Typhoid fever and paratyphoid fever combined vaccine and preparation method thereof
A technology combining vaccines and paratyphoid fever, applied in vaccines, multivalent vaccines, pharmaceutical formulations, etc., can solve problems such as loss of vaccine protection, poor reproductive ability of vaccines, uncertain genetic stability of attenuated strains, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation method of typhoid Vi polysaccharide:
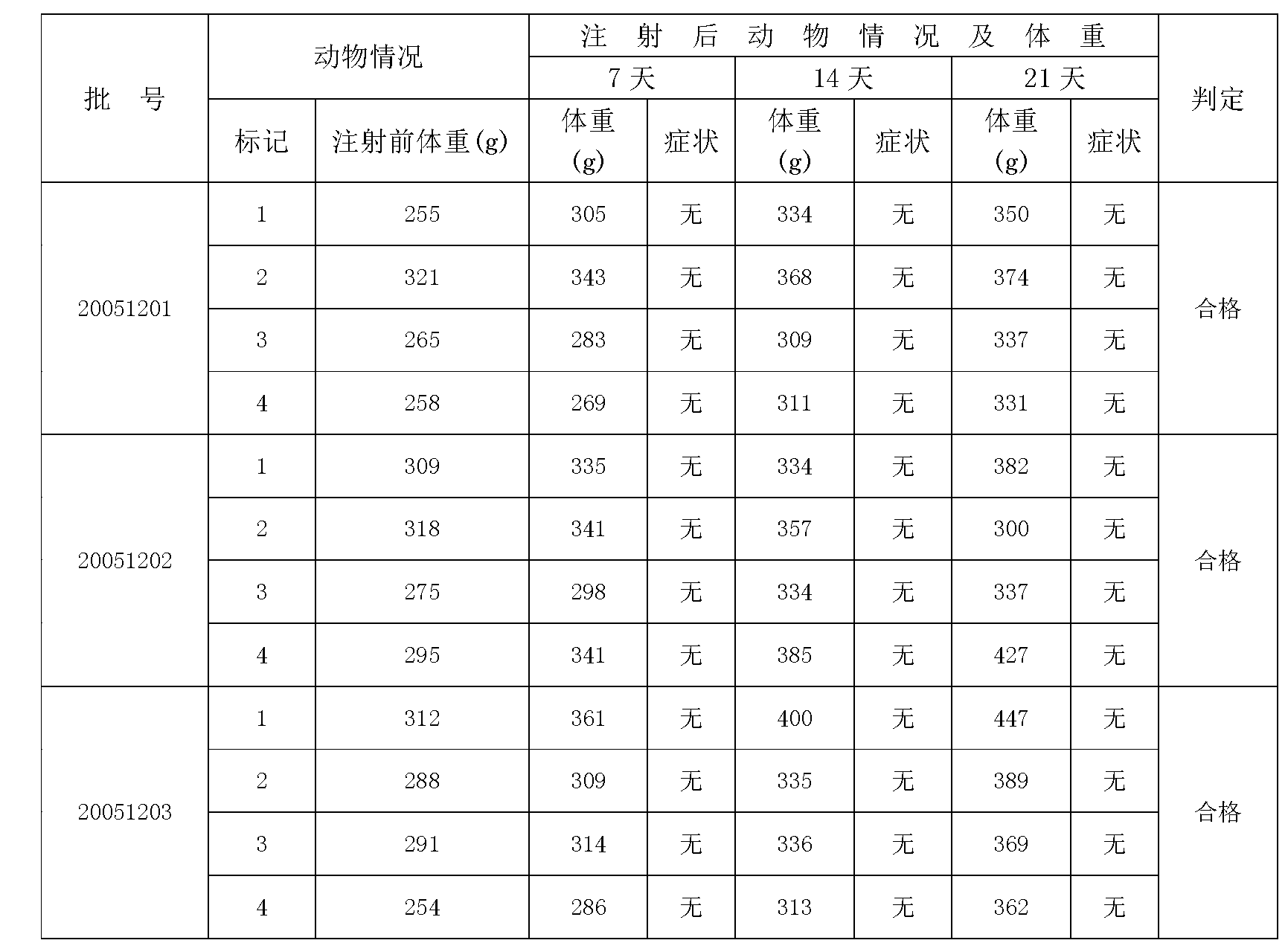
[0030] 1. Strain preparation: Inoculate Salmonella typhi (50098) in glucose broth medium, culture at 35-37°C for 16-20 hours, transfer the second generation and the third generation to normal agar slant culture under the same conditions On the base, cultivate at 35-37°C for 16-20 hours, and then collect the third-generation strains in strain tanks equipped with a certain amount of improved semi-comprehensive liquid medium, and cultivate at 35-37°C for 4-8 hours.
[0031] 2. Cultivation in large tanks: press the strains in the strain tank into a fermenter equipped with a certain amount of improved semi-comprehensive liquid medium, carry out expanded culture, and culture in deep layer at 35-37°C for 6-8 hours.
[0032] 3. Sterilization: Add 1-2% (V / V) formaldehyde solution to the tank for sterilization of the culture, stir for more than half an hour and let stand overnight.
[0033] 4. Bacteria removal: use a high-sp...
Embodiment 2
[0037] The preparation method of O-SP polysaccharide of paratyphoid fever bacterium:
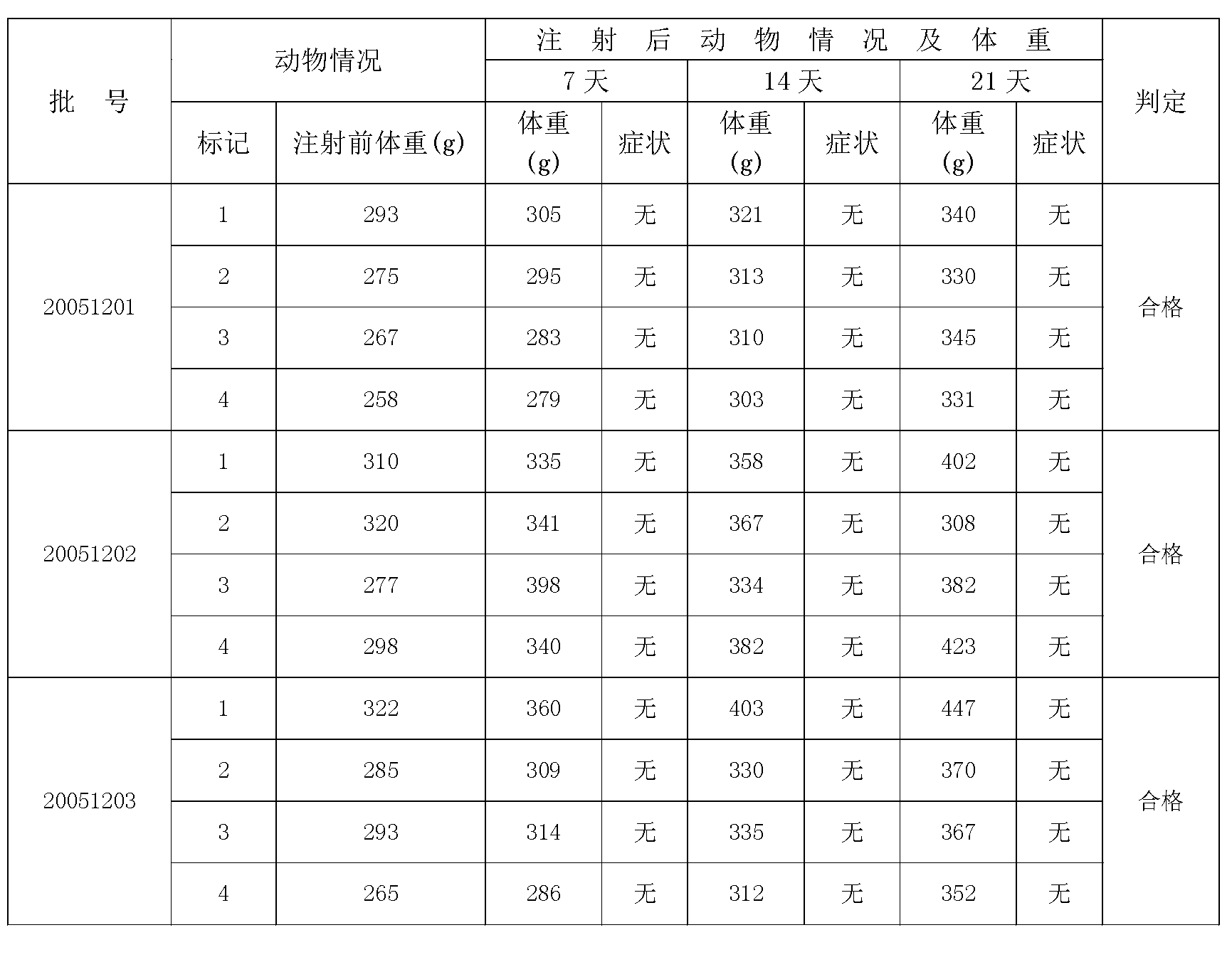
[0038] 1. Strain preparation: Inoculate Salmonella paratyphi A (50073) in glucose broth medium, culture at 35-37°C for 16-20 hours, transfer the second generation and third generation to ordinary agar under the same conditions On the slant medium, cultivate at 35-37°C for 16-20 hours, and then collect the third-generation strains in strain tanks equipped with a certain amount of improved semi-comprehensive liquid medium, and cultivate at 35-37°C for 4-8 hours.
[0039] 2. Cultivation in large tanks: press the strains in the strain tank into a fermenter equipped with a certain amount of improved semi-comprehensive liquid medium, carry out expanded culture, and culture in deep layer at 35-37°C for 6-8 hours.
[0040] 3. Sterilization: Add 1-2% (V / V) formaldehyde solution into the tank for the culture to sterilize, and stir for more than half an hour.
[0041] 4. Collect bacterial cells: colle...
Embodiment 3
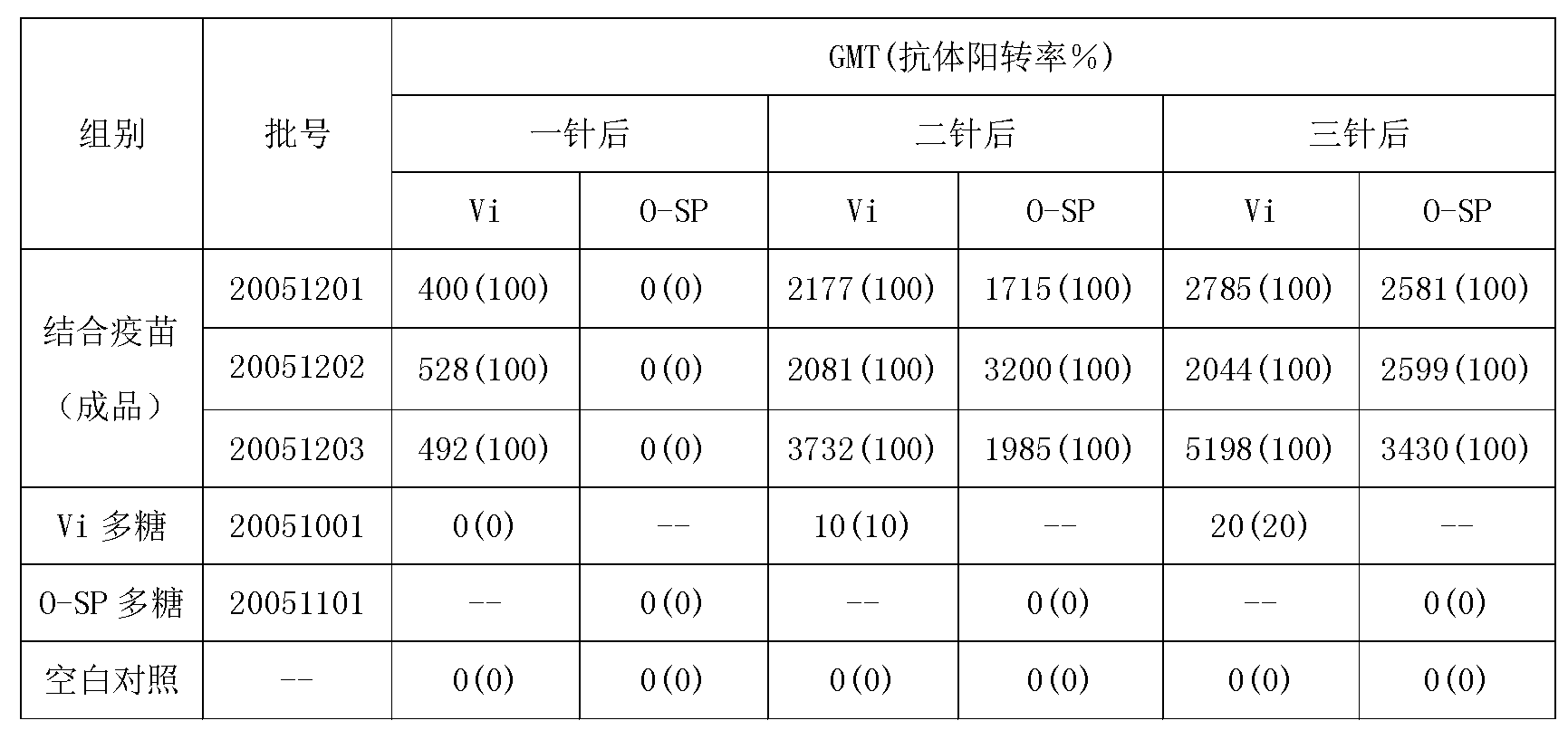
[0048] The preparation method of typhoid Vi polysaccharide-carrier protein conjugate:
[0049] Tetanus toxoid (TT) was used as the carrier protein of typhoid Vi polysaccharide.
[0050] 1. TT derivatization: Dilute TT to 10 mg / ml according to the protein amount, add 3.5 mg of 1,6-adipic dihydrazide (ADH) per mg of protein at room temperature 20-26°C, mix well, adjust the pH value to 5.75, add Carbodiimide (EDAC) makes the ratio of EDAC to protein 0.1-0.8, maintains pH 5.75 and stirs for 1 hour, and uses 30k ultrafiltration membrane to ultrafilter small molecular impurities such as ADH and EDAC. The protein content and ADH residue (-AH) content were measured, and the derivation rate was calculated.
[0051] When derivatizing proteins, EDAC / TT is set between 0.1 and 0.15, which can have a more appropriate degree of derivatization.
[0052] 2. Combination of typhoid Vi polysaccharide with derivatized carrier protein: adjust the pH value of typhoid Vi polysaccharide to 5.75 with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com