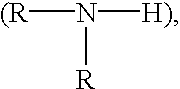Patents
Literature
308 results about "Cartilage cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cartilage is made up of cartilage cells, called chondrocytes, which aid in production, function and repair of the tissue. Cartilage cells are composed of several different materials, but contain a large amount of gelatinous collagen fibers.
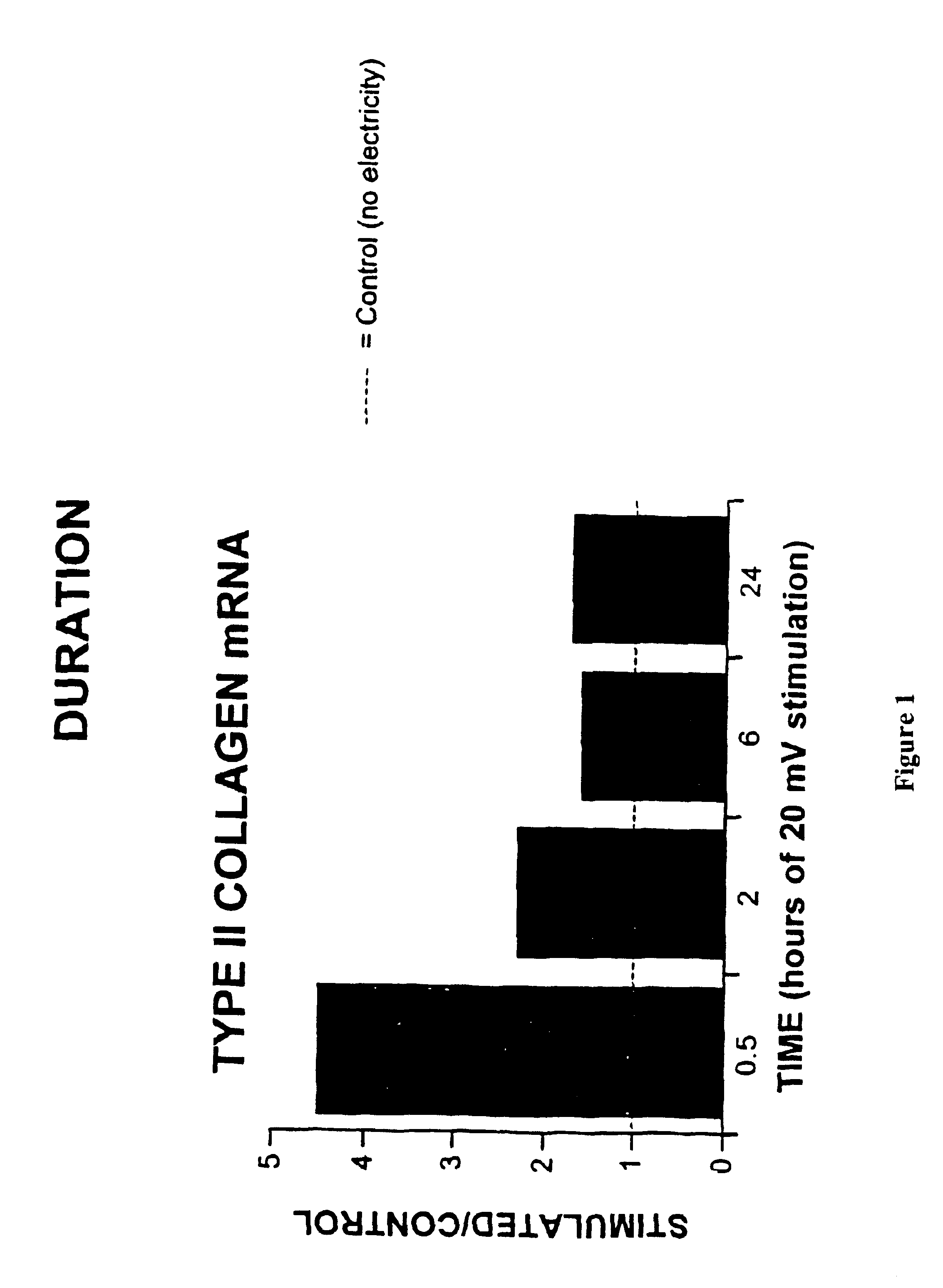
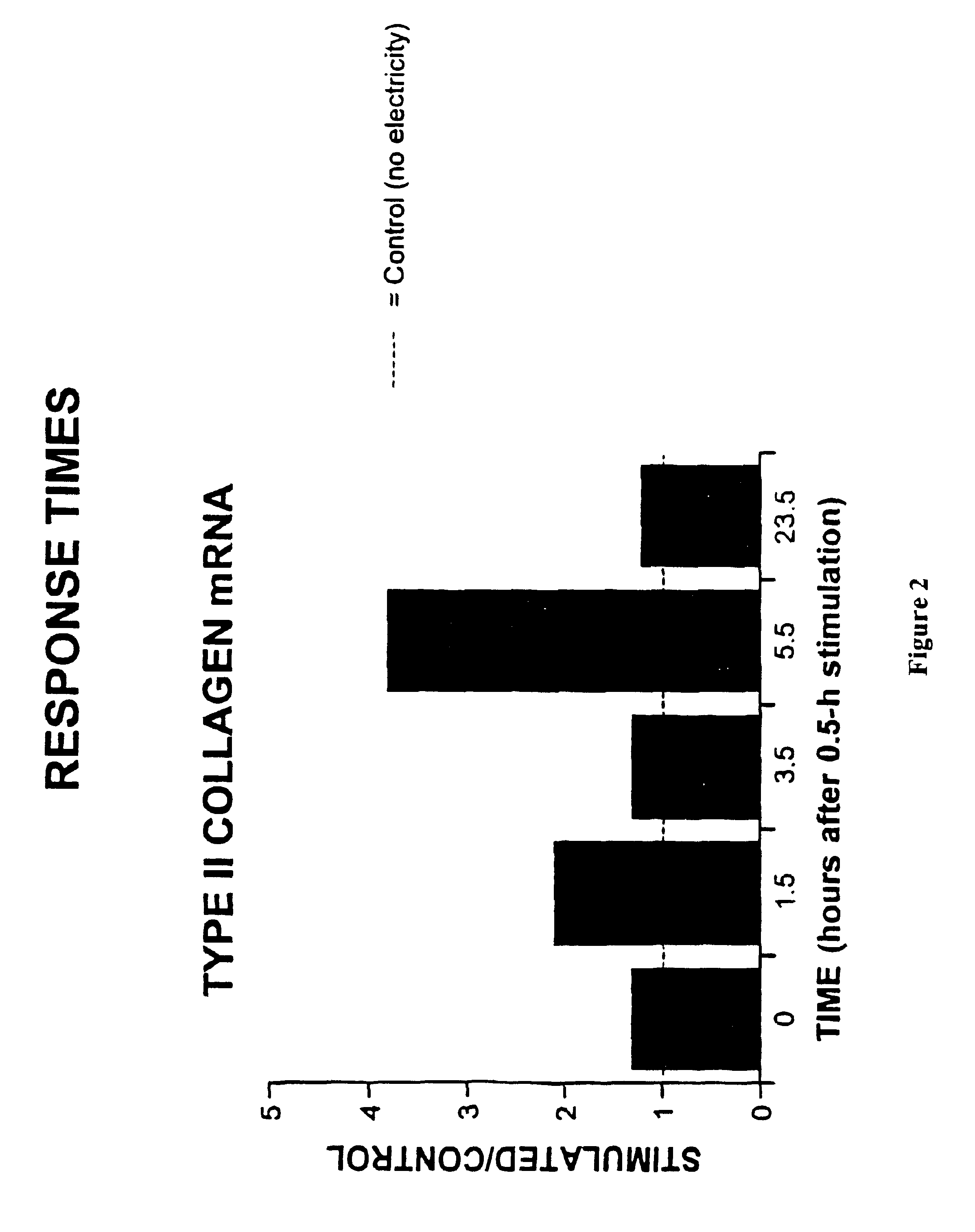
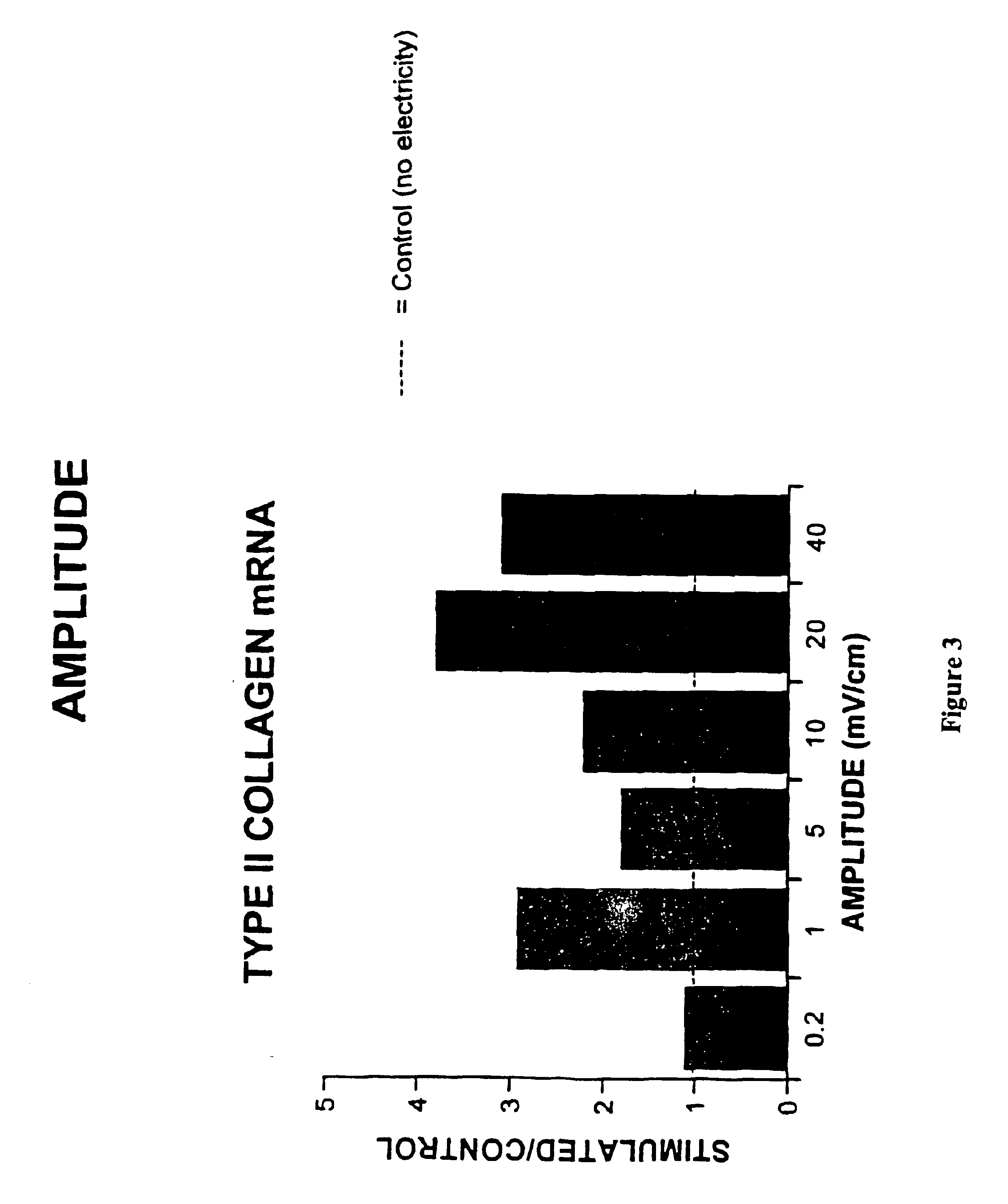
Regulation of type II collagen gene expression using specific and selective electrical and electromagnetic signals
InactiveUS6919205B2Increase typeElectrotherapySkeletal/connective tissue cellsCartilage cellsHuman DNA sequencing
Methods and devices for the regulation of type II collagen gene expression in cartilage cells via the application of specific and selective fields generated by specific and selective electric and electromagnetic signals in the treatment of diseased or injured articular cartilage. By gene expression is meant the up regulation or down regulation of the process whereby specific portions (genes) of the human genome (DNA) are transcribed into mRNA and subsequently translated into protein. Methods and devices are provided for the targeted treatment of injured or diseased cartilage tissue that include generating specific and selective electric and electromagnetic signals that generate specific and selective fields optimized for type II collagen gene expression and exposing cartilage tissue to the specific and selective fields generated by specific and selective signals so as to regulate type II collagen gene expression in such cartilage tissue. The resulting methods and devices are useful for the targeted treatment of osteoarthritis, rheumatoid arthritis, cartilage injury, and cartilage defects.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
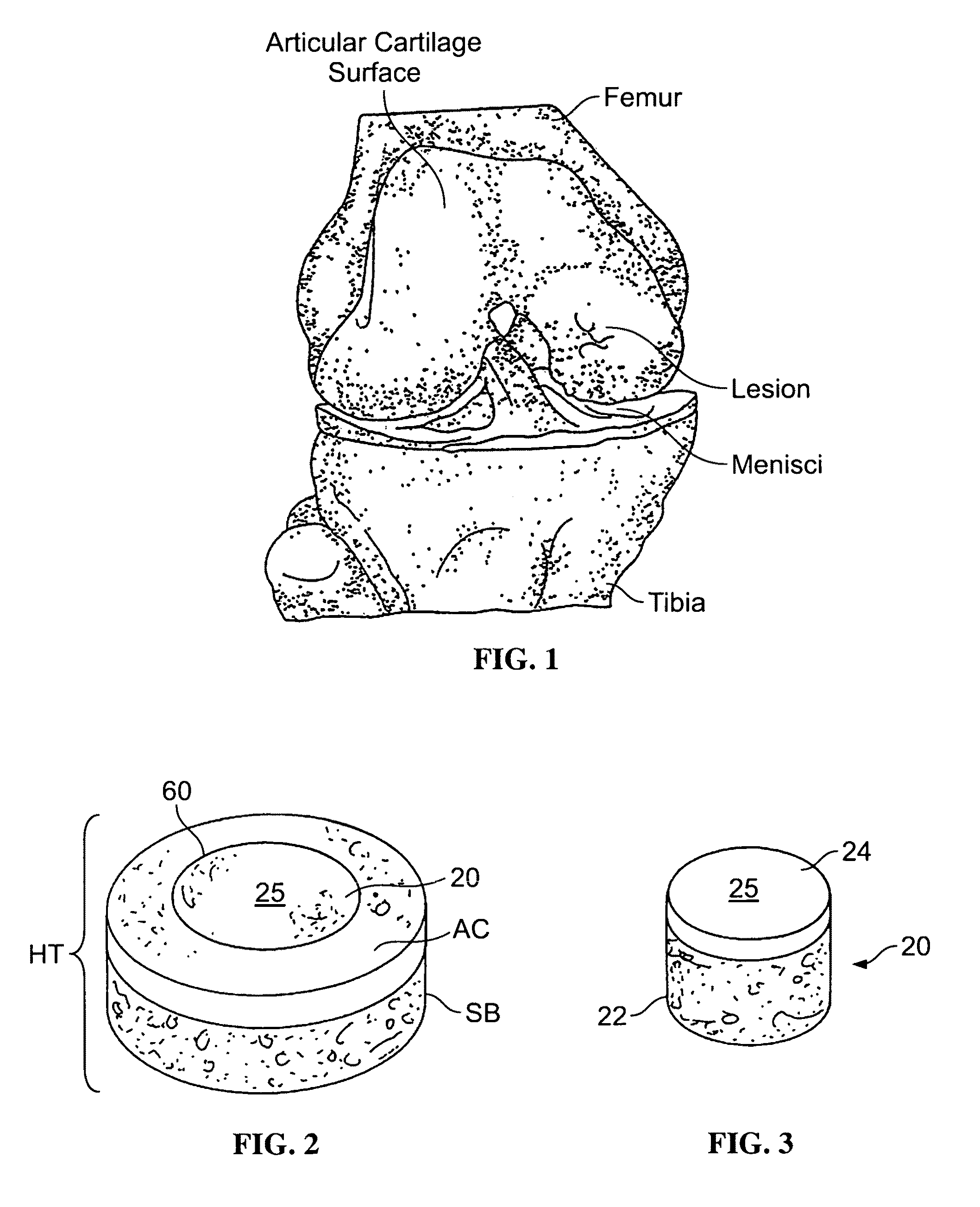
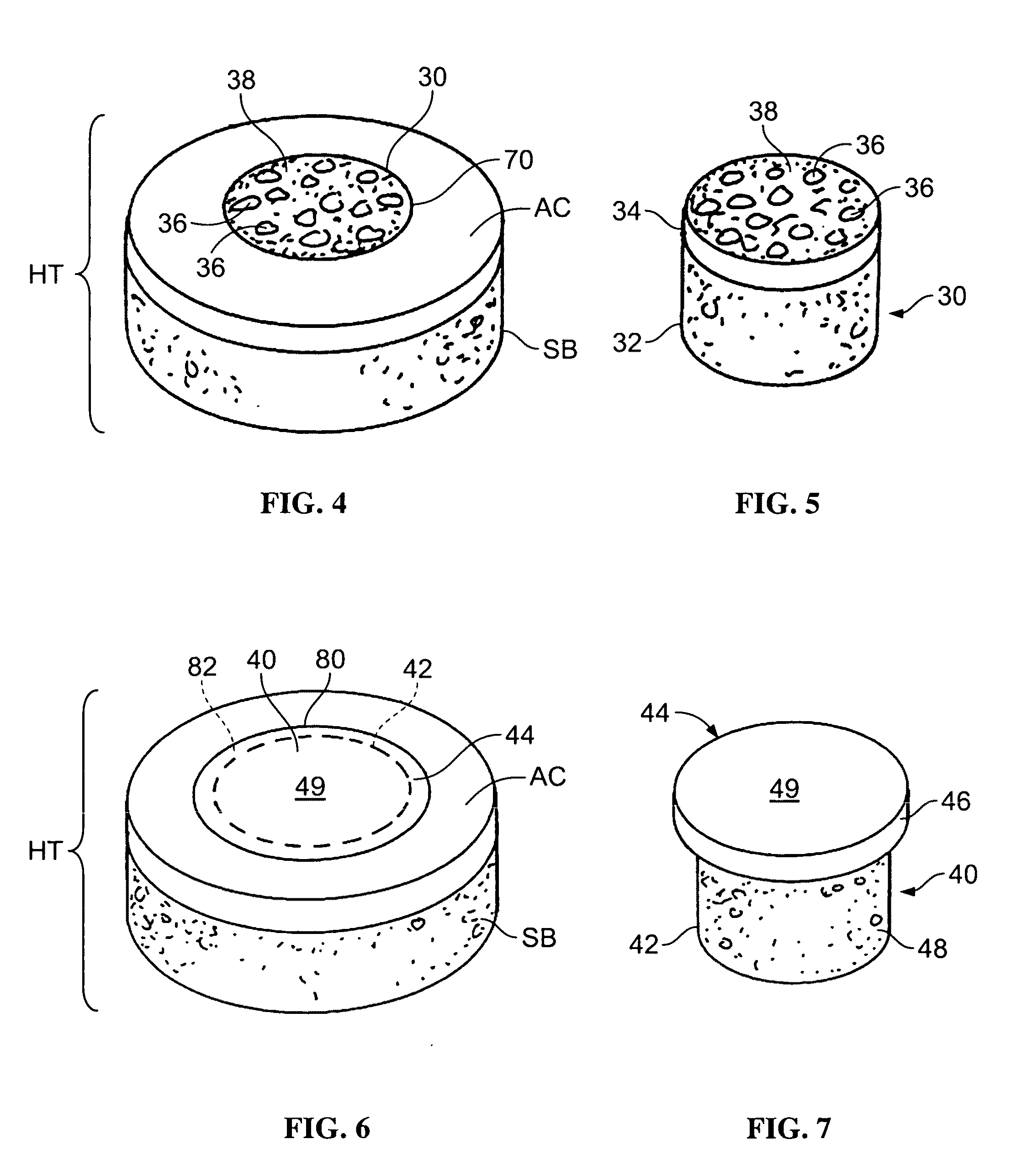
Allograft osteochondral plug combined with cartilage particle mixture
InactiveUS20090291112A1Promoting cartilage cell migration intoPromoting proliferationBiocidePeptide/protein ingredientsInterference fitSubchondral bone
An allograft osteochondral plug is combined with a mixture that includes freeze-milled cartilage particles, and such combination is used to repair defects in articular cartilage. The plug includes an subchondral bone portion and an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans. At least a portion of the plug has a lateral dimension selected to form an interference fit against a tissue layer exposed as a result of a bore formed in a defect area in articular cartilage of a host. The cartilage particle mixture is placed adjacent at least a portion of the plug for promoting cartilage cell migration into (i.e., from the adjacent host cartilage) and proliferation in the bore, and for enhancing tissue integration between the plug and patient (i.e., host) tissue when the plug is inserted into the bore. Methods for surgical implantation of the plug into a patient are also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
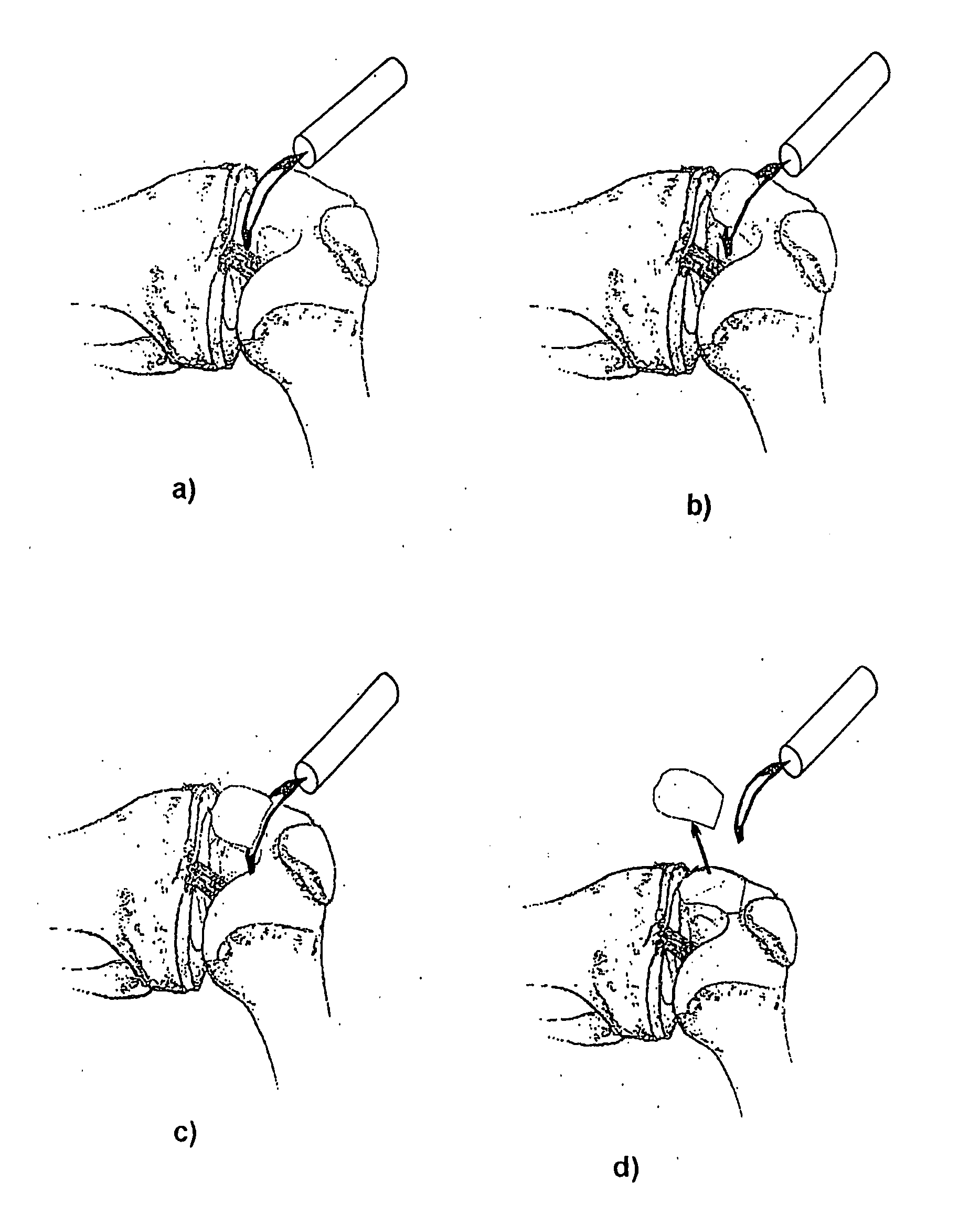
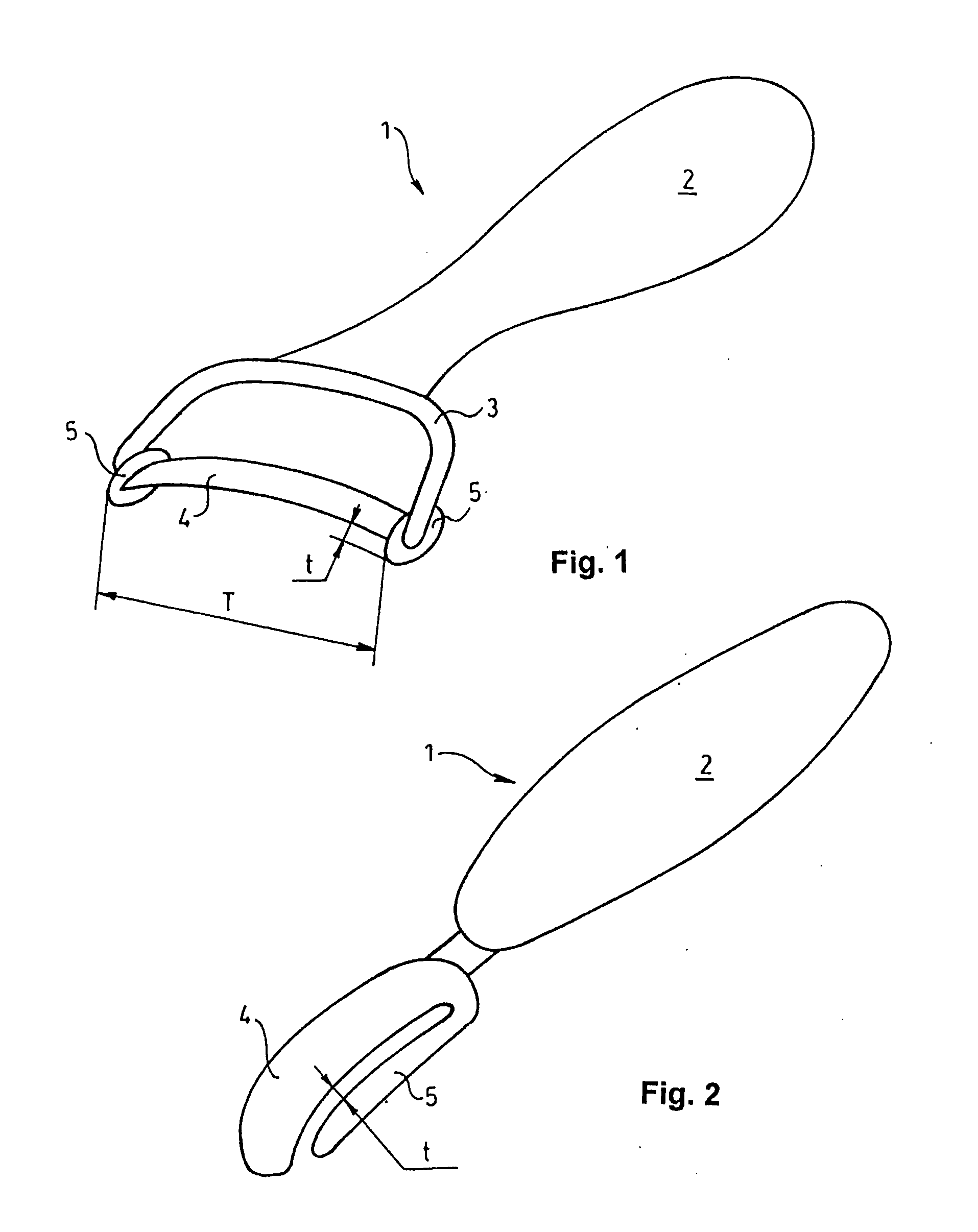
Articular cartilage, device and method for repairing cartilage defects
InactiveUS20100211173A1Easy to useSimple structureSuture equipmentsInternal osteosythesisYarnCartilage cells
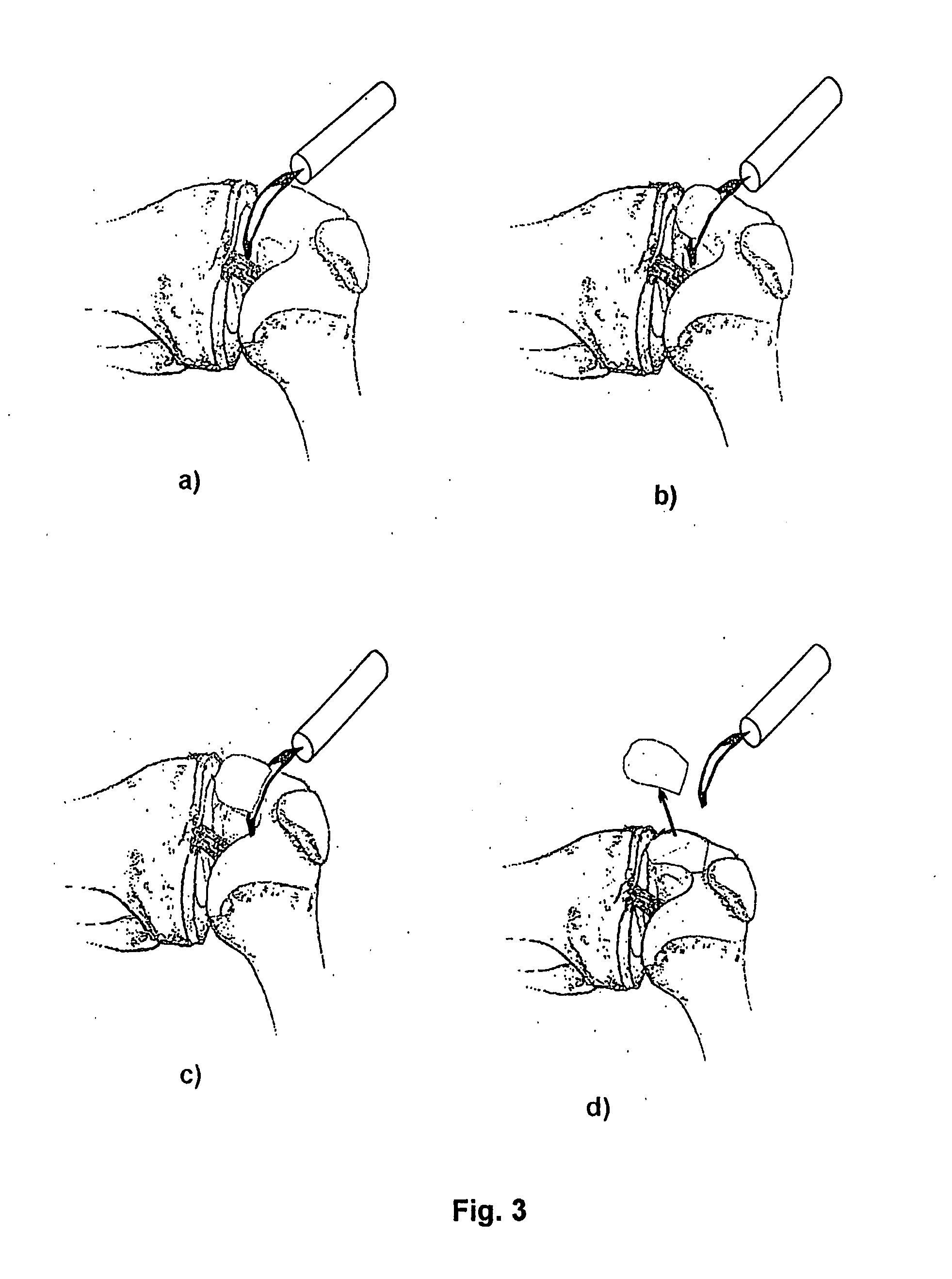
The articular cartilage according to the invention is made of pure cartilage and is provided with incisions (12) on the surface facing the bone. The cartilage cells are preferably seeded on the surface provided with incisions (12). The method for producing the articular cartilage comprises the step of collecting cartilage from joints, wherein pure cartilage is collected without bone, and incisions are made on the surface of the cartilage intended to face the bone. It is preferably fresh frozen until use. The device for harvesting articular cartilage, comprises handle and cutting blade, wherein the cutting blade (4) is curvilinear and is provided with spacer elements (5), meanwhile the device for producing incisions in articular cartilages comprises handle (2) and a bridge (3) connected to said handle (2) and being provided with one or more cutting blade(s) (4). During the method for applying the articular cartilage the articular cartilage is fixed by thin surgical yarn stitches, by fibrin glue or by small anchors (FIG. 8).
Owner:PECSI TUDOMANYEGYETEM
A gradient laminated composite supporting frame material based on bionic structures and its preparation method
The invention relates to a kind of laminated gradient composite scaffold material based on the bionic structure and its preparation method. The said material has three or more layers of porous structure, comprising of hyaluronic acid, PLGA, PLA, collagen II and nano-hydroxyapatite (nano-HA) , beta- tricalcium phosphate ( beta-TCP). The upper layer is made by collagen II / hyaluronic acid or PLGA and PLA imitating the cartilage layer. With the counterfeit the calcified cartilage layer in the middle, it is one layer or multi- sublayer, made by nano-HA or beta-TCP with collagen II / hyaluronic acid or PLGA and PLA; the bottom is made of nano-HA or beta-TCP with collagen II or PLGA and PLA. From top to bottom, the content of inorganic material increases in its layers, about 0 to 60 mass percent of layers. The aperture of the scaffold material is 50 to 450 micron, with 70 to 93 percent porosity. The scaffold material made by this invention has an adjustable degradation rate, good mechanical and biocompatible properties, can adapt to culture with cartilage bone cells and compound with growth factor and small molecular or peptides, it can be used to repair cartilage simultaneously.
Owner:HUAZHONG UNIV OF SCI & TECH
Method for preparing integrated frame fabrication of cartilage of tissue-engineered bone having function interface
InactiveCN101032430AAchieve mechanical strengthGood biocompatibilityBone implantCartilage cellsOsteoblast adhesion
The present invention discloses process of preparing integral bionic tissue engineering bone-cartilage rack with functional interface. The process includes the first establishment of CAD model of the integral bone-cartilage rack by means of bionic principle; preparing collagen / chitosan and collagen / hydroxyapatite micro polymer powder; converting the CAD model into fast forming file input into a 3D printing fast forming machine, spreading the powder with one powder spreader, spraying pentanedial adhesive selectively on the spread powder for adhering and eliminating excessive powder to obtain the integral bionic tissue engineering bone-cartilage rack with functional interface. The present invention has excellent biocompatibility, controllable cartilage rack degrading speed and mechanical strength and other advantages.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Cartilage cell epimatrix three-dimensional porous sponge stent for tissue engineering and preparation method thereof
ActiveCN101496913AFacilitate in vitro constructionPromotes regeneration in the bodyBone implantCartilage cellsCell-Extracellular Matrix
The invention discloses a three-dimensional porous cartilage extracellular matrix sponge scaffold made of natural cartilage, which can compound cells further to construct tissue engineered cartilage, and can be used for clinically repairing cartilage defects. In the invention, conditions which can fully perform cell extraction and form a porous scaffold matrix are provided by processing cartilage into cartilage microfilaments, and then the cell extraction and solidification and / or strengthening treatment are performed to obtain the three-dimensional porous cartilage extracellular matrix sponge scaffold which is completely decellurized. The antigenicity and cell components are removed in the natural cartilage, and an extracellular matrix component of the cartilage is retained to obtain the scaffold with appropriate pore diameter and porosity, suitable degradation rate, good biocompatibility and certain biomechanical strength. The scaffold has the advantages of broad material sources, low cost, simple and feasible preparation technology, and good repetitiveness; and the scaffold can be widely applied in the field of tissue engineering and has good clinical application prospect.
Owner:GENERAL HOSPITAL OF PLA
Formative agent of protein complex
InactiveUS20020119946A1Keep for a long timePromote resultsBiocidePeptide/protein ingredientsCartilage cellsCuticle
The present invention proposes formative agent of protein complex, in which a polyphenol is useful component, and the agent is useful as gene complex, cell adhesion inhibitor or immune tolerogen. The polyphenol of forming the agent is selected from catechin group consisting of epigallocatechin-gallate, tannic acids, or proanto-dianisidine, a protein of the protein complex is selected from proteins consisting of animal proteins composed of polypeptide chain of peptide-combined amino acids, vegetative proteins, nucleus proteins, glycogen proteins, lipo-proteins and metal proteins, the gene complex comprises by compositing genes by polyphenol catechins in order to introduce genes to cells of animals or human bodies, a cell composed of the cell adhesion inhibitor is selected from cells consisting of an animal cell including a stem cell, skin cell, mucosa cell, hepatocyte, islet cell, neural cell, cartilage cell, endothelial cell, epidermal cell, osteocyte or muscle cell isolated from human or animal organism, or sperm, ovum or fertilized egg of domestic animals or fishes and a tissue or an organ for transplantation of the immune tolerogen is selected from the tissue or the organ consisting of skin, blood vessel, cornea, kidney, heart, liver, umbilical cord, bowels, nerve, lung, placenta or pancreas.
Owner:BERTELSMANN MUSIC GROUP
Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
The invention relates to a human amnion mesenchymal stem cell serum-free culture medium and a culture method thereof. The culture medium is formed by adding human serum albumin, human transferrin, human insulin and sodium selenite into a DMEM / F12 basic culture medium. The culture method for the culture medium comprises the following steps of: digesting human amnion by using trypsin, then digesting the human amnion by using collagenase IV and deoxyribonuclease I, and filtering the mixture to obtain single cell suspension; and adding the human serum albumin, the transferrin, the insulin and the sodium selenite into the DMEM / F12 basic culture medium in a ratio of VDMEM to VF12 of 1:1, and putting human amnion mesenchymal stem cells in a 37 DEG C CO2 incubator with saturated humidity and volume fraction of 5 percent under the serum-free condition, wherein culture in vitro and amplification are realized by solution change and transfer of culture, potentiality of multi-direction differentiation is maintained, and the amplified cells can be induced in vitro to form cartilage cells, osteoblasts and adipocytes. The culture medium and the culture method have the characteristics of no other animal sources, wide source and no limitation of ethics.
Owner:辽宁艾米奥干细胞与再生医学研究院有限公司
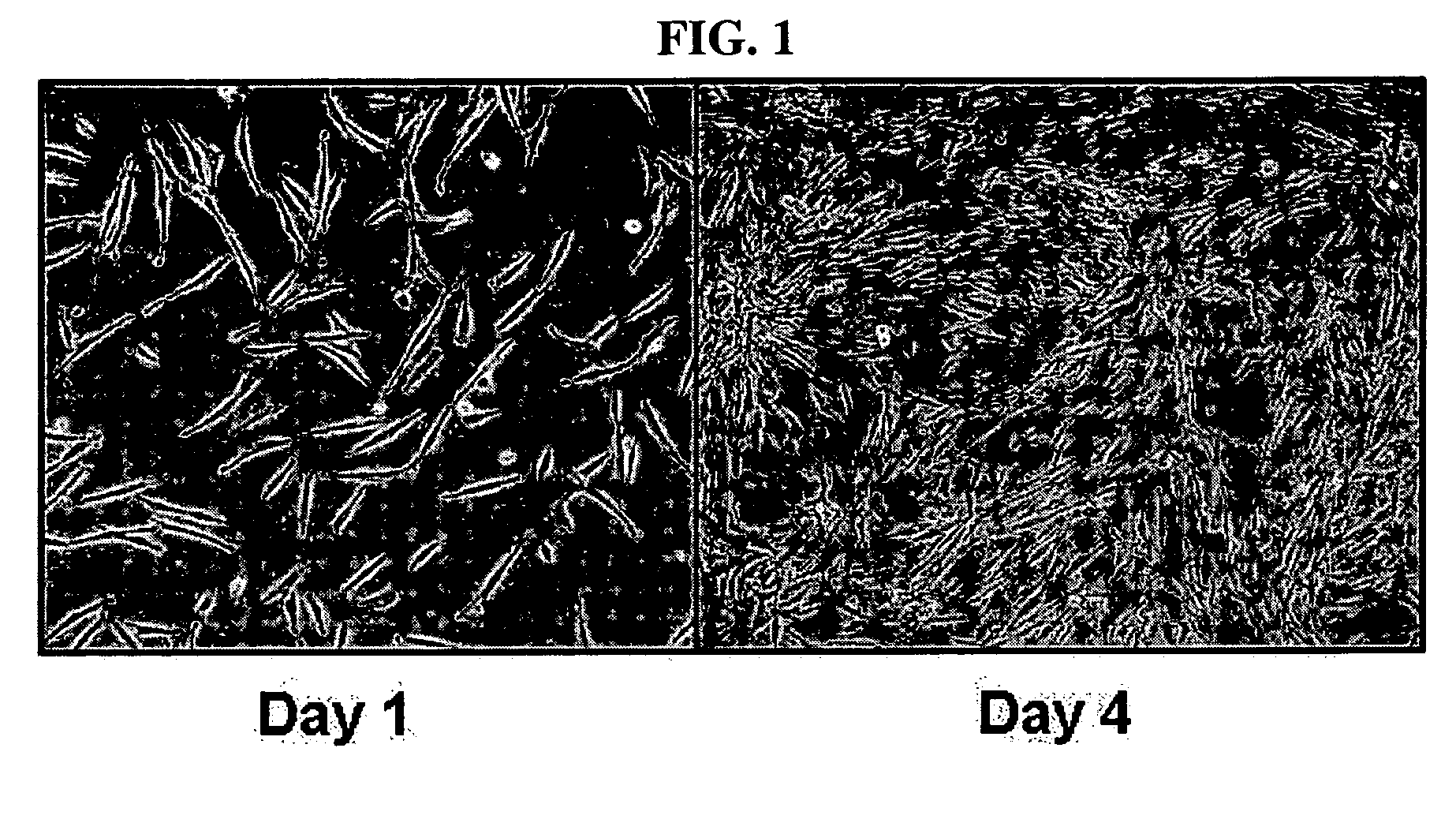
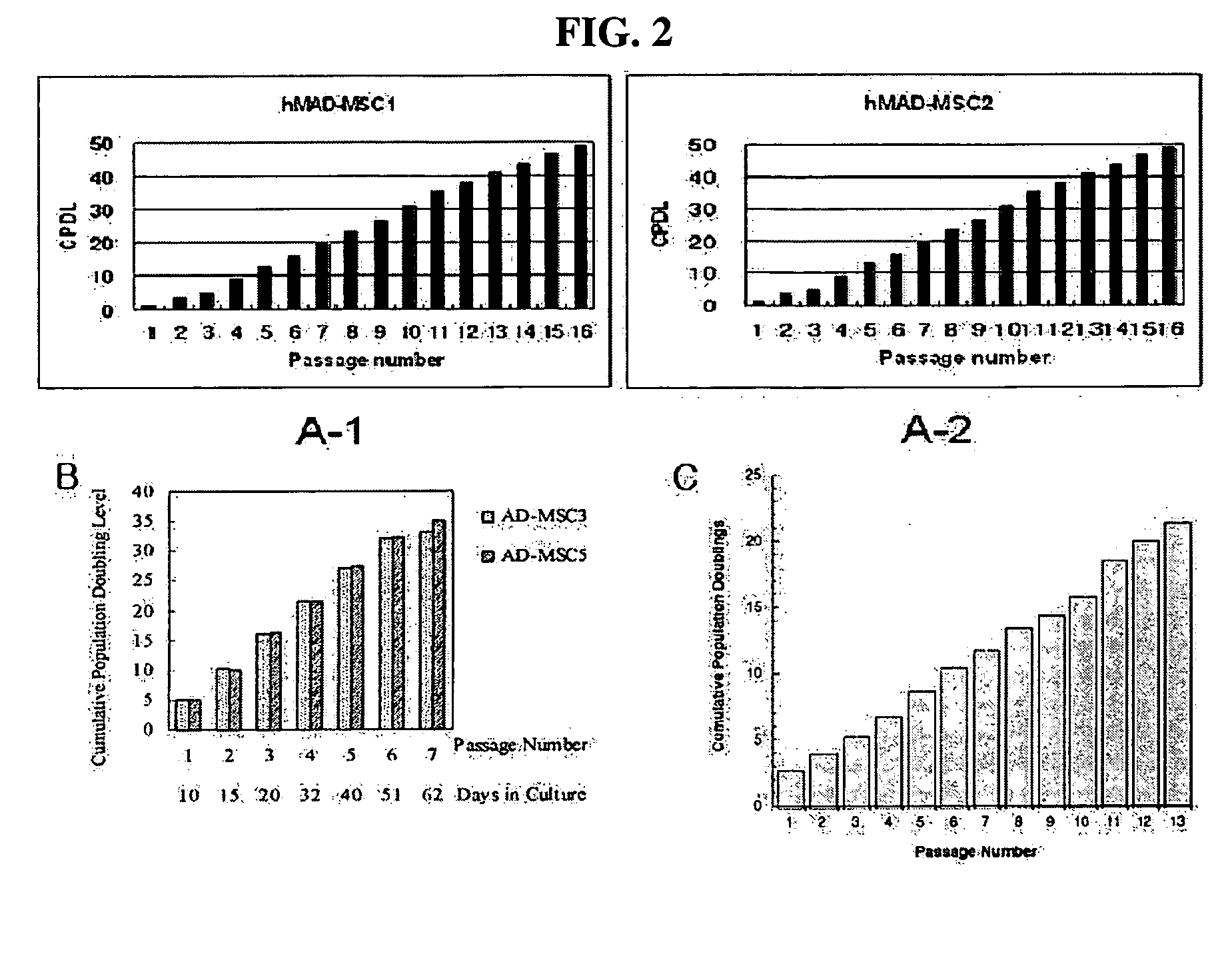
Multipotent stem cells derived from human adipose tissue and cellular therapeutic agents comprising the same
ActiveUS20070110729A1High proliferation ratePositive immunological responsesBiocideNervous disorderCartilage cellsSerum free media
This invention relates to human adipose tissue-derived multipotent adult stem cells. More particularly, the invention relates to human adipose tissue-derived multipotent stem cells, which can be maintained in an undifferentiated state for a long period of time by forming spheres and have high proliferation rates, as well as methods for isolating and maintaining the adult stem cells, and methods for differentiating the multipotent adult stem cells into nerve cells, fat cells, cartilage cells, osteogenic cells and insulin-releasing pancreatic beta-cells. Also, the invention relates to cellular therapeutic agents for treating osteoarthritis, osteoporosis and diabetes and for forming breast tissue, which contain the differentiated cells or the adult stem cells. Although the multipotent stem cells are adult stem cells, they have the ability to differentiate into osteogenic cells, nerve cells, astrocytes, fat cells, chrondrogenic cells or insulin-releasing pancreatic beta-cells, and so are effective in treating osteoporosis, osteoarthritis, nerve disease, diabetes, etc. Also, the stem cells form spheres in a serum-free medium containing CORM-2, and thus can be maintained in an undifferentiated state for a long period of time. Also, the stem cells have very high proliferation rates. Accordingly, the stem cells are useful as cellular therapeutic agents.
Owner:RNL BIO
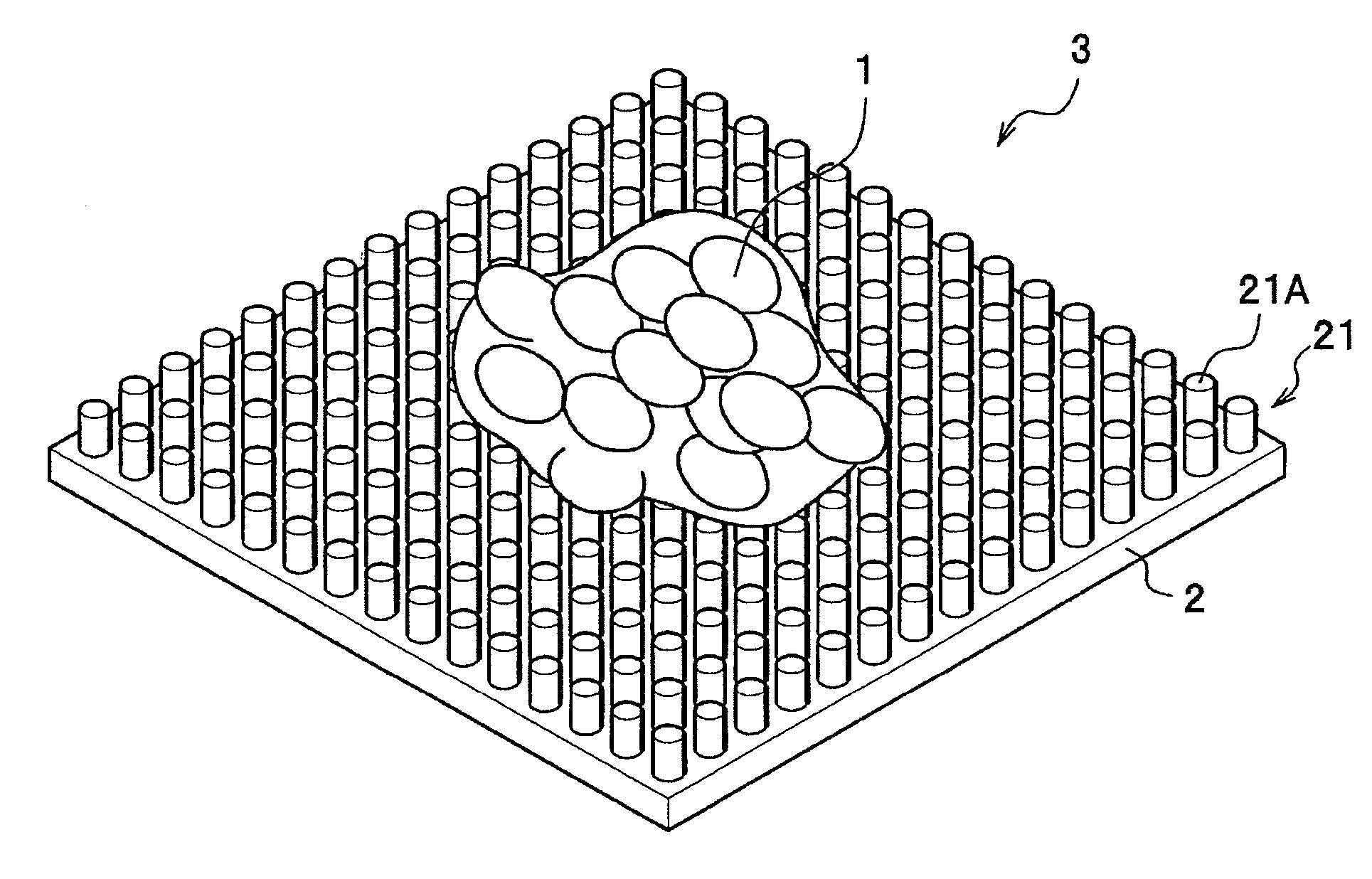
Process and substrate for culturing cartilage cell, material for reproducing biological tissue containing cartilage cell, and cartilage cell
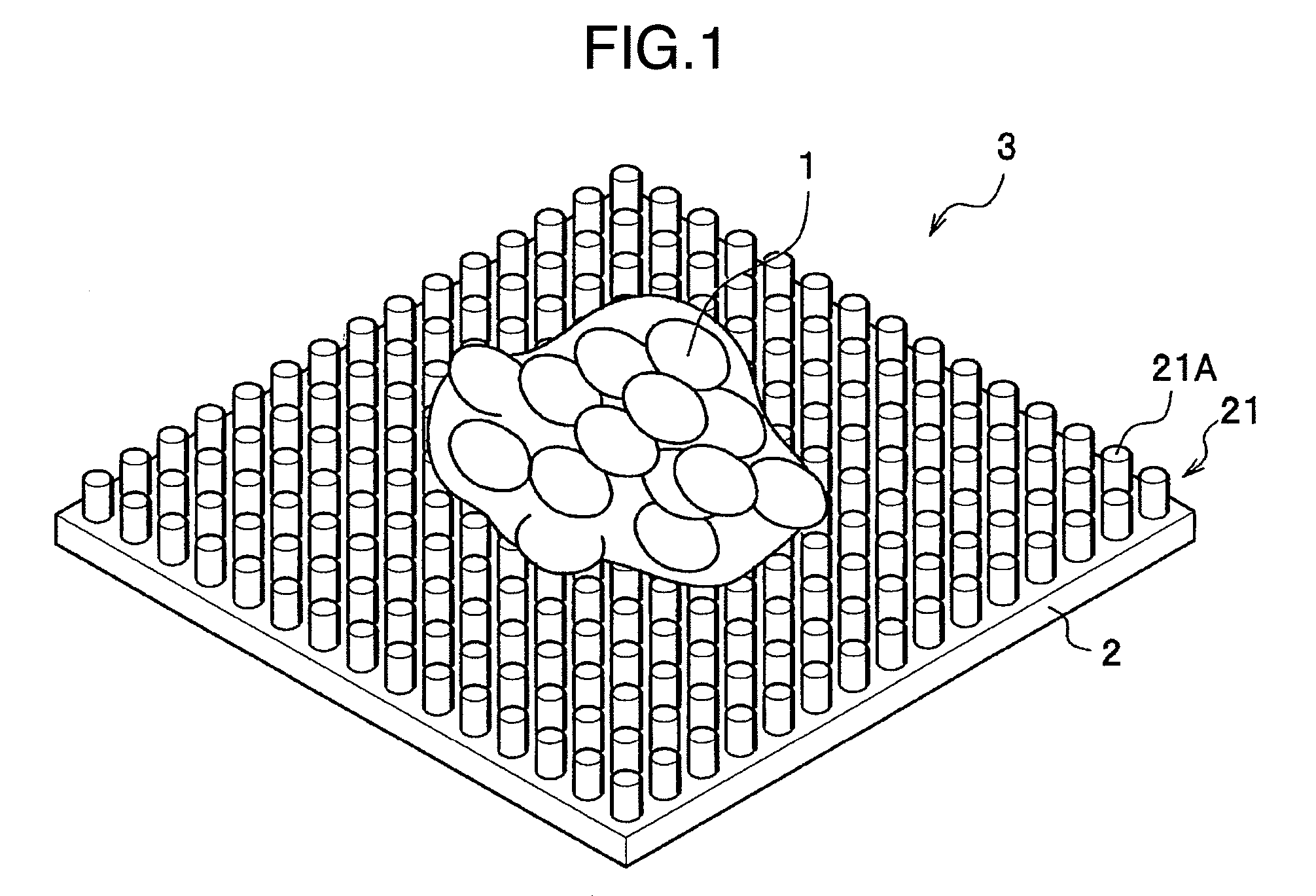
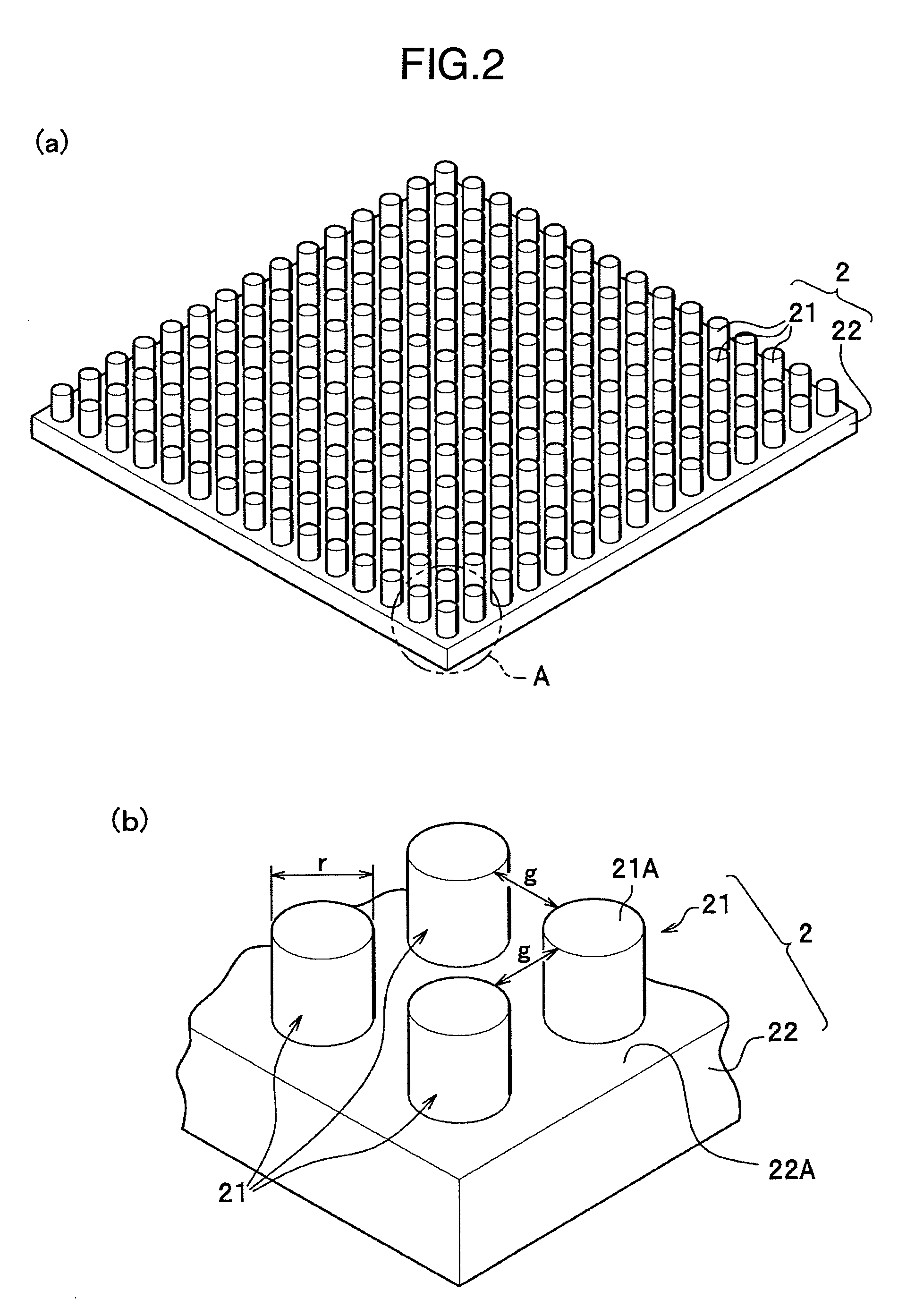
There is provided a technology for simple three-dimensional culturing of cartilage cells. The technology includes a method for culturing cartilage cells which comprises seeding cartilage cells 1 on a plurality of projected portions 21 of a culturing substrate 2 having a culture surface on which the projected portions 21 having an equivalent diameter and an interval which are smaller than the equivalent diameter of the cartilage cells 1 to be cultured, placing the culture substrate 2 having the cartilage cells 1 in a culture vessel, and culturing the cartilage cells to form aggregates of cartilage cells.
Owner:HITACHI LTD
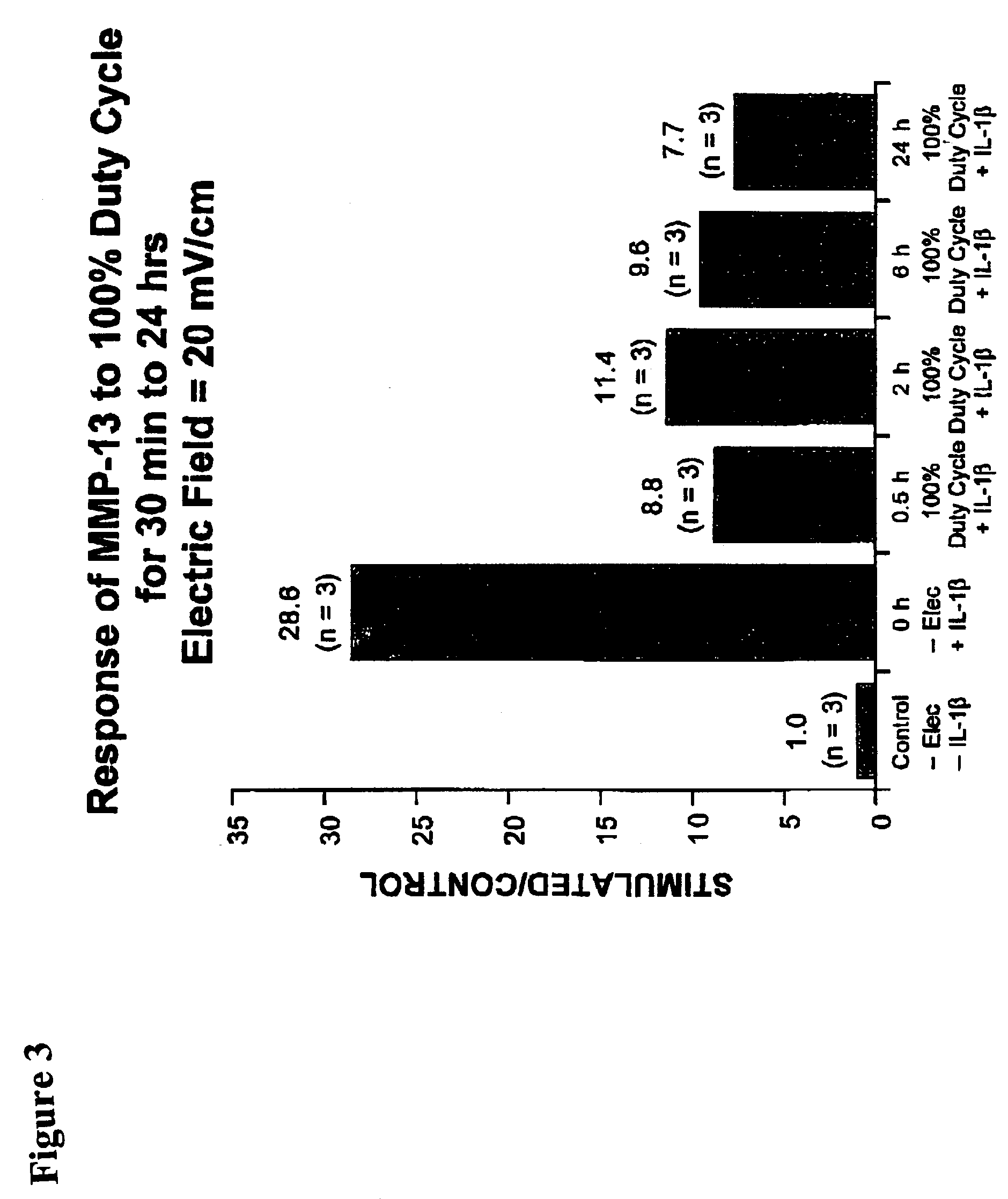
Regulation of matrix metalloproteinase gene expression using specific and selective electrical and electromagnetic signals
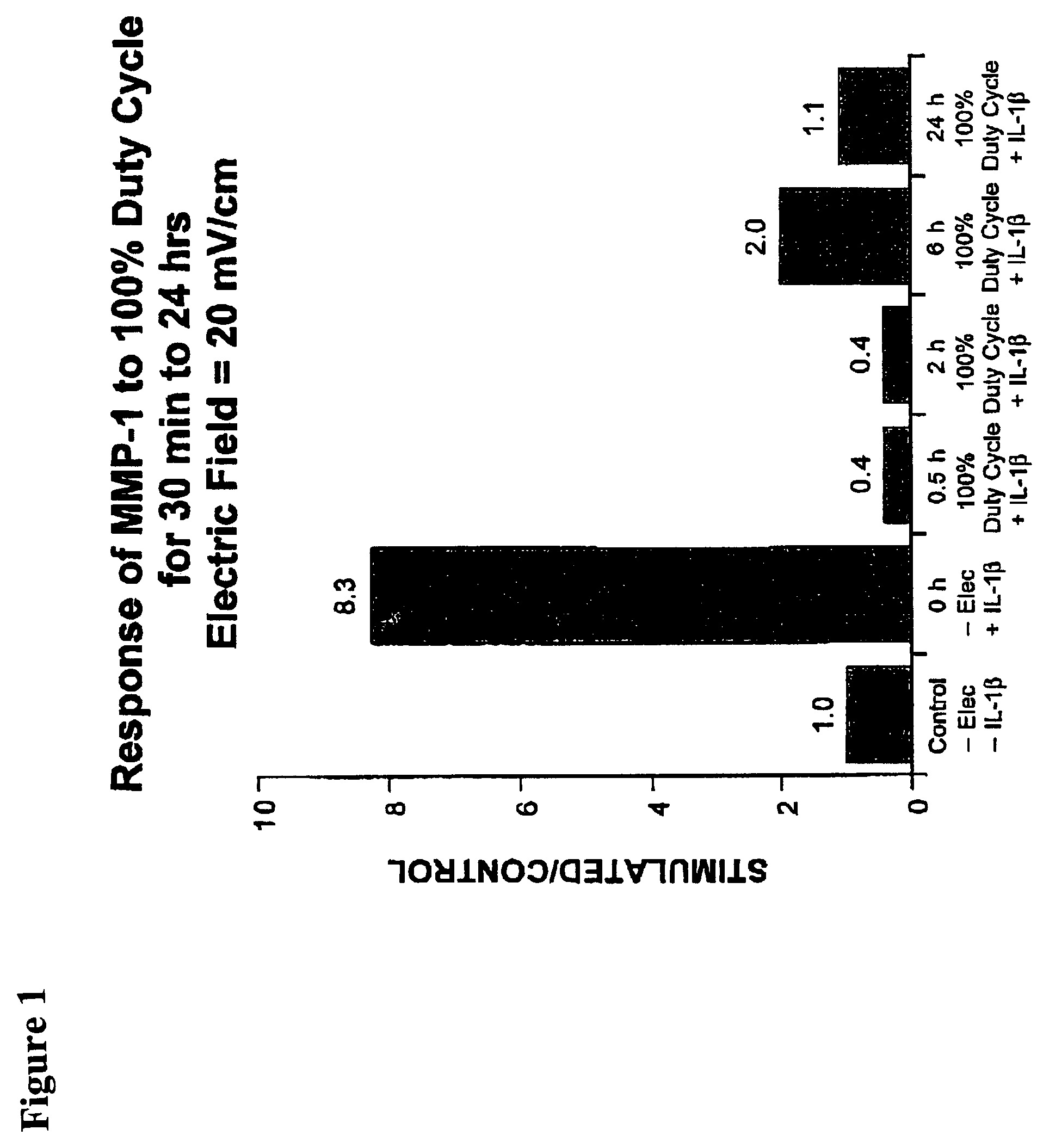
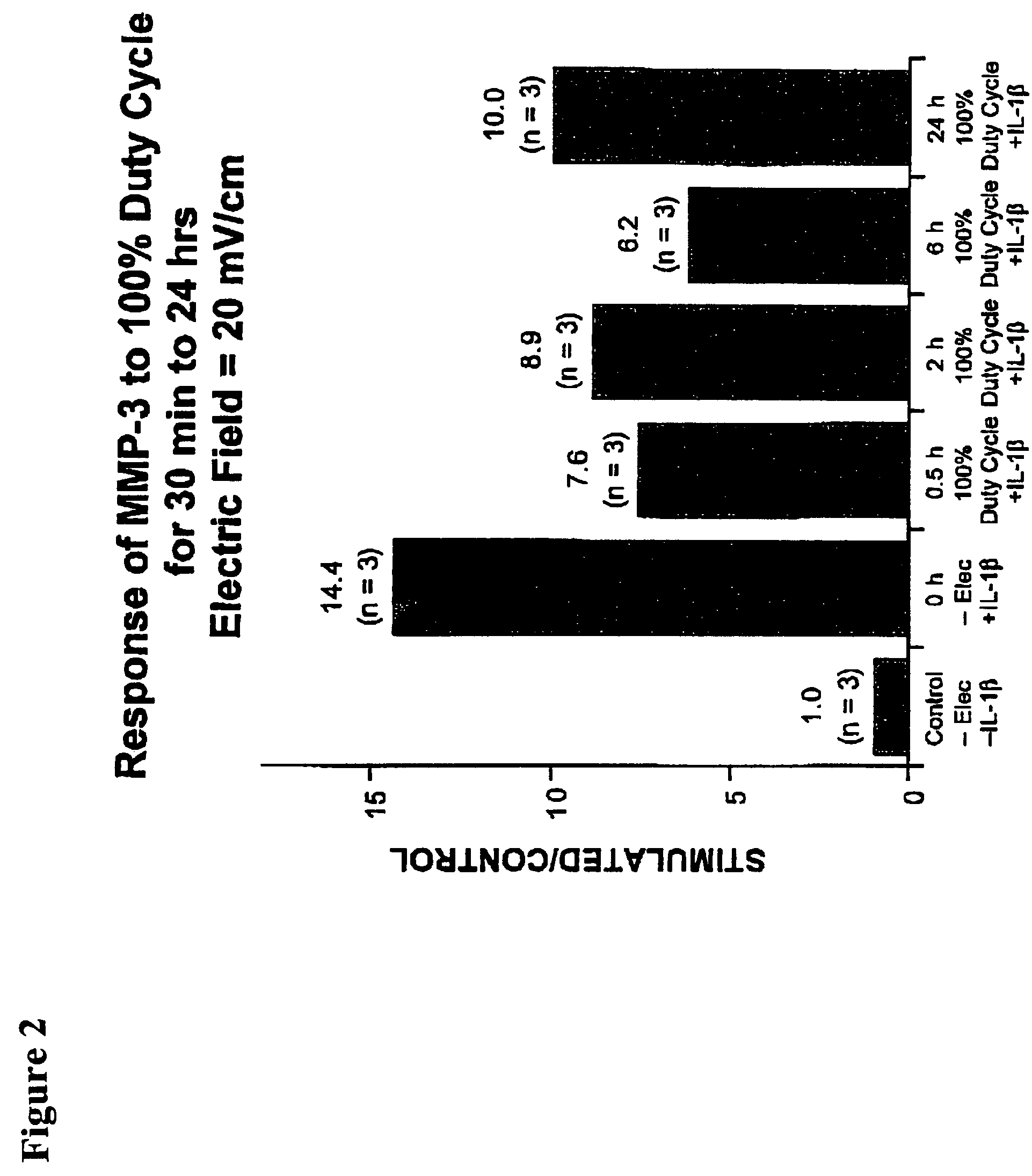
Methods and devices for the regulation of matrix metalloproteinase gene expression in cartilage cells via the application of fields generated by specific and selective electric and electromagnetic signals in the treatment of diseased or injured articular cartilage. By gene expression is meant the up-regulation or down-regulation of the process whereby specific portions (genes) of the human genome (DNA) are transcribed into mRNA and subsequently translated into protein. Methods and devices are provided for the targeted treatment of injured or diseased cartilage tissue that include generating specific and selective electric and electromagnetic signals that generate fields optimized for reduction of matrix metalloproteinase gene expression and exposing cartilage tissue to the fields generated by specific and selective signals so as to regulate matrix metalloproteinase gene expression in such cartilage tissue. The resulting methods and devices are useful for the targeted treatment of osteoarthritis, rheumatoid arthritis, cartilage injury, cartilage defects, and tumor metastasis.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Tissue engineered meat for consumption and a method for producing tissue engineered meat for consumption
A non-human tissue engineered meat product and a method for producing such meat product are disclosed. The meat product comprises muscle cells that are grown ex vivo and is used for food consumption. The muscle cells may be grown and attached to a support structure and may be derived from any non-human cells. The meat product may also comprise other cells such as fat cells or cartilage cells, or both, that are grown ex vivo together with the muscle cells.
Owner:VEIN JON
Integrated bone-cartilage repair scaffold and preparation method thereof
ActiveCN108478871ASemi-permeableGuaranteed functionTissue regenerationProsthesisCartilage cellsCartilage repair
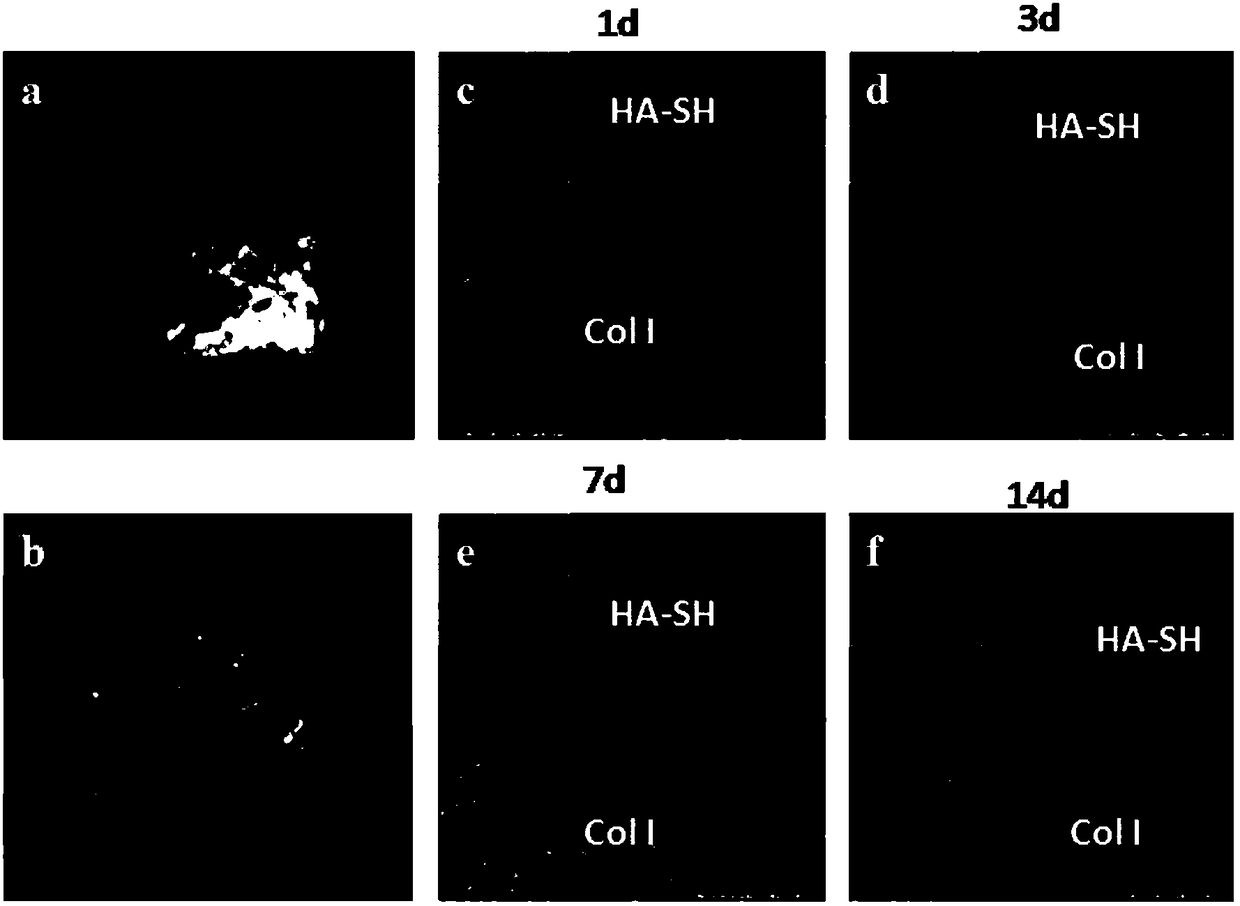
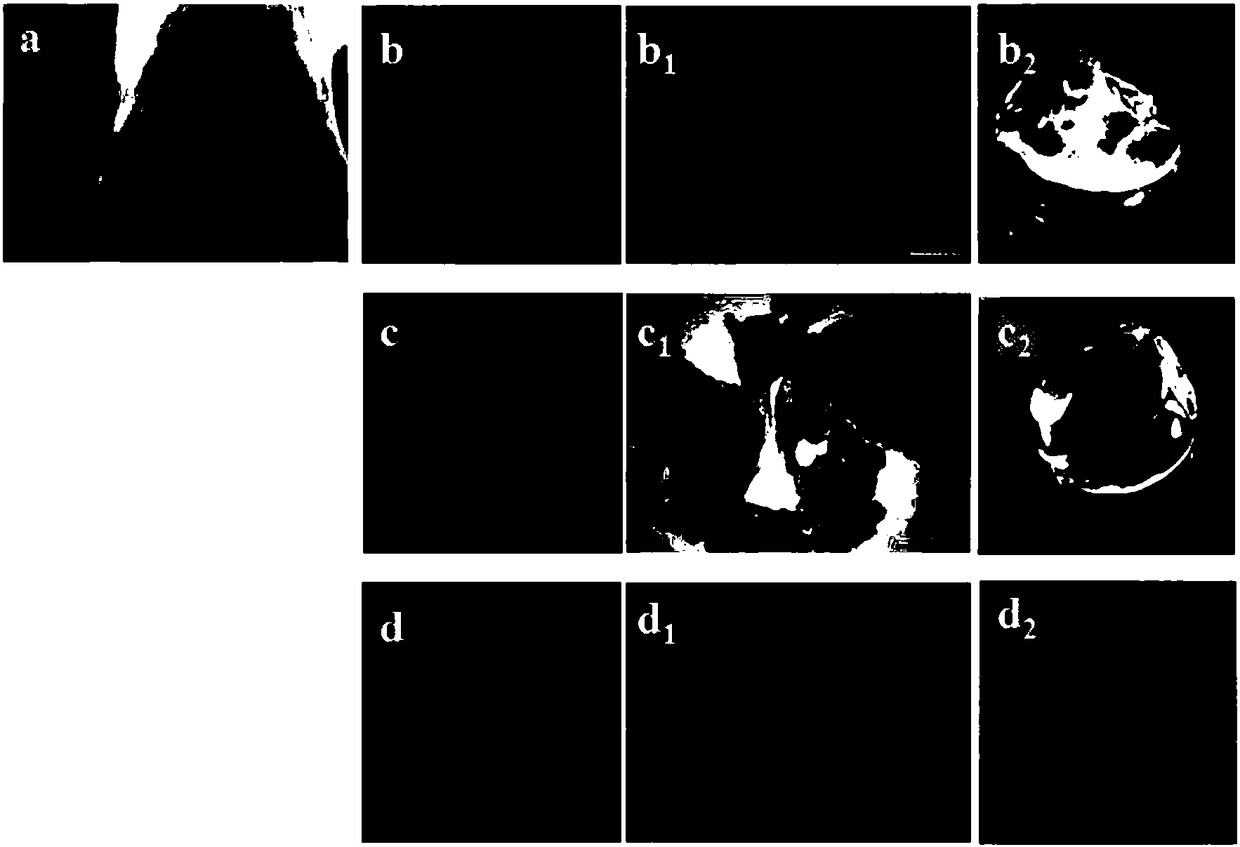
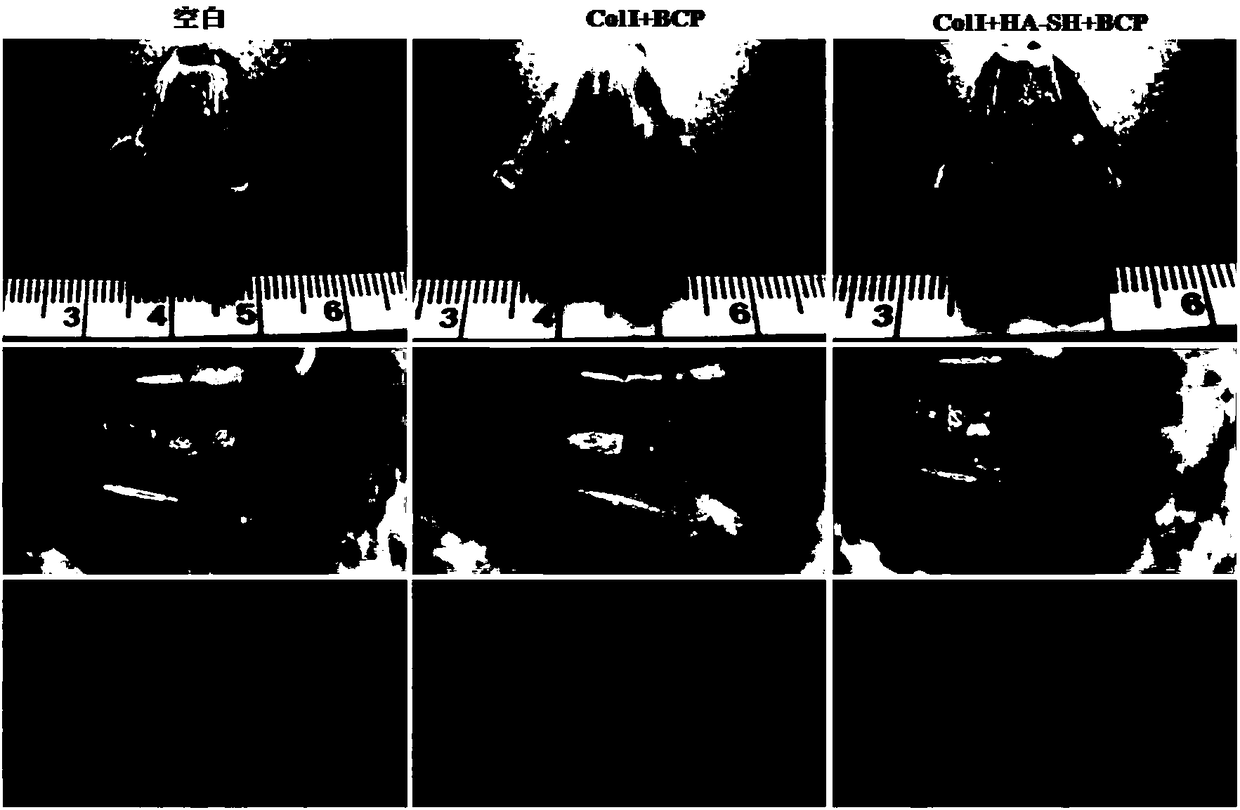
The invention provides an integrated bone-cartilage repair scaffold. The integrated bone-cartilage repair scaffold is of an integrated structure composed of a subchondral bone repair layer, a middlelayer and a cartilage repair layer, wherein the subchondral bone repair layer is fashioned porous calcium phosphate biological ceramic, the middle layer is sulfydryl-hyaluronic acid hydrogel, the cartilage repair layer is composed of I-type collagen hydrogel and chondrocytes or mesenchymal stem cells, and the middle layer is located between the subchondral bone repair layer and the cartilage repair layer and in a porous structure of the fashioned porous calcium phosphate biological ceramic to separate the subchondral bone repair layer from the cartilage repair layer. The invention further provides a preparation method of the integrated bone-cartilage repair scaffold. The repair scaffold can prevent blood vessels from invading a cartilage layer, so that the tightness and the stability of connection between a bone layer and the cartilage layer are improved, biological bonding of the bone layer and the cartilage layer is achieved, and thus a good repair effect is achieved.
Owner:SICHUAN UNIV
Biological material for repairing meniscus tear and preparation method for biological material
ActiveCN102716515APromote proliferationPromote wound healingProsthesisCartilage cellsAutologous tissue
The invention relates to a biological material for repairing meniscus tear and a preparation method for the biological material. The material consists of an acellular matrix membrane on the inner layer, a porous structure collagen matrix membrane on the middle layer, a cell layer formed by cartilage cells on the outer layer and a cell sheet. The repair material has certain elasticity and toughness and is high in biological activity; the cartilage cells can secrete cell factors per se, and nutritional ingredients can be directly obtained through articular cavity synovia, and thus, the establishment of communication link between tissue cells at both ends of the tear during the meniscus tear process is accelerated; the immigration and the infusion of the cells in the material and autologous tissue cells are promoted; the tissue regeneration and functional reconstruction time is shortened; and the ideal novel material and the preparation method for the material are provided for clinically repairing the damage of the meniscus tear (including blood supply zones), especially the tissue regeneration and the functional reconstruction of no-blood zones and low-blood zones.
Owner:西安博鸿生物技术有限公司
Composition and method for inducing mesenchymal stem cells to be differentiated to cartilage cells
The invention provides a composition and a method for inducing mesenchymal stem cells to be differentiated to cartilage cells. The composition comprises 20-80 uM of L-ascorbic acid-2-phosphate, 2-10 ng / ml of TGF beta 3, 50-150 nM of dexamethasone, 10-50 ug / ml of vitamin C, 10-50 ug / ml of proline, 0.5-5 ug / ml of indometacin, 1xITS+1premix and 0.5-10 ug / ml of chlormadinone ester. The combination when being used as an inducer of a culture medium can improve differentiation activity and differentiation directionality.
Owner:安沂华
Complex support body for regenerating bone-cartilage, method for manufacturing thereof, and composition for treating bone and cartilage related diseases comprising same as active ingredient
ActiveUS20140012393A1Regenerate bone and cartilageBone implantTissue regenerationDiseaseCartilage cells
The present invention relates to a complex support body for regenerating bone-cartilage, a method for manufacturing thereof, and a composition for treating bone and cartilage related diseases comprising the same as an active ingredient, and more particularly, to a complex support body for regenerating bone-cartilage, which comprises a bone regeneration layer consisting of a biodegradable polymer and a biocompatible ceramic, and a cartilage regeneration layer, which is coupled to the bone regeneration layer and in which cells that can be differentiated into cartilages cells are fixed; a method for manufacturing thereof; and a composition for treating bone and cartilage related diseases comprising the same as an active ingredient. The complex support body of the present invention for regenerating bone-cartilage is manufactured as a three-dimensional support body, which is similar to a living tissue, according to a bioplotting method, and exerts the effect of regeneration into a bone tissue and a cartilage tissue, respectively, depending on materials encountered in the environment where the complex support body for regenerating bone-cartilage is used.
Owner:INJE UNIV IND ACADEMIC COOP FOUND
Cartilage complex of tissue engineering bone and method for preparing same
The invention belongs to the field of cartilage tissue repair and tissue engineering and provides a cartilage complex of a tissue engineering bone and a method for preparing the same. The preparation method comprises the following steps of: performing marrow removal, degreasing and layered calcium-removal of cancellous allograft bones or xenogeneic cancellous bones to obtain the cartilage framework of tissue engineering bones, wherein the upper layer of the cartilage framework is completely decalcified and only the surface of the lower layer is decalcified; and using the framework as a primary framework, adding gel-type matters, such as fibrinogens or alginic acid, serving as a secondary framework and a distribution carrier to obtain the cartilage complex of the tissue engineering bone. Compared with the conventional tissue engineering bone cartilage, the cartilage complex of the invention has the advantages of complete natural biomaterials, complete integration, no application of the layered stacking and interface combination technology, high pressure resistant, tensile resistant and shearing resistant properties, uniform and accurate loading of cells, effective enforcement of the differentiation of seeded cells into cartilage cells, easy operation, convenient minimally invasive operation under an arthroscope, and the like.
Owner:中国人民解放军第三0九医院
Method for separating and culturing mesenchymal stem cells from umbilical cord and inducing and differentiating mesenchymal stem cells into cartilage cells
ActiveCN104988117AIncrease acquisition rateEase of mass productionSkeletal/connective tissue cellsCartilage cellsMesenchymal stem cell
The invention provides a method for separating and culturing mesenchymal stem cells from an umbilical cord and inducing and differentiating the mesenchymal stem cells into cartilage cells. The method comprises the steps that the umbilical cord is treated; the cells are separated from the umbilical cord and umbilical vessels and subjected to primary cell culture; cell subculture is conducted, and the mesenchymal stem cells are obtained; the mesenchymal stem cells are induced and differentiated into the cartilage cells, wherein in the step that the cells are separated from the umbilical cord and the umbilical vessels, the umbilical cord and the umbilical vessels are digested through digestive juice. By means of the method, the mesenchymal stem cells can be separated from the umbilical cord and cultured, and the obtained mesenchymal stem cells are subjected to further induced transformation; the method can be used for mass production of the mesenchymal stem cells and has importance significance in treatment of injured tissue which can hardly regenerate.
Owner:SHENZHEN ZHONGJIHENGRUN INVESTMENT CO LTD +1
Cartilage cell treatment comprising collagen, hyaluronic acid derivative, and stem cell derived from mammal umbilical cord
The present invention relates to a medical composite biomaterial. More specifically, the present invention relates to a medical composite biomaterial including collagen and a hyaluronic acid derivative. Also, the present invention relates to a cartilage cell treating agent using the biomaterial and stem cells derived from a mammal umbilical cord. The biomaterial does not cause an immune reaction, has superior durability, and the cartilage cell treating agent comprising the biomaterial and the stem cells enables arthroscopic surgery, thereby reducing pain of the patient and effectively treat of degenerative arthritis and cartilage damage.
Owner:CHABIO&DIOSTECH
Method for inducing differentiation from mesenchymal stem cells to cartilage cells and application of mesenchymal stem cells in osteoarthritis
InactiveCN102899287AHomogeneous differentiation stateFully contactedAntipyreticAnalgesicsCartilage cellsDexamethasone
The invention relates to a method for inducing differentiation from mesenchymal stem cells to cartilage cells and application of the mesenchymal stem cells in osteoarthritis, and provides a method for preparing umbilical cord mesenchymal stem cells differentiated to the cartilage cells, mesenchymal stem cells differentiated to the cartilage cells and application of the prepared drug prepared by the mesenchymal stem cells in treatment of the osteoarthritis. The method comprises the following steps of: inducing and culturing the mesenchymal stem cell in a 15ml taper-bottom centrifuge tube by using a globule method; and slightly shaking the centrifuge tube for one time every 24 hours to separate cell sphere from the bottom of the tube, wherein the differentiation inducing culture medium is prepared by adding 100mu g / ml of sodium pyruvate, 10ng / ml TGFbeta3, 100nM of dexamethasone, 25mu g / ml of vitamin C, 40mu g / ml of proline and 1*ITS and 1 of premix into a high-glucose dulbecco modified eagle medium (DMEM). A non-bracket cartilage built by the method can be used for repairing damaged articular cartilages.
Owner:黑龙江和泽北方生物科技有限公司
Tissue engineering cartilage construction method using bone matrix gelatin
A tissue engineering cartilage construction method using bone matrix gelatin which comprises, using cartilage cell for isolated culture or base material stem cell for isolated culture to evoke cartilage cell i.e. seed cell, fetching New Zealand rabbit or human embryon os longum and metaphysic trabecular bone to construct bone matrix gel BMG, inoculating the seed cells harvested through isolated culture onto cortex bone or cancellous bone BMG for extracorporal culture.
Owner:XI AN JIAOTONG UNIV
Preparation method of multifunctional layered joint cartilage support
InactiveCN108355174ARealize regulationReduce rejectionAdditive manufacturing apparatusTissue regenerationCartilage cellsOsteoblast
The invention provides a preparation method of a multifunctional layered joint cartilage support. The multifunctional layered joint cartilage support capable of simulating the biological property andthe mechanical property of natural cartilage is prepared by taking hydrogel as a support material, taking mesenchymal stem cells, cartilage cells and osteoblasts as inducing and developing objects andby adopting a 3D printing technology. The multifunctional layered joint cartilage support can accurately simulate the internal tissue form and the contour of each layer of the natural cartilage and can realize that different layers have different mechanical and biological characteristics. The prepared artificial cartilage support cells are derived from patients, the support serves as a degradablehydrogel material and the rejection reaction after implantation is greatly reduced. Various nutritional substances and growth factors are mixed in the hydrogel support, so that the sustained releaseeffect can be achieved and regulation and control of the growth and development of the artificial cartilage can be realized.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Cartilage derived collagen sponge scaffold and preparation method thereof
ActiveCN101890184AQuality is easy to controlEasy to useProsthesisCartilage cellsBiocompatibility Testing
The invention discloses a method for preparing a cartilage derived collagen sponge scaffold and a collagen sponge scaffold prepared by the method. The method comprises the following steps of crushing, removing cells, removing telopeptide, dissolving and crosslinking, and the obtained collagen sponge scaffold has good biocompatibility and extremely low antigenicity; and a homogenized sponge scaffold can be prepared from cartilages of a plurality of individuals from the conspecific animals, has controllable pore diameter and porosity, can provide appropriate growth and propagation environment for cartilage cells and bone mesenchymal stem cells, particularly has good effect of repairing cartilage defect parts, and is more favorable for standardized production and clinical use.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Cell-culture and polymer constructs
InactiveUS20020123142A1Perfect cartilage repair surgerySpeed up the repair processBiocideMammal material medical ingredientsCartilage cellsImplanted device
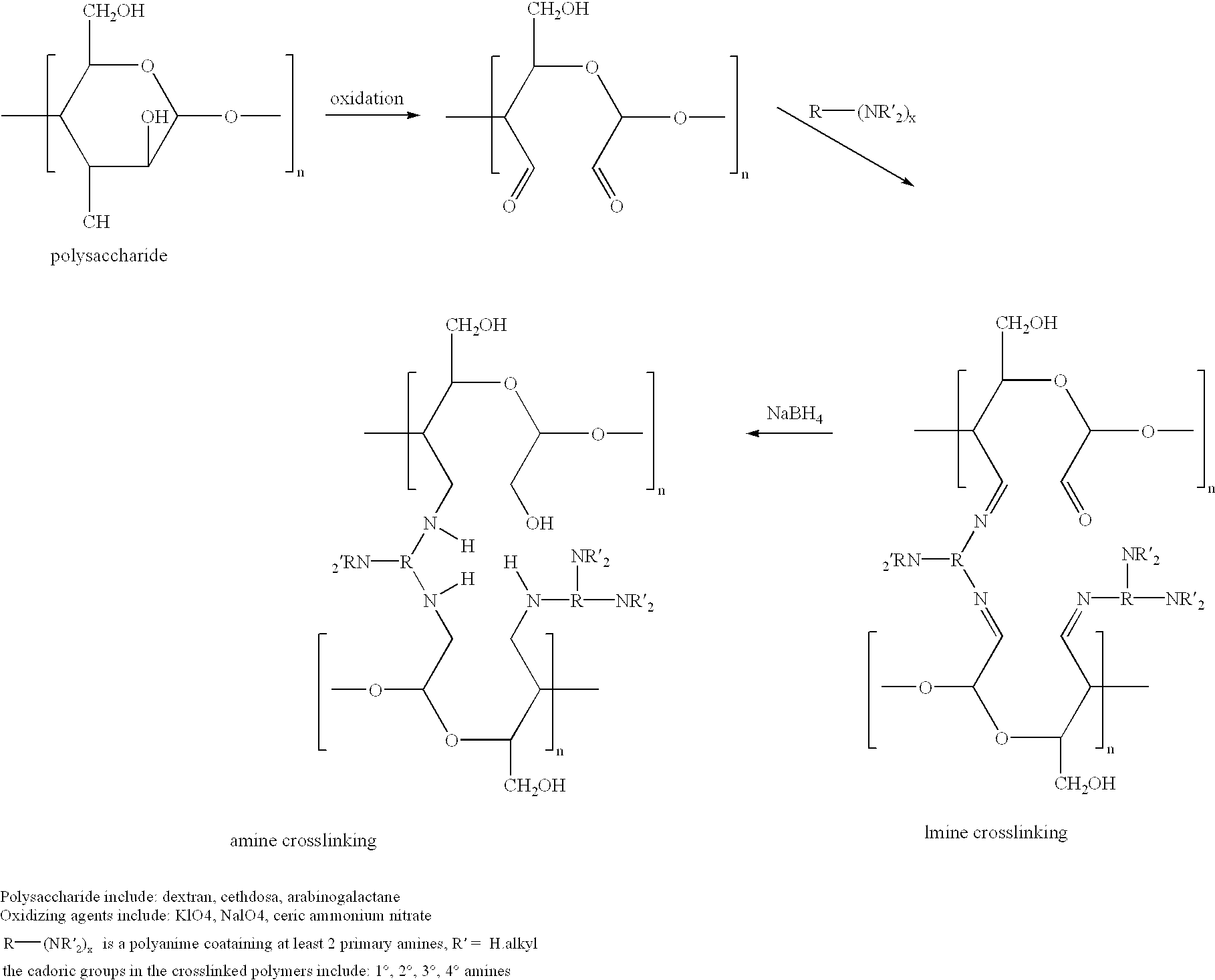
Cells grown on a microcarrier are separated from the microcarrier by enzymatically digesting the microcarrier. More specifically, chondrocytes may be grown on dextran microcarrier beadlets and then the beadlets digested using dextranase to separate the chondrocytes from the carrier. Cells can also be grown on chitosan microcarriers to be used for implantation. In addition, cells can be grown on polysaccharide polymers to be used as implant devices. Various polymers serve as scaffolds for cells to be used for implantation. The polymers can be used for cell culture as well as for preparing scaffolds useful for tissue replacement such as cartilage tissue.
Owner:HUNGERFORD DAVID S +4
Three dimensional microtissue bioprinter
ActiveUS20180243478A1Enhance cell viabilityConvenient position controlHepatocytesPharmaceutical delivery mechanismCartilage cellsEngineering
A bioprinter comprises one or more dispensing units. Each dispensing unit may include (i) a syringe including a hollow body and a plunger dimensioned to translate in the body wherein the body has an exit orifice; (ii) an actuator in contact with a proximal end of the plunger; (iii) a controller for moving the actuator; and (iv) a nozzle having a wall defining a fluid path extending from an inlet of the nozzle to an outlet of the nozzle. The inlet of the nozzle is in fluid communication with the exit orifice of the syringe body. The nozzle includes a fluid passageway in fluid communication with a source of fluid and the fluid path. The bioprinter can be used in a method of preparing microtissue comprising dispensing a bioink from one or more dispensing units of the bioprinter on a plate. The microtissue may comprise cartilage cells or tumor cells or liver cells.
Owner:THE GENERAL HOSPITAL CORP
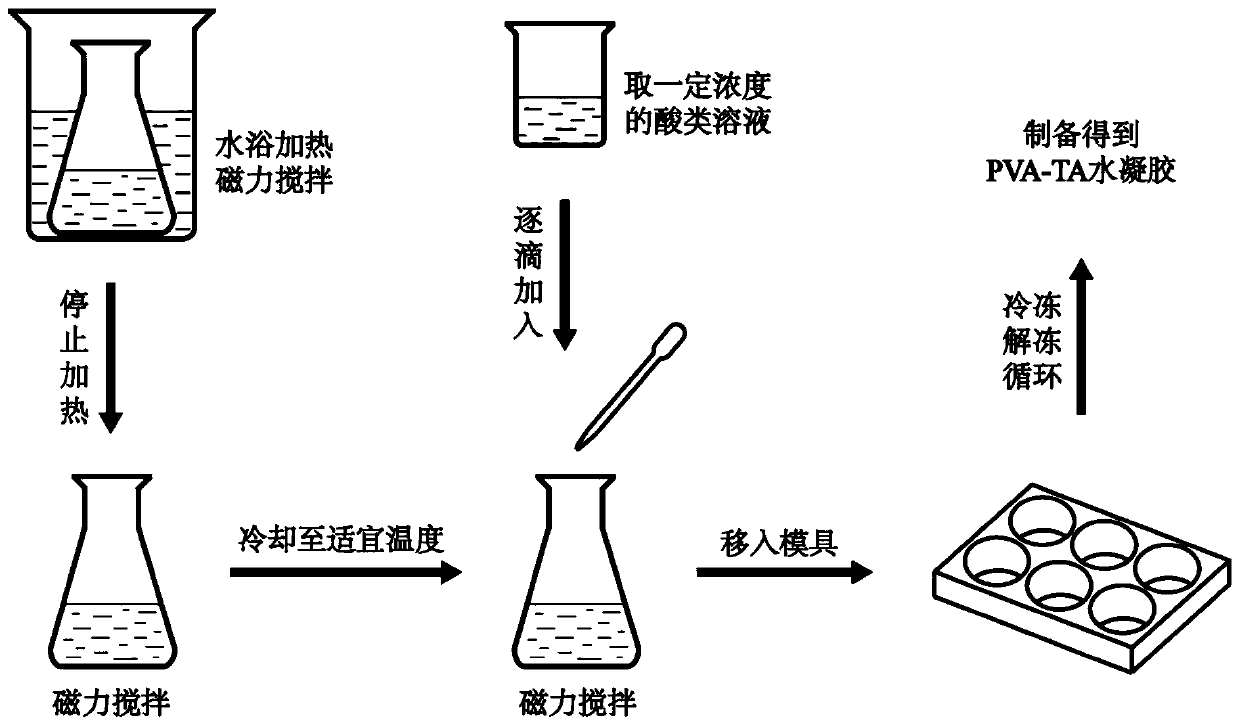
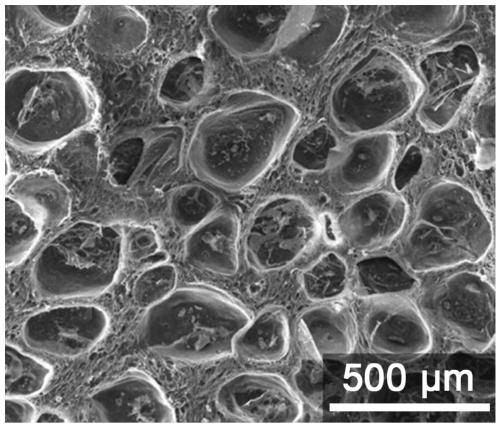
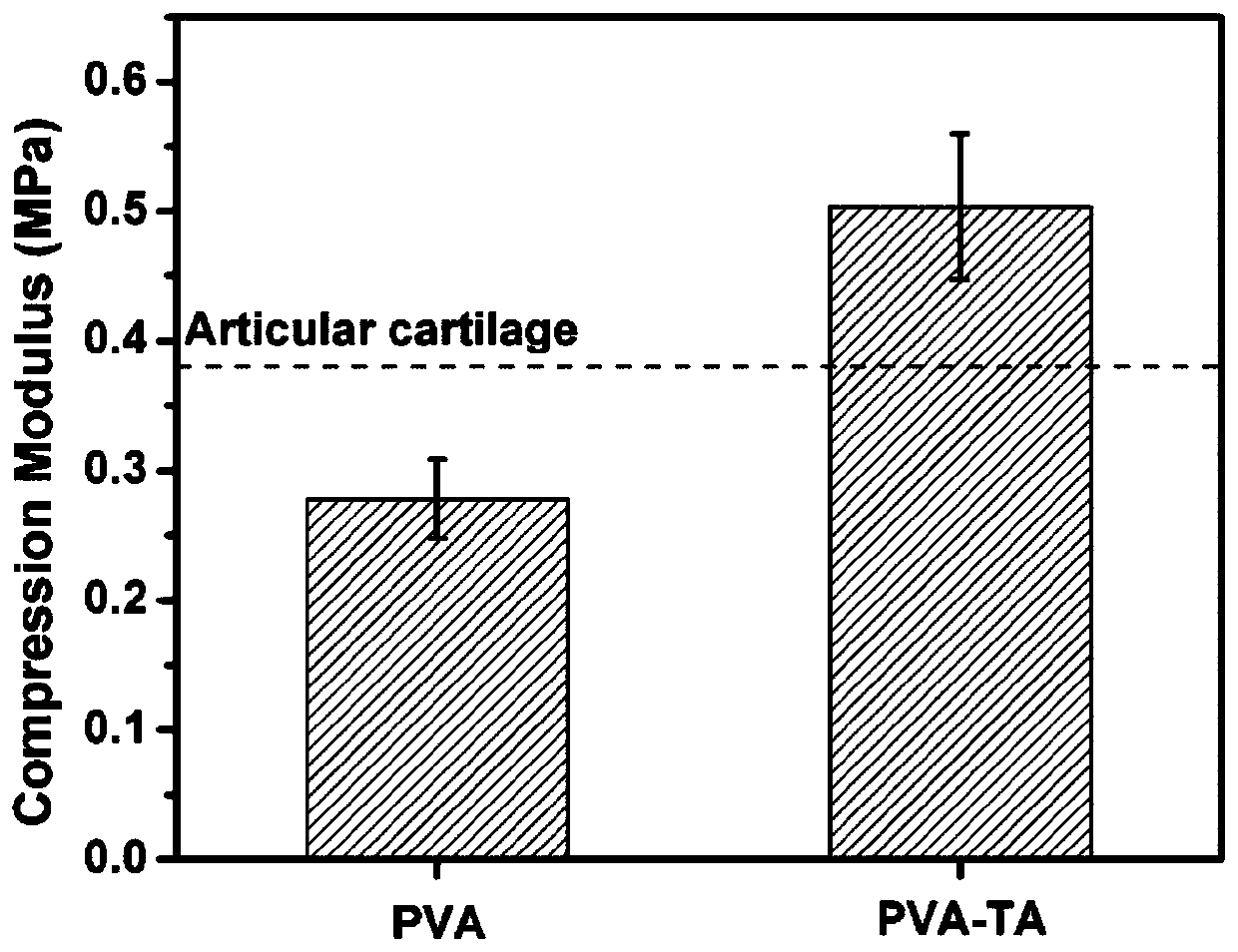
Preparation method and application of high strength and porous structure polyvinyl alcohol-gallotannic acid hydrogel
ActiveCN109762182AIncrease moisture contentImprove mechanical propertiesProsthesisCartilage cellsMicrobubbles
The invention belongs to the field of articular cartilage repair materials, and provides a preparation method and application of high strength and porous structure polyvinyl alcohol-gallotannic acid hydrogel. The hydrogel is a potential articular cartilage replacement material. According to the preparation method, polyvinyl alcohol is adopted as a substrate material, the ice crystal forming ability of a hydrogel precursor solution is improved by introducing gallotannic acid and microbubbles, and meanwhile, the mechanical property of the hydrogel is greatly enhanced due to the intermolecular interaction between the gallotannic acid and the polyvinyl alcohol. The porous structure and high strength hydrogel is prepared at one step, the preparation process is simple, repeatability is high, theobtained hydrogel has good biocompatibility, meets a porous environment for adhesion and proliferation of cartilage cells and the mechanical property similar to natural articular cartilage, and can be applied to the field of articular cartilage replacement materials.
Owner:DALIAN UNIV OF TECH
Cartilage preservation solution
InactiveCN102308787AOvercome mechanical propertiesOvercoming chondrocytesDead animal preservationCartilage cellsFiber
The invention relates to a cartilage preservation solution. Each 1000ml of the preservation solution comprises 20-30mmol of potassium dihydrogen phosphate, 80mmol of histidine or 15mmol of a histidine salt, 75-95mmol of lactobionic acid, 50-70mmol of sucrose, 0.8-1.2mmol of allopurinol, 45-55g of low molecular dextran-40, 45-55mmol of NaCl, 25-35mmol of KCl, 3-7mmol magnesium sulfate, 70-90U of penicillin, 2-4mmol of reduced glutathione, 3-7mmol of adenosine, 2-5g of trehalose, and the balance deionized water, and the pH value of the cartilage preservation solution is 7.33-7.53. The cartilagepreservation solution is packaged, sealed and preserved at 0-4DEG C. By adopting the preservation solution to preserve cartilage tissues, the death speed of cartilage cells is slowed, the activity ofcartilage fibers is maintained, the performance of the cartilage tissues is kept, and the antibiotic property is increased, so the preservation solution has a good preservation effect and a long preservation time on the cartilage tissues, and is in favor of clinic cartilage tissue transplant operations.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Preparation method of nano micrometer structure coexistence chitosan double-layer support
The invention relates to a preparation method of a nano micrometer structure coexistence chitosan double-layer support, which belongs to the biology medical material field. The preparation method is characterized in that a phase separating technology is used to prepare a three-dimensional micrometer chitosan support as a basal layer, a high voltage static static spinning technology is used for spinning nano chitosan fiber on the micrometer chitosan surface, and thereby the nano micrometer structure coexistence chitosan double-layer support can be obtained. The prepared double layer support has good mechanical strength and high amount of porosity, and is suitable for adhesion, growth and differentiation of cells. The surface layer chitosan nano fiber is suitable for growth of cartilage cells, the basal layer micrometer chitosan support is suitable for the growth of the osteoblast, and the nano micrometer structure coexistence chitosan double-layer support can be used for restoring joint position of the human body cartilage and hard bone.
Owner:QINGDAO UNIV
Method for preparing biological composite artificial trachea and application thereof
ActiveCN105056302APorous structureAvoid dependenceAdditive manufacturing apparatusTubular organ implantsCartilage cellsForeign matter
The invention provides a biological artificial trachea and a preparation method thereof in combination with a 3D printing technology. Materials adopted for the biological artificial trachea simultaneously have flexibility and a certain mechanical strength, so that physical and mechanical properties of the trachea serving as a hollow organ in the chest are met. The artificial trachea is porous and loosened in structure and favorable for nutrient diffusion and ingrowth of autologous vessels. In addition, seed cells obtained by expansion of airway epithelial cells and cartilage cells of a receptor are printed on the trachea wall, and patients' rejection reaction to cells is avoided. Besides, the seed cells are composed of mature cells in terminal differentiation, the induced differentiation problem and the tumor formation risk caused by the use of stem cells can be avoided. The spatial distribution of the cells is accurately controllable in printing, and the restorative process is accelerated according to the histology regular distribution. Finally, a trachea support can be degraded in vivo, complications caused by the fact that foreign matter remains in vivo are reduced, and the risk of taking out the foreign matter through a second operation is avoided.
Owner:SHANGHAI PULMONARY HOSPITAL +1
Articular cavity inner injecting and administering reparations containing trehalose
The present invention provides an intra-articular injection administration preparation containing trehalose, which is characterized in that the preparation contains the trehalose. The present invention can improve the capabilities of the cartilage cells to adapt to and resistant to the environmental changes through the intra-articular injection administration, at the same time, the trehalose has the significant effects of antioxidant and anti-free radicals, which can alleviate the symptoms of arthritis; in addition, the trehalose also provides the necessary sugar for cartilage repair, so as to repair and reconstruct the cartilage. The present invention is used for improving and treating the rheumatism, rheumatoid arthritis, osteoarthritis, synovitis and / or cartilage injury.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com