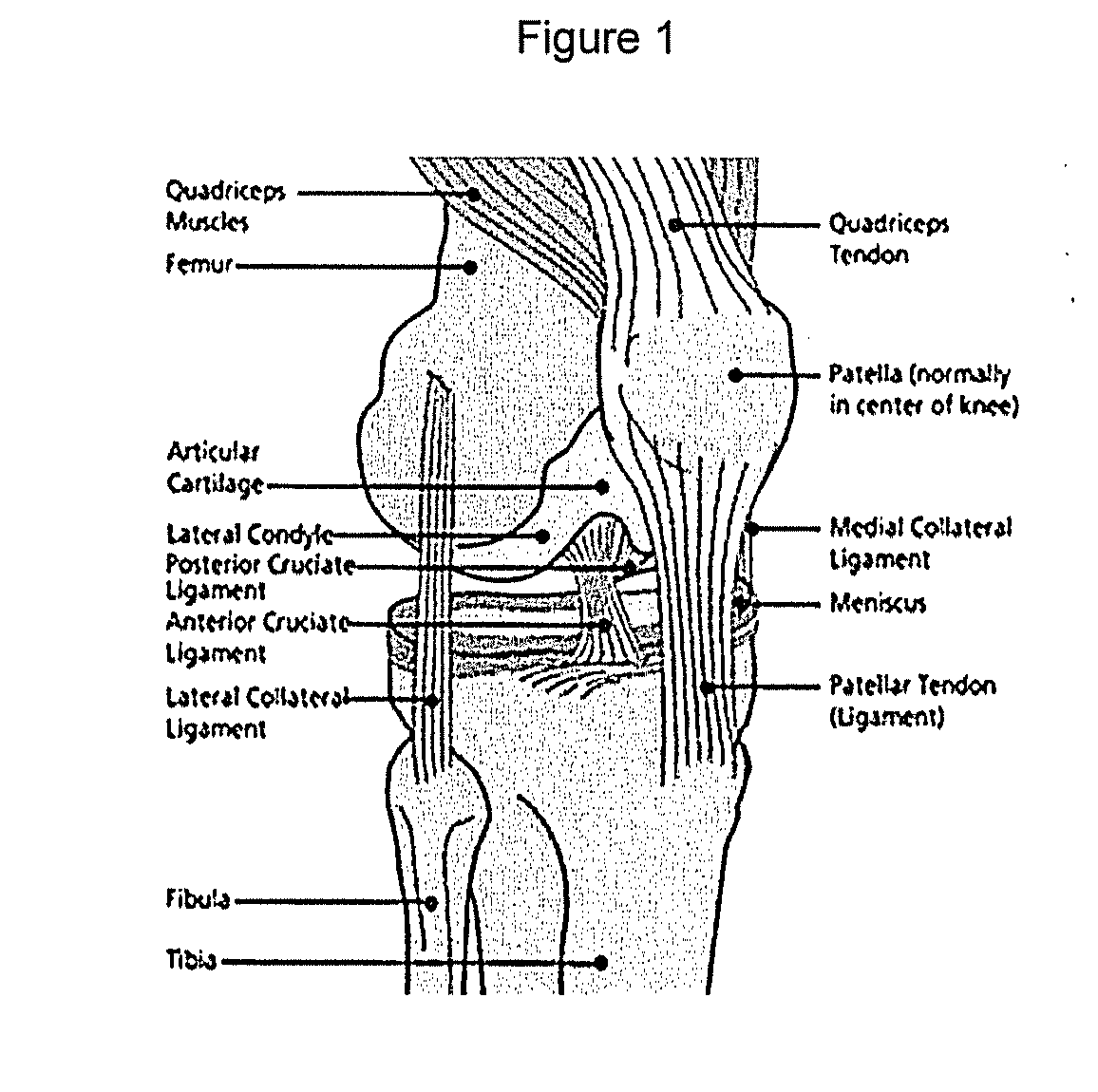
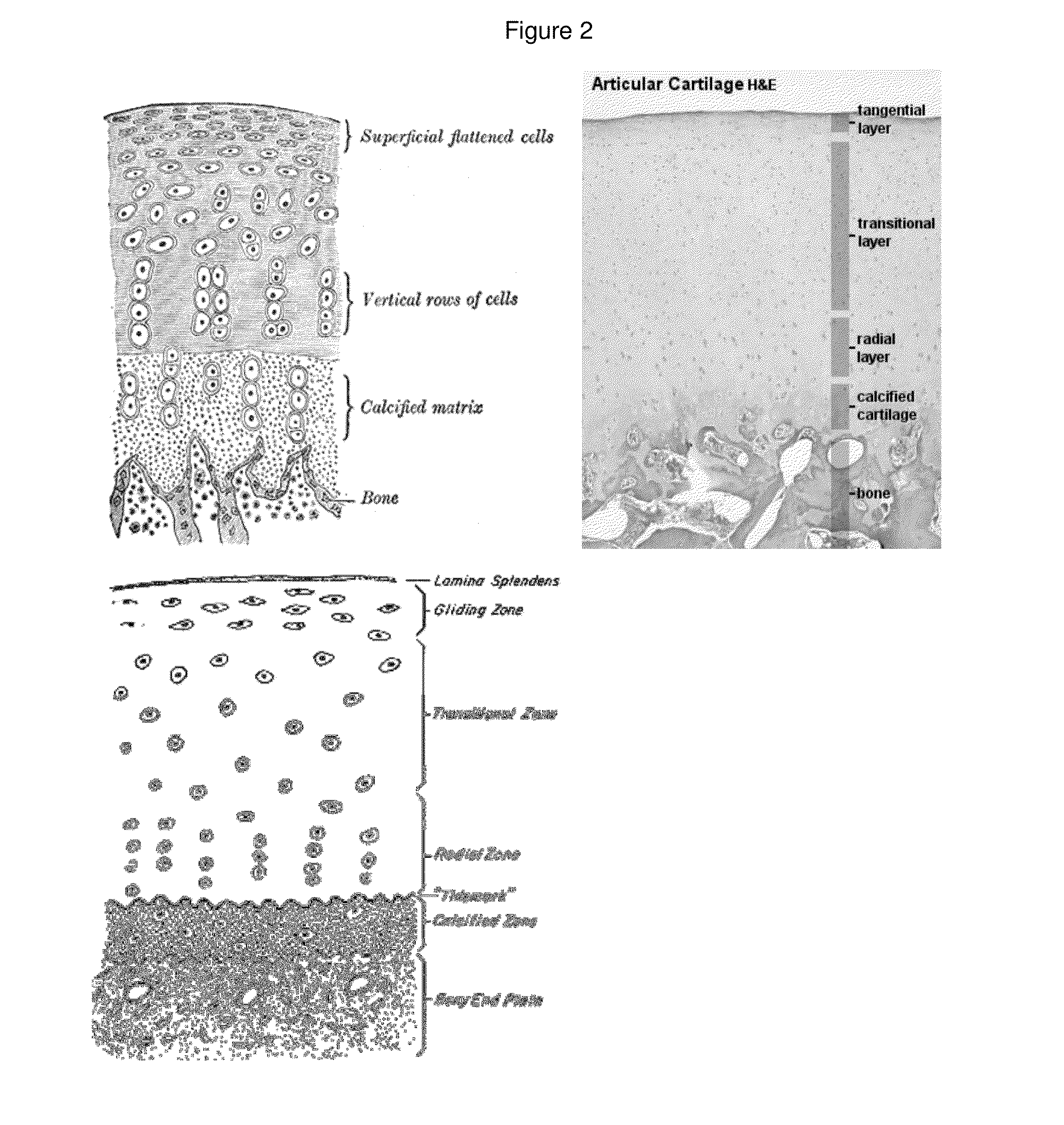
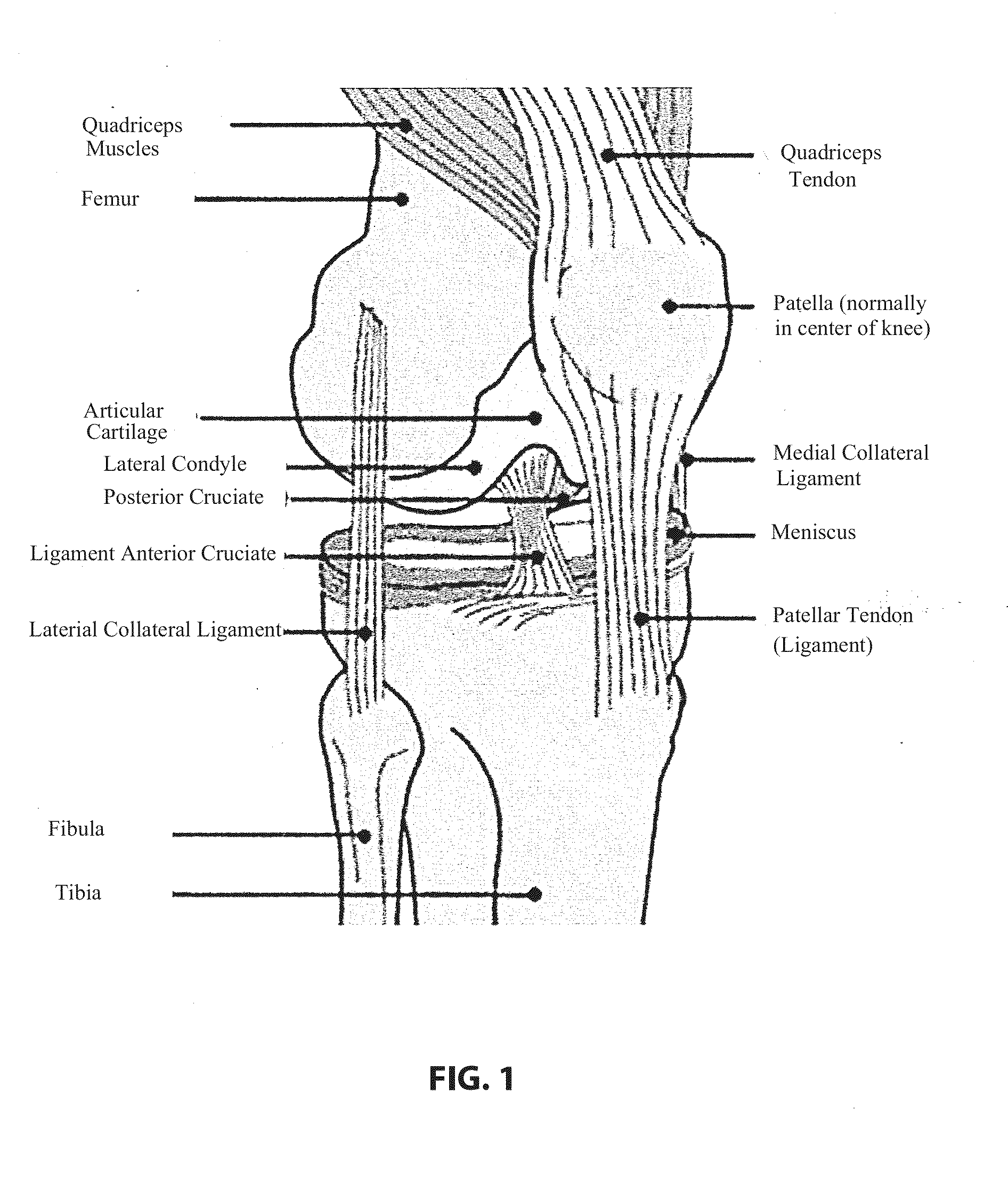
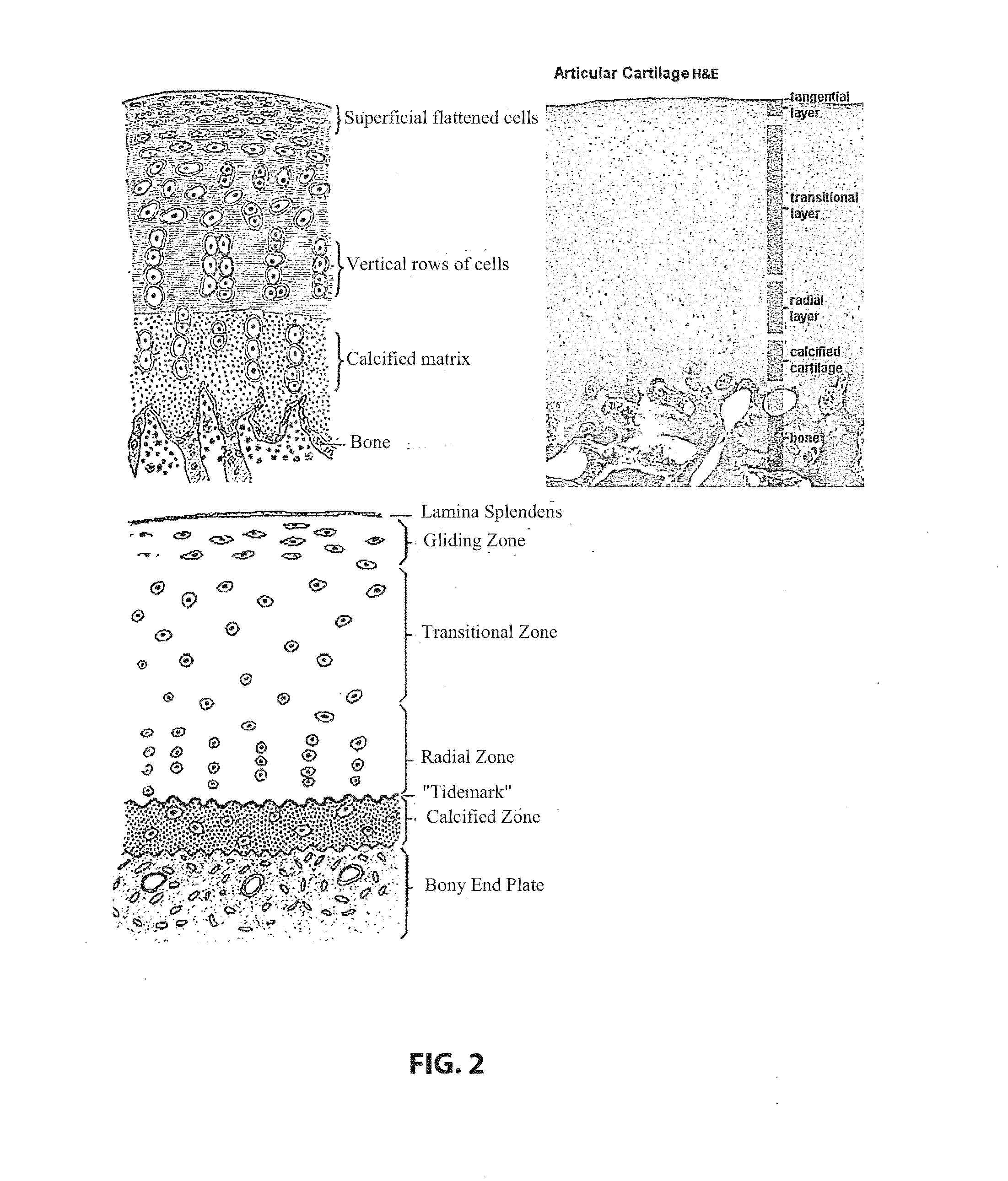
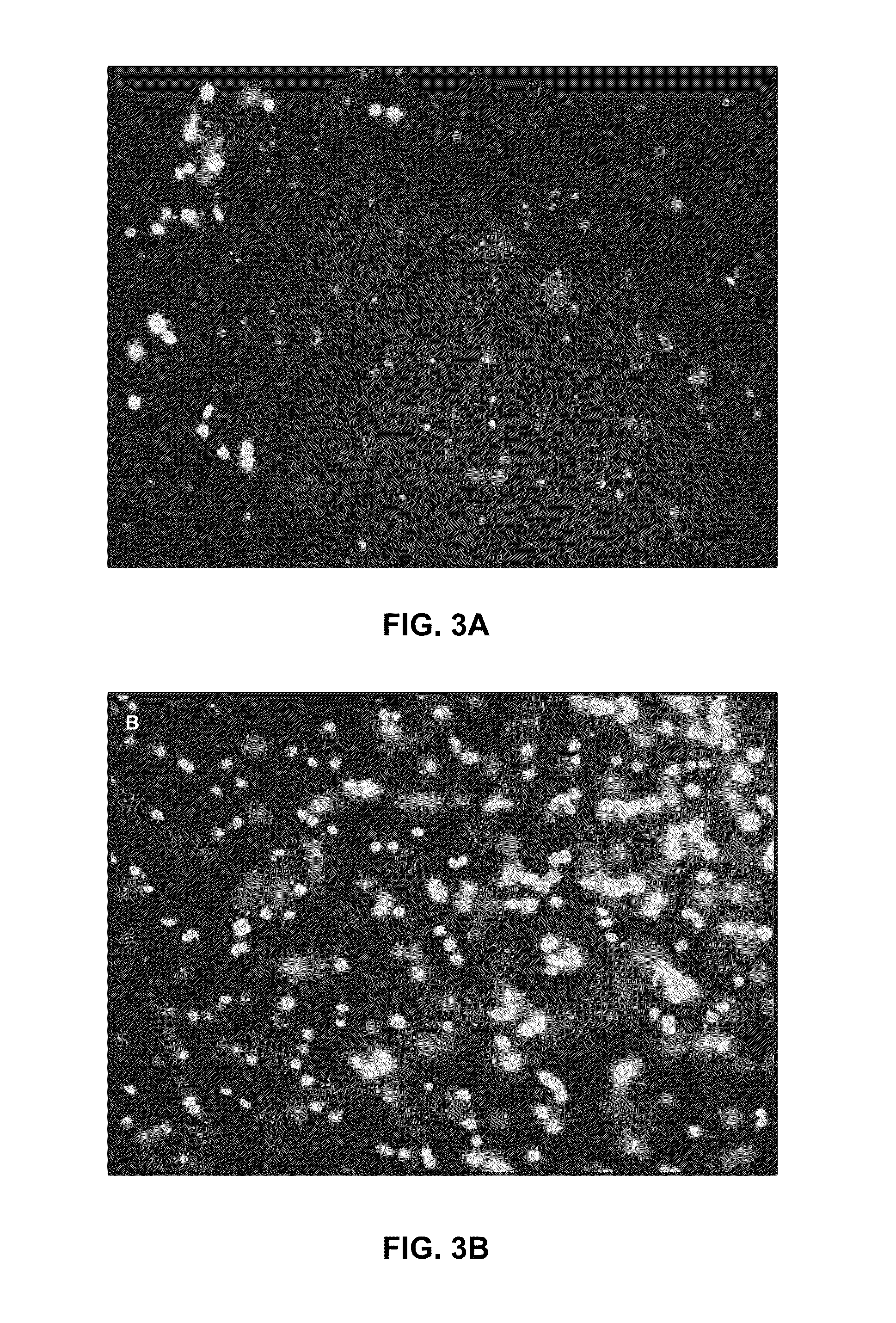
Patents
Literature
144 results about "Chondrogenesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chondrogenesis is the process by which cartilage is developed.
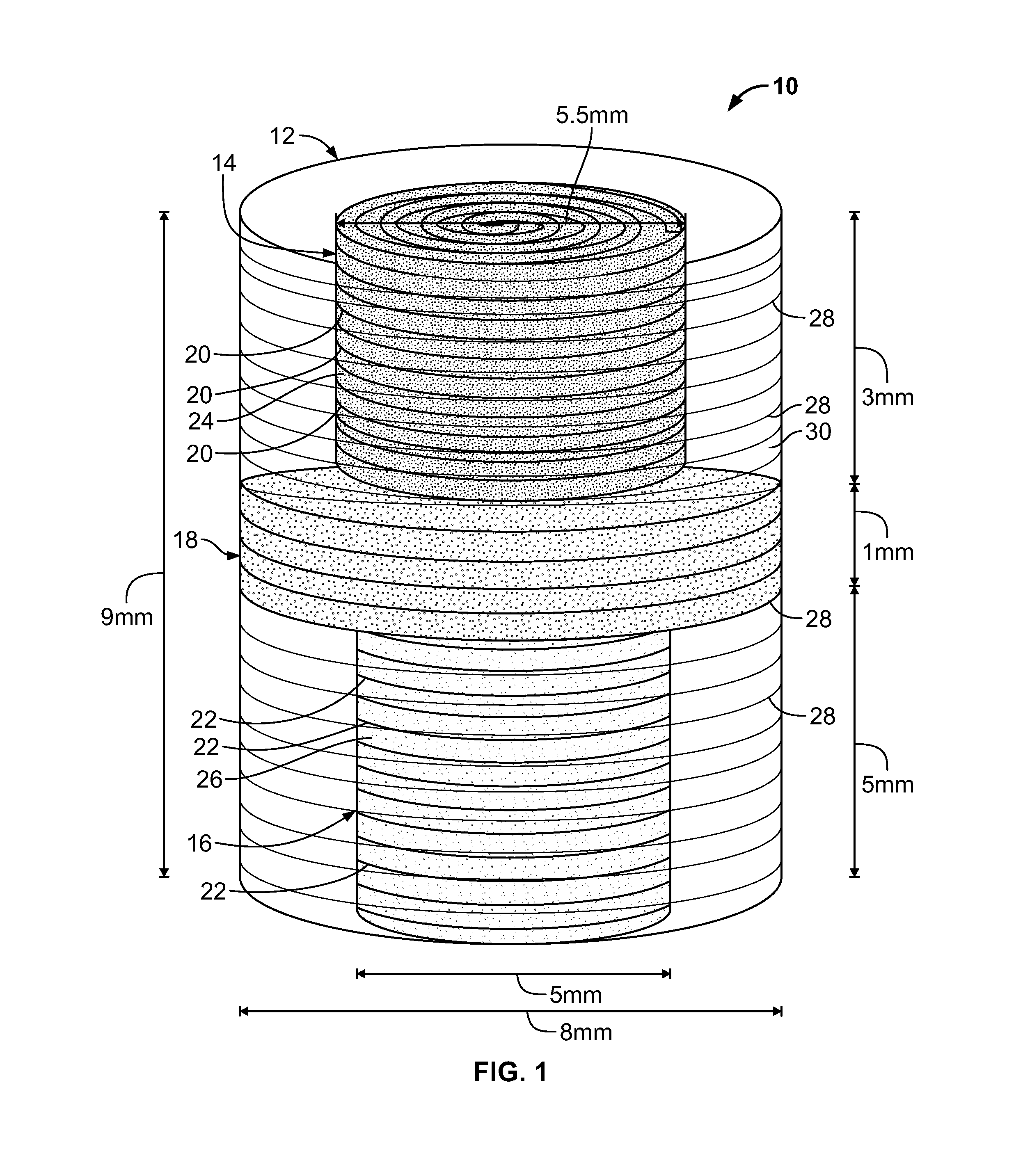
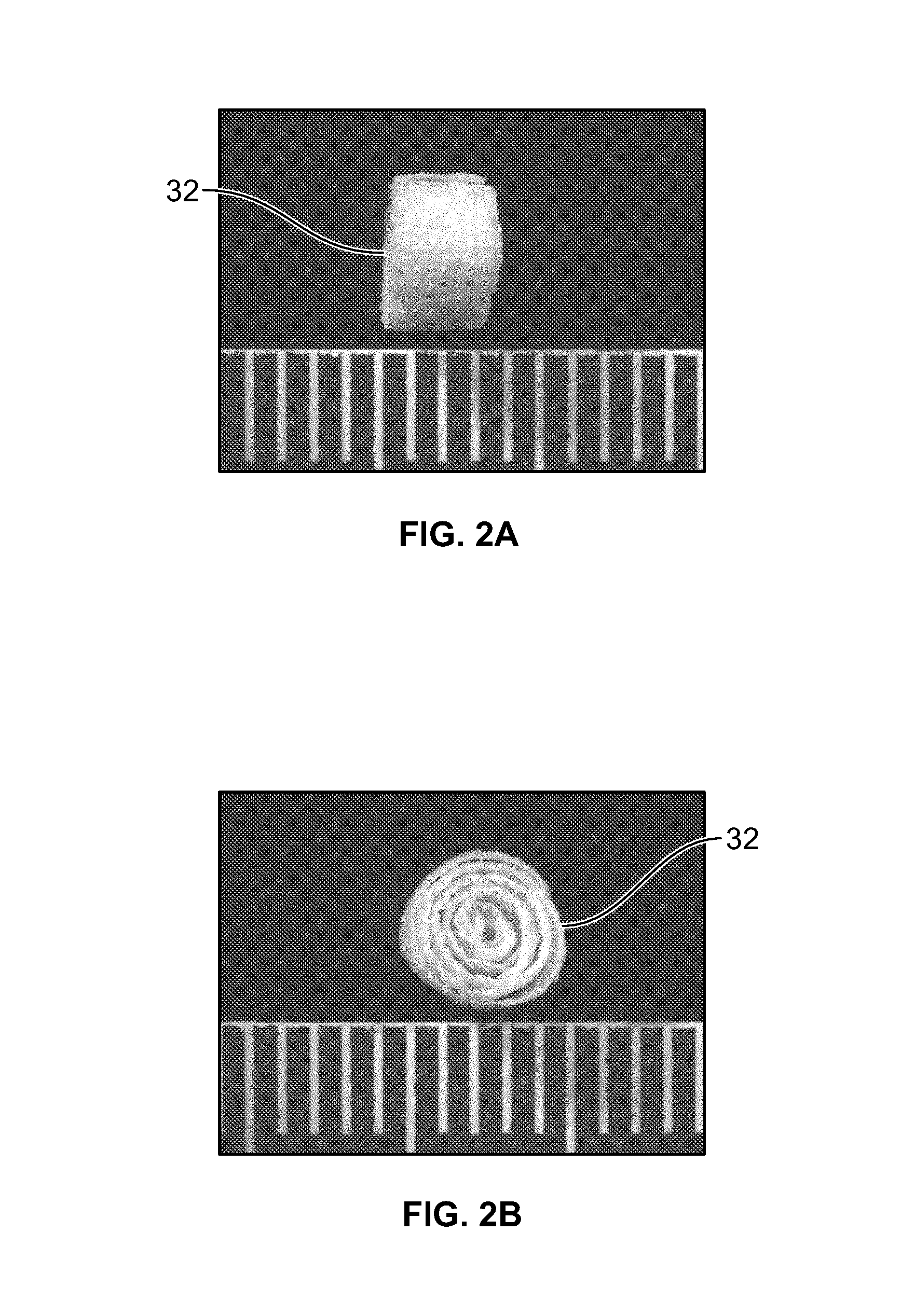
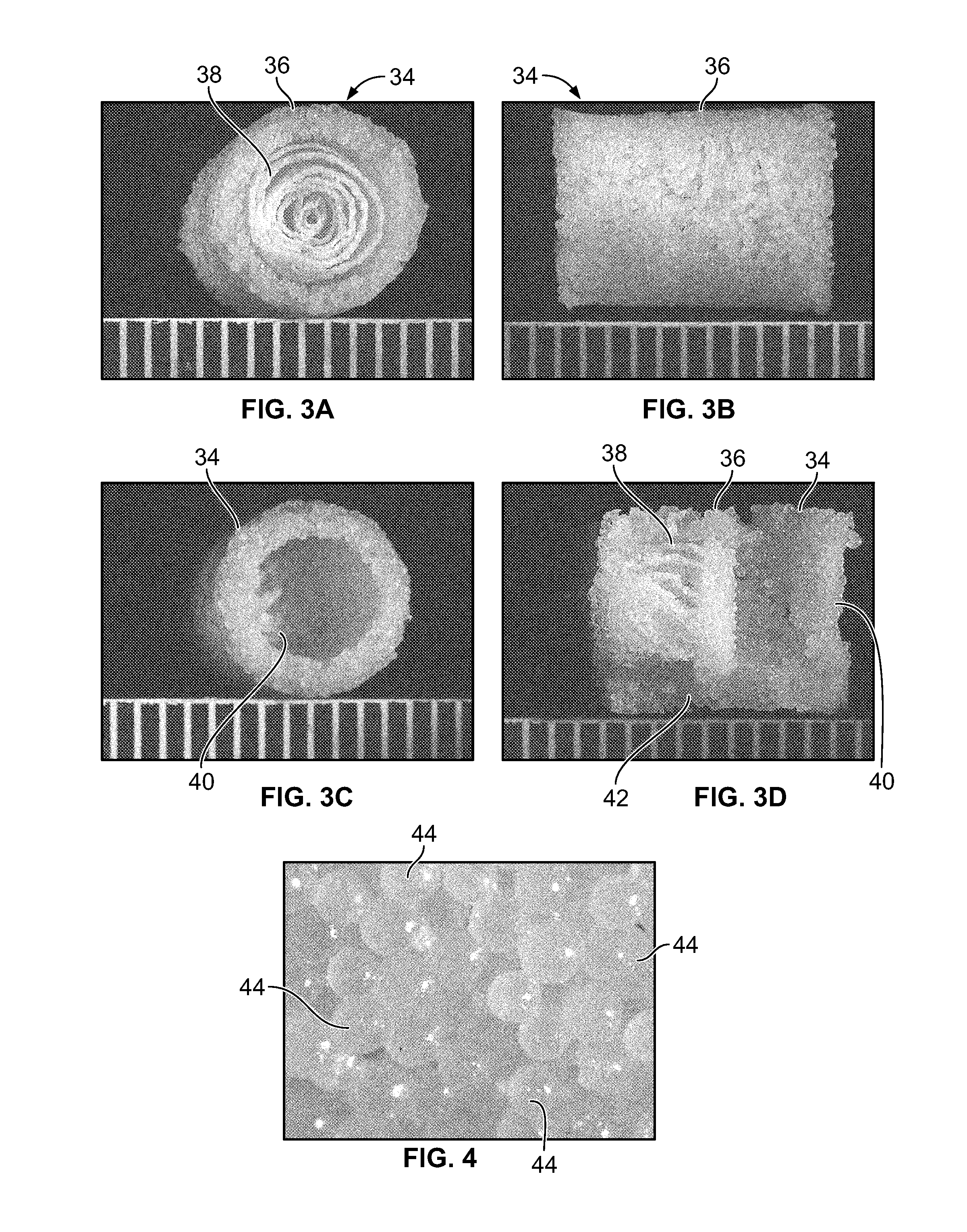
Biphasic osteochondral scaffold for reconstruction of articular cartilage
An osteochondral scaffold has a chondrogenic spiral scaffold in one end of an outer shell made of sintered microspheres, and an osteogenic spiral scaffold in the other end of the outer shell. Each spiral scaffold has nanofibers of a composition selected to promote attachment and proliferation of the desired types of cells. The nanofibers for the chondrogenic spiral scaffold have a different composition than the nanofibers for the osteogenic spiral scaffold. The nanofibers of each spiral scaffold are aligned to orient the attached cells so as to recreate the structure of the native tissue.
Owner:STEVENS INSTITUTE OF TECHNOLOGY
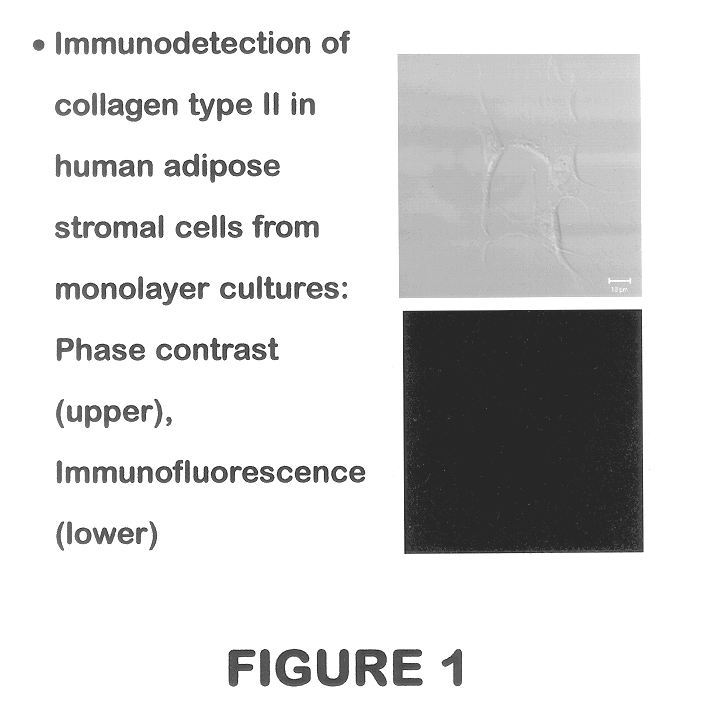
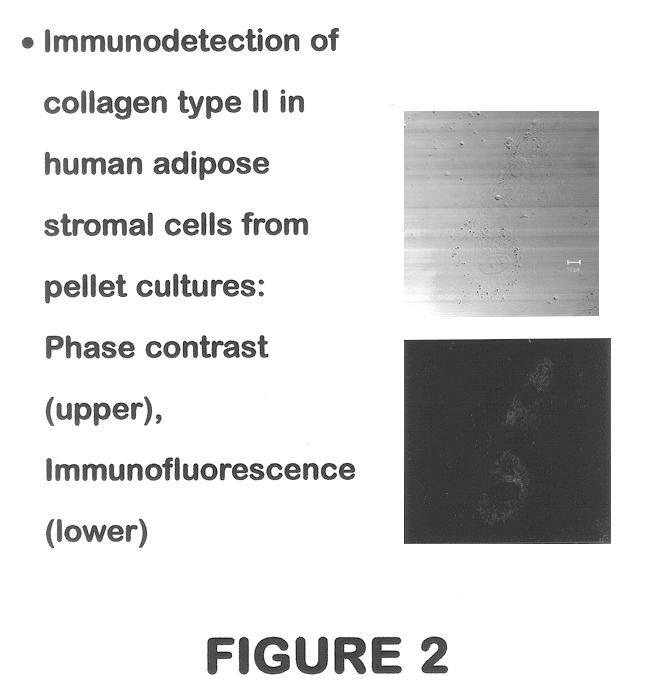
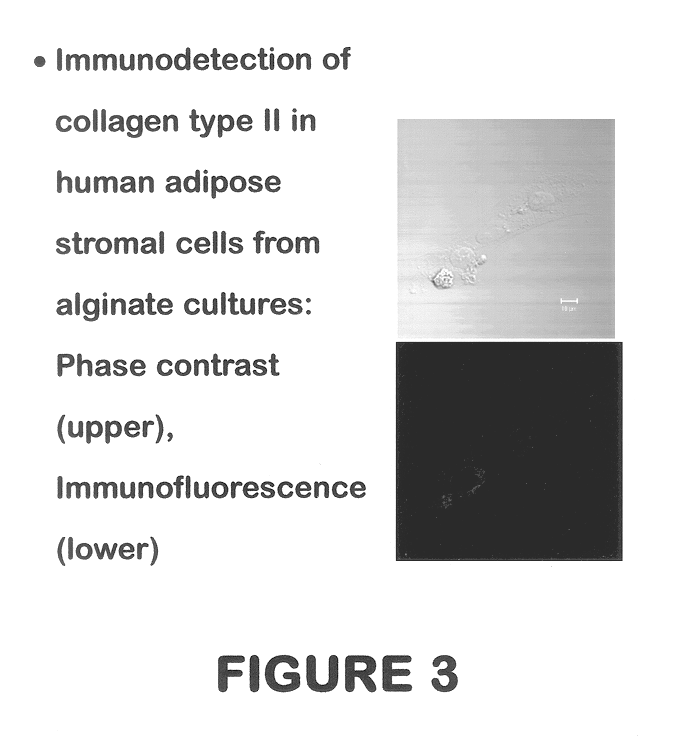
Use of adipose tissue-derived stromal cells for chondrocyte differentiation and cartilage repair
Methods and compositions for directing adipose-derived stromal cells cultivated in vitro to differentiate into cells of the chondrocyte lineage are disclosed. The invention further provides a variety of chondroinductive agents which can be used singly or in combination with other nutrient components to induce chondrogenesis in adipose-derived stromal cells either in cultivating monolayers or in a biocompatible lattice or matrix in a three-dimensional configuration. Use of the differentiated chondrocytes for the therapeutic treatment of a number of human conditions and diseases including repair of cartilage in vivo is disclosed.
Owner:COGNATE BIOSERVICES
Cartilage and bone repair and regeneration using postpartum-derived cells
Cells derived from postpartum tissue and methods for their isolation and induction to differentiate to cells of a chondrogenic or osteogenic phenotype are provided by the invention. The invention further provides cultures and compositions of the postpartum-derived cells and products related thereto. The postpartum-derived cells of the invention and products related thereto have a plethora of uses, including but not limited to research, diagnostic, and therapeutic applications, for example, in the treatment of bone and cartilage conditions.
Owner:DEPUY SYNTHES PROD INC
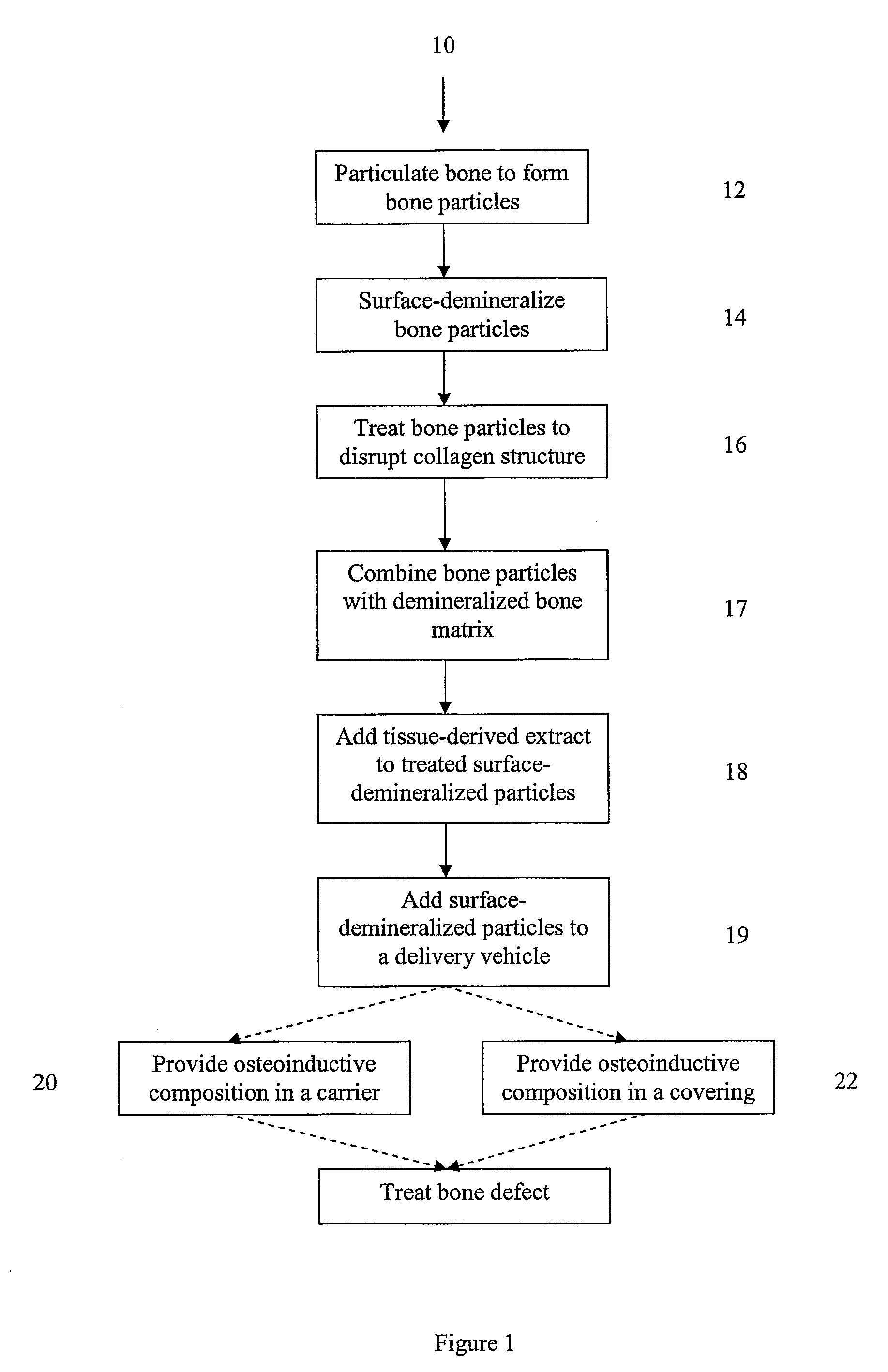
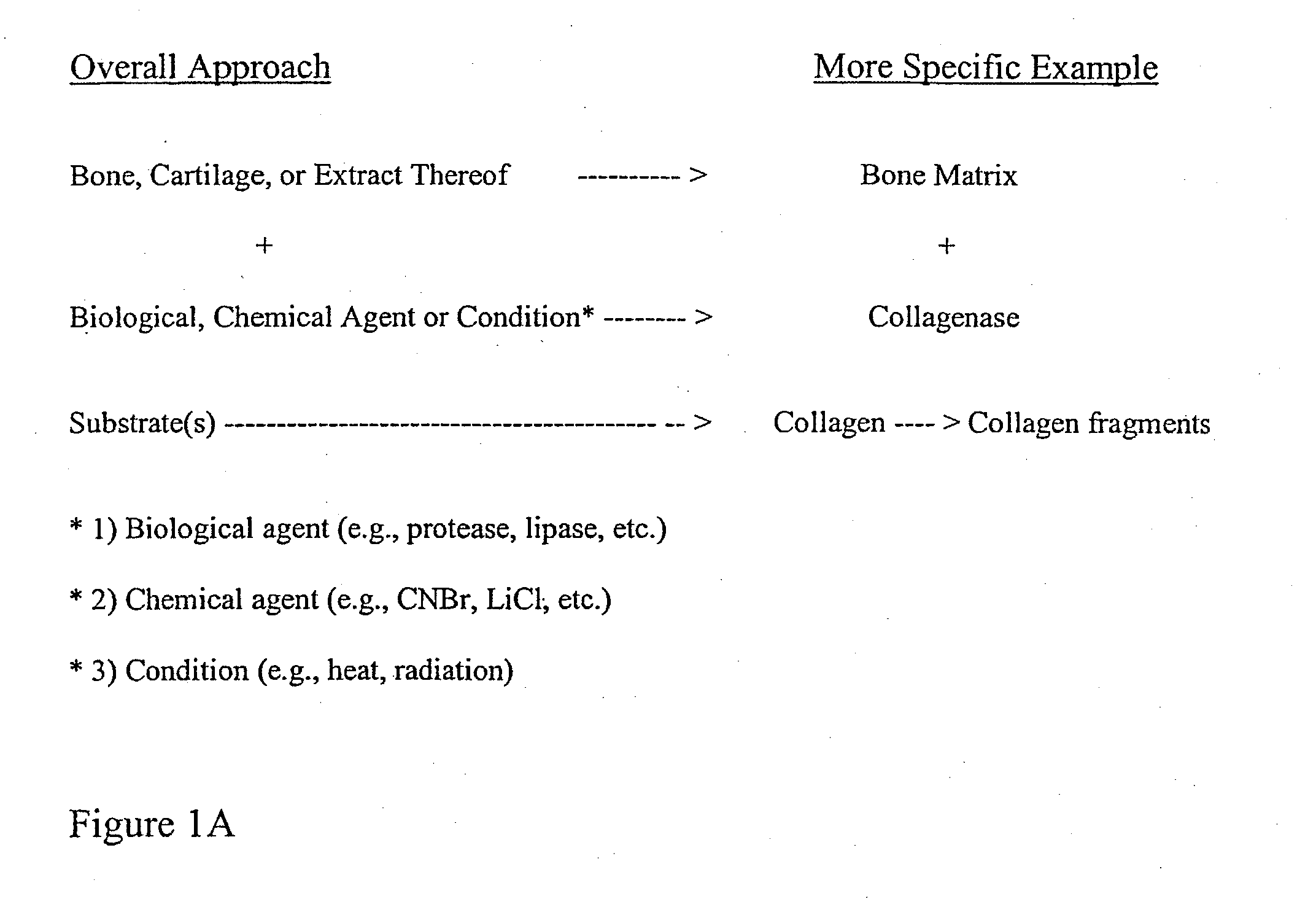
Bone matrix compositions and methods
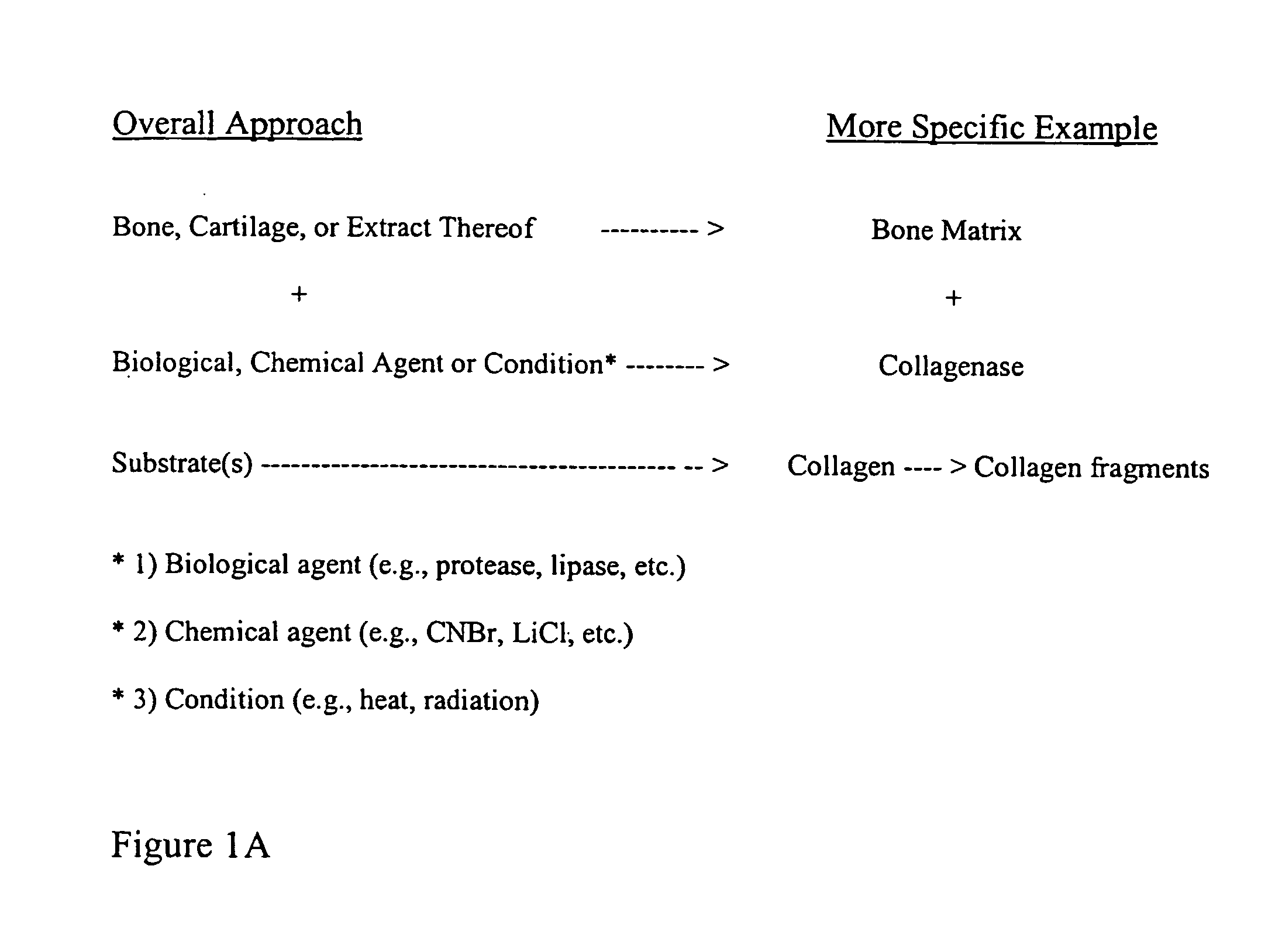
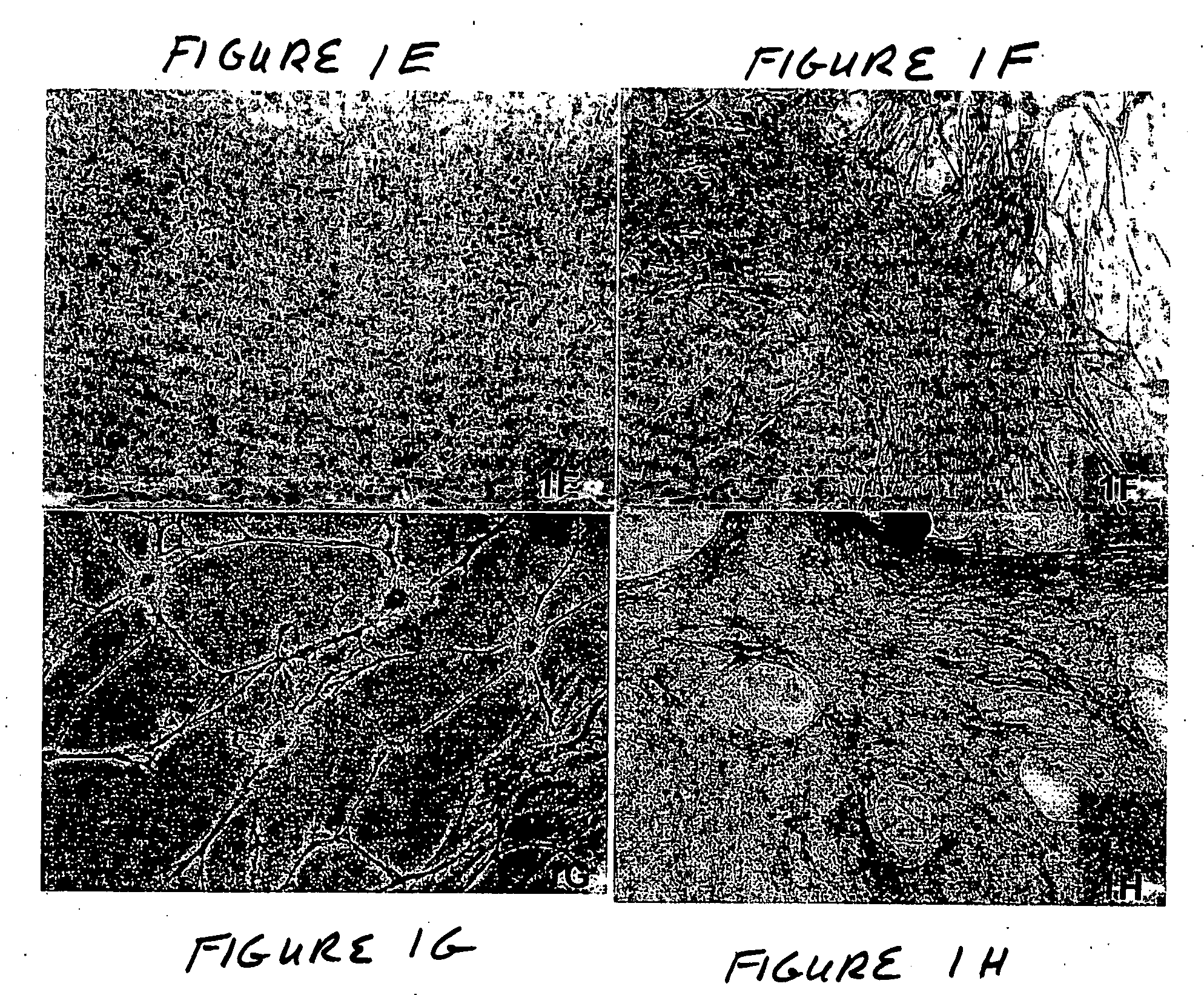
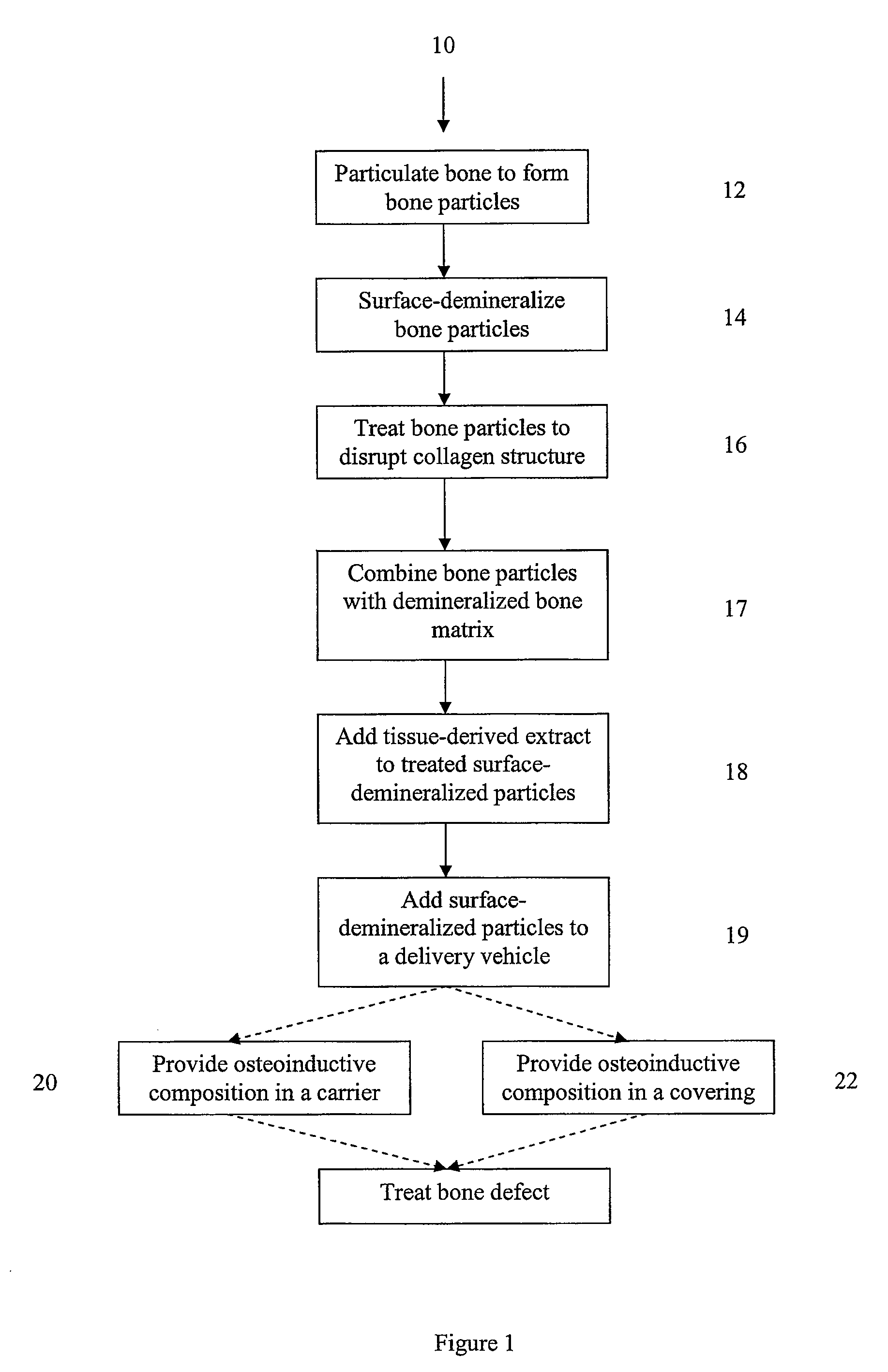
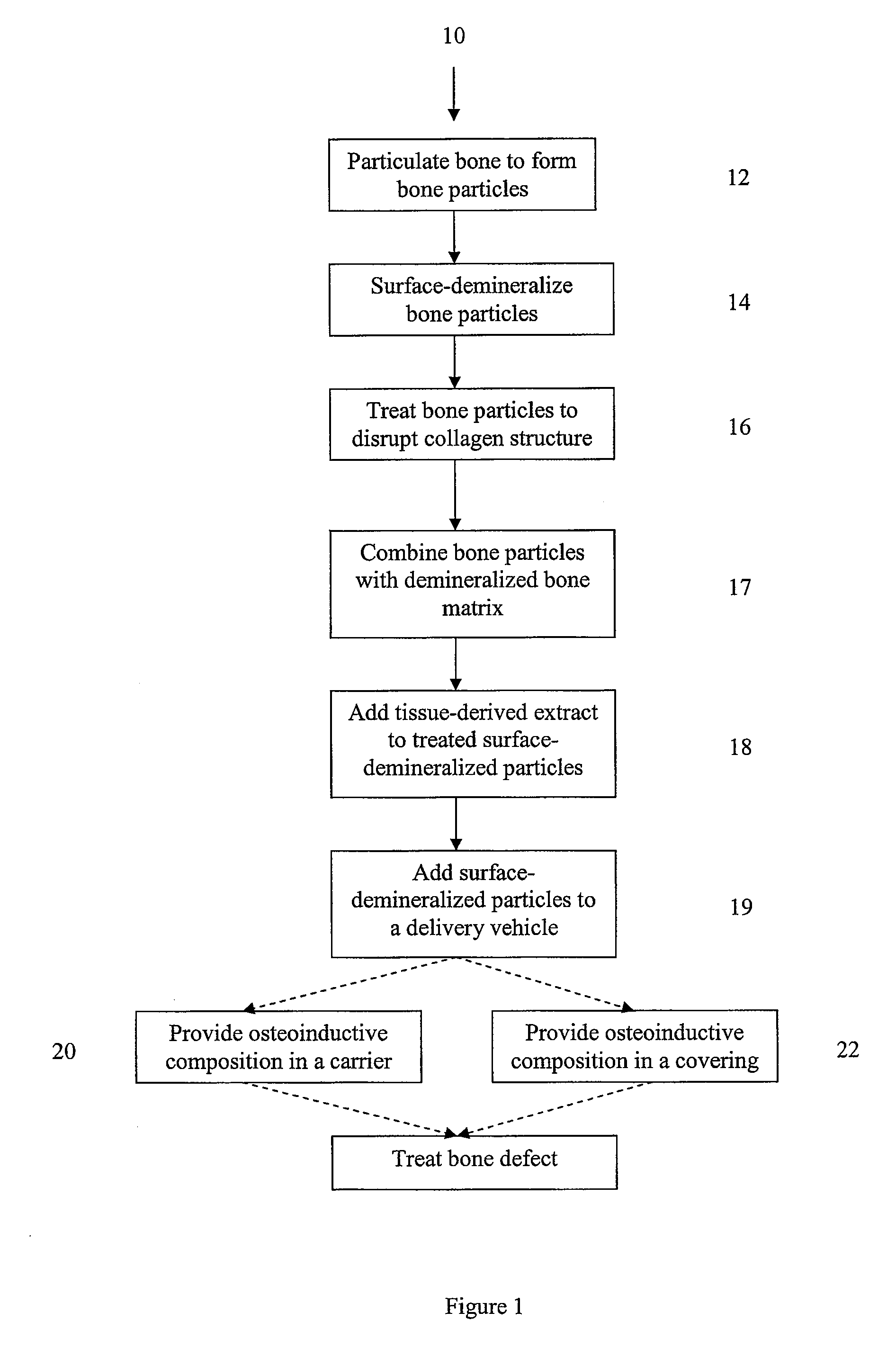
Osteoinductive compositions and implants having increased biological activities, and methods for their production, are provided. The biological activities that may be increased include, but are not limited to, bone forming; bone healing; osteoinductive activity, osteogenic activity, chondrogenic activity, wound healing activity, neurogenic activity, contraction-inducing activity, mitosisinducing activity, differentiation-inducing activity, chemotactic activity, angiogenic or vasculogenic activity, and exocytosis or endocytosis-inducing activity. In one embodiment, a method for producing an osteoinductive composition comprises providing partially demineralized bone, treating the partially demineralized bone to disrupt the collagen structure of the bone, and optionally providing a tissue-derived extract and adding the tissue-derived extract to the partially demineralized bone. In another embodiment, an implantable osteoinductive and osteoconductive composition comprises partially demineralized bone, wherein the collagen structure of the bone has been disrupted, and, optionally, a tissue-derived extract.
Owner:WARSAW ORTHOPEDIC INC
Production of tissue engineered digits and limbs
ActiveUS20060257377A1Enhance tissue maturationImprove functionalityBiocidePeptide/protein ingredientsBone formingTissue construct
The invention pertains to methods of producing artificial composite tissue constructs that permit coordinated motion. Biocompatable structural matrices having sufficient rigidity to provide structural support for cartilage-forming cells and bone-forming cells are used. Biocompatable flexible matrices seeded with muscle cells are joined to the structural matrices to produce artificial composite tissue constructs that are capable of coordinated motion.
Owner:WAKE FOREST UNIV HEALTH SCI INC
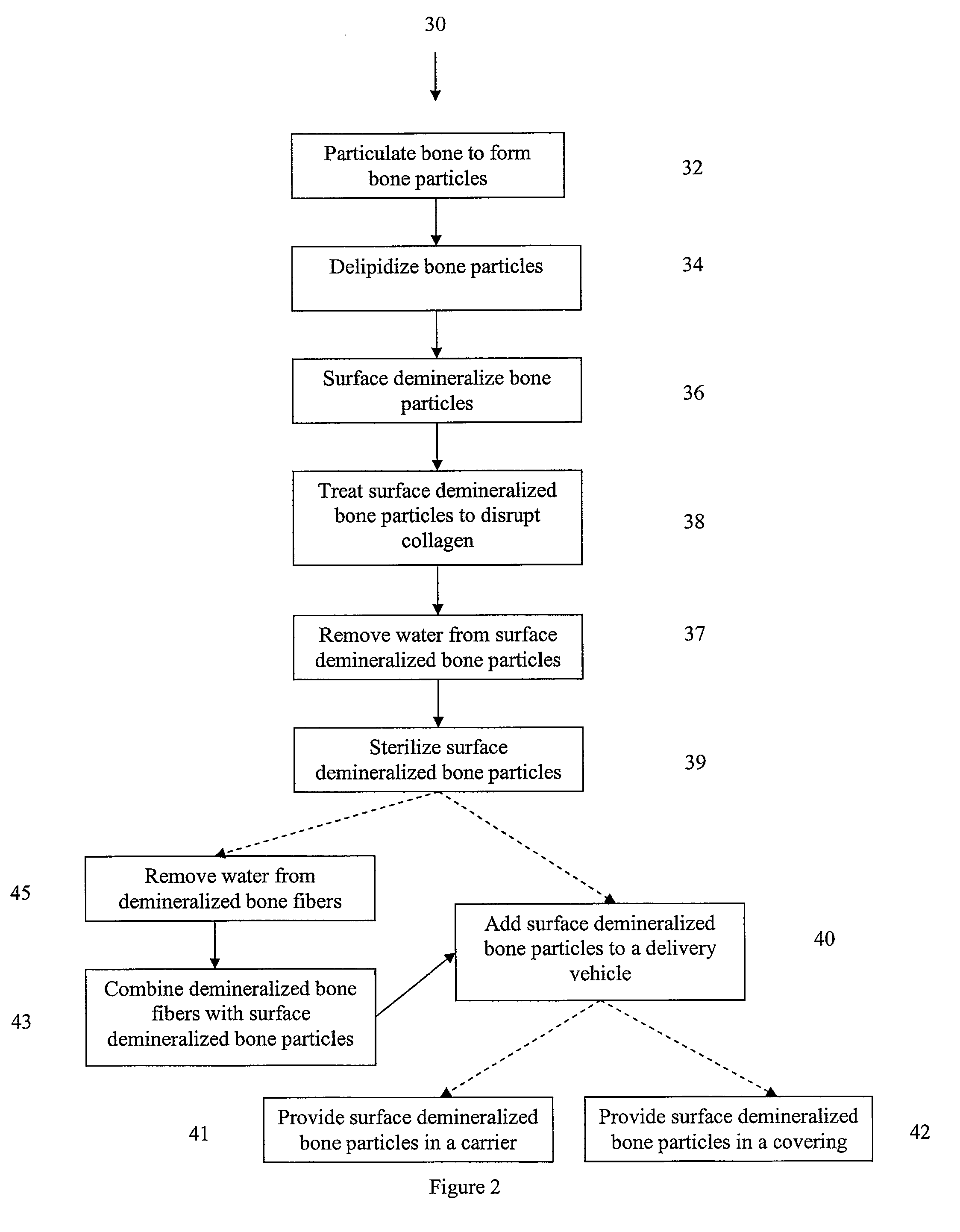
Bone matrix compositions and methods
ActiveUS20070154563A1Good osteoinductivityHigh activityHydrolysed protein ingredientsBone implantOsteoblastLine of therapy
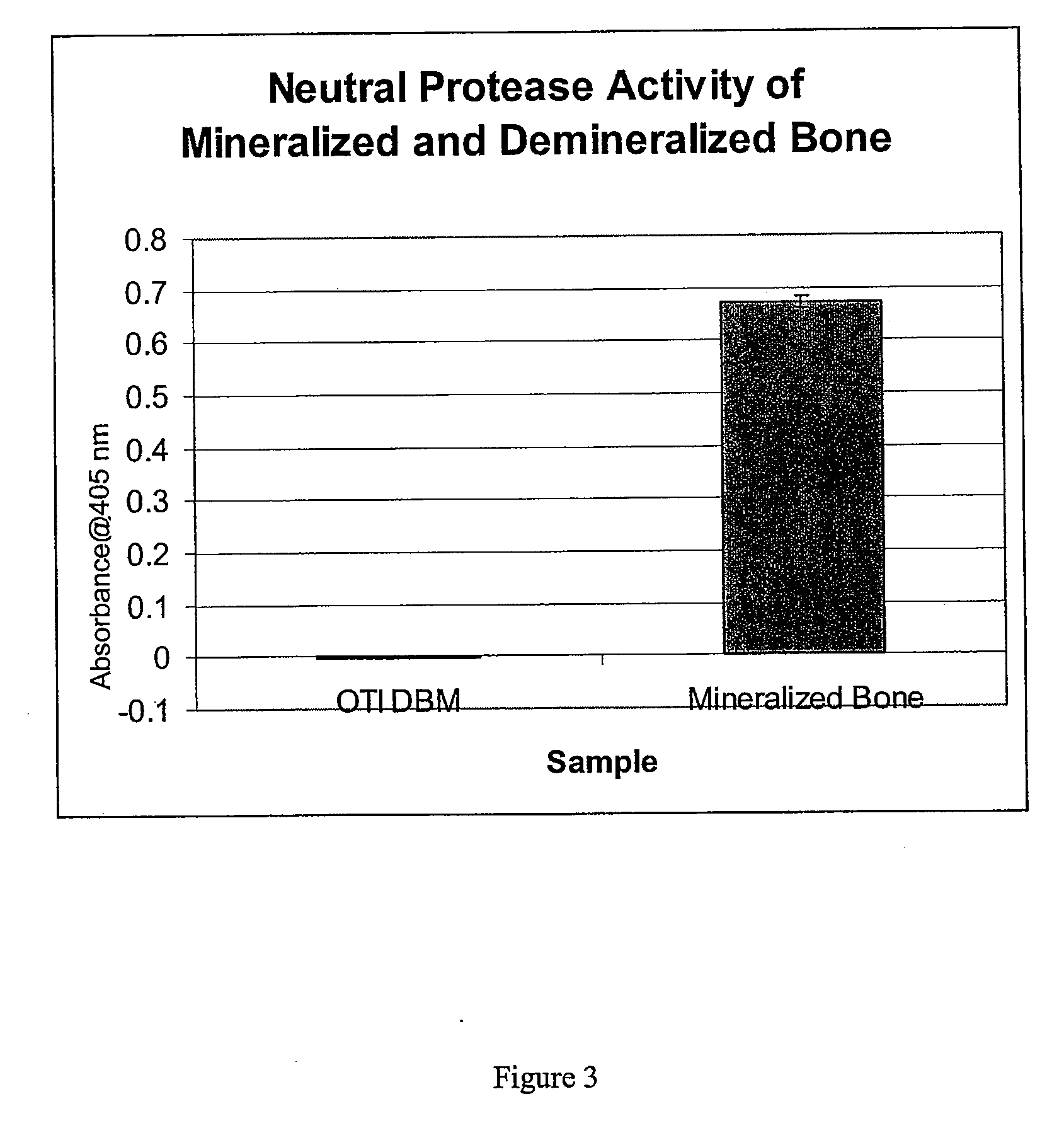
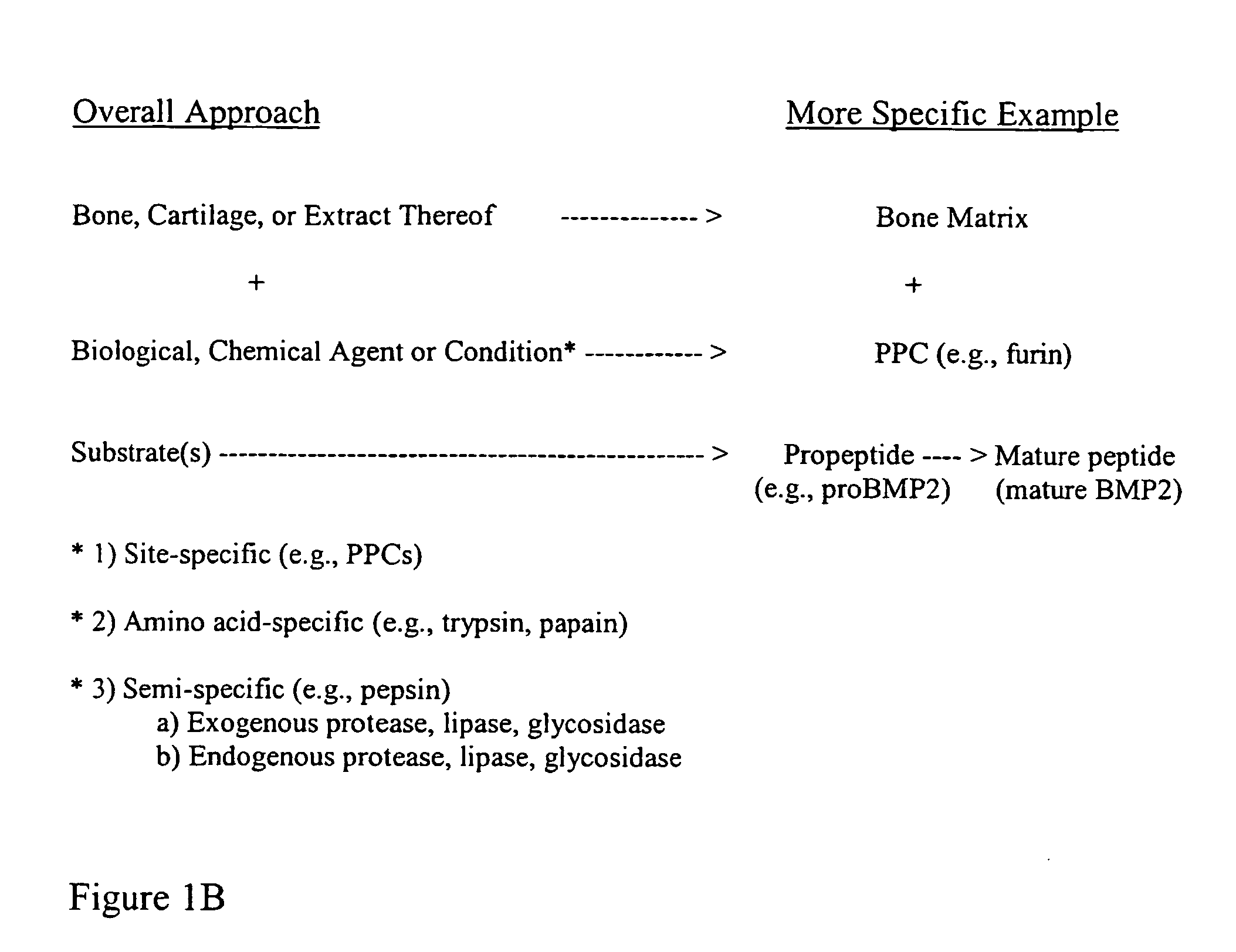
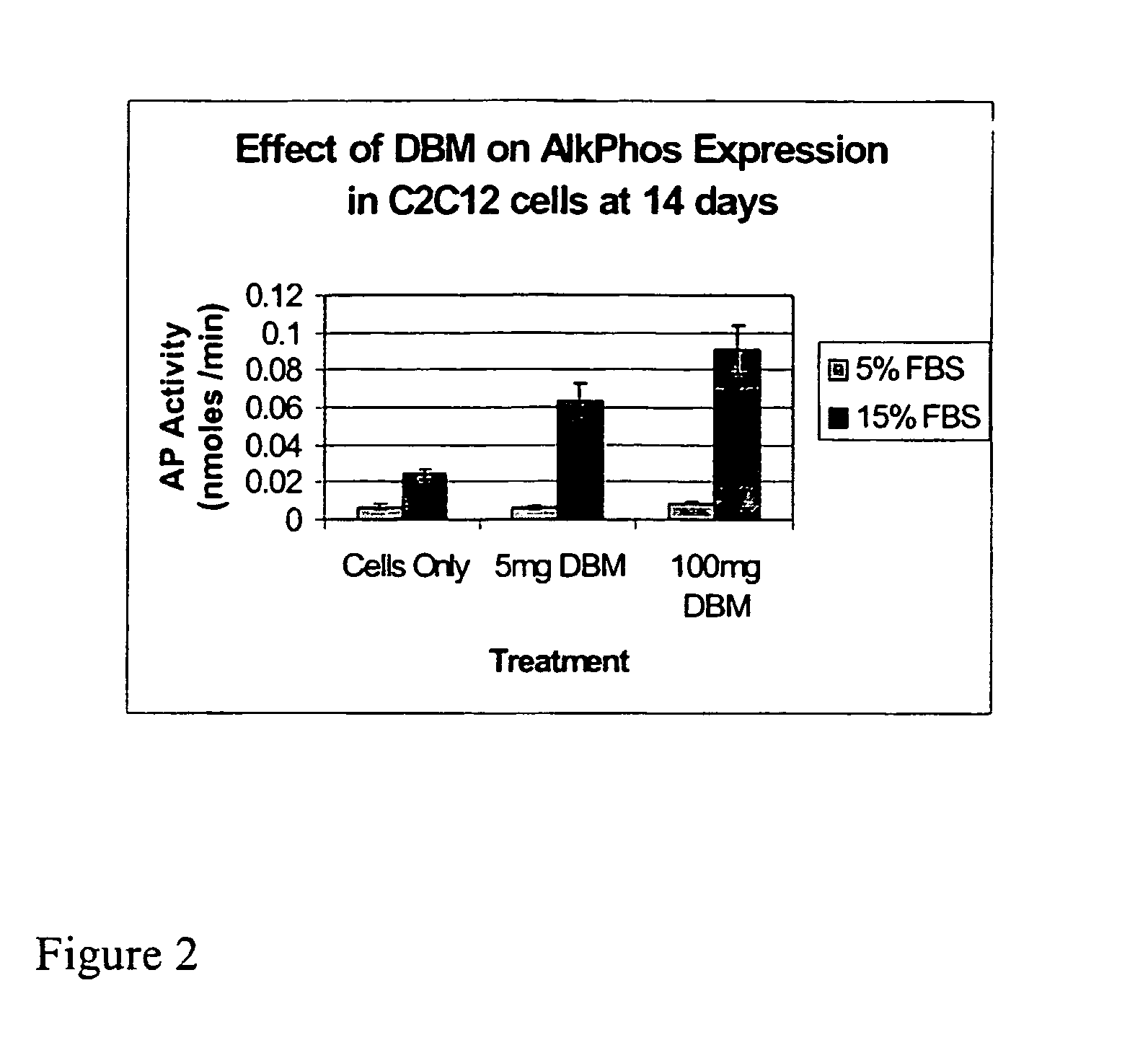
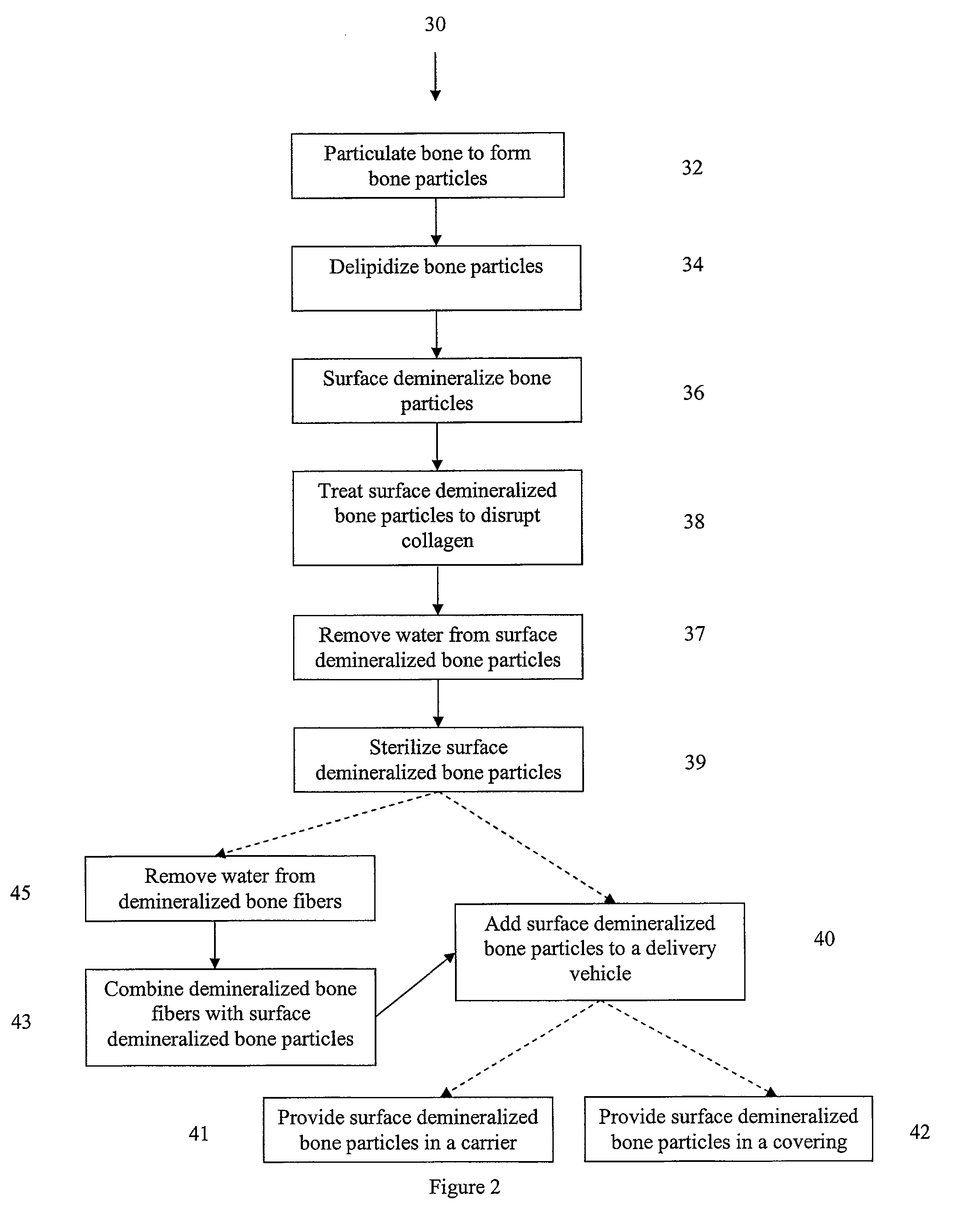
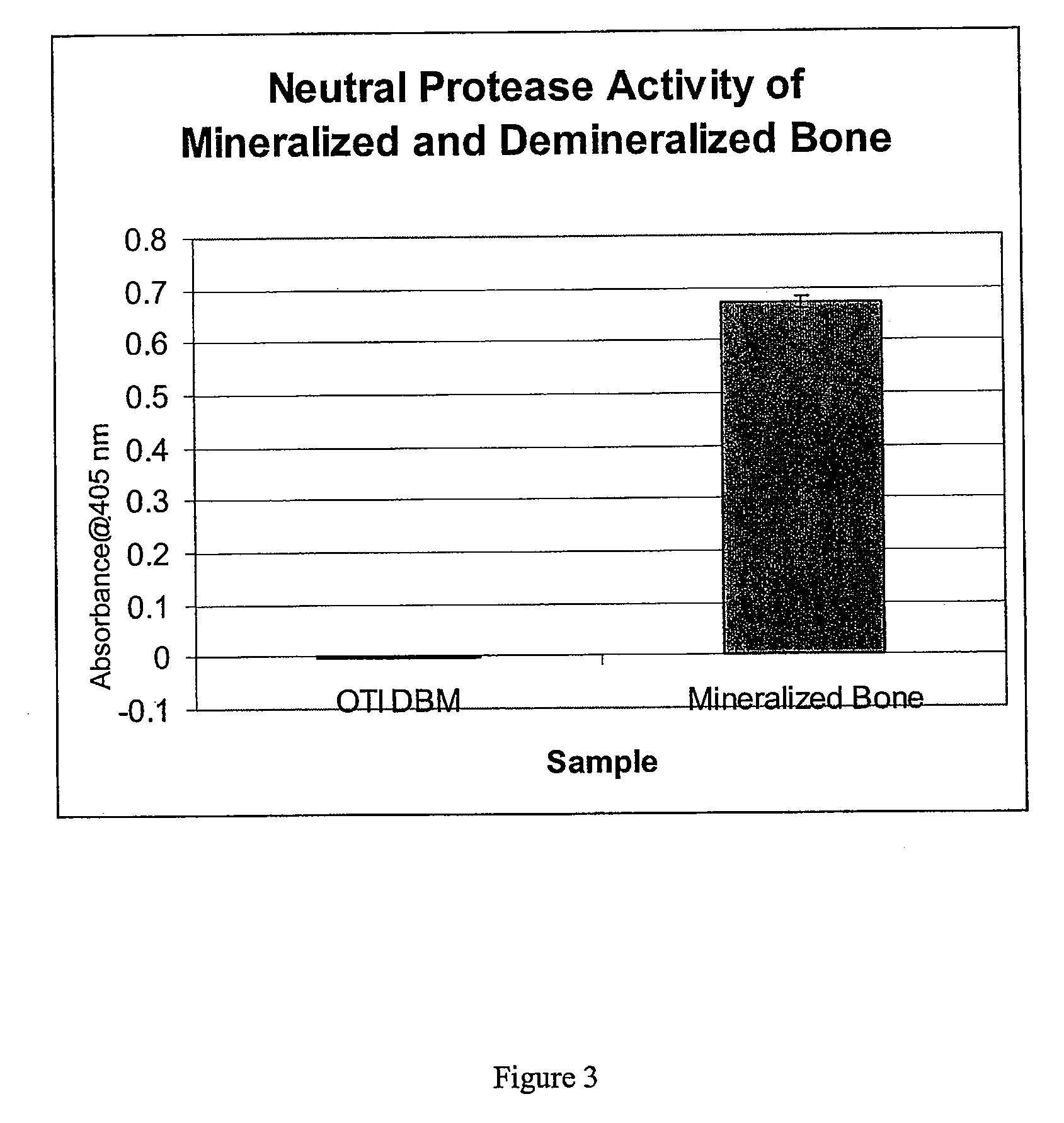
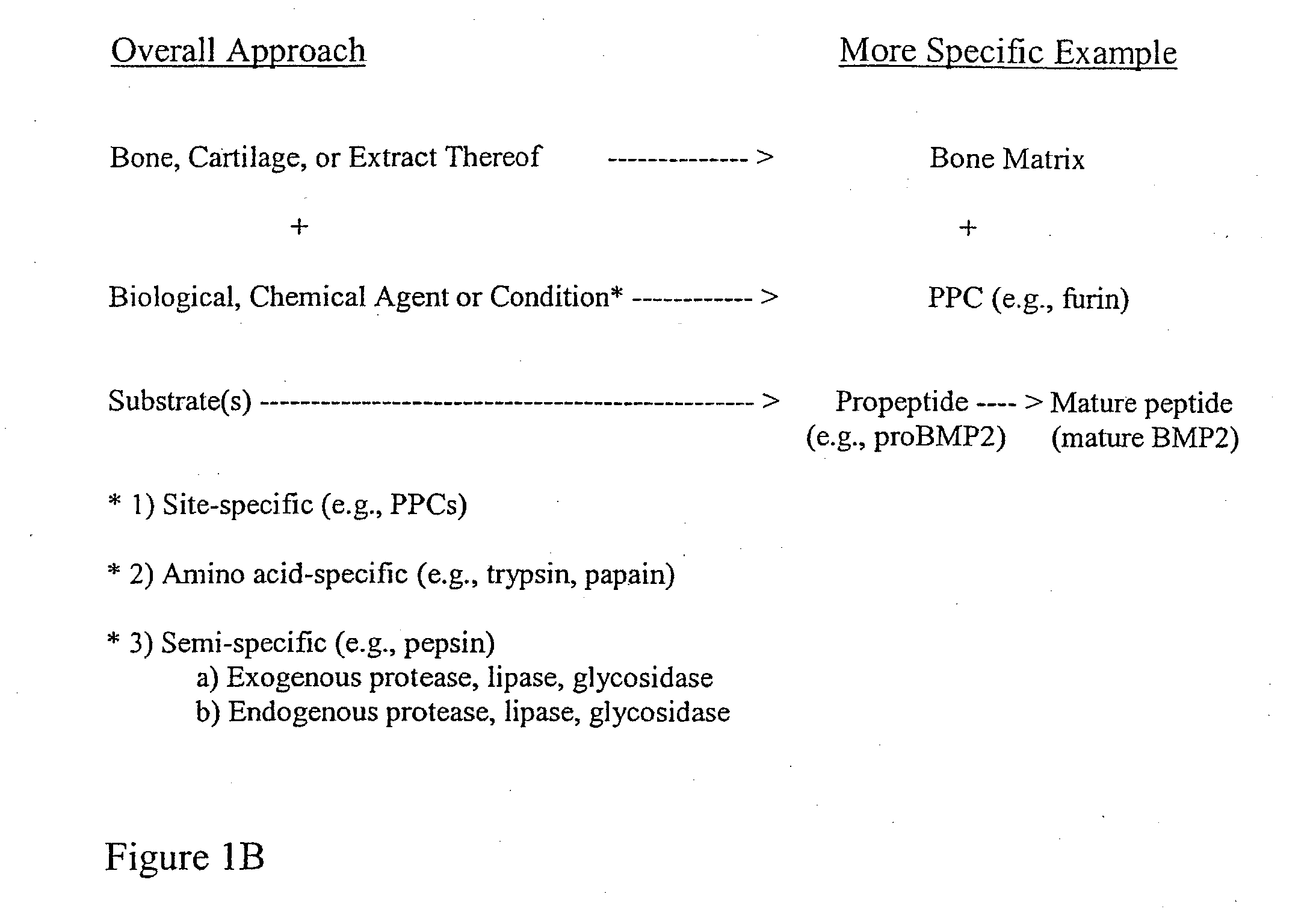
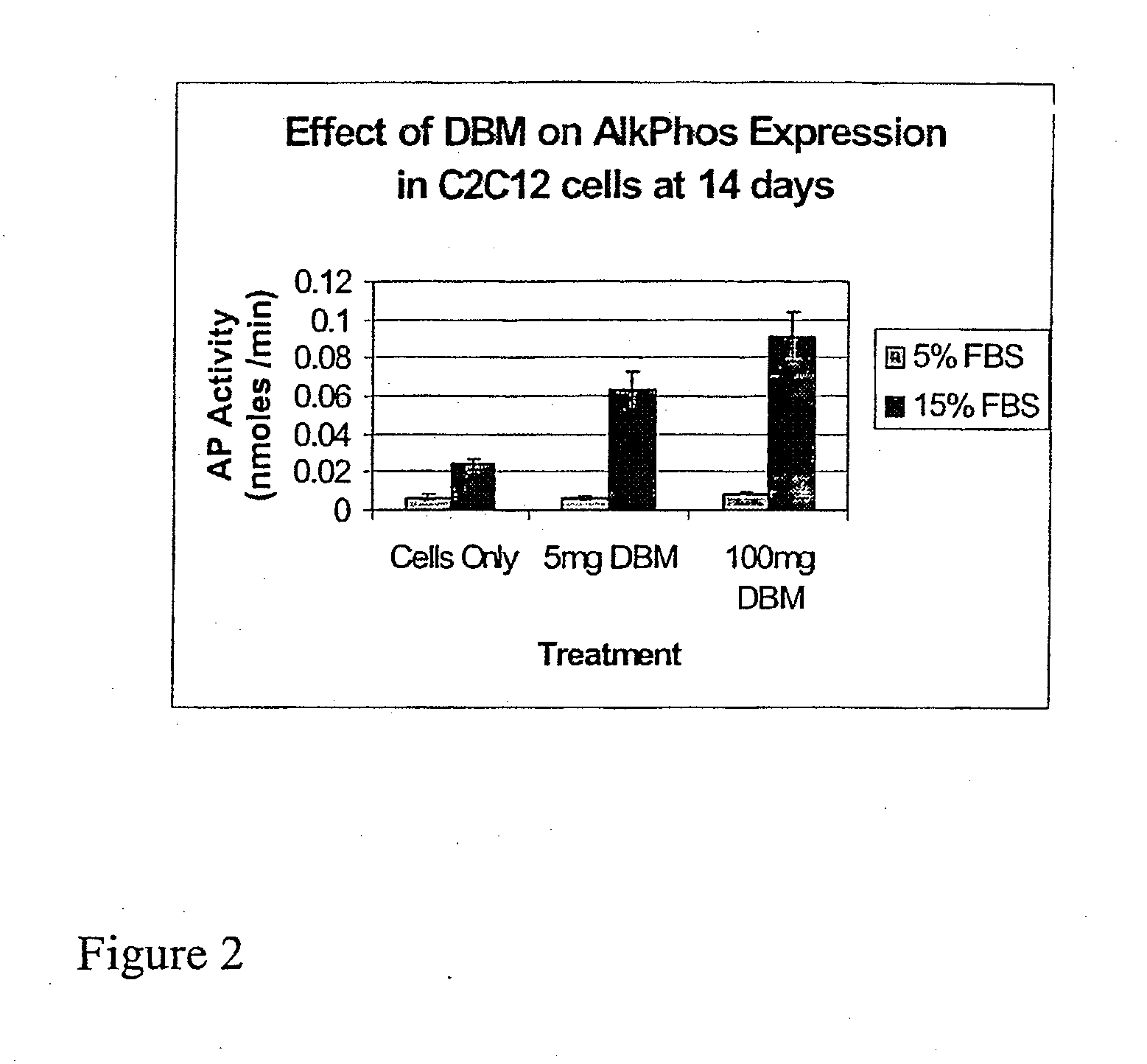
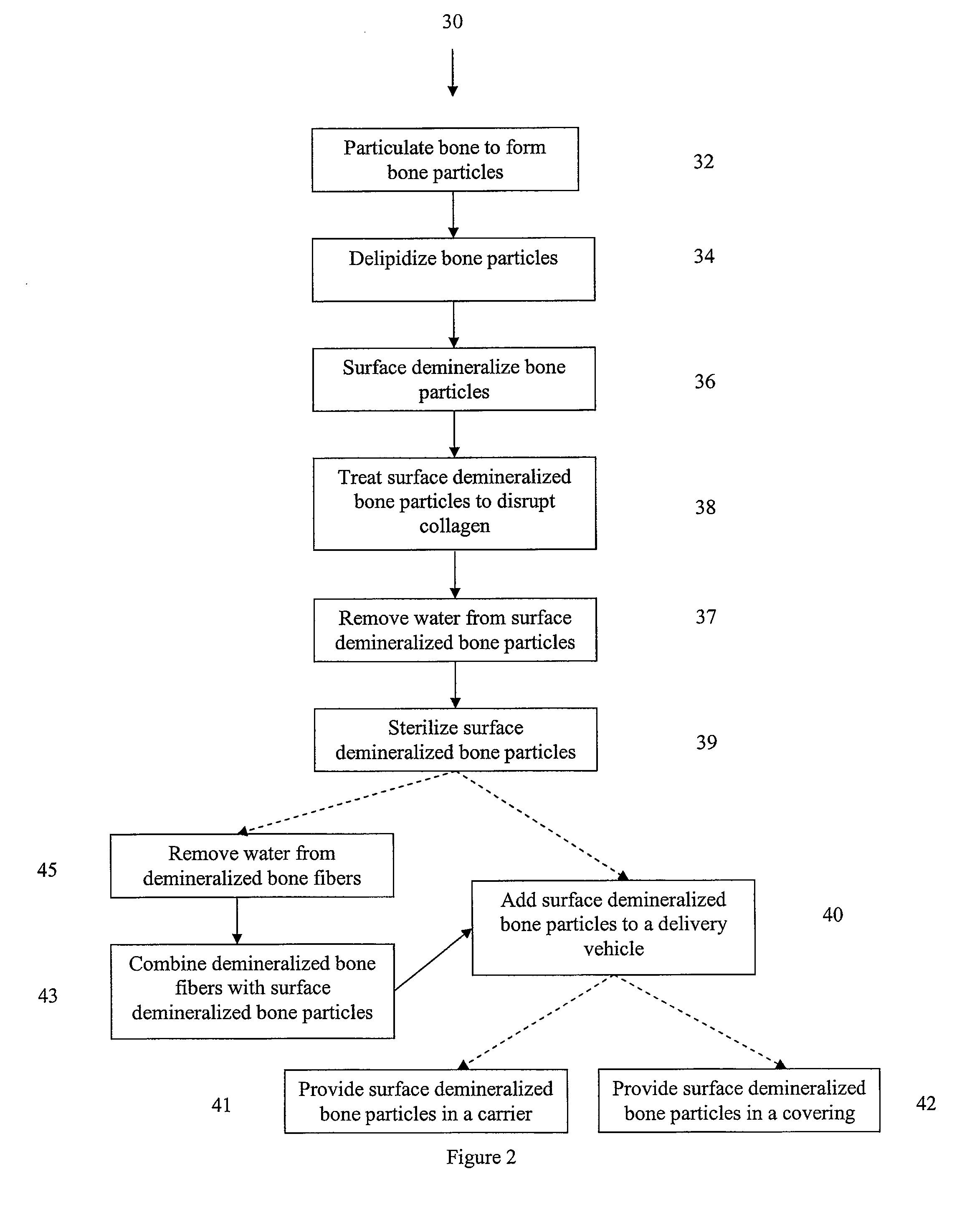
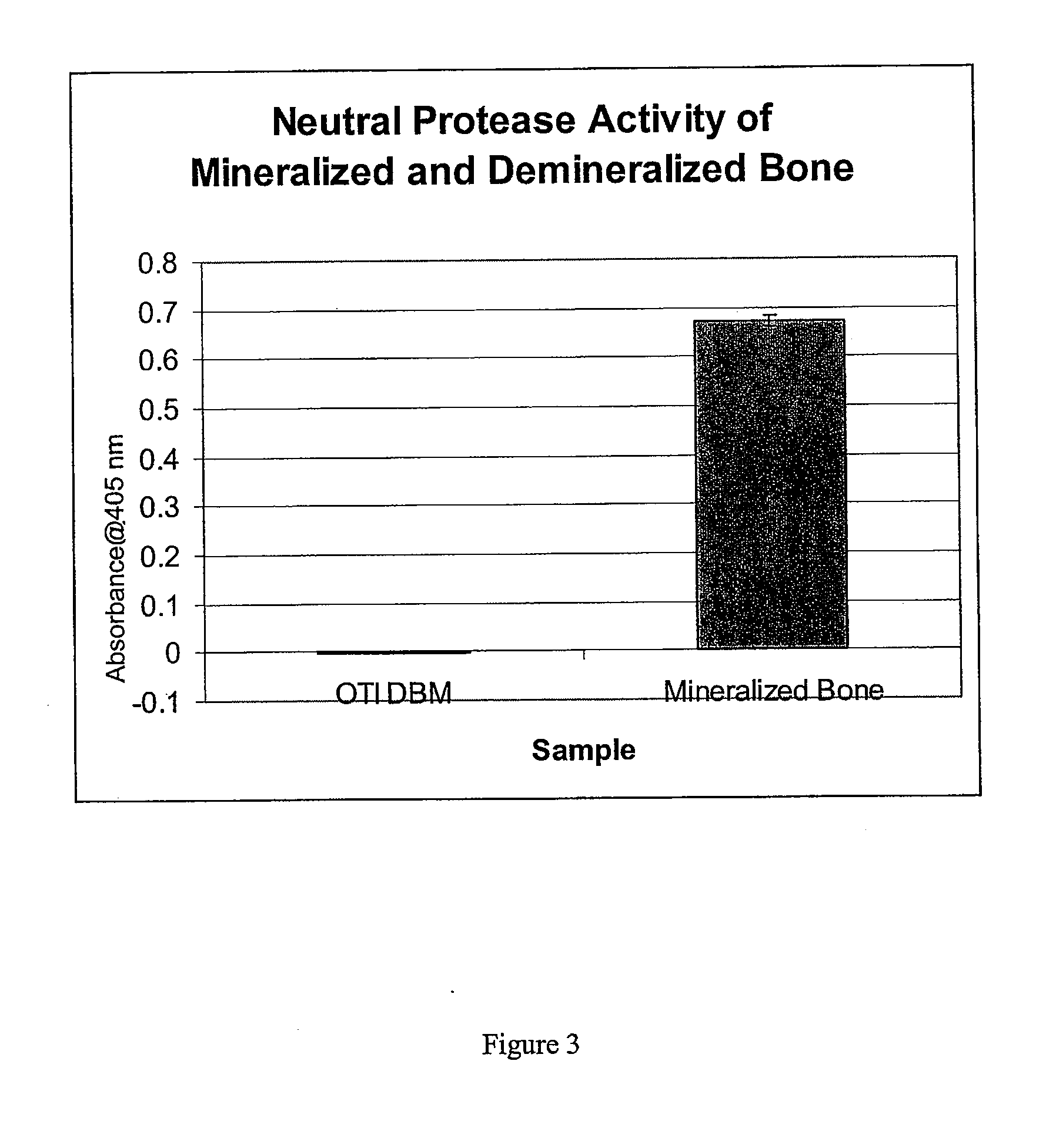
The present invention provides methods of improving the osteogenic and / or chondrogenic activity of a bone matrix, e.g., a dermineralized bone matrix (DBM), by exposing the bone matrix to one or more treatments or conditions. In preferred embodiments the bone matrix is derived from human bone. The treatment or condition may alter the structure of the bone matrix and / or cleave one or more specific proteins. Cleavage may generate peptides or protein fragments that have osteoinductive, osteogenic, or chondrogenic activity. Preferred treatments include collagenase and various other proteases. The invention further provides improved bone and cartilage matrix compositions that have been prepared according to the inventive methods and methods of treatment using the compositions. The invention further provides methods of preparing, testing, and using the improved bone matrix compositions. Ona assay comprises exposing relatively undifferentiated mesenchymal cells to a bone matrix composition and measuring expression of a marker characteristic of osteoblast or chondrocyte lineage(s). Increased expression of the marker relative to the level of the marker in cells that have been exposed to a control matrix (e.g., an inactivated or untreated matrix) indicates that the treatment or condition increased the osteogenic and / or chondrogenic activity of the bone matrix. Suitable cells include C2C12 cells. A suitable marker is alkaline phosphatase. The inventive methods increase the osteogenic and / or chondrogenic activity of human DBM when tested using this assay system.
Owner:WARSAW ORTHOPEDIC INC
Methods and compositions for smooth muscle reconstruction
This invention also provides a purified or isolated population of ADSCs that can differentiate into a cell of the leiomyogenic lineage, e.g., smooth muscle or skeletal muscle. In yet another aspect, the population additionally can be differentiated into a lineage selected from the group consisting of osteogenic, adipogenic, chondrogenic, myogenic and neuronal. This invention further provides a composition comprising a substantially homogeneous expanded population of smooth muscle cells. This invention provides a composition comprising a substantially homogeneous expanded population of skeletal muscle cells. Also provided herein is an isolated composition comprising a purified adipose-derived stem cell (ADSC) or progeny of said ADSC and an effective amount of laminin or heparin, effective to induce leiomyogenic differentiation. Diagnositic and therapeutic uses for these compositions are provided herein.
Owner:RGT UNIV OF CALIFORNIA
Biomaterials for guided tissue regeneration and drug delivery
The present invention is directed to compositions and methods of using compositions comprising a scaffold with growth factors chemically immobilized thereto for inducing chondrogenesis and / or osteogenesis when implanted in vivo or osteogenesis or chondrogenesis in cultures in vitro. The compositions and methods enhance bone and cartilage growth. Also described are compositions and methods for targeted drug delivery.
Owner:GELWELL BIOTECH CORP
Particulate cartilage compositions, processes for their preparation and methods for regenerating cartilage
InactiveUS20050196460A1Start fastEffective cartilage compositionSkeletal disorderJoint implantsParticulatesMedicine
Particulate cartilage compositions for stimulating chondrogenesis and producing cartilage regeneration and processes for their preparation are disclosed. Methods for regenerating articular cartilage are also disclosed.
Owner:MALININ THEODORE I
Multipotent stem cells derived from placenta tissue and cellular therapeutic agents comprising the same
InactiveUS20070243172A1Negative immunological responseBiocideArtificial cell constructsGerm layerDisease
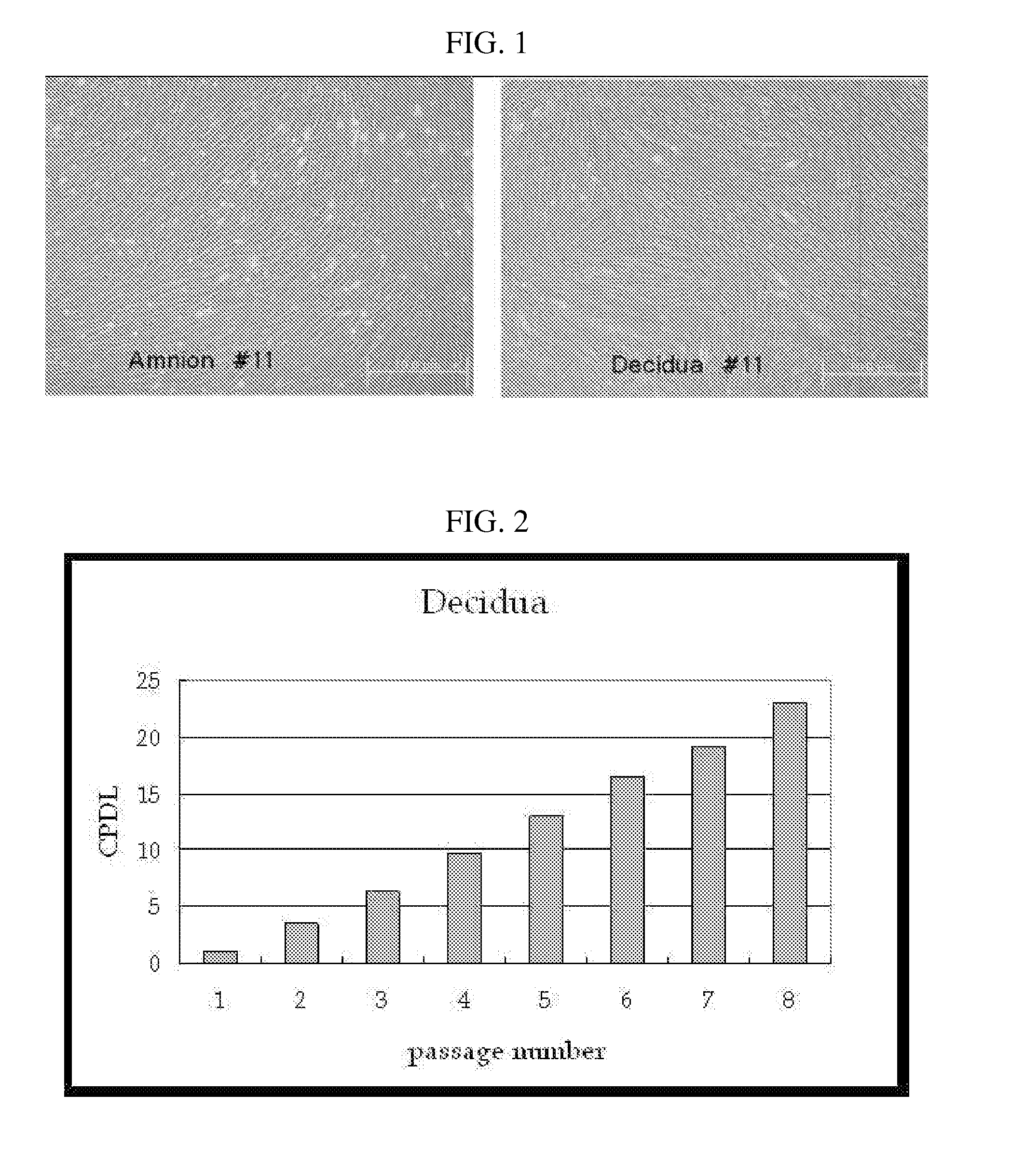
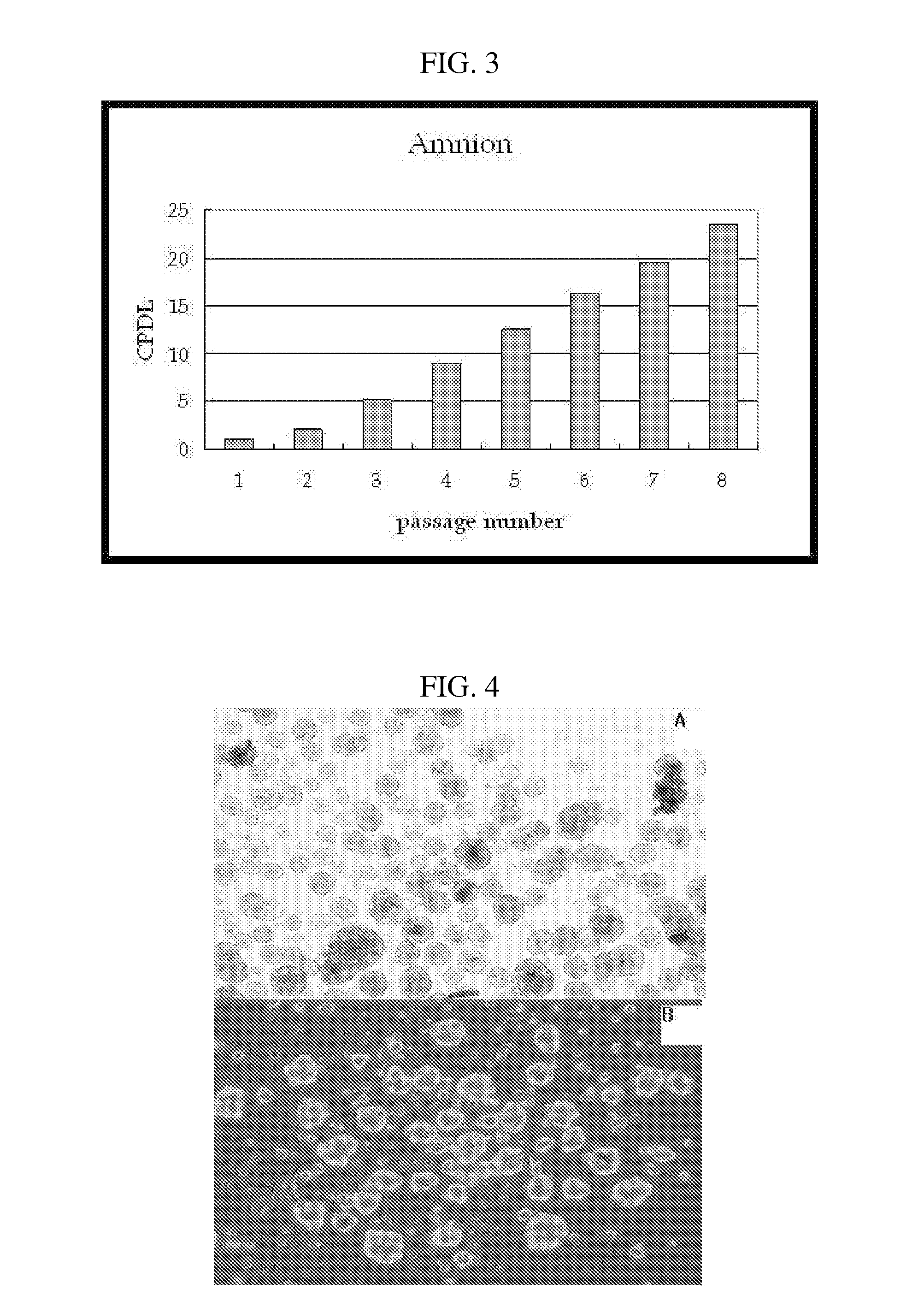
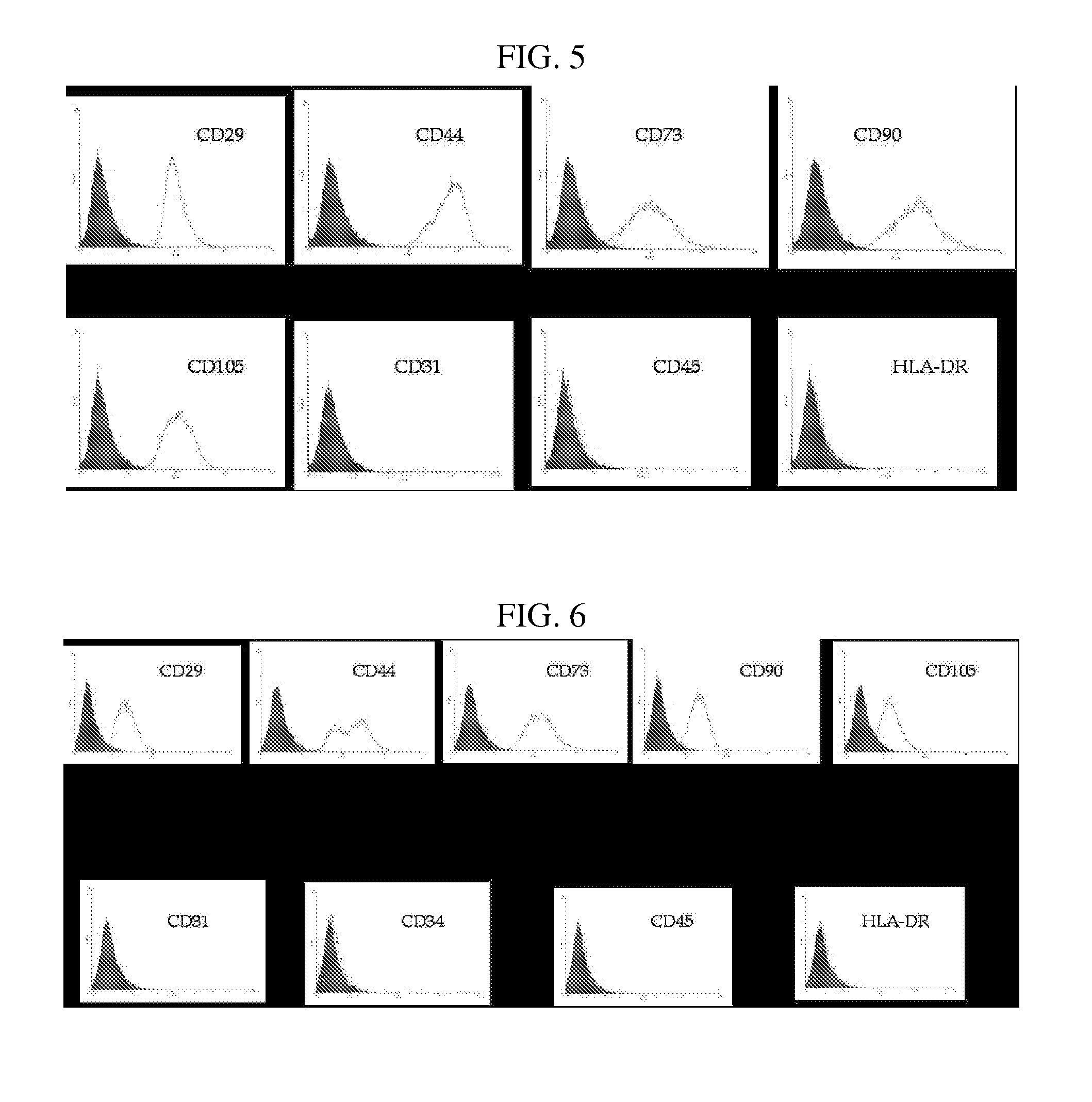
The present invention relates to placenta tissue-derived multipotent stem cells and cell therapeutic agents containing the same. More specifically, to a method for producing placenta stem cells having the following characteristics, the method comprising culturing amnion, chorion, decidua or placenta tissue in a medium containing collagenase and bFGF and collecting the cultured cells: (a) showing a positive immunological response to CD29, CD44, CD73, CD90 and CD105, and showing a negative immunological response to CD31, CD34, CD45 and HLA-DR; (b) showing a positive immunological response to Oct4 and SSEA4; (c) growing attached to plastic, showing a round-shaped or spindle-shaped morphology, and forming spheres in an SFM medium so as to be able to be maintained in an undifferentiated state for a long period of time; and (d) having the ability to differentiate into mesoderm-, endoderm- and ectoderm-derived cells. Also the present invention relates to placenta stem cells obtained using the production method. The inventive multipotent stem cells have the ability to differentiate into muscle cells, vascular endothelial cells, osteogenic cells, nerve cells, satellite cells, fat cells, cartilage-forming cells, osteogenic cells, or insuline-secreting pancreatic β-cells, and thus are effective for the treatment of muscular diseases, osteoporosis, osteoarthritis, nervous diseases, diabetes and the like, and are useful for the formation of breast tissue.
Owner:RNL BIO
Method for chondrocyte expansion with phenotype retention
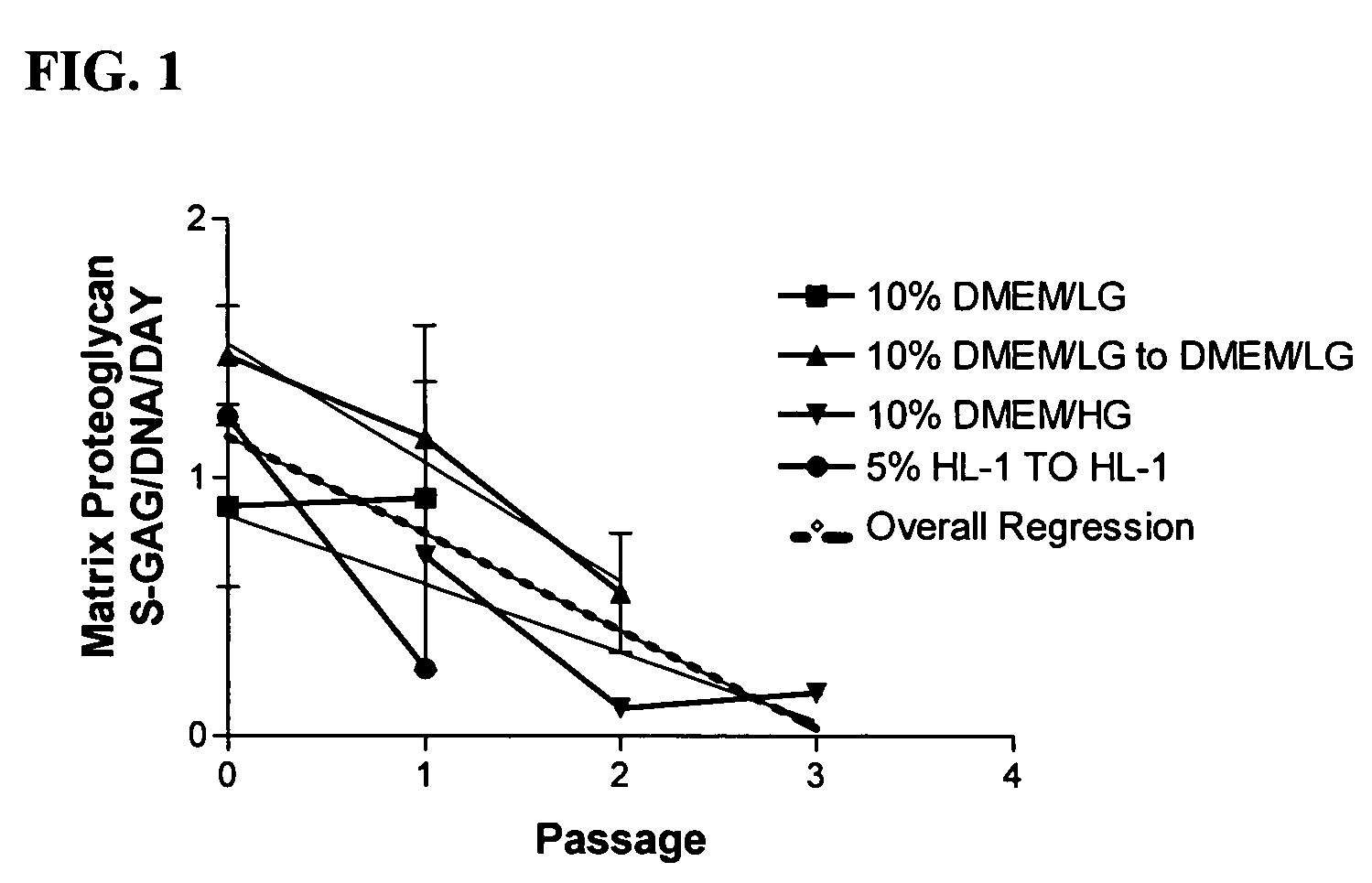
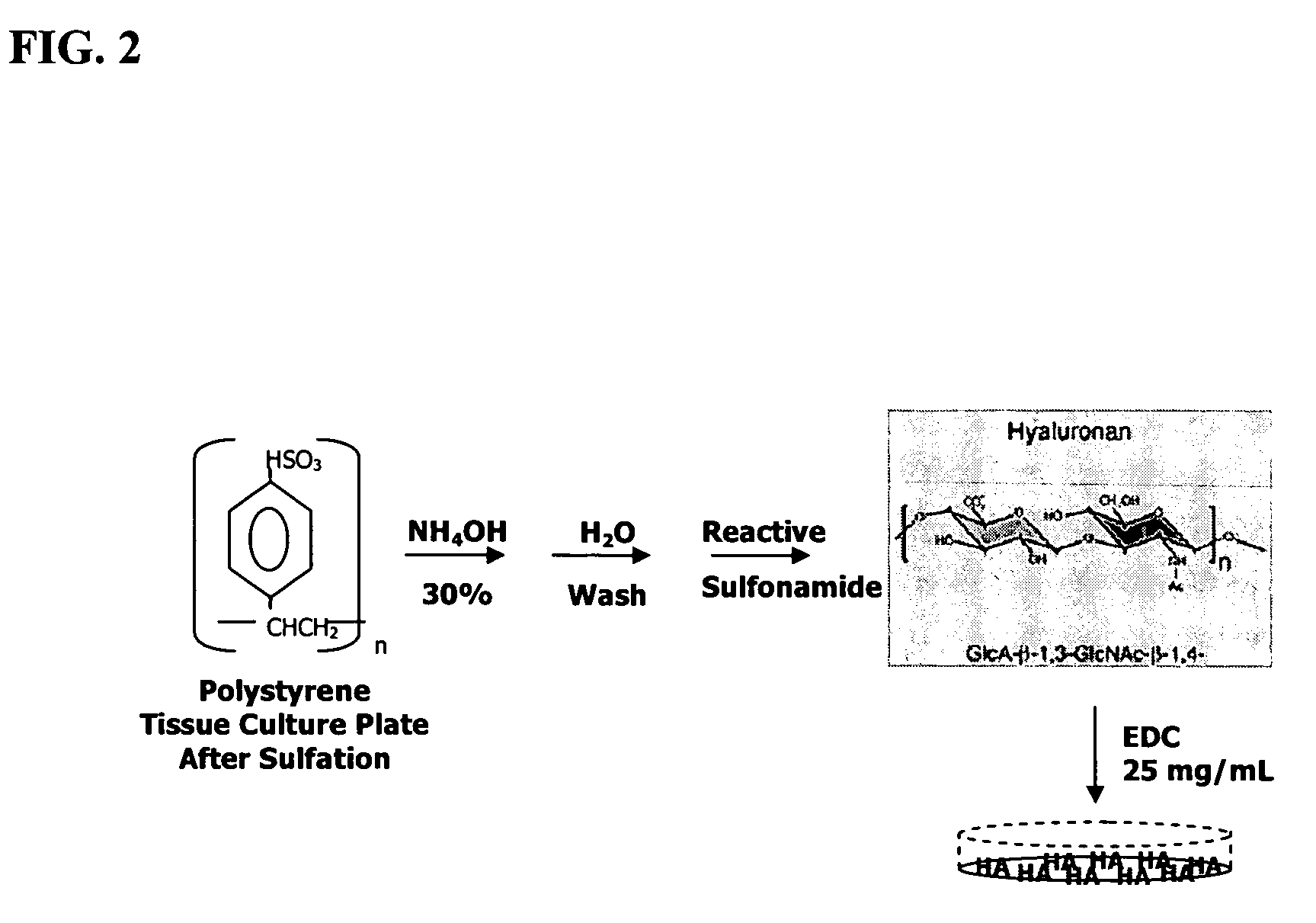
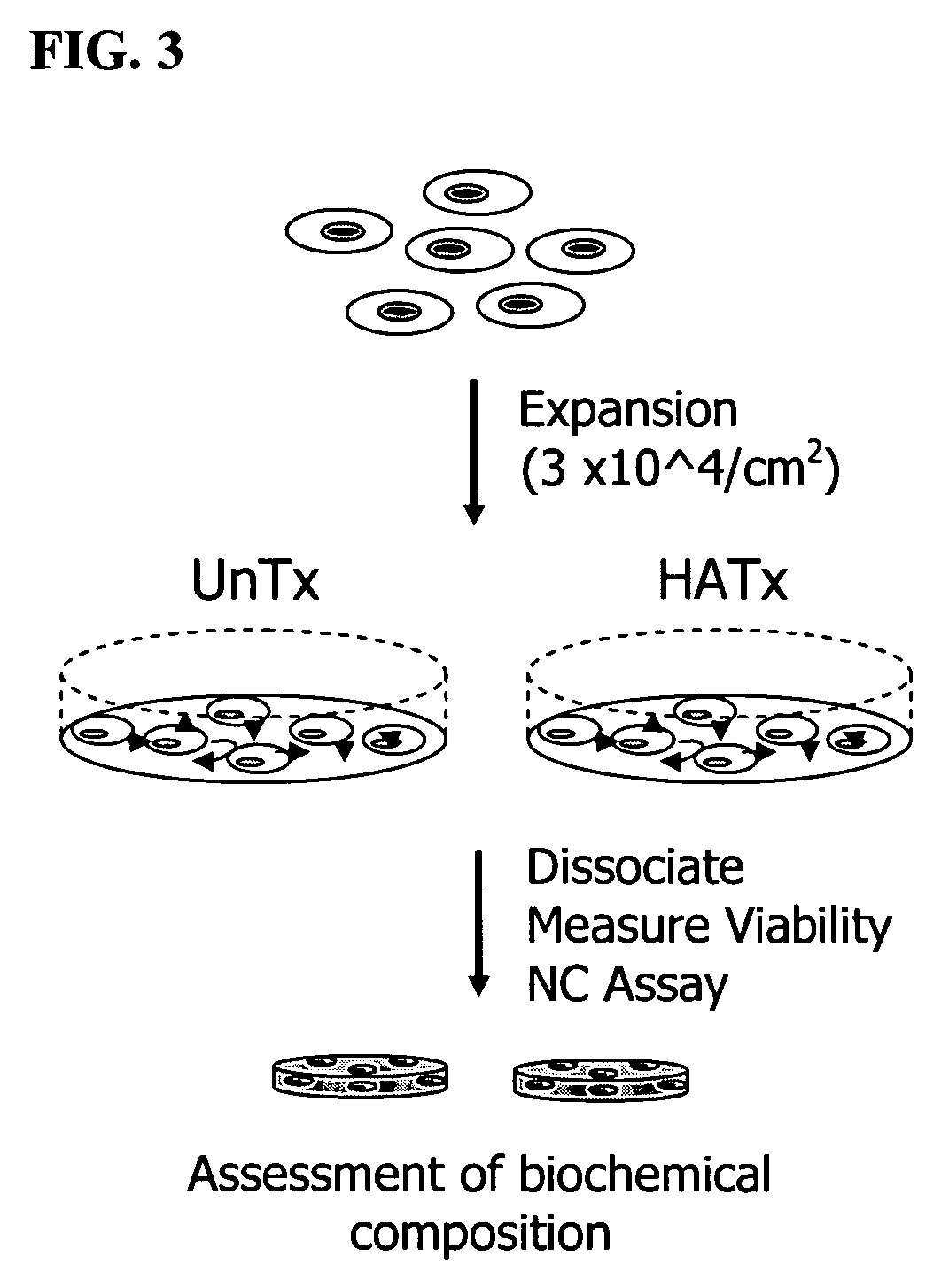
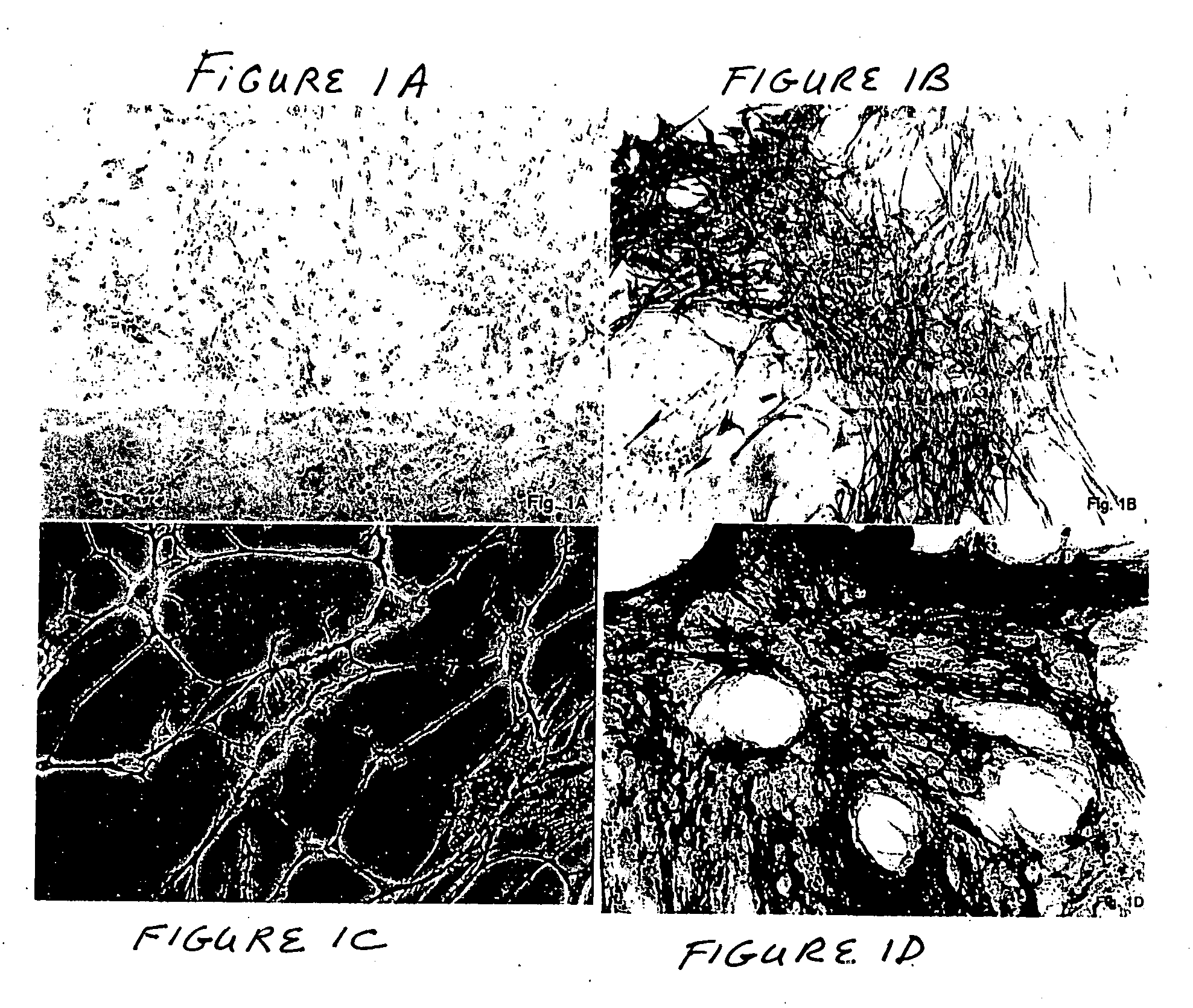
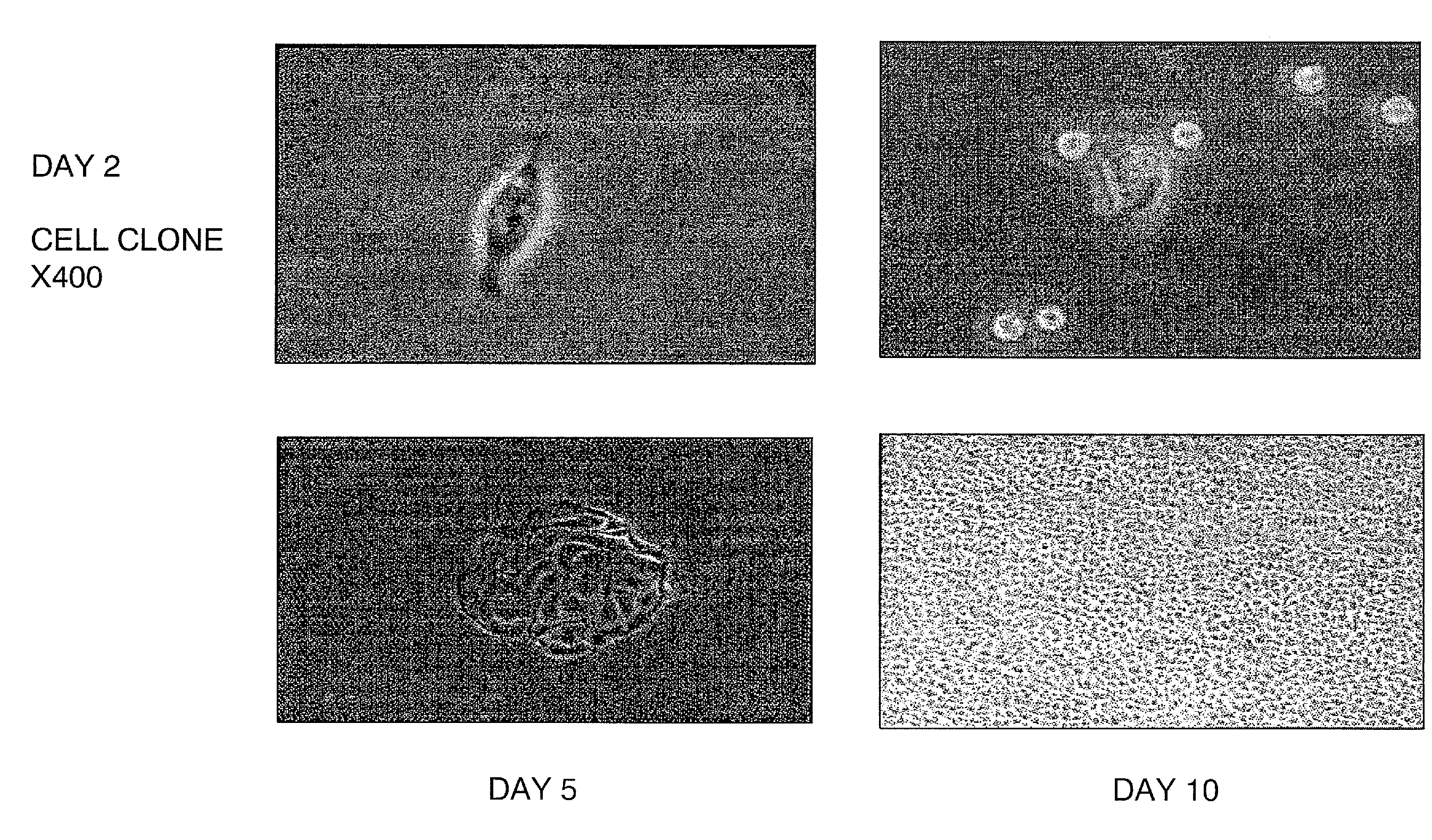
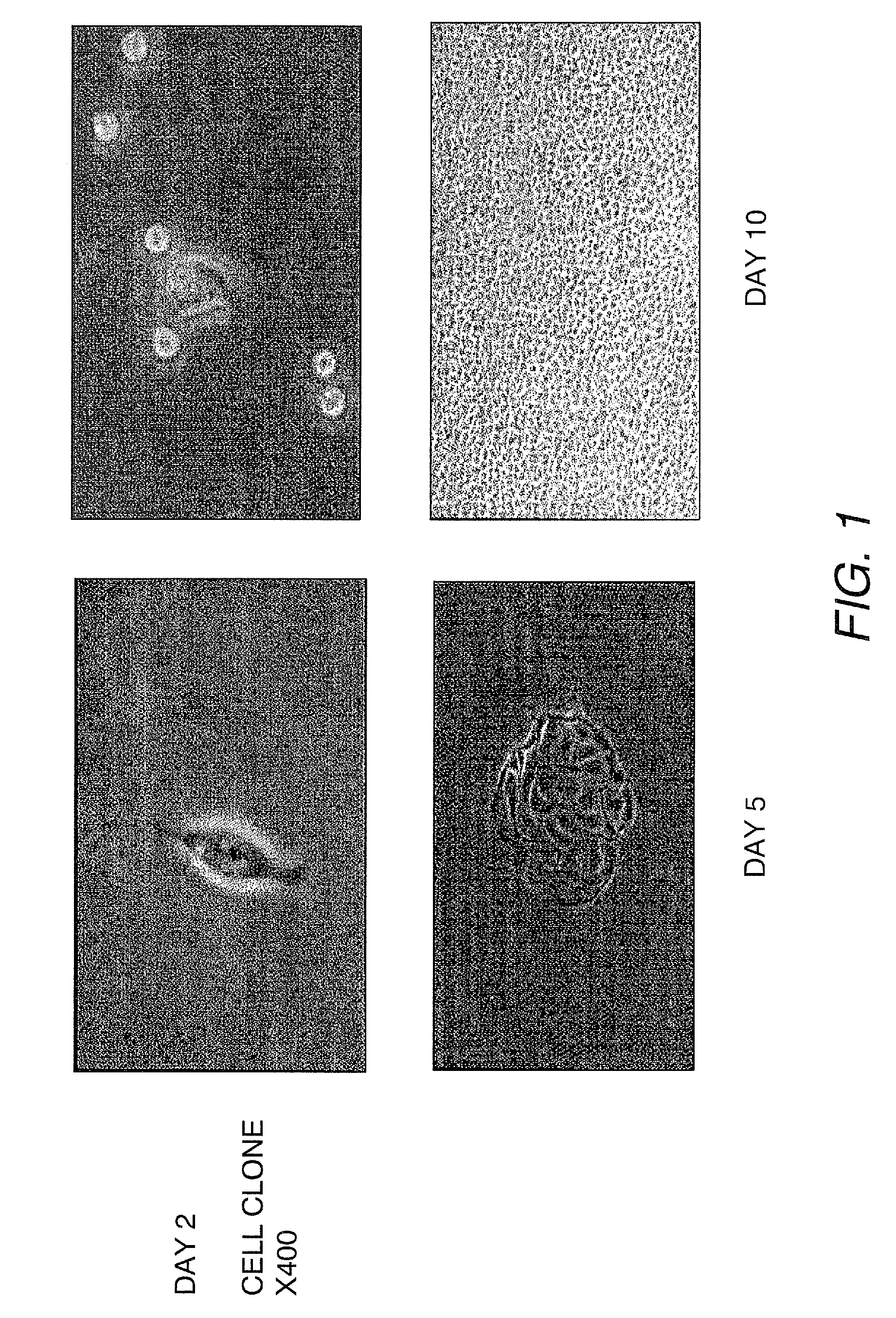
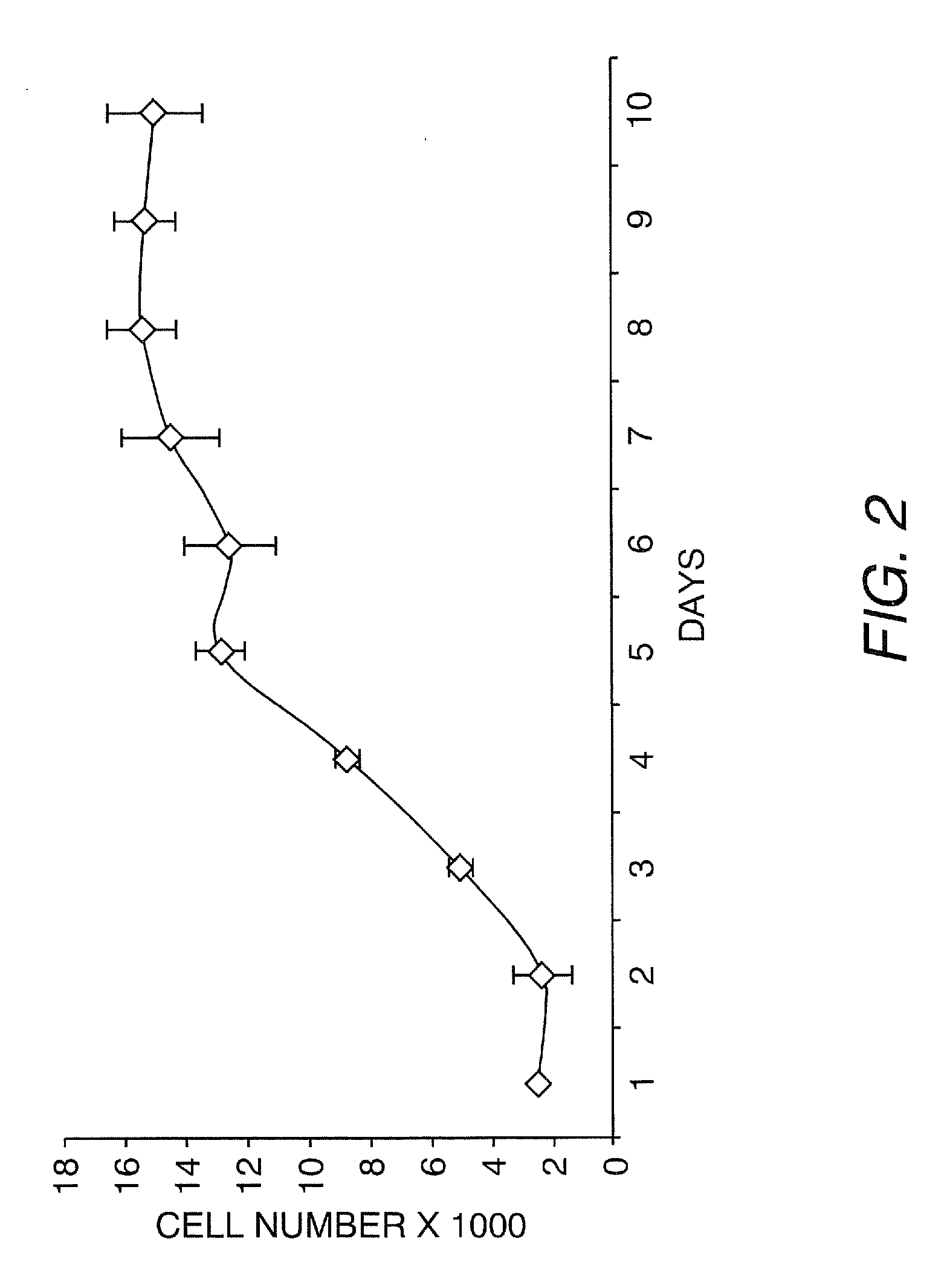
The present invention provides a method that maintains chondrocyte phenotype during serial expansion by culturing a population of chondrocytes in a defined serum-free culture medium containing cytokines and on a substrate that is modified by covalent attachment of hyaluronic acid. The underlying principle is to maintain native chondrocyte phenotype by growing the dissociated chondrocytes on a substrate modified by covalent attachment of hyaluronic acid to retain native chondrocyte morphology and function. Chondrocyte expanded in this manner can be used in various medical applications to repair cartilaginous tissues that have been injured by trauma or disease. This substratum provides a microenvironment that more closely mimics that of native articular cartilage, thereby promoting chondrogenesis in a predictable manner.
Owner:ZIMMER INC +1
Methods and compositions for smooth muscle reconstruction
This invention also provides a purified or isolated population of ADSCs that can differentiate into a cell of the leiomyogenic lineage, e.g., smooth muscle or skeletal muscle. In yet another aspect, the population additionally can be differentiated into a lineage selected from the group consisting of osteogenic, adipogenic, chondrogenic, myogenic and neuronal. This invention further provides a composition comprising a substantially homogeneous expanded population of smooth muscle cells. This invention provides a composition comprising a substantially homogeneous expanded population of skeletal muscle cells. Also provided herein is an isolated composition comprising a purified adipose-derived stem cell (ADSC) or progeny of said ADSC and an effective amount of laminin or heparin, effective to induce leiomyogenic differentiation. Diagnositic and therapeutic uses for these compositions are provided herein.
Owner:RGT UNIV OF CALIFORNIA
Bone matrix compositions and methods
Owner:WARSAW ORTHOPEDIC INC
Cartilage composites and methods of use
Disclosed are neocartilage compositions characterized by having multiple layers of cells, said cells being surrounded by a substantially continuous insoluble glycosaminoglycan and collagen-enriched hyaline extra-cellular matrix, and which neocartilage phospholipids are advantageously enriched in anti-inflammatory n-9 fatty acids, particularly 20:3 n-9 eicosatrienoic or Mead acid.Also disclosed are methods of growing neocartilage in substantially serum-free growth media and methods of producing a conditioned growth media containing compounds effective to enhance neocartilage formation.The neocartilage compositions are useful as implants and as replacement tissue for damaged or defective cartilage and as a model system for studying articular cartilage disease and response to natural and synthetic compounds.
Owner:ZIMMER INC
Disrupted cartilage products
InactiveUS20140030309A1Increase flexibilityBiocidePeptide/protein ingredientsMedicineCollagen matrices
This invention provides disrupted cartilage products, methods of manufacturing disrupted cartilage products, and methods of treating a subject comprising administering a cartilage product. The cartilage products are manufactured by a method comprising disrupting a collagen matrix, e.g. to produce a flexible cartilage product. Optionally, the cartilage products comprise viable chondrocytes, bioactive factors such as chondrogenic factors, and a collagen type II matrix. Optionally, the cartilage products are non-immunogenic.
Owner:OSIRIS THERAPEUTICS
Cartilage material
InactiveUS20080279825A1Start fastEffective cartilage fluff cartilageBiocideSkeletal disorderMedicineSacroiliac joint
Cartilage materials such as cartilage fluff and a cartilage composition comprising a particulate material are disclosed. These are suitable for stimulating chondrogenesis and / or producing cartilage regeneration. Also disclosed are processes for their preparation. Methods for regenerating articular cartilage are also disclosed, which involve, for example, placing the cartilage fluff or cartilage composition into a cartilage defect.
Owner:VIVEX BIOLOGICS GRP INC
Porated cartilage products
This invention provides porated cartilage products, methods of producing porated cartilage products, and methods of treating subjects by administering cartilage products. Optionally, the cartilage products are sized, porated, and digested to provide a flexible cartilage product. Optionally, the cartilage products comprise viable chondrocytes, bioactive factors such as chondrogenic factors, and a collagen type II matrix. Optionally, the cartilage products are non-immunogenic.
Owner:OSIRIS THERAPEUTICS
Production of tissue engineered digits and limbs
ActiveUS8728463B2Increase vascularisationEncourages recruitmentBiocidePeptide/protein ingredientsBone formingComposite tissue
The invention pertains to methods of producing artificial composite tissue constructs that permit coordinated motion. Biocompatable structural matrices having sufficient rigidity to provide structural support for cartilage-forming cells and bone-forming cells are used. Biocompatable flexible matrices seeded with muscle cells are joined to the structural matrices to produce artificial composite tissue constructs that are capable of coordinated motion.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Stem cells from urine and methods for using the same
Provided herein are stem cells and methods for producing a culture of stem cells from urine. The stem cells may be differentiated into an osteogenic, chondrogenic, adipogenic, endothelial, neurogenic or myogenic lineage. Methods of use of the cells are provided, including methods of treating a subject in need of a cell based therapy.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Repair of defects or lesions in cartilage and bone using a chondro-regulative matrix
InactiveUS20110184381A1Promote healingInduce the repair of lesions in cartilage and boneBiocidePowder deliveryProgenitorMatrix method
Methods and compositions are provided for the treatment and repair of defect in the cartilage in partial- or full-thickness defects in joints of animals, in particular humans. To induce cartilage formation, a defect in cartilage is filled with layers of thin flaps of synovium or of peritendineum, which contains chondro- and osteo-progenitor cells, with interposed layers of a matrix. The matrix contains a chondrogenic factor, which induces chondrogenesis of chondroprogenitor cells in the flaps, and an anti-hypertrophic agent, which arrest differentiation of chondrocytes in an early phase, in an appropriate delivery system. The matrix filling the bone area of a full-thickness defect may contain an osteogenic factor, which induces osteogenesis of osteoprogenitor cells. The layer of a flap between cartilage and bone areas may work as a barrier, which prevents blood vessels and associated cells from penetrating from the bone area into the cartilage area. To promote the induction of chondro- and osteo-genesis of the progenitor cells in the flaps of synovium or peritendineum effectively, the flaps may be treated with enzymes, e.g., matrix metalloproteinases or be punched by a needle before filling a defect.
Owner:UNIVERSITY OF BERN
Bone matrix compositions and methods
ActiveUS20110195052A1Improve biological activityFacilitated releaseBiocideHydrolysed protein ingredientsOsteoblastSpecific protein
The present invention provides methods of improving the osteogenic and / or chondrogenic activity of a bone matrix, e.g., a dermineralized bone matrix (DBM), by exposing the bone matrix to one or more treatments or conditions. In preferred embodiments the bone matrix is derived from human bone. The treatment or condition may alter the structure of the bone matrix and / or cleave one or more specific proteins. Cleavage may generate peptides or protein fragments that have osteoinductive, osteogenic, or chondrogenic activity. Preferred treatments include collagenase and various other proteases. The invention further provides improved bone and cartilage matrix compositions that have been prepared according to the inventive methods and methods of treatment using the compositions. The invention further provides methods of preparing, testing, and using the improved bone matrix compositions. On a assay comprises exposing relatively undifferentiated mesenchymal cells to a bone matrix composition and measuring expression of a marker characteristic of osteoblast or chondrocyte lineage(s). Increased expression of the marker relative to the level of the marker in cells that have been exposed to a control matrix (e.g., an inactivated or untreated matrix) indicates that the treatment or condition increased the osteogenic and / or chondrogenic activity of the bone matrix. Suitable cells include C2C12 cells. A suitable marker is alkaline phosphatase. The inventive methods increase the osteogenic and / or chondrogenic activity of human DBM when tested using this assay system.
Owner:WARSAW ORTHOPEDIC INC
Method of inducing or enhancing chondrogenesis with extracellular matrix containing BMP-4
InactiveUS6932977B2Induce and enhance chondrogenesisEnhancing chondrogenesisOrganic active ingredientsBiocideCell-Extracellular MatrixECM Protein
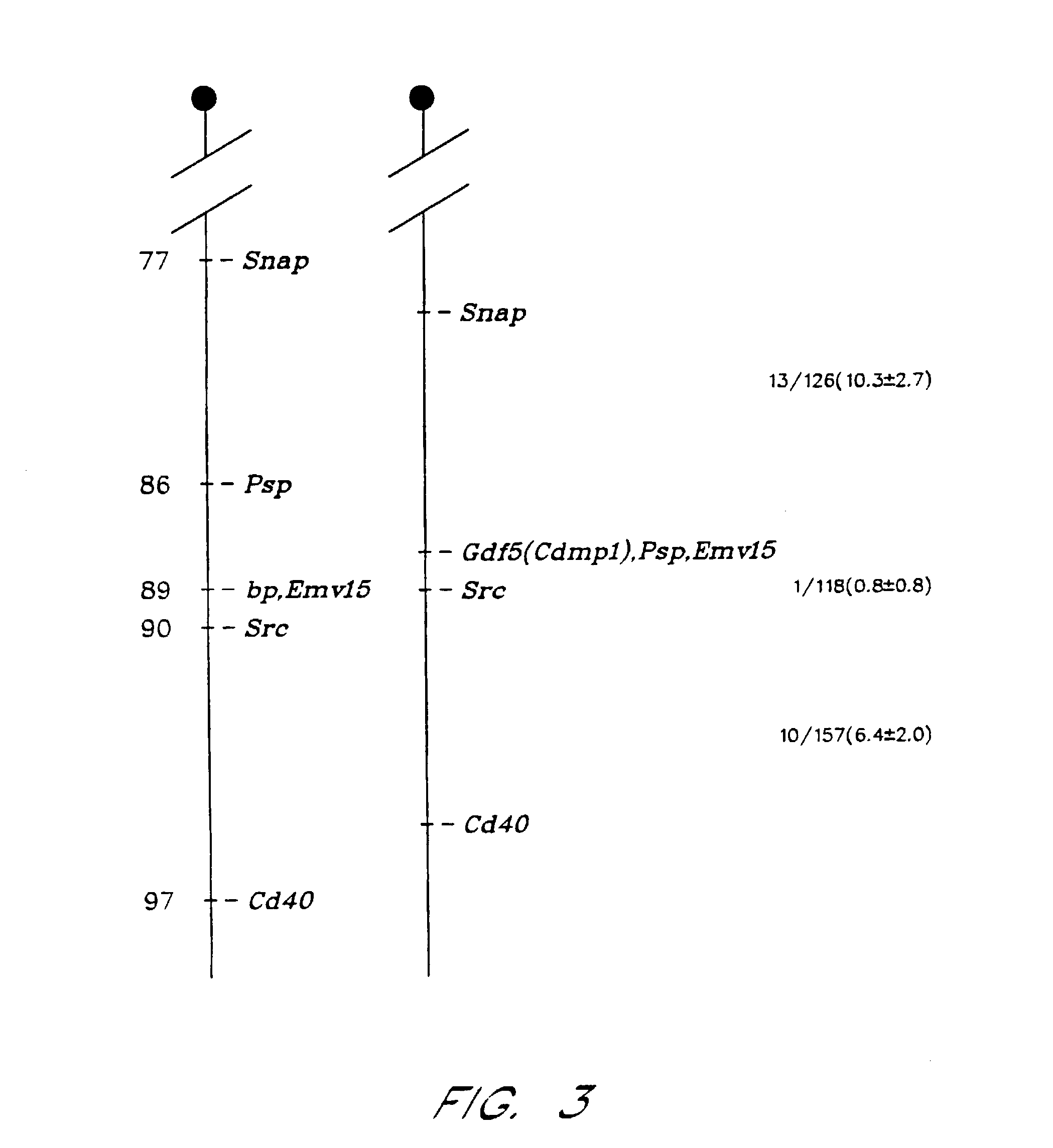
A method and composition are provided for inducing or enhancing chondrogenesis in vivo or in vitro. The method is performed by exposing the cells in vitro or in vivo to an extracellular matrix comprising of type I collagen, type II collagen or a mixture of type I collagen or type II collagen and hyaluronate and further containing BMP-4 or a combination of BMP-4 and GDF-5.
Owner:DEPUY SPINE INC (US)
Pharmaceutical composition and uses thereof
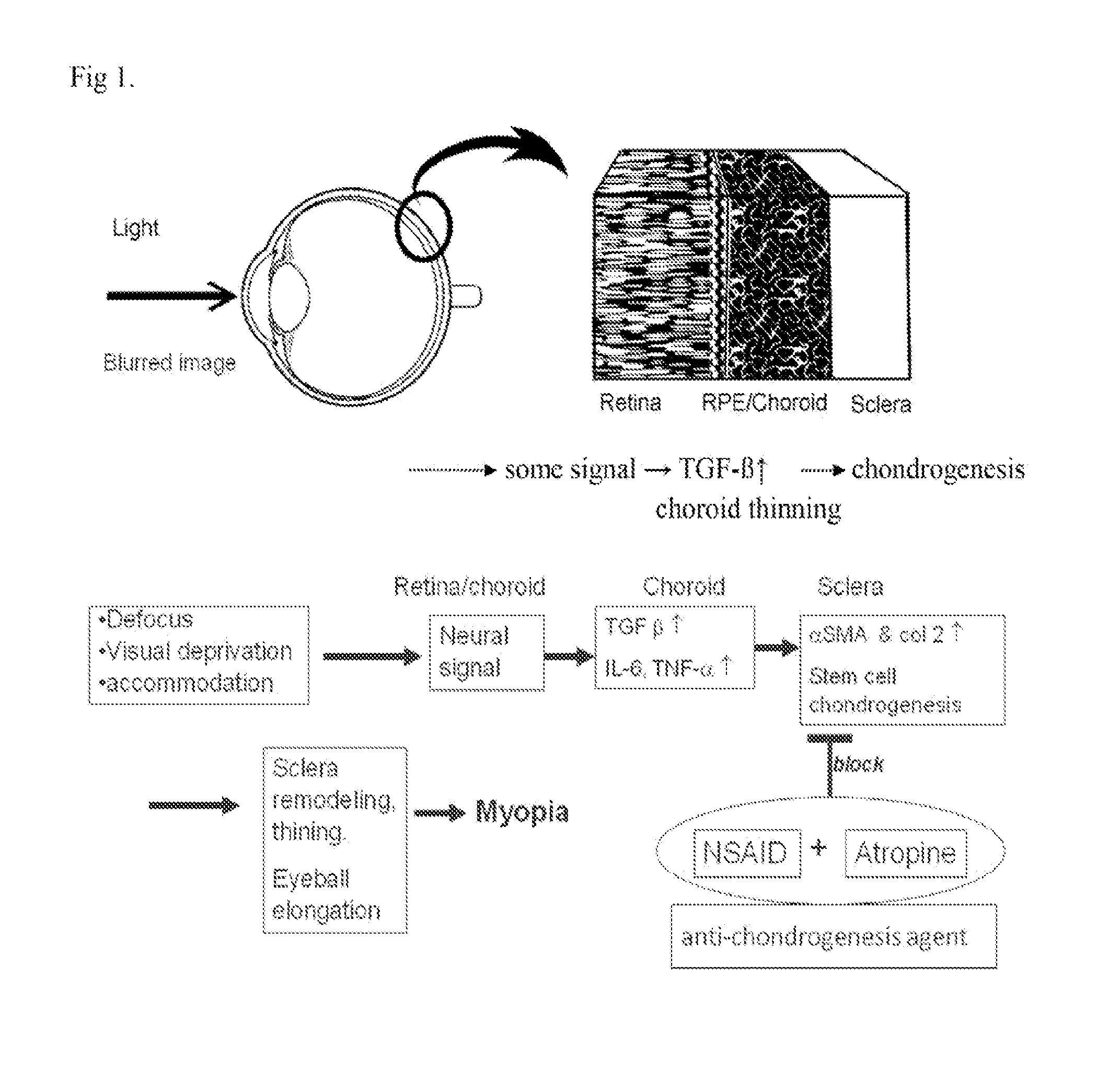
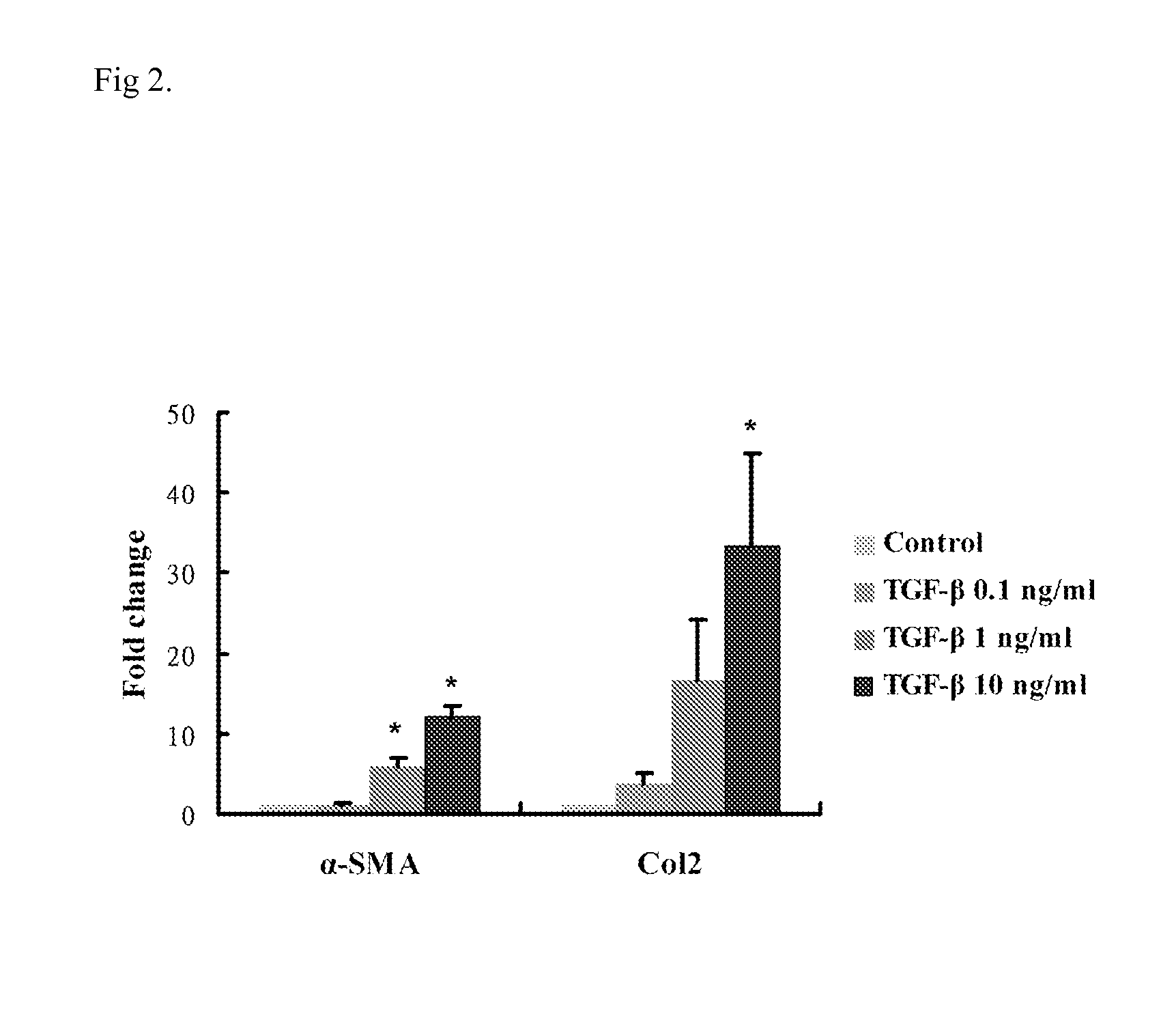
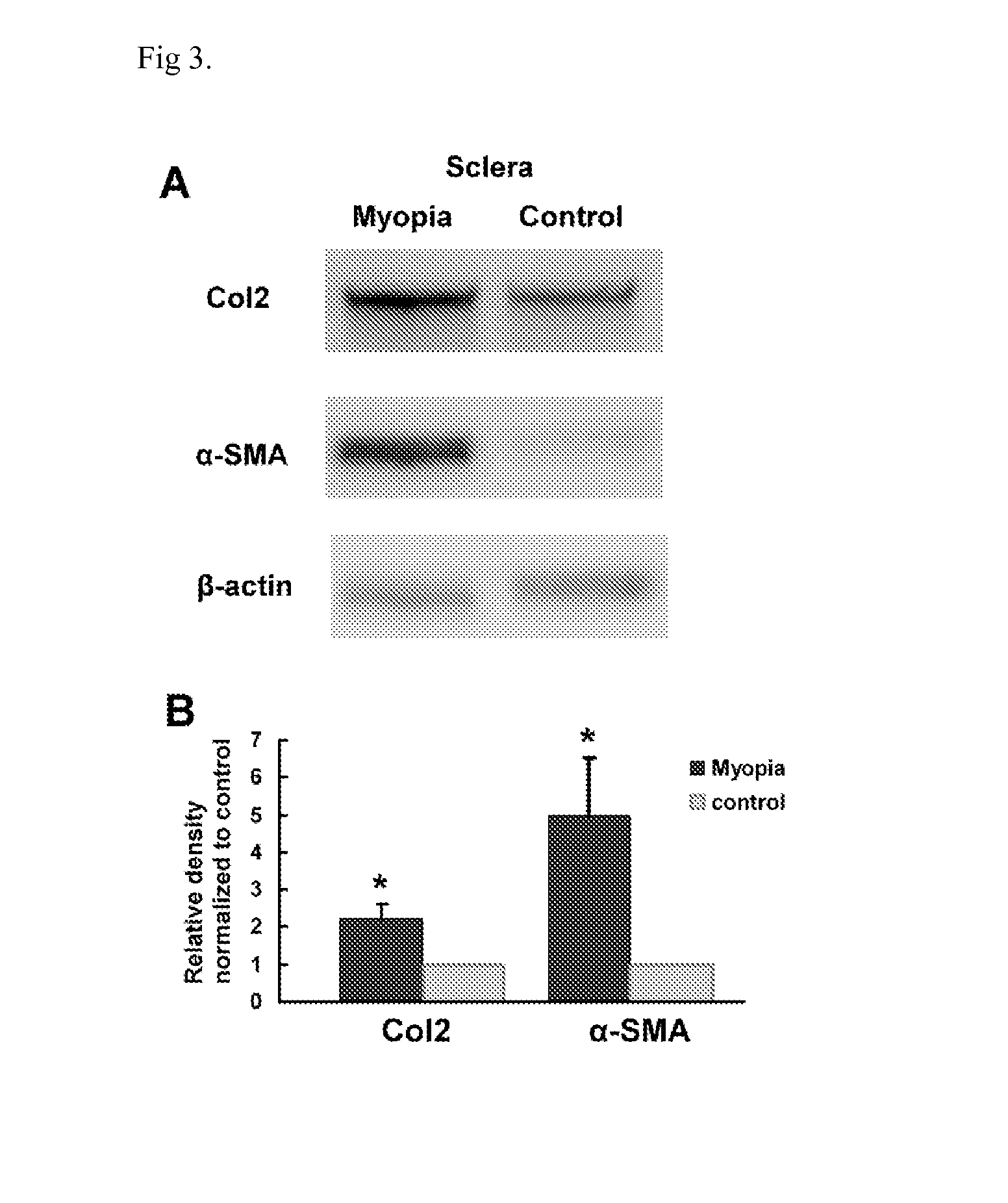
InactiveUS20160067238A1Reducing one or more chondrogenic proteinsReducing scleral chondrogenesisBiocideSenses disorderInflammationChondrogenesis
Pharmaceutical compositions containing a combination of anti-chondrogenesis agents are disclosed. Methods of reducing scleral chondrogensis, reducing one or more ocular chondrogenic proteins, reducing inflammation induced chondrogensis and treating myopia by administering an effective amount of one or more anti-chondrogensis agents are also provided. The pharmaceutical compositions are useful for treating myopia.
Owner:KAOHSIUNG CHANG GUNG MEMORIAL HOSPITAL +1
Bone Matrix Compositions and Methods
Owner:WARSAW ORTHOPEDIC INC
Methods of manufacturing cartilage products
This invention provides porated cartilage products and methods of producing porated cartilage products. Optionally, the cartilage products are sized, porated, and digested to provide a flexible cartilage product. Optionally, the cartilage products comprise viable chondrocytes, bioactive factors such as chondrogenic factors, and a collagen type II matrix. Optionally, the cartilage products are non-immunogenic.
Owner:OSIRIS THERAPEUTICS
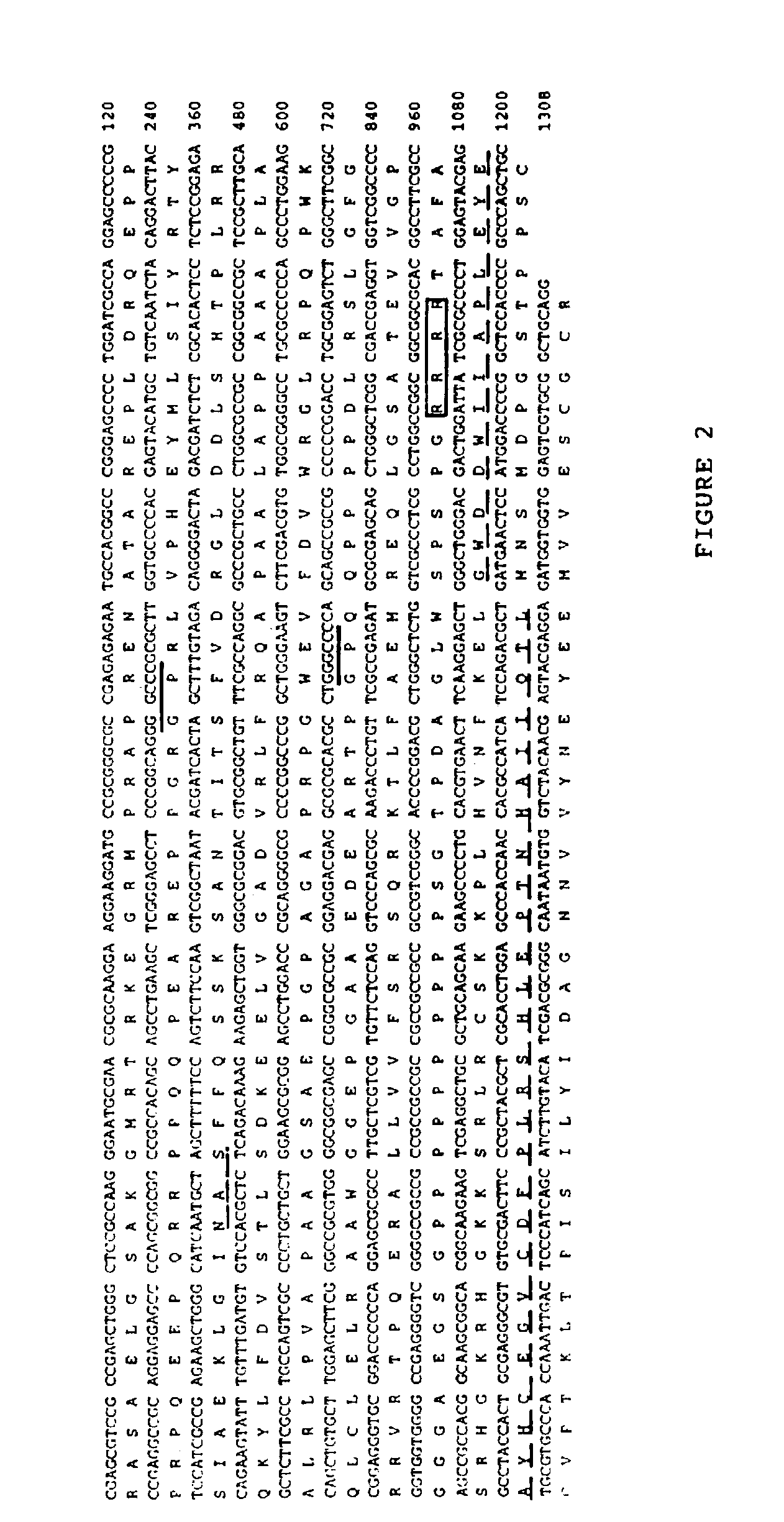
Cartilage-derived morphogenetic proteins
Nucleotide and amino acid sequences of cartilage-derived morphogenetic proteins (CDMP-1 and CDMP-2) from human and bovine cartilage extracts. These proteins exhibit chondrogenic activity and can be used to repair cartilage defects in a mammal.
Owner:UNITED STATES OF AMERICA
Calcium-mediated effects of coral and methods of use thereof
This invention is directed to coral scaffolds seeded with precursor cells in culture in the presence of a chelator and uses thereof in inducing or enhancing bone and / or cartilage formation in a subject, and kits related thereto. This invention is also directed to use of cadherin-upregulating coral for treating cancer or inhibiting cancer progression. This invention is also directed to use of aragonite or calcite-producing species for in vivo calcium release, and its application to the treatment of skin diseases, disorders or conditions.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
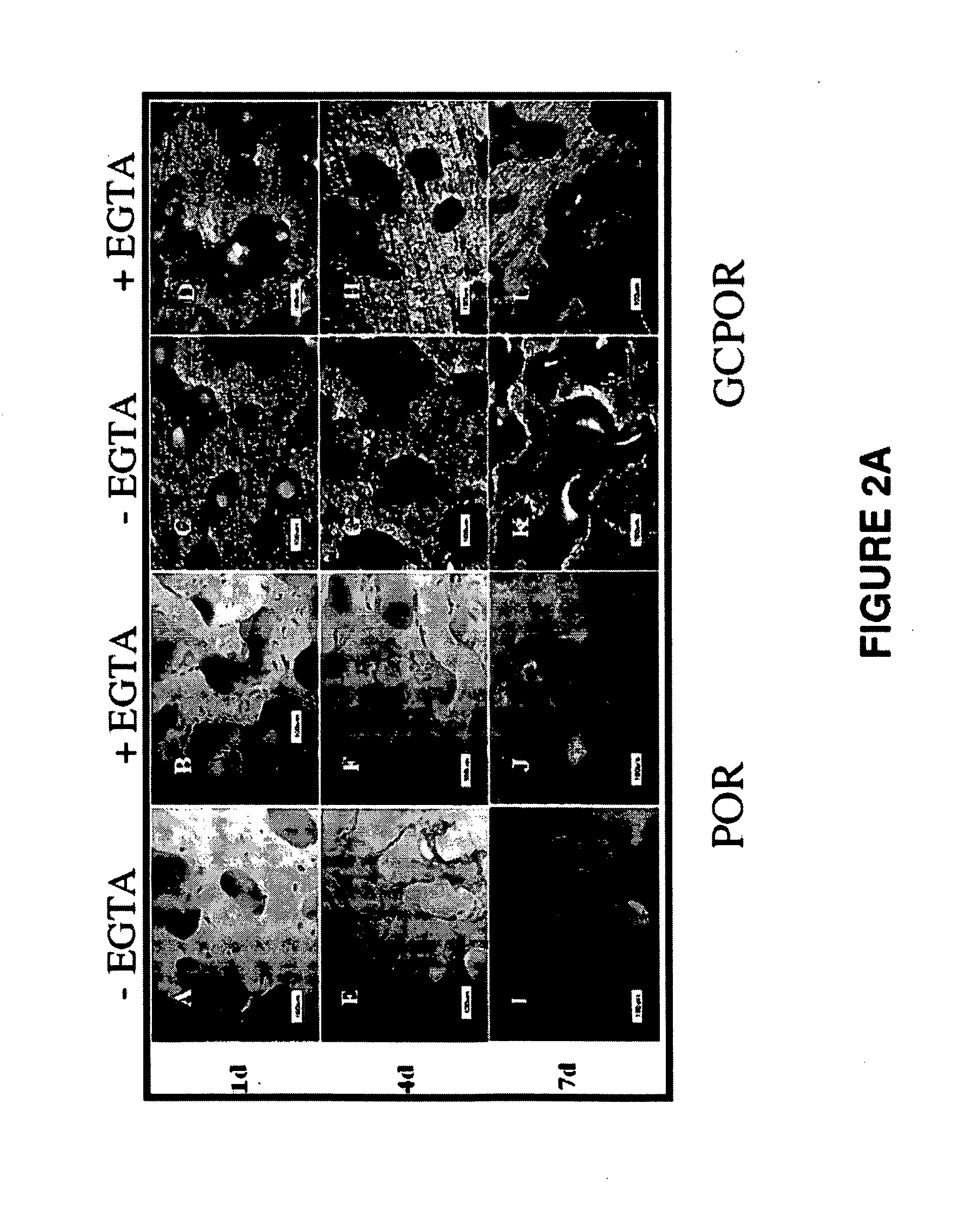
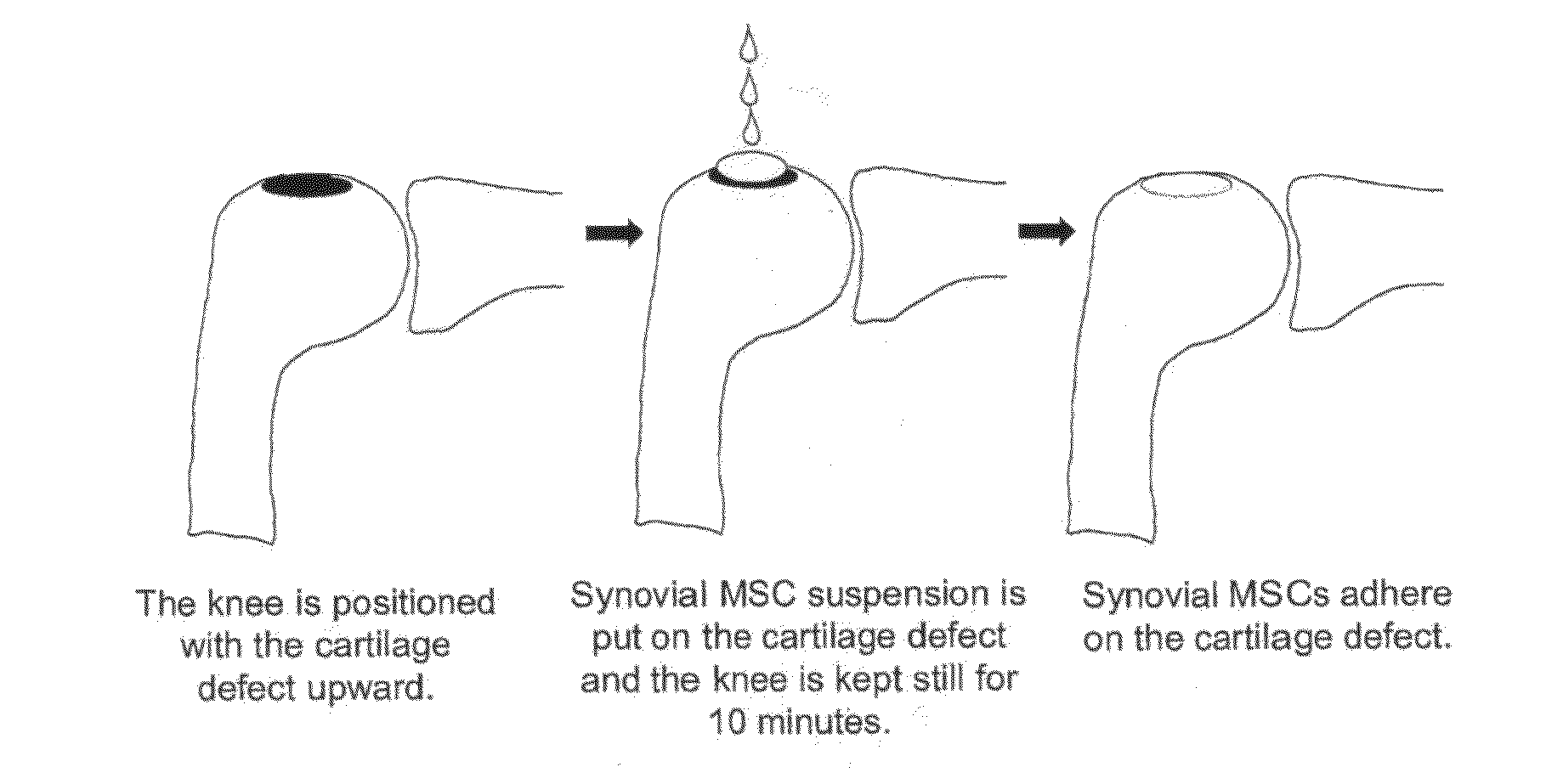
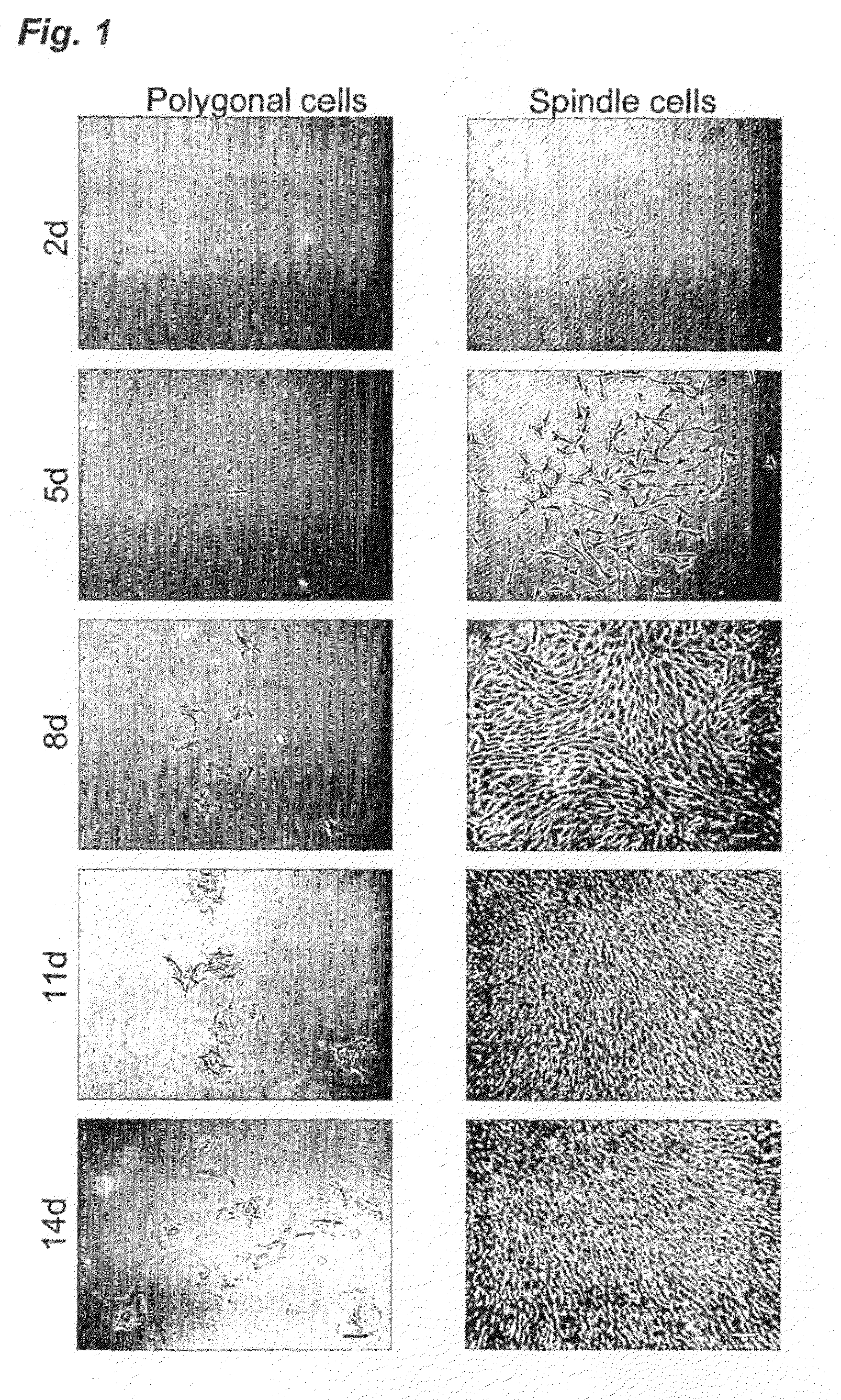
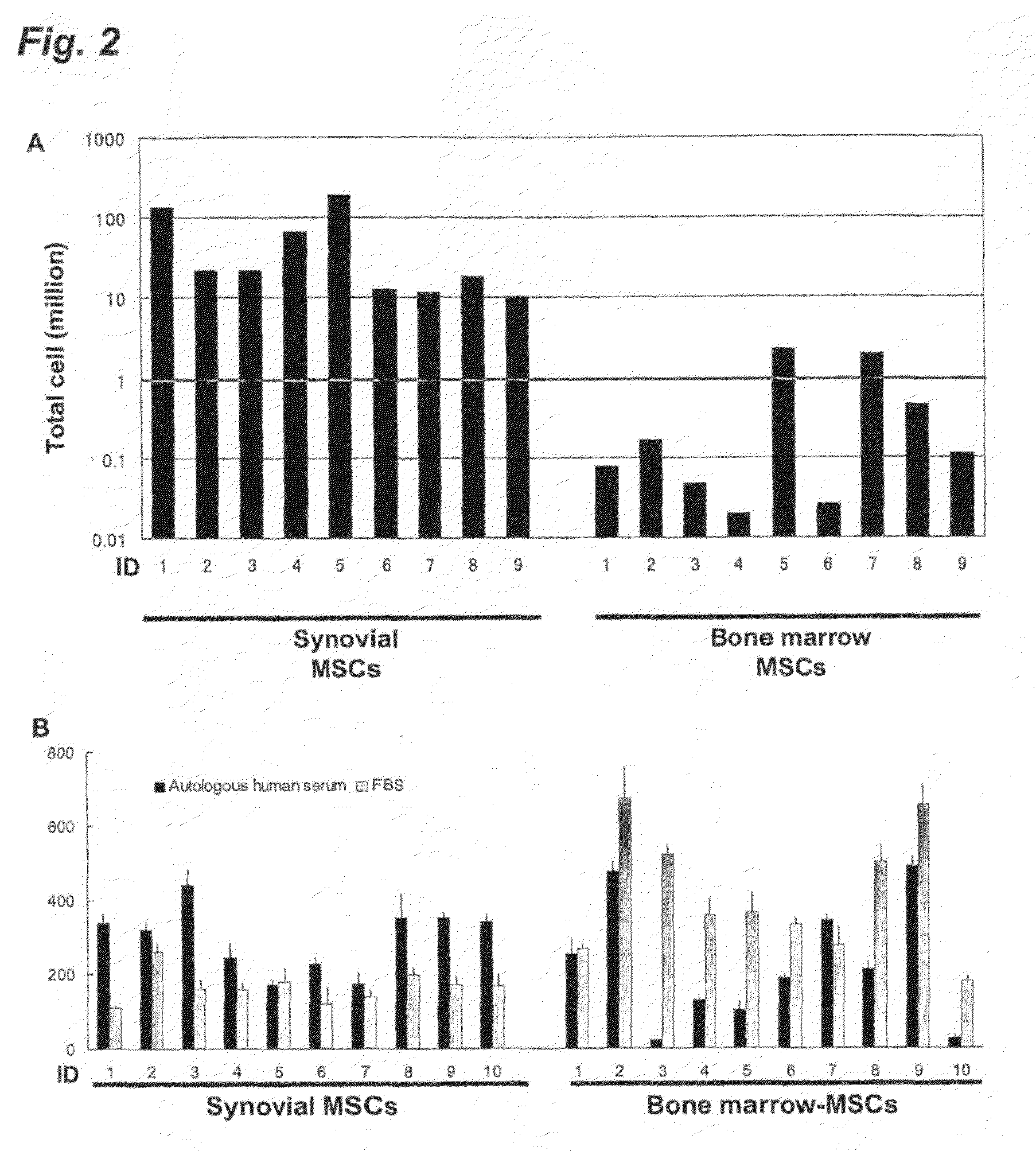
Application of synovium-derived mesenchymal stem cells (MSCS) for cartilage or meniscus regeneration
An object of the present invention is to provide a method for treating defects of articular cartilage or meniscus of a patient using in vivo chondrogenesis of synovium-derived MSCs. The present invention provides a method for treating a disease associated with defects of cartilage or meniscus. In the present invention, the method for treating a disease associated with defects of cartilage or meniscus comprises the following steps: culturing ex vivo autologous synovium-derived mesenchymal stem cells (MSCs); implanting the MSCs such that said cartilage defect site or meniscal defect site is covered by the MSCs; and regenerating cartilage tissue at the cartilage defect site or meniscal defect site in situ by differentiating the MSCs into cartilage cells.
Owner:NAT UNIV CORP TOKYO MEDICAL & DENTAL UNIV
Cartilage material
ActiveUS20110104242A1Start fastEffective compositionSkeletal disorderUnknown materialsMedicineSacroiliac joint
Cartilage materials such as cartilage fluff and a cartilage composition comprising a particulate material are disclosed. These are suitable for stimulating chondrogenesis and / or producing cartilage regeneration. Also disclosed are processes for their preparation. Methods for regenerating articular cartilage are also disclosed, which involve, for example, placing the cartilage fluff or cartilage composition into a cartilage defect.
Owner:VIVEX BIOLOGICS GRP INC
Compositions and Methods for Cartilage Repair
ActiveUS20130287753A1Optimal cartilage repairPromote formationBiocidePeptide/protein ingredientsMedicineCartilage repair
Autologous compositions and methods are provided for cartilage repair in patients in need thereof. Some aspects include combinations of platelet-based materials with chondrogenesis inducing agents in the presence or absence of cell-based therapies.
Owner:REGENEXX LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com