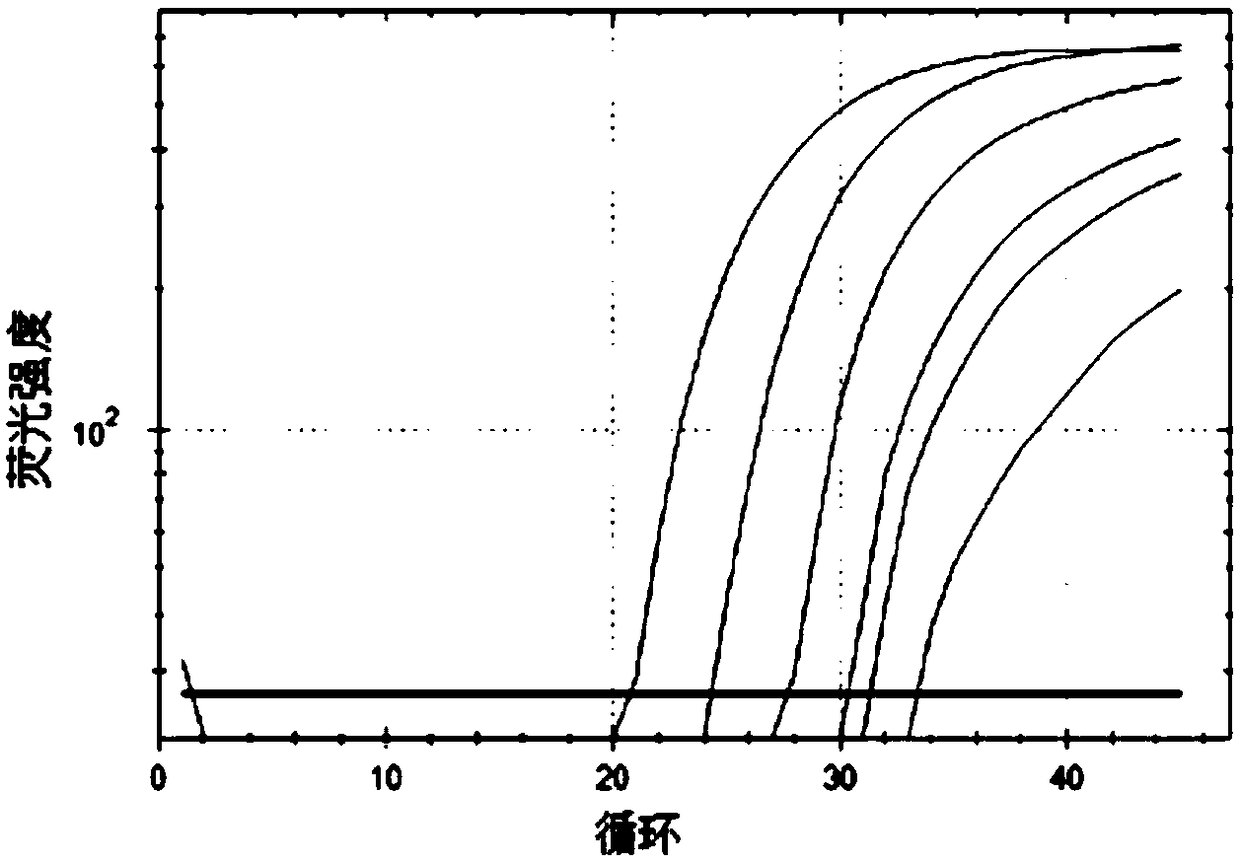
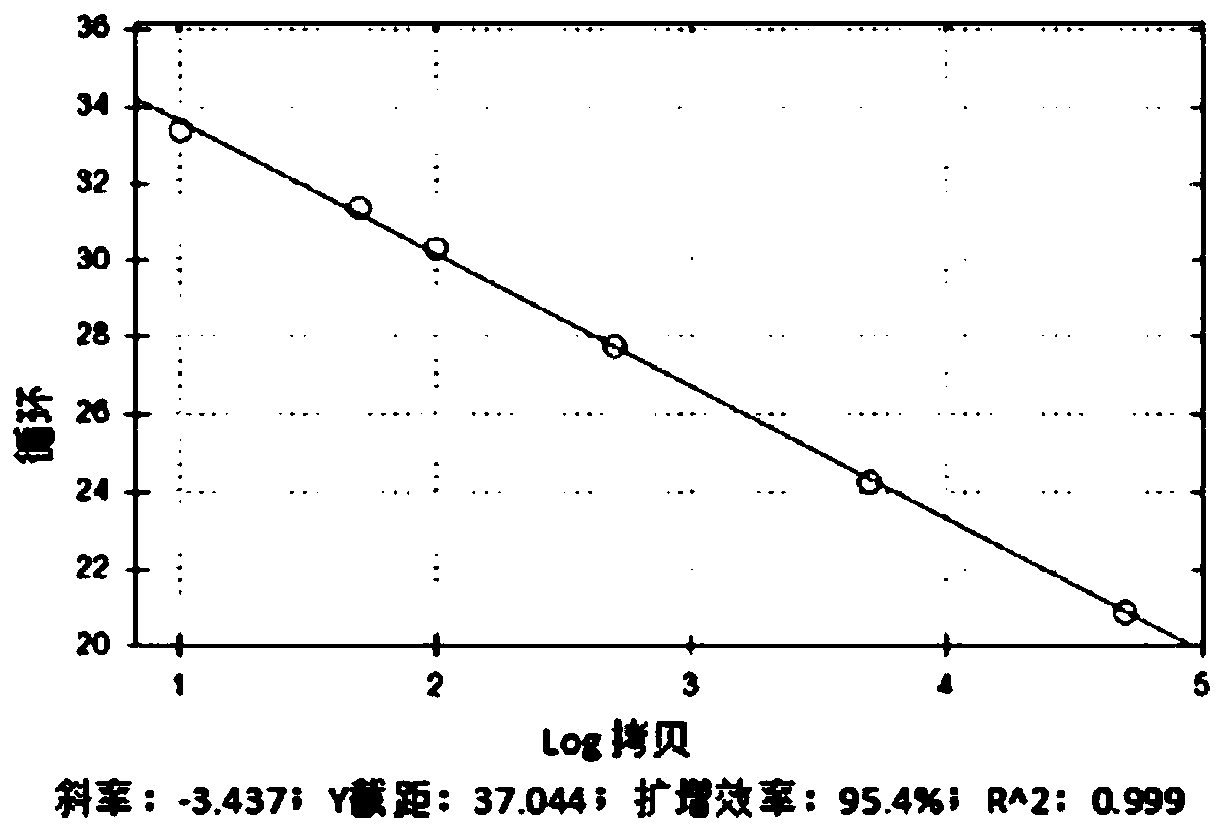
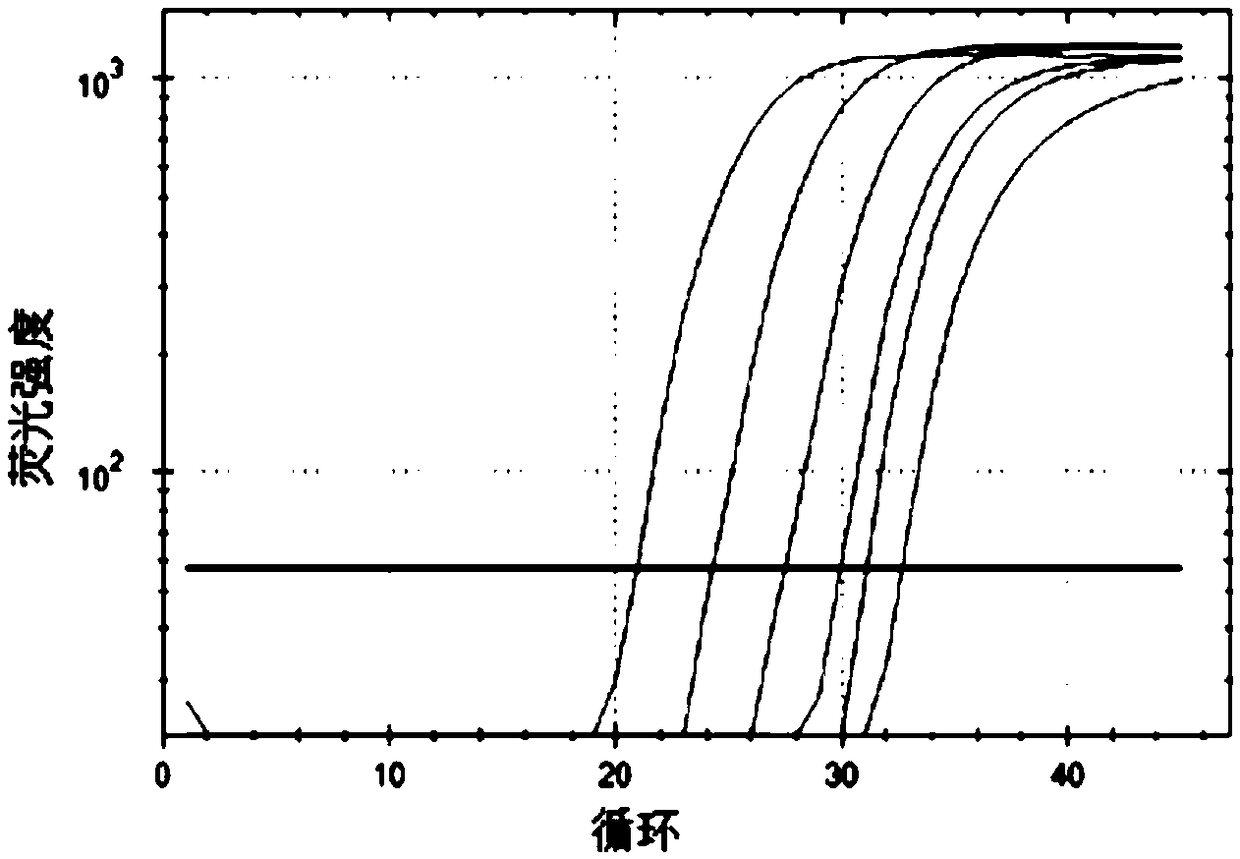
Real-time fluorescent quantitative PCR kit for detecting TRECs and KRECs genes, and application thereof
A technology of real-time fluorescence quantification and kits, which is applied in the determination/testing of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc. Advanced problems, to achieve accurate and reliable test results, reduce false positive results, and achieve high copy number effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the preparation of kit
[0049] 1. Design and synthesis of primers and probes
[0050] According to the UCSC website query TRECs, KRECs and TRAC gene sequence, use Primer 3.0 to design fluorescent quantitative PCR forward and reverse primers and probes on TRECs, KRECs and TRAC genes. The selected primers have high PCR amplification efficiency, and the selected probes have good specificity for gene binding. Both primers and probes were entrusted to Suzhou Jinweizhi Biotechnology Co., Ltd. for chemical synthesis, in which primers were purified by PAGE, and probes were purified by HPLC. The 5' end of the TRECs detection probe is labeled with a HEX fluorescent group, and the 3' end is a TAMRA quencher group; the 5' end of the KRECs detection probe is labeled with a HEX fluorescent group, and the 3' end is a TAMRA quencher group; The 5' end of the TRAC detection probe is labeled with a FAM fluorescent group, and the 3' end is a TAMRA quencher group. Primer s...
Embodiment 2
[0069] Example 2: Verification and use of the kit
[0070] 1. Collection of verification samples after kit development
[0071] The clinically confirmed positive SCID and XLA children's dry blood samples (donated by Beijing Obstetrics and Gynecology Hospital) were used as the positive control group for the kit verification, and the expected test results should be positive.
[0072] Dried blood samples (donated by Beijing Obstetrics and Gynecology Hospital) from children with no clinical phenotype and clinically judged to be normal were used as samples to be tested, and the expected test results should be negative. The dried blood samples of the positive control group and the samples to be tested were stored at 4°C. 2. Extraction of DNA from dried blood samples
[0073] The operation steps are as follows:
[0074] A. Use a clean puncher to take a dried blood piece with a diameter of 3mm, put it into a 1.5ml EP tube, add 100μl 0.5% (V / V) Triton X-100, shake at 800rpm for 10mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gene copy number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com