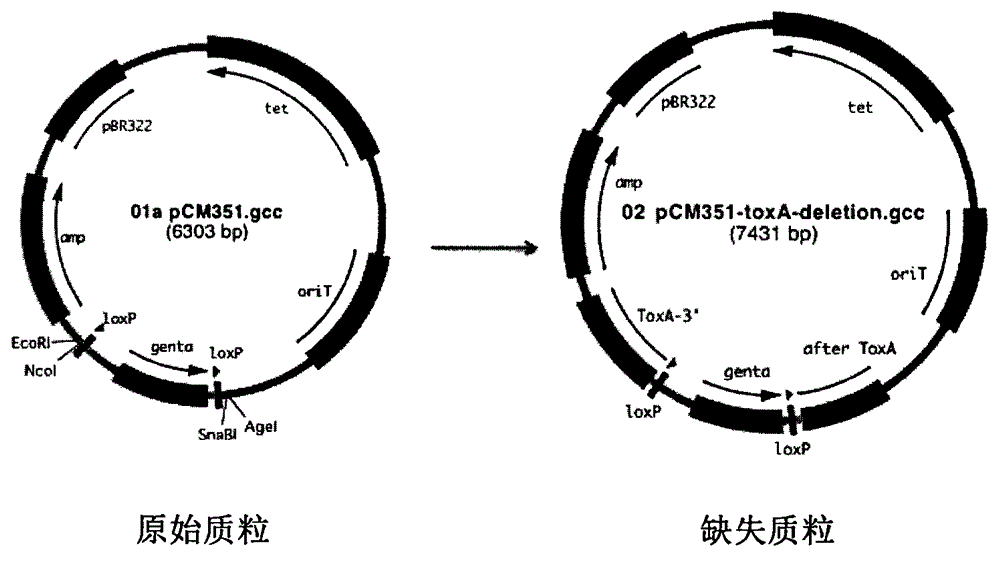
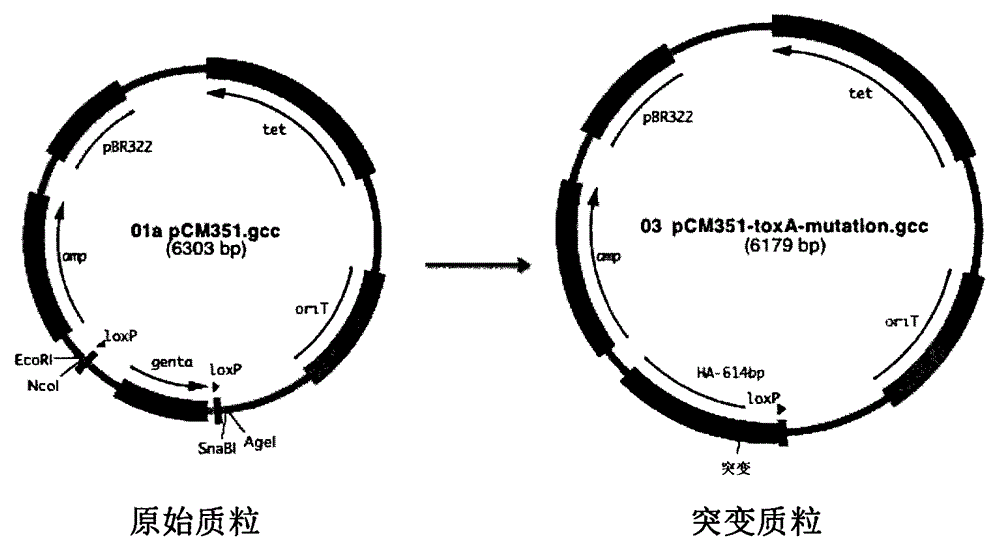
Construction method and application of pseudomonas aeruginosa mutant strain
A technology for Pseudomonas aeruginosa and mutant bacteria, which is applied in chemical instruments and methods, microorganism-based methods, biochemical equipment and methods, etc., and can solve the problem of not yet obtained attenuated strains of Pseudomonas aeruginosa exotoxin A.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1. Preparation of Pseudomonas aeruginosa exotoxin A protein Glu578Asp mutant and Glu578 deletion mutant
[0065] According to the description of the first step to the sixth step above, a mutant strain of Pseudomonas aeruginosa exotoxin A protein Glu578Asp was prepared, wherein the triparental hybridization was carried out according to the following steps:
[0066] (1) Donor bacteria Escherichia coli (toxA) and auxiliary bacteria Escherichia coli HB101 (pRK2013) (gifted by Dr. Wang Hailong, State Key Laboratory of Microbial Technology, Shandong University) were activated by streaking on LB plate solid medium containing Kan20μg / mL, Cultivate for 16-18h, then pick a single colony into 3ml LB liquid medium of Kan15μg / mL, culture overnight at 37°C, and grow to logarithmic phase.
[0067] (2) Receptor bacterium Pseudomonas aeruginosa PAO1 (purchased from the American type culture collection (American type culture collection), preservation number is ATCC15692) was acti...
Embodiment 2
[0072] Embodiment 2. toxicity test
[0073] (1) Cytotoxicity test
[0074] The cytotoxicity of recombinant PEA (Glu578Asp mutant protein) and parental PEA protein was determined by MTT assay. Recombinant PEA and parental PEA protein were isolated and purified from Pseudomonas aeruginosa exotoxin A protein Glu578Asp mutant strain and Pseudomonas aeruginosa POA1 respectively. The recombinant PEA and parental PEA proteins stored at -80°C were melted, the protein content was measured, and the protein concentration was determined for the next step of the cytotoxicity experiment. There were three gradients in the experiment, namely 250 μg / well, 325 μg / well, and 500 μg / well. Passage L929 cells in a 96-well plate. When the cell density reaches 70% fullness, change the DMEM medium containing 2% serum, and add DMEM-dissolved gradients of recombinant PEA or parental PEA protein to treat the adherent L929 cells , and treated L929 cells with the same concentration of BSA as a blank cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com