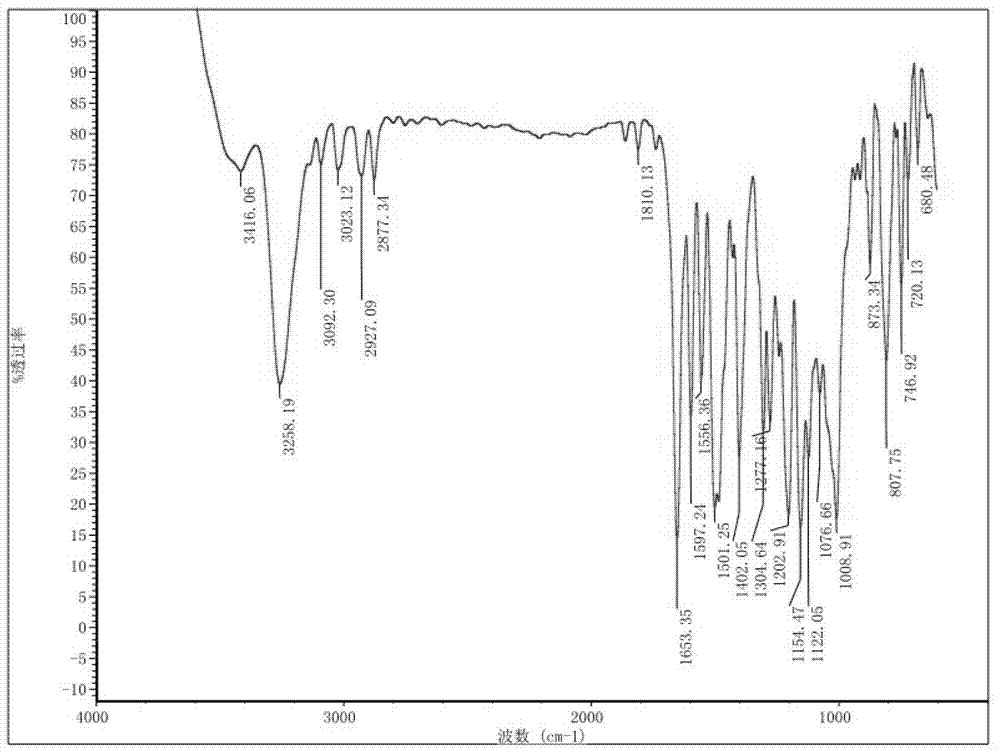
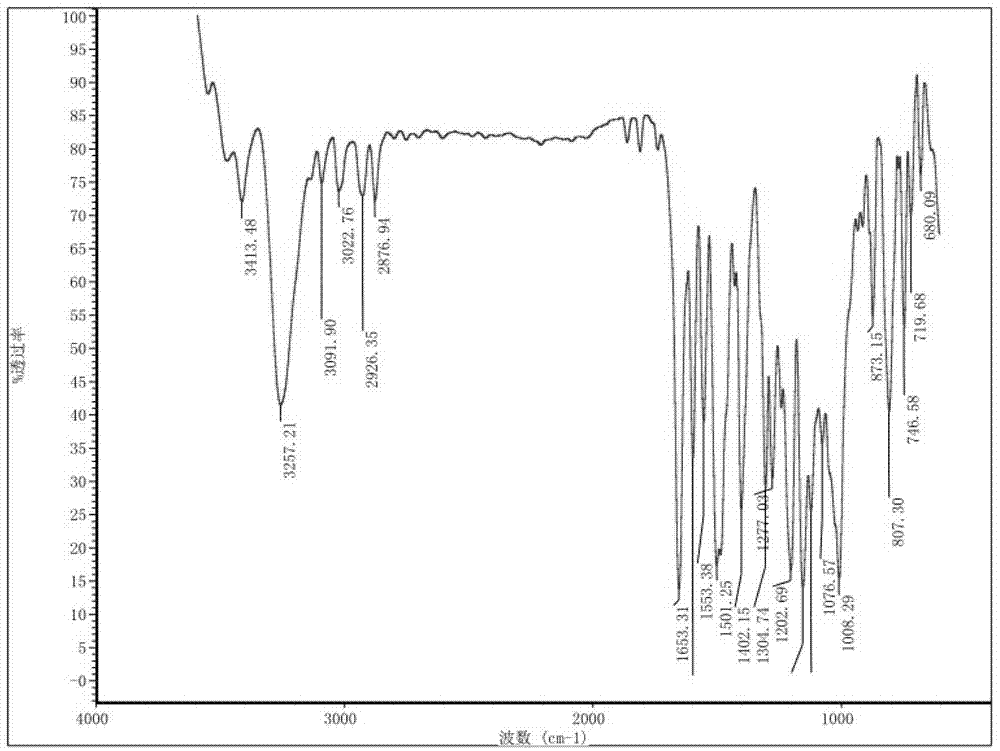
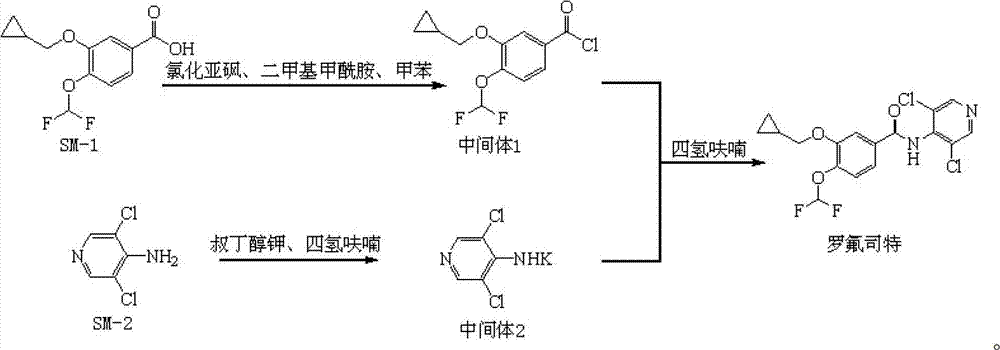
Preparation method and detection method of roflumilast material
A technology of roflumilast and a detection method, applied in the field of medicinal chemistry, can solve the problems of lack of a set of strict and reliable quality detection standards, difficult control and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0277] Embodiment 1: the synthesis of compound SM-1
[0278] Step 1, 3,4-dihydroxybenzaldehyde, sodium chlorodifluoroacetate, H 2 O, NaOH, added to dimethylformamide for heating and stirring reaction, after the reaction, the reaction solution was cooled to room temperature, diluted with water, adjusted to acidity with HCL, then extracted with isopropyl ether, the organic layer was extracted with dilute NaOH, adjusted After the pH of the aqueous layer reaches 3, extract with isopropyl ether, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and recrystallize the residue with isopropyl ether to obtain 3-hydroxy-4-difluoromethoxybenzaldehyde M1 ;
[0279] Step 2, dissolve M1 in THF, add K 2 CO 3 , bromomethylcyclopropane, the reaction solution was heated to reflux for 14h, the reaction solution was cooled to room temperature, filtered, and the solvent was distilled off under reduced pressure, and the residue was dissolved in dilute NaOH, and then...
Embodiment 2
[0281] Embodiment 2: the synthesis of compound SM-2
[0282] Dissolve 4-aminopyridine in dilute hydrochloric acid, add sodium hypochlorite aqueous solution dropwise under heating conditions, after the reaction, adjust the pH to alkaline with NaOH, solid precipitates, filter, wash the precipitate with water, and dry to obtain compound SM-2.
Embodiment 3
[0283] Embodiment 3: the synthesis of compound intermediate 1
[0284]Take 35g of 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid, 39mL of thionyl chloride, 1mL of dimethylformamide, and 350mL of toluene, add them to a 500mL three-necked flask, stir to dissolve the solid, and heat up to 80±5°C, then keep warm at 80±5°C for 3 hours, when the room temperature is naturally cooled to 50°C, transfer the reaction solution into a 500mL round bottom flask, and evaporate the solvent under vacuum to obtain 40g of intermediate 1, the yield range 90-110%, seal the round-bottom flask and set aside.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com