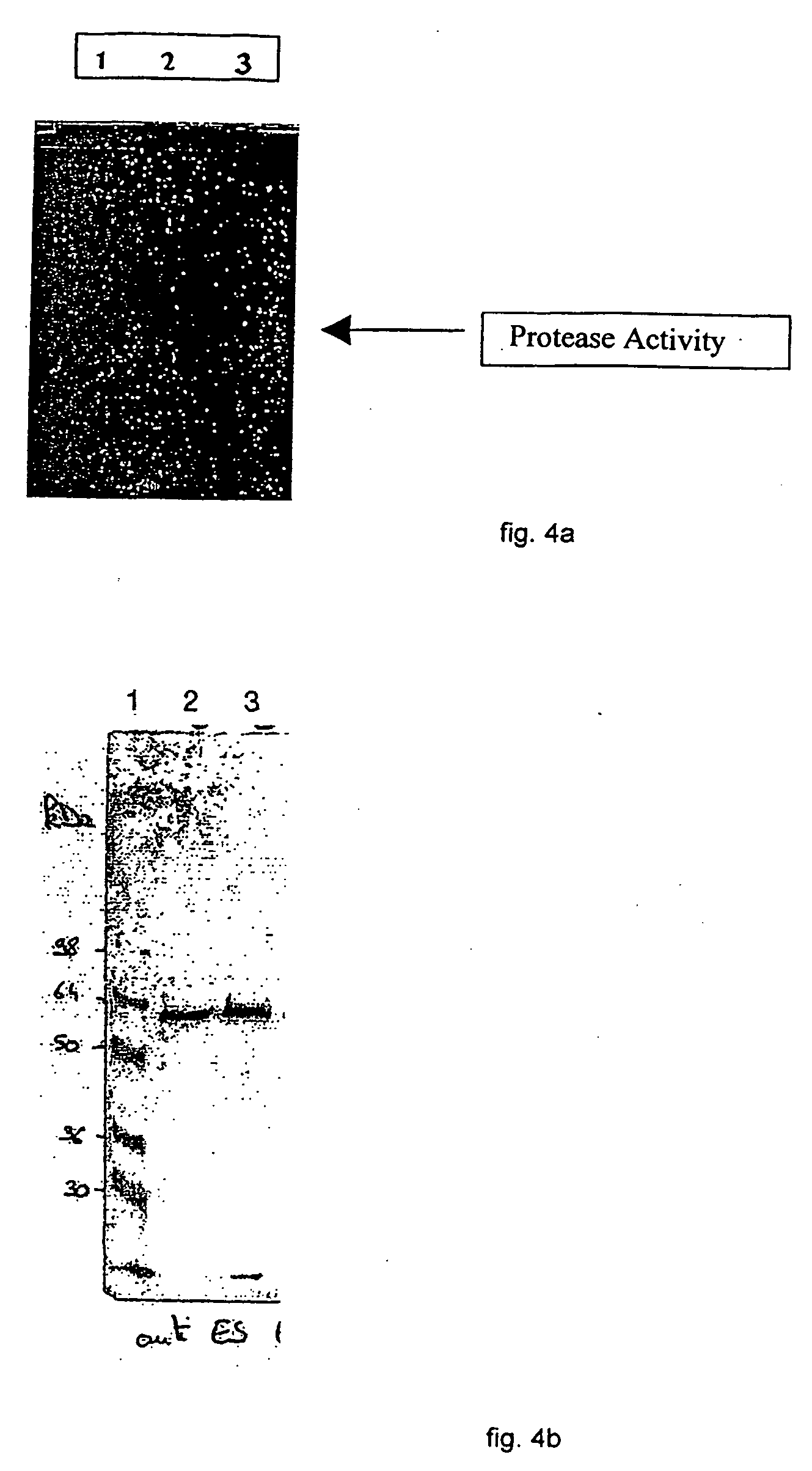
Patents
Literature
100 results about "Leishmania" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Leishmania /liːʃˈmeɪniə/ is a genus of trypanosomes that are responsible for the disease leishmaniasis. They are spread by sandflies of the genus Phlebotomus in the Old World, and of the genus Lutzomyia in the New World. At least 93 sandfly species are proven or probable vectors worldwide. Their primary hosts are vertebrates; Leishmania commonly infects hyraxes, canids, rodents, and humans.
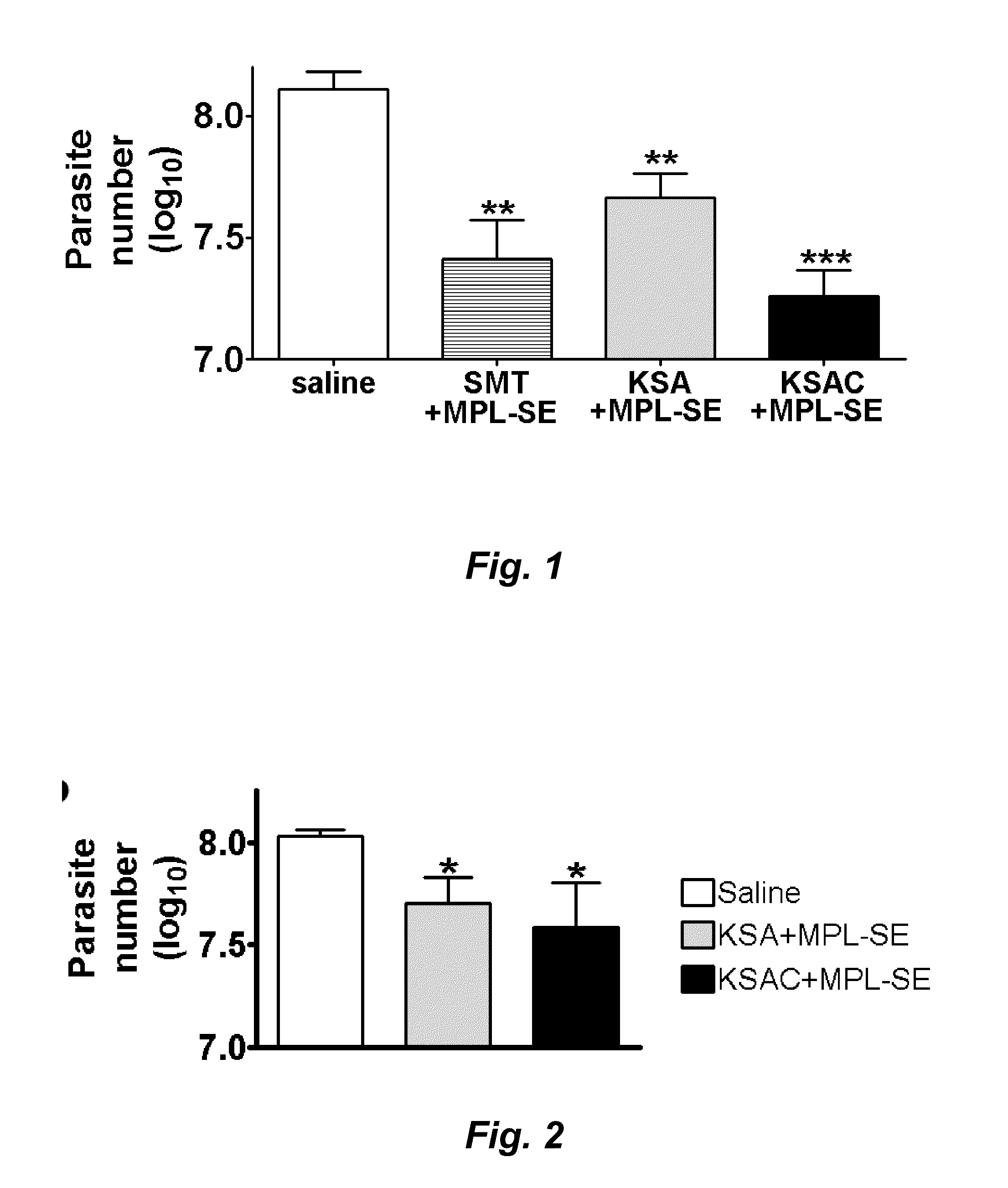
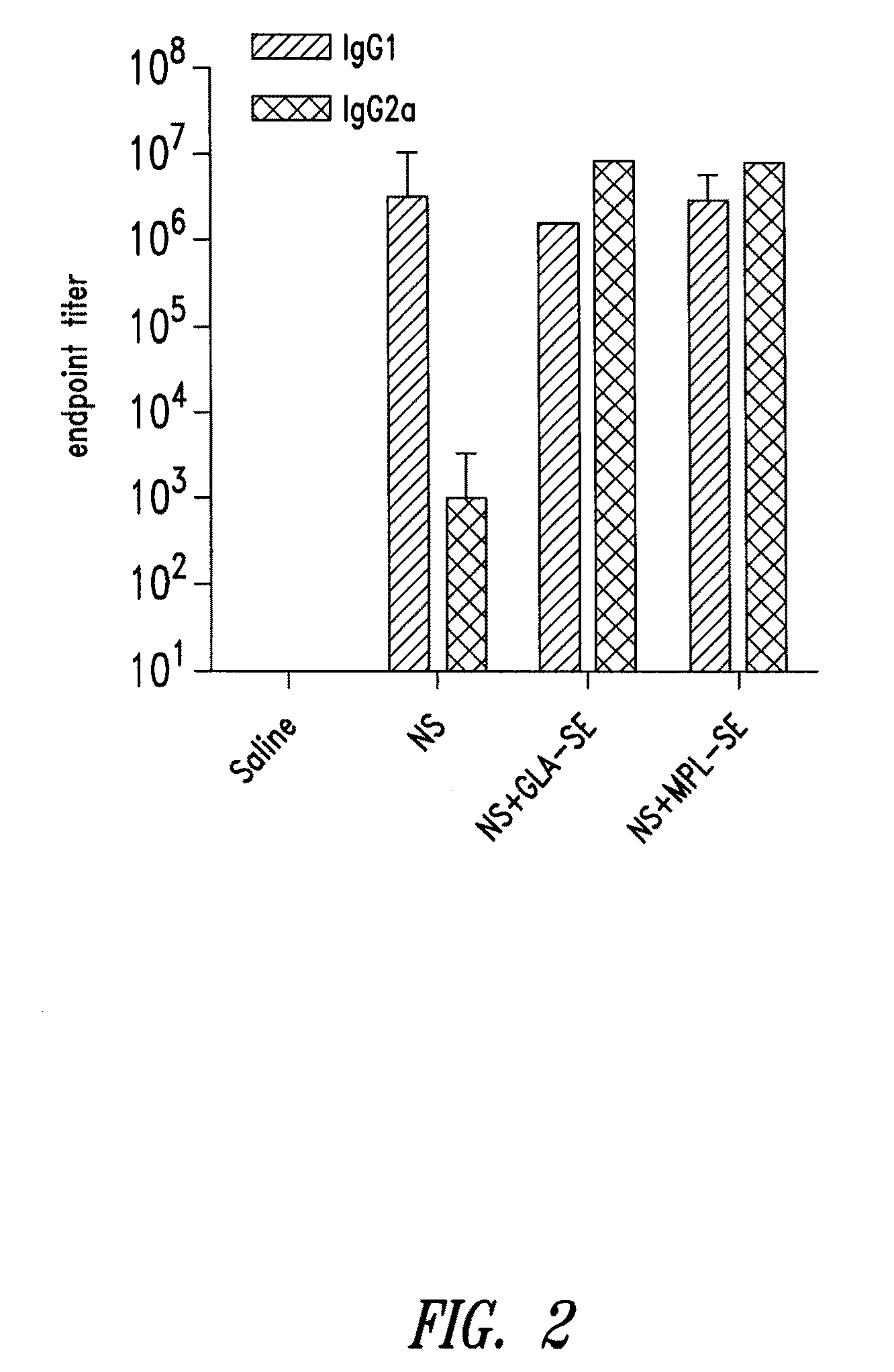
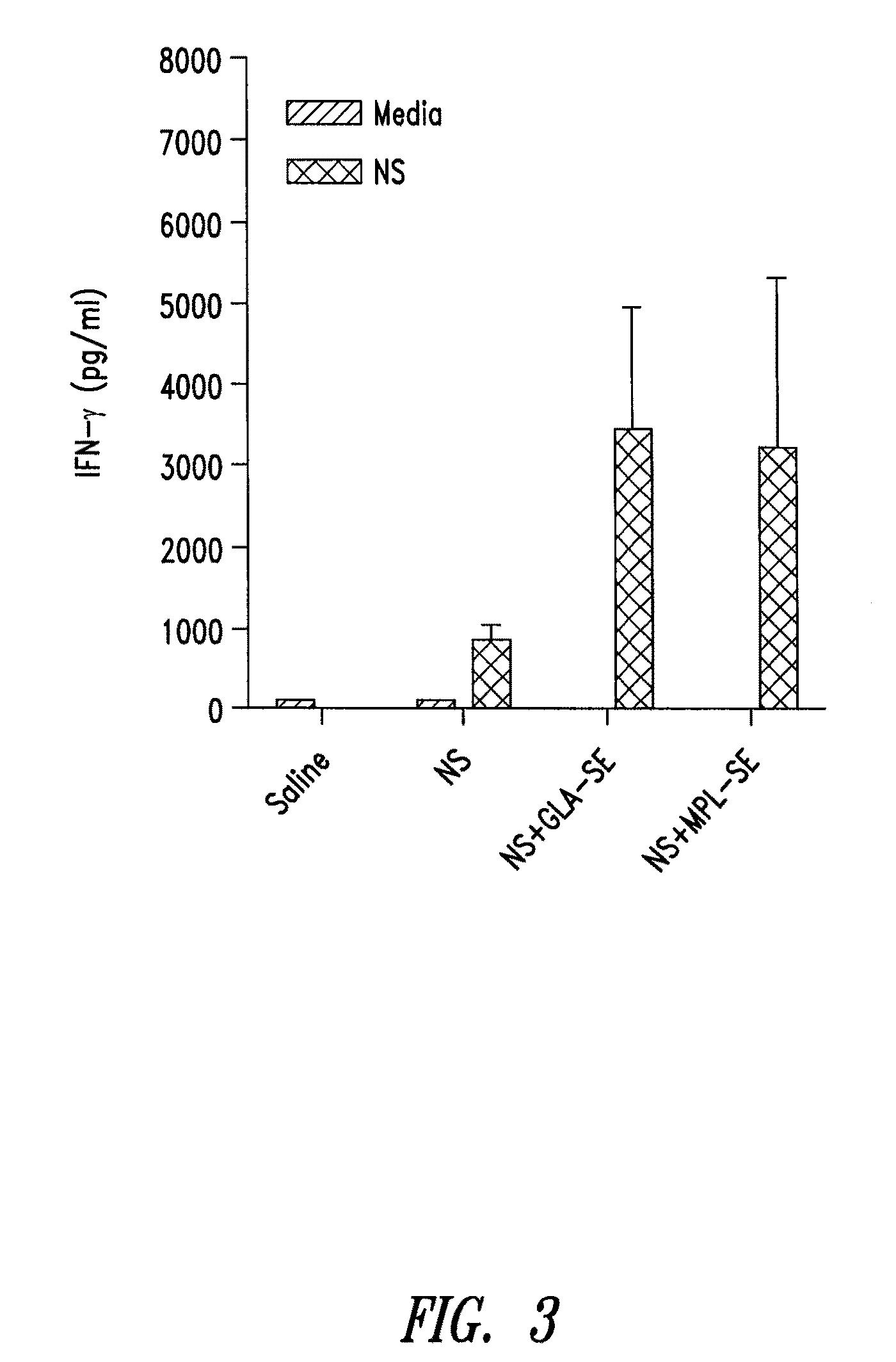
Recombinant polyprotein vaccines for the treatment and diagnosis of leishmaniasis
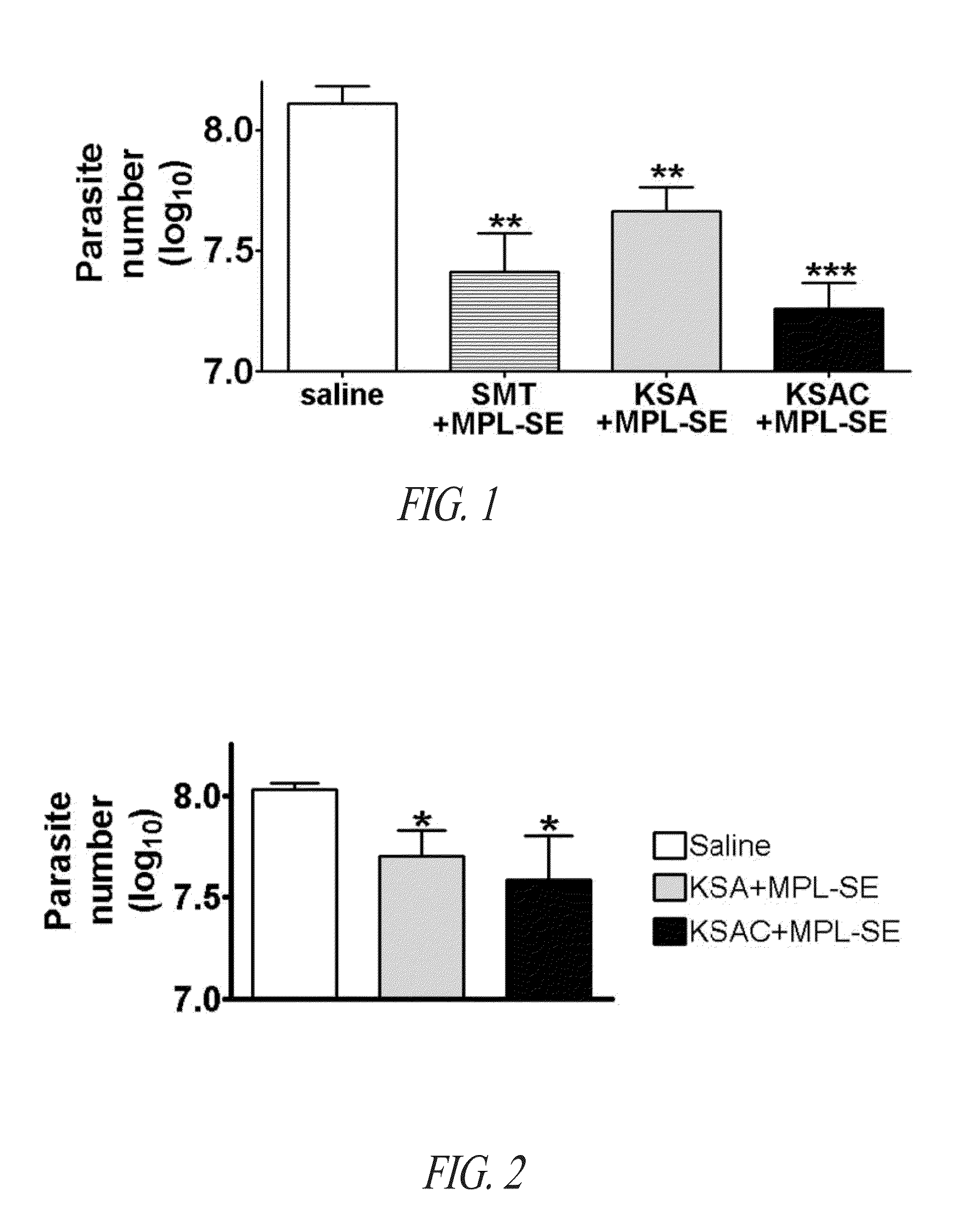
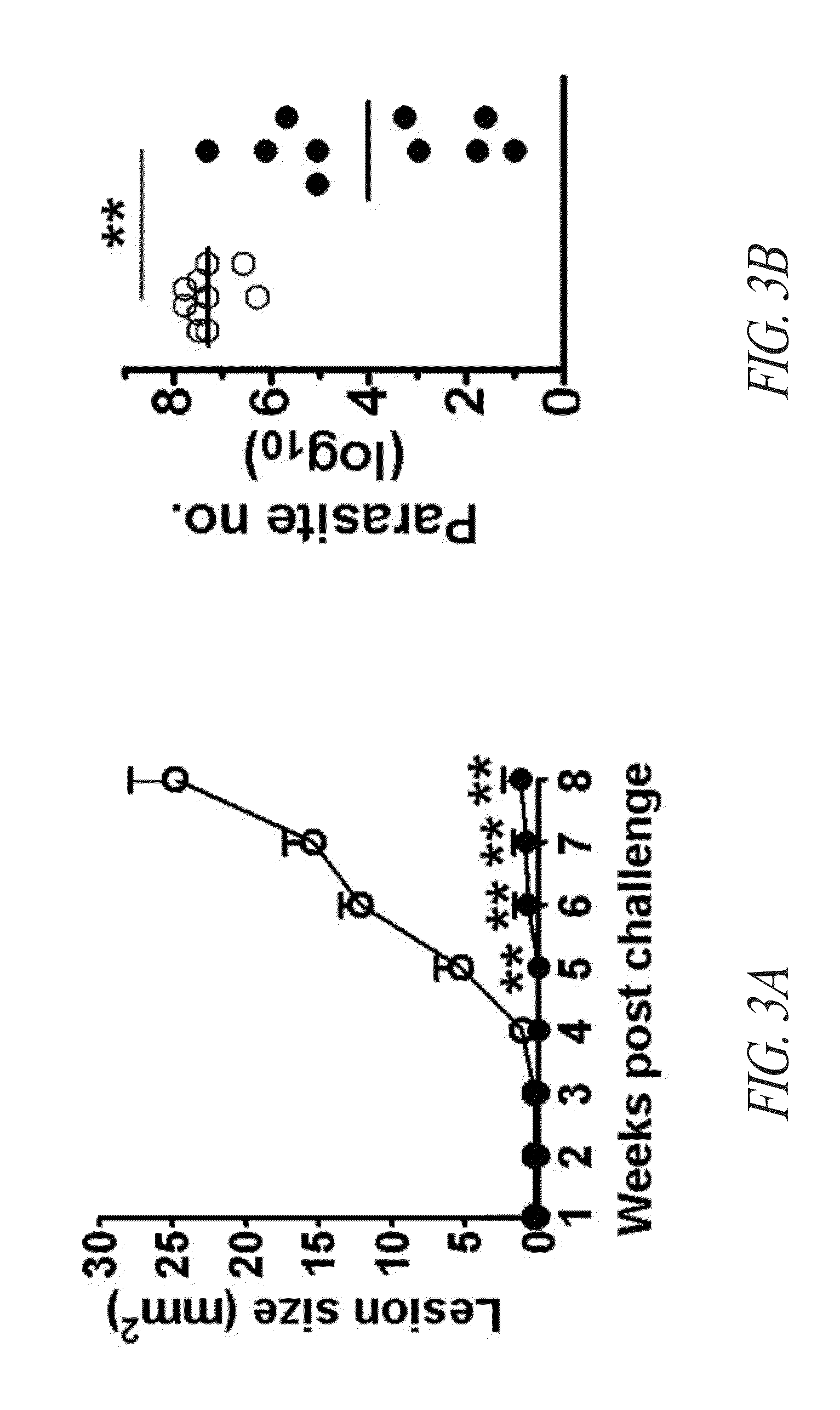
Compositions and methods for preventing, treating and detecting leishmaniasis are disclosed. The compositions generally comprise fusion polypeptides comprising multiple Leishmania antigens, in particular, KMP11, SMT, A2 and / or CBP, or immunogenic portions or variants thereof, as well as polynucleotides encoding such fusion polypeptides.
Owner:ACCESS TO ADVANCED HEALTH INST
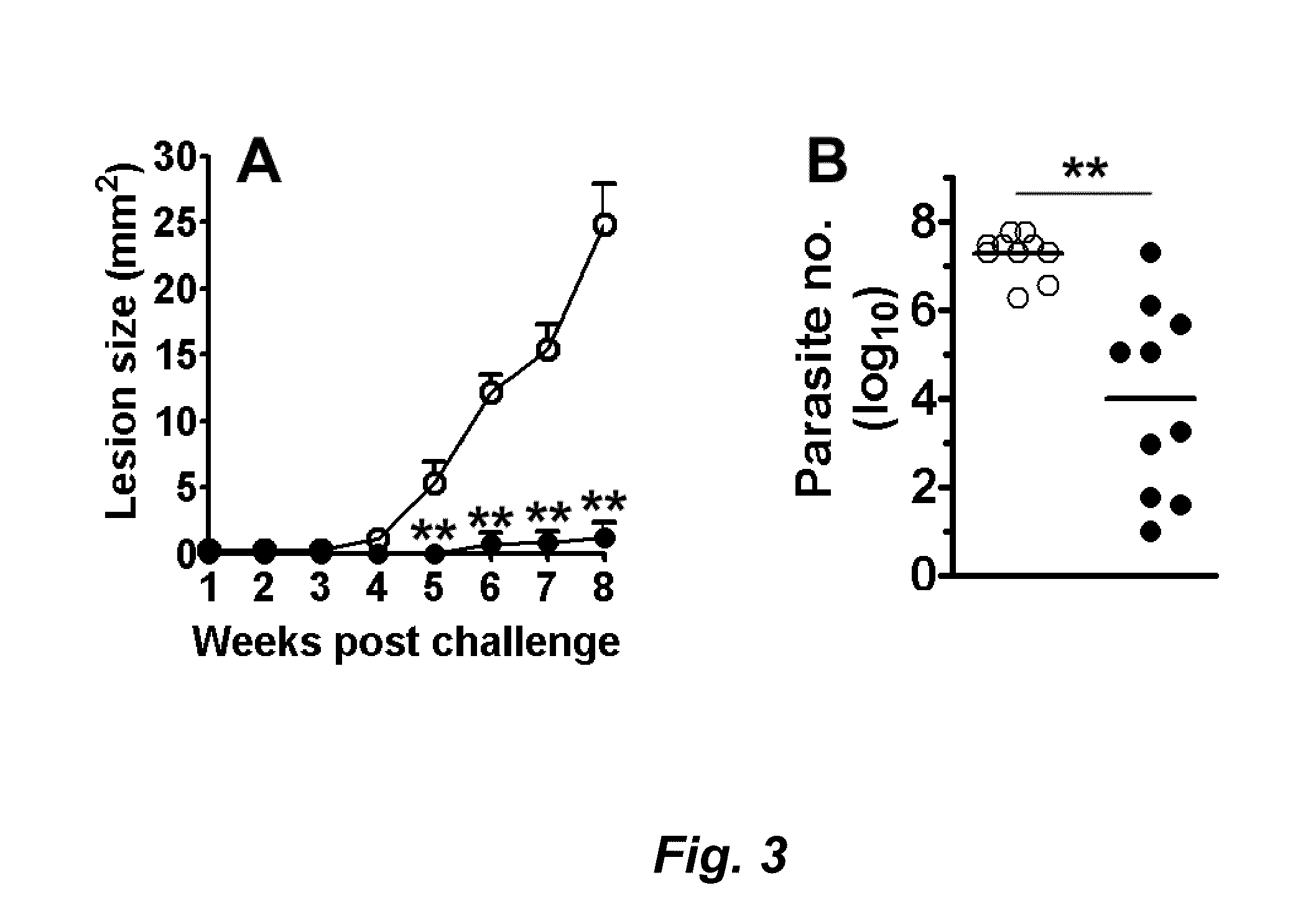
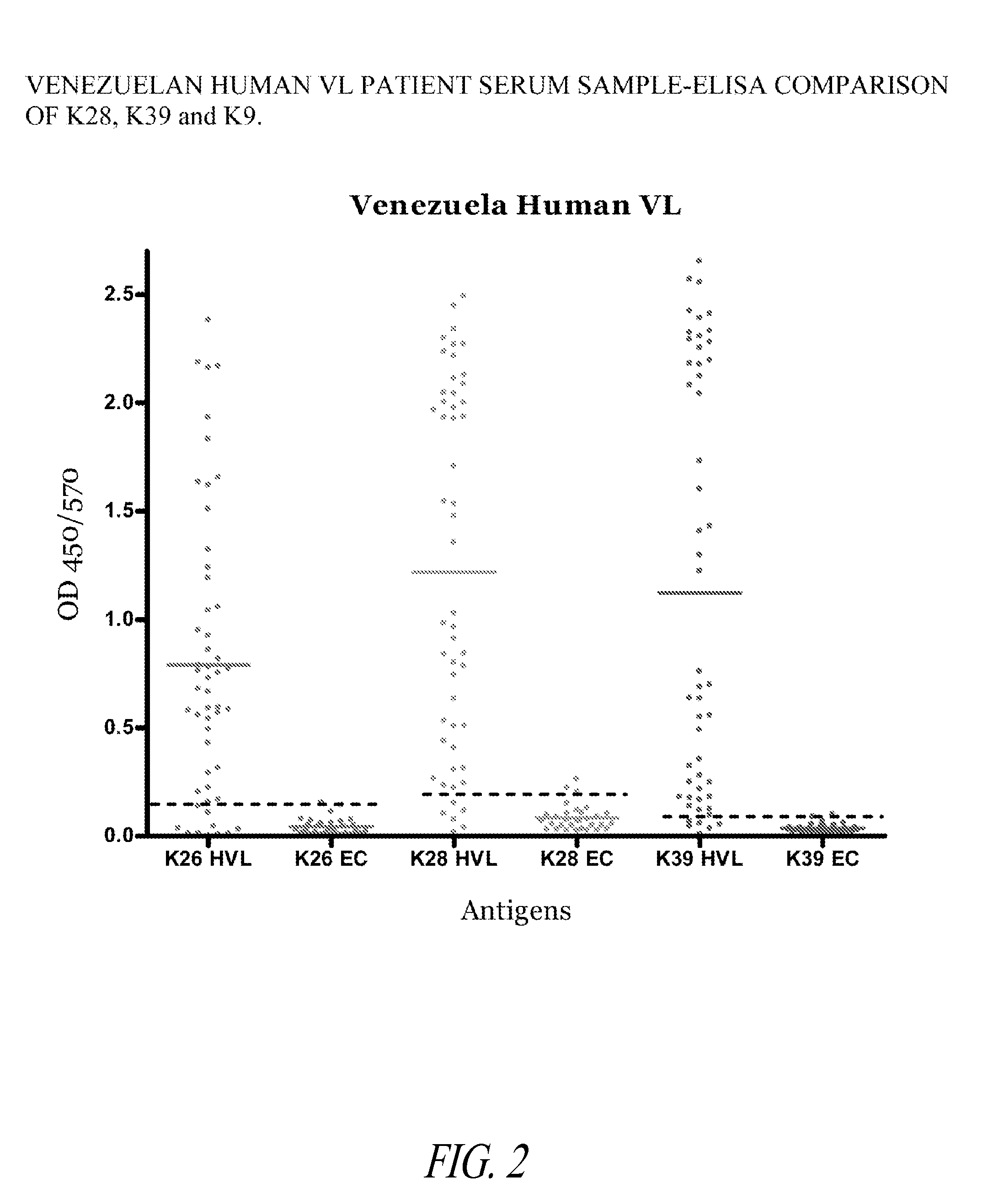
Fusion proteins and their use in the diagnosis and treatment of leishmaniasis
ActiveUS8231881B2Treatment, and prevention of leishmaniasisPeptide/protein ingredientsEnzymologyLeishmaniaVisceral leishmaniasis
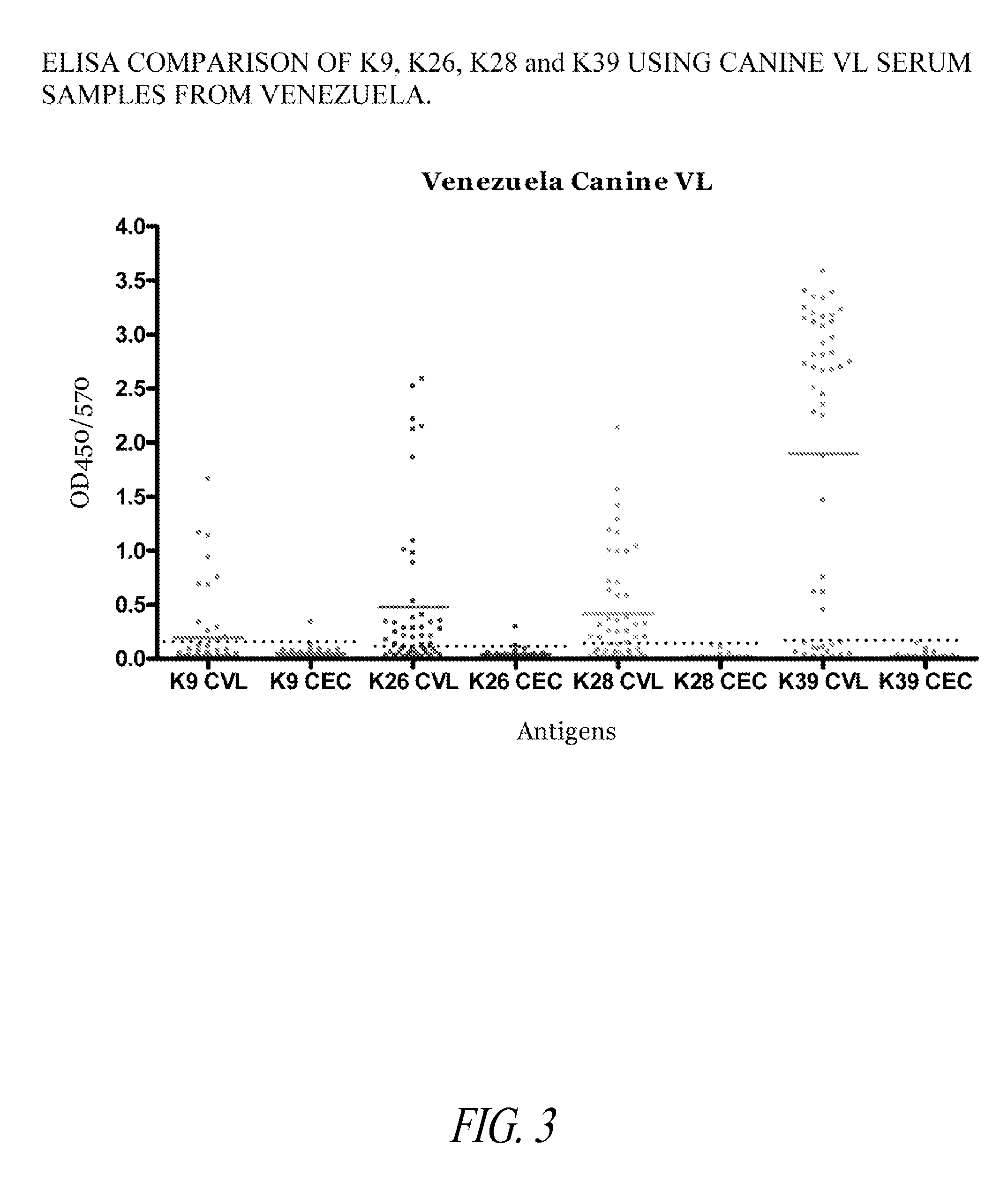
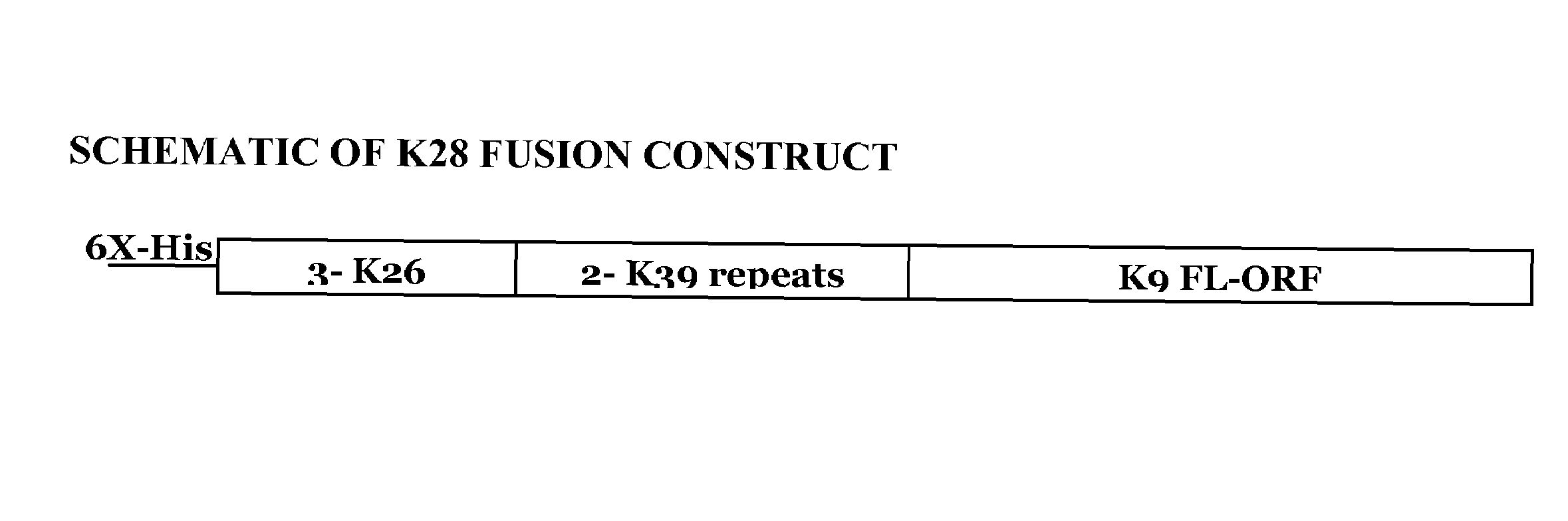
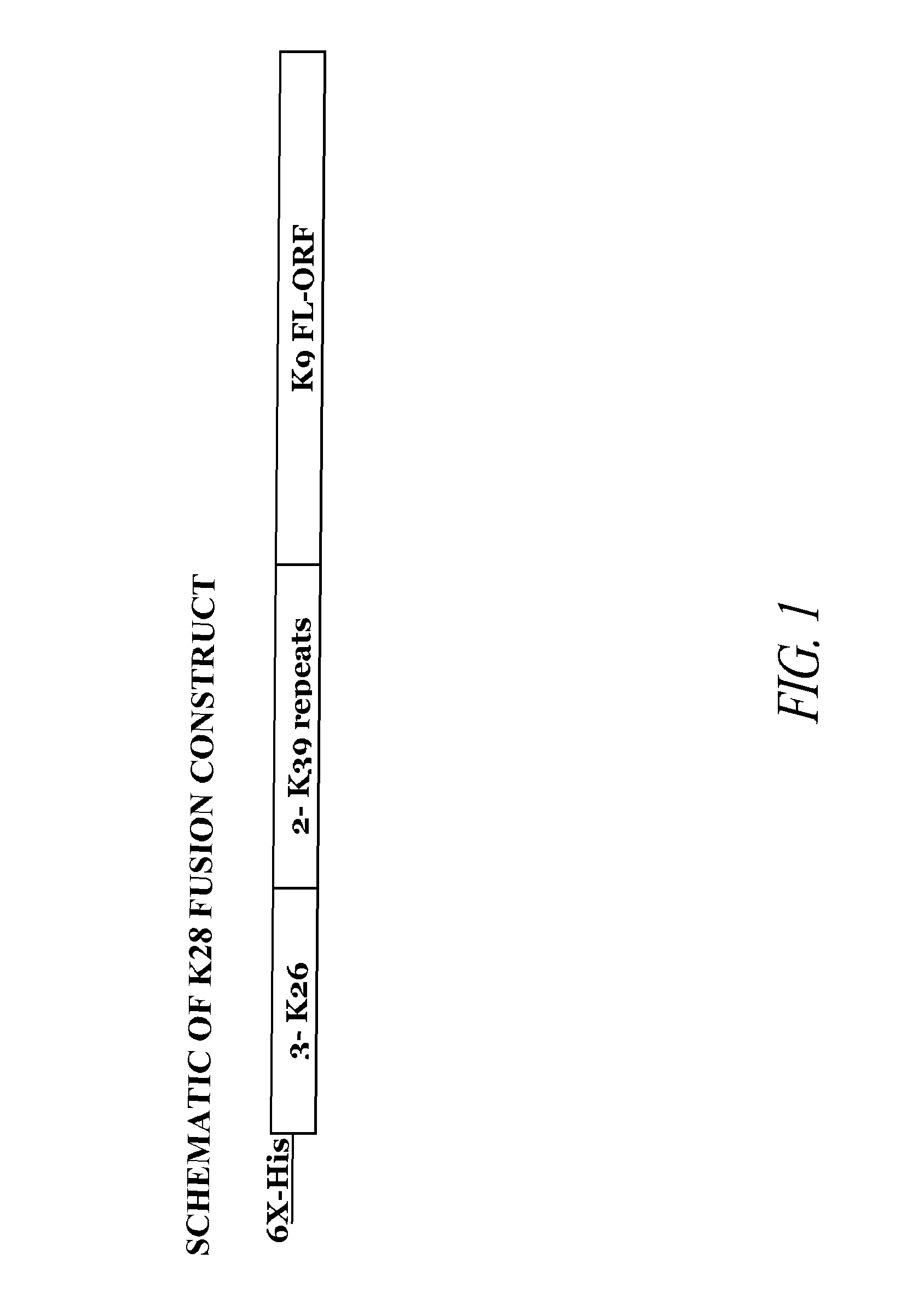
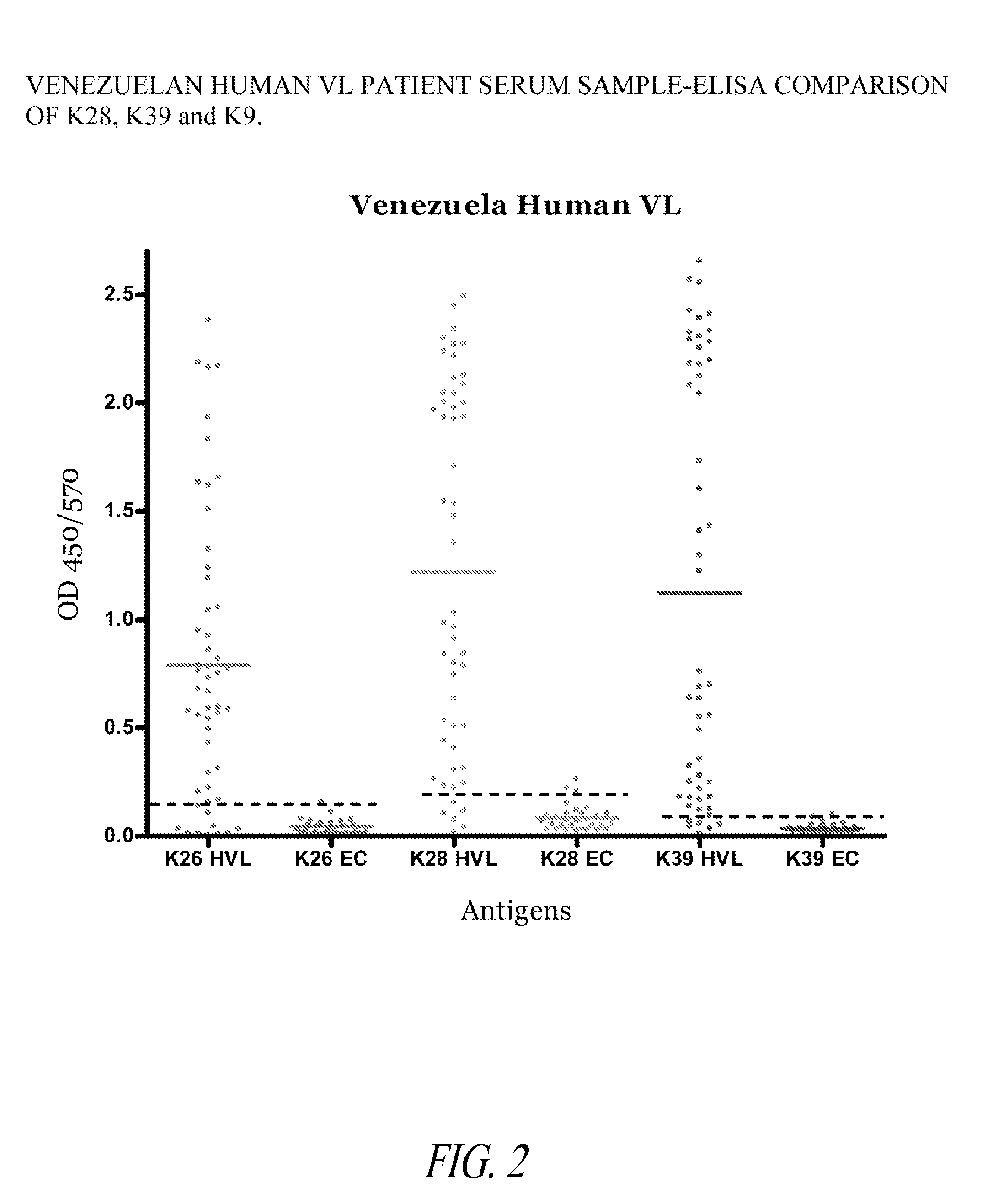
The present invention relates generally to a fusion protein made from a synthetic gene construct comprising of elements derived from the Leishmania antigens K26, K39, and K9. The fusion protein is particularly useful in the diagnosis of leishmaniasis, particularly visceral leishmaniasis in animals such as humans and dogs.
Owner:ACCESS TO ADVANCED HEALTH INST
Leishmania sterol 24-c-methyltransferase compositions for the prevention, treatment and diagnosis of leishmaniasis
ActiveUS20090041798A1Preventing and treating and detecting leishmaniasisProtozoa antigen ingredientsPeptide/protein ingredientsLeishmaniasisSterol
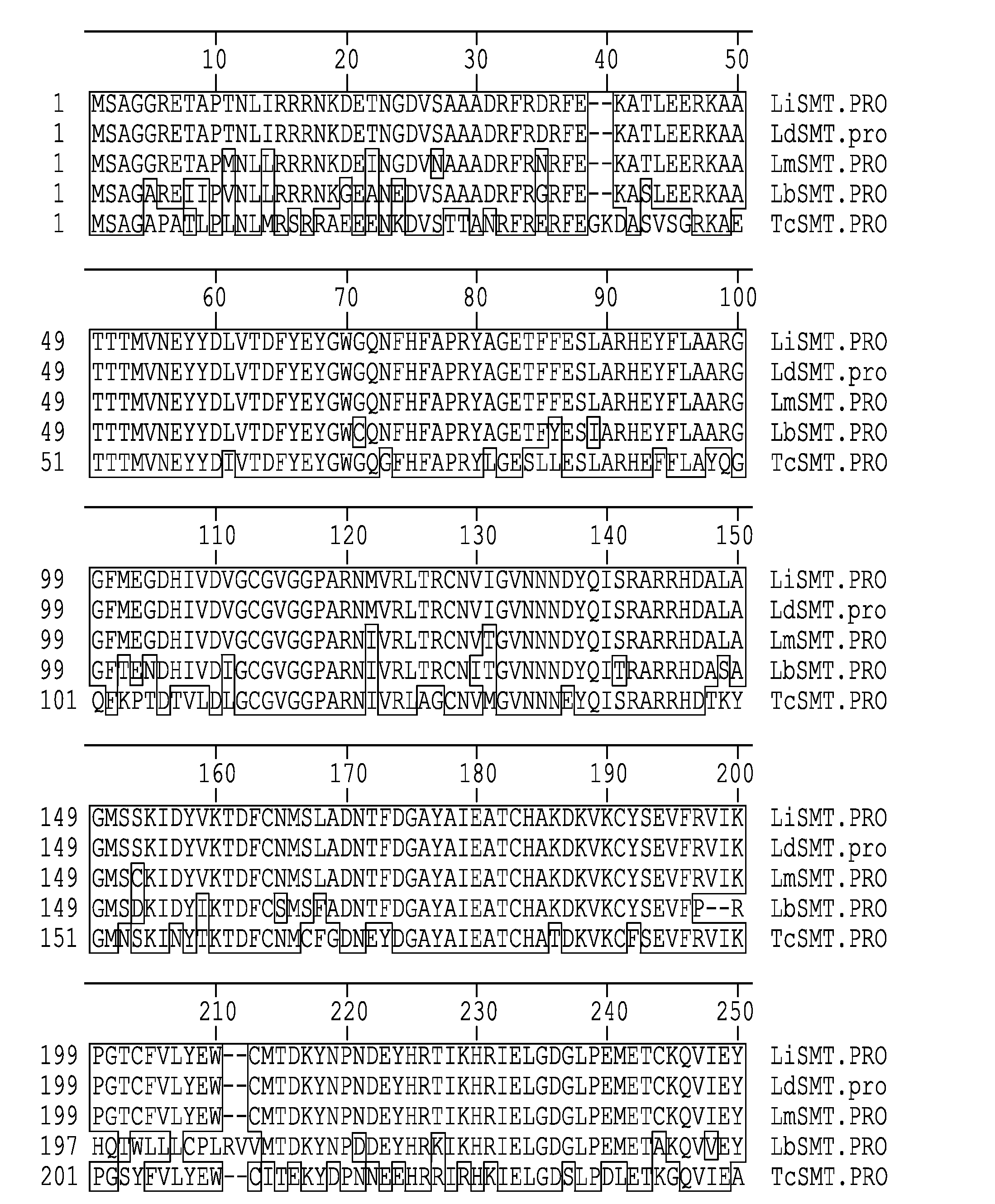
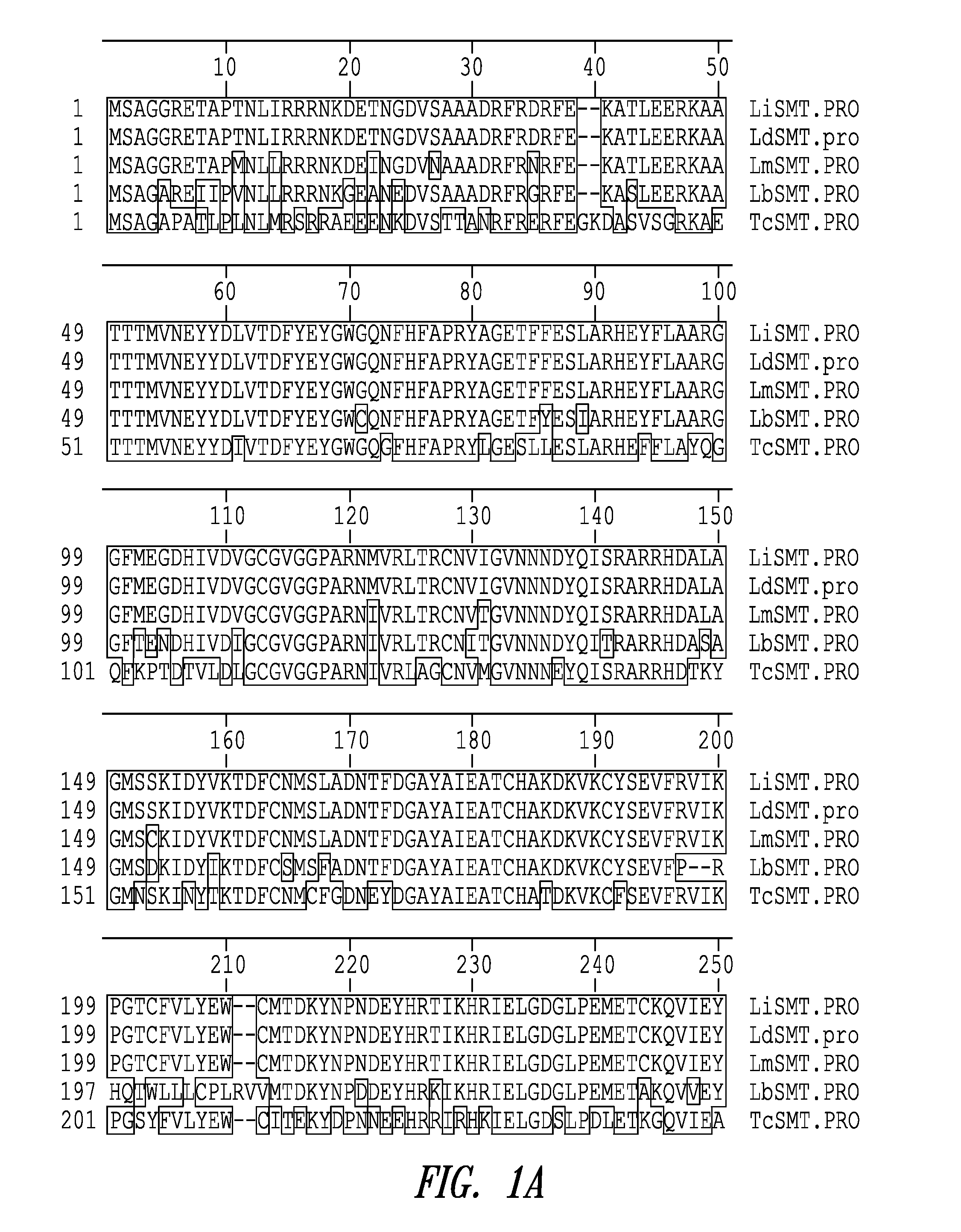
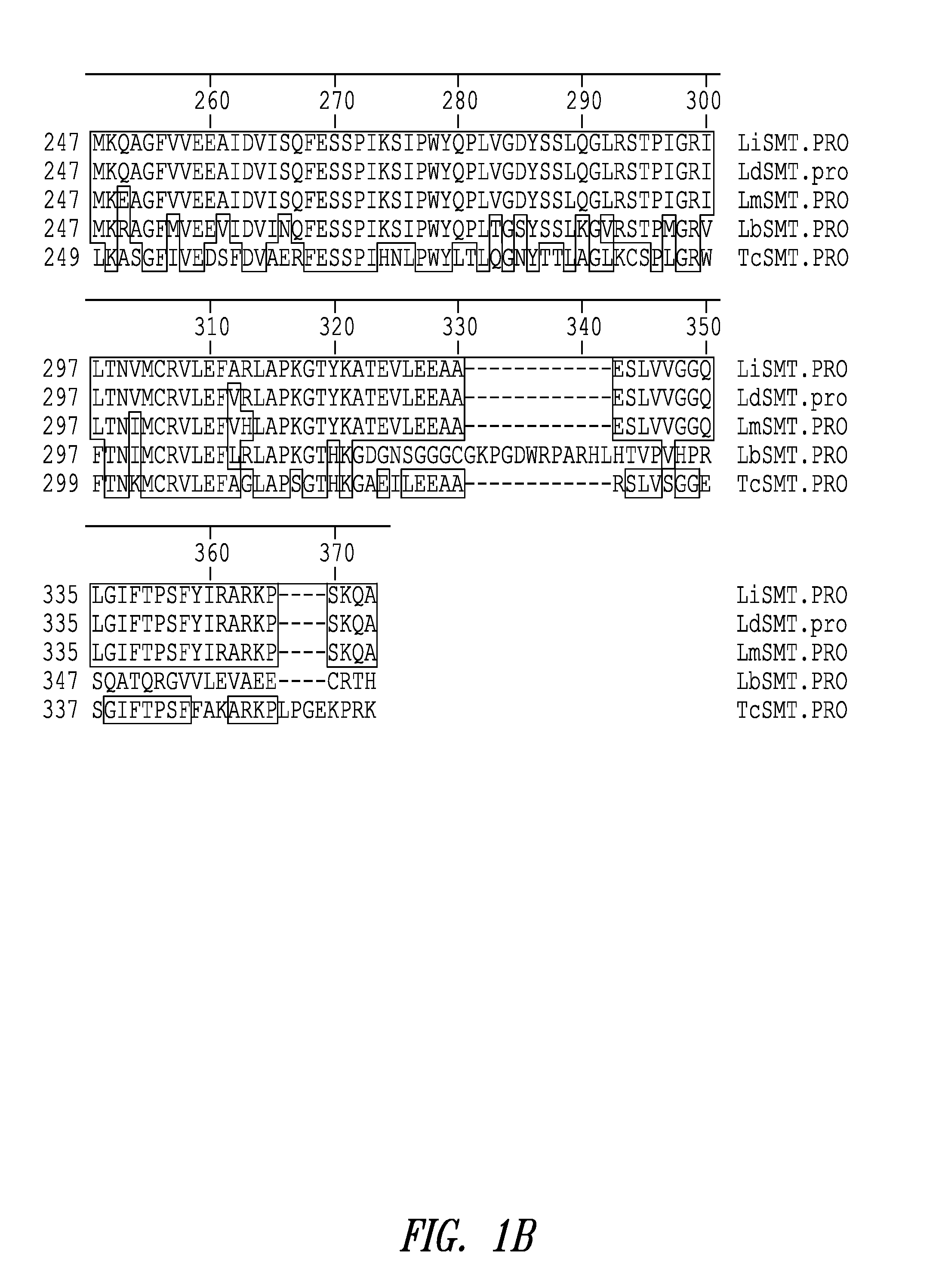
Compositions and methods for preventing, treating and detecting leishmaniasis are disclosed. The compositions generally comprise Leishmania sterol 24-c-methyltransferase (SMT) polypeptides, portions, variants and / or fusions, as well as polynucleotides encoding SMT polypeptides, portions, variants and / or fusions.
Owner:INFECTIOUS DISEASE RES INST
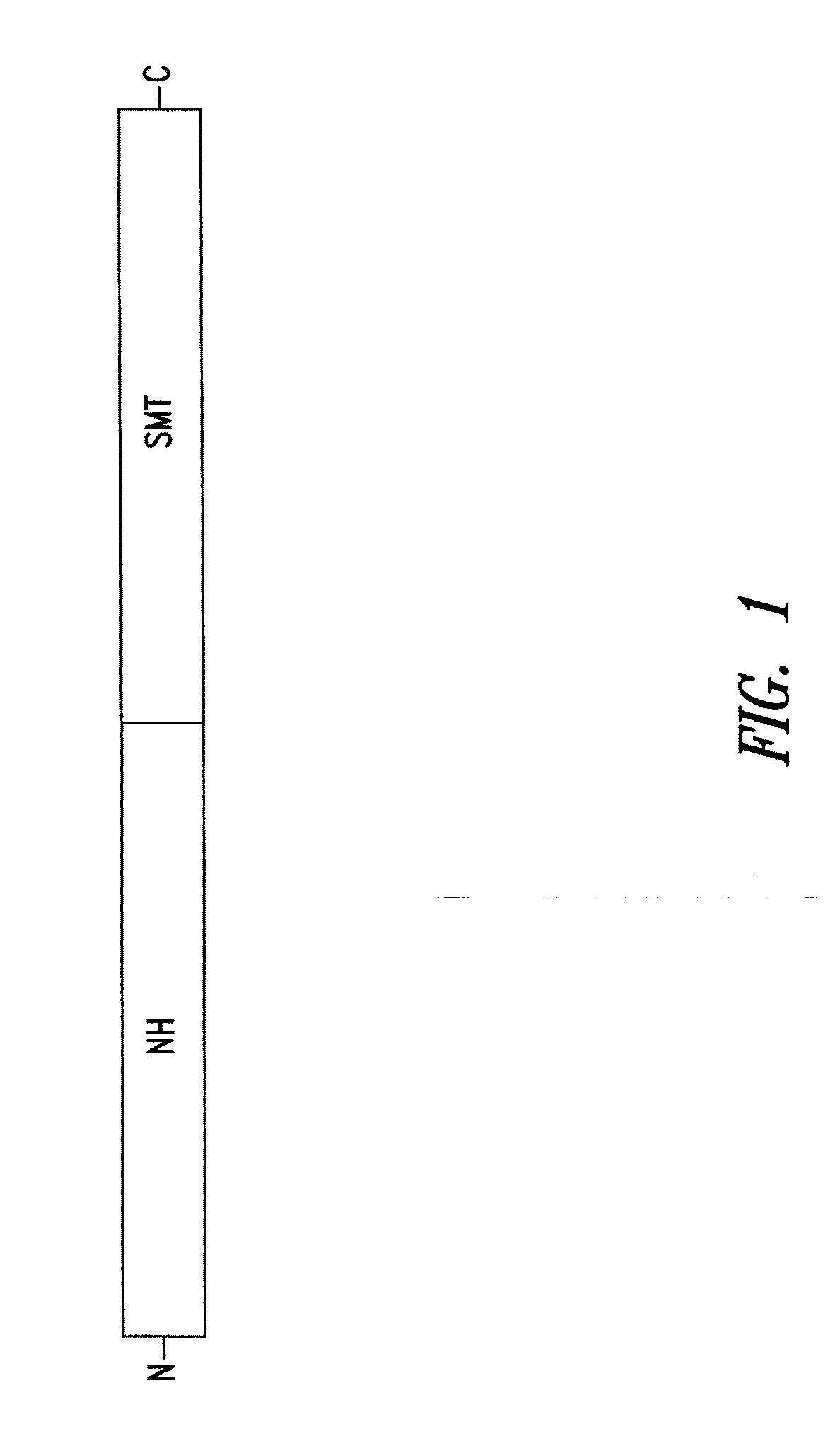
Vaccines comprising non-specific nucleoside hydrolase and sterol 24-c-methyltransferase (SMT) polypeptides for the treatment and diagnosis of leishmaniasis
InactiveUS20120114688A1Preventing and treating and detecting leishmaniasisAntibody mimetics/scaffoldsTransferasesLeishmaniaLeishmaniasis
Compositions and methods for preventing, treating and detecting leishmaniasis are disclosed. The compositions generally comprise fusion polypeptides comprising Leishmania antigens, in particular, SMT and NH antigens or immunogenic portions or variants thereof, as well as polynucleotides encoding such fusion polypeptides.
Owner:INFECTIOUS DISEASE RES INST
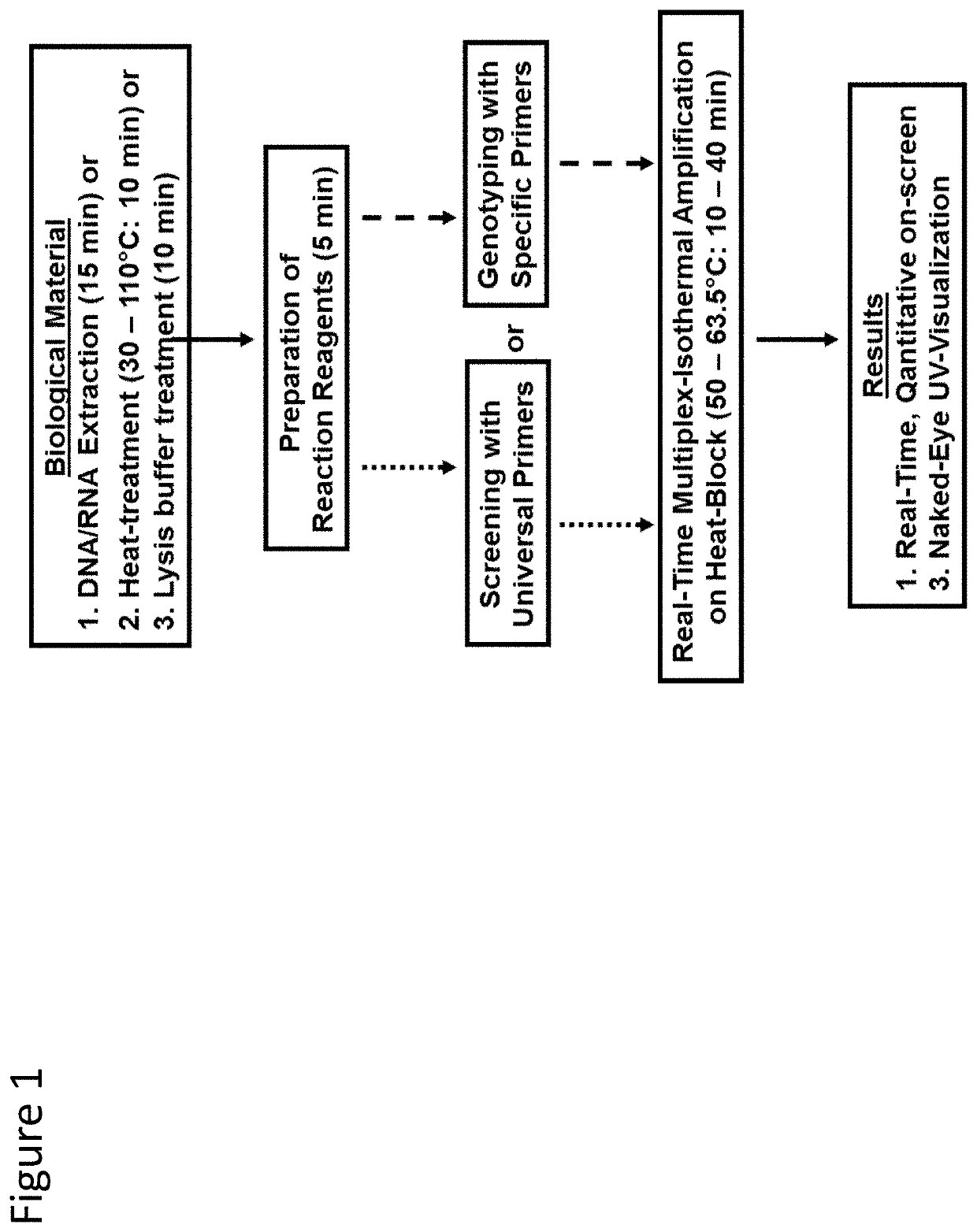
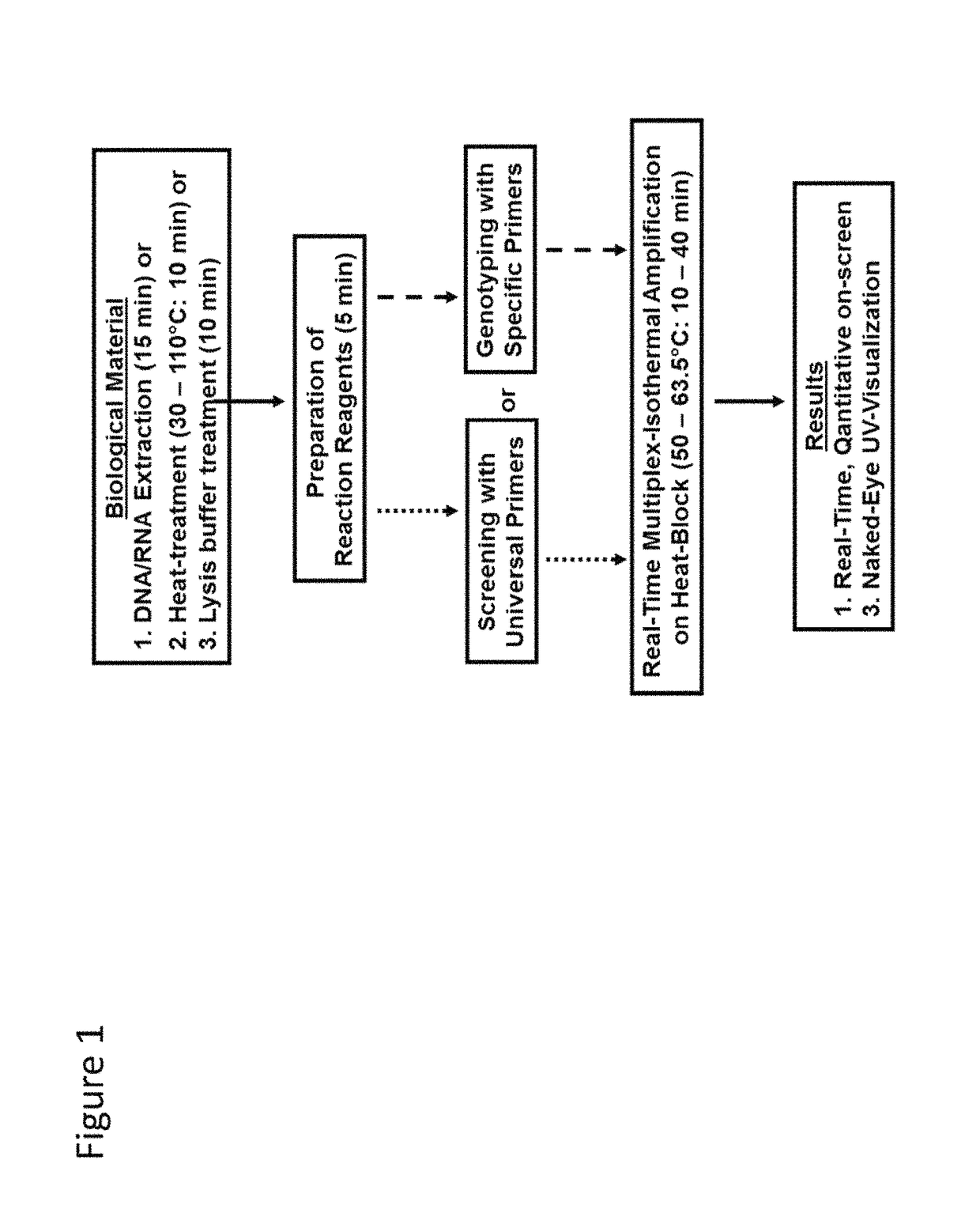
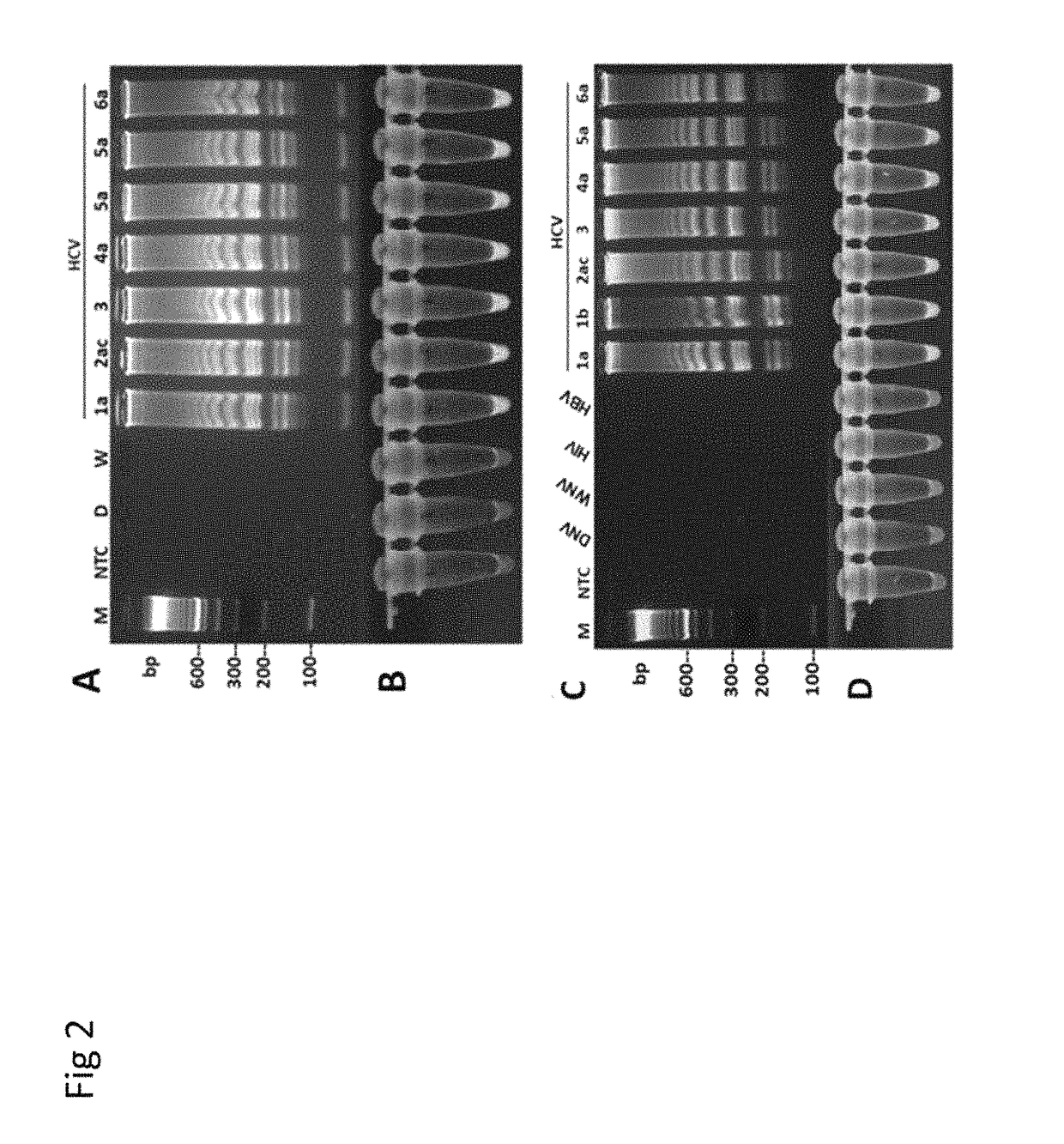
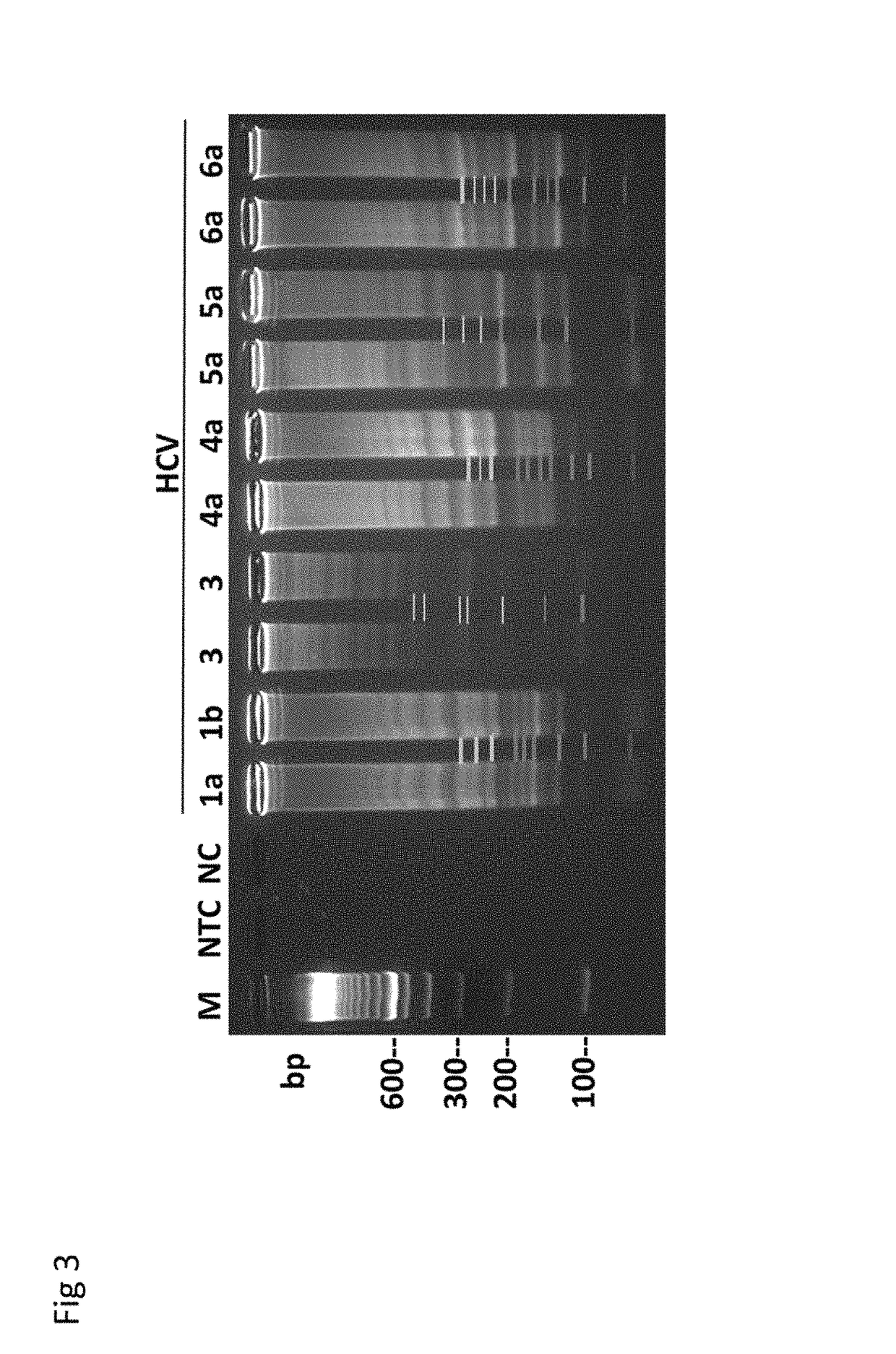
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS20200048722A1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaLoop-mediated isothermal amplification
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
Recombinant polyprotein vaccines for the treatment and diagnosis of leishmaniasis
Compositions and methods for preventing, treating and detecting leishmaniasis are disclosed. The compositions generally comprise fusion polypeptides comprising multiple Leishmania antigens, in particular, KMP11, SMT, A2 and / or CBP, or immunogenic portions or variants thereof, as well as polynucleotides encoding such fusion polypeptides.
Owner:ACCESS TO ADVANCED HEALTH INST
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS10072309B1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaFluorescence
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
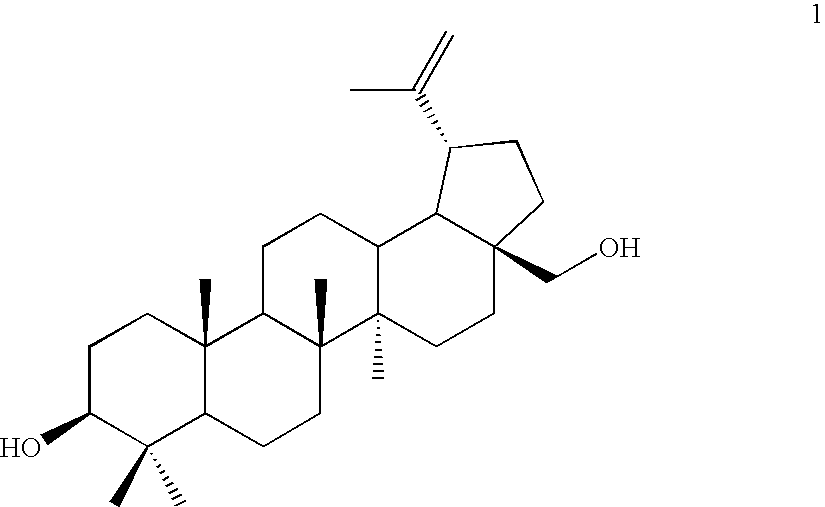
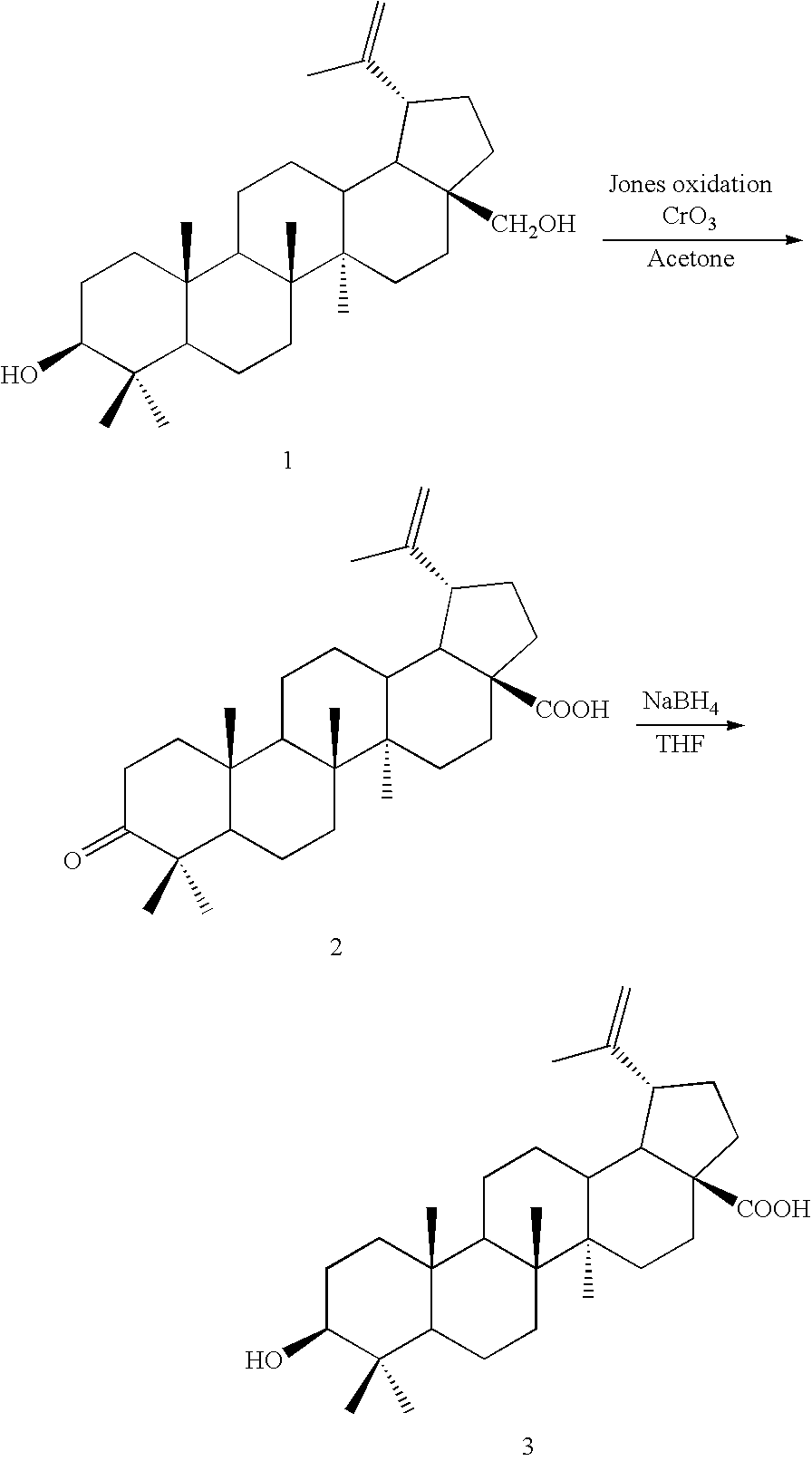
Betulin derived compounds useful as antiprotozoal agents
InactiveUS20100190795A1Improve solubilityLow toxicityAntibacterial agentsBiocideProtozoaLeishmaniasis
The invention relates to betulin derivatives, and to the use thereof as agents against protozoa of the genus Leishmania and against leishmaniasis in applications of pharmaceutical industry.
Owner:VALTION TEKNILLINEN TUTKIMUSKESKUS
Vaccine complex for preventing or treating leishmaniases
InactiveUS20060073170A1Facilitates emergenceInhibition of differentiationProtozoa antigen ingredientsAntiparasitic agentsSerum free mediaMammal
A therapeutic vaccine complex for preventing or treating leishmaniases and infections mediated by intracellular pathogenic micro-organism in mammals and in particular in humans, members of the dog, car and horse family. The invention is characterized in that it includes excretion secretion molecules derived from Leishmania sp. Promastigotes produced in a specific germ-free and serum-free medium.
Owner:BIO VETO TESTS BVT
Immunochromatographic strip for detecting viscerotropic leishmania infection and diagnosing kala-azar
InactiveCN102590508AIncreased sensitivityImprove featuresProtozoa antigen ingredientsAntiparasitic agentsCelluloseLeishmania Infections
The invention discloses an immunochromatographic strip for detecting viscerotropic leishmania infection and diagnosing kala-azar, which comprises a sample pad, a conjugate pad which is closely connected to the sample pad and contains a colloidal gold-labeled probe, a cellulose membrane closely connected with the conjugate pad and a water absorption pad closely connected to the cellulose membrane. The water absorption pad is far away from the conjugate pad, a quality control line is arranged at one end of the cellulose membrane, which is close to the water absorption pad, a detection line is positioned on the cellulose membrane between the quality control line and the conjugate pad, the detection line contains soluble antigen of the viscerotropic leishmania, and the quality control line contains antibodies specifically binding to the colloidal gold-labeled probe. The invention further provides a kit containing the immunochromatographic strip and a preparation method for the immunochromatographic strip. The immunochromatographic strip has the advantages of being simple and convenient to use, high in sensitivity and specificity, fast in detection, and simultaneously applicable to clinical and field use for human, livestock and wild animals.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Compounds and Methods for Diagnosis and Treatment of Leishmaniasis
Compounds and methods are provided for diagnosing, preventing, treating and detecting leishmaniasis infection and stimulating immune responses in patients are disclosed. The compounds disclosed are include polypeptides and fusion proteins that contain at least one immunogenic portion of one or more Leishmania antigens, or a variant thereof. Additionally, methods of screening a screening library for tandem repeat proteins that have immunogenic properties are disclosed. Vaccines and pharmaceutical compositions comprising polynucleotides, polypeptides, fusion proteins and variants thereof that may be used for the prevention and therapy of leishmaniasis, as well as for the detection of Leishmaniasis infection are described.
Owner:INFECTIOUS DISEASE RES INST
Vaccine formulations for leishmania
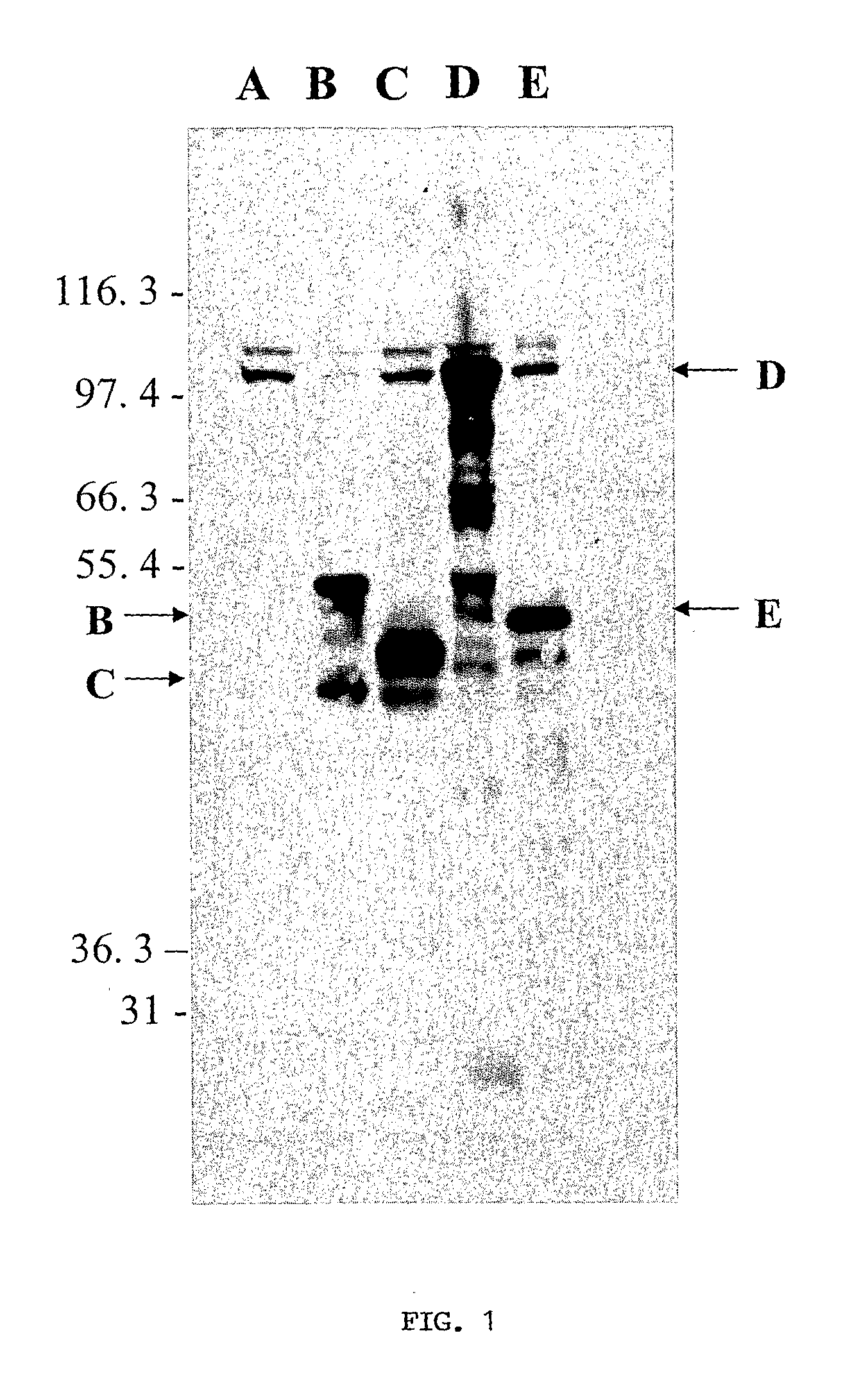
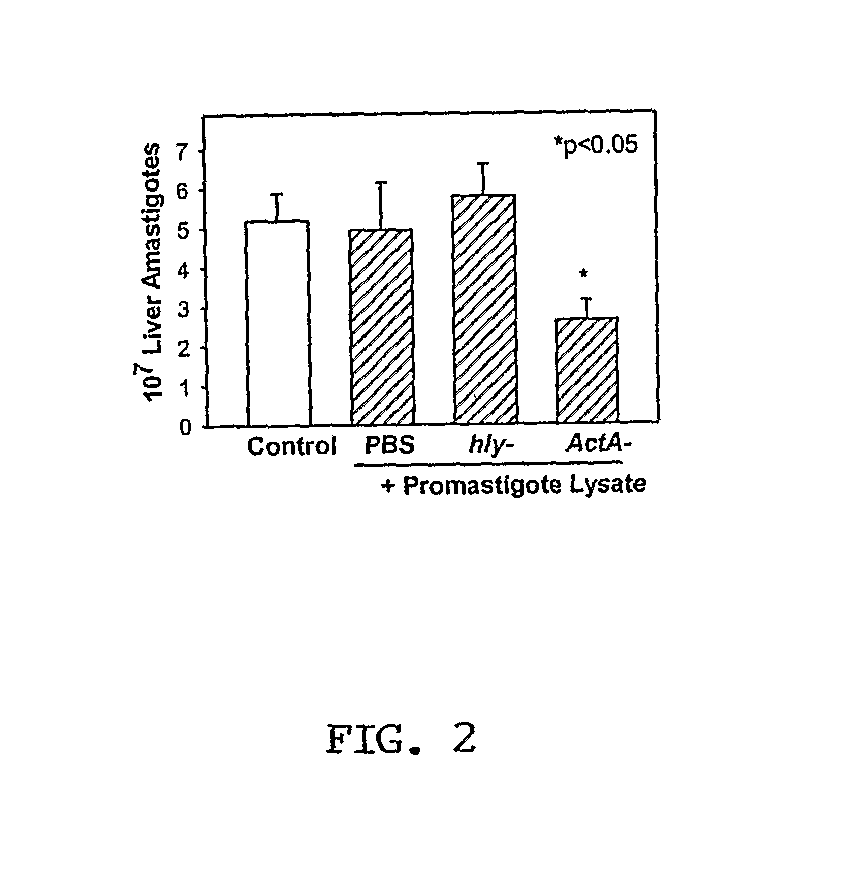
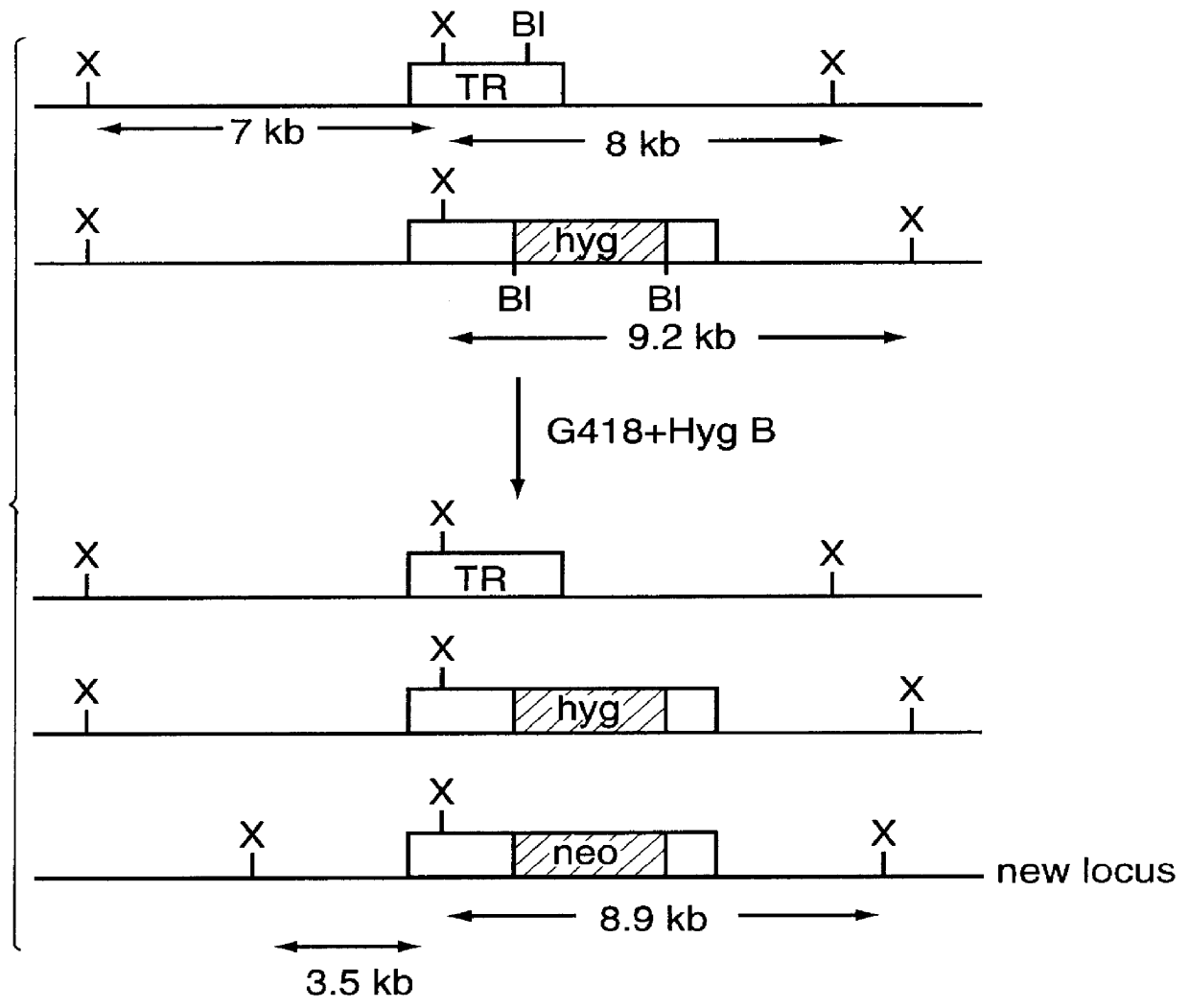
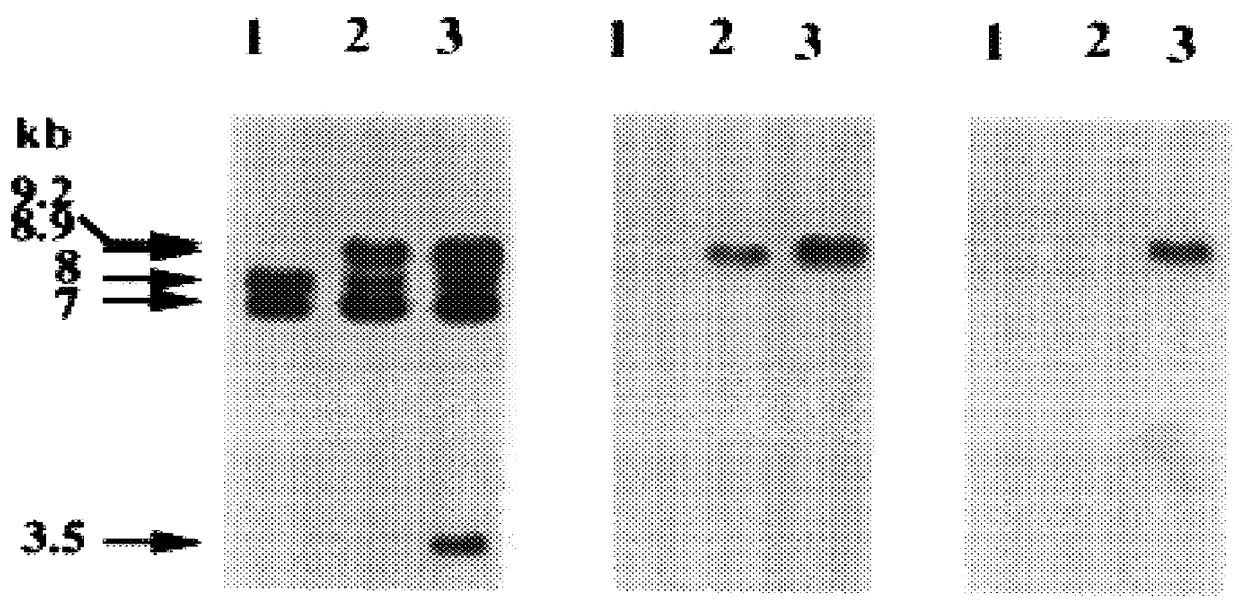
The present invention involves the use of Leishmania antigens and nucleic acids coding therefor as vaccines. It also provides a method of inducing protective immune responses in a subject through the use of specific delivery systems such as protein and / or DNA vaccines and Listeria monocytogenes vectors.
Owner:UNIV OF IOWA RES FOUND +1
Attenuated strains of leishmania and uses thereof
InactiveUS6162638ALow RNA levelReduce the number of copiesBiocideProtozoaDiseaseVirulent characteristics
Attenuated strains of Leishmania are provided in which at least one gene contributing to virulence of the strain and expressed in both the promastigote and amastigote forms of the strain is functionally disabled, such as, by deleting at least a portion of the gene or by mutagenesis of the gene. The attenuated strain may be used for administration to a host to confer protection against disease caused by a virulent Leishmania strain or as a diagnostic reagent.
Owner:UNIV LAVAL
Leishmania challenge model
Owner:MERIAL INC
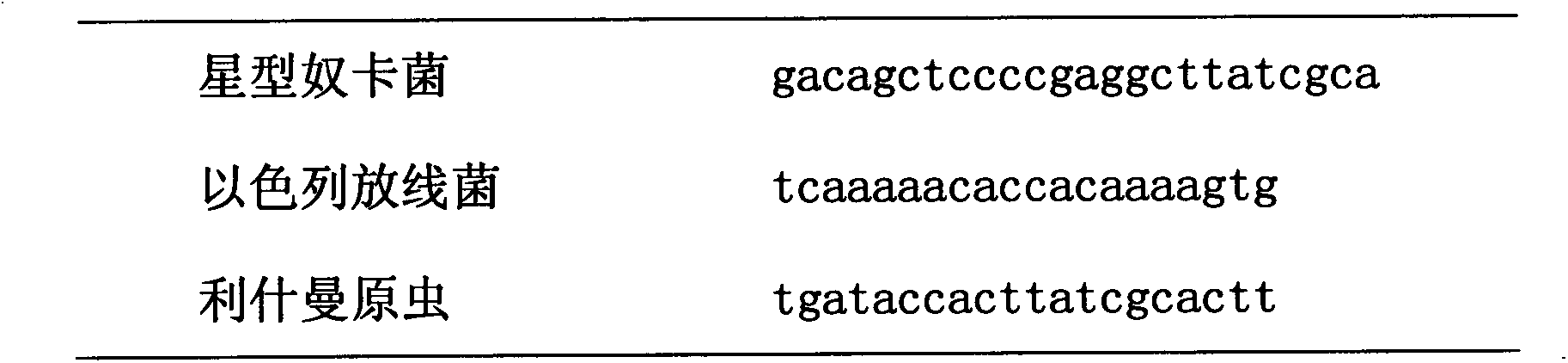
Leishmania antigens suitable for a diagnostic kit of Leishmania
A purified Leishmania infantum polypeptide comprising at least 10 consecutive amino acids of a protein is provided. The protein is mitochondrial integral ADP / ATP carrier protein, NADH-cytochrome b5 reductase, mitochondrial carrier protein, guanine nucleotide binding protein beta subunit (LACK), aldehyde reductase, ubiquinol-cytochrome-c reductase Rieske iron-sulfur protein, truncated elongation factor 1-alpha having a molecular weight as determined by SDS-PAGE of 36.4 kDa, truncated elongation factor 1-alpha having a molecular weight as determined by SDS-PAGE of 34.5 kDa, or truncated elongation factor 1-alpha having a molecular weight as determined by SDS-PAGE of 30.6 kDa. Methods for using the polypeptides for diagnosing MVL are disclosed.
Owner:INST PASTEUR
Recombinant polyprotein vaccines for the treatment and diagnosis of leishmaniasis
Compositions and methods for preventing, treating and detecting leishmaniasis are disclosed. The compositions generally comprise fusion polypeptides comprising multiple Leishmania antigens, in particular, KMP11, SMT, A2 and / or CBP, or immunogenic portions or variants thereof, as well as polynucleotides encoding such fusion polypeptides.
Owner:ACCESS TO ADVANCED HEALTH INST
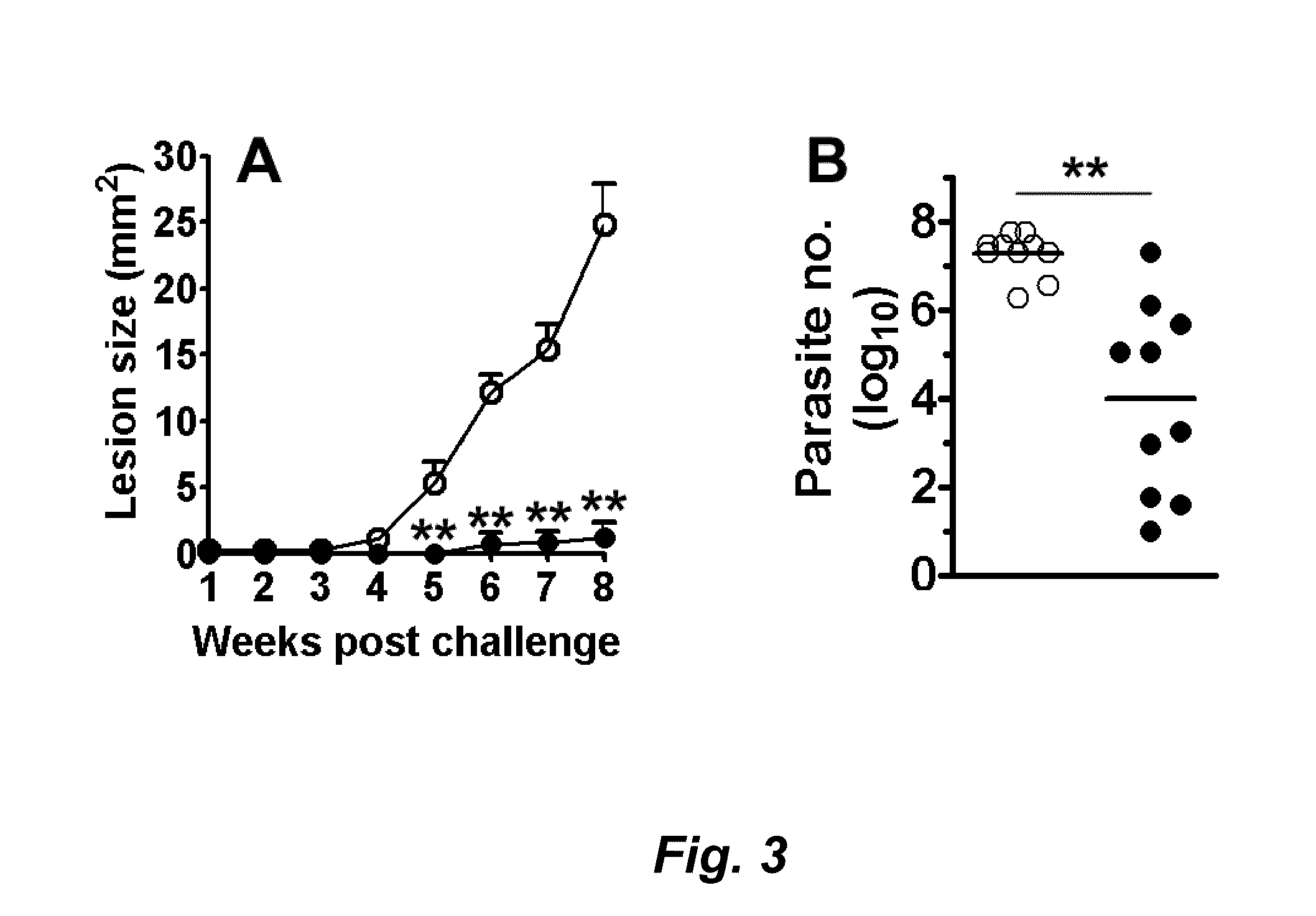
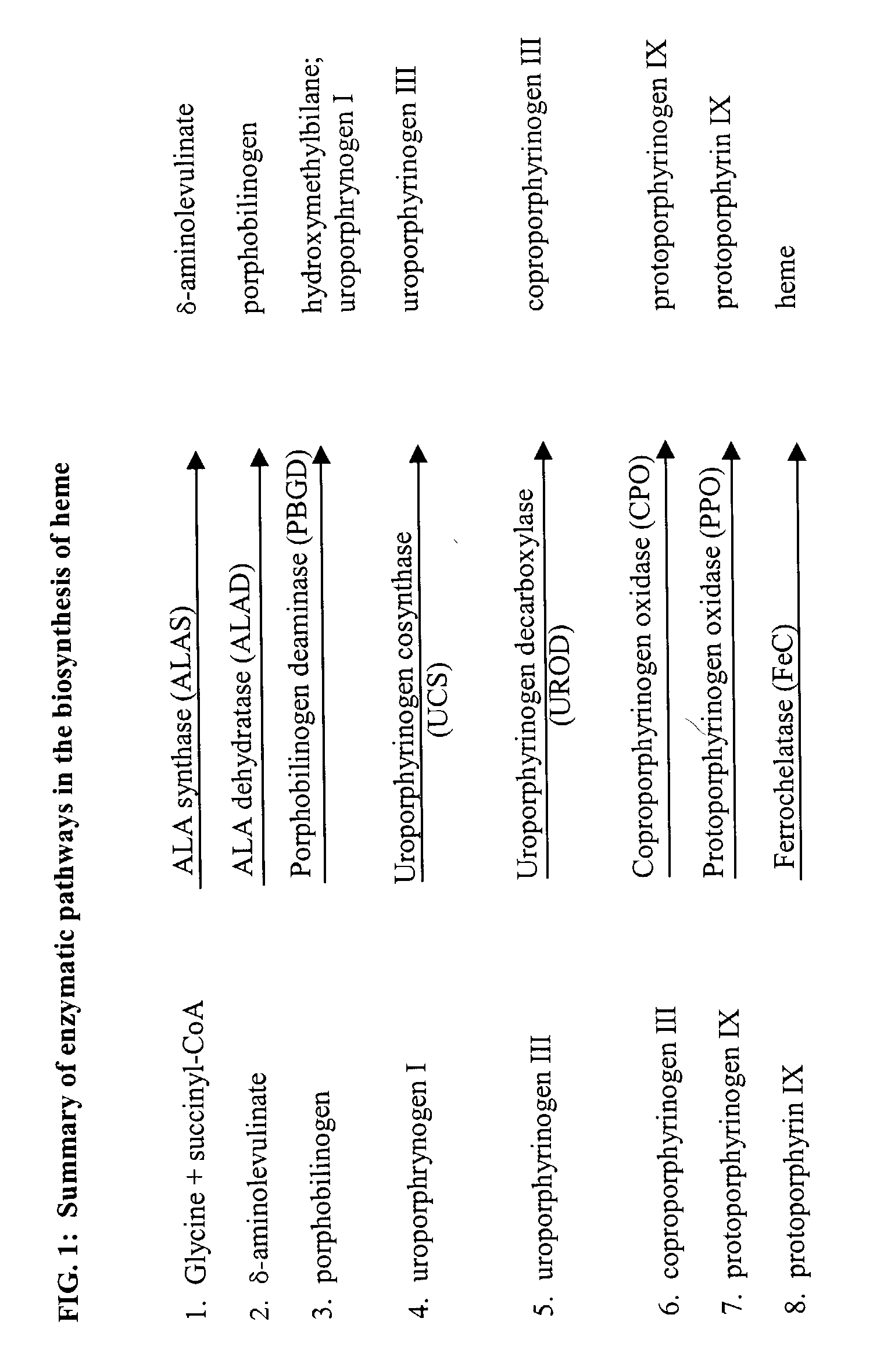
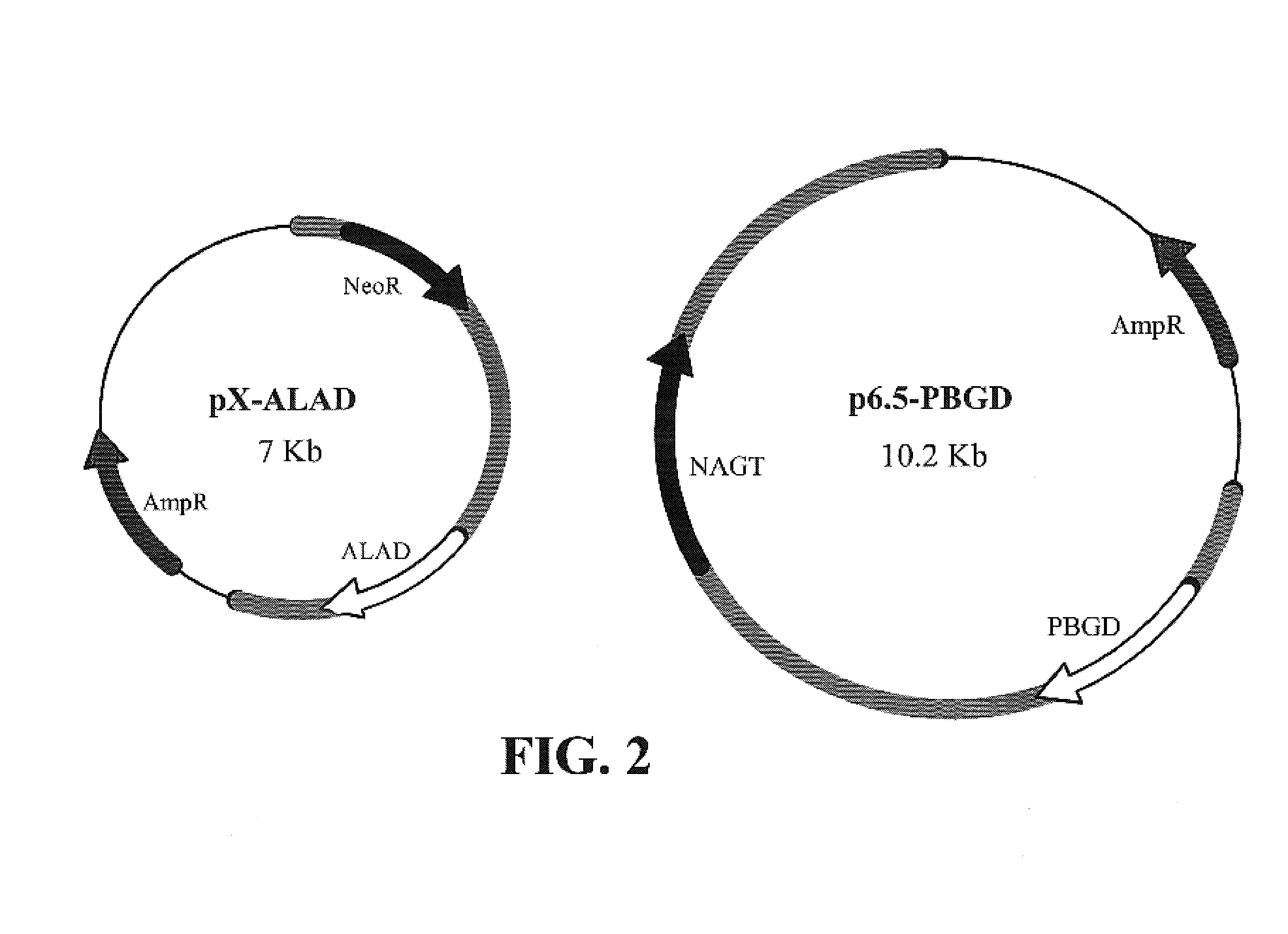
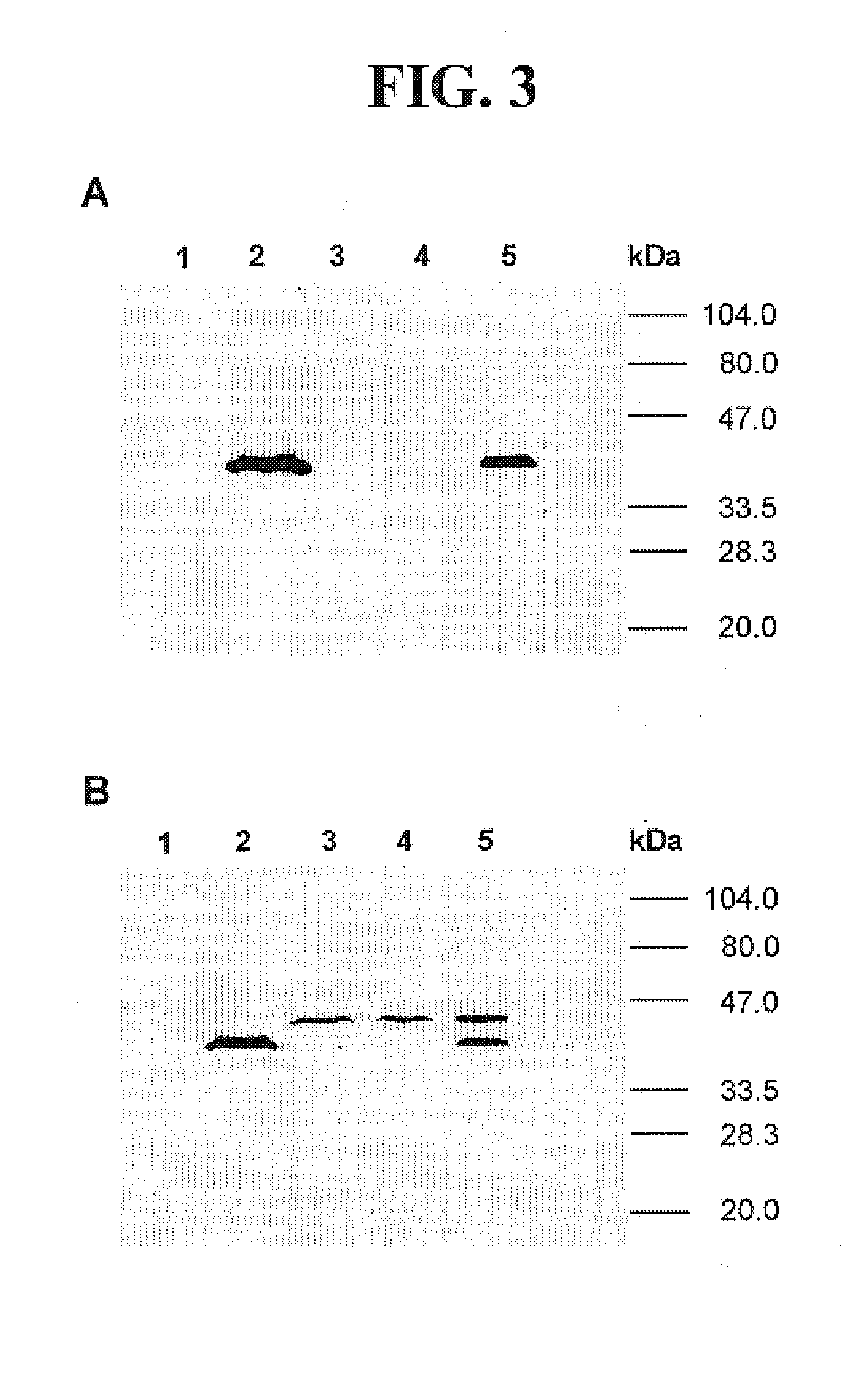
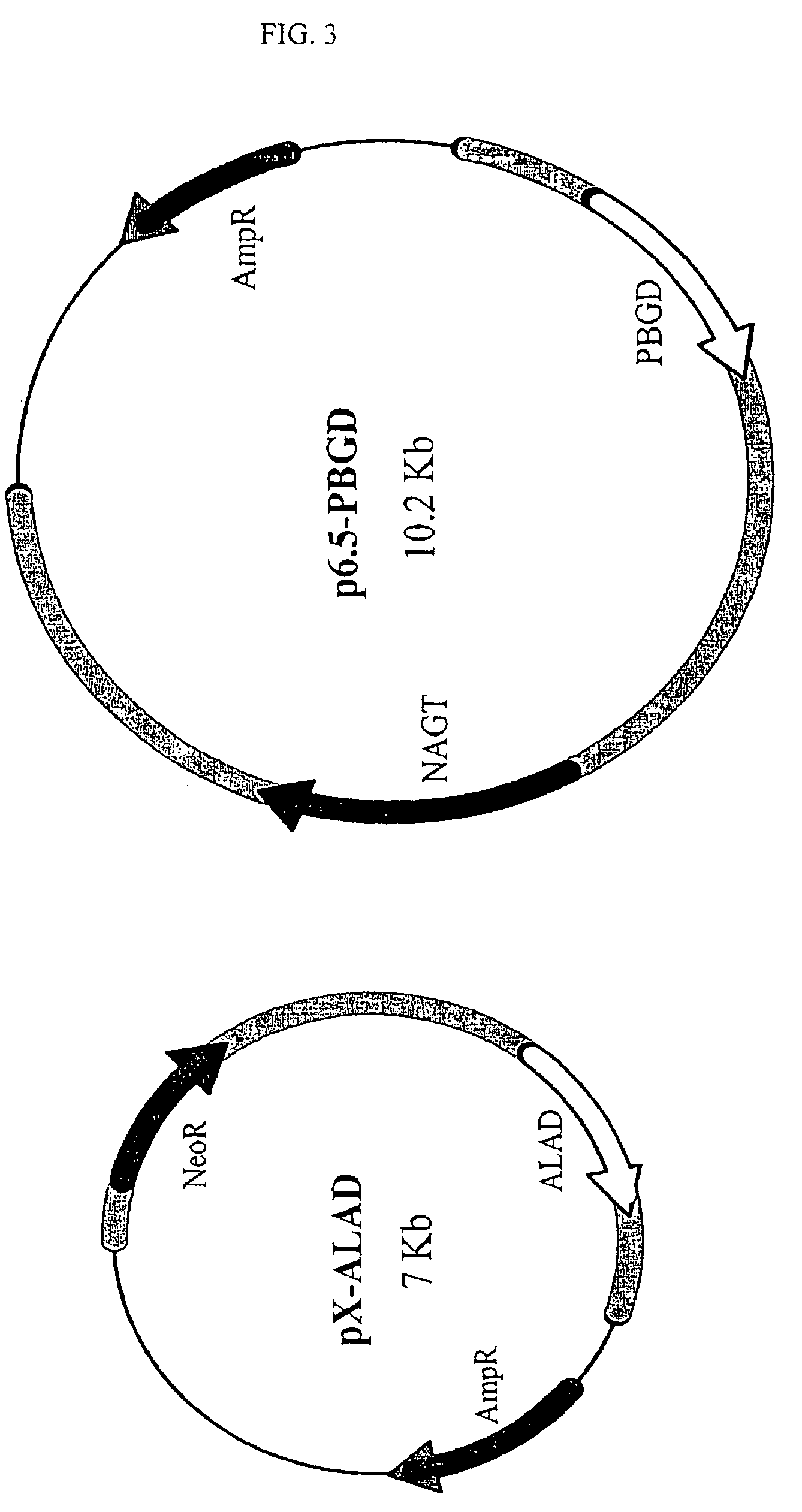
Leishmania as carriers for the delivery of proteins and peptides
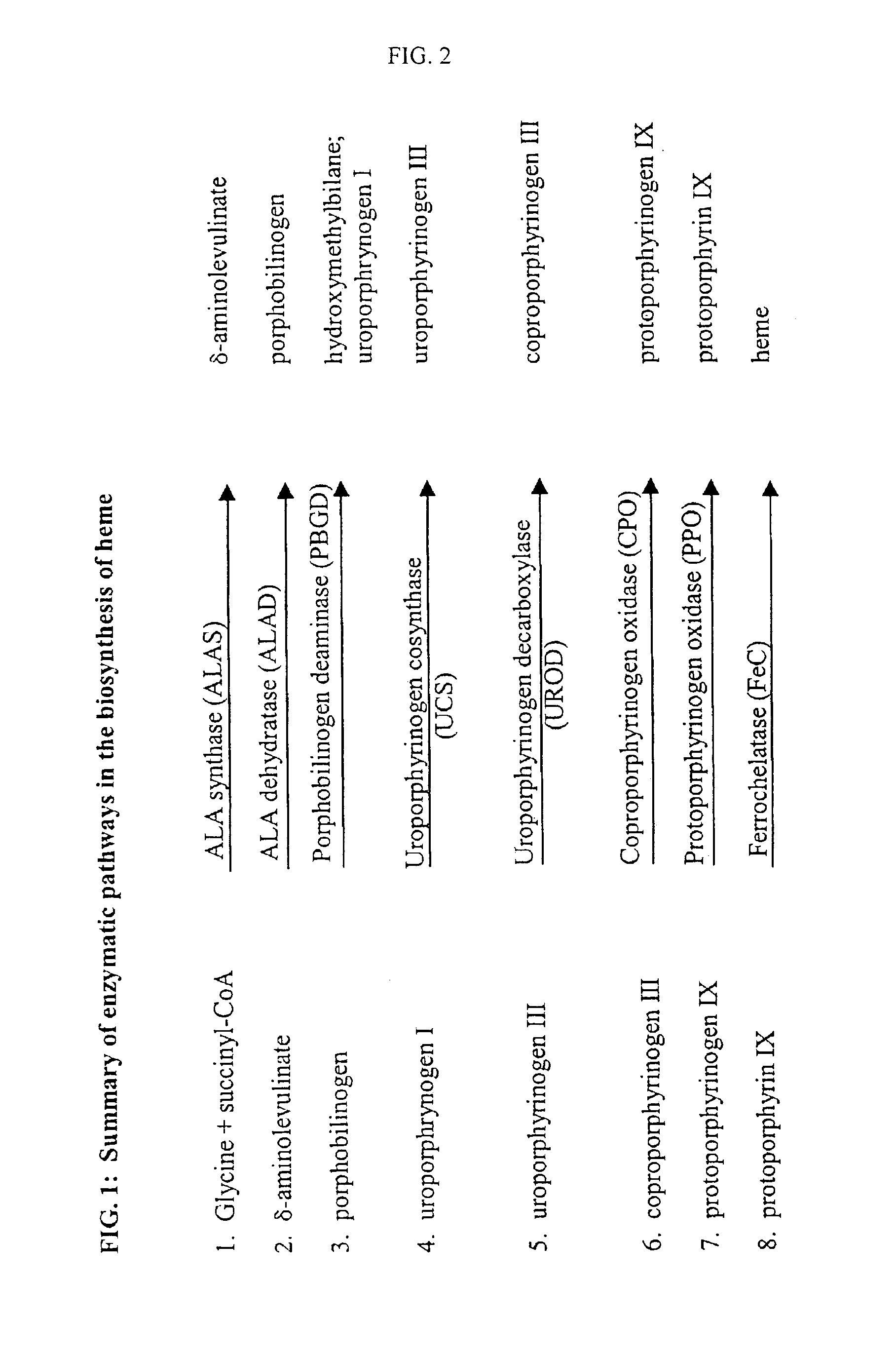
Methods for delivering potentially therapeutic or prophylactic protein and peptide agents to mammalian cells are provided. The agents are delivered by mutant trypanosomatid protozoa that have been genetically manipulated to code for such protein or peptide agents. The mutant protozoa additionally lack certain enzymes within the heme biosynthetic pathway, making the mutants susceptible to porphyria and eventual lysis.
Owner:ROSALIND FRANKLIN UNIVERSITY OF MEDICINE AND SCIENCE
Composition containing leishmania lip2a
A composition and method for stimulating an immune response against an antigen in immunised individuals or in cell groups. The composition comprises a protein called Lip2a Leishmania formed by a sequence of amino acids coded for by a sequence of DNA that comprises the nucleotides: 1-1 of nos. 778 to 1231 of SEQ. ID NO!1-2 or formed by a sequence of nucleotides able to hybridise with the sequence described in 1-1 under moderately strict hybridisation conditions and which would therefore code for a protein similar to Lip2a. The method comprises incubating said cells with said Lip2a protein in the presence or not of an antigen specific to a disease or infection.
Owner:C B F LETI SL
Compounds and methods for diagnosis and treatment of leishmaniasis
Compounds and methods are provided for diagnosing, preventing, treating and detecting leishmaniasis infection and stimulating immune responses in patients are disclosed. The compounds disclosed are include polypeptides and fusion proteins that contain at least one immunogenic portion of one or more Leishmania antigens, or a variant thereof. Additionally, methods of screening a screening library for tandem repeat proteins that have immunogenic properties are disclosed. Vaccines and pharmaceutical compositions comprising polynucleotides, polypeptides, fusion proteins and variants thereof that may be used for the prevention and therapy of leishmaniasis, as well as for the detection of Leishmaniasis infection are described.
Owner:INFECTIOUS DISEASE RES INST
Attenuated strains of leishmania and uses thereof
Attenuated strains of Leishmania are provided in which at least one gene contributing to virulence of the strain and expressed in both the promastigote and amastigote forms of the strain is functionally disabled, such as, by deleting at least a portion of the gene or by mutagenesis of the gene. The attenuated strain may be used for administration to a host to confer protection against disease caused by a virulent Leishmania strain or as a diagnostic reagent.
Owner:AVENTIS PASTEUR LTD
DNA vaccine as immunoprophylaxis against kala-azar
ActiveUS20080145385A1Reduce decreaseEfficient administrationProtozoa antigen ingredientsGenetic material ingredientsLeishmaniaVisceral leishmaniasis
A highly conserved membrane protein present in all species of Leishmania can be used as a vaccine antigen for genetic immunization against visceral leishmananiasis.
Owner:COUNCIL OF SCI & IND RES
Fusion proteins and their use in the diagnosis and treatment of leishmaniasis
ActiveUS20100008924A1Treatment, and prevention of leishmaniasisOrganic active ingredientsPeptide/protein ingredientsLeishmaniaVisceral leishmaniasis
The present invention relates generally to a fusion protein made from a synthetic gene construct comprising of elements derived from the Leishmania antigens K26, K39, and K9. The fusion protein is particularly useful in the diagnosis of leishmaniasis, particularly visceral leishmaniasis in animals such as humans and dogs.
Owner:ACCESS TO ADVANCED HEALTH INST
Compounds and methods for diagnosis and treatment of leishmaniasis
Compounds and methods are provided for diagnosing, preventing, treating and detecting leishmaniasis infection and stimulating immune responses in patients are disclosed. The compounds disclosed are include polypeptides and fusion proteins that contain at least one immunogenic portion of one or more Leishmania antigens, or a variant thereof. Additionally, methods of screening a screening library for tandem repeat proteins that have immunogenic properties are disclosed. Vaccines and pharmaceutical compositions comprising polynucleotides, polypeptides, fusion proteins and variants thereof that may be used for the prevention and therapy of leishmaniasis, as well as for the detection of Leishmaniasis infection are described.
Owner:INFECTIOUS DISEASE RES INST IN
Probe and kit for common pathogens detection of skin infectious granuloma
InactiveCN103409502AImprove diagnostic efficiencyImprove treatment efficiencyMicrobiological testing/measurementAgainst vector-borne diseasesFonsecaea pedrosoiInfective granuloma
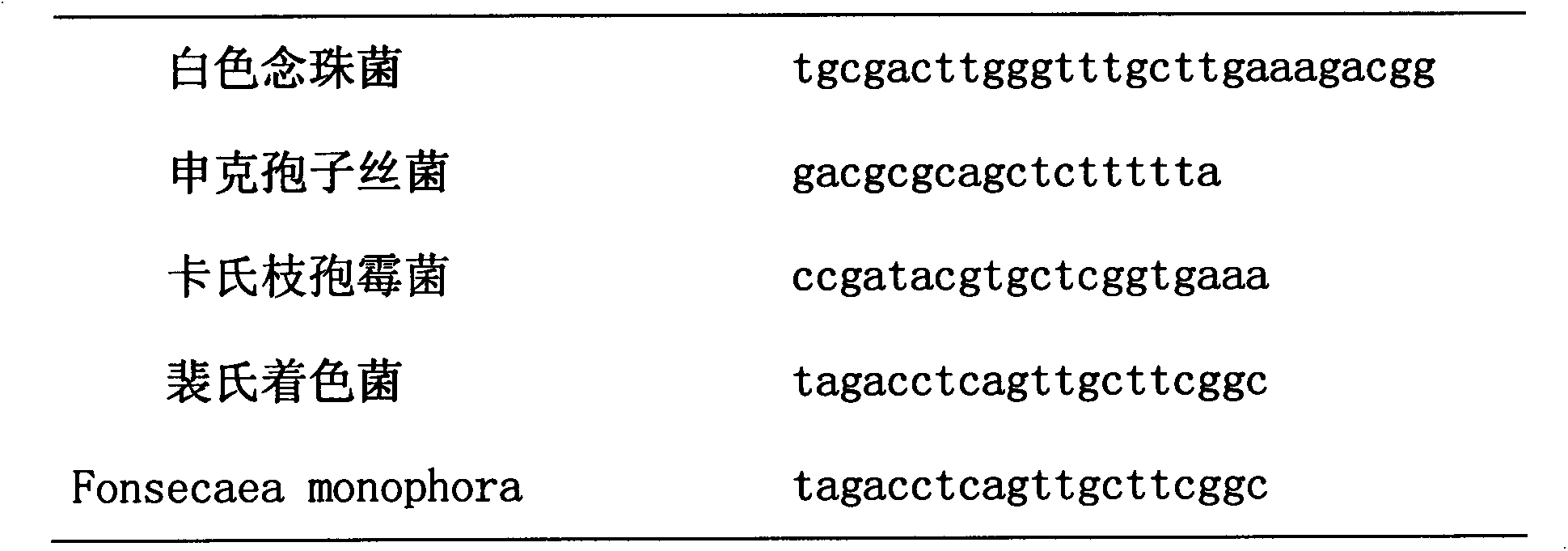
The invention discloses a probe and a kit for the common pathogens detection of skin infectious granuloma. The probe comprises a mycobacterium probe, a mycobacterium tuberculosis probe, a mycobacterium leprae probe, a mycobacterium avium probe, a mycobacterium intracellulare probe, a mycobacterium kansasii probe and a mycobacterium fortuitum probe, a candida albicans probe, a sporotrichum schenckii probe, a cladosporium trichoides probe, a Fonsecaea pedrosoi probe, and a Fonsecaea monophora probe; and a nocardia probe, an actinomycetes probe and a leishmania probe. The nucleotide sequence of each probe is shown in sequence tables SEQ ID NO.1-SEQ ID NO.15; and the kit comprises the probes and corresponding universal primers. According to the invention, through designing and optimizing the kit for carrying out pathogens screening and detection on common skin infectious granuloma, the improvement of the clinical diagnosis and the treatment efficiency is facilitated.
Owner:王洪生 +1
Suicidal mutant Leishmania vaccine
Owner:ROSALIND FRANKLIN UNIVERSITY OF MEDICINE AND SCIENCE
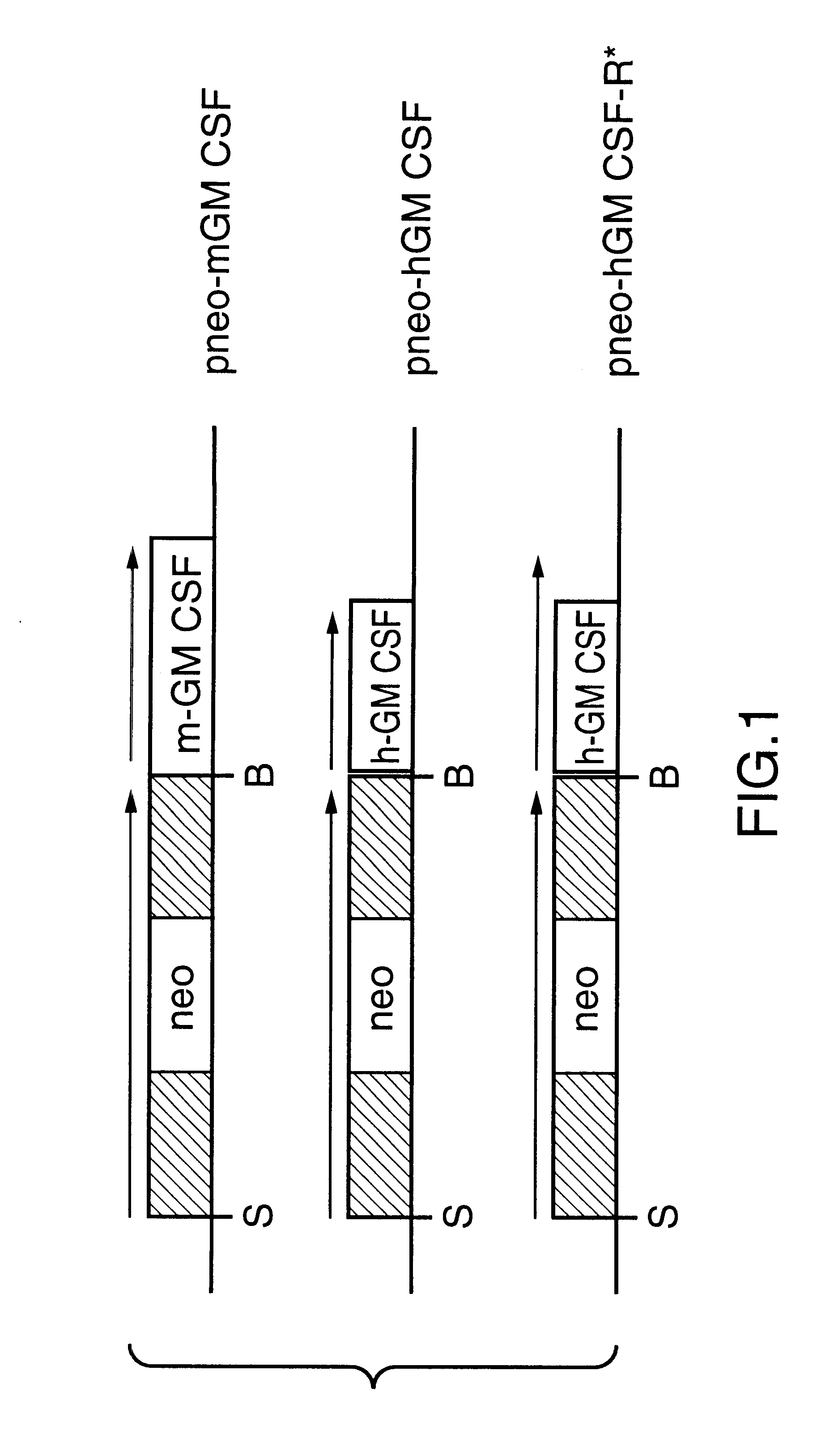
Macrophage-infecting parasites expressing a granulocyte macrophage colony stimulating factor
InactiveUS6331304B1Reduced viabilityFacilitated releaseProtozoa antigen ingredientsProtozoaVirulent characteristicsLeishmania
Strains of Leishmanda and other macrophage-infecting parasites are provided which express the GM-CSF gene which are useful in treating hosts infected by the parasite and in protecting hosts against disease caused by infection of hosts by parasites. The parasites are reduced in their ability to infect or survive in macrophages and hence are attenuated. At least one gene of the parasite contributing to the virulence thereto may be functionally disabled. The attenuated strains may be used for administration to a host (a) to treat a host infected by Leishmania or (b) to confer protection against disease caused by a virulent Leishmania strain, or as a diagnostic reagent.
Owner:UNIV LAVAL
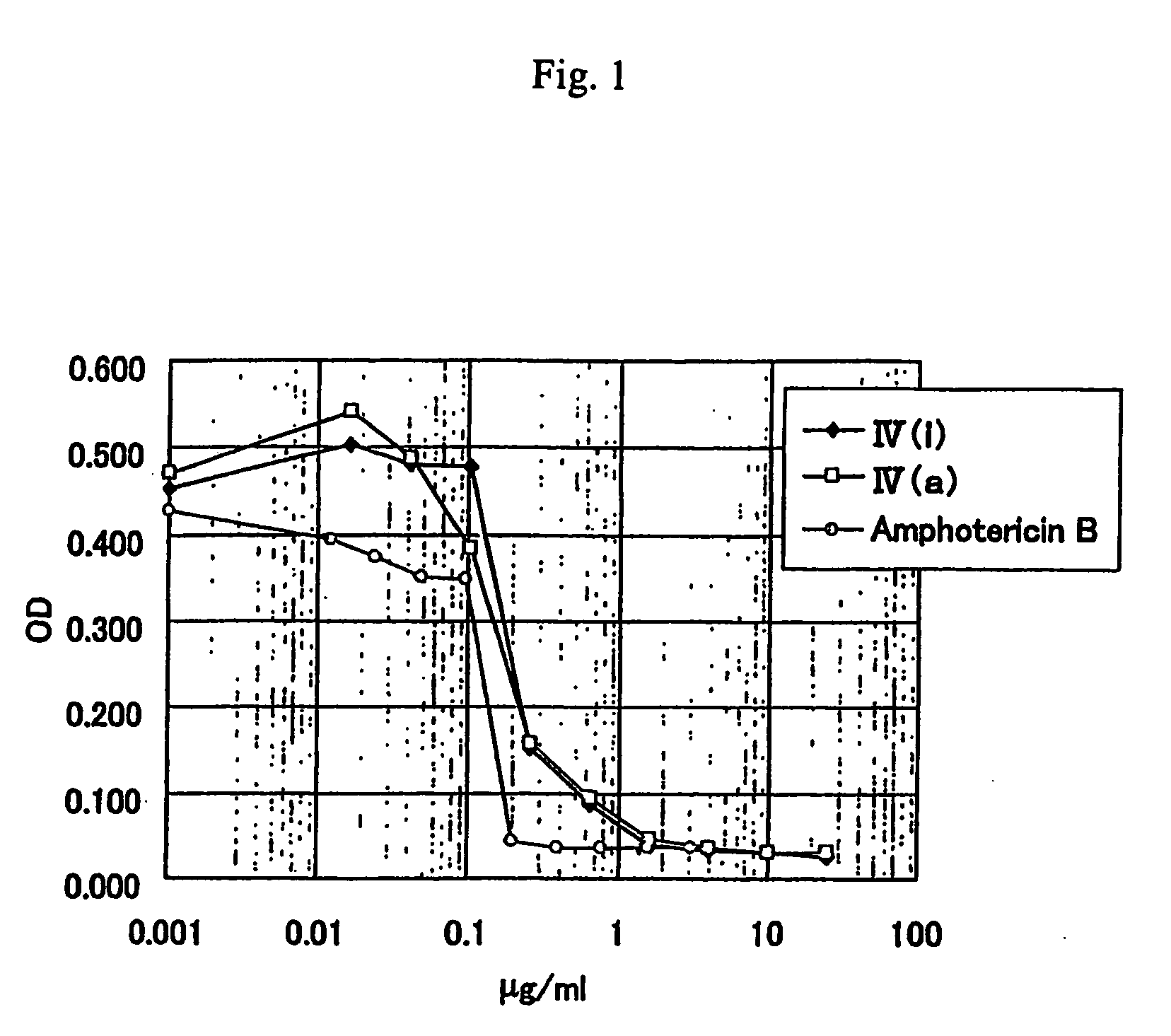
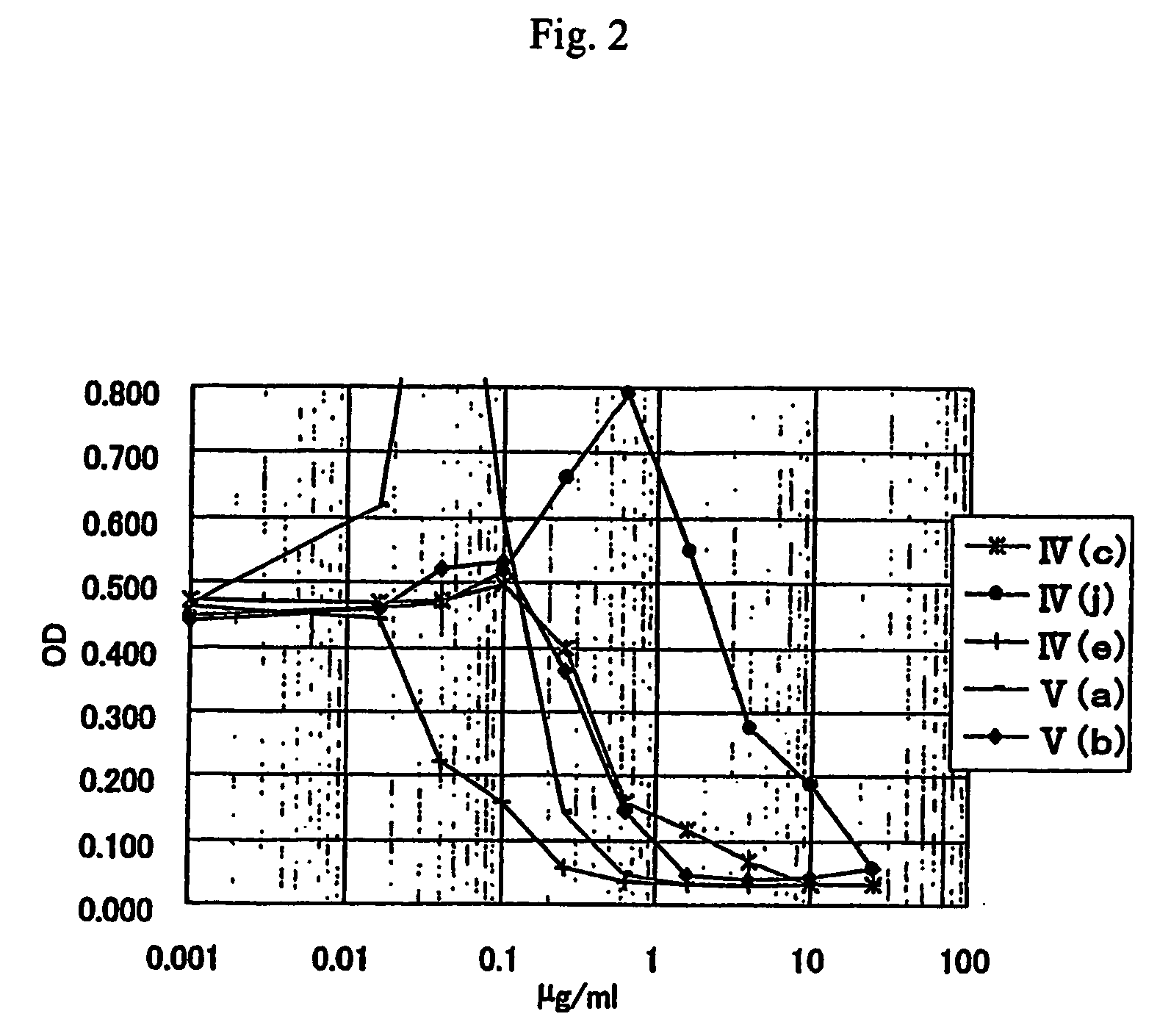
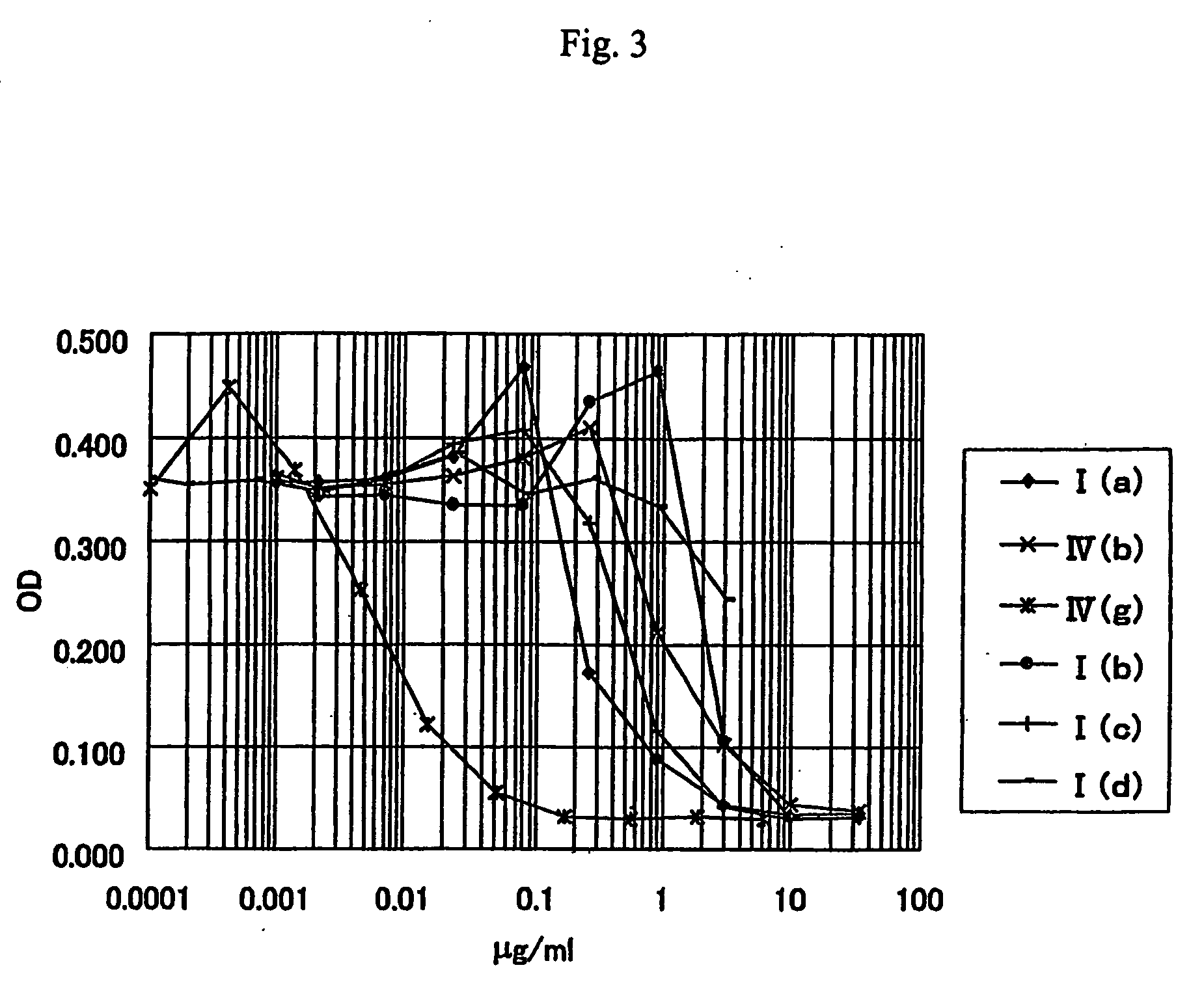
Anti-leishmania agent
The present invention is to provide a new anti-leishmania agent with fewer side effects, having a high cell proliferation-inhibiting effect to leishamia protozoa, and also easy to manufacture at a low cost. A compound wherein a heterocycle with a conjugated system and a nitrogen atom and a heterocycle with a nitrogen atom and a sulfur atom are bound by a carbon chain with an ethylene group, that is a compound wherein a specific 5- to 8-membered heterocyclic ring and a specific 5- to 8-membered heterocycle having a conjugated system are bound via a vinylene group, more particularly a specific rhodacyanine dye compound is used as an active ingredient of the anti-leishmania agent.
Owner:JAPAN SCI & TECH CORP
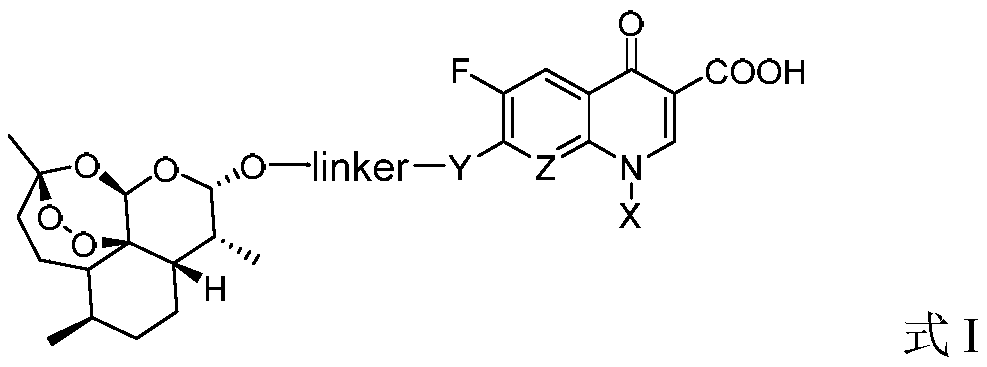
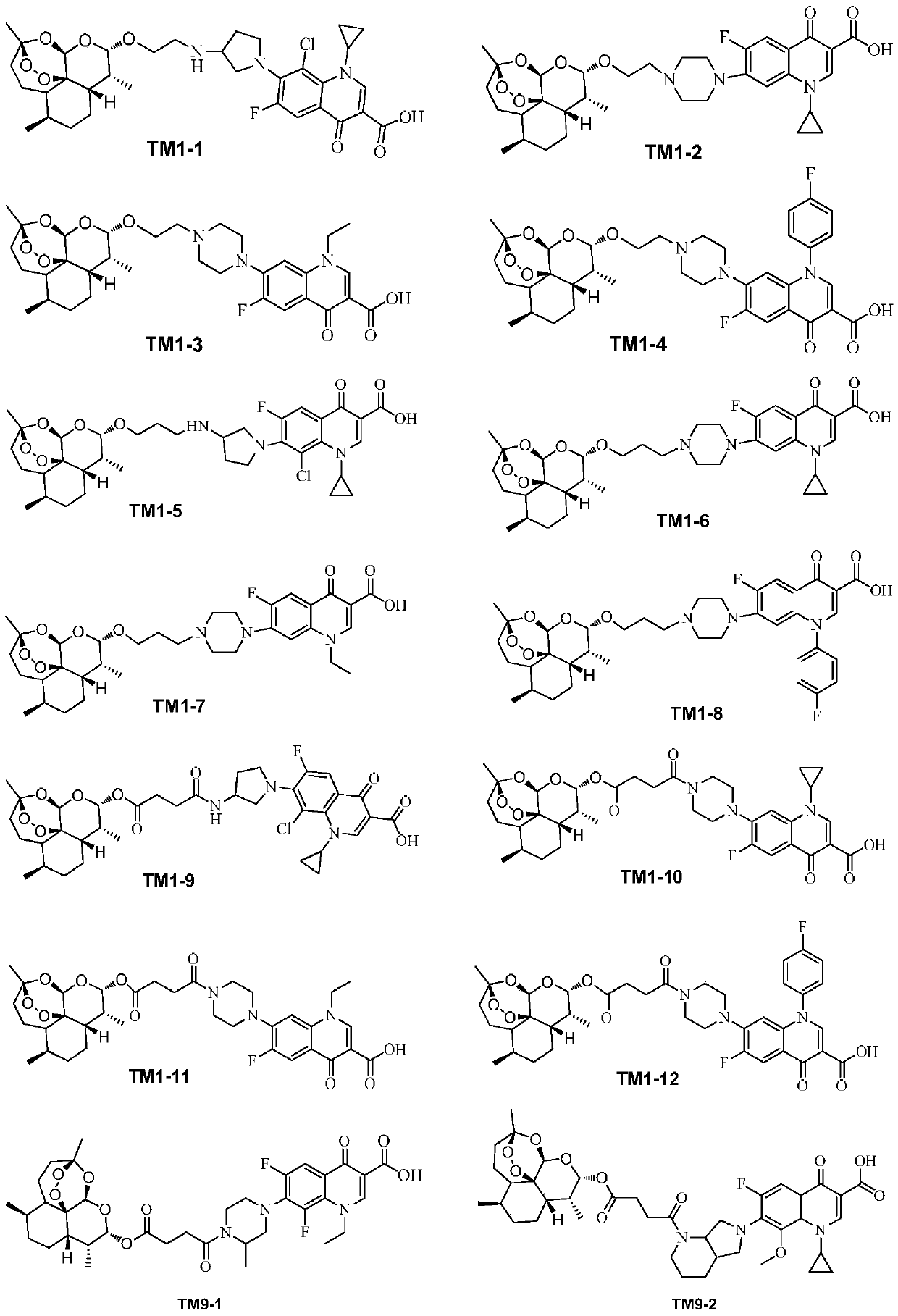
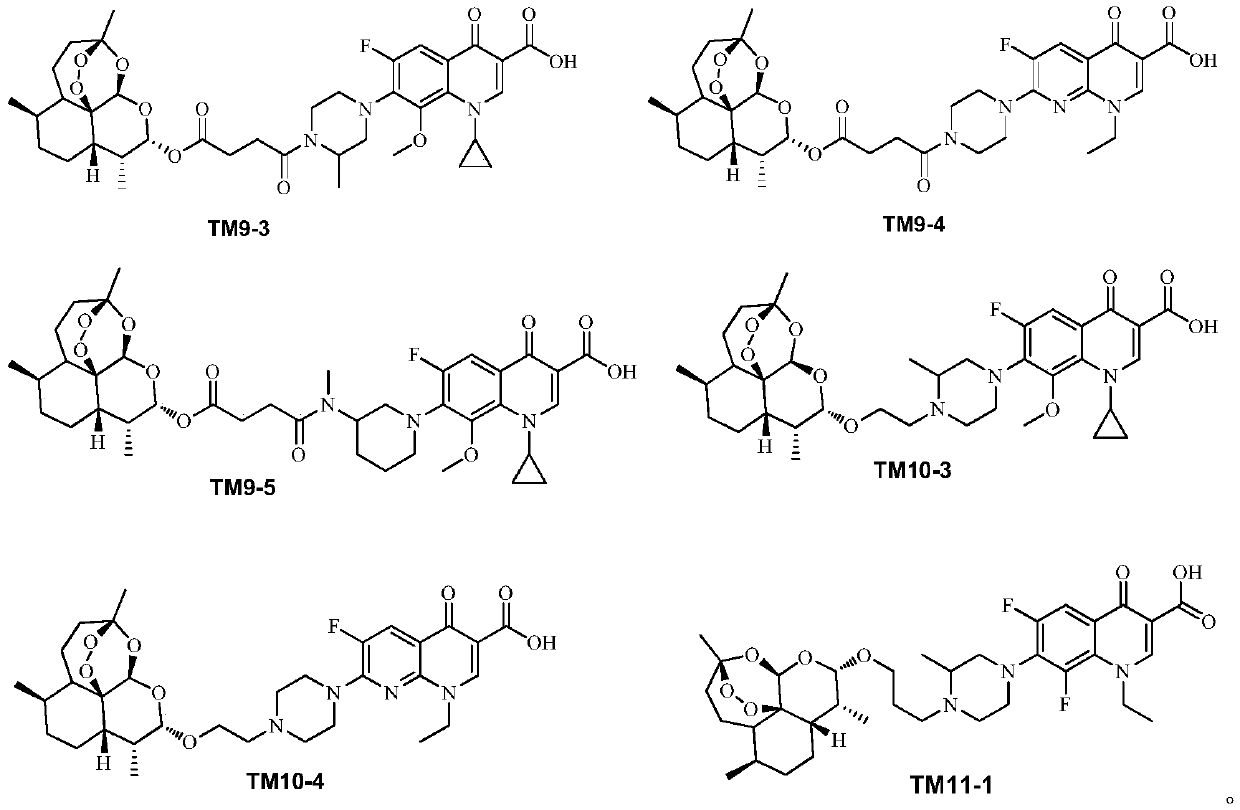
Application of dihydroartemisinin and quinolone conjugate in preparation of drugs for resisting leishmania
ActiveCN110368384ABroaden the use of pharmaceuticalsOrganic active ingredientsAntiparasitic agentsLeishmaniaQuinolone
The invention discloses application of a dihydroartemisinin and quinolone conjugate shown in a formula I in preparation of drugs for resisting leishmania. The pharmaceutical application of the dihydroartemisinin and quinolone conjugate is broadened.
Owner:SOUTHWEST UNIVERSITY
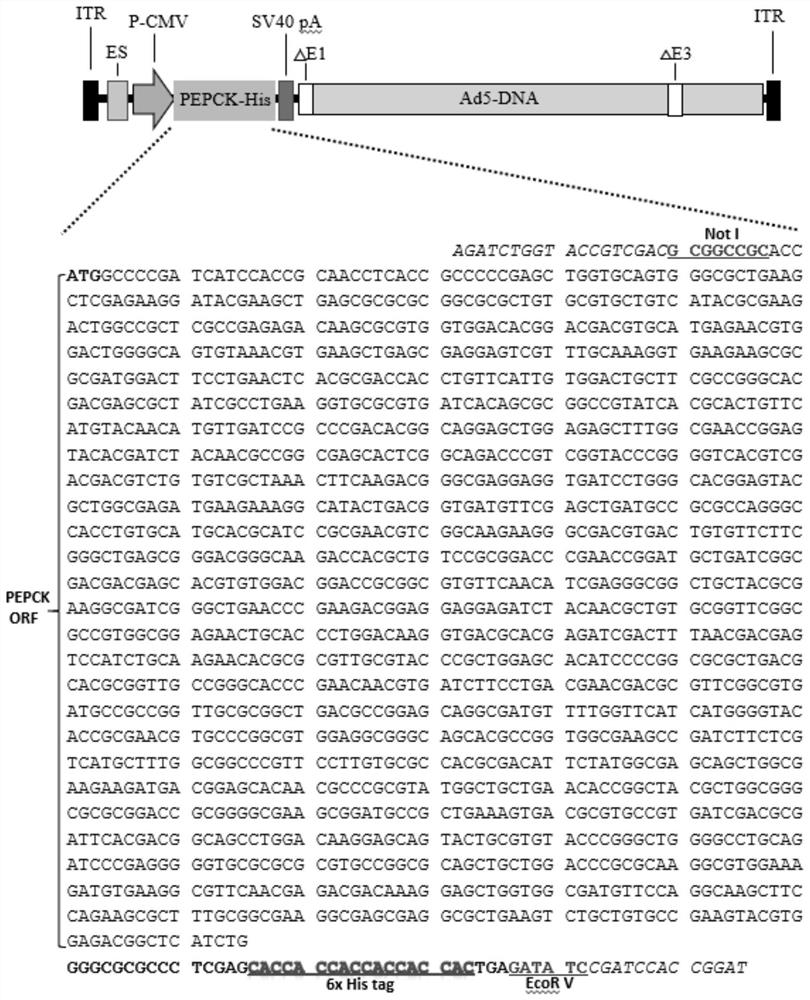
Human replication-deficient recombinant adenovirus vector for efficiently expressing leishmania PEPCK
InactiveCN113337542AImprove securityEasy to manufactureProtozoa antigen ingredientsAntiparasitic agentsAntigenDisease
The invention belongs to the field of biotechnology, and discloses a human replication-deficient recombinant adenovirus vector for efficiently expressing leishmania phosphoenolpyruvate carboxykinase (PEPCK) aiming at the current situation of leishmania. Specifically, carrying out homologous recombination on a recombinant vector pShuttle-CMV-Leish-PEPCK constructed by a modified PEPCK gene and a pShuttle-CMV vector and a pAdEasy-1 vector, carrying out PacI cutting on the obtained plasmid vector pAd5-L.m-PEPCK, and transfecting into a human embryo kidney cell 293, so as to obtain the replication-deficient recombinant adenovirus vector Ad5-L.m-PEPCK. Through Coomassie brilliant blue R250 dyeing and Western blot analysis of a specific anti-His tag antibody, it is shown that the recombinant adenovirus vector Ad5-L.m-PEPCK can efficiently express the PEPCK protein. As the leishmania phosphoenolpyruvate carboxykinase (PEPCK) is the most immunodominant leishmania disease antigen which is found recently. Therefore, the recombinant adenovirus vector Ad5-L.m-PEPCK disclosed by the invention can be used for a vaccine for the leishmaniasis, so that the prevention and control of the leishmaniasis are facilitated.
Owner:SHANXI UNIV
Use of 5-hydroxy-2-hydroxymethyl-y-pyrone (HMP) as a leishmanicidal agent
ActiveUS20110178169A1Enhance immune responseIncrease on actin filament polimerizationBiocideOrganic chemistrySuperoxideLysosome
The present invention refers to the use of HMP (a secondary metabolite obtained from Aspergillus fungi) as an agent that intensifies the mechanism of macrophage activation, leading to the death of L. (Leishmania) amazonensis, the etiologic agent of cutaneous leishmaniasis. The main mechanism of action of this agent is the activation of the microbicidal activity of host cells, through increased superoxide production, number of lysosomes, actin and microtubule filament polymerization and increased spreading, typical of activated cells. Additionally, HMP represents a molecule of easy acquisition, presents an efficient combat mechanism with no adverse reactions and capacity to inhibit the development of promastigotes and amastigotes forms. Finally, results suggest HMP to be a potential candidate for use against cutaneous leishmaniasis at a minimal concentration of 50 μg / mL.
Owner:UNIV FEDERAL DO PARA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com