Pharmaceutical aerosol formulations of formoterol and beclometasone dipropionate
a technology of beclometasone and formoterol, which is applied in the direction of organic active ingredients, respiratory disorders, medical atomizers, etc., can solve the problems of ostwald ripening, the effect of reducing the solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
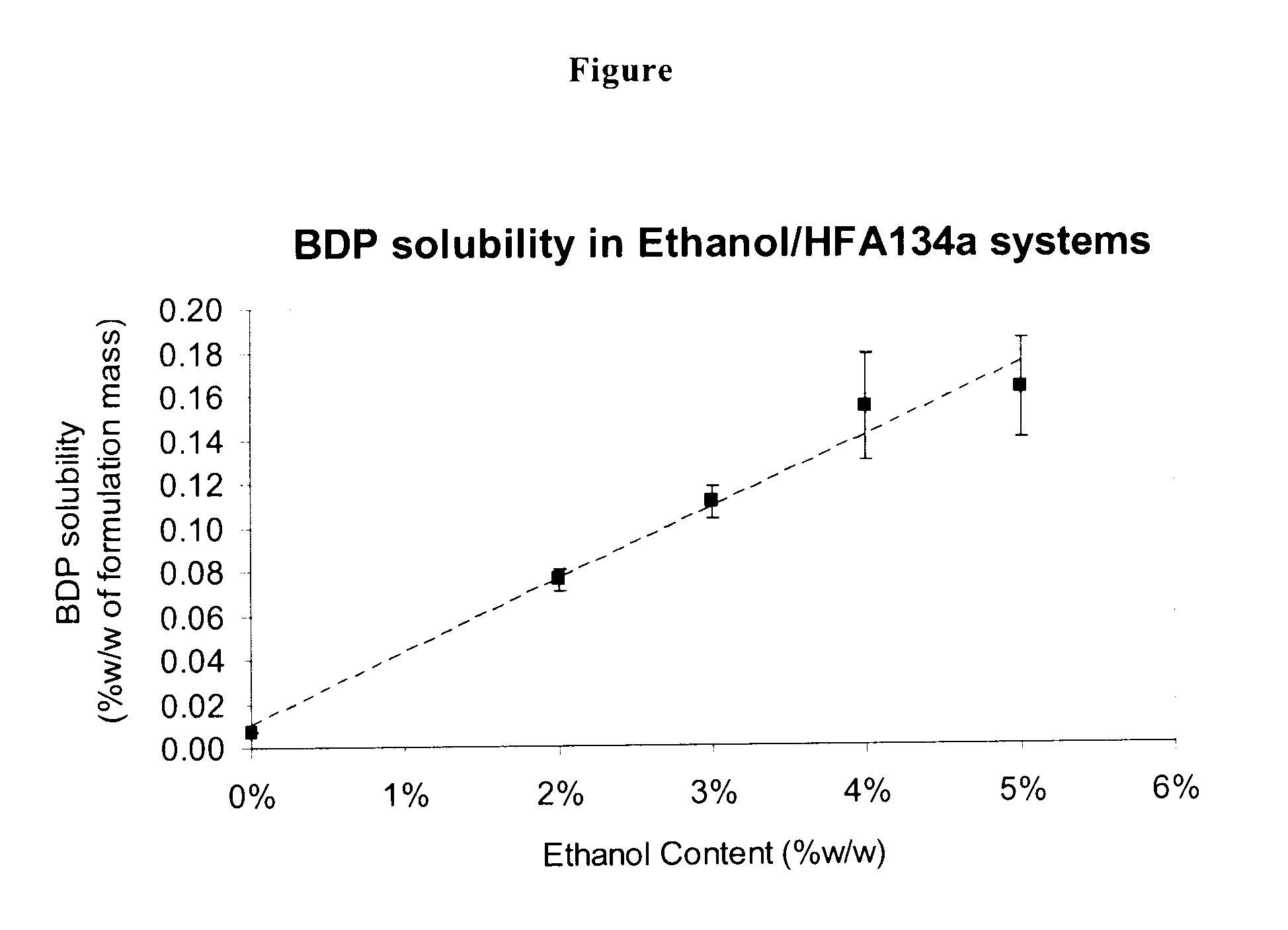
[0111]The solubility determination of BDP in the HFA134a / ethanol mixtures was determined according the method reported in Gupta A et al, J. Aerosol Medicine, 2003, 16(2), 167-174, slightly modified as follows.
[0112]Vials that contained excess BDP were prepared at 2%, 3%, 4% and 5% ethanol in HFA134a. After equilibration, the samples were filtered though a 0.2 μm PTFE filter coupled inline with Presspart standard C126 canisters fitted Bespak EPDM with a dip tube. The results are reported in the plot of FIG. 1, from which the BDP solubility can be extrapolated.
example 2
[0113]The solubility of formoterol fumarate dihydrate in HFA134a:ethanol 97.3:2.7 (w / w) in the presence and in the absence of 0.1% w / w BDP was estimated according to the method of Example 1. The formoterol fumarate dihydrate solubility at 20° C. without BDP turned out to be about 0.005 μg / μl corresponding to about 0.0005% w / w. After addition of 50 μl of 0.1 w / w BDP solution, it decreases more than half, i.e. to 0.002 μg / μl corresponding to about 0.0002% w / w.
example 5
[0116]An aerosol formulation was prepared starting from micronized formoterol fumarate dihydrate obtained by milling having a MMD comprised between 2 and micron and beclometasone dipropionate as commercially available. Said formulation has the following composition:
Formoterol fumarate dihydrate0.0095% w / wBeclometasone dipropionate 0.079% w / wEthanol 2.7% w / wHFA134to 100%
This formulation was filled into an aluminum canister under pressure and fitted with a metering valve having a 63 μl metering chamber. It is suitable for delivering 6 μg formoterol and 50 μg beclometasone dipropionate per actuation.
[0117]The aerosol performances were assessed using an Andersen Cascade Impactor according to according to the procedure described in the European Pharmacopoeia 6th edition, 2009 (6.5), part 2.09.18. Quantification of beclometasone dipropionate (BDP) and formoterol fumarate dehydrate (FF) was performed using a HPLC method. The following parameters were determined:
[0118]i) delivered dose is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dielectric constant εm | aaaaa | aaaaa |
| dielectric constant εm | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com