Vaccine protectant, hydrophobia vaccine and preparation method thereof
A kind of technology of rabies vaccine, vaccine protective agent, be applied in: [0001] The present invention relates to a kind of vaccine protective agent, the field of vaccine protective agent, can solve the problems such as promotion and use restriction, vaccine quality discount, unstable rabies vaccine, etc., to improve Stability, extended shelf life, good clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 protective agent of the present invention
[0031] 1. Preparation method
[0032] Weighing, dissolving and autoclaving are operated in a workshop with a cleanliness level of 100,000, and filtration sterilization is operated under a laminar flow of a level of 100 in a workshop with a cleanliness of 10,000, including the following steps:
[0033] (1) Prepare 1000ml of gelatin with a concentration of 11%: Weigh 110g of gelatin, dissolve it in 750ml of 0.01M PBS (pH 7.6) sterilized solution in a hot water bath at 100°C, stir in real time, and when it is completely clear, set the volume at 1000ml, sterilized at 0.11MPa for 30 minutes, then supplemented with sterilized 0.01M PBS (pH 7.6) solution to make up to 1000ml to obtain 11% gelatin solution, stored at 2-8°C, and used within one week. Used gelatin is the product of Fluka Company;
[0034] (2) Preparation of 1000ml protective agent sugar solution: Weigh 179.9g of pharmaceutical grade mal...
Embodiment 2
[0036] The preparation of embodiment 2 rabies vaccine of the present invention
[0037] 1. Vaccine preparation
[0038] (1), preparation of protective agent
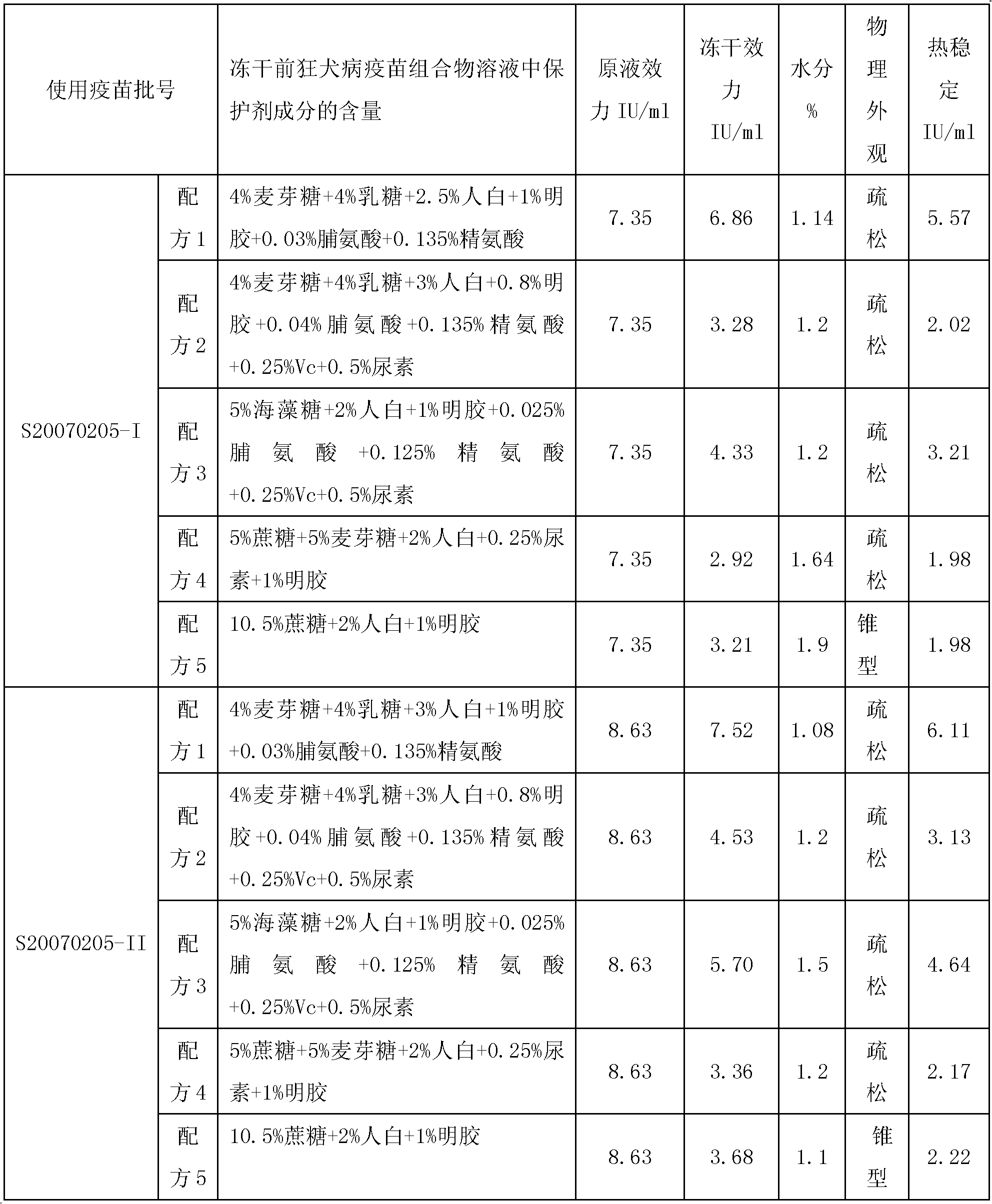
[0039] According to the formula shown in Table 1, the protective agent was prepared according to the following steps:
[0040] a. Prepare 1000ml of 11% gelatin: Weigh 110g of gelatin, dissolve it in 750ml of sterilized 0.01M PBS (pH 7.6) solution in a hot water bath at 100°C, stir in real time, and when it is completely clear, set the volume to 1000ml , 0.11MPa for 30 minutes to sterilize, then supplement the liquid with sterilized 0.01M PBS (pH 7.6) solution to make up to 1000ml to obtain 11% gelatin solution, store at 2-8°C, and use within one week. Used gelatin is the product of Fluka Company;
[0041] b. Prepare 1000ml protective agent sugar solution: weigh pharmaceutical grade polysaccharide and dissolve it in sterilized 750ml 0.01M PBS (pH 7.6) solution under 100°C hot water bath, stir in real time, and wait until...
Embodiment 3
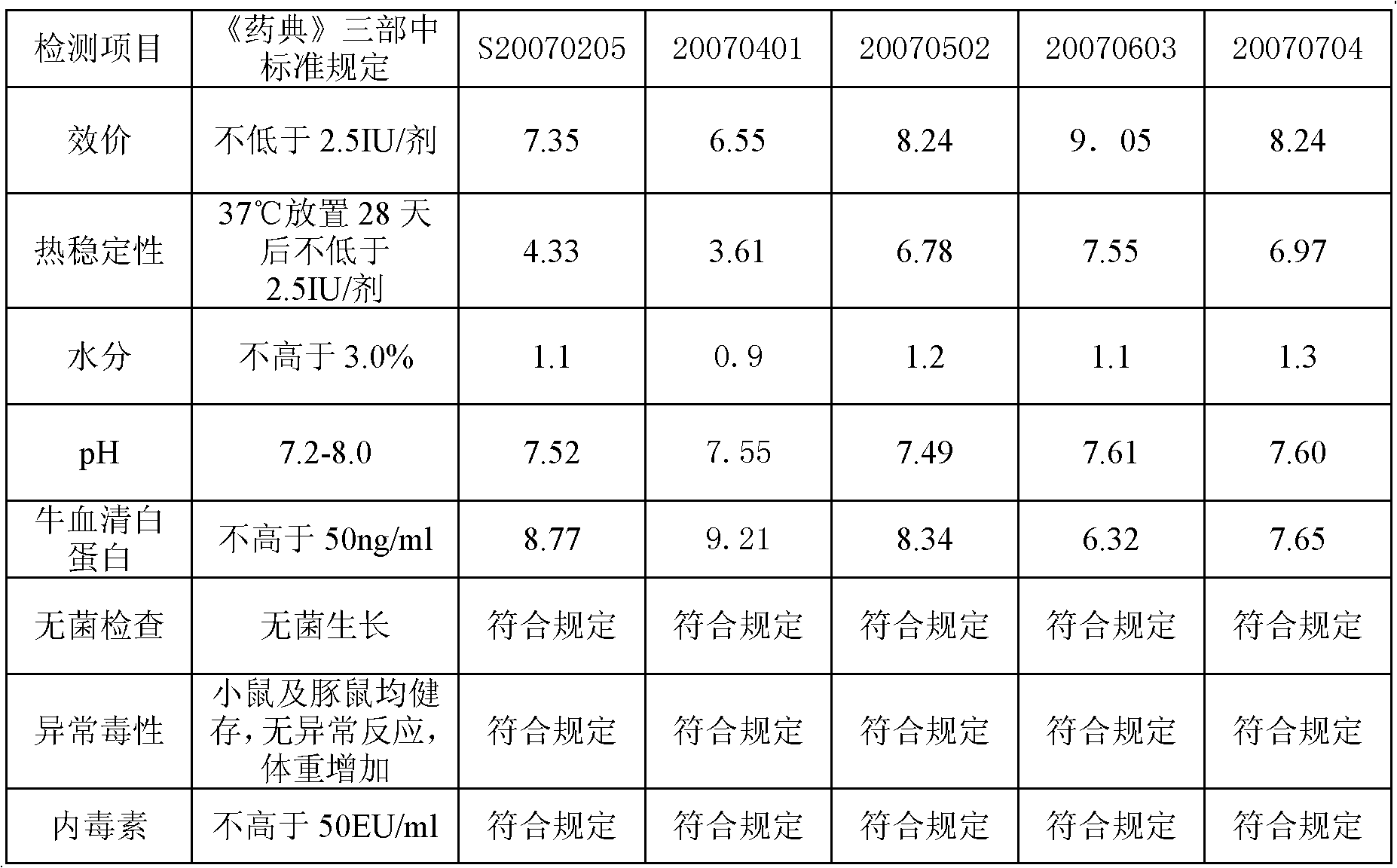
[0067] The quality detection of the vaccine that embodiment 3 protective agent of the present invention prepares
[0068] Get the vaccine that embodiment 2 formula 1 prepares, carry out following detection:
[0069] 1. Vaccine safety test, vaccine intracerebral injection test to observe safety
[0070] The rabies vaccine produced by screening the protective agent was tested for vaccine safety, vaccine intracerebral injection test, allergic test, and vaccine injection site irritation test. The results showed that after the vaccine safety test and intracerebral injection of Kunming mice, The mice showed no abnormal behavior, and all the experimental mice survived after 14 days of observation.
[0071] 2. Vaccine abnormal toxicity test
[0072] Trial freeze-dried rabies vaccine was injected intraperitoneally into mice (18-22g) and guinea pigs (250-350g). The results showed that the mice survived and gained weight after 7 days of observation.
[0073] 3. Vaccine allergy test
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com