Method for preparing proteus mirabilis-staphylococcus aureus-pseudomonas aeruginosa adsorption combined vaccine
The technology of Proteus mirabilis and Pseudomonas aeruginosa is applied in the field of preparation of Proteus mirabilis-Staphylococcus aureus-Pseudomonas aeruginosa adsorption combined vaccine, and can solve the problem that the natural structure of active components is incomplete and the recovery rate is low. , weak immunogenicity and other problems, to achieve the effect of improving immunogenicity and protection, avoiding large side effects, and covering a wide range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Proteus mirabilis cell membrane soluble antigen stock solution.
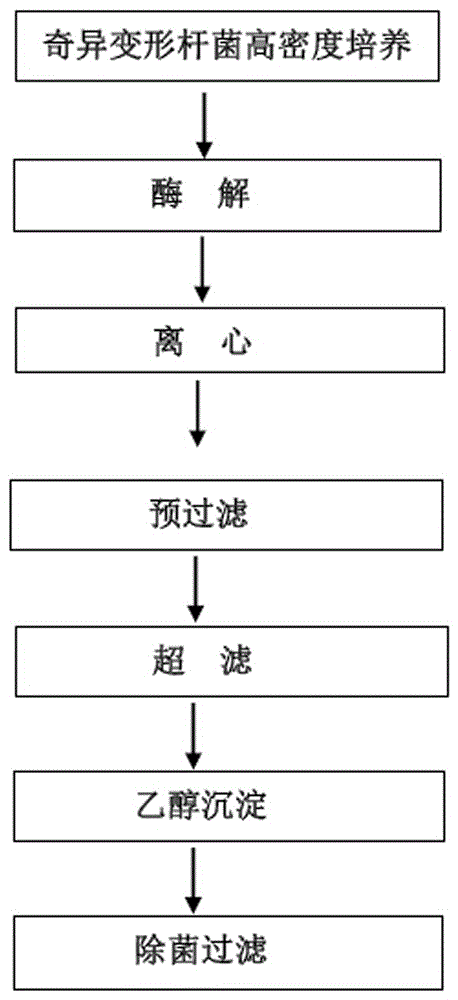
[0033] Six strains PM13, PM28, PM43, PM62, PM92 and PM104 were cultured at high density in fermenter, and the cells were collected. Through enzymatic hydrolysis of the bacterial cell membrane, centrifugation, pre-filtration, ultrafiltration, and precipitation, the soluble fraction of the Proteus mirabilis cell membrane was separated and purified to obtain a highly protective Proteus mirabilis antigen stock solution (6 strains fermentation and purification conditions the same), the process flow is as figure 1 shown.
[0034] The purification steps are as follows.
[0035] (1) Enzymolysis: mix the bacterial cell solution obtained from high-density culture of fermenter with pH 6.0-8.0 in sterile 0.9% NaCl solution, and then mix according to 4.0-6.0×10 9 Add 1-2g trypsin to the concentration of bacteria per ml, pH7.8-8.5, digest at 50-65°C for 4.5 hours.
[0036] (2) Centrifugation: Collect the...
Embodiment 2
[0040] Example 2 Staphylococcus aureus cytoplasmic antigen stock solution.
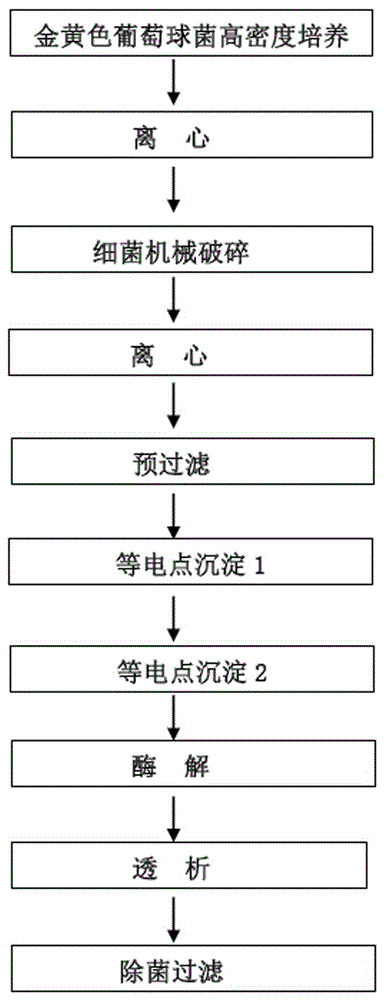
[0041] The strain SA79 was cultured in a fermenter at a high density, and the cells were collected. Through the bead milling method, the bacteria are mechanically crushed, pre-filtered, isoelectric point precipitation 1, isoelectric point precipitation 2, enzymatic hydrolysis, and dialysis are used to separate and purify the cytoplasmic soluble components of Staphylococcus aureus to obtain highly protective gold. Staphylococcus aureus cytoplasmic antigen stock solution, the process is as follows figure 2 shown.
[0042] The purification steps are as follows.
[0043] (1) Mechanical crushing: Mix the bacteria solution obtained from high-density culture in the fermenter with a pH of 6.0-8.0 in a sterile 0.9% NaCl solution, and then pump the bacterial suspension into the crusher through a peristaltic pump for continuous crushing. The bead size range is 0.45-1.25mm, and the broken rate is not less tha...
Embodiment 3
[0050] Example 3 Staphylococcus aureus toxoid stock solution.
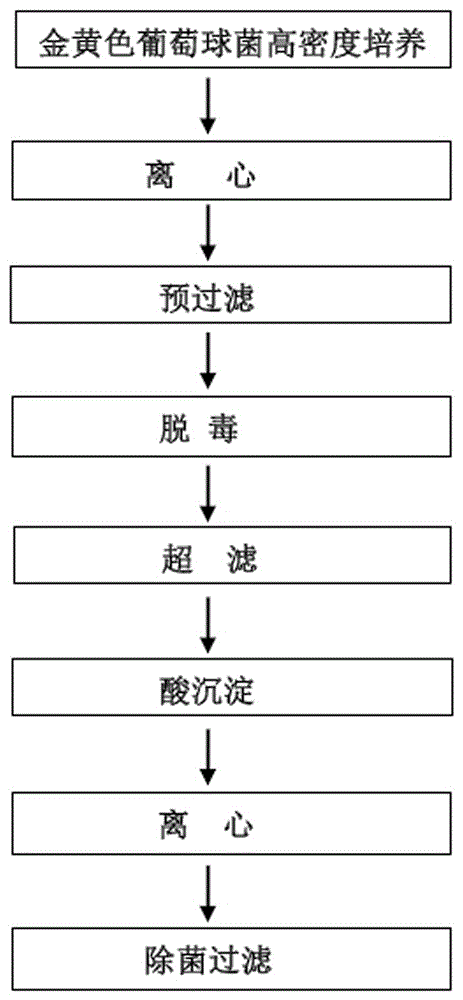
[0051] Staphylococcus aureus CMCC26002 was cultured in a fermenter at high density, and the bacterial suspension was collected. Separation and purification of Staphylococcus aureus toxoid components through centrifugation, detoxification, pre-filtration, ultrafiltration, acid precipitation, and centrifugation to obtain highly protective Staphylococcus aureus toxoid antigen stock solution. The process is as follows image 3 shown.
[0052] The purification steps are as follows.
[0053] (1) Centrifugation: Collect the supernatant by refrigerated high-speed centrifugation at 10,000-12,000 rpm, 4°C, for 10-15 minutes.
[0054] (2) Detoxification: add 0.4-1.0% formaldehyde solution or glutaraldehyde solution, 30-37°C, pH6.8-7.2 detoxification for 5-7 days.
[0055] (3) Pre-filtration: Use a 0.2+0.45um filter element to pre-filter the supernatant after centrifugation to obtain the pre-filtered clarified liquid.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| crush indicators | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com