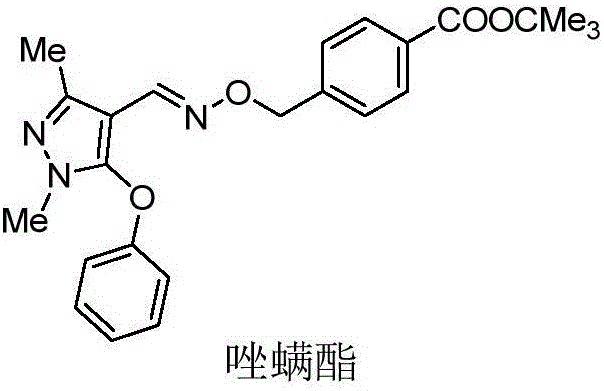
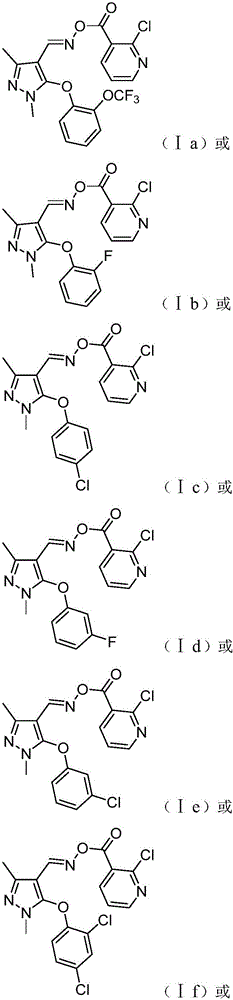
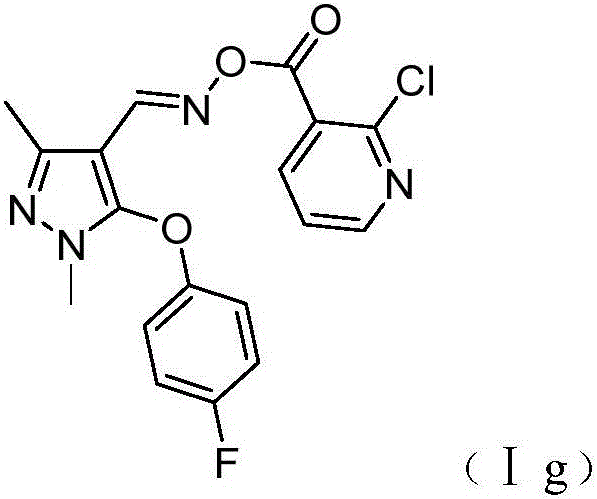
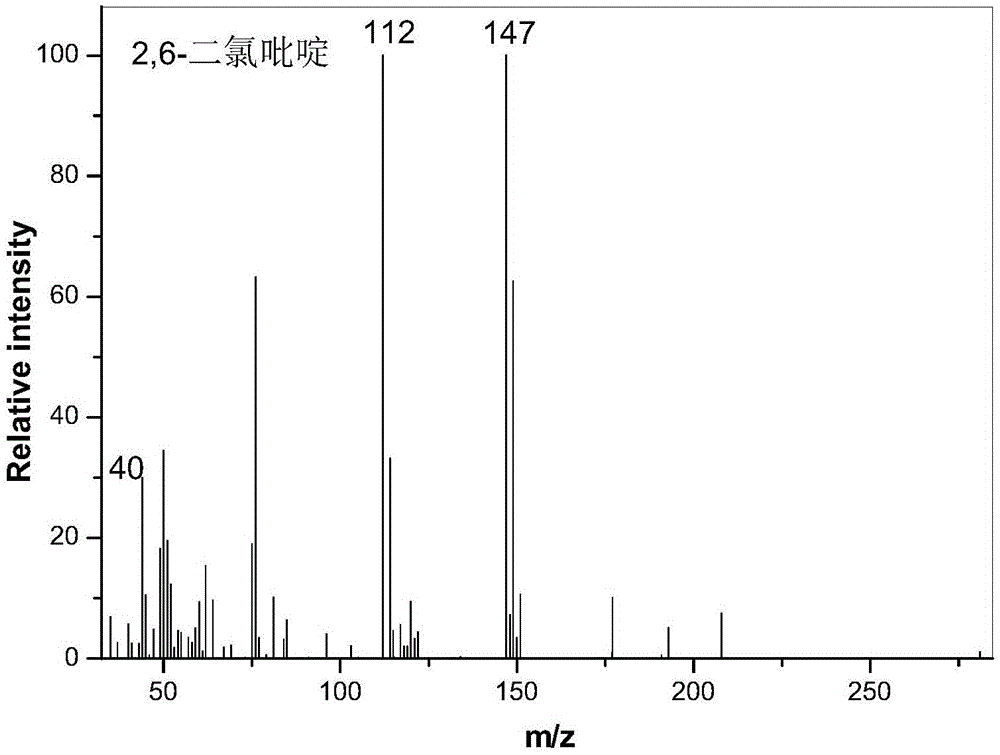
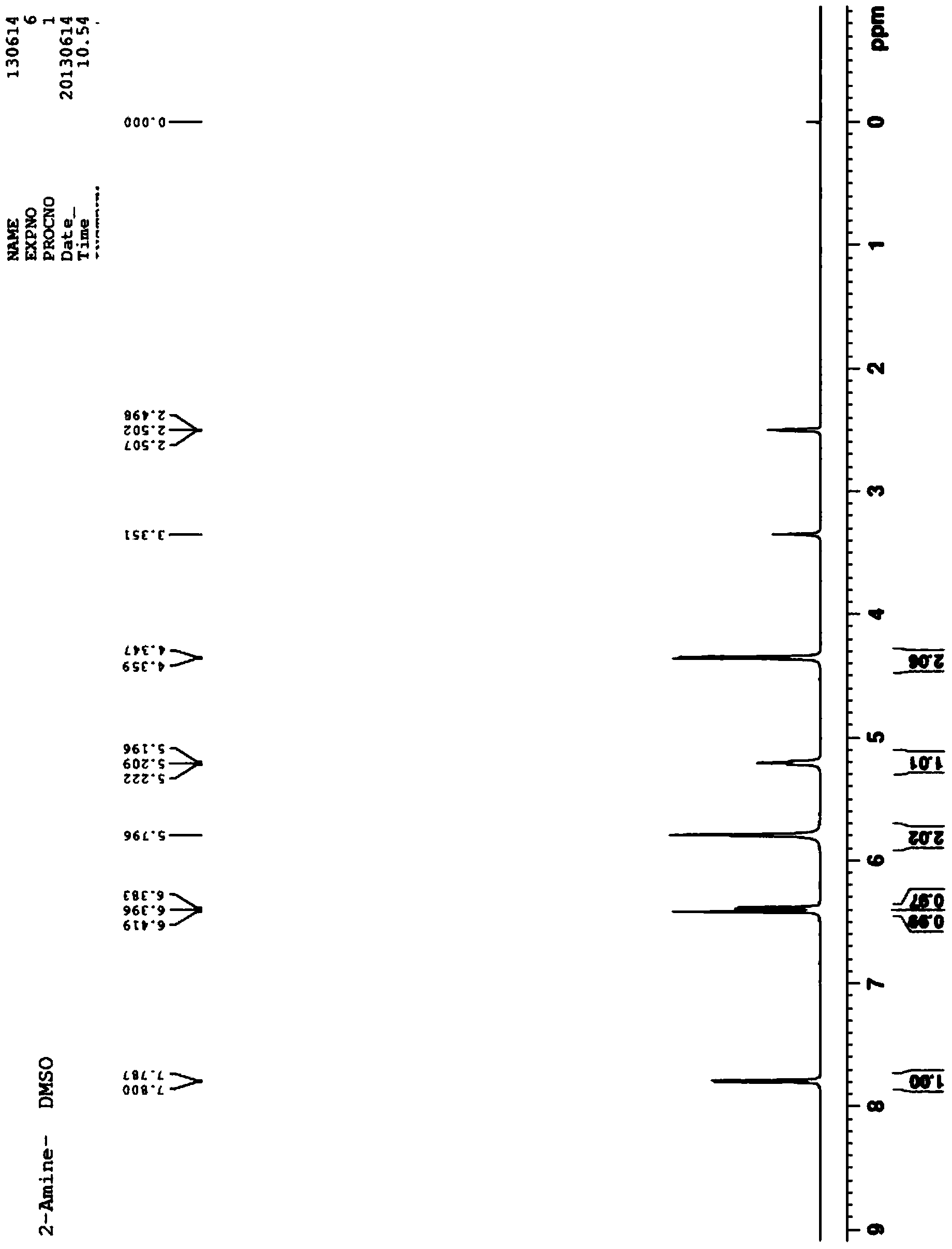
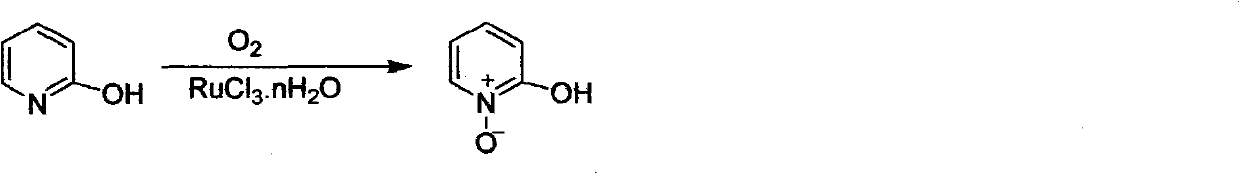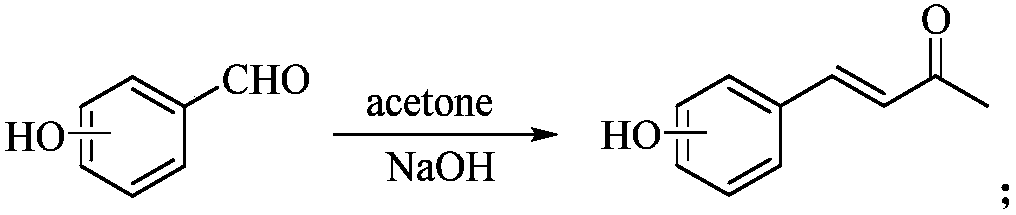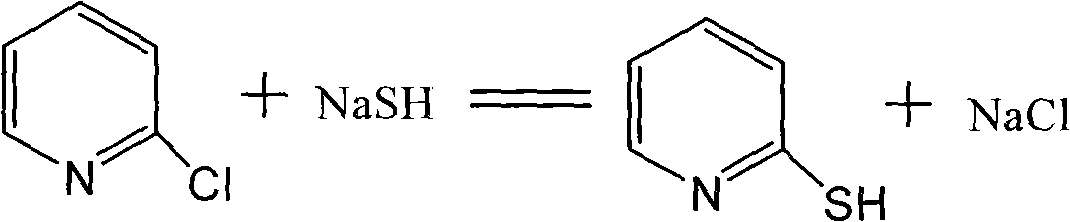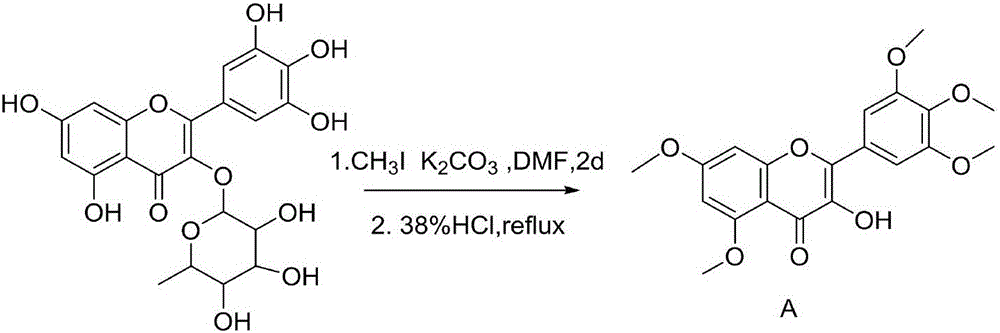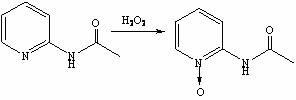Patents
Literature
90 results about "2-Chloropyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
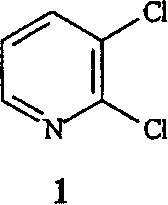
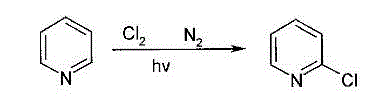
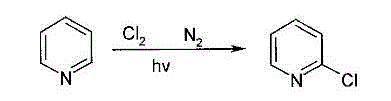
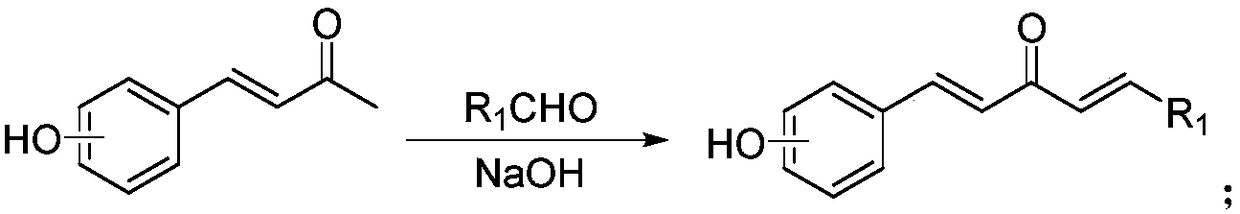
2-Chloropyridine is an organohalide with the formula C₅H₄ClN. It is a colorless liquid that is mainly used to generate fungicides and insecticides in industry. It also serves to generate antihistamines and antiarrythymics for pharmaceutical purposes.
Process for the manufacture of 2,3-dichloropyridine
A method for preparing 2,3-dichloropyridine is disclosed in which 3-amino-2-chloropyridine is contacted with an alkali metal nitrite in the presence of aqueous hydrochloric acid to form a diazonium salt; and the diazonium salt is subsequently decomposed in the presence of copper catalyst wherein at least about 50% of the copper is the copper(II) oxidation state.
Owner:FMC CORP
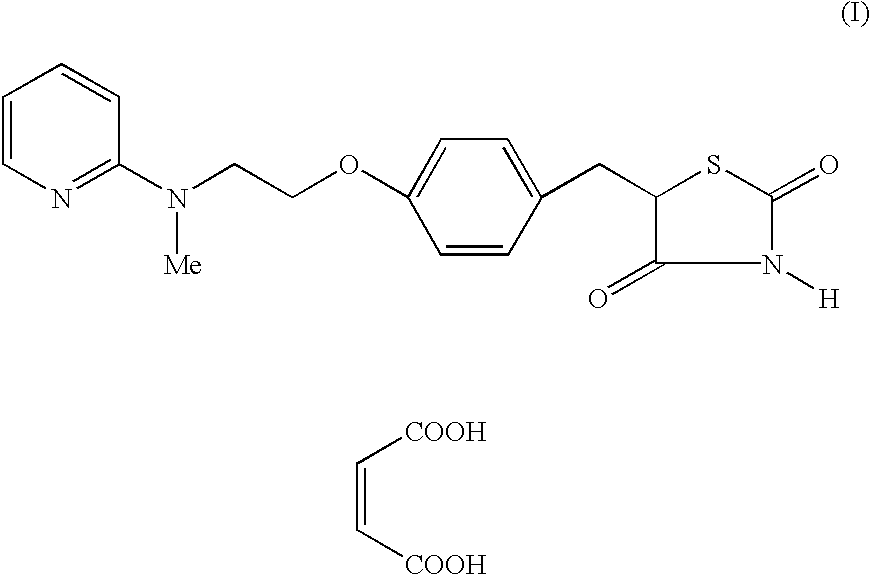
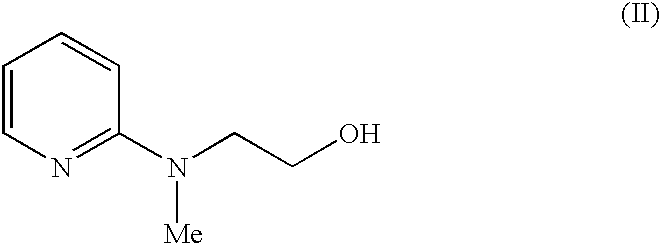
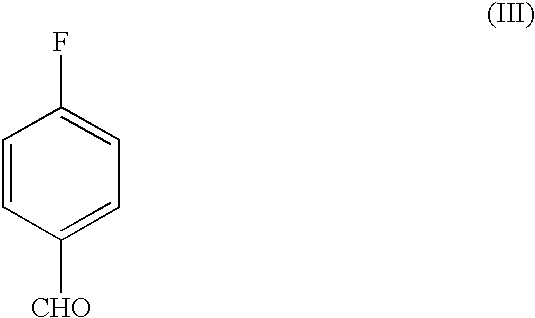
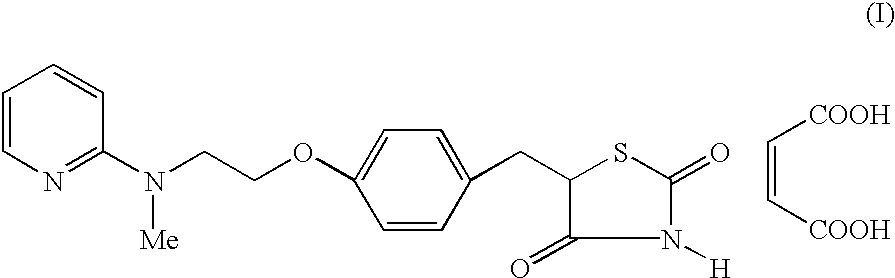
Process for the preparation of rosiglitazone maleate
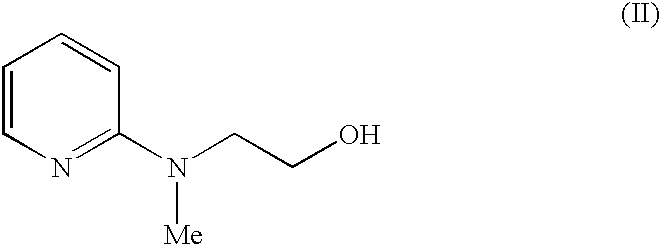
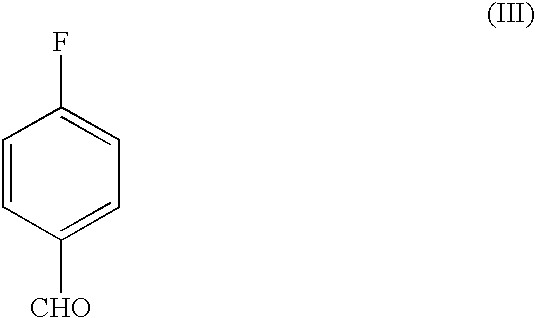
The invention discloses a process for the preparation of a pyridine derivative namely 5-{4-2(N-methyl-N(2-pyridyl)amino ethoxy]benzyl]thiazolidine-2,4-dione maleate comprising the steps of:(a) reacting 2-chloropyridine with 2-(N-methyl amino)ethanol;(b) coupling 2-(N-methyl-N-(2-pyridyl) amino)ethanol) obtained in step (a) and 4-fluorobenzaldehyde in an aprotic polar solvent with an alkali metal hydroxide or an alkali metal alkoxide as base.(c) isolating the product of the coupling reaction viz 4-[2-(N-methyl-N-(2-pyridyl) amino) ethoxy]benzaldehyde;(d) converting said isolated benzaldehyde compound of step (c) into 5-[4-[2-N-methyl-N-(2-pyridyl) amino)ethoxy]benzyl]thiazolidine-2,4-dione in a known manner and(e) converting said thiazolidine-2,4-dione compound obtained in step (d) into a pharmaceutically acceptable maleate salt.
Owner:TORRENT PHARMA LTD
Novel synthesis process of 2,2'-dipyridyl
The invention discloses a novel synthesis process of 2,2'-dipyridyl. The novel synthesis process adopting 2-chloropyridine as the raw material comprises the following steps of: coupling the material to obtain 2,2'-dipyridyl under the catalytic action of the catalyst; screening out a better alkaline hydrolysis reagent and auxiliaries to carry out alkaline hydrolysis by selecting an alkaline hydrolysis reagent and auxiliaries; and obtaining refined 2,2'-dipyridyl through the steps including extracting, acid-washing and re-alkaline hydrolyzing and the like. The novel synthesis process disclosed by the invention is simple, capable of greatly reducing the production cost, capable of reducing the reaction yield and capable of optimizing the process flow.
Owner:NANJING COSTAR BIOTECHNOLOGY RES INST CO LTD
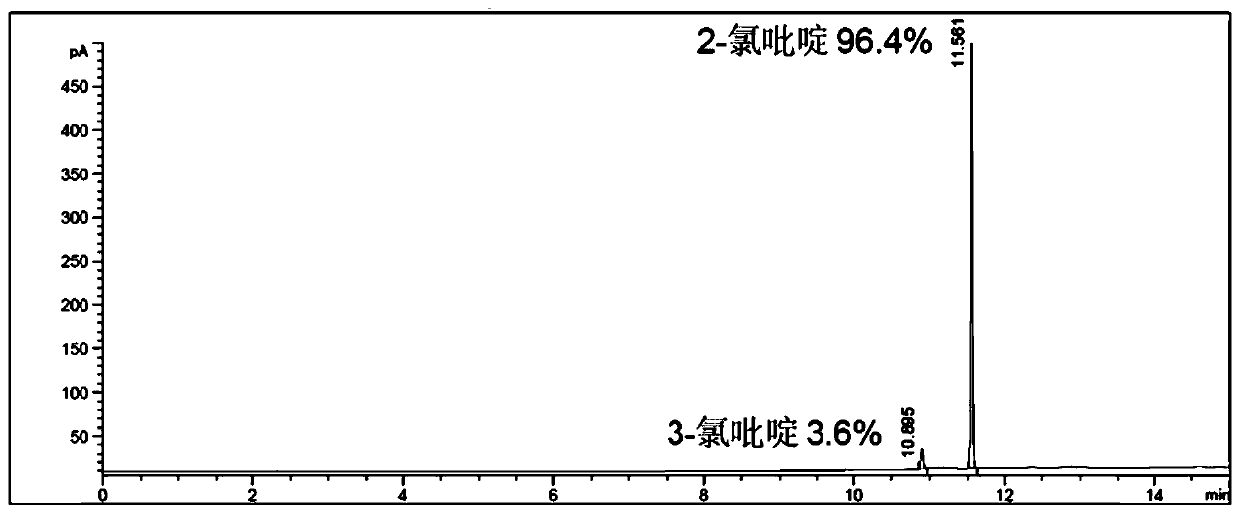
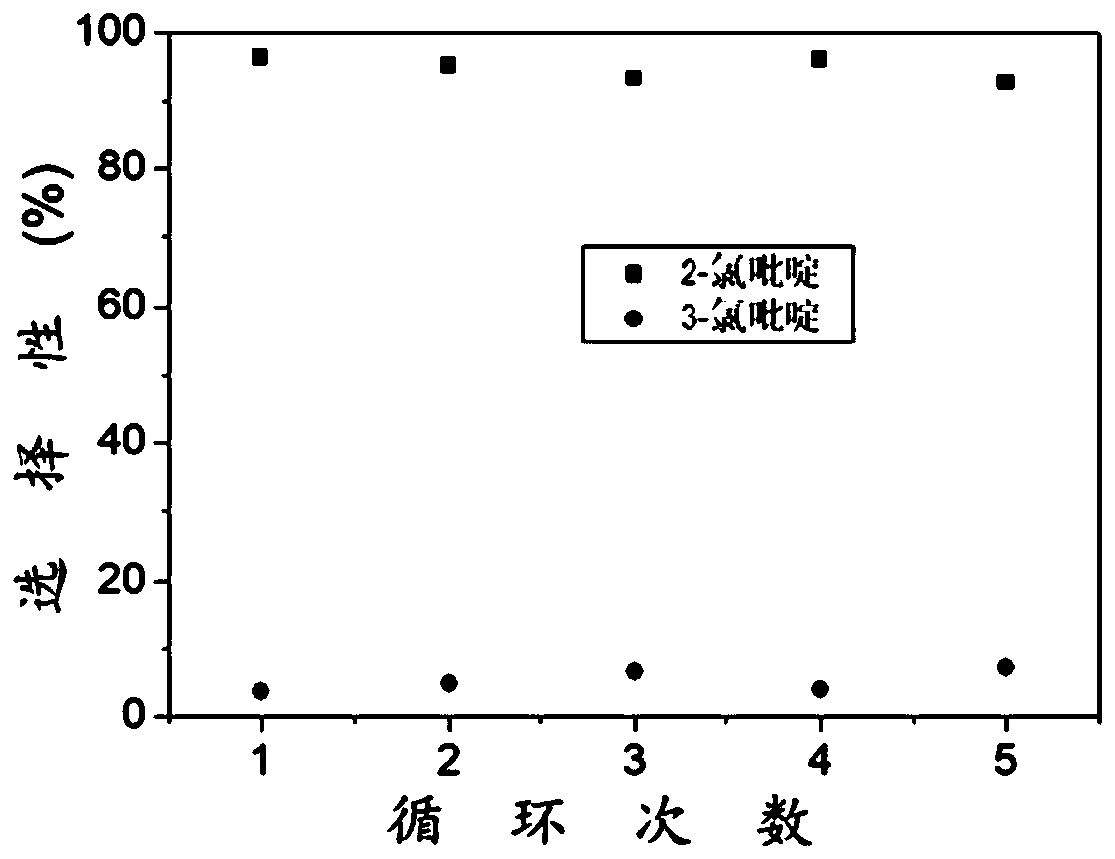
Separating method of 2-chloropyridine and 3-chloropyridine
ActiveCN110372580AImprove stabilityEasy to operateOther chemical processesOrganic compound preparationBromineEthyl Chloride
The invention discloses a separating method of 2-chloropyridine and 3-chloropyridine. A bis(dibromoethyoxypillar[6]arene) crystal material is used for adsorbing and separating a mixture of the 2-chloropyridine and the 3-chloropyridine, wherein a structural formula of the bis(dibromoethyoxypillar[6]arene) crystal material is as shown in the description. According to the separating method of the 2-chloropyridine and the 3-chloropyridine, the separating process is simple to operate, no complex equipment is needed, and the operation safety is good; no rectification operation is needed during separation, so that energy is saved, and the production cost is reduced; and the used crystal material is high in stability, and can be recycled, and a separating effect is not reduced.
Owner:ZHEJIANG UNIV
Synthesis method of copper pyrithione
ActiveCN102702094AReduce consumptionEasy to operateOrganic chemistryMolecular sieve catalystsChemical oxygen demandSynthesis methods
The invention relates to a synthesis method of copper pyrithione, which is characterized by comprising the following steps: (1) 2-chloropyridine oxidization: adding 2-chloropyridine and a catalyst into a container, and dropwisely adding hydrogen peroxide; (2) filtering the solution obtained in the step (1), carrying out vacuum drying on the filter cake, washing the filter cake, and carrying out vacuum drying on the filter cake again, wherein the obtained filter cake is the catalyst used in the step (1), and the filtrate is a 2-chloropyridine oxynitride solution; and (3) preparation of copper pyrithione. By using the synthesis method provided by the invention, the oxidization yield of 2-chloropyridine is enhanced from 80% to 98%, the total yield is enhanced from 75% to 93%, the product quality is enhanced from 95-96% to more than 98%, and the recycled catalyst can be used repeatedly; and thus, the raw material consumption is reduced, the production cost is lowered, the technological operation is simple, and the wastewater amount and COD (chemical oxygen demand) are obviously reduced.
Owner:NANTONG ACETIC ACID CHEM
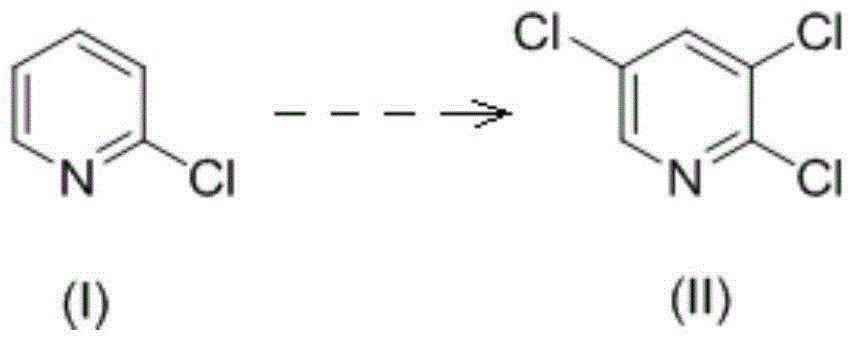
Method for preparing 2,3,5-trichloropyridine
InactiveCN108341767ARaw materials are easy to obtainMild operating conditionsOrganic chemistryCompound aHydrogen
The invention relates to the field of compound preparation, and discloses a method for preparing 2,3,5-trichloropyridine, wherein the method comprises the following steps: a. in the presence of a chlorination catalyst, a compound A is contacted with chlorine for chlorination reaction to obtain a mixture containing the 2,3,5-trichloropyridine and 2,3,4,5-tetrachloropyridine; b. the mixture obtainedin the step a is contacted with hydrogen in the presence of a hydrogenation catalyst and a solvent to carry out catalytic hydrogenation reaction; or, the 2,3,5-trichloropyridine and the 2,3,4,5-tetrachloropyridine in the mixture obtained in the step a are separated, the separated 2,3,4,5-tetrachloropyridine is contacted with the hydrogen for catalytic hydrogenation reaction; wherein the compoundA is 2-chloropyridine and / or 2-chloropyridine hydrochloride. The raw materials in the method are simple and easy to obtain, the operating conditions are mild, the product yield is high, the cost islow, the pollution is small, and the method is suitable for large-scale application.
Owner:盐城恒盛化工有限公司
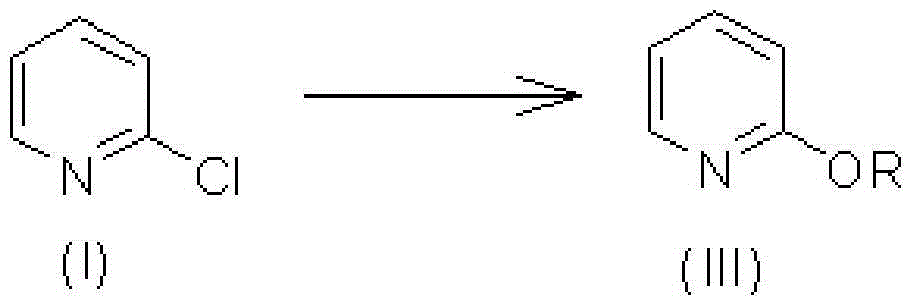
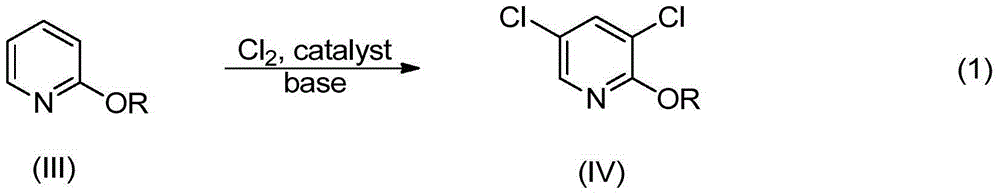
Synthetic method of 2, 3, 5-trichloropyridine
InactiveCN104478793AHigh yieldEasy to separate and purifyOrganic chemistry2-ChloropyridineRaw material
The invention discloses a synthetic method of highly selectively synthesizing 2, 3, 5-trichloropyridine with 2-chloropyridine as a raw material. The synthetic method comprises the following steps: carrying out reaction on the 2-chloropyridine and water or alcohols under base catalysis to produce 2-alkyloxypyridine, carrying out reaction on the 2-alkyloxypyridine and a chlorinating agent under the condition of base to produce 3, 5-dichloro-2-alkyloxypyridine, and finally carrying out chlorination to prepare the 2, 3, 5-trichloropyridine.
Owner:盐城恒盛化工有限公司
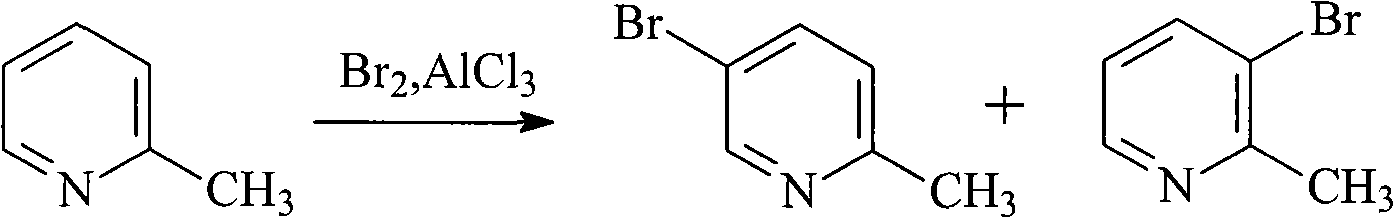
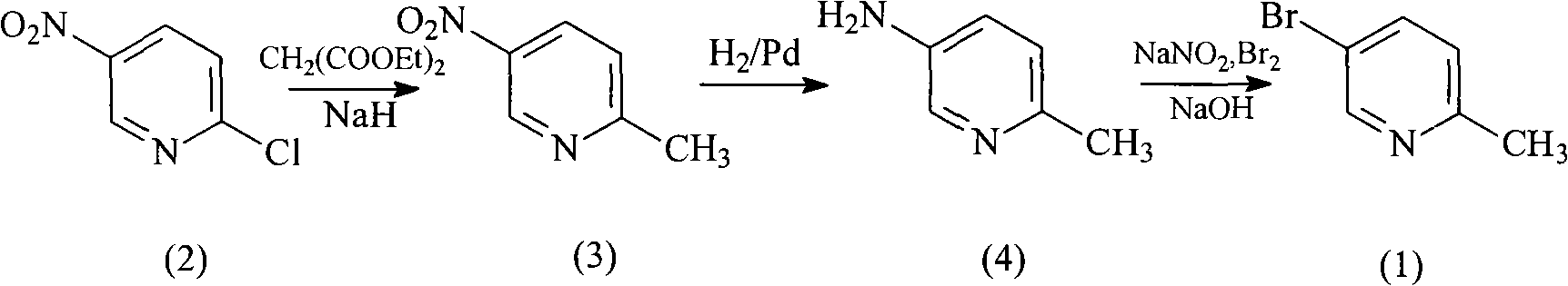
Method for preparing 5-bromo-2-methylpyridine
InactiveCN101560183AHigh yieldLow priceOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsCatalytic effectSodium nitrite
The invention discloses a method for preparing intermediate 5-bromo-2-methylpyridine. In the prior art, the dosage of aluminium trichloride is large; the catalytic effect is poor; the by-products are more; the yield of products is low; and the obtained products are difficult to separate. The method comprises the following steps: reacting diethyl malonate with alkali metal to generate salts, dripping 5-nitryl-2-chloropyridine into the salts for condensation reaction, and subsequently performing decarboxylation on the obtained product under acidic condition to obtain 5-nitryl-2-methylpyridine; performing hydrogenation reduction on the 5-nitryl-2-methylpyridine under the catalysis of Pd / C catalyst to obtain 5-amido-2-methylpyridine; and reacting the 5-amido-2-methylpyridine with acid to generate salts, dripping bromine, dripping a sodium nitrite water solution, and obtaining the 5-bromo-2-methylpyridine. The method has mild reaction conditions, easy operation, simple post-treatment, good catalytic effect, high yield of each step and high yield of final products, and is particularly suitable for industrialized production.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Method for producing 2-chloropyridine and 2,6-chloropyridine through organic solvent method
The invention discloses a method for producing 2-chloropyridine and 2,6-chloropyridine through an organic solvent method. The method comprises the following steps: mixing pyridine and carbon tetrachloride to form a pyridine solution according to a mass ratio of (0.2-2): 1, and vaporizing the pyridine solution to obtain a vaporized product; mixing the vaporized product and chlorine according to a mass ratio of (1-3): 1 and introducing the mixture into a reactor, so that the mixture reacts under the condition of 150-250 DEG C, and cooling to prepare a reaction solution after the reaction is ended; distilling the reaction solution to remove the carbon tetrachloride, performing rectification under reduced pressure under the condition of -0.08 to -0.085MPa, collecting fractions of which the temperature ranges from 110 DEG C to 113 DEG C to obtain the 2-chloropyridine, and collecting fractions of which the temperature ranges from 142 DEG C to 146 DEG C, thus obtaining the 2,6-chloropyridine. According to the method, protective gases such as nitrogen and carbon dioxide are not needed, a precious metal catalyst is not added, the pyridine is not needed to be directly chlorinated under high-pressure conditions, the yield is 90-98 percent, and the main byproduct 2,6-chloropyridine is an important chemical material, is easy to separate and has a relatively high economic value.
Owner:山东谦诚工贸科技有限公司
Method for producing uniform lamella zinc pyrithione
ActiveCN105906560AHigh specific surface areaImprove the bactericidal effectOrganic chemistry methodsSodium PyrithioneSodium hydrosulfide
The invention provides a method for producing uniform lamella zinc pyrithione. The method comprises the following steps: first step: adding 2-chloropyridine, a catalyst and deionized water in a reaction kettle, slowly dropwise adding hydrogen peroxide, and generating a 2-chloro-N-pyridine oxide solution; second step: dropwise adding a sodium hydrosulfide solution and a sodium hydroxide solution in the reaction kettle with the 2-chloro-N-pyridine oxide solution obtained from the first step to generate a 2-sulfydryl-N-pyridine oxide solution; third step: adding hydrochloric acid in the reaction kettle with the 2-sulfydryl-N-pyridine oxide solution obtained from the second step; fourth step: adding a sodium hydroxide solution in the solution obtained from the third step and adjusting pH value to be 8-10; and fifth step: adding a ZnSO4 solution in the reaction kettle with 2-sulfydryl-N-pyridine oxide sodium salt obtained from the fourth step to obtain the lamella zinc pyrithione. The small-grain-size lamella zinc pyrithione with uniform particles plays a quite important role in application in the aspects of civil coating, biocide mildewcide and the like.
Owner:江苏燎原环保科技股份有限公司
Process for the preparation of pyridine derivative
The invention discloses a process for the preparation of a pyridine derivative namely 5-{4-2(N-methyl-N(2-pyridyl) amino ethoxy]benzyl]thiazolidine-2,4-dione maleate comprising the steps of: (a) reacting 2-chloropyridine with 2-(N-methyl amino) ethanol; (b) coupling 2-N-methyl-N-(2-pyridyl)amino)ethanol) obtained in step (a) and 4-fluorobenzaldehyde in an aprotic polar solvent with an alkali metal hydroxide or an alkali metal alkoxide as base. (c) isolating the product of the coupling reaction viz 4-[2-(N-methyl-N-(2-pyridyl)amino)ethoxy]benzaldehyde; (d) converting said isolated benzaldehyde compound of step (c) into 5-[4-[2-N-methyl-N-(2-pyridyl) amino)ethoxy]benzyl]thiazolidine-2,4-dione in a known manner and (e) converting said thiazolidine-2,4-dione compound obtained in step (d) into a pharmaceutically acceptable maleate salt.
Owner:TORRENT PHARMA LTD
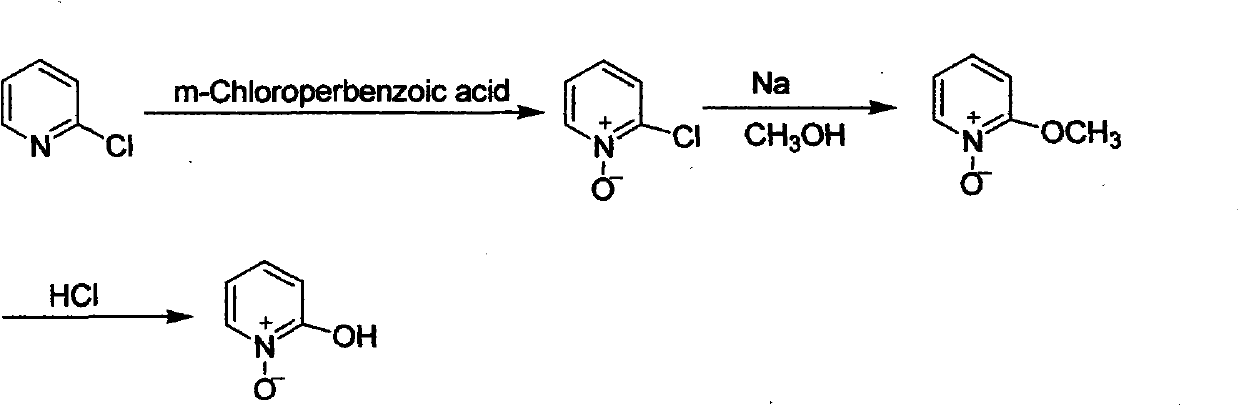
Preparation method of 2-hydroxypyridine-N-oxide
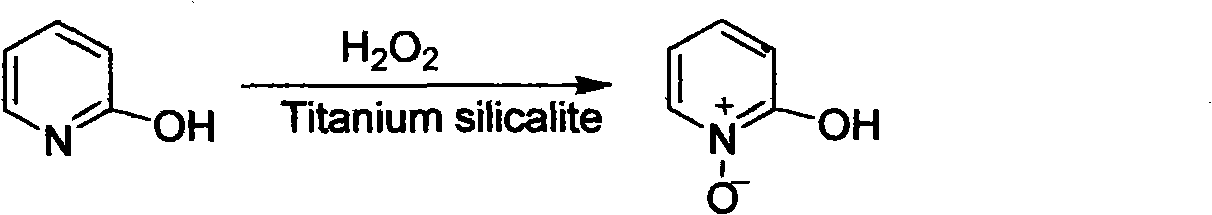
The invention discloses a preparation method of an important intermediate: 2-hydroxypyridine-N-oxide with the CAS Number of 13161-30-3. The invention reports a new synthetic route applicable to industrial production, which comprises the following steps: under catalysis of a catalyst generated in-situ, oxidizing 2-chloropyridine with hydrogen peroxide (30%) to generate an N-oxide, and then hydrolyzing under an alkaline condition to directly generate the target product. The preparation method greatly improves the yield, realizes one-pot reaction, greatly reduces generation of organic waste liquor and realizes environmental protection.
Owner:湖南欧亚药业有限公司
Method for producing 2-chloropyridine and 2,6-dichloropyridine with inorganic solvent process
The invention discloses a method for producing 2-chloropyridine and 2,6-dichloropyridine with an inorganic solvent process. The method comprises the following steps: mixing pyridine with water to form a pyridine water solution, and vaporizing the pyridine water solution to prepare a vaporized substance; mixing the vaporized substance with chlorine and introducing into a reactor to react, and cooling to prepare a reaction solution after the reaction is finished; adjusting the pH (potential of Hydrogen) value of the reaction solution to 9-11, and steaming the organic substance out to prepare the mixed solution of the organic substance and the water; (4) standing the mixed solution to layer, taking an organic phase out, rectifying with the organic phase, collecting a fraction within the temperature range of 110-113 DEG C to obtain the 2-chloropyridine, and collecting the fraction within the temperature range of 142-146 DEG C to obtain the 2,6-dichloropyridine. According to the method, direct chlorination of the pyridine can be performed under the conditions of no protective gas, no added noble metal catalyst and no high pressure, the yield is high, and the main byproduct, namely the 2,6-dichloropyridine, of the process is also an important chemical raw material, is easy to separate and has higher economic value.
Owner:山东谦诚工贸科技有限公司
Nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and preparation method and use thereof
InactiveCN103601732AGood antitumor activityImprove anti-tumor activityOrganic active ingredientsOrganic chemistryTumor cells2-Methylpyridine
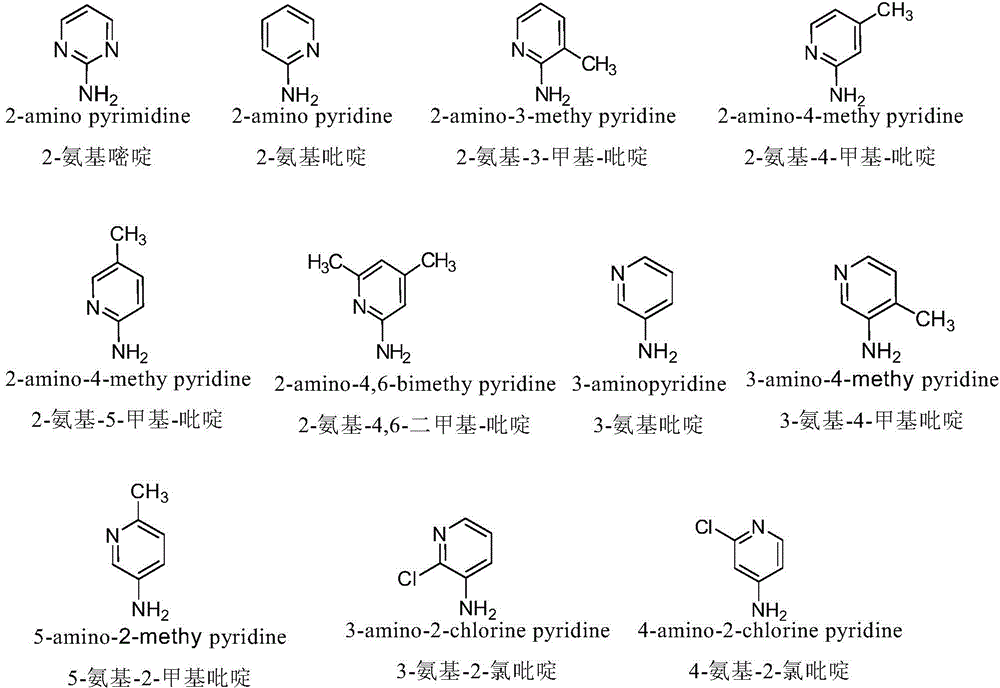
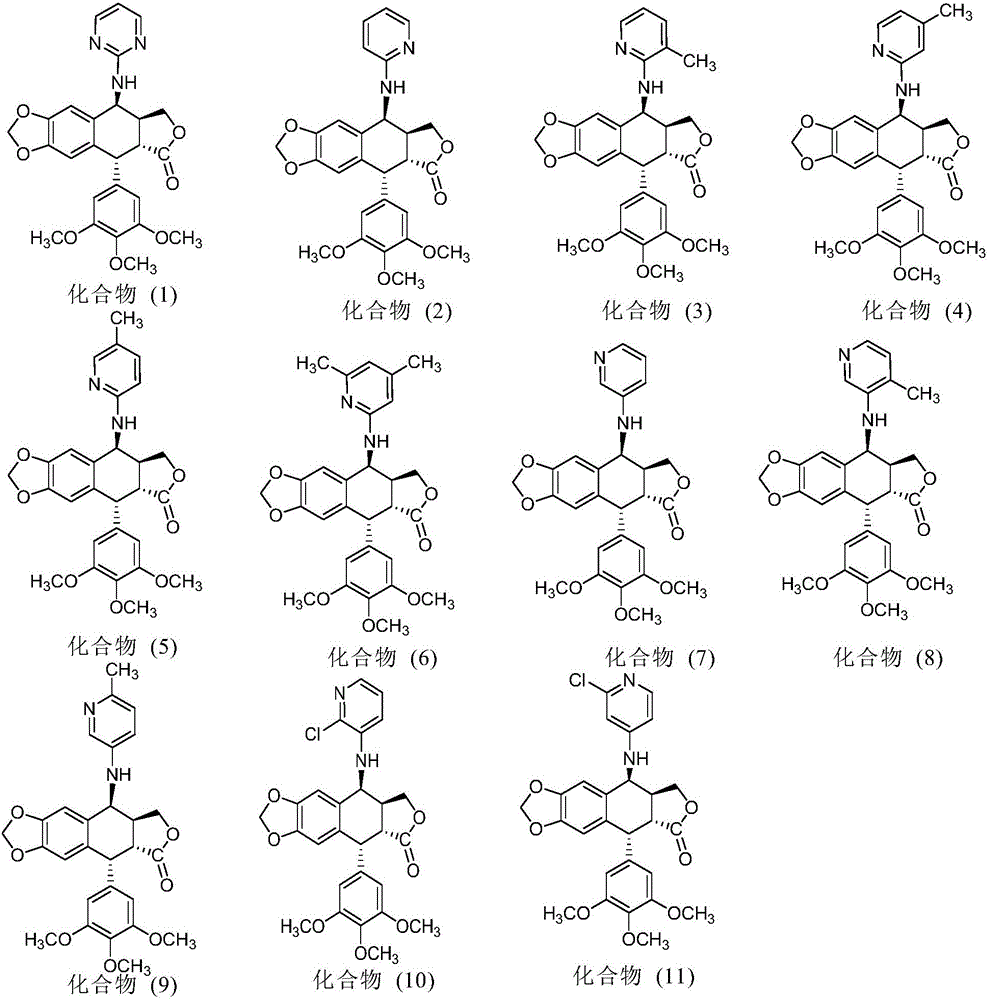
The invention discloses a nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and a preparation method and use thereof. According to the method, 2-aminopyrimidine, 2-aminopyridine, 2-amino-3-methylpyridine, 2-amino-4-methylpyridine, 2-amino-5-methylpyridine, 2-amino-4,6-dimethyl pyridine, 3-aminopyridine, 3-amino-4-methylpyridine, 5-amino-2-methylpyridine, 3-amino-2-chloropyridine or 4-amino-2-chloropyridine is respectively introduced to an activated C-ring fourth position of a podophyllotoxin compound through nitrogen substitution reaction, so as to obtain the nitrogen-substituted podophyllotoxin derivative, represented by a formula (V) shown in the specification, with excellent anti-tumor activity. The nitrogen-substituted podophyllotoxin derivative disclosed by the invention acts on tumor cells through multiple ways and multiple target points, and the anti-tumor activity of the nitrogen-substituted podophyllotoxin derivative is remarkably improved compared with that of the podophyllotoxin compound. The compound disclosed by the invention can be used for preparing anti-tumor drugs and is clinically applied to anti-tumor treatment.
Owner:HUBEI UNIV OF TECH
Insect ether and producing method thereof
InactiveCN1439262AProtection securityProtect healthBiocideAnimal repellantsEpoxyReaction temperature
A pyriproxyfen as insecticide is prepared from 4-hydroxydiphenyl ether, epoxy propane and 2-chloropyridine through dissolving 4-hydroxydiphenyl ether in alcohol, reacting on expoxy propane at 0-90 deg.C for 2-24 hr under existance of sodium hydroxide to obtain the intermediate, dissolving it in toluene, heating to 30-80 deg.C, slowly dropping 2-chloropyridine in it while reacting at 10-110 deg.C for 2-24 hr under existance of botassium hydroxide, distilling to remove toluene and refining.
Owner:沈志荣 +1
Preparation and application of pyrazole oxime ester compound containing 2-chloropyridine structure
The invention relates to a pyrazole oxime ester compound (I) containing a 2-chloropyridine structure and a preparation method and application of the pyrazole oxime ester compound (I). The pyrazole oxime ester compound (I) containing the 2-chloropyridine structure is obtained through condensation of oxime and 2-chloronicotinyl chloride, wherein oxime contains pyrazole. The pyrazole oxime ester compound containing the 2-chloropyridine structure has effective prevention and treatment effects on harmful insects and can be used for preparing pesticides in the fields such as agriculture and gardening.
Owner:SHANGHAI RUN BIOTECH CO LTD
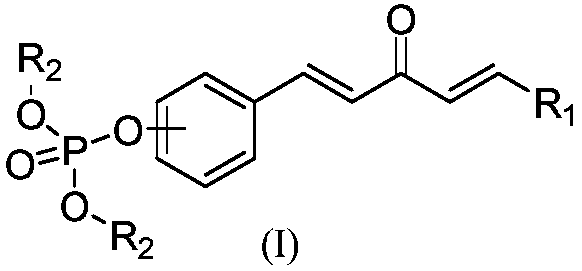
1,4-pentadiene-3-one derivative containing phosphite ester as well as preparation method and application thereof
ActiveCN108864188AImprove biological activityRaw materials are easy to getBiocideGroup 5/15 element organic compoundsBenzenePhosphite ester
The invention discloses a 1,4-pentadiene-3-one derivative containing phosphite ester as well as a preparation method and application thereof. According to the 1,4-pentadiene-3-one derivative containing phosphite ester, the general formula (I) of the 1,4-pentadiene-3-one derivative containing phosphite ester is as shown in the formula (I) in the specification, wherein R1 is a phenyl group, a substituted phenyl group or a substituted aromatic heterocyclic group, R2 is an alkyl group of C1-C6, the substituted phenyl group of the R1 is one or more methoxy group, nitro group, methyl group, trifluoromethyl group or halogen atom contained in ortho-, meta- and para- on a benzene ring or two positions of the ortho-, meta- and para- on the benzene ring, and the substituted aromatic heterocyclic group of R1 is a furan group, a pyridyl group, a thienyl group, a pyrrolyl group, a thiazolyl group, a 2-chloropyridine group or a 2-chlorothiazolyl group. The 1,4-pentadiene-3-one derivative as well as the preparation method and application thereof have the advantages that the activity of plant bacteria can be inhibited, the raw materials are easily available, the reaction conditions are mild, the post-treatment is simple, and the yield is high. The formula (I) is shown in the description.
Owner:GUIZHOU UNIV
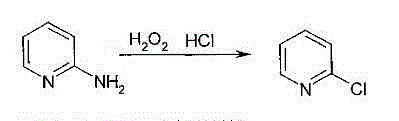
2-chloropyridine synthetic method
The invention discloses a 2-chloropyridine synthetic method, which belongs to the technical field of fine chemical engineering. The method comprises the following steps: adding pyridine into a hypochloric acid salt solution, uniformly mixing the solution, slowly adding hydrochloric acid drop by drop at room temperature, stirring a mixture and reacting the mixture for 1-2 hours; then heating the material to the temperature of 60-80 DEG C, continuously reacting the material for 1-2 hours; and finally, adding a certain amount of a NaOH solution and neutralizing the solution to a pH value of 9-11, extracting a reactant by using trichloromethane and separating the reactant; distilling an extract phase and removing a trichloromethane solvent in order, recovering the unreacted pyridine to obtain the chloropyridine product. According to the invention, a GC / MS analysis on the chloropyridine product is carried out, selectivity of 2-chloropyridine can reach 83%. The hydrochloric acid and hypochlorite by-product with low cost in industrial production can substitute a chlorinating agent such as Cl2, reaction condition is mild, selectivity is good, process is simple, cost is low, and environment and safety problems during the production process of 2-chloropyridine can be solved.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
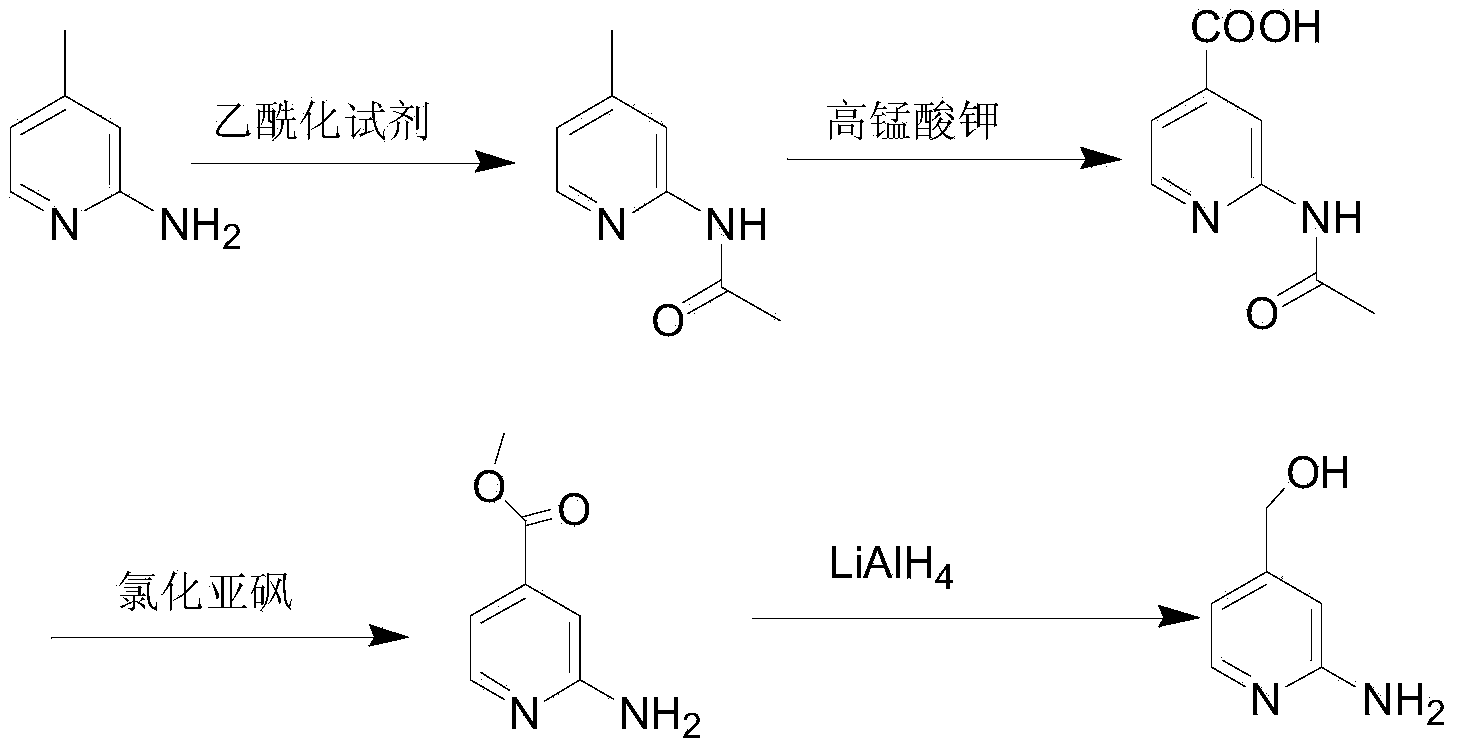
Preparation method of 2-aminopyridine-4-methyl alcohol
The invention discloses a preparation method of 2-aminopyridine-4-methyl alcohol, which comprises the following steps: 2-chloropyridine-4-carboxylic acid is used as a raw material to be in esterification reaction with micromolecule alcohol so as to obtain 2-chloropyridine-4-carboxylic acid ester under the function of sulfoxide chloride; 2-chloropyridine-4-carboxylic acid ester is reduced to 2-chloropyridine-4-methyl alcohol under the function of a reducing agent; finally, 2-chloropyridine-4-methyl alcohol and ammonia water are in ammonolysis reaction so as to obtain 2-aminopyridine-4-methyl alcohol under the catalysis of a copper catalyst. With adoption of the preparation method, the process route is short, generation of large amount of waste liquid and waste residue is avoided, and pollution to the environment is reduced; in addition, the product yield is high, the copper-class catalyst in the ammonolysis reaction can be recycled, the production cost is reduced, and the preparation method is suitable for industrial production.
Owner:ZHEJIANG UNIV +2
Industrialized method for preparing 2-mercaptopyridine
InactiveCN101941942ASolve the problems of industrial productionSimple production processOrganic chemistryRefluxSodium bisulfide
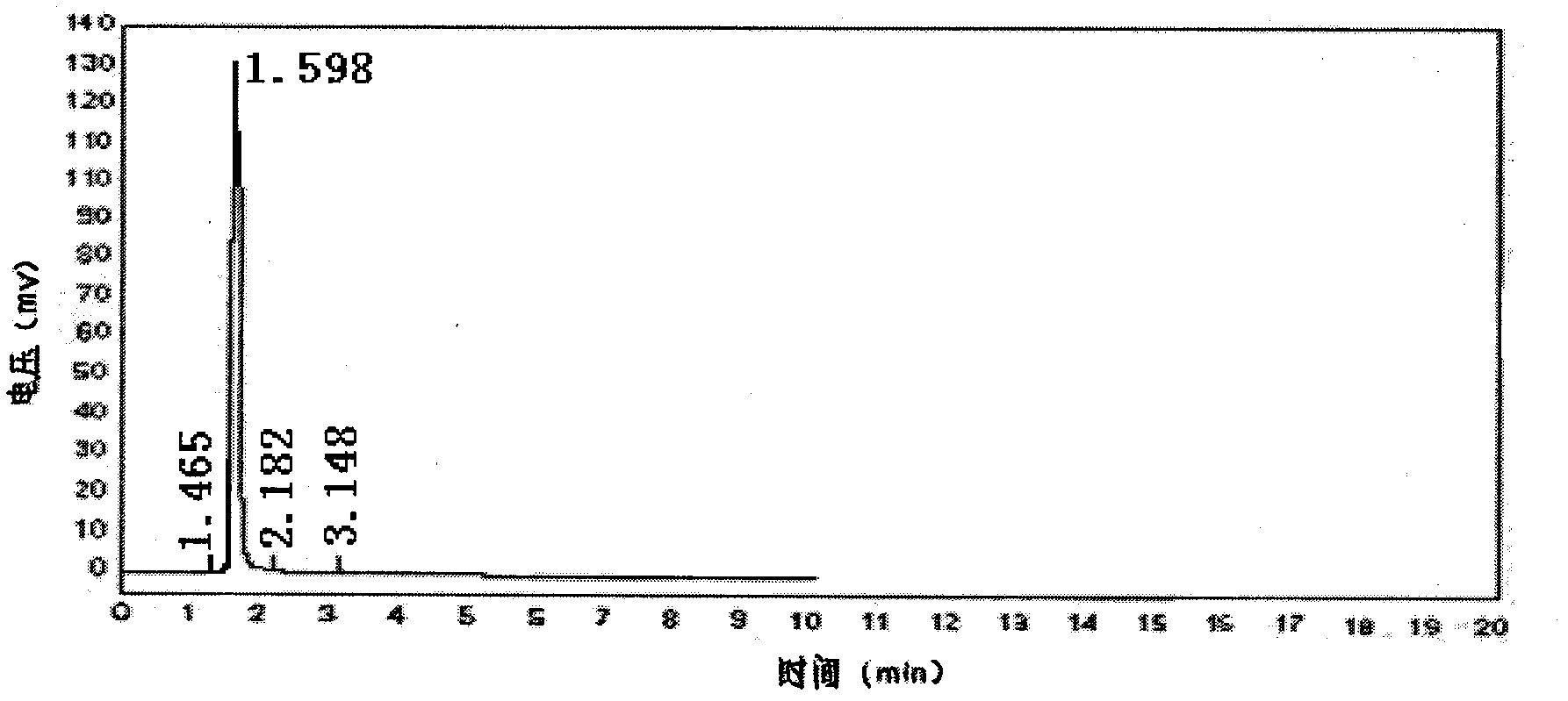
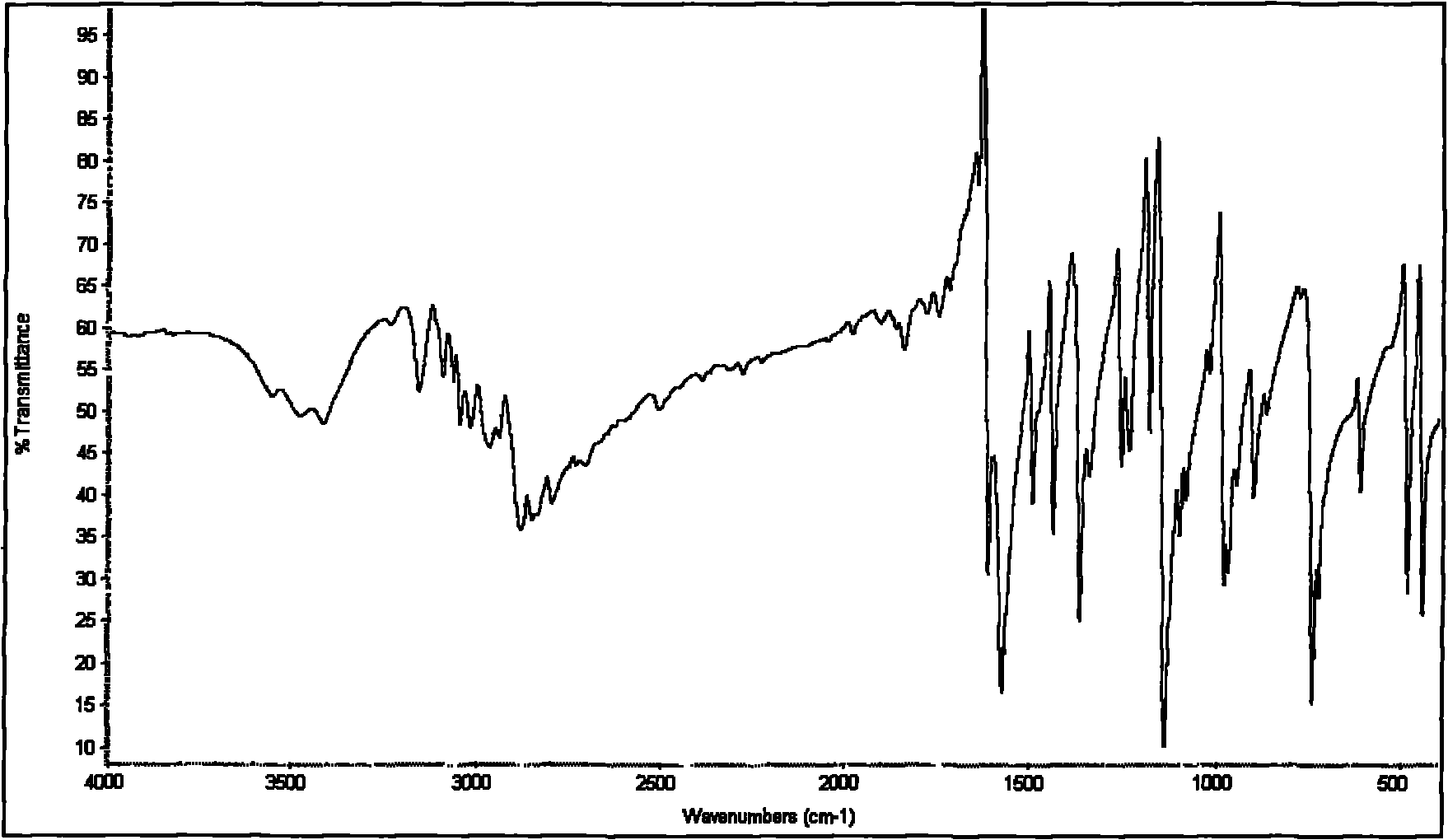
The invention discloses an industrialized method for preparing 2-mercaptopyridine, which comprises the following production processes of: adding anhydrous sodium bisulfide and an organic solvent into a conical reactor with a stirrer, a thermometer, a reflux device, a distilling device, a heating device and a charging device, heating to a certain temperature, adding 2-chloropyridine in batches from a charging opening, performing reaction for several hours until the reaction is completed, removing a solvent by distilling, adding an appropriate amount of distilled water, regulating the pH value by using acid, extracting by using the organic solvent, and removing an extracting agent by distilling to obtain faint yellow 2-mercaptopyridine crystals. The preparation method of the invention has the advantages of simple and convenient production process, mild reaction conditions, and high yield and purity of products; and the industrialized method for synthesizing the 2-mercaptopyridine in one step is provided and the problem of industrialized production of the 2-mercaptopyridine is solved.
Owner:TIANJIN NORMAL UNIVERSITY
2,3,5,6-tetrachloropyridine synthesis and separation method
InactiveCN110229095AIncrease profitReduce pollutionElectrolysis componentsOrganic chemistryElectrolysisDistillation
The invention discloses a 2,3,5,6-tetrachloropyridine synthesis and separation method, which comprises the following operation steps: (1) introducing gasified pyridine and chlorine into a primary chlorination reactor to prepare 2-chloropyridine and 2,6-dichloropyridine; (2) gasifying 2-chloropyridine and 2,6-dichloropyridine by heating to prepare 2,3,6-trichloropyridine and 2,3,5-trichloropyridine; (3) gasifying the liquid-phase 2,3,6-trichloropyridine and 2,3,5-trichloropyridine, introducing products into a high-grade chlorination reactor, introducing excessive chlorine, continuously distilling to obtain tetrachloropyridine in a crude product tower and pentachloropyridine at the tower bottom; (4) adding pentachloropyridine into a high pressure reactor, adding zinc dust and 31% industrialhydrochloric acid to obtain 2,3,5,6-tetrachloropyridine; and (5) letting the tetrachloropyridine obtained after distillation in the crude product tower undergo electrolytic reaction to prepare the 2,3,5,6-tetrachloropyridine. The 2,3,5,6-tetrachloropyridine synthesis and separation method provided by the invention has the following advantages: utilization rate of the raw materials is high, productpurity is high, the process is simple, and the environmental pollution is low.
Owner:潍坊新绿化工有限公司
Preparation method of 2-chloropyridine
InactiveCN101830844AReduced activityLow reaction temperatureOrganic chemistryGas phaseUltraviolet lights
The invention provides a preparation method of 2-chloropyridine. The method comprises the following steps of: mixing pyridine and water by a molar ratio of 1:(1.5-3.6) and adding an activating agent; vaporizing, then leading the pyridine and the water into a glass reactor by a molar ratio of 1:(0.70-0.80) to carry out a chlorination reaction for 3-5 seconds at 150-170 DEG C in the presence of an ultraviolet light source and carrying out cooling and gas-liquid separation on an obtained reaction mixture; absorbing the separated gas phase to form hydrochloric acid by using water; and neutralizing the separated liquid phase and then distilling to obtain pyridine, 2-chloropyridine and 2,6-dichloropyridine, wherein distilled water is applied to the production of a next batch. Because the activating agent is added in the process of the chlorination reaction, activation energy and reaction temperature which are required by the chlorination reaction are reduced, and the process period is shortened. By adopting the glass reactor with a built-in helical filler and the ultraviolet light source the wavelength of which is matched with the glass reactor, the probability of secondary chlorination of the 2-chloropyridine is reduced, and the conversation rate and the selectivity of raw materials are improved.
Owner:YANCHENG HUAOU IND
Myricetin derivative containing thiadiazole thioether structure and preparation method thereof
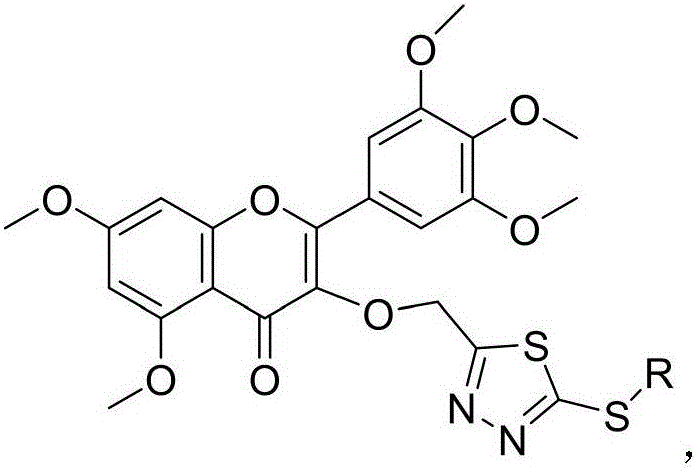
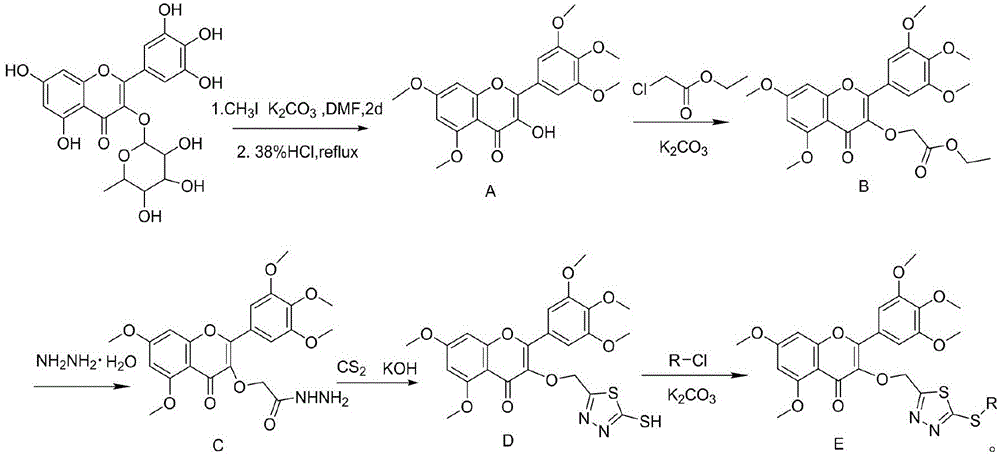
ActiveCN106674216AStrong antiviral activityInhibitory activity overOrganic chemistryDisinfectantsBenzyl group2-Chloropyridine
The invention discloses a myricetin derivative containing a thiadiazole thioether structure. The myricetin derivative is shown as a general formula in the specification, wherein R is H, alkyl, benzyl, 4-(pyridyl)methylene, 3-(pyridyl)methylene, 2-(pyridyl) methylene, 5-(2-chloropyridine)methylene, 5-(2-chlorothiazole)methylene or ortho-, meta- and para- mono-substituted or poly-substituted methoxyacetic benzyl, nitrobenzyl, methylbenzyl and halogen atom benzyl. The myricetin derivative containing the thiadiazole thioether structure has high virus resistance, and can be used as a medicament for restraining plant viruses.
Owner:GUIZHOU UNIV
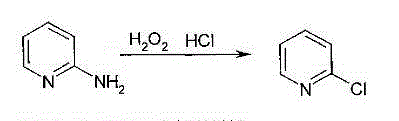
Process for synthesizing 2-chloropyridine from chlorine and pyridine
A process for synthesizing 2-chloropyridine from pyridine and chlorine gas includes such technological steps as reaction while rectification, neutralization, and refining by rectification. It features that the liquid-phase mixture of pyridine and water in mol ratio of 1 : 3 is fed into coupled rectifying tower-reactor, the mixture containing pyridine output from tower top is partially returned back into tower, residual is fed to top of reactor while the mol raatio of chlorine gas to pyridine is ensured to be 1 : 2.5-4.0, the residual and chlorine gas take part in parallel-current reation in reactor to generate 2-chloropyridine, and after neutralized it comes to central position of coupled rectifying tower with the temp at top of 94-96 deg.C and reactor temp of 140-190 deg.C. Its advantages include short technological route, high output rate and less waste liquid drainage.
Owner:TIANJIN UNIV
Synthesis method of 2,2'-dipyridyl
The invention discloses a synthesis method of 2,2'-dipyridyl, mainly comprising the following steps of: coupling 2-chloropyridine as a raw material under the catalysis effect of a catalyst to obtain 2,2'-dipyridyl; screening an alkaline hydrolysis reagent and an auxiliary agent, which have the better advantage, by selecting the alkaline hydrolysis reagent and the auxiliary agent, to carry out alkaline hydrolysis; and finally, carrying out steps of extraction, acid washing, second time of alkaline hydrolysis, second time of acid washing, alkaline hydrolysis and the like to obtain refined 2,2'-dipyridyl. The method disclosed by the invention is simple; the production cost is greatly reduced and the reaction yield is improved; and meanwhile, a process flow is further optimized.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Synthesis method of zinc pyrithione
InactiveCN107628995AReduce Sulfate LevelsReduce salt contentOrganic chemistrySodium PyrithioneThioketone
The invention discloses a synthesis method of zinc pyrithione, and belongs to the field of organic synthesis. According to the synthesis method, 2-chloropyridine is used as a raw material; a final product is obtained through three steps of reactions of nitrogen oxidation, sulfhydrylation and salt formation. The synthesis method is simple in step and convenient to operate and further, the content of an obtained product is high.
Owner:JIANGSU ZHONGBANG PHARMA
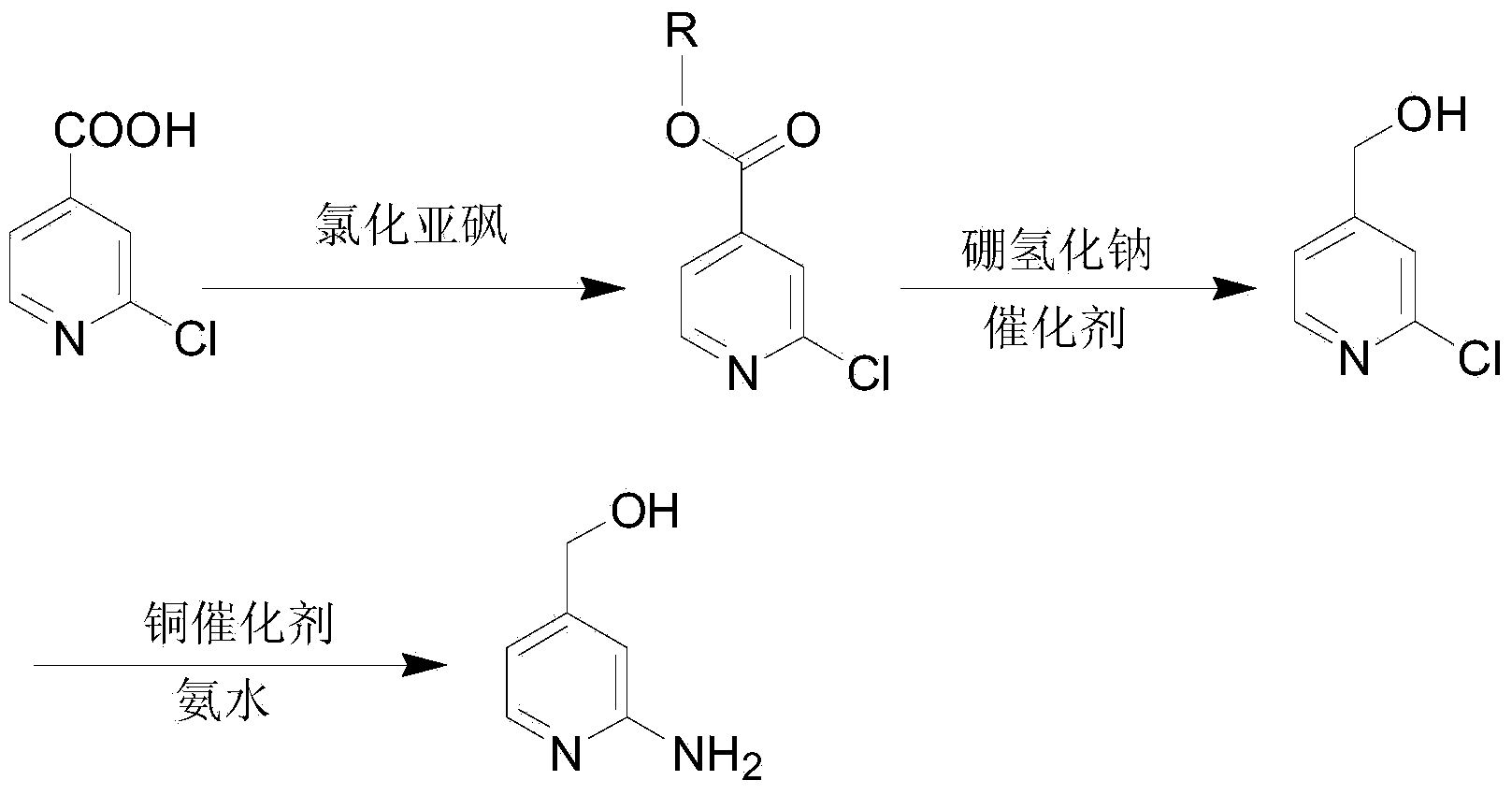
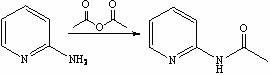
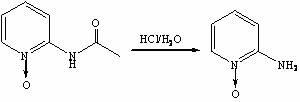
Method for synthesis preparation of 2-chloro-4-aminopyridine
InactiveCN102101841AHigh yieldMild reaction conditionsOrganic chemistryNitrogen oxides2-Chloropyridine
The invention discloses a method for preparing 2-chloro-4-aminopyridine, which comprises the following steps: 1, synthesizing 2-acetamidopyridine; 2, synthesizing a 2-acetamidopyridine nitrogen oxide; 3, synthesizing a 2-aminopyridine nitrogen oxide; 4, synthesizing a 2-aminopyridine nitrogen oxide; 5, synthesizing 2-cholorpyridine nitrogen oxide; 6, synthesizing 2-chloro-4-nitropyridine nitrogenoxide; and 6, synthesizing 2-chloro-4-pyridinamine. In the preparation method, the reaction conditions are mild, the operation is simple, the raw materials are readily available, and the total yield is high.
Owner:周玉莲
2-chloro pyridine preparation method
PendingCN105330594AReduced activityLow reaction temperatureOrganic chemistryGas phaseReaction temperature
The invention provides a 2-chloro pyridine preparation method. Pyridine and water are mixed according to the molar ratio of 1:1.5-3.6, an activator is added, pyridine and chlorine are fed into a glass reactor according to the molar ratio of 1:0.70-0.80 after vaporization, chlorination reaction is performed under the effect of an ultraviolet light source at the temperature of 150-170 DEG C, the reaction time is 3-5 seconds, and the reaction mixture undergoes cooling and gas-liquid separation. The separated gas phase is absorbed with water to produce hydrochloric acid, the separated liquid phase is neutralized and then is distilled to obtain pyridine, 2-chloro pyridine and 2,6-dichloro pyridine, and a water jacket is distilled out for the next batch of production. Due to the fact that the activator is added in the process of chlorination reaction, the activation energy and reaction temperature required by the chlorination reaction are reduced, and a process cycle is shortened. By the adoption of the glass reactor provided with a spiral packing in the inside and the ultraviolet light source matched with the glass reactor in wavelength, the probability that the 2-chloro pyridine is secondarily chlorinated is reduced, and the conversion rate and selectivity of the raw materials are improved.
Owner:山东谦诚工贸科技有限公司
Preparation method of pyriproxyfen
The invention relates to a preparation method of pyriproxyfen. The preparation method comprises the following steps of using POP (4-phenoxyphenol) as the raw material to synthesize an intermediate, namely 1-(4-phenoxyl phenoxyl)2-propanol; treating the intermediate by methylbenzene solvent without separating, and directly synthesizing with 2-chloropyridine, so as to obtain the pyriproxyfen. The preparation method has the advantages that the route and the method are simple, the conditions are moderate, the operation steps are simplified, the reaction yield rate is increased, the preparation method is more suitable for industrialized production, the total yield rate of synthesis reaches 85.5% on the basis of POP, and the content of crude drug is greater than or equal to 97%.
Owner:NANTONG PAISIDI PESTICIDE CHEM
Method for preparing 2,2'-dipyridine under palladium/carbon catalysis
ActiveCN105481761AAvoid pollutionRecyclingOrganic chemistryChemical recyclingOrganic synthesisDistillation
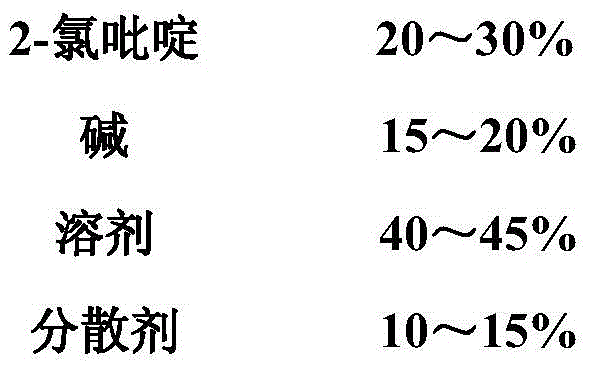
The invention discloses a method for preparing 2,2'-dipyridine under palladium / carbon catalysis, which belongs to the technical field of organic synthesis. A reaction system in the preparation method comprises the following components by mass: 20 to 30% of 2-chloropyridine, 15 to 20% of alkali, 40 to 45% of a solvent, 10 to 15% of a dispersing agent and 1.5 to 3% of a catalyst, wherein the catalyst is a palladium / carbon catalyst containing 5% by mass of palladium. According to the invention, through filtering, distillation, desalination and other treatment of reaction mother liquor, recovery and cyclic usage of mother liquor water are realized, so the water resource is saved and environmental pollution caused by sewage discharge is effectively avoided; application conditions of the palladium / carbon catalyst are mild, which is favorable for prolonging the service life of equipment; and the catalyst is convenient to regenerate, activate and recover, so production efficiency is improved and production cost is reduced.
Owner:安徽翰邦科技咨询有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com