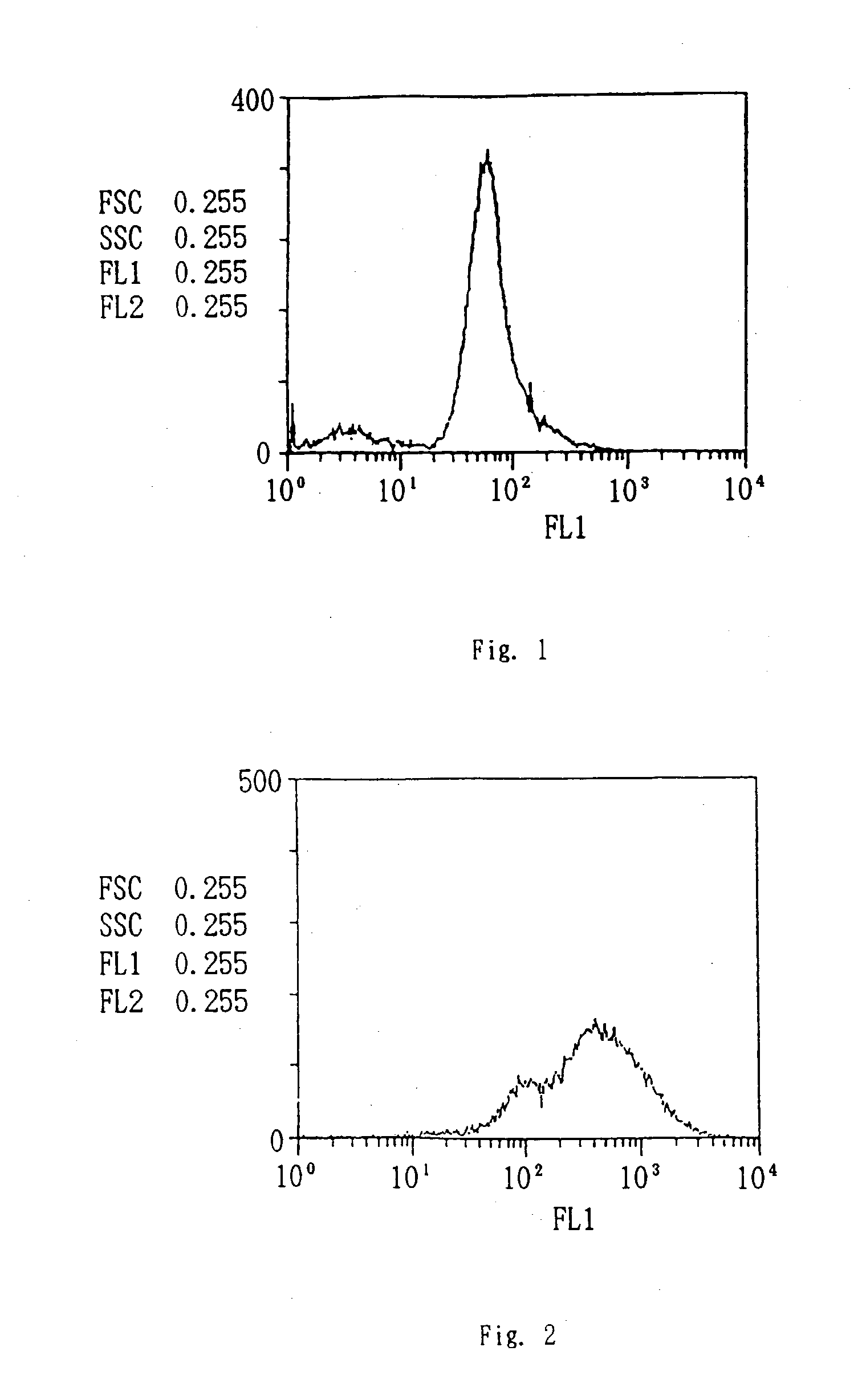
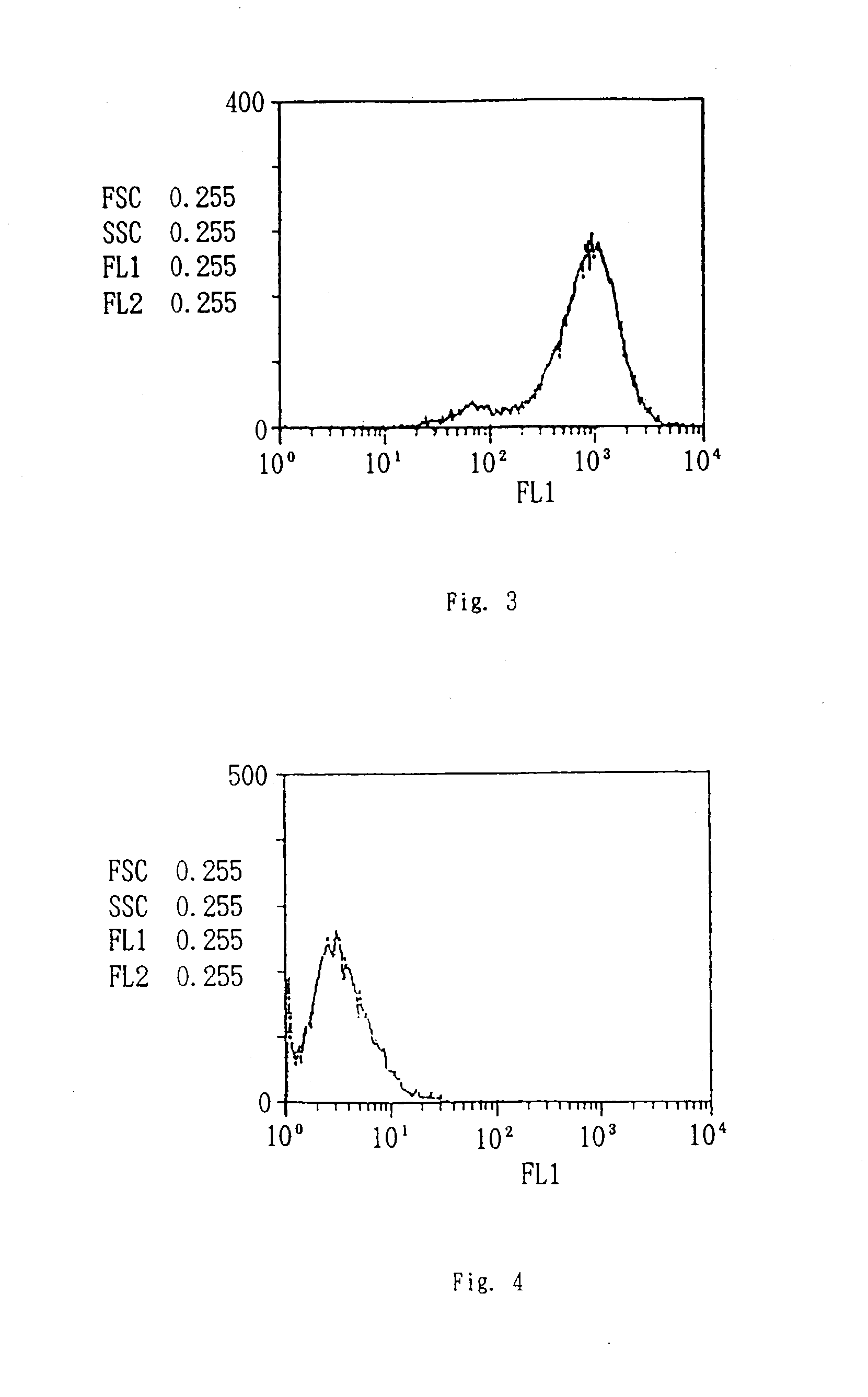
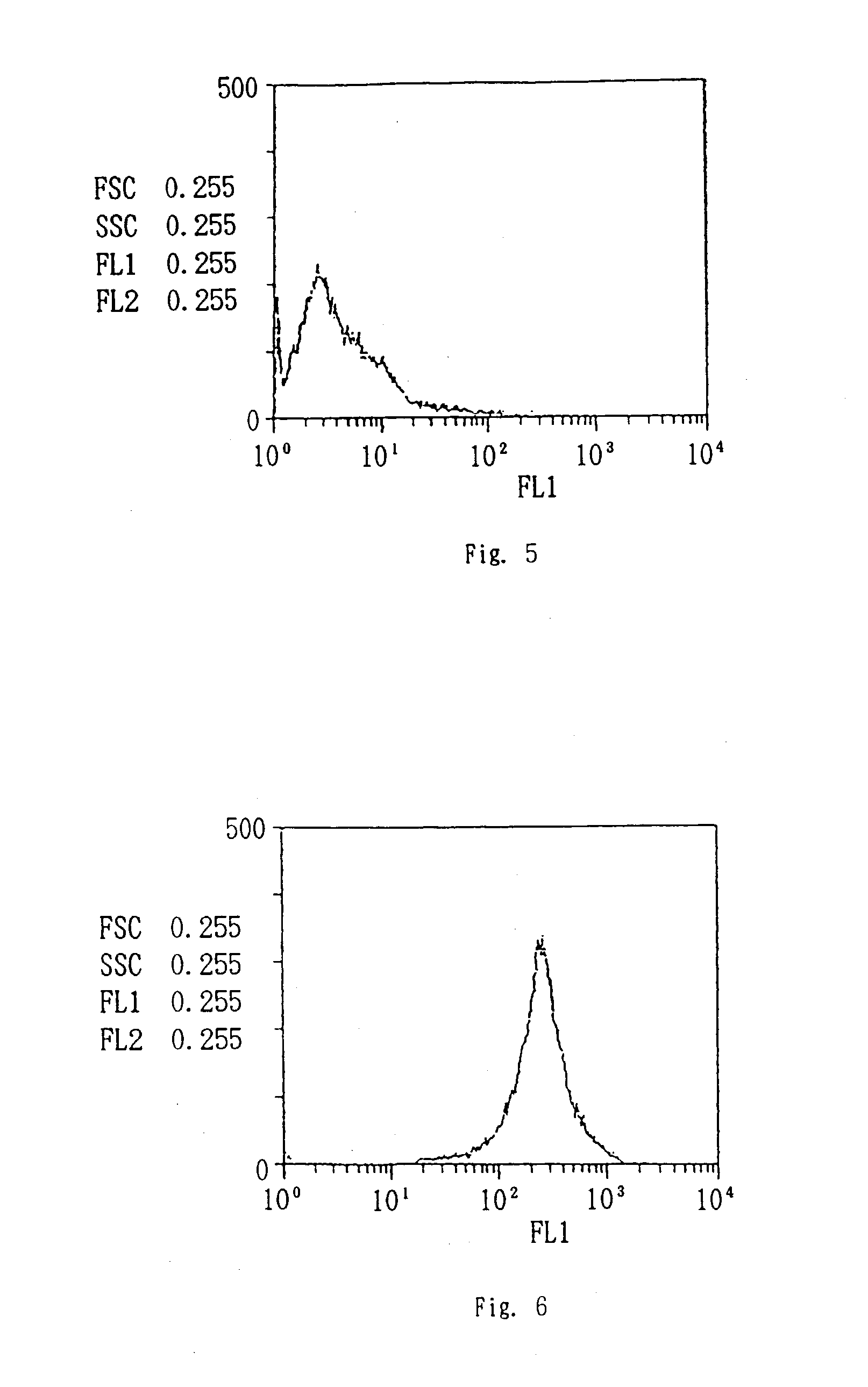
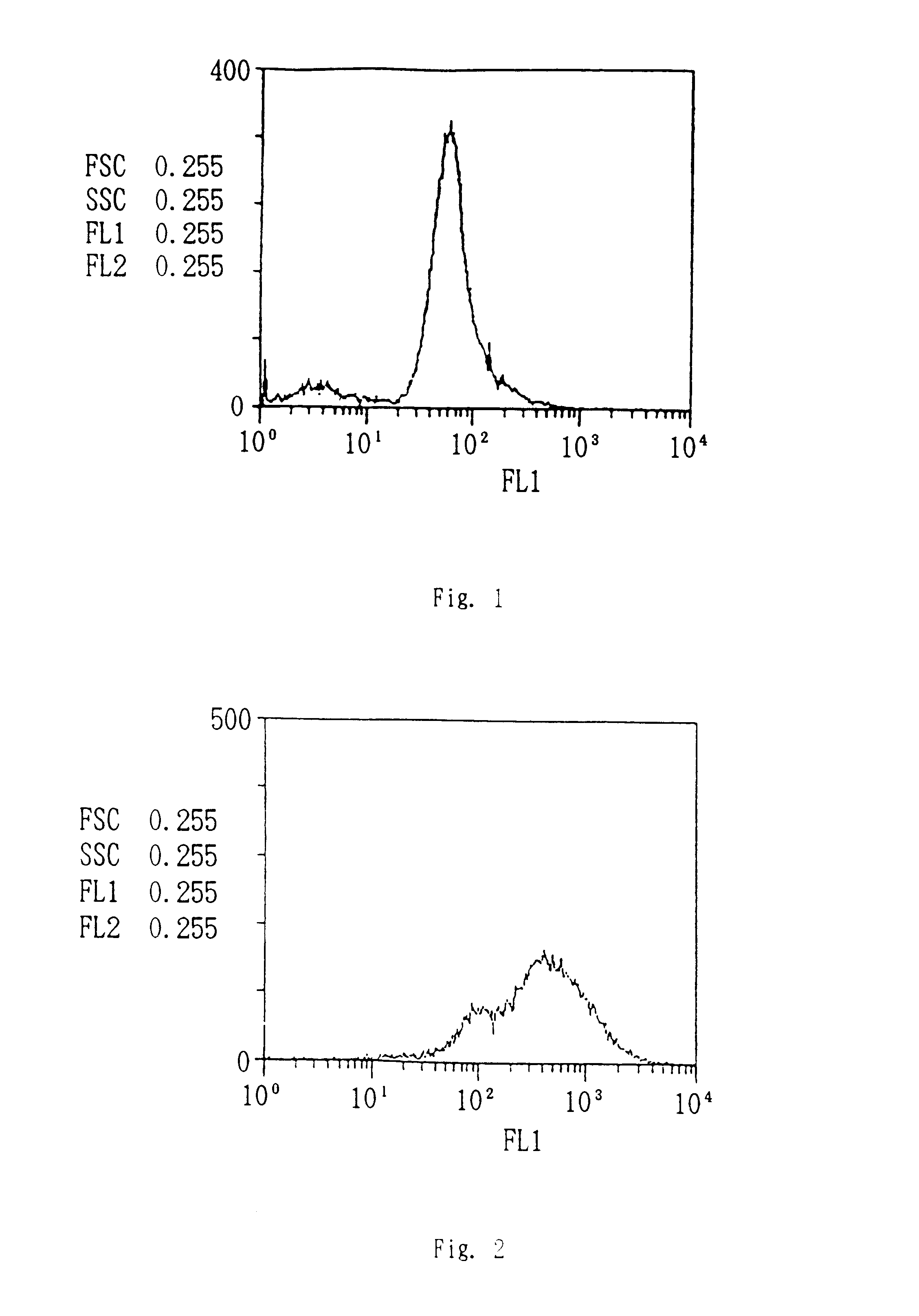
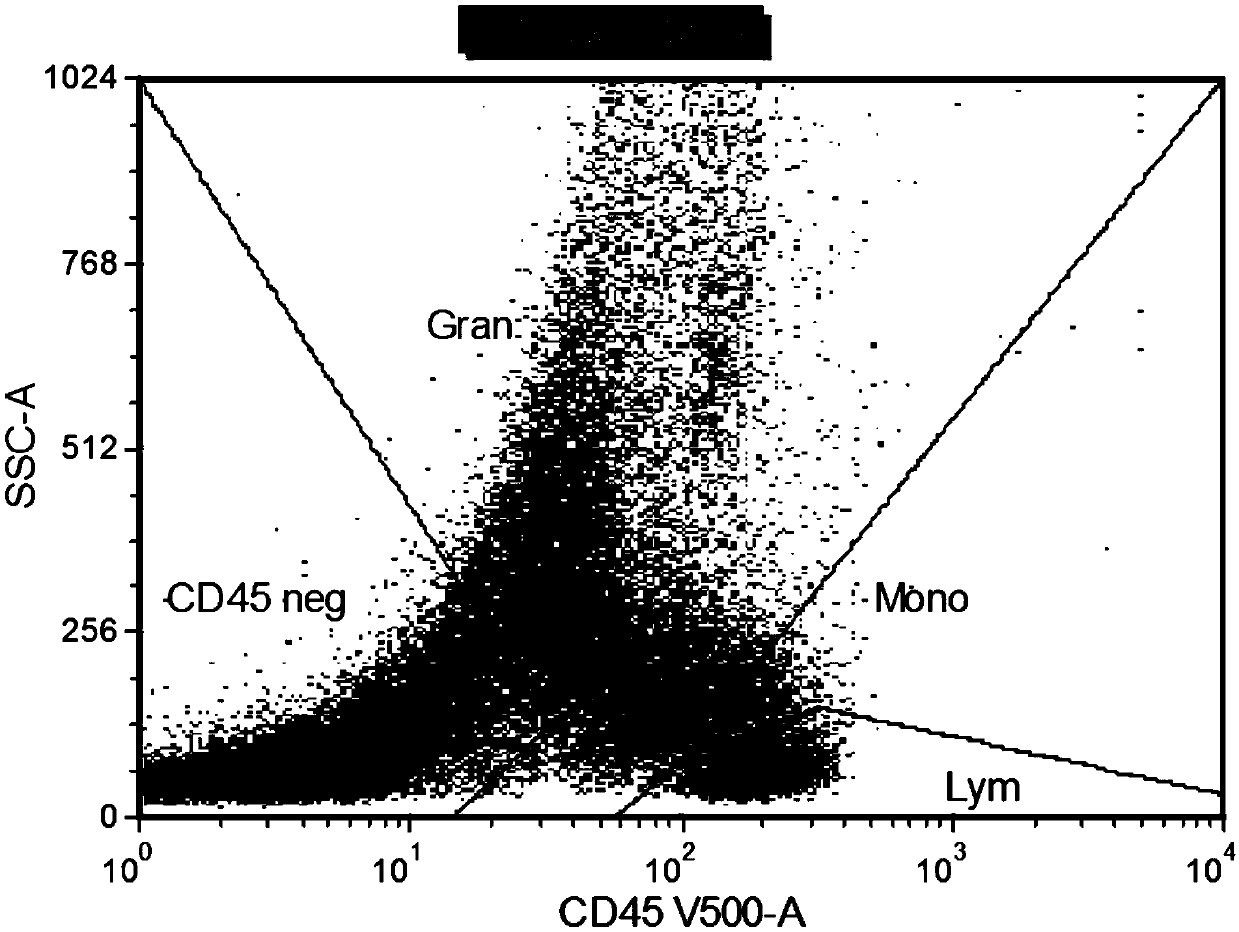
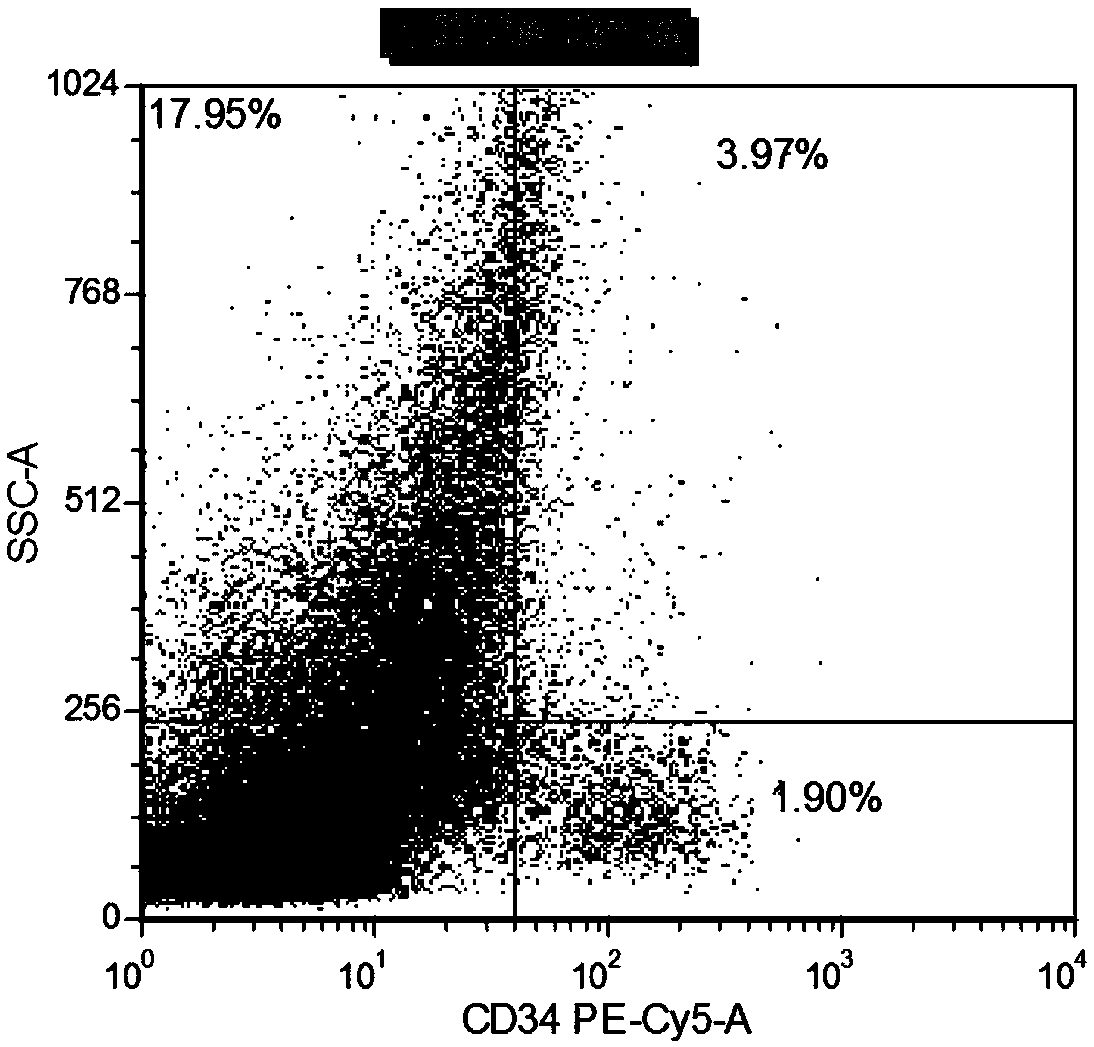
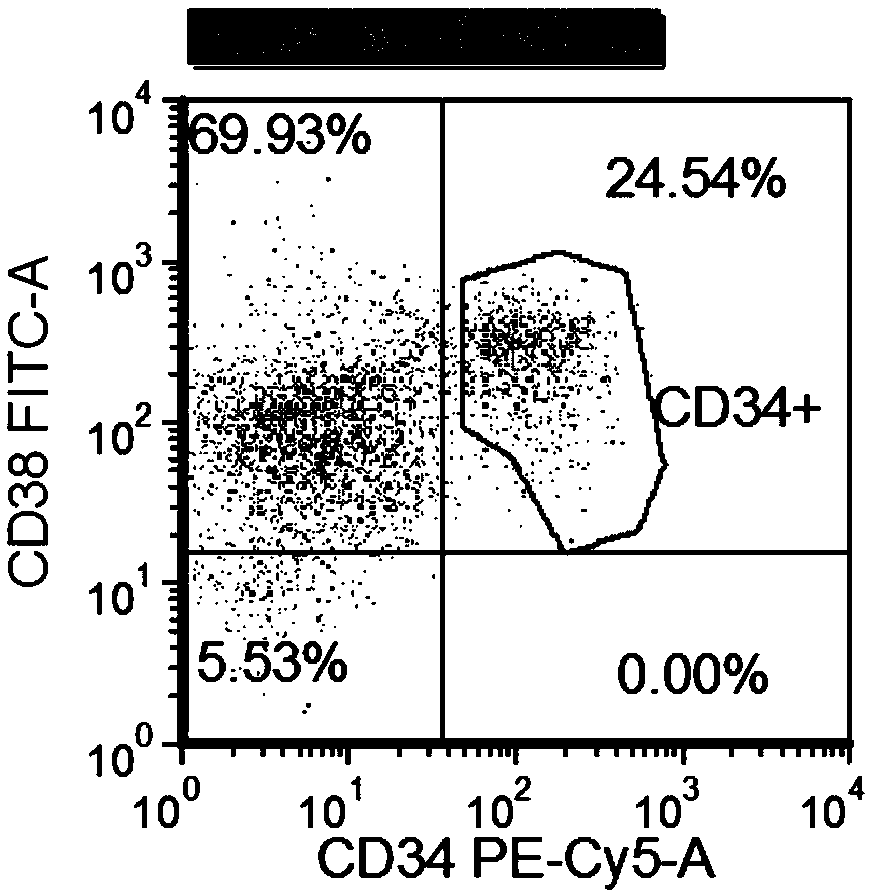
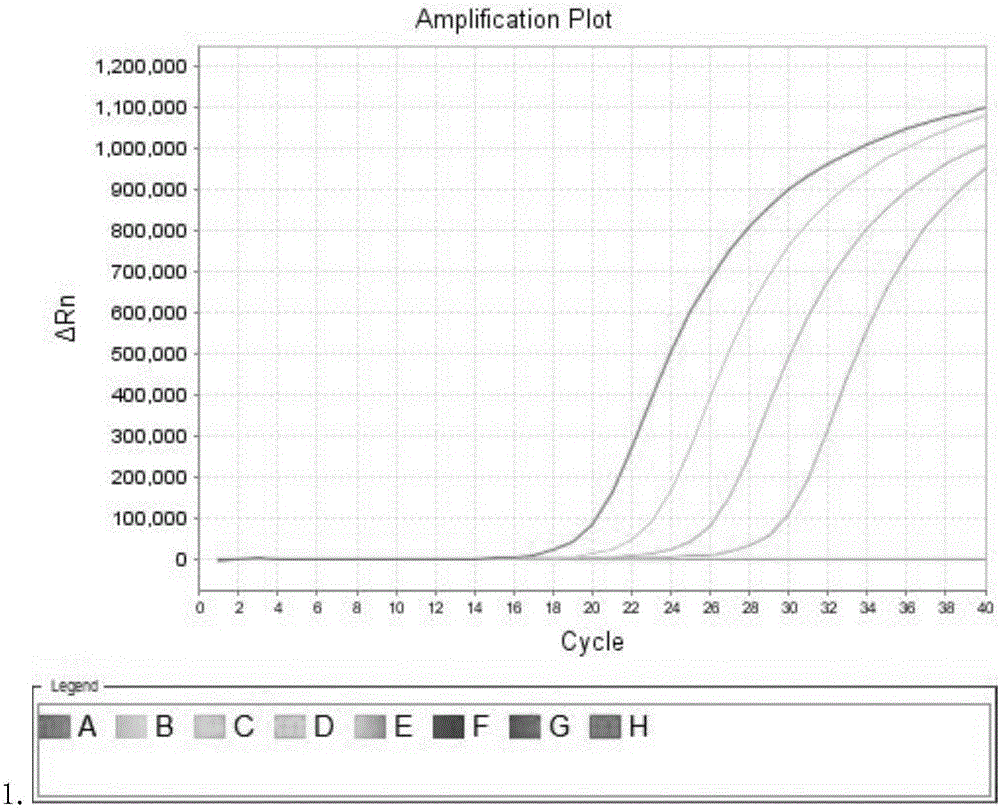
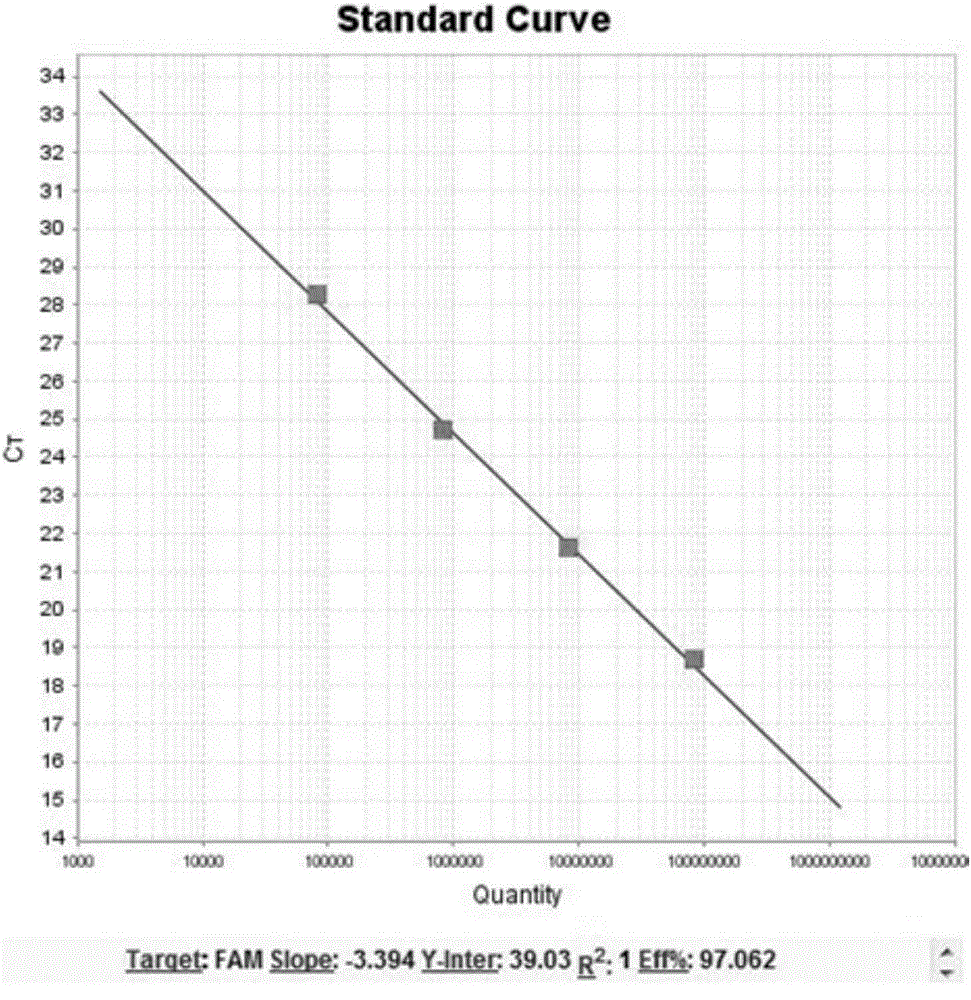
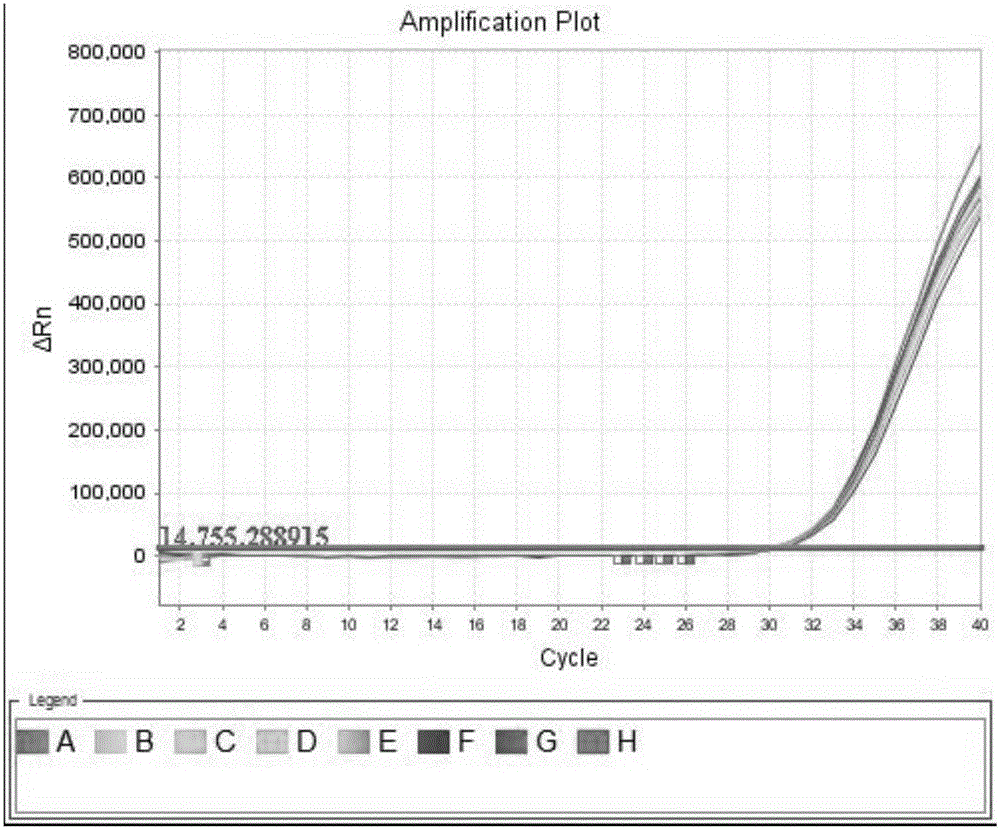
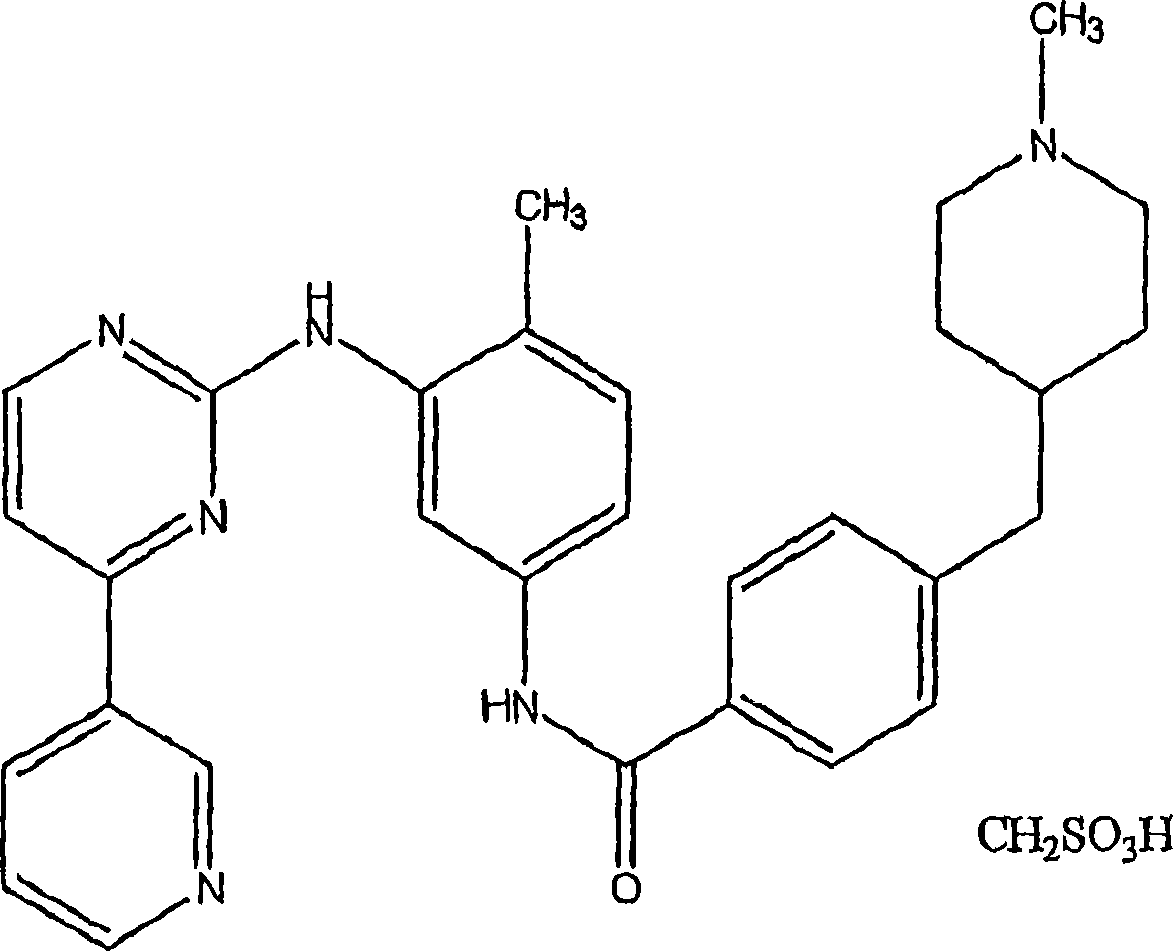
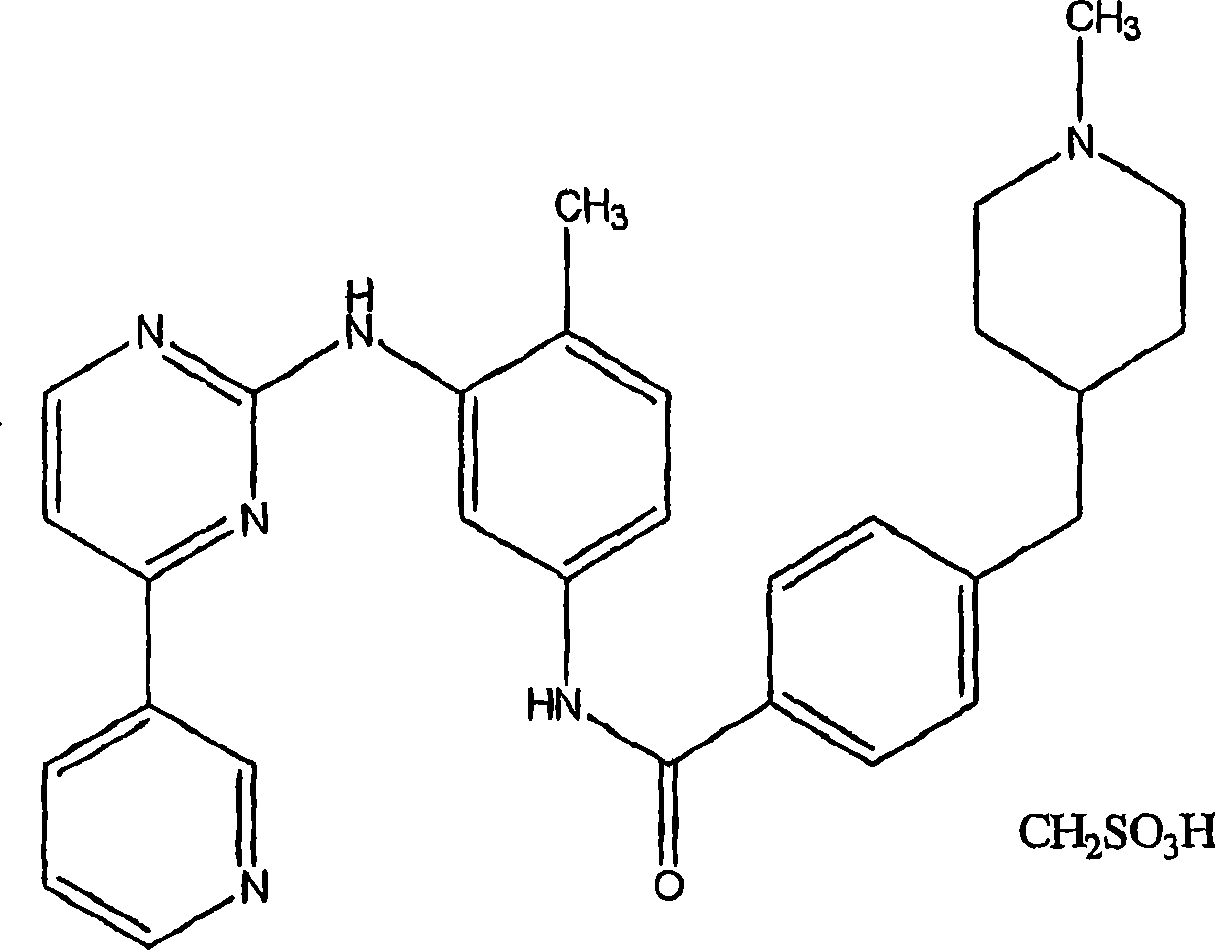
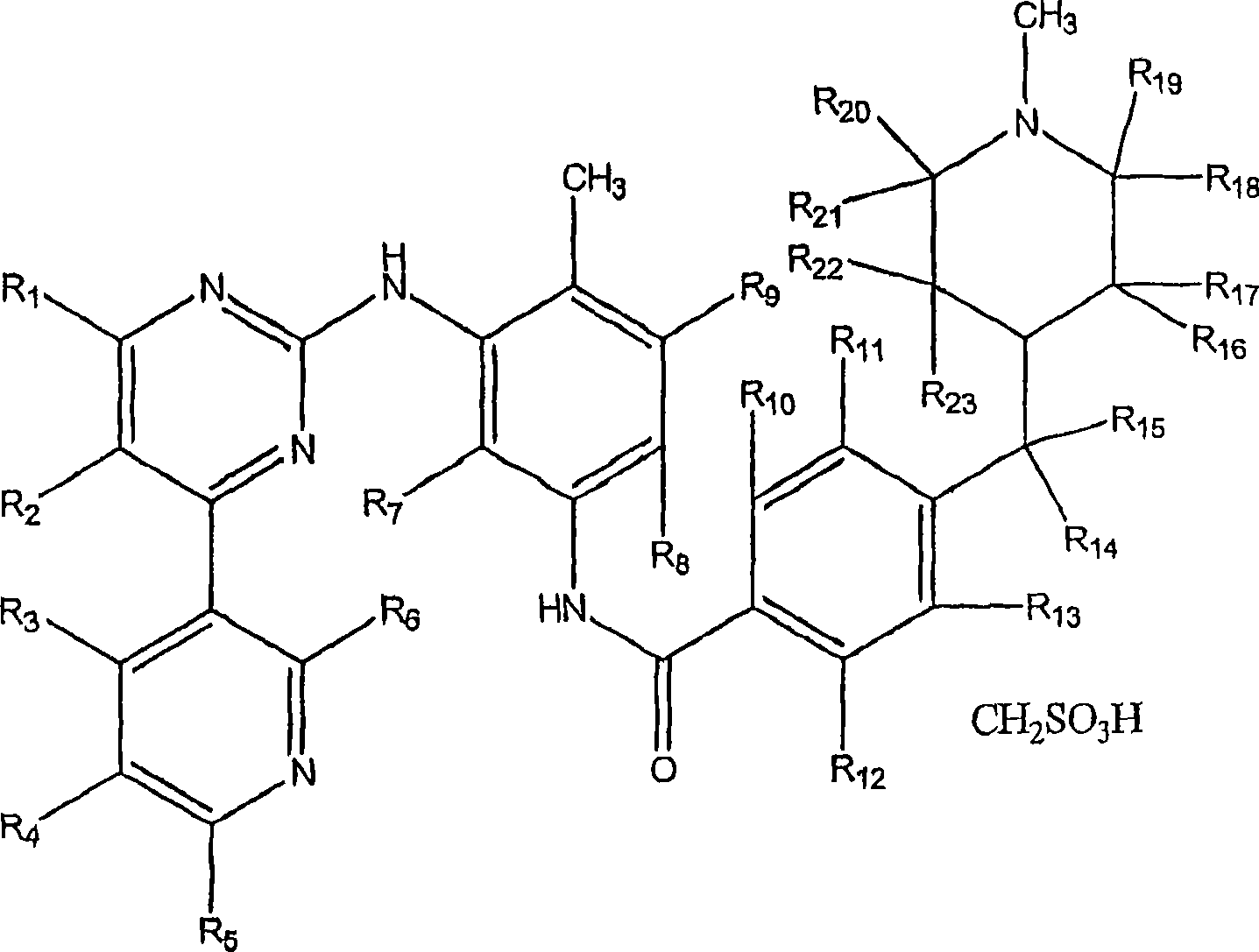
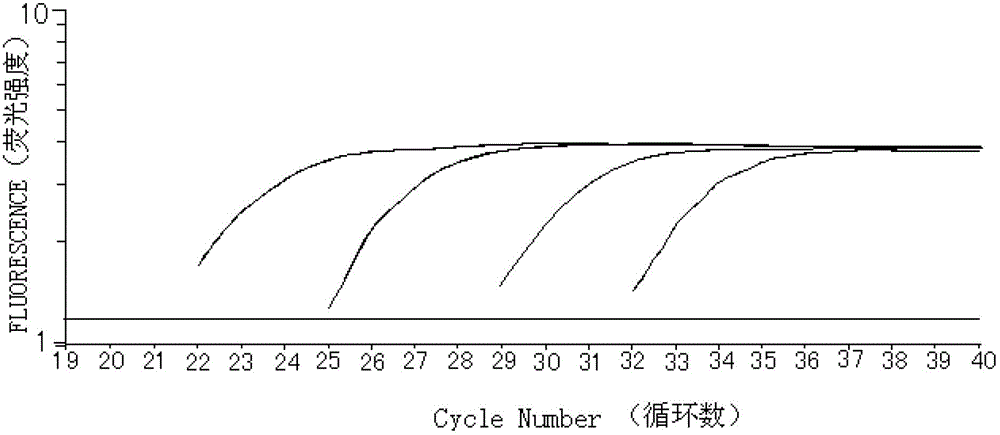
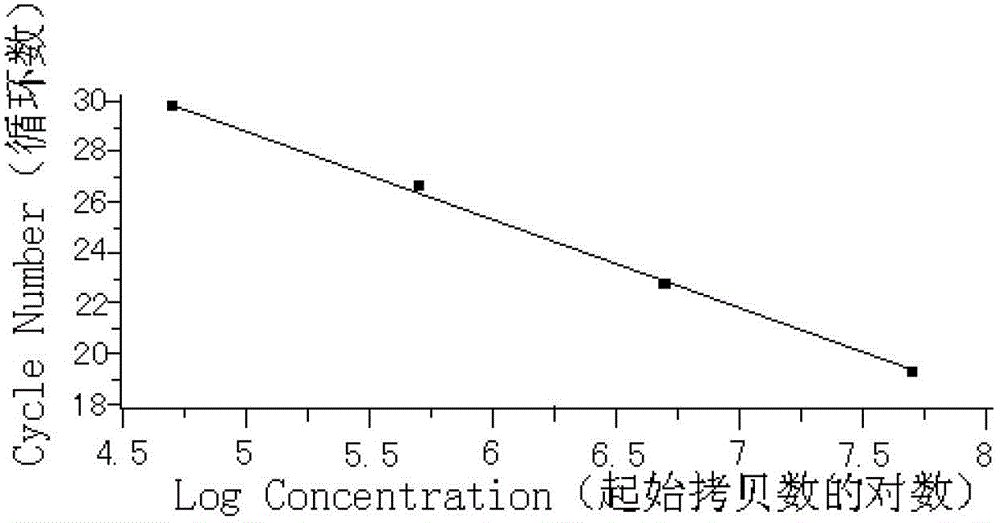
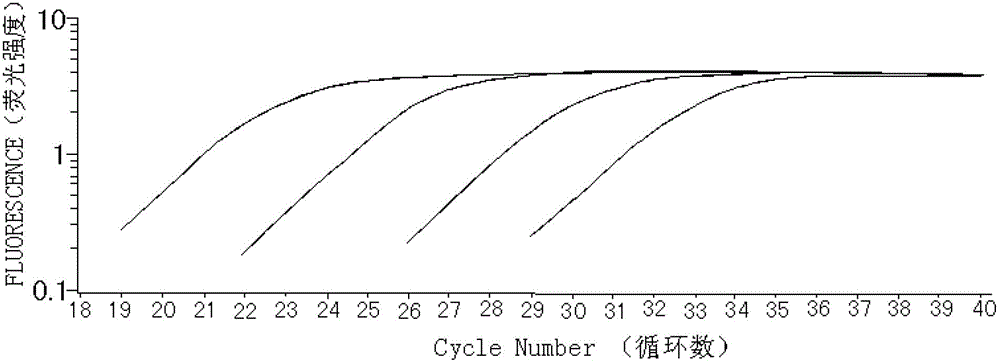
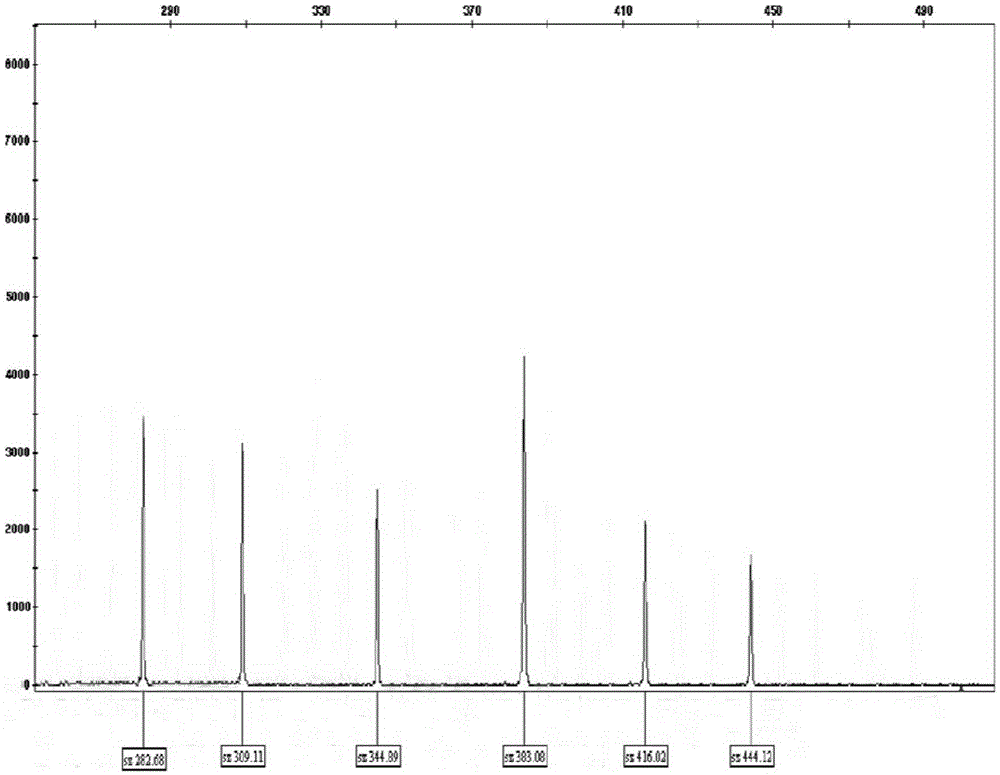
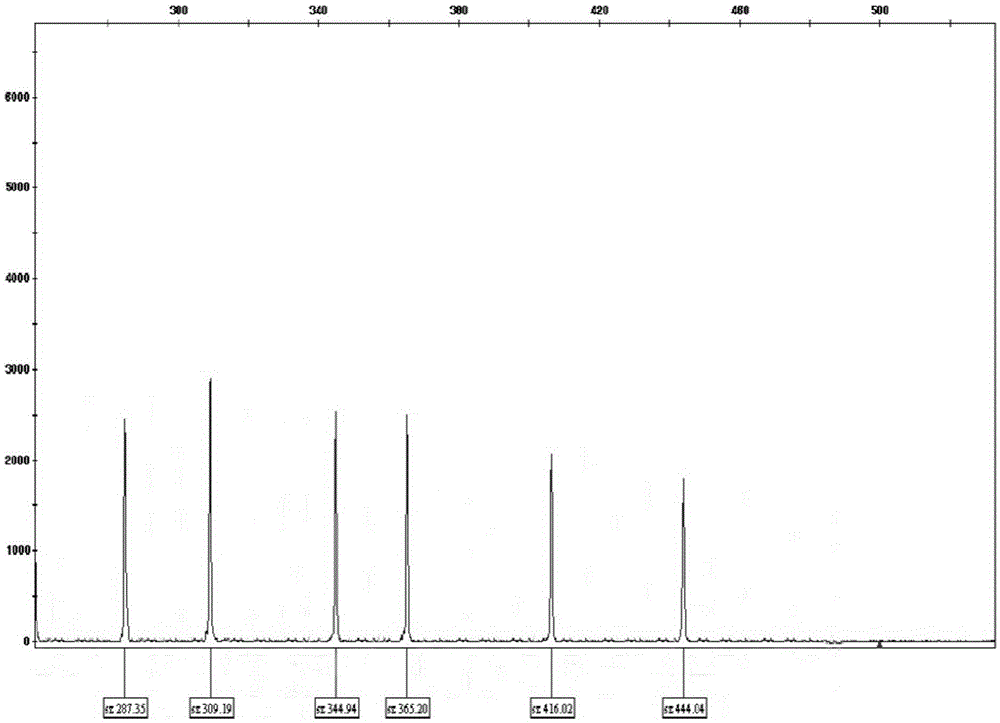
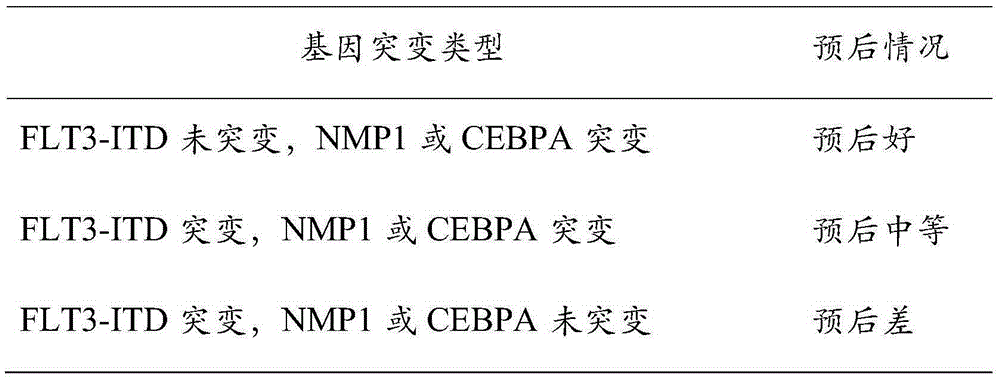
Patents
Literature
83 results about "Myelocytic leukemia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
T cell receptor-like antibodies specific for a wti peptide presented by hla-a2
The present invention provides antigen binding proteins that specifically bind to Wilms' tumor protein (WT1), including humanized, chimeric and fully human antibodies against WT1, antibody fragments, chimeric antigen receptors (CARs), fusion proteins, and conjugates thereof. The antigen binding proteins and antibodies bind to HLA-A0201-restricted WT1 peptide. Such antibodies, fragments, fusion proteins and conjugates thereof are useful for the treatment of WT1 associated cancers, including for example, breast cancer, ovarian cancer, prostate cancer, chronic myelocytic leukemia, multiple myeloma, acute lymphoblastic leukemia (ALL), acute myeloid / myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). In more particular embodiments, the anti-WT1 / A antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels.
Owner:EUREKA THERAPEUTICS INC +1
T cell receptor-like antibodies specific for a WT1 peptide presented by HLA-A2
The present invention provides antigen binding proteins that specifically bind to Wilms' tumor protein (WT1), including humanized, chimeric and fully human antibodies against WT1, antibody fragments, chimeric antigen receptors (CARs), fusion proteins, and conjugates thereof. The antigen binding proteins and antibodies bind to HLA-A0201-restricted WT1 peptide. Such antibodies, fragments, fusion proteins and conjugates thereof are useful for the treatment of WT1 associated cancers, including for example, breast cancer, ovarian cancer, prostate cancer, chronic myelocytic leukemia, multiple myeloma, acute lymphoblastic leukemia (ALL), acute myeloid / myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). In more particular embodiments, the anti-WT1 / A antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels.
Owner:EUREKA THERAPEUTICS INC +1
Method of screening apoptosis inducing substances
InactiveUS20030157577A1Differentiate, identify and screen readily and highly efficientlyCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsAnticarcinogenScreening method
The present invention provides a method of screening substances having property of causing apoptosis, and relates to a method of screening substances having property of causing apoptosis characterized by using cells which are expressing IAP (Integrin Associated Protein), and the relates to above screening method, wherein the cells used are myeloid cells, and relates to pharmaceutical compositions containing as the active ingredient the substances obtained by the above method, and the present invention makes it possible to differentiate, identify and screen readily and highly efficiently the substances, such as antibodies and the like, that have property of causing apoptosis on myeloid cells by using cells which are expressing IAP while using specific binding reactions of the substances, and the above specific substances thus obtained can be used by virtue of their characteristics as the active ingredient of pharmaceutical compositions such as anticancer agents and medicines for myelocytic leukemia and the like.
Owner:CHUGAI PHARMA CO LTD
Method of screening apoptosis inducing substances
InactiveUS6579692B1Differentiate, identify and screen readily and highly efficientlyCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsAnticarcinogenScreening method
The present invention provides a method of screening substances having property of causing apoptosis, and relates to a method of screening substances having property of causing apoptosis characterized by using cells which are expressing IAP (Integrin Associated Protein), and the relates to above screening method, wherein the cells used are myeloid cells, and relates to pharmaceutical compositions containing as the active ingredient the substances obtained by the above method, and the present invention makes it possible to differentiate, identify and screen readily and highly efficiently the substances, such as antibodies and the like, that have property of causing apoptosis on myeloid cells by using cells which are expressing IAP while using specific binding reactions of the substances, and the above specific substances thus obtained can be used by virtue of their characteristics as the active ingredient of pharmaceutical compositions such as anticancer agents and medicines for myelocytic leukemia and the like.
Owner:CHUGAI PHARMA CO LTD
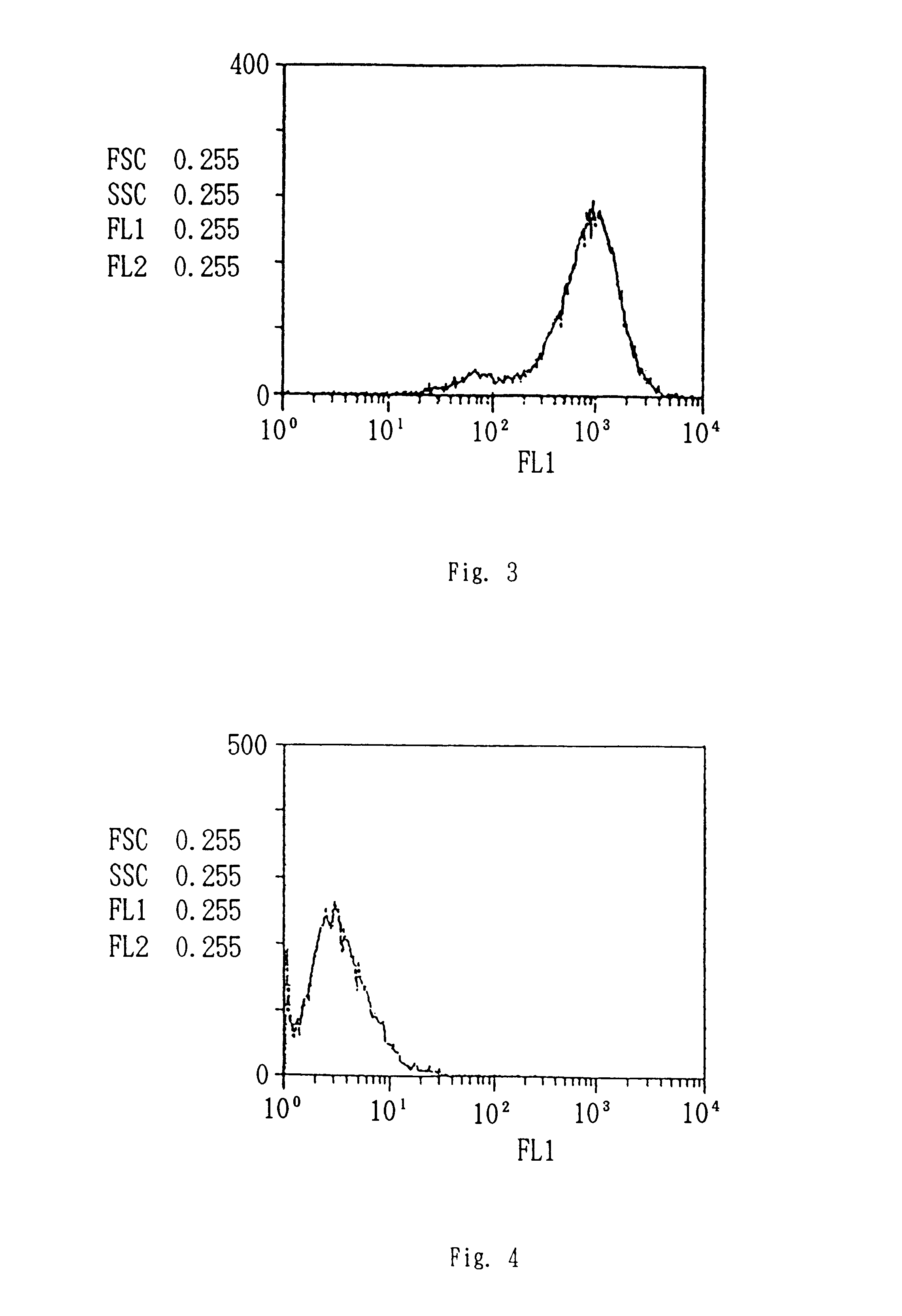
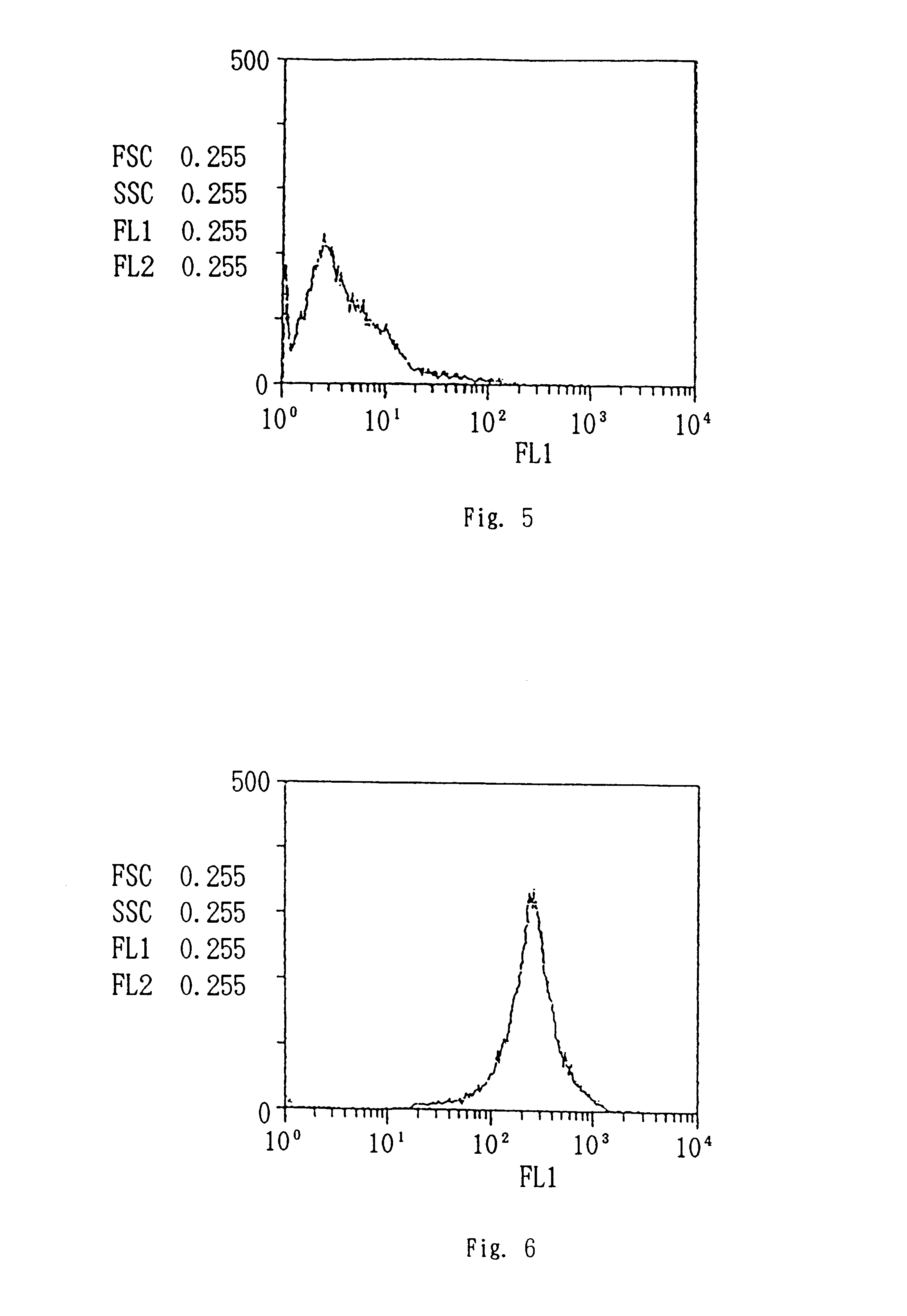
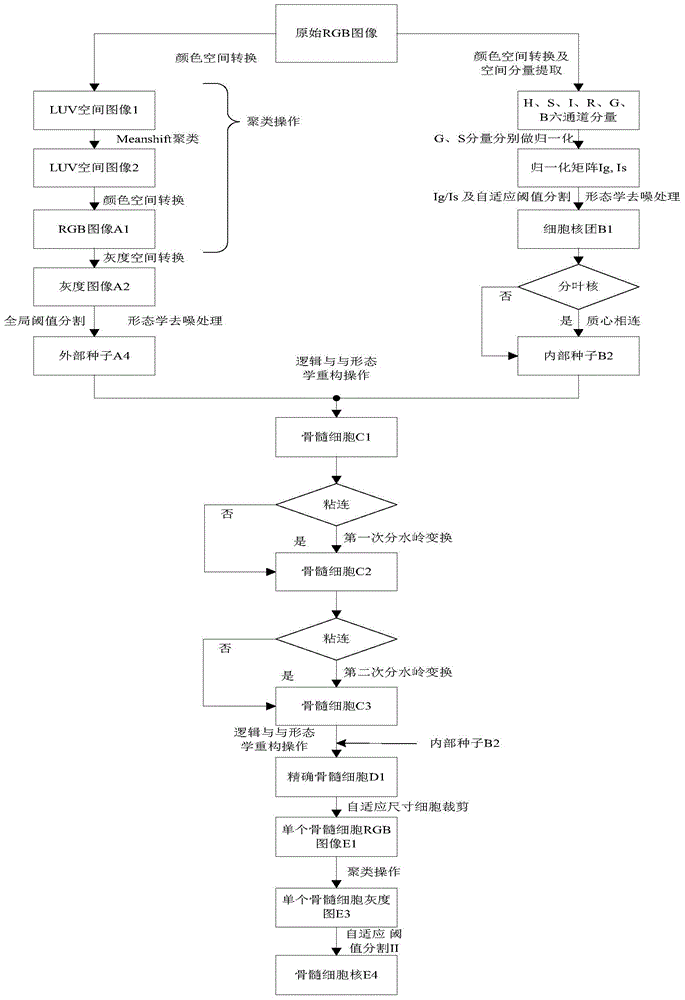
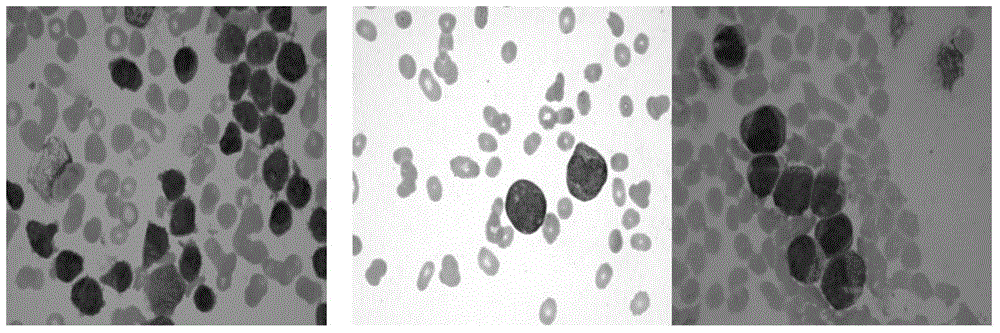
AML cell segmentation method based on Meanshift cluster and morphological operations
ActiveCN104484877ASolve the over-segmentation problemGood segmentation effectImage enhancementImage analysisBone marrow cellCell segmentation
The invention discloses an AML (Acute Myelocytic Leukemia) cell segmentation method based on Meanshift cluster and morphological operations. The algorithm is to cluster a bone marrow cell and a cell nucleus from two aspects of spatial distance and color distance, and is combined with a series of morphological operations and the modified watershed conversion technology, so as to solve the accurate segmentation problem of the adherent bone marrow cell and bone marrow cell nucleus. The algorithm is high in stability, and good in robustness for segmenting the adherent bone marrow cells with different AML types under different illumination conditions.
Owner:SHANDONG UNIV
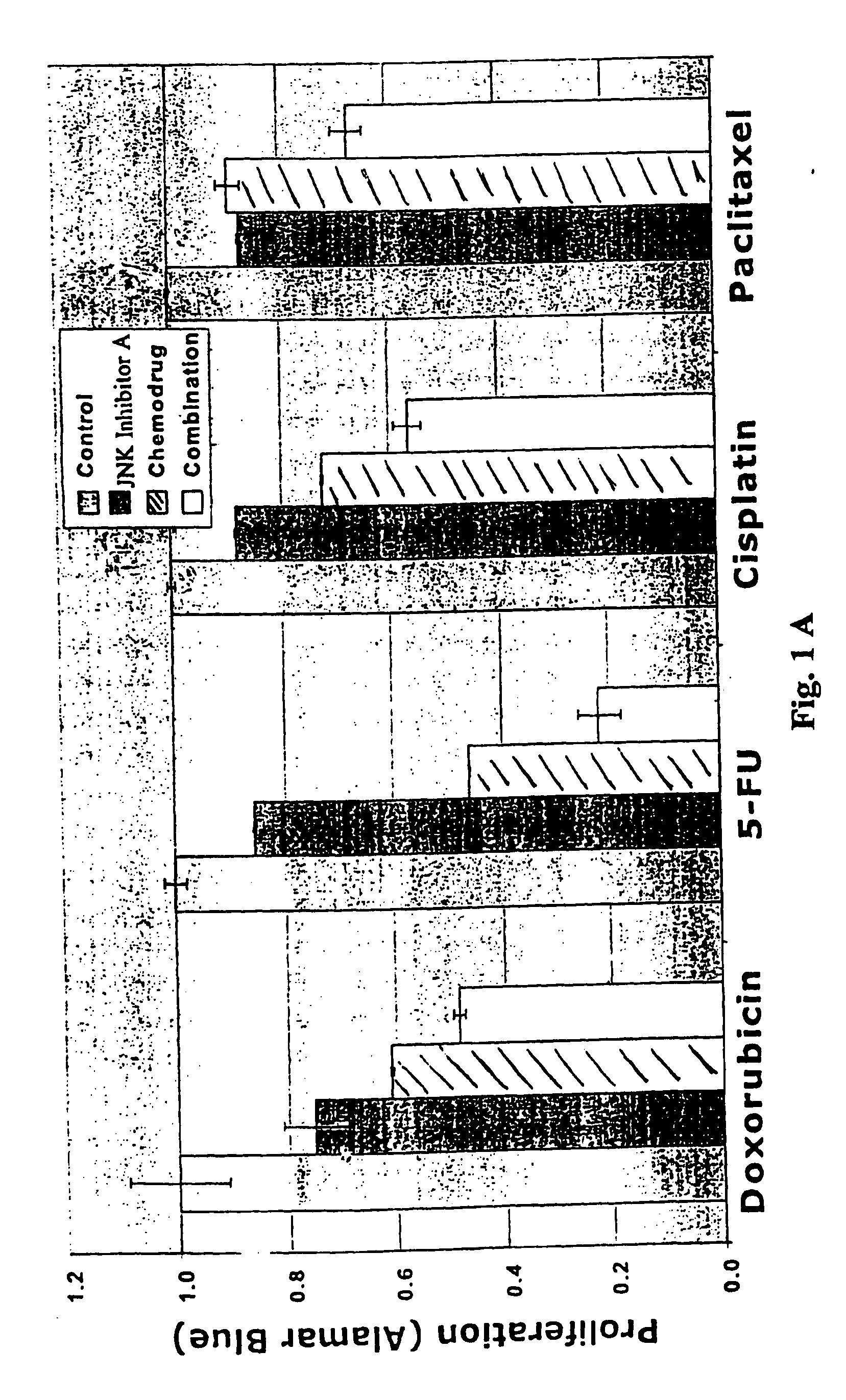
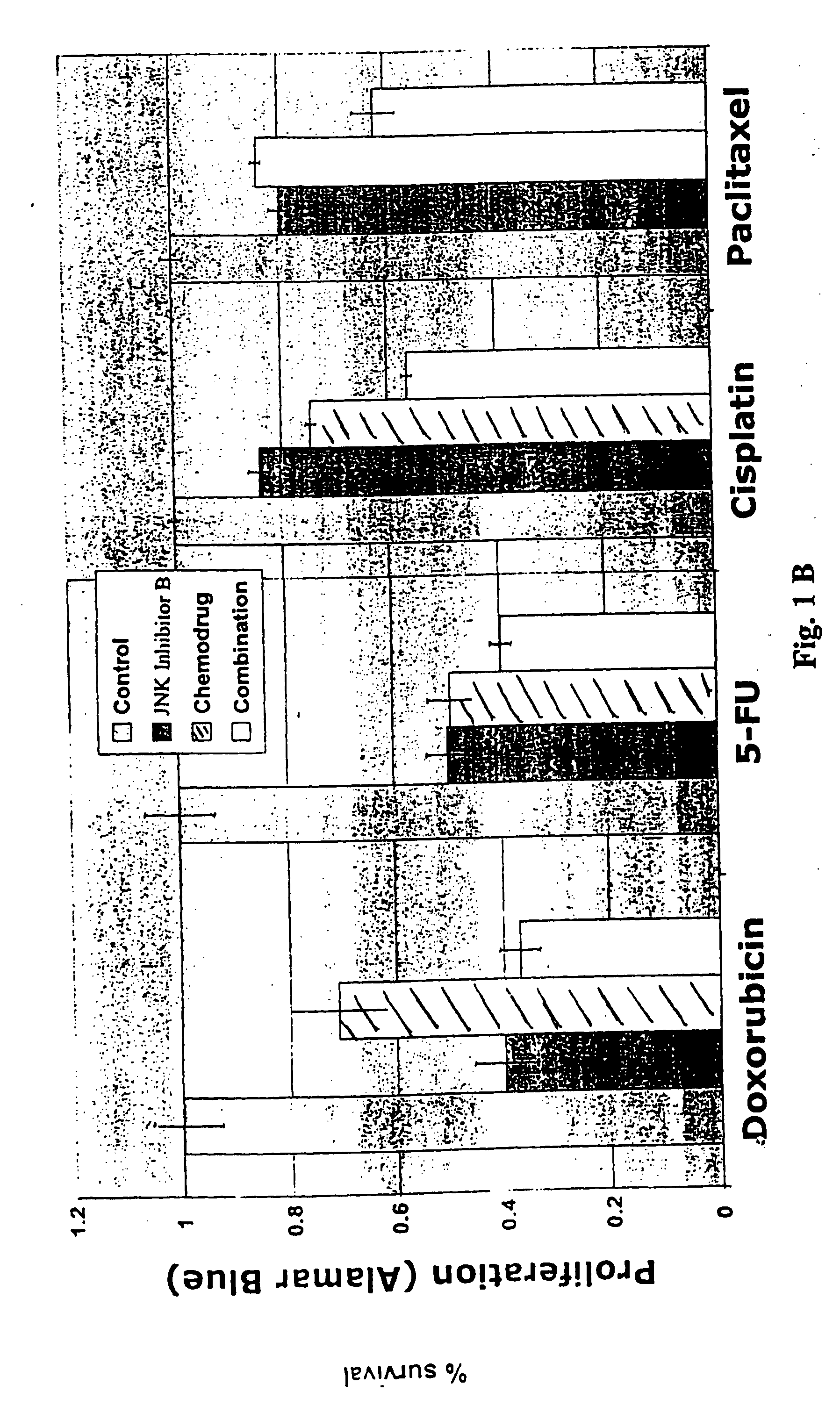
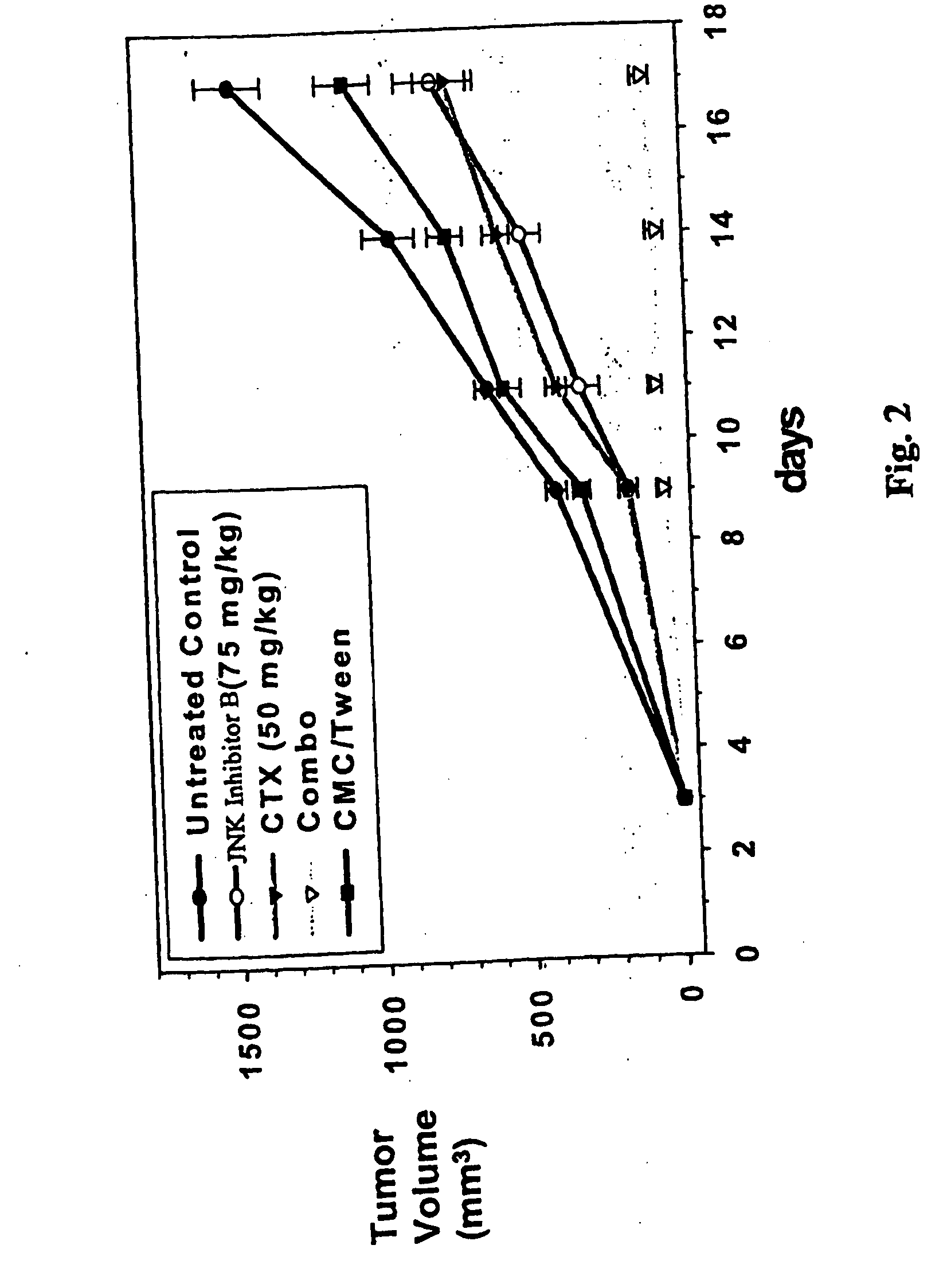
Combination therapy for treating or managing acute myelocytic leukemia
InactiveUS20070149571A1Improve efficiencyImprove toleranceHeavy metal active ingredientsBiocideCancer preventionRadical radiotherapy
Owner:SIGNAL PHARMA LLC
Combined reagent for detecting acute myelocytic leukemia cells and system thereof
ActiveCN109655616AWide coverageThere is no problem of reciprocal inhibition of expressionMaterial analysisCD33CD15
The invention relates to a combined reagent for detecting acute myelocytic leukemia cells and a system thereof, wherein the combined reagent and the system thereof belong to the field of medical technology. The combined reagent comprises at least one selected from the following antibody combinations: a first antibody combination which comprises CD38, CD13, CD34, CD117, CD33, CD19, HLA-DR and CD45antibodies; a second antibody combination which comprises CD38, CD64, CD34, CD123, CD56, CD14, HLA-DR and CD45 antibodies; and a third antibody combination which comprises CD38, CD7, CD34, CD5, CD11b,CD15 and CD45 antibodies. The antibody combinations of the invention cover the expression marks of three systems of granulocyte, single cell and lymphocyte. A normal antibody expression mode is established. Tumor cells can be identified maximally. Furthermore, through a large number of experiment data, the antibodies in each combination have no problem of mutual expression inhibition. FurthermoreAML-MRD can be comprehensively and quickly detected with high sensitivity through multi-parameter flow type cell analysis.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
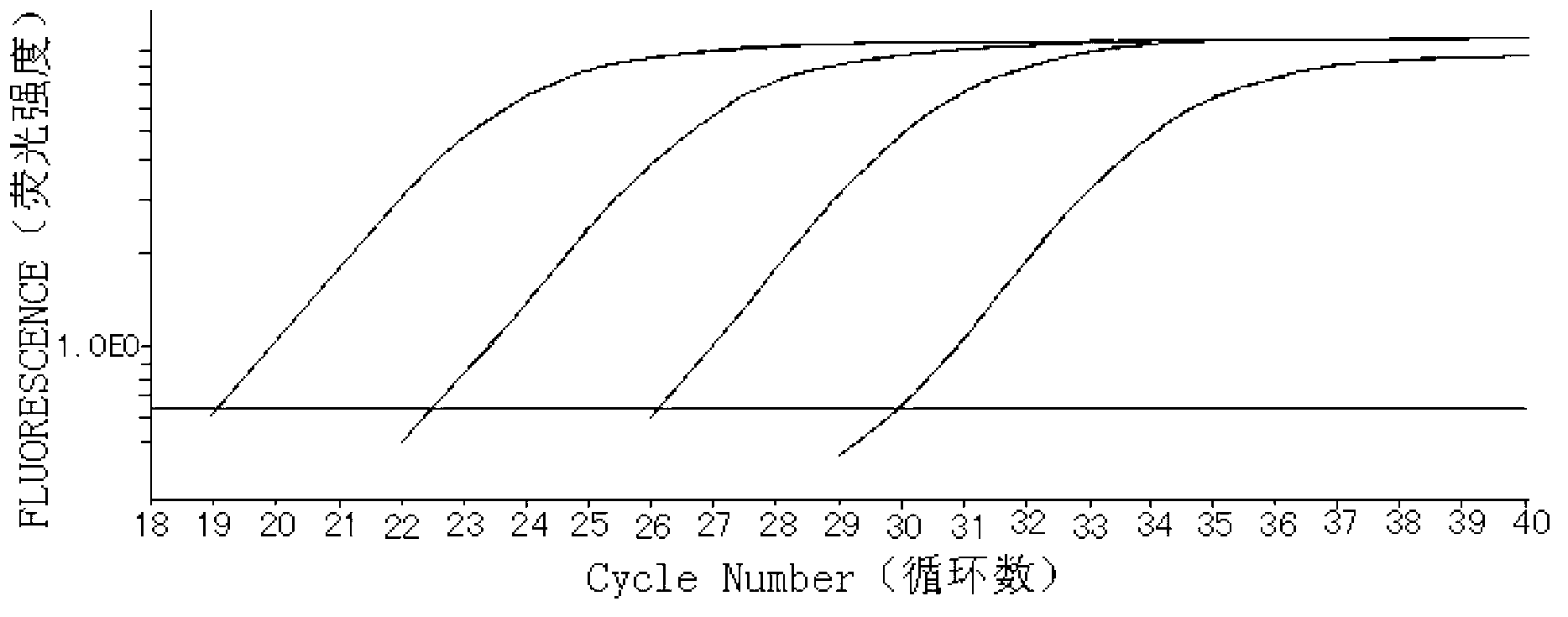
BCR/ABL fusion gene mRNA fluorescence quantitative PCR detecting kit
InactiveCN101168772AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationRNA extractionReverse transcriptase
The invention relates to a BCR / ABL fusion gene (P210bcr / abl) mRNA fluorescence quantitative PCR measurement detection reagent box which comprises lymphocyte segregating liquid, RNA extracting liquid A, RNA extracting liquid B, Oligo (dT) 12-18, RT reaction fluid, reverse transcriptase, quantitative PCR reaction fluid, standard sample, comparison sample, and DEPC water; wherein, a marked primer and a non-marked primer are contained in the quantitative PCR reaction fluid. The reagent box can accurately detect the BCR / ABL fusion gene (P210) mRNA level in the specimen to be detected by extracting the total RNA of medulla ossium or peripheral blood, obtaining cDNA through reverse transcriptase and being combined with a real-time fluorescence quantitative PCR measurement detection technology. The reagent box adopts the latest self-quenched probe technology, thereby having the advantages that the repetitiveness is good, the sensitivity is high, the cost is low, and the invention can be applied to the dynamic monitoring of chronic myelocytic leukemia diagnosis, curative effect observation, prognosis and micro residual leukemia (MRD).
Owner:冯文莉
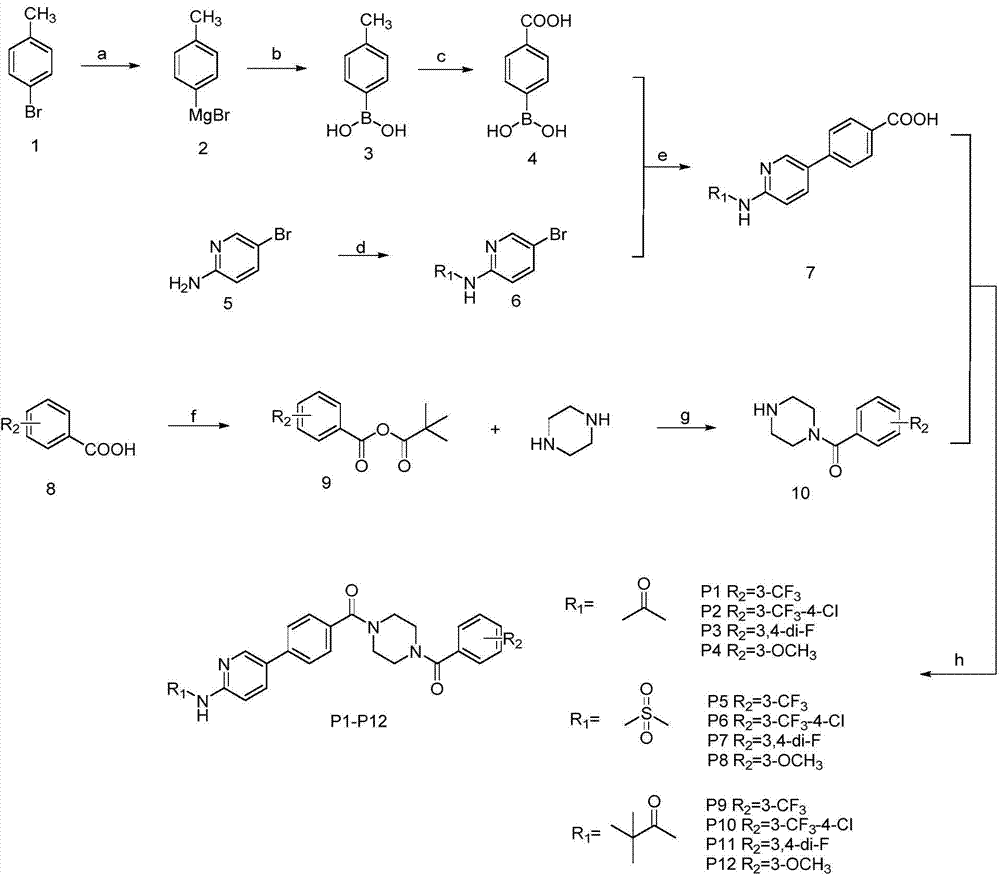
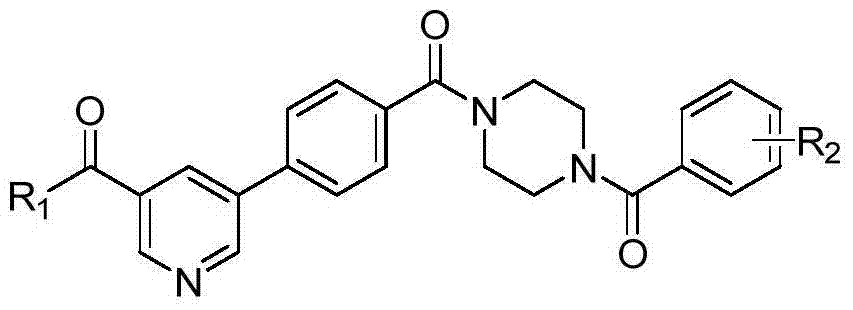
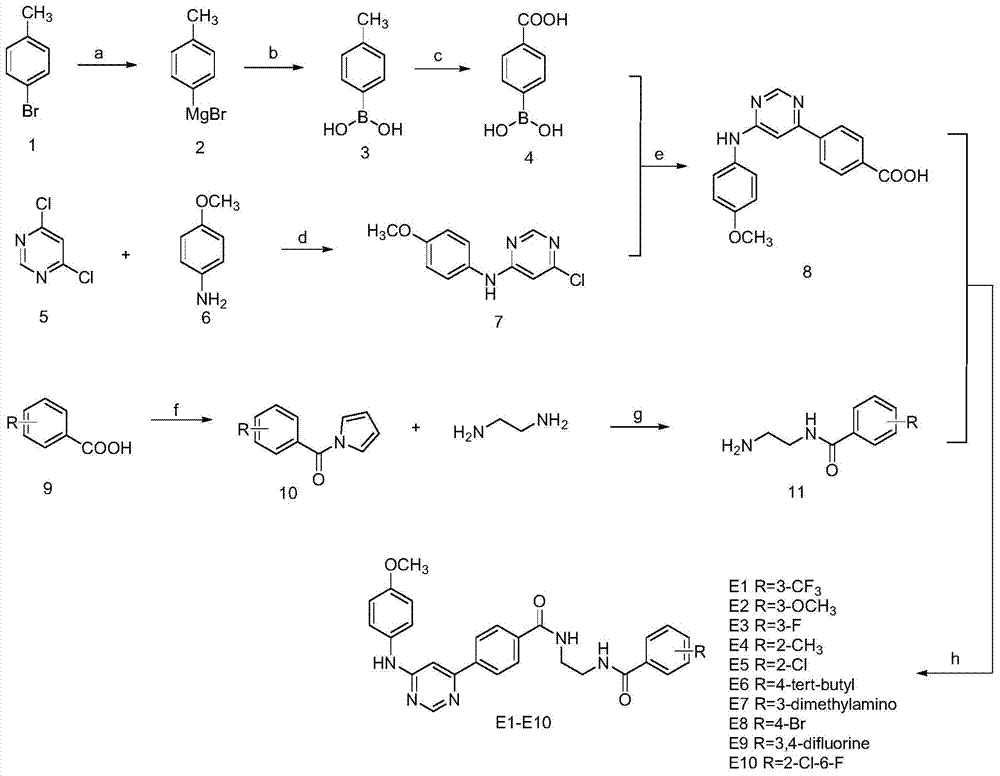
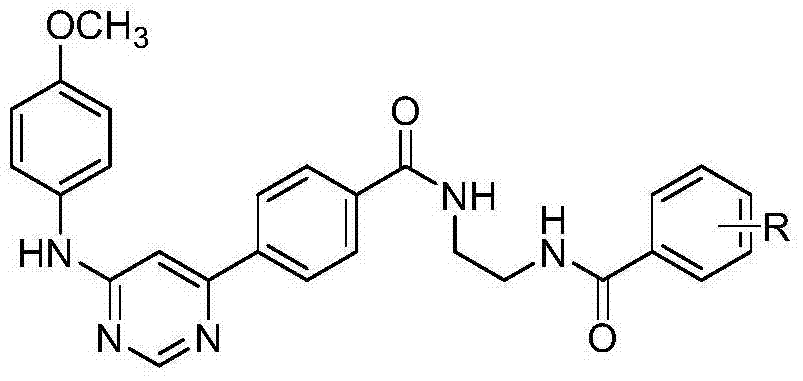
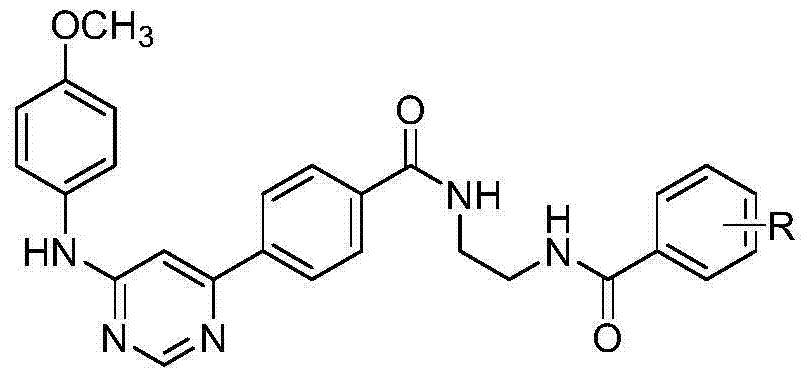
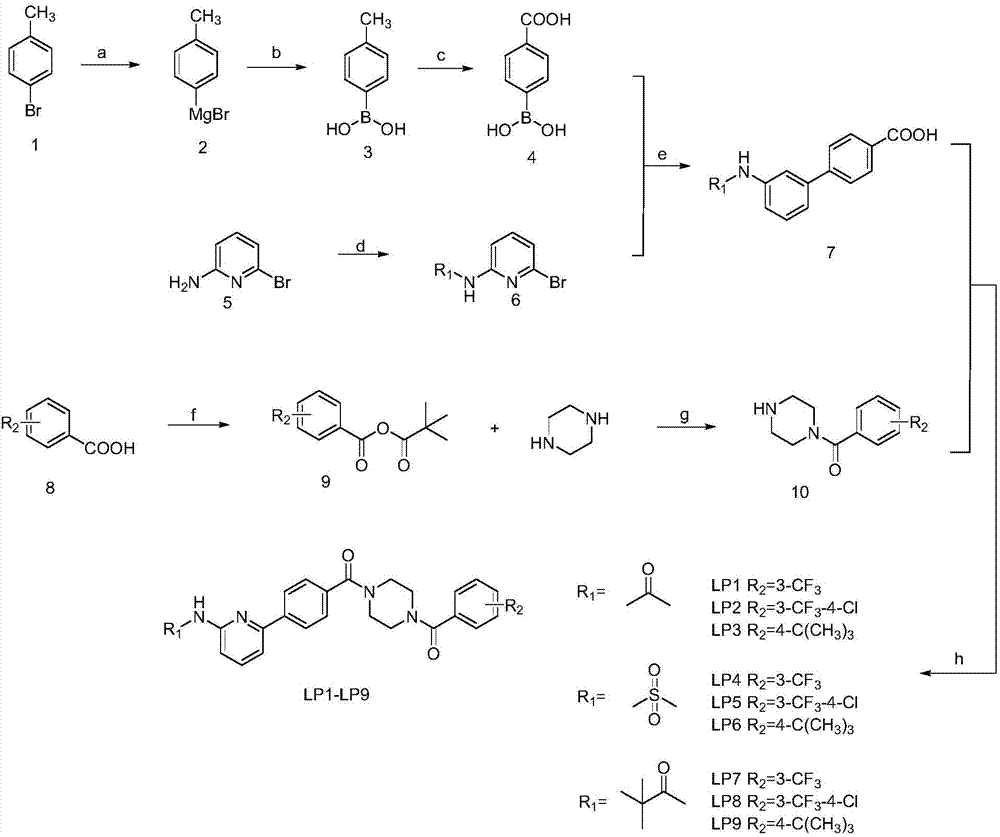
5-phenylpyridine-2-amine bcr-abl inhibitors as well as preparation method and application thereof
InactiveCN104262244AInhibitory activityHigh activityOrganic active ingredientsOrganic chemistryStructural formulaMyelocytic leukemia
Owner:XI AN JIAOTONG UNIV
Rho target protein human mDia and gene encoding same
An objective of the present invention is to provide an activated Rho protein target protein derived from a human and a gene coding for the same. The present invention provides a protein derived from a human and a derivative thereof which has the following characteristics: (1) having activated Rho protein binding activity, (2) having profilin binding activity, (3) the gene coding for the protein being located at q31.2 of chromosome 5, and (4) having a molecular weight of about 150 kDa as measured by SDS-PAGE. Respiratory tract hypersensitivity, bronchial asthma, acute myelocytic leukemia (AML) and myelodysplasia syndrome (MDS) can be diagnosed using the nucleotide sequence coding for this protein.
Owner:SHIRANKAI KYOTO UNIV FACULITY OF MEDICINE ALUMNI ASSOC
Fluorescent reverse transcription-polymerase chain reaction (RT-PCR) kit for quantitatively detecting leukemia fusion gene TEL-AML1
InactiveCN102094074AIncreased sensitivityReduce the risk of contaminationMicrobiological testing/measurementRNA extractionFluorescence
The invention discloses a fluorescent reverse transcription-polymerase chain reaction (RT-PCR) kit for quantitatively detecting a leukemia translocation ETS leukemia acute myelocytic leukemia (TEL-AML)1 fusion gene and belongs to the field of in-vitro nucleic acid diagnosis. The kit comprises a quantitative reference substance, a negative reference substance, a positive reference substance, RT-PCR enzyme, PCREnhancer, diethypyrocarbonate (DEPC) water, fluorescent PCR reaction liquid I, fluorescent PCR reaction liquid II and ribose nucleic acid (RNA) extraction liquid. The kit comprises an RT-PCR system in which a fluorescent PCR technology is taken as basis, and forward and reverse primers and a fluorescent probe aiming at AMLI and TEL-AML1, can detect the RNA of AMLI and TEL-AML1 simultaneously under the same PCR condition, detect whether TEL-AML1 gene fusion occurs in a clinical sample conveniently and quickly, and provides important basis for leukemia diagnosis, typing, clinical treatment and prognosis diagnosis, and a new train of thought for clinical treatment.
Owner:上海裕隆医学检验所股份有限公司
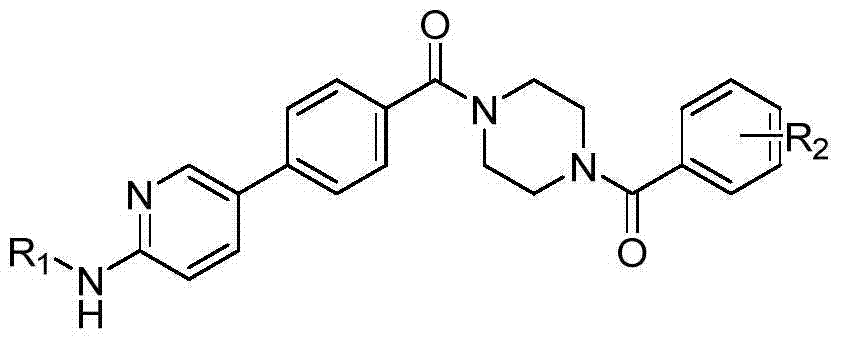
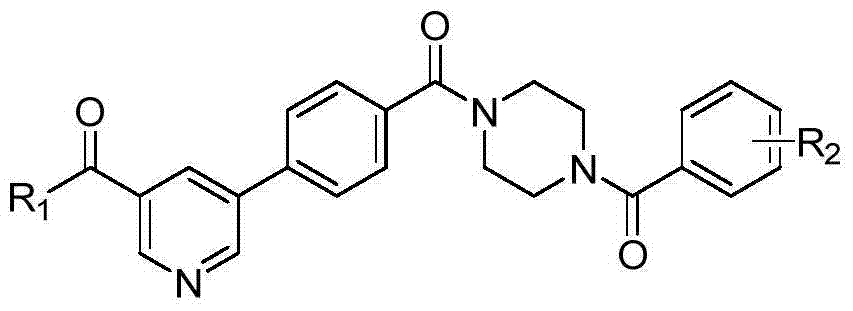
5-phenylnicotinamide bcr-abl inhibitors as well as preparation method and application thereof
ActiveCN104262246AInhibitory activityGrowth inhibitionOrganic active ingredientsOrganic chemistryStructural formulaMyelocytic leukemia
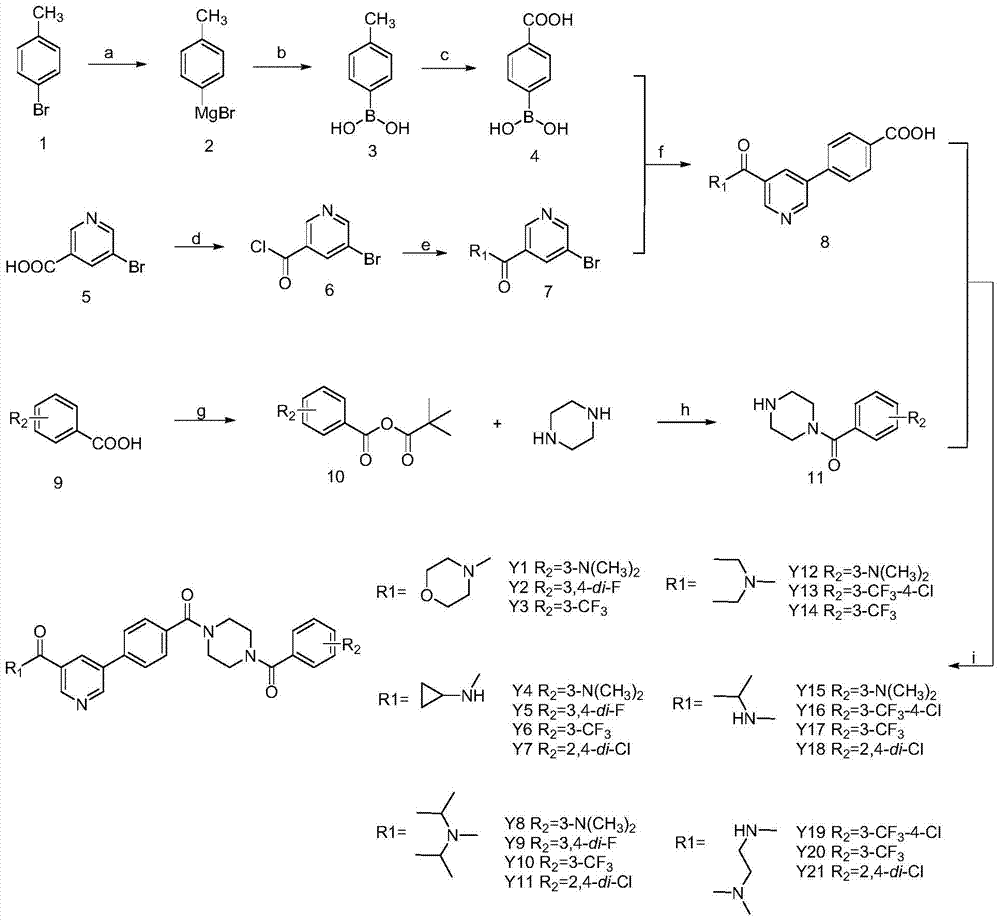
The invention discloses 5-phenylnicotinamide Bcr-Abl inhibitors as well as a preparation method and application thereof. A structural formula of the compounds is shown in the specification, wherein in the structural formula, R1 is morpholinyl, cyclopropylamino, diisopropylamino, diethylin, isopropylamino or an N,N-dimethylethylenediamine group; R2 is a mono-substituent or a di-substituent, and the substituent is tertiary amine or halogen. The series of inhibitors have a certain inhibiting effect on ABL1 kinase in vitro, can inhibit proliferation of a tumor cell K562 and can be used for preparing antitumor drugs, especially CML (chronic myelocytic leukemia) drugs. The preparation method of the 5-phenylnicotinamide Bcr-Abl inhibitors, which is provided by the invention, has the advantages of easiness in obtainment of raw materials, mild reaction conditions, simplicity in operation of reaction processes and cheap used reagents.
Owner:XI AN JIAOTONG UNIV
Real-time fluorescence quantitative PCR method for detecting NPM1 genic mutation
InactiveCN102181564AMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceNPM1 Mutation
The invention provides a method and a kit for detecting nucleophosmin (NPM1) genic mutation by using a primer specificity fluorescence quantitative polymerase chain reaction (PCR), and in particular relates to diagnosis of the NPM1 genic mutation in a tumor tissue and the peripheral blood serum of a tumor patient. In the kit, a specific oligonucleotide primer sequence and a probe sequence are designed for NPM1 gene 12 exon mutation, namely NPM1-mutation A, NPM1-mutation B, NPM1-mutation D and NPM1 wild type gene, fluorescence quantitative PCR detection is performed on each sample to be detected by a wild type PCR system and a mutation type PCR system respectively, and whether NPM1 gene 12 exon mutation, namely the NPM1-mutation A, the NPM1-mutation B or the NPM1-mutation D exists in the sample is judged by Ct value differences between a mutation type PCR and a wild type PCR. By the method and the kit, NPM1 gene mutation can be detected quickly and qualitatively or quantitatively, and reference basis is provided for the determination of diagnosis and treatment schemes and prognosis determination of acute myelocytic leukemia (AML) and myelodysplastic syndrome (MDS).
Owner:INOVOGEN TECH
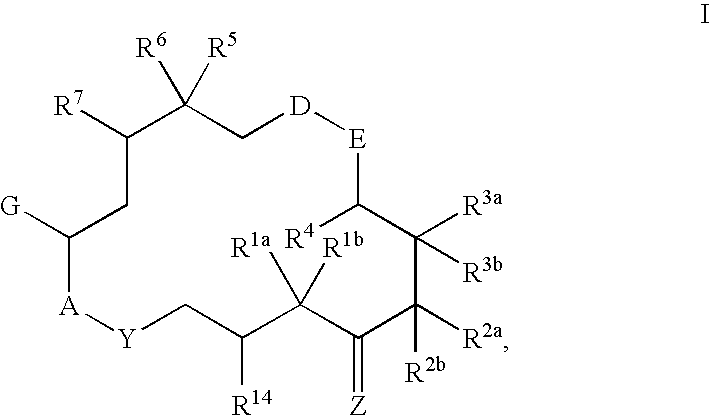
6-alkenyl-, 6-alkinyl- and 6-epoxy-epothilone derivatives, process for their production, and their use in pharmaceutical preparations
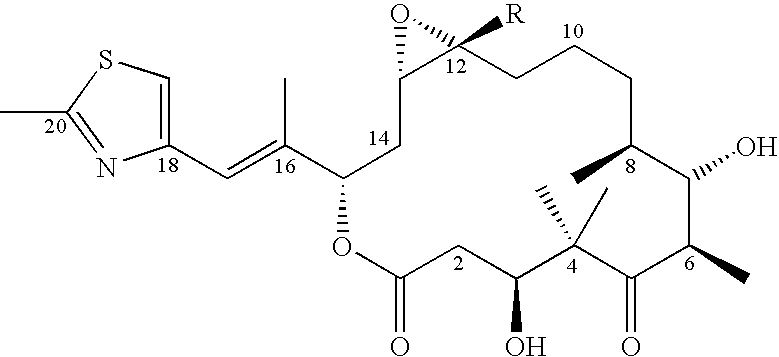
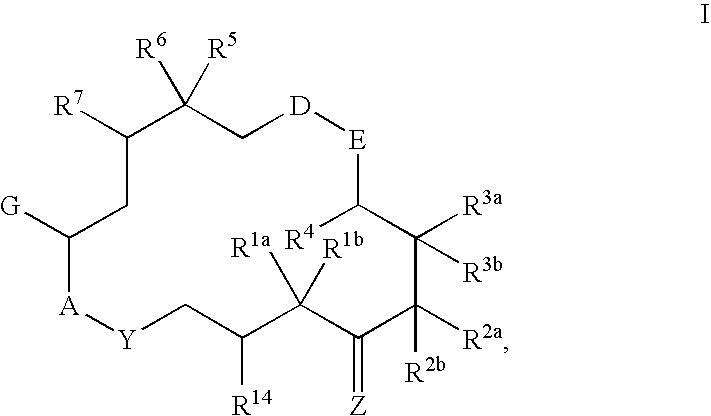
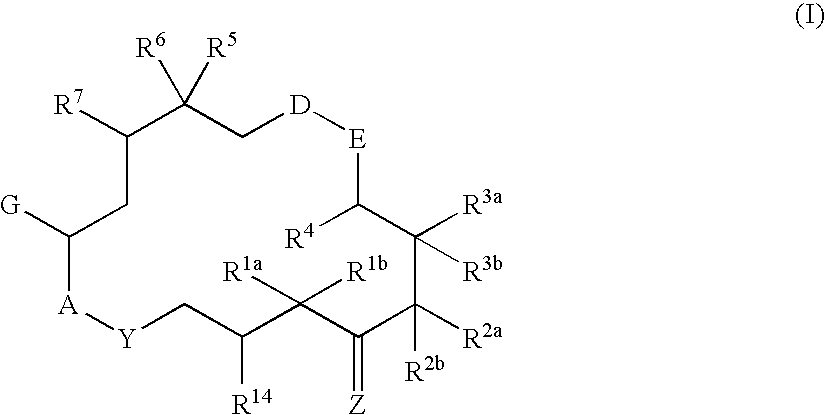
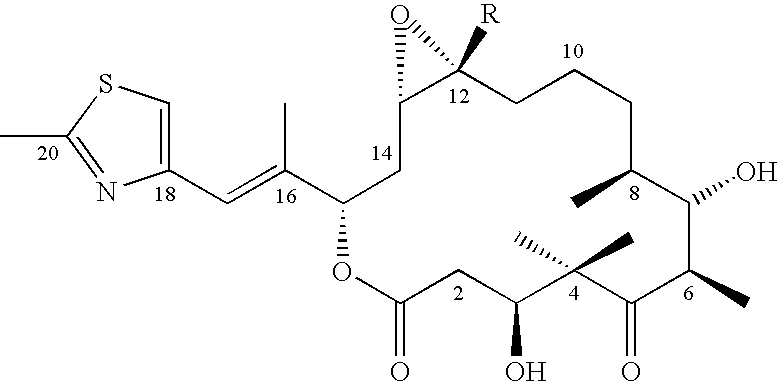
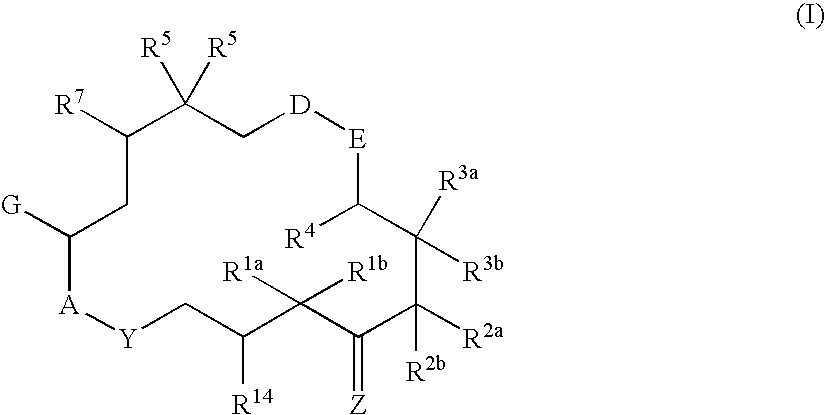
This invention describes the new 6-alkenyl- and 6-alkinyl-epothilone derivatives of general formula Iin which R1a, R1b, R2a, R3a, R3b, R4, R5, R6, R7, A, Y, D, E, G, Y and Z are as defined in the specification. The compounds are useful in treating malignant tumours, for example, ovarian, stomach, colon, breast, adeno-, head and neck carcinomas, acute lymphocytic and myelocytic leukemia. In addition, these compounds are suitable for anti-angiogenesis therapy as well as for treatment of chronic inflammatory diseases such as psoriasis and arthritis. Methods of use and preparation of the compounds are also described.
Owner:BAYER INTPROP GMBH
T cell receptor-like antibodies specific for a wti peptide presented by hla-a2
The present invention provides antigen binding proteins that specifically bind to Wilms' tumor protein (WT1), including humanized, chimeric and fully human antibodies against WT1, antibody fragments, chimeric antigen receptors (CARs), fusion proteins, and conjugates thereof. The antigen binding proteins and antibodies bind to HLA-A0201-restricted WT1 peptide. Such antibodies, fragments, fusion proteins and conjugates thereof are useful for the treatment of WT1 associated cancers, including for example, breast cancer, ovarian cancer, prostate cancer, chronic myelocytic leukemia, multiple myeloma, acute lymphoblastic leukemia (ALL), acute myeloid / myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). In more particular embodiments, the anti-WT1 / A antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT +1
Immunotherapy for chronic myelocytic leukemia
InactiveUS7323168B2Enhance and induce expressionGood curative effectIn-vivo radioactive preparationsHydroxy compound active ingredientsAntigenSide effect
Immunotherapy utilizing naked anti-granulocyte antibodies provides an effective means for treating chronic myelocytic leukemia (CML). Such antibodies can be administered alone or in combination with other therapies, such as immunoconjugates or chemotherapeutics. In either format, an effective therapy for treating CML is provided, which avoids the toxic side-effects typically associated with cancer therapy. The disclosed immunotherapy also is effective for treating acute myelocytic leukemia (AML) when co-administered with inducing agents which induce expression of antigens minimally displayed on the surface of myeloblasts.
Owner:IMMUNOMEDICS INC
BCR-ABL fusion gene amplification kit and BCR-ABL fusion gene detection kit
InactiveCN106399462AEasy to operateEasy to judgeMicrobiological testing/measurementTypingBCR-ABL Fusion Gene
The present invention provides a BCR-ABL fusion gene amplification kit and a BCR-ABL fusion gene detection kit, wherein the amplification kit has advantages of convenient use, rapid operation, and high sensitivity. According to the present invention, the BCR-ABL fusion gene can be subjected to typing and quantitation with the detection kit, such that the pathogenetic condition development and the prognosis of the chronic myelocytic leukemia patient can be monitored according to the BCR-ABL fusion gene detection result.
Owner:SHANGHAI REPODX BIOTECH CO LTD
Nanoparticulate imatinib mesylate formulations
InactiveCN101232870AReduce sizeSmall dosePowder deliveryOrganic active ingredientsStromal tumorDisease
The present invention is directed to a nanoparticulate compositions of imatinib mesylate, or a salt or derivative thereof, having improved pharmacokinetic profiles and reduced fed / fasted variability. The nanoparticulate imatinib mesylate particles of the composition have an effective average particle size of less than about 2000 nm and are useful in the treatment of chronic myeloid leukemia, gastrointestinal stromal tumors and related diseases.
Owner:ELAN PHRMA INT LTD
Kit for detecting expression index of mRNA (messager Ribose Nucleic Acid) of WT1 (Wilms Tumor 1) gene
ActiveCN102912018AStrong specificityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceNewly diagnosedDisease monitoring
The invention relates to a kit for detecting an expression index of mRNA (messager Ribose Nucleic Acid) of a WT1 (Wilms Tumor 1) gene, and belongs to the field of biotechnology. The kit comprises detection primers, a fluorescent probe, a cDNA (complementary Deoxyribose Nucleic Acid) first strand synthesis reagent, a fluorescent quantitative PCR (Polymerase Chain Reaction) mixed solution, negative reference and positive reference, wherein the detection primers and the fluorescent probe comprise a WT1 gene primer, an internal reference gene ABL primer and a Taqman fluorescent probe. The WT1 gene is related with hematopoietic tumor incidence, is of over-expression in about 80% of patients with newly diagnosed acute myelocytic leukemia and acute lymphocytic leukemia, is recognized as a leukemia marker gene, and can serve as an independent minimal residue disease monitoring and prognosis prompting index. The level of the mRNA of the WT1 gene is detected by adopting a fluorescent quantitative PCR technology with higher sensitivity and specificity, and both the specificity and the sensitivity of a detection result are remarkably improved. The kit provides a brand-new quick, simple and convenient gene diagnosis technology for prognosing the acute myelocytic leukemia and the acute lymphocytic leukemia and confirming chemotherapy regimens.
Owner:童永清 +1
Kit for prognostic stratification of AML (Acute Myelocytic Leukemia)
PendingCN106701905AQuick guideFast test resultsMicrobiological testing/measurementCell sensitivityMyelocytic leukemia
The invention provides a kit for the prognostic stratification of AML (Acute Myelocytic Leukemia), which is capable of quickly detecting multigene / multisite mutation. By using the kit, a mutant site can be completely detected; further, the detection sensitivity is high; the entire experimental process from the preparation of a sample to the issue of a report can be completed within one day; the detection is quick and the detection result is accurate; the coverage degree is high; meanwhile, the quick guidance can be effectively provided for the prognostic analysis of the AML.
Owner:ANNOROAD GENE TECH BEIJING +2
Peripheral blood TCR marker of acute myeloid leukemia and detection kit and application of peripheral blood TCR marker
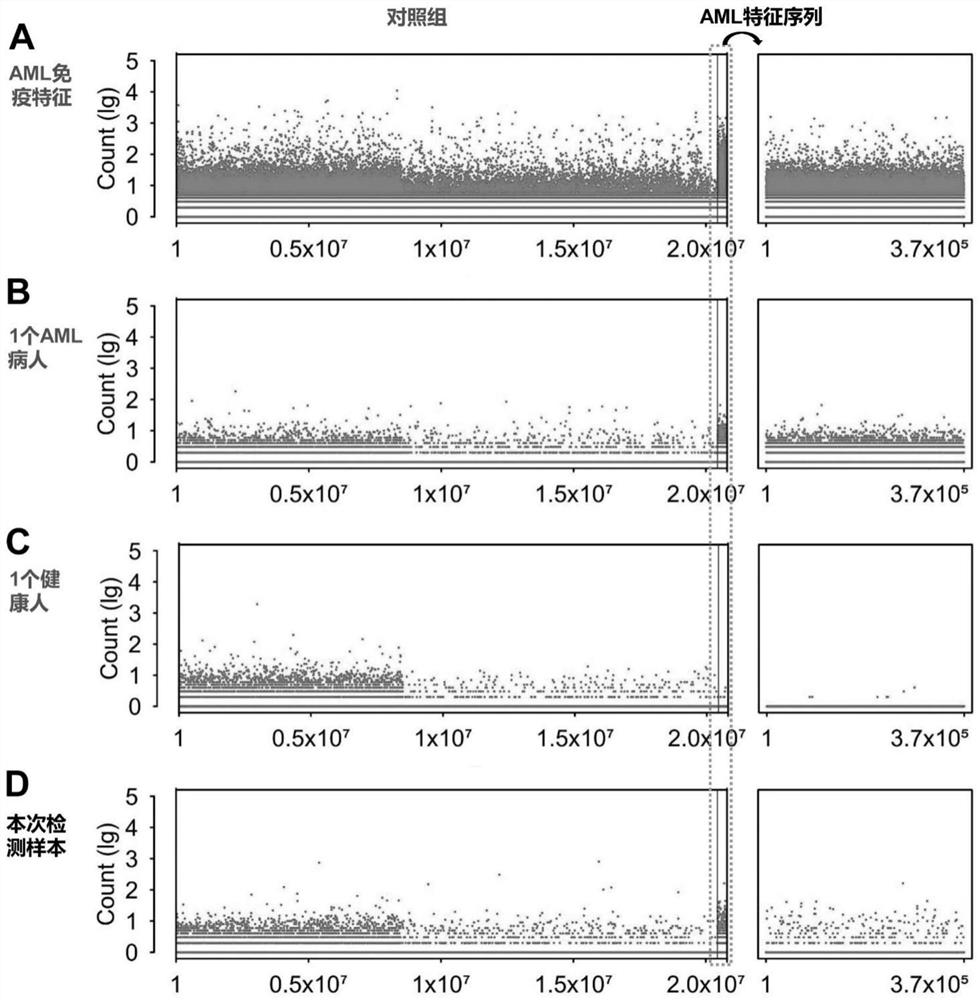
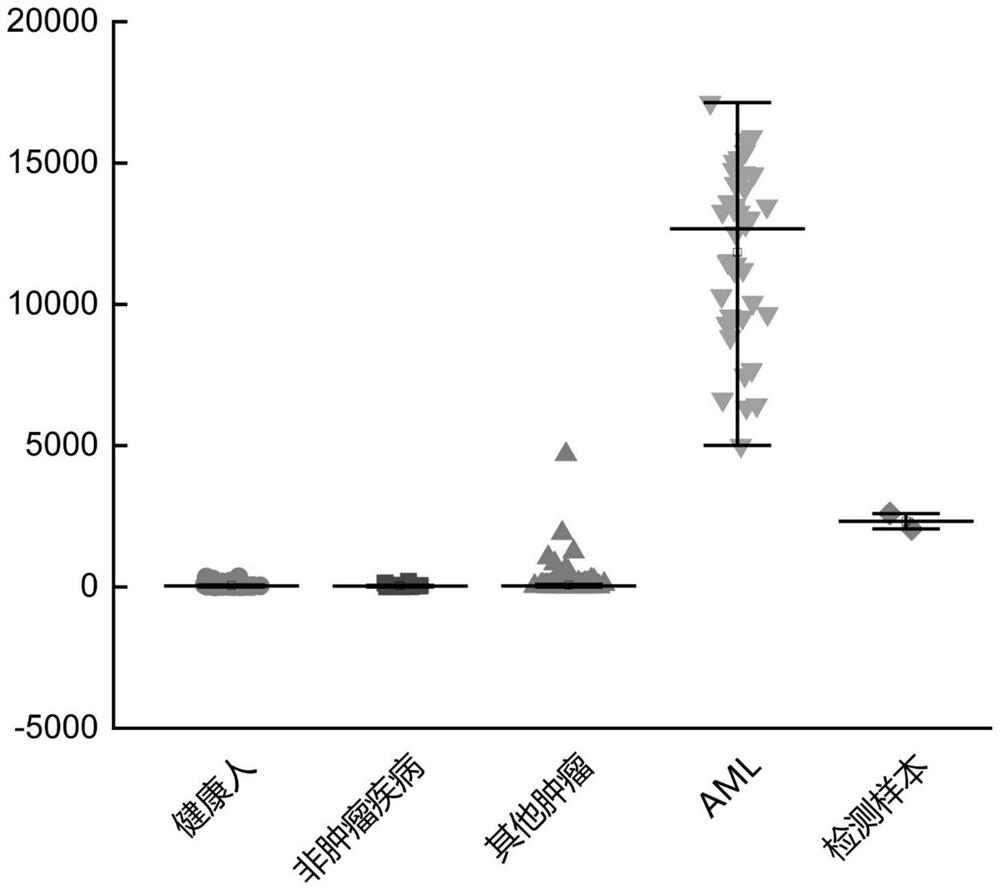
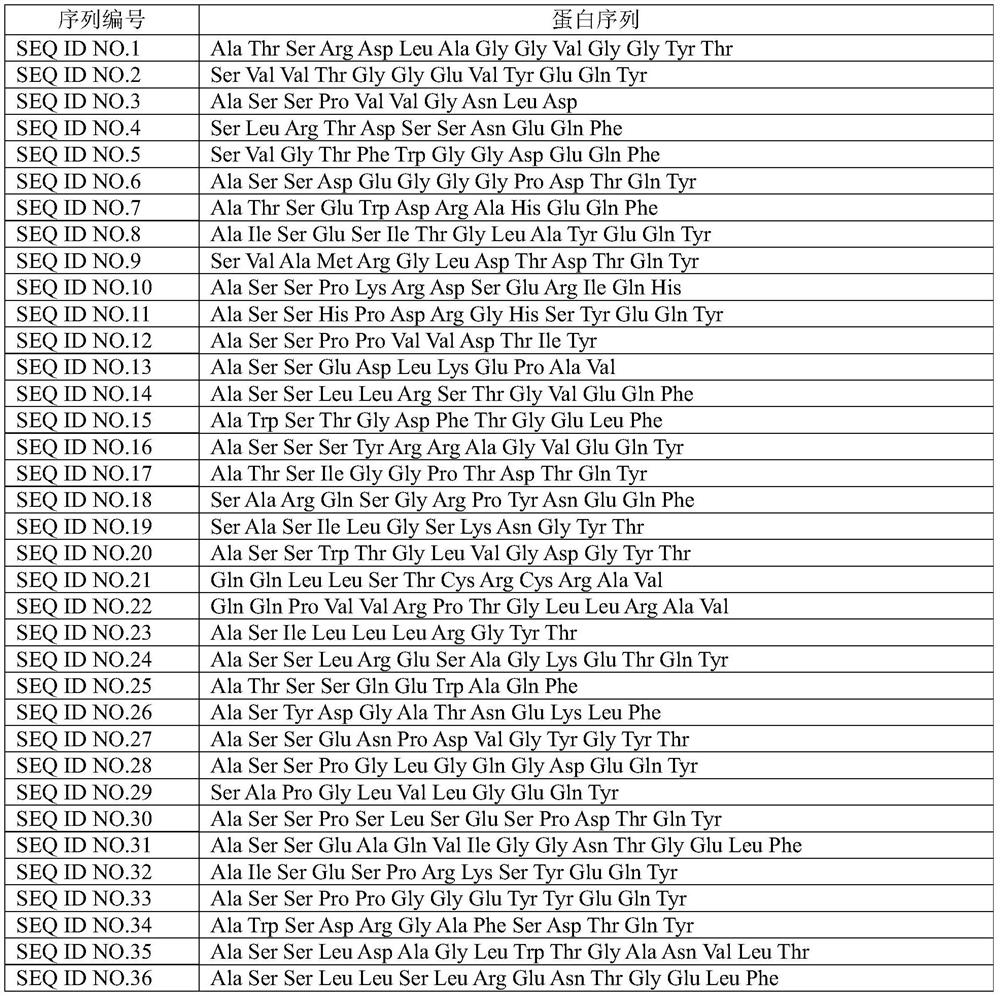
PendingCN113109564AImprove featuresImprove accuracyImmunoglobulin superfamilyBiological material analysisMyeloid leukemiaTest sample
The invention discloses a peripheral blood TCR marker of acute myeloid leukemia and a detection kit and application of the peripheral blood TCR marker. The marker comprises at least one of proteins of which the sequences are as shown in SEQ ID NO.1-100. On the basis of a high-throughput sequencing method, only a small amount of peripheral blood needs to be adopted, RNA is extracted, an immune map library is established by treating a sample, then through high-throughput sequencing and TCR data analysis, firstly, a characteristic TCR sequence in the peripheral blood of acute myeloid leukemia is determined, then a test result of a to-be-tested sample is compared with the characteristic TCR sequence. Therefore, whether the patient suffers from acute myeloid leukemia is determined. According to the kit, a huge number of acute myeloid leukemia specific TCR sequences can be compared at the same time, and compared with single detection of one or more markers, the kit has higher specificity and accuracy, and the diagnosis efficiency is improved.
Owner:CHENGDU EXAB BIOTECH CO LTD
Monoclonal antibodies having property of causing apoptosis and used as anticancer agents
It is the objective and purpose of the present invention to provide a monoclonal antibody having the property of causing apoptosis on myeloid cells.This invention relates to a monoclonal antibody having the property of causing apoptosis on myeloid cells, and fragments thereof, and furthermore relates to a hybridoma producing the monoclonal antibody.Since the monoclonal antibodies of the present invention are useful as antibodies recognizing and identifying antigens causing apoptosis on myeloid cells specifically and besides have the property of causing apoptosis on myeloid cells, they may be used as medicine useful in the field of remedies for myelocytic leukemia utilizing the property.
Owner:CHUGAI PHARMA CO LTD
Application of embellin in preparation of medicament for inhibiting angiogenesis
InactiveCN101278925AEnhanced inhibitory effectOrganic active ingredientsSenses disorderDiseaseEmbryo
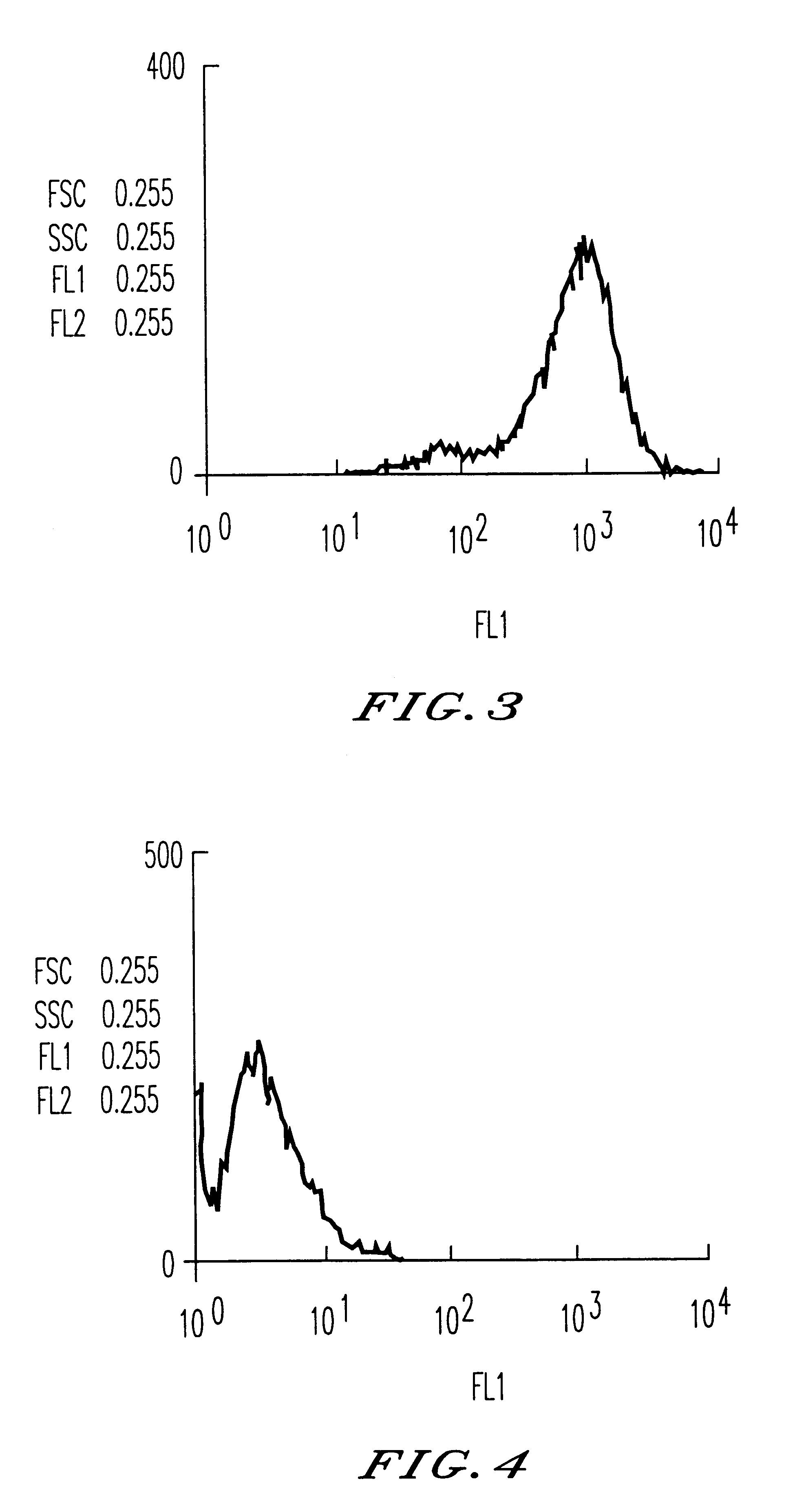
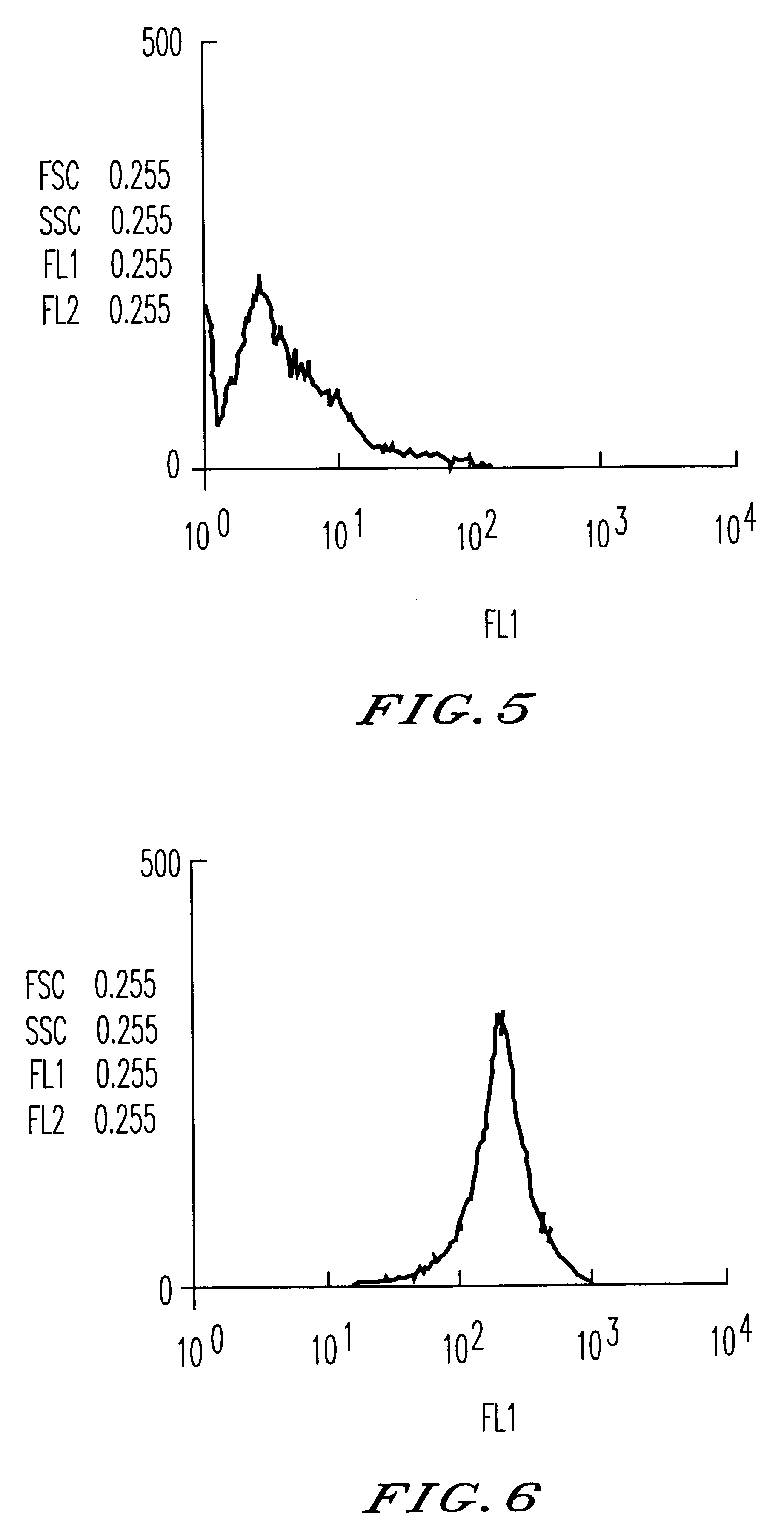
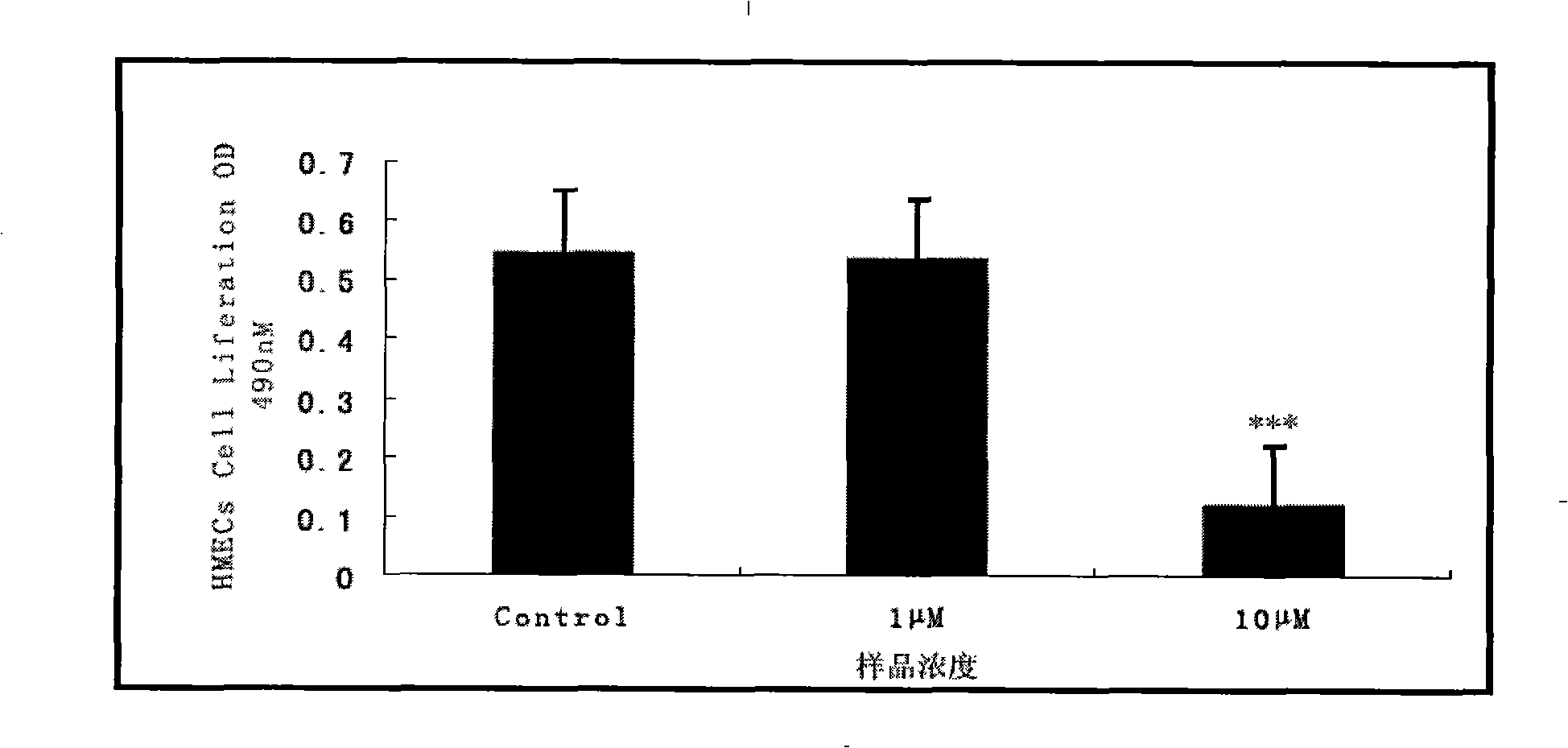
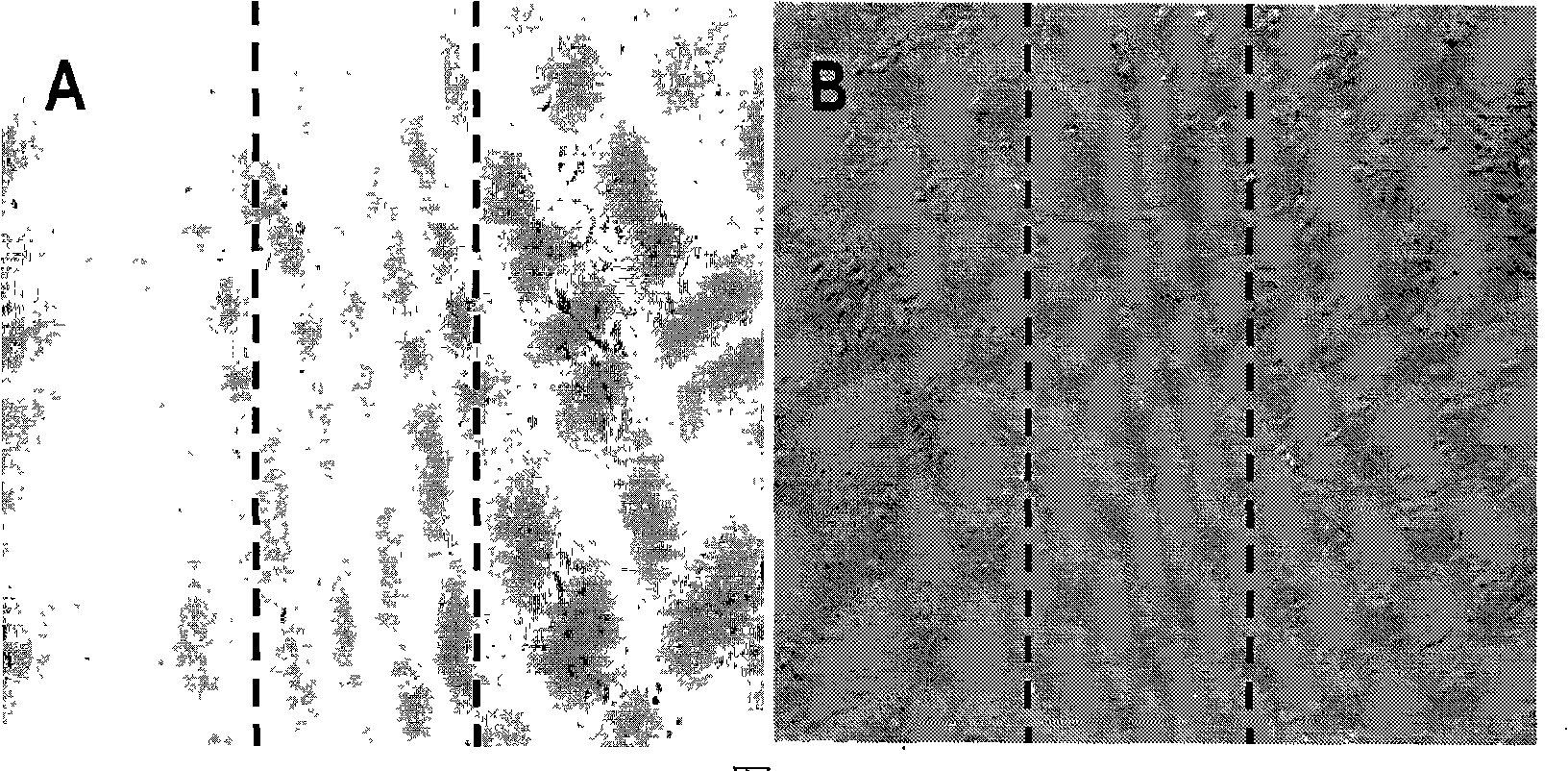
The invention relates to a novel use of Chinese herbal medicine monomer embelin, in particular to an application of the embelin in a preparation of a drug for inhibiting angiogenesis. The embelin is applied in the inhibition of angiogenesis dependent disease such as mammary cancer, colon cancer, lymphoma, acute myelocytic leukemia, rheumatoidarthritis, psoriasis, diabetic syndrome, hemangioma, etc. Experiments show that the embelin has remarkable inhibitory action in proliferation experiment, migration experiment, invasion experiment, cell tabulation experiment, the angiogenesis of chick embryo allantois, etc. in the human microvascular endothelial cells (HMEC-1), thereby being used for preparing the drug for inhibiting the angiogenesis.
Owner:EAST CHINA NORMAL UNIVERSITY
6-Alkenyl-, 6-alkinyl- and 6-epoxy-epothilone derivatives, process for their production, and their use in pharmaceutical preparations
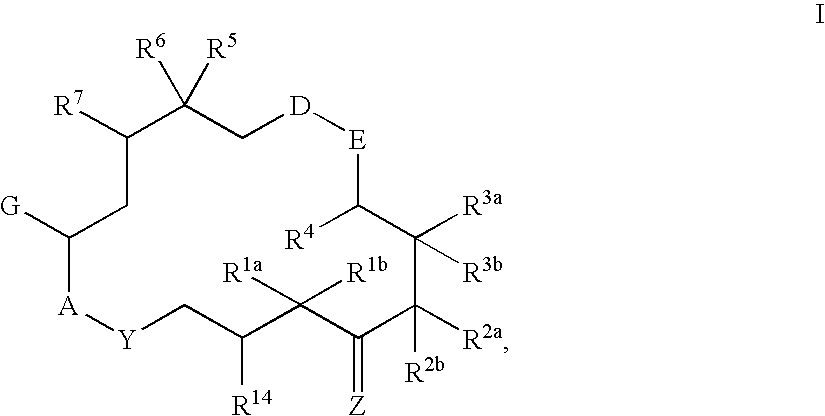
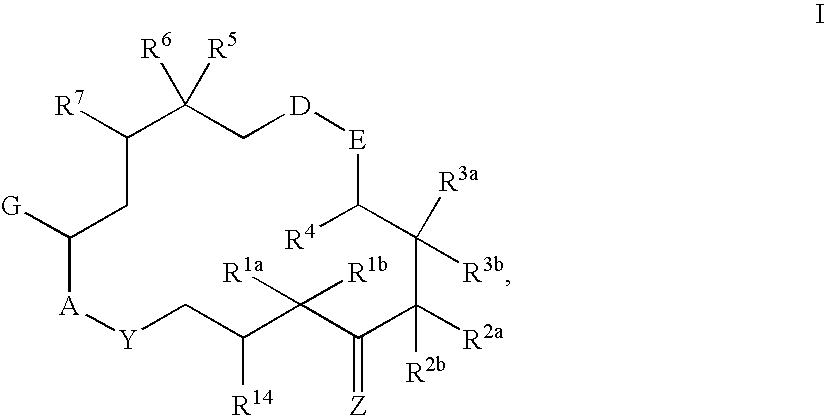
This invention describes the new 6-alkenyl- and 6-alkinyl-epothilone derivatives of general formula (I) in which R1a, R1b, R2b, R3a, R3b, R4, R5, R6, R7, A, Y, D, E, G, Y and Z have the meanings that are indicated in the description. The new compounds interact with tubulin by stabilizing microtubuli that are formed. They are able to influence the cell-splitting in a phase-specific manner and thus find use in treating diseases or conditions associated with the need for cell growth, division and / or proliferation. Thus the compounds are suitable for treating malignant tumors, for example, ovarian, stomach, colon, adeno-, breast, lung, head and neck carcinomas, malignant melanoma, acute lymphocytic and myelocytic leukemia. In addition, they are suitable for anti-angiogenesis therapy as well as for treatment of chronic inflammatory diseases (such as psoriasis, arthritis). Methods of use and preparation of the compounds are also described.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
6-Alkenyl -, 6-alkinyl- and 6-epoxy-epothilone derivatives, process for their production, and their use in pharmaceutical preparations
This invention describes the new 6-alkenyl- and 6-alkinyl-epothilone derivatives of general formula (I) in which R1a, R1b, R2a, R3a, R3b, R4, R5, R6, R7, A, Y, D, E, G, Y and Z have the meanings that are indicated in the description. The new compounds interact with tubulin by stabilizing microtubuli that are formed. They are able to influence the cell-splitting in a phase-specific manner and thus find use in treating diseases or conditions associated with the need for cell growth, division and / or proliferation. Thus the compounds are suitable for treating malignant tumors, for example, ovarian, stomach, colon, adeno-, breast, lung, head and neck carcinomas, malignant melanoma, acute lymphocytic and myelocytic leukemia. In addition, they are suitable for anti-angiogenesis therapy as well as for treatment of chronic inflammatory diseases (such as psoriasis, arthritis). Methods of use and preparation of the compounds are also described.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
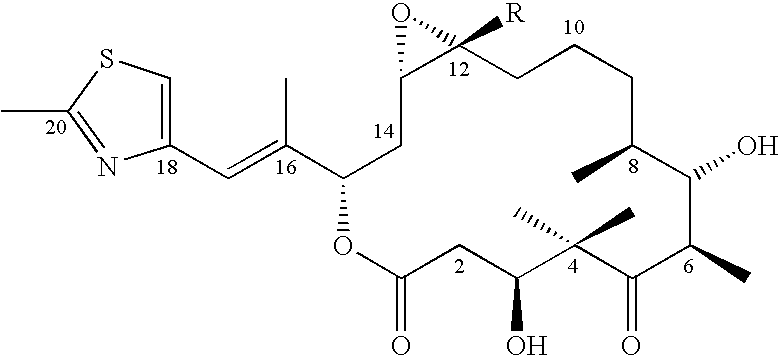
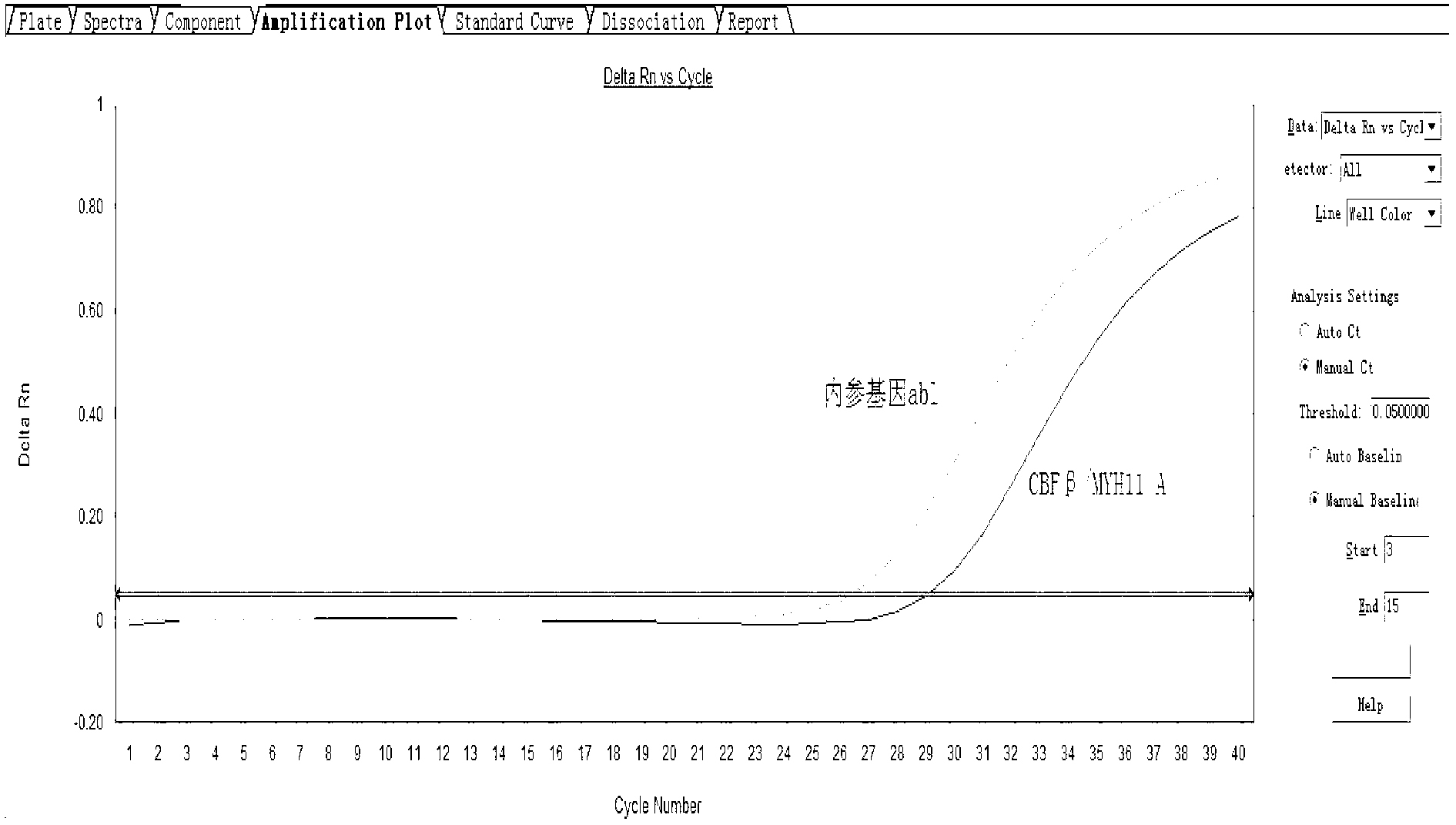
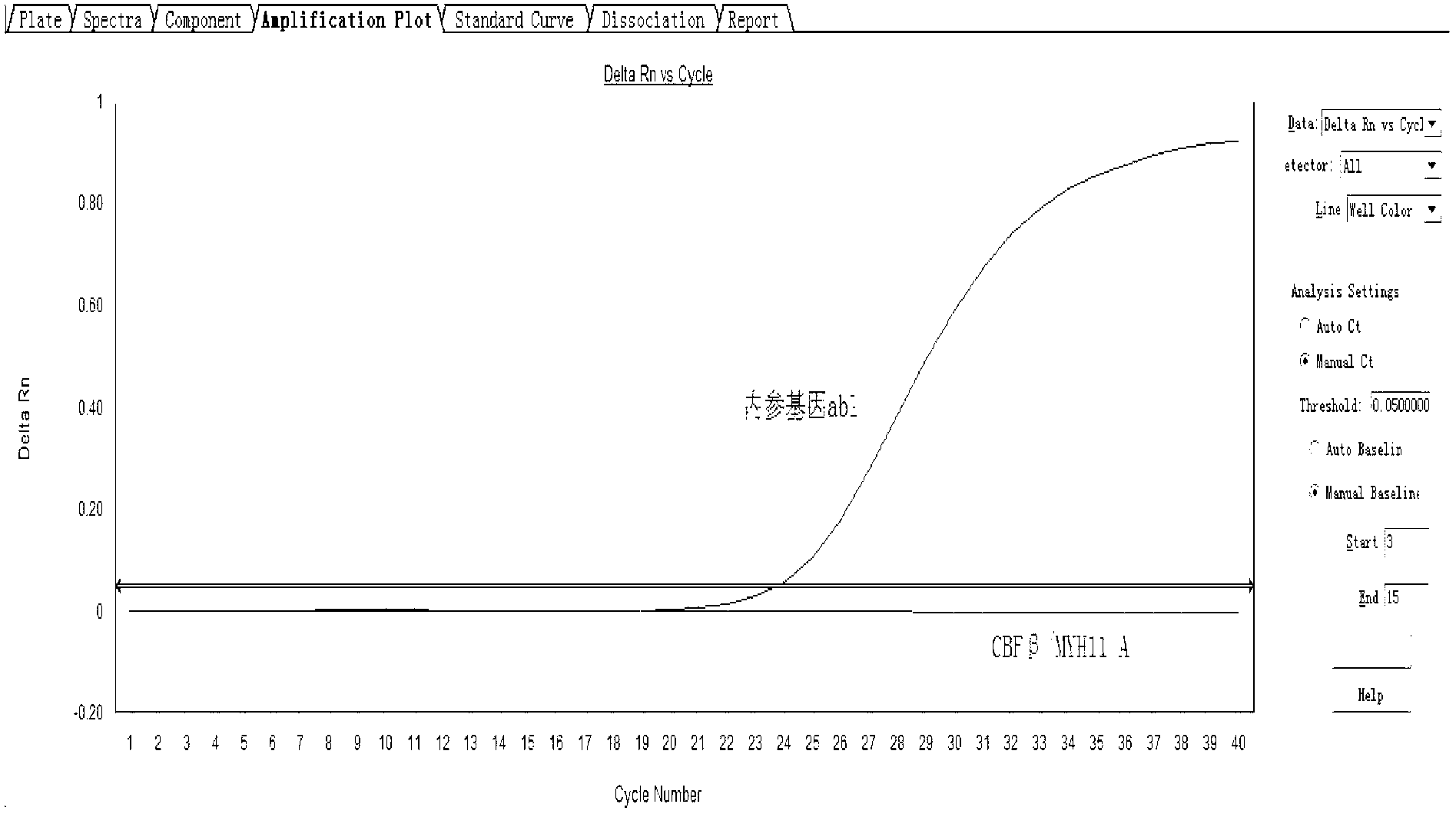
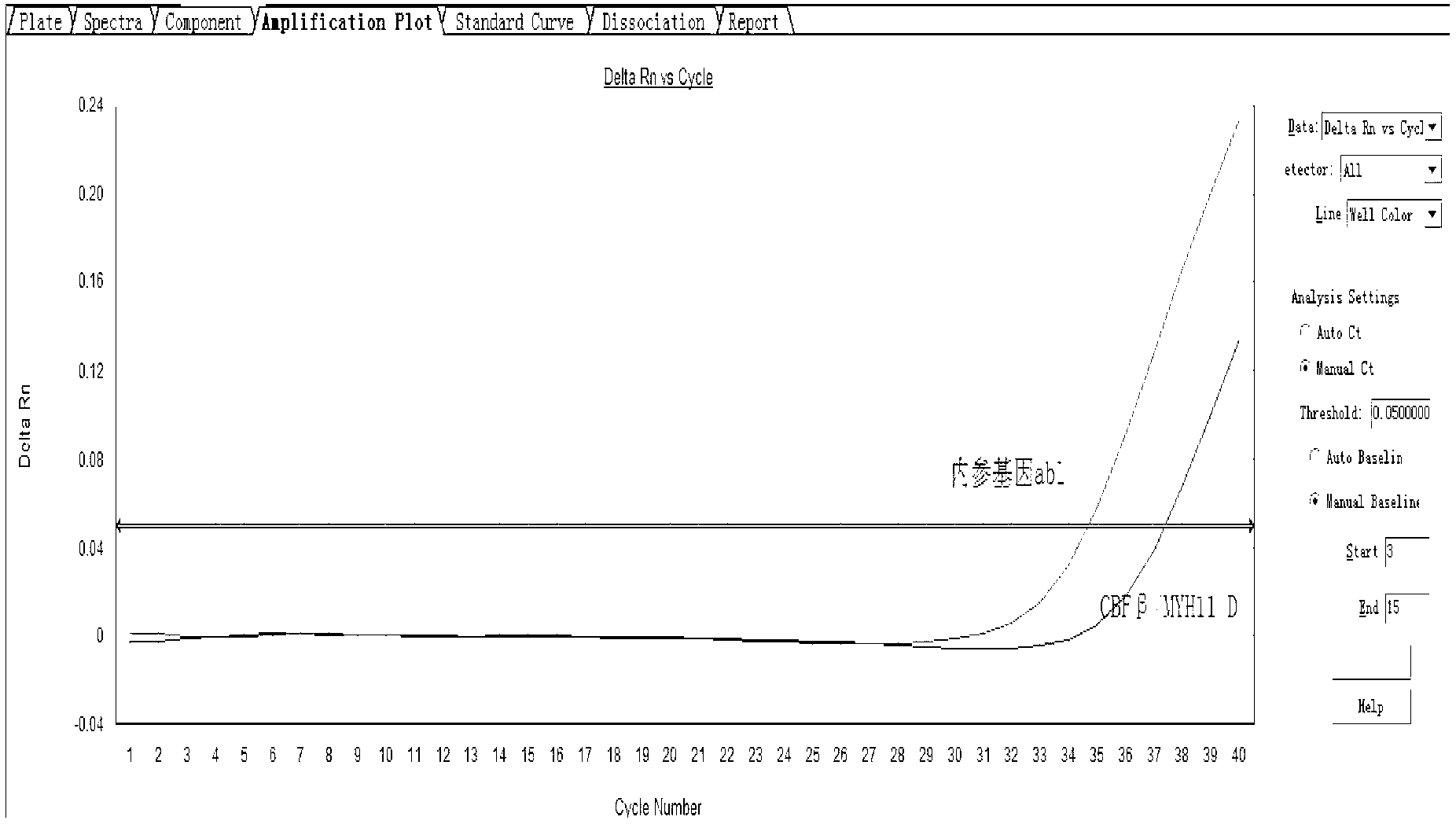
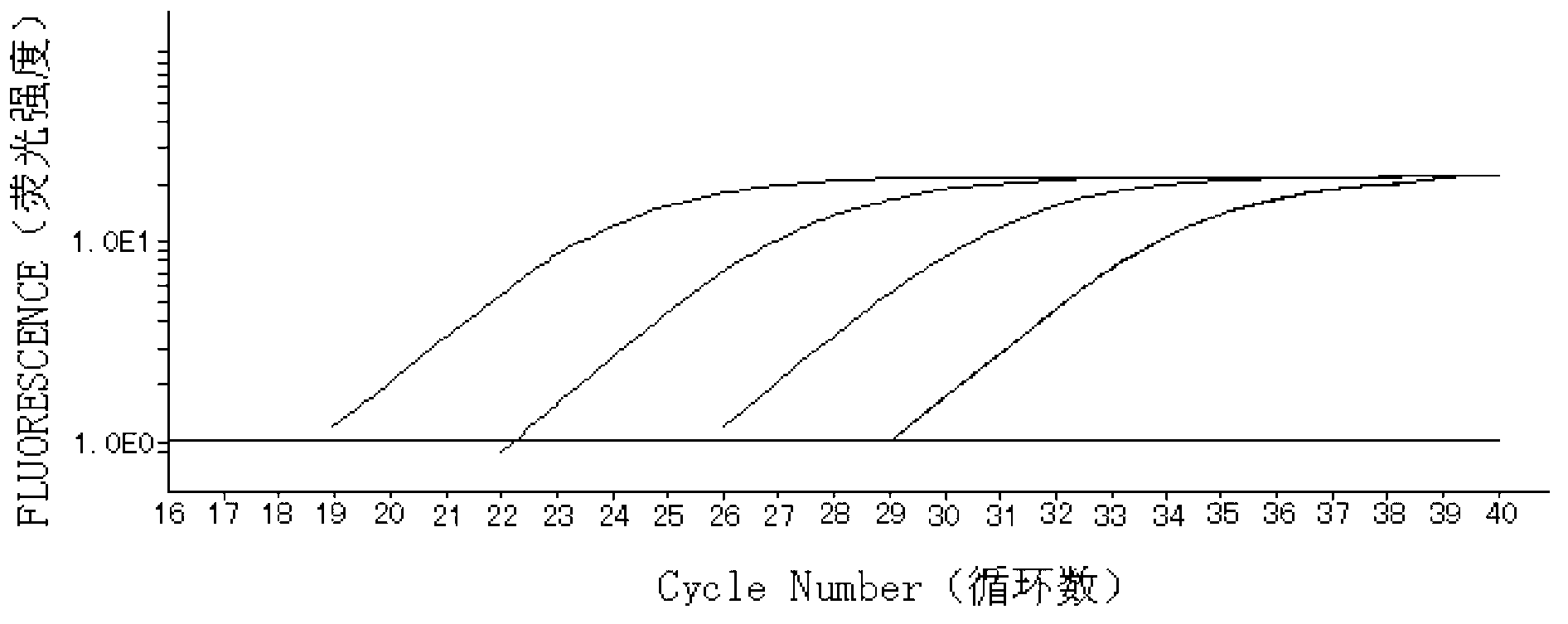
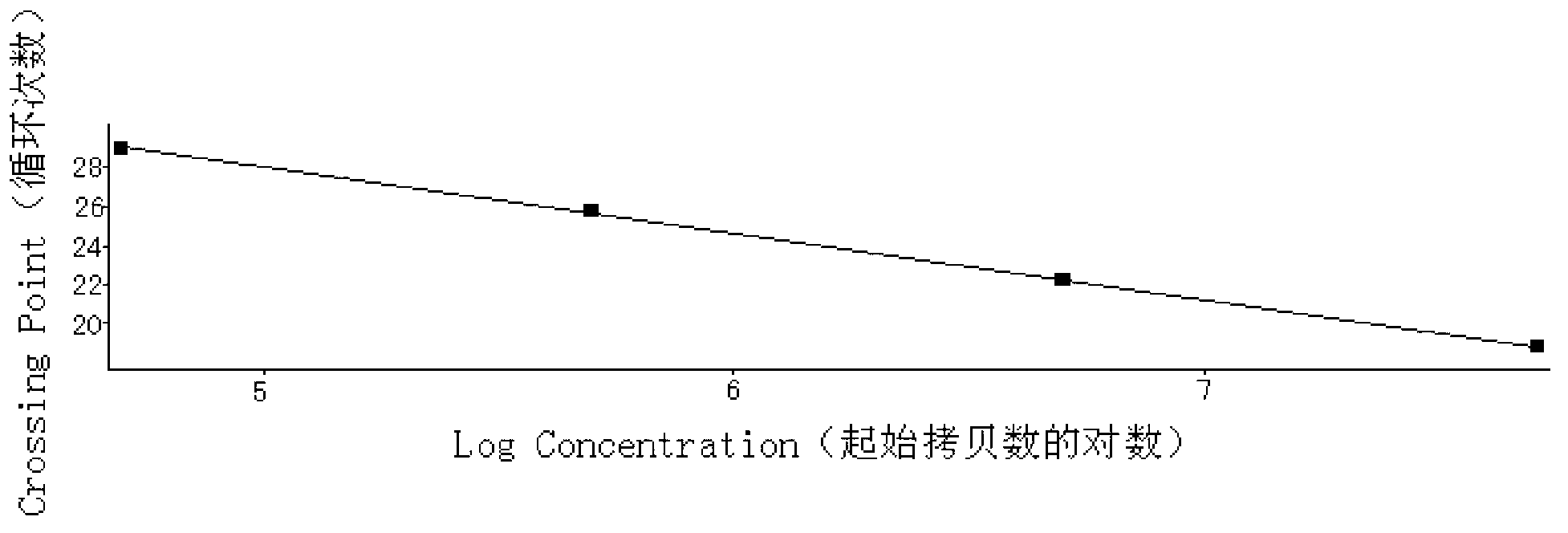
Assay kit for testing relative expression of core-binding factor (CBF) beta/myosin 11 fusion genes
InactiveCN102796818AHigh precisionStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceGene expression levelMyelocytic leukemia
The invention discloses an assay kit for testing the relative expression of core-binding factor (CBF) beta / myosin 11 fusion genes, and the kit comprises red blood cell lysis buffer, TRIzol, chloroform, absolute ethyl alcohol, ReverTraAceqPCRRTKit, testing system polymerase chain reaction (PCR) reaction solution, positive control substance and negative control substance, and is characterized in that the testing system PCR reaction solution comprises THUNDERBIRDqPCRMIX, primers of CBF beta / MYH11-F, CBF beta / MYH11-A-R, CBF beta / MYH11-D-R and CBF beta / MYH11-E-R for amplifying a target gene, probe of CBF beta / MYH11-Prob, primers of abl-F and abl-R for amplifying an internal control gene, and probe of abl-Probe. The assay kit can be used for testing the expression level of the CBF beta / myosin 11 fusion genes of a patient suffering from human acute myelognous leukemia (AML), so the test time can be effectively saved, and the test precision is improved.
Owner:南昌艾迪康医学检验实验室有限公司
Kit capable of detecting expression quantity of BAALC (brain and acute leukemia cytoplasmic) gene mRNA (Messenger Ribose Nucleic Acid)
InactiveCN103014154AIncreased sensitivityStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceReference genesMyeloid leukemia
The invention discloses a kit capable of detecting expression quantity of BAALC (brain and acute leukemia cytoplasmic) gene mRNA (Messenger Ribose Nucleic Acid), belonging to the field of biotechnology. The kit comprises a detection primer and fluorescence probe, a cDNA first strand synthesis reagent, fluorogenic quantitative PCR (Polymerase Chain Reaction) mixed liquor, negative control and positive control, wherein the primers and fluorescence probes for detection include a BAALC gene primer, a reference gene ABL primer and a Taqman fluorescence probe. The BAALC gene is a sign of progenitor cell of early hematopoietic cell, and is abnormally expressed in acute and chronic patients with AML (Acute Myelocytic Leukemia), ALL (Acute Lymphocytic Leukemia) and CML (Chronic Myeloid Leukemia). According to the embodiment of the invention, the mRNA level of the BAALC gene is detected by fluorogenic quantitative PCR with high sensitivity and specificity, and the specificity and sensitivity of the detection result are both obviously improved. The kit can provide a brandnew, fast, simple and convenient gene diagnosis technology for clinically evaluating the diagnosis, prognosis and reappearance period of acute lymphocytic leukemia.
Owner:李艳 +1
N,6 diphenylpyrimidine-4-amine Bcr-Abl inhibitors as well as preparation method and application thereof
ActiveCN104262262AInhibitory activityStrong inhibitory activityOrganic active ingredientsOrganic chemistryStructural formulaMyelocytic leukemia
The invention discloses N,6 diphenylpyrimidine-4-amine Bcr-Abl inhibitors as well as a preparation method and application thereof. A structural formula of the inhibitors is shown in the specification, wherein in the structural formula, R is a mono-substituent or a di-substituent, and the substituent is alkyl, halogen or tertiary amino. The series of inhibitors have a certain inhibiting effect on ABL1 kinase in vitro, can inhibit tumor cell proliferation and can be used for preparing antitumor drugs, especially CML (chronic myelocytic leukemia) drugs. The preparation method of the N,6 diphenylpyrimidine-4-amine Bcr-Abl inhibitors, which is provided by the invention, has the advantages of easiness in obtainment of raw materials, mild reaction conditions, simplicity in operation of reaction processes and cheap used reagents.
Owner:XIAN HONGHUI HOSPITAL
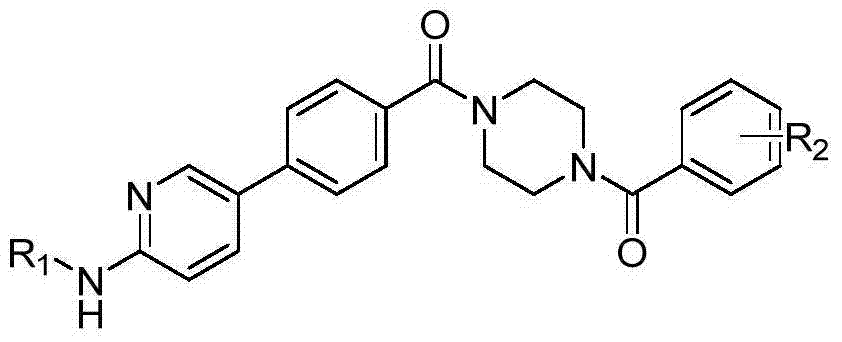
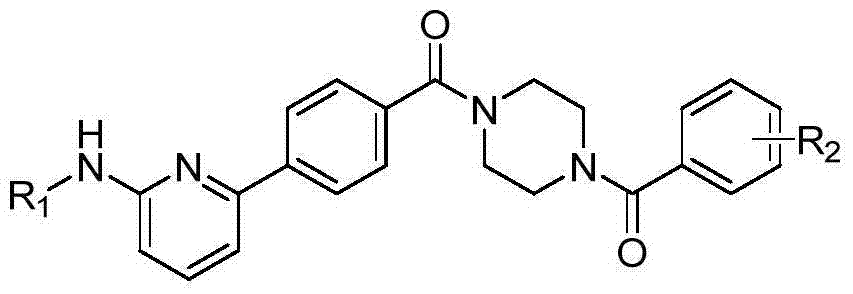
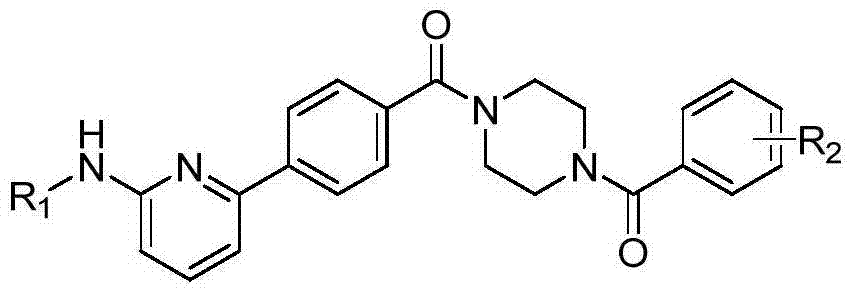
6-phenylpyridine-2-amine Bcr-Abl inhibitors as well as preparation method and application thereof
InactiveCN104262245AInhibitory activityHigh activityOrganic active ingredientsOrganic chemistryStructural formulaMyelocytic leukemia
The invention discloses 6-phenylpyridine-2-amine Bcr-Abl inhibitors as well as a preparation method and application thereof. A structural formula of the inhibitors is shown in the specification, wherein in the structural formula, R1 is acetyl, methylsulfonyl or pivaloyl; R2 is a mono-substituent or a di-substituent, and the substituent is alkyl or halogen. The series of inhibitors have a certain inhibiting effect on ABL1 kinase in vitro, can inhibit proliferation of a tumor cell K562 and can be used for preparing antitumor drugs, especially CML (chronic myelocytic leukemia) drugs. The preparation method of the 6-phenylpyridine-2-amine Bcr-Abl inhibitors, which is provided by the invention, has the advantages of easiness in obtainment of raw materials, mild reaction conditions, simplicity in operation of reaction processes and cheap used reagents.
Owner:XI AN JIAOTONG UNIV
Kit for diagnosing acute myelocytic leukemia
InactiveCN102643899AConvenient, Accurate and Sensitive DiagnosisReduce false positive rateMicrobiological testing/measurementFluorescenceReverse transcriptase
The invention discloses a kit for diagnosing acute myelocytic leukemia. The kit comprises a reverse transcription system and a PCR (Polymerase Chain Reaction) amplification system, and is characterized in that: the reverse transcription system comprises repetition T oligonucleotide Ologod T, reverse transcription reaction solution, an M-MLV reverse transcriptase, an RNA (Ribonucleic Acid) enzyme inhibitor and dNTPs (Deoxynucleotide Triphosphate), wherein the PCR amplification system comprises an SYBRGreen PCR system and a primer pair; the reverse transcription reaction solution comprises diethyl pyrocarbonate treated water and M-MLV reverse transcriptase buffer solution; the SYBRGreen PCR system comprises PCR buffer solution, template cDNA (complementary Deoxyribonucleic Acid), dNTPs and an SYBRGreen fluorescent dye; and the primer pair is one capable of amplifying human Lc3 synthetase beta3Gn-T5 and human beta-actin. According to the invention, a method for detecting expression of the Lc3 synthetase beta3Gn-T5 by using the SYBRGreen technology is constructed, and the kit capable of conveniently, accurately and sensitively diagnosing the acute myelocytic leukemia is provided.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com