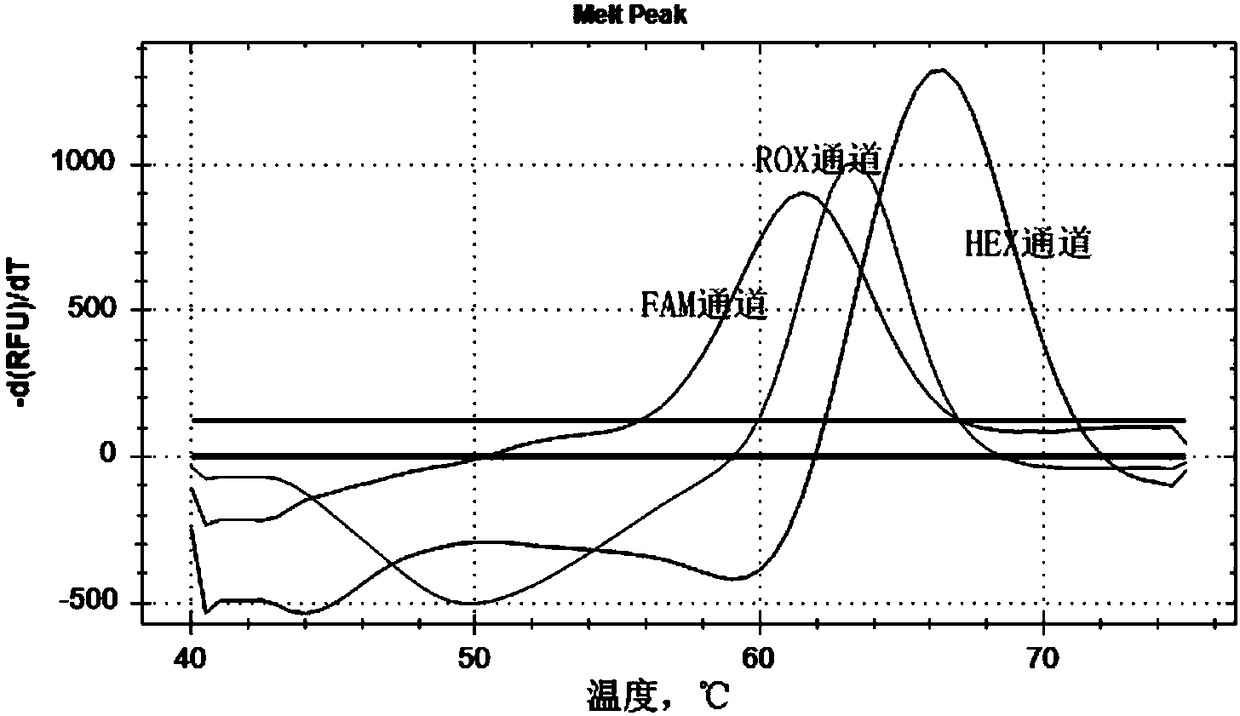
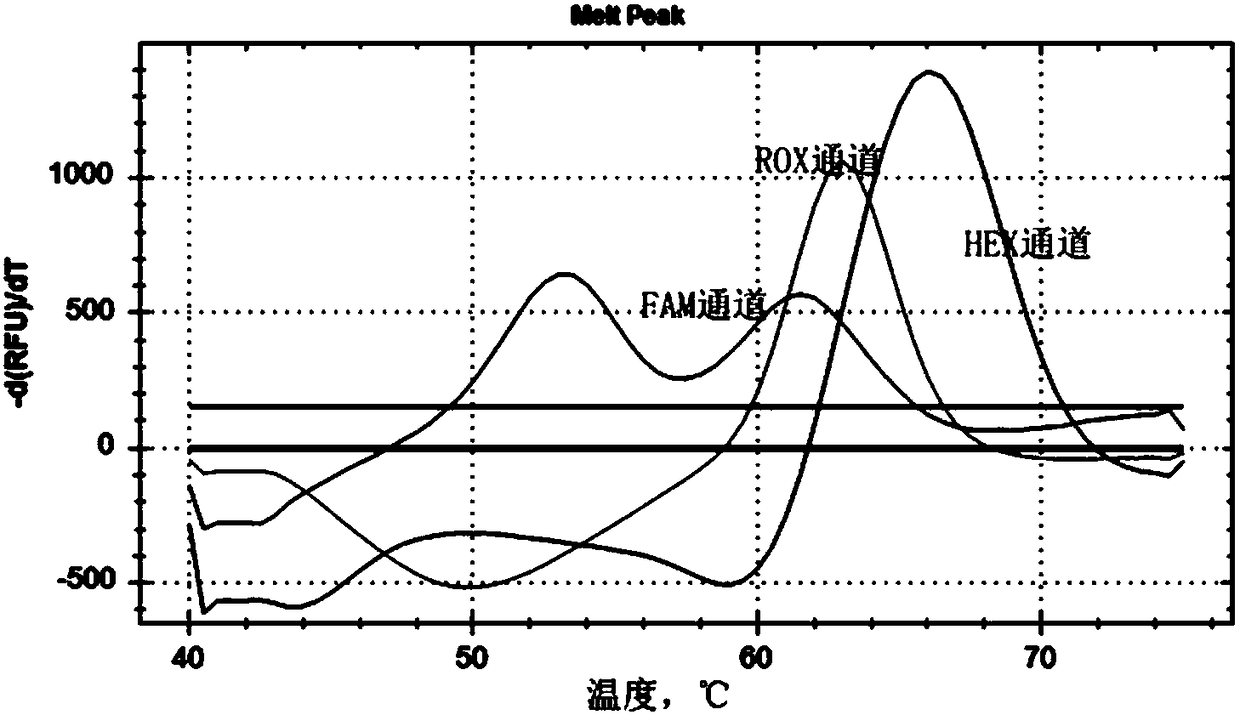
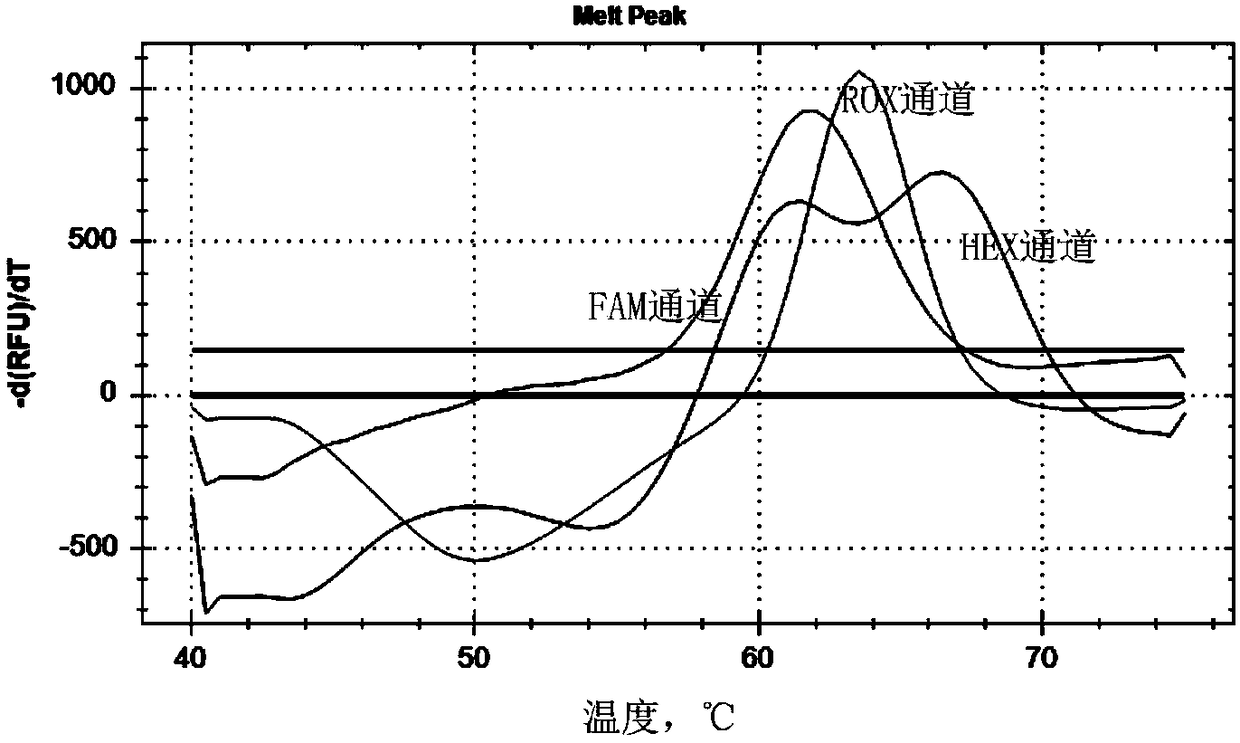
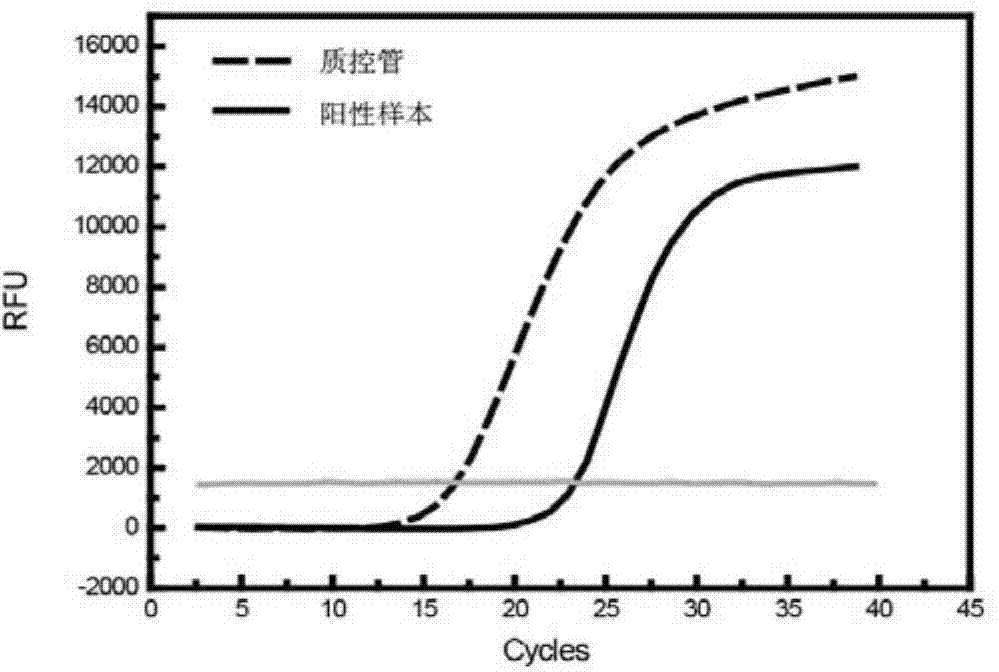
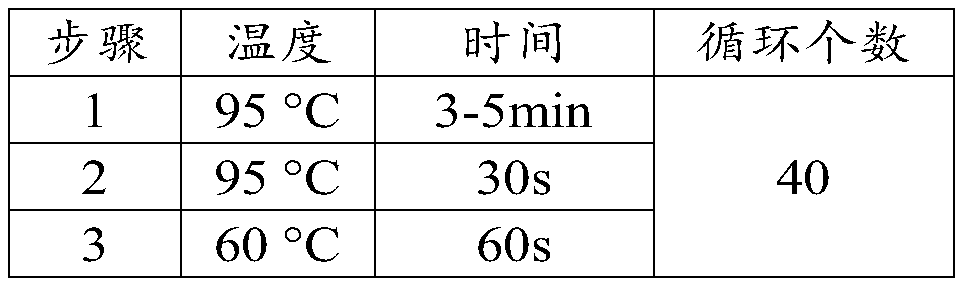
Patents
Literature
43results about How to "Reduce false negative results" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Primer probe composition and kit for detecting polymorphism of human CYP2C19 gene and application
ActiveCN108486231AEasy to readHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceMutation type
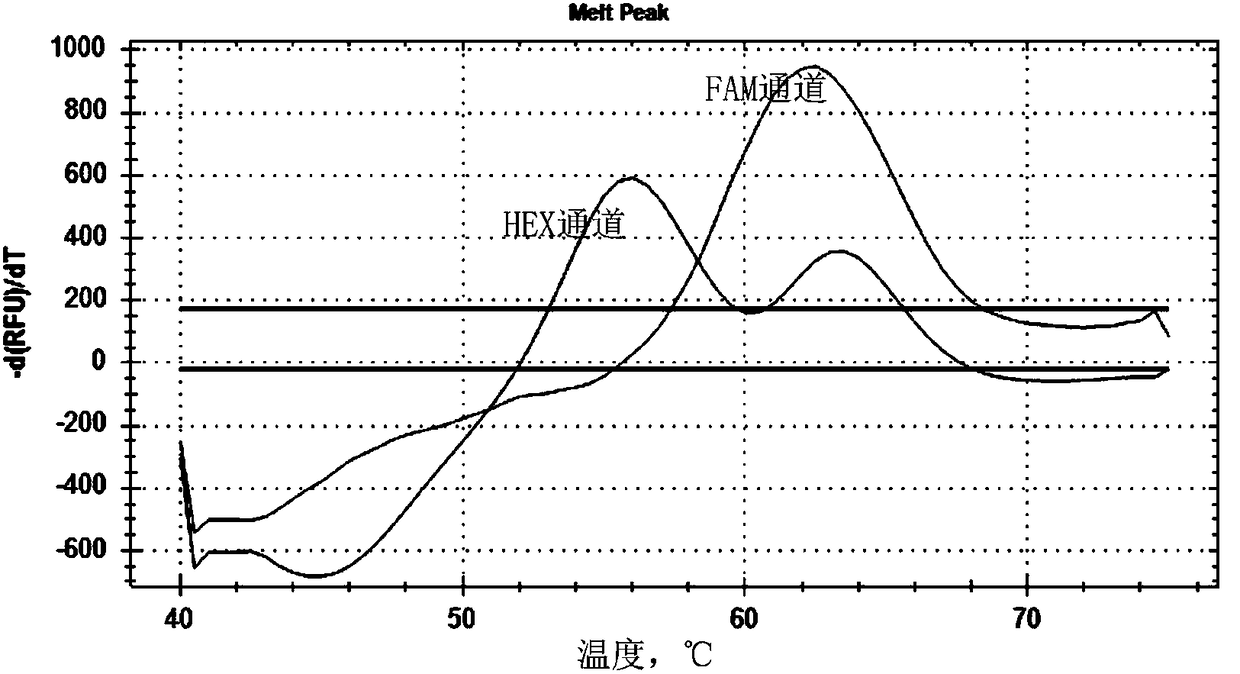
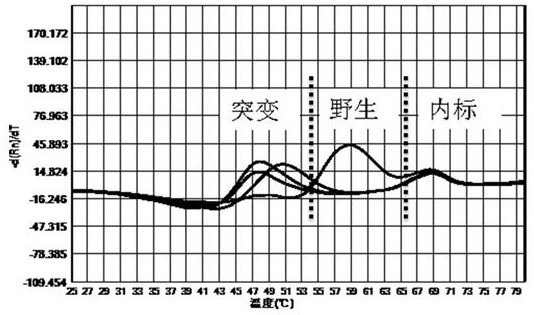
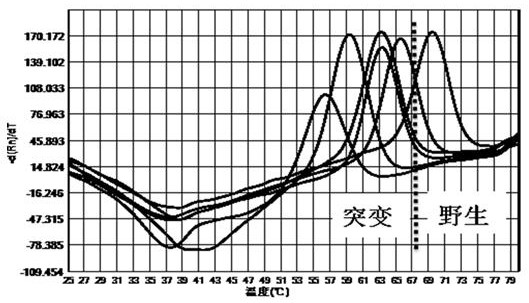
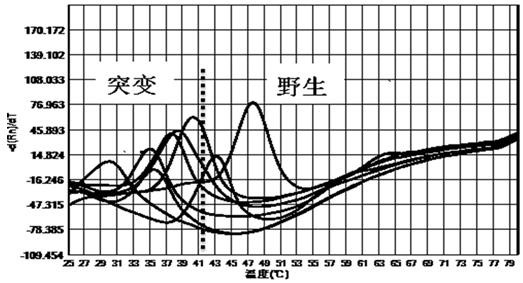
The invention discloses a primer probe composition and kit for detecting the polymorphism of a human CYP2C19 gene and application. The primer probe composition comprises three pairs of specific primers for amplifying CYP2C19*2, CYP2C19*3 and CYP2C19*17 sites and three specific fluorescent probes. The primers and the probes have high sensitivity, strong specificity and strong anti-interference capability; the polymorphism of the gene is detected by adopting a manner combining asymmetric PCR (Polymerase Chain Reaction) amplification and a fluorescent probe melting curve analysis technology; different gene types can be effectively distinguished according to the quantity of melting peaks and Tm value and result interpretation is convenient, clear and objective. Single-tube sampling can be usedfor detecting 6 mutation types of 3 gene sites at the same time; the operation is simple and convenient and the detection efficiency is improved; a large batch of samples can be detected and clinicaloperation is facilitated.
Owner:SHANDONG VIGENE BIOSCI
Primers, probes and kit for detecting human EGFR gene mutations
ActiveCN104513864AHigh sensitivityReduce false negative resultsMicrobiological testing/measurementDNA/RNA fragmentationMutationHuman DNA sequencing
The invention belongs to the field of molecular biology and particularly relates to primers, probes and a kit for detecting human EGFR gene mutations. The invention provides 7 groups of primers and probes, which can accurately detect 29 types of common human EGFR gene mutations. The kit adopting the primers and the probes has high sensitivity, can detect 1% of micro mutation templates under the background of 20 ng of wild human genomes and reduces false-negative results. Besides, the kit is simple and convenient to operate, has high controllability, can be used for detecting mass samples and is favorable for clinical operation.
Owner:SHANDONG VIGENE BIOSCI
Primer and probe composition for detecting polymorphism of human CYP2C9 and VKORC1 genes, kit and application
ActiveCN108570498AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceMicrobiology
The invention discloses a primer and probe composition for detecting polymorphism of human CYP2C9 and VKORC1 genes, a kit and application. The primer and probe composition comprises three pairs of specific primers for amplifying CYP2C9*2, CYP2C9*3 and VKORC1 sites, and three specific fluorescent probes. The primers and the probes, disclosed by the invention, have high sensitivity, strong specificity and strong anti-interference capability; a manner combining an asymmetric PCR (Polymerase Chain Reaction) amplification and fluorescent probe melting curve analysis technologies is used for detecting the gene polymorphism; different gene types can be effectively distinguished according to the quantity and Tm value of a melting peak; a result is convenient, clear and objective to judge and read.Single-tube sampling can be used for simultaneously detecting 6 mutation types of 3 gene sites; the primer and probe composition is simple and convenient to operate and the detection efficiency is improved; a large batch of samples can be detected and clinical operation is facilitated.
Owner:SHANDONG VIGENE BIOSCI
Kit for detecting helicobacter pylori drug-resistant gene polymorphism by multiple fluorescent PCR melting curve method
ActiveCN111850154AShort detection timeImprove efficiencyMicrobiological testing/measurementMicroorganism based processesMultiplexLysis
The invention discloses a kit for detecting helicobacter pylori drug resistance gene polymorphism by a multiplex fluorescence PCR melting curve method. The helicobacter pylori drug resistance gene comprises the following three genes: a 23S rRNA gene, a 16S rRNA gene and a gyr A gene, the kit comprises a nucleic acid extraction reagent and a nucleic acid amplification reagent, the nucleic acid extraction reagent comprises superparamagnetic silicon oxide nano magnetic beads, a lysis solution, a washing solution and an eluent; the nucleic acid amplification reagent comprises a primer pair and a probe which respectively correspond to the helicobacter pylori drug resistance gene 23S rRNA gene, the helicobacter pylori 16S rRNA gene, the gyr A gene and an internal standard gene human housekeepinggene beta-globin. According to the invention, three drug-resistant sites and one internal standard gene can be simultaneously detected in a single tube, so that the detection flux of a sample is improved; meanwhile, interference between multiple pairs of primer probes in a detection system is improved, and the sensitivity and specificity of reagent detection are effectively improved.
Owner:SHANG OUTDO BIOTECH CO LTD
Single tube in situ nested polymerase chain reaction method and its use
InactiveCN1858219AEasy to operateOperation specificMicrobiological testing/measurementFermentationAgricultural scienceReaction tube
The present invention relates to single tube in-situ nested PCR method and its application in nucleotide detection. The reaction liquid contains both inside primer and outer primer, and the inner primer has mismatching with target gene sequence or one section of non-specific gene sequence in the 5' end. The inner primer and the original template have highest annealing temperature lower than that of the first round reaction, while the inner primer and the matched template have the melting temperature higher than the highest allowed annealing temperature of the outer primer. The first round PCR cycle annealing temperature is controlled for only the outer primer to be capable of annealing amplification, and the second round PCR cycle annealing temperature is raised for only the inner primer to be capable of annealing amplification. Between the first round and the second round, there are two or more transition stages with lower circular annealing temperature for the inner primer and the original template to form matching template to start the second round reaction.
Owner:徐定邦 +1
Microdroplet-type digital PCR detection kit for latent hepatitis B virus
PendingCN109777893AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesConserved sequenceHepatitis B virus
The invention provides a microdroplet-type digital PCR detection kit for a latent hepatitis B virus. The kit comprises digital PCR reaction liquid and a primer probe composition, and the primer probecomposition is selected from at least one of an S gene area primer probe composition and a C gene area primer probe composition. According to the kit, primer probes are respectively designed for S andC conserved sequences of the hepatitis B virus, a duplex double-gene digital PCR technology is adopted to synchronously detect HBV DNA in the same tube, and the operation is convenient and quick; thesensitivity and specificity of a detection method for the latent hepatitis B virus are improved, the probability that the false negative result is caused due to mutation is reduced as much as possible, and the detection efficiency can be further improved.
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV
Primer probe system, kit and method for detecting common pathogenic bacteria of severe pneumonia
PendingCN111088378ARealize dynamic monitoring functionReasonable probe designMicrobiological testing/measurementMicroorganism based processesCommon diseaseNucleotide sequenc
The invention relates to the field of molecular biology, in particular to a primer probe system, kit and method for detecting common pathogenic bacteria of severe pneumonia. Firstly, the invention discloses the primer probe system for detecting the common pathogenic bacteria of the severe pneumonia. The primer probe system for detecting the common pathogenic bacteria of the severe pneumonia comprises a nucleotide sequence group with nucleotide sequences shown in SEQ ID NO: 1-24. The invention further discloses the kit comprising the primer probe system and the method for detecting the pathogenic bacteria by using the primer probe system or the kit. The kit can quickly, efficiently and sensitively detect the common pathogenic bacteria in various biological samples of patients suffering fromthe severe pneumonia. The primer probe system, kit and method provide technical support for quick diagnosis of clinical infection and further help clinicians to achieve precise medical treatment on clinical patients.
Owner:MEI HOSPITAL UNIV OF CHINESE ACAD OF SCI
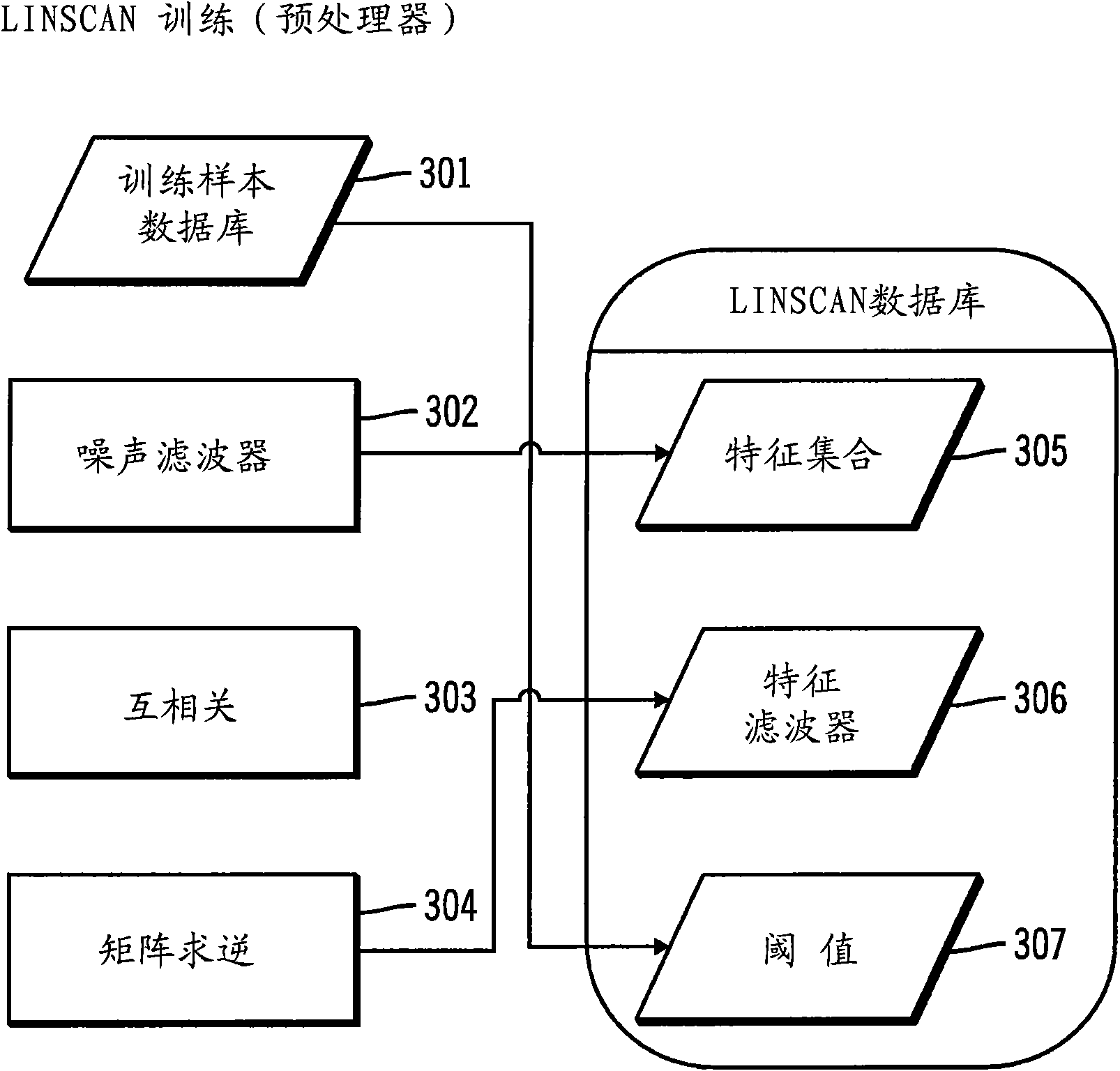
Advanced pattern recognition systems for spectral analysis
InactiveCN101632011AConvenient formalizationReduce false negative resultsSpectrum investigationColor/spectral properties measurementsRadioactive agentExplosive material
A process of rapid and highly accurate analysis of spectral data, includes both a linear scanning (LINSCAN) method and an advanced peak detection method for pattern recognition. One or both of the methods are used to support the detection and identification of chemical, biological, radiation, nuclear and explosive materials. The spectra of various targets can be analyzed by the two spectral analysis methods. These two methods can be combined for dual confirmation, greater accuracy, and to reduced false positives and false negatives, relative to what can be accomplished by either alone.
Owner:INNOVATIVE AMERICAN TECH +3
African swine fever virus triple fluorescent PCR detection kit and application thereof
PendingCN112375849AImprove accuracyReduce false negative resultsMicrobiological testing/measurementMicroorganism based processesClassical swine fever virus CSFVFluorescent pcr
The invention discloses an African swine fever virus triple fluorescent PCR detection kit as well as a preparation method and application thereof. The detection kit comprises a fluorescent PCR reaction solution, positive control, negative control, an exogenous gene standard solution and a negative extraction control solution, wherein the fluorescent PCR reaction solution is prepared from upstreamand downstream primers and probes of a VP72 gene, an exogenous gene and an endogenous gene. According to the detection kit, the endogenous gene serves as internal quality control in the whole detection process, and is used for monitoring the whole process of sample collection, extraction and amplification; and the exogenous gene serves as external positive quality control in a nucleic acid extraction process, is added into a sample nucleic acid extraction process to be subjected to nucleic acid extraction along with the sample, and the nucleic acid extraction effect of the nucleic acid extraction kit is monitored according to the change of a PCR reaction Ct value of the exogenous gene, so that the accuracy of a detection result is improved, and false negative outcome is reduced.
Owner:北京明日达科技发展有限责任公司
Novel coronavirus nucleic acid detection kit, preparation method and application
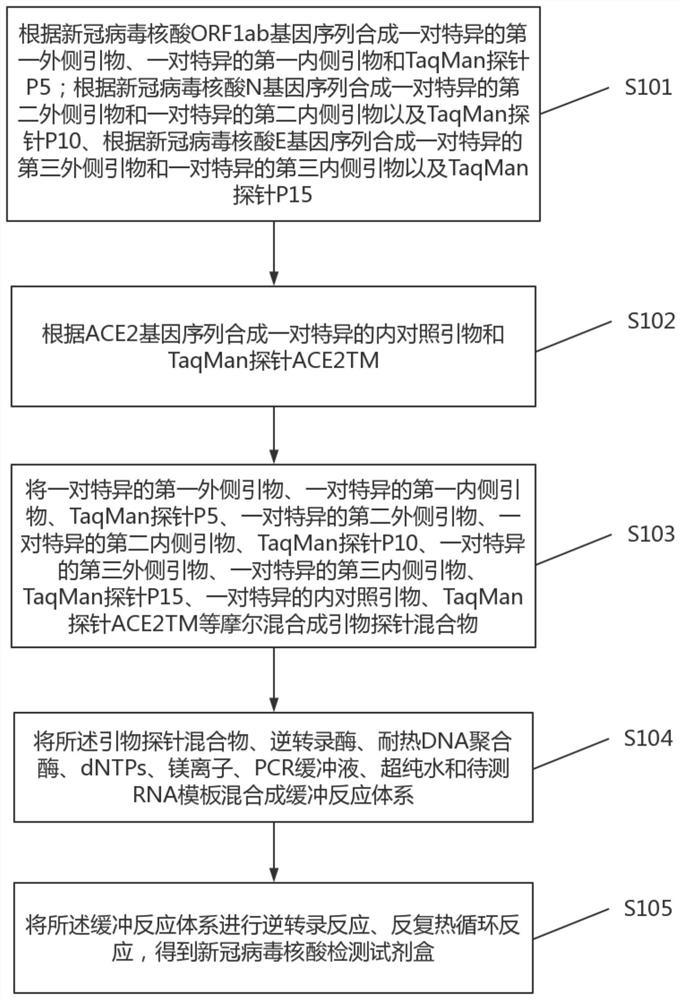
PendingCN111690772AImprove accuracyStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesMolecular biologyRNA
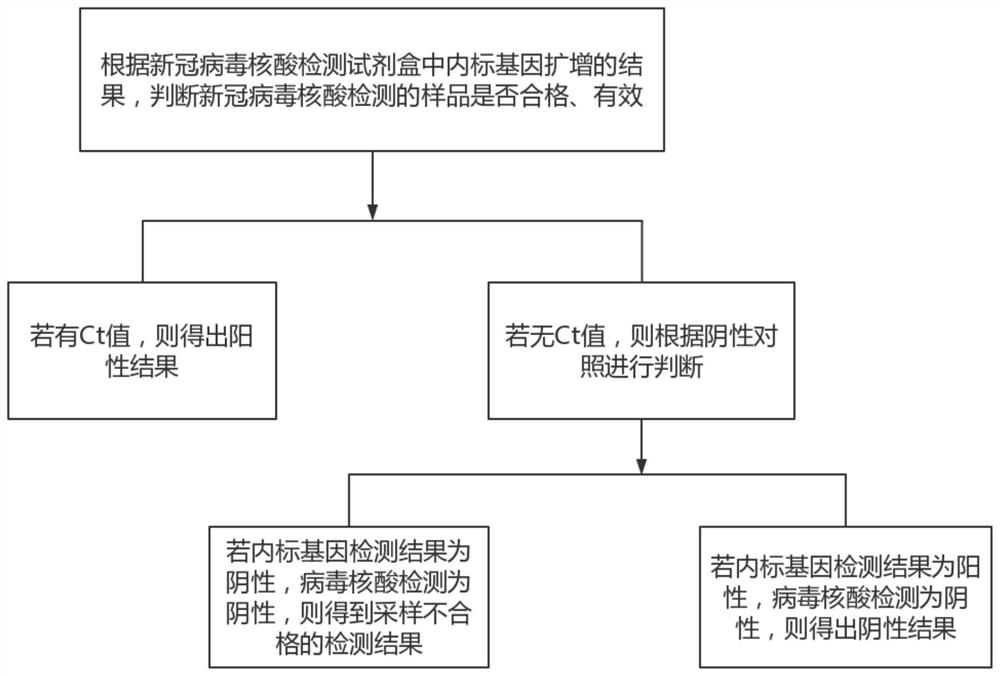
The invention discloses a novel coronavirus nucleic acid detection kit as well as a preparation method and application thereof. The novel coronavirus nucleic acid detection kit comprises primers and probes synthesized according to novel coronavirus nucleic acid ORF1ab gene, N gene and E gene sequences, a pair of specific internal control primers and TaqMan probe ACE2TM synthesized according to anACE2 gene sequence, reverse transcriptase, heat-resistant DNA polymerase, dNTPs, magnesium ions, a PCR buffer solution, ultrapure water and an RNA template to be detected; the method comprises the following steps: performing reverse transcription reaction on a buffer reaction system by a one-step method to synthesize template cDNA, performing repeated thermal cycle to obtain a PCR product, degrading a probe release fluorescein A of the PCR product, and degrading a probe release fluorescein B of the ACE2 gene. The PCR product guided by the inner primer is increased by more than two times, so that the efficiency of specific amplification can be fundamentally improved, and a false negative result caused by a sampling error can be identified, thereby improving the accuracy of a detection result.
Owner:URIT MEDICAL ELECTRONICS CO LTD
Bubble-shaped primer, reagent kit with same and application
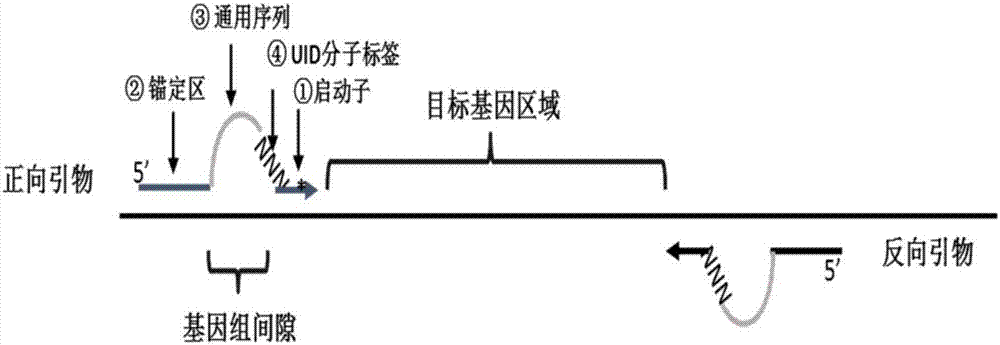
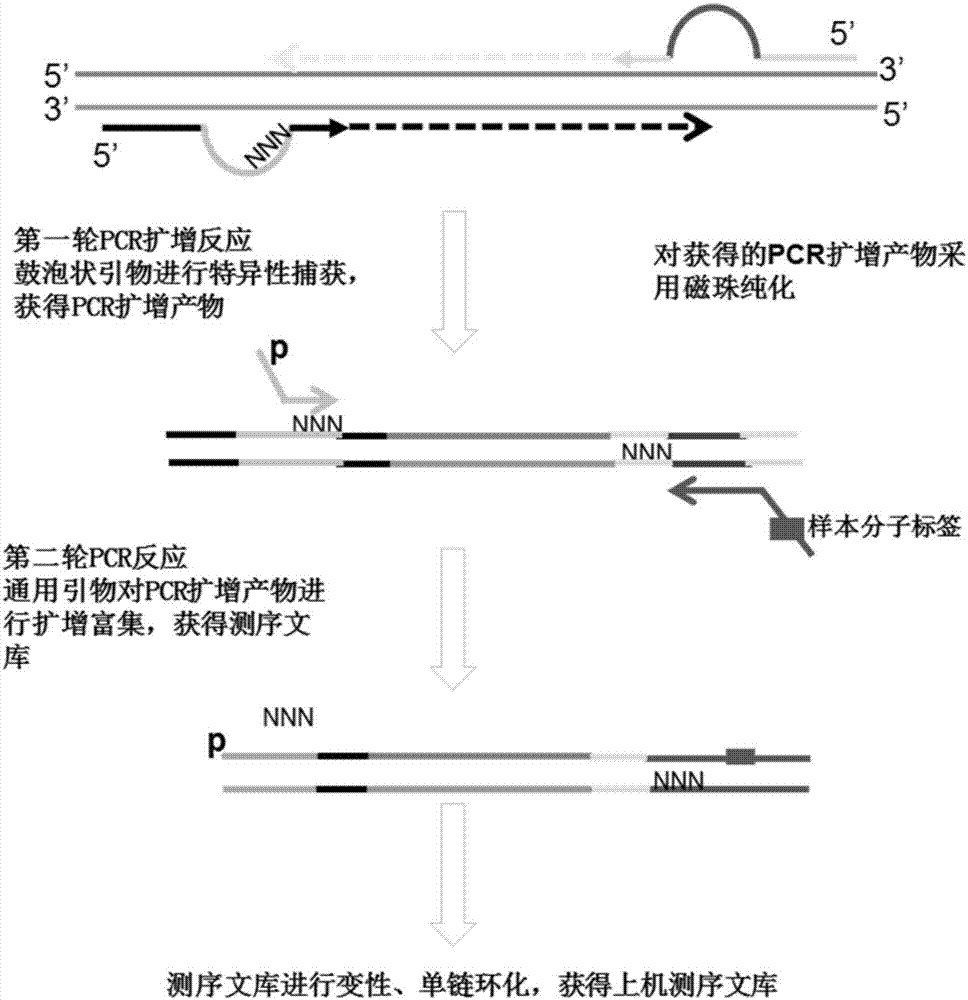
ActiveCN107365769AImprove analytical detection sensitivityTest applicableMicrobiological testing/measurementLibrary creationBase pairReagent
The invention relates to a bubble-shaped primer, a reagent kit with the same and application. The bubble-shaped primer is of a nucleic acid single-strain structure and comprises four portions. The four portions are sequentially linked with one another and include a 5p arm, a universal sequence, a molecular label and a 3p arm; the 5p arm is arranged at a 5' end of the bubble-shaped primer and can be combined with a template sequence during first-round PCR (polymerase chain reaction) according to complementary base pairing principles; the universal sequence is in the shape of protruded bubble without complementation with DNA (deoxyribonucleic acid) sequences of templates during the first-round PCR, and at least one part of the universal sequence and at least one part of a sequence of a 3' end of a universal primer are identical to each other during second-round PCR; the molecular label comprises a plurality of degeneracy bases; the 3p arm is arranged at a 3' end of the bubble-shaped primer and can be combined with the template sequence according to the complementary base pairing principles. The bubble-shaped primer, the reagent kit and the application have the advantages that the analysis and detection sensitivity of sequencing data can be greatly improved by the bubble-shaped primer, a plurality of sites and a plurality of types can be simultaneously detected, and the bubble-shaped primer and the reagent kit are high in detection sensitivity, throughput and accuracy and applicable to detecting different types of tumor.
Owner:MGI TECH CO LTD
Kit for EGFR gene mutation detection and application
InactiveCN108130362AMeet the actual needs of rapid detectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationSerum igeEGFR Gene Mutation
The invention discloses a kit for EGFR gene mutation detection and application, and belongs to the fields of biotechnology and medicine. Compared with the prior art, the kit has the advantages that aprimer, a probe and the kit of an amplification system are high in detection sensitivity, and can detect specimens with the mutation content being smaller than 0.1 percent; the detection accuracy is high, a double control detection system and a locked nucleic acid technology are adopted, and the reliability of detection results is guaranteed; only 8 reactions are needed, including EGFR quality control reaction liquid, L858R detection reaction liquid, 19del detection reaction liquid, G719X detection reaction liquid, L861Q detection reaction liquid, 3Ins20 detection reaction liquid, T790M detection reaction liquid and S768I detection reaction liquid, to be used for detecting 29 common mutant types of EGFR gene in serum or plasma and tissue samples of non-small cell lung cancer patients.
Owner:安徽安龙基因科技有限公司
Application of primer probe combination and kit thereof in HBV detection
PendingCN109852731AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationConserved sequenceTrue positive rate
The invention provides application of a primer probe combination and a kit thereof in HBV detection. The primer probe combination is at least one selected from the group consisting of an S gene regionprimer probe combination, a C gene region primer probe combination, and an X gene region primer probe. Primer probes are designed in conserved sequences of S, C and X genomes of hepatitis B virus, and uses fluorescent quantitative PCR technique to simultaneously detect HBV-DNA in a same tube; and the primer probe combination is simple and rapid, and improves the sensitivity and specificity of thedetection method of hepatitis B virus, minimizes the chance of false negative results due to mutations, and further improves detection efficiency.
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV
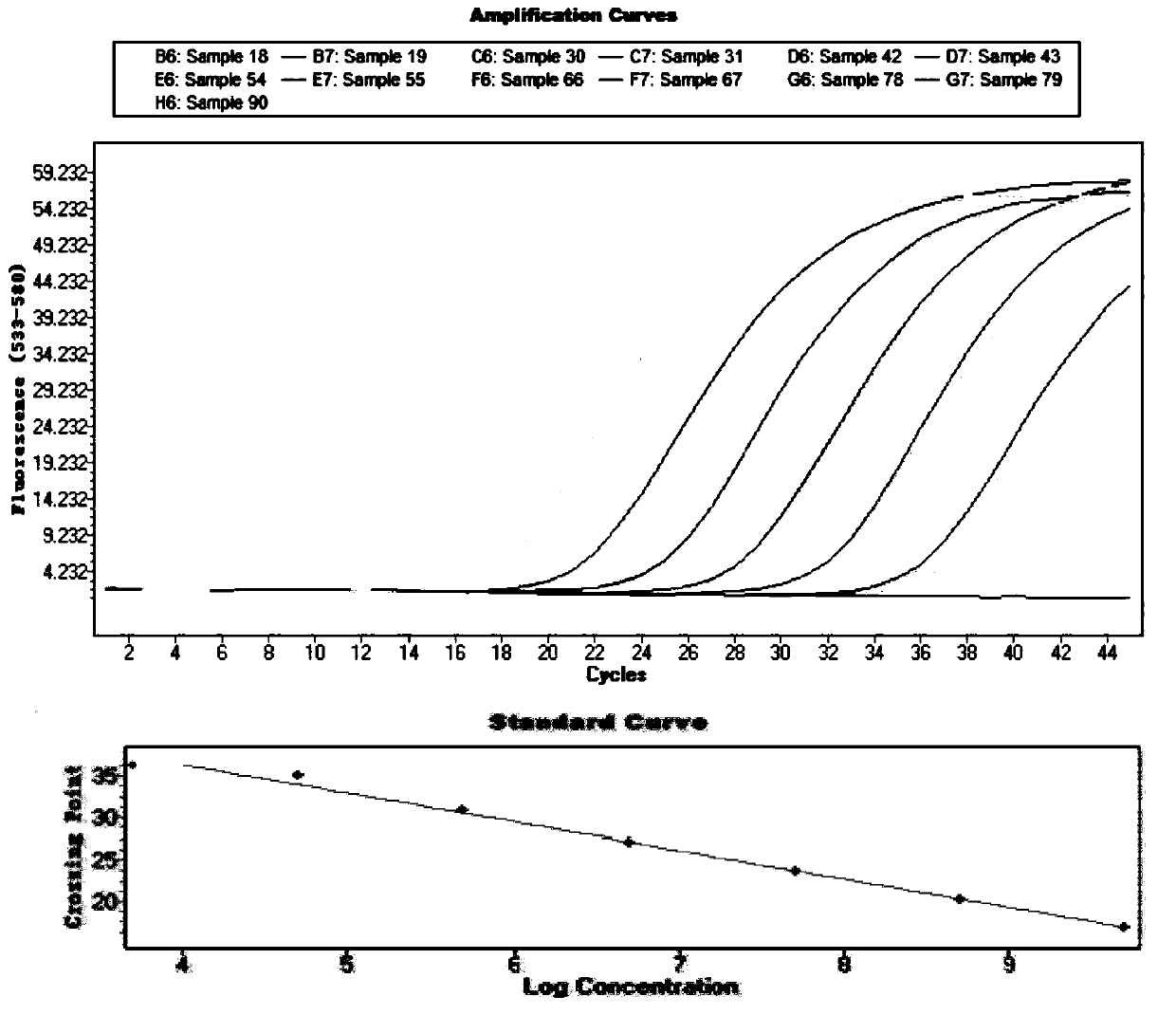
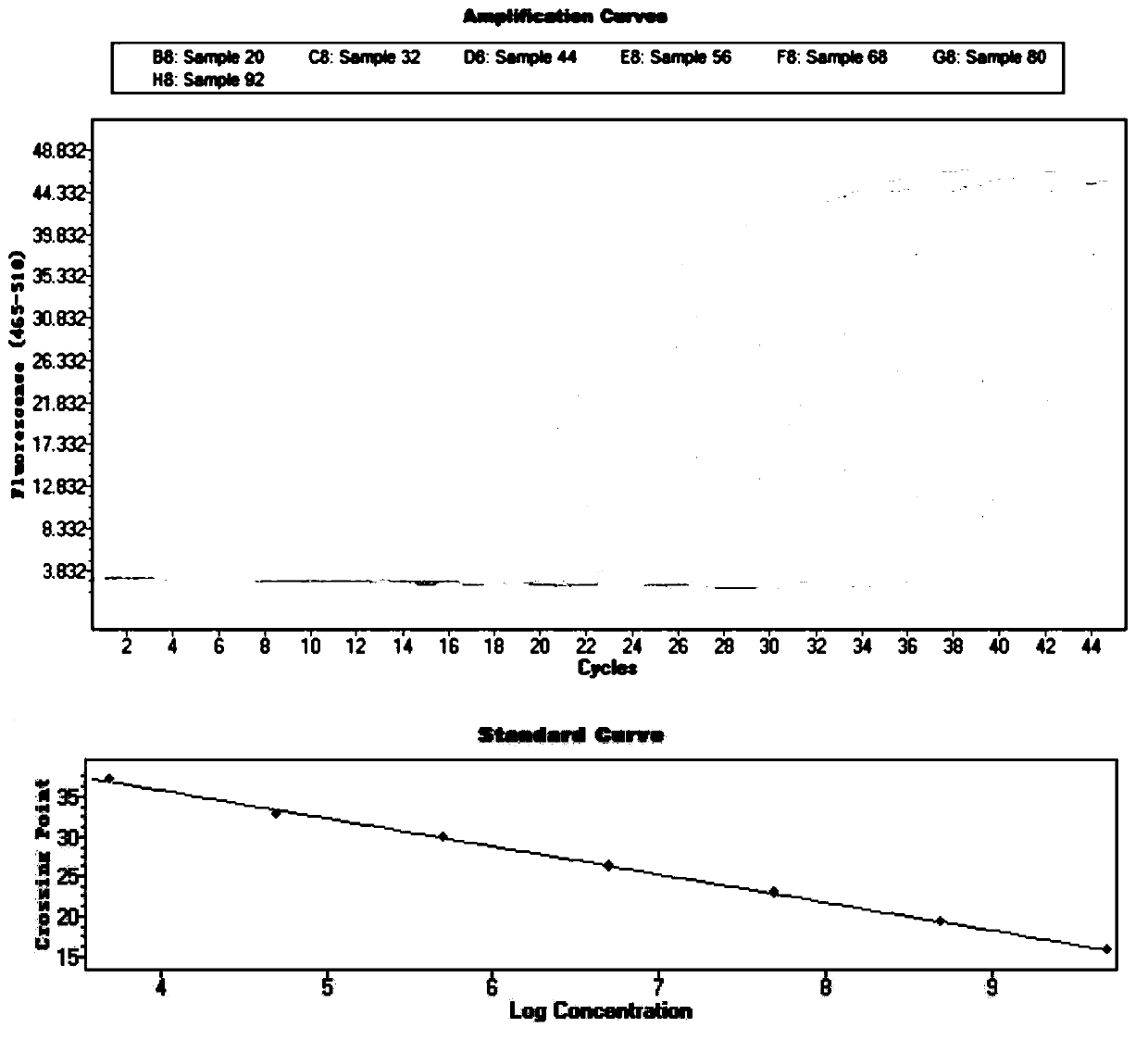
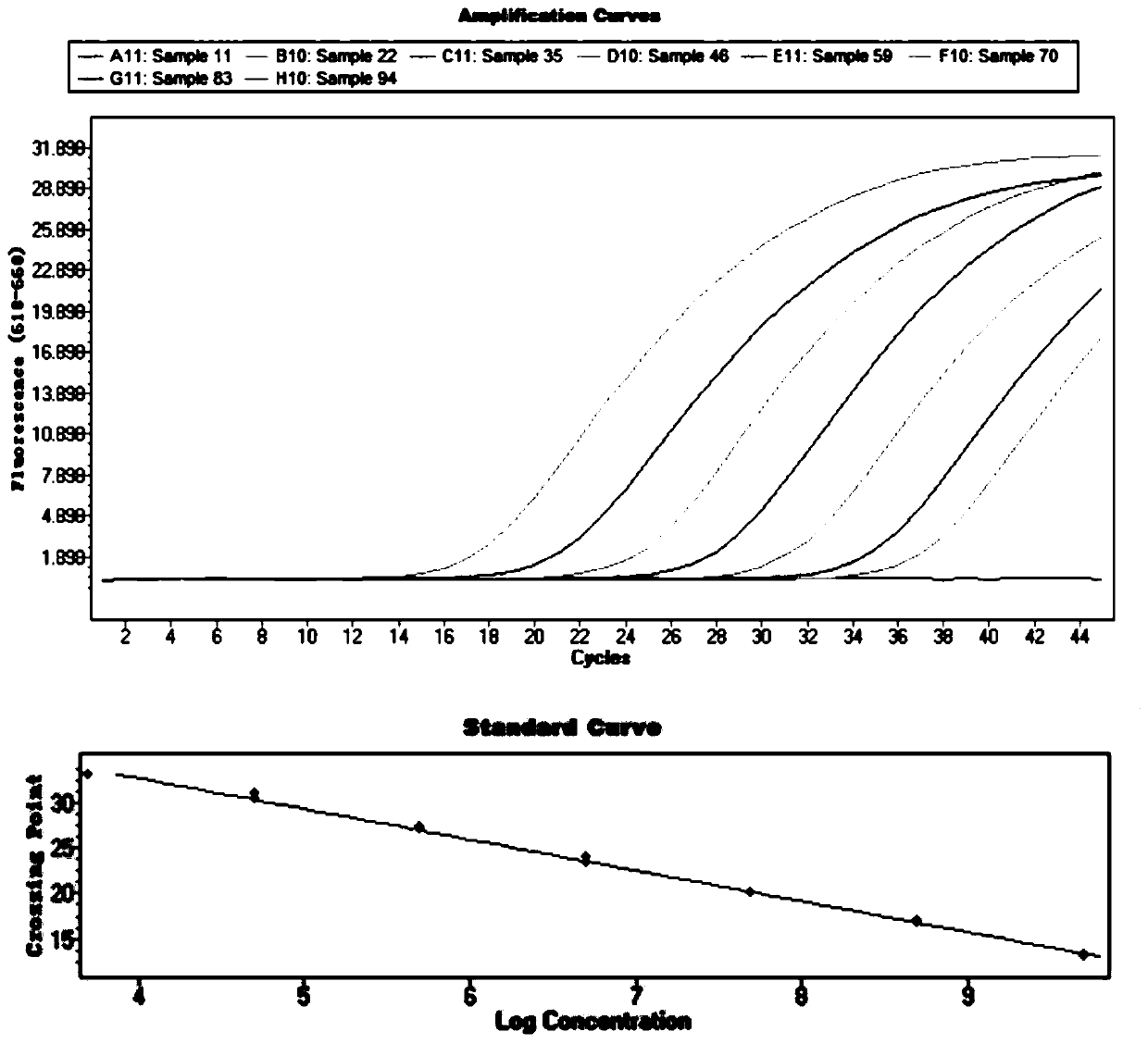
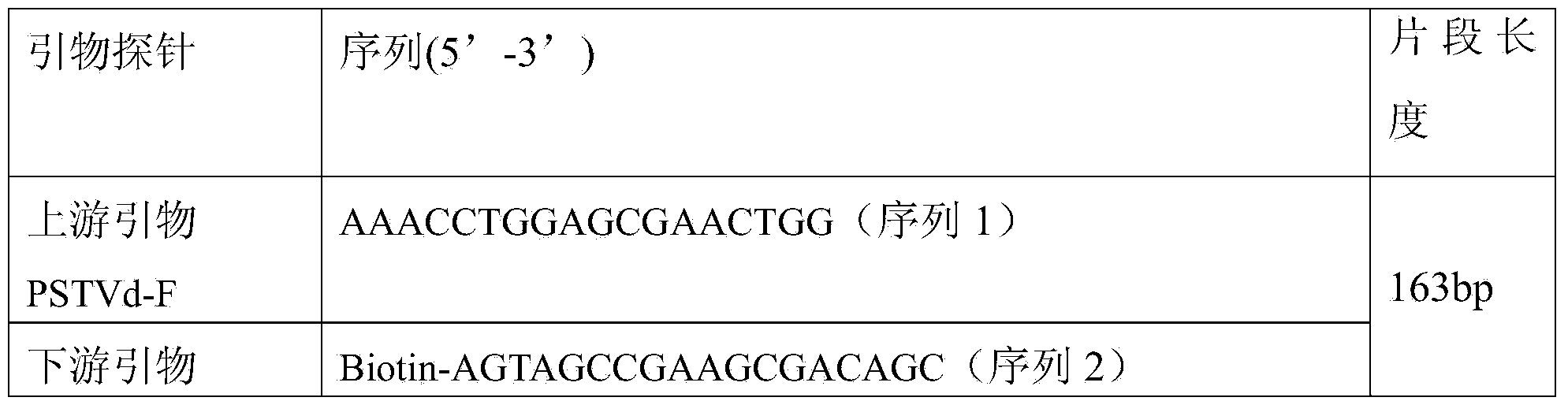
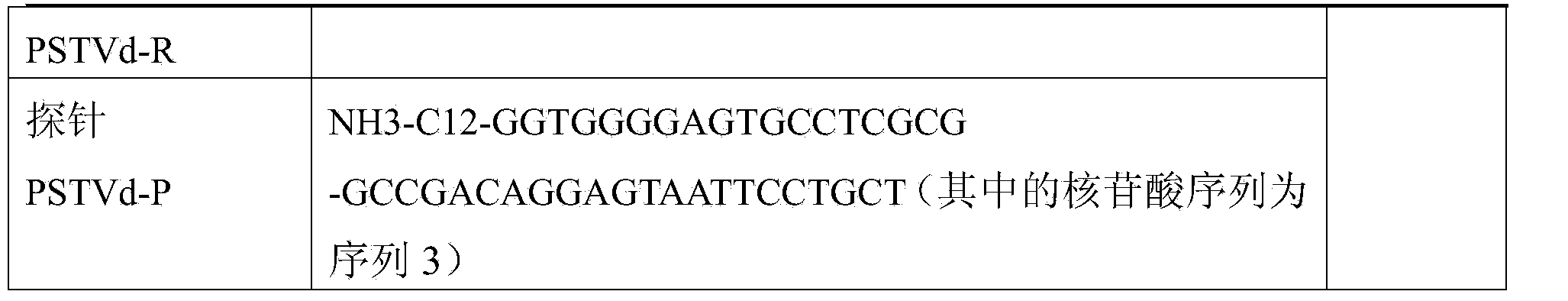
Liquid phase chip detection primer of potato spindle tuber viroid, and detection method thereof
ActiveCN103667527AStrong specificityHigh throughputMicrobiological testing/measurementDNA/RNA fragmentationNon specificBiology
The invention discloses a liquid phase chip detection primer of potato spindle tuber viroid, and a detection method thereof. The invention also provides a kit used for the liquid phase chip detection or the auxiliary detection of the potato spindle tuber viroid. The kit comprises a primer group and a probe, and the primer is composed of a primer 1 and a primer 2. Experiments prove that a sample can be rapidly detected through the detection method, and only about 4h is spent on a process from sample treatment to result obtaining; and there is no cross reaction of the primers designed in the invention and common plant viruses on potatoes, so the false negative result caused by the non-specific amplification is reduced.
Owner:哈尔滨海关技术中心
Test kit for detecting African swine fever virus and method for detecting African swine fever virus
InactiveCN111304361AImprove accuracyReduce false negative resultsMicrobiological testing/measurementDNA/RNA fragmentationClassical swine feverVirus detection
The invention provides a test kit for detecting African swine fever virus. The kit comprises a plasmid containing an ASFV structural protein gene VP73 fragment; an upstream primer of the VP73, a downstream primer of the VP73, an upstream primer of the beta-Actin and a downstream primer of the beta-Actin; a probe of VP73, a probe of beta-Actin. The test kit is easy and convenient to operate, low inprice and short in consumed time, has high sensitivity and specificity and has wide market prospects and commercial value. The invention also provides a method for detecting the African swine fever virus, which comprises the following steps: taking a plasmid containing an ASFV structural protein gene VP73 fragment as a positive control, and taking a porcine actin gene beta-Actin as an internal reference control gene; monitoring from the initial stage of detection, so that the whole virus detection process can be monitored, the effect of internal reference quality control is perfectly embodied, and the accuracy of the detection result is improved.
Owner:ZHEJIANG UNIV
Detection method for screening illegal additives in weight-reducing health food based on real-time direct analysis ion source-high resolution mass spectrum combined use
InactiveCN108020591AKeep full informationReduce extraction lossPreparing sample for investigationMaterial analysis by electric/magnetic meansData acquisitionMass analyzer
The invention establishes a detection method for screening illegal additives in weight-reducing health food. Based on a real-time direct analysis ion source, the ionization process of a target samplecan be completed in a few seconds; by use of an orbitrap high resolution mass spectrometer is for data acquisition of charged ions entering the mass spectrometer to obtain high-resolution first-and second-level mass spectrometric signals by a full-scan-based data-associated scanning mode; and the illegal additives in the weight-reducing health food can be screened by use of ToxID software to rapidly screen and qualitatively process collected data. The method has the advantages of high sensitivity, strong selectivity, strong qualitative ability, and simple and quick operation (the data collection process is completed in a few seconds), retains the complete information of the measured objects to a greater extent, and can be used for rapid screening of the illegal additives in the weight-reducing health food.
Owner:BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU INSPECTION & QUARANTINE TECH CENT
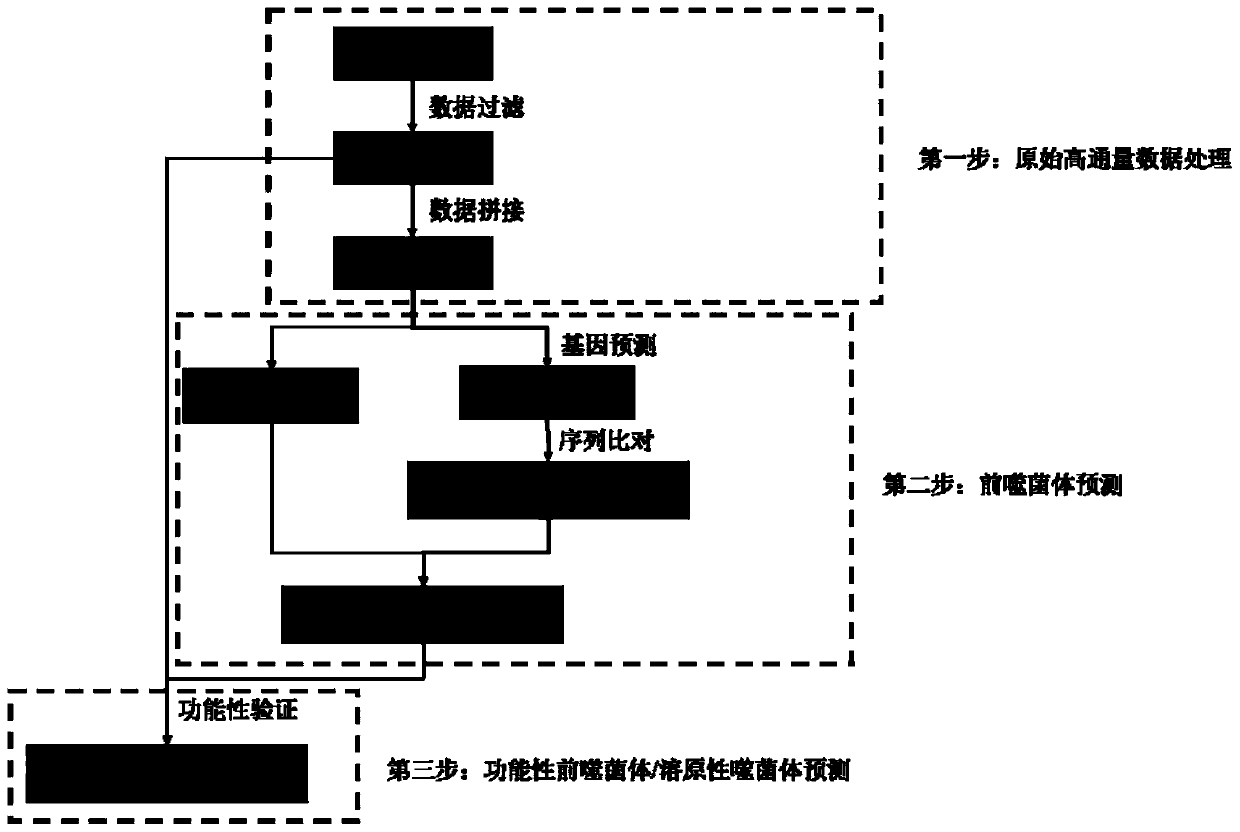
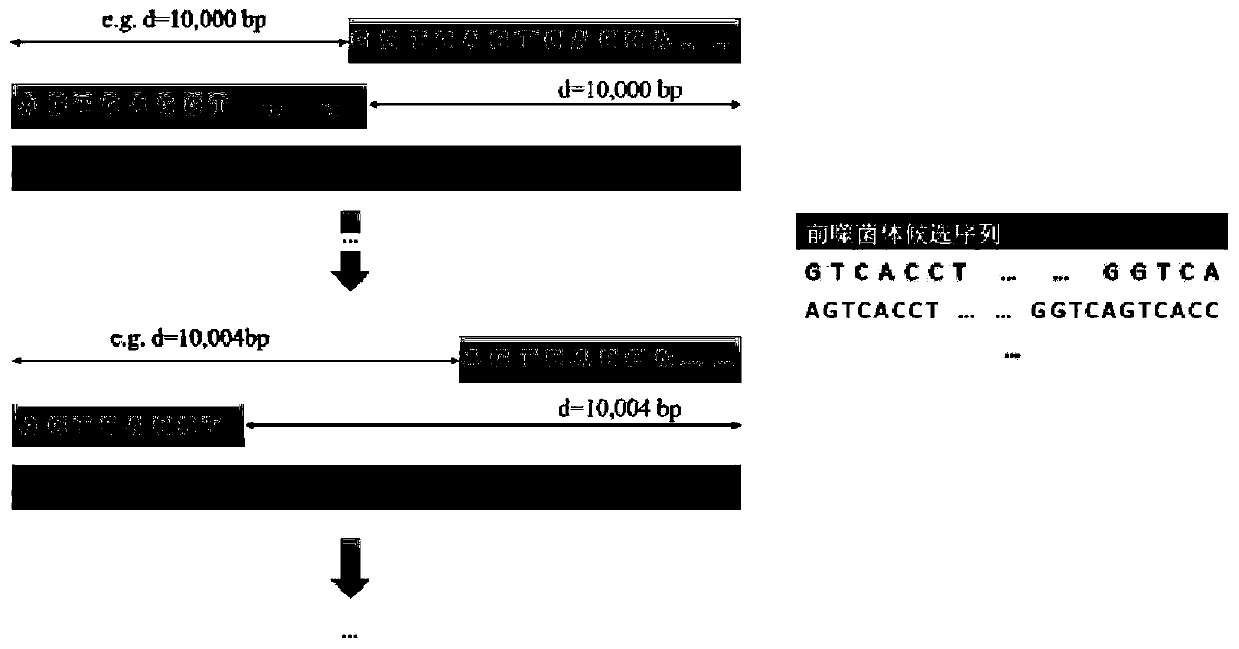
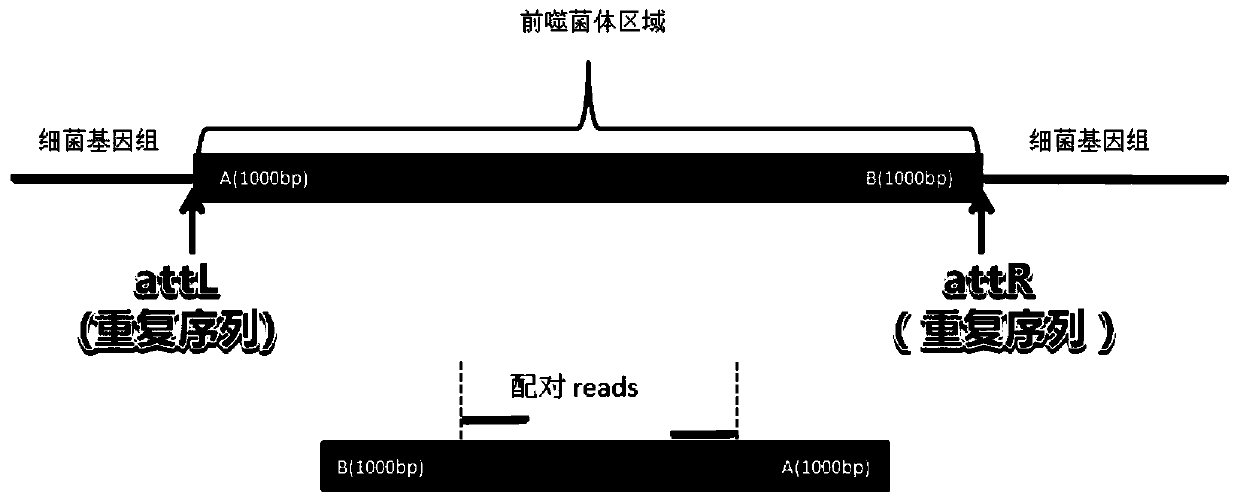
Detection method for functional prophage as well as location and sequence thereof in bacteria
PendingCN111429969AMake sure efficient and accurateSimple and fast operationProteomicsGenomicsProphageProtein
The invention discloses a detection method for functional prophage as well as location and sequence thereof in bacteria. The detection method in the invention includes steps of: predicting an openingreading frame in the genome sequencing data of to-be-detected bacteria to obtain the proteins encoded by the opening reading frame, and comparing the protein sequence with the sequences in the proteinlibrary of the phage, wherein the proteins which are matched with the phage proteins are the functional proteins; searching a positive repeating sequence on the encoding genes of the functional proteins as well as upstream and downstream thereof, wherein a sequence between two positive repeating sequences is the candidate sequence of a candidate prophage; connecting the head and the tail of the candidate sequence, wherein the candidate prophage containing the sequencing read length crossing the head-to-tail connection of the candidate sequence is the functional phage, whereas the candidate prophage without the sequencing read length crossing the head-to-tail connection of the candidate sequence is not the functional phage. The method is simple in operation and has great application prospect.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Immunochromatography kit for jointly detecting IgM (Immunoglobulin M) and IgG (Immunoglobulin G) of multiple pathogens
PendingCN114280298AAvoid mutual contaminationAchieving co-detectionMaterial analysisIgm antibodyMicrobiology
The invention discloses an immunochromatography kit for jointly detecting IgM (Immunoglobulin M) and IgG (Immunoglobulin G) of multiple pathogens, the kit comprises an upper cover and a base which are clamped with each other, a sample groove is formed in the middle of the base, a plurality of test paper clamping grooves are formed along the radial direction of the sample groove, and the test paper clamping grooves are respectively communicated with the sample groove through a guide groove; a sample adding hole and an observation window are formed in the positions, corresponding to the sample groove and the test paper clamping groove, of the upper cover respectively, a test strip is clamped in each test paper clamping groove, each test strip is used for detecting one TORCH pathogen to be detected, and each test strip comprises a bottom lining. A sample pad, a first marker combination pad, a second marker combination pad, a reaction film and a water absorption pad which are tightly connected are sequentially attached to the upper portion of the bottom lining from front to back, the reaction film is sequentially coated with a first detection line, a second detection line and a quality control line from front to back, the first detection line is coated with a mouse anti-human IgG antibody, and the second detection line is coated with a mouse anti-human IgM antibody. According to the kit, multiple pathogens can be detected at the same time only through one-time sample adding, and co-detection of the IgM antibody and the IgG antibody is achieved.
Owner:INTEC PROD INC
Kit for BRAFV600E gene mutation and detection method
InactiveCN107177682AEfficient extractionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPositive controlGene selection
The invention discloses a kit for BRAFV600E gene mutation. The kit comprises a PCR (Polymerase Chain Reaction) buffer solution, primer probe mixed liquor, an enzyme system 1, positive control and negative control. The primer probe mixed liquor comprises a mutation site gene and internal control primer probe, a quality control gene and internal control detection primer probe, a mutation and quality control probe 5' terminal marking FAM fluorescein, a 3' terminal marking MGB fluorescein, an internal control 5' terminal marking HEX fluorescein, and a 3' terminal marking BHQ1 fluorescein. Quality control gene selects a relatively conservative section of a human BRAF V600E gene, internal control selects a human beta-aCtin conservative gene, and a double control system is jointly used for monitoring a plasma sample DNA quality and PCR reaction process. Compared with the prior art, the kit utilizing the primer, the probes and an amplification system provided by the invention is high in detection sensitivity, and can be used for detecting a sample with the mutation content being 0.1 percent; the detection accuracy is high, a double-control detection system is adopted, and the reliability of a detection result is ensured.
Owner:安徽安龙基因科技有限公司
Real-time fluorescent quantitative PCR (polymerase chain reaction) kit for one-step quantitative detection of KRECs gene and its application
InactiveCN105274231AEasy accessEasy to transportMicrobiological testing/measurementFluorescenceFluorescent pcr
The invention relates to a real-time fluorescent quantitative PCR (polymerase chain reaction) kit for one-step quantitative detection of KRECs gene and its application. The kit comprises a real-time fluorescent quantitative PCR reaction system based on real-time fluorescent PCR technique. The fluorescent PCR reaction system comprises forward and reverse primers specific to KRECs and beta-actin genes, and a specific fluorescent probe. The kit allows quick screening of neonatal immune system B-cell level, has high sensitivity and stability and provides excellent reproducibility, and this method is applicable to the quantitative detection of KRECs and to the functional screening of the neonatal immune system and is worthy of practical clinical application.
Owner:SHANGHAI ADVANCED CLINICAL LAB SCI
Colloidal gold chromatography test strip for rapidly detecting lymph node metastasis of papillary thyroid carcinoma and preparation method of colloidal gold chromatography test strip
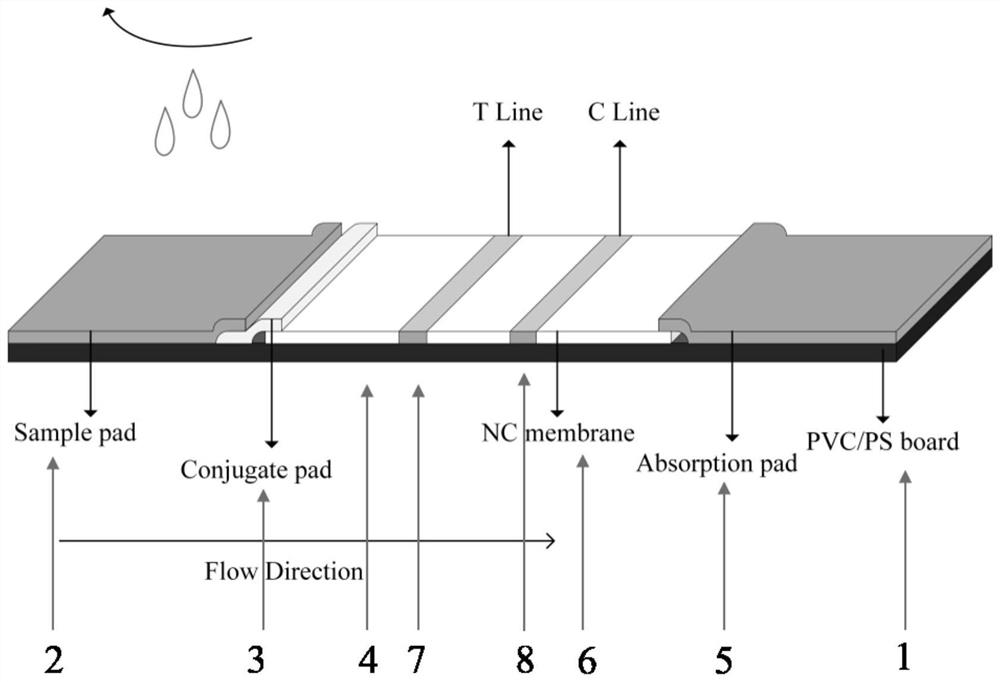
PendingCN114859038AImprove organizational utilizationImprove work efficiencyDisease diagnosisDiseasePolyester
The invention discloses a colloidal gold chromatography test strip for rapidly detecting thyroid papillary carcinoma lymph node metastasis and a preparation method of the colloidal gold chromatography test strip. A sample pad, a combination pad, a nitrocellulose membrane and an absorption pad are sequentially overlapped at one end of a bottom plate from left to right; the binding pad is a polyester film sprayed with a Cyfra21-1 / Braf V600E capture antibody-colloidal gold compound, the nitrocellulose film is coated with a detection line T and a quality control line C, an antibody in the detection line T is a Cyfra21-1 / Braf V600E monoclonal antibody, and the quality control line C is goat anti-mouse IgG. The occurrence rate of false negative is reduced by improving the tissue utilization degree, the detection speed is increased through a chromatography test strip method, finally the purposes of reducing the missed diagnosis rate, relieving the disease burden of a patient and improving the working efficiency of thyroid surgery are achieved, and important clinical significance is achieved.
Owner:THE FIRST AFFILIATED HOSPITAL OF ANHUI MEDICAL UNIV
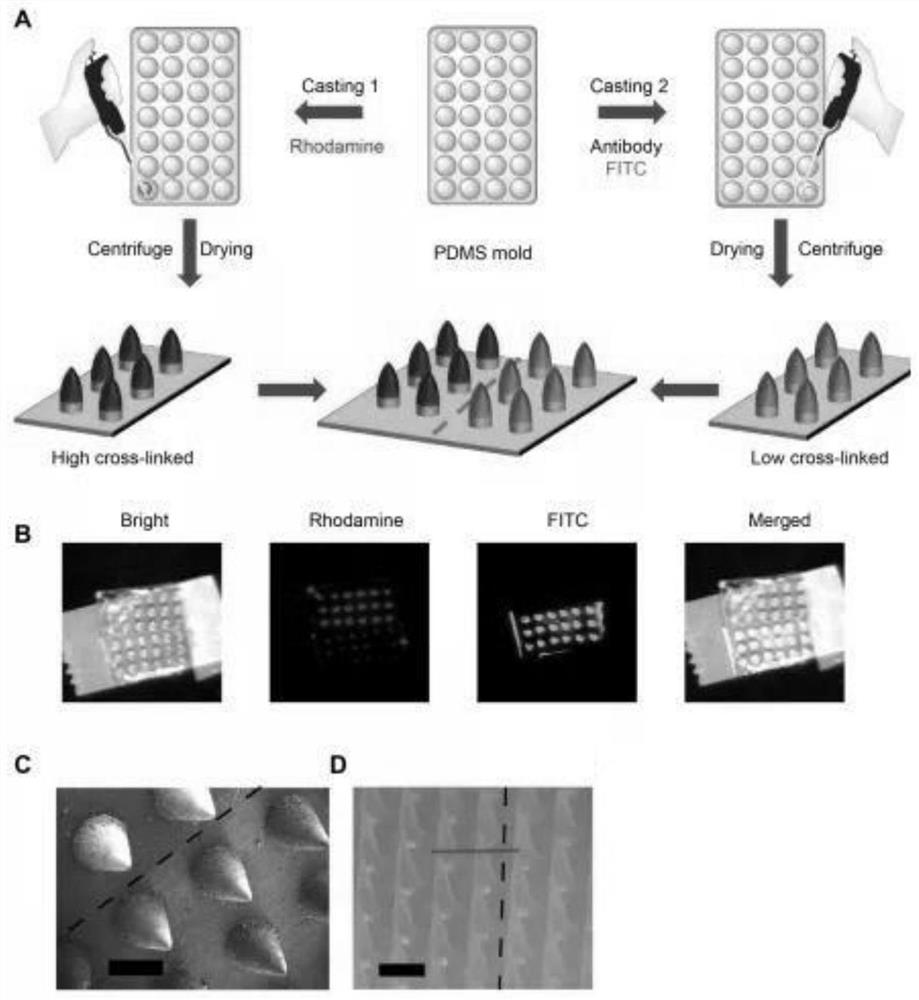
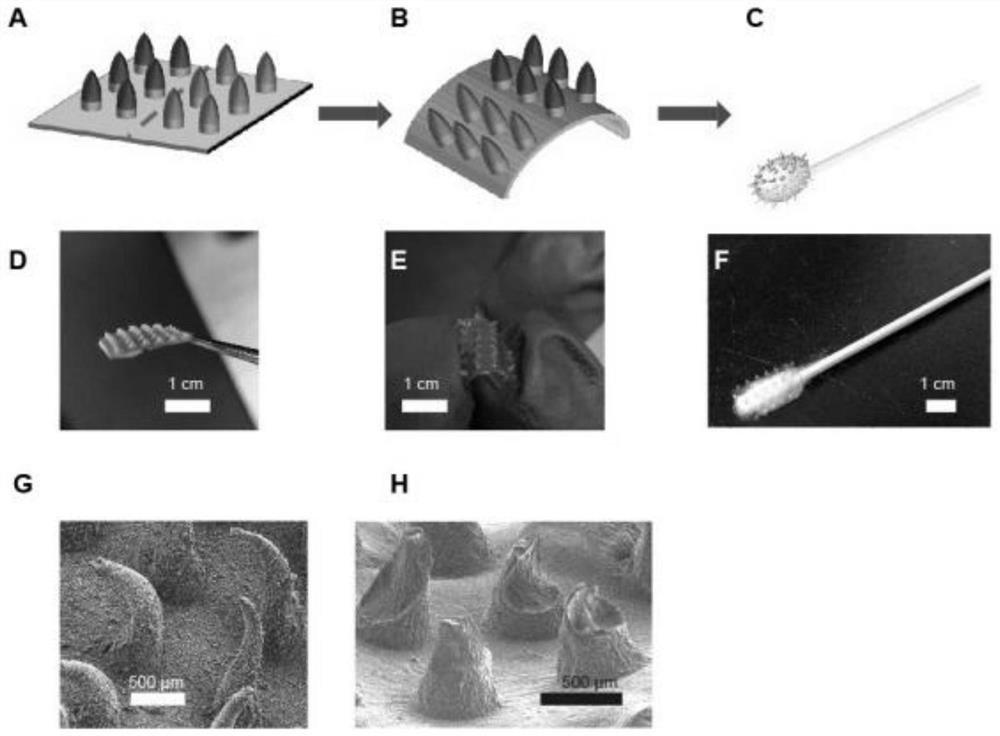
A preparation method and application of a microneedle swab capable of spontaneously enriching viruses
ActiveCN112168220BEfficient captureImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesAntigenViral antibody
The invention discloses a preparation method and application of a microneedle swab capable of spontaneously enriching viruses, comprising the following steps: (1) preparation of a microneedle mold; (2) loading of virus antibodies: preparing a virus antibody solution, and adding it into the microneedle mold; (3) construction of microneedle array; (4) construction of virus-enriched microneedle swab. The present invention utilizes minimally invasive microneedle technology combined with specific germ antibodies to construct a microneedle system capable of spontaneously enriching germs, and assembles it to the end of a medical swab. The constructed microneedle swab can penetrate the oral mucosa and reach the deep tissue, and through the interaction of antigens and antibodies, it can capture germs more efficiently, increase the difference between negative samples and positive samples, and reduce false negatives in the detection process result. The method provided by the invention is simple, efficient, has good biocompatibility and repeatability, improves the detection efficiency and accuracy of germs, and has high potential for clinical transformation.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Primers, probes and kits for detecting human egfr gene mutations
ActiveCN104513864BHigh sensitivityReduce false negative resultsMicrobiological testing/measurementDNA/RNA fragmentationHuman DNA sequencingEGFR Gene Mutation
The invention belongs to the field of molecular biology and particularly relates to primers, probes and a kit for detecting human EGFR gene mutations. The invention provides 7 groups of primers and probes, which can accurately detect 29 types of common human EGFR gene mutations. The kit adopting the primers and the probes has high sensitivity, can detect 1% of micro mutation templates under the background of 20 ng of wild human genomes and reduces false-negative results. Besides, the kit is simple and convenient to operate, has high controllability, can be used for detecting mass samples and is favorable for clinical operation.
Owner:SHANDONG VIGENE BIOSCI
Novel coronavirus detection kit and detection method thereof
PendingCN112048574AAvoid Card Issue StructuresAvoid non-specific bindingMicrobiological testing/measurementAgainst vector-borne diseasesViral testNasopharyngeal aspirate
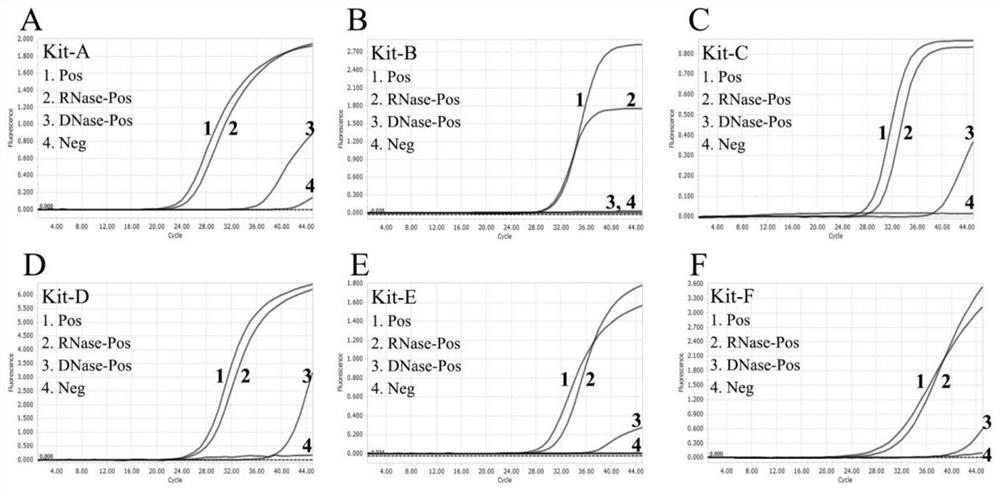
The invention provides a novel coronavirus detection kit and a detection method thereof, and belongs to the technical field of molecular biological detection. A series of primer probe groups for detecting the novel coronavirus are redesigned, an existing detection method is improved, and detection targets are further increased, so that the detection sensitivity of the novel coronavirus is improved, false negative results can be remarkably reduced especially for detection of low-copy-number samples, the sensitivity and the accuracy of detection are effectively improved, and high-efficiency, high-specificity and low-cost detection is realized. The kit can be used for qualitatively detecting the novel coronavirus genes in pneumonia suspected cases and suspected aggregated case patients infected by the novel coronavirus in vitro and samples such as nasopharyngeal swabs and sputum of other patients needing novel coronavirus infection diagnosis or differential diagnosis.
Owner:北京吉检医疗科技有限公司
Chemically modified high-stability RNA, kit and method
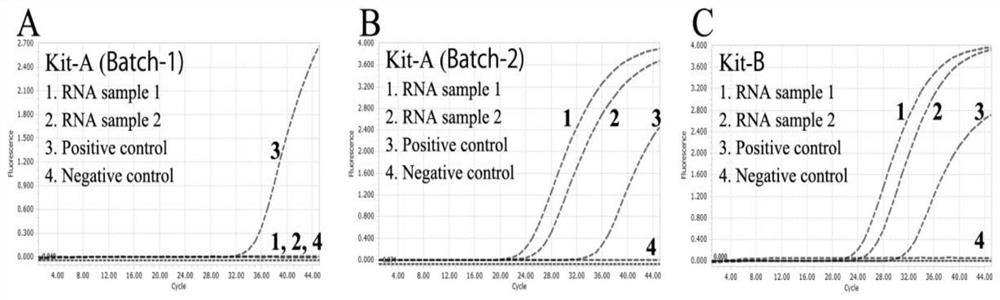
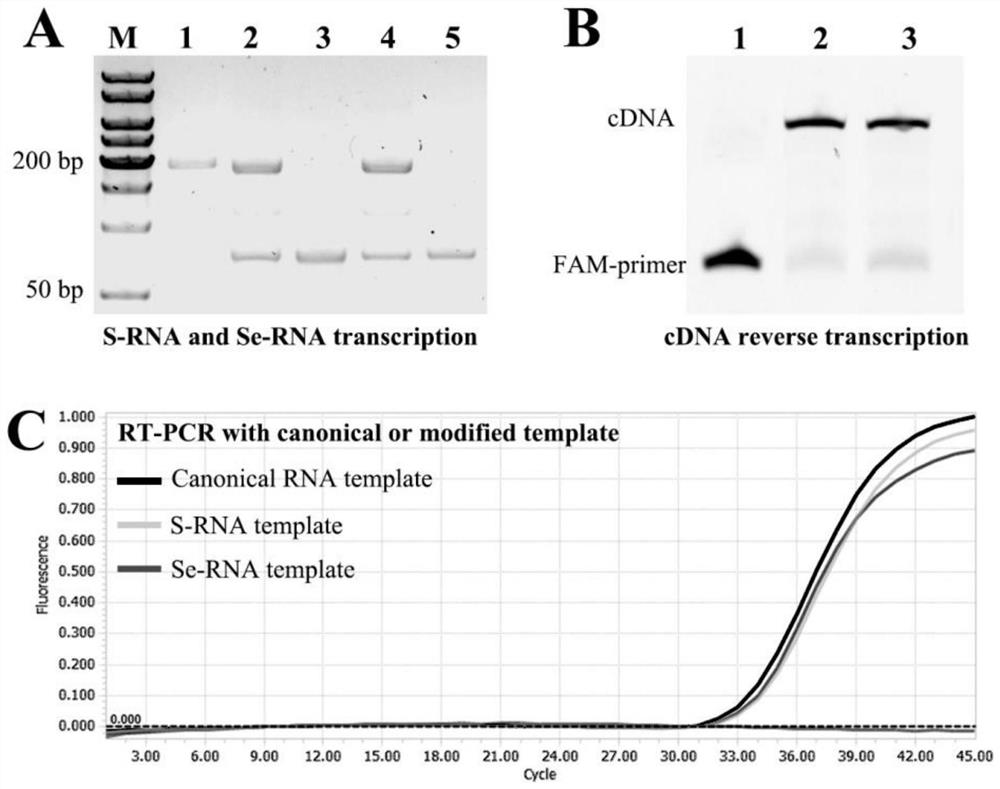
PendingCN112501166AImprove hydrolytic stabilityIncreased risk of false negativesMicrobiological testing/measurementDNA preparationNucleaseMolecular biology
The invention discloses chemically modified high-stability RNA, a kit and a method. Oxygen atoms in phosphate ester of the high-stability RNA are replaced by S or Se. According to the chemically modified high-stability RNA, the kit and the method, the high-stability RNA is used as a positive or negative control of molecular detection and molecular research, the high-stability RNA is selenophosphate RNA, phosphorothioate RNA or selenophosphorothioate RNA, Se-RNA, S-RNA or Se-S-RNA can be used as a good template for reverse transcription, the high-stability RNA has good thermal stability, biological stability, nuclease hydrolysis resistance stability and chemical stability, and also has molecular selection, exclusiveness and specificity, which indicates that the high-stability RNA has greatpotential and application prospects as positive and negative controls of a nucleic acid detection system and molecular research, and in positive and negative controls for the molecular detection and the molecular research, at least one is the high-stability RNA.
Owner:SICHUAN UNIV +1
A method and system for detecting epistasis in complex diseases based on chromatin regulatory loops
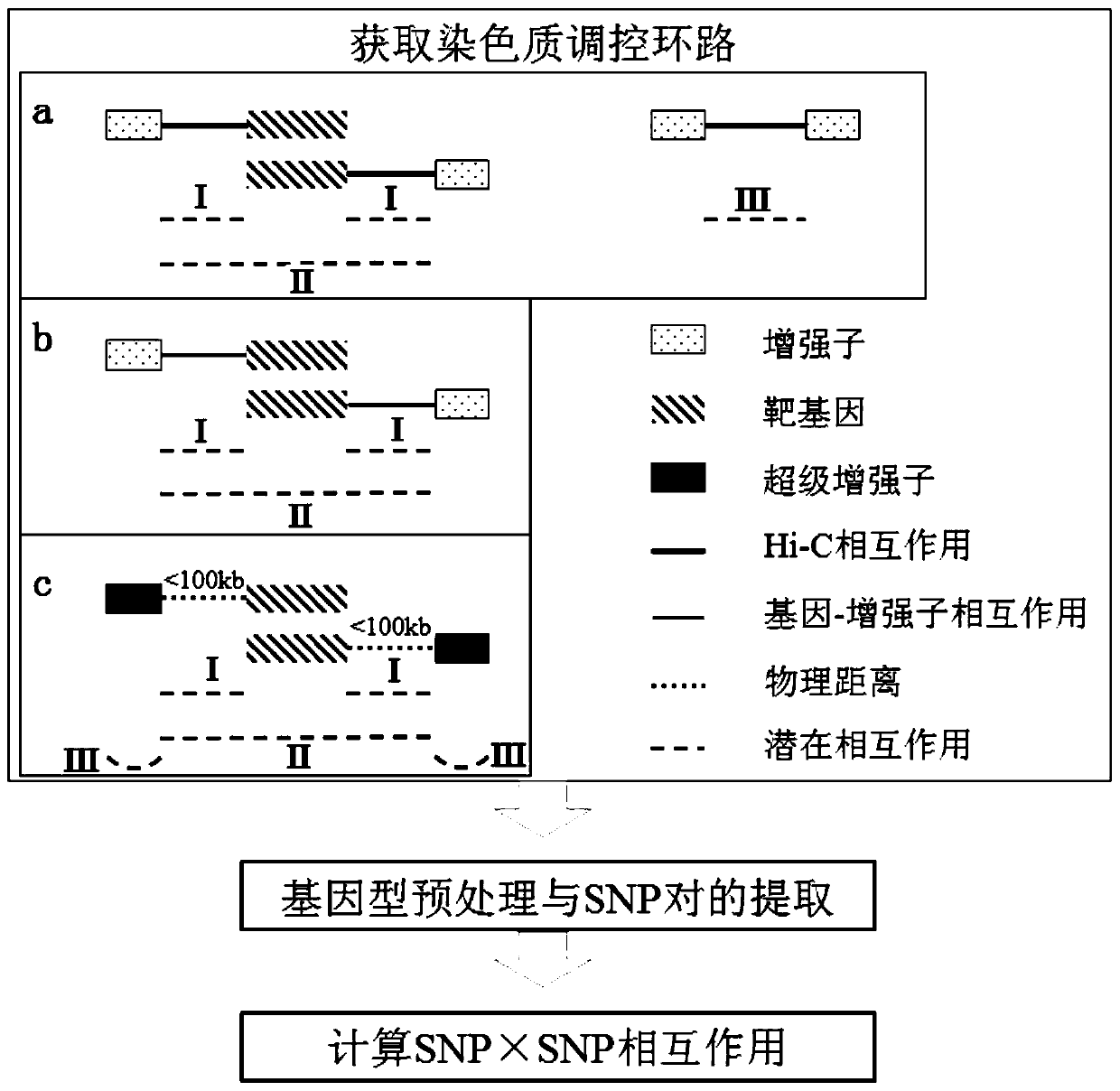
ActiveCN108334749BQuick exploreAccurate explorationBiostatisticsProteomicsPharmaceutical drugGene interaction
The invention discloses a method and a system of detecting epistasis of a complex disease on the basis of chromatin regulation loops. Chromatin remote-interaction data and chromatin segmentation status data of cell lines related to the complex disease are collected and arranged; the above-mentioned data are utilized to establish the chromatin regulation loops; and SNP (Single nucleotide polymorphism) interaction which is in the regulation loops and can influence phenotypes of the complex disease is calculated. According to the method, the data of chromatin remote-interaction and the like are utilized to establish the chromatin regulation loops based on gene interaction, and the epistasis is calculated according to the chromatin regulation loops. Compared with the prior art, the method cangreatly decrease a calculation amount, can also reduce false negative results, thus rapidly and accurately explores SNP interaction related to the complex disease, and provides potential targets for subsequent drug design and the like.
Owner:XI AN JIAOTONG UNIV
Detection reagent kit of fluorescent quantitation PCR latent hepatitis b viruses
PendingCN109852732AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationHepatitis b viralHepatitis B virus
The invention provides a detection reagent kit of fluorescent quantitation PCR latent hepatitis b viruses. The detection reagent kit comprises fluorescent quantitation reaction liquid and a primer andprobe combination, wherein the primer and probe combination comprises one or more of an S gene region primer and probe combination, and a C gene region primer and probe combination. Primers and probes are respectively designed for a hepatitis b virus S and a C consensus sequence, a dual dual-gene fluorescent quantitation PCR technique is used for performing same-pipe detection at the same time onHBVDNA. The detection reagent kit is simple and quick to operate, the sensitivity and the specificity of a latent hepatitis b virus detection method are improved, the probability of false negative results caused by mutation can be reduced to the best, and the detection efficiency can be further improved.
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV
Primer combination for detecting SARS-CoV-2 and D614G mutant strain thereof and application of primer combination
ActiveCN113604609AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesSingle strandNasopharyngeal aspirate
The invention discloses a primer combination for detecting SARS-CoV-2 and a D614G mutant strain thereof and application of the primer combination. The primer combination for detecting the SARS-CoV-2 and the D614G mutant strain thereof disclosed by the invention is composed of 15 single-stranded DNAs shown in sequences 1-15 in a sequence table. When the primer combination is used for detecting the novel coronavirus, the specificity is high, the sensitivity is high, the sample detection limit is 1-10 copy number / reaction, cross reaction with other six kinds of coronavirus infecting people is avoided, and false negative results can be remarkably reduced. The primer combination provided by the invention can be used for large-scale, rapid, in-vitro and qualitative detection of novel coronavirus genes in nasopharyngeal swab, sputum, alveolar lavage fluid and other samples of novel coronavirus infected pneumonia suspected cases, suspected aggregation case patients, and other diagnosers needing novel coronavirus infection diagnosis or identification, and has good application prospects.
Owner:ACADEMY OF MILITARY MEDICAL SCI
PCR method with unique primer and its application
InactiveCN1548543AAmplify the effectAvoid negative effectsMicrobiological testing/measurementFermentationPolymerase LPcr method
The present invention provides the PCR or RT-PCR method with unique prime and its application, and the unique primer is oligomeric nucleotide chain designed based on the template DNA chain order characteristic and has 3' end with 6-12 bases in complementary order with the template and 5' end flexibly designed. The method is superior to classical PCR method, which has one pair of primers and maybe negative effect on proliferation efficiency and the complementing between 3' end bases in forward and inverse primers. The present invention is suitable for use in the PCR or RT-PCR test of human and pathological microbe gene, in multiple PCR or RT-PCR to reduce the mutual effect between primers and as the inner reference reaction of other PCR or RT-PCR, and is favorable to obtaining quantitative or semi-quantitative results.
Owner:徐定邦 +1
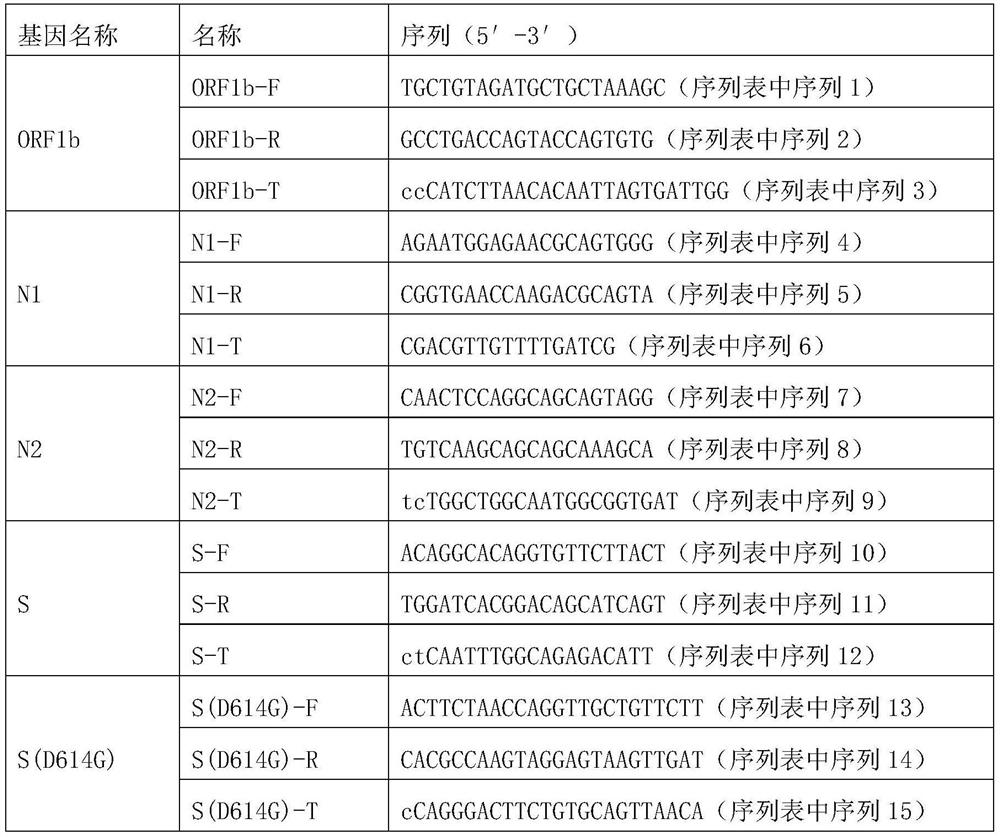
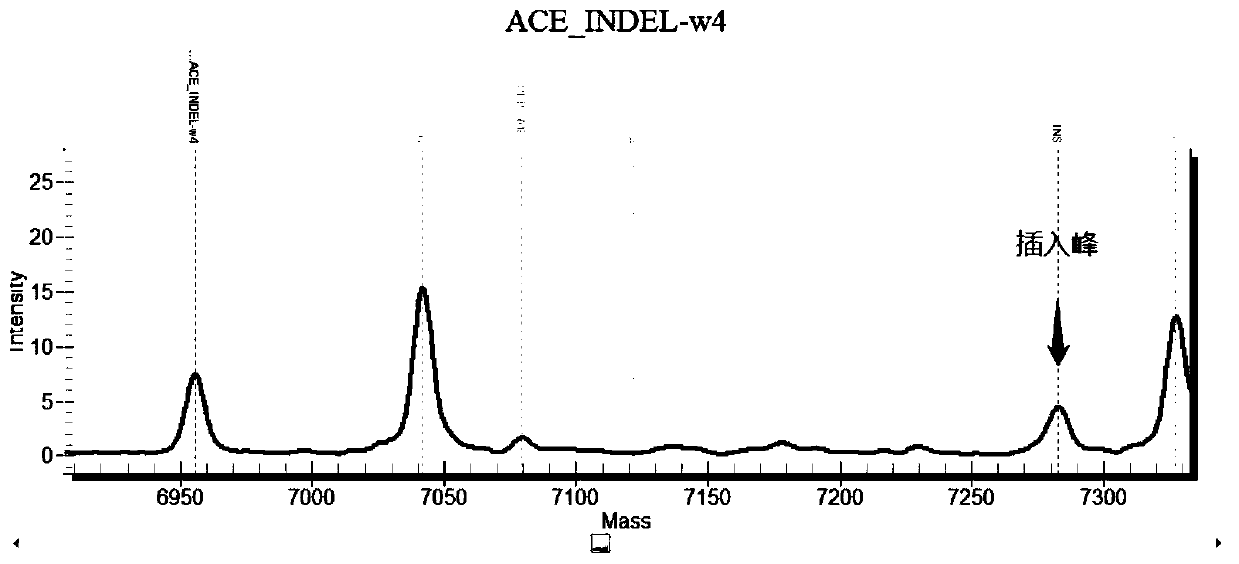
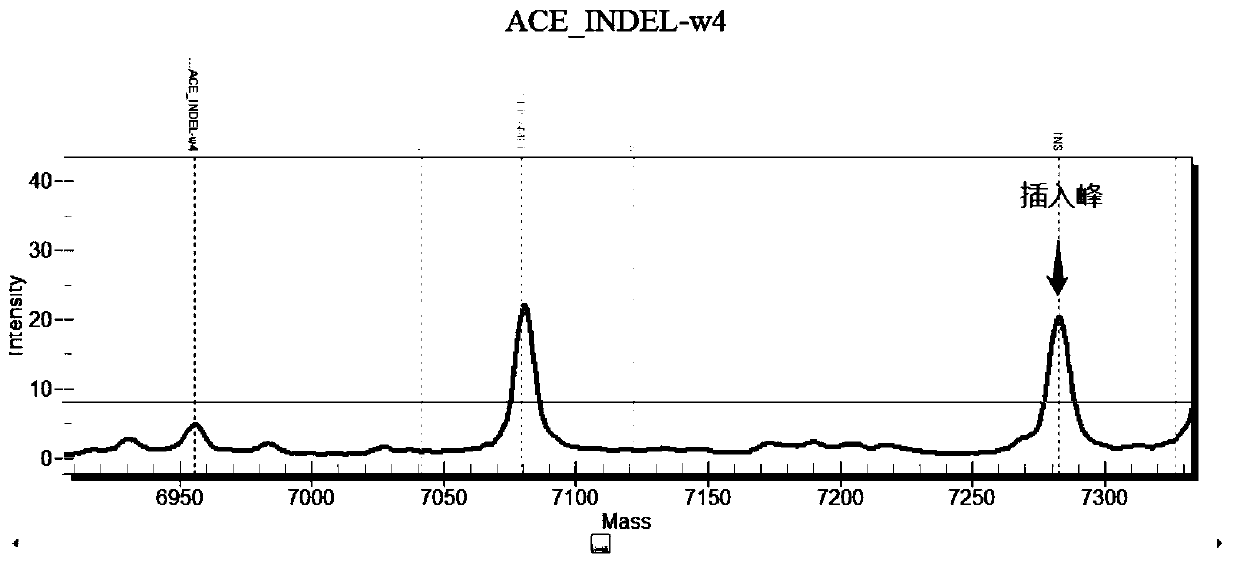
Chromosome long segment insertion detection method and long segment insert detection method based on MassARRAY platform
ActiveCN110305947AReduce consumptionReduce detection accuracyMicrobiological testing/measurementLong segmentBiology
The invention provides a chromosome long segment insertion detection method and a long segment insertion detection method based on a MassARRAY platform, and relates to the technical field of biology.The detection method includes the steps: simultaneously identifying whether samples to be detected are wild and / or mutant by a first detection unit; identifying whether the samples to be detected aremutant or not by a second detection unit; reading and discriminating identification results of the first detection unit and identification results of the second detection unit; judging whether chromosome long segments are inserted into the samples to be detected or not. By the aid of the additional arranged second detection unit, chromosome long segment insertion can be effectively detected according to the identification results of the first detection unit and the second detection unit, false negative results are effectively decreased, and false positive results can be effectively decreased.Besides, the chromosome long segment insertion detection method has the advantage that types of the samples to be detected can be expanded.
Owner:JIANGSU SIMCERE MEDICAL DEVICE CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com