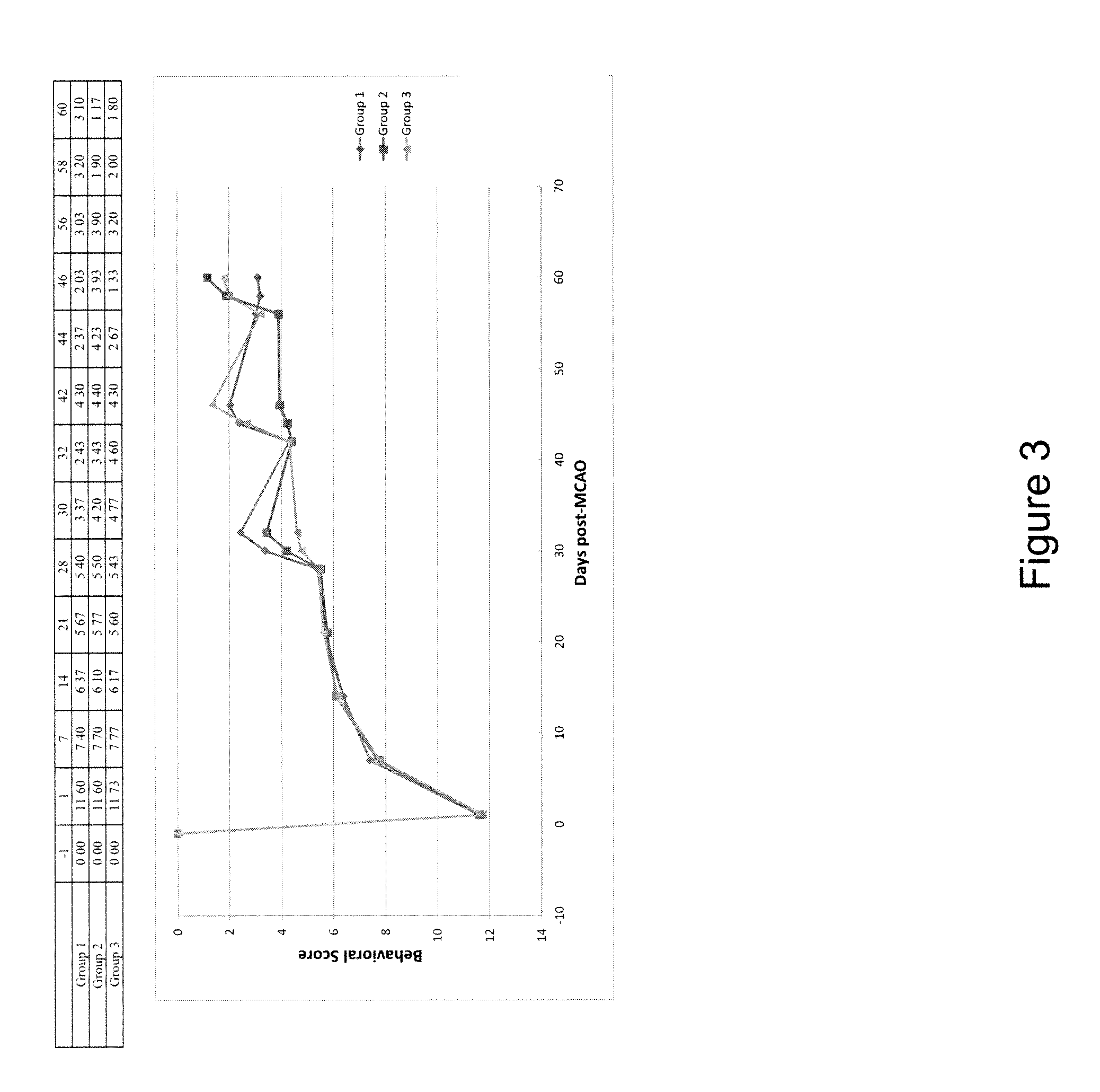
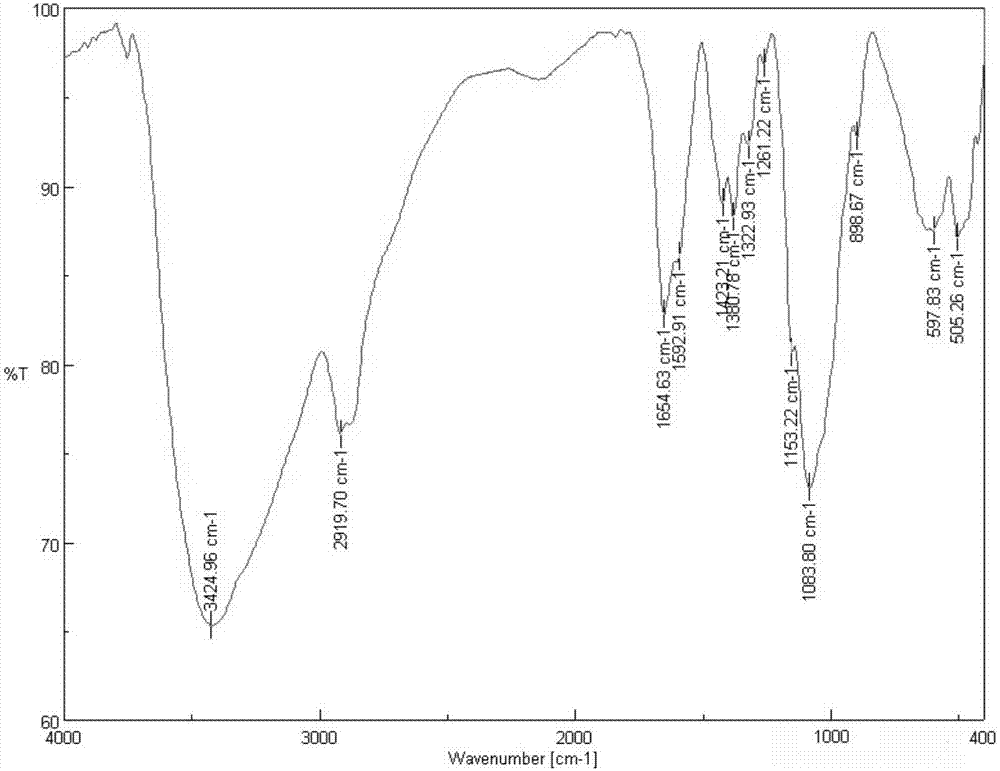
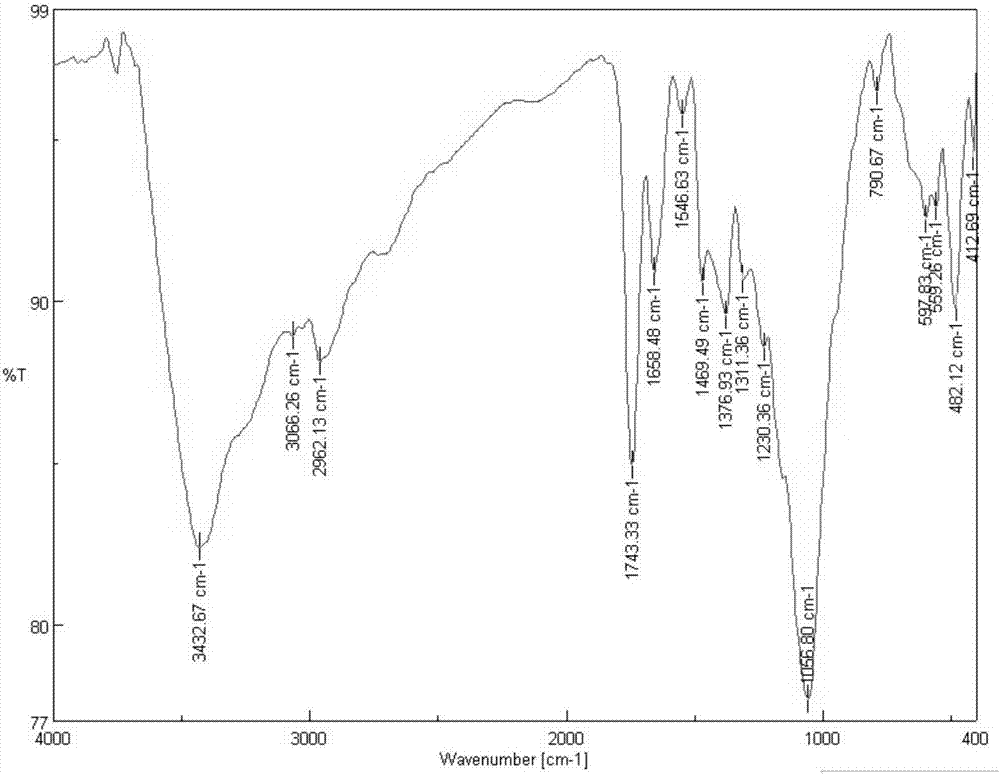
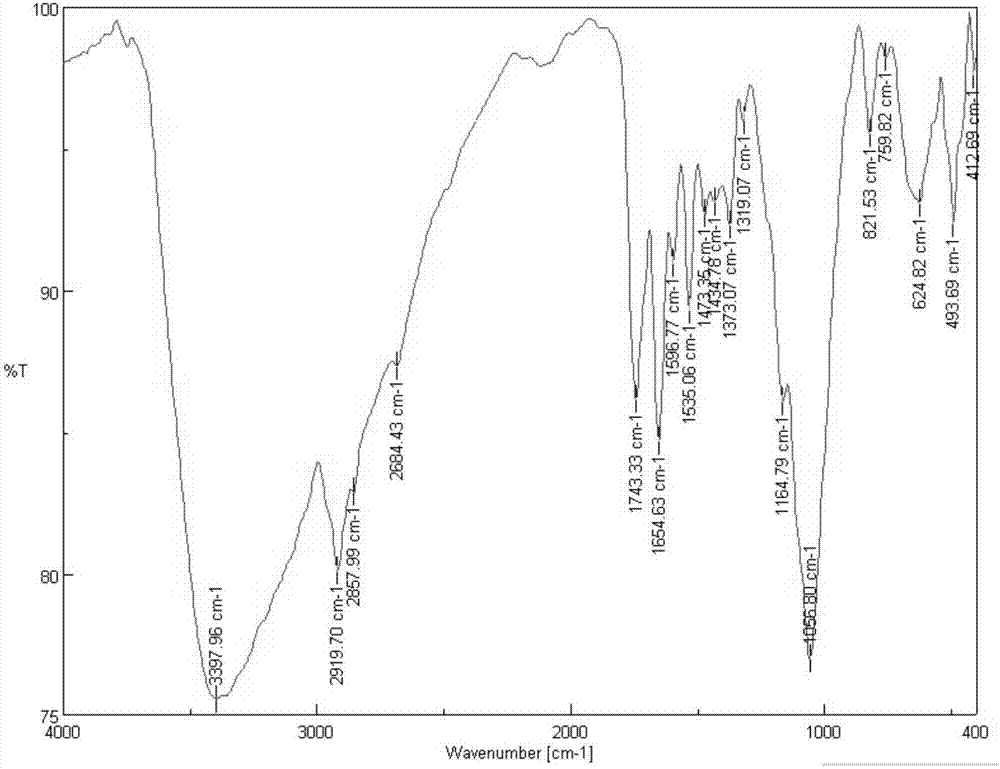
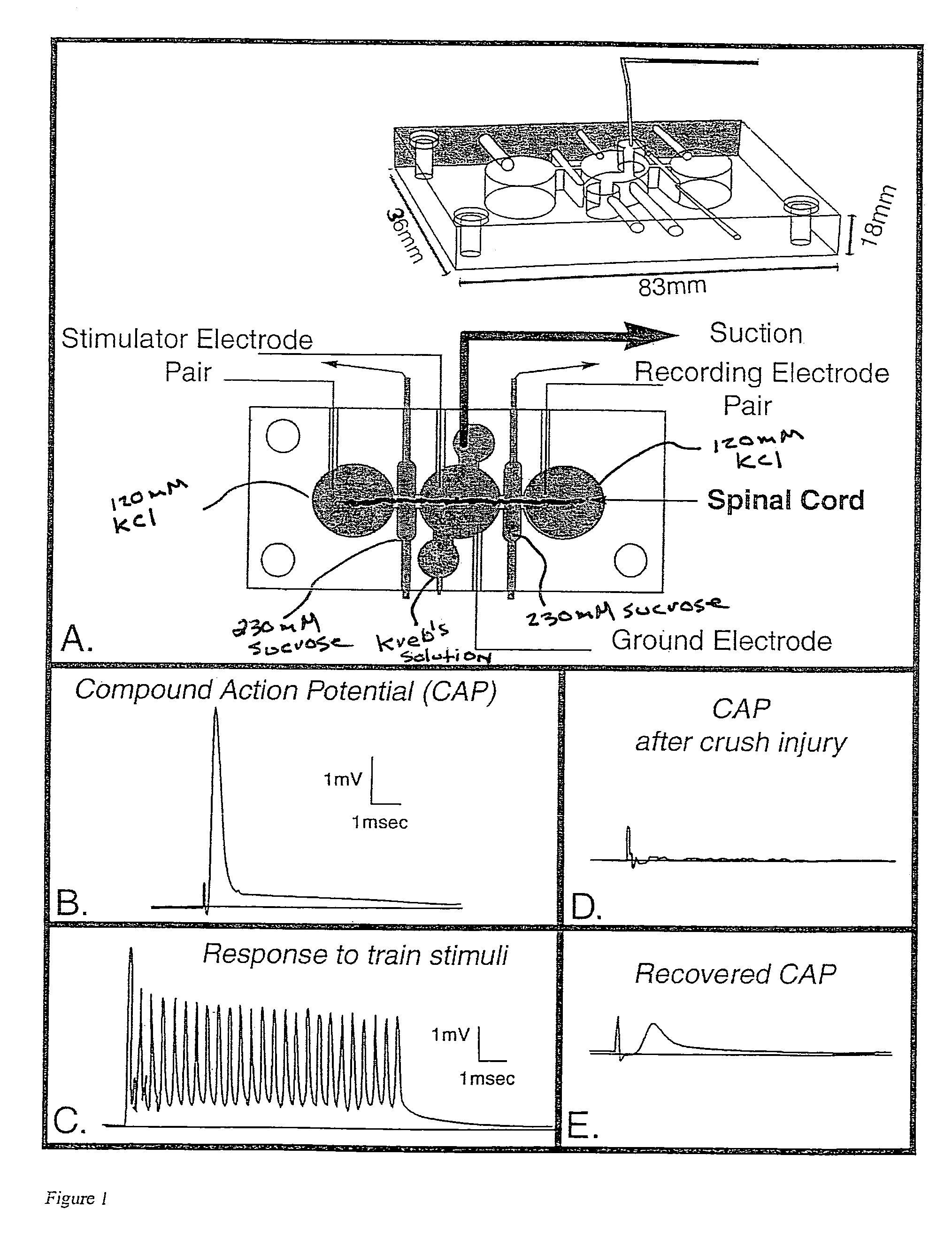
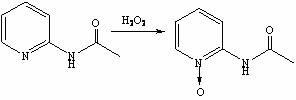Patents
Literature
116 results about "4-Aminopyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
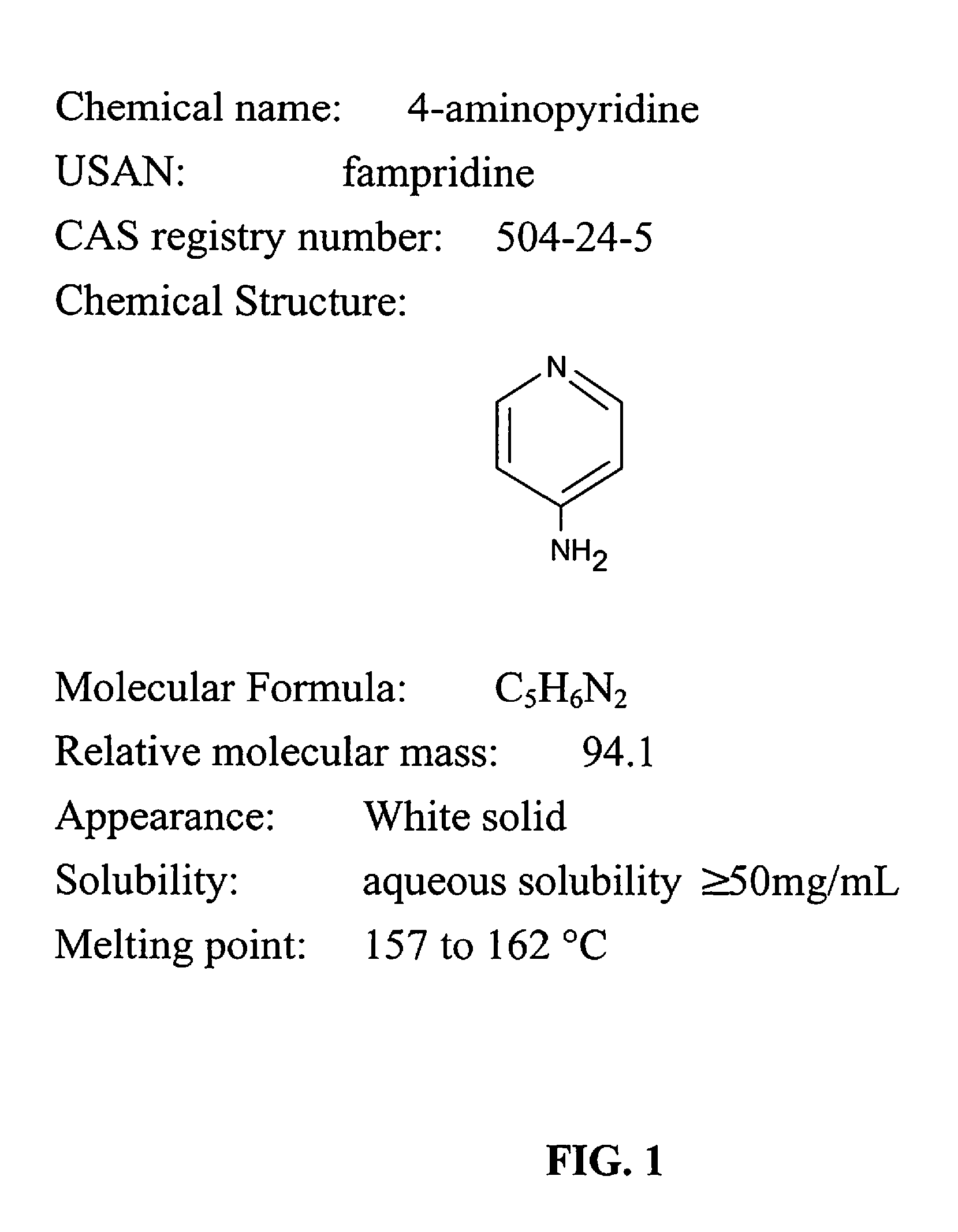
4-Aminopyridine (4-AP, fampridine, dalfampridine) is an organic compound with the chemical formula C₅H₄N–NH₂. The molecule is one of the three isomeric amines of pyridine. It is used as a research tool in characterizing subtypes of the potassium channel. It has also been used as a drug, to manage some of the symptoms of multiple sclerosis, and is indicated for symptomatic improvement of walking in adults with several variations of the disease. It was undergoing Phase III clinical trials as of 2008, and the U.S. Food and Drug Administration (FDA) approved the compound on January 22, 2010. Fampridine is also marketed as Ampyra (pronounced "am-PEER-ah," according to the maker's website) in the United States by Acorda Therapeutics and as Fampyra in Europe. In Canada, the medication has been approved for use by Health Canada since February 10, 2012.
4-aminopyridine and a pharmaceutical composition for treatment of neuronal disorders
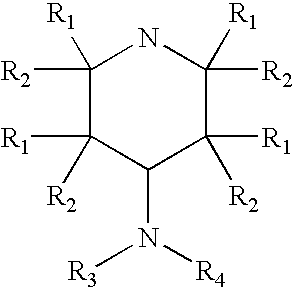
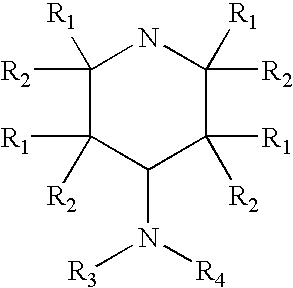
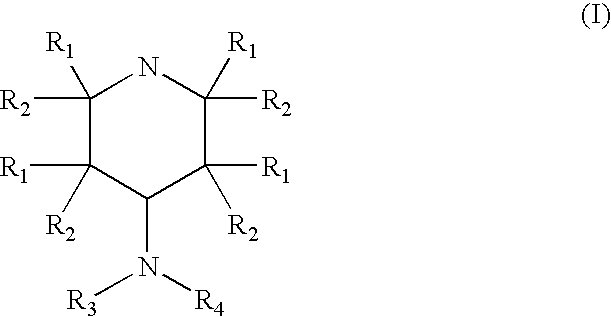
A composition is provided having the formulawhere R1 and R2 are each independently H or a C1-C4 hydrocarbon; R3 is H, and R4 is a moiety capable of crossing the blood brain barrier selected from the group consisting of: an amino acid, a peptide, transferrin, gluconate, lactate, citrate, malate, fumarate, benzoate, salicylate, pyruvate and propionate. The composition includes 4-aminopyridine and a transporter species which allows for improved transport of the aminopyridine across the blood brain barrier thereby reducing systemic side effects of aminopyridine administration.
Owner:MILLER LANDON C G
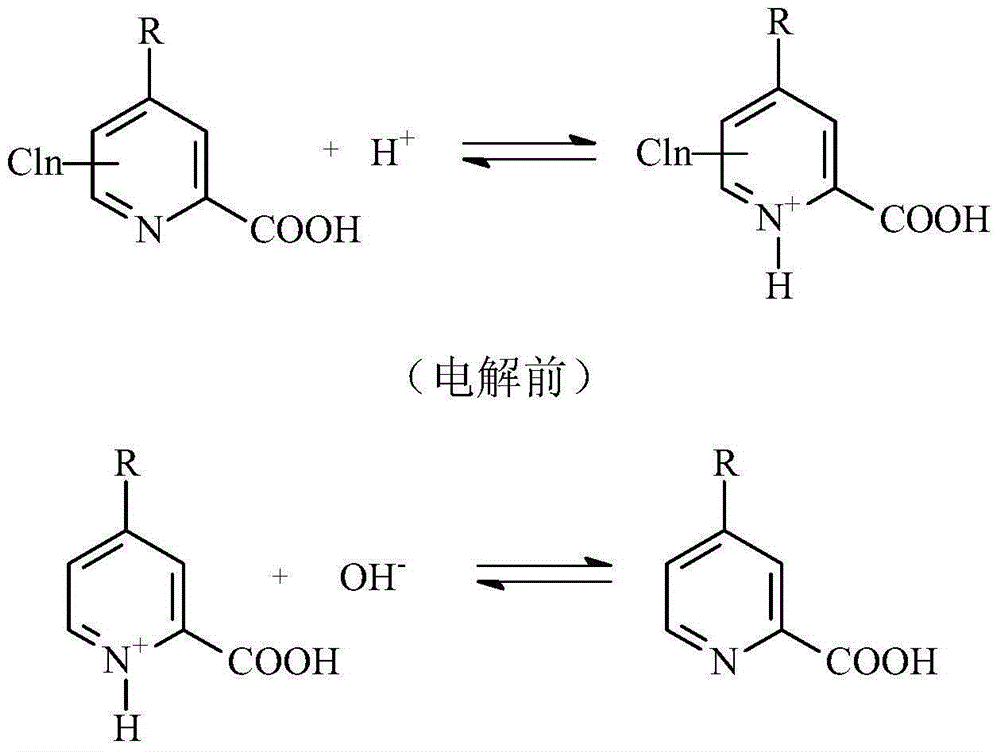
Method for preparing picolinic acid through electro-catalysis selective dechloridation of chloropicolinicacid
ActiveCN104988531AIncrease added valueSolve processing problemsElectrolysis componentsElectrolytic organic productionSupporting electrolyteElectrolysis
Owner:菏泽建数智能科技有限公司
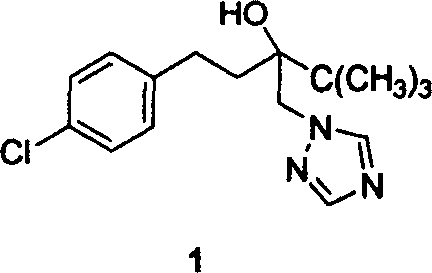
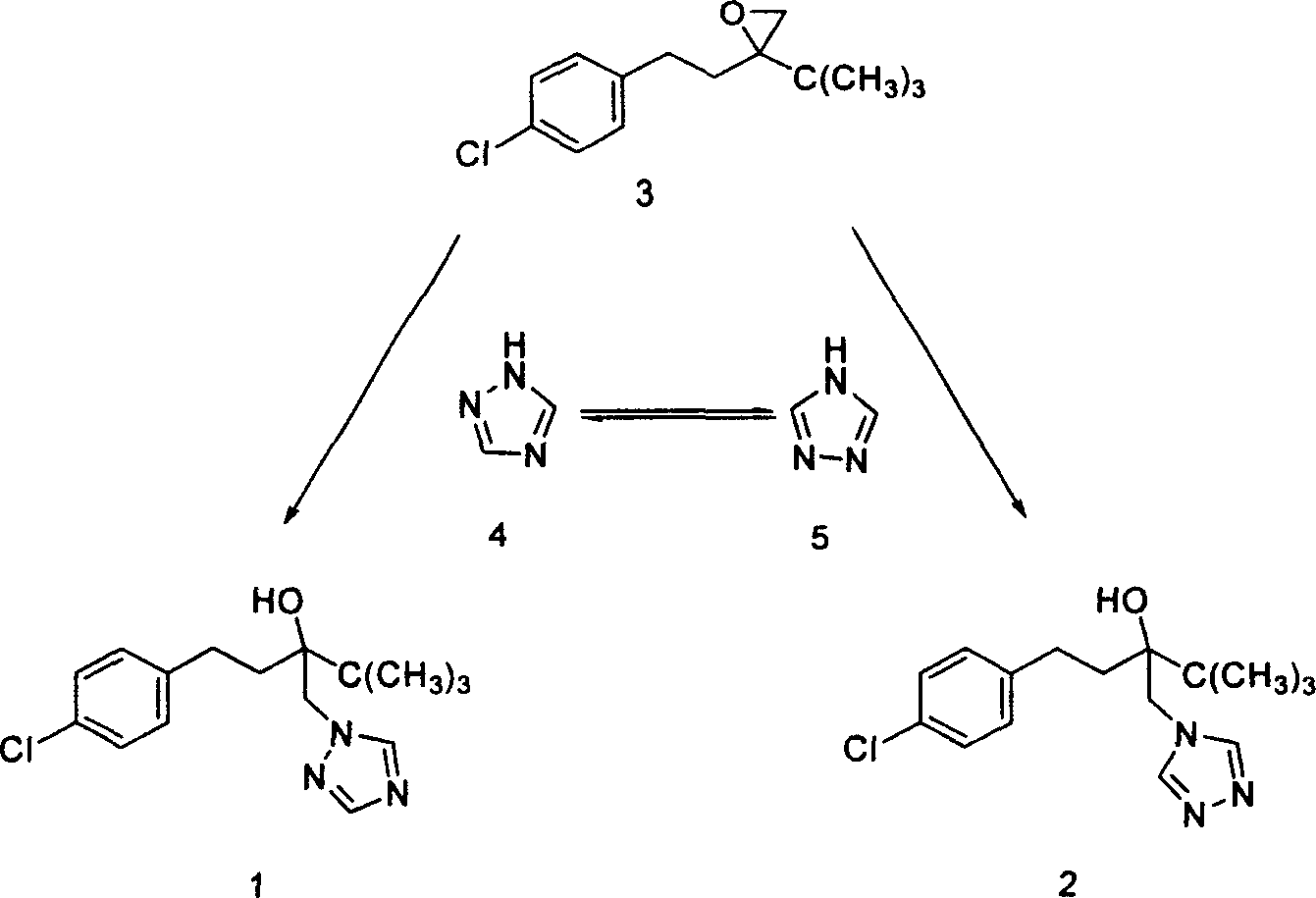
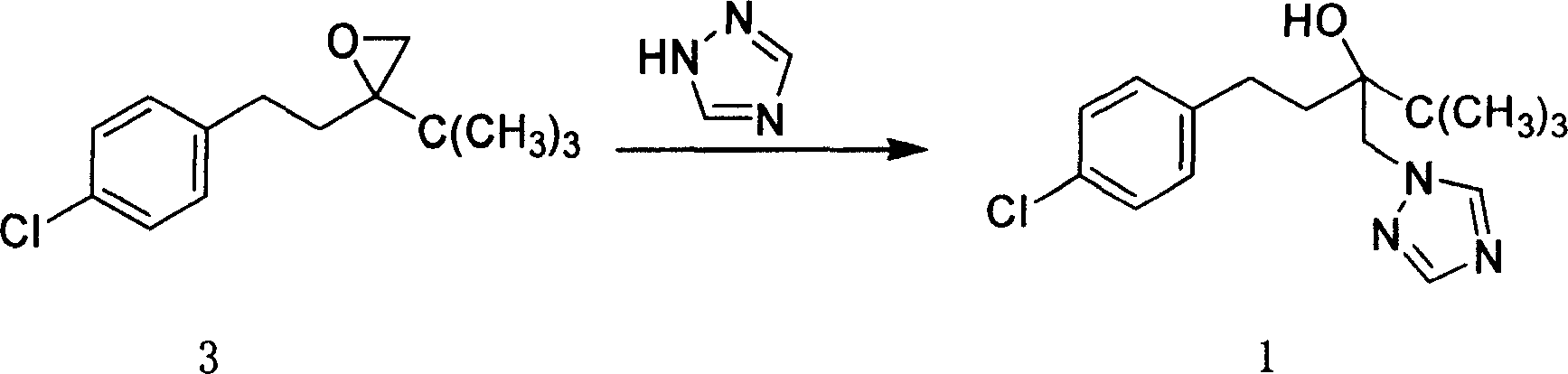
Method for preparing Tebucomazole in high purity
A process for preparing high-purity pentazolol features the reaction between 1-(4-chlorphenylethyl)-1-tert-butyl-1, 2-epoxy ethane and 1,2,4-triazole in organic polar solvent under existence of alkali while adding catalyst chosen from N,N-dimethyl-4-aminopyridine, N,N-dimethylphenylamine, methylphrrolidone, dimethyl formamide, methanol sodium, ethanol sodium, potassium hydroxide and sodium hydroxide. Its advantages are high output rate and high purity.
Owner:HUNAN CHEM RES INST +1
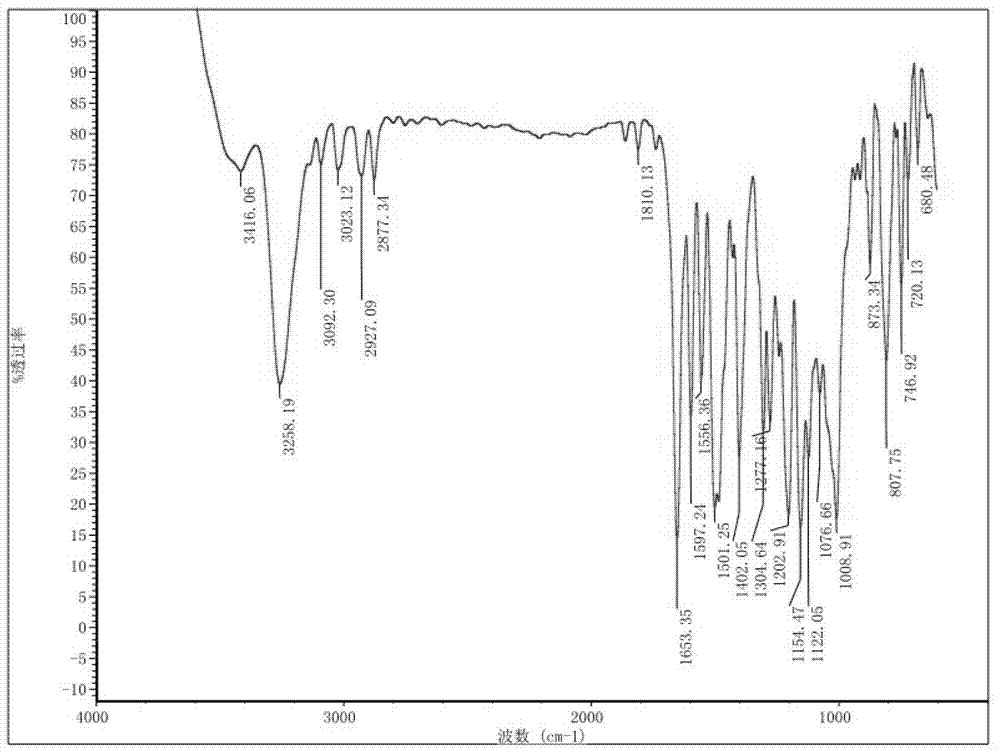
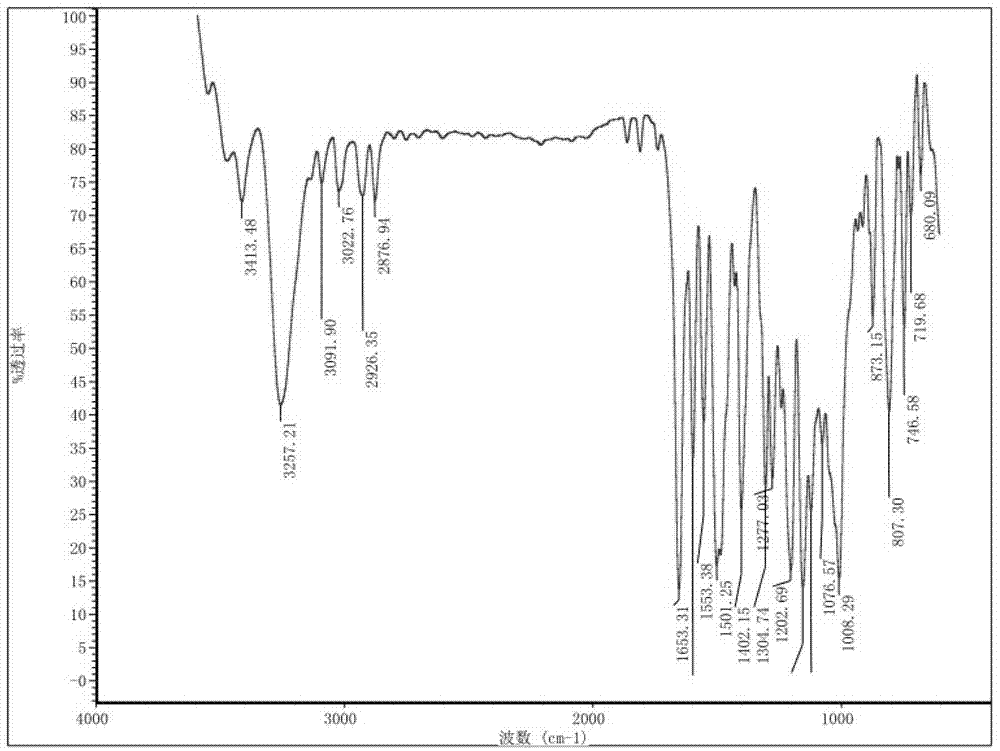
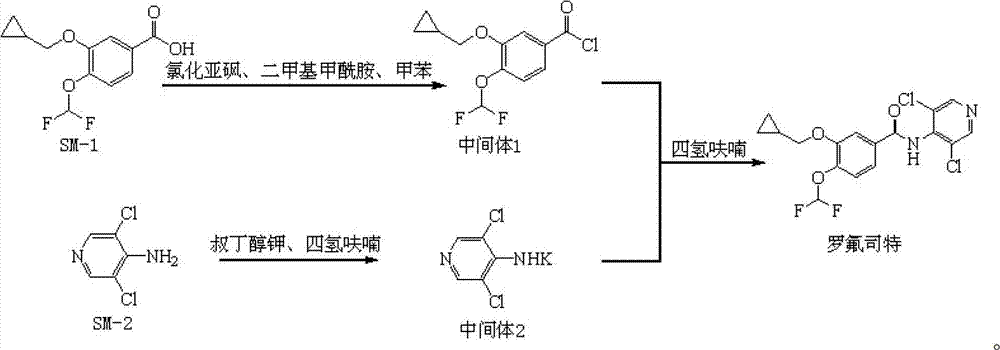
Preparation method and detection method of roflumilast material
ActiveCN102964297ASimple preparation processReduce adverse reactionsOrganic chemistryMaterial analysis by observing effect on chemical indicatorBenzoic acidDisease
The invention discloses a preparation method and a detection method of a roflumilast material. The preparation method comprises the following steps: mixing 3-cyclopropyl methoxy group-4-difluoro methoxy group benzoic acid SM-1, thionyl chloride, dimethyl formamide with toluene, and carrying out an acylating chlorination reaction to obtain a midbody 1; mixing 3,5-dichloro-4-aminopyridine SM-2, tetrahydrofuran with potassium tert-butoxide and carrying out a salt forming reaction to obtain tetrahydrofuran solution of a midbody 2; and then mixing the midbody 1 and the midbody 2 with tetrahydrofuran, carrying out amidation to obtain a crude product of roflumilast, and refining the crude product of roflumilast to prepare the roflumilast material. Aiming to overcome the shortage of the prior art, the preparation process of the roflumilast material is optimized, so that the curative effect for treating diseases such as chronic obstructive pulmonary disease (COPD) is more remarkable; and besides, a systematic, complete and effective composition identifying and content measuring method is provided, so that the quality of the medicine can be effectively controlled, and the clinical effect is ensured.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Use of potassium channel blockers to treat cerebral palsy
InactiveUS20130030025A1Signs improvedSymptoms improvedBiocideNervous disorder3-AminopyridinePotassium
Disclosed herein is the use of aminopyridines, such as 3-aminopyridine, 4-aminopyridine or 3,4-diaminopyridine, in the management and treatment of cerebral palsy patients of all ages.
Owner:ACORDA THERAPEUTICS INC
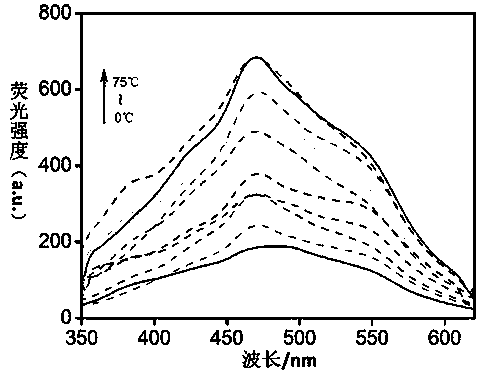
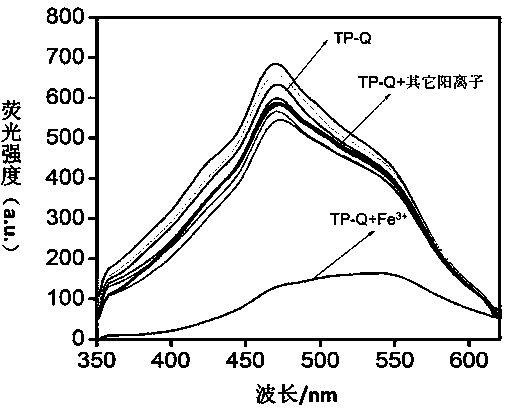
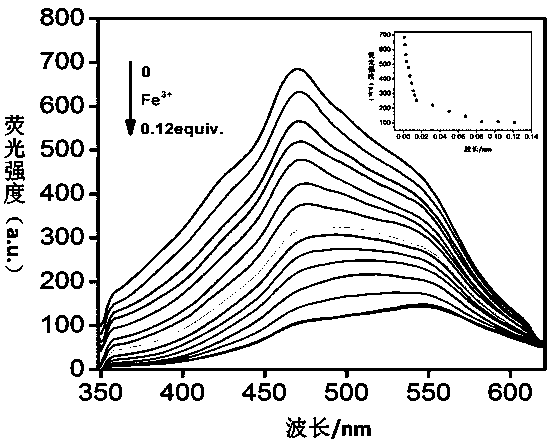
Tripodia pseudorotaxane supramolecular gel based on trimesoyl chloride and preparation and application of metal gel
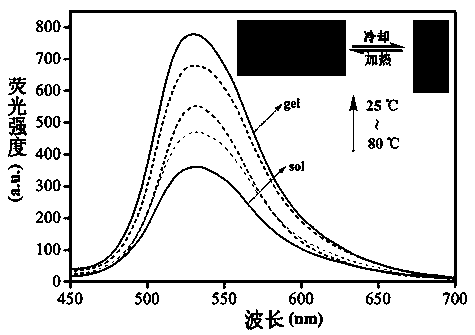
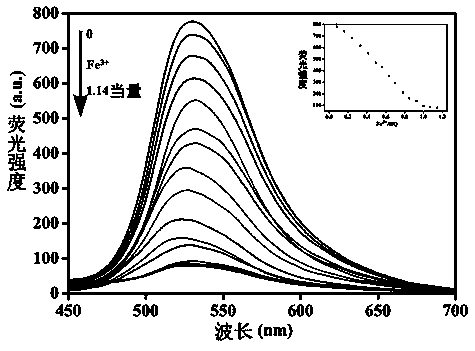
The invention discloses tripodia pseudorotaxane supramolecular organic gel based on trimesoyl chloride. According to the tripodia pseudorotaxane supramolecular gel based on the trimesoyl chloride, thepillar[5]arene and 4-aminopyridine functionalized trimesoyl chloride is fully dissolved into DMSO-H2O under the heating condition so as to obtain a transparent solution; cooling is carried out to reach room temperature so as to form the stable supramolecular organic gel TP-Q with blue and white aggregation-state fluorescence; a series of cationic solutions are added into the TP-Q, and fluorescence quenching can be realized only by adding Fe3+>, so that the TP-Q can specifically and selectively recognize the Fe3+>; and the tripodia pseudorotaxane supramolecular organic gel and ferric perchlorate hexahydrate are heated and dissolved in the DMSO-H2O, then cooling is carried out so as to obtain metal organic gel, when a series of anions are added into the metal gel separately, the fluorescence of the metal gel can be opened only by adding F<->, the fluorescence is changed into blue and white from black, and therefore high-sensitivity detection of the F<-> is realized.
Owner:NORTHWEST NORMAL UNIVERSITY
New method for preparing roflumilast
InactiveCN102617457AHigh yieldThe reaction is easy to operateOrganic compound preparationCarbonyl compound preparation3-HydroxybenzaldehydeBenzoic acid
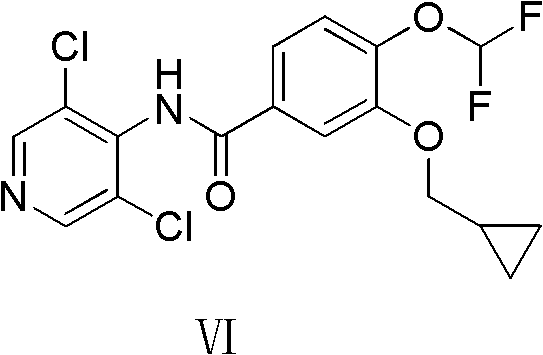
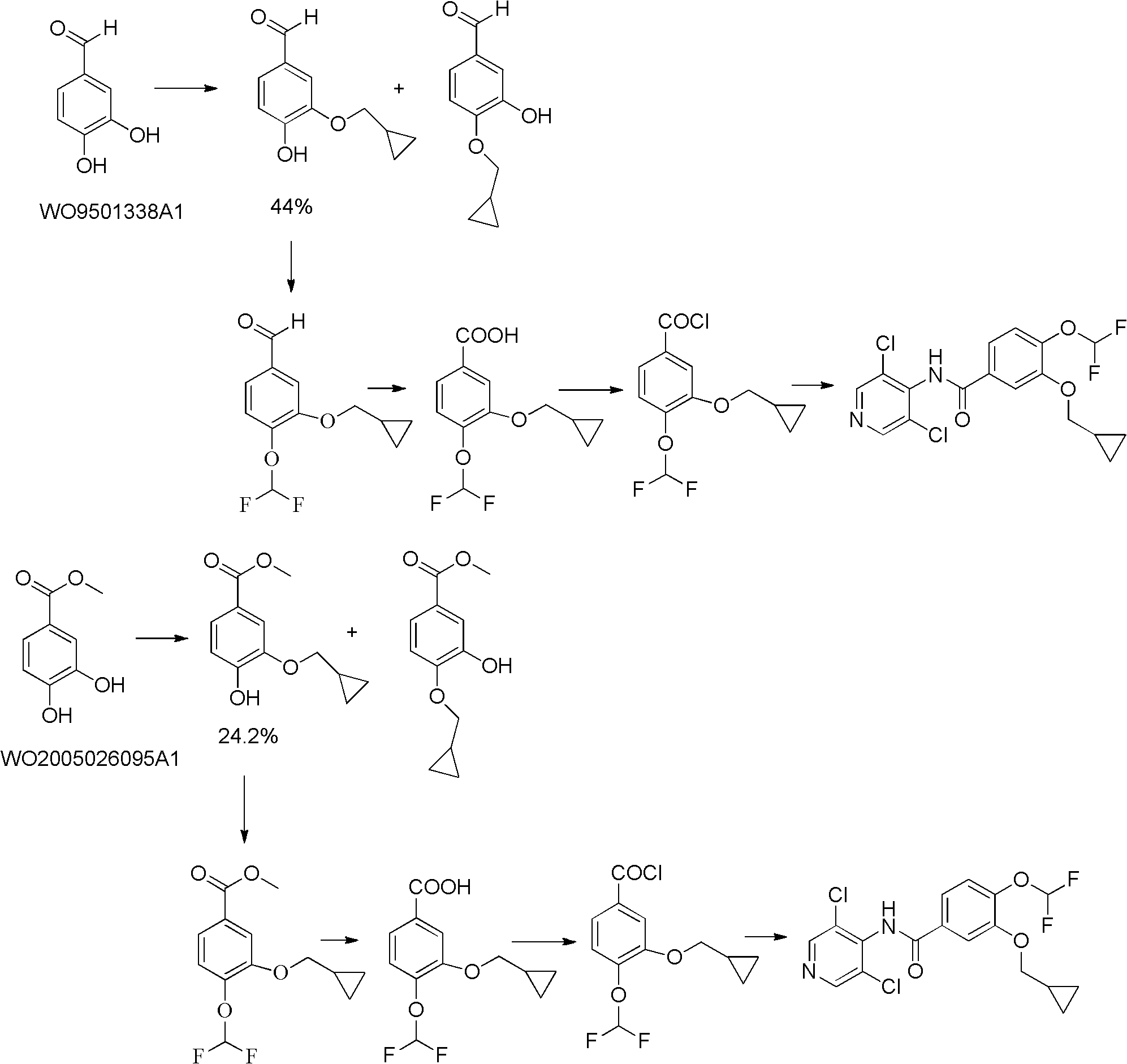
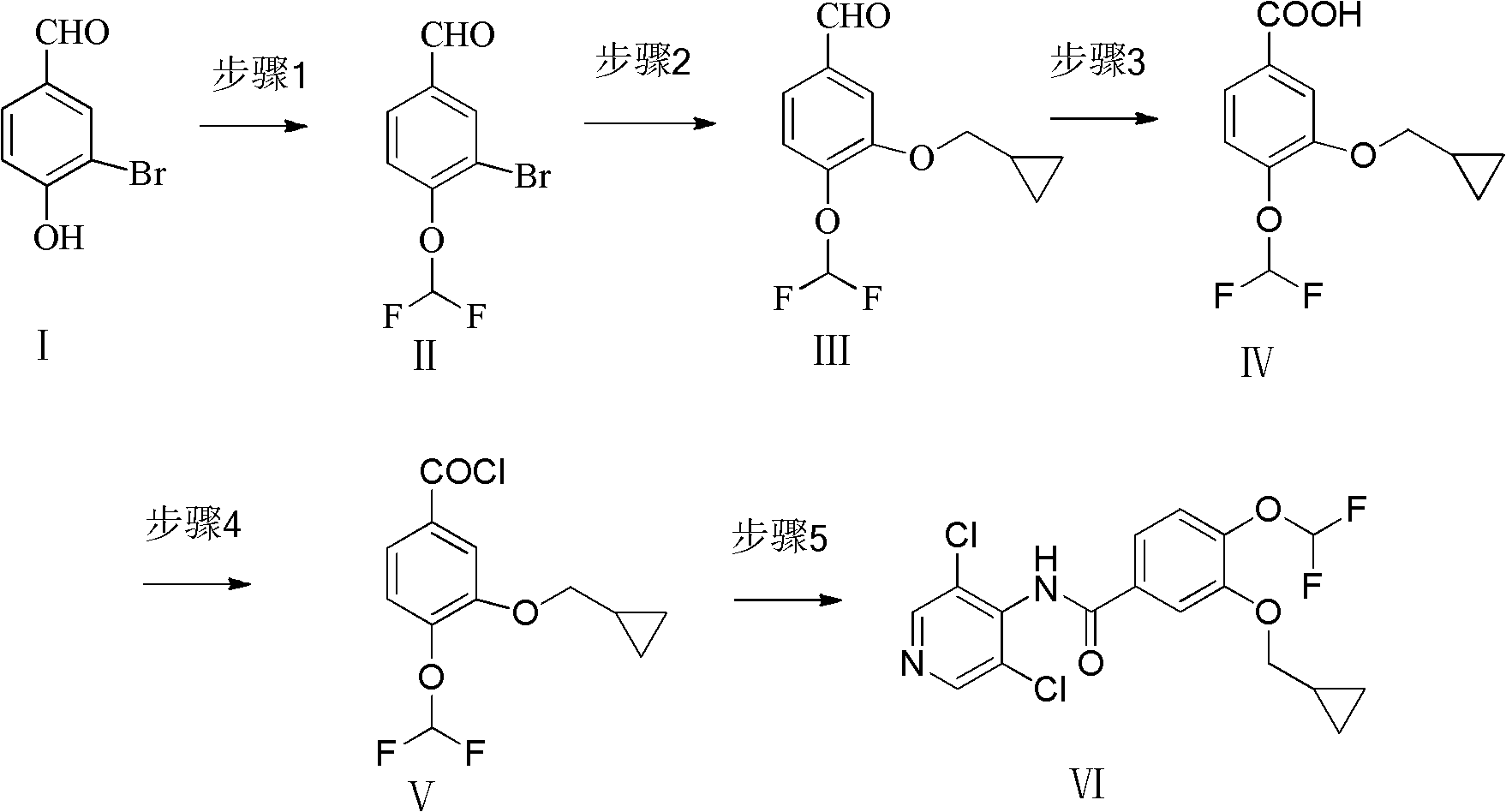
The invention provides a simple method for preparing roflumilast. According to the invention, 3-bromine-4-hydroxy-benzaldehyde (I) is etherified to obtain 4-difluoromethoxy-3-hydroxybenzaldehyde (II), the compound II is subjected to an Ullmann condensation reaction to obtain 3-cyclopropylmethoxy-4-difluoromethoxy-benzaldehyde (III), the compound III is oxidized by sodium hypochlorite to obtain 3-cyclopropylmethoxy-4-difluoromethoxy-benzoic acid (IV), the compound IV is chloridized to obtain 3-cyclopropylmethoxy-4-difluoromethoxy-benzoyl chloride (V), and the compound V and 3,5-dichloro-4-aminopyridine are acylated to obtain the roflumilast. The method of the invention has the advantages of no need of selective etherification and column chromatography purification in the preparation process, simple reaction operation, simple post-treatment, low cost, high yield, and high purity.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +1
Double-component supermolecular organic gel and preparation and application of metal gel of double-component supermolecular organic gel
InactiveCN109320454AIron group organic compounds without C-metal linkagesFluorescence/phosphorescenceRoom temperatureChloride
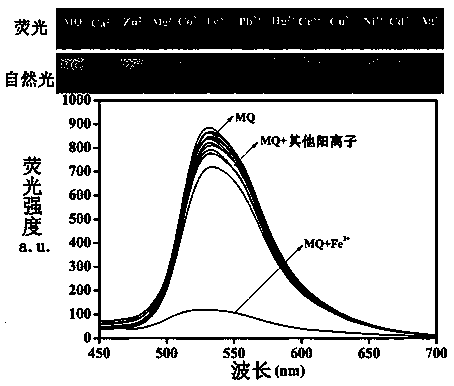
The invention discloses a double-component supermolecular organic gel factor. Schiff base functionalized naphthalimide and 4-aminopyridine functionalized trimesoyl chloride are fully dissolved in DMSO-H2O under heating, and thus a transparent solution is obtained; the transparent solution is cooled to the room temperature, thus stable double-component supermolecular organic gel MQ with orange aggregation fluorescence is formed; a series of positive solutions are added into the MQ, the fluorescence of the MQ is quenched only by adding Fe3+, and thus the MQ can identify Fe3+ in an exclusive andselective fluorescence mode; and the double-component supermolecular organic gel and ferric perchlorate hexahydrate are heated and dissolved into the DMSO-H2O, metal gel is formed after cooling, whena series of negative ions are added into the metal gel, the fluorescence of the metal gel can be opened only by adding H2PO4<->, and the fluorescence is changed from black to orange, and thus ultra-sensitive detection of the H2PO4<-> is achieved.
Owner:NORTHWEST NORMAL UNIVERSITY
Lignin/polyvinyl alcohol composite material and preparation method thereof
The invention belongs to the technical field of high molecular materials, and discloses a lignin / polyvinyl alcohol composite material and a preparation method thereof. The composite material comprisesthe following components by mass percentage: 70-99.4% of polyvinyl alcohol, 0.5-30% of lignin and 0.1-10% of additive, wherein the additive comprises at least one of 3-amino-1,2,4 triazole, 4-aminopyridine, 1-(3-aminopropyl) imidazole, 4-(2-ethylamino) benzene-1,2-diphenol, 2-amino-3-imidazolylpropionic acid, tannic acid, 3,3,3',3'-tetramethyl-1,1-helix biindolyl-5,5',6,6'-tetraol, zinc chloride,zinc acetate, ferric chloride, calcium chloride, copper chloride, iron oxide, and sodium chloride. The present invention also provides a composite material preparation method. The tensile strength ofthe lignin / polyvinyl alcohol composite material can reach up to 140 MPa, and the elongation at break of the lignin / polyvinyl alcohol composite material can reach up to 800%.
Owner:SOUTH CHINA UNIV OF TECH
4-aminopyridine and a pharmaceutical composition for treatment of neuronal disorders
A composition is provided having the formula where R1 and R2 are each independently H or a C1-C4 hydrocarbon; R3 is H, and R4 is a moiety capable of crossing the blood brain barrier selected from the group consisting of: an amino acid, a peptide, transferrin, gluconate, lactate, citrate, malate, fumarate, benzoate, salicylate, pyruvate and propionate. The composition includes 4-aminopyridine and a transporter species which allows for improved transport of the aminopyridine across the blood brain barrier thereby reducing systemic side effects of aminopyridine administration.
Owner:MILLER LANDON C G
Synthesis method of chelate fiber animal protein factor (APF) with selective adsorption on lead ions
InactiveCN103590243AOvercome the disadvantages of poor selective adsorption performanceGood choiceOther chemical processesFibre typesFiberSynthesis methods
The invention discloses a synthesis method of a chelate fiber animal protein factor (APF) with selective adsorption on lead ions. The method comprises the following steps: 1) by taking a crylic acid-modified polytetrafluoroethylene fiber as a parent, putting the crylic acid-modified polytetrafluoroethylene fiber into methylbenzene to soak for 7-9 hours; 2) adding a ligand to the product obtained in the step 1), stirring at 60-80 DEG C to react for 12-16 hours, wherein the ligand is 4-aminopyridine; the molar ratio of the parent to the ligand is (0.9-1.1):4; 3) filtering the product obtained in the step 2), drying the obtained filter cake into constant weight after rinsing, so as to obtain the chelate fiber APF with selective adsorption on the lead ions. By adopting the chelate fiber APF obtained by the method disclosed by the invention, the defect of poor selective adsorption performance of the original parent is overcome, and the obtained chelate fiber APF shows excellent selectivity on adsorption of the lead ions.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Flame retardant catalyst of metal organic frame complex and preparation method thereof
The invention discloses a flame retardant catalyst of a metal organic frame complex and a preparation method thereof. Cyanuric chloride and 4-aminopyridine are adopted to synthesize a triazine-based organic metal ligand; and then the ligand and metal acetate undergo coordination reaction, so as to obtain the flame retardant catalyst of the metal organic frame complex. A synthesis process is easy to operate, the product aftertreatment is simple, and the flame retardant catalyst is suitable for industrial production. The flame retardant catalyst can improve char formation efficiency and flame restardation of a flame-retardant system, the addition amount of a flame retardant can be effectively reduced, and the flame retardant catalyst further has the advantages of good compatibility, thermal stability, migration resistance and environmental friendliness and the like and is a good flame retardant synergist.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
Pyridines for treating injured mammalian nerve tissue
Embodiments of the present disclosure provide a novel pyridine, pharmaceutical compositions comprising such pyridine, and the use of such compositions in treating injured mammalian nerve tissue, including but not limited to an injured spinal cord. In at least one embodiment of the method, the method comprises the step of administering to the mammal in need thereof with a pharmaceutical composition, or pharmaceutically acceptable salt, comprising 4-aminopyridine-3-methanol.
Owner:PURDUE RES FOUND INC
Carbamide-containing acetylated chitosan quaternary ammonium salt, and preparation method and application thereof
The invention relates to the fields of daily use chemicals and pharmaceutical industry, in particular to carbamide-containing acetylated chitosan quaternary ammonium salt, and a preparation method and application thereof. The preparation method of the carbamide-containing acetylated chitosan quaternary ammonium salt is characterized in that firstly, chitosan reacts with iodomethane and sodium iodide under the alkaline condition for a period of time, and then reacts with chloroacetyl chloride to obtain chloracetylated chitosan quaternary ammonium salt; then, various types of phenylamine and triphosgene react to prepare isocyanate; the isocyanate and 4-aminopyridine react to prepare carbamide; finally, the chloracetylated chitosan quaternary ammonium salt and the prepared carbamide react; through purification, the carbamide-containing acetylated chitosan quaternary ammonium salt is obtained, wherein the molar weight of the chloracetyl chloride is 1 to 2 times of that of the chitosan; the molar weight of the various types of carbamide is 2 to 3 times of that of the chloracetylated chitosan quaternary ammonium salt. The reaction is simple, convenient and efficient; the popularization is easy; the required equipment and raw materials can be easily obtained. The research shows that the derivative has good water solubility and good bacteriostatic activity; the biological activity of the chitosan is enhanced; the application range of the chitosan is enlarged; the chitosan quaternary ammonium salt can be widely applied to the fields of daily use chemicals and medicine.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Pyridines for treating injured mammalian nerve tissue
The invention provides novel pyridines, pharmaceutical compositions comprising such pyridines, and the use of such compositions in treating injured mammalian nerve tissue, including but not limited to an injured spinal cord. In one embodiment, the compounds, compositions, and methods of the instant invention treat a mammalian nerve tissue injury by restoring action potential or nerve impulse conduction through a nerve tissue lesion. Significantly, in vivo application of compounds of the instant invention established, on the basis of SSEP testing, that the compounds provide longer lasting effects at lower concentrations than comparable treatment with the known agent 4-aminopyridine (4 AP).
Owner:PURDUE RES FOUND INC
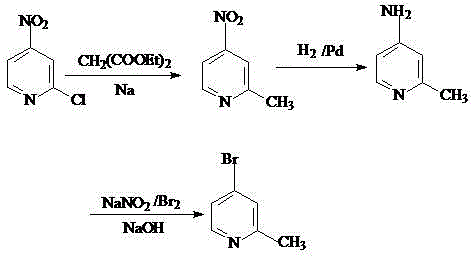
Preparation method of 2-methyl-4-bromopyridine
The invention belongs to the field of organic synthesis and in particular relates to a preparation method of 2-methyl-4-bromopyridine. The preparation method comprises the following steps: (1) reacting diethyl malonate with alkali metal to generate salt, then dropwise adding a toluene solution of 2-chloro-4-nitropyridine to carry out condensation reaction, and then carrying out decarboxylation under the acidic condition to obtain 2-methyl-4-nitropyridine; (2) carrying out hydrogenation reduction on 2-methyl-4-nitropyridine in the presence of a catalyst Pd / C by using methanol as a solvent, carrying out suction filtration and concentrating filtrate to obtain 2-methyl-4-aminopyridine; (3) firstly reacting 2-methyl-4-aminopyridine with acid to generate salt, cooling the salt to minus 10-0 DEG C, dropwise adding bromine, dropwise adding a sodium nitrite water solution after completing dropwise adding bromine, regulating the pH value of the solution to alkalinity after completing dropwise adding the water solution, and then carrying out extraction, drying and concentration to obtain 2-methyl-4-bromopyridine. The preparation method has the beneficial effects that the preparation method is mild in reaction conditions, is easy to operate, is simple in after-treatment, easily achieves large scale production and is very suitable for industrial production; the catalytic effects are good and the yield is high; the raw materials are cheap and the production cost is low.
Owner:陈吉美
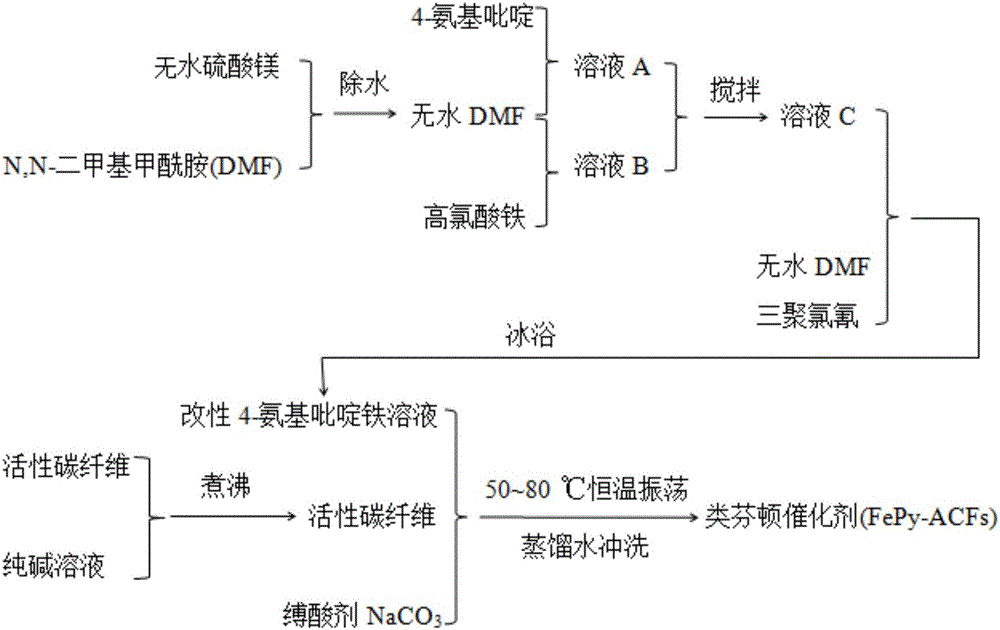
Preparation method of Fenton-like catalyst, as well as product and application of Fenton-like catalyst
InactiveCN106140300ASolve difficult to recycleEfficient degradationWater treatment compoundsOrganic-compounds/hydrides/coordination-complexes catalystsFiberFenton reaction
The invention provides a preparation method of a Fenton-like catalyst. The preparation method comprises the following steps: performing reaction on 4-aminopyridine and ferric perchlorate which serve as reaction precursors in an anhydrous N,N-dimethyl formamide solution to obtain 4-aminopyridine iron, and bonding the 4-aminopyridine iron to active carbon fibers to obtain the Fenton-like catalyst. The catalyst can quickly and effectively degrade organic pollutants, so that the problems that the pH value range for Fenton reaction is narrow and the catalyst is hard to recycle are solved.
Owner:HOHAI UNIV
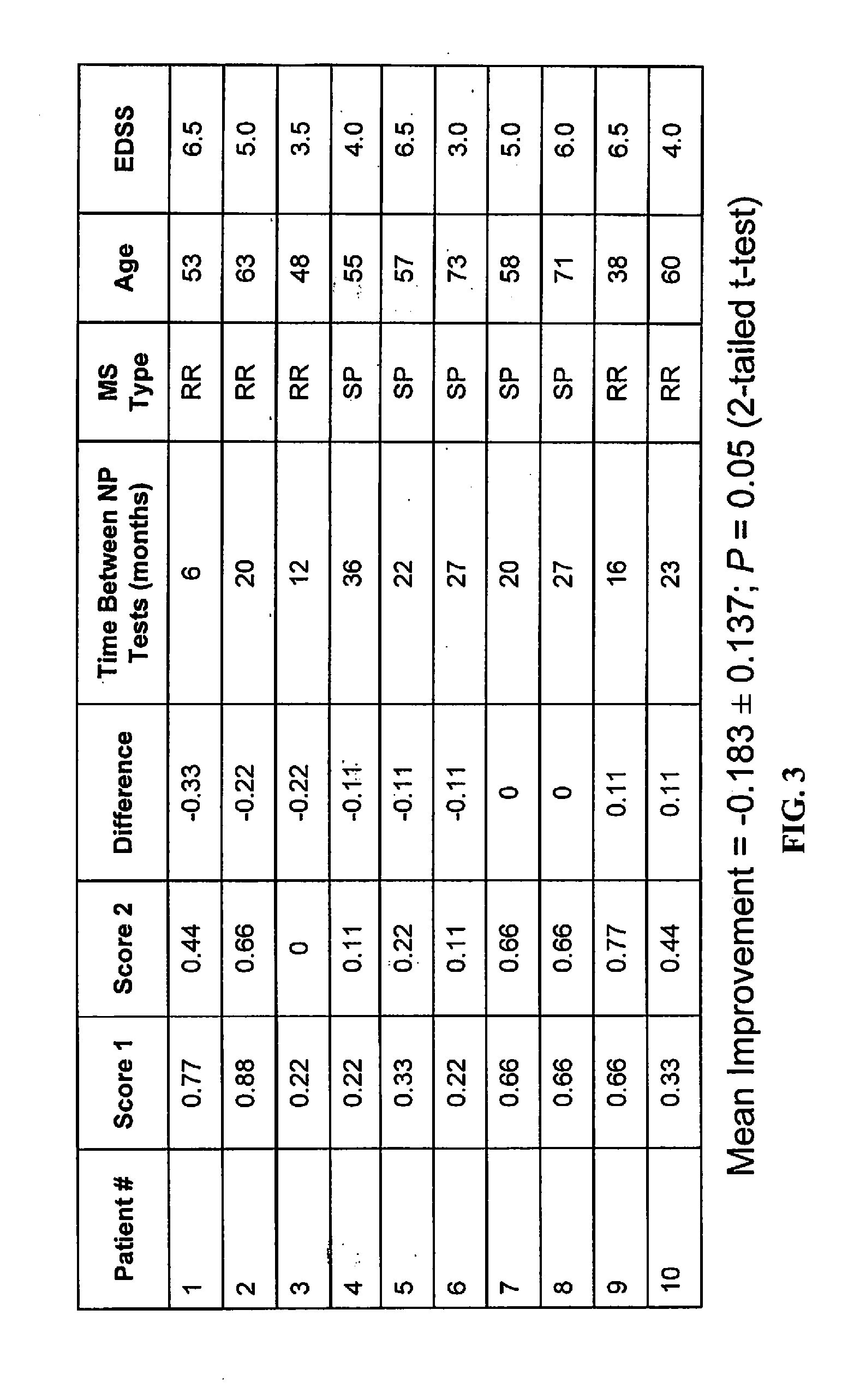
Use of 4-Aminopyridine to Improve Neuro-Cognitive and/or Neuro-Psychiatric Impairment in Patients With Demyelinating and Other Nervous System Conditions
InactiveUS20130053420A1Relieve symptomsAvoid seizuresBiocideNervous disorderNervous systemBrain traumas
Disclosed herein are methods and compositions related to use of aminopyridines, such as 4-aminopyridine, to improve the neuro-cognitive impairments and related neuro-psychiatric impairments of patients with a demyelinating condition such as MS, traumatic brain injury, cerebral palsy, post-radiation encephalopathy.
Owner:ACORDA THERAPEUTICS INC
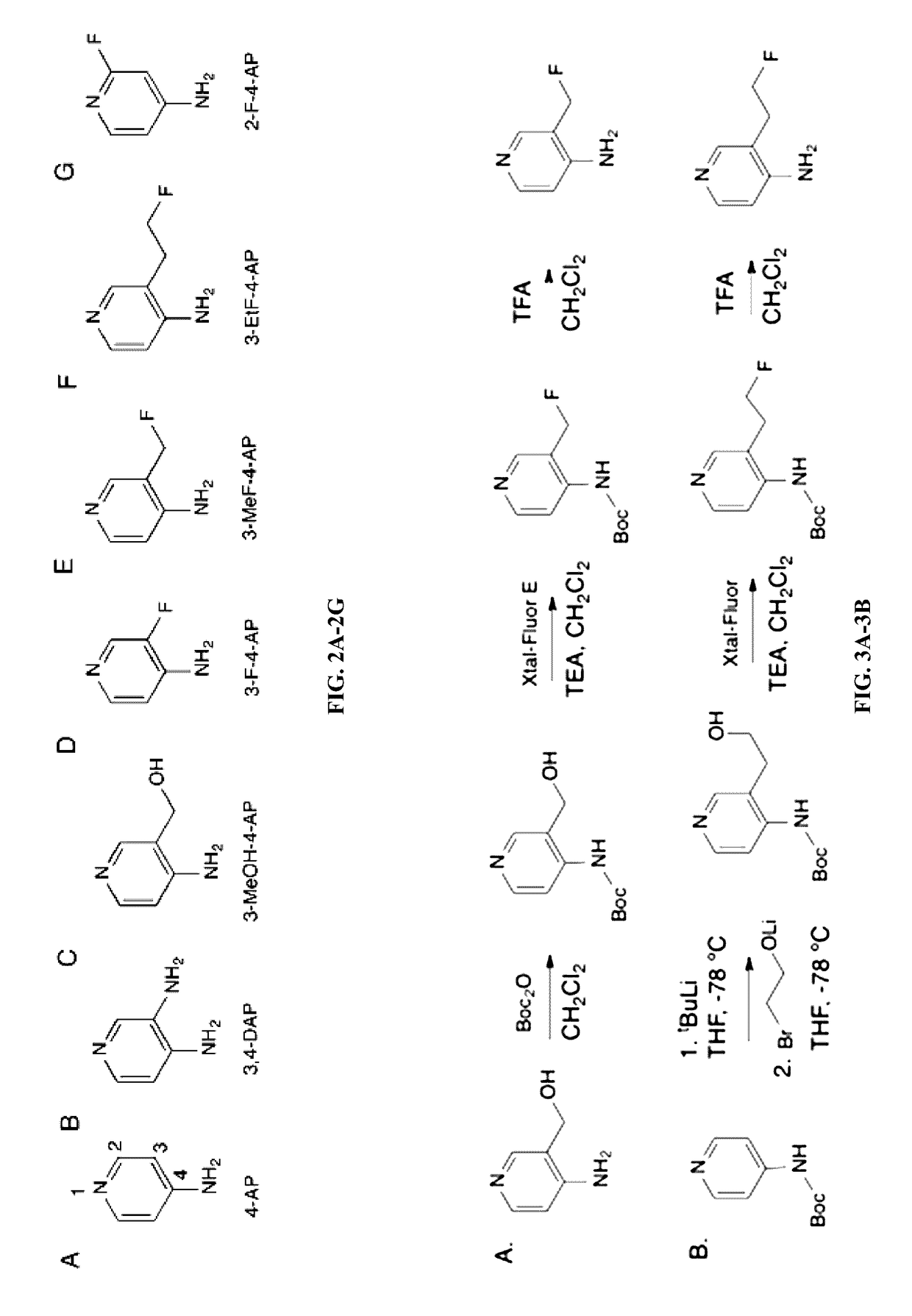
Use of fluorinated derivatives of 4-aminopyridine in therapeutics and medical imaging
ActiveUS9617215B2Isotope introduction to heterocyclic compoundsRadioactive preparation carriersPotassiumMedical imaging
The present disclosure provides novel compounds, including compounds that bind to potassium channels, methods for their manufacture, and methods for their use, including their use to diagnose and / or assess traumatic brain injury and use to treat dymeylinating diseases, and / or in vivo imaging of the central nervous system, and to diagnose and / or assess the progression of MS or other diseases.
Owner:UNIVERSITY OF CHICAGO
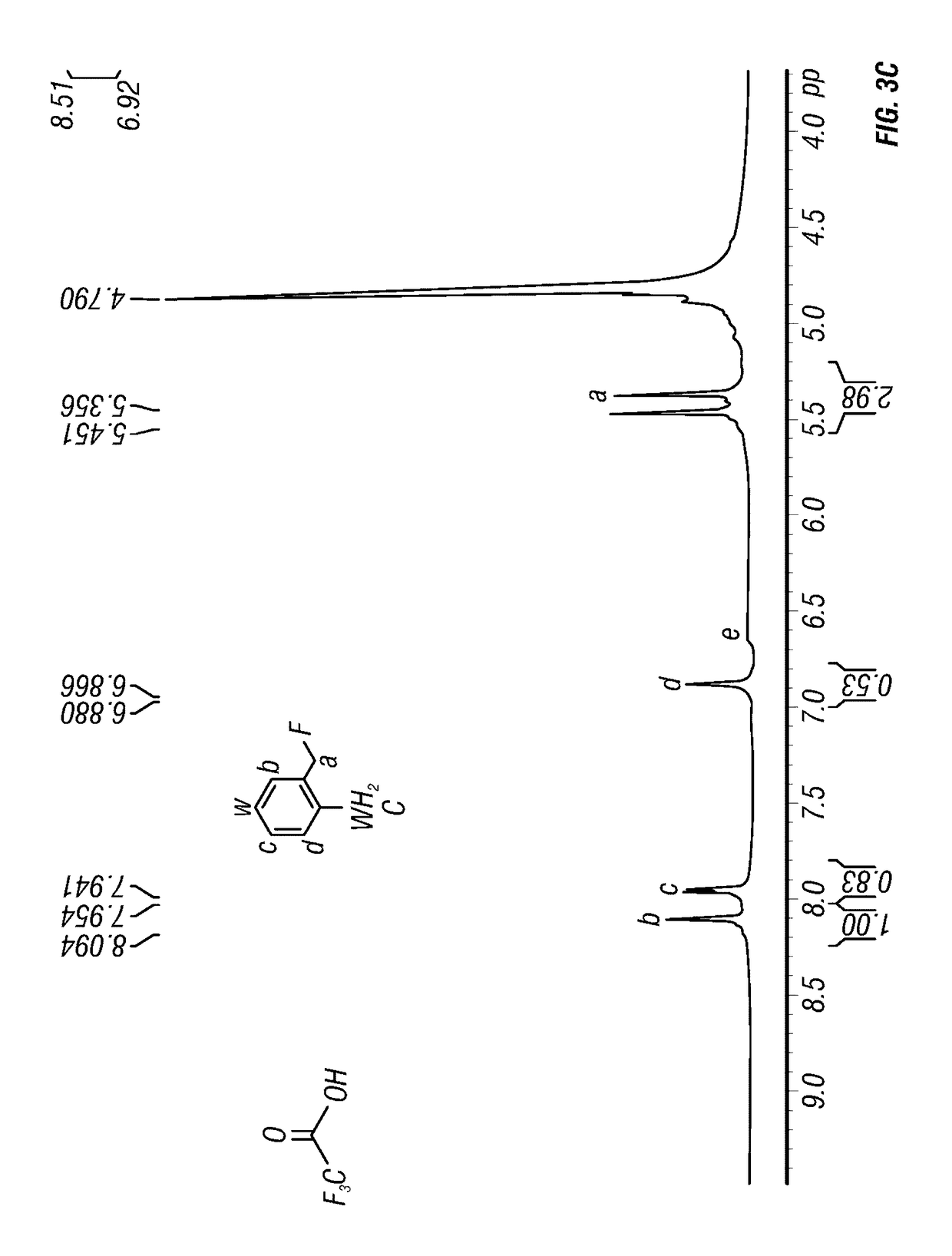
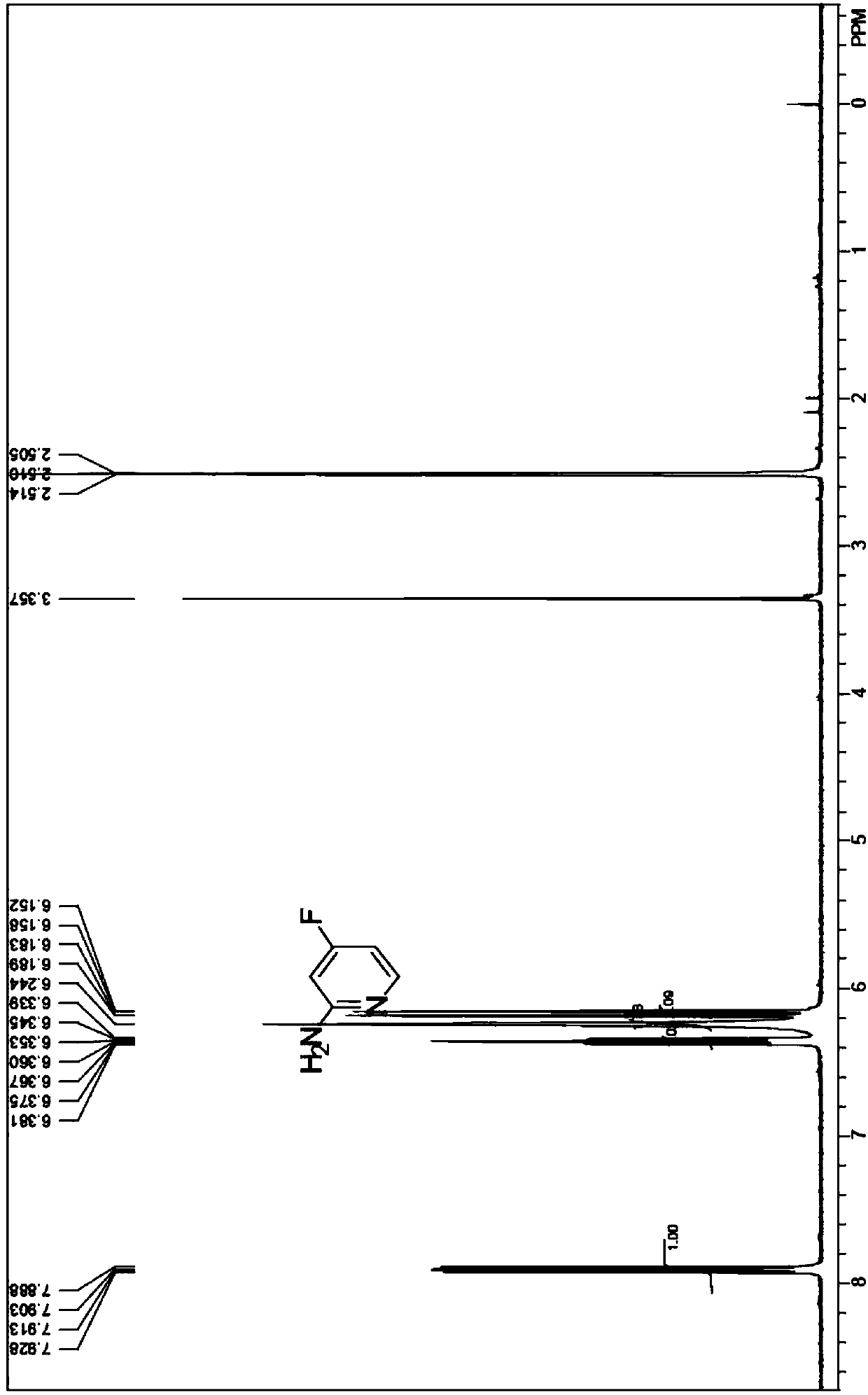
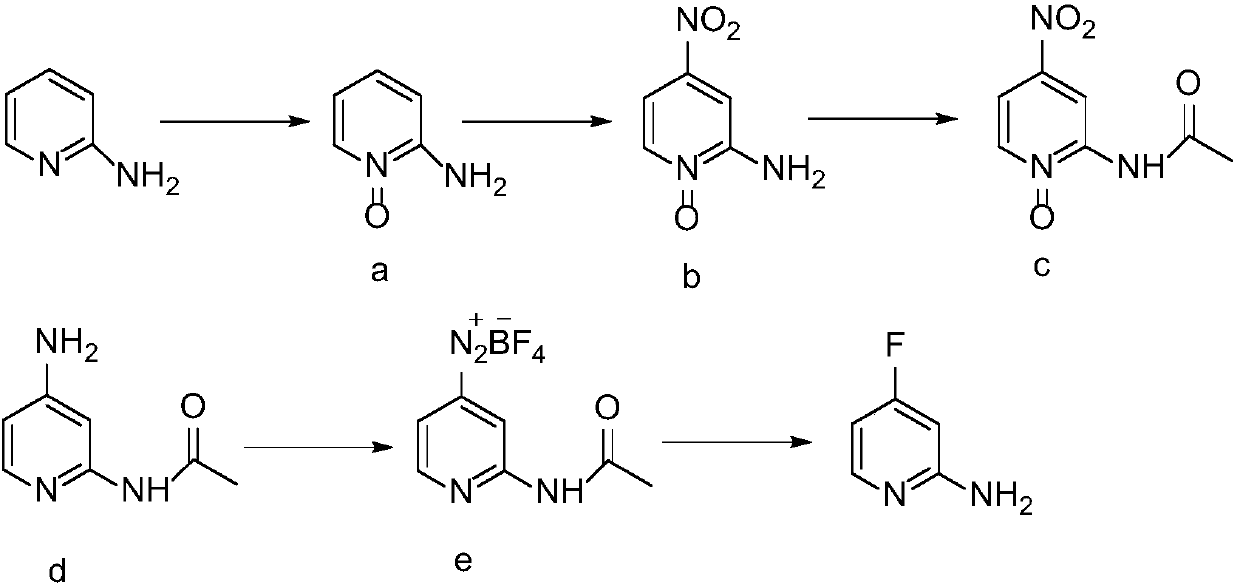
Synthesis method of 2-amino-4-fluoropyridine
InactiveCN107759515ANo pollution in the processEasy to operateOrganic chemistrySynthesis methodsNitration
The invention discloses a synthesis method of 2-amino-4-fluoropyridine. The synthesis method comprises the following synthesis steps: 1, taking diaminopyridine as the raw material, carrying out oxidation reaction to prepare 2-aminopyridine N-oxide a; 2, subjecting the compound a to nitration reaction with mixed acid to obtain 2-amino-4-nitropyridine N-oxide b; 3, carrying out acylation reaction onthe compound b and an acylation reagent to obtain 2-acetamino-4-nitropyridine N-oxide c; 4, carrying out reduction reaction on the compound c to obtain 2-acetamino-4-aminopyridine d; 5, carrying outdiazo-reaction on the compound d and HBF4 to obtain diazonium fluoroborate e; and 6, carrying out Balz-Schiemann reaction on the compound e to introduce fluorine atoms and conducting hydrolysis deacetylation to obtain 2-amino 4-fluoropyridine. The method provided by the invention solves the problems of long synthesis route, complicated operation, serious pollution, high cost, low yield and the like in existing synthesis methods.
Owner:SHANGHAI LINKCHEM TECH CO LTD
Silver cathode activation
ActiveUS20130256148A1Quick responseOrganic chemistryPhotography auxillary processesBiological activation4-Aminopyridine
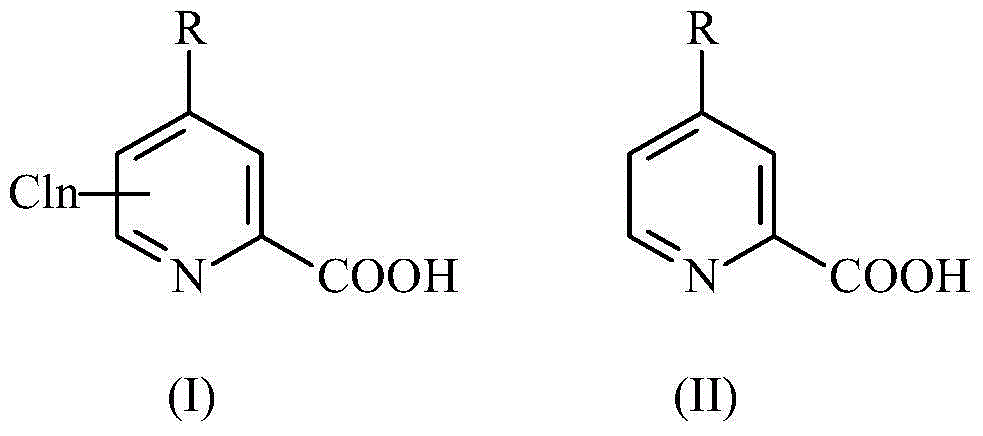
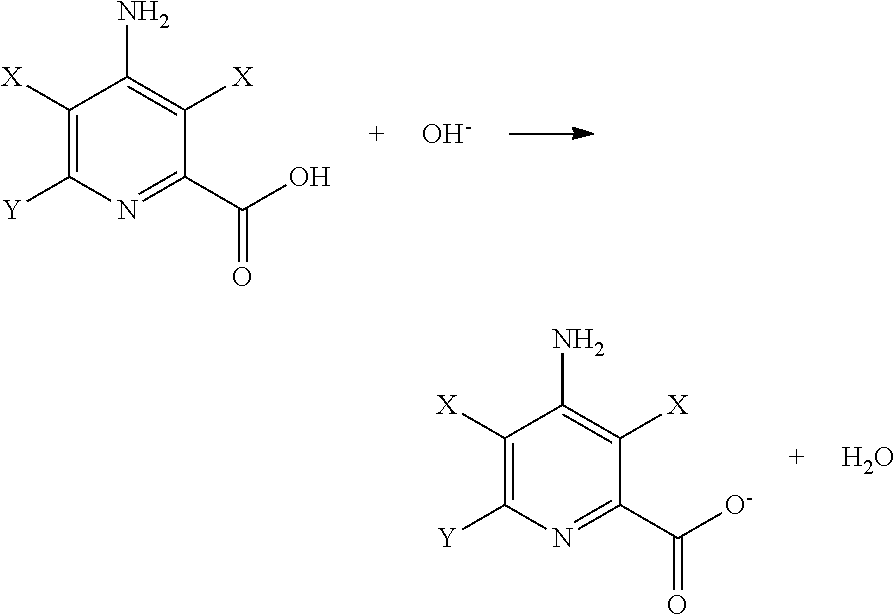
The selective electrochemical reduction of halogenated 4-aminopicolinic acids is improved by activating the cathode at a final potential from about +1.0 to about +1.8 volts.
Owner:CORTEVA AGRISCIENCE LLC
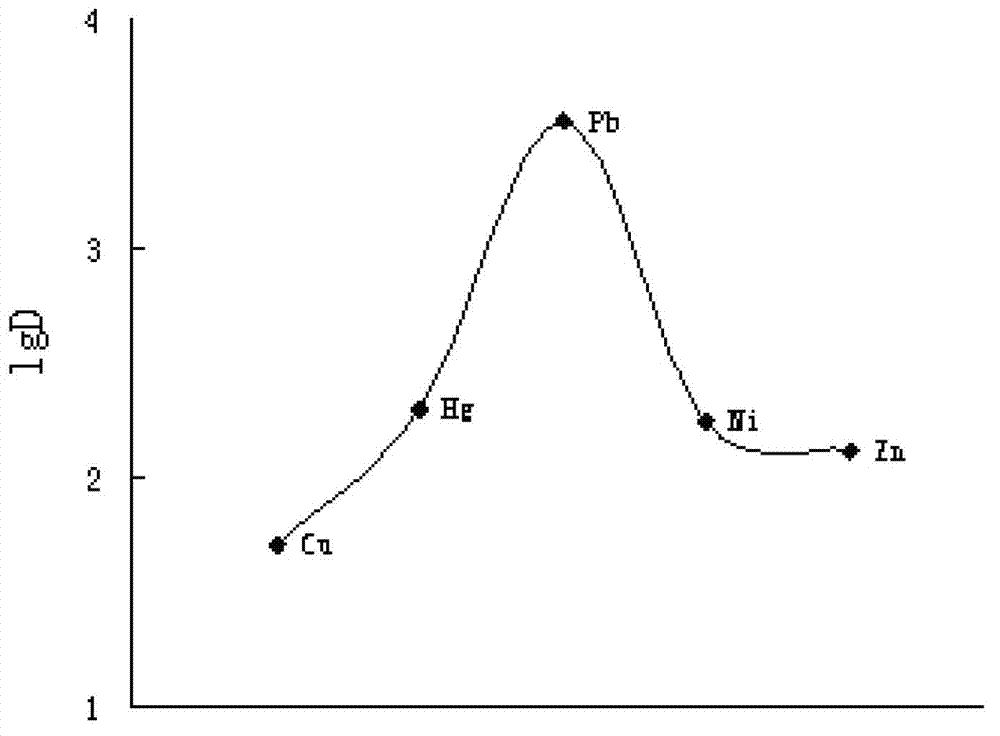
Water Detoxification by a Substrate-Bound Catecholamine Adsorbent
InactiveUS20140061135A1Increase costIon-exchanger regenerationSpecific water treatment objectivesCatecholamineLutetium
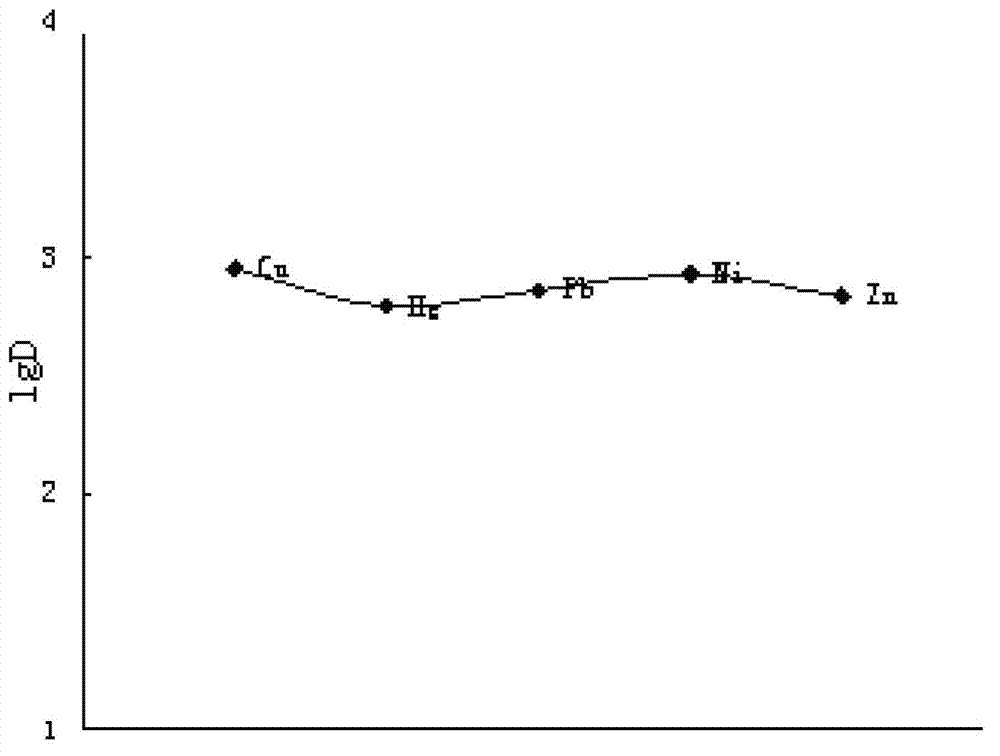
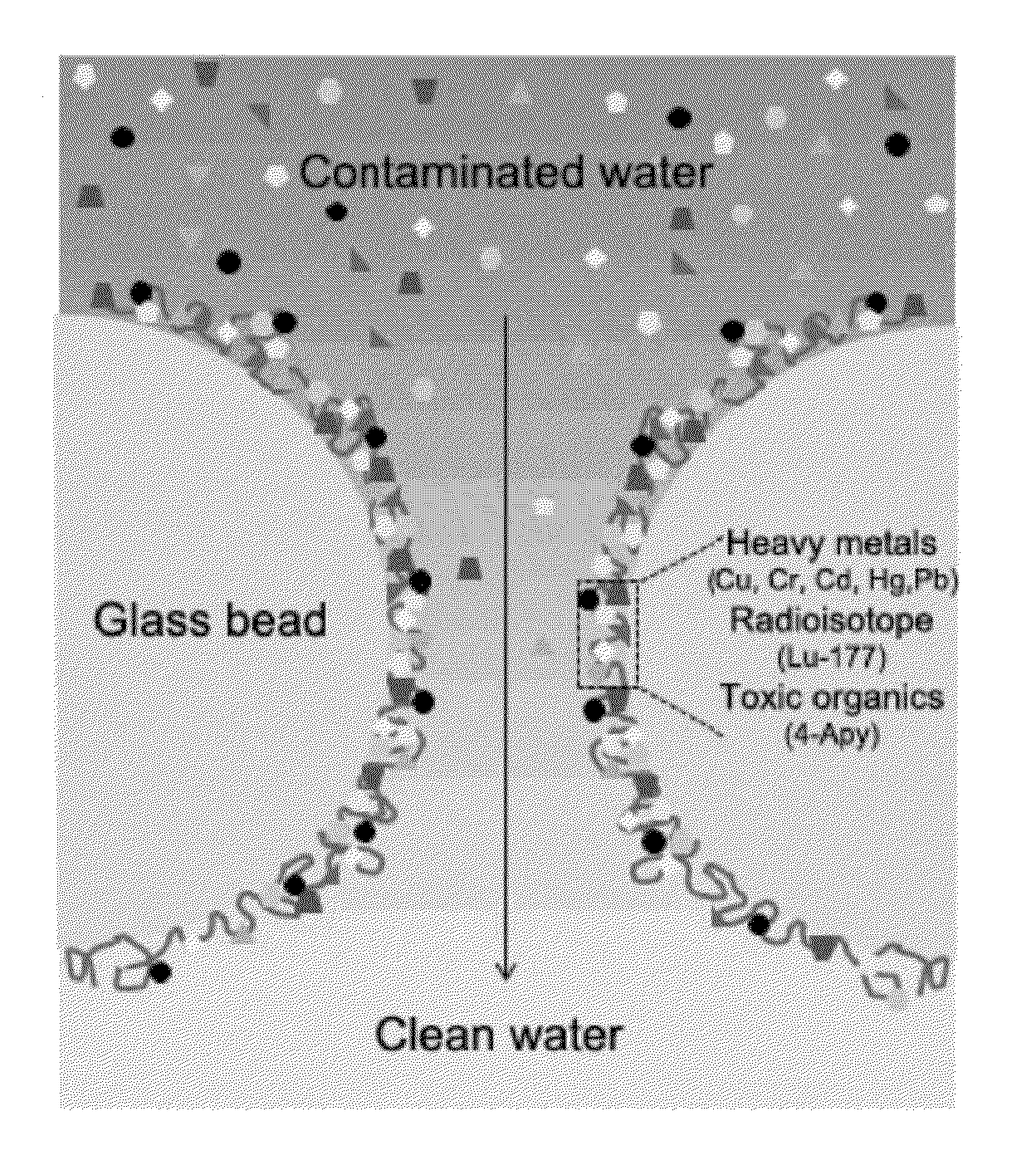
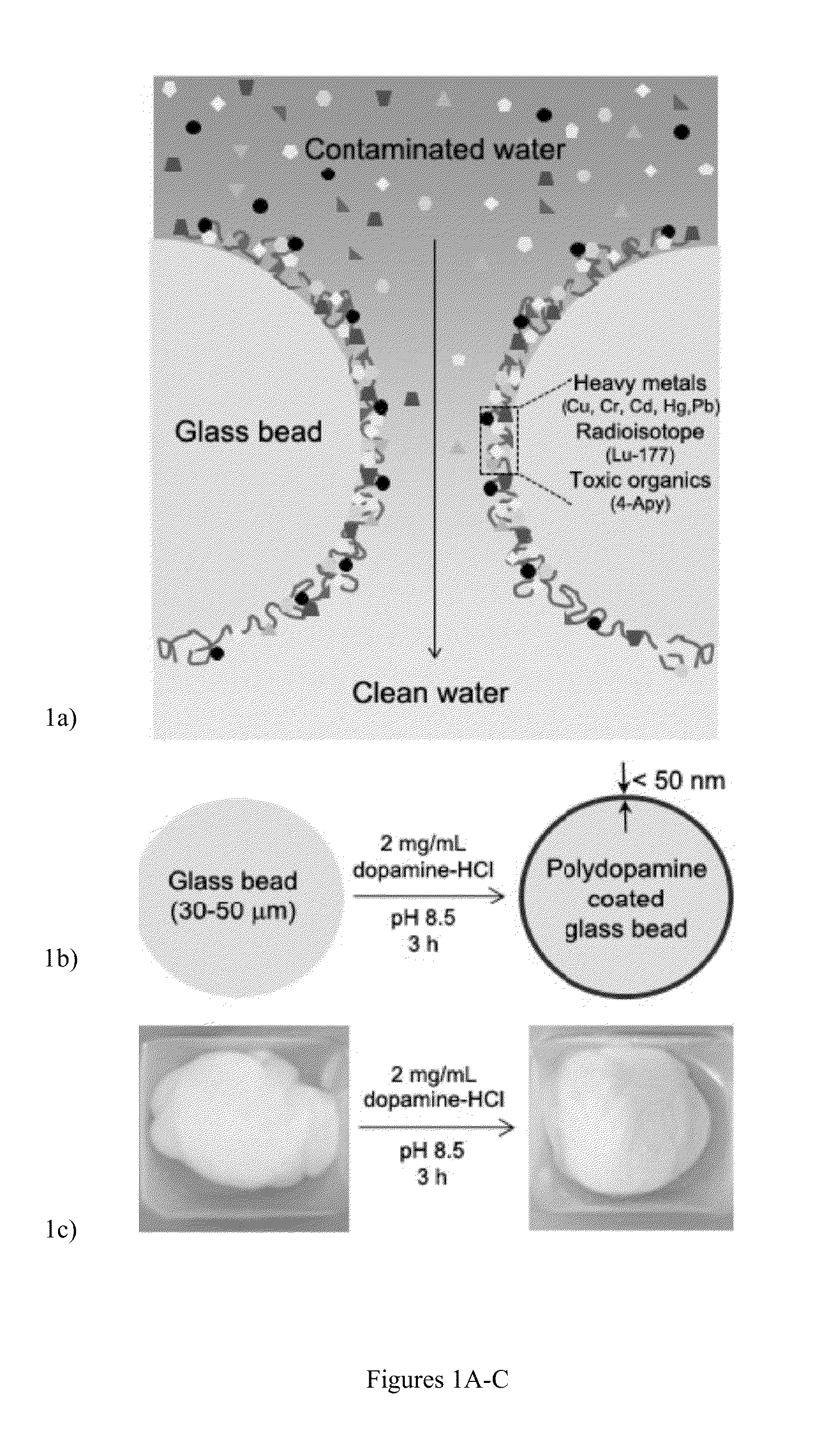
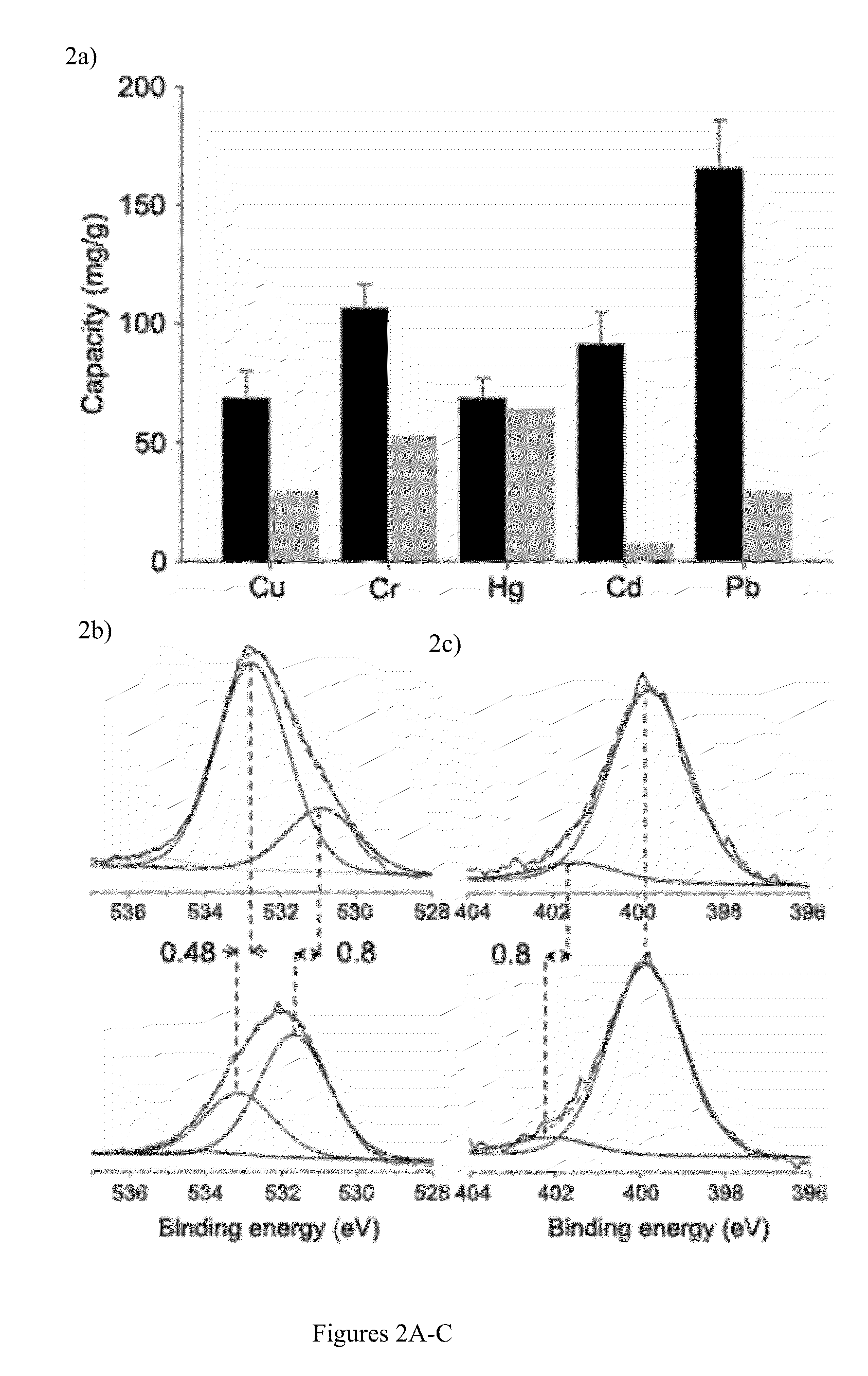
A bio-inspired method for detoxifying contaminated water is disclosed. In the method, polydopamine, a mussel-inspired adhesive catecholamine was used as an adsorbent to effectively remove from contaminated water three major classes of toxic agents: heavy metal ions (e.g., Cr, Hg, Pb, Cu, and Cd), toxic organic species (e.g., 4-aminopyridine), and radioisotopes (e.g., Lutetium-177). Furthermore, the polydopamine adsorbent was regenerated by treatment with acid or hydrogen peroxide.
Owner:NORTHWESTERN UNIV
Nicotinic acid pharmaceutical co-crystal and preparation method thereof
InactiveCN107011261APreserve drug propertiesExtend the market cycleOrganic chemistry methodsBenzoic acidSolvent
The invention discloses a nicotinic acid pharmaceutical co-crystal and a preparation method of the nicotinic acid pharmaceutical co-crystal. The nicotinic acid pharmaceutical co-crystal is characterized by mixing nicotinic acid with 2,6-dihydroxy benzoic acid or 4-aminopyridine in a weight ratio of 1:(1-8), adding methanol to be ground until a solvent is volatilized after grinding uniformly, then carrying out ultrasonic treatment in a methanol / acetone mixed solvent until being dissolved completely, and then standing for 5 to 10 days for separating out a white transparent crystal, namely the pharmaceutical co-crystal of nicotinic acid and 2,6-dihydroxy benzoic acid or nicotinic acid and 4-aminopyridine. Compared with the prior art, the nicotinic acid pharmaceutical co-crystal disclosed by the invention is simple and controllable in preparation method, short in synthesis cycle and good in repeatability, not only keeps drug characteristics of nicotinic acid, but also has obviously improved solubility, prolongs the market cycle of the original drug and has broad application prospects.
Owner:EAST CHINA NORMAL UNIV
Metal complex flame retardant and preparation method thereof
InactiveCN107344998AReduce heat release peaksImprove flame retardant performanceNickel organic compoundsGroup 2/12 organic compounds without C-metal linkagesThermal stabilityCoordination complex
The invention discloses a metal complex flame retardant and a preparation method thereof. The metal complex flame retardant is prepared by the steps of adopting hexachlorotriphosphazene and 4-aminopyridine, and synthesizing a cyclotriphosphazene-based organic metal ligand; and then carrying out coordination reaction on the ligand and metal acetate to obtain the metal complex flame retardant with high thermal stability and high char yield. A synthesis process is easy to operate, simple in aftertreatment of a product and suitable for industrial production; and the metal complex flame retardant combines the flame retardant advantages of metal catalysis and phosphazene flame retardants, can effectively lower the addition amount of the flame retardant and is an efficient halogen-free and environmental-friendly flame retardant.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
Biomimetic catalytic carbon fiber and preparation method thereof
InactiveCN104607246AAvoid gatheringEasy transferOrganic-compounds/hydrides/coordination-complexes catalystsFiberCarbon fibers
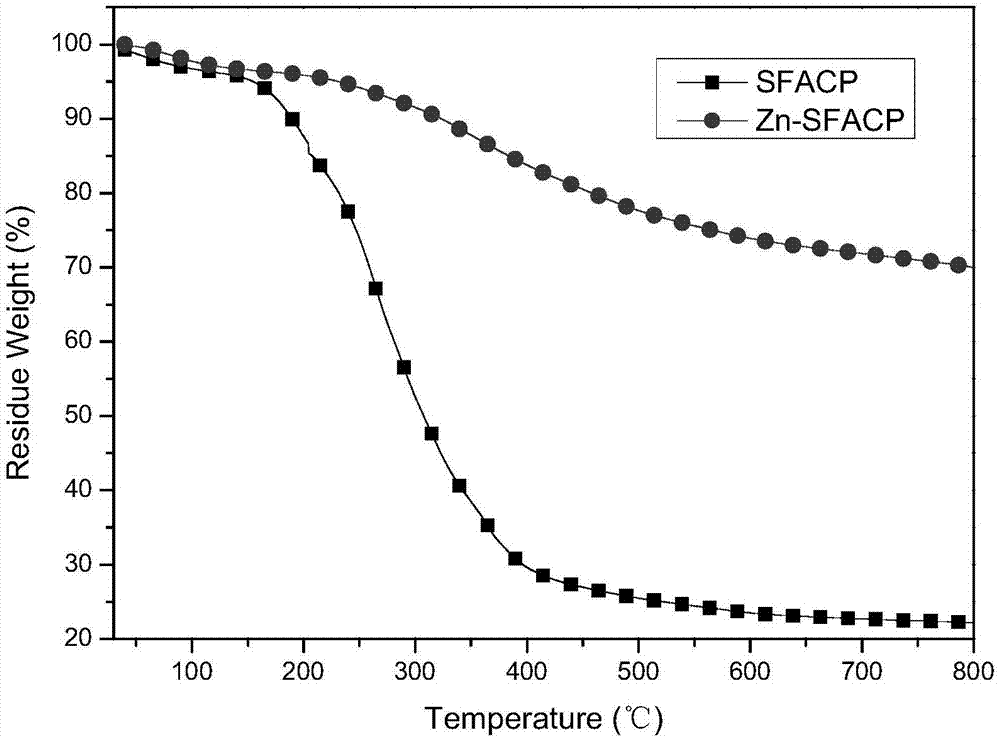
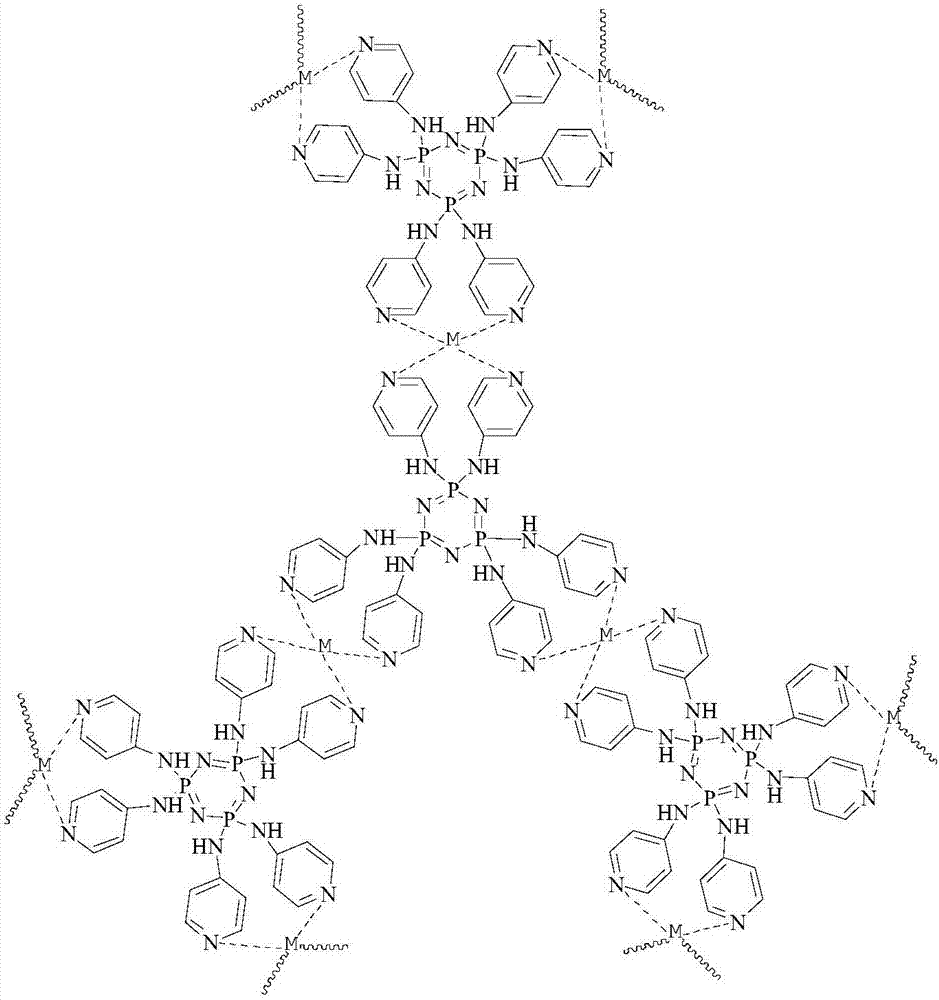
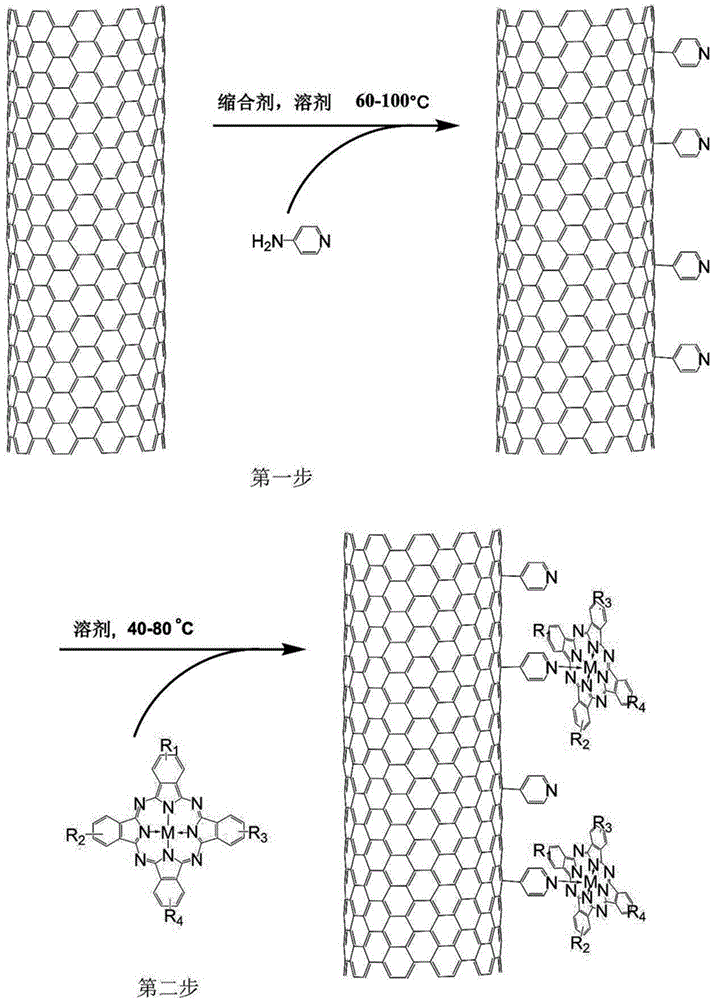
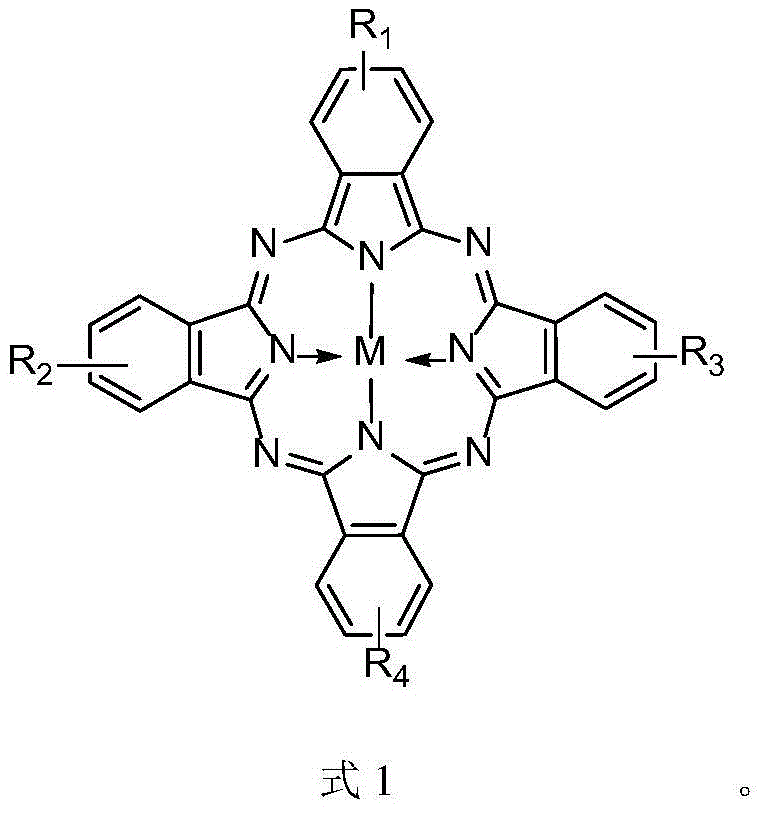
The invention relates to a biomimetic catalytic carbon fiber and a preparation method thereof. The biomimetic catalytic carbon fiber is formed by combining a modified carbon fiber containing a pyridine group and metal phthalocyanine in a coordination bond way. The preparation method of the biomimetic catalytic carbon fiber comprises the following steps: dispersing a carbon fiber into a solvent under the condition that the reaction temperature is 60-100 DEG C, adding 4-aminopyridine and a condensing agent fully dissolved in the solvent, stirring and reacting for 2-48 hours, taking out the modified carbon fiber containing the pyridine group after the completion of reaction, respectively washing with a reaction solvent, N,N-dimethyl formamide, water and ethanol and drying at a temperature of 80-100 DEG C to obtain the modified carbon fiber; successively dispersing the modified carbon fiber in the solvent at a reaction temperature of 40-80 DEG C, adding metal phthalocyanine fully dissolved in the solvent, stirring and reacting for 2-48 hours, taking out the carbon fiber loaded with the metal phthalocyanine after the completion of reaction, washing with the reaction solvent, the N,N-dimethyl formamide, the water and the ethanol and drying at a temperature of 80-100 DEG C to obtain the biomimetic catalytic carbon fiber.
Owner:ZHEJIANG SCI-TECH UNIV
Functional 1,4,5,8-naphthalimide supermolecular organogel based on 4-aminopyridine, and application
InactiveCN109053728ARealize highly sensitive detection and identificationOrganic chemistryFluorescence/phosphorescenceFluorescencePyridine
The invention designs and synthesizes a functional 1,4,5,8-naphthalimide supermolecular organogel based on 4-aminopyridine. In DMSO, a main body which is the 4-aminopyridine functional 1,4,5,8-naphthalimide and an object body which is a supramolecular compound are complexed to form an orange aggregative-state induced fluorescence supermolecule organogel through hydrogen-bond interaction and pi-piaccumulation under heating and dissolving. Fe<3+>, Hg<2+>, Ag<+>, Ca<2+>, Cu<2+>, Co<2+>, Ni<2+>, Cd<2+>, Pb<2+>, Zn<2+>, Cr<3+> and Mg<2+> are respectively added into the supermolecular organogel, and the experiments find that only through adding the Fe<3+> and the Cu<2+>, the fluorescence quenching of the supermolecular organogel can be realized, the high-sensitivity detection of the Fe<3+> andthe Cu<2+> can be realized, and the lowest detectable limits of the Fe<3+> and the Cu<2+> are 1x10<-6>M and 1x10<-7>M.
Owner:NORTHWEST NORMAL UNIVERSITY
Polymorphic substances of 4-aminopyridine, and preparation and application thereof
The invention discloses polymorphic substances I-VIII of 4-aminopyridine or solvate thereof, and a preparation method and application thereof as active ingredients of medicaments. The polymorphic substances of 4-aminopyridine or solvate thereof provide a better active medical substance state other than the amorphous state, thereby providing optimal selection for the preparation of agents for improving the walking speed of patients with multiple sclerosis and simultaneously relieving other symptoms of multiple sclerosis.
Owner:TIANJIN HEMAY BIO TECH CO LTD
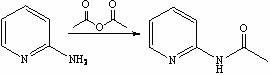
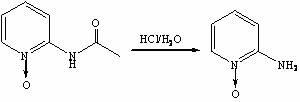
Method for synthesis preparation of 2-chloro-4-aminopyridine
InactiveCN102101841AHigh yieldMild reaction conditionsOrganic chemistryNitrogen oxides2-Chloropyridine
The invention discloses a method for preparing 2-chloro-4-aminopyridine, which comprises the following steps: 1, synthesizing 2-acetamidopyridine; 2, synthesizing a 2-acetamidopyridine nitrogen oxide; 3, synthesizing a 2-aminopyridine nitrogen oxide; 4, synthesizing a 2-aminopyridine nitrogen oxide; 5, synthesizing 2-cholorpyridine nitrogen oxide; 6, synthesizing 2-chloro-4-nitropyridine nitrogenoxide; and 6, synthesizing 2-chloro-4-pyridinamine. In the preparation method, the reaction conditions are mild, the operation is simple, the raw materials are readily available, and the total yield is high.
Owner:周玉莲
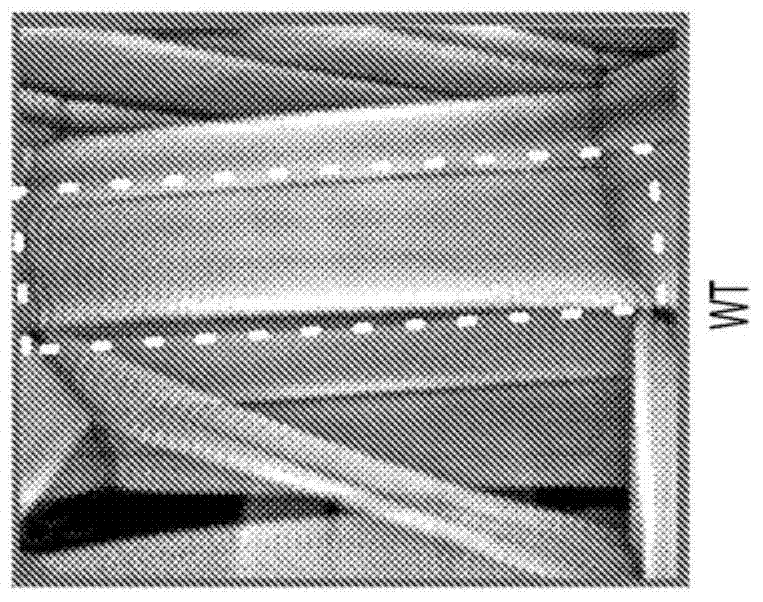
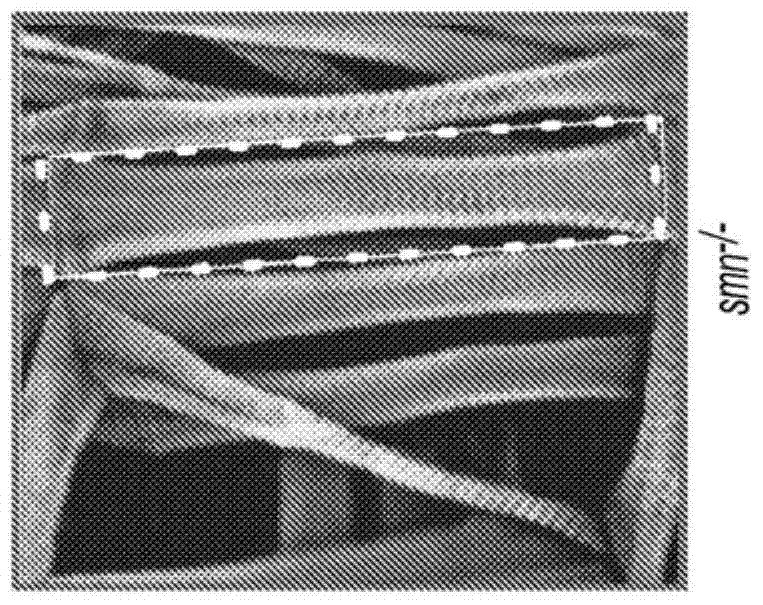
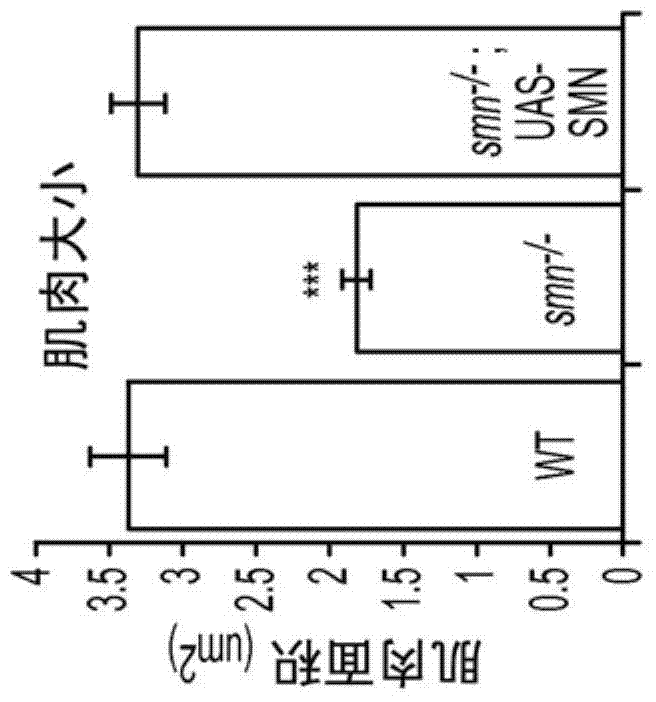
4-aminopyridine as a therapeutic agent for spinal muscular atrophy
It has been discovered that pharmacological inhibition of K+ channels (using the FDA-approved broad-spectrum K+ channel antagonist 4-AP) positively benefitted smn mutant phenotypes, a result that is consistent with the defective excitability of motor circuits by their interneuron or sensory neuron inputs being a critical consequence of SMN depletion. Based on these observations, certain embodiments of the invention are directed to methods of treatment of SMA by administering therapeutically effective amounts of one or more potassium channel antagonists, including 4-aminopyridine, 4-(dimethylamino)pyridine, 4-(methylamino)pyridine, and 4-(aminomethyl)pyridine. Other embodiments are directed to new pharmaceutical formulations comprising two or more potassium channel antagonists.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
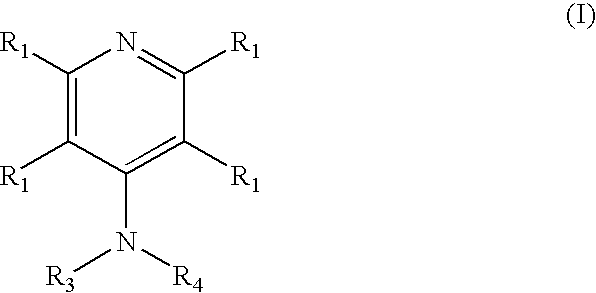
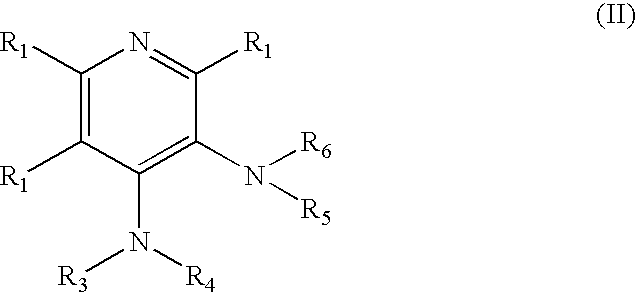
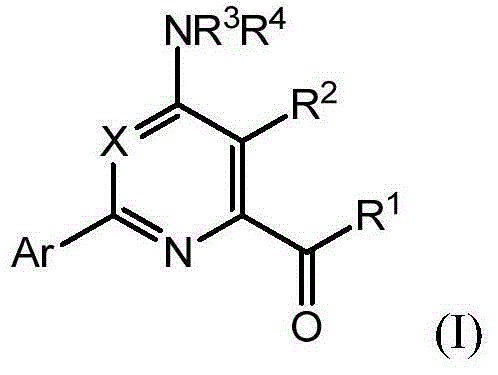
Novel 4-aminopyridine and 6-aminopyrimidine carboxylates as herbicides
4-Amino-6-(pyridyl and 2-substitutedphenyl)-picolinic acids and their derivatives; 6- amino-2-(pyridyl and 2-substitutedphenyl)-pyrimidine-4-carboxylates and their derivatives; and methods of using the same as herbicides.
Owner:DOW AGROSCIENCES LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com