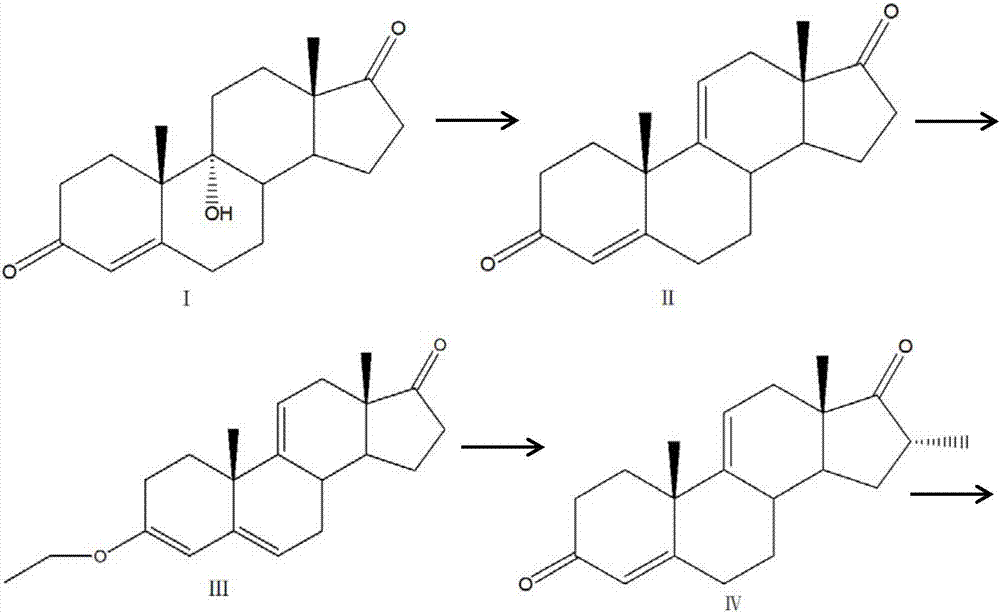
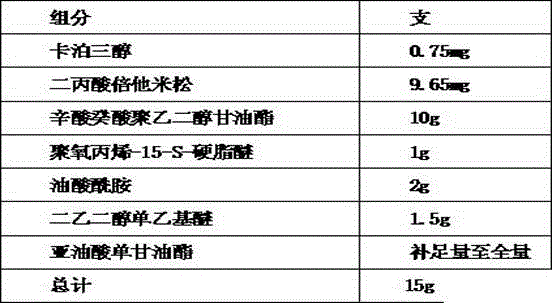
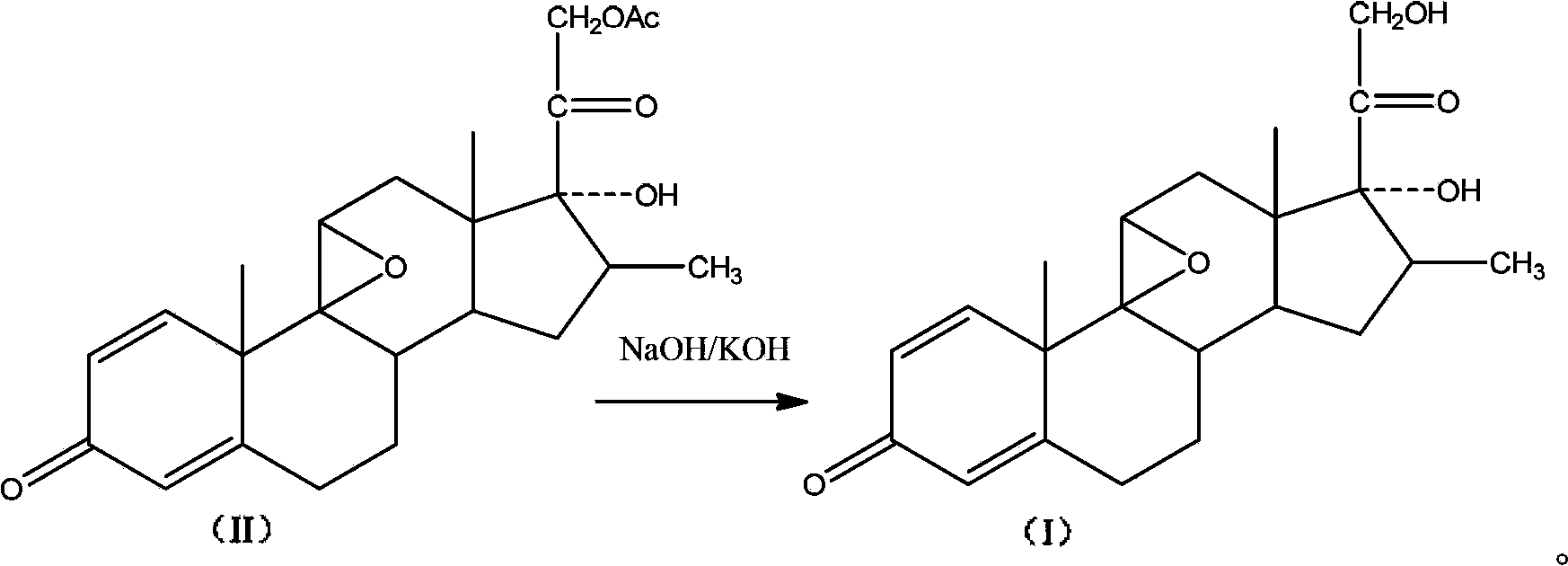
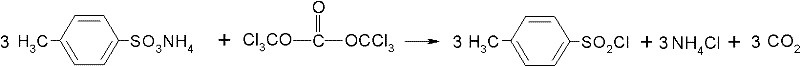Patents
Literature
143 results about "Betamethasone" patented technology
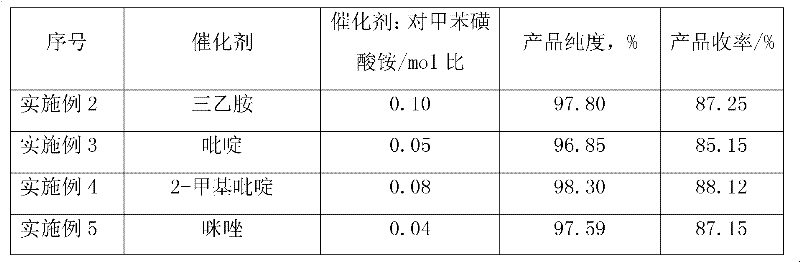
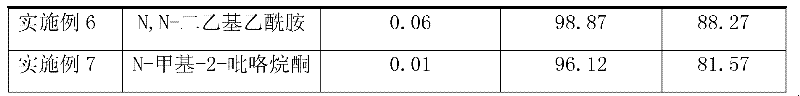
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
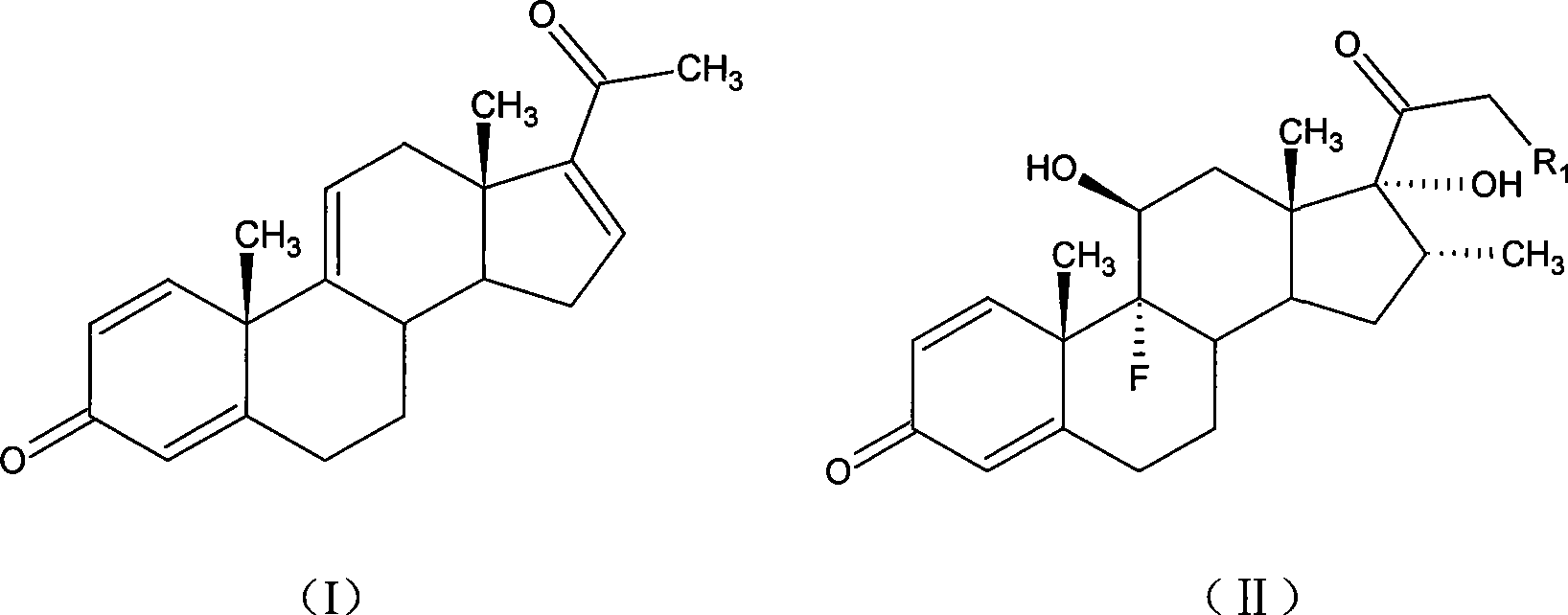
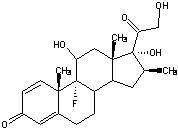
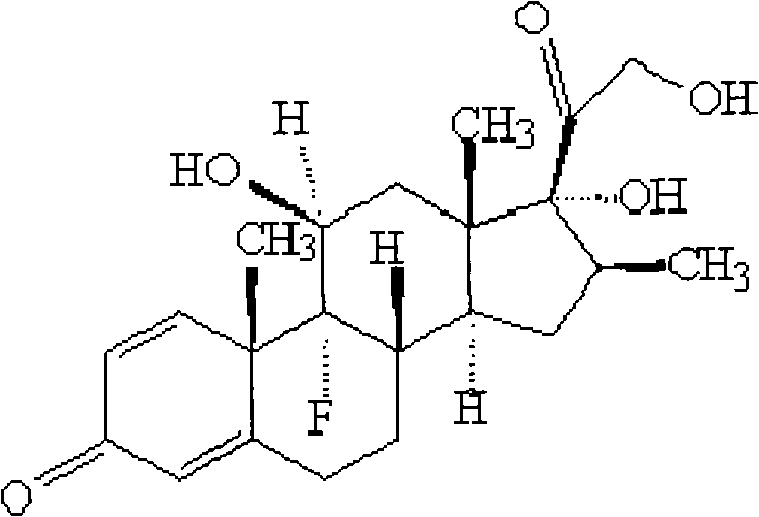
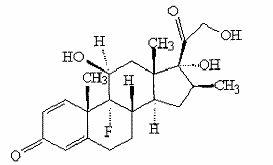
Betamethasone is a steroid medication. It is used for a number of diseases including rheumatic disorders such as rheumatoid arthritis and systemic lupus erythematosus, skin diseases such as dermatitis and psoriasis, allergic conditions such as asthma and angioedema, preterm labor to speed the development of the baby's lungs, Crohn's disease, cancers such as leukemia, and along with fludrocortisone for adrenocortical insufficiency, among others. It can be taken by mouth, injected into a muscle, or applied as a cream. When given by injection, anti-inflammatory effects begin in around two hours and last for seven days.
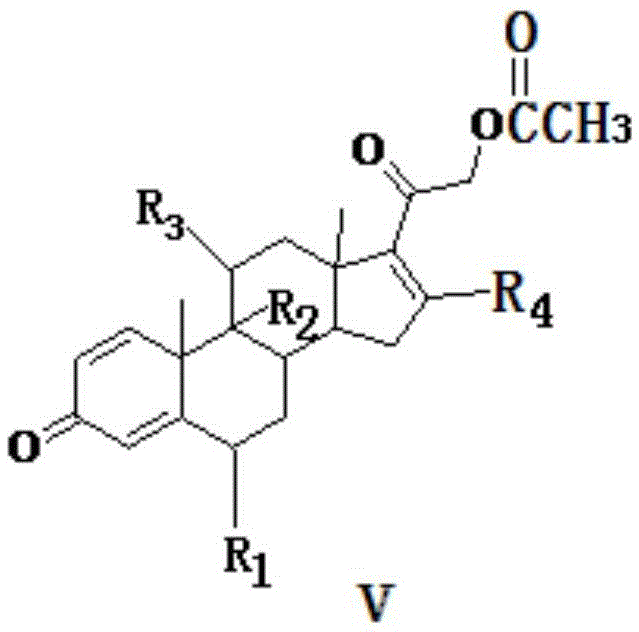
Method for preparing dexamethasone and series products thereof
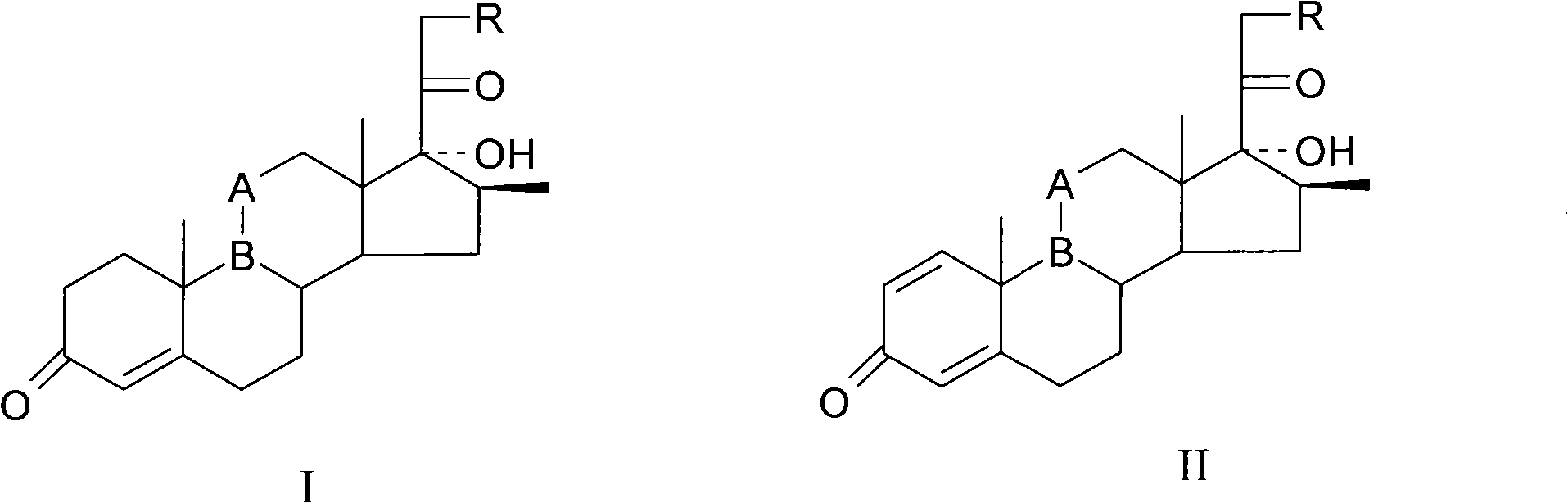
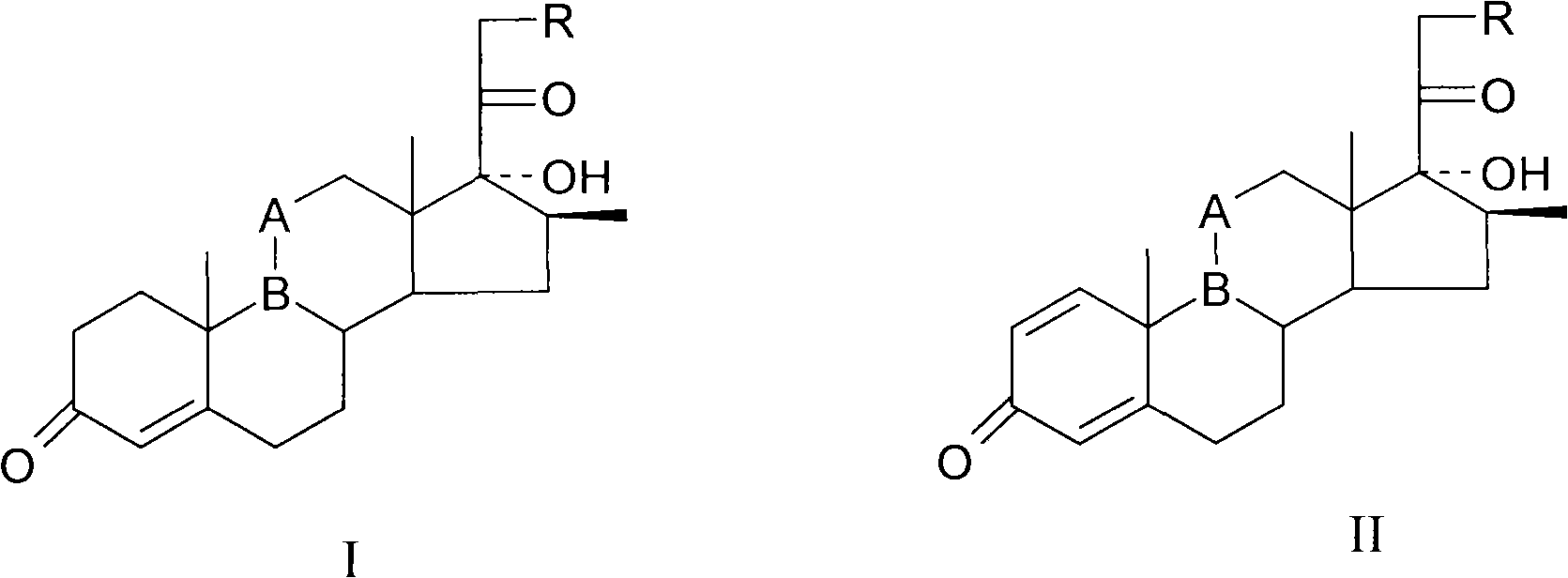
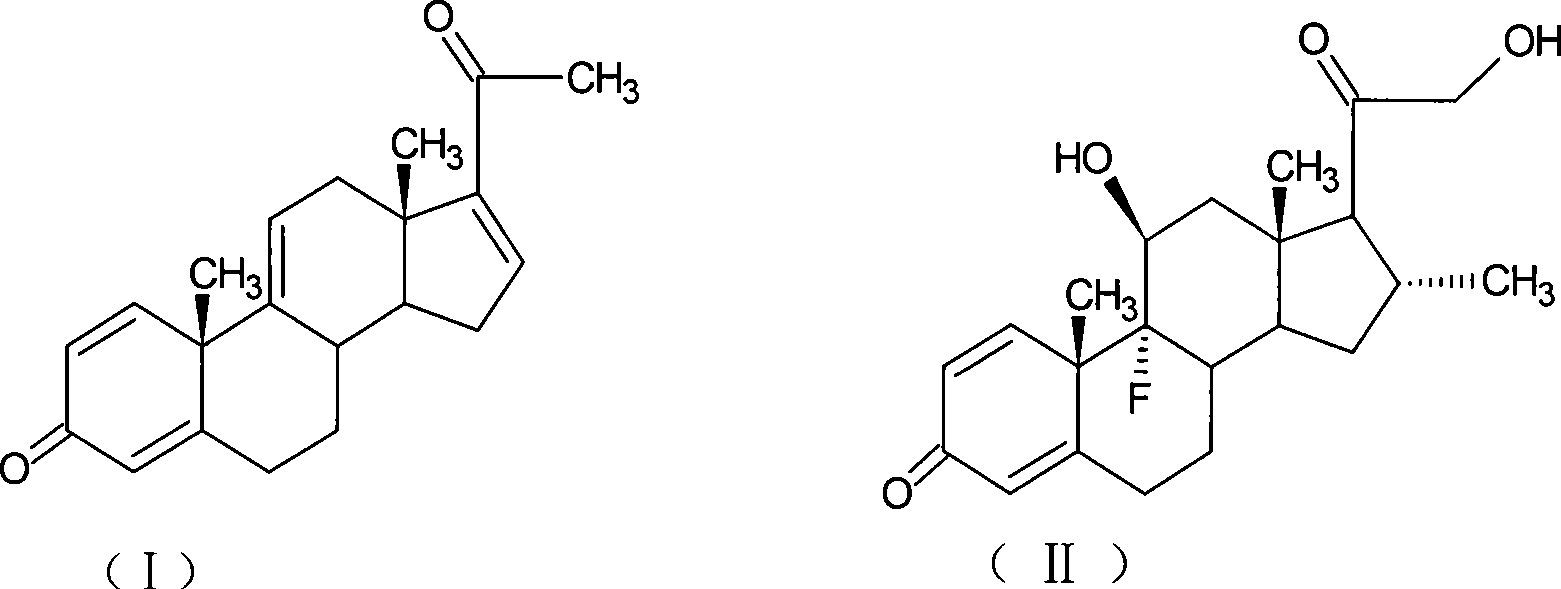
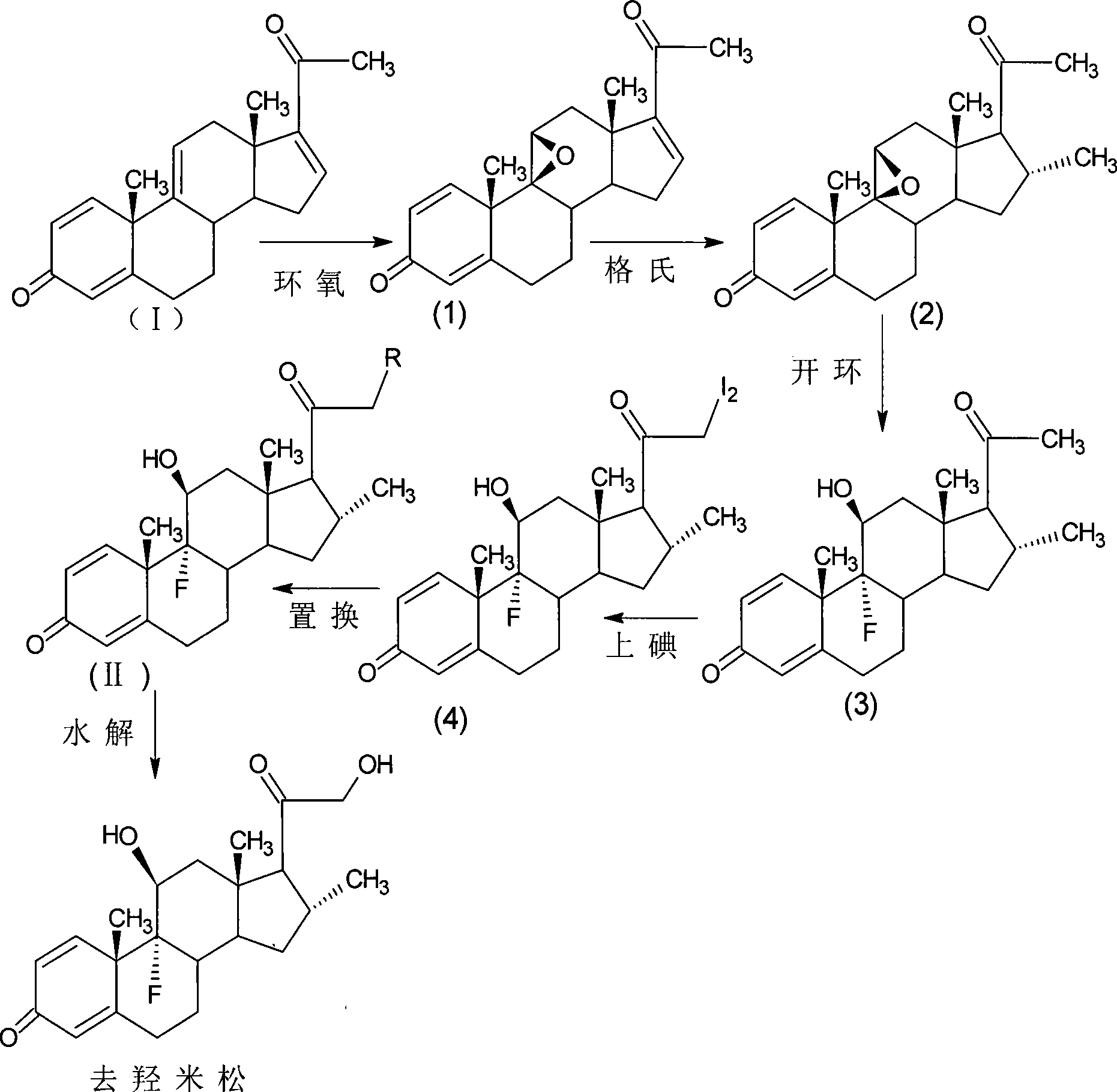
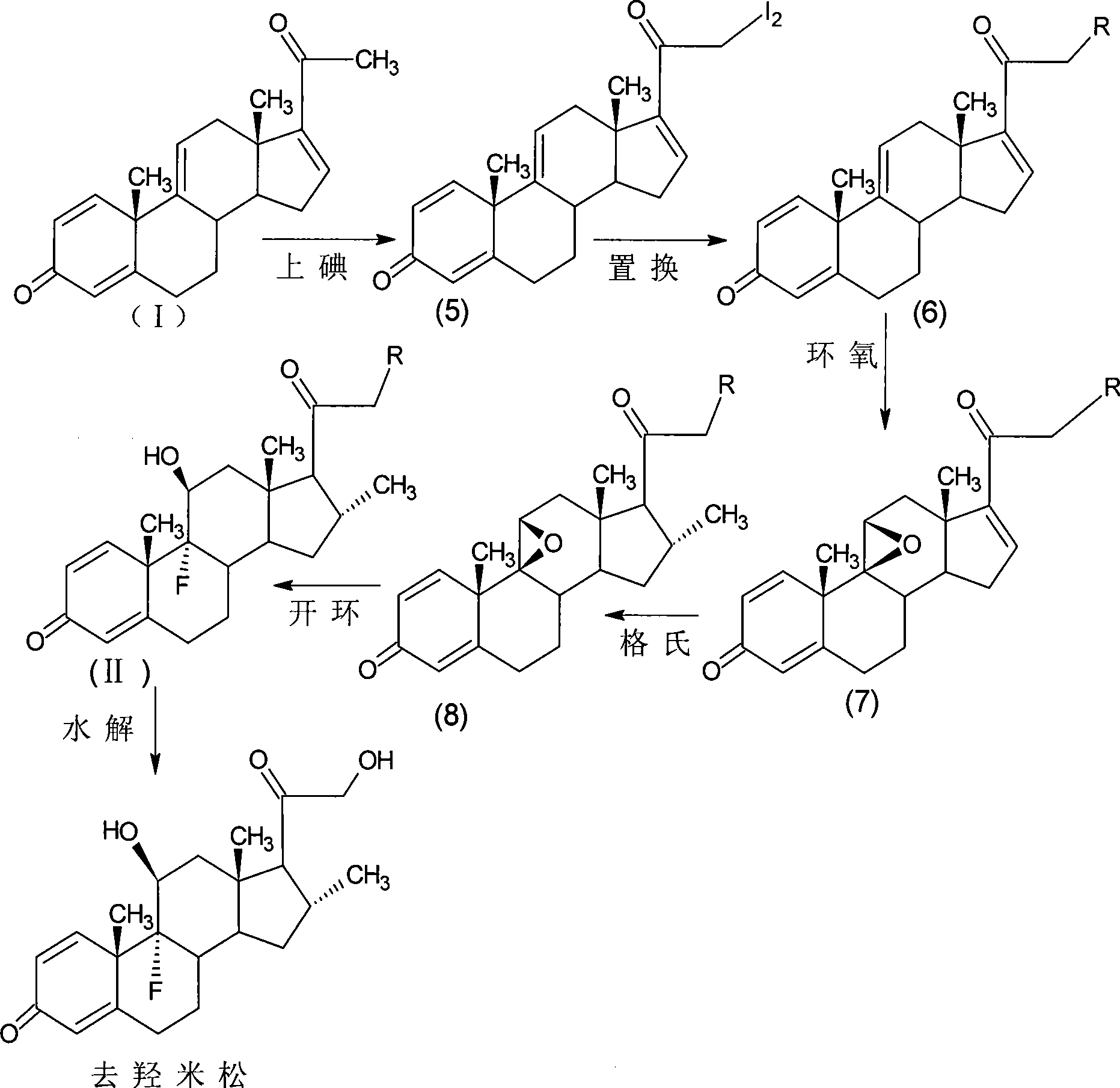
The invention provides a completely new process route for synthesizing dexamethasone and series products thereof. The invention adopts 1, 4, 9, 16-tetraene-pregna-3, 20-diketone as the original material which is modified by 9, 11bits, 16, 17 bits and 21 bits so as to obtain the dexamethasone and the series products thereof such as dexamethasone acetate and dexamethasone sodium phosphate and the like. The process has the advantage that the invention adopts the existing intermediates of manufacturers as the original material; the route is simple; the materials are available; the use of expensive accessories is avoided; the yield and the cost are dramatically better than that of the prior methods used for synthesizing the dexamethasone and derivatives thereof; moreover, the adoption of the existing intermediates realizes the combined-line prodction of the betamethasone series products and the dexamethasone series products, thus greatly reducing the manufacturing cost and the industrial manufacturing condition.
Owner:TIANJIN TIANYAO PHARM CO LTD
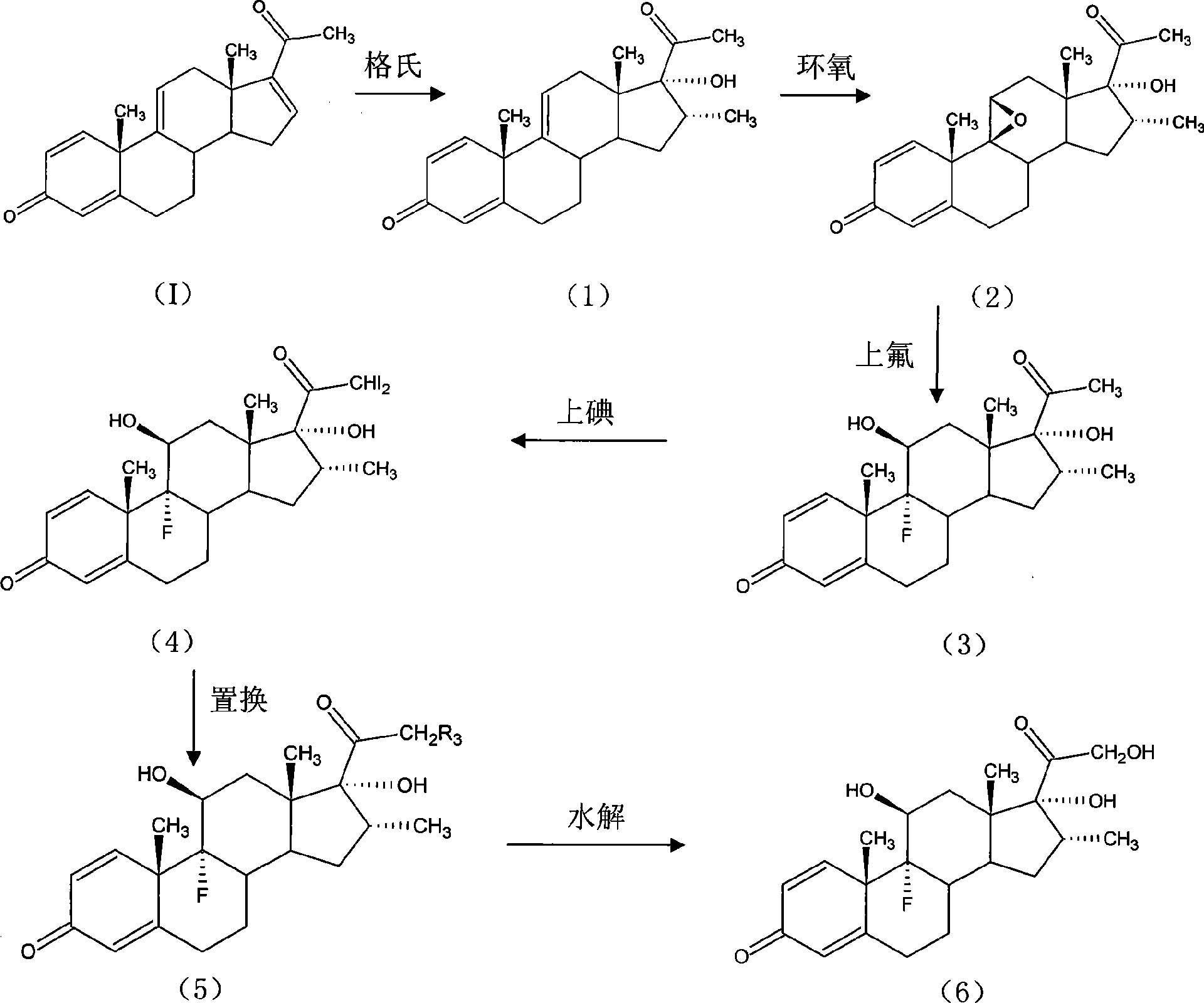
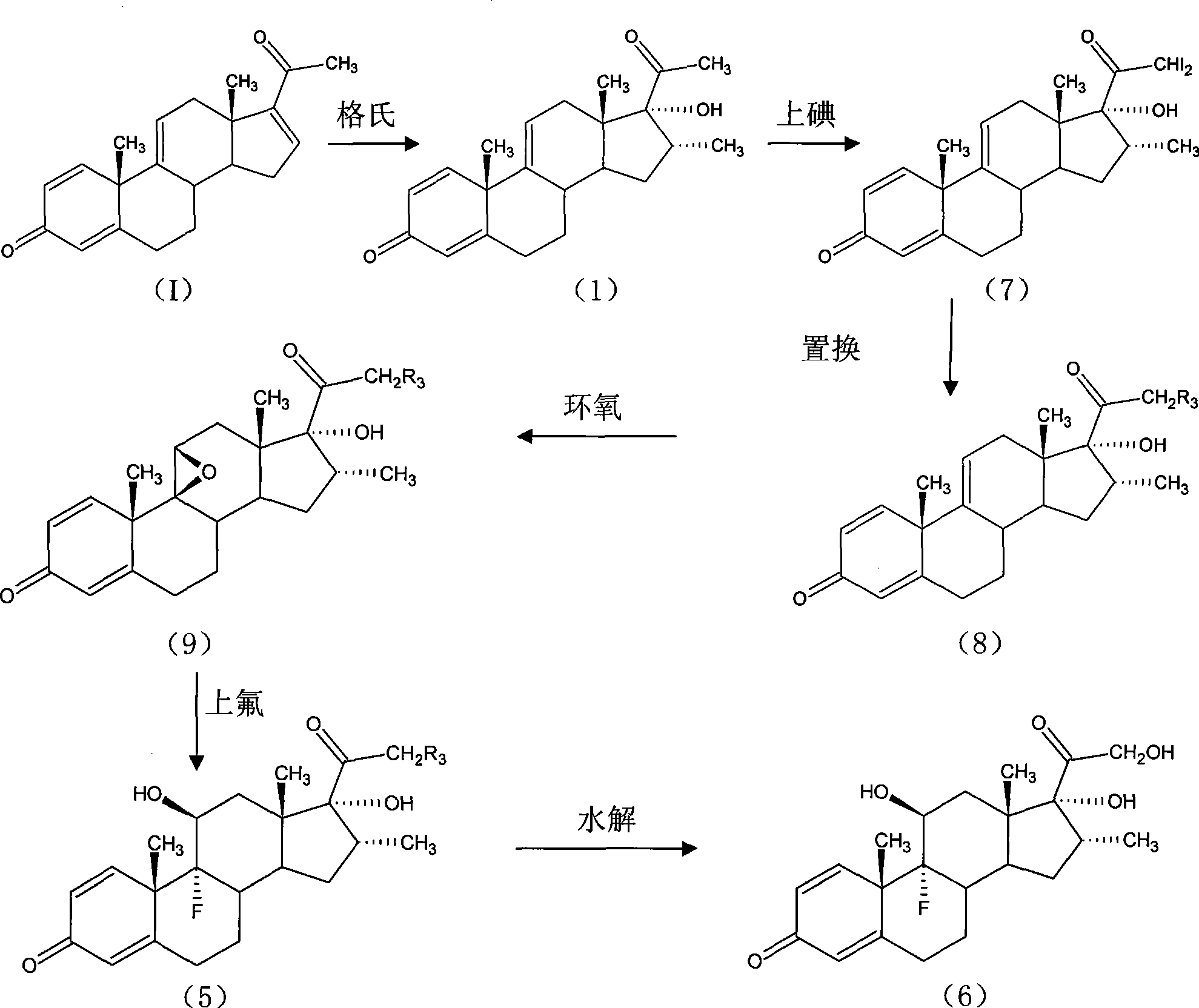
Preparation method for betamethasone intermediate or its analogue
ActiveCN103641878AStarting materials are readily availableHigh and stable yieldSteroidsSNiBetamethasone
The invention relates to a preparation method of a steroid hormone medicinal intermediate, in particular to a preparation method for a betamethasone intermediate or its analogue. The betamethasone intermediate or its analogue is prepared from a compound I by means of elimination reaction, methylation reaction, cyano-group substitution reaction, siloxy protective reaction, intramolecular nucleophilic substitution reaction and esterification reaction. The method provided by the invention has the characteristics of cheap raw materials, and high and stable yield. The reaction route is as the following, wherein R1 is Cl or Br, and R is H or hydrocarbonyl of C1-C10.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
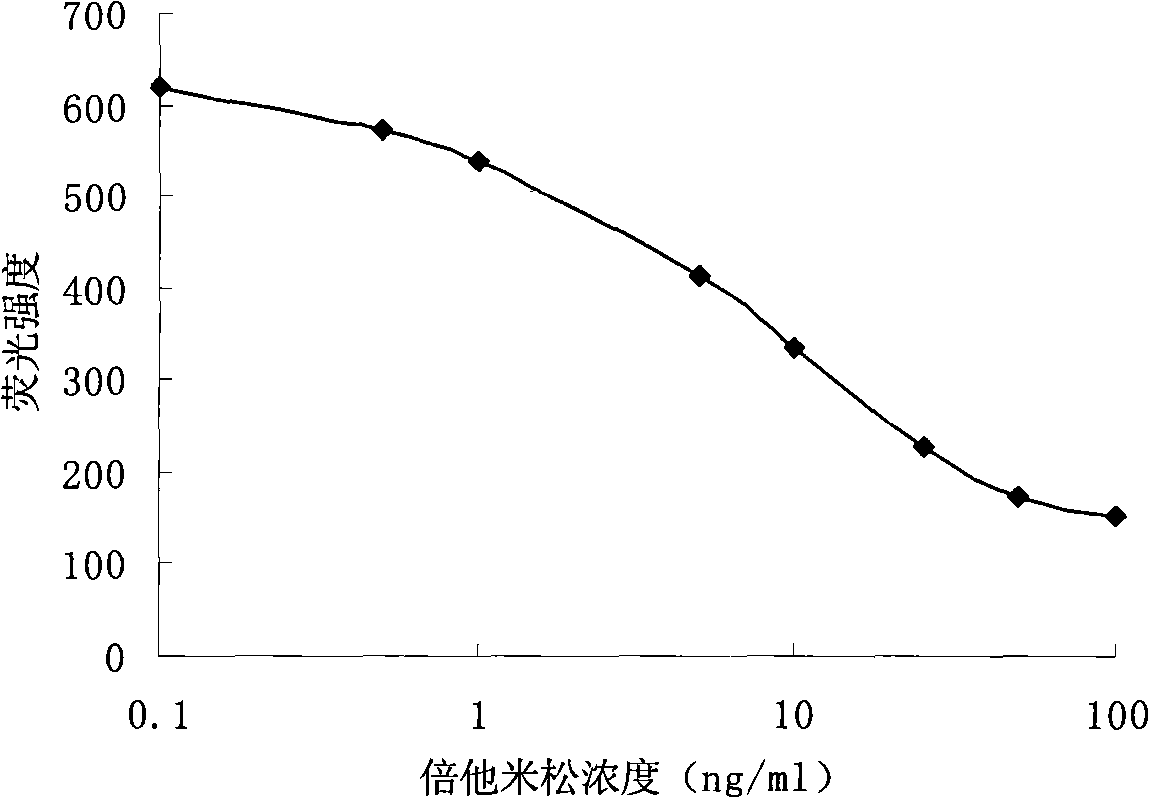
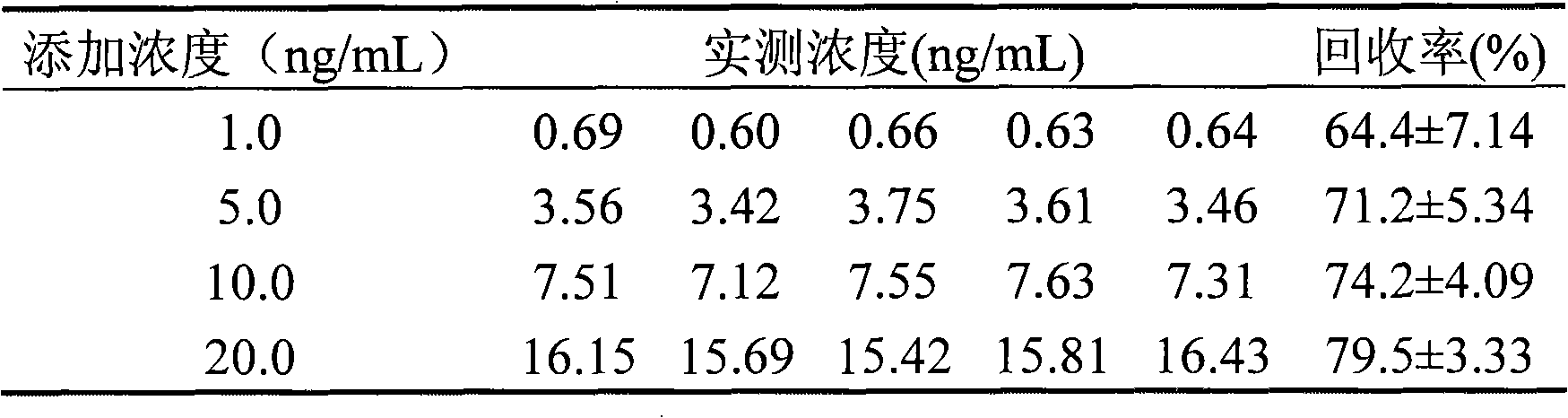
Method for quantum dot mark indirect competition fluoroimmunoassay detection for becort
InactiveCN101308146AEasy to operateHigh fluorescence intensityBiological testingFluorescence/phosphorescenceAntigenImmuno detection
Disclosed is a method of quantum dot-labeled indirect competitive fluorescence immunoassay of betamethasone, which belongs to the immunoassay method technique field. Quantum dots for labeling antibodies of the invention have the emission spectra of QD590, and the method comprises: directly covering coating antigens in micro-holes of an enzyme label plate, adding betamethasone standard solution or a sample under test to form an antigen-antibody fluorescence immunity compound body, stimulating and detecting the fluorescence intensity of the formed antigen-antibody fluorescence immunity compound body with a fluorescence enzyme-labeling instrument, and obtaining the concentration of betamethasone in the sample under test through comparing with the standard solution. The invention can detect the content of betamethasone in the sample under test without adding chromogenic substance, namely, the concentration of betamethasone in the sample under test can be detected indirectly through the fluorescence intensity of the antigen-antibody immunity compound body, and both the operation and reaction need only one step; and the quantum dots for labeling antibodies of the invention have advantages of stronger emitted fluorescence intensity and long stabilization time of fluorescence compared with the traditional fluorescence.
Owner:JIANGNAN UNIV
Fluorous synthesis method of betamethasone
InactiveCN102304163AReasonable designReduce chemical reaction timeSteroidsChemical reactionDrugs synthesis
The invention relates to a fluorous synthesis method of betamethasone, belonging to the technical field of steride medicament synthesis methods in pharmaceutical chemistry. The method comprises the following process steps: fluorinating and refining betamethasone epoxide which is used as a raw material so as to obtain betamethasone. According to the invention, the fluorous synthesis method of betamethasone is reasonable in design, thus chemical reaction time is greatly reduced, production period is shortened, a whole technical level of a product is greatly improved, cost is reduced by 15% as compared with the conventional process, and the fluorous yield of betamethasone reaches 85-90%.
Owner:ZHEJIANG XIANJU XIANLE PHARMA
Method for preparing paratoluensulfonyl chloride
The invention discloses a method for preparing paratoluensulfonyl chloride, which comprises the following step of: reacting paratoluenesulfonic acid ammonium salt with bis(trichloromethyl) carbonate (commonly called triphosgene) in an inert organic solvent under the condition that organic alkali is used as a catalyst to synthesize the paratoluensulfonyl chloride. The preparation method has the advantages that: raw materials are conveniently and readily available, the process is simple and suitable for scale-up production, reaction conditions are mild, and a product is easy to purify. The prepared paratoluensulfonyl chloride is an important fine chemical product and can be used for preparing a dye intermediate, synthesizing intermediates of more than ten kinds of antibacterial medicines and anti-inflammatory medicines such as betamethasone, sulfamylon and the like and synthesizing plastic plasticizers, resin, coatings, pesticides and light-sensitive materials.
Owner:SINOCHEM LANTIAN +1
Method for preparing tetraene acetate and derivatives thereof
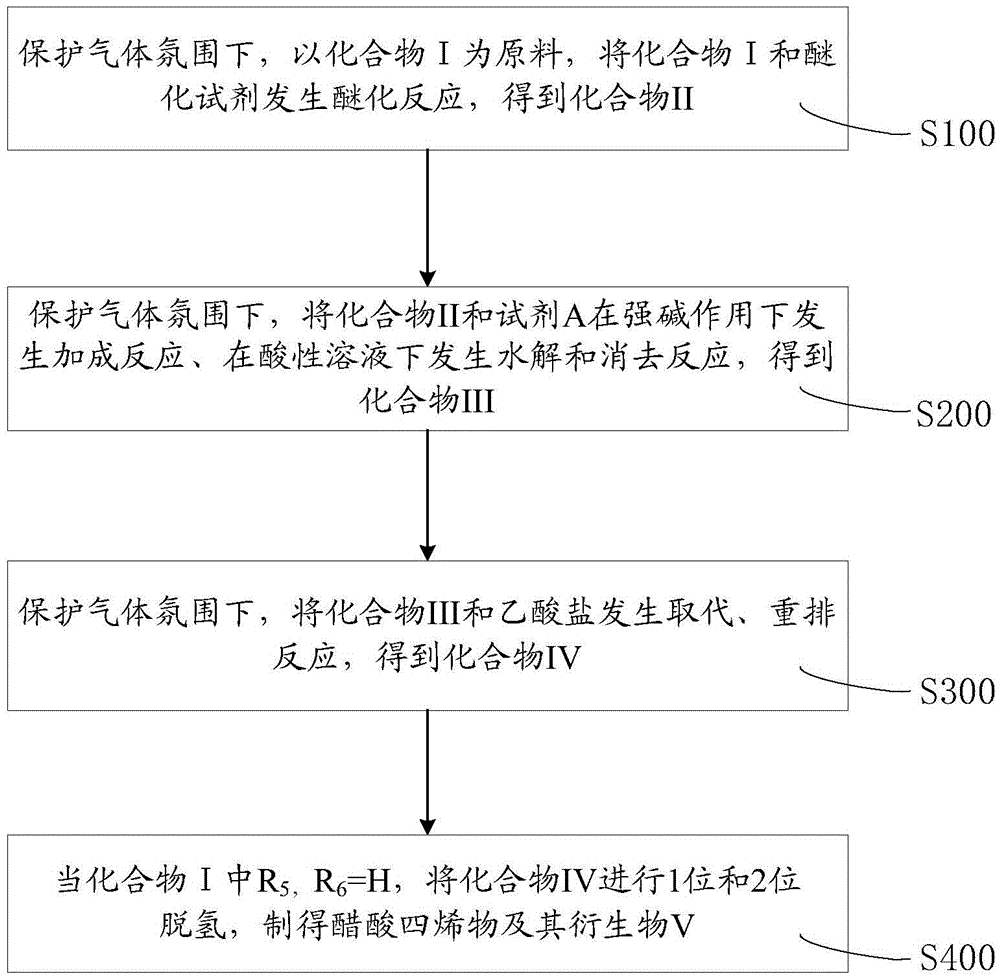
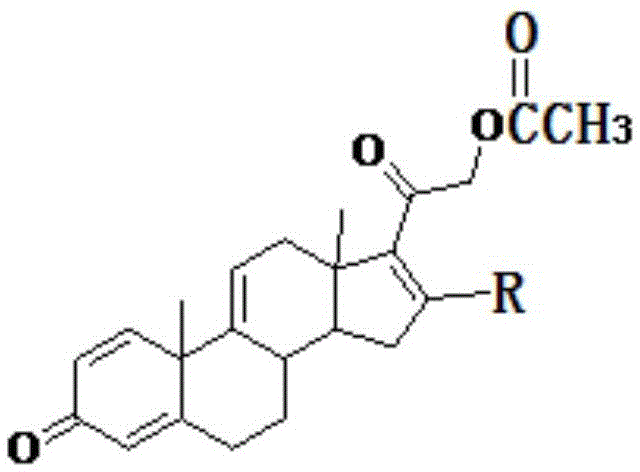
The invention relates to a method for preparing tetraene acetate and derivatives thereof. The method comprises the steps of obtaining a compound II through etherification reaction of a compound I and an etherification agent in the atmosphere of protective gases, obtaining a compound III through addition reaction of the compound II and a reagent A under the action of strong base and hydrolysis and elimination reaction of the compound II and the reagent A in the presence of an acid solution, obtaining a compound IV through substitution reaction and rearrangement reaction of the compound III and acetate, and conducting 1 position dehydrogenation and 2 position dehydrogenation on the compound IV to obtain the tetraene acetate and derivatives thereof. According to the method, the compound I is taken as the raw material and subjected to carbonyl etherification, addition, hydrolysis, elimination, rearrangement and dehydrogenation reaction to obtain the product, the raw material compound I is easy to obtain, cost is low, no precious metal is needed during preparation, reaction conditions are easy to control, operation is convenient, the method is suitable for large-scale industrial production, and the obtained tetraene acetate is an important intermediate for synthesis of dexamethasone, budesonide, betamethasone and other steroidal drugs.
Owner:湖南成大生物科技有限公司
Method for preparing sterides compound 17-alpha ester
ActiveCN101891797AMake up for the impactPromote hydrolysisSteroids preparationPrednisoloneCompound 17
The invention discloses a method for preparing a sterides compound 17-alpha ester. The method comprises the following steps of: dissolving 17 alpha, 21-dihydroxyl sterides compound serving as a raw material in a cyclic ester solvent; adding an ester and a catalyst into the mixture and reacting the mixture to prepare a cyclic ester; and dissolving the prepared cyclic ester in a hydrolysis solvent, adding an orientation reagent and a hydrolysis reagent into the mixed solution and hydrolyzing the cyclic ester into the 17-alpha ester. The sterides compound is hydrocortisone, prednisolone, prednisone, hexadecadrol or betamethasone. The method has selective hydrolysis effect,, and can effectively enhance the conversion rate of a product, greatly increase product yield and greatly promote the preparation capability of a sterides medicament.
Owner:ZHEJIANG XIANJU PHARMA
Preparation process of betamethasone intermediate
InactiveCN102071240AHigh yieldQuality improvementMicroorganism based processesFermentationCompound (substance)Betamethasone
The invention discloses a preparation process of a compound shown in a formula II. The compound is prepared from a compound shown in a formula I by biological fermentation and dehydrogenation, and in the processes of biological fermentation and dehydrogenation, an inert grinding material is added in fermentation liquid. In the invention, the fermentation manner is improved under a condition of using a common arthrobacter simplex strain; and a proper amount of inert grinding material is added in the fermentation liquid so that the yield and the quality of a compound product (namely a betamethasone intermediate) in the formula II are improved under the condition that the feeding concentration is maintained to be over 0.5%, and the production cost of betamethasone is reduced. In addition, no chemical solvent is used in the preparation process, and thus the cost for auxiliary materials is saved.
Owner:ZHEJIANG XIANJU PHARMA
Method for simultaneously detecting two glucocorticoid isomerides in animal-derived food
The invention discloses a method for simultaneously detecting two glucocorticoid isomerides in animal-derived food. The method separates two enantiomers of betamethasone, dexamethasone, fluorometholone and desoximetasone by adopting a conventional chromatographic column, and can detect the residual amount of betamethasone, dexamethasone, fluorometholone and desoximetasone in the animal-derived food and confirm. A C18 chromatic column is adopted for liquid chromatogram; the flow phases are respectively acetonitrile containing methyl alcohol and water containing methyl alcohol, and tandem mass spectrum detection is carried out; two enantiomers of betamethasone, dexamethasone, fluorometholone and desoximetasone can be subjected to chromatograph separation; the detection limit of dexamethasone is 0.2 micron g / kg; the detection limit of betamethasone, fluorometholone and desoximetasone is 0.4 micron g / kg; the residual amount regulatory restriction requirement at home and abroad is met; the method is simple, convenient, accurate, flexible and reliable.
Owner:内蒙古出入境检验检疫局检验检疫技术中心
Synthesis method of betamethasone epoxy hydrolyzate
The invention belongs to the field of steroid hormone drug synthesis, and particularly relates to a synthesis method of a betamethasone epoxy hydrolyzate, which comprises the following step: by using a betamethasone suppressor as a raw material, carrying out bromohydroxylation reaction and epoxy hydrolysis reaction to obtain the betamethasone epoxy hydrolyzate finished product, thereby enhancing the overall yield of the betamethasone epoxy hydrolyzate by reducing the elutriation running. The method reduces the hydrolysis of the bromohydroxy substance in the reaction, and integrates the steps of epoxidation and hydrolysis into one step, thereby enhancing the yield of the betamethasone epoxy hydrolyzate.
Owner:ZHEJIANG XIANJU XIANLE PHARMA
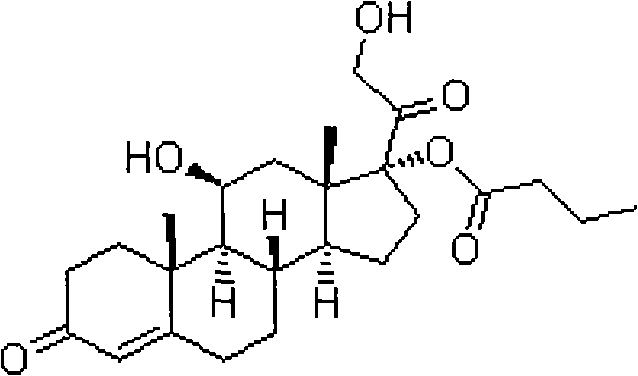
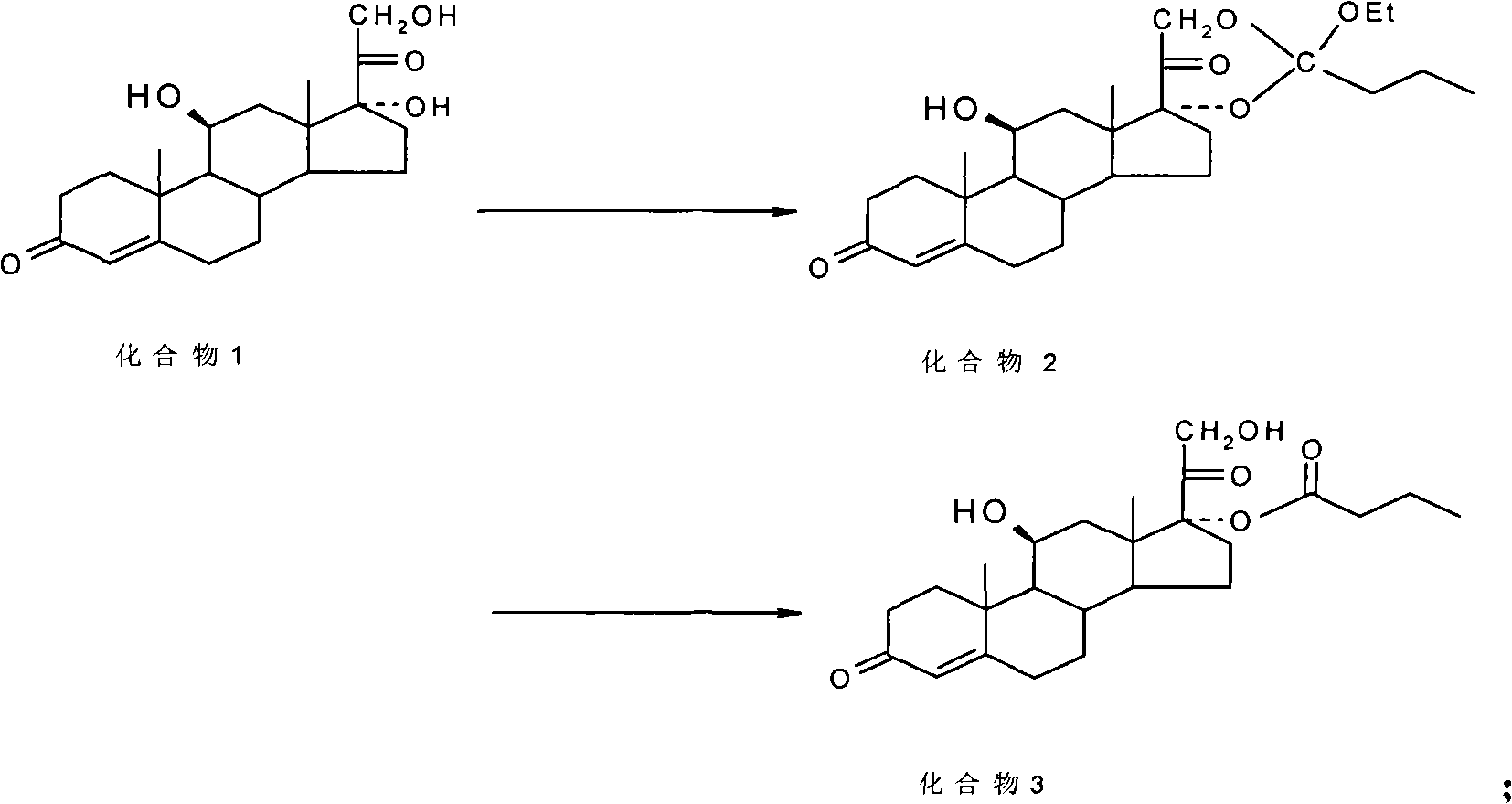
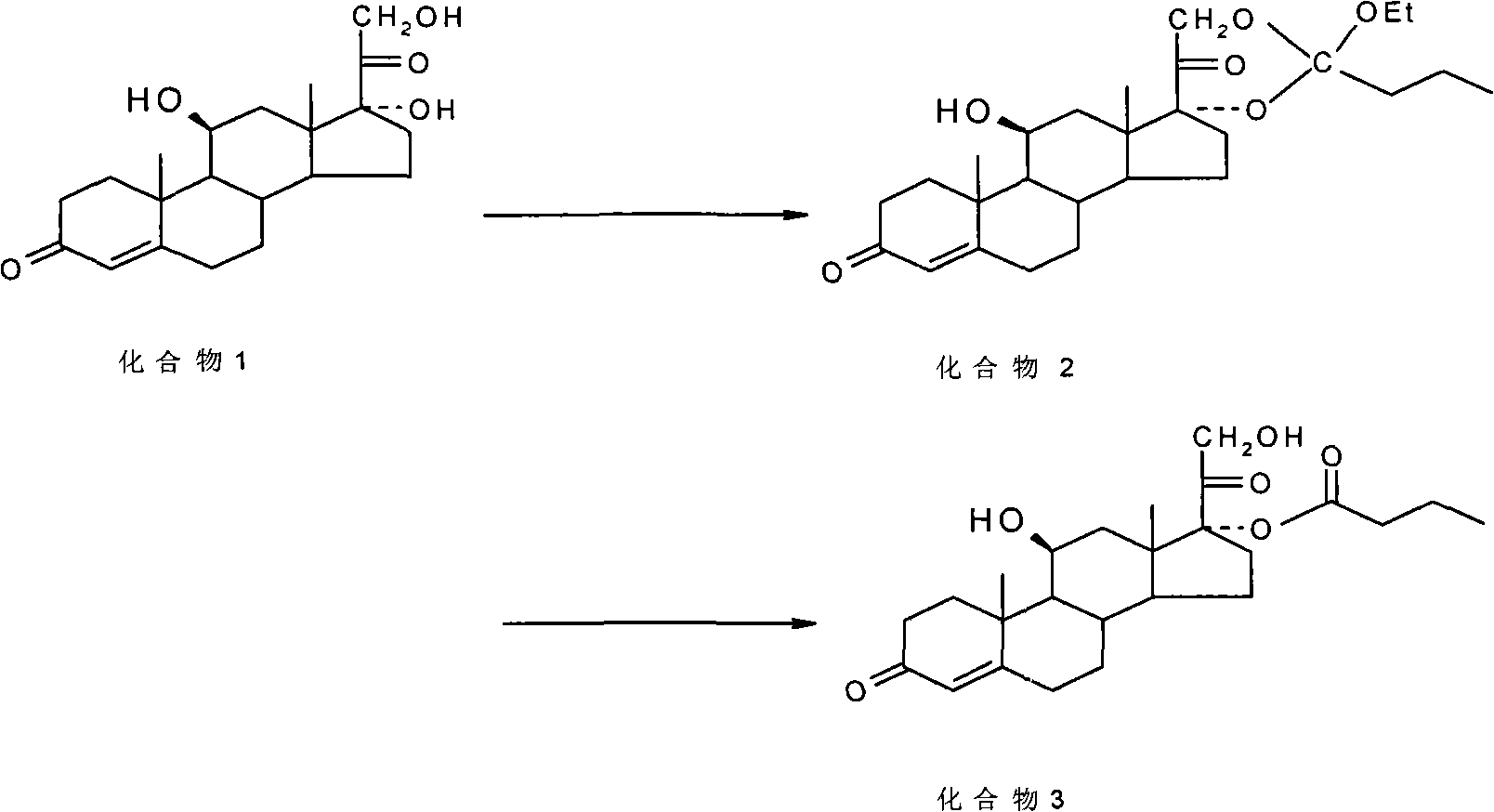
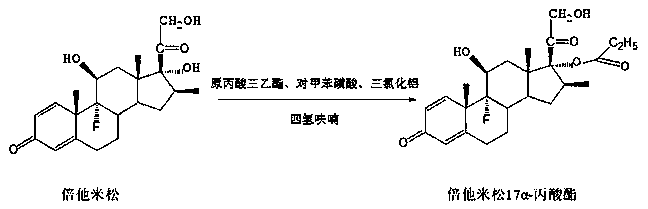
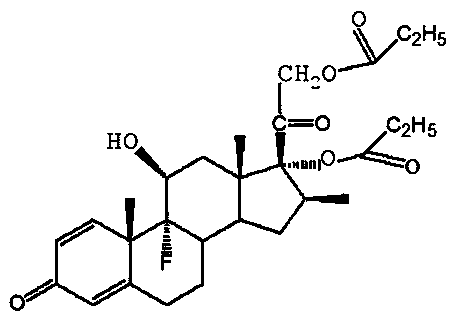
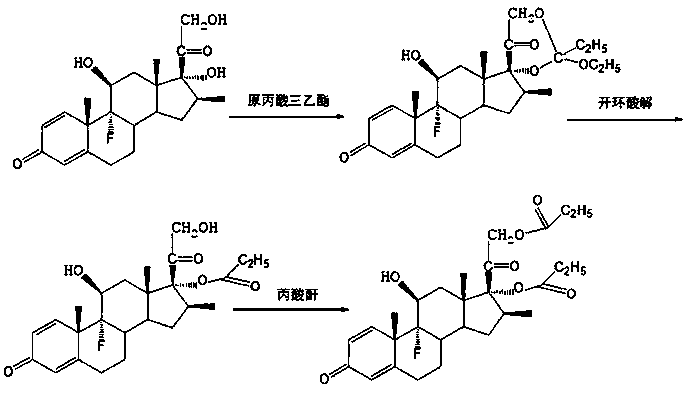
Preparation method of betamethasone 17 alpha-propionate
The invention provides a preparation method of betamethasone 17 alpha-propionate. According to the preparation method, betamethasone is subjected to a reaction with triethyl orthopropionate, the reaction is performed in a tetrahydrofuran solvent, further, p-toluenesulfonic acid is adopted as a catalyst, after the reaction is finished, an aluminum trichloride solution is dropwise added to the samesolvent system directly without discharging, a thermal insulation reaction is performed after the solution is dropwise added, and betamethasone 17 alpha-propionate is obtained with a post-treatment process. According to the preparation method of betamethasone 17 alpha-propionate, preparation process is simplified, yield is increased and purity is improved while the problem that dioxane, dimethylformamide or another solvent is adopted as the reaction solvent of betamethasone 17 alpha-propionate is solved.
Owner:HENAN LIHUA PHARMA
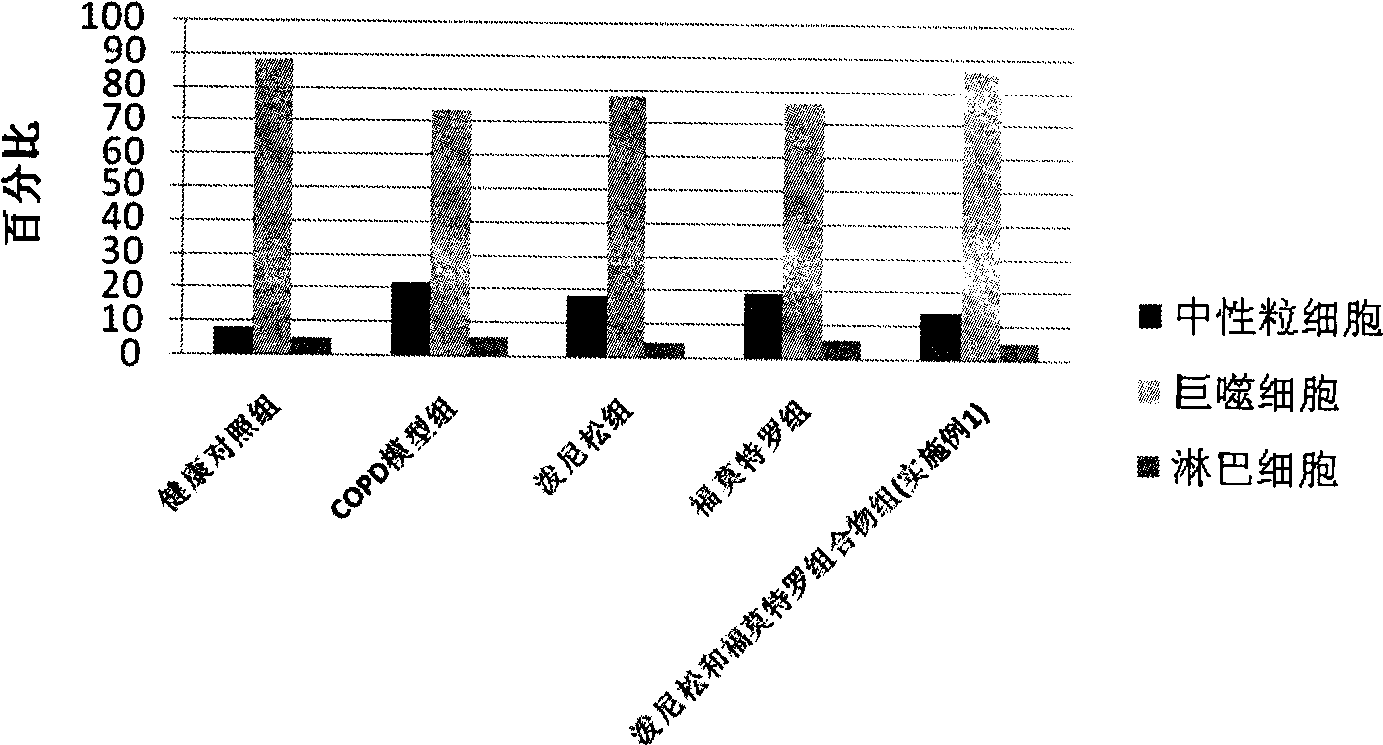
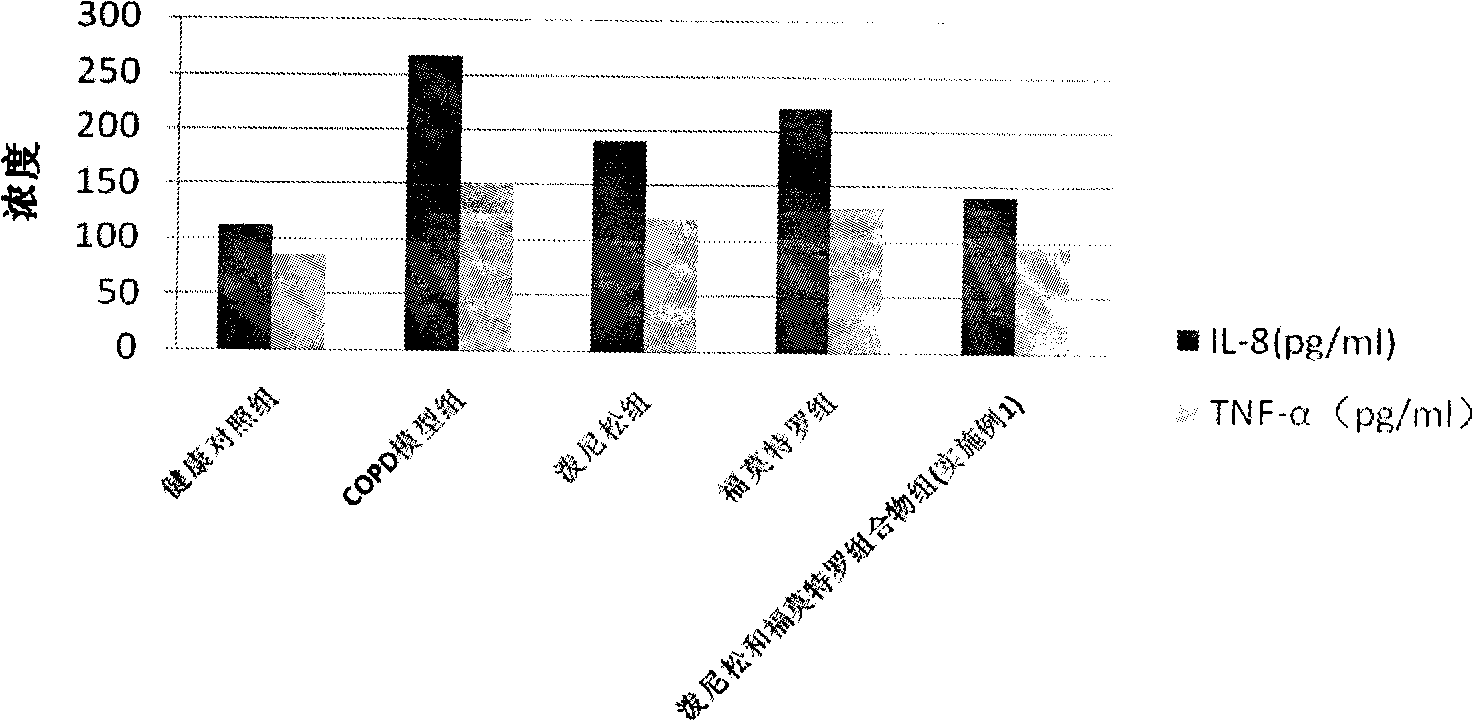
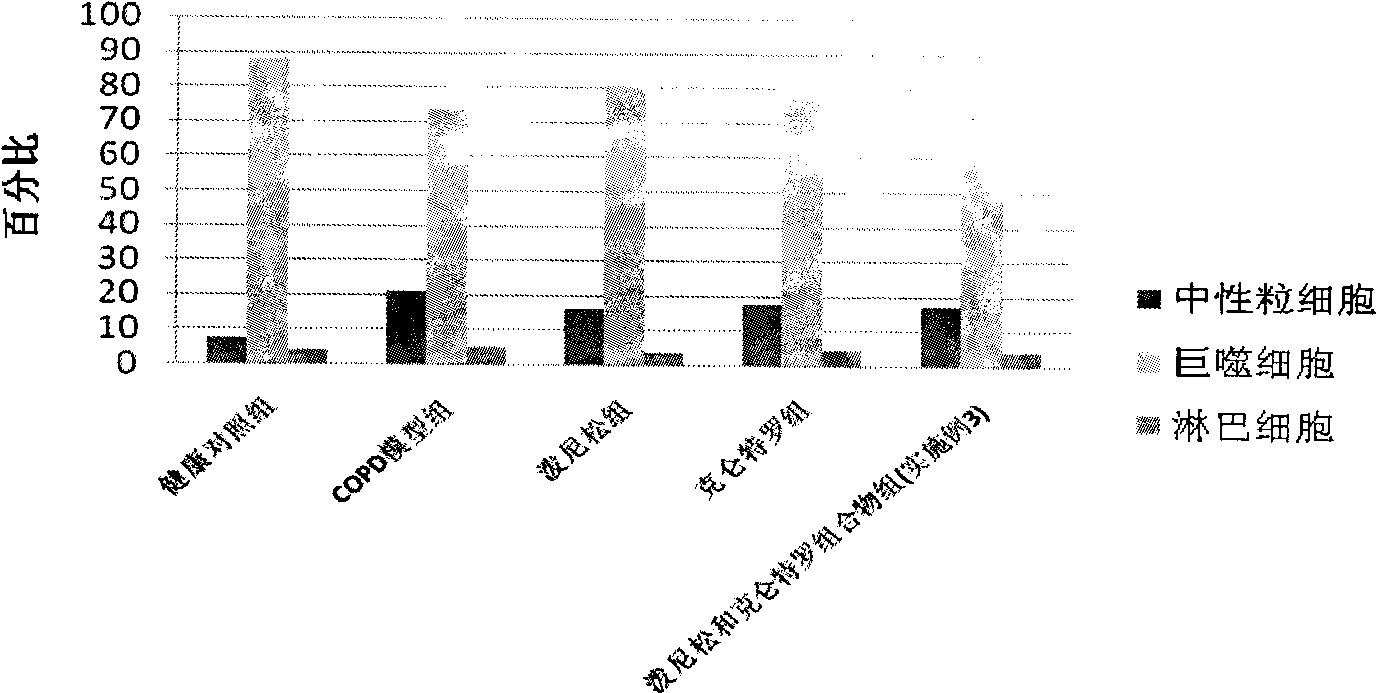
Pharmaceutical composition for the treatment of chronic obstructive pulmonary disease and bronchial asthma
InactiveCN101530618ALower doseReduce or avoid side effectsRespiratory disorderHeterocyclic compound active ingredientsDiseaseAdditive ingredient
The invention discloses a pharmaceutical composition for the treatment of chronic obstructive pulmonary disease (COPD) and bronchial asthma, which is composed of a glucocorticoid, a bronchodilator and a pharmaceutically acceptable auxiliary material or carrier; the composition is a preparation for oral use. The glucocorticoid in the inventive pharmaceutical composition is selected from prednisone, prednisolone, methylprednisolone, betamethasone, decamethasone or hydrocortisone; the bronchodilator is selected from formoterol, clenbuterol, procaterol or theophylline. The inventive composition has better therapeutic effect on the COPD and the bronchial asthma than independent administration of two ingredients, and has synergistic effect. The composition has easily-available raw materials, inexpensive price and increased medicine taking compliance as well as plays a significant role in preventing and treating the COPD and the bronchial asthma of patients in vast rural areas and patients in low-income class of the city in China.
Owner:莫始平
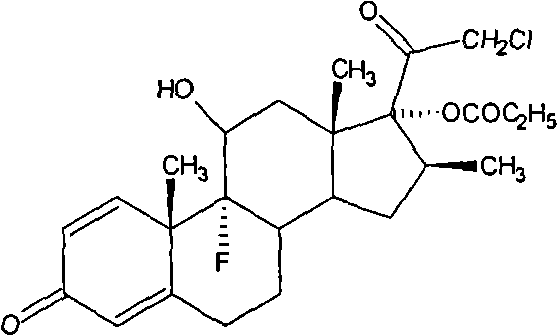
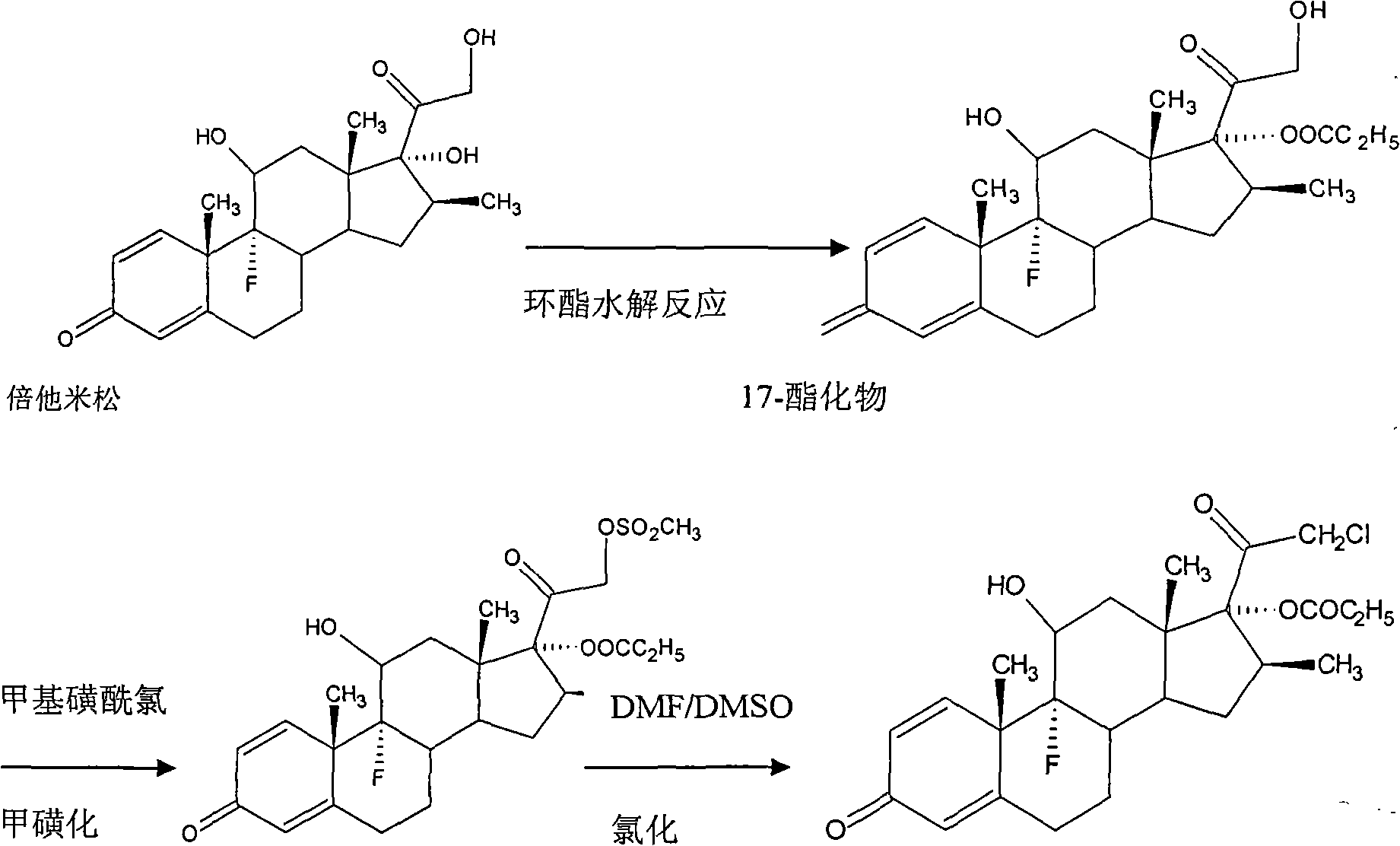
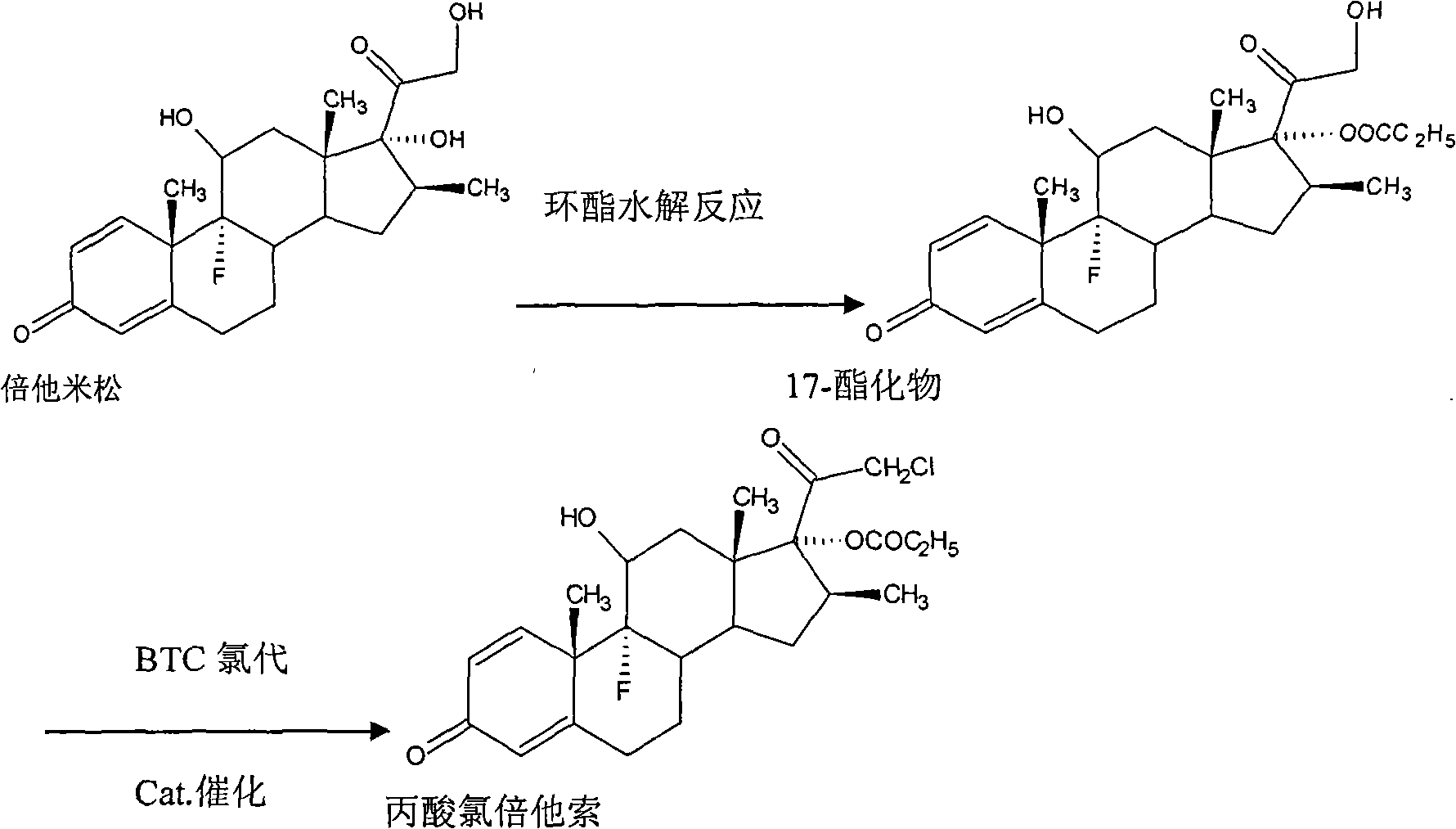
Method for synthesizing clobetasol propionate intermediate
ActiveCN101812107AReduce usageEmission reductionSteroidsReaction temperatureMethanesulfonyl chloride
The invention discloses a method for synthesizing clobetasol propionate, which belongs to the technical field of synthesis of steroid medicaments in the pharmaceutical chemistry and comprises the following steps: (1) dissolving betamethasone-17-ester into acetone solution; (2) adding a catalyst and BTC (bis(trichloromethyl) carbonate) for carrying out chloroacetic reaction at the reaction temperature of 30-50DEG C for 2-5 hours; and (3) after reaction, carrying out reduced pressure concentration, elutriation, filtering and drying. Compared with the traditional process, the yield can be improved by 6 percent, the cost is reduced by 20 percent and the quality is remarkably improved. The using amount of original auxiliary materials are reduced by 20 percent, the using amount of a toxic material of methanesulfonyl chloride can be reduced by 1 ton each year, the using amount of DMF (Dimethyl Formamide) can be reduced by 17 tons and the using amount of pyridine can be reduced by 6.3 tons, the economic cost can be totally saved by 366,000 yuan and waste water drain can be reduced by 310tons / year; and in addition, the invention greatly reduces the pressure for protecting the environment and can effectively reduce the hazard on human body and the pollution on the environment.
Owner:ZHEJIANG XIANJU XIANLE PHARMA
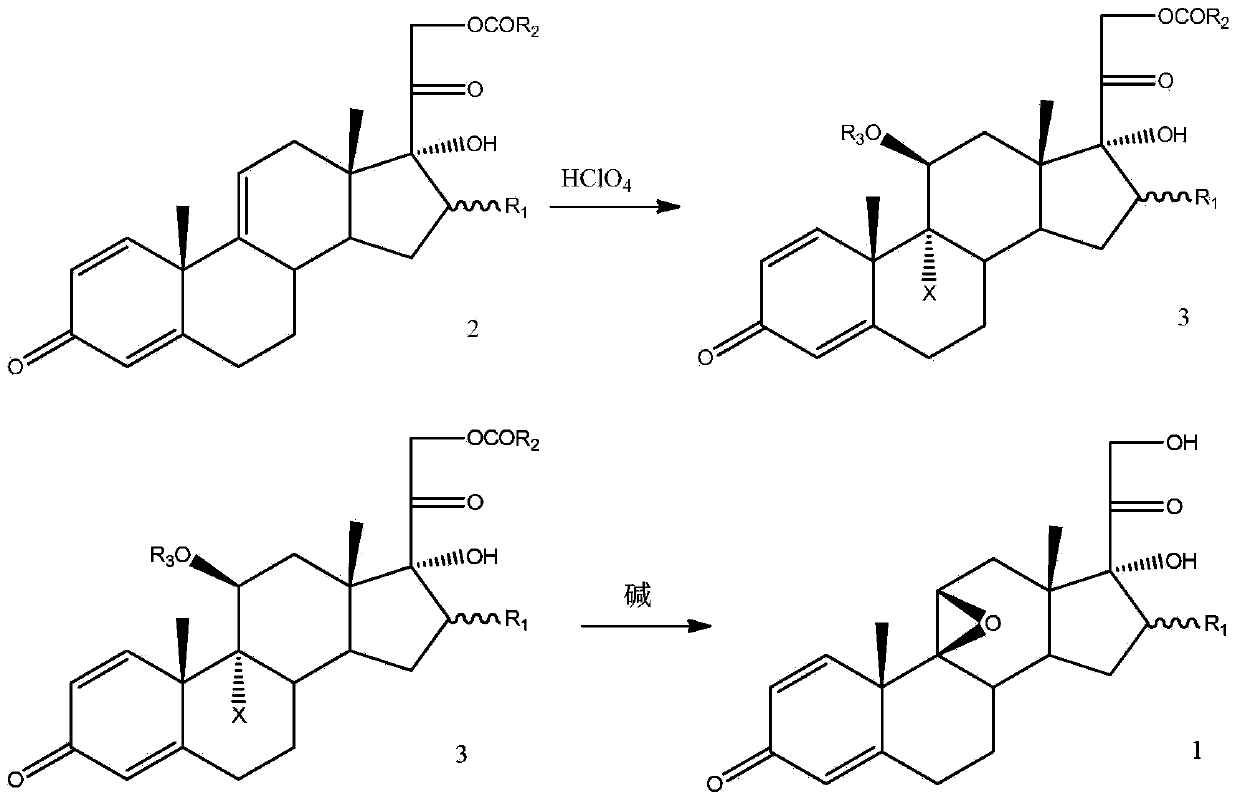
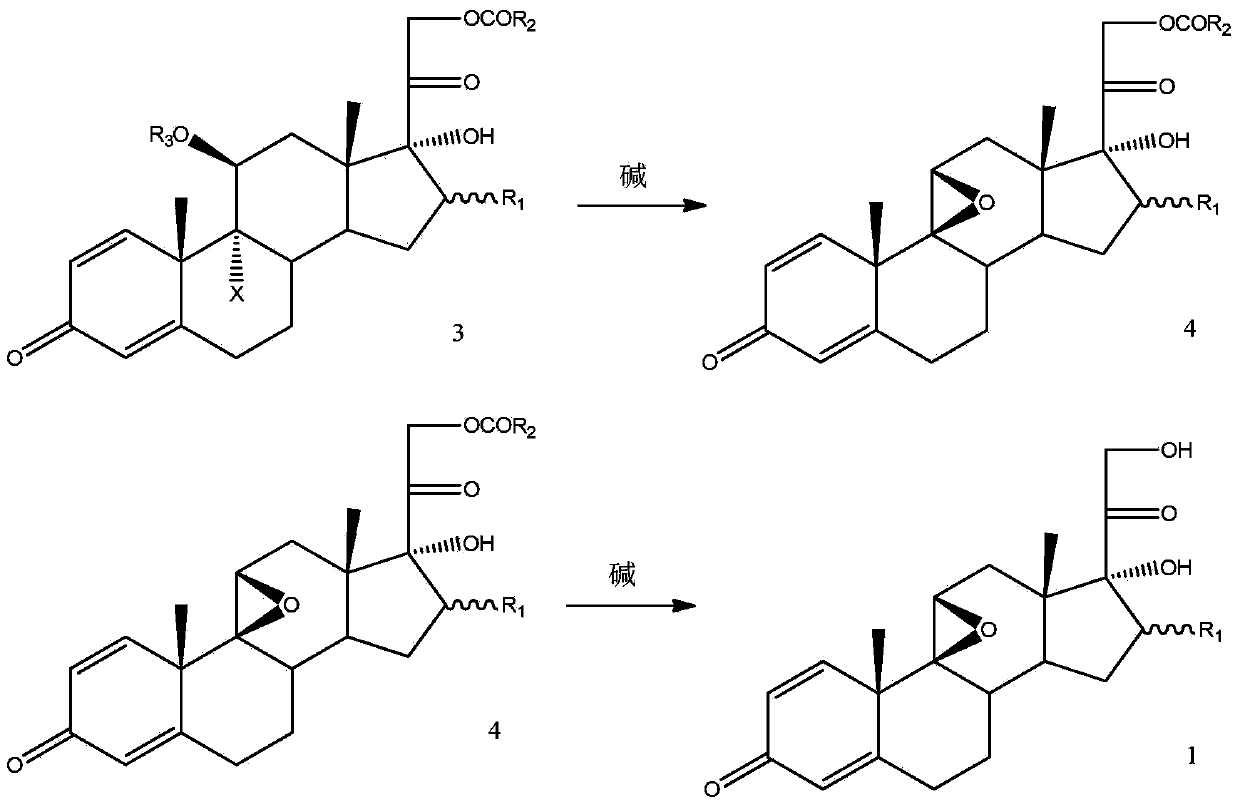
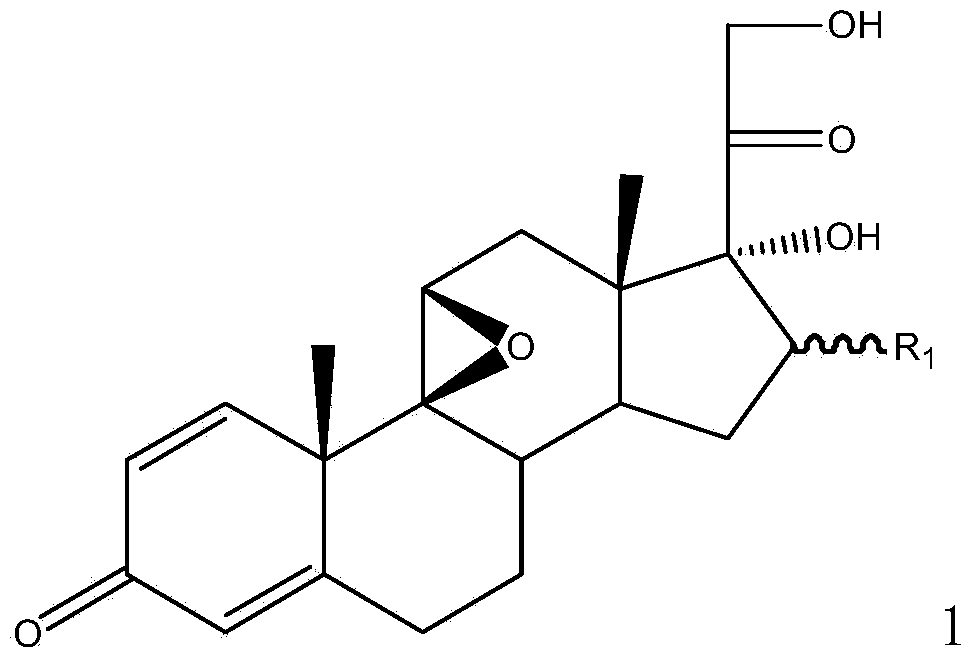
Method for preparing 9,11beta-epoxy steroid compound
The present invention provides a method for preparing a 9,11beta-epoxy steroid compound represented by a formula 1. According to the method, a 9,11-double bond-21-ester steroid compound is adopted as a raw material, and reacts with a halogenating agent in a suitable organic solvent containing 70% perchloric acid to produce a 9alpha-halo-11beta-ester-21-ester steroid compound, and the 9alpha-halo-11beta-ester-21-ester steroid compound reacts with a strong base in an organic solvent to obtain the 9,11beta-epoxy steroid compound. The 9,11beta-epoxy steroid compound can be used for preparation of dexamethasone, betamethasone and series other drugs. According to the present invention, the 9,11beta-epoxy steroid compound can be prepared from the almost equimolar amount of the 9,11-double bond-21-ester steroid compound, such that the method is the synthesis method with characteristics of economy, efficiency and environmental protection.
Owner:AURISCO PHARMACEUTICAL CO LTD
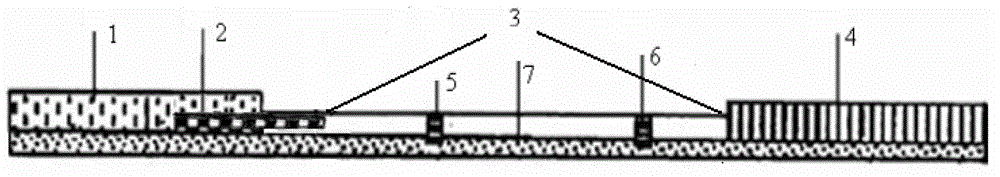
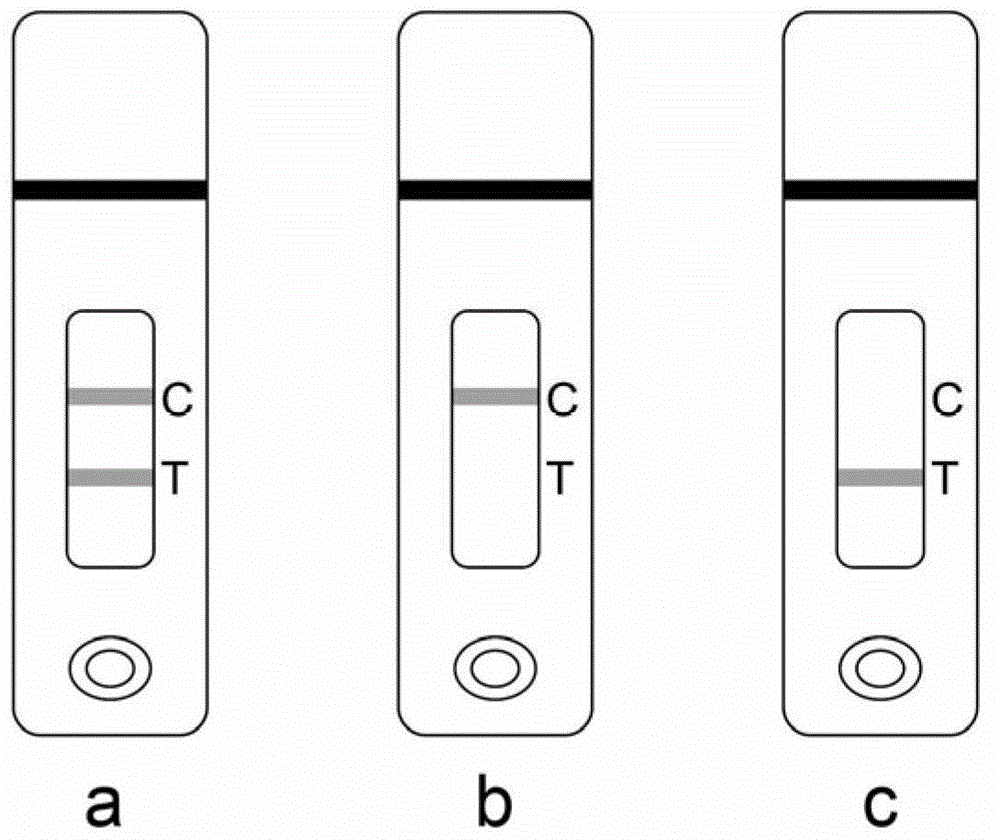
A dipstick used for testing betamethasone and application thereof
ActiveCN103940999AHigh sensitivityStrong specificityMaterial analysisMonoclonal antibodyQuality control
The invention discloses a dipstick used for testing betamethasone and application thereof. The dipstick consists of a sample absorption pad (1), a conjugate release pad (2), a reaction film (3), an absorbent pad (4) and a bottom patch (7), the said reaction film consists of a detection line (5) which is coated with betamethasone hapten carrier protein conjugates and a quality control line (6) which is coated with goat-anti-mouse antibody, the said conjugate release pad (2) is sprayed with a monoclonal antibody-colloidal gold marker. The invention also provides a testing method for betamethasone residue in cosmetics using the said betamethasone dipstick. The dipstick provided in this invention has characteristics of simple operation, high sensitivity, high testing speed, low cost and the like; the said dipstick is suitable for screening and field monitoring of a large number of samples.
Owner:BEIJING KWINBON BIOTECH
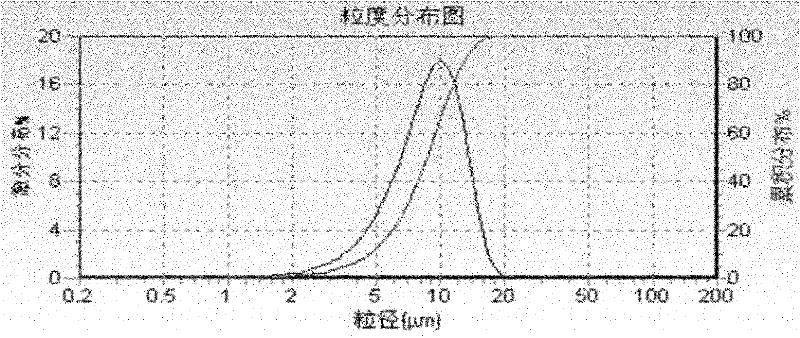
Compound betamethasone suspension injection and preparation method thereof
ActiveCN102526078AGood flocculation effectInvention effect is goodOrganic active ingredientsSolution deliveryBetamethasoneInjection solution
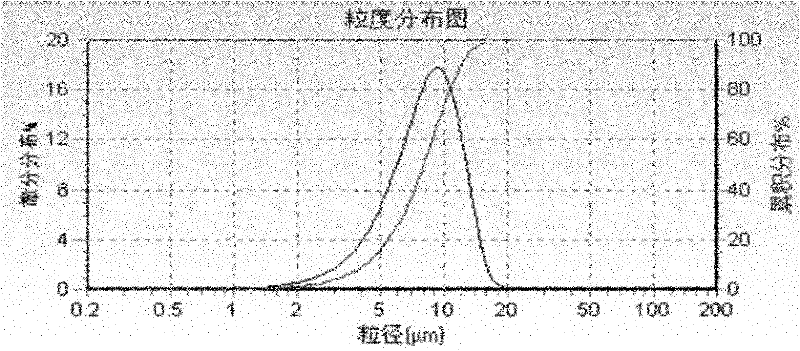
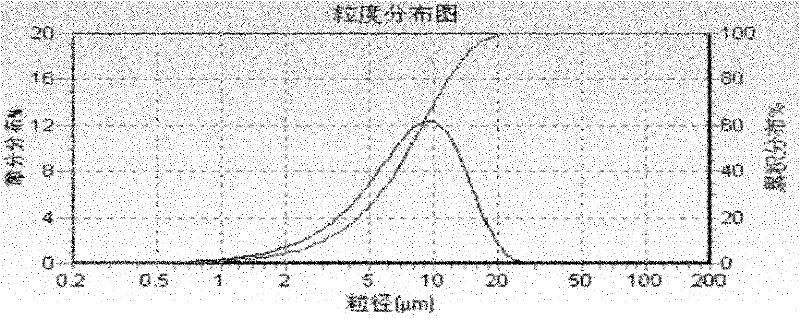
The invention finds applications of nipagin and phenylcarbinol for serving as flocculating agents in a compound betamethasone suspension injection, and screens an appropriate flocculating agent using amount. By adding the flocculating agents, the particle size, uniformity and precipitating speed of suspension fine particles can be controlled effectively, and the suspension fine particles can be vibrated and dispersed uniformly without being agglomerated after the injection is kept for a long time. The invention further provides a sterile process for producing the suspension injection by adapting to an industrial way. Due to the adoption of the process, the problems of degradation of a main medicament and agglomeration of fine particles existing in a terminal high-temperature sterilizing method are solved, and a sterile product with stable quality is finally obtained.
Owner:CHONGQING HUAPONT PHARMA
Preparation of desoximetasone
The invention relates to a preparation method of a steroid compound, in particular to the preparation of desoximetasone, which takes 1, 4, 9, 16-arachidonic-pregna-3, 20-diketone (CN1896090) as the initiator and is improved by 16, 17-grignard, 9, 11th and 21st to obtain the desoximetasone and the 21st esterified ester thereof. The process has the advantages that, as the existing intermediate of the company is adopted as the initiator, the line is concise, the material is easy to obtain, expensive auxiliary materials are saved, and the yield and the cost are obviously superior to the historical synthetic method of the desoximetasone; in addition, the adoption of the existing intermediate realizes the doubling production of the dexamethasone products, betamethasone products and the desoximetasone products, thus greatly reducing the production cost and industrial conditions.
Owner:TIANJIN PHARMA GROUP CORP
Cosmetic product betamethasone high efficiency liquid chromatography detection method
InactiveCN101169396AAnalytical method is accurateAnalysis method is simpleComponent separationPretreatment methodRetention time
The invention provides an analysis method of detecting the betamethasone in cosmetic by use of high efficiency liquid chromatographic instrument, and the invention is characterized in that octadecylsilane chemically bonded silica is adopted as the C18 column or the C8 column of filler, and an ultraviolet detector or a diode array detector are used to detect at the position of 190 to 380 nm; the mobile phase adopts methanol-water mixed solution or acetonitrile-water mixed solution; the sample is extracted by methanol-hydrochloric acid mixed solution or acetonitrile-hydrochloric acid mixed solution, extracted by ultrasound for around 20 minutes, and undergone with an ice bath, and then is centrifuged in 12000rpm / min for 5 minutes. The clear liquid is prepared, which is, if necessary, filtered by 0.45Mu meters filtering membrane for using. The qualitative analysis is conducted according to the sample retention time and the spectrogram, and the quantitative analysis is conducted according to the peak area. The invention has the advantages of high solution and sensitivity, and perfect reproducibility and selectivity as well as simple sample pre-treating method, thereby being applicable to microanalysis of betamethasone in cosmetic.
Owner:广东省保化检测中心有限公司
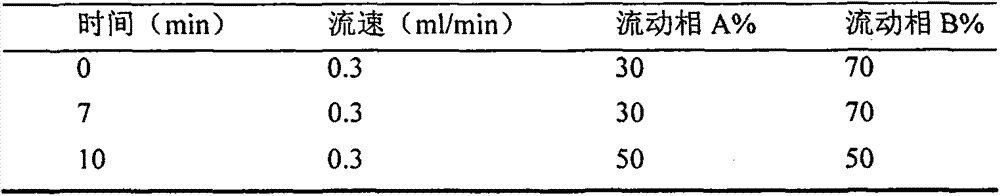
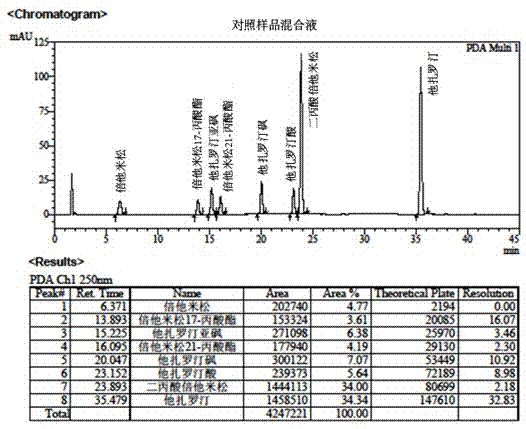
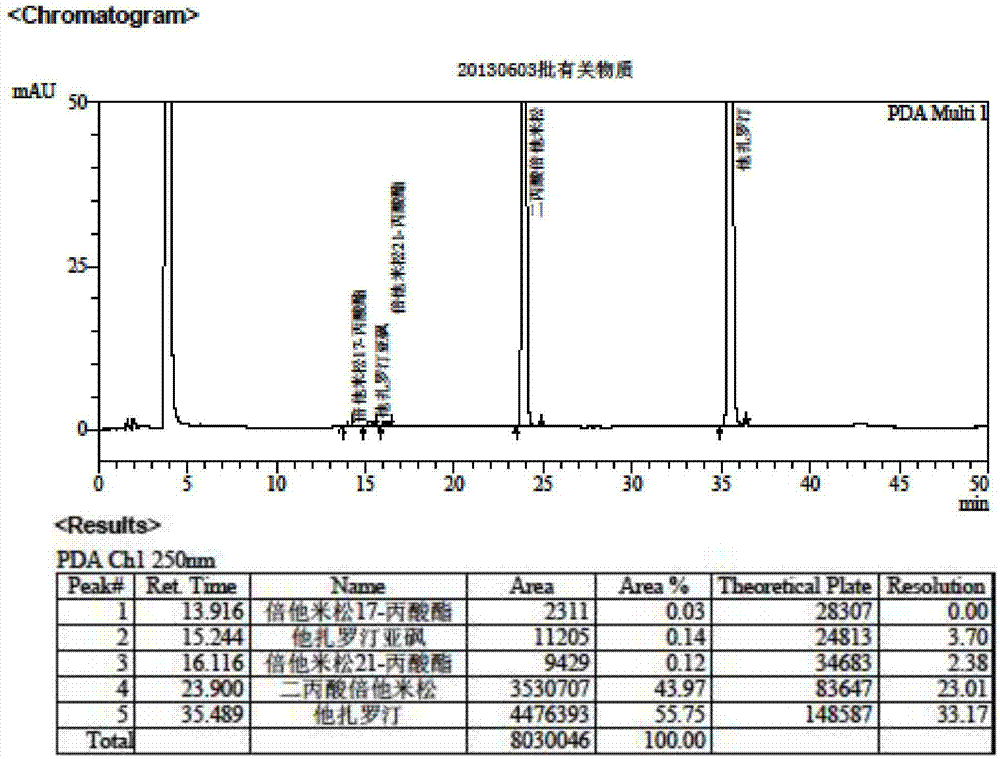
Method for separation and determination of two medicine contents and related substances in tazarotene-betamethasone cream
ActiveCN106918676AEfficient separationSeparation assay is validComponent separationBetamethasoneSolvent
The present invention belongs to the field of analytical chemistry, and relates to a method for separation and determination of two medicine contents and related substances in a tazarotene-betamethasone cream. According to the method, a reagent composition for solid-liquid separation and determination of two medicine contents and related substances in a tazarotene-betamethasone cream comprises an acidic aqueous solution system and acetonitrile, wherein the acidic aqueous solution system comprises an acidic additive, methanol and water; and by using the reagent composition, the two medicine contents and the related substances in the tazarotene-betamethasone cream can be simultaneously separated and determined through high performance liquid chromatography. According to the present invention, the method has good reproducibility, the solvent peak does not interfere with the determination of the two medicine contents and the related substances in the tazarotene-betamethasone cream during the detection, the two medicines and the related substances in the tazarotene-betamethasone cream can be effectively separated, the separation degree is more than 2.0, the detection result is accurate and reliable, and the important significance is provided for the achievement of the quality control of the tazarotene-betamethasone cream.
Owner:CHONGQING HUAPONT PHARMA
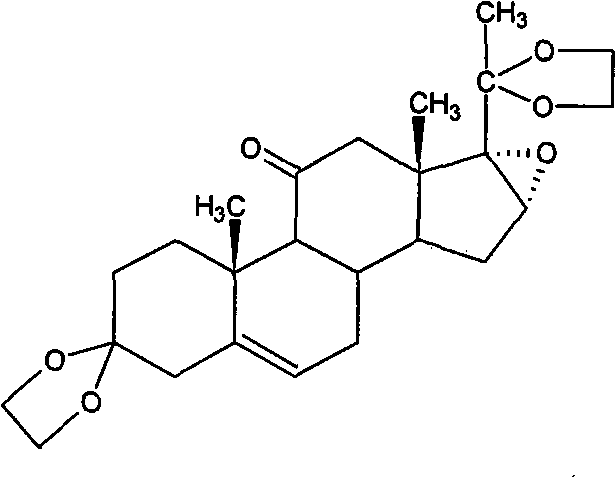
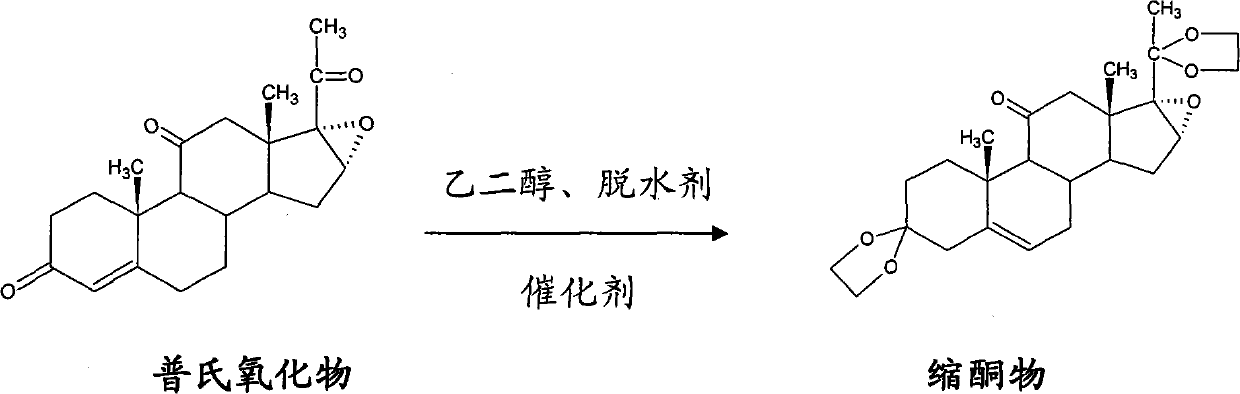
Method for preparing betamethasone ketal by homogeneous reaction at normal temperature
The invention provides a novel method for preparing betamethasone ketal by homogeneous reaction at normal temperature, which comprises the steps of: adding reagents of dichloromethane, triethyl orthoformate and boron fluoride etherate in a normal temperature homogeneous system; carrying out condensation reaction through Platts oxides and ethanediol to prepare betamethasone ketal; reacting for 2-15h, condensing filtrate again, extracting semi-condensed materials, and again putting the materials. The product content is not less than 90 percent and the yield is 115-118 percent. Compared with thetraditional process, the method has the advantages of shortening the reaction time, improving the content and the yield of the betamethasone ketal, reducing the environment pollution, discarding the benzene with huge hurt on the human body and the environment, ensuring that the production process is more environmentally friendly, lowering the labor intensity of workers, generating better social benefit, and being more beneficial to the massive industrialized production.
Owner:TIANJIN JINHUI PHARMA
Compound skin cream for treating leucoderma
InactiveCN104997790ARelieve painWith quality controllabilityAntipyreticAerosol deliveryDiseaseIrritation
The invention relates to an externally-used drug used for treating leucoderma skin disease, in particular to compound skin cream for treating leucoderma. The cream comprises, by weight, 0.20-0.50% of anisodamine, 0.10-0.50% of quercetin and 0.10-0.15% of betamethasone, the cream further comprises, by weight, 1.50-3.00% of dimethyl sulfoxide and 0.05-0.10% of 8-methoxypsoralen, and the cream further comprises oil phase raw materials, namely 15.00-25.00% of albolene, 8.00-15.00% of liquid paraffin, 5.00-9.00% of glycerin monostearate and 5.00-9.00% of octadecanol, and water phase raw materials, namely 0.50-1.20% of lauryl sodium sulfate, 5.00-10.00% of glycerinum, 0.05-0.10% of ethylparaben and 5.00-10.00% of propylene glycol; the cream further comprises 1.00-2.50% of hydrochloric acid and purified water, and the pH value ranges from 5.5 to 6.5. According to the compound skin cream for treating leucoderma, the formula is reasonable, the technique is feasible, and the cream has the advantages of being great in stability, good in effect and free of irritation.
Owner:魏传梅
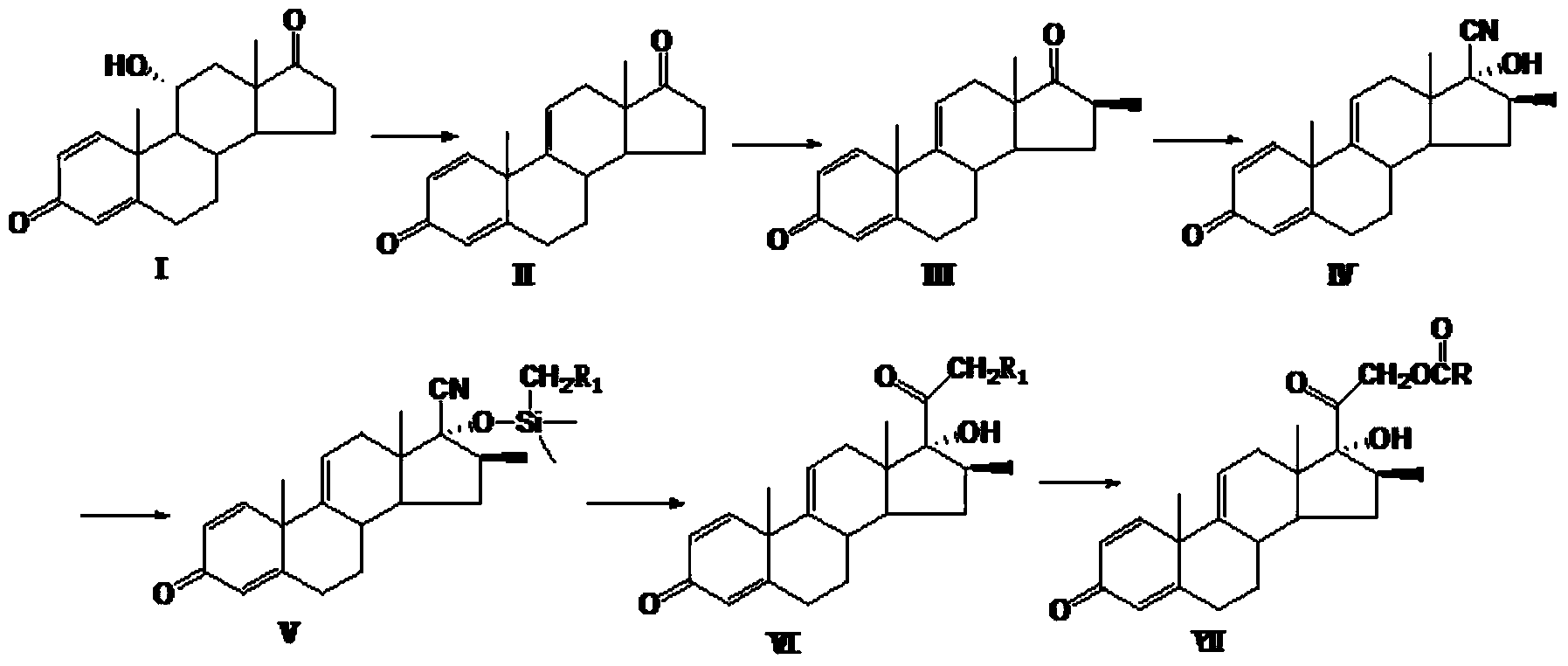
Preparation method of betamethasone intermediate
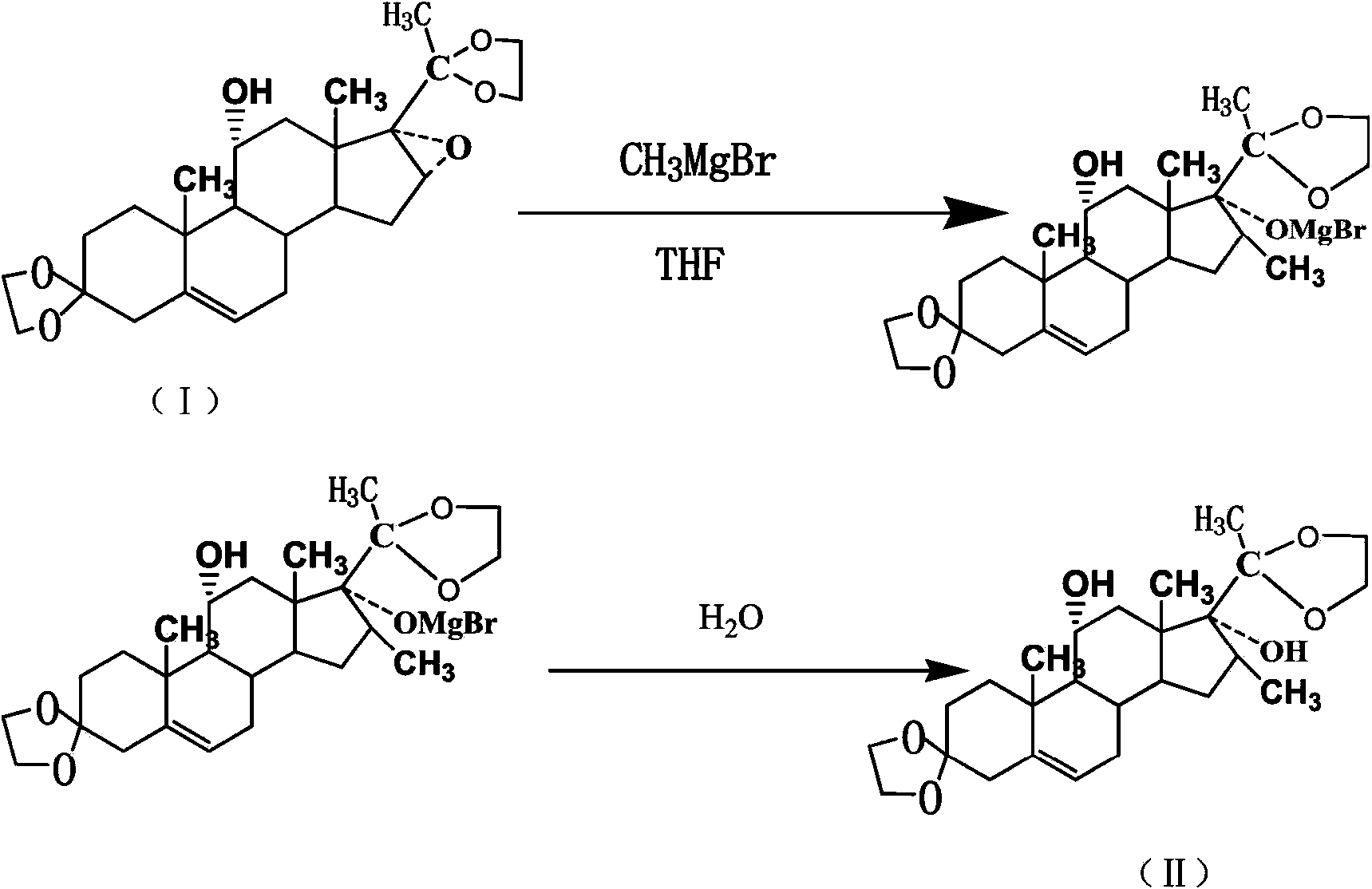
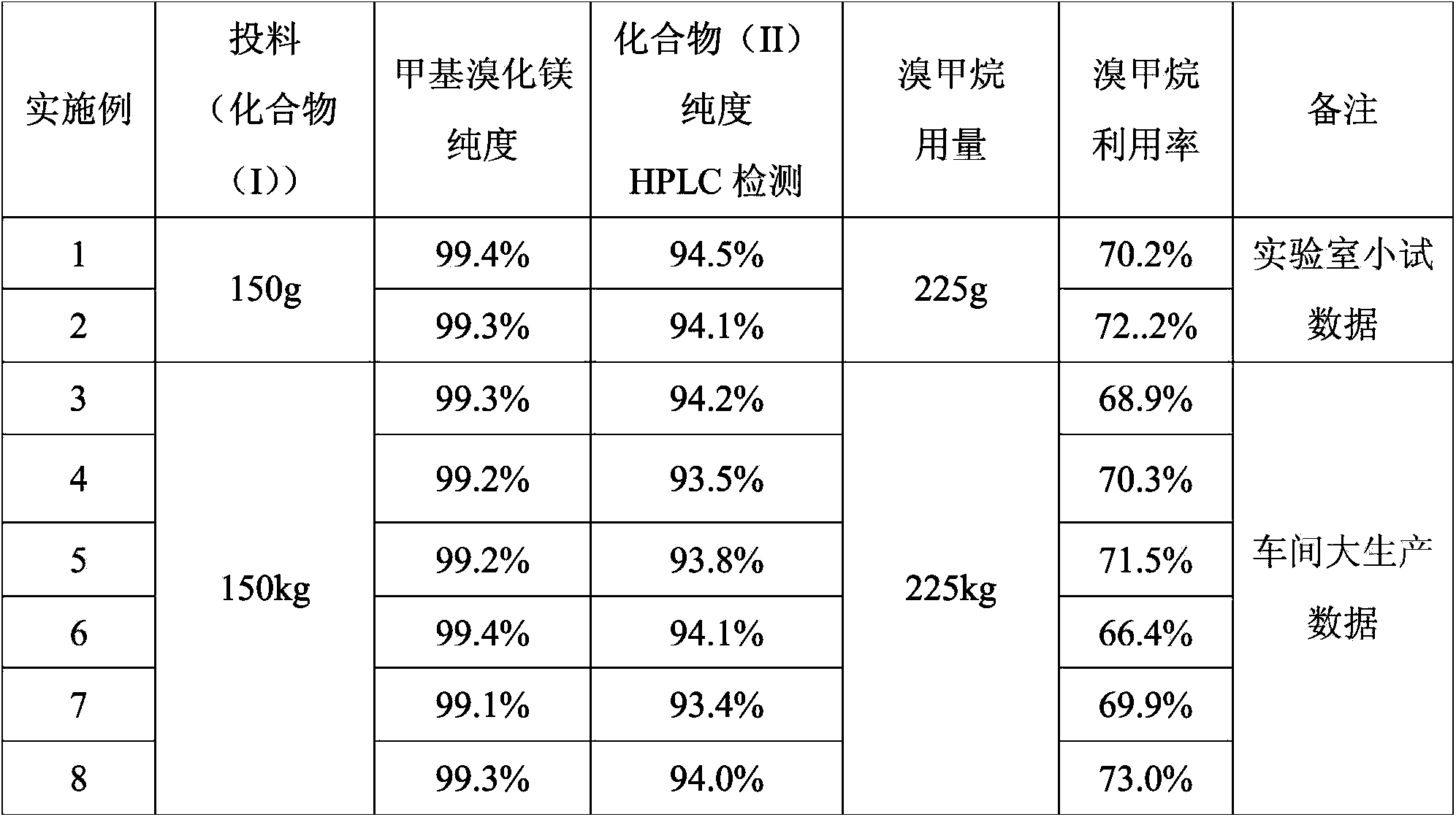
ActiveCN103819495AReduce pollutionIncrease profitSteroidsMagnesium organic compoundsEpoxyGrignard reagent
The invention provides a preparation method of a betamethasone intermediate. According to the method, magnesium granules are used as a raw material, and reacted with halogenated methane gas under the action of a proper amount of iodine granules to obtain a high-purity Grignard reagent methylmagnesium halide, preferably CH3MgBr, and the betamethasone intermediate 5-methylpregna-16beta-methyl-11alpha,17alpha-dihydroxy-3,20-diethylene ketal (II) is obtained by introducing methyl into a compound 5-methylpregna-16beta,17alpha-epoxy-11alpha-hydroxy-3,20-diethylene ketal (I). The method increases the purity of the CH3MgBr and the purity of the betamethasone intermediate (II), significantly reduces the consumption of methyl bromide gas, largely enhances reaction safety and reduces environment pollution.
Owner:SHANGHAI NEW HUALIAN PHARMA
Preparation method of betamethasone intermediate
The invention relates to a preparation process of a betamethasone intermediate. The structural formula of the betamethasone intermediate is shown as a formula I, and is prepared by undergoing a ketone group protection reaction, a Grignard reaction and a hydrolysis deprotection reaction on a compound III; and the ketone group protection reaction comprises making a compound II, diazanyl carboxylic ether and an acid react to obtain a compound IV. The method has high yield, and contributes to protecting the environment; and the use of toxic substances is avoided. The structural formula of the compound III is shown as a formula III.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Preparation method of key intermediate of dexamethasone and betamethasone
The invention discloses a preparation method of a key intermediate of dexamethasone and betamethasone. The preparation method specifically comprises the following steps: (1) firstly carrying out dehydration reaction on a compound I to remove a part of water, so as to obtain a compound II; (2) carrying out addition reaction on the compound II and ethanol or triethyl orthoformate under an acidic condition, so as to obtain 3-etherate III; (3) introducing methyl bromide into a solution of 3-etherate III under a strong base condition, so as to obtain a 16-methyl compound IV; and (4) carrying out oxidative dehydrogenation on the compound IV in the presence of a metal catalyst, so as to obtain the key intermediate of dexamethasone and betamethasone. According to the preparation method, the key intermediate of dexamethasone and betamethasone is directly prepared through dehydration reaction, addition reaction, substitution reaction and dehydrogenation reaction, and a brand-new preparation process is adopted, so that the process is simple, the material conversion rate is high, the purity of the final product is high, and the high quality of the key intermediate of dexamethasone and betamethasone is guaranteed.
Owner:ZHEJIANG PURUI PHARMA
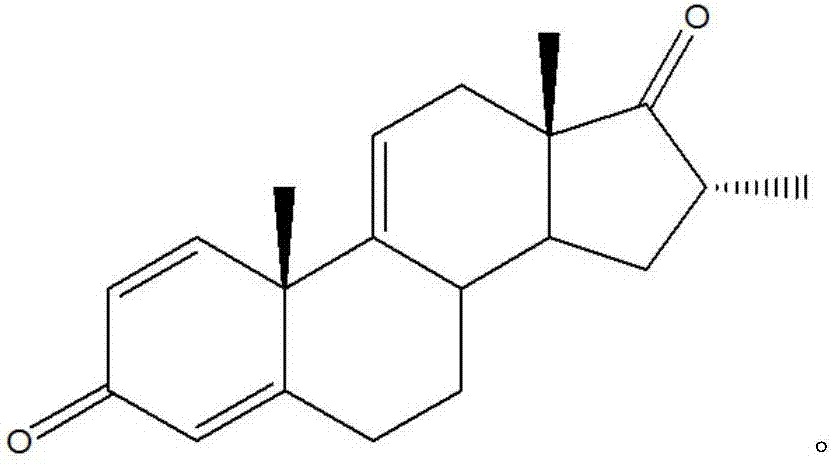
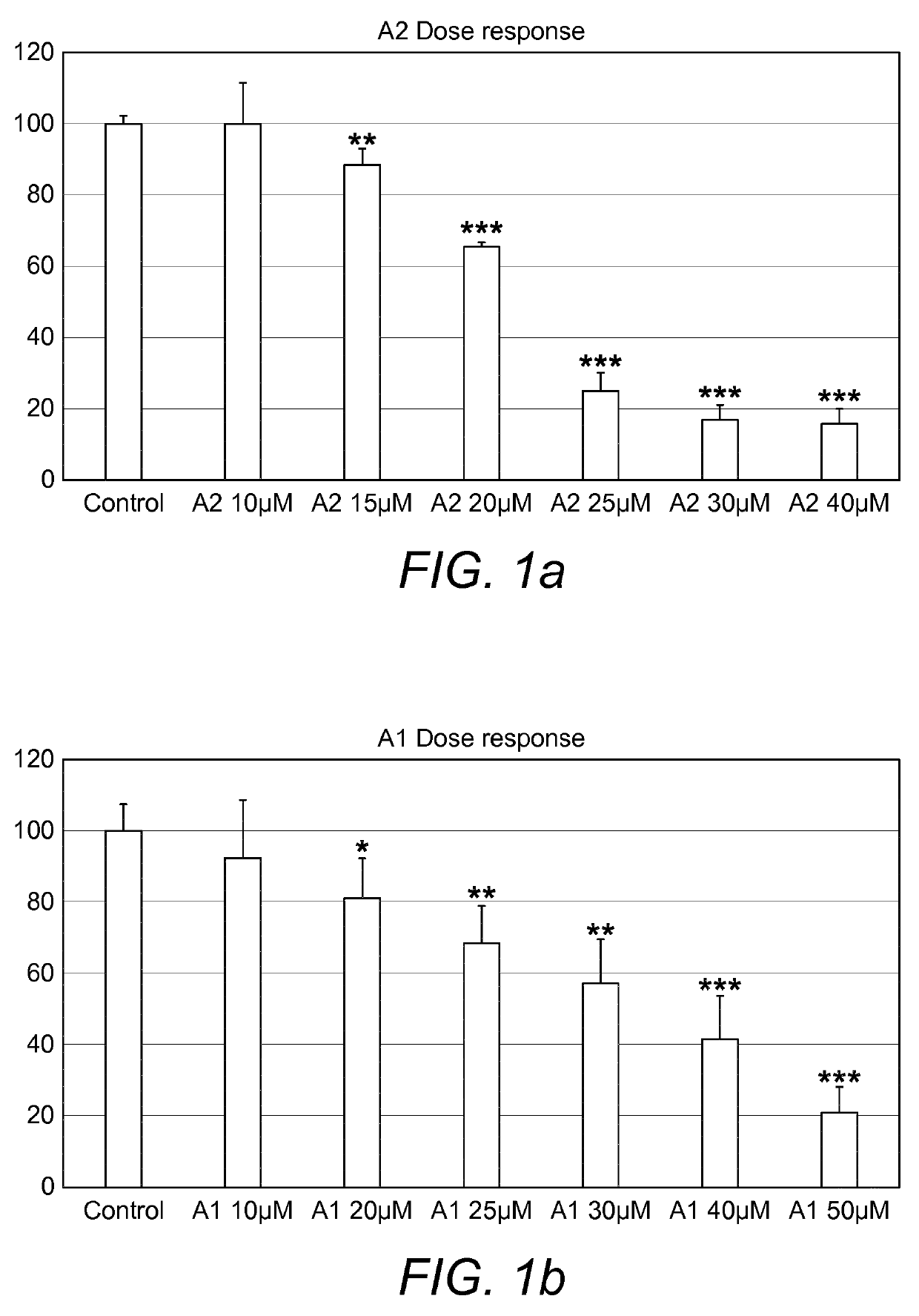
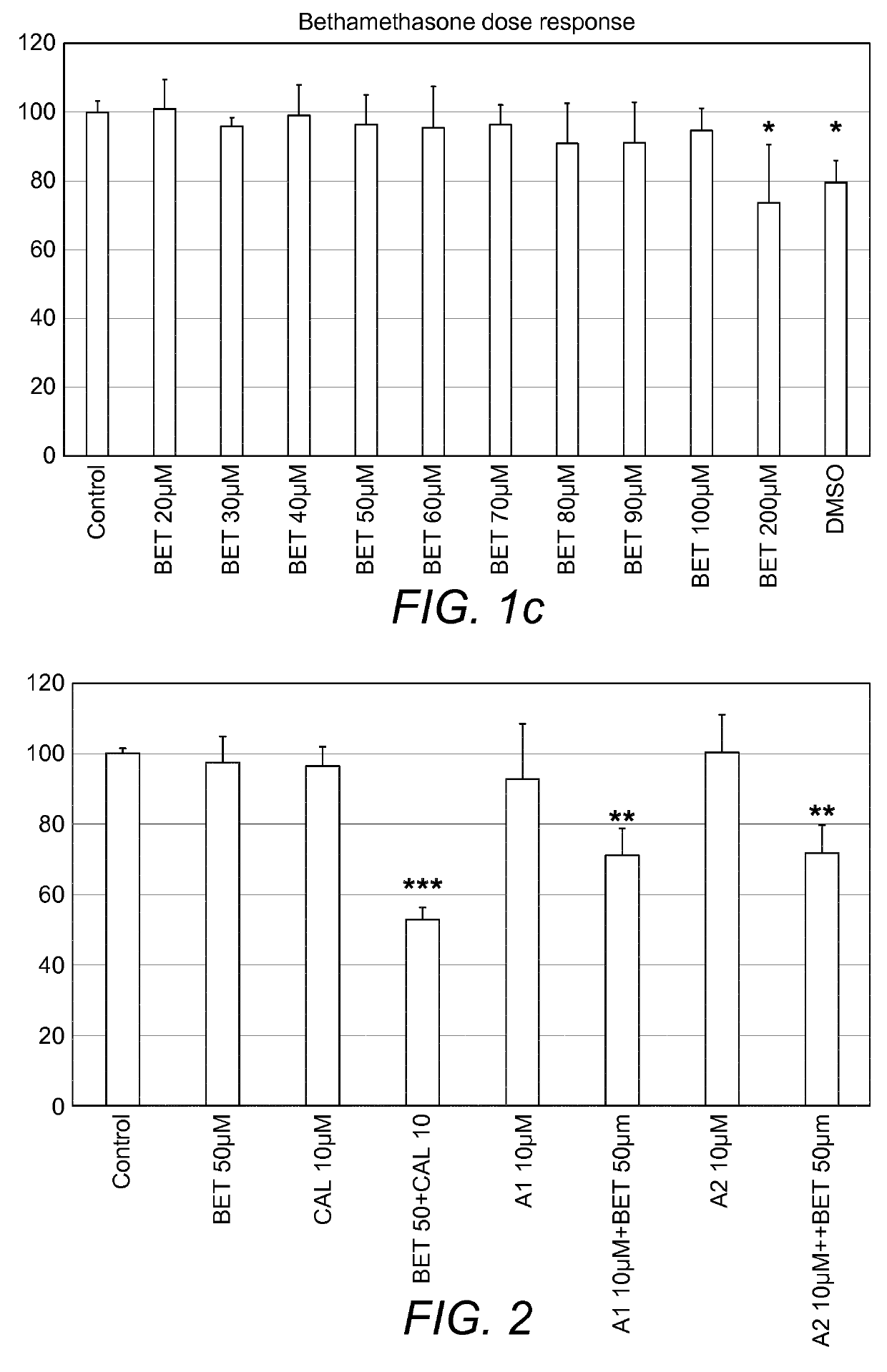
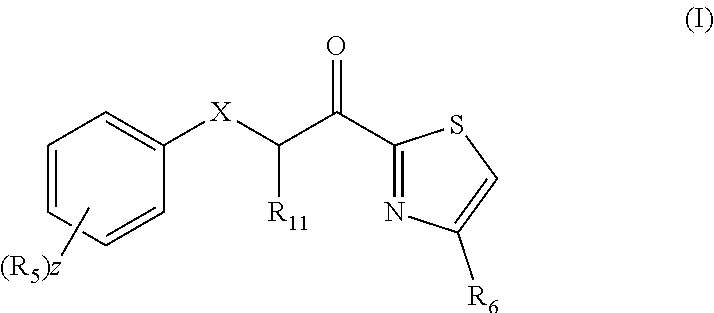
Combination therapy comprising a thiazole and a corticosteroid to treat skin conditions
InactiveUS20190255023A1Preferable effectReduce inflammation and itchinessOrganic active ingredientsAerosol deliveryFluocinoloneFluocinonide
A pharmaceutical composition comprising: (A) at least one compound of formula (I): wherein X is O or S, preferably O R6 is H, C1-6alkyl, —(CH2)pCOOH, —(CH2)pCOOC1-6alkyl, —(CH2)pCONH2, —(CH2)pCONHC1-6alkyl, —(CH2)pCON(C1-6alkyl)2, R11 is H or C1-6 alkyl; each R5 is —OC1-10alkyl, —SC1-10alkyl, —C1-12alkyl, or OAr2; wherein Ar2 is phenyl, optionally substituted with one or more halo; each p is 0 to 3; each z is 1 to 2; or a pharmaceutically acceptable salt, or a hydrate or solvate thereof; and (B) one or more corticosteroid partners, preferably selected from the group consisting of betamethasone, clobetasol, halometasone, dexamethasone, fluocortolone, desoximetasone, diflorasone, fluocinonide, flurandrenolide, halobetasol, amcinonide, halocinonide, triamcinolone, hydrocortisone, aclometasone, fluticasone, mometasone, clocortolone, fluocinolone, desonide, prednisone, prednisolone, and prednicarbate or a pharmaceutically acceptable salt, or a hydrate or solvate thereof, especially betamethasone or a pharmaceutically acceptable salt, or a hydrate or solvate thereof.
Owner:AVEXXIN
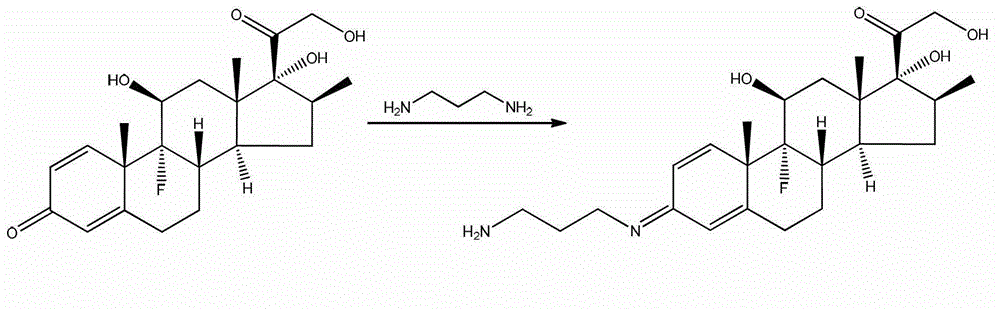
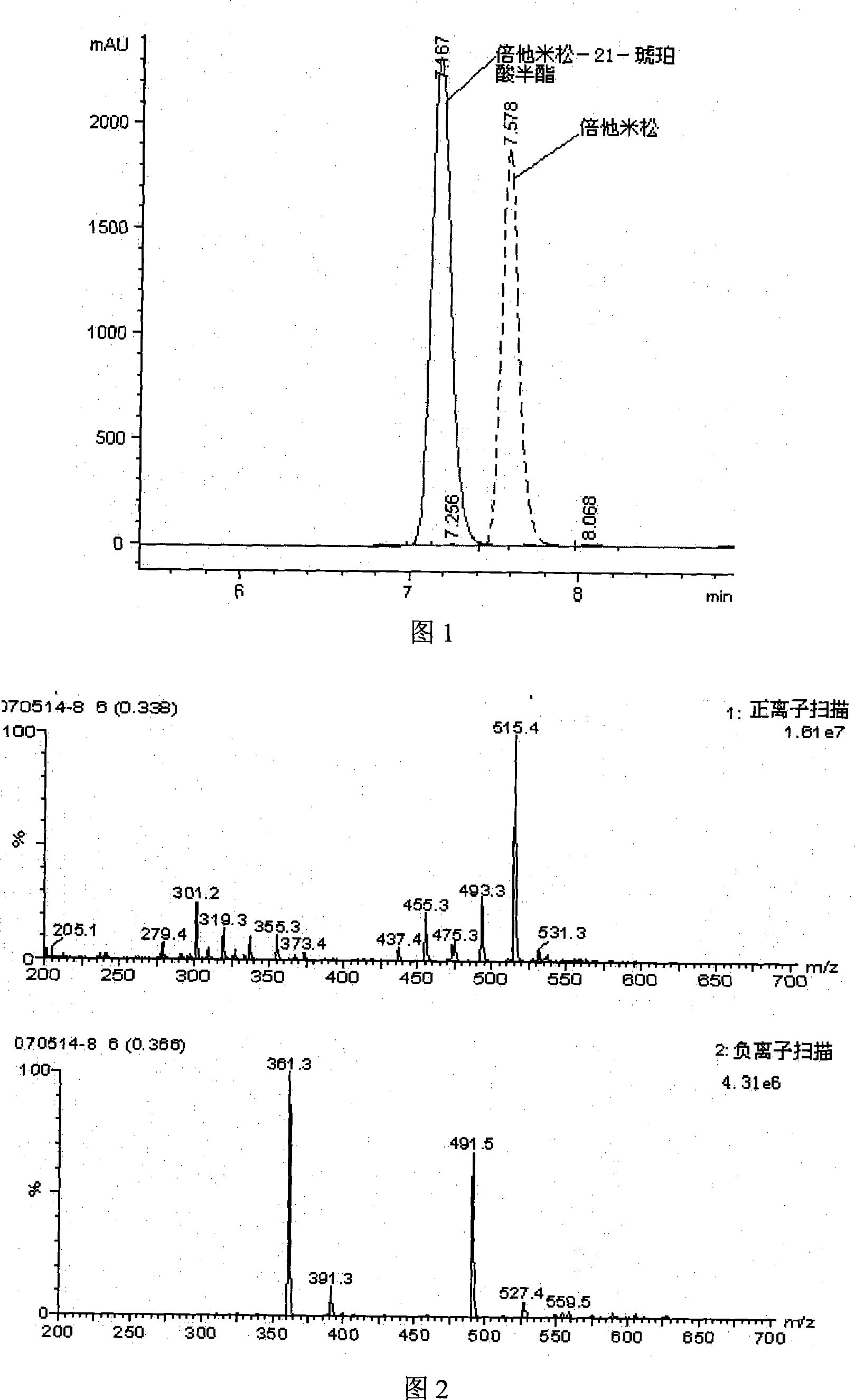
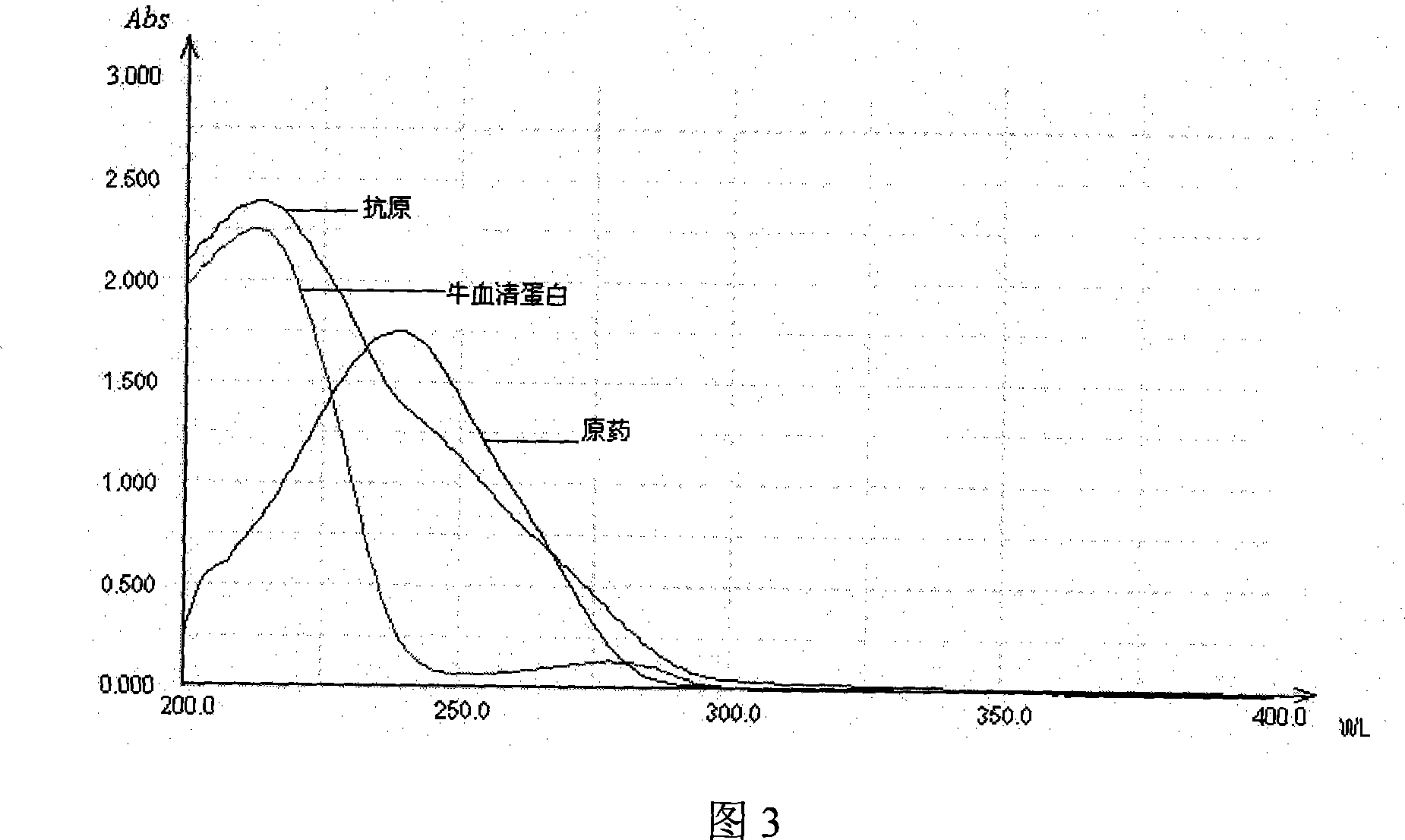
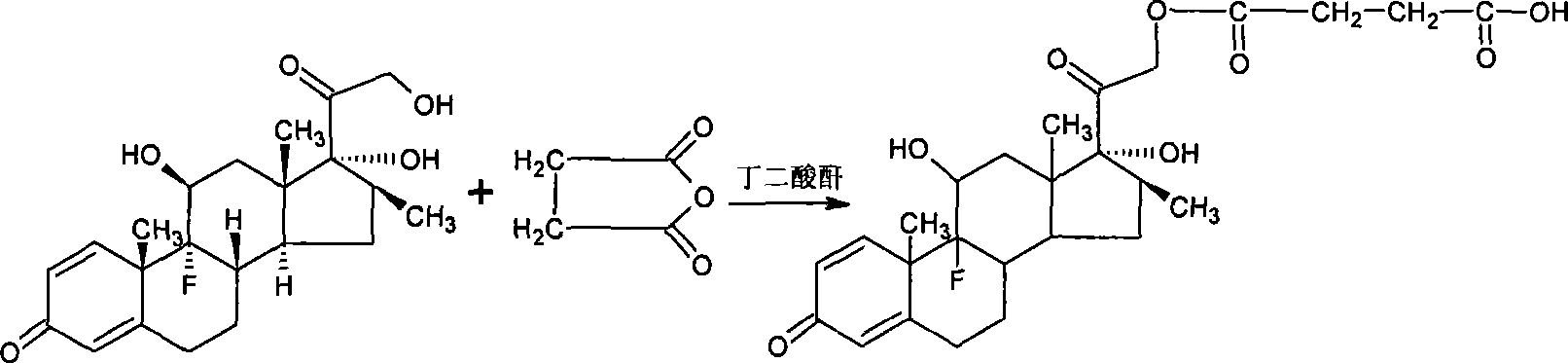
Method for preparing beta-corlan artificial antigen
InactiveCN101161680AThe synthesis steps are simpleThe synthetic step worksSerum albuminSteroidsAntigenGlucocorticoid
A preparation method of artificial antigen of betamethasone is provided, which belongs the biochemistry field. The present invention adopts betamethasone as the raw material, and utilizes the reaction between uccinic anhydride and betamethasone to produce betamethasone succinate and obtain the artificial semi-antigen, then the active ester method is used to transform the semi-antigen into a intermediate body of active ester, which is combined with bovine serum to prepare the artificial antigen of betamethasone, i.e. betamethasone bovine serum protein. The present invention synthesizes the artificial antigen of betamethasone antigen, and the prepared product can be used for researches on immunization method with glucocorticoid has simplified and effective synthetic procedures, as well as feasibility to be applied to immune analysis, thereby providing a convenient path for the further research by the people, and being capable of satisfying the domestic needs on the study on the object.
Owner:JIANGNAN UNIV
Aurosol test paper used for quickly detecting betamethasone residue and preparation method thereof
InactiveCN101609094AOvercome operational complexityOvercome operating costsMaterial analysisBetamethasonePolyclonal antibodies
The invention discloses an aurosol test paper used for quickly detecting betamethasone residue and a preparation method thereof. The test paper includes a box body, a test paper strip, a sponge holder and a holder, wherein the test paper strip absorbs envelope antigen and betamethasone polyclonal antibody labeled by the aurosol. The invention overcomes the defects of a betamethasone physical and chemical analysis method such as complex operation, high detection cost, slow speed and secondary environment pollution, and can accurately and sensitively detect the betamethasone residue in water, meat products and milk products. Besides, with little time consumed in the simple pretreatment process of samples, the invention can simultaneously and quickly detect a great quantity of samples.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Calcipotriol betamethasone self-microemulsion preparation with excellent performance
InactiveCN106265511AImprove solubilityIncrease dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsOctanoic AcidsPolyethylene glycol
The invention discloses a calcipotriol betamethasone self-microemulsion preparation with excellent performance, and the self-micromulsion preparation contains main drugs (namely the derivative or analogue of vitamin D (such as calcipotriol) and betamethasone), glycerides, C8-10 mono-and di-, propylene oxygen-15-S-stearyl ether, oleamide, oil phase and co-emulsifier. In the molecular structure of the glycerides, C8-10 mono-and di-, the degree of polymerization of the ethylene glycol is 2-4, and the composition ratio of the aliphatic acid in the molecular structure of the glycerides, C8-10 mono-and di- is that the percentage of octanoic acid is no less than 88%, the percentage of decanoic acid is no more than 10%, and the total proportions of lauric acid, aliphatic acid which are more advanced than lauric acid and aliphatic acid which are more lower than octanoic acid are no less than 2%. The stability, particularly the clinical effect of the composition, is improved.
Owner:JIANGSU SEMPOLL PHARMA
Method for refining betamethasone
The invention discloses a method for refining betamethasone, and betamethasone crude product is purified by organic solvent and water for obtaining betamethasone refined product, wherein, the organic solvent is a mixed solvent of halogenated hydrocarbon and alcohol. The method provided by the invention has the advantages of simple operation and greatly improved product yield and quality. Double-solvent and water system can effectively control impurity removal, and the refined product content of the prepared betamethasone is increased from 99.0% to 99.5% or more, and the yield is increased from 85% to 90% or more; the refining method employs purified water, which has the advantages of little danger and low cost.
Owner:SHANGHAI NEW HUALIAN PHARMA
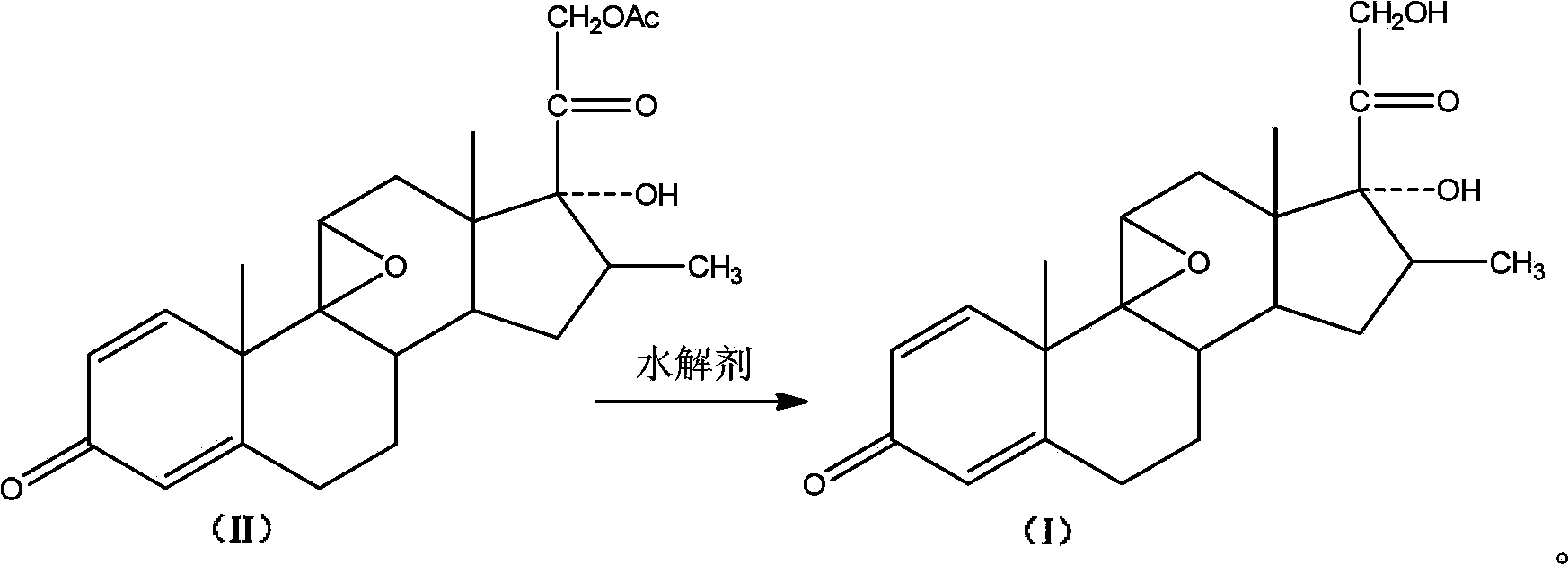
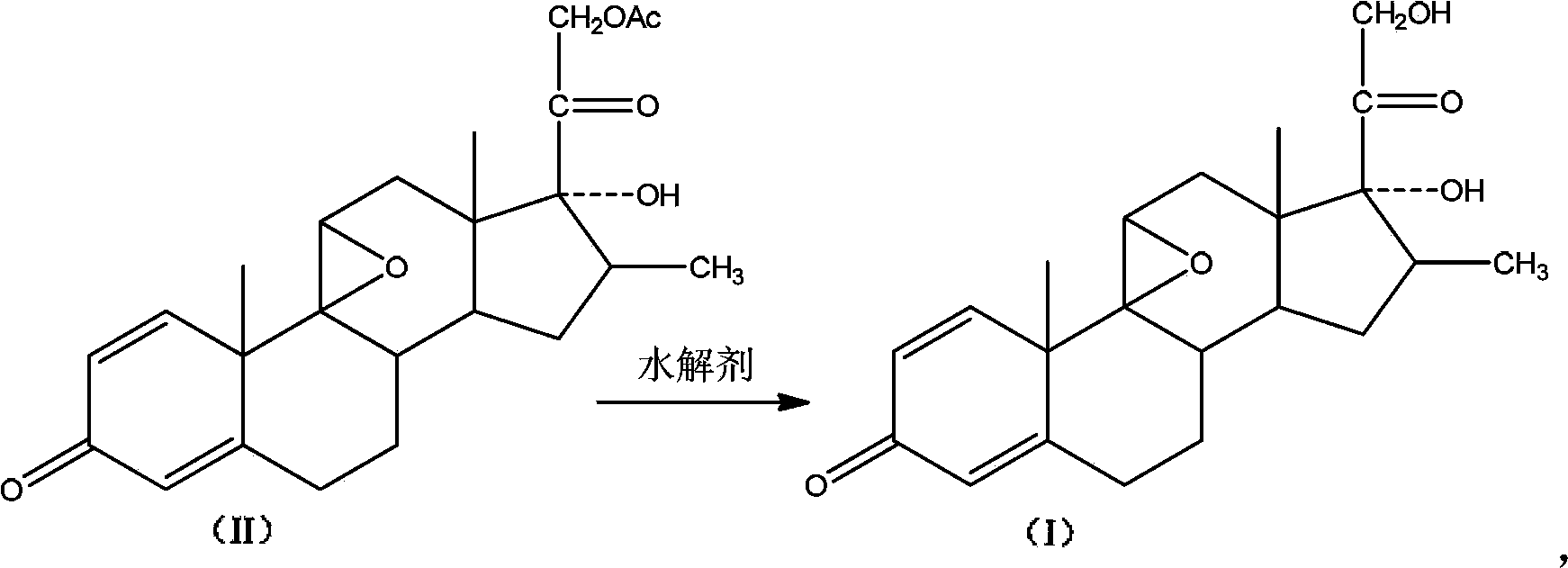
Preparation method of betamethasone intermediate
The invention discloses a preparation method of betamethasone intermediate hydrolysate (I), which employs a betamethasone substitute (II) as substrate, and employs a hydrolytic agent with reductibility to hydrolyze, and a compound in formula (I) is obtained. The reaction formula is shown in the figure. The invention employs the hydrolytic agent with reductibility to prepare the hydrolysate (I), and has the characteristics of high yield and good quality with good industrial production value.
Owner:SHANGHAI NEW HUALIAN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com