5-phenylnicotinamide bcr-abl inhibitors as well as preparation method and application thereof
A technology of phenylnicotinamide and bcr-abl, which is applied in the field of biomedicine, can solve the problems of mutation resistance and drug resistance, and achieve the effect of cheap reagents, mild reaction conditions and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
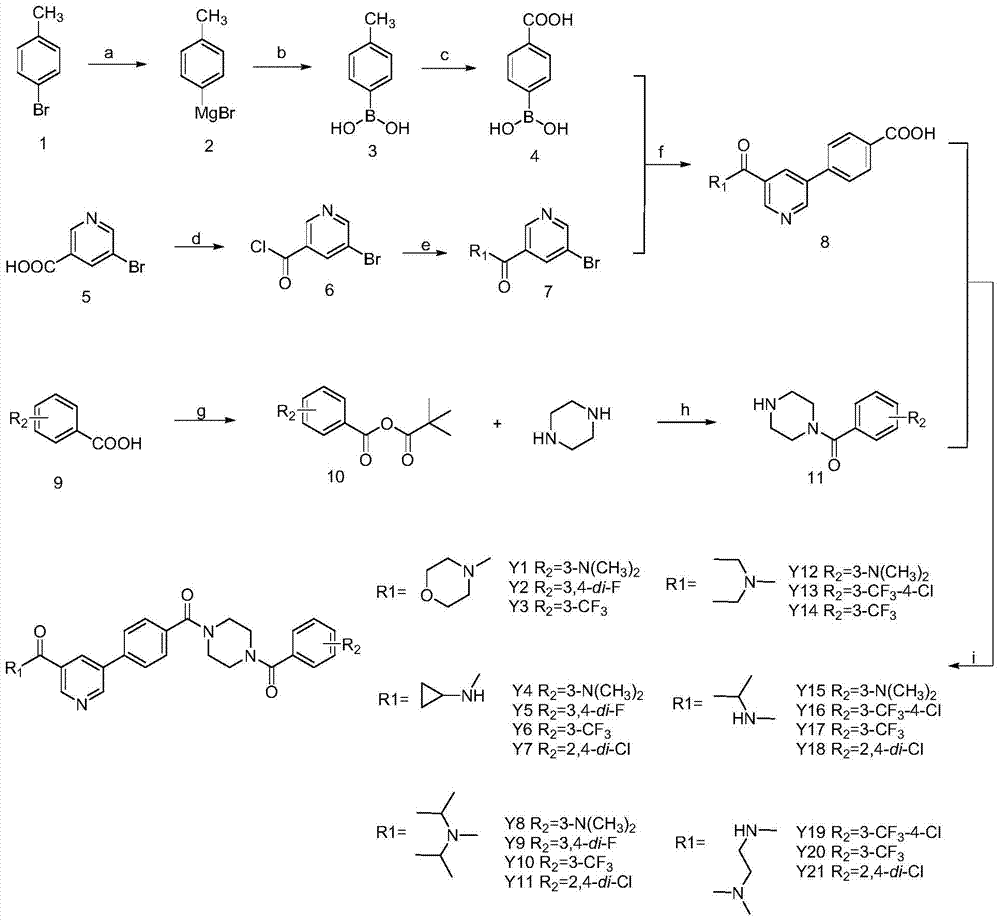
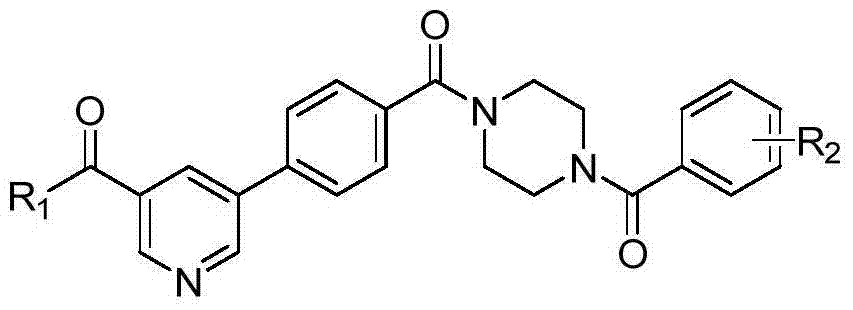
[0042] In the structural formula of 5-phenylnicotinamide Bcr-Abl inhibitors, R 1 is morpholinyl, R 2 For m-trifluoromethyl, prepared by the following steps:
[0043] 1) Preparation of p-carboxyphenylboronic acid (compound 4)
[0044] Add treated Mg strips (2.15g, 90mmol), 2 capsules of iodine into a 250ml double-necked bottle, protect with nitrogen, and vacuumize 3 times. Slowly add the anhydrous THF solution of the compound p-methylbromobenzene (compound 1, 60mmol) with a syringe under heating conditions. After the reaction is initiated, it is in a reflux state. Continue to add the remaining solution. After the addition, reflux for 5 hours to obtain the format of p-bromotoluene Reagent (compound 2), after cooling to room temperature, the reaction device was transferred to a low-temperature reactor, and the temperature was adjusted to -20 ° C. After 5 minutes, anhydrous THF solution of trimethyl borate (9.36 g, 90 mmol) was added with a syringe, After the addition, react at...
Embodiment 2
[0062] In the structural formula of the inhibitor, R 1 is cyclopropylamino, R 2 is 3,4-difluoro, prepared by the following steps:
[0063] Step 1) is the same as in Example 1, that is, p-carboxyphenylboronic acid (compound 4) is prepared from p-bromotoluene (compound 1).
[0064] 2) Preparation of 5-bromo-N-cyclopropylnicotinamide (compound 7)
[0065] Under the protection of nitrogen, 20ml of thionyl chloride was added dropwise to 5-bromonicotinic acid (compound 5, 5.0g, 24.7mmol). After the dropwise addition, reflux to clarification and react for 2 hours. After the reaction was completed, the pressure was reduced. Thionyl chloride was spin-off to obtain 5-bromonicotinoyl chloride (compound 6);
[0066] 5-Bromonicotinoyl chloride (compound 6) was dissolved in anhydrous dichloromethane (30ml), and the acid chloride solution was added dropwise to cyclopropylamine (3.77ml, 54.4mmol) in anhydrous dichloromethane ( 50ml) solution, after the dropwise addition, remove the ice ba...
Embodiment 3
[0080] In the structural formula of the inhibitor, R 1 is diisopropylamino, R 2 For m-dimethylamino, prepared by the following steps:
[0081] Step 1) is the same as step 1) in Example 1, that is, p-carboxyphenylboronic acid (compound 4) is prepared from p-bromotoluene (compound 1).
[0082] 2) Preparation of 5-bromo-N,N-diisopropylnicotinamide (compound 7)
[0083] Under the protection of nitrogen, 20ml of thionyl chloride was added dropwise to 5-bromonicotinic acid (compound 5, 5.0g, 24.7mmol). After the dropwise addition, reflux to clarification and react for 2 hours. After the reaction was completed, the pressure was reduced. Thionyl chloride was spin-off to obtain 5-bromonicotinoyl chloride (compound 6);
[0084] 5-Bromonicotinoyl chloride (compound 6) was dissolved in anhydrous dichloromethane (30ml), and the acid chloride solution was added dropwise to diisopropylamine (7.66ml, 54.4mmol) in anhydrous dichloromethane (30ml) in an ice bath. Chloromethane (50ml) soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com