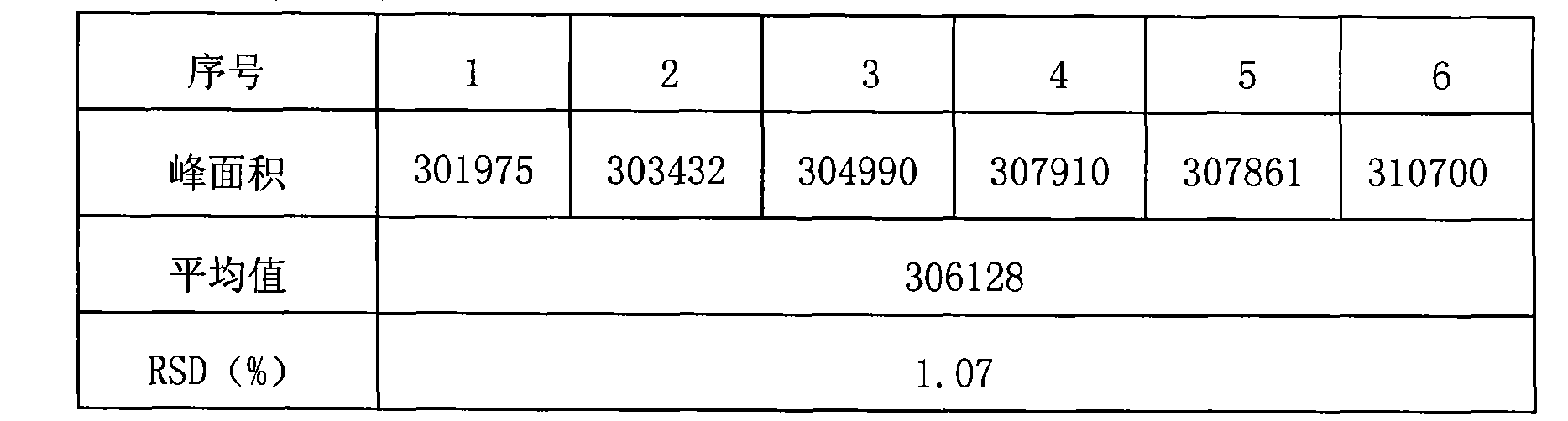
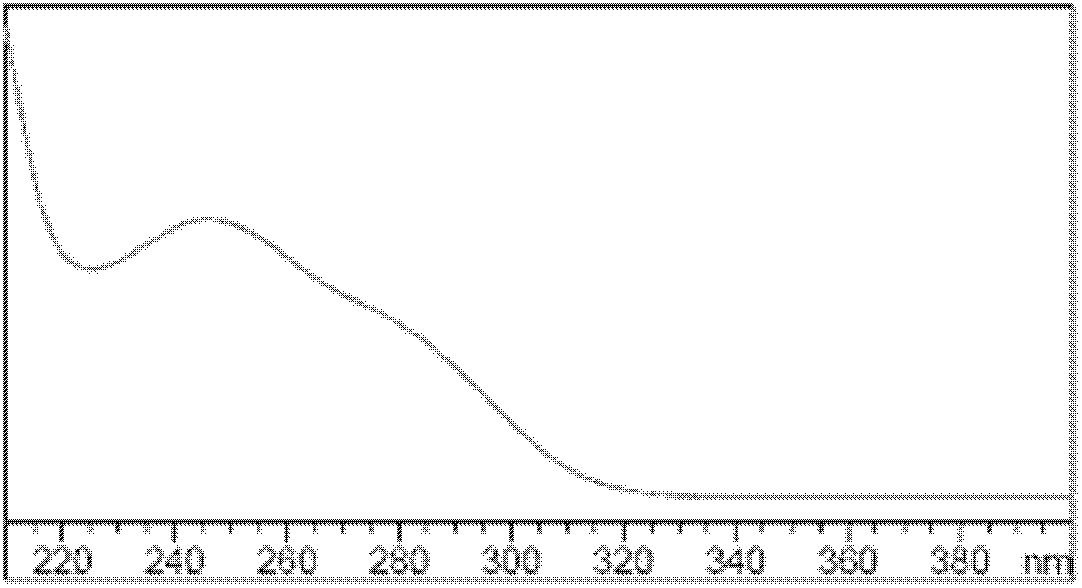
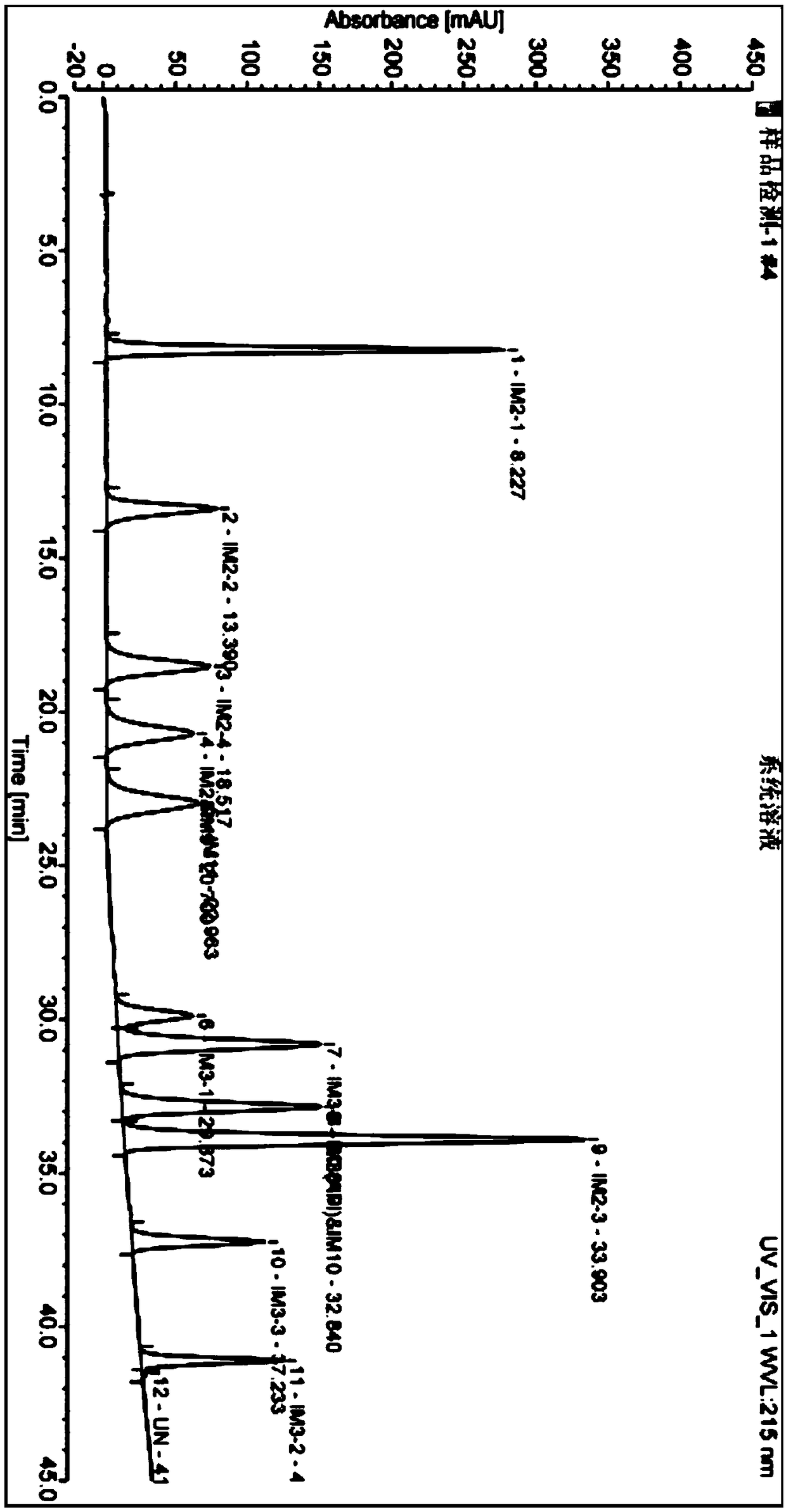
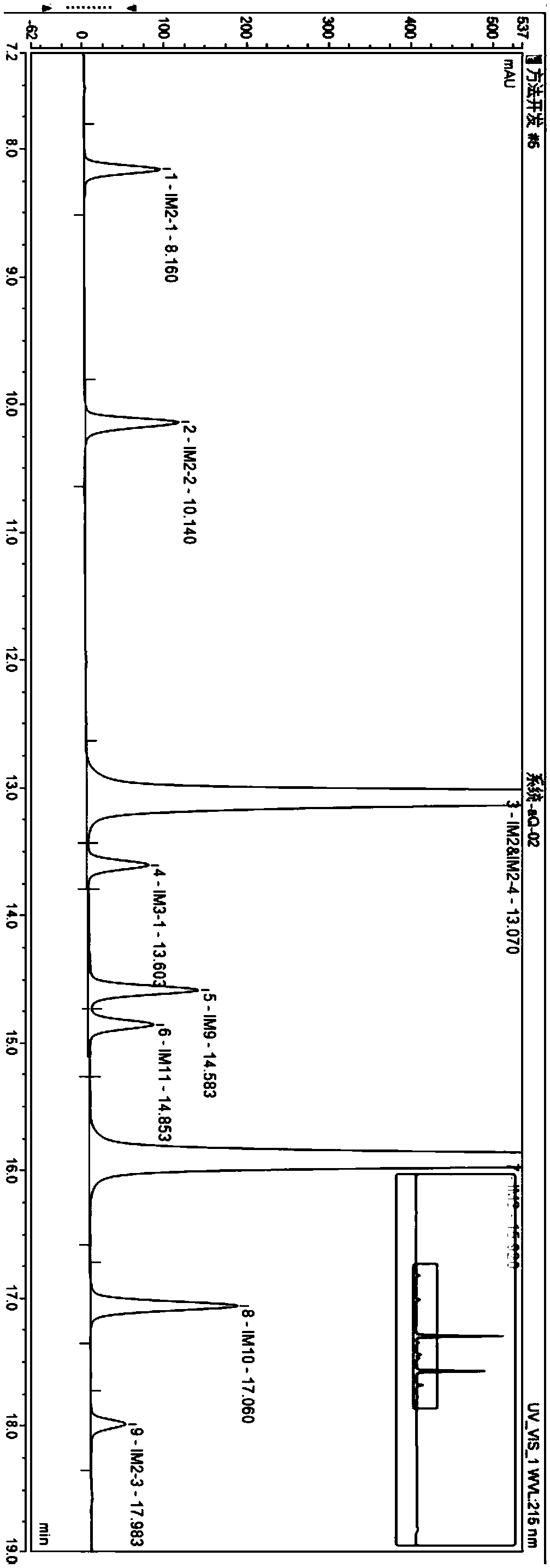
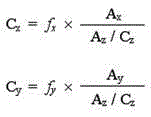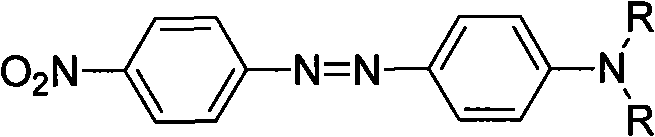Patents
Literature
327 results about "Octadecyl silane-bonded silica" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Analysis and detection method for rivaroxaban intermediate
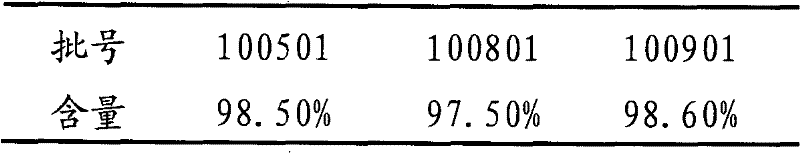
The invention relates to an analysis and detection method for a rivaroxaban intermediate, which is particularly used for mass control on the rivaroxaban intermediate. A chromatographic column (C18, 4.6mm*150mm, 5 microns) which takes octadecyl silane bonded silica gel as a packing and a perchloric acid solution and methanol as flow phases are adopted for gradient elution, and analysis and detection are performed by a high performance liquid chromatography method under the conditions that the detection wavelength is 240nm-250nm, the column temperature is 30-40 DEG C and the velocity is 0.7-1.1 ml / min. By adopting the analysis and detection method provided by the invention, the rivaroxaban intermediate can be effectively separated from impurities of the rivaroxaban intermediate, and moreover, the method has the advantages of high separation degree and sensitivity, good repeatability and durability, short analysis time, simplicity in operation and stable and reliable result.
Owner:SHANDONG NEWTIME PHARMA
Related substance detection method for trelagliptin succinate and preparation thereof
The invention provides a related substance detection method for trelagliptin succinate and preparation thereof. According to the related substance detection method for trelagliptin succinate and preparation thereof, a diode array detector is adopted, a chromatographic column adopted in the method is an octadecyl silane bonded silica gel chromatographic column, a mobile phase A is phosphate buffer solution, a mobile phase B is acetonitrile, the volume ratio of the phosphate buffer solution to acetonitrile is 60: 40 to 85: 15, the pH value of the phosphate buffer solution is 5.0 to 5.5, the concentration of the phosphate buffer solution is 0.05 to 0.1mol / L, and a detection wavelength is 278nm. The various ingredients in the analysed substances are thorough in separation and high in operability; the analysis method is high in specificity, high in accuracy and sensitivity, high in adaptability, and capable of providing a basis for the safety, effectiveness and controllability of medicines.
Owner:JINAN ORIENT KAIYUAN PHARMA TECH
Method for simultaneously detecting contents of four active ingredients in herba erigerontis preparation
InactiveCN102914609AHigh precisionImprove accuracyComponent separationPhosphoric acidBULK ACTIVE INGREDIENT
The invention relates to a method for simultaneously detecting contents of four active ingredients in a herba erigerontis preparation. Content determination of the four active ingredients is performed after preparation of reference substance solution and test product solution. The method comprises injecting the four active ingredients in a liquid chromatograph, determining, recording a chromatogram, and obtaining the contents of the four active ingredients by calculation according to peak areas in an external standard method. Octadecyl silane bonded silica gel serves as a filling agent of the liquid chromatograph, one or more of acetonitrile or methanol can serve as a mobile phase A, one or more of 0.05%-1.0% of formic acid or acetic acid or phosphoric acid can serve as a mobile phase B, and elution is performed according to the following gradients: time of 0-10min and A at a proportion of 7%-9%; time of 10-24min and A at a proportion of 8%-12%; time of 24-37min and A at a proportion of 9%-16%; time of 37-57min and A at a proportion of 10%-24%; time of 57-60min and A at a proportion of 24%-7%. The method is strong in specificity, high in precision, good in repeatability, high in accuracy and good in durability.
Owner:昆明振华制药厂有限公司
Fingerprint pattern quality control method for cordyceps sinensis bacterium powder raw material in herbs medicaments for strengthening the body resistance and activating blood and dissolving stasis
ActiveCN101293002AGuarantee normal implementationHigh sensitivityFungiComponent separationHplc fingerprintRetention time
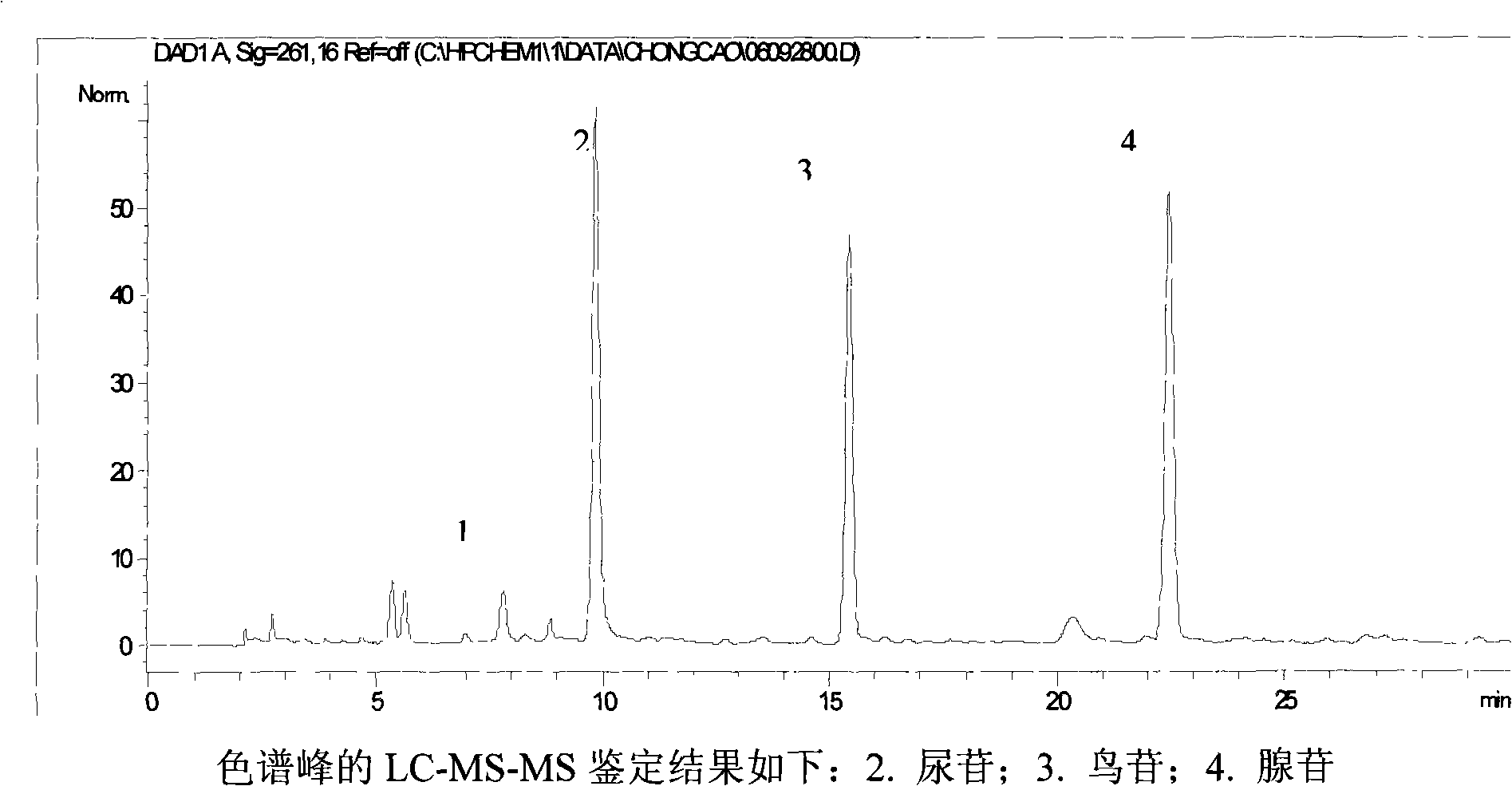
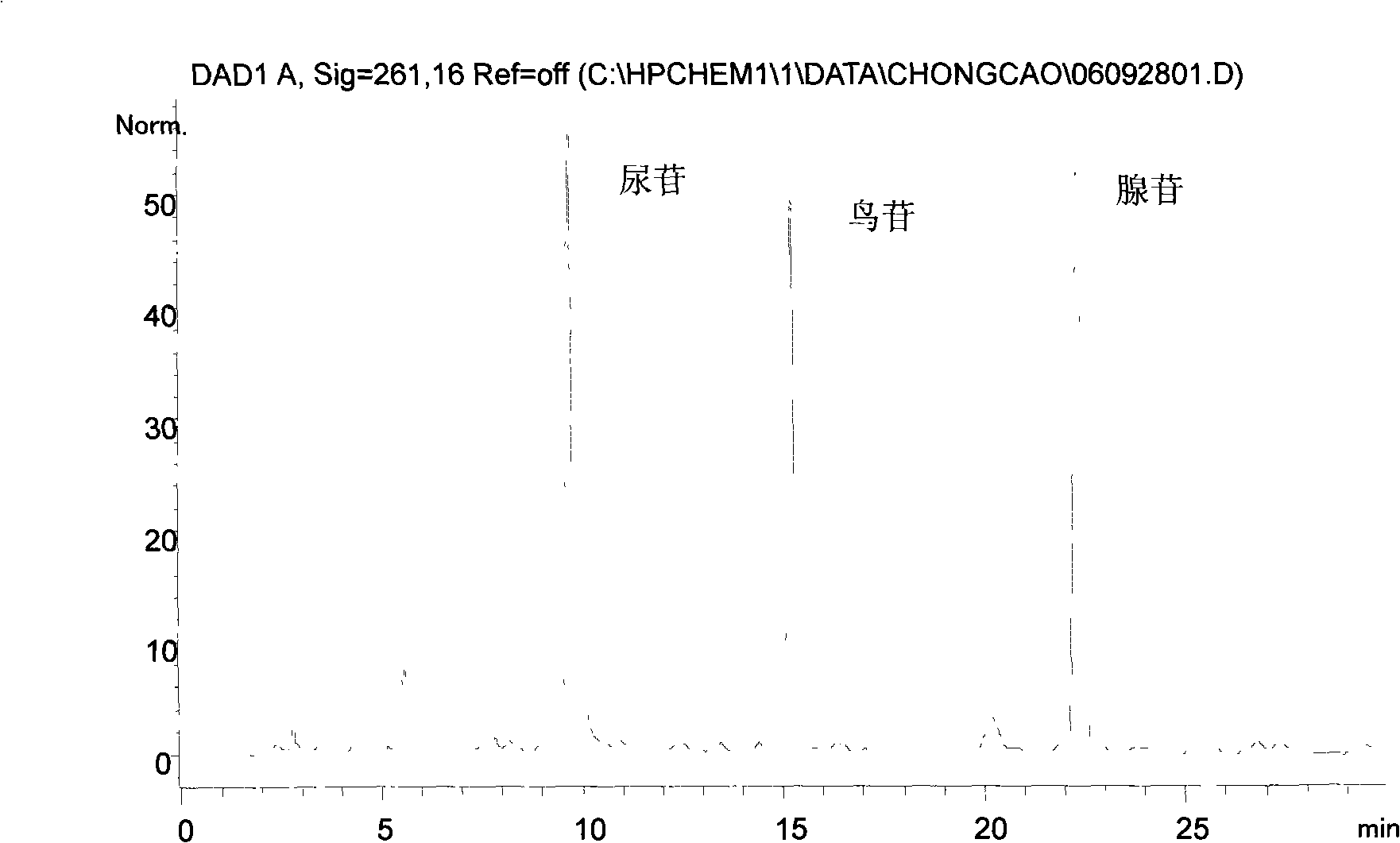
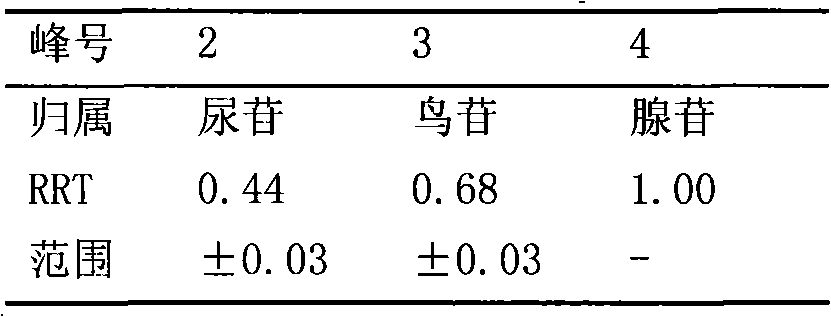
The invention relates to a control method of the fingerprint spectrum quality of cordyceps sinensis powder raw material in botanical drug for strengthening vital qi and removing blood stasis, comprising the steps that: (1) cordyceps sinensis powder is extracted: 0.100g of cordyceps sinensis powder is taken, purified water is added, the ultrasonic extraction, the filtration and the sample injection are carried out; (2) the gradient elution with mobile phase is carried out: octadecyl silane bonded silica gel is taken as a filler, water and acetonitrile are taken as mobile phase to carry out the gradient elution for 0 to 30min and 0 to 7 percent B; (3) a standard fingerprint spectrum is established: the HPLC standard fingerprint spectrum of the cordyceps sinensis powder is determined, and 3 characteristic peaks are selected; (4) the quality control of the fingerprint spectrum is carried out: the relative retention time of No.2 peak uridine, No.3 peak guanosine and No.4 peak adenosine are 0.44 plus or minus 0.03, 0.68 plus or minus 0.03 and 1.00 respectively; the HPLC fingerprint spectrum of the sample is compared with the contrast fingerprint spectrum. The similarity calculated by the 5 common peaks is not less than 0.9, (5) the preparation of the cordyceps sinensis powder raw material is carried out; the control method has good repetitivity and can fully reflect the basic characteristics of nucleoside ingredients of the cordyceps sinensis powder.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV
Method using high performance liquid chromatography (HPLC) to measure Rivaroxaban intermediate content
ActiveCN104931595AEfficient separationQuality assuranceComponent separationSilica gelAqueous solution
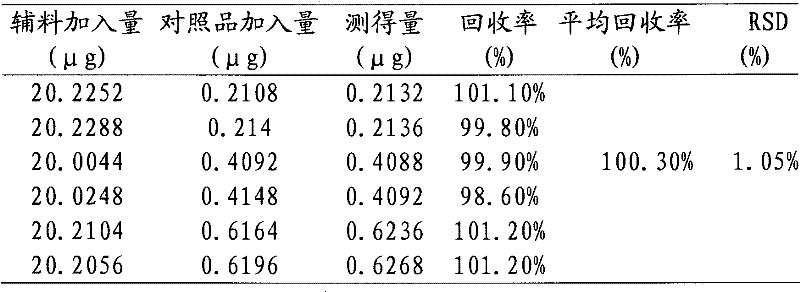
The invention relates to a method using high performance liquid chromatography (HPLC) to measure the Rivaroxaban intermediate content. The method is used to control the quality of Rivaroxaban intermediate. In HPLC, a chromatography column (4.6*150 mm, 5[mu]m) is filled with octadecyl silane bonded silica gel, and an acidic water solution and an organic modifier are taken as the mobile phase. The provided method can effectively separate Rivaroxaban intermediate from other impurities, and has the advantages of high separation degree and sensitivity, good repeatability and durability, short analysis time, simple operation, and reliable and stable results.
Owner:LUNAN PHARMA GROUP CORPORATION
Vitamin A content detection method by high performance liquid chromatography
InactiveCN101393177AAccurate Analysis RequirementsComponent separationUltraviolet detectorsVitamin A Alcohol
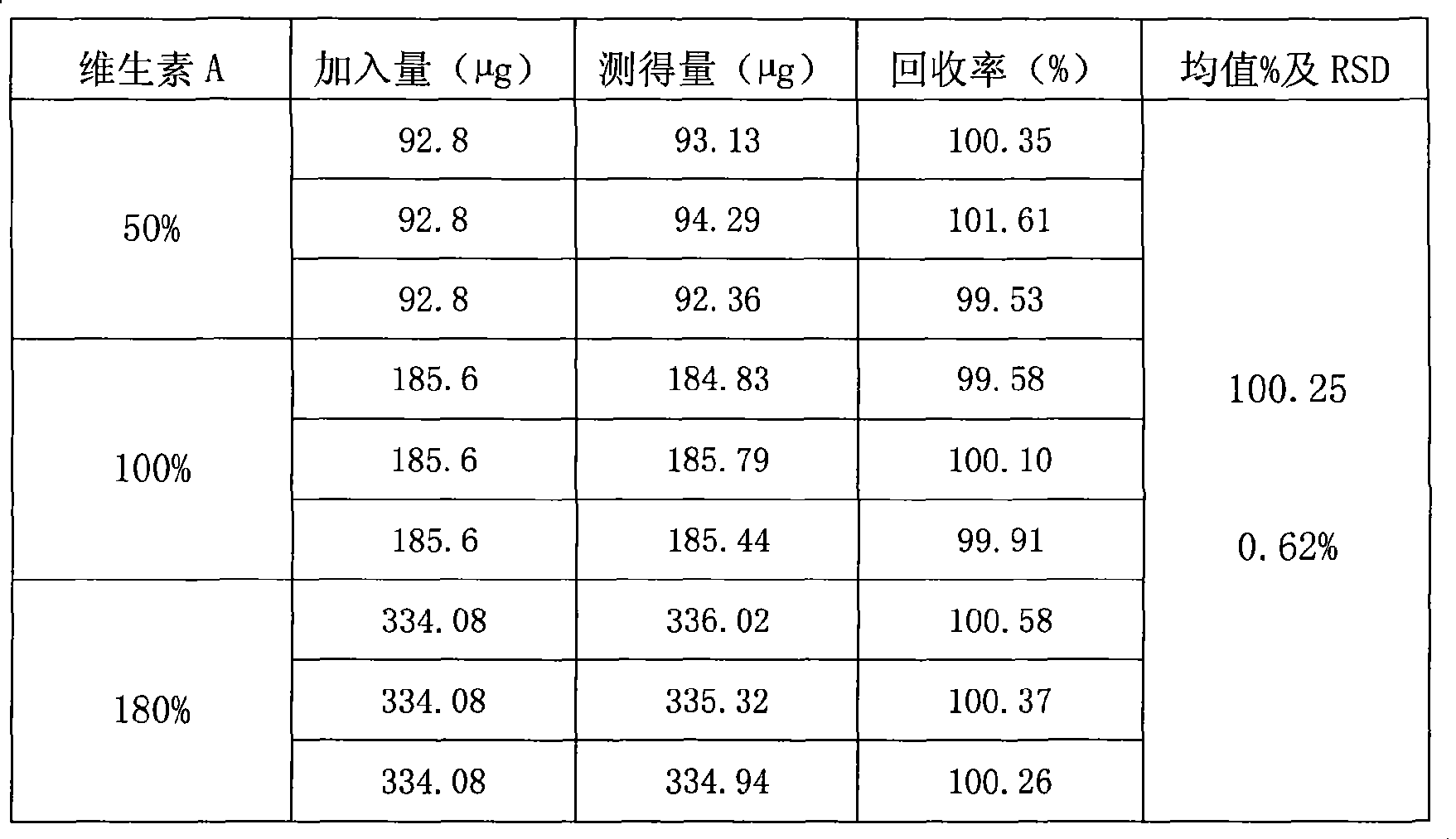
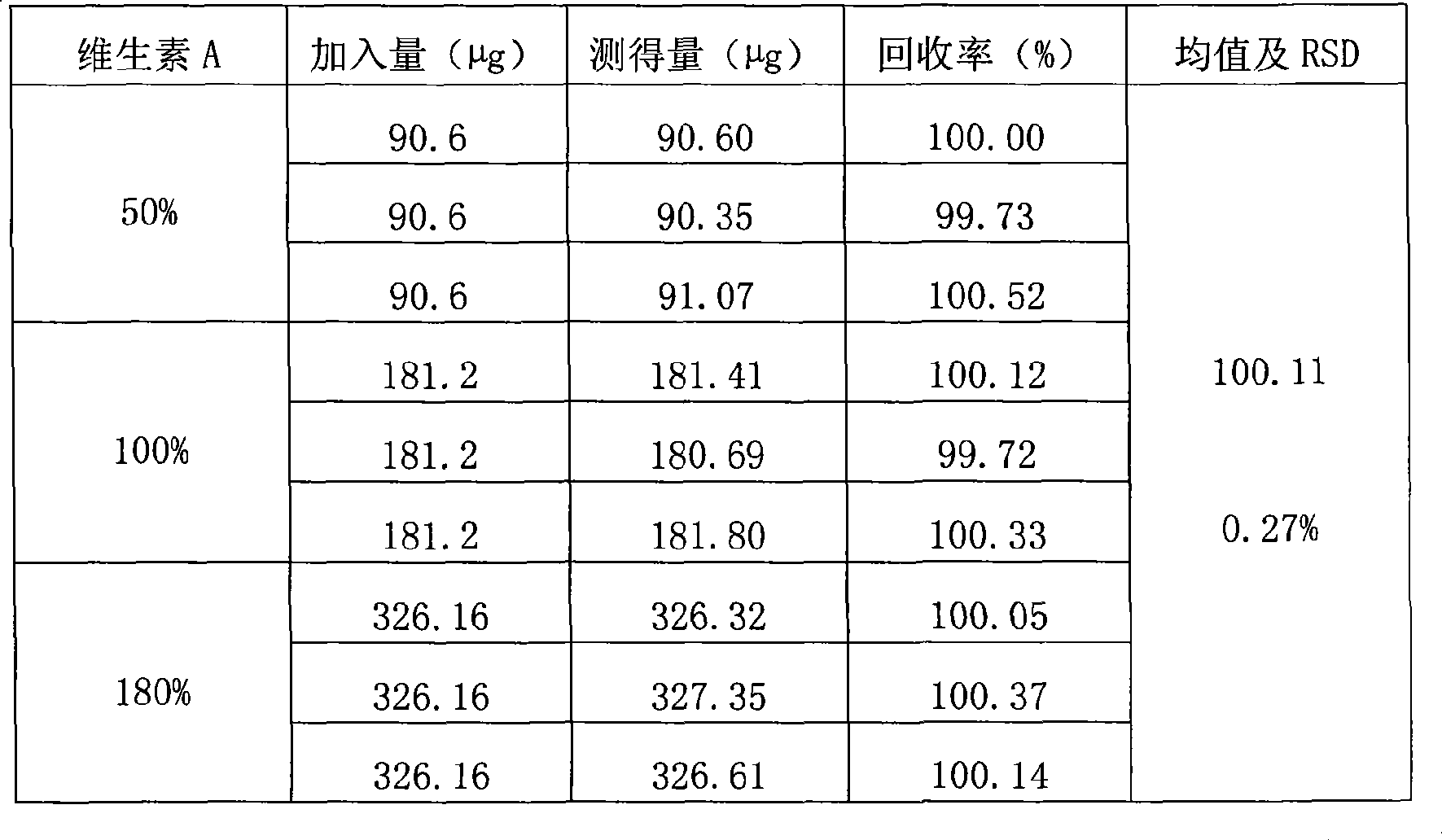
The invention relates to a method for mensurating the content of vitamin A in a high performance liquid phase chromatography method. The method has the following chromatographic conditions: a. a filling agent for a chromatographic column is octadecyl silane bonded silica gel; b. a chromatographic mobile phase is a mixed solution of methanol and water in a mixed proportion of 80-90 to 20-10 (excluding 90 to 10); and c. the detection wavelength of an ultraviolet detector is 325 nm. The flow speed of the mobile phase is 1 ml / min; and the size of sample introduction of the mobile phase is 20 mu l. The method is accurate and reliable; the linear range is between 36.72 and 68.85mu g / ml; a linear related coefficient r is equal to 0.9992; the reclaiming rate is equal to 100.3 percent; and RSD is less than 2 percent.
Owner:HANGZHOU MINSHENG HEALTHCARE CO LTD
Method for analyzing night cold flu cough allergy capsule by utilizing HPLC (High Performance Liquid Chromatography)
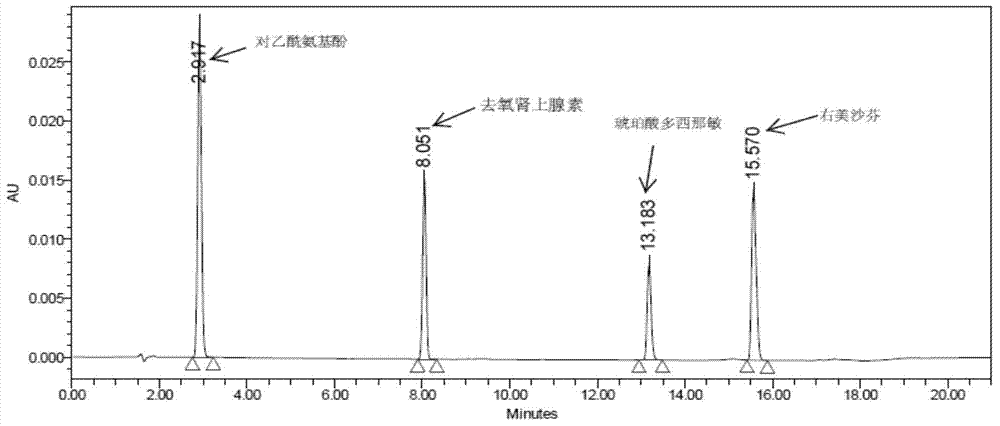
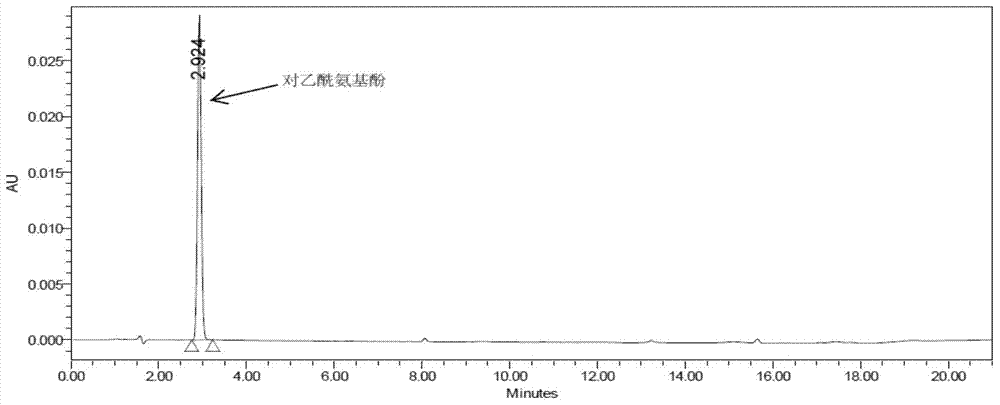
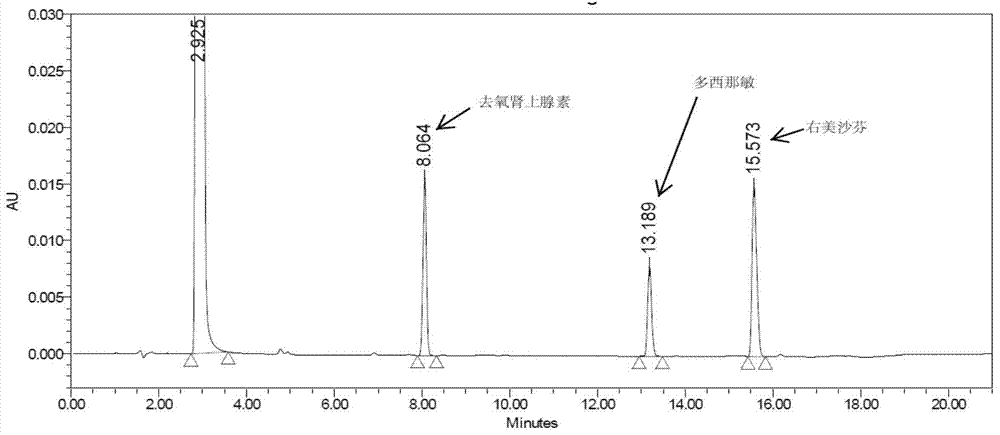
The invention discloses a method for analyzing a night cold flu cough allergy capsule by utilizing HPLC (High Performance Liquid Chromatography). The night cold flu cough allergy capsule contains acetaminophen, phenylephrine hydrochloride, succinic acid doxylamine and dextromethorphan hydrobromide. In the HPLC analysis, an octadecyl silane bonded silica gel column is adopted as a chromatographic column; a sodium 1-octanesulfonate-phosphate buffer solution with the pH of 2.0-3.0 acts as a mobile phase A; acetonitrile and a mixed solution of acetonitrile and methyl alcohol act as a mobile phase B. The method can be simultaneously and effectively used for detecting four effective ingredients in the night cold flu cough allergy capsule, is simple to operate, analyzes rapidly, is good in repeatability, has favorable specificity, and can effectively and comprehensively control the product quality of the night cold flu cough allergy capsule.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Method for detecting related substances in piperacillin sodium and sulbactum sodium for injection
ActiveCN104215697ARealize determinationEfficient separationComponent separationSilanesStability indicating
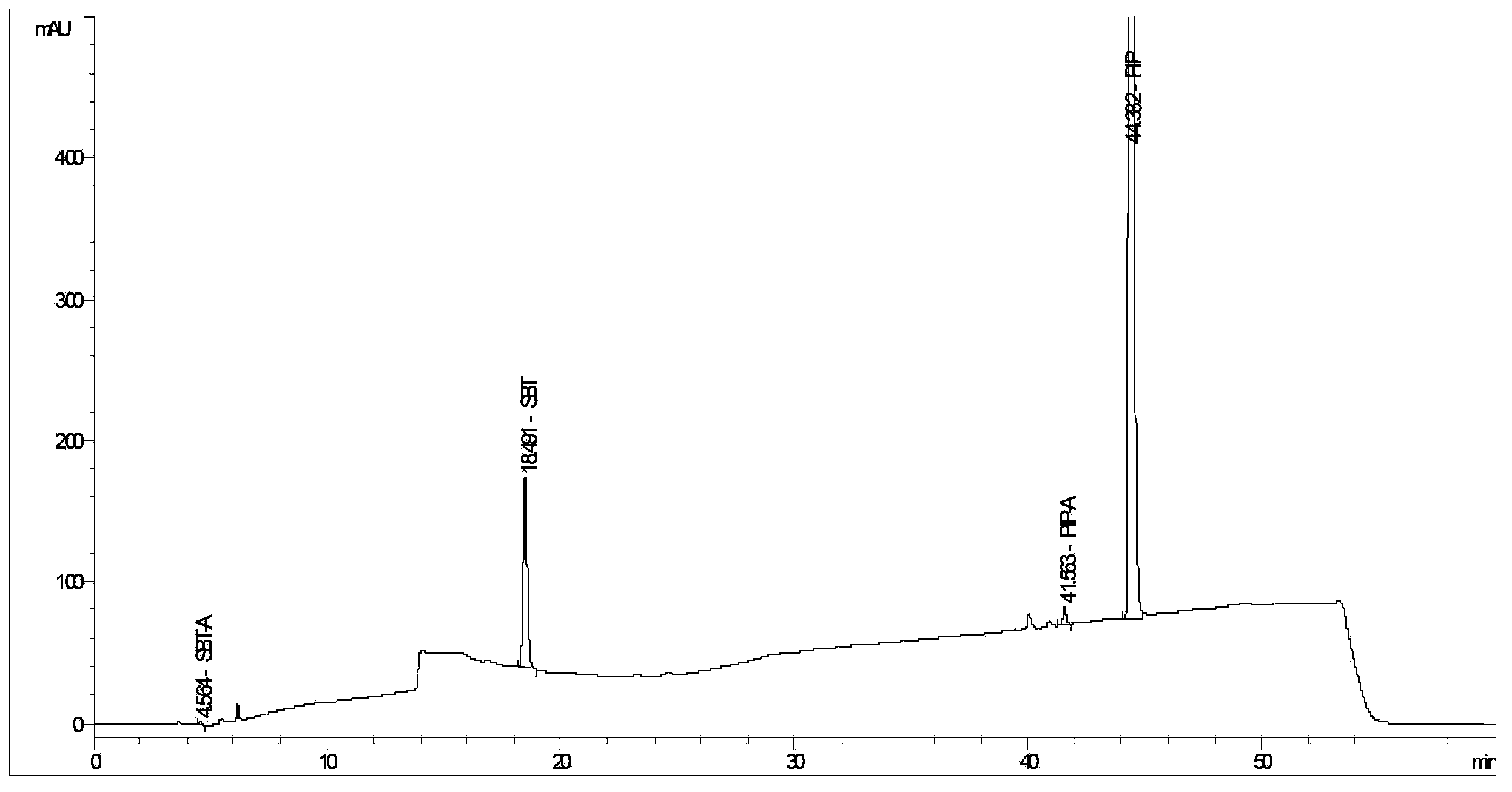
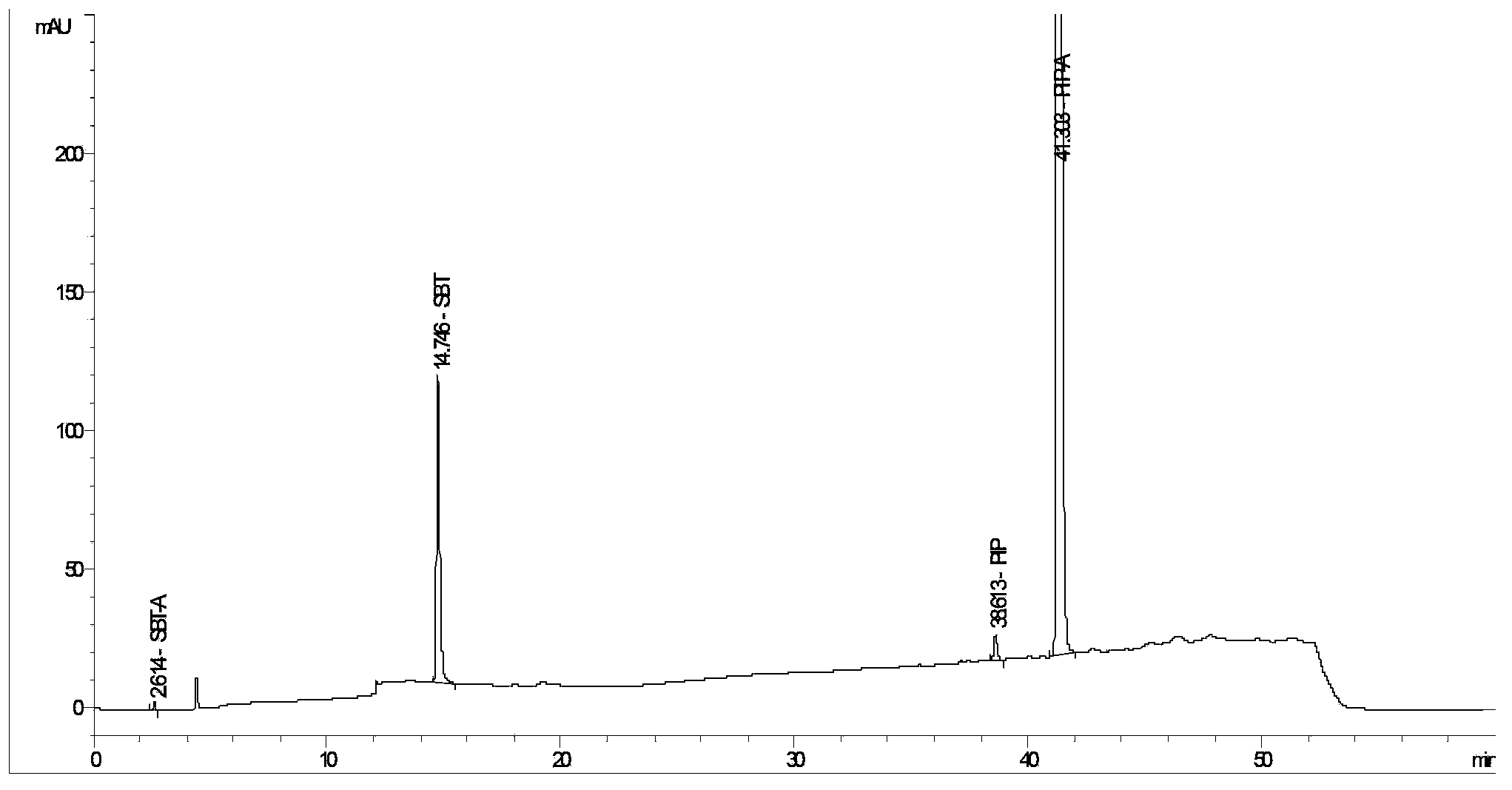
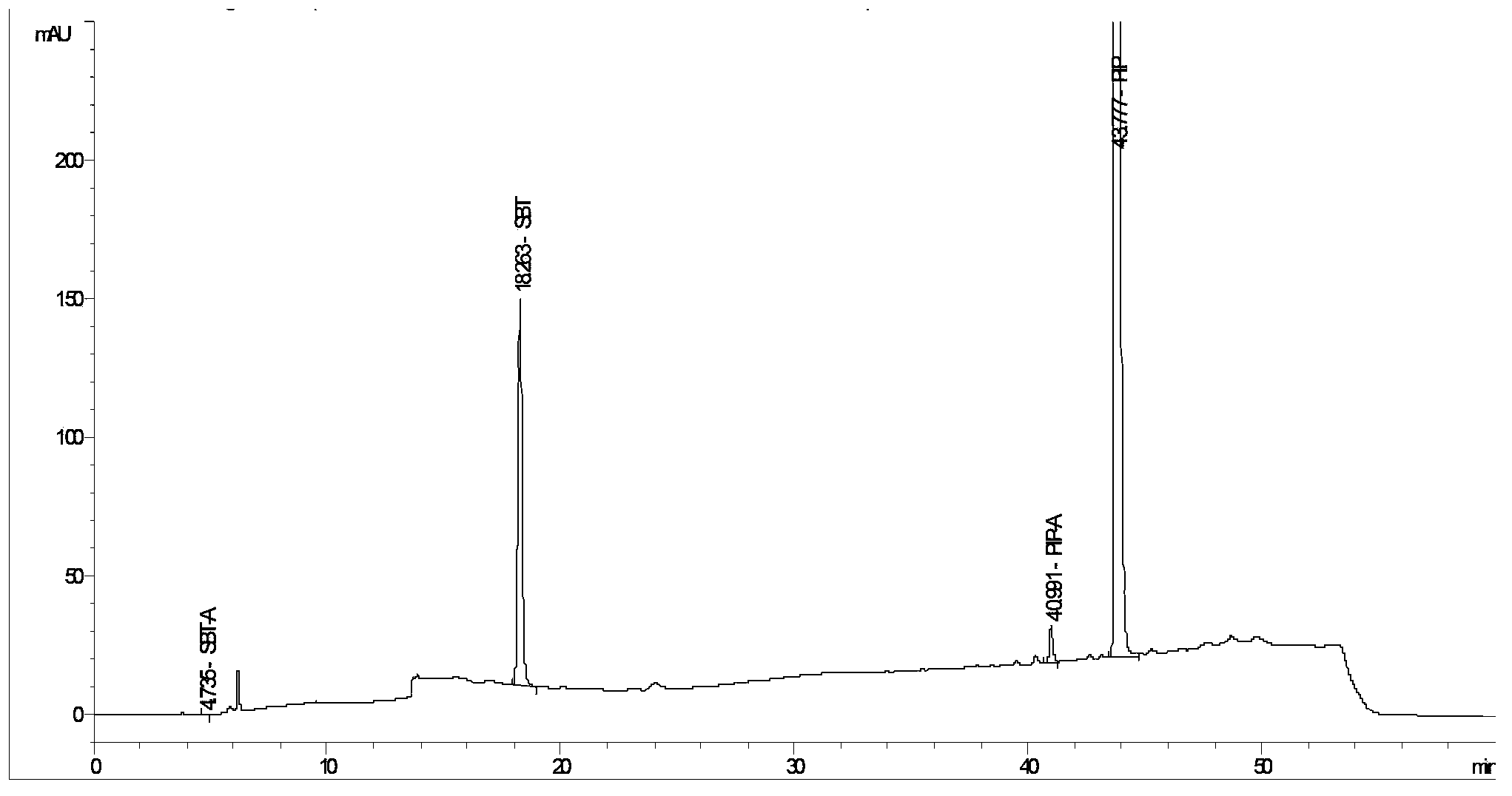
The invention provides a method for detecting related substances in piperacillin sodium and sulbactum sodium for injection. The method adopts an octadecyl silane bonded silica-gel chromatographic column to carry out gradient elution so as to rapidly and precisely complete the detection on related substances in piperacillin sodium and sulbactum sodium. In the obtained chromatogram, the main related substance (2S)-2-amino-3-methyl-3-sulfinobutyric acid and piperacillin penicilloic acid are well separated, piperacillin and sulbactum are also well separated from other related substances, and the separation degree is more than 1.5. The degradation researches and methodology on piperacillin sodium and sulbactum sodium show that the provided method has a stability indicating function, and thus the detection method can be used to control the limits of impurities in piperacillin sodium and sulbactum sodium, and can also be used to control the quality of piperacillin sodium and sulbactum sodium for injection. The method has the advantages of convenient operation and low cost, and has a good economic profit and promotion prospect.
Owner:XIANGBEI WELMAN PHARMA CO LTD +1
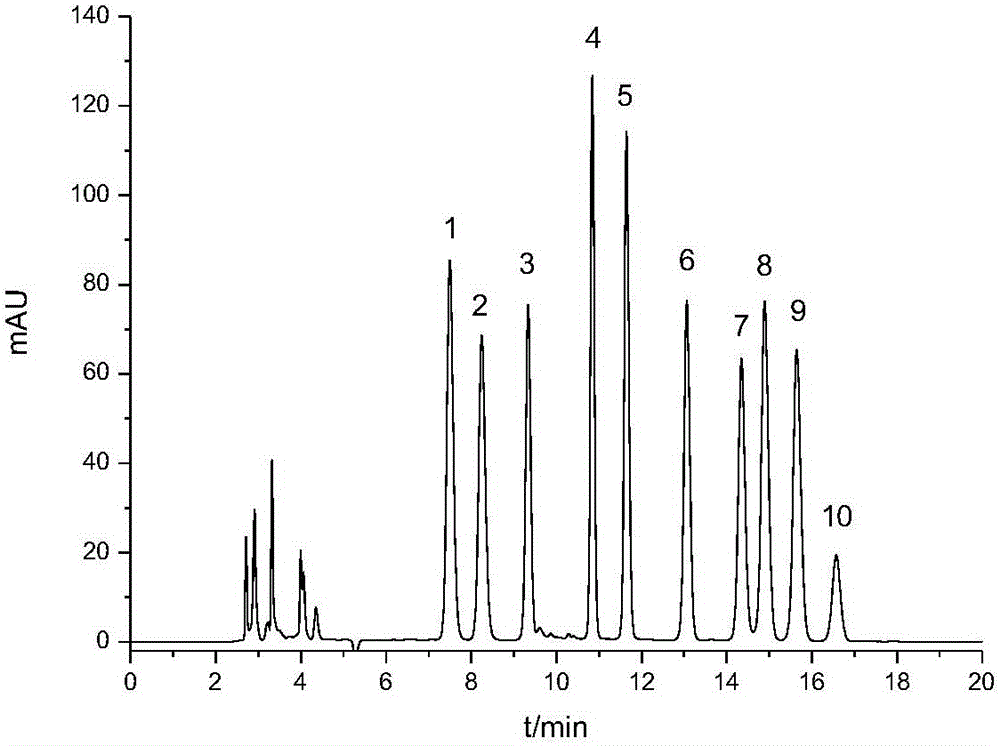
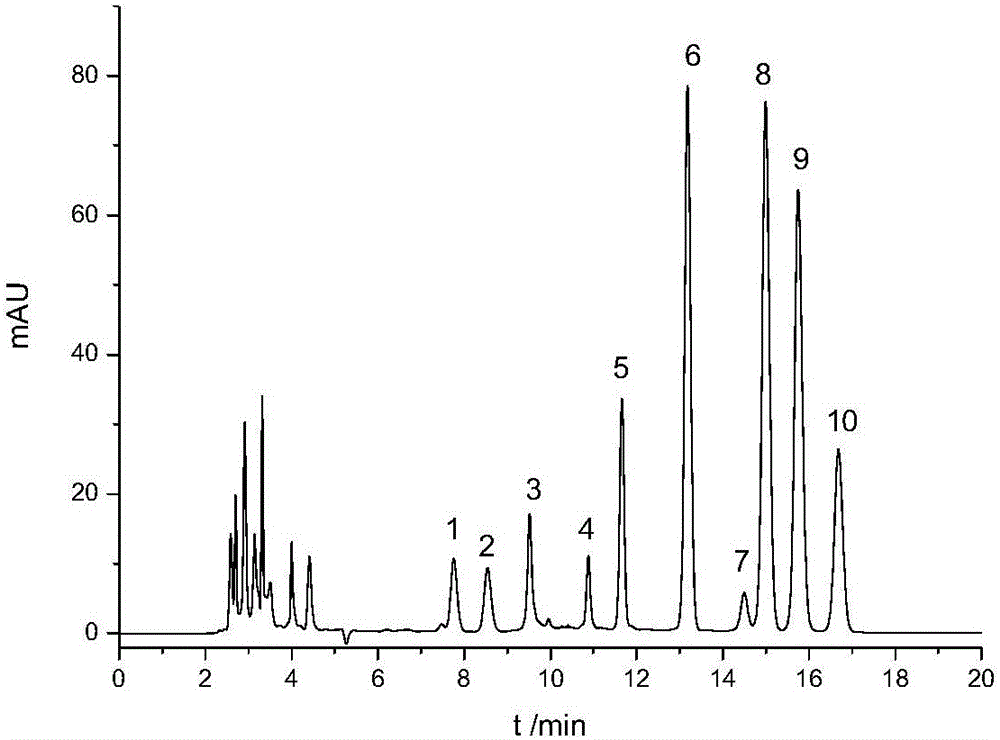
Rapid quantitative method for tea polysaccharide monosaccharide composition
InactiveCN105136951AEasy to separateMonosaccharide peaks are sharpComponent separationMonosaccharide compositionFiller Excipient
The invention discloses a rapid quantitative method for tea polysaccharide monosaccharide composition. The steps includes: (1) hydrolyzing tea polysaccharide with trifluoroacetic acid; (2) dissolving the hydrolyzed tea polysaccharid sample with ultra pure water to obtain a hydrolyzed polysaccharide solution, and adding trehalose to serve as the internal standard substance; (3) conducting derivatization on the internal standard containing hydrolyzed polysaccharide solution with 1-phenyl-3-methyl-5-pyrazolone (PMP); (4) at the same time of hydrolyzed polysaccharide derivatization, conducting derivatization on standard monosaccharide; and (5) carrying out high performance liquid chromatographic analysis, thus obtaining the content of each monosaccharide. The high performance liquid detection system conditions employed by the method include: octadecyl silane bonded silica gel is a chromatographic column of filler, the mobile phase is a buffer solution mixed according to certain ratio and an organic agent, the flow speed is 1.0ml / min, and the detection wavelength of an ultraviolet detector is 250nm. The method is simple, fast, convenient and, stable, achieves separation and analysis of monosaccharide within 20min, and improves the accuracy and precision of tea polysaccharide monosaccharide detection.
Owner:HUAZHONG AGRI UNIV
Detection method for flupenthixol and melitracen compound drug impurities, new identifiable impurities and safer compound drug
ActiveCN108362792AClear separationImprove applicabilityOrganic active ingredientsComponent separationCompounding drugsSilanes
The invention discloses a detection method for flupenthixol and melitracen compound drug impurities, new identifiable impurities and a safer compound drug, wherein the detection method comprises the process of carrying out gradient elution in a high performance liquid chromatographic column containing an octadecyl silane bonded silica gel filler in 0-60 min and analyzing a spectrogram furthermore.The detection method can identify various impurities of the flupenthixol and melitracen compound drug and the accurate contents of the impurities, and specially can separate and identify 6 new oxidative and degradable impurities, and thus safer drug control standards for the flupenthixol and melitracen compound drug are obtained.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Analytic method for related substances in raw materials and preparation of trelagliptin succinate
ActiveCN106896166AQuick monitoringEffective monitoringComponent separationColor/spectral properties measurementsIsocratic elutionPhosphate
The invention discloses an analytic method for related substances in raw material and a preparation of trelagliptin succinate. A high performance liquid chromatography is adopted, and chromatographic conditions include that a chromatographic column is octadecyl silane bonded silica gel chromatographic column, the mixed solution with the pH of 3.3-3.7 and the volume ratio of phosphate buffer aqueous solution to acetonitrile of (88-92):(8-12) is taken as a mobile phase A, the mixed solution with the pH of 3.3-3.7 and the volume ratio of phosphate buffer aqueous solution to acetonitrile of (18-22):(78-82) is taken as a mobile phase B, detection wavelength is 218-222nm and isocratic elution is carried out. The analytic method disclosed by the invention can detect many impurities and can rapidly, effectively and accurately monitor the related substances in trelagliptin succinate.
Owner:合肥拓锐生物科技有限公司
Method for simultaneously and quantitatively detecting ligustilide and senkyunolide A
The invention discloses a method for simultaneously and quantitatively detecting ligustilide and senkyunolide A. The method comprises the following steps: according to an HPLC (high performance liquid chromatography) method in which butylphthalide serves as a substitution reference substance and octadecyl silane bonded silica gel serves as a filling agent, respectively determining the peak area Ax and the peak area Ay of the ligustilide (X) and the senkyunolide A (Y) in a to-be-detected sample by using a single wavelength in a range of 275-284nm; and according to the concentration Cz and the peak area Az of the substitution reference substance, namely the butylphthalide (Z), calculating the concentration C of the detected components, namely the ligustilide and the senkyunolide A, according to a formula by using correction factors f, wherein a correction factor fx is within 0.20-0.25, and a correction factor fy is within 0.46-0.54. A detection result obtained by using the method provided by the invention is stable, and is in accordance with a detection result obtained by using a method in which the ligustilide and the senkyunolide A, freshly prepared, serve as reference substances, and the problems that the dual-wavelength detection needs to be simultaneously adopted, the requirements on HPLC equipment are high and the universality is poor in the prior art are solved.
Owner:SICHUAN ACAD OF CHINESE MEDICINE SCI
Quality control method for rhizoma-cimicifugae and kudzu-vine-root soup composition
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA +1
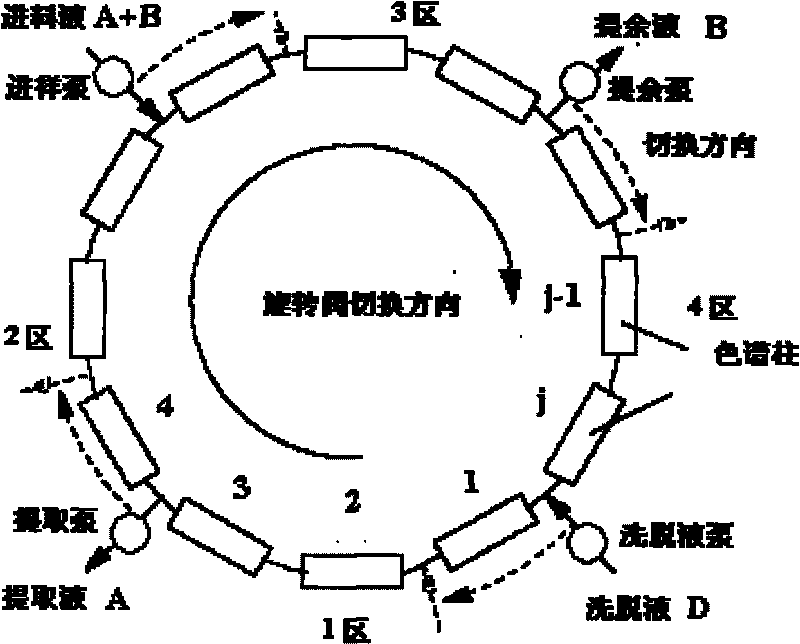
Method for separating vitamin E polyethylene glycol succinate monoester from vitamin E polyethylene glycol succinate diester
ActiveCN101721838AReduce consumptionHigh degree of automationIon-exchange process apparatusIon-exchanger regenerationSilanesPolyethylene glycol
The invention discloses a method for separating a vitamin E polyethylene glycol succinate monoester from a vitamin E polyethylene glycol succinate diester. The method takes octadecyl silane bonded silica of which the grain diameter is of between 5 and 100 mu m as a stationary phase, takes a mixture of acetonitrile and isopropanol as a mobile phase, takes the mixture of vitamin E polyethylene glycol succinate of which the monoester content is of between 20 and 80 percent as a raw material, and adopts a simulated moving bed chromatographic system to separate TPGS monoester and TPGS diester of which the purity and the yield are both over 98 percent. Because simulated moving bed chromatography is a continuous operating process, the method has the advantages of high degree of automation, high efficiency, low consumption of the stationary phase and a solvent, and suitability of industrial production.
Owner:ZHEJIANG UNIV
Method for determining prochloraz or metabolite of prochloraz in plant-derived food
InactiveCN104391067AHigh recovery rateAccurate measurementComponent separationSpecific detectionMetabolite
The invention provides a method for determining prochloraz or metabolite of prochloraz in plant-derived food. The method comprises the following steps: a, selecting the plant-derived food, adding organic solvent and sodium chloride into the plant-derived food, uniformly mixing, centrifuging the mixture, standing for layering the mixture, and taking the supernatant; b, adding anhydrous sodium sulfate into the supernatant acquired in the step a, uniformly mixing the anhydrous sodium sulfate with the supernatant, standing the mixture, and taking the supernatant; c, concentrating the supernatant acquired in the step b, making the volume constant, adding anhydrous sodium sulfate, N-propylethylenediamine and octadecyl silane bonded silica gel into the supernatant, uniformly mixing the mass, centrifuging the mixture, and taking the supernatant as a sample to be detected; and d, detecting the sample by adopting GC-MS / MS or GC-MS (Gas Chromatography-Mass Spectrometer). According to the method, a specific detection condition is adopted, linear response is good, and the residue of prochloraz or metabolite of the prochloraz can be accurately, rapidly and conveniently determined; meanwhile, the method has advantages of simplicity, rapidness, good selectivity, high sensitivity and good matrix interference resistance.
Owner:INSPECTION AND QUARANTINE TECHNOLOGY CENTER ZHONGSHAN ENTRY EXIT INSPECTION AND QUARANTINE
Method for measuring content of degraded impurity methionine sulfoxide in compound amino acid injection
ActiveCN106248853AEffective controlEasy to separateComponent separationSilanesMonopotassium phosphate
The invention discloses a method for measuring content of degraded impurity methionine sulfoxide in compound amino acid injection; an octadecyl silane bonded silica gel column is used, program setting is made by using Agilent sampler and derivatization is carried out, wavelength of 334 nm is detected after sampling, and the content is calculated according to an external standard method; for mobile phases, 0.038 mol / L of monopotassium phosphate (pH 6.9) and acetonitrile (88:12) acts as mobile phase A, acetonitrile acts as mobile phase B, and gradient diluting is carried out; test article diluent is water, and sampling concentration is 5 Mug / ml; according to the method of the invention, other amino acids and auxiliaries in the formulation are friendly to detection, the method has a good degree of separation and high specificity, the difficulty in detecting methionine sulfoxide in the amino acid compound is overcome, the methionine sulfoxide in a product is effectively controlled, and product quality is guaranteed.
Owner:SICHUAN KELUN PHARMA CO LTD
Determination method of related substances in atorvastatin calcium capsules
A detection method of atorvastatin calcium capsule related substances of the invention relates to a method which tests or analyzes a drug by separating the drug into various components by adsorption, and the method can detect a single component of related substances in an atorvastatin calcium capsule, and facilitates the improvement of quality standard of atorvastatin calcium capsules. According to the invention, a sample solution to be tested, a self-control solution, and a blank solution are weighed; the solutions are injected into a liquid chromatography, wherein the chromatographic conditions are as follows: a chromatographic column with octadecyl silane bonded silica as a filler is selected; acetonitrile-tetrahydrofuran is used as a mobile phase A; 0.56% ammonium biphosphate solution-mobile phase A is used as a mobile phase B; 0.56% ammonium biphosphate solution- mobile phase A-methanol is used as a mobile phase C; gradient elution is performed; the detection wavelength is 240-250 nm, and the column temperature is 20 DEG C-30 DEG C, wherein in the 0.56% ammonium biphosphate solution, 0.56% is a mass-volume ratio; and a chromatogram is recorded.
Owner:北京汉典中西药研究开发中心
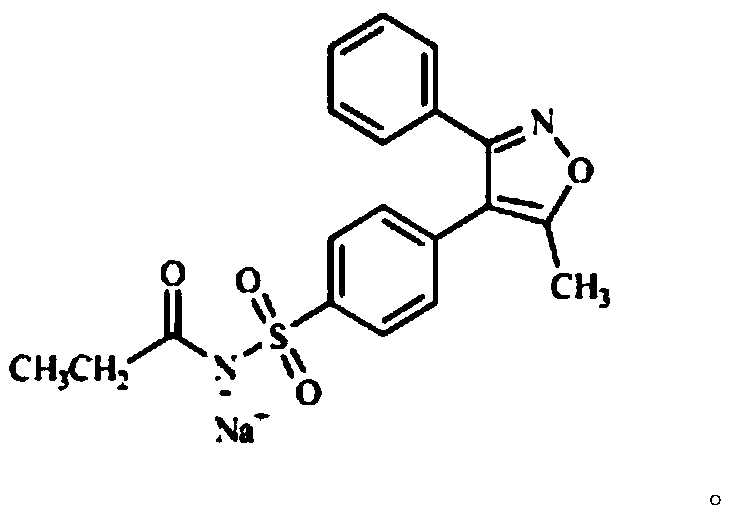
Liquid chromatography method for detecting parecoxib sodium and related substances in synthesis intermediate
The invention discloses a liquid chromatography method for detecting parecoxib sodium and related substances in a synthesis intermediate. A reverse phase efficient liquid chromatography method is adopted, pentafluorophenyl bonded silica gel and octadecyl silane bonded silica gel are taken as chromatographic column fillers, an ultraviolet detector is adopted, a mobile phase containing potassium dihydrogen phosphate is selected to carry out elution, the related substances, i.e., first related substances and second related substances, in parecoxib sodium intermediate valdecoxib and parecoxib aremeasured in two times; the mobile phases of the first related substances and the second related substances are both a mixed solution of methyl alcohol and a monopotassium phosphate aqueous solution. Two gradient methods of the liquid chromatography method are combined to finish simultaneously detecting 11 types of parecoxib sodium and the related substances of the intermediate of the parecoxib sodium, wherein fives types of position isomer impurities which are difficult to separate are contained. The liquid chromatography method has the advantages of high sensitivity, good repeatability and high accuracy.
Owner:SHANGHAI PHARMA DONGYING JIANGSU PHARMA CO LTD
Establishment of Xiasangju preparations fingerprint pattern and fingerprint pattern thereof
ActiveCN101034085AEffectively Characterize QualityMonitor qualityComponent separationPreparing sample for investigationHplc fingerprintAcetic acid
This invention relate to the found of summer mulberry chrysanthemum preparation finger print, refer methodical control of quality of Chinese traditional medicine preparation. The invention is a method of founding HPLC finger print to detect alcohol extracted thing in summer mulberry chrysanthemum preparation. The method include;(a) preparation of control article solution;(b) preparation of examining solution; (c) chromatographic condition; chromatographic column take octadecyl silane bonded silica gel as padding;adopt gradient elution, mobile phase is gradient elution liquid composed by 0.1 to 3 percent acetic acid and methanol; column temperature;25 to 50 deg; ultraviolet detecting wavelength is 285 to 295 nm;velocity of flow;0.5 to 1.5 ml per min; time;30 to 80 min;(d) measuring; high efficiency liquid chromatography to gain finger print. This invention can validly characterize quality of summer mulberry chrysanthemum preparation, in favor of monitoring product quality; possess merit of method handiness, stable, high degree of precision, good reappearance; the invention can quickly and exactly discriminate the product.
Owner:GUANGZHOU XINGQUN PHARMA
Detection method of brexpiprazole-related substance(s)
ActiveCN109307716AThe detection method is simple and fastStrong specificityComponent separationPhosphateSilanes
The present invention provides a detection method of brexpiprazole-related substance(s). The detection of the related substances is performed via a high performance liquid chromatography method. The determination condition of the liquid chromatography is: an octadecyl silane bonded silica gel is taken as a filler of a chromatographic column, a mobile phase A is acetonitrile, a mobile phase B is phosphate buffer-acetonitrile, and the detection is performed by adopting gradient elution. The method has the advantages of specificity, durability, good reproducibility and high sensitivity, and the method is suitable for the qualitative and quantitative detection of the brexpiprazole-related substance(s).
Owner:四川弘远药业有限公司
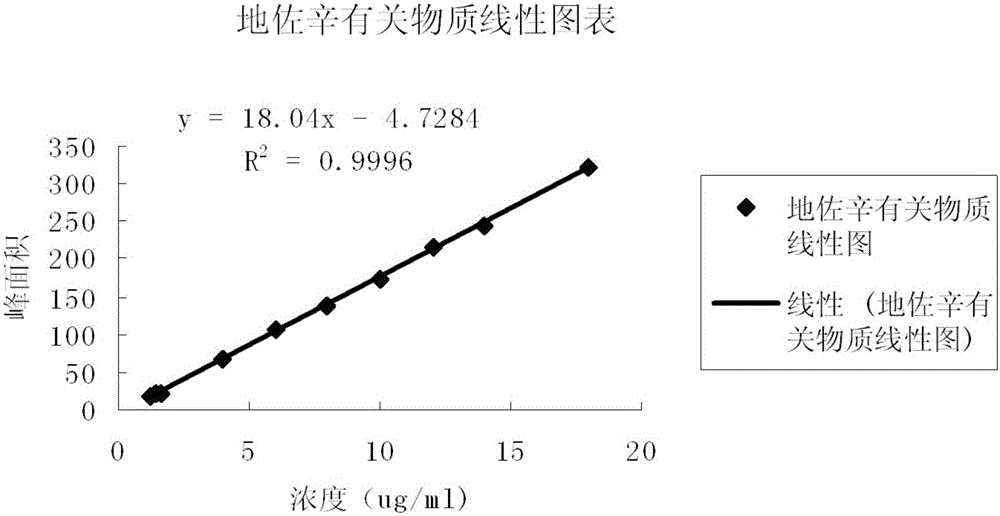
Detection method for dezocine related substances
InactiveCN106018584AIncrease concentrationImprove elutionComponent separationO-Phosphoric AcidSilica gel
The invention provides a detection method for dezocine related substances. Octadecyl silane bonded silica gel is adopted as a chromatographic column of a filling agent, and an acetonitrile-triethylamine solution is adopted as a mobile phase, wherein the acetonitrile-triethylamine solution is obtained by taking 1000 ml of water, adding 5 ml of triethylamine and 2 ml of a tetrabutyl ammonium hydroxide water solution, and adjusting pH to 3.2 through diluted phosphoric acid (1-5), and the ratio of acetonitrile to triethylamine is 200:800; the flow rate is 0.6 ml per min, and the detection wavelength is 281 nanometers. According to the detection method for dezocine related substances, the concentration of buffer salts is increased, elution is enhanced, an ion-pairing agent is added, the peak shape is improved, and the problems that due to the fact that the number of theoretical plates of an original method is small and a main peak cannot be rapidly eluted, the peak width is large and trailing is serious are solved.
Owner:JINAN LIMIN PHARMA
Quality control method of Dayuan decoction composition
InactiveCN106483214AAdvantages of quality control methodsEffective controlComponent separationSilanesPhosphoric acid
The invention discloses a quality control method of a Dayuan decoction composition, and belongs to the field of traditional Chinese medicine analysis. The method comprises the steps that high performance liquid chromatography is adopted for establishing a Dayuan decoction composition fingerprint spectrum, and according to the chromatographic conditions, an octadecyl silane bonded silica gel chromatographic column is adopted as a chromatographic column, the flow speed is 0.8 ml / min, the column temperature ranges from 25 DEG C to 35 DEG C, an ultraviolet-visible light detector is adopted as a detecting instrument, and the detecting wavelength of the fingerprint spectrum ranges from 215 nm to 240 nm; the number of theoretical plates is calculated according to a reference substance baicalin peak and should be not smaller than 3,000; a reference substance solution is a methanol solution of baicalin; a mobile phase is subjected to gradient eluting with an acetonitrile-0.1% phosphoric acid solution as a system according to the following eluting sequence, and the diagram is defined in the specification. According to the method, quality information of the Dayuan decoction composition is comprehensively reflected, and therefore the product quality can be controlled more comprehensively and effectively.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA
Method for analyzing 6-ethylchenodeoxycholic acid and synthetic intermediate thereof by high-performance liquid chromatography
ActiveCN107917972AHigh detection sensitivityGuaranteed stabilityComponent separationSilanesSilica gel
The invention belongs to the technical field of pharmaceutical analysis and relates to a method for analyzing 6-ethylchenodeoxycholic acid and a synthetic intermediate thereof by high-performance liquid chromatography. The separation and detection method provided by the invention adopts an octadecyl silane bonded silica gel packed column with the chromatographic column temperature of 35-40 DEG C and a differential detector, and a phosphate buffer solution and an acetonitrile and methanol mixed solution are used for elution. The method provided by the invention realizes simple, quick and accurate separation and detection of the 6-ethylchenodeoxycholic acid and the synthetic intermediate thereof, and solves the problem of separation and detection of 6-ethylchenodeoxycholic acid-containing raw materials and preparations, thereby ensuring the quality controllability of the 6-ethylchenodeoxycholic acid and the 6-ethylchenodeoxycholic acid-containing compositions or preparations.
Owner:JIANGSU SKYRUN PHARMA CO LTD
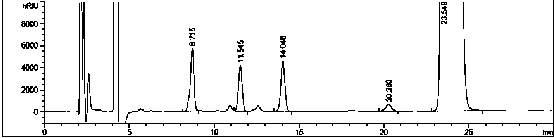
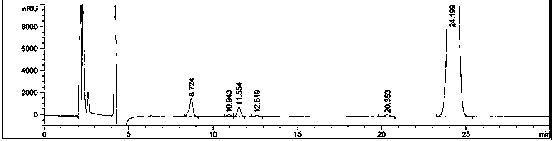
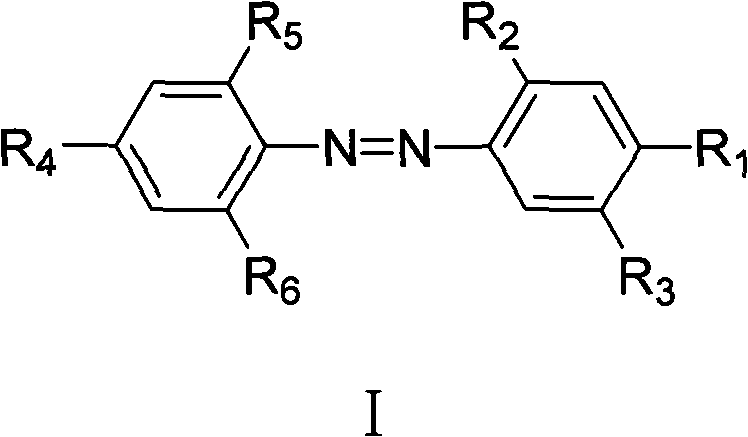
Azo allergenic disperse dye, and purifying method and application thereof
InactiveCN102020873AHigh purityLarge amount of pure productIon-exchange process apparatusIon-exchanger regenerationDisperse dyeUltraviolet detectors
The invention discloses a purifying method of an azo allergenic disperse dye shown as the formula I in the specification. The method comprises the following steps of: dissolving a commodity dye shown as the formula I into a polar solvent and purifying a compound I by a preparative high performance liquid chromatography, wherein the conditions of the preparative high performance liquid chromatography are that: gradient elution is performed by using an octadecyl silane bonded silica gel chromatographic column as a chromatographic column, and methanol and water as mobile phases, wherein the start gradient is the ratio of methanol to water of 20:80-40:60; the final gradient is the ratio of methanol to water of 95:5; the time of the gradient elution is over 7 minutes; the flow rate is 5 to 30ml / min; and the detection wavelength of an ultraviolet detector is 210 to 450nm. The invention also discloses the azo allergenic disperse dye produced by the purifying method, and application of the azo allergenic disperse dye serving as a standard sample for analysis. The purity of the product obtained by the purifying method reaches 97.6 to 99.8 percent.
Owner:SHANGHAI INST OF MEASUREMENT & TESTING TECH
Measuring method of content of butyric acid clevidipine butyrate and content of related substances
InactiveCN103134891ALow equipment requirementsCommonly available mediaComponent separationClevidipineWavelength
Disclosed is a measuring method of the content of butyric acid clevidipine butyrate and the content of related substances. A high performance liquid chromatography is adopted, and octadecyl silane bonded silica gel is used as a reversed phase column of filler; ultraviolet detection is adopted, and detection wavelength is 230-250 nm; and a mobile phase A is acetonitrile, a mobile phase B is water, and the volume of the phase A accounts for 50%-90% of that of the mobile phases.
Owner:TIANJIN JINYAO GRP
Method for determining procaine hydrochloride content in gentamycin procaine vitamin B12 particle by high performance liquid chromatography
InactiveCN102226790AThe measurement result is accurateAccurate and reliable measurement resultsComponent separationInjection volumeSilanes
The invention relates to a method for determining the procaine hydrochloride content in a gentamycin procaine vitamin B12 particle by high performance liquid chromatography, and the chromatographic conditions adopted by the method include that: an octadecyl silane bonded silica gel column is employed as a chromatographic column; a mobile phase is prepared by water, acetonitrile and triethylamine with a volume ratio of 85:15:0.02 and is adjusted by glacial acetic acid to obtain a pH of 3.9; a column temperature is 35 DEG C; a detection wavelength is 292 nm; a flow speed is 1.0 ml / min; and an injection volume is 20 microliter. The determination result of the procaine hydrochloride content in a gentamycin procaine vitamin B12 particle obtained by the method of the invention is accurate and reliable, and the method is simple and scientific. The test time is short; no interference peak appears; the theoretical plate number is up to above 2000; the resolution, the tailing factor, and the asymmetry degree all meet the requirements of Chinese pharmacopoeia; and the detection results is good.
Owner:NINGBO SHUANGWEI PHARMA
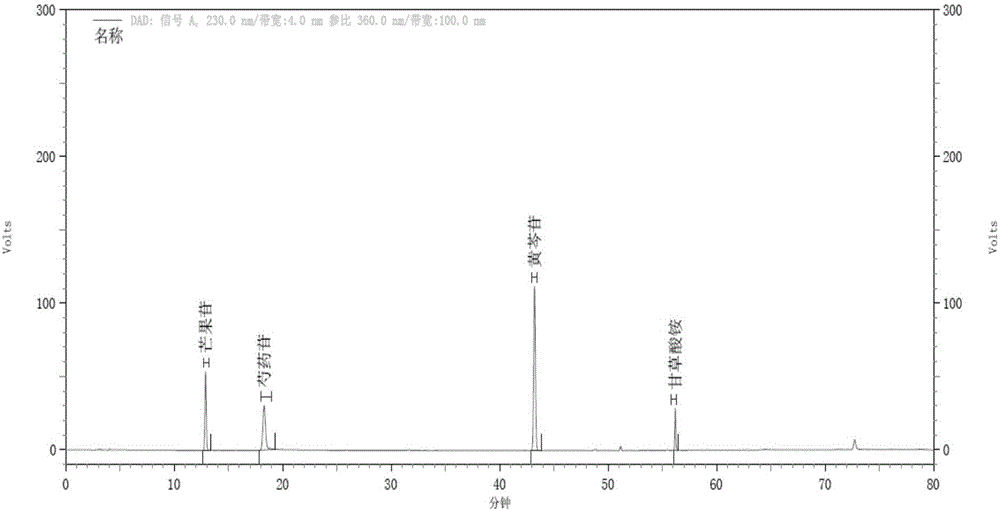
Method for testing effective ingredients of Gegen Qinlian medicine
InactiveCN101278991AHigh precisionReduce ingredient variationAntibacterial agentsAntipyreticAdditive ingredientPuerarin
The invention provides a method to detect effective ingredients of radices puerarire, radices scutellariae and coptis chinensis medicine (Gegen Qin Lian Medicine) by high efficiency liquid chromatogrophy. The method consists of the following steps: firstly, a sampling liquid to be detected and a mixing control solution are prepared, which are then respectively detected by the following methods: a chromatographic column stuffed by octadecyl silane bonded silica gel is injected by the sample size of 1 to 20ul, the column temperature is controlled between 10 to 40 DEG C, then gradient elution is carried out by a mobile phase which is composed of phase A and phase B at a flow velocity of 0.3 to 1.5ml / min according to the stipulated procedure; moreover, multi-wavelength ultraviolet is employed to detect the eluent so as to obtain the high efficiency liquid chromatograms of the detected medicines and the control solution; finally, the high efficiency liquid chromatograms of the detected medicines and the control solution are compared so as to determine the effective components of puerarin, daidzin, wogonoside, baicalin, palmatine and berberine and the contents thereof. The phase A in the method of the invention is ammonium acetate solution with the concentration of 0.01 to 0.1mol / l, and the phase B is acetonitrile.
Owner:SOUTHERN MEDICAL UNIVERSITY
Quality detection method for fingerprint spectrum method of radix astragali saponin injection
ActiveCN1844913AGood reproducibilitySimple and fast operationComponent separationAstragalosideForeign matter
The invention discloses a method for detecting the fingerprint drawing quality of astragalus root saponin injection. Said method uses octadecyl silane bonded silica gel as stuff; uses acetonitrile monohydrate as liquid, to process grad removing; the flow speed is 0.5-1.0mol / min, and the post temperature is 20-40Deg. C; getting some astragalus root saponin as comparison to be diluted with methanol as the comparison solution; concentrating some astragalus root saponin injection, using water saturated butanol as extraction solvent, using the water of saturated butanol to remove foreign matters; after the extracted matter of butanol solution is volatilized, adding methanol into the left matter to dilute, and using the methanol solution as the astragalus root saponin injection fingerprint test solution; using high-efficiency liquid spectrum to test the astragalus root saponin injection fingerprint test solution, while it has better similarity, that higher than 0.90.
Owner:SICHUAN KELUN PHARMA CO LTD
Method for measuring related substances of azithromycin capsule by high performance liquid chromatography
ActiveCN109870528AQuality improvementEasy to separateComponent separationAzithromycinFiller Excipient
The invention provides a method for measuring the related substances of an azithromycin capsule by high performance liquid chromatography. The method adopts octadecyl silane bonded silica gel as a chromatographic column of filler, and a mobile phase A is characterized in that the ratio of 0.05mol / L of dipotassium phosphate to acetonitrile is 97-99:1-3, wherein the pH value of the 0.05mol / L of dipotassium phosphate is regulated to 8.2 by 20% phosphoric acid solution; and a mobile phase B is acetonitrile, a column temperature is 20-35DEG C, a detection wavelength is 205-215nm, and a flow rate is0.8-1.1mL / min. Since a gradient elution isochromatic spectrum condition is adopted for detection, the method has the advantages of good specificity, high analysis speed and high reproducibility and can be used for accurately and sensitively detecting 15 known impurities and other unknown impurities of the azithromycin capsule, and a related substance chromatography condition is obviously superiorto domestic and oversea pharmacopeia methods.
Owner:BEIJING YUEKANGKECHUANG PHARM TECH CO LTD
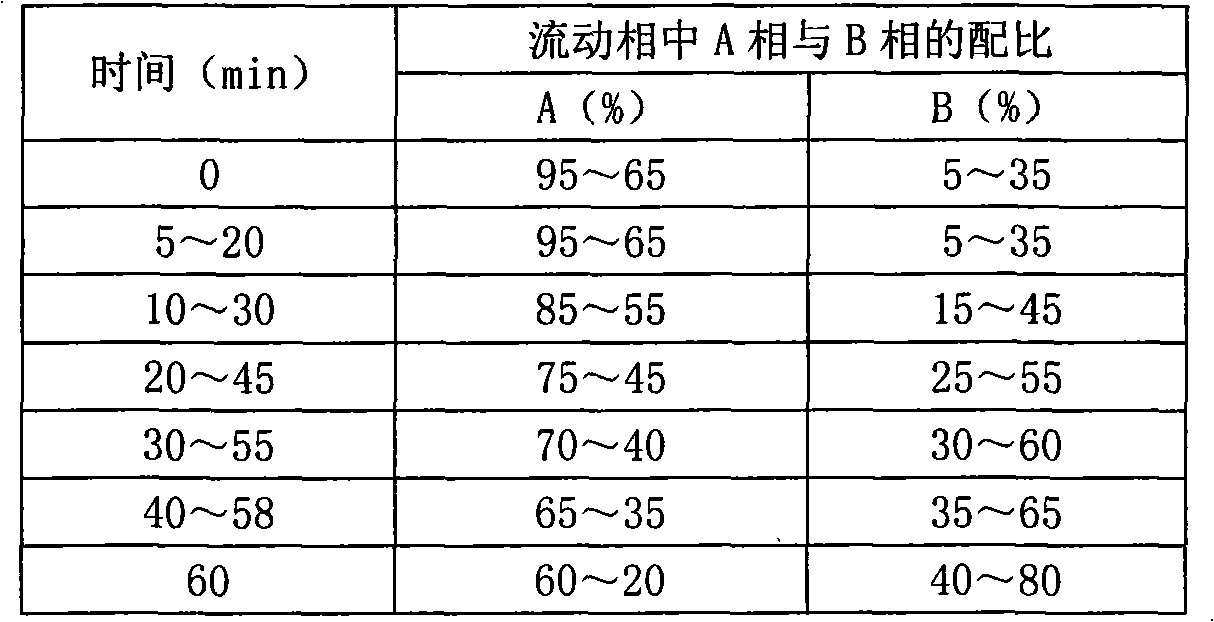
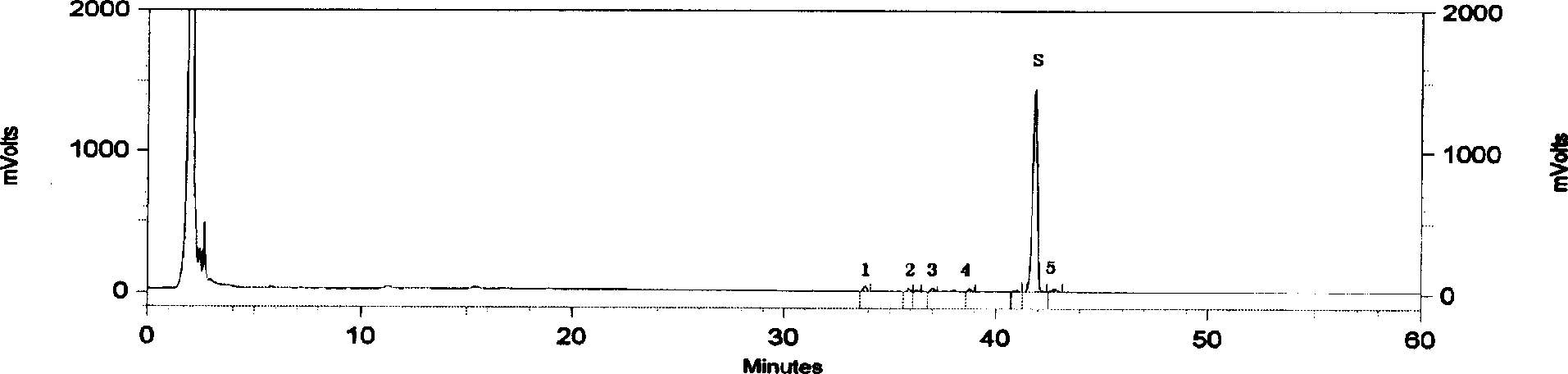
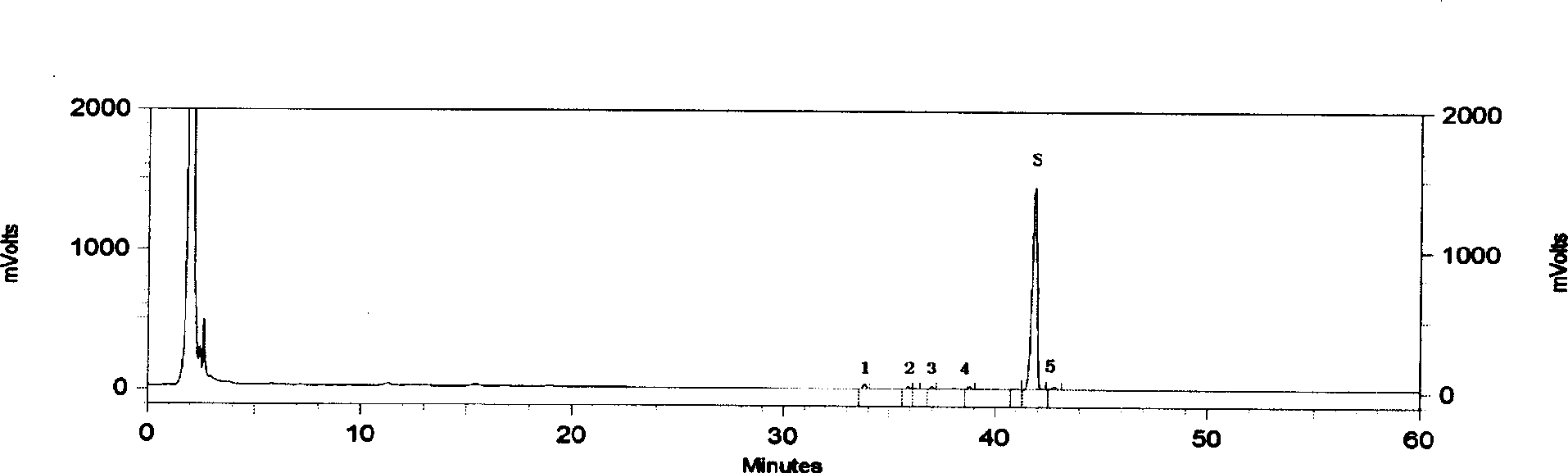
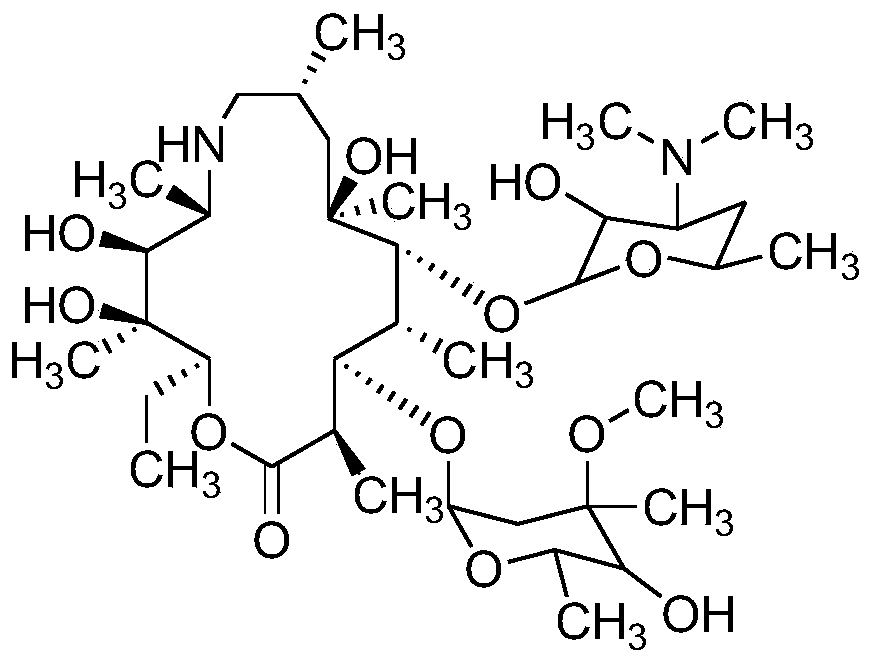
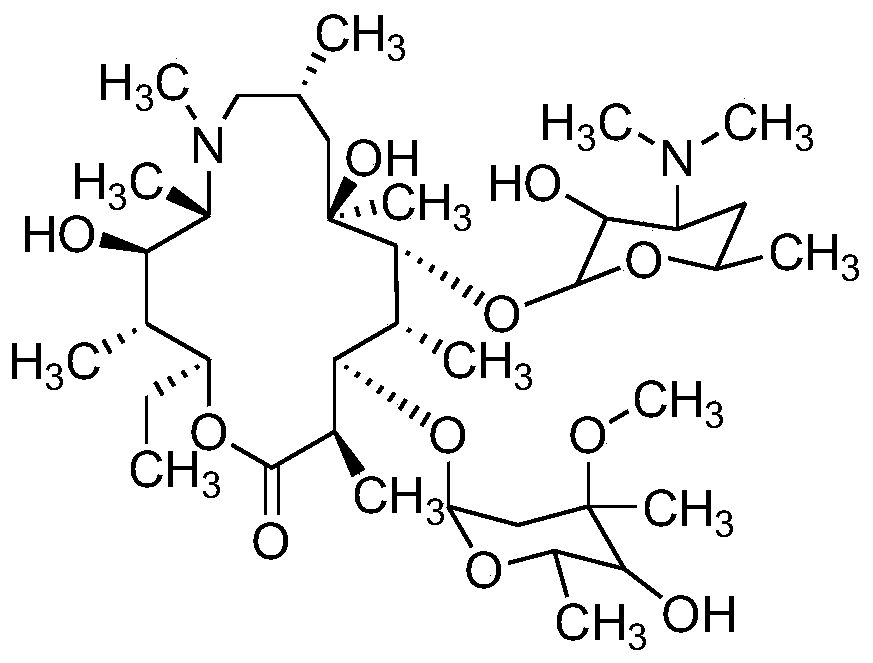
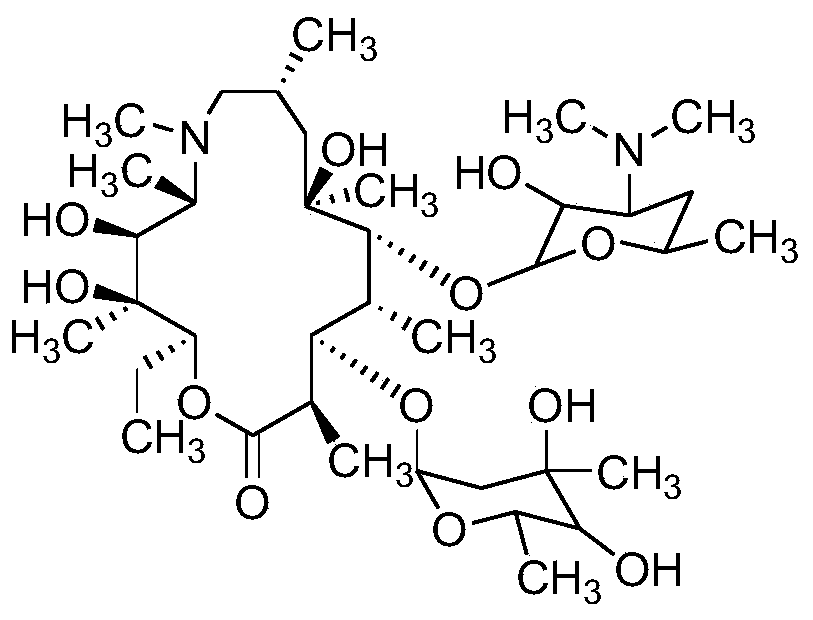
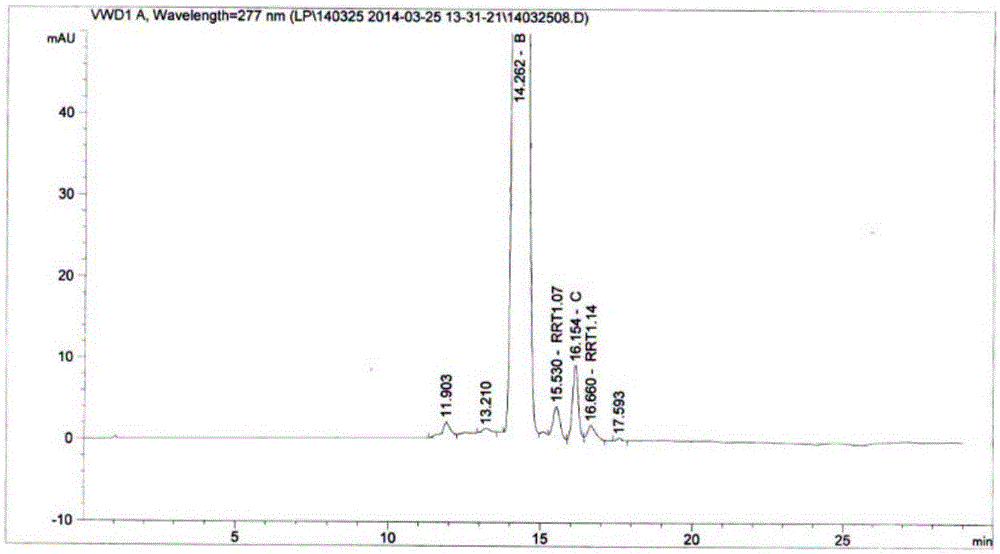
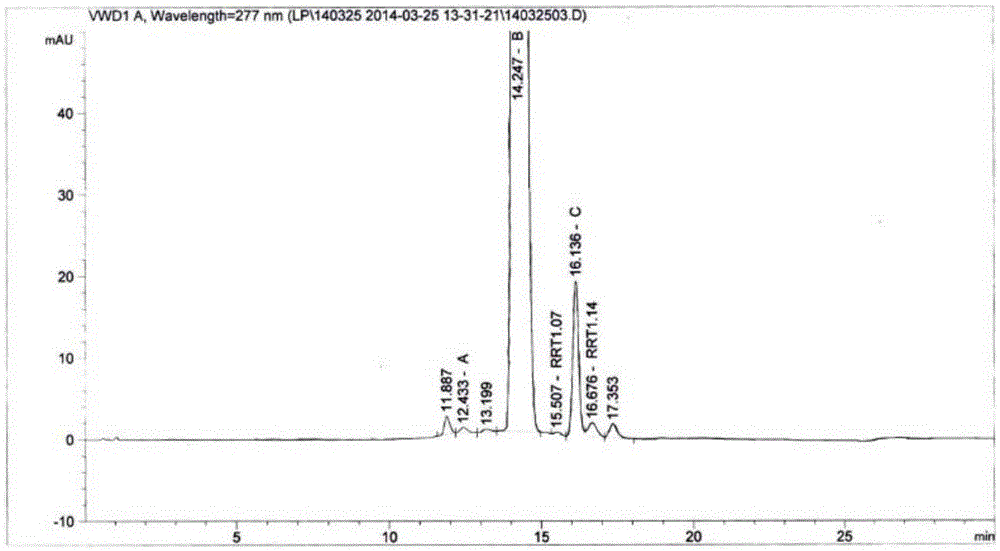
High performance liquid chromatography analysis method of sirolimus
ActiveCN105301159AImprove stabilityEfficient separationComponent separationInjection volumeMass ratio
The invention provides a high performance liquid chromatography analysis method of sirolimus. A sample solution containing the sirolimus is led into a chromatographic column, quantitative analysis is performed by adopting a reverse liquid chromatography external-reference percentage method, and the liquid chromatography conditions are as follows: the chromatographic column uses octadecyl silane bonded silica gel as a filling material, and the specifications are 4.6 mm * 150 mm and 3 microns; a mobile phase is the mixed solution of a mobile phase A and a mobile phase B with the volume ratio of 18:82-22:78 and is used for isocratic elution, the mass ratio of the phosphoric acid to sodium heptanesulfonate to water in the mobile phase A is 0.1:0.01:1, and the volume ratio of methanol to acetonitrile in the mobile phase B is 15:85; the detection wavelength is 270-280 nm; the flow velocity is 1.5-2.0 ml / min; the temperature of the column is 25-35 DEG C; the sample injection volume is 15-30 microliters. The high performance liquid chromatography analysis method has the advantages of being practical, reliable, better in stability and good in data reproducibility and can separate out impurities RRT1.07, C and RRT1.14.
Owner:WUXI FORTUNE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com