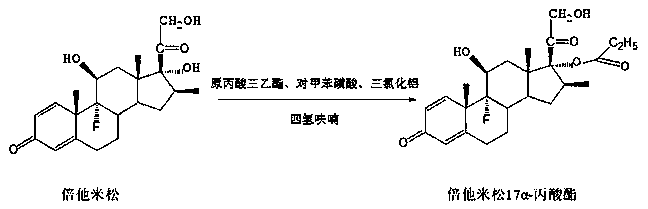
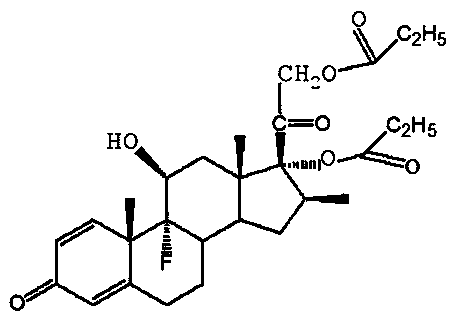
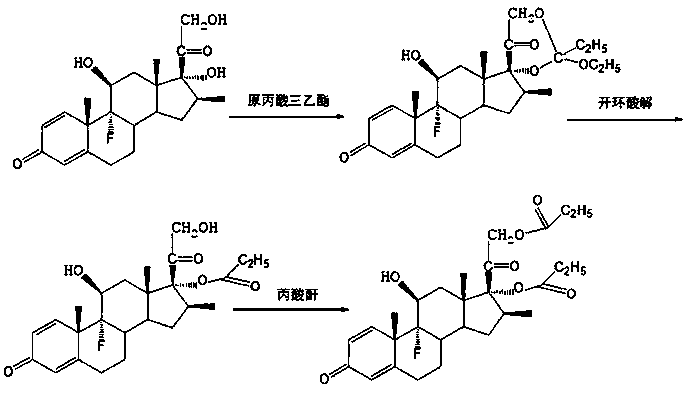
Preparation method of betamethasone 17 alpha-propionate
A technology of betamethasone and propionate is applied in the preparation of intermediates, the field of preparation of betamethasone 17α-propionate, and can solve the problems of unrecovered organic solvent, incompatible with energy saving and environmental protection, and many reaction steps, etc. Achieve the effect of guaranteed quality yield, improved production process economy and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A. Under nitrogen protection, add 200ml tetrahydrofuran and 1.6ml triethyl orthopropionate into the reaction flask, add 20g betamethasone, stir to dissolve, cool down to below 15°C, add 0.8g p-toluenesulfonic acid, control Temperature 15 ℃ ~ 20 ℃, heat preservation reaction for 2 hours;
[0022] B, TLC detects that raw material betamethasone reacts completely. Cool down to 10-15°C, slowly add aluminum trichloride solution (1.5g of aluminum trichloride) dropwise to the reaction solution, keep the temperature at 10-15°C, keep warm for about 1.5 hours, after the dropwise addition, keep warm reaction. The temperature is 25°C-30°C, and the reaction time is 15 hours;
[0023] C. TLC detects that the reaction of the raw material betamethasone-17-21 cyclic ester is complete, control the temperature at about 50°C, and the vacuum degree is not less than 0.06Mpa, concentrate under reduced pressure to produce 190ml of tetrahydrofuran, and precipitate a white solid, then add 100ml...
Embodiment 2
[0025] A. Under nitrogen protection, add 200ml tetrahydrofuran and 1.4ml triethyl orthopropionate into the reaction flask, add 20g betamethasone, stir to dissolve, cool down to below 15°C, add 0.6g p-toluenesulfonic acid, control Temperature 15 ℃ ~ 20 ℃, heat preservation reaction for 3 hours;
[0026] B, TLC detects that raw material betamethasone reacts completely. Cool down to 10-15°C, slowly add aluminum trichloride solution (1.3g of aluminum trichloride) dropwise to the reaction solution, keep the temperature at 10-15°C, keep warm and drop for about 1.5 hours, after the dropwise addition, keep warm reaction. The temperature is 25°C-30°C, and the reaction time is 18 hours.
[0027] C. TLC detects that the reaction of the raw material betamethasone-17-21 cyclic ester is complete, control the temperature at about 50°C, and the vacuum degree is not less than 0.06Mpa, concentrate under reduced pressure to produce 191ml of tetrahydrofuran, and precipitate a white solid, then ...
Embodiment 3
[0029] A. Under nitrogen protection, add 150ml tetrahydrofuran and 1.8ml triethyl orthopropionate into the reaction flask, add 20g betamethasone, stir to dissolve, cool down to below 15°C, add 0.8g p-toluenesulfonic acid, control Temperature 15℃~20℃, heat preservation reaction for 1.5 hours;
[0030] B, TLC detects that raw material betamethasone reacts completely. Cool down to 10-15°C, slowly add aluminum trichloride solution (aluminum trichloride 1.7g) dropwise to the reaction solution, keep the temperature at 10-15°C, keep warm and drop for about 1.5 hours, after the dropwise addition, keep warm reaction. The temperature is 25°C-30°C, and the reaction time is 13 hours.
[0031]C. TLC detects that the reaction of the raw material betamethasone-17-21 cyclic ester is complete, control the temperature at about 50°C, and the vacuum degree is not less than 0.06Mpa, concentrate under reduced pressure to produce 190ml of tetrahydrofuran, and precipitate a white solid, then add 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com