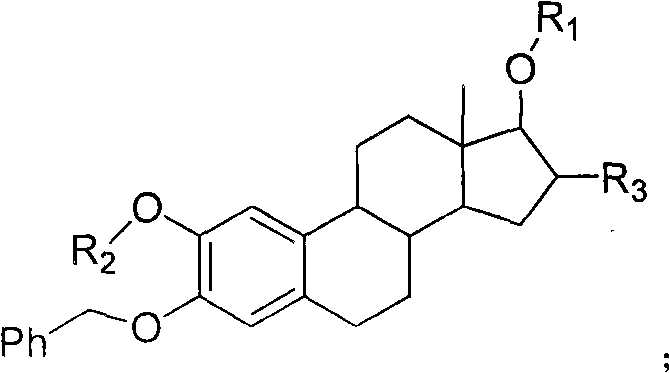
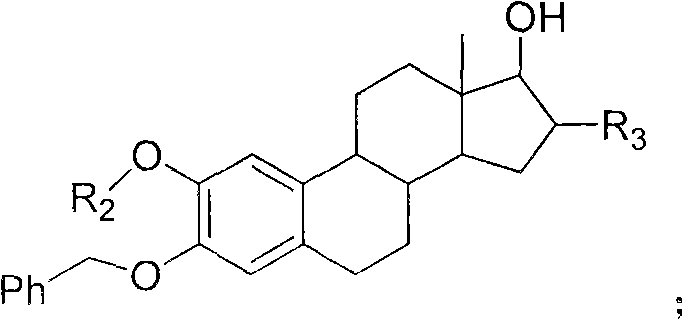
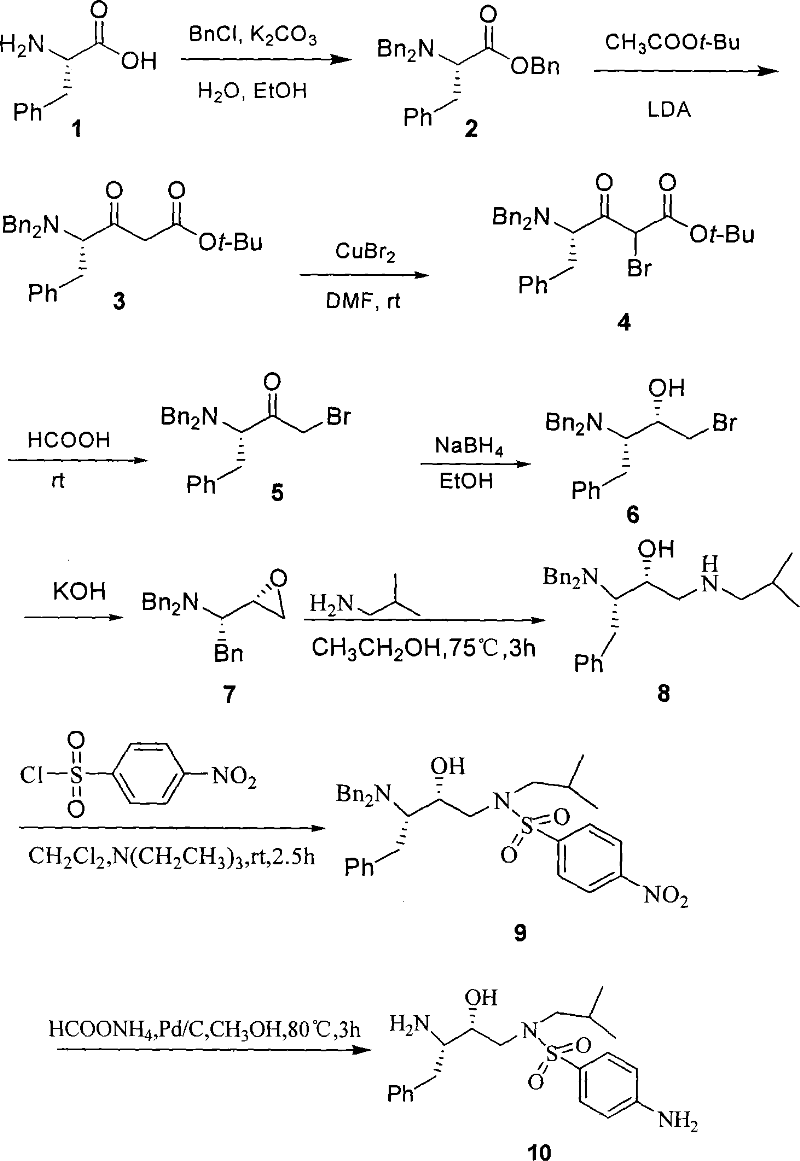
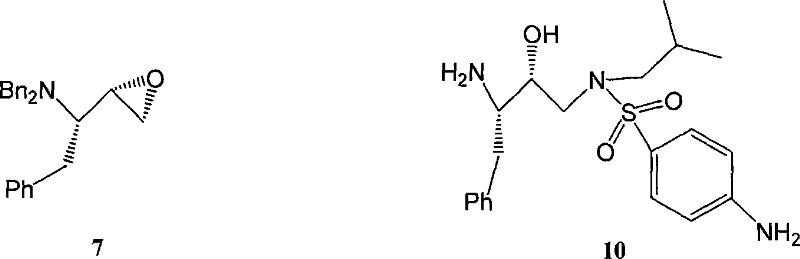
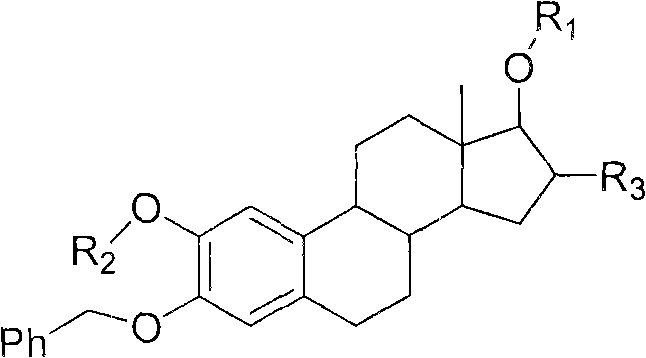
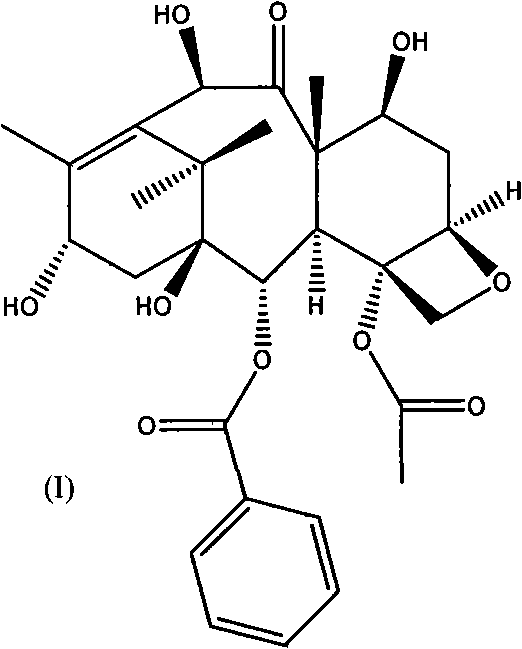
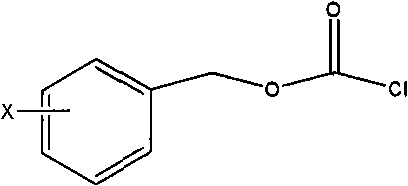
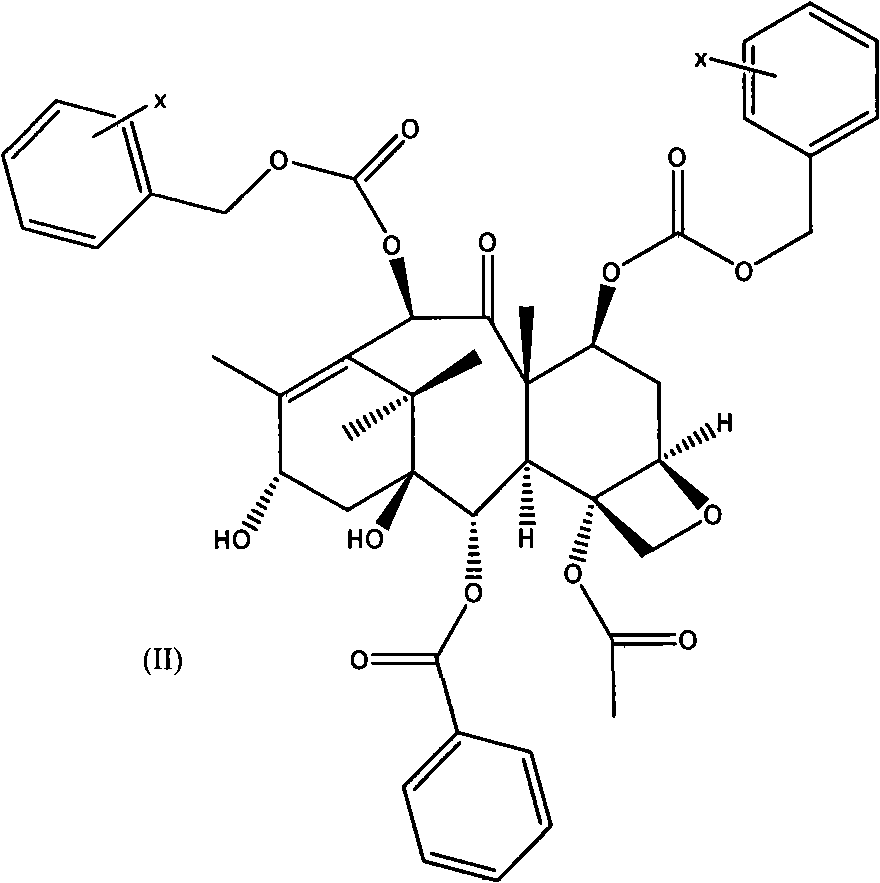
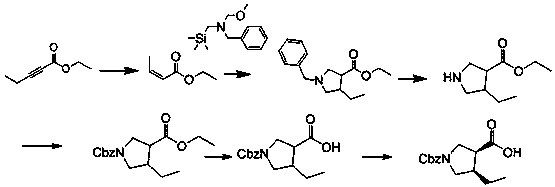
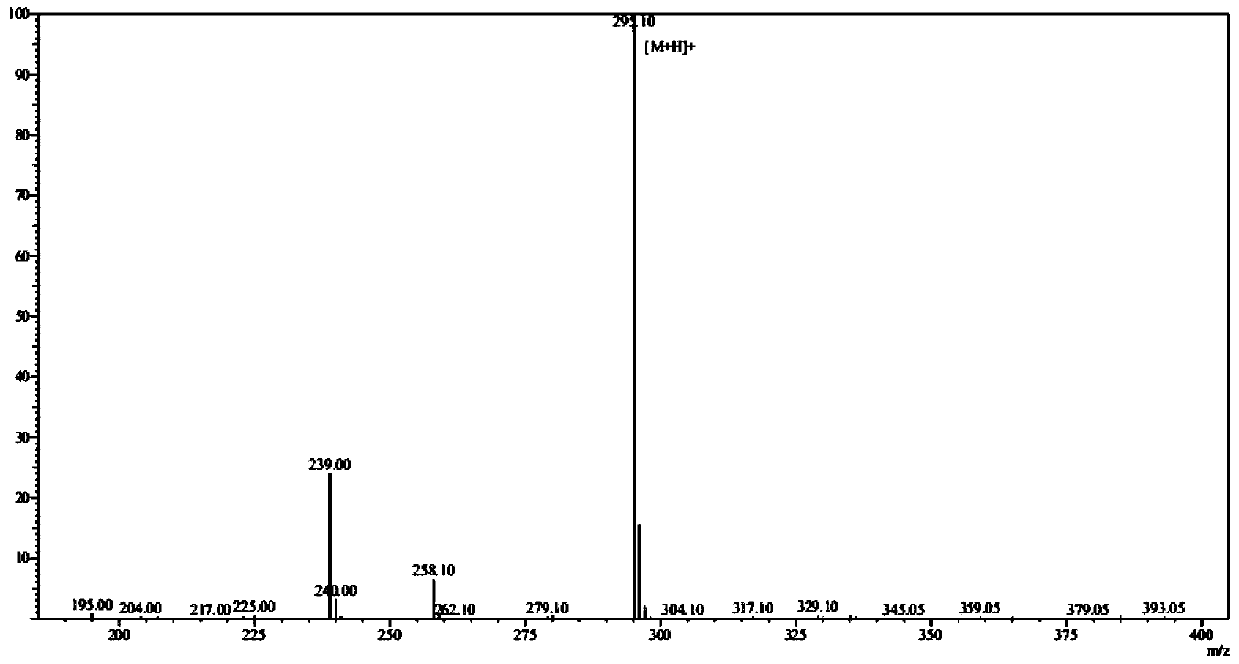
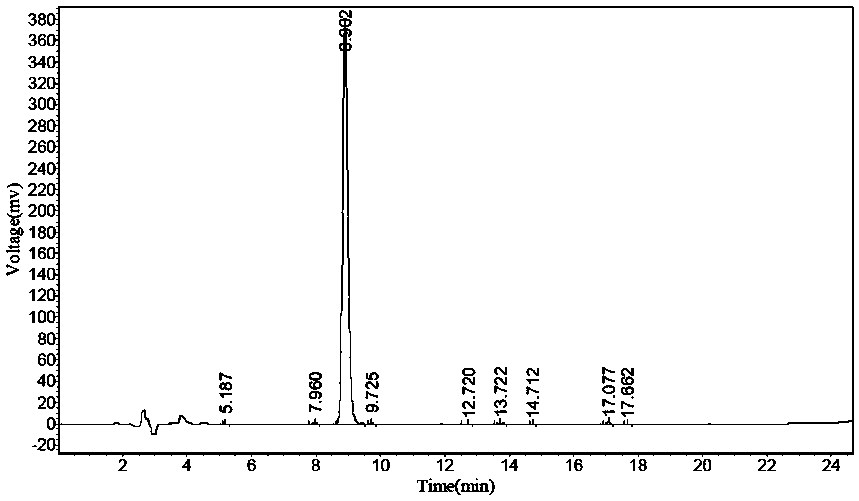
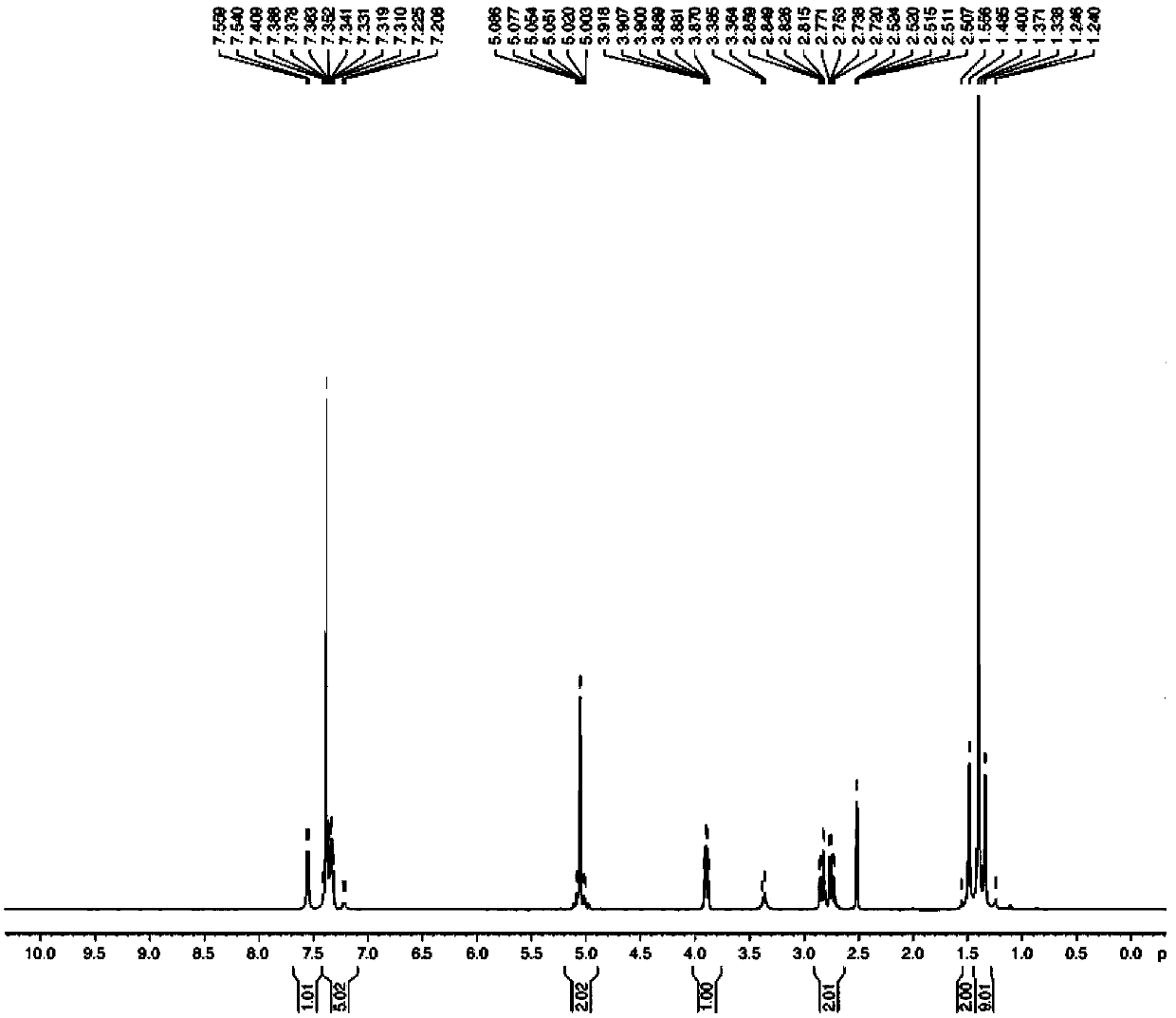
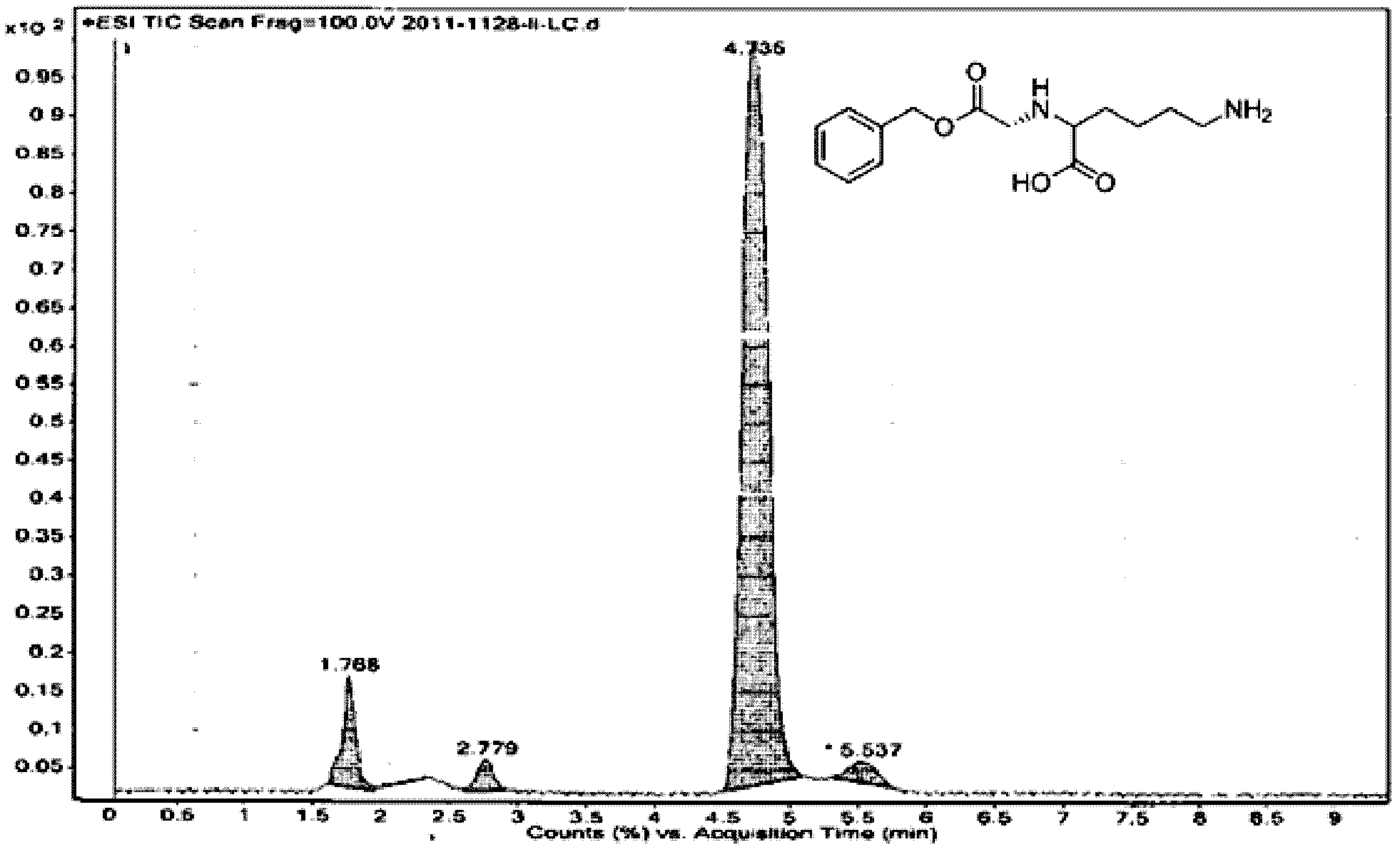
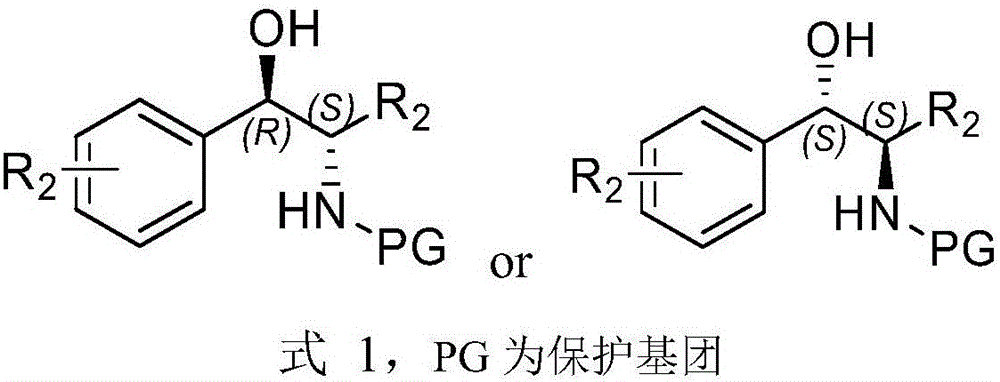
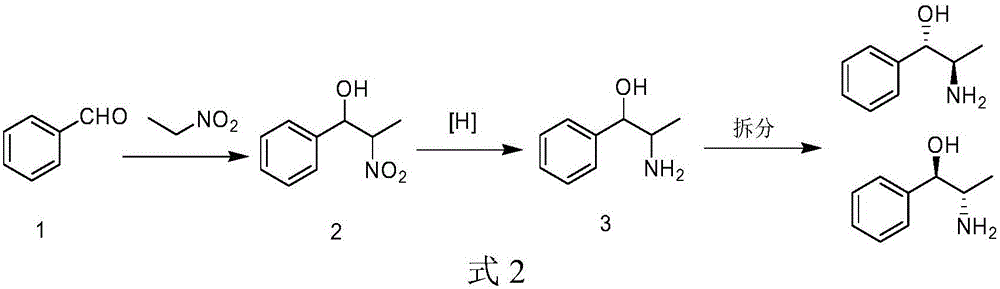
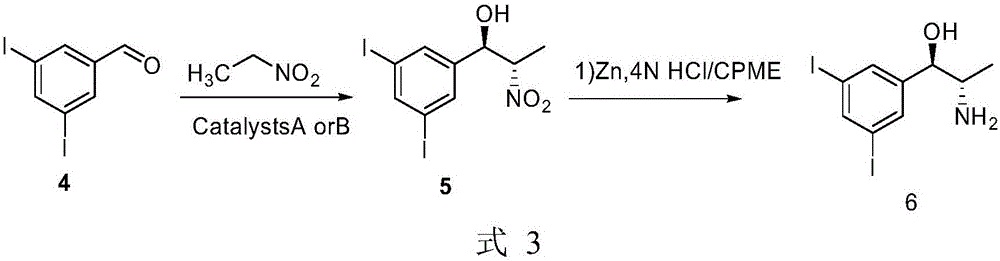
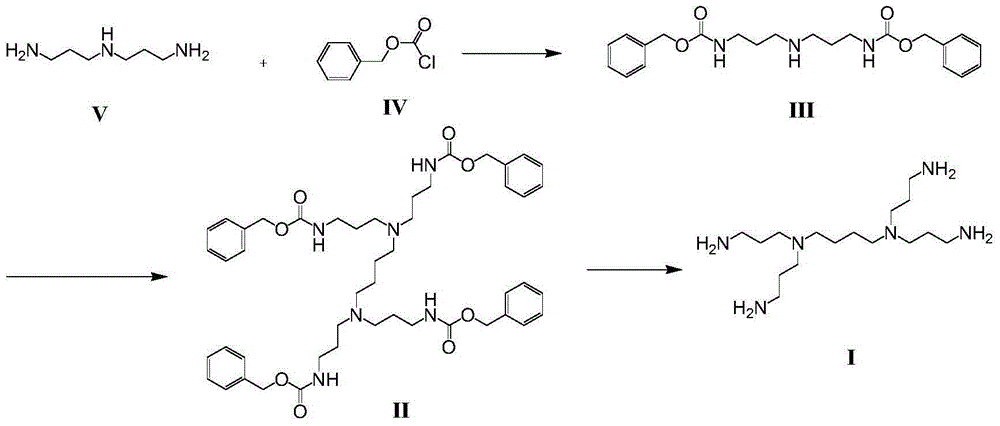
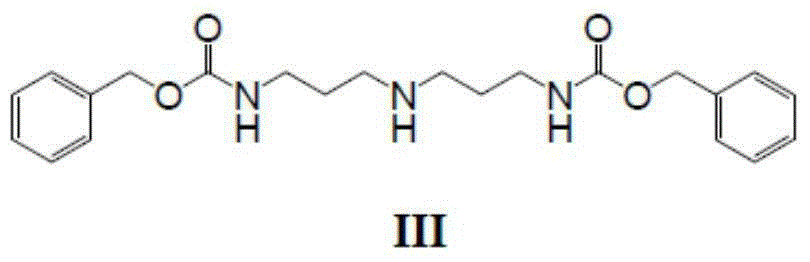
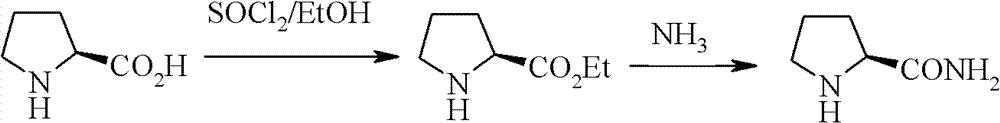
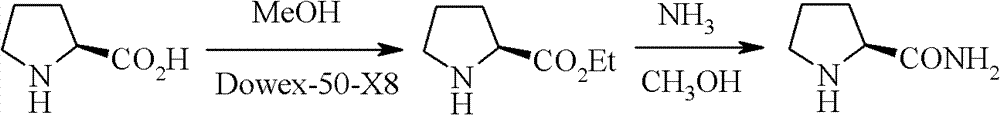
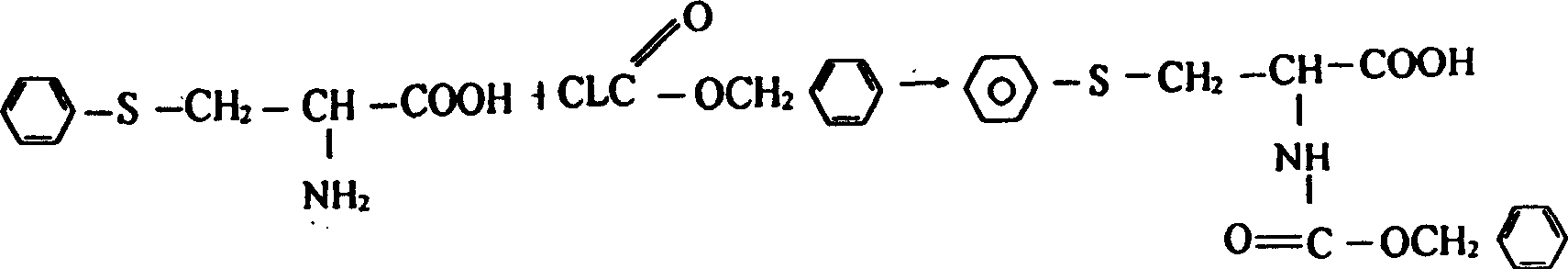Patents
Literature
97 results about "Benzyl chloroformate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Benzyl chloroformate is the benzyl ester of chloroformic acid. Also known as benzyl chlorocarbonate it is an oily colorless liquid although impure samples appear yellow. It is also known for its pungent odor. In contact with water it degrades.
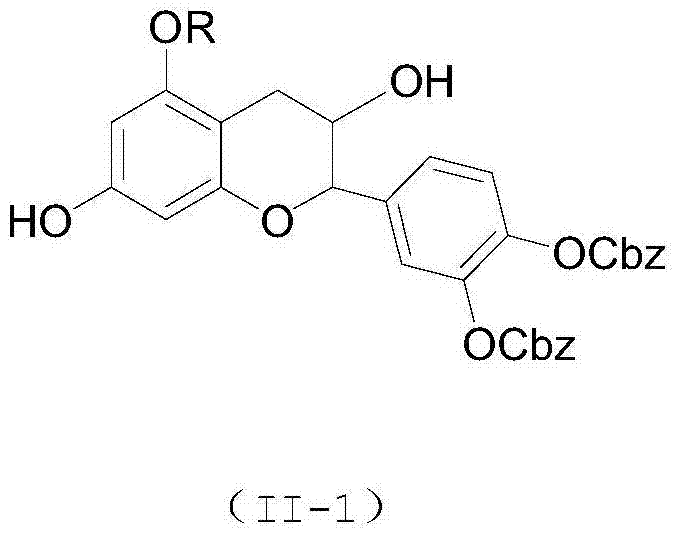
Cytarabine 5'-O-amino-acid ester, salts thereof and preparation method thereof
InactiveCN101812105AGood membrane permeabilityIncrease Absolute BioavailabilityOrganic active ingredientsSugar derivativesCytarabineSodium bicarbonate
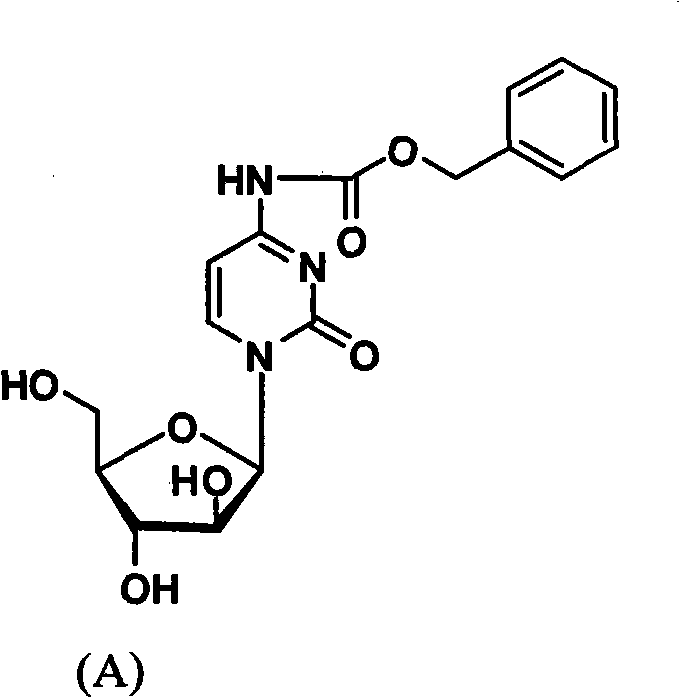
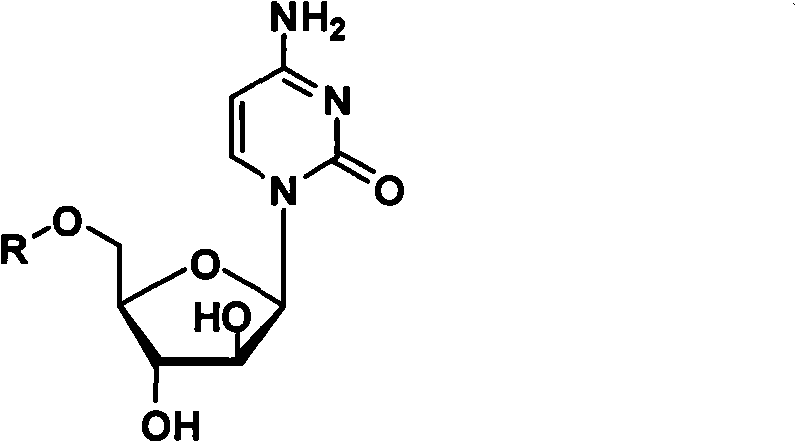
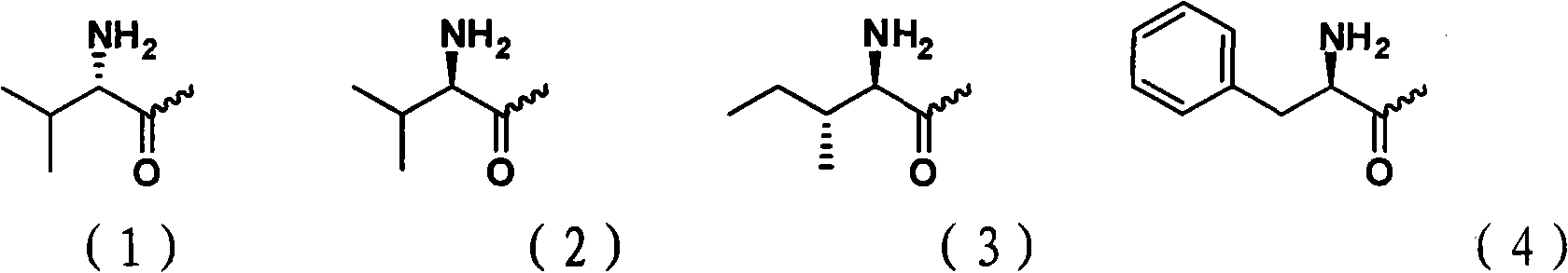
The invention belongs to the technical field of medicines and discloses cytarabine 5'-O-amino-acid ester, pharmaceutically acceptable salts thereof and a preparation method thereof. The preparation method comprises the following steps of: slowly dropping carbobenzoxy chloride into a solution formed by cytarabine, sodium bicarbonate and N,N-dimethylacetylamide, and obtaining a compound A after reacting at room temperature; using the compound A and N-butyloxy formoxyl-amino acid as raw materials; adding a reagent to the solution to carry out an esterification reaction to obtain the cytarabine 5'-O-amino-acid ester; and then adding acid to obtain a finished product. The pharmaceutically acceptable salts comprise hydrochlorides, sulfates, formates, acetates, mesylates, propionates, butyrates, p-toluene sulphonates, phosphates, bisulfates, maleates, lactates, carbonates, bicarbonates, malonates, and salts formed with acidic amino acids, and the like. The invention can obviously improve the membrane permeability of the cytarabine so as to improve the bioavailability of the cytarabine.
Owner:SHENYANG PHARMA UNIVERSITY
Aliphatic polyester-polyamino acid Y-type three-arm block copolymers and synthetic method thereof
The invention relates to a Y-shaped three-arm segmented copolymer of aliphatic polyester namely polyamino acid and a process for synthesis, which belongs to the high-polymer biological medicine material field. And the process for synthesis comprises protecting amidogens of 2-amidocyanogen-1 and 3-propylene glycol with benzyl chloroformate, ringopening and polymerizing 2-amidocyanogen-1 and 3-propylene glycol with aliphatic cyclic esters monomer in benzene or toluene dissolvent under the condition of warming up and stirring and without water and oxygen through utilizing stannous octoate as catalyst, utilizing amidogens to be macromolecule initiating agent after de-protecting, triggering alpha- amino acid-N-carboxylic acid anhydrides to ring-open and polymerize, getting Y-shaped segmented copolymer of carboxy group which is protected by benzyl group, forming relative polymer with free carboxy group through dewatering hydrogen bromide or catalyzing hydrogenating and deacidizing palladium-charcoal, and then getting the Y-shaped segmented copolymer which can be biodegraded and can have biology functionalization. The invention can be widely applied in the filed such as internal fixation of bone fracture, medicinal carrier, tissue engineering scaffold and the like.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Estrogen amino-acid ester compound with antitumor activity as well as synthetic method thereof
ActiveCN102079771AGood water solubilityGood inhibitory effectSteroidsDipeptidesSodium bicarbonateSolubility
The invention relates to an estrogen amino-acid ester compound with antitumor activity as well as a synthetic method thereof, effectively solving the preparation problems of the estrogen amino-acid ester compound with the antitumor activity. The synthetic method comprises the following steps of: dissolving amino acids or small molecule peptides to an aqueous solution of sodium hydroxide or a saturated solution of sodium bicarbonate, dripping carbobenzoxy chloride, making the solution react until the solution is not purple by developing with ninhydrin or carrying out film detection; washing with aether, removing aether, adjusting the pH value of the water phase with hydrochloric acid until milky white solid occurs and extracting with ethyl acetate; washing mixed solution with distilled water and saturated common salt solution, drying with anhydrous magnesium sulfate, carrying out suction filtration, concentrating and purifying; and then, mixing the mixed solution with sterides nucleus and dissolving the mixture to a reaction solvent, adding a condensing agent and a catalyst, supplementing an additional condensing agent, carrying out film detection, filtering, concentrating and purifying, dissolving in a dissolving solvent, adding palladium carbon for catalytic hydrogenation, detecting the film, filtering, concentrating and purifying. The estrogen amino-acid ester compound withantitumor activity, provided by the invention, has favorable water solubility and is superior to 2-methoxyestradiol in the tumor cell resistance action.
Owner:ZHENGZHOU UNIV
Method for synthesizing anti-aids drug amprenavir intermediate
InactiveCN101037403ANo pollution in the processHigh yieldOrganic chemistryBenzyl chloroformateSynthesis methods
A method for synthesizing anti - AIDS medicine amprenavir intermediate is related to a synthesis method of a compound, especially for 4-amido-N-((2R,3S)-3-amido-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfamide (amprenavir intermediate), including taking the L-phenylalanine as material, adopting the benzyl protection and esterification, reaction of bromine with CuBr2 / DMF, Pd carbon deoxidization to synthesize amprenavir intermediate. We choose the economic means of protecting the amido by benzyl so as to avoid using the benzyl chloroformate. The copper bromide for halogenated use can be reacted in room temperature so as to avoid using the expensive reagent such as chlorobromomethane and chloroiodomethane and produce no pollution to the air. The debenzylation hydrogenation is finished by Pd / C so as to avoid using the expensive palladium dydroxide. The method uses cheaper materials and has a higher yield, which is suitable for commercial process.
Owner:XIAMEN UNIV
Method for preparing halofuginone intermediate
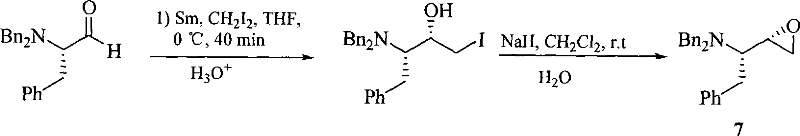
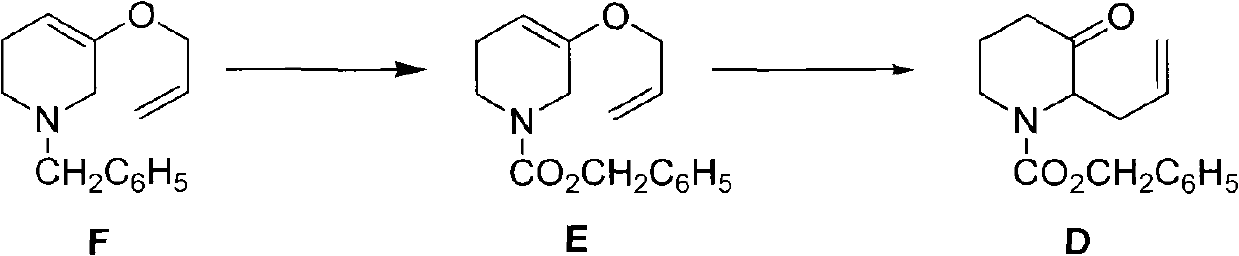
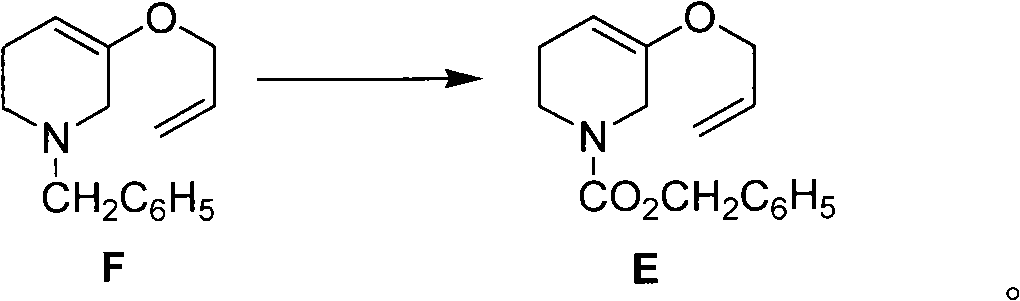
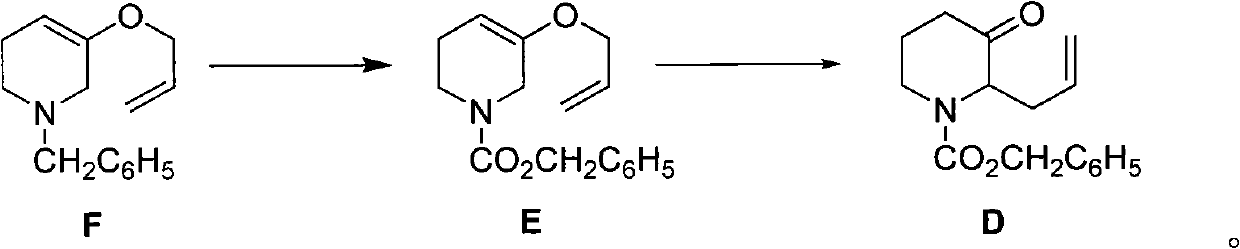
The invention discloses a method for preparing a halofuginone intermediate shown in a formula D or E. The method comprises the following steps of: (1) dropwise adding solution of a compound F into benzyl chloroformate solution, and performing Van Braun reaction to obtain a compound E; and (2) performing Claisen rearrangement reaction on the compound E obtained in the step (1). By the method, the Van Braun reaction can be completely performed, the using amount of benzyl chloroformate is reduced, the efficiency of the reaction is improved, and a 2-bit product can be selectively obtained in the subsequent Claisen rearrangement reaction. In the better embodiment of the invention, the operation process is simplified by a one-pot method, the using amount of solvents is reduced, the selectivity is improved, the cost of the reaction is reduced, the pollution to the environment is reduced, and good conditions are created for mass production of halofuginone.
Owner:南通远航医药化工有限公司 +1
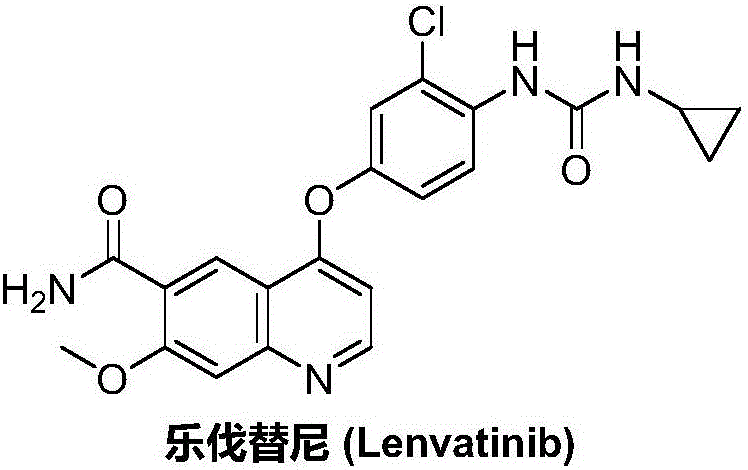
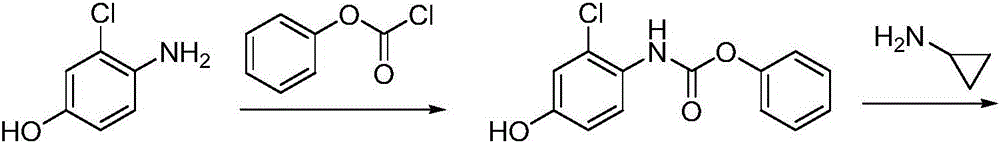
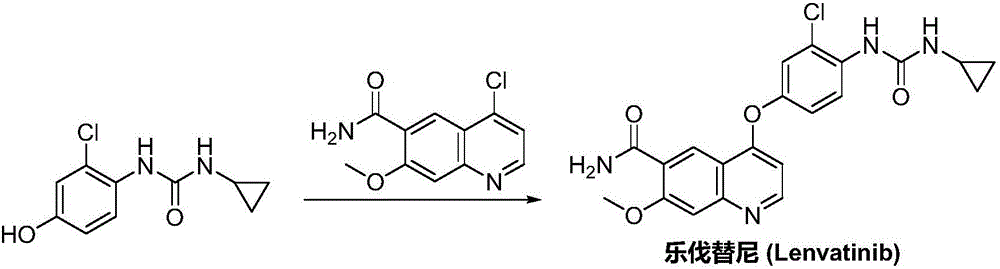
Lenvatinib synthesizing method
InactiveCN105801481AMeet the needs of useMild reaction conditionsOrganic chemistryLenvatinibBenzyl chloroformate
The invention discloses a lenvatinib synthesizing method.The method includes the steps that 4-amino-3-chlorophenol and benzyl chloroformate are subjected to amidation reaction, the obtained 4-(carbobenzoxy)amino-3-chlorophenol and 4-chlorine-7-methoxyquinoline-6-formamide are subjected to condensation reaction, the obtained 4-[3-chlorine-4-(carbobenzoxy)aminophenoxy]-7-methoxyquinoline-6-formamide and cyclopropylamine are subjected to amidation reaction, and the finished product lenvatinib is obtained.The method is short in process route step, operation is simplified, cost is low, and the method is environmentally friendly and suitable for industrial production.
Owner:湖南欧亚药业有限公司
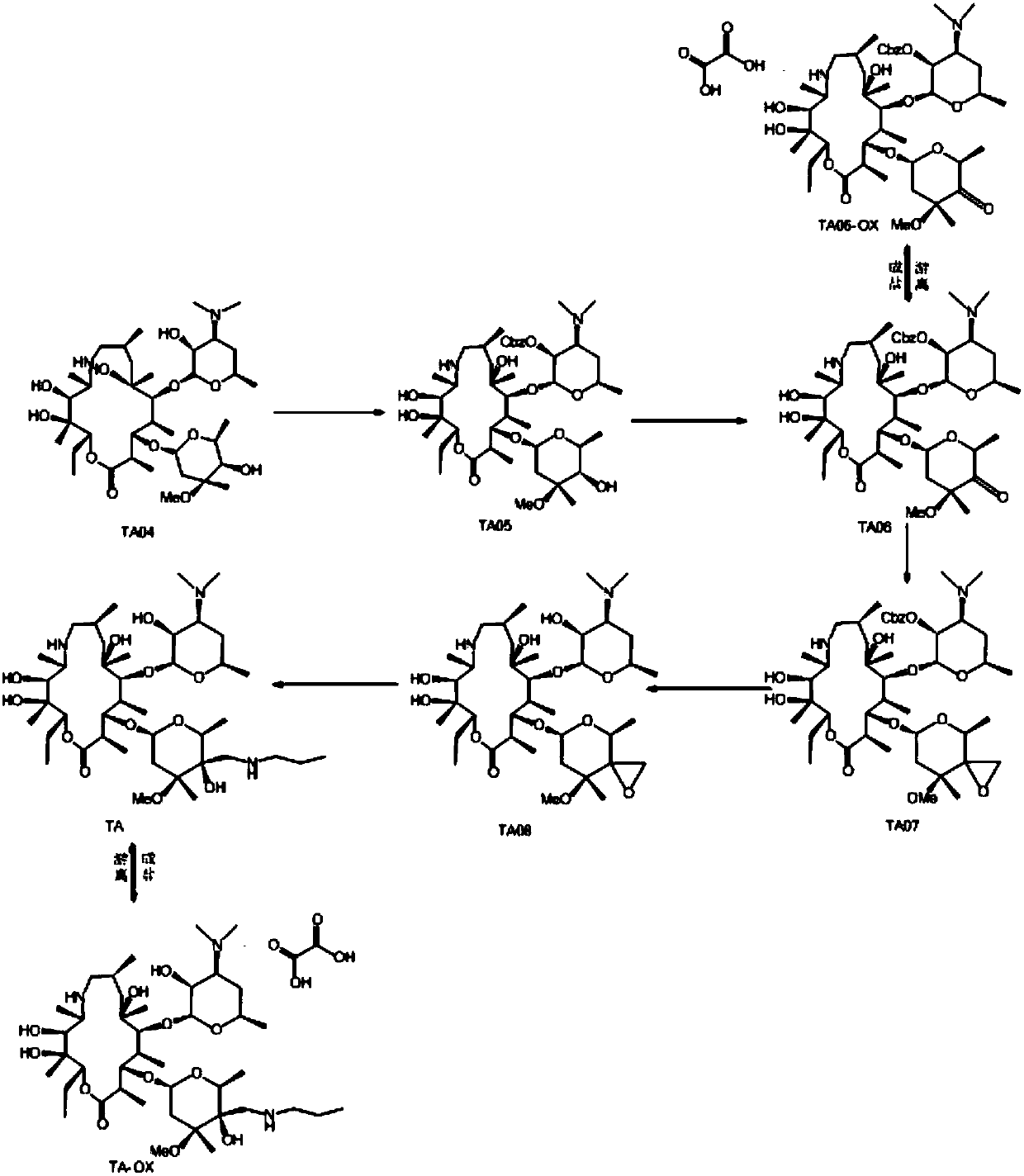
Preparation method of Draxxin
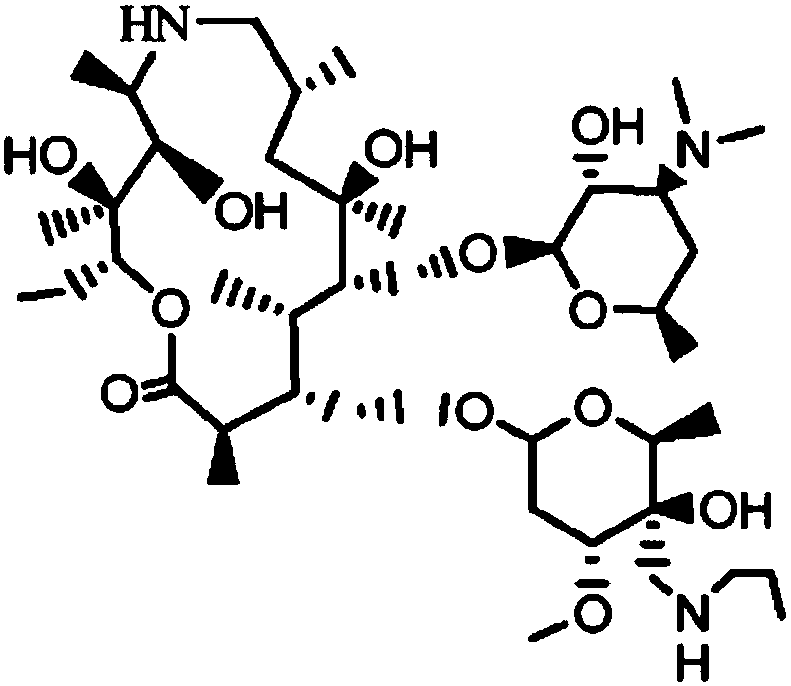
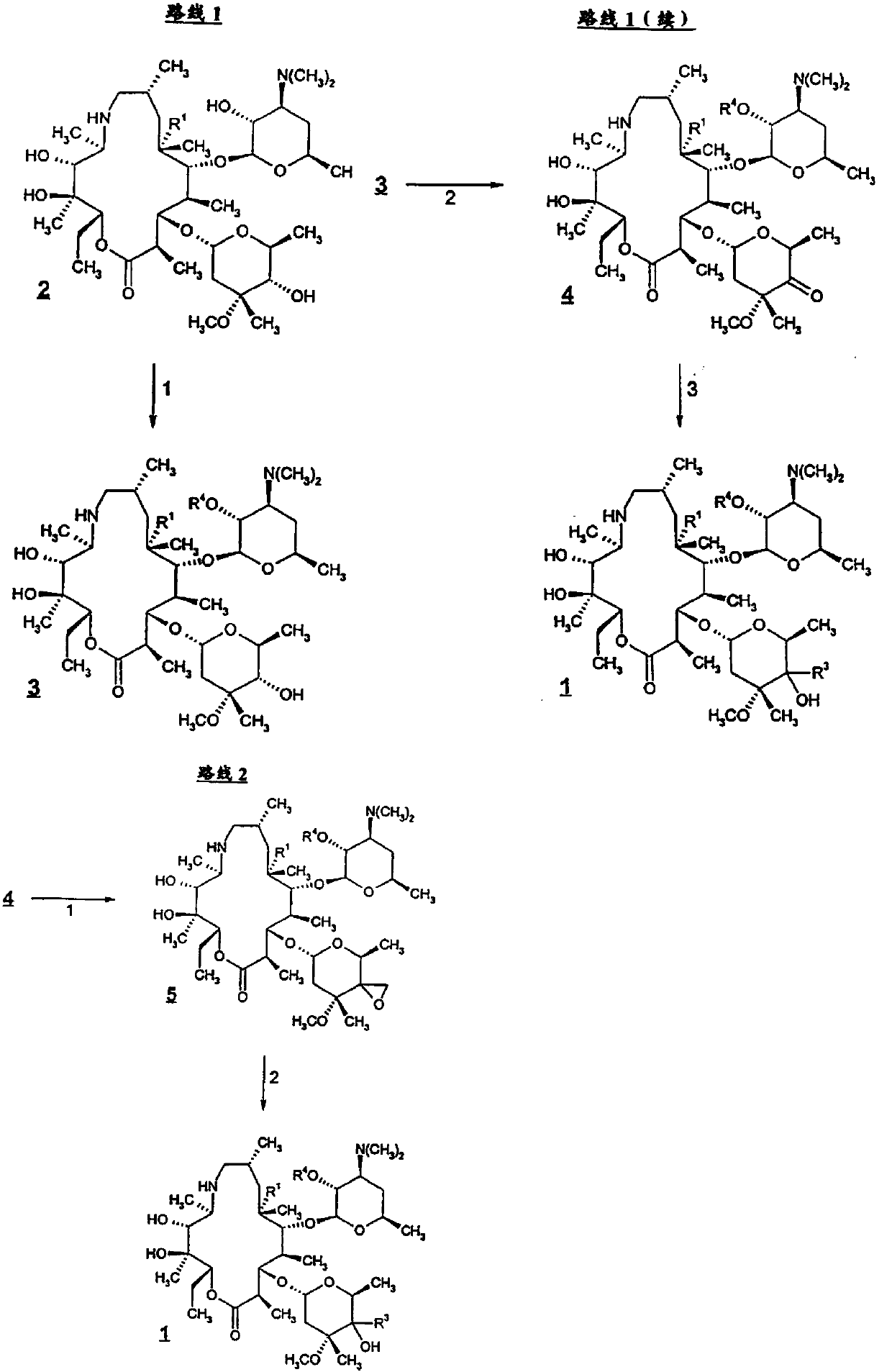
ActiveCN107556351AHigh yieldAvoid ultra-low temperature reactionsSugar derivativesSugar derivatives preparationPotassium tert-butoxideBenzyl chloroformate
The invention discloses a preparation method of Draxxin. The method comprises the following steps: enabling a reaction between an intermediate TA04 and benzyl chloroformate, so as to obtain TA05; oxidizing the TA05 with DMSO and IBX at the temperature of 20-30 DEG C, so as to obtain TA06; dissociating and performing aftertreatment after the TA06 becomes oxalate TA06-OX, so as to obtain purified TA06; oxidizing the TA06 with trimethylsulfonium bromide and potassium tert-butoxide, so as to obtain TA07; hydrogenating the TA07 to remove a protection group, so as to obtain TA08; enabling a reactionbetween the TA08 and n-propylamine, so as to obtain TA; dissociating and performing aftertreatment after the TA becomes oxalate TA-OX, so as to obtain purified end-product Draxxin. The total yield ofthe Draxxin prepared with the method is increased by above 20%.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Preparation method of taurylamine hydrochloride
ActiveCN106008283AEasy to handleSimple processSulfonic acid amide preparationSulfonic acid preparationBenzyl chloroformateReaction temperature
The invention discloses a preparation method of taurylamine hydrochloride. The preparation method comprises the following steps: by using taurine as an initial raw material, sequentially carrying out amino protecting reaction, chlorination reaction, ammonolysis reaction and deprotection-salification to obtain the taurylamine hydrochloride. The amino protecting group adopted by the amino protecting reaction is benzyl chloroformate, and the amino protecting group is carried out at 5-15 DEG C in the presence of sodium hydroxide and water. The chlorination reagent adopted by the chlorination reaction is thionyl chloride, and the chlorination reaction temperature is 60-70 DEG C. The ammonolysis reaction is carried out in tetrahydrofuran under the action of stronger ammonia water, and the ammonolysis reaction temperature is 5-15 DEG C. The total yield of the four-step reaction can reach 80% or above, the product purity can reach 99% or above, and thus, the method is suitable for industrialized mass production.
Owner:CHANGZHOU SUNLIGHT PHARMA
Intermediate compound for use in preparation of androst amino acid ester and synthesis method thereof
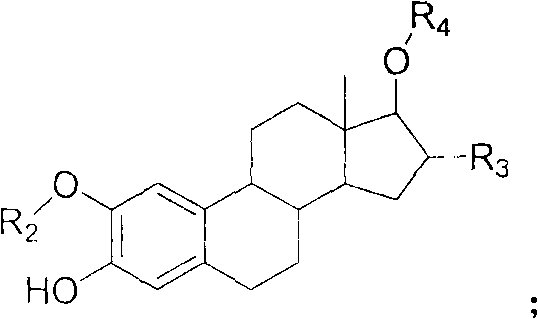
The invention relates to an intermediate compound for use in the preparation of androst amino acid ester and a synthesis method thereof, and solves the problem of the preparation of the intermediate compound that is used in the preparation of androst amino acid ester, which is a novel antitumor compound, for keeping or increasing the antitumor activity of androst amino acid ester and increasing the water solubility of androst amino acid ester. The method comprises: dissolving amino acid or small peptides in aqueous solution of sodium hydroxide or saturated solution of sodium bicarbonate; dripping benzyl chloroformate, adding ninhydrin for color developing till no purple color or performing lamina detection; washing with diethyl ether; removing diethyl ether; regulating the pH value of water phase with hydrochloric acid till milky solid appears, extracting with ethyl acetate, washing mixed extract with distilled water and saturated table salt solution, drying by anhydrous magnesium sulfate, performing suction filtration, concentrating and purifying; and dissolving a mixture of a compound A and the mother nucleus of a steride in a reaction solvent, adding a condensing agent and a catalyst, supplying extra condensing agent, performing lamina detection, filtering, concentrating and purifying. In the invention, the method is simple and the operation is convenient.
Owner:ZHENGZHOU UNIV
Synthetic method of benzyl carbazate
InactiveCN103819366AImprove qualityHigh yieldOrganic chemistryBenzyl chloroformateHydrazine compound
The invention relates to a synthetic method of benzyl carbazate. The method comprises the following steps of: a), dissolving hydrazine hydrate in a solvent and adding an alkali; b), controlling the reaction temperature to be 20-80 DEG C, dropping benzyl chloroformate; c), removing impurities after salt forming reaction of an obtained crude product; and d), performing basic dissociation of an obtained salt to obtain a pure f benzyl carbazate product. The synthetic method has the advantages of simple operation, good safety, high yield and low production cost, so as to meet a large demand of volume production of new pesticide indoxacarb and other fine chemical products on the key intermediate-benzyl carbazate.
Owner:JIANGSU HUITENG BIOMEDICAL TECH
Synthesis method of lifitegrast intermediate
InactiveCN107857728AImprove responseStarting materials are cheap and readily availableOrganic chemistryBenzyl chloroformateSynthesis methods
The invention discloses a preparation method of a lifitegrast intermediate. The preparation method comprises the following steps: (1) taking a compound (1), treating the compound (1) by using tetramethyldiethylamine and n-butyl lithium, adding benzyl chloroformate, reacting at the temperature of 70 DEG C below zero to obtain a compound (2); and (2) taking the compound (2), reacting the compound (2) with a strong alkaline solution, adjusting pH to be 3-4 after the reaction is ended, and extracting to obtain a compound (3). The prepared lifitegrast intermediate is high in process selectivity, high in controllability, high in stability and high in total yield, and is suitable for commercial large-scale production.
Owner:成都惟邦药业有限公司
Method for preparing docetaxel
InactiveCN101353334AHigh purityHigh reaction yieldOrganic chemistryAntineoplastic agentsTert-Butyloxycarbonyl protecting groupBenzyl chloroformate
The invention relates to a new method for preparing Docetaxel which includes a new synthesizing method for forming 10-deacetyl baccatin III(II) for protecting C-7, C-10-carbobenzoxy by using the 10-deacetyl baccatin III(I) for reacting with a benzyl chloroformate compound, and a new method for forming a new compound of 2'-(1-ethoxyethyl)-N-debenzoyl-N-(boc)-C-7, C-10-II-X-CBZ-10-deacetyl paclitaxel(IV) by the reaction of (II) with 1-boc-(3R,4S)-4-phenyl azetidine-2-ketone(III) as well as the new compound. Simultaneously, the invention also provides a new method for preparing a new compound of 2'- (1-ethoxyethyl)-N-debenzoyl-N-(boc)-10-deacetyl paclitaxel(V) and a new method for removing the 1-ethoxyethyl from the (IV) to form the Docetaxel.
Owner:重庆医科大学医药研究所
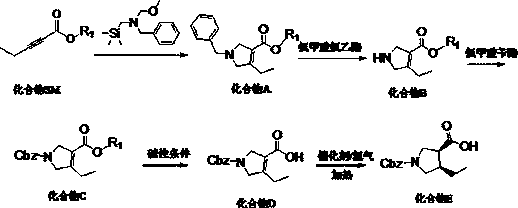
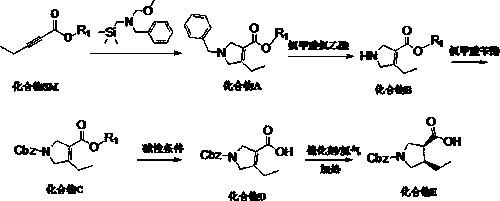
(3R,4S)-1-carbobenzoxy-4-ethylpyrrole-3-carboxylic acid synthesis method suitable for industrialization
InactiveCN110183367AEasy to operateHigh purityOrganic chemistry methodsBenzyl chloroformateCarboxylic acid
The invention discloses a (3R,4S)-1-carbobenzoxy-4-ethylpyrrole-3-carboxylic acid synthesis method suitable for industrialization. The method comprises the steps: adopting 2-pentynoate and N-methoxymethyl-N-(trimethylsilylmethyl)benzylamine as starting materials, performing a condensation cyclization reaction in a solvent under the catalysis of an acid so as to prepare an intermediate condensationproduct, adding the intermediate to chloroethyl chloroformate, performing a reaction so as to obtain another intermediate, performing an acylation reaction between the intermediate and benzyl chloroformate so as to obtain an N-protected intermediate product, performing an ester hydrolysis reaction, and performing an asymmetric reduction reaction between the hydrolyzed product and hydrogen so as to obtain (3R,4S)-1-carbobenzoxy-4-ethylpyrrole-3-carboxylic acid. The (3R,4S)-1-carbobenzoxy-4-ethylpyrrole-3-carboxylic acid with high purity and high yield can be prepared through five steps of thereactions, the problem of difficult control on the quality and yield in the prior art is solved, and sufficient raw material intermediates are provided for synthesis of upadacitinib.
Owner:南京新酶合医药科技有限公司
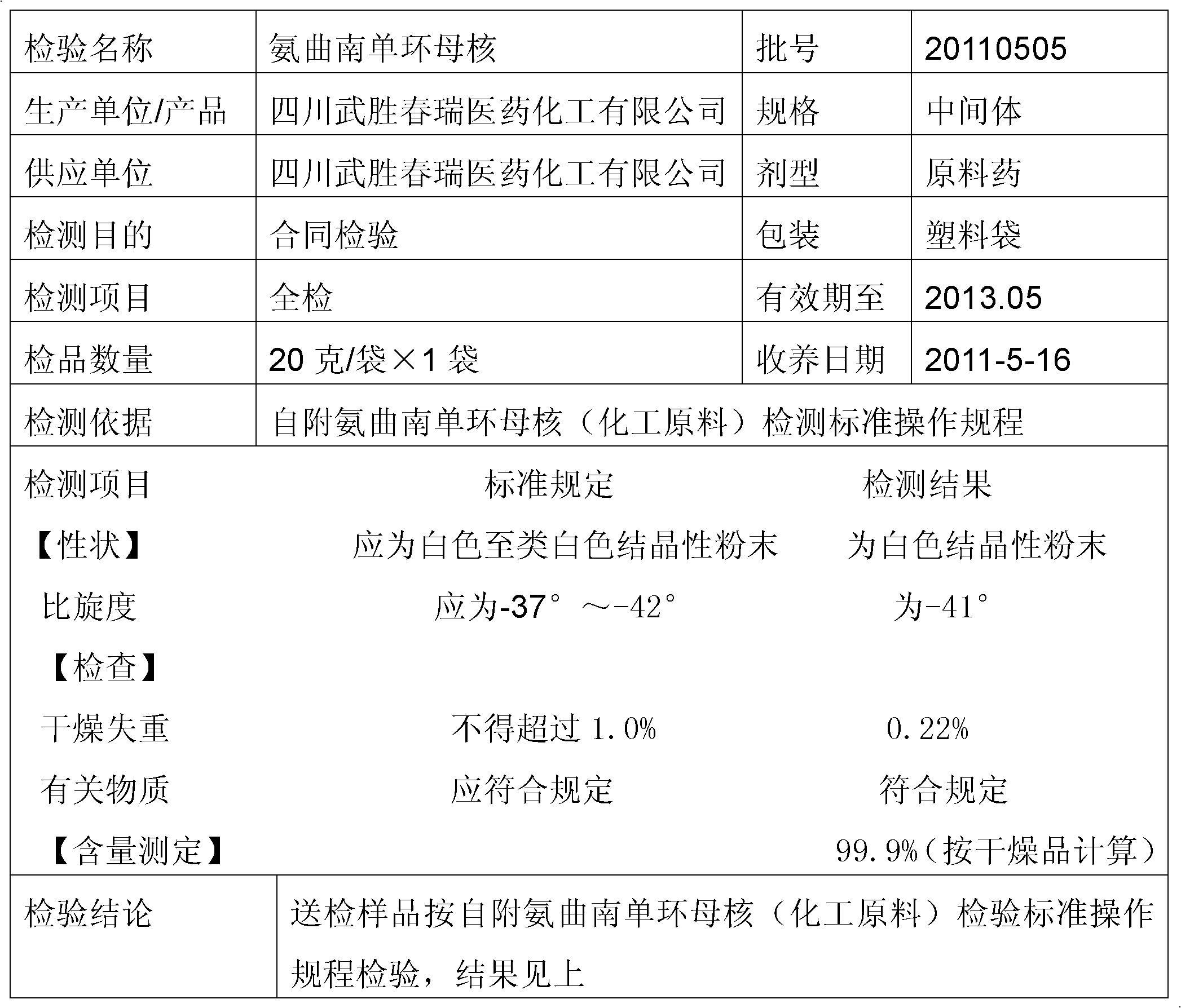
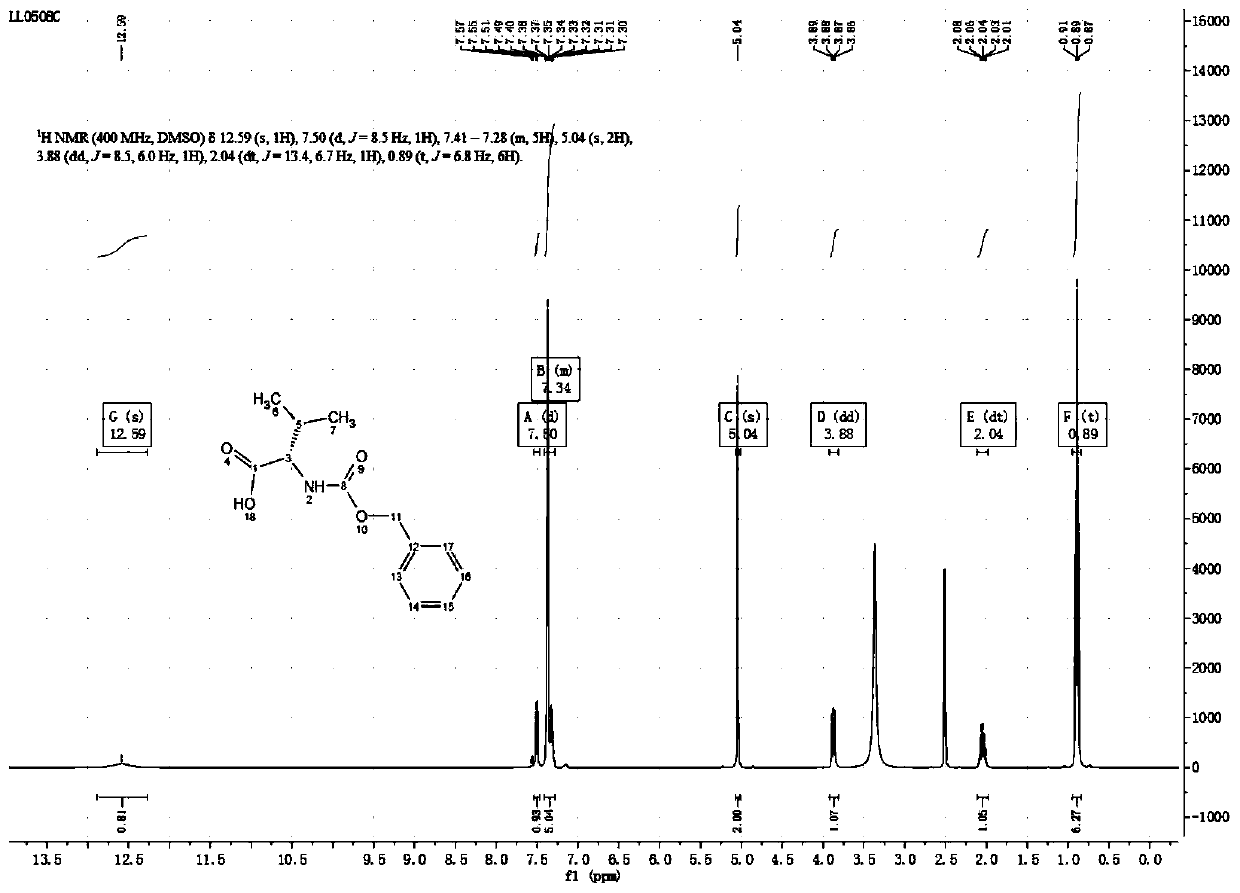
The preparation method of aztreonam monocyclic nucleus
ActiveCN102285907AGood water solubilityImprove product qualityOrganic chemistryBenzyl chloroformateAztreonam
The invention relates to a preparation method of aztreonam single-ring parent nucleus. The method uses benzyl chloroformate to protect under strong acidic conditions, and uses sodium carbonate to perform ring-closing reaction. The reaction is stable, the yield is greatly improved, and the total yield is For the 48% advantage.
Owner:SICHUAN WUSHENG CHUNRUI MEDICAL CHEM
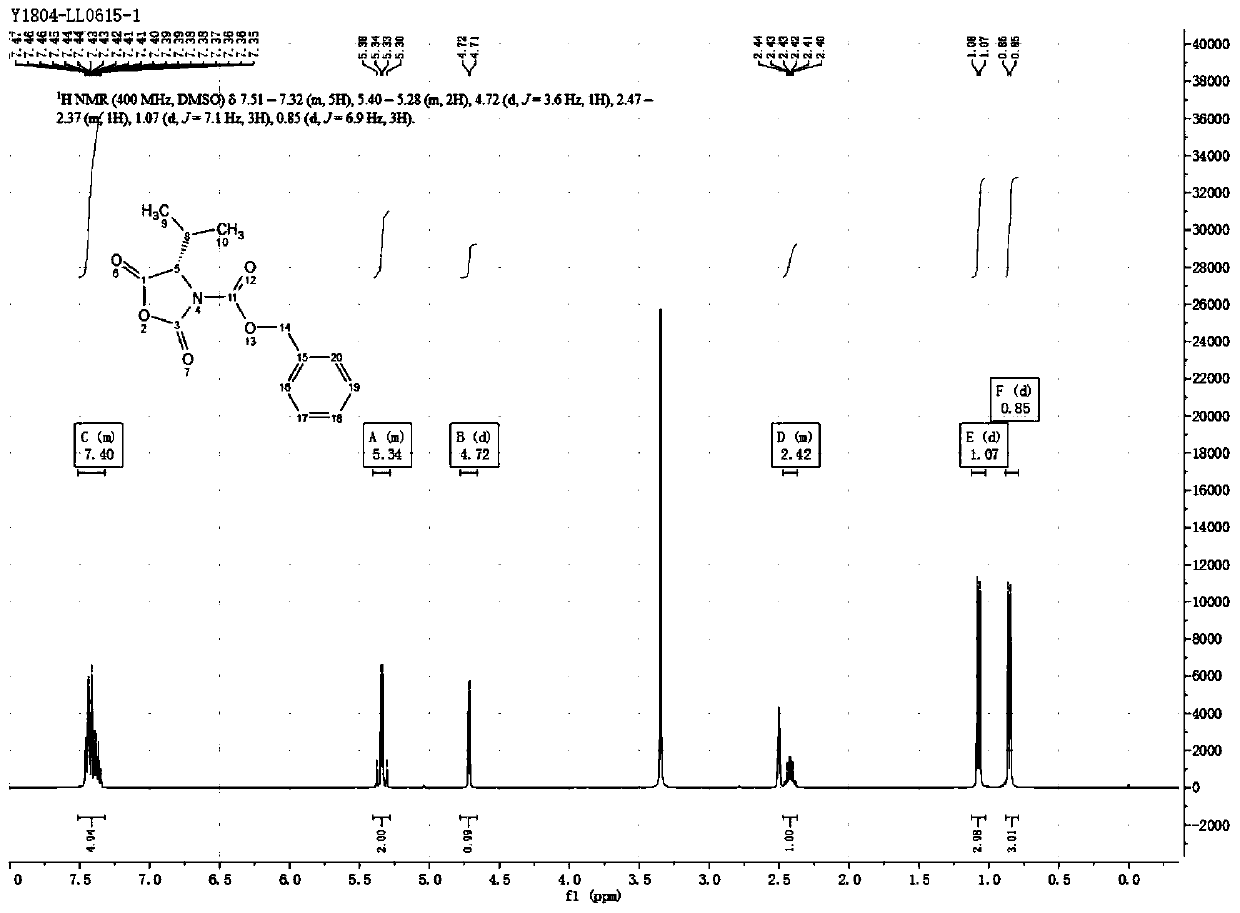
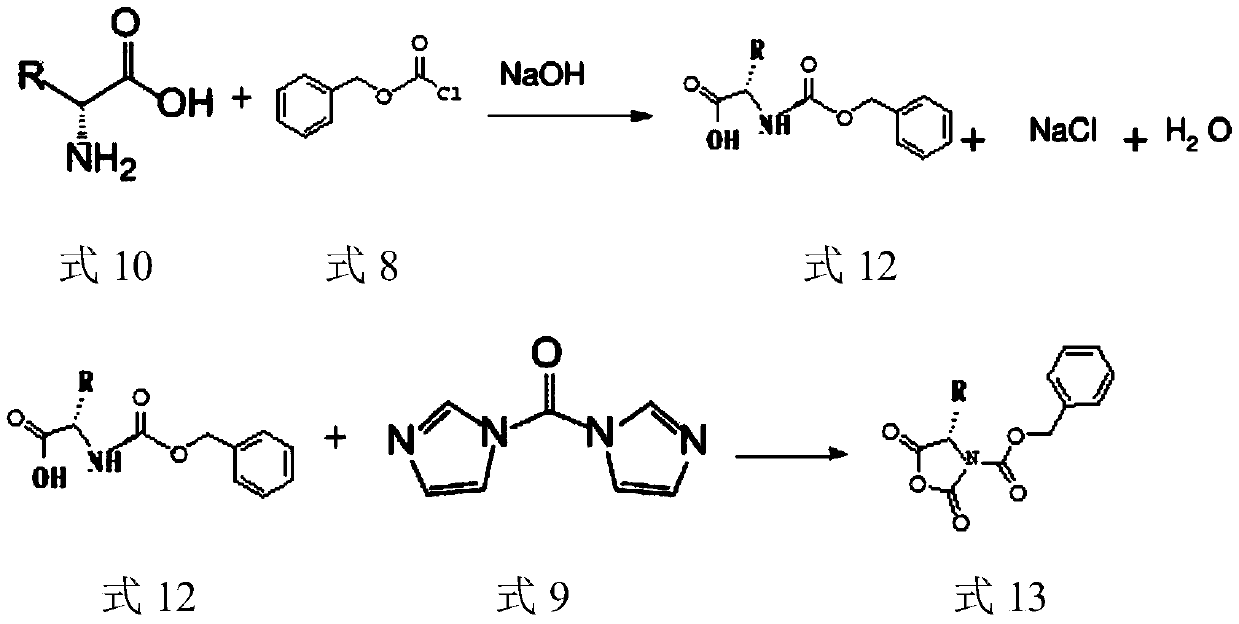
Preparation technology of (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione
ActiveCN110204505AHigh yieldLow yieldOrganic chemistry methodsBulk chemical productionOxazolidinedioneValganciclovir Hydrochloride
The invention provides a preparation technology of (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione. The (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione can serve as a valganciclovir hydrochloride intermediate. The technology includes the following operations that an L-valine starting material reacts with benzyl chloroformate to generate N-carbobenzoxy-L-valine; the N-carbobenzoxy-L-valinereacts with N,N-carbonyl diimidazole (CDI) to generate the (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione. The preparation technology is simple in method, purification is easy, the process is stable, the quality is controllable, the product yield is greatly improved, environmental pollution is not caused, and the technology is suitable for industrial mass production.
Owner:荆门医药工业技术研究院 +1
Preparation method of N-carbobenzoxy-3-amino-alanine tert-butyl ester
InactiveCN109535035AIncreased overall process yieldReduce manufacturing costCarbamic acid derivatives preparationOrganic compound preparationN dimethylformamideBenzyl chloroformate
The invention discloses a preparation method of tert-butyl N-carbobenzoxy-3-amino-alanine tert-butyl ester, and mainly solves the technical problem of low total yield and high cost in the original process. The preparation method comprises the following steps of 1, suspending asparagine into a mixed solution of water and acetone; regulating the pH value to a basic state by a sodium carbonate solution; lowering the temperature; adding carbobenzoxy chloride; then, raising the temperature for reaction; after the reaction is finished, performing acidification by hydrochloric acid; separating out solid; performing filtering to obtain an intermediate N-benzoxycarbonyl asparagine; 2, suspending the N-benzoxycarbonyl asparagine into dichloromethane; introducing isobutene; adding concentrated sulfuric acid; performing sealed reaction for two days; performing treatment to obtain N-carbobenzoxy asparagine tert-butyl ester; 3, dissolving the N-carbobenzoxy asparagine tert-butyl ester into water andN,N-dimethylformamide; adding iodobenzene diacetate for reaction first; then adding pyridine; continuously performing reaction till the completion; performing treatment to obtain a final product.
Owner:GL BIOCHEM SHANGHAI +1
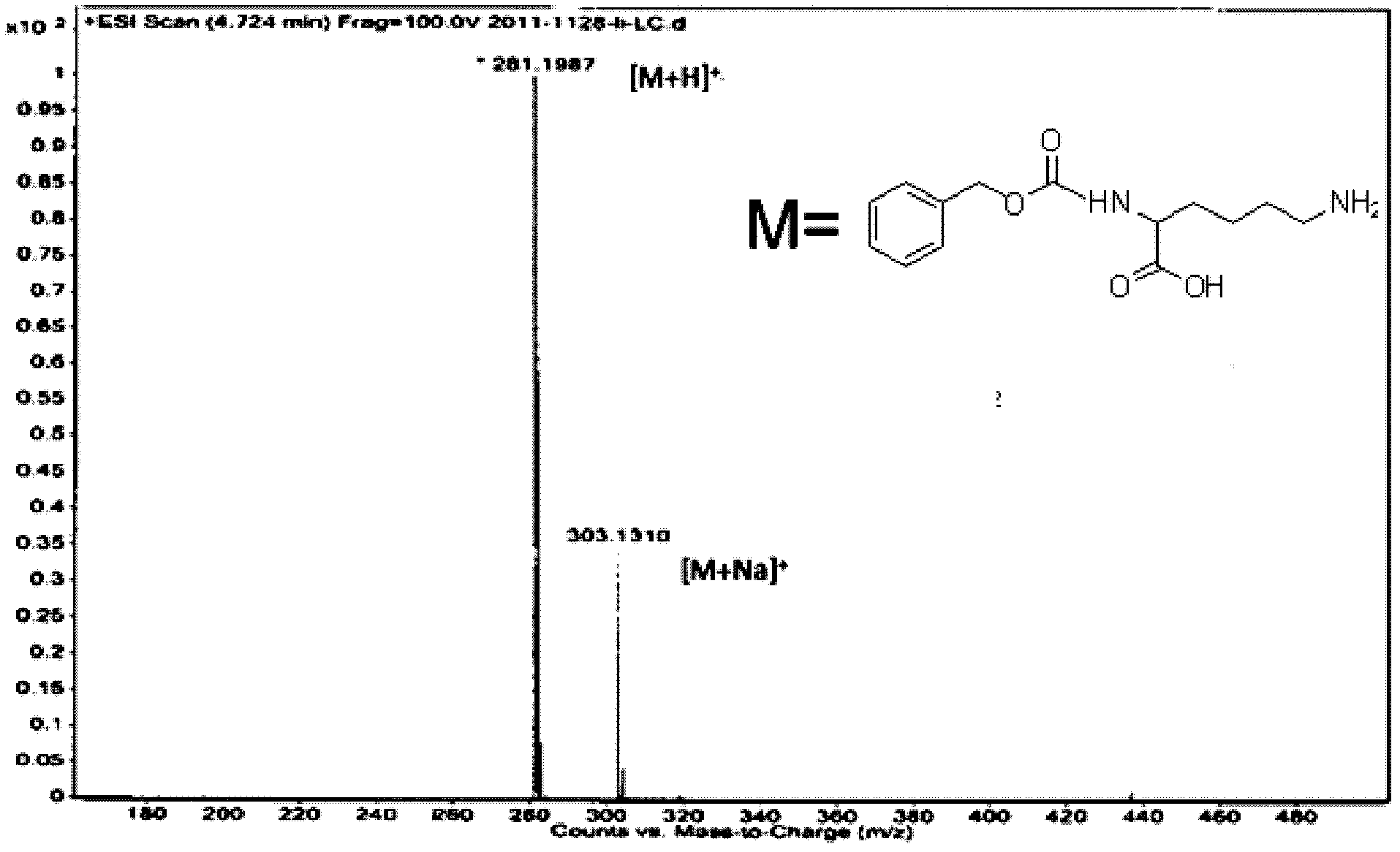
Lysine alpha-amino carbobenzoxy high-efficiency selective protection method and product thereof
InactiveCN102584633AHigh product contentIncrease contentCarbamic acid derivatives preparationOrganic compound preparationOrganic solventDistillation
The invention discloses a lysine alpha-amino carbobenzoxy high-efficiency selective protection method, which enables beta-cyclodextrin to be dissolved in water and uses alkali to adjust to alkalescence, adds in lysine for blending and dissolving, drops in benzyl chloroformate, simultaneously controls reaction temperature at 18-23 DEG C, continuously blends to obtain reaction liquid, extracts the reaction liquid by using organic solvent, combines extraction liquid, conducts decompression and distillation to obtain lysine alpha-amino carbobenzoxy pre-protection products. The lysine alpha-amino carbobenzoxy high-efficiency selective protection method is simple and convenient, practical, high in yield, low in cost, small in investment, free of the three wastes discharge, and particularly applicable to production of medium-sized and small enterprises.
Owner:SHANDONG UNIV
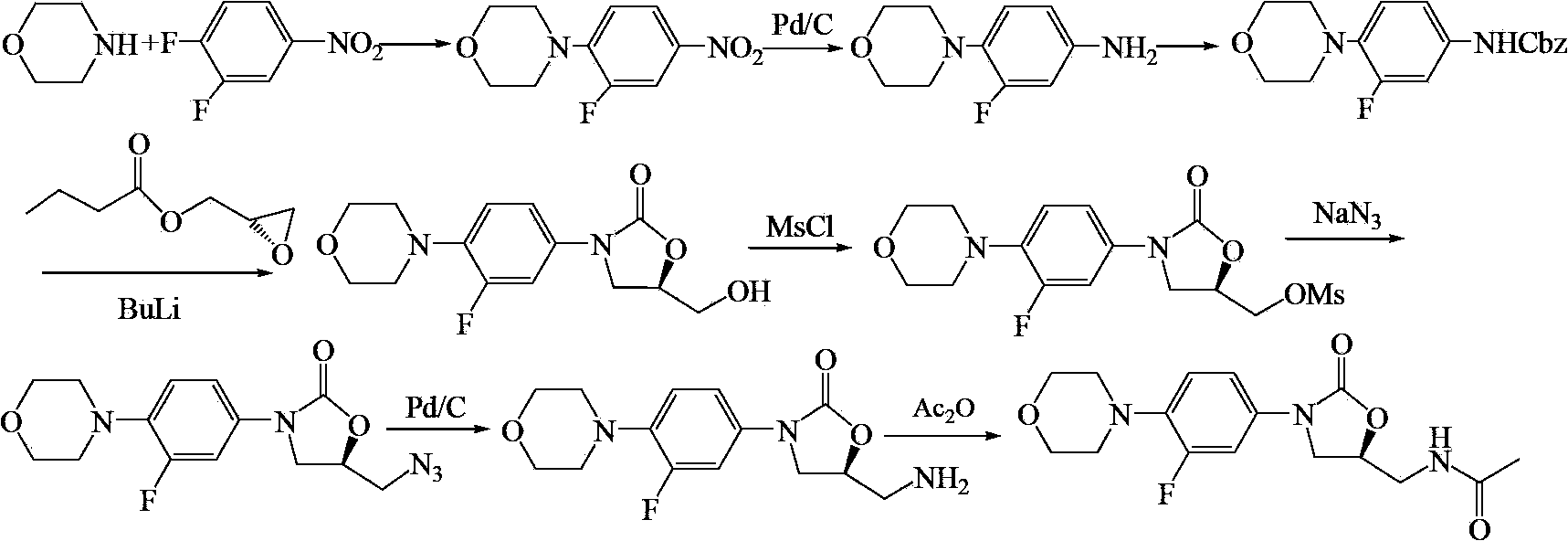
Linezolid preparation method
ActiveCN103420933AReduce the refining processMild conditionsOrganic chemistryMorpholineBenzyl chloroformate
The invention relates to a linezolid (1) preparation method. The method comprises the following steps: reacting a raw material 3,4-difluoronitrobenzene with morpholine, reducing, reacting with benzyl chloroformate to obtain N-benzyloxycarbonyl-3-fluoro-4-morpholinylaniline, carrying out a ring closure reaction of N-benzyloxycarbonyl-3-fluoro-4-morpholinylaniline and (S)-N-(2,3-epoxypropyl)phthalimide, ammonolyzing, and acetylating to obtain linezolid (1).
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing arene beta-amino alcohol of optical voidness
InactiveCN105693555ALow costSimple stepsCarbamic acid derivatives preparationOrganic compound preparationRefluxAlcohol
The invention discloses a method for preparing arene beta-amino alcohol of optical voidness. The method is characterized by comprising the following steps that a D or L-amino acid initial material reacts with benzyl chloroformate CBz-Cl or BOC acid anhydride, and a compound I-1 is obtained; the compound I-1 is subjected to reflux dewatering in solvent A through paraformaldehyde, and a compound I-2 is obtained; the compound I-2 is subjected to a Grignard reagent reaction, dilute hydrochloric acid processing is conducted, and a compound I-3 is obtained; the compound I-3 is subjected to catalytic reduction with aluminium isopropoxide, and the arene beta-amino alcohol of the optical voidness can be obtained. The method aims to overcome defects of the prior art, materials are cheap and easy to obtain, it is beneficial to lower cost, the preparation process is simple, and the obtained intermediate is a medical intermediate stable in structure.
Owner:ZHONGSHAN HAIHONG MEDICINE CO LTD
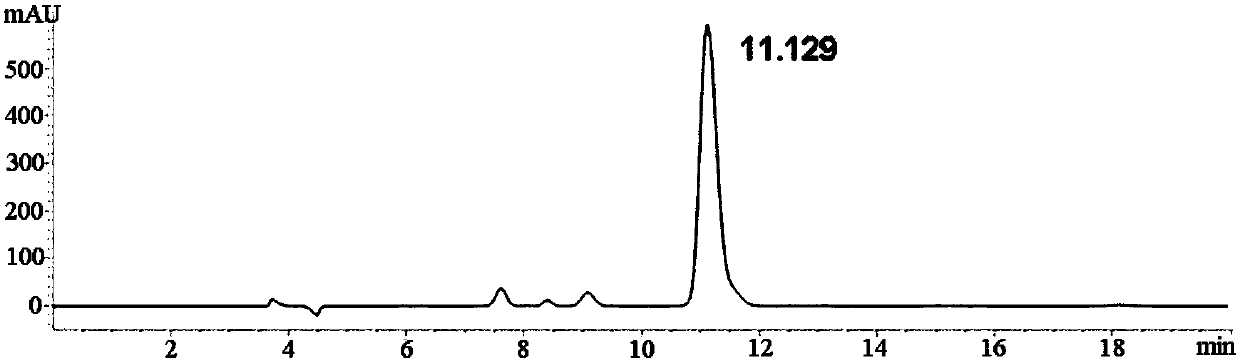
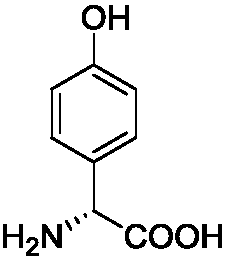
Method for separating and detecting D-p-hydroxyphenylglycine and enantiomer thereof
ActiveCN107941970AEasy to solveLow cost of analysis and detectionComponent separationBenzyl chloroformateEnantiomer
The invention discloses a method for separating and detecting D-p-hydroxyphenylglycine and an enantiomer thereof. The method comprises: step 1, carrying out a derivatization reaction through adoptionof a derivatization reagent and D-p-hydroxyphenylglycine at a certain reaction temperature in a reaction solvent to prepare a derivatized product; and step 2, analyzing the derivatized product by using normal-phase high performance liquid chromatography, and separating and detecting derivatized D-p-hydroxyphenylglycine and an enantiomer thereof. The derivatization reagent is one of di-tert-butyl dicarbonate, 9-fluorenylmethyl chloroformate, and benzyl chloroformate, and the high performance liquid chromatography takes a normal-phase chromatographic column as a separation column. According to the method, D-p-hydroxyphenylglycine and an enantiomer derivative thereof can be efficiently separated, the baseline separation is achieved, and the separation degree is more than 1.5. The baseline issmooth and steady, and the peak pattern is good. The method is great in the specialization and high in detection sensitivity and is beneficial for fast accurately detecting the content of the enantiomer in D-p-hydroxyphenylglycine.
Owner:CHANGZHOU HEQUAN PHARMA CO LTD +1
Preparation method for N-benzyloxycarbonyl-2-amino-1-propanol
InactiveCN110229074AProduct yield is highEasy to makeCarbamic acid derivatives preparationOrganic compound preparationBenzyl chloroformate1-Propanol
The invention discloses a preparation method for N-benzyloxycarbonyl-2-amino-1-propanol. The preparation method comprises the following steps: adding ammonia water into a reactor, adding a catalyst and epoxypropane after cooling, carrying out heating and pressurization, and performing heating reflux to obtain 1-amino-2-propanol; and adding 1-amino-2-propanol and benzyl chloroformate into a solvent-containing mixer to prepare a mixed solution, allowing the mixed solution to undergo a reaction via a microchannel reactor, then carrying out discharging, terminating the reaction and removing low-boiling-point substances via a degassing column so as to obtain N-benzyloxycarbonyl-2-amino-1-propanol. The preparation method of the invention realizes high preparation yield of N-benzyloxycarbonyl-2-amino-1-propanol through two-step operation and is simple in preparation process; and through fast reaction in the microchannel reactor, reaction time is greatly reduced, reaction efficiency is improved, and the conversion rate of raw materials and product yield are enhanced.
Owner:南京博源医药科技有限公司
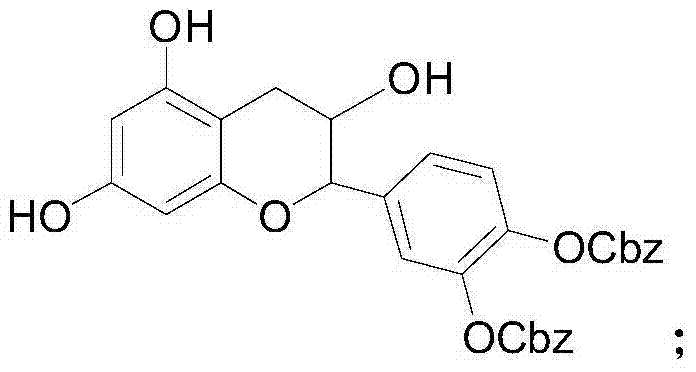
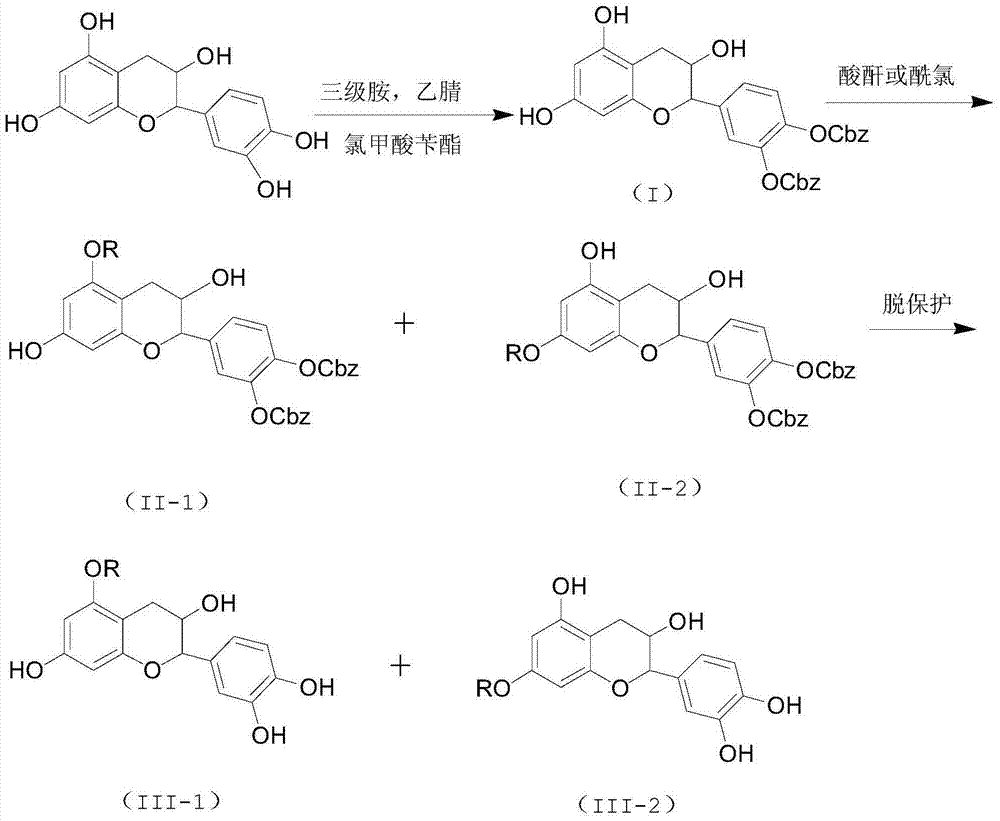
Selective preparation method for 5-site and 7-site ester catechin molecules
The present invention discloses a selective preparation method for 5-site and 7-site ester catechin molecules. The selective preparation method comprises: dissolving catechin in acetonitrile, adding benzyl chloroformate and tertiary amine, reacting to obtain 3',4'-dibenzyloxycarbonyl ester catechin, carrying out an acylation reaction of the 3',4'-dibenzyloxycarbonyl ester catechin in the presence of an acylation agent and the tertiary amine to obtain a mixture of 3',4'-dibenzyloxycarbonyl ester-5-ester catechin and 3',4'-dibenzyloxycarbonyl ester-7-ester catechin, and removing the benzyloxycarbonyl from the 3',4'-dibenzyloxycarbonyl ester-5-ester catechin and the 3',4'-dibenzyloxycarbonyl ester-7-ester catechin to obtain the 5-ester catechin and the 7-ester catechin. According to the present invention, the product obtained by adopting the preparation method has characteristics of high purity, good selectivity and good application prospect; and the preparation method has the simple operation, and is suitable for industrial production application.
Owner:ZHEJIANG UNIV
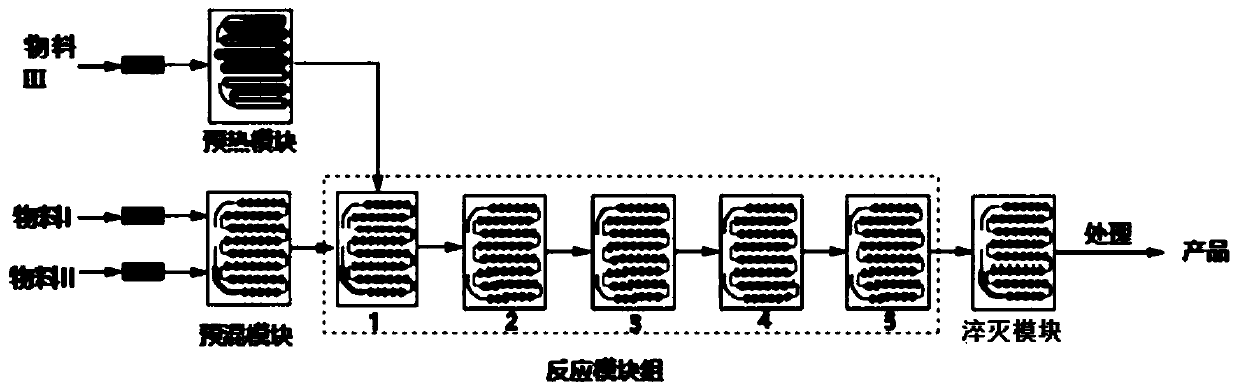
Method for synthesizing ticagrelor intermediate by micro-channel reactor
InactiveCN111574494ALow impurity contentContinuous online mixingOrganic chemistryChemical/physical/physico-chemical microreactorsCyclopenteneBenzyl chloroformate
The invention discloses a method for synthesizing a ticagrelor intermediate through a micro-channel reactor, and belongs to the technical field of cardiovascular and cerebrovascular drug synthesis. The invention aims to solve the problems of low yield, low purity, uncontrollable heat release and the like in the synthesis of an intermediate in a traditional stirring reaction kettle. The method forsynthesizing the ticagrelor intermediate comprises the following steps of: mixing a precursor (3aR, 4S, 6R, 6aS)-6-aminotetrahydro-2, 2-dimethyl-4H-cyclopentene-1, 3-dioxolane-4-ol with an organic solvent to obtain a material I; dissolving alkali required by the reaction in water to obtain a material II; and dissolving benzyl chloroformate in an organic solvent to obtain a material III, preheatingthe materials, conveying the preheated materials to the reaction module group of a micro-channel reactor, carrying out a reaction, and carrying out post-treatment on a reaction product to obtain theticagrelor intermediate. The method has the advantages of short reaction time and greatly improved safety, and is suitable for industrial production of the ticagrelor intermediate.
Owner:HEILONGJIANG XINCHUANG BIOLOGICAL TECH DEV CO LTD
Synthesis method of Fmoc-O-tert-butyl-L-threoninol
InactiveCN106631900AReduce generationMild reaction temperatureCarbamic acid derivatives preparationOrganic compound preparationBenzyl chloroformateReaction temperature
The present invention relates to a synthesis method of Fmoc-O-tert-butyl-L-threoninol, and mainly solves the technical problems that in a process of Fmoc-thr(tbu)-oh reduction by sodium borohydride, the reaction temperature is strictly required and a FMoc protecting group is decomposed, resulting in low yield and high cost. The synthesis method of the invention comprises the following steps: a. L-threonine reacts with thionyl chloride to form L-threonine methyl ester hydrochloride; b. the L-threonine methyl ester hydrochloride under the action of sodium hydroxide is reacted with benzyl chloroformate to produce z-thr-ome; c. the Z-thr-ome reacts with introduced isobutene in the presence of methylene chloride and concentrated sulfuric acid, and alkali adjustment treatment is carried out to obtain z-thr (tbu)-ome; d. in the presence of acetone and water, the Z-thr(tbu)-ome is saponified with added alkali to obtain z-thr(tbu)-oh; e. the Z-thr(tbu)-oh is reduced to z-thr(tbu)-ol by sodium borohydride in tetrahydrofuran; f. the z-thr(tbu)-ol is hydrogenated in methanol to obtain H-thr(tbu)-ol; and g. N-(9-fluorenylmethoxycarbonyloxy)succinimide is added to the H-thr(tbu)-ol to obtain the Fmoc-O-tert-butyl -L-threoninol.
Owner:GL BIOCHEM SHANGHAI
Powerful double-sided adhesive tape
ActiveCN107353839AHigh activityImprove adhesionFilm/foil adhesivesPolyureas/polyurethane adhesivesDispersityBenzyl chloroformate
The invention discloses a powerful double-sided adhesive tape and relates to the field of double-sided adhesive tapes. The powerful double-sided adhesive tape is prepared through uniformly coating both sides of a plastic film base layer with an adhesive, wherein the adhesive is prepared through mixing a nano-montmorillonite suspension, rhizoma dioscoreae surface slime and carbobenzoxy chloride, which are in the mass ratio of (10 to 12): (1 to 2): (0.1 to 0.3), and then, carrying out high-speed dispersion on a polyurethane prepolymer. The powerful double-sided adhesive tape disclosed by the invention has good water resistance and can be used in a long term in wet or water-including environments without adhesion property lowering; and the powerful double-sided adhesive tape is good in stability and dispersity, is pollution-free and odor-free and is free of influence on human health.
Owner:广东硕成科技股份有限公司
Bixalomer intermediate
ActiveCN105111089ARaw materials are cheap and easy to getThe reaction is easy to operateCarbamic acid derivatives preparationOrganic compound preparationBenzyl chloroformateCalcium Binder
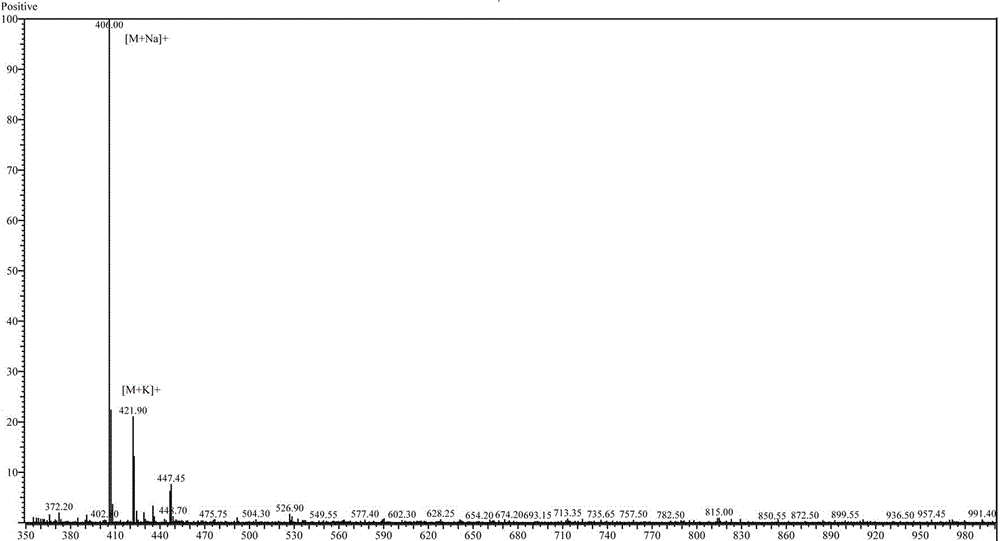
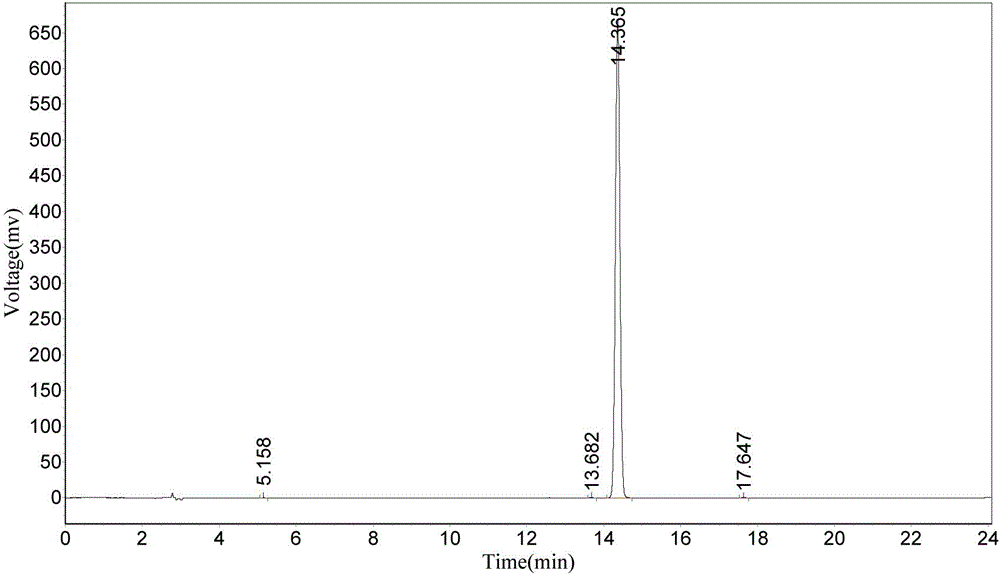
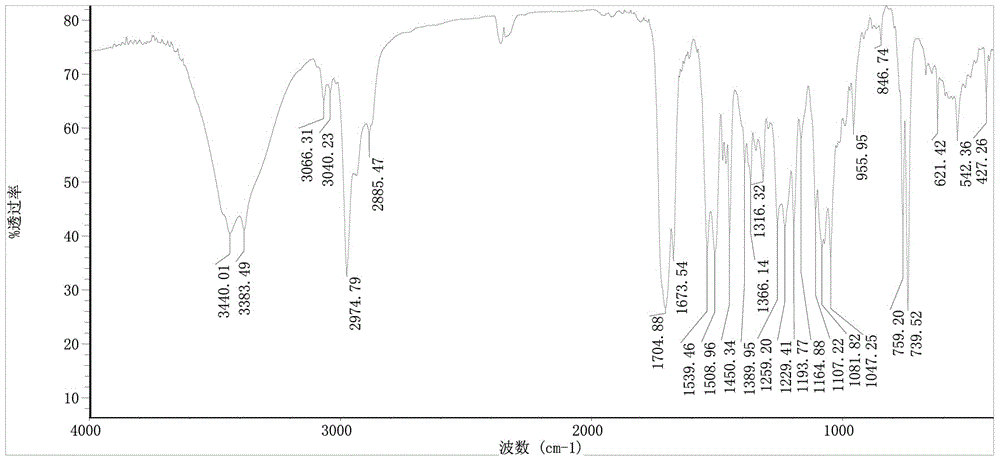
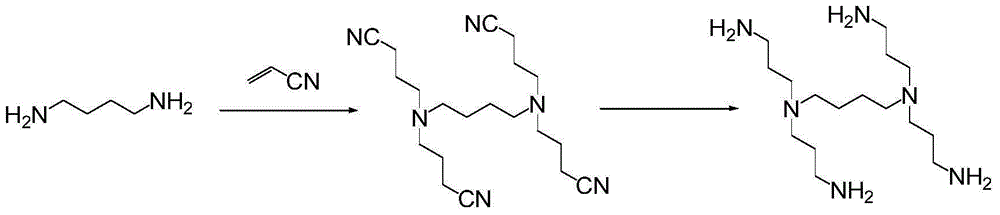
The invention particularly relates to an amine phosphate binder bixalomer intermediate and a preparation method thereof. The preparation method comprises the following steps: reacting a raw material 3,3'-diamidodipropyl amine under the action of benzyl chloroformate to obtain 3,3'-dibenzyloxyamidodipropyl amine, carrying out substitution reaction on the 3,3'-dibenzyloxyamidodipropyl amine and 1,4-dibromobutane or 1,4-dichlorobutane to obtain N,N,N',N'-tetra(3-dibenzyloxyamido)-1,4-butanediamine, and carrying out deprotection by pressure hydrogenation under the action of Pd-C to obtain N,N,N',N'-tetra(3-aminopropyl)-1,4-butanediamine. The method has the advantages of cheap and accessible raw materials, operable reaction, high yield and fewer three wastes, and thus, has excellent industrial prospects.
Owner:NANJING HUAWE MEDICINE TECH DEV
New synthetic method of high-optical activity prolinamide
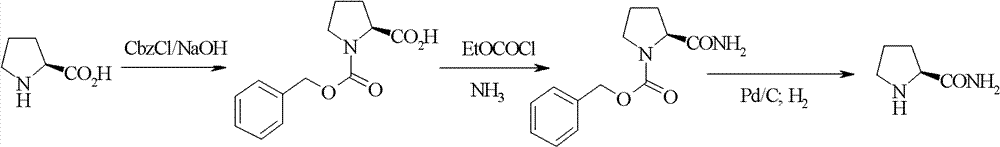
The invention relates to a synthetic method of high-optical chirality prolinamide, wherein the synthetic method has industrial prospect; no racemization is caused in the synthetic process, and only three steps of operations and reactions are carried out in the whole synthetic process; the first step, N-benzyloxycarbonyl proline methyl ester is generated from proline methyl ester and benzyl chloroformate under an alkaline condition; the second step, the N-benzyloxycarbonyl proline methyl ester is exchanged with ammonia in a protonic solvent to generate N-benzyloxycarbonyl prolinamide; the third step, the N-benzyloxycarbonyl prolinamide is oxidized, hydrogenated and deprotected in the protonic solvent, and then chiral prolinamide is obtained. Compared with the prior art, the preparation method provided by the invention has the advantages of low cost, high yield, easiness for realization of industrial operations and the like.
Owner:LIANYUNGANG DUXIANG CHEM +1
Technique for preparing N-carbobenzoxy-5 phenyl-L-cysteine
InactiveCN1660801AImprove product qualitySimple manufacturing processSulfide preparationBenzyl chloroformateAniline
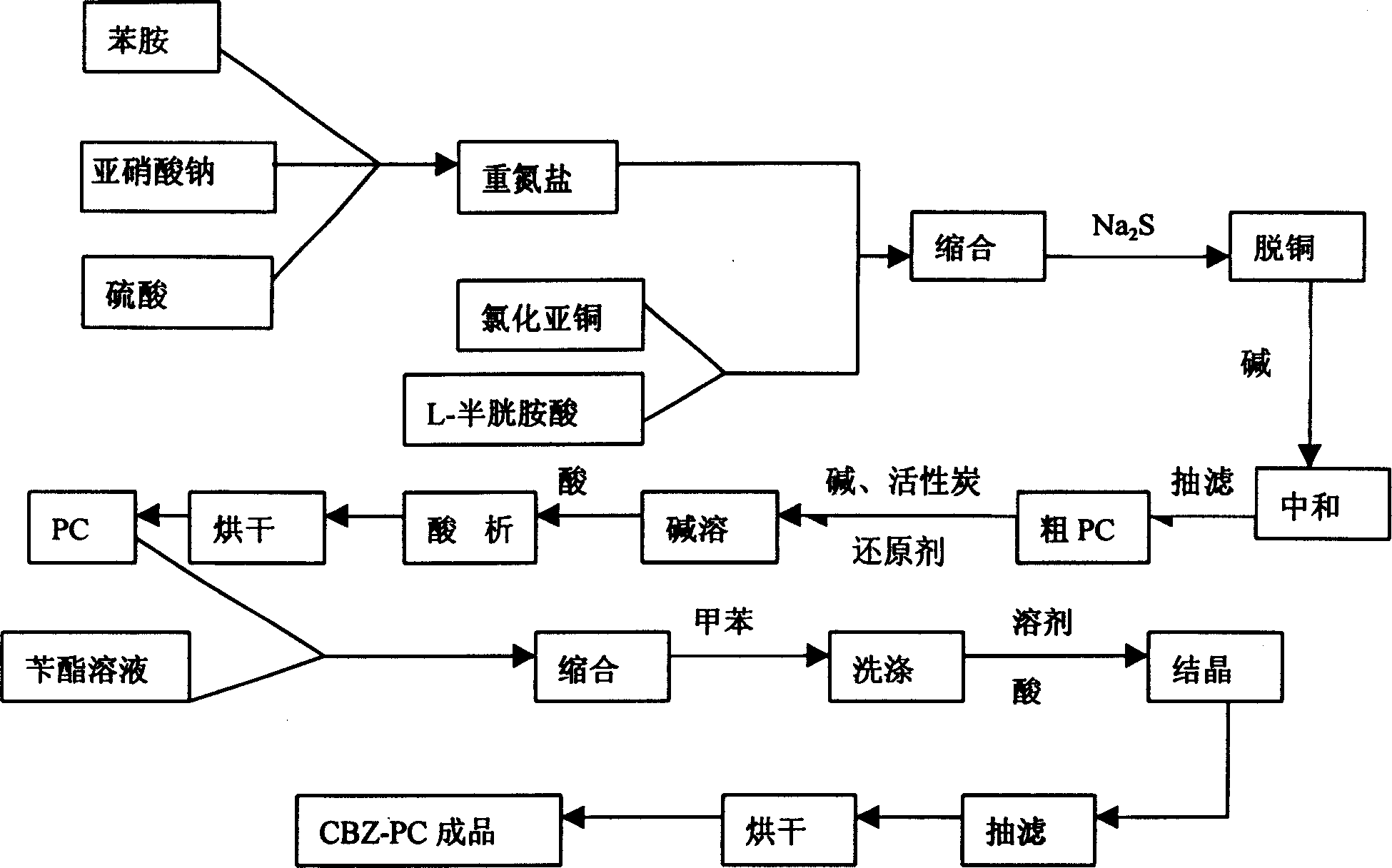
A process for preparing N-benzoxycarbonyl-S-phenyl-L-cysteine includes synthesizing phenylamine diazonium salt from sodium nitrate and phenylamine, reacting on the solution of L-cysteine to obtain coarse S-phenyl-L-cysteine (PC), refining, dissolving, reacting on the solution of benzyl chloroformate, removing solvent, washing to obtain N-benzoxycarbonyl-S-phenyl-L-cysteine, adding solvent, acidifying, filter and baking. It can be used as the basic raw material for preparing anti-AIDS and antineoplastic medicines.
Owner:高玉冠 +1
Preparation method of flame-retardant material for wires and cables
ActiveCN107503122AGuaranteed mechanical propertiesPromote environmental protectionPlastic/resin/waxes insulatorsHeat resistant fibresFiberPolymer dissolution
The invention provides a preparation method of a flame-retardant material for wires and cables. The preparation method comprises steps as follows: benzyl chloroformate modified levodopa as a monomer is subjected to a polymerization reaction, and a modified dopa polymer is prepared; the modified dopa polymer is dissolved in acetone, and a modified dopa polymer solution is prepared; sodium-based montmorillonite is placed in an ethanol solution, a sodium-based montmorillonite dispersion liquid is prepared, and the pH value of the sodium-based montmorillonite dispersion liquid is adjusted to 9.5-10 with an alkaline solution; the sodium-based montmorillonite dispersion liquid treated with a sonifier cell disrupter for 7-9 min; polyimide fibers are soaked in a hydrochloric acid water solution for 1.2-1.5 h and squeezed to be dewatered for later use, and a polyimide fiber substrate is prepared; the sodium-based montmorillonite dispersion liquid and the modified dopa polymer solution are applied to the surface of the polyimide fiber substrate alternately, the surface is dried once every time when the surface is coated, and the flame retardant material for the wires and cables is prepared finally after being dried. The flame-retardant material has the flame retardance, mechanical property and environmental protection property.
Owner:JIANGXI CHENGDA ENG CONSULTING & SUPERVISION +1
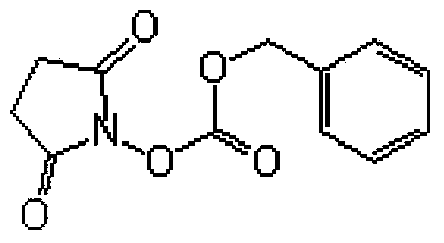
Method for preparing N-(carbobenzoxy) succinimide
The invention relates to the field of refined chemical, and in particular relates to a method for preparing N-(carbobenzoxy) succinimide. The method comprises the following steps: (1) feeding N-hydroxy succinimide, an alkali water solution and an organic solvent which is not mutually dissolved with water into a reaction device, (2) dropwise adding benzyl chloroformate at 0-60 DEG C and then further reacting for 1 hour, (3) stopping stirring when the reaction stops, standing for 1-2 hours so that the water phase at the upper layer and the water phase at the lower layer are separated; then discharging the water phase at the lower layer; adding petroleum ether after the organic phase is separated out; stirring for 1 / 2-1 hour at the room temperature to separate out a solid product, and (4) filtering to obtain a wet product, and drying to obtain the product, namely the N-(carbobenzoxy) succinimide. The technical scheme of the invention has the beneficial effects that the N-(carbobenzoxy) succinimide is produced by using a one-pot method, the operation is simple, the reaction yield is high, the product, namelyN-(carbobenzoxy) succinimide has high purity and is applicable to industrial production.
Owner:GENCHEM & GENPHARM CHANGZHOU CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com