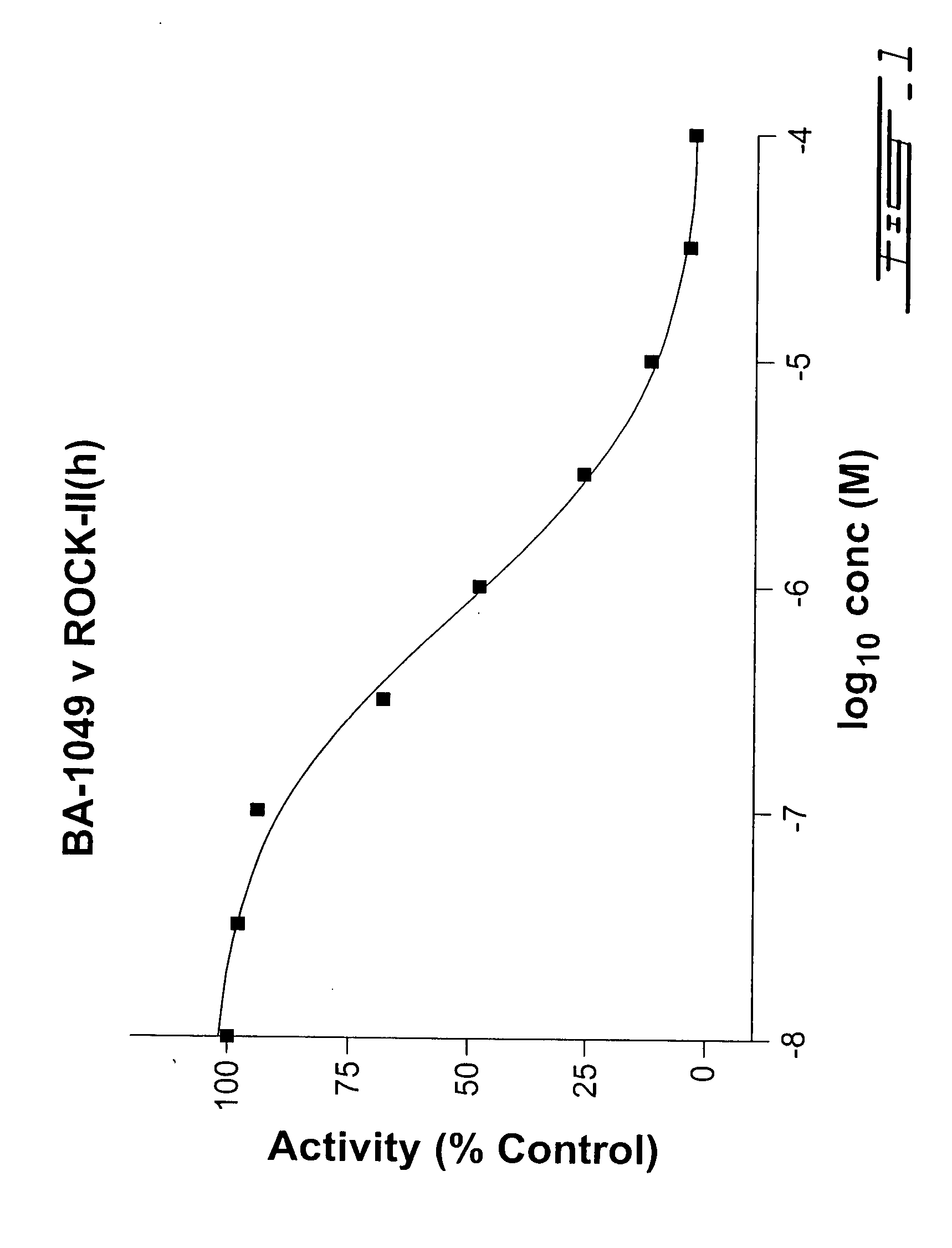
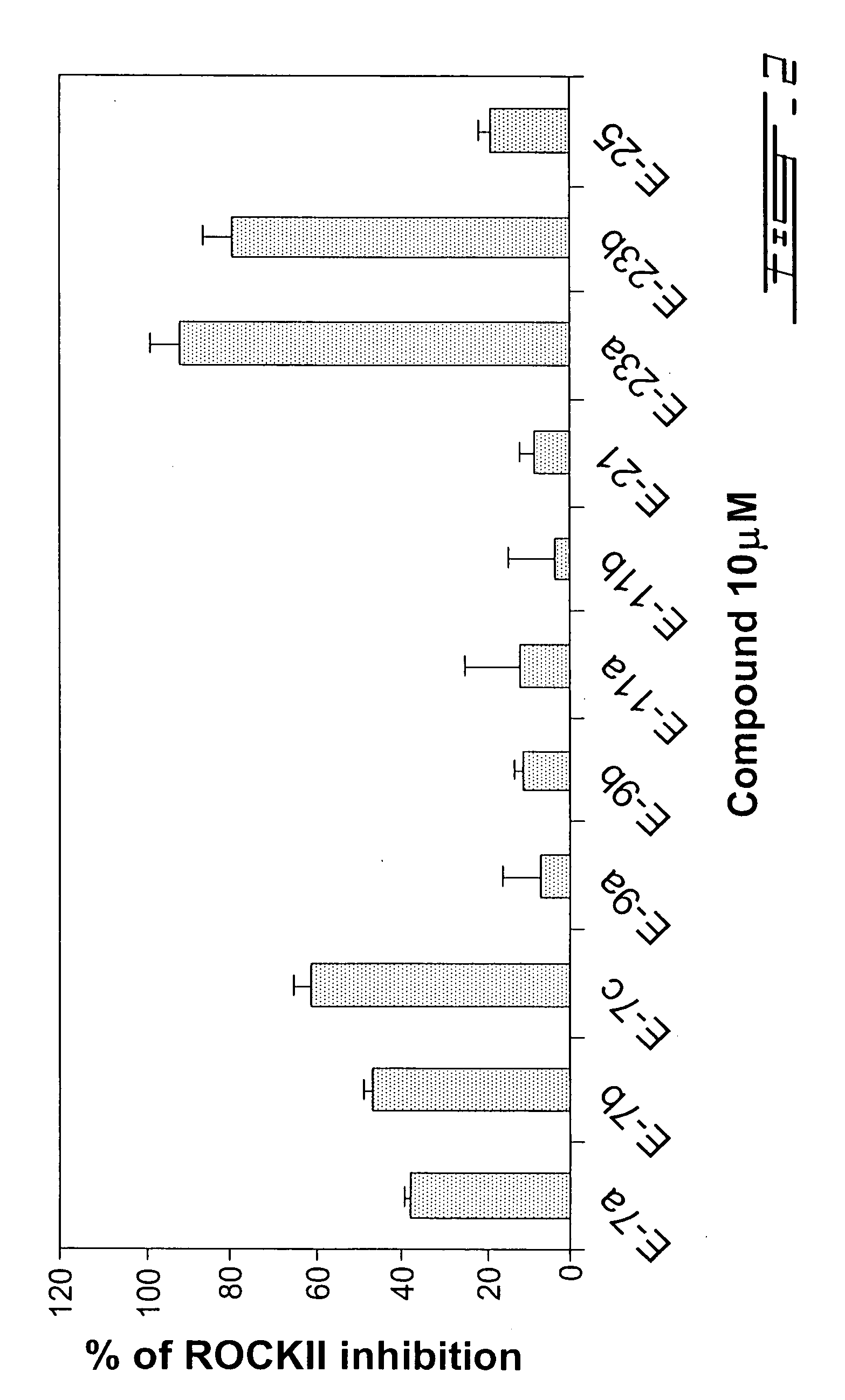
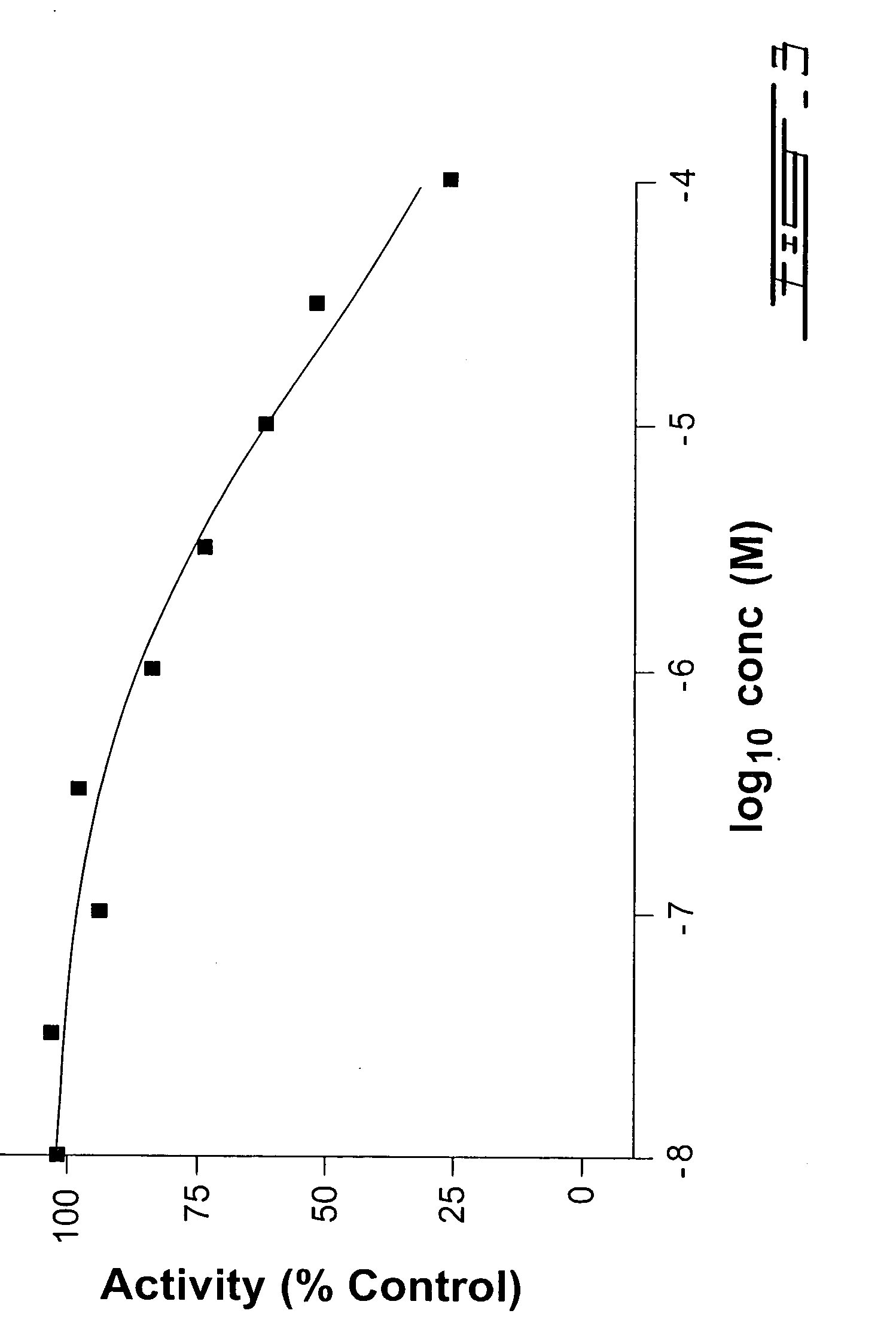
4-Substituted piperidine derivatives
a technology of substituted piperidine and derivatives, which is applied in the direction of drug compositions, antibacterial agents, immunological disorders, etc., can solve the problem that nerves in the cns have a very limited ability to repair and regrow after injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-Benzyloxycarbonyl-4-oxopiperidine (E-1)
[0515] A stirred solution of 4-oxopiperidine hydrochloride monohydrate (1.0 g, 6.5 mmol) in dry dichloromethane (DCM, 40 mL) was cooled to 0° C., treated with diisopropylethylamine (3.40 mL, 19.5 mmol), stirred for five minutes, treated over 20 minutes with benzyl chloroformate (1.54 mL, 10.7 mmol) over 20 minutes, allowed to warm to room temperature and stirred for two hours. The mixture was partitioned between DCM (25 mL) and water (15 mL). The layers were separated and the aqueous phase was extracted with DCM (2×25 mL). The combined organic phases were washed with brine (1×15 mL), dried over Na2SO4 and evaporated to a residue that was purified by column chromatography using a gradient of 20 to 40% EtOAc in hexanes as eluant. Evaporation of the collected fractions gave carbamate E-1 (1.20 g, 85%) as a clear oil: HRMS calc'd for C13H15NO3 (M+): 233.1051, found: 233.1048; 1H NMR (CDCl3) δ 2.43 (s, 4H), 3.78 (d, 4H, J=5.96), 5.16 (s, 2H), 7.3...
example 2
N-Benzyloxycarbonyl-4-[(N″-(tert-butyloxycarbonyl)hydrazono]piperidine (E-2)
[0516] To a stirred solution of N-benzyloxycarbonyl-4-oxopiperidine (E-1, 1.02 g, 4.65 mmol) in dry toluene (25 mL), tert-butyl carbazate (616 mg, 4.65 mmol) was added at room temperature. The mixture was stirred for 5 minutes, allowed to stand at room temperature for 24 hours, treated with Na2SO4 (1 g), stirred at room temperature for 3 hours and filtered. The filtrate was evaporated to give quantitatively the hydrazone E-2 as an oil which was used in the next step without further purification: HRMS calcd for C18H26N3O4 [(MH)+]: 348.1923, found: 348.1935, 1H NMR (CDCl3) δ 1.48 (s, 9H), 2.35 (m, 2H), 2.51 (m, 2H), 3.62 (m, 4H), 5.13 (s, 2H), 7.33 (m, 5H), 7.77 (br s, 1H).
example 3
N-Benzyloxycarbonyl-4-[N″-(tert-butyloxycarbonyl)hydrazino]piperidine (E-3)
[0517] A stirred solution of N-benzyloxycarbonyl-4-[(N″-(tert-butyloxycarbonyl)hydrazono]piperidine (E-2, 1.56 g, 4.49 mmol) in dry tetrahydrofuran (THF, 7.5 mL) at room temperature was treated with sodium cyanoborohydride (353 mg, 5.61 mmol) followed by bromocresol green (2 mg). The mixture was stirred vigorously at room temperature, treated with a solution of p-toluenesulfonic acid (773 mg, 4.49 mmol) in dry THF (8 mL) over two hours in order to maintain a green colored mixture. The reaction was partitioned between EtOAc (25 mL) and brine (20 mL). The phases were separated and the aqueous phase was extracted with EtOAc (3×10 mL). The combined organic phases were washed with NaHCO3 sat. (2×15 mL) and brine (1×15 mL), dried over Na2SO4 and evaporated to a residue, that was suspended in dioxane (10 mL), treated slowly with aqueous sodium hydroxide (1 N, 3 mL), stirred for 5 minutes at room temperature and par...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com