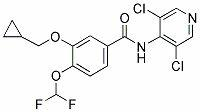
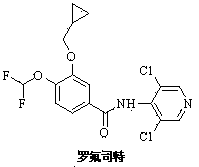
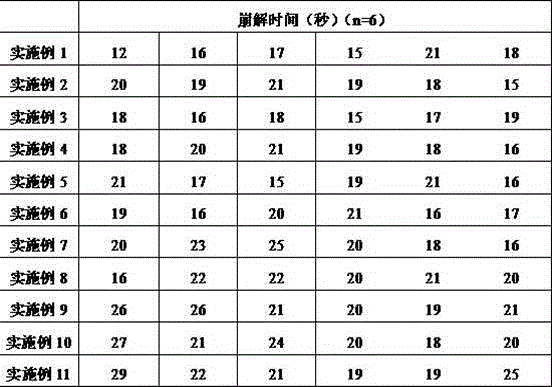
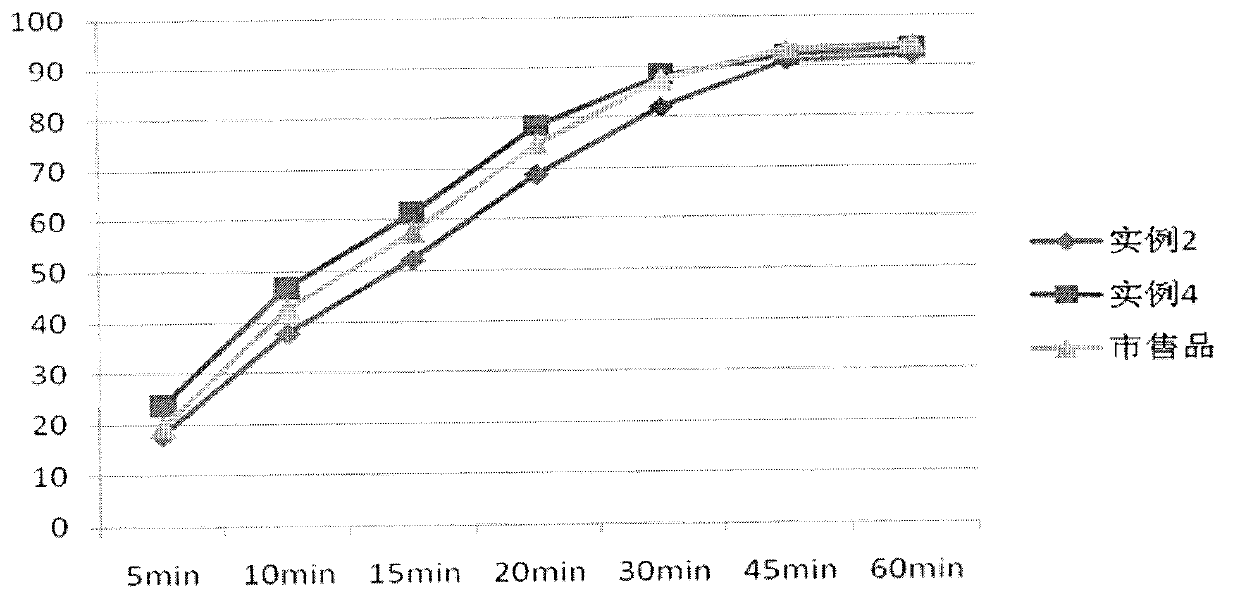
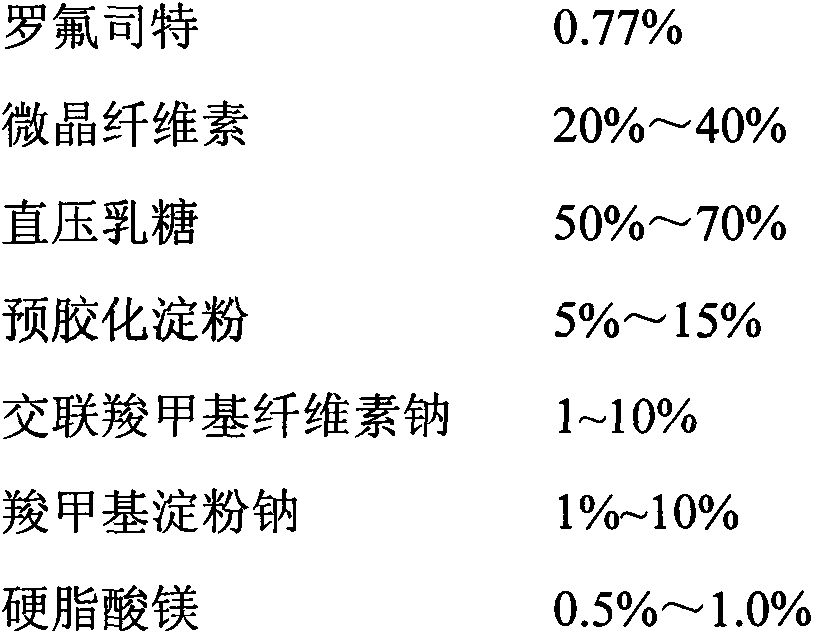
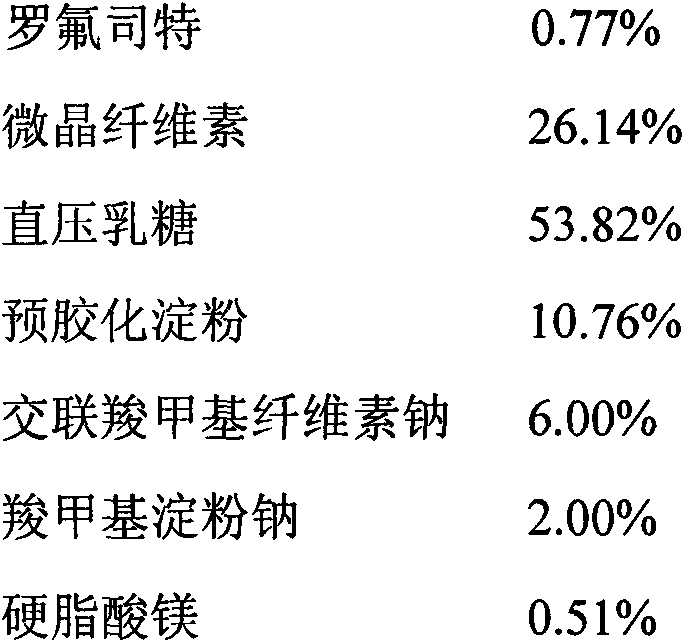
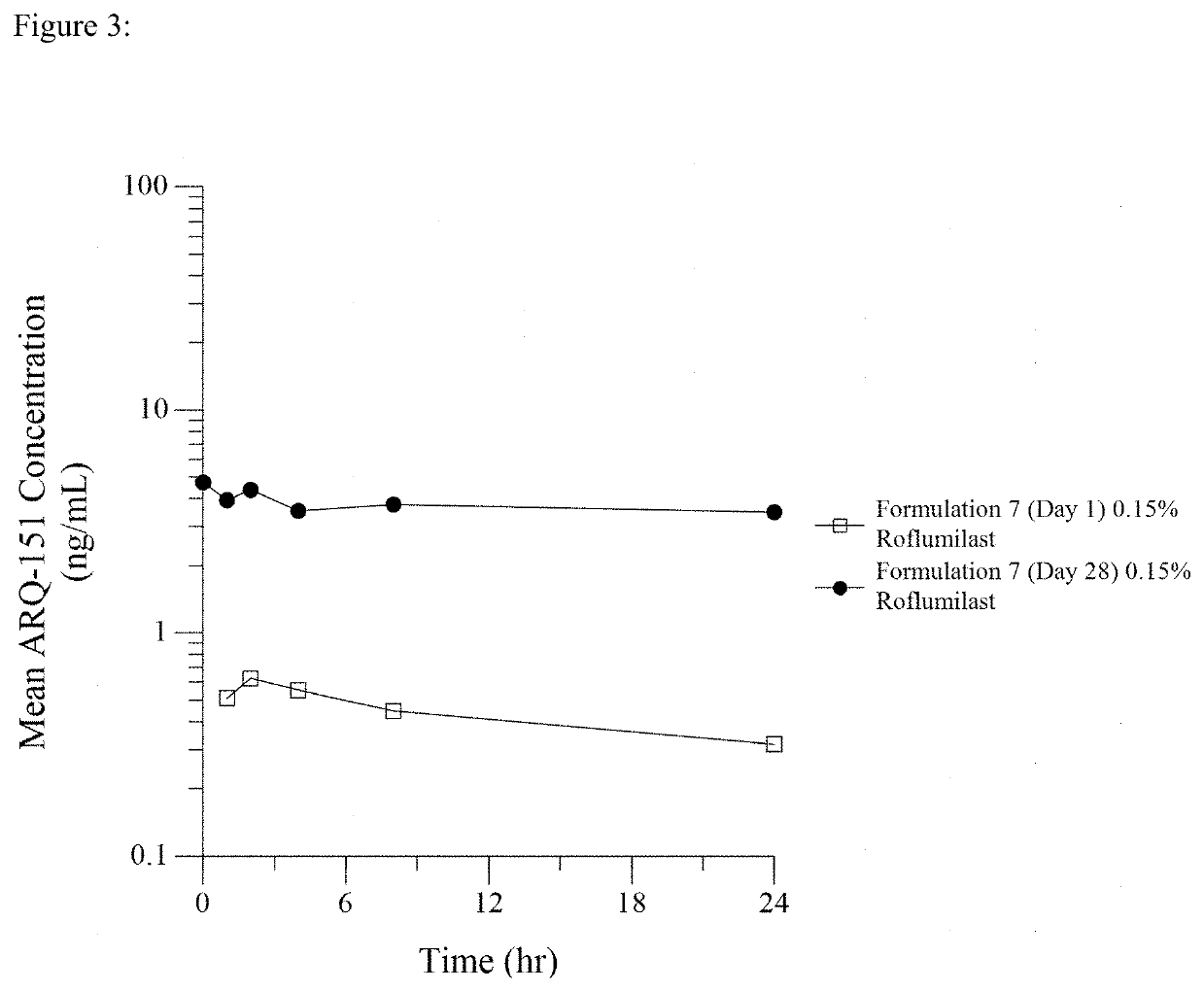
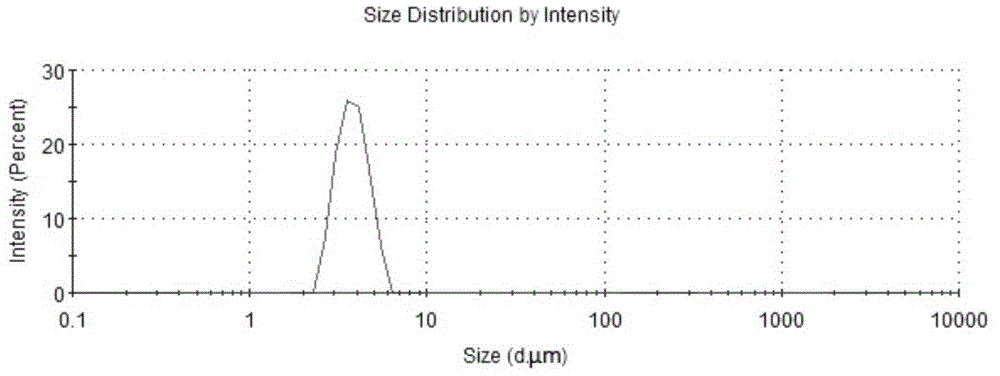
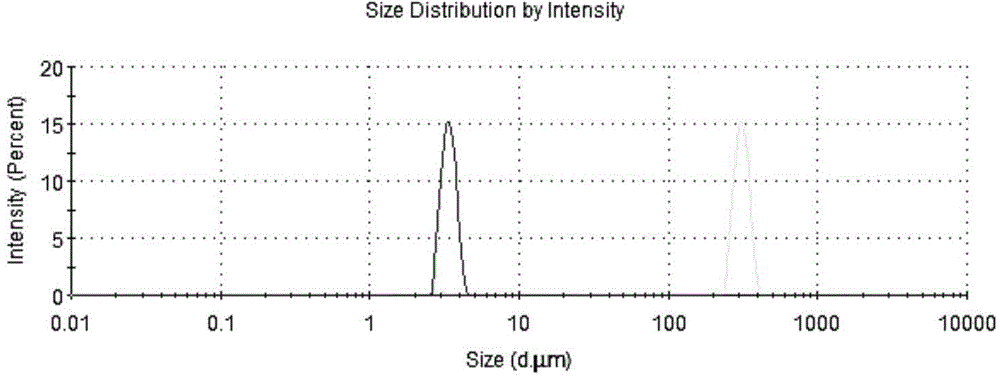
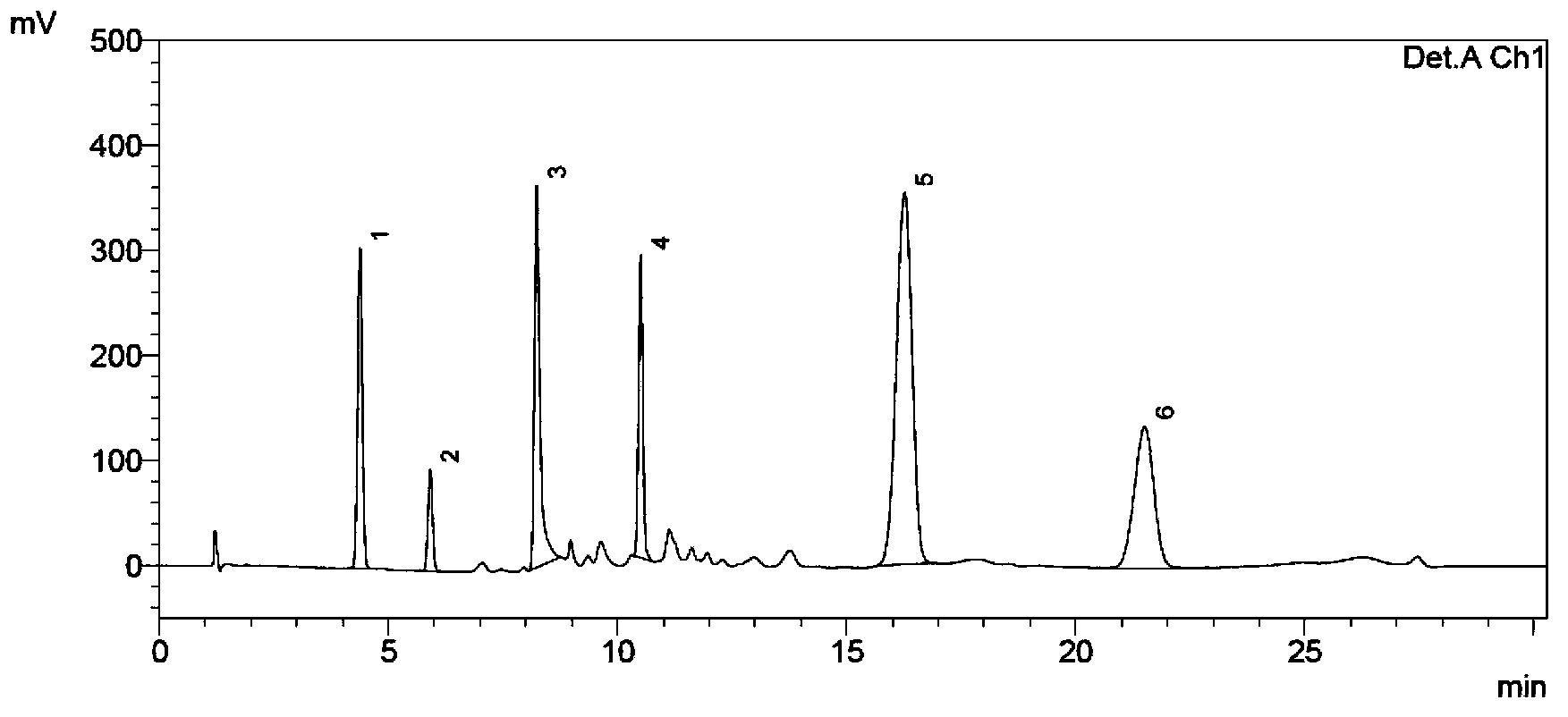
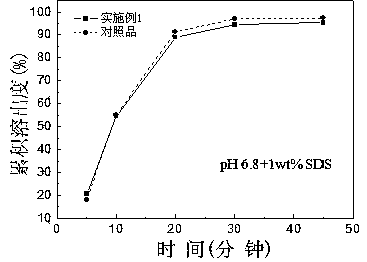
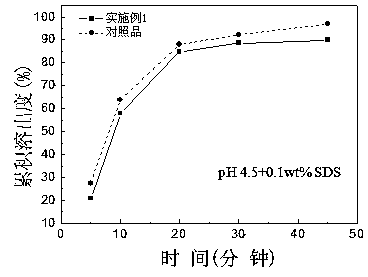
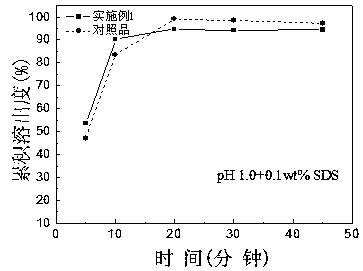
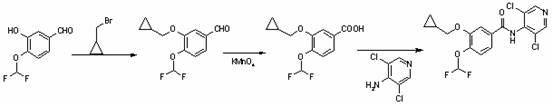
Patents
Literature
141 results about "Roflumilast" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
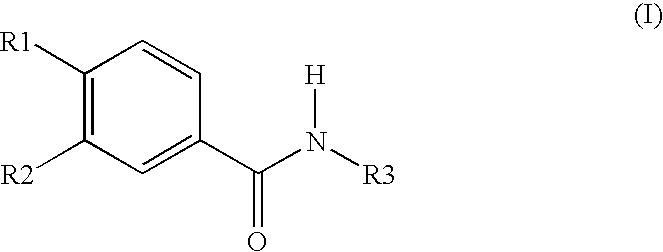
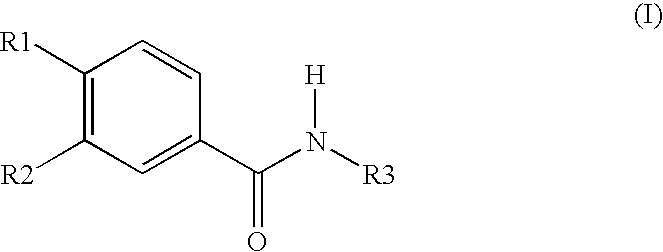
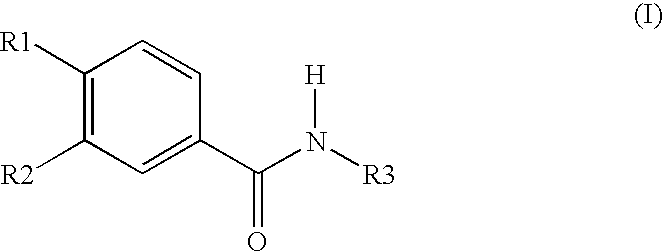
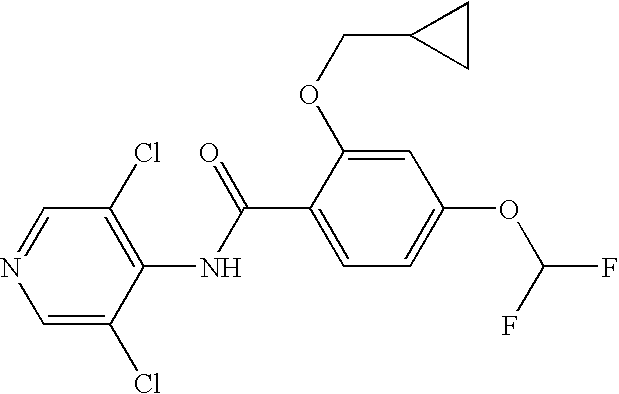
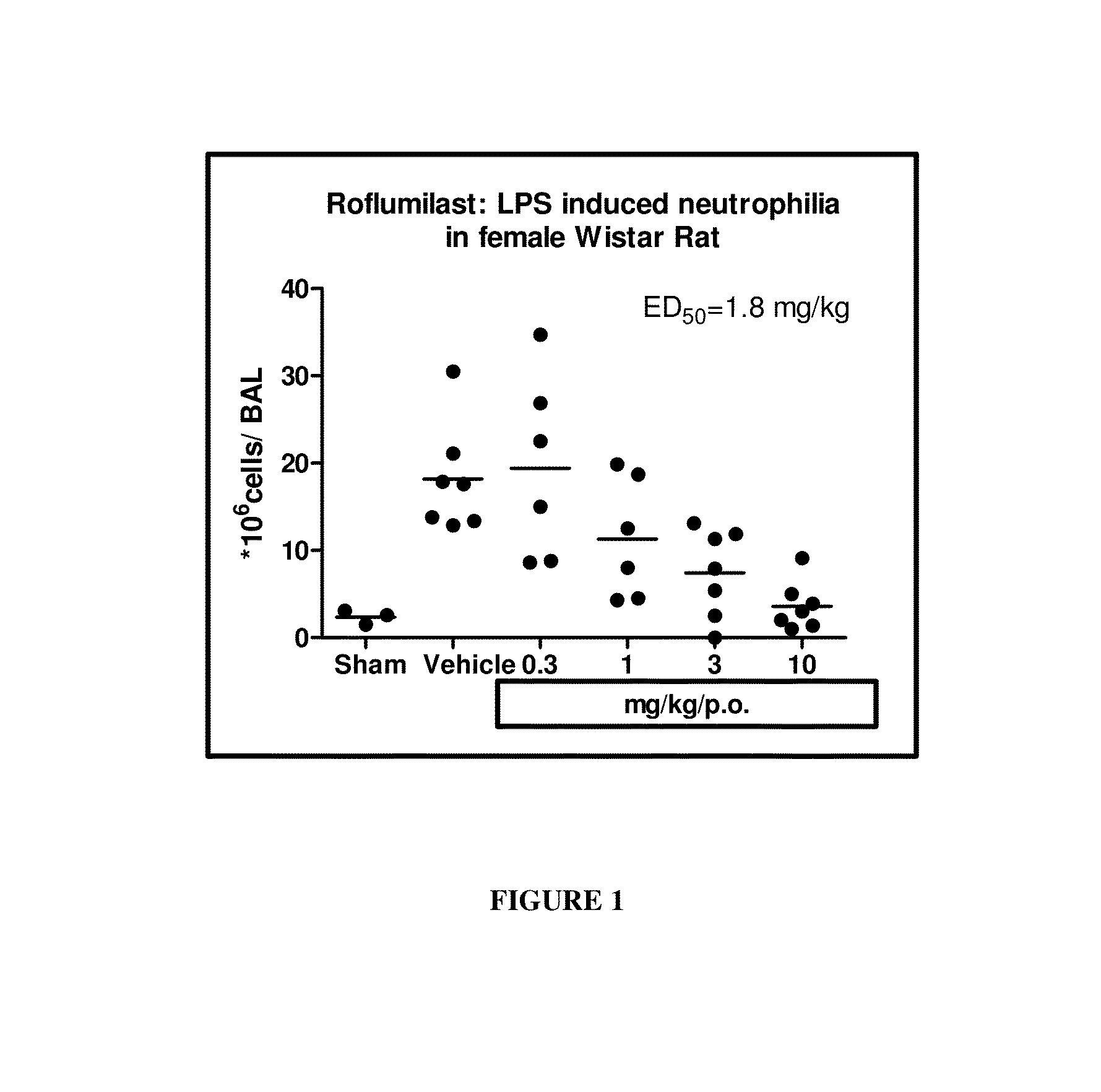
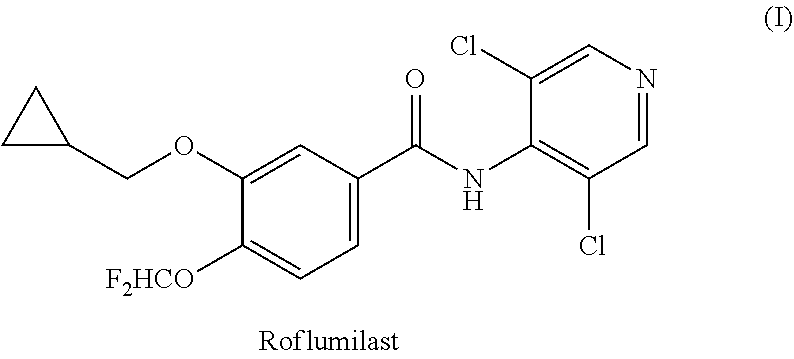
Roflumilast is used to control and prevent symptoms (wheezing and shortness of breath) caused by ongoing lung disease (chronic obstructive pulmonary disease-COPD which includes bronchitis). It should be used along with other medications (bronchodilators such as salmeterol, ipratropium) to treat COPD.
Aqueous Pharmaceutical Preparation Comprising Roflumilast
Owner:ASTRAZENECA AB
Aqueous pharmaceutical preparation comprising roflumilast
Owner:ASTRAZENECA AB
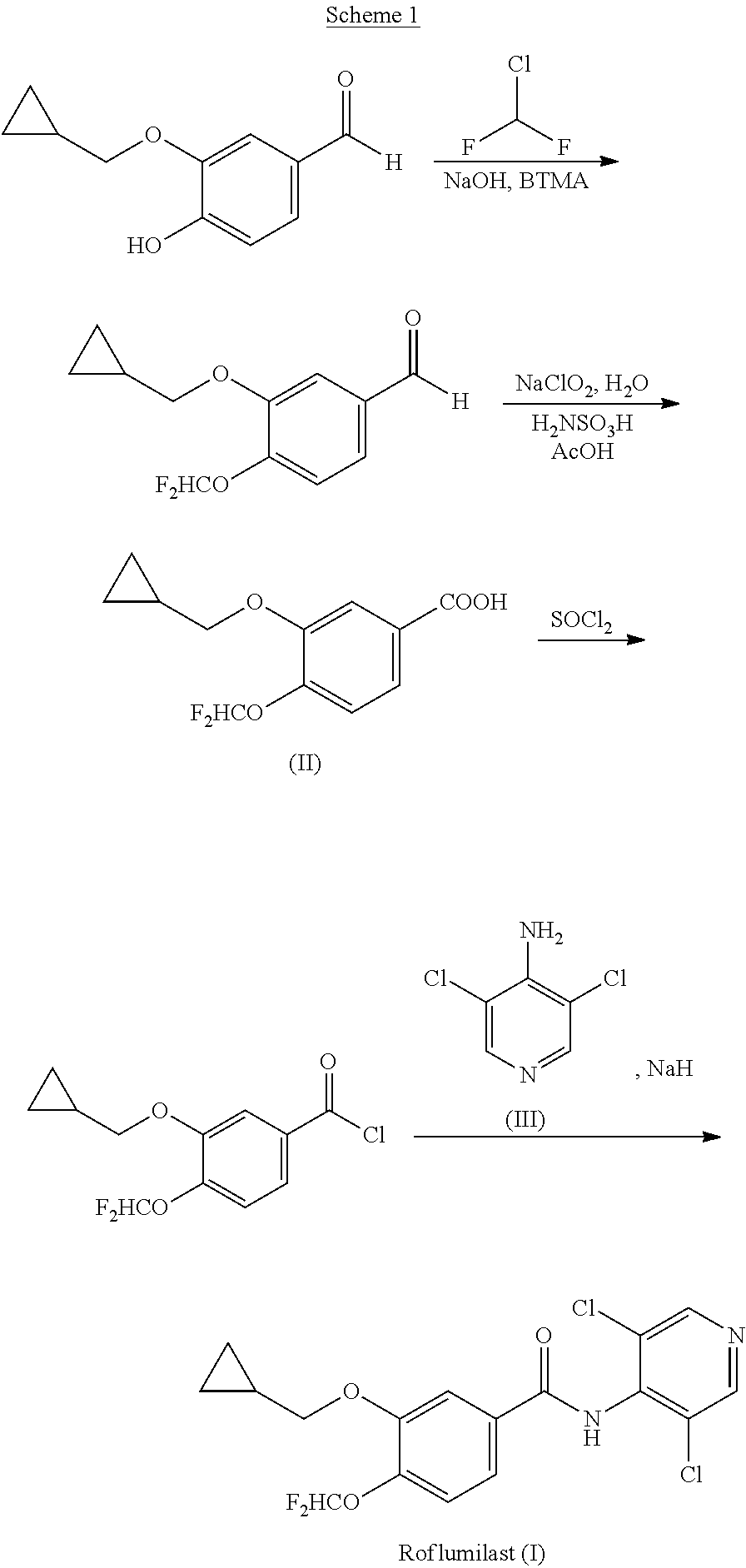
Method for preparing Roflumilast
InactiveCN102336704AShort stepsRaw materials are cheap and easy to getOrganic chemistryBenzaldehydeEthyl acetate
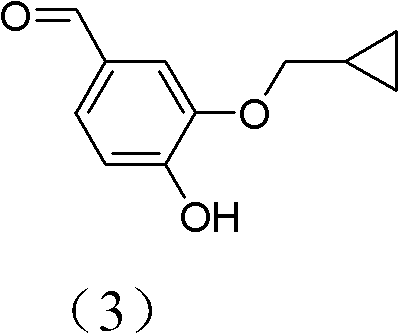
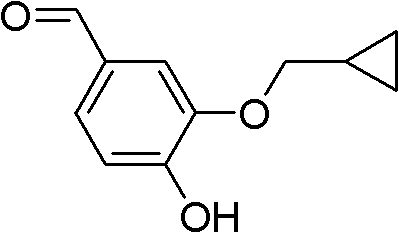
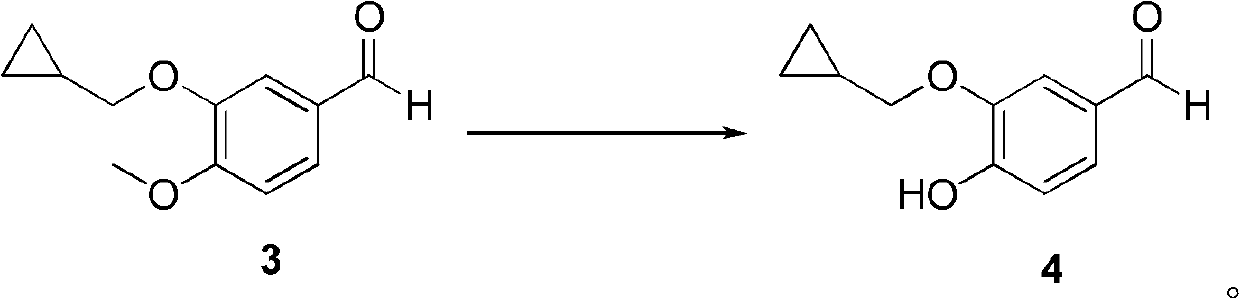
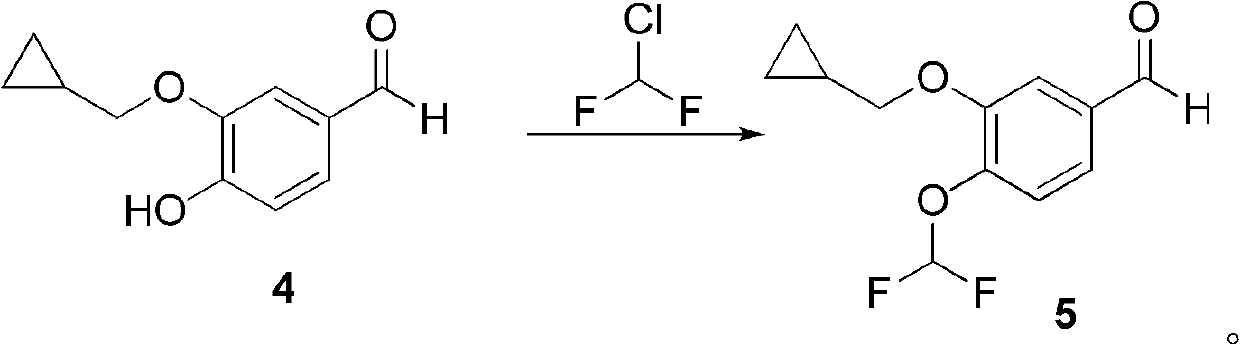
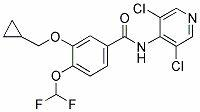
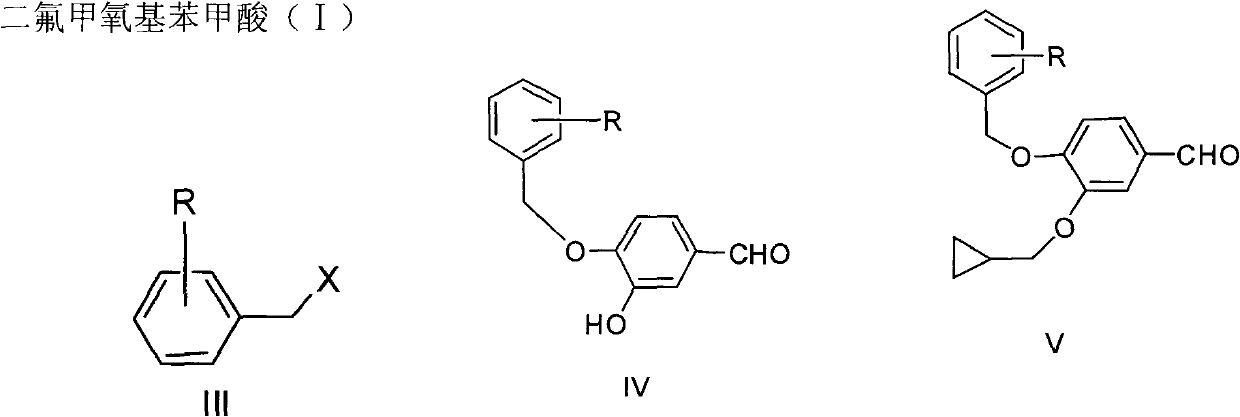
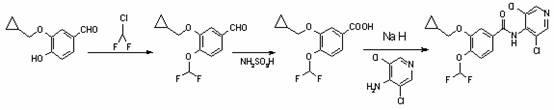
The invention discloses a method for preparing Roflumilast. The method comprises the following steps of: performing cyclopropyl methylation on isovanillin to obtain 3-cyclopropylmethoxy-4-methoxybenzaldehyde; performing demethylation to synthesize an important intermediate of the Roflumilast, namely 3-cyclopropylmethoxy-4hydroxyl-benzaldehyde; and further synthesizing a key intermediate in a formula (5) according to American patent US5712298 and finally synthesizing the Roflumilast in a formula (7). A crude product of the Roflumilast is treated by isopropanol and water, and is recrystallized by ethyl acetate and petroleum ether. The preparation method has a few steps, raw materials are readily available and cheap, the reaction selectivity is high, the yield is high and the post treatment is simple.
Owner:SHANDONG RUIHE PHARMA R&D CO LTD
Inhibition of crystal growth of roflumilast
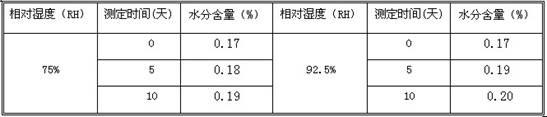
Roflumilast crystals have been shown to increase in size during storage. The size of the roflumilast crystals can affect the bioavailability and efficacy of a pharmaceutical composition. The growth of roflumilast crystals can be inhibited during storage by including hexylene glycol in the composition. The resulting composition has improved bioavailability and efficacy and can be used to inhibit phosphodiesterase 4 in patient in need of such treatment.
Owner:ARCUTIS BIOTHERAPEUTICS INC
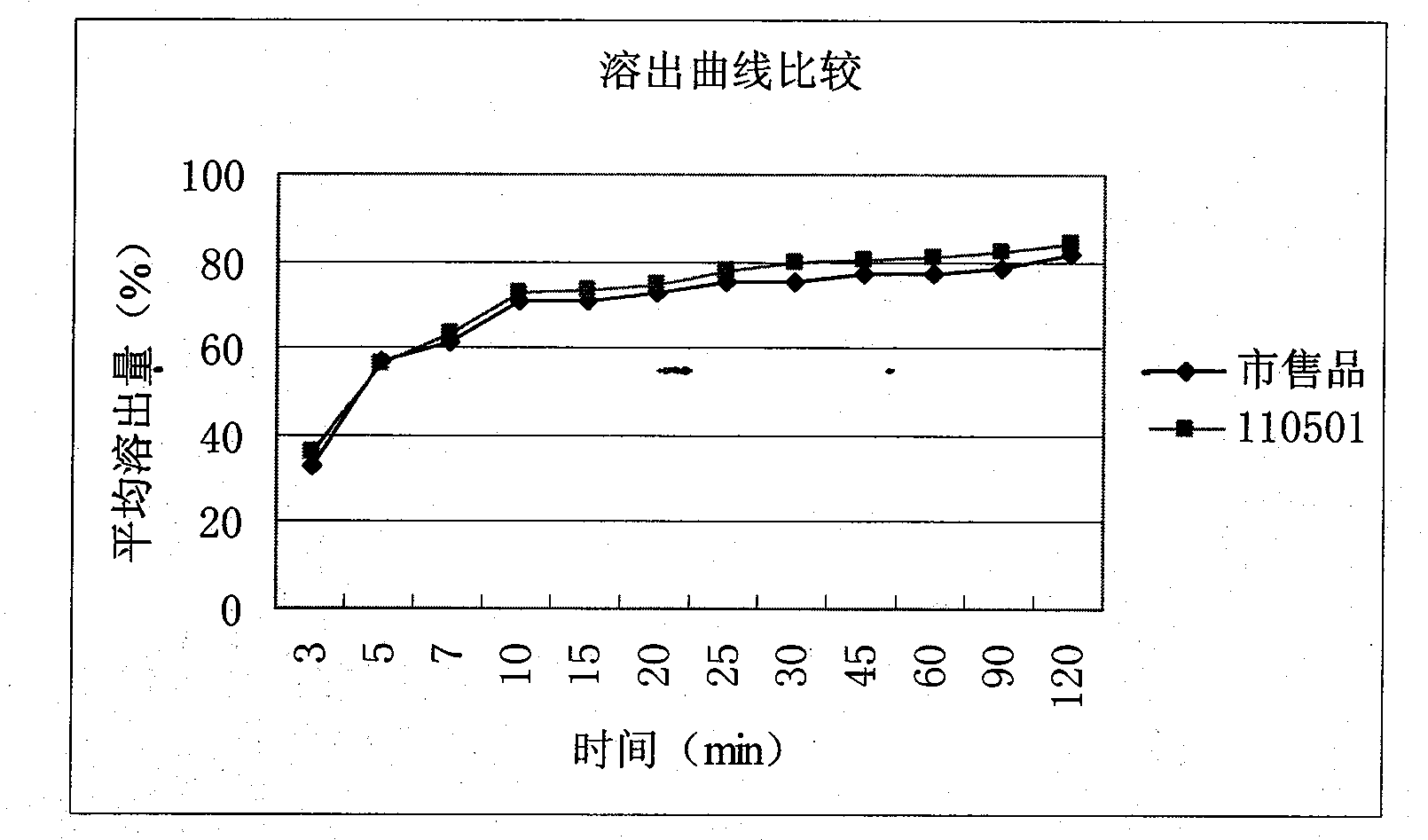
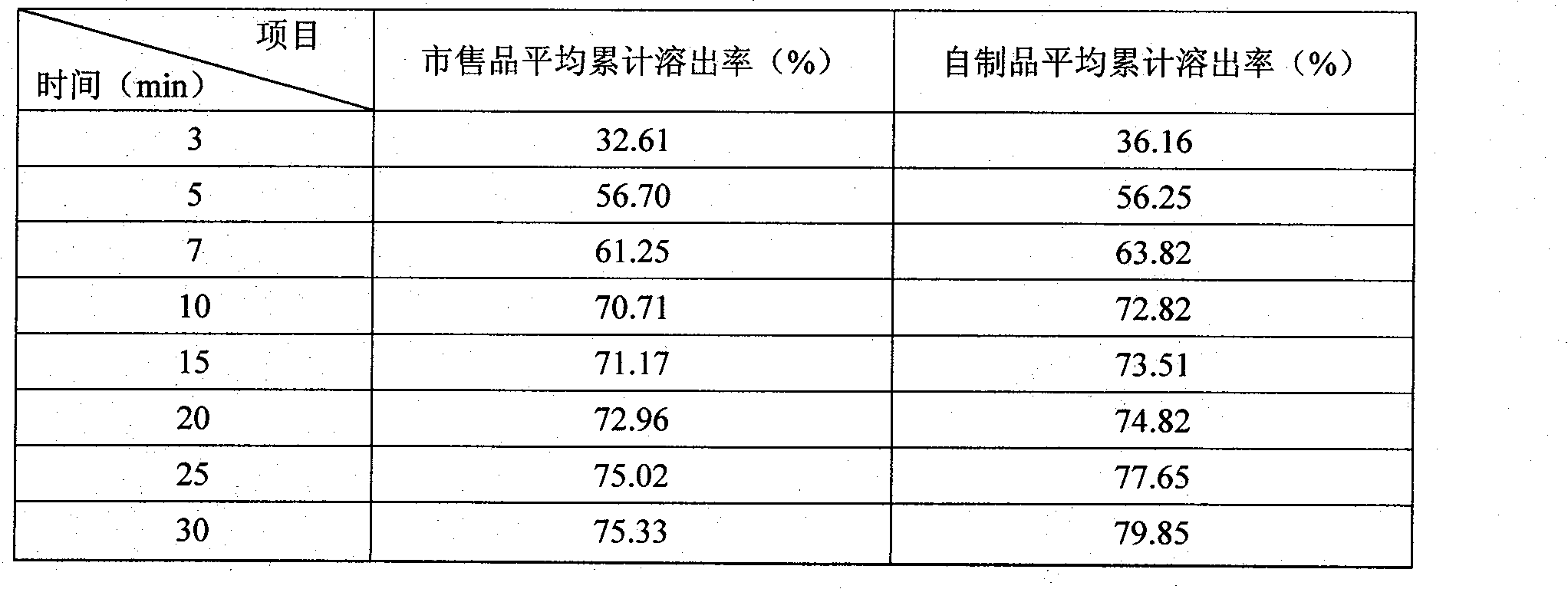
Roflumilast tablets as well as preparation method and detection method thereof
ActiveCN102949370ASimple preparation processEffective massComponent separationRespiratory disorderDiseaseClinical efficacy
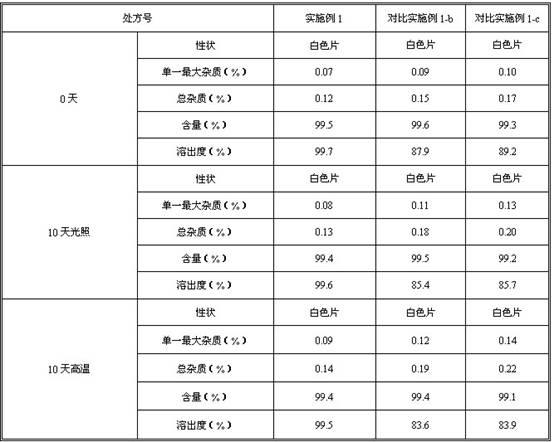
The invention provides roflumilaste tablets as well as a preparation method and a detection method thereof. The roflumilaste tables are prepared by adopting 0.5mg roflumilast as crude drugs, adding 100mg to 150mg of lactose, 80mg to 120mg of pregelatinized starch, 10mg to 30mg of povidone K30 and 0.5 to 2.6mg of magnesium stearate and adopting acetone, ethanol and pure water as solvent. Aiming at the weaknesses of the prior art, the formula and the preparation process of the roflumilast tablets are optimized, so that a remarkable effect for treating diseases such as chronic obstructive pulmonary disease (COPD) is obtained, a systematic, complete and valid component discrimination and content determination method is established, the quality of the medicine can be effectively controlled, and the clinical effect of the roflumilast tablets can be guaranteed.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Preparation method and detection method of roflumilast material
ActiveCN102964297ASimple preparation processReduce adverse reactionsOrganic chemistryMaterial analysis by observing effect on chemical indicatorBenzoic acidDisease
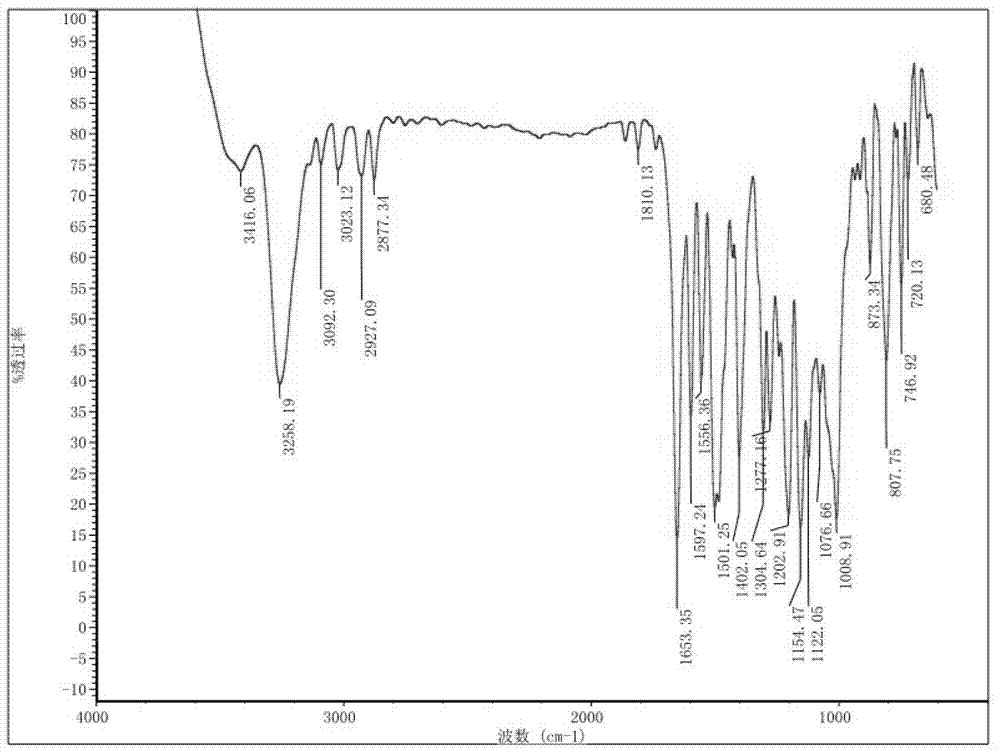
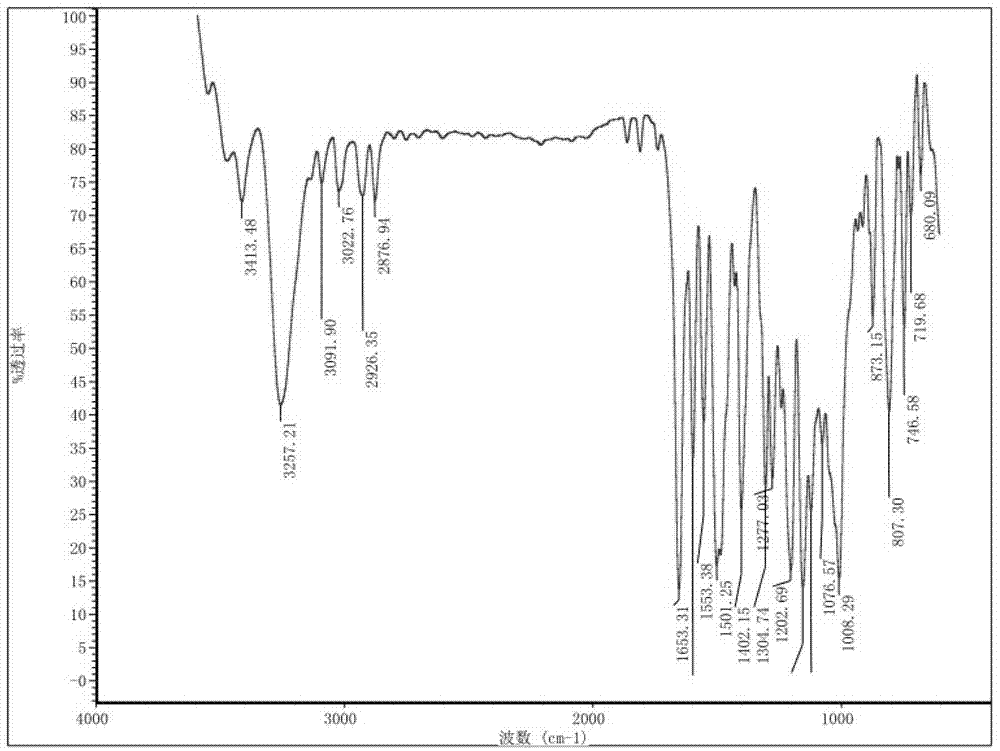
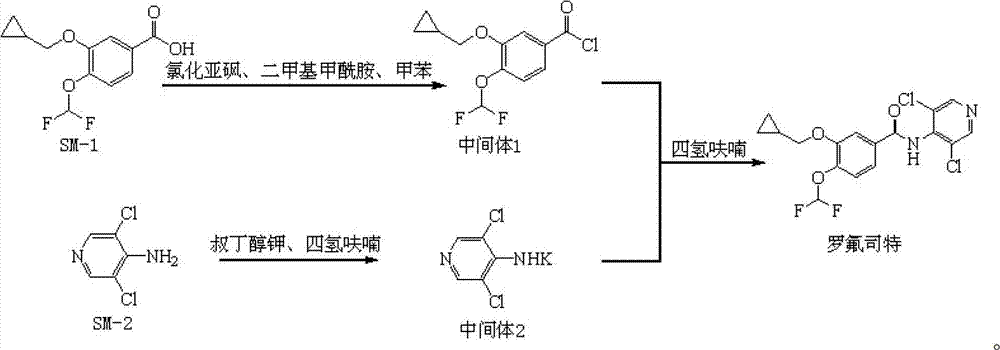
The invention discloses a preparation method and a detection method of a roflumilast material. The preparation method comprises the following steps: mixing 3-cyclopropyl methoxy group-4-difluoro methoxy group benzoic acid SM-1, thionyl chloride, dimethyl formamide with toluene, and carrying out an acylating chlorination reaction to obtain a midbody 1; mixing 3,5-dichloro-4-aminopyridine SM-2, tetrahydrofuran with potassium tert-butoxide and carrying out a salt forming reaction to obtain tetrahydrofuran solution of a midbody 2; and then mixing the midbody 1 and the midbody 2 with tetrahydrofuran, carrying out amidation to obtain a crude product of roflumilast, and refining the crude product of roflumilast to prepare the roflumilast material. Aiming to overcome the shortage of the prior art, the preparation process of the roflumilast material is optimized, so that the curative effect for treating diseases such as chronic obstructive pulmonary disease (COPD) is more remarkable; and besides, a systematic, complete and effective composition identifying and content measuring method is provided, so that the quality of the medicine can be effectively controlled, and the clinical effect is ensured.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
High-bioavailability roflumilast compound
ActiveCN102351787AAdequate responseLess side effectsOrganic chemistryAntipyreticCombinatorial chemistryBioavailability
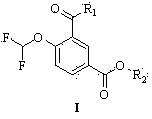
The invention belongs to the technical field of medicines, and particularly relates to a roflumilast compound shown in formula I and a preparation method thereof. Roflumilast is a new crystal form, and has the advantages that the chemical purity is high, the highest purity content is smaller than 1 per mill, the optical purity is high, roflumilast is soluble in water, and a preparation prepared from roflumilast has good stability, particularly wet stability, and high bioavailability. The compound provided by the invention has low production cost and stable quality, and is suitable for industrial production.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Method for preparing roflumilast intermediate
ActiveCN103539671AMild reaction conditionsHigh purityOrganic compound preparationCarboxylic acid esters preparationPalladium on carbonBiochemical engineering
The invention relates to a new roflumilast intermediate compound and a preparation method thereof, and application of the intermediate compound in preparing roflumilast. The roflumilast prepared from the intermediate has the advantages of mild reaction conditions, low requirements for reaction conditions and equipment, fewer byproducts, high product purity and yield and low production cost, does not use palladium on carbon, carbon monoxide or any other high-cost high-toxicity reagent, and is suitable for large-scale industrial production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Roflumilast and integrin inhibitor combination and treatement method
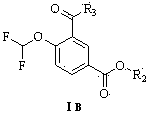
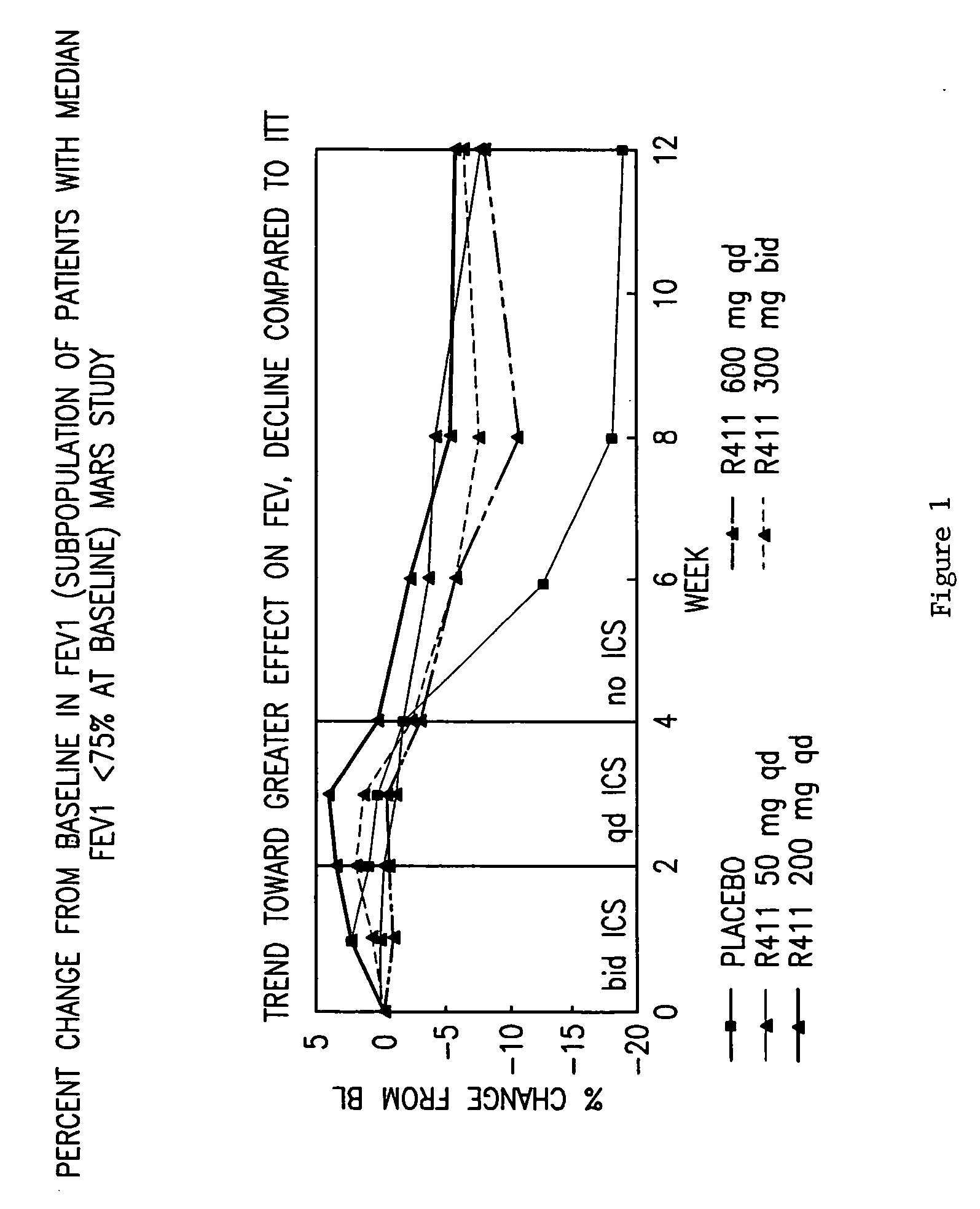
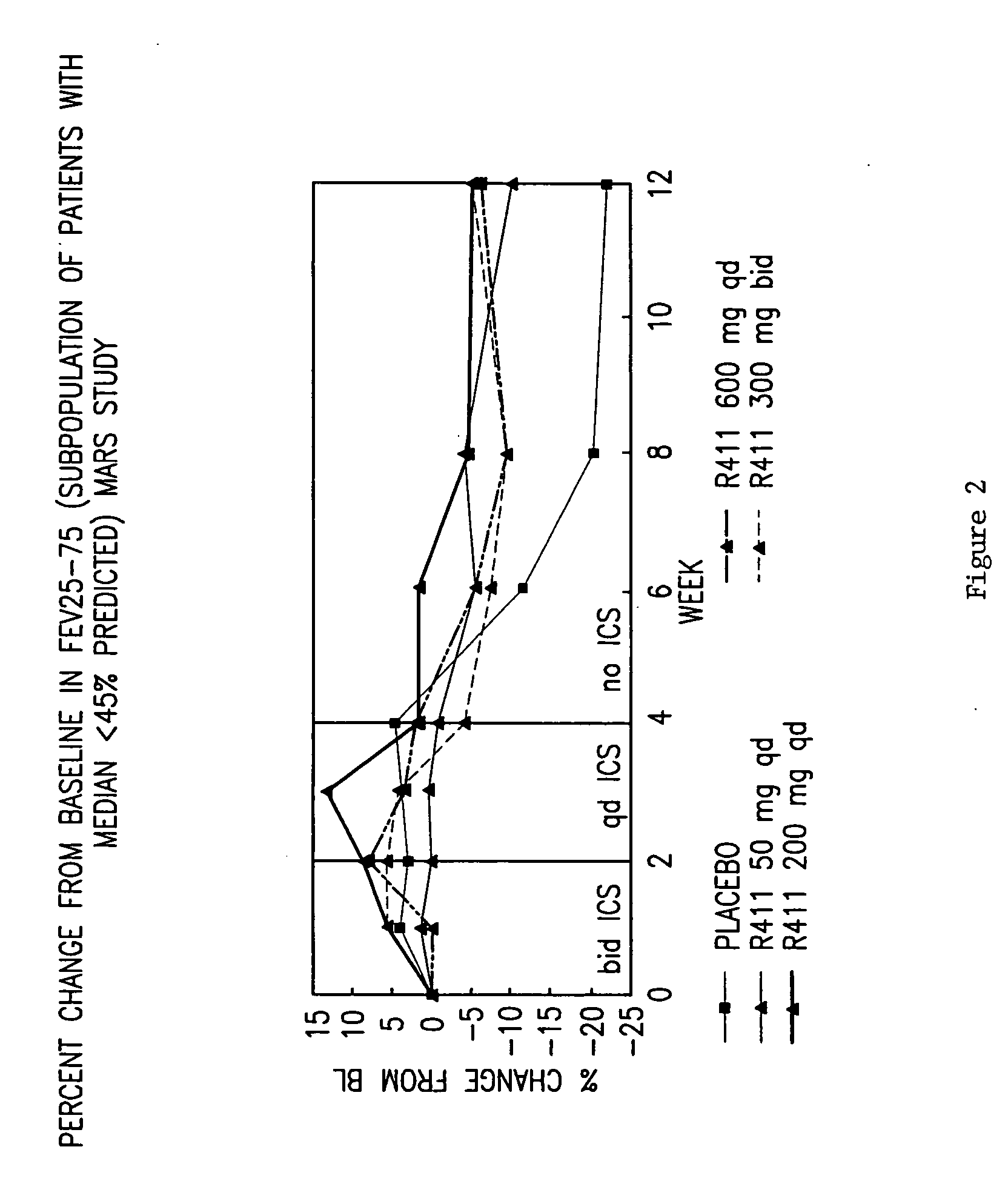
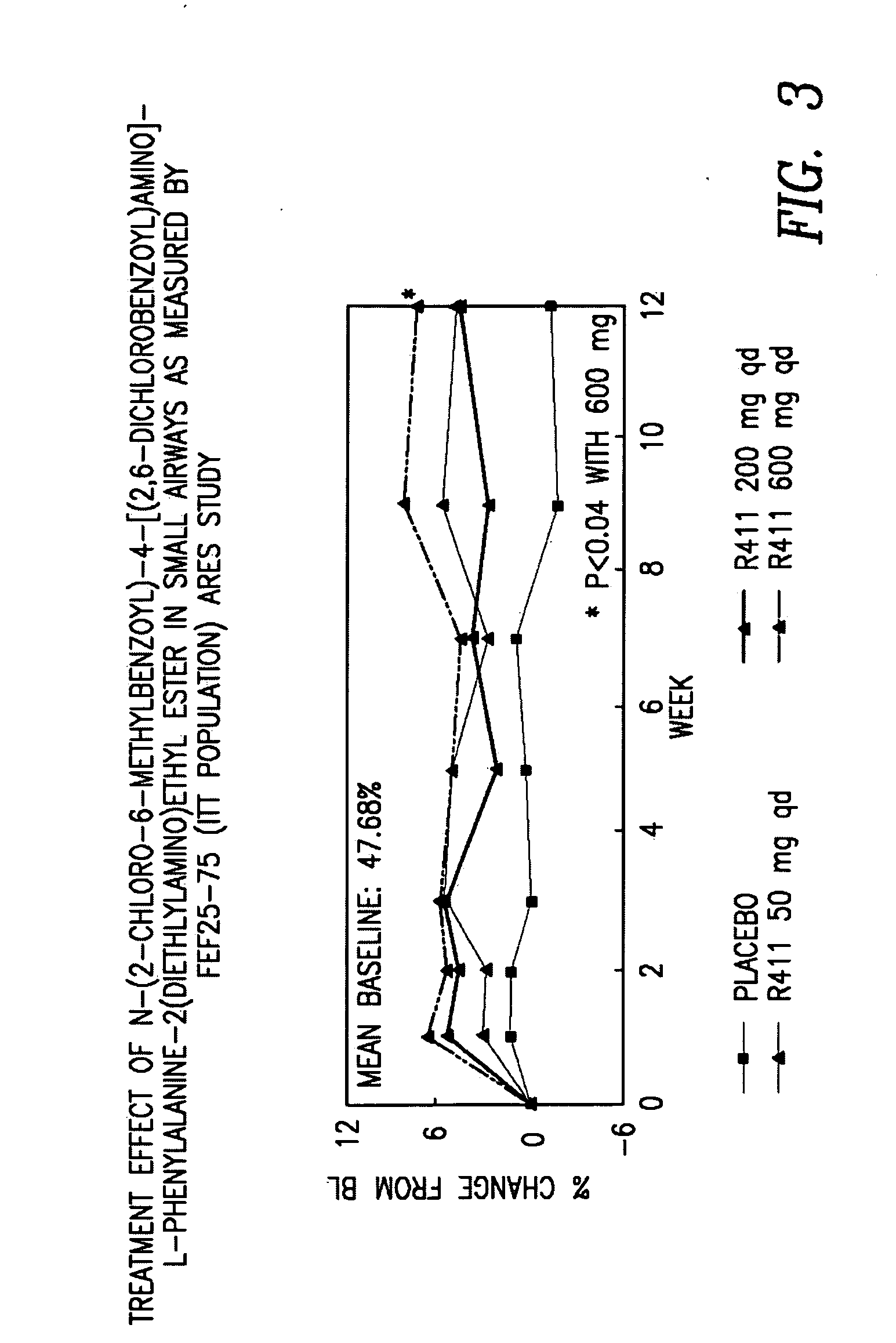
InactiveUS20060198889A1Cell receptors/surface-antigens/surface-determinantsDipeptide ingredientsOral medicationEthyl ester
The present invention provides novel solid pharmaceutical dosage forms for oral administration comprising a therapeutically active amount of roflumilast, or a pharmaceutically acceptable salt thereof, a therapeutically effective amount of N-(2-chloro-6-methylbenzoyl)-4-[(2,6-dichlorobenzoyl)amino]-L-phenylalanine-2-(diethylamino)ethyl ester, or a pharmaceutically acceptable thereof, and one or more pharmaceutically acceptable excipients. These novel solid pharmaceutical dosage forms are useful in the treatment or control of asthma. The present invention also provides a method for treating asthma employing the solid pharmaceutical dosage forms and a method for preparing the pharmaceutical dosage forms.
Owner:SANDHU HARPREET K +1
Inhibition of crystal growth of roflumilast
Roflumilast crystals have been shown to increase in size during storage. The size of the roflumilast crystals can affect the bioavailability and efficacy of a pharmaceutical composition. The growth of roflumilast crystals can be inhibited during storage by including hexylene glycol in the composition. The resulting composition has improved bioavailability and efficacy and can be used to inhibit phosphodiesterase 4 in a patient in need of such treatment.
Owner:ARCUTIS BIOTHERAPEUTICS INC
Method for preparing high-purity roflumilast
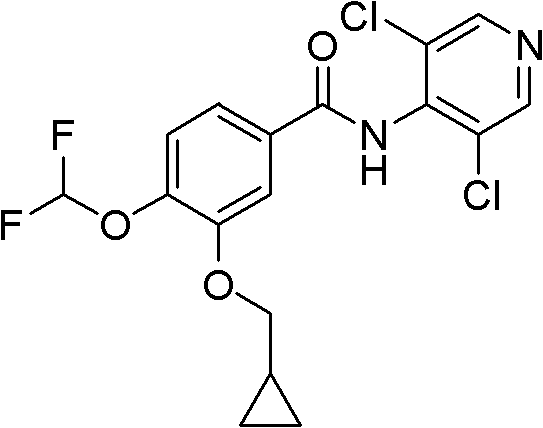
InactiveCN102603623AOrganic compound preparationCarbonyl compound preparationMedicineObstructive Pulmonary Diseases
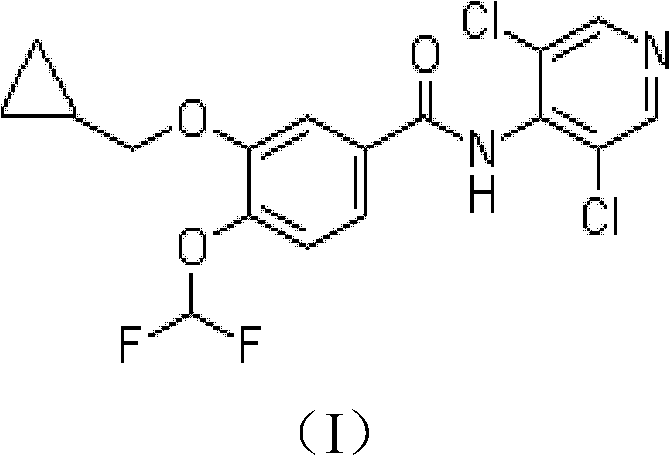
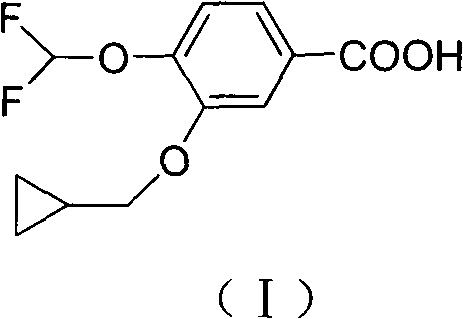
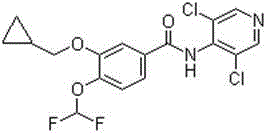
The invention discloses a method for preparing high-purity roflumilast which is a compound as shown in the formula (I). The compound is an orally taken selective phosphodiesterase 4 (PDE4) inhibitor and is proven to be capable of inhibiting inflammation related to a chronic obstructive pulmonary disease (COPD).
Owner:CENTAURUS BIOPHARMA
Roflumilast pharmaceutical composition with high bioavailability and preparation method thereof
ActiveCN102274222ASolve the problem of large differences in content uniformityRapid dissolutionRespiratory disorderMacromolecular non-active ingredientsCyclodextrinPharmaceutical drug
The invention discloses a high-bioavailability roflumilast medicinal composition, which consists of roflumilast, betacyclodextrin, lactobiose, microcrystalline cellulose and magnesium stearate. The medicinal composition is characterized in that: a weight ratio of the roflumilast to the betacyclodextrin to the lactobiose is 1:2:20; the roflumilast medicinal composition has the advantages of improving yield, reducing cost, realizing industrialization, along with high stability; and the composition is better applied clinically, and the dissolution rate and bioavailability can be improved effectively; and the high-bioavailability roflumilast medicinal composition can be used for treating chronic obstructive pulmonary diseases.
Owner:天津汉嘉医药科技有限公司
New method for preparing roflumilast
InactiveCN102617457AHigh yieldThe reaction is easy to operateOrganic compound preparationCarbonyl compound preparation3-HydroxybenzaldehydeBenzoic acid
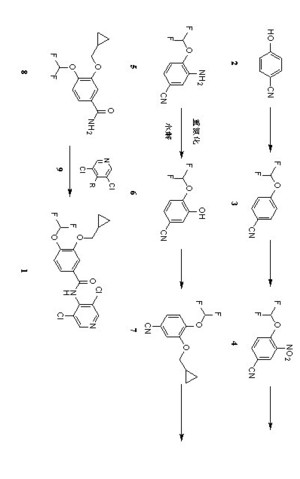
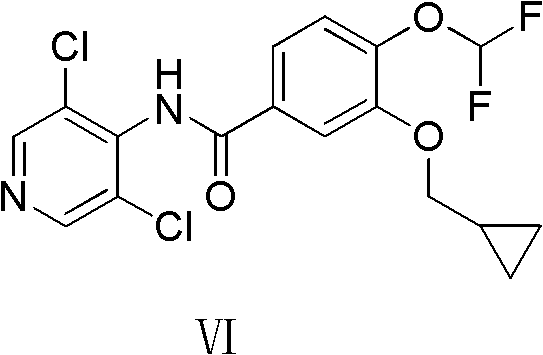
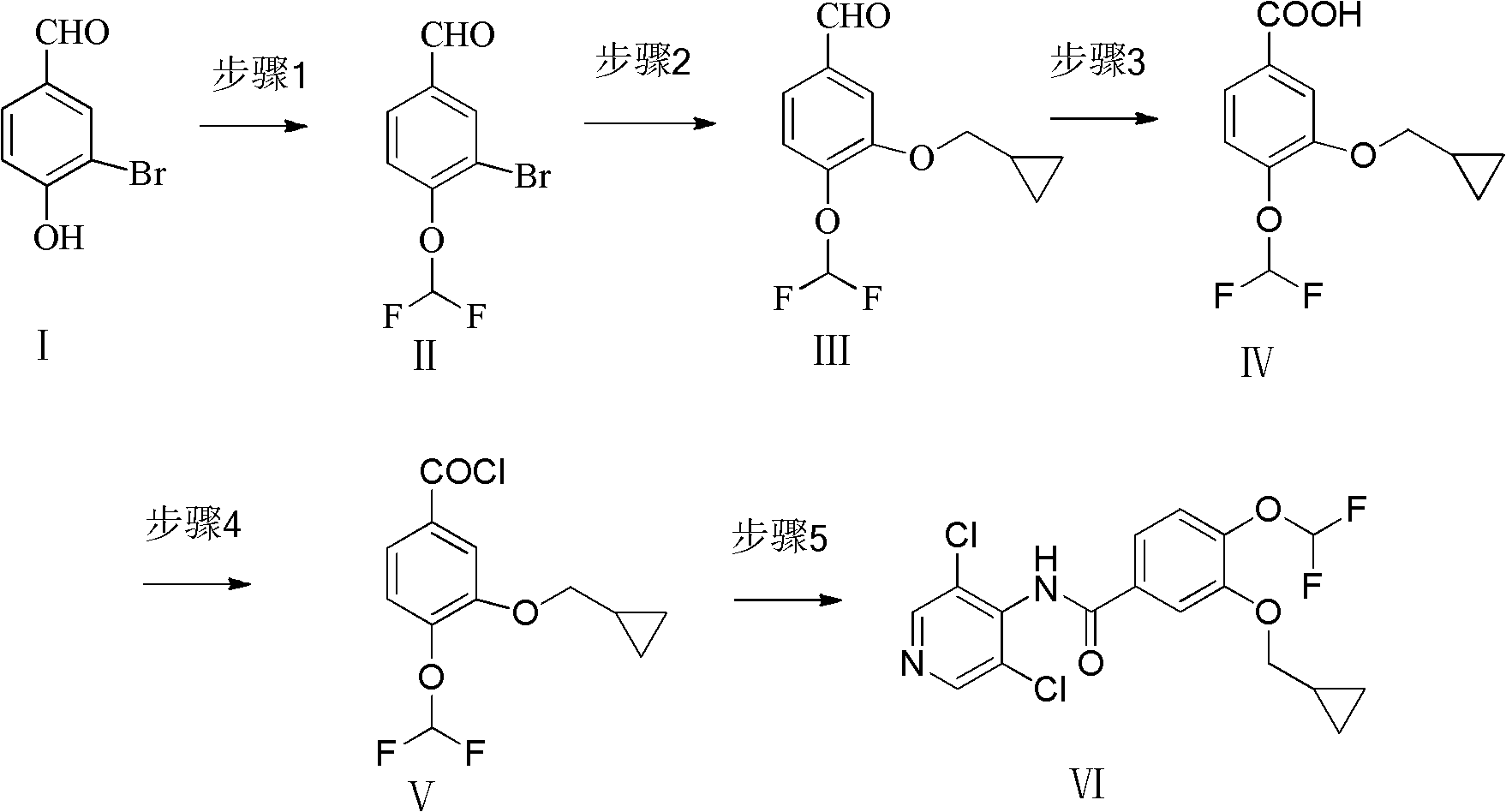
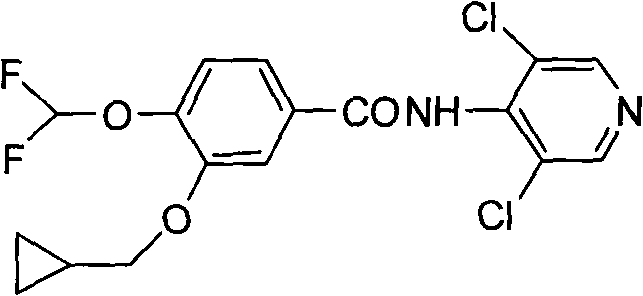
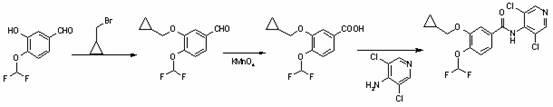
The invention provides a simple method for preparing roflumilast. According to the invention, 3-bromine-4-hydroxy-benzaldehyde (I) is etherified to obtain 4-difluoromethoxy-3-hydroxybenzaldehyde (II), the compound II is subjected to an Ullmann condensation reaction to obtain 3-cyclopropylmethoxy-4-difluoromethoxy-benzaldehyde (III), the compound III is oxidized by sodium hypochlorite to obtain 3-cyclopropylmethoxy-4-difluoromethoxy-benzoic acid (IV), the compound IV is chloridized to obtain 3-cyclopropylmethoxy-4-difluoromethoxy-benzoyl chloride (V), and the compound V and 3,5-dichloro-4-aminopyridine are acylated to obtain the roflumilast. The method of the invention has the advantages of no need of selective etherification and column chromatography purification in the preparation process, simple reaction operation, simple post-treatment, low cost, high yield, and high purity.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +1
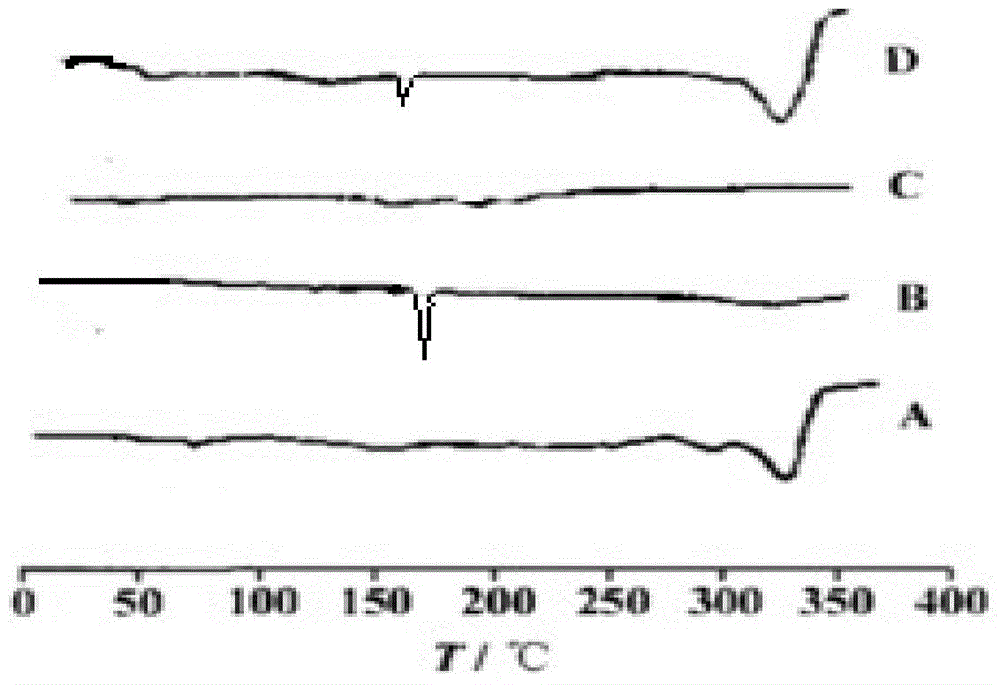
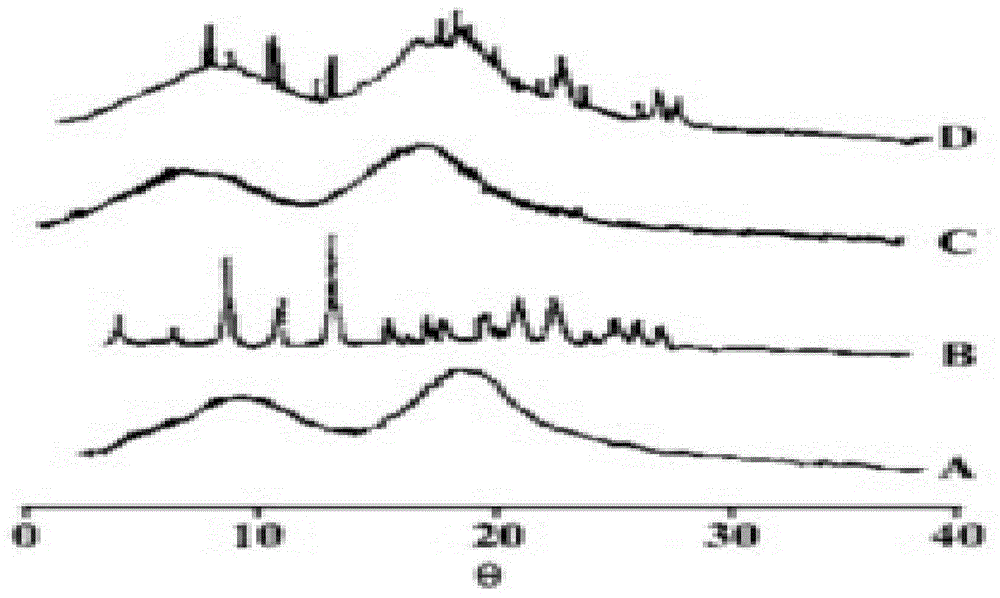
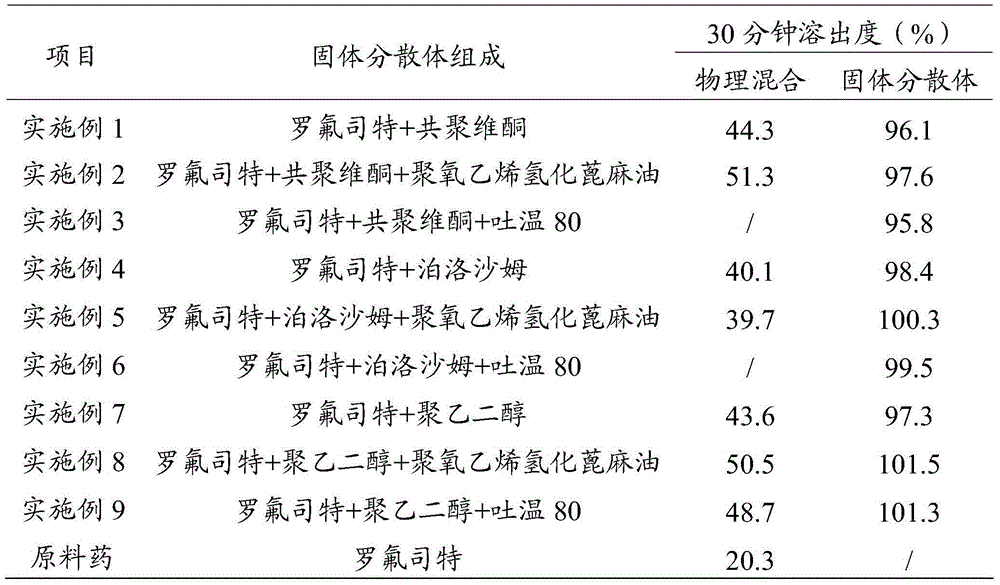
Roflumilast solid dispersoid and preparation method thereof as well as roflumilast preparation
ActiveCN104473862AHigh dissolution rate in 30 minutesImprove bioavailabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMedicinePolyethylene glycol
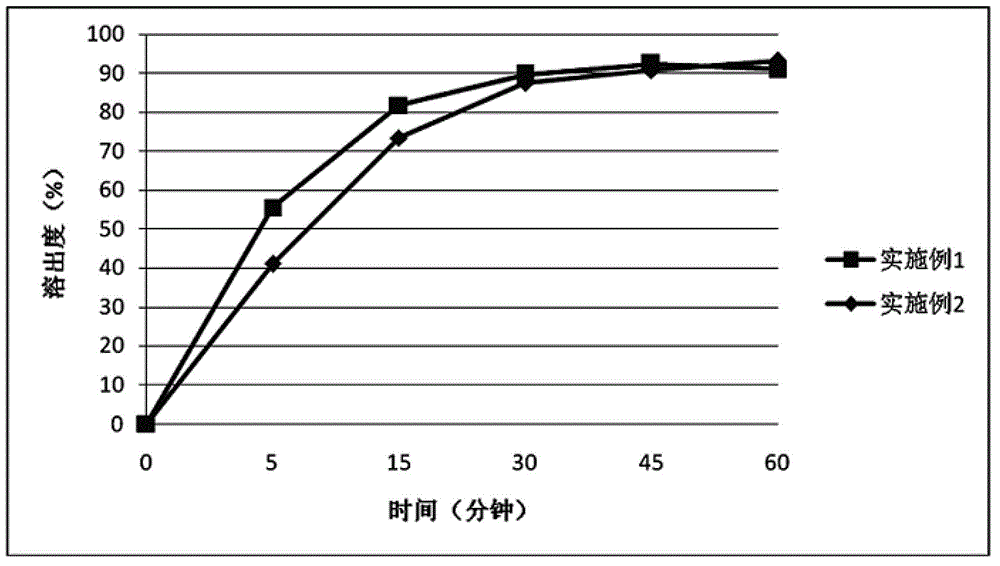
The application belongs to the field of medicines, and in particular relates to a roflumilast solid dispersoid and a preparation method thereof as well as a roflumilast preparation. The roflumilast solid dispersoid provided by the application comprises the following components in parts by weight: 1 part of roflumilast and 3-10 parts of a carrier, wherein the carrier is copovidone, poloxamer or polyethylene glycol. The roflumilast preparation provided by the invention comprises the following components in parts by weight: 0.4-12 parts of a solid dispersoid and 5-95 parts of a filling agent. Experiment results show that the 30-minute dissolution rate of the roflumilast solid dispersoid provided by the application is more than 96%; and the 30-minute dissolution rate of the roflumilast preparation provided by the invention is more than 98%, and the biological availability is more than 75%.
Owner:BEIJING COLLAB PHARMA
Eye drop containing roflumilast
InactiveUS20090209599A1Good effectGood curative effectBiocideSenses disorderBULK ACTIVE INGREDIENTActive ingredient
An object of the present invention is to enhance the efficacy of roflumilast in an eye drop containing roflumilast as an active ingredient. By formulating at least one type of viscosity-increasing agent in the eye drop containing roflumilast as an active ingredient, an eye drop in which the efficacy of roflumilast is enhanced can be prepared.
Owner:SANTEN PHARMA CO LTD
Novel crystalline state of roflumilast and preparation method thereof
The invention discloses a novel crystalline state of roflumilast and a preparation method thereof. The crystalline state is a stable high-purity roflumilast crystalline state form, and has the characteristics of easiness, convenience and practicability in operation and high adaptability to industrial mass production; particularly, the solubility of the product in a water-containing system is properly increased; and further, a medicinal preparation applying the crystalline state roflumilast prepared by the invention can still reach an effective dissolution rate under the condition of not adding PVP (Polyvinyl Pyrrolidone).
Owner:大道隆达(北京)医药科技发展有限公司
Preparation method for roflumilast intermediate
InactiveCN102503815AThe total yield of the five-step reaction is highMild reaction conditionsOrganic compound preparationCarboxylic compound preparationBenzaldehydeRoflumilast
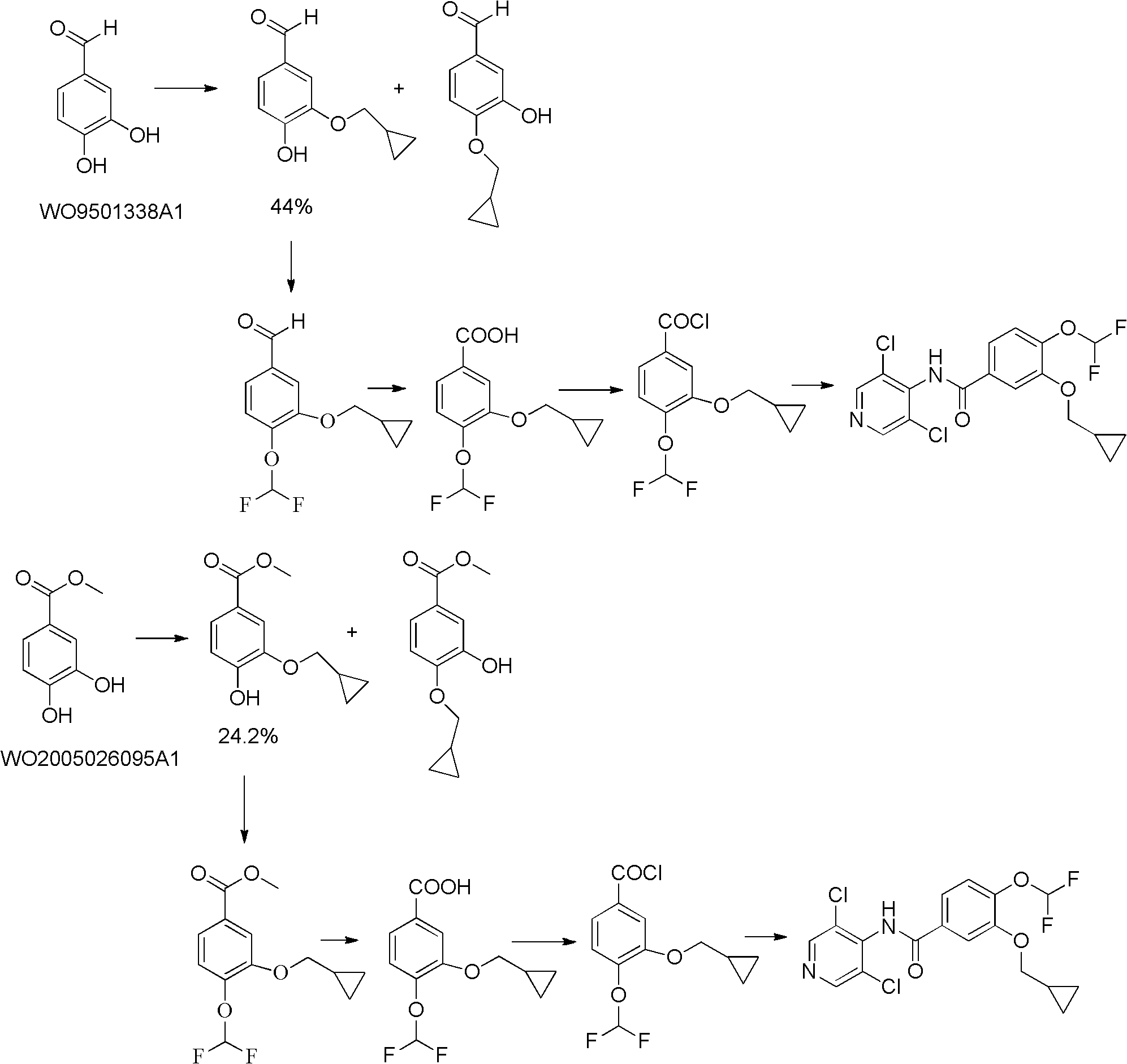
The invention discloses a preparation method for a roflumilast intermediate. The preparation method is characterized by comprising the following steps of: etherifying 3,4-dihydroxy benzaldehyde serving as a raw material and 3-hydroxyl by using benzene ring non-substituted, mono-substituted or poly-substituted benzyl protection 4-hydroxy and halogenated methyl cyclopropane; performing catalytic hydrogenolysis to obtain 4-hydroxy-3-cyclopropyl methoxy-benzaldehyde; etherifying and oxidizing the 4-hydroxy-3-cyclopropyl methoxy-benzaldehyde with difluoromonoch-loromethane to obtain 3-cyclopropyl methoxy-4-difluoromethoxybenzoic acid (I) serving as the roflumilast intermediate. The preparation method has the advantages of easiness and convenience for operating, mild reaction conditions, stable quality, simple post-treatment and no need of complex operation such as column chromatography and the like, and is suitable for industrial production.
Owner:NANJING TIANHAI MEDICAL TECH
Orally disintegrating tablet comprising roflumilast
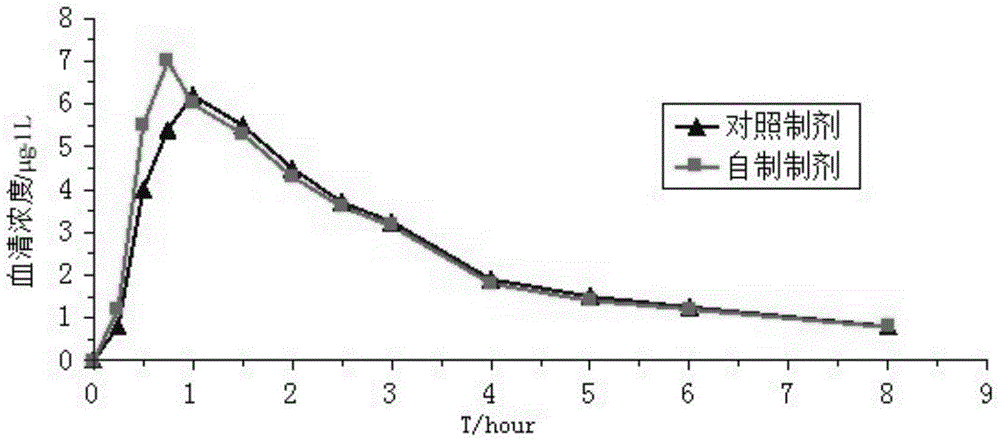
The invention belongs to the field of medicine technology, and relates to an orally disintegrating tablet comprising roflumilast and a preparation method of the orally disintegrating tablet. The orally disintegrating tablet comprises roflumilast, a hydrophilic filler, a water-soluble polymer adhesive, a disintegrating agent, a flavouring agent, a lubricant, and the like. The objective of the invention is to provide the roflumilast orally disintegrating tablet that has advantages of simple preparation technology, taking convenience, rapid effect, rapid concentration peak and obvious curative effects.
Owner:BEIJING VENTUREPHARM BIOTECH
Roflumilast tablet and preparation method thereof
InactiveCN103127011AGood lookingSmooth processPill deliveryPharmaceutical non-active ingredientsCurative effectDissolution
The present invention provides a Roflumilast tablet and a preparation method of the Roflumilast tablet. The method adopts a direct powder tabletting method, production process is easy, production cycle is shortened, equipment cost and operation cost are reduced, the prepared and obtained Roflumilast tablet is good in appearance, technical process is smooth, the method possesses good operability, product dissolution rate is high, content uniformity is qualified, the Roflumilast tablet can achieve good bioavailability in human body, and therefore good curative effects is produced.
Owner:深圳万乐药业有限公司
Roflumilast oral preparation and preparation method thereof
The invention provides a roflumilast oral preparation and a preparation method thereof. According to the invention, solvent solid dispersion technology is adopted, and the solid oral preparation is prepared by 0.1-50 wt% of roflumilast and 50-99.9 wt% of pharmaceutically acceptable auxiliary materials. Roflumilast tablets with stable and controllable quality can be produced by using common pharmaceutical equipment, which is helpful for vast patients to obtain cheap and good medicine products.
Owner:JIANGSU CAREFREE PHARM CO LTD
Methods of treating autoimmune, respiratory and inflammatory disorders by inhalation of roflumilast n-oxide
ActiveUS20140213560A1Low plasma levelLess side effectsBiocidePowder deliveryInhalationObstructive Pulmonary Diseases
The present disclosure relates to pharmaceutical compositions useful for (and to a method of) treating autoimmune, respiratory and / or inflammatory diseases and conditions. The method involves administering to a subject in need thereof roflumilast N-oxide by inhalation. The present disclosure particularly relates to the treatment of asthma and chronic obstructive pulmonary disease (COPD) by administering roflumilast N-oxide by inhalation.
Owner:INCOZEN THERAPEUTICS PVT
Method for reducing side effects from administration of phosphodiesterase-4 inhibitors
InactiveUS20200155524A1Reduce spikesProducing highAerosol deliveryOintment deliveryPhosphodiesterasePhosphoric Acid Esters
A method for altering the PK profile of a pharmaceutical formulation containing a PDE-4 inhibitor, such as roflumilast, to reduce the spike in Cmax. The spike in Cmax is reduced by topically administering the PDE-4 inhibitor in combination with one or more phosphate ester surfactants. Reducing the spike in Cmax will reduce gastrointestinal side effects and result in better patient compliance.
Owner:ARCUTIS INC
Roflumilast inhalation aerosol compound and preparation method thereof
InactiveCN104800214AReduce mouth sprayLong durationAerosol deliveryRespiratory disorderSide effectPoor adherence
The invention discloses a compound aerosol for quick-acting treatment of COPD, and a preparation method thereof. The compound aerosol comprises roflumilast and salbutamol. The compound aerosol combines respective characteristics of the long-acting COPD medicine roflumilast and the quick-acting COPD medicine salbutamol, and realizes rapid effectiveness and long lasting time in order to reduce the oral spray frequency of patients and reduce toxic side effects. A propellent changes from CFCs to HFC in order to eliminate destroys to the earth's ozone layer. Lecithin selected in the invention is in favor of realizing immersion and penetration of medicines to lung cells in order to improve the bioavailability and realize quick acting. Mentholum is added into a dispersant, so the patients have refreshing and comfortable feeling after administration, thereby the patients' compliance is improved.
Owner:成都英诺新科技有限公司
Separation and detection method for roflumilast and intermediate thereof
InactiveCN103630613AEasy to separateMeet the separation requirementsComponent separationAcetonitrileLength wave
The invention relates to a separation and detection method for roflumilast and an intermediate thereof. According to the invention, to realize separation and detection, high performance liquid chromatography and a reversed phase column are adopted, a buffer solution containing ions and having a pH value of 2 to 7.5 on a reagent is used as a mobile phase A, methanol or acetonitrile is used as a mobile phase B, detection wavelength is 215 nm, and a linear gradient elution mode is employed.
Owner:NEW FOUNDER HLDG DEV LLC +2
Roflumilast crystal form compound, preparation method, composition and applications thereof
ActiveCN103012255AQuality improvementHigh dissolution rateOrganic chemistryRespiratory disorderRefluxOrganic solvent
The present invention provides a new roflumilast crystal form compound, wherein an organic solvent and water mixed system is adopted to prepare the compound, and a high yield and good quality are obtained. The specific operation steps comprise: adopting a hot-dissolution cold-precipitation refinement method generally used by technicians in the field to dissolve a roflumilast crude product in an organic solvent solution, heating to a reflux temperature to dissolve, carrying out hot filtration, cooling the filtrate, adding water, precipitating a crystal, filtering, and carrying out vacuum drying.
Owner:天津康鸿医药科技发展有限公司
Roflumilast solid dispersion and medicinal composition containing same
InactiveCN102988297AImprove solubilityDissolution rate is fastPowder deliveryPharmaceutical non-active ingredientsSolubilityMedicine
The invention discloses a roflumilast solid dispersion and a medicinal composition containing the same. The roflumilast solid dispersion comprises (1) roflumilast, (2) one or several of crosslinked polyvinylpyrrolidone, lactose, polyethylene glycol and microcrystalline cellulose, and (3) sodium dodecyl sulfate. The medicinal composition comprises the roflumilast solid dispersion and a pharmaceutically acceptable carrier. By preparing roflumilast into a solid dispersion, the solubility of a medicine is improved, the dissolution speed of the medicine is increased, and the dissolution rate of a medicinal composition containing the roflumilast solid dispersion in a dissolution medium is greatly improved; and in a grinding process, premixing of the medicine and some accessories is realized, and the problem in the content uniformity of a small dose of preparation is greatly reduced.
Owner:WUXI HONGXING BIOMEDICAL TECH
Roflumilast tablet and preparation method thereof
InactiveCN104107173AClear release curveGood content uniformityPill deliveryPharmaceutical non-active ingredientsMagnesium stearateLactose
The invention discloses a roflumilast tablet and a preparation method thereof. The roflumilast tablet is composed of roflumilast, starch, pregelatinized starch, lactose, PVP-K90 and magnesium stearate. According to the preparation method, roflumilast is dissolved in anhydrous ethanol, so that the bulk drug is dispersed in an excipient in a solution form; and the release rate of the tablet in four media with different pH is regulated by specific proportions of the starch to the pregelatinized starch, so that the roflumilast tablet has good release curve and good content uniformity in the four media. The preparation method is simple and convenient in process; the bulk drug is in no need of micronization treatment; and the preparation method is in no need of special equipment, can be industrialized easily, is high in product yield, and has obvious advantages.
Owner:SHANDONG TAITIAN NEW MEDICINE DEV
Method for preparing Roflumilast raw material and intermediates
InactiveCN102532011AIngenious ideaSimple processOrganic chemistryChemical recyclingBenzoic acidBenzaldehyde
The invention discloses a method for preparing a Roflumilast raw material and intermediates. The method includes steps as follows:, 4-difluoromethoxy-3-hydroxy benzaldehyde reacts with cyclopropylmethylbromide under the conditions of solvent and catalytic agents, and then 4-difluoromethoxy-3-cyclopropyl methoxybenzaldehyde is obtained after extraction and recovery; 4-difluoromethoxy-3-cyclopropyl methoxybenzaldehyde reacts with potassium permanganate in a solvent environment, and 4-difluoromethoxy-3-cyclopropyl methoxybenzoic acid is obtained through post-processing; and 4-difluoromethoxy-3-cyclopropyl methoxybenzoic acid reacts with 4-amino-3,5-dichloropyridine under the conditions of solvent and thionyl chloride, and crude Roflumilast products are obtained through post-processing. The method has the advantages of skillful concept, simple process, low manufacturing cost, high product yield coefficient and no generation of harmful gas.
Owner:SICHUAN BAILI PHARM CO LTD
Tablet containing roflumilast as active ingredients and preparation method of tablet
InactiveCN102871976ADissolve fastRapid dissolutionPharmaceutical non-active ingredientsPill deliveryCellulosePharmacologic action
The invention discloses a tablet containing roflumilast as active ingredients and a preparation method of the tablet. The tablet comprises binders, other drug excipients, humectants, drug-loading solvents and the roflumilast, wherein the binders are hydroxypropyl methyl cellulose, and the humectants and drug preparations are mixed solution of ethanol and water. The tablet and the preparation method thereof have the advantages that the mixed solution of the ethanol and the water serves as the drug-loading solvents, and dissolved states of the active ingredients not subjected to micronization are controlled by utilizing difference of solubility of the roflumilast in the ethanol and the water and adjusting the proportion of the ethanol and the water; the roflumilast is dissolved out fast from preparations to achieve pharmacological action by using the hydroxypropyl methyl cellulosas as the binders and conjunctively adjusting and controlling the dissolution rate of the roflumilast from the tablets, the whole process does not need pretreatment and can be achieved by normal granulation and tabletting, and the technical process is simple while cost is saved.
Owner:CHINA RESOURCES SAIKE PHARMA
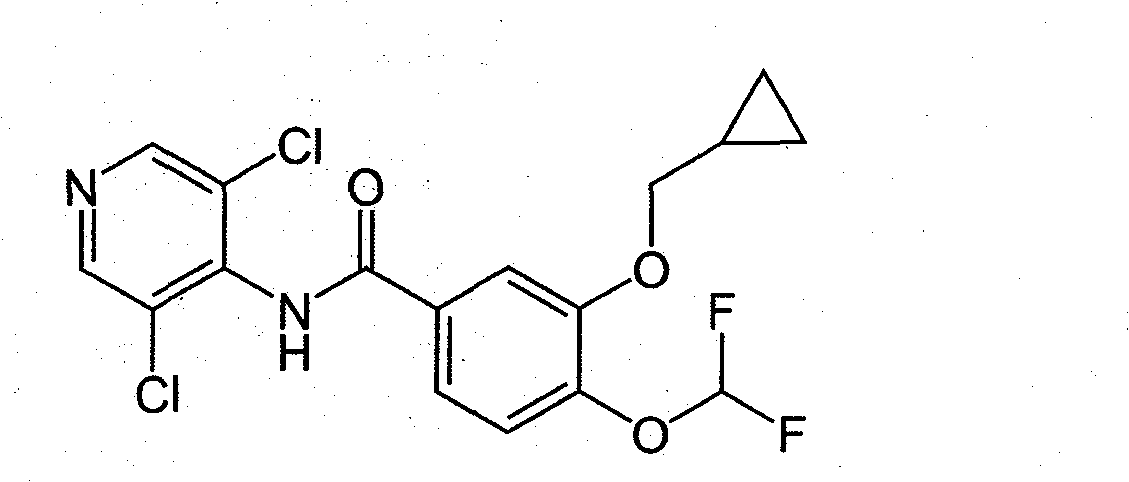
Process for preparing roflumilast
The invention relates to a process for the preparation of Roflumilast by reaction of an activated form of 3-(cyclopropylmethoxy)-4-(difluoromethoxy)-benzoic acid with an activating agent selected from (a) carbonyldiimidazole (CDI), (b) 1,1′-carbonyl-di-(1,2,4-triazol) (CDT), (c) 1,1′-carbonyl-bis-(2-methylimidazol), (d), 1′-carbonyl-dipyperidin, (e) N,N′-dicyclohexylcarbodiimide (DCC), (f) N,N′-diisopropylcarbodiimide (DIC), (g) 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and a combination of one of the previous (a)-(g) with (h) N-hydroxysuccinimide or (i) N-hydroxyphthalimide, and the subsequent reaction with 3,5-dichloropyridine-4-amine in the presence of an inorganic base. The invention also relates to the synthesis intermediates.
Owner:INTERQUIM SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com