reference substance mixture of the four impurities: take about 25 mg of the reference substances of impurity A, impurity B, impurity C and impurity D respectively, put them in 25ml measuring bottles, add methanol to dissolve and dilute to the mark, shake well , to obtain impurity A stock solution, impurity B stock solution, impurity C stock solution and impurity D stock solution. Precisely pipette 2.5ml of the above four stock solutions, place them in a 100ml measuring bottle, add diluent to dilute to the mark, and shake well to obtain the reference substance mixture of the four impurities with a concentration of 25 μg / ml.
[0066] The preparation of test sample m...
Embodiment 2
[0071] Example 2 Palonosetron Hydrochloride Injection Auxiliary Materials Influence on the Determination of Palonosetron Hydrochloride
[0072] Take 1 stick of palonosetron hydrochloride injection (manufacturer: Chongqing Huabang Pharmaceutical Co., Ltd.) (containing 0.25 mg of palonosetron hydrochloride) as the test solution.
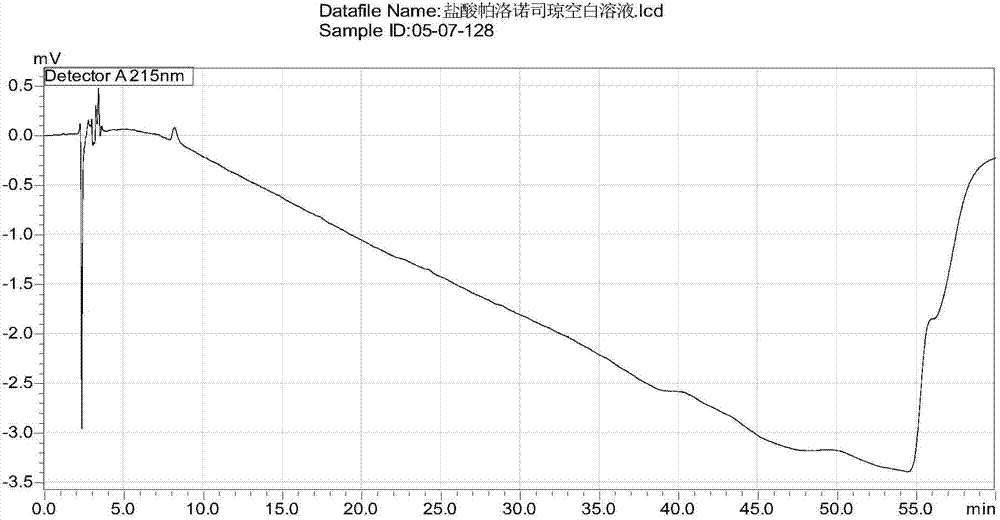
[0073] Take the test solution, inject samples according to the above chromatographic conditions, record the chromatogram, and carry out the blank excipient test in the same way, the result shows that no other peaks appear in the HPLC spectrum of the blank excipient after 4 minutes, indicating that the blank excipient does not interfere with palonosetron hydrochloride determination, see image 3 , Figure 4 .
[0074] Conclusion: Blank excipients do not interfere with the determination of this product, indicating that the method of the present invention can be used for the quality detection of palonosetron hydrochloride injection. The analysis method...
Embodiment 3
[0075] Example 3 Determination of Oxidative Degradation Products of Palonosetron Hydrochloride
[0076] Take 25mg of palonosetron hydrochloride, accurately weigh it, put it in a 25ml measuring bottle, add 2.0ml of 10% hydrogen peroxide, bathe in water at 60°C for 4 hours, take it out, cool it, dissolve it with a diluent and dilute to the mark, shake it up, and use it as a degradation product solution for measurement.
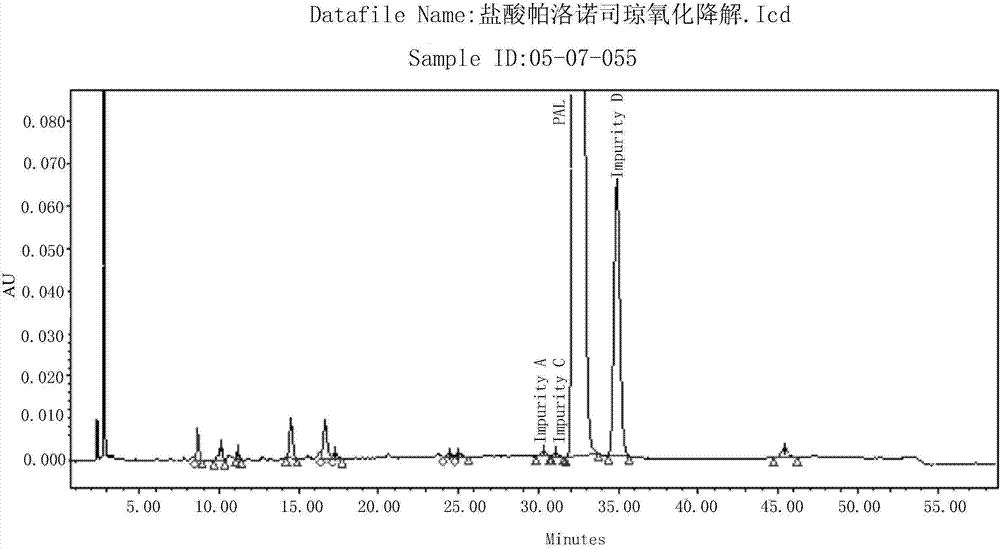
[0077] Take the solution for the determination of the above-mentioned degradation products and inject samples, record the chromatogram, and do a blank test at the same time, record the chromatogram, such as Figure 5 As shown, the impurity content was calculated according to the area normalization method. The test results are shown in Table 3.
[0078] Table 3 Oxidative Degradation Test Determination Results
[0079]
[0080] Conclusion: Under the condition of oxidative degradation, impurity D was produced in water bath at 60°C for 4 hours, with a level of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com