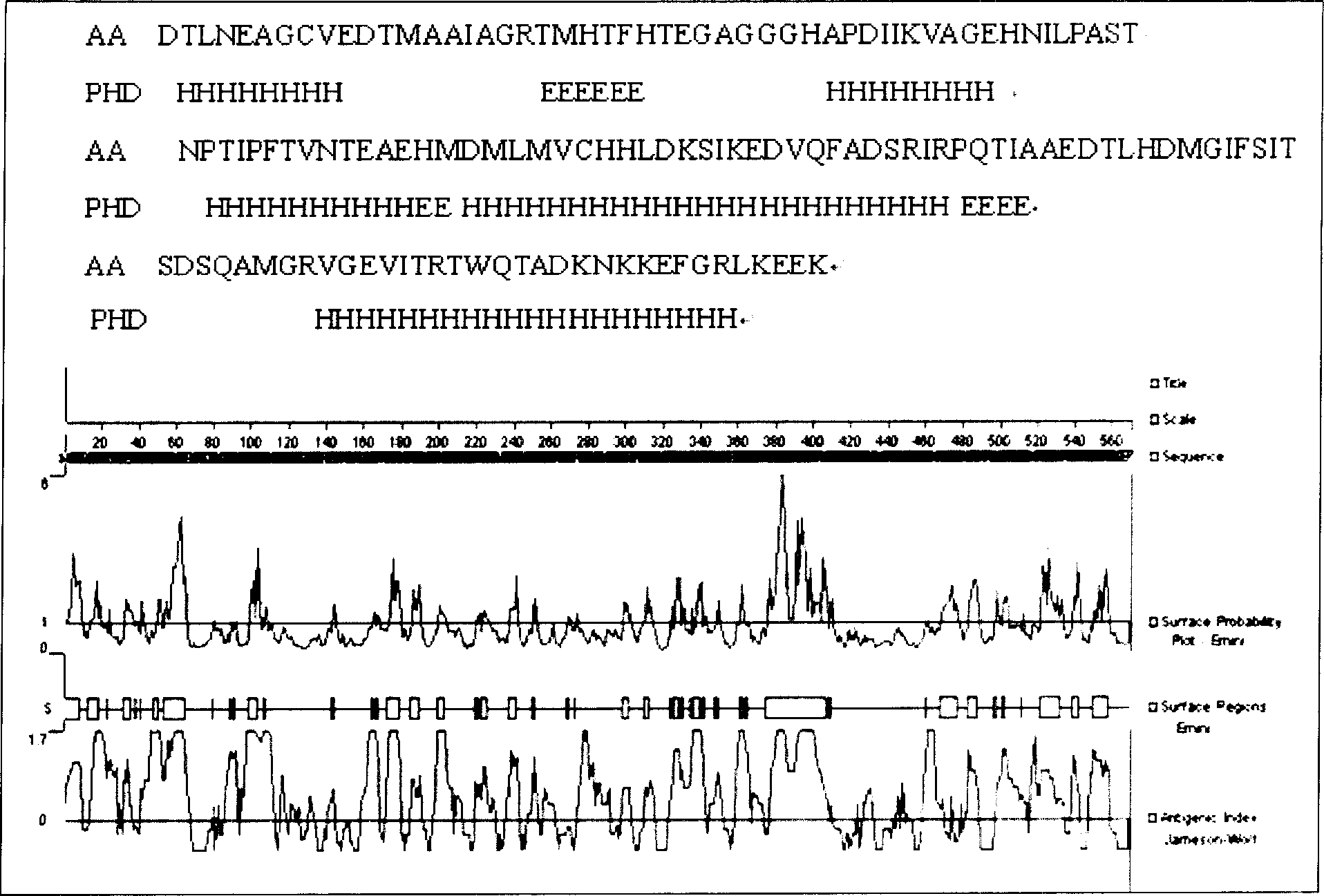
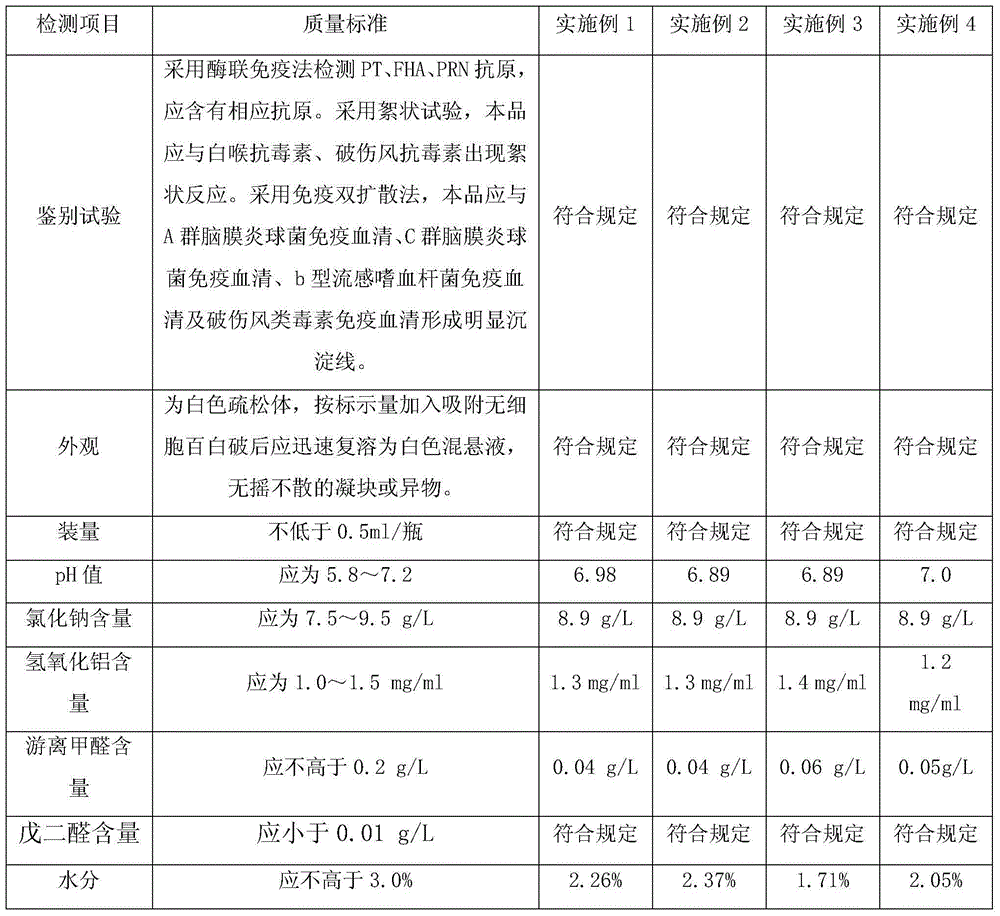
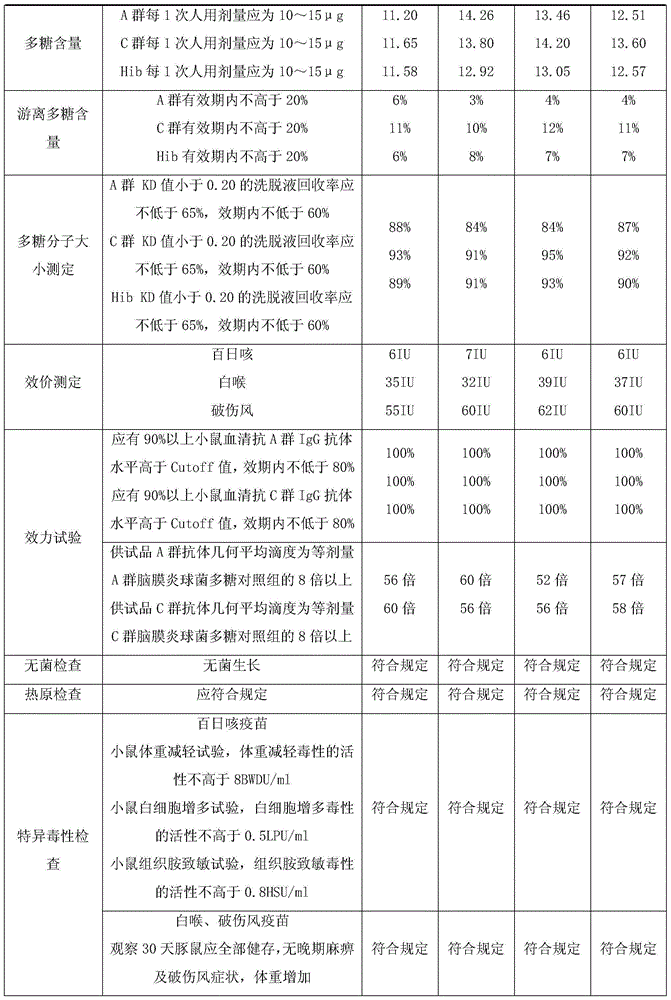
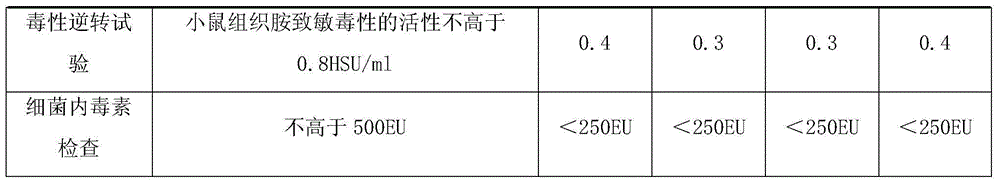
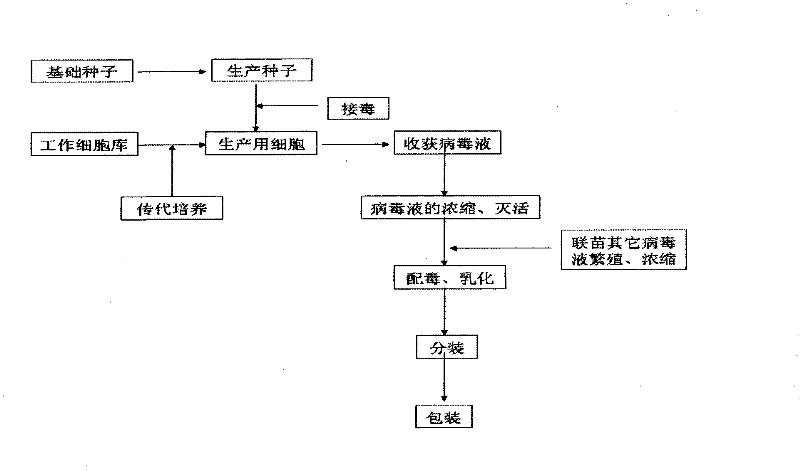
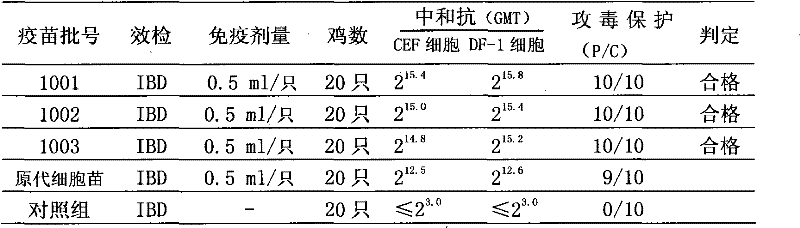
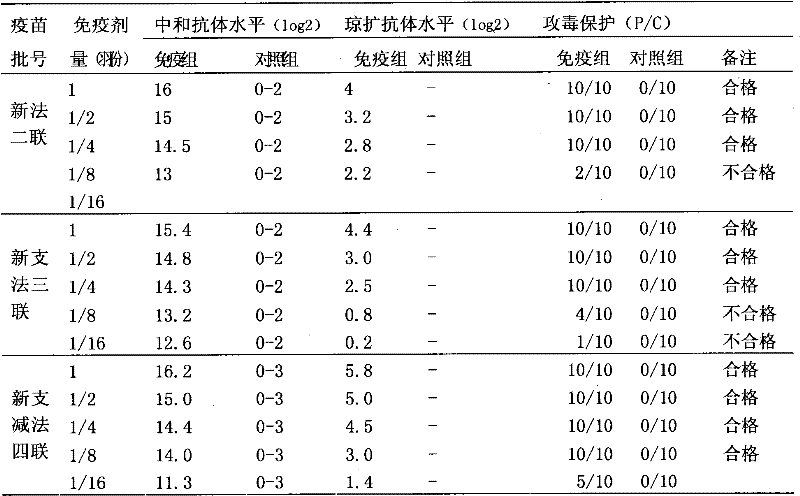
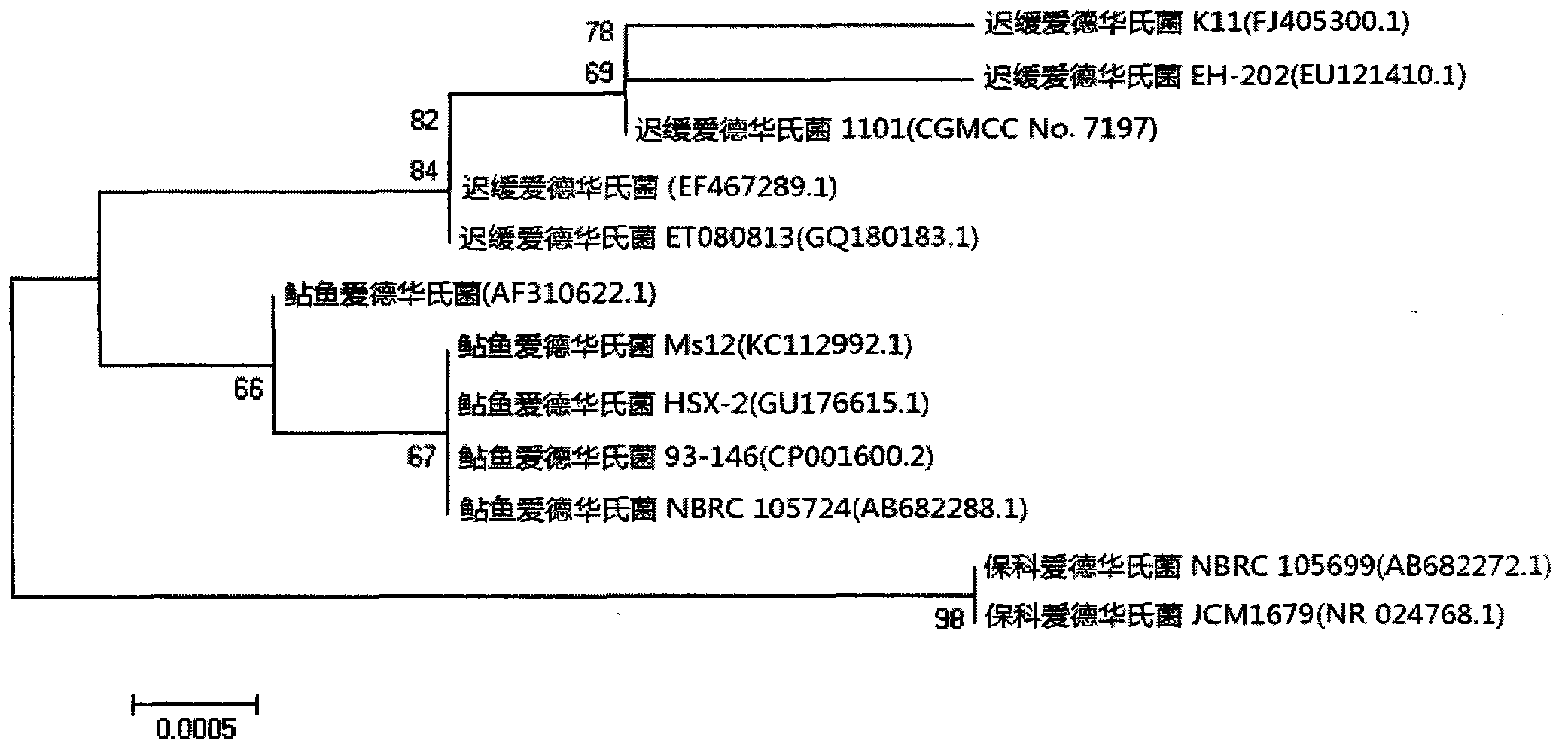
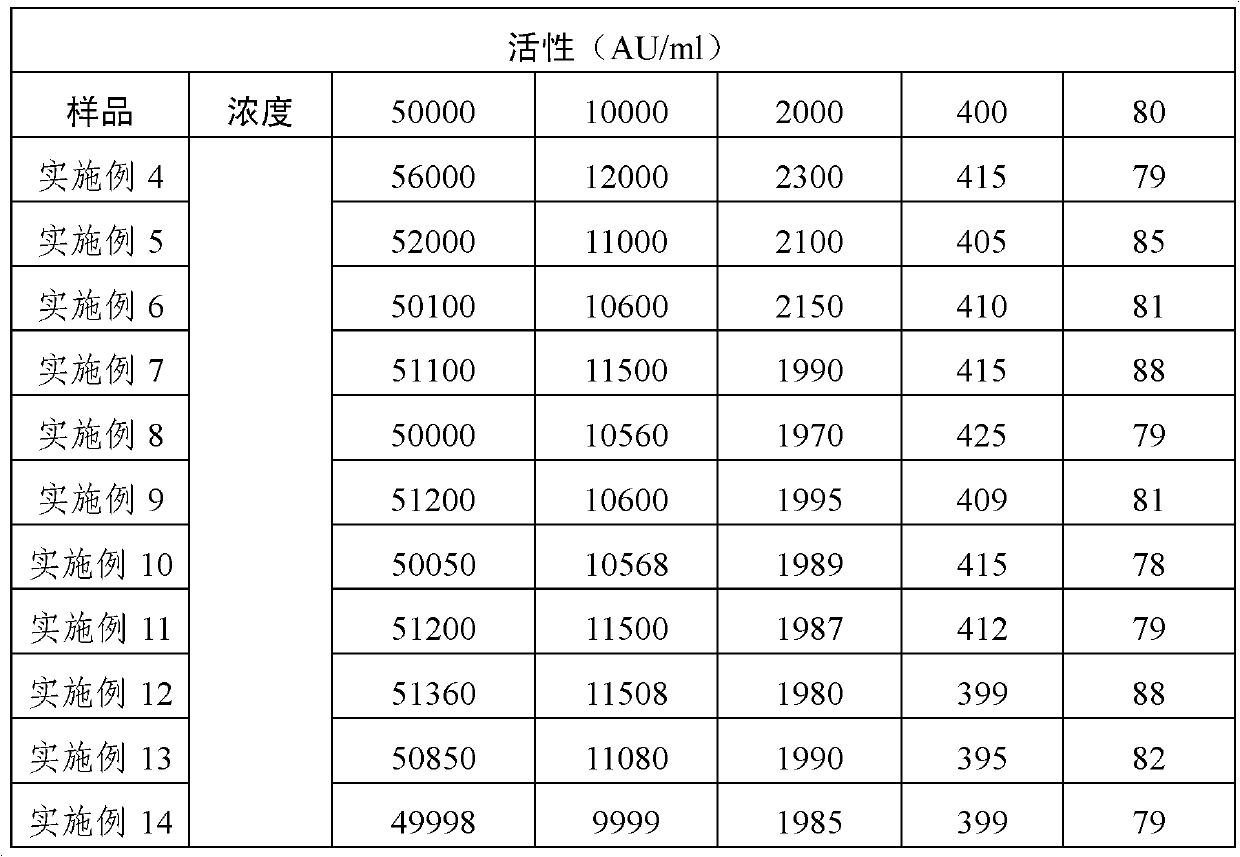
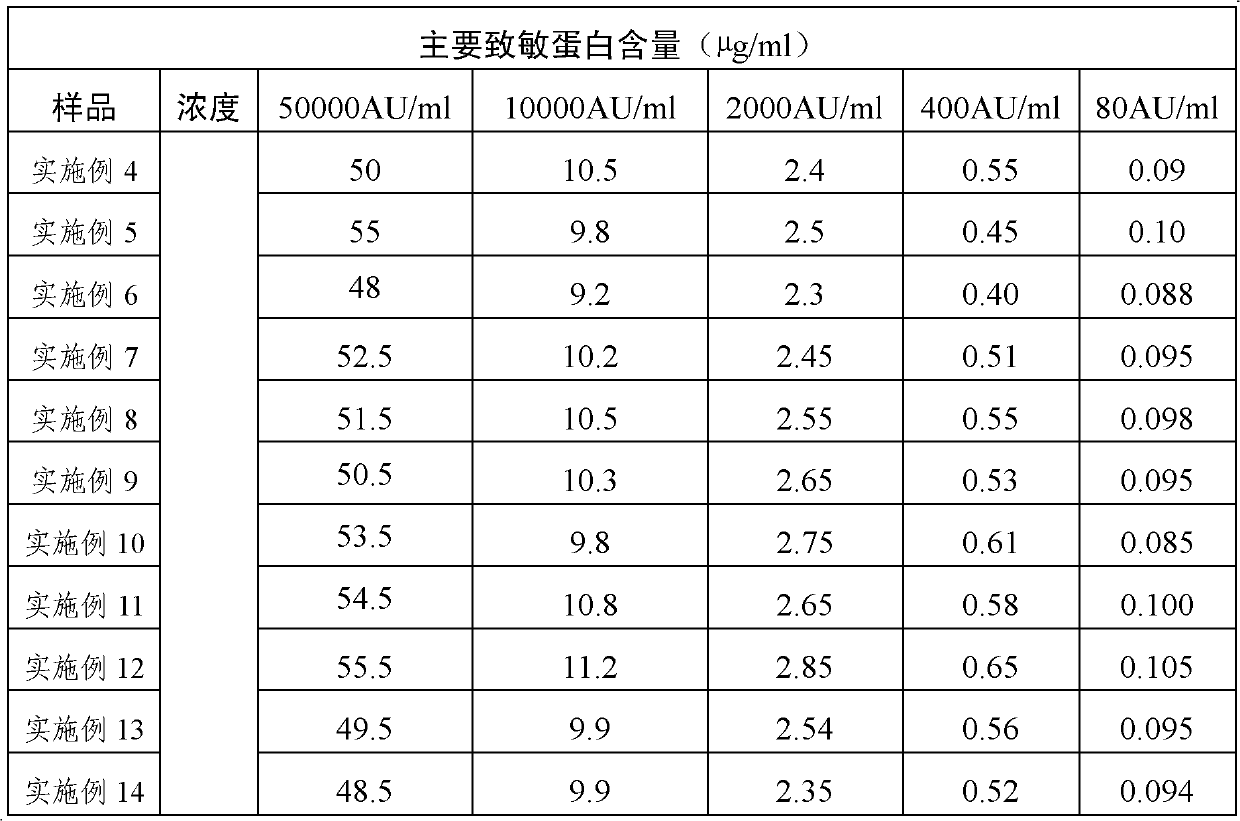
Patents
Literature
291 results about "Combined Vaccines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Licensed combination vaccinesCombination or combined vaccineA vaccine that consists of two or more antigens in the same preparation (e.g., MMR, DTP).
Porcine pseudorabies virus virulent strain, and gene deletion vaccine strain thereof and applications thereof
ActiveCN102994458AEffective preventionEffective therapeuticMicroorganism based processesAntiviralsRabiesMicrobacterium
The invention discloses a porcine pseudorabies virus virulent strain, and a gene deletion vaccine strain thereof and applications thereof. The porcine pseudorabies virus virulent strain is named as HeN1, the microbial preservation number of the porcine pseudorabies virus virulent strain is CGMCC NO.6656, the deleted gE gene obtaines the gene deletion vaccine strain rPRV-gE-EGFP+ on the basis of the virulent strain HeN1, and the microbial preservation number is CGMCC NO.6657. The virulent strain can be prepared into inactivated vaccine (single vaccine or combined vaccine), the gene deletion vaccine strain rPRV-gE-EGFP+ can be prepared into activated vaccine or inactivated vaccine (single vaccine or combined vaccine) and the like, so that porcine pseudorabies can be effectively prevented or cured, or the gene deletion vaccine strain rPRV-gE-EGFP+ can be prepared into a diagnosis reagent for diagnosing the porcine pseudorabies. The gene deletion vaccine strain rPRV-gE-EGFP+ has the advantages of being good in safety, high in protection efficiency, beneficial to differential diagnosis.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
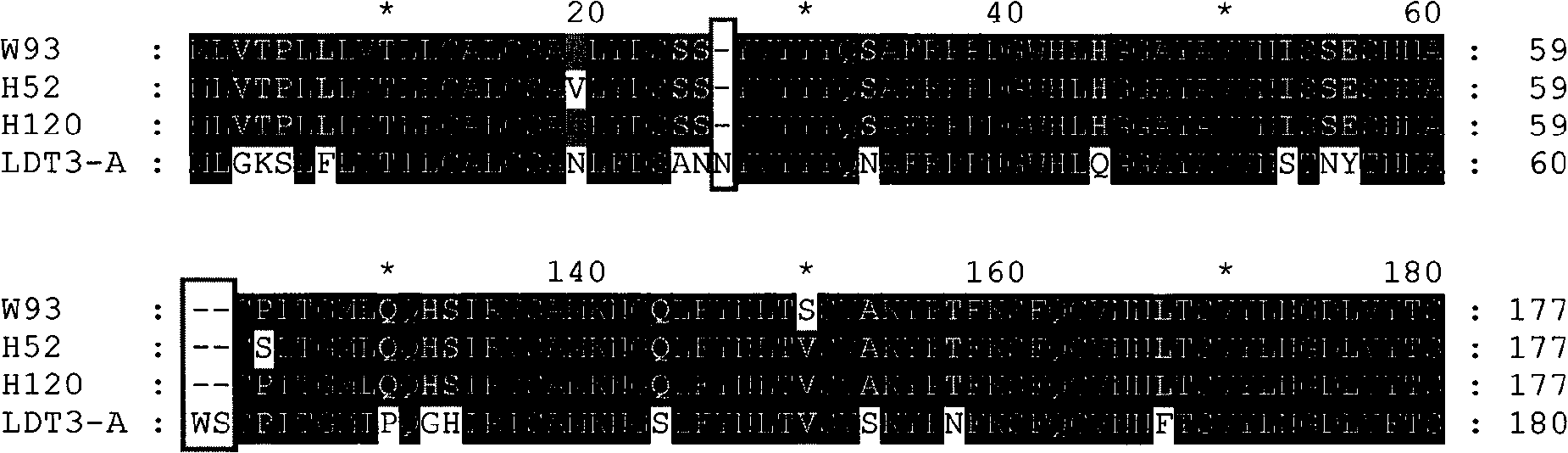
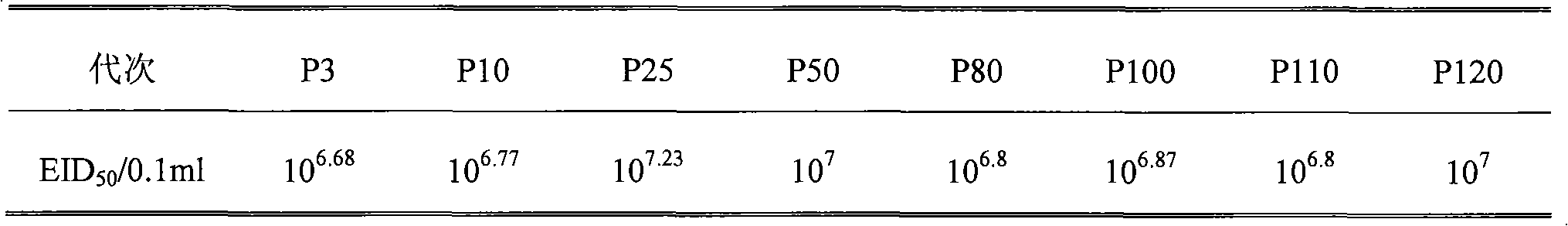
Chicken infectivity bronchitis virus attenuated vaccine strain and application thereof
ActiveCN101514334AImprove securityNo side effectsInactivation/attenuationMicroorganism based processesInfectious bronchitisMicroorganism
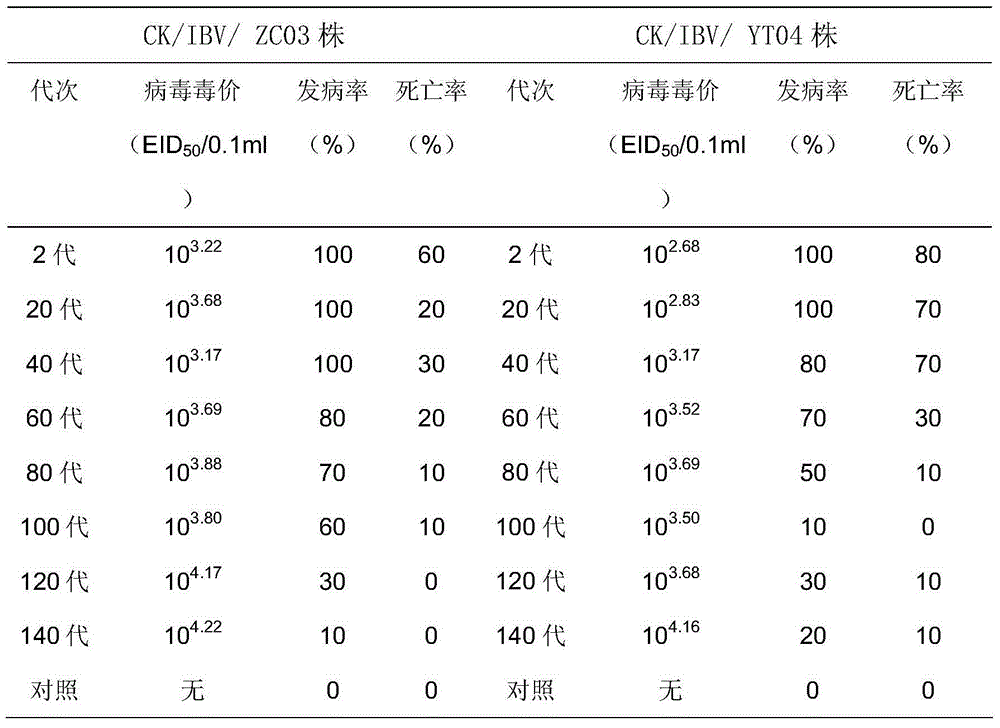
The invention discloses a infectivity bronchitis attenuated vaccine strain LDT3-A strain, and discloses application and application effect thereof in preventing and curing chicken infectivity bronchitis. The microorganism accession number of the attenuated vaccine strain is CGMCC-2902. The attenuated vaccine strain of the present invention has good safety and good immunization protection effect to the chicken infectivity bronchitis. The attenuated strain can be prepared into single vaccine or combined vaccine for preventing or curing infectivity bronchitis virus.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Helicobacter pylori vaccine based on urease B subunit active segment and its prepn process
InactiveCN1887349AGood immune protectionAntibacterial agentsBacterial antigen ingredientsProtective antigenAntigen
The present invention provides one kind of genetic engineering multivalent subunit vaccine for preventing and treating human helicobacter pylori infection and its preparation process. The vaccine consists of active helicobacter pylori UreB fragment UreB414 as the central antigen component, other combined protective antigens, and intramolecular or extramolecular adjuvant. Compared with univalent vaccine, the multivalent combined vaccine can stimulate the body to generate more powerful and more comprehensive helicobacter pylori resisting specific immune reaction.
Owner:ARMY MEDICAL UNIV
Polyvalent bacteria capsule polysaccharide-protein conjugate combined vaccine
ActiveCN1709505AImproving immunogenicityReduce the number of vaccinationsBacterial antigen ingredientsHaemophilusMedicine
The present invention relates to a polyvalent bacterial capsule polysaccharide-protein conjugate combined vaccine preparation, in particular, it is a combined vaccine containing group A, group C, group Y and group W135 epidemic cerebrospinal meningitis coccal capsule polysaccharide-protein conjugate and b type haemophilus influenzal capsule polysaccharide-protein conjugate.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM
Separated and purified acellular pertussis-diphtheria-tetanus, b-type haemophilus influenzae and A-group and C-group meningococcus combined vaccine and preparation method thereof
InactiveCN104689309ARelieve painReduce the burden onAntibacterial agentsBacterial antigen ingredientsHemagglutininAluminium hydroxide
The invention discloses a combined vaccine and a preparation method thereof. The combined vaccine is formed by a component A and a component B, wherein the component A is a liquid preparation and composed of pertussis toxin, filamentous hemagglutinin, pertussis adhesion, diphtheria toxoid, tetanus toxoid, aluminium hydroxide and sodium chloride; the component B is a freeze-drying preparation and composed of A-group meningitis polysaccharide conjugate, C-group meningitis polysaccharide conjugate, b-type haemophilus influenzae polysaccharide conjugate and lactose. The preparation method of the vaccine includes the steps of preparing of acellular pertussis-diphtheria-tetanus vaccine semi-finished products, A-group and C-group meningococcocci and b-type haemophilus influenzae combined vaccine semi-finished products, split charging and packaging. The vaccine has the characteristics of being safe, effective, controllable and capable of preventing diseases through an injection, the preparation method is easy to operate, preparation is facilitated, cost is low, and the combined vaccine is suitable for industrialized mass production.
Owner:CHENGDU OLYMVAX BIOPHARM
Pneumo-streptococcal-polysaccharide adventitia jointed vaccine and preparing method
InactiveCN101024079AEasy to manufactureImprove immunityAntibacterial agentsBacterial antigen ingredientsAntigenDisease
The present invention relates to a streptococcus pneumoriae polysaccharide-outer membrane protein combined vaccine and its preparation method, belonging to the field of streptococcus pneumoriae vaccine. The main antigen component of said vaccine is a streptococcus pneumoriae capsular polysaccharide-outer membrane protein combined product obtained by covalently connecting the capsular polysaccharide produced by streptococcus pneumoriae with its outer membrane protein. Said capsular polysaccharide is the capsular polysaccharide of one or several kinds of streptococcus pneumoriae, its molecular weight is about 200-500 KDa, every polysaccharide molecule has about 300-700 repeating units. The outer membrane protein is the outer membrane protein of one or several kinds of streptococcus pneumoriae, its molecular weight is about 30-100 Kda. Said vaccine can be used for preventing or curing the diseases induced by streptococcus pneumoriae.
Owner:CHANGHUI BIOLOGICAL ENG FUZHOU
Immunization method against Neisseria meningitidis serogroups A and C
The present invention describes methods of immunizing a patient with a combined vaccine that offers protection against meningococcal disease caused by the pathogenic bacteria Neisseria meningitidis serogroups A and C. The vaccine comprises at least two distinct polysaccharide-protein conjugates that are formulated as a single dose of vaccine. The purified capsular polysaccharides of Neisseria meningitidis serogroups A and C are chemically activated and selectively attached to a carrier protein by means of a covalent chemical bond, forming polysaccharide-protein conjugates capable of eliciting long-lasting immunity to a variety of N. meningitidis strains in infants.
Owner:SANOFI PATEUR
Combination vaccines with 1-hydroxy-2-phenoxyethane preservative
Processes for preparing combination vaccines that include diphtheria and tetanus toxoids, where these two toxoids are used in the processes as a single component containing both toxoids, and also containing 1-hydroxy-2-phenoxyethane.
Owner:NOVARTIS AG
Combined measles-human papilloma vacine
The present invention relates to combined vaccines against measles and human papilloma virus (HPV). In particular, the invention relates to recombinant measles virus vectors containing heterologous nucleic acid encoding single or several antigens derived from HPV, preferably, the major capside antigen L1, the minor capside antigen L2, the early gene E6 and the early gene E7 oncoproteins of HPV type 16, and optionally of types 18, 6 and 11. In a first embodiment, prophylactic vaccines are generated expressing HPV antigens, preferably L1 and / or L2 such that they induce a potent long-lasting immune response in mammals, preferably humans, to protect against HPV and MV infection. In another embodiment, therapeutic vaccines are generated expressing E6 and E7 proteins, and optionally L1 and L2, such that they induced strong immune responses will resolve persistent HPV infections at early or late stages, including HPV-induced cervical carcinoma. In a preferred embodiment, the combined vaccines are easy to produce on a large scale and can be distributed at low cost.
Owner:CADILA HEALTHCARE LTD
Infectious bursal virus and method for propagating bursal virus with chicken embryo cell line and bioreactor to prepare inactivated vaccine and combined vaccine
InactiveCN102260649AImprove adaptabilityImproving immunogenicityViral antigen ingredientsMicroorganism based processesTGE VACCINEEmbryo cell
The invention relates to an infectious bursal disease virus (IBDV) HQ strain CGMCC NO.4935 and a preparation method of inactivated vaccines and combined vaccines for infectious bursal disease (IBD). The preparation method mainly comprises the following steps of: (1) carrying out chain amplification on cell seeds; (2) adding cell growth-promoting liquid into a sterilized bioreactor, and inoculating cells for preparing vaccines for suspension culture; (3) replacing maintenance liquid containing an IBDV strain when cells are of the maximum density, and continuing to culture; (4) collecting viruses in time, and measuring virus titer; and (5) inactivating virus liquid, and preparing inactivated vaccines and combined vaccines thereof for the IBD according to different proportions. The method provided by the invention increases the cell density, improves the virus titer, improves the vaccine titer, reduces the side reaction, reduces the labor intensity, lowers the production cost, improves the controllability of production processes, and ensures the uniformity and stability of product quality. The produced inactivated vaccines and combined vaccines thereof for the IBD have the advantagesof good safety and high immune efficiency and have a complete protective effect on the IBDV attack.
Owner:POULTRY DISEASE RES INST OF HENAN AGRI UNIV
Combination vaccines with lower doses of antigen and/or adjuvant
InactiveUS20140112950A1Without loss of immunoprotective effectMany solutionsAntibacterial agentsBacterial antigen ingredientsAdjuvantImmunogenicity
Combination vaccine compositions as well as methods for their manufacture have a relatively low amount of antigen and / or a relatively low amount of aluminium, but they can nevertheless have immunogenicity which is comparable to combination vaccines with a relatively high amount of antigen and / or a relatively high amount of aluminium. Aluminium-free combination vaccine compositions are also provided e.g. compositions which are adjuvanted with an oil-in-water emulsion adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
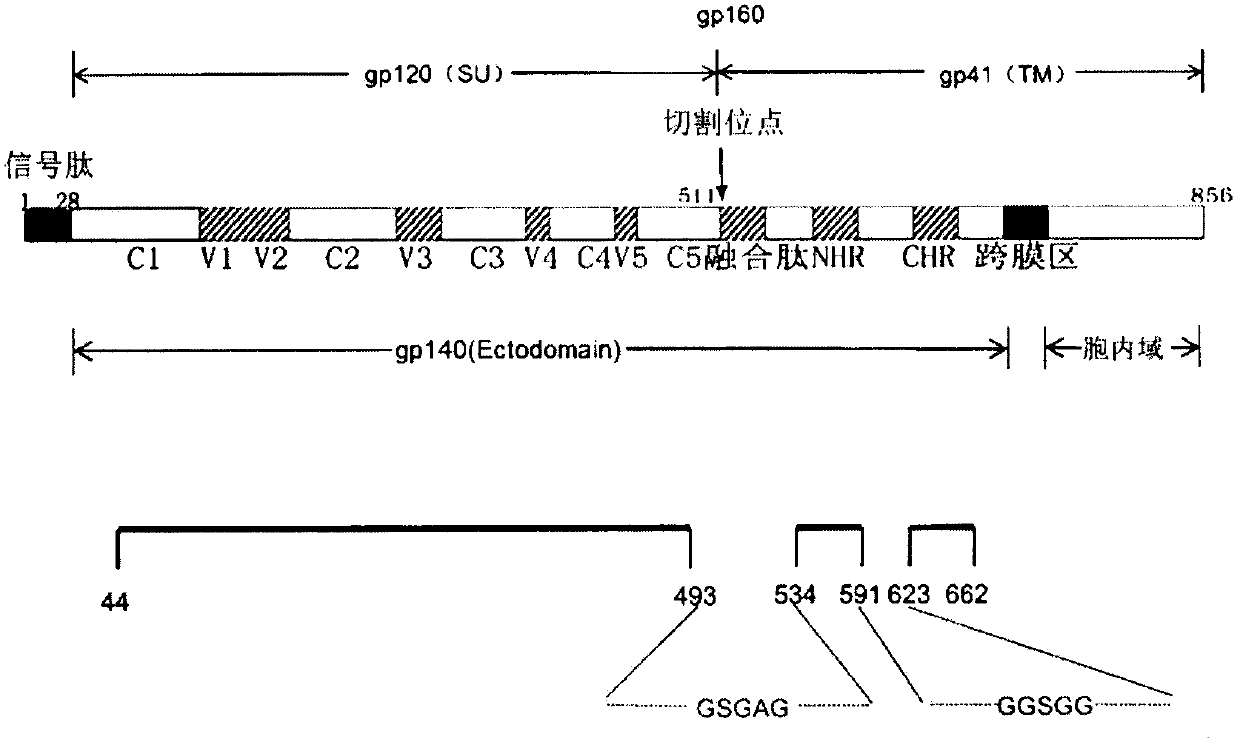
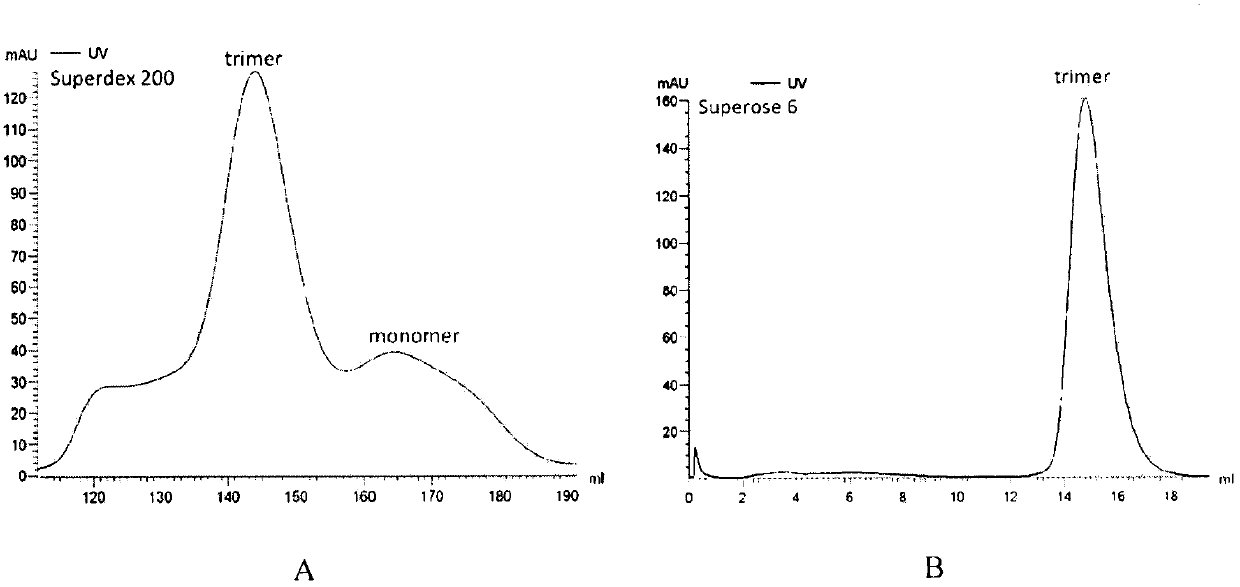
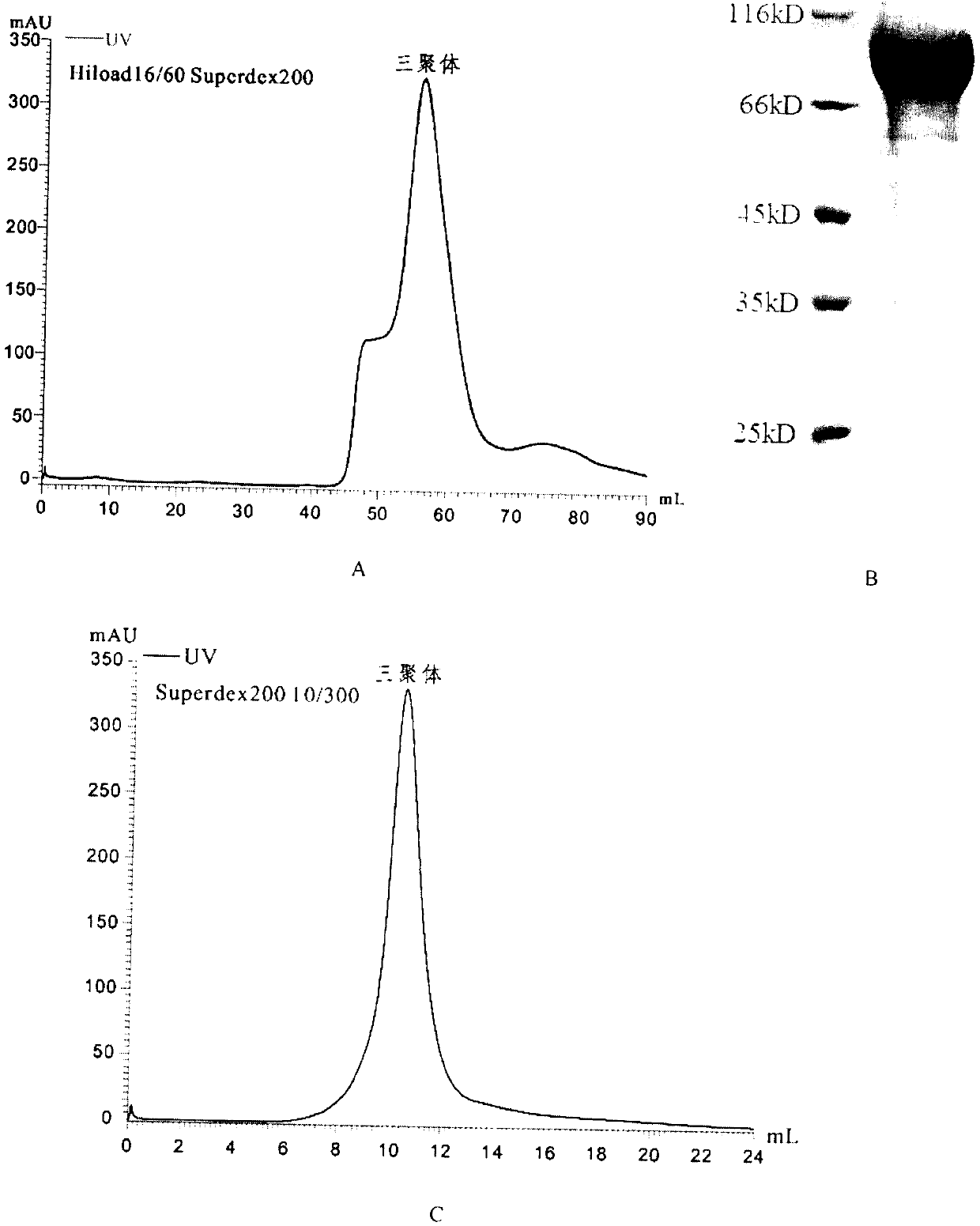
Potential preparation method for highly-efficient recombinant HIV-1 CRF07-BC gp140 immunogen
InactiveCN103992396AImprove uniformityHigh antigen reactivityVirus peptidesDepsipeptidesAntigenEndotoxin removal
The invention discloses a potential preparation method for a highly-efficient recombinant HIV-1 CRF07-BC gp140 immunogen. The immunogen is designed based on the structure of HIV-1 envelope protein crystals which have been published internationally and obtained in our lab. The specific method uses an overlap extension PCR technology to obtain gp140 gene segments, and comprises the steps that target genes are cloned into an eukaryotic expression vector pMT, endotoxin is removed through extraction of a large amount of plasmids, the gp140 gene segments and resistance screening plasmids pCoBlast are co-transfected into drosophila melanogaster Schneider2 (S2) cells together, blasticidin (Blasticidin S) is used for positive clone screening, S2 cell lines for stably and efficiently secreting and expressing gp140 are screened, and after enlarged cultivation and through two steps of purification of nickel column affinity chromatography and gel filtration chromatography, the gp140 with high purity can be obtained. A series of biochemical and biophysical technologies indicate that the gp140 is uniform in polymeric states, and high in antigenic reactivity, and is quite fittingly used as the immunogen for the research and the development of AIDS subunit vaccines or multivalent combined vaccines.
Owner:NANKAI UNIV
Combined vaccines for prevention of porcine virus infections
ActiveUS20140093535A1SsRNA viruses positive-senseViral antigen ingredientsProtection sexProtective immunity
The present disclosure provides vaccine compositions comprising a PRRSV vaccine and a second porcine vaccine, which are substantially free from immuno-inhibition against each other. The second porcine virus vaccine can be CSFV and / or PRV. The preparation methods for the vaccines and the formulations are also provided. The vaccine compositions provided herein confer protective immunity to pigs against porcine reproductive and respiratory syndrome, classical swine fever, and / or pseudorabies.
Owner:SINOVET JIANGSU BIOPHARM CO LTD
Production technique of dual vaccine of chicken new castle disease and infectious bronchitis
InactiveCN101491674ASimple production processSave timeViral antigen ingredientsAntiviralsDiseaseAntigen
The invention relates to a method for producing a combined vaccine, in particular to a method for producing a combined vaccine against Newcastle disease and infective bronchitis. The method comprises that: before culture in the same embryo, purity of breeding virus of the Newcastle disease and the infective bronchitis is improved; and during culturing in the same embryo, concentrations of two antigens in the combined vaccine against the Newcastle disease and the infective bronchitis are adjusted to reach reasonable combination. The vaccine prepared by the method is vaccinated with chicken flocks to generate ND and IB immunity without any interference. Furthermore, the vaccine strains are reasonably matched to effectively solve the problem of serious interference in simultaneous vaccination of the Newcastle disease living vaccine and infective bronchitis living vaccine, improve immunization quality and reduce the number of immunization.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
Protein of acinetobacter baumannii hypothetical protein A1S_1523 as well as preparation method and application of protein
ActiveCN104877019ADefend against deadly infectionElicit a protective immune responseAntibacterial agentsBacterial antigen ingredientsAdjuvantAmino acid
The invention relates to recombinant protein of A1S_1523 as well as a preparation method and application of the recombinant protein. The recombinant protein comprises A1S_1523 mature peptide, and an amino acid sequence of the recombinant protein is shown in SEQ ID NO. 3. The recombinant protein disclosed by the invention is high in expression quantity and convenient to separate and purify, is efficient and safe, can be directly matched with adjuvants for use, and can be used for preparing acinetobacter baumannii infection resistant subunit vaccines and related detection kits; proven by animal experiments, gene engineering recombinant monovalent subunit vaccines have good immune protective effects on acinetobacter baumannii infection resistance; and the recombinant protein can be used for laying a foundation for further researching combined vaccines and multi-subunit fusion vaccines, and can also play important roles in the development and application of prevention and treatment vaccines and diagnostic kits.
Owner:ARMY MEDICAL UNIV
Method for Propagating Infectious Bursal Virus with Chicken Embryo Origin Cell Line to Prepare Inactivated Vaccine and Combined Vaccine
InactiveCN102258777AGuaranteed to be pureAvoid Biosafety HazardsViral antigen ingredientsAntiviralsVaccine ProductionEmbryo
The invention relates to a method for preparing a vaccine by breeding infectious bursal disease virus (IBDV) by a chicken embryo source cell line. The method mainly comprises the following steps of: 1) subculturing cells DF-1 for preparing vaccines; 2) breeding IBDV HQ cell seeds; 3) breeding virus liquid for preparing the vaccines; 4) concentrating and inactivating the virus liquid for preparingthe vaccine; 5) preparing other virus liquid of newcastle disease method, newcastle disease-infectious bronchitis virus method, and newcastle disease-infectious bronchitis virus-egg drop syndrome method combined vaccine and concentrating; and 6) proportioning inactivated combined vaccine, emulsifying and sub-packaging. The production process is simple, and stable and is easy to operate, eliminates biological potential safety hazard existing in the conventional vaccine production, and overcomes the defects that large-scale production of the vaccines is limited by supply of chicken embryos; cost and batch-to-batch variation are reduced; the virus titer and the quality of vaccine are improved; basis is laid for culturing virus liquid on large scale by a suspension culture technology in vaccine industry; and the produced IBDV inactivated vaccine and combined vaccine have high safety and immune efficacy, and have the complete immune protection effect on IBDV attack.
Owner:POULTRY DISEASE RES INST OF HENAN AGRI UNIV
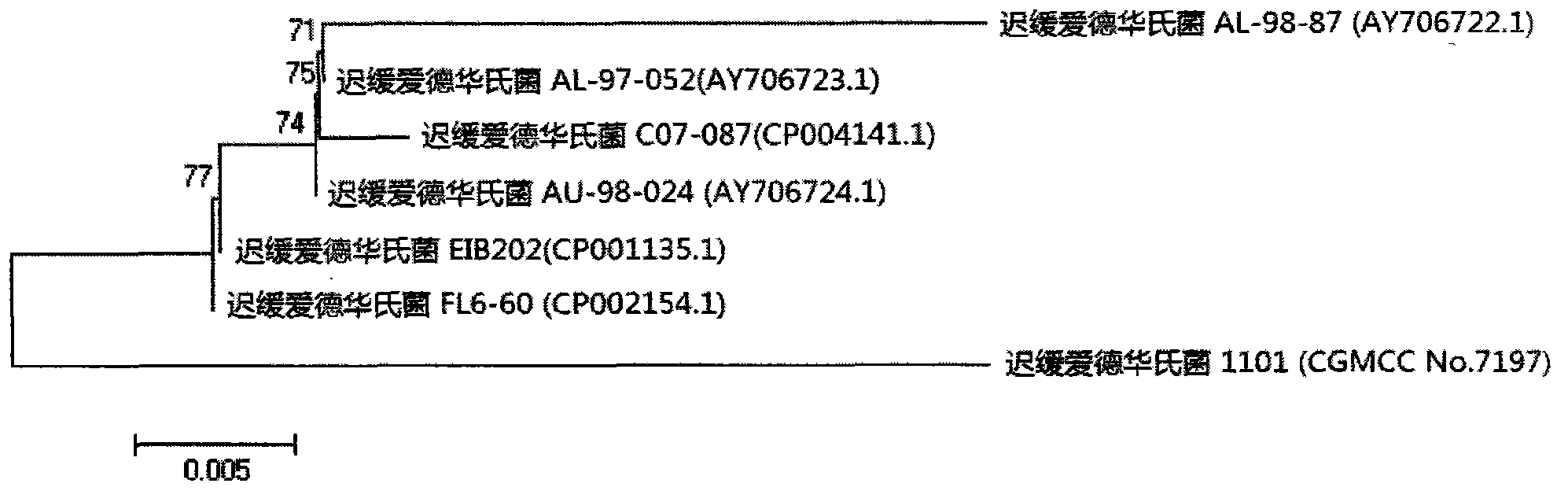
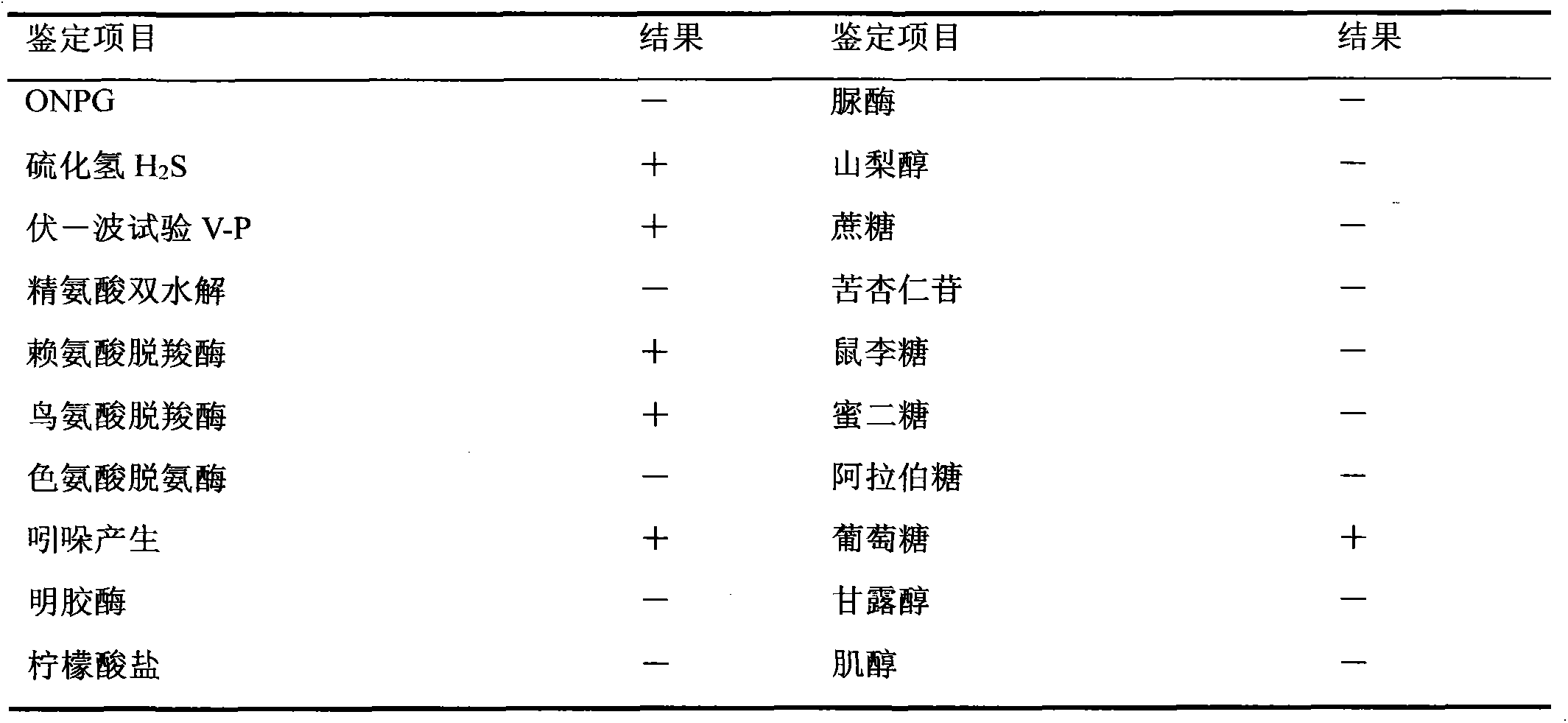
Velogenic Edwardsiella tarda vaccine strain and application thereof
ActiveCN103255089AStrong drug resistanceReduce lossesAntibacterial agentsBacterial antigen ingredientsBacteroidesProtective antigen
The invention relates to an Edwardsiella tarda strain and an application method thereof. The Edwardsiella tarda strain is separated from a turbot adult fish body and is a wild strain with strong virulence, and the preservation number of the Edwardsiella tarda strain is CGMCC No.7197. Preparation modes of an antigen of the Edwardsiella tarda strain comprise any one or more than one of an inactivated thallus, a bacteruak ghost ingredient, an attenuated strain, a protective antigen, an antigen subunit and an expression product of an antigen determinant or an antigen gene expression carrier; the produced vaccine can be a single ingredient of the antigen prepared by utilizing the Edwardsiella tarda strain and can also be a combined vaccine produced by mixing the antigen prepared by utilizing the Edwardsiella tarda strain with antigens of other bacteria, and the prepared single or combined vaccine antigen is added with an adjuvant to produce the vaccine; and an inoculation mode of the vaccine in immunization application can adopt injection immunization, wound immunization, immersion bath immunization or oral administration immunization.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Chicken new castle disease-infectious bronchitis trivalent combined vaccine and preparation method thereof
InactiveCN103599533AImprove the chance of preventionMeet the needs of preventionPowder deliveryViral antigen ingredientsDiseaseAntigen
The invention discloses a chicken new castle disease-infectious bronchitis trivalent combined vaccine and a preparation method thereof. The trivalent combined vaccine comprises antigens and a freeze-drying protective agent. The antigens comprise a chicken kidney-type infectious bronchitis virus K136 strain having an accession number of CCTCC-V201320, a chicken breath-type infectious bronchitis virus H120 strain, and a chicken new castle disease virus La Sota strain. A mode of inoculating the K136 strain and the La Sota strain in a homeomorphism manner and inoculating the H120 strain individually is adopted for preparing the antigens. Based on the mode, after culture products are mixed according to a ratio to prepare the antigens, a product of the vaccine is obtained by adding the freeze-drying protective agent. The trivalent combined vaccine can prevent the chicken new castle disease and the chicken infectious bronchitis simultaneously, thus solving effectively a problem that vaccines at present can only provide a part of protection or cannot provide protection for the kidney-type IB, and achieving an objective that one injection can be used for preventing two diseases.
Owner:TIANJIN RINGPU BIO TECH
Freeze-drying hepatitis A varicella attenuation combined vaccine and producing method thereof
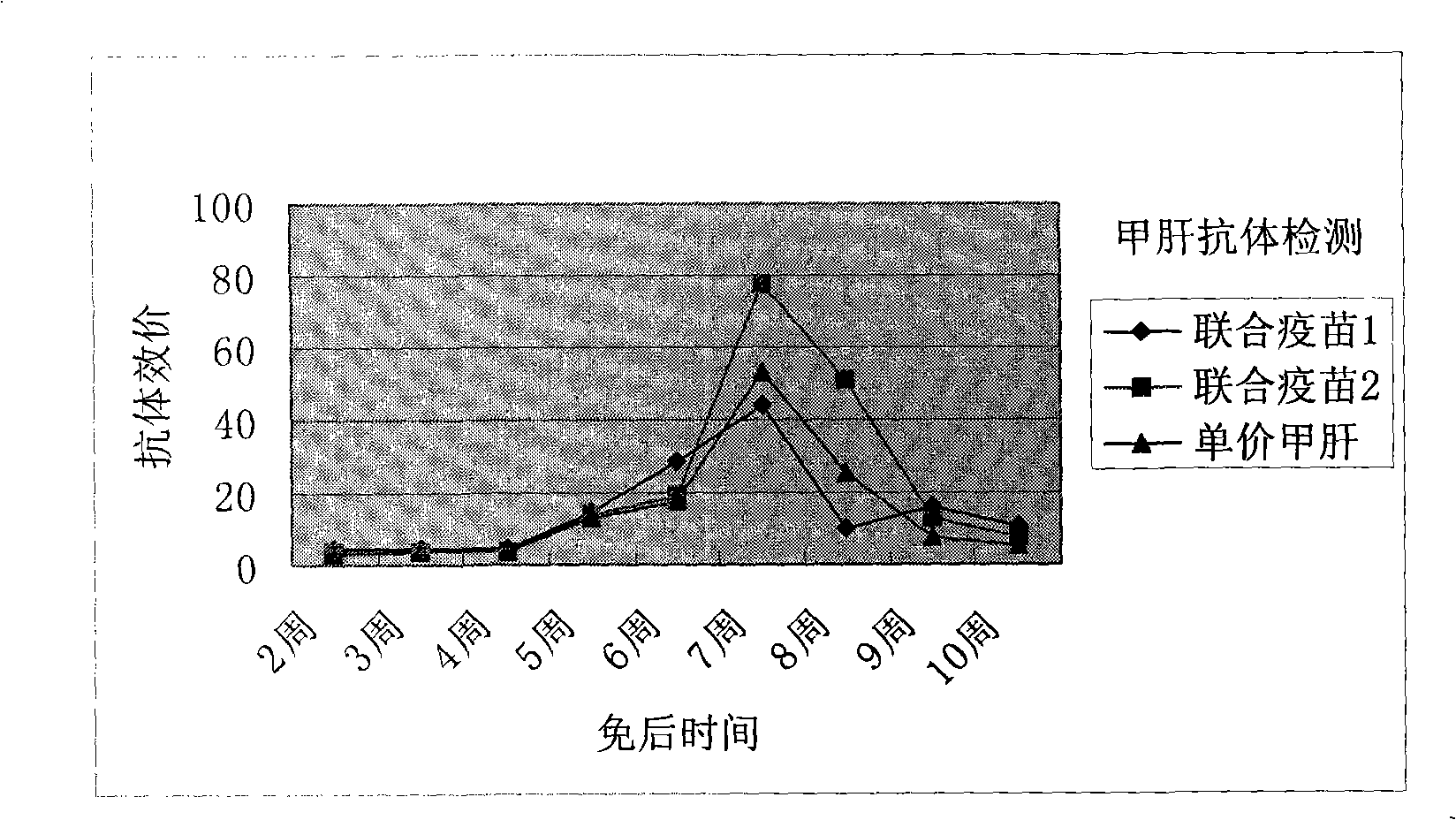
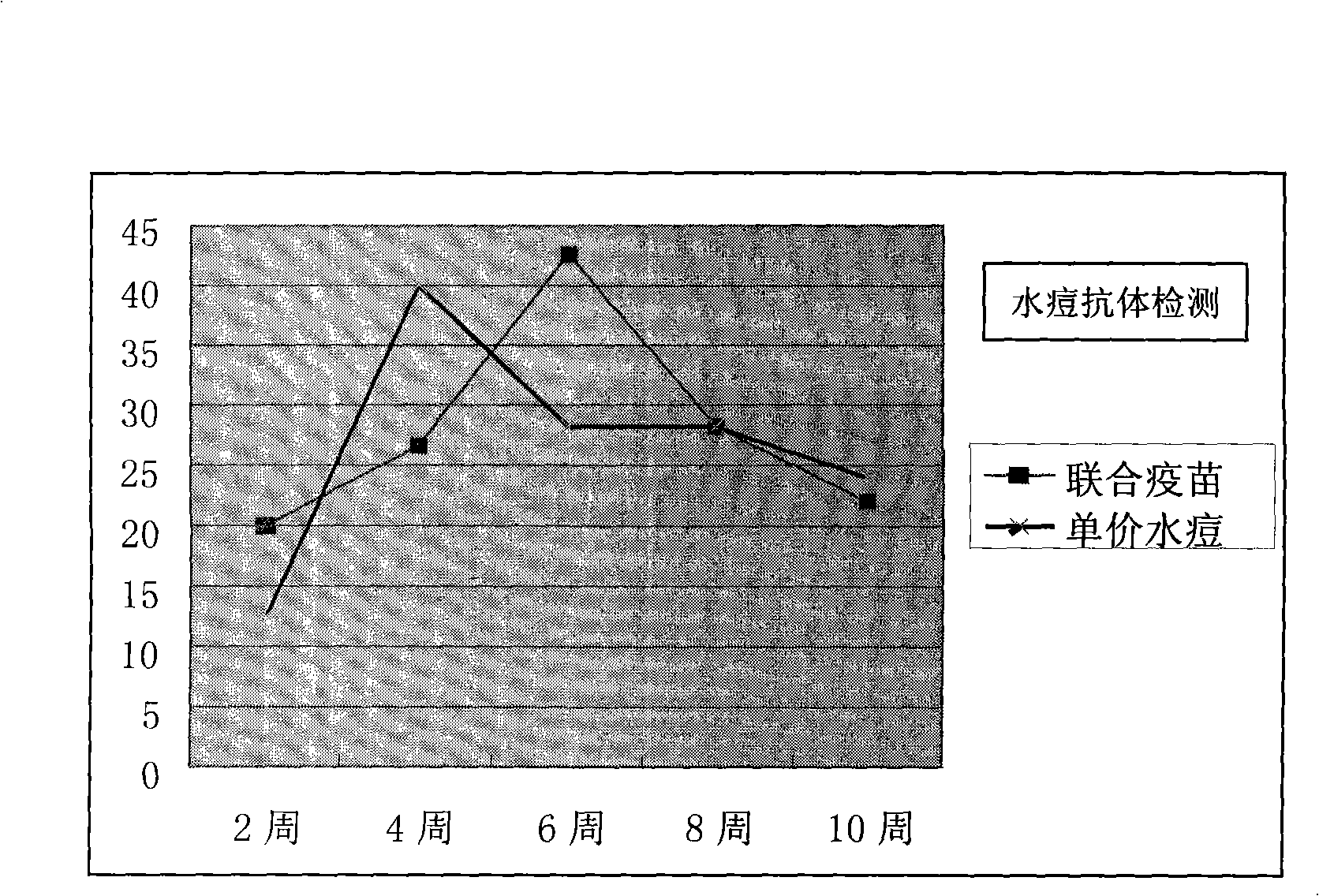
ActiveCN101259269AImprove securityImproving immunogenicityPowder deliveryDigestive systemDiseaseUltrasound attenuation
The present invention provides a freeze-dried hepatitis-A-chicken-pox attenuation combined vaccine and a production thereof. The combined vaccine has the security and the immunogenicity as good as the univalent vaccine and can effectively prevent the hepatitis A and the chicken pox. Two kinds of diseases can be effectively prevented just by one immunization, which can reduce vaccine cost, improve vaccine inoculation efficiency and reduce the bad reaction caused by a plurality of times of injections, and can reduce the vaccine inoculation times but realize the purpose of preventing various diseases.
Owner:长春生物制品研究所有限责任公司
Porcine pseudorabies virus gene deletion attenuated vaccine strain for passage via low temperature of cell and attenuation via drug screening and application thereof
ActiveCN106282128AImprove securityImprove protection efficiencyViral antigen ingredientsMicroorganism based processesLarge fragmentVariant strain
The invention discloses a porcine pseudorabies virus gene deletion attenuated vaccine strain for passage via low temperature of a cell and attenuation via drug screening and an application thereof. The attenuated vaccine strain is prepared through the following steps: based on a porcine pseudorabies virus variant strain (named as strain HeN1, of which the microbial preservation serial No. is CGMCC No. 6656), firstly, carrying out low-temperature passage and screening on a Vero cell to obtain large fragments of deleted viruses including gI, gE, Us9, Us2 and part of inverted repeated sequence which exist in zone US through, and then making the TK gene thereof partially deleted through drug screening. The gene-deleted attenuated vaccine strain is named as strain PRV TP, of which the microbial preservation serial No. is CGMCC No. 12300. A live vaccine or an inactivated vaccine (a single vaccine or combined vaccine) can be prepared from the attenuated vaccine strain disclosed by the invention, and can prevent porcine pseudorabies effectively, and a reagent for diagnosing or treating porcine pseudorabies can be prepared from the attenuated vaccine strain too. According to the porcine pseudorabies virus gene deletion attenuated vaccine strain for passage via low temperature of a cell and attenuation via drug screening and the application thereof, the porcine pseudorabies attenuated vaccine strain PRV TP has the advantages of good safety, efficient protection, convenient differential diagnosis and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Artemisia pollen allergen vaccine, and preparation method and application thereof
InactiveCN102552900AImprove effectivenessGood curative effectAllergen ingredientsImmunological disordersAllergic dermatitisDisease
The invention provides an artemisia pollen allergen vaccine, and a preparation method and application thereof. The artemisia pollen allergen vaccine comprises an artemisia sieversiana pollen allergen, an artemisia annua pollen allergen and an artemisia argyi pollen allergen. Compared with a single artemisia pollen allergen vaccine, the combined vaccine has the advantages that the effectiveness of treatment is improved while the safety can be ensured; and the artemisia pollen allergen vaccine can be used for treating allergic dermatitis, chronic urticaria, allergic rhinitis and allergic asthma which are caused by allergy to multiple artemisia pollens.
Owner:北京新华联协和药业有限责任公司
Chicken Marek's disease Meq gene deleted vaccine strain, construction method thereof, and application thereof
ActiveCN102363769ANon-pathogenicGenetic stabilityMicrobiological testing/measurementMicroorganism based processesOncogeneWild strain
The invention discloses a chicken Marek's disease (MD) Meq gene deleted vaccine strain, a construction method thereof, and an application thereof. The chicken Marek's disease rMS delta Meq gene deleted vaccine strain has a microbe reservation number of CGMCC No. 4612. According to the invention, on a basis of a parent strain which is MDV MS strain, a 468bp base sequence on a front part of a main oncogene is deleted thorough two times of homologous recombination, such that the chicken Marek's disease Meq gene deleted vaccine strain is obtained. The invention also relates to the application of the gene deleted vaccine strain in preparation of medicine used for controlling chicken Marek's disease, and in detection and disgnosis methods used for distinguishing a vaccine strain and a chicken Marek's disease wild strain, wherein the methods are designed aiming at the deleted gene sequence of the gene deleted strain. The gene deleted vaccine strain provided by the invention has good safety and good immuno-protection effect upon chicken Marek's diseases. The vaccine strain can be used for preparing monovalent vaccines or combined vaccines used for controlling chicken Marek's disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Combined inactivated vaccine for avian influenza virus and fowl adenovirus
ActiveCN105582533AImprove securityHigh potencySsRNA viruses negative-senseViral antigen ingredientsImmunogenicityTGE VACCINE
The invention provides a combined inactivated vaccine for an avian influenza virus and a fowl adenovirus. An H9 subtype avian influenza virus QDY strain and an I-group 4-type aviadenovirus YBAV-4 new strain used by the vaccine are high in TCID50 / EID50 valence and good in immunogenicity and can well withstand attacks of the H9 avian virus and various local separate viruses. The vaccine prepared by the invention is good in safety, and free of local and whole-body adverse effects caused by the vaccine. Through analysis of characters, a safety test and efficacy test data in a storage-life test, the combined vaccine is free of an obvious difference from a single vaccine of a similar product in effect, and is stable and effective; the efficacy test result proves that the antibodies of the combined vaccine and two single vaccines are kept at a high level and are faster in antibody generation of similar products; and a control group antibody is negative.
Owner:YEBIO BIOENG OF QINGDAO
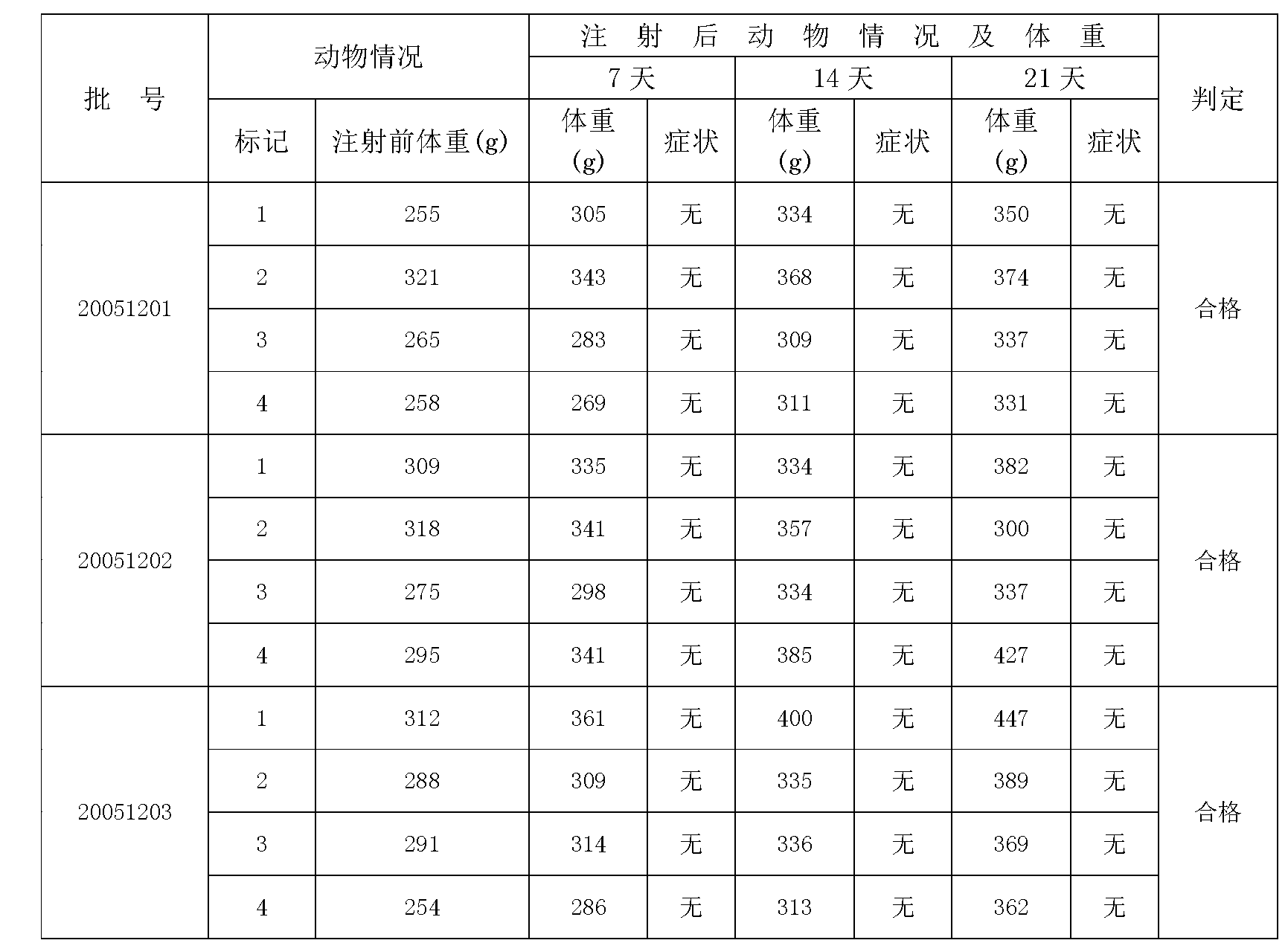
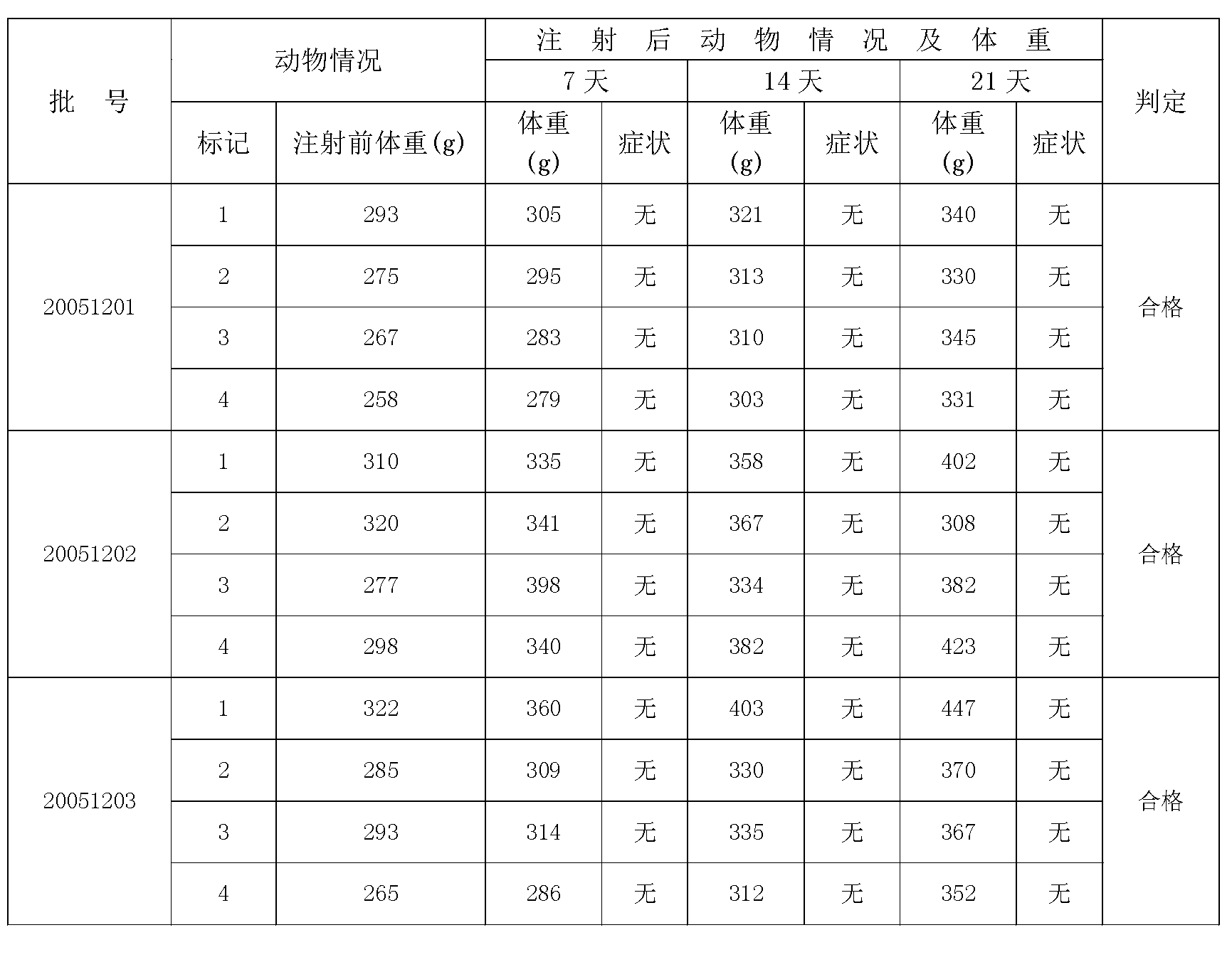
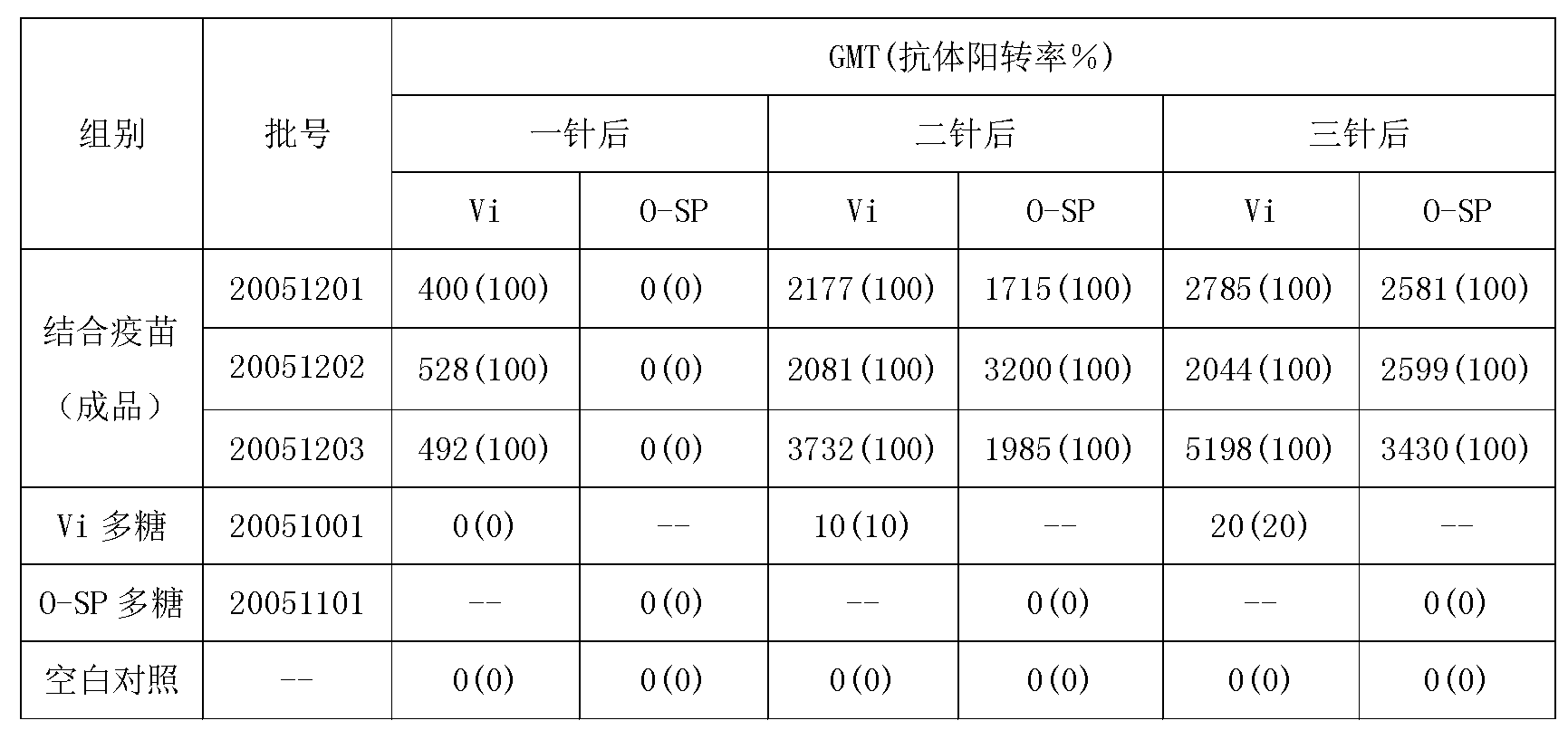
Typhoid fever and paratyphoid fever combined vaccine and preparation method thereof
ActiveCN102935226ASame immune responseAntibacterial agentsBacterial antigen ingredientsAcute toxicity testingBiology
The invention discloses a typhoid fever and paratyphoid fever combined vaccine. The typhoid fever and paratyphoid fever combined vaccine comprises typhoid Vi polysaccharide and paratyphoid fever thallus specific polysaccharide O-SP. The invention further discloses a preparation method of the vaccine. The preparation method comprises the steps of preparing the typhoid Vi polysaccharide, preparing the paratyphoid fever thallus specific polysaccharide O-SP, preparing typhoid Vi polysaccharide-carrier protein conjugate, preparing paratyphoid fever thallus specific polysaccharide O-SP-carrier protein conjugate and preparing end products. Animal experiments prove that the typhoid fever and paratyphoid fever combined vaccine can induce high-level anti-typhoid Vi polysaccharide and anti-paratyphoid fever lipopolysaccharide (LPS) antibody, has immunologic memory reaction, and has no acute toxicity reactions.
Owner:罗益(无锡)生物制药有限公司
Dog parvovirus attenuated vaccine strain and application thereof
InactiveCN101942419AStrong immunityImprove protectionDigestive systemInactivation/attenuationDiseaseMicroorganism
The invention discloses a dog parvovirus attenuated vaccine strain and application thereof. In the invention, the separated dog parvovirus virulent strain is cultivated in a cell subculture mode to obtain the dog parvovirus attenuated vaccine strain. The microbial preservation number is CGMCC No.3841. The test on safety and immunogenicity indicates that the attenuated vaccine strain CPV-YNR can protect the dog subjected to the attack of homologous virulent strain and has favorable immunogenicity. The attenuated vaccine strain CPV-YNR can be used for preparing a single vaccine or combined vaccine, and can effectively prevent or treat diseases caused by dog parvovirus. The attenuated vaccine strain CPV-YNR has the advantages of stable heritability, enduring immunity, favorable effect, high safety and reliability, long storage time and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Multivalent immunogenic composition
ActiveCN103394082AImprove securitySave the number of seedsBacterial antigen ingredientsViral antigen ingredientsHemagglutininTetanus toxoids
The invention provides a multivalent immunogenic composition, which includes an inactivated hepatitis A antigen and an inactivated poliovirus. The composition also can further include over one or two of a purified pertussis antigen, diphtheria toxoid, tetanus toxoid, filamentous hemagglutinin, Haemophilus influenzae type b polysaccharide, Neisseria meningitidis capsular polysaccharide, a hepatitis B virus antigen, enterovirus 71 and a coxsackievirus A16 antigen, and a physiologically acceptable carrier. The composition involved in the invention is employed to immunize the inoculated population in the form of a bivalent vaccine or more combined vaccines. Without reducing the immune effects of each immunizing antigen, the inoculation number of times can be reduced at the same time, and the time and human resources can also be saved.
Owner:SINOVAC BIOTECH
Serotype 5 haemophilus parasuis (HPs) vaccine strain
ActiveCN103194412AStable biological propertiesEpidemic preventionAntibacterial agentsBacteriaMortality rateHaemophilus Vaccines
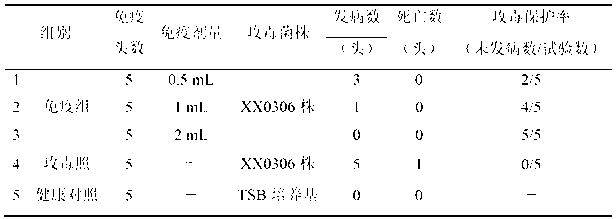
The invention relates to the field of vaccines of Haemophilus parasuis (HPs) in veterinary biological products and discloses a serotype 5 HPs vaccine strain. The class name of the vaccine strain is HPs. The strain number is XX0306. The vaccine strain has been collected in the China Center for Type Culture Collection, with collection number being CCTCC NO:M2013095 and collection date being March 21, 2013. The serotype 5 HPs strain XX0306 has stronger pathogenicity toward swine and has good immunogenicity. The inactivated vaccines prepared from the vaccine strain are safe and reliable and have quite good protective effects on the swine challenging homological strains. The morbidity and mortality of the immunized swinery are obviously reduced. Either the single vaccine or the combined vaccine has the immune effects equivalent or superior to the immune effects of the existing commercialized vaccines and can effectively prevent prevalence of HPs.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Multivalent Meningococcal Polysaccharide-Protein Conjugate Vaccine
InactiveUS20130216571A1Antibacterial agentsBacterial antigen ingredientsConjugate vaccineCarrier protein
Owner:SANOFI PASTEUR INC
Serotype 4 haemophilus parasuis (HPs) vaccine strain
ActiveCN103194413AStable biological propertiesEpidemic preventionAntibacterial agentsBacteriaMortality rateSerotype
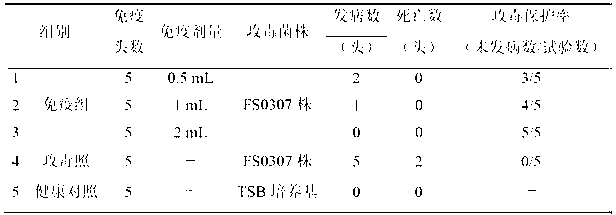
The invention relates to the field of vaccines of Haemophilus parasuis (HPs) in veterinary biological products and discloses a serotype 4 HPs vaccine strain. The class name of the vaccine strain is HPs. The strain number is FS0307. The vaccine strain has been collected in the China Center for Type Culture Collection, with collection number being CCTCC NO:M2013094 and collection date being March 21, 2013. The serotype 4 HPs strain FS0307 has stronger pathogenicity toward swine and has good immunogenicity. The inactivated vaccines prepared from the vaccine strain are safe and reliable and have quite good protective effects on the swine challenging homological strains. The morbidity and mortality of the immunized swinery are obviously reduced. Either the single vaccine or the combined vaccine has the immune effects equivalent or superior to the immune effects of the existing commercialized vaccines and can effectively prevent prevalence of HPs.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Method of preparing newcastle disease, infectiousness bronchitis bigeminy killed vaccine
InactiveCN101108248AReduce usageReduce stepsViral antigen ingredientsRespiratory disorderInfectious bronchitisAdjuvant
The invention relates to a method to prepare a combined inactive vaccine of newcastle disease and infectious bronchitis vaccine for chicks. The invention is characterized in that: firstly, concentrate and inactivate the NDVLaSota virus strains (10 per cent to 22 per cent weight ratio) and IBVM41 virus strains (10 per cent to 22 per cent weight ratio) respectively; and then add white oil and immune additives including tween, span and stearic acid to prepare the combined vaccine.
Owner:PU LIKE BIO ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com