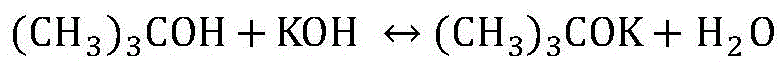Patents
Literature
307 results about "Potassium tert-butoxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Potassium tert-butoxide is the chemical compound with the formula K⁺(CH₃)₃CO⁻. This colourless solid is a strong base (pKa of conjugate acid around 17), which is useful in organic synthesis. It exists as a tetrameric cubane-type cluster. It is often seen written in chemical literature as potassium t-butoxide. The compound is often depicted as a salt, and it often behaves as such, but it is not ionized in solution.
Poly-beta-peptides from functionalized beta-lactam monomers and antibacterial compositions containing same
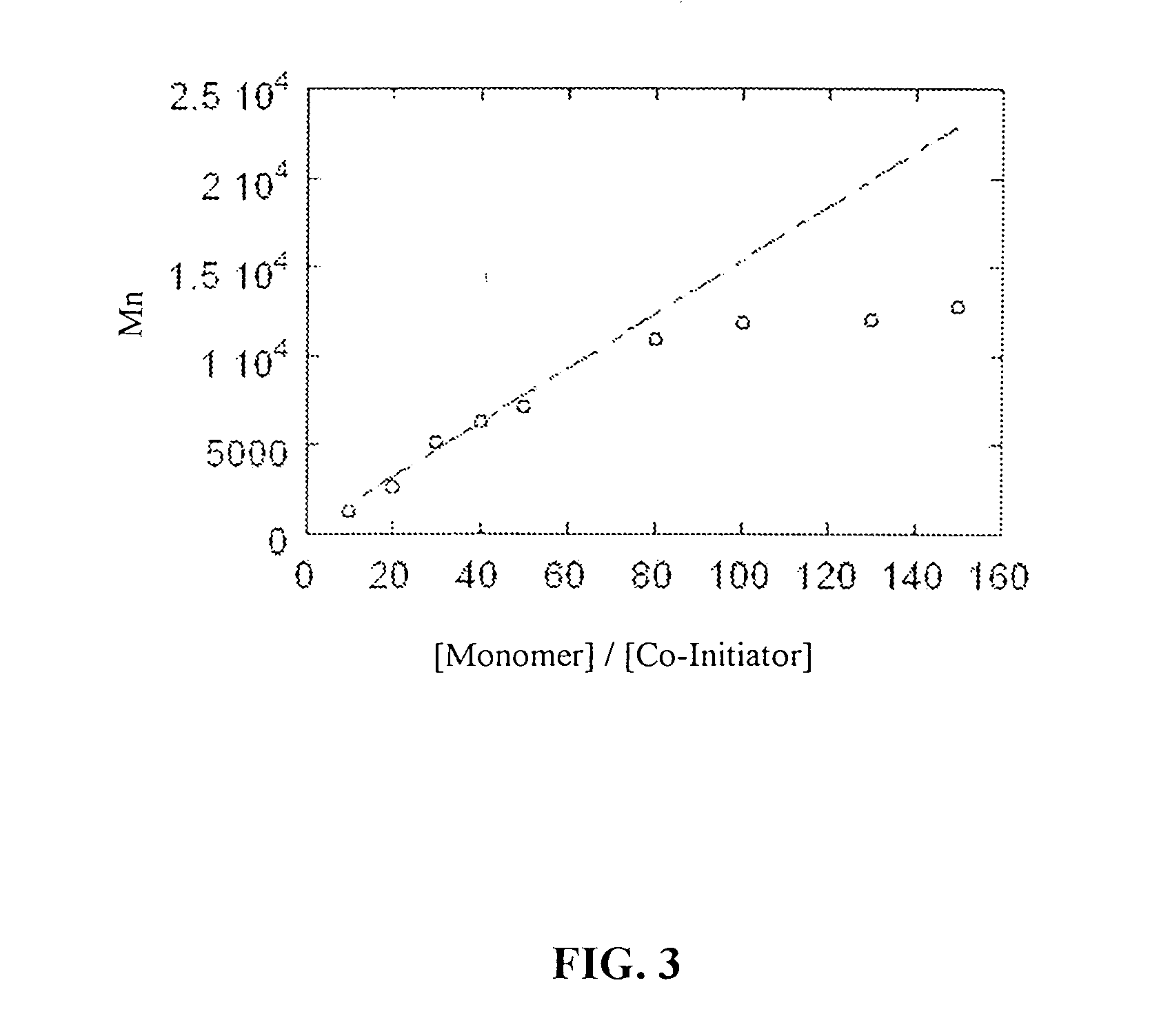
InactiveUS20070087404A1Control over polymerization conditionLarge molecular weightAntibacterial agentsPeptide/protein ingredientsMonomerTetrahydrofuran
Disclosed is a method of making β-polypeptides. The method includes polymerizing β-lactam-containing monomers in the presence of a base initiator and a co-initiator which is not a metal-containing molecule to yield the product β-polypeptides. Specifically disclosed are methods wherein the base initiator is potassium t-butoxide, lithium bis(trimethylsilyl)amide (LiN(TMS)2), potassium bis(trimethyl-silyl)amide, and sodium ethoxide, and the reaction is carried out in a solvent such as chloroform, dichloromethane, dimethylsulfoxide, or tetrahydrofuran.
Owner:WISCONSIN ALUMNI RES FOUND
Solid basic catalyst, preparation method of solid basic catalyst and application of solid basic catalyst in ester exchange reaction
InactiveCN102698811AImprove stabilityImprove catalytic performanceOrganic-compounds/hydrides/coordination-complexes catalystsPreparation from organic carbonatesOrganic baseSilicon oxide
The invention discloses a solid basic catalyst, a preparation method of the solid basic catalyst and an application of the solid basic catalyst in ester exchange reaction. The solid basic catalyst catalyzes the ester exchange reaction by the reaction of metal organic compound and hydroxyl on a carrier under the moderate conditions to synthesize dimethyl carbonate. The solid basic catalyst provided by the invention consists of metal organic alkali and the carrier. The metal organic alkali is linked to the carrier in bond-forming manner; the metal organic alkali is one or more of lithium methoxide, lithium ethoxide, lithium isopropoxide, lithium n-butoxide, lithium tert-butoxide, sodium methoxide, sodium ethoxide, sodium isopropoxide, sodium n-butoxide, sodium tert-butoxide, potassium methoxide, potassium ethoxide, potassium isopropoxide, potassium n-butoxide and potassium tert-butoxide; the carrier is one or more of silicon oxide, aluminium oxide, titanium oxide, zirconia, mesoporous silicon oxide synthesized by the template method, mesoporous aluminium oxide synthesized by the template method, mesoporous titanium oxide synthesized by the template method, and mesoporous zirconia synthesized by the template method.
Owner:NANJING UNIV OF TECH
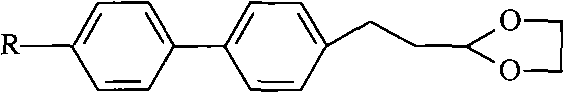
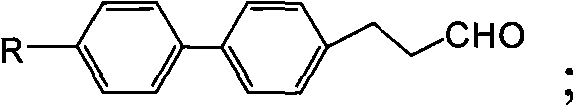
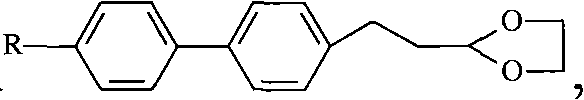
Method for synthesizing (trans)-4-alkyl-3-alkene biphenyl derivative monomer liquid crystals
ActiveCN102010287AReduce usageMild reaction conditionsLiquid crystal compositionsHydrocarbon by isomerisationSolventMonomer
The invention discloses a method for synthesizing (trans)-4-alkyl-3-alkene biphenyl derivative monomer liquid crystals, and belongs to the field of the preparation of monomer liquid crystal materials. The method comprises the following steps of: performing condensation of para-bromo cinnamaldehyde or 3-(4-bromo-phenyl)-propionaldehyde serving as a raw material and alcohol to prepare acetal, and performing a cross-coupling reaction of the acetal and 4-alkylbenzene borate in solvent under the catalytic action of palladium to obtain a biphenyl intermediate; and removing protective group alcohol of the biphenyl intermediate to produce an intermediate, performing a witting reaction of the intermediate and alkyltriphenylphosphonium halide in the presence of potassium tert-butoxide to produce a (cis, trans)-4-alkyl-3-alkene biphenyl derivative, and performing the inversion of cis and trans configuration to obtain the (trans)-4-alkyl-3-alkene biphenyl derivative monomer liquid crystals. The method has the advantages of low cost, high quality of products and mild reaction condition, and the expanded production is easy to perform. Therefore, the method is particularly suitable for the industrial production of the (trans)-4-alkyl-3-alkene biphenyl derivative monomer liquid crystals.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
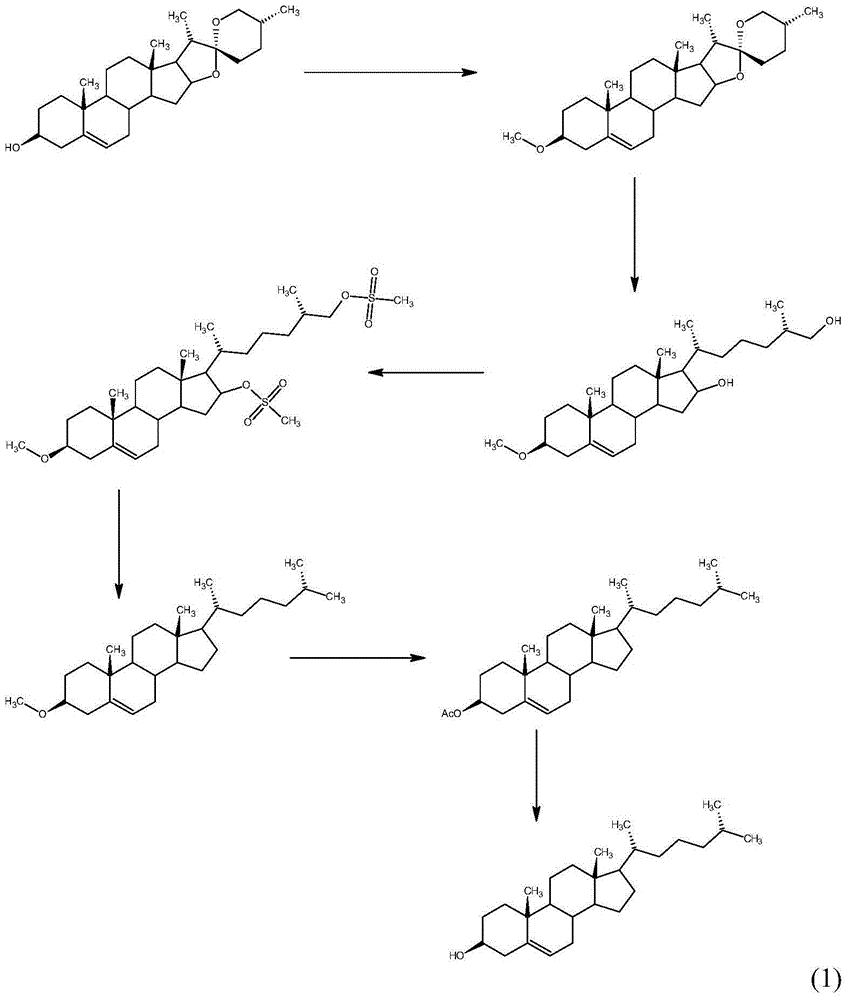
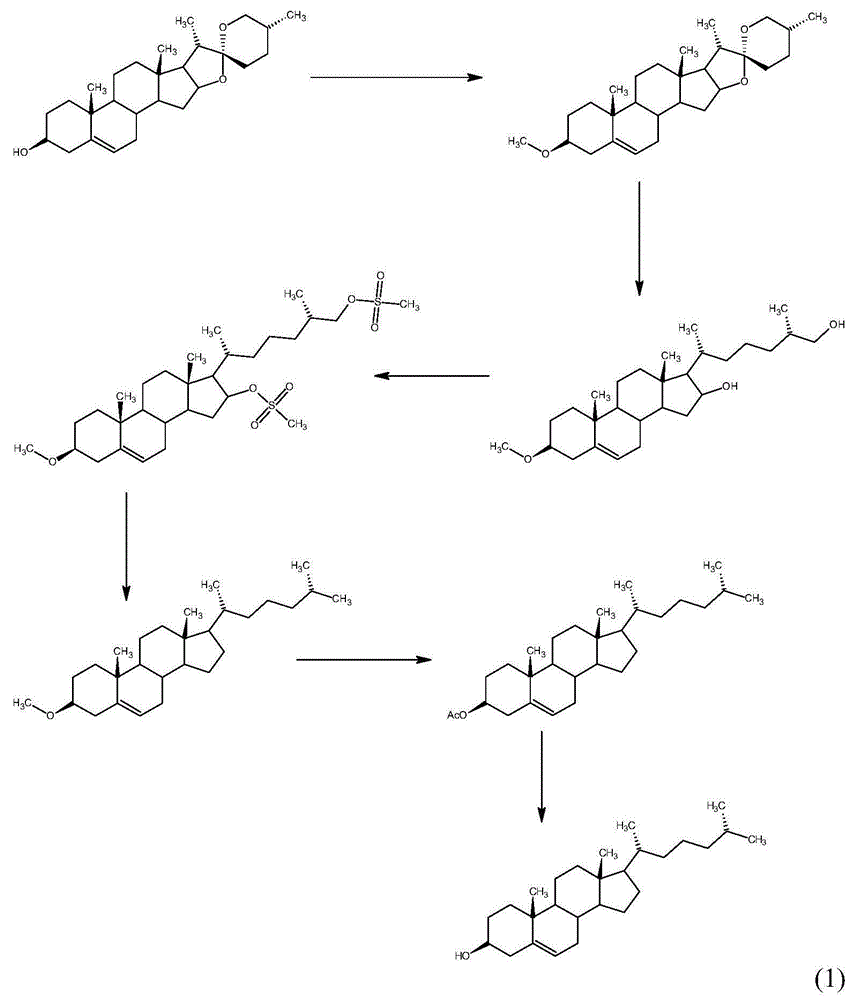
Method for synthesizing cholesterol by using stigmasterol degradation products as raw materials
The invention provides a method for synthesizing cholesterol by using stigmasterol degradation products as raw materials. The method comprises the following steps: 1) performing an etherification reaction on 3-carbonyl-4-pregnene-22-aldehyde and triethyl orthoformate to obtain 3-ethyoxyl-3,5-pregnadiene-22-aldehyde; 2) preparing a 3-methylbutyltriphenyl phosphonium chloride solution; 3) adding potassium tert-butoxide into the 3-methylbutyltriphenyl phosphonium chloride solution, performing a wittig reaction on the 3-methylbutyltriphenyl phosphonium chloride solution and the 3-ethyoxyl-3,5-pregnadiene-22-aldehyde to obtain 3-ethyoxyl-3,5,22-triene cholestane; 4) catalyzing the 3-ethyoxyl-3,5,22-triene cholestane to perform a selective hydrogenation reaction to obtain 3-ethyoxyl-5-ene cholestane; 5) performing a reaction on the 3-ethyoxyl-5-ene cholestane and acetic anhydride to obtain 3-acetyl-5-ene cholestane; 6) performing a hydrolysis reaction on the 3-acetyl-5-ene cholestane to obtain the cholesterol. The synthesizing method is simple in process, and the mole yield of the cholesterol exceeds 85 percent by using the stigmasterol degradation products which are cheap and easily obtained as the raw materials; the production cost is low, the process is environmentally friendly, and the method is economical and environmentally friendly, and facilitates industrial implementation.
Owner:HUNAN KEREY BIOTECH
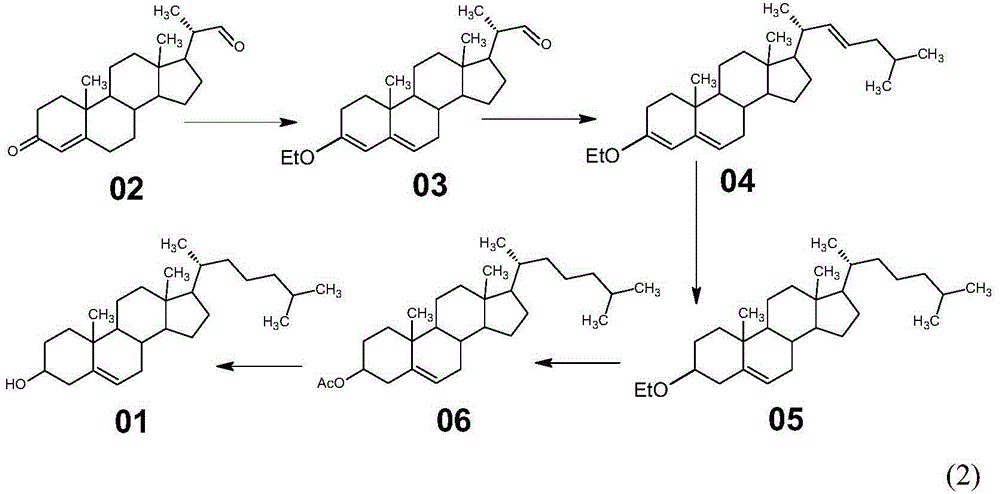
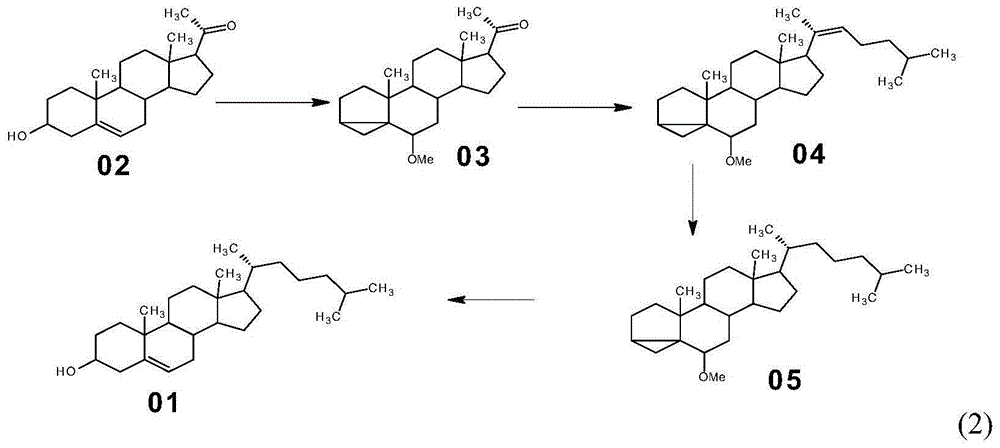
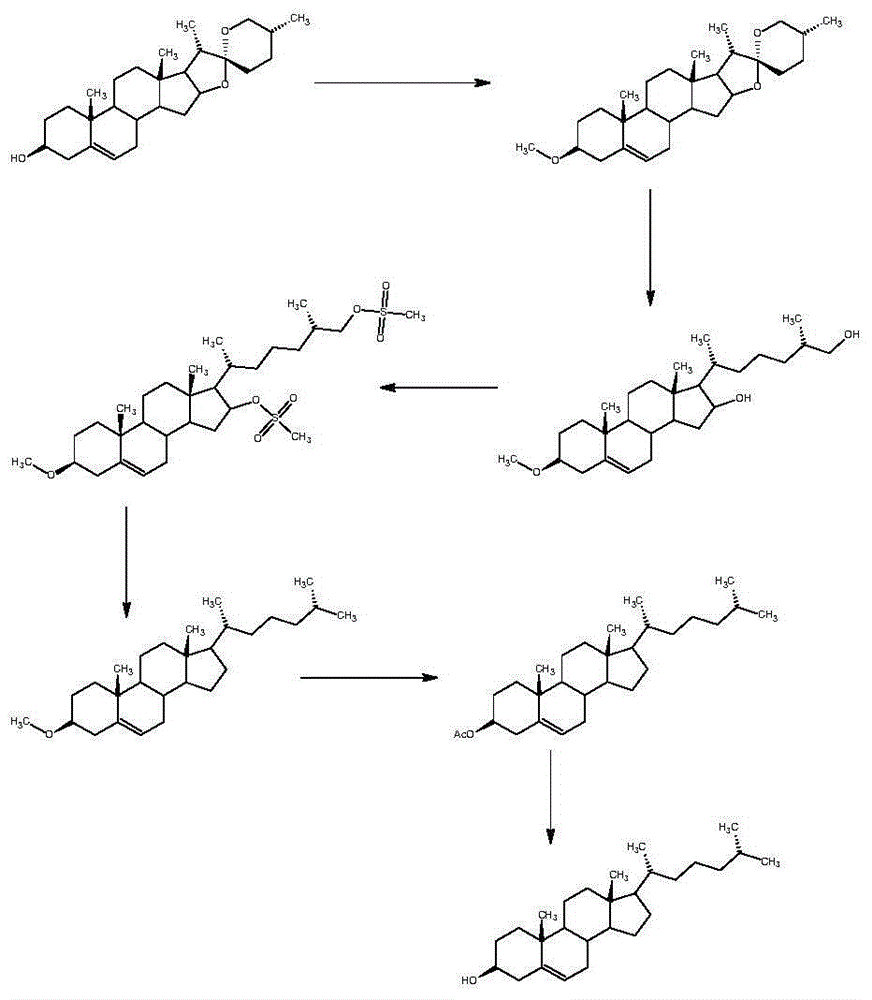
Method for synthesizing cholesterol by using pregnenolone as raw material
The invention provides a method for synthesizing cholesterol by using pregnenolone as a raw material. The method comprises the following steps: 1) adding potassium acetate into methyl alcohol, and performing a reaction on sulfonate to obtain 6-methoxyl-3,5-cyclo-5alpha-pregn-20-one; 2) performing a reaction on triphenylphosphine and 1-chloro-4-methylpentane in an aprotic solvent to obtain a 4-methylbutyltriphenyl phosphonium chloride solution; 3) adding potassium tert-butoxide into the 4-methylbutyltriphenyl phosphonium chloride solution, and performing a wittig reaction; 4) under the catalysis of a rhodium catalyst, performing an asymmetric hydrogenation reaction to obtain 6-methoxyl-3,5-cyclo-5alpha-cholestane; 5) performing a catalytic hydrolysis deprotection reaction by using sulfuric acid to obtain the cholesterol. The method provided by the invention has the advantages that six-step reactions in the conventional method are simplified into four-step reactions, and a ring-opening reaction in which a great number of hydrochloric acid and a large number of zinc powder are consumed in a route of using saponin as an initial raw material. The synthesizing method is simple in process, the consumption of the raw material and auxiliary materials is low, and the mole yield is high; the method is economical and environmentally friendly, and facilitates industrial implementation.
Owner:HUNAN KEREY BIOTECH
Synthesis method for pyridone
InactiveCN104030972AOptimize the synthetic routeReduce manufacturing costOrganic chemistrySynthesis methodsMorpholine
The invention discloses a synthesis method for pyridone, and relates to the technical field of synthesis of a heterocyclic compound containing three heterocyclic rings one of which takes nitrogen and oxygen as the only one heteroatom. The synthesis method comprises the following steps: reacting tetrahydrofuran, ursol, triethylamine and 5-chlorin valeryl chloride which are taken as raw materials, and after the reaction, adding potassium tert-butoxide for reacting again, thereby obtaining a monomer 1 after reaction; reacting the monomer 1 with phosphorus pentachloride in a dichloromethane solvent, thereby obtaining a monomer 2 after reaction; reacting the monomer 2 with morpholine to obtain a final product I. The synthesis method has the advantages that the raw materials are cheap and easily available, and the reaction process is greatly shortened in comparison with that of the prior art, and the synthesis method is mild and safe in reaction conditions, good in reaction reproducibility, low in cost, and high in efficiency, and has simple and easy operations in the reaction.
Owner:河北序能生物技术有限公司
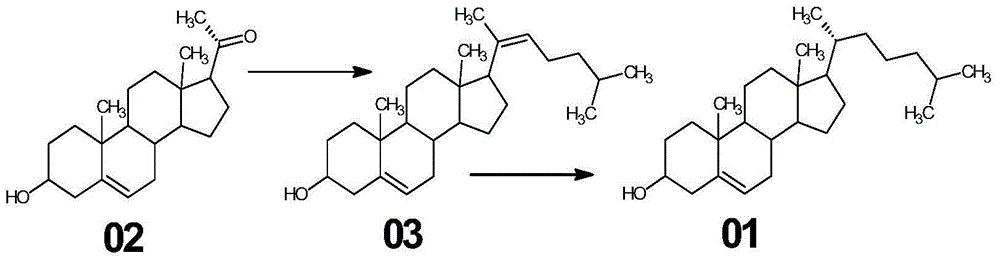
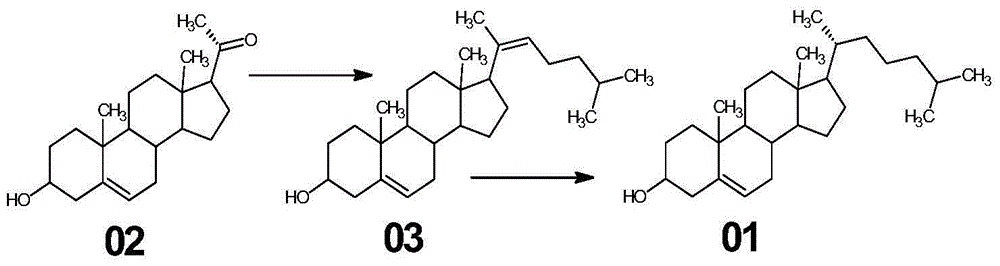
Synthetic method of cholesterol
InactiveCN104961788AReduce consumptionOmit ring opening reactionSteroidsCholesterolTriphenyl phosphonium
The invention relates to a synthetic method of cholesterol. The synthetic method of the cholesterol specifically comprises the following steps that 1, firstly, triphenylphosphine and 1-chlorine-4-methylpentane are used to react to obtain a 4-methyl pentyl triphenyl phosphonium chloride solution, then potassium tert-butoxide or sodium tert-butoxide is added to react to obtain a witting reagent, a witting reaction occurs by adding pregnenolone into the witting regent, and a compound 03 is obtained through extracting after complete reaction; 2, under the action of chirality phosphine ligand and a rhodium catalyst, an asymmetric hydrogenation reaction occurs to the compound to obtain the cholesterol. According to the synthetic method of the cholesterol, a 6-step reaction of an existing method is simplified into a 2-step reaction, and meanwhile the ring-opening reaction consuming a large amount of hydrochloric acid and zinc powder is omitted. The technology is simple, the consumption of raw materials is less, the yield is high, the cost is low, environmental protection is achieved, so that the economical effect and the environmental protection are achieved, and the industrial implementation is facilitated.
Owner:HUNAN KEREY BIOTECH
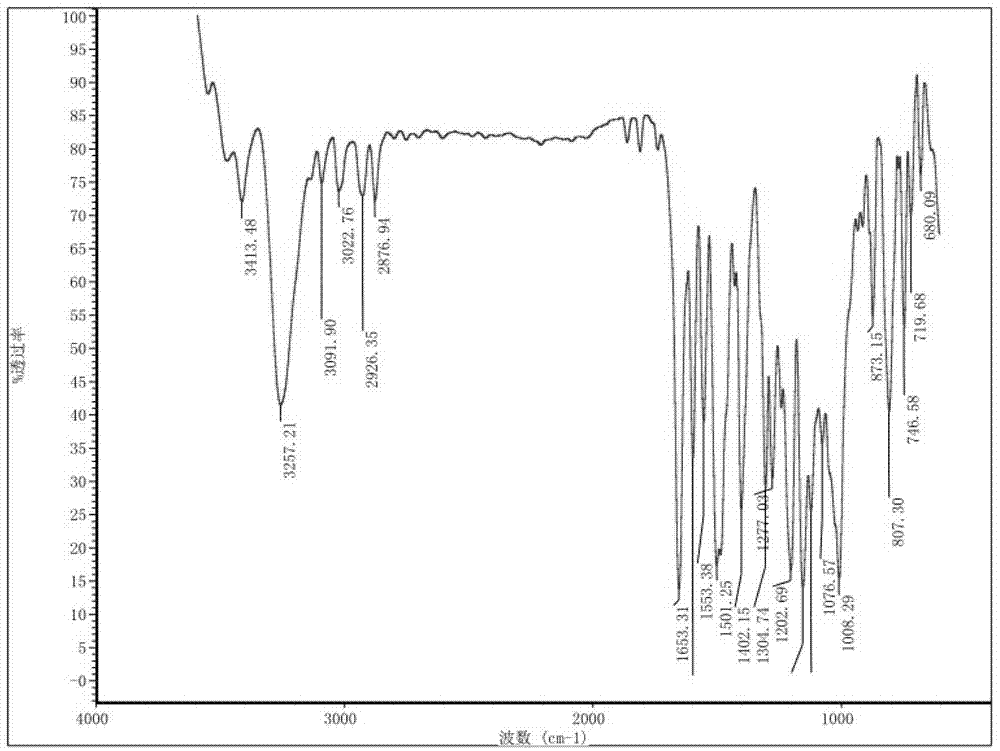
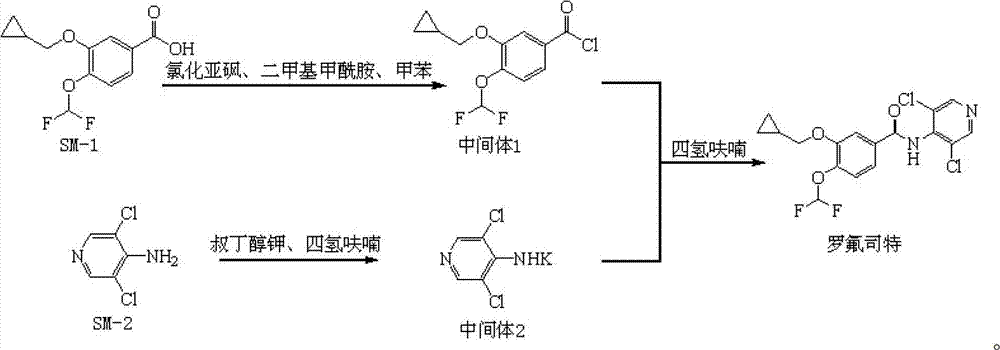
Preparation method and detection method of roflumilast material
ActiveCN102964297ASimple preparation processReduce adverse reactionsOrganic chemistryMaterial analysis by observing effect on chemical indicatorBenzoic acidDisease
The invention discloses a preparation method and a detection method of a roflumilast material. The preparation method comprises the following steps: mixing 3-cyclopropyl methoxy group-4-difluoro methoxy group benzoic acid SM-1, thionyl chloride, dimethyl formamide with toluene, and carrying out an acylating chlorination reaction to obtain a midbody 1; mixing 3,5-dichloro-4-aminopyridine SM-2, tetrahydrofuran with potassium tert-butoxide and carrying out a salt forming reaction to obtain tetrahydrofuran solution of a midbody 2; and then mixing the midbody 1 and the midbody 2 with tetrahydrofuran, carrying out amidation to obtain a crude product of roflumilast, and refining the crude product of roflumilast to prepare the roflumilast material. Aiming to overcome the shortage of the prior art, the preparation process of the roflumilast material is optimized, so that the curative effect for treating diseases such as chronic obstructive pulmonary disease (COPD) is more remarkable; and besides, a systematic, complete and effective composition identifying and content measuring method is provided, so that the quality of the medicine can be effectively controlled, and the clinical effect is ensured.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
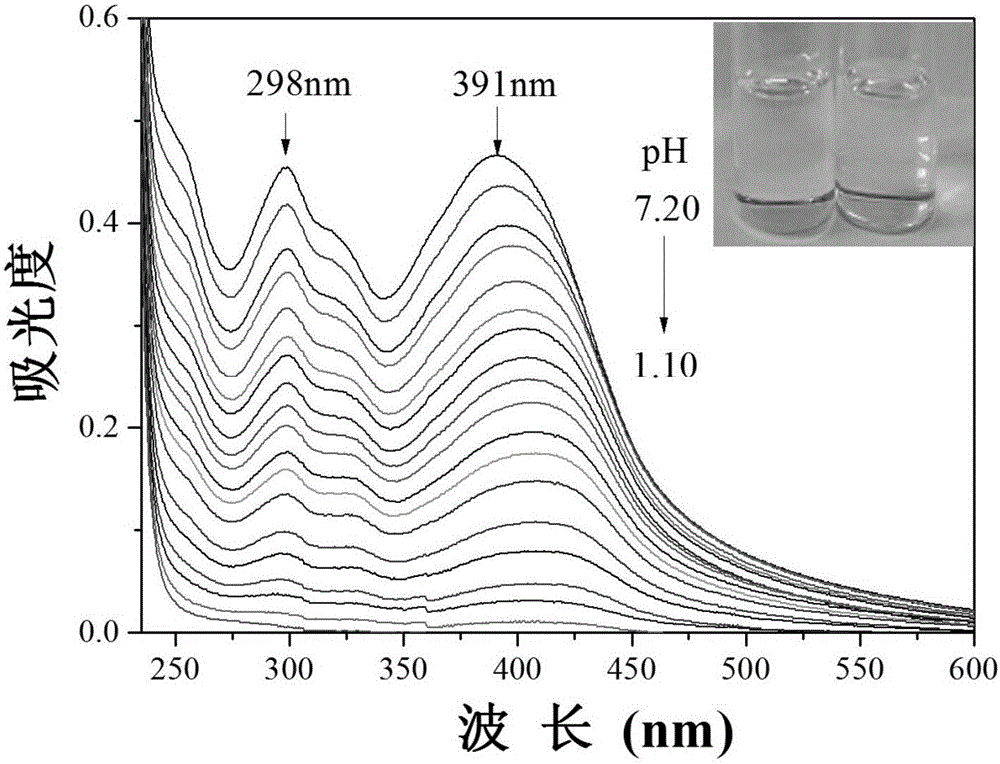
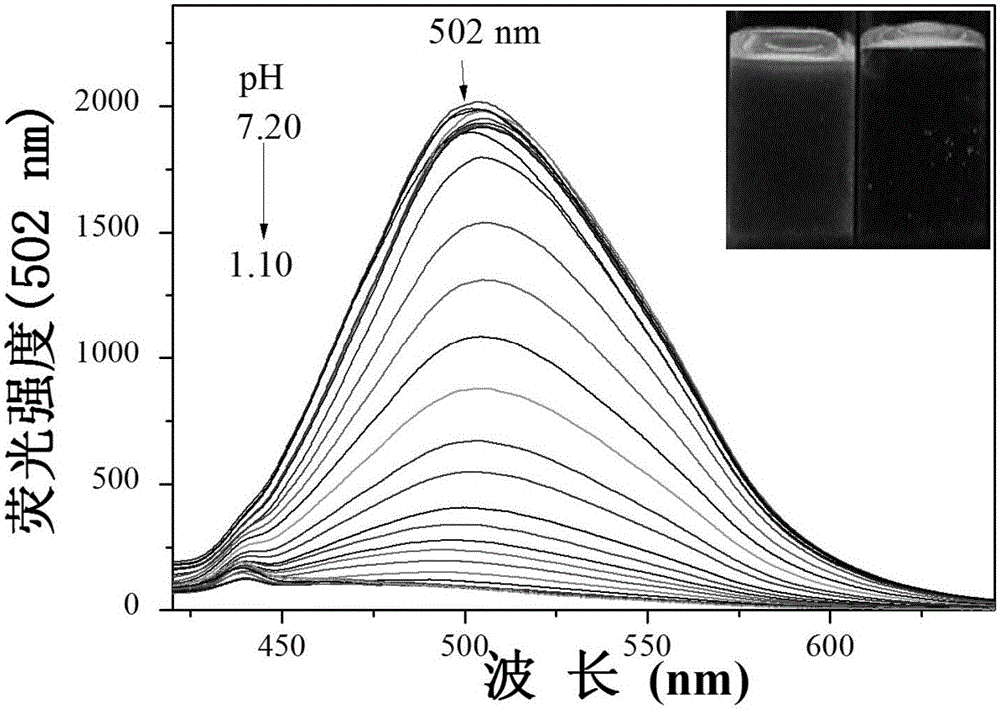
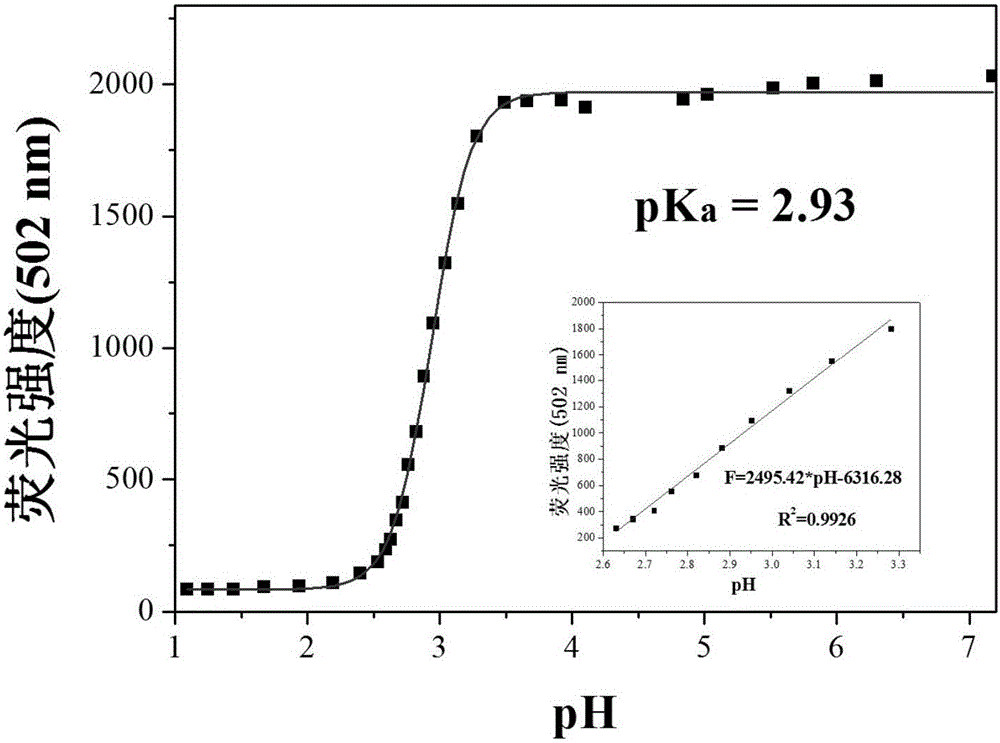
Extremely-acidic carbazole pH fluorescent probe and preparing method and application thereof
InactiveCN106083816AThe synthesis steps are simpleLow costOrganic chemistryFluorescence/phosphorescencePotassium tert-butoxideCarbazole
The invention discloses an extremely-acidic carbazole pH fluorescent probe and a preparing method and application thereof. The fluorescent probe is 3-(9-butyl carbazole-3-cyanovinyl) pyridine. The preparing method includes the steps that carbazole and bromobutane are dissolved in a DMSO / KOH mixed solution, stirring and heating are carried out, and 9-butyl carbazole is generated; DMF serves as a solvent, POCl3 and the 9-butyl carbazole are reacted, and 3-formyl-9-butyl carbazole is obtained; then the obtained 3-formyl-9-butyl carbazole and 3-pyridyl acetonitrile are mixed in methyl alcohol, potassium tert-butoxide is added, the mixture is drawn, filtered and dried, a yellow solid product is obtained, and the extremely-acidic carbazole pH fluorescent probe can be obtained. The probe has the advantages that the low pKa value is achieved, the high sensitivity, the high selectivity and the good stability for H+ are shown, the detection process is easy, convenient and rapid, and the detection result is accurate. In addition, according to the laser confocal scanning microscopical technology, the novel fluorescent probe can be used for detecting biological sample pH change at the extremely-acidic environment.
Owner:SHANXI UNIV
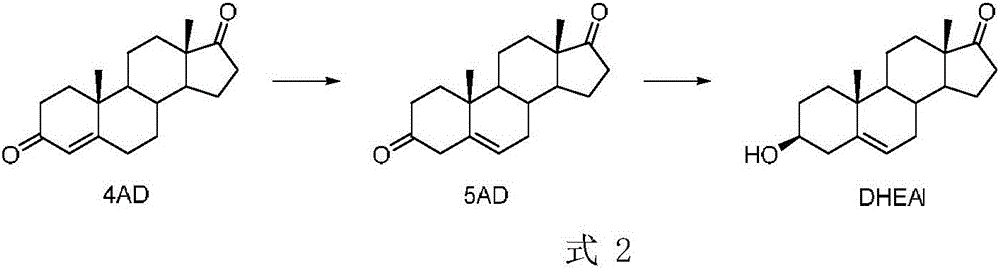
Method for preparing dehydroepiandrosterone through chemical-enzyme method
The invention discloses a method for preparing dehydroepiandrosterone through a chemical-enzyme method. The method comprises that dehydroepiandrosterone is prepared from 4-androstenedione as an initial substrate orderly through a chemical method and a biological method. In preparation, the two reaction processes are optimized. In the chemical method-based 5-androstenedione preparation process, a reaction solution is added into an aqueous solution with sodium ascorbate and acetic acid so that reaction conditions are mild. In the second biological method-based dehydroepiandrosterone preparation process, a ketoreductase is used as a catalyst so that the product yield and purity are improved. In the whole reaction processes, use amounts of a coenzyme and potassium tert-butoxide are low and a high practical value is obtained.
Owner:ENZYMEWORKS
Method of synthesizing imine and amine compounds by means of borrowing-hydrogen reduction coupling
ActiveCN108689786ASimple and fast operationMild conditionsPhysical/chemical process catalystsCarboxylic acid nitrile preparationChemical industryPtru catalyst
The invention belongs to energy and chemical industry and particularly relates to a method of synthesizing imine and amine compounds by means of borrowing-hydrogen reduction coupling by using a nitrogen-doped hierarchical-porous biomass-based carbon material supported catalyst. The method includes the steps of: under a seal reaction condition, adding a nitro-aromatic hydrocarbon compound, benzyl alcohol compounds with different substituent groups, the supported catalyst, methylbenzene and potassium tert-butoxide; performing a reaction at 50-150 DEG C for 4-24 h, cooling the product to room temperature and filtering a reaction liquid to obtain the imine compound represented in the formula (1) or the amine compound represented in the formula (2). The raw materials of the catalyst are regenerable resources, are widely distributed, are green and environment-friendly, are easy to prepare and abundant in sources, and are low in cost; the catalyst can be recycled without deactivation and is stable to air, water and heat. By means of the supported metal catalyst, the conversion rate of the borrowing-hydrogen reduction coupling reaction on the nitro-compound and alcohol to prepare the iminecompounds is higher than 99%, and yield can reach 90-60%; the conversion rate of same to prepare the amine compounds is higher than 99%, and yield can reach 90-60%.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
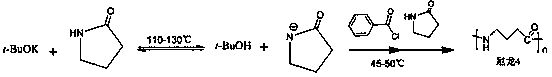
Synthetic process of poly-alpha-pyrrolidone based on anion open loop polymerization
InactiveCN108047443ASuitable for large-scale productionSimple production processPotassium tert-butoxideBenzoyl chloride
The invention provides a synthetic process of poly-alpha-pyrrolidone based on anion open loop polymerization, which is characterized in that the mass ratio of butyrolactam, potassium tert-butoxide andbenzoyl chloride applied in the synthetic process is 15-20: 0.9-0.14: 0.7-3-0.99. The synthetic process includes processing of a reaction container, nitrogen filling, activation, polymerization, andpreparation of a finished production; the molecular weight of the prepared poly-alpha-pyrrolidone is 15000-20000, the product yield is 60-90%.
Owner:CHTC BIO BASED MATERIAL ENG & TECH NINGBO CO LTD
Cavity transfer material with triphenylamine-bipyridyl structure and preparation method thereof
InactiveCN102181282ADifferent structureSimple preparation processOrganic chemistryLuminescent compositionsChromatographic separationPotassium tert-butoxide
The invention relates to the field of photoelectric materials, in particular to a cavity transfer material with a triphenylamine-bipyridyl structure and a preparation method thereof. The method comprises the following steps of: adding corresponding triphenylamine aldehyde, a Hornet reagent and dry tetrahydrofuran into a reactor under the protection of N2; stirring until all the materials are dissolved and cooling to 0 DEG C in an ice bath; dropwise adding a tetrahydrofuran solution of potassium tert-butoxide; fully stirring and reacting reaction liquid under an ice bath condition and removing a solvent by performing rotary steaming; dissolving with dichloromethane; washing twice with water and drying with anhydrous MgSO4; removing a solvent by performing rotary steaming; and separating a crude product with column chromatography to obtain a target product (the structural formula is shown in the specification). The invention provides a novel cavity transfer material with a triphenylamine-bipyridyl structure as well as a preparation method and photoelectric property research thereof. By adopting the cavity transfer material, the research contents of a small molecular cavity transfer material are enriched and the design concept of the novel cavity transfer material is expanded. The cavity transfer material plays important theoretical and practical roles in researching organic photoelectric cavity transfer materials.
Owner:TIANJIN UNIV
Preparation technology of telmisartan active pharmaceutical ingredient
InactiveCN101983962AThe preparation process route is simpleThe preparation process is matureOrganic chemistryBenzoic acidNitration
The invention relates to a preparation technology of a telmisartan active pharmaceutical ingredient, which comprises the technological routes: taking 3-methyl-4-nitryl-benzoic acid as an initial raw material; preparing 4-amino-3-methyl benzoic acid methyl ester through esterification and reduction; preparing 4-methyl-2-propyl-benzimidazole-6-carboxylic acid through acylation, nitration of fuming nitric acid, reduction, cyclization and hydrolysis; producing 4-methyl-2-propyl-6-(1-benzimidazole-methylbenzimidazole-2-yl)-benzimidazo through the condensation with N-methy-o-phenylenediamine; and condensing with 4'-brooethyl biphenyl-2-carboxylic methyl ester in the presence of potassium tert-butoxide and preparing a telmisartan crude product through the hydrolysis of a sodium hydroxide solution-methanol system. The technological routes are simple and mature. The total molar yield can finally reach 23.2% through improving the traditional technological routes; and reaction operation is more easier, the process is simpler, serious three-waste pollution is not generated and the preparation technology is convenient for industrial production.
Owner:FUZHOU NEPTUNUS FUYAO PHARMA
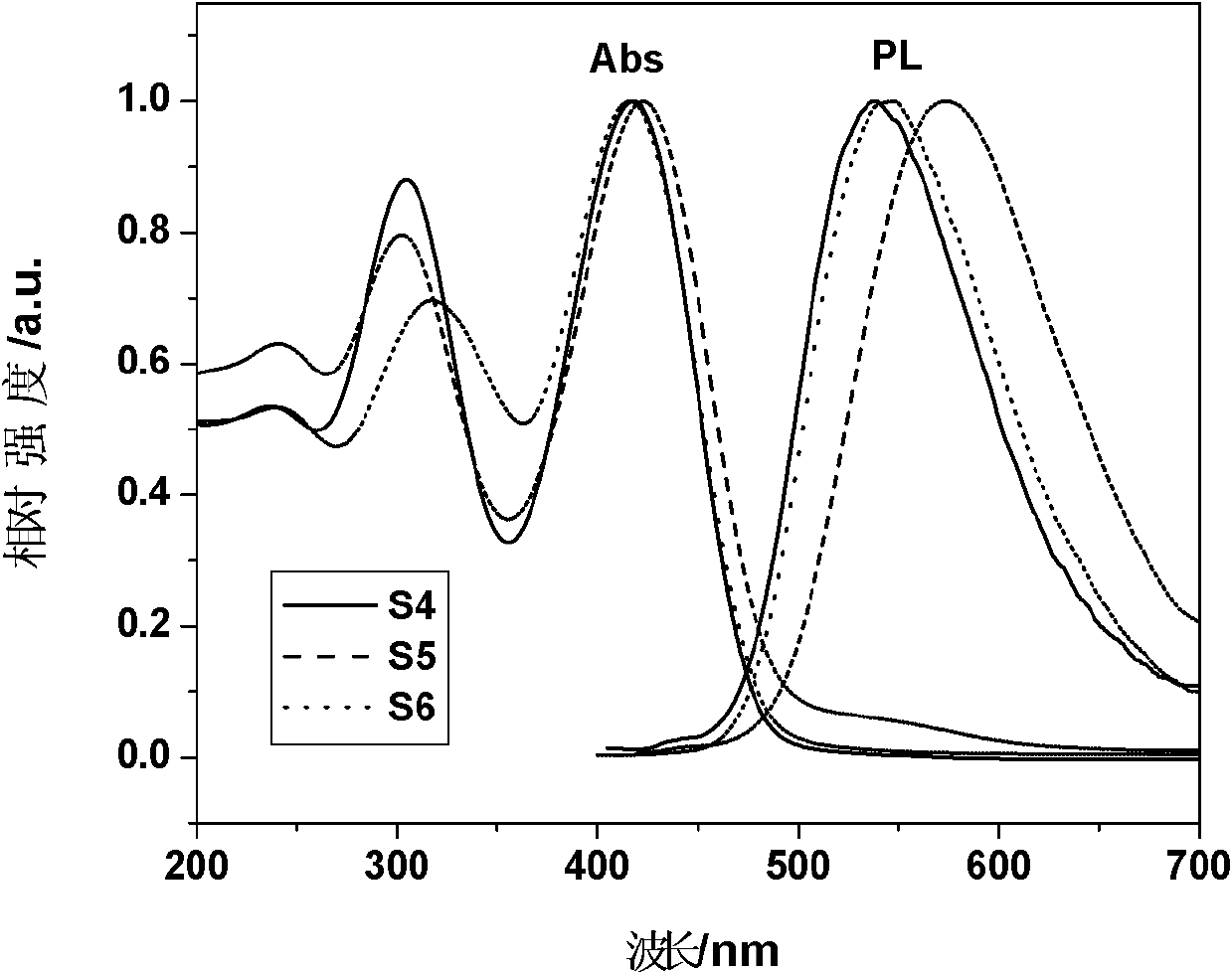
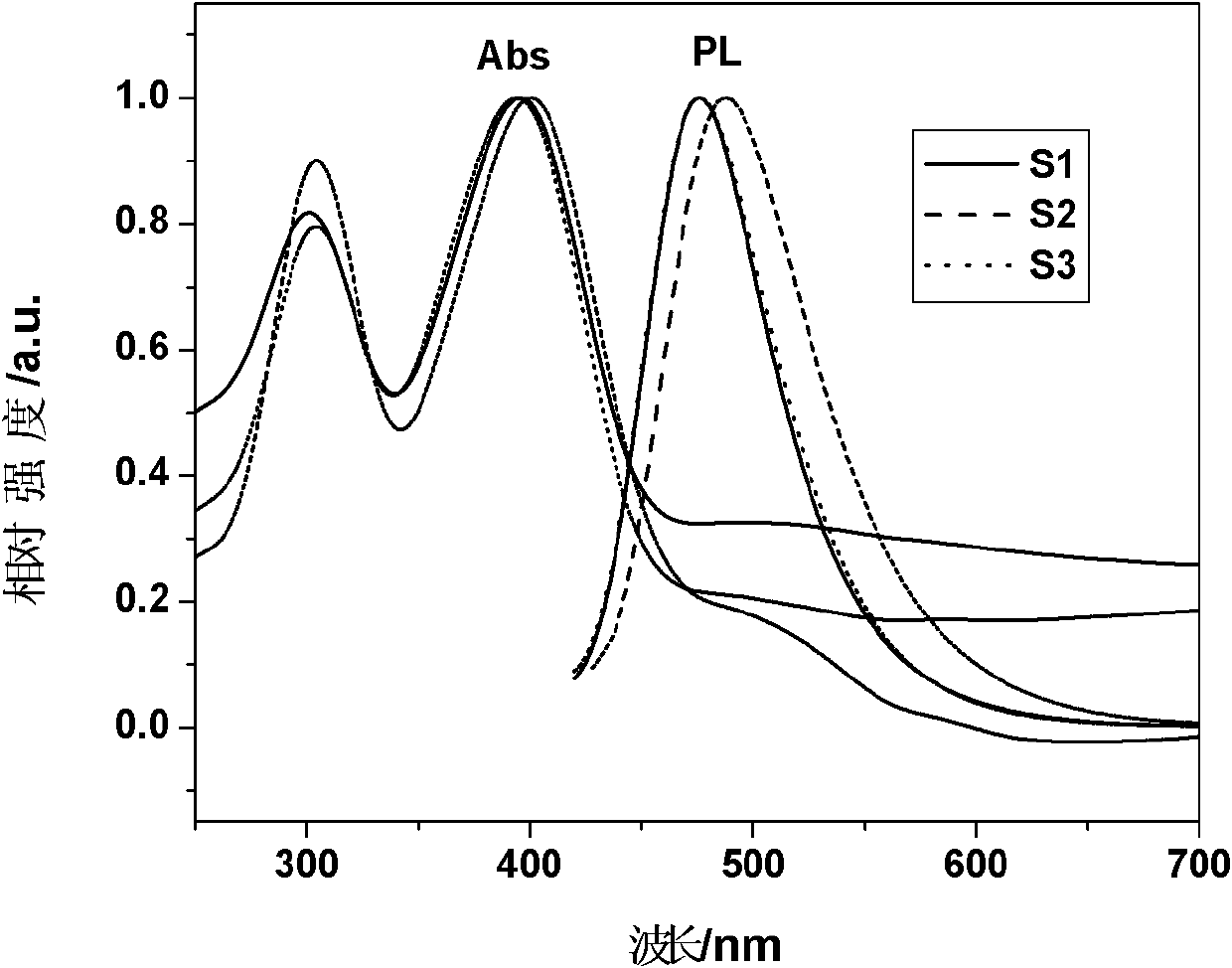
Polyalkenyl-terminated copolyether bonding agent and synthesis method thereof
ActiveCN107739588AImprove flexibilityImprove mechanical propertiesPolyether adhesivesElastomerPotassium tert-butoxide
The invention provides a polyalkenyl-terminated copolyether bonding agent and a synthesis method thereof. The polyalkenyl-terminated copolyether bonding agent is shown as a structural formula (I). Thesynthesis process comprises the following steps of (1) using hydroxyl-terminated oxirane-tetrahydrofuran copolyether as an initiator and 3-hydroxymethyl-3-methyloxetane as monomers to perform cationic ring-opening polymerization reaction to obtain polyalkenyl-terminated oxirane-tetrahydrofuran copolyether; (2) using the polyalkenyl-terminated oxirane-tetrahydrofuran copolyether and allyl bromideas raw materials and potassium tert-butoxide as a catalyst to obtain the polyalkenyl-terminated copolyether bonding agent through etherification reaction. The synthesis method is simple; the polyalkenyl-terminated copolyether bonding agent can give strong mechanical property to polyisoxazoline elastomers. The formula I is shown in the description, wherein x+y is equal to1 to 6, and is an integer;m is 10 to 60, and is an integer; n is 10 to 60, and is an integer.
Owner:XIAN MODERN CHEM RES INST
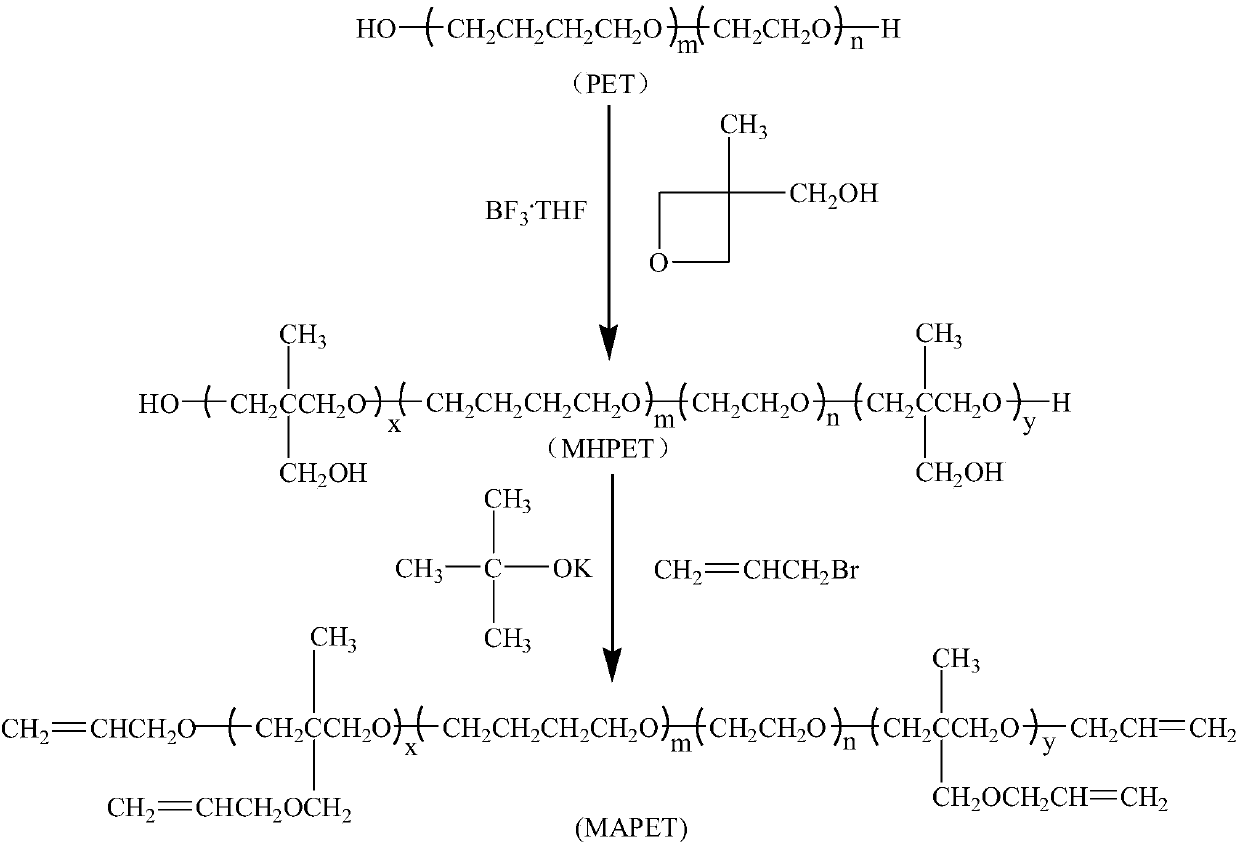
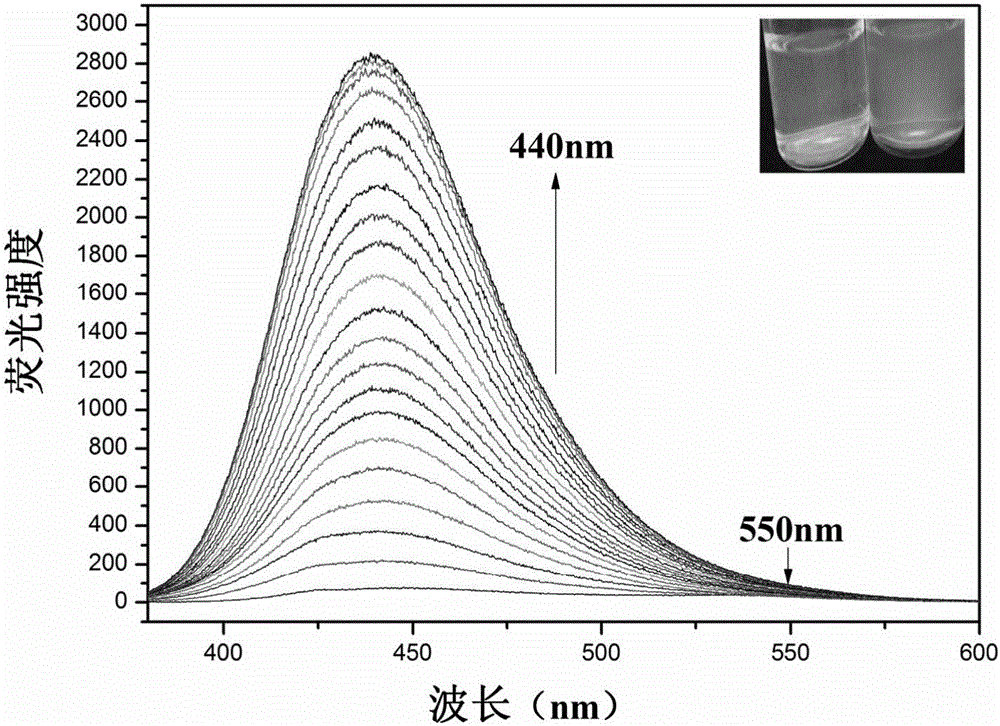
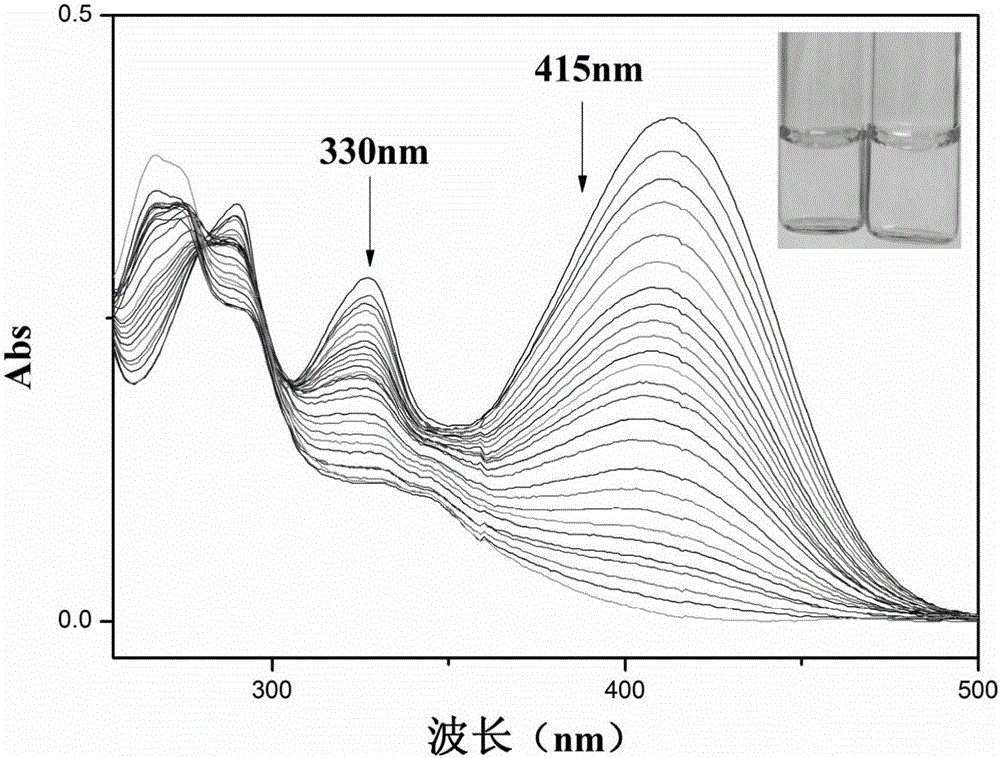
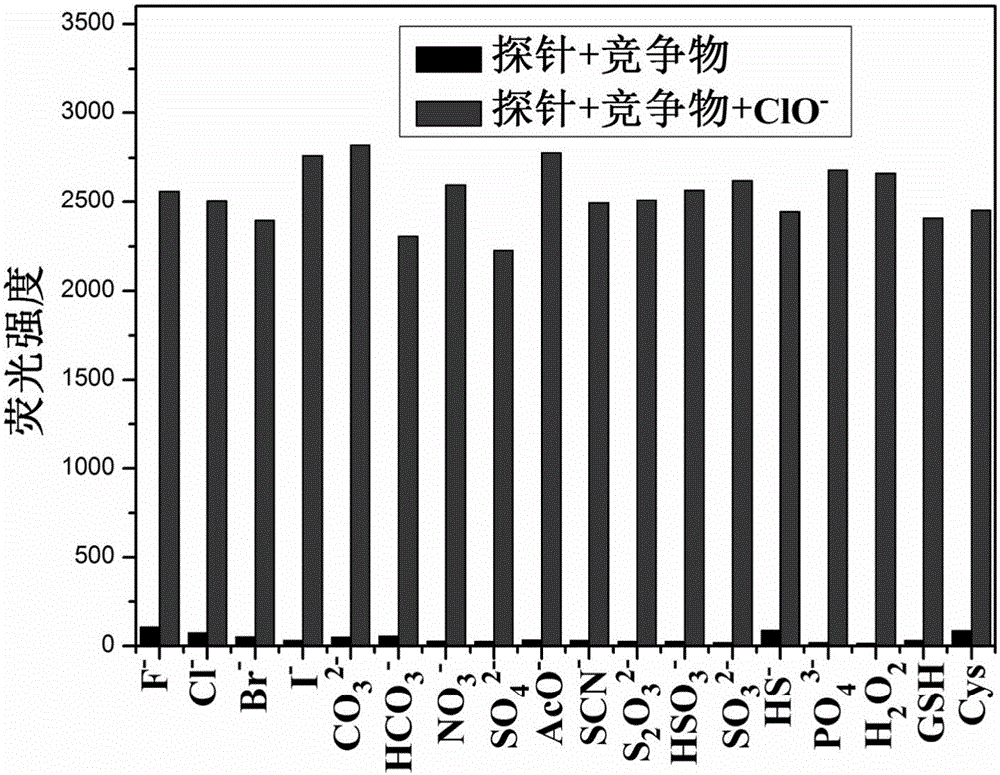
Carbazole fluorescent probe for detecting ClO- and preparation method and application thereof
InactiveCN105968094ACell membrane penetration is goodSimple reaction conditionsOrganic chemistryFluorescence/phosphorescenceFiltrationSolvent
The invention discloses a carbazole fluorescent probe for detecting ClO- and a preparation method and application thereof. The preparation method of the fluorescent probe comprises the steps that carbazole and bromobutane are dissolved in a DMSO / KOH mixed solution, stirred and heated, and 9-butyl carbazole is generated; a DMF is used as a solvent, POC13 reacts with the product, and 3-formoxyl-9-butyl carbazole is obtained; the 3-formoxyl-9-butylcarbazole and 3-pyridine acetonitrile are mixed in methyl alcohol, potassium tert-butoxide is added, and 1-methyl-3-(9-butylcarbazole-3-cyanogen vinyl)pyridine is generated; the product is dissolved in HCC13, CH3I is added, suction filtration and drying are carried out, and a saffron yellow solid product is obtained. The probe displays high sensitivity and selectivity for CIO- and displays large Stocks displacement when detecting CIO- ions, the detection process is easy and convenient, and the detection result is accurate. By means of laser confocal scanning microscopy, a fluorescence image of the novel fluorescent probe in living cells can be obtained.
Owner:SHANXI UNIV
Hydrosilylation of rhodium complex catalyzed alkene in room-temperature ion liquid/super-critical CO2 medium
InactiveCN101671356AEasy separationHigh selectivitySilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsAdjuvantSilanes
The invention relates to a hydrosilylation of rhodium complex catalyzed alkene in room-temperature ion liquid / super-critical CO2 medium. The technical problem to be solved is characterized in that thehydrosilylation condition is moderate and safe, the reaction conversion rate is high, the outcome Beta-addition product of the hydrosilylation has strong selection, no outgrowth paraffin hydrocarbonis produced, the outcome and the catalyst are convenient to be separated from each other, and the catalyst can be reclaimed and re-utilized. The hydrosilylation is characterized in that the alkene andhydrogen-contained silanes are taken as raw materials, rhodium compound is taken as the catalyst, the potassium tert-butoxide is taken as adjuvant, and the room-temperature ion liquid / supercritical CO2 is taken as reaction medium; and the mixture is heated and mixed so as to react sufficiently, the outcome is collected by reducing pressure, thus obtaining the hydrosilylation products.
Owner:HANGZHOU NORMAL UNIVERSITY
Preparation method of negative liquid crystal compound
ActiveCN107779202AStrong negative dielectric anisotropyModerate refractive indexLiquid crystal compositionsCompound aPotassium tert-butoxide
The invention belongs to the technical field of liquid crystal compounds, and relates to a preparation method of a negative liquid crystal compound. The preparation method comprises the following steps: (1) by taking a compound a as a raw material, carrying out reaction under existence of BuLi / DMF to obtain a compound 1; (2) under existence of triphenylphosphine bromide / potassium tert-butoxide, carrying out one-step reaction on the compound 1 to obtain an intermediate B; (3) by taking a compound b as a raw material, under a condition that an alkaline reagent and a reaction solvent exist, carrying out reaction on the compound b and the obtained intermediate B to obtain the negative liquid crystal compound as shown in Formula (I). The synthesis route of the method is obviously shortened, andthe related reactions can be all quantitatively and completely implemented; the quantity of impurities is small, and the intermediate is liable to decompress, distill and purify, so that mass production is facilitated, and the cost is reduced.
Owner:HEBEI MAIERSTON ELECTRONICS MATERIAL
Synthetic method of intermediate cyclopropyl acetylene of anti-aids drug efavirenz
InactiveCN103664465AProcess raw materials are easy to getSimple processHydrocarbon from halogen organic compoundsPtru catalystEthyl group
The invention discloses a synthetic method of intermediate cyclopropyl acetylene of an anti-aids drug efavirenz, and relates to the technical field of synthesis of cyclopropyl acetylene. The synthetic method comprises the following steps: reacting in an organic solvent to produce alpha, alpha-ethyl dichloride cyclopropane by taking cyclopropyl methyl ketone as a raw material and taking phosphorus pentachloride as a chlorinating agent; and obtaining the cyclopropyl acetylene by adding the alpha, alpha-ethyl dichloride cyclopropane into strong base aqueous liquor through a phase transfer catalyst. The synthetic method has the beneficial effects that the organic solvent and inorganic strong base are not needed to be adopted, expensive reagents such as the organic solvent and potassium tert-butoxide are avoided; moreover, process raw materials are easily available, process is simple, operation is convenient, cost is lowered and the three wastes are reduced, and therefore, the synthetic method is suitable for industrial production.
Owner:JIUJIANG ZHONGTIAN PHARMA
Preparation method of betrixaban
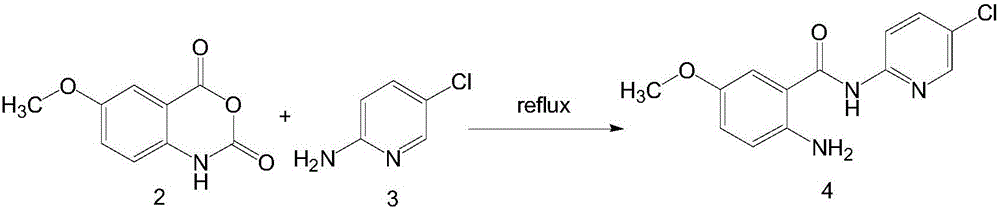
InactiveCN105732490AHigh yieldLow impurity contentOrganic chemistryNucleophilic additionRaw material
The invention provides a method for preparing betrixaban. The method comprises the following steps: performing the nucleophilic addition elimination on 5-methoxyisatoic anhydride used as a raw material and 2-amino-5-chloropyridine under the condition of potassium tert-butoxide to obtain N-(5-chloro-2-pyridyl)-5-methoxyl-2-aminobenzamide, allowing a compound 4 to react with cyanobenzoyl chloride to obtain N-(5-chloro-2-pyridyl)-2-[(4-cyano benzoyl) amino]-5-methoxyl benzamide hydrochloride, and finally performing the nucleophilic addition with dimethylamine to obtain a target product betrixaban, wherein the total yield is 57.8 percent. By adopting the preparation method, the raw materials are low in price and easy to get, the reaction conditions are moderate, the reaction controllability is high, and the industrialized application prospect is good.
Owner:CHONGQING MEDICAL UNIVERSITY
Preparation method of Draxxin
ActiveCN107556351AHigh yieldAvoid ultra-low temperature reactionsSugar derivativesSugar derivatives preparationPotassium tert-butoxideBenzyl chloroformate
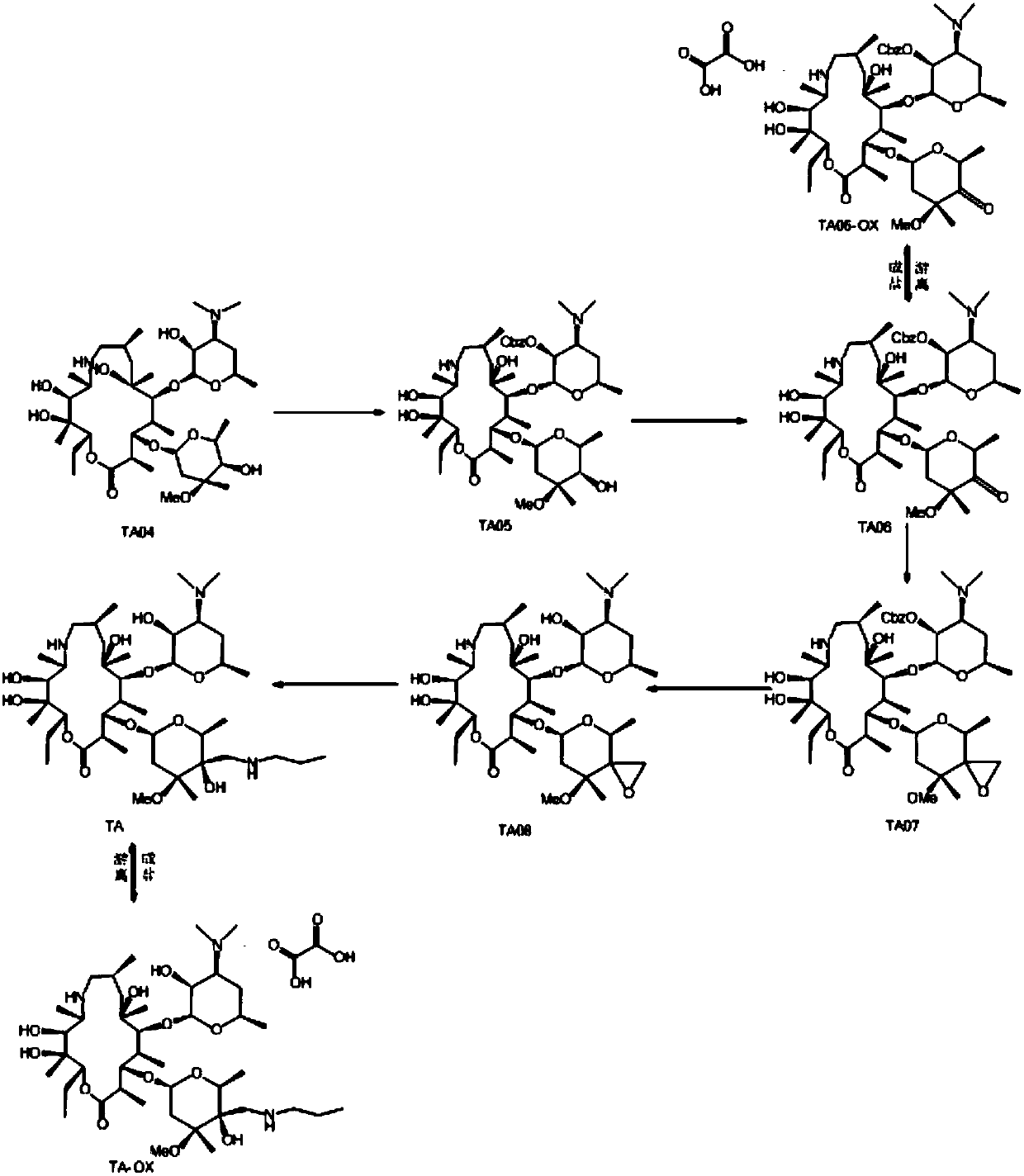
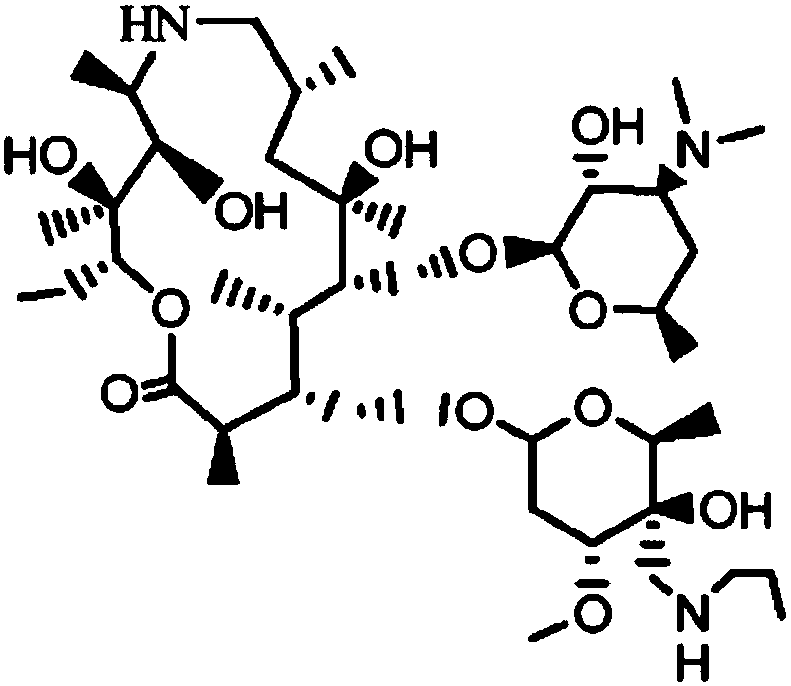
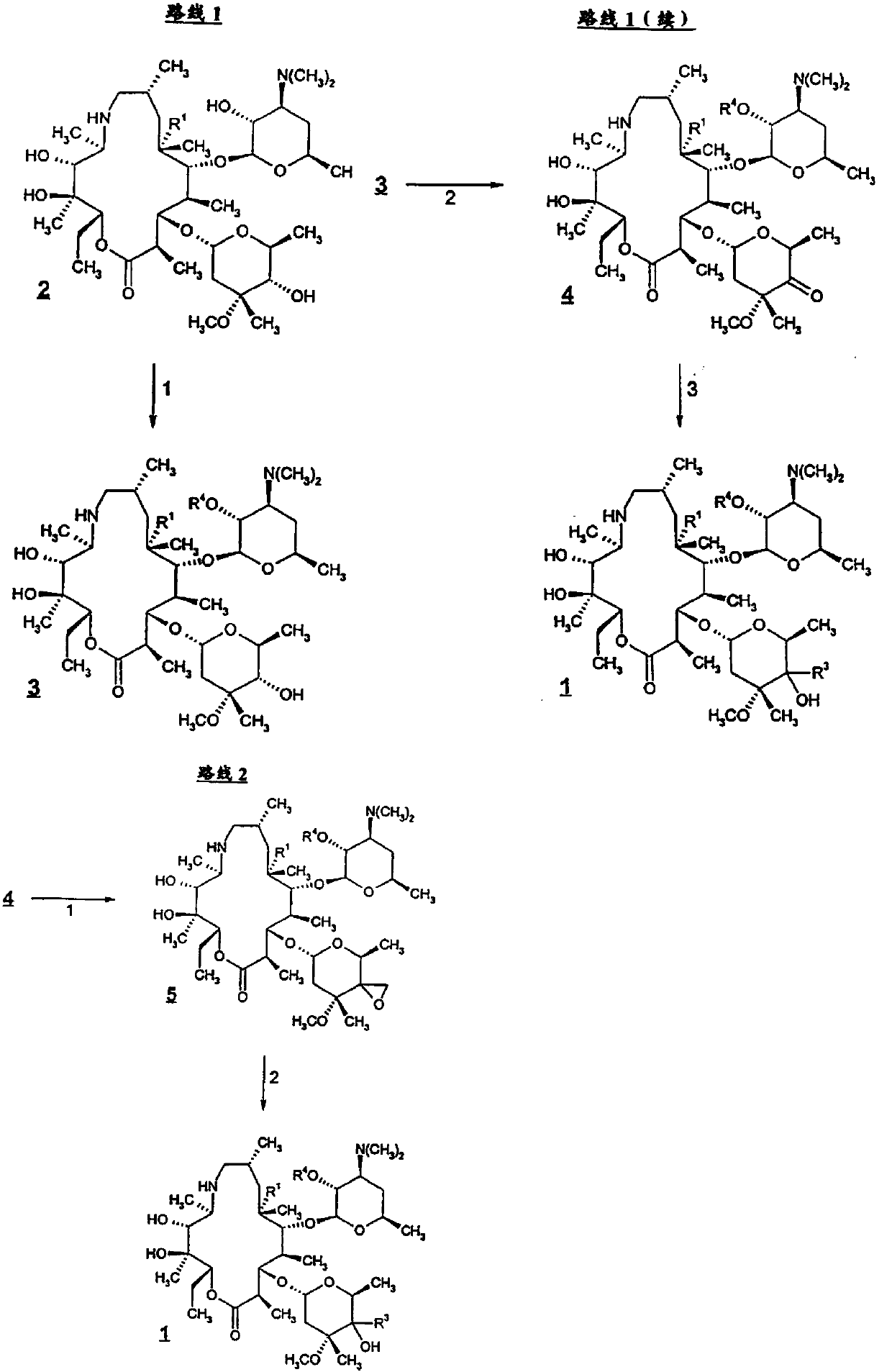
The invention discloses a preparation method of Draxxin. The method comprises the following steps: enabling a reaction between an intermediate TA04 and benzyl chloroformate, so as to obtain TA05; oxidizing the TA05 with DMSO and IBX at the temperature of 20-30 DEG C, so as to obtain TA06; dissociating and performing aftertreatment after the TA06 becomes oxalate TA06-OX, so as to obtain purified TA06; oxidizing the TA06 with trimethylsulfonium bromide and potassium tert-butoxide, so as to obtain TA07; hydrogenating the TA07 to remove a protection group, so as to obtain TA08; enabling a reactionbetween the TA08 and n-propylamine, so as to obtain TA; dissociating and performing aftertreatment after the TA becomes oxalate TA-OX, so as to obtain purified end-product Draxxin. The total yield ofthe Draxxin prepared with the method is increased by above 20%.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative
ActiveCN104788361AThe reaction route has short stepsEasy to operateOrganic chemistryAlkalinitySulfonate
The invention provides a synthetic method for a 5-azaspiro[2.4]heptane-6-formic acid derivative. The synthetic method comprises the following steps: taking 1,1-cyclopropane dimethanol as a starting material, reacting with dichlorosulfoxide, performing oxidization to obtain a sulfonate compound, condensing the sulfonate compound and imine prepared from glycine methyl ester in the presence of potassium tert-butoxide, finishing hydrolysis, cyclization and amino protection with a one-pot procedure by adjusting the acidity and alkalinity of a system to obtain a racemization product, and finally performing resolution to obtain a finished product; the total yield reaches more than 30%; a reaction route has short steps; a used reagent is safe; the method is simple to operate, low in reaction cost, relatively high in yield and suitable for industrialized production.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Method for synthesizing hypericin
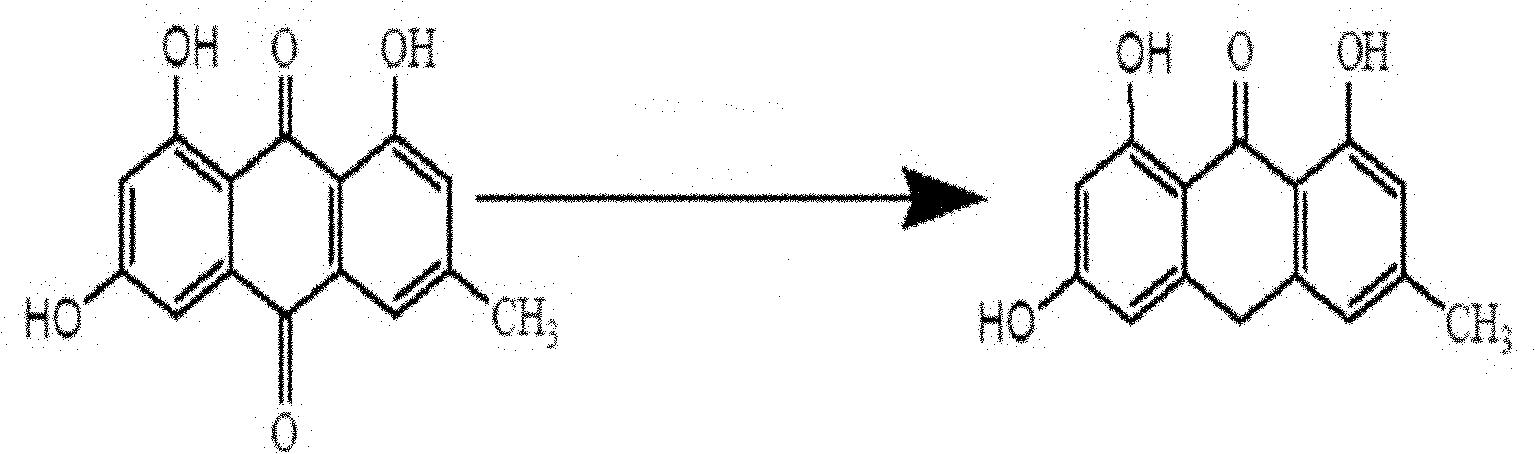
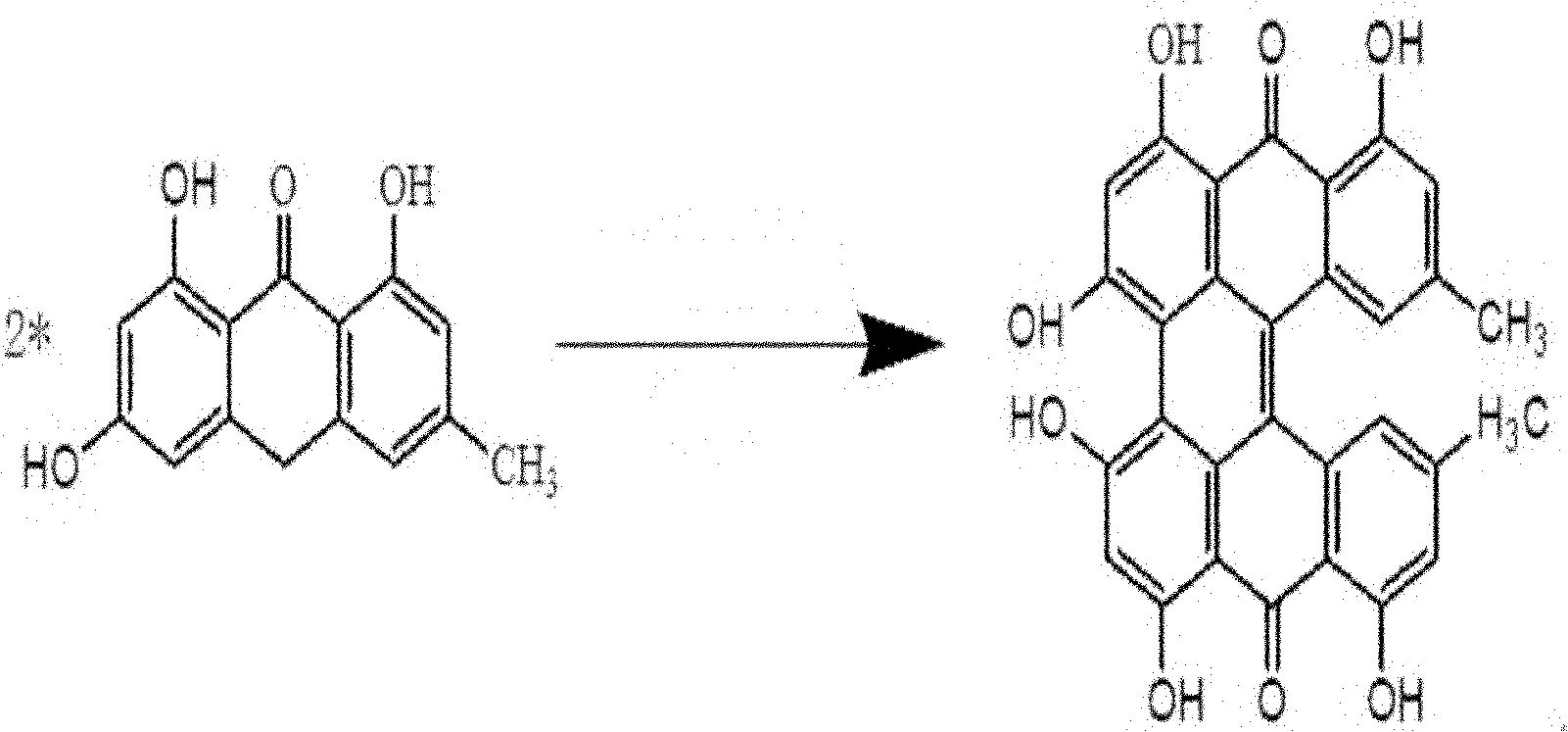
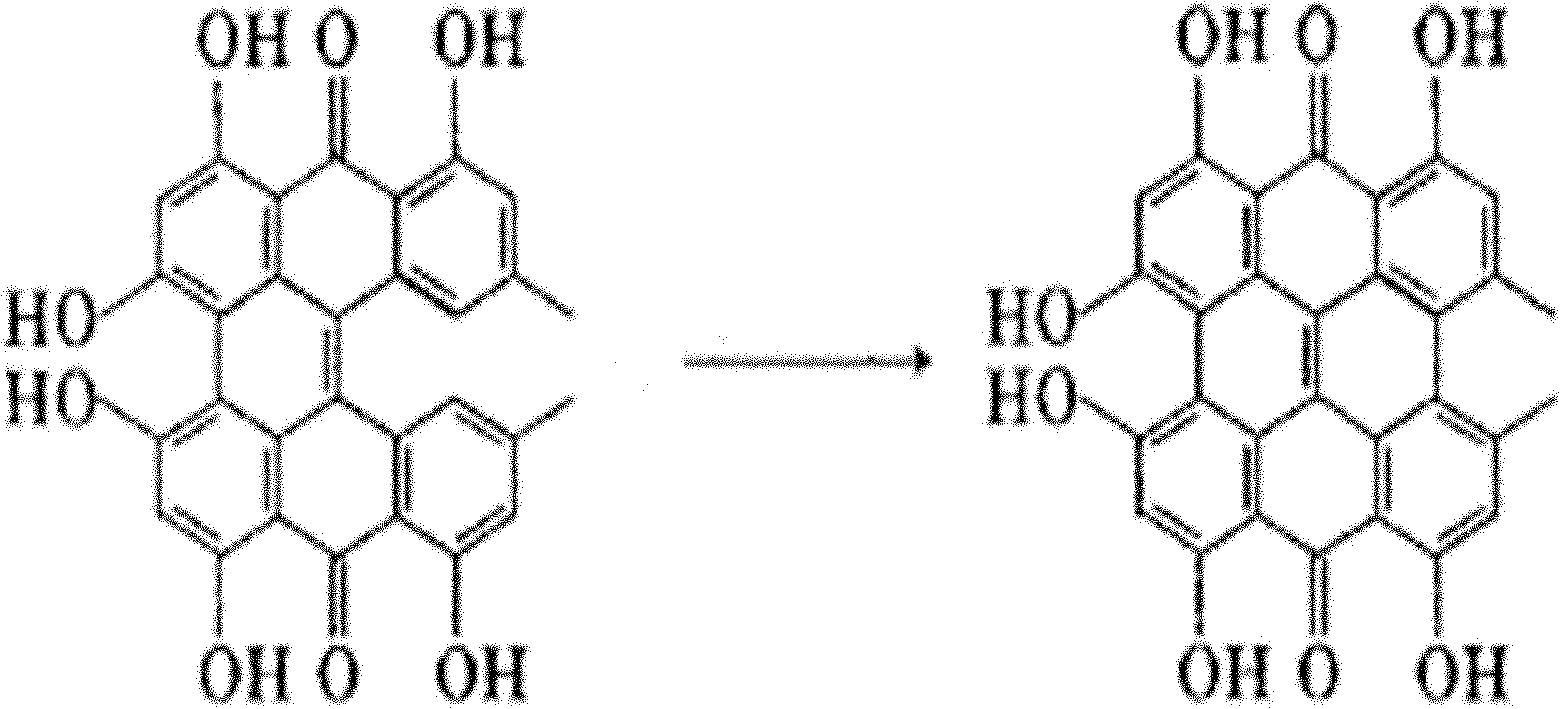
InactiveCN102126942AEasy to operateReduce manufacturing costOrganic compound preparationQuinone preparationAcetic acidProtohypericin
The invention relates to a method for synthesizing hypericin. The method comprises the following steps of: (1) dissolving emodin and SnCl2.2H2O in glacial acetic acid, raising the temperature to 100-125 DEG C, adding concentrated hydrochloric acid with the concentration of between 36 percent and 40 percent for reaction of 1-3 hours and cooling to obtain emodin anthrone; (2) dissolving emodin anthrone and potassium tert-butoxide in DMF (Dimethyl Formamide), carrying out reaction in a microwave solid-liquid phase synthesis / extraction instrument for 30-90 minutes and cooling to obtain protohypericin; and (3) dissolving the protohypericin in acetone, irradiating with a halogen lamp for 15-24 hours, concentrating and precipitating to obtain the hypericin. The method has the advantages of easiness in operating, low cost, little pollution, high yield, high purity and the like.
Owner:WUHAN MAIDESEN MEDICAL TECH
Preparation method of novel polyphenols antioxidant
InactiveCN107827713AGood compatibilityInhibition of oxidative degradationOrganic chemistryOrganic compound preparationPolystyreneEthyl acetate
The invention relates to the field of preparation of antioxidants, in particular to a preparation method of a novel polyphenols antioxidant. The preparation method comprises the following steps: preparing an intermediate which adopts 3,5-di-tert-butyl-4-hydroxy-benzyl alcohol: adding 7.27 g of paraformaldehyde, 1.08 g of potassium tert-butoxide and 100 mL of tertiary butanol into a round-bottom flask with the volume of 500 mL, heating the flask under a condition of 70 DEG C till the paraformaldehyde is depolymerized; adding 20 g of 2,6-di-tert-butylphenol into the reaction liquid for reactionunder a normal temperature condition for 3 h, then adding 200 mL of deionized water into the reaction liquid, adding 100 mL of an ethyl acetate extraction organic phase, drying the mixture with a drying agent for 2 h, and carrying out suction filtering to remove the drying agent. The preparation method is simple in process and convenient to operate; a product is relatively high in heat stability and oxidization resistance, is high in compatibility with a polymer, can inhibit oxidative degradation of polypropylene, polyethylene, acrylonitrile-butadiene-styrene resin and polystyrene, and also can be used for synthesizing natural rubber, plastic and lubricating oil.
Owner:SHAANXI QIYUAN TECH DEV
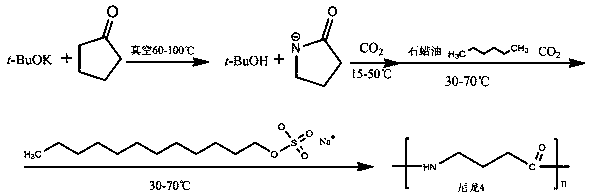
Preparation method of polybutyrolactam through anionic ring-opening polymerization
ActiveCN109851778AHigh molecular weightEasy to control polymerization ratePotassium tert-butoxideFiltration
The invention provides a preparation method of polybutyrolactam through anionic ring-opening polymerization. The preparation method comprises potassium tert-butoxide activation, carbon dioxide triggering, and addition of paraffin oil and sodium dodecyl sulfate. 2-pyrrolidone is taken as the primary raw material, after potassium tert-butoxide activation, carbon dioxide triggering, and addition of paraffin oil and sodium dodecyl sulfate, polymerization is performed, then n-hexane is added and stirred, and suction filtration is carried out to obtain polybutyrolactam. The preparation method has the advantages that the polymerization speed is slow and controllable, the polymer discharge is convenient, the molecular weight of polymers is high, and the requirements of massive production can be met.
Owner:TIANE CHEM FIBER GROUP CORP BAODING +1
Novel preparation method of pranoprofen
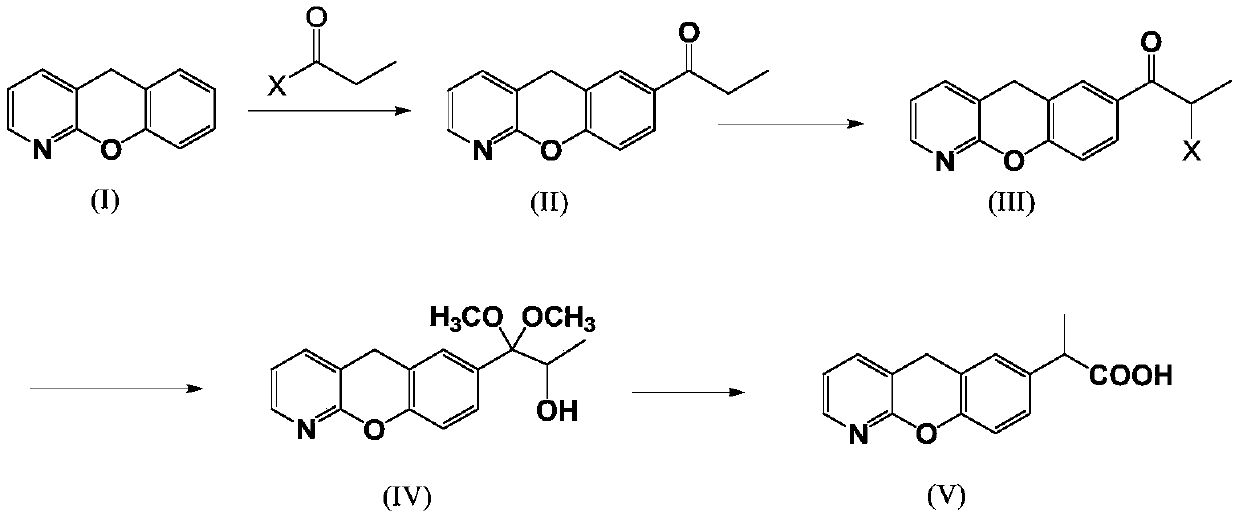
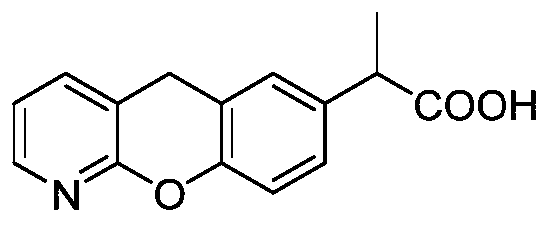
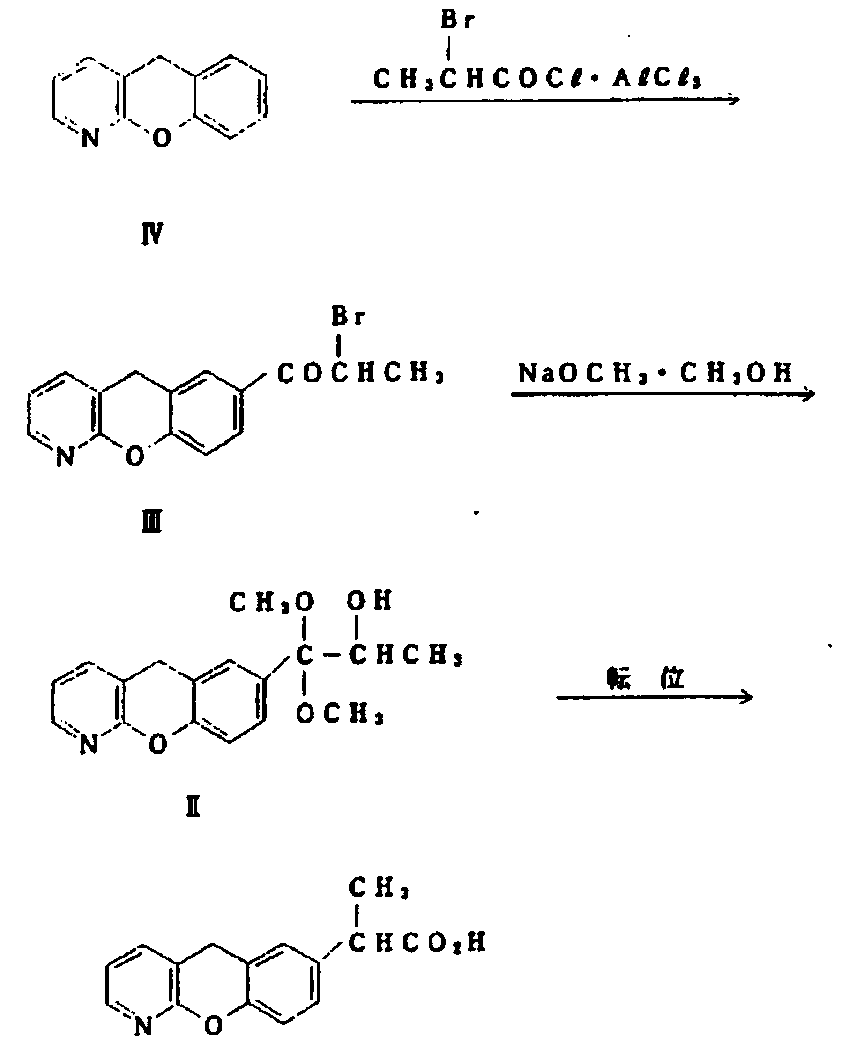
The invention discloses a novel preparation method of pranoprofen. The novel preparation method of the pranoprofen has the advantages of being high in yield, having less impurities, and being safe andenvironmentally friendly. The novel preparation method of the pranoprofen comprises the following steps that step 1, 5H-[1]-benzopyran[2,3-b]pyridine and propionyl chloride are directly acylated; step 2, bromination is carried out; step 3, potassium tert-butoxide or sodium tert-butoxide is used for hydrolysis, and finally, rearrangement is carried out to obtain the pranoprofen.
Owner:GUANGDONG XIANQIANG PHARMA +2
Synthetic method of pyronine derivative dye
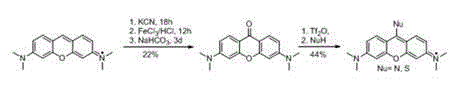
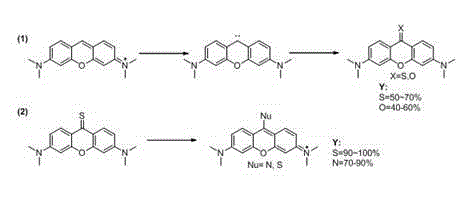
The invention discloses a synthetic method of a pyronine derivative dye. The synthetic method comprises the following steps: with pyronine as a raw material, producing carbine serving as an intermediate under the action of potassium tert-butoxide, carrying out reaction on carbine and sulfur or oxygen to produce pyronine thione or ketone, and then carrying out reaction on pyronine thione and amine or halohydrocarbon to produce the pyronine derivative dye. The synthetic method is safe and simple in operation, short in reaction time and good in yield and purity of the pyronine derivative dye.
Owner:山东埃米森生物科技有限公司
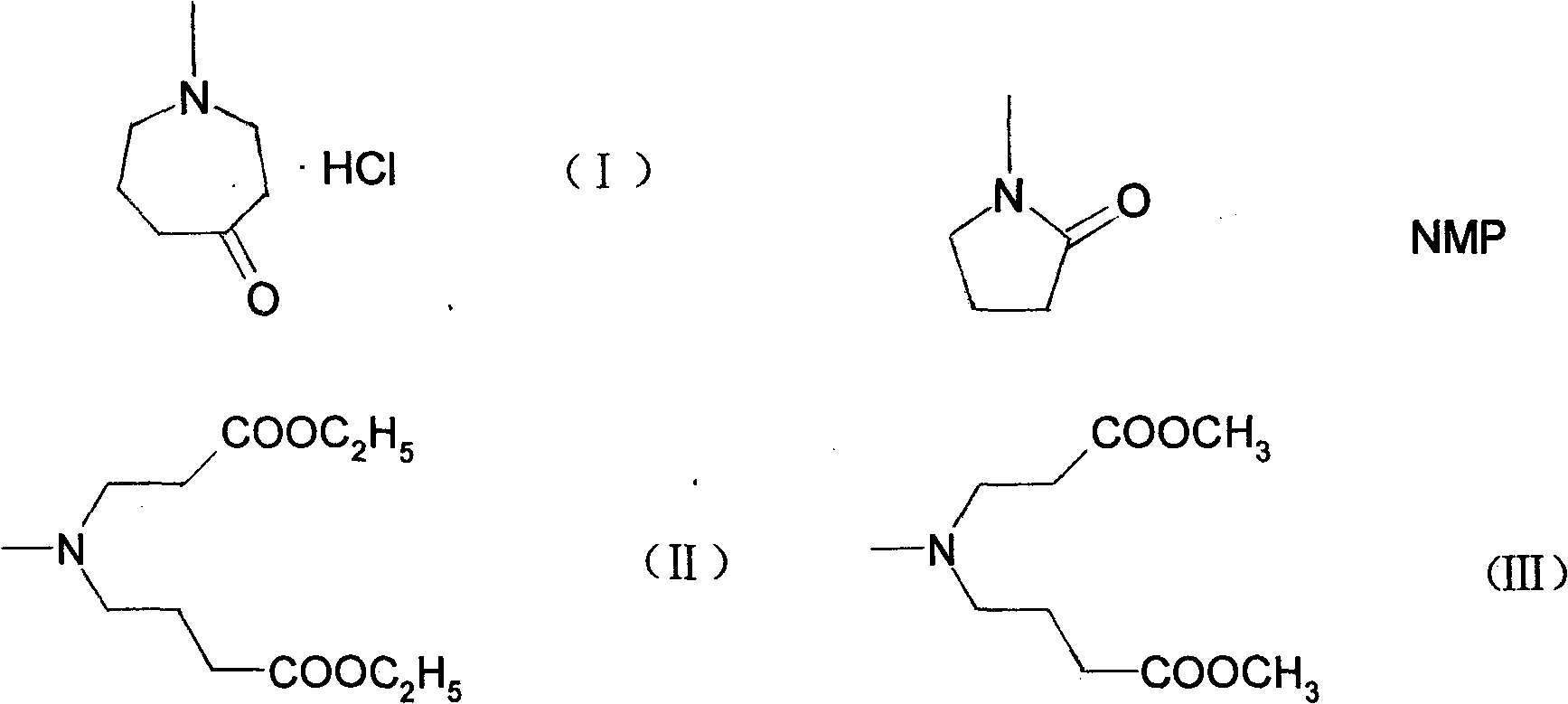
Method for synthesizing N-methylhexahydroazepin-4-one hydrochloride, azelastine hydrochloride intermediate
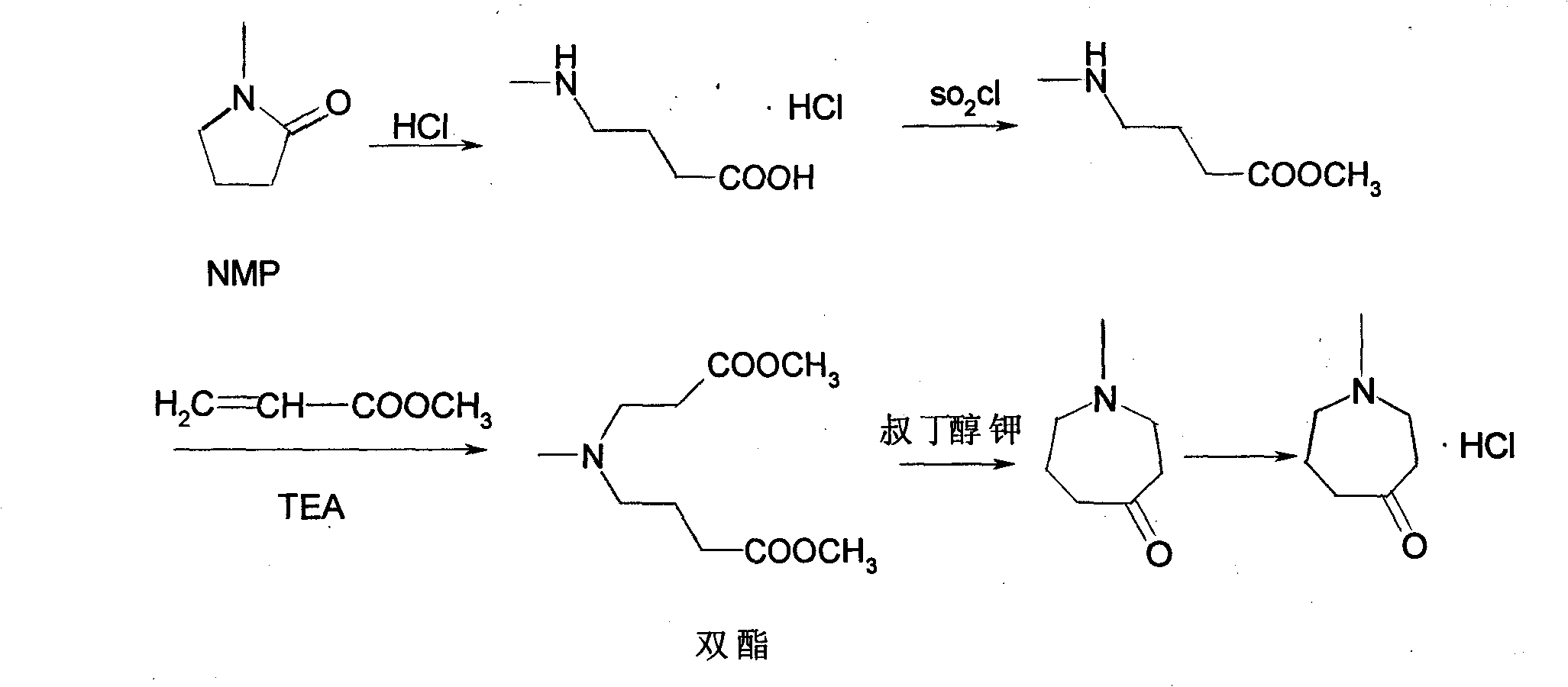
The invention relates to a method for synthesizing N-methylhexahydroazepin-4-one hydrochloride. In the method, N-methyl-2-pyrrolidone (NMP) is taken as the raw material. The method is characterized by heating and carrying out reflux on NMP in the presence of hydrochloric acid to prepare 4-methylaminobutyric acid hydrochloride, adding 4-methylaminobutyric acid hydrochloride into methanol and thionyl chloride to carry out mono-esterification reaction to prepare 4-methylaminobutyric acid methyl ester hydrochloride, then adding 4-methylaminobutyric acid methyl ester hydrochloride into the solution of methyl acrylate, triethylamine and methanol to react to prepare diester, carrying out cyclization reaction on the diester and metal organic alcohols such as potassium tert-butoxide to obtain N- methylhexahydroazepin-4-one and finally salifying and crystallizing N- methylhexahydroazepin-4-one to obtain the N-methylhexahydroazepin-4-one hydrochloride. The product has high purity and yield, the process is simple and convenient to operate, a single solvent can be used in the whole reaction process, and N-methylhexahydroazepin-4-one hydrochloride is convenient to recycle, small in wastewater quantity, low in comprehensive cost and suitable for large-scale production.
Owner:山东众诚生物医药股份有限公司
Quinazoline ketone anticoccidial medicament
InactiveCN101255138AHas anti-coccidia effectOrganic active ingredientsOrganic chemistrySodium methoxideKetone
A series quinazoline ketones compounds with halogeno- quinazolone ring, propa-2-ketone group or buta-2ketone group or guaiacyl are synthesized by condensing halogeno- quinazoline-4-ketone and bromium-3-(2-methoxylbenz)propa-2-ketone or 1-bromium-4-(2-methoxyl benz )buta-2-ketone at 70-80 degree C for 1-7h under existing condition of sodium hydride, potassium carbonate, potassium tert butoxide, sodium methoxide or sodium ethylate using DMSO or DMF as a dissolvent. Proved by bioactivity experiment, the compounds have anticoccidial activity.
Owner:SICHUAN UNIV
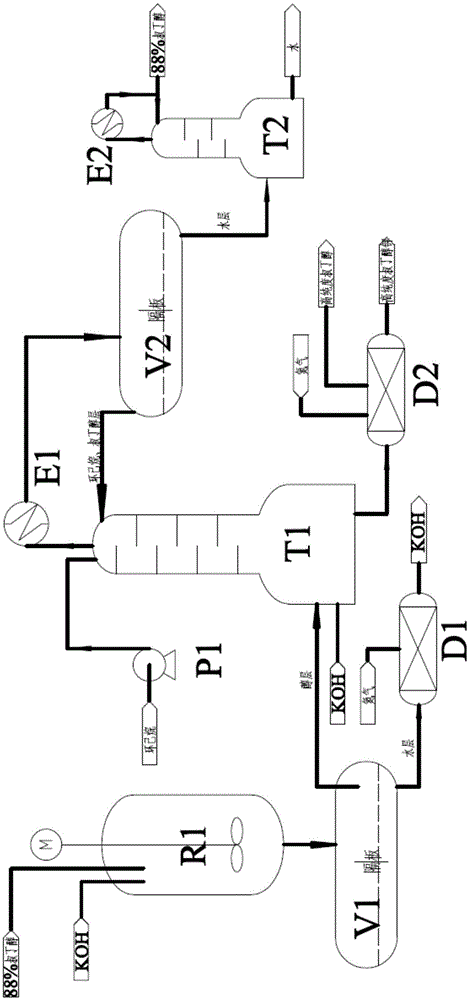
Method for preparing high-purity potassium tert-butoxide with tert-butanol-water azeotropic solution and potassium hydroxide
InactiveCN105541550AOrganic compound preparationPreparation of metal alcoholatesPotassium tert-butoxideSalting out
The invention discloses a method for preparing high-purity potassium tert-butoxide with a tert-butanol-water azeotropic solution and potassium hydroxide. The potassium hydroxide serves as a salting-out water separating agent to concentrate the tert-butanol-water azeotropic solution, and the concentrated tertiary butanol solution and a certain amount of potassium hydroxide are reacted in a semi-batch rectifying tower kettle; meanwhile, cyclohexane and tertiary butanol serve as water carrying agents, and water generated in the reaction process is separated and brought out in real time. After the reaction is over, the generated tertiary butanol solution of the potassium tert-butoxide is dried to be crystallized under the protection of nitrogen, the potassium tert-butoxide with the purity of 98.2wt% or above can be obtained, and the tertiary butanol with the purity of 99.0wt% or above can be obtained in a vapor condensation mode; overhead products of a semi-batch rectifying tower is layered through standing, the cyclohexane and the tertiary butanol serve as the water carrying agent, returns to the semi-batch rectifying tower and is recycled, and after the tert-butanol-water solution is rectified, the obtained tert-butanol-water azeotropic solution is recycled.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

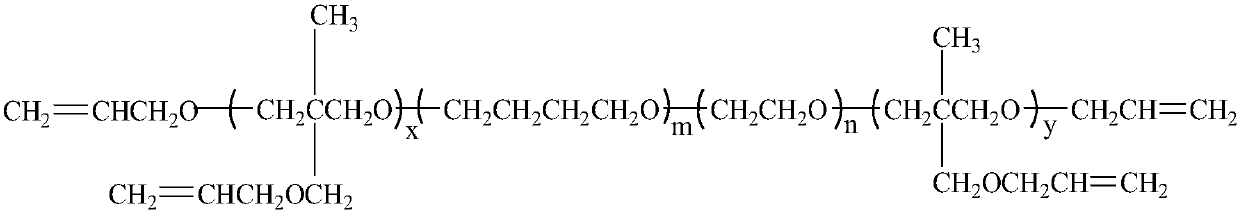
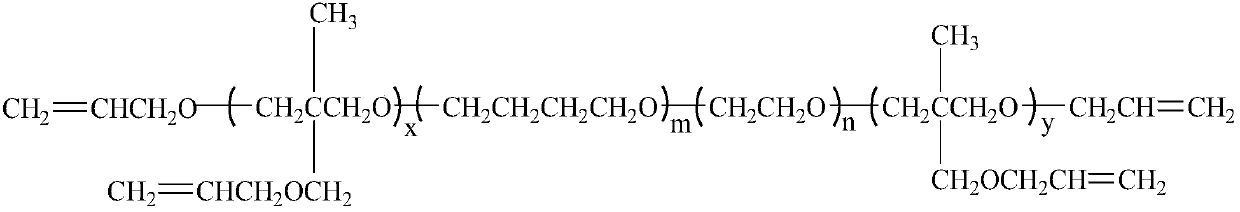
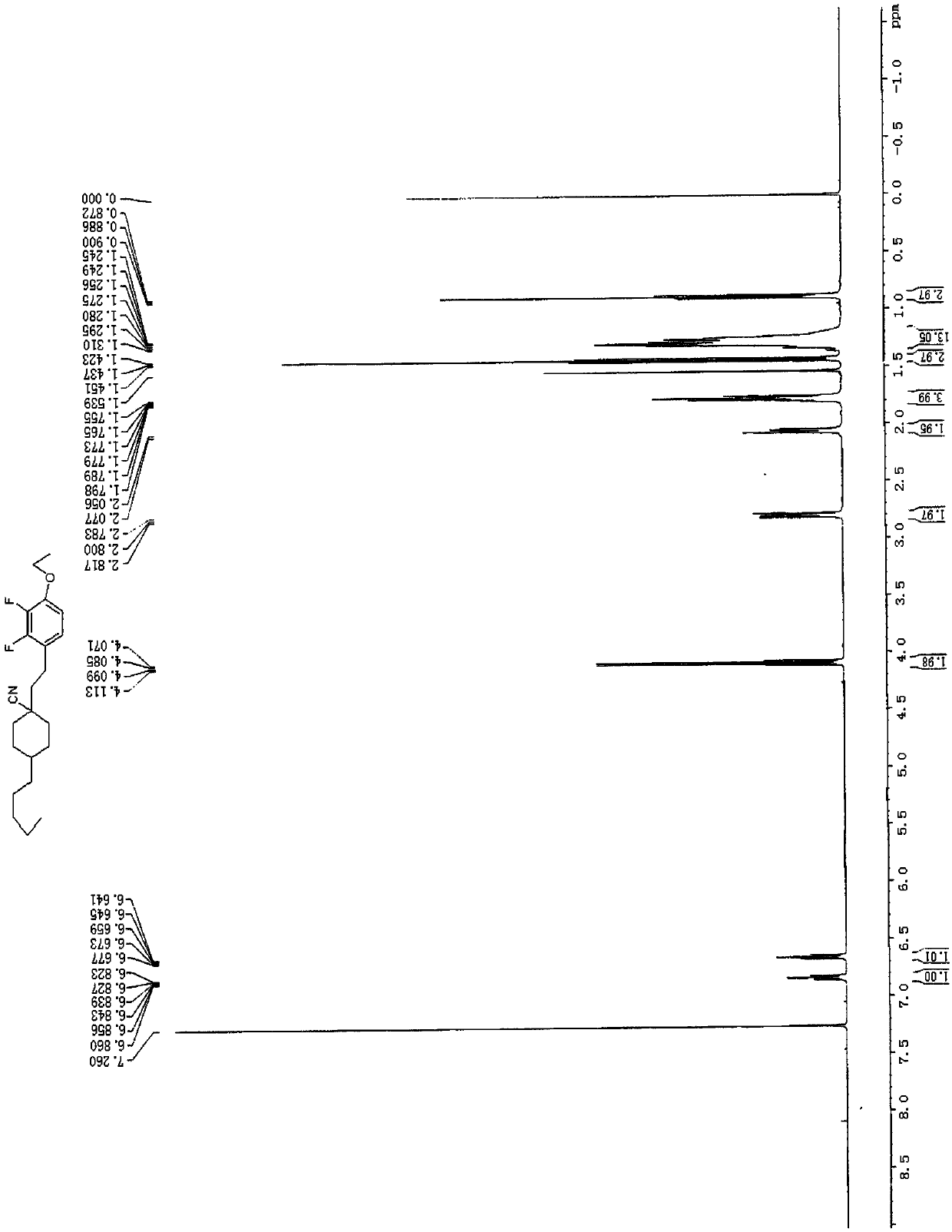

![Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative](https://images-eureka.patsnap.com/patent_img/5a858dde-8c3b-4e46-ae81-05b747950675/BDA0000703241120000012.PNG)
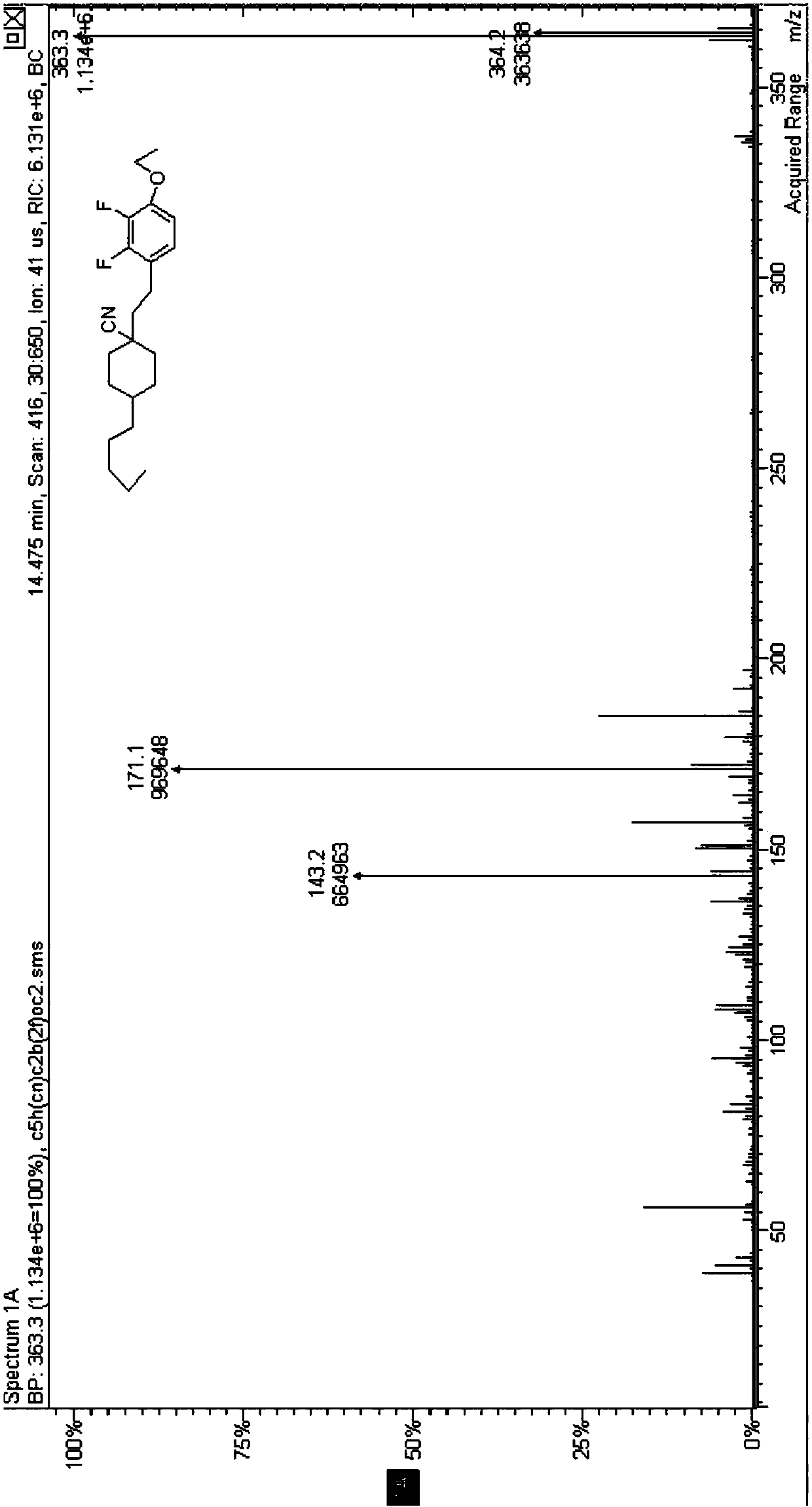
![Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative](https://images-eureka.patsnap.com/patent_img/5a858dde-8c3b-4e46-ae81-05b747950675/BDA0000703241120000021.PNG)
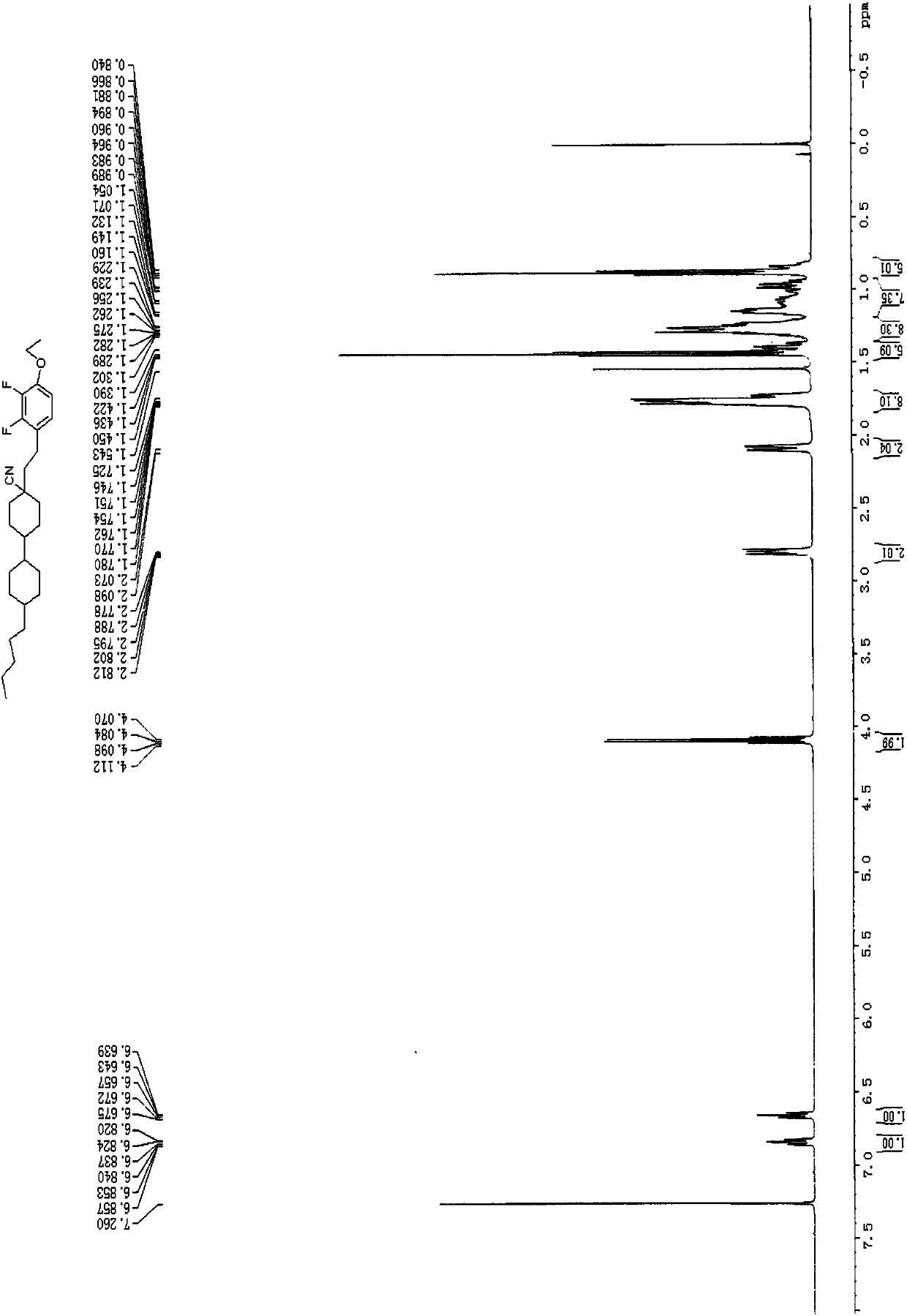
![Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative Synthetic method for 5-azaspiro[2.4]heptane-6-formic acid derivative](https://images-eureka.patsnap.com/patent_img/5a858dde-8c3b-4e46-ae81-05b747950675/BDA0000703241120000022.PNG)