Selective preparation method for 5-site and 7-site ester catechin molecules
A technology of molecular selectivity and ester-based catechin, applied in organic chemistry and other fields, can solve the problem of no esterification, etc., and achieve good selectivity, simple operation, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
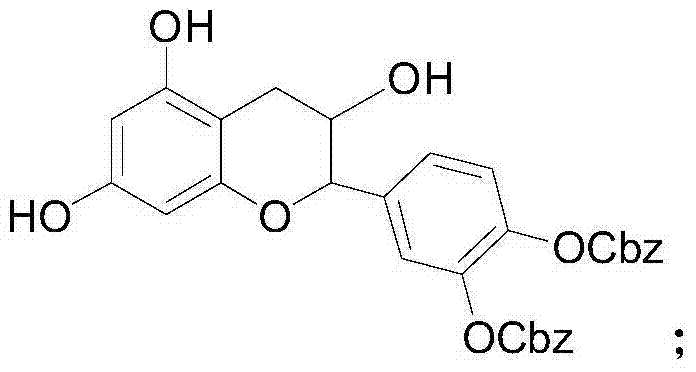
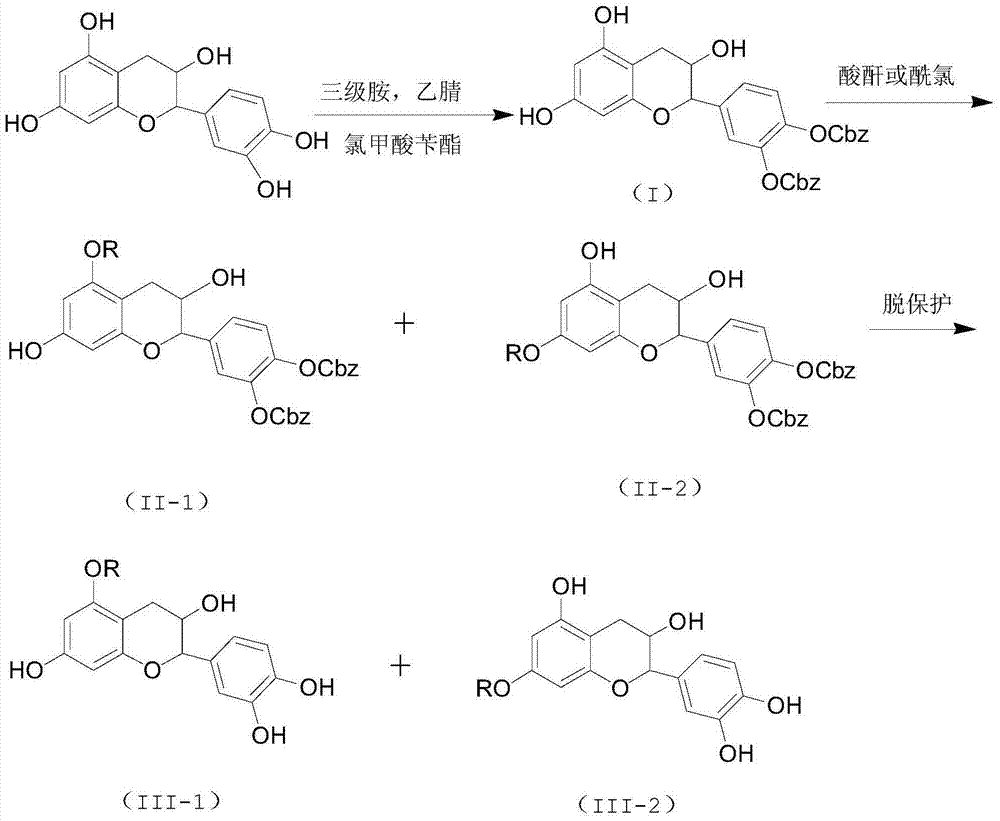
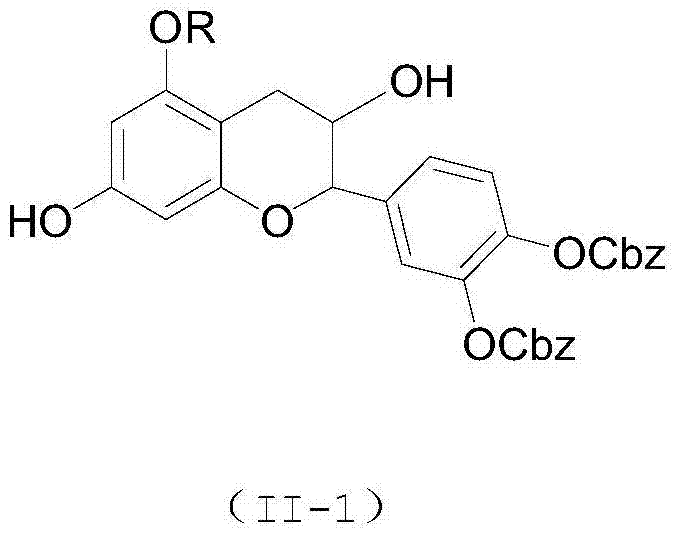
[0038] Catechin (29 mg), benzyl chloroformate (40 mg) and triethylamine (30 mg) were stirred and reacted at room temperature for 2 hours, extracted with ethyl acetate after the reaction, and purified by column chromatography to obtain 3',4 '-Dibenzyloxycarbonyl ester catechin, 3',4'-dibenzyloxy carbonyl ester catechin was dissolved in an organic solvent, added lauryl chloride (22mg) and triethylamine (15mg) at room temperature The reaction was stirred for 2 hours. After the reaction was completed, it was extracted with ethyl acetate and purified by column chromatography to obtain 3',4'-dibenzyloxycarbonyl-5-dodecanoyl catechin and 3' ',4'-dibenzyloxycarbocarboxy-7-dodecanoyl catechin catechin mixture. Then dissolve in methanol, add 5% palladium carbon (11 mg) and triethylsilane (120 mg), stir and react at room temperature for 12 hours, extract with ethyl acetate after the reaction is completed, and obtain 5- Lauryl catechin (yield: 15%, eluent is chloroform methanol mixed sol...
Embodiment 2
[0043] Catechin (29 mg), benzyl chloroformate (34 mg) and tripropylamine (36 mg) were stirred at room temperature for 2 hours, extracted with ethyl acetate after the reaction, and purified by chromatography to obtain 3',4'-di Benzyloxycarbonyl ester catechins. Dissolve 3',4'-dibenzyloxycarbonyl ester catechin in an organic solvent, add acetic anhydride (11mg) and N,N-diisopropylethylamine (15mg) and stir at room temperature for 2 hours. After extraction with ethyl acetate, after purification by column chromatography, 3',4'-dibenzyloxycarbonyl-5-acetyl catechin and 3',4'-dibenzyloxycarbonyl- 7-Acetoxylate catechin mixture. Then it was dissolved in methanol, 5% palladium carbon (1.4 mg) and triethylsilane (120 mg) were added, and the reaction was stirred at room temperature for 12 hours. After the reaction was completed, it was extracted with ethyl acetate, and purified by column chromatography to obtain 5 - Acetyl ester catechin (yield: 11%, the eluent is a mixed solvent of c...
Embodiment 3
[0045] Catechin (29mg), benzyl chloroformate (40mg) and N,N-diisopropylethylamine (33mg) were stirred and reacted at room temperature for 2 hours, extracted with ethyl acetate after the reaction was completed, and purified by chromatography The 3',4'-dibenzyloxycarbonyl ester catechin was obtained. Dissolve 3',4'-dibenzyloxycarbonyl ester catechin in an organic solvent, add acetic anhydride (11 mg) and tripropylamine (17 mg) and stir at room temperature for 2 hours, and extract with ethyl acetate after the reaction is complete. After purification by column chromatography, 3',4'-dibenzyloxycarbonyl-5-acetoyl catechin and 3',4'-dibenzyloxycarbonyl-7-acetyl catechin were obtained mixture. Then it was dissolved in methanol, 5% palladium carbon (1.4 mg) and triethylsilane (120 mg) were added, and the reaction was stirred at room temperature for 12 hours. After the reaction was completed, it was extracted with ethyl acetate, and purified by column chromatography to obtain 5 - Acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com