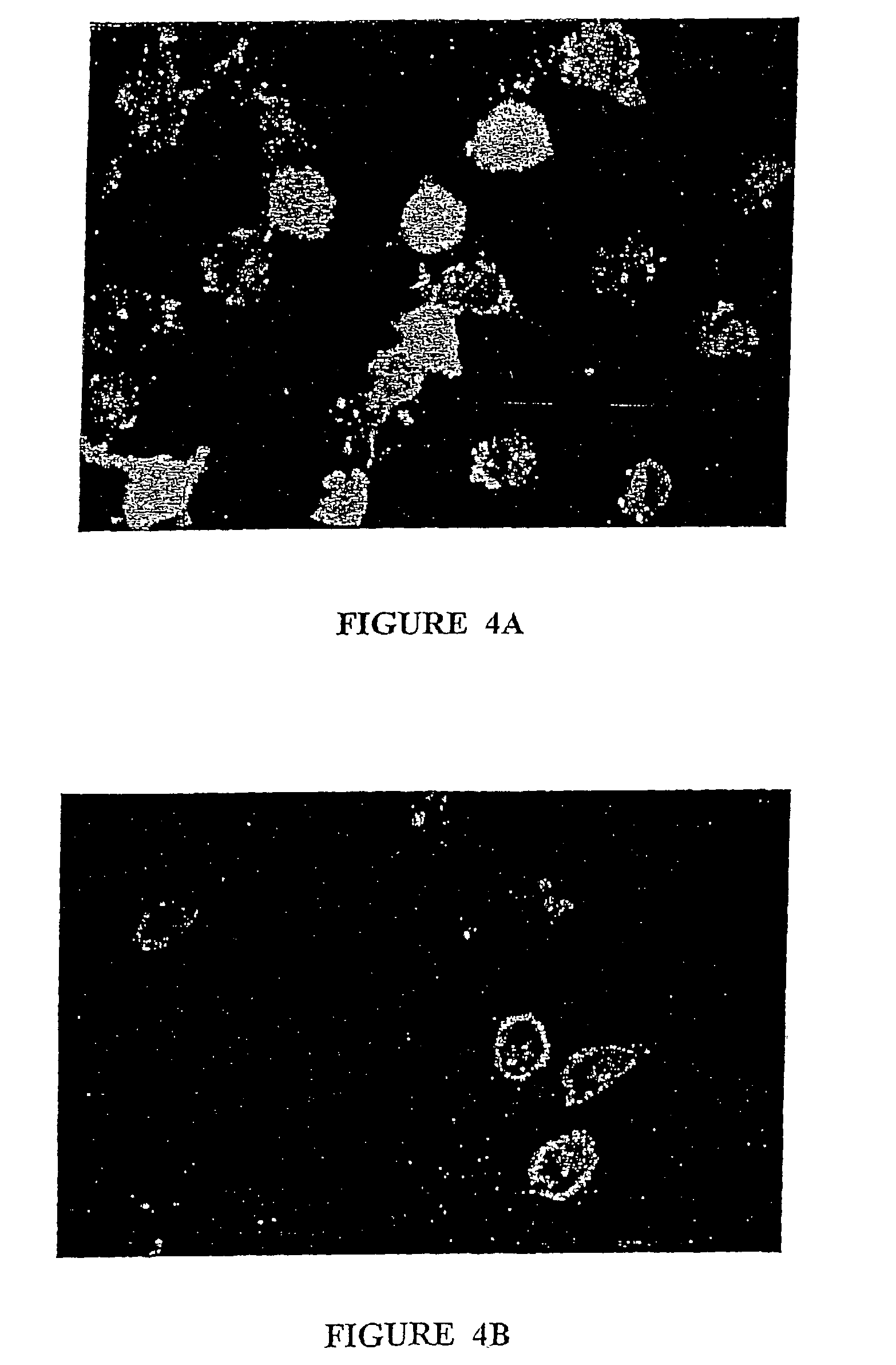
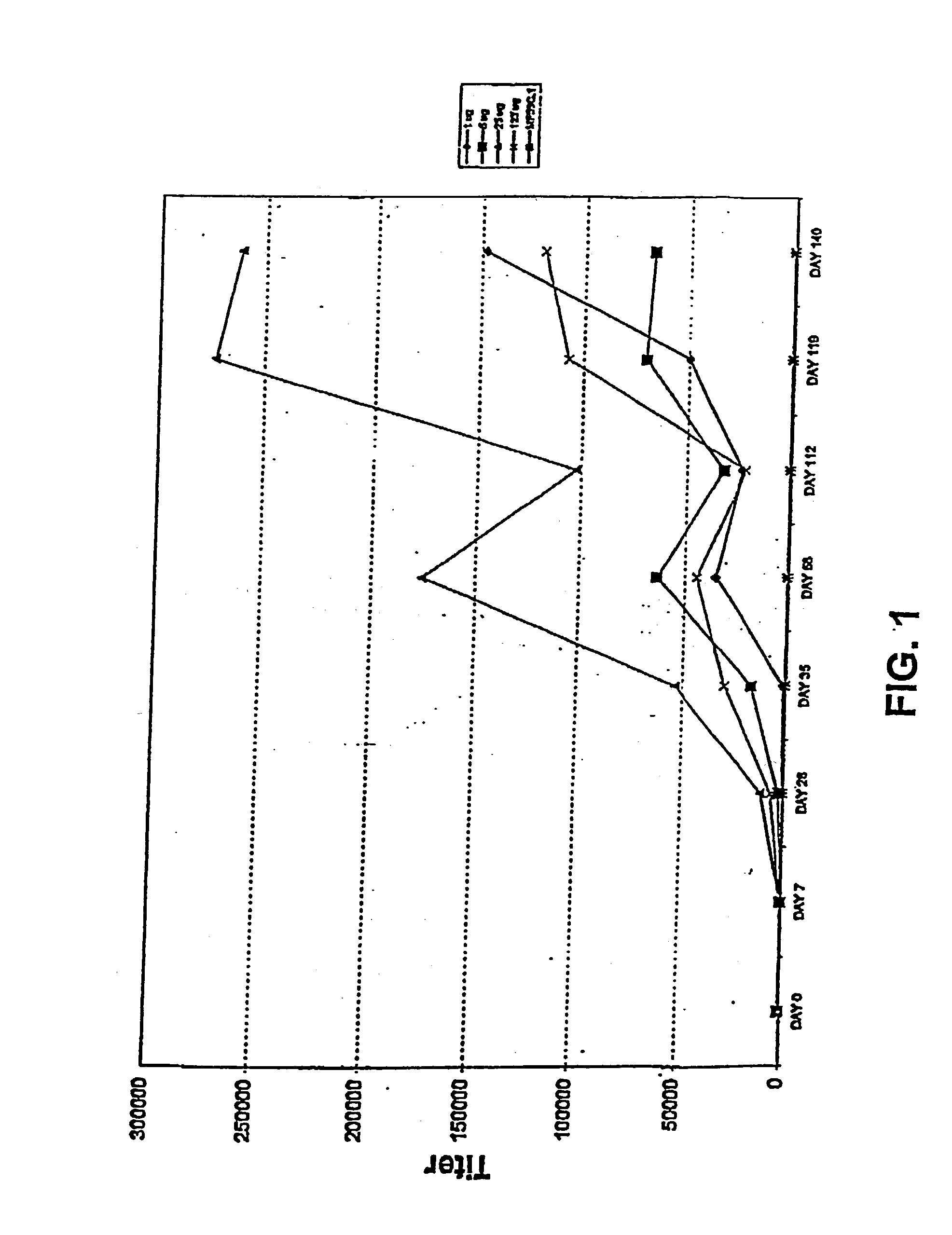
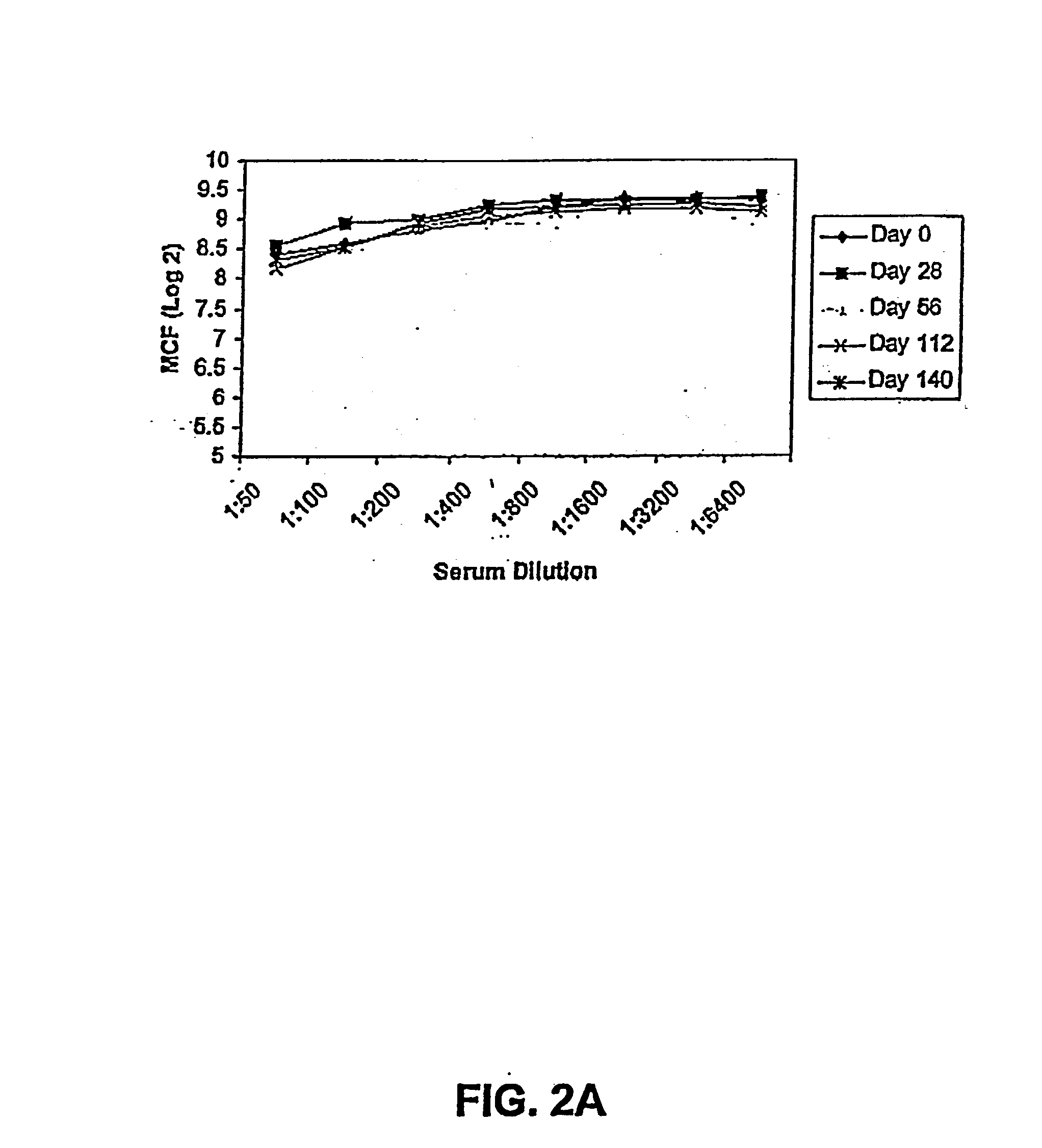
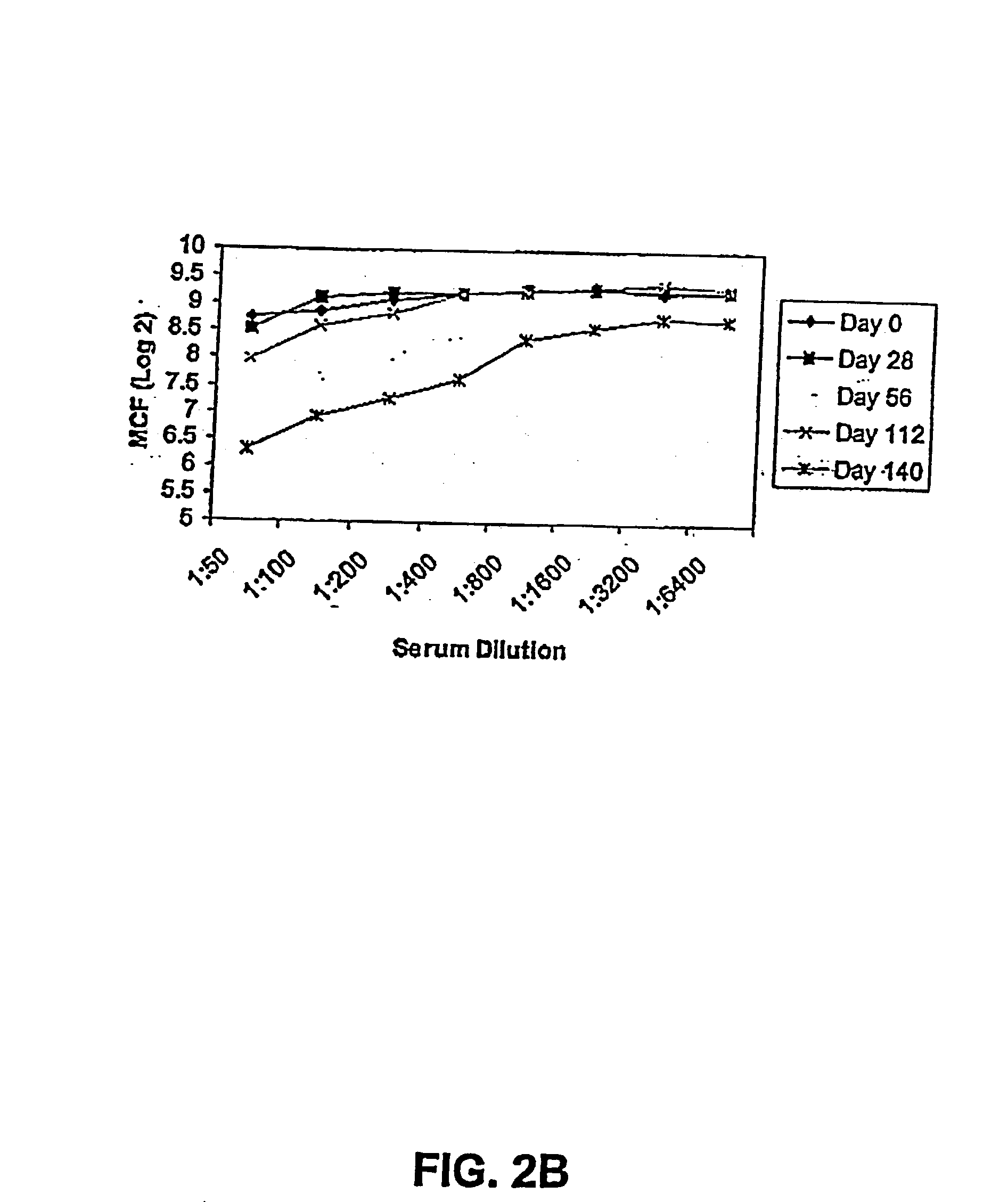
Patents
Literature
226 results about "Primate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A primate (/ˈpraɪmeɪt/ PRY-mayt) (from Latin primat-, from primus: "prime, first rank") is a eutherian mammal constituting the taxonomic order Primates. Primates arose 85–55 million years ago from small terrestrial mammals (Primatomorpha), which adapted to living in the trees of tropical forests: many primate characteristics represent adaptations to life in this challenging environment, including large brains, visual acuity, color vision, altered shoulder girdle, and dexterous hands. Primates range in size from Madame Berthe's mouse lemur, which weighs 30 g (1 oz), to the eastern gorilla, weighing over 200 kg (440 lb). There are 190–448 species of living primates, depending on which classification is used. New primate species continue to be discovered: over 25 species were described in the first decade of the 2000s, and eleven since 2010.
Serum albumin binding proteins with long half-lives
InactiveUS20070269422A1Increased serum half-lifeReduce dosing frequencyOrganic active ingredientsPeptide/protein ingredientsSerum igePrimate
The present invention relates to amino acid sequences that are capable of binding to serum albumin; to compounds, proteins and polypeptides comprising or essentially consisting of such amino acid sequences; to nucleic acids that encode such amino acid sequences, proteins or polypeptides; to compositions, and in particular pharmaceutical compositions, that comprise such amino acid sequences, proteins and polypeptides; and to uses of such amino acid sequences, proteins and polypeptides. Particularly, the amino acid sequences and compounds of the present invention bind to or otherwise associate with serum albumin in such a way that, when the amino acid sequence or compound is bound to or otherwise associated with a serum albumin molecule in a primate, it exhibits a serum half-life of at least 50% of the natural half-life of serum albumin in said primate.
Owner:ABLYNX NV
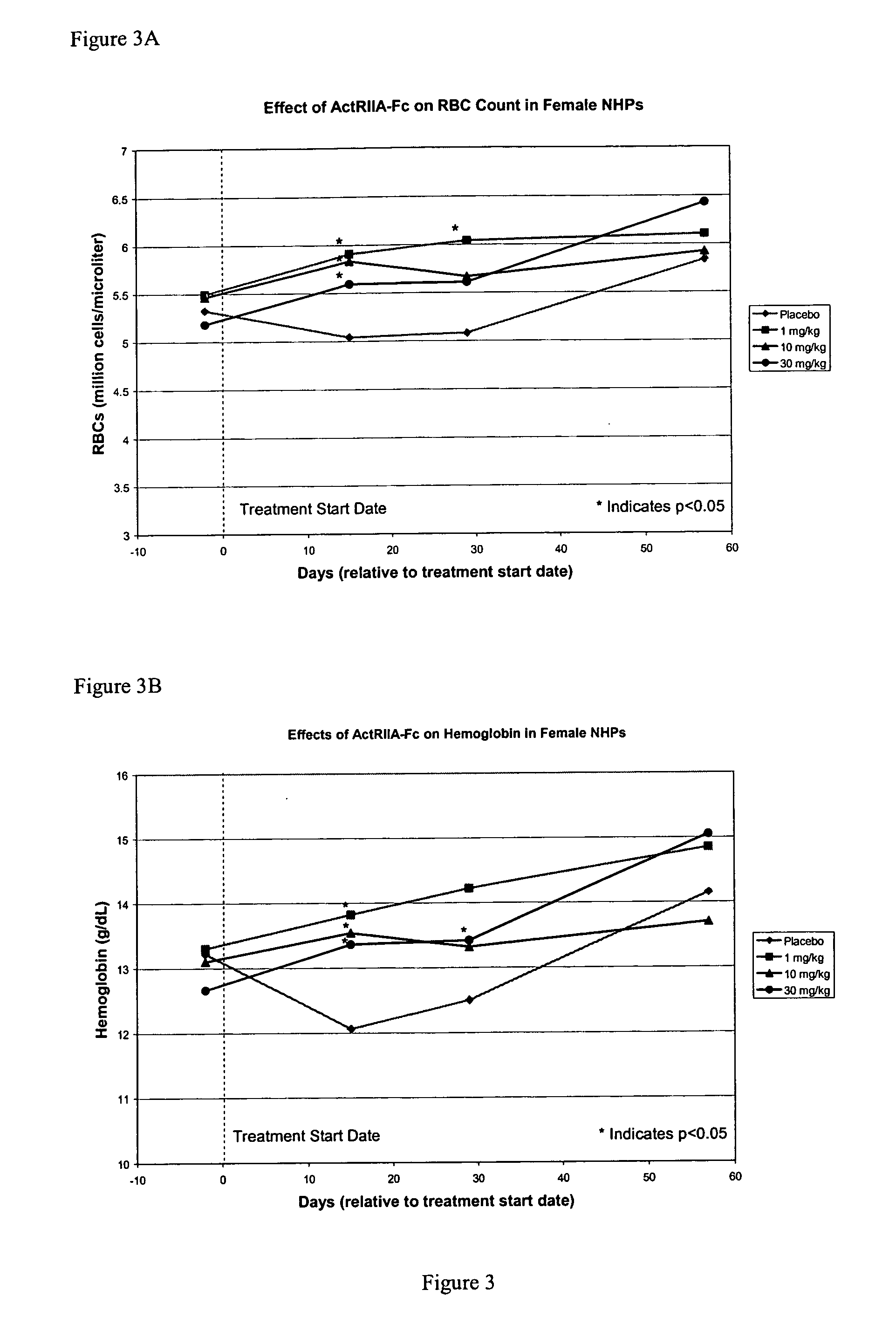
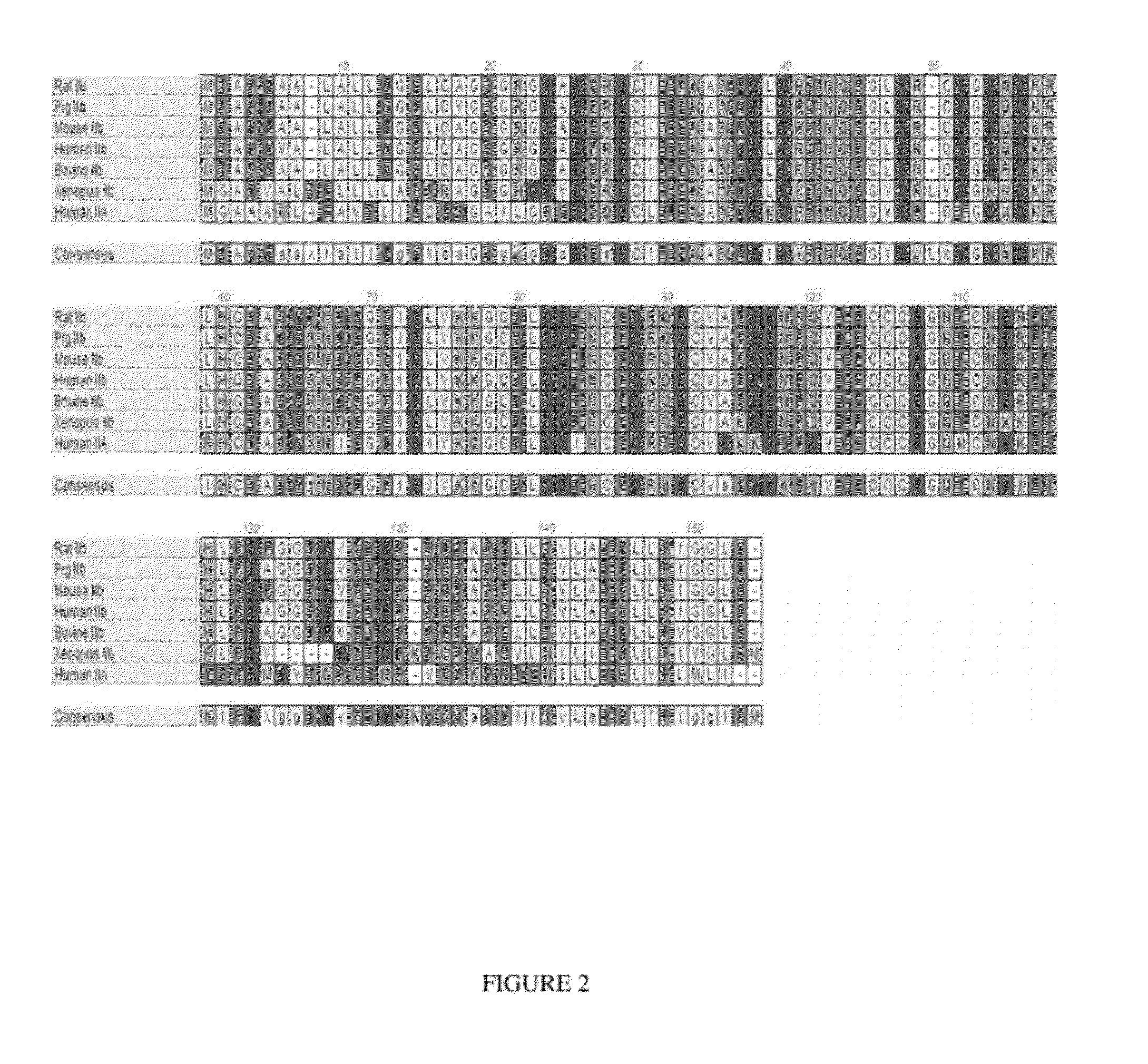
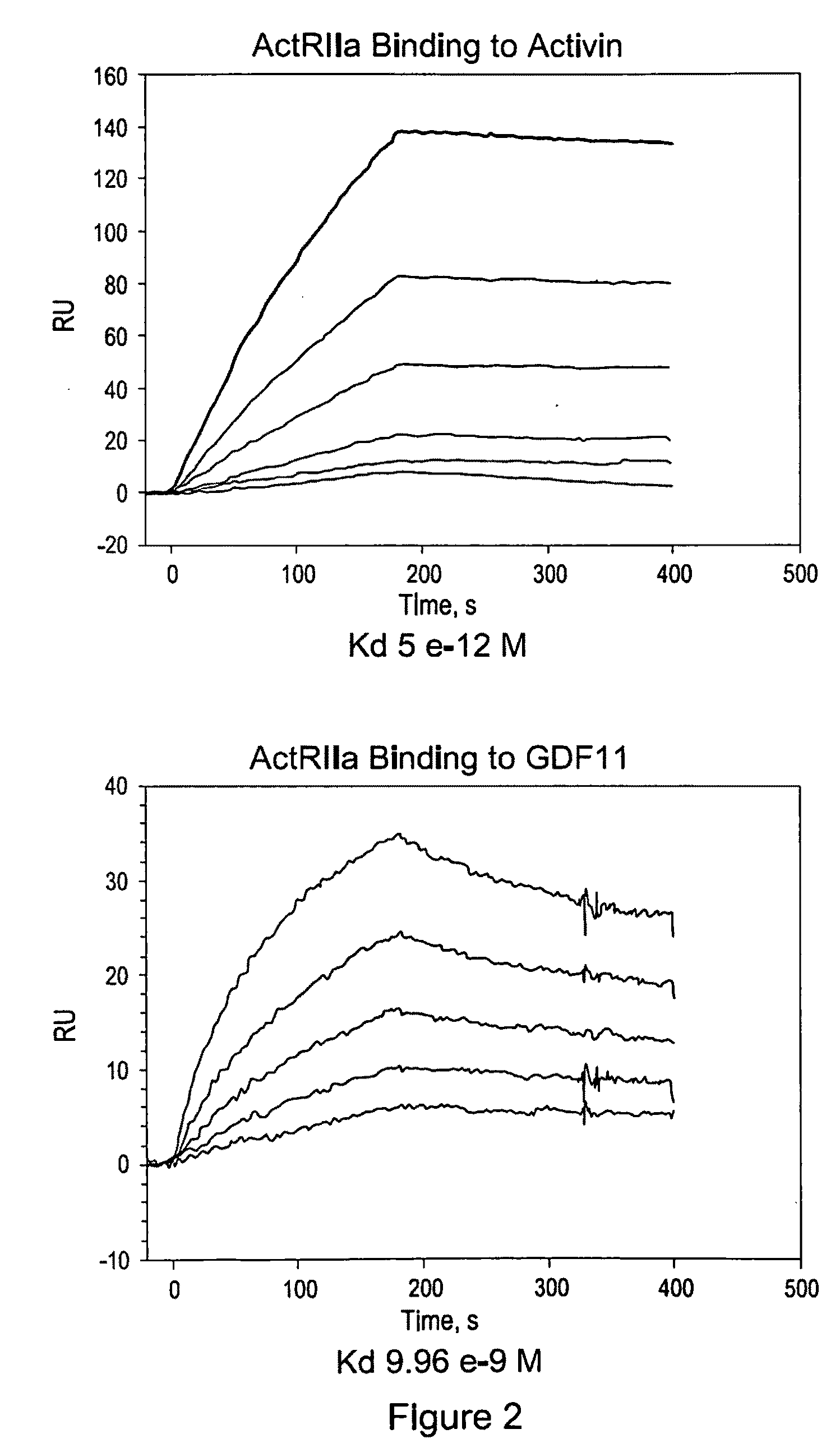
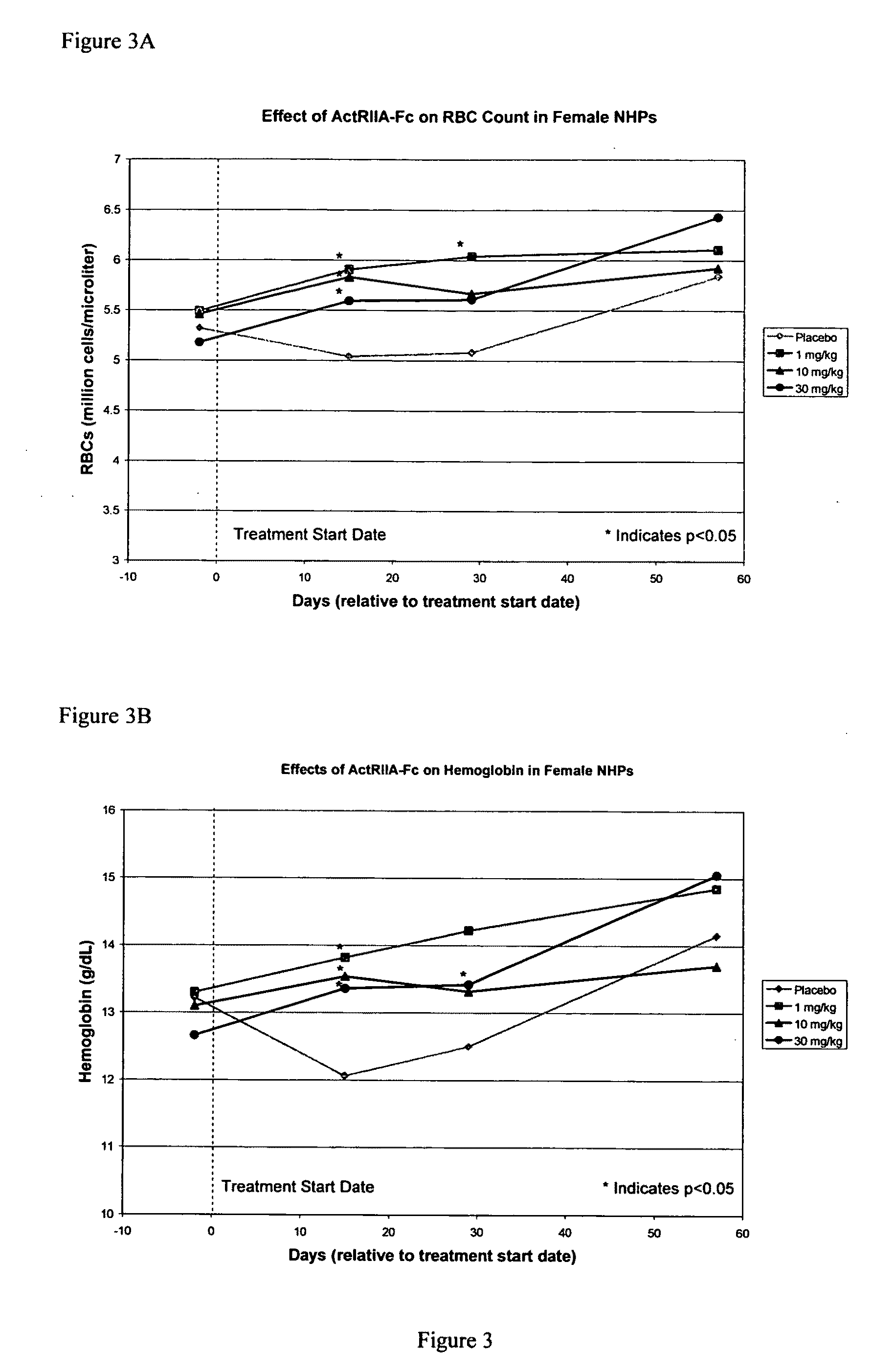
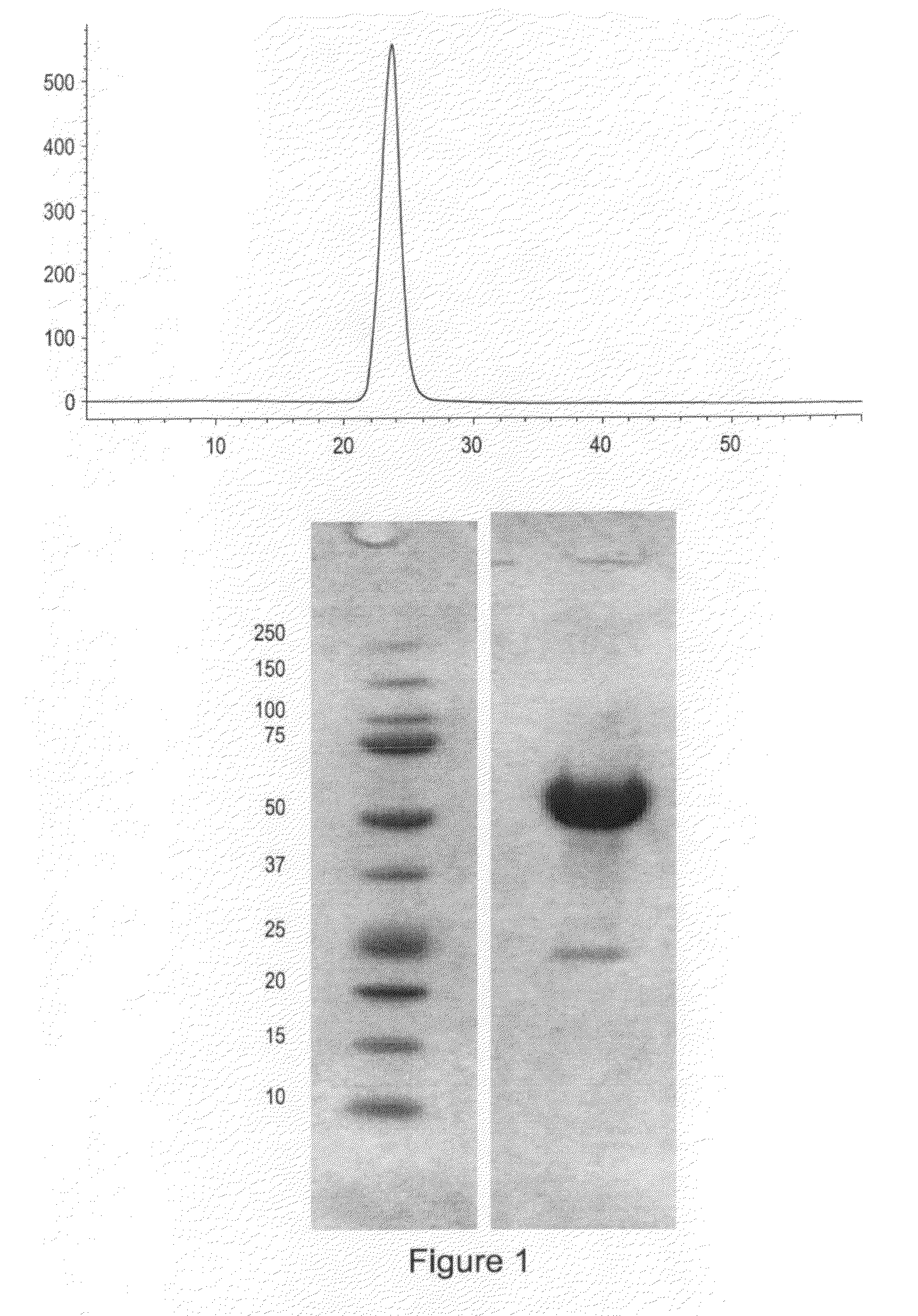
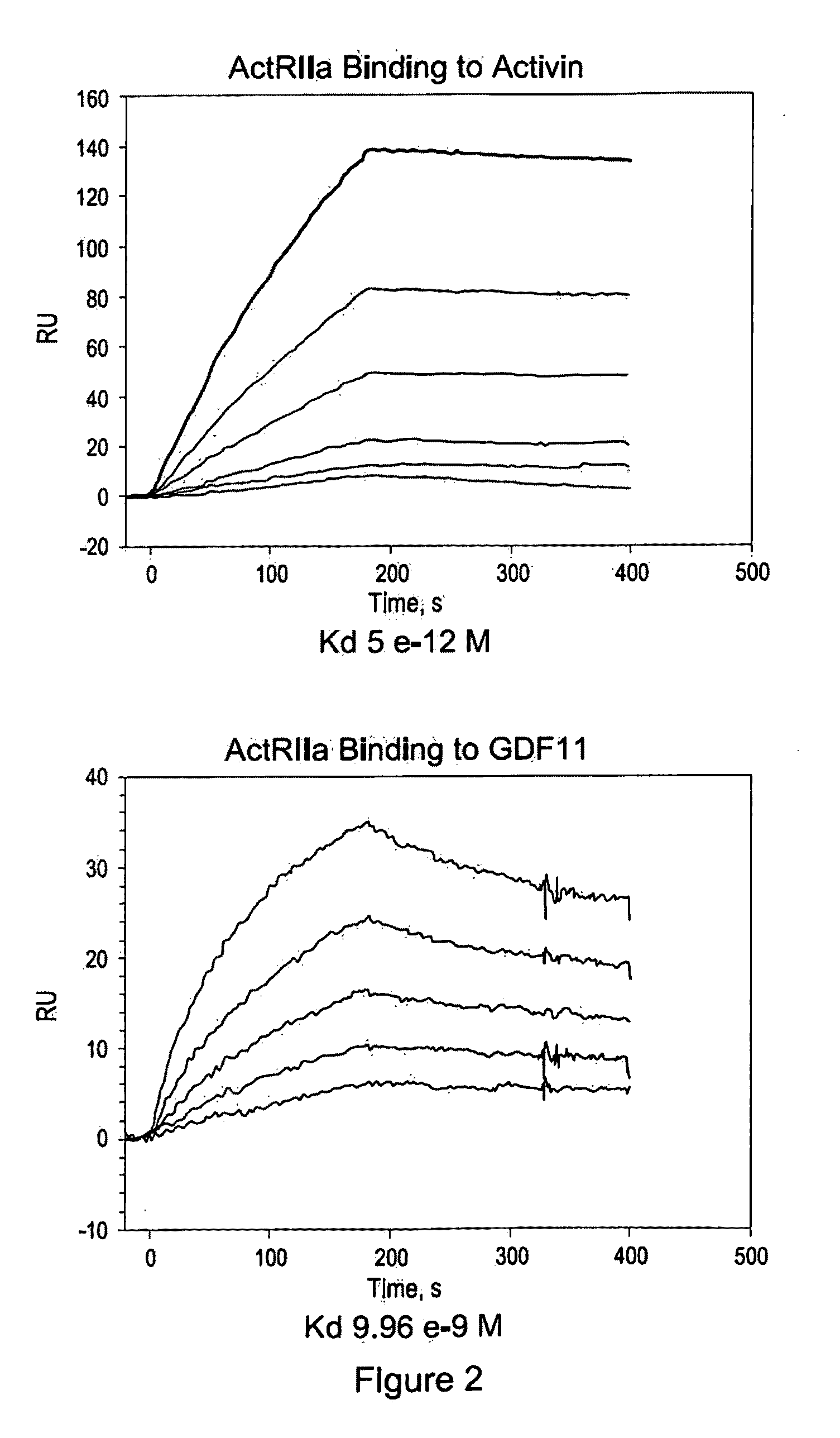
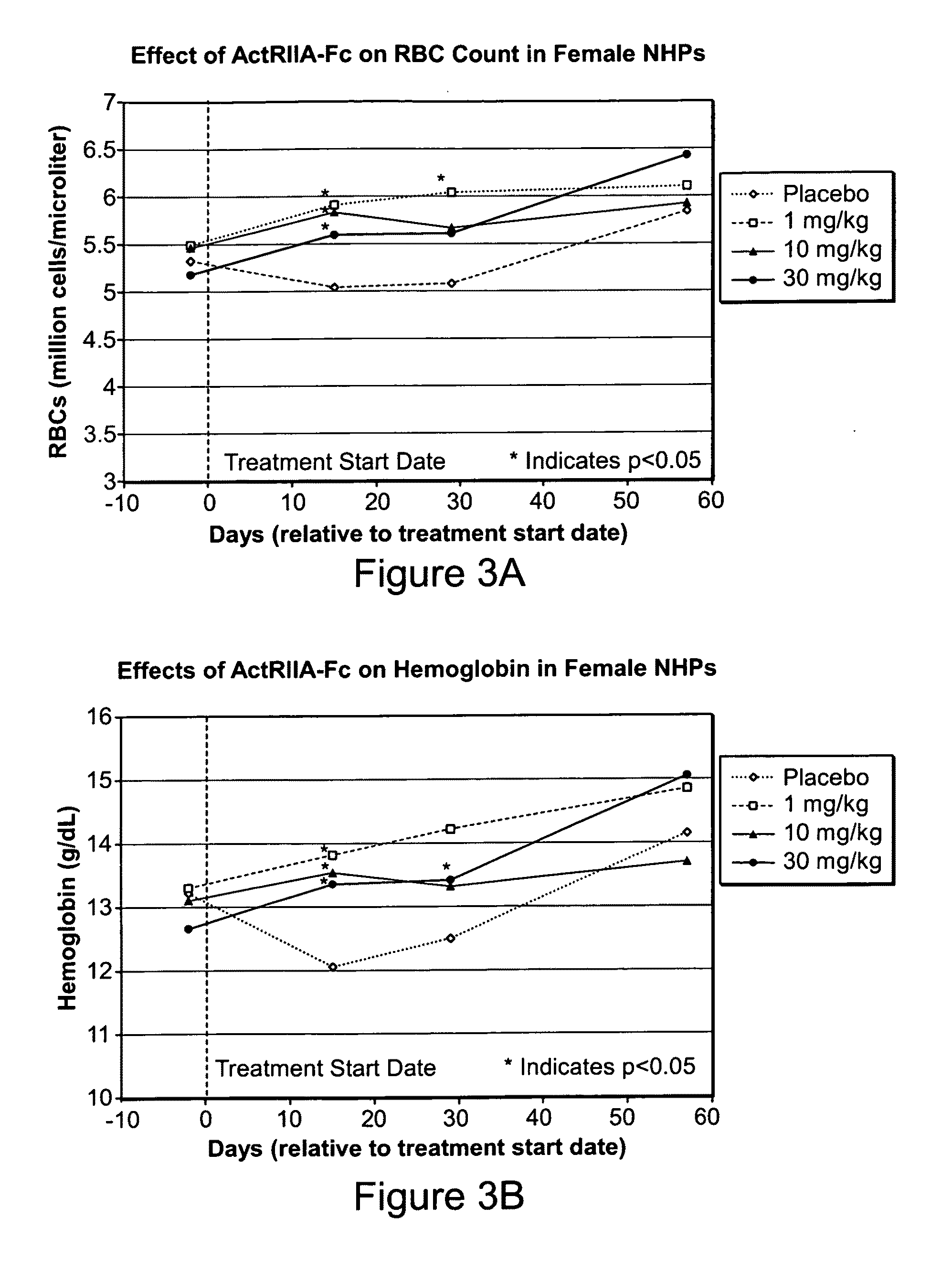
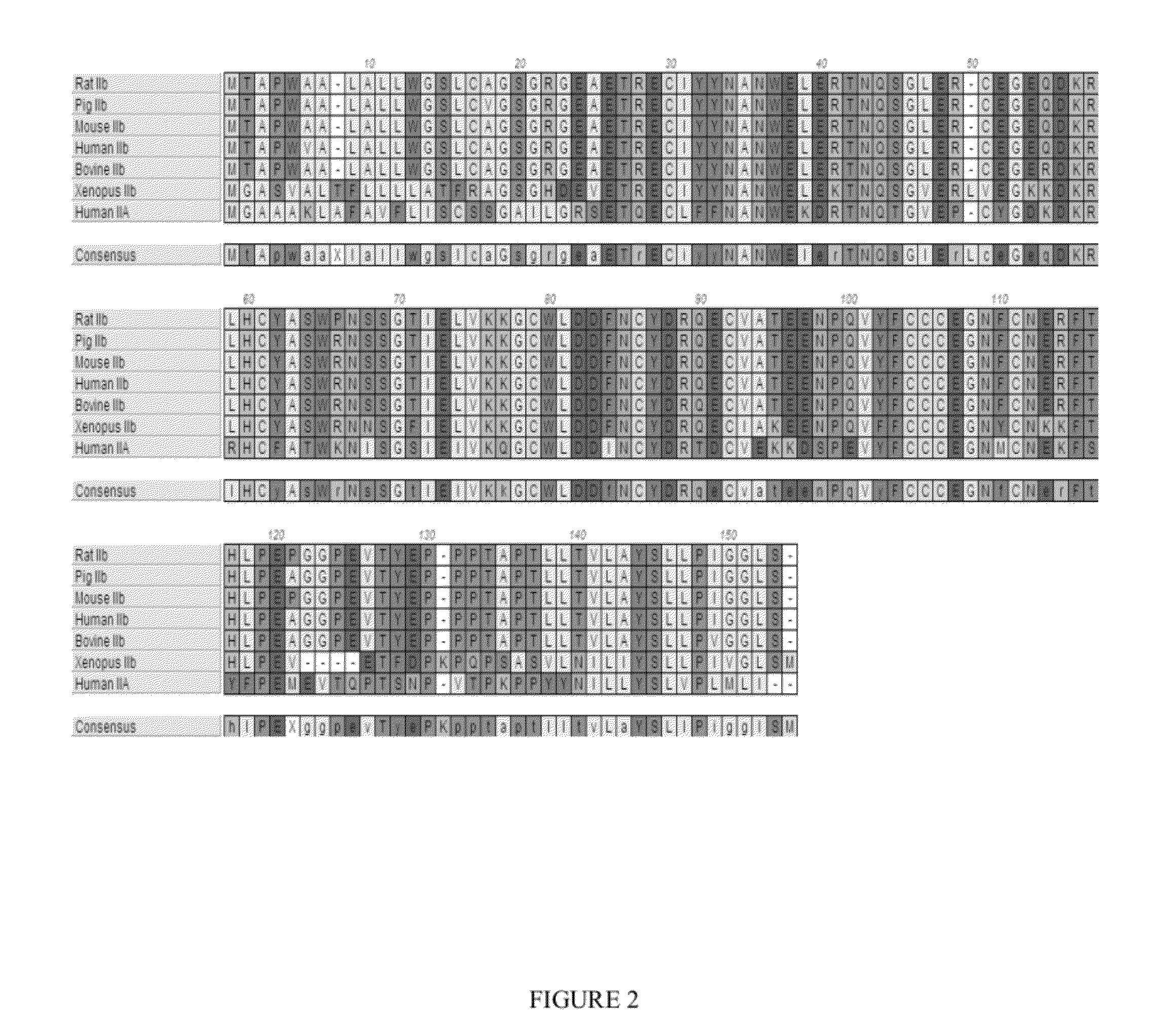
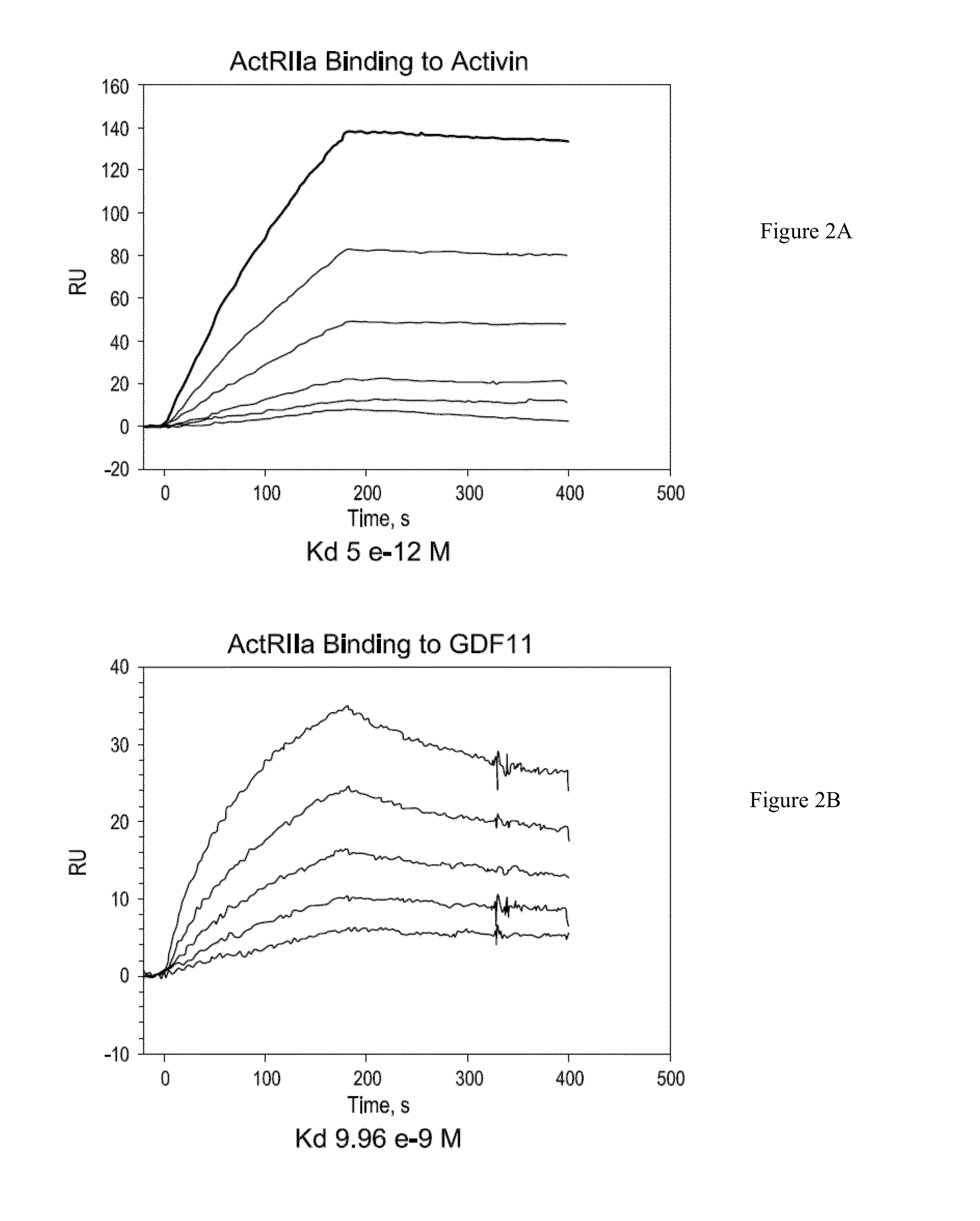
Use of GDF traps to increase red blood cell levels
ActiveUS20100068215A1Improve pharmacokineticsImprove purification effectPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed Cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Activin-ActRII antagonists and uses for increasing red blood cell levels
ActiveUS20090047281A1Easy to produceReduce expressionCompound screeningApoptosis detectionPrimateRed Cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Gynogenetic or androgenetic production of pluripotent cells and cell lines, and use thereof to produce differentiated cells and tissues
InactiveUS20040014206A1Rejection is prevented and reducedEliminate, orMammal material medical ingredientsSkeletal/connective tissue cellsPrimateEmbryo
Methods for obtaining pluripotent (embryonic stem) cells from parthenogenetic embryos, especially primates, are provided. These cells are useful for producing differentiated cells, tissues and organs, especially human and non-human primate cells, tissues and organs.
Owner:UNIV OF MASSACHUSETTS
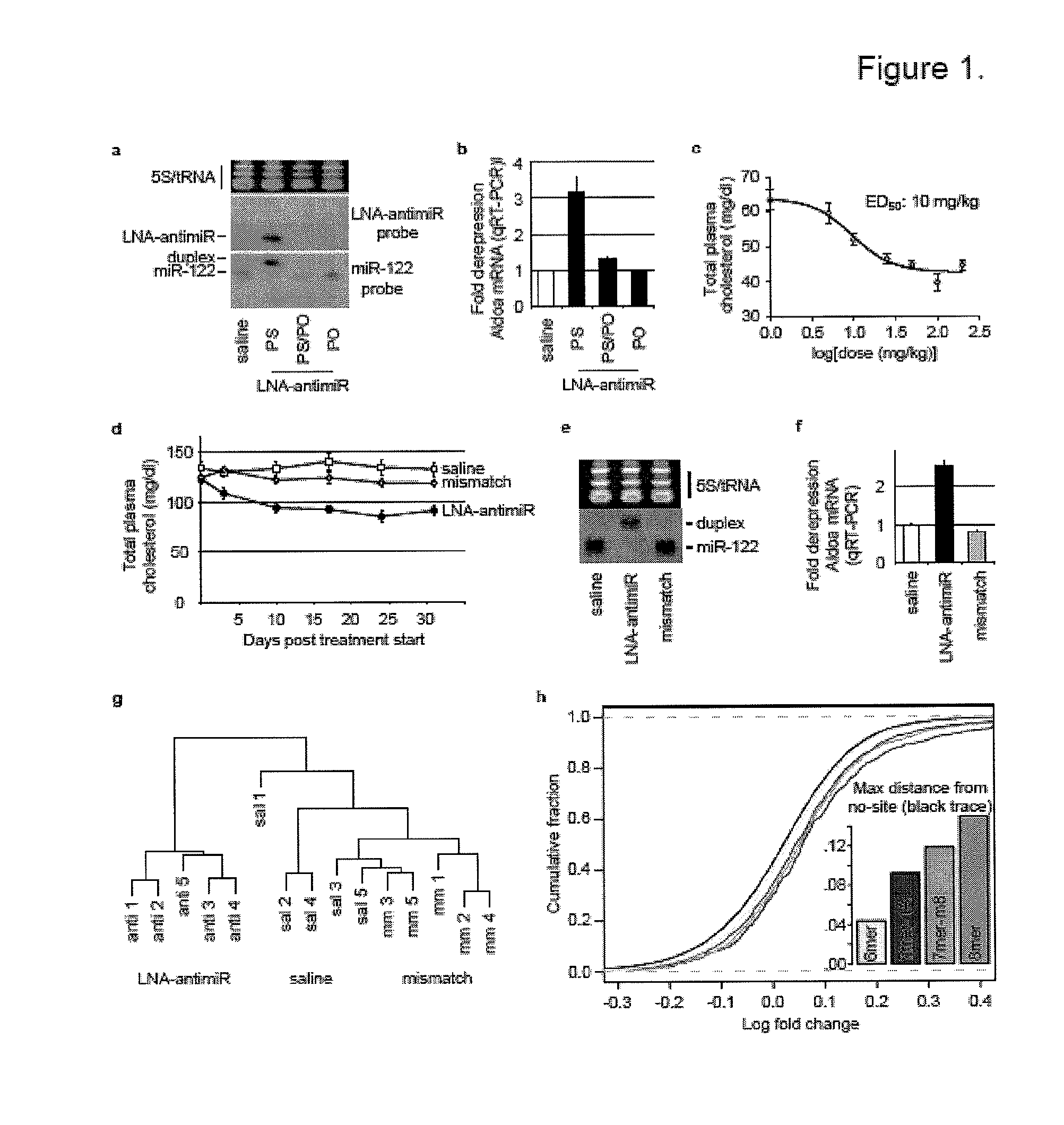
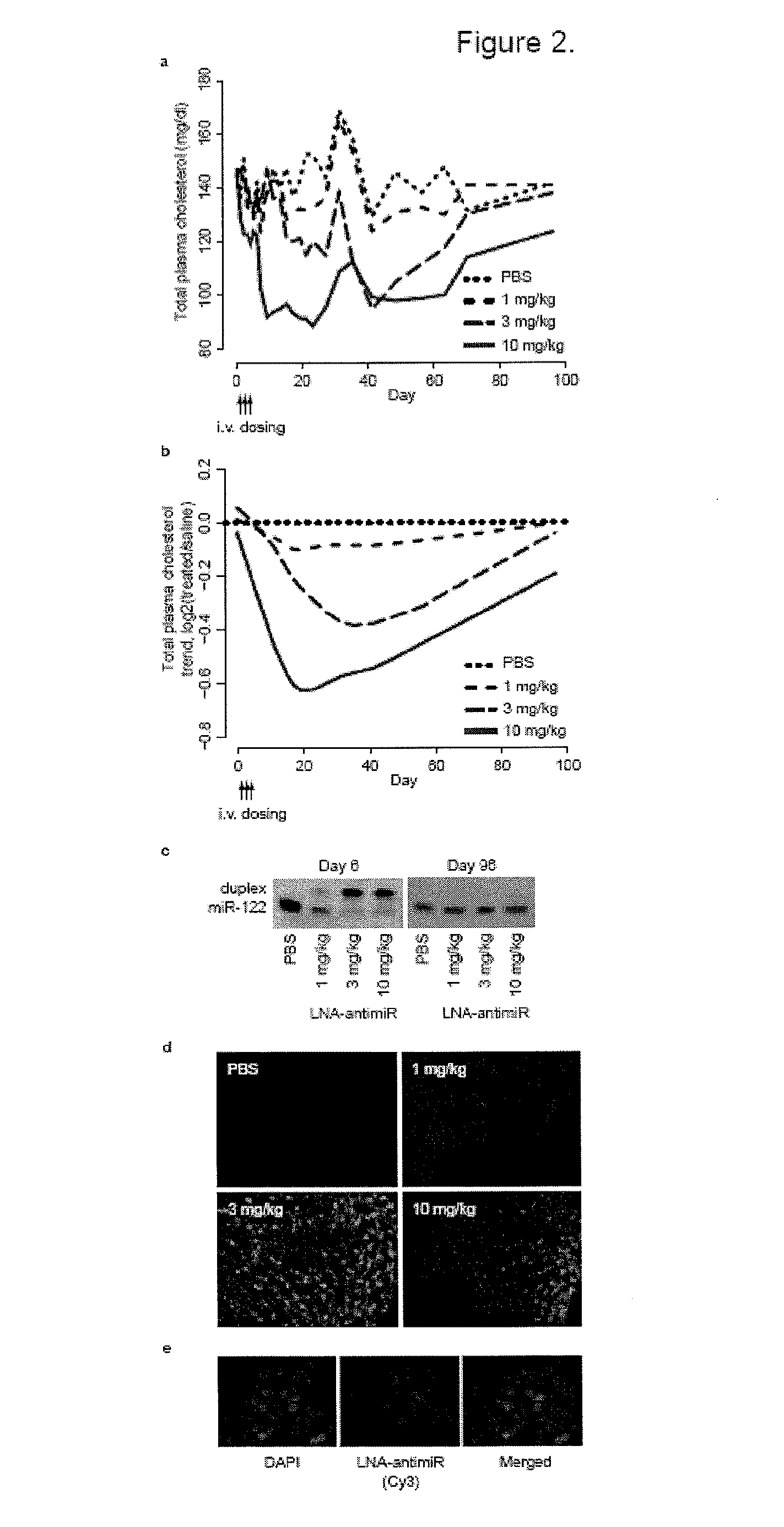
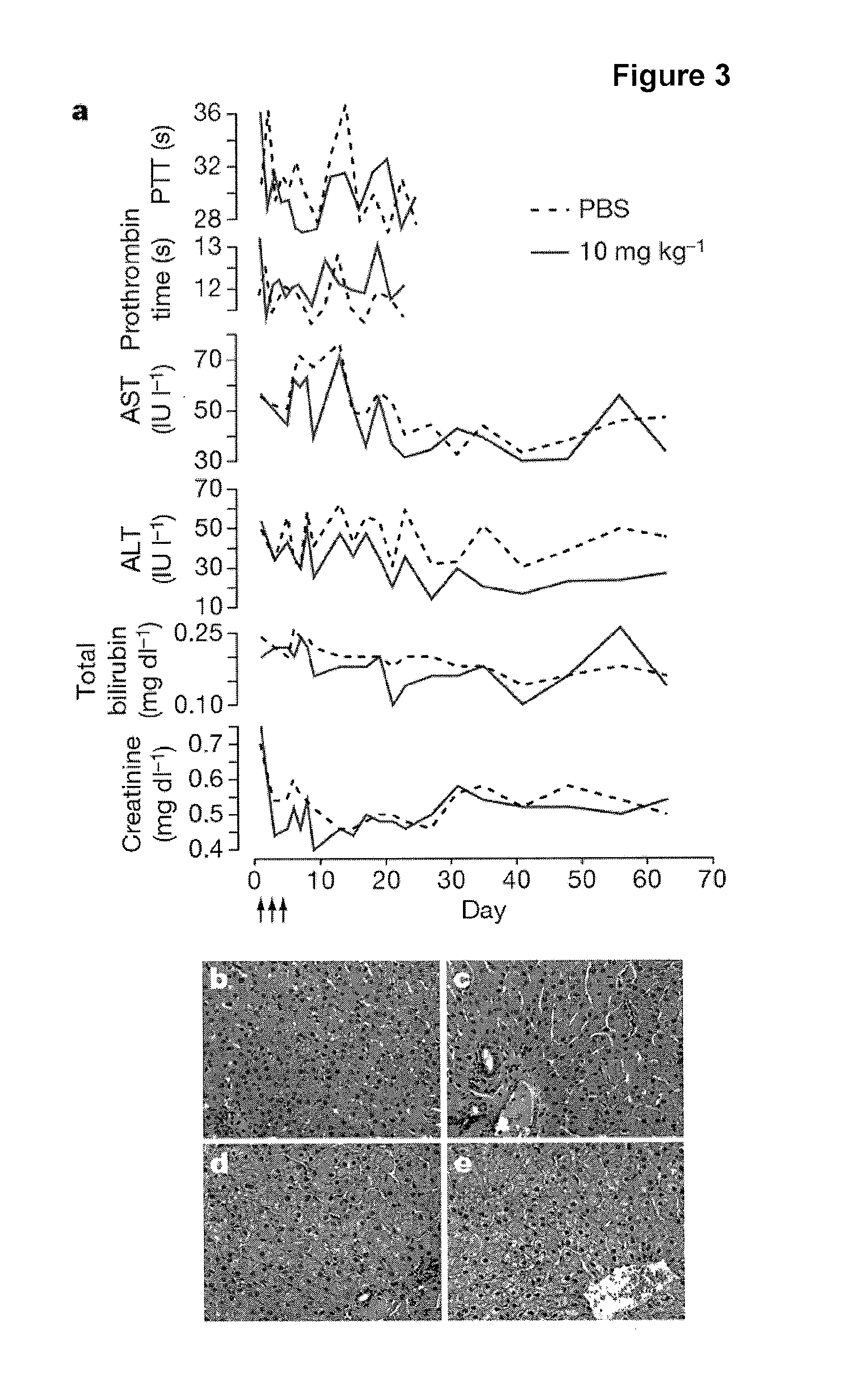
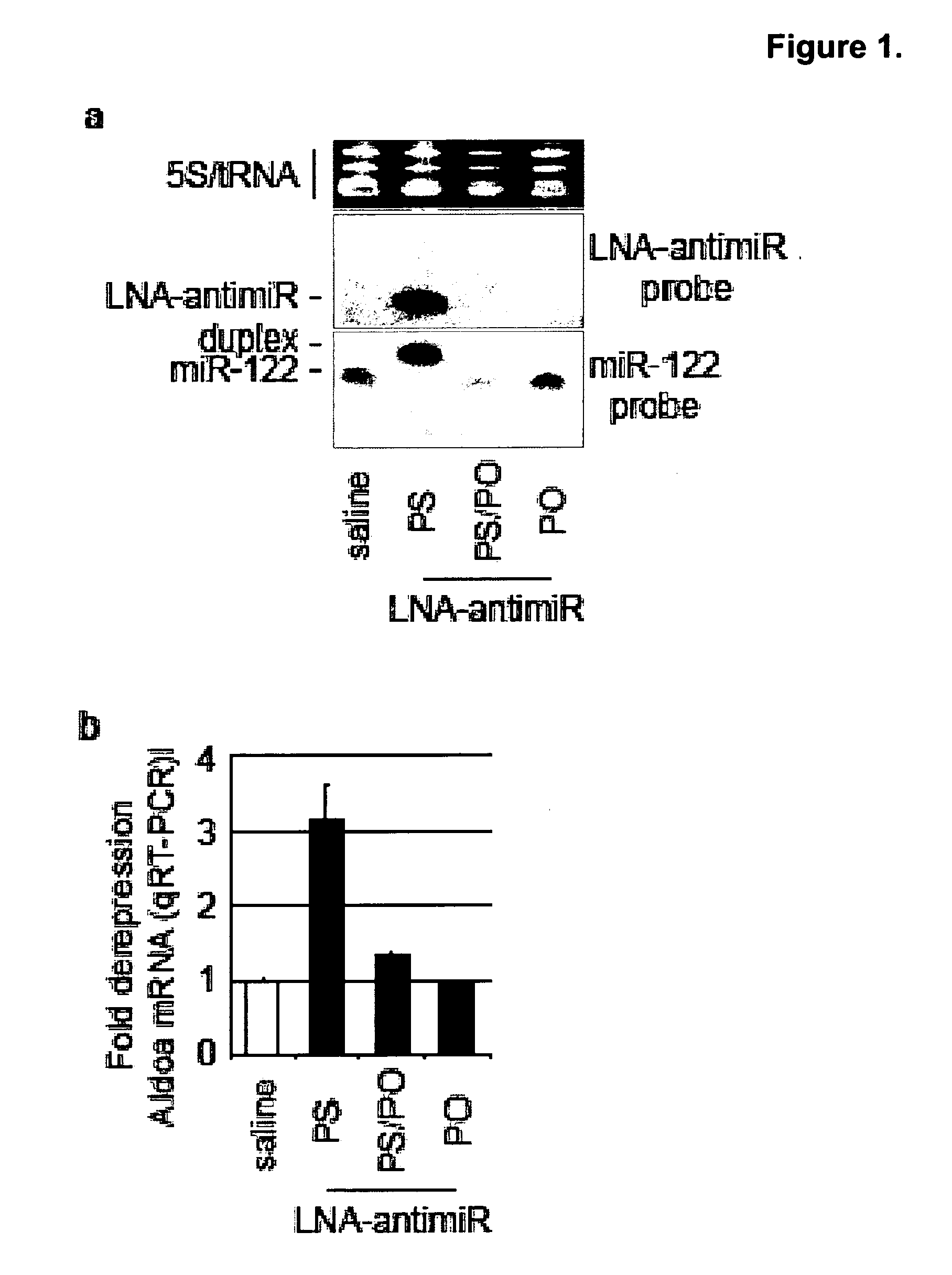
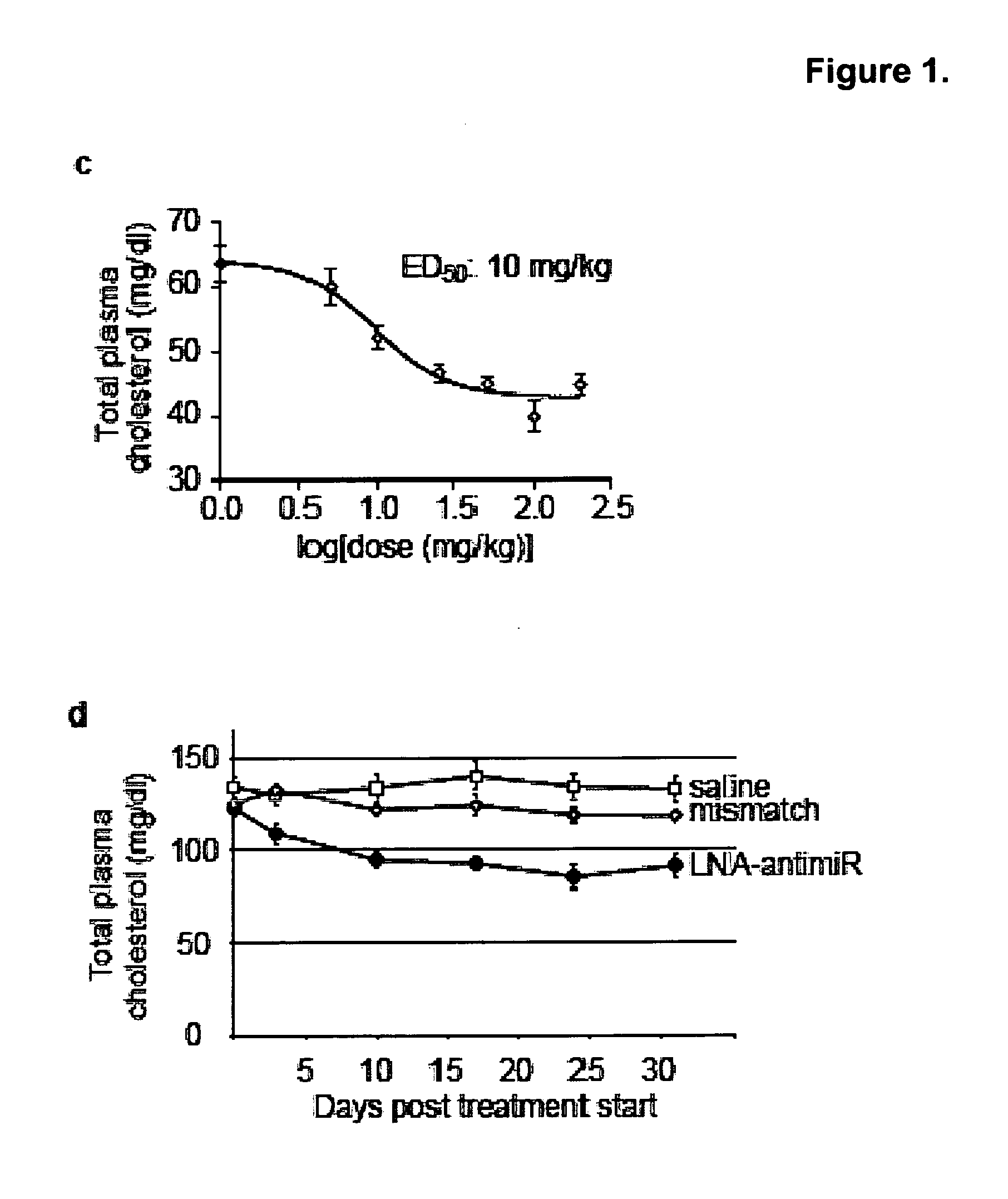
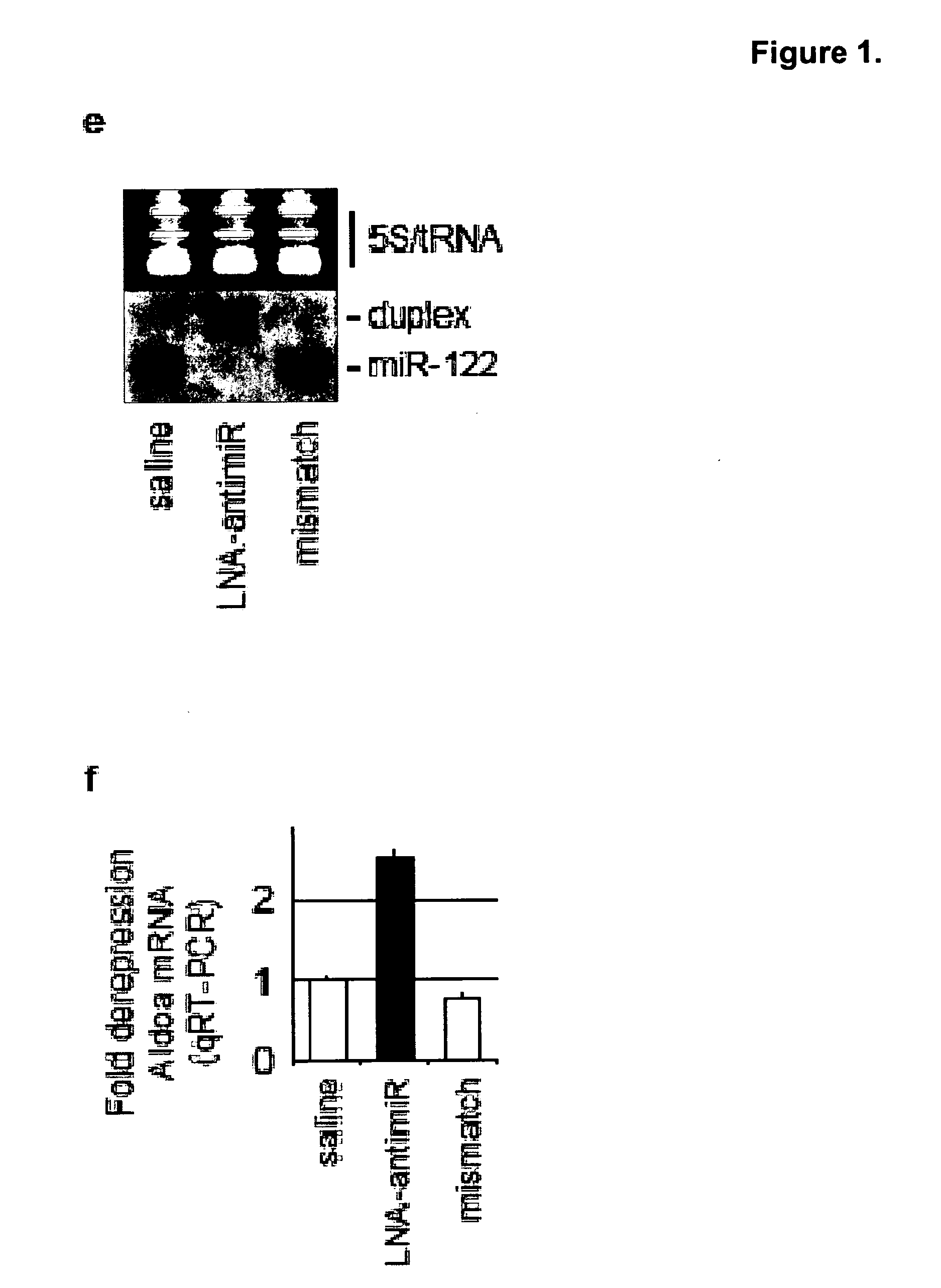
Pharmaceutical compositions for treatment of microRNA related diseases
InactiveUS20090298916A1Inhibitory activityOrganic active ingredientsMetabolism disorderDiseasePrimate
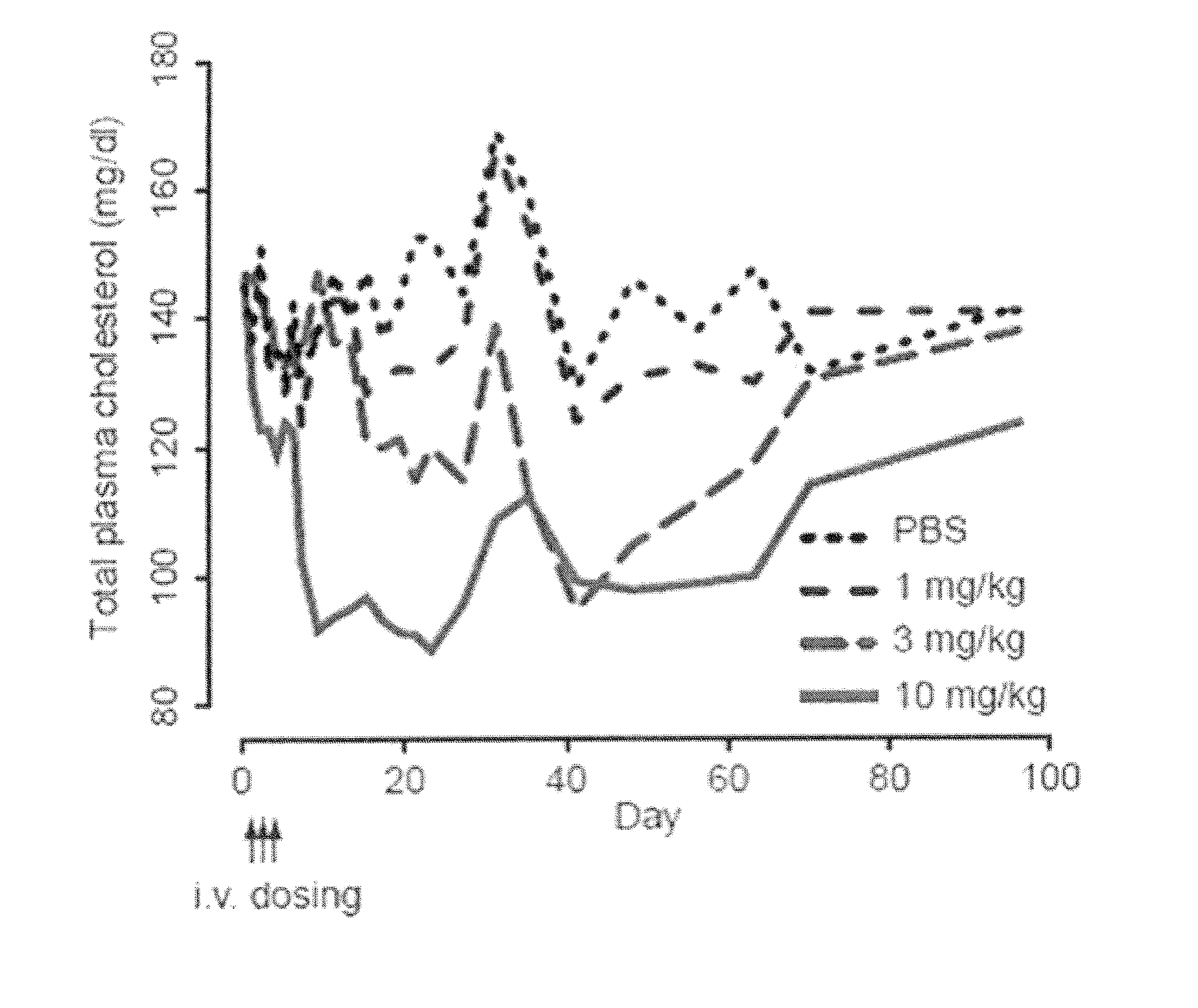
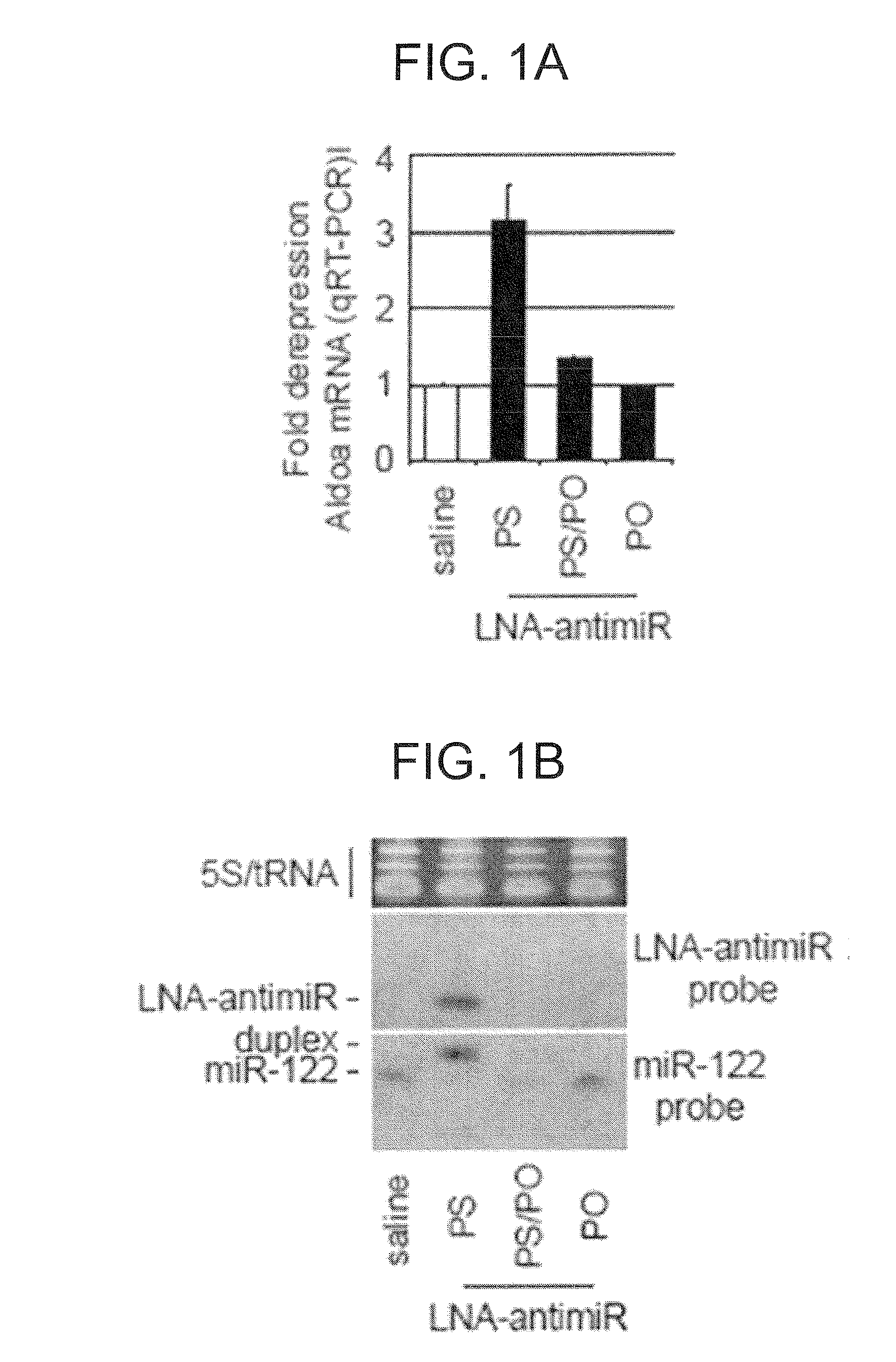
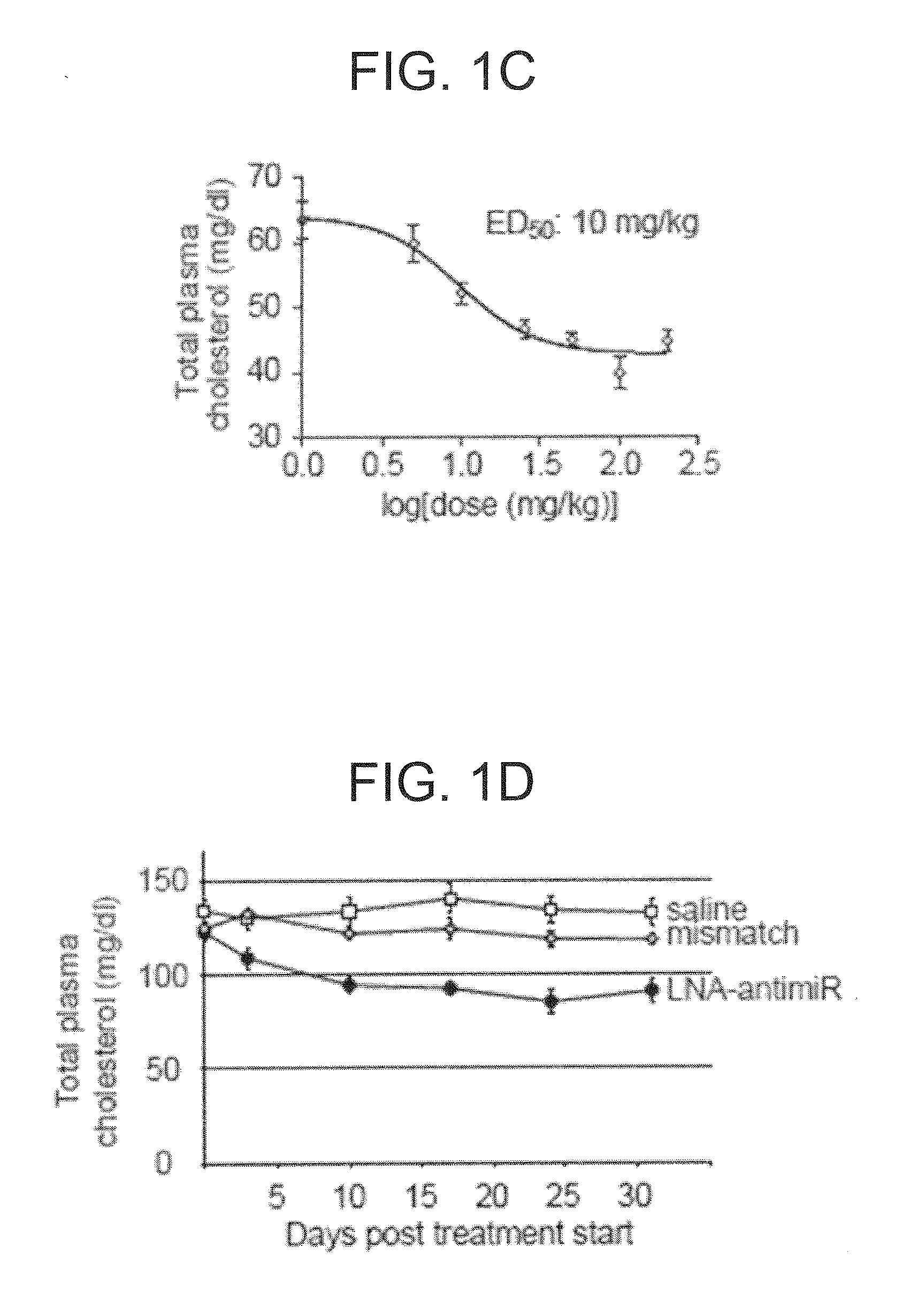
The present invention provides compositions and methods of treatment of diseases that are sensitive to drugs that downregulate the function of microRNA's, mRNA, non-coding RNA, or viral genomes. In particular, it has been discovered that a very long term effect of an anti microRNA oligonucleotide may be obtained when administered to a primate. Therefore, the present invention relate to pharmaceutical compositions and methods for treatment of primates, including humans wherein the compositions are administered with a long time interval.
Owner:ROCHE INNOVATION CENT COPENHAGEN
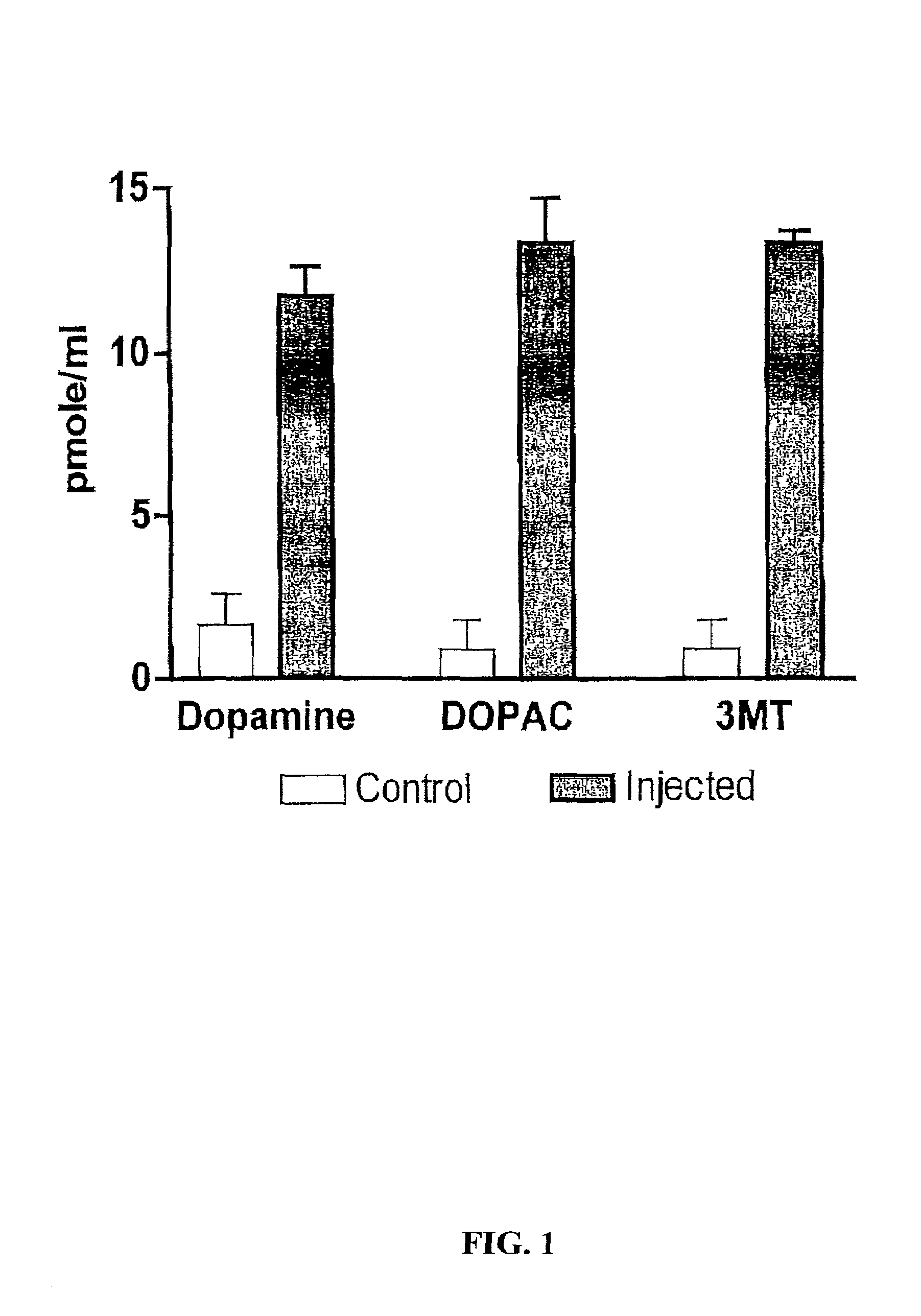
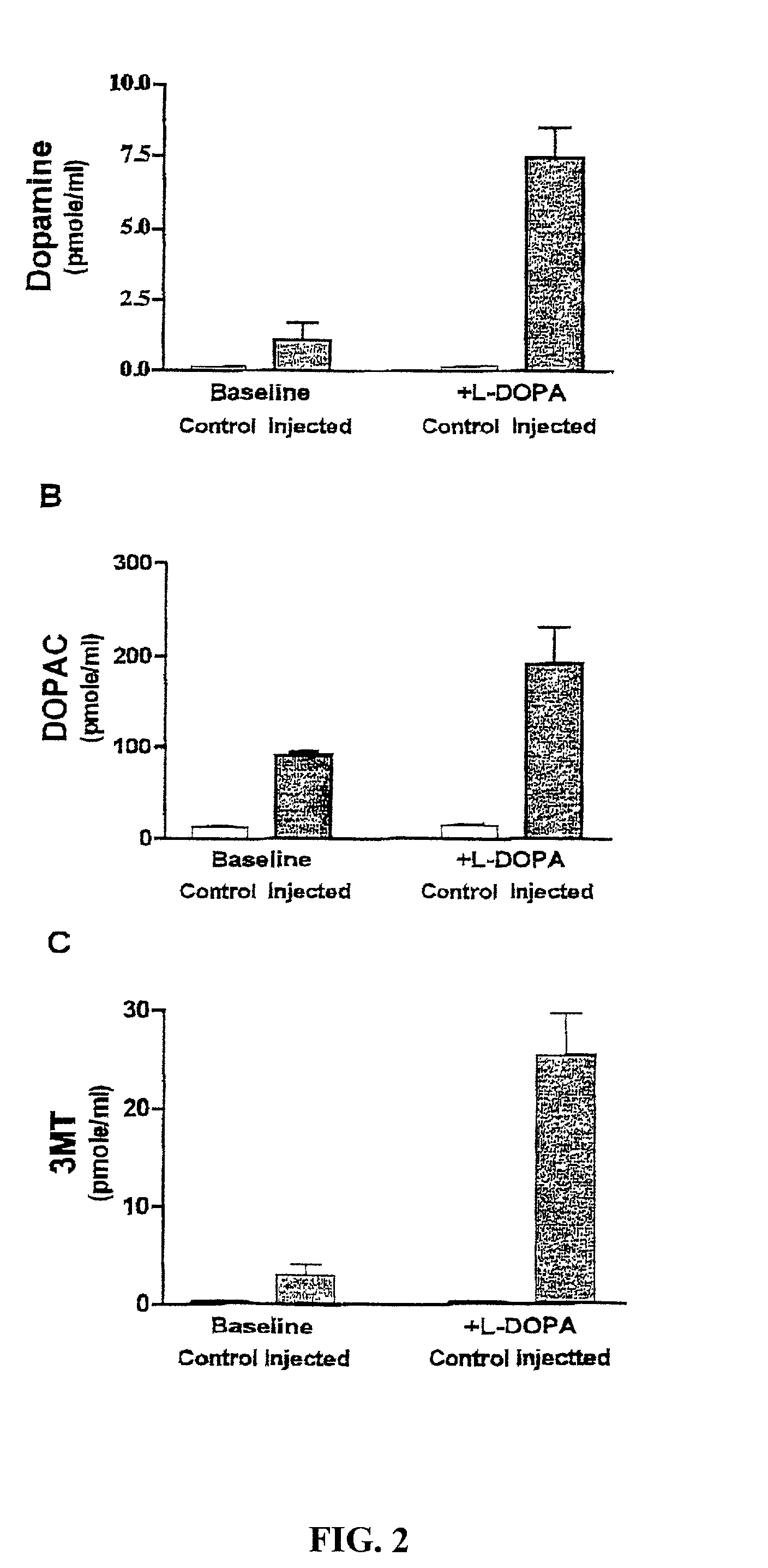
Methods of treating Parkinson's disease using recombinant adeno-associated virus virions
ActiveUS7588757B2Reduce deliveryIncrease in fine motor taskingBiocidePeptide/protein ingredientsGene deliveryDisease
Methods for treating Parkinson's disease (PD) are provided. Recombinant adeno-associated virus (rAAV) virions are used to deliver genes encoding dopamine-synthesizing enzymes to the central nervous system of a primate. Once delivered, the genes are expressed, which then results in dopamine synthesis and amelioration in the clinical signs and symptoms of PD. The methods of the present invention can be used to deliver the three central dopamine synthesizing enzymes: tyrosine hydroxylase, aromatic L-amino acid decarboxylase, and guanosine triphosphate cyclohydrolase I thereby enhancing dopamine biosynthesis and providing for enhanced therapeutic efficacy.
Owner:GENZYME CORP
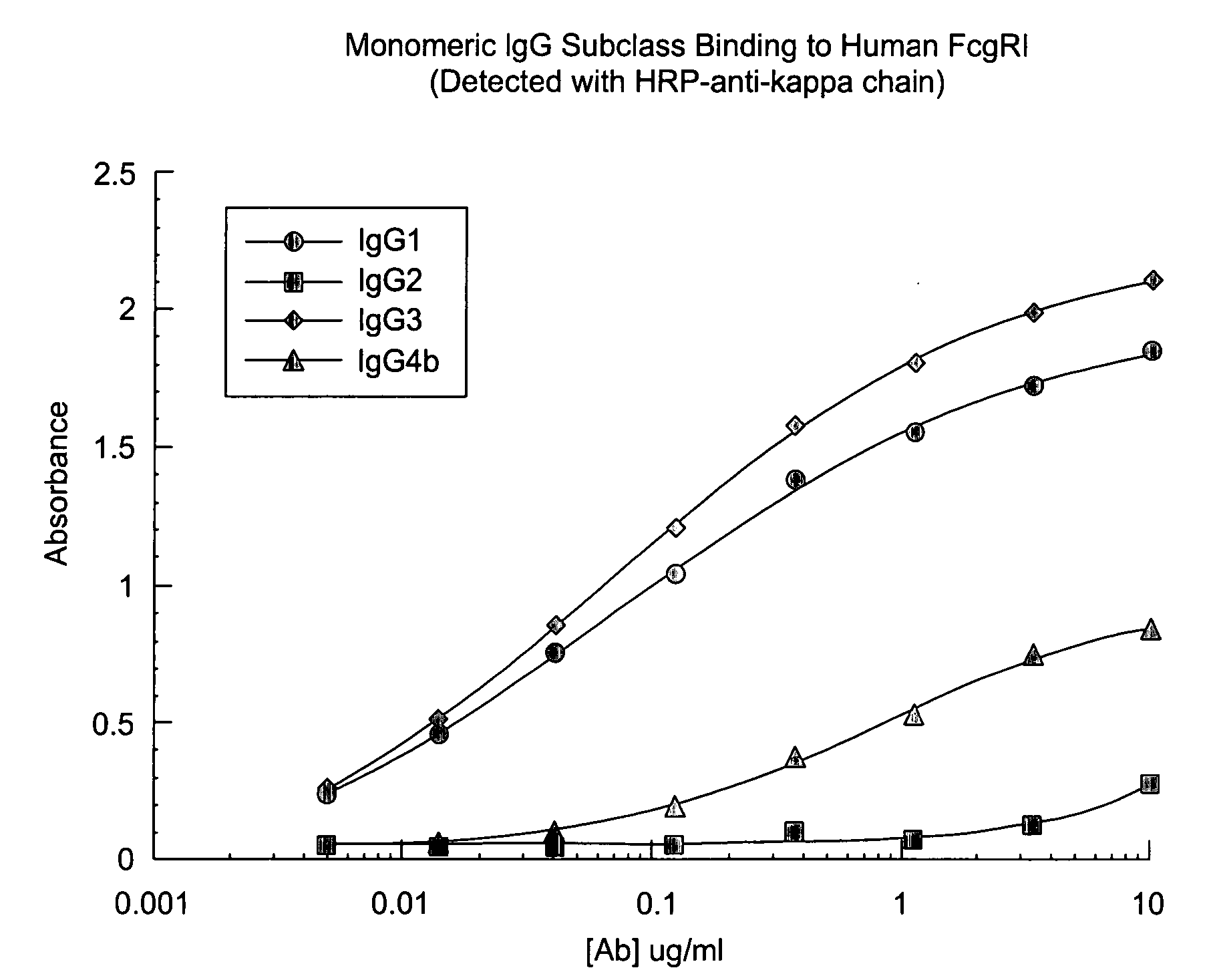
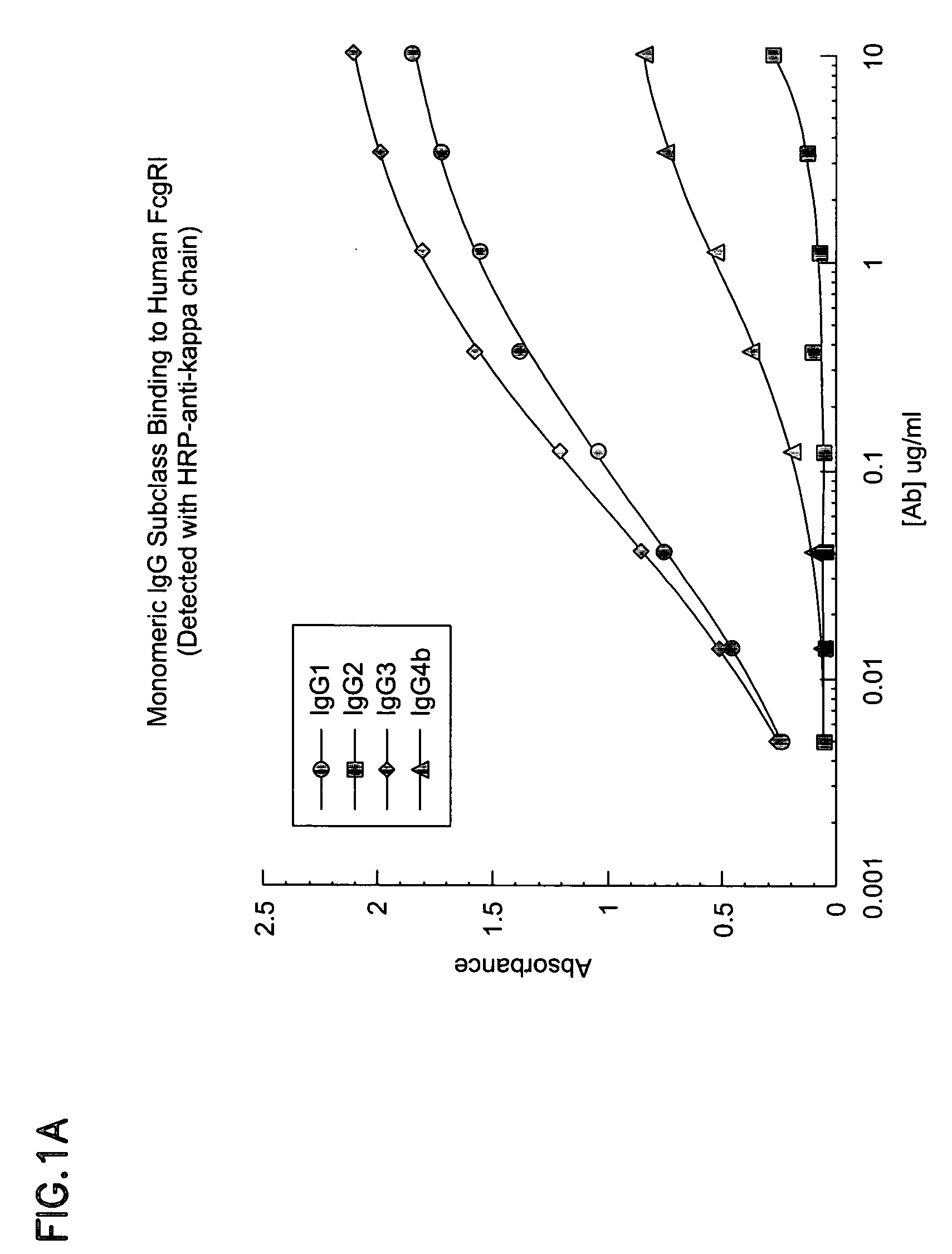
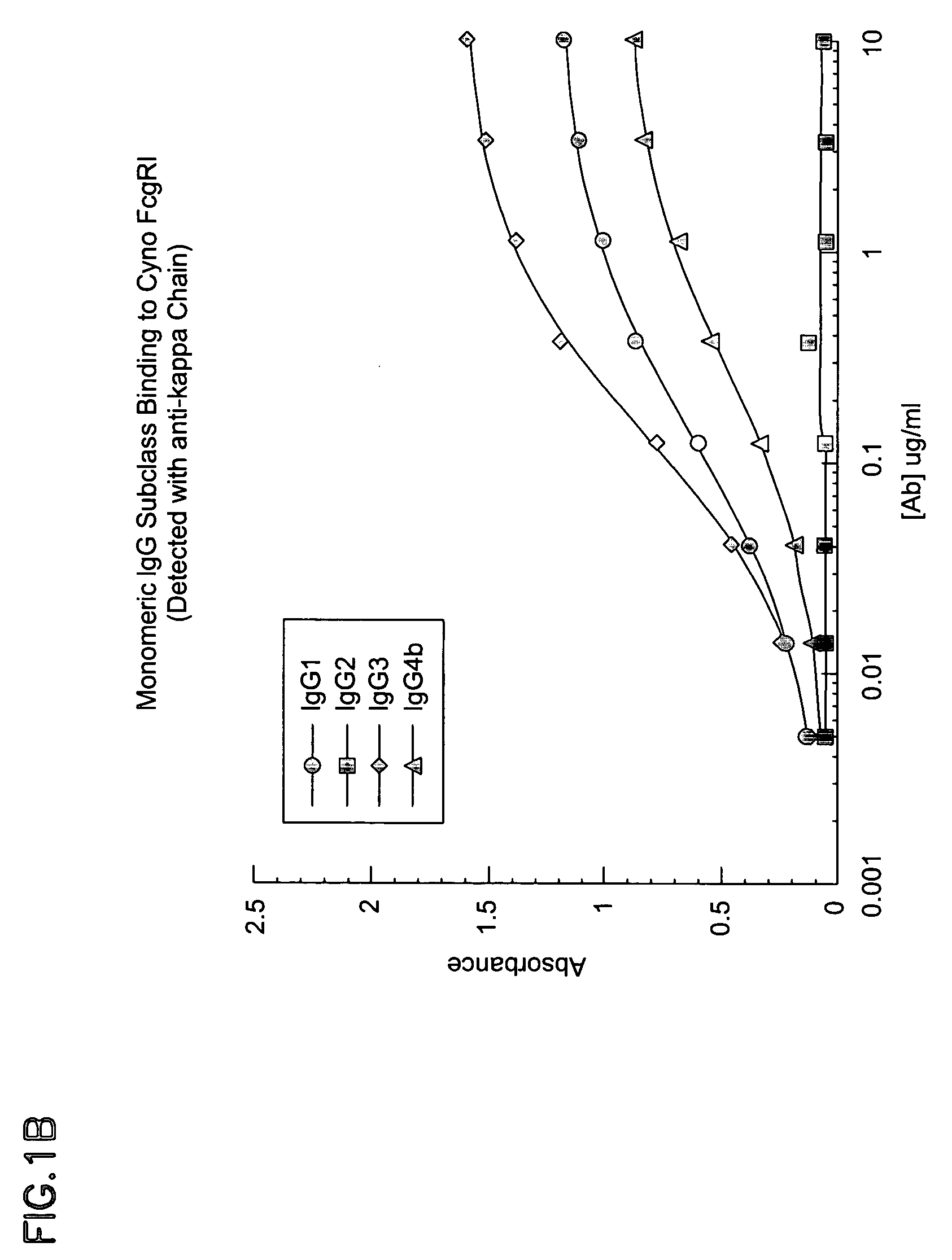
Non-human primate Fc receptors and methods of use
InactiveUS20050054046A1Improve stabilityFacilitate oligomerizationAntibody mimetics/scaffoldsImmunoglobulinsFc(alpha) receptorFc receptor
The invention provides isolated non-human primate Fc receptor polypeptides, the nucleic acid molecules encoding the Fc receptor polypeptides, and the processes for production of recombinant forms of the Fc receptor polypeptides, including fusions, variants, and derivatives thereof. The invention also provides methods for evaluating the safety, efficacy and biological properties of Fc region containing molecules using the non-human primate Fc receptor polypeptides.
Owner:GENENTECH INC
Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators
ActiveUS8216997B2Increased formationImprove the level ofOrganic active ingredientsPeptide/protein ingredientsPrimatePhysiology
Owner:ACCELERON PHARMA INC
Activin-actrii antagonists and uses for increasing red blood cell levels
ActiveUS20090163417A1Reduce expressionIncrease redCompound screeningApoptosis detectionPrimateRed Cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Method of increasing red blood cell levels or treating anemia in a patient
ActiveUS8058229B2Low affinityReduce impactPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed Cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
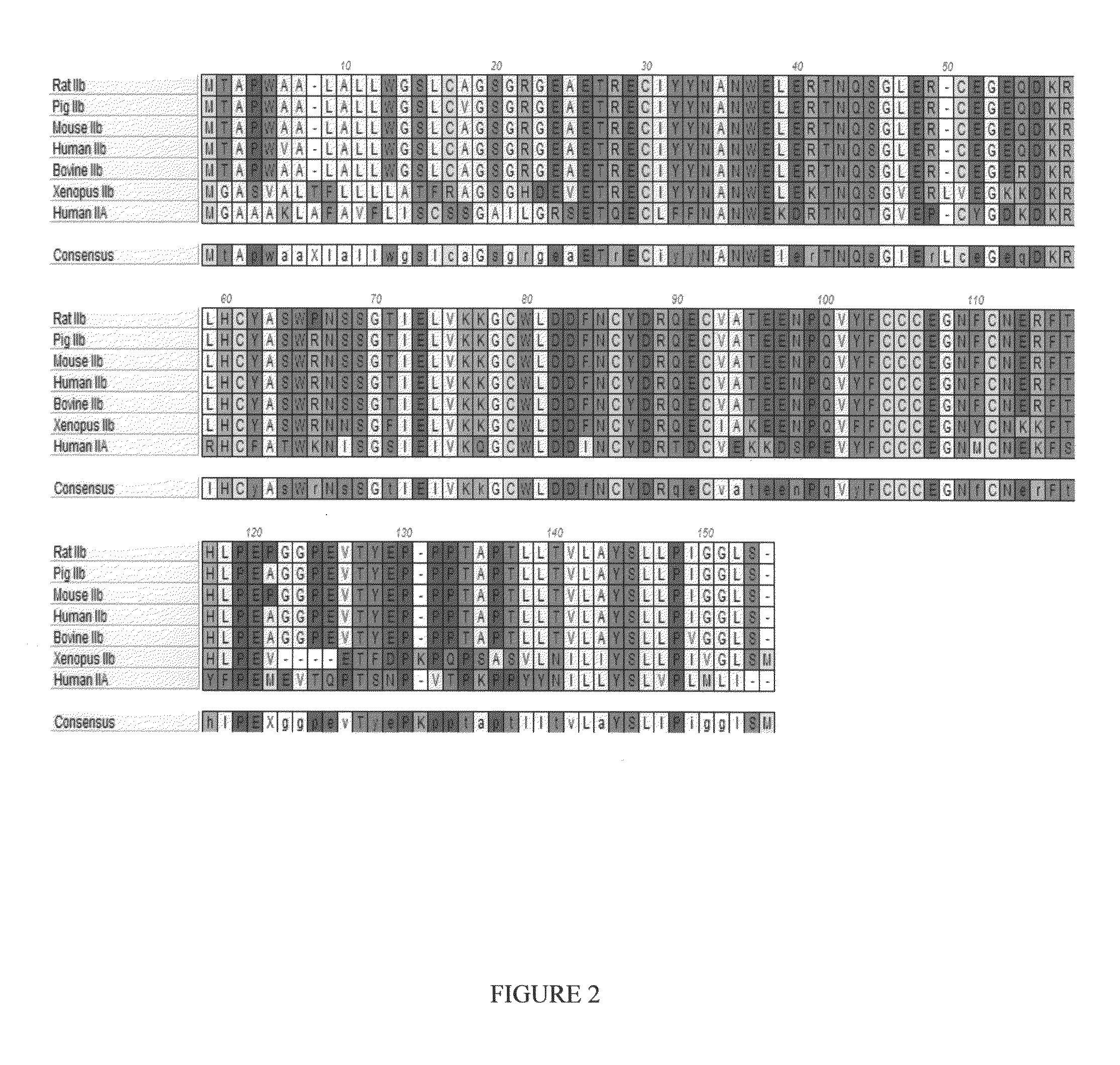
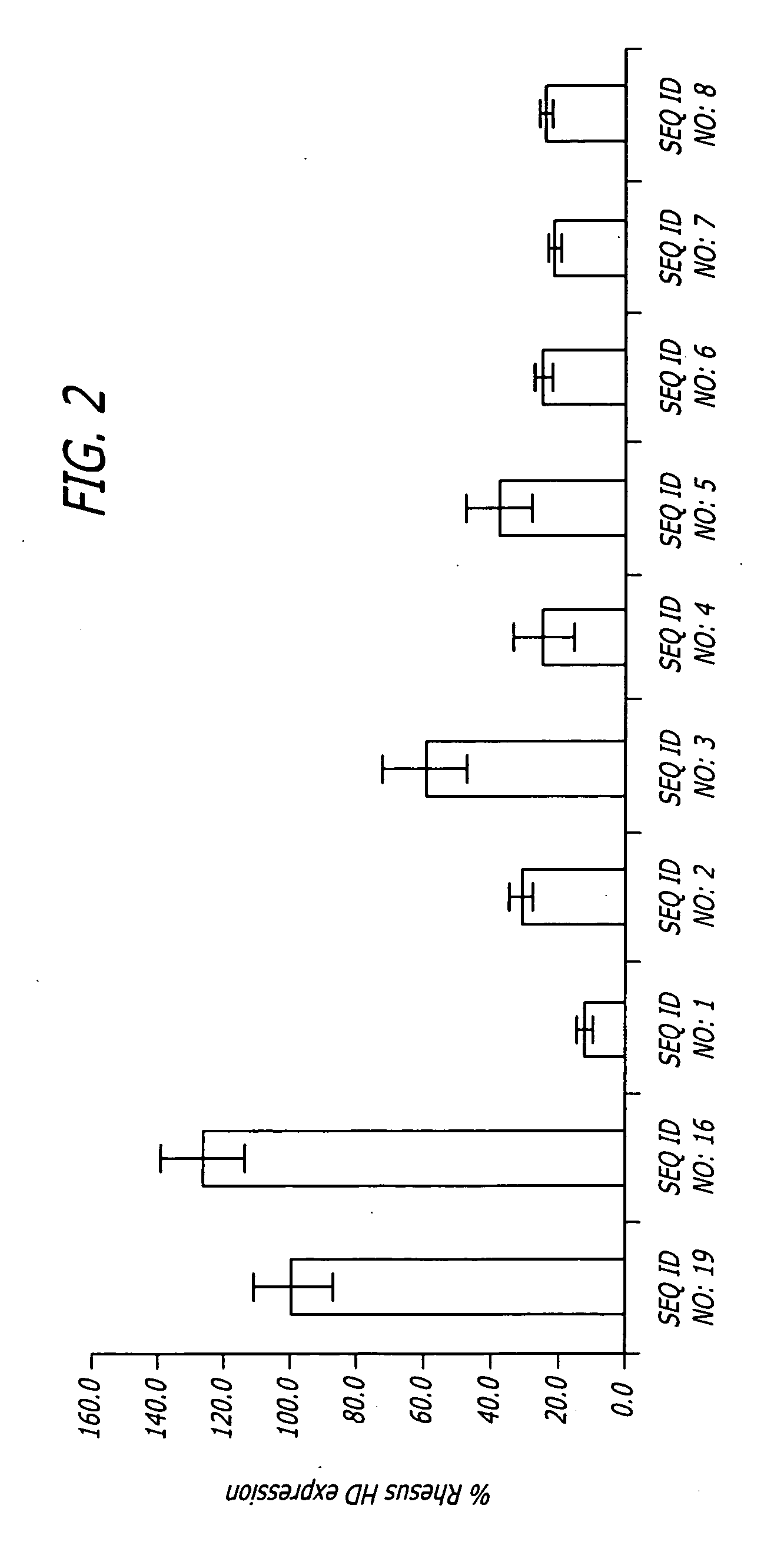
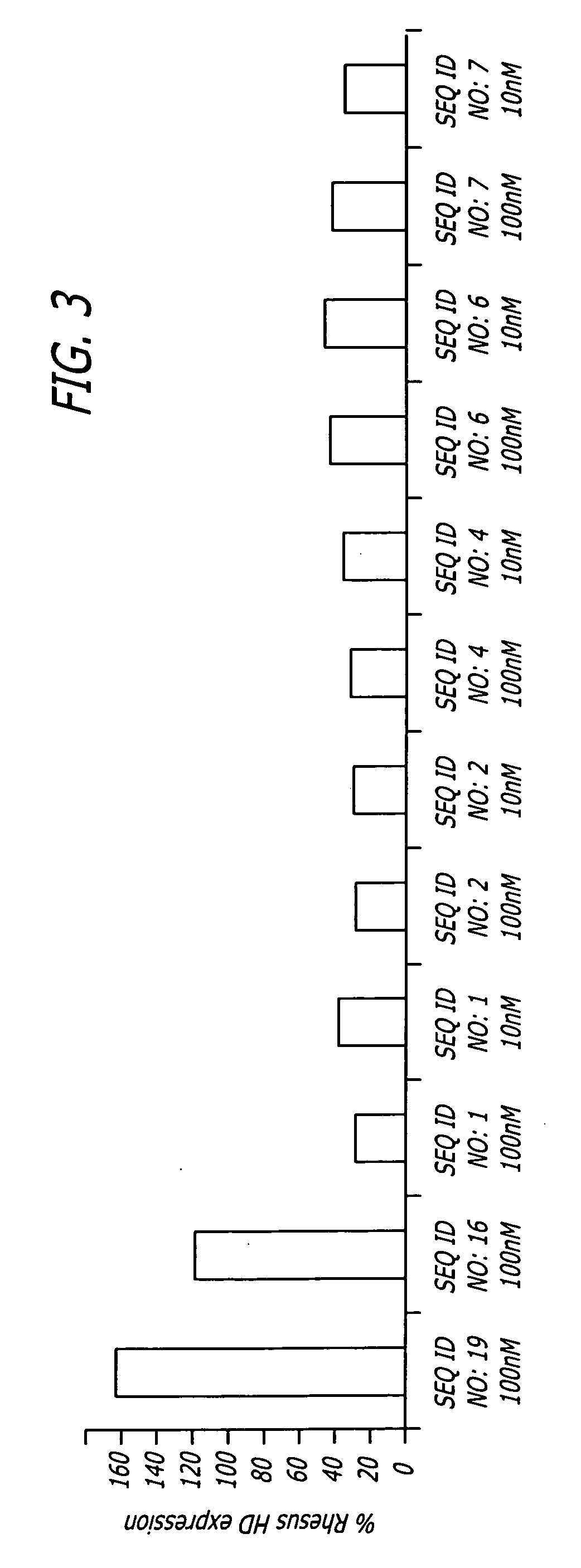
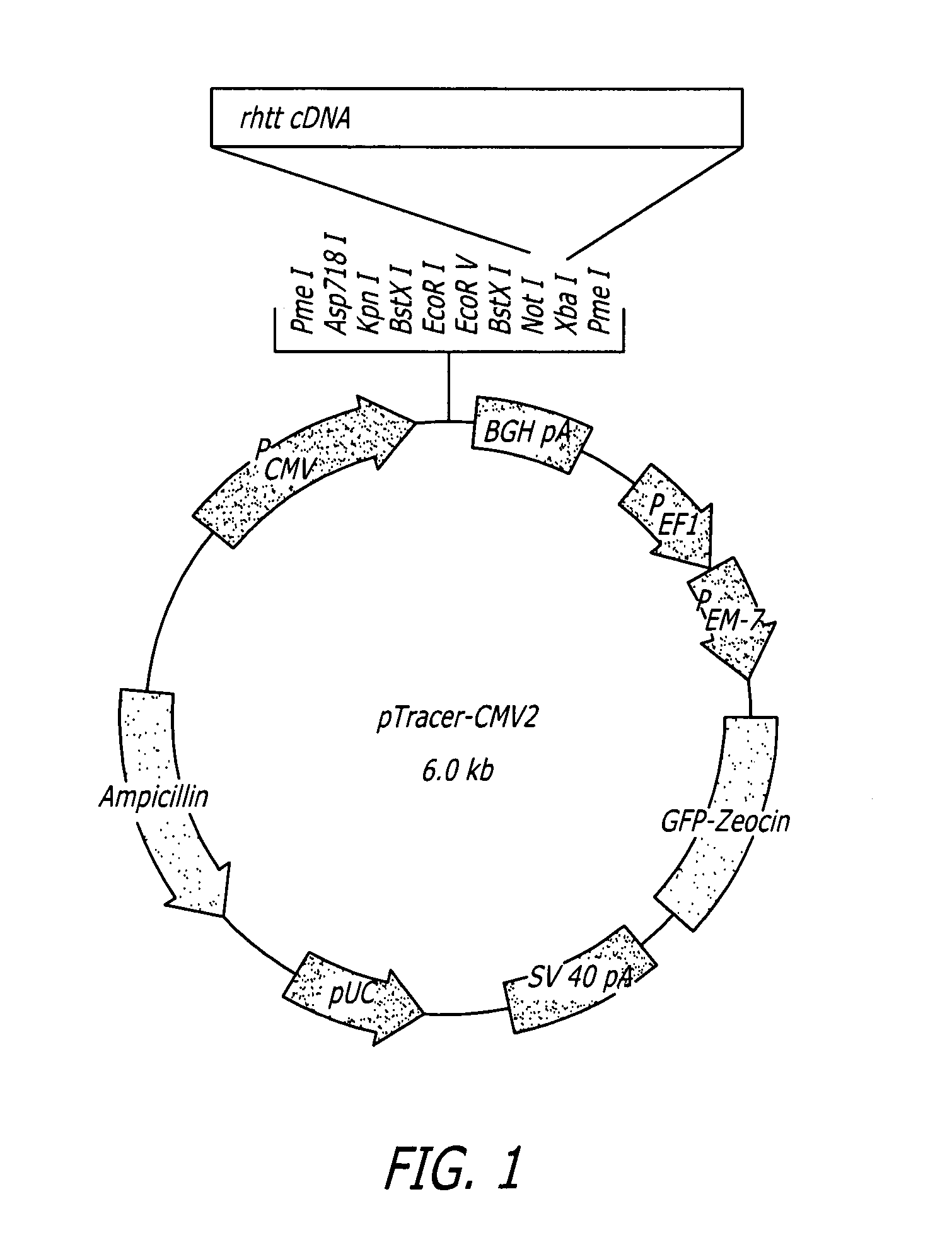
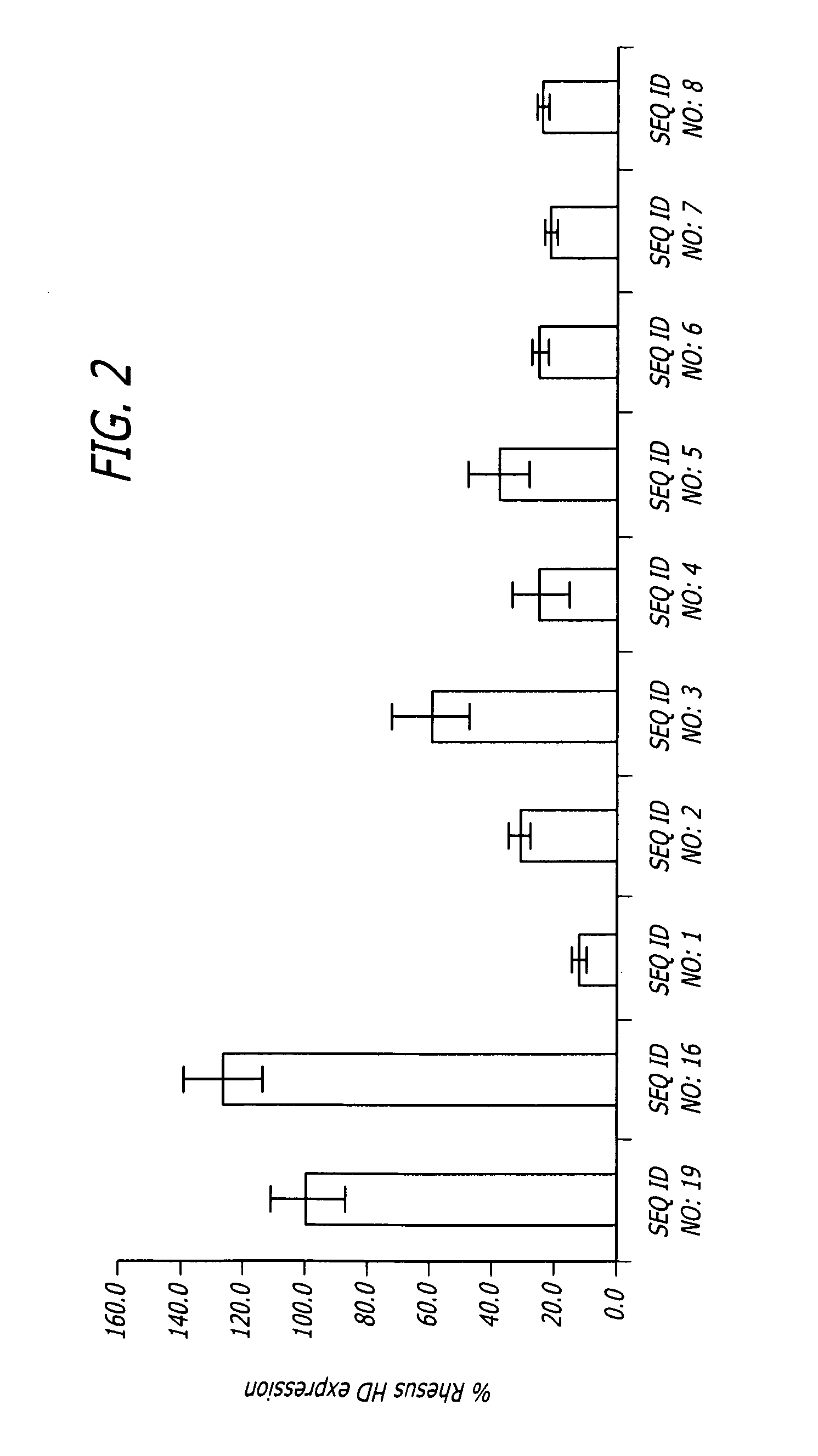
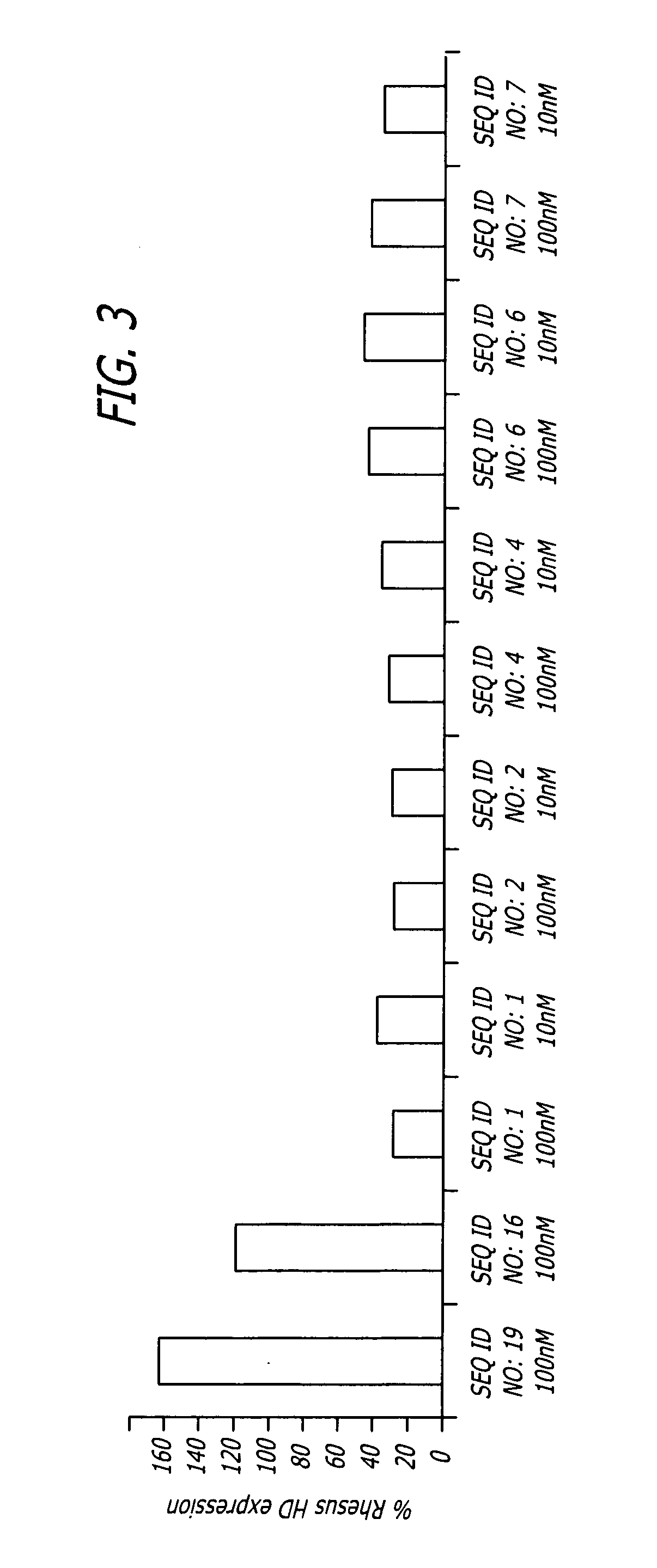
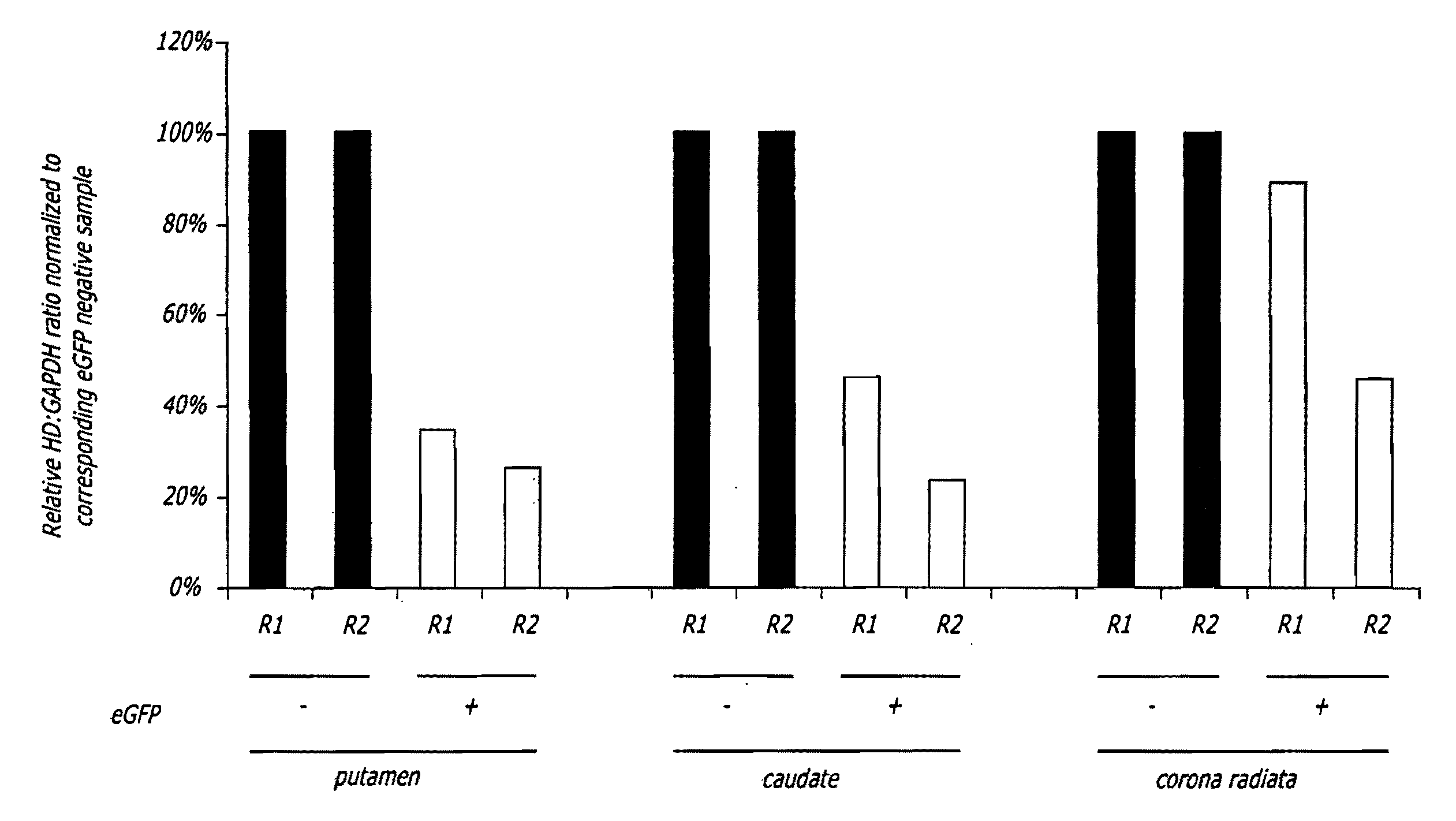
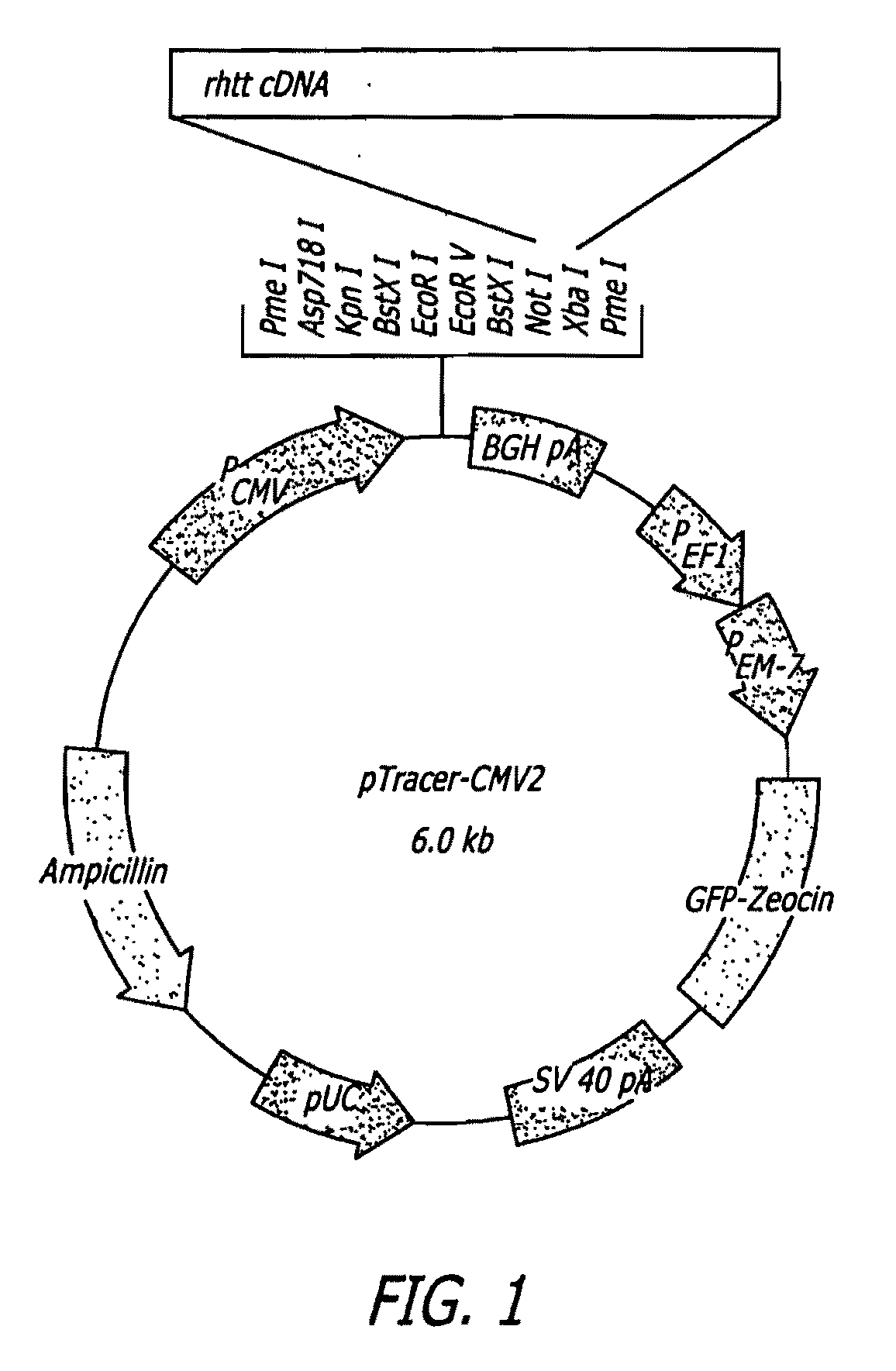
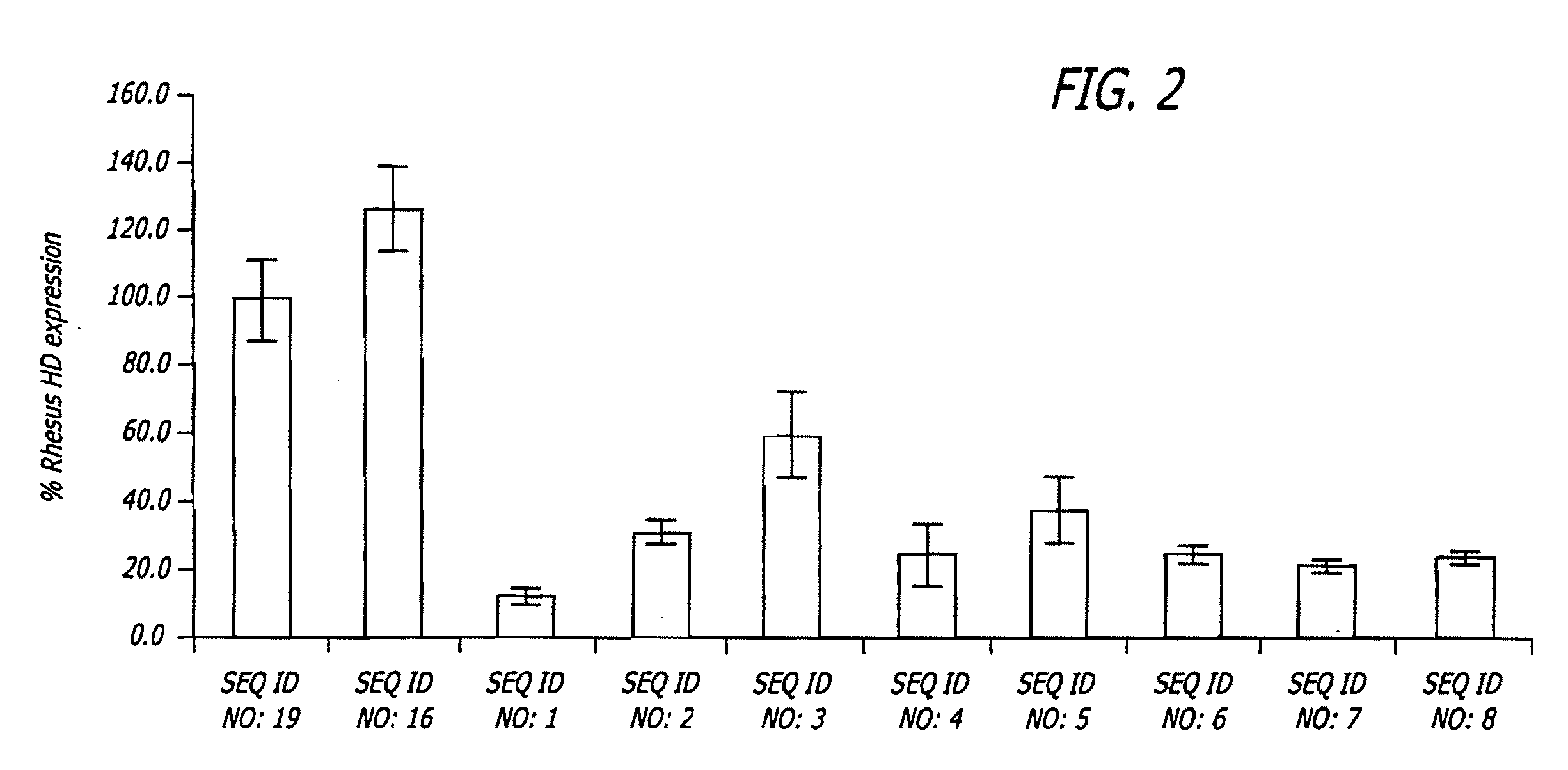
Methods and sequences to suppress primate huntington gene expression
InactiveUS20060257912A1Inhibit expressionLower Level RequirementsGenetic material ingredientsFermentationPrimateGene
Disclosed herein are sequences, molecules and methods used to suppress the expression of HD genes encoding for huntingtin protein in primates including Macaca mulatta and Homo sapiens. These sequences, molecules and methods aid in the study of the pathogenesis of HD and can also provide a treatment for this disease.
Owner:MEDTRONIC INC
Antagonists of activin-actriia and uses for increasing red blood cell levels
ActiveUS20100028331A1Easy to produceReduce expressionPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed Cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Use of gdf traps to increase red blood cell levels
ActiveUS20120015877A1Distribution moreMany formatsPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed Cell
Owner:ACCELERON PHARMA INC
Vaccines against cancer and infectious diseases
InactiveUS6440416B1Promote antibody productionPromote productionOrganic active ingredientsImmunoglobulins against animals/humansProtozoaPrimate
A method of stimulating an immune response in a human against malignant cells or an infectious agent comprises the step of administering to the human an immunogenic amount of a primate anti-idiotype antibody or antibody fragment that acts as an immunogenic functional mimic of an antigen produced by or associated with a malignant cell or an infectious agent. Sub-human primate anti-idiotype antisera, especially from baboons, are preferred. Such anti-idiotype antibodies are used to make vaccines for inducing preventive immunity or a therapeutic immune response against tumors, viruses, bacteria, rickettsia, mycoplasma, protozoa, fungi and multicellular parasites.
Owner:IMMUNOMEDICS INC
Pharmaceutical Compositions for Treatment of MicroRNA Related Diseases
The present invention provides compositions and methods of treatment of diseases that are sensitive to drugs that downregulate the function of microRNA's, mRNA, non-coding RNA, or viral genomes. In particular, it has been discovered that a very long term effect of an anti microRNA oligonucleotide may be obtained when administered to a primate. Therefore, the present invention relate to pharmaceutical compositions and methods for treatment of primates, including humans wherein the compositions are administered with a long time interval.
Owner:ROCHE INNOVATION CENT COPENHAGEN
Antagonists of activin-actriia and uses for increasing red blood cell levels
ActiveUS8895016B2Reduce expressionIncrease red blood cell and hemoglobin levelPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed blood cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Antagonists of actriib and uses for increasing red blood cell levels
InactiveUS20120003218A1Distribution moreMany formatsHormone peptidesPeptide/protein ingredientsPrimateRed Cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
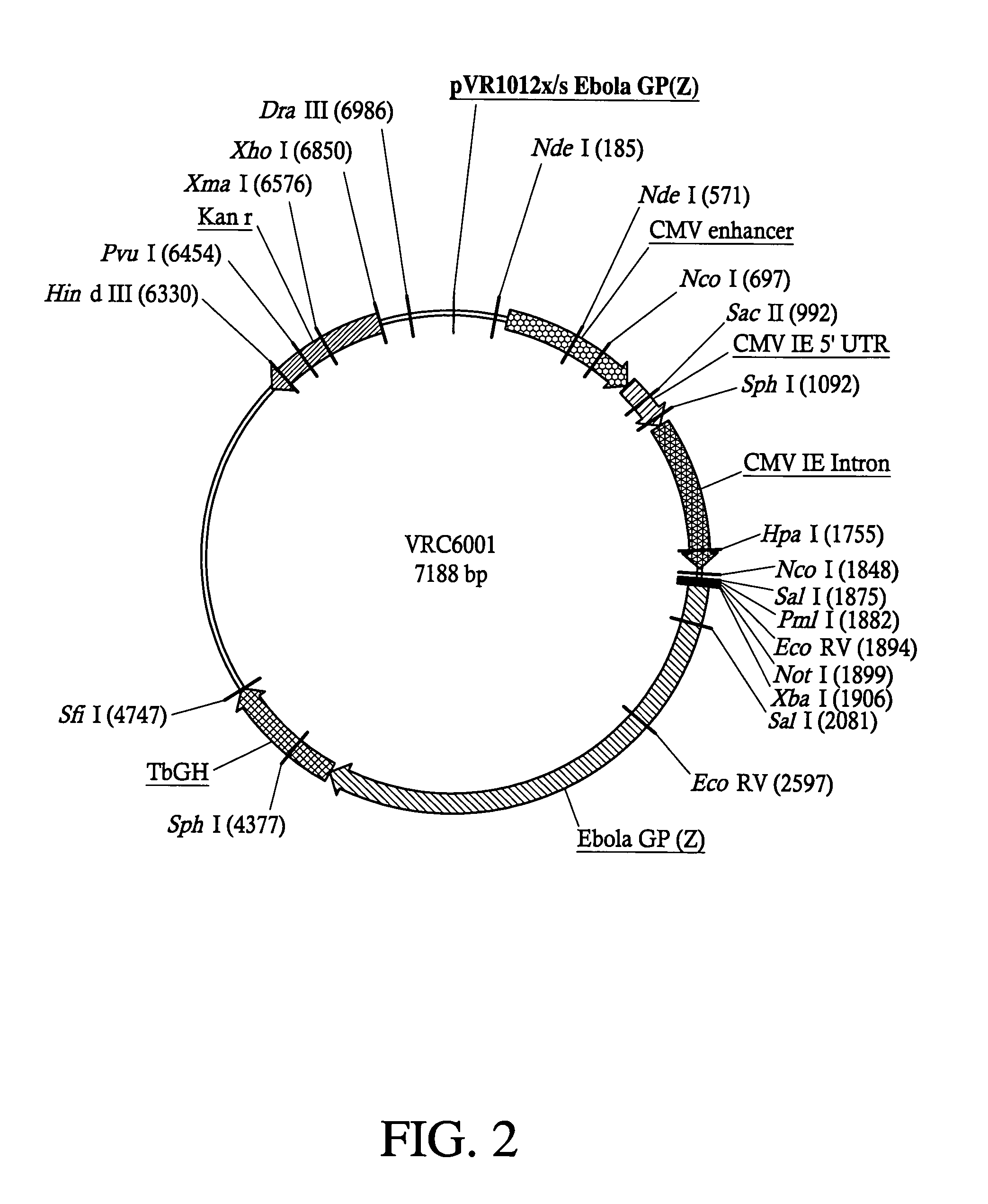
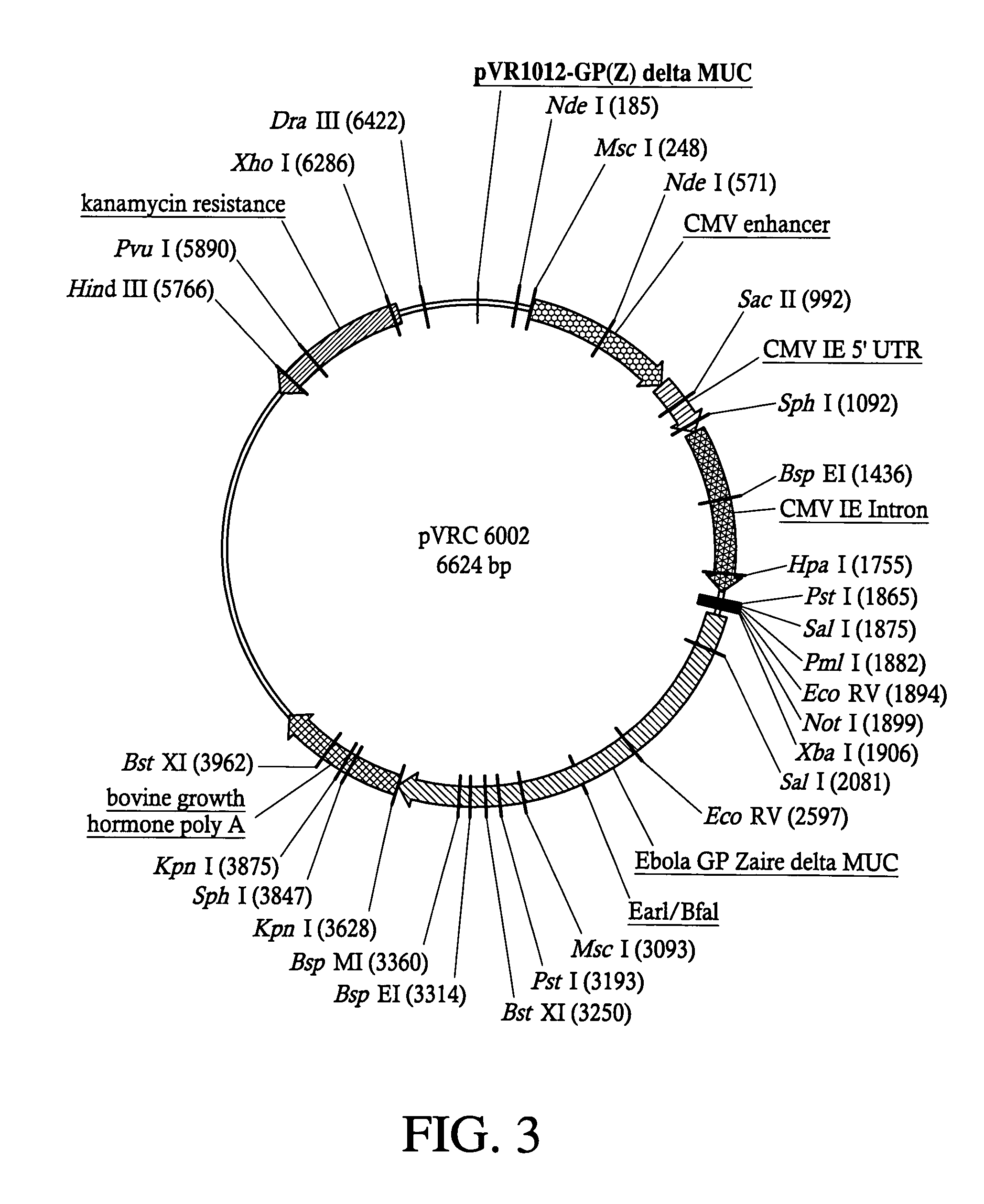
Development of a preventive vaccine for filovirus infection in primates
The present invention relates generally to viral vaccines and, more particularly, to filovirus vaccines and methods of eliciting an immune response against a filovirus or disease caused by infection with filovirus.
Owner:UNITED STATES OF AMERICA
Cryopreservation medium for primate embryo stem cells and cryopreservation method
InactiveUS20050026133A1Easy to controlLow costArtificial cell constructsDead animal preservationPrimateMicrobiology
A cryopreservation medium and a cryopreservation method which make it possible to cryopreserve ES cells from primates simply with high viability are provided. A cryopreservation medium containing a cryoprotectant at a Concentration of from 12% (W / V) to 50% (W / V) and a cryopreservation method for primate embryonic stem cells, which comprises a step of suspending primate embryonic stem cells in the cryopreservation medium, and a refrigeration step of freezing the suspension of the primate embryonic stem cells in the cryopreservation medium by cooling it to −80° C. or below at a rate of from 0.5° C. to 10° C. per minute, and a preservation step of storing the frozen suspension of primate embryonic stem cells in the cryopreservation medium enable simple cryopreservation of primate embryonic stem cells with high viability.
Owner:ASAHI TECHNO GLASS CORP
Engineered antibodies with new world primate framework regions
InactiveUS20080095767A1Low immunogenicityAntibacterial agentsNervous disorderPrimateComplementarity determining region
The present invention provides an antibody or antigen-binding portion thereof having a variable region comprising at least two complementarity determining regions (CDRs) and at least three framework regions. The the framework regions are, or are derived from New World primate framework regions, and at least one of the CDRs is a non-New World primate CDR.
Owner:CEPHALON AUSTRALIA
Methods and sequences to suppress primate huntington gene expression in vivo
InactiveUS20100325746A9Inhibit expressionLower Level RequirementsBiocideNervous disorderDiseasePrimate
Owner:MEDTRONIC INC
Methods and sequences to suppress primate huntington gene expression
ActiveUS20100008981A1Inhibit expressionLower Level RequirementsOrganic active ingredientsSugar derivativesDiseasePrimate
Owner:MEDTRONIC INC
Therapeutic agents—I
InactiveUS7449492B2Antibacterial agentsOrganic active ingredientsChemical fractionChemical synthesis
The present invention relates generally to chemical agents useful in the treatment and prophylaxis of infection by pathogenic or potentially pathogenic entities, or entities capable of opportunistic infection in mammals, including humans and primates, non-mammalian animals and avian species. More particularly, the present invention provides a chemical agent of the macrocyclic diterpene family obtainable from a member of the Euphorbiaceae family of plants or botanical or horticultural relatives thereof or derivatives or chemical analogues or chemically synthetic forms of the agents for use in the treatment or prophylaxis of infection by pathogenic entities in mammalian, animal and avian subjects. The present invention further contemplates a method for the prophylaxis and / or treatment in mammalian, animal or avian subjects of infection or potential infection by pathogenic entities by the topical or systemic administration of a macrocyclic diterpene obtainable from a member of the Euphorbiaceae family of plants or their botanical or horticultural derivatives or a derivative, chemical analogue or chemically synthetic form of the agent. The chemical agent of the present invention may be in the form of a purified compound, mixture of compounds, a precursor form of one or more of the compounds capable of chemical transformation into a therapeutically active agent or in the form of a chemical fraction, sub-fraction, preparation or extract of the plant.
Owner:AF 30 APRIL 2003 +1
Method of administering FimH protein as a vaccine for urinary tract infections
InactiveUS20030138449A1Reduce morbidityInhibit bindingAntibacterial agentsBacterial antigen ingredientsDiseaseBacteroides
The present invention relates to methods of stimulating an immune response in a primate utilizing compositions comprising bacterial adhesin proteins and / or immunogenic fragments thereof. The compositions are useful for the prevention and treatment of bacterial induced diseases involving bacterial adherence to a target cell, such as diseases of the urinary tract. More specifically, the invention relates to the vaccination of primates, preferably humans, with protein complexes, such as a purified FimH polypeptides, a purified FimC-FimH (FimCH) polypeptide complex, or immunogenic fragments thereof, to stimulate protective immunity in the recipient against infection by pathogenic bacteria, including all types of Enterobacteriaceae, preferably E. coli to produce specific immunoglobin molecules in the serum and urine or mucosal secretions of the subject.
Owner:MEDIMMUNE LLC
Pharmaceutical Compositions for Treatment of HCV Patients that are Poor-Responders to Interferon
InactiveUS20100330035A1Inhibitory activityOrganic active ingredientsPeptide/protein ingredientsInterferon therapyPrimate
The present invention provides compositions and methods of treatment of HCV infected subjects that are not sensitive to interferon treatment. Further, compositions and methods are provided for prevention of organ transplant rejection. The compositions of the invention comprise an anti microRNA-122 oligonucleotide, and are made for administration to a primate.
Owner:ROCHE INNOVATION CENT COPENHAGEN
Gynogenetic or androgenetic production of pluripotent cells and cell lines, and use thereof to produce differentiated cells and tissues
InactiveUS20030129745A1Rejection is prevented and reducedEliminate, orMammal material medical ingredientsSkeletal/connective tissue cellsPrimateEmbryo
Methods for obtaining pluripotent (embryonic stem) cells from parthenogenetic embryos, especially primates, are provided. These cells are useful for producing differentiated cells, tissues and organs, especially human and non-human primate cells, tissues and organs.
Owner:ROBL JAMES M +2
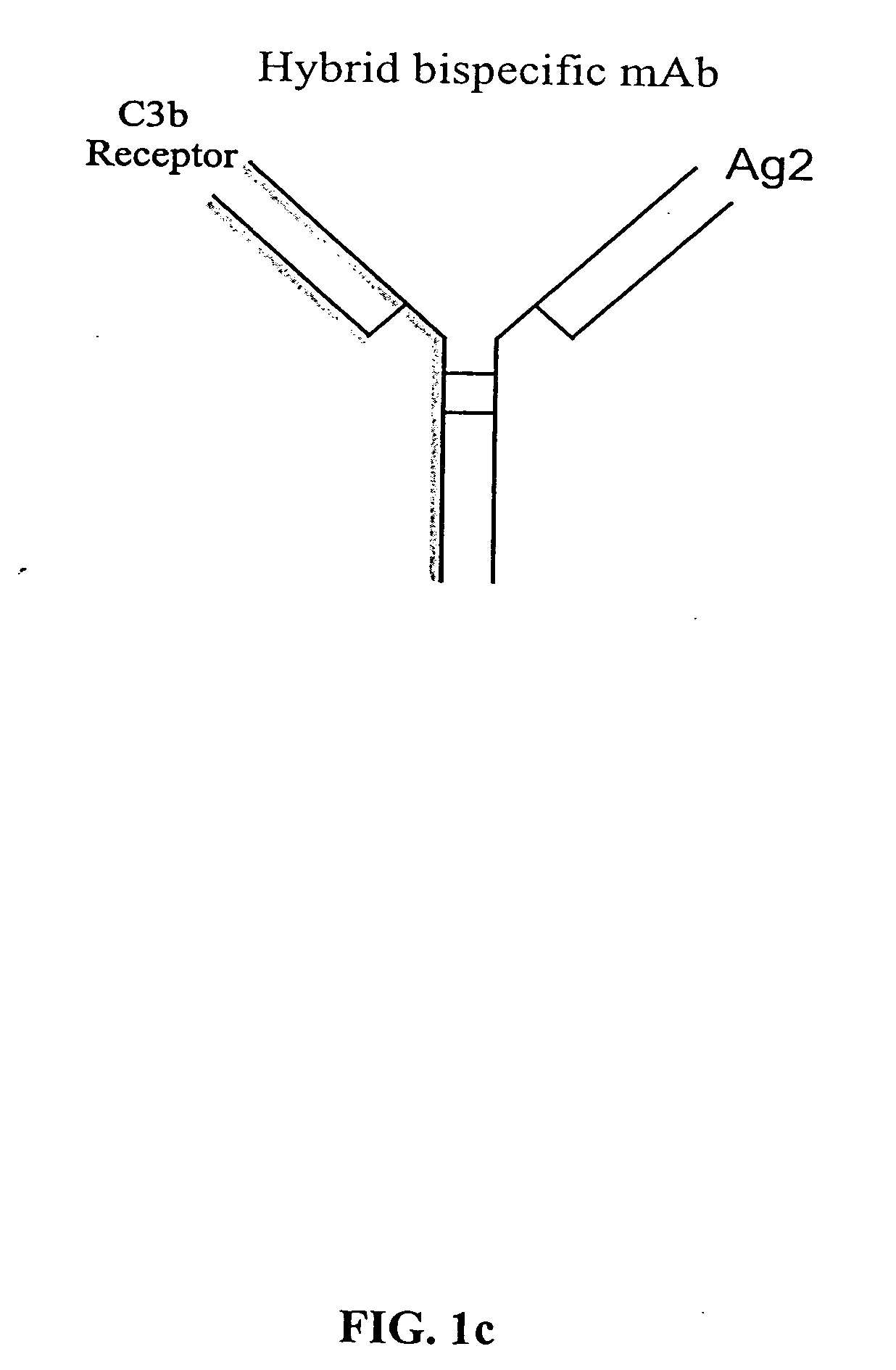
Bispecific molecules and uses thereof
InactiveUS20040180046A1Reduce clearanceRapidly and efficiently clearedFungiBacteriaCross-linkPrimate
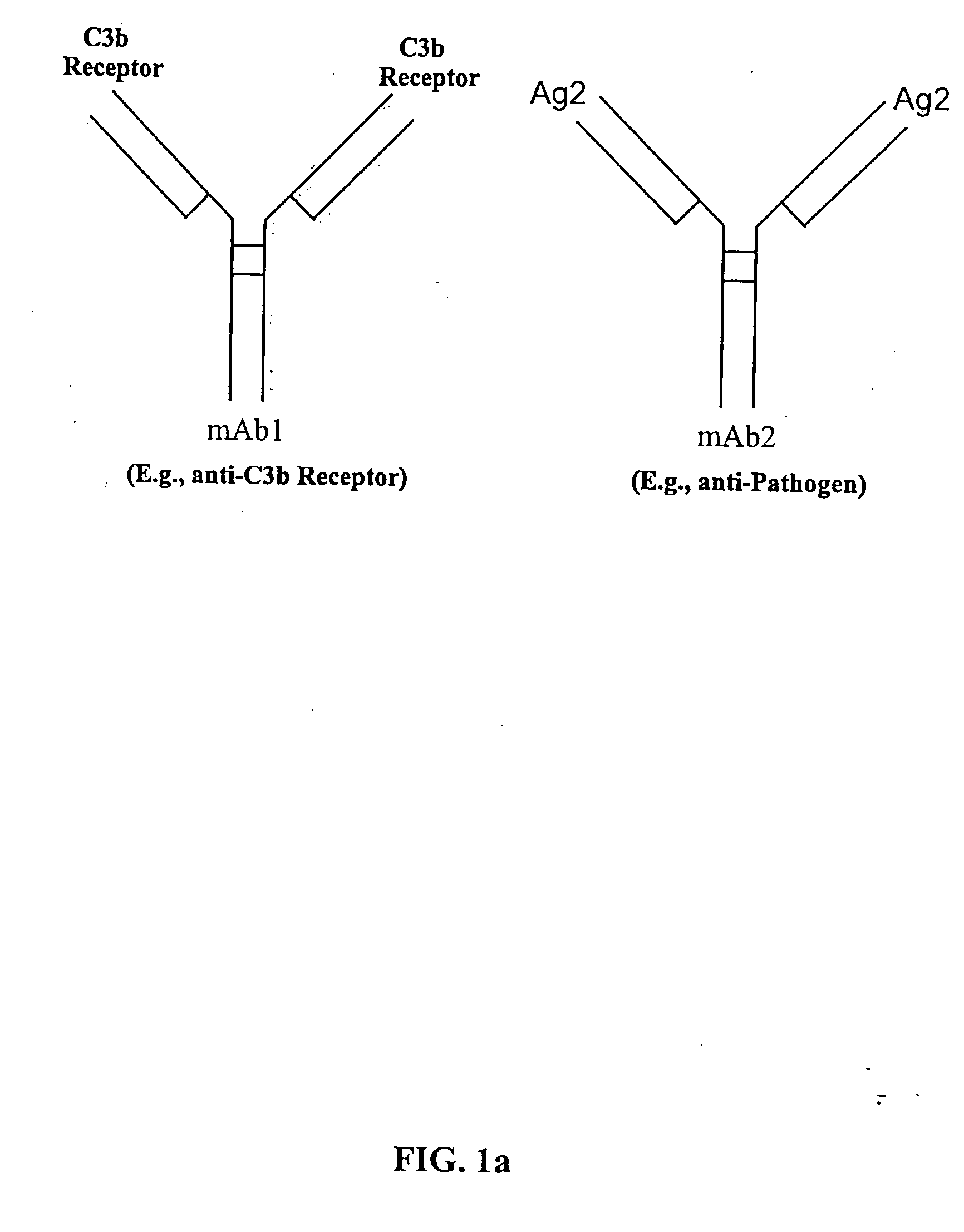
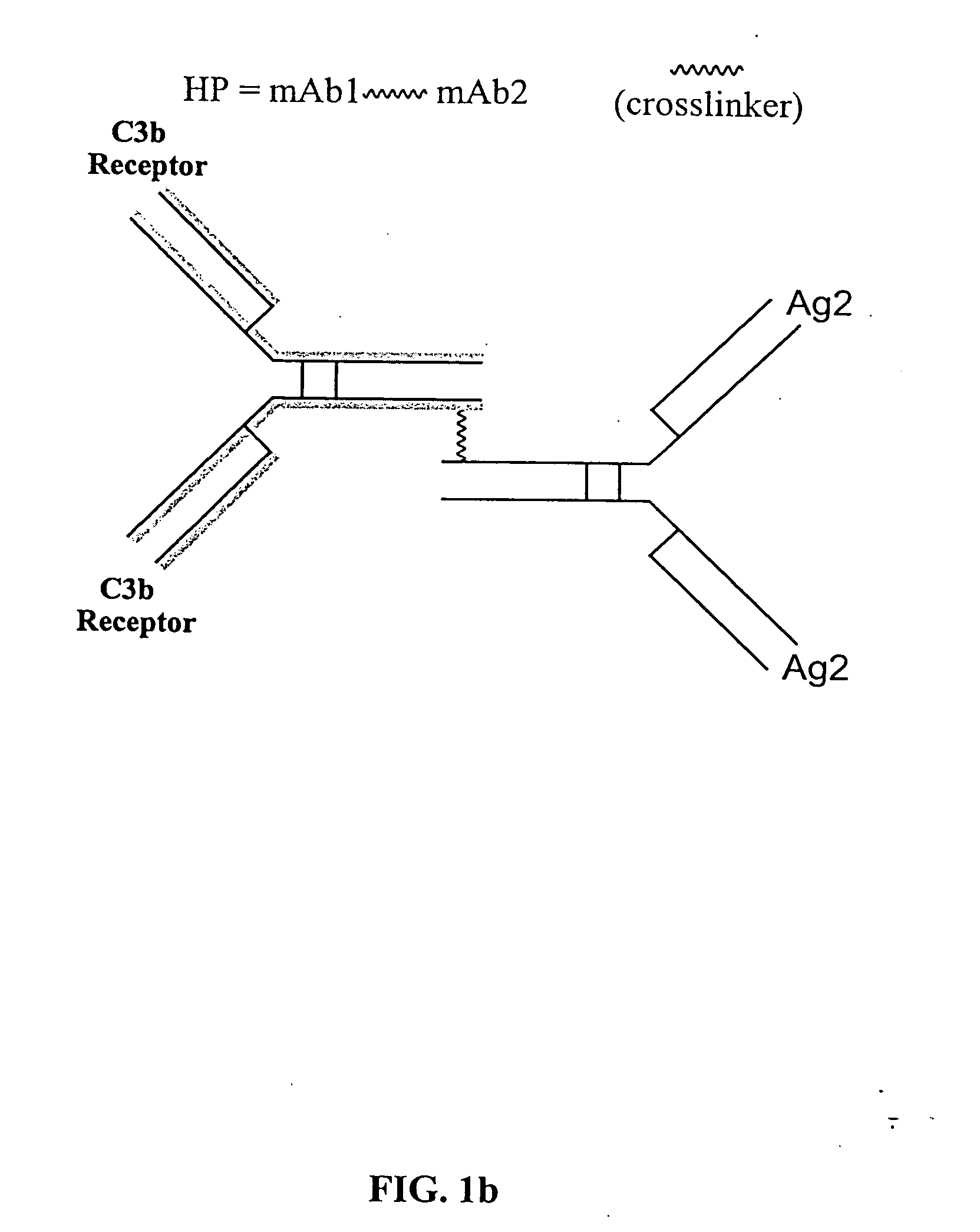
The present invention relates to bispecific molecules that are characterized by having a first binding domain which binds an antigen present in the circulation of a mammal and a second binding domain which binds the C3b-like receptor (known as complement receptor 1 (CR1) or CD35 in primates). The bispecific molecules do not consist of a first monoclonal antibody to CR1 that has been chemically cross-linked to a second monoclonal antibody. The invention also relates to methods of making the bispecific molecules and therapeutic uses thereof, as well as to kits containing the bispecific molecules. The invention further provides polyclonal populations of bispecific molecules, which comprise populations of bispecific molecules with different antigen recognition specificities. Such polyclonal populations of bispecific molecules can be used for targeting multiple epitopes of a pathogenic antigenic molecule and / or multiple variants of a pathogenic antigenic molecule.
Owner:ELUSYS THERAPEUTICS
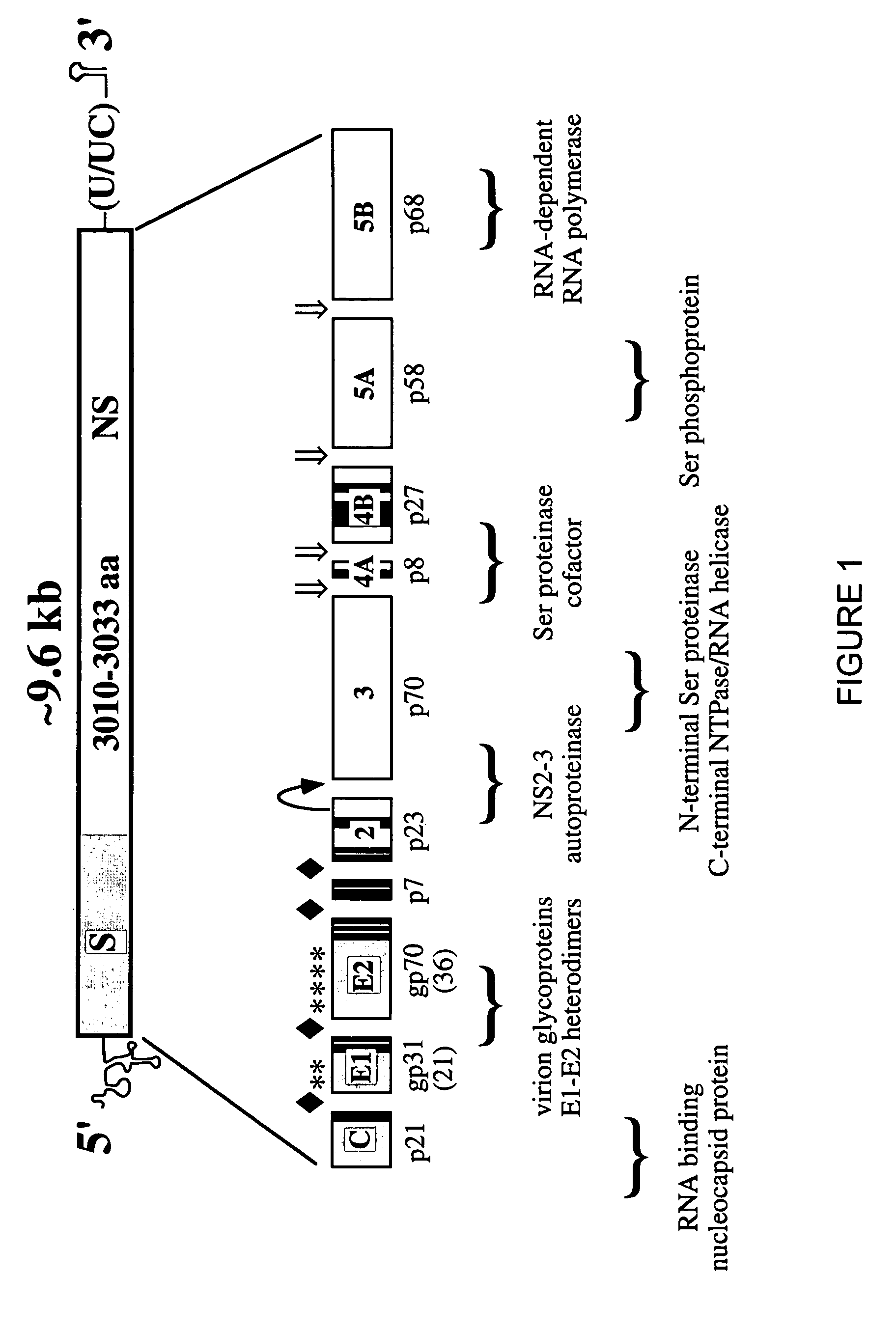
HCV variants
InactiveUS7049428B1Increased ability to establish replicationSsRNA viruses positive-senseSugar derivativesPrimateNucleotide
HCV variants are described. The variants include polynucleotides comprising non-naturally occurring HCV sequences and HCV variants that have a transfection efficiency and ability to survive subpassage greater than HCV that have wild-type polyprotein coding regions. Expression vectors comprising the above polynucleotides and HCV variants are also described, as are the provision of cells and host cells comprising the expression vectors. Methods for identifying a cell line that is permissive for infection with HCV are also provided, as are vaccines comprising the above polynucleotides in a pharmaceutically acceptable carrier. Additionally, methods for inducing immunoprotection to HCV in a primate are described, as are methods for testing a compound for inhibiting HCV replication.
Owner:WASHINGTON UNIV IN SAINT LOUIS
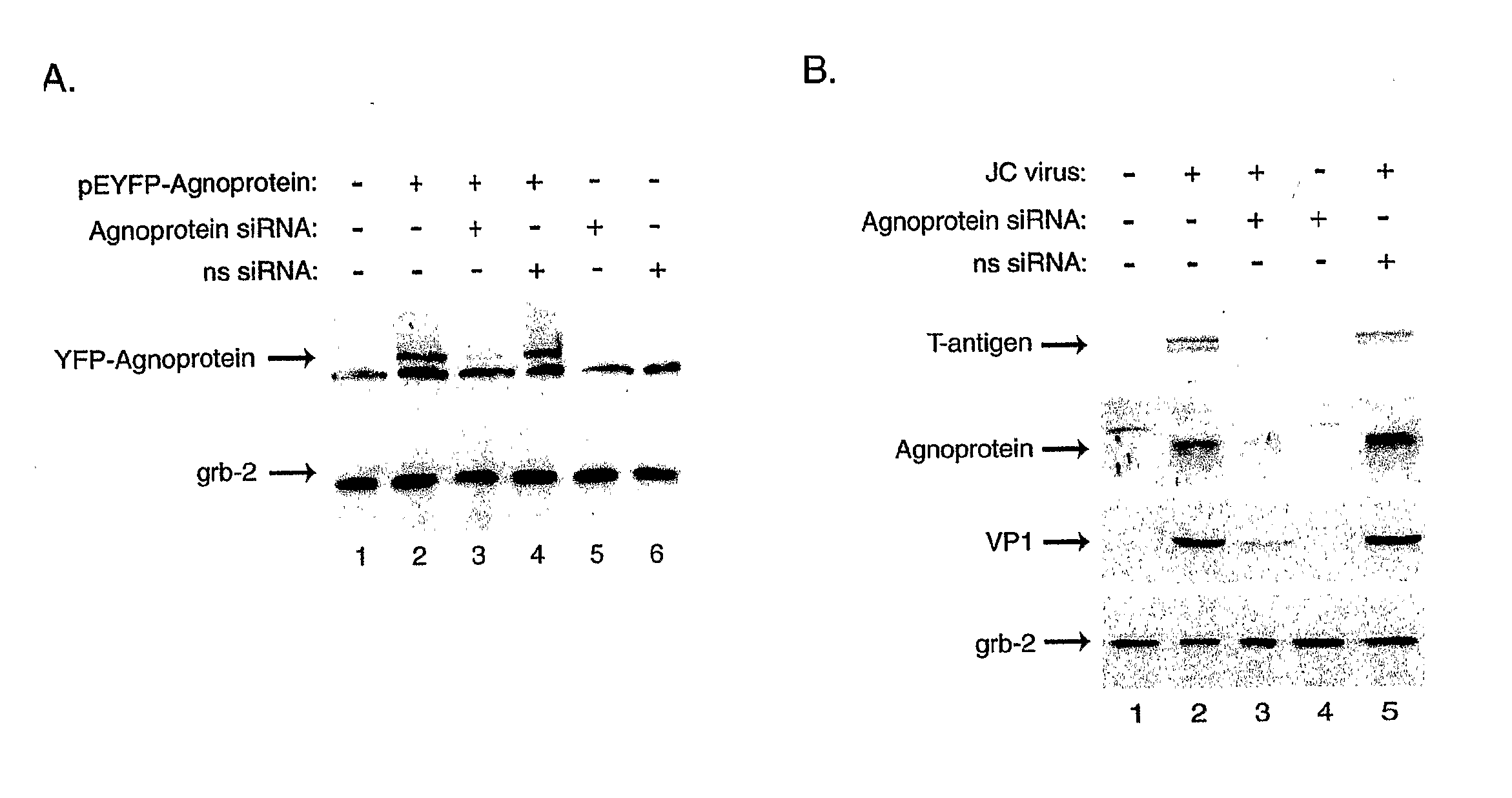
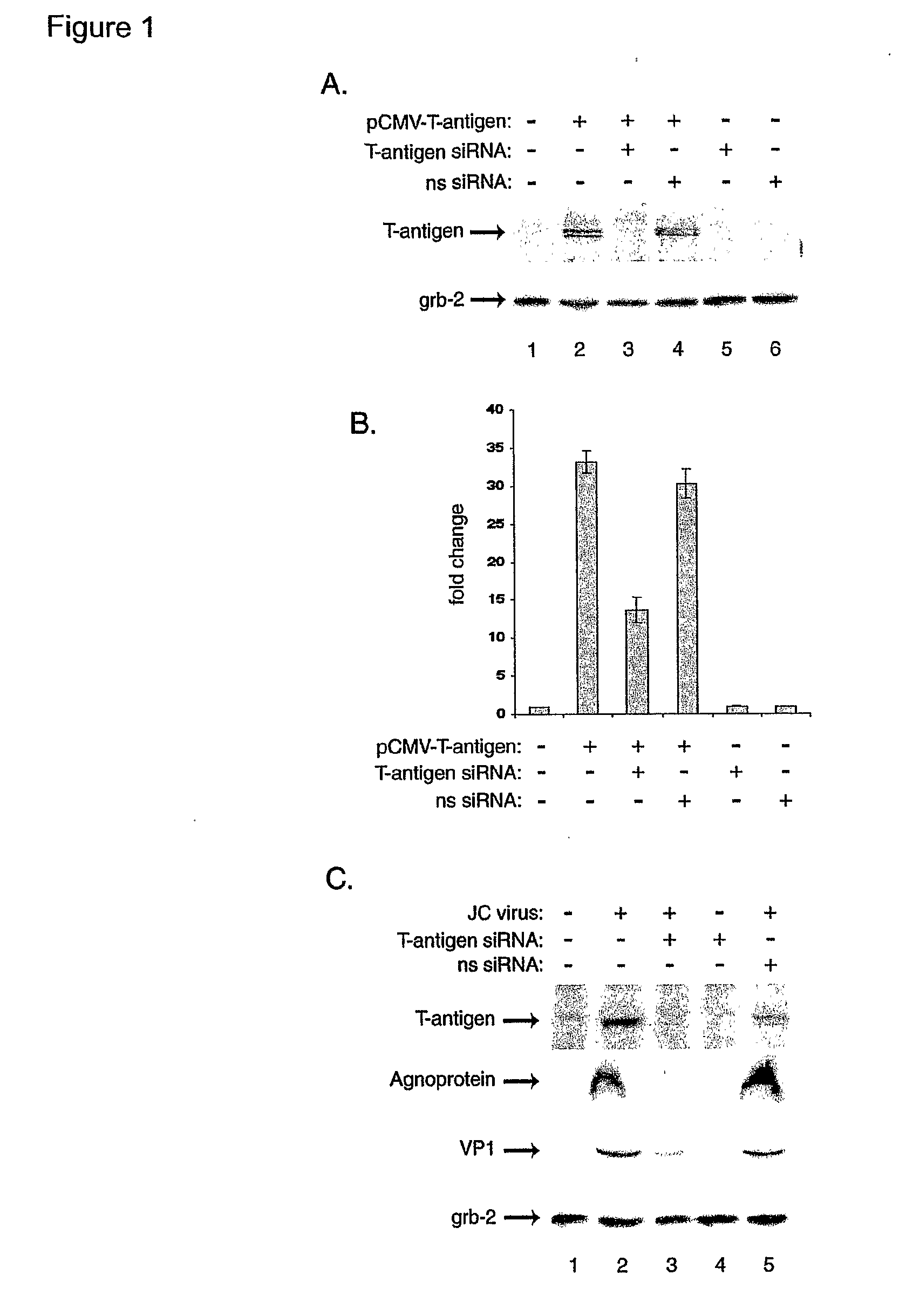
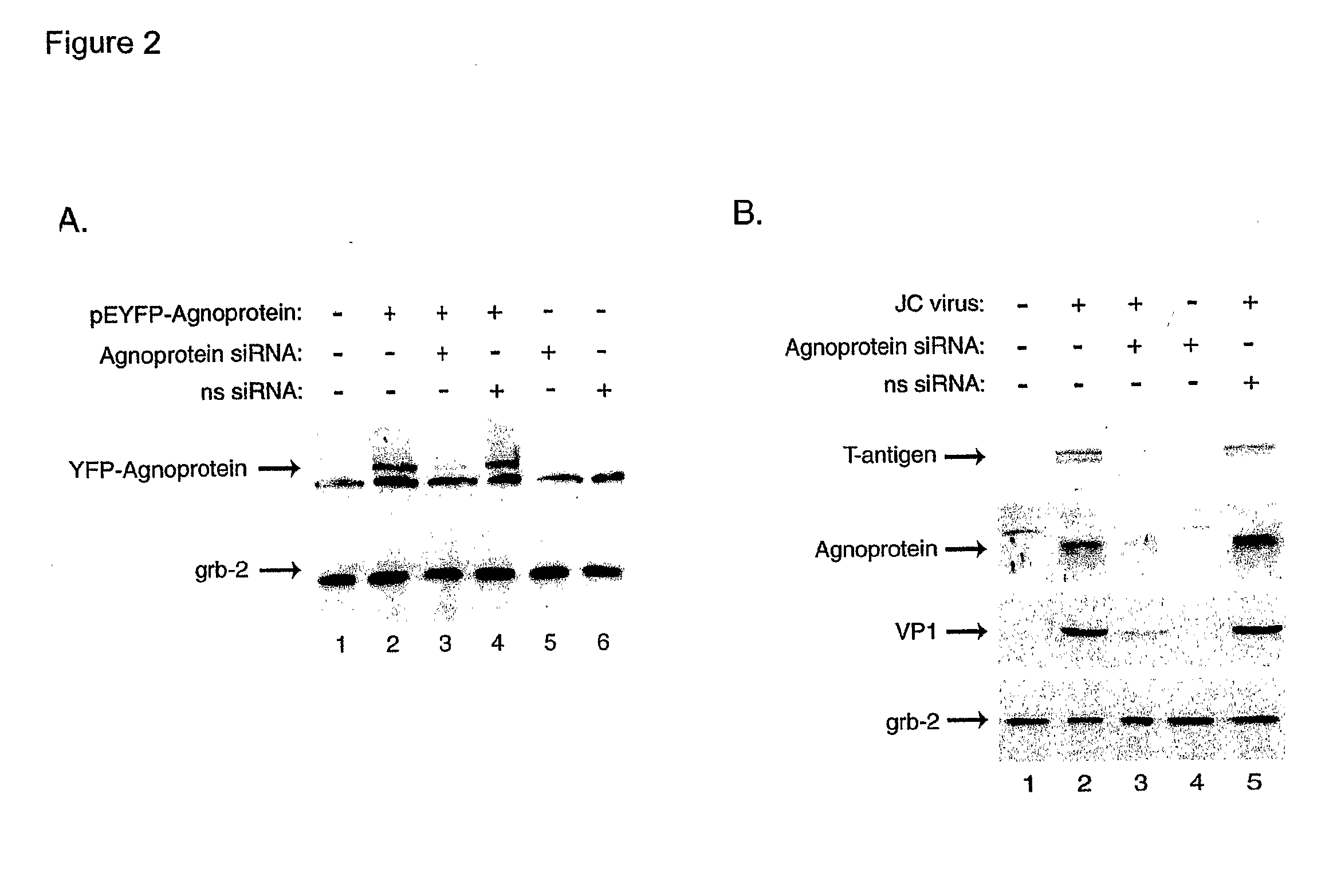
Compositions and Methods for Sirna Inhibition of Primate Polyomavirus Genes
InactiveUS20070249552A1Inhibit expressionAvoid infectionOrganic active ingredientsBiocideAntigenPrimate
RNA interference using small interfering RNAs which are specific for mRNA produced from the JCV agnoprotein and large T antigen genes inhibits expression of these and other primate polyomavirus genes. Primate polyomavirus infection, and diseases which are associated with primate polyomavirus infection, can be treated by administering the small interfering RNAs.
Owner:KHALILI KAMEL +1
Chimeric antibodies
The present invention provides a chimeric antibody or an antigen-binding portion thereof. The antigen-binding portion comprises at least two complementarity determining regions (CDR) and at least three framework regions, wherein at least one CDR is a New World primate CDR.
Owner:ARANA THERAPEUTIC LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com