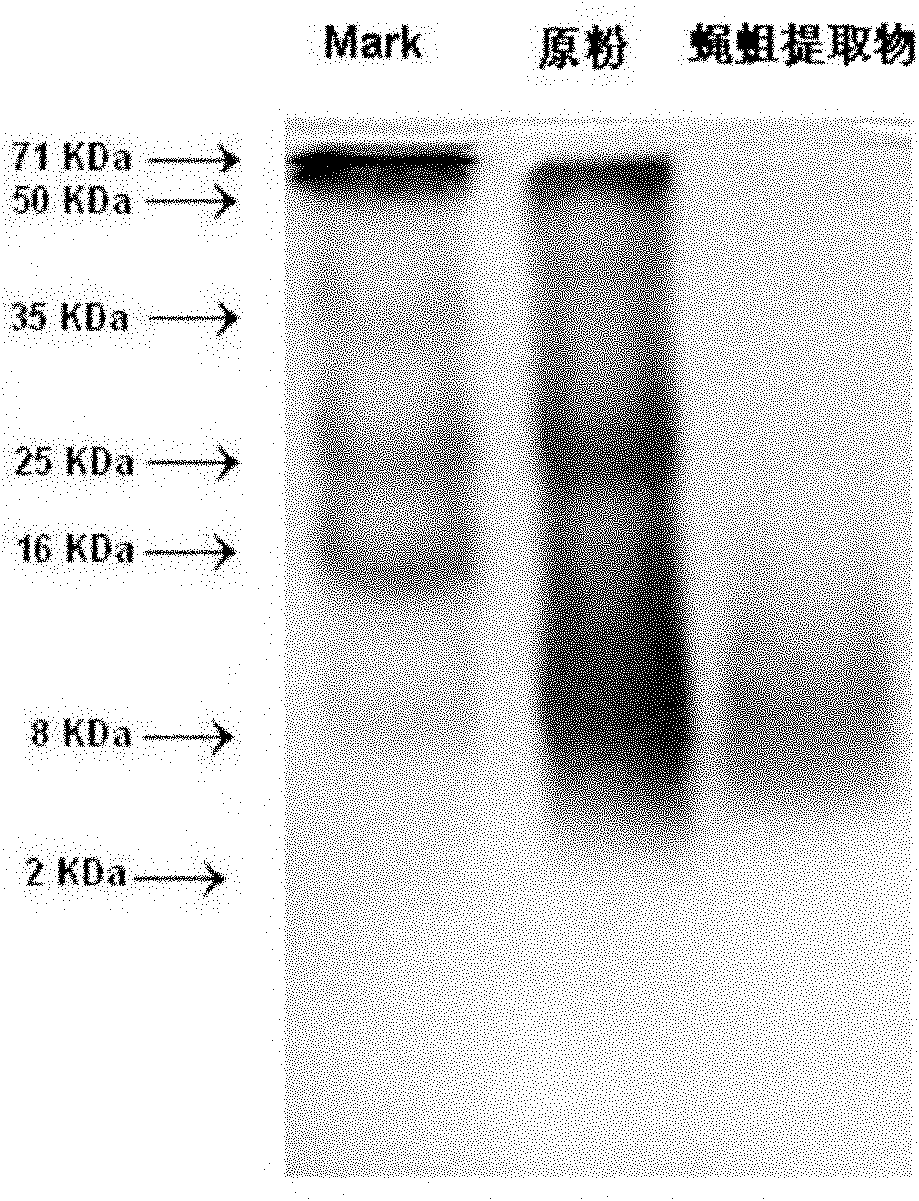Patents
Literature
715 results about "Leukaemia cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Leukaemia is a cancer of cells in the bone marrow (the cells which develop into white blood cells). Cancer is a disease of the cells in the body.
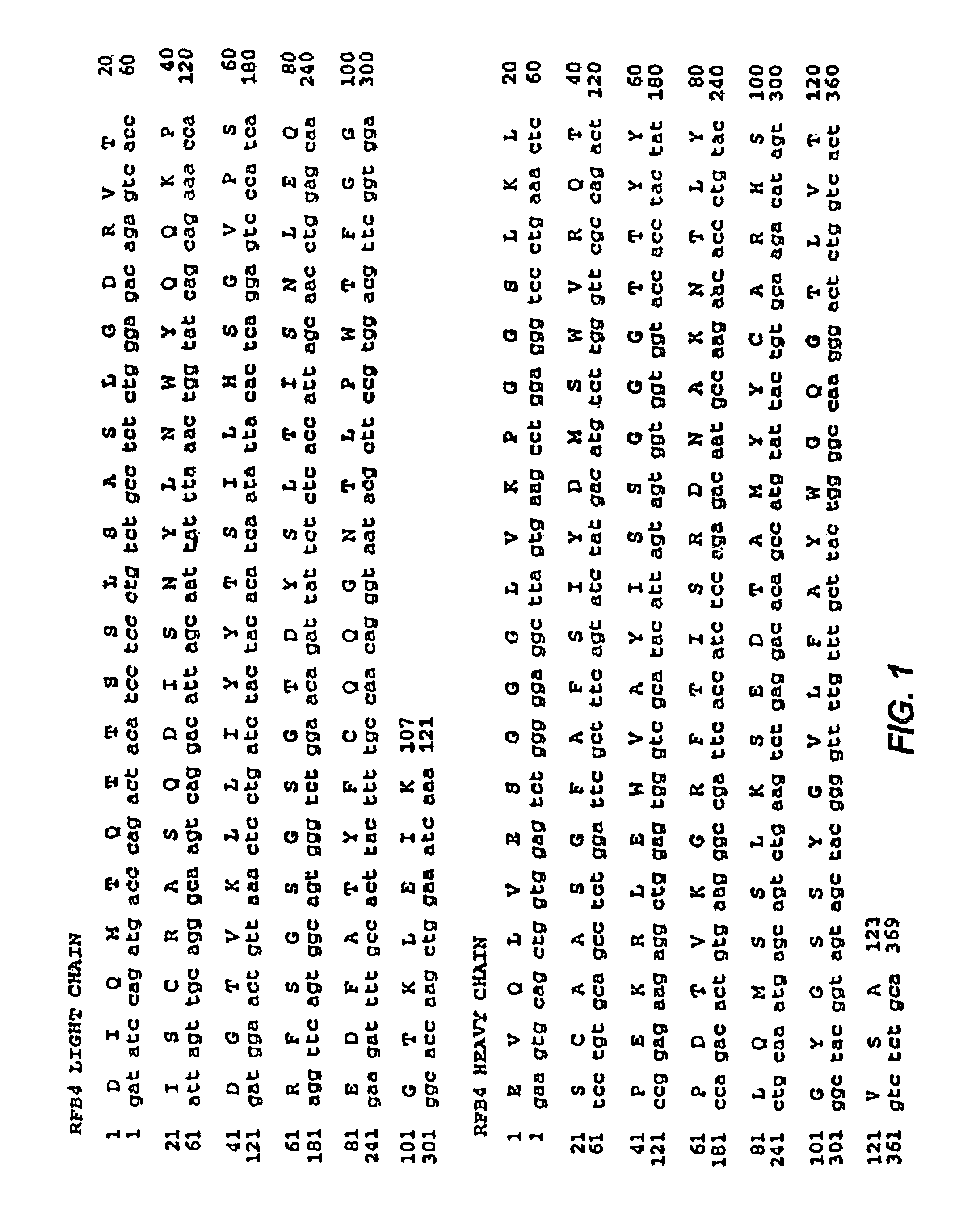
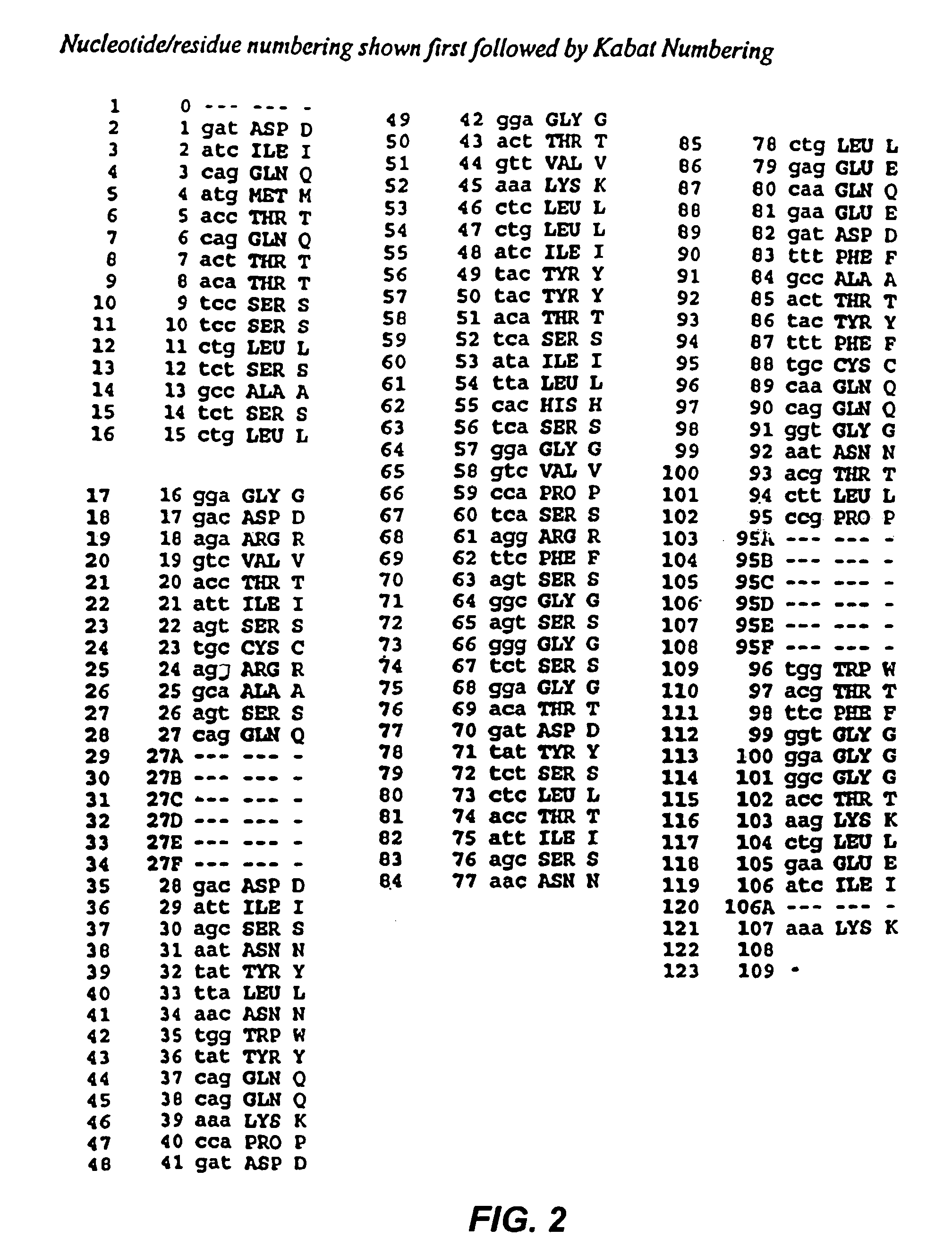
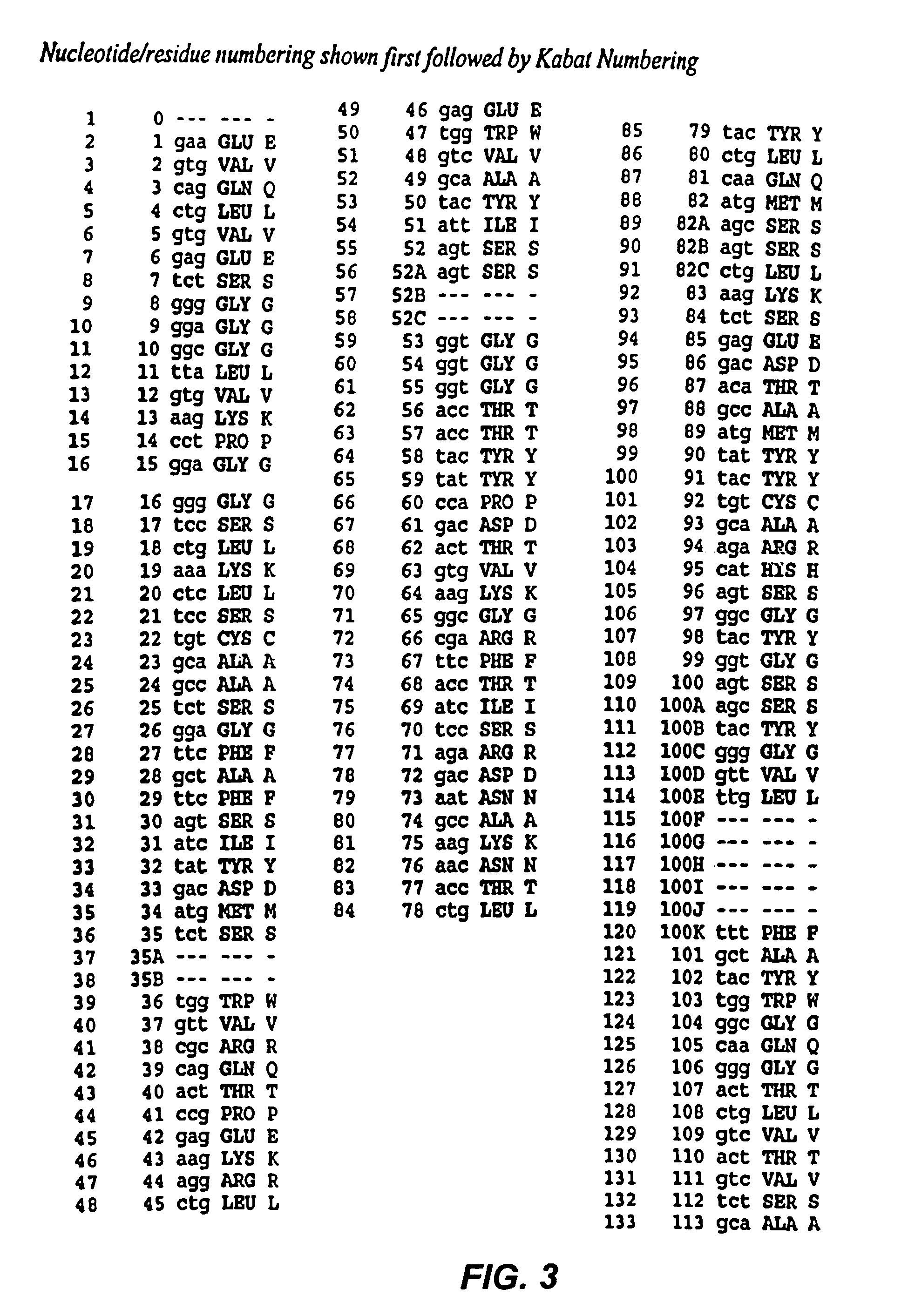
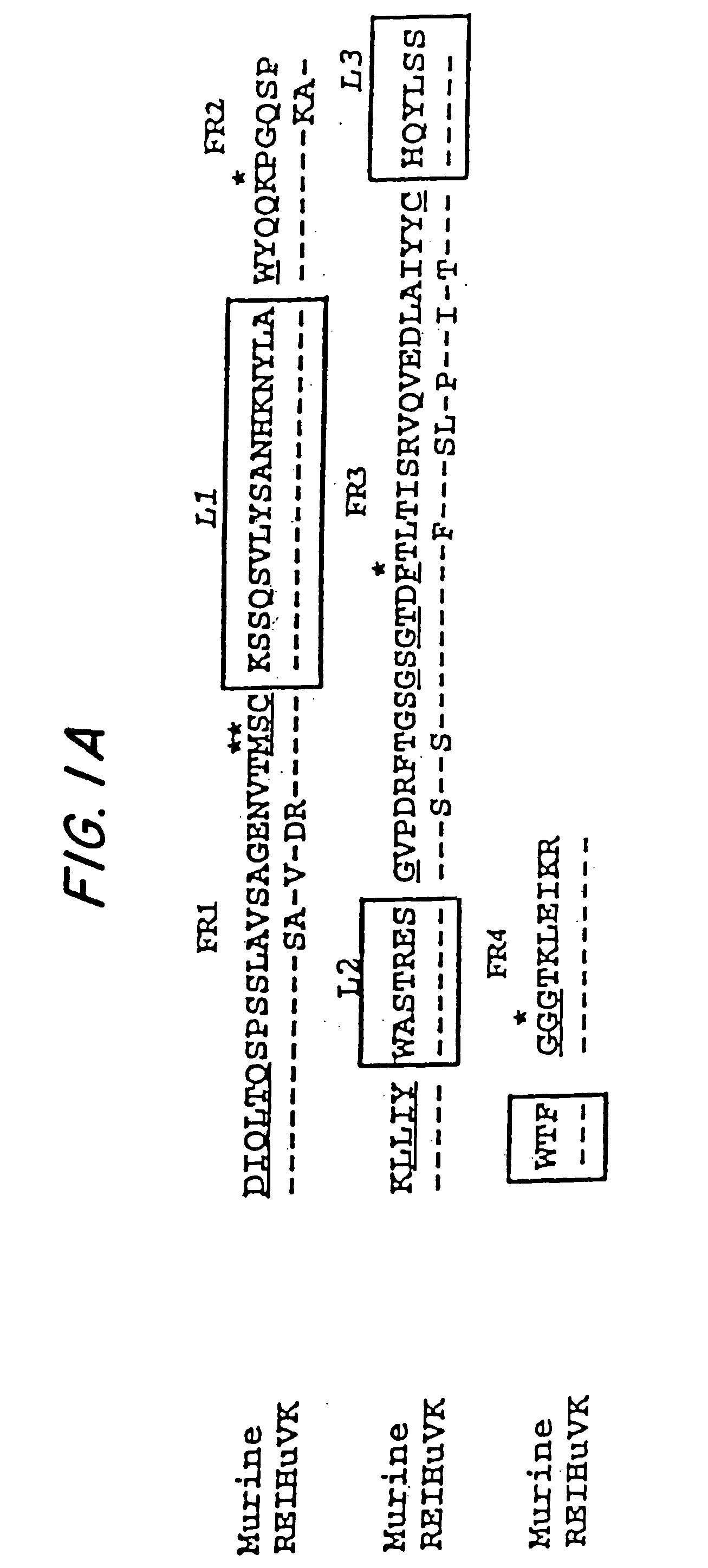
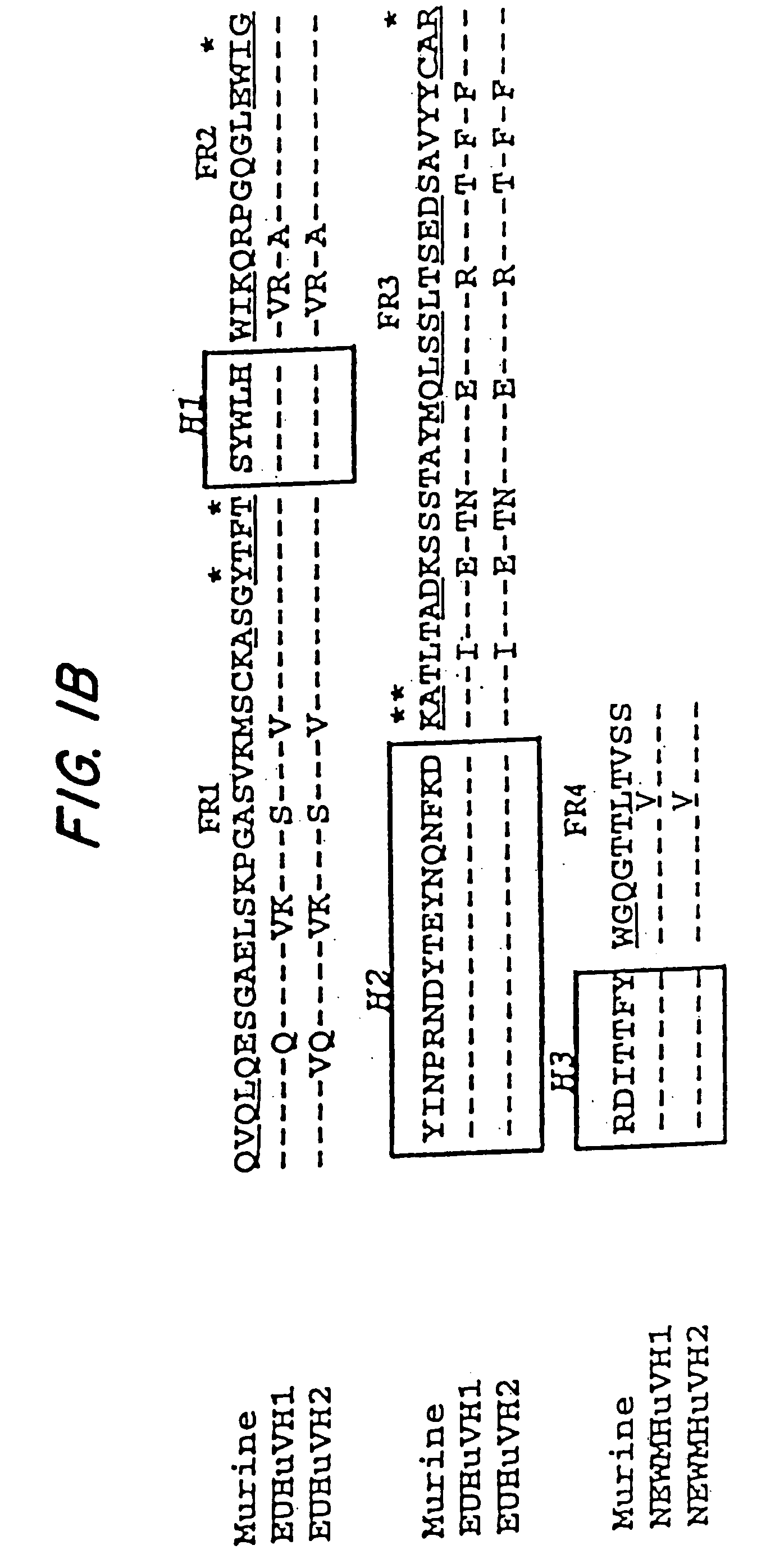
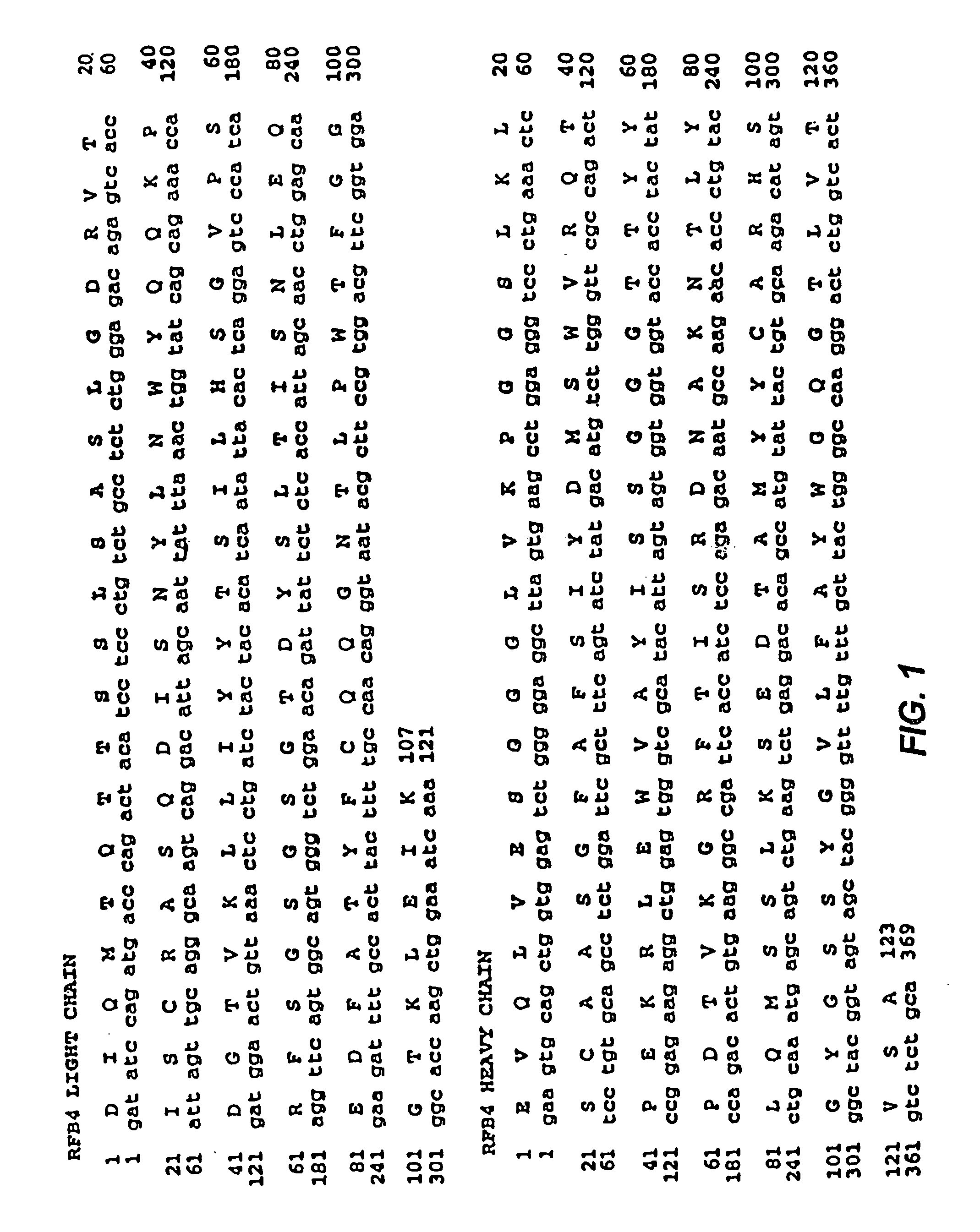
Mutated anti-CD22 antibodies with increased affinity to CD22-expressing leukemia cells
ActiveUS7355012B2Snake antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenBacteroides
Recombinant immunotoxins are fusion proteins composed of the Fv domains of antibodies fused to bacterial or plant toxins. RFB4 (Fv)-PE38 is an immunotoxin that targets CD22 expressed on B cells and B cell malignancies. The present invention provides antibodies and antibody fragments that have improved ability to bind the CD22 antigen of B cells and B cell malignancies compared to RFB4. Immunotoxins made with the antibodies and antibody fragments of the invention have improved cytotoxicity to CD22-expressing cancer cells. Compositions that incorporate these antibodies into chimeric immunotoxin molecules that can be used in medicaments and methods for inhibiting the growth and proliferation of leukemia and lymphoma cells.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES
Immunoconjugates and humanized antibodies specific for B-cell lymphoma and leukemia cells
InactiveUS20050106108A1Lowered HAMA reactionReduced responsePeptide/protein ingredientsAntibody mimetics/scaffoldsHuman antimouse AntibodyComplementarity determining region
A chimeric LL2 monoclonal antibody is described in which the complementarity determining regions (CDRs) of the light and heavy chains of the murine LL2 anti-B-lymphoma, anti-leukemia cell monoclona lantibody has been recombinantly joined to the human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-cell lymphoma and leukemia cell internalization capacity of the parental murine LL2 monoclonal antibody, and which has the potential of exhibiting reduced human anti-mouse antibody production activity. A humanized LL2 monoclonal antibody is described in which the CDRs of the light and heavy chains have been recombinantly joined to a framework sequence of human light and heavy chains variable regions, respectively, and subsequently linked to human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-lymphoma and leukemia cell internalization capacities of the parental murine and chimeric LL2 monoclonal antibodies, and which has the potential for exhibiting reduced human anti-mouse antibody production activity. Vectors for producing recombinant chimeric and humanized chimeric monoclonal antibodies are provided. Isolated DNAs encoding the amino acid sequences of the LL2 variable light and heavy chain and CDR framework regions are described. Conjugates of chimeric and humanized chimeric LL2 antibodies with cytotoxic agents or labels find use in therapy and diagnosis of B-cell lymphomas and leukemias.
Owner:IMMUNOMEDICS INC
Biological compositions and methods for treatment of leukemia
The present invention relates to pharmaceutical compositions and dietary supplement comprising yeast cells that can produce a healthful benefit in a subject inflicted with leukemia. The biological compositions can be used to reduce the number of leukemia cells and / or prolonging the time of survival of the subject. The invention also relates to methods for manufacturing the biological compositions.
Owner:ULTRA BIOTECH
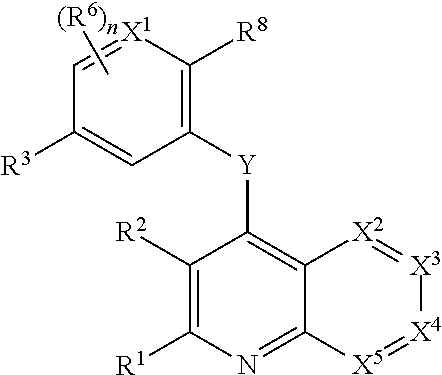
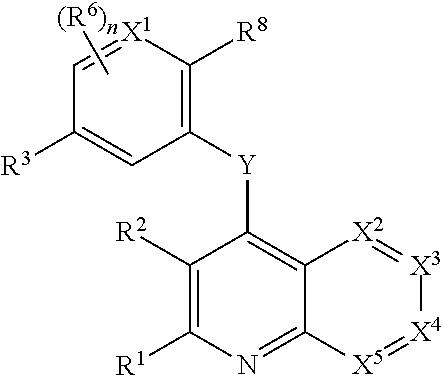
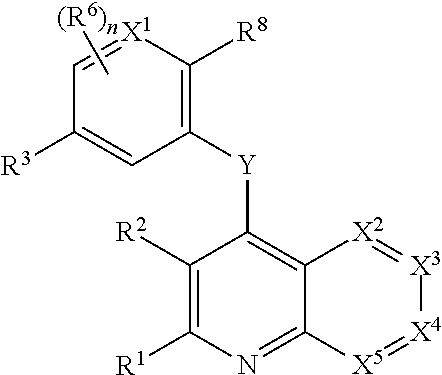
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjogren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
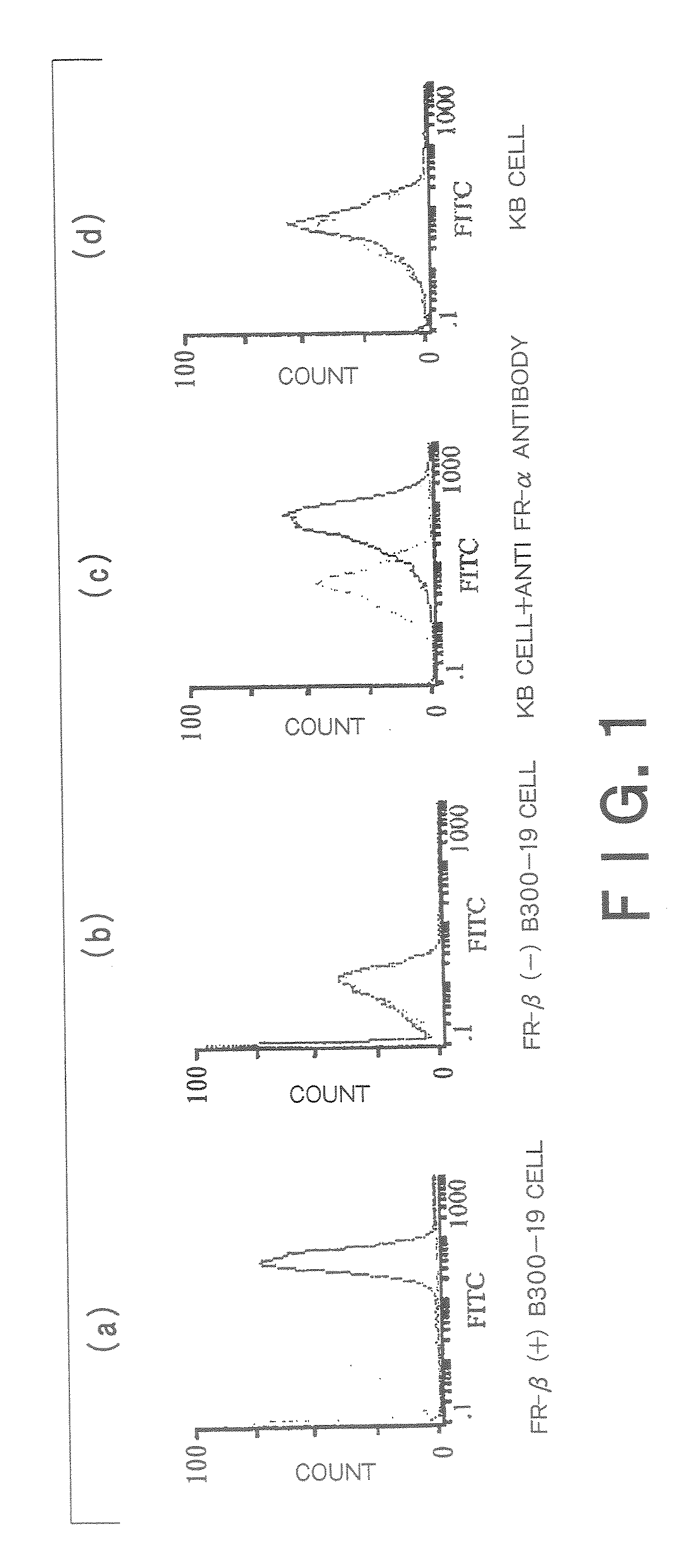
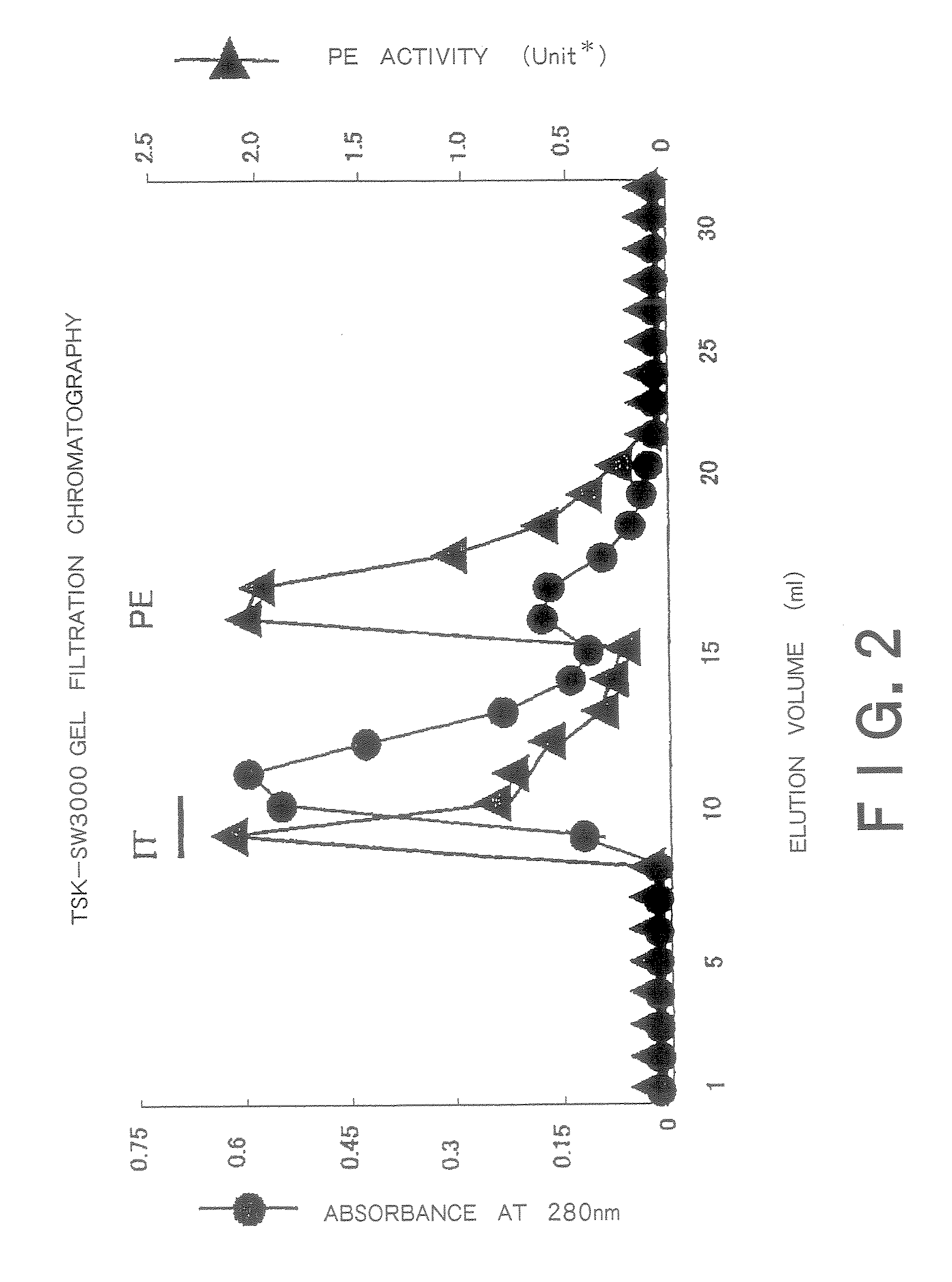
Therapeutic Medicine Containing Monoclonal Antibody Against Folate Receptor Beta (Fr-Beta)
InactiveUS20080260812A1Good chemical stabilitySufficient amountPeptide/protein ingredientsAntipyreticMacrophage activation syndromeArthritis therapy
An objective of the present invention is to provide a therapeutic agent for treating rheumatoid arthritis, juvenile rheumatoid arthritis, macrophage activation syndrome, septicemia, and FR-β expressing leukemia, which induces apoptosis in activated macrophages and folate receptor beta (FR-β) expressing leukemia cells to specifically destroy these cells. An FR-β monoclonal antibody of the present invention is preferably an IgG-type monoclonal antibody which specifically reacts with a human-type FR-β antigen and is produced from a clone resulting from immunization with an FR-β expressing B300-19 cell. The FR-β monoclonal antibody of the present invention specifically reacts with the FR-β antigen of activated macrophages and FR-β expressing leukemia cells and a therapeutic agent of the present invention contains an FR-β antibody immunotoxin which causes apoptosis in activated macrophages and FR-β expressing leukemia cells, as an active ingredient. Further, suitable administration form for the therapeutic agent of the present invention includes intravenous injection as well as joint injection in the case of therapeutic agents for rheumatoid arthritis and juvenile arthritis.
Owner:TAKAMI MATSUYAMA KAGOSHIMA UNIV +1
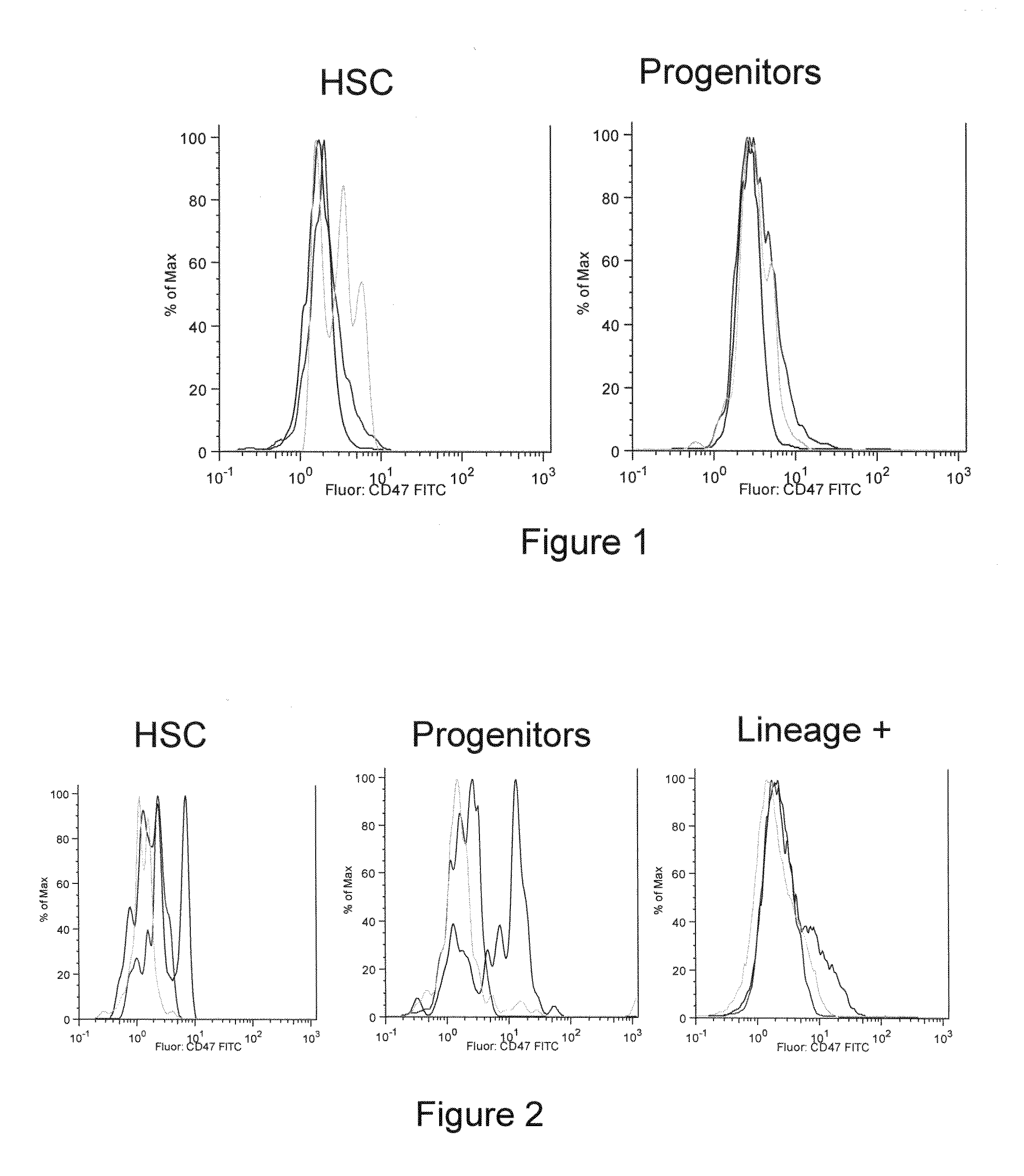
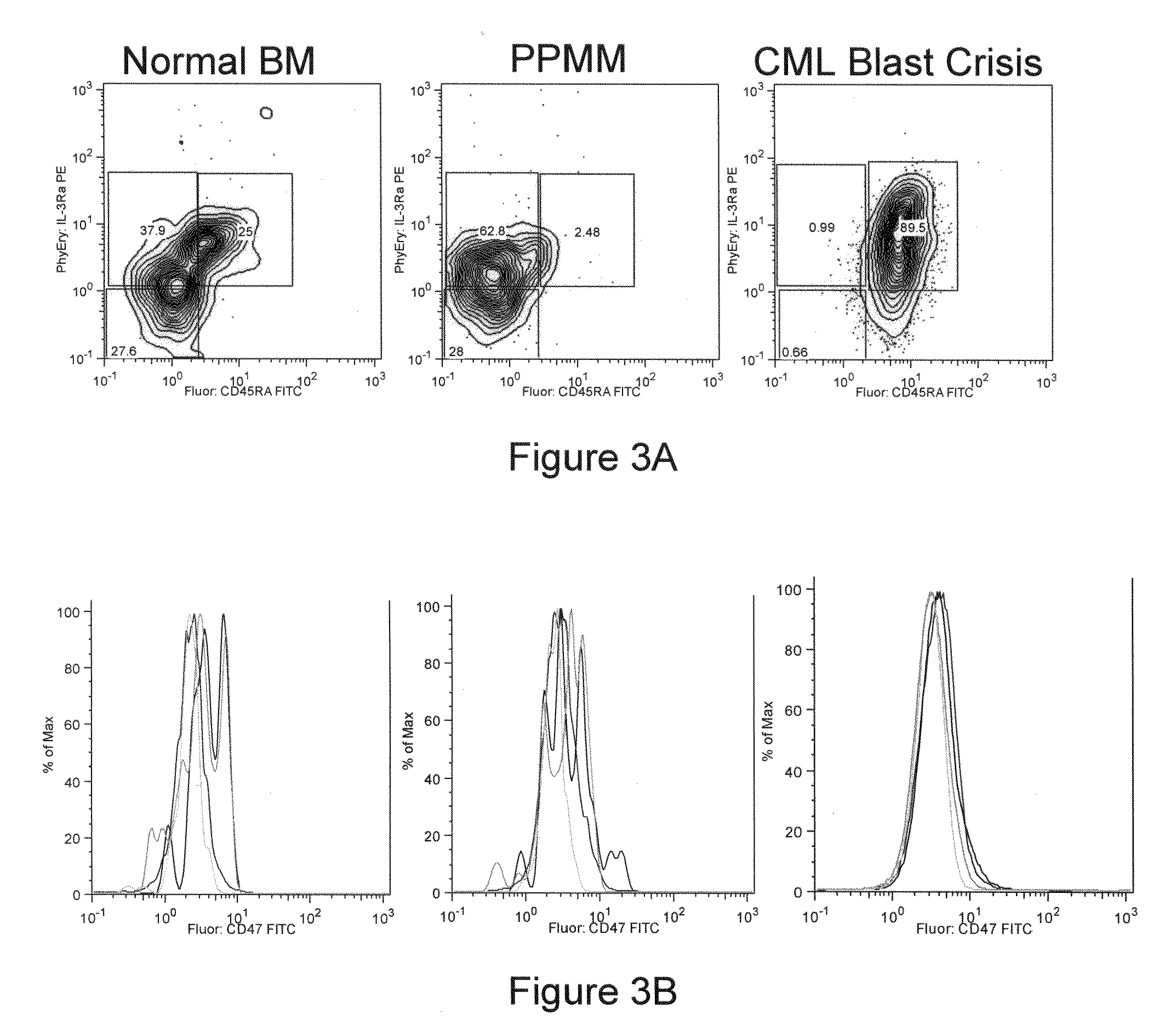
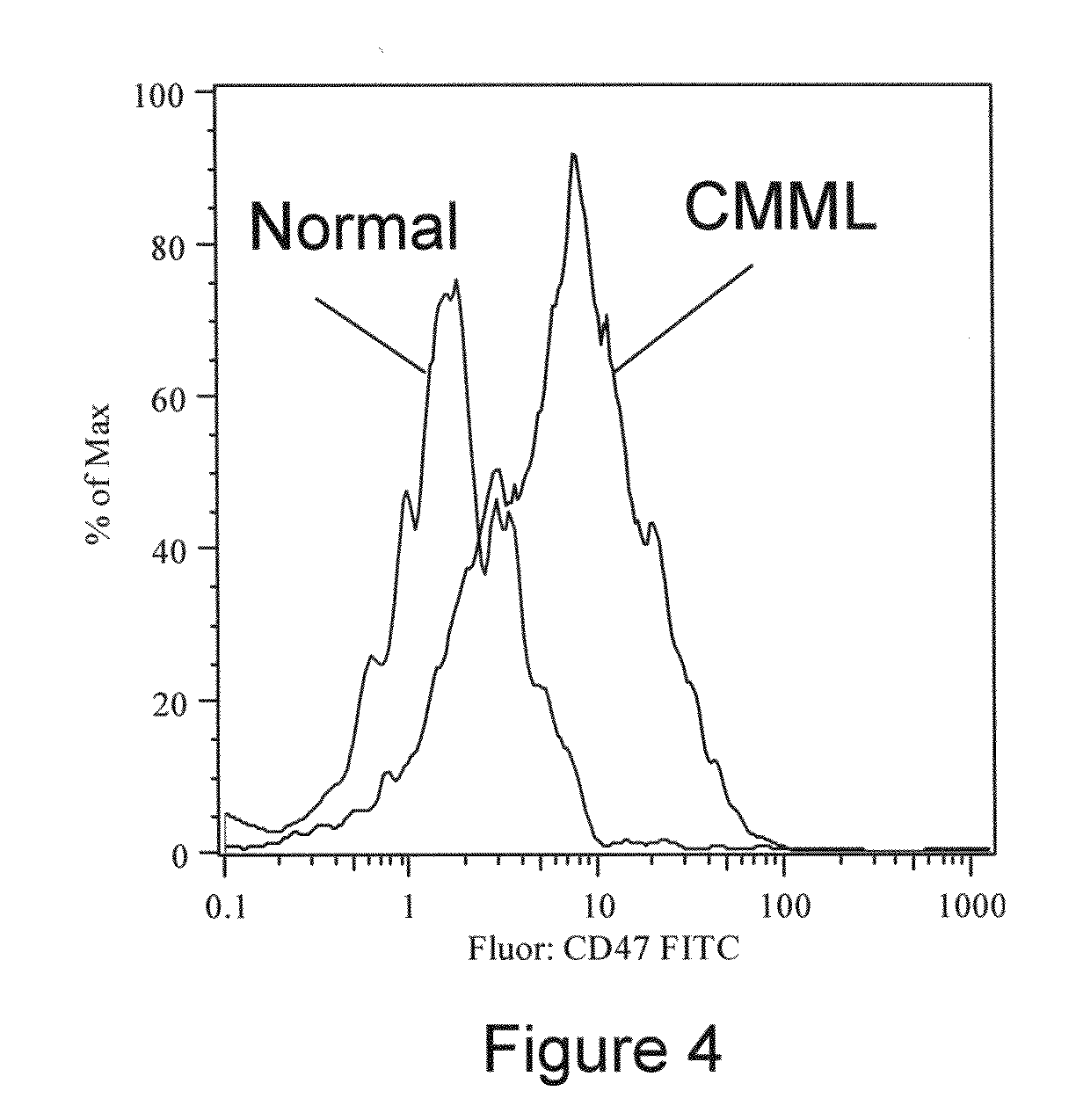
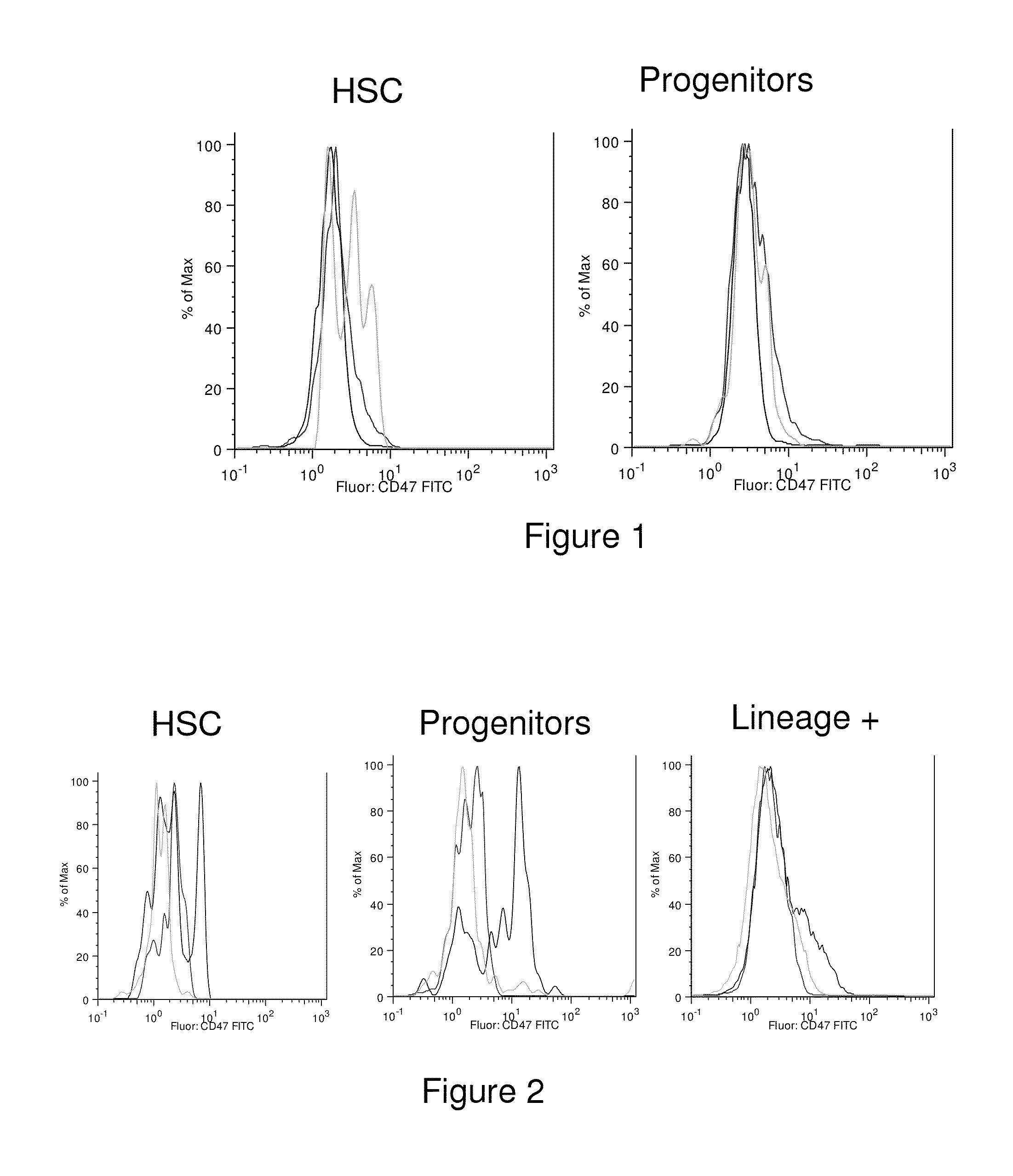
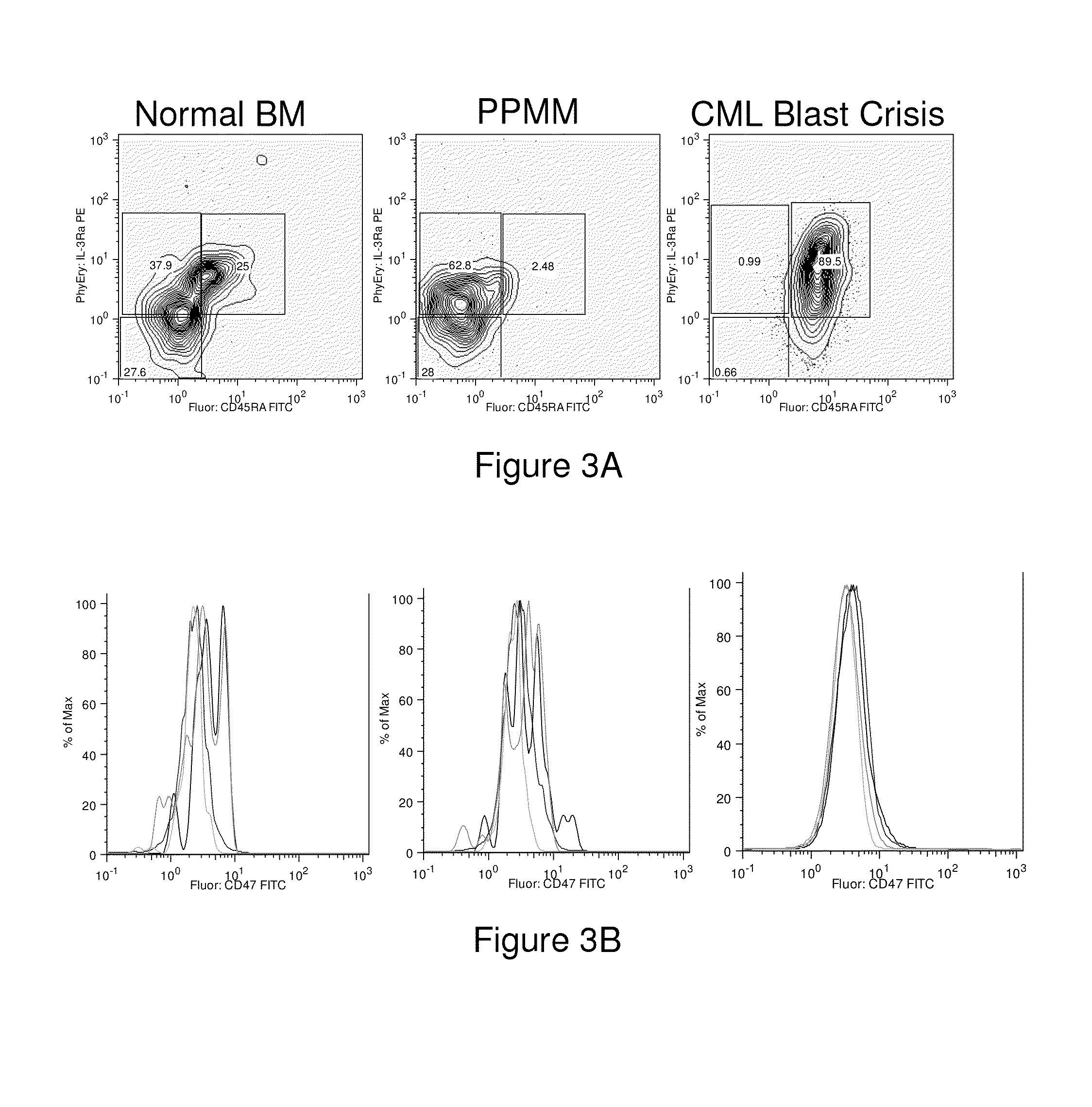
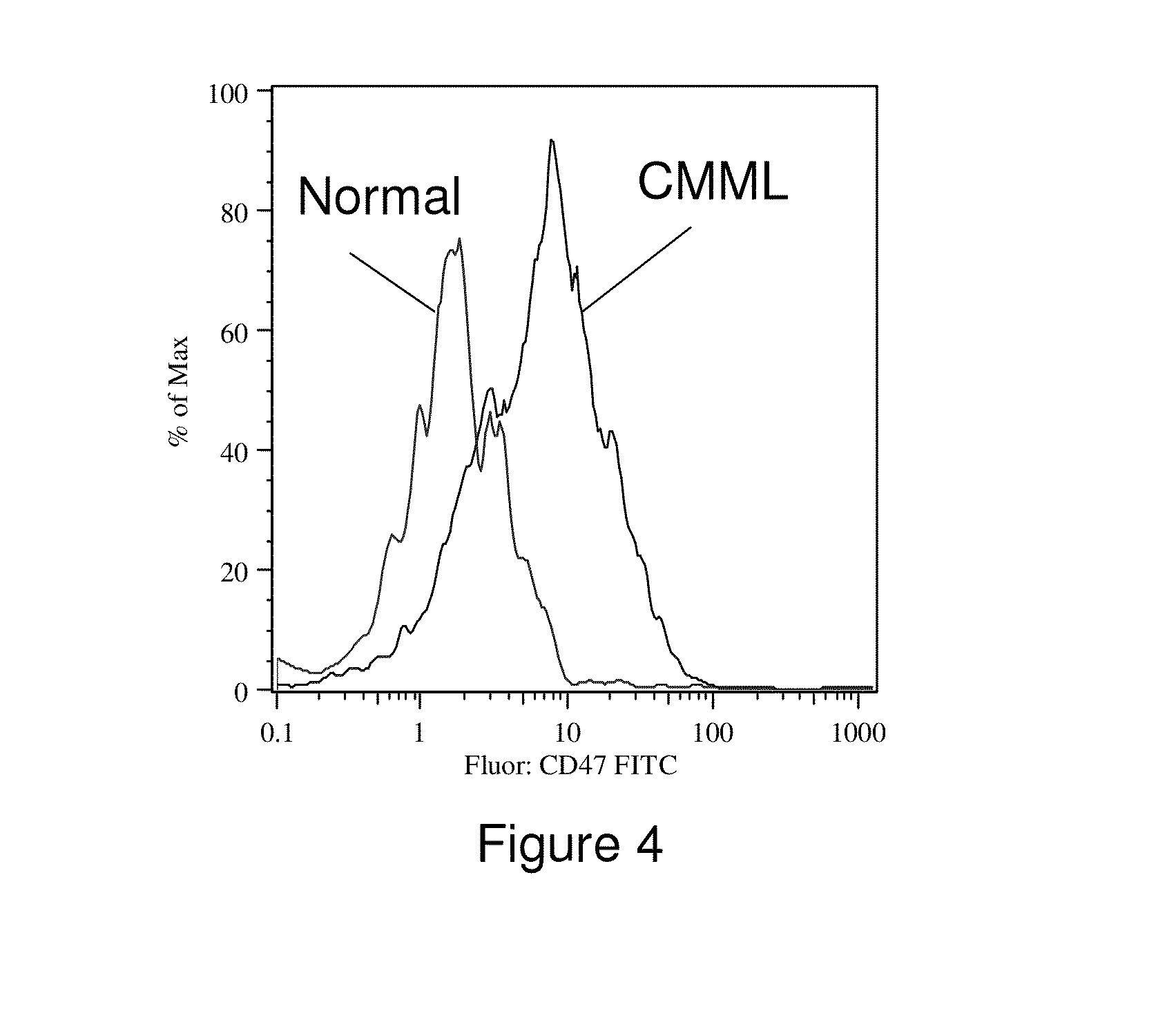
Methods for manipulating phagocytosis mediated by CD47
InactiveUS20090191202A1Enhance phagocytosisPeptide/protein ingredientsMammal material medical ingredientsHematopoietic cellSolid tumor
Methods are provided to manipulate phagocytosis of cells, including hematopoietic cells, e.g. circulating hematopoietic cells, bone marrow cells, etc.; and solid tumor cells. In some embodiments of the invention the circulating cells are hematopoietic stem cells, or hematopoietic progenitor cells, particularly in a transplantation context, where protection from phagocytosis is desirable. In other embodiments the circulating cells are leukemia cells, particularly acute myeloid leukemia (AML), where increased phagocytosis is desirable.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Mutated anti-cd22 antibodies with increased affinity to cd22-expressing leukemia cells
Recombinant immunotoxins are fusion proteins composed of the Fv domains of antibodies fused to bacterial or plant toxins. RFB4 (Fv)-PE38 is an immunotoxin that targets CD22 expressed on B cells and B cell malignancies. The present invention provides antibodies and antibody fragments that have improved ability to bind the CD22 antigen of B cells and B cell malignancies compared to RFB4. Immunotoxins made with the antibodies and antibody fragments of the invention have improved cytotoxicity to CD22-expressing cancer cells. Compositions that incorporate these antibodies into chimeric immunotoxin molecules that can be used in medicaments and methods for inhibiting the growth and proliferation of leukemia and lymphoma cells.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES
Potentiation of anti-CD38-Immunotoxin cytotoxicity
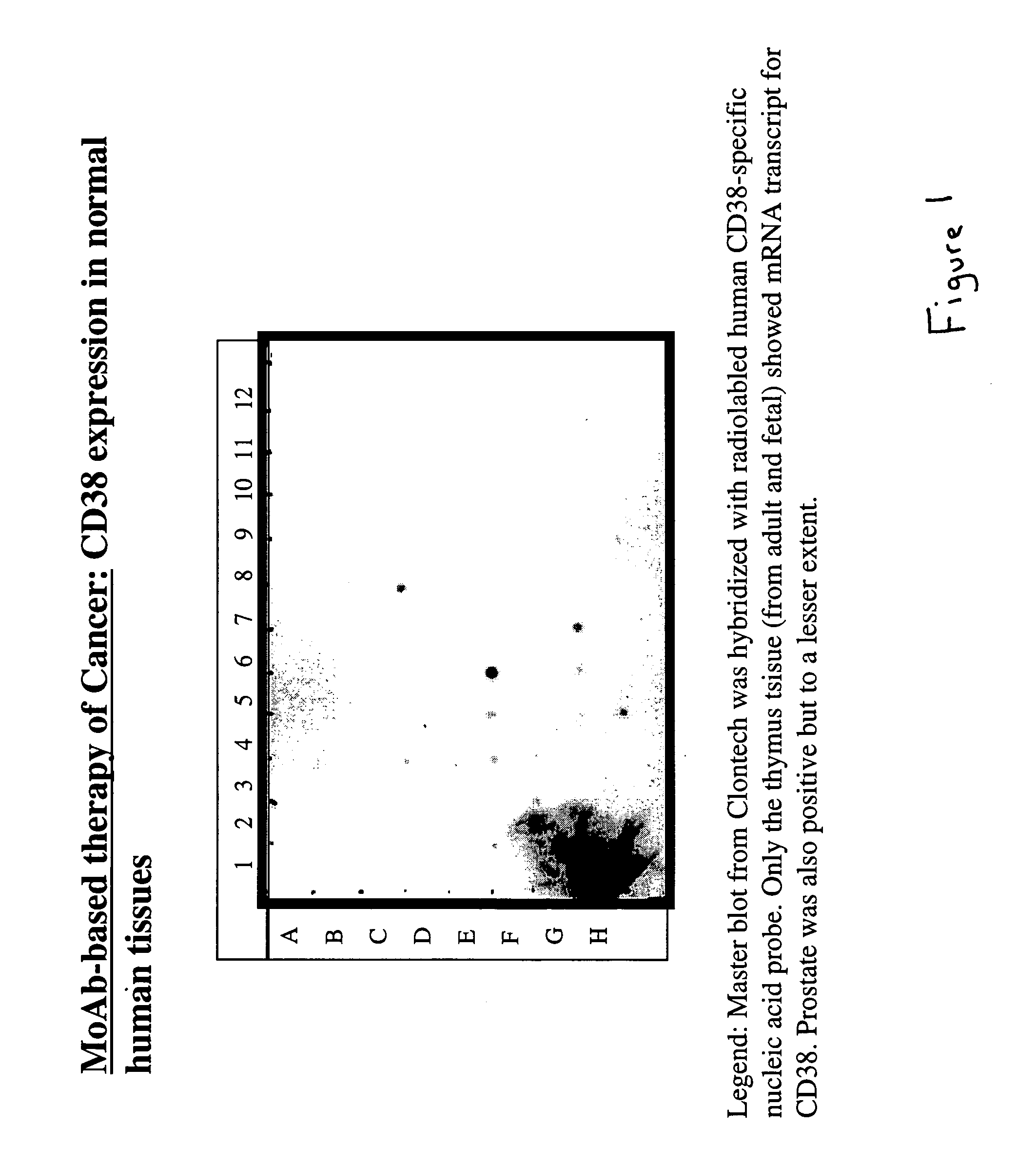
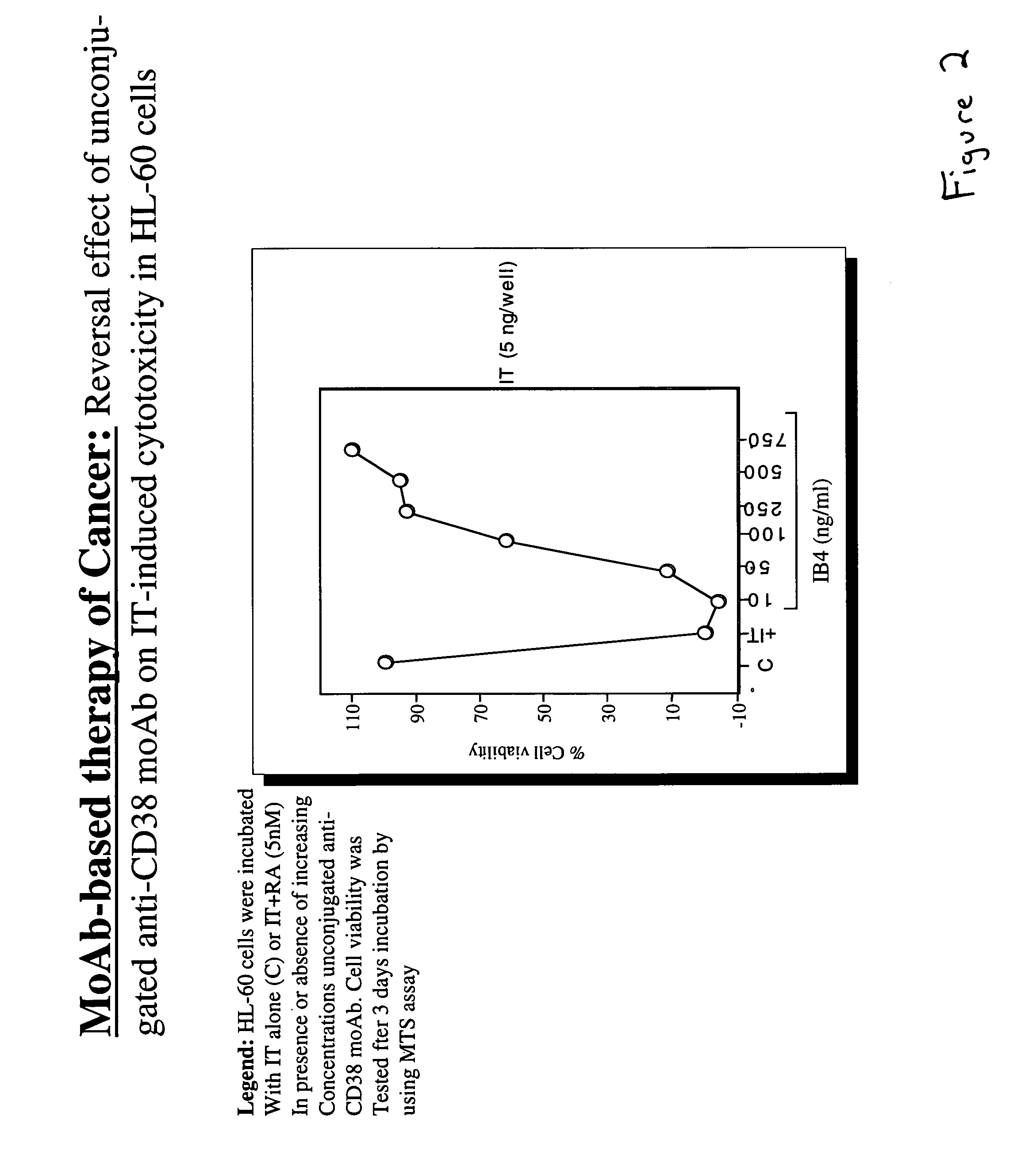
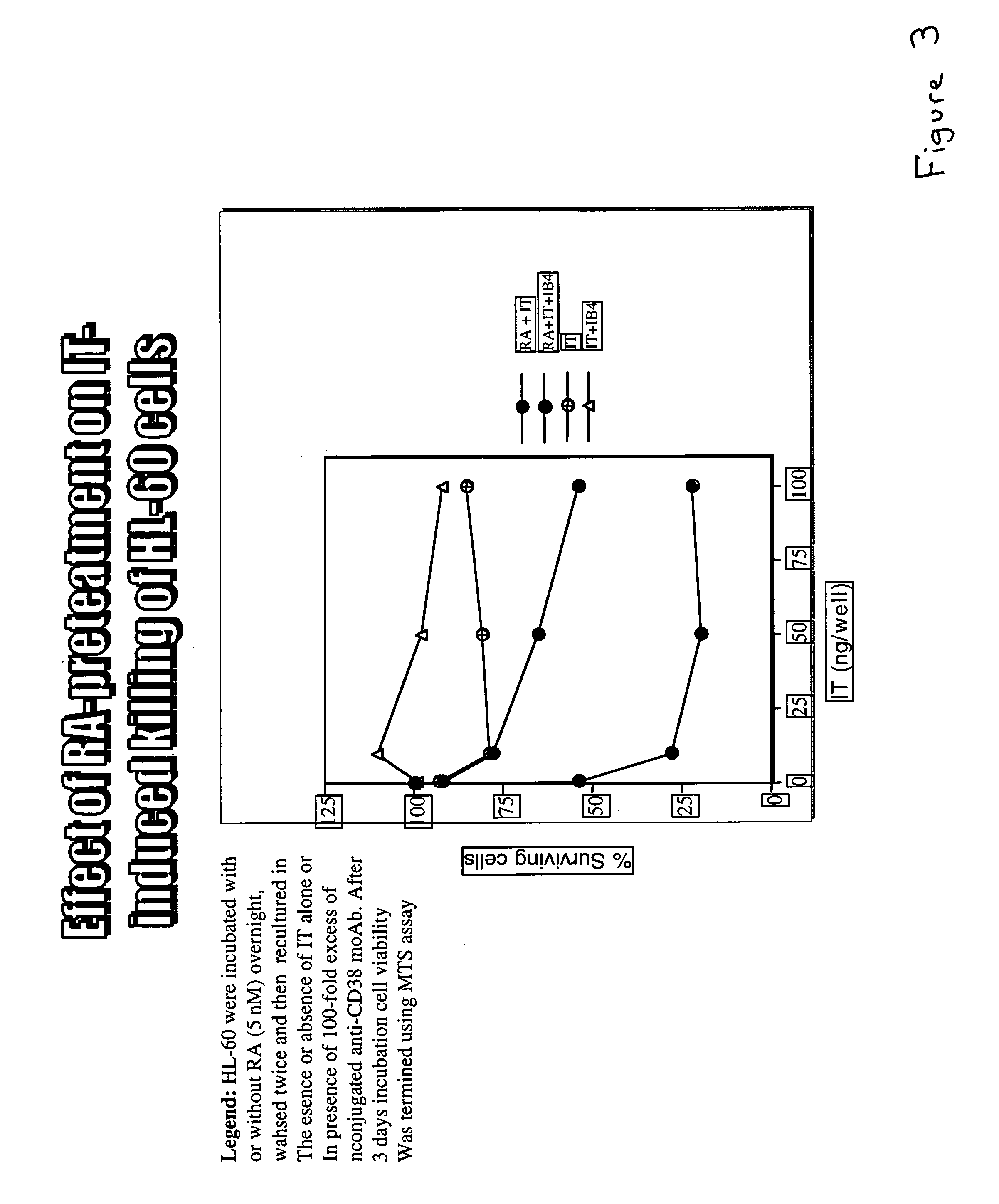
The present invention is directed to the use of agents that induce high levels of cell surface molecules to provide targets for immunotoxins directed against the same cell surface molecules. A specific example is given in which all-trans-retinoic acid (RA) is used to induce high levels of CD38 cell surface antigen expression in several myeloid and lymphoid leukemia cells. CD38 was then used as target for delivering plant toxin (gelonin) to leukemia cells. Treatment of leukemia cells with RA induced high levels of CD38 in those cells that otherwise had low CD38 expression. The RA-induced leukemia cells then became exquisitely sensitive to an immunotoxin constructed from an anti-CD38 monoclonal antibody conjugated to the plant toxin gelonin.
Owner:BOARD OF REGENTS
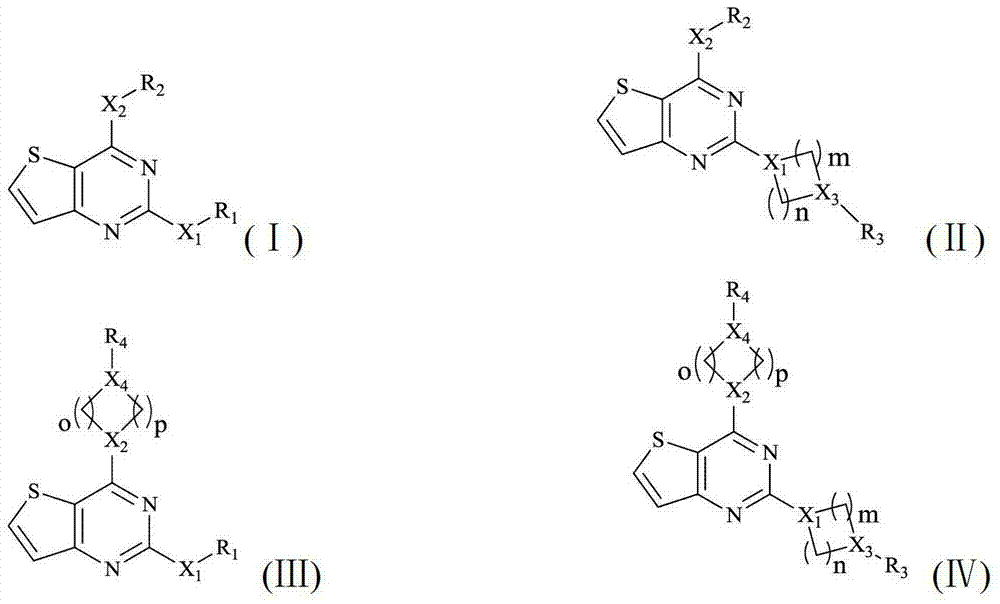
Thieno 2,4-substituted pyrimidine compound, and pharmaceutical composition and application thereof
InactiveCN103242341AInhibit abnormal expressionPrevent proliferationOrganic active ingredientsNervous disorderDiseaseCancer cell
The invention discloses a thieno 2,4-substituted pyrimidine compound of which the general formulas are (I), (II), (III) and (IV), or pharmaceutically acceptable salt or stereisomer or a prodrug molecule, and a pharmaceutical composition and application thereof. The thieno 2,4-substituted pyrimidine compound can effectively restrain abnormal expression of Aurora kinase, has specific inhibited effect on Aurora-A and Aurora-B, can be applied to a novel field of molecular targeting treatment, and has strong inhibitory activity on an excessive hyperplasia disease, especially cervical tumor cells, human macrophage line leukemia cell, and human t-lymphocyte line cancer cell.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
CD19 targeting chimeric antigen receptor and NKT cell, and preparation method thereof and applications thereof
ActiveCN105418765AEnhance specific killing activityProlong survival timePeptide/protein ingredientsGenetic material ingredientsAntigenHinge region
The present invention discloses a chimeric antigen receptor, a gene and a recombinant expression vector thereof, an engineered CD19 targeting NKT cell and applications thereof. The chimeric antigen receptor is CD19ScFv-CD8-CD137-CD3 zeta, and consists of a hinge region and a transmembrane region of the CD19ScFv and CD8, an intracellular signal structural domain of CD137, and an intracellular signal structural domain of CD3 zeta, and the the hinge region, the transmembrane region, the intracellular signal structural domain of CD137 and the intracellular signal structural domain of CD3 zeta are connected in series. When used to treat advanced stage CD19-positive B-cell acute lymphocytic leukemia, the NKT cell modified by the chimeric antigen receptor CD19ScFv-CD8-CD137-CD3 zeta provided by the present invention has good specific killing activity on leukemic cells, and has certain therapeutic effect on advanced stage CD19-positive B-cell acute lymphocytic leukemia patients who are repeatedly subjected to therapy such as radiotherapy, chemotherapy and symptomatic therapy by other drugs but cannot recover obviously.
Owner:CELLULAR BIOMEDICINE GRP SHANGHAI +1
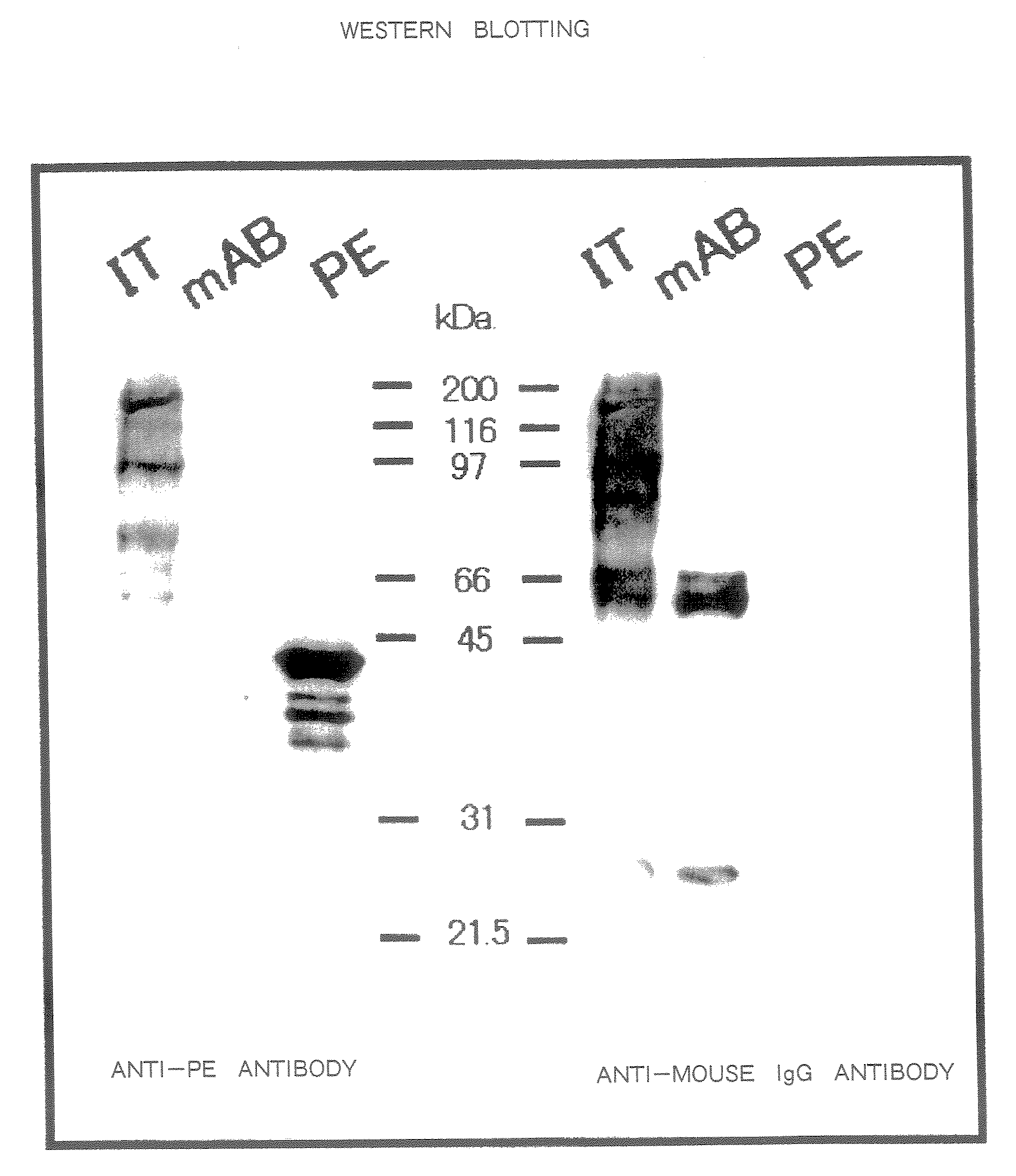
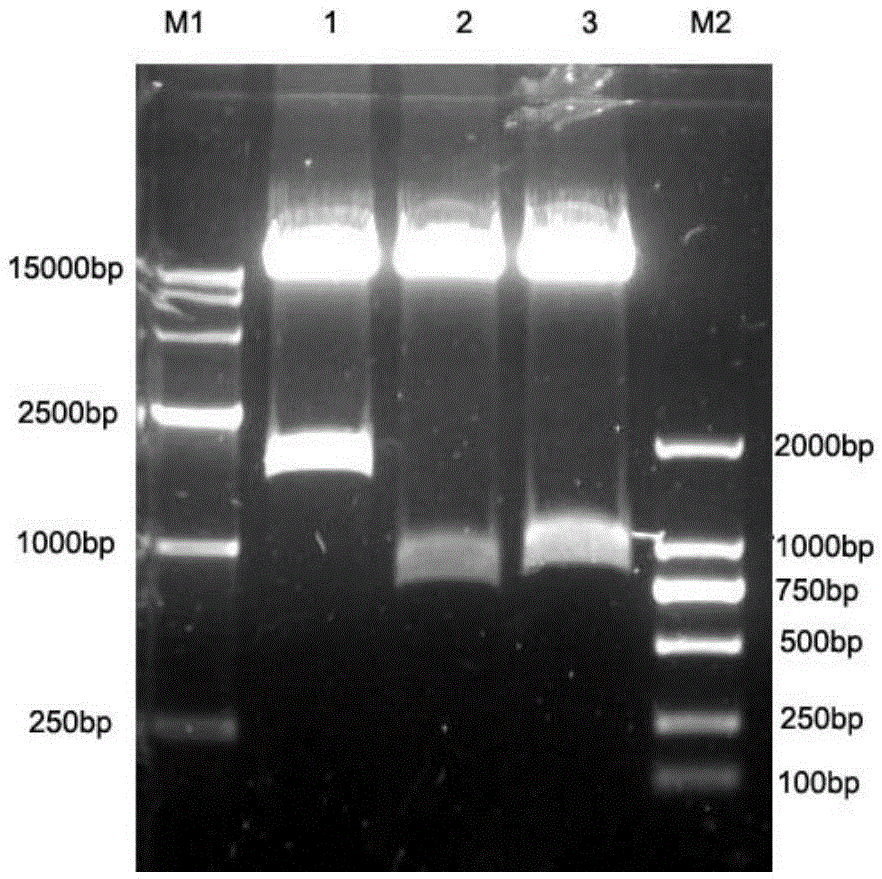
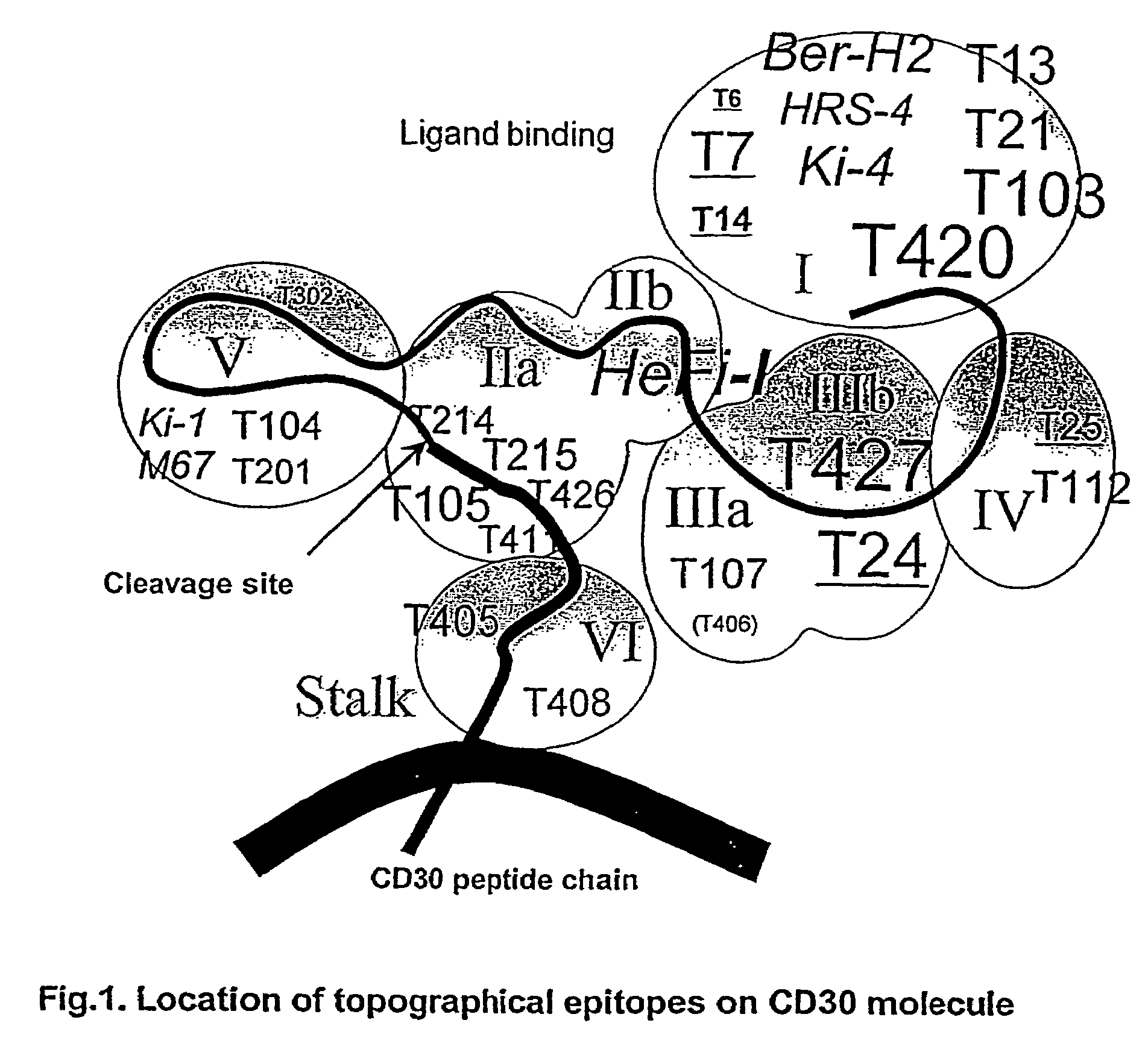
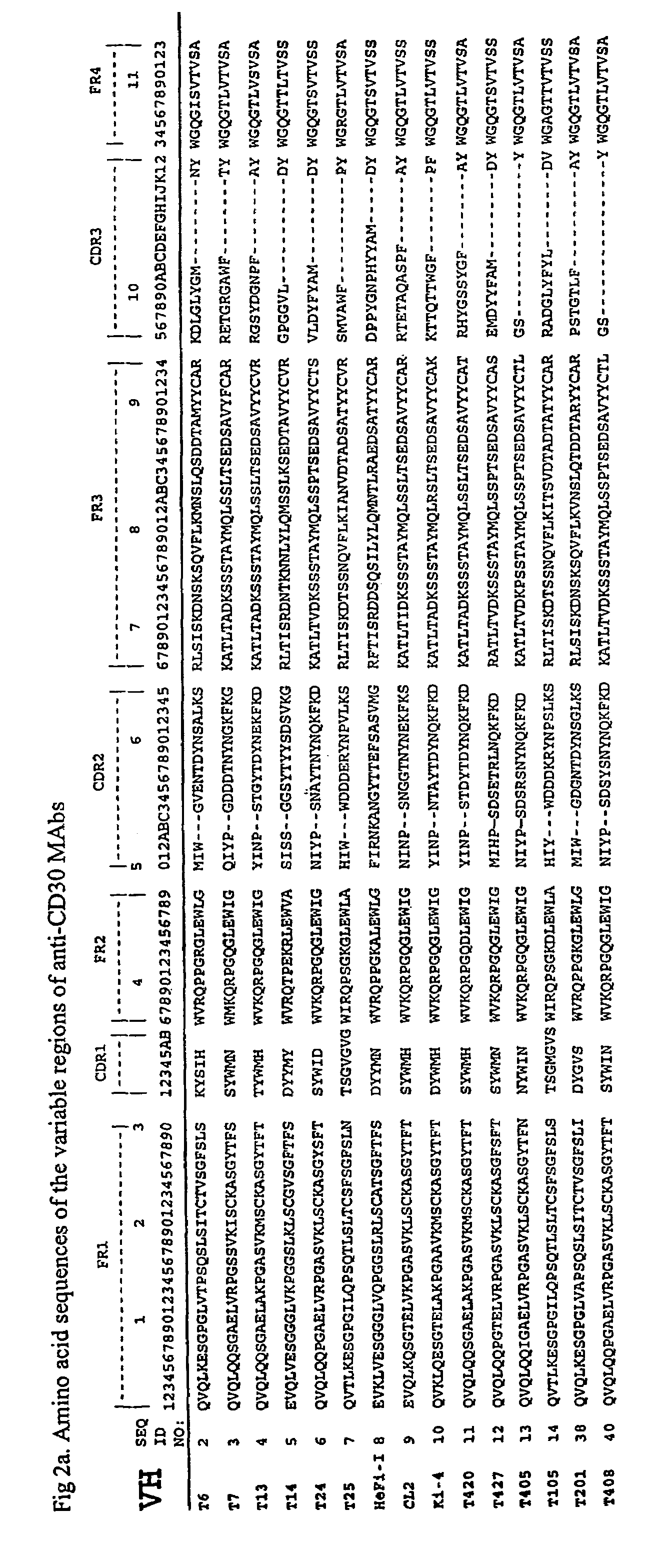
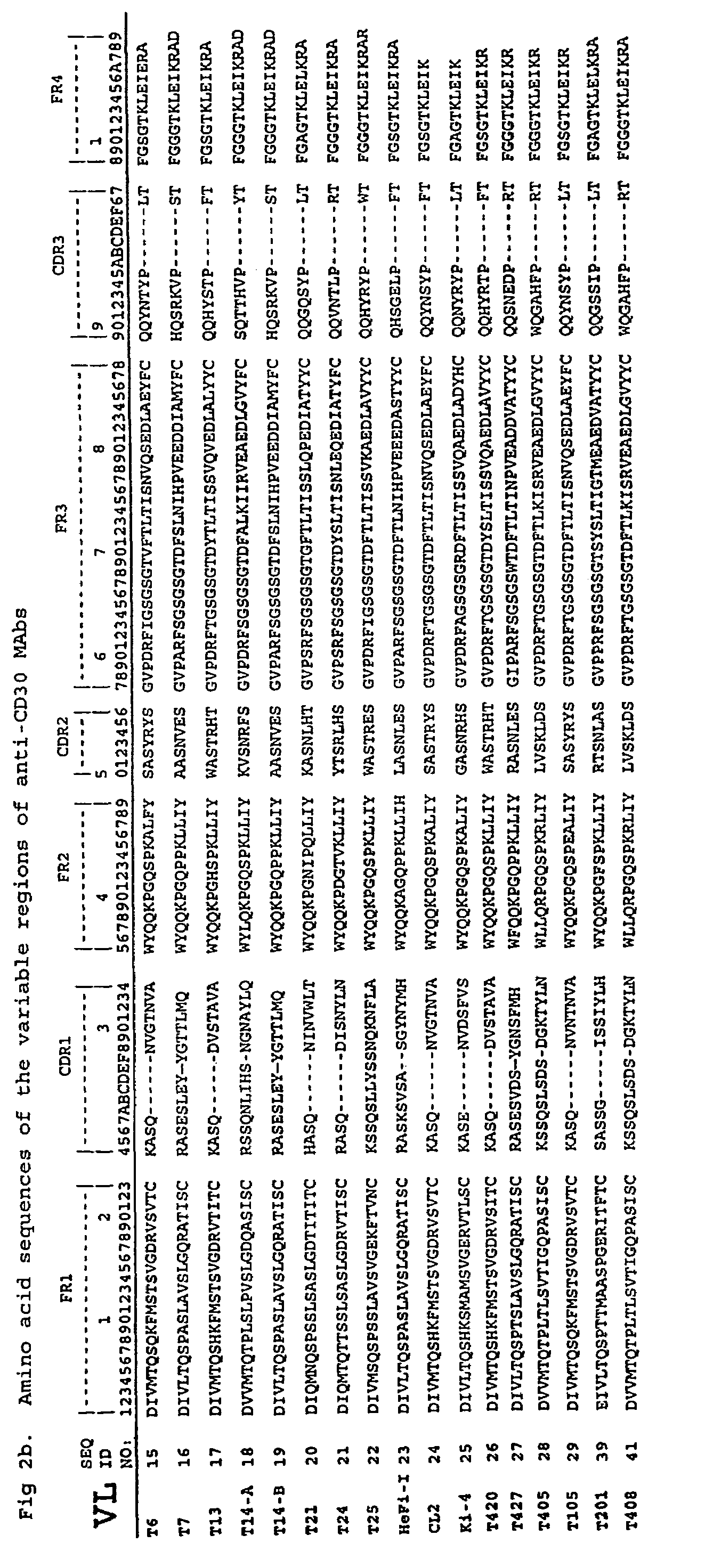
Anti-CD30 stalk and anti-CD30 antibodies suitable for use in immunotoxins
InactiveUS7470775B2Low immunogenicityGrowth inhibitionAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseCancer cell
Owner:UNITED STATES OF AMERICA
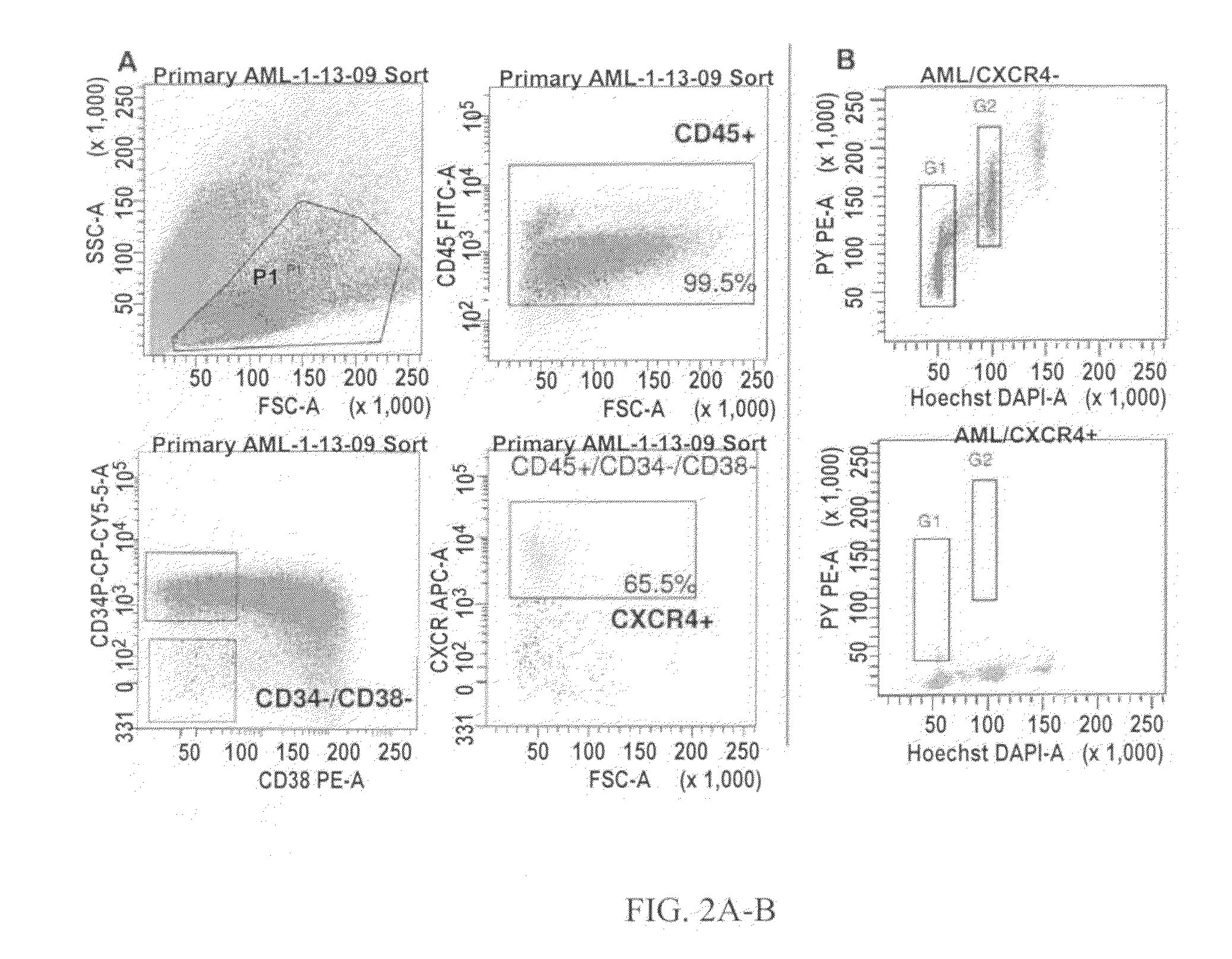
Methods for identifying leukemia stem cells and distinguishing them from normal hematopietic stem cells in patients with acute myeloid leukemia: uses in diagnosis, treatment, and research
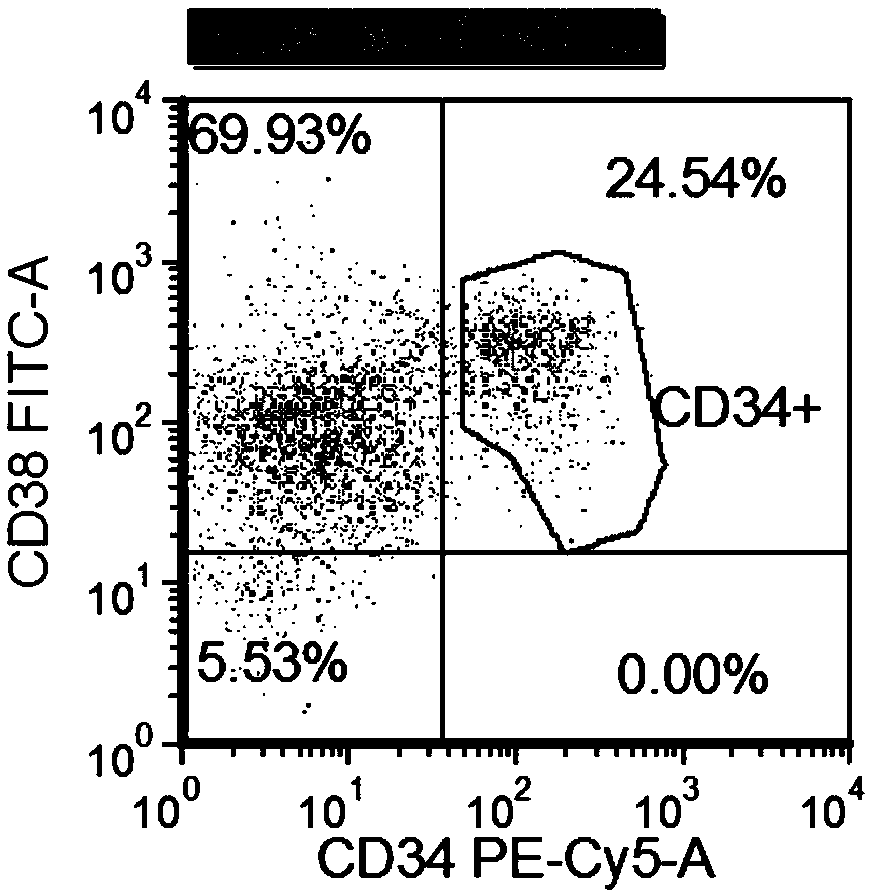
ActiveUS20130079424A1Increase relapse riskIncrease riskBiocideMicrobiological testing/measurementCD34Minimal residual disease
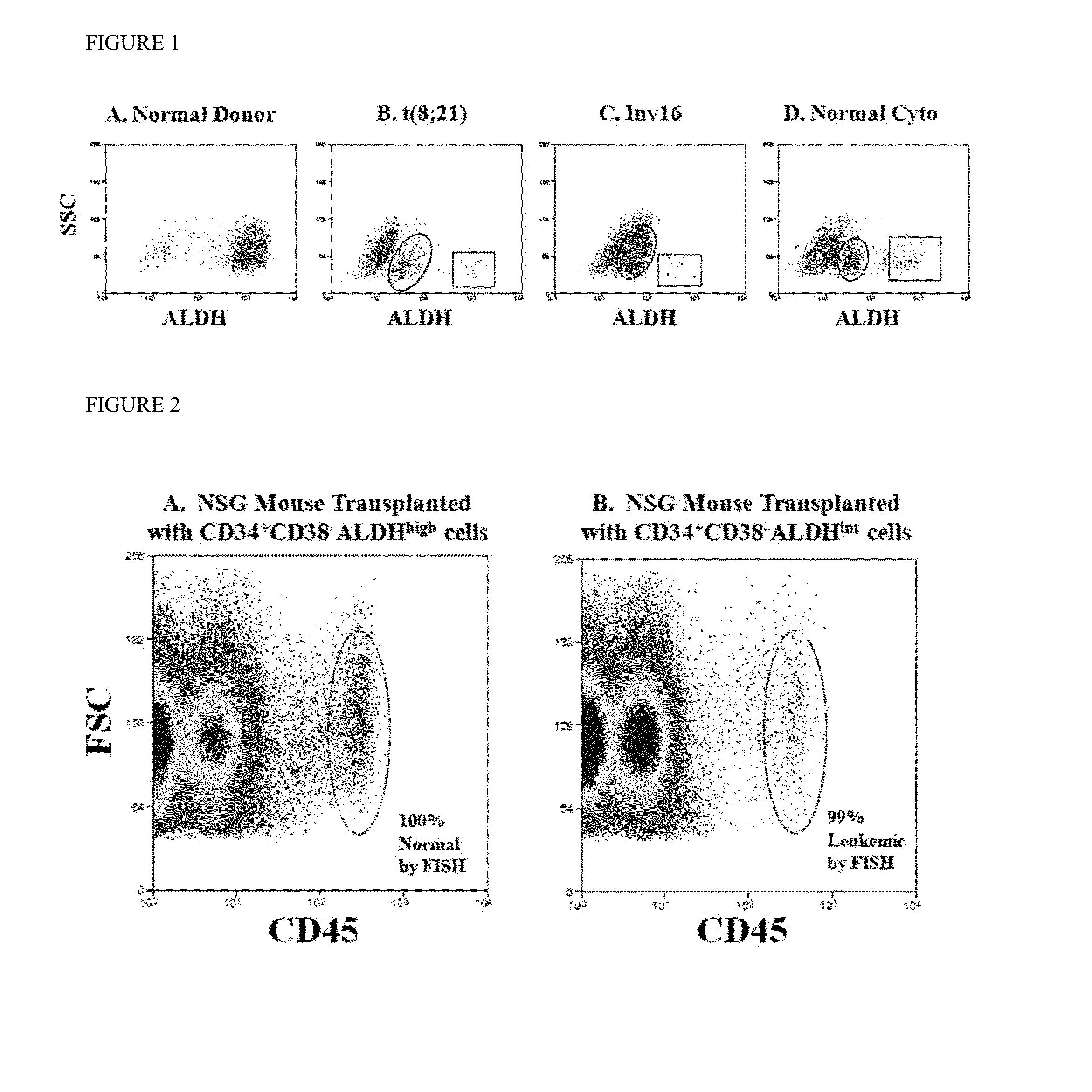
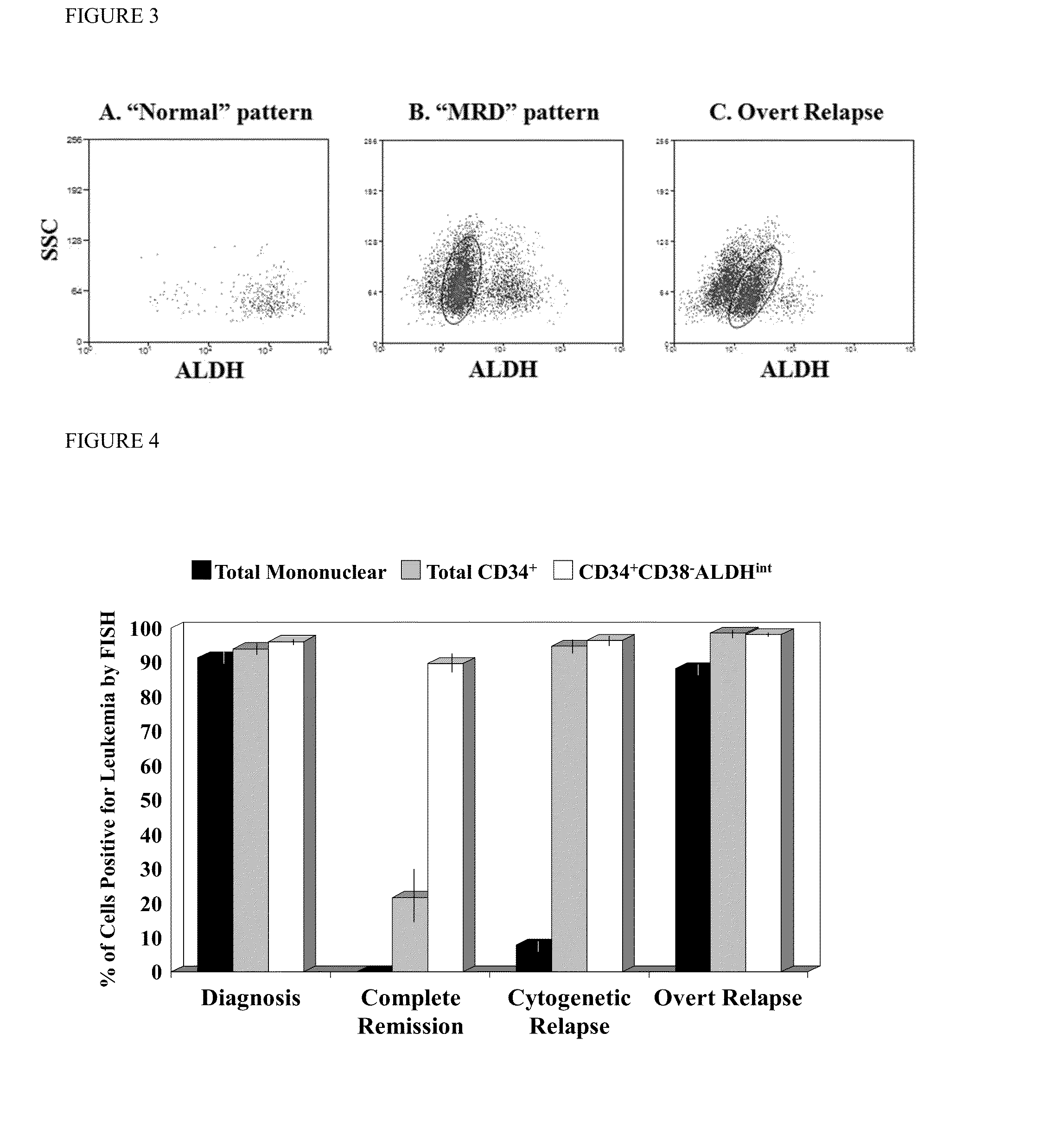
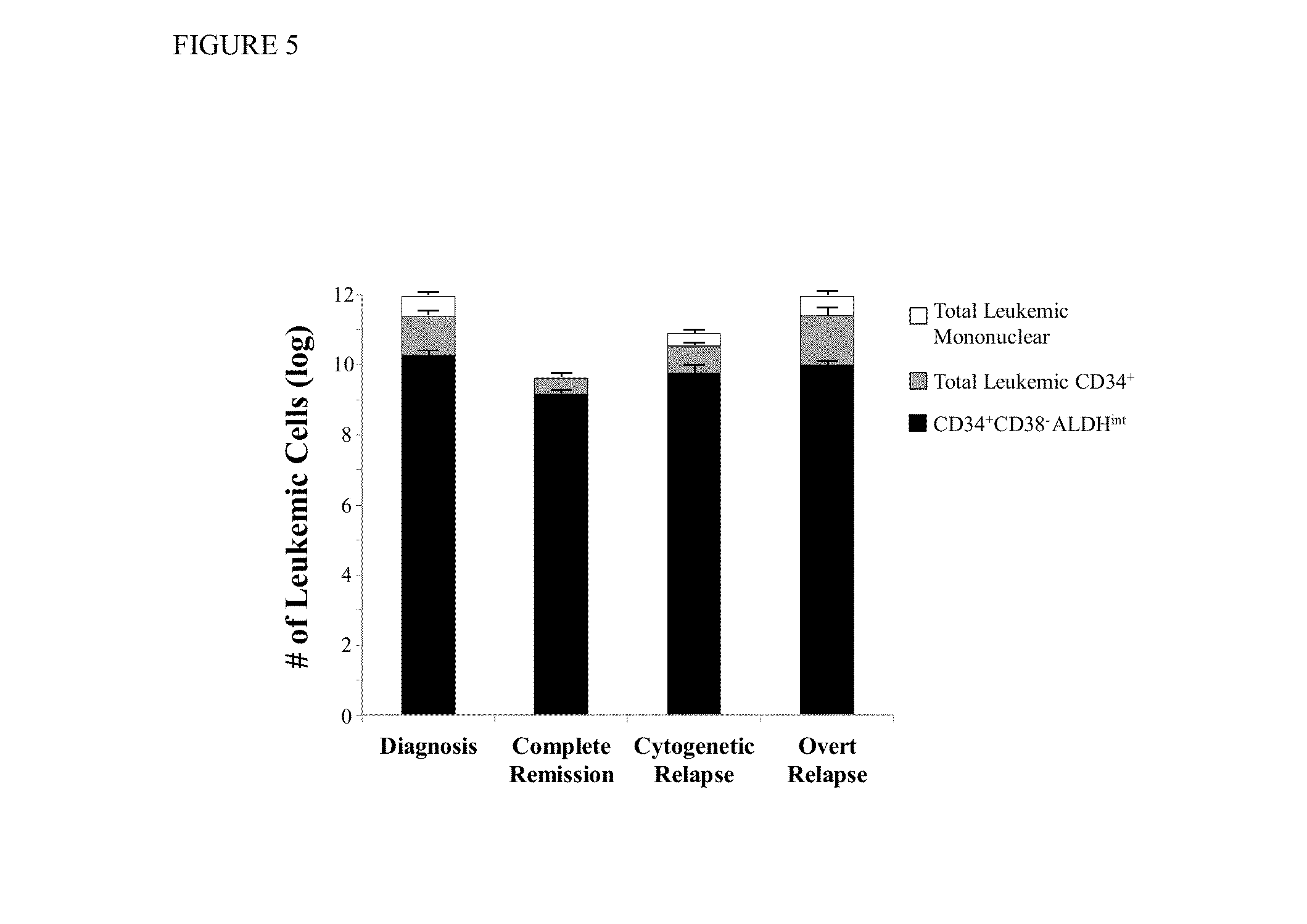
Using the methods of the present invention, intermediate (int) levels of aldehyde dehydrogenase (ALDH) activity reliably distinguished leukemic CD34+CD38− cells capable of engrafting immunodeficient mice, from residual normal hematopoietic stem cells that exhibited relatively higher ALDH activity. Minimal residual disease (MRD) detected during complete remission was enriched for the CD34+CD38−ALDHint leukemic cells, and the presence of these cells after therapy highly correlated with subsequent clinical relapse. The methods of the present invention can distinguish normal from leukemic CD34+CD38− cells, and identifies those AML cells associated with relapse. Methods of prediction of relapse of AML patients and methods of treatment are also provided.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
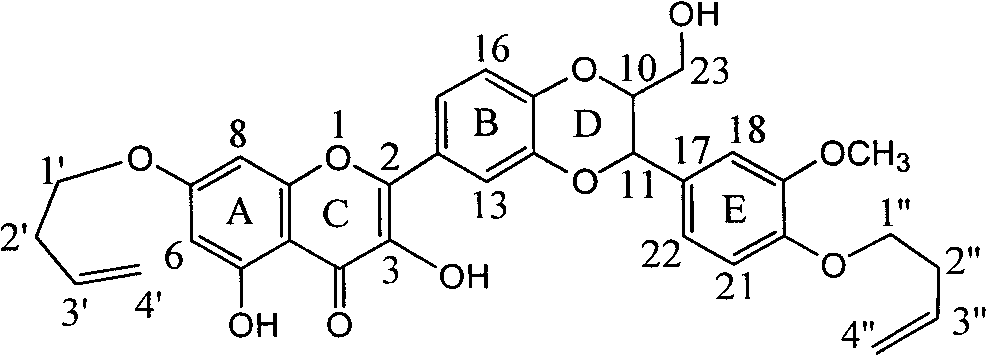
Application of dehydrogenated silibinin diether in preparation of medicaments for preventing and treating leukemia
InactiveCN101785767ASimple methodShort stepsOrganic active ingredientsAntineoplastic agentsMyeloid leukemiaLeukemia
The invention provides application of dehydrogenated silibinin diether in the preparation of medicaments for preventing and treating leukemia and relates to medical application of lignin flavone silibinin to the prevention and treatment of chronic myeloid leukemia. Particularly, the invention relates to application of dehydrogenated silibinin diether, of which the seventh bit and the twentieth bit are substituted by 1-butylene, or pharmaceutically acceptable salts thereof in the preparation of medicaments for preventing and treating chronic myeloid leukemia. Natural products of the silibinin are treated by simple steps to synthesize the compound. The pharmacological tests prove that the compound can potently inhibit the in-vitro proliferation of human chronic myeloid leukemia cell strains(K562) and adriamycin drug-resistant strains (K562 / ADR), and the IC50 values are 11.9+ / -1.6 micromoles and 15.9+ / -1.2 micromoles respectively.
Owner:DALI UNIV
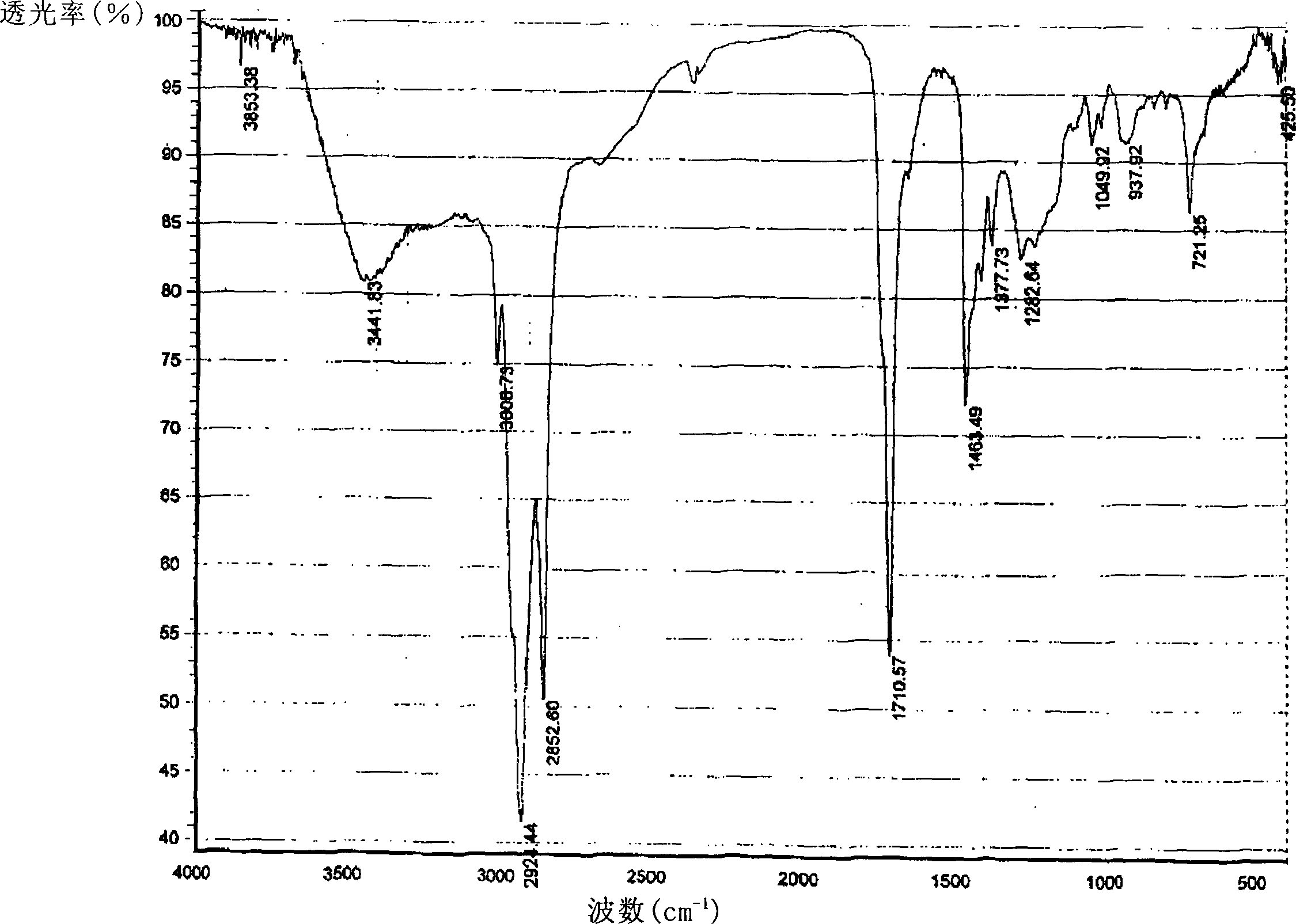
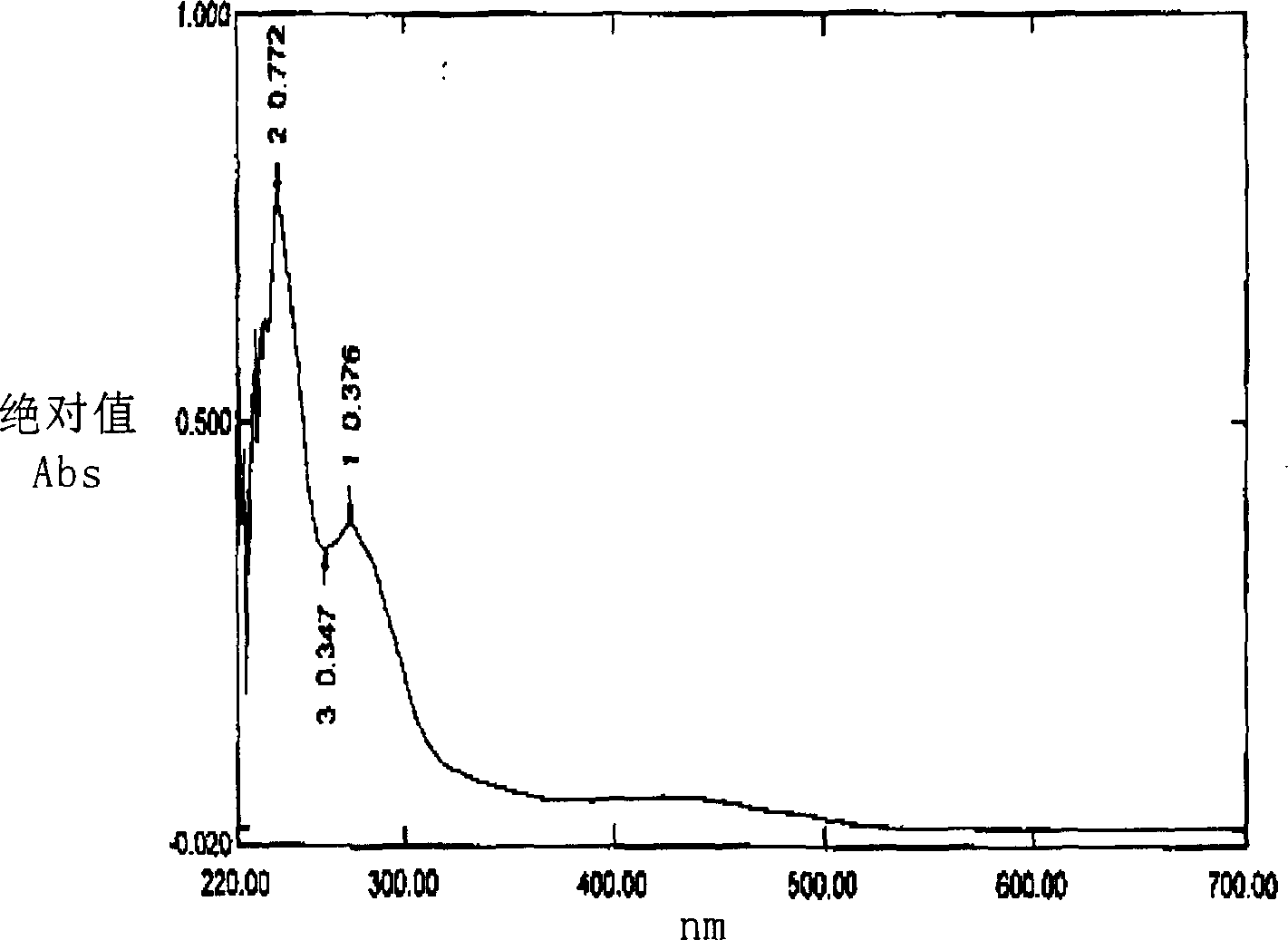
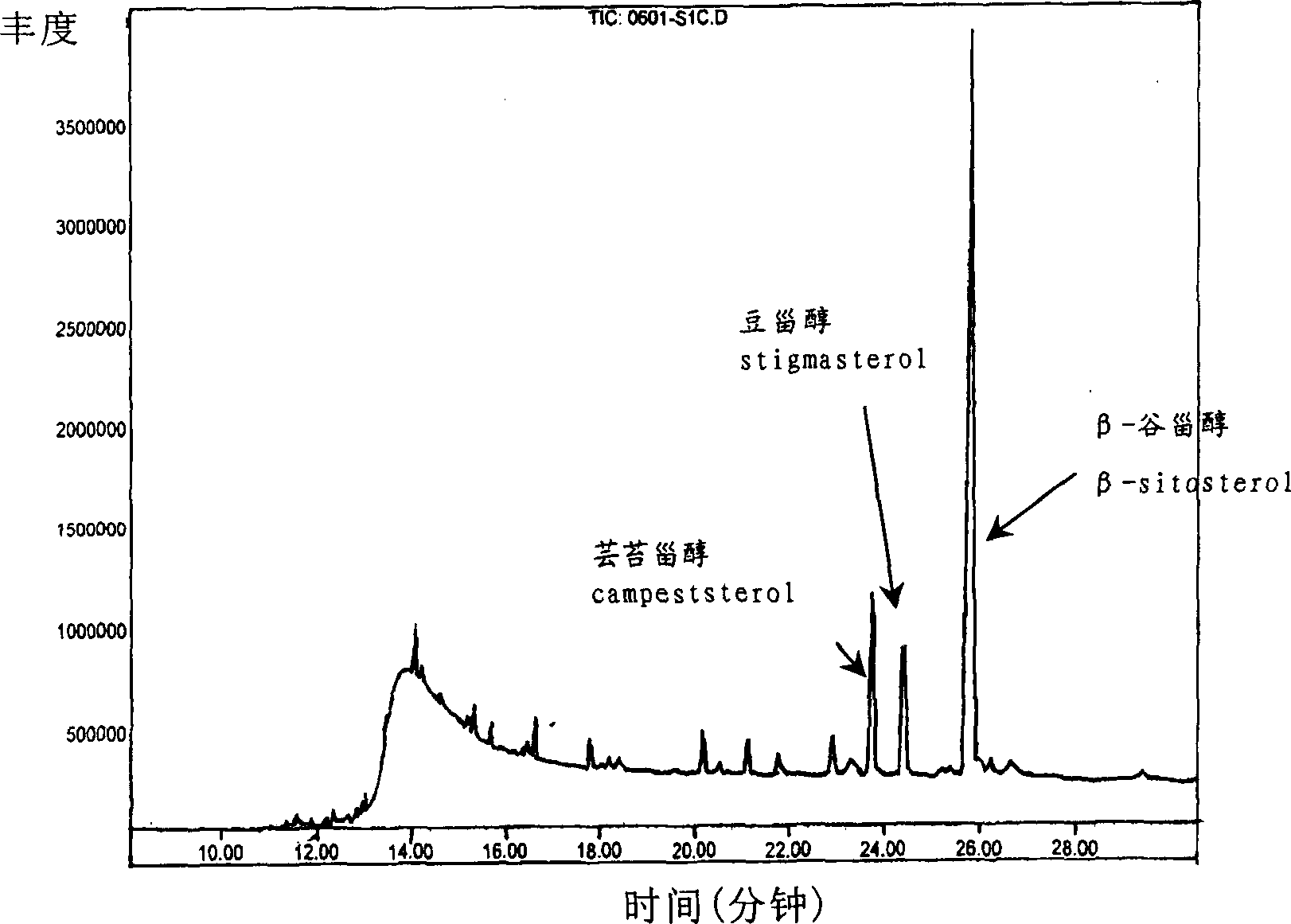
Extractive of phytosterol extracted from bamboo shoot, preparation method and application
InactiveCN1796400AWide variety of sourcesClear compositionOrganic active ingredientsCosmetic preparationsHistiocytosisLeukemia
This invention publishes a phytosterol extract from bamboo shoots, its preparation method and application. The phytosterol extract from bamboo shoots mentioned in this invention contains 5~50% sterols, 10~40% of which is beta-sitosterol. The ratio between beta-sitosterol, stigmasterol and campesterol is 10~40:1~3:2~5. The phytosterol extract from bamboo shoots mentioned in this invention performs significant anti-inflammatory and leukemia histiocytosis inhibitory activities and is thus applicable in cosmetics, foods, health products and medicines.
Owner:ZHEJIANG UNIV HANGZHOU LEAF BIO TECH +1
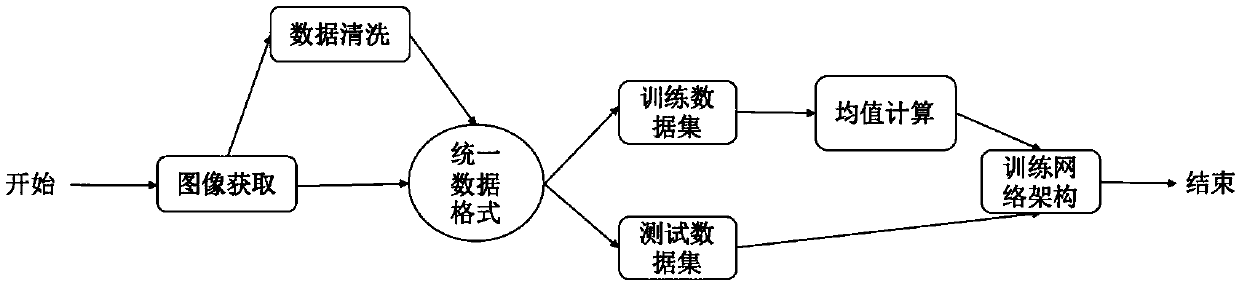
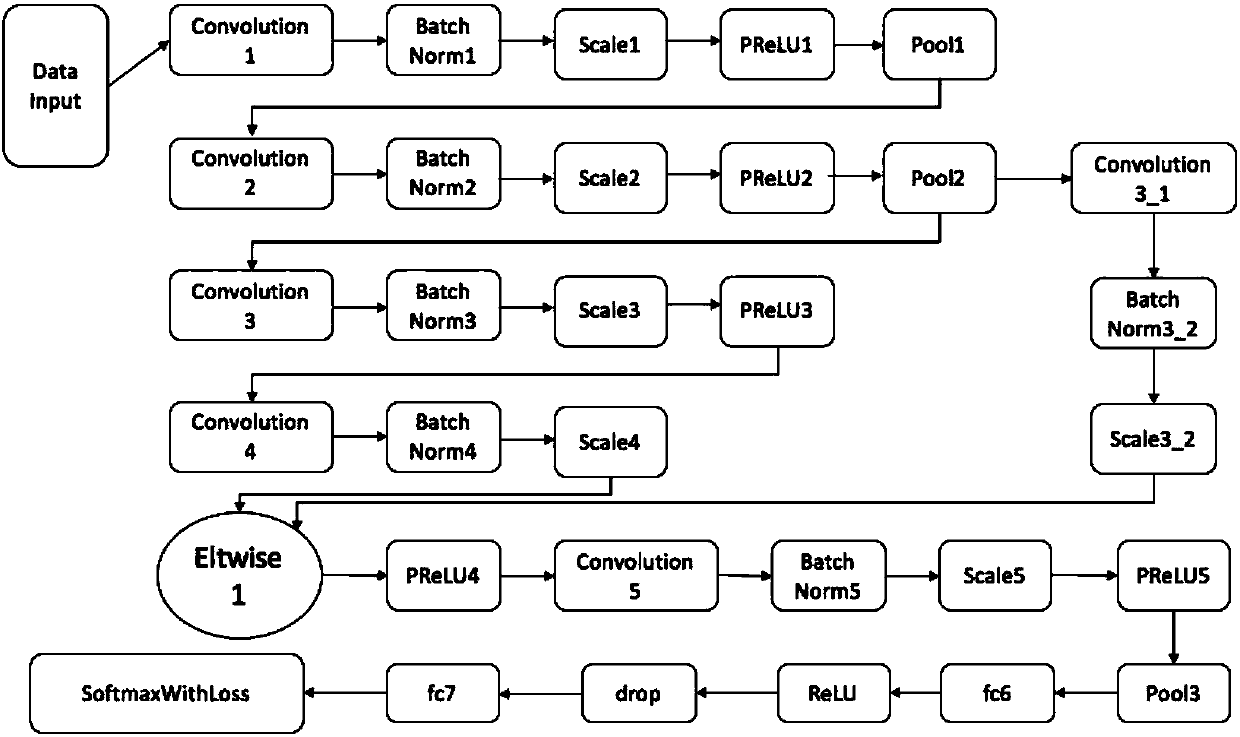
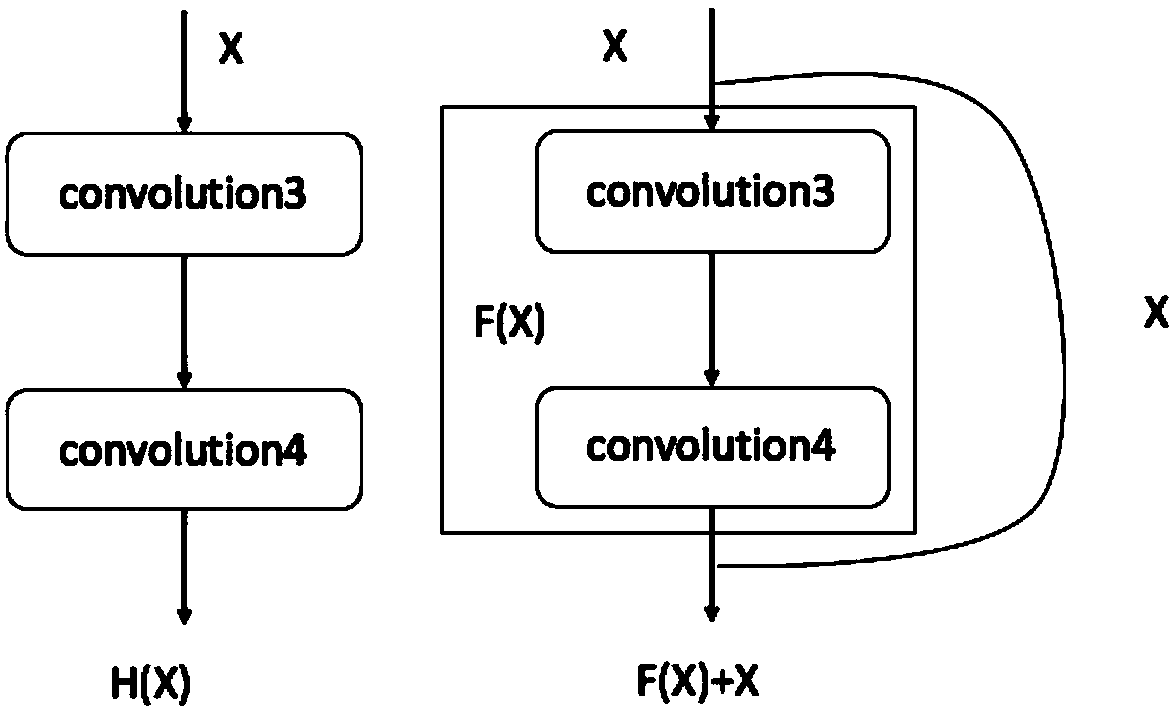
White blood cell multi-classification identification method based on deep residual network
InactiveCN107784324ARun fastFit closelyCharacter and pattern recognitionNeural architecturesMicroscopic imageNerve network
The invention discloses a white blood cell multi-classification identification method based on deep residual network. The invention provides a network configuration based on deep learning convolutionnerve network technology, and the residual design concept is merged in the network configuration. Strange white blood cell images can be identified and classified by using quite good fitting model generated by the network configuration. A multi-classification network configuration capable of conducting end-to-end learning can be designed by means of convolution nerve network in deep learning having residual configuration, and a classification model having quite good fitting can be trained based on the configuration. As the running speed of the configuration is fast, the fitting model of the training data can be rapidly obtained. The multi-classification model can conduct accurate identification and classification on strange white blood cell microscopic images. The white blood cell multi-classification identification method has quite a high accuracy in the field of leukemia cell recognition and classification.
Owner:HANGZHOU DIANZI UNIV
Methods for Manipulating Phagocytosis Mediated by CD47
Methods are provided to manipulate phagocytosis of cells, including hematopoietic cells, e.g. circulating hematopoietic cells, bone marrow cells, etc.; and solid tumor cells. In some embodiments of the invention the circulating cells are hematopoietic stem cells, or hematopoietic progenitor cells, particularly in a transplantation context, where protection from phagocytosis is desirable. In other embodiments the circulating cells are leukemia cells, particularly acute myeloid leukemia (AML), where increased phagocytosis is desirable.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Immunoconjugates and humanized antibodies specific for b-cell lymphoma and leukemia cells
InactiveUS20070172920A1Peptide/protein ingredientsAntibody mimetics/scaffoldsHuman antimouse AntibodyComplementarity determining region
A chimeric LL2 monoclonal antibody is described in which the complementarity determining regions (CDRs) of the light and heavy chains of the murine LL2 anti-B-lymphoma, anti-leukemia cell monoclonal antibody has been recombinantly joined to the human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-cell lymphoma and leukemia cell internalization capacity of the parental murine LL2 monoclonal antibody, and which has the potential of exhibiting reduced human anti-mouse antibody production activity. A humanized LL2 monoclonal antibody is described in which the CDRs of the light and heavy chains have been recombinantly joined to a framework sequence of human light and heavy chains variable regions, respectively, and subsequently linked to human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-lymphoma and leukemia cell internalization capacities of the parental murine and chimeric LL2 monoclonal antibodies, and which has the potential for exhibiting reduced human anti-mouse antibody production activity. Vectors for producing recombinant chimeric and humanized chimeric monoclonal antibodies are provided. Isolated DNAs encoding the amino acid sequences of the LL2 variable light and heavy chain and CDR framework regions are described. Conjugates of chimeric and humanized chimeric LL2 antibodies with cytotoxic agents or labels find use in therapy and diagnosis of B-cell lymphomas and leukemias.
Owner:IMMUNOMEDICS INC
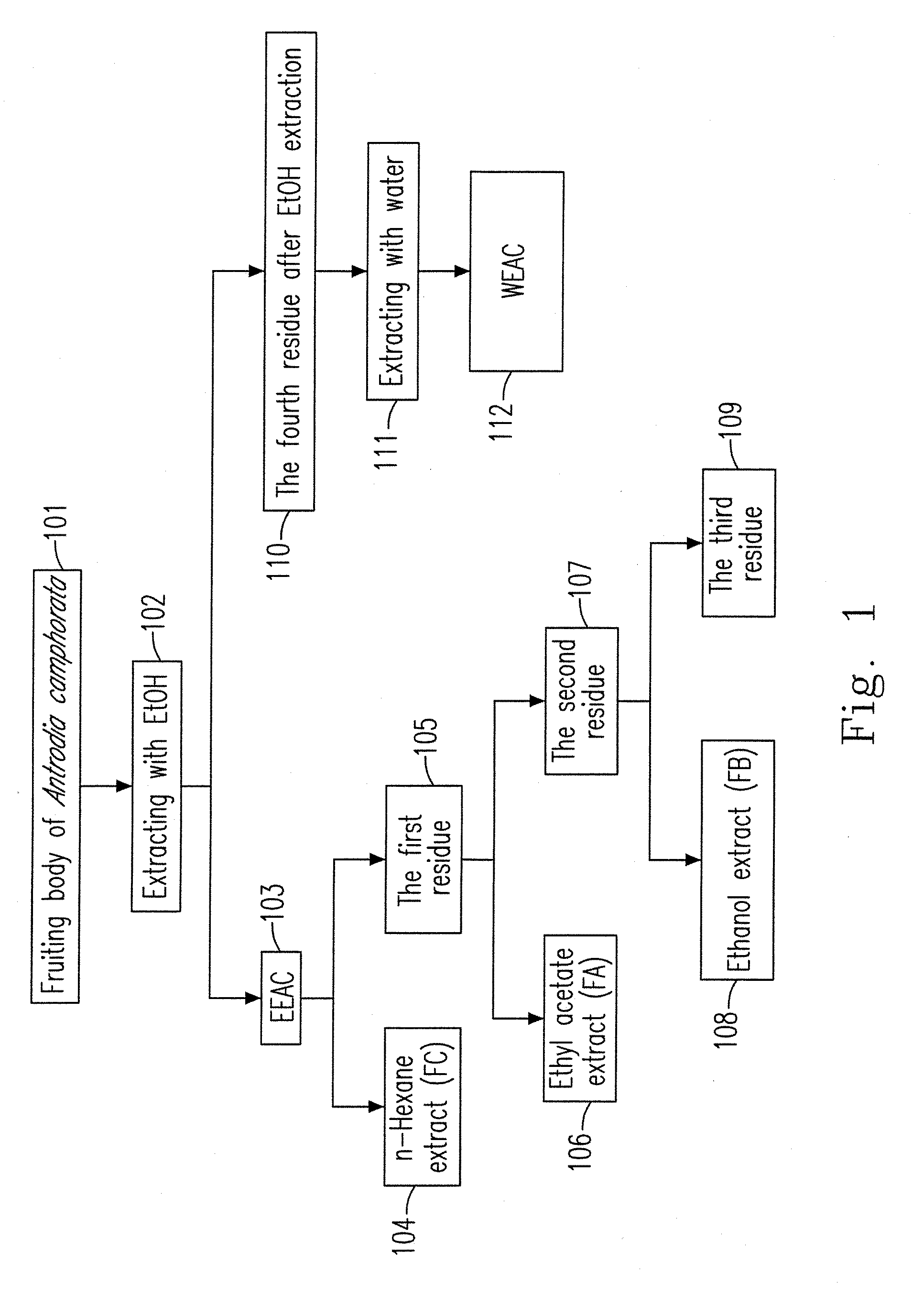
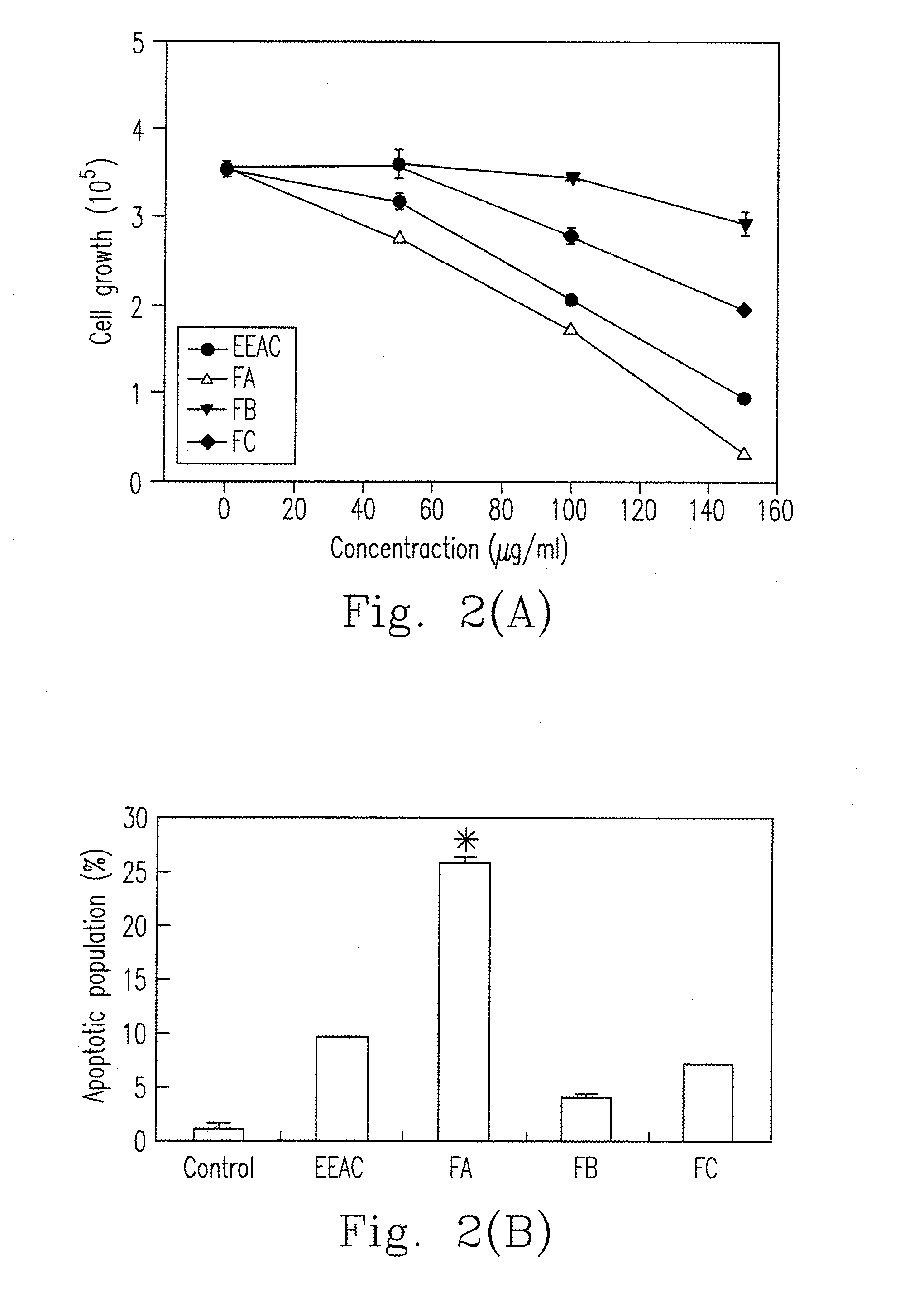
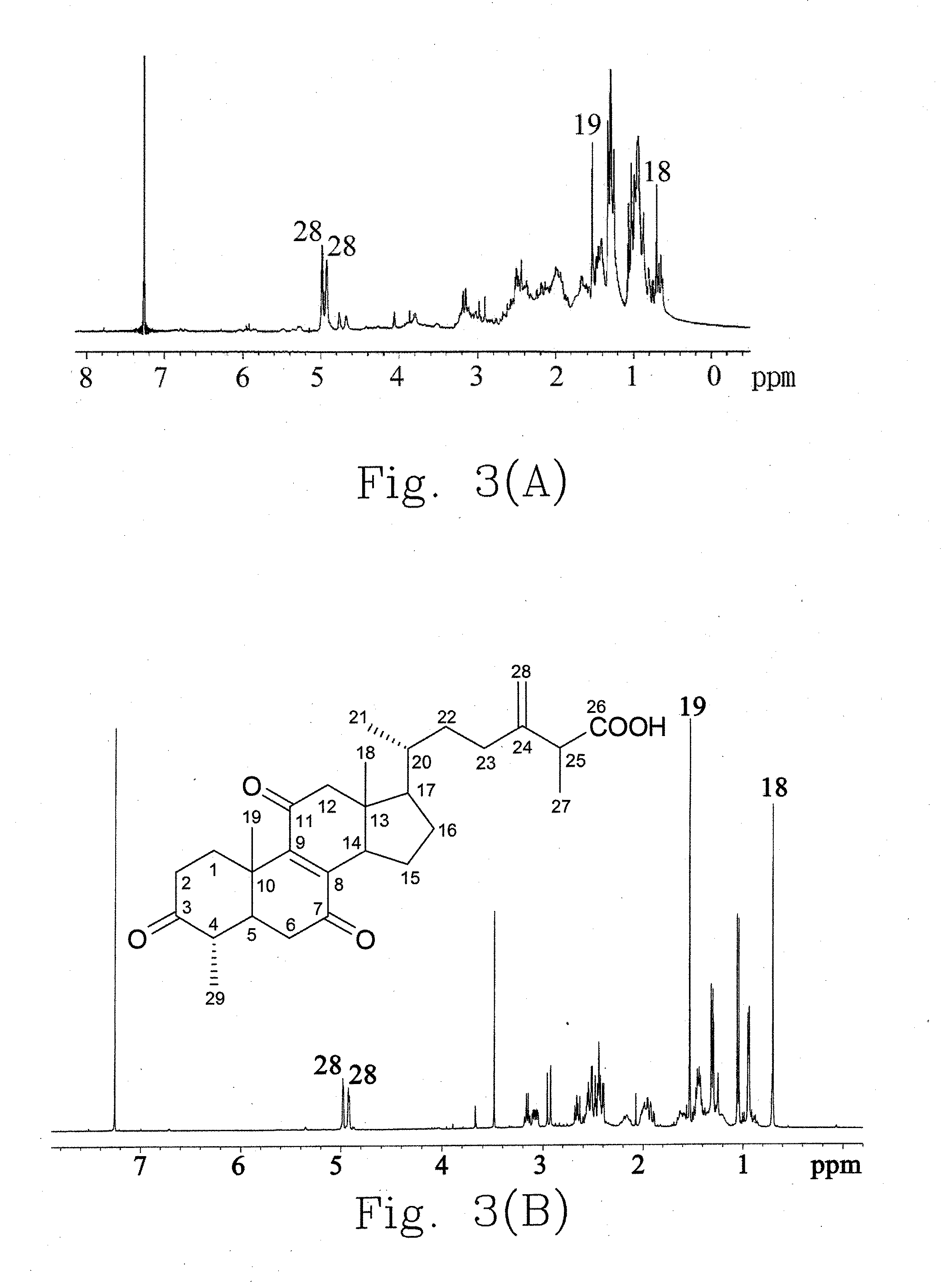
Ethanol extract of antrodia camphorata for inducing apoptosis and preparation method thereof
InactiveUS20100210869A1Effectively inhibit growth of leukemiaIncrease polarityOrganic compound preparationFungi medical ingredientsEthyl acetateDrug biological activity
A preparation method for an ethanol extract of the fruiting body of Antrodia camphorata (EEAC) is provided. The preparation method includes steps of: (a) providing the fruiting body of A. camphorata (AC); (b) extracting the fruiting bodies with a first ethanol solution; and (c) obtaining EEAC. EEAC further can be sequentially extracted or fractioned by n-hexane, ethyl acetate and ethanol, and an n-hexane fraction (FC), an ethyl acetate fraction (FA) and an ethanol fraction (FB) respectively are generated. The growth inhibition and apoptosis induction of leukemia cell line HL 60 are effectively mediated by FA product, in which zhankuic acid A is the bioactive marker. The amount of triterpenoid in the fruiting body of AC can be determined by NMR and HPLC analysis.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
Demibodies: dimerization-activated therapeutic agents
InactiveUS8623356B2Strong specificityEnhanced immunophenotypic selectionAntibacterial agentsPeptide/protein ingredientsAntigenCancer cell
The present invention relates generally to a set of synthetic immunointeractive molecules referred to herein as “demibodies” which are useful in targeting particular cells in a subject. More particularly, the present invention provides a set of demibodies wherein at least two molecules from within the set, each specific for a different antigen on a target cell, are required to interact together at the cell surface in order to form an active complex which directs demibody-mediated cellular or complement mediated cytotoxicity and / or reporter function and / or therapeutic activity. The demibodies of the present invention are useful in the targeting of particular cells such as cancer cells including leukemic cells, pathogens including malarial, bacterial and viral agents, and stem cells including embryonic and adult stem cells and pathogen cells. The present invention provides, therefore, methods of treatment, diagnosis and undertaking research and compositions comprising demibodies useful for same.
Owner:THE UNIV OF SYDNEY
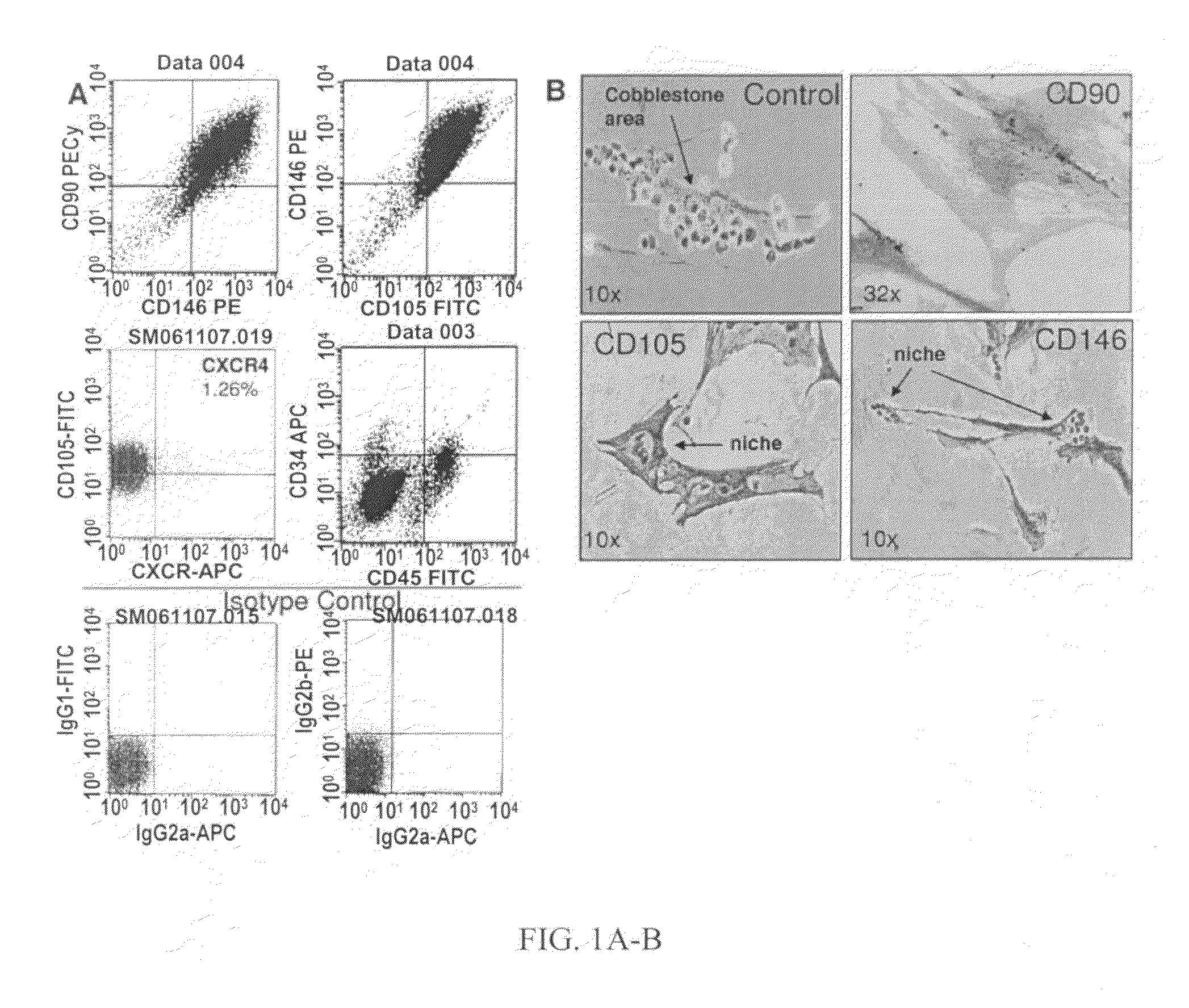
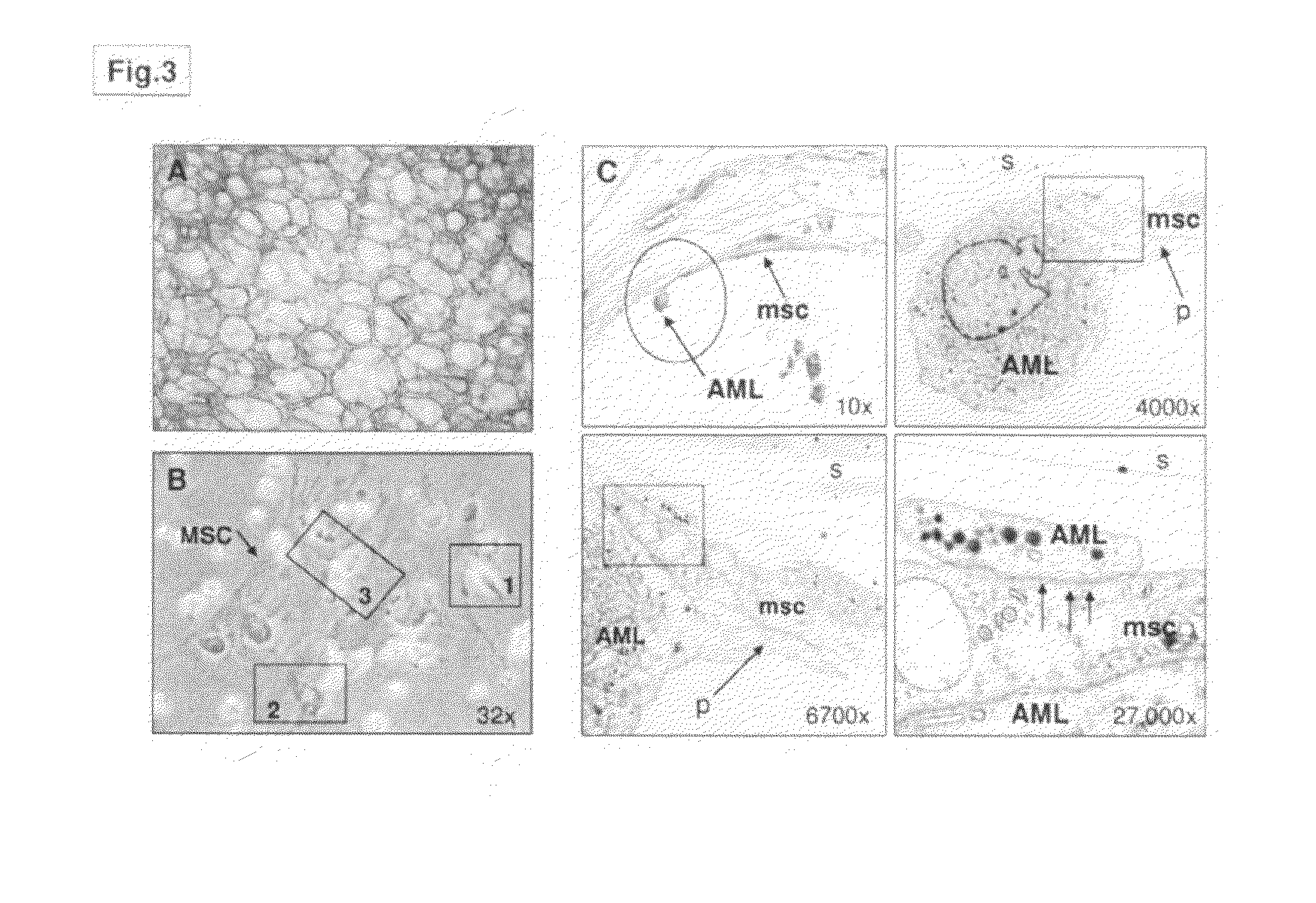
Human bone marrow microenvironments and uses thereof
The present invention is directed to an in vitro cultured permissive niche, or human bone marrow microenvironment, comprising a scaffold coated with human mesenchymal stem cells and a culture medium, wherein the stem cells are viable and proliferate in culture and the niche is permissive for the establishment of introduced hematopoietic or leukemic cell populations. The present invention is also directed to establishment of a permissive niche in a non-human animal model comprising a scaffold coated with human mesenchymal stem cells introduced into the animal ectopically, wherein the niche and the model are permissive for the establishment of introduced hematopoietic or leukemic cell populations. The implanted scaffold forms an ectopic human bone marrow microenvironment to study the mesenchymal leukemic stem cell niche. In addition, the present invention is directed to methods of using the in vitro cultured human bone marrow microenvironment and the non-human animal model to evaluate an agent for anti-leukemic properties.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
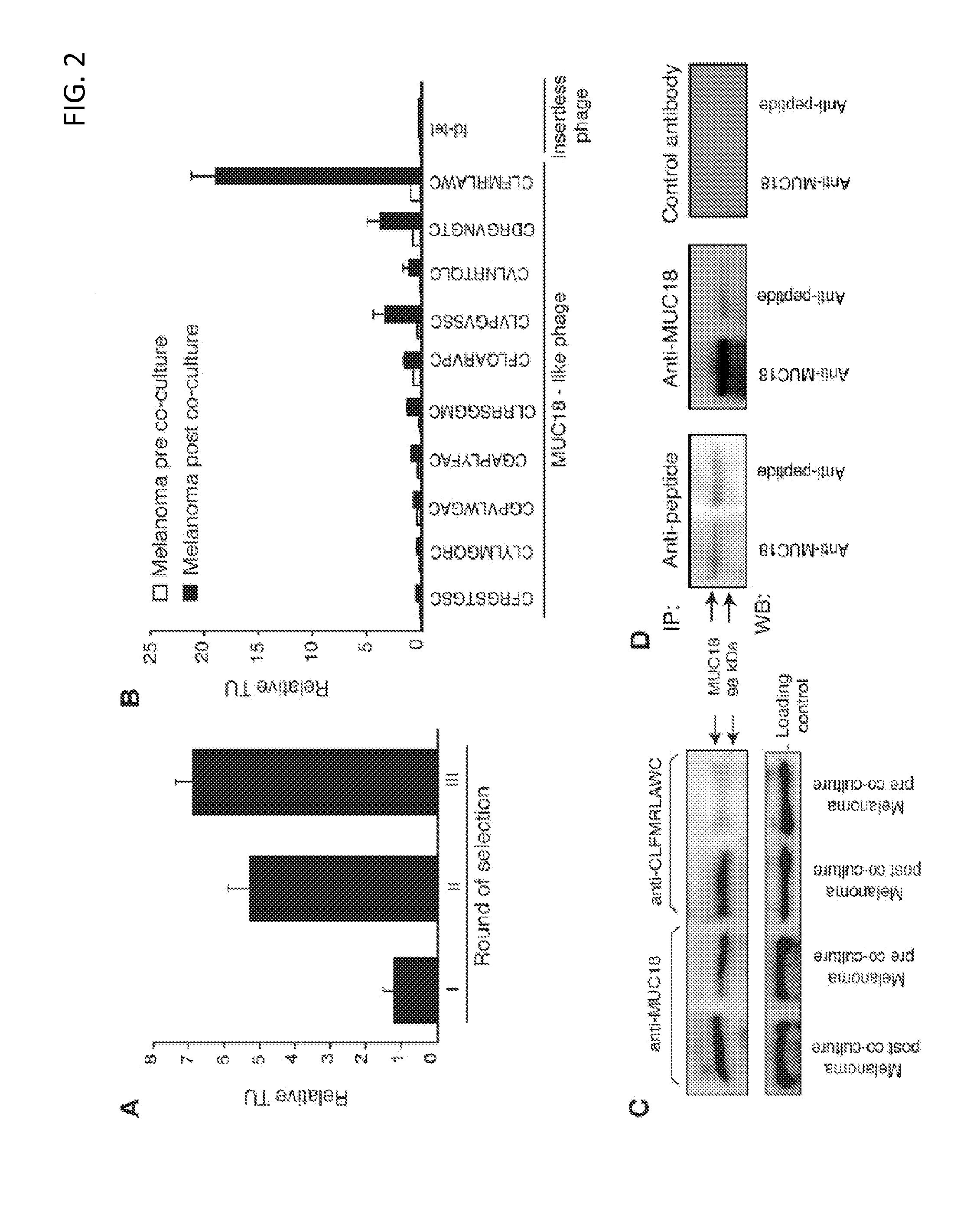
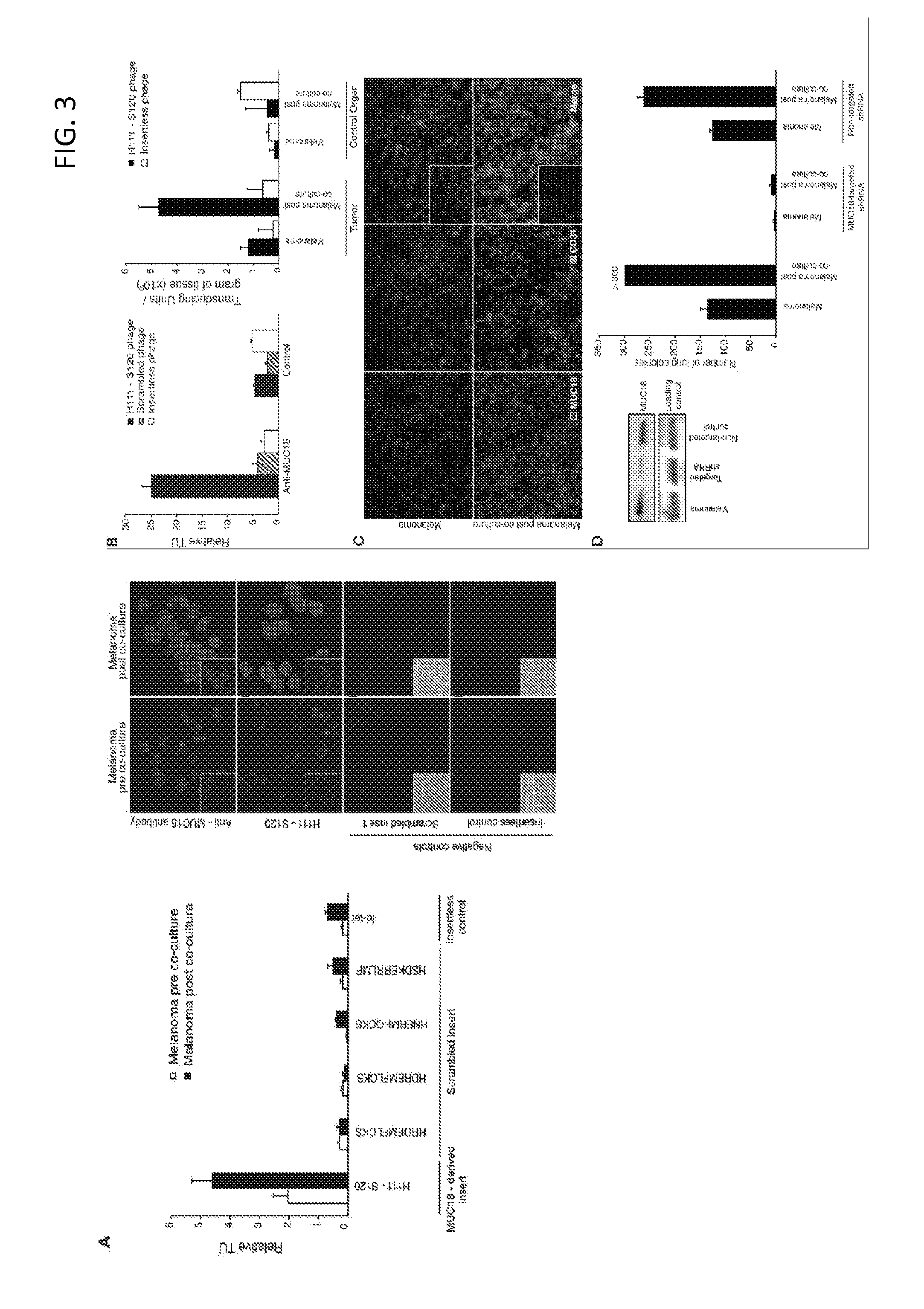
MUC18 targeting peptides
ActiveUS8450278B2Preventing and decreasing interactionEasy transferTumor rejection antigen precursorsIn-vivo radioactive preparationsMelanomaTarget peptide
Provided are MUC 18 targeting peptides which may be used, e.g., to therapeutically target B-I lymphocytes to reduce the influence of these cells on the metastatic potential of melanoma cells and / or to target cancerous cells, including certain melanoma and leukemia cells. MUC 18 targeting peptides may be comprised in fusion constructs, imaging constructs, and / or therapeutic constructs such as fusion constructs which may be used for diagnosing or treating a cancer.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
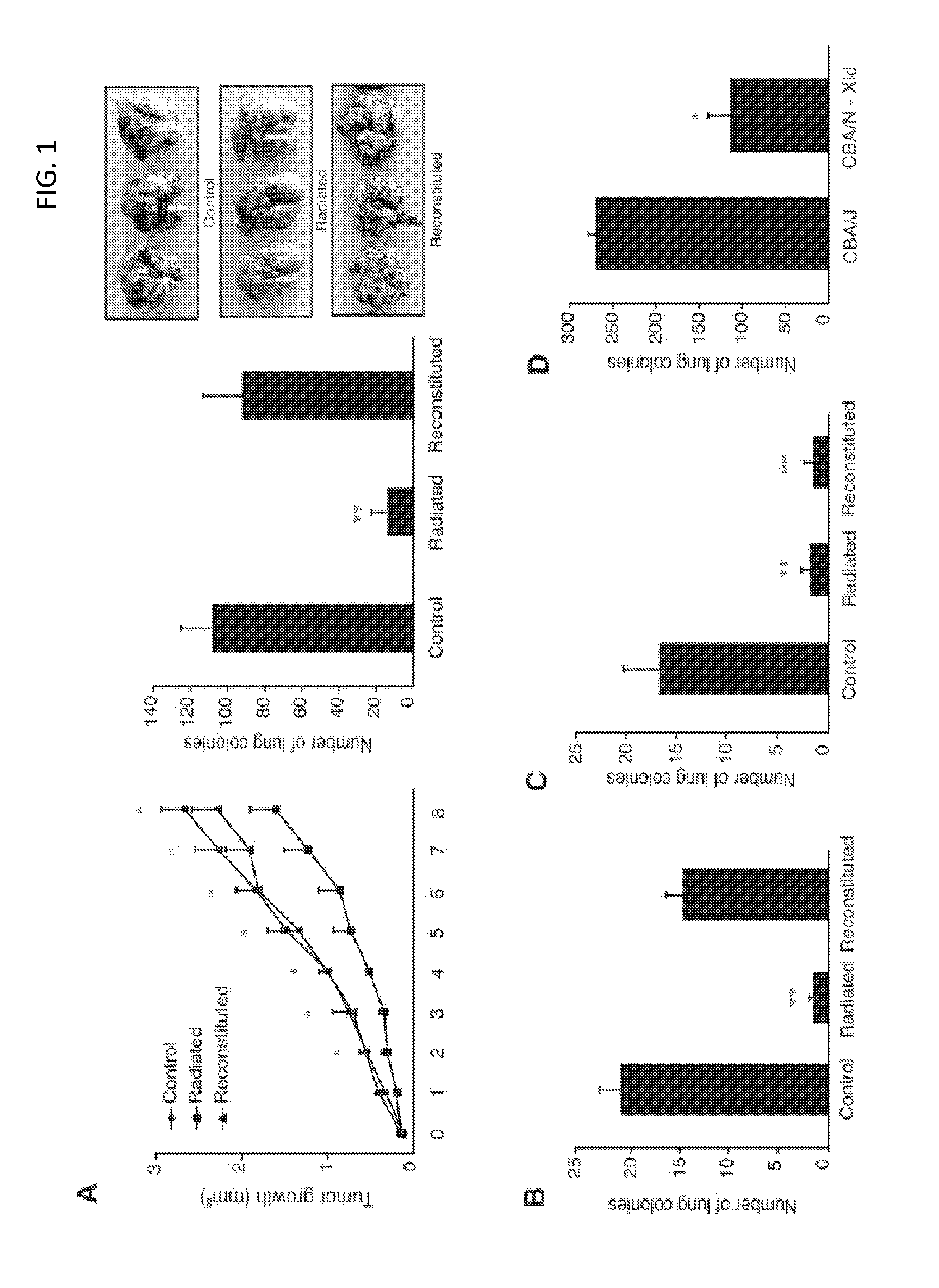
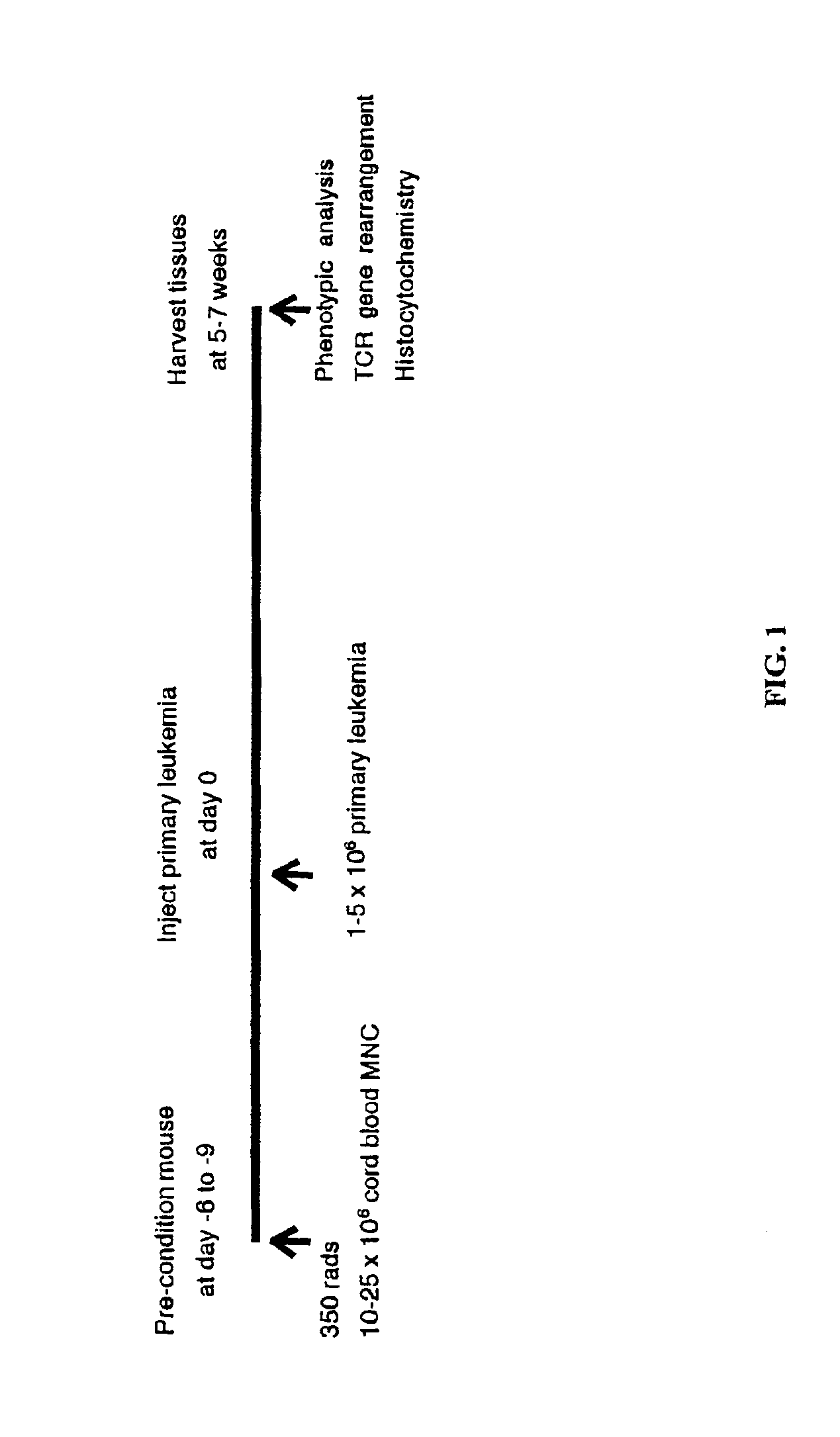
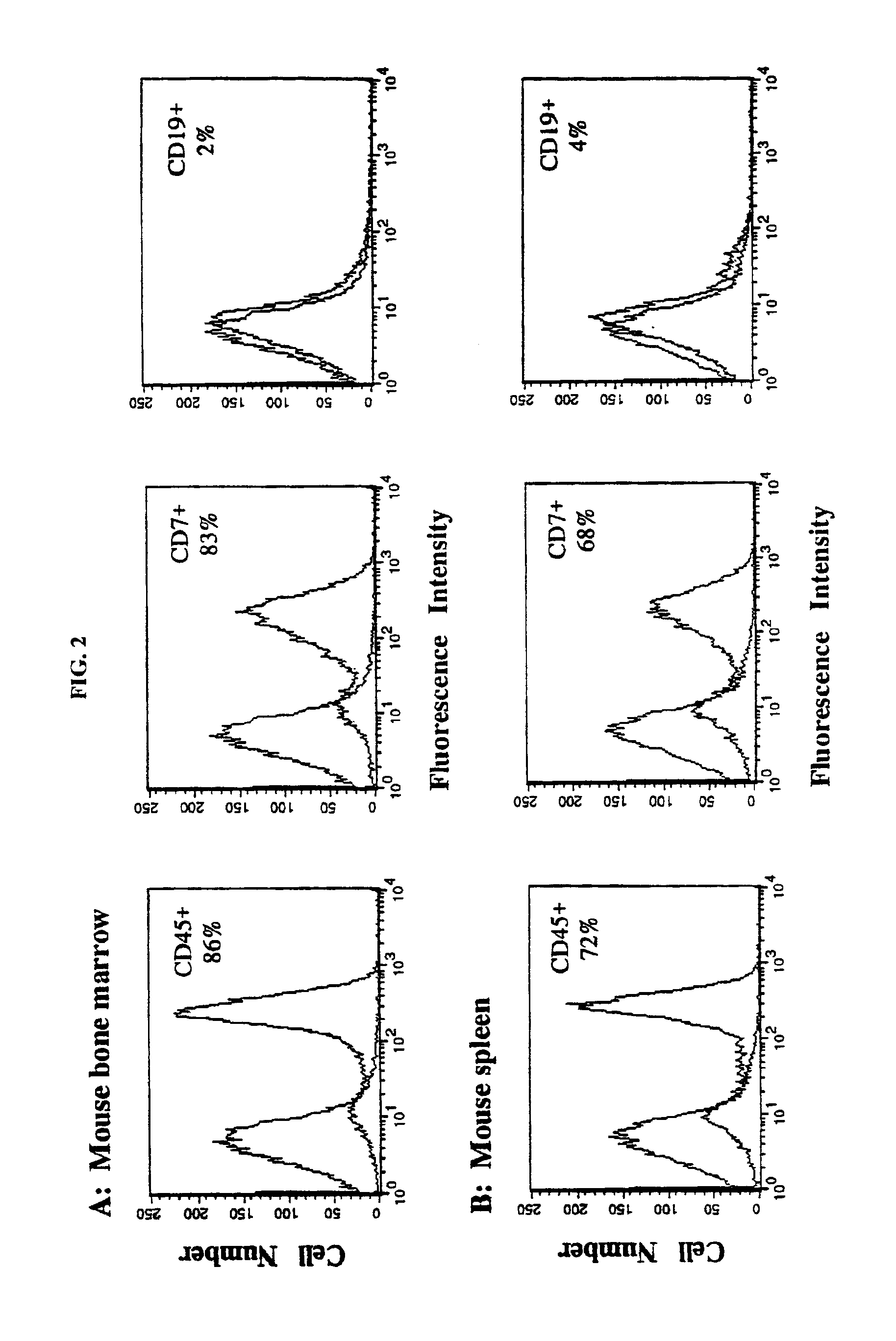
In vivo animal model of human leukemia
InactiveUS6930222B2Improve the level ofIncrease the number ofAnimal husbandryCord blood stem cellHuman leukemia
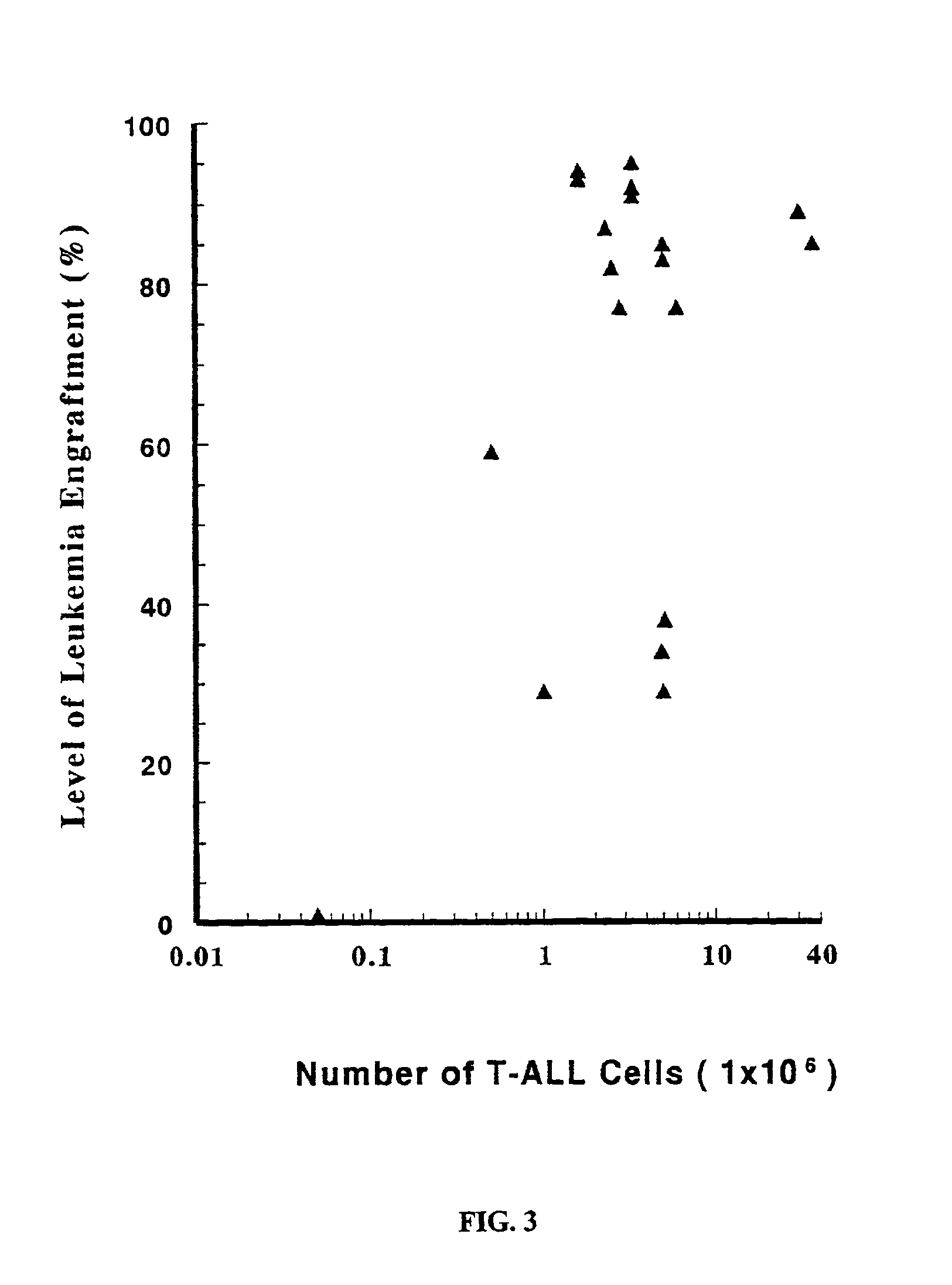
The present invention provides a process for making an in vivo model of human leukemia. The process includes the steps of: pre-conditioning an immunodeficient rodent by administering to the rodent a sub-lethal dose of irradiation and injecting the rodent with an effective pre-conditioning amount of human fetal cord blood mononuclear cells; maintaining the rodent for from about 5 to 10 days; and injecting the rodent with an effective engrafting amount of primary human leukemia cells. An in vivo and in vitro model of human leukemia are also provided.
Owner:THE SCRIPPS RES INST
Compositions for the treatment of cancer, and methods for testing and using the same
ActiveUS20090075883A1Rapidly and effectively determinedHigh activityPeptide/protein ingredientsDisease diagnosisBacteroidesProtein isolate
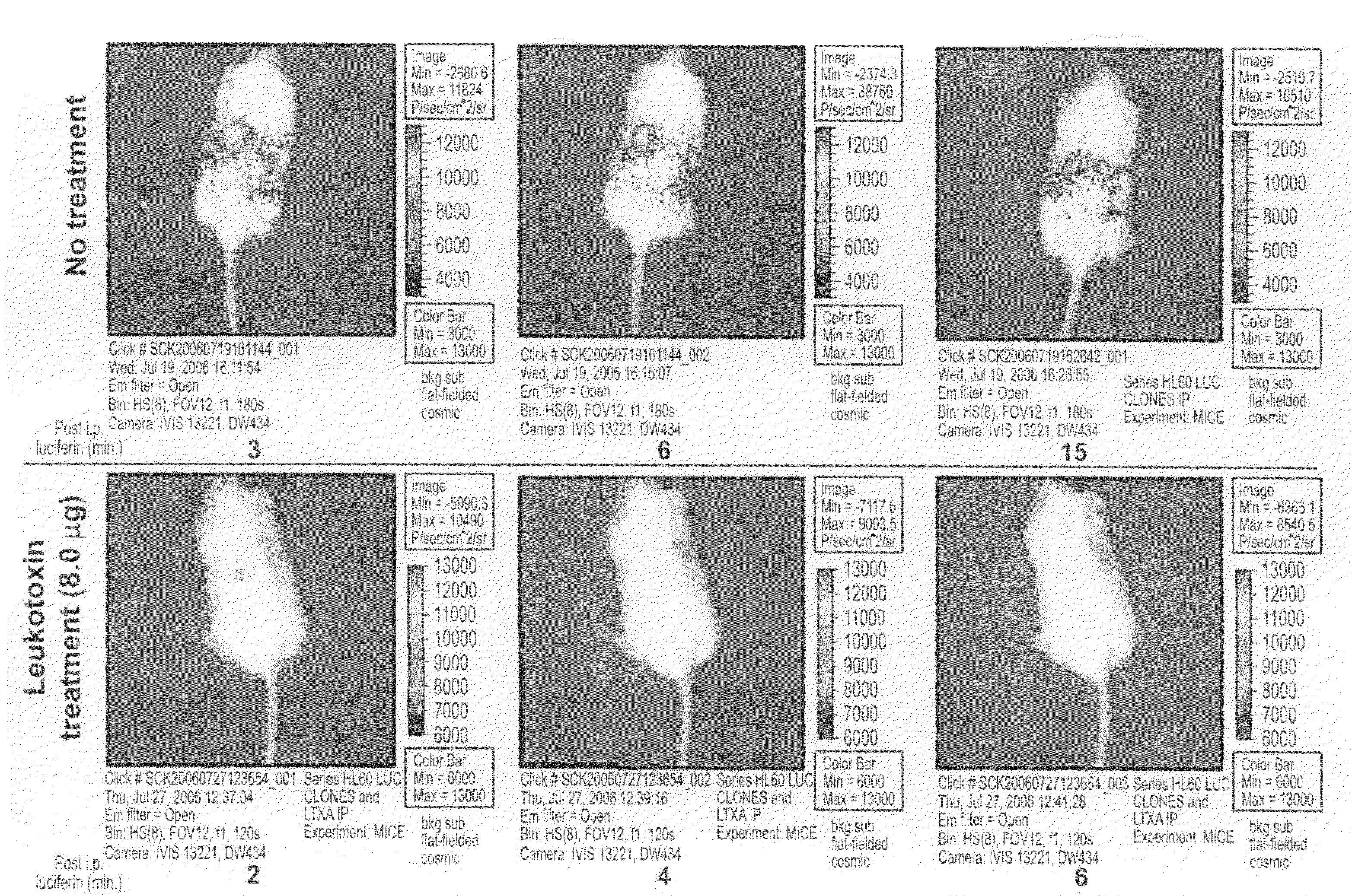
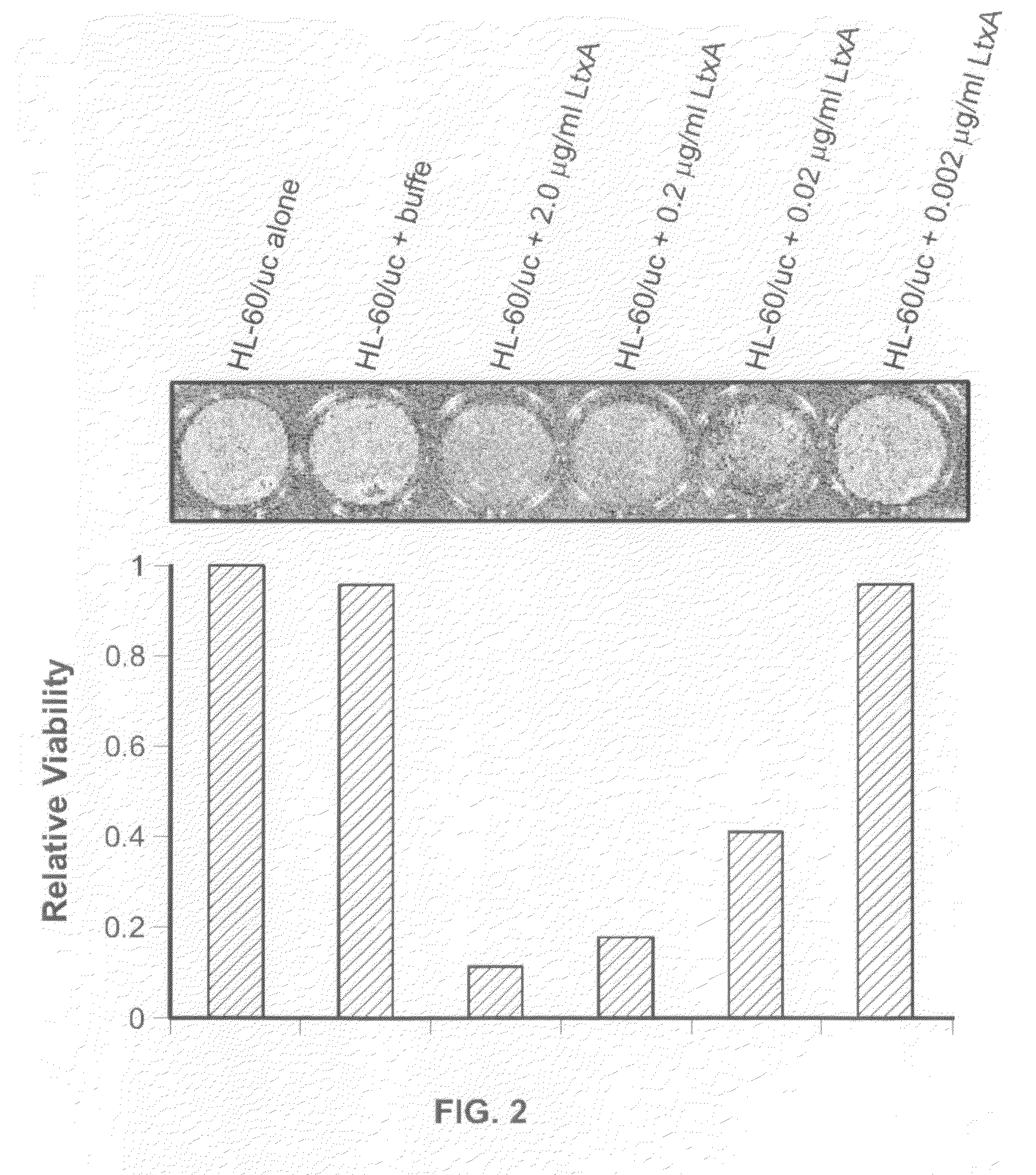
A composition comprising leukotoxin proteins isolated from a bacterium is provided. In this composition, greater than 85% of the leukotoxin proteins are chemically modified at a basic amino acid residue, and the proteins induce cell death in myeloid leukocytes, while remaining substantially non-toxic to lymphoid leukocytes, lymphocytes, and red blood cells. Also provided is a method of selectively inducing cell death in myeloid leukocytes. The method comprises contacting the myeloid leukocytes with a composition comprising leukotoxin proteins. These leukotoxin proteins may be isolated from the NJ4500 strain of Actinobacillus actinomycetemcomitans. A method of purifying leukotoxin protein from the NJ4500 strain of Actinobacillus actinomycetemcomitans is also provided, as well as an assay that allows for the rapid determination of the activity of a given drug against leukemic cells either taken from a patient or derived from a cell line. The assay is performed in the presence of whole blood or serum.
Owner:RUTGERS THE STATE UNIV
Fly maggot extractive as well as preparation method and application thereof
ActiveCN101991608AEnhance the body's humoral immunitySimple and efficient operationAnthropod material medical ingredientsAntineoplastic agentsMaggotWater soluble
The invention discloses a fly maggot extractive containing the principal components of water-soluble proteins, and the water-soluble proteins contained in the fly maggot extractive have the mass percentage of 50-70 percent and the molecular weight of 2-16 KDa. The fly maggot extractive not only has obvious inhibiting effect on human promyelocytic leukemia HL-60 cells, human erythroleukemia K562 cells, human liver cancers SMMC-7721, mouse leukemia P388 cells, human lung adenocarcinoma A549 cells, human nasopharyngeal darcinoma CNE cells, human prostatic carcinoma PC3 cells, human cervical carcinoma HeLa in vitro, but also has outstanding inhibiting effect on mouse S180 sarcomas and mouse Heps liver cancer solid tumors, and also has the effect on enhancing the humoral immunity of organisms. The invention also discloses a preparation method of the fly maggot extractive. The preparation method is easy and convenient for operation and control and low in cost and is suitable for industrialized production.
Owner:浙江佰科堂生物科技股份有限公司
Biosensing method for detecting leukemia by graphene/mimetic peroxidase double-signal amplification
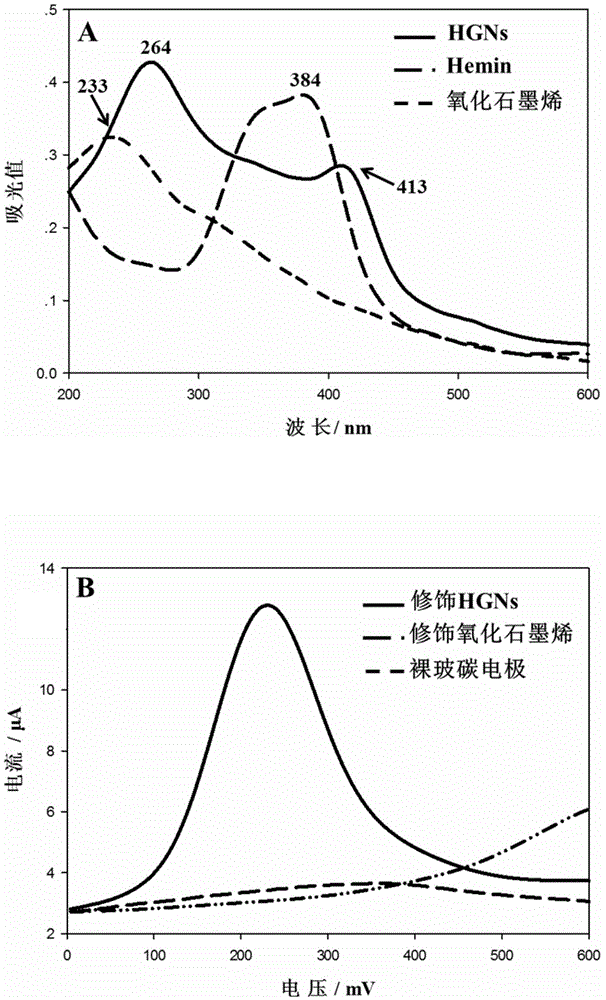
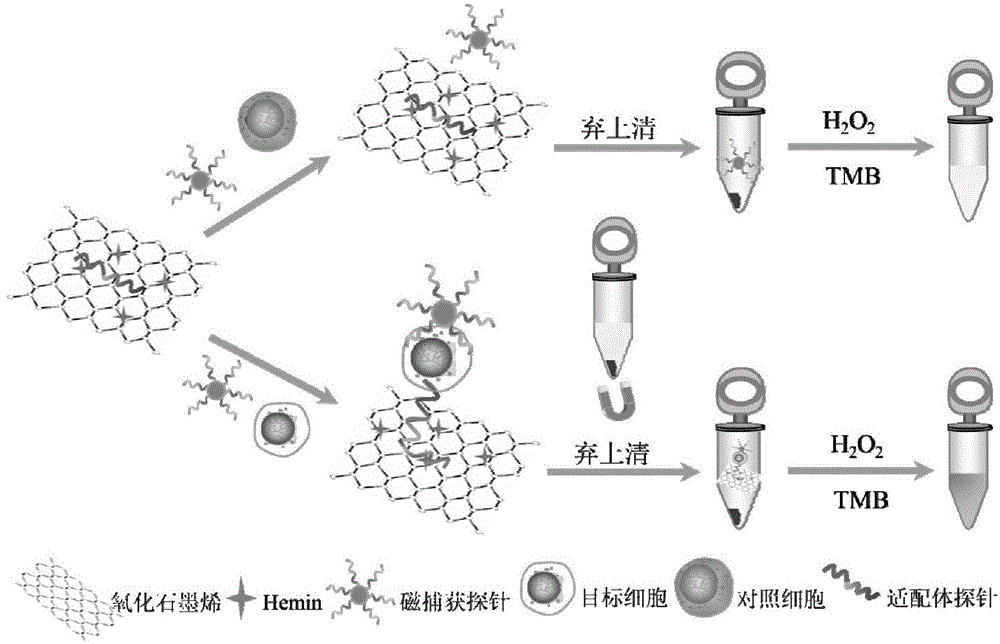
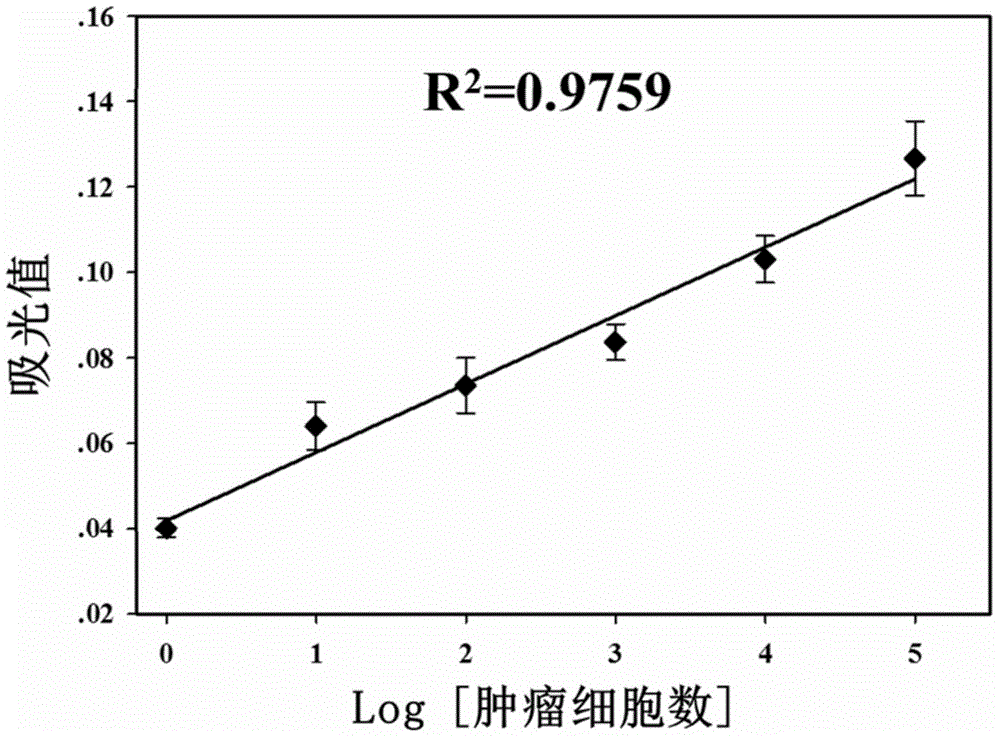
The invention discloses a biosensing method for detecting leukemia by graphene / mimetic peroxidase double-signal amplification. The invention provides a kit for detecting leukemia cells; the kit comprises (a) a magnetic bead capture probe and (b) an aptamer functionalization Hemin-graphene composite nanomaterial, wherein the magnetic bead capture probe comprises a magnetic bead and a capture probe fixed on the magnetic bead; the capture probe is a single-stranded deoxyribonucleic acid (DNA) molecule shown by sequence 1; the aptamer functionalization Hemin-graphene composite nanomaterial is composed of graphene modified by Hemin, and an aptamer probe fixed on the graphene modified by the Hemin; the aptamer probe is a single-stranded DNA molecule shown by sequence 2. After the biosensing method is used, the target leukemia cells with lower concentration can be captured and enriched within a short time, and a detection signal is amplified, so that the leukemia cells can be rapidly, accurately and sensitively detected.
Owner:GUANGXI MEDICAL UNIVERSITY
Combined reagent for detecting acute myelocytic leukemia cells and system thereof
ActiveCN109655616AWide coverageThere is no problem of reciprocal inhibition of expressionMaterial analysisCD33CD15
The invention relates to a combined reagent for detecting acute myelocytic leukemia cells and a system thereof, wherein the combined reagent and the system thereof belong to the field of medical technology. The combined reagent comprises at least one selected from the following antibody combinations: a first antibody combination which comprises CD38, CD13, CD34, CD117, CD33, CD19, HLA-DR and CD45antibodies; a second antibody combination which comprises CD38, CD64, CD34, CD123, CD56, CD14, HLA-DR and CD45 antibodies; and a third antibody combination which comprises CD38, CD7, CD34, CD5, CD11b,CD15 and CD45 antibodies. The antibody combinations of the invention cover the expression marks of three systems of granulocyte, single cell and lymphocyte. A normal antibody expression mode is established. Tumor cells can be identified maximally. Furthermore, through a large number of experiment data, the antibodies in each combination have no problem of mutual expression inhibition. FurthermoreAML-MRD can be comprehensively and quickly detected with high sensitivity through multi-parameter flow type cell analysis.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
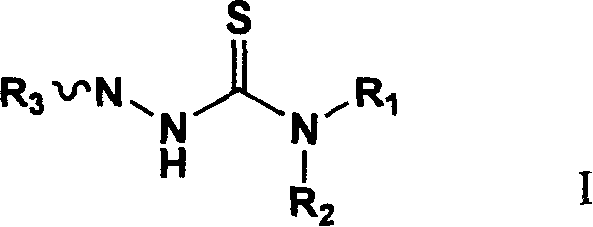
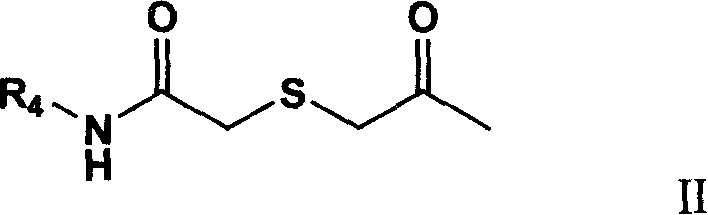
1-substituted-4,4-2 substituted thiosemicarbazide compounds, production method and uses of the same
InactiveCN101195597AStrong anti-cell proliferation effectOrganic chemistryAntineoplastic agentsIntestinal CancerAdenocarcinoma lung cancer
The invention relates to a 1-substituted-4, 4-bis-substituted thiosemicarbazide compound, a relative preparation method and application thereof, wherein the compound is represented as formula I, R1, R2 and R3 are defined as description. The inventive compound can restrain A-549 (human lung adenocarcinoma), P388 (mouse leukemia cell line), and HT-29 (human intestinal cancer cell line), with strong inhibition of cell proliferation, which can be used to develop new anti-cancer drug.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Thioxothiazolidinone Compounds For Use As Pharmaceuticals
InactiveUS20080108677A1Suppressed leukocyte migrationEnhance and inhibit primary adhesionBiocideOrganic active ingredientsWhite blood cellIntegrin
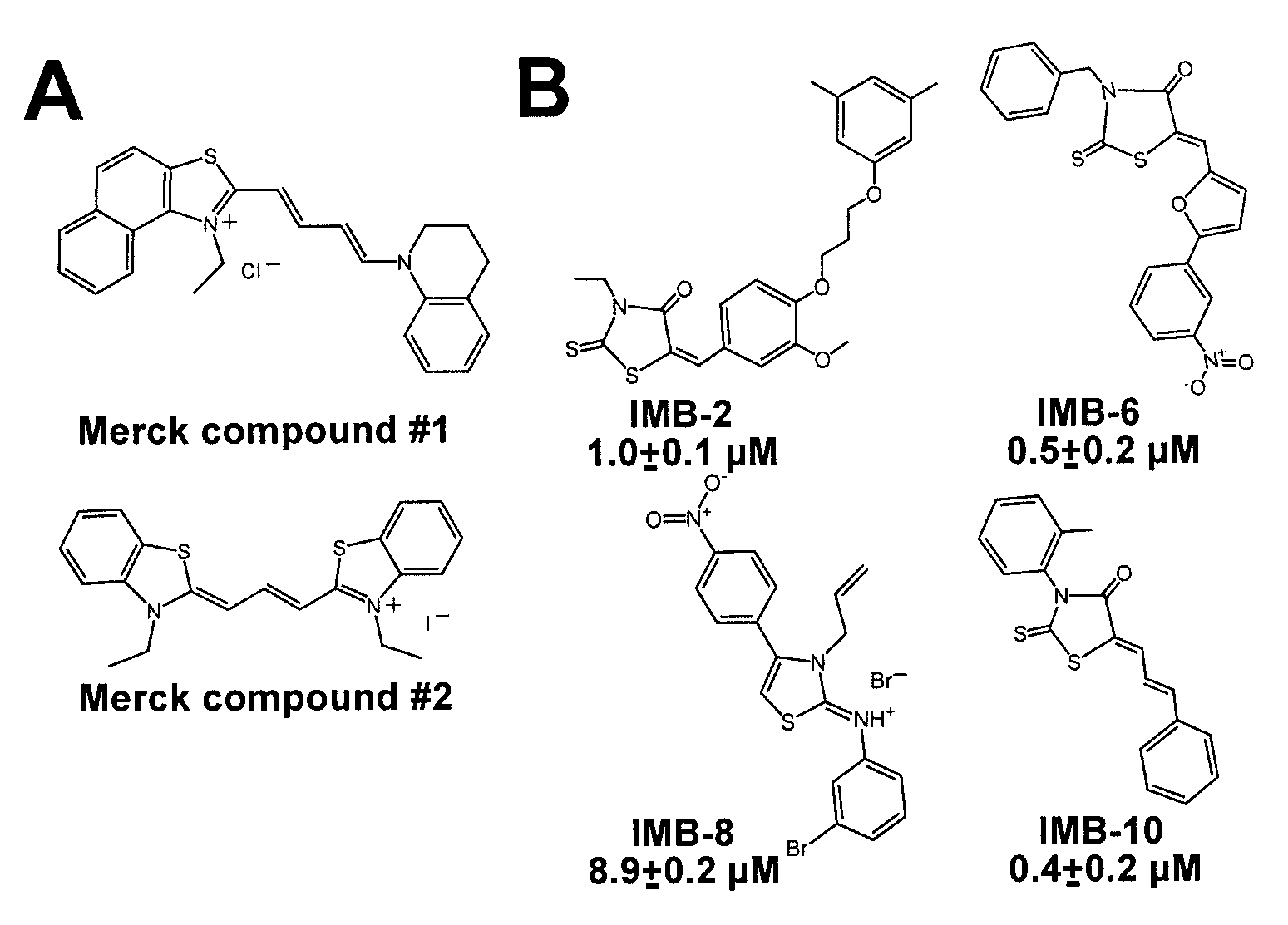
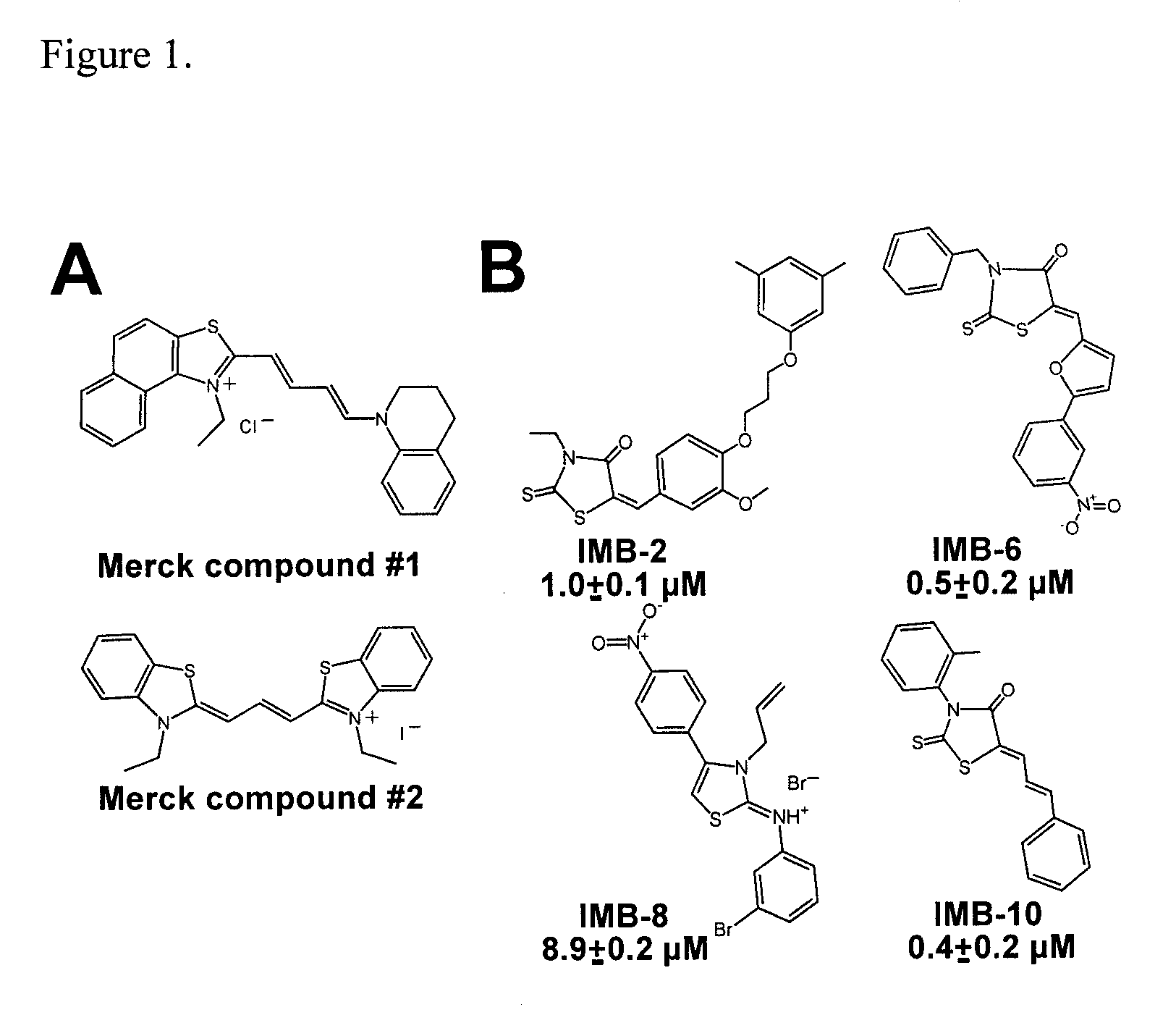
The present invention relates to thioxothiazolidinone compounds for use as pharmaceuticals, to pharmaceutical compositions comprising these compounds, and to the use of said small-molecule compounds for the manufacture of pharmaceutical compositions for the treatment of conditions dependent on leukocyte cell migration, such as leukaemia and inflammatory diseases. Said compounds inhibit leukaemia cell migration by stabilizing the active conformation of the αM integrin I domain.
Owner:KARYON CTT
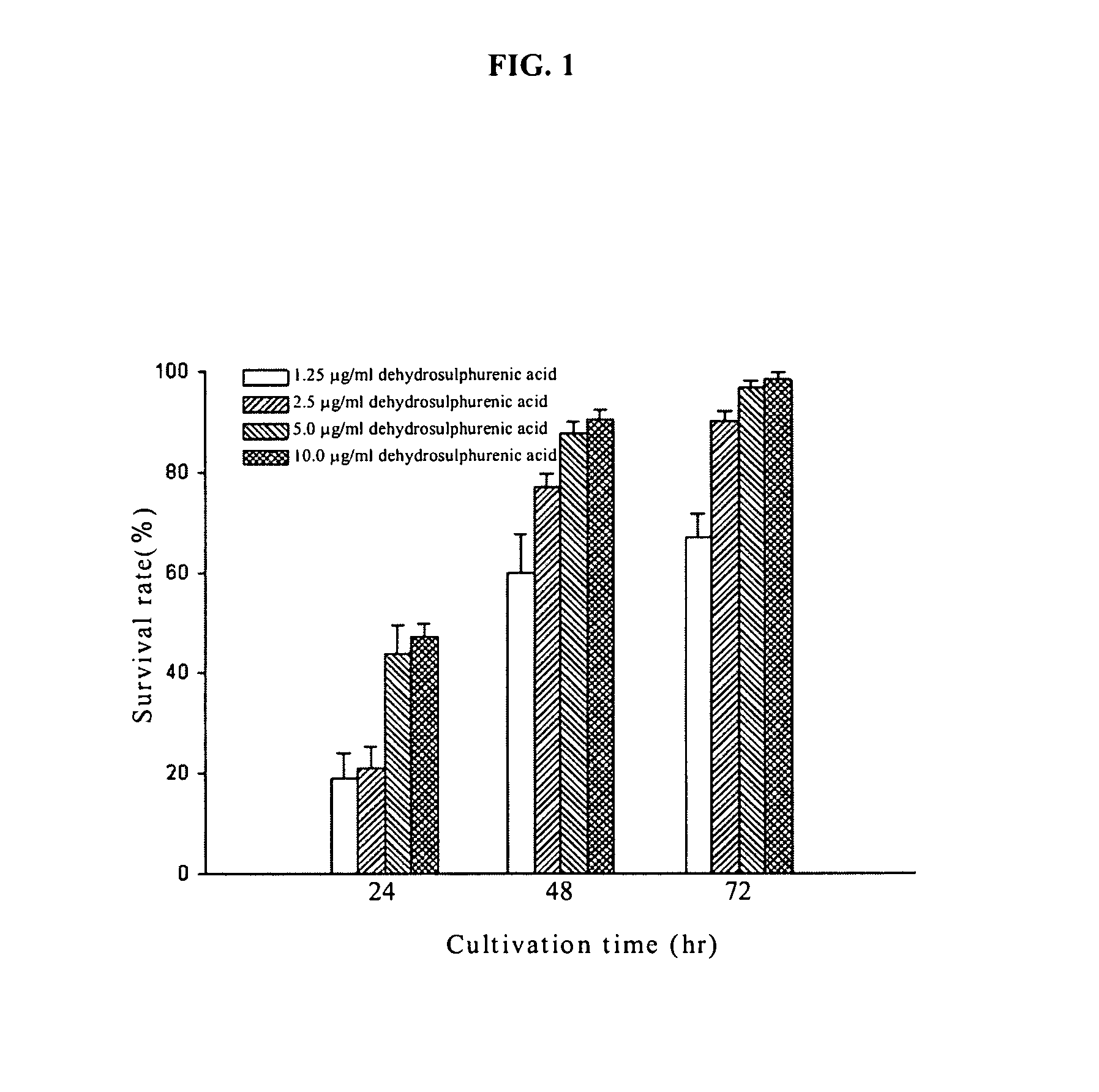
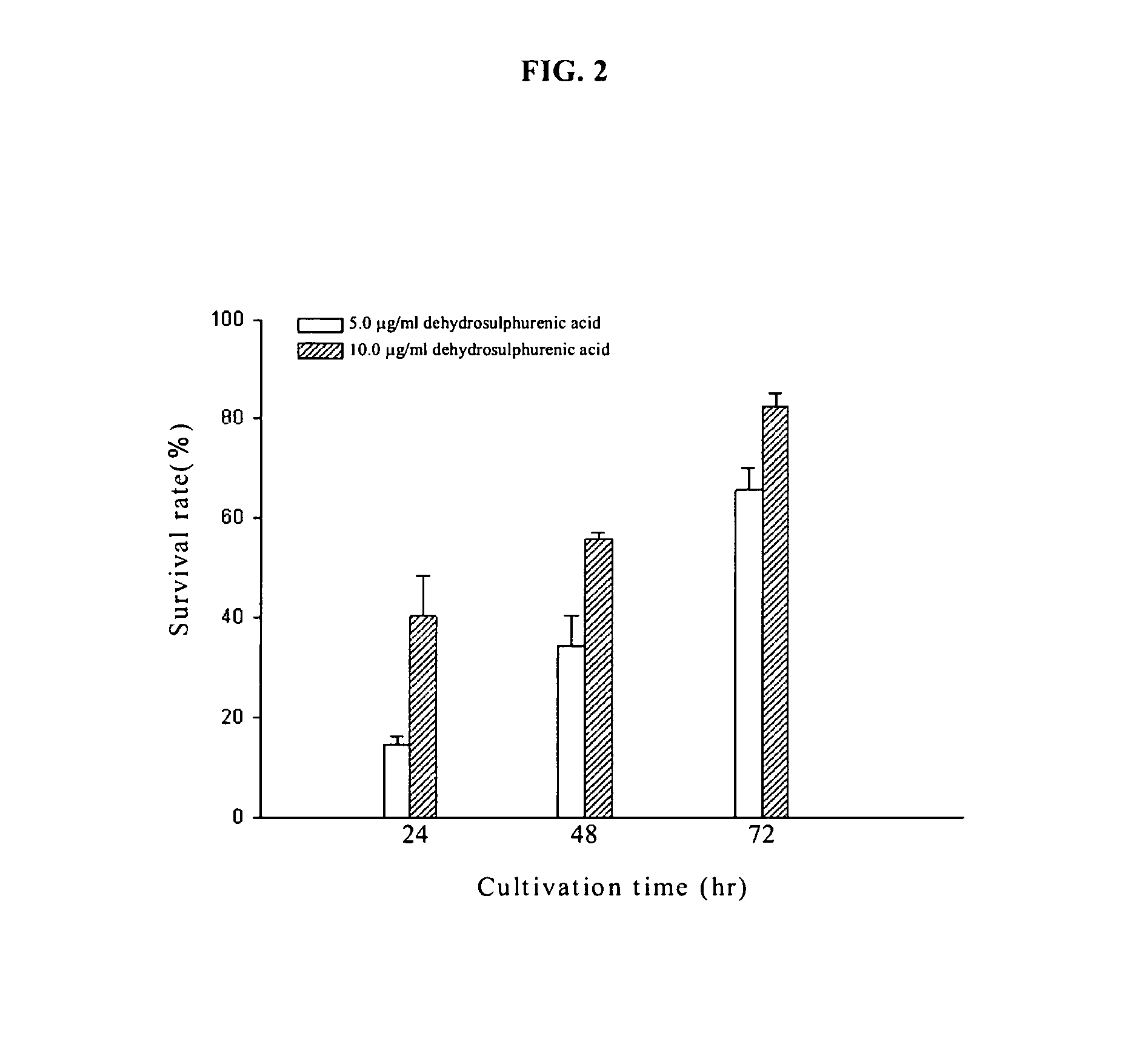
Method for inhibiting tumor growth with dehydrosulphurenic acid extracted from Antrodia cinnamomea
The present invention relates to a method for inhibiting tumor growth, in particular to the method using dehydrosulphurenic acid to inhibit the growth of leukemia cell or pancreatic cancer cell by a compound extracted and purified from Antrodia cinnamomea. Dehydrosulphurenic acid of the invention can be used as a pharmaceutical composition to inhibit the tumor growth of leukemia or pancreatic cancer.
Owner:MACKAY MEMORIAL HOSPITAL
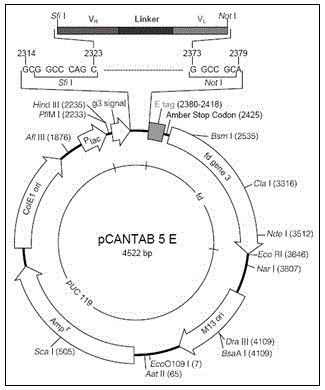
Leukemia single-chain antibody library, as well as construction method and application thereof
ActiveCN102978713AIncrease library capacityGood varietyPeptide librariesAntibody ingredientsCell specificLeukemia associated antigens
The invention provides a leukemia bacteriophage single-chain antibody library, which uses phasmid pCANTA-5E as a carrier, has storage capacity of 1.5*10<9>, and has excellent diversity in proving of DNA (deoxyribonucleic acid) sequencing. A leukemia cell specific completely humanized single-chain antibody can be obtained conveniently in multiple screenings by using a specific leukemia related antigen as a target through a bacteriophage representation technology from the completely humanized single-chain antibody library. The antibody library provided by the invention has high storage capacity and excellent diversity, and comprises a combination of complete humanized single-chain antibodies; and a single-chain antibody is formed by connection of a heavy chain variable region VH and a light chain variable region VL through a connecting peptide (GGGGS)3, and contains a complete antigen-binding site. The method has the advantages of strong operability and high storage capacity, and can be used for conveniently screening the leukemia cell specific completely humanized single-chain antibody; and the completely humanized single-chain antibody can be used for preparing a target medicament, which is used for treating leukemia.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com