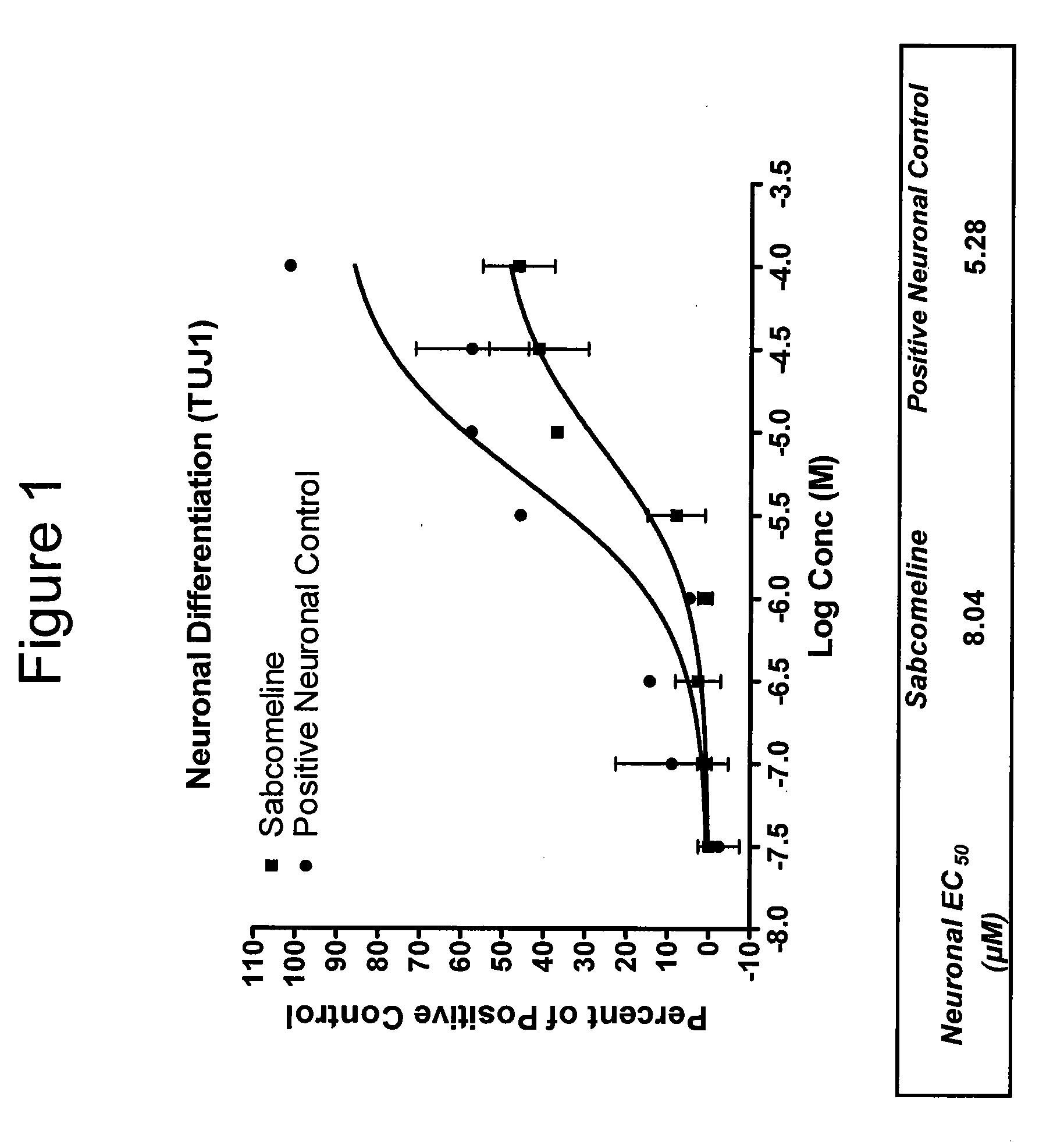
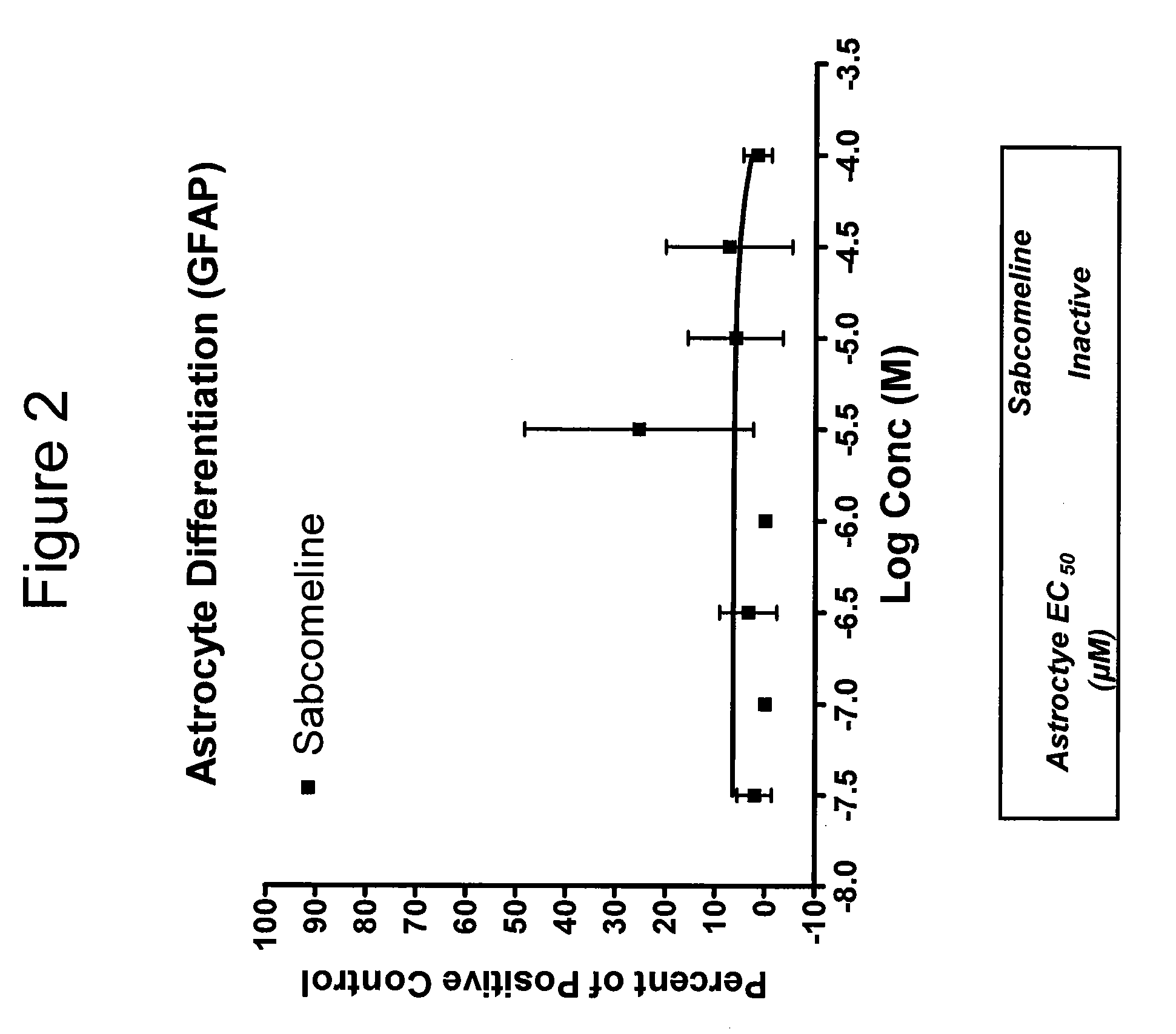
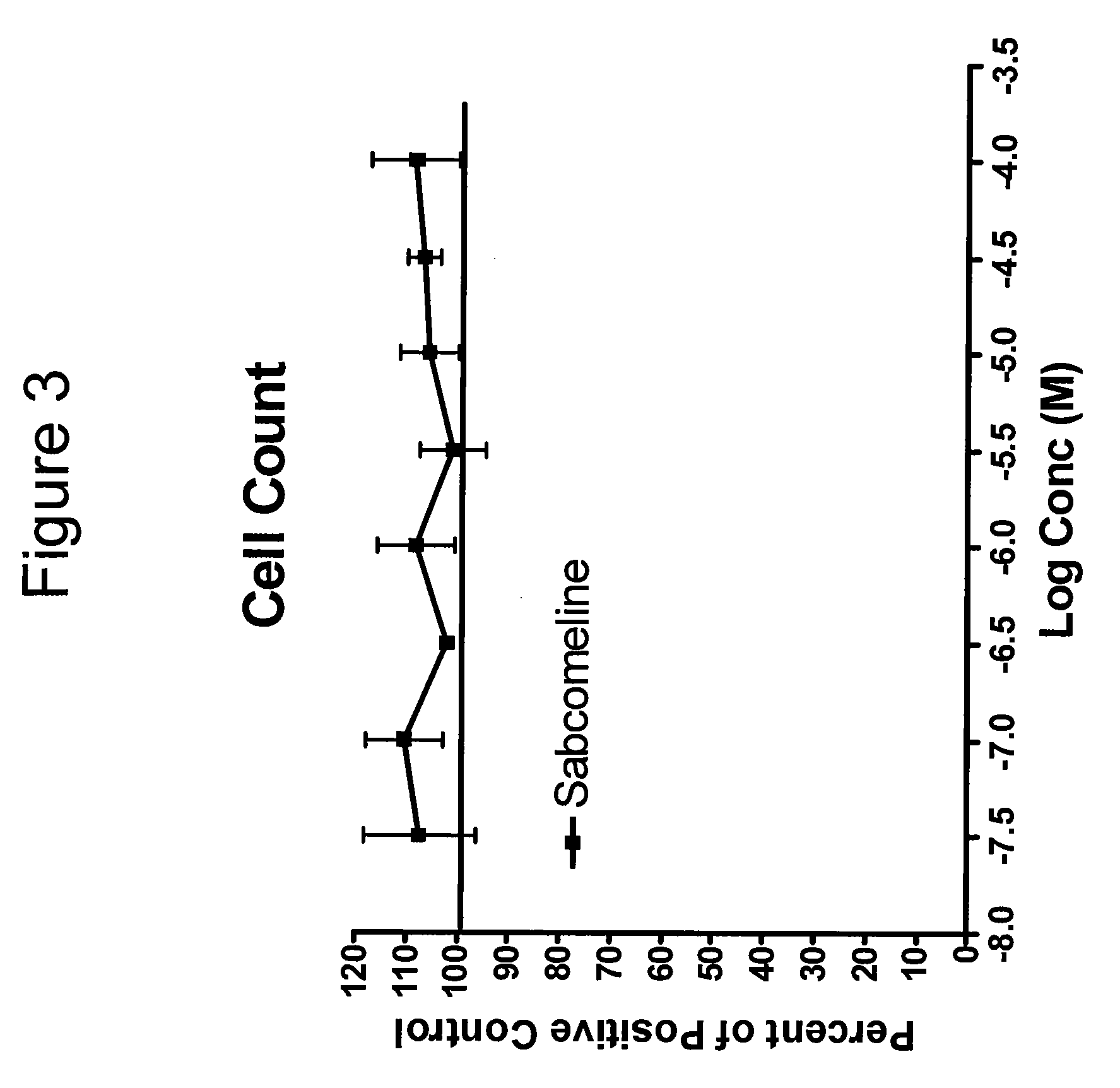
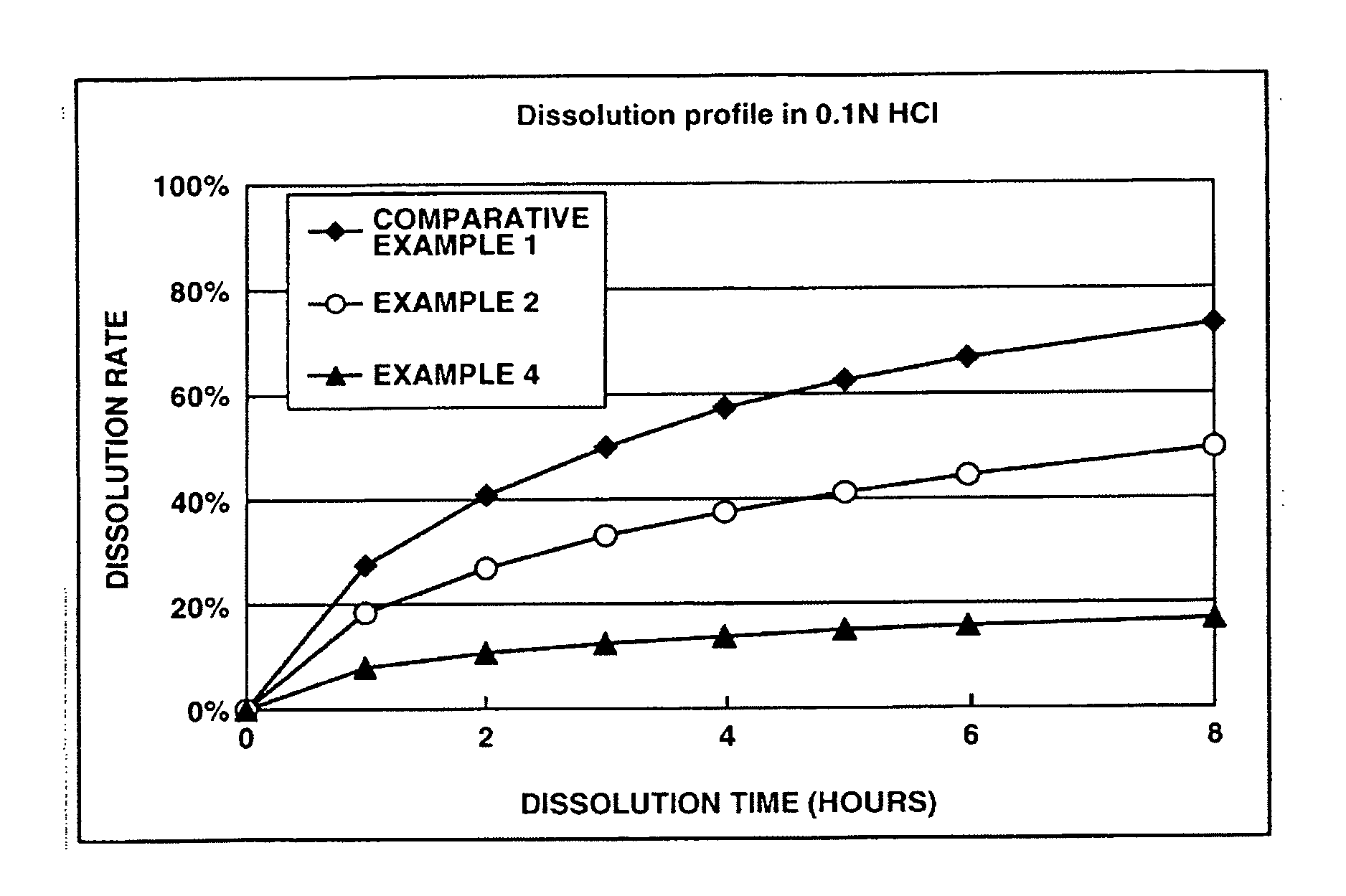
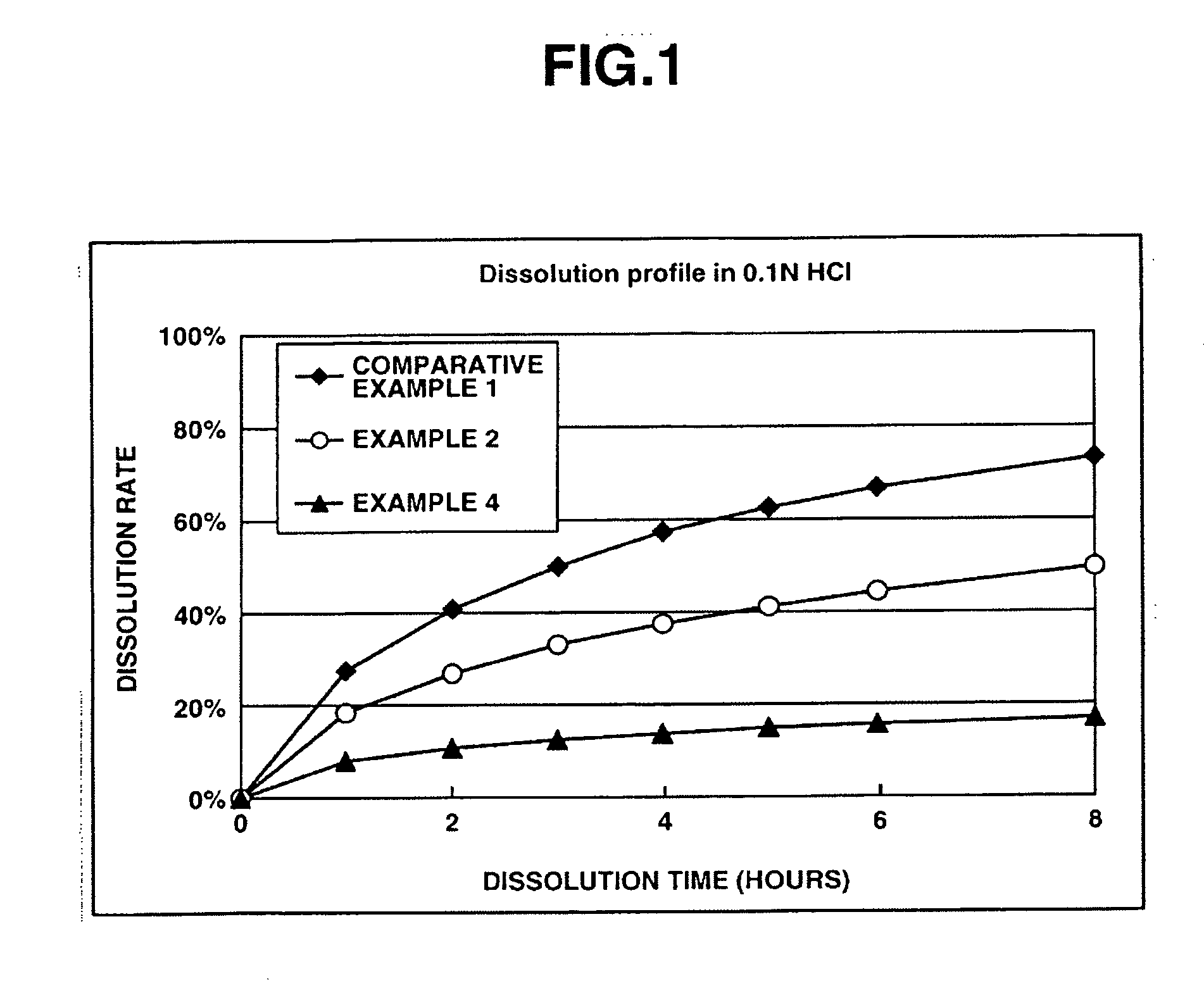
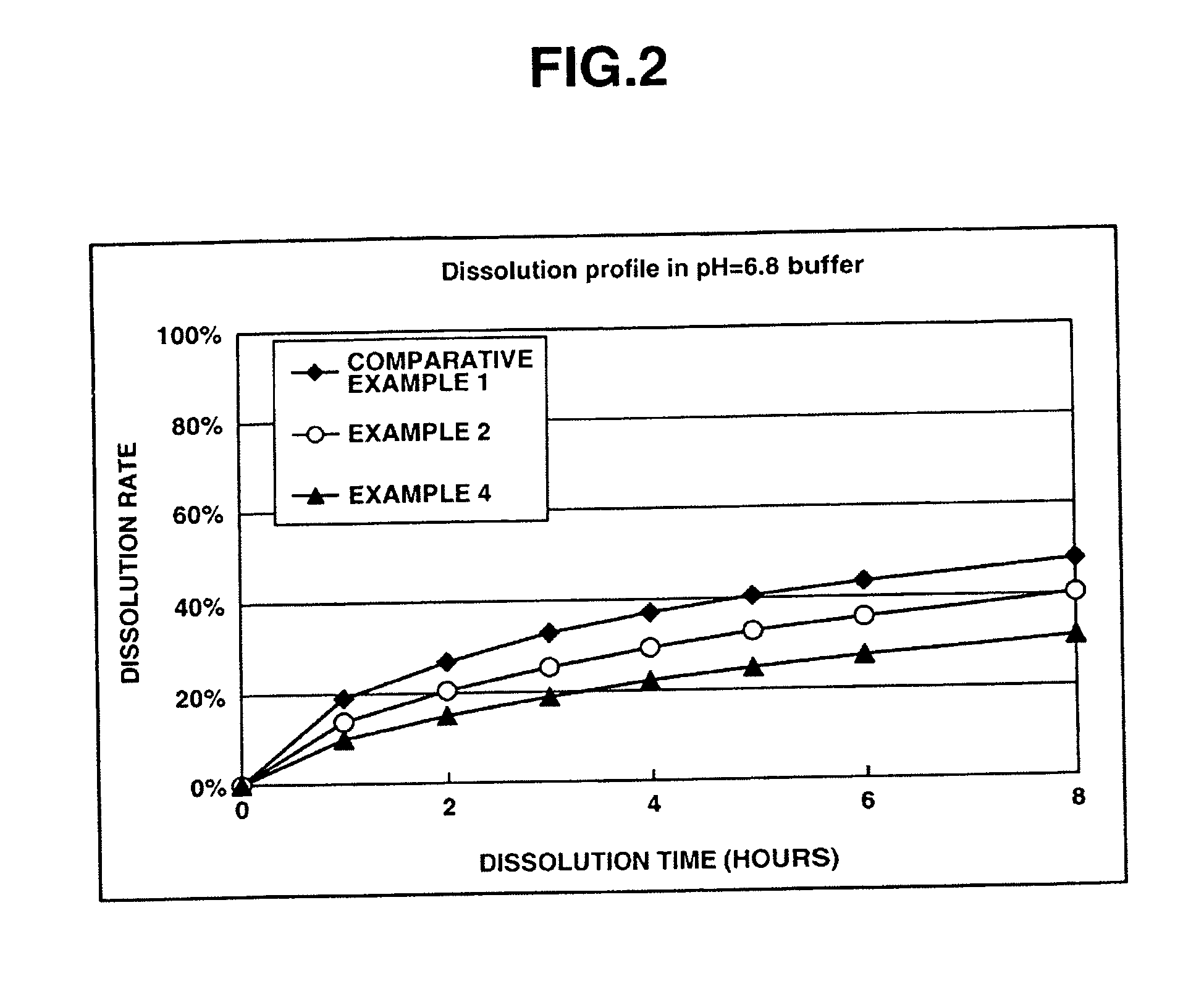
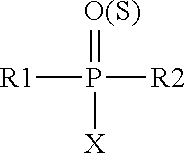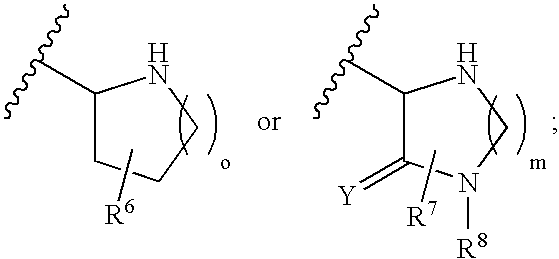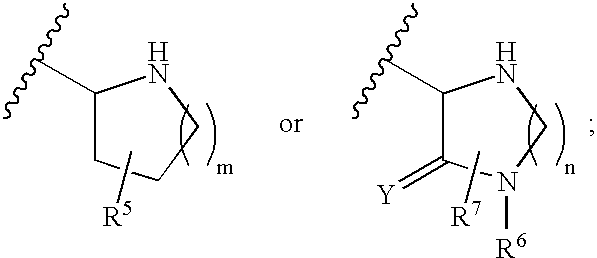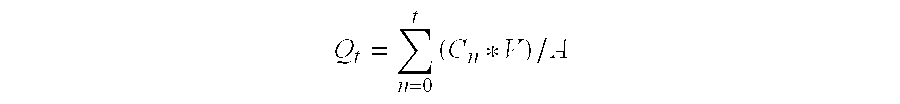Patents
Literature
763 results about "Cholinesterase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In biochemistry, a cholinesterase or choline esterase is a family of esterases that lyses choline-based esters, several of which serve as neurotransmitters. Thus, it is either of two enzymes that catalyze the hydrolysis of these cholinergic neurotransmitters, such as breaking acetylcholine into choline and acetic acid. These reactions are necessary to allow a cholinergic neuron to return to its resting state after activation. For example, in muscle contraction, acetylcholine at a neuromuscular junction triggers a contraction; but for the muscle to relax afterward, rather than remaining locked in a tense state, the acetylcholine must be broken down by a choline esterase. The main type for that purpose is acetylcholinesterase (also called choline esterase I or erythrocyte cholinesterase); it is found mainly in chemical synapses and red blood cell membranes. The other type is butyrylcholinesterase (also called choline esterase II or plasma cholinesterase); it is found mainly in the blood plasma.
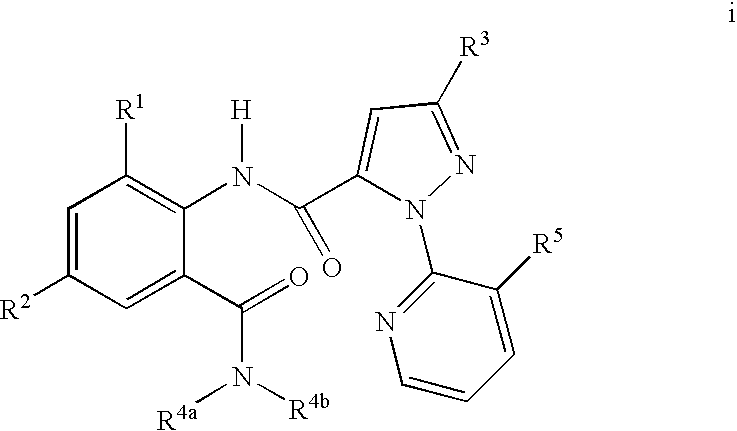
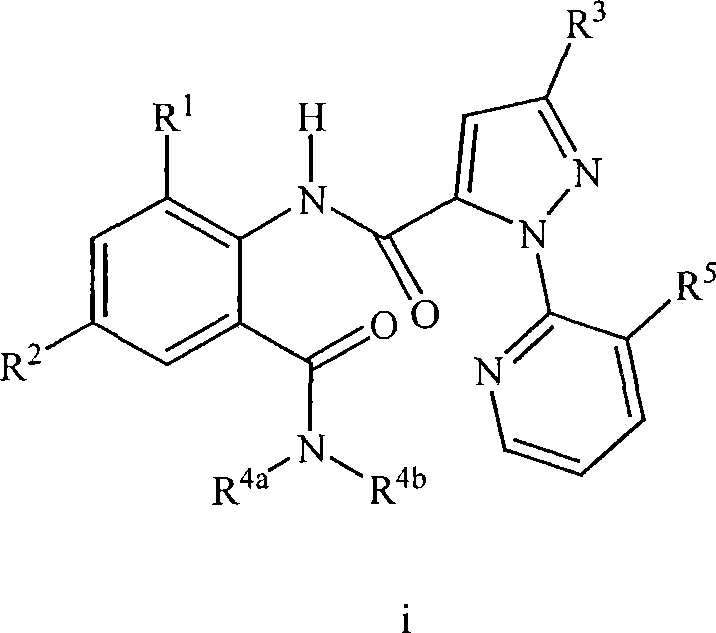
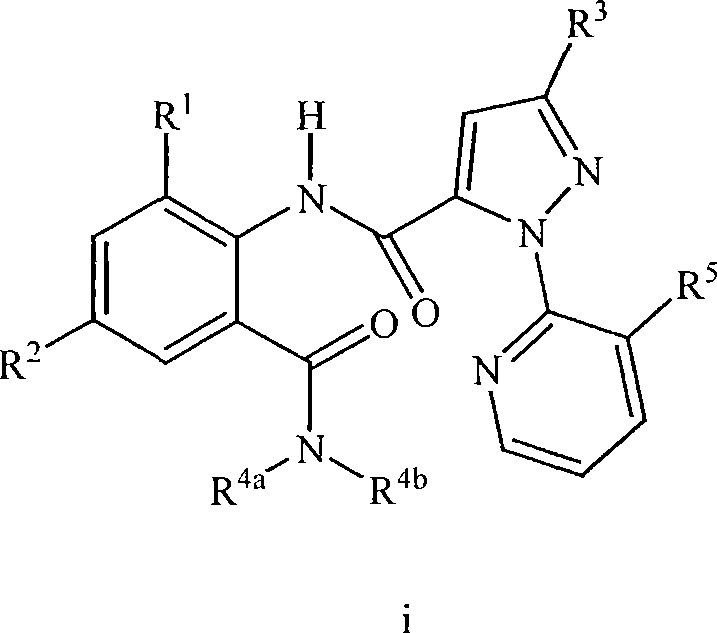
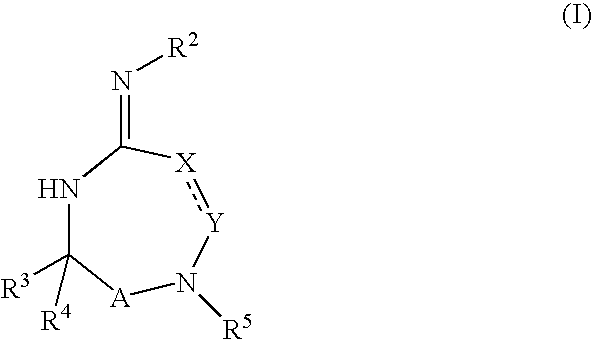
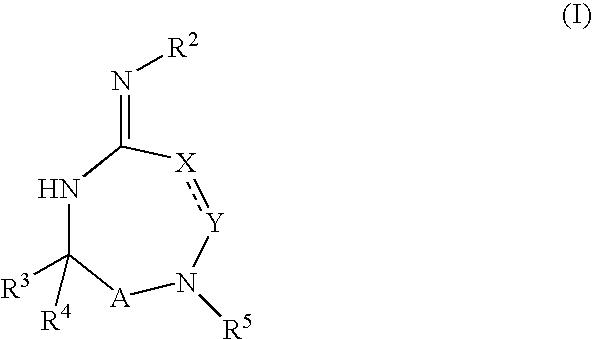
Heterocyclic aspartyl protease inhibitors
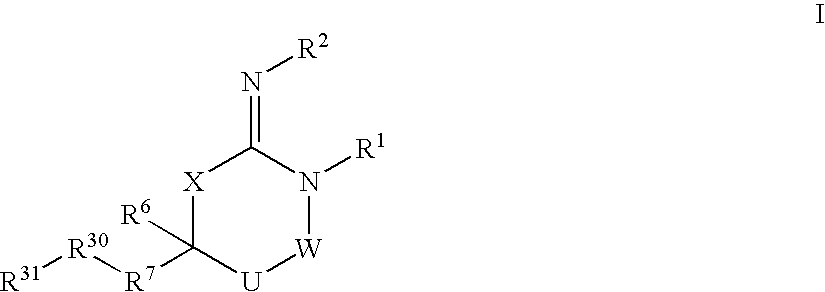
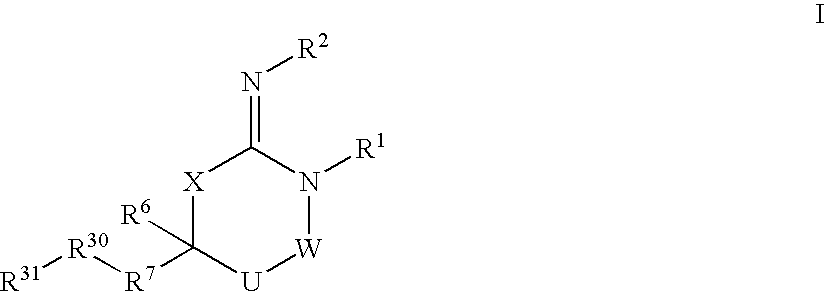
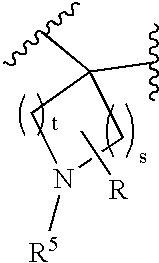
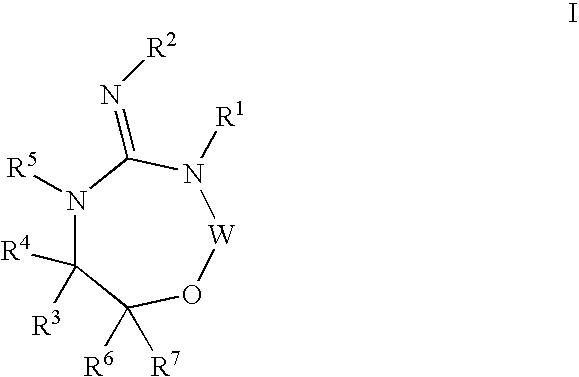
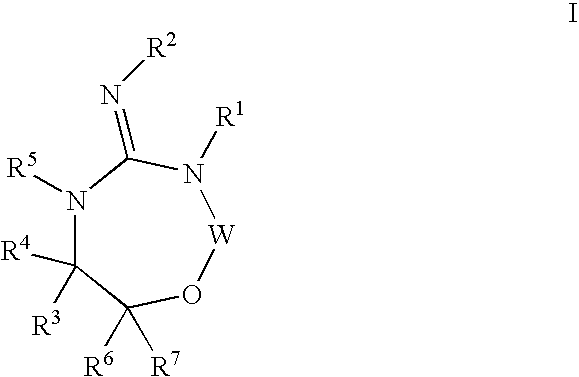
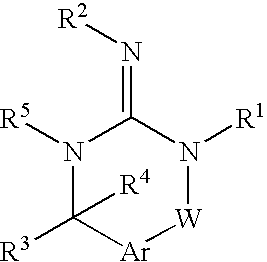
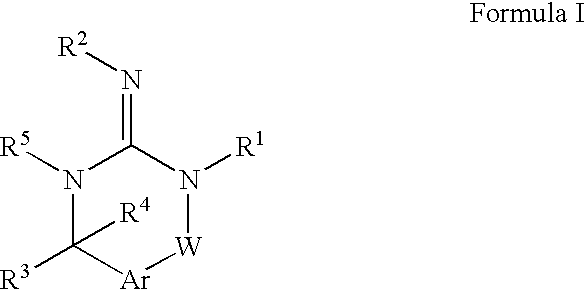
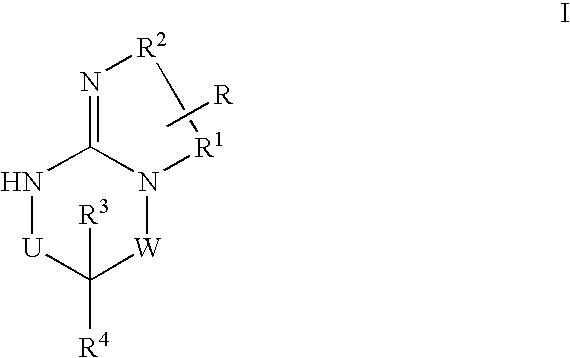
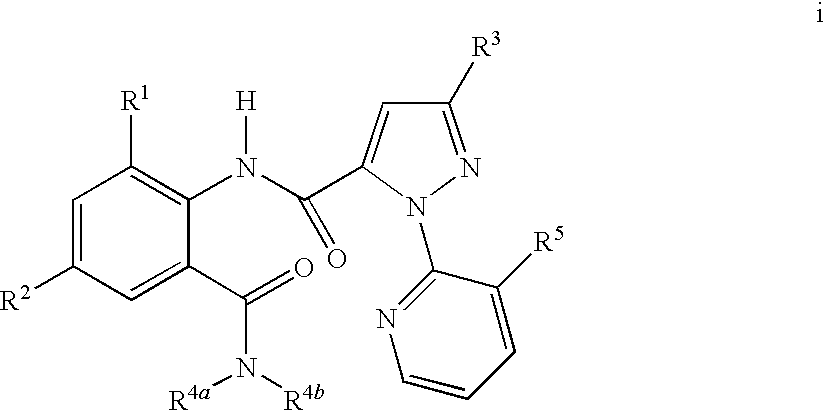
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein W is a bond, —C(═S)—, —S(O)—, —S(O)2—, —C(═O)—, —O—, —C(R6)(R7)—, —N(R5)— or —C(═N(R5))—; X is —O—, —N(R5)— or —C(R6)(R7)—; provided that when X is —O—, U is not —O—, —S(O)—, —S(O)2—, —C(═O)— or —C(═NR5)—; U is a bond, —S(O)—, —S(O)2—, —C(O)—, —O—, —P(O)(OR15)—, —C(═NR5)—, —(C(R6)(R7))b— or —N(R5)—; wherein b is 1 or 2; provided that when W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, U is not —S(O)—, —S(O)2—, —O—, or —N(R5)—; provided that when X is —N(R5)— and W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, then U is not a bond; and R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:PHARMACOPEIA DRUG DISCOVERY +1
Synergistic Mixtures of Anthranilamide Invertebrate Pest Control Agents
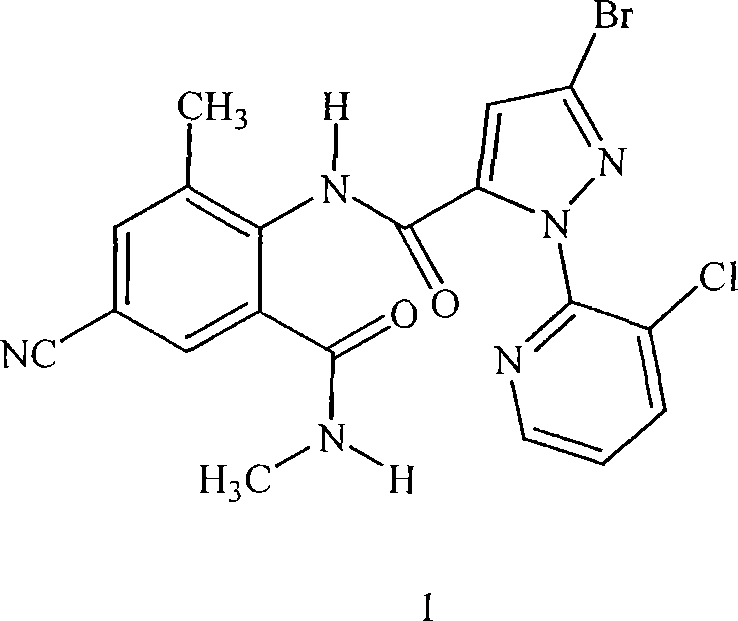
Disclosed are mixtures and compositions for controlling invertebrate pests relating to combinations comprising (a) 3-bromo-N-[4-chloro-2-methyl-6-[(methylamino)carbonyl]phenyl]-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxamide, and its N-oxides, and suitable salts thereof and a component (b) wherein the component (b) is at least one compound or agent selected from neonicotinoids, cholinesterase inhibitors, sodium channel modulators, chitin synthesis inhibitors, ecdysone agonists, lipid biosynthesis inhibitors, macrocyclic lactones, GABA-regulated chloride channel blockers, juvenile hormone mimics, ryanodine receptor ligands, octopamine receptor ligands, mitochondrial electron transport inhibitors, nereistoxin analogs, pyridalyl, flonicamid, pymetrozine, dieldrin, metaflumizone, biological agents, and suitable salts of the foregoing. Also disclosed are methods for controlling an invertebrate pest comprising contacting the invertebrate pest or its environment with a biologically effective amount of a mixture or composition of the invention.
Owner:FMC CORP
Neurogenesis by muscarinic receptor modulation
The instant disclosure describes methods for treating diseases and conditions of the central and peripheral nervous system by stimulating or increasing neurogenesis. The disclosure includes compositions and methods based on muscarinic receptor modulation, such as via inhibition of acetylcholine esterase (AChE) activity, alone or in combination with another neurogenic agent to stimulate or activate the formation of new nerve cells.
Owner:BRAINCELLS INC
Aspartyl protease inhibitors
Disclosed are compounds of formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, U, W, X, R1, R2, R6, R7, R30 and R31 are as described above in the specification. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:MERCK SHARP & DOHME LLC
Aspartyl protease inhibitors
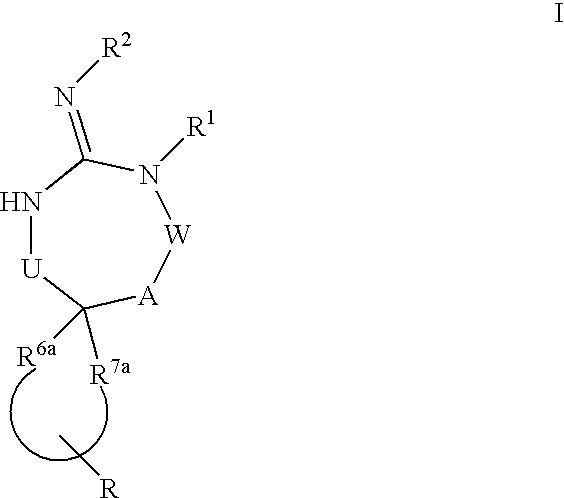
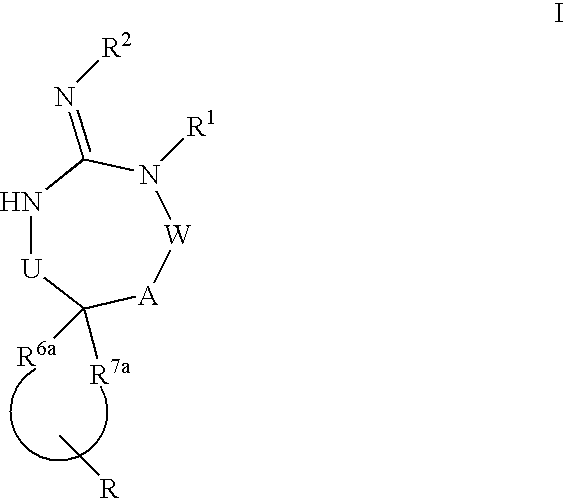
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein U, W, A, R, R1, R2, R6a and R7, are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:MERCK SHARP & DOHME LLC +1
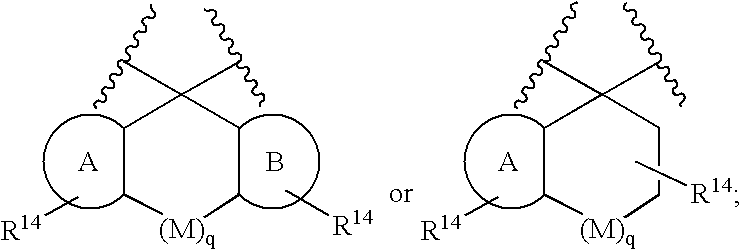
Macrocyclic heterocyclic aspartyl protease inhibitors
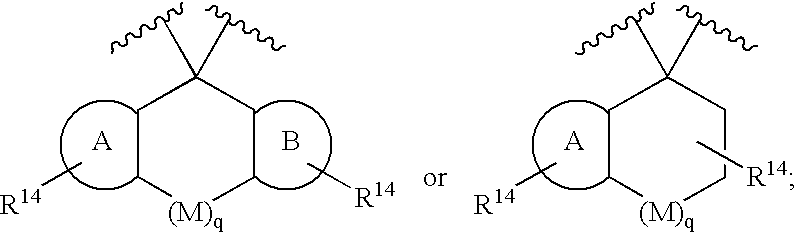
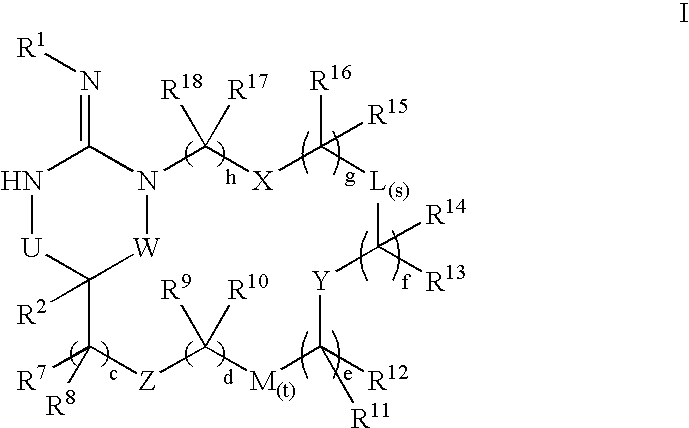
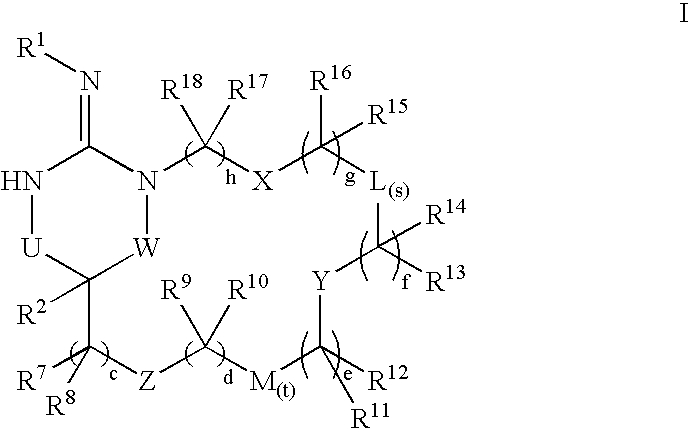
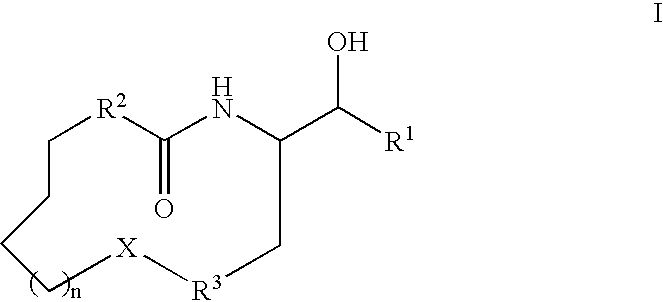
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt, solvate or ester thereof, wherein U, W, X, L, Y, M, Z, c, d, e, f, g, h, s, t, R1, R2, R7, R8, R9, R10, R11, R12, R13, R14, R15, R16, R17 and R18 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:MERCK SHARP & DOHME LLC
Aspartyl protease inhibitors
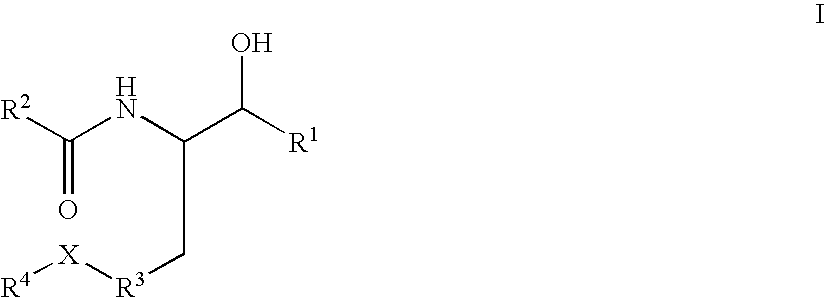
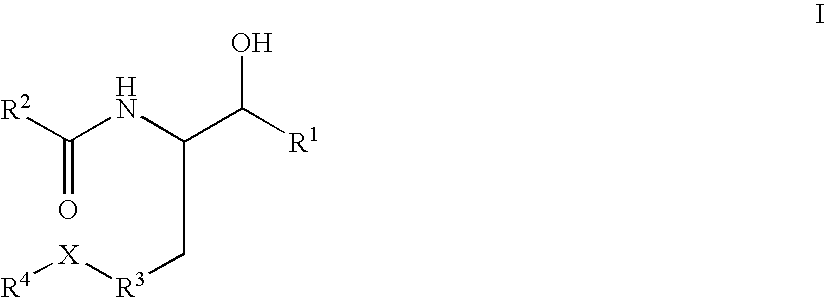
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein W, R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic antagonist.
Owner:SCHERING CORP
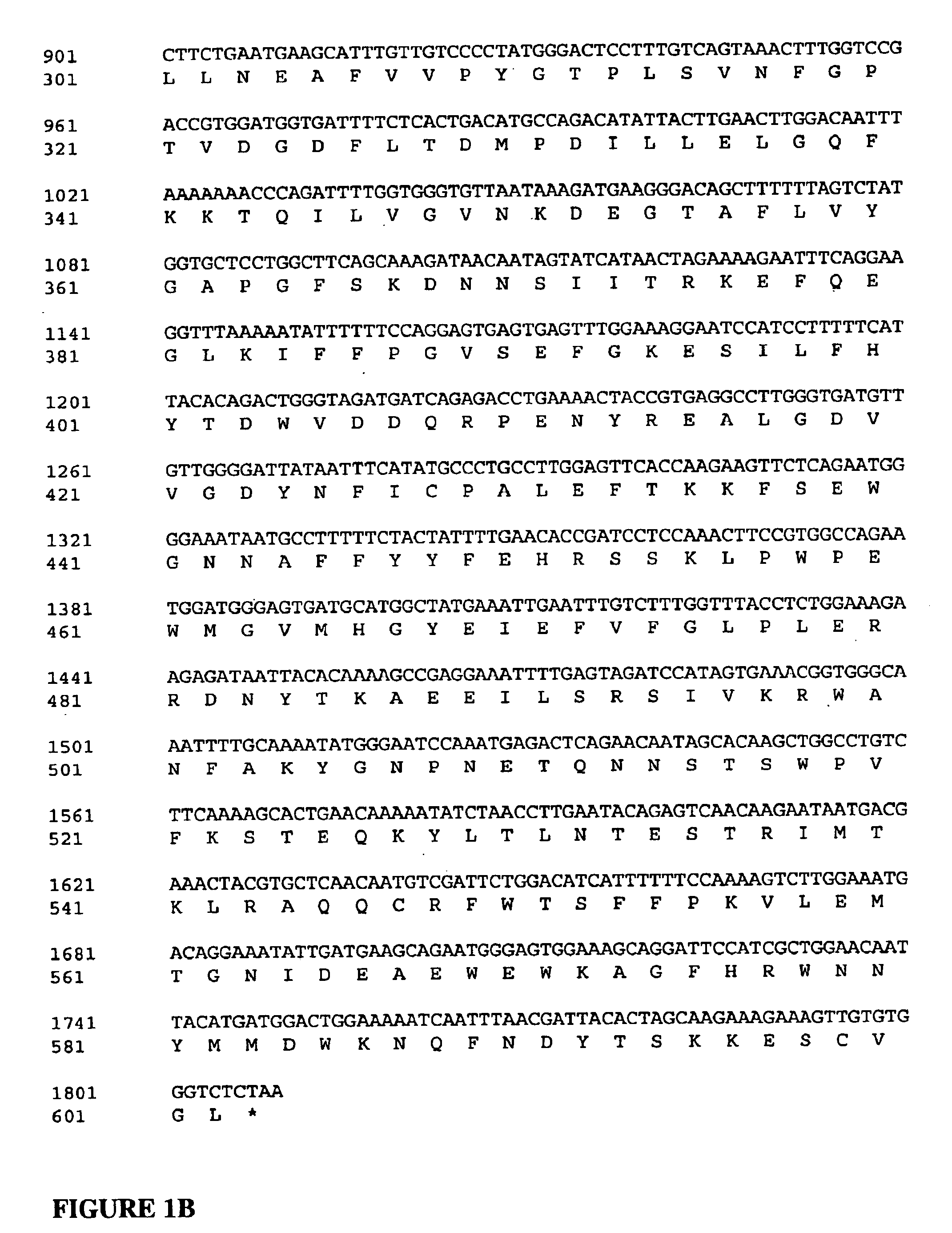
Production of hSA-linked butyrylcholinesterases in transgenic mammals
InactiveUS20060253913A1Promote formationImprove stabilityAnimal cellsVectorsMammalButyrylcholinesterase
The present invention provides methods for the large-scale production of recombinant butyrylcholinesterase fused to human serum albumin in cell culture, and in the milk and / or urine of transgenic mammals. The recombinant butyrylcholinesterase-albumin fusion protein of this invention can be used to treat and / or prevent organophosphate pesticide poisoning, nerve gas poisoning, cocaine intoxication, and succinylcholine-induced apnea.
Owner:PHARMATHENE
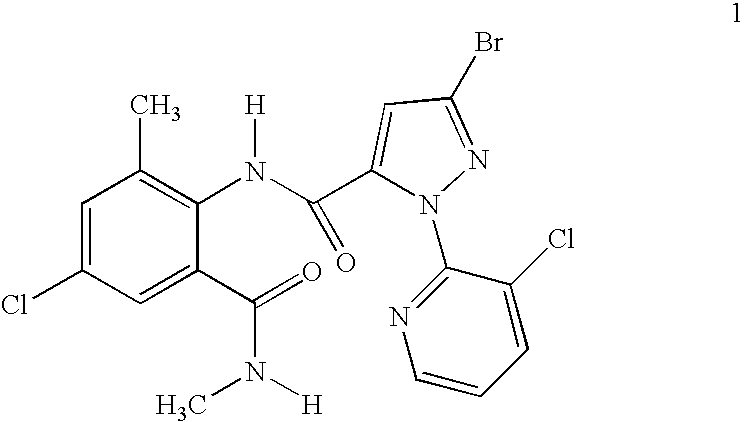
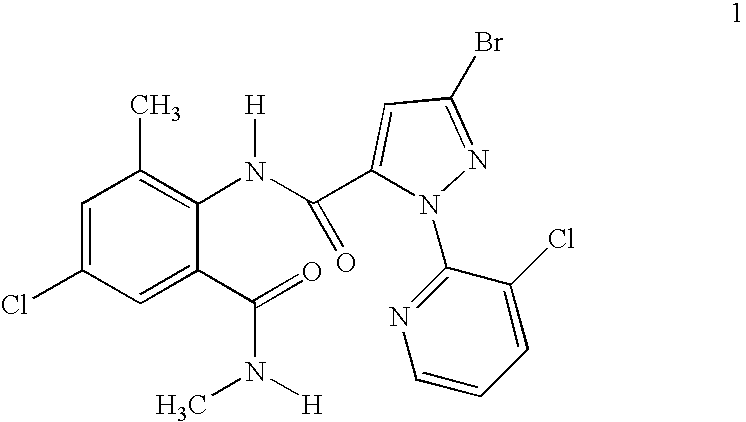
Mixtures of anthranilamide invertebrate pest control agents
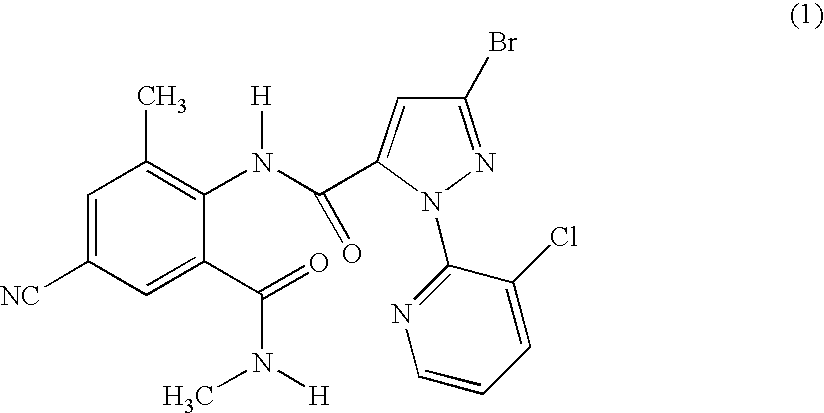
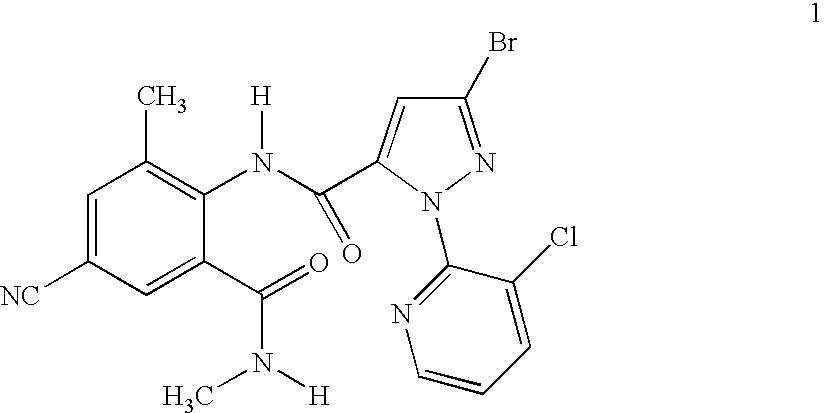
Disclosed are mixtures and compositions for controlling invertebrate pests relating to combinations comprising (a) 3-bromo-N-[4-cyano-2-methyl-6[(methylamino)carbonyl]phenyl]-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxamide, an N-oxide, or a salt thereof, Formula (1) and (b) at least one invertebrate pest control agent selected from neonicotinoids, cholinesterase inhibitors, sodium channel modulators, chitin synthesis inhibitors, ecdysone agonists, lipid biosynthesis inhibitors, macrocyclic lactones, GABA-regulated chloride channel blockers, juvenile hormone mimics, ryanodine receptor ligands, octopamine receptor ligands, mitochondrial electron transport inhibitors, nereistoxin analogs, pyridalyl, flonicamid, pymetrozine, dieldrin, metaflumizone, biological agents, and salts of the foregoing. Also disclosed are methods for controlling an invertebrate pest comprising contacting the invertebrate pest or its environment with a biologically effective amount of a mixture or composition of the invention.
Owner:FMC AGRO SINGAPORE PTE LTD +1
Heterocyclic aspartyl protease inhibitors
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein Ar, R1, R2, R3, R4 and R5 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:MERCK SHARP & DOHME LLC
Aspartyl protease inhibitors
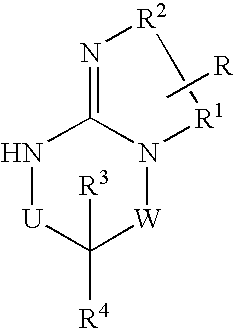
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein U, W, R, R1, R2, R3 and R4 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:MERCK SHARP & DOHME LLC
Treatment of diseases with combinations of alpha 7 Nicotinic Acetylcholine Receptor agonists and other compounds
The present invention relates to compositions and methods to treat diseases or condition with an α7 nAChR full agonist and an inhibitor of cholinesterase, and or beta secretase and or gamma secretase.
Owner:CORBETT JEFFREY W +1
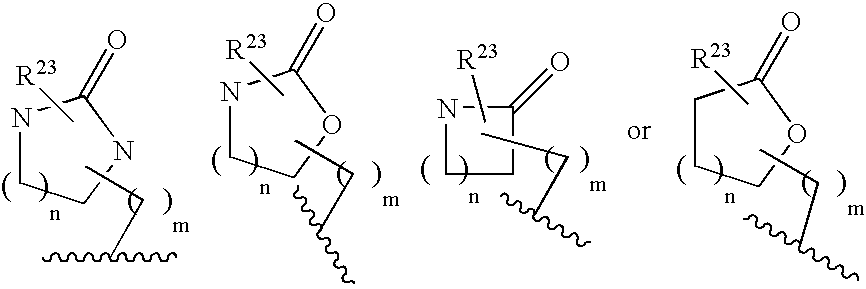
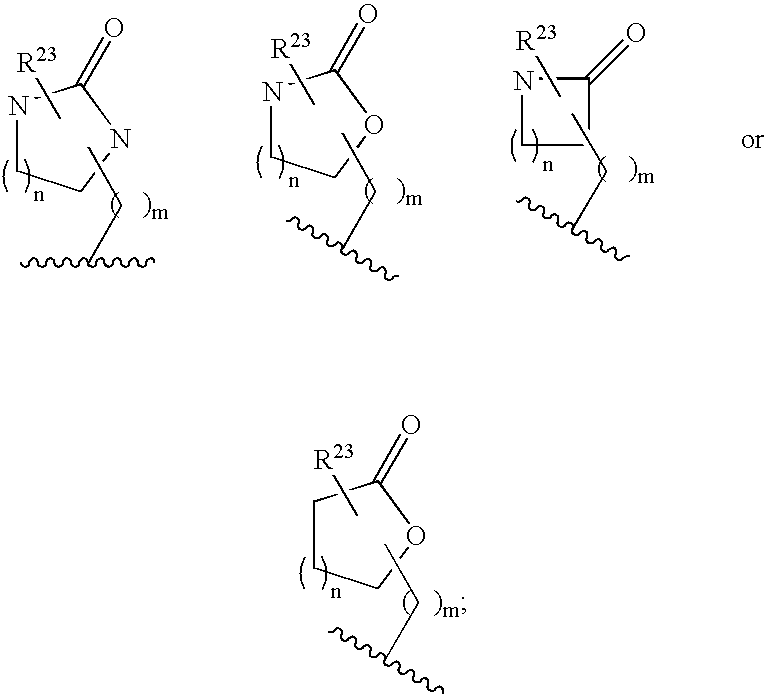
Cyclic amine bace-1 inhibitors having a benzamide substituent
ActiveUS20050119227A1Inhibition formationBiocideNervous disorderHMG-CoA reductaseNeuro-degenerative disease
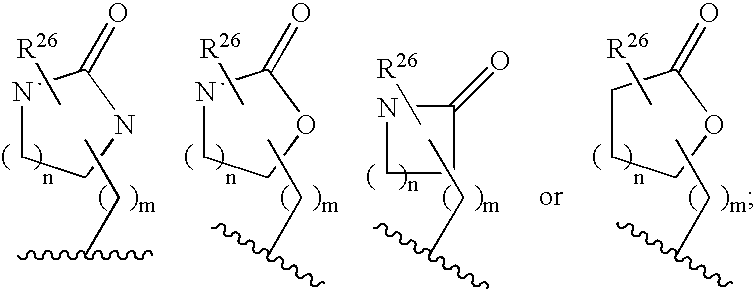
Disclosed are compounds of the formula or a pharmaceutically acceptable salt or solvate thereof, wherein [0001]R1 is [0002]R is —C(O)—N(R27)(R28) or and the remaining variables are as defined in the specification. Also disclosed are pharmaceutical compositions comprising the compounds of formula I. Also disclosed are methods of treating cognitive or neurodegenerative diseases such as Alzheimer's disease. Also disclosed are pharmaceutical compositions and methods of treating cognitive or neurodegenerative diseases comprising the compounds of formula I in combination with a β-secretase inhibitor other than those of formula I, an HMG-CoA reductase inhibitor, a gamma-secretase inhibitor, a non-steroidal anti-inflammatory agent, an N-methyl-D-aspartate receptor antagonist, a cholinesterase inhibitor or an anti-amyloid antibody.
Owner:PHARMACOPEIA DRUG DISCOVERY +1
Preparations and methods for ameliorating or reducing presbyopia
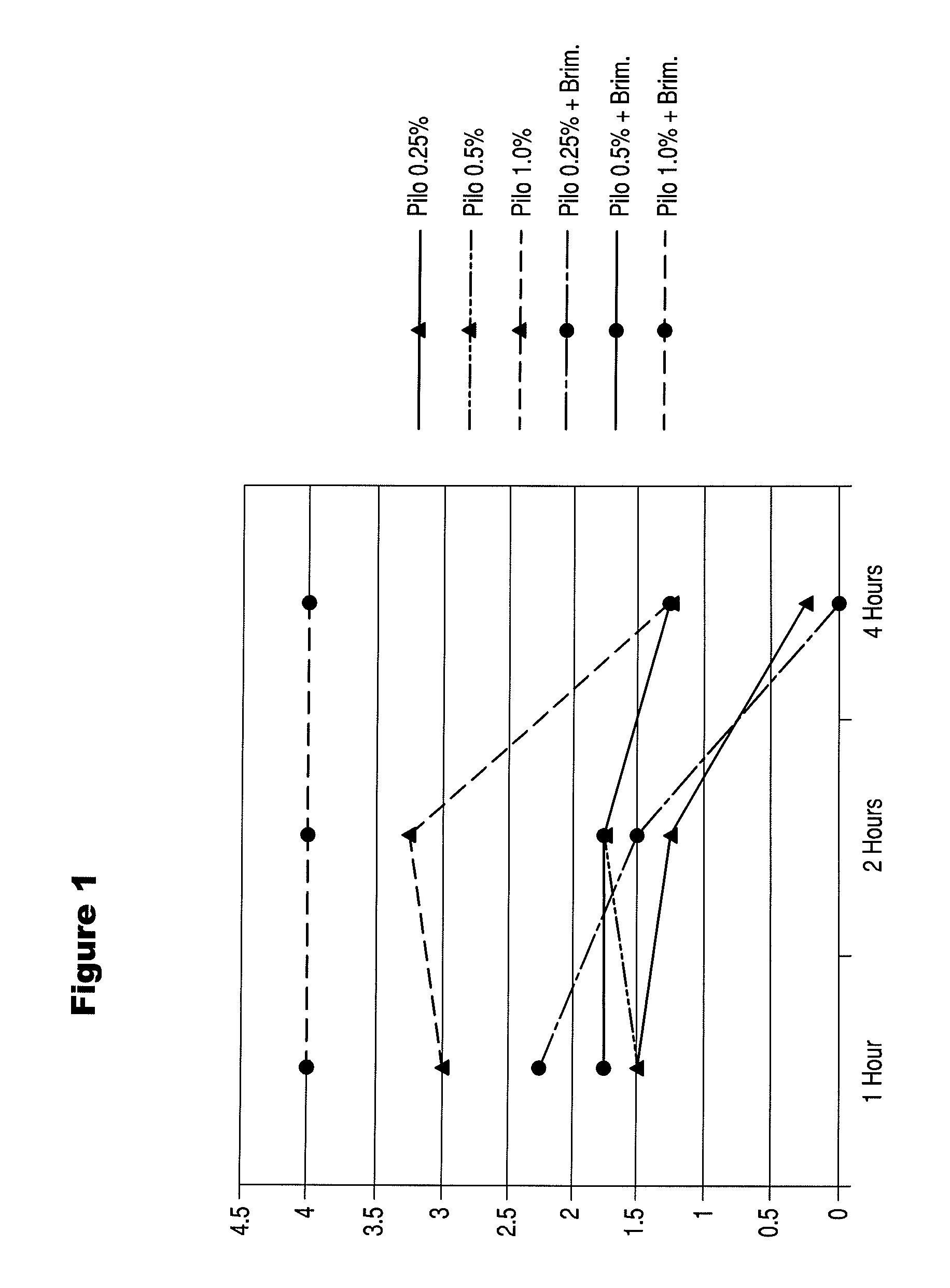
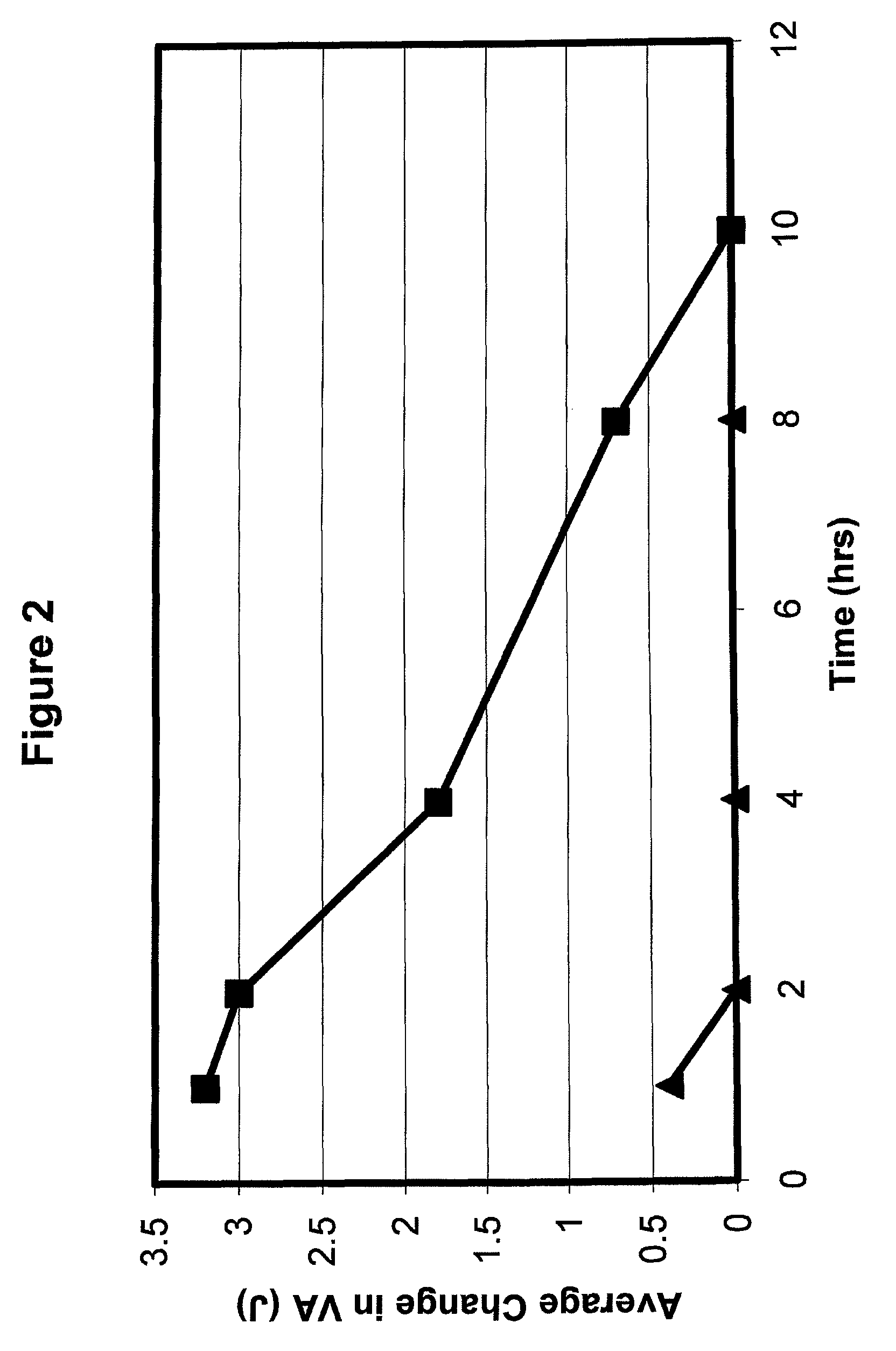
This application relates to the use of one or more parasympathomimetic drugs in combination with one or more alpha agonists to create optically beneficial miosis to, for example, temporarily treat presbyopia. The invention provides a pharmaceutical preparation comprising a therapeutically effective amount of one or more parasympathomimetic drugs or cholinesterase inhibitors, or a pharmaceutically acceptable salt thereof, in combination with one or more alpha agonists or antagonists, or a pharmaceutically acceptable salt thereof. The invention further provides for a method for treating, ameliorating or reducing presbyopia of a patient having an eye, comprising administering to said eye a pharmaceutically effective amount of the ophthalmic preparation.
Owner:VISUS THERAPEUTICS INC
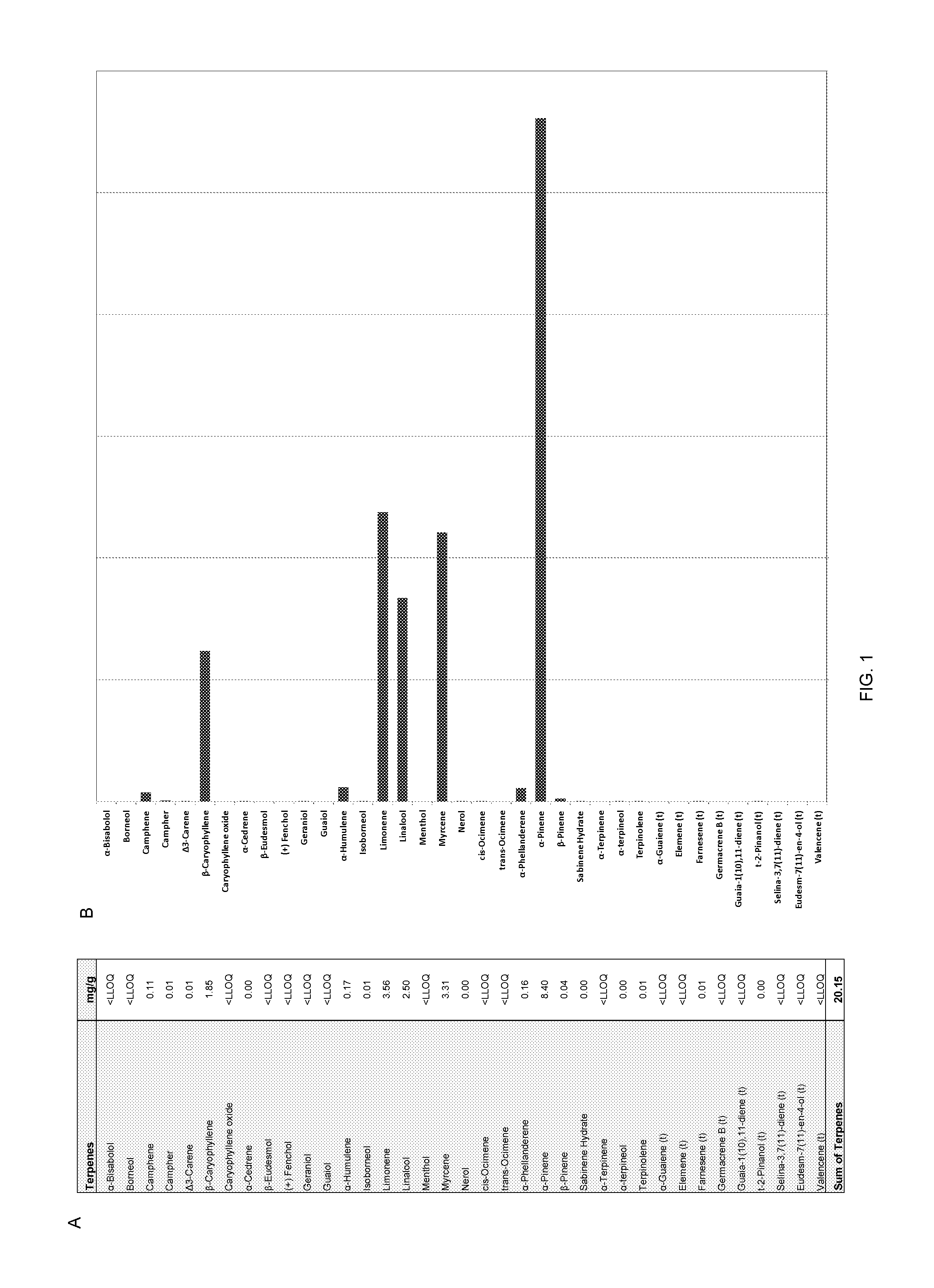
Use of cannabinoids and terpenes for treatment of organophosphate and carbamate toxicity
InactiveUS20150313868A1Prevent crashExtended duration of actionBiocideHydrocarbon active ingredientsDiseaseCarbamate
Pharmaceutical compositions in which isolated cannabinoid receptor modulators are optionally combined with terpene blends in a pharmaceutically acceptable carrier. Methods for treating or preventing a disease, disorder, dysfunction or condition caused by exposure to an organophosphate or carbamate acetylcholineesterase inhibitor with the inventive compositions are also disclosed.
Owner:KOTZKER CONSULTING
Large-Scale Production of Human Serum Butyrylcholinesterase as a Bioscavenger
InactiveUS20070184045A1Treating and preventing and inhibiting toxicityPeptide/protein ingredientsHydrolasesCholinesteraseButyrylcholinesterase
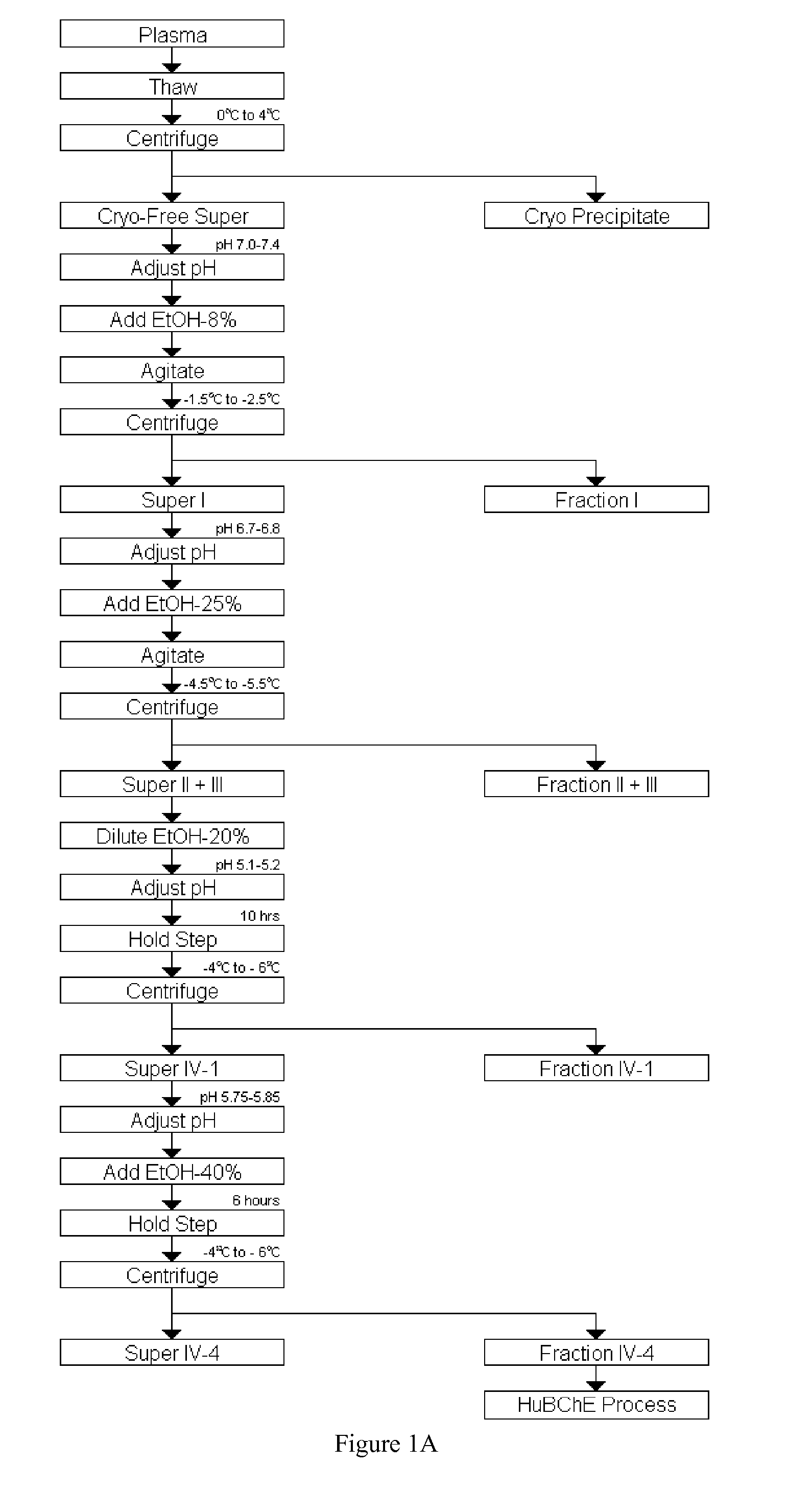
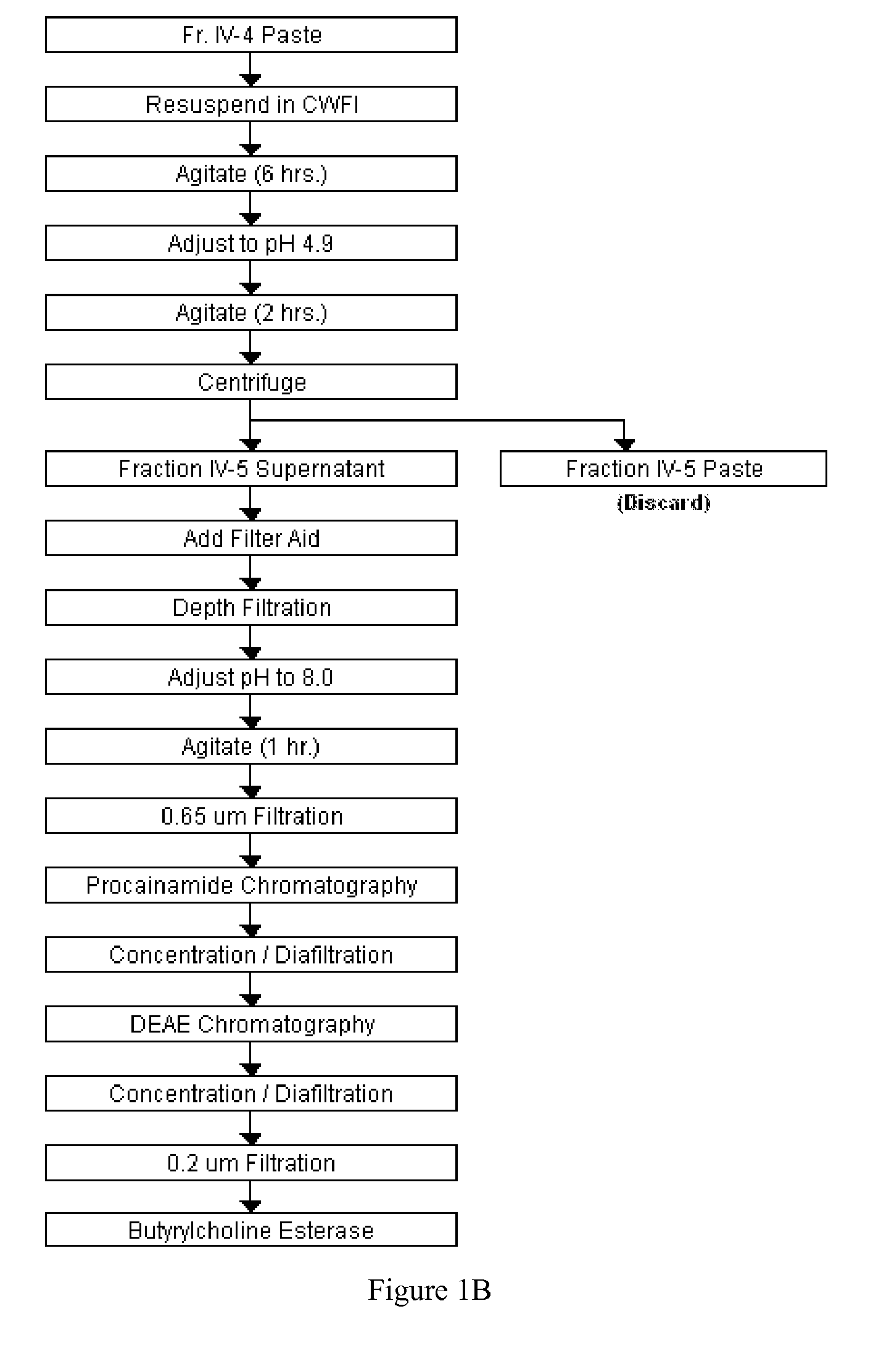
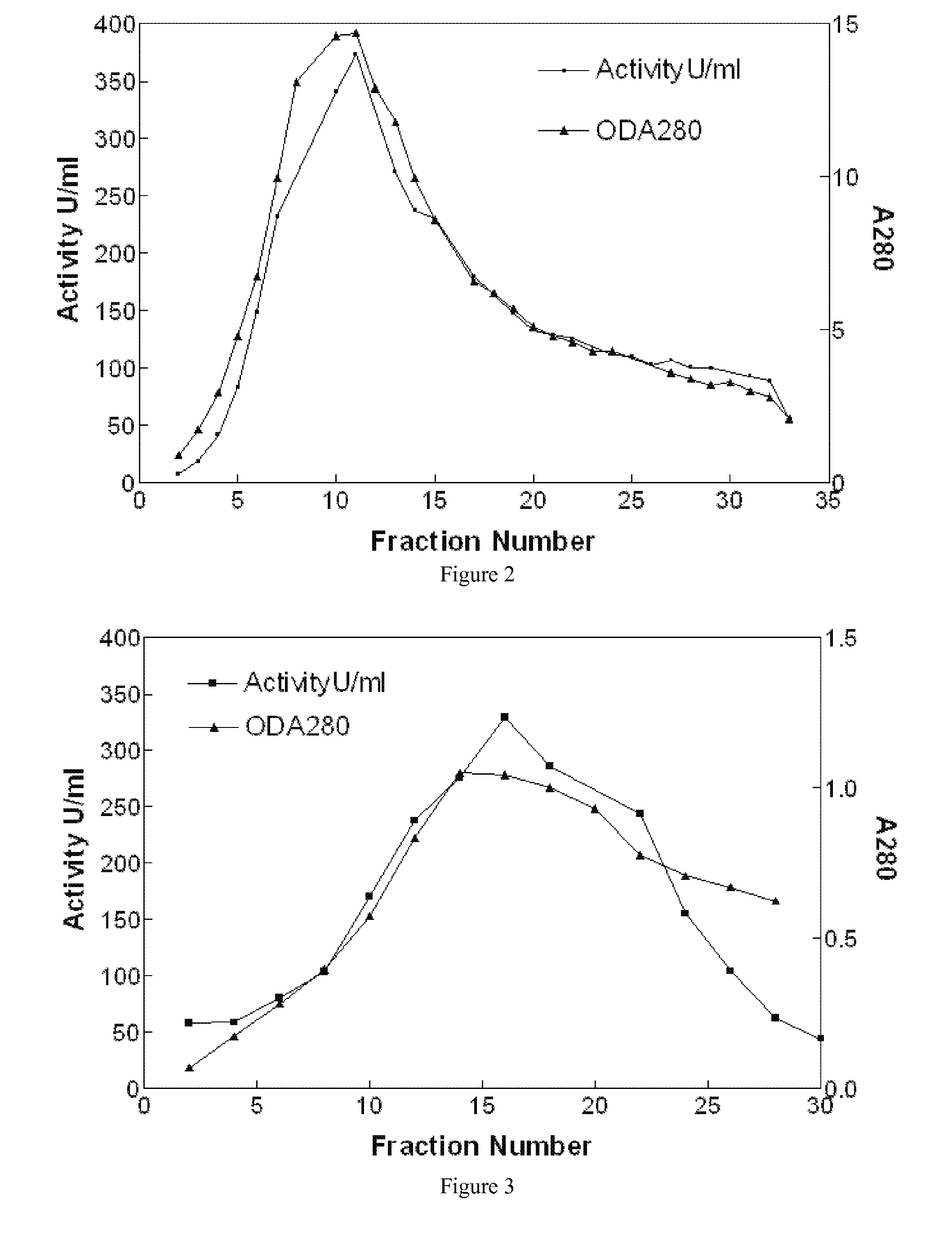
Disclosed herein are methods for the large-scale preparation of human butyrylcholinesterase (HuBChE) preparations from Cohn Fraction IV-4. As disclosed, the methods provide HuBChE preparations that are about 99% or more pure with recovery yields of about 60%. Also disclosed are the pharmacokinetics, safety and toxicity, stability and efficacy of the HuBChE preparations.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
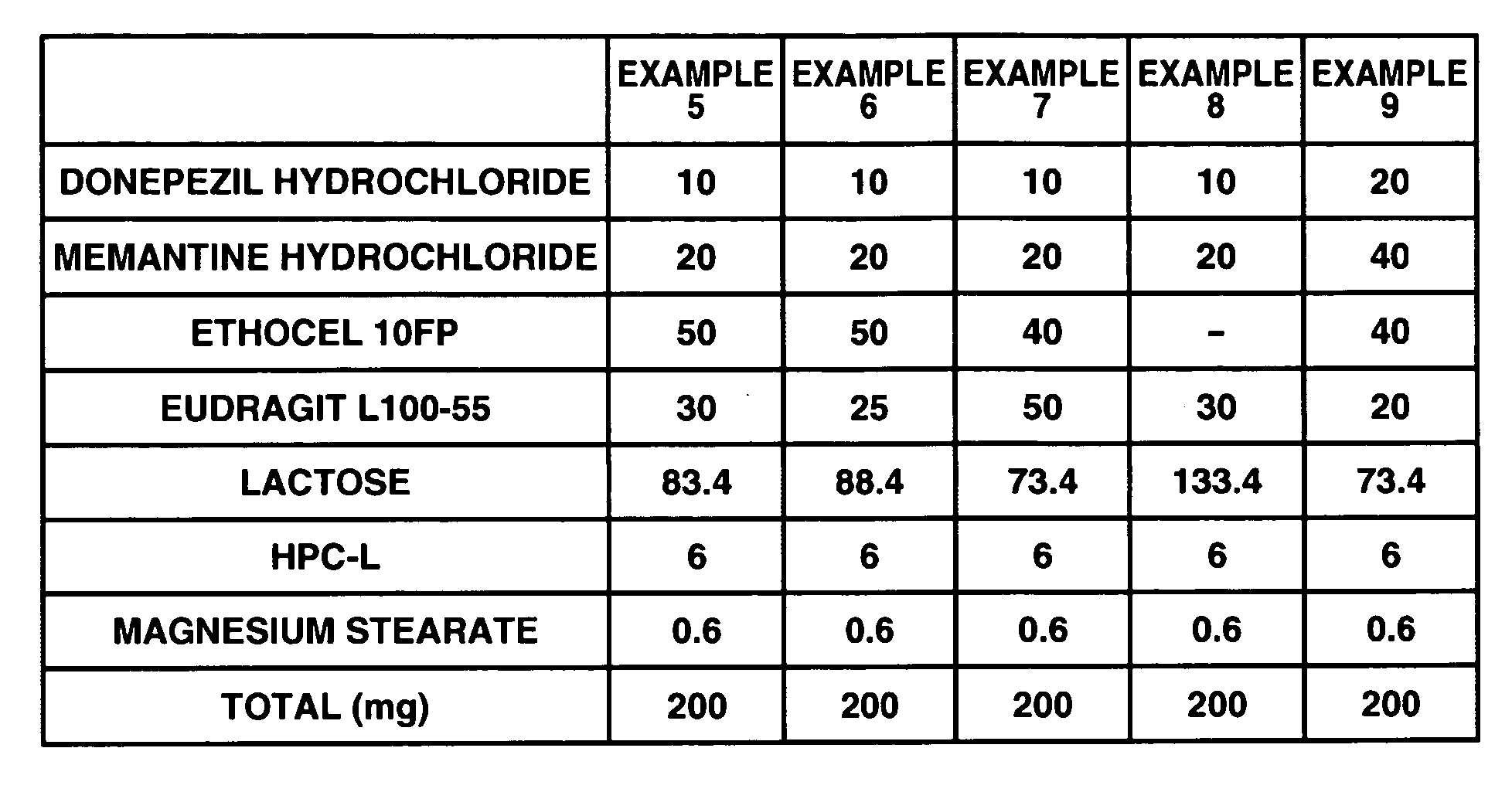
Sustained release formulations
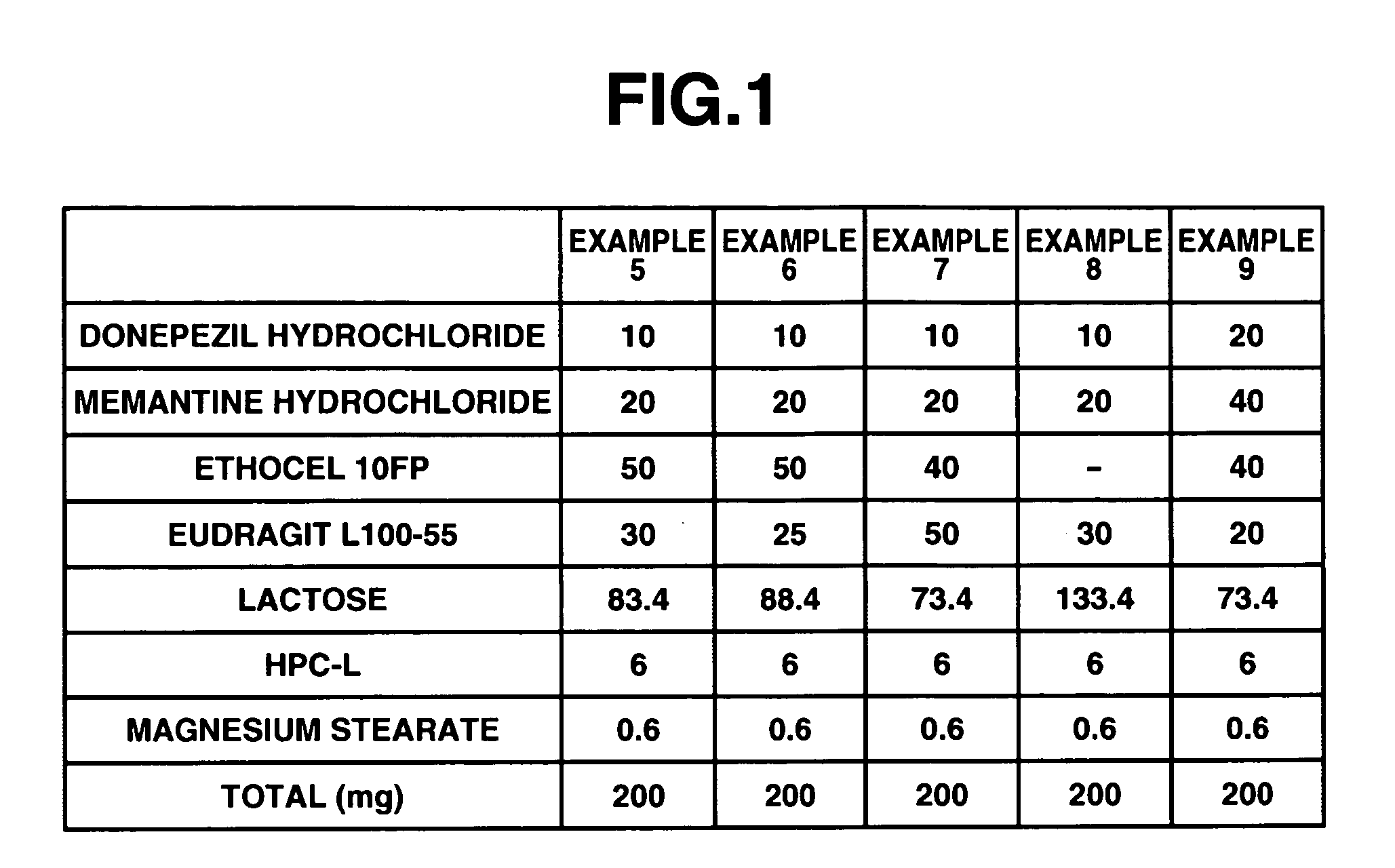
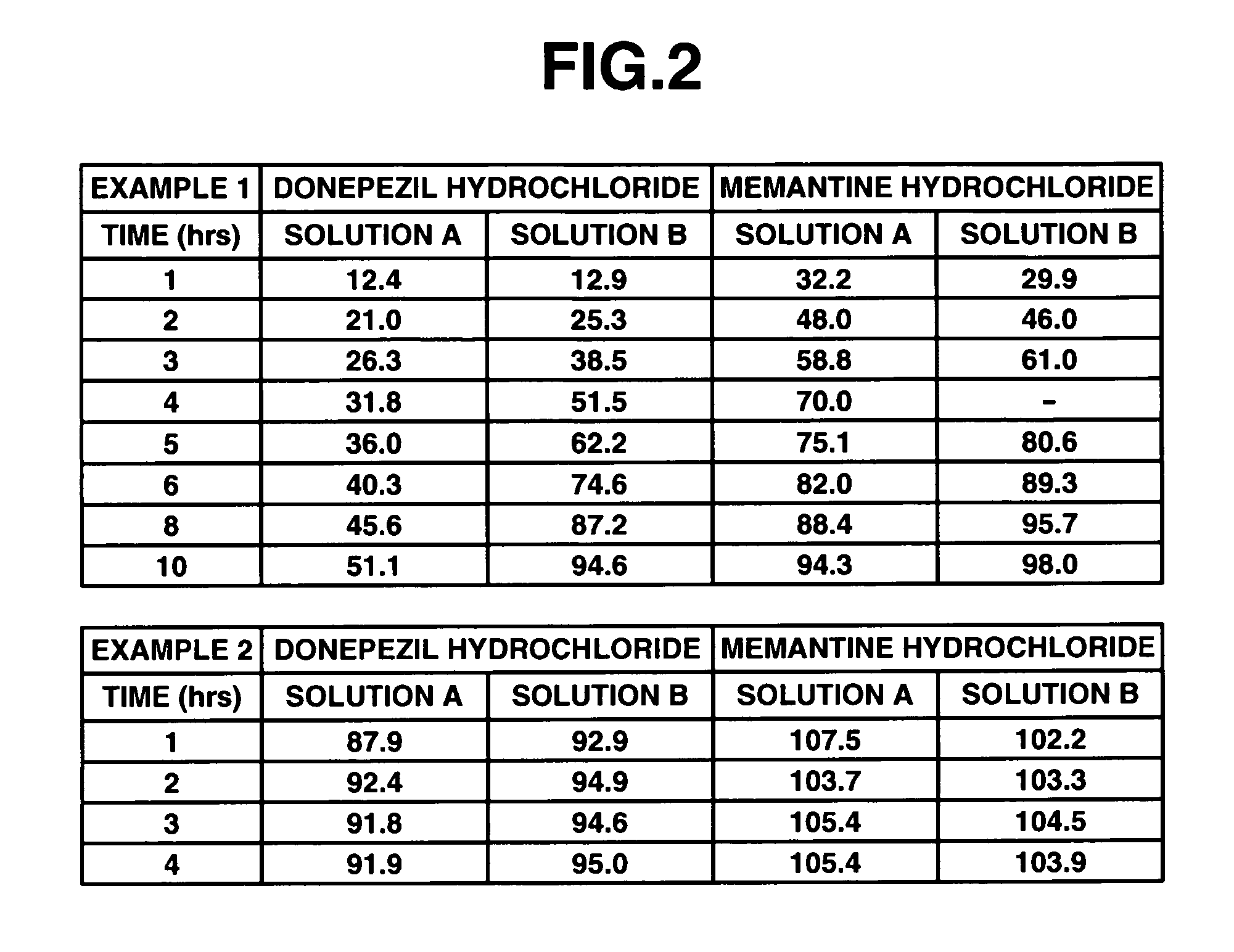
InactiveUS20060280789A1Reduce in quantityImprove complianceBiocidePill deliveryDonepezilCholinesterase
The invention provides sustained release formulations of basic drugs, stereoisomers of basic drugs, pharmaceutically acceptable salts of basic drugs, and pharmaceutically acceptable salts of stereoisomers of basic drugs. The basic drugs may be anti-dementia drugs, such as cholinesterase inhibitors or memantine. In one embodiment, the cholinesterase inhibitor is donepezil.
Owner:EISAI CO LTD
New uses for quaternary ammonium anticholinergic muscarinic receptor antagonists in patients being treated for cognitive impairment or acute delirium
ActiveUS20080114014A1Maximizing beneficial effectMaximize the effectBiocideNervous disorderSolubilityFecal incontinence
A method for treating the adverse effects of acetyl-cholinesterase inhibitors used in the treatment of cognitive disorders such as acute delirium and cognitive impairment in elderly human patients. The administration of a clinically effective amount of a quaternary ammonium anti-cholinergic muscarinic receptor antagonist having very low lipid solubility substantially eliminates the adverse effects of urinary and / or fecal incontinence, nausea, bradycardia, bronchorrhea or brochospasm caused by the acetyl-cholinesterase inhibitors, without affecting the beneficial activity of the acetyl-cholinesterase inhibitors. This permits the administration of the optimum effective dosing of acetyl-cholinesterase inhibitors to provide maximum benefit to the patient with the added benefit of reducing or eliminating the unwanted side effects of fecal and urinary incontinence. Further, the combination of rivastigmine and glycopyrrolate has been effective in significantly improving cognitive function in patients suffering from acute dementia or cognitive impairment.
Owner:QAAM PHARMA LLC
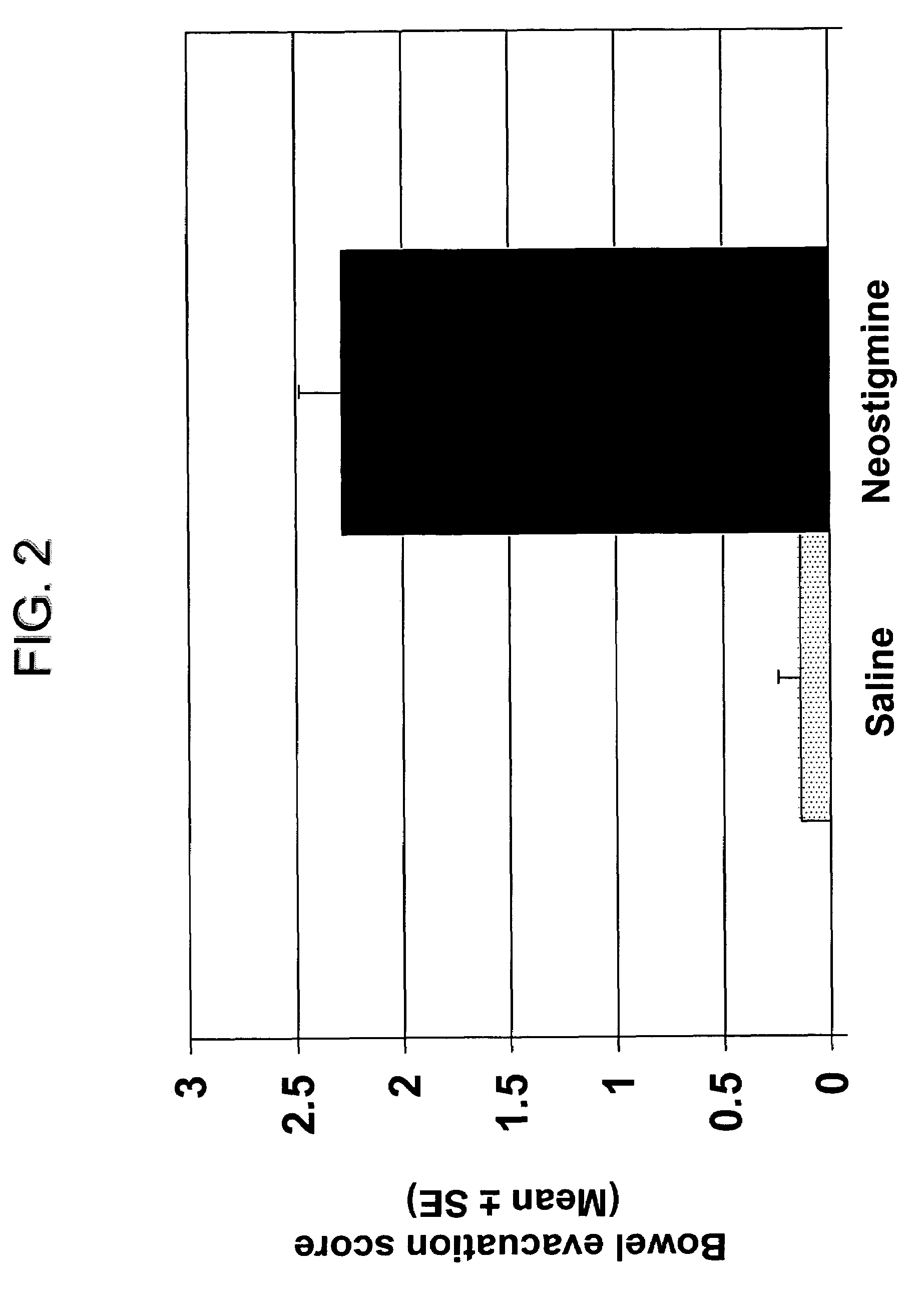
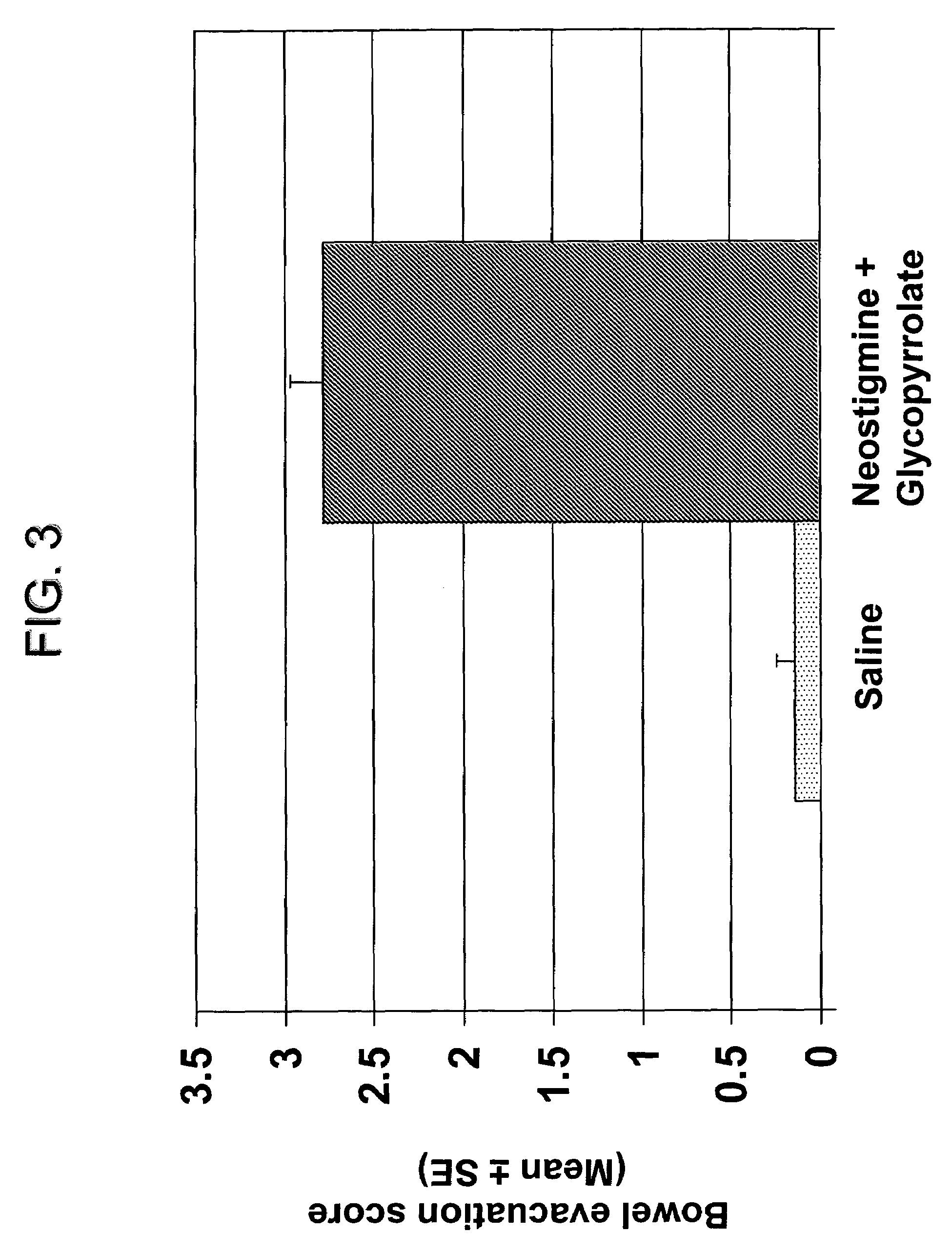
Compositions and methods for bowel care in individuals with chronic intestinal pseudo-obstruction
ActiveUS7635709B2Shorten the construction periodDifficult to administerBiocideAmine active ingredientsBowel careSide effect
The present disclosure provides compositions and methods for on-going bowel care for persons with chronic intestinal pseudo-obstruction. The compositions and methods can be administered in a non-clinical setting. The compositions comprise acetylcholinesterase inhibitors for stimulating motility of the bowel in combination with anti-cholinergic agents to counteract the potentially dangerous cardiac side effects of the acetylcholinesterase inhibitor. In some examples, the acetylcholinesterase inhibitor, neostigmine, and the anti-cholinergic agent, glycopyrrolate, are combined in a pharmaceutical composition. Certain examples also provide the frequency and duration of administration of the disclosed drug combinations.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
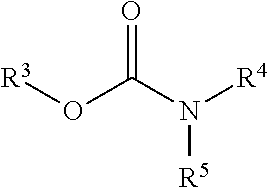
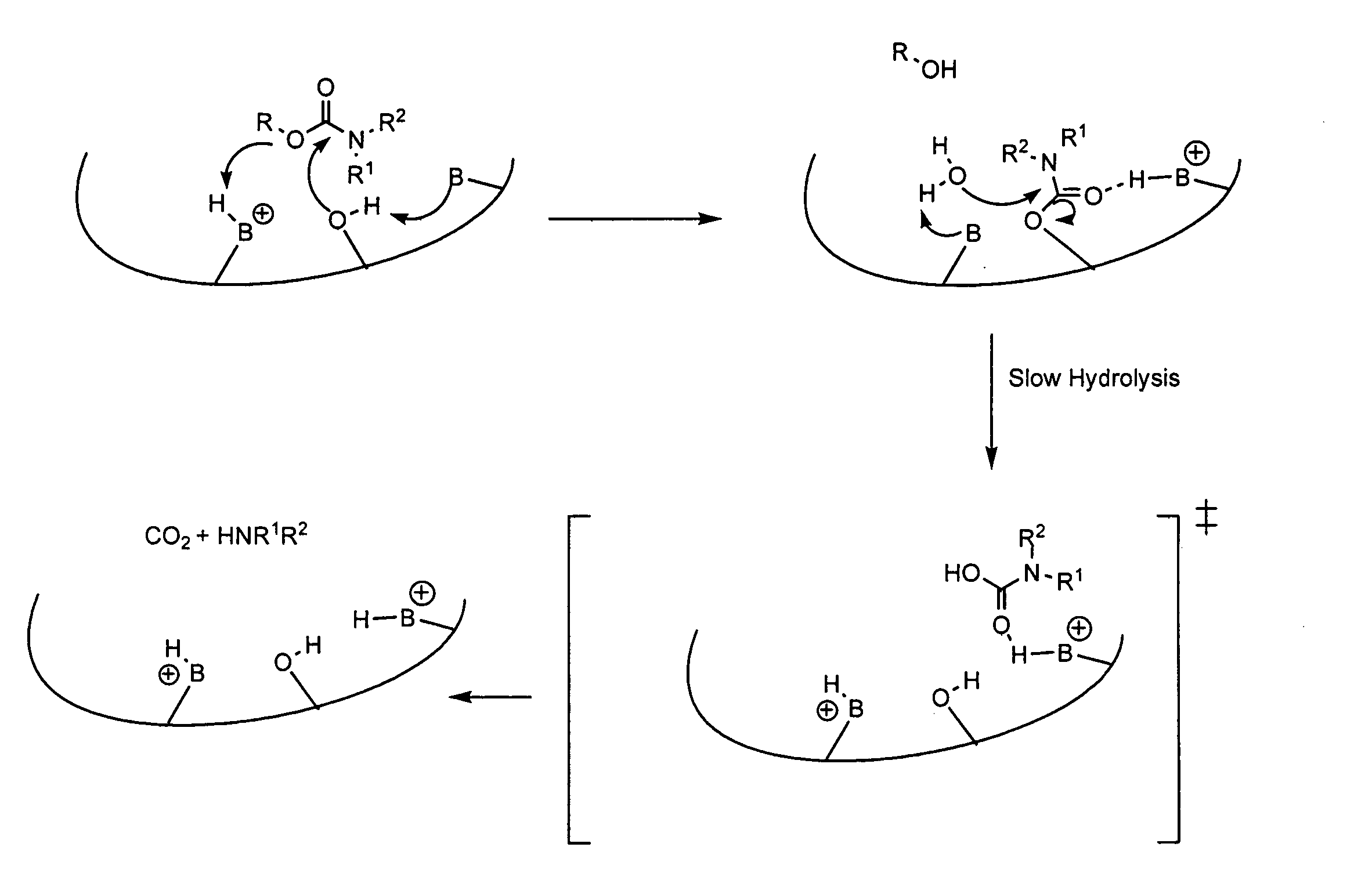
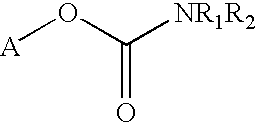
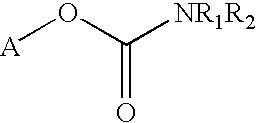
Carbamoyl esters that inhibit cholinesterase and release pharmacologically active agents
Carbamoyl esters inhibit cholinesterase activity and, upon hydrolysis release a pharmacologically active agent. In one embodiment, the carbamoyl ester has the following structure: wherein A is selected from the group consisting of an unsubstituted aryl, a substituted aryl, an unsubstituted heteroaryl and a substituted heteroaryl. The carbamoyl esters are employed in methods to treat an individual. The pharmacologically active agent obtained by hydrolysis of the carbamoyl esters can treat, for example, a nervous system condition, a cholinergic deficiency and conditions or diseases associated with a deficiency in a pharmacologically active agent, such as acetylcholine.
Owner:COLUCID PHARM INC
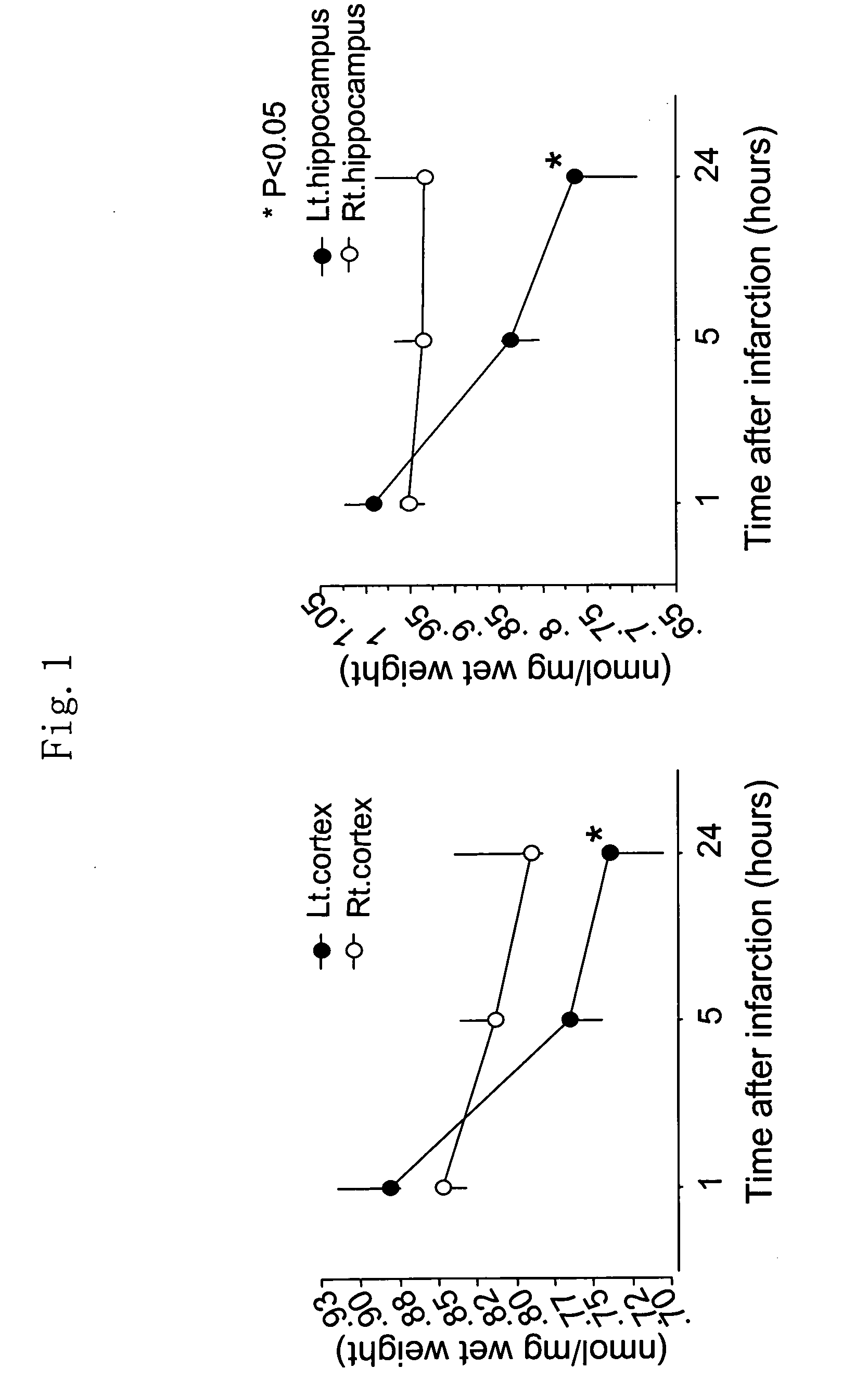
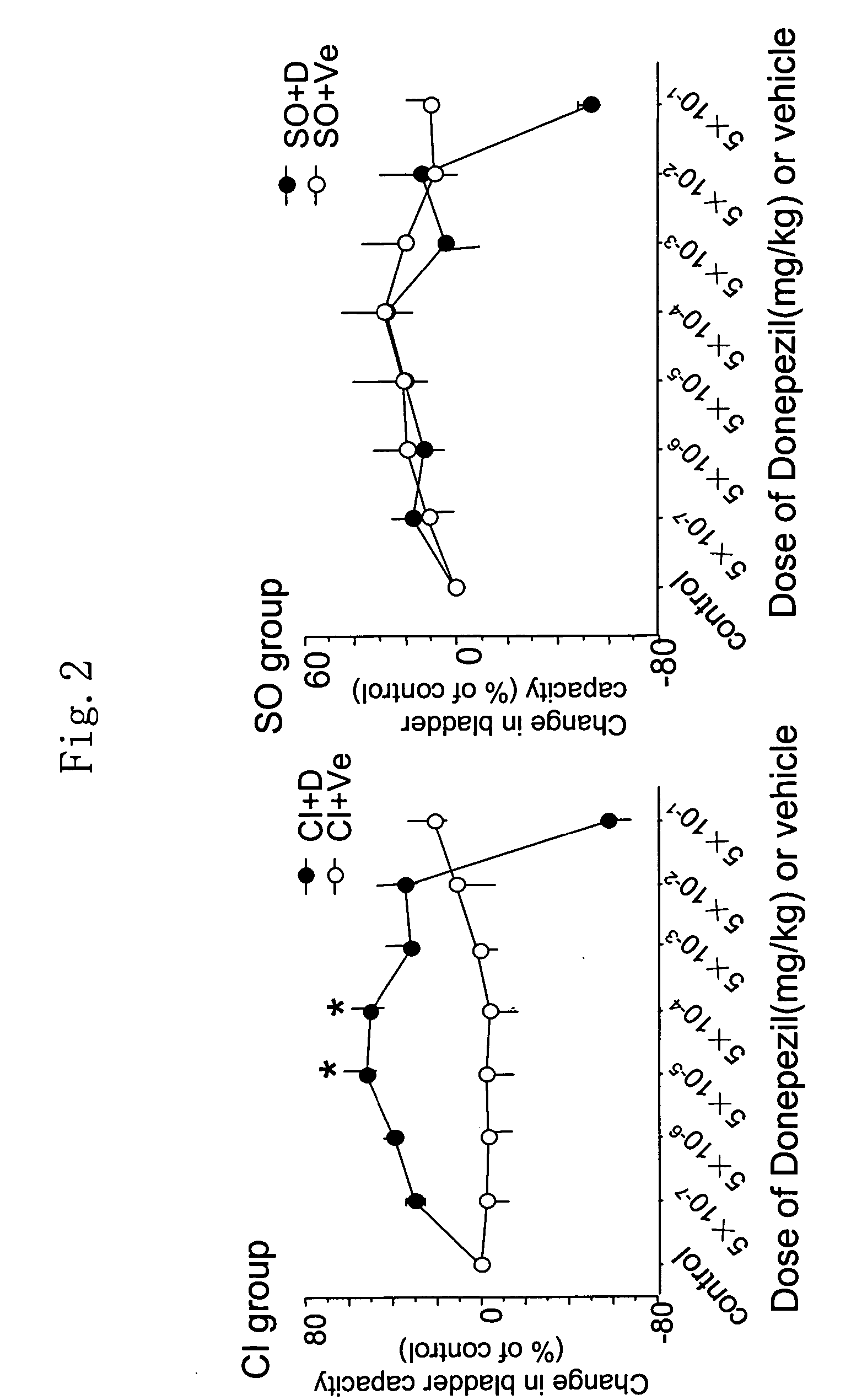
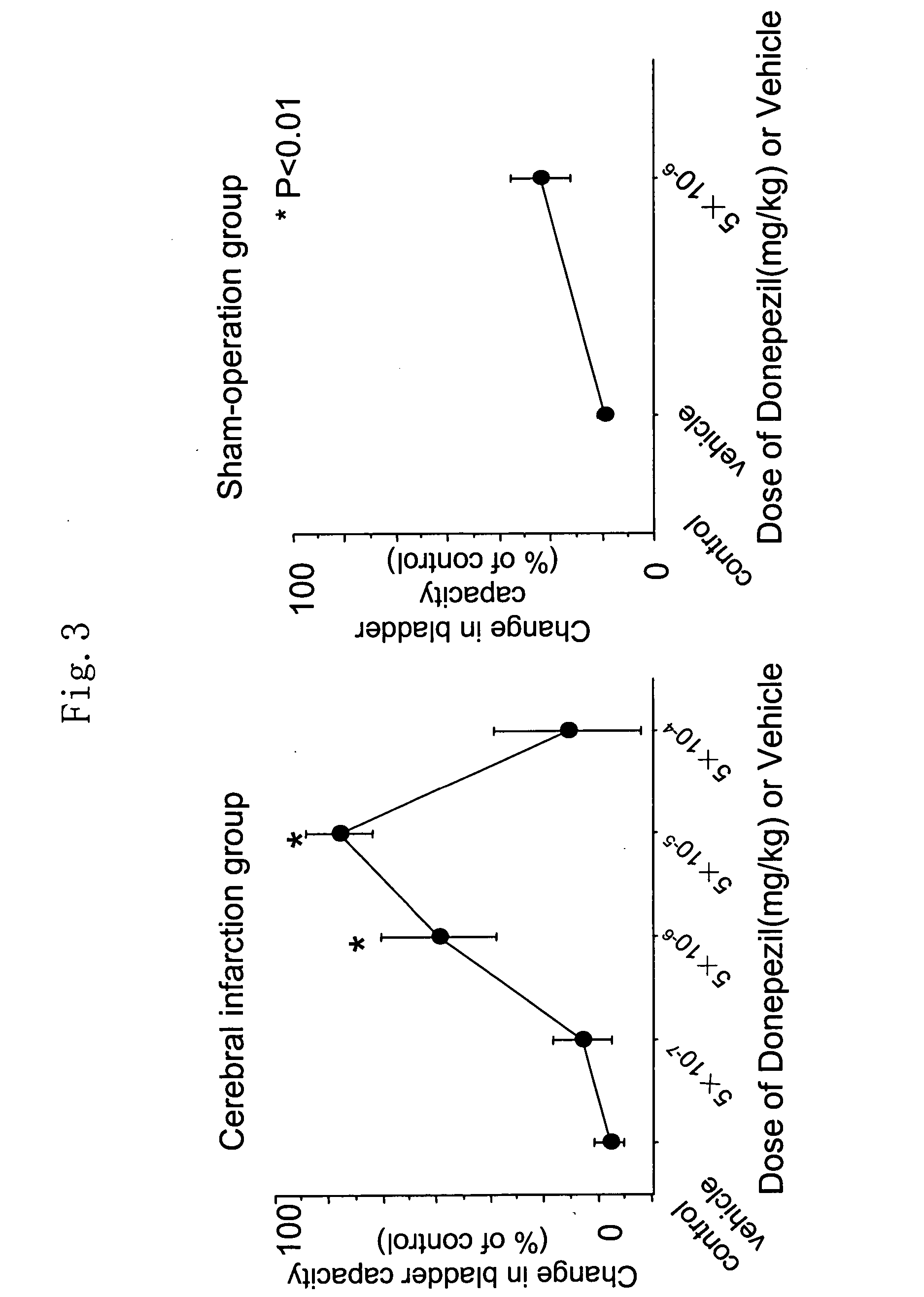
Therapeutic agent for overactive bladder resulting from cerebral infarction
An agent for treating overactive bladder resulting from cerebral infarction, comprising administrating a compound having a cholinesterase inhibitory activity or a pharmacologically acceptable salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
Preparation and use of compounds as aspartyl protease inhibitors
ActiveUS20070010667A1Nervous disorderMetabolism disorderImmunodeficiency virusNeuro-degenerative disease
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein A is a bond, —C(O)—, or —C(R3′)(R4′)—; X is —N(R1)— or —C(R6)(R7)—; Y is —S(O)2—, —C(═O)—, —PO(OR9) or —C(R6′R7′)—; is a single or double bond and R, R1, R2, R3, R4, R3′, R4′, R5, R6, R6′, R7 and R7′ are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic antagonist.
Owner:MERCK SHARP & DOHME LLC
Heterocyclic aspartyl protease inhibitors
Disclosed are compounds of the formula Ior a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, whereinW is a bond, —C(═S)—, —S(O)—, —S(O)2—, —C(═O)—, —O—, —C(R6)(R7)—, —N(R5)— or —C(═N(R5))—;X is —O—, —N(R5)— or —C(R6)(R7)—; provided that when X is —O—, U is not —O—, —S(O)—, —S(O)2—, —C(═O)— or —C(═NR5)—;U is a bond, —S(O)—, —S(O)2—, —C(O)—, —O—, —P(O)(OR15)—, —C(═NR5)—, —(C(R6)(R7))b— or —N(R5)—; wherein b is 1 or 2; provided that when W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, U is not —S(O)—, —S(O)2—, —O—, or —N(R5)—; provided that when X is —N(R5)— and W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, then U is not a bond;and R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I.Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes.Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:PHARMACOPEIA INC +1
Use of cholinesterase antagonists to treat insulin resistance
InactiveUS20050049293A1Insulin resistance in skeletal muscle relating to insufficient responseGood release effectBiocideNervous disorderCholinesteraseAcetylcholine esterase
There is provided a method of reducing insulin resistance in a mammalian subject comprising administering a suitable acetylcholine esterase antagonist.
Owner:UNIVERSITY OF MANITOBA
Macrocyclic beta-secretase inhibitors
Disclosed are novel compounds of the formula or a pharmaceutically acceptable salt or solvate thereof, wherein R1, R2, R3, n and X are as defined in the specification. Also disclosed are pharmaceutical compositions comprising the compounds of formula I. Also disclosed are methods of treating cognitive or neurodegenerative diseases such as Alzheimer's disease. Also disclosed are methods of treating a cognitive or neurodegenerative disease comprising administering to a patient I need of such treatment a combination of at least one compound of formula I and at least one compound selected from the group consisting of β-secretase inhibitors other than those of formula I, HMG-CoA reductase inhibitors, gamma-secretase inhibitors, non-steroidal anti-inflammatory agents, N-methyl-D-aspartate receptor antagonists, cholinesterase inhibitors and anti-amyloid antibodies.
Owner:MERCK SHARP & DOHME LLC
Substituted amide beta secretase inhibitors
Disclosed are novel compounds of the formula or a pharmaceutically acceptable salt or solvate thereof, wherein R1, R2, R3, R4 and X are as defined in the specification. Also disclosed are pharmaceutical compositions comprising the compounds of formula I. Also disclosed are methods of treating cognitive or neurodegenerative diseases such as Alzheimer's disease. Also disclosed are methods of treating a cognitive or neurodegenerative disease comprising administering to a patient I need of such treatment a combination of at least one compound of formula I and at least one compound selected from the group consisting of β-secretase inhibitors other than those of formula I, HMG-CoA reductase inhibitors, gamma-secretase inhibitors, non-steroidal anti-inflammatory agents, N-methyl-D-aspartate receptor antagonists, cholinesterase inhibitors and anti-amyloid antibodies.
Owner:MERCK SHARP & DOHME LLC
Compositions and methods of treatment using L-type calcium channel blockers and cholinesterase inhibitors
InactiveUS20050153953A1Good curative effectProlong the action timeBiocideNervous disorderDonepezilCholinesterase
The present invention relates to compositions comprising at least one L-type calcium channel blocker, particularly the compound (+)-isopropyl 2-methoxyethyl 4-(2-chloro-3-cyano-phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3,5-dicarboxylate, in combination with at least one cholinesterase inhibitor, particularly donepezil, and uses thereof in methods of treatment.
Owner:MEMORY PHARMA CORP
Mixtures of Anthranilamide Invertebrate Pest Control Agents
Disclosed are mixtures and compositions for controlling invertebrate pests relating to combinations comprising (a) 3-bromo-N-[4-cyano-2-methyl-6-[(methylamino)carbonyl]phenyl]-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxamide, an N-oxide, or a salt thereof, Formula (1) and (b) at least one invertebrate pest control agent selected from neonicotinoids, cholinesterase inhibitors, sodium channel modulators, chitin synthesis inhibitors, ecdysone agonists, lipid biosynthesis inhibitors, macrocyclic lactones, GABA-regulated chloride channel blockers, juvenile hormone mimics, ryanodine receptor ligands, octopamine receptor ligands, mitochondrial electron transport inhibitors, nereistoxin analogs, pyridalyl, flonicamid, pymetrozine, dieldrin, metaflumizone, biological agents, and salts of the foregoing. Also disclosed are methods for controlling an invertebrate pest comprising contacting the invertebrate pest or its environment with a biologically effective amount of a mixture or composition of the invention.
Owner:HUGHES KENNETH ANDREW +4
Composition containing anti-dementia drug
InactiveUS20060246003A1Improve complianceReduce the burden onIn-vivo radioactive preparationsPill deliveryCholinesteraseCholinesterase inhibition
An object of the present invention is to provide, for the case of implementing a therapeutic method in which at least two kinds of anti-dementia drugs are used together, a composition that has a good therapeutic effect on dementia, and also gives excellent compliance. Another object of the present invention is to provide a composition containing at least two kinds of anti-dementia drugs, in which release of the anti-dementia drugs from the composition is controlled, whereby a combined effect of the anti-dementia drugs can be achieved well. Still another object of the present invention is to provide a composition for which the frequency of administration and the amount taken are reduced and hence compliance can be improved, and a method of manufacturing such a composition. According to the present invention, there is provided a composition containing at least two kinds of anti-dementia drugs; such a composition containing at least one sustained-release portion containing an anti-dementia drug; and such a composition containing at least one cholinesterase inhibitor, and at least one N-methyl-D-aspartate receptor antagonist.
Owner:EISIA R&D MANAGEMENT CO LTD
Transdermal administration of huperzine
The present invention provides a composition of transdermally administered huperzine for improving memory and cognitive function. In one aspect, huperzine is delivered in a sufficient amount to achieve and maintain a blood plasma Huperzine level of about 0.5 ng / mL to about 30 ng / mL. Huperzine may be delivered by itself, or in combination with other elements, such as additional cholinesterase inhibitors, drugs or treatment agents, or positive health promoting substances. Various formulations for the transdermal delivery of huperzine are disclosed, and may include selected penetration enhancers.
Owner:XEL HERBACEUTICALS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com