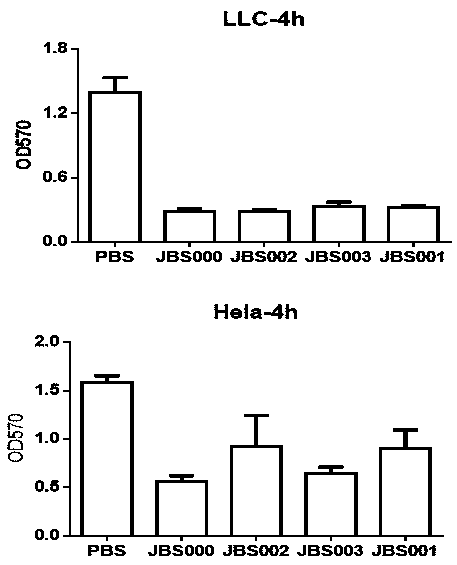
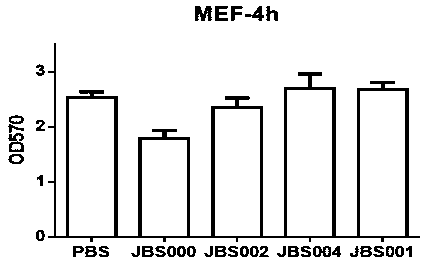
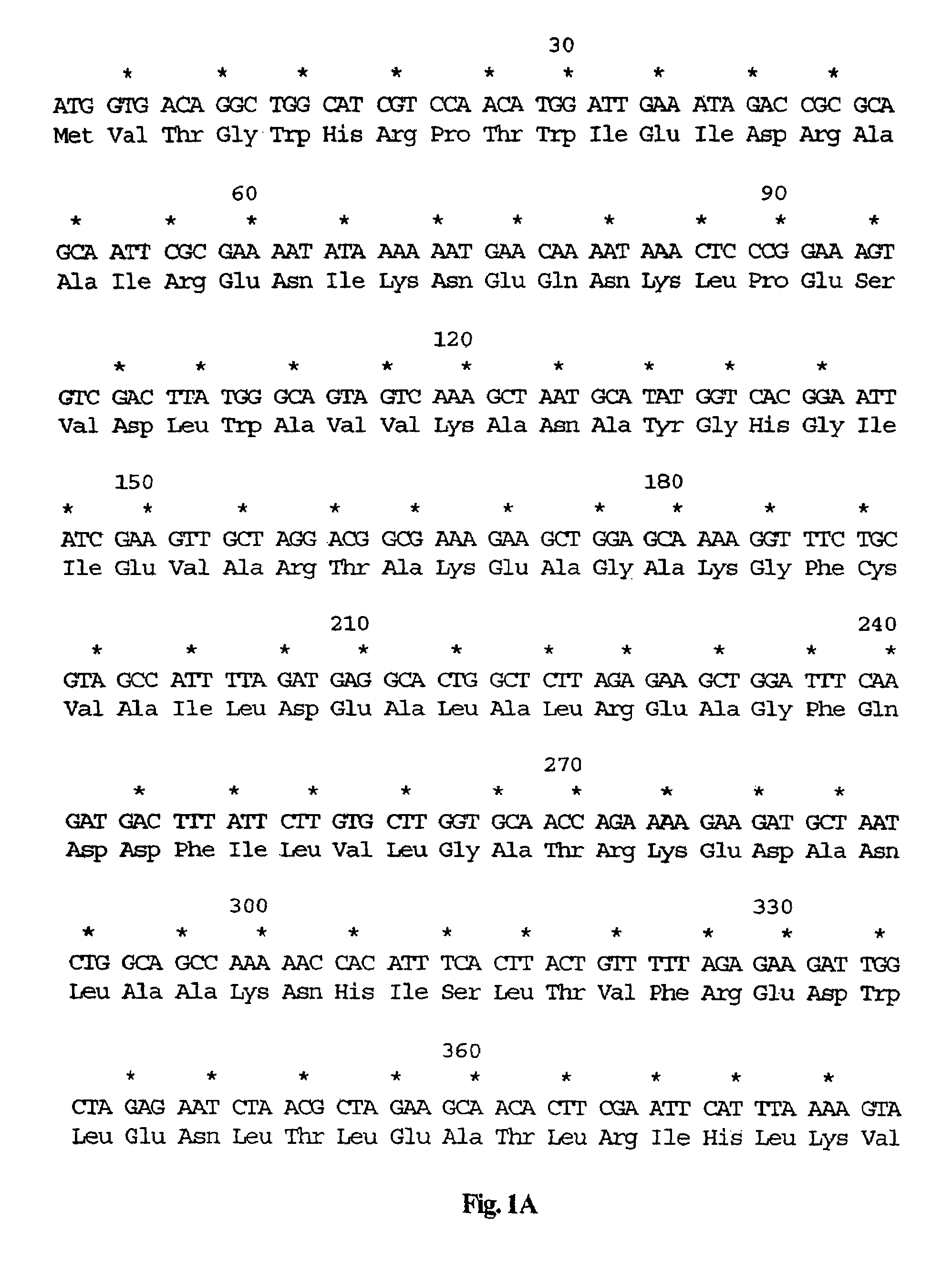Patents
Literature
424 results about "Attenuated strain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Attenuated strain NADL-2 and virulent strain NADL-8 of porcine parvovirus (PPV) were titrated in vivo and in vitro under similar conditions to provide a better understanding of some of the factors involved in virulence of PPV in causing maternal reproductive failure of swine.
Immunogenic compositions comprising DAL/DAT double mutant, auxotrophic attenuated strains of listeria and their mehods of use
InactiveUS20050048081A1Bacterial antigen ingredientsViral antigen ingredientsMicrobiologyGenus Listeria
The invention includes auxotrophic attenuated mutants of Listeria and methods of their use as vaccines.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Composition and method for the treatment of carcinoma
InactiveUS20070134273A1Good effectConvenient treatmentAntibacterial agentsBiocideMycobacterial antigenCompound (substance)
The present invention relates to compositions and methods useful for treating a carcinoma or viral infection in mammals, including humans. The methods and compositions typically comprise use of an immunogenic or immunomodulatory compound, and a gamma delta T cell activator, such that the composition is effective for treating a carcinoma or viral infection. In a preferred aspect of the invention, the methods comprise use of a gamma delta T cell activator and a Mycobacterium antigen, which for example is an attenuated strain of Mycobacterium bovis (Bacillus Calmette-Guerin (BCG)).
Owner:ROMAGNE FRANCOIS +1
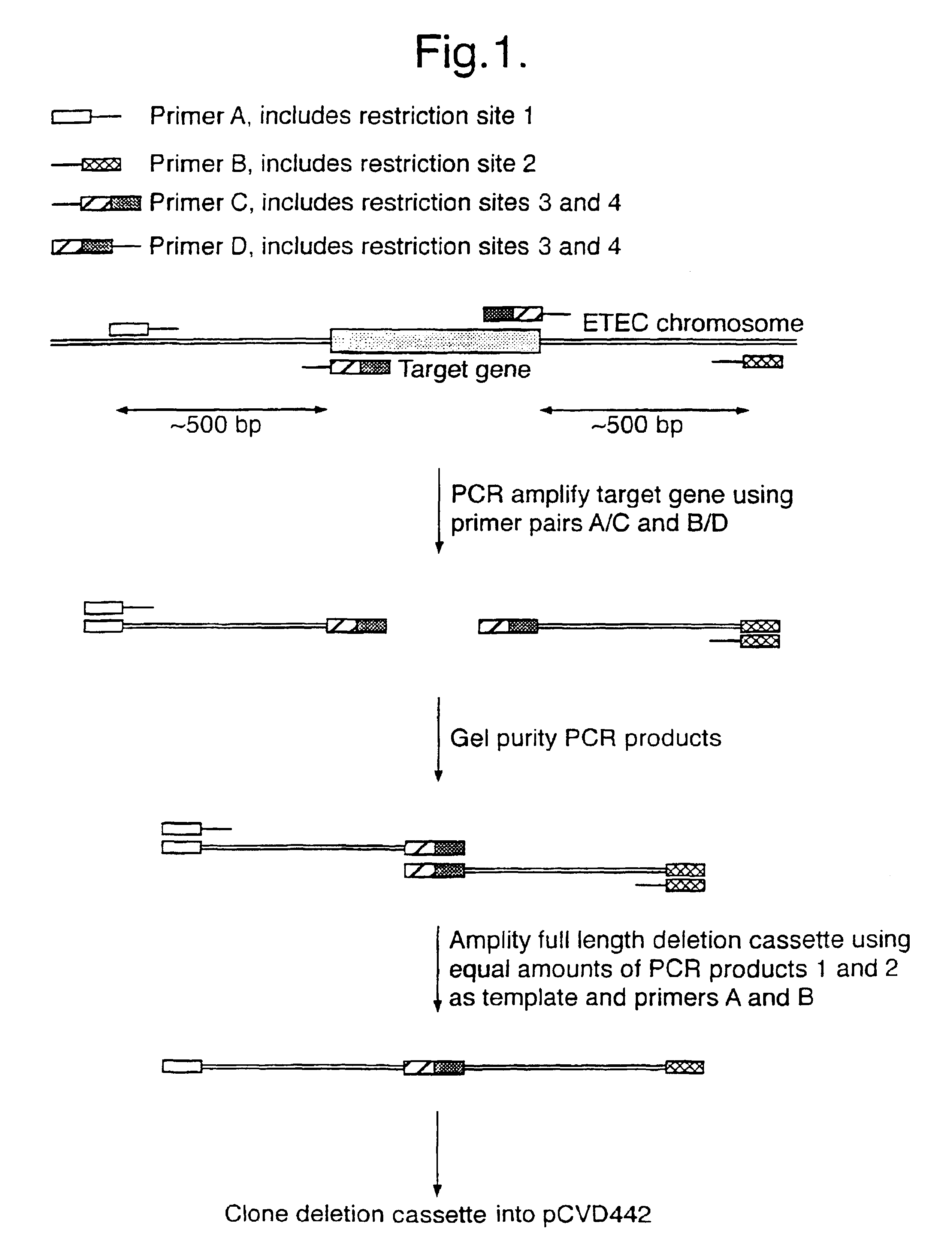
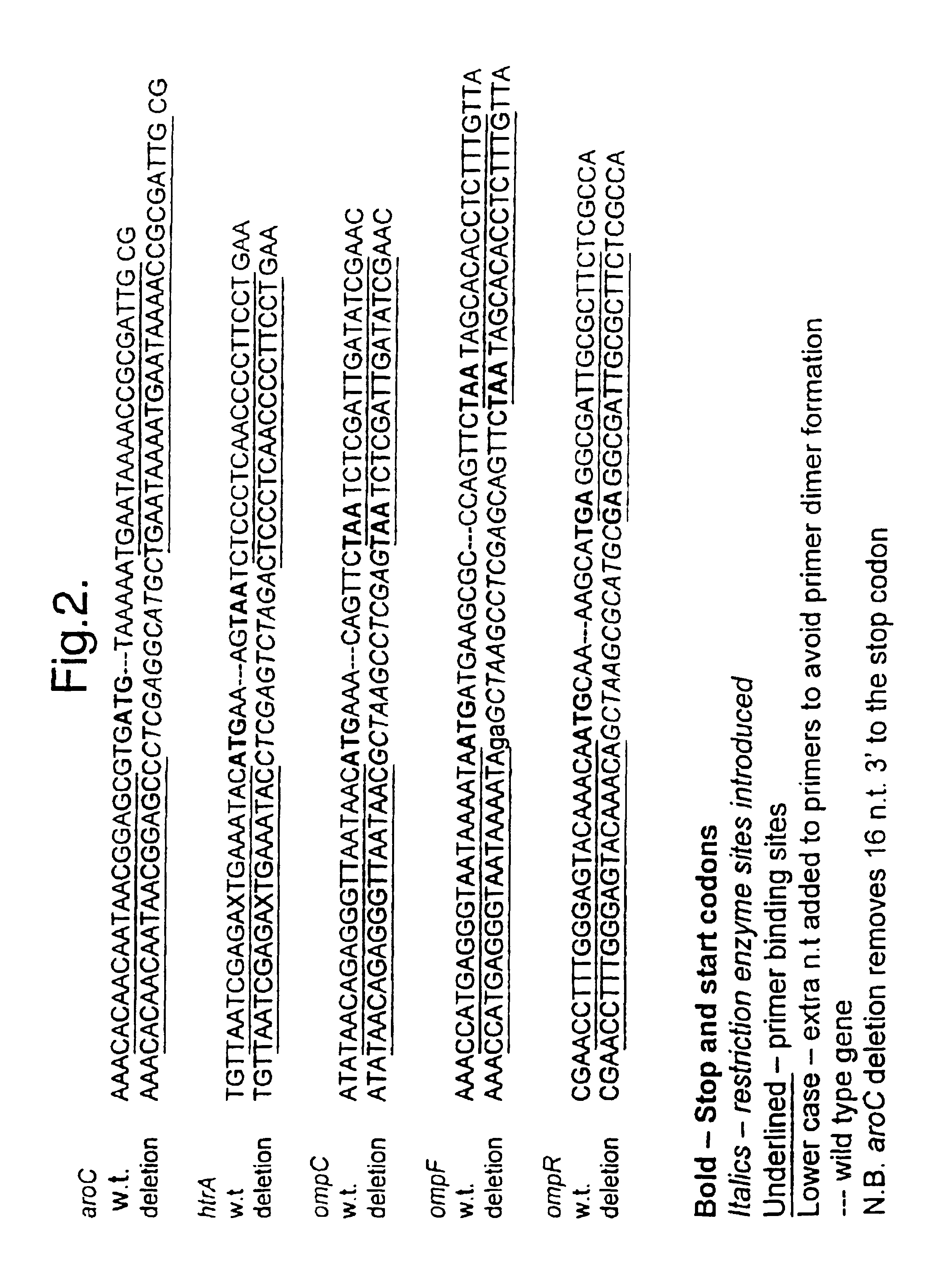
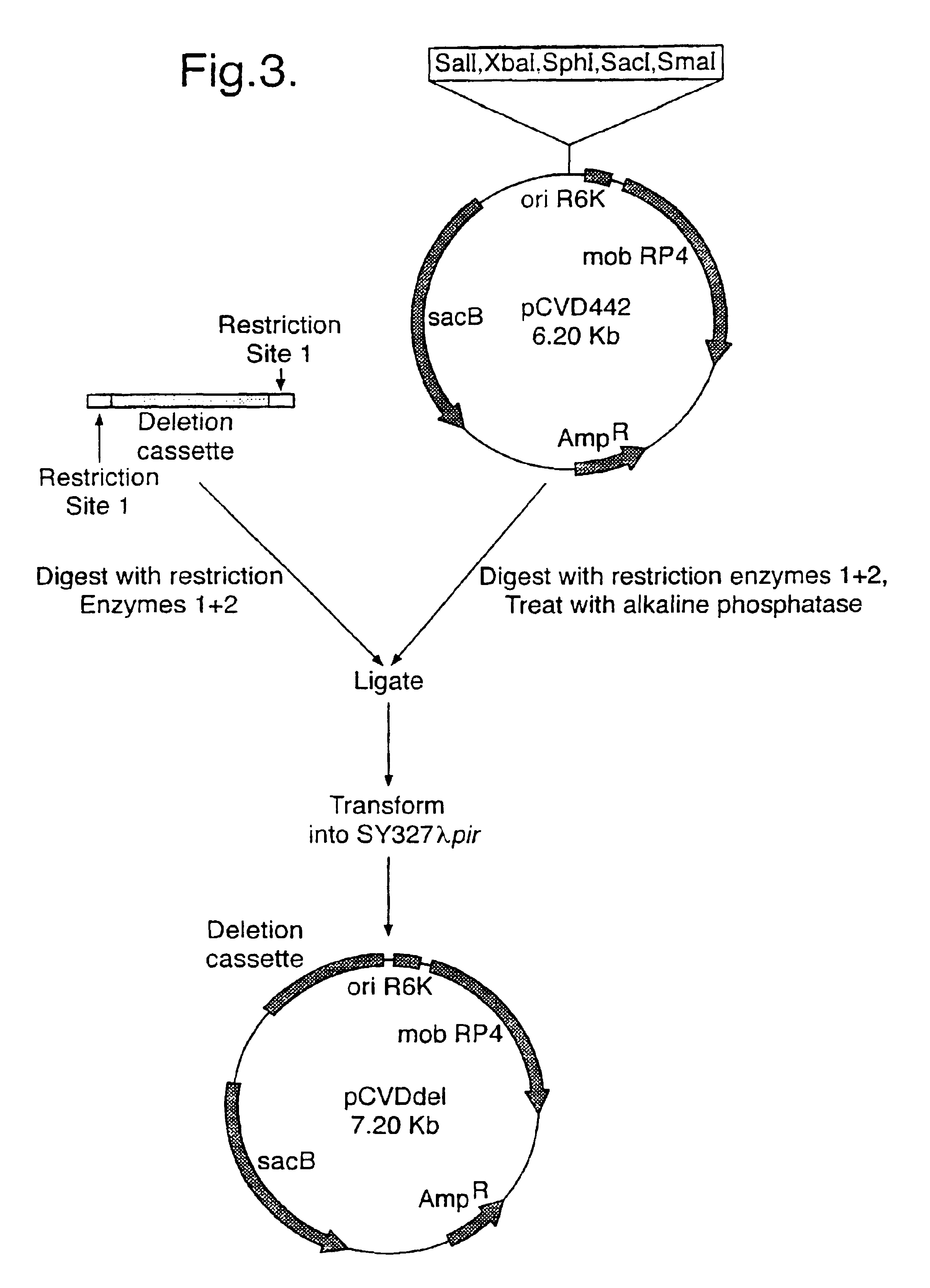
Bacteria attenuated by a non-reverting mutation in each of the aroC, ompF and ompC genes, useful as vaccines
InactiveUS6902906B1Low immunogenicitySpeed up the descentAntibacterial agentsBiocideBacteroidesEscherichia coli
The invention provides a bacterium attenuated by a non-reverting mutation in each of the aroC gene, the ompF gene and the ompC gene. The bacterium is useful as a vaccine. The bacterium may, for example, be an attenuated strain of E. coli useful in vaccination against diarrhoea.
Owner:ACAMBIS RES LTD
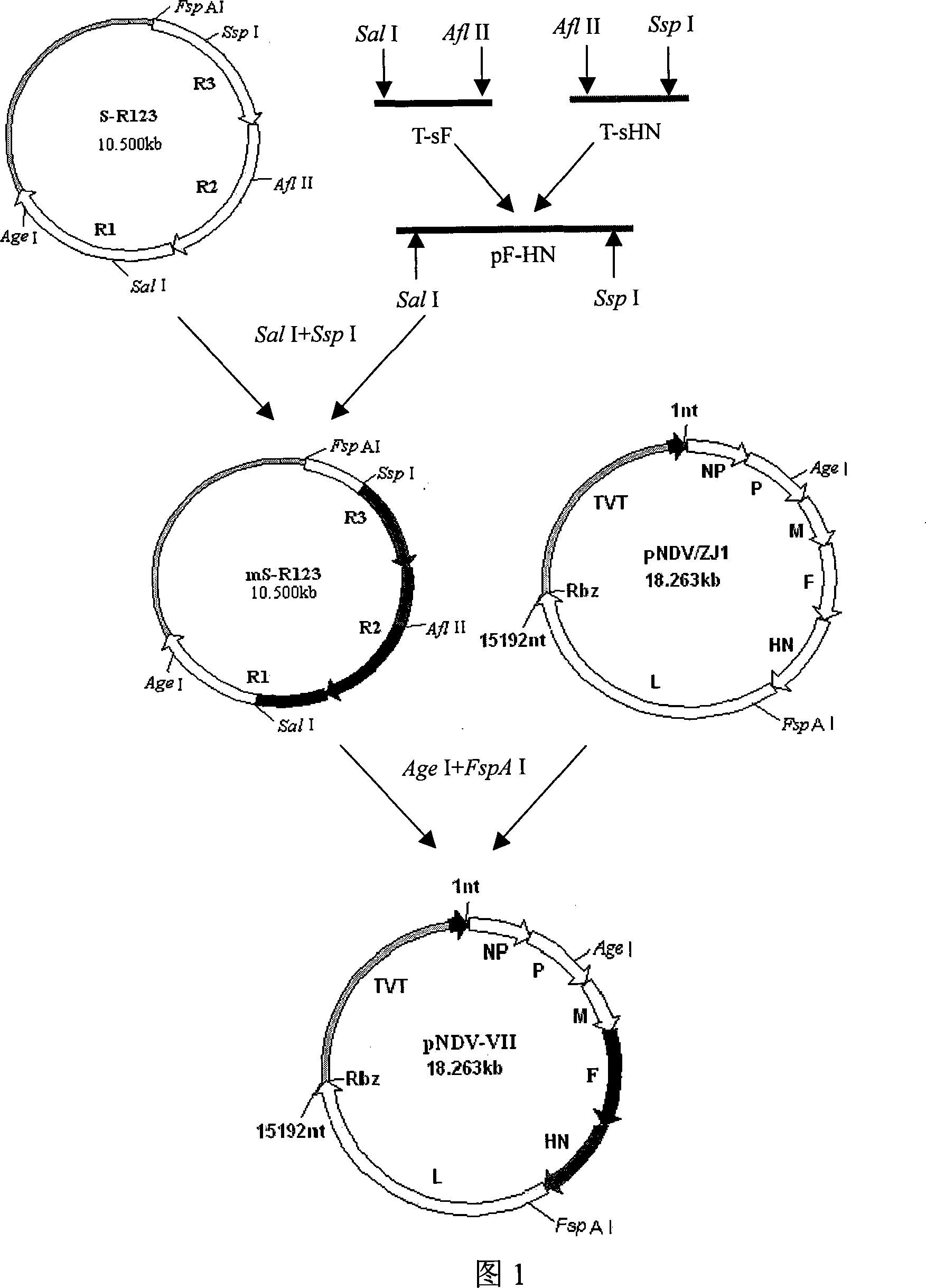
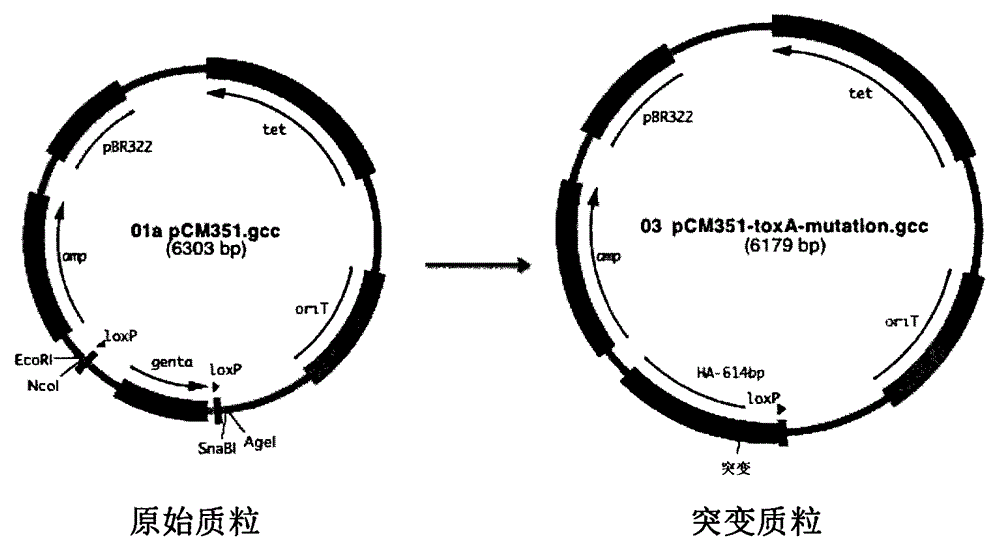
Gene ó¸ type new castle disease virus weakening strain A-NDV-ó¸ and construction method thereof
ActiveCN101182494AHigh reproductive titerSuitable for mass productionInactivation/attenuationNewcastle disease virus NDVGene type
A VII gene type of an attenuated strain of Newcastle disease virus A-NDV-VII and a construction method are disclosed. The invention relates to the application of reverse genetics technique. The invention uses the constructed reverse genetics platform of ZJ1 strain of Newcastle disease virus of goose origin. The invention replaces two envelope glycoprotein gene fragments F and HN of an isolated strain JS-5-05-Go of Newcastle disease virus with high reproductive performance with the corresponding fragments of the ZJ1 strain of Newcastle disease virus of goose origin, so that the recombinant virus NDV-VII is obtained. The VII gene type of Newcastle disease virus A-NDV-VII which is highly attenuated is rescued after the attenuated mutation of the F gene of the recombinant virus. And the virus has a higher reproduction titer on chicken embryo. The invention is suitable for a mass production of vaccine, which can be used for the manufacture of vaccine.
Owner:YANGZHOU UNIV
Chicken infectivity bronchitis virus attenuated vaccine strain and application thereof
ActiveCN101514334AImprove securityNo side effectsInactivation/attenuationMicroorganism based processesInfectious bronchitisMicroorganism
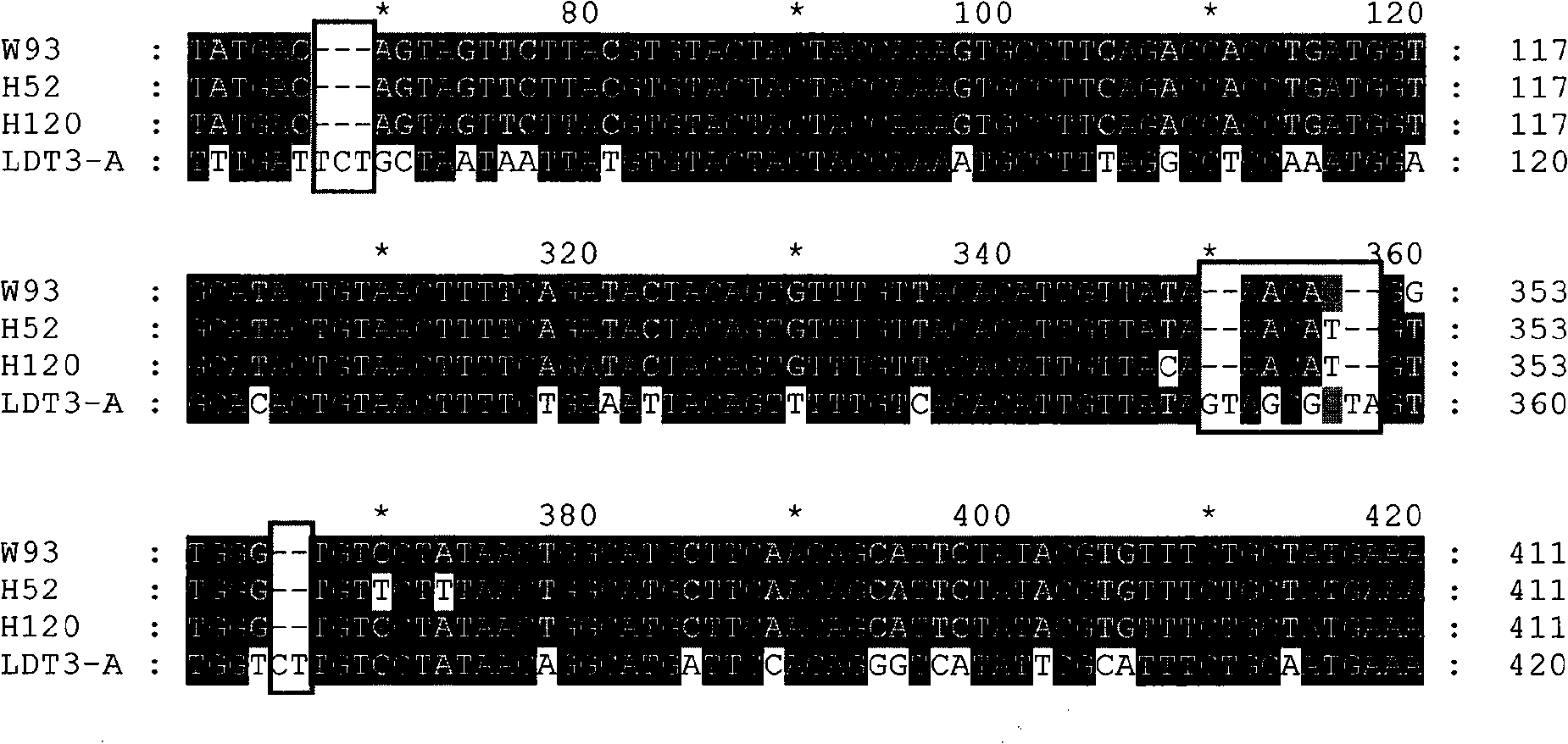
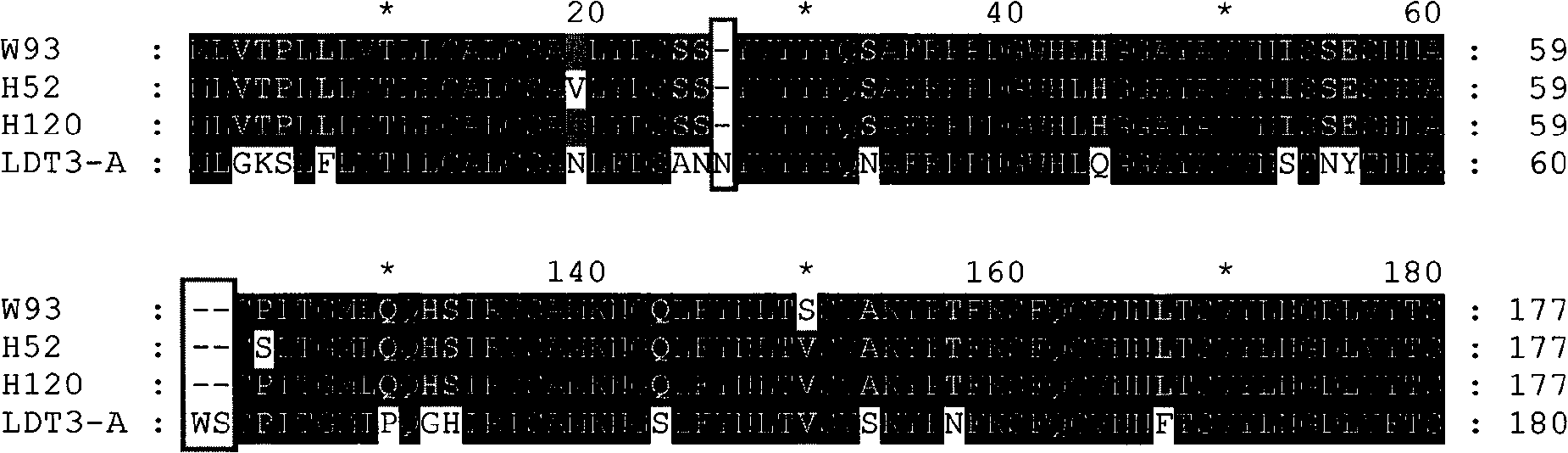
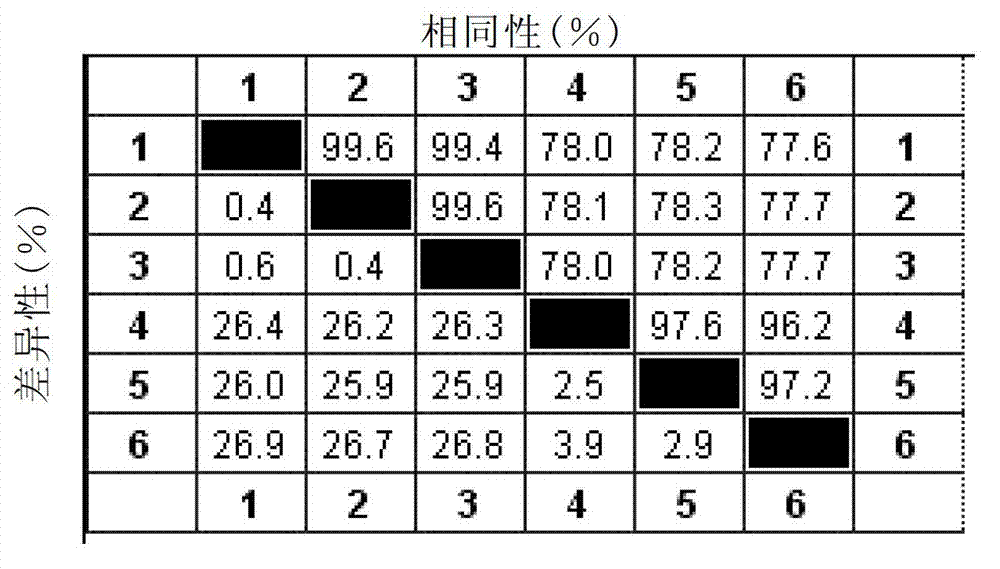
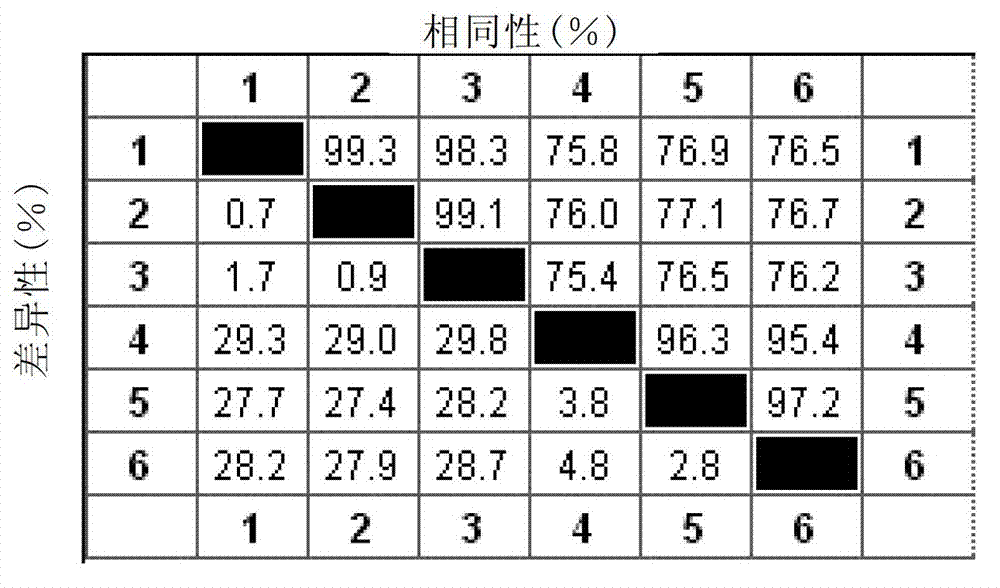
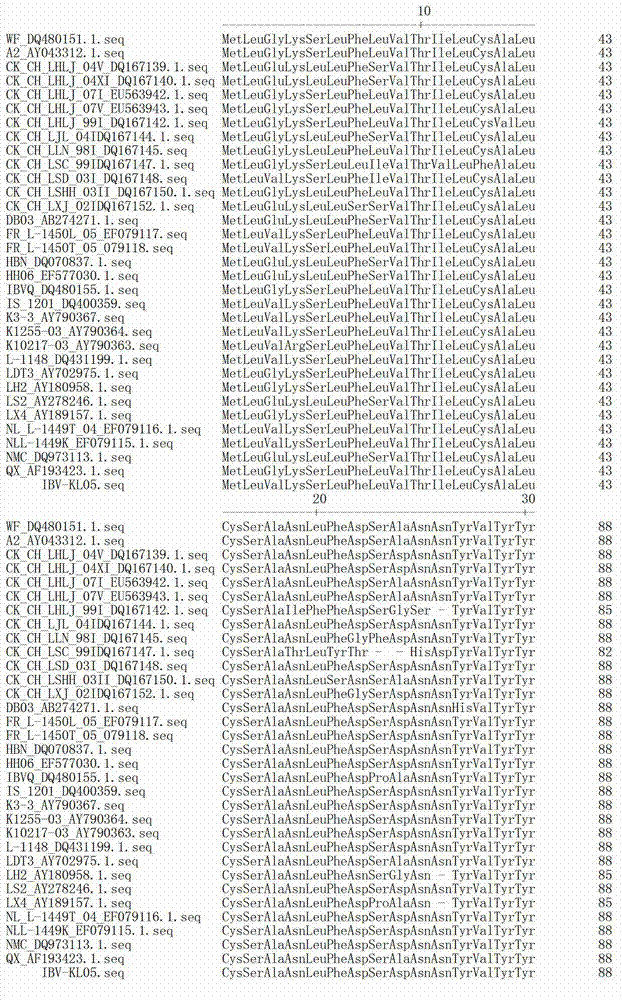
The invention discloses a infectivity bronchitis attenuated vaccine strain LDT3-A strain, and discloses application and application effect thereof in preventing and curing chicken infectivity bronchitis. The microorganism accession number of the attenuated vaccine strain is CGMCC-2902. The attenuated vaccine strain of the present invention has good safety and good immunization protection effect to the chicken infectivity bronchitis. The attenuated strain can be prepared into single vaccine or combined vaccine for preventing or curing infectivity bronchitis virus.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
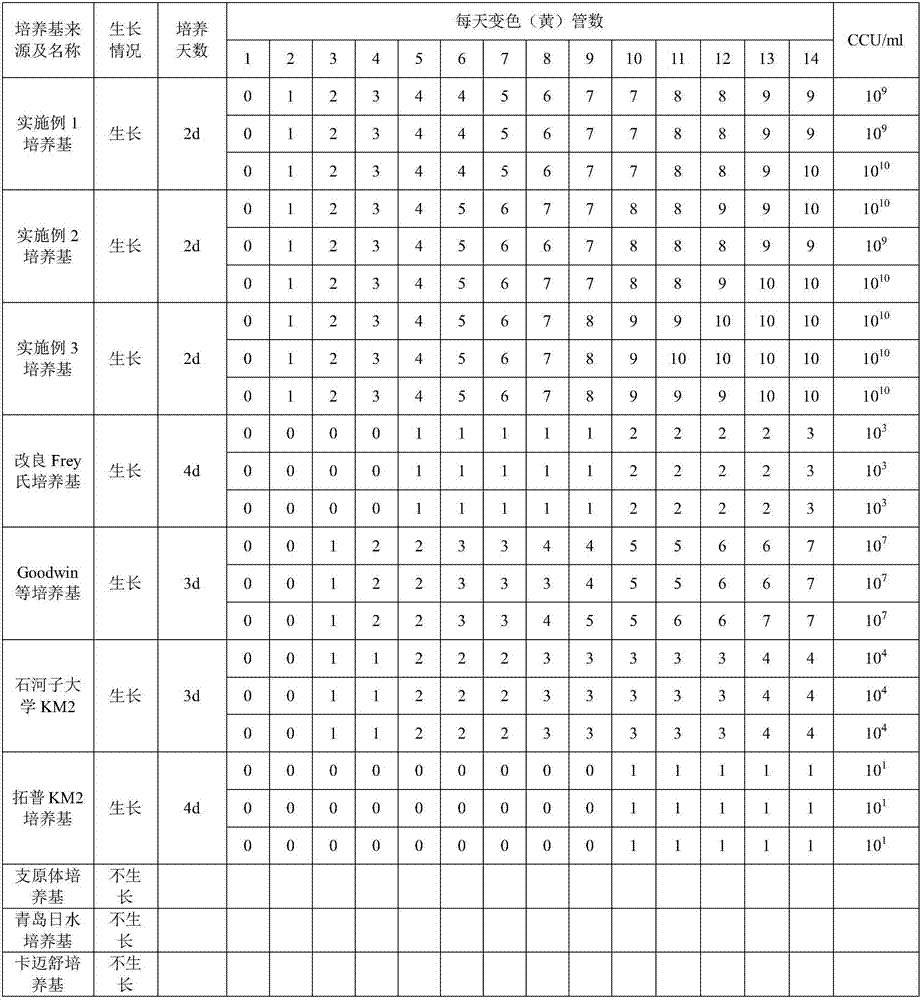
Low serum efficiency mycoplasma gallisepticam attenuated strain culture medium and preparation method thereof
ActiveCN103074246AIncrease the titer of live bacteriaReduce allergic reactionsBacteriaMicroorganism based processesAntigenCulture mediums
The present invention relates to a low serum efficiency mycoplasma gallisepticam attenuated strain culture medium and a preparation method thereof, and belongs to the technical field of veterinary biology. The culture medium comprises: (1) a base culture medium; and (2) an auxiliary culture medium, wherein the auxiliary culture medium mainly comprises MEM, yeast extract powder, tryptone, glucose, an inorganic salt, and the like, and growth, high titer and stability of the semi-finished product can be ensured with the auxiliary culture medium. According to the present invention, a titer of the semi-finished product bacterial liquid prepared by using the preparation method is up to 10<11> CCU / ml; and the culture medium adopts reduced pig serum to culture mycoplasma gallisepticam so as to reduce allergic stress reactions on chicken by heterologous pig serum, consider animal biosafety, improve antigen titer, and reduce production cost.
Owner:兆丰华生物科技(南京)有限公司
F genotype mumps virus attenuated strain as well as construction method and application thereof
The invention provides an F genotype mumps virus attenuated strain as well as a construction method and application thereof. Specifically, the invention provides an F genotype mumps virus attenuated strain and the attenuated strain is a mumps virus QS-F-SH2 with an accession number of CCTCC NO: V201950. The invention also provides a vaccine composition containing the F genotype mumps virus attenuated strain as an active ingredient and a preparation method thereof. The mumps virus attenuated vaccine strain disclosed by the invention can match the F type mumps virus predominantly popular in China, and the level of the mumps virus attenuated vaccine strain is equivalent to that of the current vaccine strain in the aspects of growth characteristics, immunogenicity, neurotoxicity and the like.In addition, the mumps virus genetic engineering attenuated strain screened by the invention can be stably produced in chick embryo cells, and the safety is high.
Owner:SHANGHAI KING CELL BIOTECHNOLOGY CO LTD +1
Attenuated vaccine strain for avian infectious bronchitis virus and application thereof
InactiveCN102851257AImprove securityInactivation/attenuationMicroorganism based processesInfectious bronchitisFowl
The method relates to an attenuated vaccine strain for avian infectious bronchitis virus and application thereof. According to the invention, avian infectious bronchitis virus (IBV) is attenuated, so as to successfully obtain an IBV attenuated strain CCTCC.V201232 and a derivative virus strain thereof. The attenuated strain and derivative virus strain provided by the invention can be used in preparation of a vaccine composition for prevention of infectious bronchitis. Experiments show that the attenuated strain and vaccine composition provided by the invention can be inoculated to immature birds, so as to effectively activate immune system in the birds and well prevent avian infectious bronchitis.
Owner:SHANGHAI QISHENG BIOTECH CO LTD
Mycoplasma bovis attenuated strain and application thereof
ActiveCN102220263AGreat clinical valueEasy to makeAntibacterial agentsBacterial antigen ingredientsSocial benefitsUltrasound attenuation
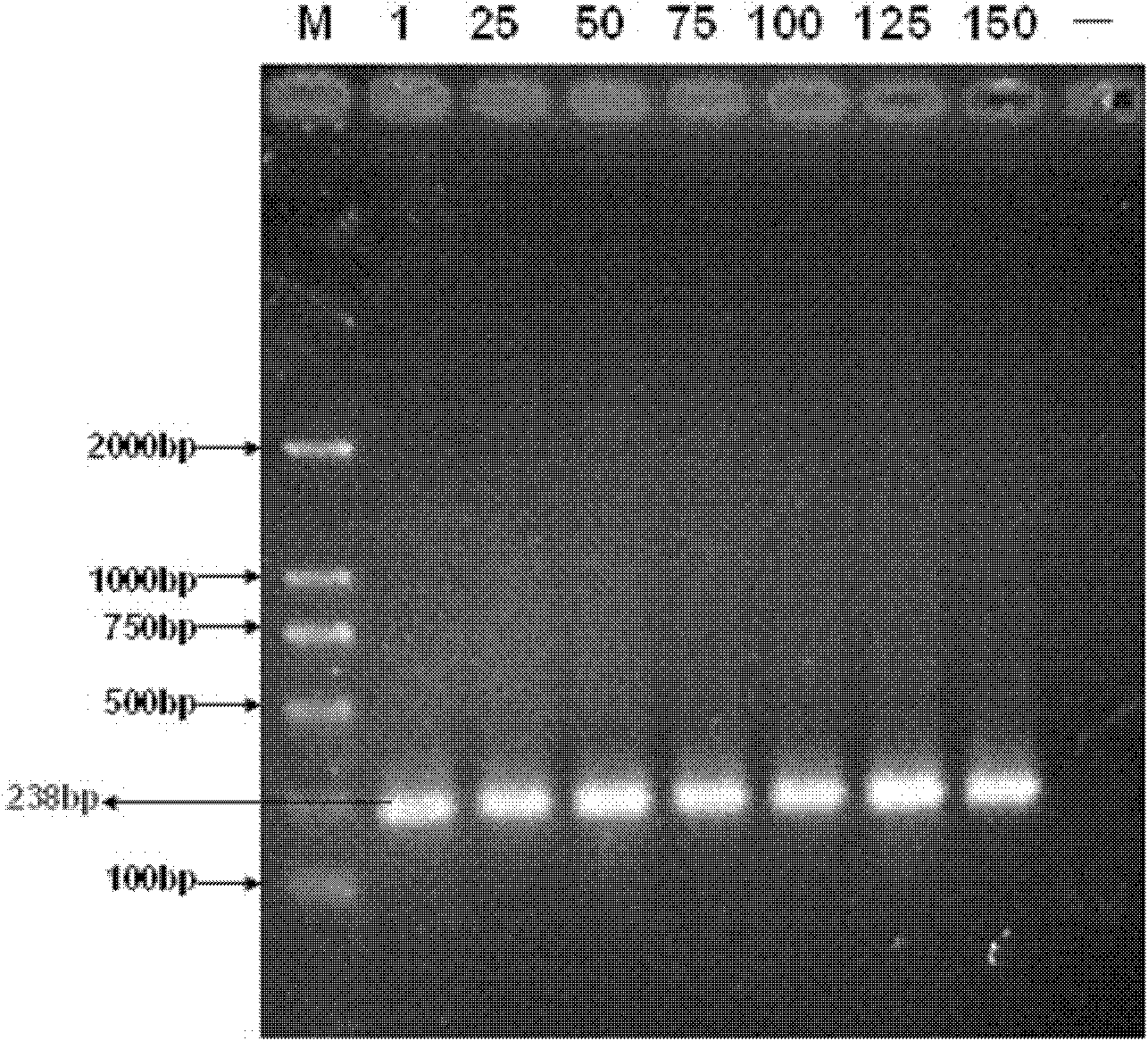
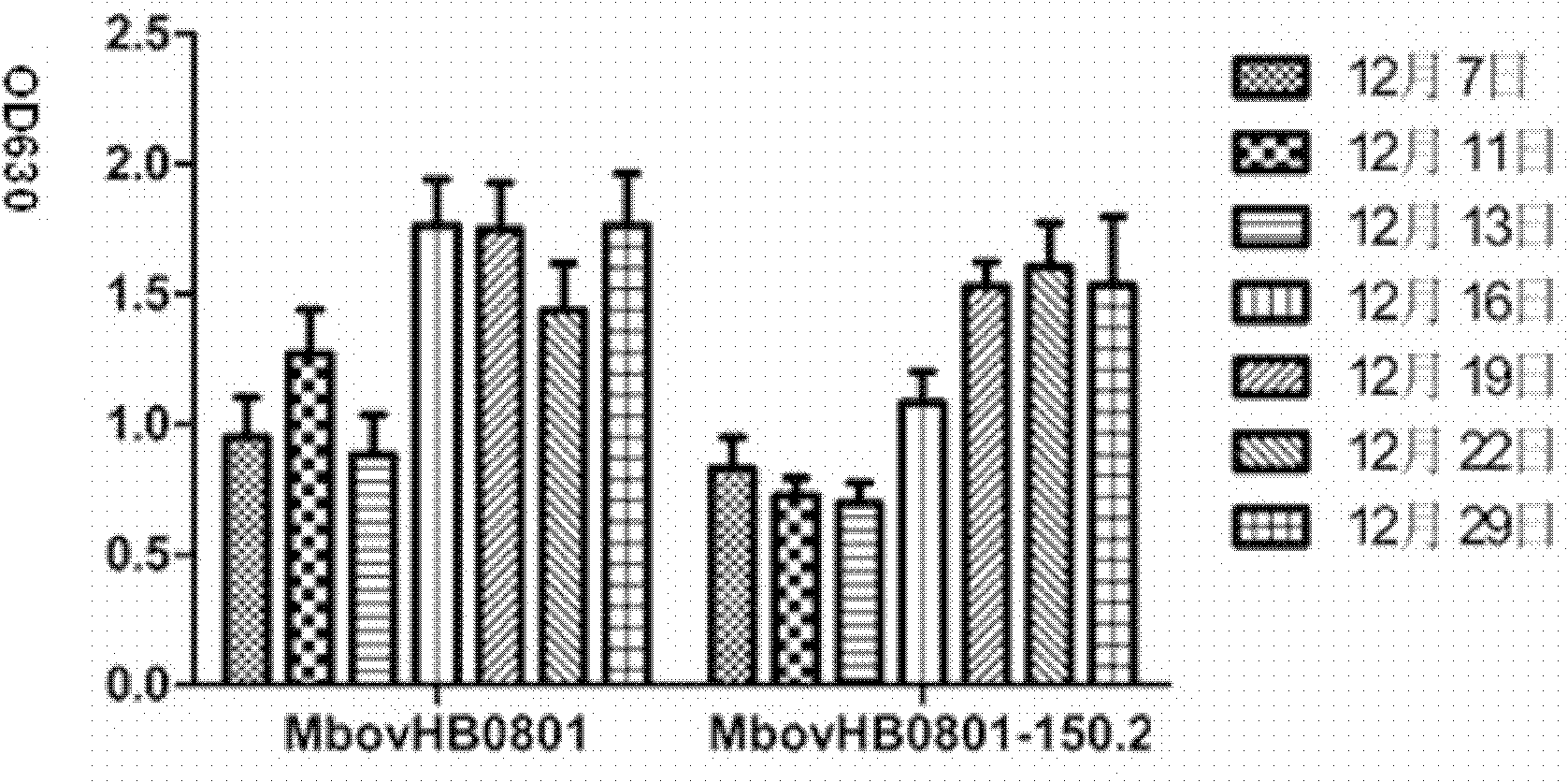
The invention discloses a mycoplasma bovis attenuated strain and application thereof. The mycoplasma bovis attenuated strain is prepared by the following steps: A. a mycoplasma bovis virulent strain is separated and determined: by pathogeny separation culture and PCR (Polymerase Chain Reaction) detection, the pathogeny is determined to be mycoplasma bovis; B. the mycoplasma bovis virulent strain is cultured: the mycoplasma bovis which is obtained by separation is inoculated with a liquid drug culture medium, and is then cultured in an incubator, wherein the culture medium becomes bright yellow from red, and the previous generation of bacterium liquid of 1mul is taken for inoculating PPLO (pleuropneumonia-like organism) culture medium and culturing; C. the full attenuation of mycoplasma bovis can be shown by the experiments, and by the detection of morphology, and the generation 150 of strain Mbov HB0801-150.2 cultured by mycoplasma bovis Mbov HB0801 can be verified by the experiment result; and D. the preservation number of the mycoplasma bovis Mbov HB0801-150.2 is CCTCC M20111102. The invention has good vaccine development prospective, wide clinic application value, low cost and small irritant to animals, and can produce huge economic benefits and social benefits.
Owner:HUAZHONG AGRI UNIV
Canine distemper live vaccine and preparation method thereof
ActiveCN101612396AReduced risk of introducing other live virusesAntiviralsAntibody medical ingredientsDiseaseCanine distemper virus CDV
The invention relates to canine distemper live vaccine and a preparation method thereof. The preparation method takes a canine distemper virus natural attenuated strain CGMCC No.3201 with excellent immunogenicity and obtained by the inventor through field separation as a production strain to prepare the safe, effective and single-component canine distemper live vaccine. The canine distemper live vaccine effectively reduces the risk of importing other live viruses during canine distemper vaccine immunization and provides conditions for controlling the spread of fur-bearing animal diseases.
Owner:QILU ANIMAL HEALTH PROD
Marker-free gene deletion attenuated mutant strain of Edwardsiella tarda wild strain as well as relevant preparations and application thereof
ActiveCN101974472AGood control effectImprove immunityAntibacterial agentsBacterial antigen ingredientsChorismic acidAttenuated Live Vaccine
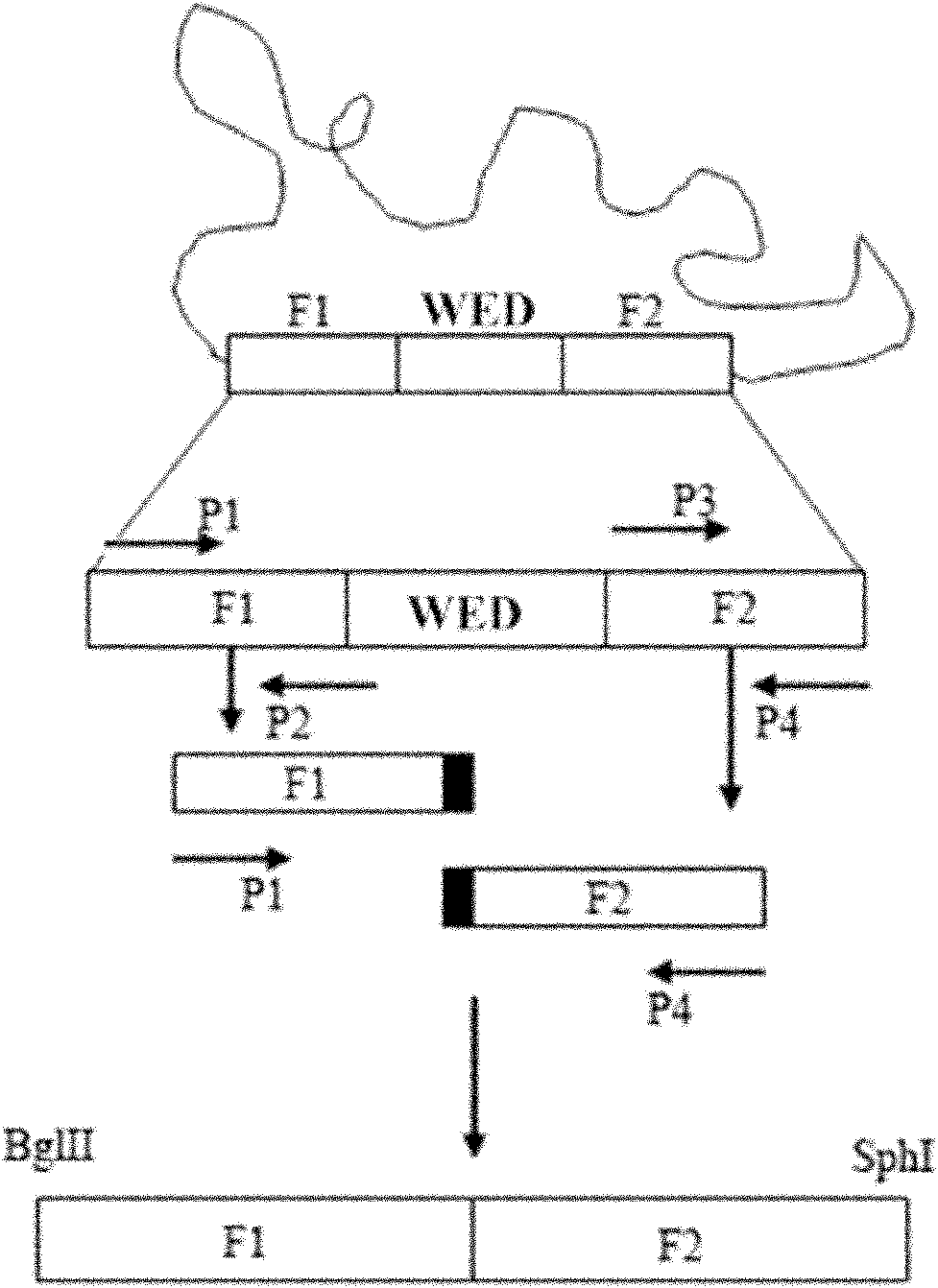
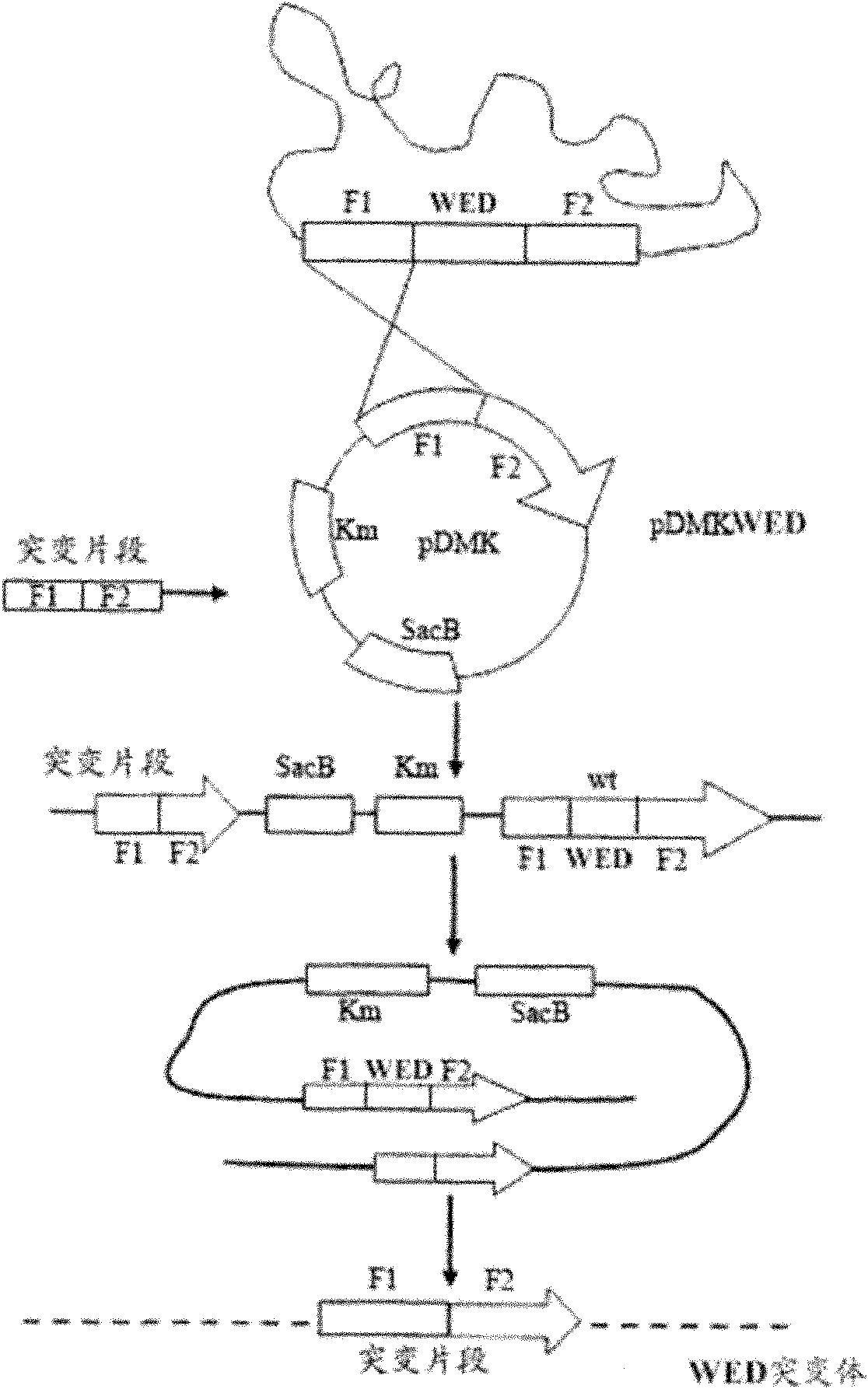
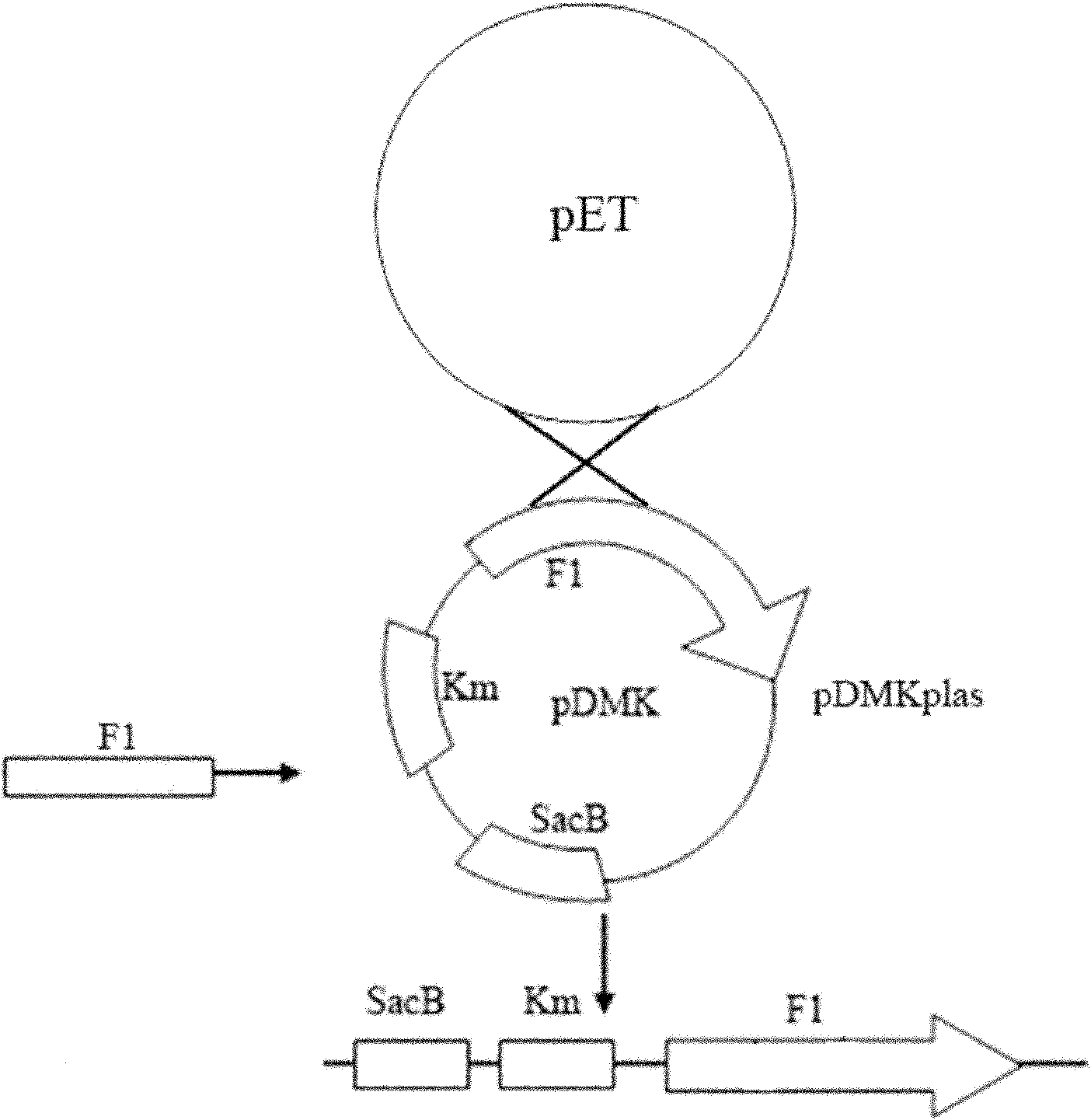
The invention relates to a marker-free gene deletion attenuated mutant strain of an Edwardsiella tarda wild strain. The marker-free gene deletion attenuated mutant strain is an attenuated live vaccine of an Edwardsiella tarda virulent strain, which deletes the chorismic acid synthase gene aroC of the Edwardsiella tarda virulent strain, three types of secretion system response element genes of eseB, escA, eseC and eseD and an endogenous plasmid, preferably, the Edwardsiella tarda virulent strain is an Edwardsiella tarda virulent strain EIB202 with the preservation number of CCTCC No:M208068; the endogenous plasmid is a plasmid of pEIB202; and the marker-free gene deletion attenuated mutant strain of the Edwardsiella tarda virulent strain is an attenuated strain WED with the preservation number of CCTCC No:M2010278. The invention also provides relevant preparations and application of the marker-free gene deletion attenuated mutant strain. The attenuated mutant strain or relevant preparations eliminate the potential environment and safety risk of products existing in the traditional attenuated live vaccines generally and is a safe, effective and economic vaccine aiming at Edwardsiella tarda diseases of cultured fishes.
Owner:EAST CHINA UNIV OF SCI & TECH
Attenuated strain of Leishmania
Differentially expressed Leishmania genes and proteins are described. One differentially expressed gene (A2) is expressed at significantly elevated levels (more than about 10 fold higher) in the amastigote stage of the life cycle when the Leishmania organism is present in macrophages than in the free promastigote stage. The A2 gene encodes a 22 kD protein (A2 protein) that is recognized by kala-azar convalescent serum and has amino acid sequence homology with an S-antigen of Plasmodium falcilparum Vietnamese isolate VI. Differentially expressed Leishmania genes and proteins have utility as vaccines, diagnostic reagents, as tools for the generation of immunological reagents and the generation of attenuated variants of Leishmania.
Owner:MCGILL UNIV
Methods of inducing immune responses through the administration of auxtrophic attenuated dal/dat double mutant Listeria strains
The present invention includes a method of eliciting a T-cell immune response to an antigen in mammal. The method of eliciting a T-cell immune response includes administering mammal an auxotrophic attenuated strain of listeria which expresses the antigen. The auxotrophic attenuated strain of listeria includes a mutation in at least one gene whose protein product is essential for growth of bacteria.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Pseudorabies virus gene-deleted attenuated strain as well as preparation method and application thereof
ActiveCN104152416AEffective controlNo clinical symptomsMicrobiological testing/measurementMicroorganism based processesNucleotide sequencingVirus
The invention discloses a pseudorabies virus gene-deleted attenuated strain. The deleted gene sequences of the attenuated strain include a nucleotide sequence for coding No.198 to No.366 amino acids of gI protein of a pseudorabies virus and a nucleotide sequence for coding No.1 to No.404 amino acids of gE protein of the pseudorabies virus. The invention further discloses a preparation method and an application of the pseudorabies virus gene-deleted attenuated strain. The pseudorabies virus gene-deleted attenuated strain has a good immune protection effect on the pseudorabies virus and can be used as a vaccine candidate strain for preventing and treating pseudorabies.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Velogenic Edwardsiella tarda vaccine strain and application thereof
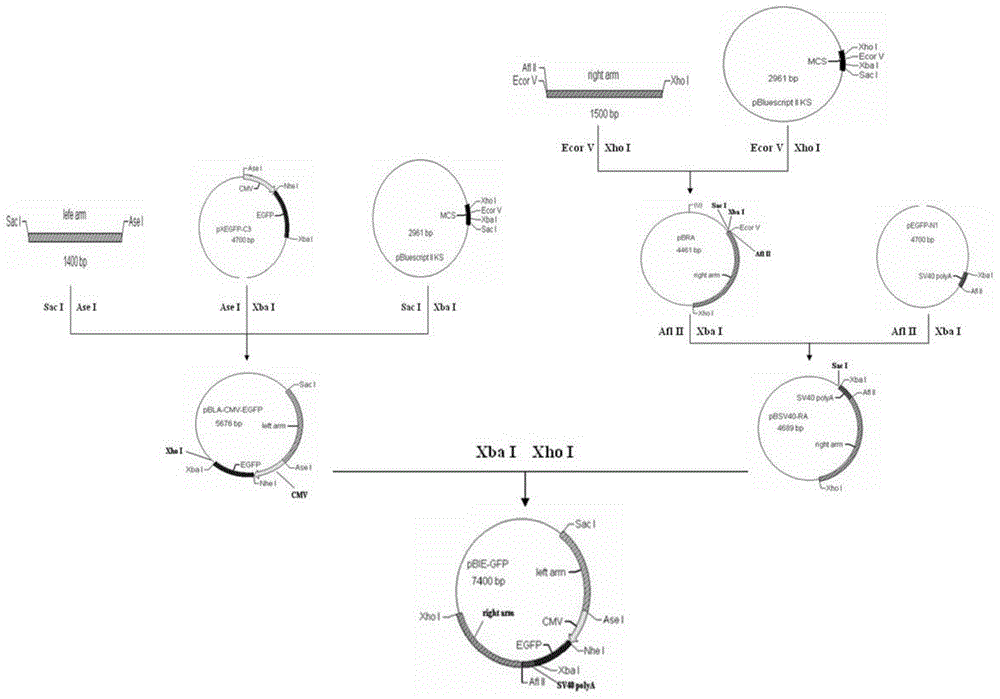
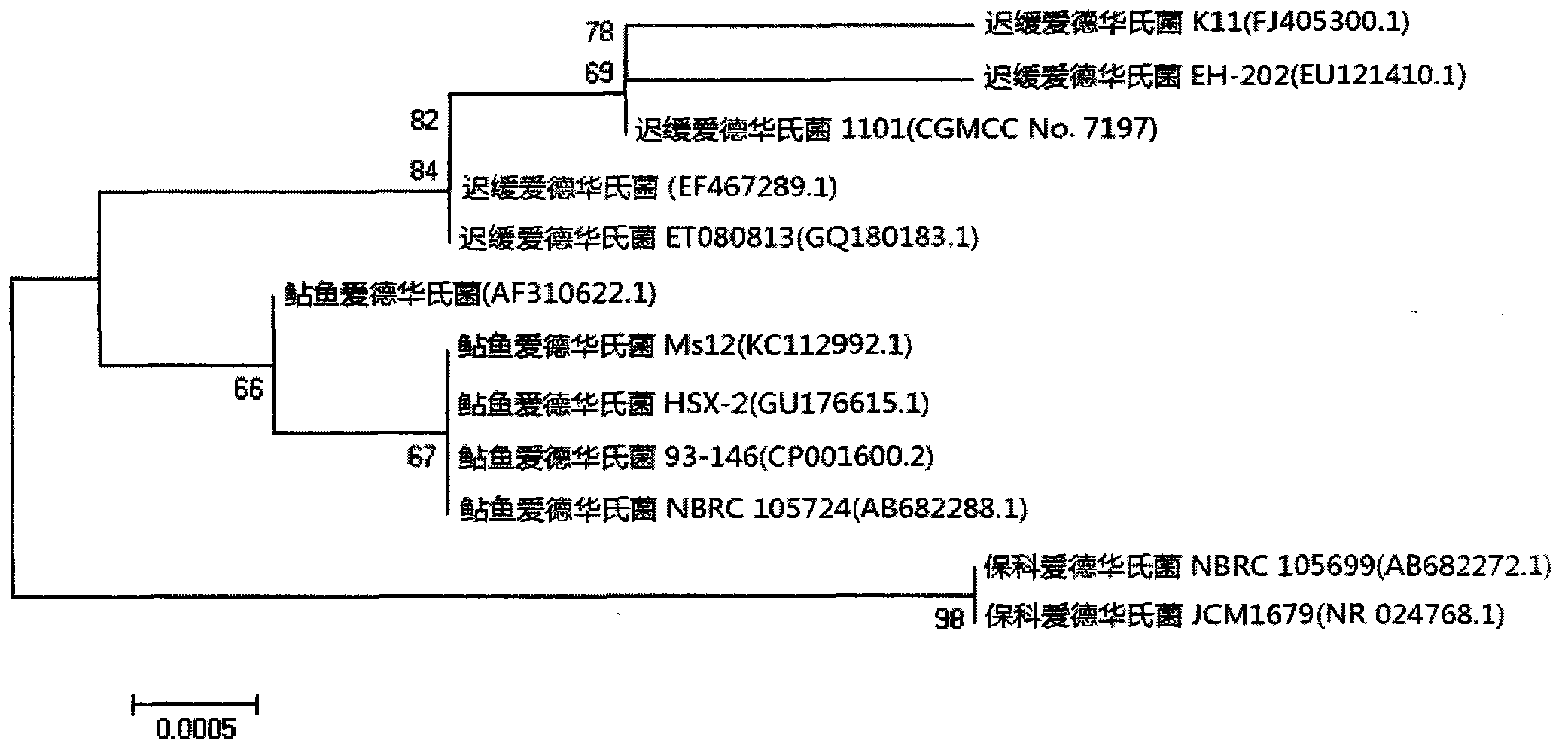
ActiveCN103255089AStrong drug resistanceReduce lossesAntibacterial agentsBacterial antigen ingredientsBacteroidesProtective antigen
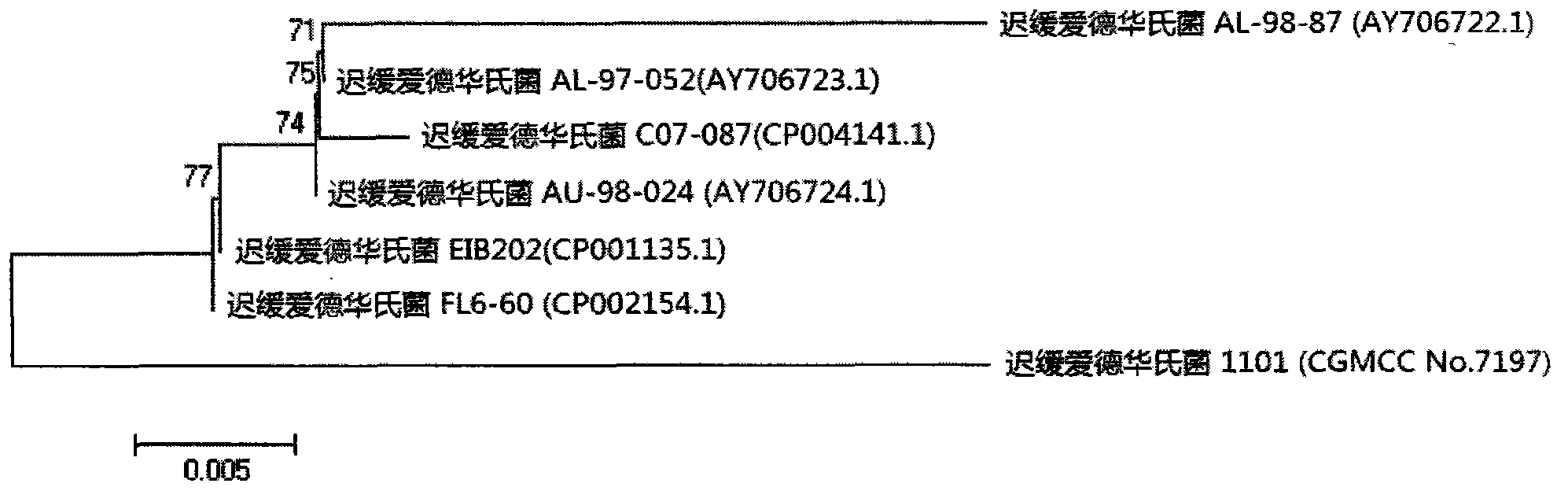
The invention relates to an Edwardsiella tarda strain and an application method thereof. The Edwardsiella tarda strain is separated from a turbot adult fish body and is a wild strain with strong virulence, and the preservation number of the Edwardsiella tarda strain is CGMCC No.7197. Preparation modes of an antigen of the Edwardsiella tarda strain comprise any one or more than one of an inactivated thallus, a bacteruak ghost ingredient, an attenuated strain, a protective antigen, an antigen subunit and an expression product of an antigen determinant or an antigen gene expression carrier; the produced vaccine can be a single ingredient of the antigen prepared by utilizing the Edwardsiella tarda strain and can also be a combined vaccine produced by mixing the antigen prepared by utilizing the Edwardsiella tarda strain with antigens of other bacteria, and the prepared single or combined vaccine antigen is added with an adjuvant to produce the vaccine; and an inoculation mode of the vaccine in immunization application can adopt injection immunization, wound immunization, immersion bath immunization or oral administration immunization.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
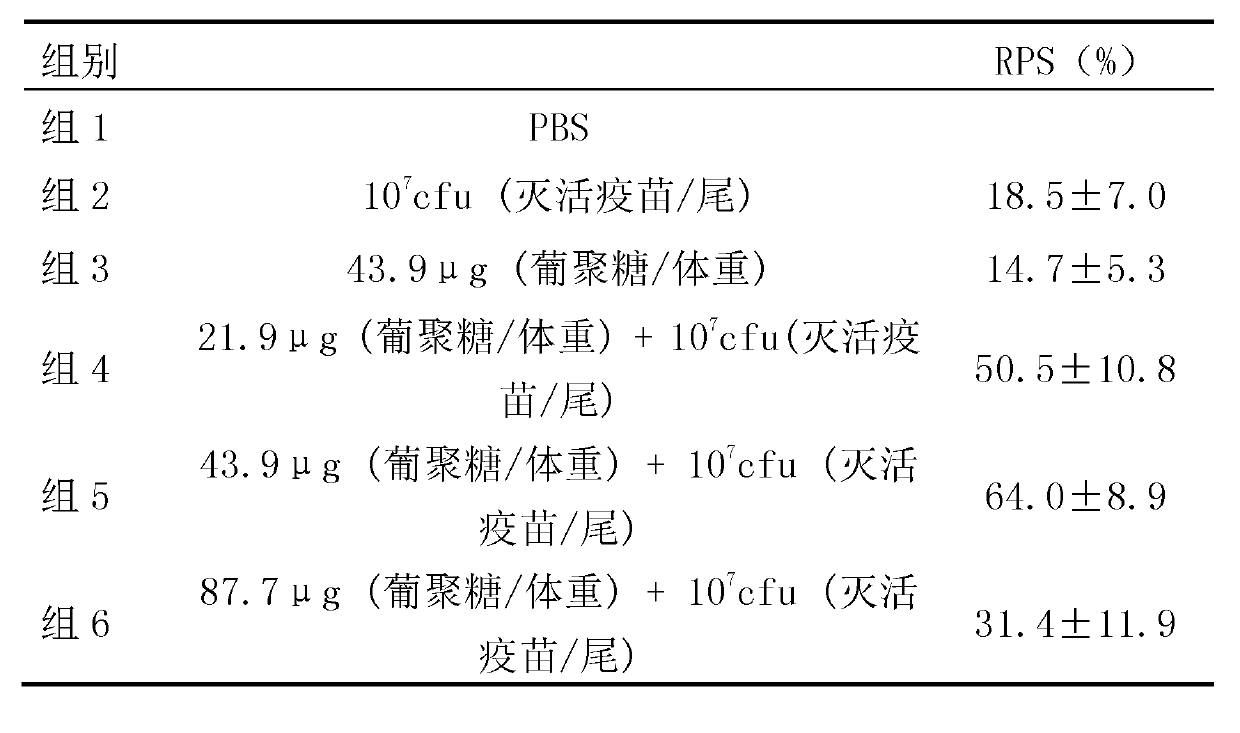
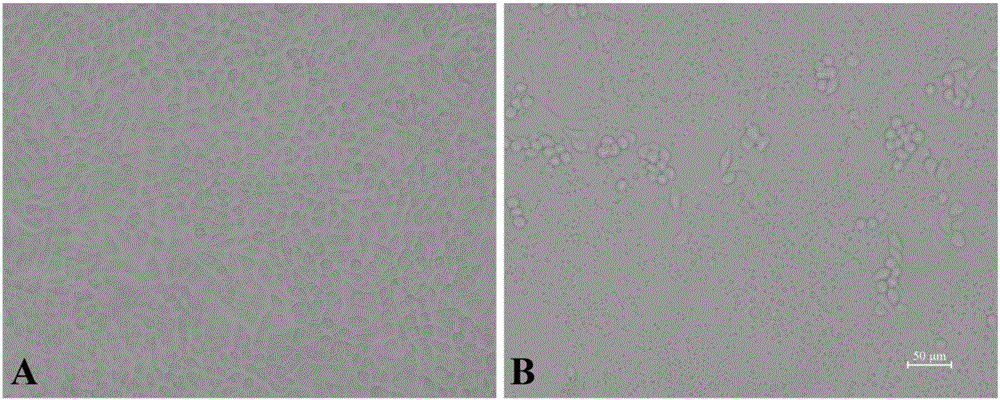
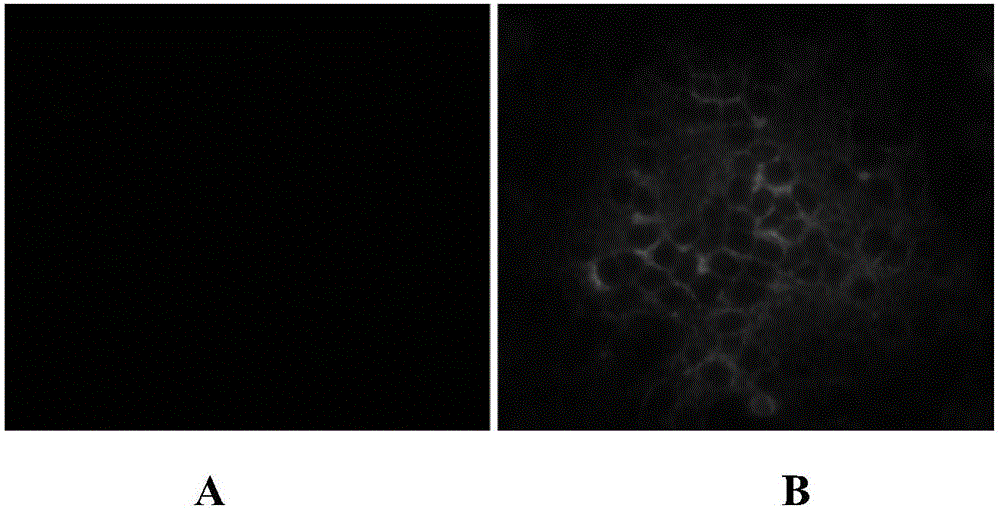
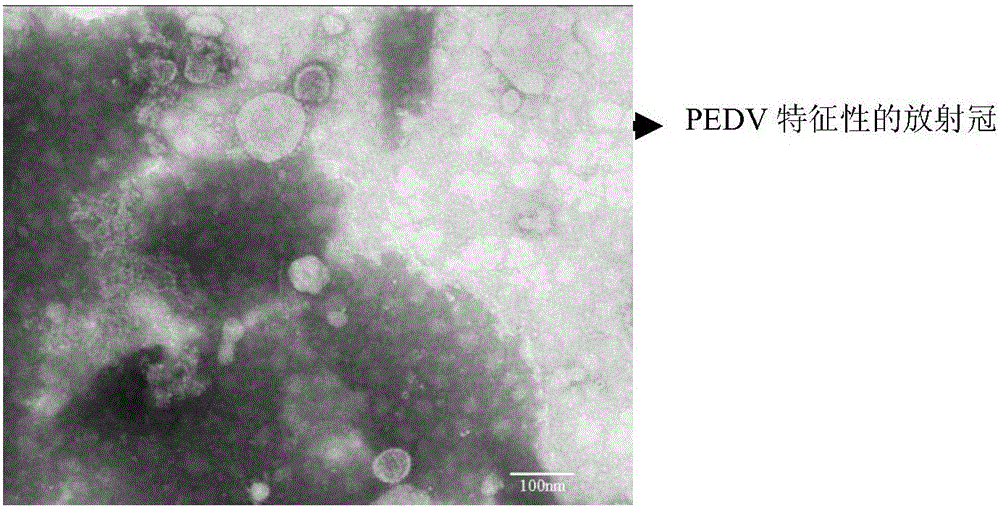
Porcine epidemic diarrhea virus attenuated strain, vaccine composition prepared therefrom, and application
The invention relates to a porcine epidemic diarrhea virus attenuated strain. The porcine epidemic diarrhea virus attenuated strain is a nucleotide fragment or termination translator in a porcine epidemic diarrhea virus S gene encoding sequence, for encoding last nine amino acids EAFEKVHVQ or a homologous fragment thereof. The porcine epidemic diarrhea virus attenuated strain is a porcine epidemic diarrhea virus epidemic strain attenuated strain, and has the advantages of substantially reduced pig pathogenicity, no return after pig immunization, good immunogenicity and realization of effective resistance of immunized pigs to virulent attack. The invention also relates to a vaccine composition obtained by adopting the porcine epidemic diarrhea virus attenuated strain as a live virus antigen, a porcine epidemic diarrhea virus attenuated strain mutation S protein, and a preparation method of the porcine epidemic diarrhea virus attenuated strain.
Owner:PU LIKE BIO ENG
Gene VII type Newcastle disease virus attenuated strain, vaccine composition and application of vaccine composition
ActiveCN107281479AImproving immunogenicityGuaranteed not to detoxSsRNA viruses negative-senseViral antigen ingredientsAntigenNewcastle disease virus NDV
The invention relates to a vaccine composition. The vaccine composition comprises an immunizing dose gene VII type Newcastle disease virus attenuated strain or an antigen of a culture of the immunizing dose gene VII type Newcastle disease virus attenuated strain and a pharmaceutically acceptable carrier. The vaccine composition completely protects against the attacking toxins of the classic virus strain and the existing prevalent strains, and a vaccine prepared for protecting the relatively classic virus strain is more comprehensive. The invention also provides a vaccine prepared by using the gene VII type Newcastle disease virus attenuated strain or the antigen of the culture of the gene VII type Newcastle disease virus attenuated strain and other pathogen antigens together, and various antigen ingredients in the vaccine respectively protect the corresponding pathogens.
Owner:PU LIKE BIO ENG +1
Live vaccine for human immunodeficiency virus
InactiveUS7189402B1Bacterial antigen ingredientsAntibody mimetics/scaffoldsSalmonella wienHIV Proteins
The present invention discloses development of a model live vaccine for HIV, using an attenuated strain of Salmonella engineered to surface express specific HIV proteins and testing of this vaccine in mice. There are provided two recombinant plasmids, containing the Lpp-OmpA genes required for surface exposure, followed by the genes for the HIV-1 proteins, Reverse Transcriptase or Transactivating protein (Tat). These plasmids are electroporated into an attenuated strain of Salmonella, and antigen expression is verified. These live vaccines are then used to orally inoculate mice and the vaccinated mice are tested for fecal IgA response and helper T cell response specific for the HIV antigens.
Owner:RES DEVMENT FOUND
Pig transmissible gastroenteritis virus vaccine strain and application thereof
ActiveCN101235363AImprove securitySafe and no side effectsViral/bacteriophage medical ingredientsAntiviralsMicroorganism preservationMicroorganism
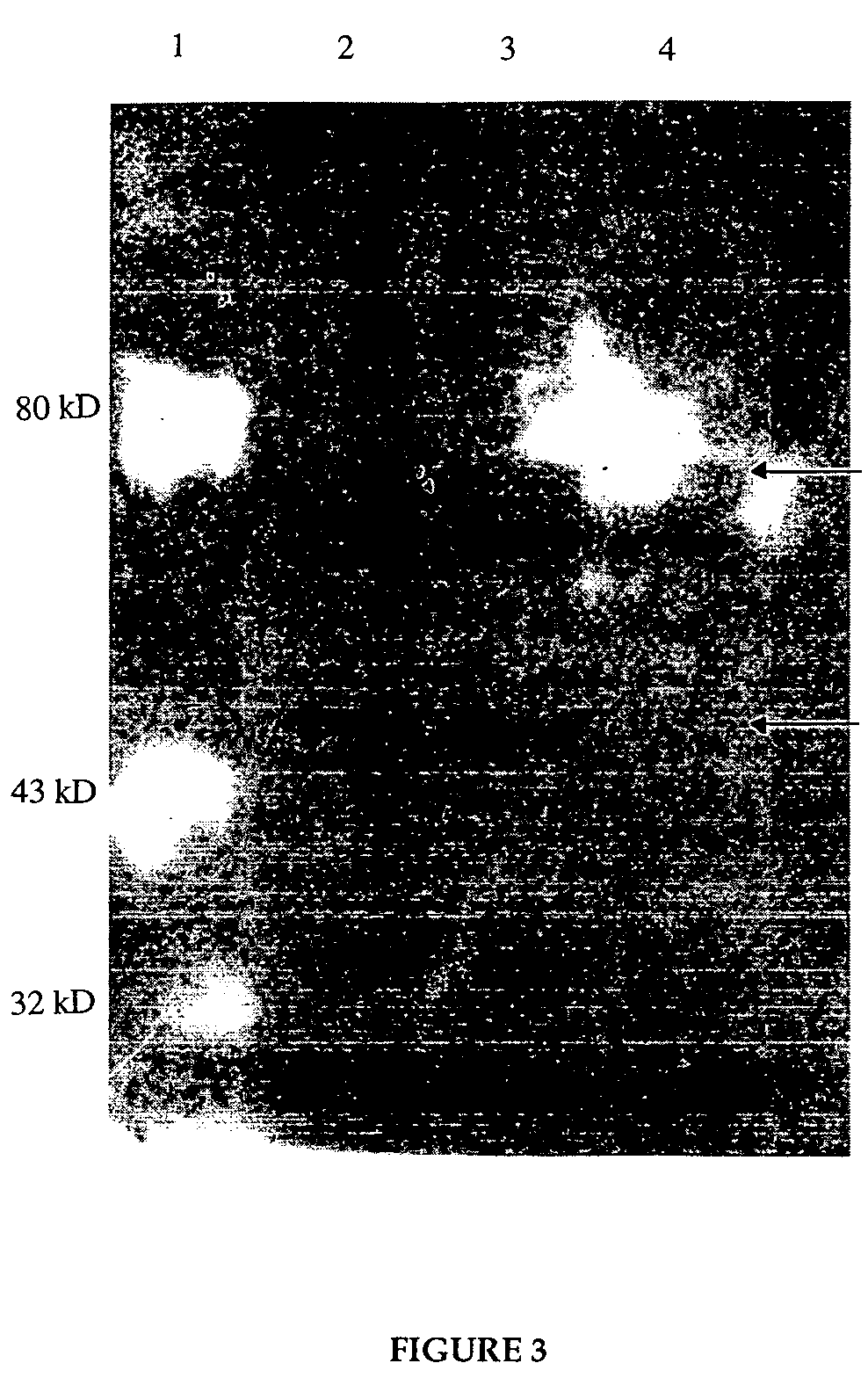
The invention discloses a transmissible gastroenteritis virus (H) attenuated vaccine strain and the application. The microorganism preservation number of the attenuated vaccine strain of the invention is CCTCC-V200609. The safety of attenuated strain TGEV of the invention is excellent, which has excellent immune protective rate. The attenuated strain of the invention can be applied in preparing diagnostic reagent for diagnosing transmissible gastroenteritis, and also can be applied in preparing single vaccine or mixed vaccine (active or inactivated vaccine) and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
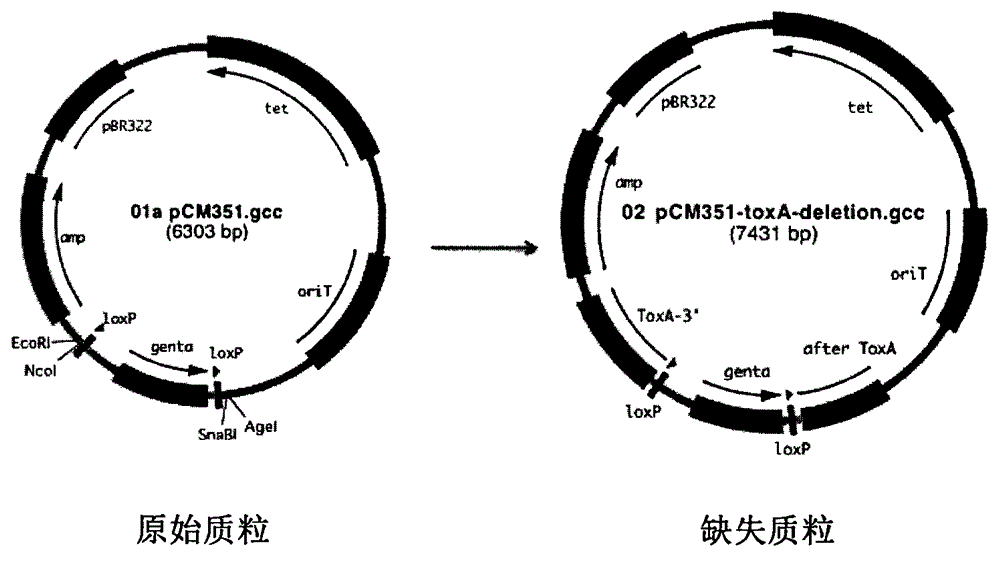
Construction method and application of pseudomonas aeruginosa mutant strain
The invention relates to a pseudomonas aeruginosa mutant strain genetic engineering mutant strain, in particular to a pseudomonas aeruginosa mutant strain exotoxin A attenuated strain, and further relates to a preparation method of the mutant strain and application of the mutant strain or the attenuated strain in preparing vaccines or adjuvant therapy drugs.
Owner:BENHEALTH BIOPHARMACEUTIC SHENZHEN CO LTD
Mycoplasma hyopneumoniae culture medium and preparation method thereof
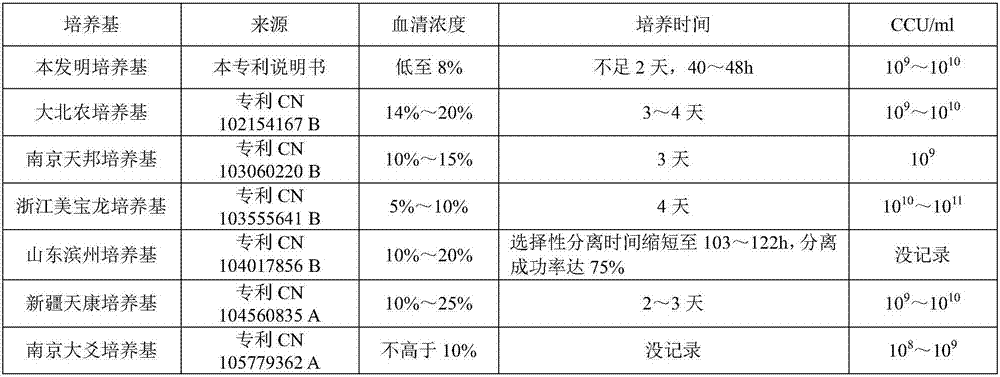
The invention relates to a mycoplasma hyopneumoniae culture medium and a preparation method thereof. The mycoplasma hyopneumoniae culture medium consists of a basic culture medium and an auxiliary culture medium, wherein the basic culture medium has major components of Hank's liquid, lactoprotein hydrolysate, a yeast extract and an ox heart extract, sterilization can be performed through autoclaving, and a pollution risk caused by filtration sterilization is greatly reduced; and the auxiliary culture medium comprises pig serum, argenine, cysteine, phenol red solution, penicillium and the like, the pig serum is sterilized through cobalt radiation, and the rest components are mixed with inactivated pig serum after filtration sterilization. The titer of a lapinized attenuated strain of the mycoplasma hyopneumoniae cultured with the culture medium provided by the invention is 109-1010 CCU, the minimum culture time may be 40 h, the generation of old bacteria and aged bacteria is greatly reduced, the usage amount of the pig serum can be as low as 8%, the allergic stress reaction caused by the pig serum is reduced, and the production cost of an enterprise is lowered.
Owner:JIANGSU NANNONG HI TECH
Gene VIb subtype Rubulavirus Newcastle disease virus attenuated strain VIbI4 and construction method thereof
ActiveCN102776156AImprove reproductive performanceSuitable for mass productionMicroorganism based processesViruses/bacteriophagesNewcastle disease virus NDVEncephalitis Viruses
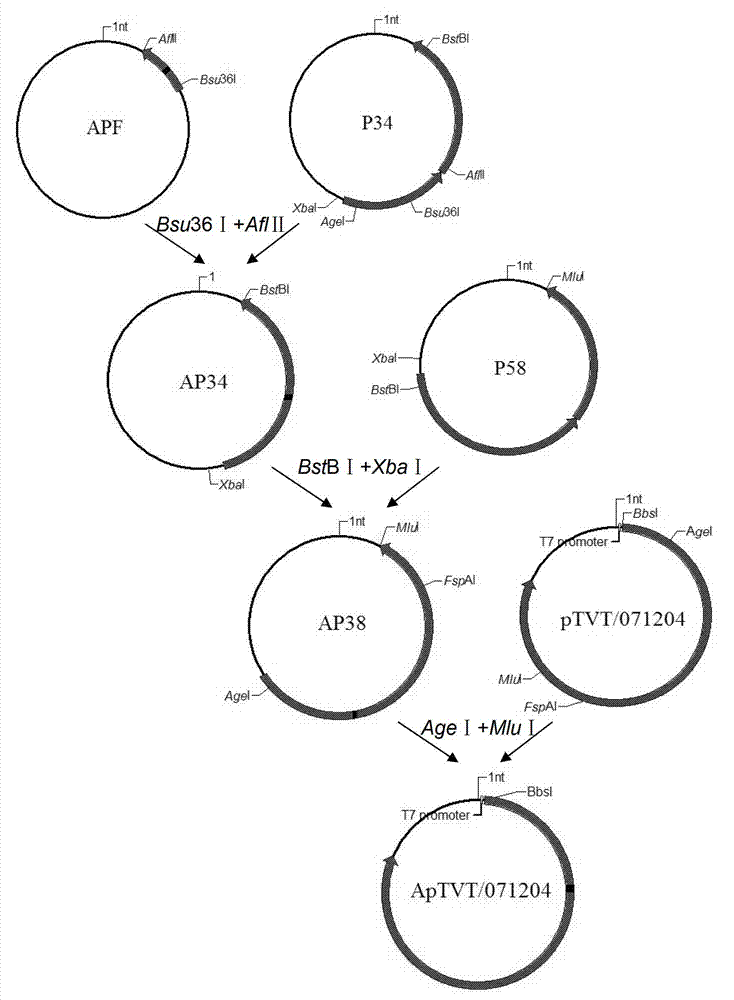
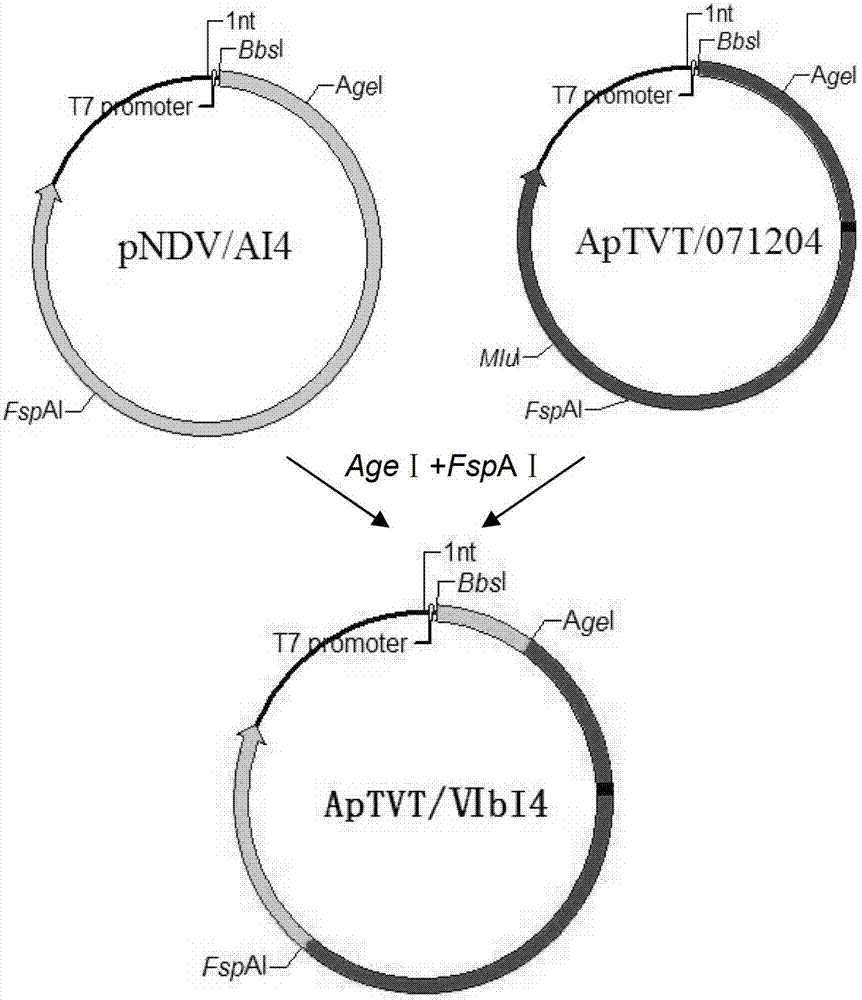
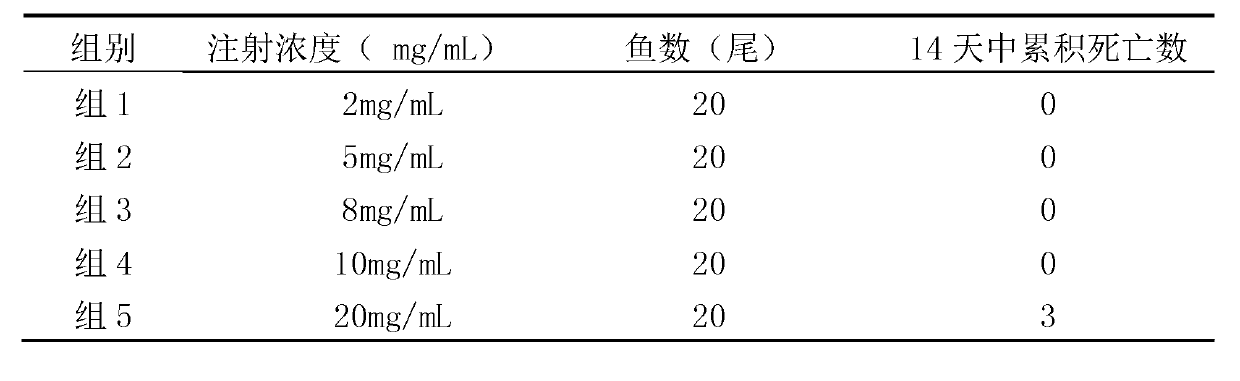
The invention relates to gene VIb subtype Avian pneumo-encephalitis virus attenuated strain VIbI4 and a construction method thereof. The preserving number of the gene VIb subtype scriber set Avian pneumo-encephalitis virus attenuated strain VIbI4 is CGMCCNo: 6149. The gene VIb subtype Rubulavirus Newcastle disease virus attenuated strain VIbI4 and the construction method thereof relate to a technology for applying reverse genetics; and the method comprises the following steps: carrying out mutation on an F gene by using a transcription carrier Ptvt / 071204 containing Avian pneumo-encephalitis virus JS / 07 / 04 / Pi full gene genome from pigeons so as to obtain to a transcription carrier ApTVT / 071204, and then substituting corresponding parts on the ApTVT / 071204 by the NP, P and L genetic fragments of Avian pneumo-encephalitis virus rNDV / I4 so as to obtain recombinant plasmids ApTVT / VIbI4, wherein the plasmids successfully save a recombinant virus VIbI4 after transfecting a BSR-T7 / 5 cell together with auxiliary plasmids. The virus is relatively high in propagating titre, is suitable for large-scale production of vaccines and can be used for manufacturing vaccines.
Owner:YANGZHOU UNIV
Adjuvant for improving immunization effect of Edwardsiella vaccine and use method of adjuvant
ActiveCN102988981AGood immune protectionSafe to useImmunological disordersAntibody medical ingredientsProtective antigenAdjuvant
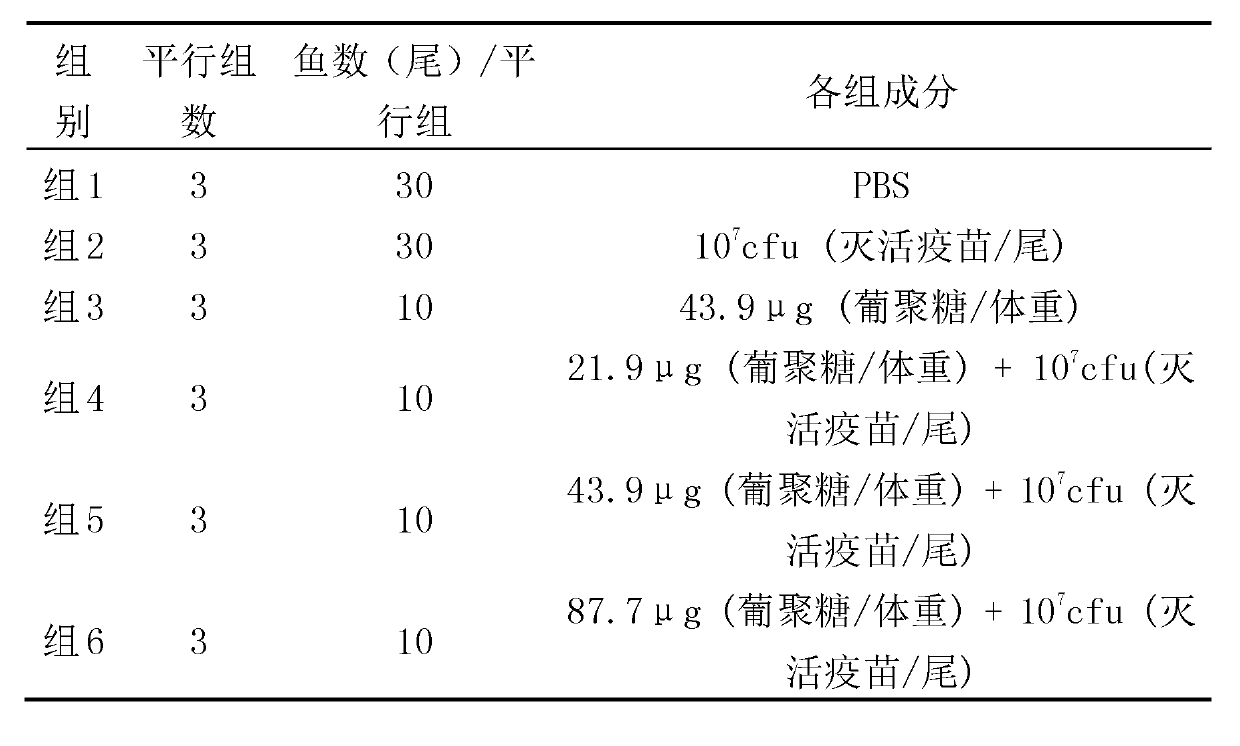
The invention relates to an adjuvant for improving the immunization effect of Edwardsiella vaccine and a use method of the adjuvant. The adjuvant is characterized by being extracted from raw materials including grain, yeast and a part of fungi or algae. The effective component of the adjuvant is beta-1,3-glucan or the natural, artificially modified or synthetic product of the beta-1,3-glucan. The adjuvant can be used for increasing the specific immune protection ratio of any one or more inculcated Edwardsiella vaccine antigens including the inactivated bacteria, the bacteria disintegration component, the less-virulent strain, the attenuated strain, the protective antigen, the antigen subunit, the antigenic determinant clusters or the expression product of the antigen cell expression vector of the Edwardsiella, can be used together or not together with the vaccine antigen and can be prepared into a single preparation which is used together with the vaccine antigen.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Attenuated strain YN150 of variant porcine epidemic diarrhea virus and applications thereof
InactiveCN105821006AVariation features are obviousEasy to makeSsRNA viruses positive-senseViral antigen ingredientsMicroorganismVero cell
The invention discloses an attenuated strain YN150 of variant porcine epidemic diarrhea virus and applications thereof. The attenuated strain is prepared by consecutively passing strain YN144 (microbial preservation number: CCTCC V201547) for six generations in Vero cells in the presence of pancreatin (10 [mu]g / mL). The PEDV attenuated strain is originated from variant porcine epidemic diarrhea virus, has good safety, and is safe to various pigs. The provided vaccine can stimulate the pigs to generate protective immune response so as to resist variant porcine epidemic diarrhea virus and effectively prevent the infection caused by variant porcine epidemic diarrhea virus.
Owner:HUAZHONG AGRI UNIV
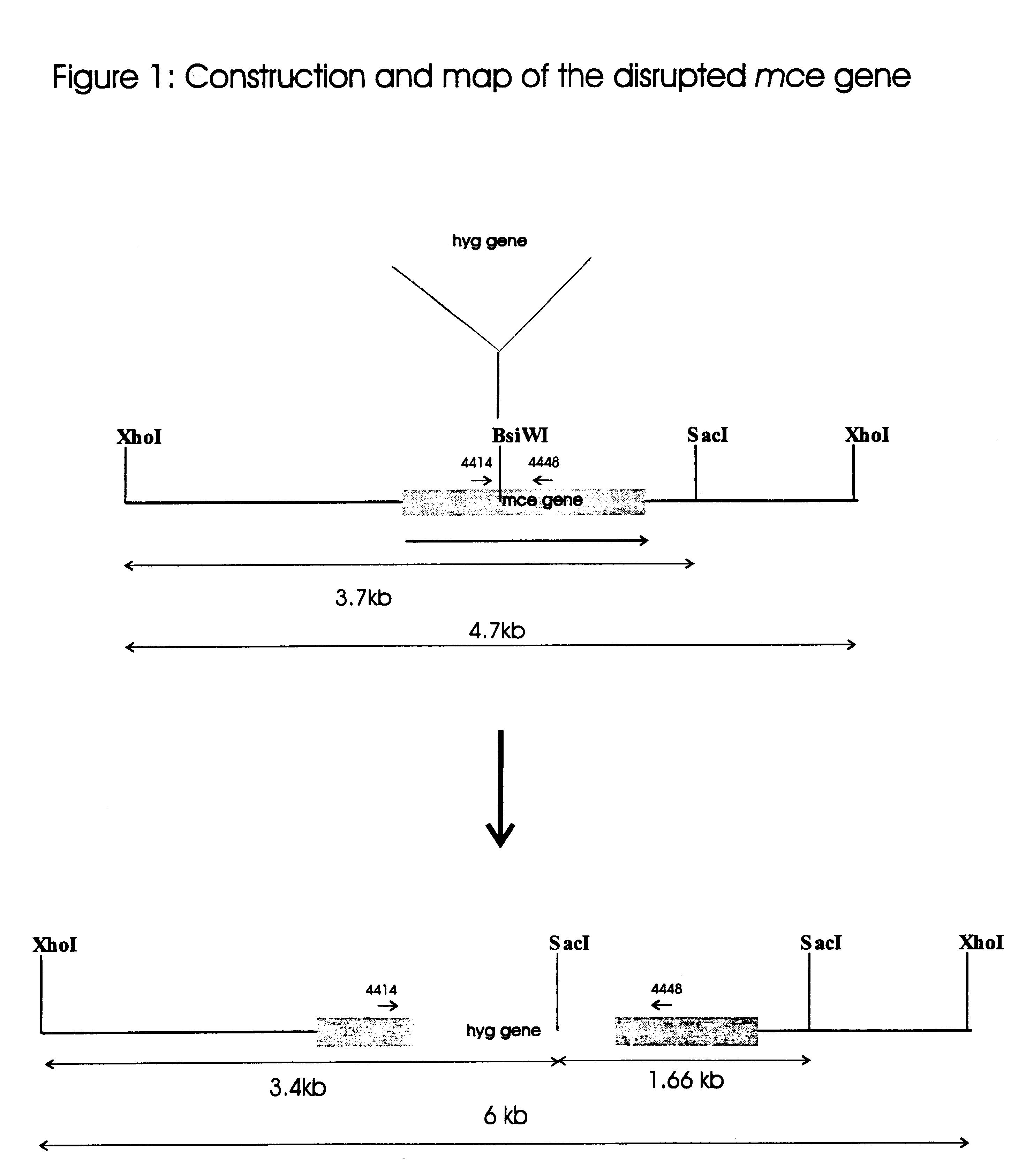
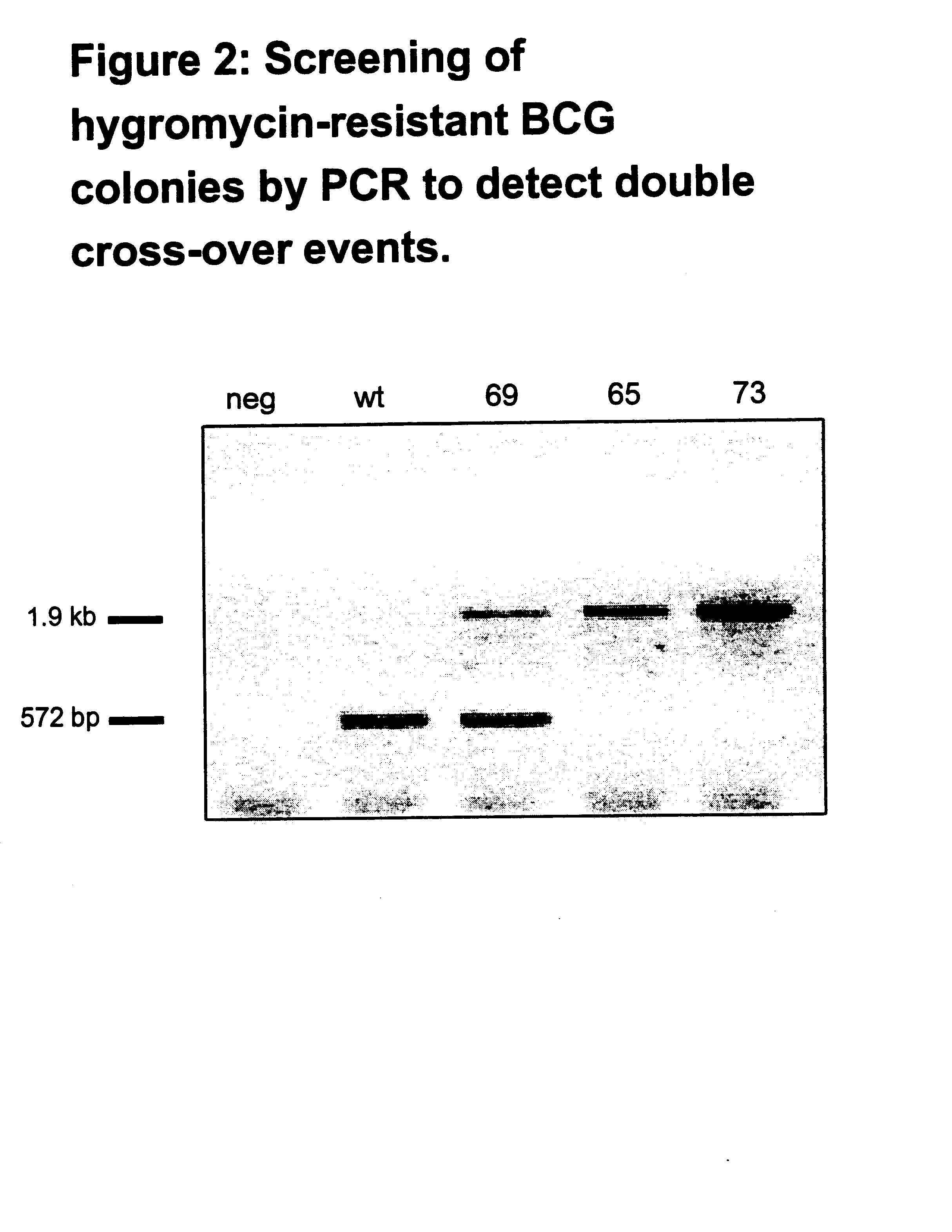
Attenuated strains of mycobacteria
Attenuated strains of Mycobacterium, particularly species of the tuberculosis complex, have the mycobacterial cell entry (mce) gene functionally disabled. The gene may be disabled by an insertion into the gene which disrupts the mycobacterial cell entry function thereof of a selectable marker which is used for screen for homologous recombinants in which a double cross-over event has been effected. The attenuated strains may be used in the immunization of hosts against Mycobacterium disease.
Owner:AVENTIS PASTEUR LTD
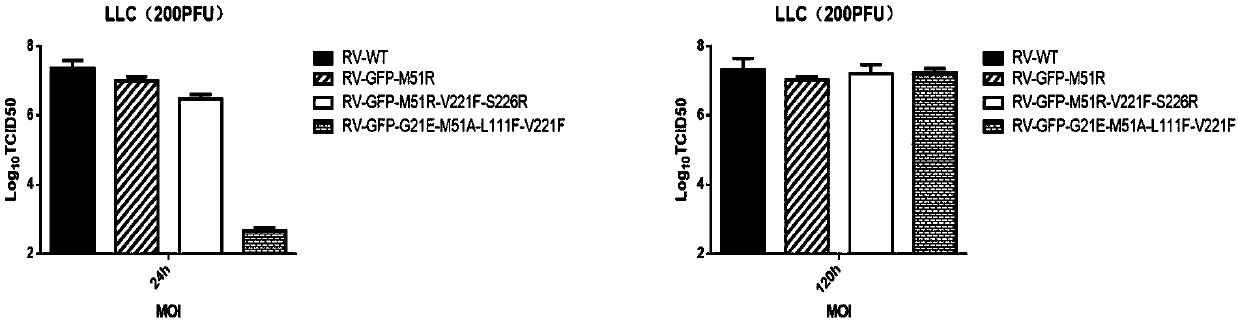
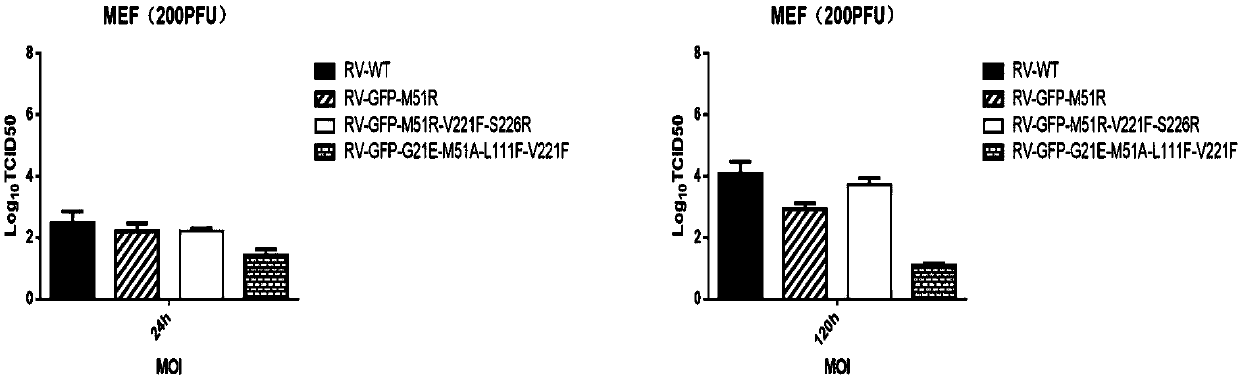
Attenuated strain of oncolytic rhabdovirus and its application in tumor therapy
ActiveCN110305198ALow toxicityGood oncolytic effectSsRNA viruses negative-senseVirus peptidesDrugImmunosuppression
The present disclosure relates to a modified matrix protein of a recombinant oncolytic rhabdovirus, an attenuated strain of the oncolytic rhabdovirus having the mentioned modified matrix protein, a composition comprising the mentioned attenuated strain, and an application thereof for preparing a drug for killing abnormal proliferative cells, inducing an anti-tumor immune response or eliminating microenvironment immunosuppression of tumor tissue. The attenuated strain of oncolytic rhabdovirus has sustained replication expression and high titer, and stimulates the immune response of the local microenvironment of the tumor, has the low toxicity to normal cells while maintaining high selectivity in infecting tumor cells, and has great significance in the clinical treatment of tumors.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
Oral delivery of nucleic acid-based gene interfering agents by salmonella
The present invention provides vectors, including a novel attenuated strain of Salmonella, for efficient gene transfer into an animal, e.g. a mammalian host.
Owner:RGT UNIV OF CALIFORNIA
Magnetic Nanoparticles for Imaging
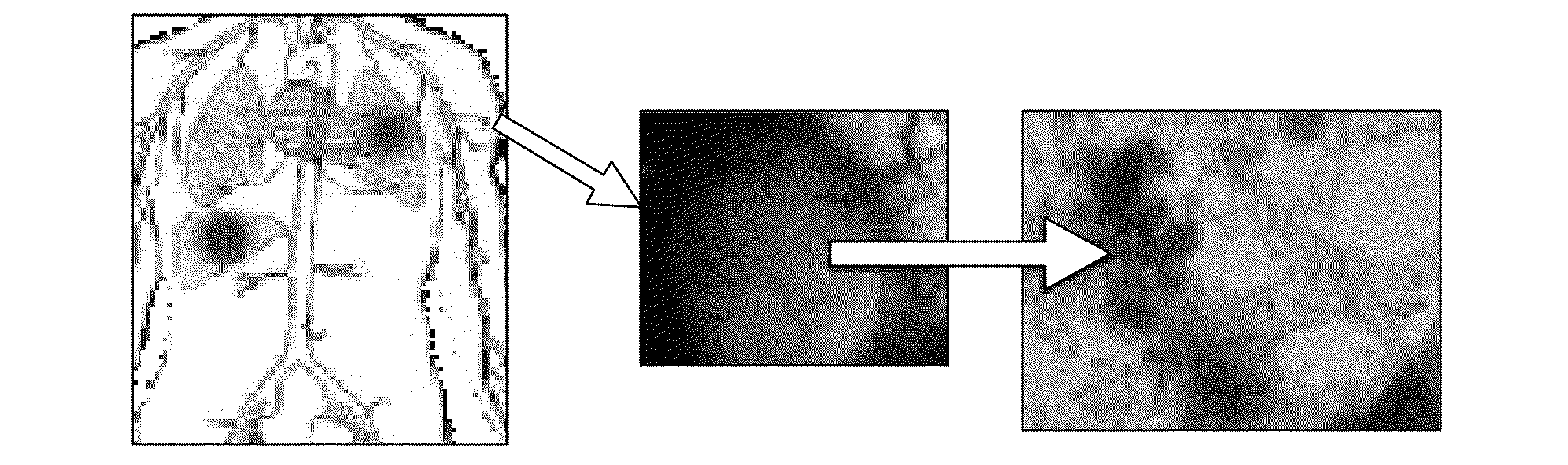
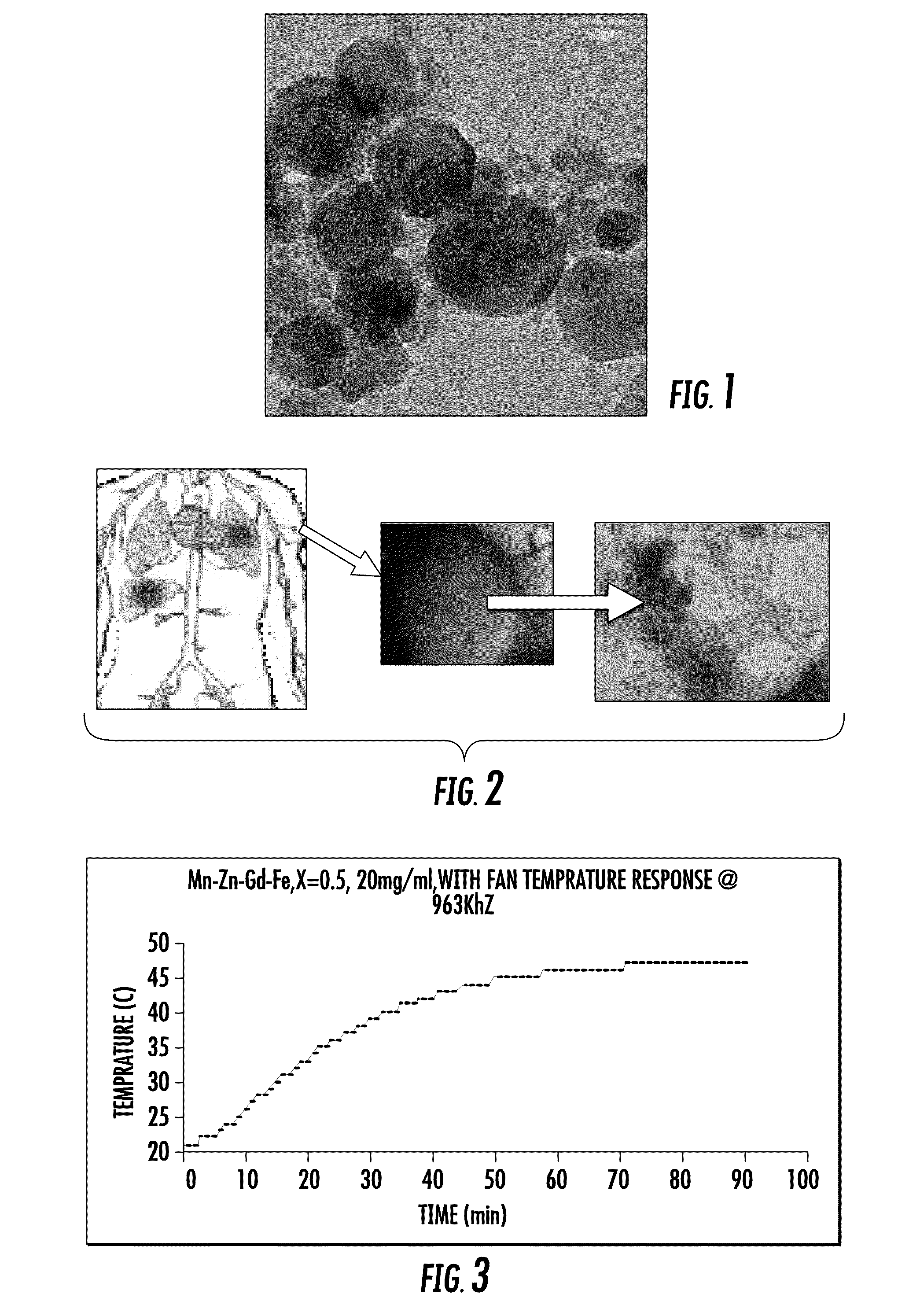
InactiveUS20090068112A1Reduction of septic shock potentialReduced virulenceNanomedicineNMR/MRI constrast preparationsTumor siteAttenuated strain
A medical imaging system that enables the discovery of malignant tissue utilizing contrast agents and heating agents made of magnetic nanoparticles that are delivered to tumor sites utilizing attenuated strains of bacteria that seek and reside at tumor sites is disclosed. The thermal contrast agents may be temperature self-controlled magnetic nanoparticles that may be encapsulated in a biocompatible coating. The thermal contrast agents may be uploaded into attenuated strains of bacteria that seek and reside in tumor tissue when placed into a bloodstream of a patient. An alternating magnetic field device with a prescribed frequency range may be used to induce heating of the magnetic nanoparticles in the patient, and a thermal scan may be utilized to identify tumors. In another embodiment, the contrast agent may be formed from magnetic nanoparticles having distinct magnetic moment profiles, and a MRI system may be utilized to identify tumors with such contrast agent.
Owner:UNIVERSITY OF NORTH CAROLINA AT GREENSBORO
Newcastle disease chimeric virus marker vaccine strain as well as construction method and application thereof
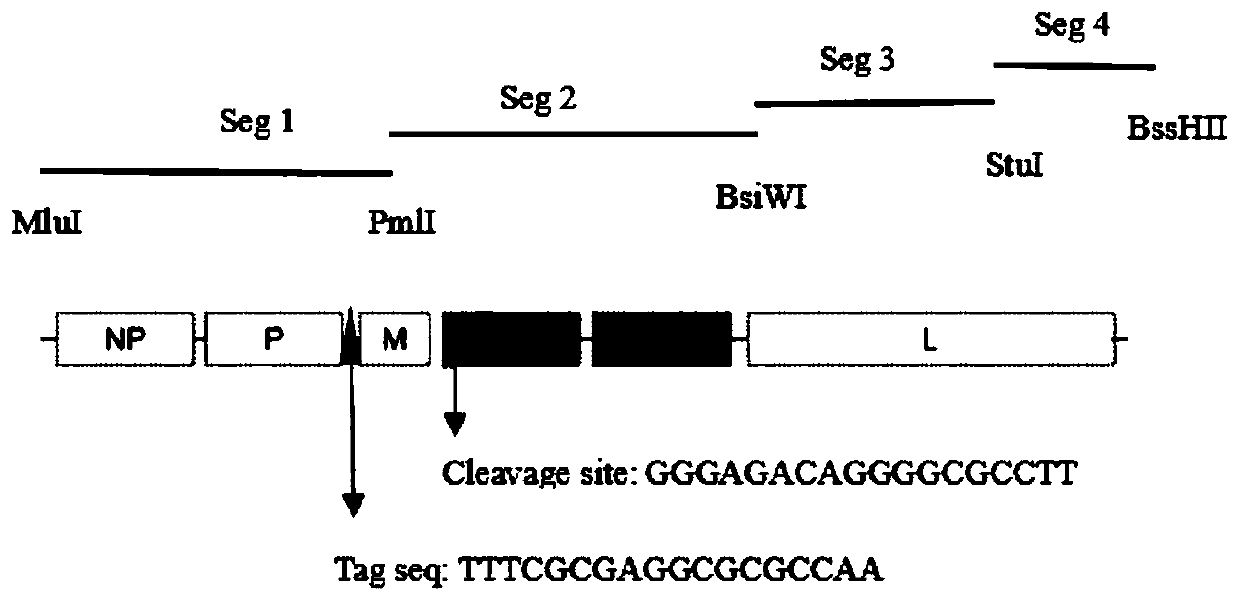
ActiveCN111575247AImprove growth characteristicsImproving immunogenicitySsRNA viruses negative-senseViral antigen ingredientsF proteinChick embryos
The invention relates to a Newcastle disease chimeric virus marker vaccine strain as well as a construction method and application thereof, and belongs to the field of rescue and application of Newcastle disease chimeric vaccine strains. A Newcastle disease virus reverse genetic operation platform is utilized to mutate an F protein cleavage site of a Newcastle disease gene GVII type strain into acleavage site of an attenuated strain, F and HN genes of a mutated gene VII type Newcastle disease strain and NP, P, M and L of a gene II type NDV La Sota strain construct a full-length chimeric cDNAsequence, and a 18bp nucleotide marker sequence is inserted into a non-coding region between P and M. A Newcastle disease chimeric virus NDV DC strain is obtained through transfection cell rescue. Theconstructed chimeric virus can reach relatively high culture titer in chick embryos and cells. The chimeric strain contains envelope surface glycoprotein of the gene VII type Newcastle disease strain, contains a skeleton of the gene II type strain, and has immunogenicity of the Newcastle disease gene VII type strain and high reproduction and high safety characteristics of the gene II type La Sotastrain.
Owner:ZHEJIANG VBIOSCI INC +1
Oncolytic virus vaccine and medicine for treating tumors by combining oncolytic virus vaccine with immune cells
ActiveCN111286493AEffective treatmentHigh cure rateSsRNA viruses negative-senseMammal material medical ingredientsTumor therapyOncology
The invention belongs to the technical field of biology, and particularly relates to an oncolytic virus vaccine and a medicine for treating tumors by combining the oncolytic virus vaccine with immunecells. The invention provides a brand-new oncolytic virus attenuated strain by carrying out site-specific mutagenesis on the VSV wild type virus matrix protein M. The gene sequence of the matrix protein M is shown as SEQ ID NO 3. The attenuated strain can be independently used as a drug for treating tumors, and is superior to wild type viruses and other known attenuated strains in safety and curerate. On the basis of the oncolytic virus attenuated strain, NY-ESO-1 is inserted into the attenuated strain, and the invention further provides a vaccine capable of being applied to tumor treatment.The vaccine is high in cure rate and high in biological safety. On the basis of the vaccine, the vaccine and TCR-T cells are combined for application, and a medicine capable of efficiently treating various tumors is provided. On a mouse lung cancer model, the cure rate can reach the surprising rate of 95 percent.
Owner:JOINT BIOSCIENCES (SH) LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com