Disrupted cartilage products
a cartilage product and dislocation technology, applied in the field of cartilage products, can solve the problems of suboptimal yield, painful joint movement, and severe restriction of joint movement, and achieve the effect of increasing the flexibility of the cartilage sampl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of Femoral Condyles and Tibial Plateau
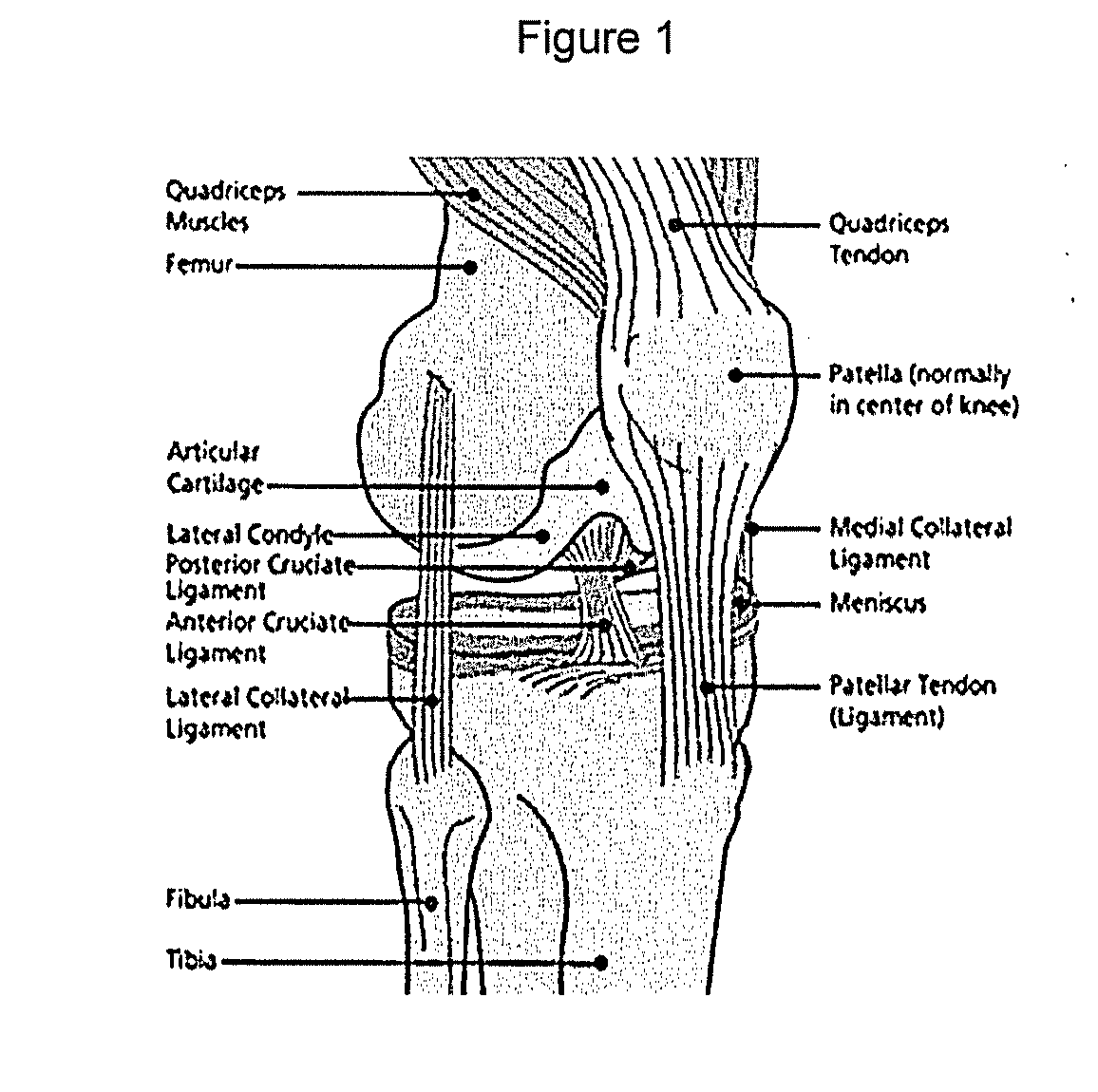
[0272]A human knee joint was obtained, as depicted in FIG. 1.
[0273]The outer surfaces of the knee joint were cleaned with iodine (10% povidione-iodine solution, Purdue, “Betadine”) without contacting the cartilage with iodine. The knee joint was dissected to separate the femur, tibia and fibula without damaging the cartilage surfaces. Soft tissue (adipose, muscle, fascia, ligaments and tendons) were removed to expose the articular cartilage surfaces on tibial plateau and femoral condyles.
[0274]The portions containing the articular cartilage (tibial plateau and the condyles of the femur) were chilled by placing in chilled saline (0.9% Sodium Chloride irrigation solution, USP) on a cold plate.
example 2
Isolating Cartilage Plugs
[0275]Femur condoyles and tibial plateau were obtained as detailed in Example 1. Osteochondral plugs having diameters of about 1 cm or about 2 cm were obtained from the femur condoyles and tibial plateau. During isolation of the plugs, the condoyles and tibial plateau were kept moist and chilled by periodic immersion in chilled saline or wiped with a wipe soaked in chilled saline. The isolated plugs were then chilled by placement in chilled saline.
[0276]The osteochondral plugs were obtained using a tissue punch while avoiding any areas of damaged cartilage. Specifically, tissue punches with diameters of 1 cm or 2 cm were used to remove whole plugs of cartilage and underlying bone from the articular surface.
example 3
Isolating a Cartilage Sample from Subchondral Bone and Calcified Cartilage
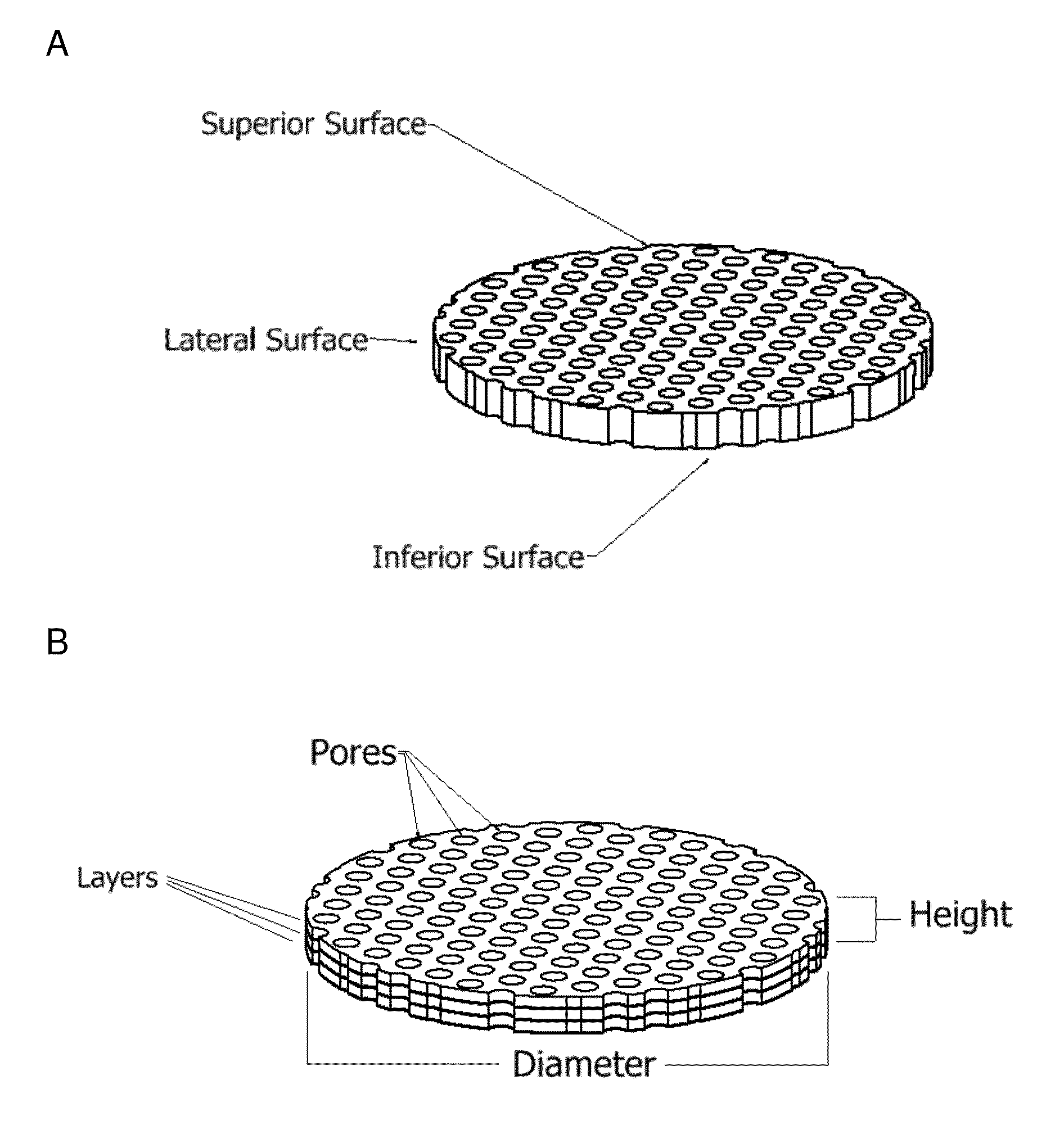
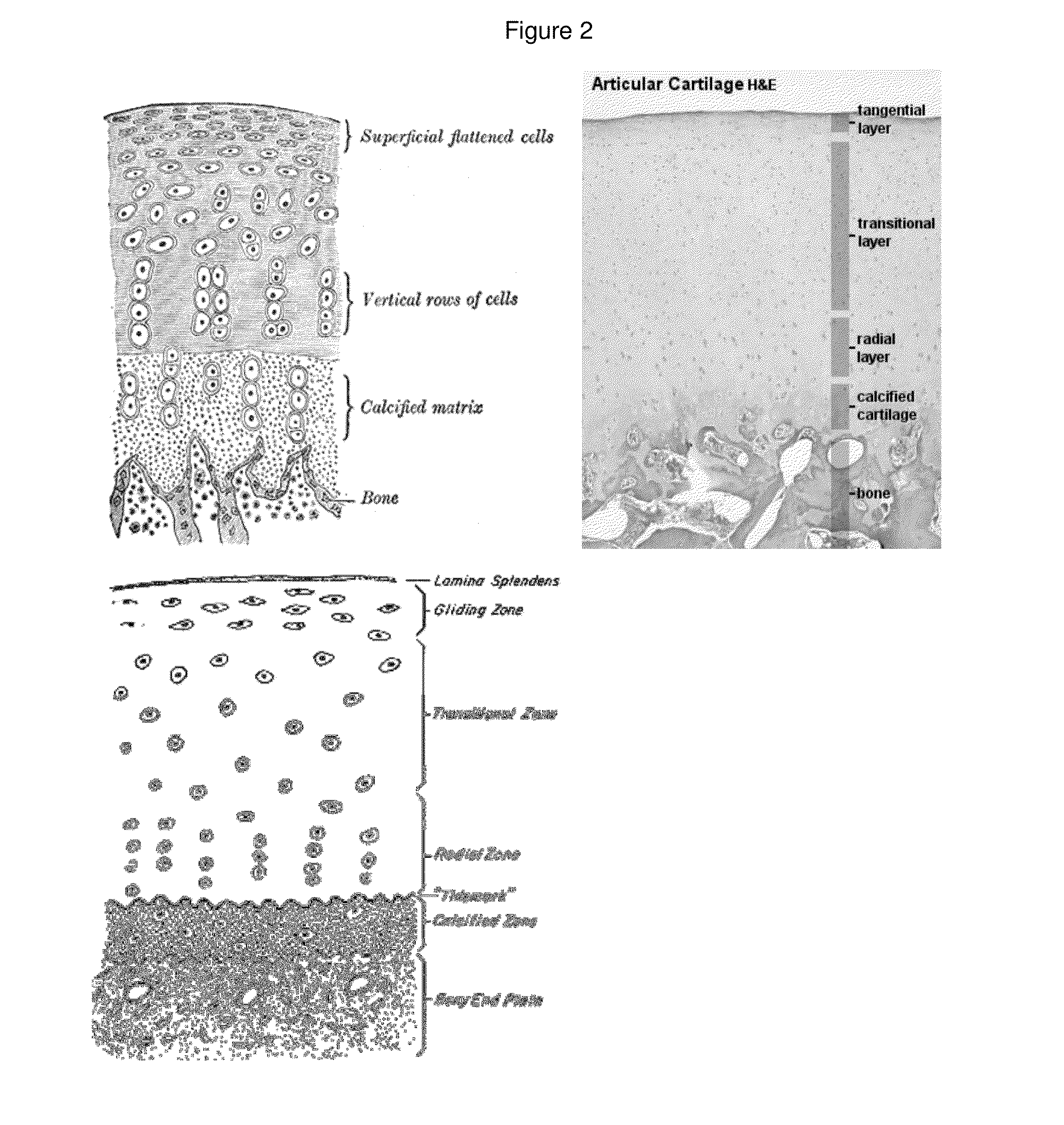
[0277]Osteochondral plugs were obtained as detailed in Example 2. The subchondral bone and calcified cartilage was removed from the osteochondral plugs to provide cartilage samples in the form of cartilage disks. During this process, the cartilage was chilled periodically with chilled saline to prevent overheating.
[0278]Specifically, each osteochondral plug was held securely and the subchondral bone layer was cut (removed) using a sagittal saw with a bent angle blade from the layer of cartilage. Once the subchondral bone was removed, any remaining bone and calcified cartilage was shaved from the underside of the cartilage discs. To prevent overheating, the tissue was frequently immersed in chilled saline throughout the sawing and shaving process. This process was repeated for each of the cartilage disks.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com