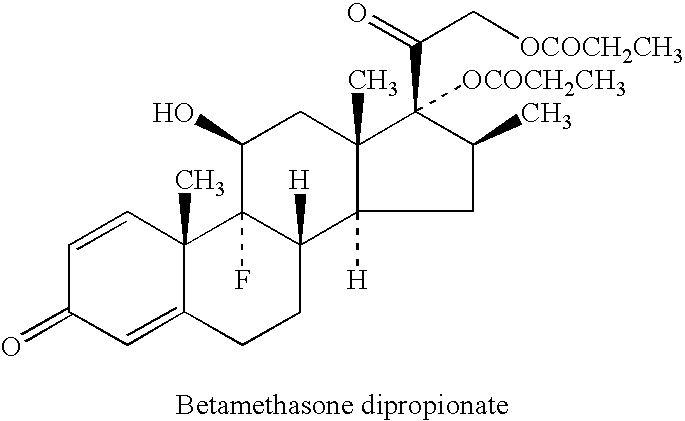
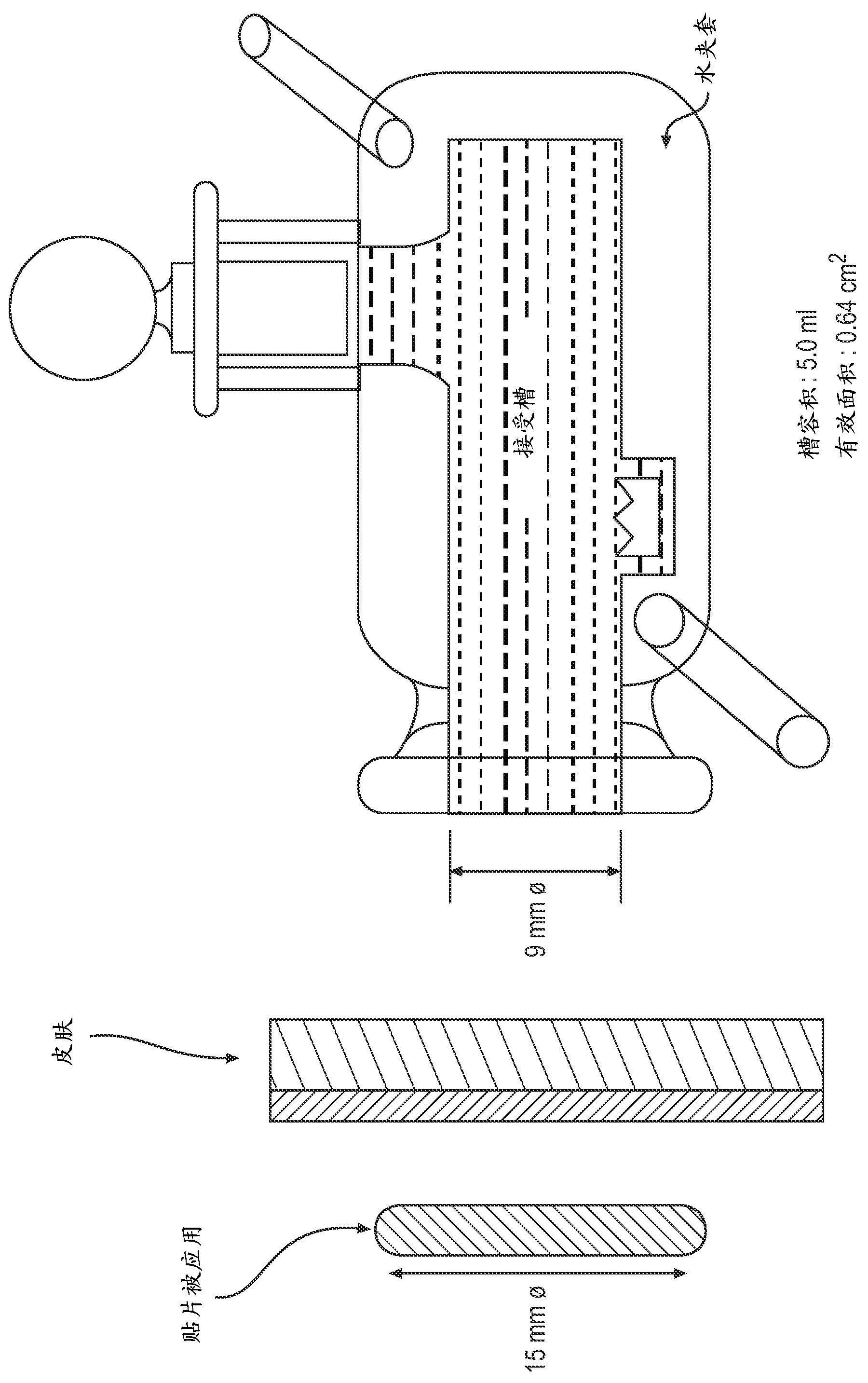
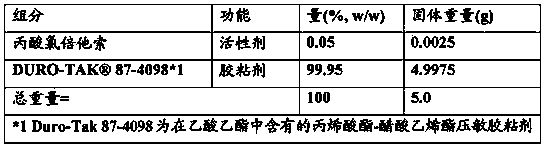
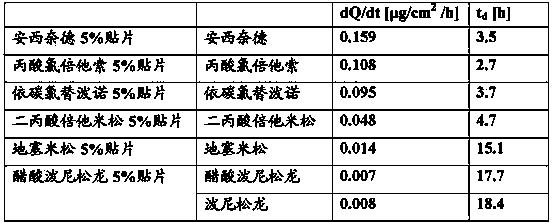
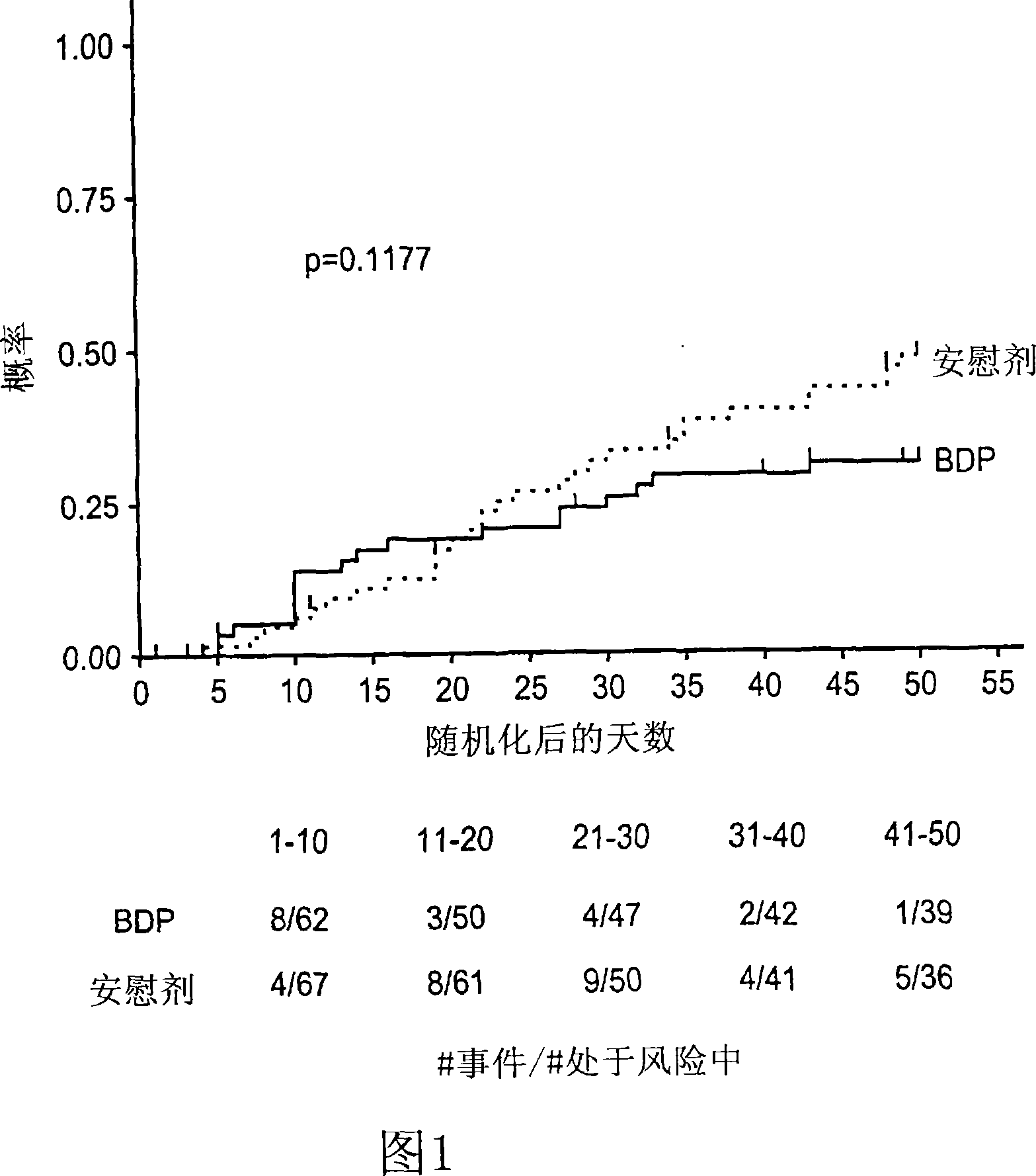
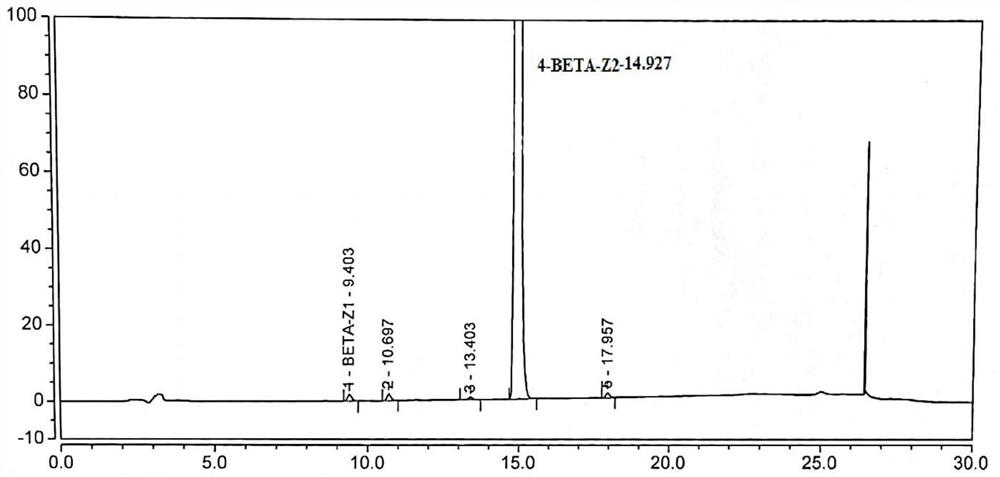
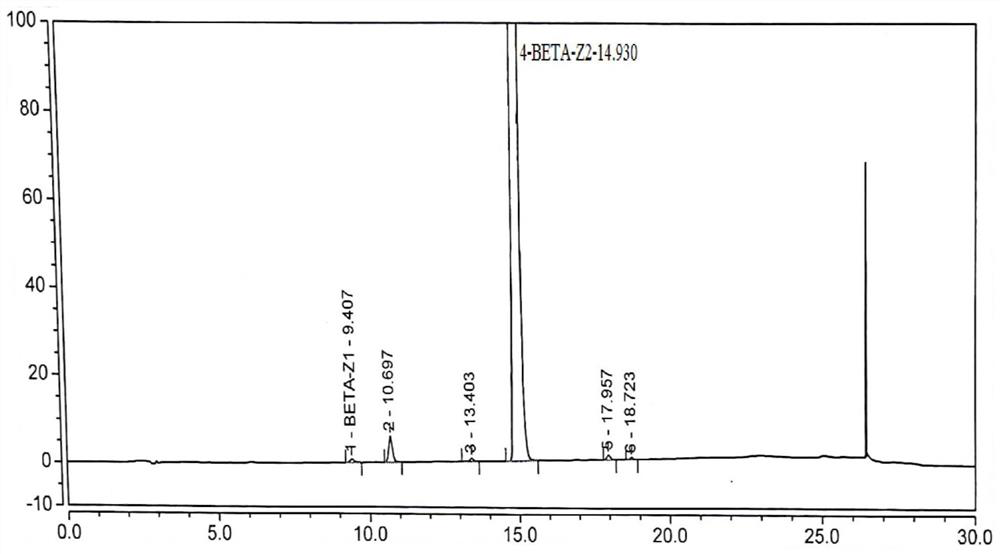
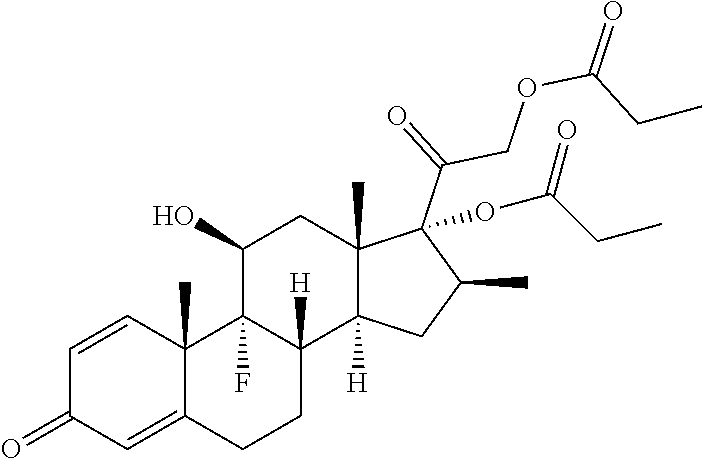
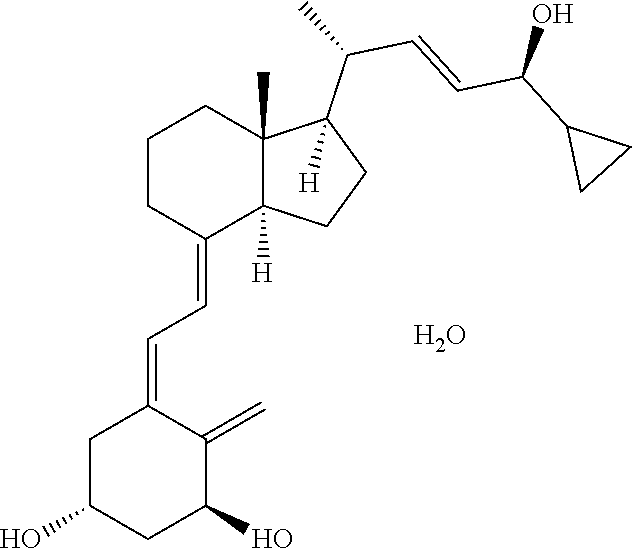
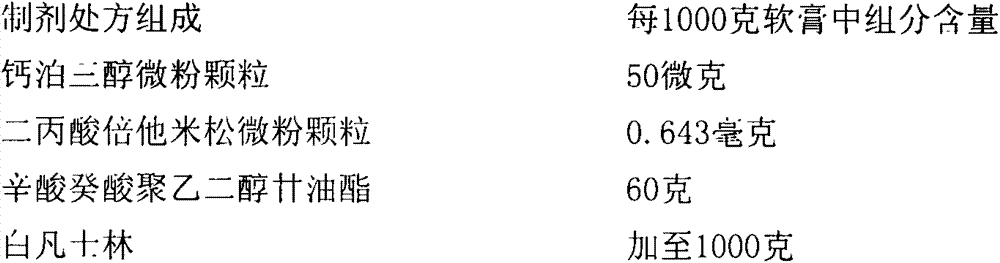Patents
Literature
51 results about "Betamethasone dipropionate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
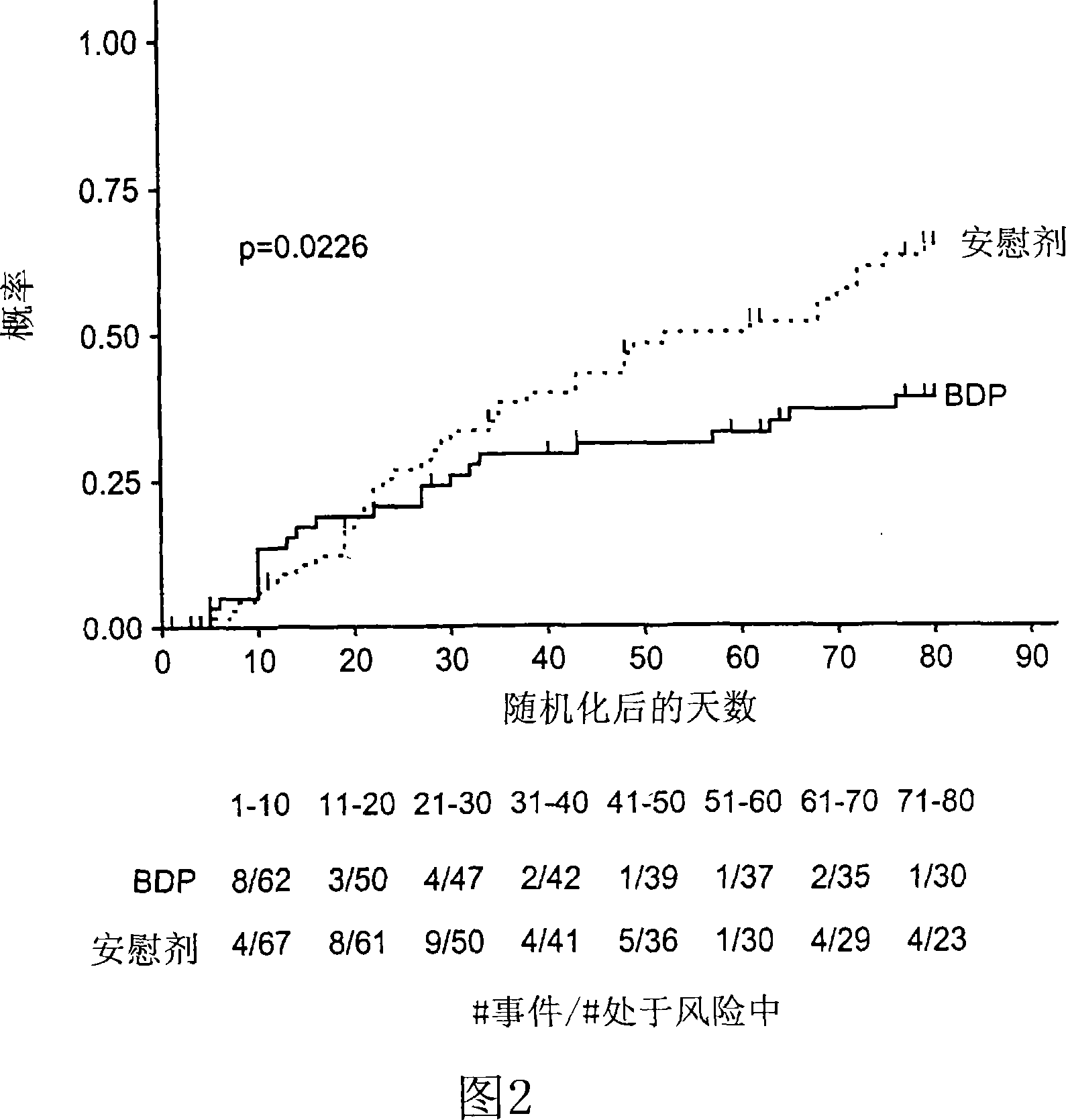
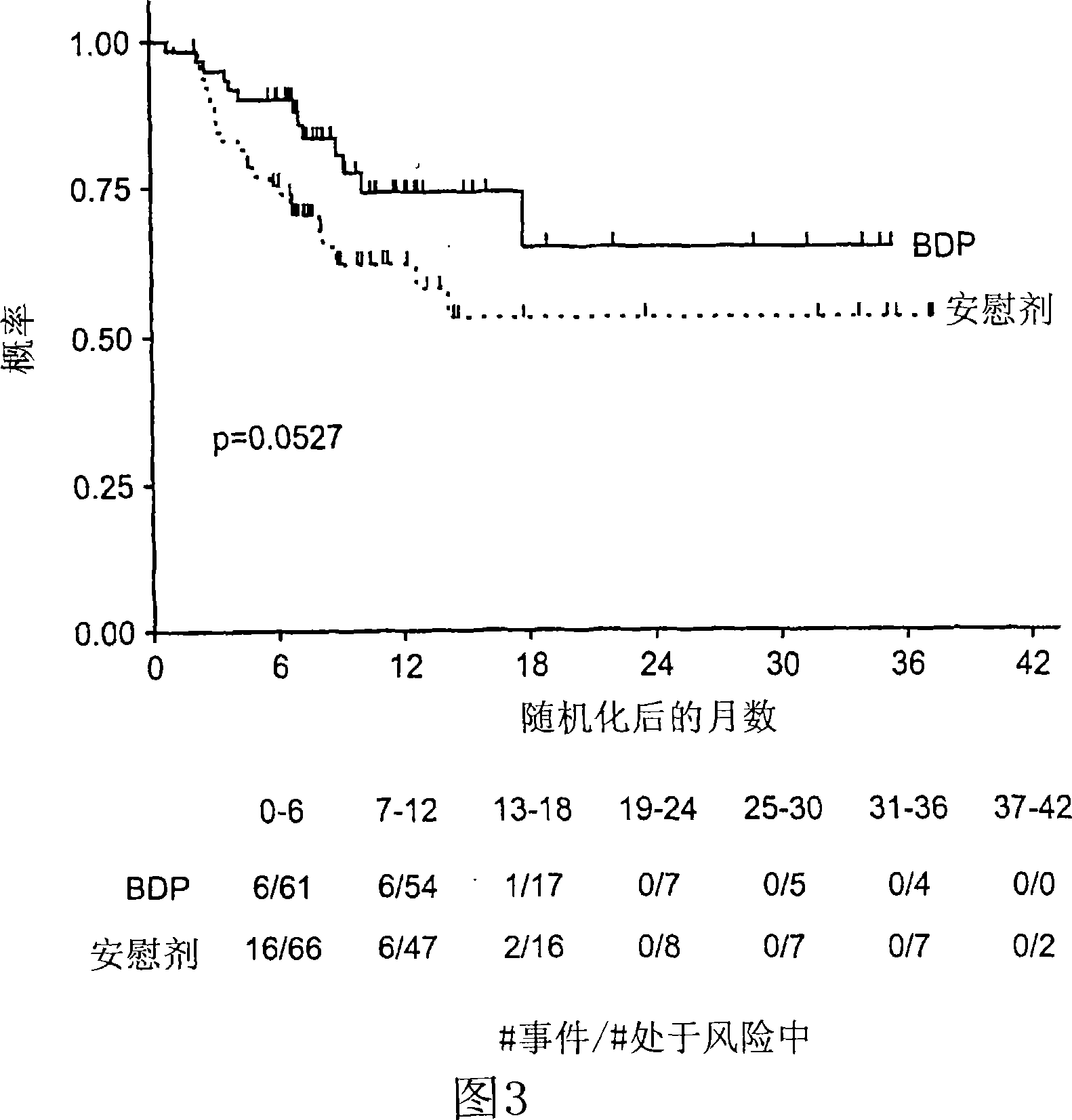
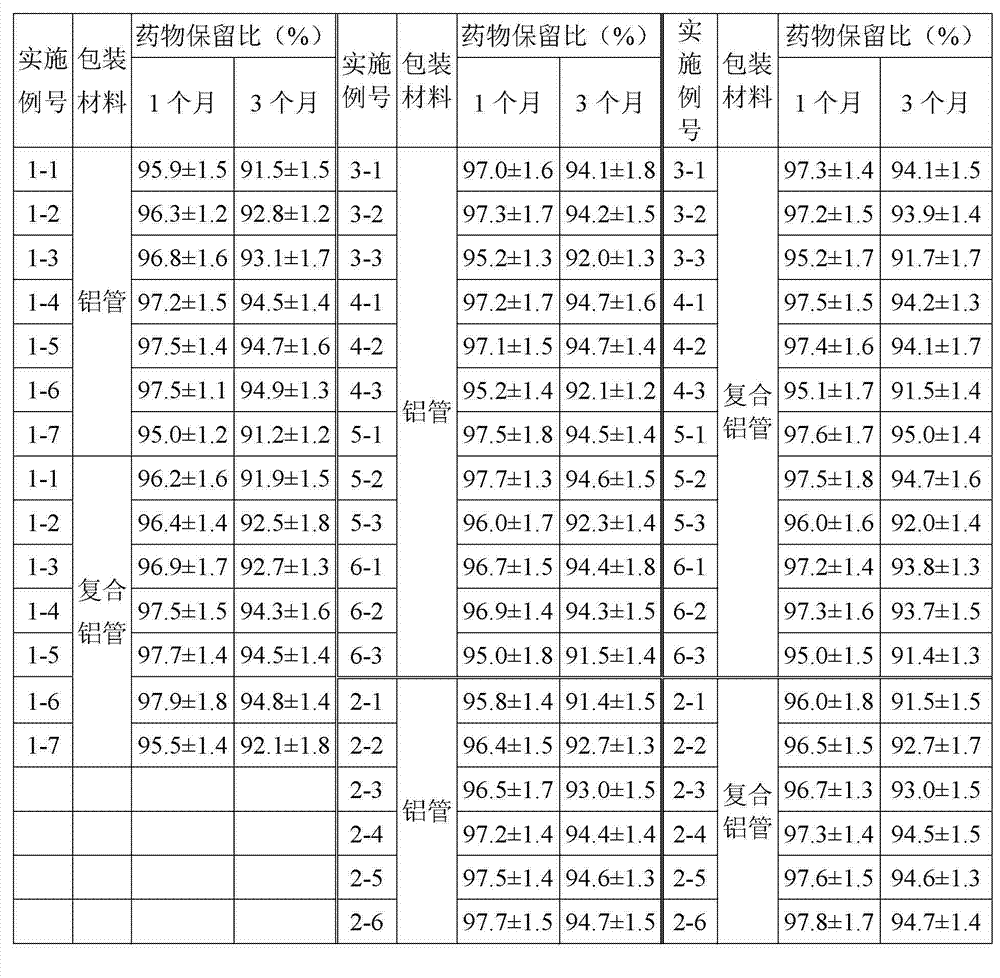
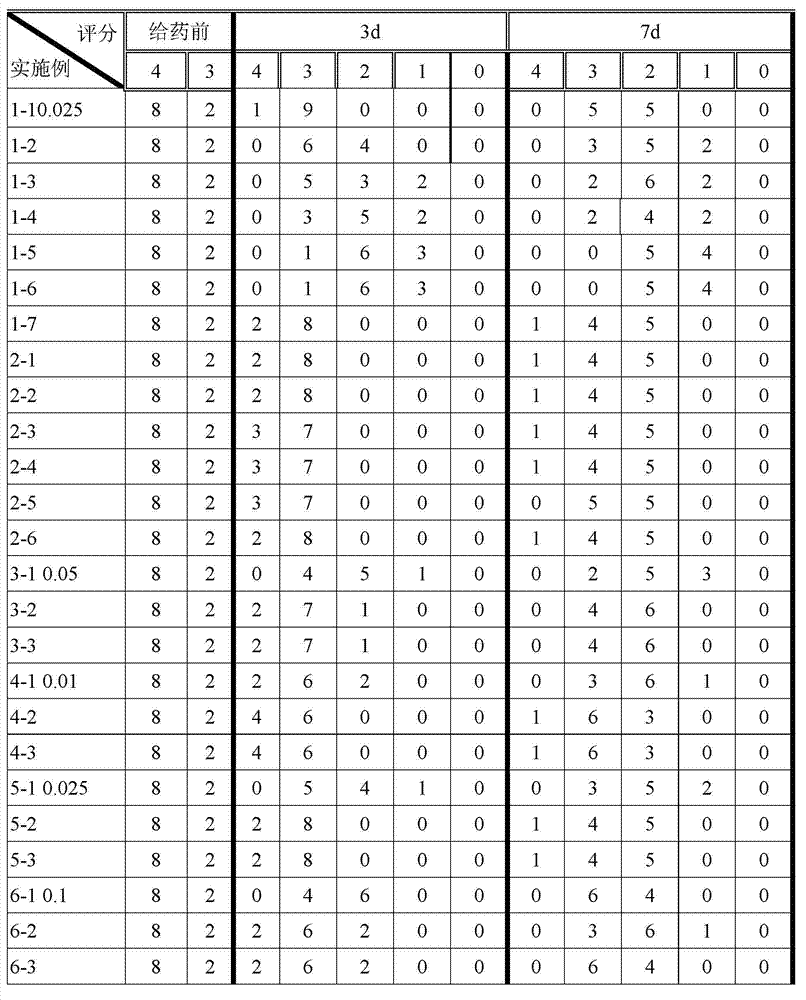
This medication is used to treat a type of skin condition (plaque psoriasis).
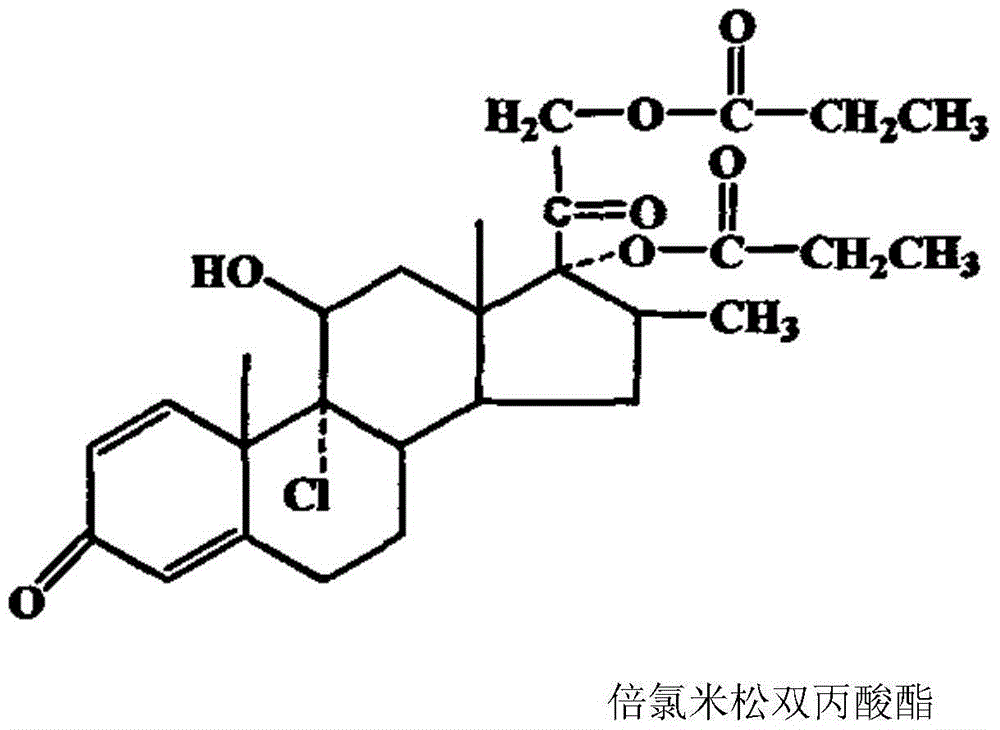
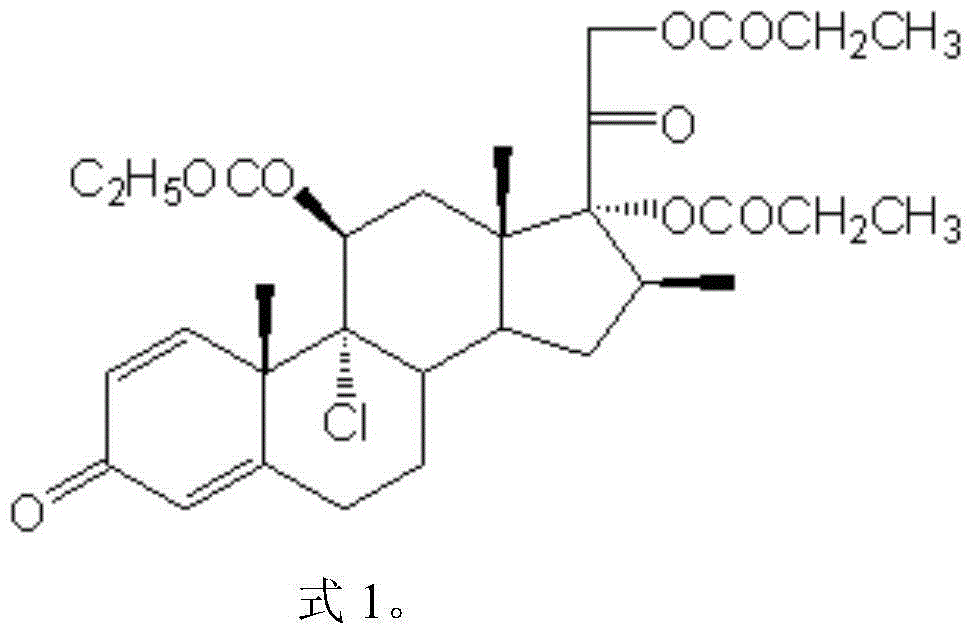
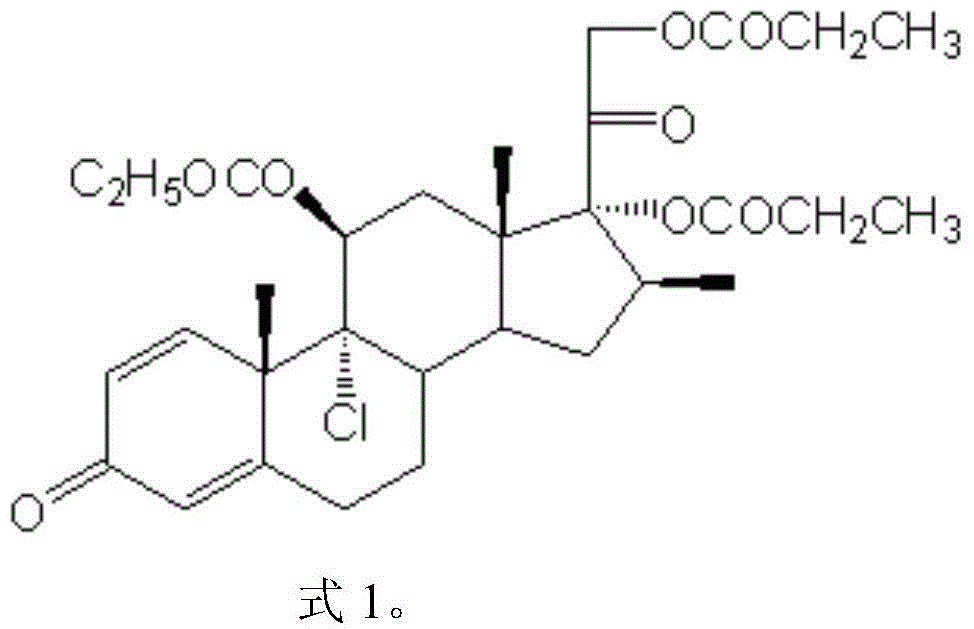
Compound betamethasone suspension injection
InactiveCN101167730A"block" does not appearGood injectabilityOrganic active ingredientsComponent separationSolubilityCollagen disease
The invention provides a compound betamethasone suspension injection, which is a compound preparation composed of low-solubility betamethasone dipropionate and high-solubility betamethasone sodium phosphate. Long-lasting, it can be used for the treatment of musculoskeletal and cartilage tissue diseases, allergic diseases, skin diseases, collagen diseases, tumors and other clinical diseases.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Calcipotriol betamethasone ointment and preparation method thereof
InactiveCN103110648AOrganic active ingredientsAerosol deliveryBetamethasone propionatePropanoic acid
The invention a calcipotriol betamethasone ointment prepared from caprylocaproyl macrogolglycerides. Under the conditions of high speed cutting and a homogeneous state, calcipotriol micronized particles with particle size range being 5-110 microns and the micronized particles of betamethasone dipropionate are uniformly dispersed to caprylocaproyl macrogolglycerides, affinities of caprylocaproyl macrogolglycerides and a commonly used ointment matrix are utilized to obtain the uniform ointment with uniformly distributed particle size. Furthermore, the stability of resisting high temperature (50 DEG C) is better, and is beneficial to improving the quality and the stability of a medicine.
Owner:JIANGSU SEMPOLL PHARMA
Pharmaceutical aerosol formulations of formoterol and beclometasone dipropionate
ActiveUS20110081301A1Improve stabilityReduce solubilityOrganic active ingredientsPowder deliveryPharmaceutical formulationBeclometasone dipropionate
Pharmaceutical formulations comprising beclometasone dipropionate and a salt of formoterol exhibit improved stability and are useful in pressurised metered dose inhalers (pMDIs).
Owner:CHIESI FARM SPA
Preparation method of betamethasone dipropionate micro-particle
ActiveCN101849910ALarge particle sizeImprove uniformityOrganic active ingredientsPowder deliveryAnti solventSemi solid
The invention relates to a preparation method of betamethasone dipropionate micro-particle, which is characterized in that betamethasone dipropionate is dissolved in a solvent, and then the mixture is added into an anti-solvent at the mixing state to be continuously mixed and crystallized. Through the micro crystallization method, the betamethasone dipropionate micro-particle which has small grain size and homogeneity satisfying the preparation requirement is prepared. The micro-particle can be further prepared into semi-solid preparations such as cream and suspension preparations such as injection, spray, aerosol and the like.
Owner:CHONGQING HUAPONT PHARMA
Preparation containing calcipotriol and betamethasone dipropionate
ActiveCN104666312AEnsure safetyGuaranteed curative effectOrganic active ingredientsAerosol deliveryForeign - SimilarPharmaceutical formulation
The invention belongs to the field of medicine preparations, and particularly relates to a preparation technology of calcipotriol. According to the preparation technology, the problem of the stability of calcipotriol and betamethasone dipropionate is solved. Benzyl alcohol and triethanolamine are added to auxiliary materials. The paste prepared by the technology is stable in property. Compared with foreign similar products, the cost is lower; and the method is suitable for industrialized production.
Owner:CHONGQING HUAPONT PHARMA
Medicinal composition for treating psoriasis
InactiveCN1478478ASignificant effectLow incidence of adverse reactionsOrganic active ingredientsDermatological disorderMedicineAdrenal gland
A composite medicine in the form of ointment and gels for treating psoriasis contains Tazhuluoding, betamethasone dipropionate and pharmacologically receptable auxiliary. Its advantages are high curative effect and high safety.
Owner:CHONGQING HUAPONT PHARMA
Transdermal drug delivery system and method of using the same
InactiveUS8900626B2Effective treatmentImprove permeabilityOrganic active ingredientsBiocideDiseaseActive agent
A transdermal drug delivery system comprising a steroid as an active agent, wherein the steroid may be clobetasol propionate, betamethasone dipropionate, amcinonide, or loteprednol etabonate. The transdermal drug delivery system also comprises a pressure-sensitive adhesive layer and a support, wherein the steroid is present in the pressure-sensitive adhesive layer, and wherein the pressure-sensitive adhesive layer is provided on a support. The transdermal drug delivery system may be applied onto the eyelid of a patient in need thereof, in order to treat a disease of the eyelid, such as chalazion, blepharitis or meibomian gland dysfunction.
Owner:SENJU USA
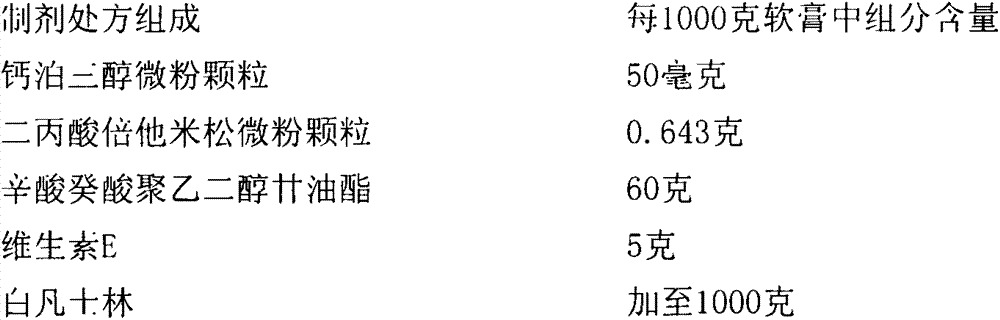
Pharmaceutical composition including a corticosteroid and a vitamin d analog having improved stability
InactiveUS20100286101A1Low dipoleSignificant degreeBiocideOrganic active ingredientsEtherVitamin D Analog
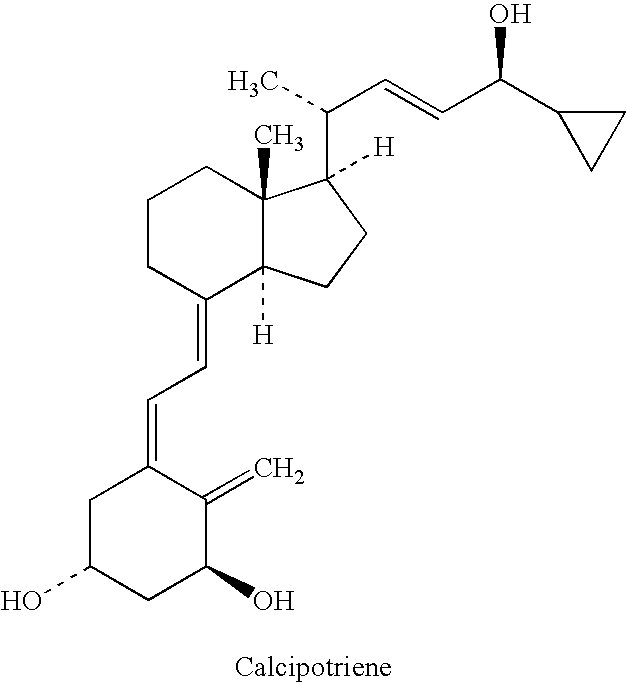
A composition that includes a corticosteroid (e.g., betamethasone dipropionate), a vitamin D analog (e.g., calcipotriene) and an acyl ester of 1,2,3-trihydroxypropane or an ether thereof; and a method of treating a dermatologic condition (e.g., psoriasis vulgaris) in a mammal (e.g., human); are provided. The method includes topically administering to a mammal in need of such treatment an effective amount of the composition, to the affected topical area, for a period of time effective to treat the dermatologic condition.
Owner:CARBOL JASON +1
Stabilized composition for treating psoriasis
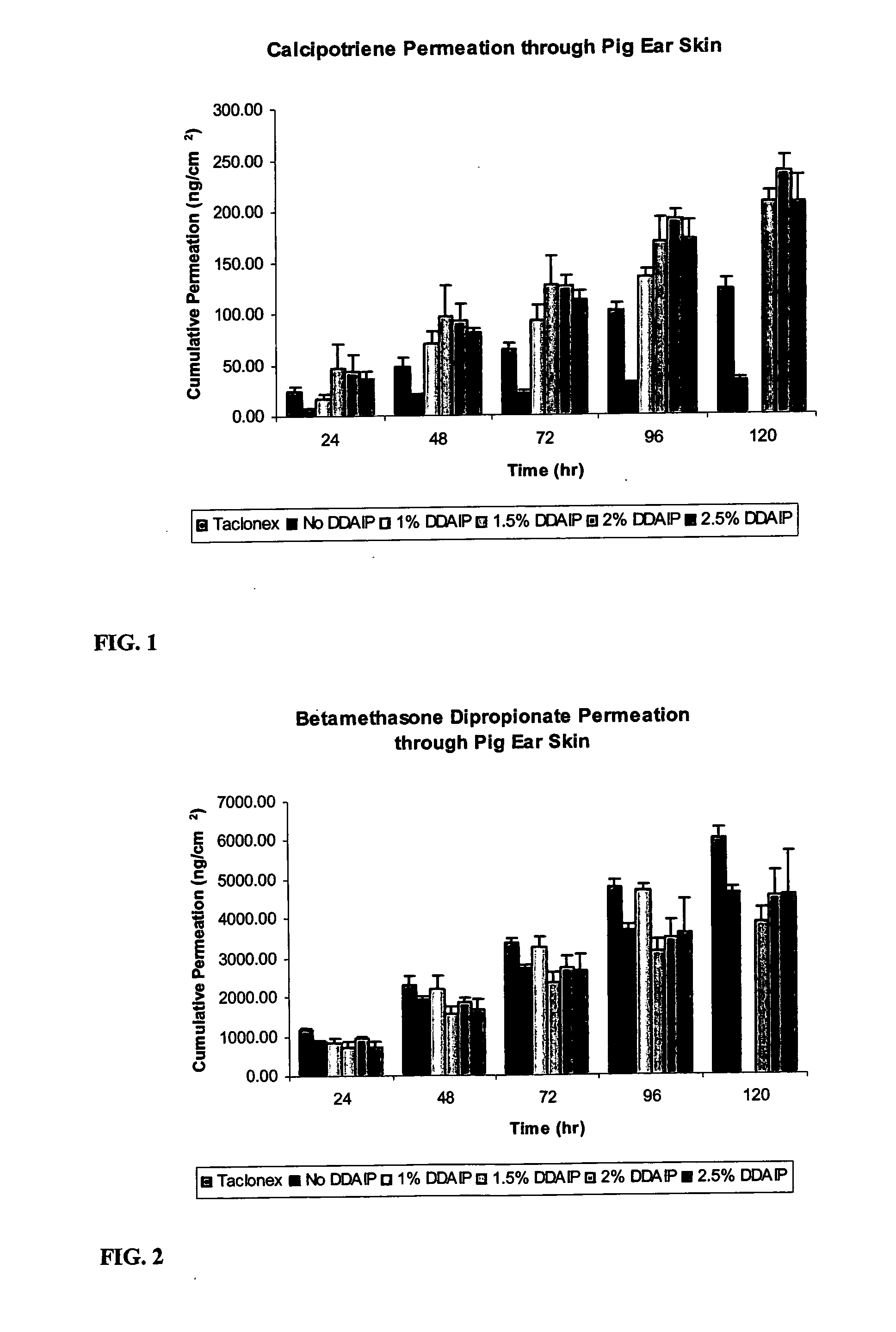
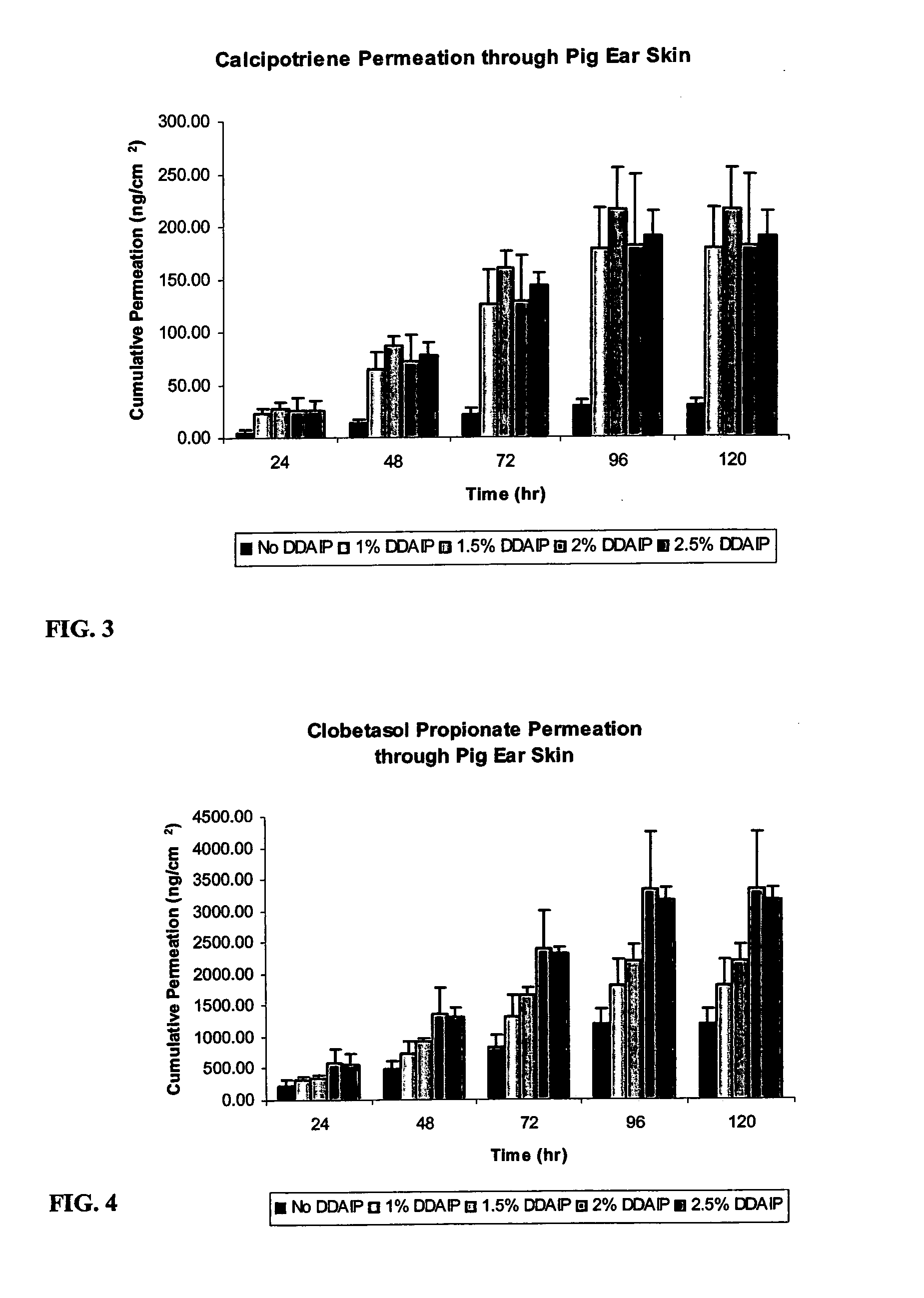
A storage stable ointment of the present invention comprises a vitamin D compound, a corticosteroid, and an N,N-di(C1-C8)alkylamino substituted, (C4-C18)alkyl(C2-C18)carboxylic ester a (C1-C4)-alkyl(C8-C22)carboxylic ester in a petrolatum ointment base, and optionally containing mineral oil and / or tocopherol. Preferably, the vitamin D compound is calcipotriene, the corticosteroid is selected from the group consisting of clobetasol propionate and betamethasone dipropionate, and the N,N-di(C1-C8)alkylamino substituted, (C4-C18)alkyl(C2-C18) carboxylic ester comprises dodecyl 2-(N,N-dimethylamino)-propionate (DDAIP).
Owner:NEXMED HLDG INC
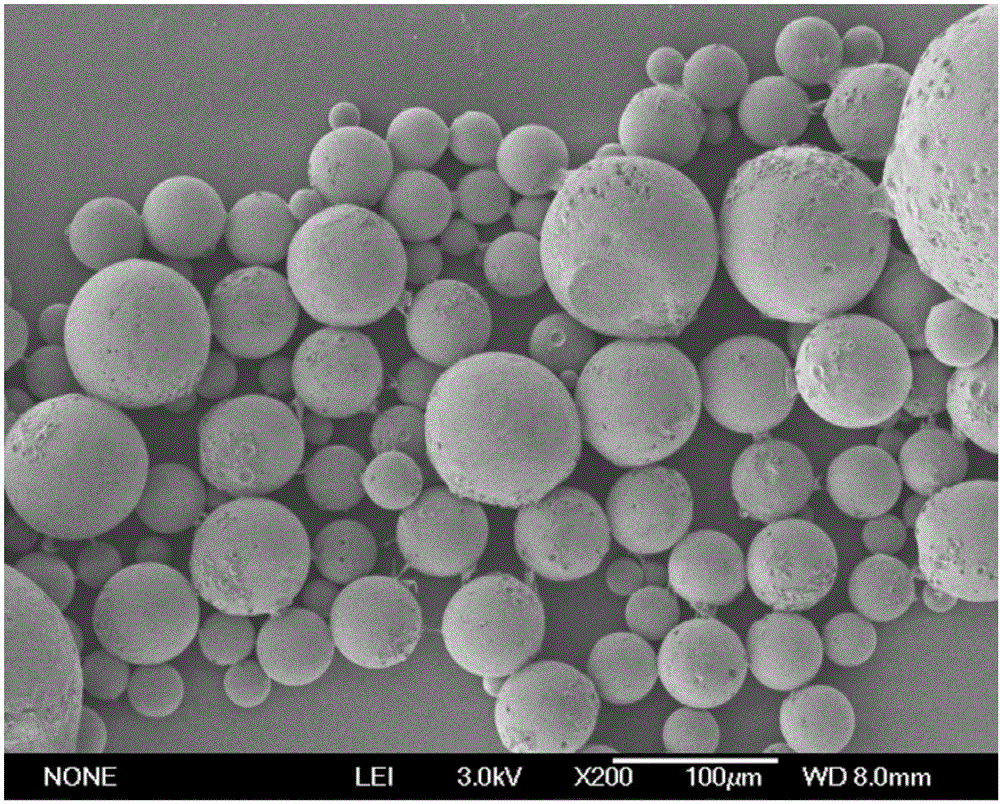
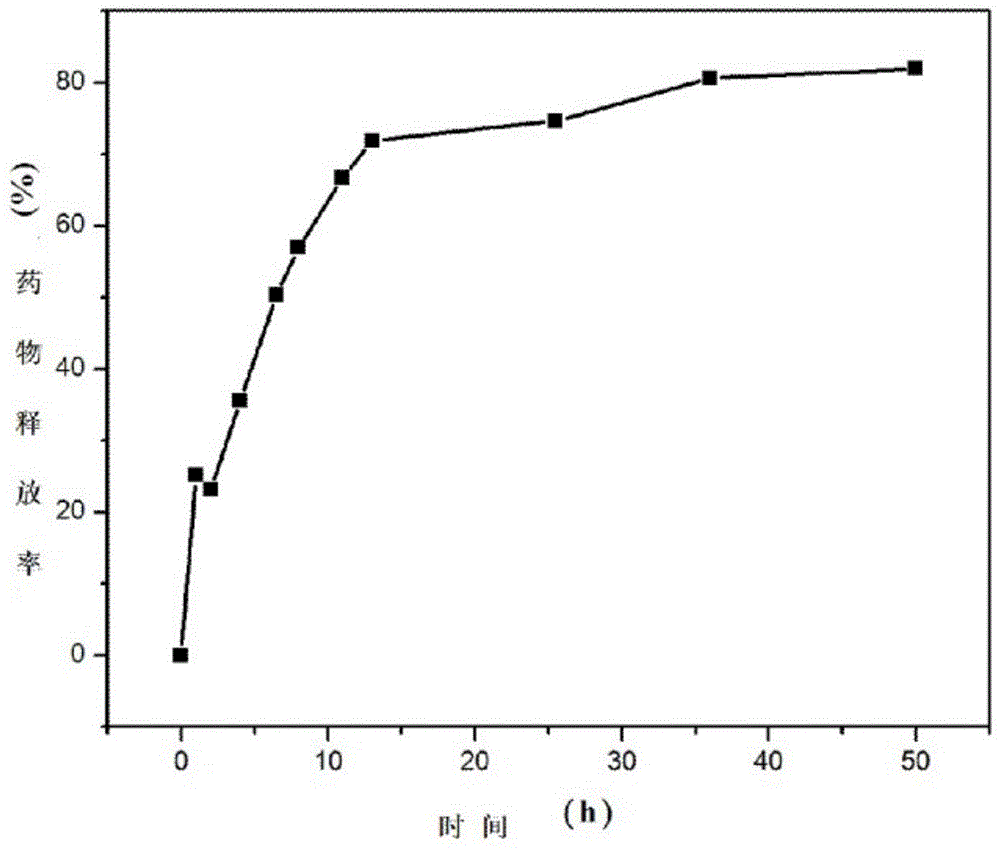
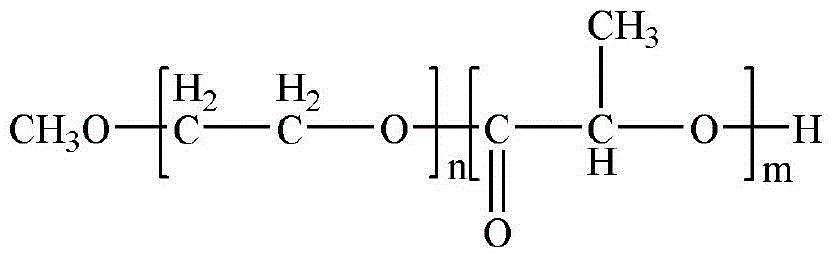
Sustained release microspheres of polyethylene glycol-polylactic acid entrapped betamethasone dipropionate and preparation method thereof
ActiveCN103599075AProlong the action timeLittle side effectsOrganic active ingredientsAntipyreticSide effectMicrosphere
The invention relates to sustained release microspheres of polyethylene glycol-polylactic acid entrapped betamethasone dipropionate and a preparation method thereof. The sustained release microspheres are prepared from degradable high-molecular biomaterial polyethylene glycol-polylactic acid entrapped betamethasone dipropionate by an O / W type emulsification-solvent evaporation process, and are used for treating inflammatory diseases. The sustained release microspheres of polyethylene glycol-polylactic acid entrapped betamethasone dipropionate, provided by the invention, can regulate the release of a drug, prolong the action time of the drug, reduce the administration times, reduce the side effects of the drug and improve the drug effect. The preparation method provided by the invention is simple and reliable, can guarantee the product quality and application effect, and facilitates industrial production.
Owner:SHANDONG UNIV
Synthetic method of beclomethasone dipropionate
The invention relates to a synthetic method of beclomethasone dipropionate. According to the method, beclomethasone 11, 17, 21-tripropionate is taken as the raw material and reacts in an organic solvent with a gluconic acid solution or an aqueous solution of ester capable of being converted into gluconic acid in water to prepare beclomethasone dipropionate.
Owner:TIANJIN JINYAO GRP
Methods and compositions for dermatological use comprsing betamethasone and biopolymers
Disclosed are compositions comprising betamethasone, such as betamethasone dipropionate or betamethasone valerate, and a biopolymer in a cream base, wherein the cream base comprises a primary and a secondary emulsifier, a waxy material, a co-solvent, a preservative, an acid, a chelating agent, a buffering agent, and water. The biopolymer comprises chitosan. The compositions disclosed herein are suitable for the treatment of dermatological conditions including but not limited to healing wounds and treatment of dermatitis.
Owner:APEX LAB PRIVATE LTD
Emulsifiable paste preparation containing alclometasone dipropionate
ActiveCN108158992AWide dispersion temperature rangeGood dispersionOrganic active ingredientsAntipyreticFast releaseMedicine
The invention belongs to the field of medicine preparations and particularly relates to a preparation process of alclometasone dipropionate. According to the preparation process, in order to solve a stability problem of alclometasone dipropionate, cetostearyl alcohol is adopted as a dispersing agent of alclometasone dipropionate serving as an active ingredient, and main medicine particles are evenly dispersed and not dissolved in a system to prepare a suspended type emulsifiable paste. The granularity D90 of the emulsifiable paste prepared by means of a formula and a method is smaller than orequal to 30 microns, and the emulsifiable paste is uniform, fine and smooth and easy to apply. Alclometasone dipropionate in a fine particular state in the prepared emulsifiable paste exists in the system; compared with a solution-type emulsifiable paste, the emulsifiable paste has the advantages that the chemical stability of main medicine is remarkably improved on the one hand, and on the otherhand, prolonging of medical effects is facilitated since medicine particles are stored and continuously released so that irritation caused to the skin since the concentration of partial medicine is excessively high due to fast release of medicine can be reduced at the same time. The process procedures are obtained by means of optimization, the operation is simple and feasible, and the process hasreproducibility and is applicable to industrial production.
Owner:CHONGQING HUAPONT PHARMA
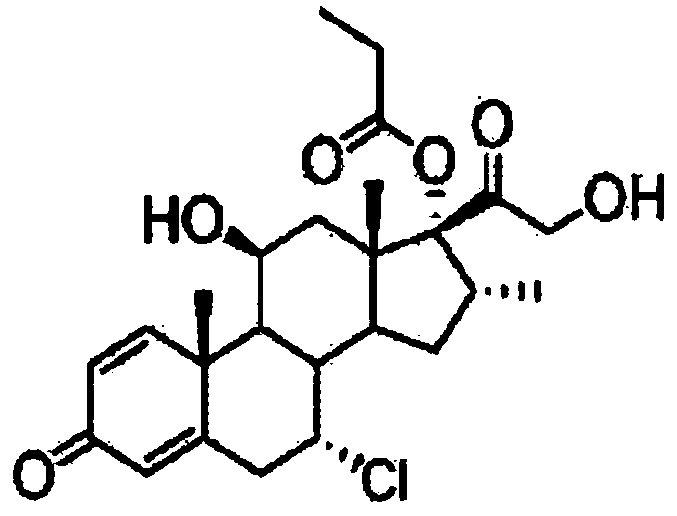
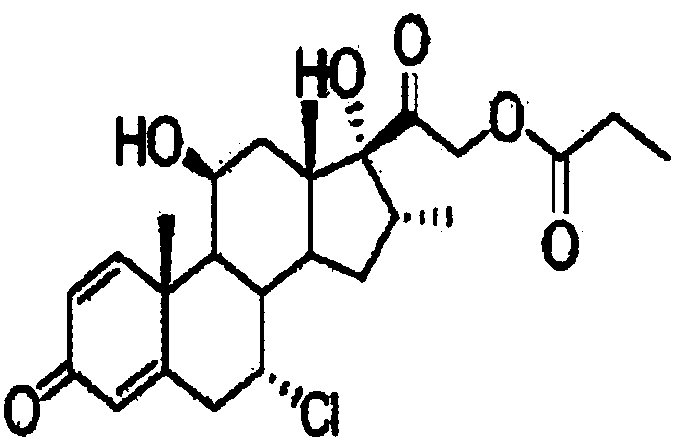
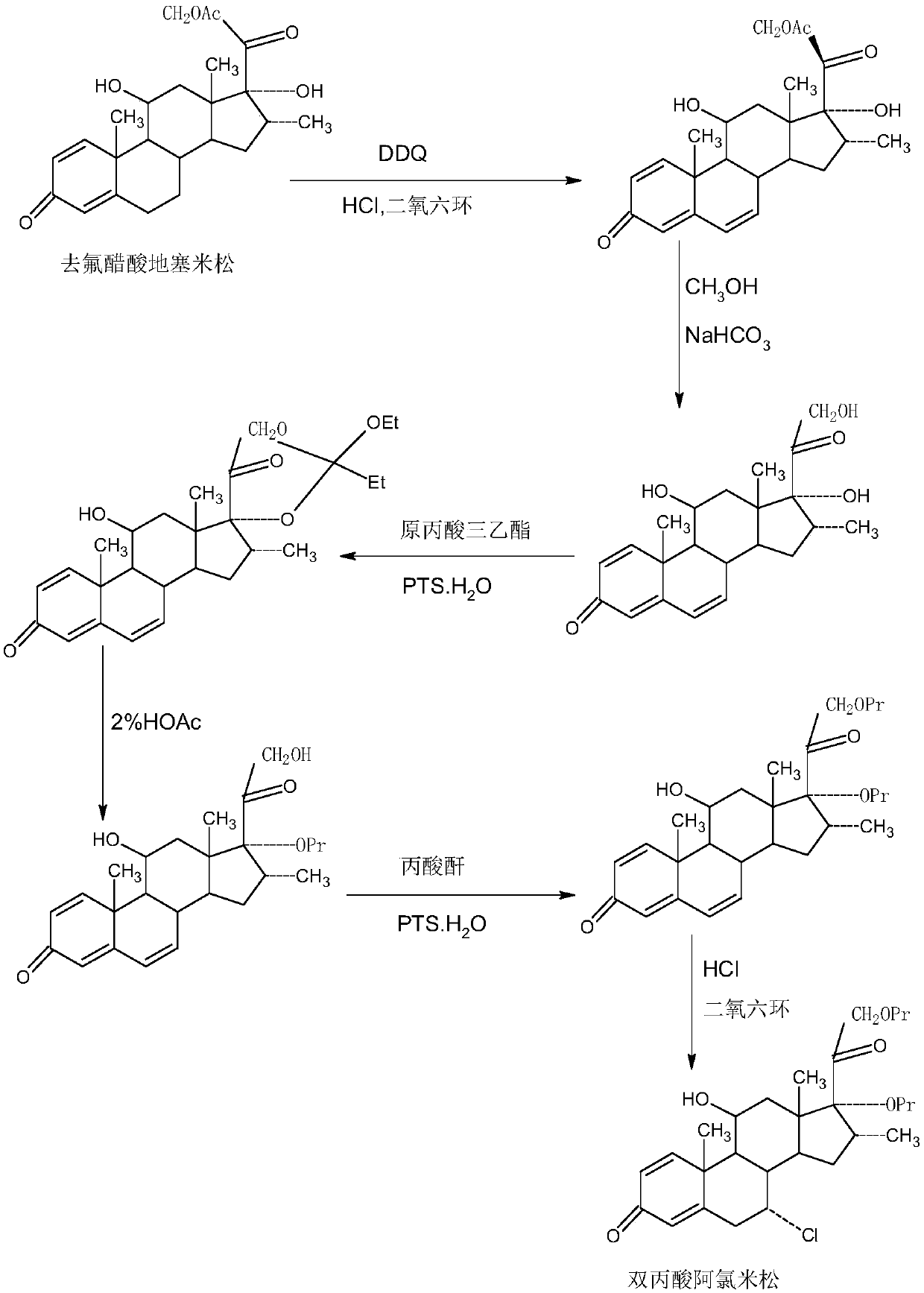
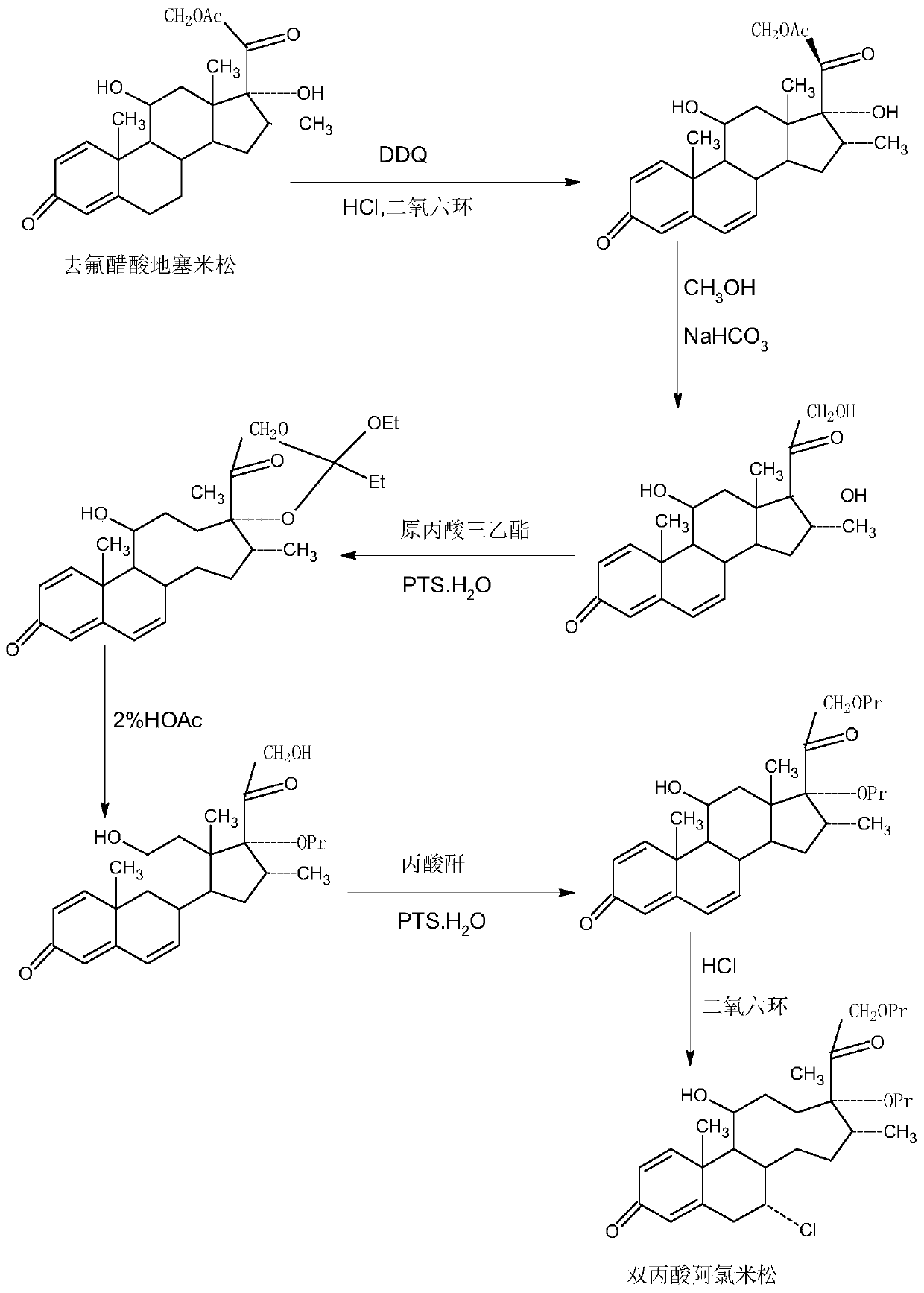
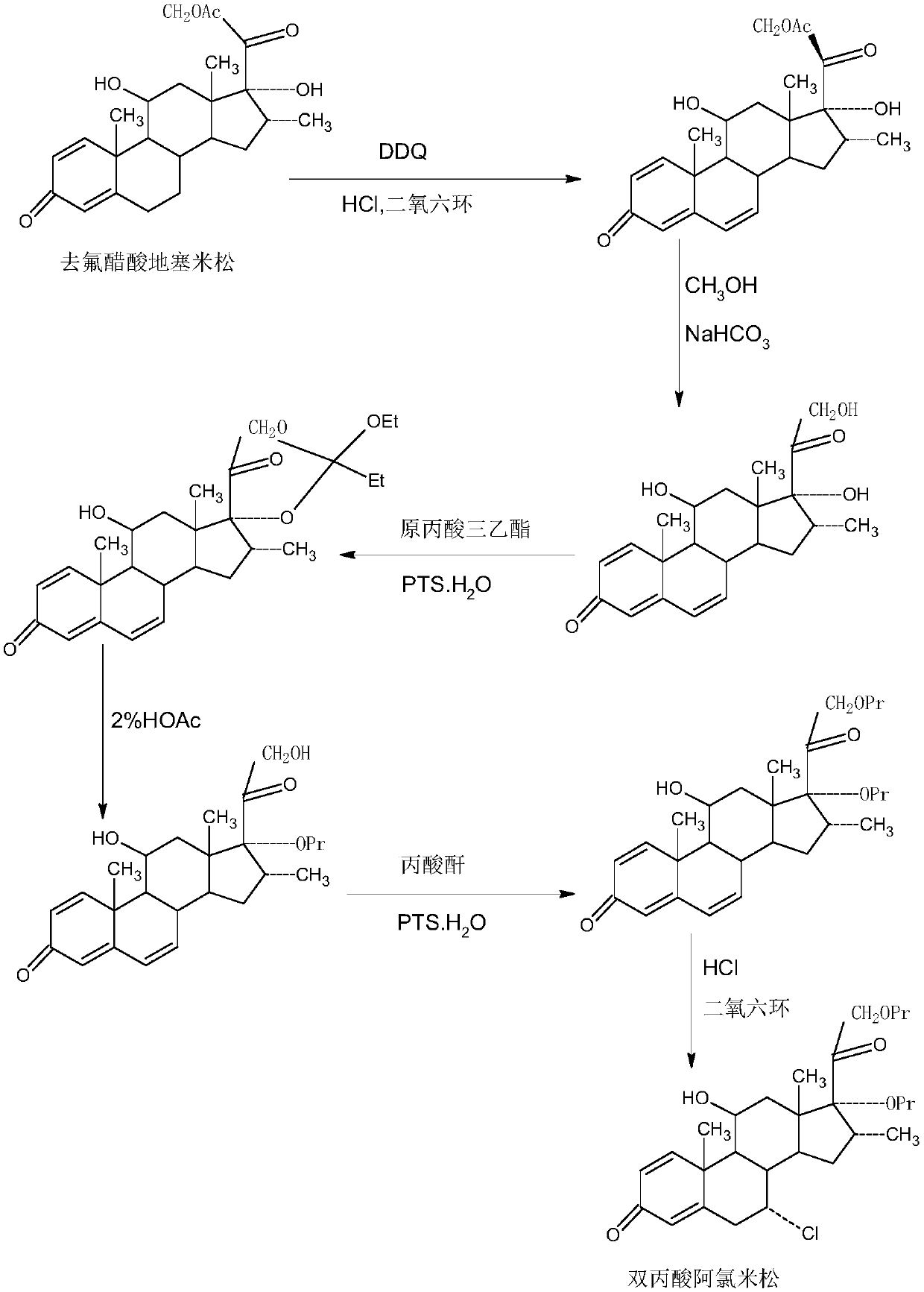
Method for preparing alclometasone dipropionate
ActiveCN109678920AShort synthetic routeProcess economy and environmental protectionSteroidsDexamethasonePropanoic acid
The invention provides a method for preparing alclometasone dipropionate. According to the method, alclometasone dipropionate is synthesized by the following seven reaction steps: esterification of 16a-methyl epihydrocortisone as a raw material with propionic acid at position 21; double-oxidization at positions 7 and 11 to obtain diketone; esterification with propionic acid at position 17; enolization and etherification protection at position 3; reduction and acid hydrolysis for deprotection with diketone at positions 7 and 11; dehydrogenation with DDQ (Dichlorodicyanobenzoquinone) at position1; and chlorine substitution at position 7. According to the method for preparing alclometasone dipropionate, the alclometasone dipropionate is synthesized by taking 16a-methyl epihydrocortisone as the raw material through seven steps; compared with a convention method with dexamethasone fluoroacetate as the raw material, the process has the advantages of short synthetic route and economy and environmental protection, simple production and operation, high product yield and the like; in the alclometasone dipropionate produced with the method, the total synthetic yield is increased from original 2.678% to 32-35%, the production cost is less than 20% of that of the traditional method; the solvent used in production can be recycled and reused; the industrial production is easy to implement.
Owner:HUNAN KEREY BIOTECH
Transdermal drug delivery system and method of using the same
A transdermal drug delivery system comprising a steroid as an active agent, wherein the steroid may be clobetasol propionate, betamethasone dipropionate, amcinonide, or loteprednol etabonate. The transdermal drug delivery system also comprises a pressure-sensitive adhesive layer and a support, wherein the steroid is present in the pressure-sensitive adhesive layer, and wherein the pressure-sensitive adhesive layer is provided on a support. The transdermal drug delivery system may be applied onto the eyelid of a patient in need thereof, in order to treat a disease of the eyelid, such as chalazion, blepharitis or meibomian gland dysfunction.
Owner:SENJU USA
Treatment of graft-versus-host disease and leukemia with beclomethasone dipropionate and prednisone
Owner:SOLIGENIX INC
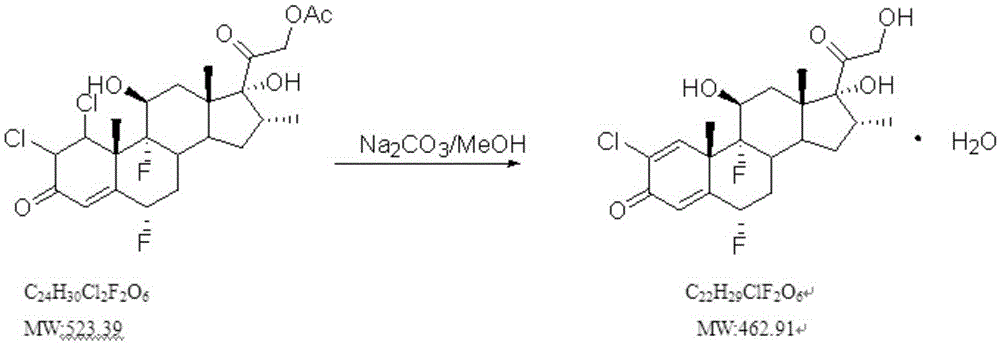
Purifying method of betamethasone phosphate
The invention discloses a purifying method of betamethasone phosphate. The method comprises the following steps: (A) adding pure water into a purifying tank, adding a crude product of betamethasone phosphate, adding a sodium hydroxide solution, and adjusting the pH value to 8.0-10.0, and waiting until the betamethasone phosphate is totally dissolved; (B) adding aluminum sulfate, stirring and measuring the pH value; if the pH value is 8.0-8.5, adjusting the pH value to 7.5-7.0 with hydrochloric acid, stirring and measuring the pH value again; if the pH value does not change, heating to 50-70 DEG C; and cooling and standing for at least 8 hours; and (C) natural filtering: after the filtrate in a filter funnel is totally filtered, checking the clarity of the filtrate, adjusting the temperature in the tank to 10-20 DEG C, adding hydrochloric acid, adjusting the pH value to 0.5-1.0, standing for at least 8 hours, and filtering to obtain pure betamethasone phosphate. The turbidity of the betamethasone phosphate purified by the method is obviously improved.
Owner:HENAN LIHUA PHARMA
Beclomethasone dipropionate emulsifiable paste
The invention discloses beclomethasone dipropionate emulsifiable paste. The beclomethasone dipropionate emulsifiable paste comprises beclomethasone dipropionate adopted as the active component, an oil-phase component, a humectant, an emulsifier, an aseptic and water, and is characterized by also comprising edetate calcium disodium.
Owner:TIANJIN JINYAO GRP
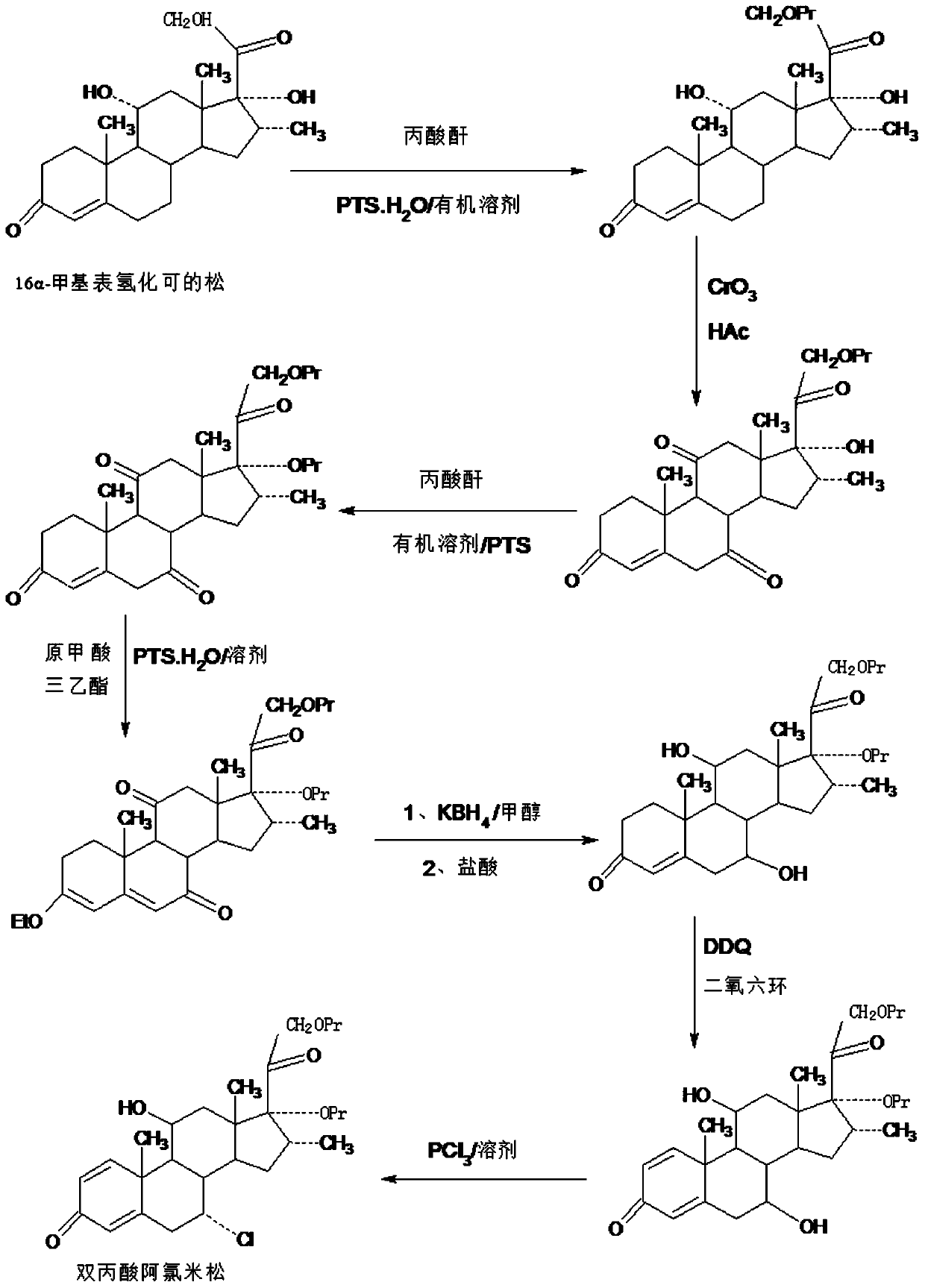
Method for preparing alclometasone dipropionate by using etherified intermediate
ActiveCN109796514AShort synthetic routeProcess economy and environmental protectionSteroidsDexamethasone acetateDehydrogenation
The invention provides a method for preparing alclometasone dipropionate by using an etherified intermediate. According to the method, the etherified intermediate 3-vinyl alcohol ether-16a-methyl-17a,21-bipropionyloxy-pregna-3,5-diene-7,11,20-triketone serves as the raw material and is reduced with 7-site and 11-site diketone and subjected to three reactions including acid hydrolysis deprotection,1-site DDQ dehydrogenation and 7-site chlorine substitution, and then alclometasone dipropionate is synthesized. According to the method, the etherified intermediate 3-vinyl alcohol ether-16a-methyl-17a,21-bipropionyloxy-pregna-3,5-diene-7,11,20-triketone serves as the raw material, alclometasone dipropionate is synthesized through three steps of reactions, compared with a traditional method using de-fluorated dexamethasone acetate as the raw material, the technology has the advantages that a synthetic route is short, the process is economical and environmentally friendly, the production operation is simple and convenient and the product yield is high; solvents used during production can be recycled, and industrial production is easy to implement.
Owner:HUNAN KEREY BIOTECH
One-step method for synthesizing halometasone from diclomethasone ethyl ester
The invention discloses a method for synthesizing halometasone from ethyl dichloroflumethasone by one step. Under the condition that inert gas is introduced, ethyl dichloroflumethasone which is taken as a raw material reacts with methanol which is taken as a solvent by one step to obtain halometasone under the action of alkali. According to the method, operation steps are simplified, the yield is increased, and the use of a toxic and harmful solvent is reduced.
Owner:HUNAN MINGRUI PHARMA
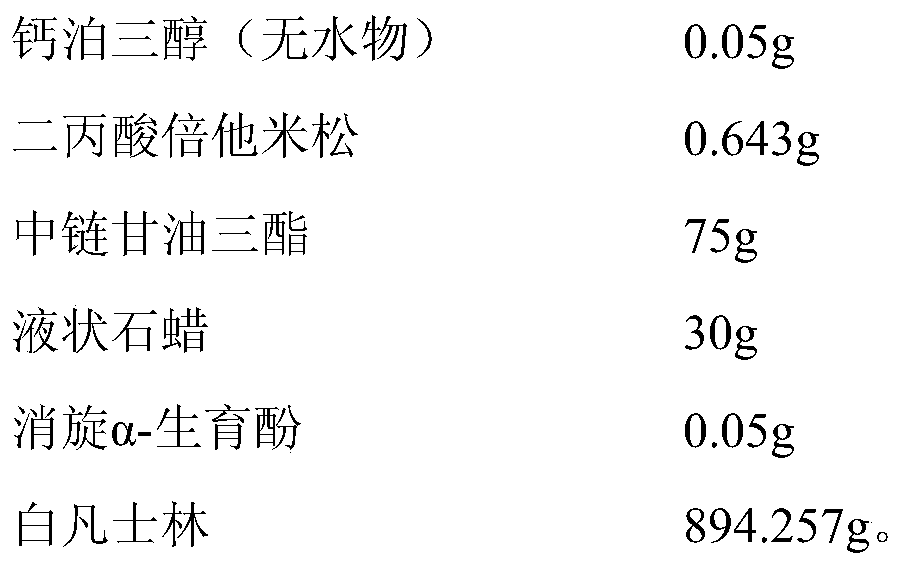
A pharmaceutical composition for external use
ActiveCN105708842BOrganic active ingredientsOrganic non-active ingredientsPropanoic acidTG - Triglyceride
Owner:SICHUAN HAISCO PHARMA CO LTD
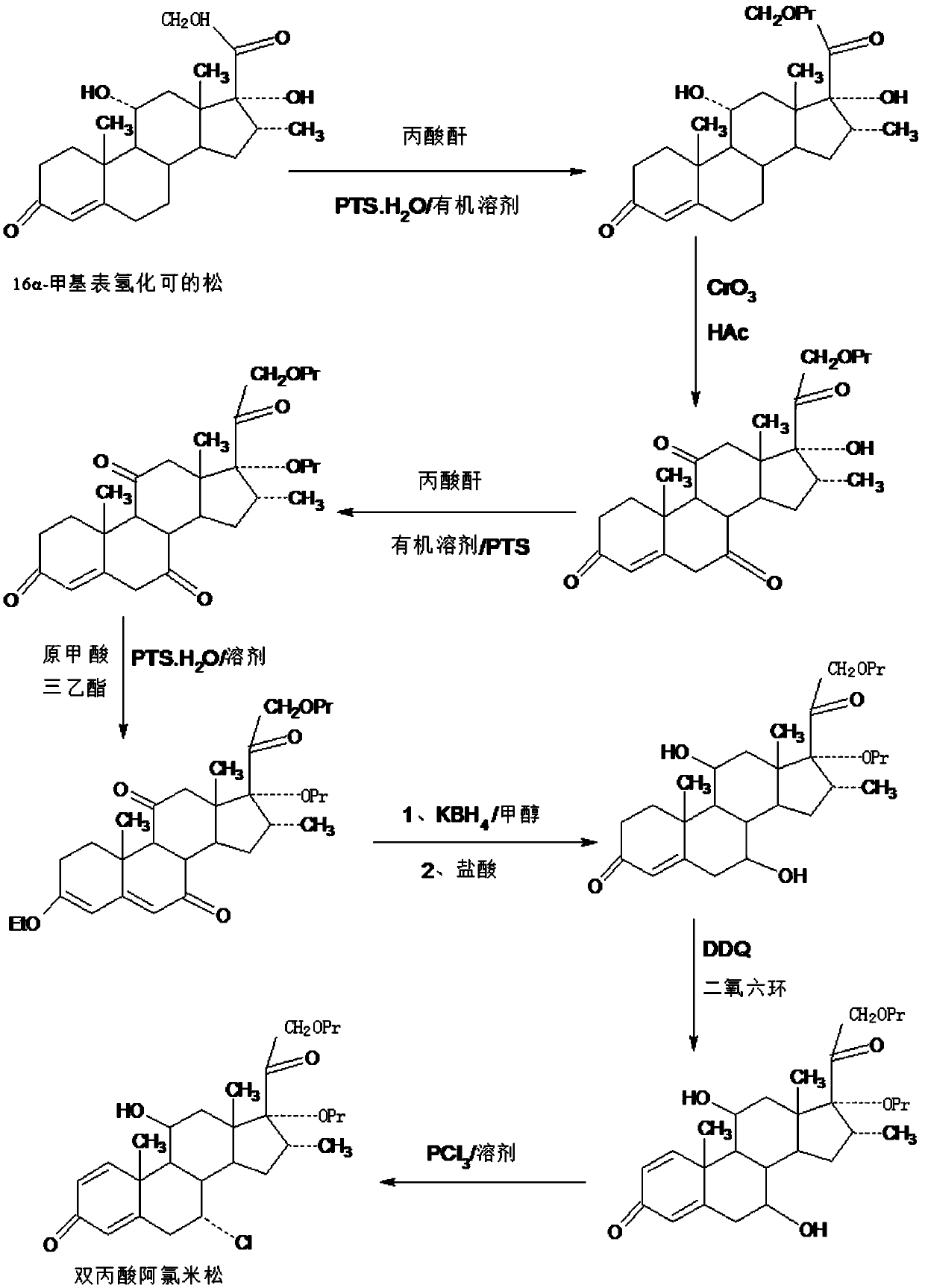
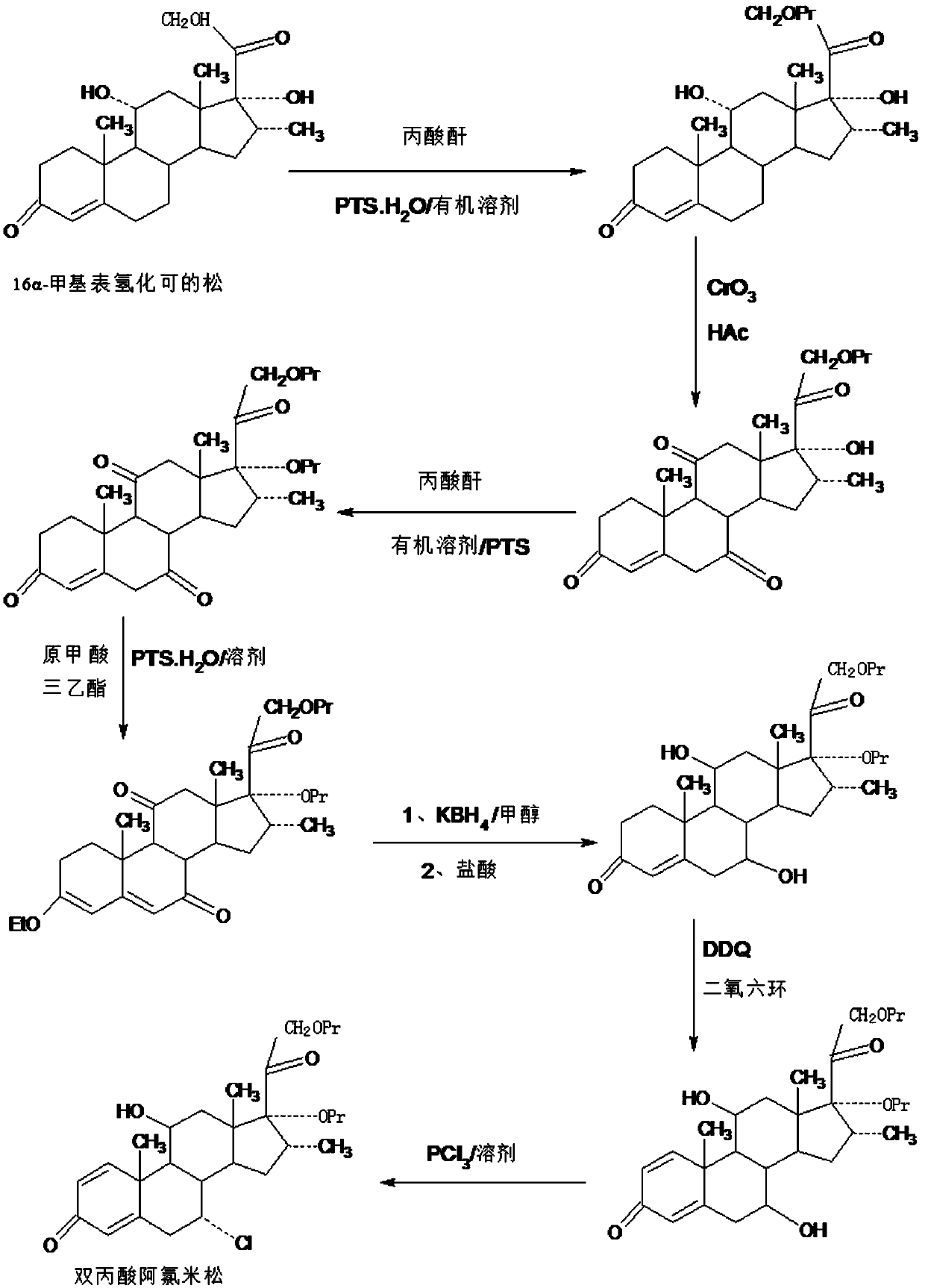
Method for preparing reduced intermediate product for aclomethasone dipropionate
ActiveCN109651476AShort synthetic routeProcess economy and environmental protectionSteroidsDiketonePropanoic acid
The invention provides a method for preparing a reduced intermediate product for aclomethasone dipropionate. The method comprises the steps that with 16a-methyl pihydrocortisone as a raw material, a crude reduced intermediate product for aclomethasone dipropionate is synthesized through a five-step reaction of 21-site propionic acid esterification, 7,11-site double-oxidation into diketone, 17-sitepropionic acid esterification, 3-site enolization etherification protection and 7,11-site diketone reduction and acid hydrolysis deprotection, then the reduced intermediate product for aclomethasonedipropionate is obtained through refining. By means of the method, with 16a-methyl pihydrocortisone as the raw material, the reduced intermediate product for aclomethasone dipropionate is synthesizedthrough the five-step reaction; the process has the advantages of being short in synthesis route, economical, environmentally friendly, simple in production operation, high in product yield and the like; a solvent used in production can be recycled and applied, and industrial production is easy to implement.
Owner:HUNAN KEREY BIOTECH
A kind of beclomethasone dipropionate cream
A beclomethasone dipropionate cream contains beclomethasone dipropionate as an active ingredient, an oil phase component, a moisturizing agent, an emulsifier, a preservative and water, and is characterized in that it also contains calcium sodium edetate.
Owner:TIANJIN JINYAO GRP
Medicinal composition for treating psoriasis
InactiveCN1210034CSignificant effectLow incidence of adverse reactionsOrganic active ingredientsDermatological disorderCurative effectAdrenal gland
The invention provides a pharmaceutical composition for treating psoriasis, and its preparation forms include ointment and gel. The pharmaceutical composition contains 0.05%-0.5% (w / w) of tazarotene, 0.05%-2.5% (w / w) of adrenocorticosteroids and other pharmaceutically acceptable auxiliary agents. The adrenocortical hormone drug is preferably betamethasone dipropionate. The pharmaceutical composition of the invention has remarkable curative effect on psoriasis and good safety.
Owner:CHONGQING HUAPONT PHARMA
Aerosol medicine composition containing beclomethasone dipropionate
Owner:TIANJIN JINYAO GRP
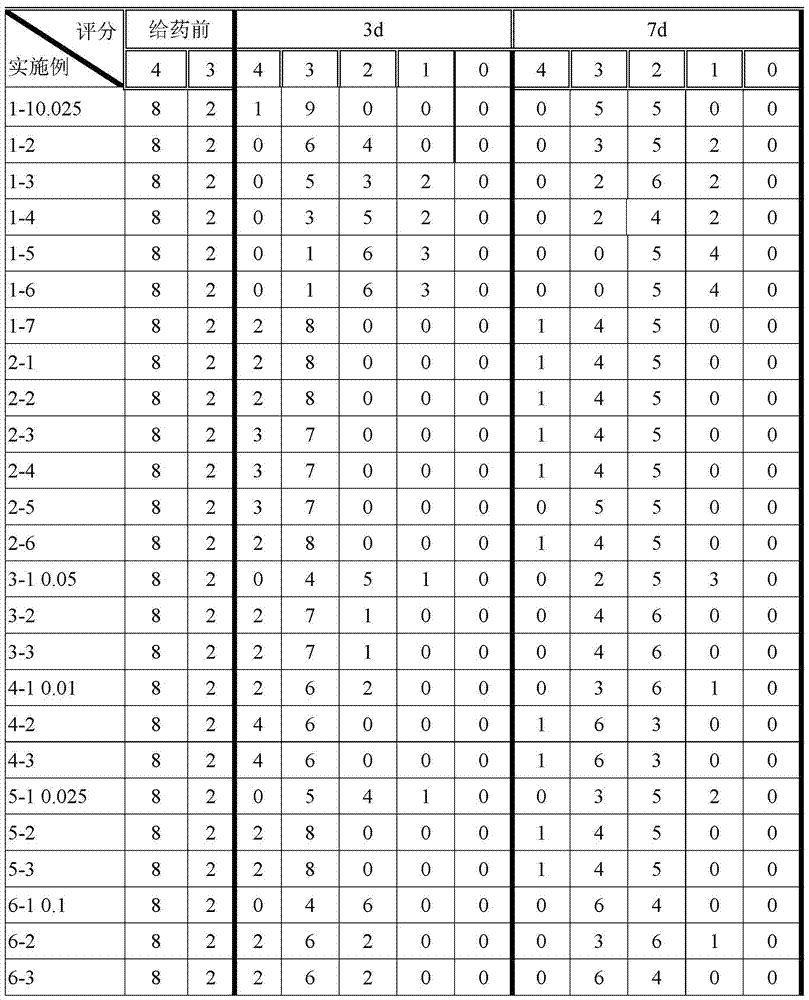
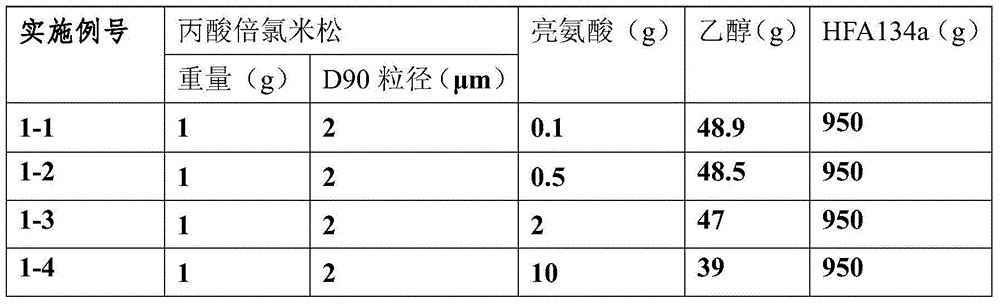
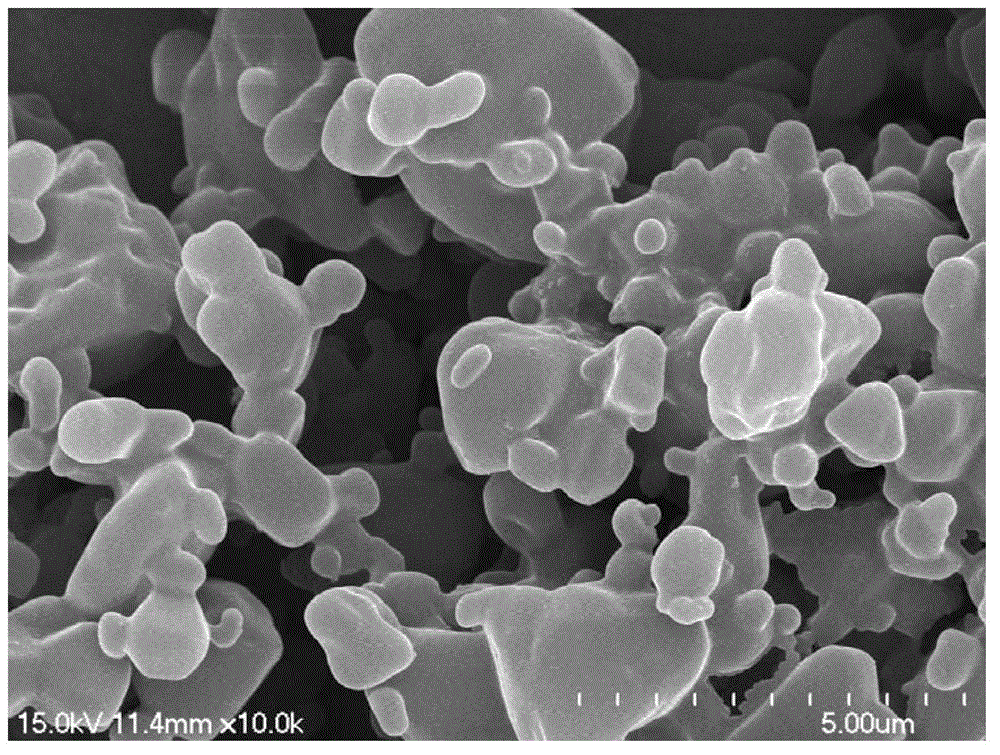
Betamethasone dipropionate nano freeze-dried powder injection and preparation method thereof
ActiveCN113057943AEffectively eliminate degradation reactionsExclude degradation reactionsPowder deliveryOrganic active ingredientsPeristaltic pumpPropanoic acid
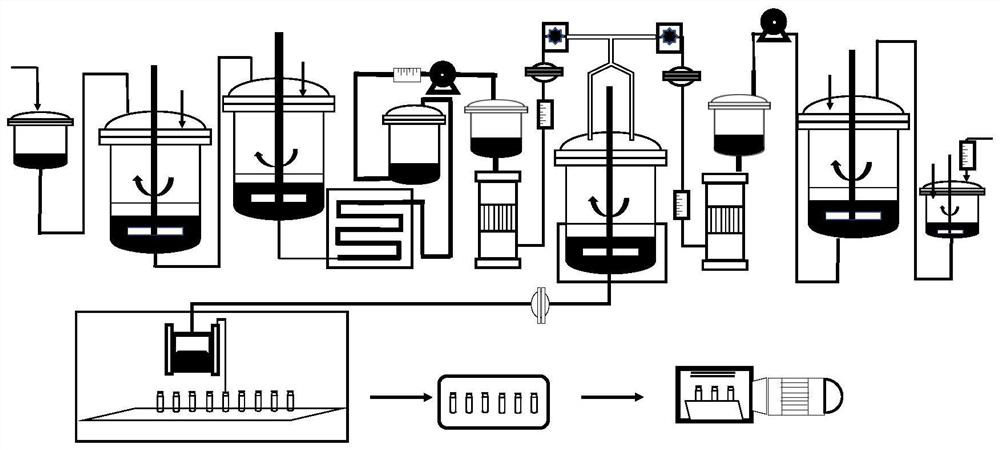
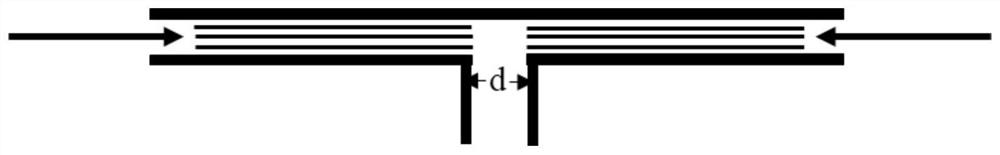
The invention discloses a betamethasone dipropionate nano freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection comprises the following components in percentage by weight: 6%-12% of betamethasone dipropionate, 20%-54% of a supporting agent, 11%-37% of a suspending aid and 21%-50% of a freeze-drying protective agent. The preparation method comprises the following steps: taking a good solvent containing betamethasone dipropionate and a supporting agent as a good solvent phase, taking an aqueous solution containing a suspending aid and a freeze-drying protective agent as an anti-solvent phase, respectively pumping out the good solvent phase and the anti-solvent phase through a peristaltic pump, colliding with fluid in the micro-channel, collecting in a container, stirring, and performing microfiltration, filling, pre-freezing and freeze-drying to obtain the product. The product prepared by the invention is small in active drug particle size and loose in texture, and can be quickly dissolved after being added with water to recover the original characteristics of the liquid medicine; the product improves the curative effect of the medicine, expands the range of dosage forms, is convenient to store and transport, and fully meets the requirements of storage and treatment.
Owner:SOUTHEAST UNIV
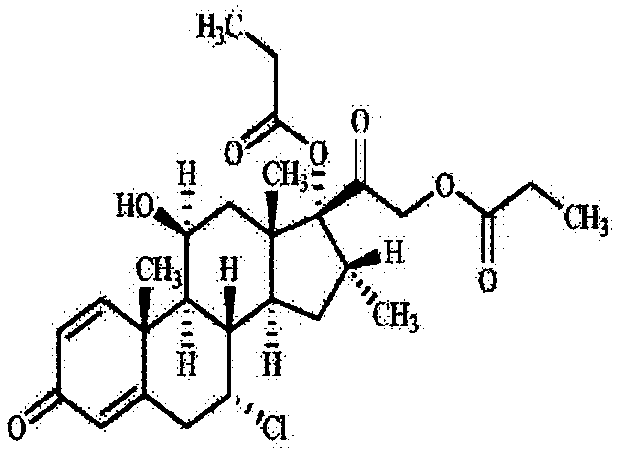
Preparation method of betamethasone dipropionate
PendingCN112851734ASimple preparation processModerate reaction temperatureSteroidsBulk chemical productionChemical synthesisPropanoic acid
The invention belongs to the technical field of chemical synthesis, and particularly relates to a preparation method of betamethasone dipropionate. According to the method, betamethason-17-propionate is taken as a raw material, propionic anhydride is taken as a raw material, under the condition that 4-dimethylaminopyridine is taken as a catalyst, betamethasone dipropionate is generated through reaction, and high-purity betamethasone dipropionate is obtained through refining by adopting absolute ethyl alcohol, dichloromethane and normal hexane. The preparation process is simple, the reaction temperature is moderate, and ultralow-temperature or high-temperature reaction is avoided; the solvent or reagent used in the preparation is cheap and easy to obtain, convenient to charge and easy to transport and store; the method has the advantages of small dosage of reaction catalyst, high yield, less generated waste liquid, environmental friendliness and suitability for commercial production.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Container system and pharmaceutical foam composition comprising betamethasone
The present invention relates to a pharmaceutical foam composition comprising a corticosteroid and a Vitamin D analogue for topical administration to a patient in need thereof, such as for the treatment of plaque psoriasis. The present invention also relates to a process for preparing the composition and a suitable container system for administration of the composition. Preferably, the invention relates to the topical administration of betamethasone dipropionate and calcipotriene with one or more pharmaceutically acceptable excipients and propellants where the composition is stable in the container system coated with a coating material selected from the group comprising of epoxyphenol resin, modified polyesters, microflex coating or polyacrylates.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Nasal formulation
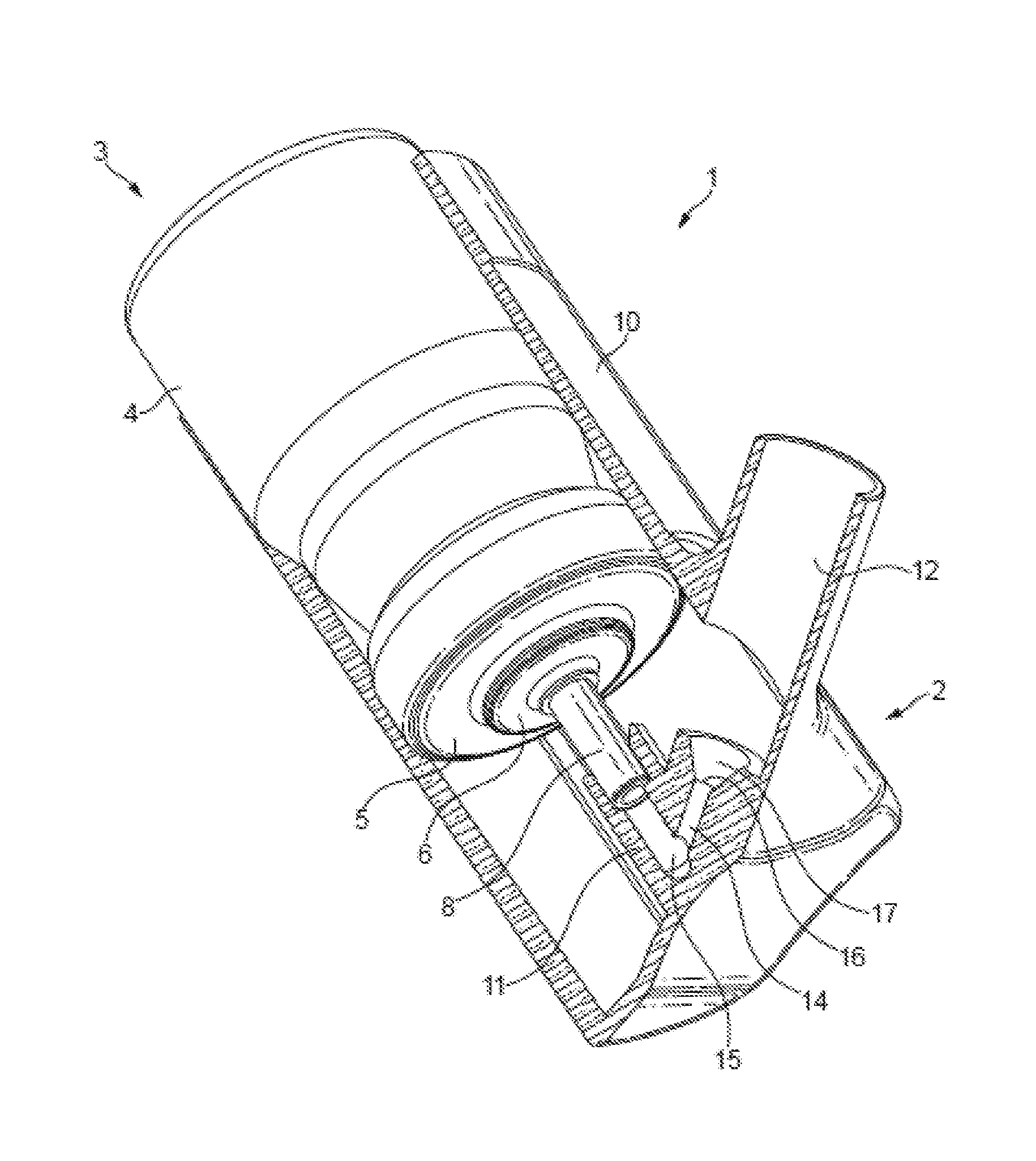
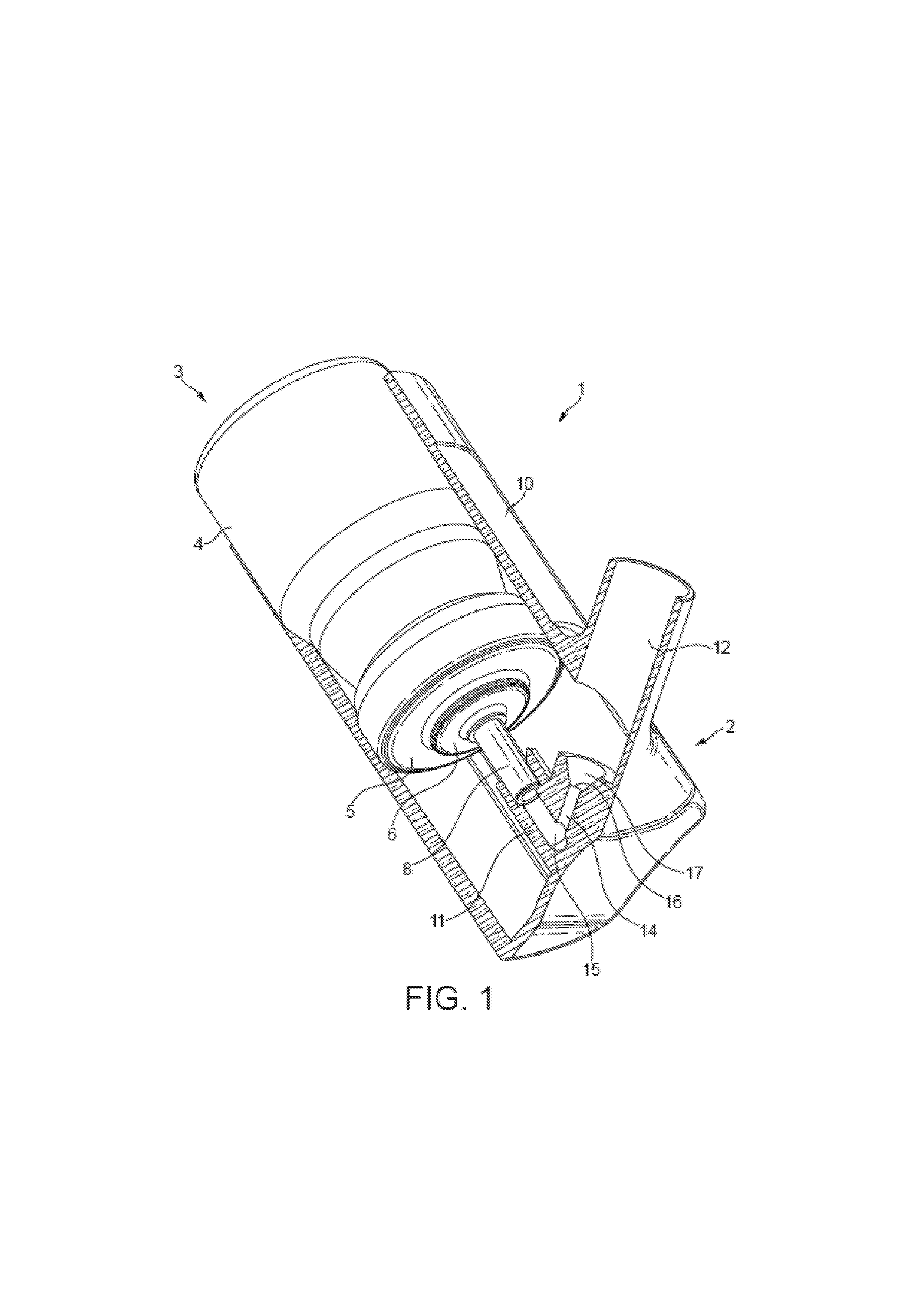
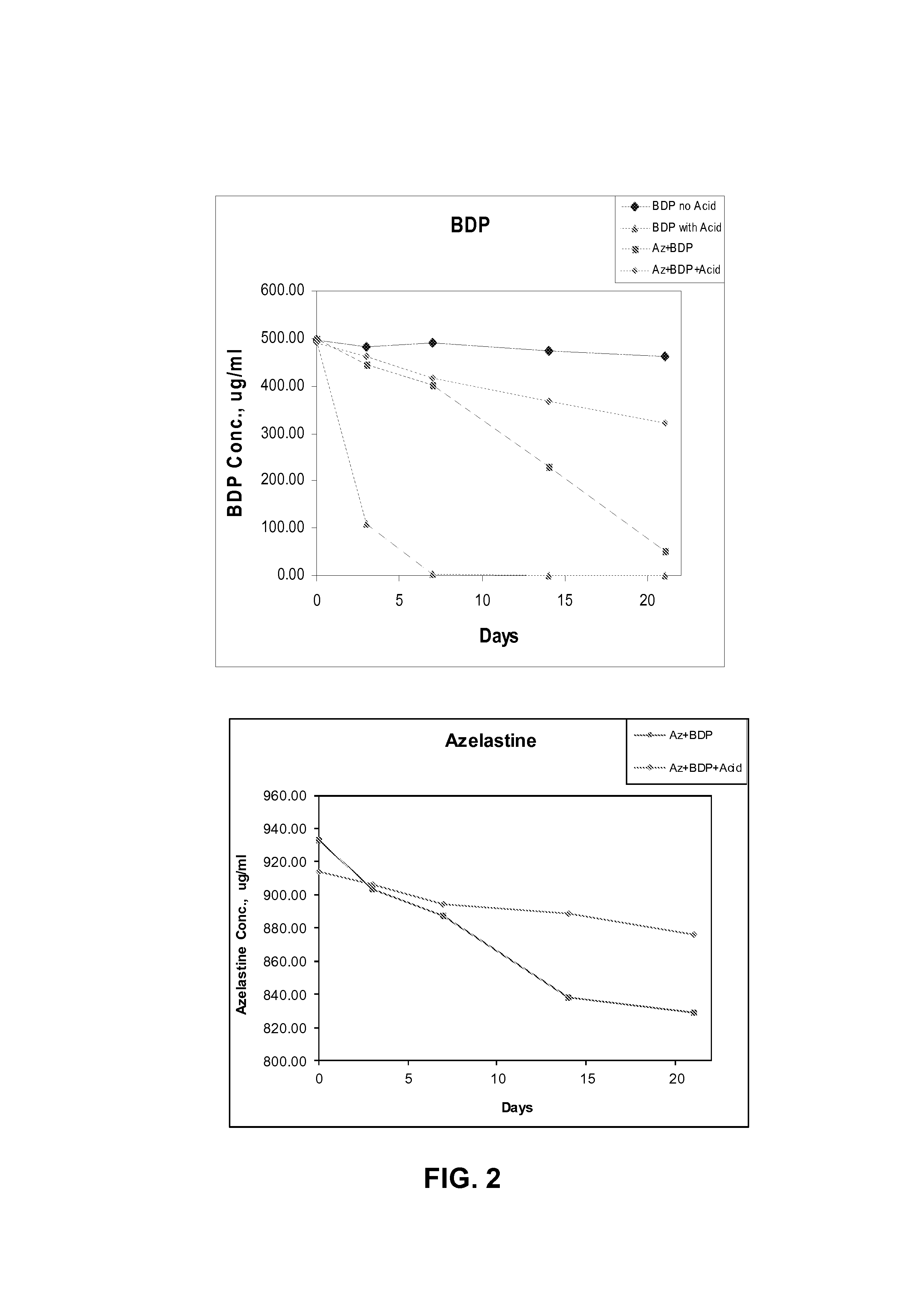
This invention relates to a solution formulation for nasal administration comprising azelastine, beclomethasone dipropionate, a co-solvent, an acid and an HFA propellant.
Owner:TEVA BRANDED PHARMA PROD R & D
Method for preparing betamethasone dipropionate atomization inhalant
InactiveCN102973539AShort duration of actionHigh lung deposition rateOrganic active ingredientsSolution deliveryDispersion stabilitySide effect
The invention relates to the field of medical products, in particular to a method for preparing a betamethasone dipropionate atomization inhalant. The method comprises the following steps: dispersing betamethasone dipropionate into water to obtain suspension, homogenizing, sterilizing, and thus obtaining the betamethasone dipropionate atomization inhalant, wherein the homogenizing pressure is 200 to 2,000 bar. The preparation method is green and environment-friendly, simplifies the operation steps, can realize continuous operation, and is short in acting time, sterile, pollution-free and suitable for industrialized production. The prepared betamethasone dipropionate atomization inhalant has the advantages of high dispersion stability, high lung deposition rate, low medicament consumption, high bioavailability and low toxic or side effect.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com