Test kit for detecting African swine fever virus and method for detecting African swine fever virus
A technology of African swine fever virus and kit, applied in the field of biological detection, can solve the problems of reducing the accuracy of detection results, false positives, false negatives, etc., achieve broad market prospects and commercial value, easy operation, and short time-consuming effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
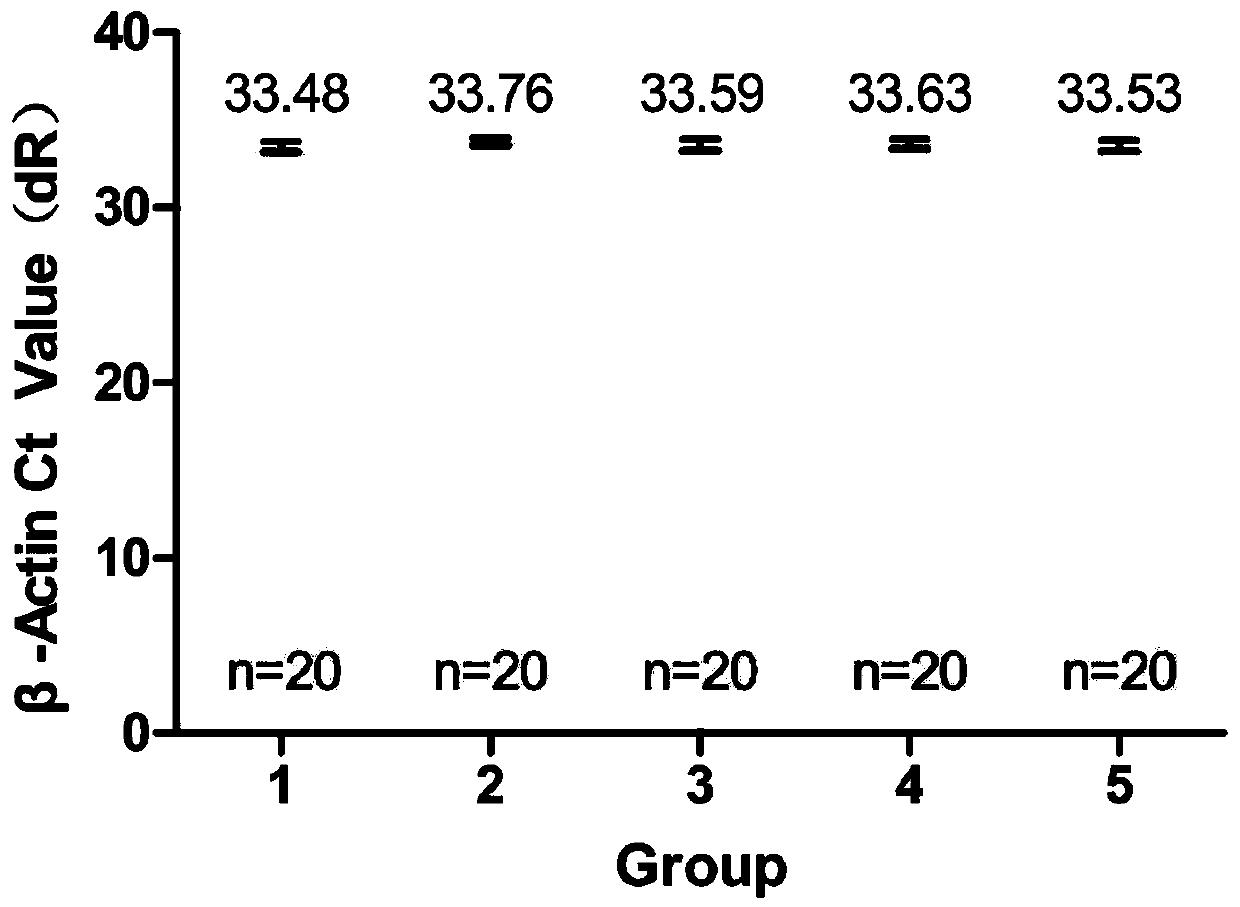
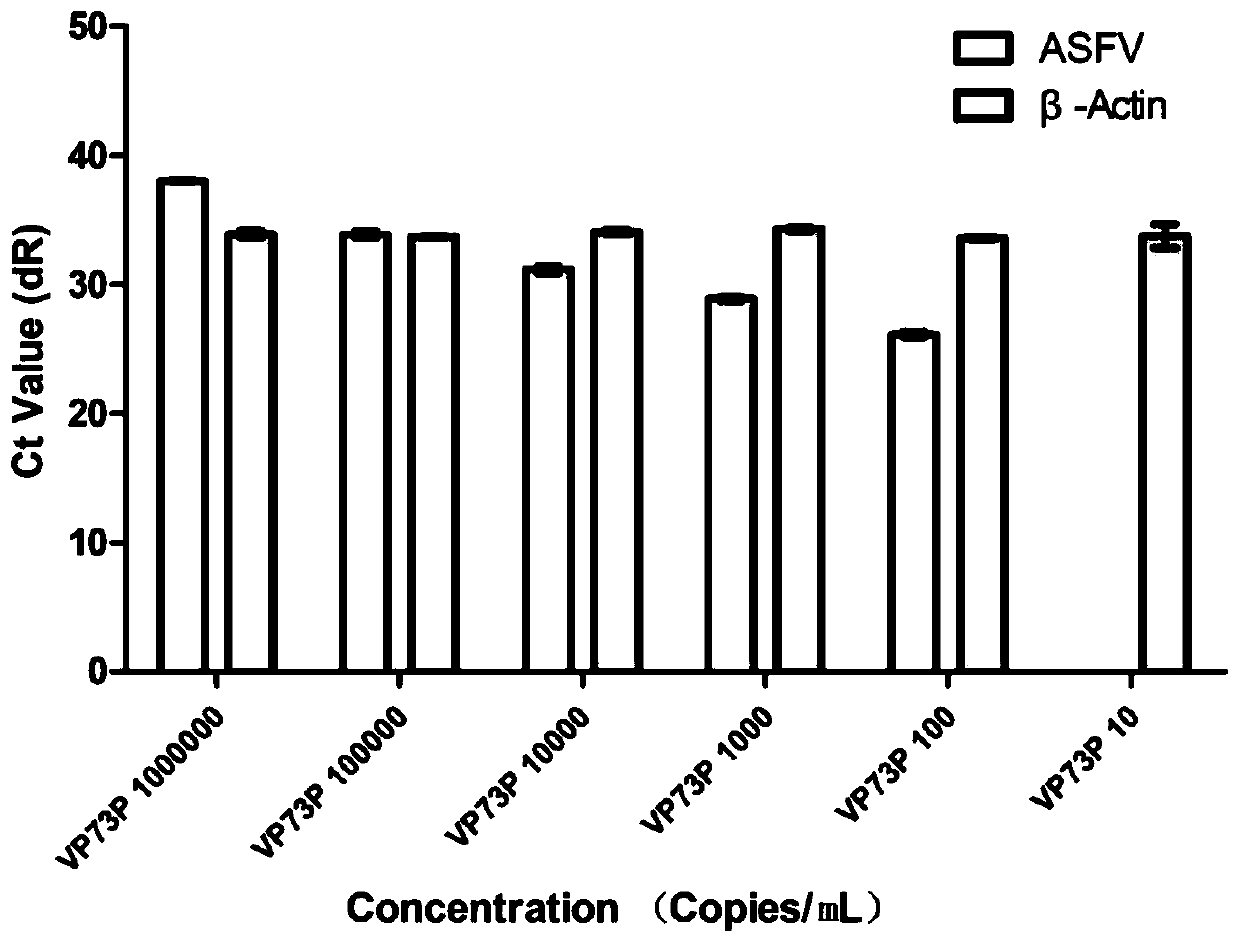
[0037] The invention provides a kit for detecting African swine fever virus. The kit uses the plasmid of the VP73 fragment of the ASFV structural protein gene as a positive control, and uses β-Actin as an internal reference control gene. The kit uses the β-Actin gene as an internal quality control, which not only monitors the extraction process of the sample nucleic acid but also monitors the gene amplification reaction process, which increases the accuracy of the test results and reduces false negative results.
[0038] Specifically, a kit for detecting African swine fever virus comprises: a plasmid containing a fragment of ASFV structural protein gene VP73, an upstream primer of VP73, a downstream primer of VP73, an upstream primer of β-Actin, a downstream primer of β-Actin, In some preferred modes, the kit also includes VP73 probes and β-Actin probes.
[0039] In this example, the plasmid containing the VP73 fragment of the ASFV structural protein gene is a freeze-dried pla...
Embodiment 2
[0062] The invention provides a method for detecting African swine fever virus, adopting the kit described above, using the plasmid solution containing the ASFV structural protein gene VP73 fragment as a positive control standard, and using the pig actin gene β-Actin as an internal reference control gene , including the following steps:
[0063] (1) extract the nucleic acid of the sample to be tested;
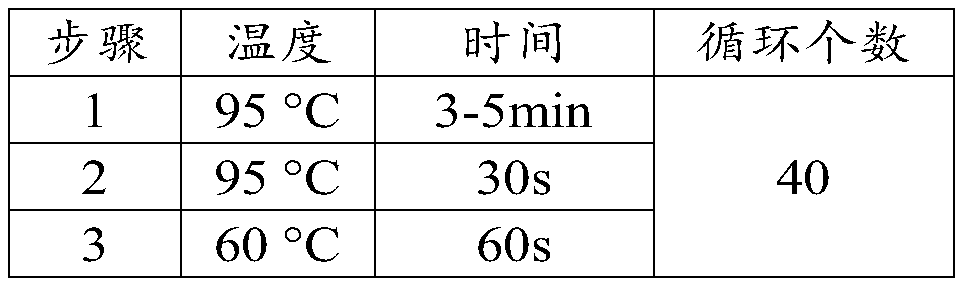
[0064] (2) carry out gene amplification reaction with the nucleic acid of sample to be tested and the plasmid containing ASFV structural protein gene VP73 fragment as template;
[0065] (3) Analyze the amplification results.
[0066] In this method, a plasmid containing the VP73 fragment of the ASFV structural protein gene was selected as a positive control, and the pig actin gene β-Actin was used as an internal control gene. Monitoring from the initial stage of detection can monitor the entire virus detection process, which perfectly reflects the role of internal reference g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com