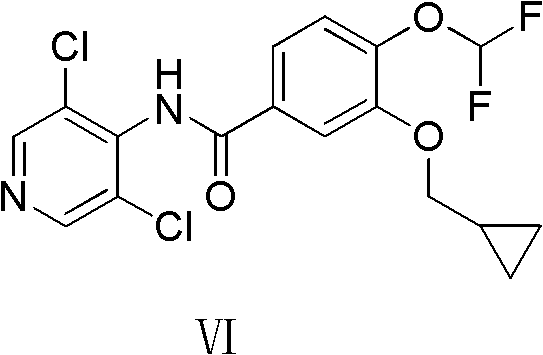
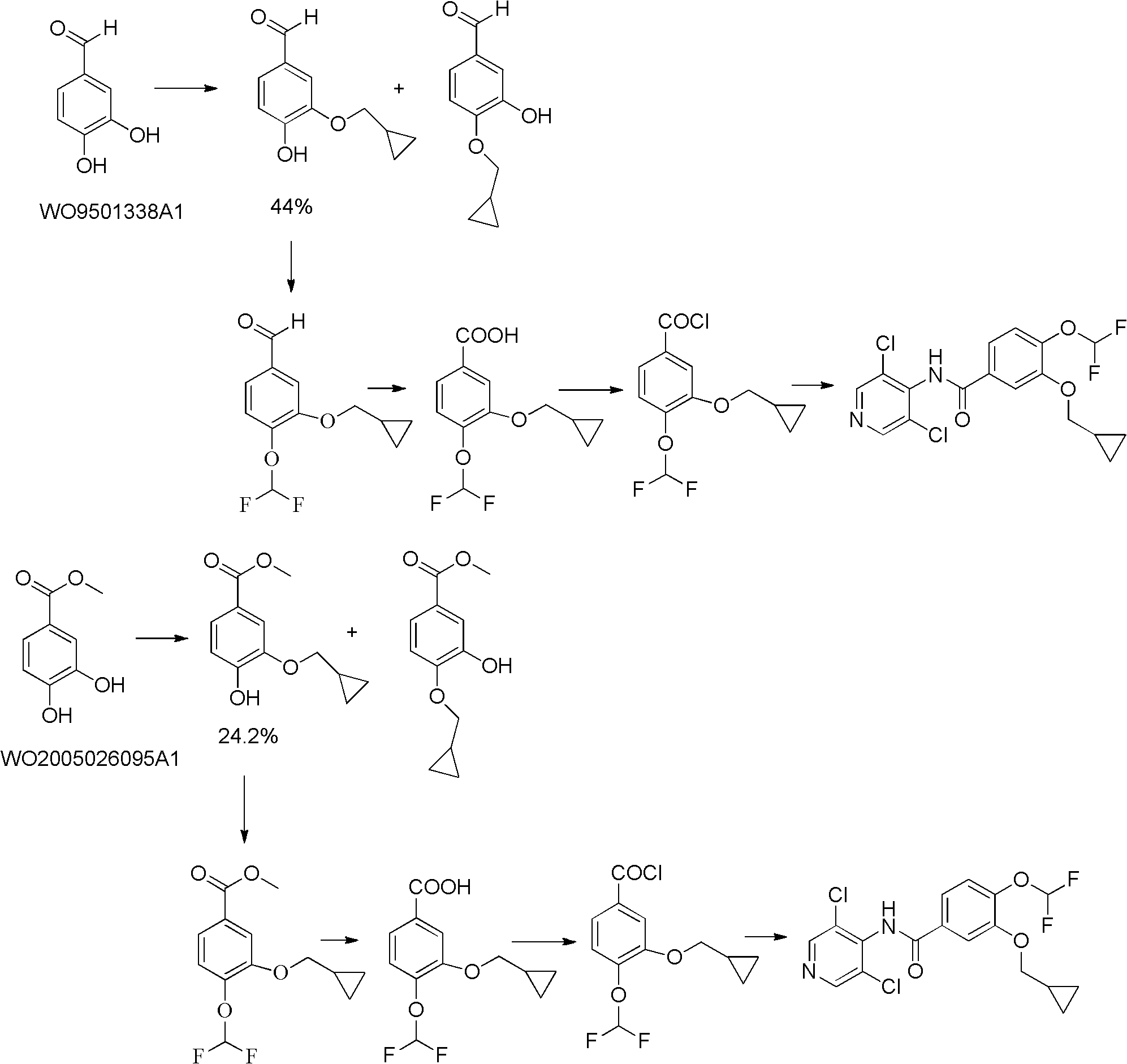
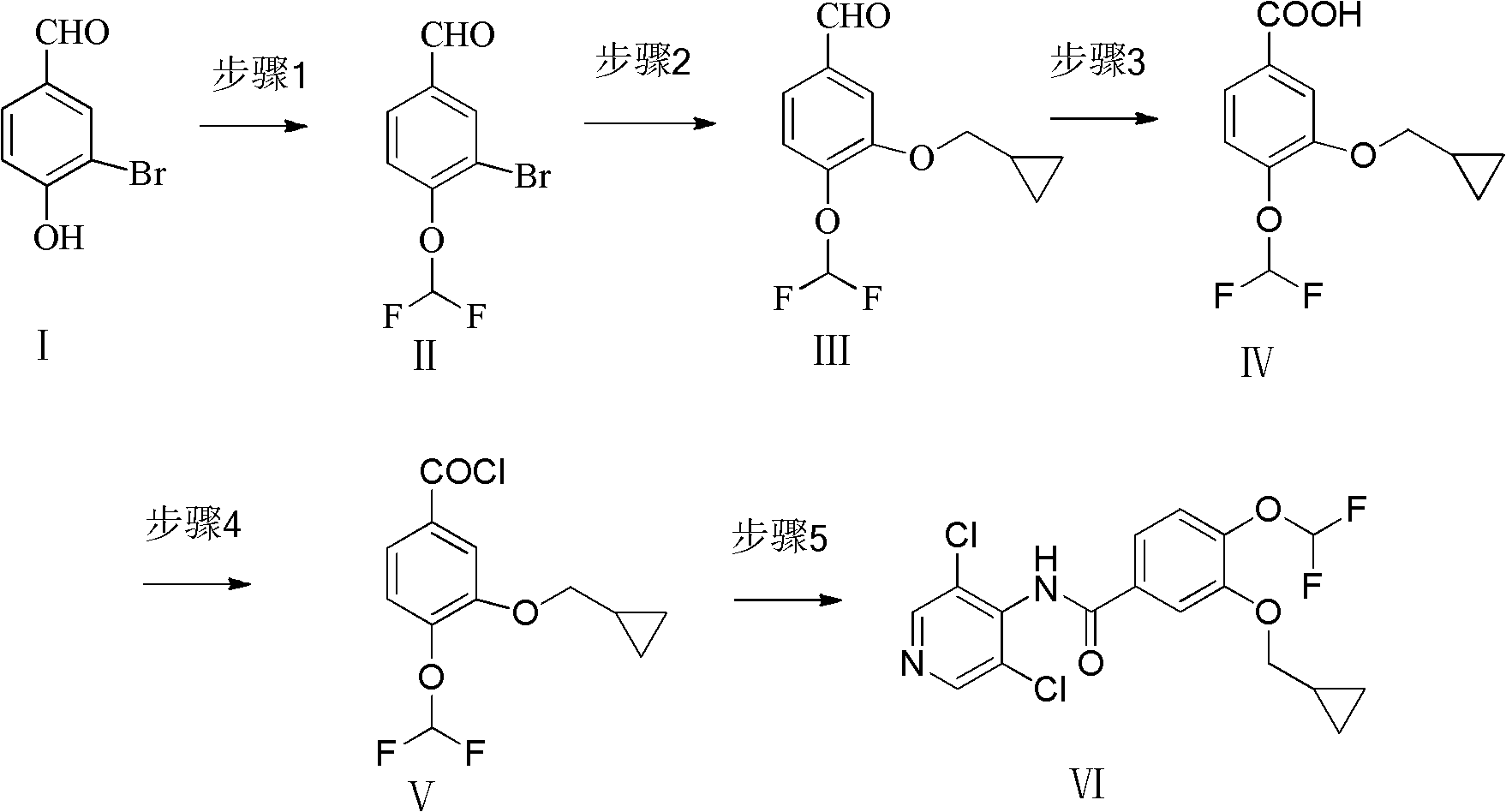
New method for preparing roflumilast
A technology of roflumilast and difluoromethoxy, which is applied in the field of improvement of the preparation method of industrially synthesized roflumilast, can solve problems such as poor selectivity, and achieve the effects of low cost, high yield, and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14- 2
[0019] The synthesis of embodiment 14-difluoromethoxy-3-hydroxybenzaldehyde (II)
[0020] In a 100mL single-necked bottle, 3-bromo-4-hydroxybenzaldehyde (1.66g, 8.3mmol), sodium chlorodifluoroacetate (1.27g, 8.3mmol), sodium hydroxide (0.48g, 12mmol), DMF ( 15mL) and water (0.3mL), heated at 120°C, reacted for 2 hours, and distilled off the solvent. Add 10mL of hydrochloric acid solution, extract with ethyl acetate, evaporate to dryness, add water, adjust the pH value to about 13-14 with sodium hydroxide, extract with ethyl acetate, dry over magnesium sulfate, filter and evaporate to dryness to obtain a brown oil, which turns white when placed at low temperature 1.90 g of needle-like crystals, yield 91.6%. mp 68-70°C; 1H NMR (400MHZ DMSO): δ7.239(s, 0.2H, CHF2), 7.420(s, 0.5H, CHF2), 7.474-7.495(d, 1H, J=8.4, ArH), 7.601(s, 0.3H, CHF2), 7.933-7.959(q, 1H, J=2, J=8.4, ArH), 8.164-8.169(d, 1H, J=2, ArH), 9.920(s, 1H, CHO), ESI-MS: m / z 251 [M+H]+.
[0021] With reference to t...
Embodiment 23
[0023] Synthesis of Example 23-cyclopropylmethoxy-4-difluoromethoxy-benzaldehyde (III)
[0024] In a 100mL single-necked bottle, add 4-difluoromethoxy-3-hydroxybenzaldehyde (0.25g, 1.0mmol), cyclopropylmethanol (0.30g, 4.2mmol), 8-hydroxyquinoline (0.04g, 0.28mmol), cuprous iodide (0.04g, 0.22mmol), potassium carbonate (0.69g, 5.0mmol), DMF (10mL), heated at 140°C, reacted for 20 hours, and evaporated the solvent. Extracted with ethyl acetate, dried over magnesium sulfate, filtered and evaporated to dryness to obtain 0.15 g of yellow oil with a yield of 62.0%. 1H NMR (400MHZ DMSO): δ0.563-0.609(m, 2H, CH2), 0.912-0.929(m, 2H, CH2), 1.224-1.286(m, 1H, CH), 3.991-4.008(d, 2H, J=6.8, CH2), 7.239(s, 0.2H, CHF2), 7.420(s, 0.5H, CHF2), 7.474-7.495(d, 1H, J=8.4, ArH), 7.601(s, 0.3H, CHF2 ), 7.933-7.959 (q, 1H, J=2, J=8.4, ArH), 8.164-8.169 (d, 1H, J=2, ArH), 9.920 (s, 1H, CHO), ESI-MS: m / z243[M+H]+.
[0025] With reference to the method of Example 2, the experimental conditions ...
Embodiment 33
[0028] Synthesis of Example 33-cyclopropylmethoxy-4-difluoromethoxy-benzoic acid (IV)
[0029] In a 100mL single-necked bottle, add 3-cyclopropylmethoxy-4-difluoromethoxy-benzaldehyde (1.54g, 6.4mmol), sulfamic acid (0.90g, 9.3mmol), sodium hypochlorite (1.00g, 11.0mmol), glacial acetic acid (6mL), water (2mL), reacted at room temperature for 1 hour, a solid precipitated, added 30mL of water, filtered, and dried to obtain 1.40g of white crystals, with a yield of 87.5%. mp 129-130°C; 1H NMR (400MHZ DMSO): δ0.563-0.609 (m, 2H, CH2), 0.912-0.929 (m, 2H, CH2), 1.224-1.286 (m, 1H, CH), 3.991- 4.008(d, 2H, J=6.8, CH2), 7.239(s, 0.2H, CHF2), 7.420(s, 0.5H, CHF2), 7.474-7.495(d, 1H, J=8.4, ArH), 7.601( s, 0.3H, CHF2), 7.933-7.959 (q, 1H, J=2, J=8.4, ArH), 8.164-8.169 (d, 1H, J=2, ArH), 12.969 (s, 1H, COOH) , ESI-MS: m / z 259 [M+H]+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com