Synthetic method of pyronine derivative dye
A synthesis method and derivative technology are applied in the field of synthesis of Pylonine derivative dyestuffs, which can solve the problems of low yield and long reaction time, and achieve the effects of reducing production cost, high product yield and reducing pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
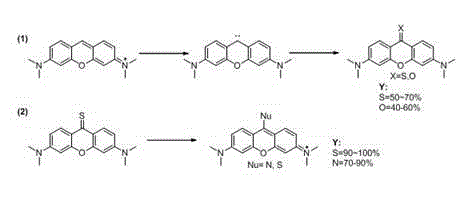
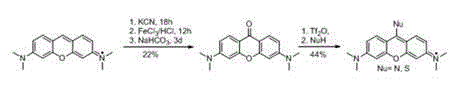
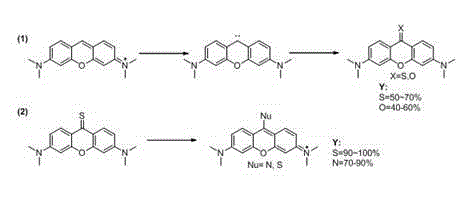
[0017] 1. Synthesis of pyronine thione or ketone, taking thione as an example
[0018] Steps: Add 0.5 (1.66 mmol) pyronin Y and 0.53 g (16.6 mmol) sulfur to a 50 ml round bottom flask at room temperature, then add 20 ml of refined tetrahydrofuran, then add 0.056 g (4.98 mmol) while stirring Potassium tert-butoxide, after the addition, put the reaction bottle into an oil bath, and heat under reflux at 70°C for 10 hours. Then the above reaction system was cooled to room temperature, filtered, the filter cake was washed with dichloromethane, and the filtrate was spin-dried and purified by column chromatography (eluent: PE:DCM=1:1) to obtain pyroninthione 0.27 g, the yield is 55%. 1 H NMR (300 MHz, CDCl 3 ) δ 8.70 (d, J = 9.2 Hz, 2H), 6.75 (dd, J = 9.2, 2.3 Hz, 2H), 6.43 (d, J = 2.3Hz, 2H), 3.13 (s, 12H). 13 C NMR (75 MHz, CDCl 3 ) δ 196.26, 154.58 , 153.03 , 131.80 , 120.07 , 110.52 , 96.06 , 40.24 . ESI-MS: [M+H] + =299.2.
[0019] 2. Synthesis of Thiopyronine Der...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com