Patents
Literature
1423 results about "Human serum albumin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human serum albumin is the serum albumin found in human blood. It is the most abundant protein in human blood plasma; it constitutes about half of serum protein. It is produced in the liver. It is soluble in water and monomeric.
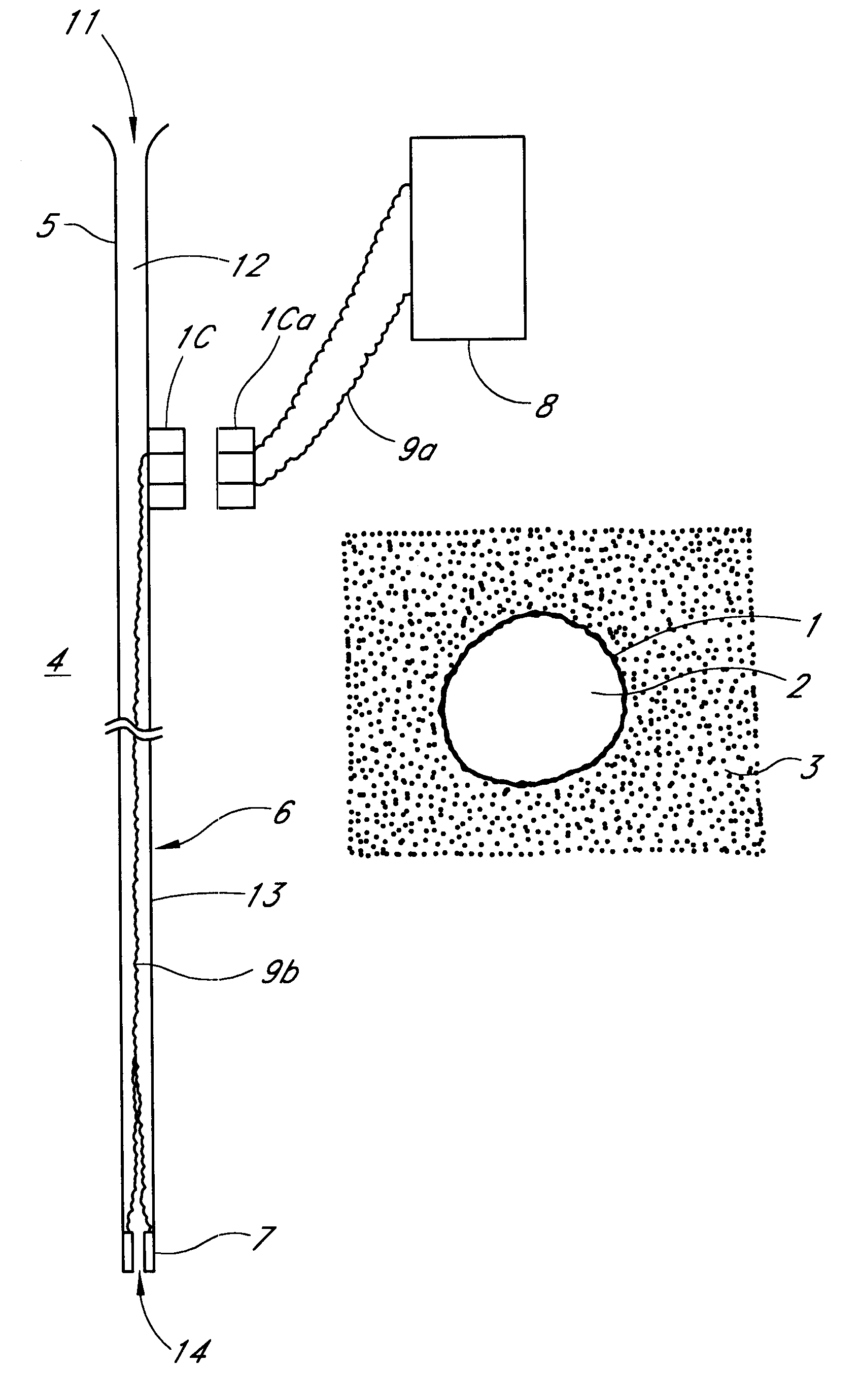
Controlled delivery of therapeutic agents by insertable medical devices
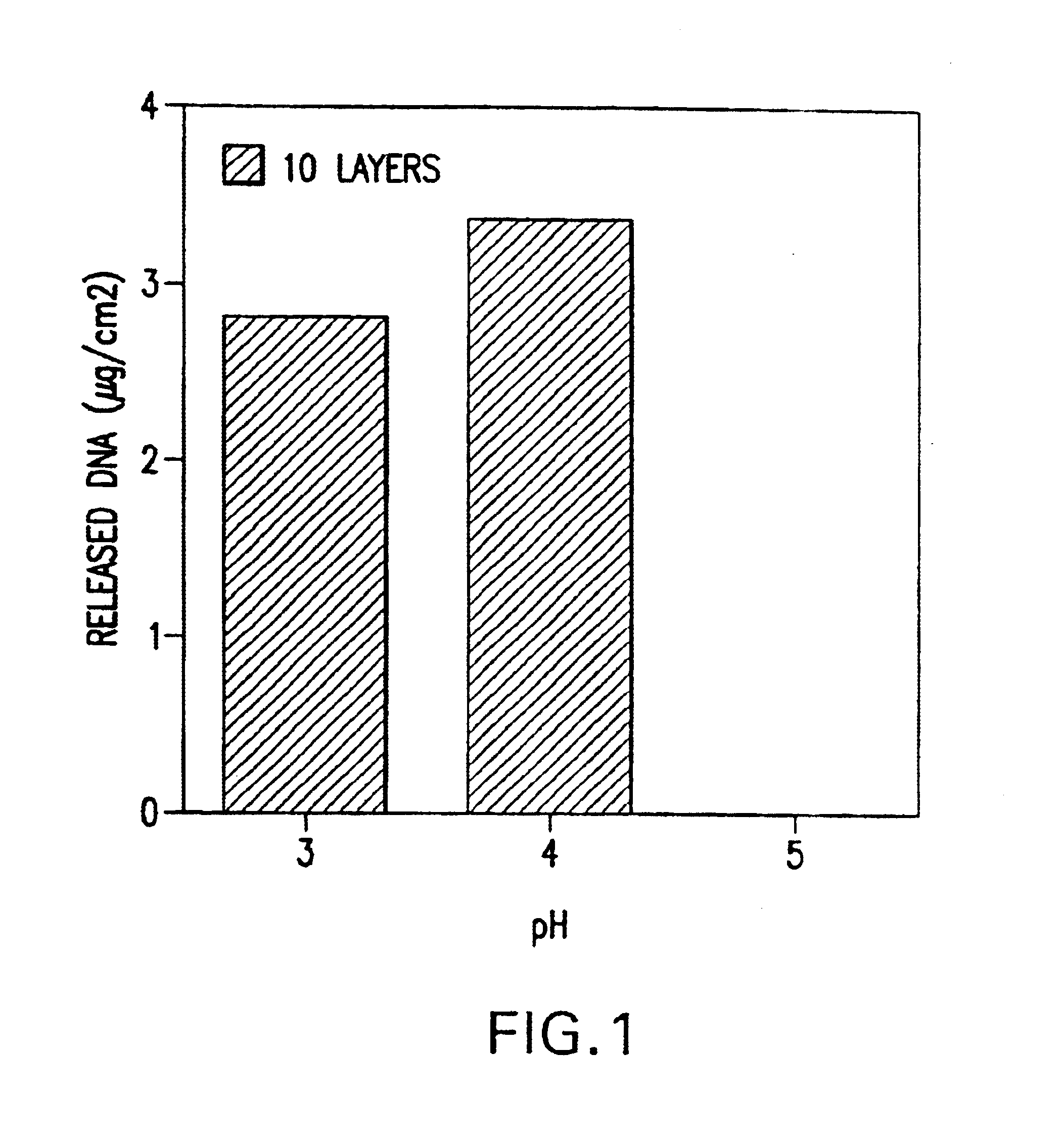
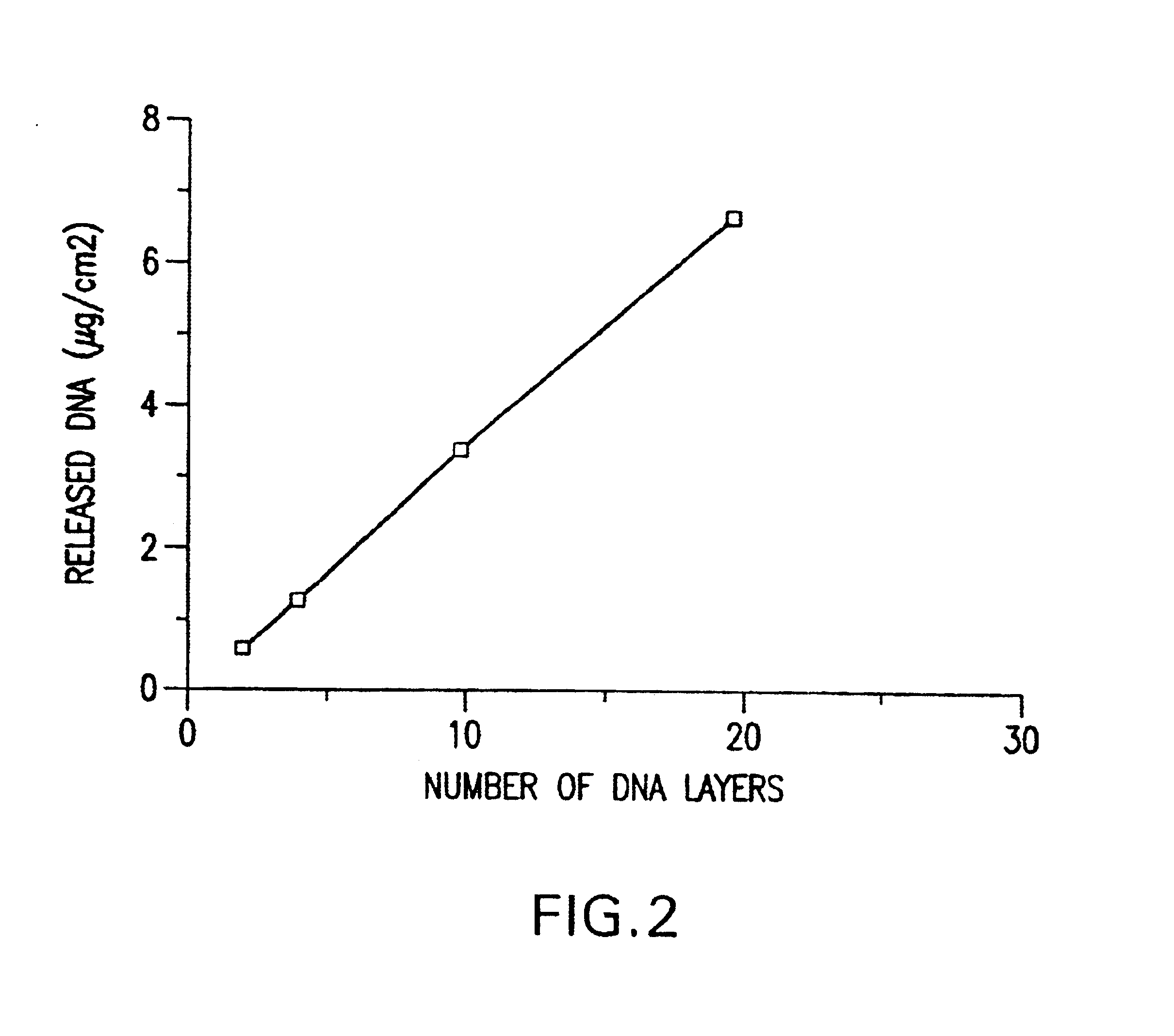
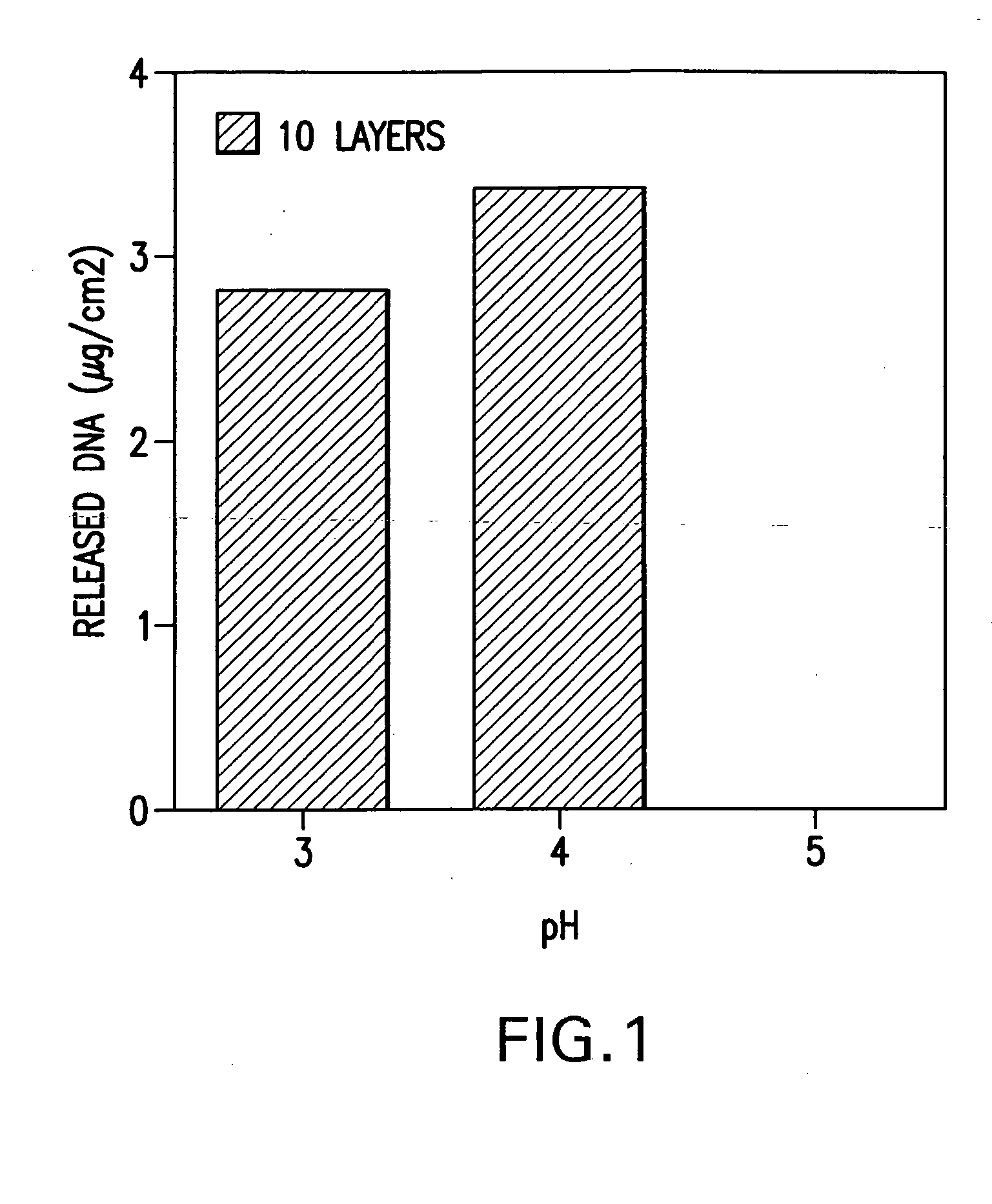
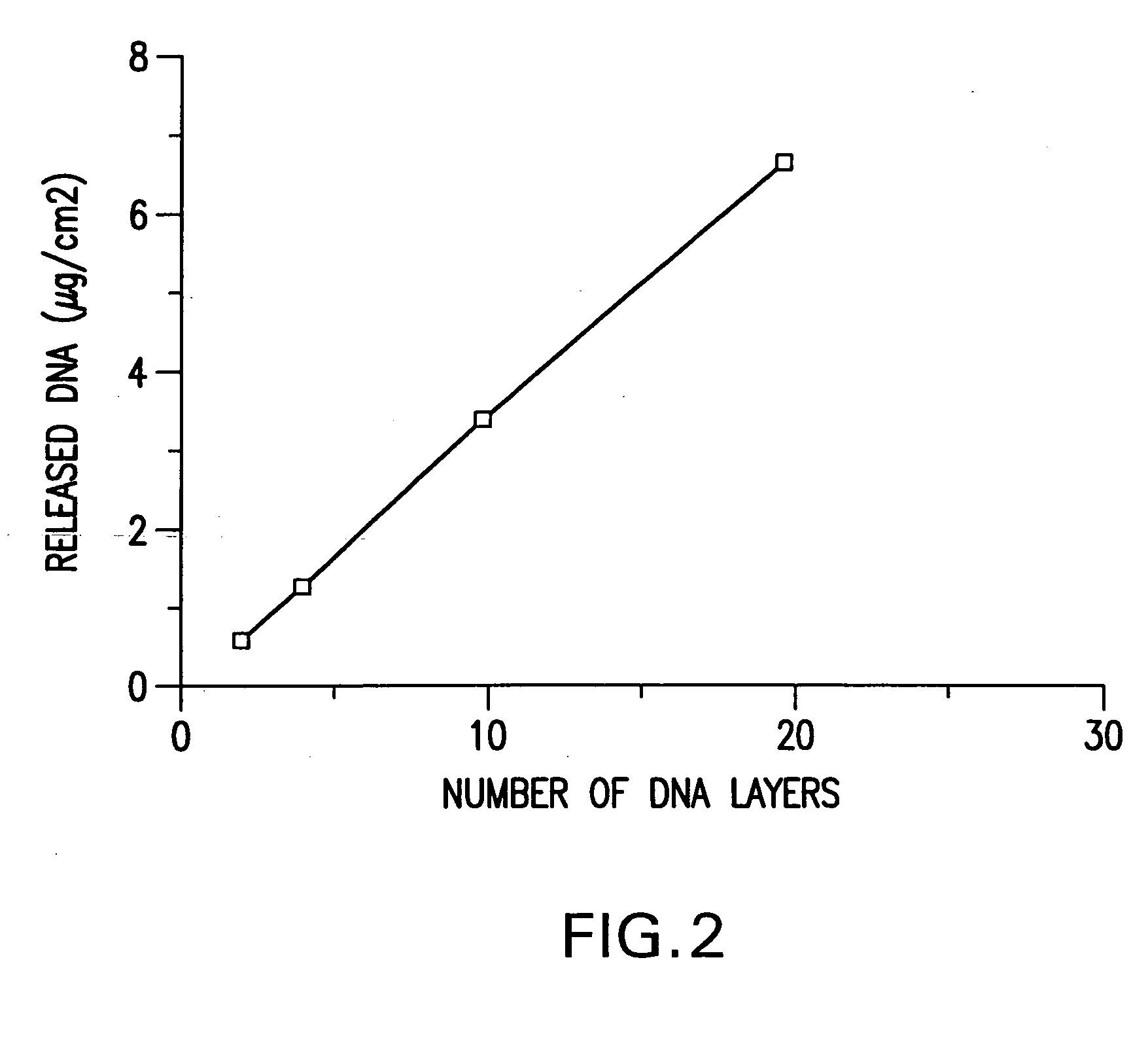
A medical device and method for transportation and release of a therapeutic agent into a mammalian body are disclosed. The medical device is coated with alternating layers of a negatively charged therapeutic agent and a cationic polyelectrolyte, following a controlled adsorption technique. The method is simple, with minimal perturbation to the therapeutic agent and uses clinically acceptable biopolymers such as human serum albumin. The amount of the therapeutic agent that can be delivered by this technique is optimized by the number of the layers of the therapeutic agent adsorbed on the surface of medical device. There is a washing step between alternate layers of the therapeutic agent and cationic polyelectrolyte carrier, so that the amount of the therapeutic agent on the insertable medical device represents the portion that is stably entrapped and adsorbed on to the medical device. The insertable medical device and method according to this invention are capable of reproducibly delivering therapeutic agent to a site in a mammalian body, and allow for a highly reproducible and controllable release kinetics of the therapeutic agent.
Owner:SCI MED LIFE SYST
Compositions and methods of delivery of pharmacological agents
InactiveUS20050004002A1Reducing one or more side effectsInhibiting oxidation in the pharmaceutical compositionAntibacterial agentsOrganic active ingredientsSide effectPharmaceutical formulation
The present invention relates to a pharmaceutical composition comprising a pharmaceutical agent and a pharmaceutically acceptable carrier, which carrier comprises a protein, for example, human serum albumin and / or deferoxamine. The human serum albumin is present in an amount effective to reduce one or more side effects associated with administration of the pharmaceutical composition. The invention also provides methods for reducing one or more side effects of administration of the pharmaceutical composition, methods for inhibiting microbial growth and oxidation in the pharmaceutical composition, and methods for enhancing transport and binding of a pharmaceutical agent to a cell.
Owner:ABRAXIS BIOSCI LLC
Serum albumin binding proteins with long half-lives
InactiveUS20070269422A1Increased serum half-lifeReduce dosing frequencyOrganic active ingredientsPeptide/protein ingredientsSerum igePrimate
The present invention relates to amino acid sequences that are capable of binding to serum albumin; to compounds, proteins and polypeptides comprising or essentially consisting of such amino acid sequences; to nucleic acids that encode such amino acid sequences, proteins or polypeptides; to compositions, and in particular pharmaceutical compositions, that comprise such amino acid sequences, proteins and polypeptides; and to uses of such amino acid sequences, proteins and polypeptides. Particularly, the amino acid sequences and compounds of the present invention bind to or otherwise associate with serum albumin in such a way that, when the amino acid sequence or compound is bound to or otherwise associated with a serum albumin molecule in a primate, it exhibits a serum half-life of at least 50% of the natural half-life of serum albumin in said primate.
Owner:ABLYNX NV
Method and apparatus for enhancing the integrity of an implantable sensor device
InactiveUS7162289B2Inhibited DiffusionExtended service lifeImmobilised enzymesBioreactor/fermenter combinationsProtein materialsLubrication
A method and apparatus for enhancing the integrity of an implantable sensor. Voids formed between an outer tubing and a sensor substrate or spacing element may be back-filled with a curable, implantable material, minimizing the extent to which unwanted fluids diffuse within the sensor. An enzyme or protein matrix pellet below the sensor window may be pre-treated with a reducing agent to enhance its bond stability, and to reduce undesired swelling that may cause the sensor window to detach or leak. The bonding between the enzyme pellet and a hydrogel layer may be reinforced by application of an intervening bonding layer of a protein material, such as human serum albumin (HSA). The size of the window may be minimized by minimizing the size of an underlying electrode, providing reduced flux and lengthening sensor. A coating may be deposited on the surface of the sensor leads, providing stiffening and lubrication.
Owner:MEDTRONIC MIMIMED INC
Composition for therapy of diseases with ultrasonic and pharmaceutical liquid composition containing the same
InactiveUSRE36939E1Good effectWeaken energySonopheresisUltrasonic/sonic/infrasonic diagnosticsDiseaseTreatment effect
A booster comprising a plenty of microbubbles of a gas in a liquid, e.g. about 4x107 cells / ml of microbubbles of a gas having a diameter of 0.1 to 100 mu m in a 3 to 5% human serum albumin solution, and a pharmaceutical liquid composition comprising the booster as set forth above and a medicament, which are useful for the therapy of various diseases together with exposure of ultrasonic, where the therapeutic effects of the medicament is enhanced by the application of ultrasound in the presence of the booster.
Owner:EKOS CORP
Production of hSA-linked butyrylcholinesterases in transgenic mammals
InactiveUS20060253913A1Promote formationImprove stabilityAnimal cellsVectorsMammalButyrylcholinesterase
The present invention provides methods for the large-scale production of recombinant butyrylcholinesterase fused to human serum albumin in cell culture, and in the milk and / or urine of transgenic mammals. The recombinant butyrylcholinesterase-albumin fusion protein of this invention can be used to treat and / or prevent organophosphate pesticide poisoning, nerve gas poisoning, cocaine intoxication, and succinylcholine-induced apnea.
Owner:PHARMATHENE
Improved fibronectin-based binding molecules and uses thereof
InactiveUS20110038866A1Simple and efficientProlong lifePeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeFibronectins
The invention provides fibronectin type III (Fn3)-based binding molecules that bind to a specific target antigen. The invention further provides bispecific Fn3-based binding molecules that bind to two or more targets simultaneously. The Fn3-based binding molecules of the invention can also be linked together to form multispecific Fn3-based binding molecules, and / or can be conjugated to a non-Fn3 moiety, such as, Human Serum Albumin (HSA), for improved half life and stability. The invention also provides methods for generating, screening and using Fn3-based binding molecules in a variety of therapeutic and diagnostic applications.
Owner:NOVARTIS AG
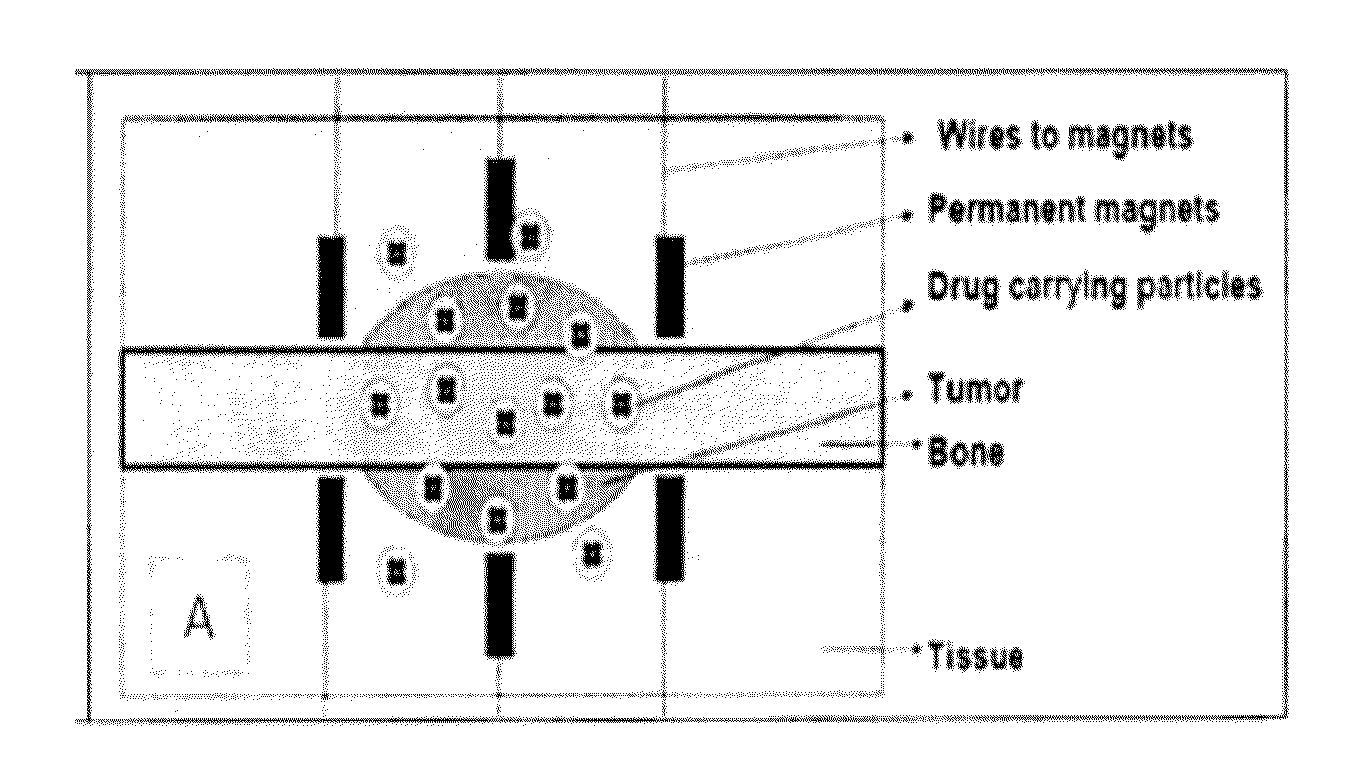
Composite magnetic nanoparticle drug delivery system
ActiveUS20120265001A1Accurate placementLimit deliveryBiocideHeavy metal active ingredientsDiseaseOil emulsion
A composite magnetic nanoparticle drug delivery system provides targeted controlled release chemotherapies for cancerous tumors and inflammatory diseases. The magnetic nanoparticle includes a biocompatible and biodegradable polymer, a magnetic nanoparticle, the biological targeting agent human serum albumin, and a therapeutic pharmaceutical composition. The composite nanoparticles are prepared by oil-in-oil emulsion / solvent evaporation and high shear mixing. An externally applied magnetic field draws the magnetic nanoparticles to affected areas. The biological targeting agent draws the nanoparticles into the affected tissues. Polymer degradation provides controlled time release delivery of the pharmaceutical agent.
Owner:WICHITA STATE UNIVERSITY
Compositions and methods of delivery of pharmacological agents
InactiveUS20070129448A1Eliminate side effectsInhibit microbial growthBiocideHydroxy compound active ingredientsSide effectPharmaceutical formulation
The present invention relates to a pharmaceutical composition comprising a pharmaceutical agent and a pharmaceutically acceptable carrier, which carrier comprises a protein, for example, human serun albumin and / or deferoxamine. The human serum albumin is present in an amount effective to reduce one or more side effects associated with administration of the pharmaceutical composition. The invention also provides methods for reducing one or more side effects of administration of the pharmaceutical composition, methods for inhibiting microbial growth and oxidation in the pharrmaceutical composition, and methods for enhancing transport and binding of a pharmaceutical agent to a cell.
Owner:ABRAXIS BIOSCI LLC
Serum albumin binding proteins with long half-lives
InactiveUS20100113339A1Increased serum half-lifeReduce dosing frequencyNervous disorderPeptide/protein ingredientsHalf-lifeSerum albumin
The present invention relates to amino acid sequences that are capable of binding to serum albumin; to compounds, proteins and polypeptides comprising or essentially consisting of such amino acid sequences; to nucleic acids that encode such amino acid sequences, proteins or polypeptides; to compositions, and in particular pharmaceutical compositions, that comprise such amino acid sequences, proteins and polypeptides; and to uses of such amino acid sequences, proteins and polypeptides.
Owner:ABLYNX NV
Process for promoting proper folding of human serum albumin using a human serum albumin ligand
InactiveUS20050209441A1Reduce concentrationSerum albuminPeptide preparation methodsHuman serum albuminProtein refolding
The present invention is a process for refolding and renaturing human serum albumin protein to substantially native conformation by the addition of a human serum albumin refolding ligand to a solution containing the protein under conditions conducive to refolding of the protein.
Owner:BLUE MOUNTAIN TEHNOLOGY DEV LAB
Production of recombinant human serum albumin with rice-embryo milk cell as biological reactor
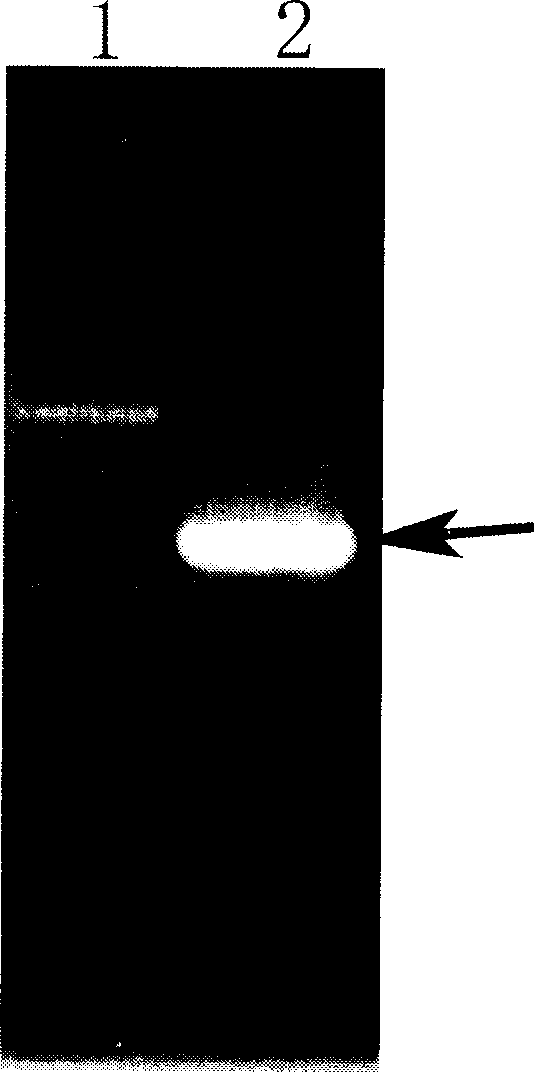
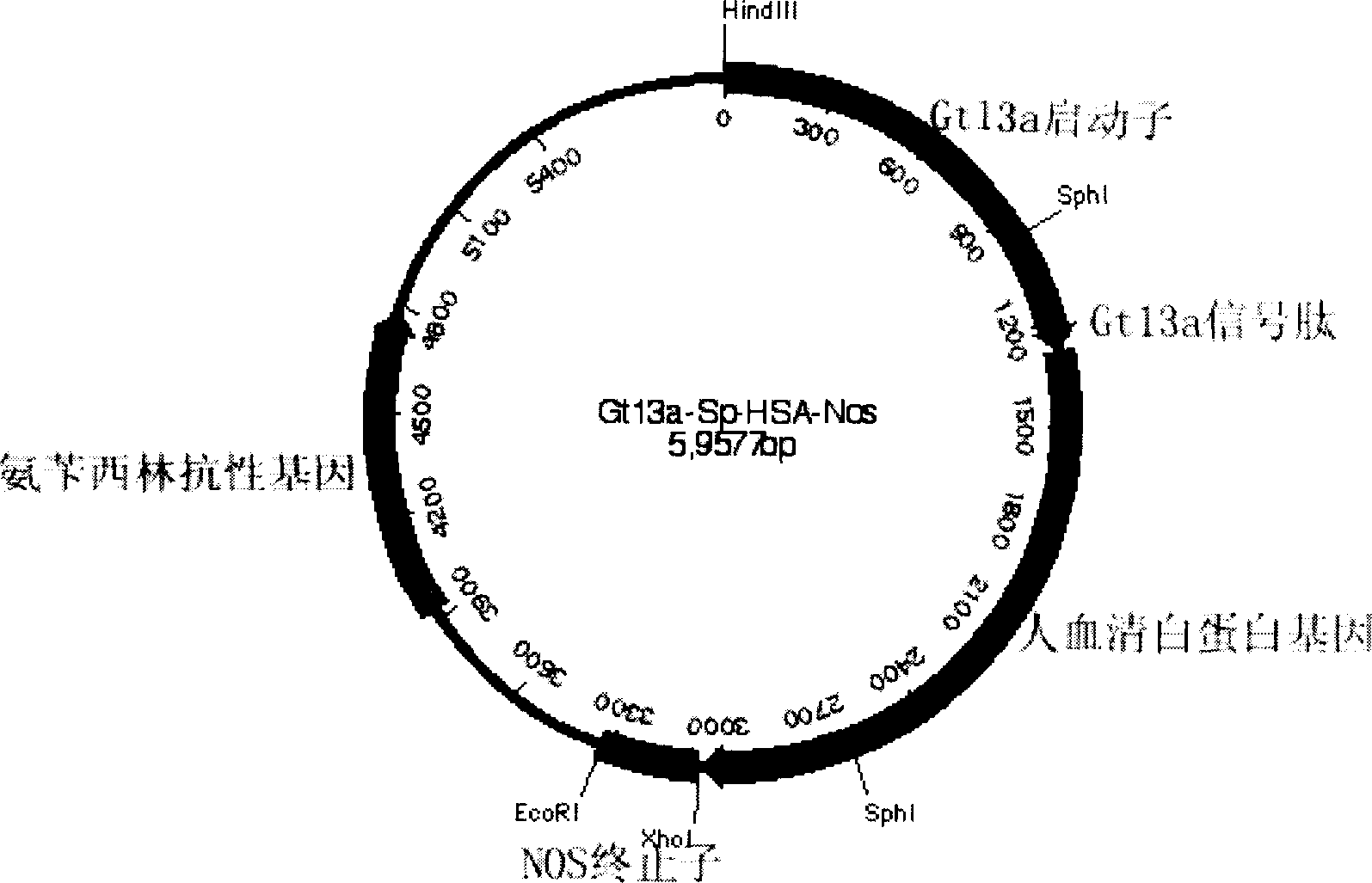
ActiveCN1896239ANo pollution in the processEfficient expressionSerum albuminFermentationSerum protein albuminEmbryo
Production of recombinant human serum albumin with rice albuminous cell as biological reactor is carried out by taking rice albuminous cell protein as storage site for recombinant protein, taking rice albuminous specific expression starter and signal peptide, entering inductive recombinant human serum albumin into inner-film system of rice serum albumin cell and storing it in rice albuminous protein. It is cheap, has more yield and higher expression.
Owner:WUHAN HEALTHGEN BIOTECHNOLOGY CORP
Booster for therapy of disease with ultrasound and pharmaceutical liquid composition containing the same
A booster comprising a plurality of microbubbles of a gas in a liquid, e.g. about 4x107 cells / ml of microbubbles of a gas having a diameter of 0.1. to 100 mum in a 3 to 5% human serum albumin solution, and a pharmaceutical liquid composition comprising the booster as set forth above and a medicament, which are useful for the therapy of various diseases together with exposure of ultrasonic, where the therapeutic effects of the medicament is enhanced by the application of ultrasound in the presence of the booster.
Owner:EKOS CORP
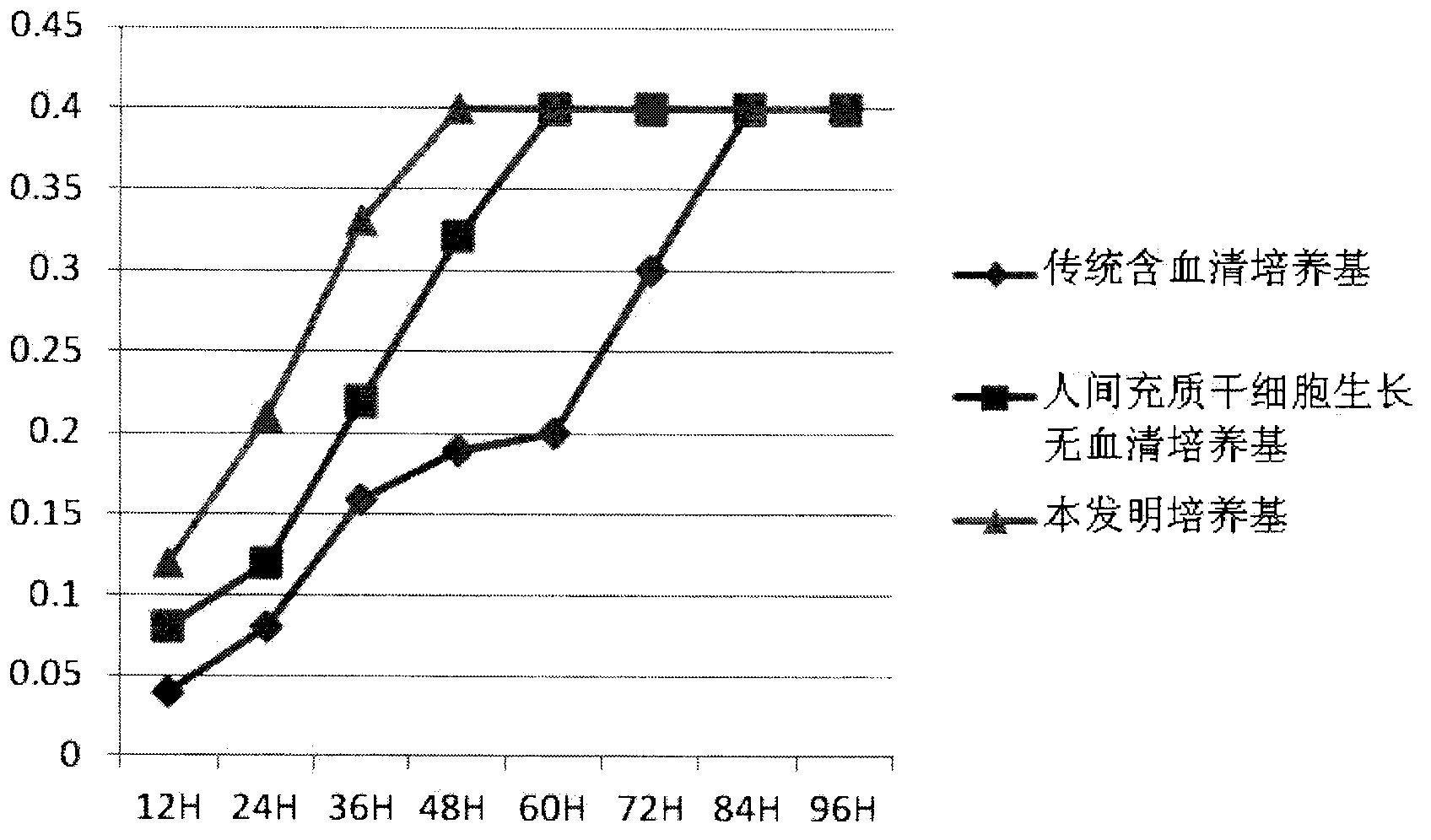
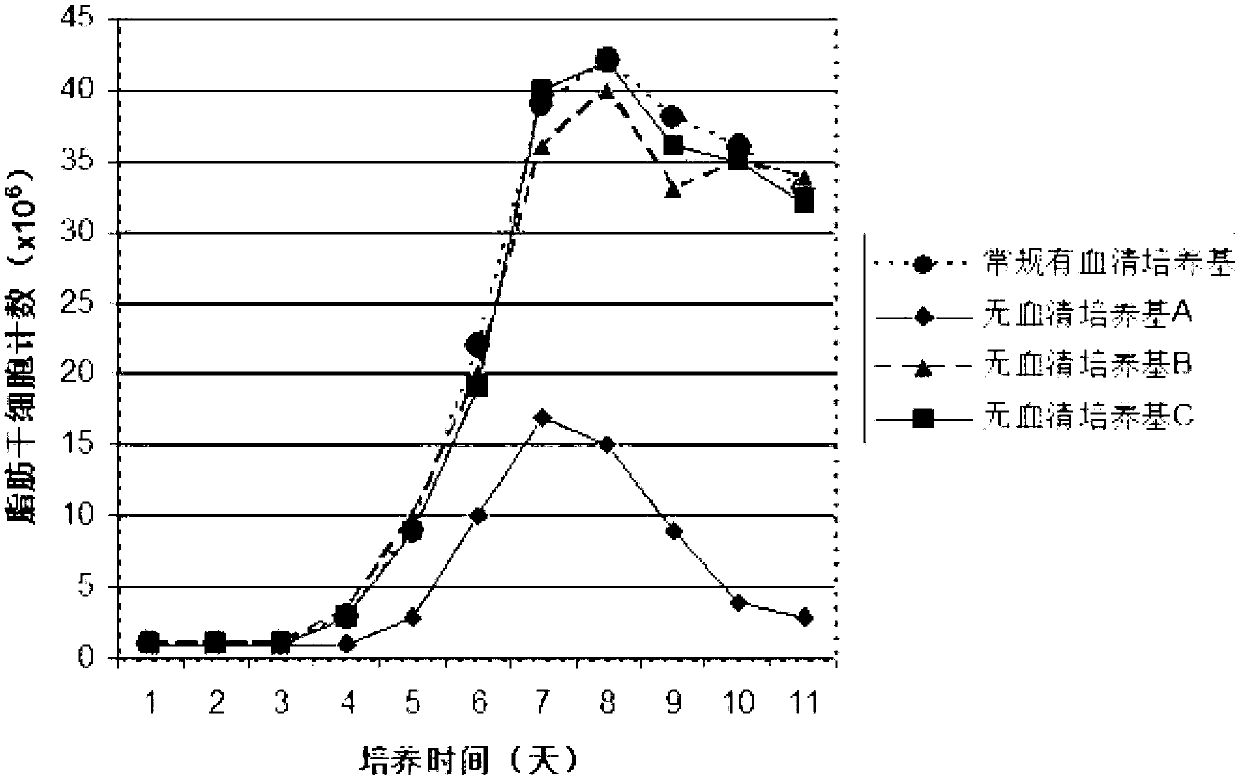
Clinical-grade human mesenchymal stem cell serum-free complete medium
ActiveCN103243071APromote growthLow toxicitySkeletal/connective tissue cellsInsulin-like growth factorCuticle
The invention relates to a human mesenchymal stem cell culture medium. According to the culture medium, the basal culture medium comprises the following components based on the final concentration: 1-2g / L of human serum albumin, 5-10mg / L of transferring, 2-8mg / L of fibronectin, 1-4mg / L of laminin, 50g / L of Fe(NO3)3.9H2O, 417g / L of FeSO4.7H2O, 1-3mu g / L of estradiol, 2-5mu g / L of testosterone, 1-3mu g / L of progesterone, 39.25-117.74 mu g / L of dexamethasone, 5-10mg / L of insulin, 376.36mg / L of riboflavin, 80.96-242.87mg / L of coenzyme A, 4.41-6.17mg / L of butanediamine, 1-2mg / L of taurine, 0.61-1.85mg / L of aminoethanol, 8.81-26.42mg / L of pyruvic acid, 3.78-7.56mu g / L of sodium selenate, 292.3-584.6mg / L of L-glutamine, 2-8mu g / L of vascular endothelial growth factor, 4-10mu g / L of epidermal growth factor, 4-10mu g / L of basic fibroblast growth factor, 1-5mu g / L of leukaemia inhibitory factor, 1-5mu g / L of insulin-like growth factor-I and 2-8mu g / L of stem cell factor. The culture medium does not contain the animal serum, the potential animal endogenous endotoxin or virus of the animal serum is eliminated, and the culture medium is conveniently applied to clinics.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
Serum albumin binding molecules
ActiveUS20110305663A1Improve solubilityReduce aggregationBacteriaPeptide/protein ingredientsSerum igeSerum protein albumin
The present invention relates to an antibody-like protein based on the tenth fibronectin type III domain (10Fn3) that binds to serum albumin. The invention further relates to fusion molecules comprising a serum albumin-binding 10Fn3 joined to a heterologous protein for use in diagnostic and therapeutic applications.
Owner:BRISTOL MYERS SQUIBB CO
Controlled delivery of therapeutic agents by insertable medical devices
A medical device and method for transportation and release of a therapeutic agent into a mammalian body are disclosed. The medical device is coated with alternating layers of a negatively charged therapeutic agent and a cationic polyelectrolyte, following a controlled adsorption technique. The method is simple, with minimal perturbation to the therapeutic agent and uses clinically acceptable biopolymers such as human serum albumin. The amount of the therapeutic agent that can be delivered by this technique is optimized by the number of the layers of the therapeutic agent adsorbed on the surface of medical device. There is a washing step between alternate layers of the therapeutic agent and cationic polyelectrolyte carrier, so that the amount of the therapeutic agent on the insertable medical device represents the portion that is stably entrapped and adsorbed on to the medical device. The insertable medical device and method according to this invention are capable of reproducibly delivering therapeutic agent to a site in a mammalian body, and allow for a highly reproducible and controllable release kinetics of the therapeutic agent.
Owner:BOSTON SCI SCIMED INC
Pharmaceutical proteins, human therapeutics, human serum albumin, insulin, native cholera toxic b submitted on transgenic plastids
InactiveUS20030204864A1Eliminate needLarge biomassBiocidePeptide/protein ingredientsEscherichia coliInsulin-like growth factor
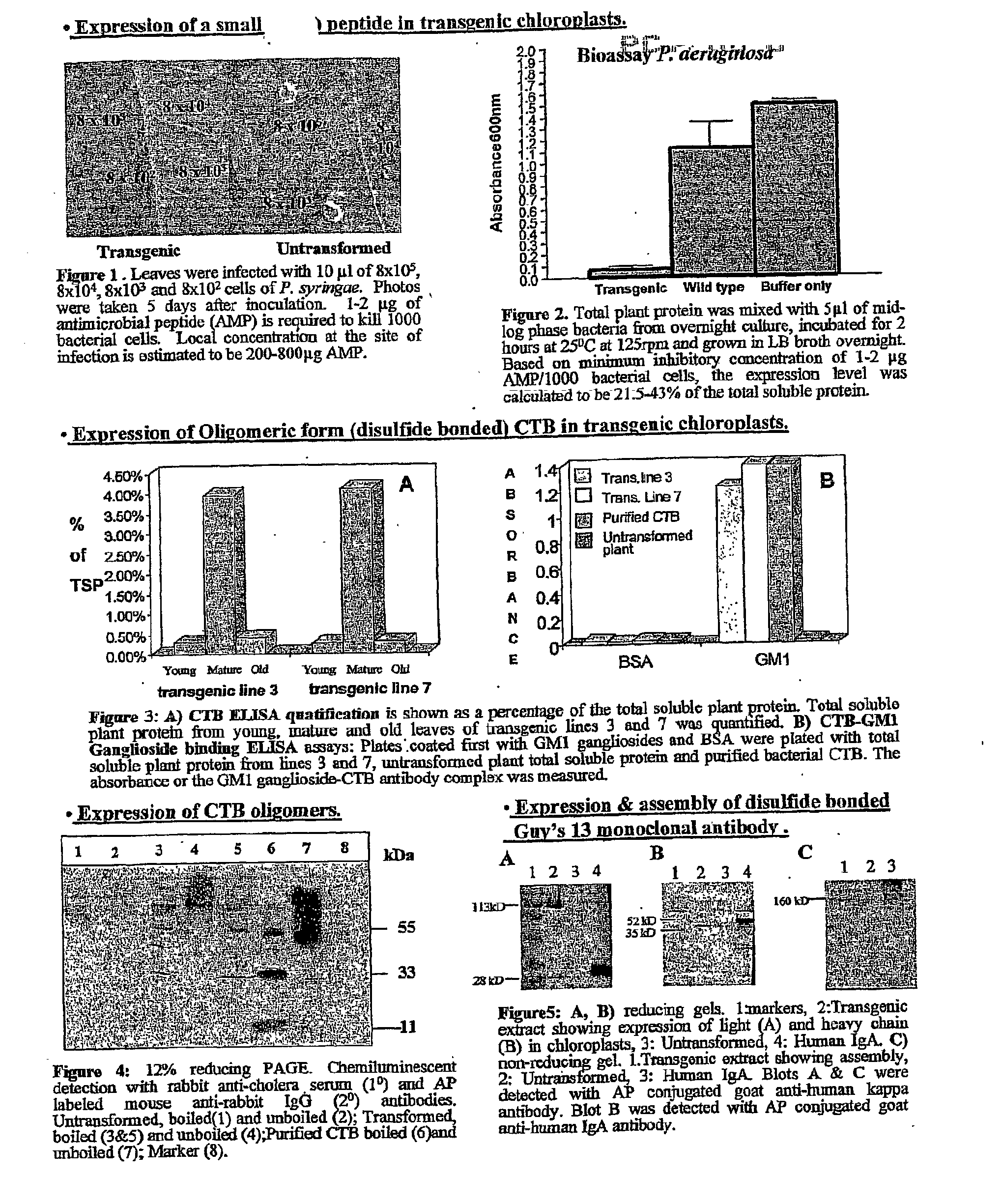
Transgenic chloroplast technology could provide a viable solution to the production of Insulin-like Growth Factor I (IGF-I), Human Serum Albumin (HSA), or interferons (IFN) because of hyper-expression capabilities, ability to fold and process eukaryotic proteins with disulfide bridges (thereby eliminating the need for expensive post-purification processing). Tobacco is an ideal choice because of its large biomass, ease of scale-up (million seeds per plant), genetic manipulation and impending need to explore alternate uses for this hazardous crop. Therefore, all three human proteins will be expressed as follows: a) Develop recombinant DNA vectors for enhanced expression via tobacco chloroplast genomes b) generate transgenic plants c) characterize transgenic expression of proteins or fusion proteins using molecular and biochemical methods d) large scale purification of therapeutic proteins from transgenic tobacco and comparison of current purification / processing methods in E. coli or yeast e) Characterization and comparison of therapeutic proteins (yield, purity, functionality) produced in yeast or E. coli with transgenic tobacco f) animal testing and pre-clinical trials for effectiveness of the therapeutic proteins. Mass production of affordable vaccines can be achieved by genetically engineering plants to produce recombinant proteins that are candidate vaccine antigens. The B subunits of Enteroxigenic E. coli (LTB) and cholera toxin of Vibrio cholerae (CTB) are examples of such antigens. When the native LTB gene was expressed via the tobacco nuclear genome, LTB accumulated at levels less than 0.01% of the total soluble leaf protein. Production of effective levels of LTB in plants, required extensive codon modification. Amplification of an unmodified CTB coding sequence in chloroplasts, up to 10,000 copies per cell, resulted in the accumulation of up to 4.1% of total soluble tobacco leaf protein as oligomers (about 410 fold higher expression levels than that of the unmodified LTB gene). PCR and Southern blot analyses confirmed stable integration of the CTB gene into the chloroplast genome. Western blot analysis showed that chloroplast synthesized CTB assembled into oligomers and was antigenically identical to purified native CTB. Also, GM1,-ganglioside binding assays confirmed that chloroplast synthesized CTB binds to the intestinal membrane receptor of cholera toxin, indicating correct folding and disulfide bond formation within the chloroplast. In contrast to stunted nuclear transgenic plants, chloroplast transgenic plants were morphologically indistinguishable from untransformed plants, when CTB was constitutively expressed. The introduced gene was stably inherited in the subsequent generation as confirmed by PCR and Southern blot analyses. Incrased production of an efficient transmucosal carrier molecule and delivery system, like CTB, in transgenic chloroplasts makes plant based oral vaccines and fusion proteins with CTB needing oral administration a much more practical approach.
Owner:AUBURN UNIV +1
Fragments or polymers of albumin with tunable vascular residence time for use in therapeutic delivery and vaccine development
InactiveUS20070041987A1Maximize the effect of treatmentMaximizing abilityPeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeResidence time
An isolated fusion protein is provided which is a conjugate of a therapeutic polypeptide and an albumin fragment, such as a fragment including an individual domain or subdomain of albumin, or a polymer of albumin (e.g., dimers, trimers, etc.) and which is used to optimize the half-life of that therapeutic agent in the bloodstream in a tunable fashion based on the molecular weight of the fragment or polymer. Albumin fragments useful in the invention include fragments containing any of the individual domains and subdomains of human serum albumin, as well as fragments including specific combinations of binding regions or subdomains. The present invention thus provides fragments or polymers which will allow for optimizing half-lives of therapeutic polypeptides depending on their molecular weight, and this will optimizes protein and vaccine therapeutics to have desired half lives for their greatest effectiveness.
Owner:NEW CENTURY PHARMA INC
Latex enhanced immunoturbidimetry kit for detection of asymmetric dimethylarginine content
ActiveCN102628868AThe detection process is fastHigh detection specificityBiological testingCompetitive bindingLatex particle
The invention relates to a latex enhanced immunoturbidimetry kit for the detection of asymmetric dimethylarginine content. Specifically, the invention relates to a latex enhanced immunoturbidimetry kit for the detection of asymmetric dimethylarginine (ADMA) content in human blood samples. The kit comprises a mouse anti-human monoclonal antibody solution, an ADMA-human Serum albumin complex crosslinked latex particle expansion and an ADMA calibrator. According to the method, by the utilization of competitive binding of free ADMA in blood and ADMA crosslinked on the surface of latex particles to ADMA monoclonal antibody, turbidity formed by the reaction between the ADMA-crosslinked latex particles and ADMA monoclonal antibody is reduced. Therefore, the content of ADMA is detected through the reduction degree of turbidity. The kit provided by the invention can be applied in a biochemical analyzer commonly-used in clinic, is convenient and rapid to operate and has strong specificity. Both sensitivity and detection range of the kit can satisfy clinic application needs.
Owner:BEIJING STRONG BIOTECH INC
Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
The invention relates to a human amnion mesenchymal stem cell serum-free culture medium and a culture method thereof. The culture medium is formed by adding human serum albumin, human transferrin, human insulin and sodium selenite into a DMEM / F12 basic culture medium. The culture method for the culture medium comprises the following steps of: digesting human amnion by using trypsin, then digesting the human amnion by using collagenase IV and deoxyribonuclease I, and filtering the mixture to obtain single cell suspension; and adding the human serum albumin, the transferrin, the insulin and the sodium selenite into the DMEM / F12 basic culture medium in a ratio of VDMEM to VF12 of 1:1, and putting human amnion mesenchymal stem cells in a 37 DEG C CO2 incubator with saturated humidity and volume fraction of 5 percent under the serum-free condition, wherein culture in vitro and amplification are realized by solution change and transfer of culture, potentiality of multi-direction differentiation is maintained, and the amplified cells can be induced in vitro to form cartilage cells, osteoblasts and adipocytes. The culture medium and the culture method have the characteristics of no other animal sources, wide source and no limitation of ethics.
Owner:辽宁艾米奥干细胞与再生医学研究院有限公司
Technique for separating and purifying recombination human serum albumin and fusion protein thereof
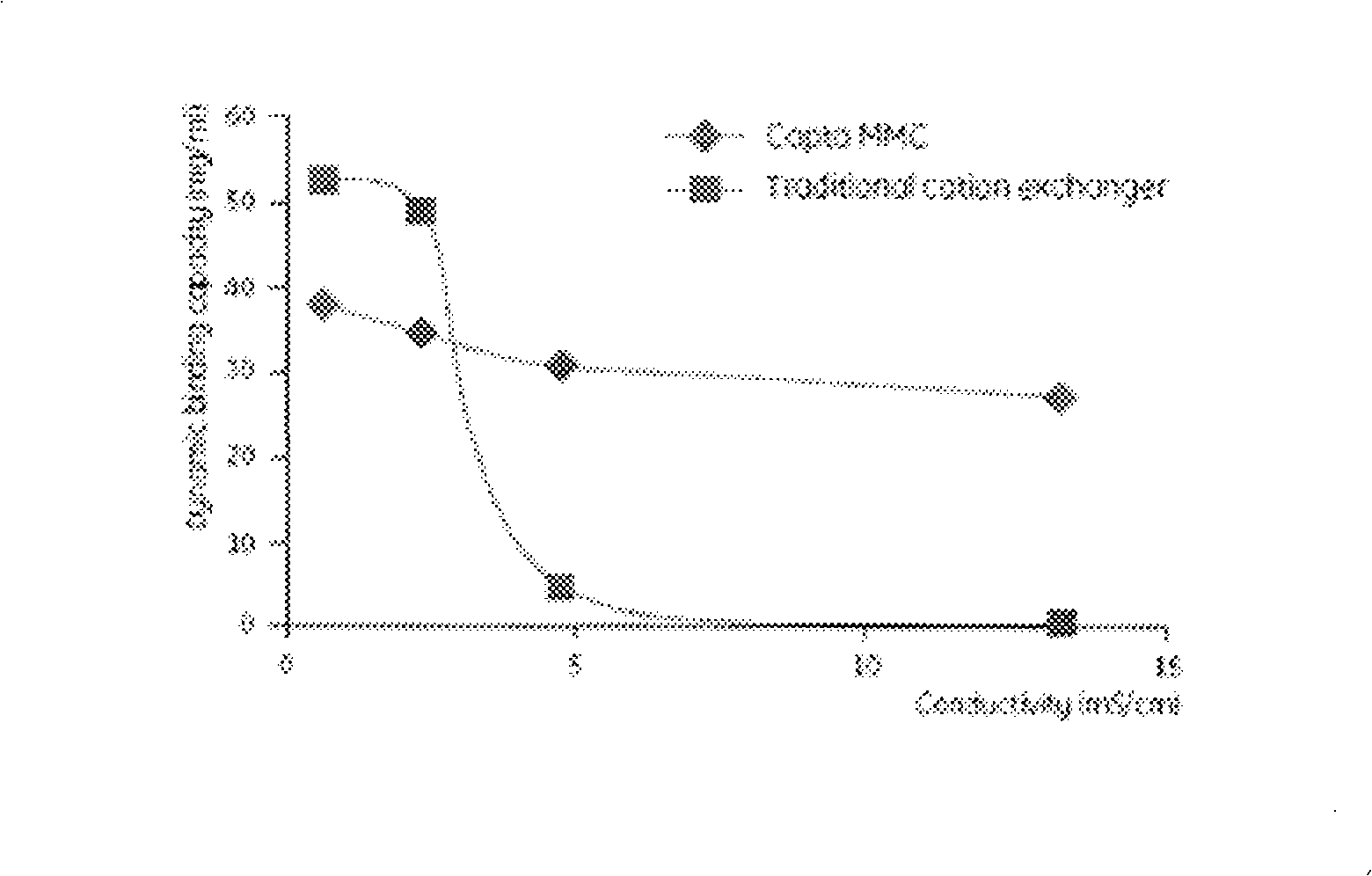
ActiveCN101260145ASimplified purification stepsUniquePeptide preparation methodsHybrid peptidesProtein targetIon exchange
The invention provides a technological flow with versatility and high efficiency for reforming human serum albumins and fusion proteins of human serum albumins by separating and purifying target proteins. The steps of the method are as follows: the inorganic salt culture medium is used to acquire the genetic engineering microzyme fermented supernatant containing target proteins, and the serum-free culture medium is used to acquire a culture solution of reforming vertebrate cells, and ion exchange type affinity column chromatography medium Capto MMC fillers with higher salinity resistance and high capacity are directly used for separation and purification. The technological flow of the invention has the advantages of simple purification program step, uniqueness, low costs and convenient industrialization.
Owner:TIANJIN SINOBIOTECH
Recombinant human albumin fusion proteins with long-lasting biological effects
ActiveUS7244833B2Good for healthImprove stabilityBacteriaPeptide/protein ingredientsDiseaseHuman albumin
Compositions, kits and methods are provided for promoting general health or for prevention or treatment of diseases by using novel recombinant fusion proteins of human serum albumin (HSA) and bioactive molecules. The bioactive molecules may be a protein or peptide having a biological function in vitro or in vivo, and preferably, having a therapeutic activity when administered to a human. By fusing the bioactive molecule to HSA, stability of the bioactive molecule in vivo can be improved and the therapeutic index increased due to reduced toxicity and longer-lasting therapeutic effects in vivo. In addition, manufacturing processes are provided for efficient, cost-effective production of these recombinant proteins in yeast.
Owner:YU ZAILIN +1
Technique for preparing amalgamation protein skin-protection product containing albuminar and skin cell growth factor, and uses of the same
ActiveCN101172091AIncrease productionEasy to grow on a large scaleCosmetic preparationsPeptide/protein ingredientsWrinkle skinFactor ii
The present invention provides a recombinant fusion protein which stimulates the rejuvenation and reactivation of skin and epidermal cells for improving skin appearance, smoothing wrinkles and freckles, and whitening skin. Particularly, the present invention provides various types of products for improving skin, which contain recombinant fusion protein of human serum albumin (HSA) with cytokine peptides (EGF, FGF, KGF, HGH, HGF, PDGF, GCSF, interferon, IL-11 or IGF) by genetic engineering technology. The fusion protein can be used independently or in a combination or combination with yeast fermentation products, or with varied emulsifiers, thickeners, moisturizer, preservatives, yeasts and ferments.
Owner:BEIJING MEIFUYUAN BIO PHARM TECH +2
Stable pharmaceutical compositions
Pharmaceutical composition for parenteral administration comprising a glucagon-like peptide and human serum albumin or a variant thereof.
Owner:NOVO NORDISK AS
Metal enhanced fluorescence-based sensing methods
InactiveUS7939333B2Fluorescence enhancementAnalysis using chemical indicatorsAnalysis by electrical excitationFluorescenceNucleotide sequencing
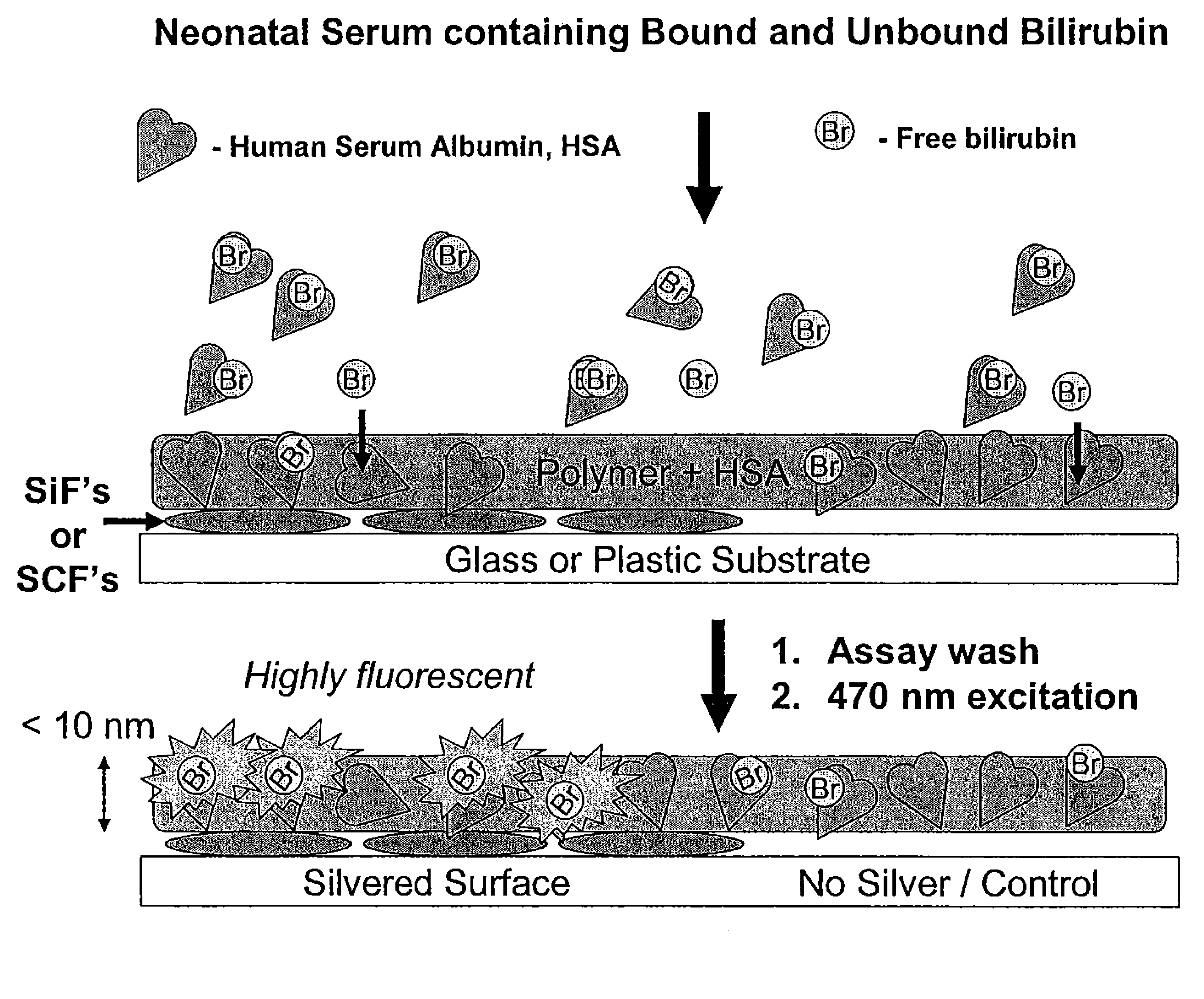
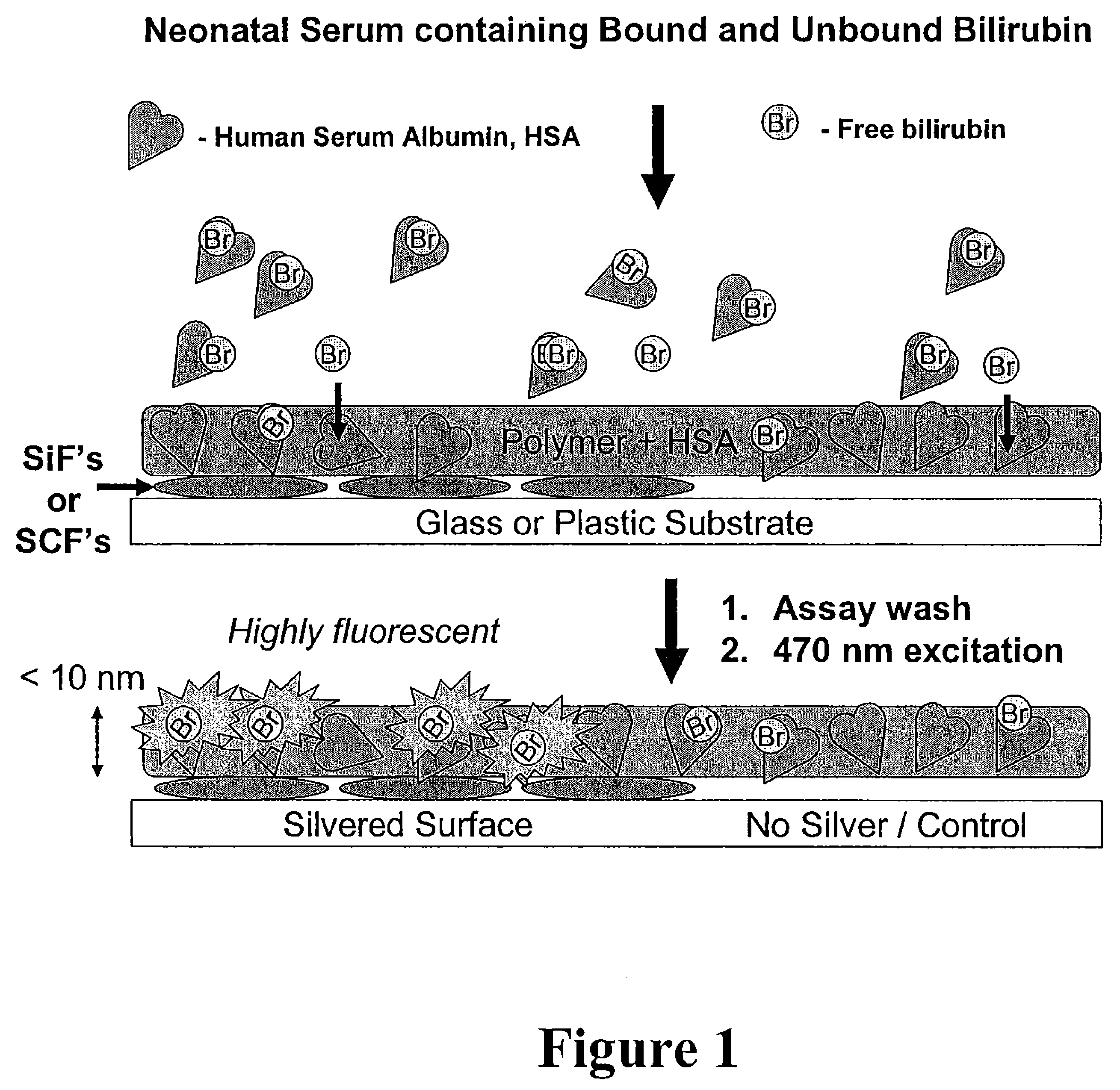
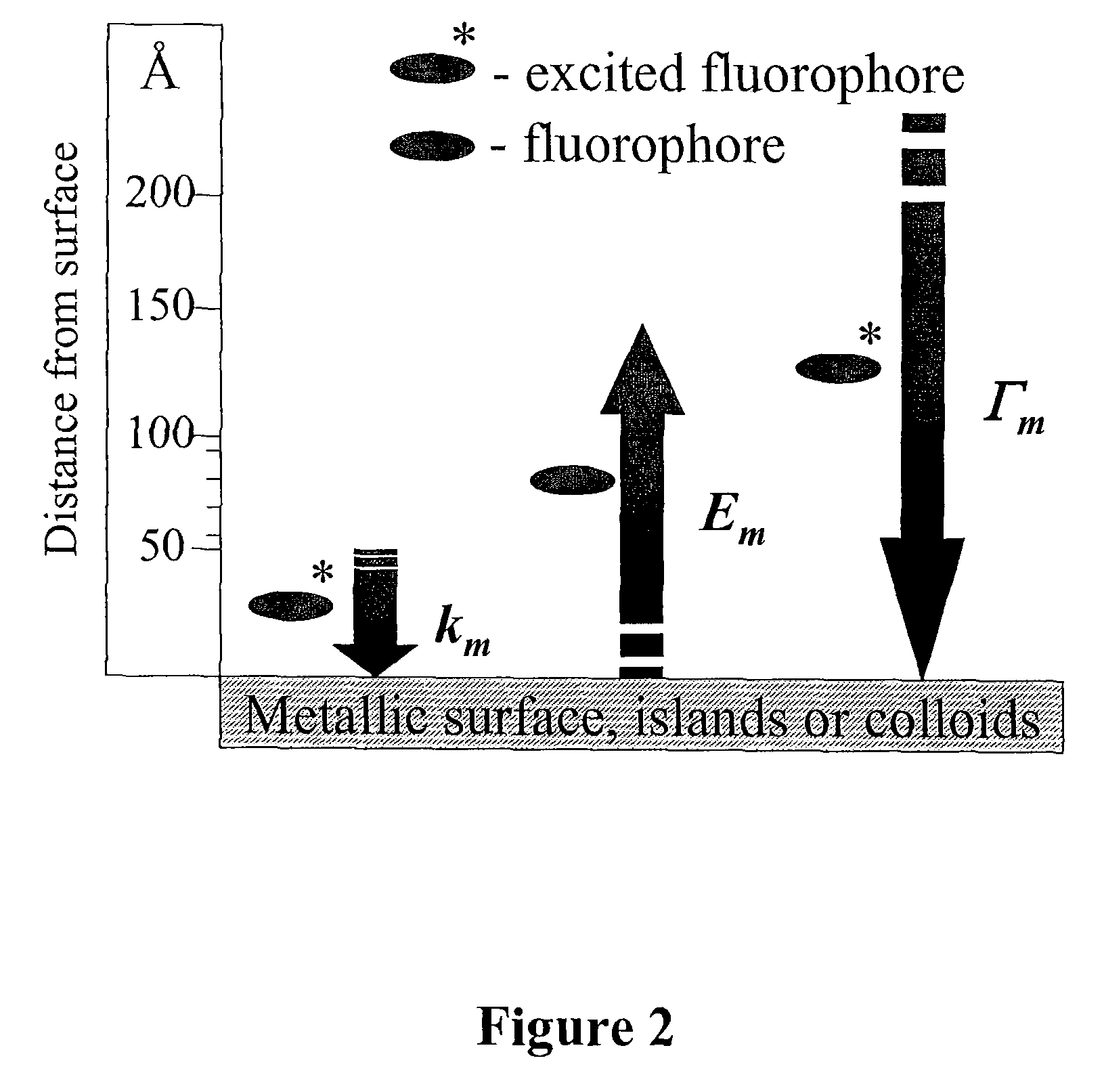
The present invention relates to metallic-surface detection systems for determining target substances including free bilirubin in neonatal serum in the presence of a predominantly high background of bilirubin bound Human Serum Albumin (HSA) or sensing and isolating target nucleotide sequences wherein a fluorescence signal is enhanced by close proximity of the target substances near metallic surfaces.
Owner:UNIV OF MARYLAND BALTIMORE +1
Human adipose-derived stem cell serum-free basic medium
ActiveCN102732477BOvercome riskSkeletal/connective tissue cellsMineral ascorbatesAscorbic acid 2-sulfate
The invention discloses a human adipose-derived stem cell serum-free basic medium. The medium uses a serum substitute, and is composed of a high glucose type DMEM basic medium, human serum albumin, transferrin, taurine, reduced glutathione, ceruloplasmin, L-ascorbic acid-2-sulfate, alpha-tocopherol succinate, linoleic acid, alpha-ketoglutarate and selenium. The serum-free basic medium can exempt potential threats caused by animal serum in conventional serum-containing mediums to human health, and the adipose-derived stem cells cultured by the medium is more suitable for clinical application.
Owner:JIANGSU RE STEM BIOTECH
Dried blood factor composition comprising trehalose
A stable blood factor composition contains a stabilising amount of trehalose in the absence of human serum albumin to provide a product stable at up to 60° C.
Owner:QUANDRANT HLDG CAMBRIDGE LTD
Paclitaxel-based antitumor formulation
InactiveUS20070020337A1Simplifies plantFinal yieldPowder deliveryBiocideNanoparticle ProductionAlbumin solution
Antitumor formulation based on nanoparticles of paclitaxel and human serum albumin as obtained by the addition of a biocompatible acid to an aqueous albumin solution before this is mixed with paclitaxel during the nanoparticle production process, the injectable solutions of this formulation having a pH between 5.4 and 5.8 and having stability and inalterability with time.
Owner:ABRAXIS BIOSCI LLC
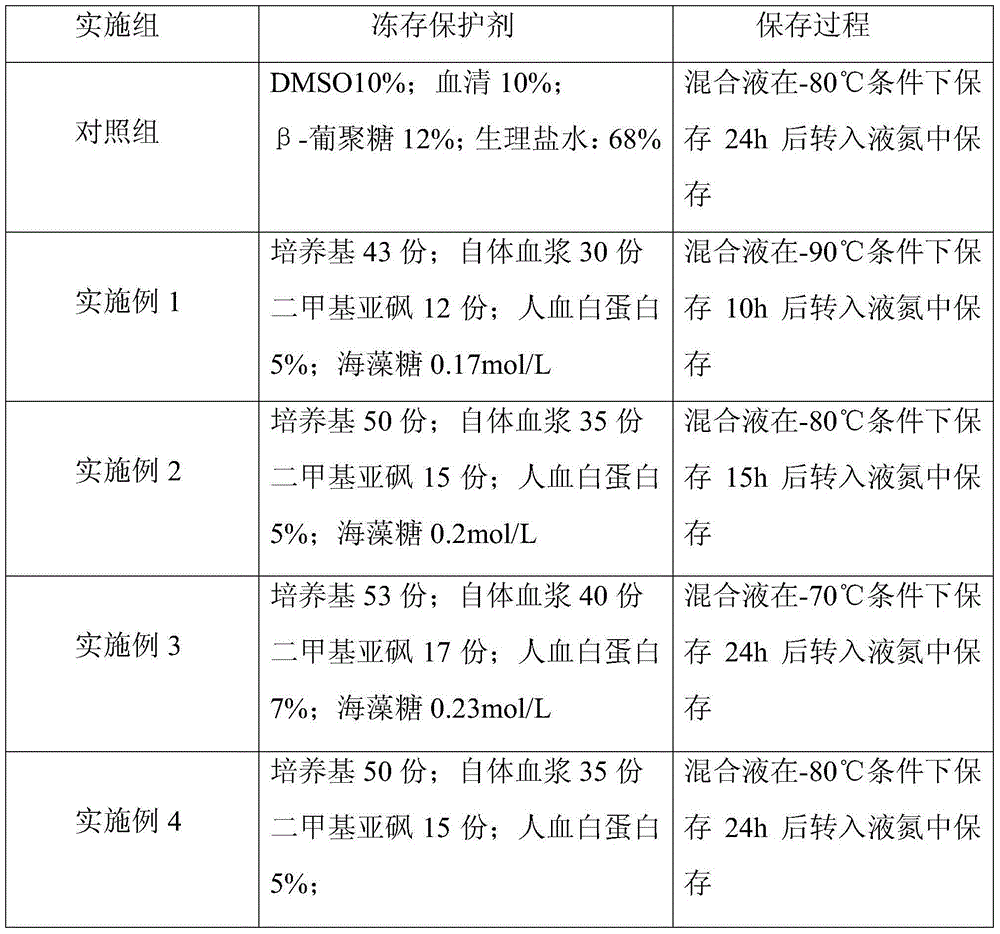
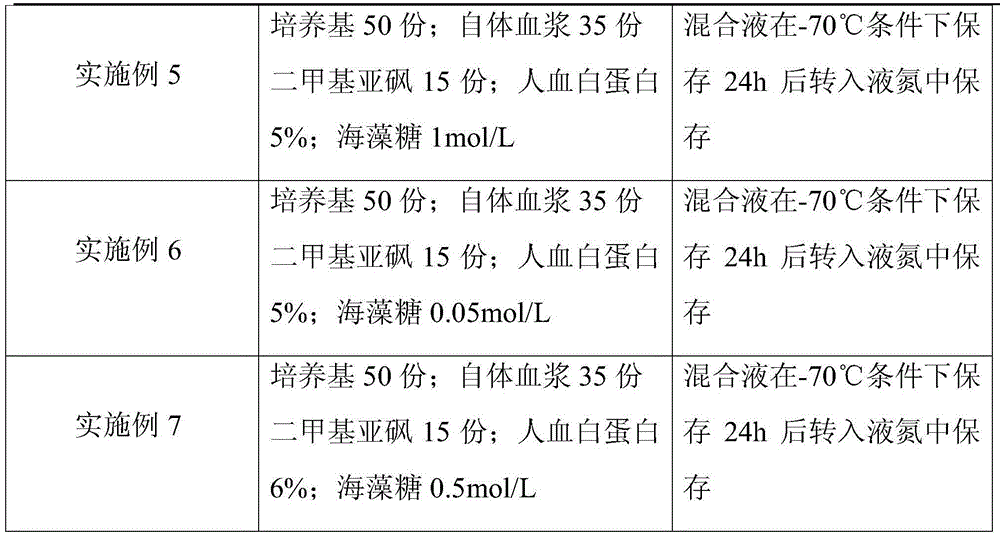
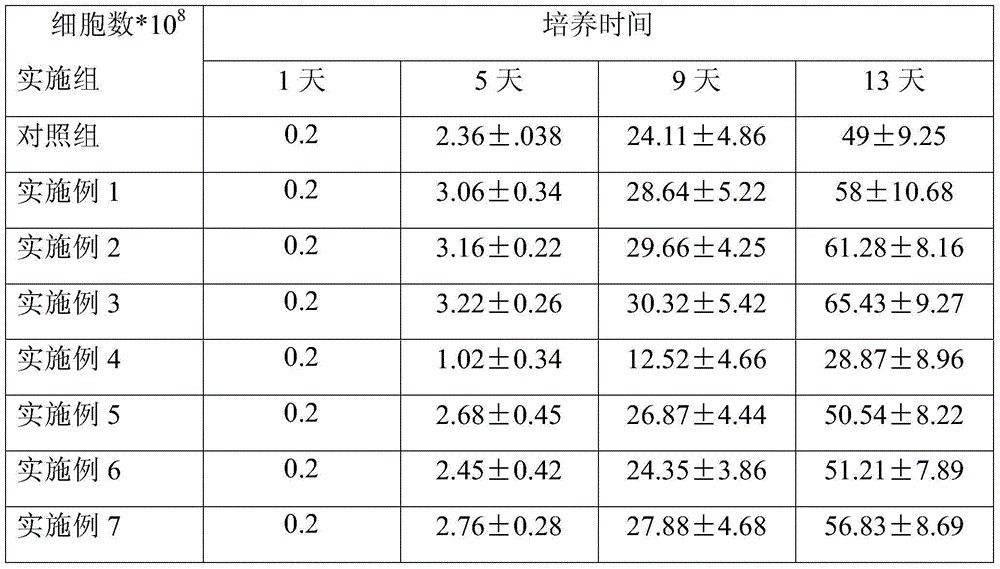
Cryoprotective agent of peripheral blood mononuclear cells and preservation method of cryoprotective agent
ActiveCN104082277AImprove survival rateImprove cell activityDead animal preservationPeripheral blood mononuclear cellMotility
The invention discloses a cryoprotective agent of peripheral blood mononuclear cells. The cryoprotective agent is prepared from the following ingredients in parts by weight: 43-53 parts of a culture medium, 30-40 parts of auto-plasma, 12-17 parts of dimethyl sulfoxide, 5-7% of human serum albumin, and the balance being 0.17-0.23mol / L trehalose. The invention discloses a preservation method of the cryoprotective agent. The preservation method comprises the steps of collecting fresh anticoagulation blood, preparing peripheral mononuclear cells into cell suspension by using human serum albumin, adding the cryoprotective agent with the same volume as that of the cell suspension, mixing uniformly, preserving for 10-20h at below 90-below-70-below DEG C, and preserving in liquid nitrogen. According to the cryoprotective agent and the preservation method thereof, a cryopreserved cell has the high motility rate and the strong cell activity and can shorten the Cytokine Induced Killer (CIT) cell induced amplification period.
Owner:CHENGDU QINGKE BIOTECH
Therapeutic composition with a botulinum neurotoxin
ActiveUS7879341B2Low immunogenicityImprove stabilityCosmetic preparationsSenses disorderGenetics manipulationHuman serum albumin
The present invention pertains to pharmaceutical compositions which comprise a botulinum neurotoxin from Clostridium botulinum, the neurotoxin being free of the complexing proteins naturally present in the botulinum neurotoxin complex or being chemically modified or being modified by genetic manipulation. Moreover the pharmaceutical compositions of the instant invention have good stability and are advantageously formulated free of human serum albumin.
Owner:MERZ PHARMA GMBH & CO KGAA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com