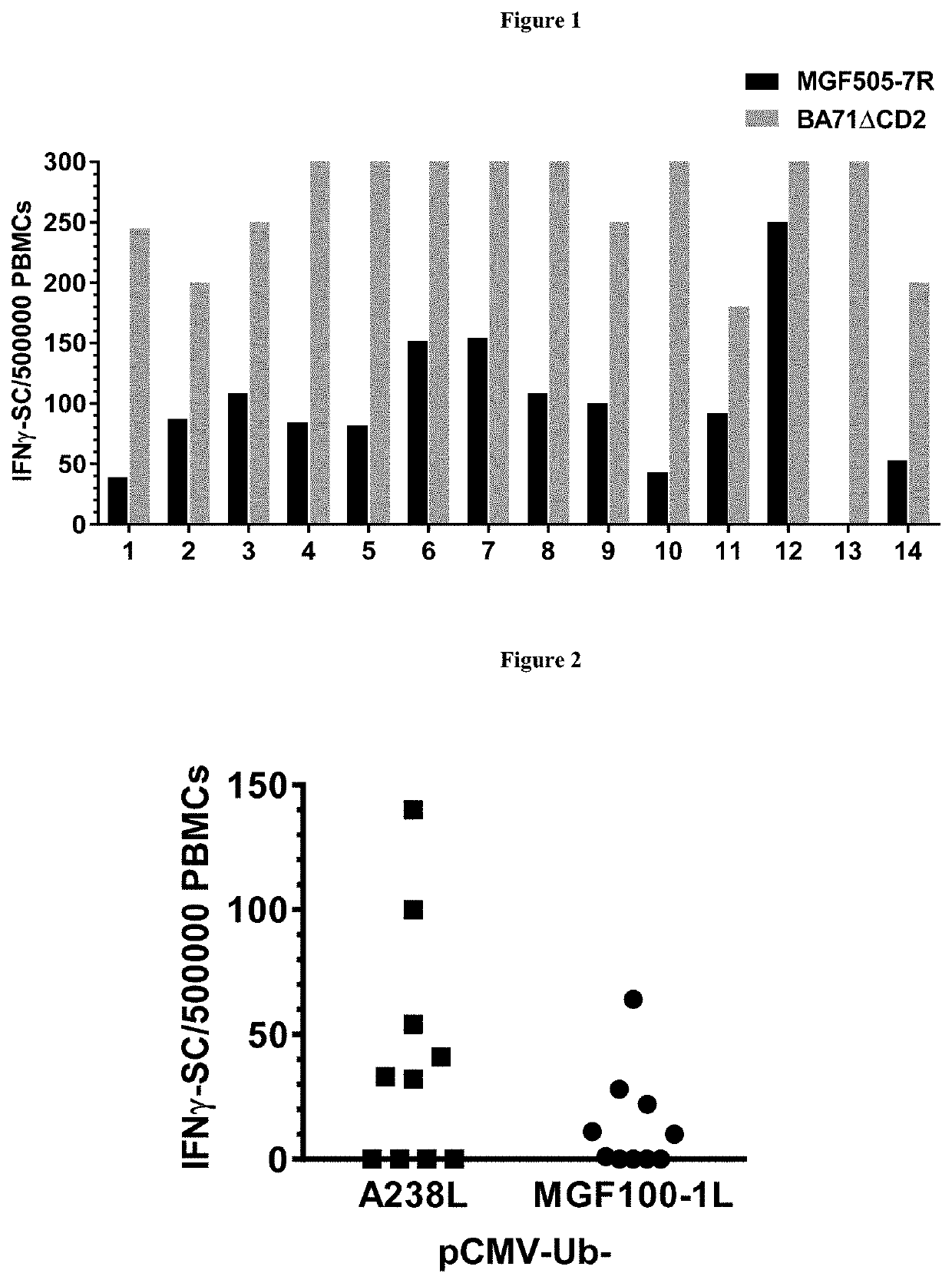
Patents
Literature
919 results about "Classical swine fever" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Classical swine fever (CSF) or hog cholera (also sometimes called pig plague based on the German word Schweinepest) is a highly contagious disease of swine (Old World and New World pigs).
Gene deletion attenuated African swine fever virus and application thereof as vaccine
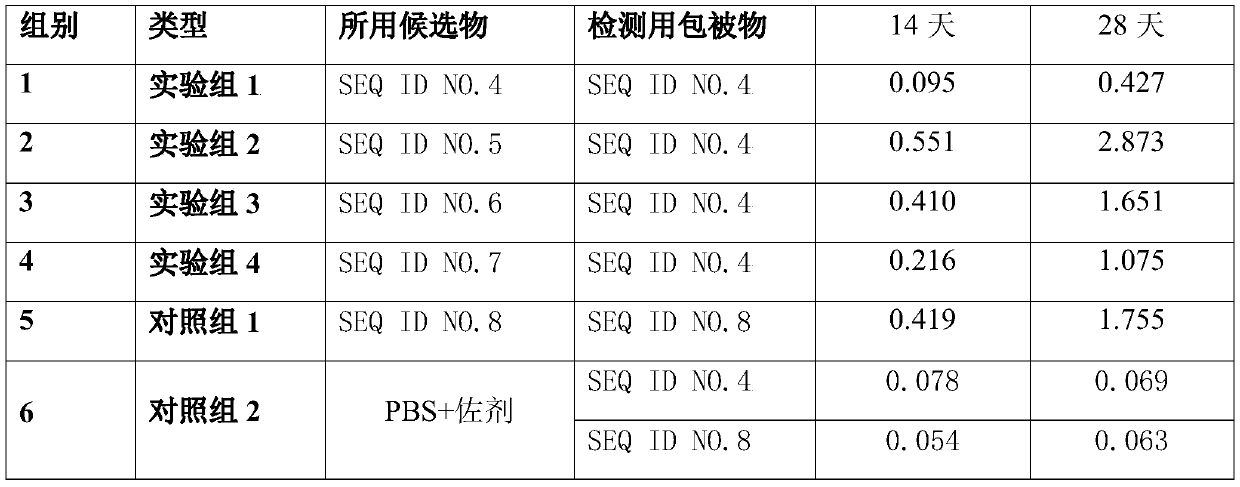
ActiveCN110093324AGood immune protectionFull Poison Attack ProtectionViral antigen ingredientsVirus peptidesAfrican swine feverGenetic engineering
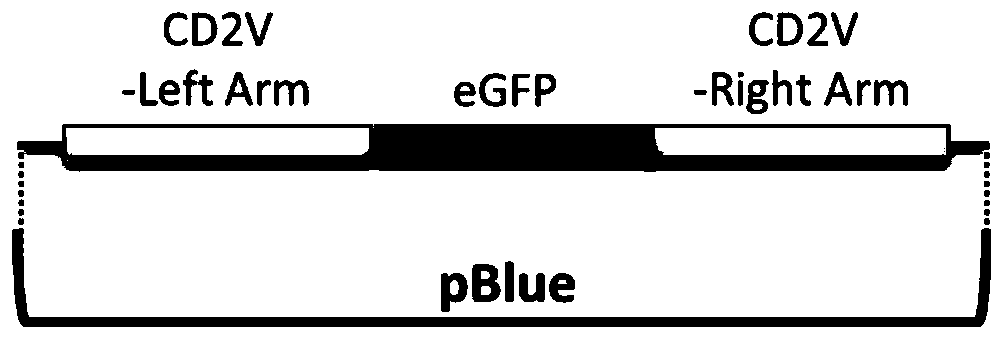
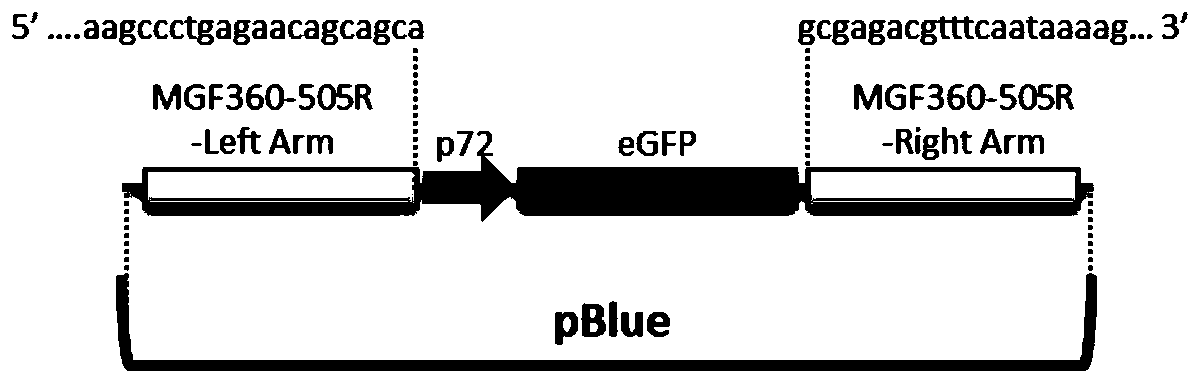
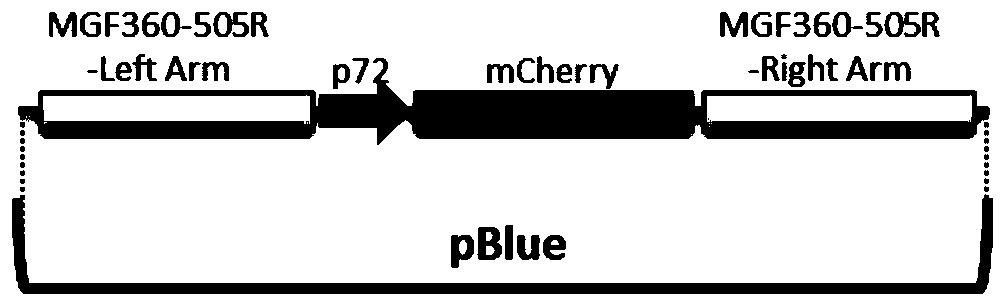
The invention relates to a gene deletion attenuated African swine fever virus as a vaccine and the vaccine, and a construction method thereof. An African swine fever Chinese epidemic strain Pig / CN / HLJ / 2018 is adopted, a virulence gene of the African swine fever virus is deleted by a genetic engineering technology, and the gene deletion virus of MGF360-505R deletion and joint deletion of CD2V and MGF360-505R is obtained. Experiments show that the two virus strains can provide 100% immune protection against the African swine fever Chinese epidemic virulent strains, can be used as vaccines for safe and effective prevention and control of African swine fever in China, and have great social value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Traditional Chinese medicine mixture for treating livestock and poultry virosis and preparation method thereof
ActiveCN101554414AEasy to takeAbsorb quickly and playDigestive systemAntiviralsHigh grade feverRadix isatidis
The invention relates to a traditional Chinese medicine mixture for treating livestock and poultry virosis and a preparation method thereof. The traditional Chinese medicine mixture contains the contents according to the parts by weight: 10-40 of radix isatidis, 10-40 of folium isatidis, 7-20 of honeysuckle, 3-9 of radix bupleuri, 1-6 of radix lithospermi, 5-20 of forsythia, 5-20 of scutellaria baicalensis, 3-9 of lightyellow sophora root, 2-7 of coptis chinensis, 4-11 of raidx astragali and 1-6 of liquorice. The traditional Chinese medicine mixture has reasonable compositions, convenient mixture taking, obvious effects and no mixture residues, conforms to the safe requirement of animal-derived foods and can be effectively applied to preventing and curing the infective livestock and poultry virosis of chicken flu, newcastle diseases, duck hepatitis viruses, swine fever, virus diarrhea, and the like. The traditional Chinese medicine mixture has the advantages of low cost, simple preparation process and no environmental pollution and is suitable for industrial production.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH
Four-gene-deletion weak-toxin strain for African swine fever viruses and application of four-gene-deletion weak-toxin strain
InactiveCN110551695AEasy to solveViral antigen ingredientsMicrobiological testing/measurementAfrican swine feverToxin
The invention discloses a four-gene-deletion weak-toxin strain for African swine fever viruses. The weak-toxin strain is the four-gene-deletion weak-toxin strain for an African swine fever virus SY18separation strain, and the following gene function protein is deleted: CD2v gene coding products and three multigene family genes( MGF360-12L, MGF360-13L and MGF360-14L ) coding products. The invention further discloses an application of the weak-toxin strain of the African swine fever viruses to preparation of vaccines for preventing or treating African swine fever. The weak-toxin strain of the African swine fever viruses can provide complete immunoprotection effect on attack of ASFV parent toxin strains, is high in safety, and is suitable for being used as vaccine candidate strains for preventing the African swine fever.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Composition for widely treating viral diseases and application thereof
The invention relates to a composition for widely treating viral diseases. The composition contains the following raw materials, or extracts of the following raw materials, or derivatives of the extracts of the following raw materials in chemically-acceptable forms, wherein the raw materials include processed products prepared from the raw materials. The constituents of the composition are as follows: 100 parts of Baikal skullcap root, 50-150 parts of radix bupleuri and 50-150 parts of kudzu root; and according to specific circumstances, one or more selected from (but not limited to) the following raw materials can be added on this basis: radix isatidis, honeysuckle flower, Fructus Forsythiae, rhubarb, phellodendron bark, rhizoma anemarrhenae, Cyrtomium fortunei, gardenia, coptis, rhizoma cyperi, fritillaria, balloonflower and licorice root. The composition provided by the invention can be prepared into powder, liquid, paste, granules, capsules or other pharmaceutically-acceptable physical forms. The composition provided by the invention is used for preventing and treating avian influenza, epidemic hemorrhagic fever, epidemic parotitis, nameless hyperpyrexia, Newcastle diseases, swine fever, blue-ear diseases and other multiple human and animal viral diseases characterized by hemorrhage or body temperature rise, and reducing the degree of pathological damage to the body.
Owner:GUANGDONG ZIJIN ZHENGTIAN PHARMA
Method for producing swine fever live vaccine with cell line
ActiveCN101181637AGuaranteed to be pureEnsure safetyAntiviralsAntibody medical ingredientsQuality controlSeedling
The invention discloses a method for producing a live swine fever vaccine by using a cell line. The present invention comprises the following technical steps: (1) selecting a cell line as the cells for making seedlings; (2) subculture and cultivation of cells for making seedlings; (3) breeding of cytotoxic species; (4) breeding of venom for making seedlings; 5) Mixing seedlings, subpackaging and freeze-drying. The invention has the advantages of simple and stable production process, easy operation, high virus content, small difference between batches, easy quality control, and can significantly improve the yield and quality of vaccines. The live swine fever vaccine produced by the invention has good safety and high immune efficacy, and has complete immune protection against the virulent attack of swine fever.
Owner:CHINA INST OF VETERINARY DRUG CONTROL +1
CHO (Chinese hamster ovary) cell strain with high-efficiency expression of CD2V protein of African swine fever (ASF)
ActiveCN110078801AHigh expressionEasy to purifyVirus peptidesMicroorganism based processesAfrican swine feverChinese hamster
The invention provides CD2V protein of African swine fever (ASF) capable of being expressed with high-efficiency in a CHO (Chinese hamster ovary) cell strain. The amino acid sequence of the CD2V protein is shown in SEQ ID NO:4; a recombinant plasmid constructed by the invention is used for expressing the CD2V protein of an African swine fever virus in CHO cells; the invention further provides a recombinant CHO cell strain prepared by transfecting the CHO cells through the recombinant plasmid, the recombinant CHO cell strain can be used to prepare the CD2V protein, and the prepared protein canbe used for differential diagnosis of the African swine fever. According to the cell strain with the expression of the CD2V protein of the African swine fever, the expression quantity is high, purification is easy, the cell strain can be used for the differential diagnosis, and a solid foundation is laid for the production of subunit vaccines and diagnostic reagents of the African swine fever.
Owner:YEBIO BIOENG OF QINGDAO
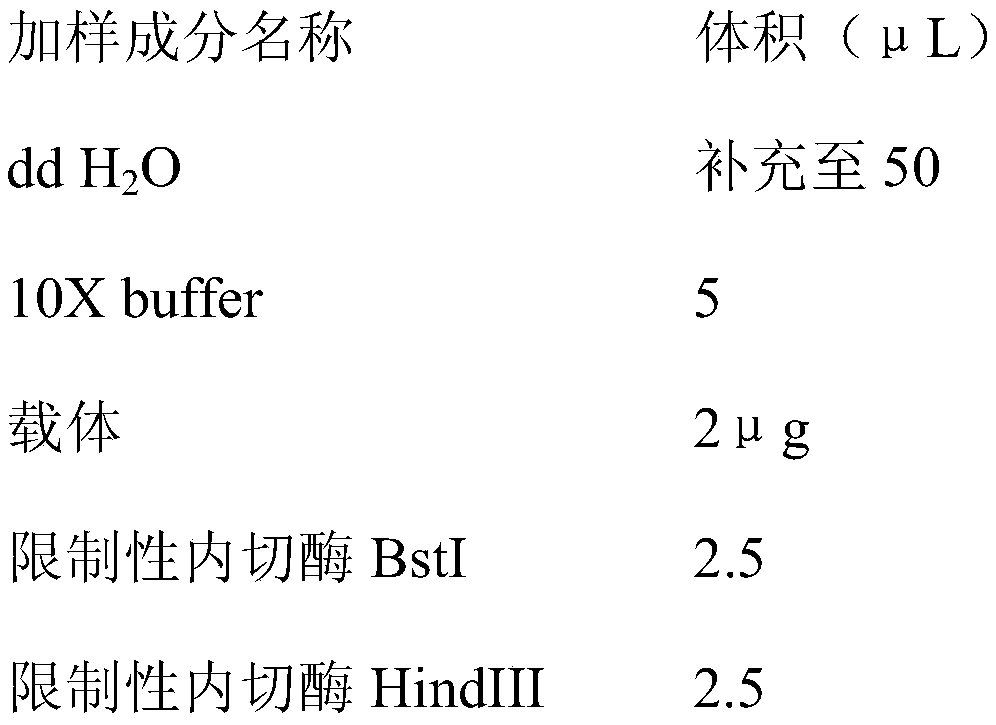
Establishing method of pig immunoglobulin Fc fragment-swine classical fever E2 fusion protein in CHO cell strain, as well as preparation method and application of fusion protein
ActiveCN106519041ASsRNA viruses positive-senseAntibody mimetics/scaffoldsFusion Protein ExpressionVaccine Production
The invention relates to a vaccine production technology in the technical field of biology, in particular to a CHO cell strain which is established by utilizing a gene engineering means and is used for expressing recombinant protein PigFC-pigSCFVE2, and a preparation method and application of the recombinant protein. The recombinant fusion protein PigFC-pigSCFVE2 provided by the invention is A1) or A2) shown as follows, wherein A1) is protein of which the amino acid sequence is as shown in SEQ ID No.2, and A2) is protein which is obtained by substituting, losing and / or adding one or several amino acid residues in the amino acid sequence of the protein of the A1) and has PigFC-pigSCFVE2 activity. A monoclonal cell strain which is obtained through the method and capable of carrying out secretory expression on PigFC-pigSCFVE2 is higher in fusion protein expression quantity, fusion protein obtained through affinity separation and purification of an antibody can be combined with a monoclonal antibody, animals can be immunized, the immunity of a generated neutralizing antibody is higher than that of a present market product, the fusion protein can be used for swine classical fever preventive vaccine, and the production cost and the immunity failure loss can be reduced.
Owner:TANGSHAN YIAN BIOLOGICAL ENG CO LTD
A real-time isothermal recombinase-polymerase amplification detection kit for African swine fever viruses
ActiveCN106521027AQuick checkRapid diagnosisMicrobiological testing/measurementMicroorganism based processesForward primerAfrican swine fever
A real-time isothermal recombinase-polymerase amplification detection kit for African swine fever viruses is disclosed. A forward primer sequence for detecting the African swine fever viruses through a method provided by the kit is shown as SEQ ID NO:1, a reverse primer sequence is shown as SEQ ID NO:2 and a probe sequence is shown as SEQ ID NO:3. A real-time isothermal recombinase-polymerase amplification method provided by the invention for ASFV detection is simple and convenient in operation, rapid in reaction and low in detection cost, can be used for ASFV detection in a laboratory and on site, particularly ASFV detection in quarantine ports, airports and epidemic disease outbreak sites, and provides a novel and reliable technique support for ASF control in China.
Owner:HANGZHOU ZHONGCE BIO SCI&TECH CO LTD
Targeted genetic engineering vaccine for African swine fever immune system
ActiveCN110760006APrevent Dependency (ADE) EffectsGood immune effectAntibody mimetics/scaffoldsVirus peptidesImmune effectsAfrican swine fever
The invention belongs to the technical field of vaccines, and particularly relates to a targeted genetic engineering vaccine for an African swine fever immune system. The main raw material of the African swine fever immune system targeted genetic engineering vaccine is African swine fever fusion protein. The African swine fever fusion protein comprises a fragment selected from p72 protein, a fragment selected from p54 protein and a fragment selected from p30 protein. Wherein the fragment selected from the p72 protein at least comprises a sequence as shown in SEQ ID NO.1, the fragment selectedfrom the p54 protein at least comprises a sequence as shown in SEQ ID NO.2, and the fragment selected from the p30 protein at least comprises a sequence as shown in SEQ ID NO.3. The African swine fever fusion protein has the advantages of good immune effect and the like.
Owner:河南省生物工程技术研究中心 +1
African swine fever B and T cell tandem epitope fusion vaccine
InactiveCN111018995AGood immune effectAvoid the risk of accelerated viral infectionAntibody mimetics/scaffoldsViral antigen ingredientsAfrican swine feverTGE VACCINE
The invention, which belongs to the technical field of vaccines, particularly relates to an African swine fever B and T cell tandem epitope fusion vaccine. The main component of the African swine fever B and T cell tandem epitope fusion vaccine is African swine fever tandem epitope fusion protein. The African swine fever tandem epitope fusion protein comprises a B cell neutralizing epitope peptidefragment and a T cell epitope; and the B cell neutralizing epitope peptide comprises the following fragments: at least one neutralizing epitope peptide of each of p72, p54, p30 proteins. When the African swine fever tandem epitope fusion protein is used as a vaccine, the immune effect is good; and the antibody level significantly higher than that of a control group can be detected after one immunization. Since the non-neutralizing epitope is reduced as much as possible in the fusion protein, the risk of accelerating virus infection (ADE effect and antibody dependence enhancement effect) by anon-neutralizing antibody after immunization can be avoided.
Owner:河南省生物工程技术研究中心 +1
Biologically safe Africa swine fever antigen multifactorial serum for ELISA diagnosis
The invention relates to a biologically safe Africa swine fever antigen multifactorial serum for ELISA (enzyme-linked immuno sorbent assay) diagnosis. A technical scheme adopted in the invention includes: adopting gene-expressed structural protein P72, K205R, P54, and A104R, conducting chemical purification, carrying out coating with a Freund's incomplete adjuvant, performing intramuscular immunization on laboratory swine in three batches, collecting swine blood after one month, separating serum, implementing serological testing, and conducting subpackaging and preservation. The serum is subpackaged into ELISA kits to undergo test according to conventional ELISA test methods.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
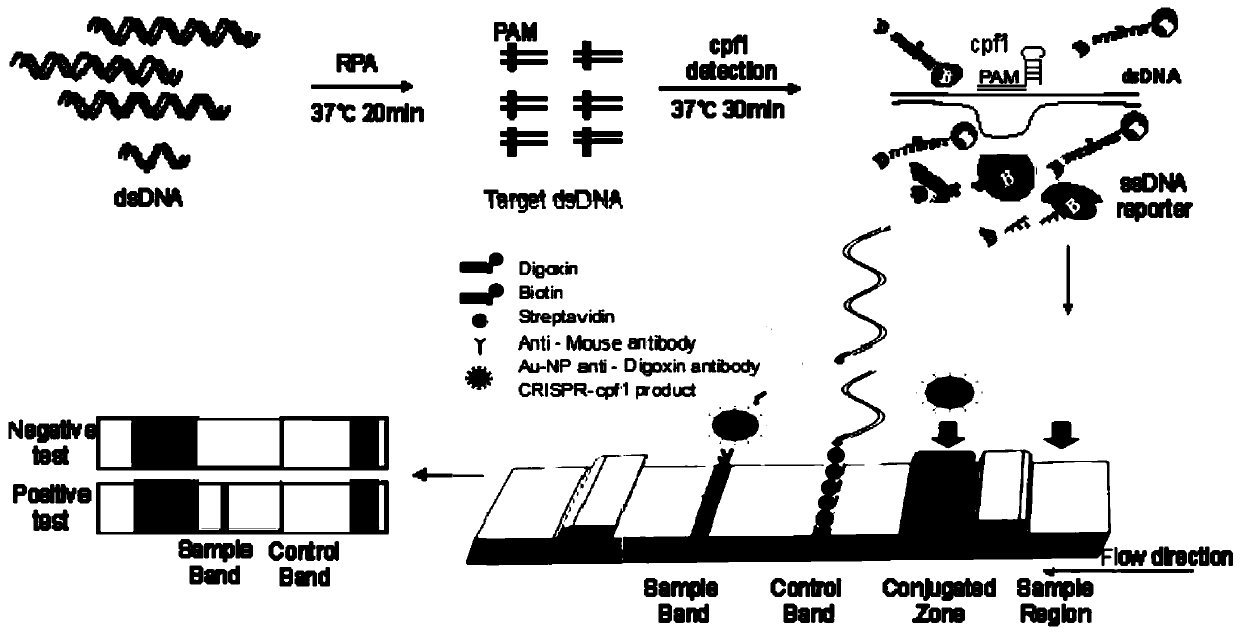
Cpf1 reagent kit and detection method for quickly detecting nucleic acid of African swine fever virus
ActiveCN110551846AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationAfrican swine feverFluorescence
The invention discloses a Cpf1 reagent kit for quickly detecting nucleic acid of an African swine fever virus. The Cpf1 reagent kit comprises a Cpf1 detection system suitable for quickly detecting theAfrican swine fever virus, and an immune colloidal gold test strip, wherein the Cpf1 detection system comprises specific crRNA protein, specific Cpf1 protein and a single-chain DNA(ssDNA) reporting system in accordance with a p72 gene of the African swine fever virus, the specific crRNA is one or more of crRNAs from ASFV P72 crRNA1 to ASFV P72 crRNA10, and the sequence of the specific crRNA is SEQ NO.4 to SEQ NO.13; and the single-chain DNA(ssDNA) reporting system comprises ssDNA FQreporter for fluorescence detection of a microplate reader and / or ssDNA DB reporter for detecting the immune colloidal gold test strip. According to the Cpf1 reagent kit disclosed by the invention, for the first time, the Cpf1 is used for detecting the African swine fever virus, and has the advantages of beinghigh in sensitivity, high in specificity, short in time consumption, high in flux, independent of large-scale experiment equipment and the like. The advantages enable a detection method based on the immune colloidal gold test strip developed by the invention to be conveniently used in basic laboratories and breeding enterprises to be used for performing detection, identification and diagnosis on basic quick detection of the African swine fever.
Owner:SHANGHAI TECH UNIV
Method for preparing live vaccines of hog cholera and product thereof
InactiveCN101879311ASmall batch-to-batch quality varianceStable production processInactivation/attenuationAntiviralsVaccine ProductionFreeze-drying
The invention discloses a method for preparing live vaccines of hog cholera and a product thereof. The preparation method comprises the following steps of: (1) culturing porcine passage cell lines; (2) inoculating the porcine passage cell lines with live vaccine production seed viruses of the hog cholera to obtain attenuated vaccine strains of the hog cholera; (3) performing virus multiplication on the attenuated vaccine strains of the hog cholera; (4) measuring the virus titer of multiplication virus suspension by adopting an immunofluorescence method; and (5) adding a freeze-drying protective agent and antibiotics into the virus suspension which is detected to be qualified for vaccine matching and freeze-drying. The preparation method has the advantages of producing the live vaccines of the hog cholera by using the cell lines so as to achieve small quality differences among batches and the characteristics of simple and stable process, easy operation, high yield, low cost, the feasibility and extendibility of industrial production and the like, and measuring the virus titer of the multiplication virus suspension by adopting the immunofluorescence method so as to achieve sensitive, fast, specific and accurate detection, high repeatability and reliable results. The live vaccines of the hog cholera prepared by the method can completely protect pigs from the attacks of violent hog cholera viruses.
Owner:武华
ELISA kit for distinctively detecting antibodies of classical swine fever (CSF) vaccine immunity and wild virus infection and preparation method thereof
InactiveCN101900731AConvenient prevention and controlImprove purification effectMaterial analysisElisa kitStructural protein
The invention relates to an ELISA kit for distinctively detecting antibodies of classical swine fever (CSF) vaccine immunity and wild virus infection and a preparation method thereof. An indirect ELISA kit or a blockage ELISA kit is formed by expressing and purifying classical swine fever virus (CSFV) non-structural protein NS3, coating a solid-phase carrier and assembling with other matched reagents. The kit has the characteristic of distinctively detecting different antibodies generated by the CSF vaccine immunity and the wild virus infection. The kit can distinctively diagnose the CSF vaccine immunity and the wild virus infection.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
PCR primer for detecting African swine fever virus, kit and application thereof
ActiveCN105695634AAccurate diagnosisEasy to distinguishMicrobiological testing/measurementDNA/RNA fragmentationAfrican swine feverNucleotide sequencing
The invention discloses a PCR primer for detecting an African swine fever virus, a kit and application thereof, and belongs to the detection field of the African swine fever virus. According to a conserved region of an ASFV p72 gene on the GenBank, four pairs of primers are designed, the primer with strong specificity and high sensitivity is screened out from the four pairs of primers, and the primer is composed of nucleotide sequences as shown in SEQ ID No.1 and SEQ ID No.2. The invention further discloses a kit prepared from the primer and used for detecting the African swine fever virus, and a corresponding PCR detection method is established. The detection method established by the invention is more specific and sensitive in comparison with two African swine fever virus PCR detection methods recommended by OIE; and the clinical sample detection result shows that the PCR detection method disclosed by the invention is simple in operation, low in cost, good in specificity and high in sensitivity, and can be effectively applied to the screening and fast diagnosis of the African swine fever.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains
PendingCN110184390ARapid identificationImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationTonsilAfrican swine fever
The invention provides an amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains. A P72 gene of ASFV and a 5'UTR noncoding region of CSFV arerespectively used as an amplification target area, a pair of specific primers and a TaqMan MGB probe (SEQ ID NO:1-6) are designed, a real-time fluorescent quantitation PCR(FQ-PCR) technique is used, and identification and detection of ASFV and CSFV are realized. The detection reagent kit provided by the invention is suitable for detecting viral nucleic acid in samples of serum, spleen, lymph nodes, tonsil, kidney and the like of suspected ASFV or CSFV infected pigs, the sensitivity can reach 1.0*10<1>copy / [mu]L, and the detection reagent kit does not have any cross reactions with other pathogens which are liable to be in mixed infection with the ASFV and the CSFV or of which the infection symptoms are similar such as PRRSV, PRV, PCV2, PPV, JEV and HPS.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Hybridoma cell line of monoclonal antibody against African swine fever virus and secreted monoclonal antibody thereof
InactiveCN101831407AHigh utility valueMicroorganism based processesImmunoglobulins against virusesBALB/cPurification methods
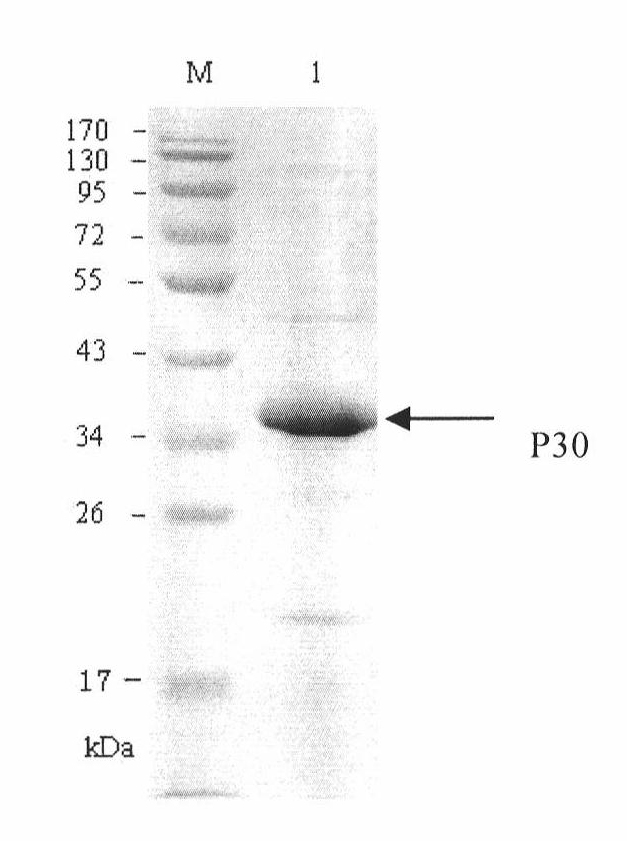
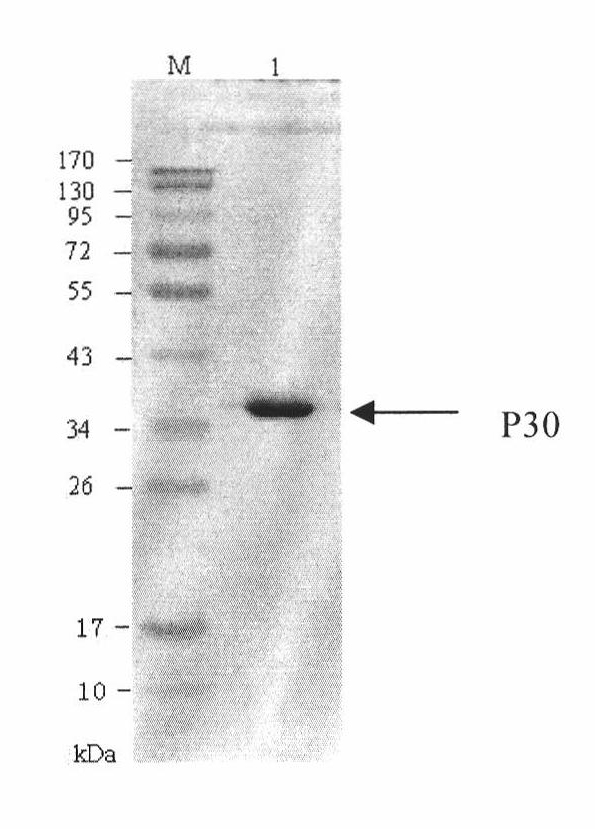
The invention discloses a hybridoma cell line of a monoclonal antibody against African swine fever virus and the secreted monoclonal antibody thereof. The preparation method of the invention comprises the following steps: preparing a recombined P30 soluble antigen by prokaryotic expression; immunizing a BALB / c mouse; and finally fusing, screening and cloning by a hybridoma technology to obtain the hybridoma cell line which can stably secrete the monoclonal antibody against African swine fever virus P30 protein. The invention further discloses a method for preparing the monoclonal antibody with the cell line, an antibody purification method and a labeling method for horseradish peroxidase of the antibody. The monoclonal antibody can be used in detecting the African swine fever viral antibody in pig serum.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Real-time fluorescent LAMP detection primer group, kit and detection method of African swine fever virus non-structural gene
InactiveCN106947838ASensitiveImprove featuresMicrobiological testing/measurementMicroorganism based processesGenotypeFluorescent pcr
The invention provides a real-time fluorescent LAMP detection primer group, a kit and a detection method of an African swine fever virus (ASFV) non-structural gene. The primer group is designed based on a non-structural DNA polymerase G1211R gene and comprises an FIP primer, a BIP primer, an F3 primer and a B3 primer. A detection result shows that a typical S-shaped nucleic acid amplification curve, and an amplification product has a specific melting curve. An ASFV70 strain virus nucleic acid is taken as a template, and LAMP detection is better than a fluorescent PCR (Polymerase Chain Reaction) method in sensitivity. Intra-assay and inter-assay variable factors of repetitive testing LAMP detection are both smaller than 5 percent. An ASFVArm07 stain is used for preparing various clinic simulated samples, and detected positive rate is up to 17.31 percent. The detection method provided by the invention can provide a new technological means for preventing and controlling African swine fever virus, and detection on different genetic stains and quick screening for exit and entry are facilitated.
Owner:TECH CENT OF GUANGZHOU CUSTOMS
Immunogenic compositions and vaccines comprising african swine fever virus peptides and proteins and uses thereof
The present invention relates to African swine fever virus (ASFV) peptides and / or polypeptides as well as immunogenic fragments thereof, corresponding encoding AFSV oligonucleotides and / or polynucleotides as well as immunogenic fragments thereof, immunogenic compositions, vaccines and uses thereof.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
DNA sequence encoding African swine fever virus antigen, composition of antigen encoded thereby and use thereof in immunological detection
ActiveCN110093356ALow detection limitHigh sensitivityVirus peptidesGenetic engineeringAntigenAfrican swine fever virus Antigen
The invention provides a DNA sequence encoding African swine fever virus antigen, a composition of the antigen encoded thereby and use thereof in immunological detection. and also provides an application of the composition of the antigen encoded by the above DNA sequence, and the above antigen composition for an immunological detection method for detecting the African swine fever virus. A codon optimization method capable of eliminating rare codons is used for optimizing the DNA sequences of CP204L, PK205R, PB602L, CP530R, E183L, and PB646L in the major membrane proteins and capsid proteins encoding the African swine fever virus, the above-described codon-optimized nucleotide sequence can efficiently express the above-mentioned main membrane proteins and capsid proteins in a suitable expression system, and an established immunological detection method can detect Africa swine fever virus quickly and simply on a large scale.
Owner:SHENZHEN ANIEASY BIOTECHNOLOGY CO LTD +1
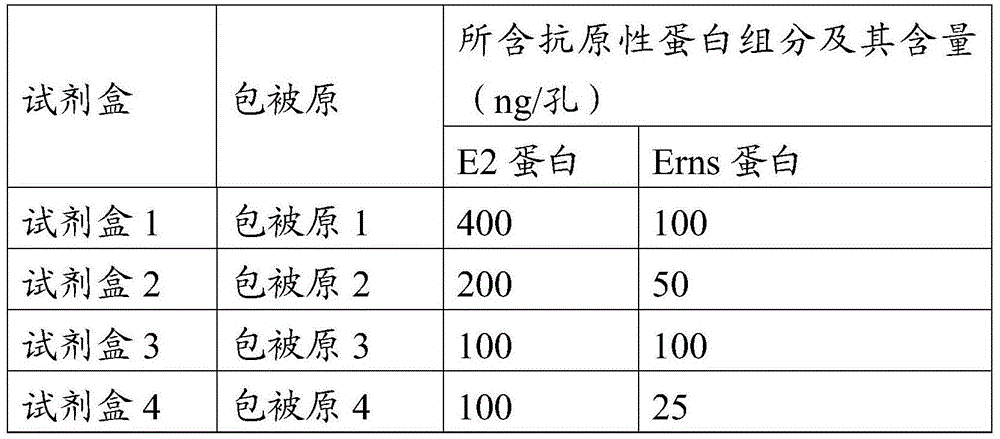
CSFV antibody detection system and preparation method thereof
ActiveCN105527442AHigh detection rate sensitivityImprove capture efficiencyBiological testingSerum igeE2 protein
The invention provides a CSFV antibody detection system and a preparation method thereof. A coating antigen of the detection system contains CSFV E2 protein and Erns protein. The CSFV E2 protein and Erns protein are recombinant proteins expressed by eucaryon, correct spatial conformation and posttranslational modification process can be guaranteed, antigen is capable of effectively combining with the antibody in serum, and specificity, sensitivity and repeatability of detection can be increased. The system and the method can be sued for diagnosis of CSFV antibody in prevention and control of CSFV as well as immunization evaluation of a CSFV vaccine.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Preparation method and application of electrochemical immunosensor based on HS-beta-CD-Ag-GOD conjugate
ActiveCN104459124ACatalytic reductionDoubly magnifiedBiological material analysisMaterial analysis by electric/magnetic meansImmune profilingBovine serum albumin
The invention belongs to the technical fields of immunoassay and biosensing and discloses a preparation method and application of an electrochemical immunosensor based on an HS-beta-CD-Ag-GOD conjugate. The electrochemical immunosensor is used for rapidly detecting a hog cholera virus antigen CSFV. The manufacturing scheme comprises the following steps: modifying a working electrode a bare platinum carbon electrode by using MWCNTs-CD-Fc-Ab1, and sequentially adding bovine serum albumin, hog cholera virus antigen CSFV and an Ab2-HS-beta-CD-Ag-GOD conjugate. The HS-beta-CD-Ag-GOD conjugate can convert glucose into gluconic acid, two protons and two electrons are transferred to oxygen molecules, hydrogen peroxide is generated, and an HS-beta-CD-Ag nanometer material serves as a mimic enzyme, the reduction of the hydrogen peroxide is catalyzed, the dual amplification of an electrochemical signal is realized, the high sensitivity is realized, and the detection limit can be low to 0.33pg / mL.
Owner:UNIV OF JINAN
Traditional Chinese medicine composition for treating disease of swine fever
The invention discloses a traditional Chinese medicine composition for treating a disease of swine fever, which relates to the technical field of veterinary medicines in biomedicine. The traditional Chinese medicine composition for treating the disease of the swine fever is prepared from the following raw materials according to parts by weight: 12 to 16 of rhizoma coptidis, 18 to 20 of baikal skullcap root, 15 to 18 of yellow-corktree bark, 18 to 20 of rhubarb, 15 to 18 of honeysuckle, 15 to 17 of gardenia, 12 to 14 of gypsum, 15 to 18 of rhizoma anemarrhenae, 20 to 30 of dandelion, 14 to 18 of garden burnet, 22 to 28 of white peony root, 15 to 18 of tuckahoe, 22 to 28 of officinal magnolia, 12 to 15 of poncirus trifoliata, 13 to 15 of dark plum fruit, 18 to 20 of realgar and 22 to 28 of licorice. The traditional Chinese medicine composition for treating the disease of the swine fever has the effects of clearing heat, expelling toxin, removing heat from the blood, purging intense heat, removing dampness by diuresis, relieving restlessness, washing bowels, removing the stagnancy of the fu-organs, regulating qi, strengthening the body resistance, regulating the functions of the liver and the spleen and the like. The traditional Chinese medicine composition for treating the disease of the swine fever has the characteristics that the traditional Chinese medicine composition for treating the disease of the swine fever is suitable for treating the disease of the swine fever, the curative effect is high and the like. The traditional Chinese medicine composition for treating the disease of the swine fever not only can be used for treating the disease of the swine fever with various epidemic situations but also can be used for preventing the disease of the swine fever.
Owner:高天生
Traditional Chinese medicine for treating swine fever
The invention discloses a traditional Chinese medicine for treating swine fever, which is characterized in that the traditional Chinese medicine is prepared by the following components according to weight portions: 30 to 50 portions of realgar, 20 to 50 portions of fineleaf schizonepeta herb, 20 to 50 portions of honeysuckle flower, 20 to 50 portions of radix bupleuri, 20 to 50 portions of radix puerariae, 20 to 50 portions of baical skullcap root, 20 to 50 portions of akebia stem, 20 to 50 portions of radix isatidis, 20 to 50 portions of liquoric root, 20 to 50 portions of indigowoad leaf and 20 to 50 portions of dried ginger. The traditional Chinese medicine has more obvious effect for preventing and treating swine fever.
Owner:张玉国
Preparation method and application of classical swine fever virus recombinant subunit vaccine
InactiveCN104826100ANo risk of contaminationImprove securityAntiviralsAntibody medical ingredientsProtein targetVaccine Production
The invention discloses a preparation method and application of a classical swine fever virus recombinant subunit vaccine with the amino acid sequence shown as SEQ ID No.1. The preparation method of the classical swine fever virus recombinant subunit vaccine typically includes the following steps: classical swine fever E2 truncated protein (TE2) coding gene is cloned into baculovirus vector pFastBacTM1, and is then transfected into Sf9 insect cells to obtain recombinant baculovirus capable of expressing protein TE2. The high five insect cells in logarithmic growth phase are infected by the recombinant baculovirus, so that a large amount of the protein TE2 can be expressed in a cell culture supernatant. Finally, the cell culture supernatant is recovered and purified to obtain a large amount of the recombinant protein TE2 with the purity more than 90%. According to the method, the target protein can be harvested from the cell culture supernatant, the time of protein purification is reduced, consumption of a large amount of time can be avoided, and the vaccine production process can be simplified. Under the premise of simplification of the vaccine production process, the recombinant protein TE2 has the advantages of strong immunogenicity and high safety, and the animal experiments prove that the recombinant protein can effectively stimulate the body to produce a highly effective humoral immune response.
Owner:NOVO BIOTECH CORP
Attenuated African swine fever virus with gene deletion and its application as a vaccine
ActiveCN110093324BGood immune protectionFull Poison Attack ProtectionViral antigen ingredientsVirus peptidesAfrican swine feverGenetic engineering
The invention relates to a gene deletion attenuated African swine fever virus as a vaccine and the vaccine, and a construction method thereof. An African swine fever Chinese epidemic strain Pig / CN / HLJ / 2018 is adopted, a virulence gene of the African swine fever virus is deleted by a genetic engineering technology, and the gene deletion virus of MGF360-505R deletion and joint deletion of CD2V and MGF360-505R is obtained. Experiments show that the two virus strains can provide 100% immune protection against the African swine fever Chinese epidemic virulent strains, can be used as vaccines for safe and effective prevention and control of African swine fever in China, and have great social value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
LAMP kit for detecting hogcholera virus and preparation method thereof
InactiveCN101358246AEasy to operateJudging whether to expand or notMicrobiological testing/measurementFood safetyQuarantine
The invention belongs to the field of sanitary examination, and relates to a LAMP kit for testing classical swine fever virus and an establishing method and an application thereof. The kit contains a test system which is composed of the LAMP reaction liquid of six LAMP primers. The tests prove that the kit of the invention has good specificity and sensitivity, fast amplification speed, high efficiency and simple and convenient identification. The test system of the invention can rapidly and conveniently test the classical swine fever virus in high-efficiency, high-specificity and high-sensitivity under the isothermal condition of 65 DEG C without complicate instruments, can better satisfy the spot tests for the classical swine fever virus, provides a novel technical platform for food safety testing, can better meet the urgent requirements for the spot testing of foot and mouth diseases at present, is used for the spot testing of import and export quarantine, food sanitary departments, animal breeding farms, etc, and is easy to be popularized in a wide range.
Owner:SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C
Live pig farming method
InactiveCN105660524APromote growthGood adaptation to growthFood processingAnimal feeding stuffIron supplementPig farming
The invention discloses a live pig farming method. The live pig farming method comprises the following steps of 1 pig house construction; 2 feeding management, wherein piggies are born, teeth and tails are cut, the piggies are injected with iron supplementing injections in 7 days, castrated after 12 days, injected with swine fever vaccines after 21 days and weaned to be fed with piggy feed, and the piggies are fed with conservation feed after being fed for 10 days, fed with piglet feed when the piggies are fed to be about 30 jin, fed with traditional Chinese medicine pig feed when the piggies about 70 jin until the piggies are fed to be about 200 jin and fed with fattening feed until the pigs grow to be about 250 jin; 3 feed management; 4 environmental management. According to the live pig farming method, through scientific control over the pig house size, feeding management, feed management and environmental management, it is guaranteed that the pig house environment can well adapt to live pig growth, and occurrence of swine diseases can be reduced; in addition, it can be guaranteed that feed nutrition is well absorbed by the pigs by reasonably mixing feed and adopting a Chinese herbal medicine feeding mode, and therefore rapid growth of the pigs is guaranteed.
Owner:孙坤
African swine fever fluorescent PCR (polymerase chain reaction) assay reagent, African swine fever fluorescent PCR assay kit and application thereof
ActiveCN106957927AIncreased sensitivityHigh homologyMicrobiological testing/measurementMicroorganism based processesFluorescenceAfrican swine fever
The invention relates to an African swine fever fluorescent PCR (polymerase chain reaction) assay reagent, an African swine fever fluorescent PCR assay kit and application thereof, which belong to the field of bioengineering. With the p72 gene as a reference, a pair of specific PCR primers and a TaqMan fluorescent probe are designed and optimized. Moreover, the primers are designed with the full length of the p72 gene to amplify the p72 gene, and the PCR product is cloned into a pGEM-T vector. Standard curves are drawn with a positive plasmid as a standard product, and the assay range is 10 to 1.0*10<7> copies. In the whole process of assaying African swine fever by an African swine fever fluorescent PCR method disclosed by the invention, only 2.5 to 3 hours are taken from DNA extraction to the appearance of an assay result, manual operation is greatly reduced, and time is greatly shortened. Furthermore, a plurality of samples can be assayed each time, and particularly assay of a large batch of samples can be realized.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
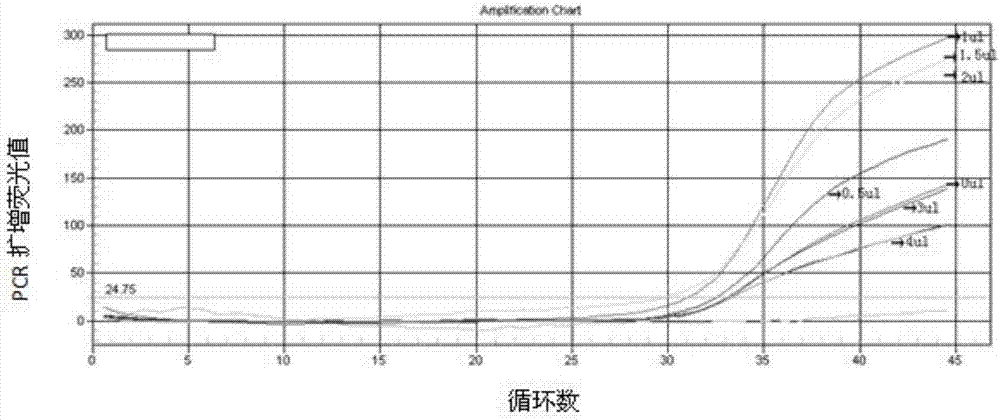
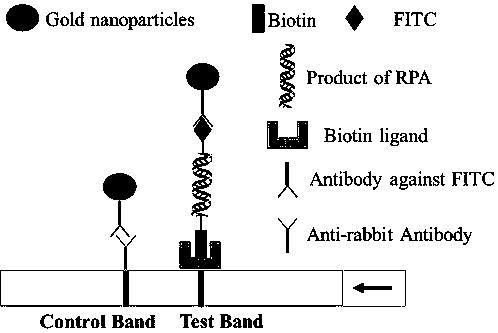
RPA (recombinase polymerase amplification) primer and detection kit for rapidly detecting African swine fever viruses
PendingCN109797246AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesQuarantinePorcine reproductive and respiratory syndrome virus
The invention discloses an RPA (recombinase polymerase amplification) primer and a detection kit for rapidly detecting African swine fever viruses. Target genes can be effectively amplified, specificity is 100%, detection sensitivity is 102 copy / reaction, and sensitivity is equivalent to that of fluorescent quantitative PCR (polymerase chain reaction). Cross reaction between the RPA amplificationprimer and one of classical swine fever viruses, vesicular exanthema swine viruses I, porcine reproductive and respiratory syndrome viruses, porcine circoviruses and the like is omitted. A RPA isothermal amplification system is rapidness in reaction and wide in temperature range, effective amplification of the target genes can be achieved at the temperature of 38-46 DEG C, and the detection kit can rapidly, efficiently and sensitively detect the African swine fever viruses and has the advantages that the kit is simple to operate, high in specificity, safe and free from pollution, reaction results are easily observed and the like. Effective technical means are provided for on-site rapidness detecting and screening of infection nucleic acid of the African swine fever viruses, and the RPA amplification primer has great significance for control of infection spreading of the African swine fever viruses in China and inspection and quarantine in infected areas and entry and exit ports.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com