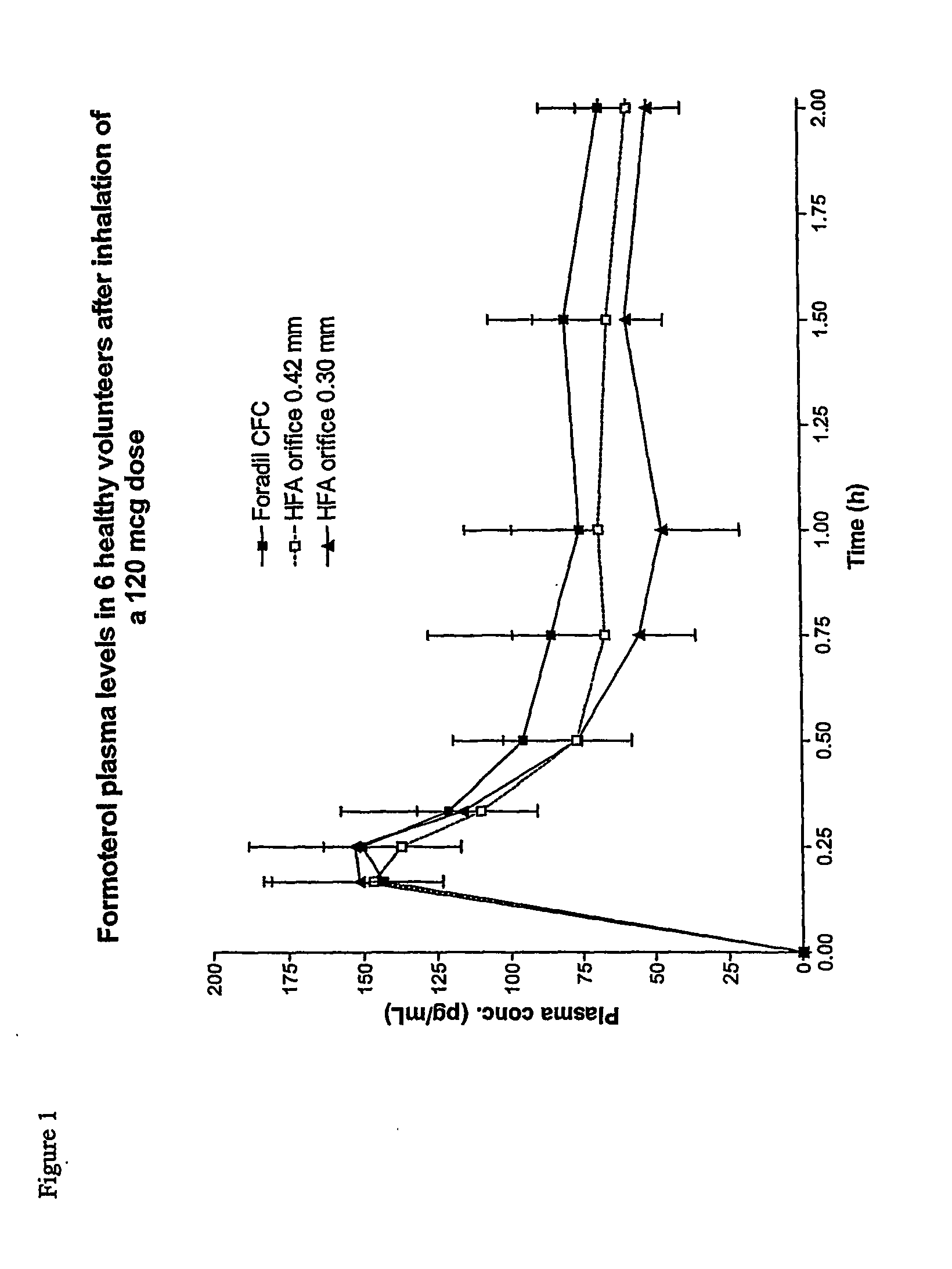
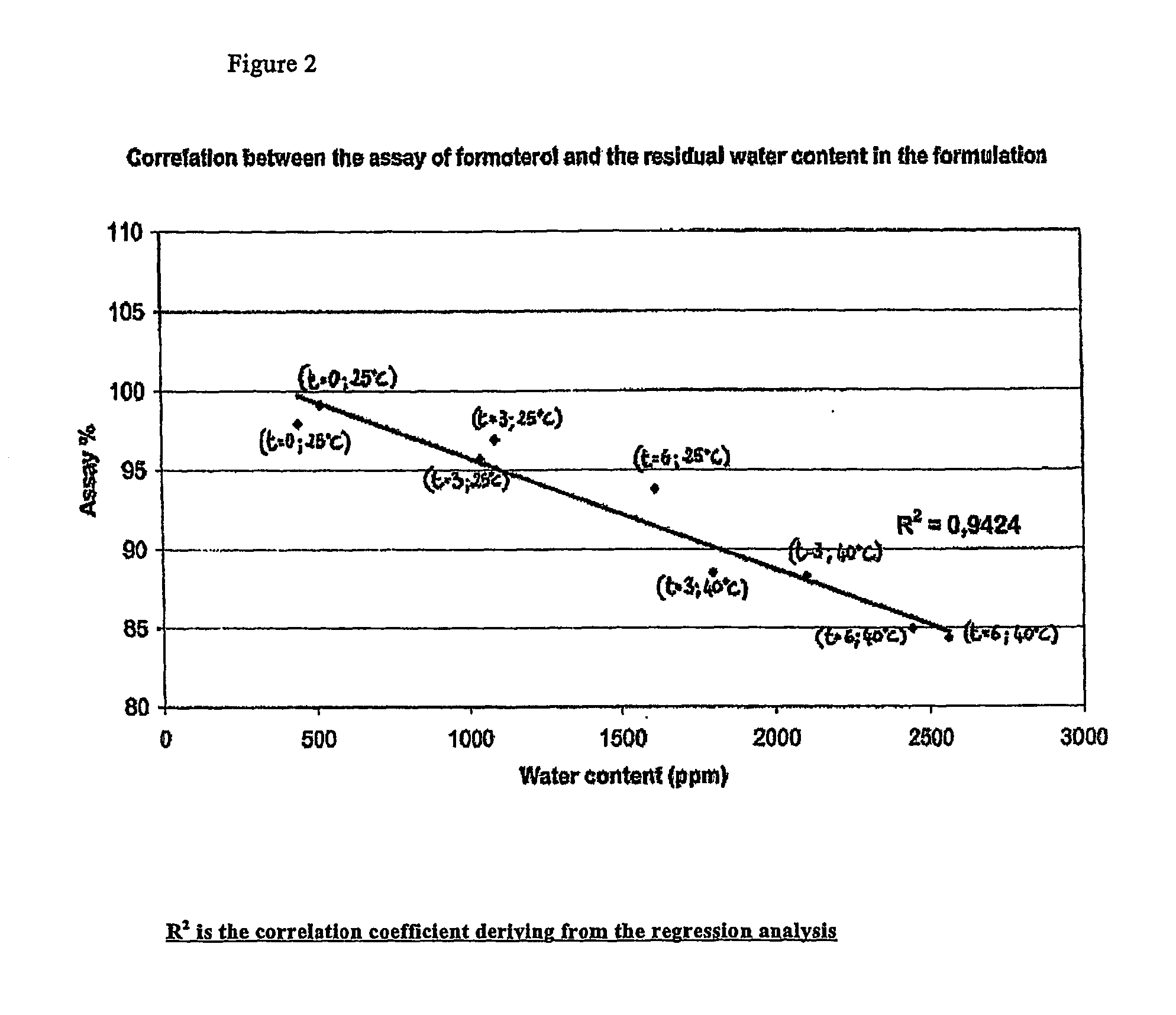
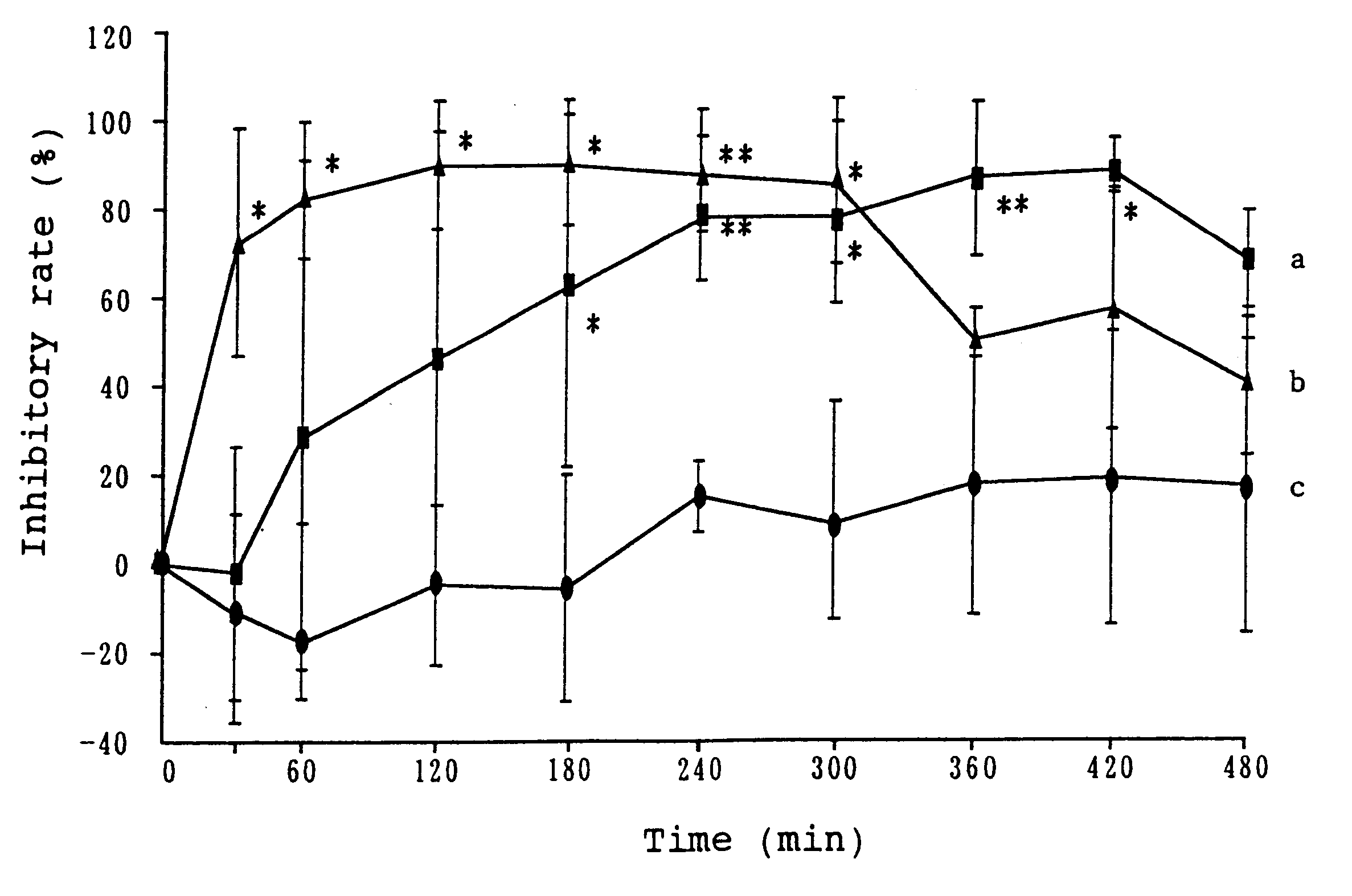
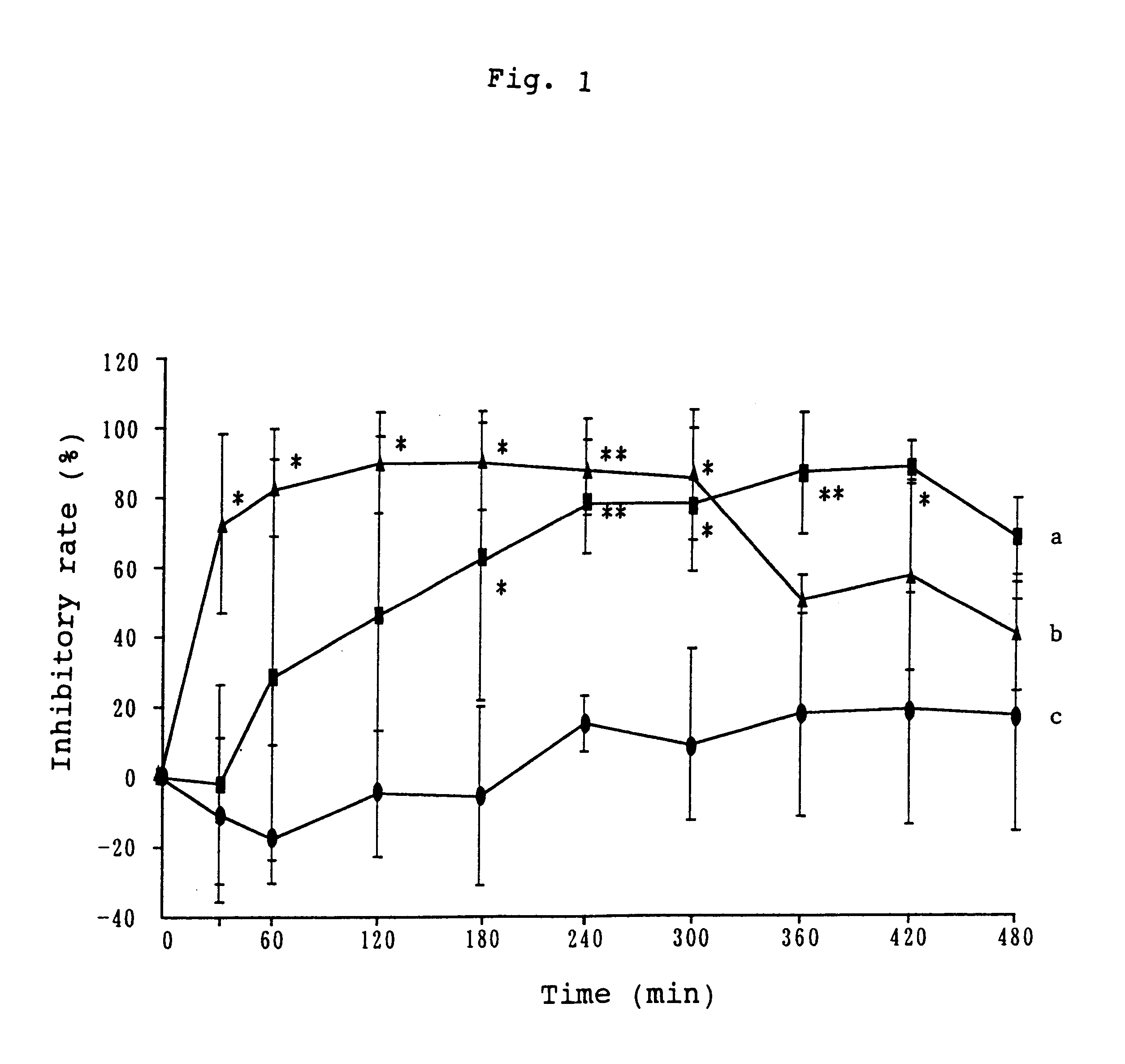
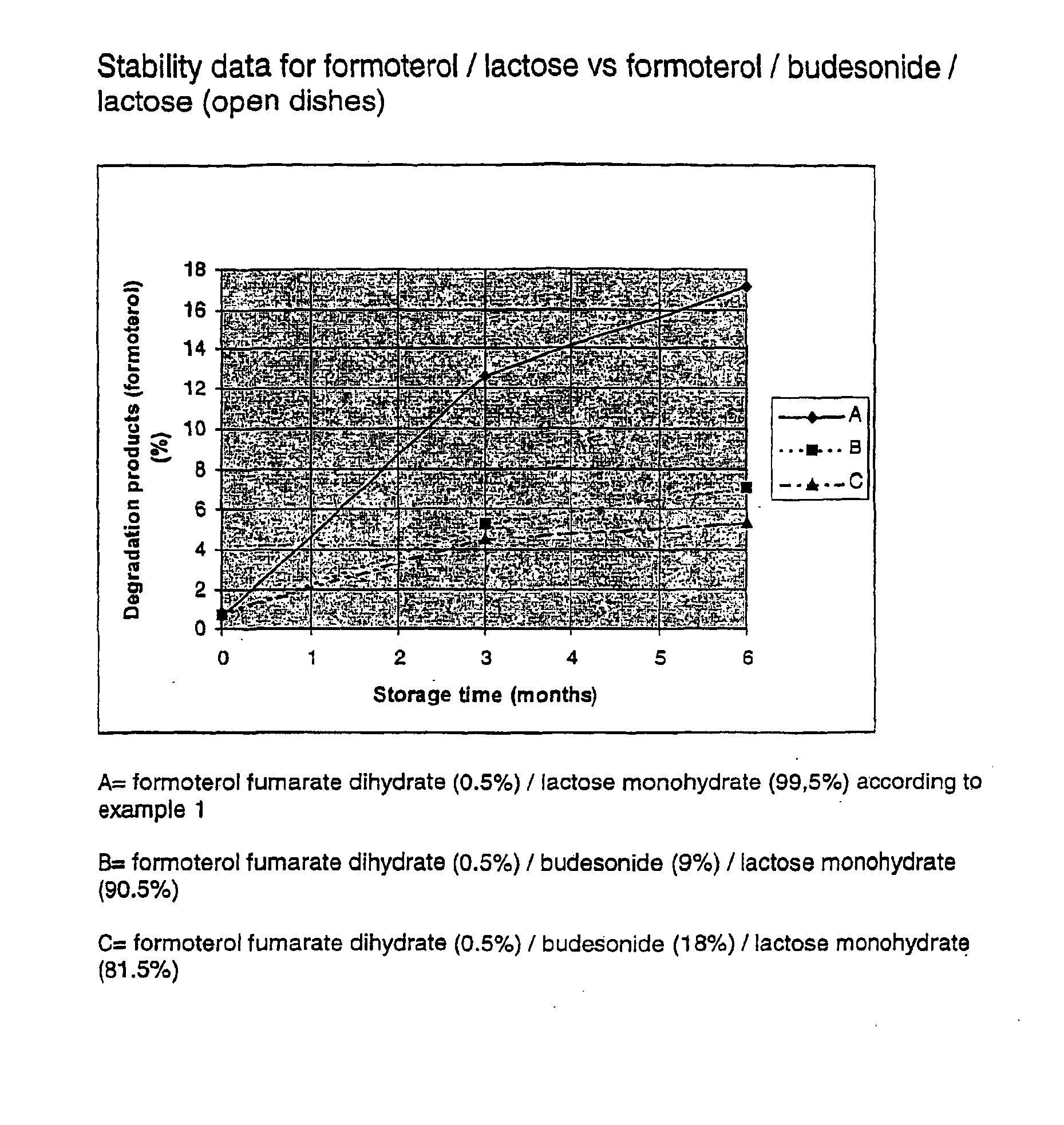
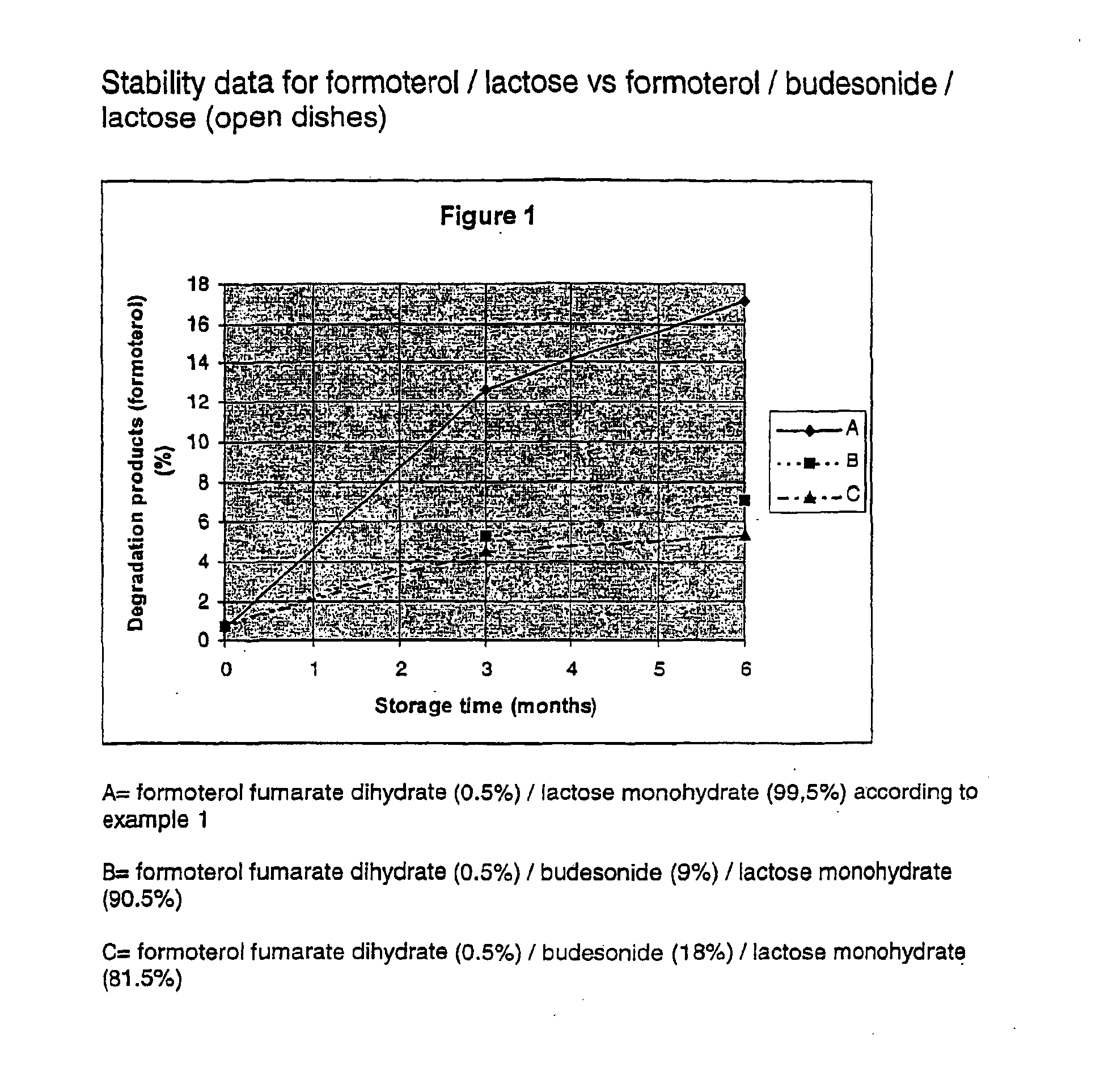
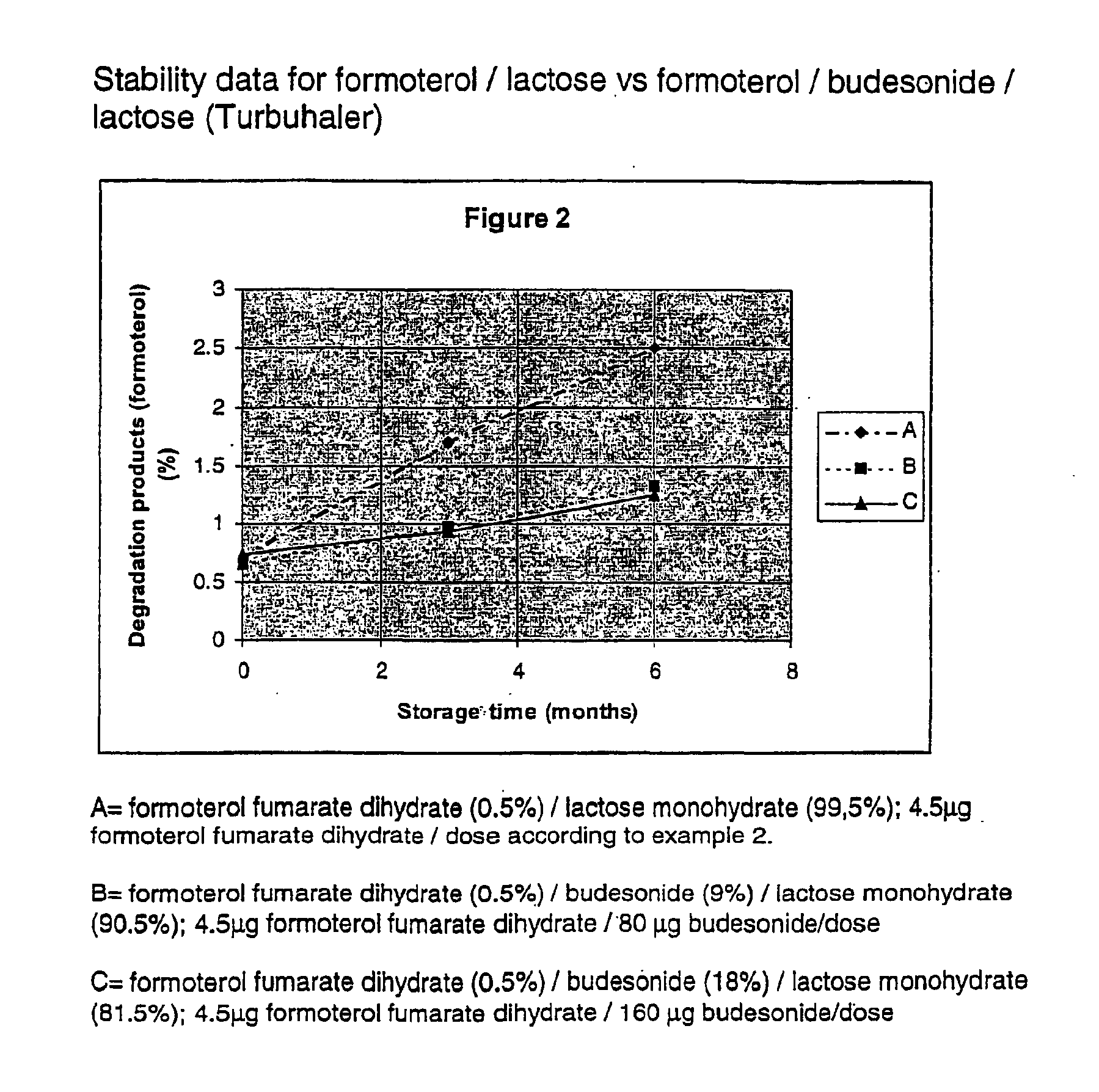
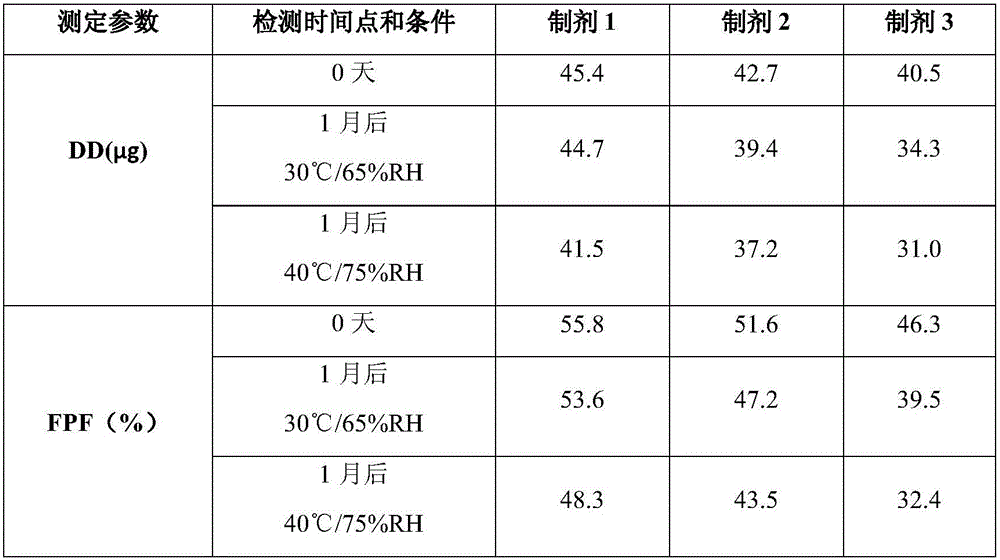
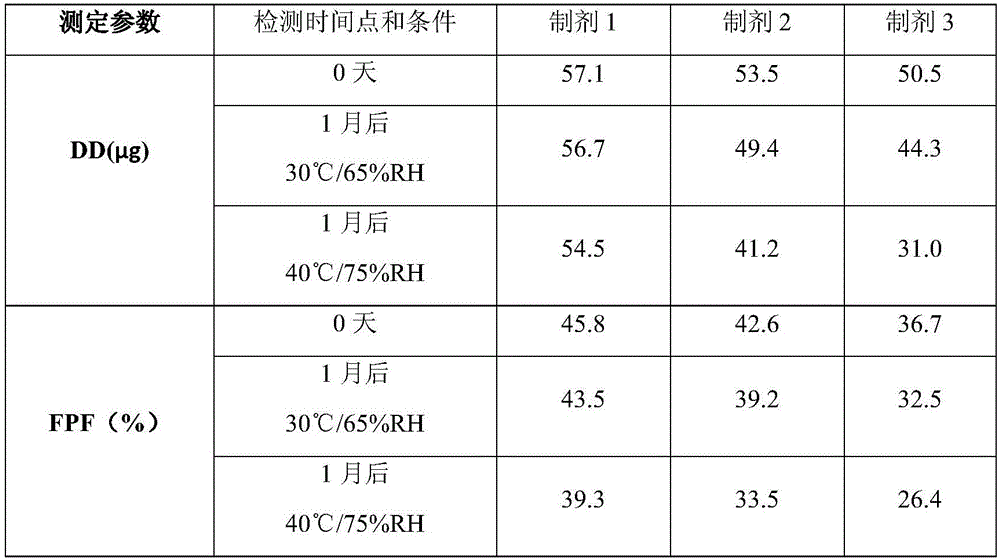
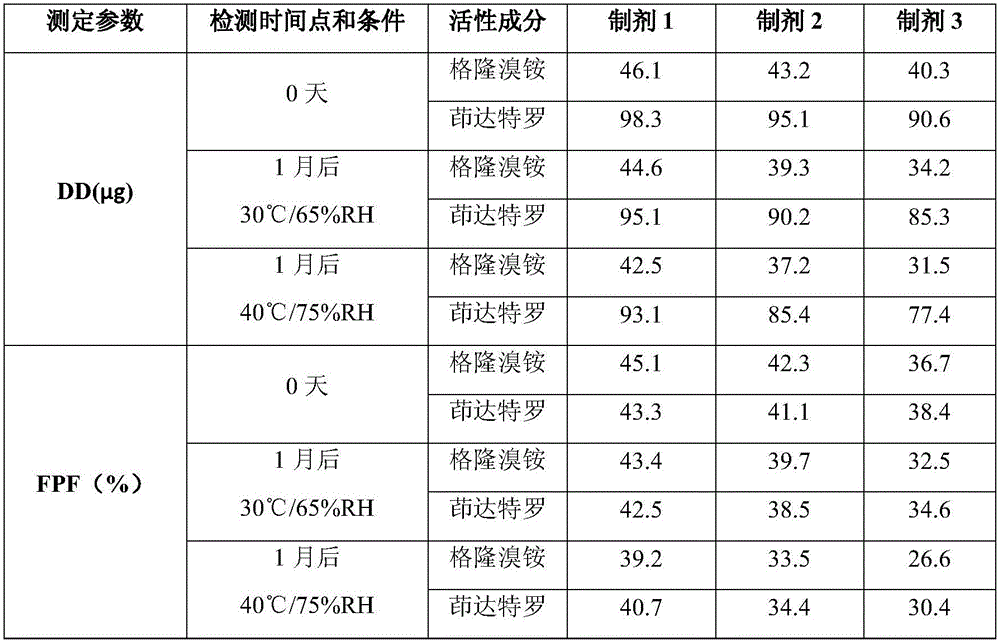
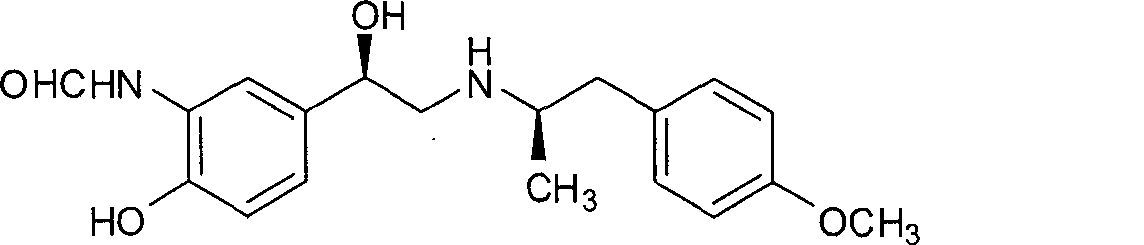
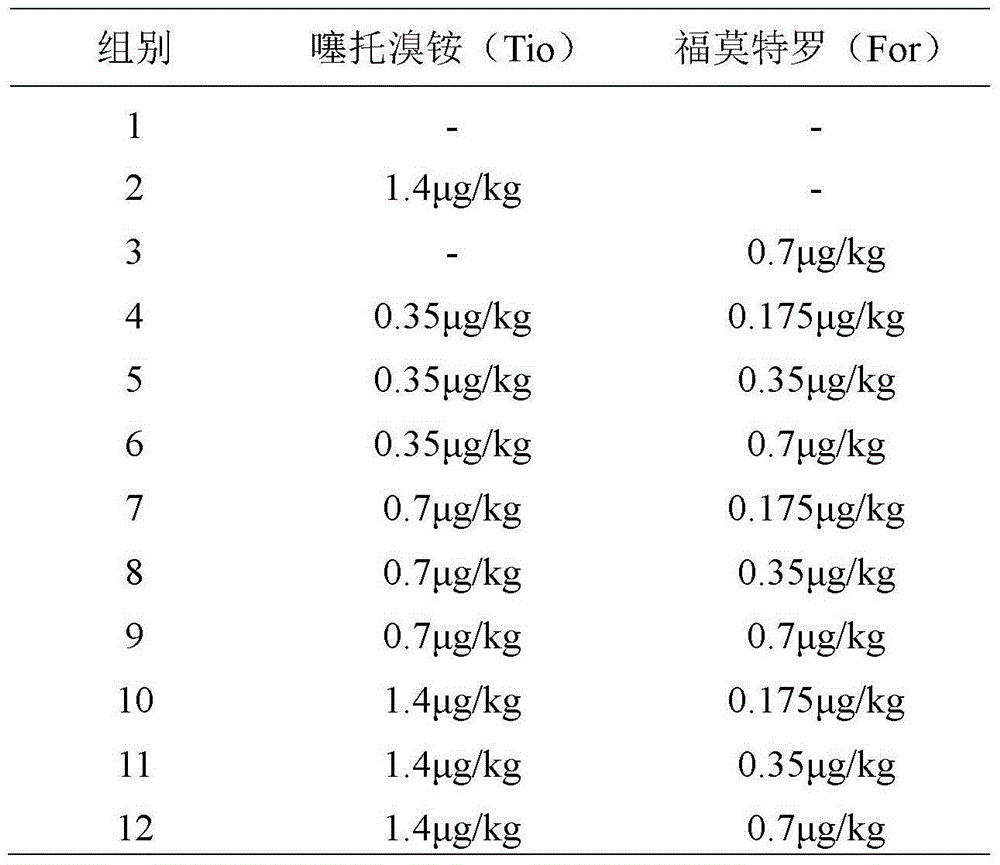
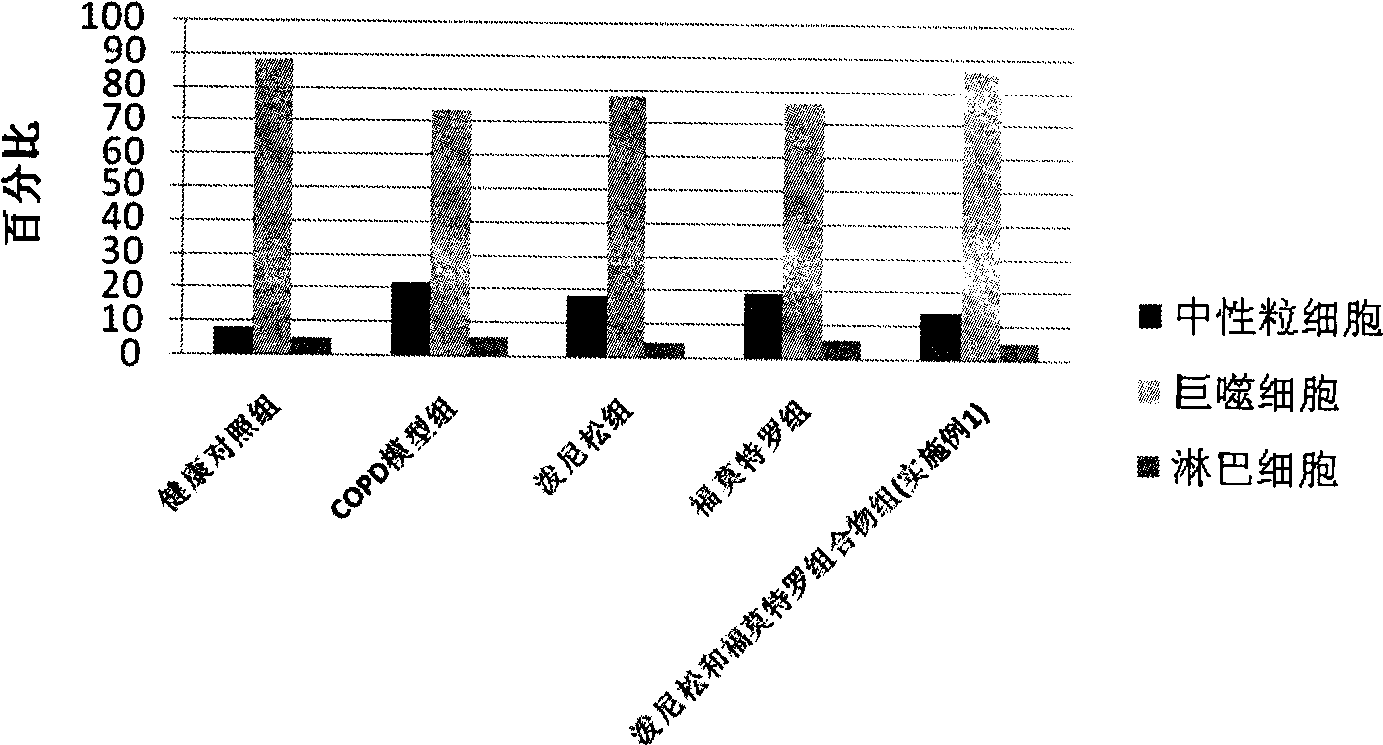
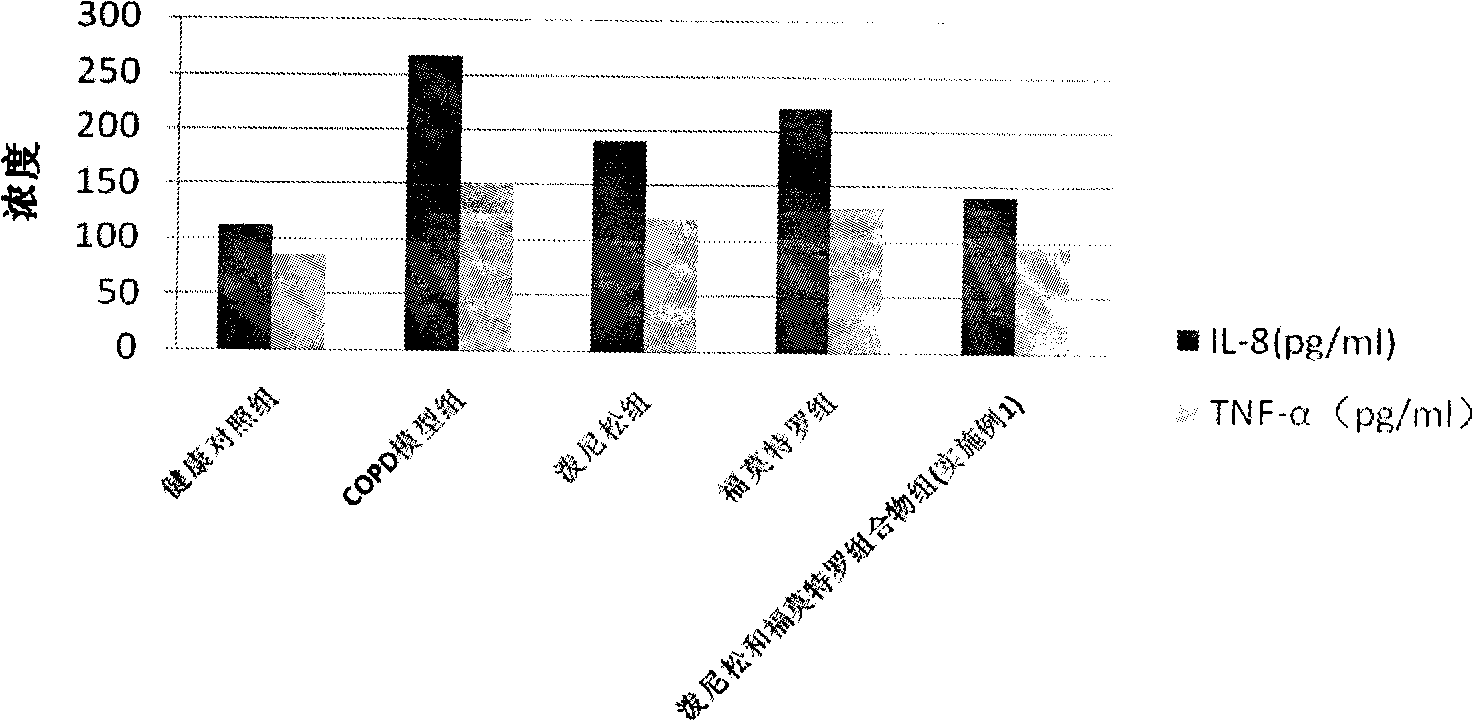
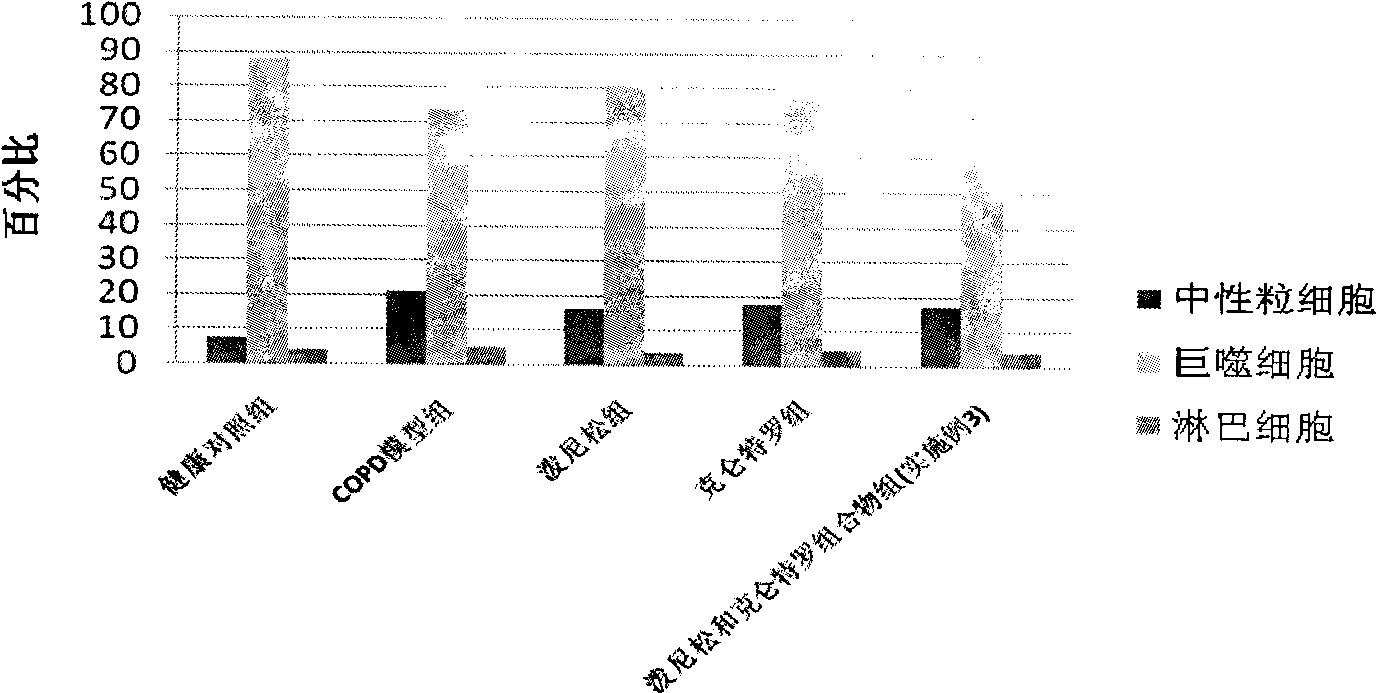
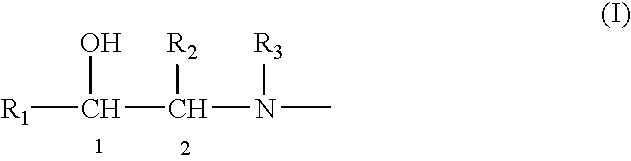Patents
Literature
96 results about "Formoterol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Formoterol is a long-acting bronchodilator used as a long-term (maintenance) treatment to prevent or decrease wheezing and trouble breathing caused by asthma or ongoing lung disease (chronic obstructive pulmonary disease-COPD, which includes chronic bronchitis and emphysema). It should only be used long-term if your asthma symptoms are not controlled by your other asthma medications (such as inhaled corticosteroids). Formoterol must not be used alone to treat asthma.
Formoterol superfine formulation
InactiveUS20050152846A1Improve toleranceInhibited DiffusionPowder deliveryOrganic active ingredientsDiseaseObstructive Pulmonary Diseases
The present invention relates to a pharmaceutical formulation for use in the administration of a long-acting β2-agonist by inhalation. In particular this invention relates to a chemically stable highly efficient formoterol HFA solution formulation to be administered by pressurised metered dose inhalers (pMDIs) characterized by a deep lung penetration. The invention also relates to methods for the preparation of said formulation and to its use in respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD).
Owner:CHIESI FARM SPA
Formoterol and mometasone aerosol formulations
ActiveUS20050255049A1Improve homogeneityHigh physical stabilityOrganic active ingredientsBiocideDrug aerosolMometasone
A pharmaceutical aerosol formulation comprising particles of (a) formoterol or a pharmaceutically acceptable salt, solvate or physiologically functional derivative thereof and (b) mometasone or a pharmaceutically acceptable salt, solvate, or physiologically functional derivative, thereof dispersed in a propellant selected from the group consisting of 1,1,1,2-tetrafluoroethane, 1,1,1,2,3,3,3-heptafluoropropane and a mixture thereof, and a bulking agent having a mass median diameter of less than one micron.
Owner:KINDEVA DRUG DELIVERY LP
Formulation for inhalation
A dry powder composition comprising formoterol and a carrier substance, both of which are in finely divided form, wherein the formulation has a poured bulk density of from 0.28 to 0.38 g / ml is useful in the treatment of respiratory disorders.
Owner:ASTRAZENECA AB
Methods and compositions for treating pulmonary disorders using optically pure (R,R)-formoterol
InactiveUS6866839B2Reduce adverse effectsReduced Tolerance RequirementsPowder deliveryBiocideTolerabilityPulmonary disease
A method and composition are disclosed utilizing the pure (R,R) isomer of formoterol, which is a potent bronchodilator with reduced adverse effects, a low incidence of the development of tolerance and an increased duration of action, as compared to racemic formoterol.
Owner:SUNOVION PHARMA INC
Combination therapy for COPD
InactiveUS20110150782A1Reduce usageUseful therapyDispersion deliveryAerosol deliveryObstructive Pulmonary DiseasesBeclometasone dipropionate
Aerosol formulations comprising glycopyrronium bromide in combination with formoterol are useful for the prevention or treatment of chronic obstructive pulmonary disease. The formulation further comprises a HFA propellant, a co-solvent, and an amount of inorganic acid sufficient to stabilize both the glycopyrronium bromide and the formoterol components. Optionally the formulation may further comprise beclometasone dipropionate.
Owner:CHIESI FARM SPA
Patch
InactiveUS6211425B1Improve long-term stabilitySmooth releaseOrganic active ingredientsThin material handlingTransdermal patchDisease
A transdermal patch containing a drug comprising formoterol and / or a salt, a solvent for the drug, a pressure-sensitive adhesive comprising an ethylene / vinyl acetate copolymer resin and at least one of a filler and a plasticizer. The patch is substantially water-free. The patch serves to promote percutaneous absorption of the formoterol and / or a salt thereof and allow the efficacy of the drug to stably persist for a long period of time. The patch is thus effective for diseases such as asthma, which can be cured or prevented by exciting beta-receptors.
Owner:SAITAMA DAIICHI SEIYAKU
High storage stability inhalable compositions
Inhalable pharmaceutical compositions are provided, for use in the treatment of respiratory disorders such as asthma, rhinitis and chronic obstructive pulmonary disease (COPD). These compositions have high storage stability, and include formoterol and a corticosteroid.
Owner:ASTRAZENECA AB
Stable pressurised aerosol solution composition of glycopyrronium bromide and formoterol combination
Aerosol solution compositions intended for use with a pressurized metered dose inhaler, comprising glycopyrronium bromide and formoterol, or a salt thereof or a solvate of said salt, optionally in combination with one or more additional active ingredients, and stabilized by a selected amount of a mineral acid, exhibit improved stability when contained in a can internally coated by a resin comprising a fluorinated ethylene propylene (FEP) polymer.
Owner:CHIESI FARM SPA
Formoterol tartrate polymorph
A method of preparation of a highly pure salt of R,R-formoterol L-tartrate is disclosed. The process provides the most thermodynamically stable polymorph by recrystallization of a novel polymorph.
Owner:SUNOVION PHARMA INC
Delivery of a combination therapy for asthma and chronic obstructive pulmonary disease
InactiveUS20070293460A1Adequate levelShortness of breathRespiratorsBiocideObstructive Pulmonary DiseasesCombination therapy
A method of delivery of a combination therapy to the pulmonary system that includes providing a nebulizer and an aqueous solution comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic, and administering the solution to the patient using the nebulizer. The corticosteroid is budesonide, the beta-agonist is formoterol and the anticholinergic is tiotropium. A pharmaceutical composition is also described for the treatment of respiratory conditions and diseases comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic, and administering the solution to the patient using the nebulizer.
Owner:CMPD LICENSING
Nebulizer Formulation
A sterile nebulizer formulation contains formoterol and budesonide in about 2 ml or less of saline and is for treatment of COPD and asthma and other airways diseases and disorders.
Owner:BREATH
High storage stability inhalable compositions
Inhalable pharmaceutical compositions are provided, for use in the treatment of respiratory disorders such as asthma, rhinitis and chronic obstructive pulmonary disease (COPD). These compositions have high storage stability, and include formoterol and a corticosteroid.
Owner:ASTRAZENECA AB
Delivery of a combination therapy for asthma and chronic obstructive pulmonary disease
A method of delivery of a combination therapy to the pulmonary system that includes providing a nebulizer and a fluid comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic in a pharmaceutically acceptable vehicle, and administering the solution to the patient using the nebulizer. The corticosteroid is budesonide, the beta-agonist is formoterol and the anticholinergic is tiotropium in a an aqueous solution, suspension or emulsion suitable for administration with the nebulizer.
Owner:RICHIES PHARMACY & MEDICAL SUPPLY
Stable pressurised aerosol solution composition of glycopyrronium bromide and formoterol combination
Aerosol solution compositions intended for use with a pressurized metered dose inhaler, comprising glycopyrronium bromide and formoterol, or a salt thereof, optionally in combination with one or more additional active ingredients, and stabilized by a selected amount of a mineral acid, exhibit improved stability when contained in an aerosol can provided with a metering valve having at least a butyl rubber gasket.
Owner:CHIESI FARM SPA
Use of composition comprising Formoterol and Budesonide for prevention or treatment of acute condition of asthma
InactiveCN1305380AOffset deteriorationIncrease inflammationPowder deliveryHydroxy compound active ingredientsChronic asthmaMedicine
Owner:ASTRAZENECA AB
Combination therapy for COPD
Aerosol formulations comprising glycopyrronium chloride in combination with formoterol may be administered by means of a pressurized metered dose inhaler (pMDI) for the prevention or treatment of chronic obstructive pulmonary disease. The formulation further comprises a HFA propellant, a co-solvent, and an amount of inorganic acid sufficient to stabilize both the glycopyrronium chloride and the formoterol components. Optionally, the formulation may further comprise beclometasone dipropionate.
Owner:CHIESI FARM SPA
Formulations for treatment of adipose tissue, cutaneous tissue and disorders, and muscular tissue
Compositions, formulations, methods, and systems for treating regional fat deposits and fat-related conditions, dermal conditions, and muscular conditions. Methods comprise administering a composition comprising at least one compound that reduces desensitization of beta adrenergic receptors, for example a glucocorticosteroid, and / or at least one long-acting beta-2 adrenergic receptor agonist, for example, formoterol. Compositions to be administered include sustained release formulations comprising at least one long-acting beta-2 adrenergic receptor agonist, at least one compound that reduces desensitization of beta adrenergic receptors, or both in a sustained crystalline microparticle form.
Owner:LITHERA
Formoterol and mometasone aerosol formulations
ActiveUS8252268B2Improve homogeneityImprove stabilityPowder deliveryOrganic active ingredientsMometasoneCombinatorial chemistry
A pharmaceutical aerosol formulation comprising particles of (a) formoterol or a pharmaceutically acceptable salt, solvate or physiologically functional derivative thereof and (b) mometasone or a pharmaceutically acceptable salt, solvate, or physiologically functional derivative thereof dispersed in a propellant selected from the group consisting of 1,1,1,2-tetrafluoroethane, 1,1,1,2,3,3,3-heptafluoropropane and a mixture thereof, and a bulking agent having a mass median diameter of less than one micron.
Owner:KINDEVA DRUG DELIVERY LP
Combination therapy for COPD
ActiveUS20150328144A1Dispersion deliveryAerosol deliveryObstructive Pulmonary DiseasesCombination therapy
Owner:CHIESI FARM SPA
Methods for inducing mitochondrial biogenesis
Methods and compositions for inducing mitochondrial biogenesis are provided. In some aspects, methods for the treatment of diseases such as acute kidney disease (AKI) or a muscle wasting disease by administering tomoxetine, nisoxetine, fenoterol, formoterol, or procaterol to an individual are provided.
Owner:MUSC FOUND FOR RES DEV
Unsymmetrical hydrogen migration synthesizing method for (R, R)-formoterol
InactiveCN101468954AIn line with the concept of green chemistryLow costOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsPhotochemistry
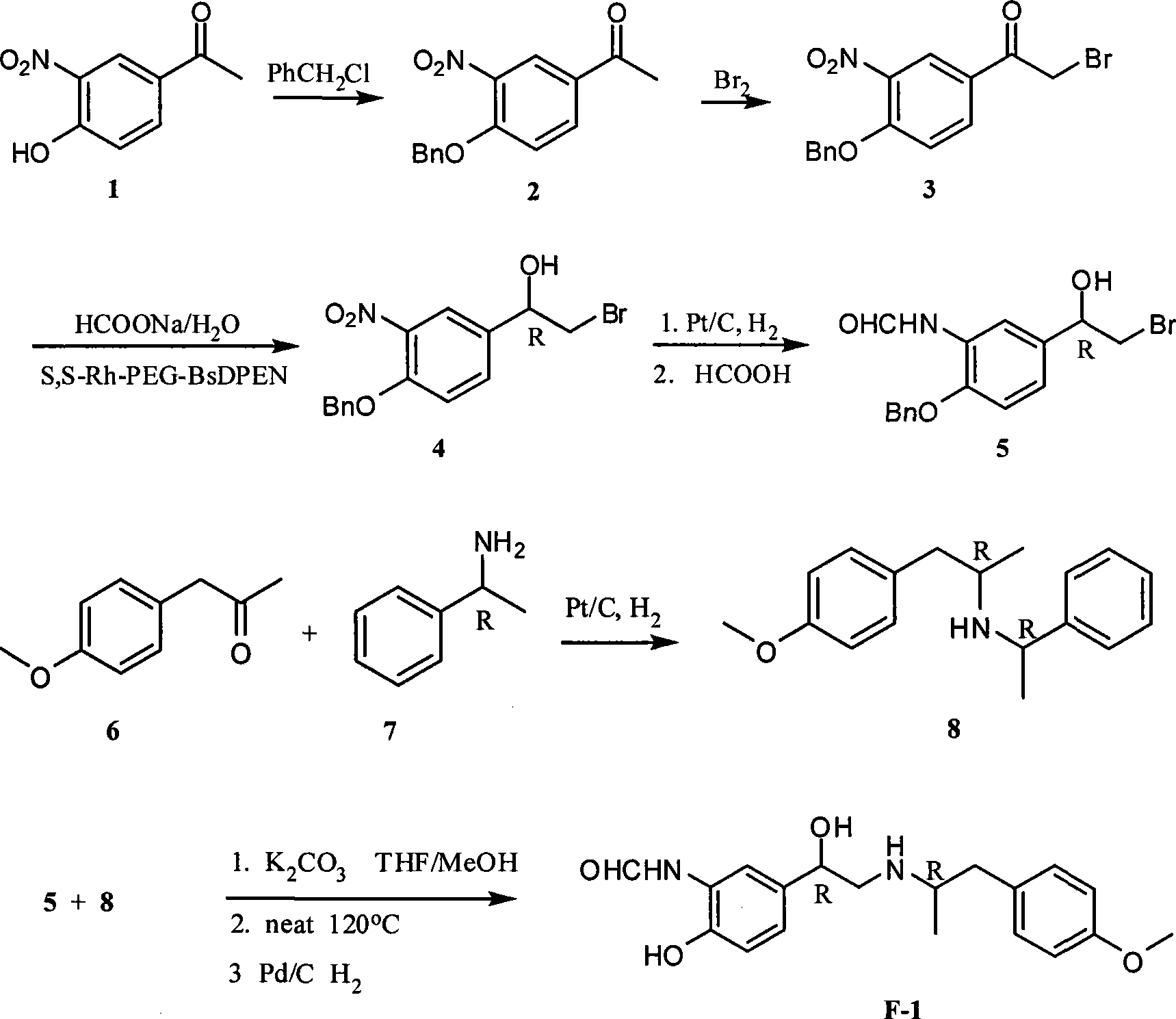
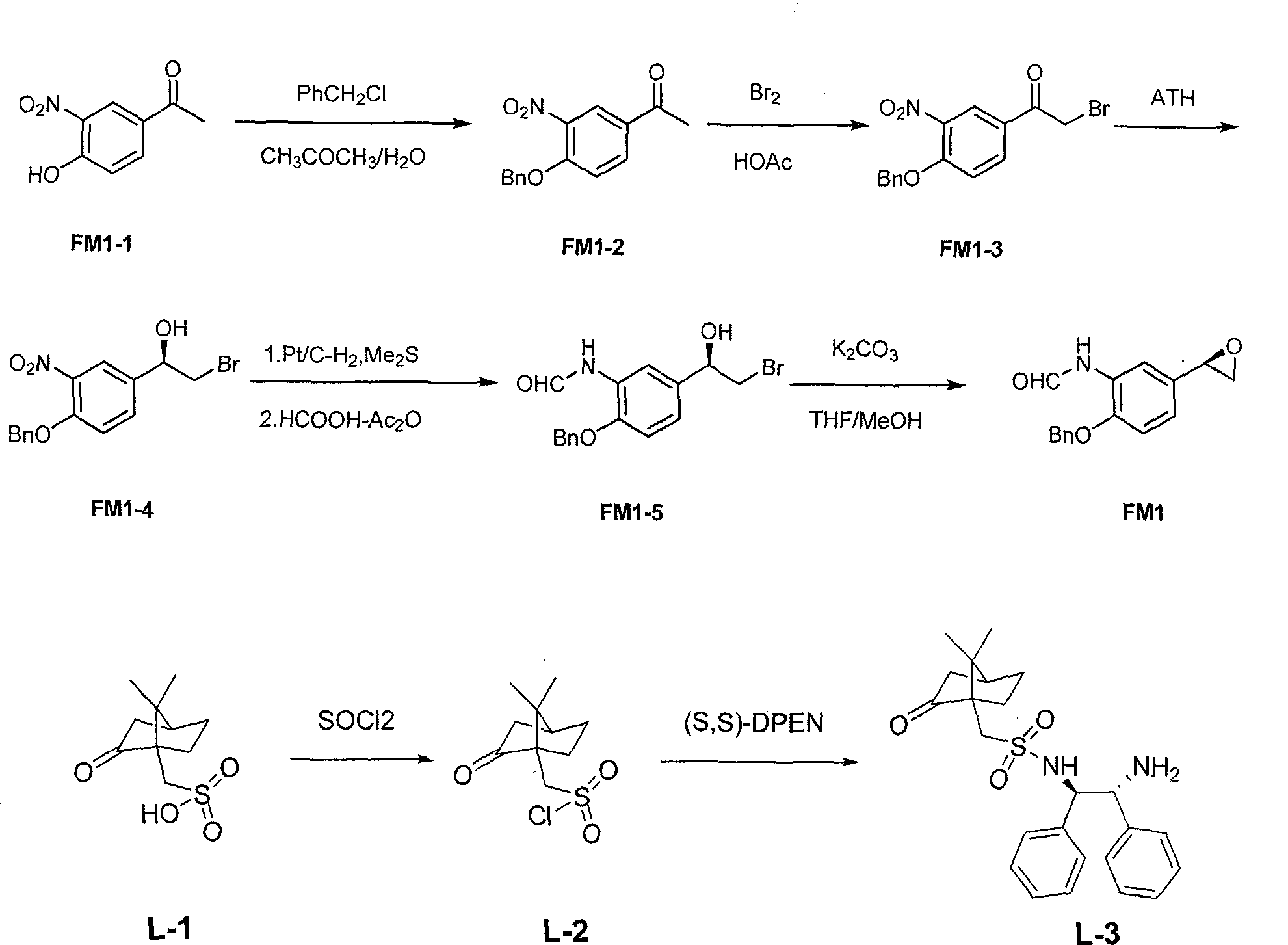
The invention relates to an asymmetric hydrogen transfer synthesis method for (R,R)-formoterol, and relates to a novel method for synthesizing an optical pure beta 2-adrenoreceptor excitant, namely formoterol. The method comprises: firstly, taking 4-hydroxyl-3 nitroacetophenone as a raw material, using benzyl groups to protect phenolic hydroxyl groups, and obtaining alpha-bromo keto after bromination; secondly, taking (S,S)-Rh-PEG-BsDPEN as a catalyst and formic acid and derivatives of the formic acid as hydrogen sources, and synthesizing chiral alcohol intermediate by an asymmetric hydrogen transfer method; thirdly, using (R)-alpha-methyl phenylethylamine and methoxyl phenylacetone to generate imine compounds, and obtaining chiral amine intermediate through hydrogenation reduction under the catalysis of Pt / C; and fourthly, reacting and coupling the chiral alcohol intermediate and the chiral amine intermediate, removing protective groups, and obtaining the (R,R)-formoterol. The invention uses the asymmetric hydrogen transfer method and a chiral auxiliary reagent to synthesize the (R,R)-formoterol, and has high yield and good ee value. Compared with a method for synthesizing chiral formoterol through chemical splitting, the method has the advantages of high total yield, mild reaction conditions, low cost and so on, and is favorable for industrial production.
Owner:SUN YAT SEN UNIV
Pharmaceutical aerosol formulations of formoterol and beclometasone dipropionate
ActiveUS20110081301A1Improve stabilityReduce solubilityOrganic active ingredientsPowder deliveryPharmaceutical formulationBeclometasone dipropionate
Pharmaceutical formulations comprising beclometasone dipropionate and a salt of formoterol exhibit improved stability and are useful in pressurised metered dose inhalers (pMDIs).
Owner:CHIESI FARM SPA
Use for budesonide and formoterol
The invention provides the use of formoterol and budesonide in the treatment of chronic obstructive pulmonary disease.
Owner:ASTRAZENECA AB
Methods and compositions for treating pulmonary disorders using optically pure (R,R)-formoterol
InactiveUS20050131072A1Reduce adverse effectsDecreased tolerance and hypersensitivityPowder deliveryBiocideTolerabilityBronchodilator
A method and composition are disclosed utilizing the pure (R,R) isomer of formoterol, which is a potent bronchodilator with reduced adverse effects, a low incidence of the development of tolerance and an increased duration of action, as compared to racemic formoterol.
Owner:SUNOVION PHARMA INC
Budesonide and formoterol spray inhalation suspension and preparation method thereof
InactiveCN105748447AAvoid potential hazardsDelivery dose is accurate and controllableOrganic active ingredientsDispersion deliveryMedicinePowder Aerosol
The invention discloses a budesonide and formoterol spray inhalation suspension and a preparation method thereof.The budesonide and formoterol spray inhalation suspension comprises, per 1000 ml, the following components: 0.5-5 g of budesonide, 10-100 mg of formoterol, 0.1-0.5 g of a dispersant, 1-100 g of isoosmotic adjusting agent, 0.1-1 g of metal ion complexing agent, 0.1-20 g of pH buffer, pH regulator to adjust pH of a system to 4.0-6.0, and the balance of water, adding up to 1000 ml.The potential hazard of a lactose vector in powder aerosols can be avoided, delivery dosage is accurate and controllable, the requirement on ventilation flow is low, and compliance is good; the preparation method is simple to perform, required equipment is simple, and the requirement for asepsis of products can be met.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Dry powder inhalation medicine composition and preparation method thereof
InactiveCN105982880AAvoid stimulationImproving In Vitro Assay ParametersPowder deliveryPharmaceutical non-active ingredientsIndacaterolAdditive ingredient
The invention provides a dry powder inhalation medicine composition and a preparation method thereof. The composition is prepared from a coating agent with a specific particle size characteristic, lactose monohydrate with a specific particle size characteristic for a carrier and a micronized medicinal active ingredient, wherein the coating agent is an inhaling magnesium stearate or a mixture of the inhaling magnesium stearate and micronized lactose monohydrate; and the medicinal active ingredient is selected from at least one of glycopyrronium bromide, umeclidinium, indacaterol, formoterol, vilanterol, fluticasone and pharmaceutically available salt of the active ingredients. The preparation method comprises the following steps of sufficiently mixing and coating the coating agent and the lactose monohydrate, and uniformly mixing with the micronized medicinal active ingredients.
Owner:SICHUAN HAISCO PHARMA CO LTD
Asymmetric synthesis method of (R,R)-formoterol tartrate
InactiveCN103664677AHigh yieldHigh enantioselectivityCarboxylic acid amides optical isomer preparationCarboxylic acid salt preparationSynthesis methodsBenzaldehyde
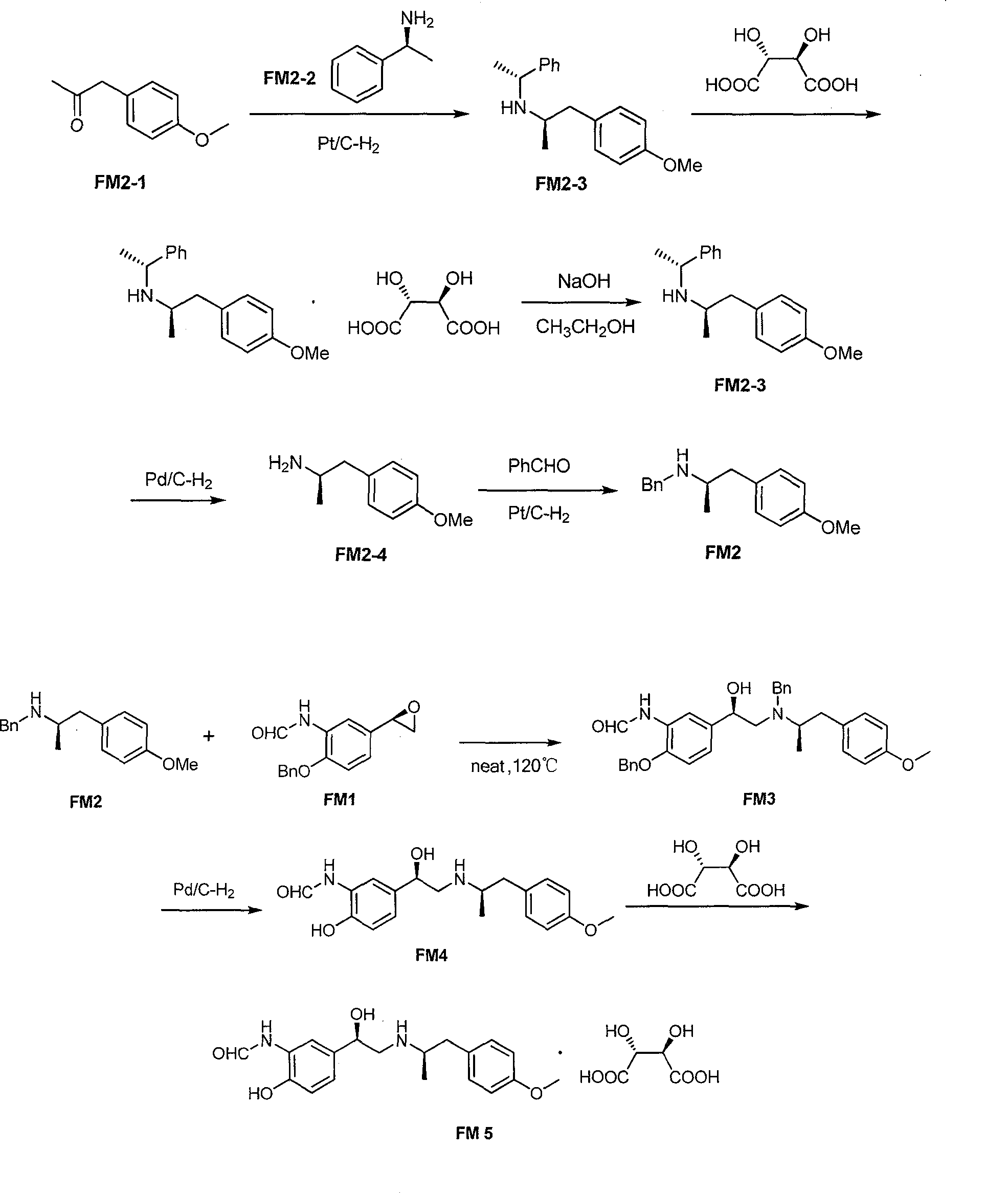
The invention relates to an asymmetric synthesis method of (R,R)-formoterol tartrate, which comprises the following steps: by taking (S,S)-CsDPEN and transition metal complex as a catalyst, performing asymmetric hydrogen transfer reaction on alpha-bromoketone used as a raw material, thus obtaining a chiral alcohol intermediate compound; performing reaction steps of nitro-reduction, formylation, cyclization and the like, thus obtaining a key intermediate compound FM 1; by taking Pt / C as a catalyst and alpha-methylphenylethylamine as a chiral assistant, synthesizing an intermediate compound FM 2-3; performing tartaric acid salification, ionization and alpha-methylphenethyl removal, and reacting with benzaldehyde, thus preparing a chiral amine intermediate compound FM 2; reacting and coupling the two key intermediate compounds, and performing protective group removal to obtain (R,R)-formoterol FM 4; and performing tartaric acid salification on the FM 4, thus preparing the target product (R,R)-formoterol tartrate FM 5. According to the invention, the (R,R)-formoterol is synthesized through an asymmetric hydrogen transfer method by means of the chiral assistant, and high yield and favorable ee value are achieved. Compared with a chemical resolution method for synthesizing chiral formoterol, the method provided by the invention has the advantages of high overall yield, mild reaction conditions, low cost and the like, thereby being beneficial to industrial production.
Owner:SUN YAT SEN UNIV +1
Medicine composition with tiotropium bromide and formoterol, application of medicine composition and preparation
InactiveCN105125542AEnhance diastolic abilityReduce omissionAntipyreticAnalgesicsTiotropium bromideObstructive Pulmonary Diseases
The invention relates to the technical field of medicine preparations, in particular to a medicine composition with tiotropium bromide and formoterol, application of the medicine composition and a preparation. The medicine composition comprises the tiotropium bromide or pharmaceutically acceptable salt of the tiotropium bromide and the formoterol or pharmaceutically acceptable salt of the formoterol. The medicine composition with the tiotropium bromide and the formoterol is applied to preparing medicine for treating respiratory and lung inflammatory diseases. The medicine composition is preferably a tiotropium bromide and formoterol composition with synergistic additive effects. The medicine composition, the application and the preparation have the advantages that as proved by pharmacodynamic experiments, the synergistic additive effects can be realized by the tiotropium bromide and the formoterol which are jointly applied, and accordingly bronchiectasis can be forcefully and quickly realized; the medicine composition and the preparation are used for treating the respiratory and lung inflammatory diseases, and preferably used for treating dyspnea such as bronchial asthma and chronic obstructive pulmonary diseases (COPD) due to bronchial constriction.
Owner:HANGZHOU ZIJIN PHARMA TECH
Pharmaceutical composition for the treatment of chronic obstructive pulmonary disease and bronchial asthma
InactiveCN101530618ALower doseReduce or avoid side effectsRespiratory disorderHeterocyclic compound active ingredientsDiseaseAdditive ingredient
The invention discloses a pharmaceutical composition for the treatment of chronic obstructive pulmonary disease (COPD) and bronchial asthma, which is composed of a glucocorticoid, a bronchodilator and a pharmaceutically acceptable auxiliary material or carrier; the composition is a preparation for oral use. The glucocorticoid in the inventive pharmaceutical composition is selected from prednisone, prednisolone, methylprednisolone, betamethasone, decamethasone or hydrocortisone; the bronchodilator is selected from formoterol, clenbuterol, procaterol or theophylline. The inventive composition has better therapeutic effect on the COPD and the bronchial asthma than independent administration of two ingredients, and has synergistic effect. The composition has easily-available raw materials, inexpensive price and increased medicine taking compliance as well as plays a significant role in preventing and treating the COPD and the bronchial asthma of patients in vast rural areas and patients in low-income class of the city in China.
Owner:莫始平
Stable pressurized aerosol solution composition of glycopyrronium bromide and formoterol combination
Aerosol solution compositions intended for use with a pressurized metered dose inhaler, comprising glycopyrronium bromide and formoterol, or a salt thereof or a solvate of said salt, optionally in combination with one or more additional active ingredients, and stabilized by a selected amount of a mineral acid, exhibit improved stability when contained in a can internally coated by a resin comprising a fluorinated ethylene propylene (FEP) polymer.
Owner:CHIESI FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com