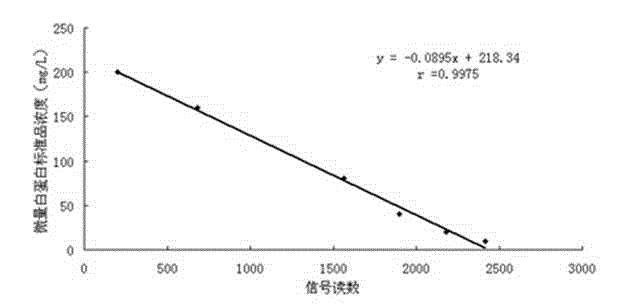
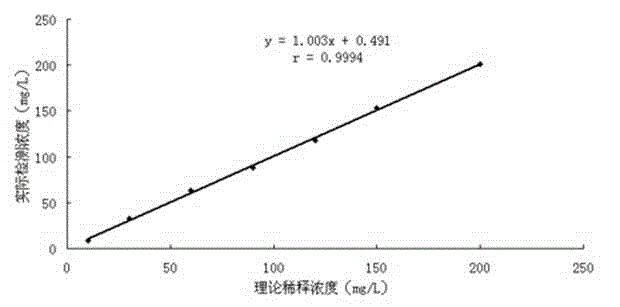
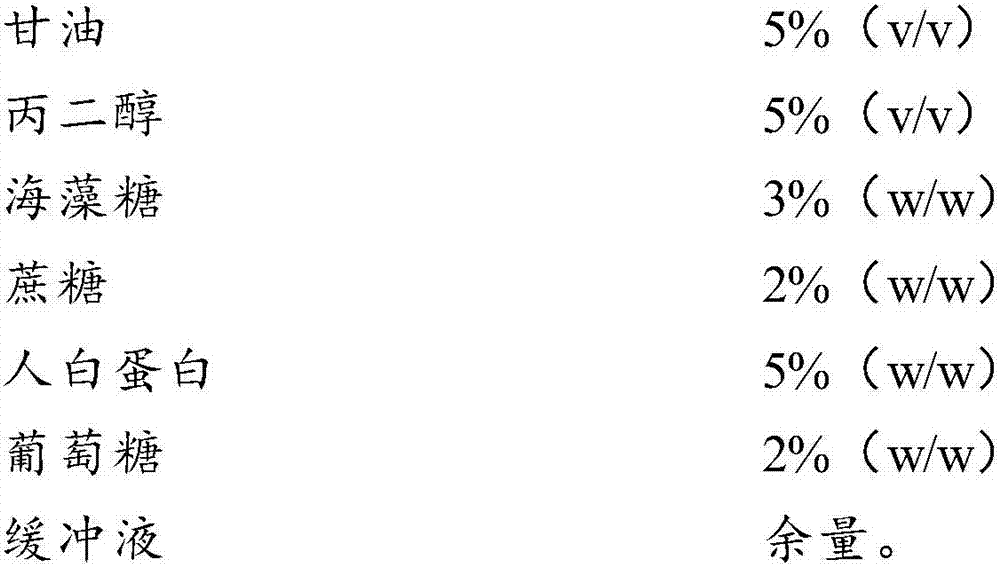Patents
Literature
336 results about "Human albumin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
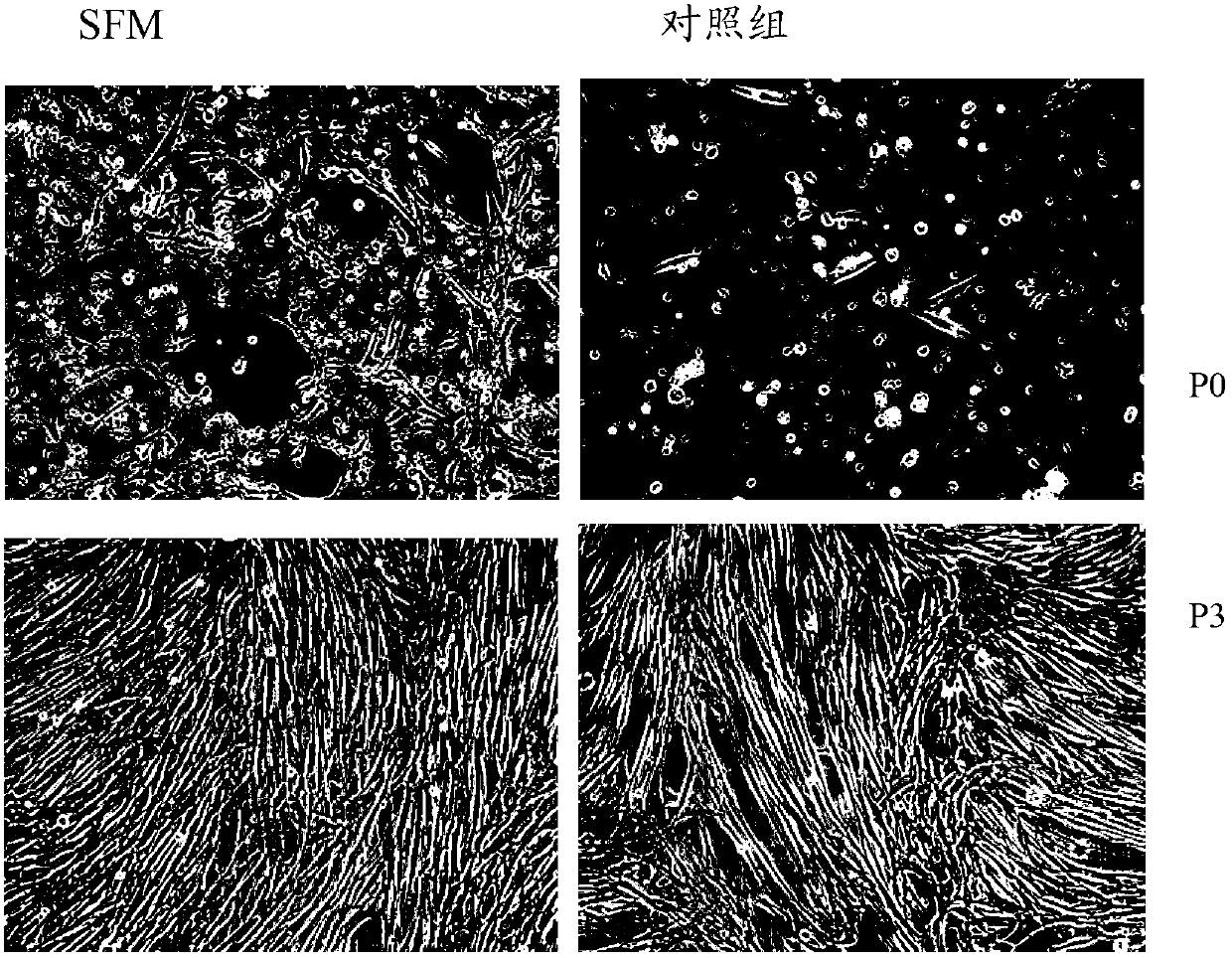
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Mesenchyme stem cell preserving fluid and use thereof
ActiveCN101210232AImprove survival rateReduce adhesionDead animal preservationTissue culturePhosphate ionCell mass
The invention discloses a preservation solution for mesenchymal stem cells and application thereof. The cell preservation solution contains human albumin and heparin as the main components, and other auxiliary reagents such as human cytokine, phosphate ion, metal ions or monosaccharide are contained in a buffer solution for preserving human mesenchymal stem cells. The preservation solution can keep high survival rate of human mesenchymal stem cells in transportation process, reduce adhesion between cells and between the cell and the inner wall of a container, and reduce the possible occurrence of cell mass embolism in blood vessel while clinically infusing human mesenchymal stem cells. The mesenchymal stem cells can be maintained in a state of single-cell suspension at an environment temperature of 4 to 15 DEG C for 24 h, thus greatly enlarging the clinic application range of the human mesenchymal stem cells. The components used in the solution accord with the clinic application, and can meet the requirement for clinic use of the human mesenchymal stem cells.
Owner:TIANJIN AMCELLGENE ENG
Cell cryo-preserved liquid, application, and immune cell cryo-preservation method
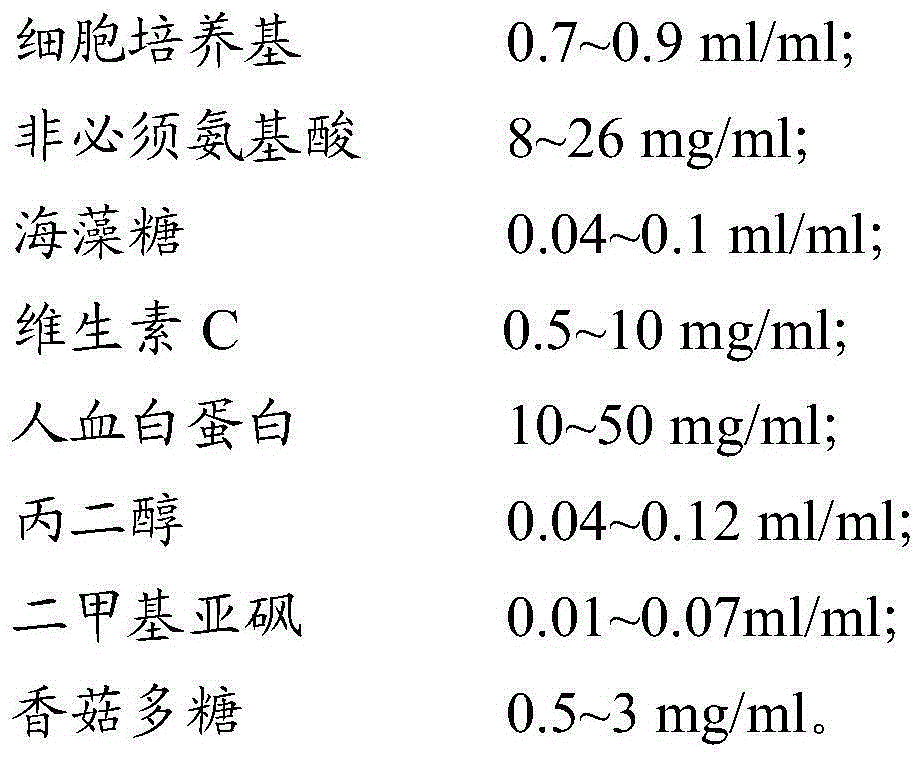
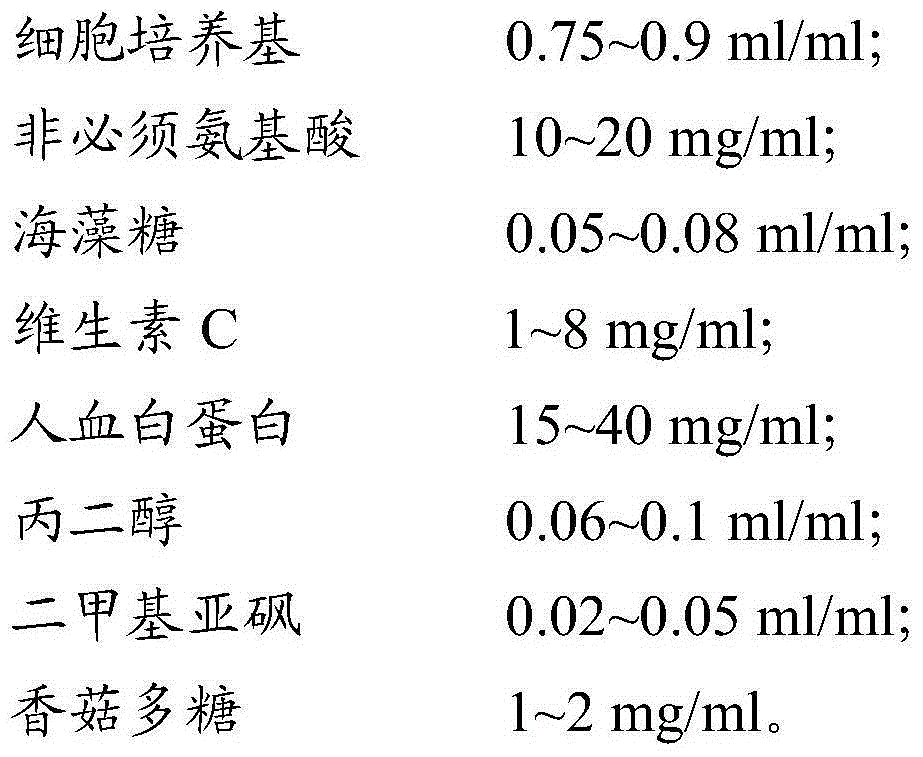
The invention provides a cell cryo-preserved liquid, an application, and an immune cell cryo-preservation method. The cell cryo-preserved liquid includes 0.7-0.9 ml / ml of a cell culture medium, 8-26 mg / ml of non-essential amino acids, 0.04-0.1 ml / ml of trehalose, 0.5-10 mg / ml of vitamin C, 10-50 mg / ml of human albumin, 0.04-0.12 ml / ml of propylene glycol, 0.01-0.07 ml / ml of dimethyl sulfoxide and 0.5-3 mg / ml of lentinan. Compared with a cell cryo-preserved liquid in the prior art, the human albumin can replace fetal calf serum to achieve the effects of the fetal calf serum in the cell cryo-preserved liquid, and meanwhile, the cell cryo-preserved liquid is added with the non-essential amino acids, the vitamin C and the lentinan, thereby ensuring cell proliferation vitality after recovery is not influenced. The trehalose and the propylene glycol can replace a protective effect of dimethyl sulfoxide, thereby ensuring moisture in cells to be not crystallized during approach of freezing point. The components are cooperated with each other to achieve cryo-preservation of immune cells.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Cell freezing medium
The invention provides a cell freezing medium which can be clinically safely used and is used for freezing human cells. The cell freezing medium comprises: 2-8wt% of human albumin, 5-10 volume% of dimethyl sulfoxide, 3.5-5.1wt% of dextranum-40 and 2.75-4.25wt% of glucose.
Owner:BEIJING YONGTAI IMMUNITY APPL TECH
Recombinant human albumin fusion proteins with long-lasting biological effects
ActiveUS7244833B2Good for healthImprove stabilityBacteriaPeptide/protein ingredientsDiseaseHuman albumin
Compositions, kits and methods are provided for promoting general health or for prevention or treatment of diseases by using novel recombinant fusion proteins of human serum albumin (HSA) and bioactive molecules. The bioactive molecules may be a protein or peptide having a biological function in vitro or in vivo, and preferably, having a therapeutic activity when administered to a human. By fusing the bioactive molecule to HSA, stability of the bioactive molecule in vivo can be improved and the therapeutic index increased due to reduced toxicity and longer-lasting therapeutic effects in vivo. In addition, manufacturing processes are provided for efficient, cost-effective production of these recombinant proteins in yeast.
Owner:YU ZAILIN +1
Human umbilical cord mesenchymal stem cell (HUMSC) anti-hepatic fibrosis injection and preparation method thereof
ActiveCN102008507ALong shelf lifeRepair damageDigestive systemUnknown materialsRingers solutionMedicine
The invention discloses a human umbilical cord mesenchymal stem cell (HUMSC) anti-hepatic fibrosis injection and a preparation method thereof. The injection is composed of 10%-50% of human albumin stock solution, 49.5%-89.5% of acetated Ringer's solution and 0.5% of heparin calcium, wherein, the concentration of the human albumin stock solution is 10%, and 1ml of the injection contains 6*10<5>-7*10<5> HUMSCs. The preparation method comprises the following steps: providing an MSC preserving fluid and pre-cooling the MSC preserving fluid for later use, wherein, the MSC preserving fluid comprises the human albumin stock solution, the Acetated Ringer's solution and the heparin calcium; providing the HUMSCs and adding the HUMSCs to the MSC preserving fluid; and adjusting the number of the HUMSCs added to the MSC preserving fluid to be 6*10<5>-7*10<5> HUMSCs / ml by means of re-suspending. The injection provided by the invention has the advantages of obvious curative effect and high stability, and is convenient in storage and transport and safe in use, thus being capable of bringing gospel to patients with hepatic fibrosis.
Owner:中源协和生物细胞存储服务(天津)有限公司
Mesenchymal stem cell injection, preparation method and application thereof in preparing medicine for treating diabetes
InactiveCN102920735AIncrease productionEasy quality controlCell dissociation methodsPeptide/protein ingredientsVitamin CSide effect
The invention discloses a mesenchymal stem cell injection, a preparation method and application of the mesenchymal stem cell injection in preparing a medicine for treating diabetes; the mesenchymal stem cells are derived from human umbilical cord and placenta; the mesenchymal stem cell injection consists of components: human mesenchymal stem cells, human albumin, low molecular heparin calcium, compound amino acid, vitamin C of 0.5% and dissolving medium; and the dissolving medium can be a compound electrolyte solution or glucose or normal saline. According to the mesenchymal stem cell injection, the preparation method and the application of the mesenchymal stem cell injection in preparing the medicine for treating the diabetes, the mesenchymal stem cell injection is used for repairing injured islet beta cells, and the blood sugar is reduced by secretion of the endogenous insulin, therefore, a purpose of foundational treating the diabetes is achieved. A disease course of the diabetes can be reversed, patients are helped in escaping out of inconvenience and toxic and side effects of taking the endogenous drug and injecting the insulin as well as serious complications caused by poor control of the blood sugar; and the 1-type and 2-type diabetes are treated thoroughly.
Owner:青岛奥克生物开发有限公司
Recombined human hyaluronidase, production and purification method and preparations thereof, use method and application
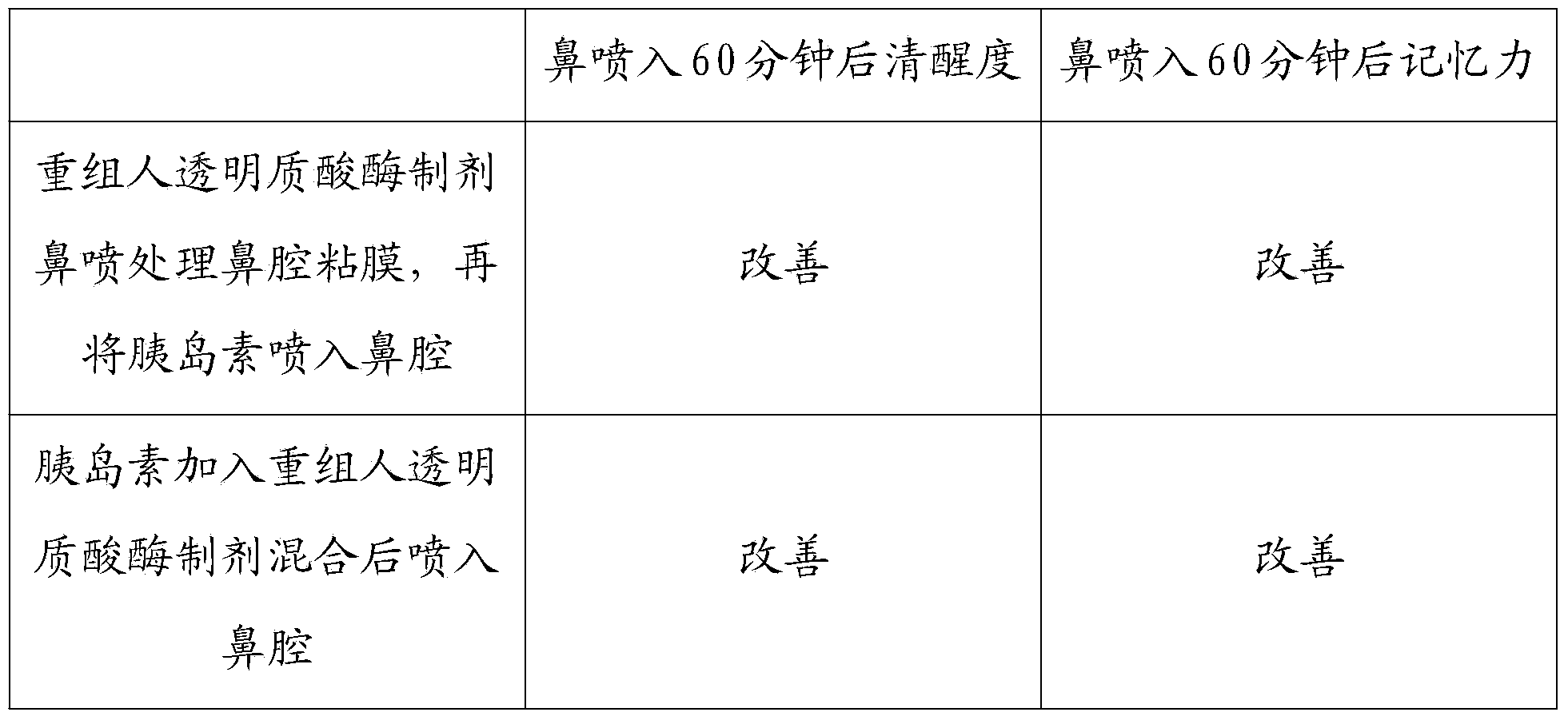
InactiveCN103468662AImprove permeabilityPharmaceutical non-active ingredientsEnzymesNasal cavityDisease
The invention discloses a recombined human hyaluronidase, a production and purification method and preparations of the recombined human hyaluronidase, a use method and application. Recombined human hyaluronidase PH20 or human hyaluronidase human albumin fusion protein PH20-HSA or human hyaluronidase human immunoglobulin IgG2Fc fragment fusion protein PH20-IgFc is adopted by the recombined human hyaluronidase and used in the mucosa or the surface of the skin. The preparations of the recombined human hyaluronidase can be made into different types such as membrane preparations, spray preparations, lotion and freeze-dried powder spray and used for skin infiltration promotion of beauty nutrient substances, skin mucosa infiltration promotion of surface anesthetic, infiltration promotion of skin disease therapeutic medicine, mucosa infiltration promotion of biological tranquillizer, mucosa skin infiltration promotion of growth factors, mucosa infiltration promotion of hypoglycemic drug, mucosa nasal cavity infiltration promotion of nervous centralis nutrient substances and the like.
Owner:惠觅宙
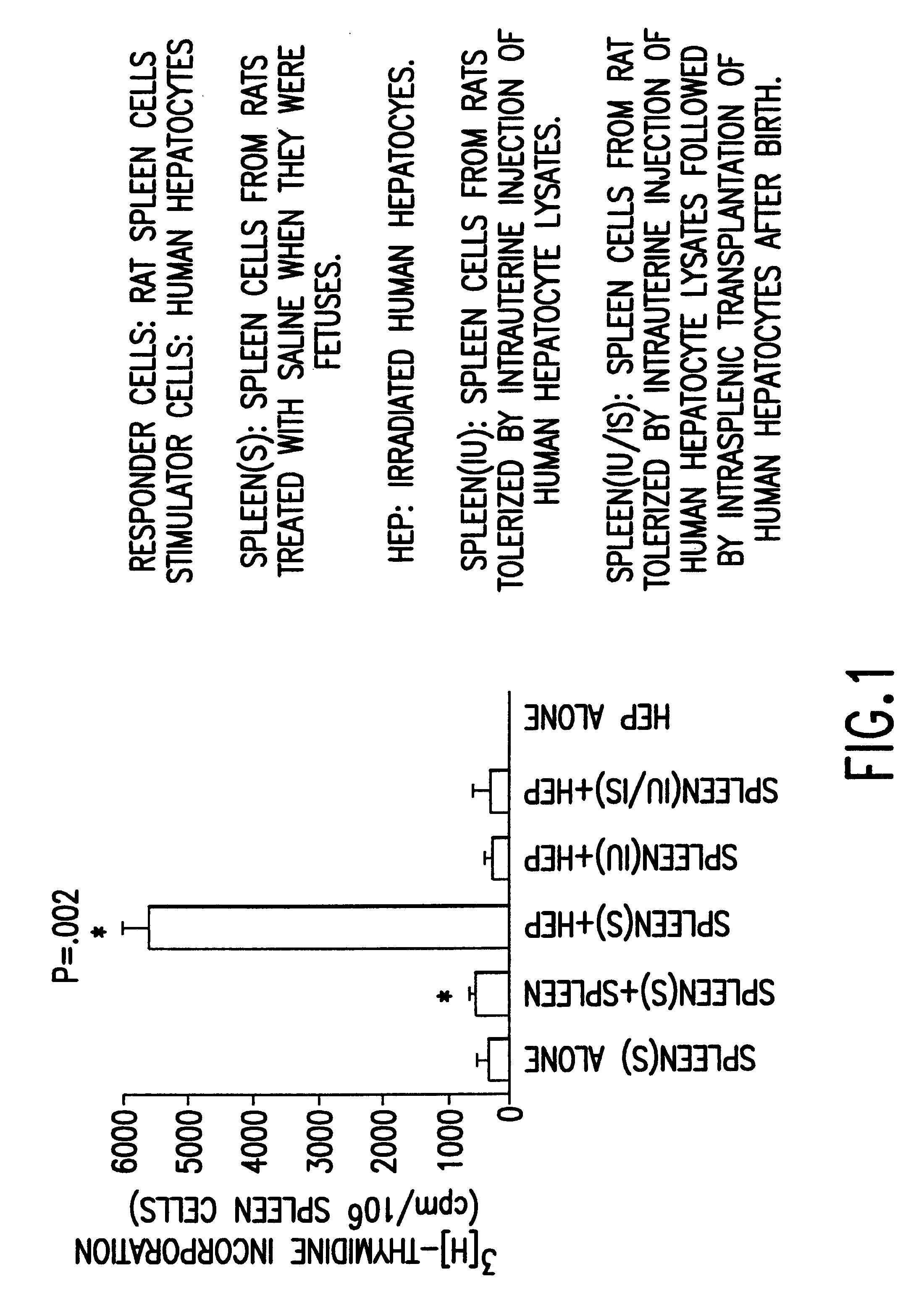
Propagation of human hepatocytes in non-human mammals
The present invention relates to the preparation of non-human animals having chimeric livers, whereby some or substantially all of the hepatocytes present are human hepatocytes. It is based, at least in part, on the discovery that rats, tolerized in utero against human hepatocytes, were found to serve as long-term hosts for human hepatocytes introduced post-natally, and the introduced hepatocytes maintained their differentiated phenotype, as evidenced by continued production of human albumin.
Owner:UNIV OF CONNECTICUT
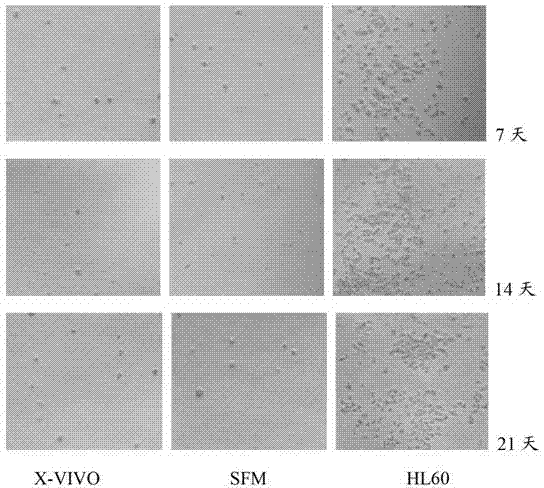
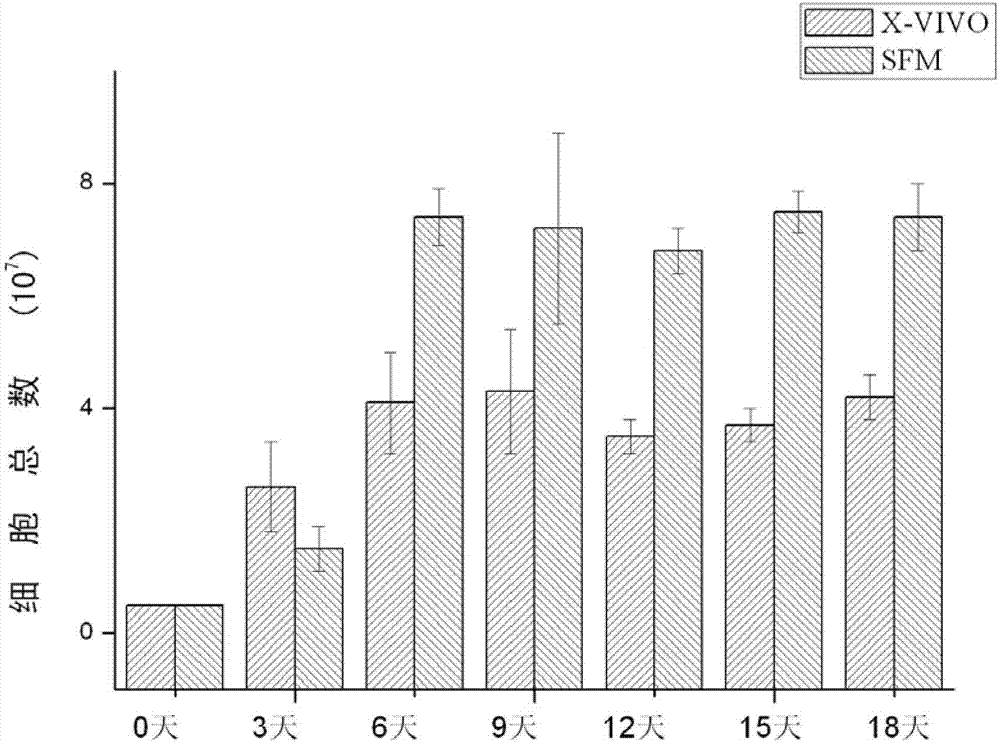
SFM (serum-free medium) for culturing MSCs (mesenchymal stem cells)
ActiveCN103555665AClear ingredientsAvoid heterogeneous contaminationSkeletal/connective tissue cellsSodium bicarbonateSerum free media
The invention relates to an SFM (serum-free medium) for culturing MSCs (mesenchymal stem cells). Based on volume, the SFM comprises the following components: 10.2 grams per liter of alpha-MEM (alpha-minimum essential medium), 2.4 grams per liter of sodium bicarbonate, 1 to 5 millimoles of L-glutamine, 50 to 300 milligrams per liter of poloxamer 188, 2 to 8 grams per liter of recombinant human albumin, 10 to 20 milligrams per liter of recombinant human transferrin, 2 to 10 milligrams per liter of recombinant human insulin, 1 to 5 millimoles per liter of Hepes, 50 nanomoles of beta-mercaptoethanol, 0.1 to 1 milligram per liter of lipid, 1 to 5 milligrams per liter of trace element, 0.1 to 5 milligrams per liter of glutathione, 0.5 to 5 milligrams per liter of para-aminobenzoic acid, 1 to 50 nanograms per milliliter of hydrocortisone, 20 to 50 milligrams per liter of vitamin PP, 5 to 50 milligrams per liter of vitamin C, 2 to 10mu M of compound shown in a formula I, 5 to 20mu M of compound shown in a formula II, 10 to 20 nanograms per milliliter of progestin, 1 to 10 milligrams per liter of putrescine, 1 to 10 international units per liter of heparin, 1 to 10 nanograms per milliliter of EGF (epidermal growth factor), 1 to 10 nanograms per milliliter of b-FGF (b-fibroblast growth factor), 1 to 10 nanograms per milliliter of HGF (hepatocyte growth factor) and 1 to 10 nanograms per milliliter of VEGF (vascular endothelial growth factor). The SFM for culturing the MSCs is a BPS-SFM which has determinate chemical components and is free of animal-derived substances.
Owner:BEIJING DONGFANG HUAHUI BIOMEDICAL TECH
Vaccine cryoprotectant without composition of gelatin and human albumin
ActiveCN102657870ALow costLow endotoxin contentPharmaceutical non-active ingredientsAntibody medical ingredientsMonosodium glutamateSucrose
The invention discloses a vaccine cryoprotectant without composition of gelatin and human albumin. The vaccine cryoprotectant comprises dextran (40), cane sugar, lactose, mannitol, glycine, arginine, sodium glutamate, urea and 199 synthetic medium. The invention does not contain ingredients of animal origin and the content of endotoxin of vaccine is greatly decreased, thus irritation and harm to a human body are greatly decreased and the vaccine has better safety and stability. Meantime, the cryoprotectant disclosed by the invention does not contain expensive raw materials such as trehalose and the like, thus the cryoprotectant has the advantage of low cost and is more applicable to industrialization.
Owner:CHANGCHUN KEYGEN BIOLOGICAL PROD
Umbilical cord mesenchymal stem cell injection as well as preparation method and application thereof
InactiveCN104873542AImprove the quality of lifeReduce releaseDigestive systemPharmaceutical delivery mechanismHydroxyethyl starchClinical grade
The invention provides umbilical cord mesenchymal stem cell injection as well as a preparation method and application thereof. The umbilical cord mesenchymal stem cell injection comprises mesenchymal stem cells and a frozen stock solution, wherein the frozen stock solution comprises the following components: a balanced electrolyte solution with the volume percentage of 25-70 percent, hydroxyethyl starch 130 / 0.4 with the mass / volume percentage (g / ml) of 1-10 percent, triphosadenine disodium magnesium chloride with the volume percentage of 5-20 percent, clinical-grade DMSO with the volume percentage of 5-20% and 20% human albumin with the volume percentage of 1-50 percent. The prepared umbilical cord mesenchymal stem cell injection is free of animal serum, safe, high in cell viability after cryopreservation resuscitation, can be used for treating inflammatory bowel disease, has a good effect and has no toxic or side effects.
Owner:北京青藤谷禧干细胞科技研究院有限公司
Mesenchymal stem cell low-temperature preserving fluid and preparation method thereof
InactiveCN106922648AHigh activityProlong the duration of activityDead animal preservationSodium acetateSide effect
The invention discloses mesenchymal stem cell low-temperature preserving fluid and a preparation method thereof. The mesenchymal stem cell low-temperature preserving fluid is prepared from human albumin injection and compound electrolyte injection according to a volume ratio of (1:100) to (1:5). Per 25ml of human albumin injection contains 5g of human albumin; per 1000 mL of the compound electrolyte injection contains 5.26g of sodium chloride, 5.02g of sodium gluconate, 3.68g of sodium acetate, 0.37g of potassium chloride and 0.30g of magnesium chloride. The invention also discloses an injection prepared from the low-temperature preserving fluid and a preparation method thereof. The use of freeze storage protection agents having the toxic and side effects and causing injury on cells is avoided; the freeze storage and unfreezing steps are avoided; the clinic safety, simplicity and convenience are greatly improved; meanwhile, the activity maintenance time of stem cells is prolonged; the condition that the cell activity can still meet the clinic use requirements after the 24h transportation. The mesenchymal stem cell low-temperature preserving fluid has the advantages that the safety is high; the transportation is convenient; the cell activity is high; the cost is low.
Owner:浙江新生泉细胞科技有限公司
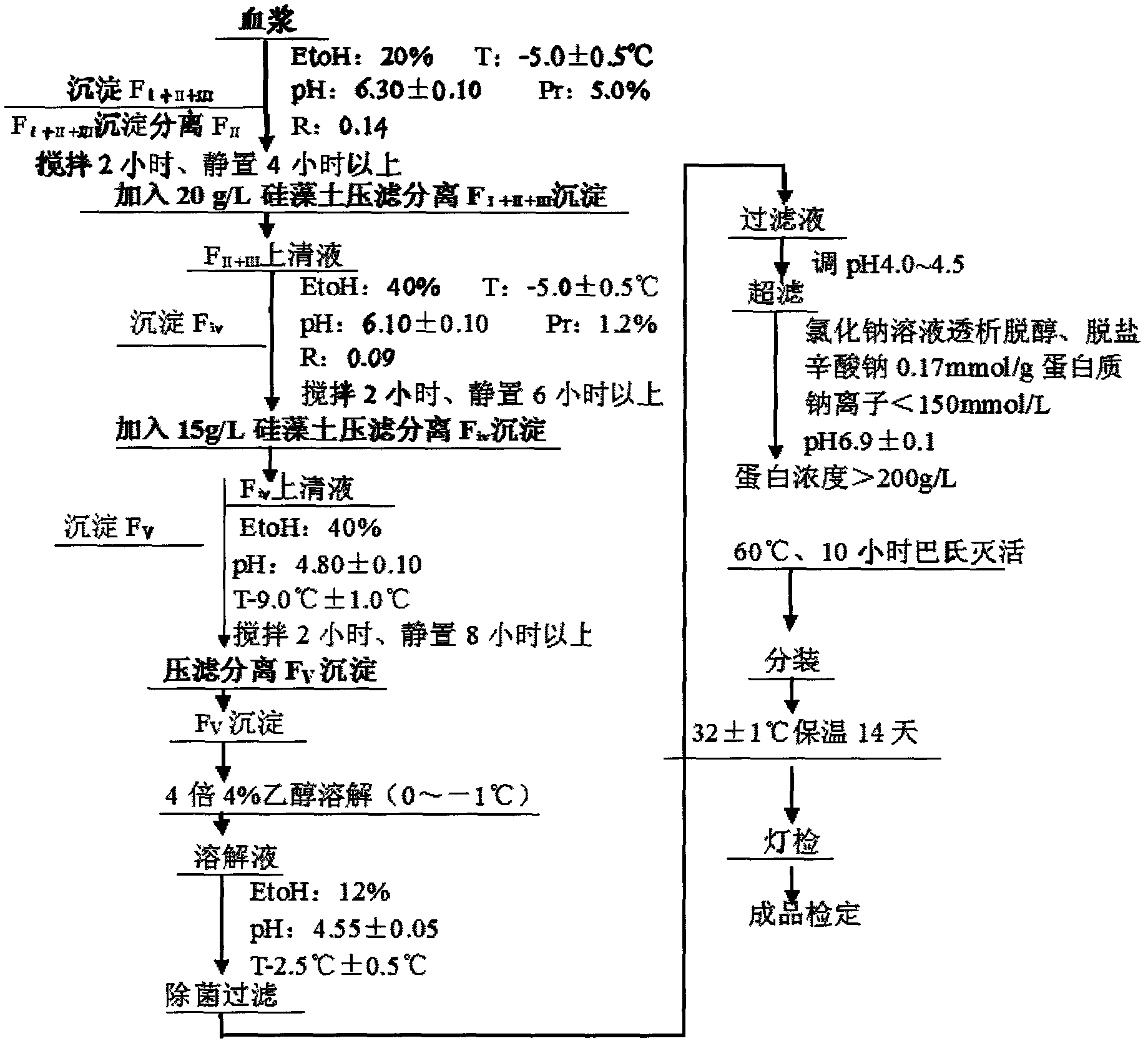
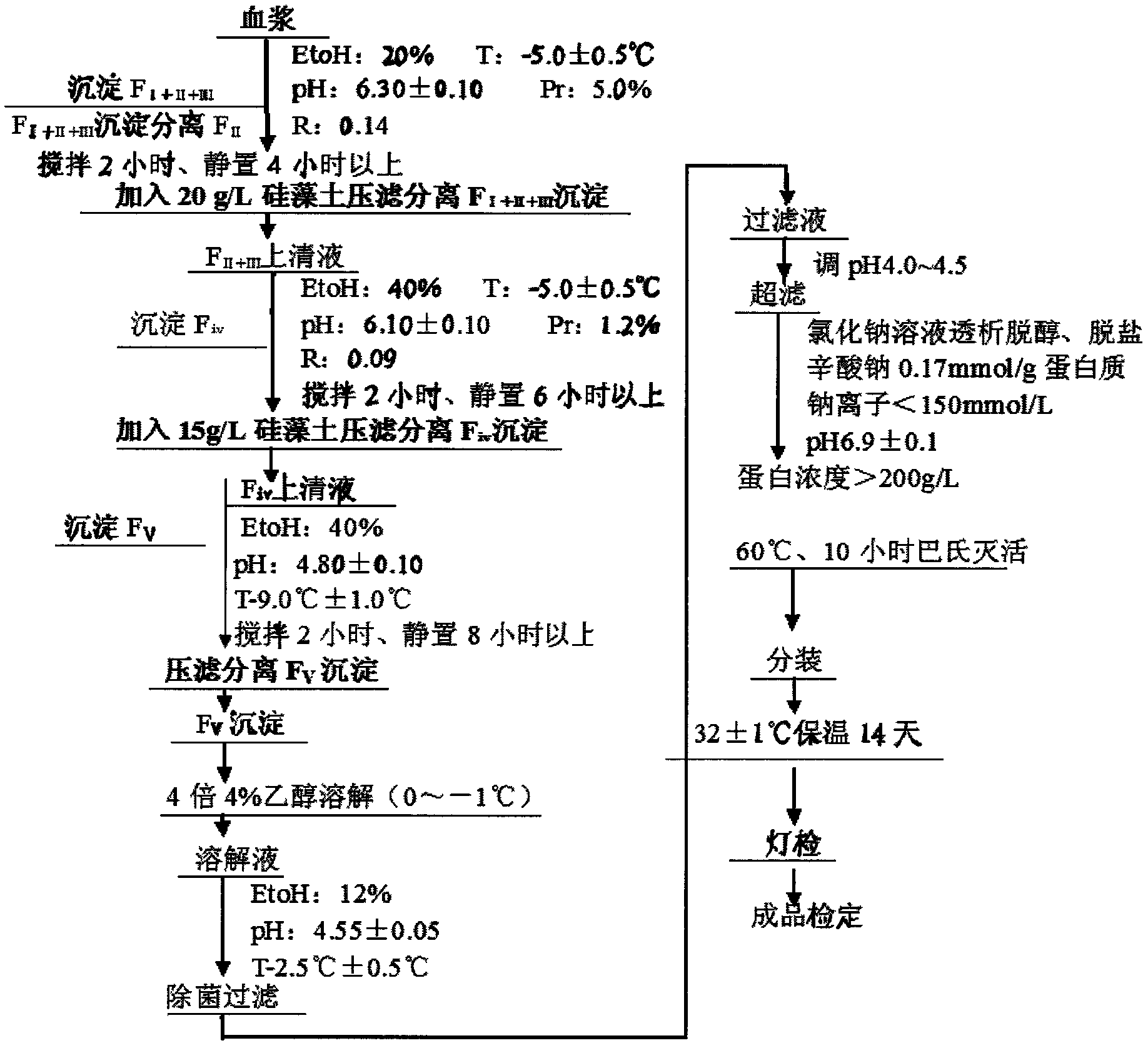
Preparation method of human serum albumin
InactiveCN102532304AStructure has no effectQuality improvementSerum albuminPeptide preparation methodsAluminum IonProtein molecules
The invention relates to a method for separating and extracting protein in a biological product technology, in particular to a preparation method of human serum albumin. The preparation method comprises the following steps of: separating components I, II and III, separating a component IV, separating a component V, and refining and purifying; and performing ultrafiltration, pasteurization and sterilized filling, examining a finished product, and packaging the qualified product by pasting a label, boxing and the like. According to the preparation method disclosed by the invention, a relatively-moderate pressure-filtration separation method is adopted, so that the molecular structure of the protein is not influenced, and the human serum albumin with high quality is ensured to be prepared; moreover, the product yield is greatly increased, the human serum albumin which is low in aluminum ion content or even does not contain aluminum ions can be provided for clinical application, and the quality of the human serum albumin is improved.
Owner:WUHAN ZHONGYUAN RUIDE BIOLOGICAL PROD CO LTD
Hepatic stem cell preserving solution and applications of hepatic stem cell preserving solution
ActiveCN102948413AImprove stabilityGood curative effectDead animal preservationSodium acetateSide effect
The invention discloses hepatic stem cell preserving solution and applications of the hepatic stem cell preserving solution. The hepatic stem cell preserving solution provided by the invention is prepared by fixing the volume of injection solution including 0.1 to 1g of human albumin, 2.60 to 4.97g of sodium chloride, 2.48 to 4.74g of sodium gluconate, 1.82 to 3.40g of sodium acetate, 0.18 to 0.35g of potassium chloride, 0.15 to 0.28g of magnesium chloride, and 0.4 to 0.7mL of heparin calcium based on effective dose by water to be reach to 100mL. The hepatic stem cell preserving solution provided by the invention is used for storing hepatic stem cells, and the survival rate of the hepatic stem cell is more than 85% and even more than 95% within 12 hours. The cell suspension which is obtained by floating the hepatic stem cells on the hepatic stem cell preserving solution can be used as the medicine for treating diabetes mellitus, and has the characteristics of being high in stability, good in curative effect, high in safety, being without toxic or side effect, convenient to store and transport, applicable to massive clinical use, and broad in application prospect. The hepatic stem cell preserving solution brings good news for diabetic patients, and provides a new way for clinical stem cell use.
Owner:北京清美联创干细胞科技有限公司
Storage solution capable of storing human mesenchymal stem cell at 4 DEG C
InactiveCN108651442AMeet short-distance transportation needsConvenient for clinical operationDead animal preservationHuman bodySodium lactate
The invention relates to a storage solution capable of storing a human mesenchymal stem cell at 4 DEG C, the storage solution comprises the ingredients of human albumin, trehalose, sodium chloride, glucose, sodium lactate ringer's injection, heparin, L-glutamine, EDTA (Ethylene Diamine Tetraacetic Acid), a phosphate buffer solution, sodium selenite, an essential amino acid, and a non-essential amino acid. The provided formula guarantees that more than 80% of the vitality and more than 70% of the adhesion rate of the cell are kept after 48 hours in the storage and transportation, the requirement on the short-distance transportation of the fresh mesenchymal stem cell is met, the storage solution uses the clinical medicine having clear compositions, is safe to the human body, can be directlyinjected when recovered to the room temperature, and greatly facilitates the clinical application of the mesenchymal stem cell after the short-distance transportation.
Owner:广东佰鸿干细胞再生医学有限公司
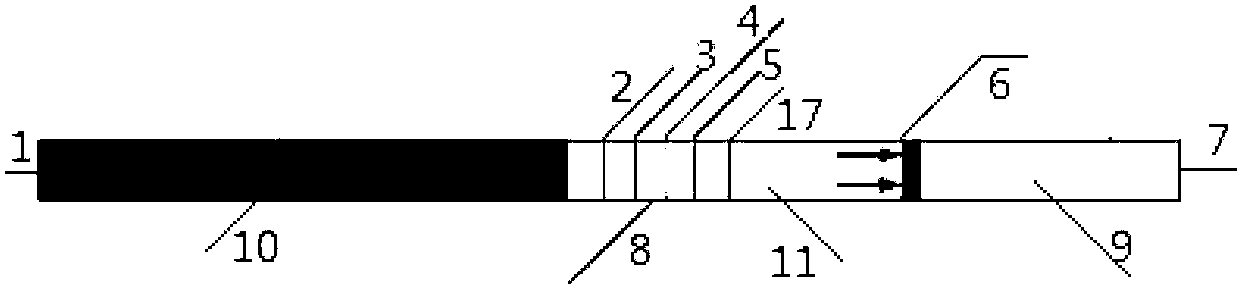
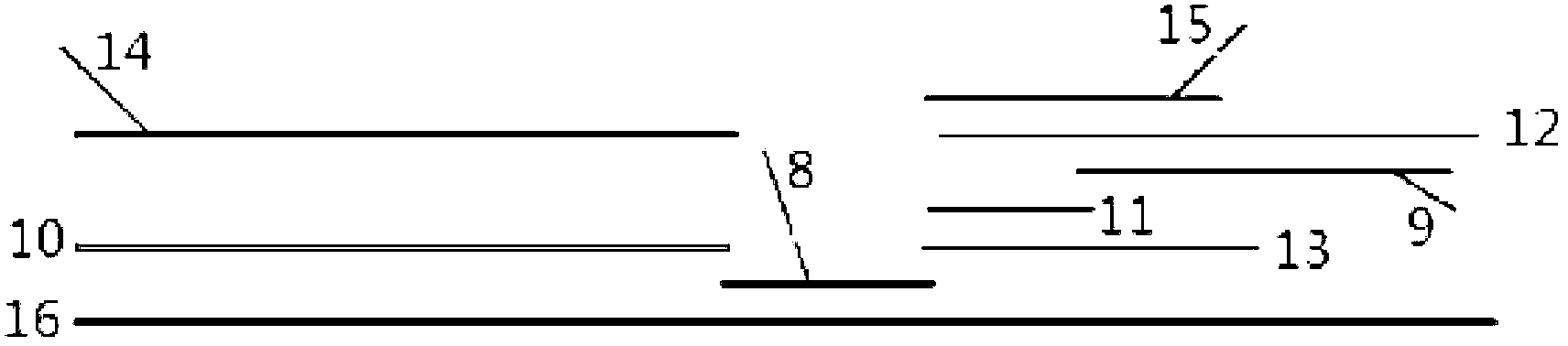
Test strip for semi-quantitative detection of microalbuminuria
The invention discloses a test strip for semi-quantitative detection of microalbuminuria, belonging to medical test consumables. The test strip is characterized in that the test strip for semi-quantitative detection of microalbuminuria is a colloidal gold test strip. An anti-human albumin antibody and rabbit IgG with acolloidal gold label is enveloped on a gold label fiber pad; anti-rabbit IgG antibody is enveloped on a quality control line C, and human albumin is enveloped on a detection line T. The test strip is simple, sensitive and accurate, can be used for semi-quantitative detection of microalbuminuria in human urine and suitable for detection of early renal injury patients.
Owner:蓝十字生物药业(北京)有限公司
Mesenchymal stem cell injection and preparation method thereof as well as application in preparation of medicine for treating ulcerative colitis
InactiveCN102920734AIncrease productionEasy quality controlDigestive systemMammal material medical ingredientsSide effectUlcerative colitis
The invention discloses a mesenchymal stem cell injection and a preparation method of the mesenchymal stem cell injection and an application in preparation of a medicine for treating ulcerative colitis, wherein the mesenchymal stem cell injection consists of the following components: 2*105-1*107 pieces / ml of mesenchymal stem cells, 5-10% of dimethyl sulfoxide (DMSO) by volume, 1-6% of human albumin by mass-to-volume ratio, and 1% of low molecular dextran and compound electrolyte solution. According to the mesenchymal stem cell injection, the preparation method and the application of the mesenchymal stem cell injection in preparation of the medicine for treating ulcerative colitis, the mesenchymal stem cell injection is used for correcting immune dysfunction of a patient of ulcerative colitis, and preventing the autoimmune dysfunction from continuously damaging the intestinal tract; meanwhile, many cell growth factors and repair factors are secreted, to promote pathologically changed intestinal ulcer to heal, therefore, the purpose of foundational treating the ulcerative colitis is achieved. The disease course of the ulcerative colitis can be reversed, the patient can be helped in escaping out of influence of stomachache and diarrhea on the life as well as toxic and side effects of the medicine, so as to treat the ulcerative colitis thoroughly.
Owner:青岛奥克生物开发有限公司
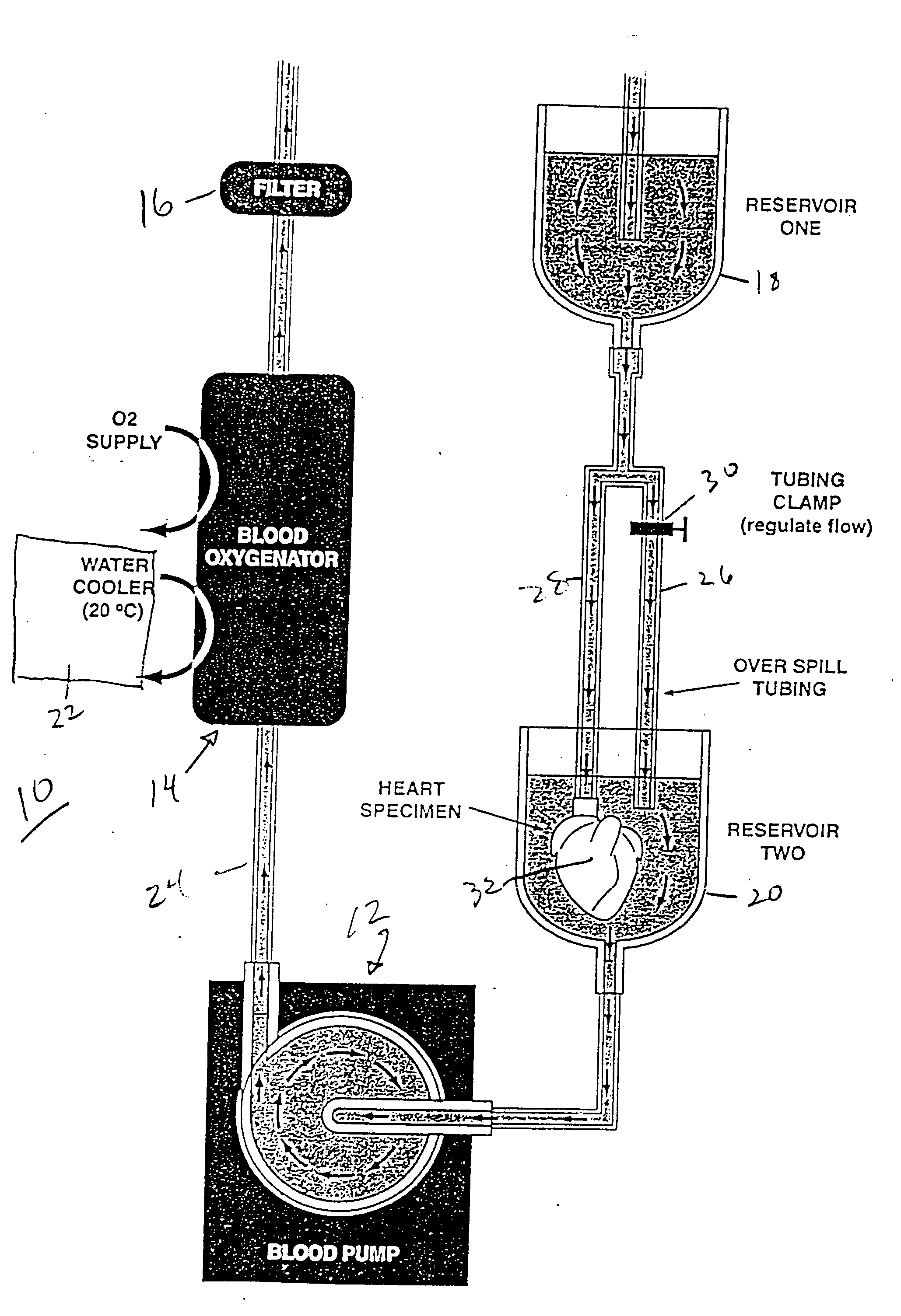
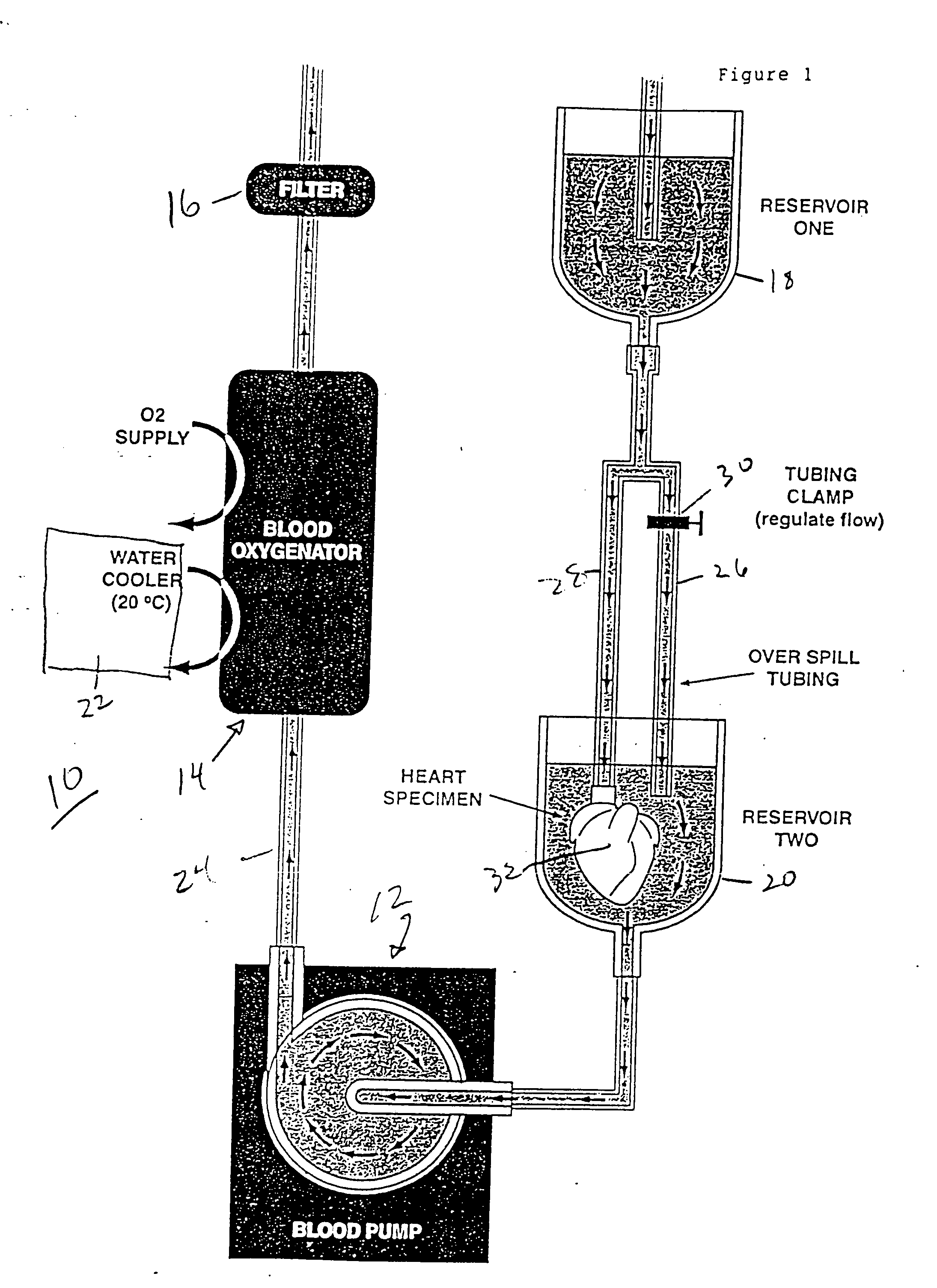
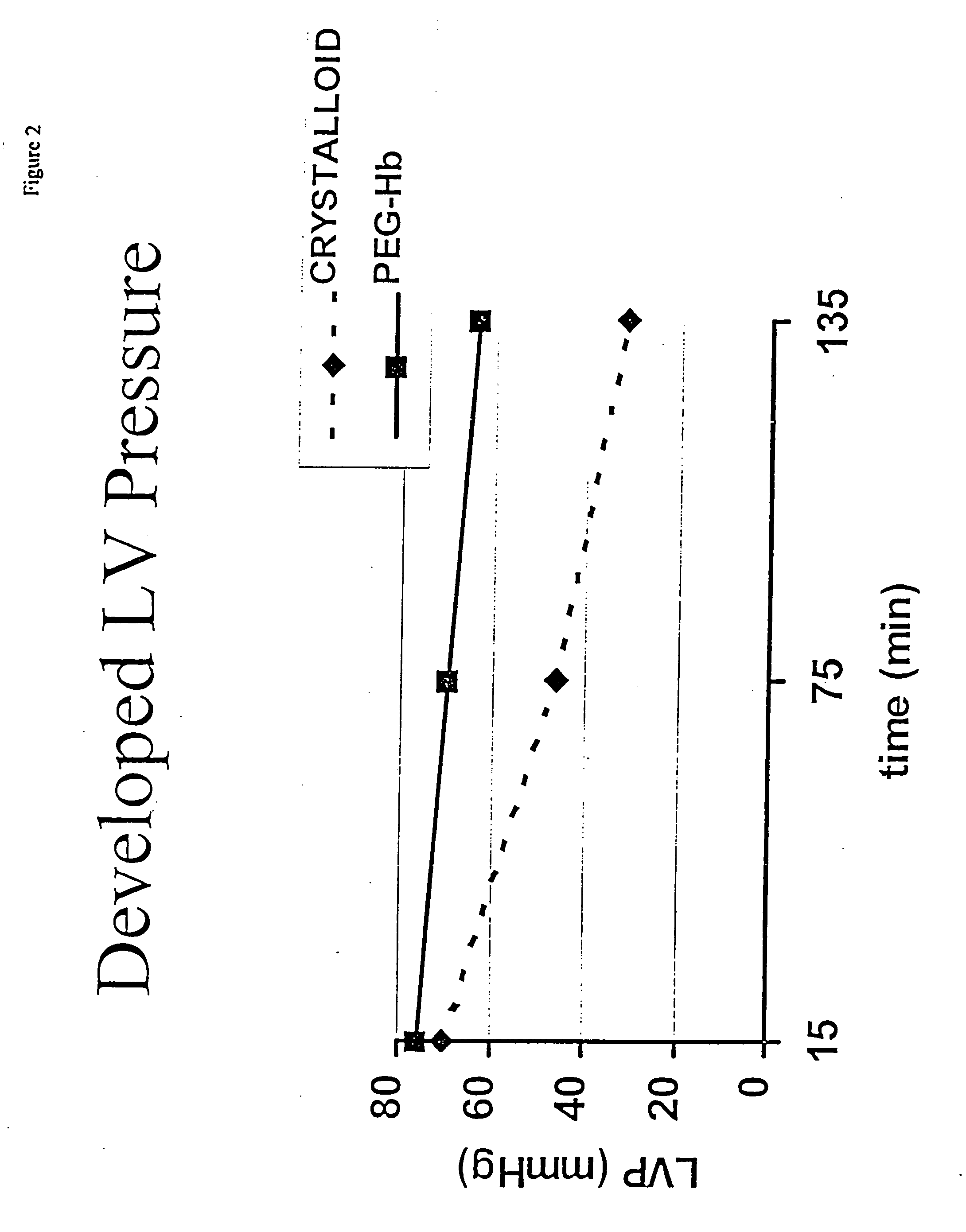
Continuous cardiac perfusion preservation with PEG-HB for improved hypothermic storage
InactiveUS20070243518A1Extended storage timeEasy to storeDead animal preservationHuman albuminPotassium
Efforts to extend myocardial preservation for transplantation by perfusion with prior crystalloid based solutions have been limited by edema and compromised function. Hypothermic perfusion preservation with a polyethylene glycol (PEG) conjugated hemoglobin solution extends preservation times. The polyethylene glycol (PEG) conjugated hemoglobin solution comprises PEG-Hb, and at least one of the constituents selected from the group of human albumin, dextrose, heparin sodium, lidocaine HCl, MgSO4, KCl, CaCl2, Tromethamine (THAM) solution, NaCl, NaHCO3, and Na2HPO4 / NaH2PO4. Comparison of cardiac function after continuous perfusion using a hypocalcemic normokalemic crystalloid perfusate is made with and without the addition of PEG-Hemoglobin (Hb).
Owner:RGT UNIV OF CALIFORNIA
General serum-free crypreservation solution for MSCs (mesenchymal stem cells) and preparation method of crypreservation solution
InactiveCN109090102AResuscitative activity is not lowAvoid reactionDead animal preservationSerum free mediaHydroxyethyl starch
The invention discloses a general serum-free crypreservation solution for MSCs (mesenchymal stem cells) and a preparation method of the crypreservation solution. The crypreservation solution is prepared from components in percentage by volume as follows: 0.5%-10% of a compound dextran 40 injection , 1%-10% of hydroxyethyl starch (130 / 0.4), 5%-30% of glycerin, 25%-70% of a serum-free medium, 0.5%-1% of a serum substitute, 20%-40% of human albumin, 0.05%-1% of vitamin C, 0.2%-1% of vitamin E and 0.5%-3% of nonessential amino acid. By the aid of the crypreservation solution, on the premise that the cell activity of MSCs under the deep hypothermia cryopreservation condition is not lower than that in existing methods, potential risks caused by low toxicity and complement reactions of certain components in existing general methods are avoided.
Owner:成都汇欣生命科技有限公司
Mesenchymal stem cell injection, as well as preparation method and application in preparing medicament for treating children dilated cardiomyopathy
InactiveCN102908364AIncrease productionEasy quality controlPeptide/protein ingredientsMammal material medical ingredientsClinical gradeLife quality
The invention provides mesenchymal stem cell injection, as well as a preparation method and application in preparing a medicament for treating children dilated cardiomyopathy. The mesenchymal stem cell injection comprises 2*10<5>-1*10<7> mesenchymal stem cells in every milliliter, clinical grade DMSO (dimethylsulfoxide) with volume ratio of 5 to 8 percent, human albumin with mass volume ratio of 1 to 6 percent, and multi-electrolyte liquid. The mesenchymal stem cell injection is prepared from placenta and umbilical cord, has the advantages of being high in yield, and being easy to realize industrialization, the preparation system quality is easy to control; the injection can be directly frozen through program-controlled freezing procedure and directly used in clinical injection after being recovered, and is safe and reliable. According to the injection, the dilated cardial structure can be changed, the function of heart can be recovered, the water supply state of each tissue in the whole body can be improved, the living quality of a patient can be improved, and the dilated cardiomyopathy can be treated fundamentally, so that the patient can get rid of serious complications caused by massive medicine administration, living action limitation and poor disease control.
Owner:青岛奥克生物开发有限公司
Application of human interferon containing pharmaceutical composition in preparation of medicine for preventing and treating virus infection of respiratory tract
InactiveCN1927389AExact epidemiological effectLittle side effectsPeptide/protein ingredientsAntiviralsDiseaseRespiratory tract disease
The invention relates to a medicine compound with interferon. Wherein, said compound can be used to prepare the medicine that prevents and resists virus infection of respiratory tract. Said compound contains interferon and other auxiliary materials. Wherein, said interferon can be alpha2b, alpha1b, beta, Omega, k, lambada, epsilon and other I type; said auxiliary materials contains protector and baffle solution; and said interferon can be recombined interferon or / and the ones made by other methods; the auxiliary materials can be acidic buffer, protein, manna sugar, polyose, and benzoic alcohol to realize baffle, stabilize and corrosion-resistant functions.
Owner:北京金迪克生物技术有限责任公司 +1
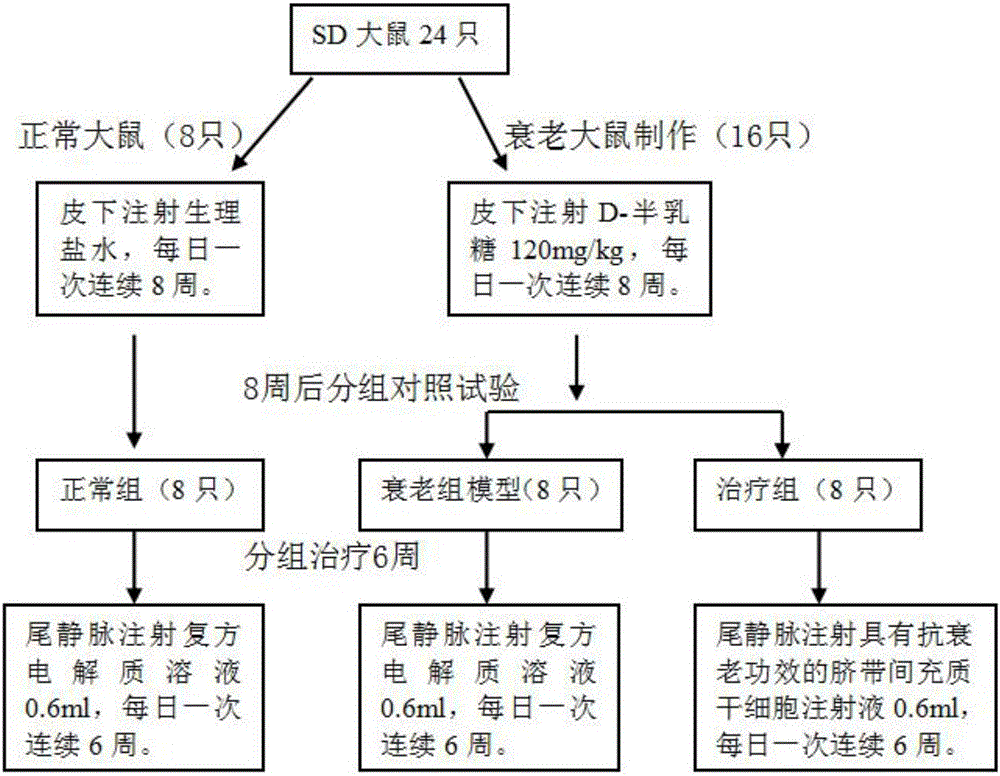
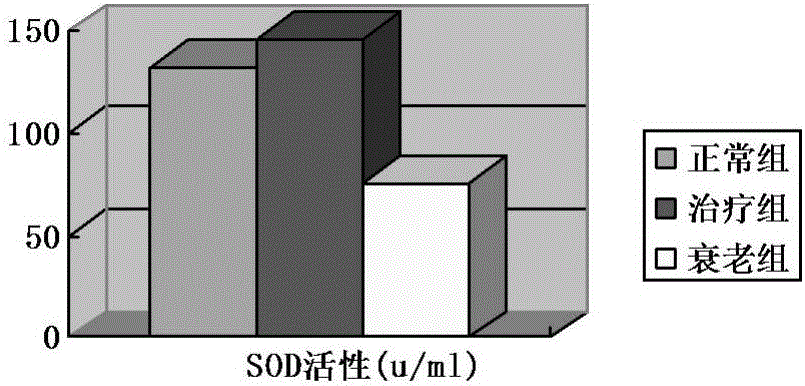
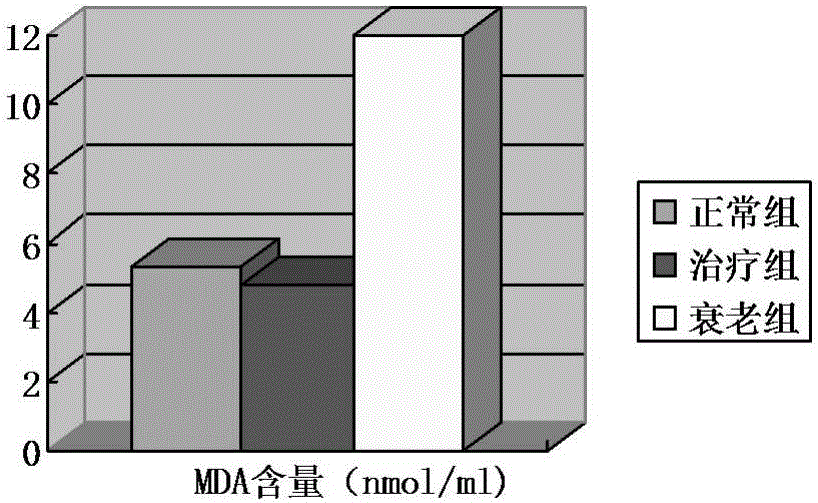
Umbilical cord mesenchymal stem cell injection with anti-aging function and preparation method thereof
InactiveCN105920042AWith amplificationDirected differentiationCell dissociation methodsCulture processTissue repairSelf recovery
The invention provides an umbilical cord mesenchymal stem cell injection with an anti-aging function and a preparation method thereof. The injection comprises the following components: umbilical cord mesenchymal stem cell (5*10<5> to 10*10<5> / mL), 2 wt% of human albumin, and 98 wt% of compound electrolyte solution. The produced umbilical cord mesenchymal stem cells can rapidly repair and replace damaged cells and tissues, and have the characteristics of amplification and directional cell differentiation; and after the stem cells receive the signals from damaged parts, the stem cells will move to the damage parts, amplify in the damage parts, carry out induced differentiation, promote the self recovery function of stem cells, rapidly repair and replace damaged cells and tissues, rapidly secrete cell factors, growth factors, and tissue related factors in time, thus improve the blood circulation, enhance the metabolism functions, improve the tissue repairing function and immunity, and finally achieve the anti-aging goal.
Owner:SHANGHAI HUAYAN MEDICINE TECH CO LTD
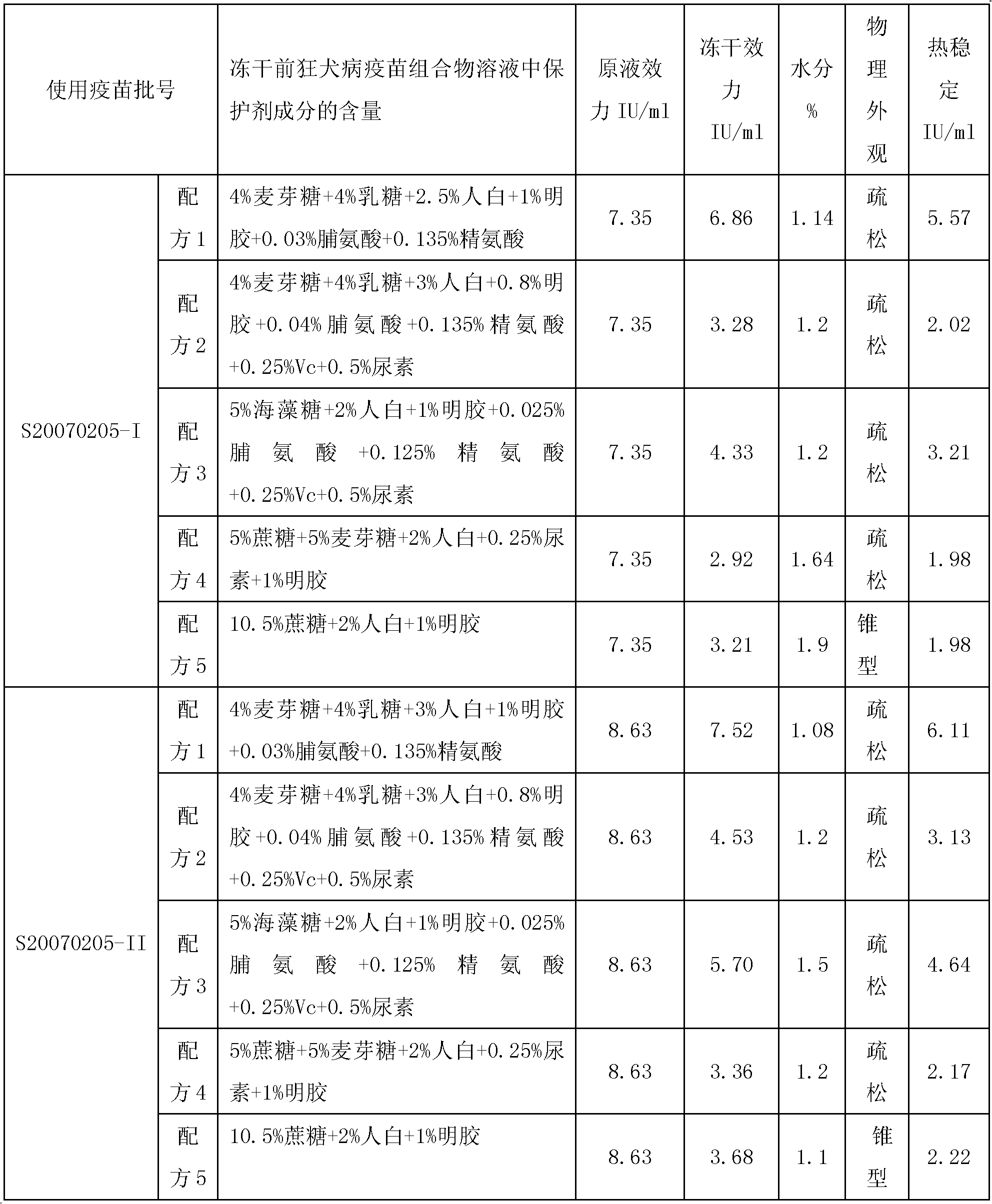
Vaccine protectant, hydrophobia vaccine and preparation method thereof
The invention provides a vaccine protectant, comprising the following components, by percentage composition: 50-80 parts of disaccharide, 20-30 parts of human albumin, 10 parts of gelatin and 1.5-1.65 parts of amino acid. The invention also provides a hydrophobia vaccine and a preparation method thereof. The vaccine protectant of the invention can effectively enhance thermal stability of a hydrophobia vaccine lyophilized preparation and has huge application value.
Owner:CHENGDU INST OF BIOLOGICAL PROD
Human albumin animal models for drug evaluation, toxicology and immunogenicity studies
InactiveUS6949691B2Improve assessmentMass productionSerum albuminDrug compositionsImmunogenicity StudyCarcinogen
An animal model is provided which is genetically engineered to express human serum albumin, and such animals may be advantageously used in assessing drugs, vaccines or other therapeutic compounds that may be used in humans. In addition, an animal model is provided which does not manufacture its own albumin and which has been injected with human serum albumin. Through the use of these animal models, drugs and other chemicals can be more accurately assessed in physiological environments that reflect the conditions to be expected in humans, and such models will be useful in assessing new drugs and evaluating toxic substances for potential dangers as carcinogens, mutagens, etc. Other applications include evaluating immunological properties of various albumin-engineered proteins which might be administered to humans as therapeutics or vaccines, and research of disease states, such as genetic diseases, to provide further insight in treating these diseases.
Owner:NEW CENTURY PHARMA INC
Recombinant human albumin fusion proteins with long-lasting biological effects
InactiveUS20080009446A1Good for healthImprove stabilityFungiBacteriaManufacturing technologyHuman albumin
Compositions, kits and methods are provided for promoting general health or for prevention or treatment of diseases by using novel recombinant fusion proteins of human serum albumin (HSA) and bioactive molecules. The bioactive molecules may be a protein or peptide having a biological function in vitro or in vivo, and preferably, having a therapeutic activity when administered to a human. By fusing the bioactive molecule to HSA, stability of the bioactive molecule in vivo can be improved and the therapeutic index increased due to reduced toxicity and longer-lasting therapeutic effects in vivo. In addition, manufacturing processes are provided for efficient, cost-effective production of these recombinant proteins in yeast.
Owner:YU ZAILIN +1
Non-animal-source serum-free culture medium for umbilical cord blood stem cells
ActiveCN102827810AAvoid instabilityClear natureBlood/immune system cellsCell phenotypeLipid formation
The invention relates to the field of biology, and discloses a non-animal-source serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, phytohaemagglutinin (PHA), lipid, amino acid, vitamins, trace elements, interleukin-3(IL-3), stem cell factor, (SCF), Fit3-L, IL-6 and granulocyte colony-stimulating factor (G-CSF). The non-animal source serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation, avoids the doped animal components and unstability of batches, and the results of cultured umbilical cord blood stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the non-animal-source serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Cell freezing medium and application thereof
InactiveCN107996558AImprove survival rateSmall batch-to-batch varianceDead animal preservationSucroseFrost
The invention relates to the field of cells, in particular to a cell freezing medium and application thereof. The cell freezing medium is prepared by dissolving glycerol, propanediol, trehalose, sucrose, human albumin, glucose and the like in PBS buffer solution, wherein permeable protection fluid comprises the glycerol and the propanediol, and impermeable protection fluid comprises the trehalose,the sucrose and the human albumin, meanwhile the glucose is used for proving energy to cells, and the PBS buffer solution is used for maintaining pH stability of the cell freezing medium. According to the cell freezing medium, components are fixed, batch-to-batch variation is small, and pollutant sources such as germs and viruses are not introduced, the permeable and impermeable liquids are usedsimultaneously to provide frost resisting capability for the cells to improve survival rate of the cells, the glucose is further added to provide energy for the cells to further improve the survival rate of the cells, and finally the PBS buffer solution is used for maintaining the pH stability of the cell freezing medium, so that environment influence on the cells is reduced to improve the survival rate of the cells.
Owner:湖南丰晖生物科技有限公司
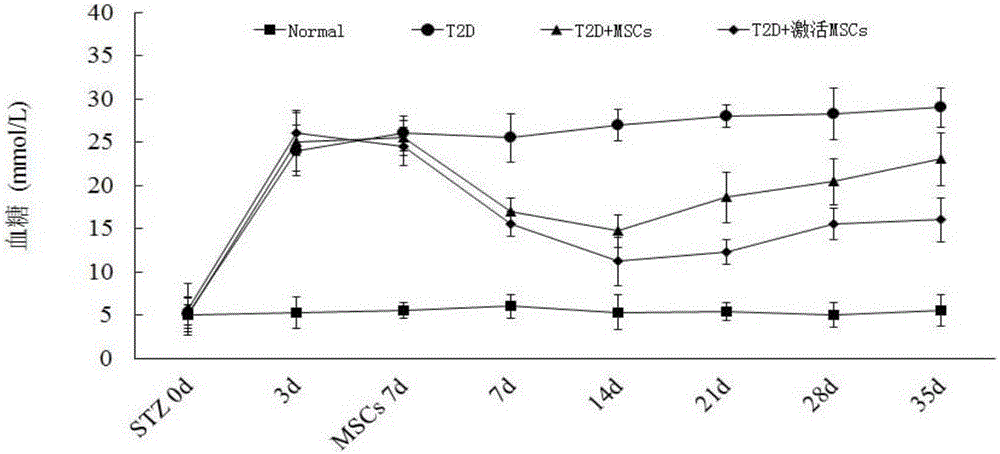
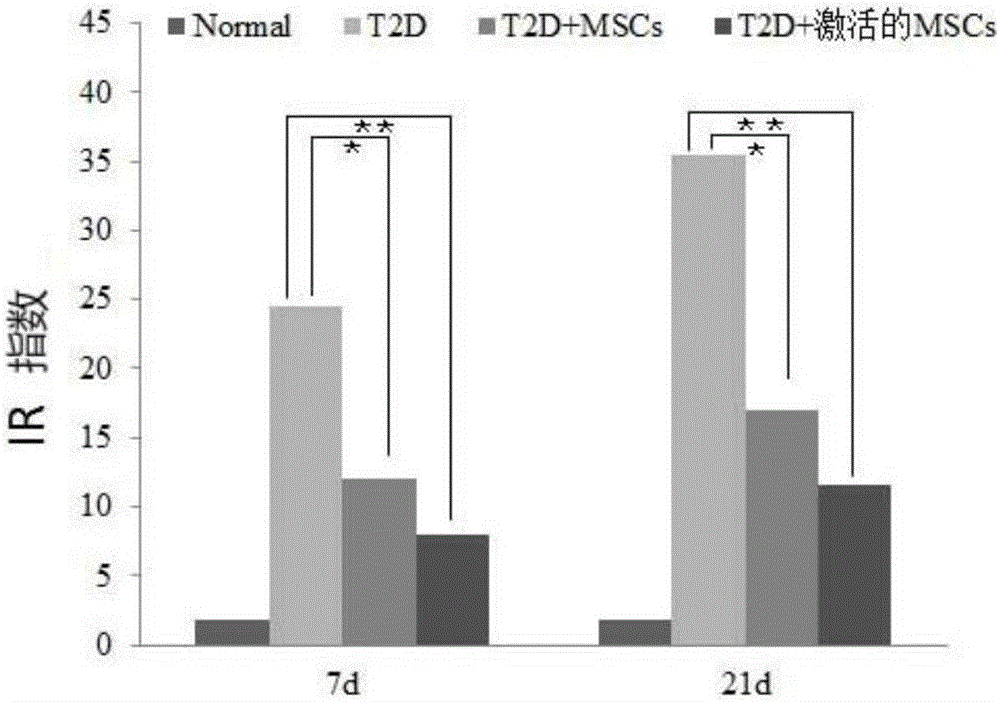
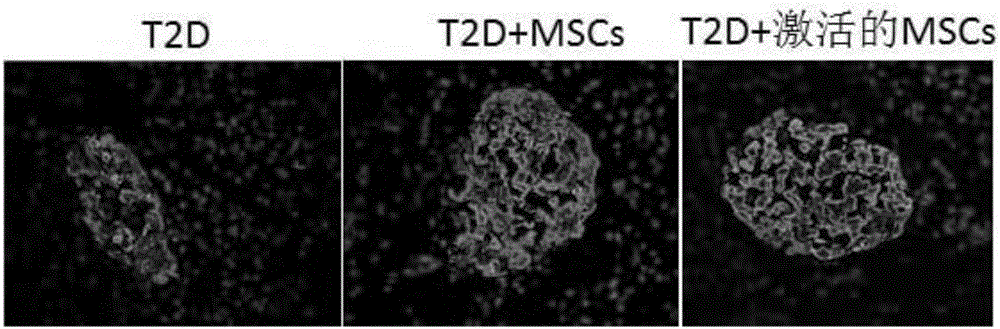
High-glucose activated mesenchymal stem cell injection and application thereof for diabetic drugs
InactiveCN106361771AEnhance the effect of anti-T2DImprove the immunityMetabolism disorderCulture processMANNITOL/SORBITOLMedicine
The invention provides a preparation method of high-glucose activated mesenchymal stem cells, and relates to a high-glucose activated mesenchymal stem cell injection and application thereof in preparation of diabetic drugs. The preparation method of the high-glucose activated mesenchymal stem cells comprises the following steps: when culturing an umbilical cord, bone marrow or the mesenchymal stem cells which are extracted separately until fusion reaches 40-50%; adding a glucose solution of which the final concentration is 20-50 mmol / L; culturing for 48+ / - 2 hours; and when fusion of the mesenchymal stem cells reaches 75-80%, digesting, neutralizing, washing and collecting the high-glucose activated mesenchymal stem cells. The high-glucose activated mesenchymal stem cell injection comprises the high-glucose activated mesenchymal stem cells, human albumin, compound vitamin, mannitol, glucose and normal saline. The high-glucose activated mesenchymal stem cell injection can be used for treating type-2 diabetes.
Owner:北京恒峰铭成生物科技有限公司
Fluorescence immunoassay quantitative detection kit of microalbuminuria, and preparation method thereof
InactiveCN102879586ASimple and fast operationImprove accuracyBiological testingAntigenFluorescence immunoassay
The invention relates to a fluorescence immunoassay quantitative detection kit of microalbuminuria, and a preparation method thereof. A sample pad, a combination pad, a nitrocellulose membrane and absorbent paper are pasted on the bottom liner of the quantitative detection kit in a successively lapped manner, wherein the combination pad is coated by fluorescent latex microsphere-marked human albumin antibodies; and the nitrocellulose membrane is coated by a detection line of albumin recombinant antigens and a quality control line of rabbit anti-mouse IgG antibodies. The fluorescence immunoassay quantitative detection kit can detect the content of microalbuminuria in human urine accurately and quantitatively, and has the characteristics of simple operation, high accuracy, high sensitivity, low cost, etc.
Owner:GETEIN BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com