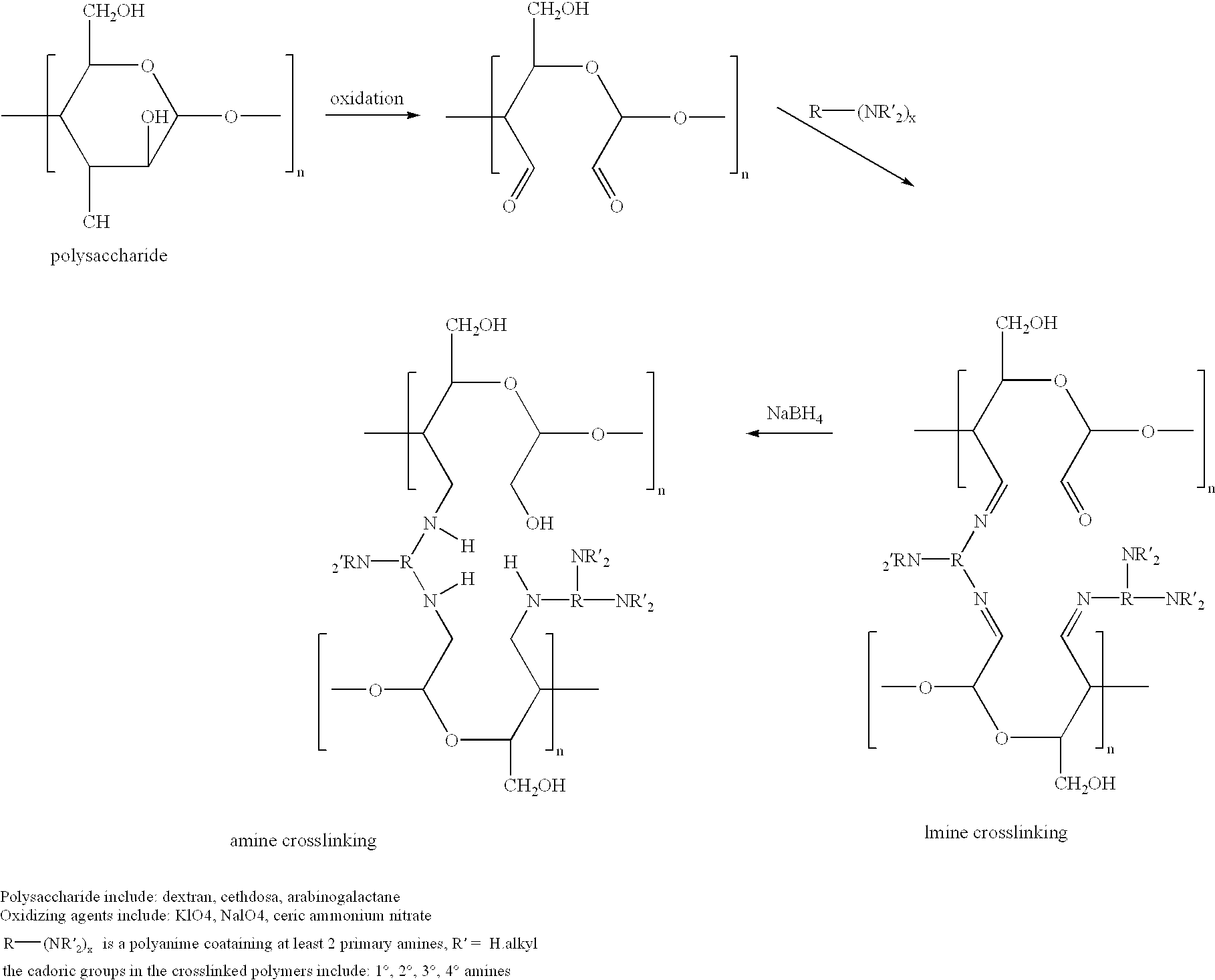
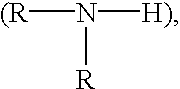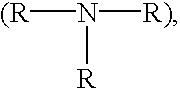Cell-culture and polymer constructs
a cell culture and polymer technology, applied in the field of cell culture and polymer constructs, can solve the problems of insufficient cell culture, insufficient cell culture, and insufficient cell culture, and achieve the effect of efficient cell propagation in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0140] We have discovered that human chondrocytes and other cells types such as osteoblastic cells can be successfully incorporated into chitosan constructs. The cells remain viable and are metabolically active indicated by their continued protein synthesis. They do not proliferate but continue to produce tissue specific matrix components. Chondrocytes produce collagen type II whereas osteoblasts produce collagen type I. The section below describes the (1) examples of cells that can be used in chitosan constructs, (2) types of chitosan tested in our laboratory, and (3) method of preparing the construct.
DESCRIPTION AND OPERATION
[0141] Differentiated chondrocytes or undifferentiated precursor cells from the bone marrow, periosteum and perichondrium can be incorporated into the chitosan scaffold described below. Several types of chitosan have been tested and characterized in our laboratory as shown in Table I Below. These chitosans were purchased from different sources.
5TABLE IV Commer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume capacity | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
| volume capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com