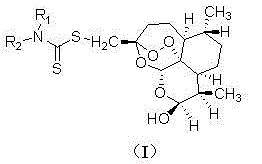
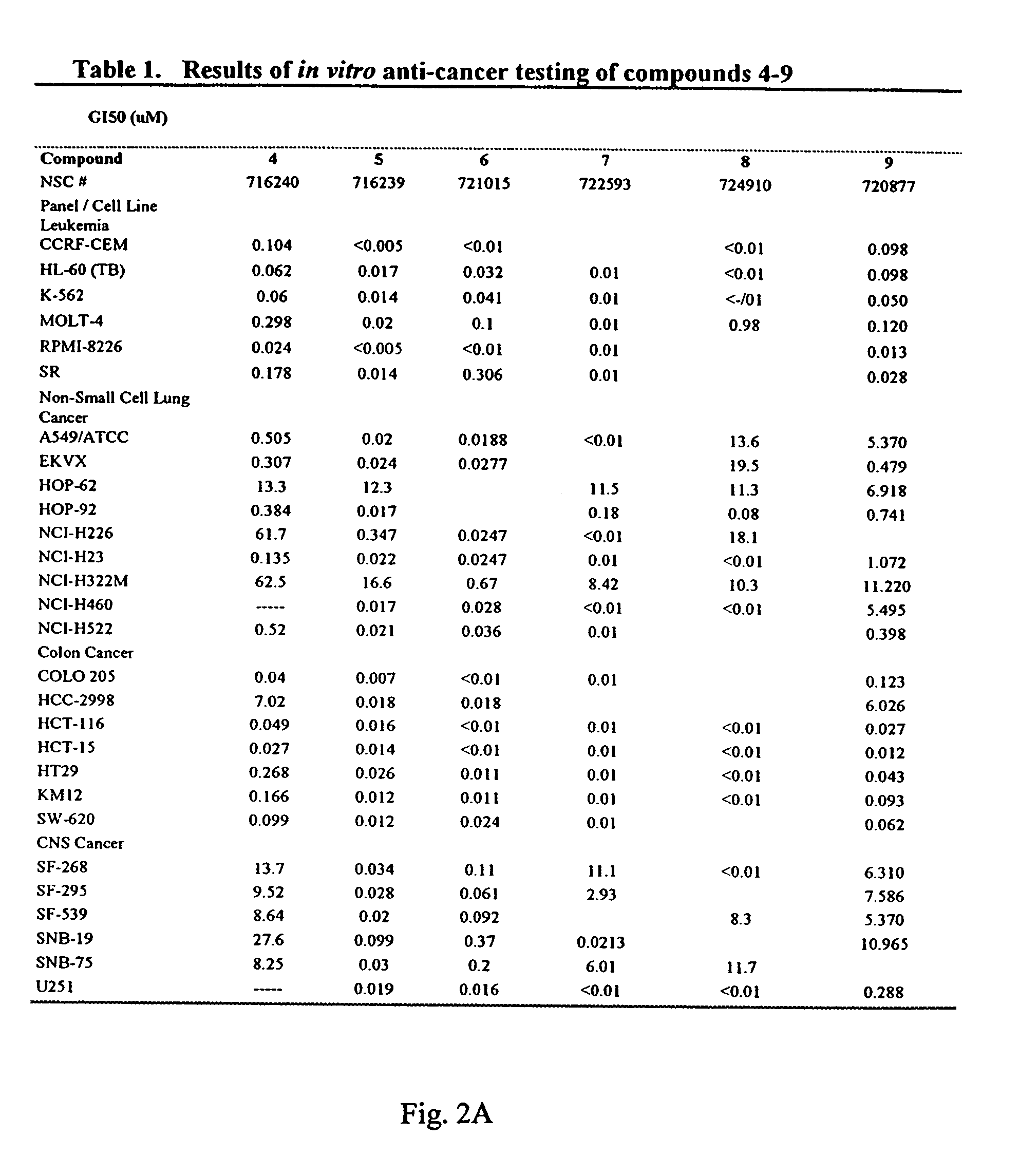
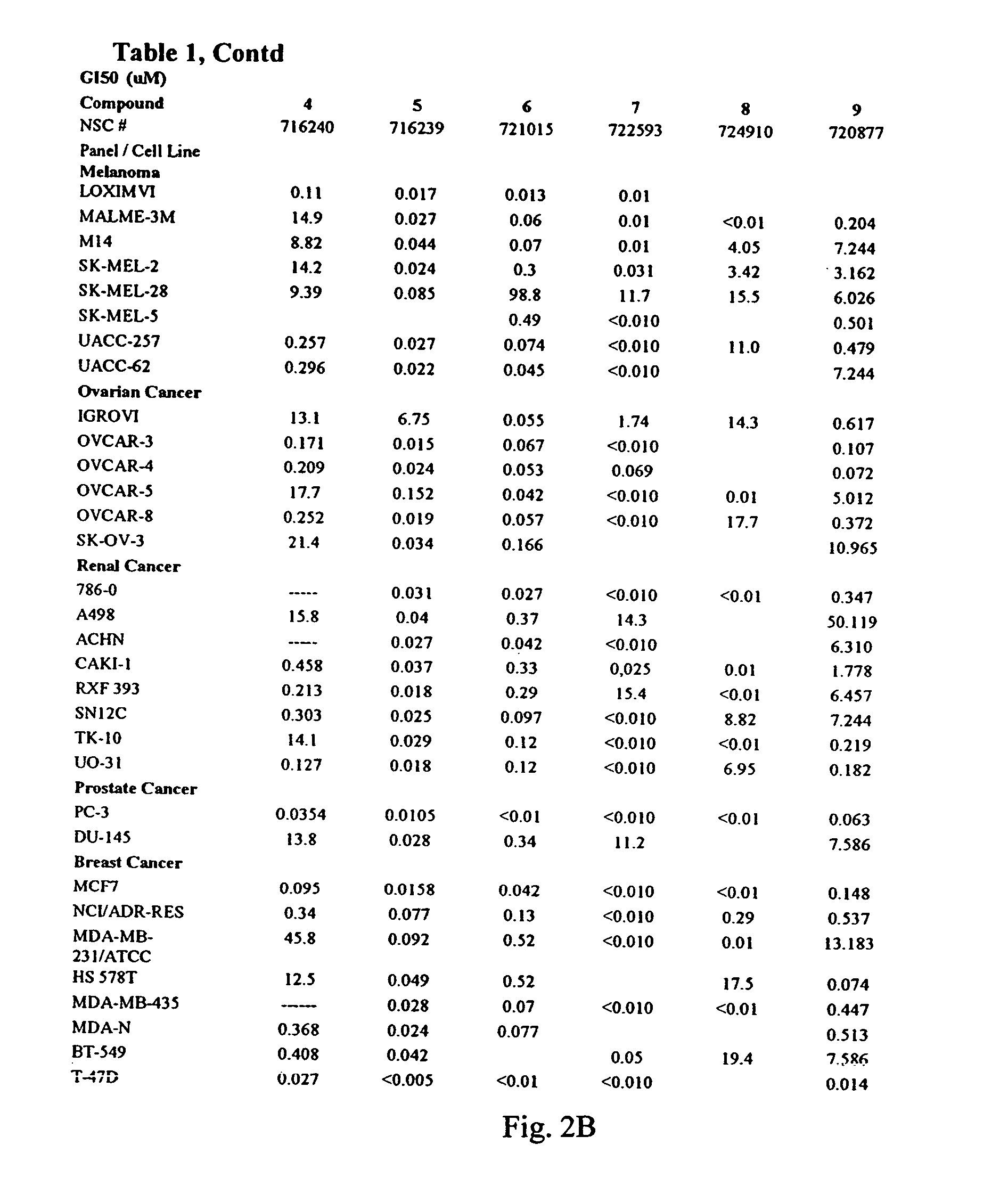
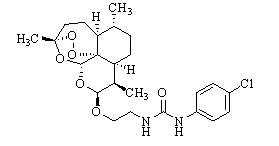
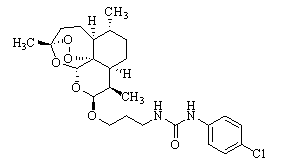
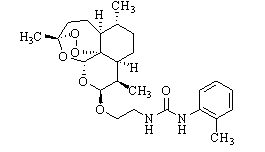
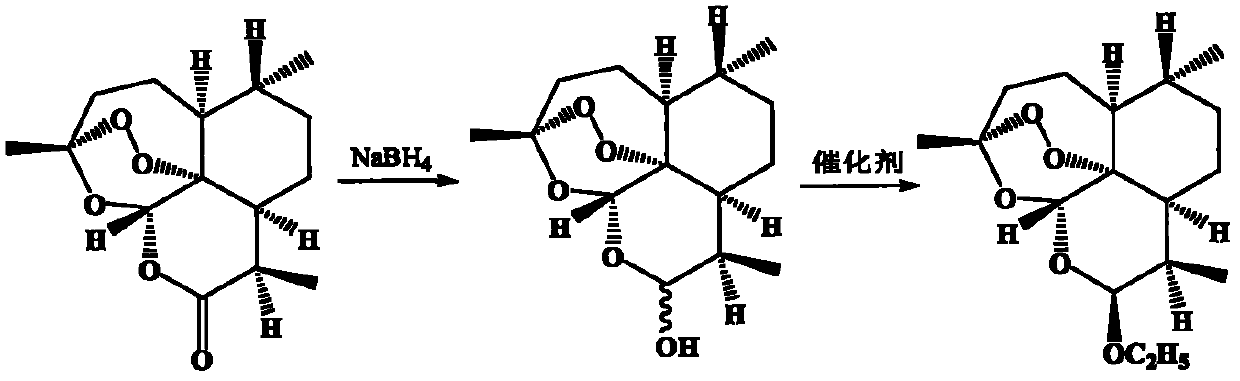
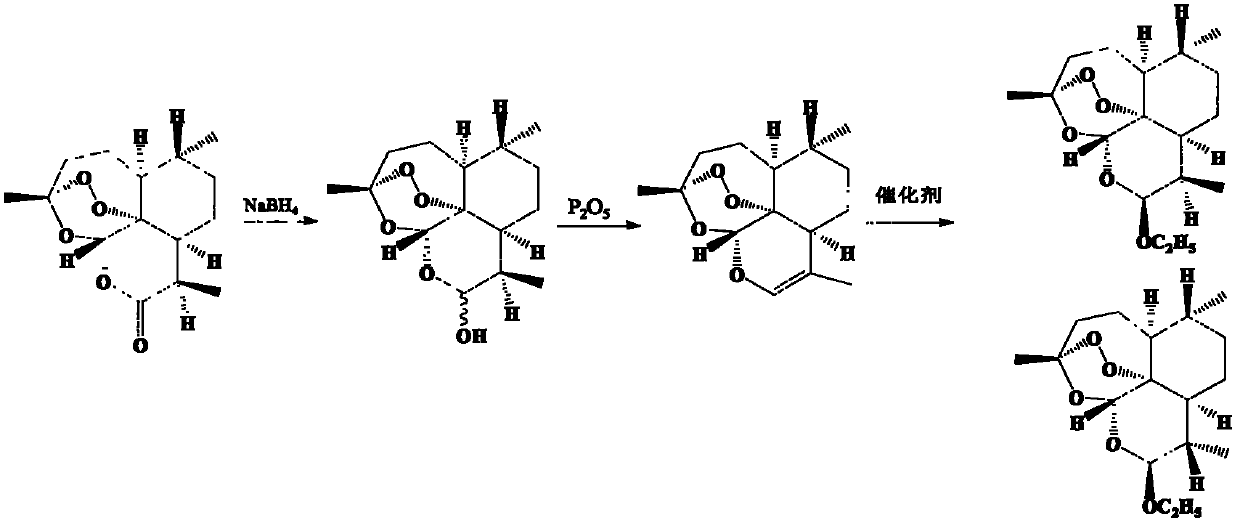
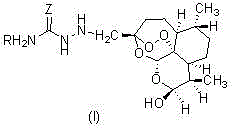
Patents
Literature
268 results about "Dihydroartemisinin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dihydroartemisinin (also known as dihydroqinghaosu, artenimol or DHA) is a drug used to treat malaria. Dihydroartemisinin is the active metabolite of all artemisinin compounds (artemisinin, artesunate, artemether, etc.) and is also available as a drug in itself. It is a semi-synthetic derivative of artemisinin and is widely used as an intermediate in the preparation of other artemisinin-derived antimalarial drugs. It is sold commercially in combination with piperaquine and has been shown to be equivalent to artemether/lumefantrine.
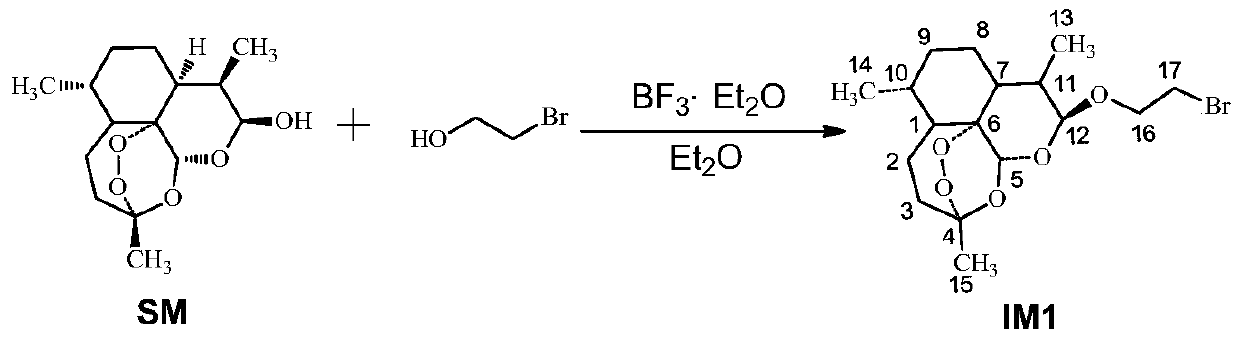
Industrial stereospecific synthesis of beta-artemether by using artemisinin as raw material
InactiveCN101857599AReduce usageReduce intermediate processOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSodium bisulfateBeta-Artemether
The invention discloses stereospecific synthesis of beta-artemether by using artemisinin as a raw material through a one-pot method, which comprises the following steps of: in a system of alkaline solution of dichloromethane, taking sodium borohydride as a reducing agent and aluminium tert-butoxide as a catalyst, and reducing to obtain dihydroartemisinin; and separating out a water phase, adding methanol, sodium acid sulfate, aluminum perchlorate nonahydrate, nickelousperchlorate and the like serving as catalysts for methyl etherification, and reacting at room temperature for 2 hours to obtain the beta-artemether, wherein the maximum yield rate of the beta-artemether is 85 percent and the maximum total yield rate of artemether is 93.5 percent. The method has the characteristics of low cost, high yield, short time and simple and safe operation, and is completely suitable for industrial production.
Owner:GUANGZHOU SWELLXIN SCI & TECH
Process for the preparation of arteethers from dihydroartemisinin
InactiveUS6346631B1High purityReduce in quantityOrganic active ingredientsBiocideOrganic solventSulphate Ion
Owner:COUNCIL OF SCI & IND RES
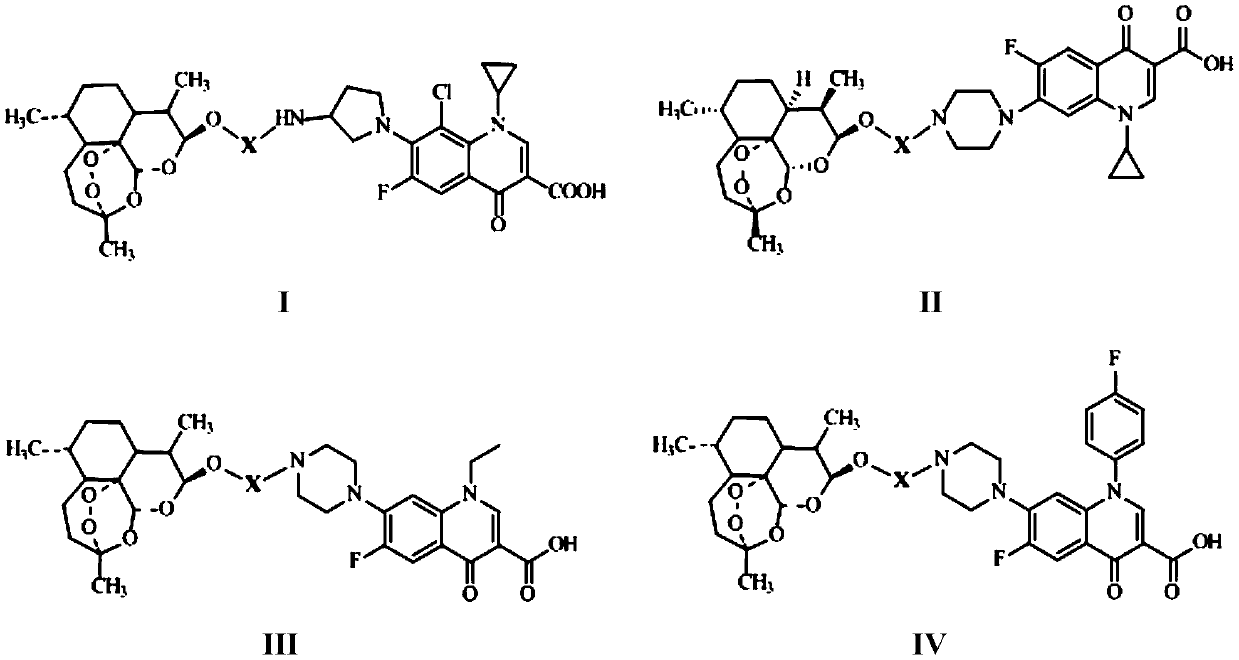
Conjugates of dihydroartemisinin and quinolones compounds as well as preparation method and application thereof
ActiveCN104418864AGood antibacterial effectEasy to prepareAntibacterial agentsOrganic active ingredientsAntituberculosis drugDihydroartemisinin
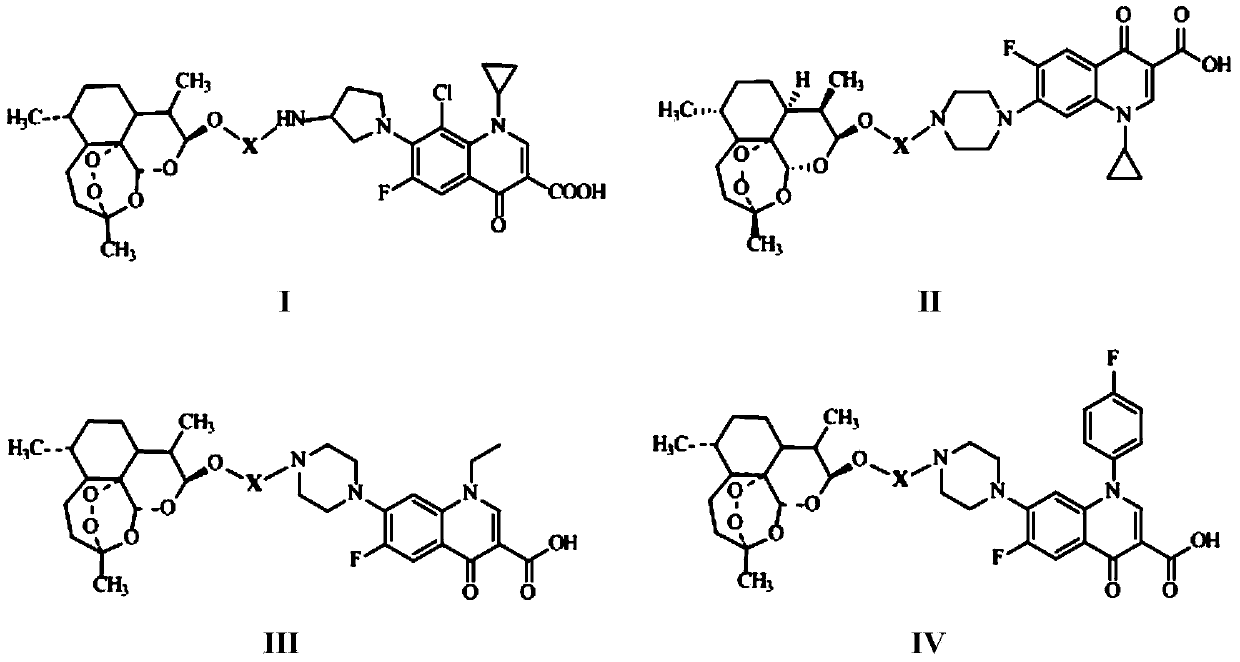
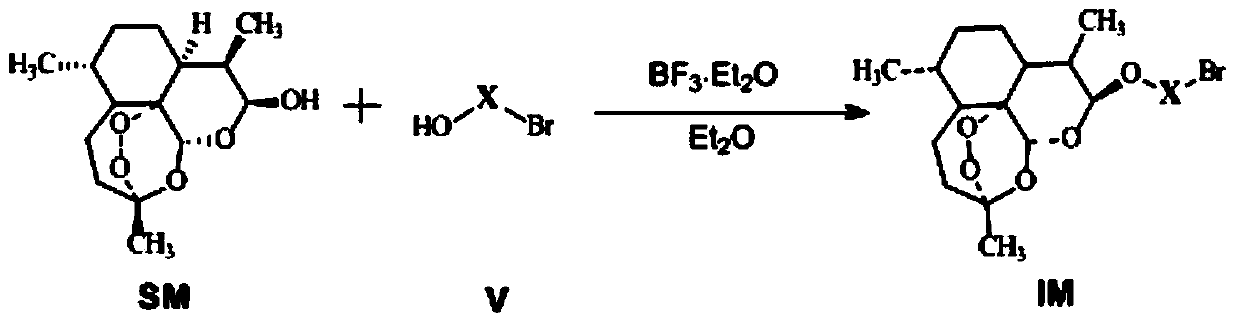
The invention discloses conjugates of dihydroartemisinin and quinolones compounds shown in formulae I to IV and officinal salt thereof, wherein X is -CH2CH2-, -CH2CH2CH2- or -COCH2CH2CO-. The conjugates have a certain antibacterial effect on a mycobacterium tuberculosis standard sensitive strain, a clinical separating sensitive strain and a clinical separating drug resistance strain, and especially the inhibitory effects of conjugates of dihydroartemisinin and clinafloxacin on the mycobacterium tuberculosis standard sensitive strain, the clinical separating sensitive strain and part of the clinical separating drug resistance strain are stronger than those of independent dihydroartemisinin and clinafloxacin. The conjugates can be used for preparing antituberculosis drugs and have a potential application prospect in tuberculosis prevention and cure field. The formulae I to IV are as shown in the specification.
Owner:SOUTHWEST UNIVERSITY
Clathrate compound of artemisinin series and alkaline cyclodextrin and method for preparing same
InactiveCN102716491APromote formationEasy to prepareOrganic active ingredientsAntiparasitic agentsArtemisininsCarboxyl radical
The invention discloses a clathrate compound of artemisinin series (artemisinin, dihydroartemisinin and artesunate) and alkaline cyclodextrin and a method for preparing the same. The alkaline cyclodextrin in the clathrate compound refers to an amido-substituted cyclodextrin; and due to the amido substituting of the cyclodextrin, an alkaline environment is formed in an aqueous solution apart from clathration between the cavity of the cyclodextrin and the artemisinin series, and forms ionic interaction with hydroxyls or carboxyls on the artemisinin series; and therefore, the artemisinin series can be dissolved in water to form a solution within an extremely wide concentration range so that liquid artemisinin series preparations can be formed. The clathrate compound provided by the invention is high in stability, high in bioavailability, simple in preparation, easy for operation, moderate in condition, and suitable for industrial production.
Owner:KUNMING UNIV OF SCI & TECH
Conjugated product of dihydroartemisinin and quinolones, preparation method and application thereof
ActiveCN104418864BGood antibacterial effectEasy to prepareAntibacterial agentsOrganic active ingredientsQuinoloneAntituberculosis drug
The invention discloses conjugates of dihydroartemisinin and quinolones compounds shown in formulae I to IV and officinal salt thereof, wherein X is -CH2CH2-, -CH2CH2CH2- or -COCH2CH2CO-. The conjugates have a certain antibacterial effect on a mycobacterium tuberculosis standard sensitive strain, a clinical separating sensitive strain and a clinical separating drug resistance strain, and especially the inhibitory effects of conjugates of dihydroartemisinin and clinafloxacin on the mycobacterium tuberculosis standard sensitive strain, the clinical separating sensitive strain and part of the clinical separating drug resistance strain are stronger than those of independent dihydroartemisinin and clinafloxacin. The conjugates can be used for preparing antituberculosis drugs and have a potential application prospect in tuberculosis prevention and cure field. The formulae I to IV are as shown in the specification.
Owner:SOUTHWEST UNIV
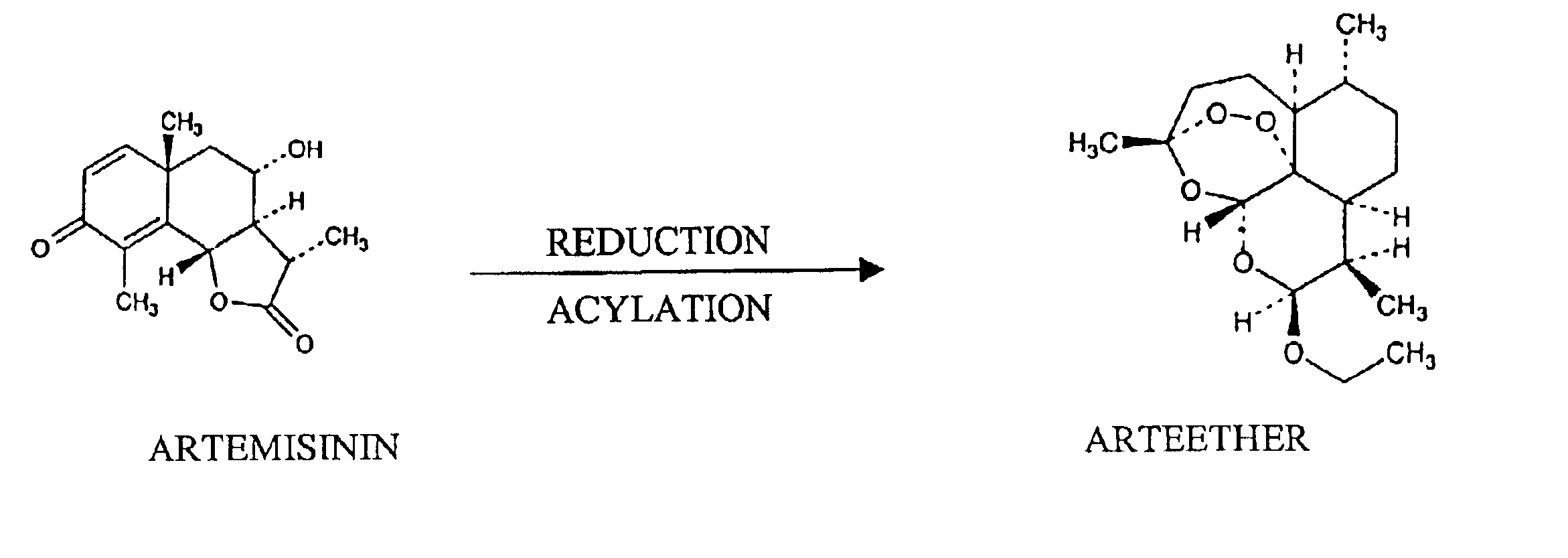
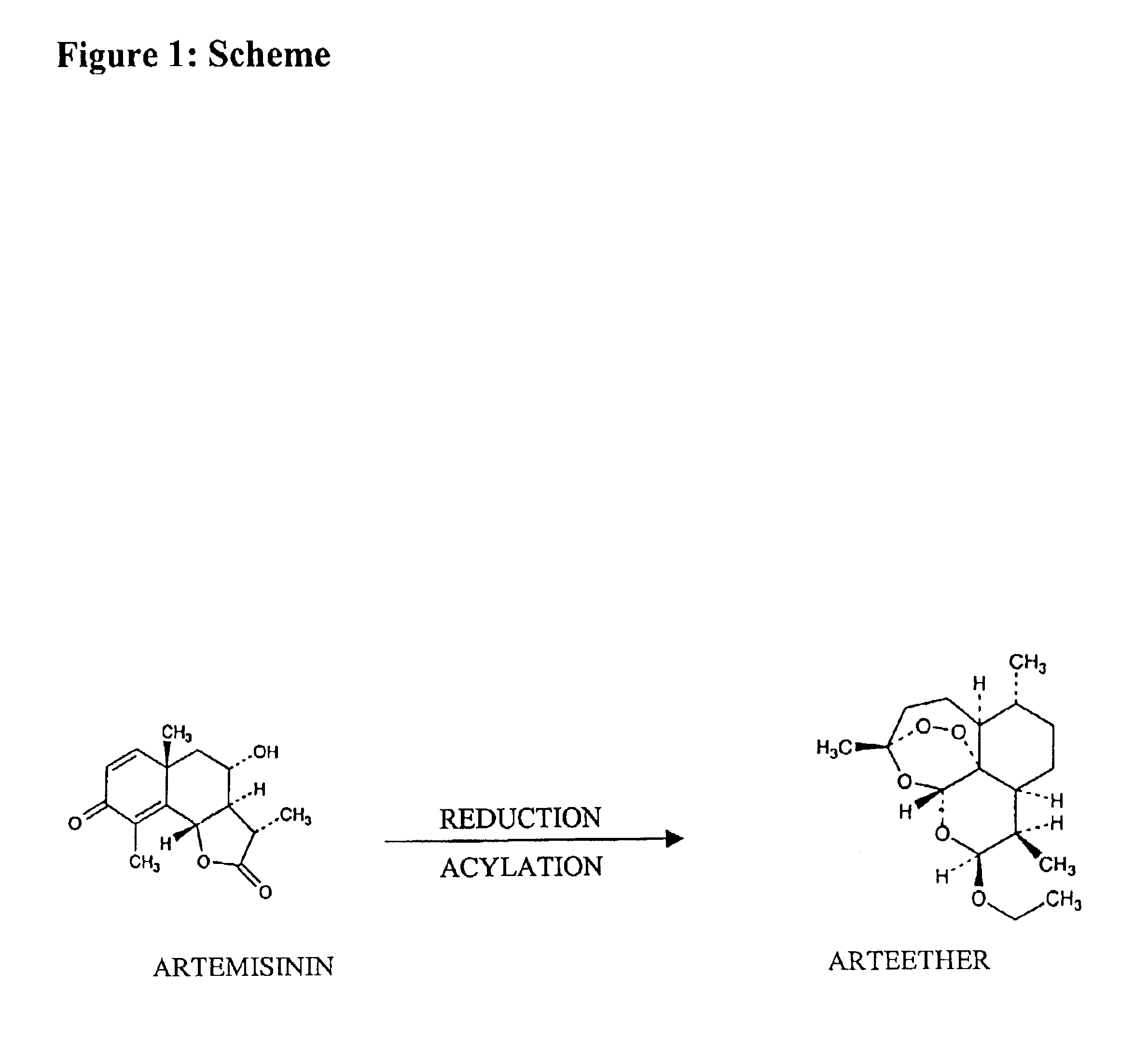
Single pot conversion of artemisinin into arteether
The present invention provides a method for the preparation of arteether from artemisinin in one pot in just about 4 hours comprising reduction of artemisinin into dihydroartemisinin by less quantity of sodium borohydride in ethanol at room temperature in the presence of a novel polyhydroxy catalyst, acylation of dihydroartemisinin in the presence of an acid catalyst, extraction of arteether from an aqueous reaction mixture using 1% ethyl acetate in n-hexane followed by workup and purification of the impure arteether to yield 80-86% (w / w) pure alpha, beta arteether.
Owner:COUNCIL OF SCI & IND RES
Formulation of dihydroartemisinin for the control of wide spectrum of malaria
The present invention relates to a synergistic formulation useful for the control of wide spectrum of malarial infections, which comprises a pharmaceutically effective amount of dihydroartemisinin and a vegetable oil.
Owner:COUNCIL OF SCI & IND RES
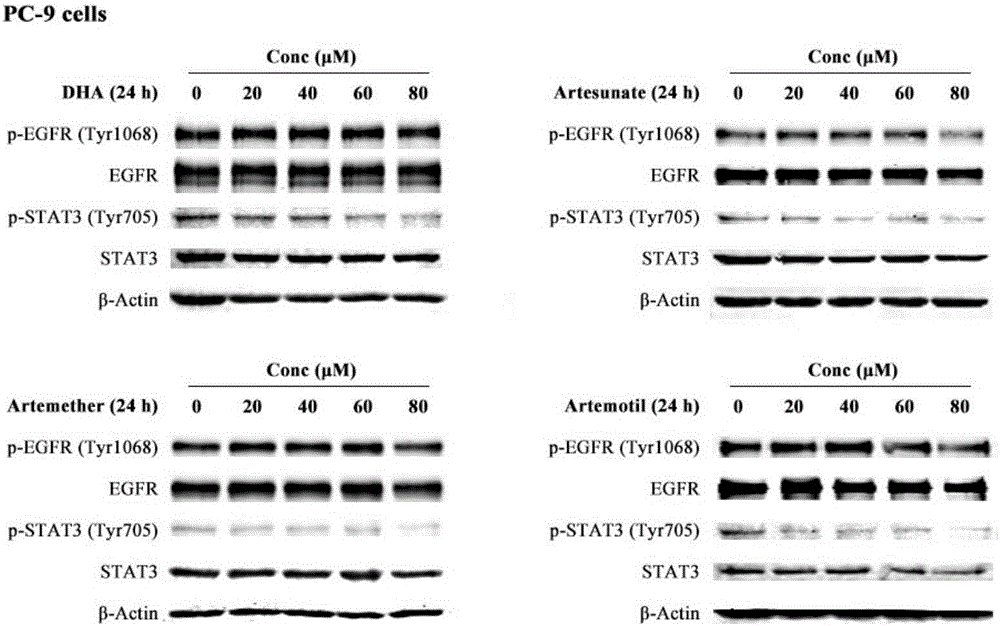
Medicinal composition for resisting non-small cell lung cancer, and application thereof
InactiveCN106668866ASolve the problem of partial drug resistanceInhibition of phosphorylation levelsOrganic active ingredientsAntineoplastic agentsActive componentDihydroartemisinin
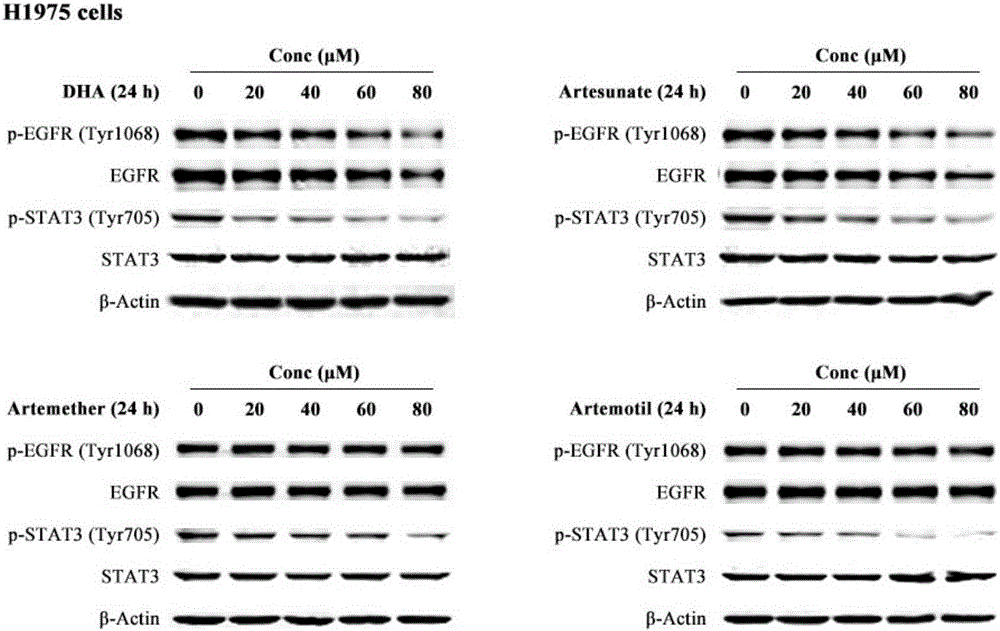
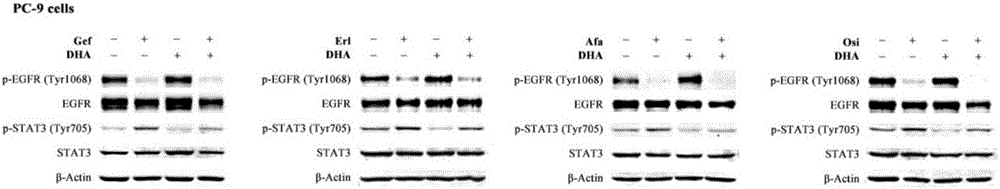
The invention discloses a medicinal composition for resisting non-small cell lung cancer. The active components of the medicinal composition comprise an artemisinin derivative and EGFR-TKI (epidermal growth factor receptor-tyrosine kinase inhibitor), wherein the artemisinin derivative is selected from one of dihydroartemisinin, artesunate, artemether and arteether; and the EGFR-TKI is selected from one of gefitinib, erlotinib, afatinib and osimertinib. The invention also discloses application of the medicinal composition to preparation of medicines for treating and resisting the non-small cell lung cancer. When the medicinal composition provided by the invention is used for treating the non-small cell lung cancer, the medicinal effect which is more excellent than that of the singly used EGFR-TKI can be achieved, the sensitizing effect is achieved, the problem of partial medicine resistance of the non-small cell lung cancer EGFR-TKI is solved, and a scientific basis is provided for the development of new medicines.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Dihydroartemisinin dithiocarbamate as well as preparation method and application of dihydroartemisinin dithiocarbamate
InactiveCN104892633AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryArylCarboxyl radical
The invention discloses dihydroartemisinin dithiocarbamate (general formula I) or a pharmaceutically-acceptable hydrate and salt of the dihydroartemisinin dithiocarbamate, wherein R1 and R2 are respectively independent H and alkyls; and the alkyls can be randomly substituted by substituent groups of halogens, amino groups, substituted amino groups, hydroxyls, carboxyls, ester groups, cyano groups, nitryls, aryls and substituted aryls. The dihydroartemisinin dithiocarbamate disclosed by the invention has a remarkable effect on inhibiting rat sarcoma S180 cells so as to be applied to preparation of anti-tumor drugs. The invention discloses a preparation method of the dihydroartemisinin dithiocarbamate.
Owner:SHIJIAZHUANG UNIVERSITY
Use of artemisinin and its derivatives in cancer therapy
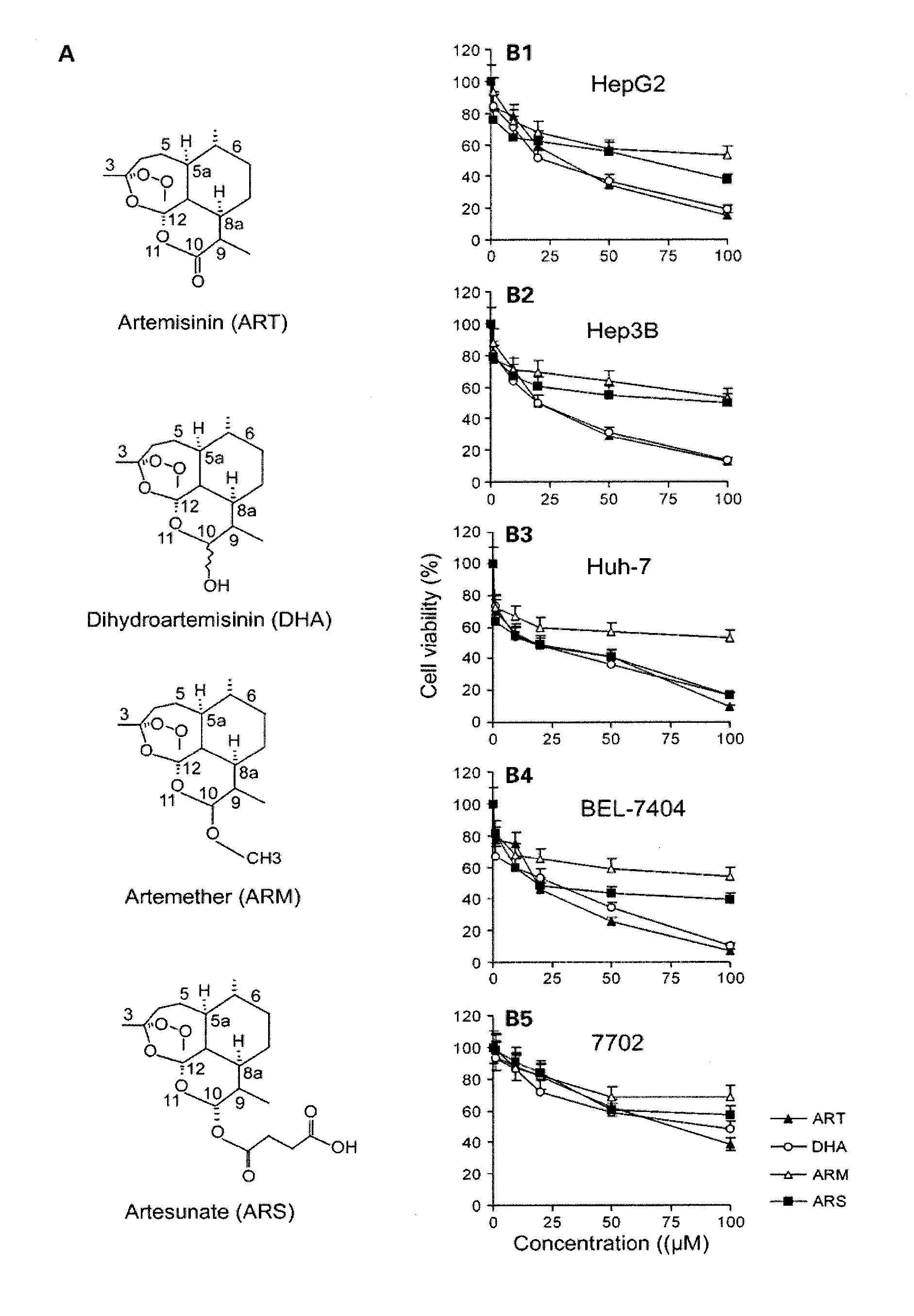
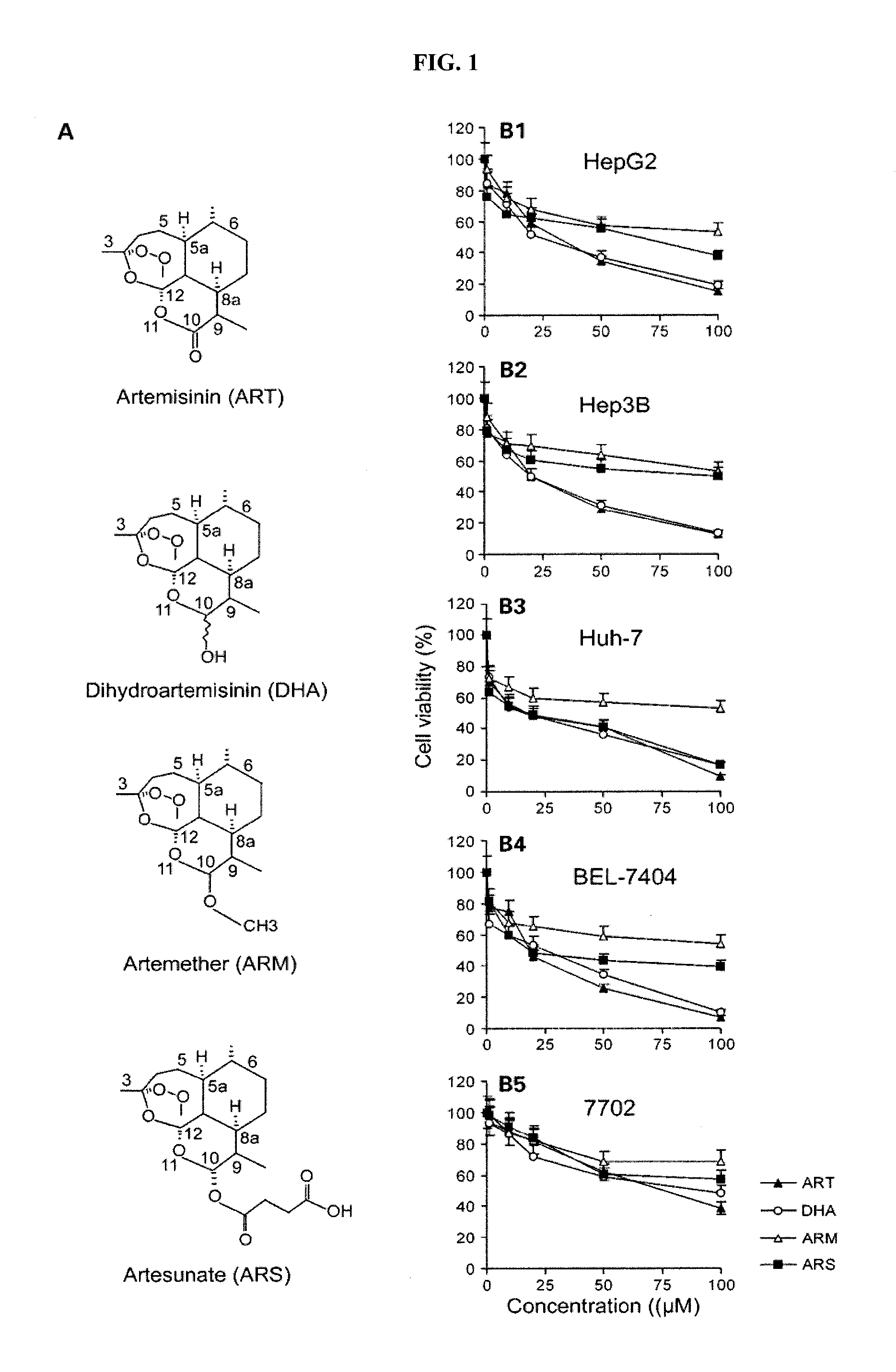
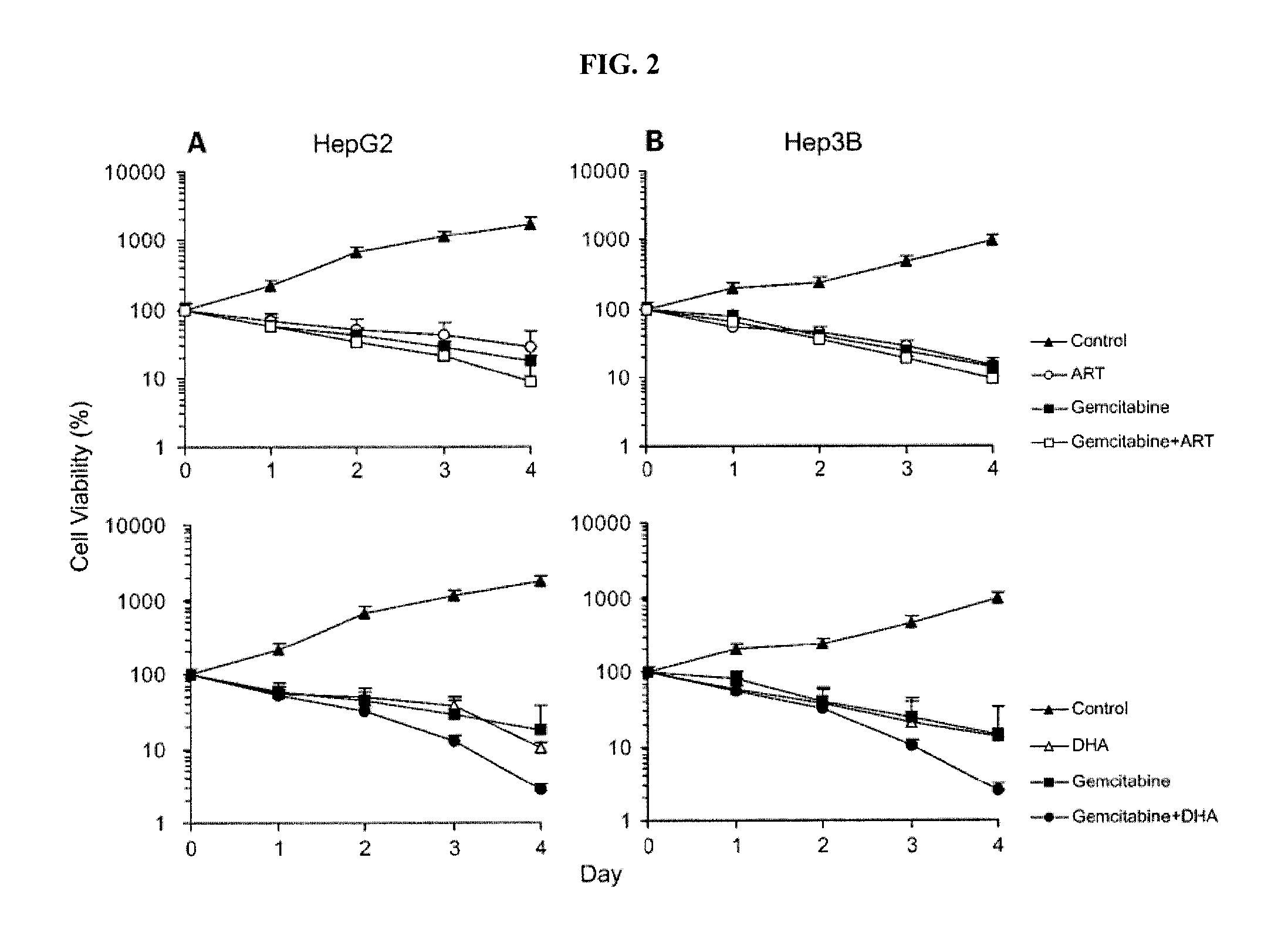
A method for treating cancer in a mammal includes administering to the mammal in need thereof a therapeutically effective amount of artemisinin (ART) or its derivative, such as dihydroartemisinin (DHA), artemether (ARM), or artesunate (ARS) alone or in combination with a chemotherapeutic agent, such as gemcitabine and carboplatin. A method for inhibiting tumor cell proliferation includes contacting a tumor cell with ART or its derivative, such as DHA, ARM, and ARS, in an amount effective to inhibit tumor cell proliferation or in combination with a chemotherapeutic agent, such as gemcitabine and carboplatin.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
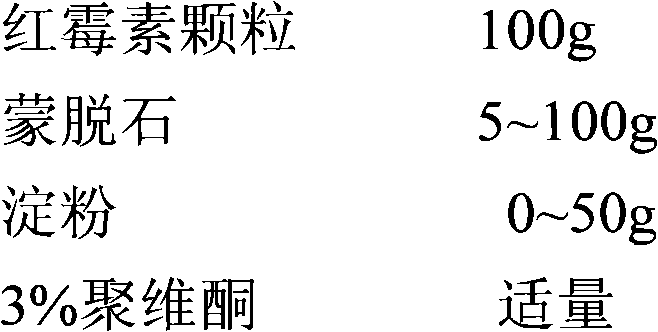
New preparation of erythrocin and relevant drug thereof and preparation method of new preparation
The invention relates to a preparation method of new preparation of erythrocin, which is characterized in that an endothelin core of erythrocin is prepared, and then an isolating layer, a protective layer, a second isolating layer and an improved enteric-coating material layer are applied one by one. In this way, new preparation of the erythrocin which has certain feature of releasing (dissolving) in acid solution (hydrochloric acid solution 9 to 1000) can be formed. The technology of the new preparation can also be widely applied to drugs which, like erythrocin, when being taken orally by a patient, cause the patient to suffer the side effects of stimulation, sickness and the like after degradation in the stomach of the patient or contact with the stomach of the patient, and drugs which the patient needs to take orally to let the blood concentration to reach the peak value in a short time. Such drugs include macrolides of azithromycin, metronidazole of nitroimidazoles, tinidazole, acyclovir as an antiviral drug, ammonium chloride as a phlegm eliminating drug, bromhexine, chloroquine as an antimalarial, nitroquine, artemisinin, dihydroartemisinin, artesunate, primaquine, pyrimethamine, carbarsone and emetine amebicides and so on.
Owner:胡昌勤 +1
Dihydroartemisinin diploid derivative, and medicine composition and application thereof
ActiveCN106928274ANo obvious in vivo toxicityGood parasite killing effectOrganic active ingredientsAntipyreticDiseaseDihydroartemisinin
The invention discloses a dihydroartemisinin diploid derivative, and a medicine composition and application thereof. The derivative is artemisinin diploid, and is applied to a medicine for treating or preventing malaria caused by plasmodium, a medicine for treating autoimmune diseases such as lupus erythematosus, and an antitumor medicine; the medicine composition is prepared from the dihydroartemisinin diploid derivative and a pharmaceutically acceptable carrier.
Owner:SOUTHEAST UNIV
Preparation method of dihydroartemisinin liquid preparation
InactiveCN101732250AGood dispersionGood water solubilityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityPEG 400
The invention discloses a preparation method of a dihydroartemisinin liquid preparation, which belongs to the field of medicine. The preparation method comprises the following steps: firstly, dissolving dihydroartemisinin into dimethyl sulfoxide, then adding ethanol and polyethylene glycol 400 and further diluting the dihydroartemisinin to obtain the dihydroartemisinin liquid preparation which can be mixed and dissolved with water in any proportion. Because of enhancing the dispersion degree of the dihydroartemisinin greatly, the water solubility of the dihydroartemisinin liquid preparation is obviously enhanced. The dihydroartemisinin liquid preparation is used as an anti-tumour preparation and can obviously suppress the growth and the multiplication of the cells of a leukaemia cell strain K562 which is cultured in vitro, so that the anti-tumour effect of the dihydroartemisinin liquid preparation is obviously enhanced.
Owner:林杨
Use of dihydroartemisinin in preparation of drug for inhibition of tumour growth
InactiveCN104739824AReduce financial burdenGood anticancer effectHeavy metal active ingredientsAntineoplastic agentsIntestinal CancerSide effect
The invention discloses use of dihydroartemisinin in preparation of a drug for inhibition of tumour growth, and the tumour is liver cancer tumor, breast cancer tumor, melanoma or intestinal cancer tumor. The liver cancer tumor is human liver cancer HepG-2, human liver cancer QGY and human liver cancer SMMC. The breast cancer tumor is human breast cancer Bcap-37.The intestinal cancer tumor is human colon carcinoma Colo-205. The drug for inhibition of tumour growth may also include one of artemisinin and artemisinin derivative. The dihydroartemisinin can be used in preparation of the drug for inhibition of tumour growth, and is obvious in anti-cancer effect, small in side effect of chemotherapy, safe to use, and low in drug cost.
Owner:KUNSHAN DAREN BIOLOGICAL PHARMA CO LTD
Preparation method of beta-artemether
ActiveCN102731523AMild conditionsSuitable for industrial productionOrganic chemistryDihydroartemisininRoom temperature
The invention provides a preparation method of beta-artemether. The method comprises the following steps: (1) artemisnin reacts to generate dihydroartemisinin in the action of a reducing agent; (2) the dihydroartemisinin reacts with p-toluenesulfonic acid to generate beta-artemether; and (3) refining: methanol, ethanol, ethylene glycol or isopropyl alcohol which is equivalent to 2 to 4 times the weight of the artemether crude product is added into the artemether crude product, the mixture is heated to a temperature of between 35 and 45 DEG C and dissolved, cooled to room temperature in a stirring condition, cooled to a temperature of between 0 and 5 DEG C, and crystallized for 3 to 5 hours, the mixture is filtered, the prepared filter cake is cleaned with frozen methanol, ethanol, ethylene glycol or isopropyl alcohol the weight of which is equivalent to 0.5 to 1 time that of the filter cake at a temperature of between 0 and 5 DEG C, and the filter cake is baked at a temperature of between 35 and 45 DEG C to a constant weight. The preparation method of the beta-artemether has mild condition and environmental friendliness and is suitable for industrial production; and the product yield is over 90 percent, and the purity is 99.2 percent.
Owner:湖南莱崔尔生物科技有限公司
Application of dihydroartemisinin to preparation of tumor cell autophagy induction medicament
InactiveCN102038678AGrowth inhibitionInhibition of newbornsOrganic active ingredientsAntineoplastic agentsPharmaceutical formulationWilms' tumor
The invention provides application of dihydroartemisinin to preparation of a tumor cell autophagy induction medicament. The molecular formula of the dihydroartemisinin is C15H24O5. The medicinal preparation comprises a preparation-allowable medicinal excipient or carrier. The excipient is in the form of a solid preparation. The medicinal preparation provided by the invention can be used as a mitochondrion targeting autophagy inductor, which has a specific anti-tumor effect and can provide a therapeutic medicament for overcoming medicament resistance and improving prognosis for treatment of tumors. The effects and the mechanisms of effective monomer components in a traditional Chinese medicine are research and described under the background of a cell autophagy theory; and an important basis is provided for the development of new theory of the traditional Chinese medicine and new acting target of the medicament, and a new research direction for developing the theory of the traditional Chinese medicine is provided.
Owner:ZHEJIANG UNIV
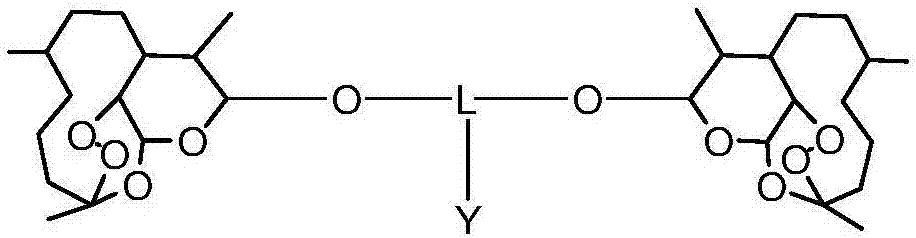
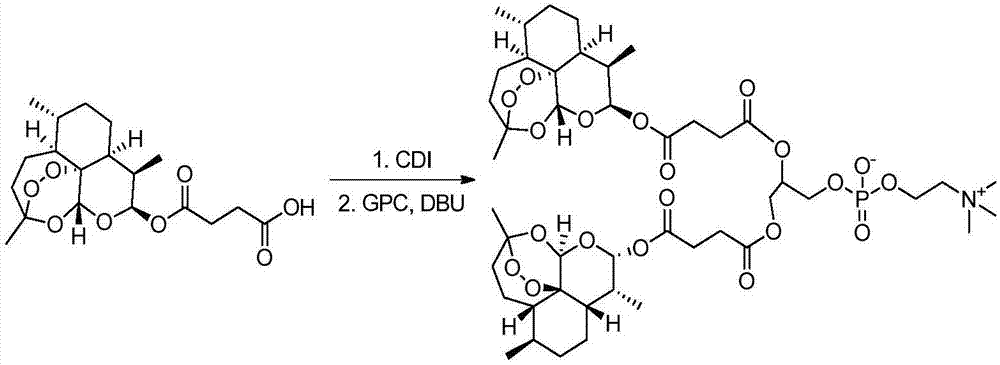
Dihydroartemisinin and dihydroartemisitene dimers as anti-cancer and anti-infective agents
This invention comprises compositions containing dihydroartemisinin and dihydroartemisitene dimers with activity as anticancer agents and anti-protozal, including anti-malarial and anti-leishmanial properties. This invention also describes methods of preparation of these compositions and methods of use of such compositions for the treatment of cancer, and protozoal infections, including malaria, or leishmaniasis.The compounds of this invention represent a potential new class of anti-tumor agents, one that has shown promising activity against solid tumors, and with a pattern of selectivity that suggests a possible new mechanism of action.
Owner:ELSOHLY LAB
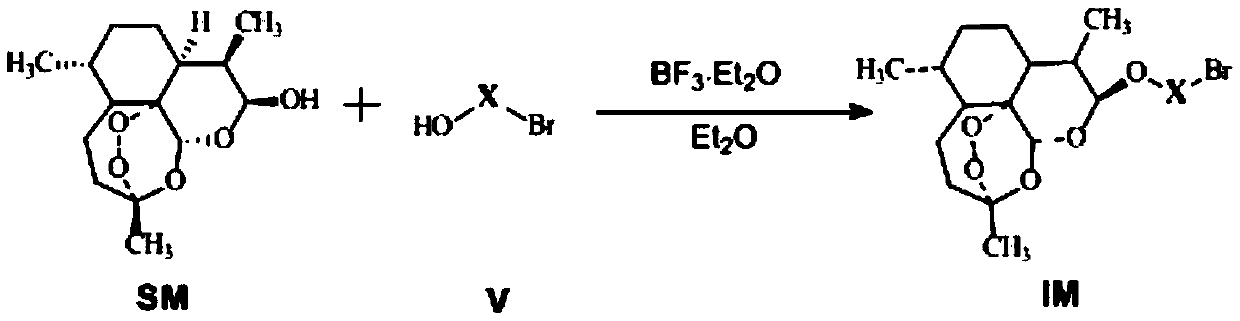
C-10 site carbamido substituted artemisinin derivative, preparation method and application
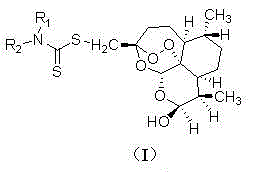
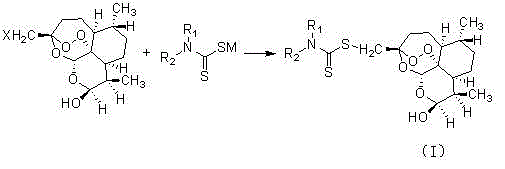
The invention relates to a C-10 site carbamido substituted artemisinin derivative (I), and relates to a structure, a preparation method and an application of the novel artemisinin 10 site derivative. The structural formula is shown as a formula I, and relates to its pharmaceutically acceptable salt, a solvate, an optical isomer or a polymorphic substance and a pharmaceutical composition which takes the compound as an active component. Being as a new antimalarial agent, an antitumor agent and an antifungal agent, the compound can be used for treating or preventing malaria, fungal infection, malignant tumor and the like. The compound takes substituted carbamide as an initial raw material, and is obtained by reacting with dihydroartemisinin under catalysis of Et2O.BF3.
Owner:SHENYANG PHARMA UNIVERSITY +1
New process for preparing beta-arteether by single reaction kettle method by taking artemisinin as raw material
The invention provides a method for semi-synthesizing beta-arteether by a single reaction kettle method by taking artemisinin as a raw material. In absolute ethyl alcohol or a moderate polarity ether solvent system such as tetrahydrofuran, borohydride such as potassium borohydride and the like is taken as a reducing agent to reduce artemisinin so as to obtain dihydroartemisinin. Without separation or purification, methanesulfonic acid, phosphoric acid or the like is added to adjust the pH value to neutral, and then, a catalyst such as Lewis acid ZnBr2 and the like is continuously added to react to prepare arteether. After reaction, separation and purification are directly carried out by way of extraction, crystallization and recrystallization to obtain beta-arteether with high yield (0.85k of beta-arteether is prepared from 1kg of artemisinin), wherein the content of beta-arteether is more than 99.0%; the content of the related substance alpha-arteether is less than 0.2%; the content of dihydroartemisinin is less than 0.1%; and the content of the total impurities is less than 0.5% of the quality product. The method has the advantages of simple process flow, low equipment requirement, convenience in operation, high atom economy, low carbon and environment friendliness, and is very suitable for industrial production.
Owner:江苏斯威森生物医药工程研究中心有限公司
Semicarbazide dihydroartemisinin derivative as well as preparation method and application of semicarbazide dihydroartemisinin derivative
InactiveCN104892626AGood inhibitory effectEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryCarboxyl radicalDihydroartemisinin
The invention relates to a semicarbazide dihydroartemisinin derivative (general formula I) or a pharmaceutically-acceptable hydrate and salt of the semicarbazide dihydroartemisinin derivative, wherein R is H and various kinds of alkyls; the alkyls can be randomly substituted by substituent groups of halogens, amino groups, substituted amino groups, carboxyls, hydroxyls, ester groups, cyano groups, nitryls, aryls and substituted aryls; and Z is O, S and NH. The semicarbazide dihydroartemisinin derivative disclosed by the invention has a remarkable effect on inhibiting Hela and A549 cells so as to have a relatively-good anti-tumor effect. The invention discloses a preparation method of the semicarbazide dihydroartemisinin derivative.
Owner:SHIJIAZHUANG UNIVERSITY
Application of dihydroartemisinin in reinforcing chemotherapy medicine antitumor curative effect
InactiveCN101125140AGood treatment effectOrganic active ingredientsPharmaceutical delivery mechanismPharmacometricsTumor chemotherapy
The present invention provides an application of dihydroartemisinin in the preparation of the drugs to enhance the anti-tumor efficacy of the chemotherapeutic drugs. The pharmaceutical preparation contains preparation allowable pharmaceutical excipients or carriers. The form of the preparation is solid preparation. The pharmaceutical preparation provided by the present invention can enhance the anti-tumor efficacy of the chemotherapeutic drugs and can be used in the adjuvant therapy during the tumor chemotherapy process. The present invention takes the theory that the angiogenesis inhibitor can change the micro-environment of tumor as the background, studies and clarifies the function and the mechanism that the effective monomer in the traditional Chinese medicine can enhance the anti-tumor efficacy of the chemotherapy drugs, provides a pharmacological basis for the development of the new usages of the artemisinin drugs and provides an important basis for the development of the new clinical values of the angiogenesis inhibitor, so the present invention is a new research direction for the development of the theory of traditional Chinese medicine.
Owner:ZHEJIANG UNIV
Dihydroartemisinin beta-cyclodextrin inclusion compound, preparation method thereof and antimalarialdrug with same
ActiveCN101954090AImprove solubilityImprove water insoluble propertiesOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityDihydroartemisinin
The invention discloses a dihydroartemisinin beta-cyclodextrin inclusion compound, a preparation method thereof and an antimalarialdrug with the inclusion compound. The inclusion compound is composed of dihydroartemisinin and beta-cyclodextrin based on the weight ratio of 1:5-80. The preparation method comprises the following the steps: dissolving the dihydroartemisinin in ethanol or acetone, obtaining a solution for later use, and preparing a beta-cyclodextrin saturated aqueous solution; and adding the dihydroartemisinin ethanol or acetone solution under the stirring condition, then continuously stirring or performing ultrasound for 1 to 4 hours, standing the mixture and passing the night, volatilizing the ethanol or acetone, filtering and drying the filtrate in a freezing mode or a decompression and low temperature mode to obtain the dihydroartemisinin beta-cyclodextrin inclusion compound. Compared with the prior art, in the invention, the inclusion compound is prepared from the dihydroartemisinin and the beta-cyclodextrin, thereby increasing the solubility of the dihydroartemisinin in water greatly, improving the stability of the dihydroartemisinin, and being beneficial for the follow-up operation of the dihydroartemisinin in pharmacy.
Owner:GUILIN PHARMA
Nanoparticle liposome for releasing different medicines in programmed manner and preparation and use thereof
InactiveCN102188439AIncrease profitReverse drug resistanceOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolDidodecyldimethylammonium bromide
The invention discloses a nanoparticle liposome for releasing different medicines in programmed manner and preparation and use thereof. The nanoparticle liposome is mainly prepared from the following components in part by mass: 19.34 to 50.79 parts of polylactic-co-glycolic acid, 5.78to 25.73 parts of dihydroartemisinin and phospholipid composite, 0.01 to 25.27 parts of phospholipid, 0.01 to 15.38 parts of didodecyldimethylammonium bromide, 0.01 to 20.0 parts of cholesterol, and 0.01 to 10.16 parts of adriamycin, wherein the molecular weight of the polylactic-co-glycolic acid is 5,000 to 300,000, and the phospholipid is natural phospholipid or synthetic phospholipid. The nanoparticle liposome for loading medicines of different natures can be used for treating breast cancer and leukocythemia, which have resistance to adriamycin, inverse the medicine resistance of tumors, improve the utilization rate of medicines and reduce pain in patients and the medical cost of patients.
Owner:ZHEJIANG UNIV
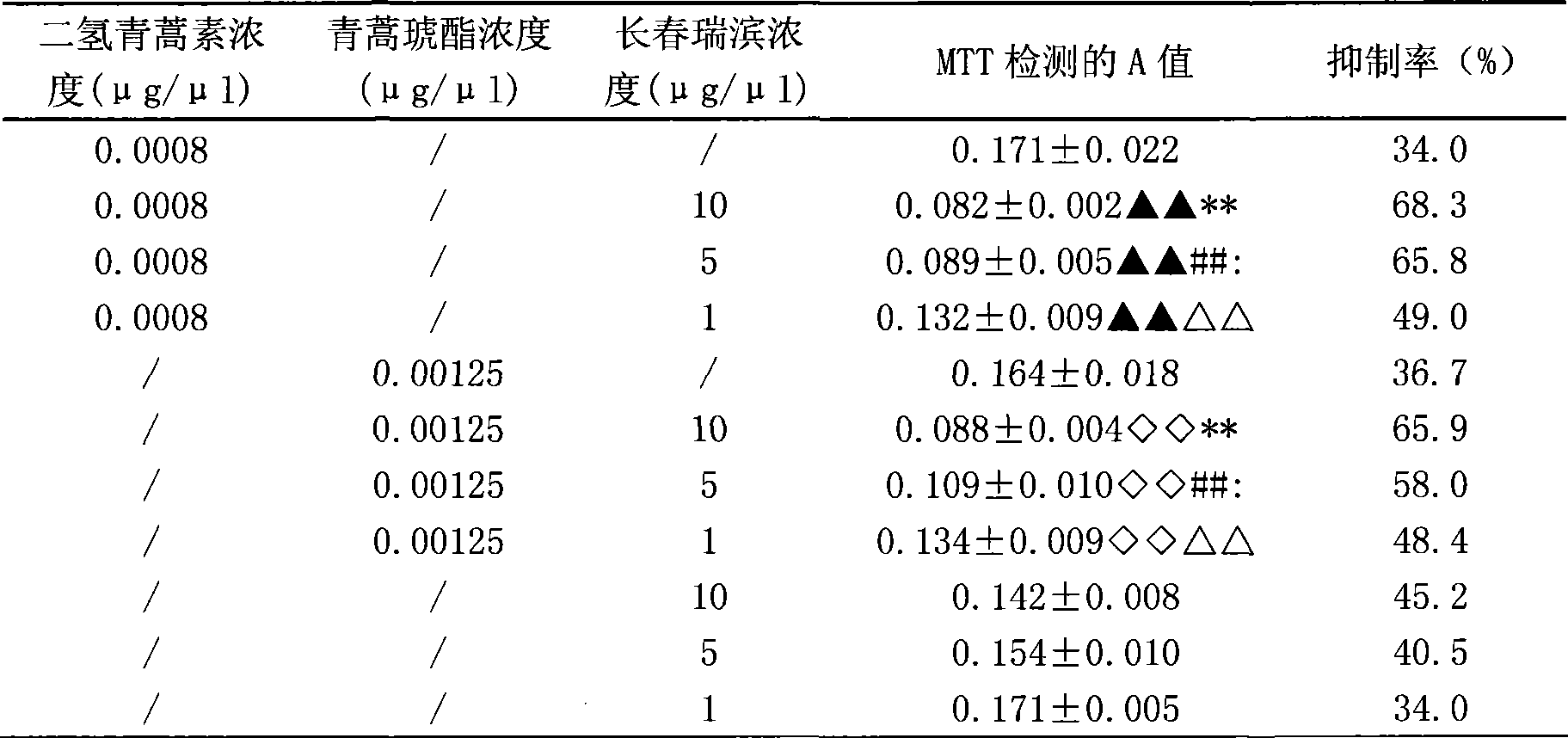
Composition of artemisinin derivative and vinorelbine, and use thereof
InactiveCN101380325AReduce dosageSynergisticOrganic active ingredientsAntineoplastic agentsAdjuvantOral medication
The invention relates to a composite of an artemisinin derivative and vinorelbine in the technical field of pharmaceuticals and the application thereof; wherein, the ratio of the artemisinin derivative (including dihydroartemisinin or artesunate) and the vinorelbine is 1:500 to 500:1. The composite can be applied to preparing antineoplastics and made into the dosage forms of injection administration and oral administration by adding pharmaceutically acceptable adjuvant carriers. The two effective components (the artemisinin derivative and the vinorelbine) of the composite not only have obvious synergistic effect and but also can be cooperatively applied so as to reduce the amount of the vinorelbine and lower the production cost. By adding the pharmaceutically acceptable adjuvant carriers to prepare the dosage forms of injection administration and oral administration, the composite can be applied to the anti-tumor field.
Owner:SHANGHAI JIAO TONG UNIV
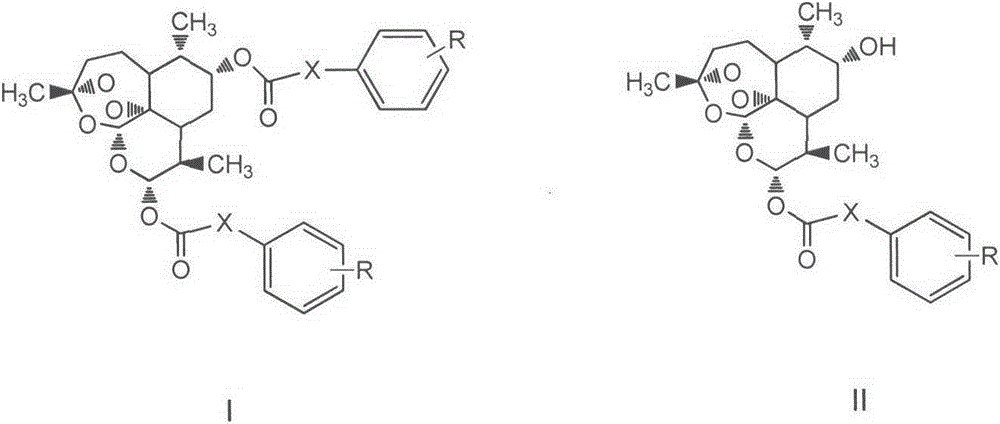
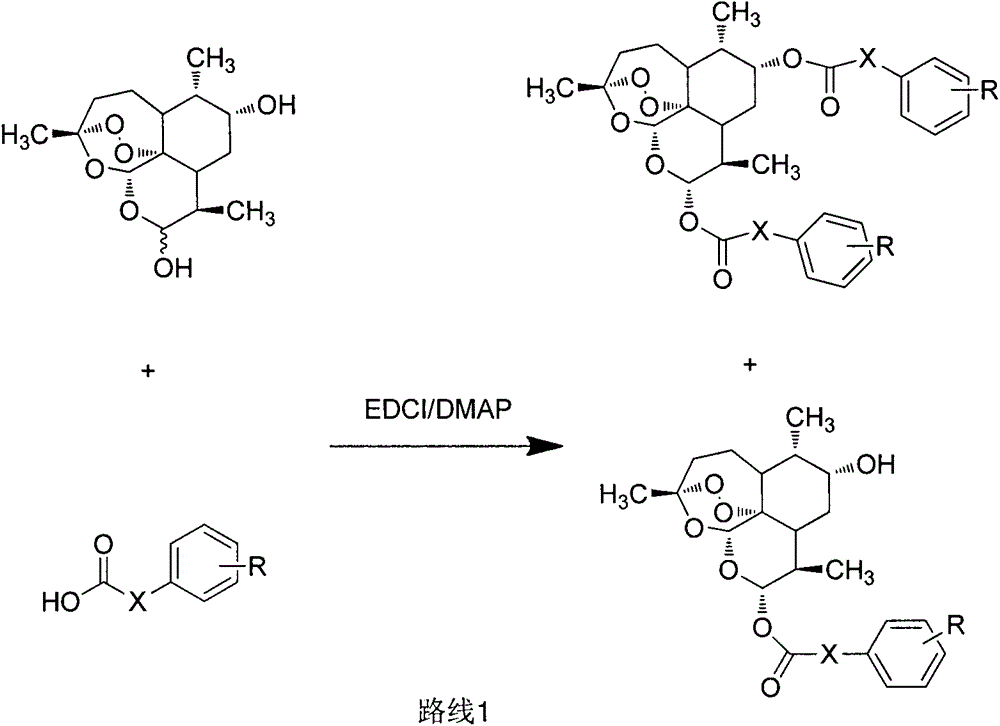
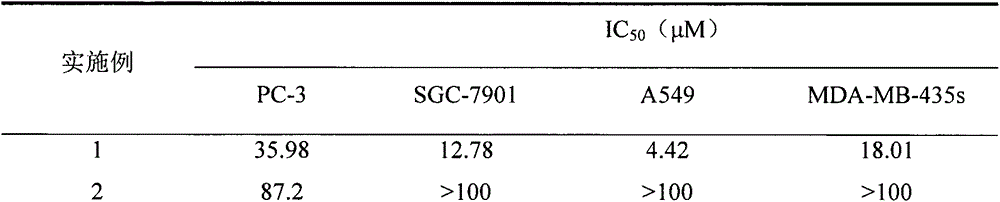
Novel hydroxyl dihydroartemisinin derivative and application thereof
InactiveCN105037384AAntitumor activityEasy to prepareOrganic active ingredientsOrganic chemistryImmunologic disordersAutoimmune condition
The invention belongs to the field of chemical medicines and relates to a novel hydroxyl dihydroartemisinin derivative and an application thereof. In particularly, the invention discloses the hydroxyl dihydroartemisinin derivative represented as the formula I and the formula II or a pharmaceutically acceptable salt thereof, or an enantiomer, a diastereoisomer or a racemate thereof, wherein X and R are defined as the specification. The invention also discloses the application of the compound in drugs of preventing and / or treating cancer and preventing and / or treating autoimmune diseases.
Owner:CHINA PHARM UNIV
Self-assembled targeting drug carrier nanoparticle and preparation method thereof
InactiveCN105879050AProlonged Circulatory Half-LifeHigh drug loadingPowder deliveryOrganic active ingredientsTarget–actionFreeze-drying
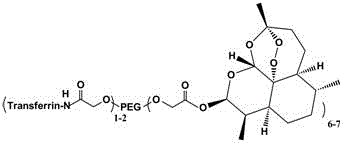
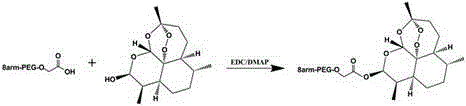
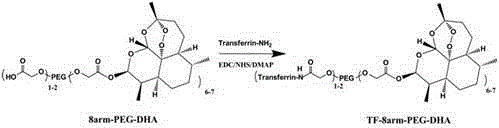
The invention discloses a method for preparing self-assembled targeting nano drug carrier particles, comprising: esterifying eight-armed carboxypolyethylene glycol with dihydroartemisinin to obtain eight-armed polyethylene glycol-dihydroartemisinin The eight-arm polyethylene glycol-dihydroartemisinin conjugate is activated by NHS; the activated conjugate is further chemically linked to the targeting molecule transferrin by using an amide bond; the eight-arm modified transferrin The polyethylene glycol-dihydroartemisinin conjugate removes impurities by dialysis, is filtered and freeze-dried; self-assembles to obtain transferrin-modified nano drug carrier particles. The nanoparticle has a double-layer structure, the outer layer is hydrophilic polyethylene glycol, and the inner layer is hydrophobic small molecule drug dihydroartemisinin. The advantages of the present invention: the use of eight-arm carboxypolyethylene glycol greatly increases the drug loading; the targeting effect of drugs on tumor cells and the pH-sensitive release in tumor cells can be realized; the targeted therapy reduces the toxic and side effects on normal tissues ; The preparation process is simple and easy to operate.
Owner:BEIJING FORESTRY UNIVERSITY
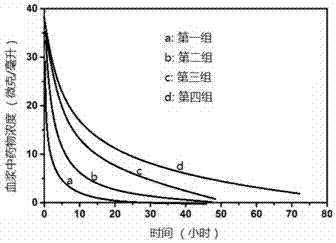
A method of simultaneously detecting the content of artesunate and the content of dihydroartemisinin in animal blood plasma
InactiveCN103940918AHigh purityImprove concentrationComponent separationSolid phase extractionSolvent
The invention discloses a method of simultaneously detecting the content of artesunate and the content of dihydroartemisinin in animal blood plasma. The method comprises steps of: extracting a blood plasma sample with a solvent, processing the sample liquid with a solid phase extraction technology, adding the sample liquid into a small processed solid-phase extraction column, washing the column with acetic acid and a methanol-acetic acid solution, eluting with ethyl acetate and 1-chorobutane, collecting the eluate, blowing the eluate to dry with nitrogen, dissolving residue with methanol so as to obtain a sample solution to be tested, and accurately measuring the content of the artesunate and the content of the dihydroartemisinin in the sheep blood plasma by utilization of HPLC-MS / MS. The lowest detectable limit of the artesunate is 0.1 ng*mL<-1>. The lowest detectable limit of the dihydroartemisinin is 1 ng*mL<-1>. The method reduces influences of impurities, enriches the sample concentration and is high in specificity and good in separation effect. Linearity, stability, reproducibility, and recovery tests of the method satisfy good technical requirements. The method lays methodology foundations for research of pharmacokinetics and bioequivalence of the medicine inside animals.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Application of artemisinin-typed compound in preparation of drug for treating and preventing hyperlipidemia
ActiveCN104398505AWide variety of sourcesLow priceOrganic active ingredientsMetabolism disorderPharmacologic actionSecondary hyperlipidemia
The invention discloses an application of an artemisinin-typed compound in preparation of a drug for treating and preventing hyperlipidemia. The artemisinin-typed compound is artemisinin, artesunate, dihydroartemisinin or artemether. The artemisinin-typed compound can be prepared into any pharmaceutically-acceptable preparation. The invention develops new medical applications of the artemisinin-typed compound. The application overcomes defect of a drug for treating and preventing the hyperlipidemia in the prior art. The drug can achieve a treatment effect from inflammatory resistance and blood fat reducing. Meanwhile, the artemisinin-typed compound is a drug which is firstly developed in our country, which means that our country is a main producing country, so that the artemisinin-typed compound is wide in sources and is low in price. In addition, the artemisinin-typed compound is strong in pharmacologic action, is high in safety, and provides an economical and safe drug for patients suffered from cardiovascular diseases.
Owner:SHANGHAI JIAO TONG UNIV
Multifunctional traditional Chinese medicinal anticancer nano-carrier and application thereof
ActiveCN105920611AOrganic active ingredientsPharmaceutical non-active ingredientsMedicineDihydroartemisinin
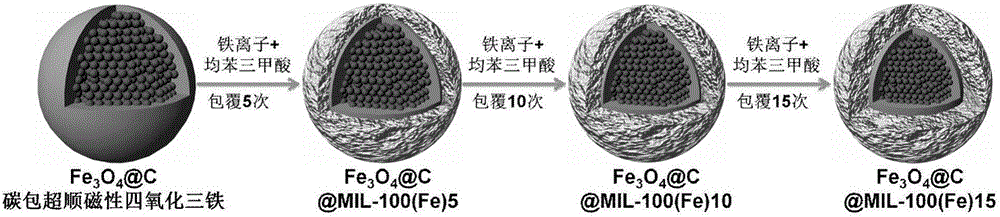
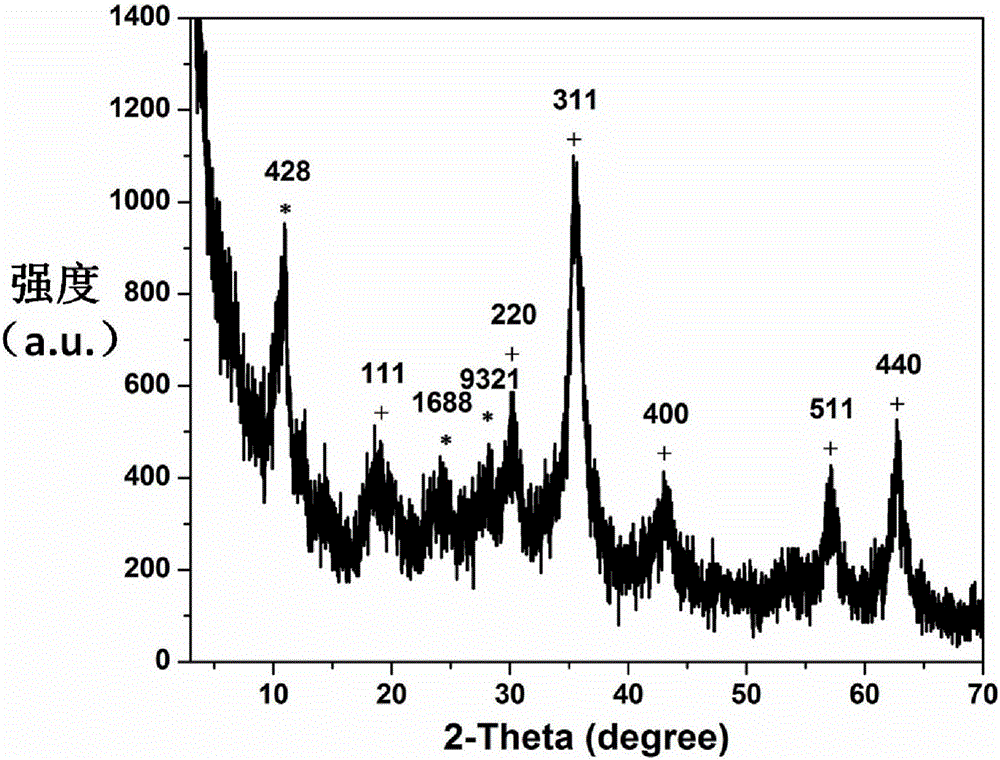
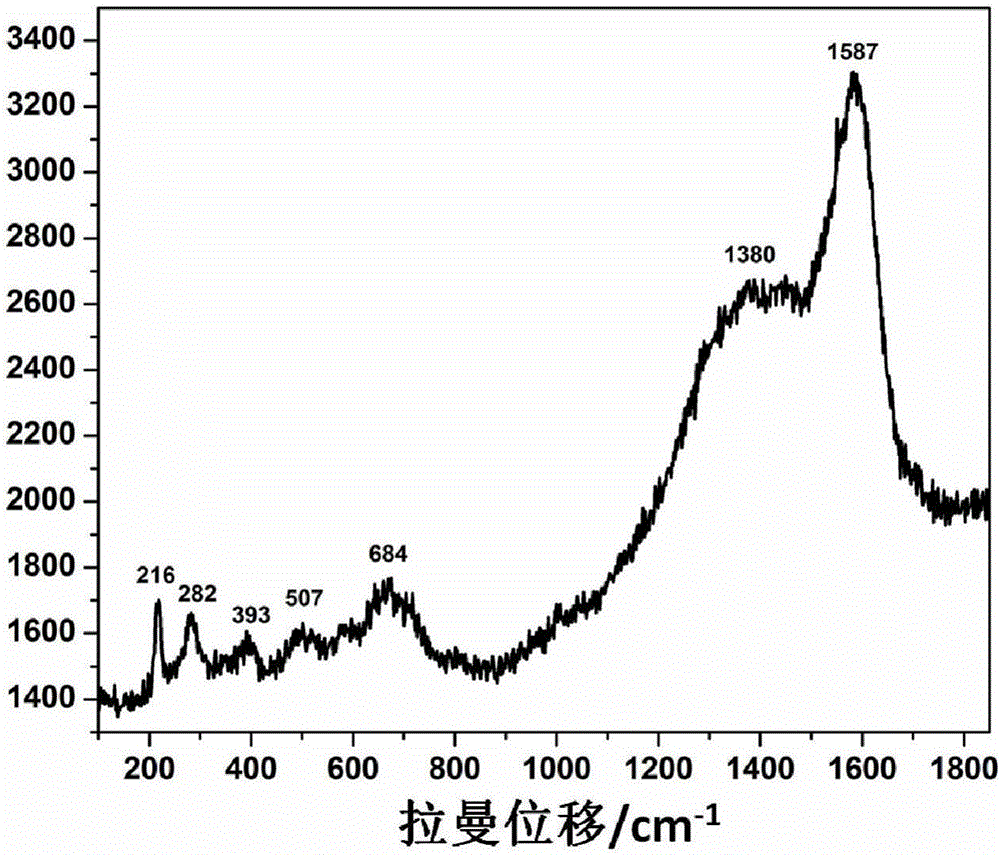
The invention discloses a multifunctional traditional Chinese medicinal anticancer nano-carrier and an application thereof, wherein the multifunctional traditional Chinese medicinal anticancer nano-carrier is in the form of carbon-coated superparamagnetic ferroferric oxide nanoparticles covered by an iron-based porous metal organic frame. The iron-based porous metal organic frame has a capacity of efficiently loading dihydroartemisinin; meanwhile, the iron-based porous metal organic frame, which covers the surfaces of the carbon-coated superparamagnetic ferroferric oxide nanoparticles, is degradable in a tumor cell slightly acidic environment, so that ferric ions and the dihydroartemisinin are synchronously released; the ferric ions are further reduced into bivalent ferrous ions by virtue of ferric reductase in cell, and the bivalent ferrous ions are capable of catalyzing the dihydroartemisinin to generate free radicals, so that the tumor cells are killed.
Owner:UNIV OF SCI & TECH OF CHINA
Fatty artemisinin emulsion and its prepn and application
InactiveCN1771909AExtended half-lifeImprove targetingOrganic active ingredientsEmulsion deliveryMedicineDihydroartemisinin
The present invention discloses one kind of fatty artemisinin emulsion and its preparation and application. The fatty artemisinin emulsion includes oil for injection, smulsifier, solubilizer and isotonizer, and has artemisinin or dihydroartemisinin well dissolved in oil and coated in emulsified oil phase, so that it has high stability, strengthened medicinal effect, slow releasing and targeting administration effect, prolonged medicine residence time in blood and raised bioavailability.
Owner:裴蕾
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com