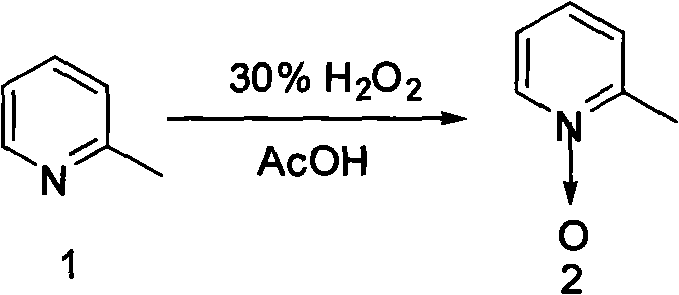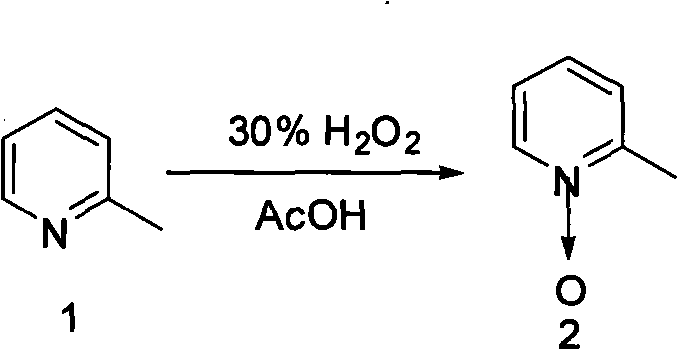Synthesis method of 2-pyridine formaldoxime
A synthetic method, the technology of pyridine formaldehyde, applied in the direction of organic chemistry, etc., can solve the problems of long steps, low yield, long time consumption, etc., and achieve the effect of short production time, high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 1) Mix and heat 205 g of 2-picoline, 700 mL of glacial acetic acid, and 320 mL of 30% hydrogen peroxide, and react at 70° C. for 3.0 hours. Then add 150mL of 30% hydrogen peroxide, continue to react for 8.0 hours, distill off the liquid under reduced pressure and cool down, add 150mL of chloroform and 150mL of 15% sodium carbonate aqueous solution, stir and mix thoroughly, let stand to separate layers, and then use trichloromethane to dissolve the water layer. Methane extraction (100 mL x 3). The organic layers were combined, and after the chloroform was distilled off, the distillate at 120°C (5mmHg) was collected under reduced pressure to obtain 212g of a colorless oily liquid, with a yield of 96%.
[0015] The synthetic formula of this step is:
[0016]
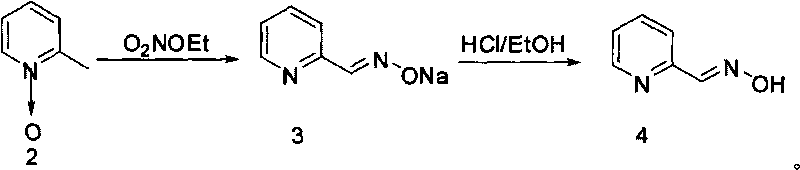
[0017] 2) Add 218g (2mol) of 2-picoline nitrogen oxide into 150mL ethanol, add 273g (3mol) of sodium nitrite, and then slowly drop in 90mL of a mixture of ethanol and concentrated sulfuric acid to carry out nitro...
Embodiment 2
[0021] 1) Mix and heat 150 g of 2-picoline, 400 mL of glacial acetic acid, and 200 mL of 30% hydrogen peroxide, and react at 70° C. for 3 hours. Then add 90mL of 30% hydrogen peroxide, continue to react for 5 hours, distill off the liquid under reduced pressure and cool down, add 60mL of chloroform and 60mL of 15% aqueous sodium carbonate solution, stir and mix thoroughly, let stand to separate layers, and then use trichloromethane Methane extraction (60 mL x 3). The organic layers were combined, and after the chloroform was distilled off, the distillate at 120° C. (5 mmHg) was collected under reduced pressure to obtain 141 g of a colorless oily liquid, with a yield of 93%.
[0022] The synthetic formula of this step is:
[0023]
[0024] 2) Add 190g of 2-picoline nitrogen oxide into 120mL of ethanol, add 226g of sodium ethyl nitrite, then slowly drop in 70mL of a mixture of ethanol and concentrated sulfuric acid to carry out nitrosation reaction to obtain sodium salt, and...
Embodiment 3
[0028] 1) Mix and heat 180 g of 2-picoline, 500 mL of glacial acetic acid, and 250 mL of 30% hydrogen peroxide, and react at 70° C. for 4 hours. Then add 110mL of 30% hydrogen peroxide, continue to react for 6 hours, distill off the liquid under reduced pressure and cool down, add 100mL of chloroform and 100mL of 15% aqueous sodium carbonate solution, stir and mix thoroughly, let stand to separate layers, and then use trichloromethane to dissolve the water layer. Methane extraction (70 mL x 3). The organic layers were combined, and after the chloroform was distilled off, the distillate at 120° C. (5 mmHg) was collected under reduced pressure to obtain 168 g of a colorless oily liquid, with a yield of 91%.
[0029] The synthetic formula of this step is:
[0030]
[0031] 2) Add 200g of 2-picoline nitrogen oxide into 140mL of ethanol, add 240g of sodium nitrite, then slowly drop in 81mL of a mixture of ethanol and concentrated sulfuric acid to carry out nitrosation reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com