Fluorescence immunoassay quantitative detection kit of microalbuminuria, and preparation method thereof
A technology of microalbumin and fluorescent immunoassay, which is applied in the field of microalbumin fluorescent immunoassay quantitative detection kit and its preparation, can solve the problems of low accuracy, limited reliability, poor stability, etc., and achieve high sensitivity and high accuracy , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 The preparation method of microalbumin fluorescent immunoassay kit is as follows:
[0034] 1) Preparation of fluorescent latex microspheres
[0035] Preparation of fluorescent latex microspheres: Dilute latex microspheres with a particle size of 153 nm to a final concentration of 20 mg / ml with an adsorption buffer (50 mM, citrate buffer at pH 5.8) to obtain a latex microsphere suspension ; add an appropriate amount of fluorescein Cy5-labeled streptavidin (purchased from Shanghai Yanjing Biotechnology Co., Ltd.) in the adsorption buffer, the final volume is 5ml; the above-mentioned latex microsphere suspension is added to the above-mentioned containing fluorescein-labeled chain In the adsorption buffer of mycoavidin, a mixed solution was prepared; the resulting mixed solution was incubated at room temperature for 1-2 hours, and stirred continuously, and then centrifuged to collect the precipitate, and the precipitate was stored in a buffer solution (containing ...
Embodiment 2
[0050] Example 2 The preparation method of another microalbumin fluorescence immunoquantitative detection kit is as follows:
[0051] 1) Preparation of fluorescent latex microspheres
[0052] Preparation of fluorescent latex microspheres: Dilute latex microspheres with a particle size of 500 nm to a final concentration of 30 mg / ml with an adsorption buffer (50 mM, citrate buffer at pH 5.8) to obtain a latex microsphere suspension ; add an appropriate amount of red fluorescein rhodamine-labeled streptavidin (purchased from Shanghai Enzyme Biotechnology Co., Ltd.) in the adsorption buffer, the final volume is 6ml; the above-mentioned latex microsphere suspension is added to the above-mentioned containing red fluorescent In the adsorption buffer of sulhodamine-labeled streptavidin, a mixed solution was prepared; the resulting mixed solution was incubated at room temperature for 1-2 hours, and kept stirring, then centrifuged, and the precipitate was collected, and the precipitate ...
Embodiment 3
[0059] Example 3 Microalbumin Fluorescence Immunoquantitative Assay Test Strip Quantitative Detection
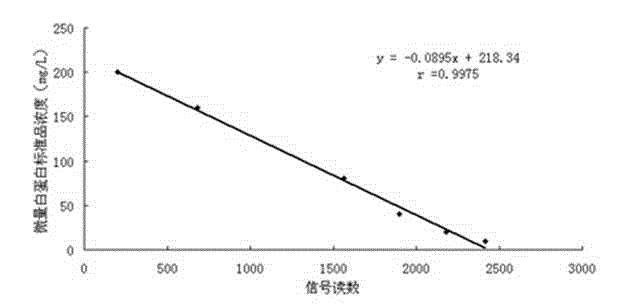
[0060] 1) Draw a standard curve
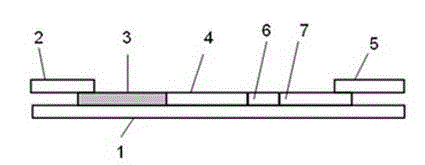
[0061] Add different concentrations of albumin antigen standard substance (take six different concentrations, respectively 10, 20, 40, 80, 160, 200 mg / L) on the microalbumin fluorescent immunoquantitative assay test strip sample pad prepared by Example 1 , with 3 replicates for each concentration), the signals of detection line 7 and quality control line 8 were read by the fluorescence immunoquantitative analyzer Getein1100 of Nanjing Gedan Biotechnology Co., Ltd. The experimental results and analysis are shown in Table 1.
[0062] Table 1 Albumin Standard Test Results
[0063]
[0064] Draw a standard curve with the concentration of the microalbumin antigen standard substance and the average value of the determined signal, such as figure 2 shown.
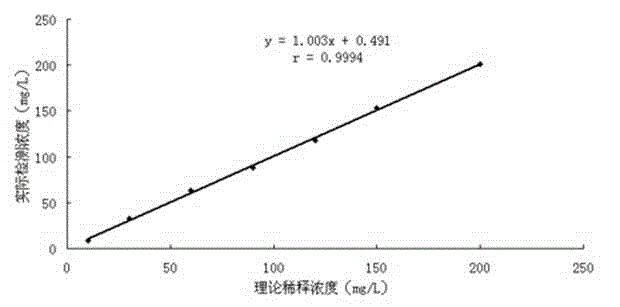
[0065] 2) Detection of linear range
[0066] The test strip prepared in Example 1 of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com