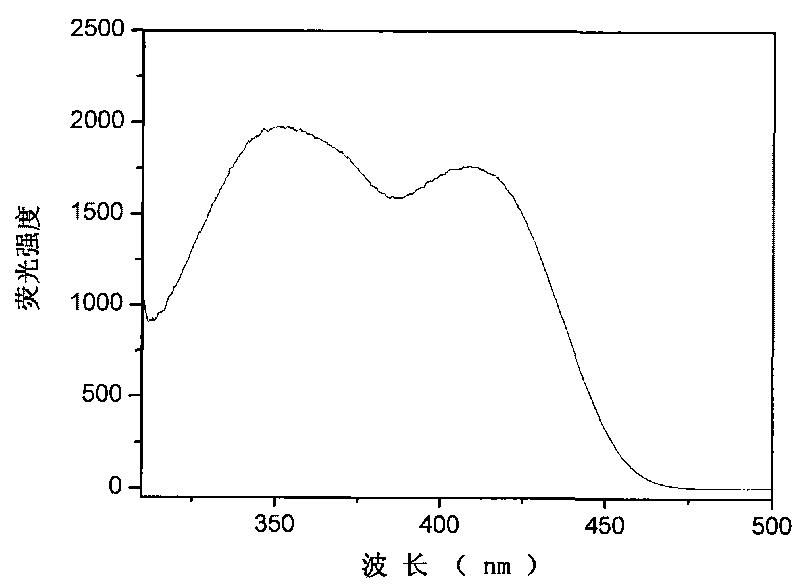
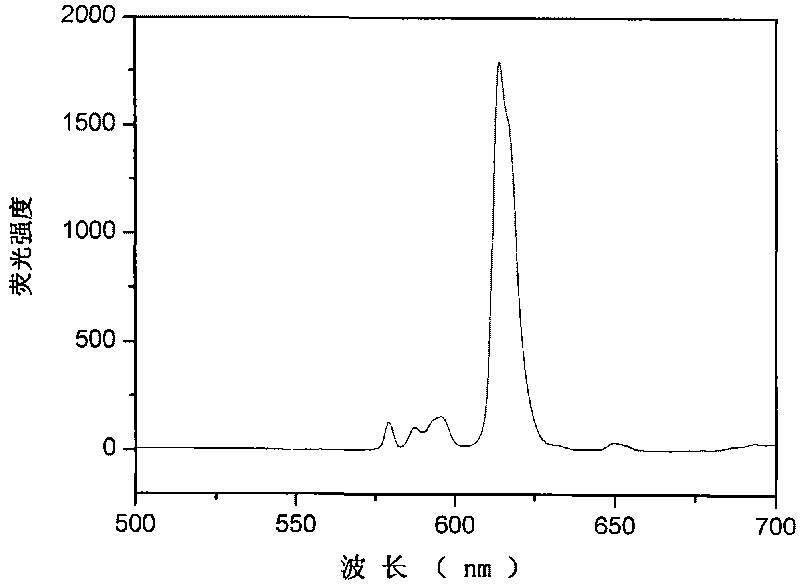
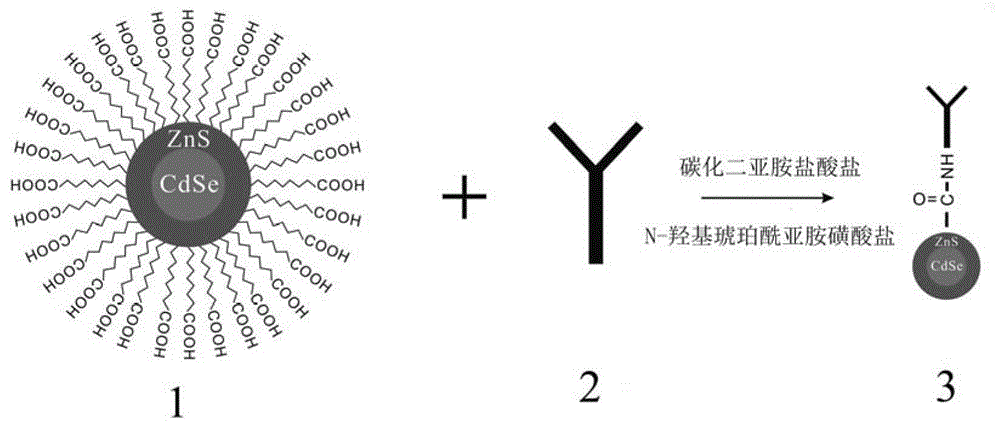
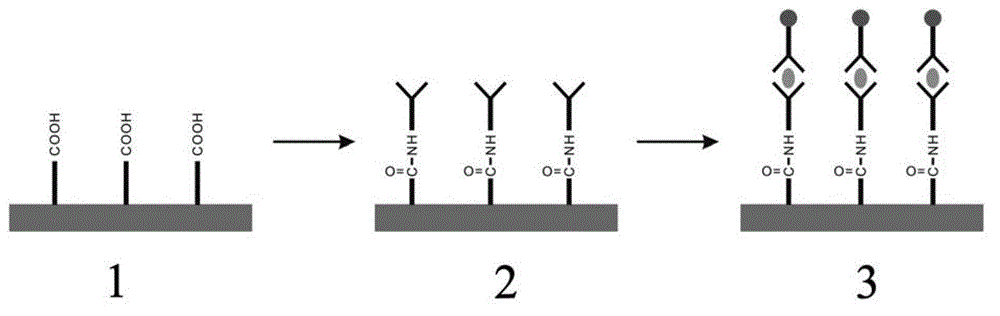
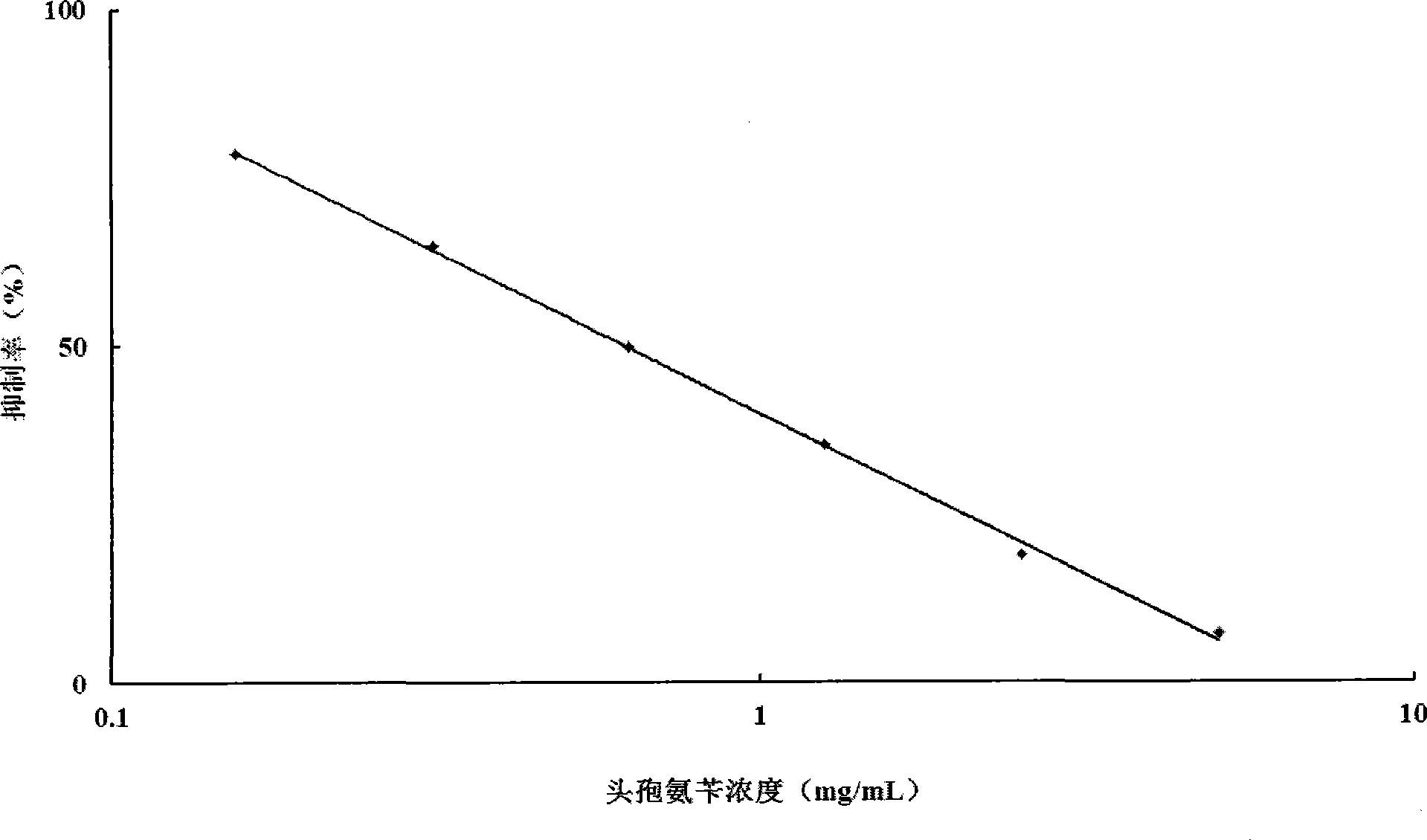
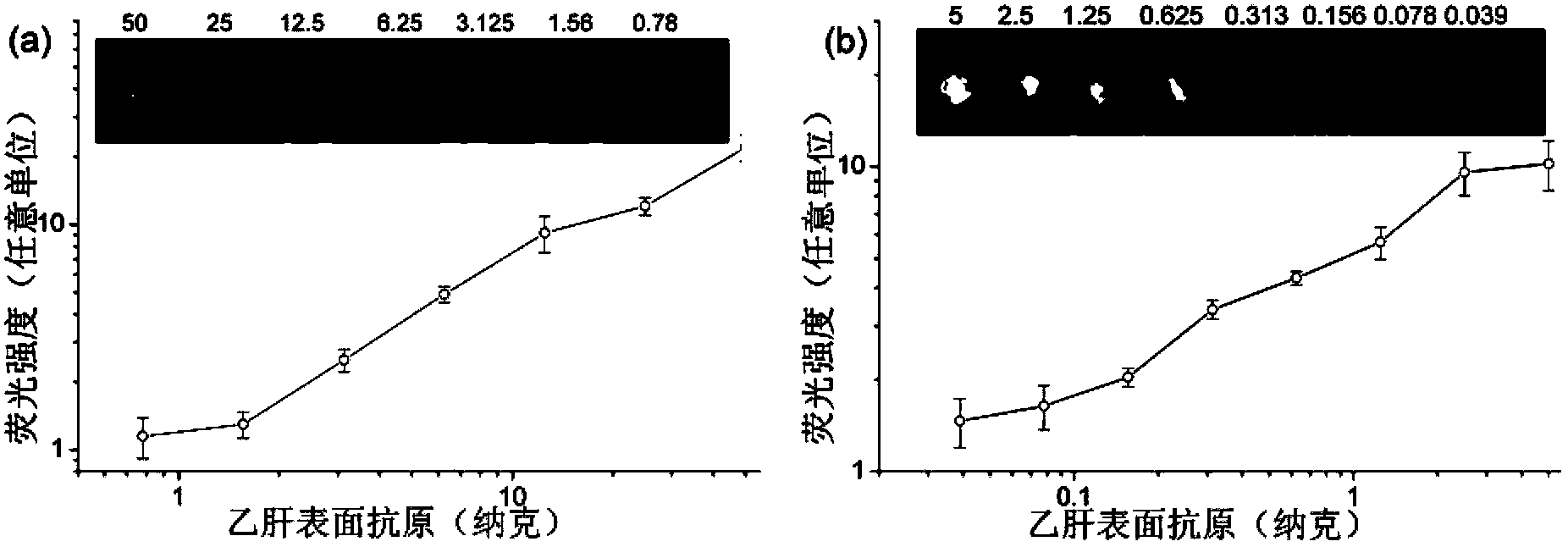
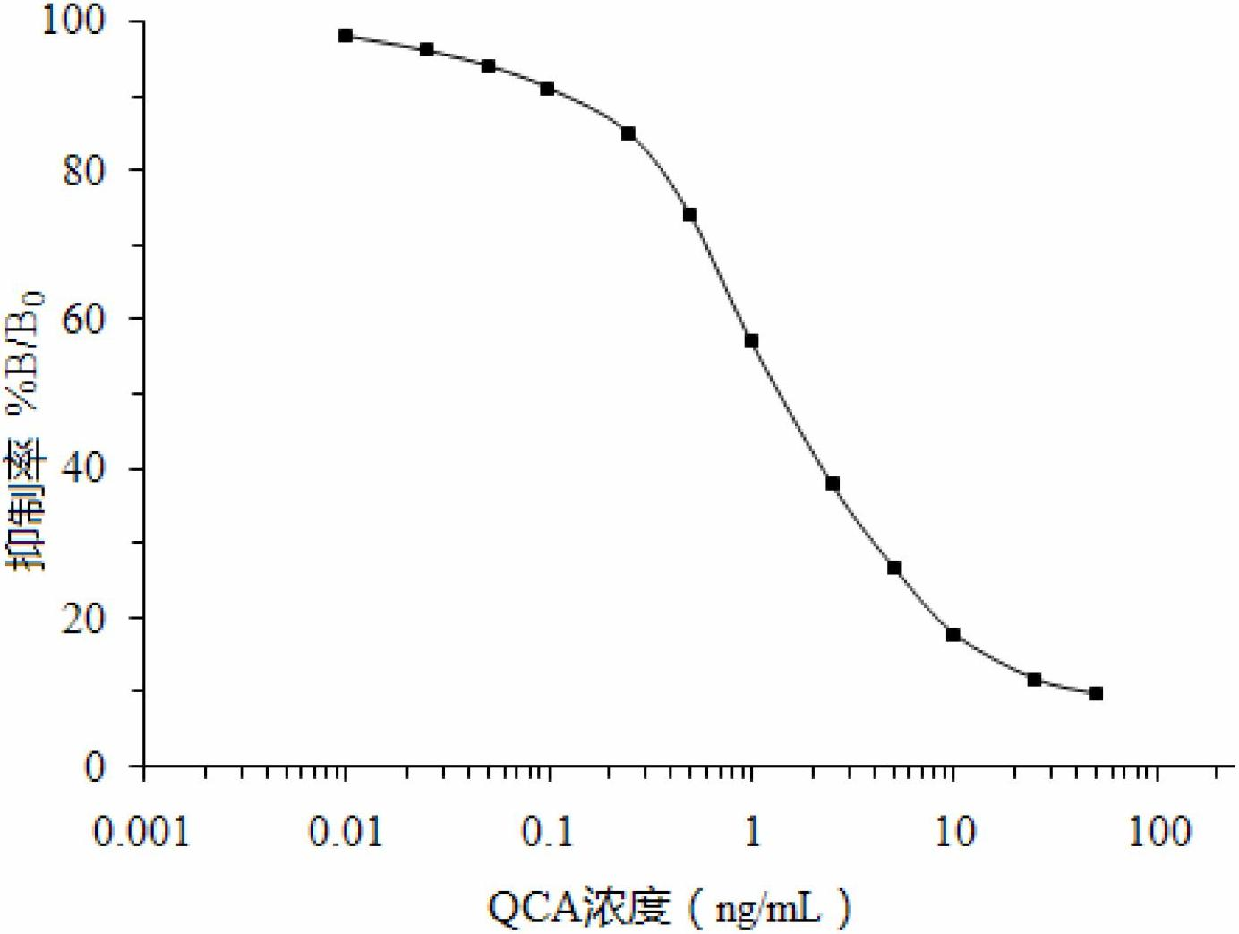
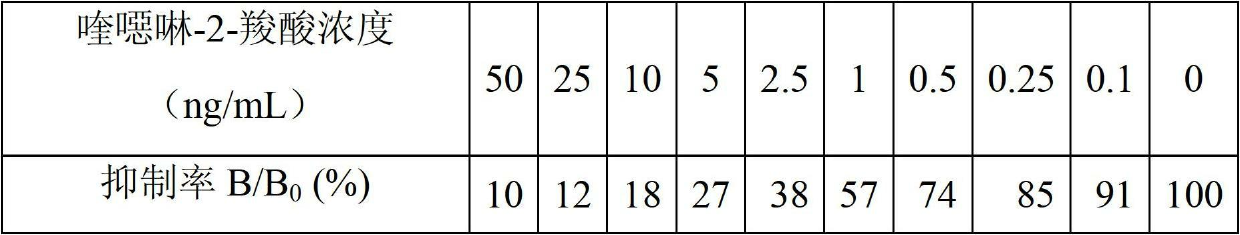
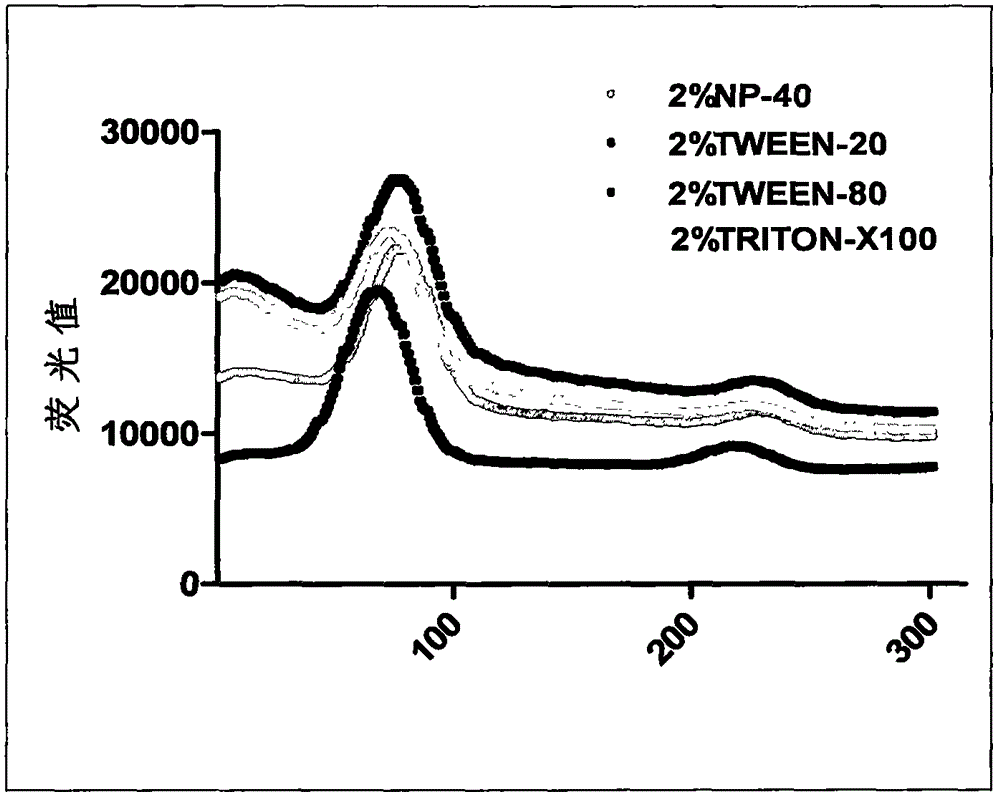
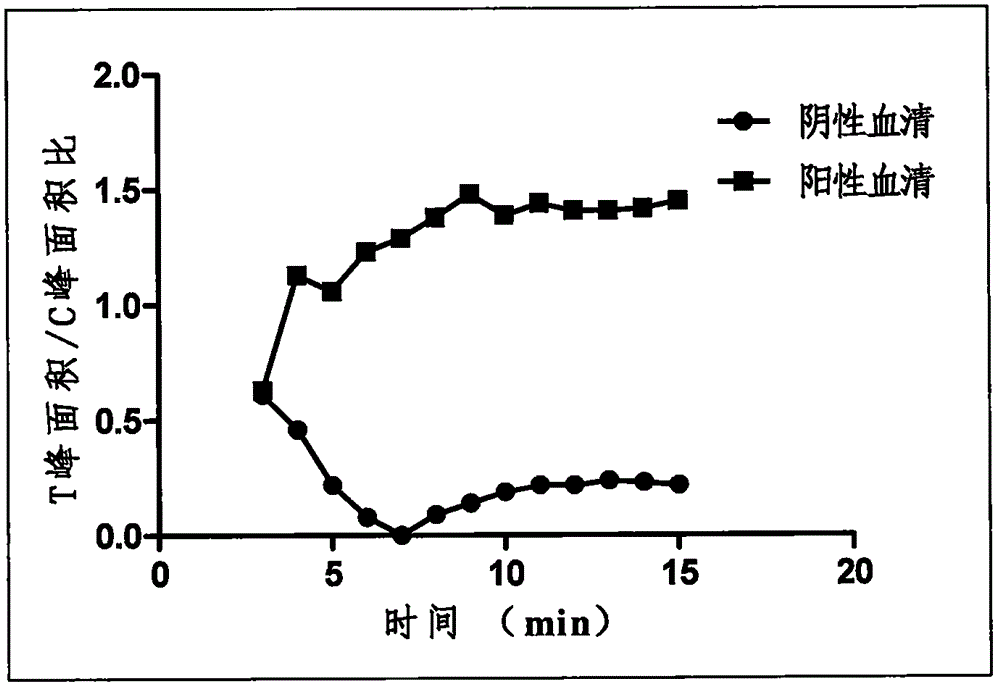
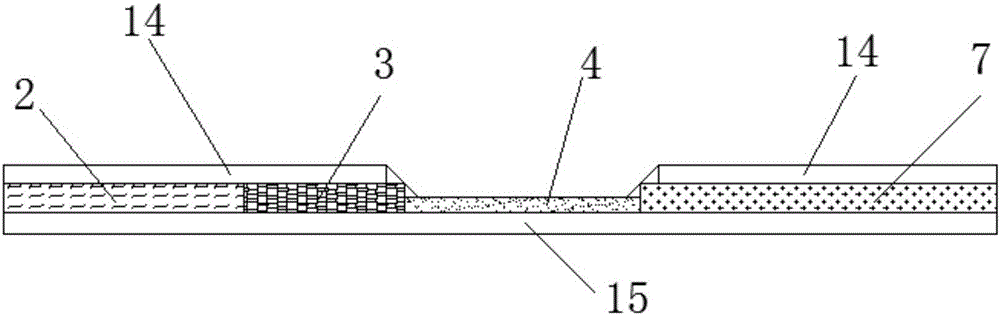
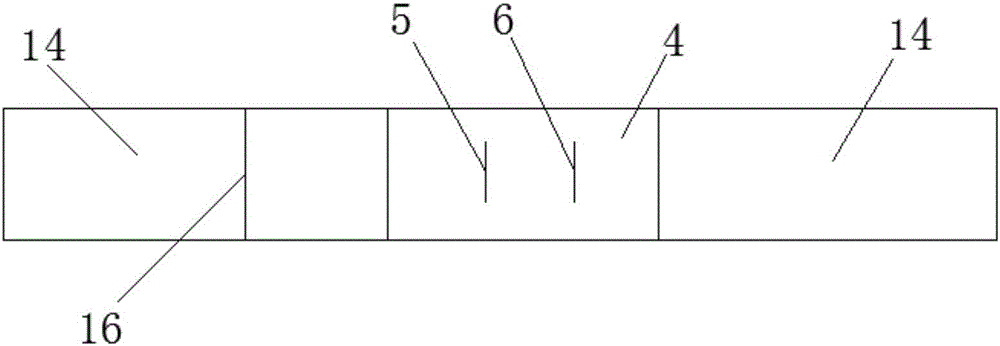
Patents
Literature
268 results about "Fluorescence immunoassay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
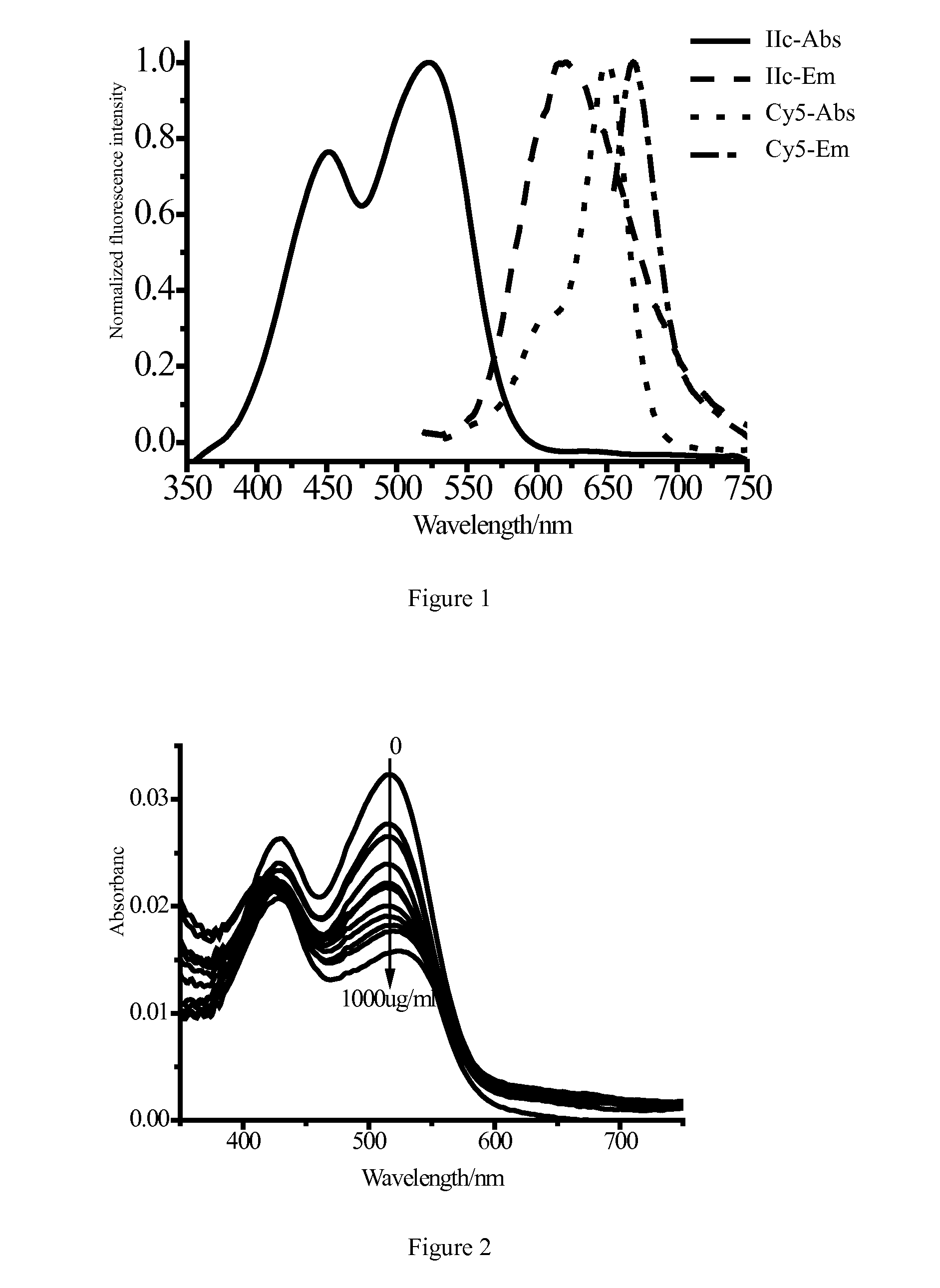
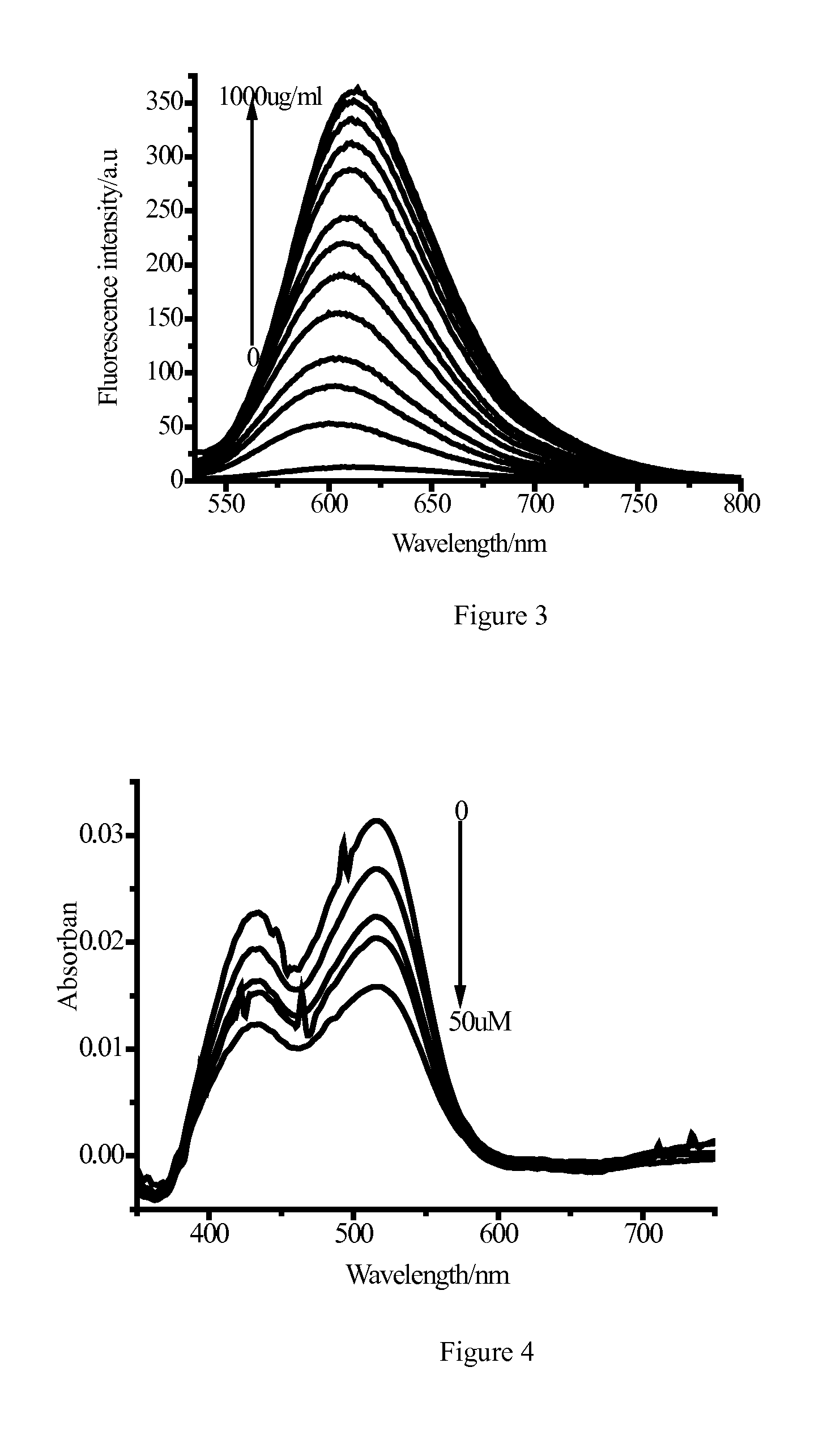
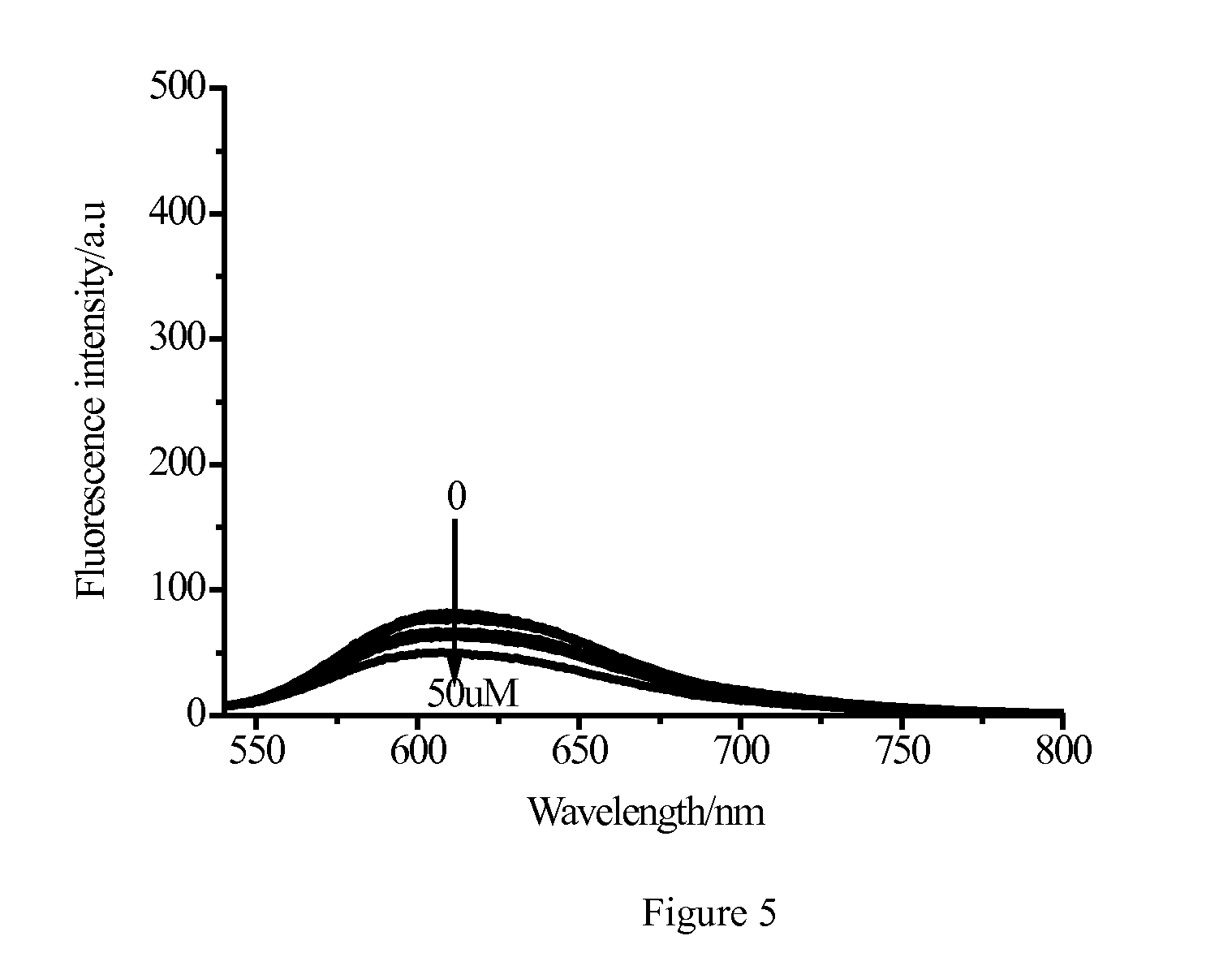
Fluorescent Immunoassays (FIA) Fluorescent Immunoassays are simply a different type of immunoassay. ... A modern fluorescent based immunoassay uses as the detection reagent a fluorescent compound which absorbs light or energy (excitation energy) at a specific wavelength and then emits light or energy at a different wavelength. The difference between the wavelength of the excitation light and the emission light is called the Stokes shift.
High throughput multi-antigen microfluidic fluorescence immunoassays
InactiveUS20060263818A1Reduce total integrated backgroundIncreased signal noiseBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenPoint of care
The development of a high-throughput multi-antigen microfluidic fluorescence immunoassay system is illustrated in a 100-chamber PDMS (polydimethylsiloxane) chip which performs up to 5 tests for each of 10 samples. Specificity of detection is demonstrated and calibration curves produced for C-Reactive Protein (CRP), Prostate Specific Antigen (PSA), ferritin, and Vascular Endothelial Growth Factor (VEGF). The measurements show sensitivity at and below levels that are significant in current clinical laboratory practice (with SIN>8 at as low as 10 pM antigen concentration). The chip uses 100 nL per sample for all four tests and provides an improved instrument for use in scientific research and “point-of-care” testing in medicine.
Owner:SCHERER AXEL +3
Fluorescent nanoparticle and preparation method and application thereof
InactiveCN101701151ANarrow emission peakHigh strengthLuminescent compositionsMaterial analysisPhotoluminescenceHydrophobic polymer
The invention discloses a class of fluorescent nanoparticles and a preparation method and an application thereof. The fluorescent nanoparticle comprises matrix material and fluorescent dye dispersed in the matrix material, wherein the matrix material is the compound composed of hydrophobic polymer main chain of which is hydrocarbon chain, organo-siloxane and organosilicon-polymer, and the fluorescent dye is rare earth complex with photoluminescence performance. The preparation method of the fluorescent nanoparticle comprises the following steps: dissolving rare earth complex, hydrophobic polymer and compound RSi (OR') 3 into organic solvent miscible with water; adding the mixture into aqueous solution containing surfactant; using precipitation and hydrolytic condensation reaction to form nanoparticles. The nanoparticle of the invention has favourable luminous performance and favourable stability, can have a silicon oxide shell and a surface functional group and can be used for bondingbiomolecules; biological probes based on the fluorescent nanoparticle have wide application prospects on the aspects of high-sensitivity fluorescence immunoassay, imaging and the like.
Owner:PEKING UNIV
Multi-functional quick fluorescence immunoassay test method using functional substrate as media and using single color and multi-color quantum dots as mark
InactiveCN103149360AShort color development (fluorescence) timeStable storageFluorescence/phosphorescenceAntigenImmuno detection
The invention relates to a novel, quick, high-sensitivity, qualitative and quantitative clinical test method which uses a chemically modified functional polymorphic substrate as media and combines inorganic fluorescent semiconductor nano materials (quantum dots) and immunology means. The multi-functional quick fluorescence immunoassay test method using a functional substrate as media and using single color and multi-color quantum dots as a mark is suitable for a single or compound qualitative and quantitative test of various hormone, protein, nucleic acid and pathogens. Compared with traditional enzyme-linked immunoassay and colloidal gold test paper, the multi-functional quick fluorescence immunoassay test method has the advantages of being simple, quick, high in sensitivity, long in storage time and the like. The multi-functional quick fluorescence immunoassay test method can meet requirements of different testing conditions and samples, can conduct the single or compound qualitative and quantitative test on different antigens on the same substrate media at the same time, and has great potential on aspects of clinical applications.
Owner:HENAN UNIVERSITY
Kit suitable for rapidly detecting AMH and INHB by using double-tagging time resolution fluorescence immunoassay method and use method of kit
InactiveCN104730247AReduce dosageReduce detection stepsBiological testingFluorescence/phosphorescenceFluorescence immunoassayFluorescence
The invention provides a kit suitable for rapidly detecting AMH and INHB by using a double-tagging time resolution fluorescence immunoassay method. The kit comprises (1) a solid phase carrier coated by an AMH coating antibody and an INHB coating antibody; (2) a mixed calibration product of AMH and INHB; (3) an AMH detection antibody marked by using a lanthanide element 1; (4) an INHB detection antibody marked by a lanthanide element 2; (5) an immunoreaction accelerant liquid; (6) a concentrated washing liquid; and (7) a reinforcing liquid. The invention further provides a use method of the kit for rapidly detecting AMH and INHB by using the double-tagging time resolution fluorescence immunoassay method. By adopting a double-tagging time resolution fluorescence immunoassay technique, the defect that conventional AMH and INHB respectively need a single kit, that is, one kit can be only used for detecting AMH or INHB can be overcome, the detection steps can be reduced, the sampling time can be shortened, and the use amount of a blood sample can be reduced.
Owner:GUANGZHOU FENGHUA BIOENG
Streptococcus-A quantum dot immunochromatographic detection reagent card and preparation method thereof
ActiveCN103926403AImprove the detection rateOptimal treatment timeBiological material analysisFluorescence immunoassayFluorescence
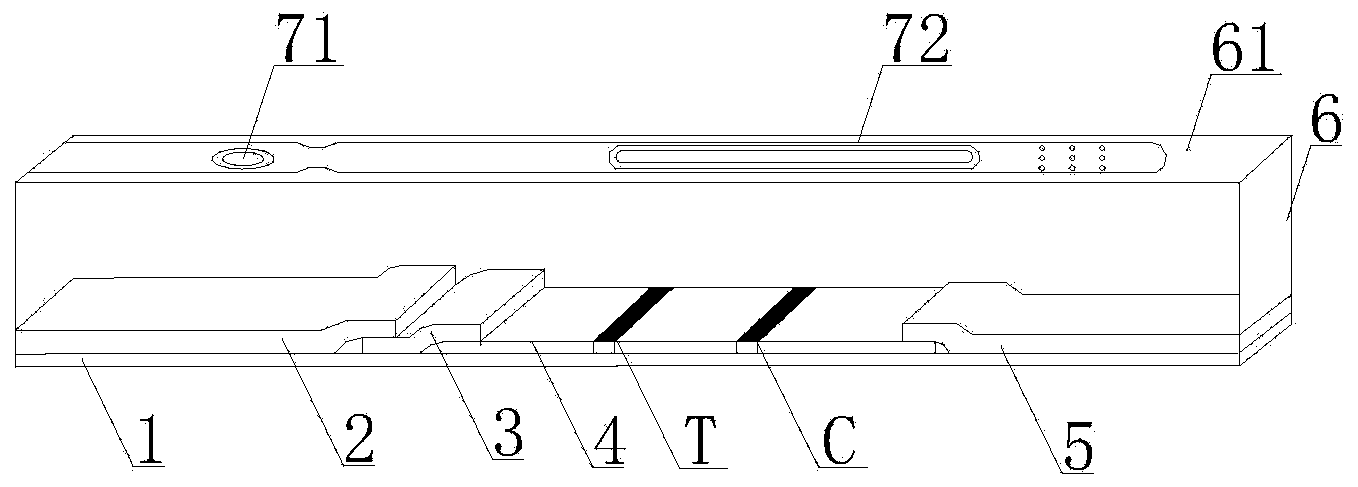
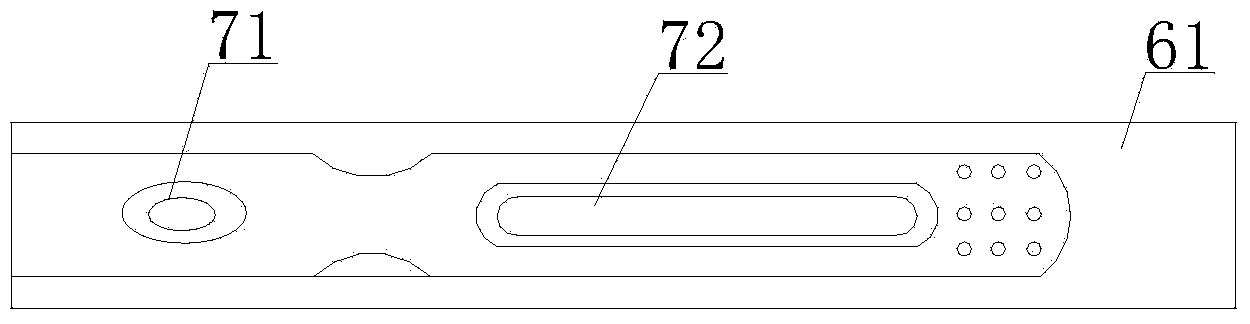
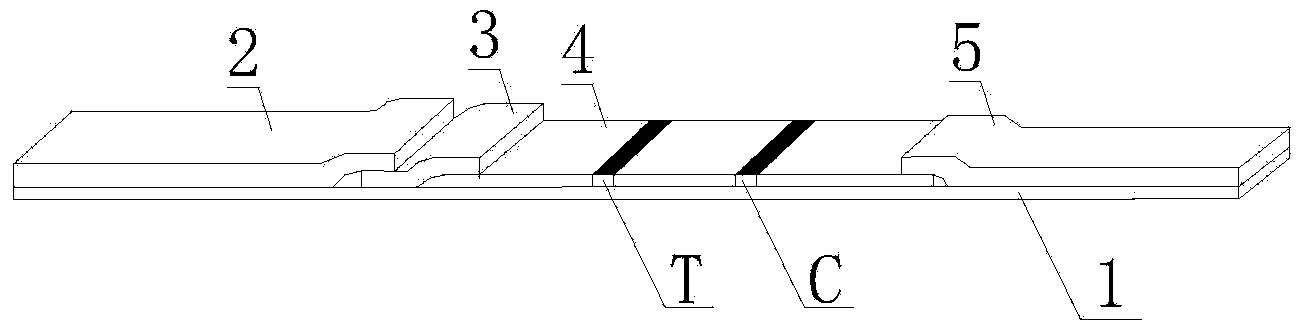
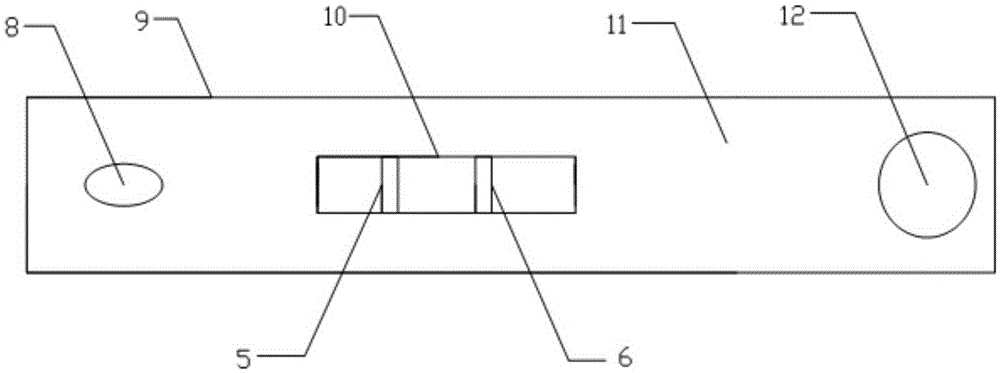
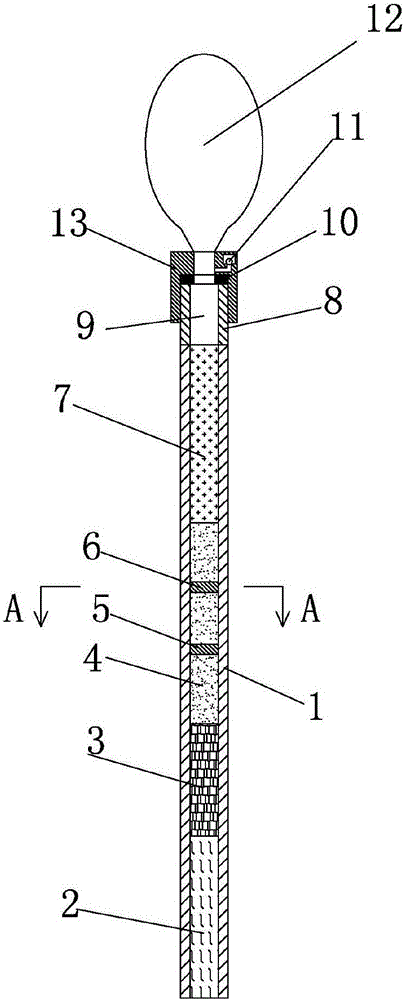
The invention discloses a streptococcus-A quantum dot immunochromatographic detection reagent card and a preparation method of the streptococcus-A quantum dot immunochromatographic detection reagent card, and aims to provide the streptococcus-A quantum dot immunochromatographic detection reagent card which is simple, high in sensitivity and reliable in detection results. The detection reagent card comprises a box body (6), wherein a piece of detection test paper is arranged in the box body (6); the detection test paper comprises a bottom plate (1); a sample pad (2), an anti-streptococcus-A quantum dot anti-body markup pad (3), a coating film (4) and a water absorption pad (5) are connected with the bottom plate (1) in sequence; a streptococcus-A quantum antibody detection area line T and a goat anti-rabbit polyclonal anti-body quality control area line C are arranged on the coating film (4); the two lines are arranged in parallel; the streptococcus-A quantum dot immunochromatographic detection reagent card and the preparation method thereof belong to the field of fluorescence immunoassay.
Owner:GUANGZHOU WEIMI BIOLOGICAL SCI & TECH
Kit for quantitative detection on O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles
InactiveCN108107220AAdequate responseIncrease binding areaBiological testingBiotin-streptavidin complexSorbent
The invention discloses a kit for quantitative detection on an O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles. The kit consists of O type foot-and-mouth disease virus antibody negative serum, O type foot-and-mouth disease virus antibody positive serum, VP1 coating magnetic beads, a biotinylation goat-anti-pig antibody, a streptavidin marking fluorescent substance, a cleaning solution and an enhancing solution. The magnetic beads used in the kit have relatively large binding areas, so that the detection range is greatly increased, the reaction time is shortened, and the sensitivity is improved. The kit has a relatively wide stimulation spectrum and a relatively narrow emitting spectrum, the cost can be reduced, and the sensitivity can be improved; compared with a conventional fluorescent substance, the kit is relatively wide in detection range and relatively good in specificity. Due to adoption of a streptavidin-biotin signal amplification system, the detection sensitivity is further improved, and the kit is relatively high in sensitivity when being compared with ELISA (Enzyme-Linked Immuno Sorbent Assay) and chemiluminiscence. Together with a full-automatic detector, on-site automatic operation can be achieved, one or more samples can be simultaneously detected, and the kit is simple, convenient and rapid to operate and low in price.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
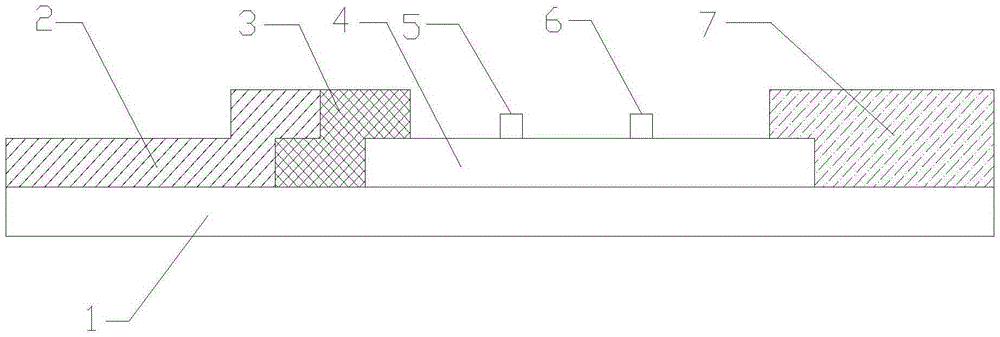
Fluorescence immunoassay chromatography test paper for cefalexin residue and preparation of test paper
ActiveCN103901201AEasy to handleThe determination method is simpleMaterial analysisGlass fiberCefalexin
The invention discloses a piece of fluorescence immunoassay chromatography test paper which is rapid, sensitive, simple and convenient to operate to test the residual quantity of cefalexin, and preparation of the test paper. The test paper comprises a sample pad, a binding pad, a nitrocellulose membrane and a water absorbing pad, which are adhered to a substrate in a mutual lap joint manner in sequence, wherein the nitrocellulose membrane is coated with a detection line and a quality control line; the binding pad is coated with a cefalexin monoclonal antibody with a fluorescent mark. The preparation method of the fluorescence immunoassay chromatography test paper for cefalexin residue comprises the following steps: synthesizing cefalexin-ovalbumin coating antigen, preparing a goat anti-mouse immune globulin antibody, coating the cefalexin-ovalbumin coating antigen and the goat anti-mouse immune globulin antibody onthe nitrocellulose membrane to serve as the detection line and the quality control line, preparing a fluorescence nano-particle mark cefalexin monoclonal antibody, then coating glass fiber to serve as the binding pad, sequentially adhering the sample pad, the binding pad, the nitrocellulose membrane and the water absorbing pad to a back plate in the lap joint manner in sequence, cutting the obtained test paper into the width of 4mm, and preserving at normal temperature.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Preparation method of signal amplifying type quantum dot immune fluorescent probe and application of signal amplifying quantum dot immune fluorescent probe
InactiveCN103361064ALittle effect on fluorescence performanceUniform particle sizeLuminescent compositionsMaterial analysisFluorescence immunoassayFluorescence
The invention belongs to technical field of immunoassay, and particularly relates to a preparation method of a signal amplifying type quantum dot immune fluorescent probe and an application of the signal amplifying type quantum dot immune fluorescent probe. At first, According to the method, a microemulsion solvent evaporation method is adopted for preparing a quantum dot-polymer composite nanosphere with controllable grain size and good dispersibility by utilizing quantum dot and polymer solution; and an antibody is covalently coupled on the surface of the composite nanosphere to obtain the signal amplifying type quantum dot immune fluorescent probe. The invention provides an application of the quantum dot immune fluorescent probe in a fluorescence immunoassay method or a fluorescence immunoassay detection kit. Compared with traditional quantum dot immune fluorescent probe, the quantum dot immune fluorescent probe prepared by the method provided by the invention has stronger signals and good detection sensitivity.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Detecting reagent kit used for detecting quinoxalinone-2-carboxylic acid and method
InactiveCN102654500AEasy to handleDetection suitable forMaterial analysisFreeze-dryingCarboxylic acid
The invention discloses a time-resolved fluorescence immunoassay reagent kit which is used for detecting quinoxalinone-2-carboxylic acid and a detection method of the time-resolved fluorescence immunoassay reagent kit. The reagent kit comprises a porous coated plate, a buffer solution, quinoxalinone-2-carboxylic acid standard, freeze-dried antibodies of quinoxalinone-2-carboxylic acid, europium-marked goat anti mouse antibodies, a cleaning solution and an enhancement solution. By measuring fluorescence intensity of counts per second (cps), the content of quinoxalinone-2-carboxylic acid in samples is calculated by a standard curve. The kit provided by the invention has the advantages of simple structure, easiness in assembly, corrosion resistance, light weight, low cost, wide application and the like. A valve core has good centering performance and can move flexibly and reliably and can play a good water-plugging role.
Owner:CHONGQING ACADEMY OF SCI & TECH
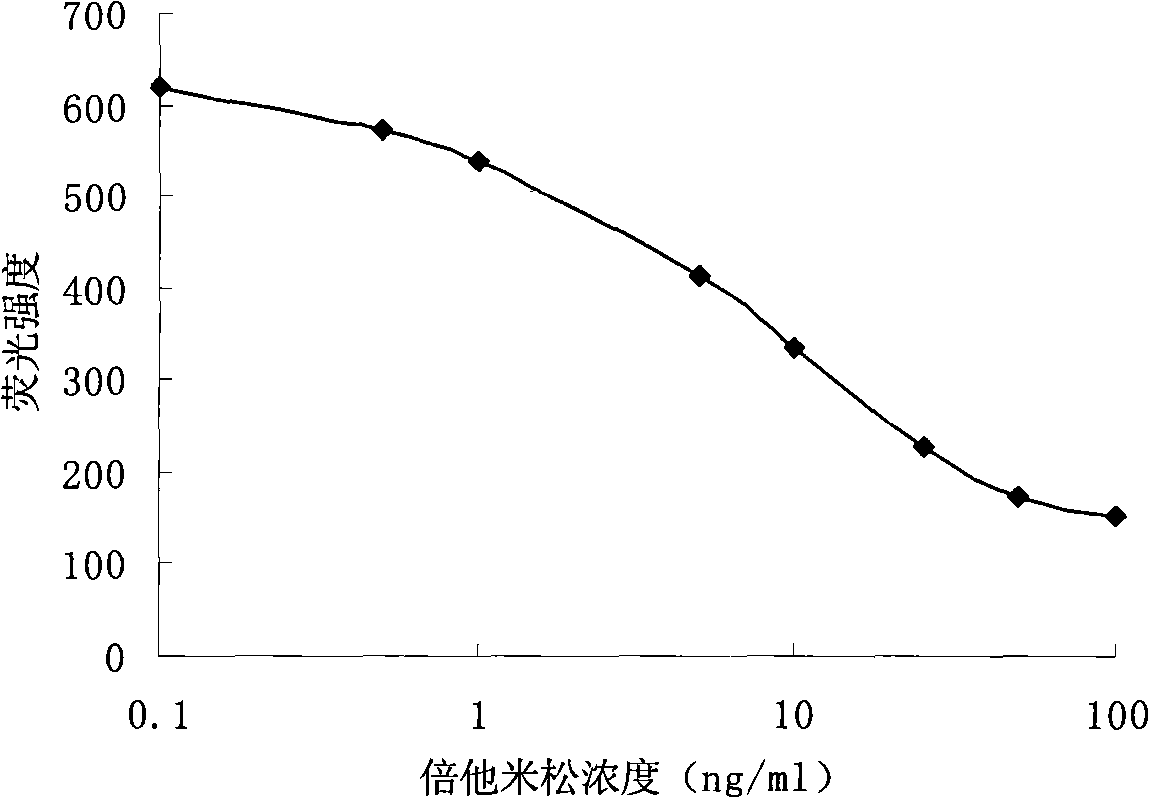
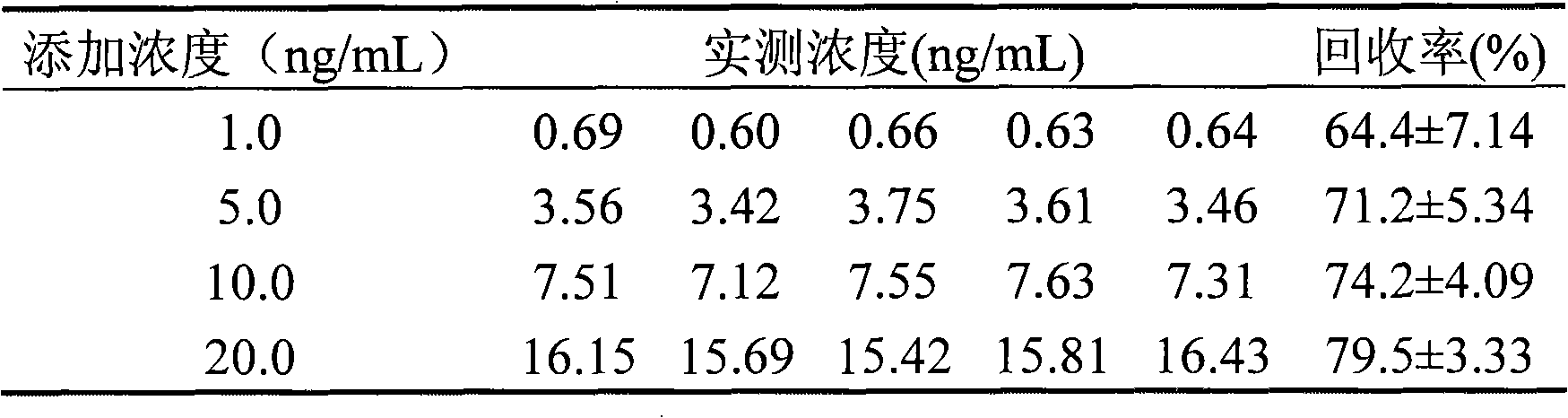
Method for quantum dot mark indirect competition fluoroimmunoassay detection for becort
InactiveCN101308146AEasy to operateHigh fluorescence intensityBiological testingFluorescence/phosphorescenceAntigenImmuno detection
Disclosed is a method of quantum dot-labeled indirect competitive fluorescence immunoassay of betamethasone, which belongs to the immunoassay method technique field. Quantum dots for labeling antibodies of the invention have the emission spectra of QD590, and the method comprises: directly covering coating antigens in micro-holes of an enzyme label plate, adding betamethasone standard solution or a sample under test to form an antigen-antibody fluorescence immunity compound body, stimulating and detecting the fluorescence intensity of the formed antigen-antibody fluorescence immunity compound body with a fluorescence enzyme-labeling instrument, and obtaining the concentration of betamethasone in the sample under test through comparing with the standard solution. The invention can detect the content of betamethasone in the sample under test without adding chromogenic substance, namely, the concentration of betamethasone in the sample under test can be detected indirectly through the fluorescence intensity of the antigen-antibody immunity compound body, and both the operation and reaction need only one step; and the quantum dots for labeling antibodies of the invention have advantages of stronger emitted fluorescence intensity and long stabilization time of fluorescence compared with the traditional fluorescence.
Owner:JIANGNAN UNIV
Fluorescence immunoassay detection method and kit for HER-2
The invention relates to fluorescence immunoassay detection method and kit for HER-2. The method comprises the following steps: preparation of a probe fixing pad; preparation of an immunoassay test strip; preparation of sample diluent; and inspection of the sample. HER-2 monoclonal antibody fluorescence latex particles and goat anti-rabbit IgG fluorescence latex particles are mixed and then diluted by using gold diluent, and the diluted mixture is sprayed on glass fiber so as to prepare the probe fixing pad; and a detection line is coated by an HER-2 monoclonal antibody, and a control line is coated by goat anti-rabbit IgG so as to prepare the fluorescence immunoassay test strip. The detection kit provided by the invention comprises a PVC liner plate, a sample pad, the probe fixing pad, a cellulose nitrate membrane and absorbent paper. The probe fixing pad is prepared by mixing and drying the HER-2 monoclonal antibody fluorescence latex particles and the goat anti-rabbit IgG fluorescence latex particles. Thus, the fluorescence immunoassay detection method and kit have the advantages of high sensitivity, high accuracy, simple operation and low cost.
Owner:SUZHOU BIONANOTECH CO LTD
Micro-fluidic chip based on fluorescence immunoassay joint detection as well as preparation method and application of micro-fluidic chip
ActiveCN108181458AFully combinedHigh sensitivityMaterial analysisFluorescence immunoassayMicrosphere
The invention relates to a micro-fluidic chip based on fluorescence immunoassay joint detection. The micro-fluidic chip comprises a chip substrate and an upper layer cover plate covering the upper part of the chip substrate; a sample adding zone, an antibody coating zone, a micro mixing zone, at least two detection zones, a quality control zone and a waste liquid collecting zone which are sequentially communicated by a capillary microchannel are arranged on the chip substrate; a sample adding hole, at least two detection windows, a quality control window and a ventilation hole are formed in the upper layer cover plate and sequentially correspond to the sample adding zone, the detection zones, the quality control zone and the waste liquid collecting zone. The invention also provides a preparation method and the application of the micro-fluidic chip. According to the micro-fluidic chip disclosed by the invention, immunomagnetic microspheres are arranged in the detection zone and the quality control zone, and to-be-detected substances are favorably fixed in the corresponding detection zones, so that the interference caused by non-specificity is reduced and the sensitivity is improved.
Owner:BEIJING ELCOTEQ BIO TECH
Fluorescence immunoassay quantitative detection kit of microalbuminuria, and preparation method thereof
InactiveCN102879586ASimple and fast operationImprove accuracyBiological testingAntigenFluorescence immunoassay
The invention relates to a fluorescence immunoassay quantitative detection kit of microalbuminuria, and a preparation method thereof. A sample pad, a combination pad, a nitrocellulose membrane and absorbent paper are pasted on the bottom liner of the quantitative detection kit in a successively lapped manner, wherein the combination pad is coated by fluorescent latex microsphere-marked human albumin antibodies; and the nitrocellulose membrane is coated by a detection line of albumin recombinant antigens and a quality control line of rabbit anti-mouse IgG antibodies. The fluorescence immunoassay quantitative detection kit can detect the content of microalbuminuria in human urine accurately and quantitatively, and has the characteristics of simple operation, high accuracy, high sensitivity, low cost, etc.
Owner:GETEIN BIOTECH
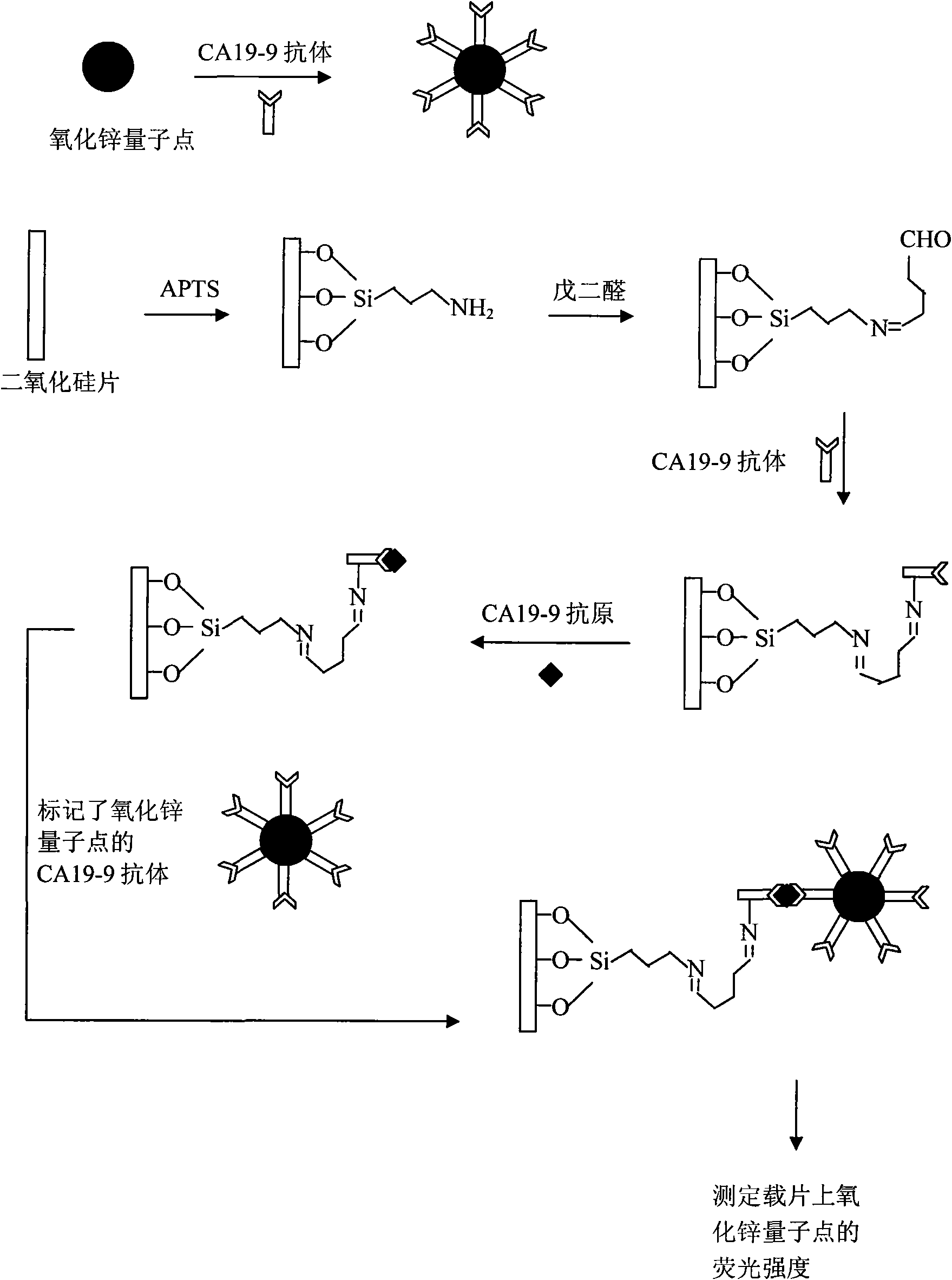
Fluorescence immunoassay method of using zinc oxide quantum dots to mark antibody
InactiveCN101587071AUniform particle sizeConditions are easy to controlPreparing sample for investigationBiological testingDiagnostic methodsFluorescence intensity
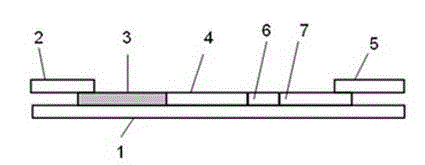
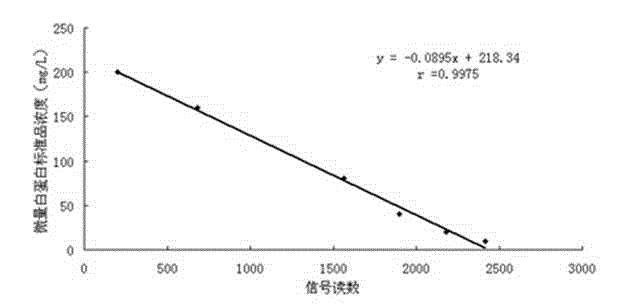
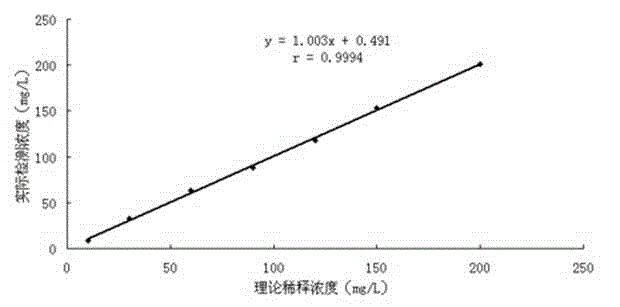
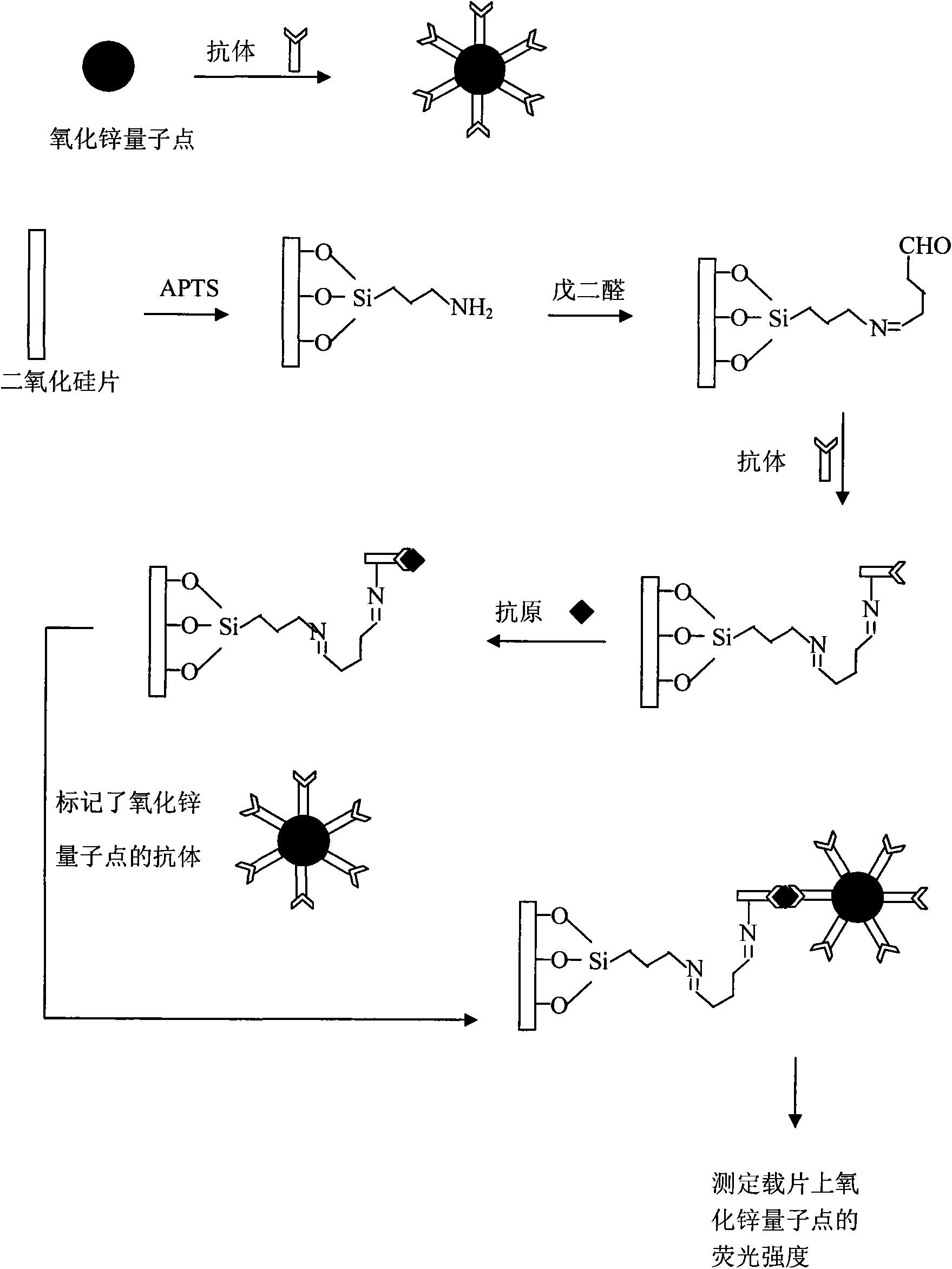
Fluorescence immunoassay method of using zinc oxide quantum dots to mark antibody is a method of combining zinc oxide quantum dots marked antibody with the antibody fixed on slide through antigen specific reaction, using fluorescent characteristic of the zinc oxide quantum dots to measure fluorescence strength of the zinc oxide fixed on slide, establishing standard curve of relationship between fluorescence strength of the zinc oxide quantum dots and antigen concentration so as to quantificationally determineantigen concentration of actual measured sample. The zinc oxide quantum dots prepared by the invention provided method has uniform particle diameter, good dispensability, stable combination with antibody, reliable and stable testing results and good repeatability, which providing a rapid and reliable detection approach for early detection of disease antigen in medical science clinical.
Owner:南通市京山锦纶有限公司 +1
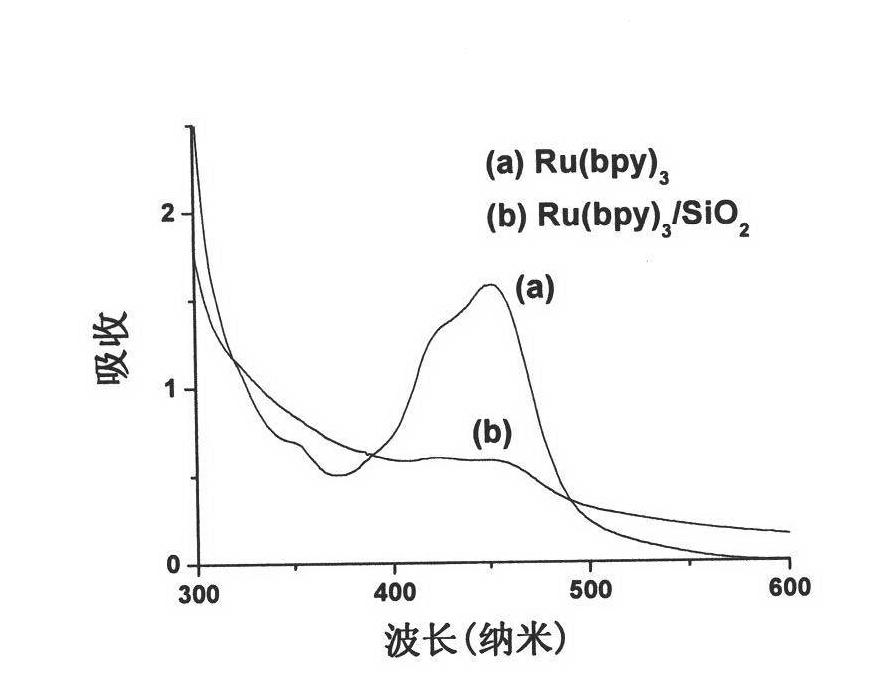
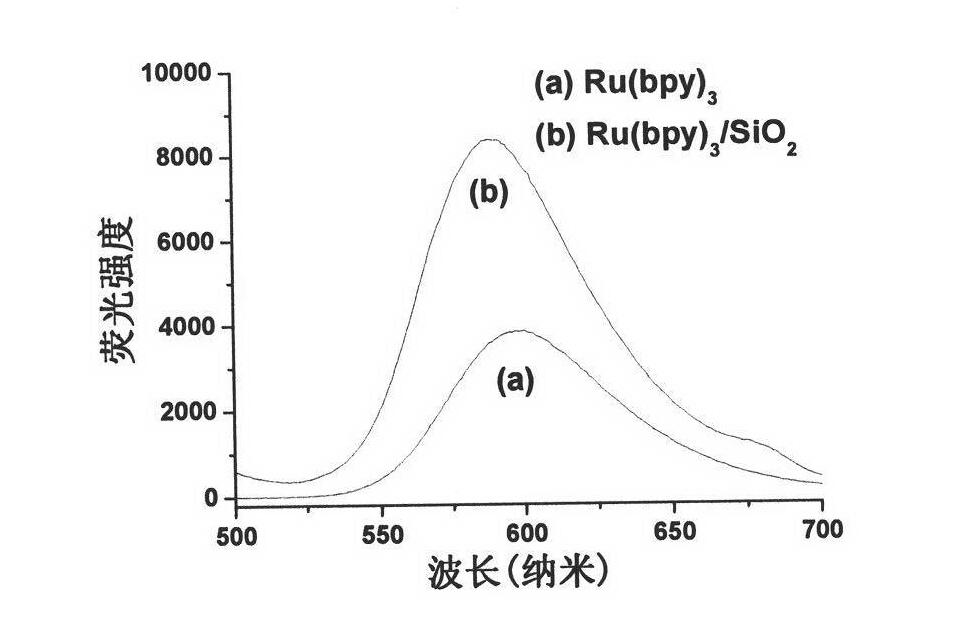
Fluorescent nanoparticles Ru(bpy)3/SiO2, preparation method and application thereof
InactiveCN101864291ABiological testingLuminescent compositionsMean diameterBiotin-streptavidin complex
The invention relates to fluorescent nanoparticles Ru(bpy)3 / SiO2, a preparation method and application thereof. The fluorescent nanoparticles have nuclear shell structures; the nuclear shell structure is formed by taking tris(2,2'-bipyridyl)ruthenium as a core, covering silicon dioxide with netlike structure on the surface of the tris(2,2'-bipyridyl)ruthenium and carrying active amino groups on the surface of the silicon dioxide, wherein the mass ratio of the tris(2,2'-bipyridyl)ruthenium to the silicon dioxide is 1:5 to 1:8; and every milligram of nanoparticles comprises 385nmol of amino group. The silicon fluorescent nanoparticles Ru(bpy)3 / SiO2 have the advantages of uniform size, high monodispersity, mean diameter of 70+ / -6nm, high light stability and difficult dye leakage in aqueous solution. The fluorescent probe is applied to a protein microarray chip to detect HIV p24 antigen after marking streptavidin; the analysis method is a sandwich fluorescence immunoassay method; and the result shows that the fluorescence intensity is in good positive relationship with p24 concentration and the analytical sensitivity is 3.1ng / mL. The result shows that the nanoparticles, serving as a novel fluorescent probe, can be applied to the systems of the protein microarray chip and fluorescence immunoassay and the like for high flexibility detection.
Owner:SHANGHAI UNIV
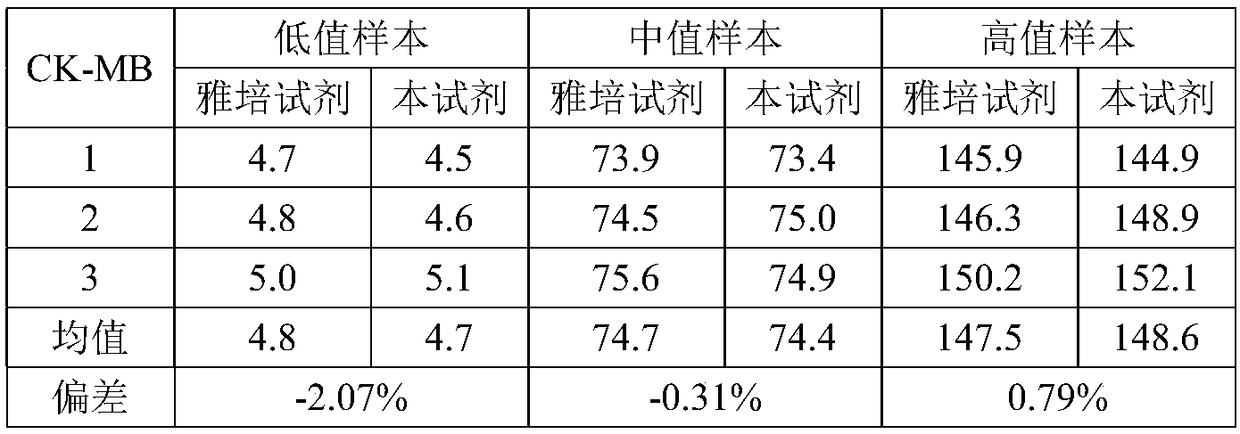
Magnetic bead time resolution fluorescence immunoassay quantitative determination CK-MB (creatine kinase-MB) kit
The invention discloses a magnetic bead time resolution fluorescence immunoassay quantitative determination CK-MB kit. The CK-MB kit comprises an immunomagnetic bead coating a CK-MB monoclonal antibody, a CK-MB standardized product solution, a europium-marked CK-MB monoclonal antibody solution, washing liquid and enhancement liquid. The immunomagnetic bead coating the CK-MB monoclonal antibody isa covalent conjugate of a superpara magnetic bead modified by a functional group and with the diameter being 1-3 microns and the CK-MB monoclonal antibody. The kit has the high sensibility, the sensibility of CK-MB is 1ng / mL, and a blood serum (plasma) does not need to be diluted; the determination time is short, and a report can be resulted within 30 minutes; the demanding amount of the sample isless, and only 50 microliters are needed for one-time sample loading; and the kit is equipped with a full-automatic time resolution immune analysis meter, operation is easy, no artificial error exists, and labor is saved. The kit reasonably utilizes the space of a reagent strip, the structure of the reagent strip is more compact, the reagent strip can be transported more easily, and used conveniently, the operation is simple, and the stability is good.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
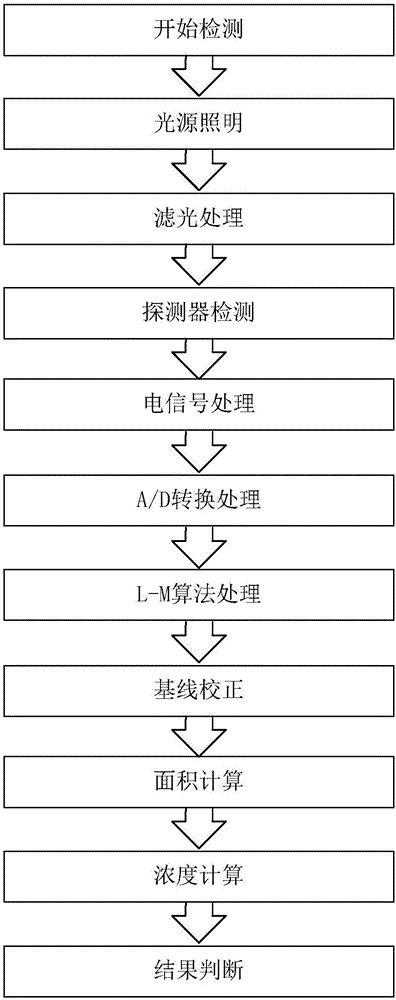
Quantitative detection and computation method based on fluorescence immuno-chromatographic technology
InactiveCN106841133ALess power consumptionIncrease heatFluorescence/phosphorescenceElectricityFluorescence immunoassay
The invention belongs to the field of immuno-chromatographic detection, and in particular relates to a quantitative detection and computation method based on the fluorescence immuno-chromatographic technology, comprising the following steps: inserting a fluorescence immunostrip dripped with a sample solution to be detected into a fluorescence immunoassay instrument, and sequentially performing irradiation with an LED light source, optical filtering processing with an optical filter, detection with a photoelectric detector, electrical signal processing, AD conversion processing, baseline correction, L-M algorithm processing, area computation and concentration computation. The quantitative detection and computation method stresses on signal reconstruction by using an algorithm, eliminates influence of noise and various interferences, ensures the signal processing course undistorted, and can obtain high-precision and low-error results, thereby quickly, conveniently and accurately determining the concentration result.
Owner:BIOHIT BIOTECH HEFEI
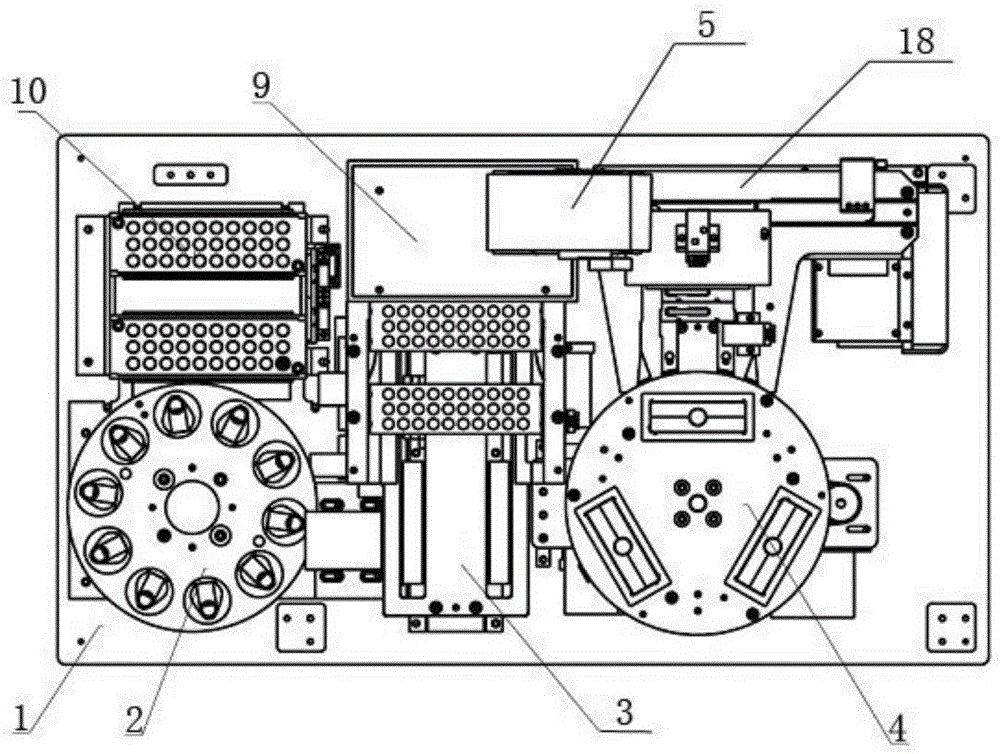
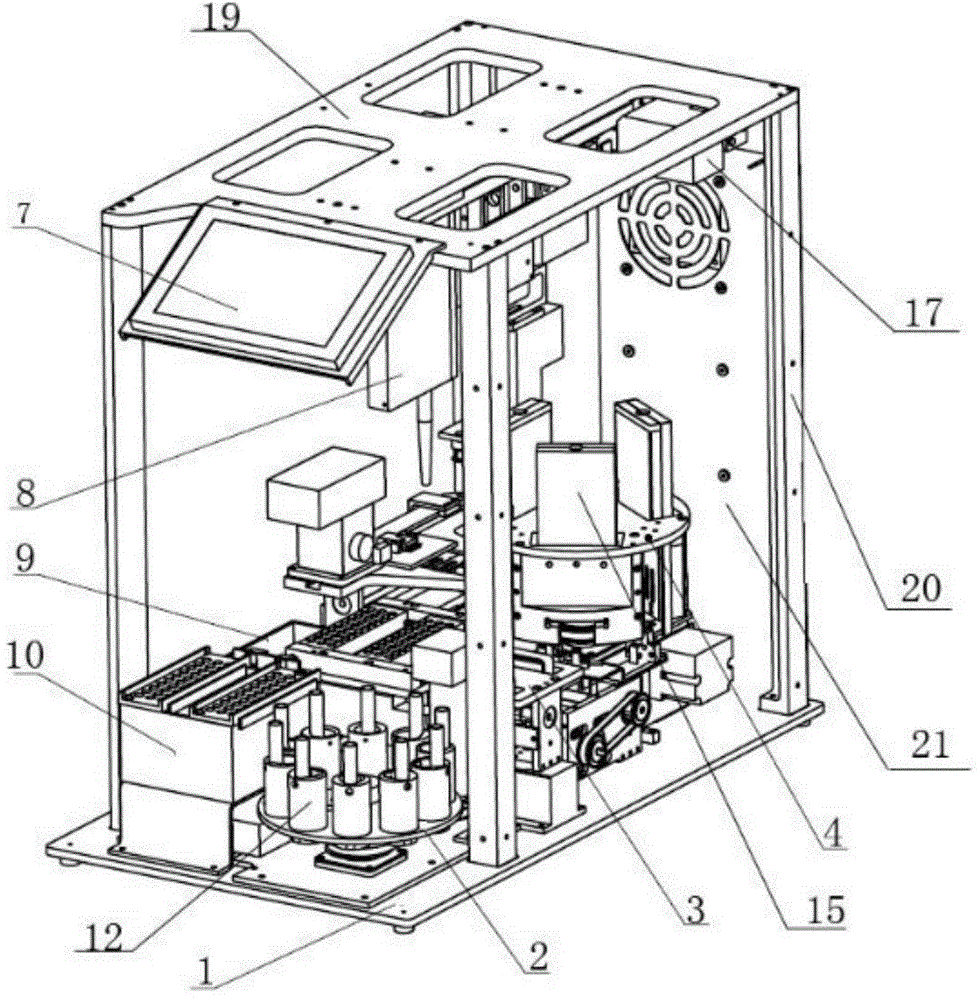
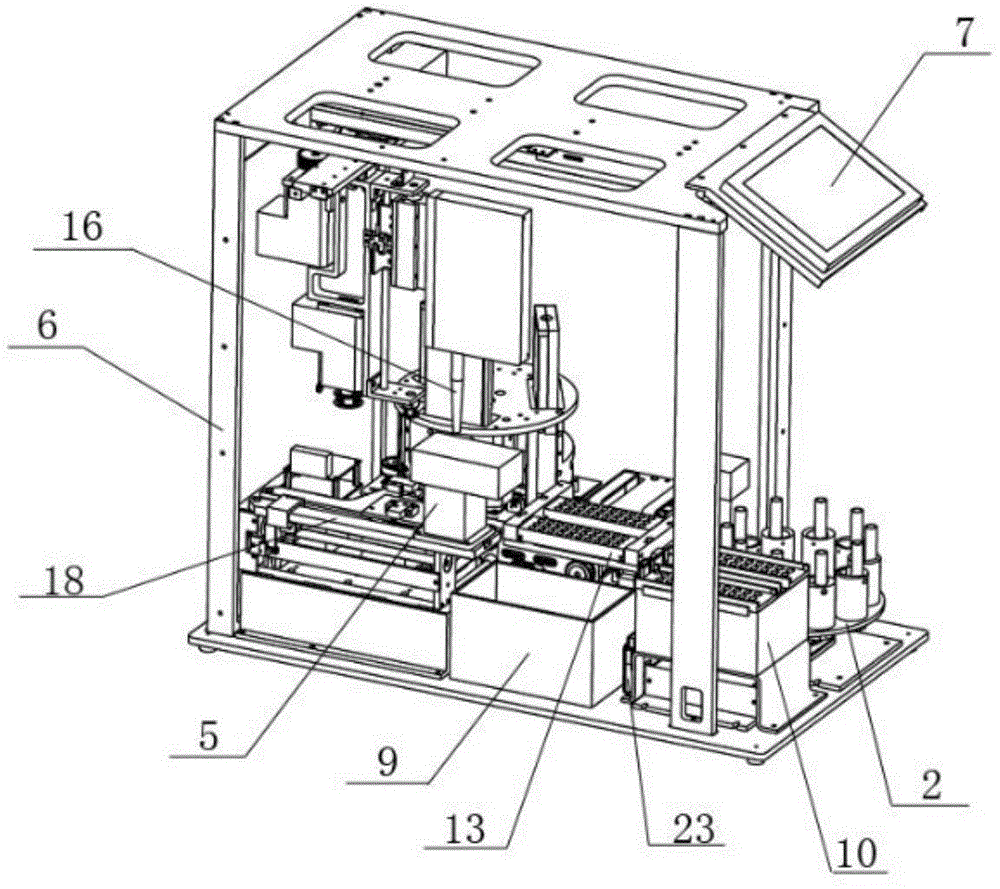
Fully-automatic fluorescence immunoassay system
ActiveCN104634984AReduce intensityReduce error rateMaterial analysisFluorescence immunoassayFluorescence
The invention discloses a fully-automatic fluorescence immunoassay system comprising a suction head placing frame, a sample uniformly-shaking unit, a buffer solution feeding unit, a test paper sheet conveying unit, a three-dimensional sample adding unit, a detection unit and a signal processing control unit, wherein a suction head is placed on the suction head placing frame; the sample uniformly-shaking unit is used for uniformly shaking a to-be-detected sample; the buffer solution feeding unit is used for conveying a buffer solution plate to a working position, and the buffer solution plate is abandoned after running out; the test paper sheet conveying unit is used for pushing out test paper sheets from a test paper box and respectively conveying the test paper sheets to a sample adding position and a detection position; the three-dimensional sample adding unit is used for loading the suction head; and the detection unit is used for detecting the test paper sheets which are subjected to sample adding, and the test paper sheets are abandoned after detection ends. According to the fully-automatic fluorescence immunoassay system disclosed by the invention, the strength and error rate of detection operations can be significantly reduced by virtue of fully-automatic operations, the detection throughput can reach 60 tests per hour, and thus the requirements of large batch detection can be met; and according to the fully-automatic fluorescence immunoassay system, the suction and mixing of the samples and the sample adding of the test paper sheets are performed by using the disposable suction heads, so that the cross contamination among the samples can be avoided, and the accuracy of results can be improved.
Owner:SUZHOU TOPMEDLAB MEDICAL SCI & TECH CO LTD
Method for detecting imidacloprid by quantum-dot-marked sandwich fluorescence immunoassay
InactiveCN103323587ASensitive detectionQuick checkFluorescence/phosphorescenceSolubilityFluorescence immunoassay
The invention discloses a method for detecting imidacloprid by quantum-dot-marked sandwich fluorescence immunoassay. According to the technical scheme, the method comprises the following steps of: 1, preparing a nuclear shell quantum dot and modifying the water solubility of a fat-soluble quantum dot by modification of different macromolecules; 2, preparing and passivating a quantum probe; and 3, constructing an imidacloprid antibody quantum dot immunoassay method. The method is applied to preparation, passivation and performance representation of the quantum dot and detection of precipitate imidacloprid residues and is a fluorescence immunoassay method for sensitively and quickly detecting the imidacloprid in vegetables based on the quantum dot and conjugates of IgG-HRP.
Owner:TIANJIN AGRICULTURE COLLEGE
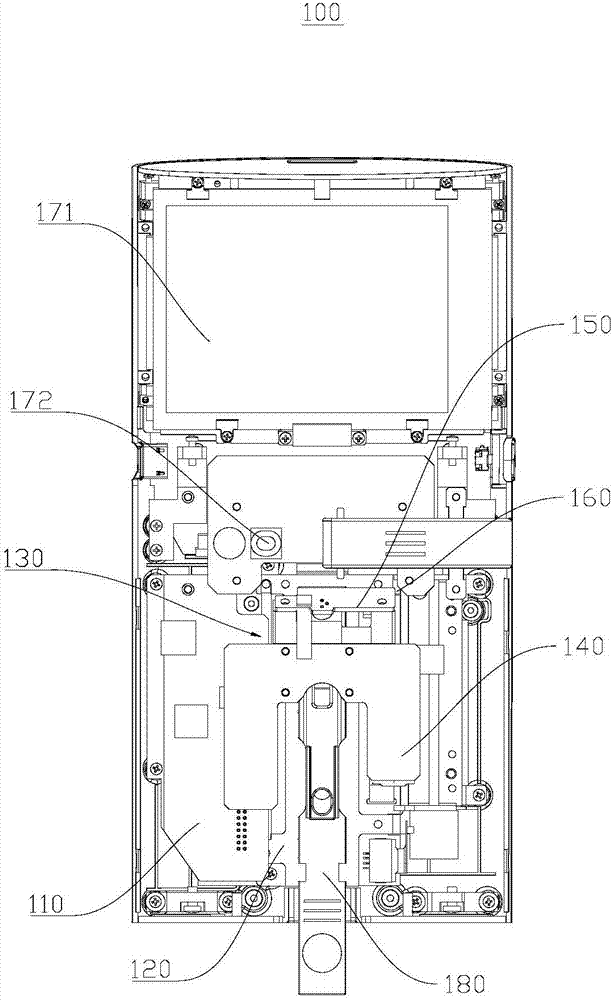
Biochemical and immunological analysis meter and biochemical and immunological detecting method
ActiveCN107449765ACompact structureEasy to carryFluorescence/phosphorescenceFluorescence immunoassayFluorescence
The invention relates to the field of medical detection and relates to a biochemical and immunological analysis meter and a biochemical and immunological detecting method. The biochemical and immunological analysis meter comprises a test board, a supporting base, a fluorescent scanning light path component and a display component; a reagent card which is fixedly inserted into a supporting part is subjected to dry biochemical analysis, and the fluorescent scanning light path component can be driven by a driving mechanism to drive a moving component to slide along the length direction of a groove, thereby carrying out fluorescent scanning analysis on the fixed reagent card and outputting a detection result by the display component. The biochemical and immunological analysis meter has the functions of fluorescence immunoassay quantitation and dry biochemical analysis, the range of detection items is widened, and detection time is saved. According to the method, the biochemical and immunological analysis meter is adopted for detecting, and further simultaneous measurement of fluorescence immunoassay quantitative analysis and dry biochemical quantitative analysis is realized; in addition, two measurement methods are in mutually non-intervention and are separated from each other.
Owner:GETEIN BIOTECH
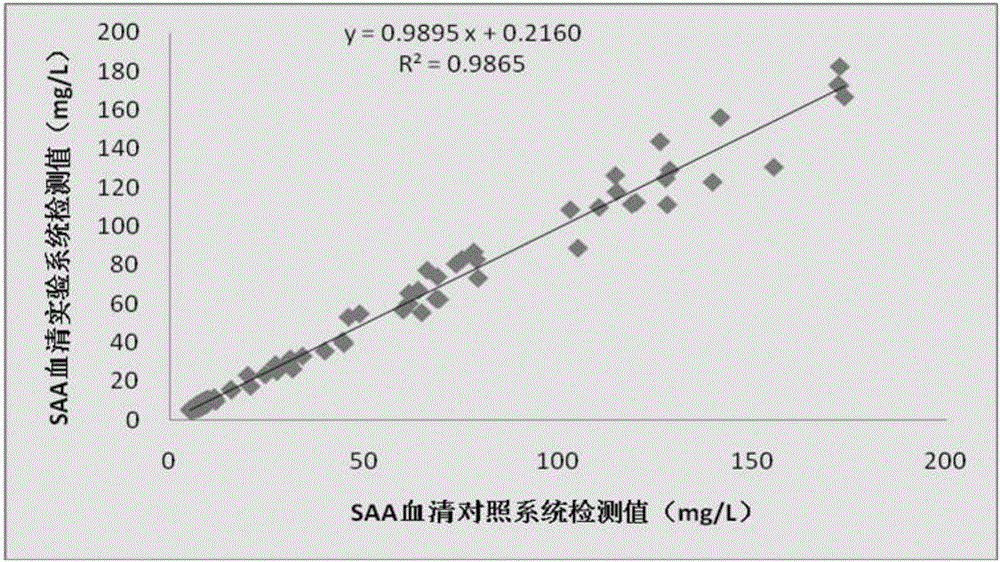
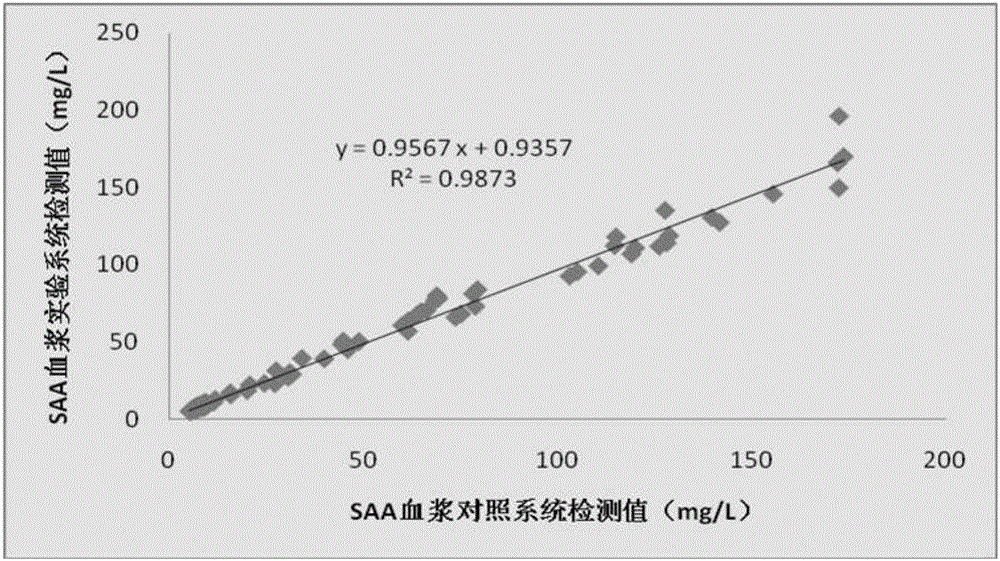
Serum amyloid A testing kit and manufacturing method
InactiveCN106706926ASuitable for detectionSuitable for economical practicalityBiological testingAntigenLanthanide
The invention relates to the technical field of fluorescence immunoassay in medical immunology, and in particular relates to a serumamyloid A (SAA) testing kit (fluorescence immunochromatographic method) and a manufacturing method. The kit is provided with a test paper card. The kit is characterized in that a PVC (Polyvinyl Chloride) plate, a sample pad, a conjugate pad, a nitrocellulose membrane and a water absorbing pad are sequentially arranged on the test paper card from bottom to top; a rare-earth Eu<3+> fluorescent microsphere marked serum amyloid A monoclonal antibody is adsorbed on the conjugate pad; the diameter of a rare-earth fluorescent microsphere is 200nm; the rare-earth fluorescent microsphere contains a rare-earth lanthanide element Eu<3+>; the rare-earth fluorescent microsphere is stable in a ground state and emits fluorescence of which the wavelength is 615nm under the stimulation action of laser of 337nm; and the monoclonal antibody is a monoclonal antibody which is purified and mixed, and comes from a monoclonal antibody cell strain for 2-6 different serum amyloid A epitopes. The kit has the advantages of being simple and convenient to operate, rapid in reaction, high in sensitivity, good in specificity and the like.
Owner:WEIHAI NEOPROBIO
Fluorescence immunoassay test strip used for detecting tumor marker CA72-4 and preparation method of fluorescence immunoassay test strip
The invention provides a fluorescence immunoassay test strip used for detecting a tumor marker CA72-4 and a preparation method of the fluorescence immunoassay test strip. The test strip comprises a sample cushion, a quantum dot marker combination pad, a nitrocellulose membrane and a piece of water absorption paper which are sequentially laid on a bottom plate in an overlapped mode. The double-antibody sandwich immunochromatographic method is adopted. A CA72-4 resistant monoclonal antibody CC49 coating provided with a quantum dot marker is arranged on the quantum dot marker combination pad. A detection line and a quality control line are arranged on the nitrocellulose membrane. A CA72-4 monoclonal antibody B72.3 is arranged on the detection line. A goat-anti-mouse IgG is arranged on the quality control line. The preparation method mainly relates to preparation of the quantum dot marker antibody. After immunochromatography is conducted through the test strip, the quantitative result of CA72-4 is obtained by conducting analysis through a fluorescence immunochromatographic chip detection instrument, operation is easy and convenient, bedside timely and rapid detection can be conducted, and stability and flexibility are high.
Owner:SHANGHAI JIAO TONG UNIV
Pentamethine Cyanine Fluorescent Dye with N-substituting at Beta-Position of Conjugated Chain
ActiveUS20120296098A1Fluorescence enhancementGood cell permeabilityOrganic chemistryMethine/polymethine dyesMembrane permeabilityFluorescence immunoassay
A pentamethine cyanine fluorescent dye with N-substituting at β-position of conjugated chain is disclosed. The dye reacts with biosubstrate including protein, liposome and DNA, and shows excellent selectivity. It has good living cell membrane permeability and shows good selectivity in living cell staining In addition, the dye has good stability and low background fluorescence, and can be used together with other fluorescent dyes having consistent wavelengths for single-channel excitation and multi-channel detection. The dye can be used in the fields of protein labeling and detecting, fluorescence immunoassay and living cell selective imaging and the like.
Owner:DALIAN UNIV OF TECH +1
Porcine trichinosis antibody test strip, and preparation method and application thereof
InactiveCN110221067AIncreased sensitivityImprove featuresMaterial analysis by observing effect on chemical indicatorFluorescence/phosphorescenceAdult stageFluorescence immunoassay
The present invention relates to a porcine trichinosis antibody test strip, a preparation method and application thereof, and belongs to the technical field of fluorescence immunoassay. In order to detect trichinella infection more quickly, stably and accurately, the present invention provides a porcine trichinosis antibody test strip. The porcine trichinosis antibody test strip comprises a samplepad, a binding pad, a chromatographic membrane, an absorbent pad and a bottom plate; the binding pad is marked with time-resolved fluorescent microspheres coupled with the goat anti-porcine IgG; andthe chromatographic membrane is provided with a detection line and a quality control line, wherein the detection line is sprayed with the trichinella cocktail antigen composed of a recombinant musclelarval stage Ts-WM5 antigen expressed by prokaryotic cells, an intestinal infectious larval stage Ts-WN10 antigen, an adult stage Ts-ZH68 antigen and a newborn larval stage Ts-T668-C antigen, and thequality control line is sprayed with the rabbit anti-goat IgG. The test strip is prepared after combination of the components. The technical scheme of the present invention can be used for early rapiddetection of porcine trichinella infection.
Owner:JILIN UNIV
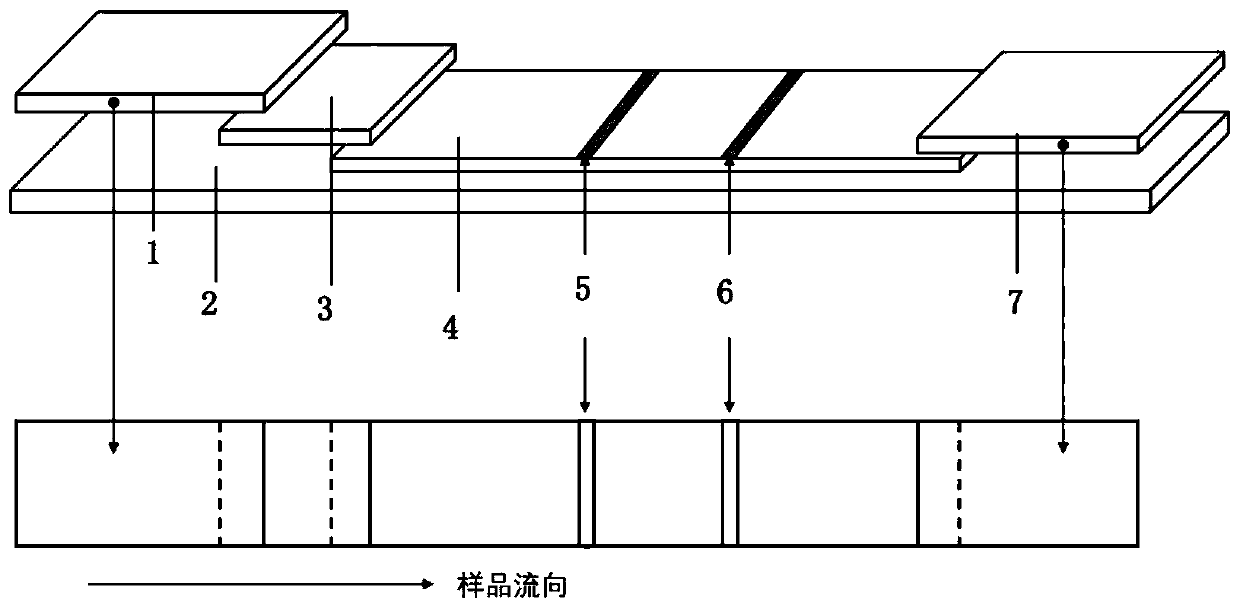
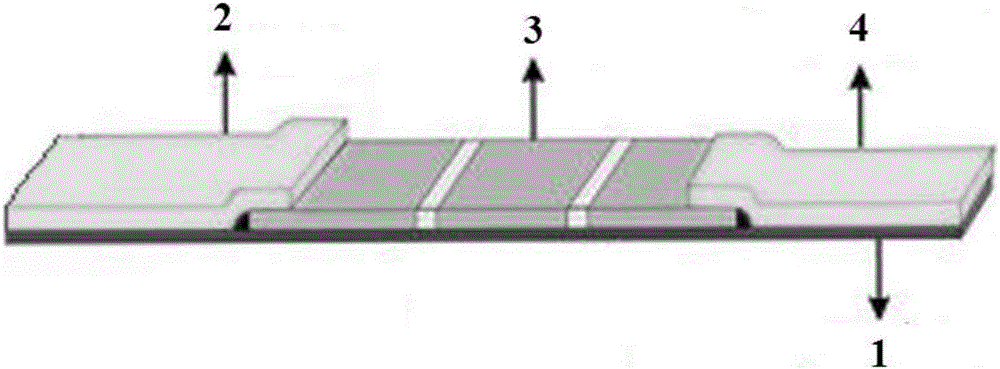
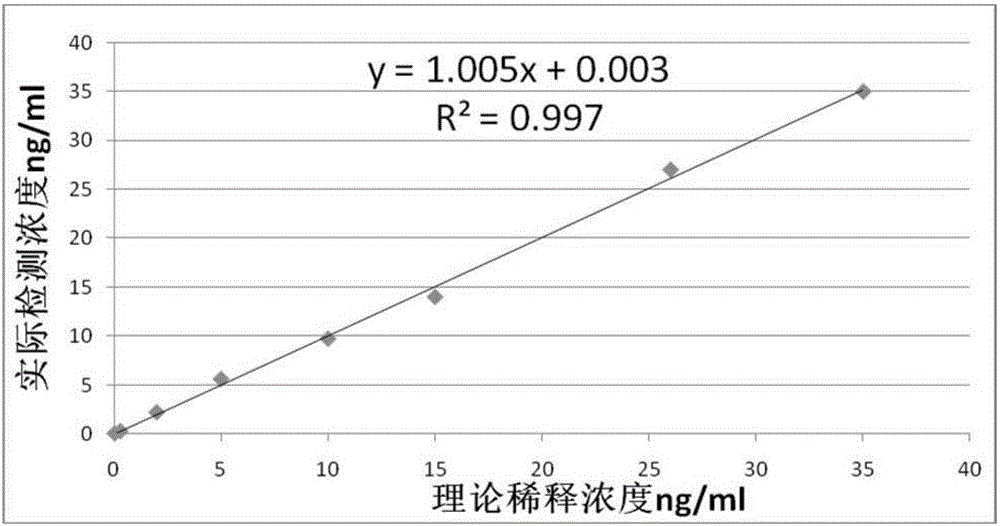
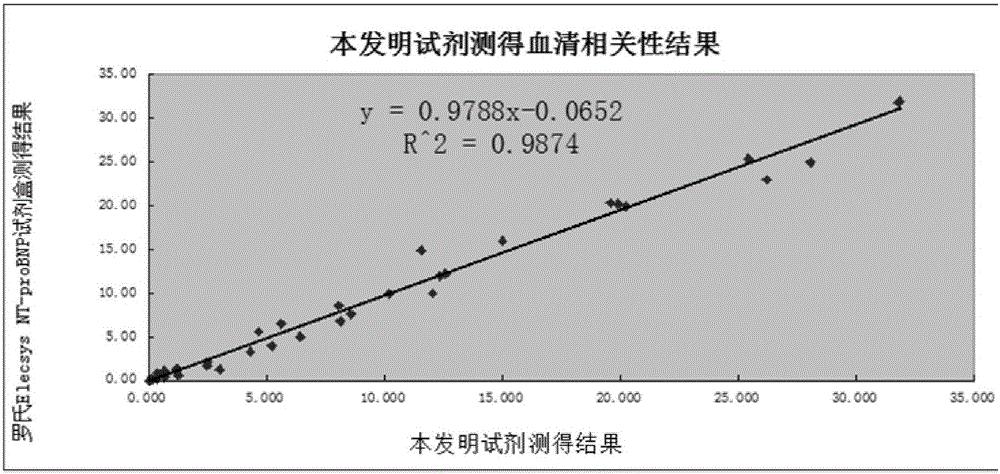
NT-proBNP fluorescence immunoassay reagent and preparing method thereof
ActiveCN105092832AThe composition formula is reasonableHigh sensitivityMaterial analysisSerum igePluronic F68
The invention relates to an NT-proBNP fluorescence immunoassay reagent and a preparing method thereof, which belong to the technical field of medical detection. The NT-proBNP fluorescence immunoassay reagent comprises an NT-proBNIP antibody for marking, a goat anti-chicken IgY antibody for marking, an NT-proBNP2 antibody for coating, a chicken IgY antibody for coating, a particle resuspension and a sample pad conditioning fluid, wherein the particle heavy suspension is Tris-HCl heavy suspension with 100-200mM Tris (containing 250-500mu.g / ml mouse IgG, 1-5% of calf serum, 0.1-0.5% of Pluronic F68, 0.5-1% of casein, 0.9% of NaCl, 0.05% of NaN3) and has a pH being 8-9, the sample pad conditioning fluid contains 10mM PBS (containing 0.1-0.5mg / ml of phytolectin) and has a pH being 7-8. The invention specifically discloses a preparing method of the NT-proBNP fluorescence immunoassay reagent, comprising specific particle marking, sample pad pretreatment, antibody coating on an NC film, and test paper assembling. The NT-proBNP fluorescence immunoassay reagent is high in sensitivity, wide in application range and capable of reducing interference in a serum test, and has a good interception effect on red cells.
Owner:NINGBO RUI BIO TECH
Time resolution fluorescence immunoassay method and kit for fast detecting gutter oil
InactiveCN104849251ATo achieve the purpose of identifying waste oilUnique research methodFluorescence/phosphorescenceGutter oilFluorescence immunoassay
The invention discloses a time resolution fluorescence immunoassay method and kit for fast detecting gutter oil. The method starts with the germ types massively propagated in the gutter oil, and advantageous germs polluting the gutter oil are analyzed, and specific components of the advantageous germs are determined to achieve the aim of identifying the gutter oil; the research method is very unique; lipopolysaccharide of the advantageous germs in the gutter oil is used for screening monoclonal antibodies and a TRFIA detection method of the lipopolysaccharide is established. The method has the advantages of high specificity and high detection sensitivity for the gutter oil, and has very important significance in identification and detection of the gutter oil.
Owner:GUANGZHOU CENT FOR DISEASE CONTROL & PREVENTION (GUANGZHOU HYGIENE INSPECTION CENT GUANGZHOU CENT FOR FOOD SAFETY RISK SURVEILLANCE & ASSESSMENT INST OF PUBLIC HEALTH OF GUANGZHOU MEDICAL UNIV)
Mycoplasma pneumoniae detection kit
InactiveCN105759034AQuick checkAccurate detectionBiological material analysisSerum igeImmunofluorescence
Owner:山东德诺生物科技有限公司
Fluorescence immunoassay kit used for detecting porcine circovirus type 2
ActiveCN103472225AStrong specificityHigh sensitivityFluorescence/phosphorescencePositive controlPhosphate
The invention provides a fluorescence immunoassay kit used for detecting porcine circovirus type 2 (PCV2), which comprises a single factor serum antibody used for detecting PCV2, an FITC (fluorescein isothiocyanate) labeled goat anti-mouse IgG (immunoglobulin G), a positive control sample, stationary liquid and PBS (phosphate buffer) washing liquor, wherein the single factor serum antibody used for detecting PCV2 is prepared from a PCV2 Cap protein antigen immunized mouse with an amino acid sequence of SEQ ID NO: 1. The main ingredient in the kit used for detecting PCV2 prepared in the invention is a single factor serum antibody of mouse anti-porcine circovirus type 2 and is prepared from the antigen immunized mouse with the amino acid sequence of SEQ ID NO: 1, and the prepared antibody can generate a specific reaction with PCV2. The fluorescence immunoassay kit of porcine circovirus type 2 created on the basis of the single factor serum can be used for detecting antigen in the diseased tissues of an PCV2 infected pig on clinic and judging whether the pig is infected with PCV2, and the fluorescence immunoassay kit is strong in specificity, high in sensitivity, good in repeatability and high in detection speed.
Owner:YEBIO BIOENG OF QINGDAO
Fluorescence immunoassay chromatography test strip for detecting vomitoxin
InactiveCN105866419AStrong specificityHigh sensitivityFluorescence/phosphorescenceCelluloseFluorescence
The invention discloses a fluorescence immunoassay chromatography test strip for detecting vomitoxin. The fluorescence immunoassay chromatography test strip comprises a supporting body and an adsorption layer fixed to the supporting body. The adsorption layer sequentially comprises an adsorption fiber layer, a fluorescent antibody fiber layer, a cellulose membrane layer and a water absorption material layer starting from the test end. Invisible detection prints printed by a coupling DON carrier protein solution and invisible contrast prints printed by a goat anti-mouse IgG or rabbit anti-mouse IgG or goat anti-rabbit IgG antibody solution are arranged on the cellulose membrane layer. The fluorescent antibody fiber layer is made from glass fiber cotton for adsorbing fluorescent antibodies. The fluorescent antibodies are graphene oxide fluorescent nanometer materials or NaYF4:Yb or Tm nano-particles or DON monoclonal antibodies or polyclonal antibodies marked by NaGd(WO4)2:Eu3+ nano-particles. The test strip has the advantages of being high in specificity, sensitivity and stability, good in safety and easy, convenient and rapid to use, can achieve on-site quantitative detection under a portable fluorescent reading device and can meet the requirements of people of different levels.
Owner:ZHONGZHOU UNIV
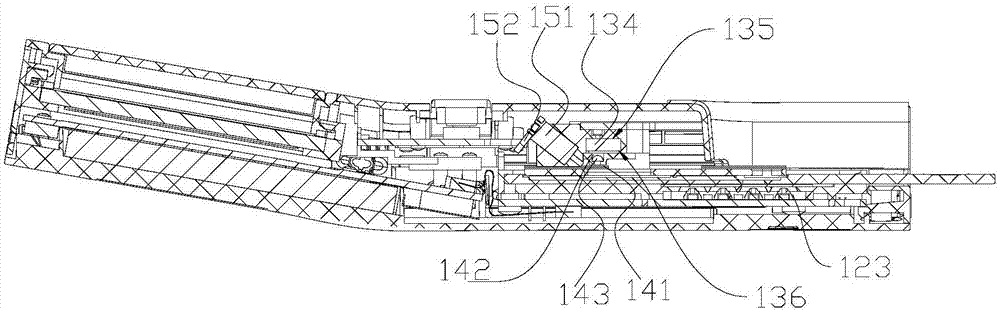
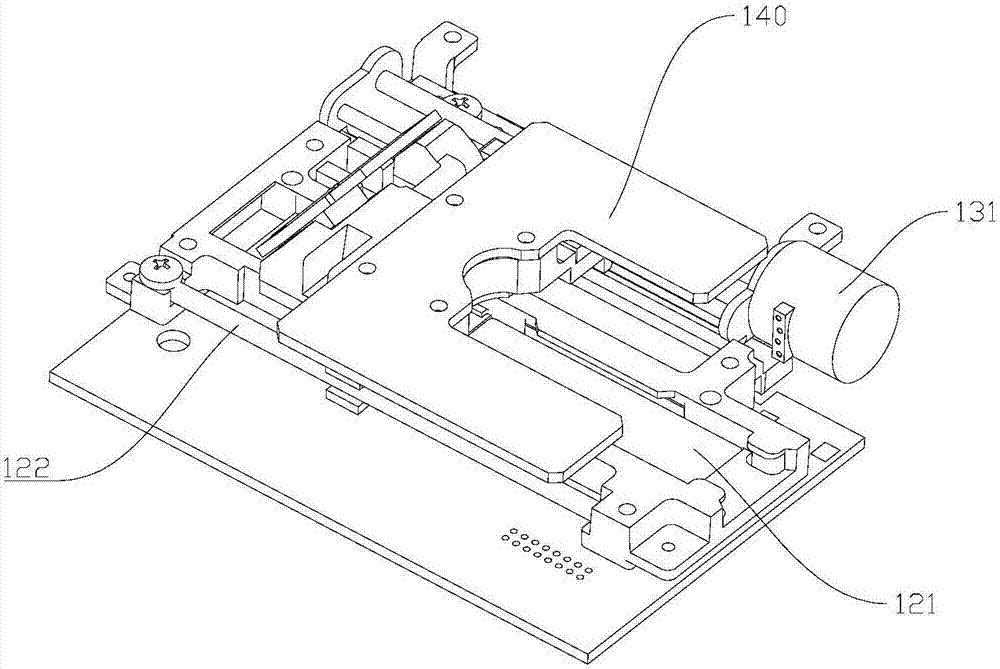
Monochromatic fluorescence detection device
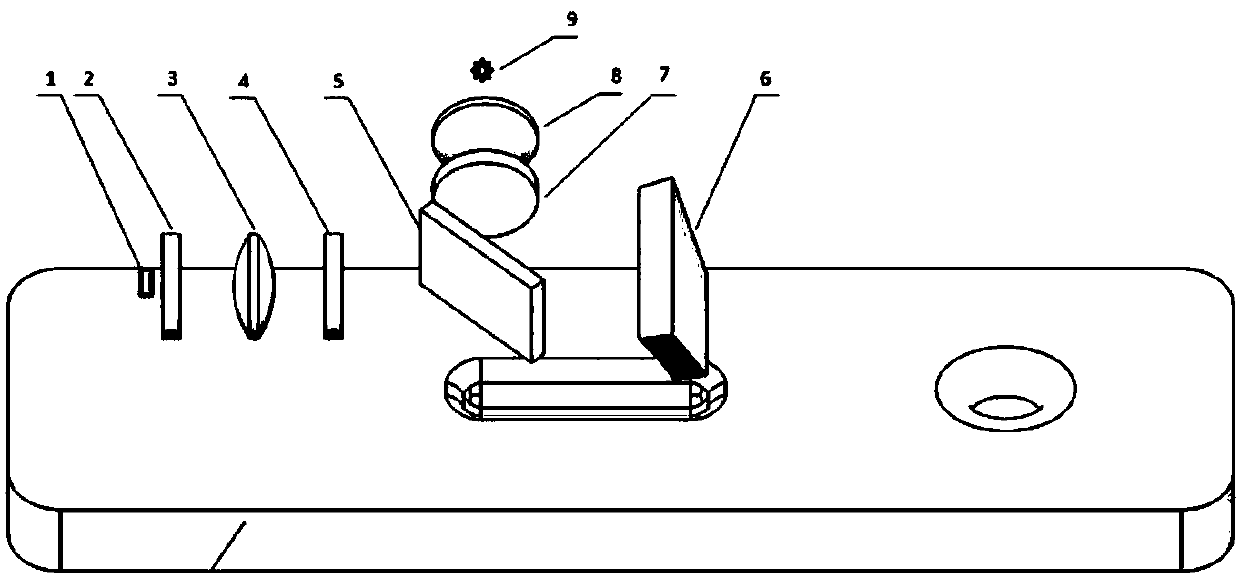
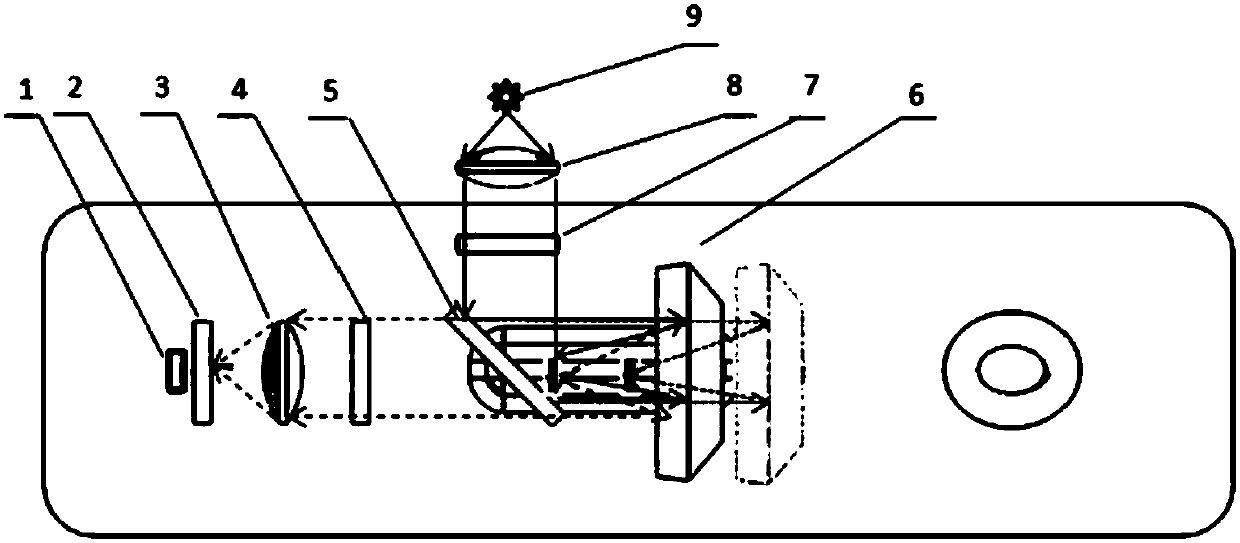
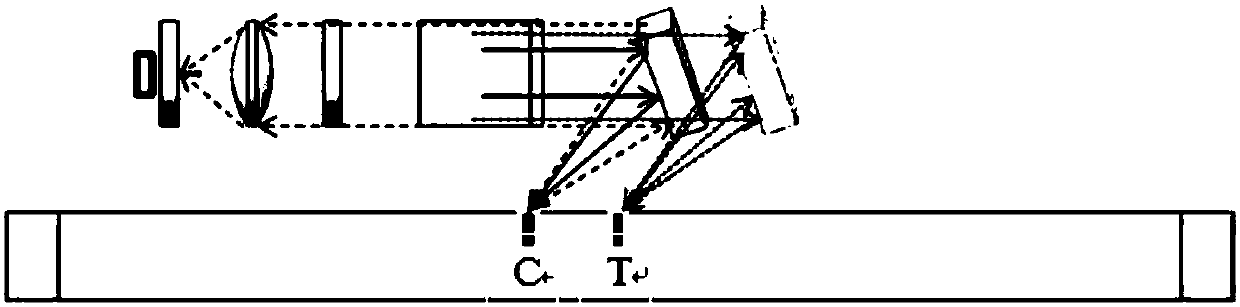
The invention discloses a monochromatic fluorescence detection device. The monochromatic fluorescence detection device comprises a photoelectric detector, a micropore diaphragm, a focusing lens, a first optical filter, a dichroic mirror, a parabolic reflector, a second optical filter, a collimating lens and an LED light source. The photoelectric detector, the micropore diaphragm, the focusing lensand the first optical filter are sequentially arrayed in an optical axis coincided manner to form a receiving light path. Optical axes of the dichroic mirror and the receiving light path form a 45-degree angle horizontally. The second optical filter, the collimating lens and the LED light source behind the dichronic mirror are sequentially arrayed in an optical axis coincided manner to form an excitation light path. The detection device for temporal separation of C line and T line optical signals on the basis of an optical path conversion structure of a parabolic reflector micromotion mechanism is small in size, high in measurement precision, portable and convenient for onsite detection and can be applied to researching of fluorescence immunoassay quantitative detection instruments.
Owner:NANJING INST OF ADVANCED LASER TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com