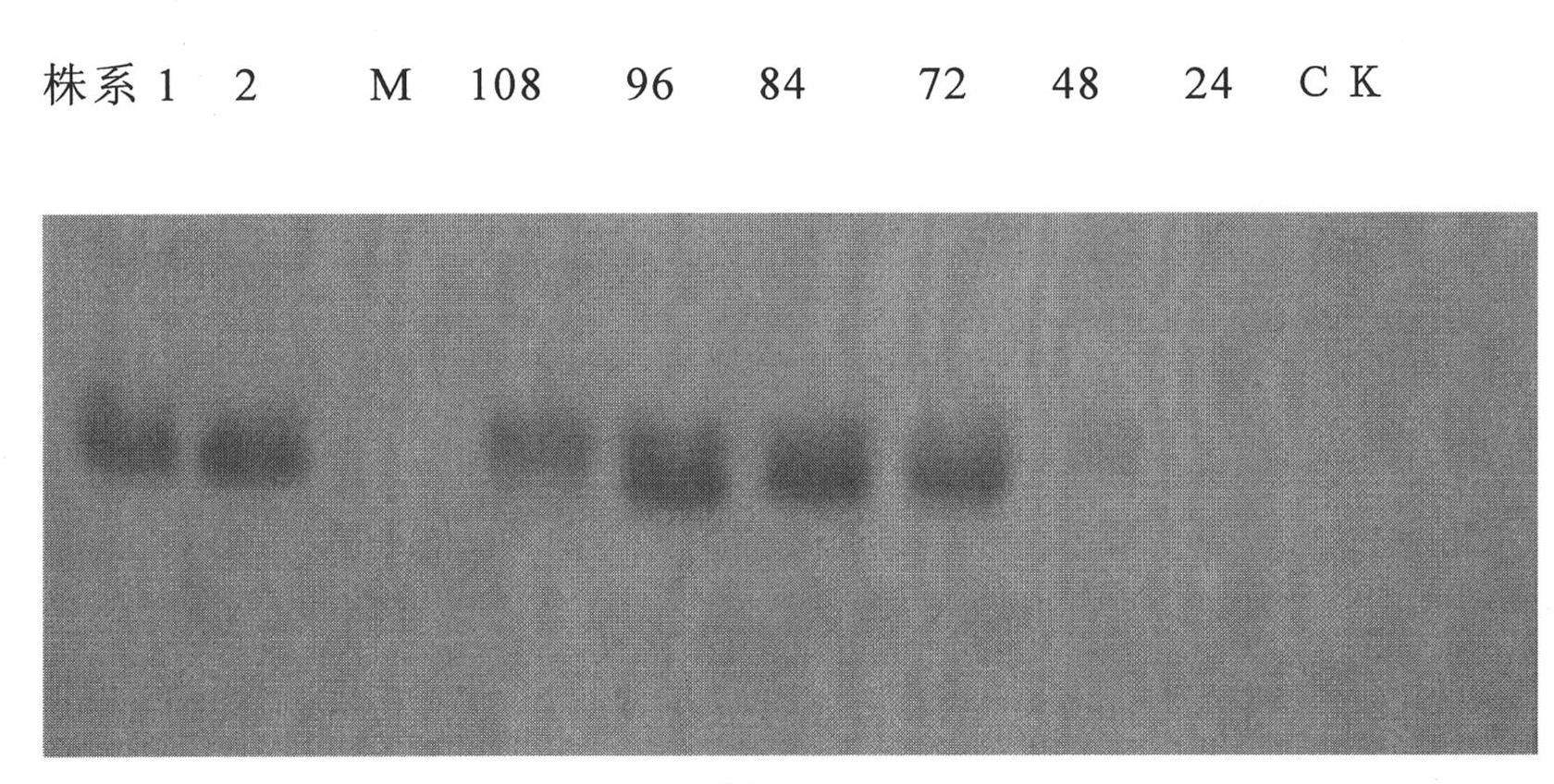
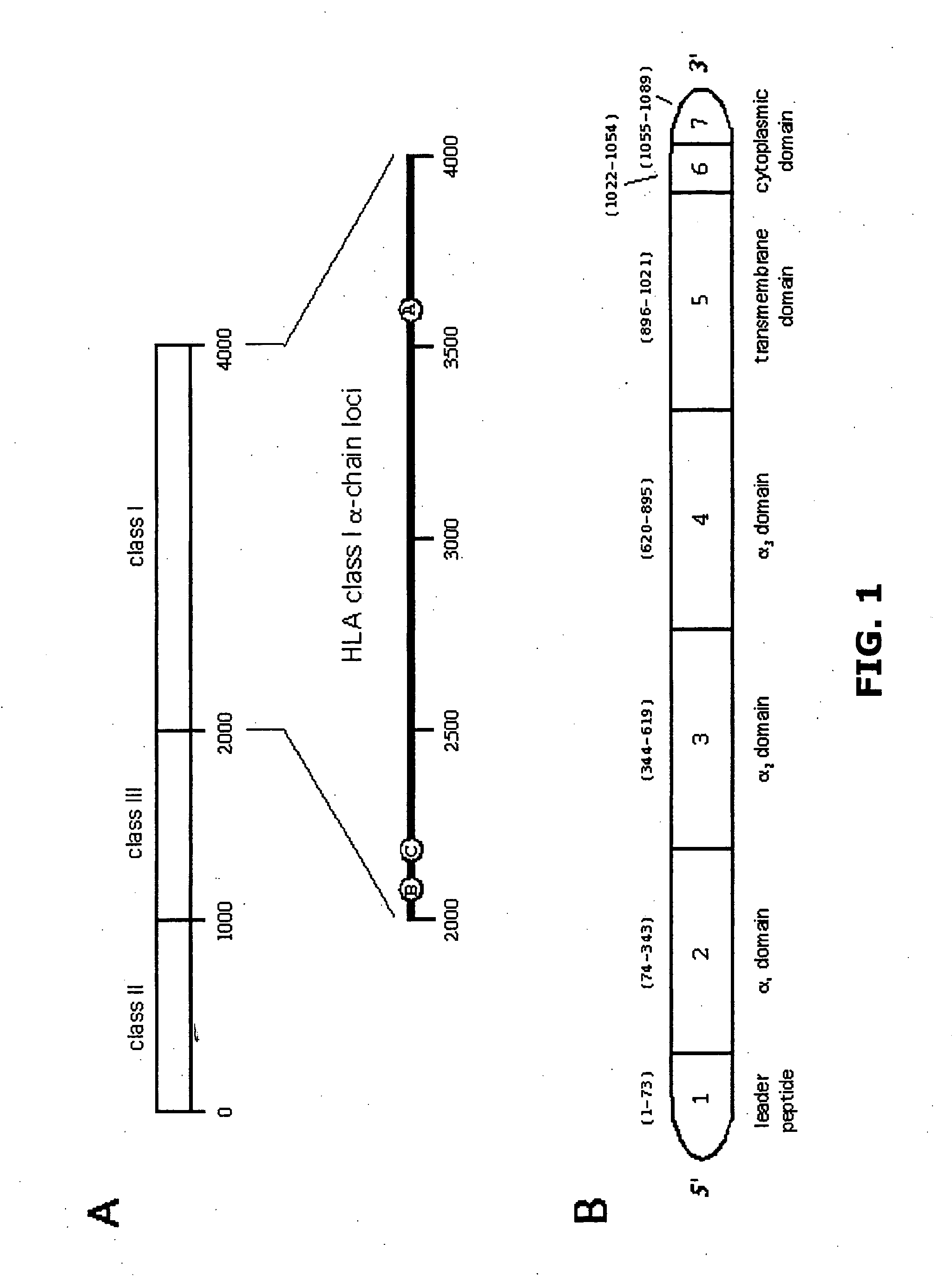
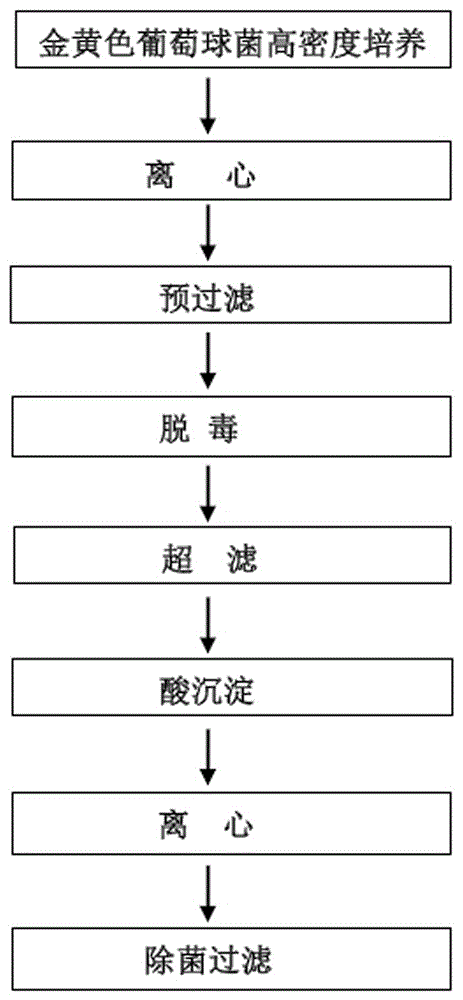
Patents
Literature
69 results about "Soluble antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An antigen dissolved in a liquid. A soluble antigen is recognized by B lymphocytes but cannot be detected by T lymphocytes until it has been processed by an antigen-presenting cell.
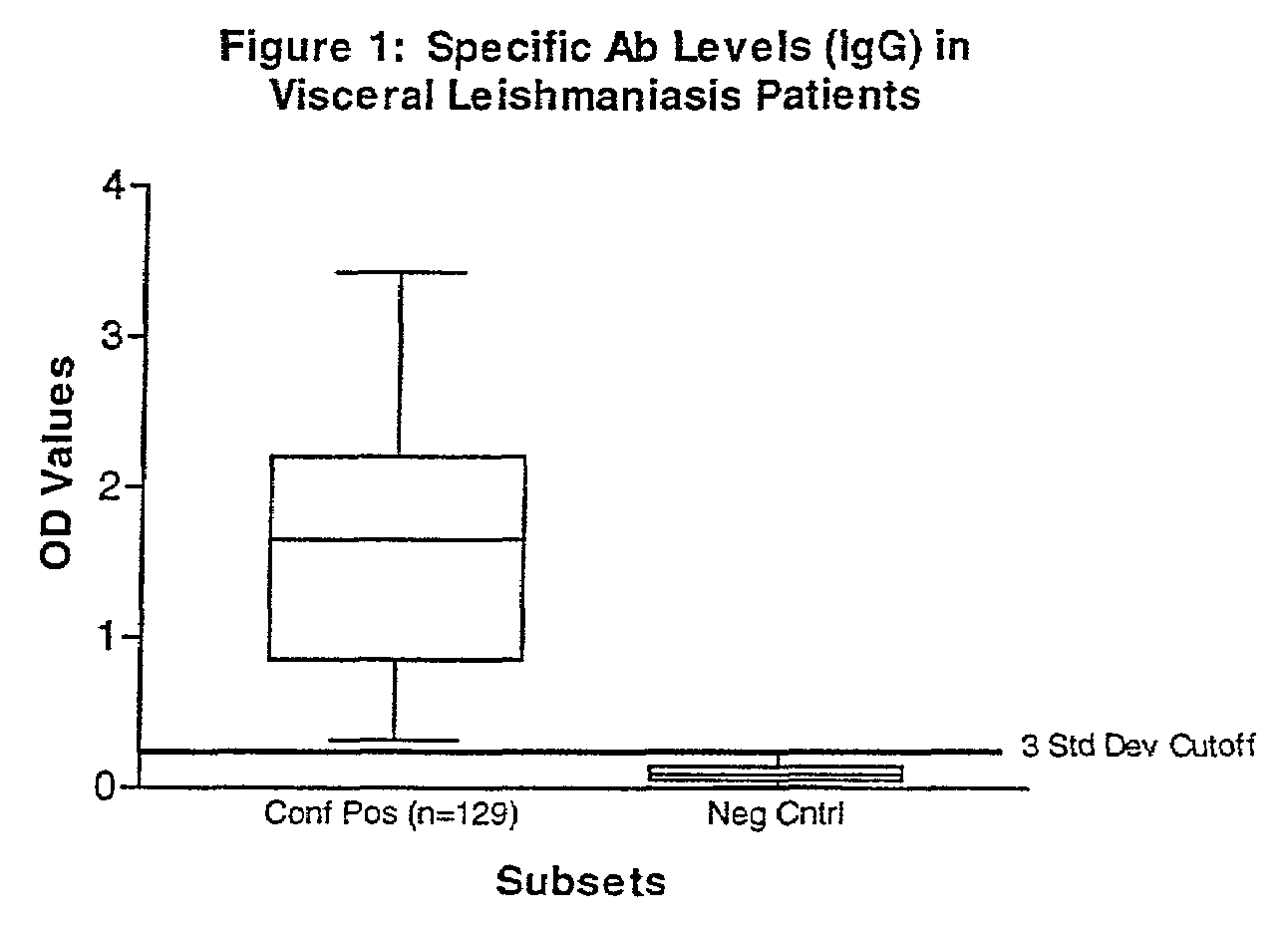
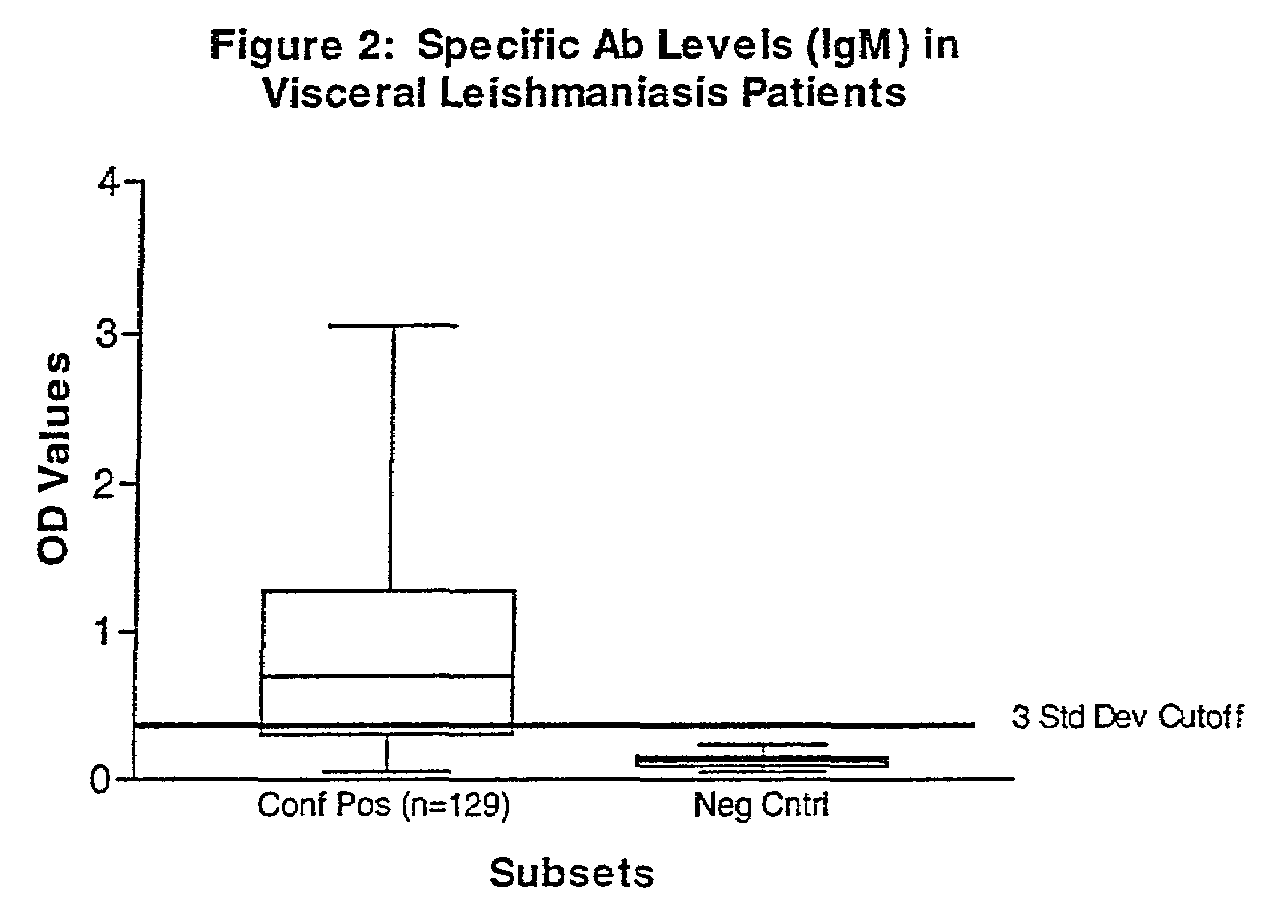
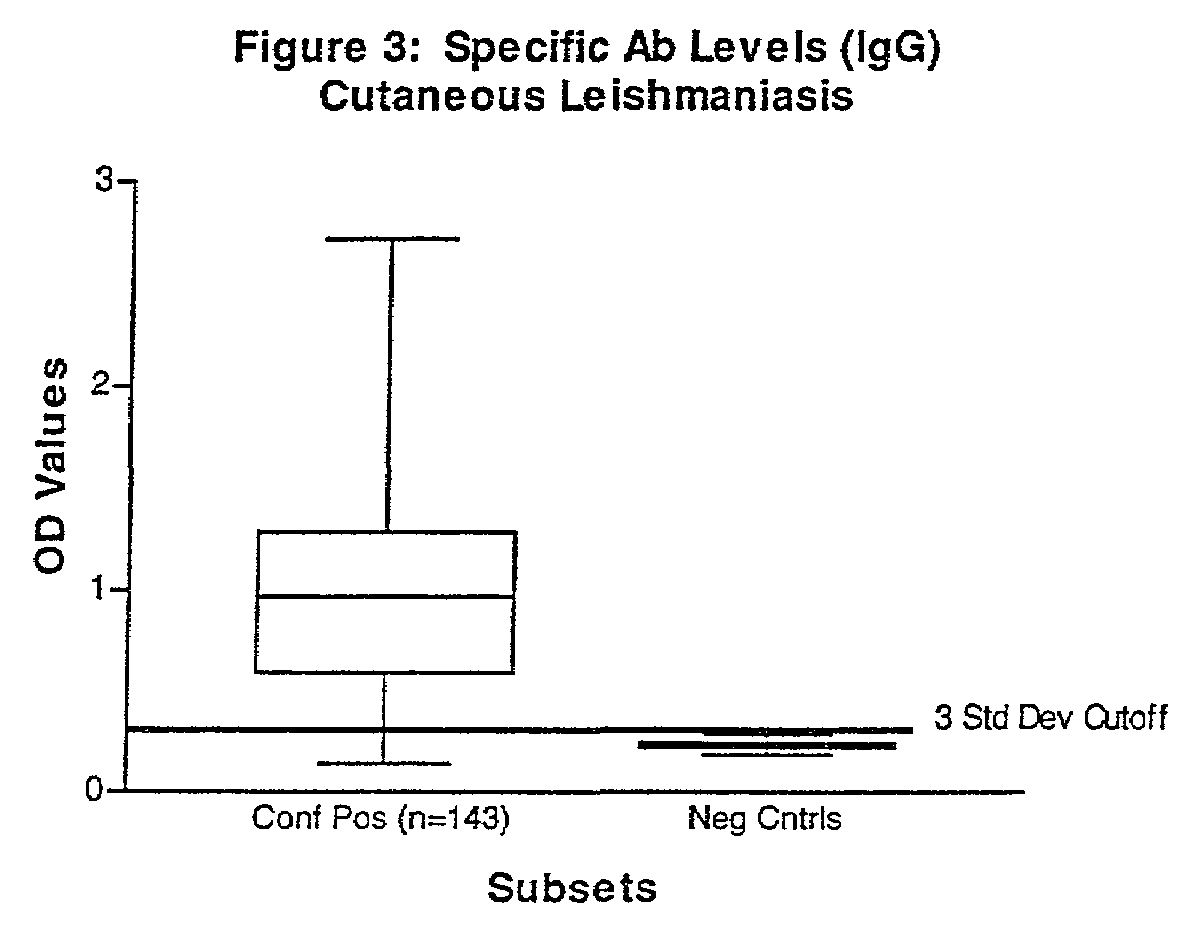
Practical serological assay for the clinical diagnosis of leishmaniasis
InactiveUS7008774B2Improve purification effectPurification is easy and lessBiocideProtozoa antigen ingredientsProtozoaAntigen capture
Methods for the diagnosis of visceral, cutaneous and canine leishmaniasis in a subject suspected of being infected with the parasitic protozoa Leishmania is disclosed. Disclosed are antibody-capture enzyme-linked immunosorbent assays (ELISAs) for the detection of antibodies to Leishmania parasite soluble antigens and antigen-capture ELISAs for the detection of Leishmania parasite soluble antigens in host samples. Also disclosed are immunodiagnostic kits for the detection of Leishmania parasite circulating antigens or IgM and IgG antibodies in a sample from subject having visceral, cutaneous or canine leishmaniasis. In these methods and kits, detection may be done photometrically or visually. The methods and kits also allow the visualization of Leishmania amastigotes or promastigotes in a sample.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
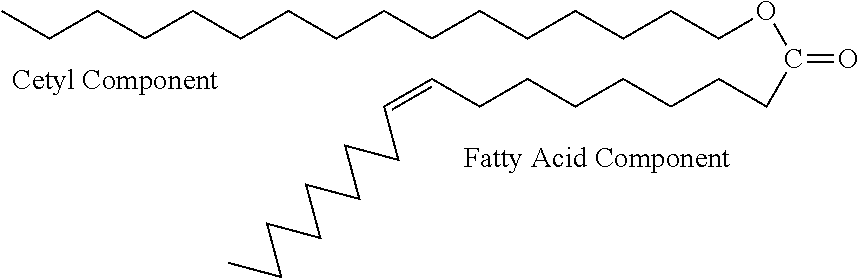
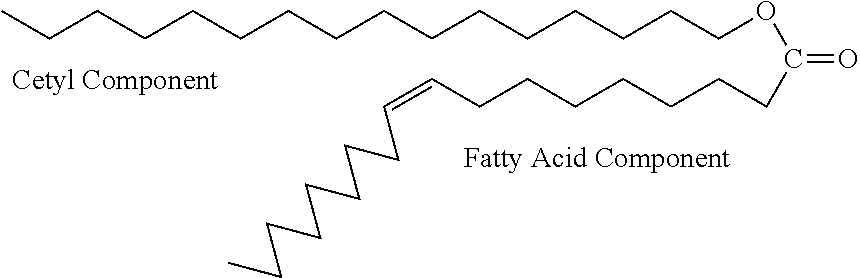
Transdermal delivery of medicaments with combinations of cetylated fatty ester penetrant complexes
This invention describes a topical delivery mechanism that contains a mixture of cetylated fatty esters that act as transdermal carriers of desired therapeutic molecules. The proposed cetyl fatty ester penetrant-complex (Base CFEP-complex) contains specific cetyl fatty esters, polar solvents, a carrier base (gel, cream, lotion, patch or stick gel), antioxidants and the desired pharmaceutical, cosmetic or antigenic response eliciting molecules that are efficaciously delivered by selectively varying component ratios in the complex. The invention proposes the use of transdermal delivery of medications such as those used in treatment of urinary incontinence, testosterone deficiency, arthritic and joint pain and other pains such as pain in the neck, lower back, back, knees, headaches, and other types of inflammatory pains, peripheral neuropathic pain, pain associated with repetitive strain injuries such as myofacial pain, rapid treatment of epileptic seizures, soluble antigens in the immuno-therapeutic treatment of allergies, actives in the treatment of foot cracks and elbow cracks, actives in the treatment of facial and other wrinkles in the form of anti-aging creams and gels and other topically delivered therapies.
Owner:CYMBIOTICS
Transdermal delivery of medicaments with combinations of cetylated fatty fatty ester penetrant complexes
InactiveUS20110065627A1Facilitate more efficacious permeationReduce resistanceCosmetic preparationsBiocideWrinkle skinAntioxidant
This invention describes a topical delivery mechanism that contains a mixture of cetylated fatty esters that act as transdermal carriers of desired therapeutic molecules. The proposed cetyl fatty ester penetrant-complex (Base CFEP-complex) contains specific cetyl fatty esters, polar solvents, a carrier base (gel, cream, lotion, patch or stick gel), antioxidants and the desired pharmaceutical, cosmetic or antigenic response eliciting molecules that are efficaciously delivered by selectively varying component ratios in the complex. The invention proposes the use of transdermal delivery of medications such as those used in treatment of urinary incontinence, testosterone deficiency, arthritic and joint pain and other pains such as pain in the neck, lower back, back, knees, headaches, and other types of inflammatory pains, peripheral neuropathic pain, pain associated with repetitive strain injuries such as myofacial pain, rapid treatment of epileptic seizures, soluble antigens in the immuno-therapeutic treatment of allergies, actives in the treatment of foot cracks and elbow cracks, actives in the treatment of facial and other wrinkles in the form of anti-aging creams and gels and other topically delivered therapies.
Owner:CYMBIOTICS
Immune chromatographic paper strip and method for quick detecting pathogen and toxin in food
The present invention discloses a kind of immunogical chromatographic paper strip for sensitive, specific and fast detection of common pathogen and toxin in food and the detection method. The present invention is the new technology for detecting pathogen and toxin in food and its application. Now, there are great amount of reports on applying colloidal gold immunogical chromatographic test paper in detecting soluble antigen and bacteria as granular antigen are detected after being treated via extraction or other processes. The present invention features that special chromatographic film and optimized conditions are adopted so that the immunogical chromatographic paper strip may be used directly in detecting bacteria. The present invention may be used in the fast detection of common pathogen in food and the fast diagnosis of toxin in food.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
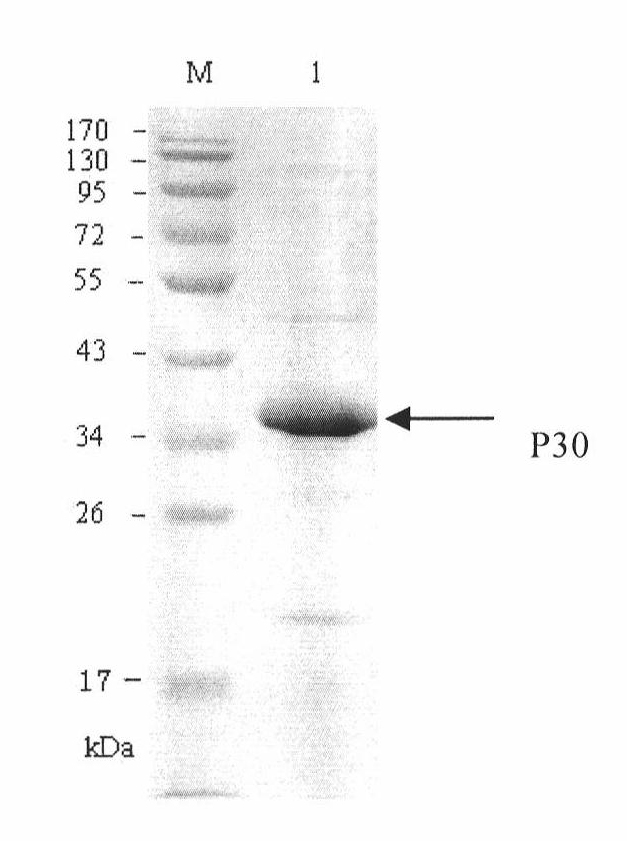
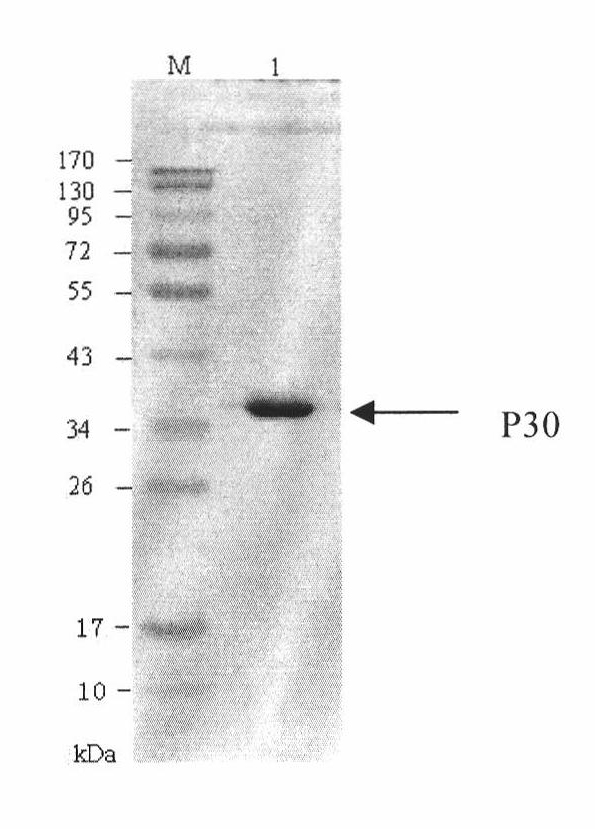
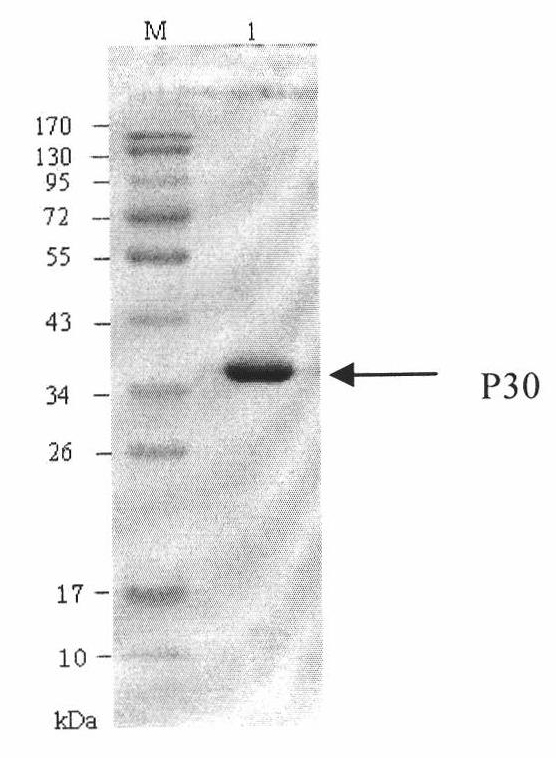
Hybridoma cell line of monoclonal antibody against African swine fever virus and secreted monoclonal antibody thereof
InactiveCN101831407AHigh utility valueMicroorganism based processesImmunoglobulins against virusesBALB/cPurification methods
The invention discloses a hybridoma cell line of a monoclonal antibody against African swine fever virus and the secreted monoclonal antibody thereof. The preparation method of the invention comprises the following steps: preparing a recombined P30 soluble antigen by prokaryotic expression; immunizing a BALB / c mouse; and finally fusing, screening and cloning by a hybridoma technology to obtain the hybridoma cell line which can stably secrete the monoclonal antibody against African swine fever virus P30 protein. The invention further discloses a method for preparing the monoclonal antibody with the cell line, an antibody purification method and a labeling method for horseradish peroxidase of the antibody. The monoclonal antibody can be used in detecting the African swine fever viral antibody in pig serum.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Animal model for allergy
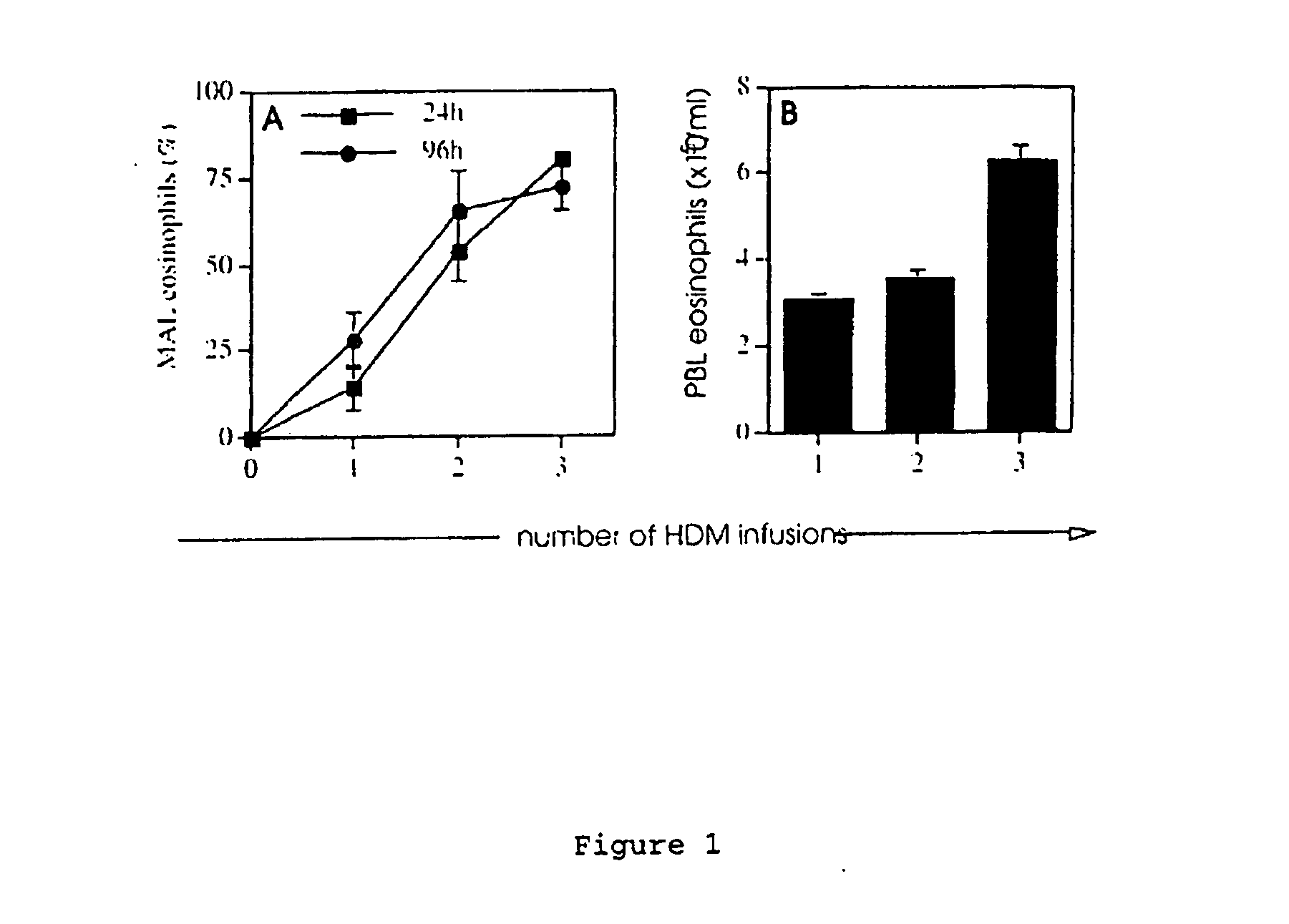
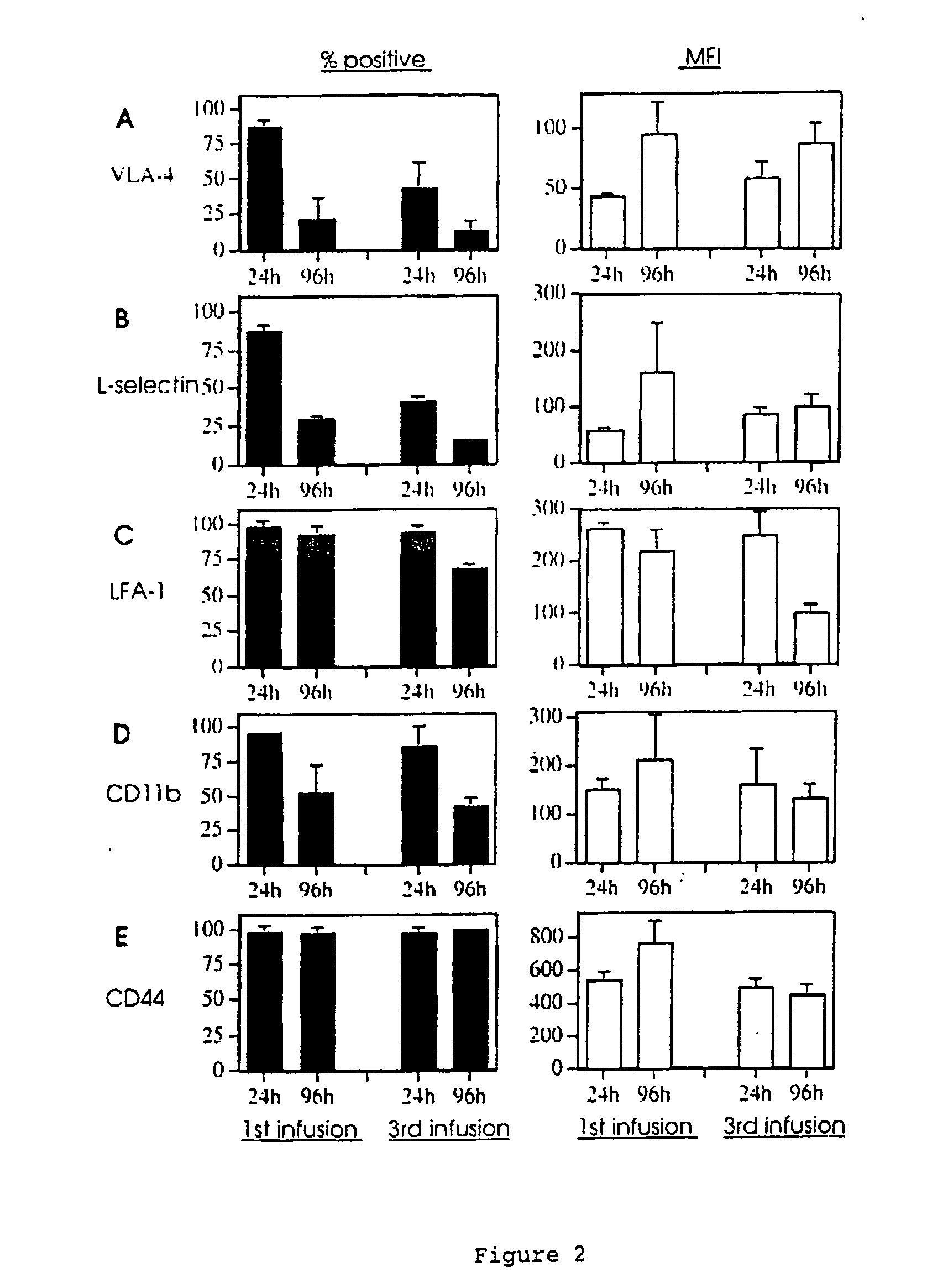
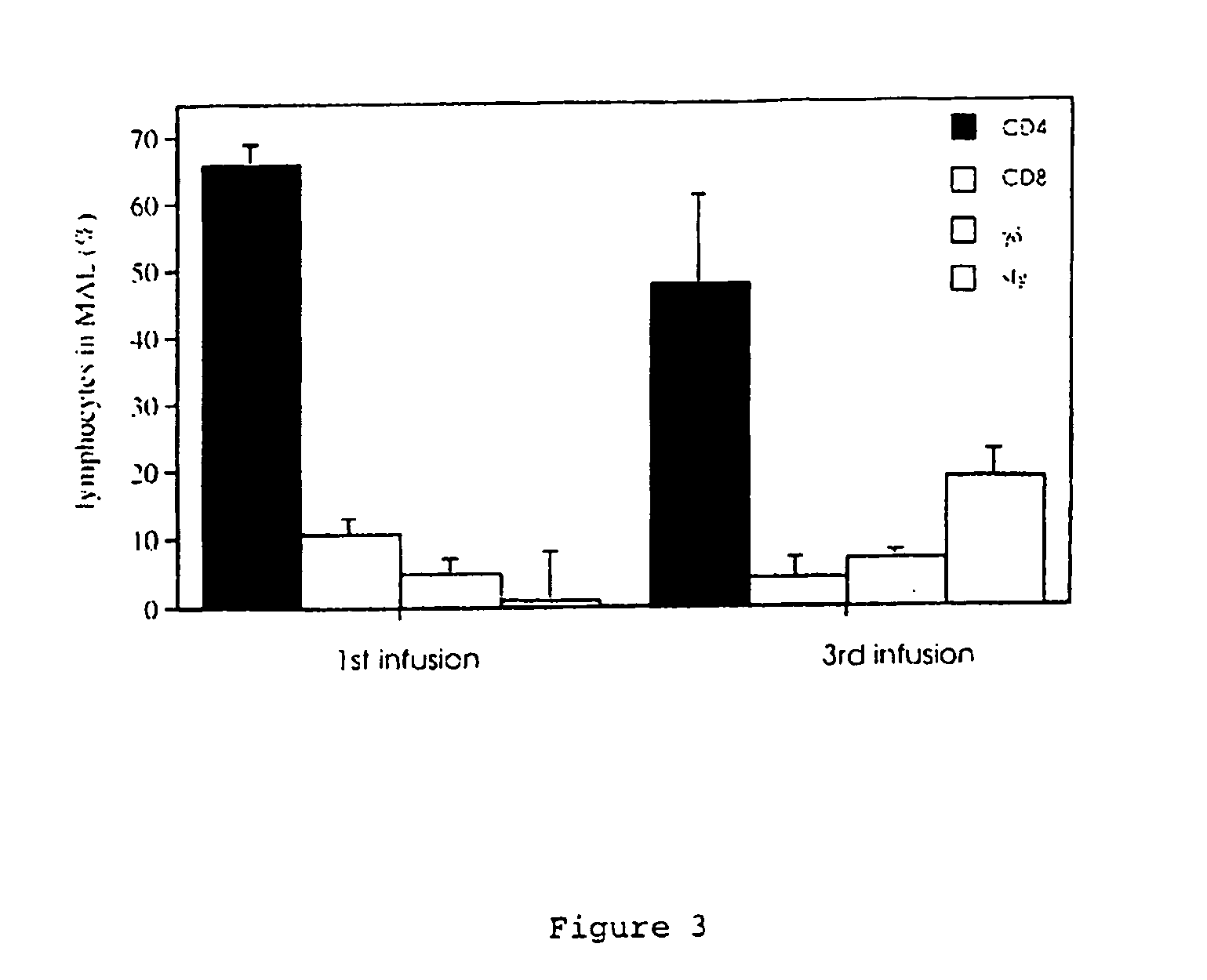
The invention relates to model systems for allergic conditions, and in particular to in vivo model systems in a large animal. The model systems of the invention are especially useful for providing large numbers of activated or non-activated eosinophils, for the discovery and evaluation of novel anti-inflammatory drug targets and for providing a model for the in vivo study of asthma and the effects of allergy treatments. In a preferred embodiment the animal is a sheep. In one embodiment, repeated infusion of house dust mite allergen (HDM) into the mammary gland is used to induce a specific allergic response, which is characterised by the recruitment of inflammatory cells, particularly eosinophils, into the mammary lumen; these cells can be harvested from peripheral blood and mammary lavage (MAL). In a second embodiment, the mammal is immunised with soluble antigen, for example by repeated subcutaneous immunisation, and then subjected to a single challenge with the same antigen administered directly to the lung.
Owner:ALLERGENIX
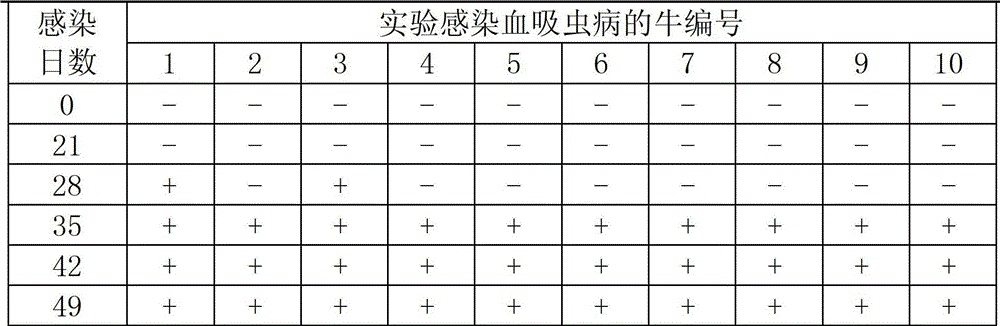
Kit for detecting circulating antigen indirect hemagglutination of schistosomiasis and manufacturing method thereof
InactiveCN101893628AHigh utility valueSimple and fast operationBiological testingFreeze-dryingDiluent
The invention provides a kit for detecting circulating antigen indirect hemagglutination of schistosomiasis and a manufacturing method thereof. The kit is composed of freeze-drying anti-SEA-IgY sensitized erythrocyte, sensitized erythrocyte diluent, sample diluents, positive comparison products and negative comparison products. The method for manufacturing the kit for detecting circulating antigen indirect hemagglutination of schistosomiasis comprises the steps of preparing a schistosoma japonica soluble antigen, performing antigen immunization and collecting eggs, extracting and purifying specific anti-SEA-IgY, preparing hydroformylated red cells, preparing tanned red cells, preparing a specific anti-SEA-IgY antibody sensitized erythrocyte, and preparing freeze-drying red cells. The kit in the invention has the advantages of simple and convenient operation, rapidness, high sensibility, strong specificity and good repeatability.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Measuring circulating therapeutic antibody, antigen and antigen/antibody complexes using ELISA assays
The present invention relates to the field of immunology and hyperproliferative diseases. More specifically, the present invention relates to a method of detecting and monitoring therapeutic antibody:antigen complex, soluble antigen and soluble therapeutic antibody, wherein a patient has undergone at least one course of immunotherapy. Yet further, levels of therapeutic antibody:antigen complexes, soluble antigens or soluble therapeutic antibodies may be measured and used to stage or monitor a hyperproliferative disease.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Immunochromatographic strip for detecting viscerotropic leishmania infection and diagnosing kala-azar
InactiveCN102590508AIncreased sensitivityImprove featuresProtozoa antigen ingredientsAntiparasitic agentsCelluloseLeishmania Infections
The invention discloses an immunochromatographic strip for detecting viscerotropic leishmania infection and diagnosing kala-azar, which comprises a sample pad, a conjugate pad which is closely connected to the sample pad and contains a colloidal gold-labeled probe, a cellulose membrane closely connected with the conjugate pad and a water absorption pad closely connected to the cellulose membrane. The water absorption pad is far away from the conjugate pad, a quality control line is arranged at one end of the cellulose membrane, which is close to the water absorption pad, a detection line is positioned on the cellulose membrane between the quality control line and the conjugate pad, the detection line contains soluble antigen of the viscerotropic leishmania, and the quality control line contains antibodies specifically binding to the colloidal gold-labeled probe. The invention further provides a kit containing the immunochromatographic strip and a preparation method for the immunochromatographic strip. The immunochromatographic strip has the advantages of being simple and convenient to use, high in sensitivity and specificity, fast in detection, and simultaneously applicable to clinical and field use for human, livestock and wild animals.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Measuring circulating therapeutic antibody, antigen and antigen/antibody complexes using ELISA assays
The present invention relates to the field of immunology and hyperproliferative diseases. More specifically, the present invention relates to a method of detecting and monitoring therapeutic antibody:antigen complex, soluble antigen and soluble therapeutic antibody, wherein a patient has undergone at least one course of immunotherapy. Yet further, levels of therapeutic antibody:antigen complexes, soluble antigens or soluble therapeutic antibodies may be measured and used to stage or monitor a hyperproliferative disease.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Chicken yolk antibody-magnetic bead ELISA (Enzyme-Linked Immuno Sorbent Assay) method for detecting schistosoma japonica circulating antigen
The invention provides a nano magnetic bead labeled by chicken yolk immune globulin IgY and used for resisting the soluble antigen of schistosoma japonica ovums and a chicken yolk antibody-magnetic bead ELISA (Enzyme-Linked Immuno Sorbent Assay) method for detecting schistosoma japonica circulating antigen. The method comprises the steps of: immunizing a hen with specific antigens of schistosoma japonica with a method of subcutaneous multi-point injection, and extracting, purifying and identifying specific IgY from an egg yolk; and coupling the polyclone IgY on a magnetic bead, and detecting the circulating antigen of the schistosoma by using the magnetic bead-IgY polyclone antibody as a capture antibody and using the specific monoclone IgY antibody as a detecting antibody. The method not only can be used for capturing more antigens by using the magnetic bead and the IgY to increase the sensibility, but also benefits the increase of detecting specificity by using the monoclonal antibody. Thus, the high combination and coordination of sensibility and specificity, required in immunology diagnosis, are realized.
Owner:HUAZHONG UNIV OF SCI & TECH
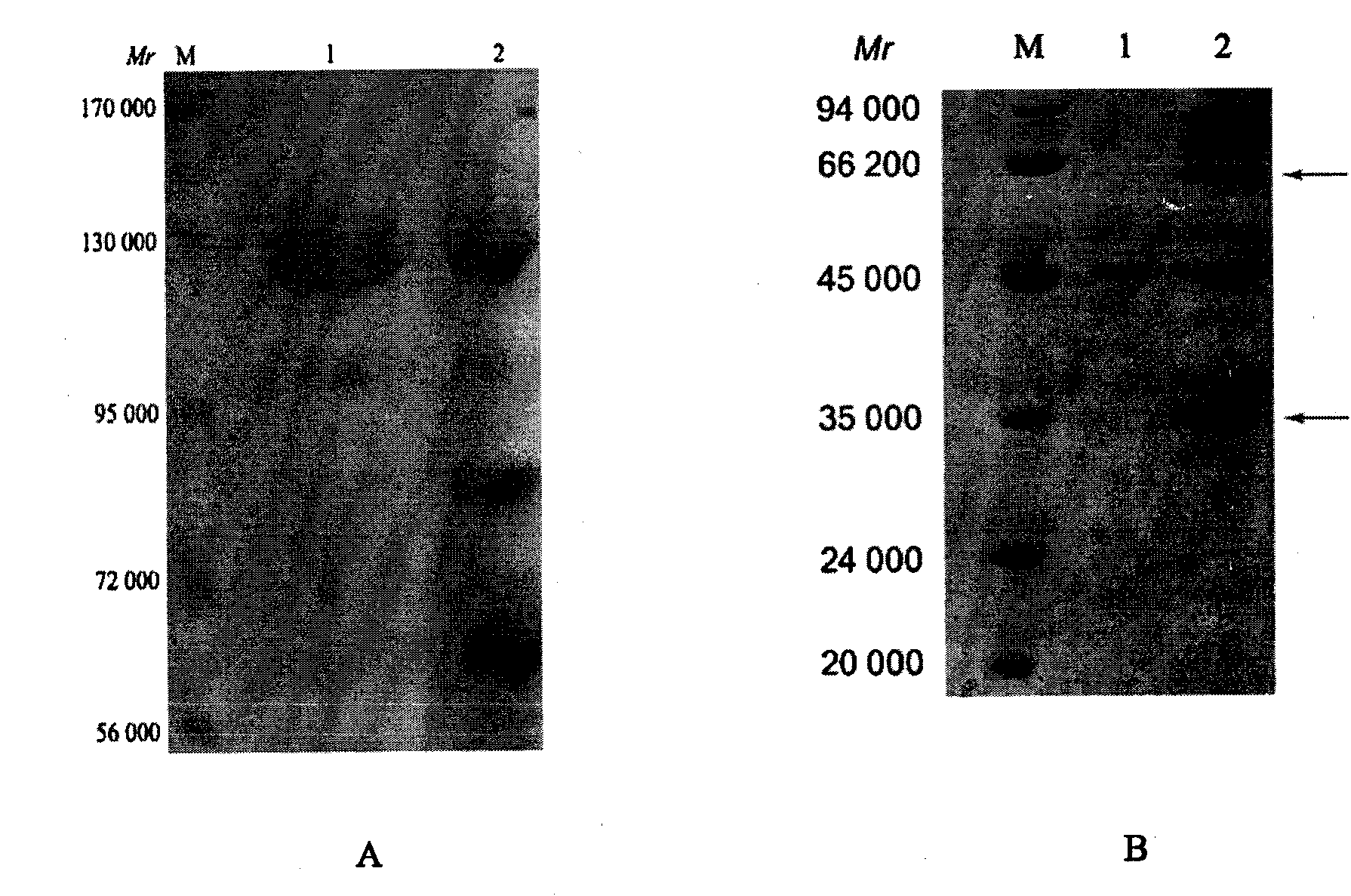
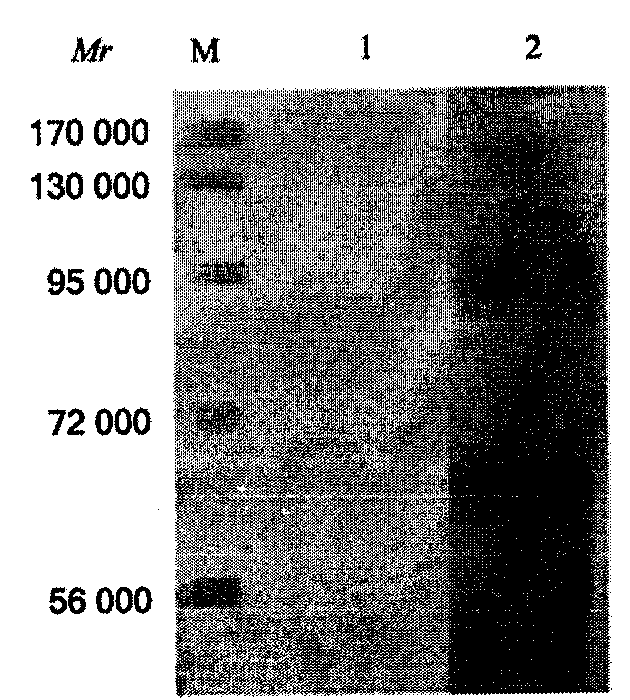
Liver fluke rapid immune detection kit for fresh water fishes and detection method
The invention discloses a fresh water fish liver fluke fast immunity test kit and a test method. The kit box comprises an enzyme linked reaction board, an enzyme conjugate, washing liquid, a substrate solution, a color developer, a diluent and an end solution, wherei the enzyme linked reaction board is made by extracting and purifying soluble antigen from liver fluke and enveloping the soluble antigen; the enzyme conjugate is made by labeling anti-fish immunoglobulin via horseradish peroxidase. The invention utilizes the sensitivity, the specificity, the practicality and the economy of the fast immunity test technique to establish a fresh water fish liver fluke fast immunity test method, is practical and simple while the kit can be opened and used instantly, without preparation or dilution, with simple operation; the result can be judged quantitatively by instruments and can be judged by eyes, thus is suitable of the on-site detection of different conditions; the invention is safe and environment friend, which adopts the harmless enzyme linked immunity color developer as tetramethylbenzidine to avoid the harm and pollution of general methods.
Owner:SHENZHEN COMBINED BIOTECH
Antibody-secreting cell assay
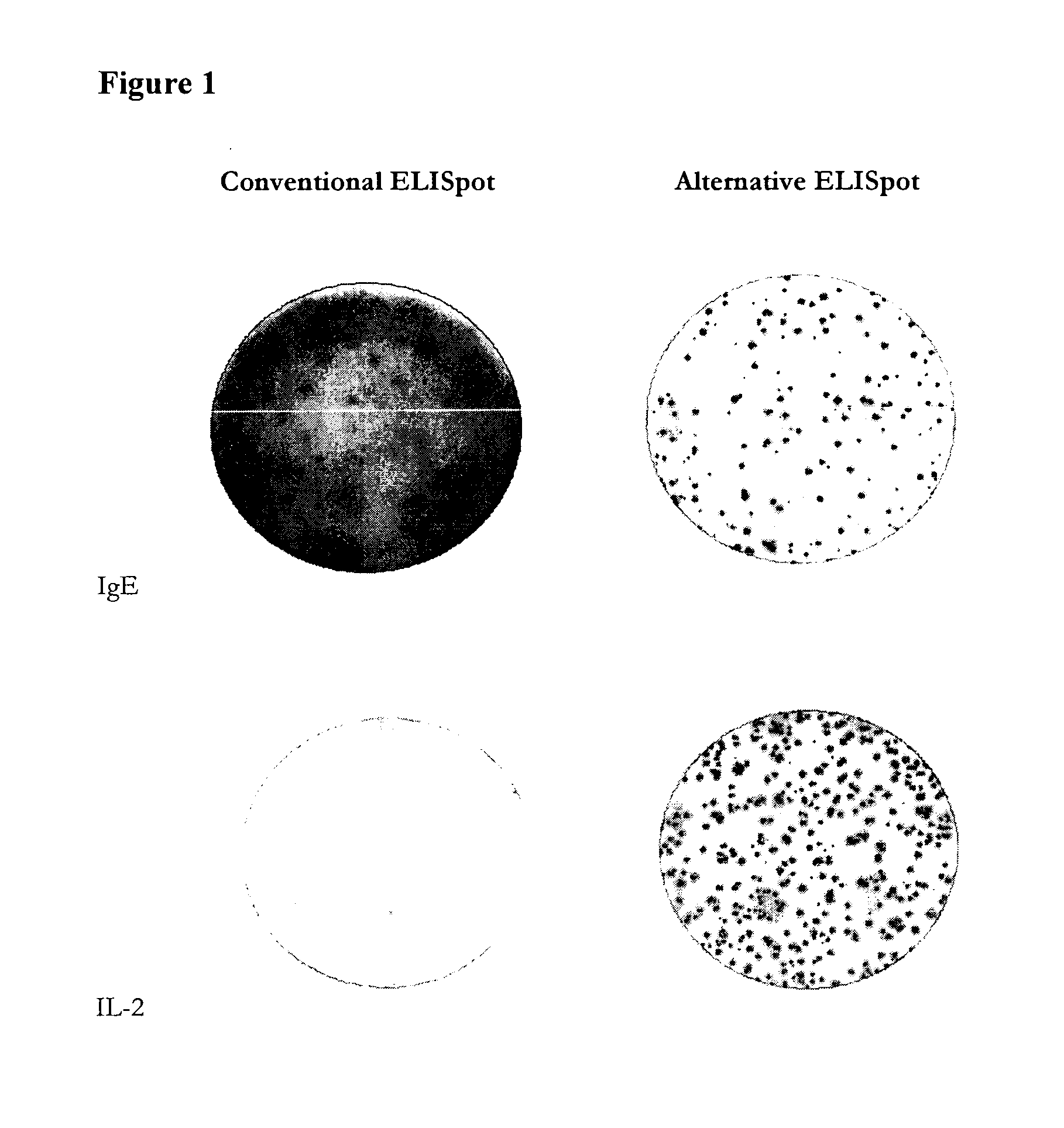
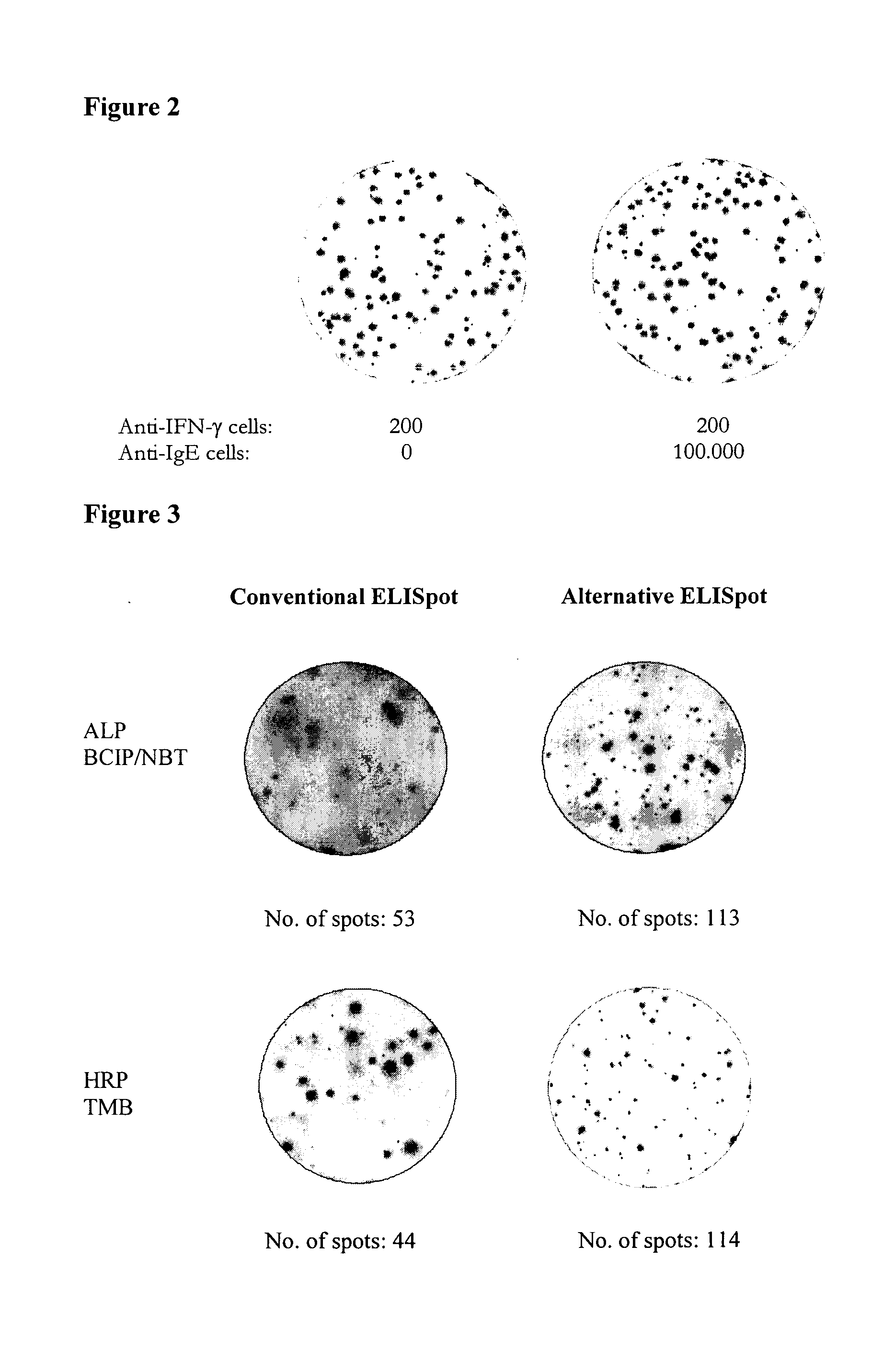
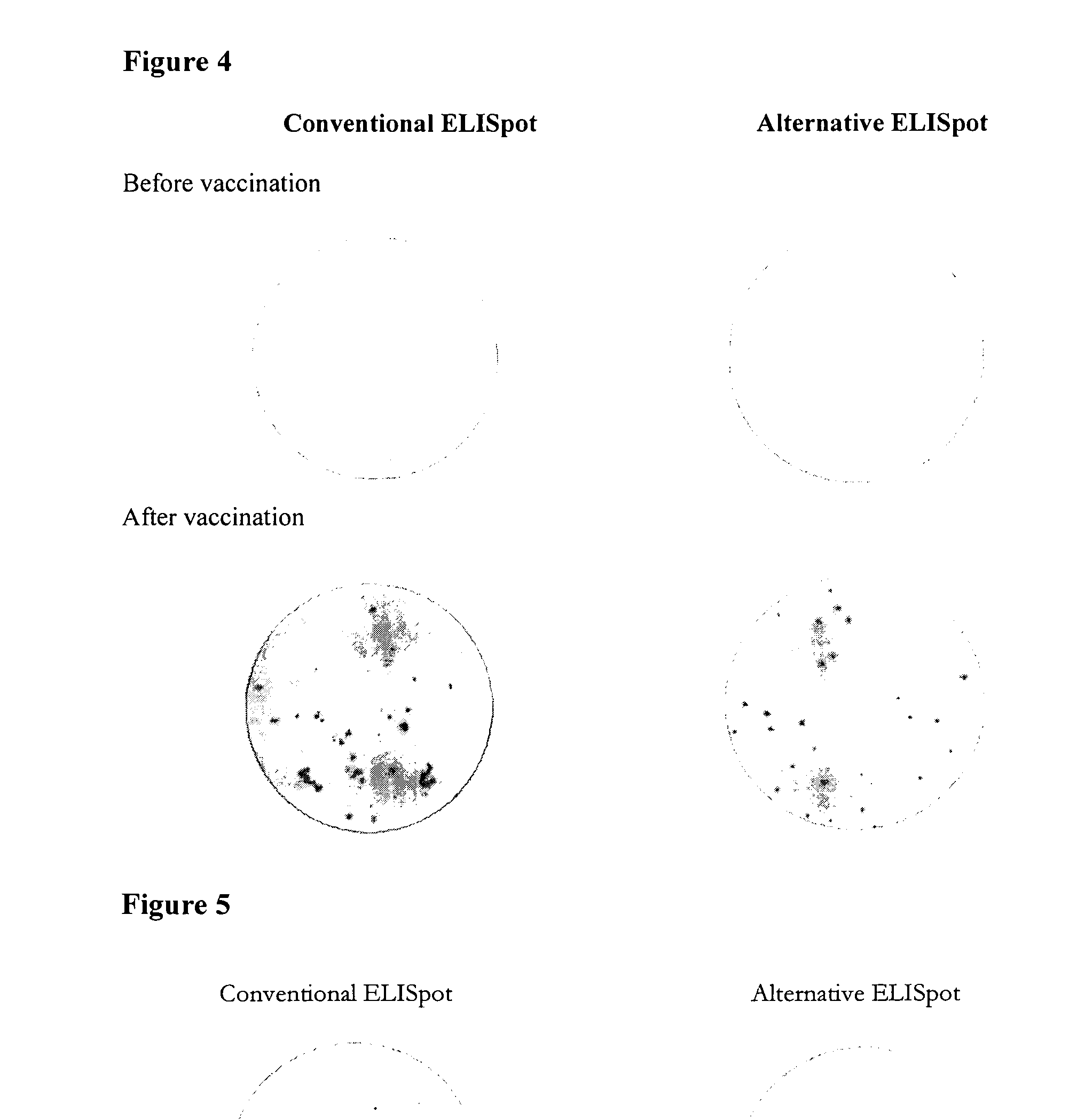
ActiveUS20110244477A1Less antigenLimited valueDisease diagnosisBiological testingEpitopeAntigen binding
An improved assay is described where a surface is provided with immobilized anti-Ig antibodies rather than antigen and where specific antibody-secreting cells (ASC) are detected using soluble antigen probes containing one of several possible labels. The method gives improved sensitivity with less background and is also more representative because antigen binding does not employ immobilized antigen. The assay is particularly effective for measuring antibody secreting cells against HIV, for determining whether an infection is acute as opposed to old or latent, for mapping epitopes and for measuring for ASCs against different antigens in the same reaction.
Owner:MABTECH
Method for detecting human tumor antigen P 185 Her-2 and its application in diagnosing tumor
InactiveCN1397803AStrong specificityImprove purification efficiencyPeptide preparation methodsRecovery/purificationNeoplasm diagnosisHuman tumor
A dual-antibody sandwith ELISA detecting method for human soluble p185HER-2 antigen features that the purified and coated P185HER-2 monoclonal antibody is bound to solid supporter, the specimen containing soluble p185HER-2, the purified standard p185HER-2 antigen and the coated antibody are incubated, and another purified and coated p185HER-2 monoclonal antibody marked by horseradish perioxidase is used as detecting antibody. It can be used for serum diagnosis of tumor.
Owner:UNIV OF SCI & TECH OF CHINA
Rapid detection chromatography technology for pathogenic bacteria in water environment
The invention discloses an immunologic paper chromatographic strip for specifically and rapidly detecting common pathogenic bacteria in a water environment and a specific and rapid detection method thereof. The immunologic paper chromatographic strip of the invention which is used for detecting the common pathogenic bacteria in the water environment is new technology and application for rapidly detecting the common pathogenic bacteria in water. At present, a large number of reports on detection of soluble antigen by colloidal gold immunochromatographic test paper are available at home and abroad; and the bacteria serving as particulate antigen are extracted or processed by other methods and then detected abroad. In the technology, a special chromatographic film is selected and used and other conditions are optimized, so that the immunochromatographic strip can be directly used for detecting the bacteria. The technology can be used for rapidly detecting the common pathogenic bacteria in the water environment.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Preparation of test strip for detection of antibody of bovine schistosoma japonicum katsurada and application method thereof
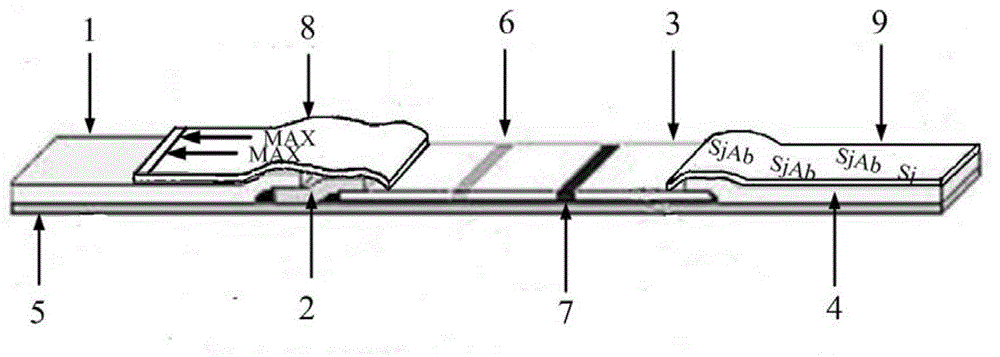
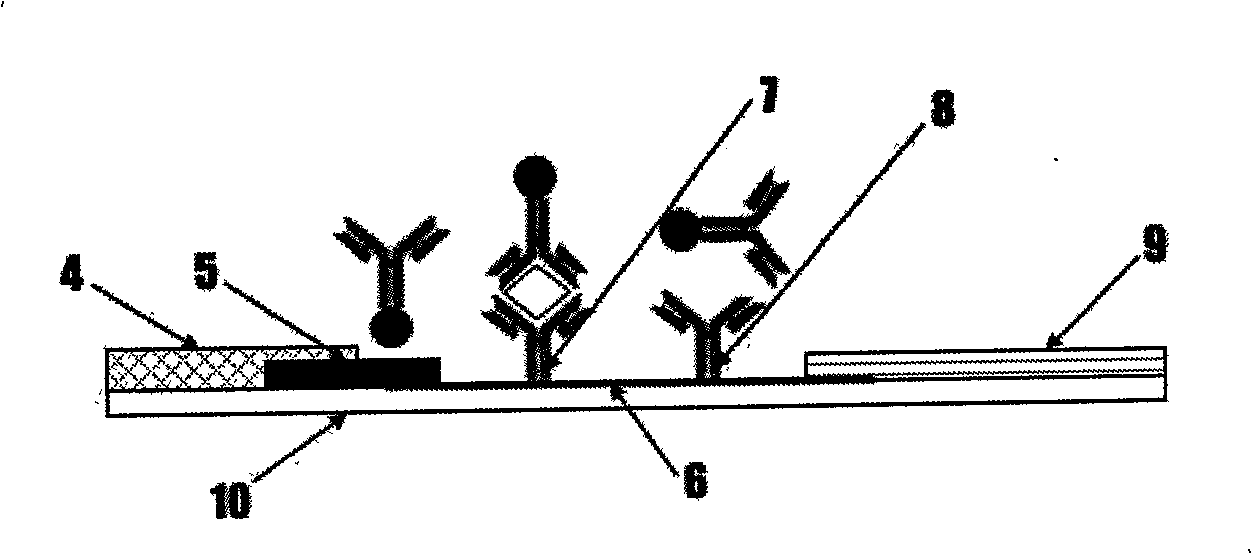
The invention discloses preparation of a test strip for detection of antibody of bovine schistosoma japonicum katsurada and an application method thereof, which belong to the technical field of diagnosis of animal parasitic diseases. The test strip consists of a sample gasket, an immune probe dry plate, a chromatographic film, water absorbing paper, a PVC (Polyvinyl Chloride) base plate, and an arrow label coating and a handle label coating which are combined. The immune probe dry plate is prepared by forming a covalent bond conjugate by carboxyl of polystyrene latex beads and amido of rabbit anti-bovine IgG antibody protein and absorbing on a polyester film. The chromatographic film is prepared by using a schistosome soluble antigen anchored on a nitrocellulose membrane as a detection line and goat anti-rabbit IgG antibody as a quality control line. The schistosome antibody in bovine serum can be quickly detected. The method is simple and convenient to operate, and the results are intuitional and accurate. The method can be widely applied to diagnosis, general survey and quarantine of bovine schistosome.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Gold mark reagent kit for detecting angiostrongyliasis cantonensis and preparing method thereof
InactiveCN101408547ASimple and fast operationIncreased sensitivityMaterial analysisSerum igeCurative effect
The invention relates to a gold-labeled kit for detecting angiostrongylus cantonensis disease and a preparation method thereof. The method comprises the preparation of angiostrongylus cantonensis soluble antigen, the preparation and purification of monoclonal antibodies, the preparation of colloidal gold-labeled anti-angiostrongylus cantonensis monoclonal antibodies, and the preparation of an angiostrongylus cantonensis gold-labeled rapid diagnosis kit. The kit comprises an angiostrongylus cantonensis gold-labeled detection plate; the detection plate comprises a PVC liner plate, an absorbent pad, a gold-labeled pad which is coated by colloidal gold-labeled angiostrongylus cantonensis monoclonal antibodies, a detection line which coats angiostrongylus cantonensis monoclonal antibodies inside and a quality control line of goat anti-mouse IgG or IgM. The kit is simple and rapid in operation, requiring only 5 to 15min; the kit has high sensitivity and strong specificity, and the detection rate of the rat serum which is experimentally infected by angiostrongylus cantonensis and the detection rate of the angiostrongylus cantonensis patient serum are both 100 percent. The kit and the preparation method can play an important role in rapid diagnosis and efficacy assessment of angiostrongylus cantonensis.
Owner:SHENZHEN CENT FOR DISEASE CONTROL & PREVENTION
Enzyme immunological method for quickly detecting rubella virus antibody
InactiveCN1434297AFor point-of-care testingImproved color development timeBiological testingViral antibodyRubella virus antibody
The invention discloses an enzyme immunological method of rapidly detecting the antibody of the measles virus, mainly using PEG to speed up the antigen-antibody reaction, thus shortening the reactiontime of routine ELISA. Firstly, wrap the soluble antigen in the enzyme-mark board; secondly made up the PBS lotion, PH9.6, and the sample diluent containing PEG; thirdly use the sample diluent to dilute the measured blood serum and the positive and negative comparison blood serum, fourthy add the diluted blood serum into the hole of the enzyme-mark board for warm cultivation; fifthly throw off the antigen solution in the hole, and use PBS lotion to wash; sixthly use the sample diluent to dilute the known enzyme-mark antibody; seventhly add the diluted enzyme-mark antibody in the hole for warmcultivation.
Owner:WUHAN UNIV
Method for preparing magnetized and hydroformyled sheep red blood cell
The invention discloses a method for preparing a magnetized and hydroformyled sheep red blood cell. The method comprises the following steps of: preparing nano magnetic beads; preparing a hydroformyled sheep red blood cell; preparing a magnetized and hydroformyled sheep red blood cell; coating protein on the magnetized and hydroformyled sheep red blood cell; detecting a sample; and observing the result. The prepared magnetized and hydroformyled sheep red blood cell can be used as an indicator cell of an agglutination test, a carrier, enzyme and a chemiluminescence matter of an antigen antibody agglutination test, or a carrier detected in a fluorescent material marking test. The magnetized and hydroformyled sheep red blood cell prepared by the method is long in retention period, simultaneously has paramagnetism, can achieve rapid aggregation and redispersion, avoids complicated centrifuge processes in the traditional agglutination test, and can be used for preparing artificial granular antigen antibodies with uniform sizes; coating and detection reaction are carried out in a uniform phase after a solid-phase carrier is replaced; and the method is full in action, high in sensitivity, low in cost than an elisa plate, and is mainly applied to a screening or quantitative detection test of a special red blood cell antibody and other soluble antigen antibodies.
Owner:SUZHOU GUOKE MEDICAL TECH DEV CO LTD
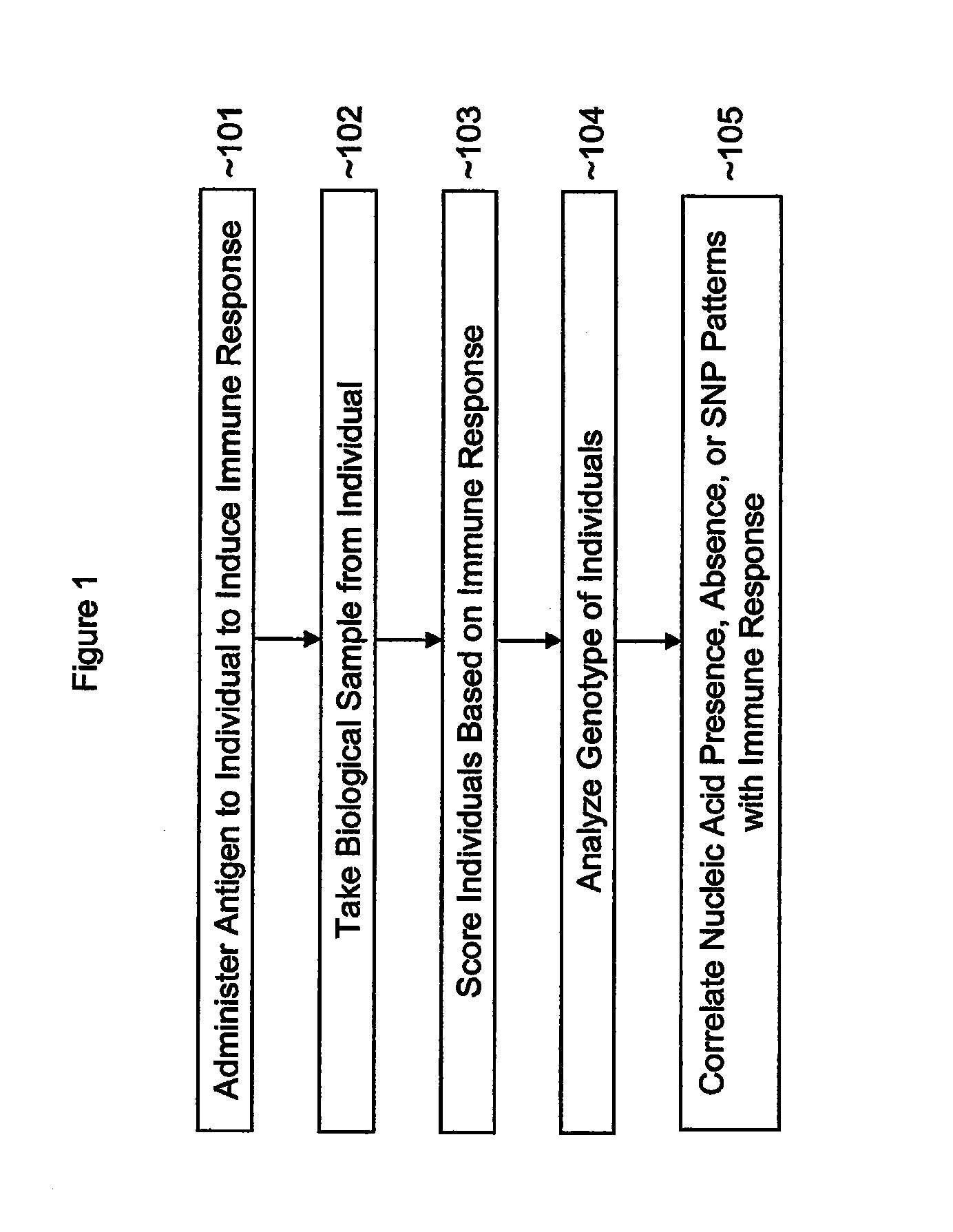
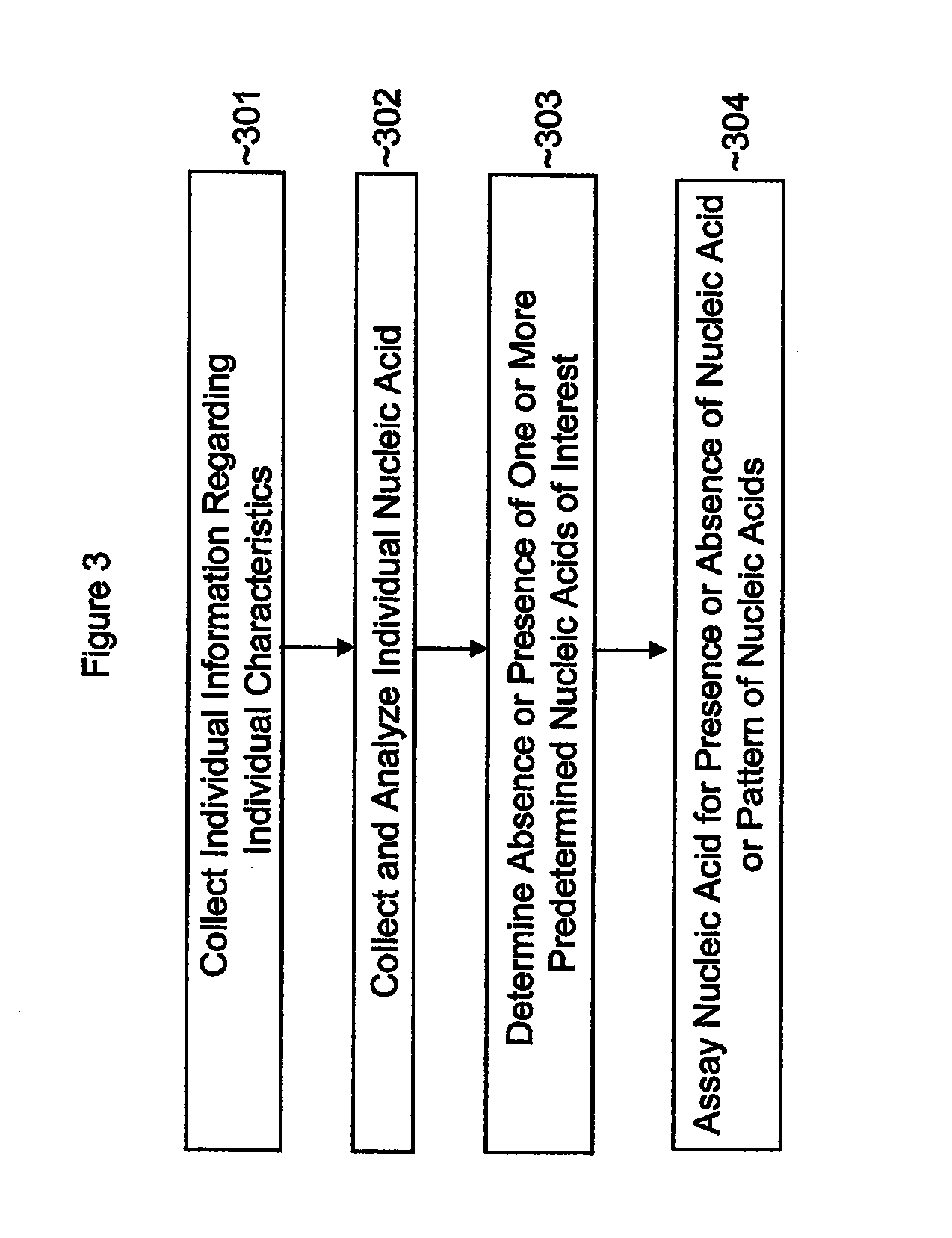
Single nucleotide polymorphisms (SNP) and association with resistance to immune tolerance induction
InactiveUS20120135014A1Convenient inductionEasily toleratedPeptide/protein ingredientsMicrobiological testing/measurementNucleic acid sequencingImmune tolerance
This application discloses methods, systems and kits for correlating the presence or absence of certain nucleic acid sequences within a population with the ability to create immune tolerance in that same population. Tolerance can be induced by solo or repeated administration of antigen, including soluble antigens administered either intravenously or sublingually. This application also discloses methods for detecting variants. In addition the application addresses the use or avoidance of non steroidal anti inflammatory drugs in therapy.
Owner:UNIV OF TENNESSEE RES FOUND
Hydatidovis soluble antigen preparation method and product thereof
ActiveCN102311957AReduce processing costsImproved genetic structurePeptide/protein ingredientsAntiparasitic agentsPichia pastorisEscherichia coli
The invention discloses a hydatidovis soluble antigen preparation method and a product thereof. The preparation method comprises: expressing gene represented by SEQ ID No.1, SEQ ID No.3 or SEQ ID No.5 in an insect bioreactor or a pichia pastoris bioreactor; and collecting and purifying the antigen to obtain the hydatidovis soluble antigen. In the method disclosed by the invention, a domestic silkworm bioreactor or the pichia pastoris bioreactor is used to prepare the hydatidovis antigen, the product is soluble, immune experiments prove the antigen has high antigen performance, the unit expression is 10 to 100 times higher than that of Escherichia coli, and animal experiments prove the antigen has high insect reducing efficiency. When the method is used, the production cost of the hydatidovis antigen can be reduced greatly, and the purification process of the conventional production of antigen by Escherichia coli is simplified; and the method has the advantages of safety, high efficiency, low cost and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
A diagnostic kit for detecting paragonimiasis and its preparation method
InactiveCN102279259AFacilitate early diagnosisAids in prognosisMaterial analysis by observing effect on chemical indicatorDiseaseNitrocellulose
The invention provides a diagnostic kit for detecting Paragonimiasis, which contains a rabbit polyclonal antibody against the soluble antigen of Paragonia wescheri adult worm, an enzyme-labeled rabbit polyclonal antibody against the soluble antigen of Paragonimus westermani adult worm, Nitrocellulose membrane, bovine serum albumin, PBST washing solution, substrate chromogenic solution, positive control sample, negative control sample and polyethylene reaction plate. The invention also provides a preparation method of a diagnostic kit for detecting paragonimiasis. The invention adopts the double-antibody sandwich method Dot-ELISA to detect the circulating antigen of Paragonimus westermani, has high sensitivity and strong specificity, and can realize rapid diagnosis and curative effect assessment of Paragonimiasis.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Method and apparatus for the production of soluble MHC antigens and uses thereof
The field of the invention relates in general to at least one method and apparatus for the production of soluble MHC antigens and more particularly, but not by way of limitation, to at least one method and apparatus for the production of soluble Class I and II HLA molecules. The field of the invention also includes such produced soluble Class I and II HLA molecules and their use. According to the methodology of the present invention, the soluble Class I and II HLA molecules can be produced from either gDNA or cDNA starting material.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Method for preparing proteus mirabilis-staphylococcus aureus-pseudomonas aeruginosa adsorption combined vaccine
InactiveCN105709218ARich varietySimple processAntibacterial agentsBacterial antigen ingredientsStaphylococcus cohniiUltrafiltration
The invention discloses a method for preparing a proteus mirabilis-staphylococcus aureus-pseudomonas aeruginosa adsorption combined vaccine. The method includes the steps that firstly, a proteus mirabilis culture solution is subjected to in-situ digestion, high-speed centrifugation, pre-filtering, ultrafiltration and precipitation, and a proper proteus mirabilis cell membrane soluble antigen raw solution is obtained; secondly, a staphylococcus aureus bacterium suspension is subjected to centrifugation, smashing, re-centrifugation, filtering, precipitation, enzymolysis and dialysis, and a proper staphylococcus aureus cytoplast antigen raw solution is obtained; thirdly, a staphylococcus aureus and pseudomonas aeruginosa culture solution is subjected to centrifugation and pre-filtering, formalin is added for detoxification, then purification is conducted, and a proper inactivated staphylococcus aureus and pseudomonas aeruginosa toxin raw solution is obtained. The vaccine is used for preventing burn, scalding and infection caused by one or more of conditioned pathogens including proteus mirabilis, staphylococcus aureus and pseudomonas aeruginosa before and after operations in an intramuscular deep injection mode.
Owner:LIAONING CHENGDA BIOTECH
Oriental schistosomiasis resistant natural numerator vaccine specific single-chain antibody
The invention relates to the field of genetic engineering antibody, and builds schistosoma japonicum immature egg soluble antigen (SIEA) single-chain antibody library for the first time. By screening the SIEA single-chain antibody library through an immuno-affinity screening technique, a specific single-chain antibody aiming at anti-schistosomiasis-japonica effective natural molecular vaccine SIEA26-28kDa is obtained. The obtainment of the specific single-chain antibody provides a foundation for treating schistosomiasis japonica, utilizing the specific single-chain antibody as a probe to screen schistosoma japonicum cDNA libraries and obtaining a corresponding gene sequence aiming at anti-schistosomiasis-japonica effective vaccine molecules more conveniently and rapidly.
Owner:何卓 +1
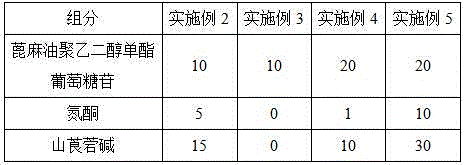
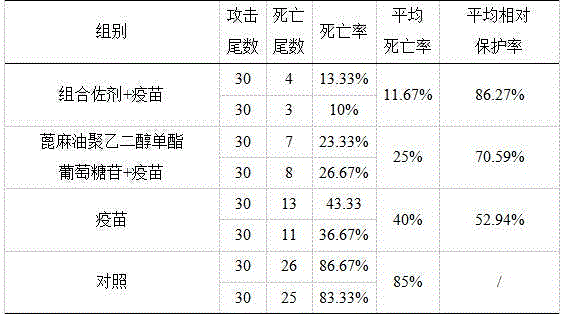
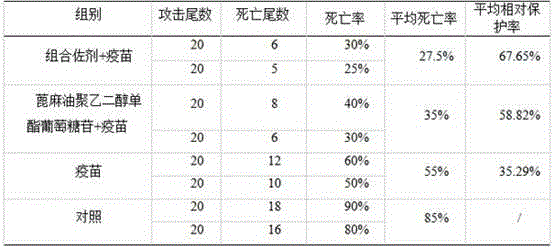
Adjuvant for improving immersion immunization effect of fishery vaccines and application of adjuvant
InactiveCN104667274APromote degradationReduce interfacial tensionImmunological disordersAntibody medical ingredientsAdjuvantPolyethylene glycol
The invention discloses an adjuvant for improving immersion immunization effect of fishery vaccines and an application of the adjuvant. The adjuvant is castor oil polyethylene glycol monoester glucoside or a composition of castor oil polyethylene glycol monoester glucoside, azone and anisodamine. The application of the adjuvant comprises the following steps: dissolving an appropriate amount of adjuvant into water and then uniformly mixing with a vaccine solution in certain volume ratio, putting to-be-immune fishes into the mixed solution, filling gas and soaking for 15-30 minutes. According to the adjuvant disclosed by the invention, the castor oil polyethylene glycol monoester glucoside has good effects of biodegradability, non-toxicity, low interface tension, good water solubility, effects of dispersing, emulsifying and the like, so that oils or in-soluble antigens which are not dissolved in water are uniformly dispersed, and the immune effects of the vaccines are effectively improved. The application method of the adjuvant is simple, safe to apply, environment-friendly and free of adverse interferences on growth of fishes in culture production.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI +1
Tetragenous diagnosis kit for food born parasite disease of human body
The invention refers to a multi-connection rapid diagnosing reagent box, including reaction board, antigen solid-phase film, golden mark coloration system, and buffering system; the antigens: 1% lung-fluke imaglo soluble antigen, liver fluke imago antigen, arched worm rapid-breeding sub-ectoblast antigen, and pig bursa cercaria coarse antigen; the solid-phase; nitric acnd acetic acid mixed fibrinfilm, aperture 0.65 micron m; the coloration system, auric chloride acid mark staphylococcus A albumen; the buffering system includes phosphate buffering solution (PSB), pH 7.2 and concentration 0.02mol / L and bull serum albumen (BSA), the content 1-5%, as well as Tris buffering solution (TBS), the content 0.5-1%, and pH 8.2.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
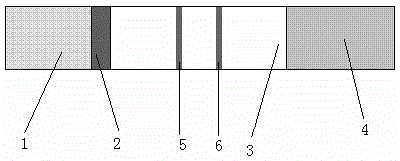
Immunochromatographic test strip for rapid detection of ancylostomiasis infection and preparation method thereof
InactiveCN105044335AIncreased sensitivityImprove featuresBiological material analysisCelluloseAdult worm
The invention relates to an immunochromatographic test strip for rapid detection of ancylostomiasis infection. The test strip includes: a sample pad, a gold labeled pad that is closely connected to the sample pad and contains a colloidal gold labeled probe, a cellulose membrane closely connected to the gold labeled pad and a water absorption pad closely connected to the other end of the cellulose membrane. One cellulose membrane end far from the gold labeled pad is provided with a quality control line, the cellulose membrane between the quality control line and the gold labeled pad is equipped with a detection line, which is composed of a hookworm purification worm antigen, and the hookworm purification adult worm antigen is a soluble antigen solution of Necator Americanus adult worms. The colloidal gold labeled probe is staphylococcus aureus A protein or s streptococcus G protein. The control line is composed of the antibody specifically binding with the colloidal gold labeled probe. The invention also provides a preparation method of the immunochromatographic test strip. The immunochromatographic test strip provided by the invention has the advantages of simplicity, sensitivity, specificity and rapidity, and can be applied to clinical and field use of human, livestock and wild animals at the same time.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Therapeutic compositions that produce an immune response
The invention provides a method for stimulating the production of antibodies to a cryptic epitope on a soluble antigen by administering to a patient having such a cryptic epitope a binding agent that binds to the soluble antigen and forming a complex between the binding agent and the soluble antigen, wherein the cryptic epitope is exposed and the patient generates antibodies that bind to the cryptic epitope.
Owner:ONCOQUEST INC
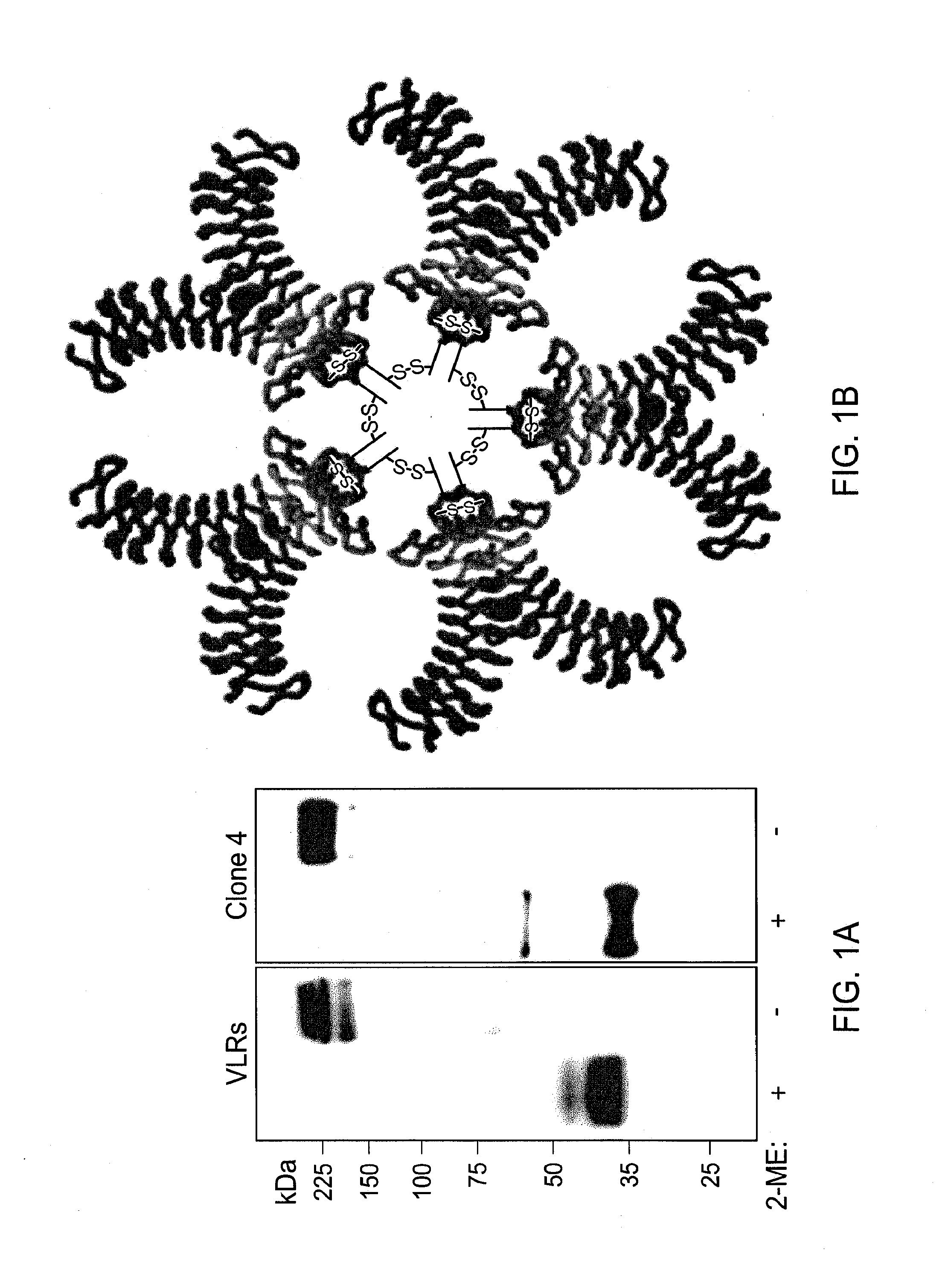
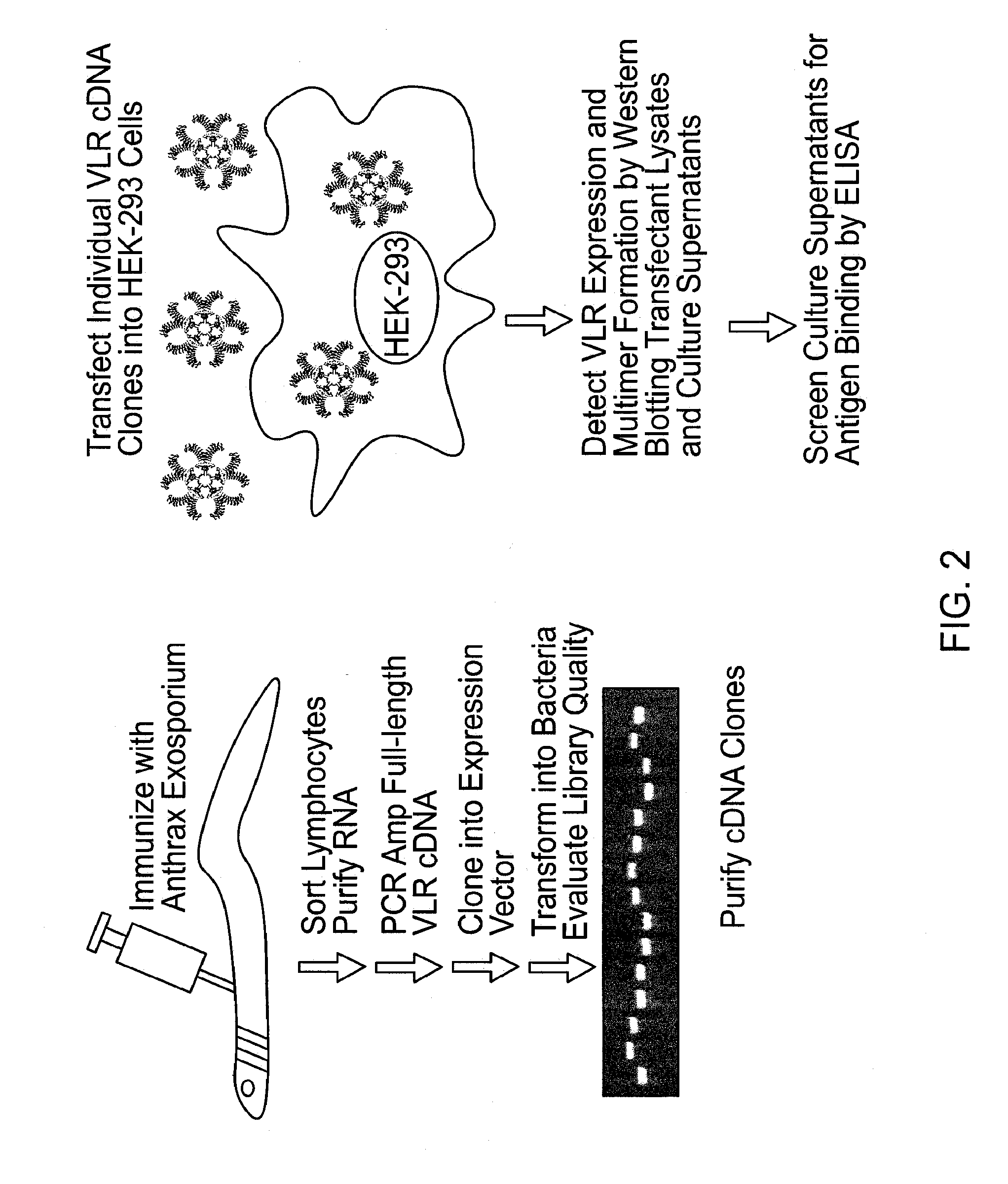
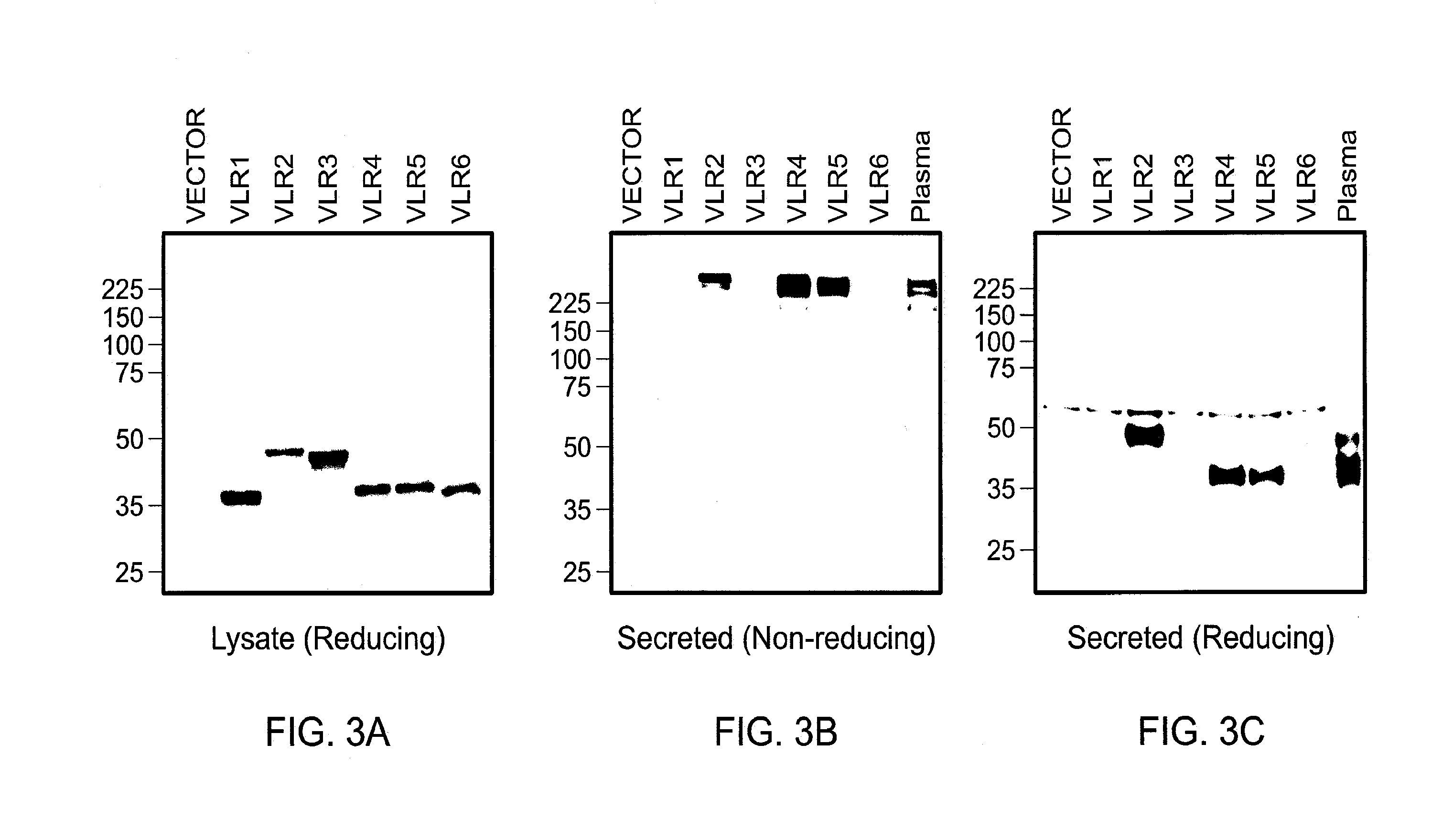
Methods and compositions related to soluble monoclonal variable lymphocyte receptors of defined antigen specificity
ActiveUS20160376348A1Immunoglobulins against blood group antigensImmunoglobulins against bacteriaLymphocyteVariable lymphocyte receptor
Disclosed are compositions and methods related to variable lymphocyte receptors (VLRs). More particularly, disclosed are a variety of antigen specific polypeptides, including soluble, monoclonal, and multivalent forms, as well as methods of using the polypeptides, antibodies that bind the antigen specific polypeptides, and nucleic acids, vectors and expression systems that encode the polypeptides. Antigen specific polypeptides that selectively bind pathogens, like anthrax, and carbohydrates, like blood group determinants, are specifically disclosed.
Owner:UAB RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com