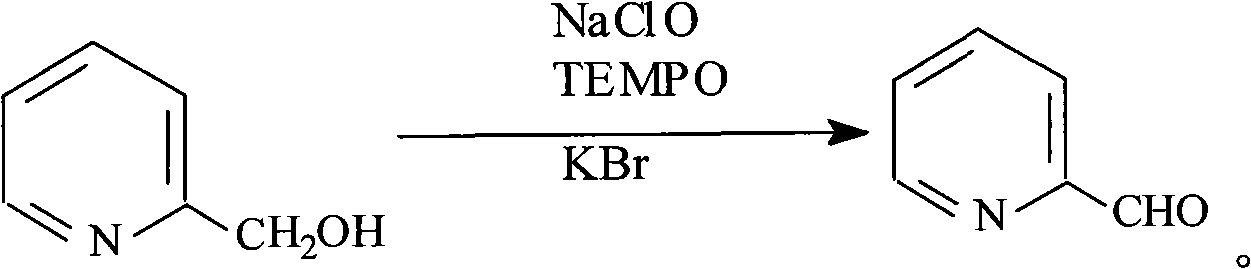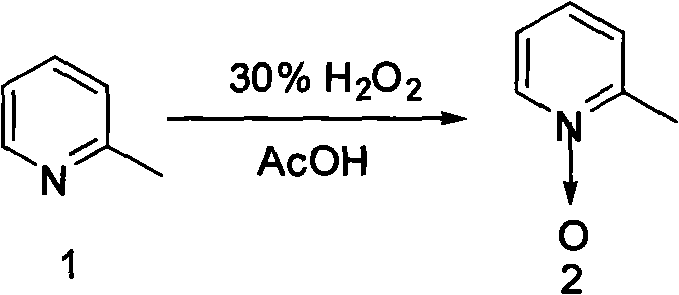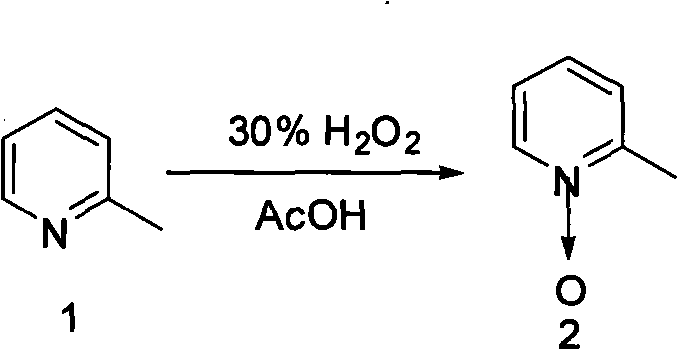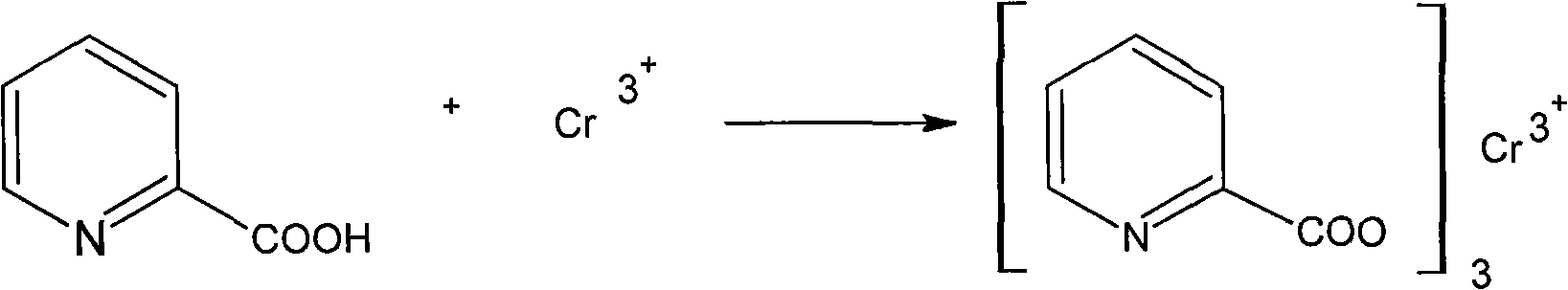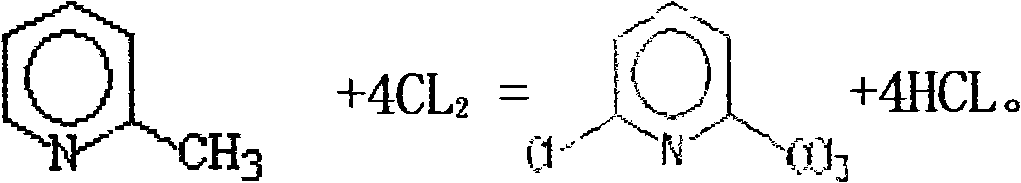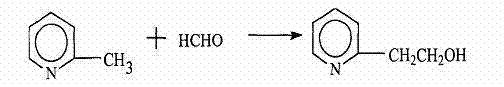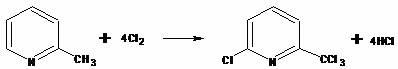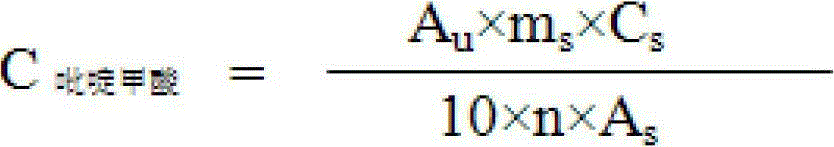Patents
Literature
142 results about "2-Methylpyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
2-Methylpyridine, or 2-picoline, is the compound described with formula C₆H₇N. 2-Picoline is a colorless liquid that has an unpleasant odor similar to pyridine. It is mainly used to make vinylpyridine and the agrichemical nitrapyrin.
Preparation method of 2-pyridine carboxaldehyde
InactiveCN101906068AMild oxidation conditionsThorough responseOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsNitric oxide2-Methylpyridine
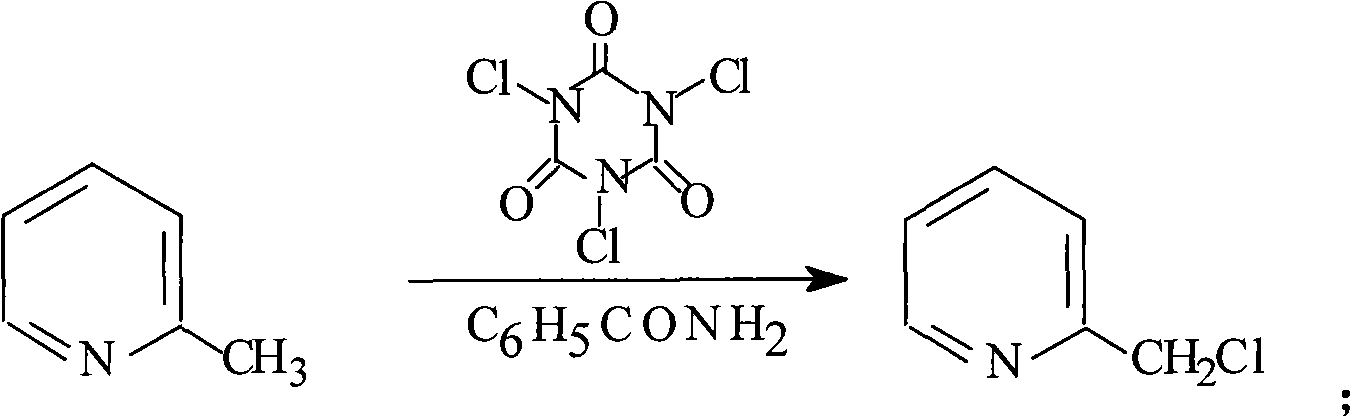
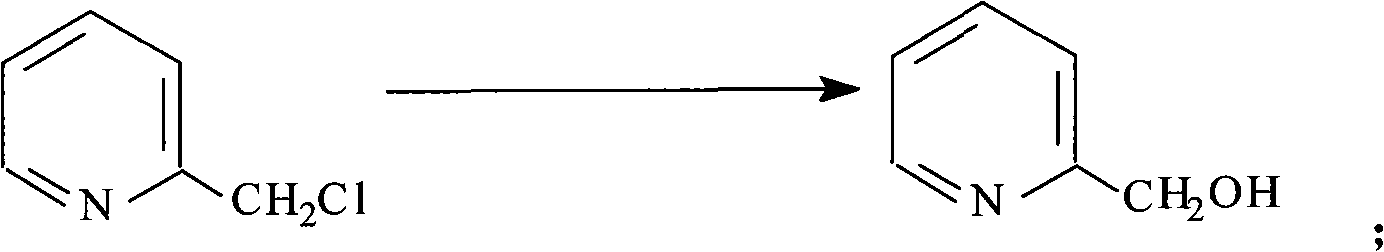
The invention provides a preparation method of 2-pyridine carboxaldehyde, which comprises the following steps of: (1) carrying out the temperature rise reflux reaction of 2-methylpyridine in the presence of halohydrocarbon used as a solvent, benzoyl amide used as a catalyst and trichloro isocyanate used as a chlorinating agent to obtain 2-chloromethyl pyridine; (2) carrying out hydrolysis on 2-chloromethyl pyridine under the alkalinity condition and rising the temperature to obtain 2-pyridinemethanol; (3) cooling 2-pyridinemethanol to -10-0 DEG C in presence of halohydrocarbon used as a solvent and 2,2,6,6-tetramethylpiperidine nitric oxide and potassium bromide used as catalysts, dipping 10 percent by weight of sodium hypochlorite solution used as a oxidizing agent, and keeping the temperature at 10-25 DEG C after the dipping to obtain 2-pyridine carboxaldehyde. The preparation method provided by the invention has the advantages of high yield, low cost, mild reaction condition and easy industrialization production.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
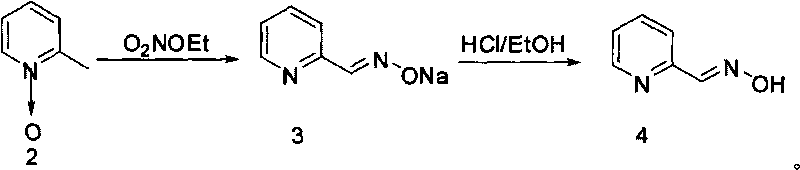
Synthesis method of 2-pyridine formaldoxime
InactiveCN101698659ASimple processShort production timeOrganic chemistrySynthesis methodsNitric oxide
The invention discloses a synthesis method of 2-pyridine formaldoxime, comprising the following steps: firstly mixing 2-methyl pyridine, glacial acetic acid and hydrogen peroxide for heating, reacting at the temperature of 60-70 DEG C for 3-5 hours; secondly, adding hydrogen peroxide, continuously reacting for 5-8 hours, and then separating and purifying to obtain the nitric oxide of 2-methyl pyridine; thirdly, adding alcohol, concentrated sulfuric acid into the nitric oxide of 2-methyl pyridine to carry out nitritation reaction on sodium nitrite to obtain sodium salt, and then using concentrated hydrochloric acid to neutralize alcohol solution to pH=3-4, filtering out sodium chloride, reducing the pressure of filter liquor and then concentrating to recover alcohol so as to obtain the pralidoxime chloride crude product, then dissolving by distilled water, and carrying out active carbon decoloration and recrystallization to obtain the finished product. The invention has simple process, short production time, high yield and easy realization of industrialization.
Owner:董婧
Method for synthesizing and preparing 2-vinyl pyridine
InactiveCN102863375AReduce energy consumptionImprove securityOrganic chemistry2-VinylpyridineGas phase
The invention relates to a method for synthesizing and preparing 2-vinyl pyridine by synthesizing 2-methyl pyridine and formaldehyde, which comprises the following steps of: adding the raw materials 2-methyl pyridine and formaldehyde (36%) into a high pressure reactor according to the weight ratio of 1: 0.03, stirring and heating for reaction to obtain 2-hydroxyethyl pyridine solution; slowly adding the obtained 2-hydroxyethyl pyridine solution into a dehydration kettle filled with sodium hydroxide (50%) solution through a measuring tank (1 ton) to obtain the crude product 2-vinyl pyridine; and sending the obtained crude product 2-vinyl pyridine into a washing kettle through a pipeline, adding sodium hydroxide (95%) solution into the washing kettle, and carrying out cut fraction on the crude product 2-vinyl pyridine which is separated by washing to obtain the 2-vinyl pyridine with the gas phase chromatography test content of more than 98.5%. The method adopts novel catalyst, so that the unit comprehensive energy consumption is reduced by 20%; the production process is automatically controlled, the product purity is greatly improved, and the product content reaches up to more than 98.5%; and the whole process route is clean and environment-friendly.
Owner:沙文茜
Synthesis method of 2-chromium picolinate
ActiveCN101602716AThe synthetic route is simpleProcess safetyOrganic chemistrySynthesis methodsSodium salt
The invention discloses a synthesis method of 2-chromium picolinate, which is implemented by hydrolyzing 2-cyanopyridine to obtain sodium salt solution of 2-chromium picolinate, adding chromium trichloride for complexation to obtain 2-chromium picolinate product. Compared with traditional method for synthesizing 2-chromium picolinate with 2-methylpyridine as raw material, the invention is simple in synthesis route, sate and reliable in process procedure, strong in maneuverability, high in reaction yield and good in product quality.
Owner:NANTONG ACETIC ACID CHEM
2-pyridinemethanol and synthetic method thereof
InactiveCN105153019ASimplify synthetic production stepsEmission reductionOrganic chemistryChemical recyclingAcetic acidAcetic anhydride
The invention discloses 2-pyridinemethanol and a synthetic method thereof. According to the method, 2-picoline and hydrogen peroxide are taken as raw materials, glacial acetic acid is taken as a solvent, the mixture has a reaction for 3-5 hours at the temperature of 70 DEG C-80 DEG C under the action of a catalyst, and 2-picoline nitrogen oxide is obtained after separation; 2-picoline nitrogen oxide and acetic anhydride react for 3-6 hours under the reflux condition, and acetic acid-2 pyridine methyl ester is obtained after separation; acetic acid-2 pyridine methyl ester is directly hydrolyzed under the alkali separating condition by sodium hydroxide, and a product 2-pyridinemethanol is obtained after separation. 2-pyridinemethanol and the synthetic method have the benefits as follows: the selectivity of process target products is high, material loss is low, the product content and the total yield can reach 98.5% and 65% respectively, and 2-pyridinemethanol is suitable for industrial mass production.
Owner:ANHUI COSTAR BIOCHEM CO LTD
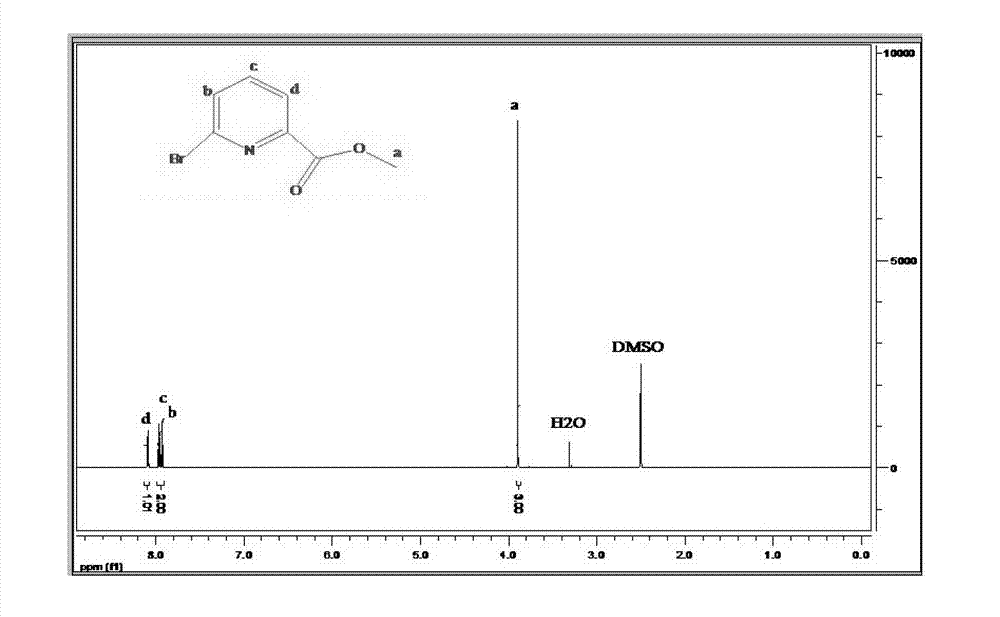
Preparation method of 6-bromine-2-pyridine methyl formate
ActiveCN103086964AOvercome the deficiency of many side effectsLess side effectsOrganic chemistryCarboxylic acidSolvent
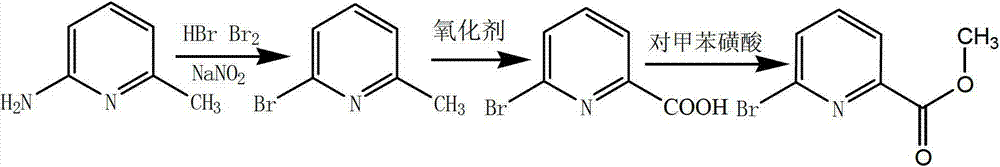
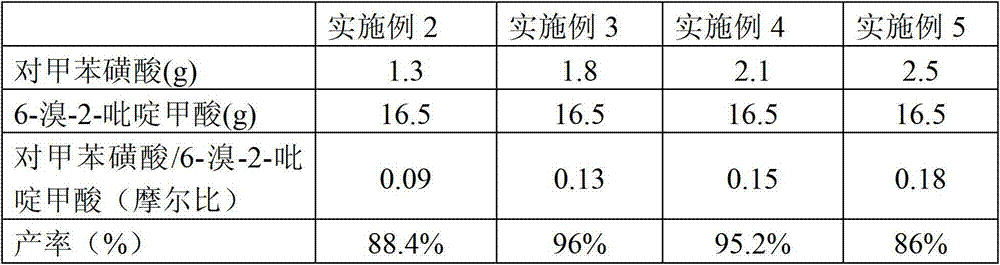
The invention relates to a preparation method of 6-bromine-2-pyridine methyl formate. The preparation method comprises the step of catalyzing esterification reaction of 6-bromine-2-pyridine carboxylic acid by taking p-toluenesulfonic acid as a catalyst, to be specific, heating and fluxing absolute methanol, the 6-bromine-2-pyridine carboxylic acid and the p-toluenesulfonic acid for 2-8 hours under stirring, cooling to a room temperature after reaction is ended, rotary drying a reaction system, dissolving solids in an organic solvent, washing, drying, filtering, concentrating, recrystallizing a concentrated product by a mixed solvent to obtain the 6-bromine-2-pyridine methyl formate, wherein a preferable molar ratio of the 6-bromine-2-pyridine carboxylic acid and the p-toluenesulfonic acid is 1: (0.1-0.16). According to one embodiment of the invention, the 6-bromine-2-pyridine carboxylic acid is obtained through diazotization, bromination and oxidation of 6-amino-2-methylpyridine. The preparation method is few in side reaction and simple in aftertreatment and is suitable for industrial production; and the product is easy to separate and has high yield, high purity and good quality.
Owner:BEIJING GREENCHEM TECH
Preparation for 6-chlorine-2-trichloromethyl pyridine
InactiveCN101314588AIncrease nutritionDelayed nitrificationOrganic chemistryTemperature controlStable state
The invention discloses a method for preparing 6-chloro-2-trichloromethyl pyridine. The method includes the steps as follows: gaseous chlorine and 2-methylpyridine are adopted as the raw materials; initiator is added to a first reactor, and excessive gaseous chlorine is introduced into the reactor; the gaseous chlorine and the initiator react to produce HCl gas which rises into a second reactor, and is in convection with 2-methylpyridine to produce 2-methylpyridine hydrochloride under a temperature-control condition; the hydrochloride returns to the first reactor and reacts with the excessive gaseous chlorine to produce 2-trichloromethylpyridine which reacts with continuously-introduced excessive gaseous chlorine at a certain temperature until the reaction reaches a stable state to obtain a volatile 6-chloro-2-trichloromethyl pyridine enriched mixture; the mixture is extracted from the kettle bottom and refined to obtain 6-chloro-2-trichloromethyl pyridine compound; and a tail-gas recovery device is connected with the second reactor. The method has the advantages of high selectivity, high yield and environment friendliness.
Owner:ZHEJIANG AOFUTUO CHEM
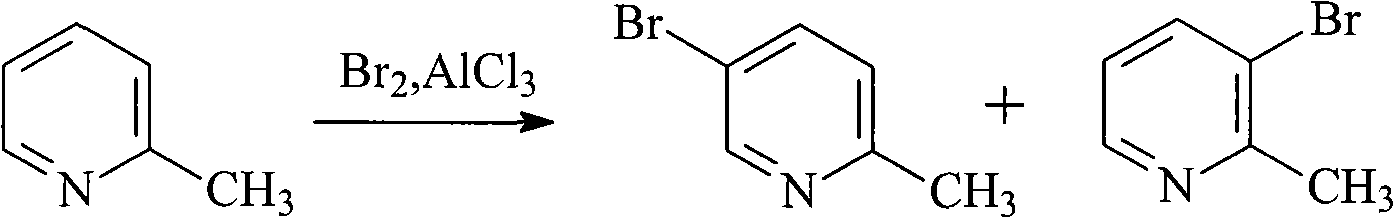
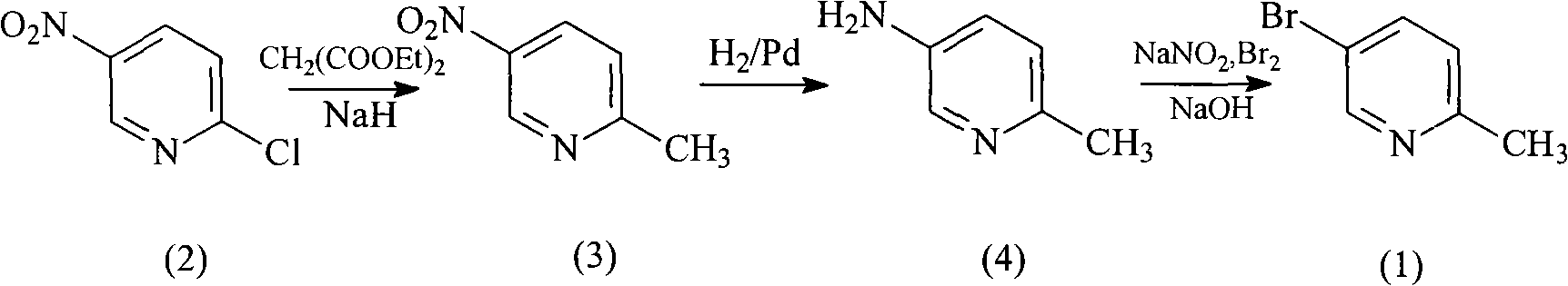
Method for preparing 5-bromo-2-methylpyridine
InactiveCN101560183AHigh yieldLow priceOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsCatalytic effectSodium nitrite
The invention discloses a method for preparing intermediate 5-bromo-2-methylpyridine. In the prior art, the dosage of aluminium trichloride is large; the catalytic effect is poor; the by-products are more; the yield of products is low; and the obtained products are difficult to separate. The method comprises the following steps: reacting diethyl malonate with alkali metal to generate salts, dripping 5-nitryl-2-chloropyridine into the salts for condensation reaction, and subsequently performing decarboxylation on the obtained product under acidic condition to obtain 5-nitryl-2-methylpyridine; performing hydrogenation reduction on the 5-nitryl-2-methylpyridine under the catalysis of Pd / C catalyst to obtain 5-amido-2-methylpyridine; and reacting the 5-amido-2-methylpyridine with acid to generate salts, dripping bromine, dripping a sodium nitrite water solution, and obtaining the 5-bromo-2-methylpyridine. The method has mild reaction conditions, easy operation, simple post-treatment, good catalytic effect, high yield of each step and high yield of final products, and is particularly suitable for industrialized production.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
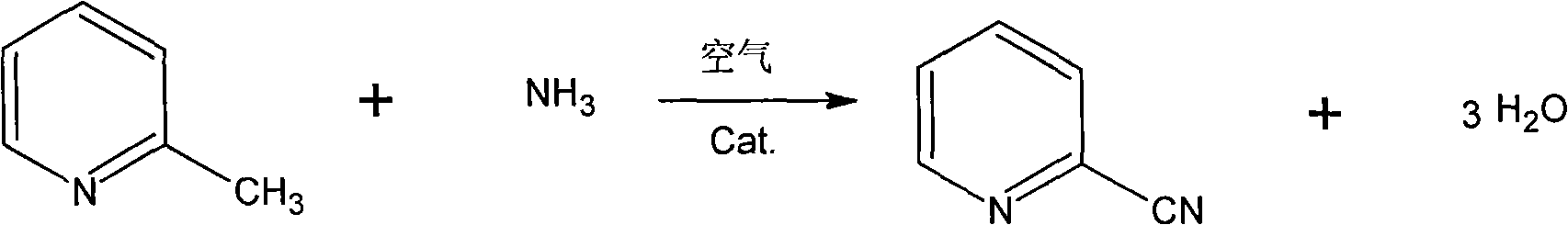
Synthesis method of 2-cyanopyridine
InactiveCN101602720ASimple processEasy to operateOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsSynthesis methodsAmmonia
The invention discloses a synthesis method of 2-cyanopyridine, which is characterized in that 2-methylpyridine is vaporized and mixed with ammonia and air; the 2-methylpyridine reacts with the ammonia and air in the presence of a catalyst, and then the finished product of 2-cyanopyridine is obtained after absorption, extraction and rectification. The method features simple process and easy operation; the conversion rate of the 2-methylpyridine is more than 97% and the yield of the 2-cyanopyridine is more than 85%.
Owner:NANTONG ACETIC ACID CHEM
Method for rectification dehydration of 2-methylpyridine
InactiveCN106866504AImprove general performanceEasy to separateOrganic chemistryDistillation regulation/controlRefluxChemical separation
The invention discloses a method for rectification dehydration of 2-methylpyridine and belongs to the field of chemical separation and purification. The method comprises introducing aqueous 2-methylpyridine into an azeotropic rectification tower, condensing rising steam at the tower top through a condenser, feeding the condensed steam into a laminator, dividing the liquid in the laminator into two phases, carrying out reflux on the organic phase from the tower top, feeding the water phase into a water recovery tower, and collecting 2-methylpyridine at the bottom of the tower. 2-methylpyridine and water form a heterogeneous azeotrope so that a solvent is avoided. The heterogeneous azeotropic rectification technology realizes complete separation of 2-methylpyridine and water so that 2-methylpyridine having a mass separation fraction of greater than 99.5% and water are obtained. The method has the characteristics of simple equipment, strong universality and good separation effect.
Owner:CHANGZHOU UNIV
2-vinylpyridine produced by catalyst distillation of heteropoly acid
InactiveCN101225071AEfficient use ofNo pollution in the processOrganic chemistryPhysical/chemical process catalysts2-VinylpyridineHeteropoly acid
The invention discloses a method of using heteropolyacid catalyst to prepare 2-vinylpyridine, which comprises: (1). 2-methylpyridine and formaldehyde or paraformaldehyde with molar ratio of 1:2 to 3 are used as raw material; mass ratio between added heteropolyacid catalyst and the raw material is 0.001 to 0.008; 4-stage series type stirring reactor is arranged and material feeding speed is 100 to 500kg / h; (2). The raw material and the catalyst are reacted in the 4-stage series type stirring reactor to collect final fraction product with boiling range of 120 to 150 DEG C, thereby getting 2-vinylpyridine; (3). The rest fractions are collected and returned to stage-1 reactor to increase conversion rate of 2-methylpyridine. The conversion rate of 2-methylpyridine reaches 95% and the selectivity of the 2-vinylpyridine reaches 97.00%, thereby realizing effective use of byproduct. The preparation method for 2-vinylpyridine by using heteropolyacid catalyst has the advantages of simple technique and non-pollution upon environment.
Owner:于景东
Preparation method of pyridine-2-formaldehyde
The invention relates to a preparation method of pyridine-2-formaldehyde. The preparation method comprises the following two steps: preparing an acidity-regulator-containing supported catalyst with titanium dioxide used as a carrier, molybdenum bismuth oxide used as a main body and transition metal oxide used as an auxiliary, carrying out gas-phase oxidation reaction on 2-methyl pyridine, oxygen and water used as raw materials in a fixed bed catalytic reactor at 250-350 DEG C to obtain a crude pyridine-2-formaldehyde product, extracting the crude product with dichloromethane, carrying out pressure-reduced distillation on the extract to remove dichloromethane, and then rectifying to obtain a pure product with a pyridine-2-formaldehyde content more than 98%. The preparation method provided by the invention has the advantages of simple preparation process, high efficiency, low cost, high catalytic reaction activity and selectivity, easy separation of main products and by-products and high purity.
Owner:ZHEJIANG UNIV
Method for preparing 2-chloro-6-trichloromethylpyridine
InactiveCN102391176ASimple production processImprove productivityOrganic chemistryPyridine2-Methylpyridine
The invention discloses a method for preparing 2-chloro-6-trichloromethylpyridine. In the method, 2-methyl pyridine is used as a raw material and a multi-stage liquid-phase continuous chlorination process is adopted; therefore, the method has the advantages of simplicity, high efficiency, environment friendliness and low cost.
Owner:LANGFANG BEIXIN CHEM
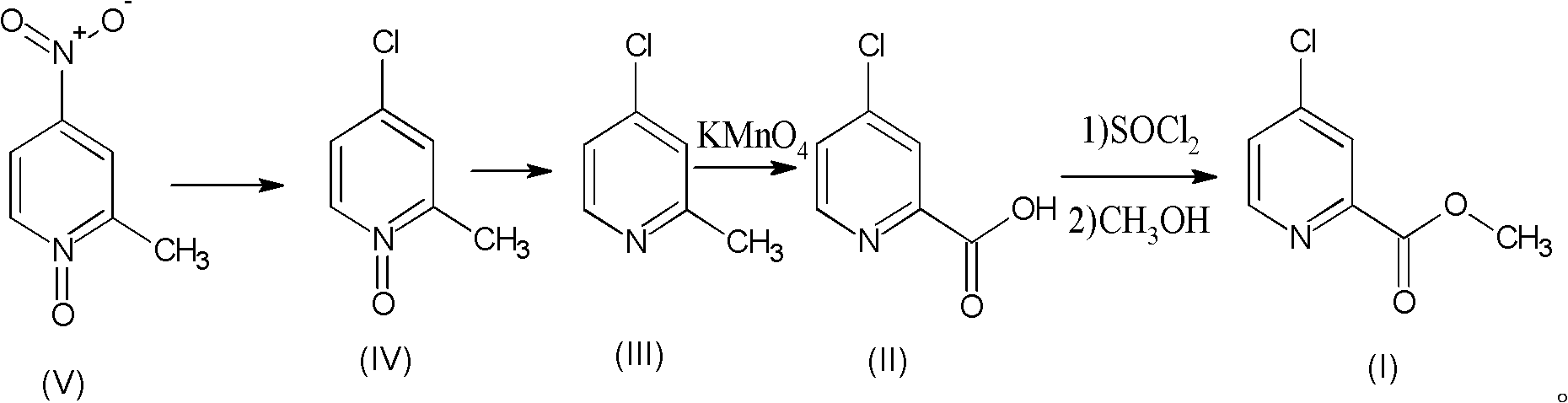
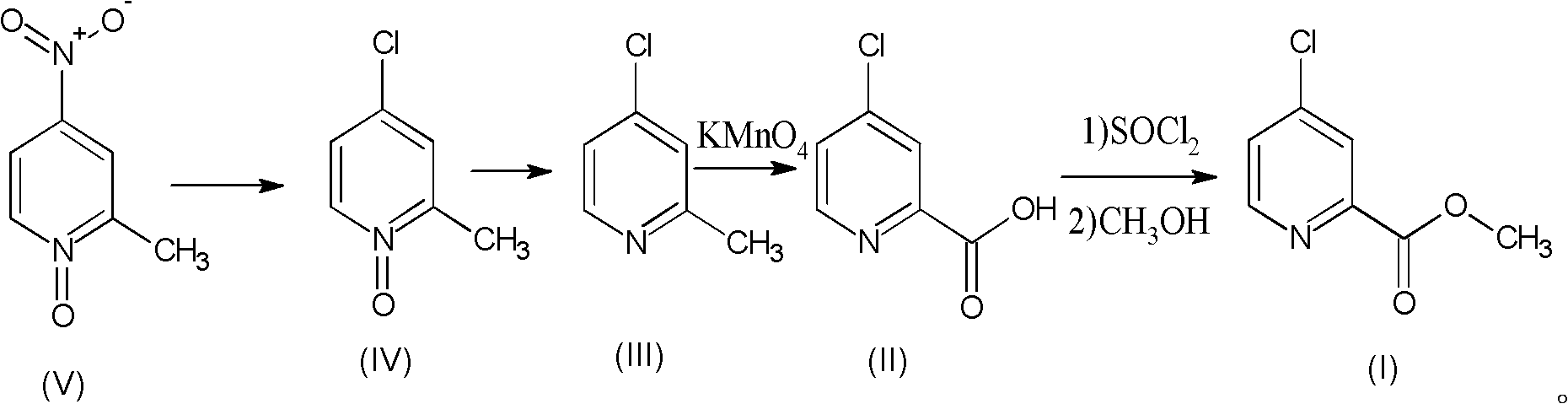
Preparation process of high-purity 4-chloro-2-pyridinecarboxylate hydrochloride
The invention relates to a preparation process of high-purity 4-chloro-2-pyridinecarboxylate hydrochloride, which comprises the following steps of reacting 2-methyl-4-nitropyridine-N-oxide taken as a starting raw material with hydrochloric acid to obtain 4-chloro-2-methyl-pyridine-N-oxide; then dripping phosphorus trichloride into an organic solvent, carrying out reaction for generating 4-chloro-2-methylpyridine, further adding potassium permanganate into water for carrying out oxidation reaction, thus obtaining 4-chloro-2-picolinate; and mixing the 4-chloro-2-picolinate with the catalytic amount of DMF (dimethyfumarate), dripping thionyl chloride, and finally carrying out esterification reaction with methanol so as to obtain the 4-chloro-2-pyridinecarboxylate hydrochloride. The total yield can be up to 36.8%, and the purity can be 99.8% according to the HPLC (high performance liquid chromatography) detection. The process has the advantages of being rich in raw material resources, small in whole process pollution, conductive to controlling three wastes and easy for realizing industrial production.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Synthesis method of 2-picoline
InactiveCN105218431AImprove the industrial chainImprove survivabilityOrganic chemistryHeterogenous catalyst chemical elementsAlcoholSynthesis methods
The invention discloses a synthesis method of 2-picoline. According to the synthesis method, pyridine and methyl alcohol serve as raw materials, and Fe-MnOx-Yb serves as a catalyst. The synthesis method has the advantages that 2-picoline is prepared through one-step synthesis with pyridine and methyl alcohol as the raw materials, a pyridine industry chain is fully completed, the market dependency of pyridine bases of enterprises is reduced, controllable self-adjustability is achieved, and the survival ability of the enterprises is improved; the catalytic activity of the catalyst doped with Yb metal elements is improved by 3-5 times under influences of the characteristics (structure and electron cloud) of the Yb metal elements, the reaction period is shortened, and efficiency is improved; the synthesis method is efficient, the reaction ingredients are simple and easy to treat, energy is saved, and cost is lowered. The synthesis method is an environment-friendly and efficient synthesis technology, the product yield reaches up to 88%, and the pyridine conversion rate reaches 95%.
Owner:ANHUI COSTAR BIOCHEM CO LTD
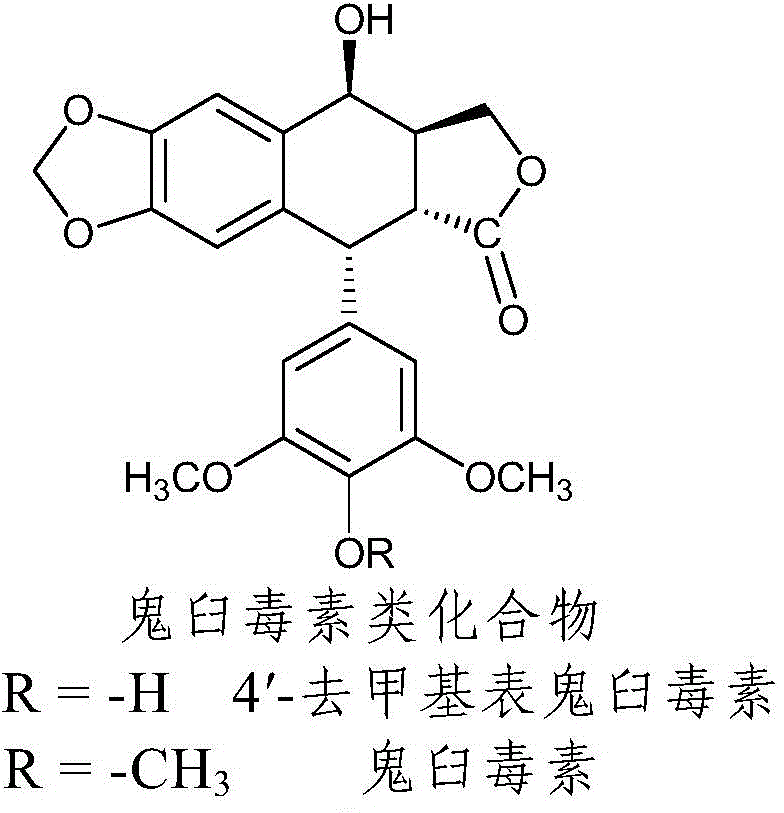
Nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and preparation method and use thereof
InactiveCN103601732AGood antitumor activityImprove anti-tumor activityOrganic active ingredientsOrganic chemistryTumor cells2-Methylpyridine
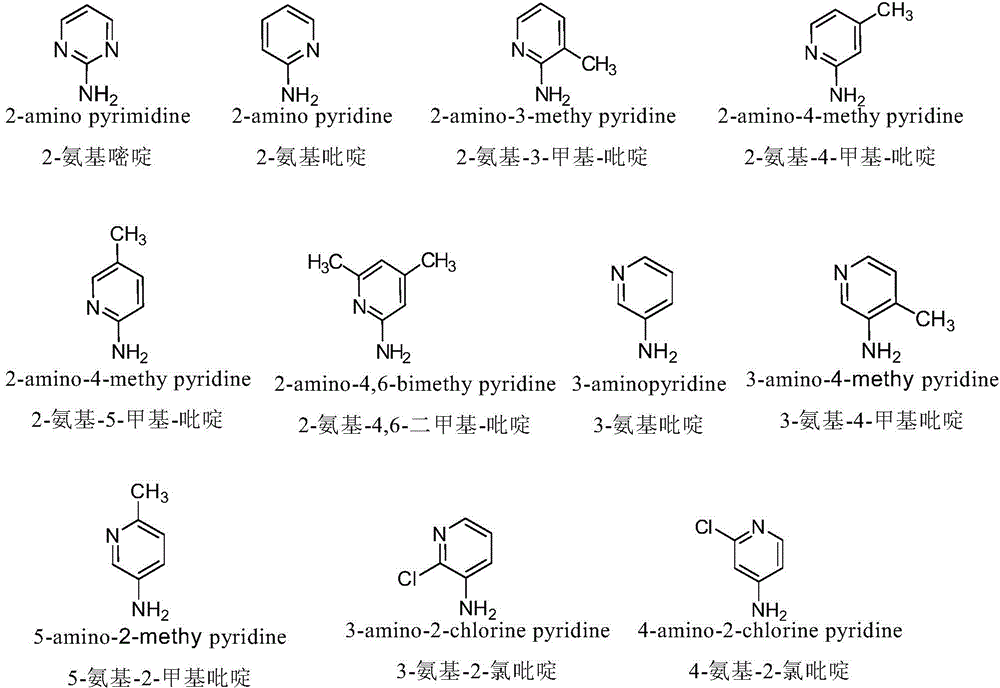
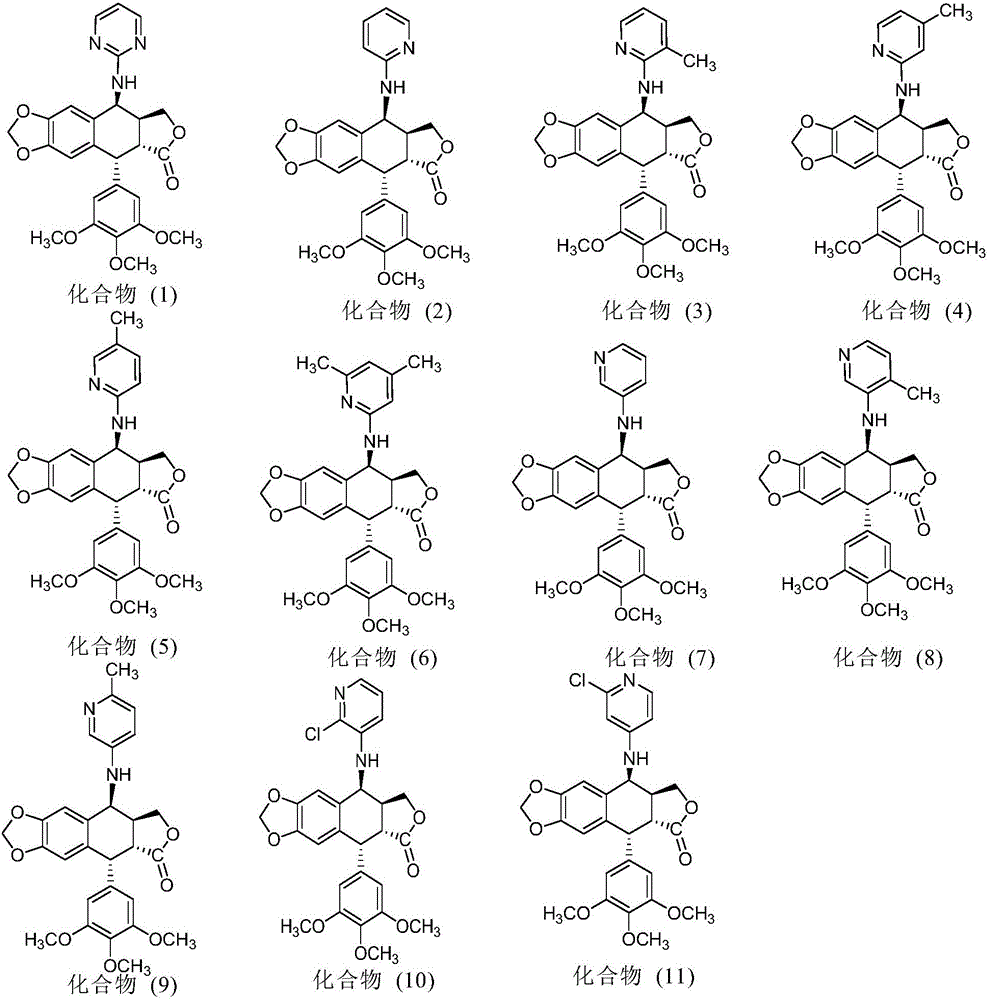
The invention discloses a nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and a preparation method and use thereof. According to the method, 2-aminopyrimidine, 2-aminopyridine, 2-amino-3-methylpyridine, 2-amino-4-methylpyridine, 2-amino-5-methylpyridine, 2-amino-4,6-dimethyl pyridine, 3-aminopyridine, 3-amino-4-methylpyridine, 5-amino-2-methylpyridine, 3-amino-2-chloropyridine or 4-amino-2-chloropyridine is respectively introduced to an activated C-ring fourth position of a podophyllotoxin compound through nitrogen substitution reaction, so as to obtain the nitrogen-substituted podophyllotoxin derivative, represented by a formula (V) shown in the specification, with excellent anti-tumor activity. The nitrogen-substituted podophyllotoxin derivative disclosed by the invention acts on tumor cells through multiple ways and multiple target points, and the anti-tumor activity of the nitrogen-substituted podophyllotoxin derivative is remarkably improved compared with that of the podophyllotoxin compound. The compound disclosed by the invention can be used for preparing anti-tumor drugs and is clinically applied to anti-tumor treatment.
Owner:HUBEI UNIV OF TECH
Preparation method of 2-ethoxyl pyridina
InactiveCN102731372ALow equipment requirementsReduce manufacturing costOrganic chemistryPyridineParaformaldehyde
The invention discloses a preparation method of 2-ethoxyl pyridina, which comprises the steps of: (1) using tetrahydrofuran as a solvent, enabling 2-methyl pyridine and organic alkali to completely react in the solvent at -60-60 DEG C to prepare a reaction liquid; (2) adding paraformaldehyde into the reaction liquid, agitating and reacting for 0.5-5 hours at 10-60 DEG C, then dropping water into the mixture under the reaction condition, and agitating continuously and reacting for 0.5-1 hours at 10-60 DEG C after dropping water to prepare a product liquid; and (3) removing water in the product liquid, then removing tetrahydrofuran in the product liquid to obtain the 2-ethoxyl pyridine. The method provided by the invention is simple in process operation, short in synthesizing time of product, low in production cost, high in production efficiency, less in secondary reaction, high in product yield and high in purity.
Owner:SHANDONG TIANYI CHEM
Production method of 6-chloro-2-(trichloromethyl)pyridine
The invention relates to a production method of a nitrogen fertilizer synergist-6-chloro-2-(trichloromethyl)pyridine, belonging to the crop production field. The production method comprises the following steps: taking 2-methylpyridine as a staring material; in the presence of solvents such as chlorobenzene, dichlorobenzene, chloro-trifluorotoluene, nitrobenzene and the like, introducing excessive chlorine at the temperature of 130-205 DEG C for chlorination to obtain a crude product of the 6-chloro-2-(trichloromethyl)pyridine; and purifying the crude product through rectification to obtain the high-purity product of the 6-chloro-2-(trichloromethyl)pyridine. The production method can overcome the defects of low yield, easy generation of a tar polymer, pipe blockage caused by materials and the like of the existing production method, thus achieving the purpose of improving reaction selectivity and product yield, reducing waste discharge and being easy to be industrialized.
Owner:SHANDONG SHENGBANG LUNAN PESTICIDE
Synthetic method of 2-mercaptobenzothiazolyl-(Z)-(2-aminothiazol-4-yl)-2-(tert-butoxycarbonyl) isopropoxyiminoacetate
The invention belongs to the field of a medical technology and specifically relates to a synthetic method of 2-mercaptobenzothiazolyl-(Z)-(2-aminothiazol-4-yl)-2-(tert-butoxycarbonyl) isopropoxyiminoacetate. The synthetic method comprises the following steps: ethyl 2-(2-aminothiazole-4-yl)-2-(1-tert-butoxycarbonyl-1-methylethoxyimino)acetate and 2,2'-dibenzothiazole disulfide are added into a mixed liquor of benzene and acetonitrile; aniline and 2-methylpyridine are successively added; triphenyl phosphite is added dropwise to carry out a reaction; and after the reaction, a crude product is obtained, and the crude product is refined with methanol so as to obtain refined active ester. The synthetic method provided by the invention has simple process and is easy to implement. By the synthetic method, purity of the product reaches more than 99%, and yield reaches more than 94%.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD +1
Method for preparing pyridine bases
InactiveCN102020603AReduce consumptionEasy to operateOrganic chemistryChemical industryChemical synthesisFixed bed
The invention relates to a method for preparing pyridine bases. The pyridine bases are prepared by taking formaldehyde, acetaldehyde and ammonia as raw materials through the steps of carrying out chemical synthesis on the formaldehyde, the acetaldehyde and the ammonia in a fixed bed reactor in the presence of a catalyst at a temperature of 350 to 550 DEG C so as to obtain a high-temperature gas containing the pyridine bases; condensing the high-temperature gas so as to obtain a solution containing the pyridine bases, and carrying out ammonia absorption and steam stripping on the uncondensable ammonia-containing gas; then recovering the obtained ammonia and feeding the ammonia to the fixed bed reactor to be used as raw materials; extracting the obtained solution by benzene, then respectively obtaining a benzene solution containing the pyridine bases and a water solution containing a small amount of ammonia, benzene and pyridine bases, etc., after the solutions are processed, feeding the solutions to a combustion furnace to burn at a temperature of 1000 to 1200 DEG C; then discharging the solutions; feeding the benzene solution containing the pyridine bases into a benzene stripper to carry out steam stripping, then respectively obtaining benzene and pyridine base liquor, feeding the pyridine base liquor into different rectifying towers to carry out rectification under normal pressure or negative pressure, then respectively obtaining pyridine, 3-picoline and 2-picoline. The method in the invention has the advantages of low material consumption, high total yield, less catalyst consumption, and low energy consumption, and is simple in operation.
Owner:郑廷来
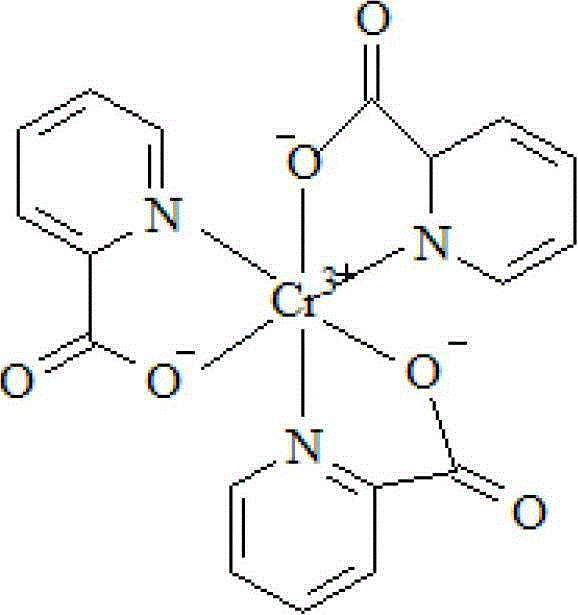
Synthesis method of chromium 2-pyridylformate
ActiveCN102875458AReduce pollutionTake advantage ofOrganic chemistryQuaternary ammonium cationPhosphonium
The invention discloses a synthesis method of chromium 2-pyridylformate, which comprises the following steps: by using 2-methylpyridine, potassium permanganate and chromium chloride alcoholic solution as raw materials, adding a phase-transfer catalyst to synthesize the chromium 2-pyridylformate, wherein the phase-transfer catalyst is a quaternary ammonium salt or quaternary phosphonium salt in onium salt phase-transfer catalysts, preferably ammonium tetrabutylbromide, ammonium tetrabutylchloride or phosphonium methyltriphenylbromide. The method disclosed by the invention uses the phase-transfer catalyst to enable the sufficient reaction of 2-methylpyridine in an inorganic reaction environment, thereby enhancing the reaction yield (up to higher than 71%); the methanol or ethanol is used as the solvent to enhance the yield of the reaction step; and the solvent is recyclable, thereby saving the raw material cost, promoting the effective performance of the complex reaction, enabling the product to precipitate sufficiently, enhancing the maximum product yield by nearly 40%, and ensuring the yield and quality of the product.
Owner:HONGFENG CHEM GUAN COUNTY HEBEI PROV
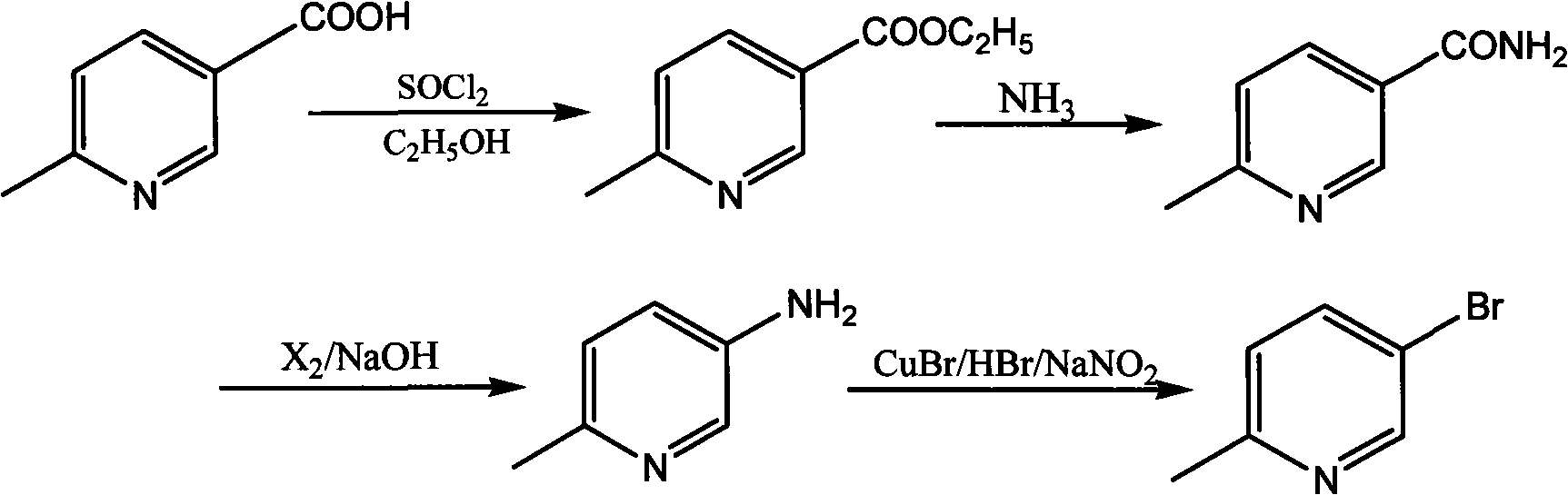
Synthetic method for 5-bromine-2-methylpyridine
InactiveCN101514184AReduce wasteMild reaction conditionsOrganic chemistryPhotochemistryPicolinic acid
The invention discloses a synthetic method for 5-bromine-2-methylpyridine: using 6-methyl-3-picolinic acid as the raw material to react with ethyl alcohol to generate 6-methyl-3-picolinic acid ethyl ester; carrying out ammonolysis reaction by aqueous ammonia on the 6-methyl-3-picolinic acid ethyl ester to generate 6-methyl-3-pyridine carboxamide; carrying out Hofmann degradation reaction to obtain 6-methyl-3-aminopyridine; and finally reacting the 6-methyl-3-aminopyridine with a bromizing reagent to generate 5-bromine-2-methylpyridine. In the method, the process reaction condition is mild, the yield is high, the raw material is available, the cost is lower, and no 3-subsidary products are generated in the whole process, the load of post separation is eliminated and the prospect of industrialization is good.
Owner:NANJING UNIV OF TECH
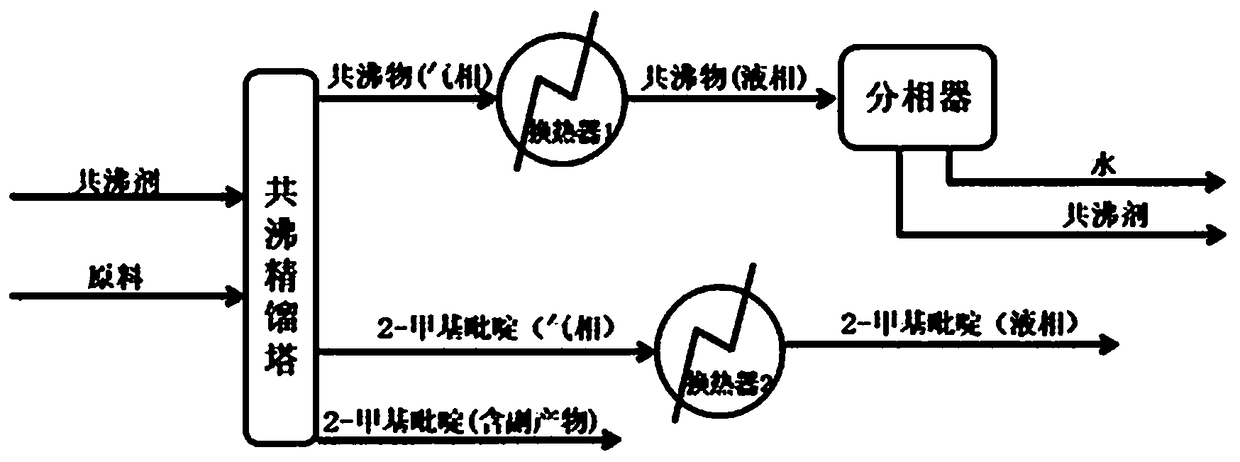
Method for realizing 2-picoline dehydration by side withdrawal from azeotropic distillation column
ActiveCN108191743AEasy to operateReduce cost inputOrganic chemistryChemical industryDistillation methodAzeotropic distillation
The invention provides a method for realizing 2-picoline dehydration by side withdrawal from an azeotropic distillation column. According to the method, cyclohexane is taken as an entrainer, a new lowazeotrope is formed from cyclohexane and water and breaks azeotropy of 2-picoline and water. A cyclohexane and water gas-phase mixture is obtained from top of the azeotropic distillation column, vapor rich in 2-picoline is withdrawn from the side of a column plate close to the column bottom and condensed into a liquid by a heat exchanger, a small amount of 2-picoline containing impurities is withdrawn from the column bottom, and accordingly, separation of 2-picoline and water is realized with the distillation method. The method is applicable to continuous distillation operation, 2-picoline with higher purity can be obtained by side withdrawal, 2-picoline is prevented from being heated at the column bottom for too long time to produce side effects, only one substance, namely, the entraineris introduced in the whole process, the entrainer can be efficiently recycled, and the method is a separation method with high efficiency and low energy consumption and cost.
Owner:淄博高新技术产业开发区精细化工和高分子材料研究院 +1
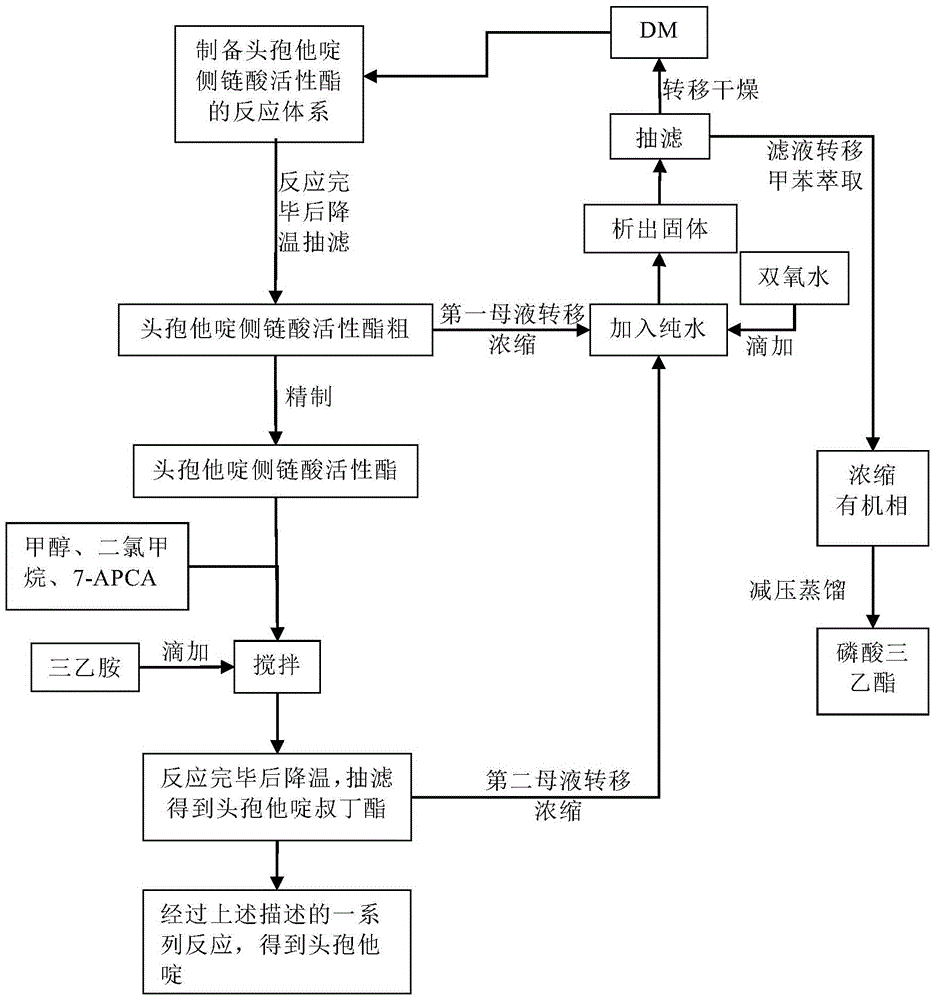
Original development quality ceftazidime and medicine preparation thereof
The invention discloses original development quality ceftazidime and a medicine preparation thereof. The third-generation cephalosporin antibiotics active ester midbody key technology and industrialization obtains the second prize of National Scientific and Technological Progress Award. The cephalosporin antibiotics active ester belongs to a key factor for influencing the internal quality of the cephalosporin. A preparation method comprises the following steps that (a) mixed solvents are added into ceftazidime side chain acid, dibenzothiazyl disulfide, aniline and 2-picoline; triethyl phosphate is dripped for reaction; (b) a coarse product is refined to obtain ceftazidime side chain acid active ester, and the first mother liquid is recovered; (c) the material is added into a mixed solvent for neutralizing 7-APCA; triethylamine is dripped; the temperature reduction is performed for crystal separation and filtering to obtain ceftazidime tert-butyl ester; the second mother liquid is recovered; (d) the ceftazidime tert-butyl ester is subjected to hydrolysis and purification, and then, the ceftazidime is obtained. The original development quality ceftazidime has the advantages that high-toxicity triphenylphosphine is not used; waste liquid and waste slag can be sufficiently recovered and reutilized; the method is safe; the cost is low; the yield is high; the industrial production is facilitated.
Owner:广东金城金素制药有限公司 +1
Method for preparing wormer sec-Butyl 2-(2-hydroxyethyl)piperidine-1-carboxylate
ActiveCN101698658AReduce generationReduce manufacturing costBiocideOrganic chemistryReaction temperatureSolvent
The invention discloses a method for preparing a wormer sec-Butyl 2-(2-hydroxyethyl)piperidine-1-carboxylate, which comprises the following three steps: firstly, taking 2-methylpyridine as a starting material to undergo a condensation reaction with paraformaldehyde to produce 2-hydroxyethyl pyridine in the presence of a catalyst sodium carbonate or potassium carbonate; secondly, dissolving the 2-hydroxyethyl pyridine into a solvent and adding a high-efficient hydrogenation catalyst for reaction with hydrogen for a certain time at a certain temperature and under a certain pressure to produce 2-hydroxyethyl piperidine; and finally, placing the 2-hydroxyethyl piperidine and aqueous slkali into a reaction bulb, controlling the reaction temperature, dropwise adding sec-butyl chloroformate dropwise under the condition of no solvent, and after reacting for certain time, performing acid cleaning, water rinsing and drying to directly produce a finished product of the sec-Butyl 2-(2-hydroxyethyl)piperidine-1-carboxylate. The method is low in production cost, moderate in reaction conditions, less in side reaction, high in yield, good in product quality, simple and convenient in process and easy in operation.
Owner:FUJIAN SENFA BIOTECH
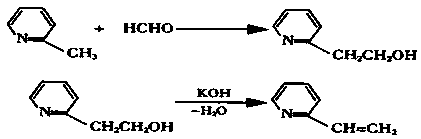
Preparation method of orthographic optimizing betahistine hydrochloride
The invention discloses a preparation method of orthographic optimizing betahistine hydrochloride, and relates to the field of drug preparation. The preparation method includes the steps: adding raw materials into a reaction bottle according to the feeding ratio (molar ratio) of 2-methylpyridine to paraformaldehyde of 1:0.57, leading in nitrogen, stirring mixture for 20 hours at the temperature of125 DEG C and at 4 barometric pressure, performing reduced pressure distillation, and collecting distillation cut at the temperature of 130-145 DEG C and under the pressure of 16mm mercury columns toobtain light-yellow oily 2-hydroxyethyl pyridine; adding the 2-hydroxyethyl pyridine into a three-opening bottle, adding sodium hydroxide according to the feeding ratio of 2-(2-Hydroxyethyl)pyridineto sodium hydroxide of 1:0.05, heating the mixture to reach 95-100 DEG C, stirring the mixture for 2 hours, removing a water layer, performing reduced pressure distillation on an oil layer, and collecting the distillation cut at the temperature of 65-70 DEG C and under the pressure of 17mm mercury columns to obtain 2-vinylpyridine. The preparation method is simple in technological process, safe tooperate and mild in reaction, production efficiency and product quality can be greatly improved, and production cost is reduced.
Owner:SHAANXI QIYUAN TECH DEV
Eco-friendly process for the preparation of 2-Chlorobenzylidene-Malononitrile (CS)
ActiveUS7732631B2Reduce sewage loadSpeed up the processCarboxylic acid nitrile preparationOrganic compound preparation4-MethylpyridineMorpholine
An improved process for the preparation of 2-chlorobenzylidenemalononitrile (CS) comprising of the steps of: preparing malononitrile suspension by adding 5-20% (wt %) preferably 12-14% malononitrile to water while constantly stirring and then adding 0.05-0.5% (v / v) preferably 0.1-0% of a catalyst like piperidine, pyridine, 2-picoline, 3-picoline, 4-picoline or morpholine preferably piperidine piperidine with constant stirring at 20-30° C.; condensing the malononitrile suspension prepared in step (a) with 2-chlorobenzaldehyde by adding 10-15% (w / v) preferably 25-30%, of 2-chlorobenzaldehyde cover a period at 30-45 minutes so that the temperature of the reaction mixture remains below 50° C., constantly stirring for 20-40 minutes, then filtering the CS and drying it at 20-30° C. under water vacuum for 3-5 hrs.
Owner:DIRECTOR GENERAL DEFENCE RES & DEV ORG
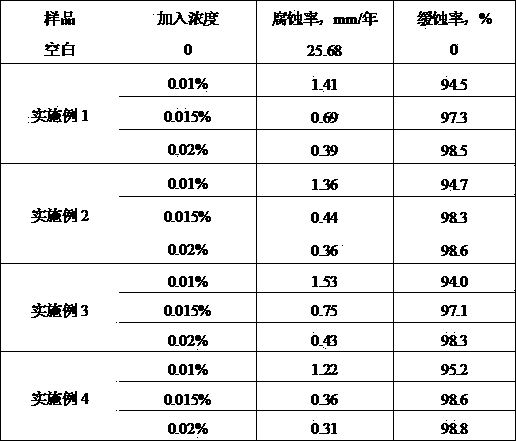
Corrosion inhibitor for biodiesel production process by pyrolysis and application thereof
The invention relates to a corrosion inhibitor for a biodiesel production process by pyrolysis. The corrosion inhibitor comprises the following components in parts by weight: 20-50 parts of naphthenic acid imidazoline amide, 2-20 parts of 2-methylpyridine, 2-15 parts of quaternary ammonium surfactant, 2-15 parts of benzotriazole, 10-30 parts of organic amine and 20-50 parts of solvent. The corrosion inhibitor disclosed by the invention is simple in component, convenient to prepare, strong in stability, small in dosage in the production process, and good in corrosion inhibition performance, and the high and low temperature corrosion problems of equipment such as a pyrolysis reaction kettle, a pipeline and the like can be solved.
Owner:HUNAN HUAYUAN NEW ENERGY CO LTD
Preparation method of pyridine-2-formaldehyde
The invention discloses a preparation method of pyridine-2-formaldehyde. The preparation method comprises the following steps of: preparing a catalyst containing an acidity regulator and a transition metal oxide carried on a carrier; carrying out a gas-phase oxidizing reaction in a fixed bed catalytic reactor at 250-400 DEG C to generate a crude target product by using 2-picoline, oxygen and water as raw materials; extracting the crude product through dichloromethane and then decompressing and distilling extract liquor to remove the dichloromethane; and then rectifying to obtain a pure product with the pyridine-2-formaldehyde content higher than 98.5 percent. The method has simple preparation process, low raw material cost, high catalytic oxidation reaction selectivity, easy separation of main products and byproducts and high purity.
Owner:ZHEJIANG UNIV
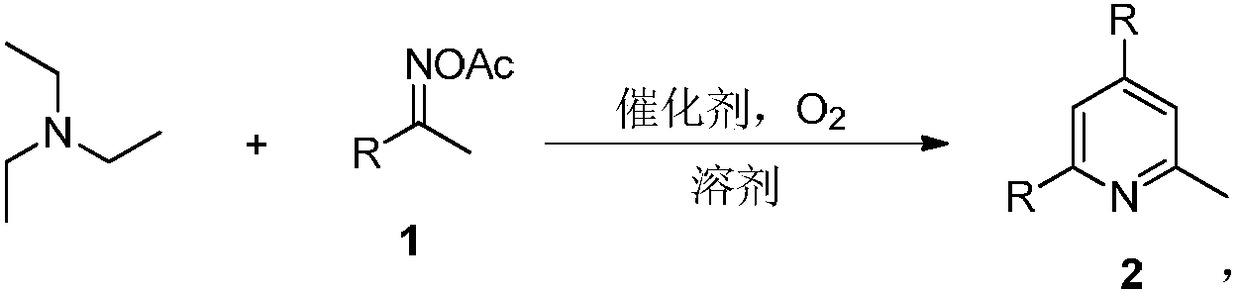
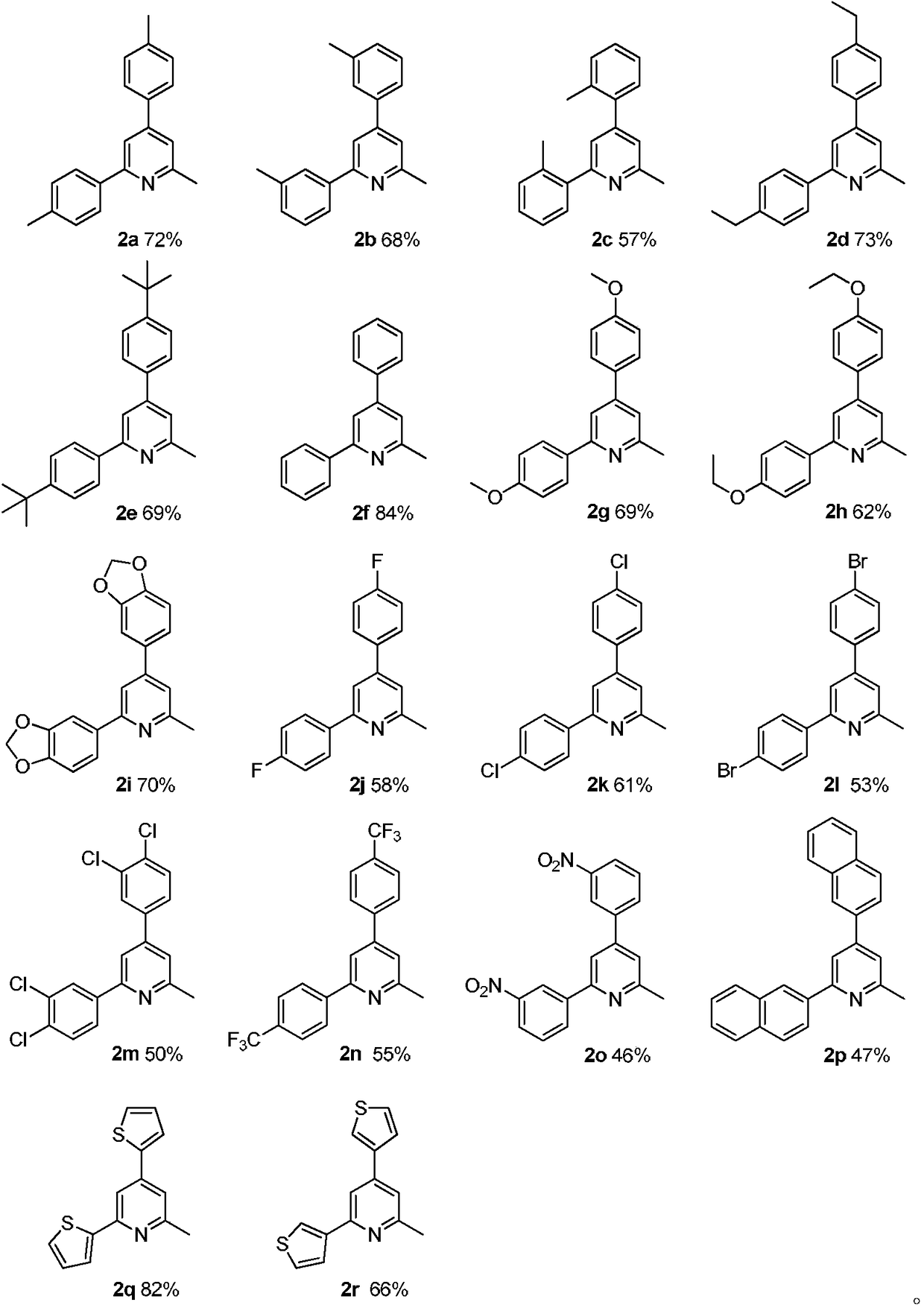
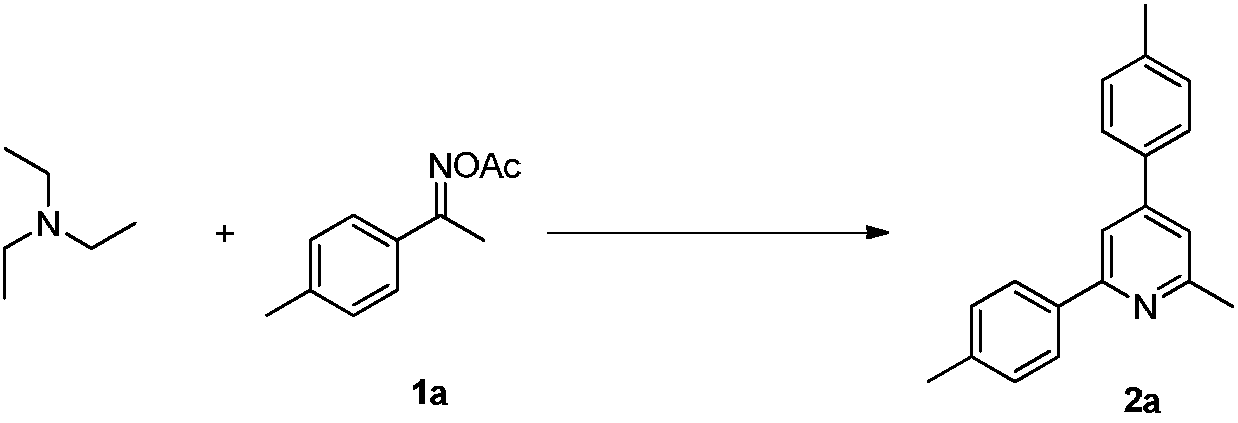
Method for synthesizing 2-methylpyridine compound
ActiveCN108314642AAvoid pollutionSimple processOrganic chemistryOrganic synthesisReaction intermediate
The invention discloses a method for synthesizing a 2-methylpyridine compound and belongs to the technical field of organic synthesis. According to key points of the technical scheme, the method for synthesizing the 2-methylpyridine compound in the invention comprises the following specific steps: dissolving triethylamine and oxime acetate compounds into a solvent, adding a catalyst, and reactingin an oxygen atmosphere at a temperature of 120 to 160 DEG C, thereby obtaining the 2-methylpyridine compound. The synthetic process is simple and efficient, and the 2-methylpyridine compound is directly prepared in one step by virtue of a one-pot cascade reaction without transition metal catalysis, so that waste of resources and environmental pollution caused by usage of multiple reagents in multi-step reactions and purification of an intermediate in each reaction step and the like can be avoided, the reaction conditions are mild, and the substrate application range is wide. Meanwhile, by taking the triethylamine as the raw material, the production cost is greatly reduced.
Owner:XINXIANG MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com