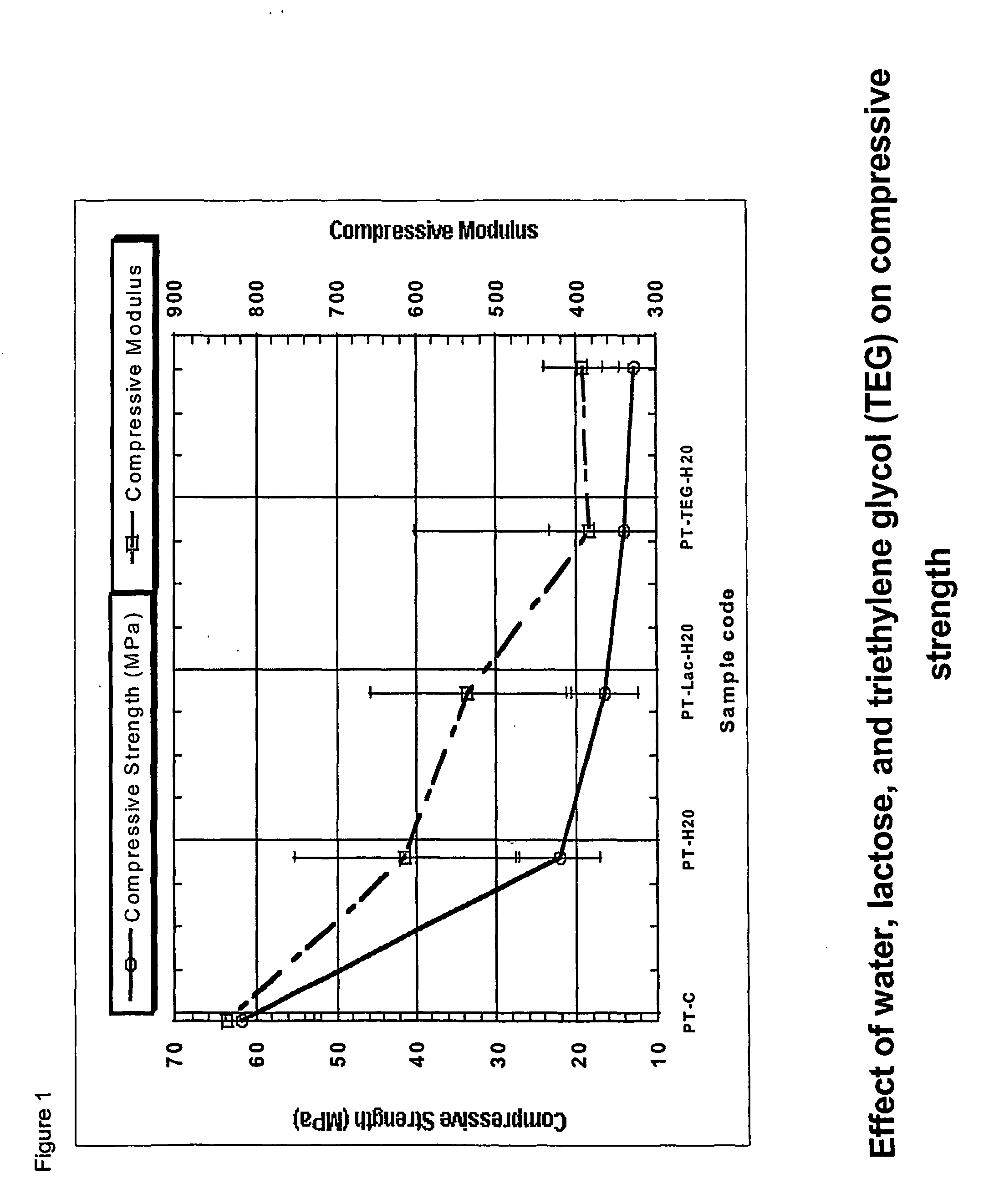
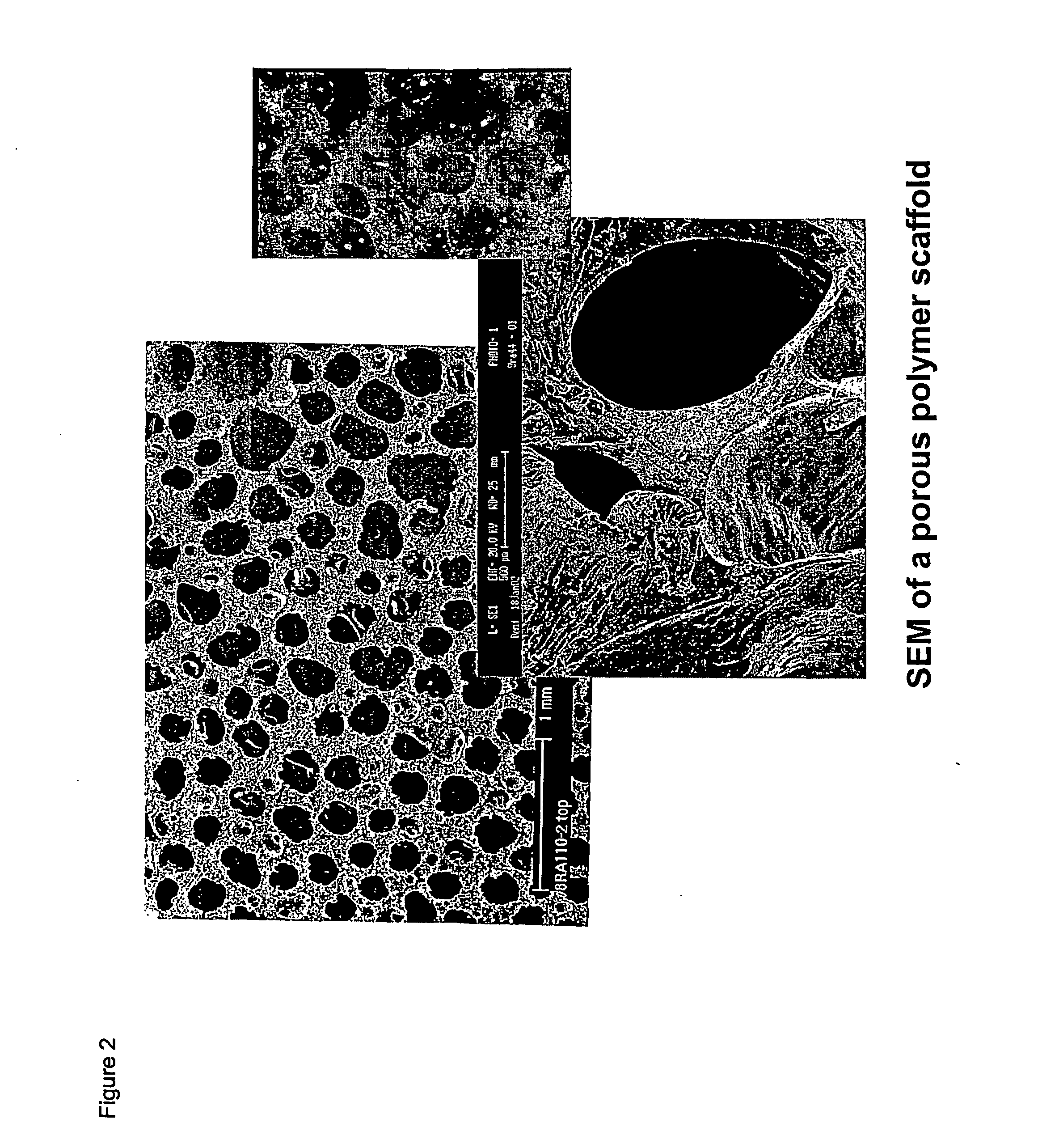
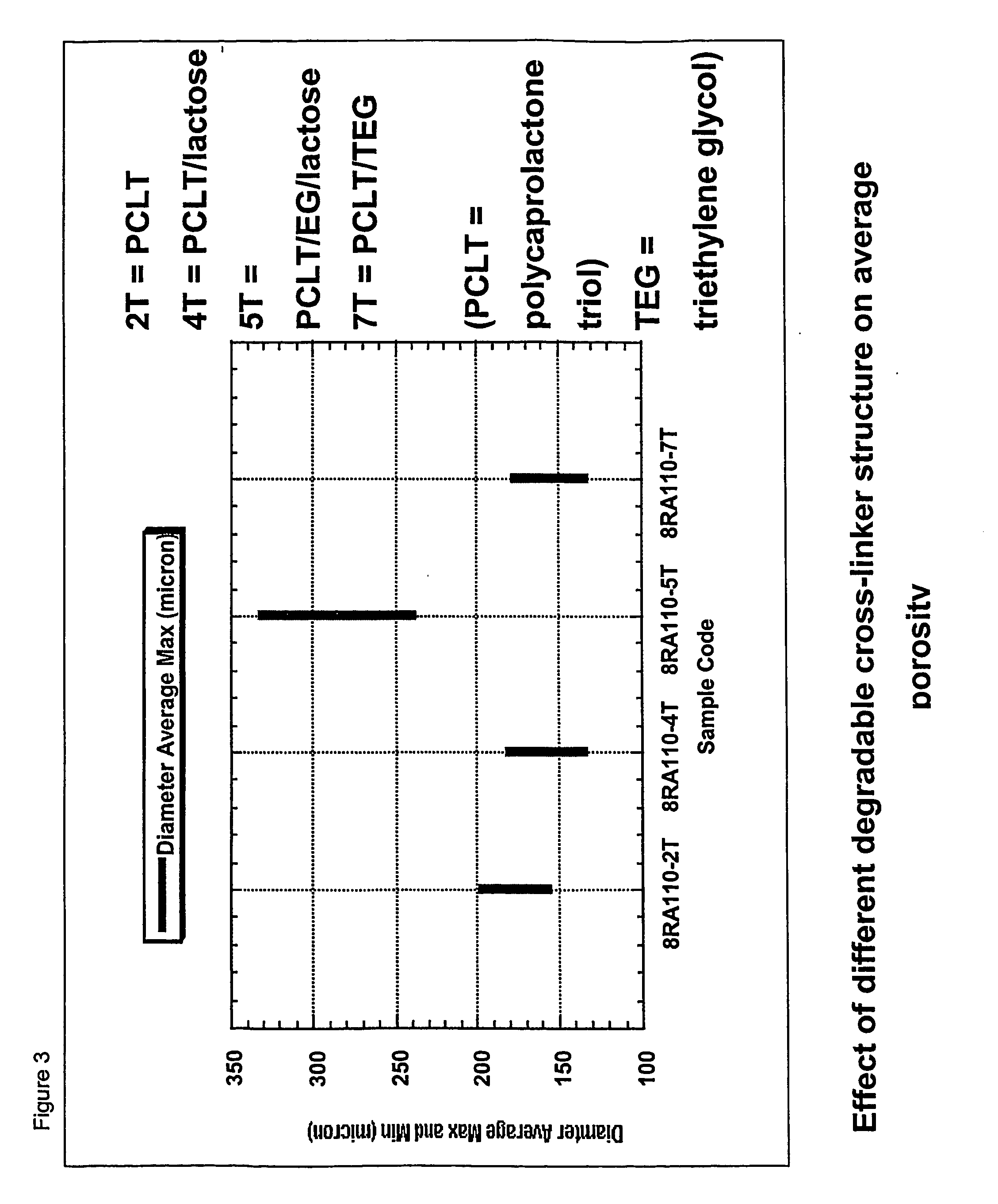
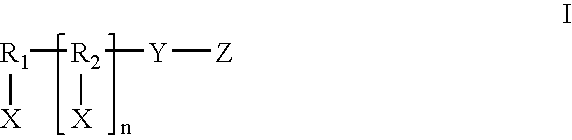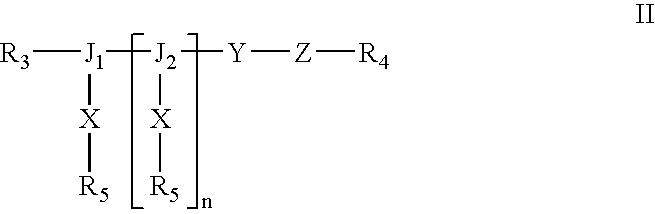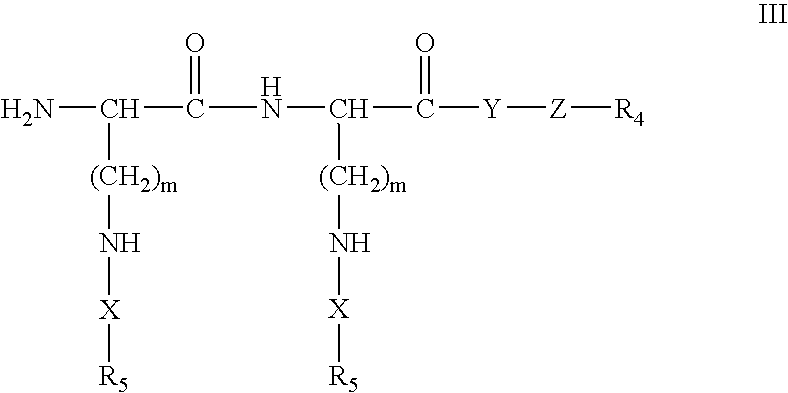Patents
Literature
407 results about "Cartilage repair" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
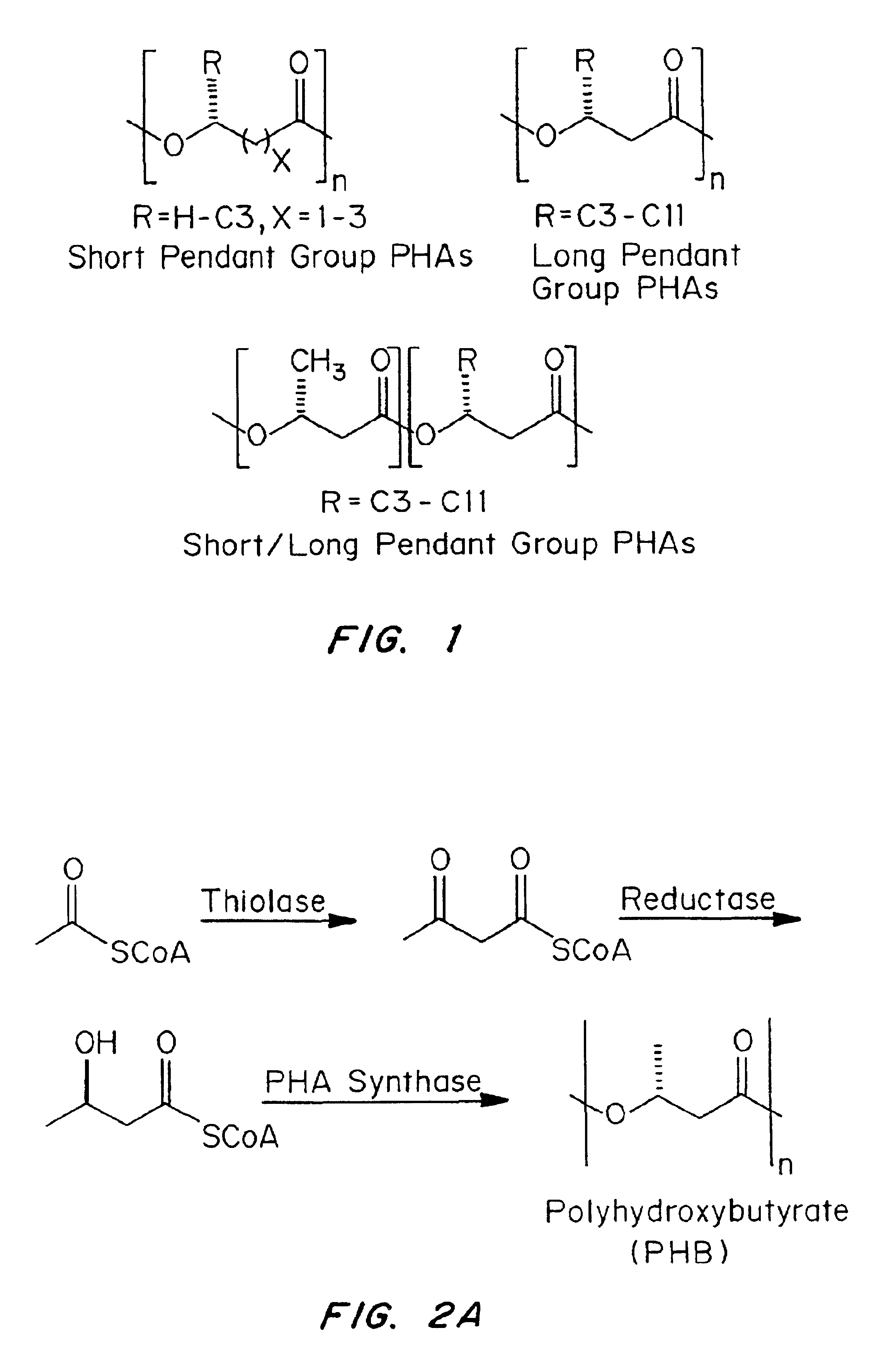
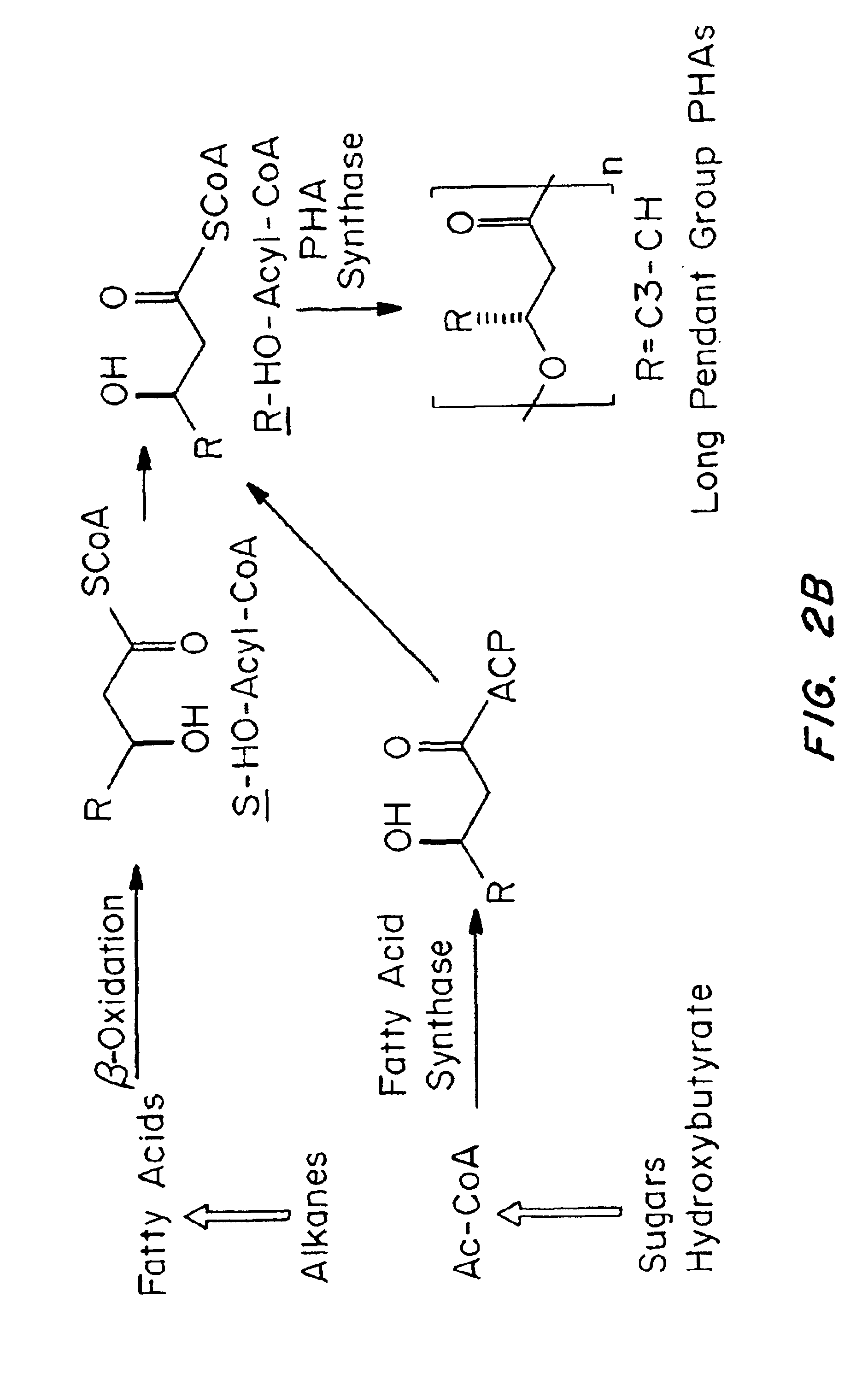
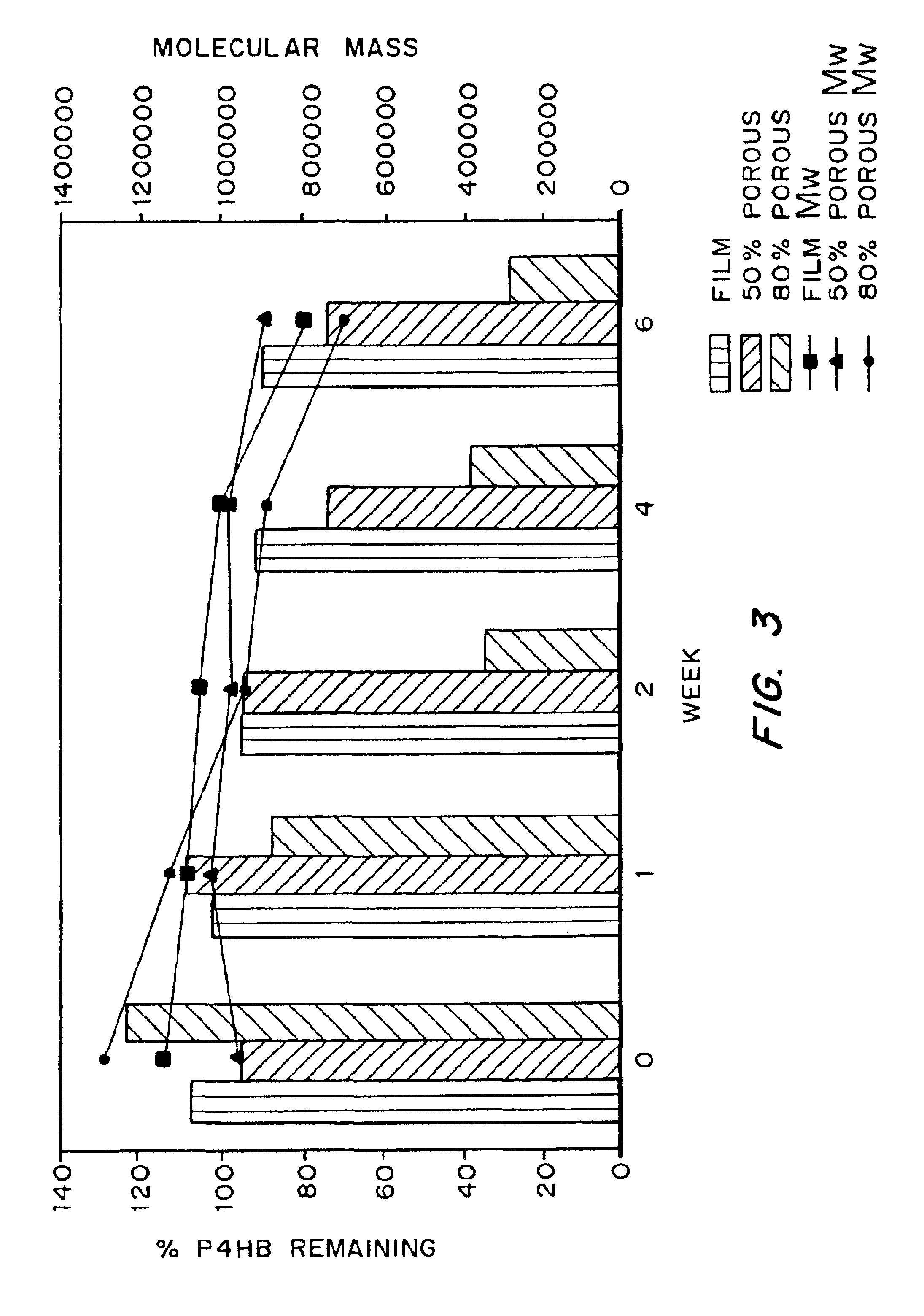
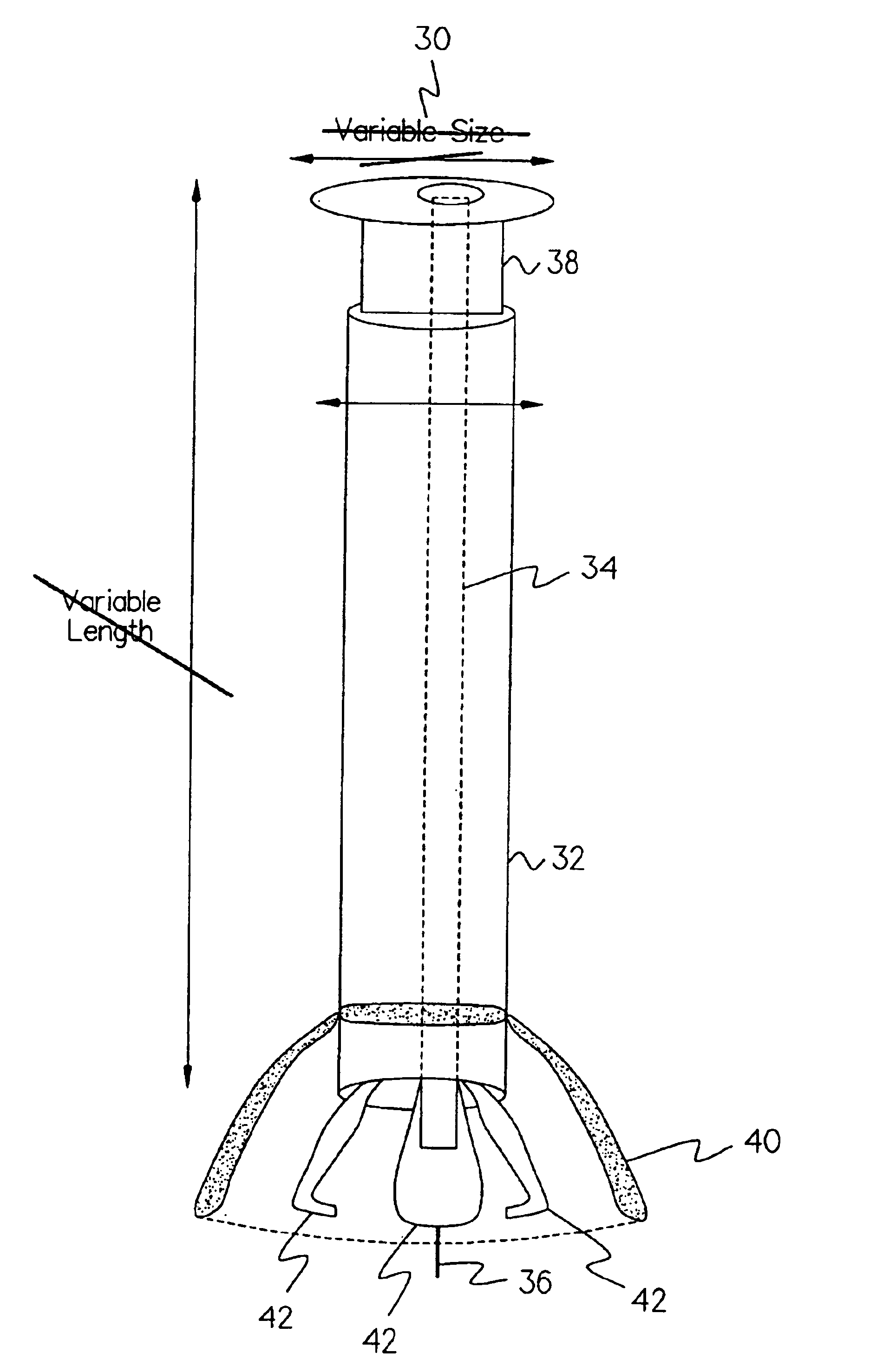
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
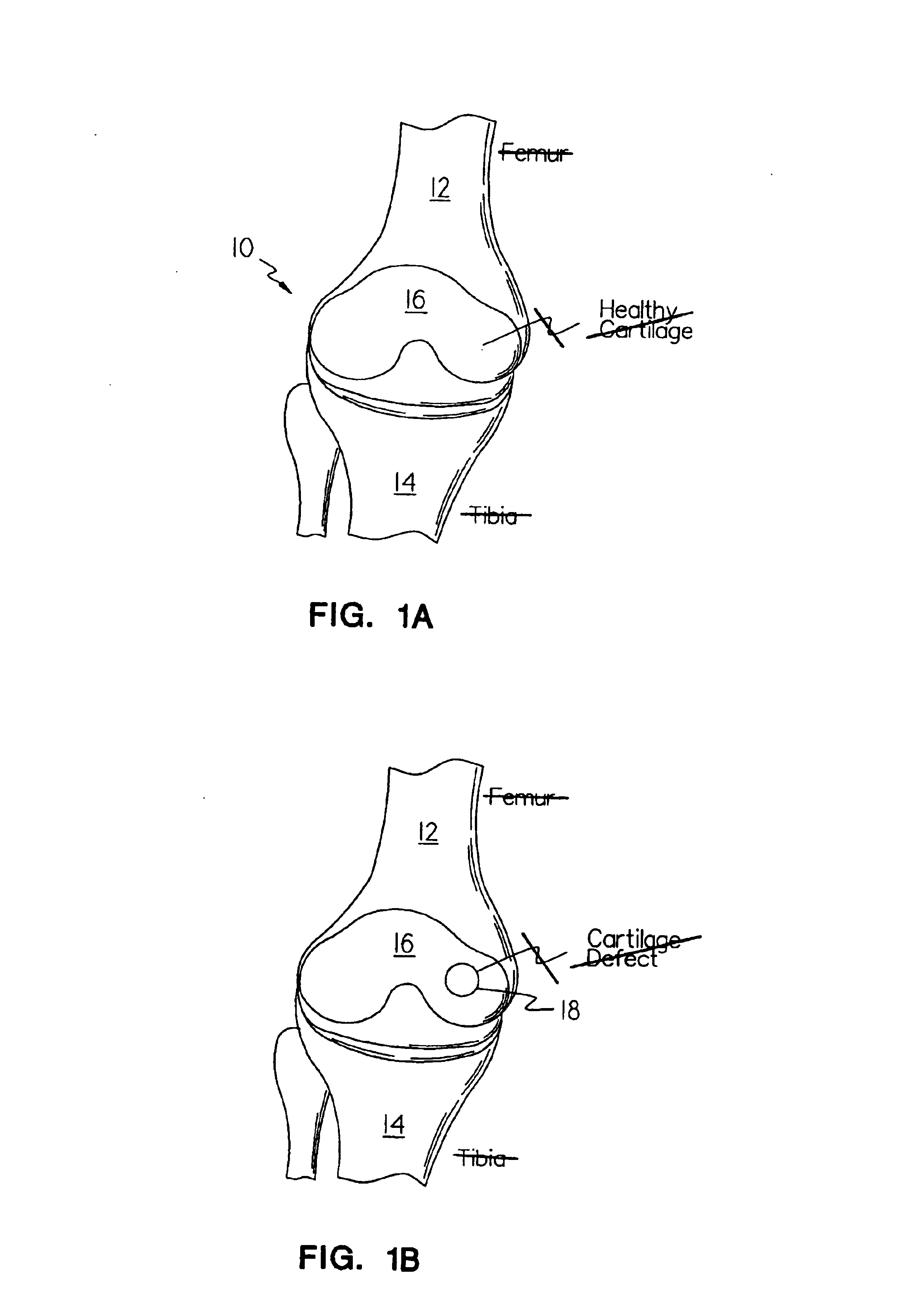
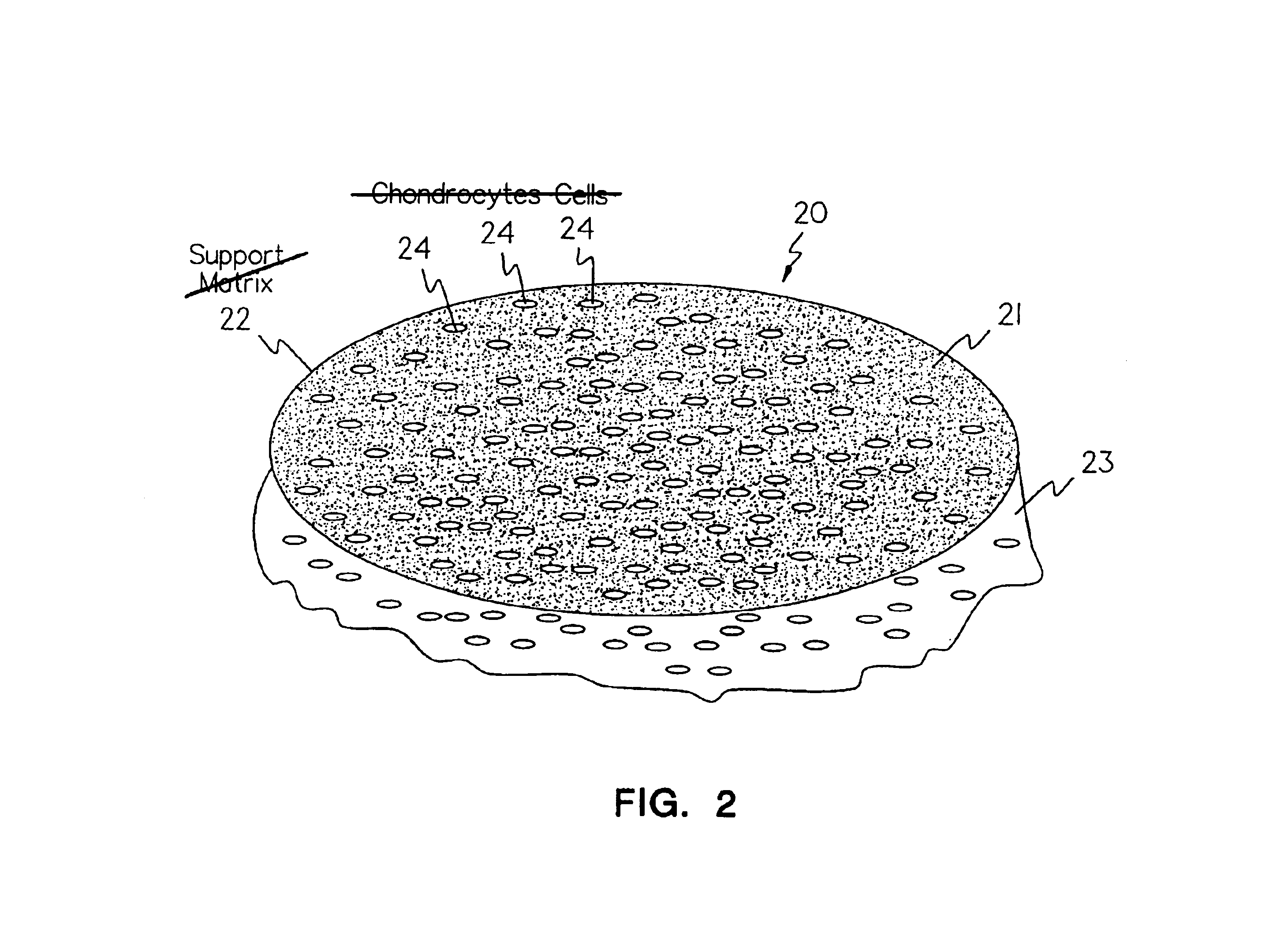
Methods, instruments and materials for chondrocyte cell transplantation
InactiveUS6866668B2Effective treatmentSuture equipmentsSurgical adhesivesSupport matrixTreated animal
A method for the effective treatment of articulating joint surface cartilage in an animal by the transplantation of an implantable article including chondrocyte cells retained to an absorbable support matrix. An instrument for placing and manipulating the implantable article at the site of implantation, and a retention device for securing the implantable article to the site of implantation. An implantable article for cartilage repair in an animal, the implantable article including chondrocyte cells retained on an absorbable support matrix, and a method of making same. An article comprising an absorbable flexible support matrix for living cells grown and adhered thereto.
Owner:VERIGEN TRANSPLANTATION SERVICE INT
Cartilage repair implant with soft bearing surface and flexible anchoring device
InactiveUS9050192B2Strong and more permanent fixationSoft and bendableJoint implantsHip jointsCartilage repairSurgical implant
A surgical implant for replacing hyaline cartilage in a knee or other articulating synovial joint has an anchoring side on one side of the implant adapted for fixing the implant to one of the bones in the joint, and a bearing surface on the opposite side of the implant for lubricious rubbing and sliding contact with another bone in the joint. The anchoring side can be configured with an irregular surface for tissue ingrowth. The bearing side can include hydrogel. The implant can be rolled up from an original shape and surgically inserted by arthroscopic means, and opens into its original shape when released inside the joint.
Owner:FORMAE
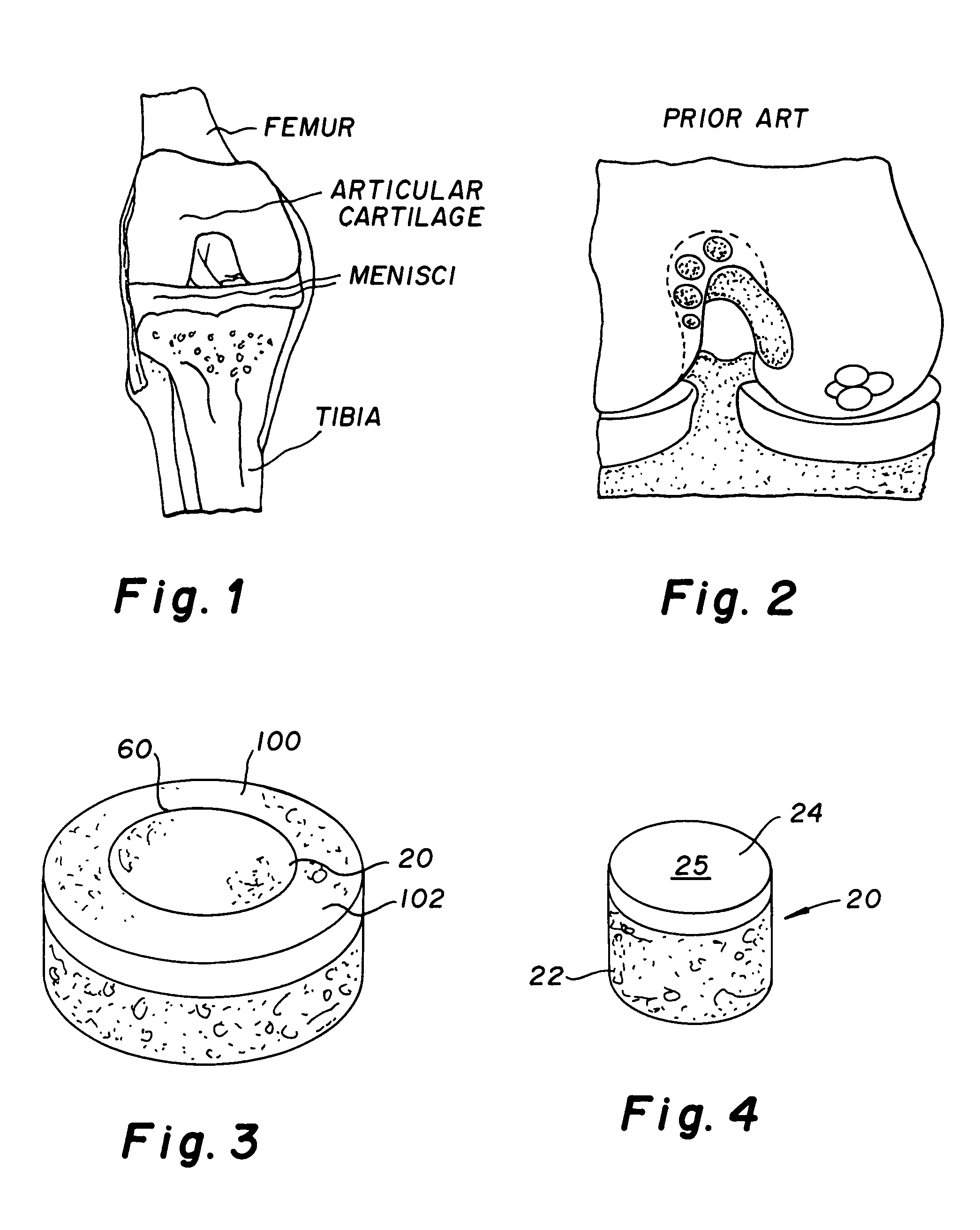
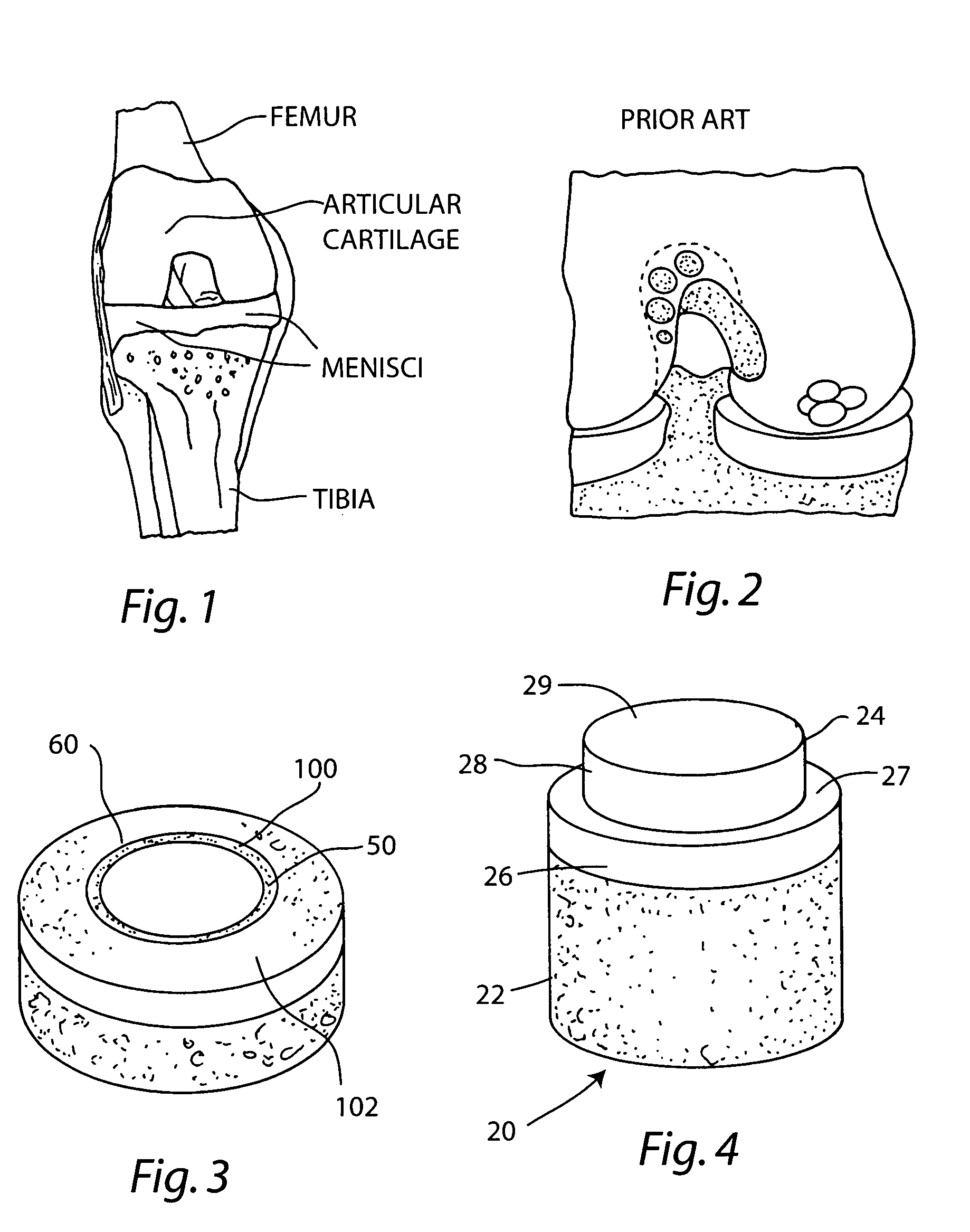
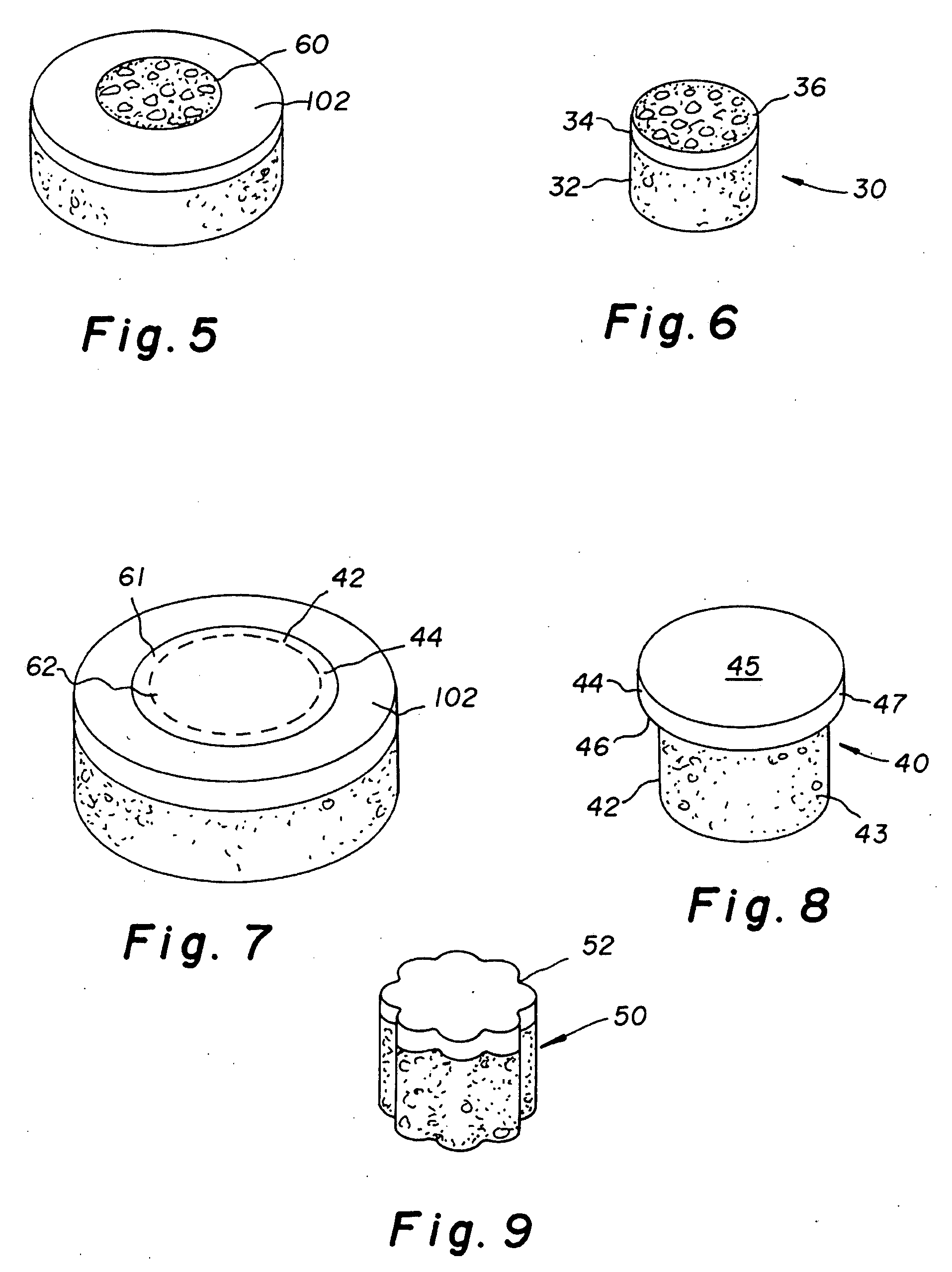
Cartilage repair plug
A cartilage plug, which is made from a biocompatible, artificial material, that is used to fill a void in natural cartilage that has been resected due to traumatic injury or chronic disease. Alternatively, the plug may be relied upon to anchor a flowable polymer to subchondral bone. The plug is prefabricatable in any size, shape, and contour and may be utilized either singly or in a plurality to fill any size void for any application. The plug may be formed of a laminated structure to match the physiological requirements of the repair site. A plurality of anchoring elements may share a single upper layer.
Owner:ABS
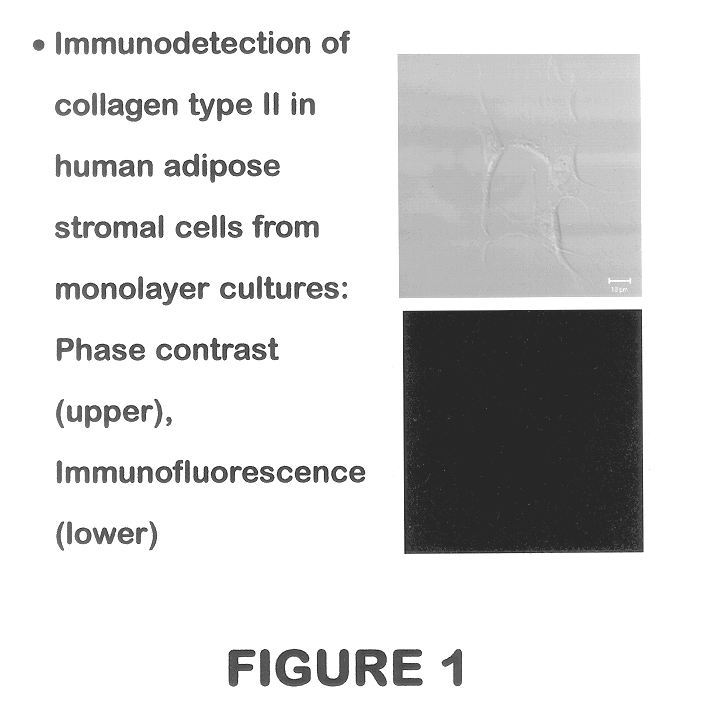
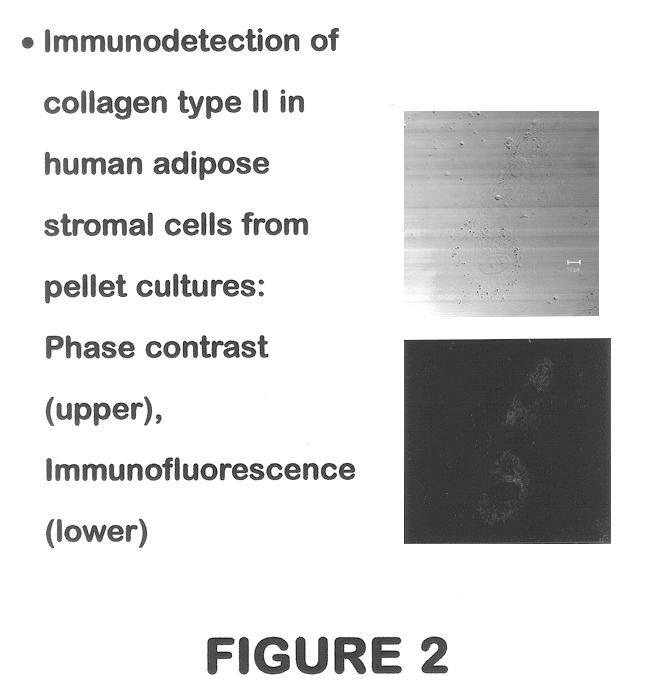
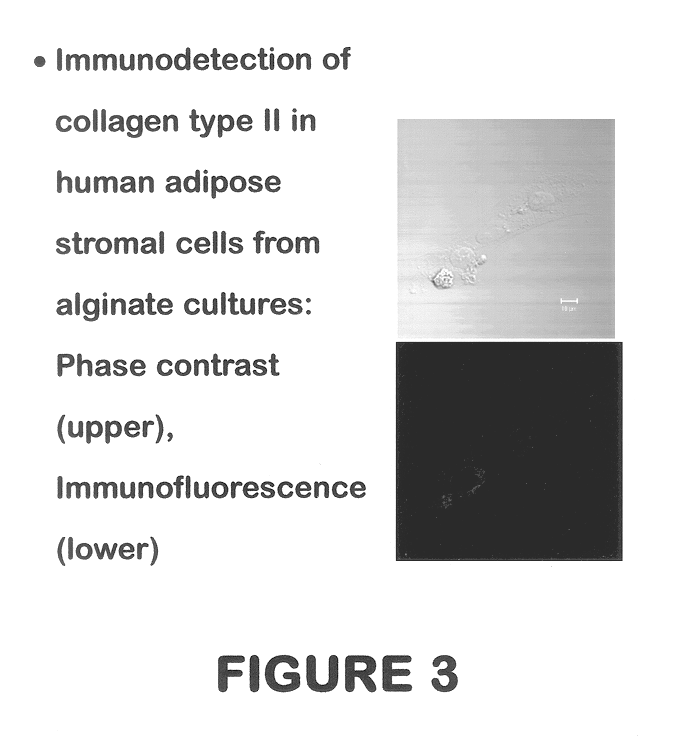
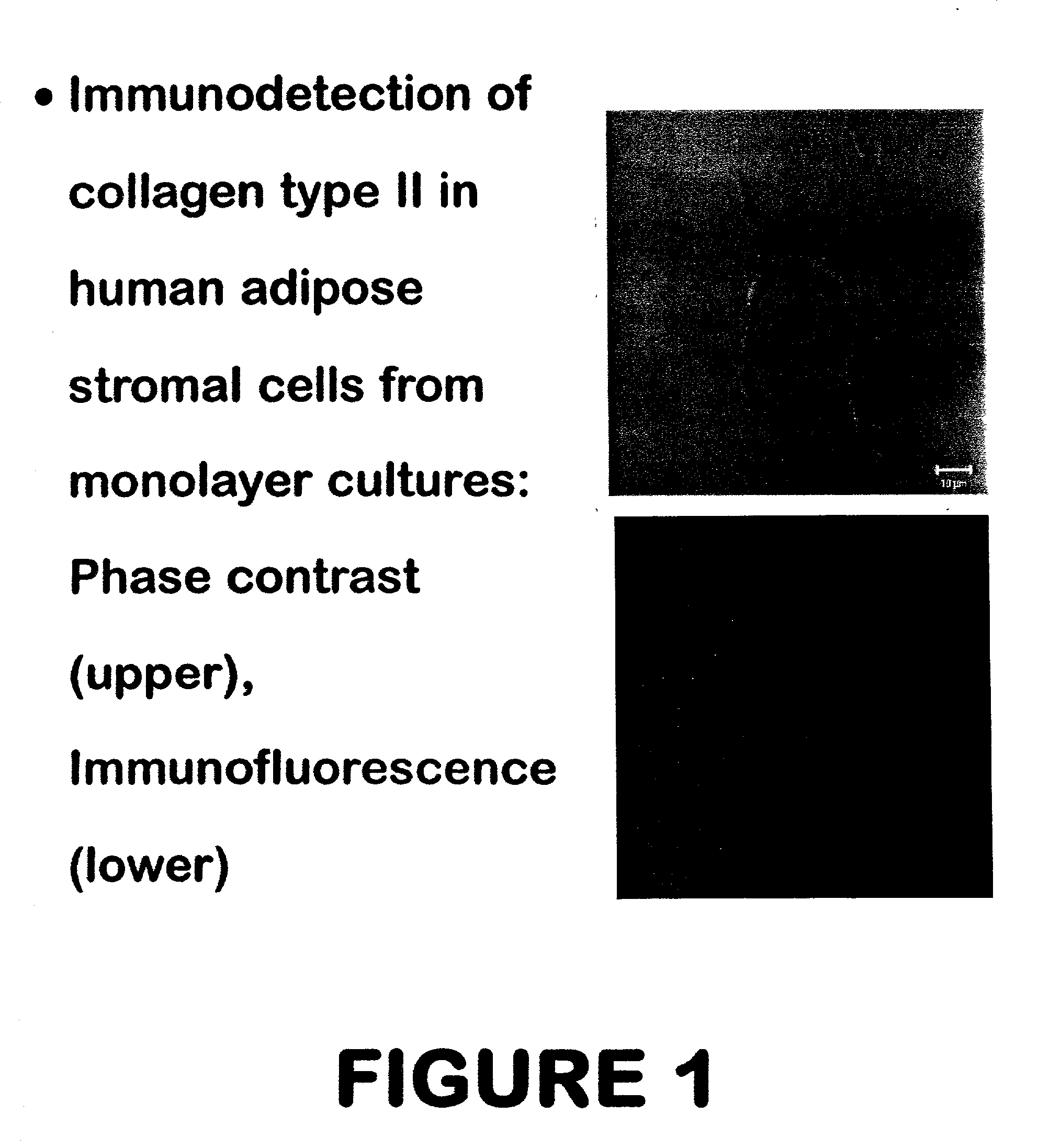
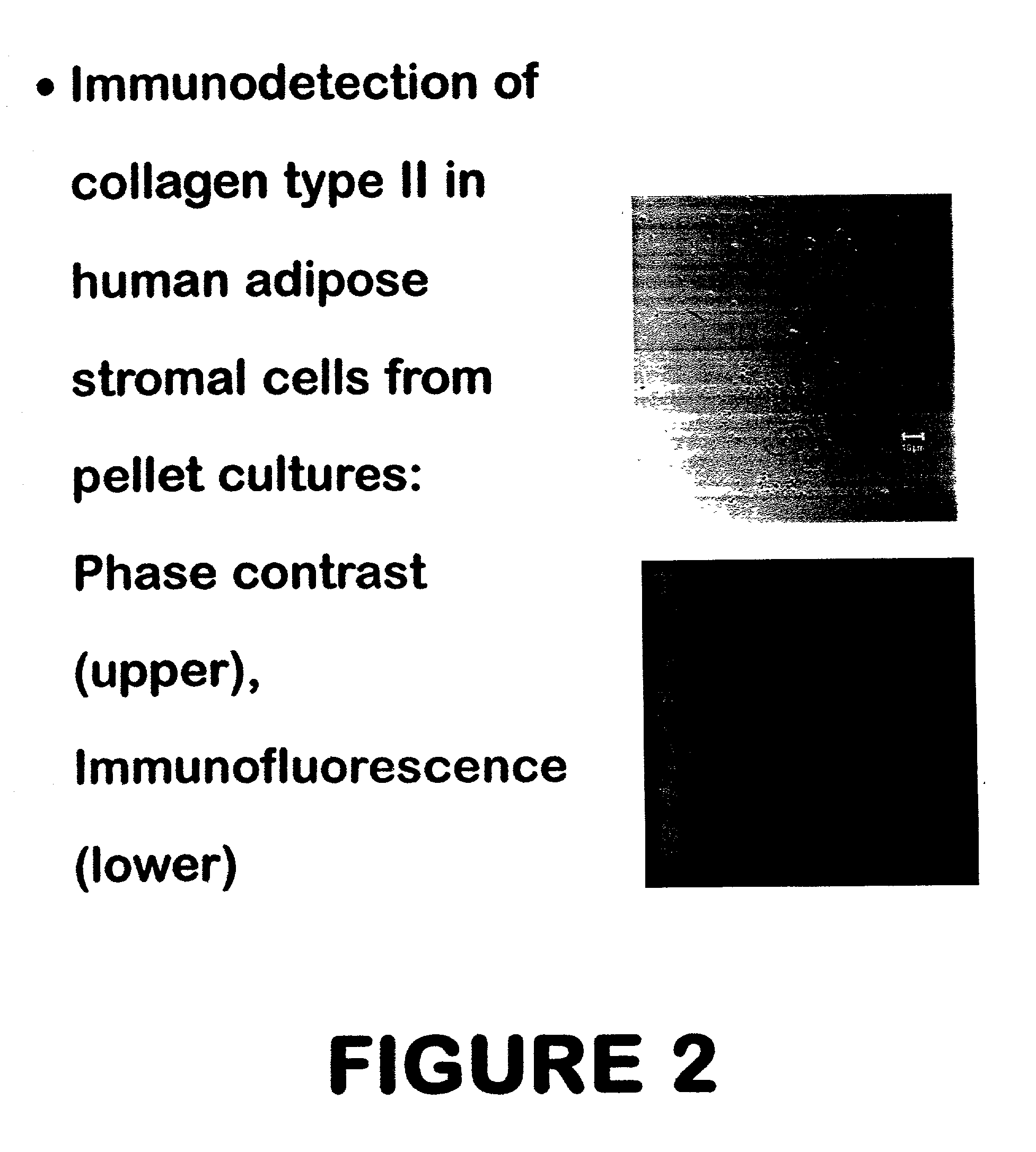
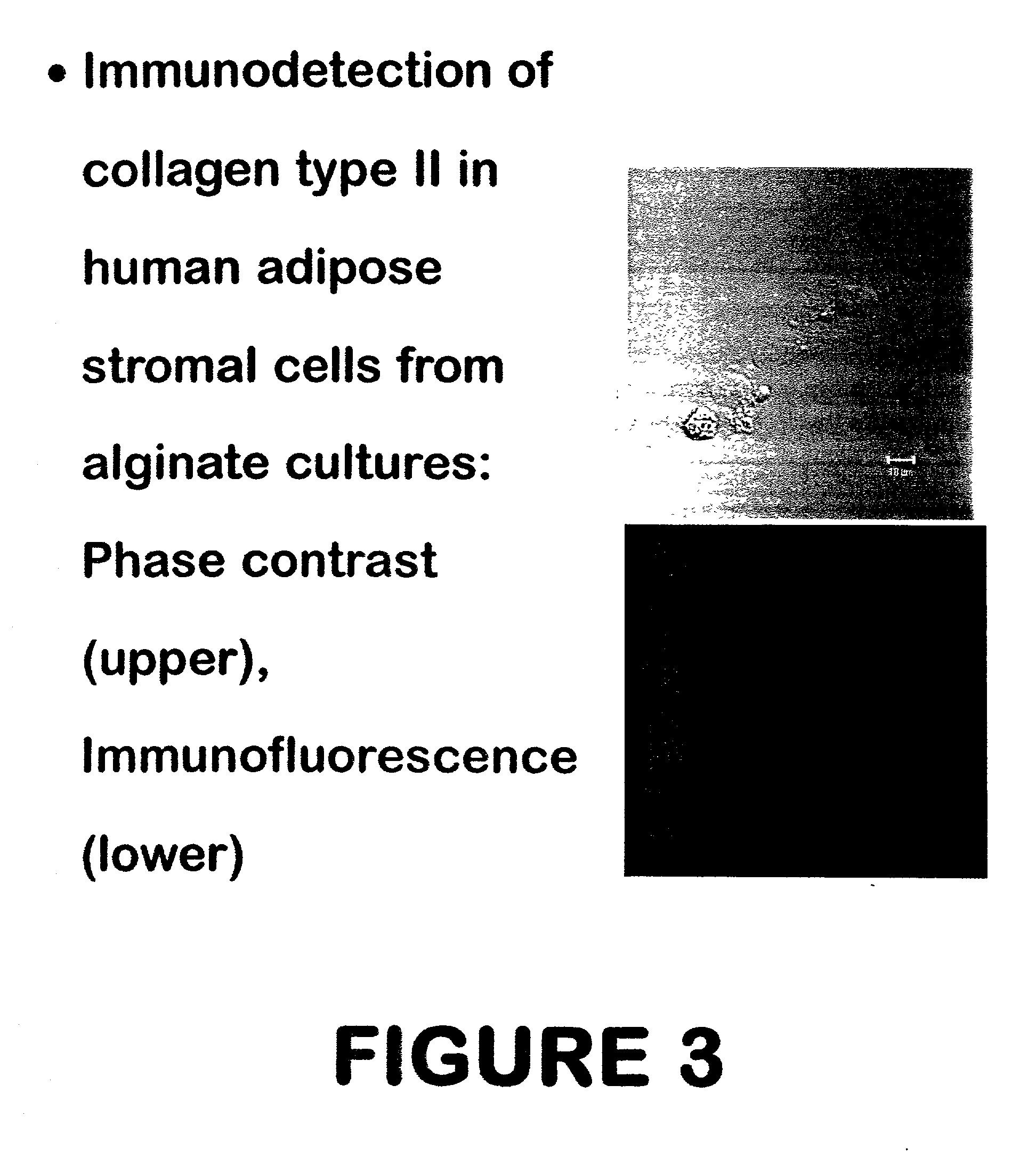
Use of adipose tissue-derived stromal cells for chondrocyte differentiation and cartilage repair
Methods and compositions for directing adipose-derived stromal cells cultivated in vitro to differentiate into cells of the chondrocyte lineage are disclosed. The invention further provides a variety of chondroinductive agents which can be used singly or in combination with other nutrient components to induce chondrogenesis in adipose-derived stromal cells either in cultivating monolayers or in a biocompatible lattice or matrix in a three-dimensional configuration. Use of the differentiated chondrocytes for the therapeutic treatment of a number of human conditions and diseases including repair of cartilage in vivo is disclosed.
Owner:COGNATE BIOSERVICES
Compositions for regeneration and repair of cartilage lesions
InactiveUS6511958B1Increase ratingsImprove repair qualitySuture equipmentsPowder deliveryMedicineCartilage lesion
Disclosed is a cartilage repair product that induces both cell ingrowth into a bioresorbable material and cell differentiation into cartilage tissue. Such a product is useful for regenerating and / or repairing both vascular and avascular cartilage lesions, particularly articular cartilage lesions, and even more particularly mensical tissue lesions, including tears as well as segmental defects. Also disclosed is a method of regenerating and repairing cartilage lesions using such a product.
Owner:ZIMMER ORTHOBIOLOGICS
Articular cartilage repair implant delivery device and method of use
A technique for the arthroscopic delivery and fixation of an articular cartilage repair device or implant is provided. The technique includes the use of a cannula tube that functions as both a cartilage cutter and a guide to pass instruments into the body arthroscopically. One such instrument is an end-cutting reamer that both prepares the subchondral bone by re-surfacing it down to a specified depth and also simultaneously drills a pilot hole in the subchondral bone to accept the cartilage repair device. A delivery device is utilized to hold and deliver the cartilage repair device to the delivery site.
Owner:DEPUY PROD INC
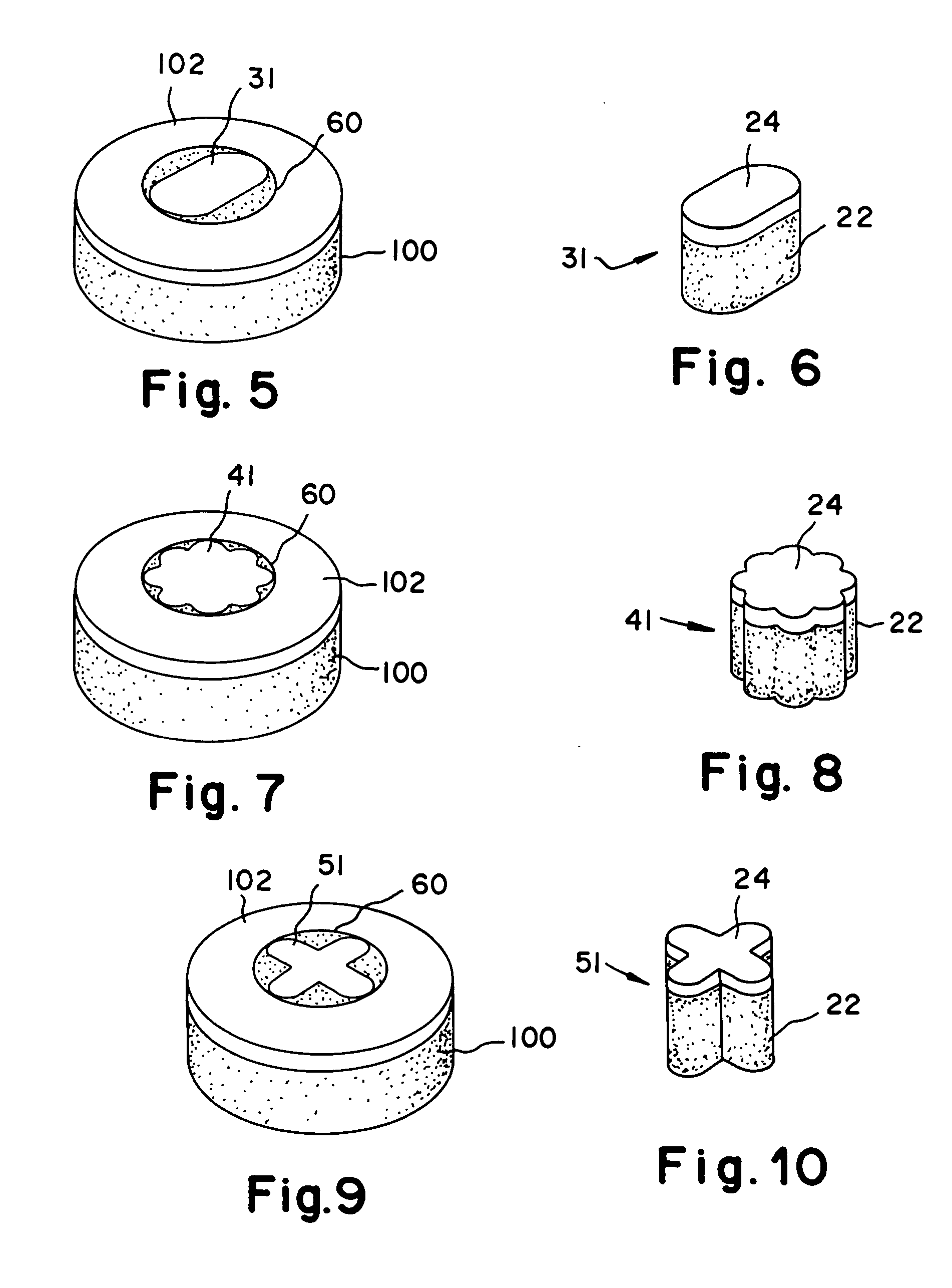
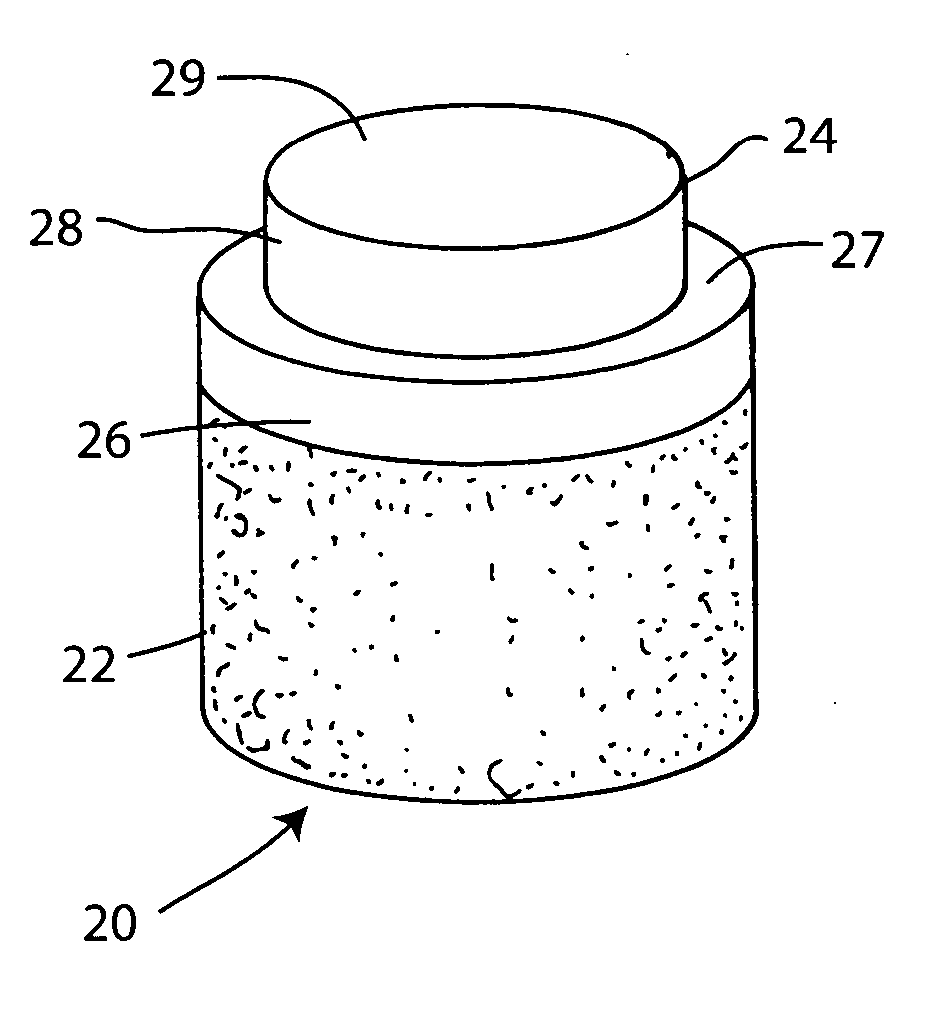
Cartilage repair plug
A cartilage plug, which is made from a biocompatible, artificial material, that is used to fill a void in natural cartilage that has been resected due to traumatic injury or chronic disease. Alternatively, the plug may be relied upon to anchor a flowable polymer to subchondral bone. The plug is prefabricatable in any size, shape, and contour and may be utilized either singly or in a plurality to fill any size void for any application. The plug may be formed of a laminated structure to match the physiological requirements of the repair site. A plurality of anchoring elements may share a single upper layer.
Owner:ABS
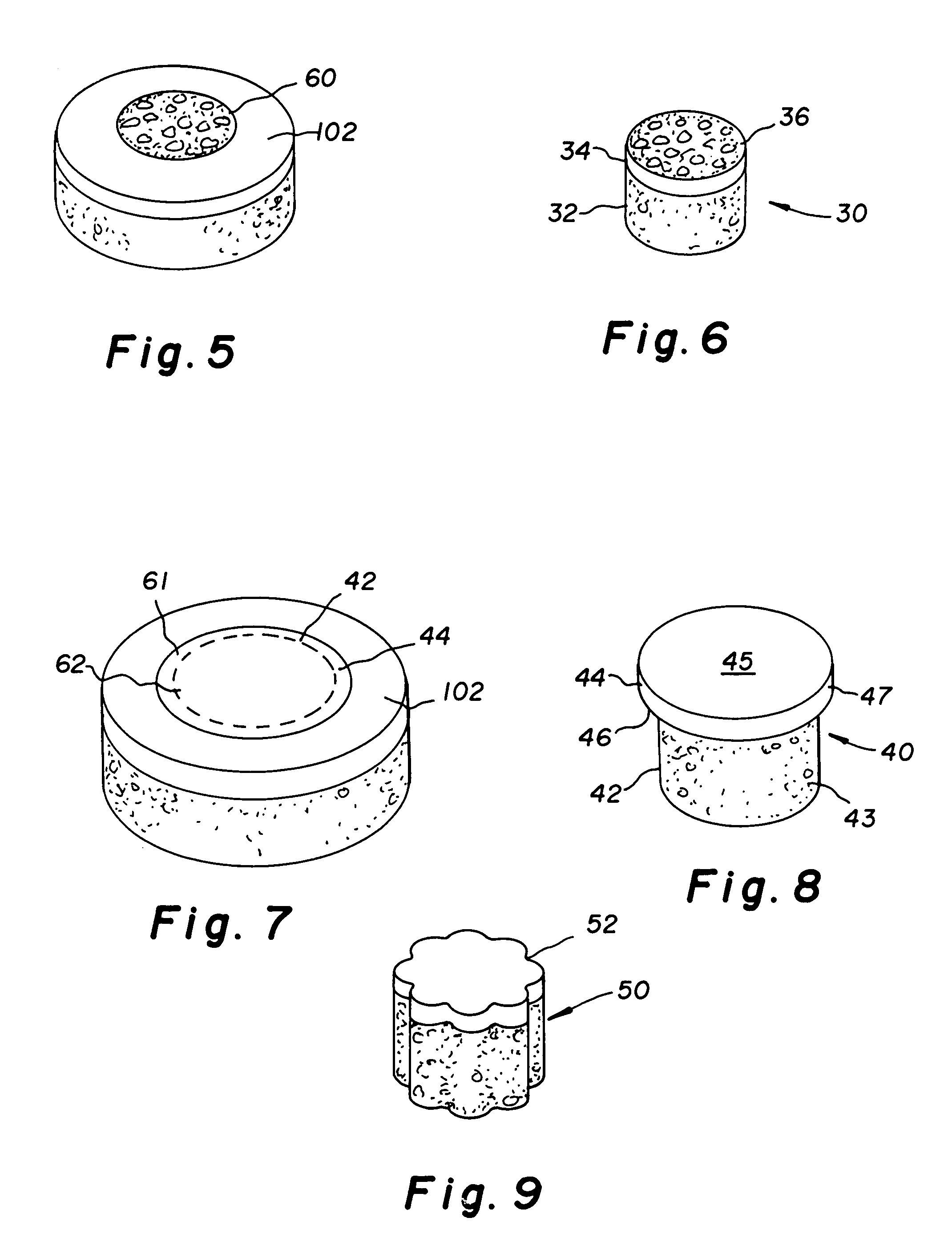
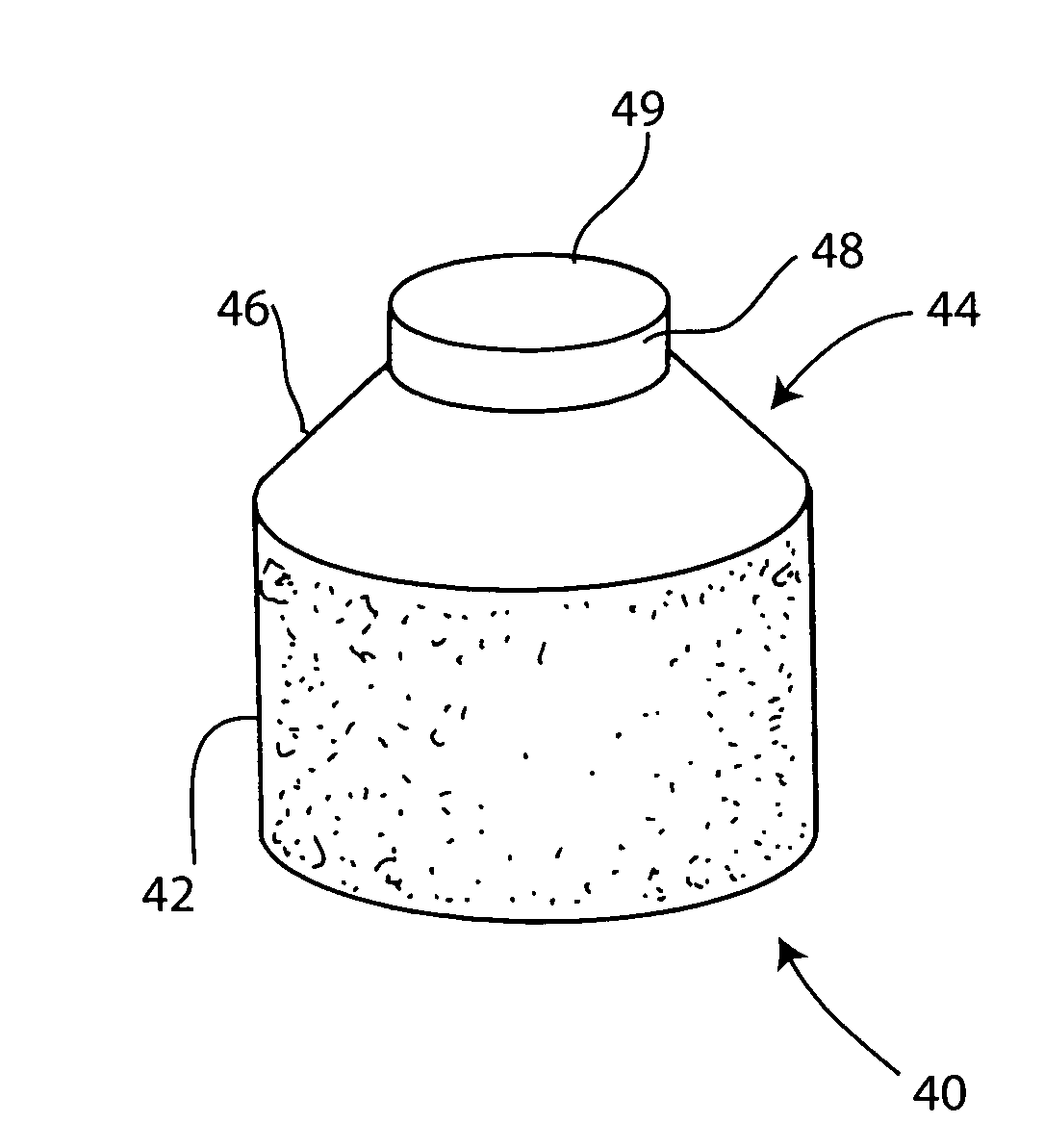
Cartilage repair plug
InactiveUS6632246B1Facilitate cell ingrowthMechanically fixedBone implantDiagnosticsCartilage repairSurgical department
A cartilage plug, which is made from a biocompatible, artificial material, that is used to fill a void in natural cartilage that has been resected due to traumatic injury or chronic disease is disclosed. Alternatively, the plug may be relied upon to anchor a flowable polymer to subchondral bone. The plug is prebricatable in any size, shape, and contour and may be utilized either singly or in a plurality to fill any size void for any application. The plug may be formed of a laminated structure to match the physiological requirements of the repair site. Additionally, ridges may be formed about the periphery of each plug to facilitate its anchoring to surrounding cartilage, bone and / or adjacent plugs. A procedure for resecting damaged or diseased cartilage and for implanting a replacement plug or plugs according to this invention, as well as a set of instruments for effecting the procedure, and a self-contained system for orthopedic surgeons, which includes a variety of differently sized and shaped plugs, as well as a set of instruments for the procedure are also disclosed.< / PTEXT>
Owner:ABS
Use of adipose tissue-derived stromal cells for chondrocyte differentiation and cartilage repair
Methods and compositions for directing adipose-derived stromal cells cultivated in vitro to differentiate into cells of the chondrocyte lineage are disclosed. The invention further provides a variety of chondroinductive agents which can be used singly or in combination with other nutrient components to induce chondrogenesis in adipose-derived stromal cells either in cultivating monolayers or in a biocompatible lattice or matrix in a three-dimensional configuration. Use of the differentiated chondrocytes for the therapeutic treatment of a number of human conditions and diseases including repair of cartilage in vivo is disclosed.
Owner:COGNATE BIOSERVICES
Cartilage implant assembly and method for implantation
InactiveUS20050222687A1Recovery functionRelief the painBone implantSurgerySubchondral boneInterference fit
The invention is directed toward a cartilage repair assembly comprising a shaped structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that said shaped bone and cartilage cap when centered in the bore does not engage the side wall of the bore in an interference fit and is surrounded by milled cartilage and carrier. A method for inserting the assembly into a cartilage defect area is disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC +1
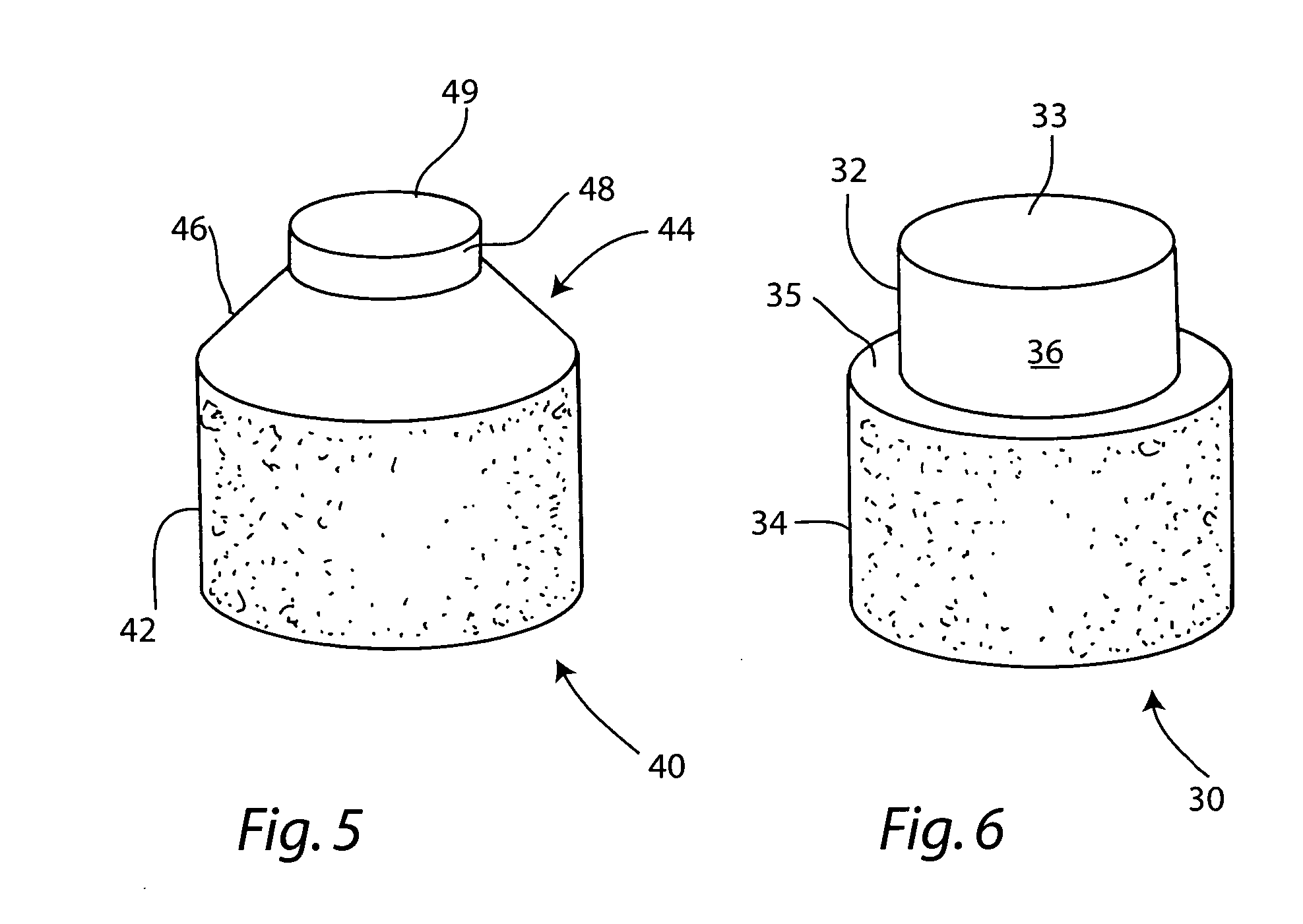
Cartilage allograft plug
InactiveUS20050251268A1Increase chondrocyte migrationIncreased proliferationBone implantLigamentsInterference fitSubchondral bone
The invention is directed toward a cartilage repair assembly comprising a cylindrically shaped allograft structure of subchondral bone with an integral overlying smaller diameter cartilage cap which is treated to remove cellular debris and proteoglycans. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that the subchondral bone of the structure engages the side wall of the bone portion of the drilled bore in an interference fit while the cartilage cap is spaced from cartilage portion of the side wall of the drilled bore forming a gap in which a milled cartilage and biocompatible carrier mixture is placed allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Biodegradable polyurethane/urea compositions
The present invention relates to biocompatible, biodegradable polyurethane / urea polymeric compositions that are capable of in-vivo curing with low heat generation to form materials suitable for use in scaffolds in tissue engineering applications such as bone and cartilage repair. The polymers are desirably flowable and injectable and can support living biological components to aid in the healing process. They may be cured ex-vivo for invasive surgical repair methods, or alternatively utilized for relatively non-invasive surgical repair methods such as by arthroscope. The invention also relates to prepolymers useful in the preparation of the polymeric compositions, and to methods of treatment of damaged tissue using the polymers of the invention.
Owner:POLYNOVO BIOMATERIALS PTY LTD
Biodegradable therapeutic implant for bone or cartilage repair
The exemplary embodiments of the present invention relate to an at least partially biodegradable implant suitable for implantation into a subject for repairing a bone or cartilage defect, comprising a matrix forming an open-celled structure having a plurality of interconnected spaces, whereas the channels of the matrix are substantially completely filled with metallic material particles, and wherein at least one of the metallic material or the matrix material is at least partially degradable in-vivo. Furthermore, the present invention relates to a method for repairing a bone or cartilage defect in a living organism, comprising implanting an implant according to the exemplary embodiments of the present invention into the defective bone or cartilage, or replacing the defective bone or cartilage at least partially.
Owner:CINVENTION AG
Articular Cartilage Fixation Device and Method
ActiveUS20080195205A1Easy to fixMaximizing pull-through forceSuture equipmentsStaplesCartilage repairSacroiliac joint
An articular cartilage fixation device and method for repairing and regenerating diseased or injured soft tissue such as articular cartilage of the knee, hip, shoulder, and the like. A cartilage flap or cartilage repair device is retained within the chondral or osteochondral defect for a an sufficient amount of time such that the cartilage repair device can perform its function and facilitate an appropriate healing response. A plurality of biocompatible anchors are attached to a plurality of flexible members and are shaped to seat into the tissue beneath or near the defect such that the cartilage flap is retained within the defect.
Owner:SCHWARTZ BIOMEDICAL LLC
Tracked cartilage repair system
ActiveUS20110208256A1Reduce removalAdditive manufacturing apparatusDiagnosticsData setCustom made implant
A system and method for repairing an area of defective tissue reduces the removal of healthy tissue at the margins of the defect. During excision of diseased or damaged tissue, the system tracks the movement and function of a tissue resection tool within a monitored surgical space. This movement is continuously recorded to create a three-dimensional set of data points representative of the excised volume of tissue. This data set is then communicated to a custom implant forming device which creates a custom implant sized to fit the void created by the excision. The system and method of the present disclosure allows a surgeon to exercise intraoperative control over the specific shape, volume and geometry of the excised area. Moreover, the surgeon may utilize a “freehand” resection method to excise only that tissue deemed to be diseased and / or damaged, because the custom-formed implant will accommodate an irregularly-shaped resection volume.
Owner:ZIMMER INC
Prosthetic Devie for Cartilage Repair
InactiveUS20070244484A1Improve mechanical stabilityRapid cartilage growthBone implantLigamentsFiberMedicine
A prosthetic device for repairing or replacing cartilage or cartilage-like tissue is described. The prosthetic device comprises at least one layer of highly oriented fibers, a base component and a stabilization area provided in between. Said fibers are aligned to more than 50% in a direction perpendicular to the base component.
Owner:STIFUNG DR H C ROBERT MATHYS
Glue for cartilage repair
ActiveUS7067123B2Promote migrationIncreased proliferationBiocidePeptide/protein ingredientsMedicineBone marrow cell
The invention is directed toward a sterile cartilage defect implant material comprising milled lyophilized allograft cartilage pieces ranging from 0.01 mm to 1.0 mm in size in a bioabsorbable carrier taken from a group consisting of sodium hyaluronate, hyaluronic acid and its derivatives, gelatin, collagen, chitosan, alginate, buffered PBS, Dextran or polymers with allogenic chondrocytes or bone marrow cells in an amount exceeding the natural occurrence of same in hyaline cartilage and adding a cell growth additive.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Assembled Cartilage Repair Graft
Owner:RTI BIOLOGICS INC
Cartilage allograft plug
InactiveUS7901457B2Promote migrationIncreased proliferationBone implantJoint implantsSubchondral boneInterference fit
The invention is directed toward a cartilage repair assembly comprising a shaped allograft structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled allograft cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that either the shaped bone or the cartilage cap engage the side wall of the drilled bore in an interference fit and is in contact with a milled cartilage and biocompatible carrier mixture allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Cartilage allograft plug
InactiveUS7488348B2Guaranteed functionEasily placed in defect areaBone implantLigamentsInterference fitSubchondral bone
The invention is directed toward a cartilage repair assembly comprising a cylindrically shaped allograft structure of subchondral bone with an integral overlying smaller diameter cartilage cap which is treated to remove cellular debris and proteoglycans. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that the subchondral bone of the structure engages the side wall of the bone portion of the drilled bore in an interference fit while the cartilage cap is spaced from cartilage portion of the side wall of the drilled bore forming a gap in which a milled cartilage and biocompatible carrier mixture is placed allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
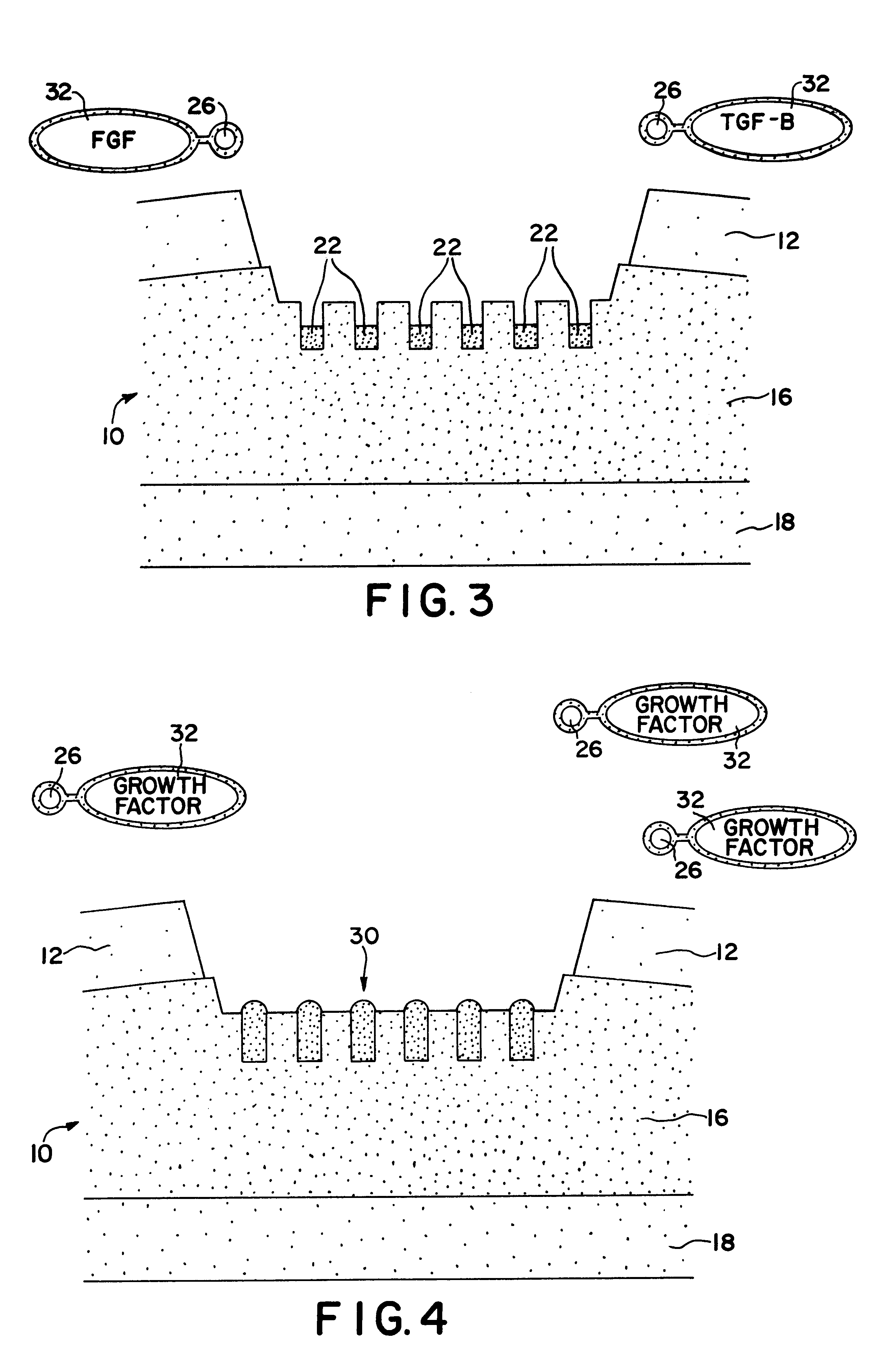
Formulations and methods for delivery of growth factor analogs
Formulations, kits and methods for bone or cartilage repair, including treatment of osteogenic defects, including formulations of synthetic heparin-binding growth factor analogs, non-ionic polymers, gelling agents and calcium-containing agents.
Owner:BROOKHAVEN SCI ASSOCS +1
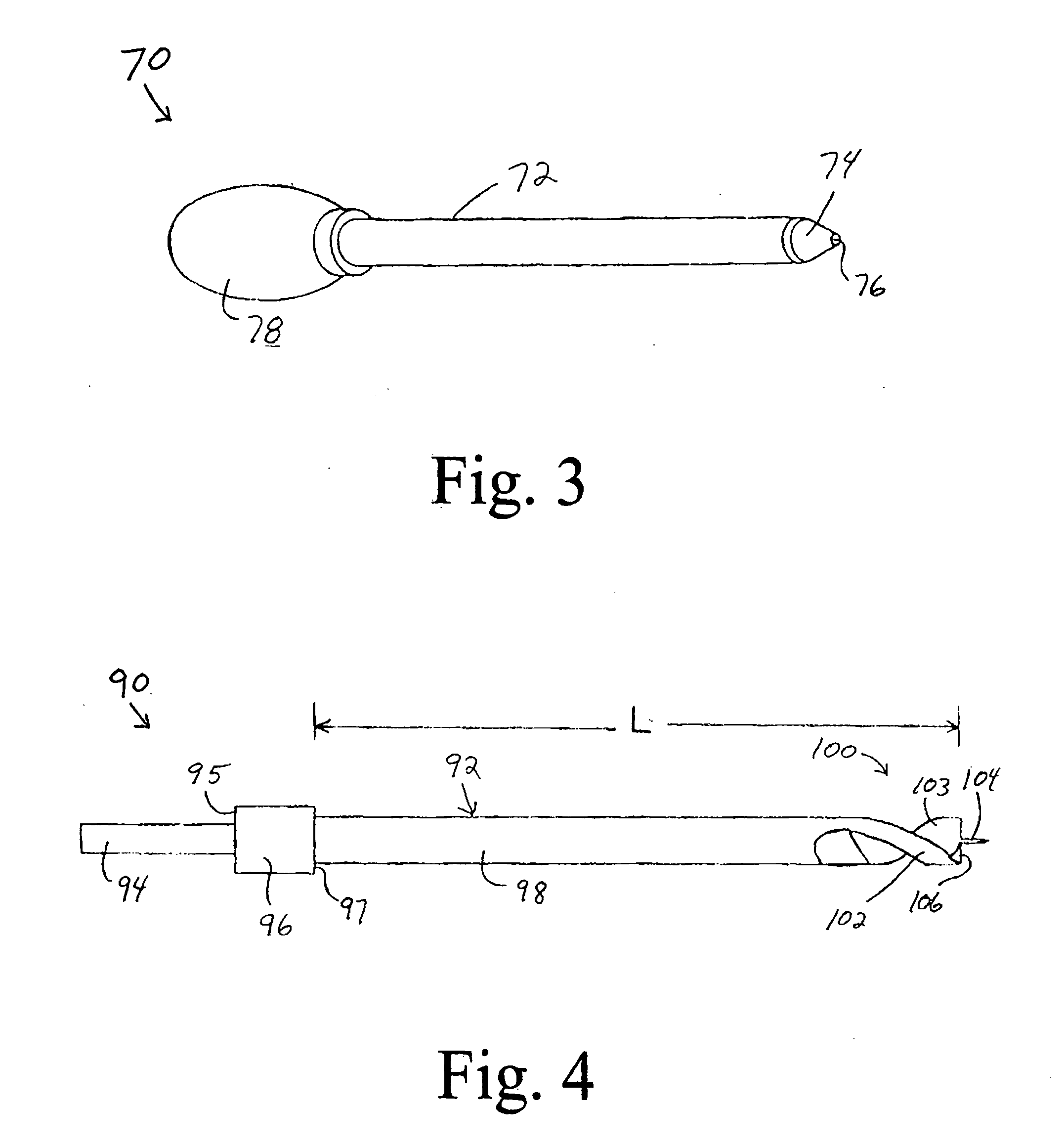
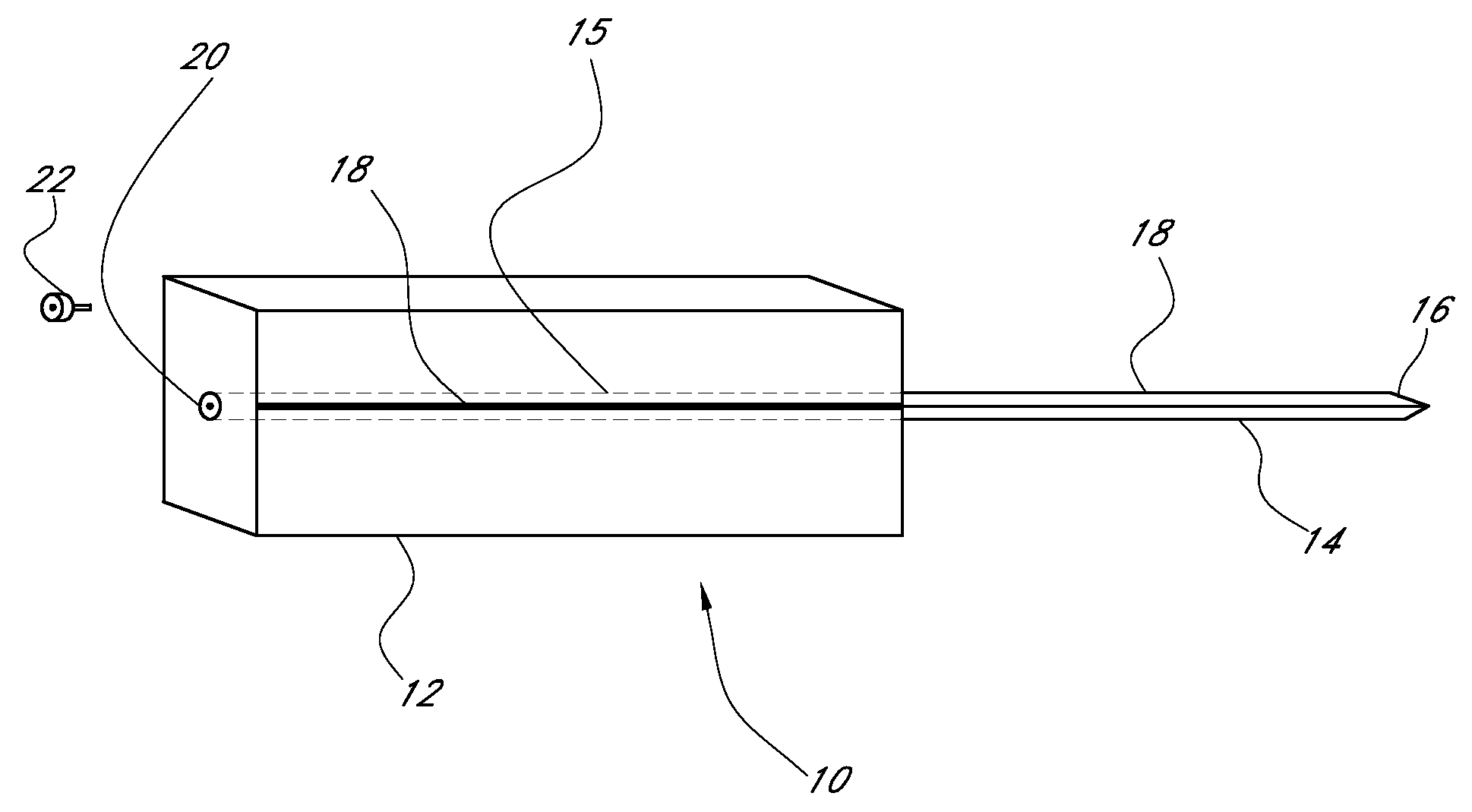
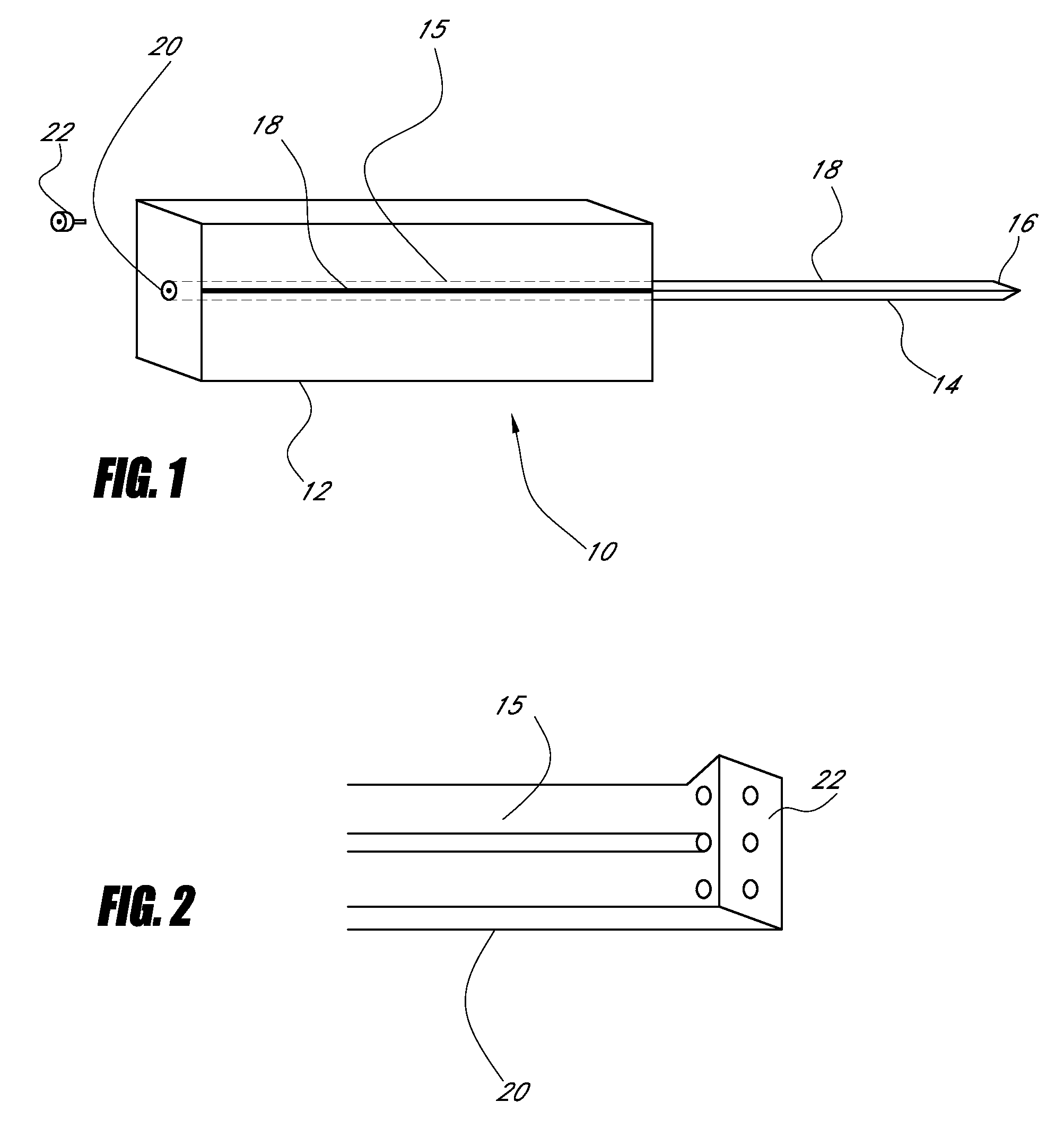
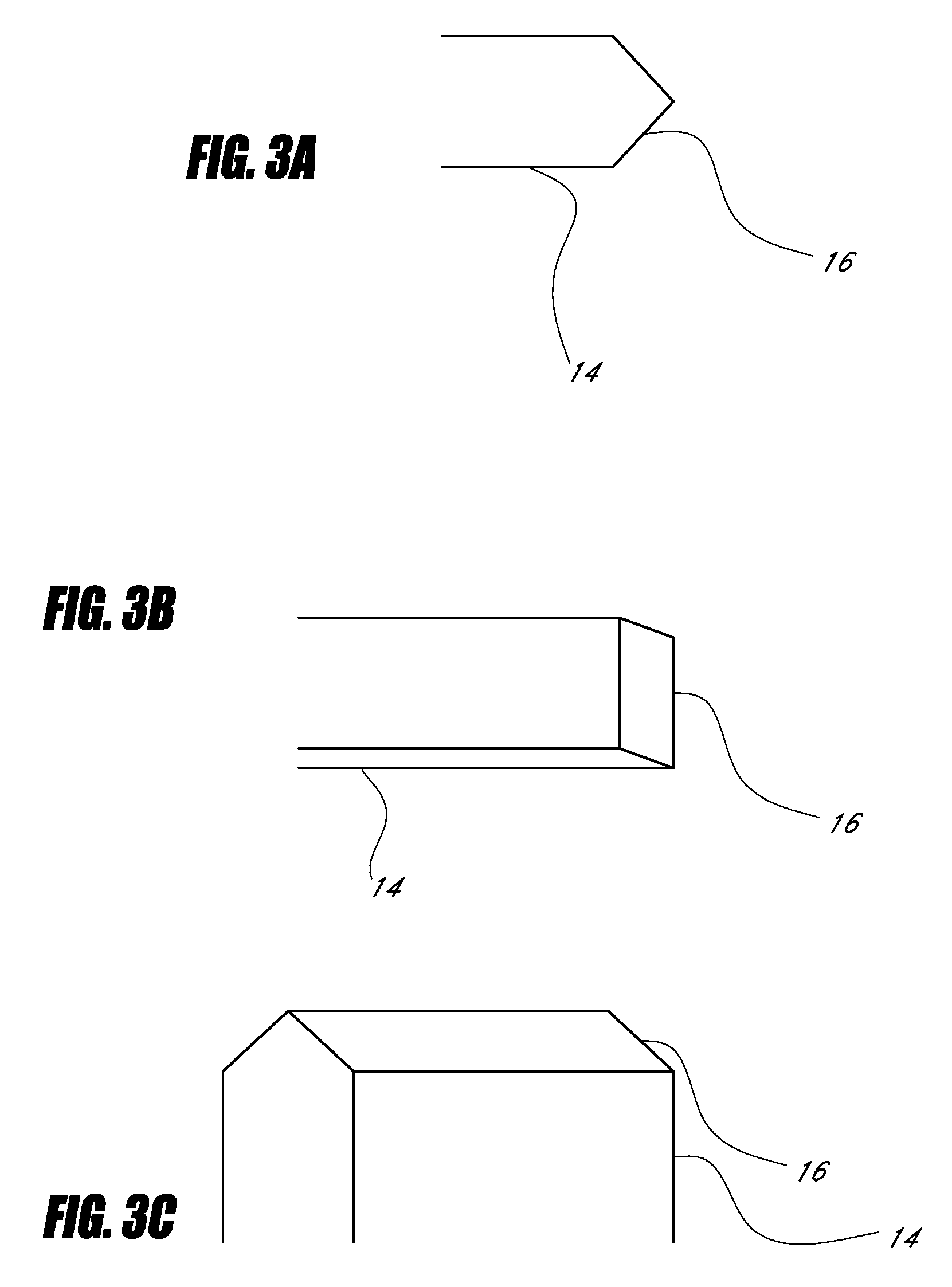
Device for cartilage repair
A surgical awl is disclosed for tissue repair. The surgical awl includes an internal lumen through which bioactive substances, such as platelet-rich plasma, may be delivered to a site of tissue or organ disease or dysfunction. In particular, the surgical awl is useful as a microfracture awl for cartilage repair augmented by delivery of platelet-rich plasma through the lumen of the device.
Owner:MISHRA ALLAN
Methods and Crosslinked Polymer Compositions for Cartilage Repair
InactiveUS20090117070A1Promote growthGood adhesionBiocidePeptide/protein ingredientsDamages tissueActive agent
A method of repairing damaged cartilage and soft tissue in a patient is provided using a biocompatible, non-immunogenic composition. The composition comprises a hydrophilic polymer and a plurality of crosslinkable components having reactive functional groups. The composition used in the method may be loaded with biologically active agents for delivery to the damaged tissues. Kits for use in carrying out the method of the invention are also provided.
Owner:ANGIOTECH PHARMA US
Means for cartilage repair
InactiveUS6179871B1Minimize extraneous effectEvenly spacedPowder deliveryElectrotherapyChondral defectGrowth promoting
A method for repairing a defect in cartilage, comprising the provision of apertures in the cartilage by drilling holes at the base of the cartilage defect, which holes may enter the mesenchymal depot, introducing a porous scaffold material containing a plurality of magnetic particles into the apertures, and subsequently and sequentially injecting magnetically-tagged cartilage growth promoting materials such as various growth factors or chondrocytes into the area of the defect. The magnetically tagged growth promoting material is then drawn into the apertures by magnetic attraction of the magnetic particles contained in the porous scaffold material, either by virtue of the particles being permanently magnetic, or by the imposition of an external magnetic field. The present application claims the biodegradable porous scaffold material containing the plurality of magnetic particles.
Owner:HALPERN ALAN A
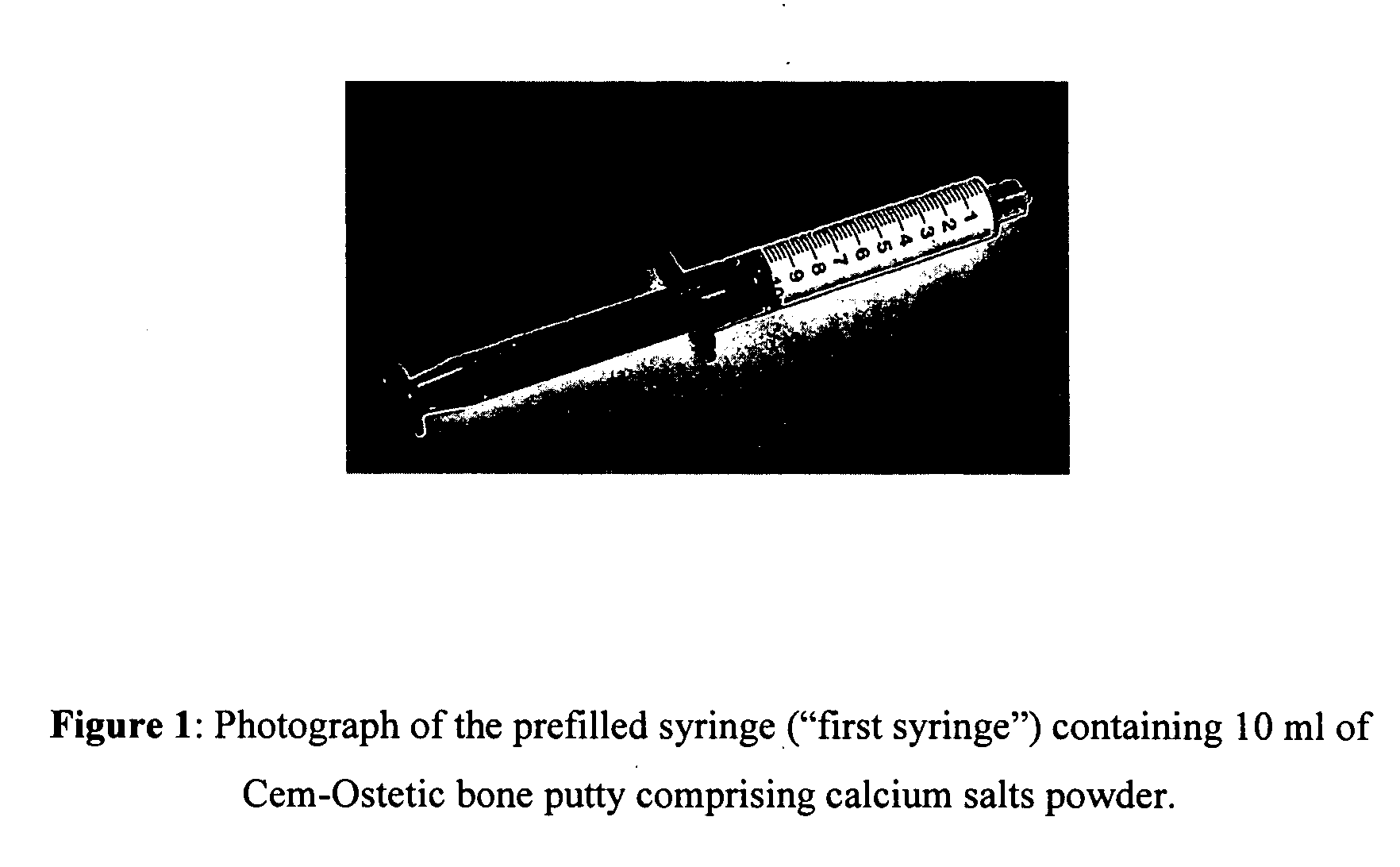
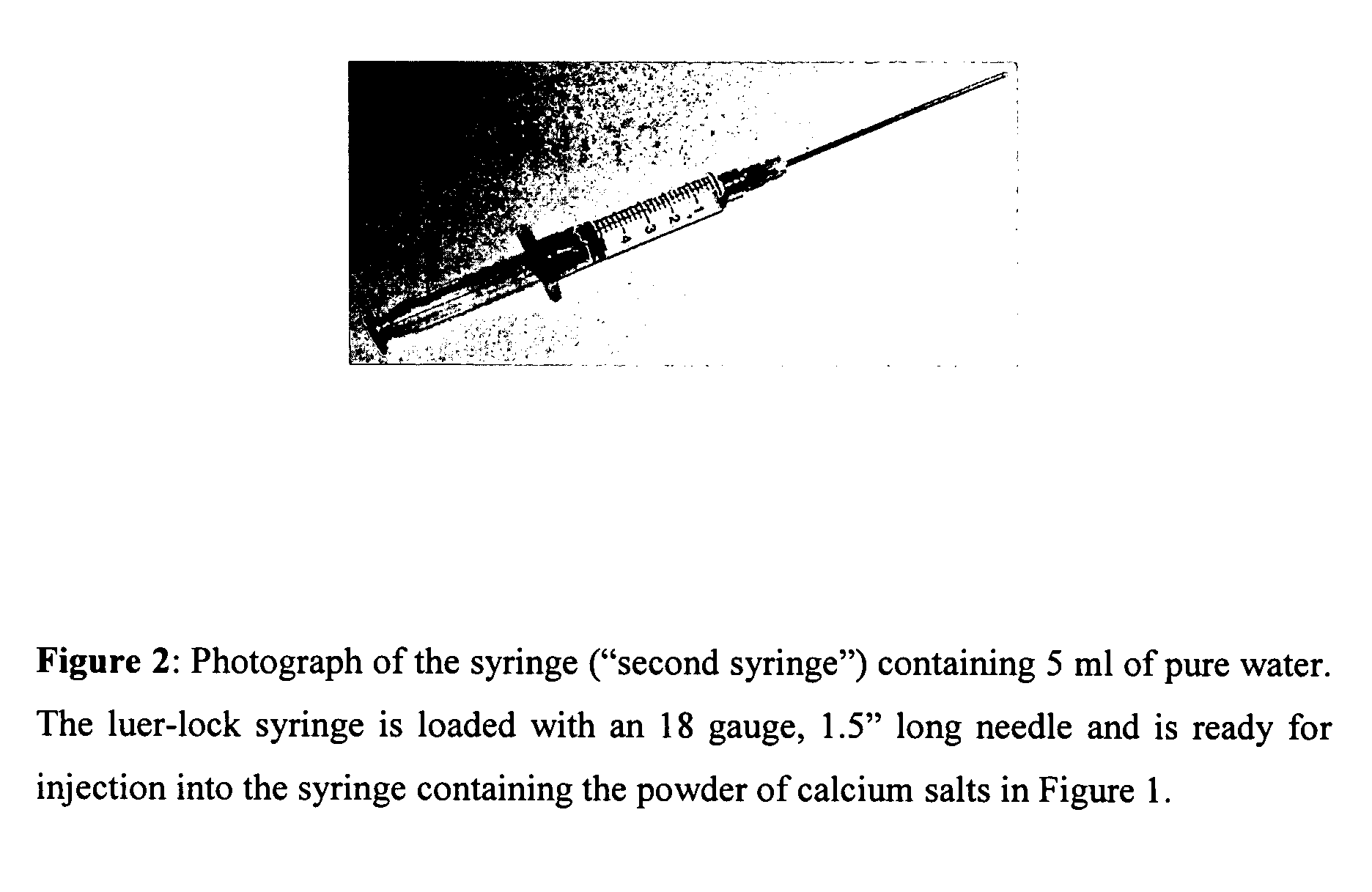
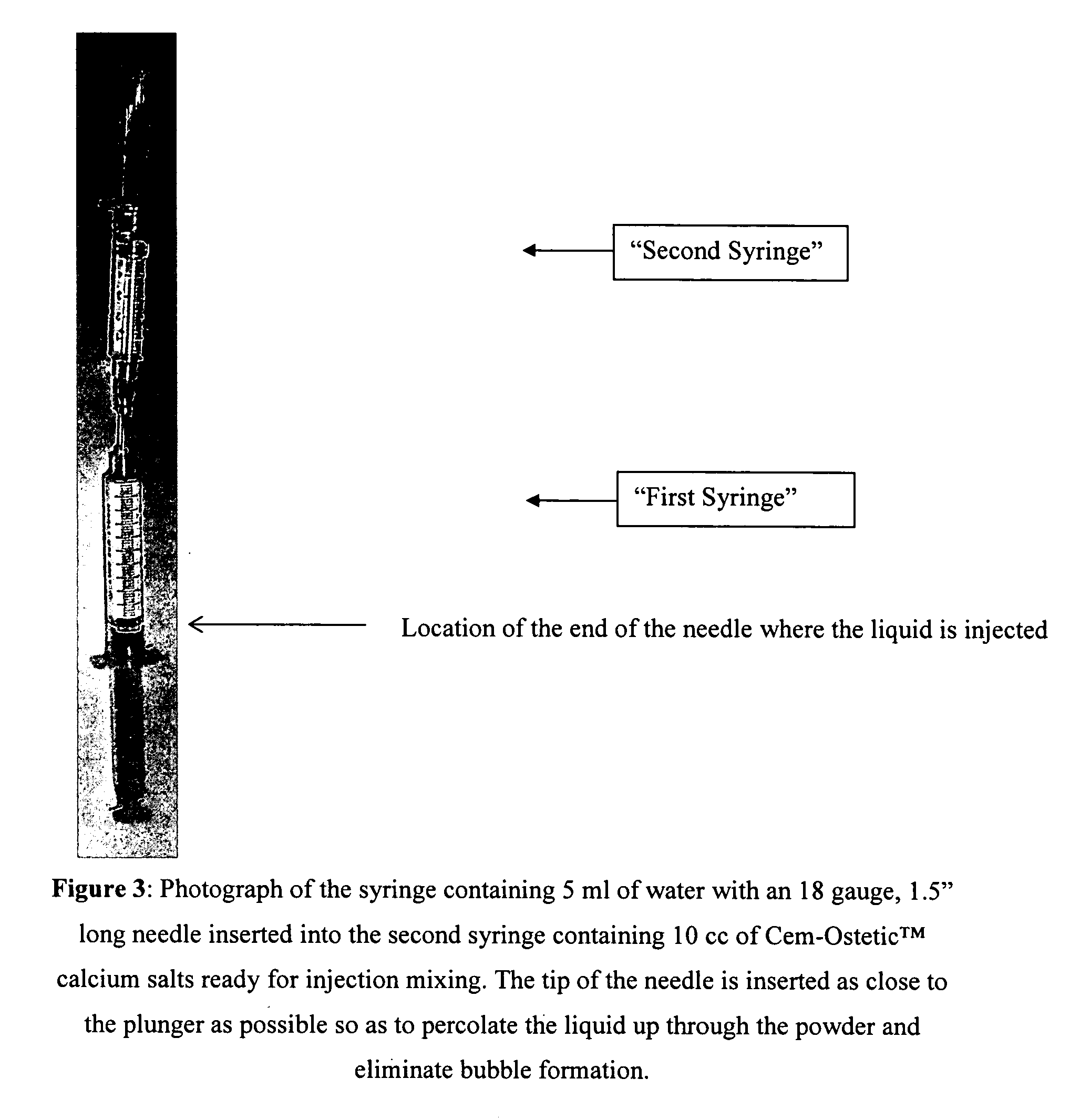
Method of preparing rheological materials for bone and cartilage repair
InactiveUS20070026030A1Volume maximizationEliminate exposureBiocideInternal osteosythesisCartilage repairAnimal body
Methods of mixing delivering biocompatible cement, paste, putty, or gel for bone and cartilage repair are described in this invention. Powder-like solid materials are loaded into a first syringe. Liquids are loaded into one or multiple syringes. The liquids are injected into the first syringe containing the solid materials. To force the liquids through the solid, prevent bubble formation and provide intimate intermixing, the liquids are injected in the very proximity of the plunger end of the syringe containing the solid materials. The first syringe is preferably held vertical with the tip facing up so as to avoid bubble formation that in turn could cause back-pressure build-up and plug the first syringe during injection. The described methods of mixing the liquids with the solids allows to form a rheological paste, cement, putty, or gel in the first syringe. As injection into the human or animal body proceeds, the paste then flows without complications often caused by entrapped bubbles or improper / heterogeneous mixing. The preparation and injection processes can be conducted at temperatures that do not damage live tissue or denature proteins. The paste, cement, putty, or gel can be injected into bone through the cannula by hand or with a pressurizing system. The method reduces the amount of time needed to prepare the paste and load it into the syringe and provides a device that is easily prepared for injection.
Owner:BERKELEY ADVANCED BIOMATERIALS
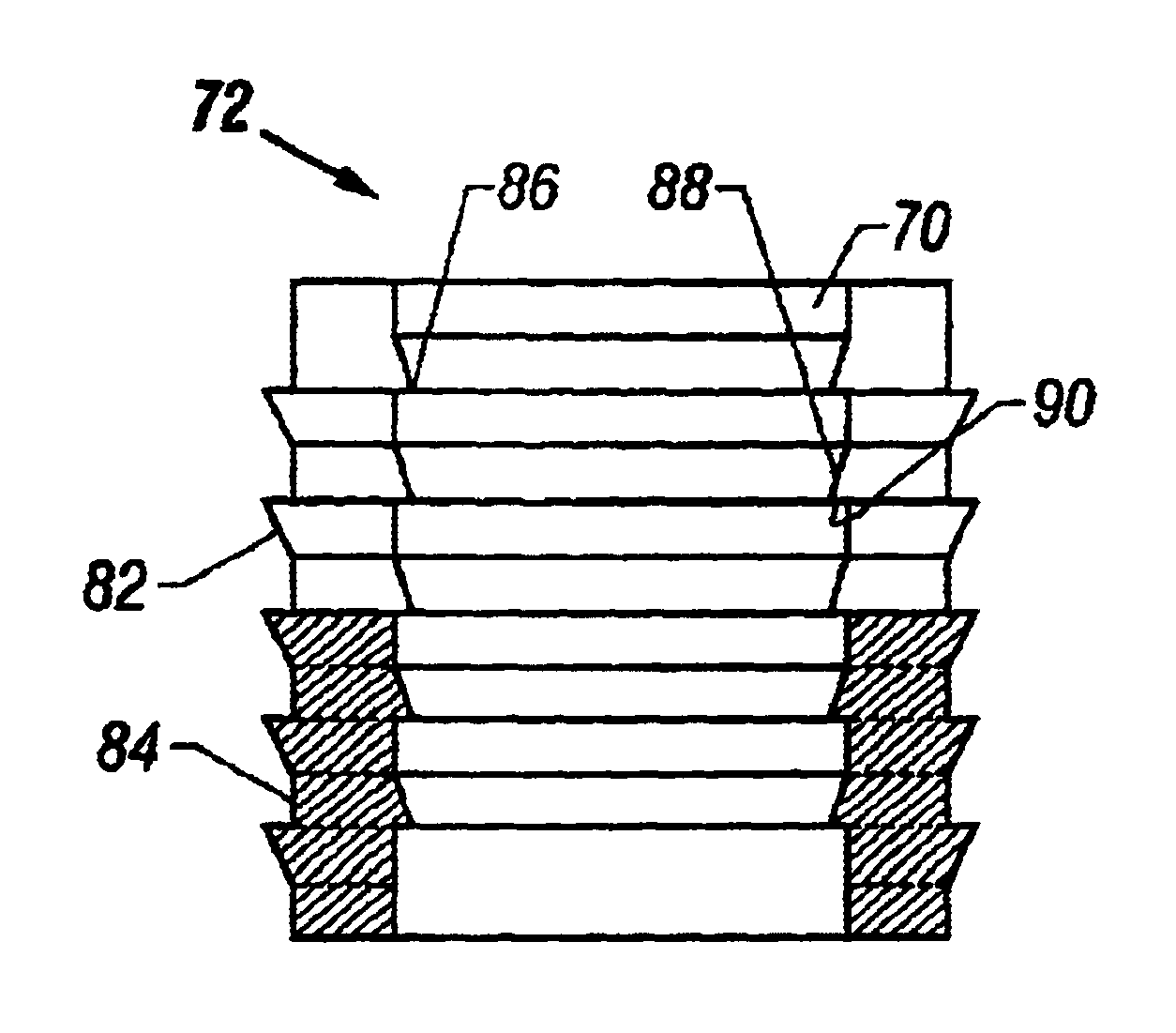
Cartilage repair plug
InactiveUS20040162622A1Easy inflowEasy to fixBone implantDiagnosticsCartilage repairSurgical department
A cartilage plug, which is made from a biocompatible, artificial material, that is used to fill a void in natural cartilage that has been resected due to traumatic injury or chronic disease is disclosed. Alternatively, the plug may be relied upon to anchor a flowable polymer to subchondral bone. The plug is prefabricatable in any size, shape, and contour and may be utilized either singly or in a plurality to fill any size void for any application. The plug may be formed of a laminated structure to match the physiological requirements of the repair site. Additionally, ridges may be formed about the periphery of each plug to facilitate its anchoring to surrounding cartilage, bone and / or adjacent plugs. A procedure for resecting damaged or diseased cartilage and for implanting a replacement plug or plugs according to this invention, as well as a set of instruments for effecting the procedure, and a self-contained system for orthopaedic surgeons, which includes a variety of differently sized and shaped plugs, as well as a set of instruments for the procedure are also disclosed.
Owner:ABS
Two piece cancellous construct for cartilage repair
ActiveUS7837740B2Guaranteed functionEasily placed in defect areaBone implantTissue regenerationInterference fitChondral defect
The invention is directed toward a cartilage repair assembly comprising a shaped allograft two piece construct with a demineralized cancellous cap and a mineralized cylindrical base member defining a blind bore with a through going transverse bore intersecting the blind bore. The demineralized cancellous cap has a cylindrical top portion and a smaller diameter cylindrical stem extending away from the top portion which fits into the blind bore of the mineralized base member. The cap stem defines a transverse through going bore which is aligned with the through going bore of the base member to receive a cylindrical cortical pin holding the cap within the base member. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that the assembly engages the side wall of the drilled bore in an interference fit.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Cartilage repair methods
The present application discloses methods for repairing hyaline cartilage defects. The methods comprise a combination of introducing autologous bone mesenchymal stem cells to a joint, and applying to the joint a membrane comprising a polyester entangled with a polysaccharide. In some aspects, the bone mesenchymal stem cells are mesenchymal stem cells originating in bone underlying the joint. In these aspects, contact between the joint and the mesenchymal stem cells can be effected by introducing apertures through the bone using standard surgical techniques such as microfracture, abrasion, or drilling. Cartilage which forms in response to application of these methods is hyaline cartilage rather than fibrocartilage.
Owner:ISTO TECH II LLC
Cartilage allograft plug
ActiveUS20090069901A1Increase chondrocyte migrationIncreased proliferationBone implantJoint implantsSubchondral boneInterference fit
The invention is directed toward a cartilage repair assembly comprising a shaped allograft structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled allograft cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that either the shaped bone or the cartilage cap engage the side wall of the drilled bore in an interference fit and is in contact with a milled cartilage and biocompatible carrier mixture allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com