Detection method of brexpiprazole-related substance(s)
A detection method, the technology of ebiprazole, applied in the field of high performance liquid chromatography analysis, can solve the problems of fast ebiprazole peak time and inability to separate ebiprazole, and achieve simple and convenient detection method, high sensitivity, good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] 1. Sample preparation
[0134] Impurity localization solution: appropriate amount of BPZ-impurity reference substance, put them in measuring bottles respectively, dissolve with methanol and dilute to the mark to get ready.
[0135] Table 6. BPZ-impurity concentration
[0136]
[0137] Sample solution: BPZ (151101) 11.32mg, put in a 50ml measuring bottle, dissolve with methanol and dilute to the mark.
[0138] Sample plus impurity control solution: Take the sample solution and add 50 μl of impurity localization solution.
[0139] Blank: Methanol
[0140] Note: BPZ-Impurity II preparation: dissolve and dilute to volume with dimethyl sulfoxide.
[0141] Preparation of BPZ-Impurity VII: Dissolve and dilute to volume with N,N dimethylformamide.
[0142] 2. Detection
[0143] Use octadecylsilane bonded silica gel as filler (5μm, 250*4.6mm); use acetonitrile as mobile phase A, pH value is 6.7, concentration is 0.02mol / L NaH 2 PO 4 Buffer-Acetonitrile (90:10) is the m...
Embodiment 2
[0151] 1. Sample preparation
[0152] With comparative example 1.
[0153] 2. Detection
[0154] Use octadecylsilane bonded silica gel as filler (5μm, 250*4.6mm); flow rate is 1.0ml per minute; column temperature is 35°C; detection wavelength is 254nm; phosphate is 0.02mol / L NaH 2 PO4 buffer, other conditions are as follows:
[0155] Table 8. Some HPLC chromatographic conditions
[0156]
[0157] Measure 50 μl of the solution and inject it into the liquid chromatograph for elution according to the following conditions:
[0158]
[0159]
[0160] 3. Results
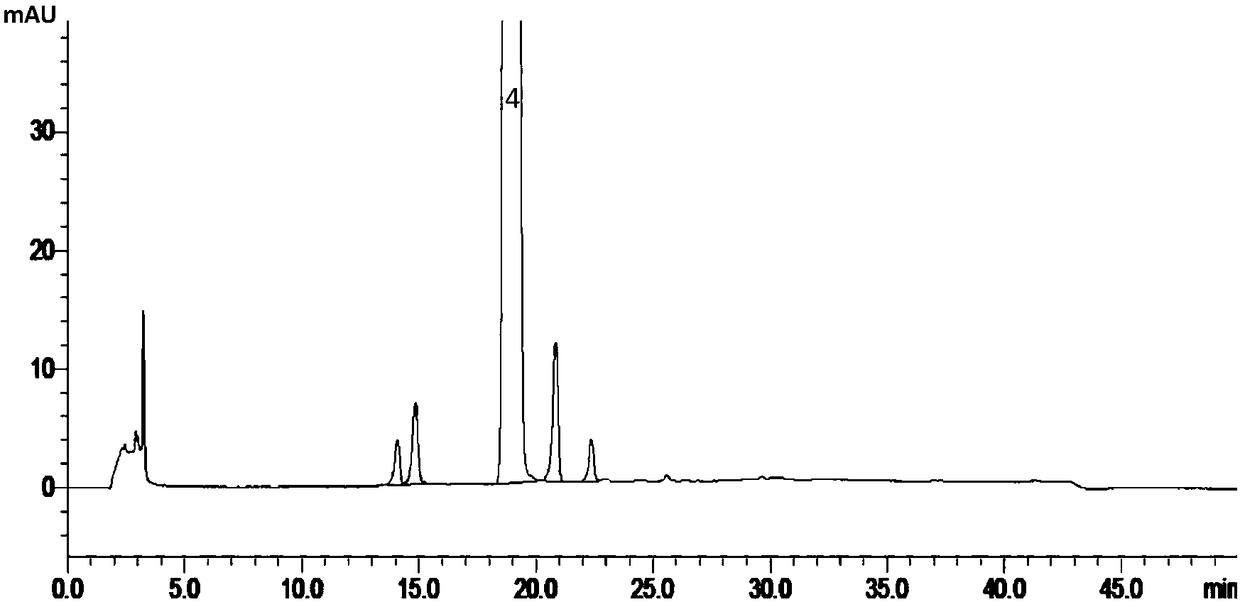
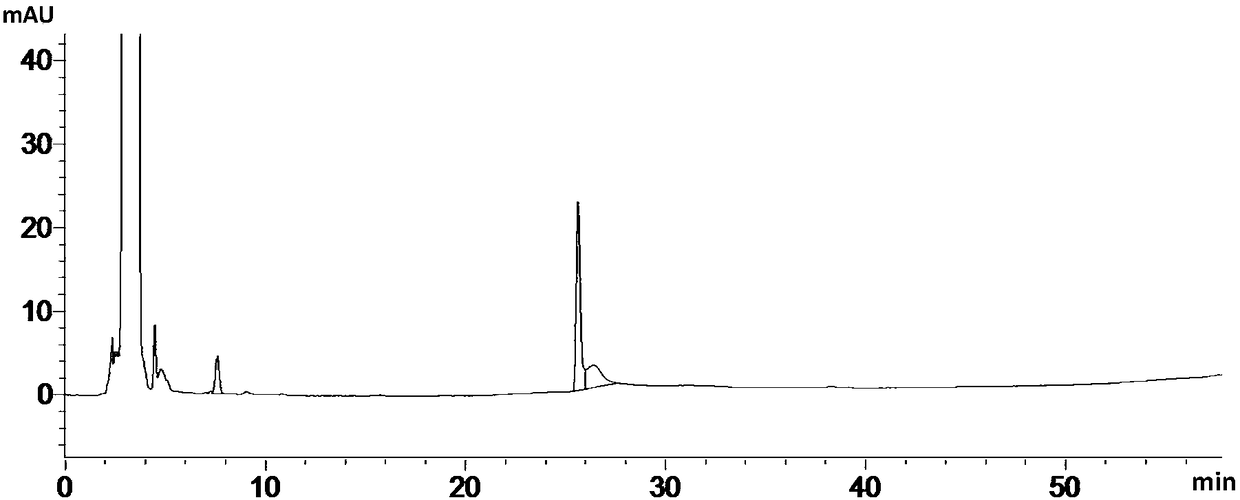
[0161] The results under the detection conditions of experiment numbers 1-4 are as follows Figure 14 , 15 , 13, and 16. In the pH range of the buffer solution from 6.5 to 6.9, the separation between ebiprazole and adjacent impurities, as well as between each impurity, is greater than 1.5; the separation between ebiprazole and adjacent impurity peaks decreases as the pH value decreases, When the pH value i...
Embodiment 3
[0163] 1. Sample preparation
[0164] With comparative example 1.
[0165] 2. Detection
[0166] Use octadecylsilane bonded silica gel as filler (5μm, 250*4.6mm); mobile phase A is acetonitrile, mobile phase B is 0.02mol / L NaH without or containing a certain proportion of acetonitrile 2 PO4 buffer; the flow rate is 1.0ml per minute; the column temperature is 35°C; the detection wavelength is 254nm; the ratio of acetonitrile is as follows:
[0167] Table 9. Some HPLC chromatographic conditions
[0168]
[0169] Measure 50 μl of the solution and inject it into the liquid chromatograph for elution according to the following conditions:
[0170] time min
Mobile phase A%
Mobile phase B%
0
25
75
55
70
30
85
70
30
85.01
25
75
90
25
75
[0171] 3. Results
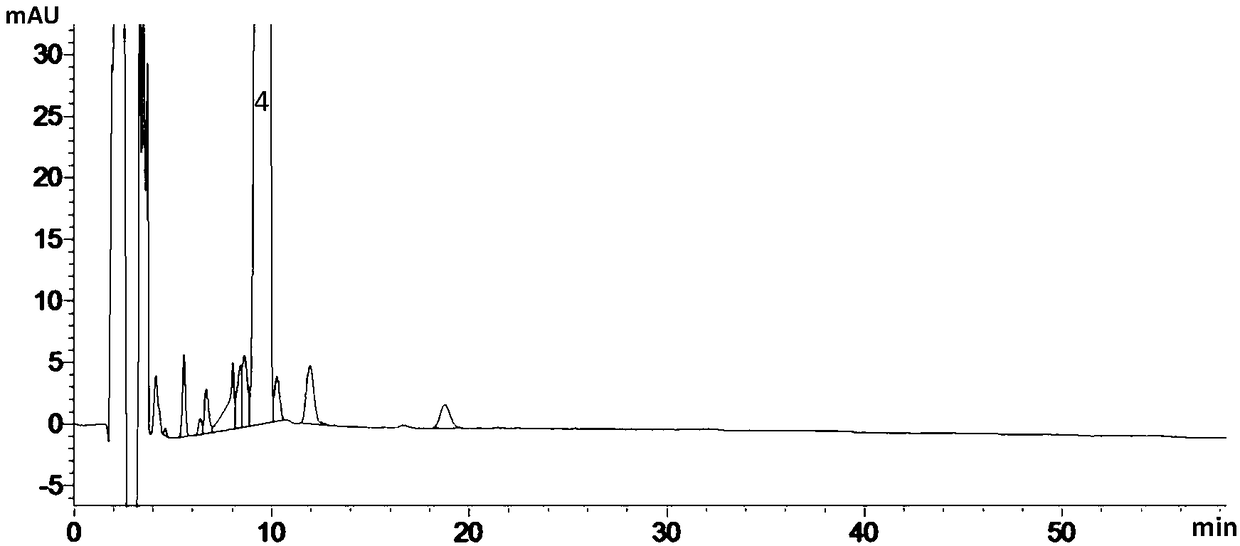
[0172] Carry out high-performance liquid chromatography detection according to above-mentioned condition, number is the experimental result u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com