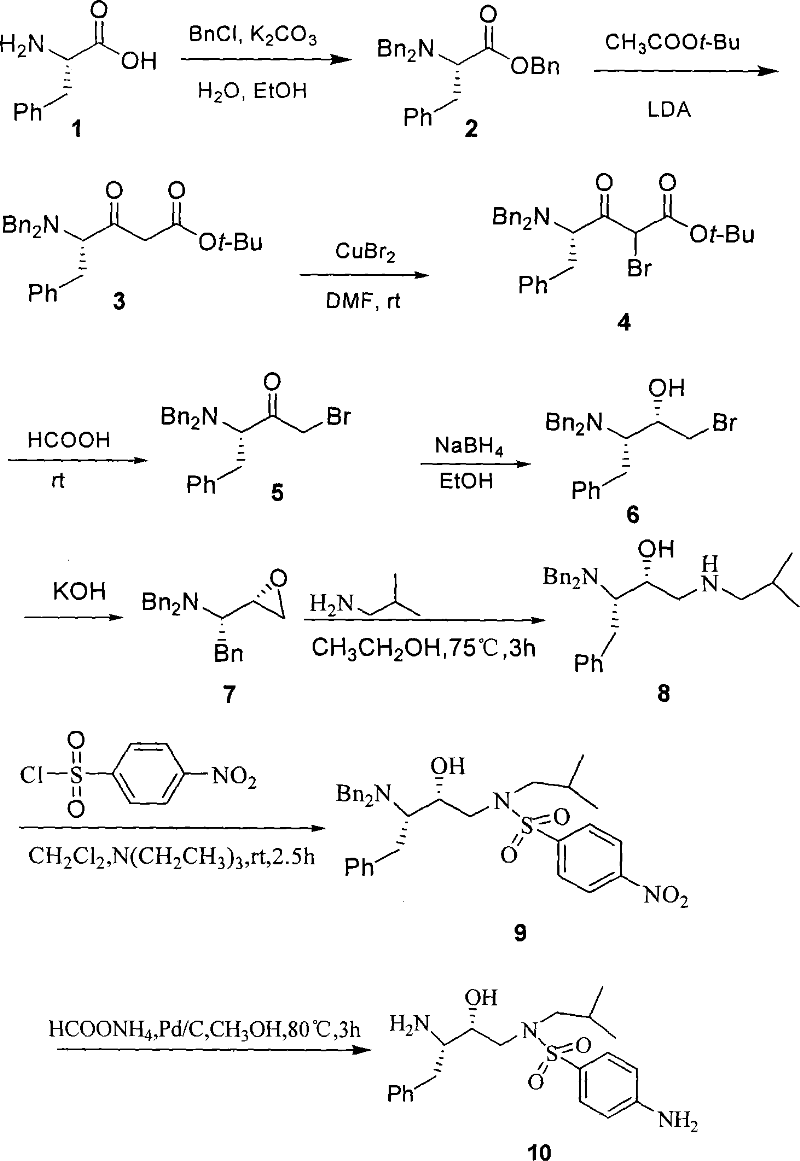
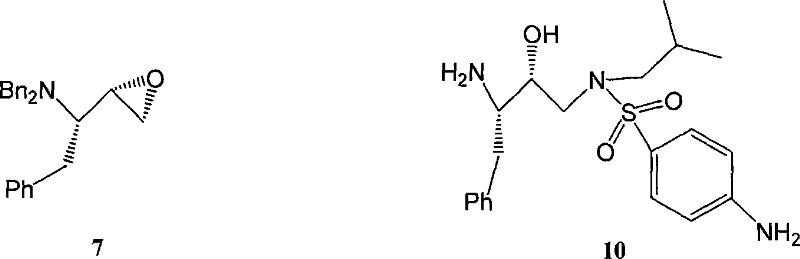
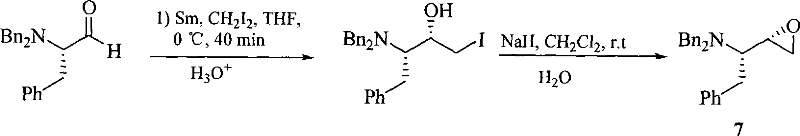
Method for synthesizing anti-aids drug amprenavir intermediate
An anti-AIDS, intermediate technology, applied in the direction of organic chemistry, can solve the problem of high cost, achieve the effect of high yield, safe operation and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Step 1 (S)-2-(Dibenzylamino)-3-phenyl-propionic acid benzyl ester 2
[0036] L-phenylalanine 1 (20.0g, 122.7mmol), K 2 CO 3 (60.0g, 606mmol), H 2 The mixture of O (90 mL), EtOH (45 mL), and BnCl (50.0 g, 393.7 mmol) was heated to 90° C., and the reaction was stopped after 15 h. After the reaction, the aqueous layer was removed, and 100 mL of n-hexane was added to the organic layer, washed with 500 mL of water, dried, filtered, and spin-dried to obtain light yellow liquid compound 2 (51.2 g, 96%). 1 H-NMR (CDCl 3 , 500MHz): δ3.20 (dd, J=8.4, 14.4Hz, 2H, PhCH 2 C), 3.60 (d, J=15.0Hz, 2H, 2PhCHaHbN), 3.80 (dd, J=8.5, 8.5Hz, 1H, NCH), 4.00 (d, J=15.0Hz, 2H, 2PhCHaHbN), 5.20(d , J=13.5Hz, 1H, PhCHaHbO), 5.30 (d, J=13.5Hz, 1H, PhCHaHbO), 7.50~7.00 (m, 20H, 4PhH) ppm. 13 C-NMR (CDCl 3 , 125MHz): δ35.6.54.3, 62.3, 66.0, 126.2, 126.9, 128.1, 128.2, 128.4, 128.5, 128.6, 129.4, 135.9, 138.0, 139.2, 172.0ppm.MS (ESI, m / z): 436.0 ( M H + ).
[0037] Step 2 (S)-tert-butyl 4...
Embodiment 2
[0054] Step 1 (S)-2-(Dibenzylamino)-3-phenyl-propionic acid benzyl ester 2
[0055] L-phenylalanine (50.0g, 302.1mmol), NaOH (24.2g, 605.0mmol) and K 2 CO 3 Dissolve in 500ml of water, heat to 97°C, slowly add BnBr (219.0ml, 1210mmol) dropwise to the above solution, react at this temperature for 10h under nitrogen protection, add toluene (2×250ml) after the reaction is complete, The organic layer was washed with water and saturated brine successively, dried, filtered, and spin-dried to obtain light yellow liquid compound 2 (124.8 g, 95%).
[0056] Step 2 (S)-tert-butyl 4-(dibenzylamino)-3-oxo-5-phenylpentanoate 3
[0057] With embodiment 1.
[0058] Step 3 (S)-tert-butyl 4-(dibenzylamino)-2-bromo-3-oxo-5-phenylpentanoate 4
[0059] Copper bromide (0.9g, 4.0mmol), compound 3 (0.44g, 1.0mmol) and Et 3 N (1.7mL, 1.2g, 12mmol) was dissolved in ethyl acetate (4mL), and the reaction was stopped after stirring for 40h at room temperature. After the reaction, water (10 mL) was a...
Embodiment 3
[0073] Step 1 (S)-2-(Dibenzylamino)-3-phenyl-propionic acid benzyl ester 2
[0074] With embodiment 1.
[0075] Step 2 (S)-tert-butyl 4-(dibenzylamino)-3-oxo-5-phenylpentanoate 3
[0076] With embodiment 1.
[0077] Step 3 (S)-tert-butyl 4-(dibenzylamino)-2-bromo-3-oxo-5-phenylpentanoate 4
[0078] With embodiment 1.
[0079] Step 4 (S)-1-bromo-3-(dibenzylamino)-4-phenyl-2-butanone 5
[0080] Dissolve (S)-4-(dibenzylamino)-2-bromo-3-oxo-5-phenylpentanoic acid tert-butyl ester 4 solution (0.522g, 1mmol) in dilute sulfuric acid (4mL, 15% ), and stop the reaction after stirring at room temperature for 15h. After the reaction, 10 mL of water was added to the system and extracted with EtOAc (3×10 mL), the organic layers were combined, dried, filtered, and spin-dried to obtain white solid 5 (0.23 g, 56%).
[0081] Step 5 (2S,3S)-1-bromo-3-(dibenzylamino)-4-phenyl-2-butanol 6
[0082] Put (S)-1-bromo-3-(dibenzylamino)-4-phenyl-2-butanone 5 solution (4.22g, 10mmol, in 20mL EtOH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com