Patents
Literature
145 results about "Meter dose inhaler" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
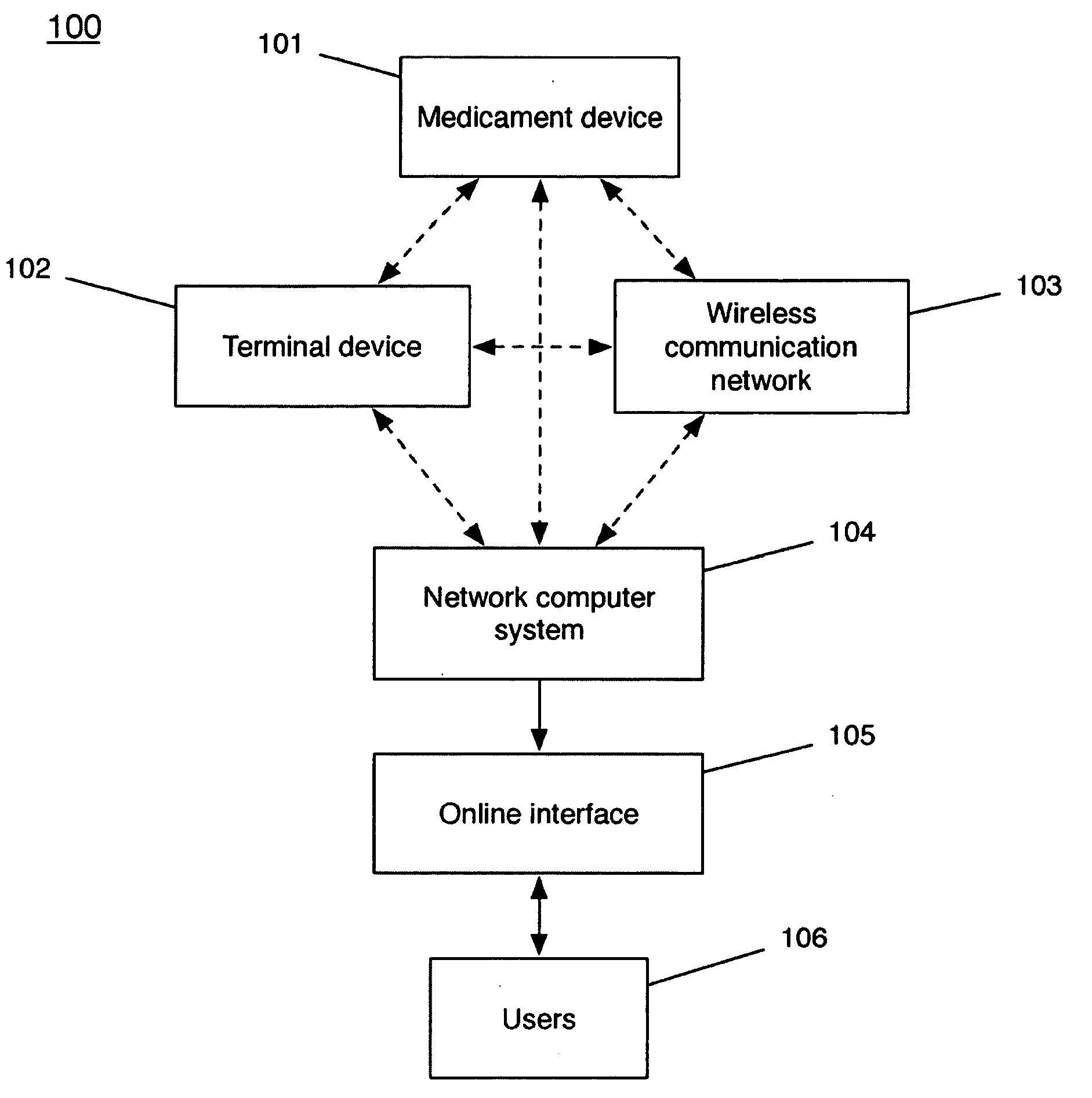
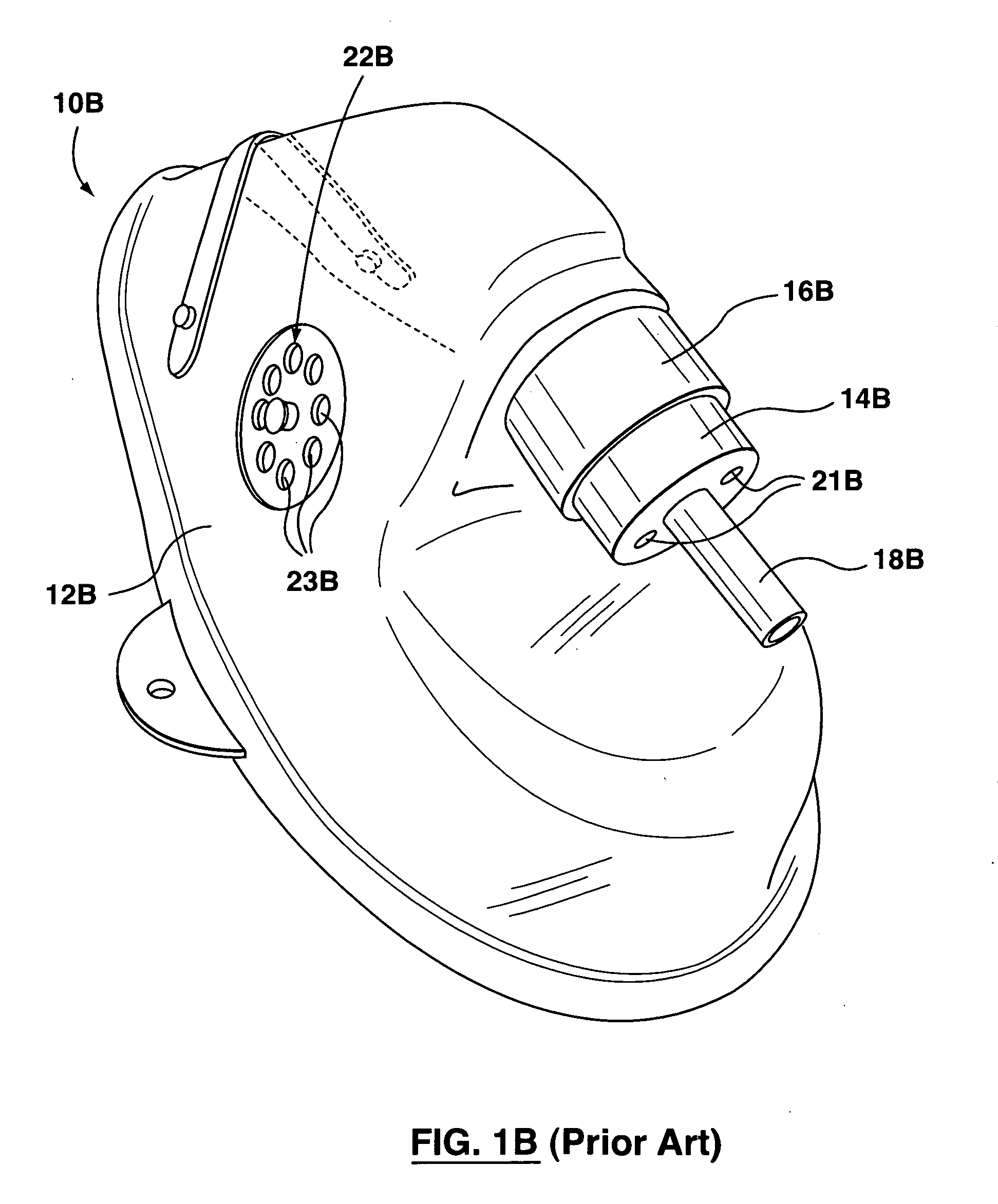
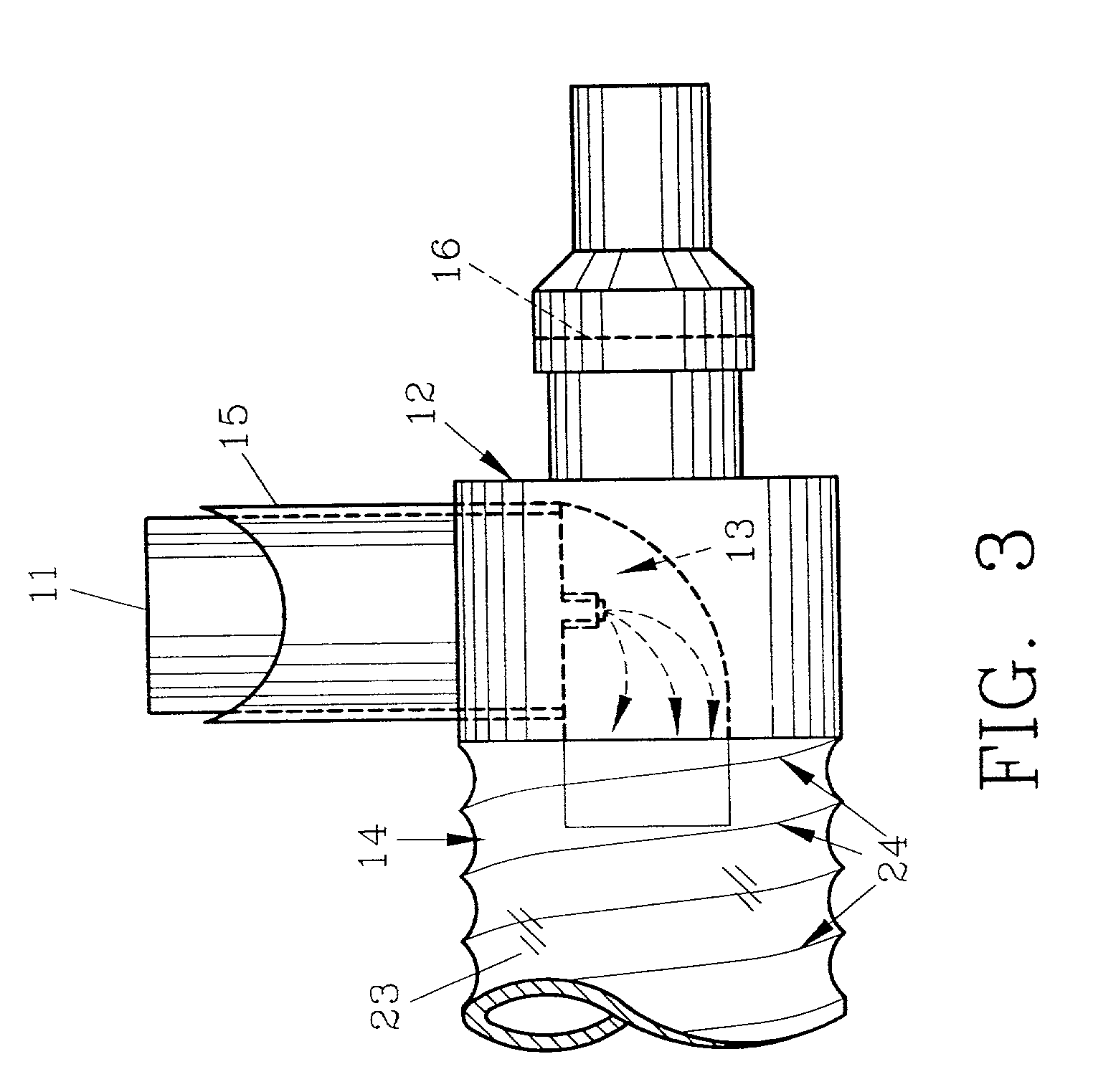
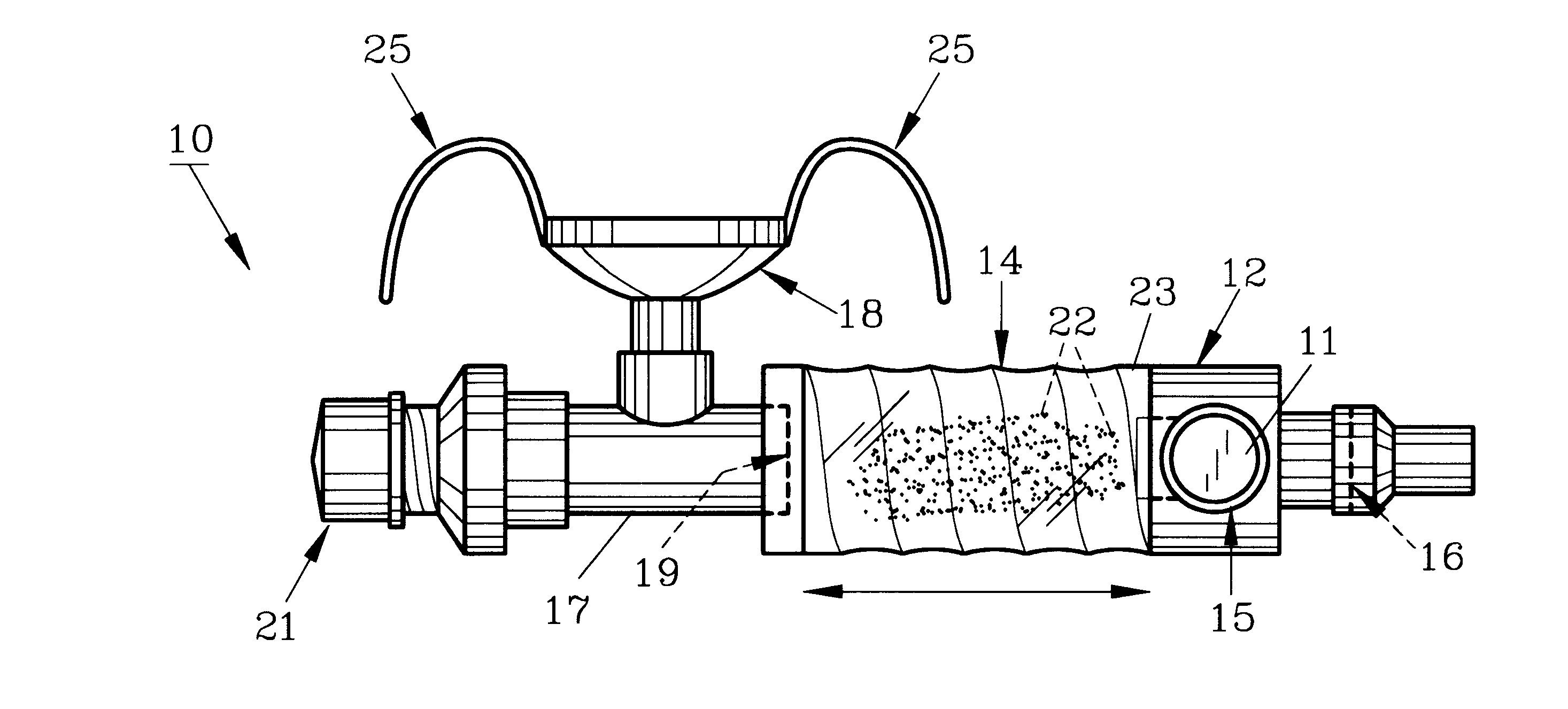
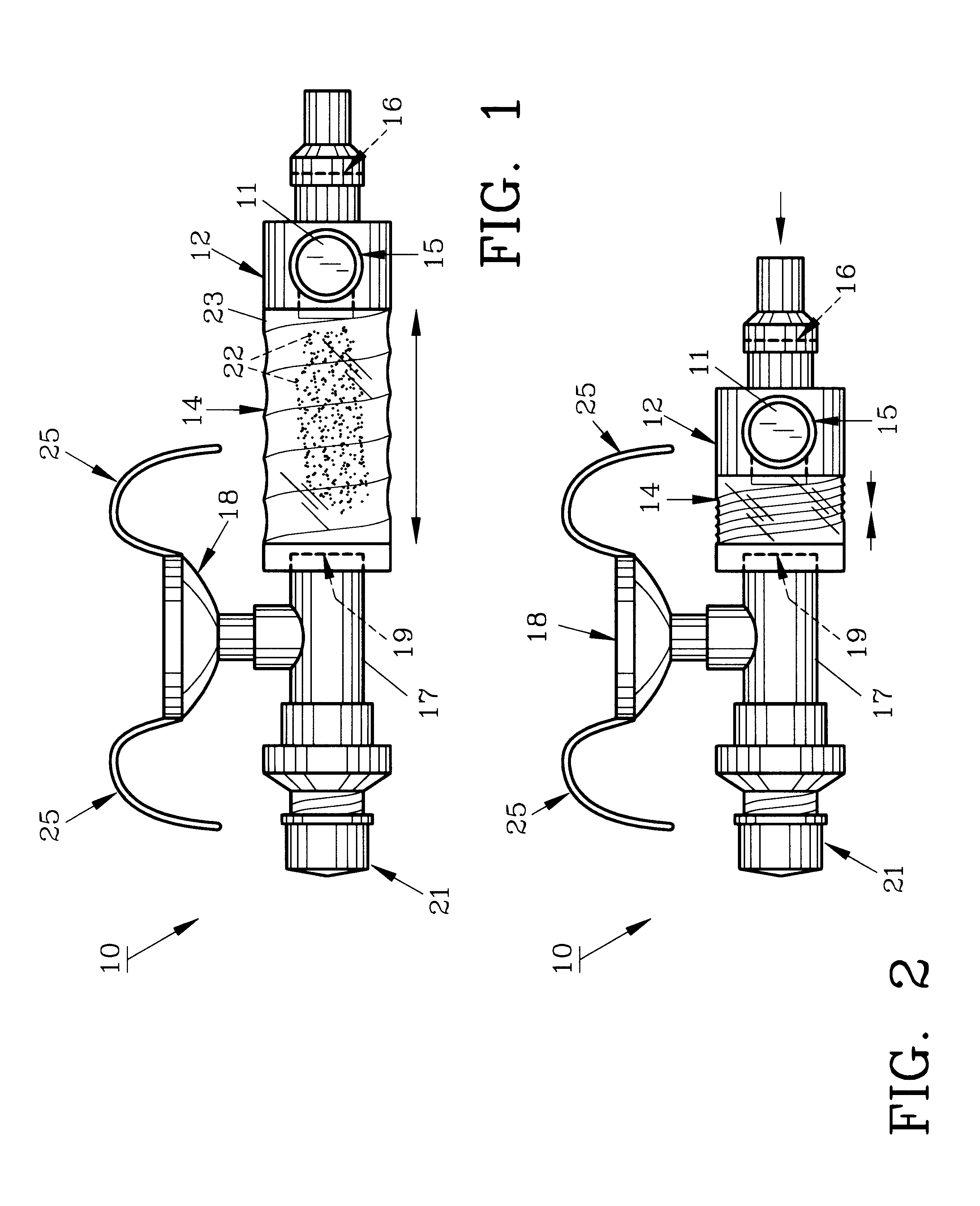
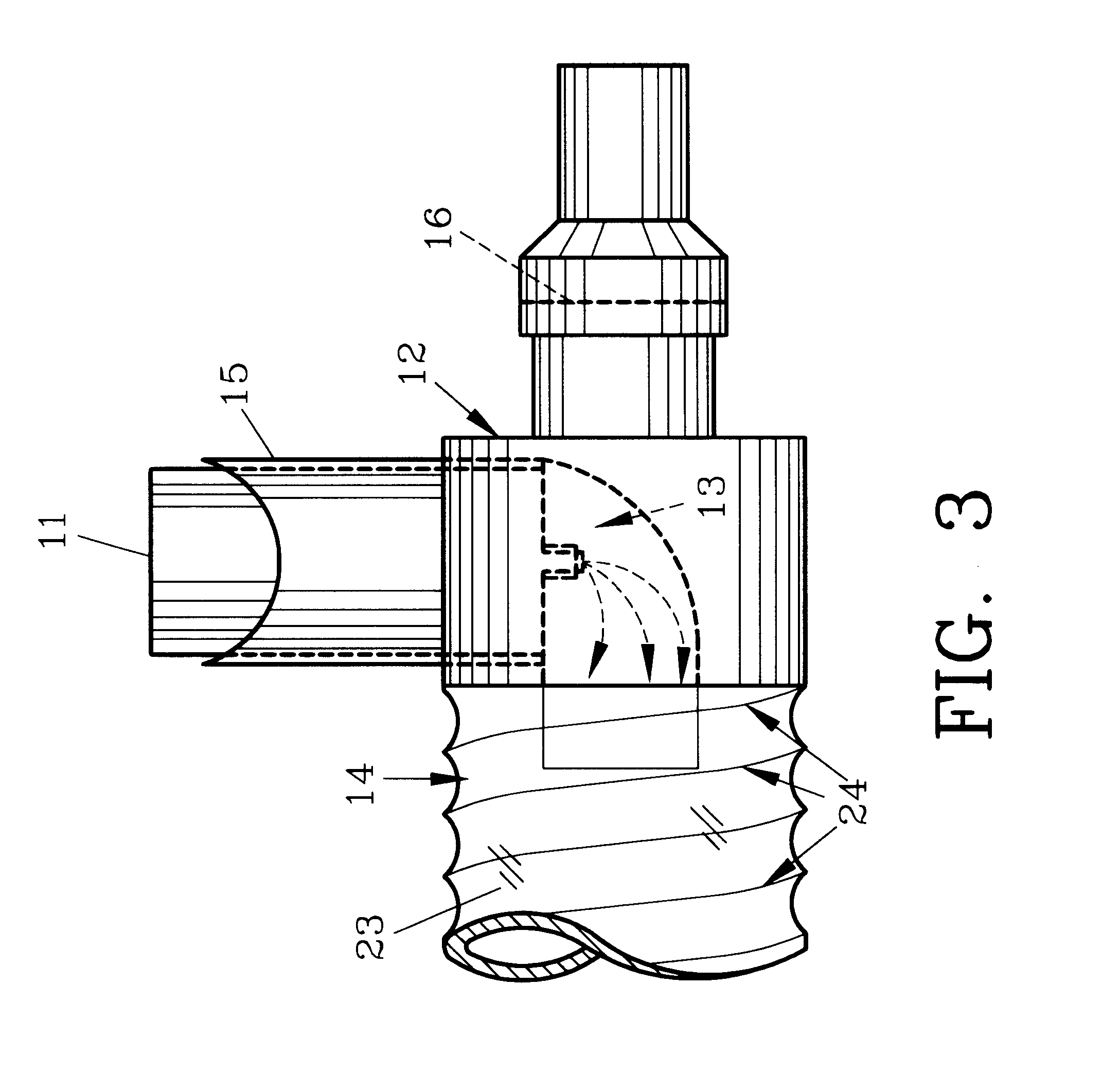
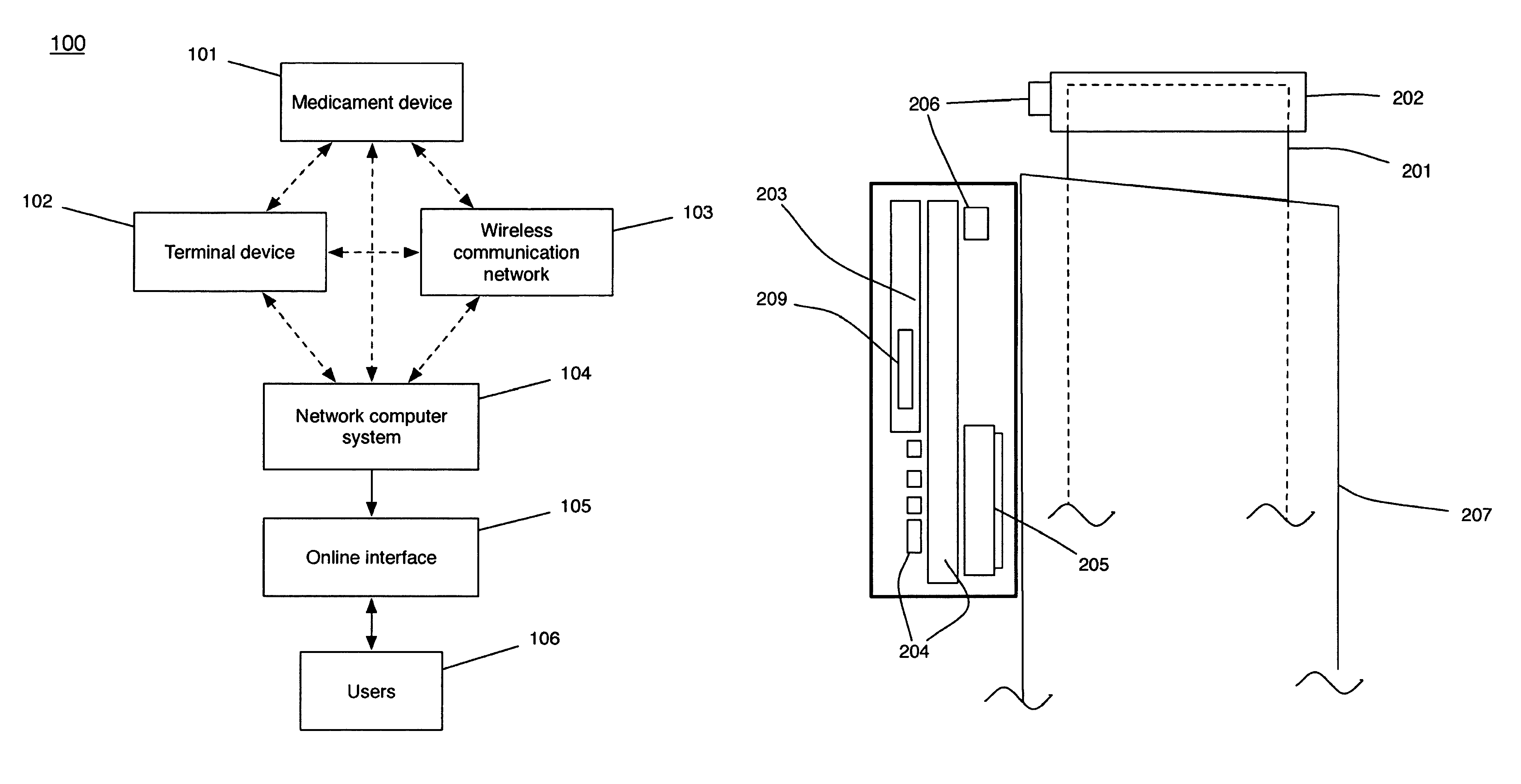
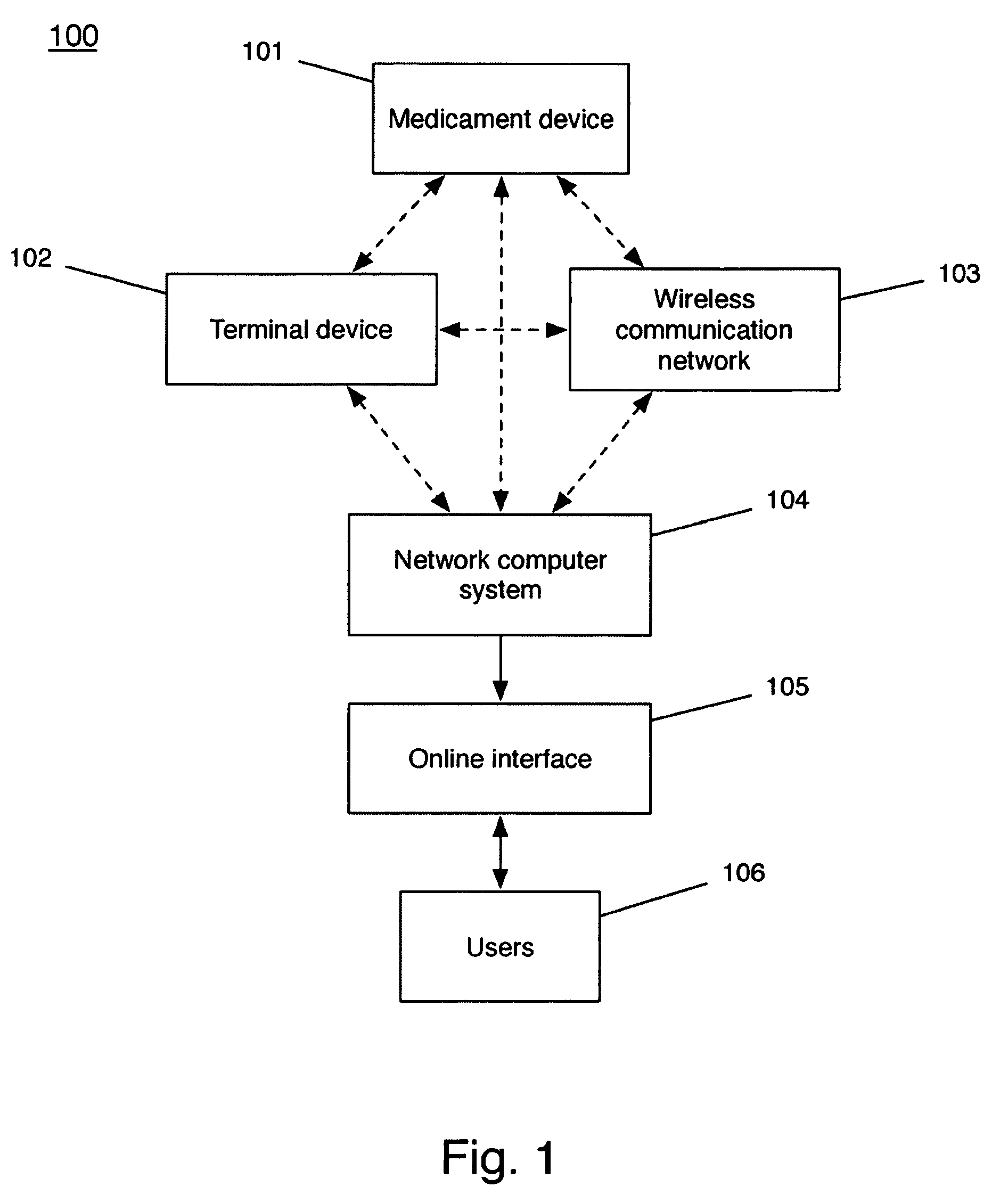
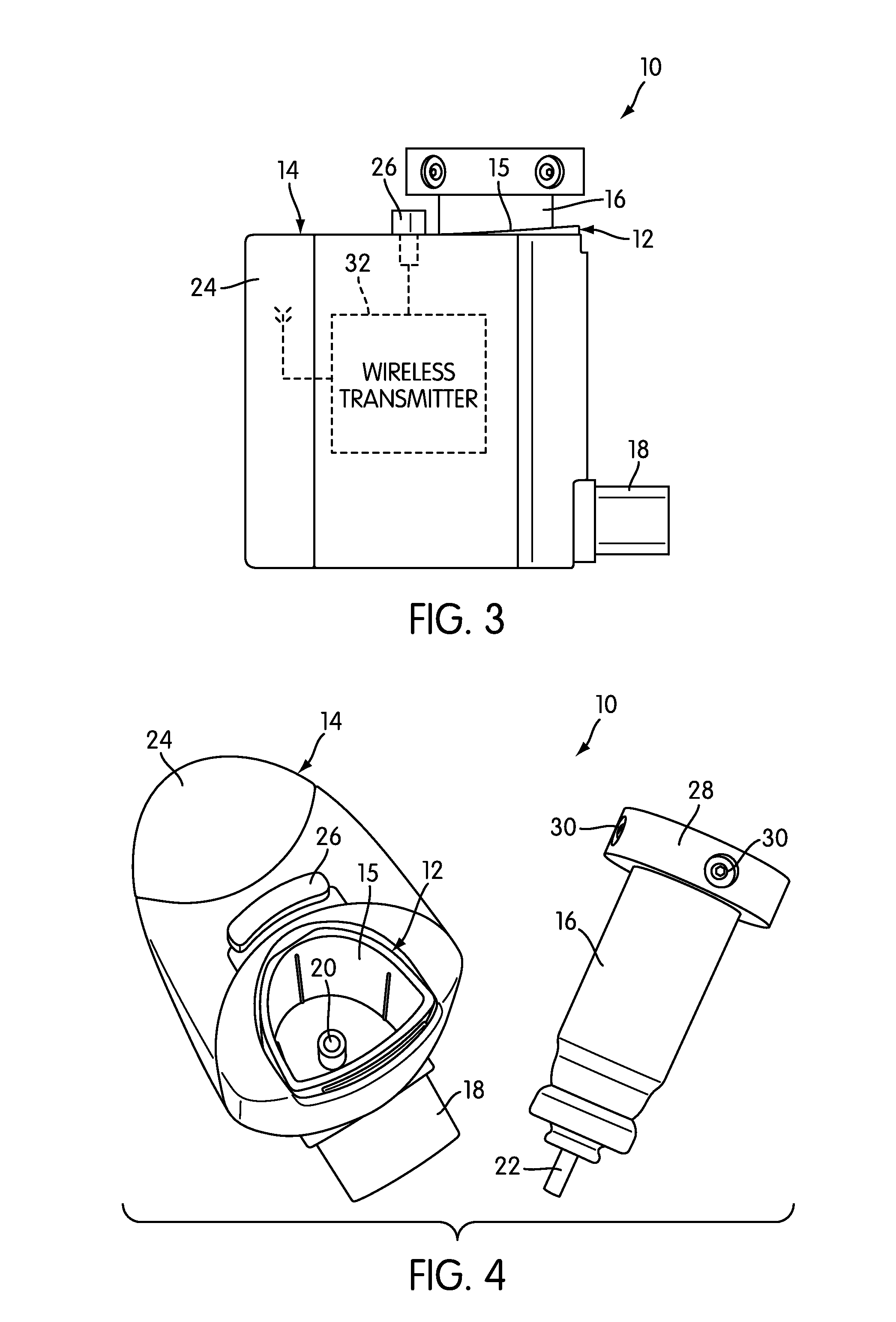
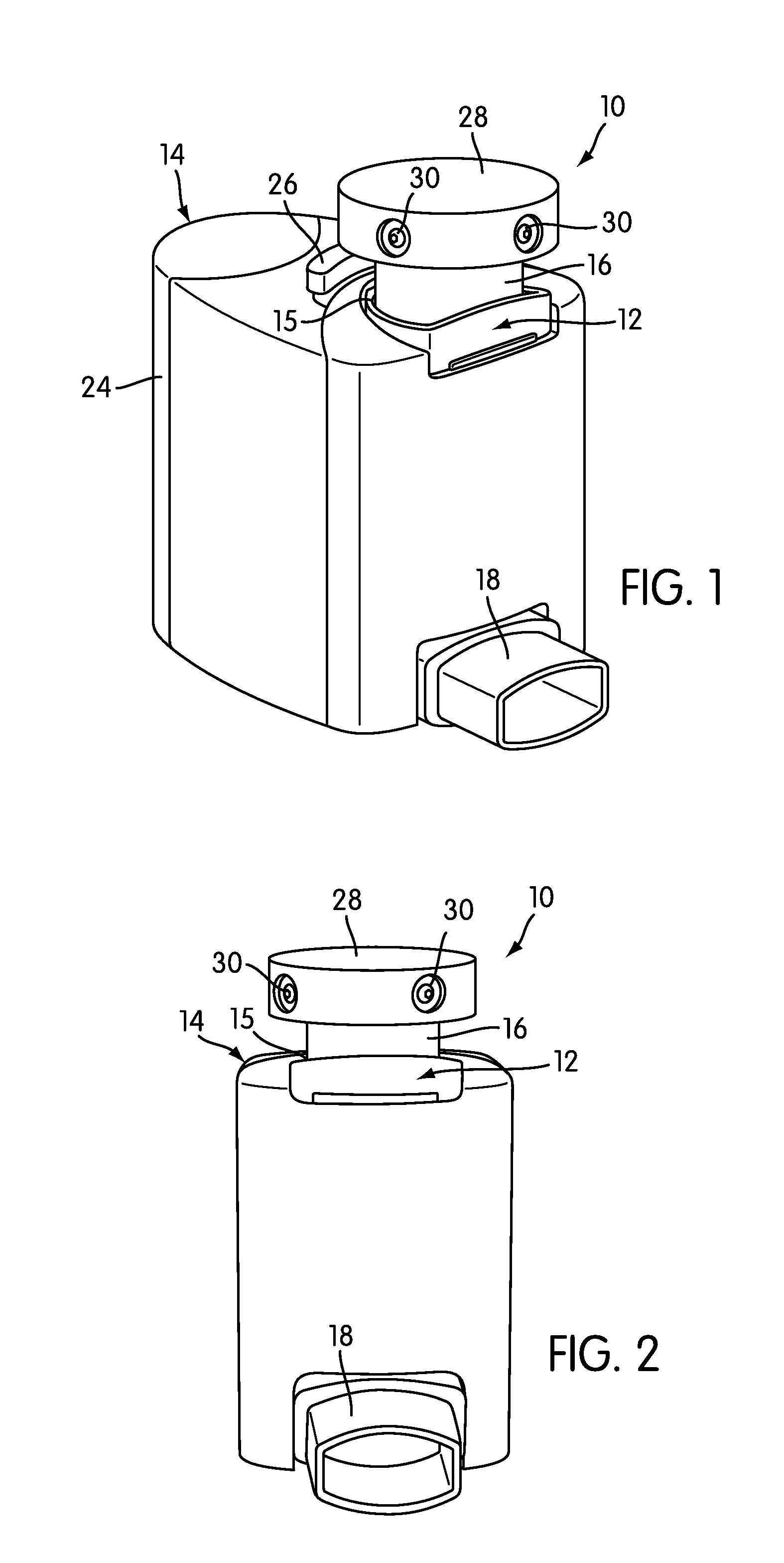
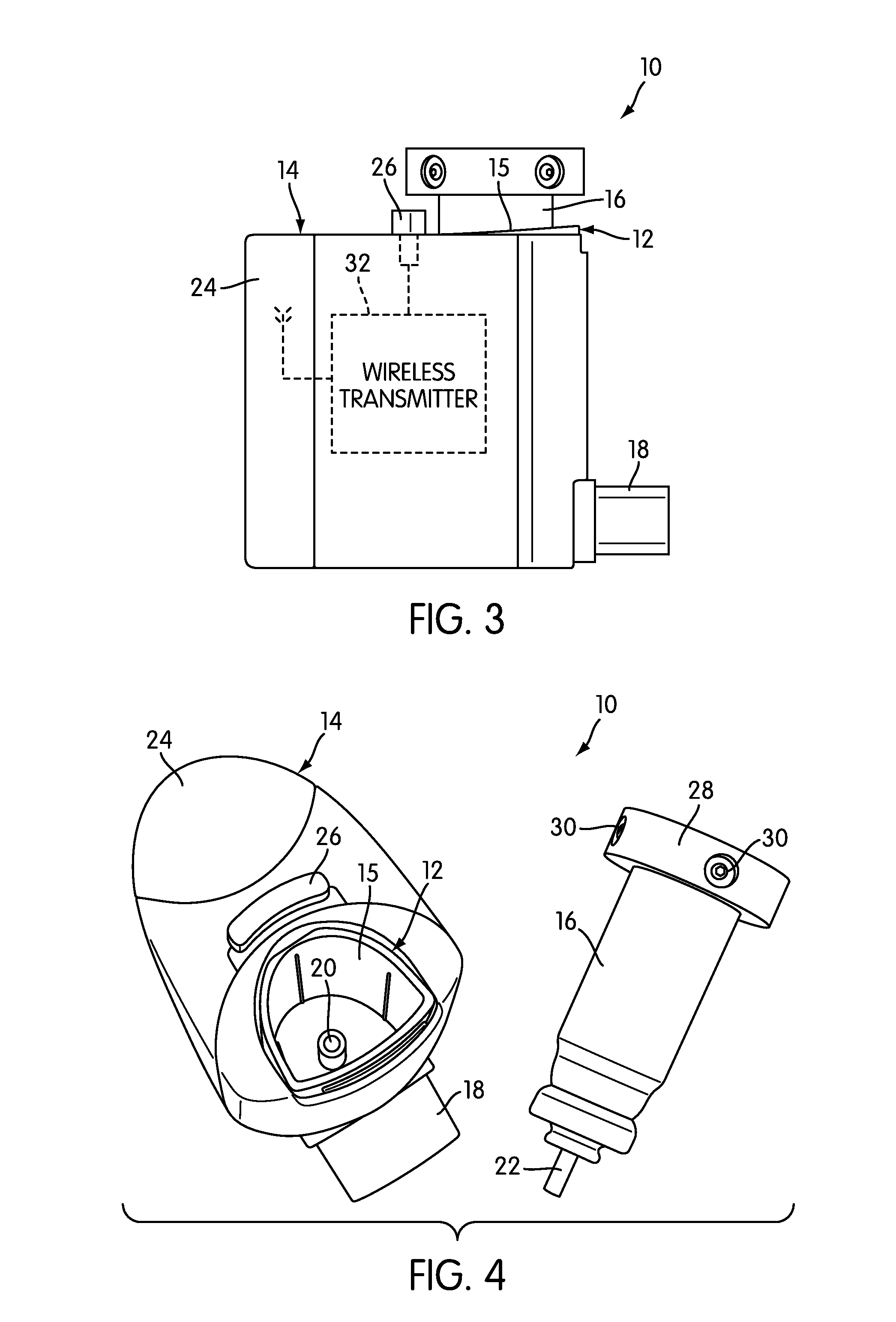
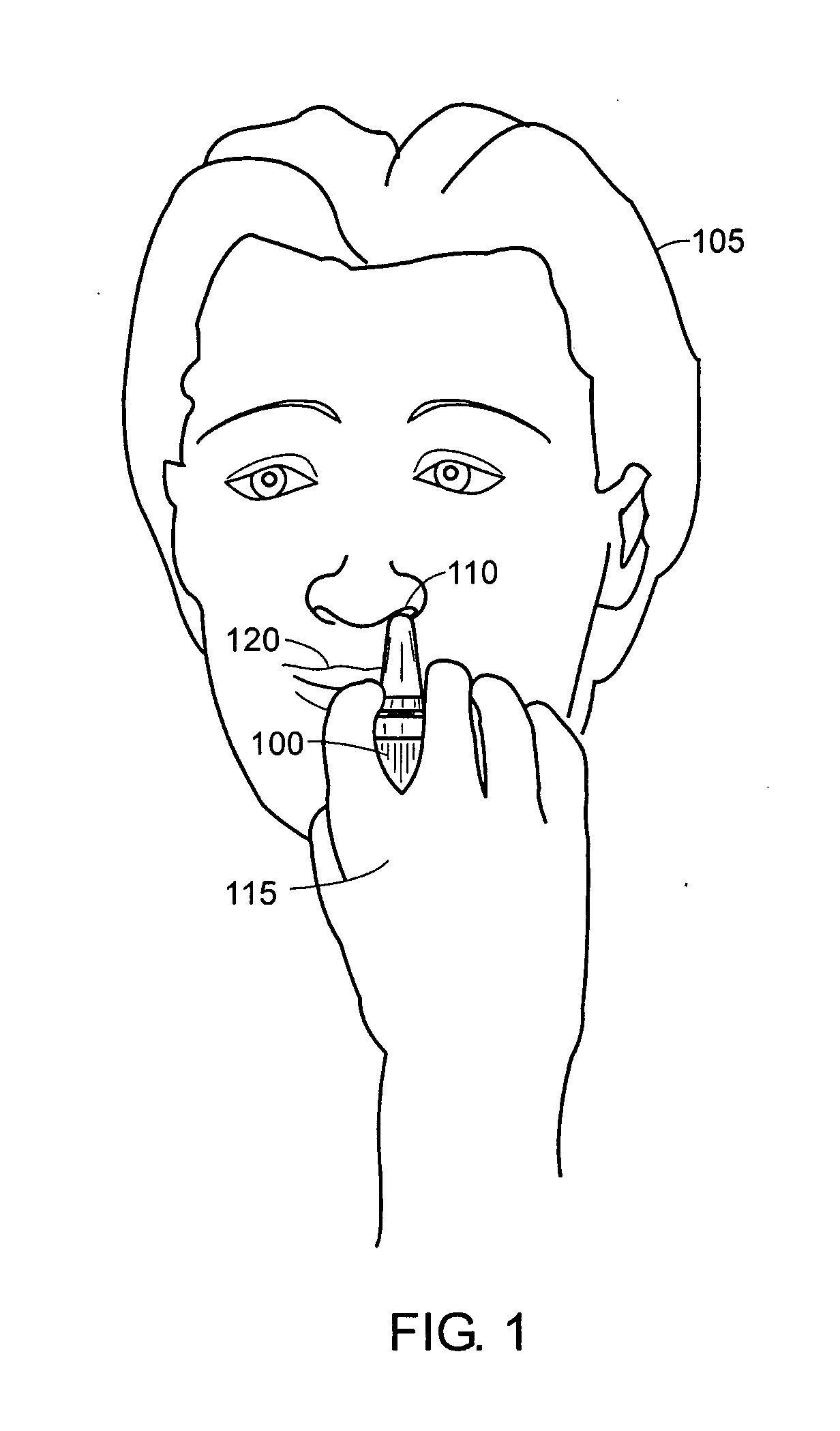
Device and method to monitor, track, map, and analyze usage of metered-dose inhalers in real-time
ActiveUS20090194104A1Easy to manageEasy to identifyRespiratorsDrug and medicationsClinical careComputer science
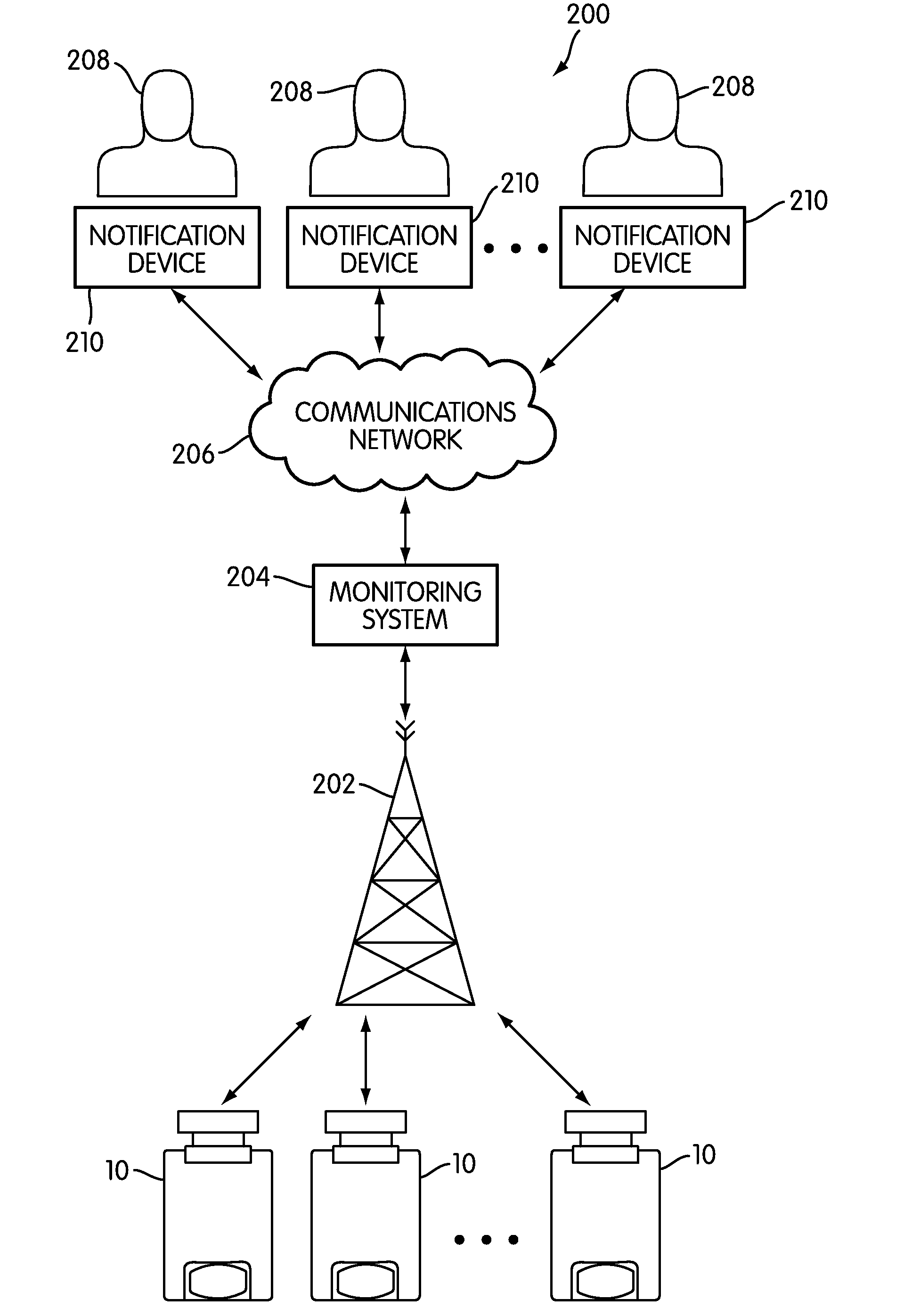
A system and method for accurately and reliably determining and recording the time, date and location where a medication is used, and a system and method for transmitting, collecting, and using that data to improve clinical care, disease management, and public health surveillance. The device allows information concerning drug usage, including the time, date and location of use, to be transmitted to a remote network computer system so that the data can be evaluated to determine current impairment and future risk, and to identify changes in the frequency, timing, or location of medication usage indicative of change in disease control or management, and to examine spatial, temporal or demographic patterns of medication use or absence of use among individuals and groups. In addition, the device may further be configured to transmit signals indicative of its status, condition or other results to the remote network computer system.
Owner:RECIPROCAL LABS CORP D B A PROPELLER HEALTH
Engineered particles and methods of use
InactiveUS7306787B2Reduce deliveryLess attractivePowder deliveryOrganic active ingredientsNebulizerActive agent
Engineered particles are provided may be used for the delivery of a bioactive agent to the respiratory tract of a patient. The particles may be used in the form of dry powders or in the form of stabilized dispersions comprising a nonaqueous continuous phase. In particularly preferred embodiments the particles may be used in conjunction with an inhalation device such as a dry powder inhaler, metered dose inhaler or a nebulizer.
Owner:NOVARTIS AG
Multipurpose therapeutic face mask
A therapeutic face mask comprises a face-engaging portion and a single connector having a mask-engaging end and a single treatment-receiving end which has a single attachment mounting for detachably sealingly receiving a treatment attachment, such as an oxygen reservoir bag or a nebulizer. A one-way inhalation valve in the connector permits fluid flow from the treatment-receiving end to the mask-engaging end during inhalation and inhibits fluid flow in the other direction. The mask also includes a valve-governed exhalation port and an anti-asphyxia valve assembly configured to permit fluid flow from ambient to the face-engaging portion during inhalation only when inspiratory effort during inhalation exceeds fluid flow to the treatment-receiving end of the connector. Also provided is an oxygen reservoir bag having a neck shaped for removable coupling to a mating connector of a therapeutic face mask. An oxygen reservoir bag may have a metered-dose inhaler port defined in its neck.
Owner:FLYNN SR STEPHEN DONALD
Breath actuated inhaler
InactiveUS7219664B2Overcome deficienciesReduce deliveryMedical devicesSpray nozzlesMedicineInhalation
A breath actuated metered dose inhaler including a housing, a mouthpiece positioned at one end of the housing, and a mechanical release mechanism positioned at another end of the housing. The release mechanism is triggered by a diaphragm and the inhaler is configured such that the air inhalation pathway is unimpeded by the release mechanism.
Owner:ABBOTT RESPIRATORY LLC
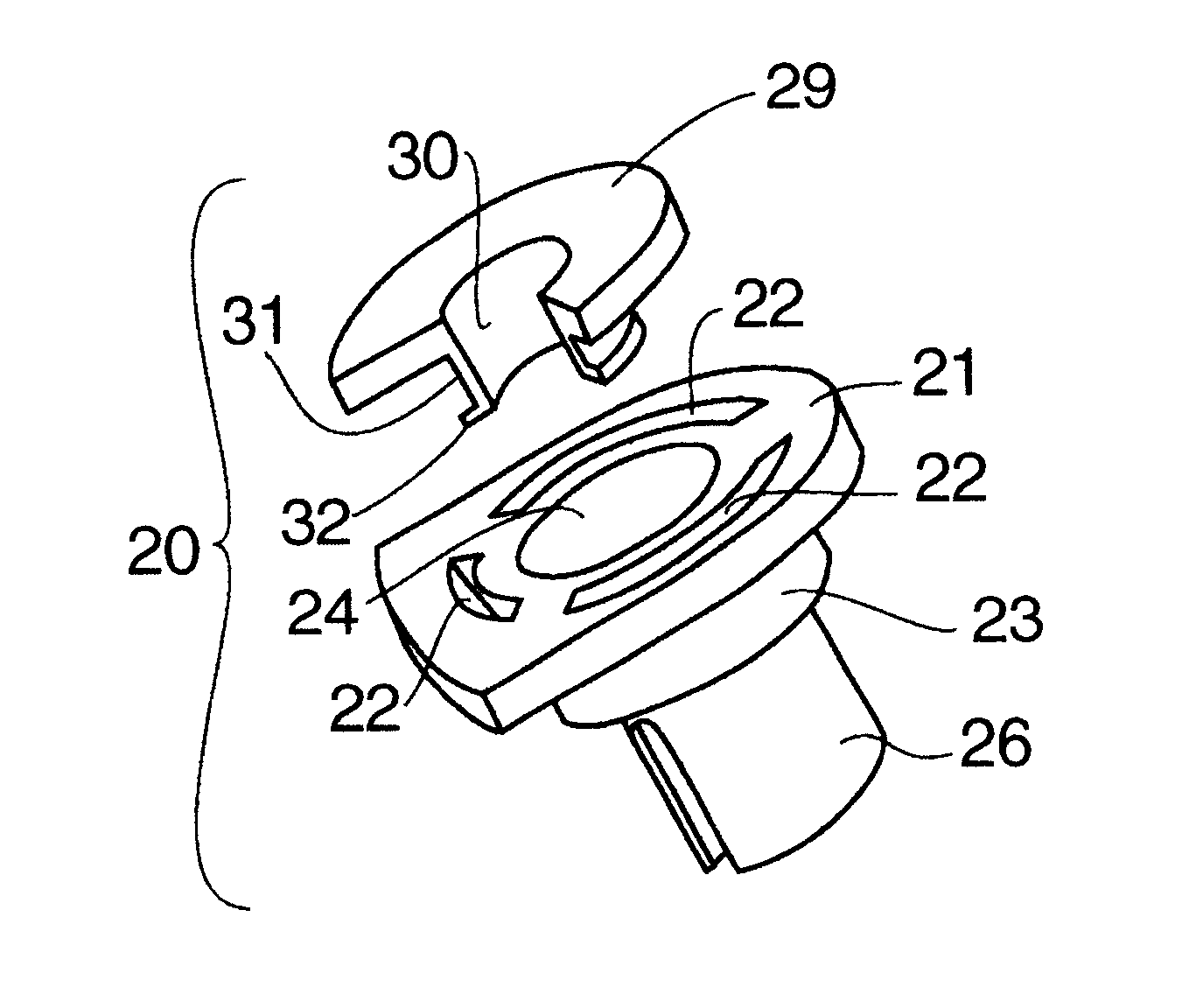
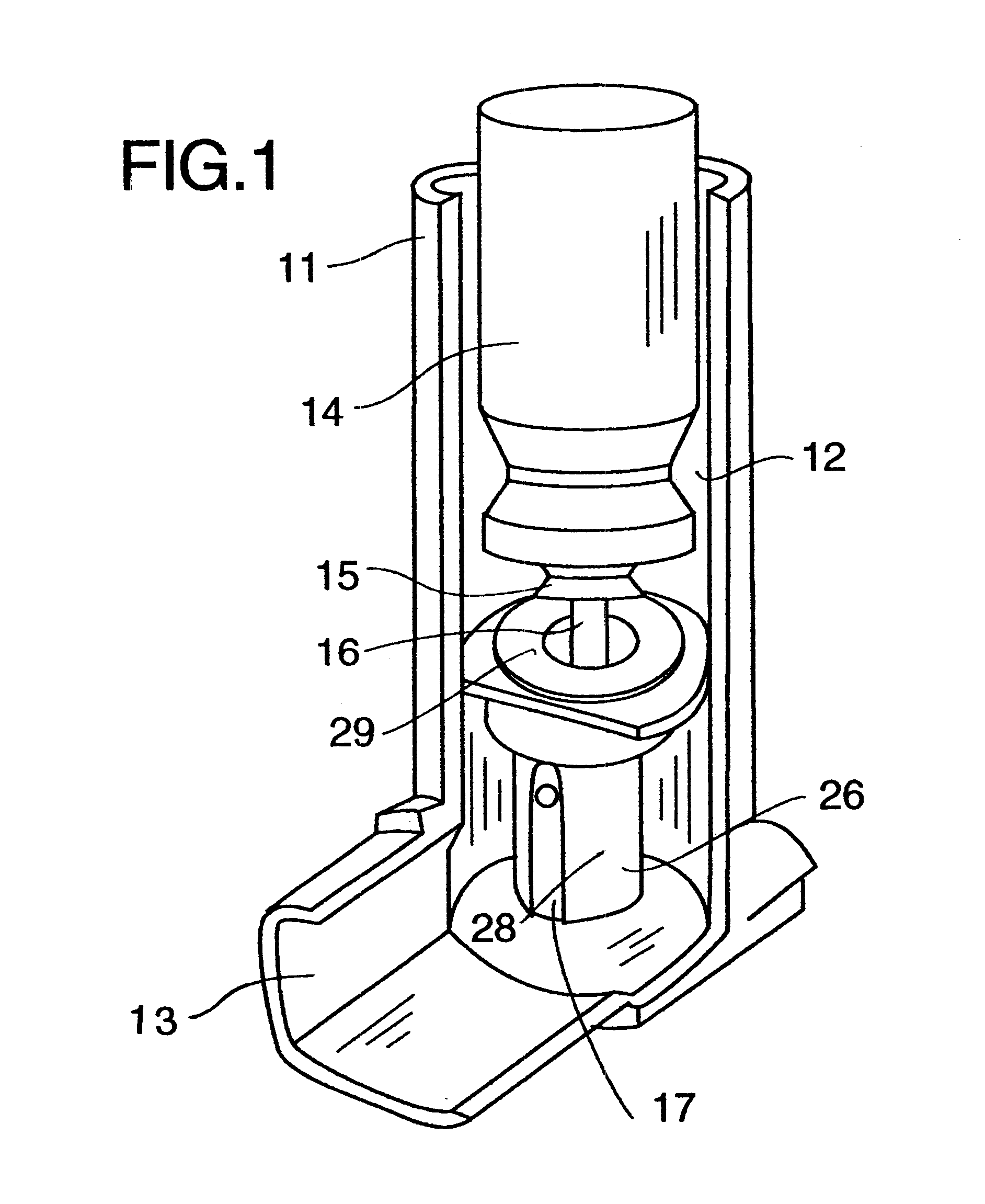
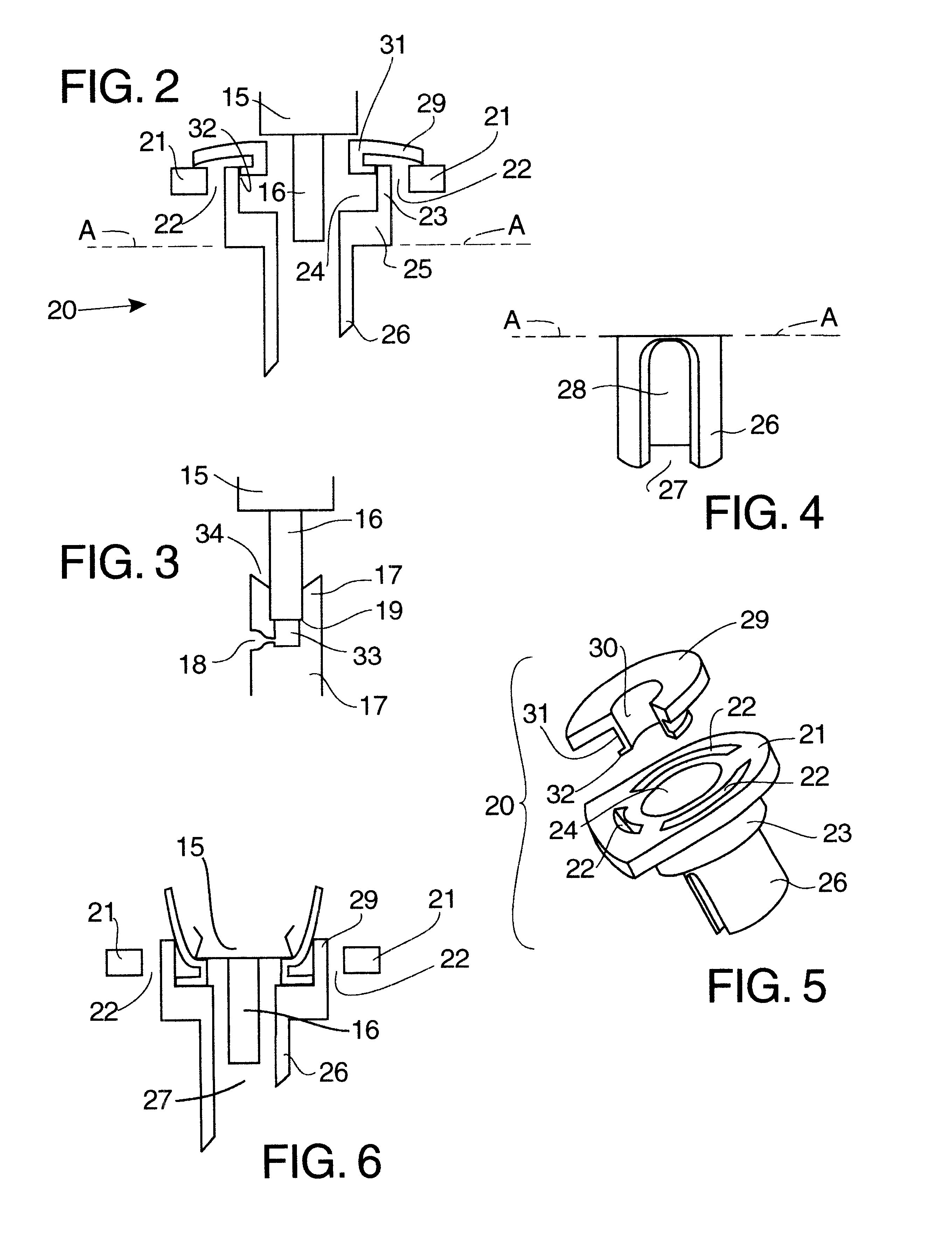
Dose counting in metered dose inhaler
A counter for indicating the number of doses left in a canister that is suitable for use in a metered dose inhaler. The counter is affixed to the canister and includes a module for providing an indication of the number of doses left in the canister and a triggering mechanism for updating the indication in response to activation of the inhaler.
Owner:GENOVA PERRY +2
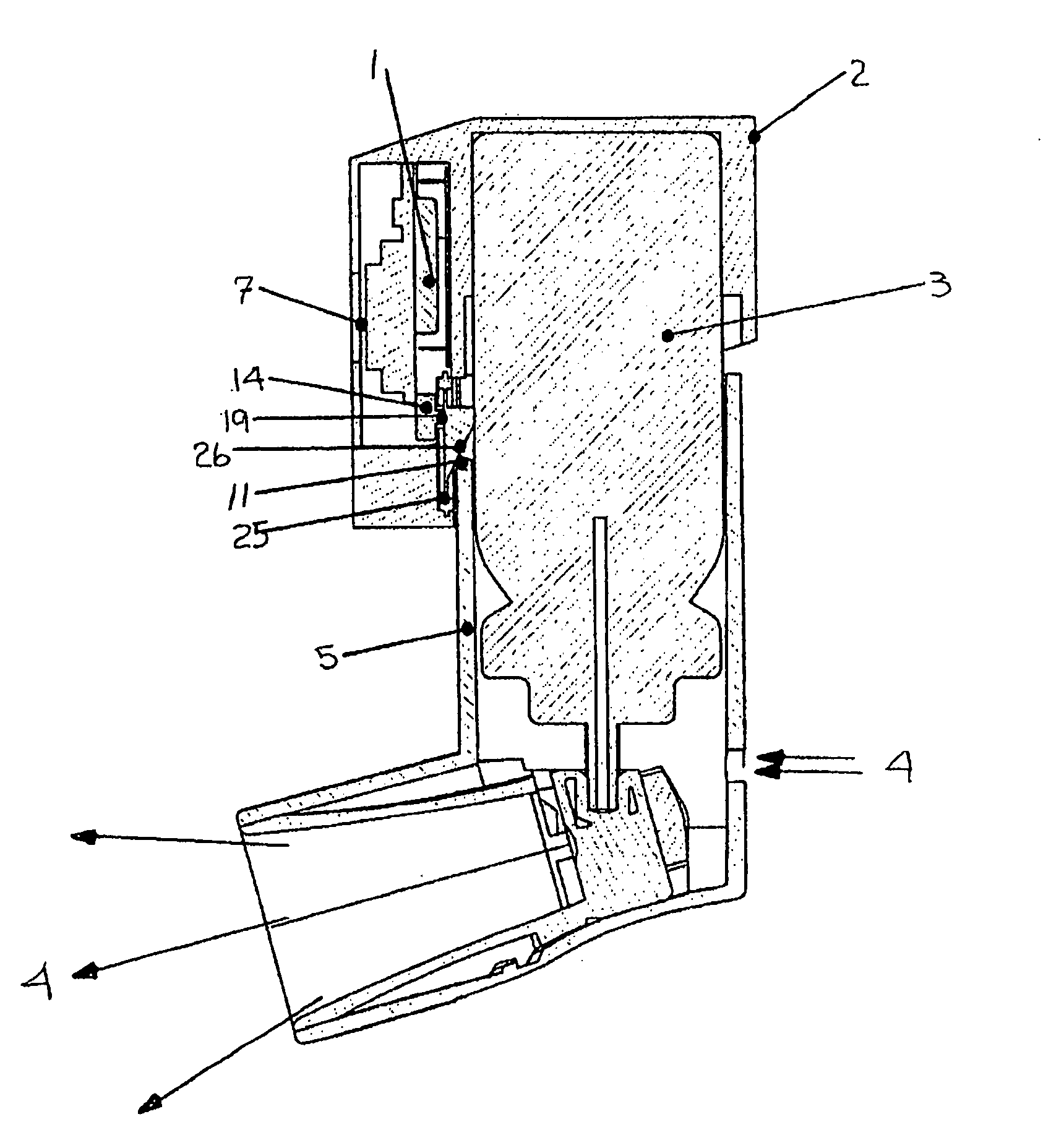
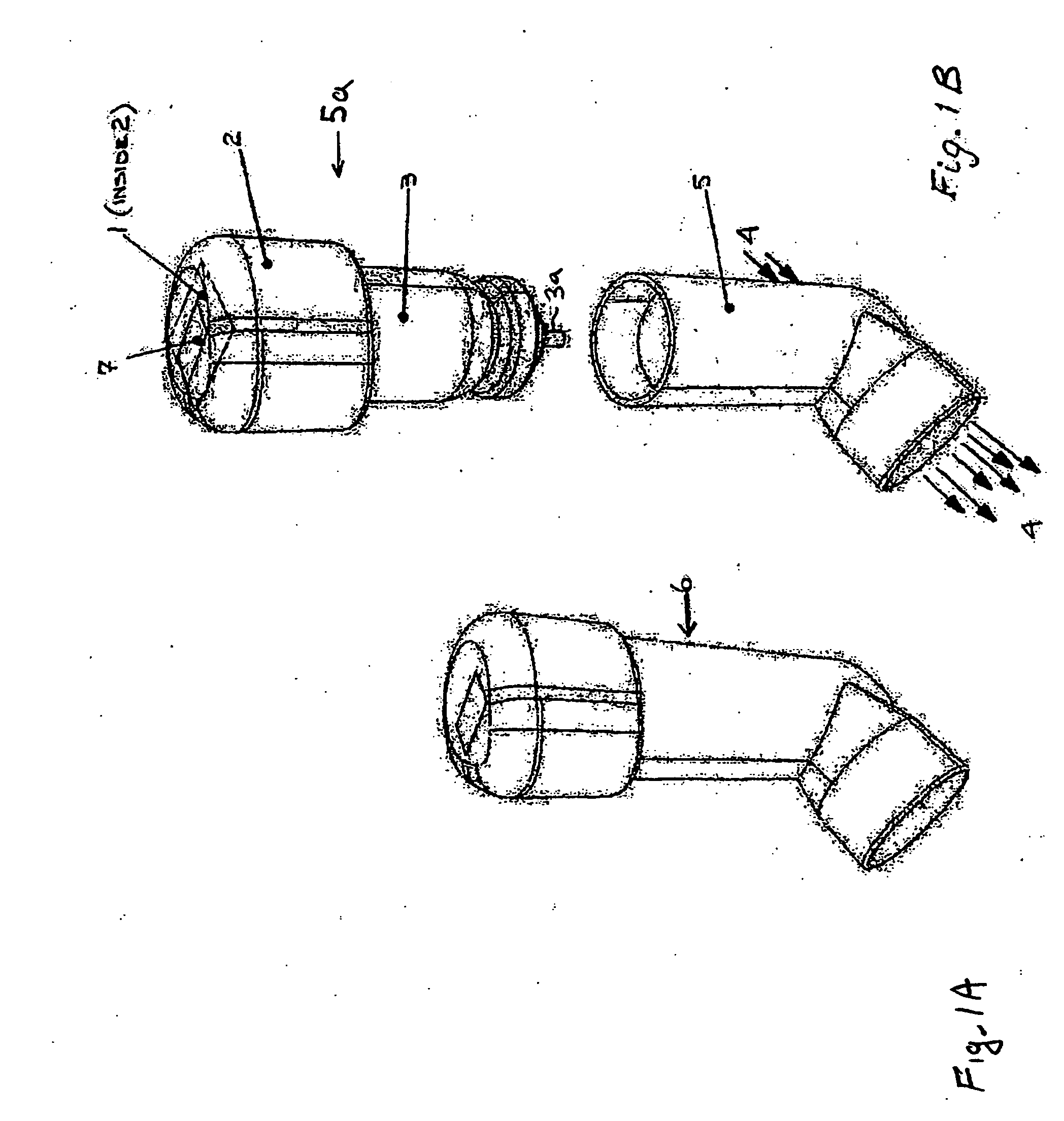
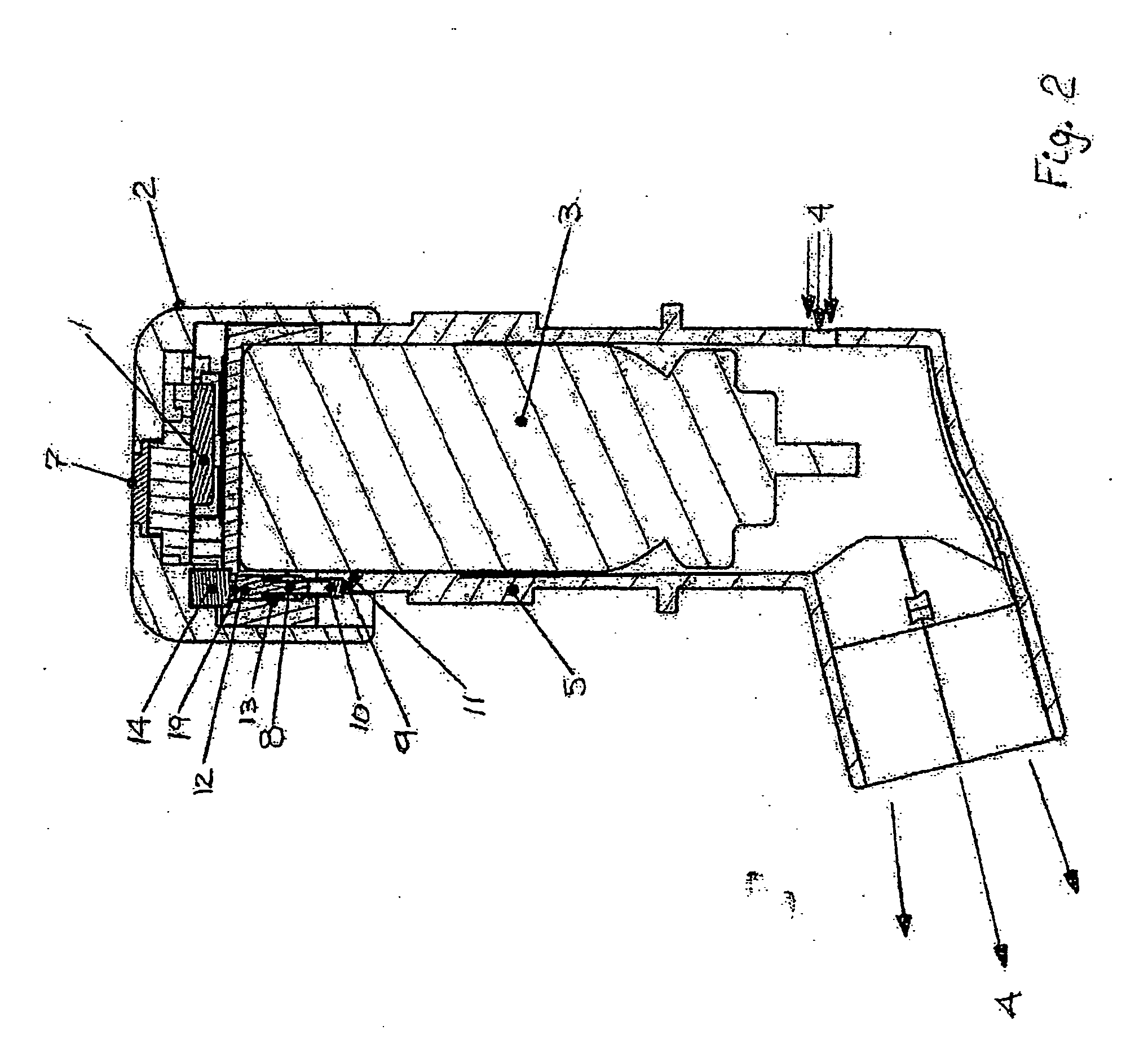
Pharmaceutical solution formulations for pressurised metered dose inhalers
A method for delivering two or more active drug substances to the lungs by inhalation from a single pressurized metered dose inhaler product, said inhaler containing a HFA / cosolvent based solution formulation wherein all the active drug substances are fully dissolved in the formulation.
Owner:CHIESI FARM SPA
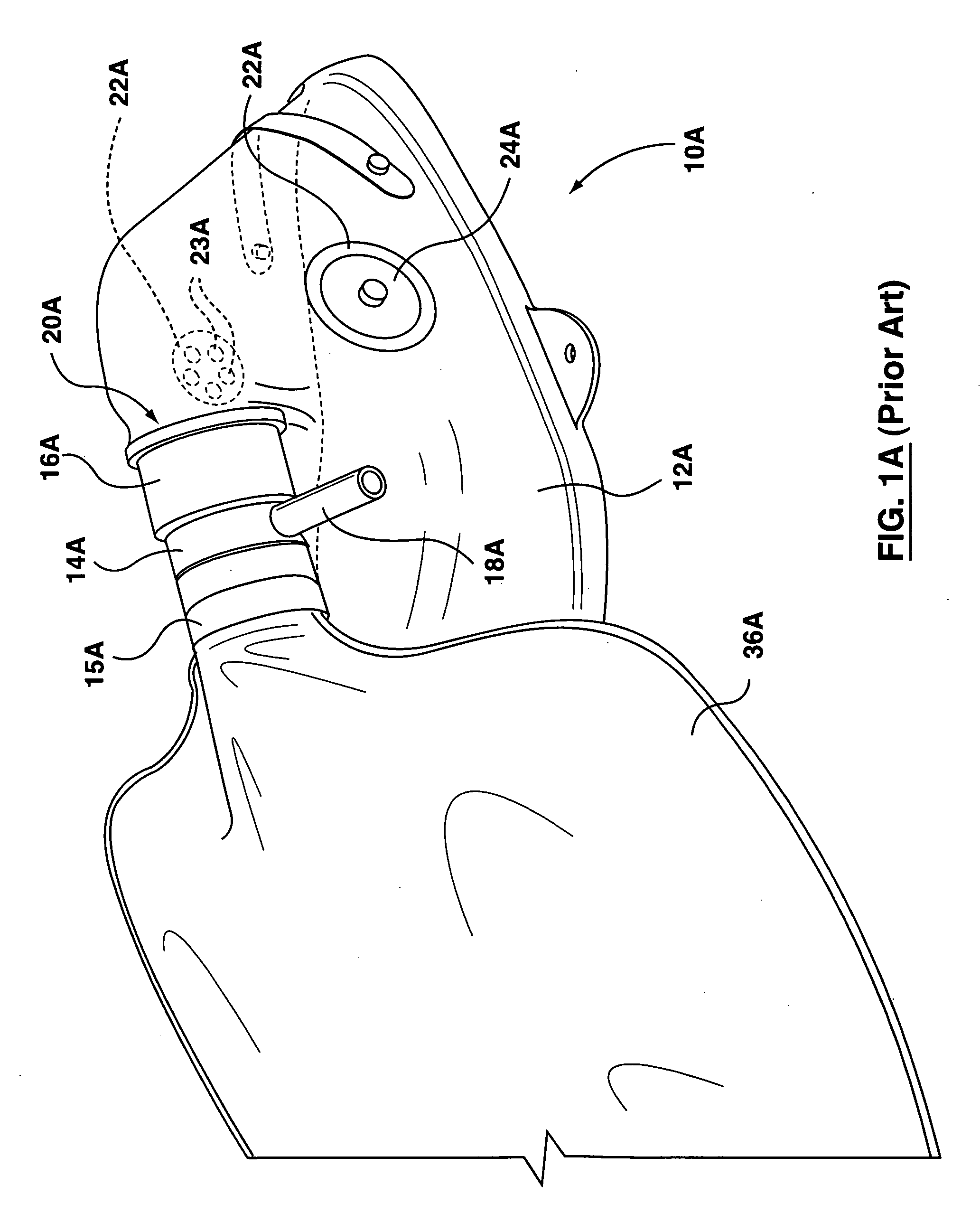
Inhalation therapy assembly and method
InactiveUS20020069870A1Conveniently and easily allowChemical protectionLighting and heating apparatusDose deliveryInhalation
The inhalation therapy assembly and method of use described herein increases the efficiency of metered dose inhalers by allowing delivery of the doses to a collapsible reservoir which can be manually pumped, ensuring that medicants contained therein are properly and completely delivered to the patient. Terminal and proximal valves of the one-way diaphragm type allow flow of the aerosol medicants while preventing improper expulsion. An exhalation valve is adjustable to ensure the patient exhales suitably to permit proper medicant absorption. A conventional metered dosage inhaler having an approved FDA canister provides proper dosage to the patient and is joined to the collapsible reservoir by a connector having a plurality of apertures for receiving the MDI and an accessory T-fitting.
Owner:FARMER MEDICAL
Inhalation therapy assembly and method
InactiveUS6494202B2Conveniently and easily allowBreathing masksRespiratory masksInhalationDose delivery
The inhalation therapy assembly and method of use described herein increases the efficiency of metered dose inhalers by allowing delivery of the doses to a collapsible reservoir which can be manually pumped, ensuring that medicants contained therein are properly and completely delivered to the patient. Terminal and proximal valves of the one-way diaphragm type allow flow of the aerosol medicants while preventing improper expulsion. An exhalation valve is adjustable to ensure the patient expires suitably to permit proper medicant absorption.
Owner:FARMER MEDICAL
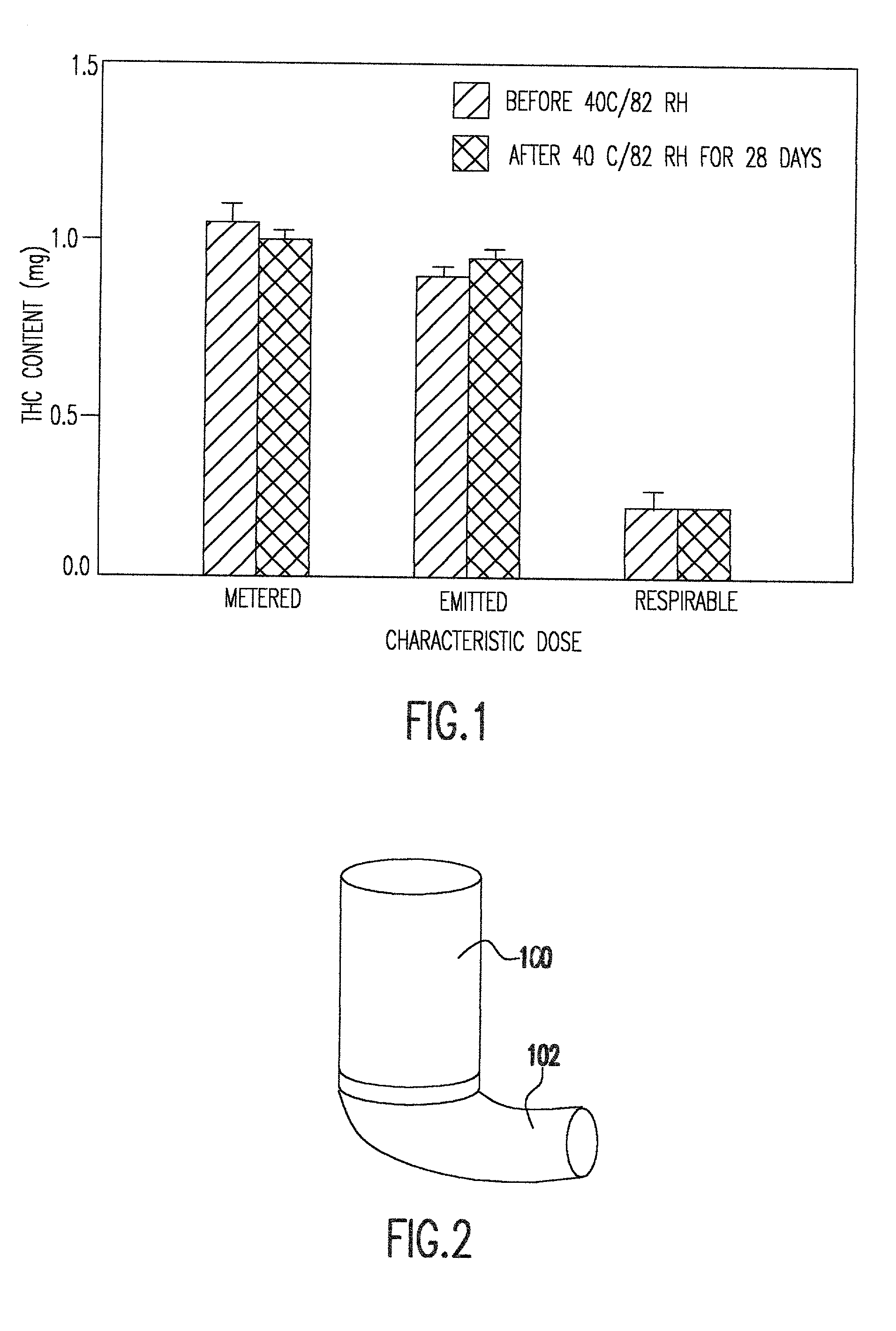
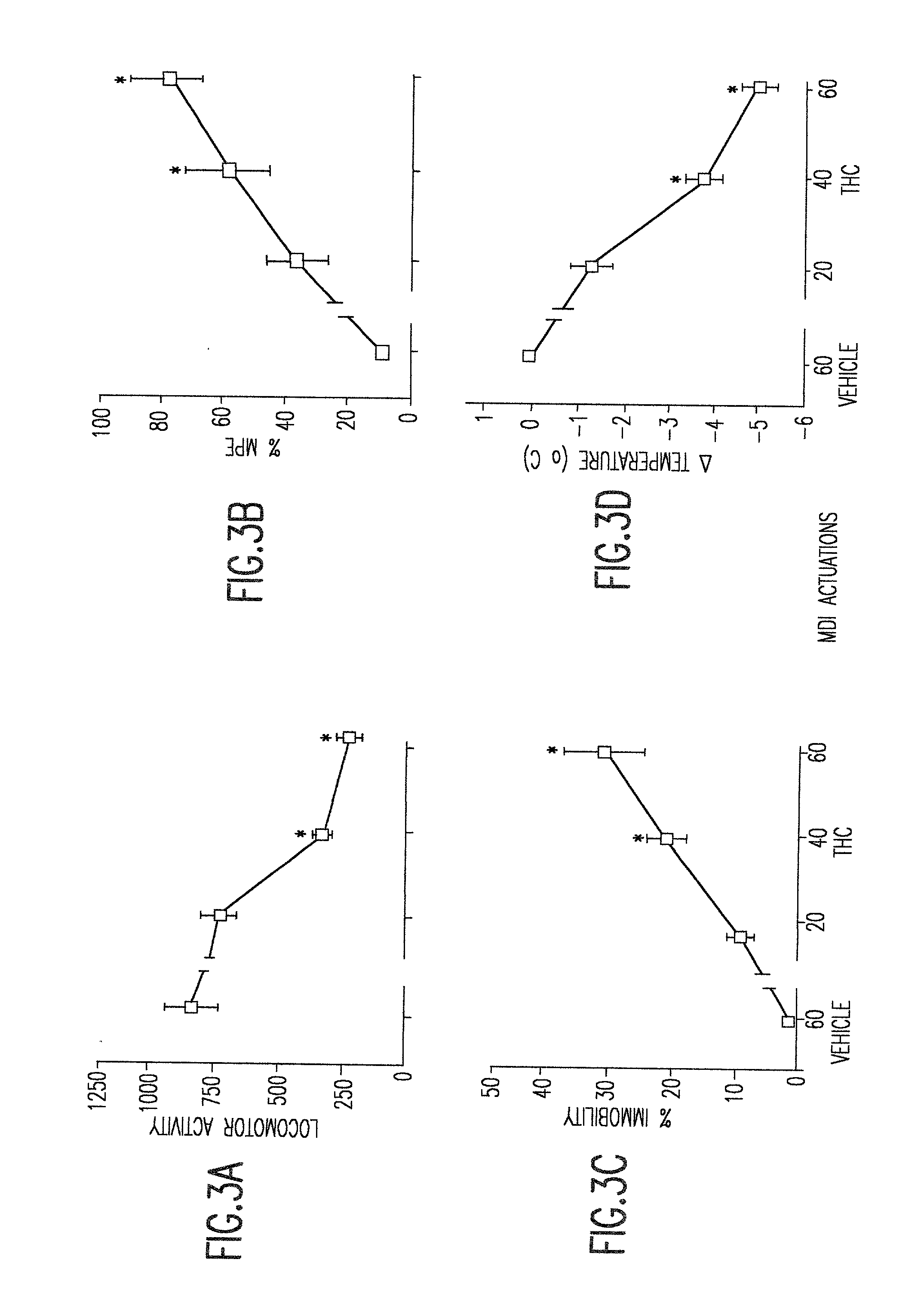
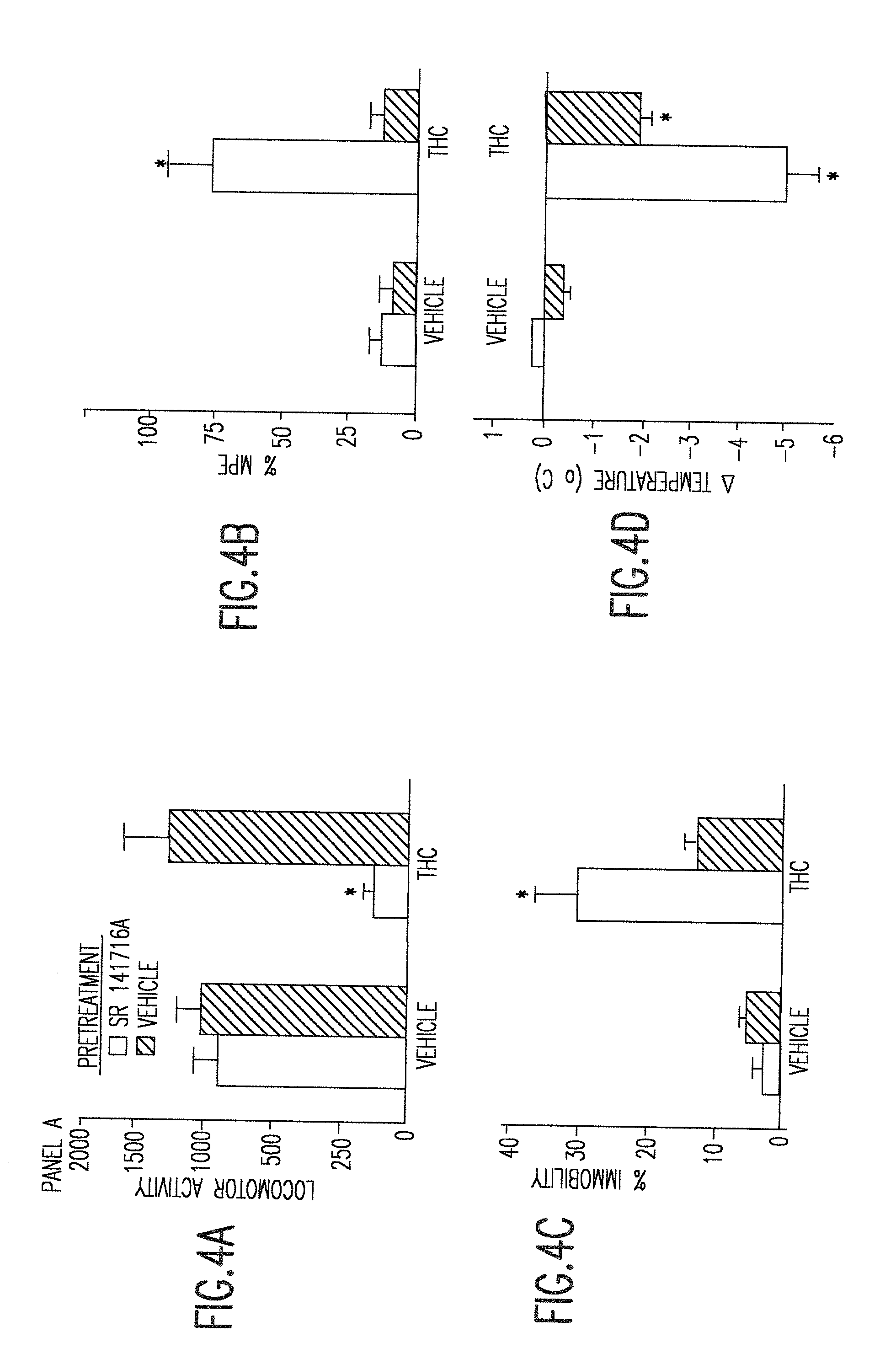
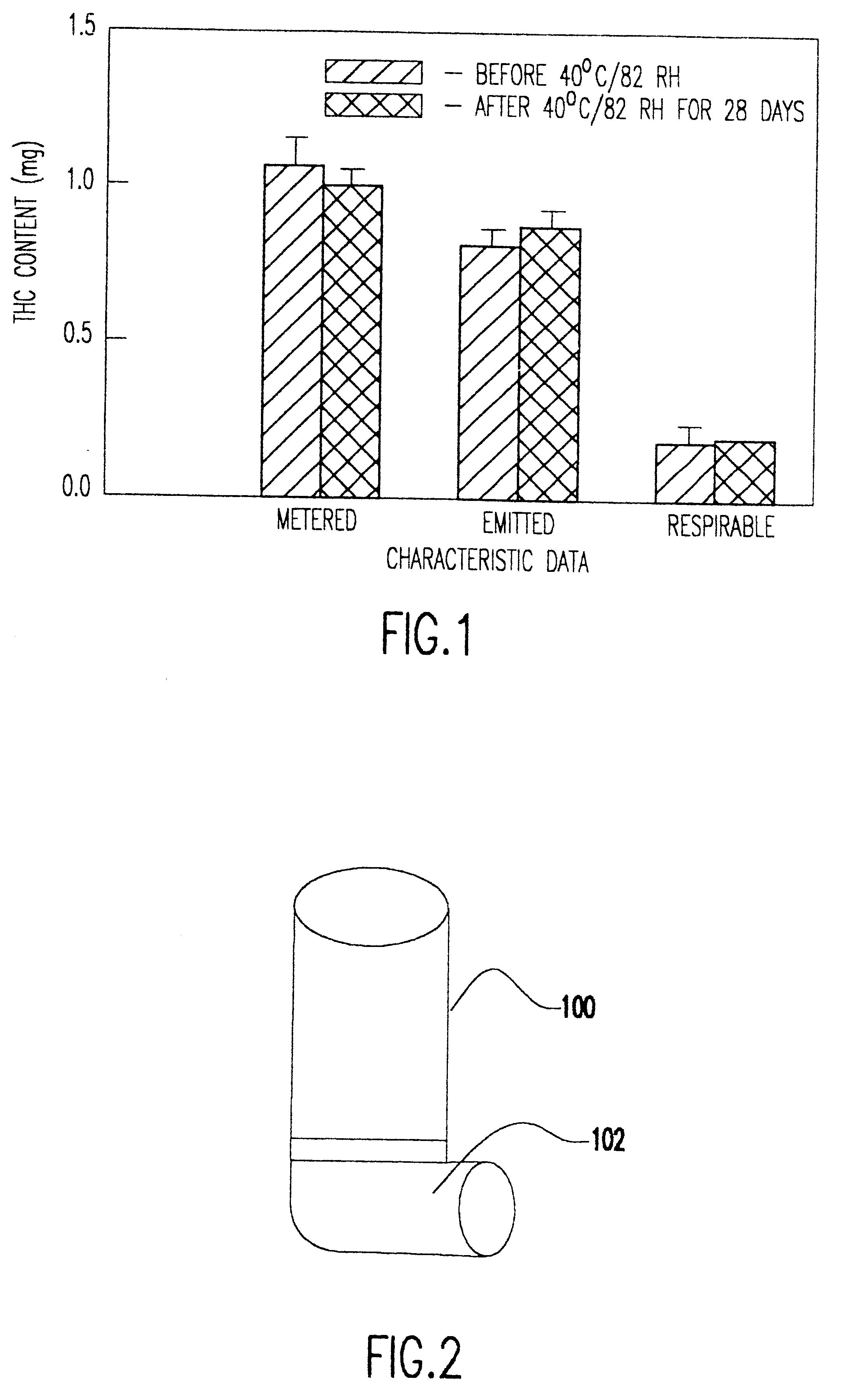
Delta9 tetrahydrocannabinol (Delta9 THC) solution metered dose inhalers and methods of use
The present invention provides therapeutic formulations for solutions of DELTA9-tetrahydrocannabinol (DELTA9 THC) to be delivered by metered dose inhalers. The formulations, which use non-CFC propellants, provide a stable aerosol-deliverable source of DELTA9 THC for the treatment of various medical conditions, such as: nausea and vomiting associated with chemotherapy-muscle spasticity; pain; anorexia associated with AIDS wasting syndrome, epilepsy; glaucoma; bronchial asthma; and mood disorders.
Owner:VIRGINIA COMMONWEALTH UNIV
DELTA9 Tetrahydrocannabinol (DELTA9 THC) solution metered dose inhaler
The present invention provides therapeutic formulations for solutions of DELTA9-tetrahydrocannabinol (DELTA9 THC) to be delivered by metered dose inhalers. The formulations, which utilize non-CFC propellants, provide a stable aerosol-deliverable source of DELTA9 THC for the treatment of various medical conditions, such as: nausea and vomiting associated with chemotherapy; muscle spasticity; pain; anorexia associated with AIDS wasting syndrome; epilepsy; glaucoma; bronchial asthma; and mood disorders.
Owner:VIRGINIA COMMONWEALTH UNIV
Self closing diaphragm type valve with primary peripheral and secondary central openings
A valve, shaped and sized for metered dose inhalers, consisting of a mount, a central body and top plate with peripheral air passages and central opening for medication delivery. A flexible elastic diaphragm closes the air passages and a central wiper seal on the diaphragm isolates the center opening from the air passageway. Pressure on the rim of the central opening of the diaphragm causes it to open the air passages timed with injection of medication through the central opening.
Owner:STAGE JACK W
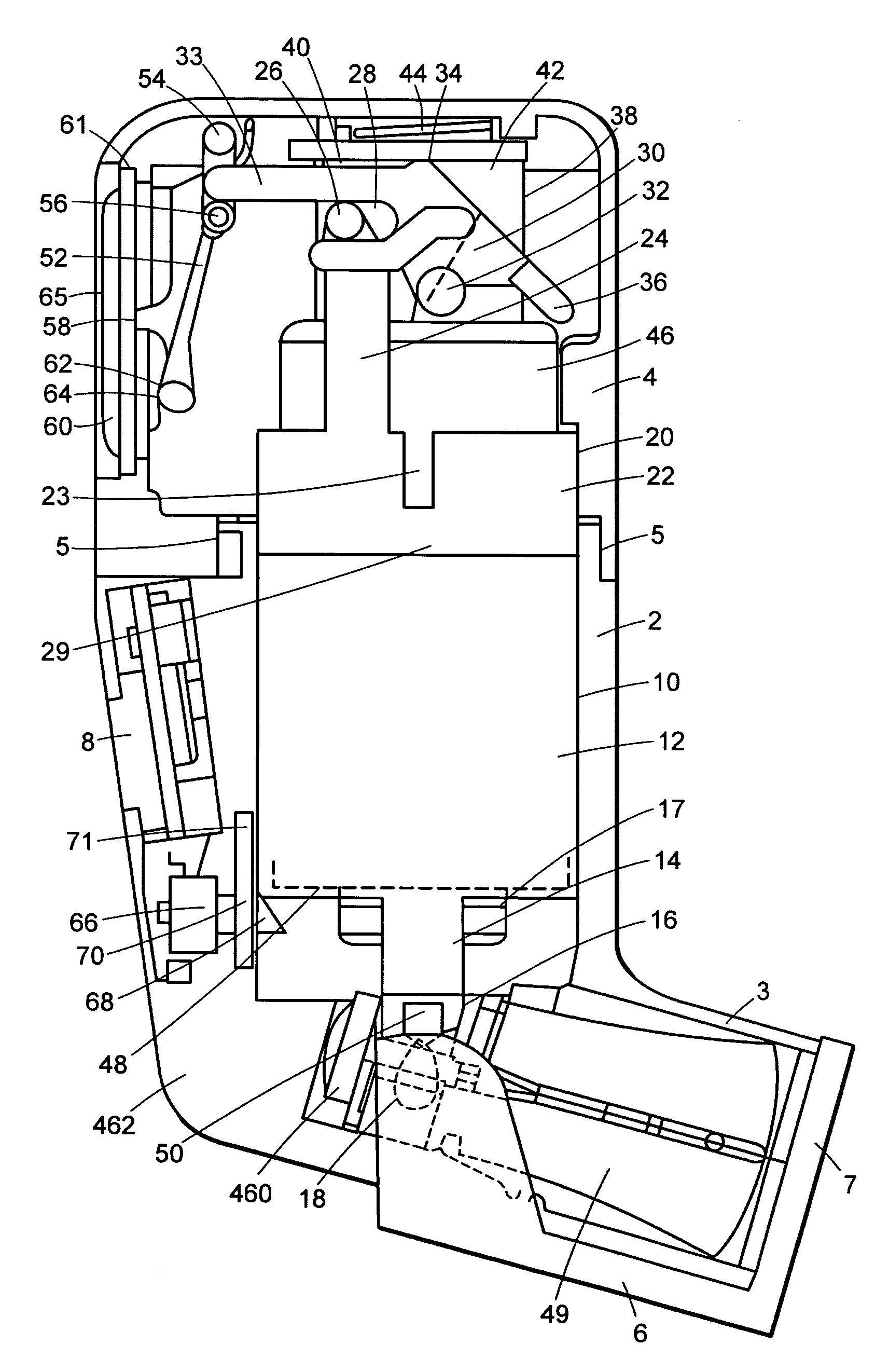
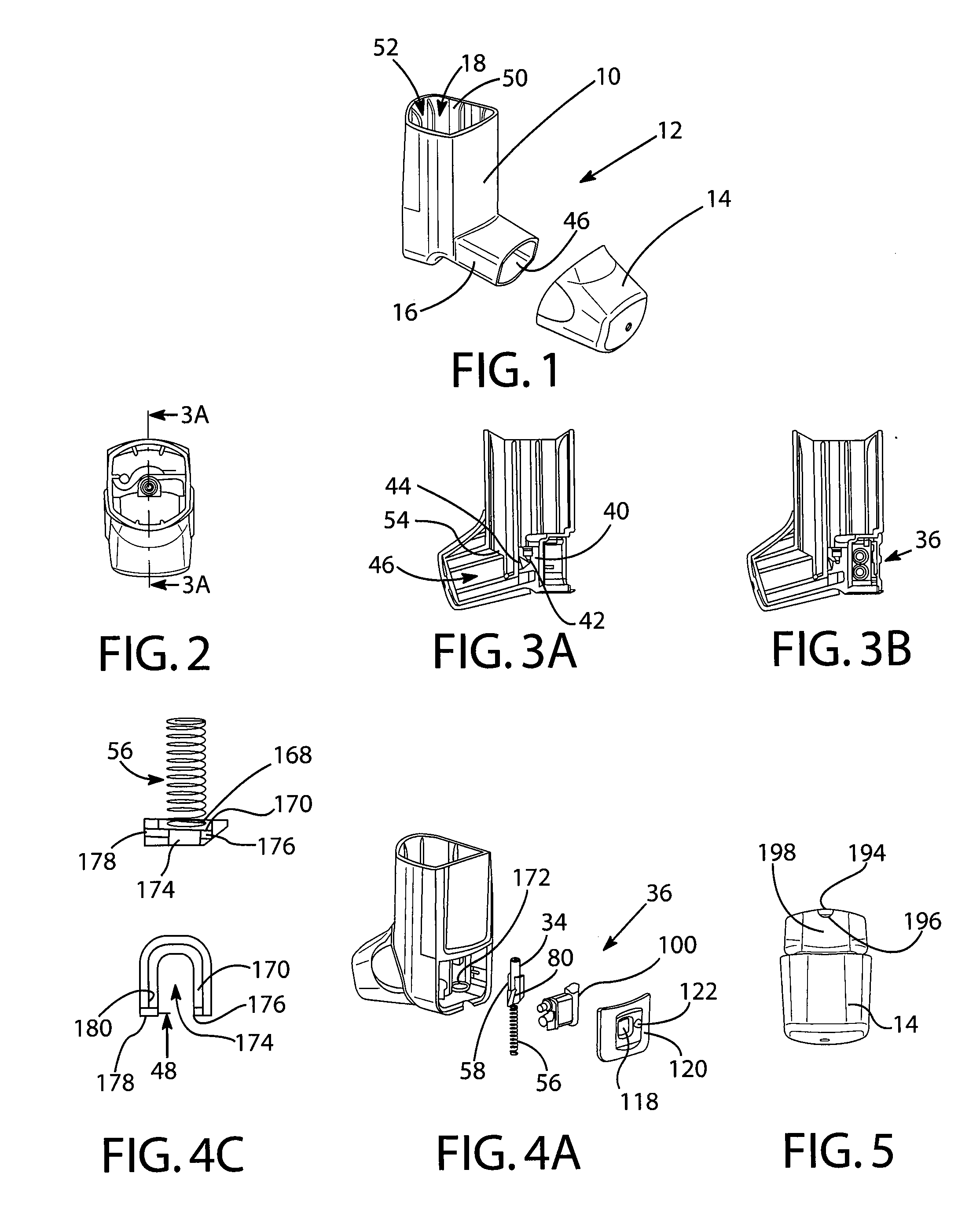
Dose counters for inhalers, inhalers and methods of assembly thereof
ActiveUS20110283997A1Prevent unwanted motionReliable countingMedical devicesLiquid transferring devicesBobbinEngineering
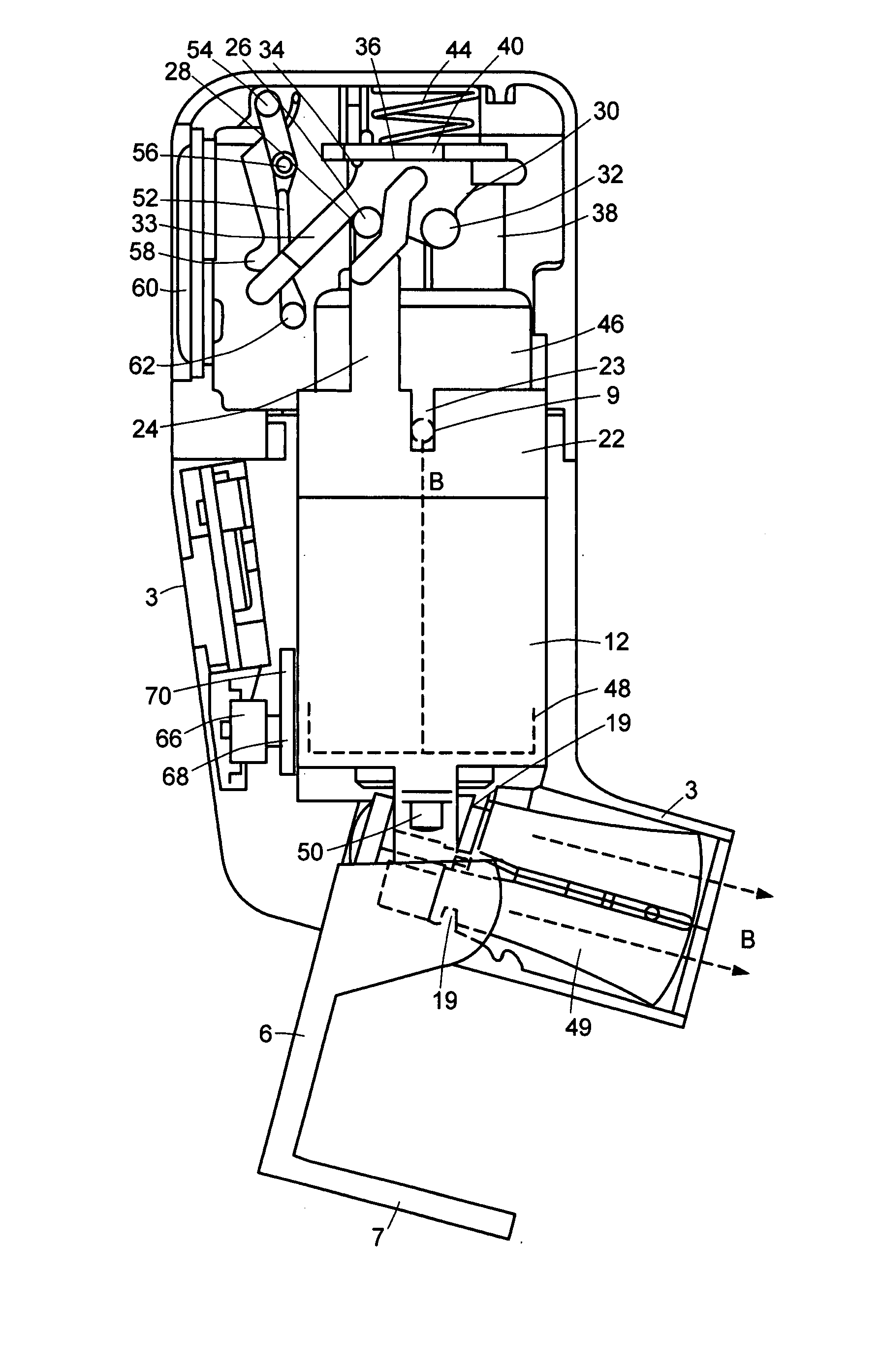
A manually operated metered dose inhaler includes a dose counter chamber including a dose display tape driven by a ratchet wheel which is driven in turn by an actuator pawl actuated by movement of a canister, the tape unwinding from a stock bobbin during use of the inhaler, a rotation regulator being provided for the stock bobbin and including a wavelike engagement surface with concavities which engage against control elements in the form of protrusions on resilient forks of a split pin thereby permitting incremental unwinding of the stock bobbin yet resisting excessive rotation if the inhaler is dropped onto a hard surface.
Owner:TEVA PHARMA IRELAND +2
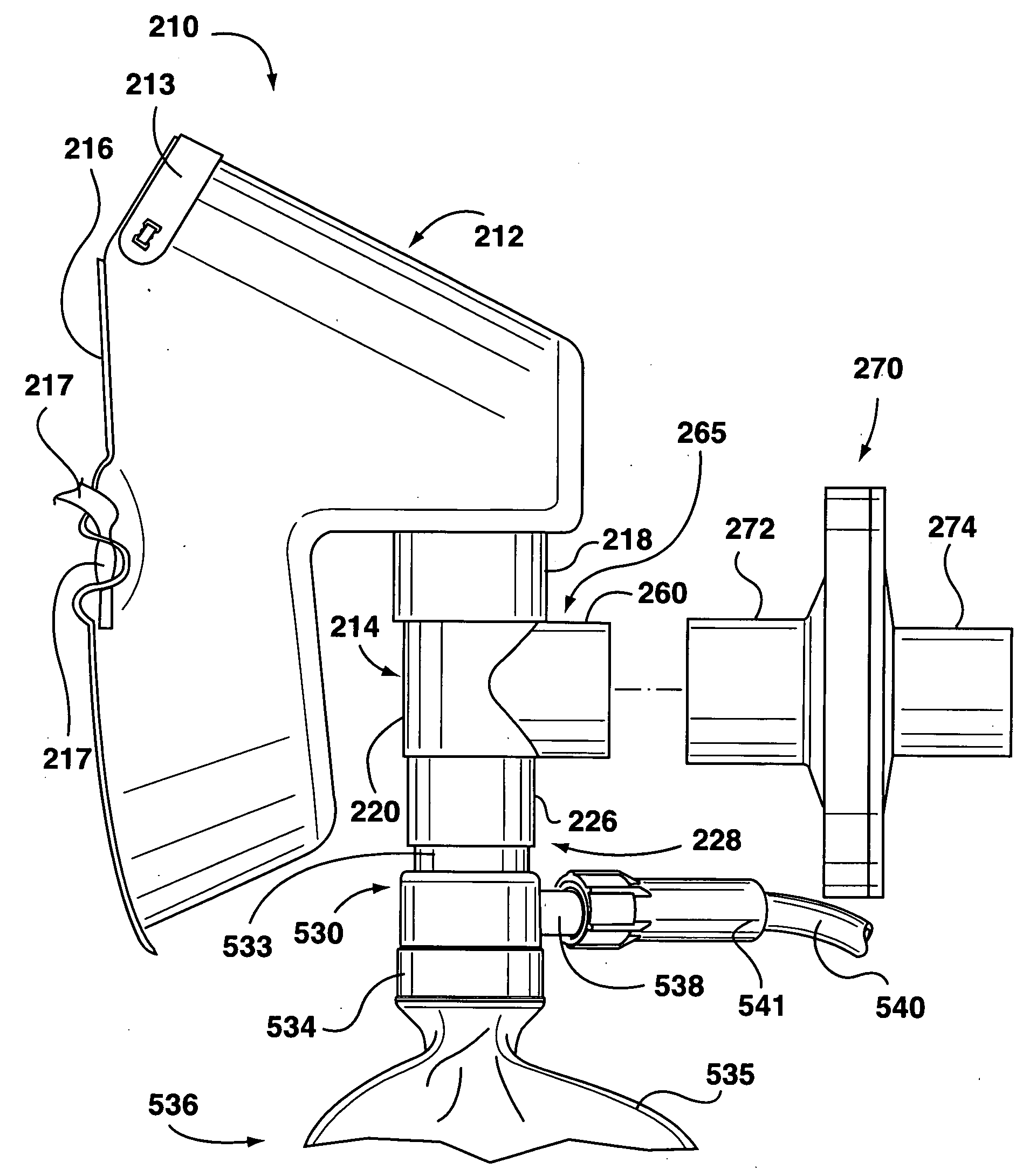
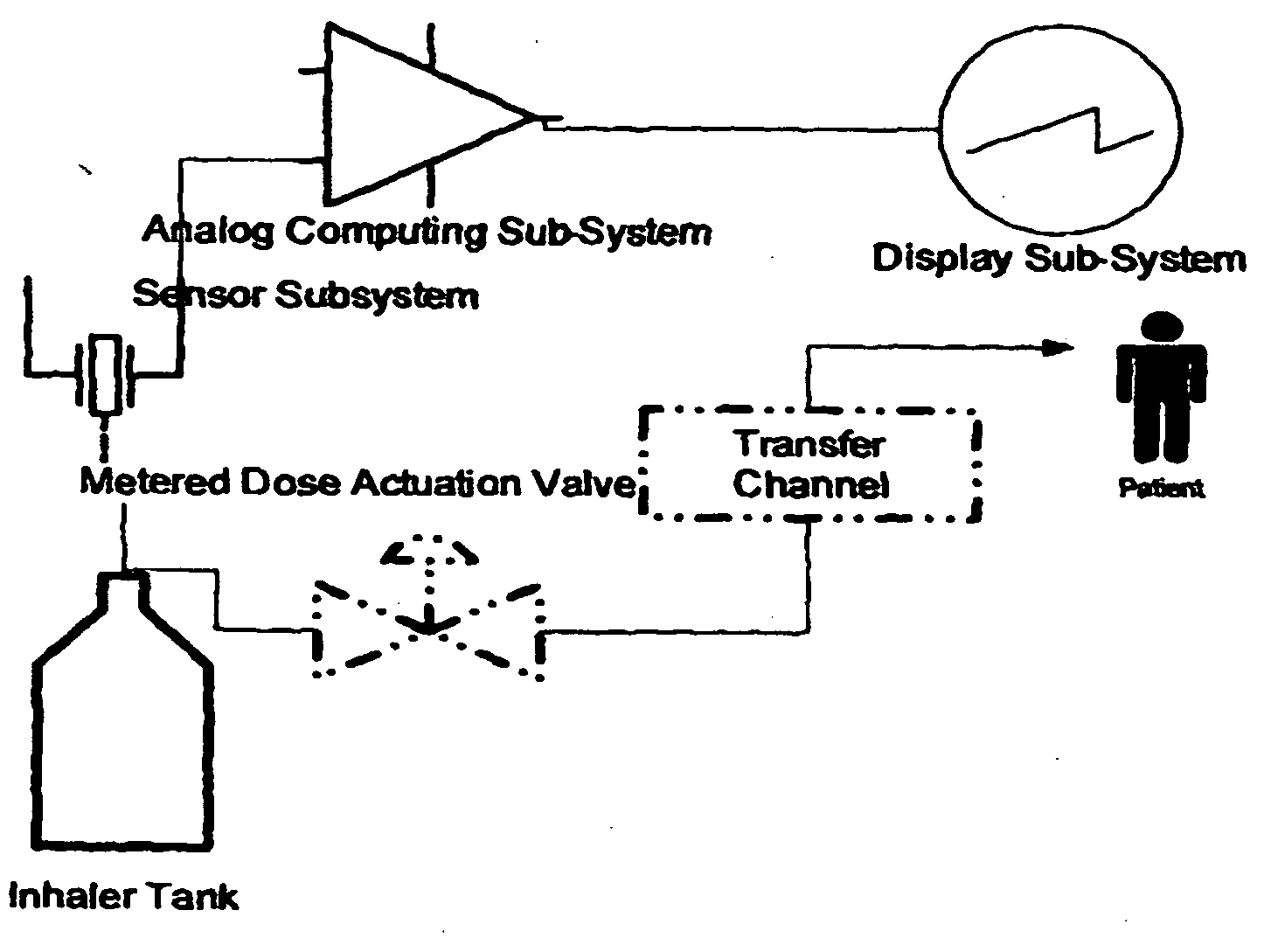
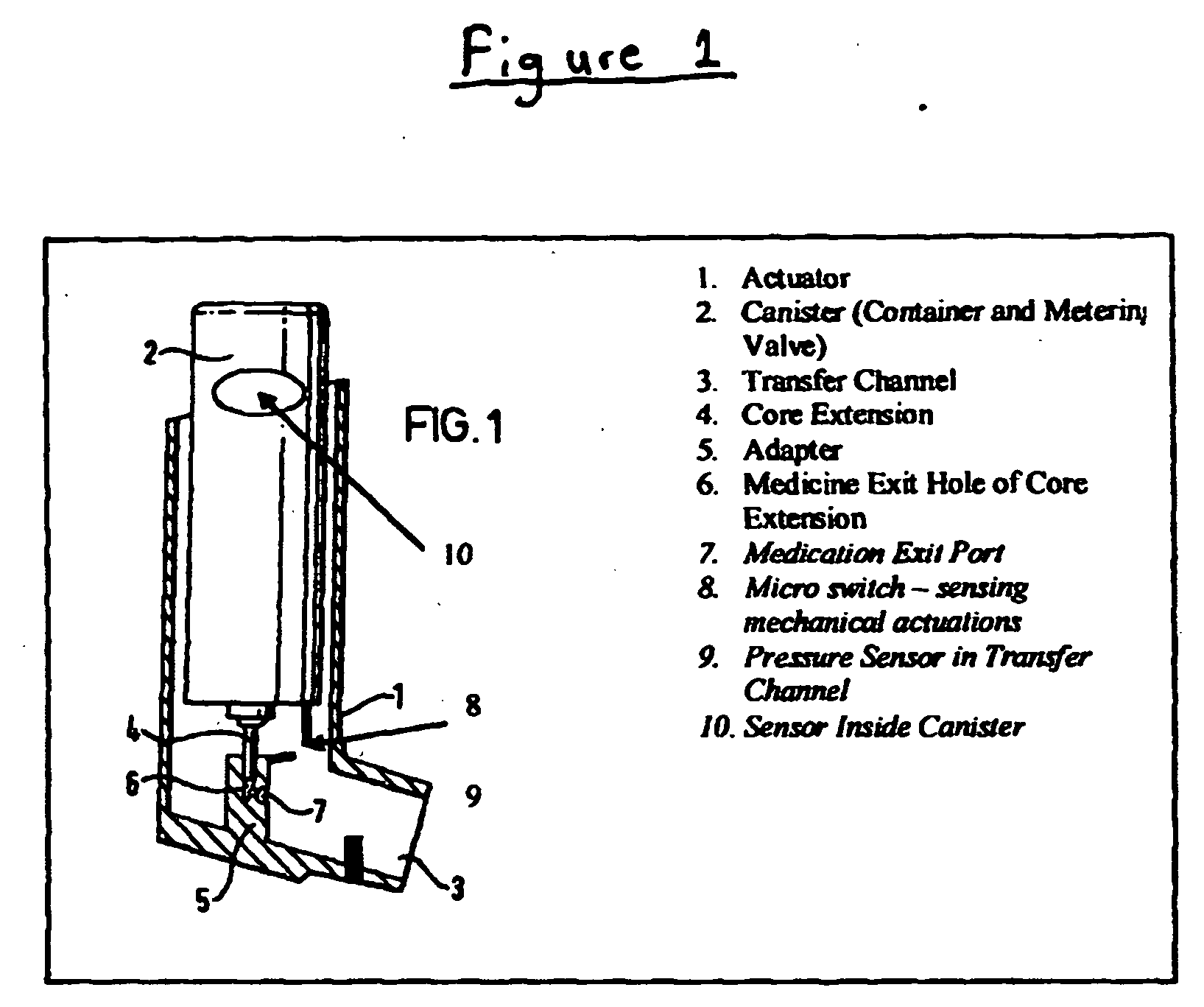
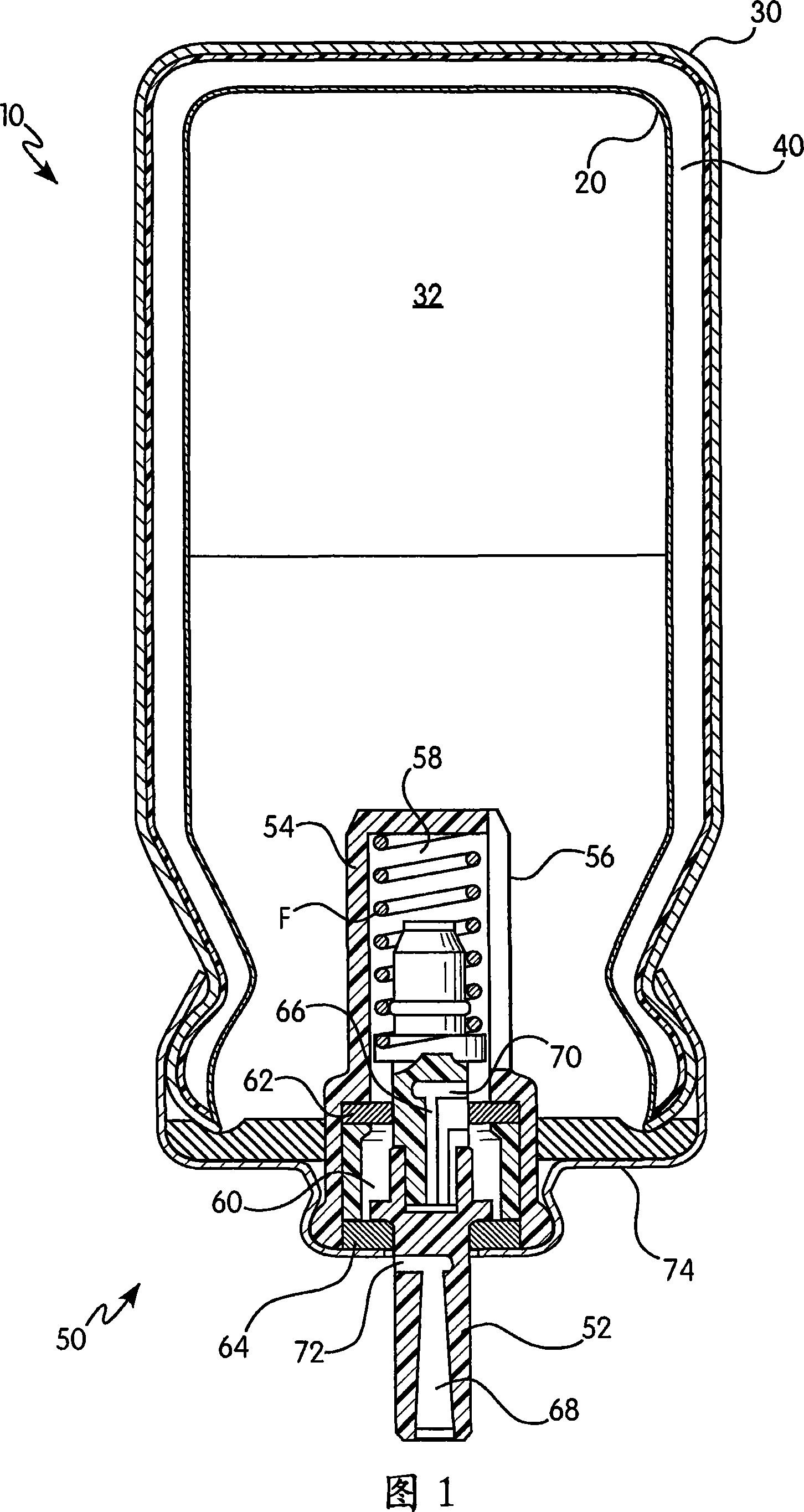
Apparatus for dispensing pressurized contents
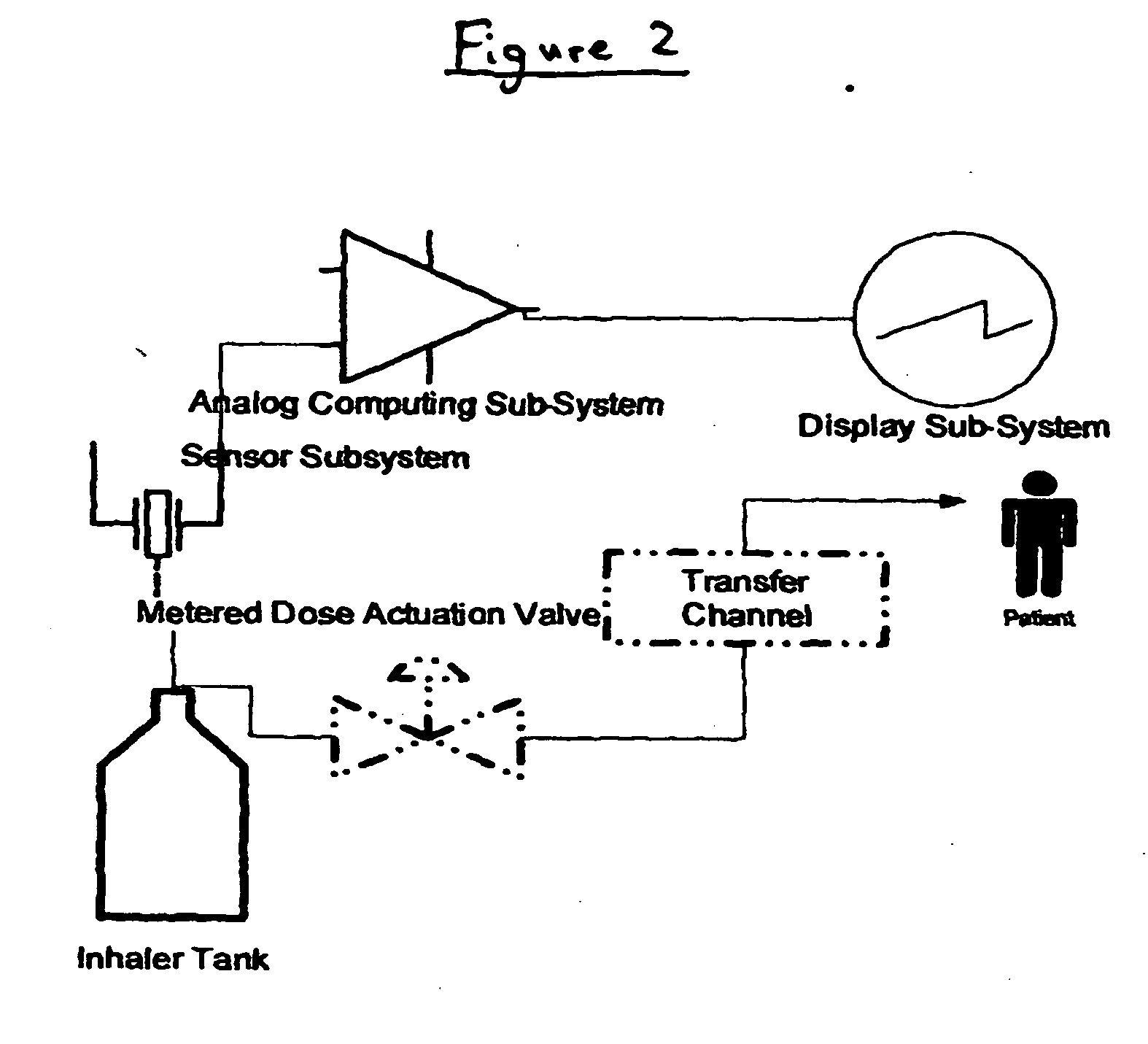
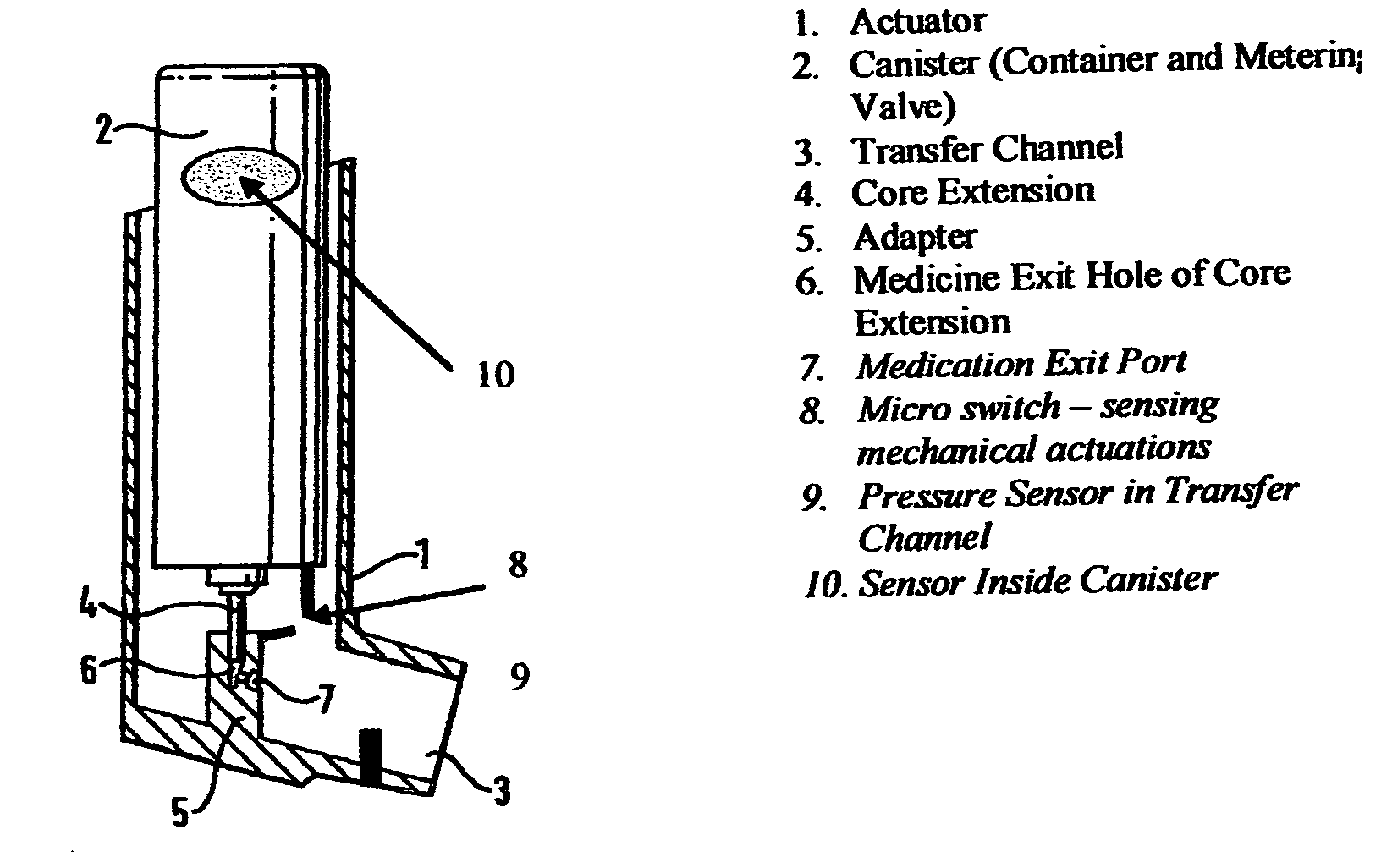
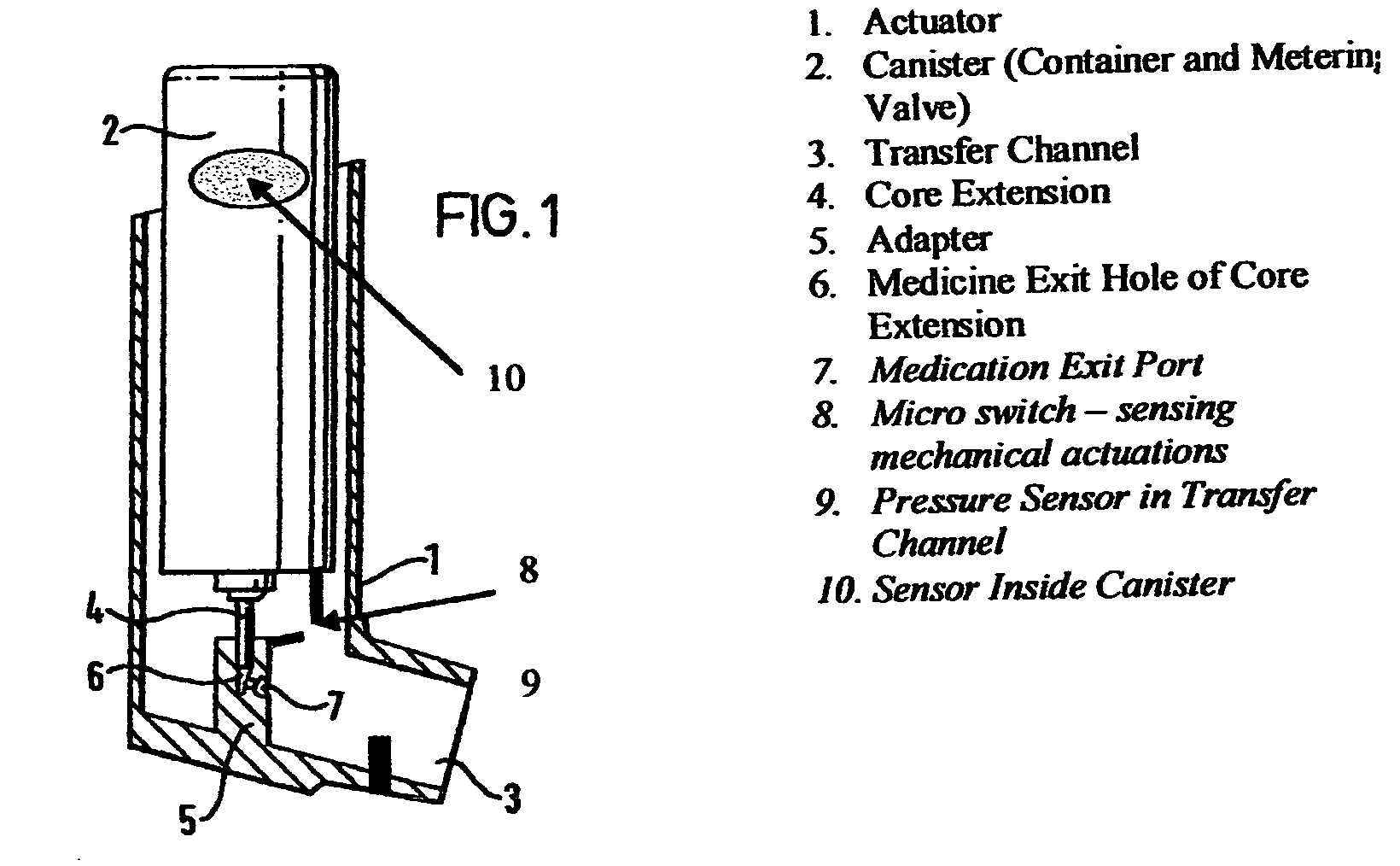
Containers for incrementally dispensing pressurized contents are described. The containers comprise a vessel suited to contain pressurized contents, a port integral with the vessel and through which pressurized contents contained in the vessel can be released from the vessel, preferably incrementally in approximately equal amounts, and a measuring device disposed in or otherwise associated with the vessel such that the quantity or amount of contents in the vessel can be measured or assessed. The measuring device senses ambient conditions in the vessel and, directly or with other components, indicates, for example, the amount of pressurized contents remaining in the vessel and displaying it to an observer. With appropriate ancillary components, the measuring device also enables the amount of contents actually released from the vessel during a particular actuation to be compared to a theoretical constant. Also, additionally, the devices of the invention may also include time logging capability (alone or in conjunction with the capability to log other data) the actuation of the dispensing valve for comparison to a prescribed method. Accordingly, devices for incrementally dispensing pressurized contents from such containers are also described. Such devices further comprise a metering value for dispensing pressurized contents from the vessel. In preferred embodiments, each actuation of the metering valve results in release of a pre-determined quantity of the vessel contents. For example, in embodiments wherein the pressurized contents comprise a therapeutic composition, the instant devices include improved Metered Dose Inhalers (MDIs) for delivery of predetermined doses of a therapeutic composition to a patient, wherein the MDIs provide for sensing of the amount, for example, of the therapeutic composition remaining in the vessel after each actuation of the metering valve.
Owner:SCHECHTER ALAN M +1
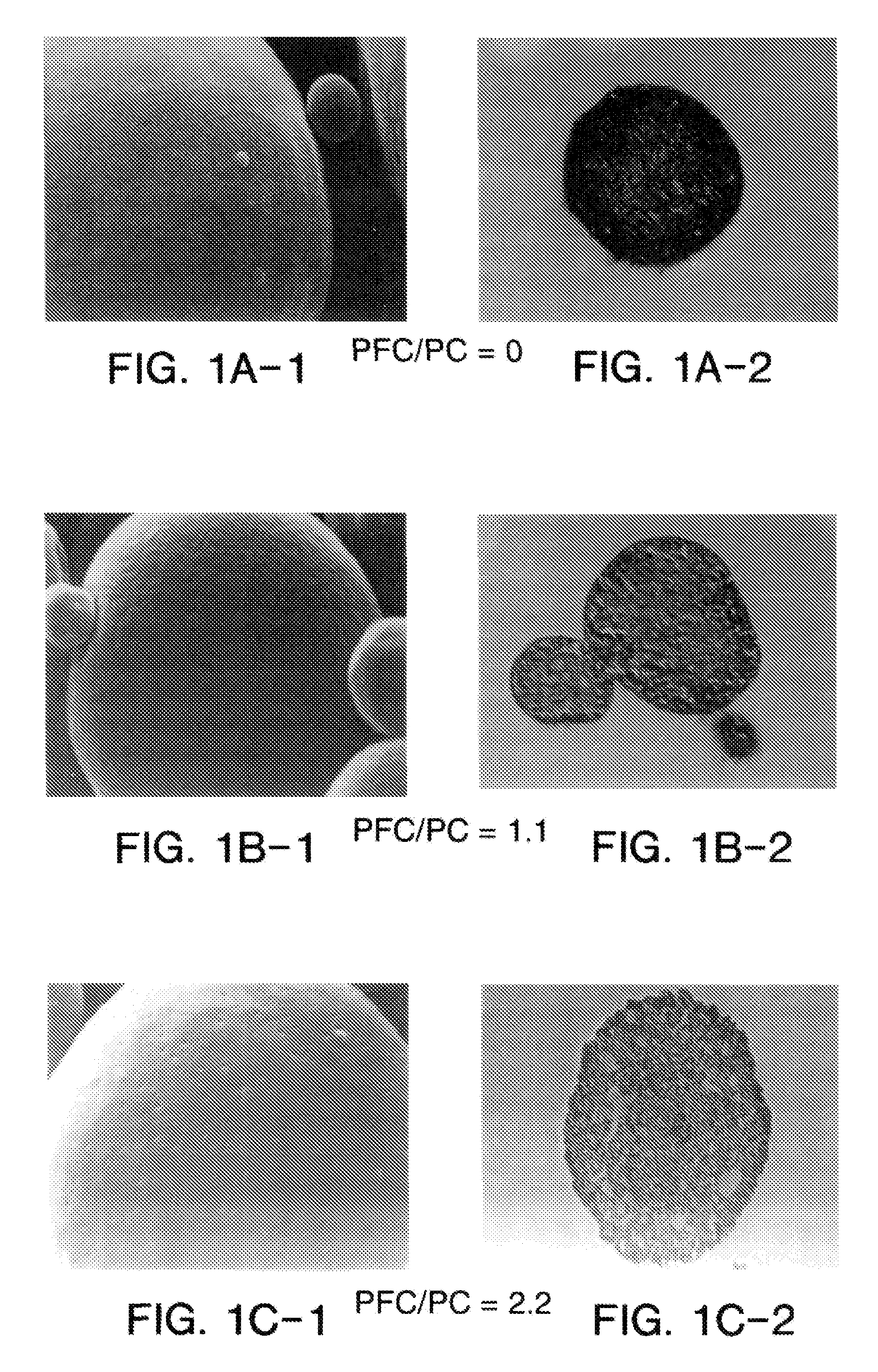
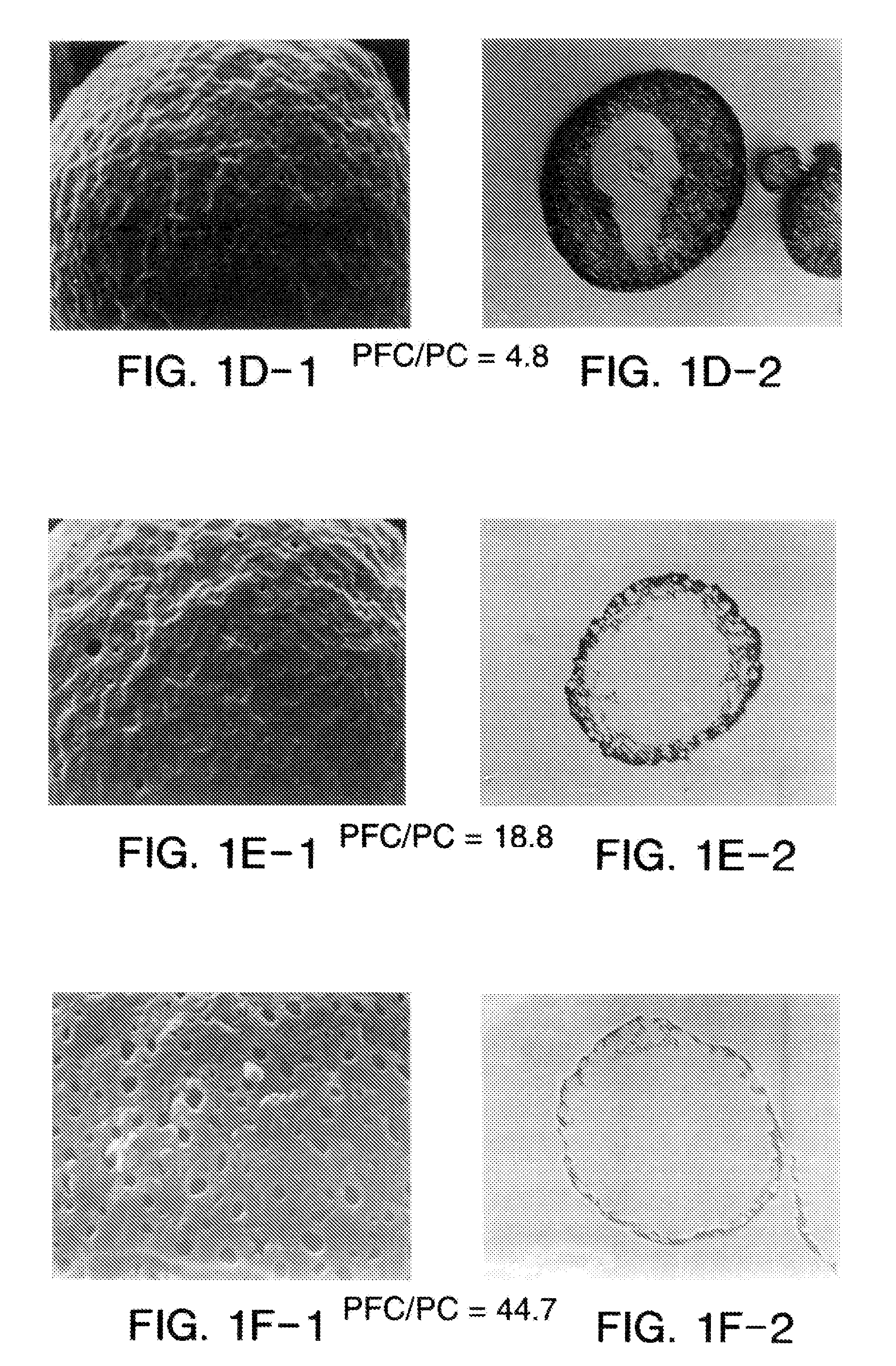
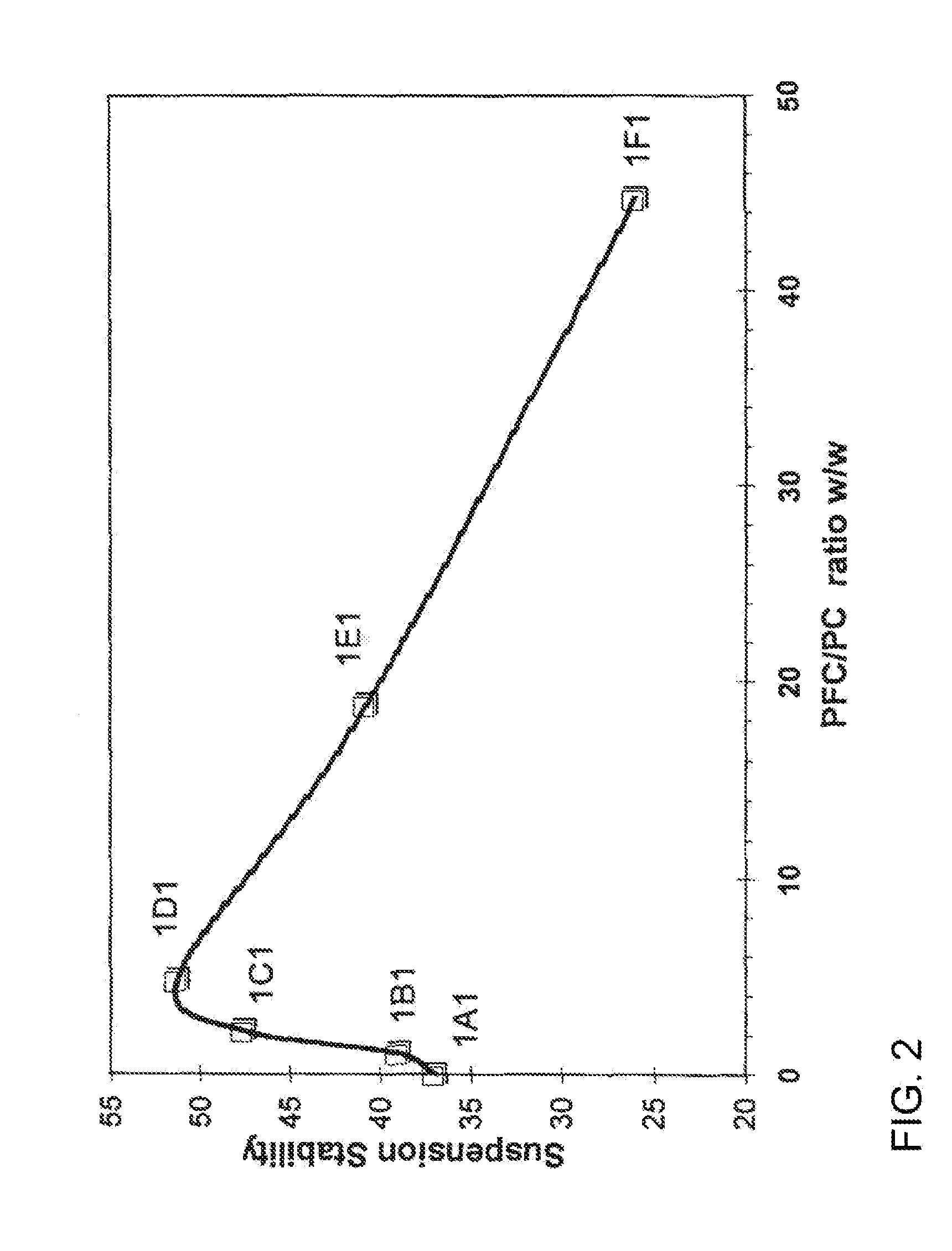
Suspension formulations
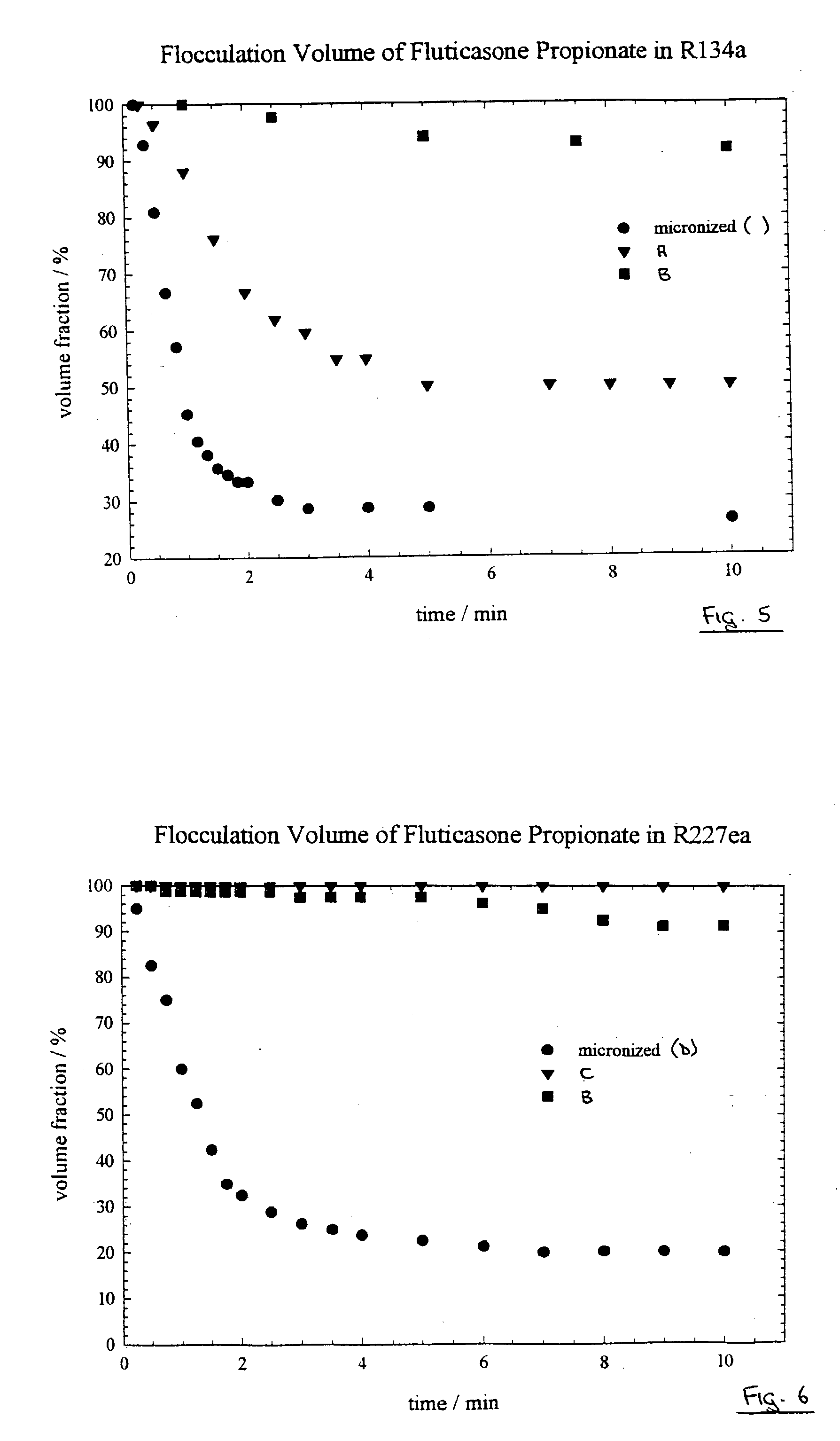
ActiveUS20110182997A1Reduced tendency to flocculateLong shelf lifeBiocideDispersion deliveryPulmonary inhalationNasal cavity
The present invention relates to suspension formulations, especially those for delivering a pharmaceutically active agent in aerosol form using a spray or aerosol device, such as a pressurised metered dose inhaler (pMDI). The formulations may be for pulmonary, nasal, buccal or topical administration, but are preferably for pulmonary inhalation.
Owner:INNOVATA BIOMED +1
Breath actuated inhaler
InactiveUS20080017189A1Overcome deficienciesReduce deliveryMedical devicesMedical atomisersMedicineInhalation
A breath actuated metered dose inhaler including a housing, a mouthpiece positioned at one end of the housing, and a mechanical release mechanism positioned at another end of the housing. The release mechanism is triggered by a diaphragm and the inhaler is configured such that the air inhalation pathway is unimpeded by the release mechanism.
Owner:ABBOTT LAB INC
Chlorofluorocarbon-free mometasone furoate aerosol formulations
The invention relates to suspension aerosol formulations which exhibit stable particle sizes, containing mometasone furoate, about 1 to about 10 weight percent ethanol and 1,1,1,2,3,3,3-Heptafluoropropane as the propellant. A surfactant, such as oleic acid, can also be included. These formulations are suitable for use in metered dose inhalers.
Owner:MERCK SHARP & DOHME CORP
Apparatus for dispensing pressurized contents
InactiveUS7832394B2Liquid surface applicatorsPowdered material dispensingMarine engineeringVALVE PORT
Containers for incrementally dispensing pressurized contents are described. The containers comprise a vessel suited to contain pressurized contents, a port integral with the vessel and through which pressurized contents contained in the vessel can be released from the vessel, preferably incrementally in approximately equal amounts, and a measuring device disposed in the vessel. The measuring device senses ambient conditions in the vessel and, directly or with other components, indicates, for example, the amount of pressurized contents remaining in the vessel and displaying it to an observer; methods for comparing the amount of contents actually released from the vessel compared to a theoretical constant; and methods for time logging (alone or in conjunction with the logging of other data) the actuation of the dispensing valve for comparison to a prescribed method. Devices (including metered dose inhalers) for incrementally dispensing pressurized contents from such containers are also described.
Owner:SCHECHTER ALAN M +1
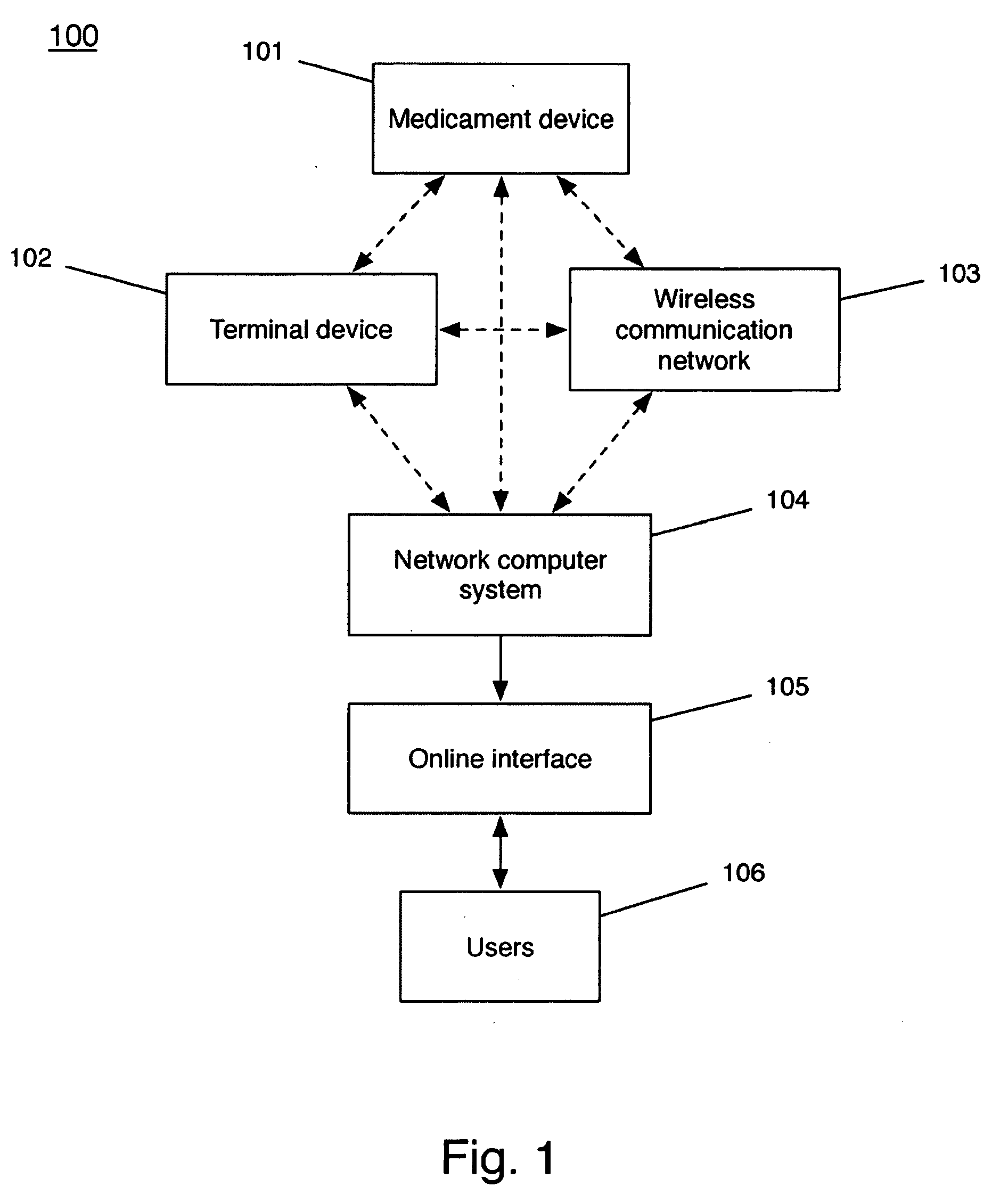
Device and method to monitor, track, map, and analyze usage of metered-dose inhalers in real-time
ActiveUS9550031B2Easy to manageEasy to identifyRespiratorsDrug and medicationsClinical careComputer science
A system and method for accurately and reliably determining and recording the time, date and location where a medication is used, and a system and method for transmitting, collecting, and using that data to improve clinical care, disease management, and public health surveillance. The device allows information concerning drug usage, including the time, date and location of use, to be transmitted to a remote network computer system so that the data can be evaluated to determine current impairment and future risk, and to identify changes in the frequency, timing, or location of medication usage indicative of change in disease control or management, and to examine spatial, temporal or demographic patterns of medication use or absence of use among individuals and groups. In addition, the device may further be configured to transmit signals indicative of its status, condition or other results to the remote network computer system.
Owner:RECIPROCAL LABS CORP D B A PROPELLER HEALTH
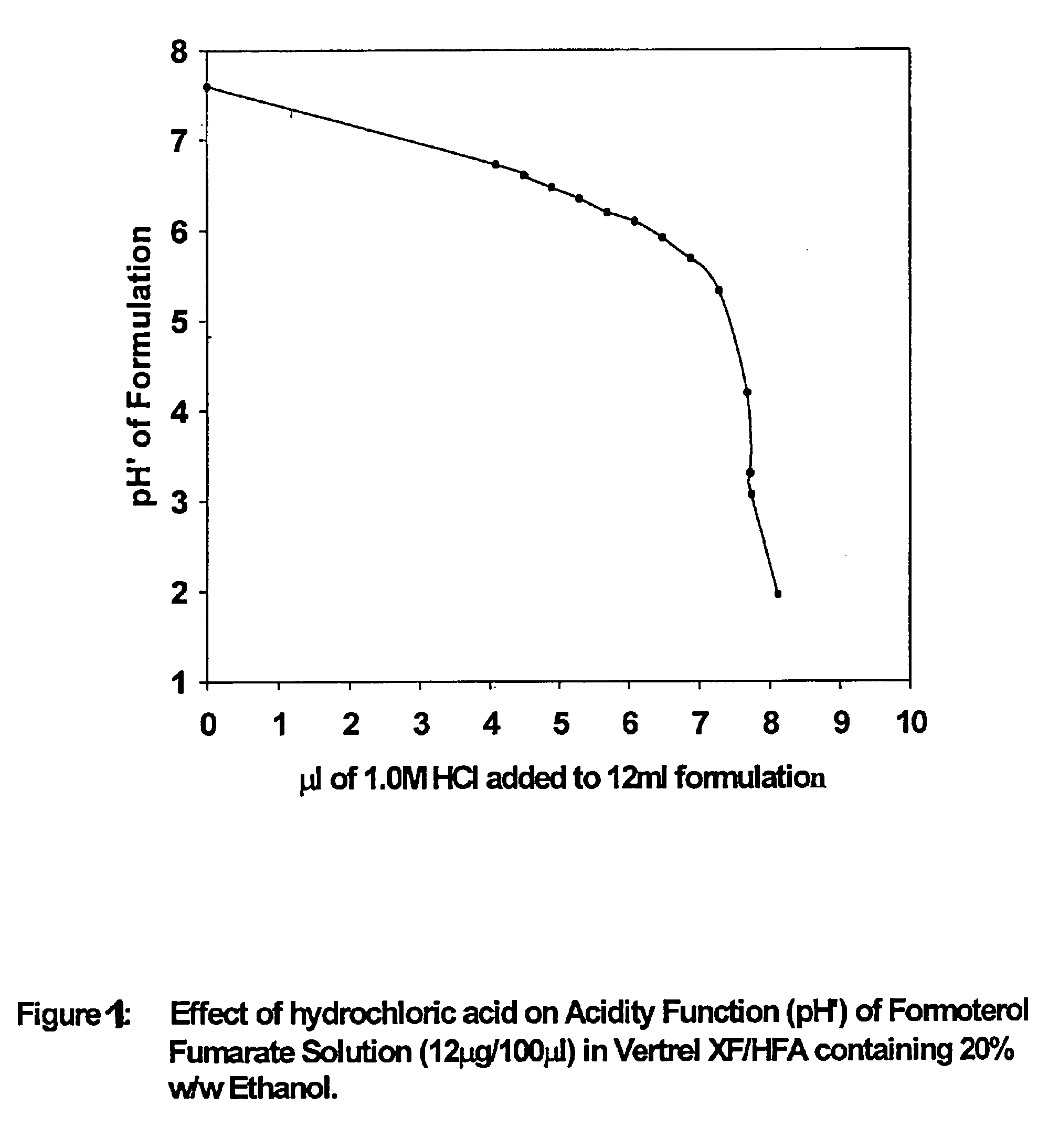
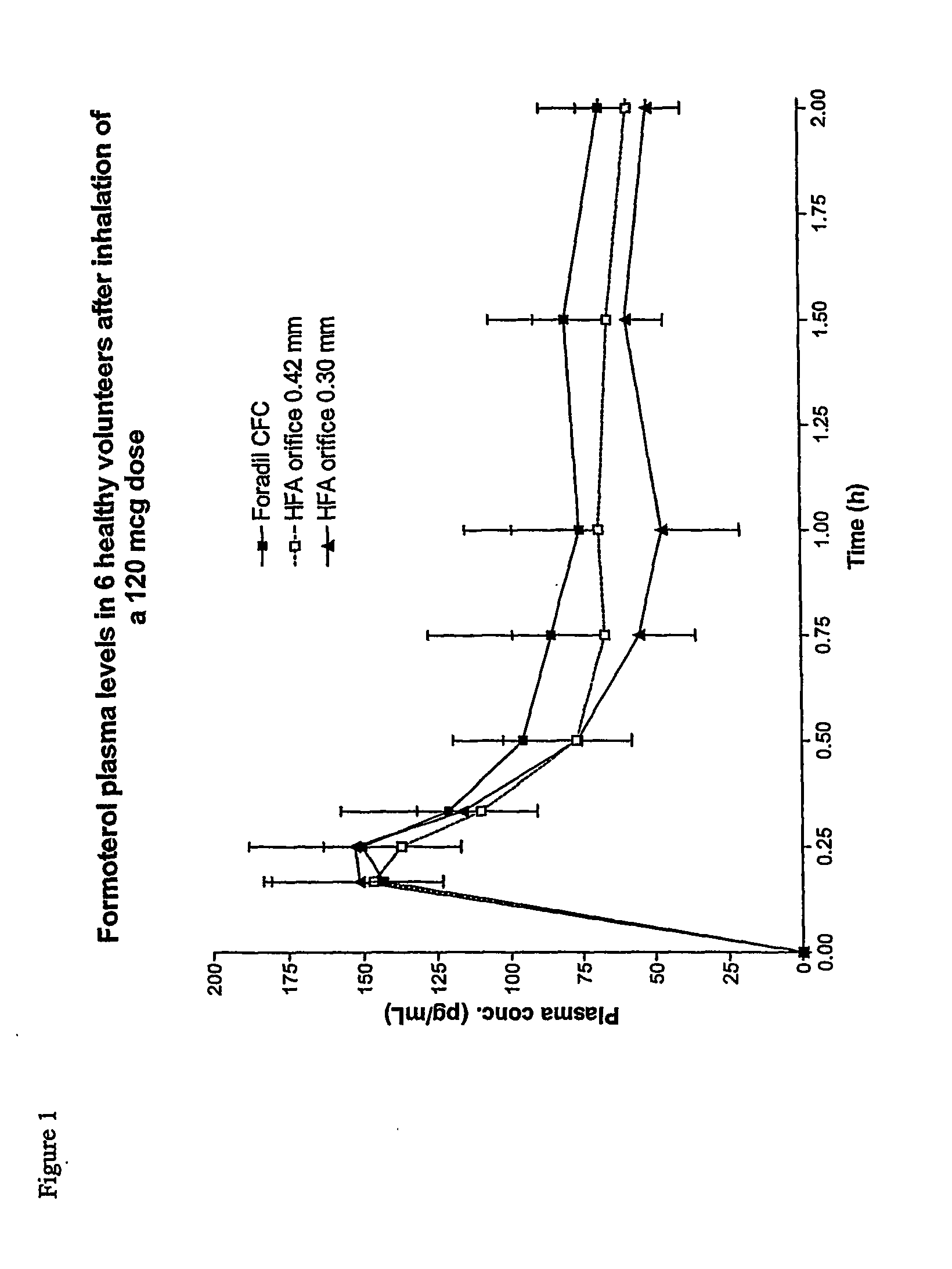
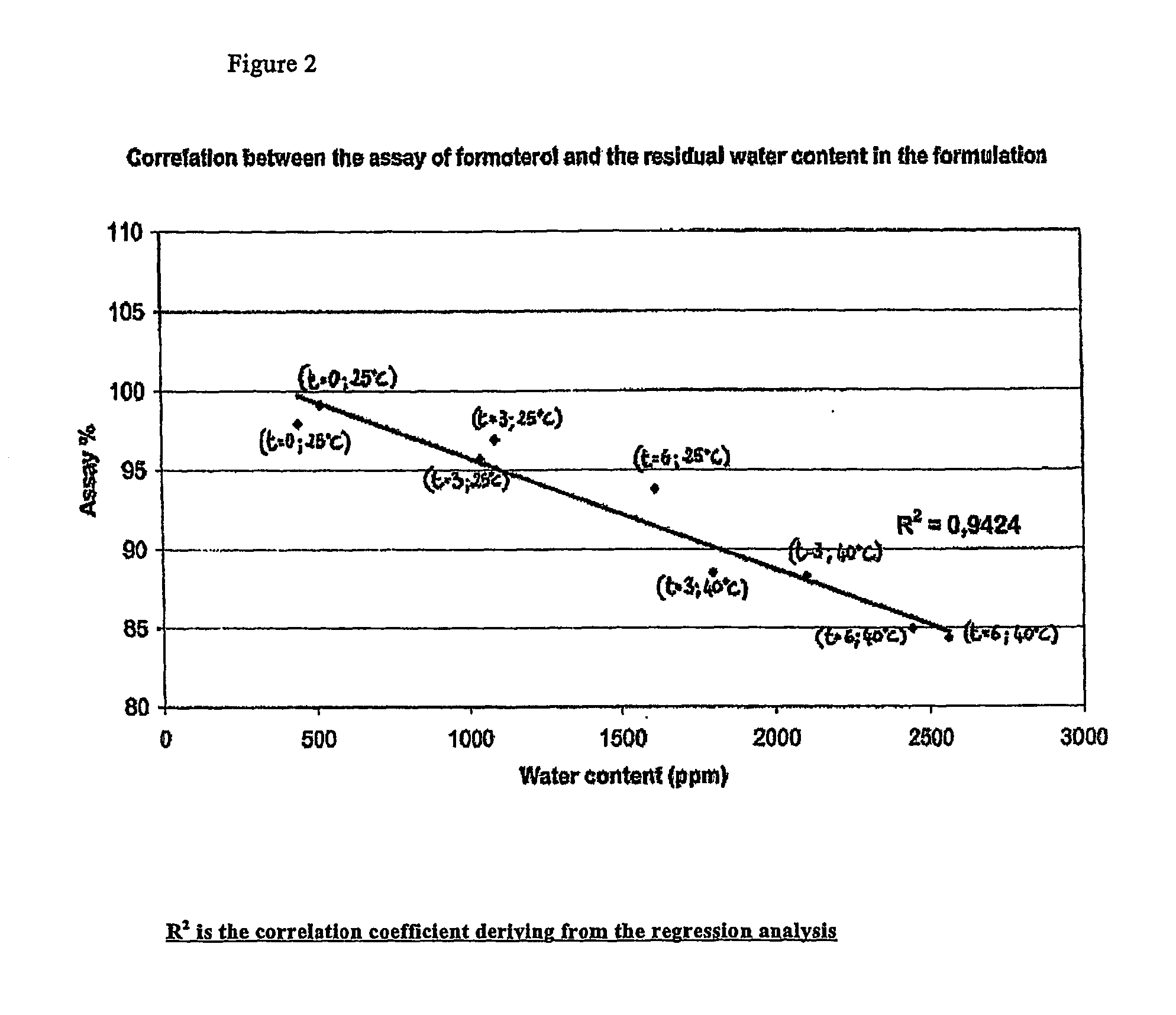
Formoterol superfine formulation
InactiveUS20050152846A1Improve toleranceInhibited DiffusionPowder deliveryOrganic active ingredientsDiseaseObstructive Pulmonary Diseases
The present invention relates to a pharmaceutical formulation for use in the administration of a long-acting β2-agonist by inhalation. In particular this invention relates to a chemically stable highly efficient formoterol HFA solution formulation to be administered by pressurised metered dose inhalers (pMDIs) characterized by a deep lung penetration. The invention also relates to methods for the preparation of said formulation and to its use in respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD).
Owner:CHIESI FARM SPA
Counter for metered dose inhaler
InactiveUS20110265788A1Easy to readReduce consumptionLiquid transferring devicesMedical atomisersInterior spaceEngineering
A dose counter is provided for coupling with a metered dose inhaler. The dose counter includes a top cover that has a top forming an observation window. The top cover forms an interior space receiving therein a circuit board and an electrical cell supplying electrical power. The circuit board includes a counting circuit and a display screen that is arranged under the window. As such, in the use of the inhaler, depression of the top cover actuates the inhaler and causes the display screen to display the number of times of actuation so as to allow a user to realize the remaining number of times for use of the inhaler thereby improving safety of medication use.
Owner:INTECH BIOPHARM
Engineered particles and methods of use
InactiveUS20050074498A1Reduce deliveryLess attractivePowder deliveryOrganic active ingredientsNebulizerActive agent
Engineered particles are provided may be used for the delivery of a bioactive agent to the respiratory tract of a patient. The particles may be used in the form of dry powders or in the form of stabilized dispersions comprising a nonaqueous continuous phase. In particularly preferred embodiments the particles may be used in conjunction with an inhalation device such as a dry powder inhaler, metered dose inhaler or a nebulizer.
Owner:NOVARTIS FARMA
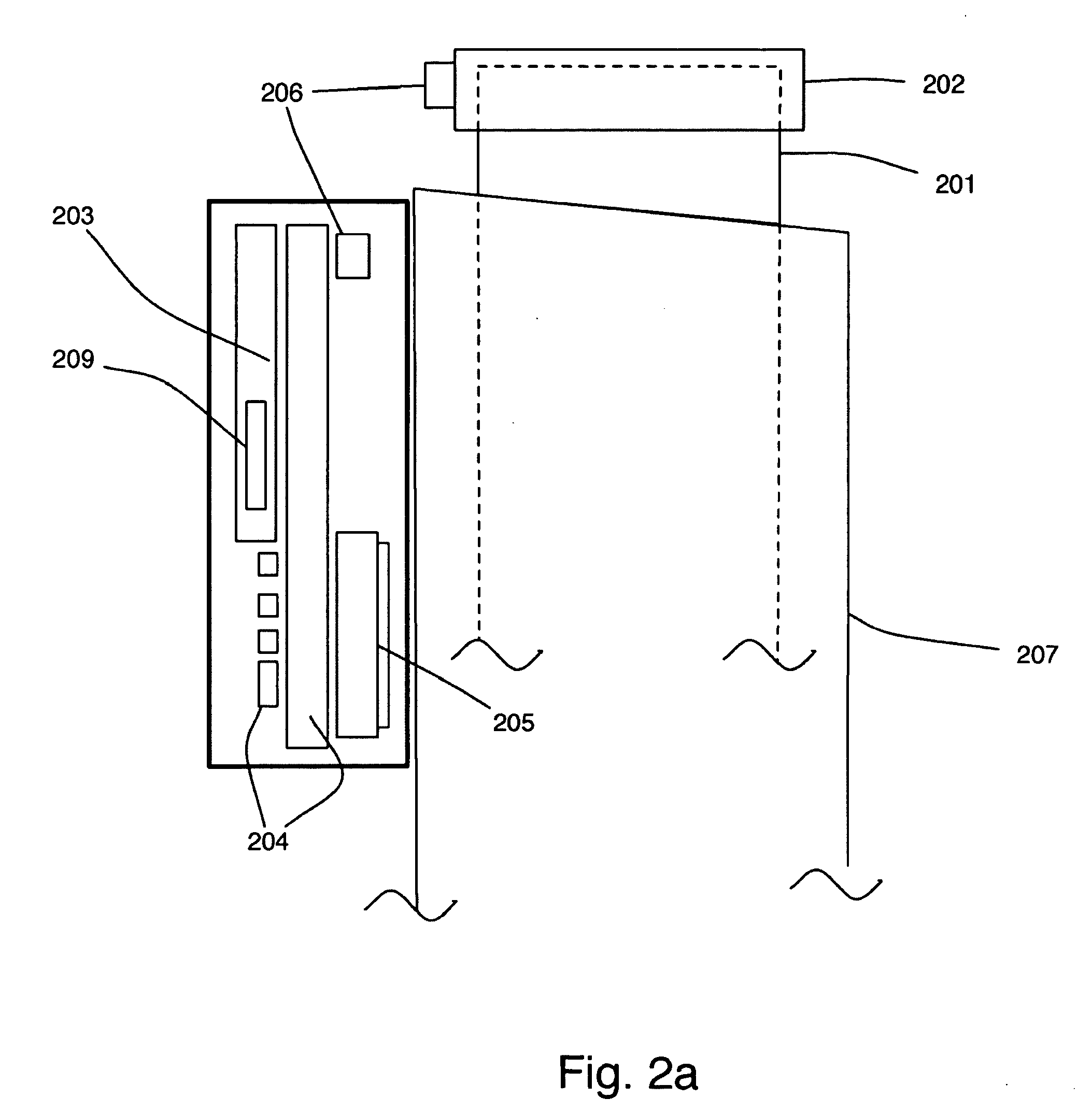
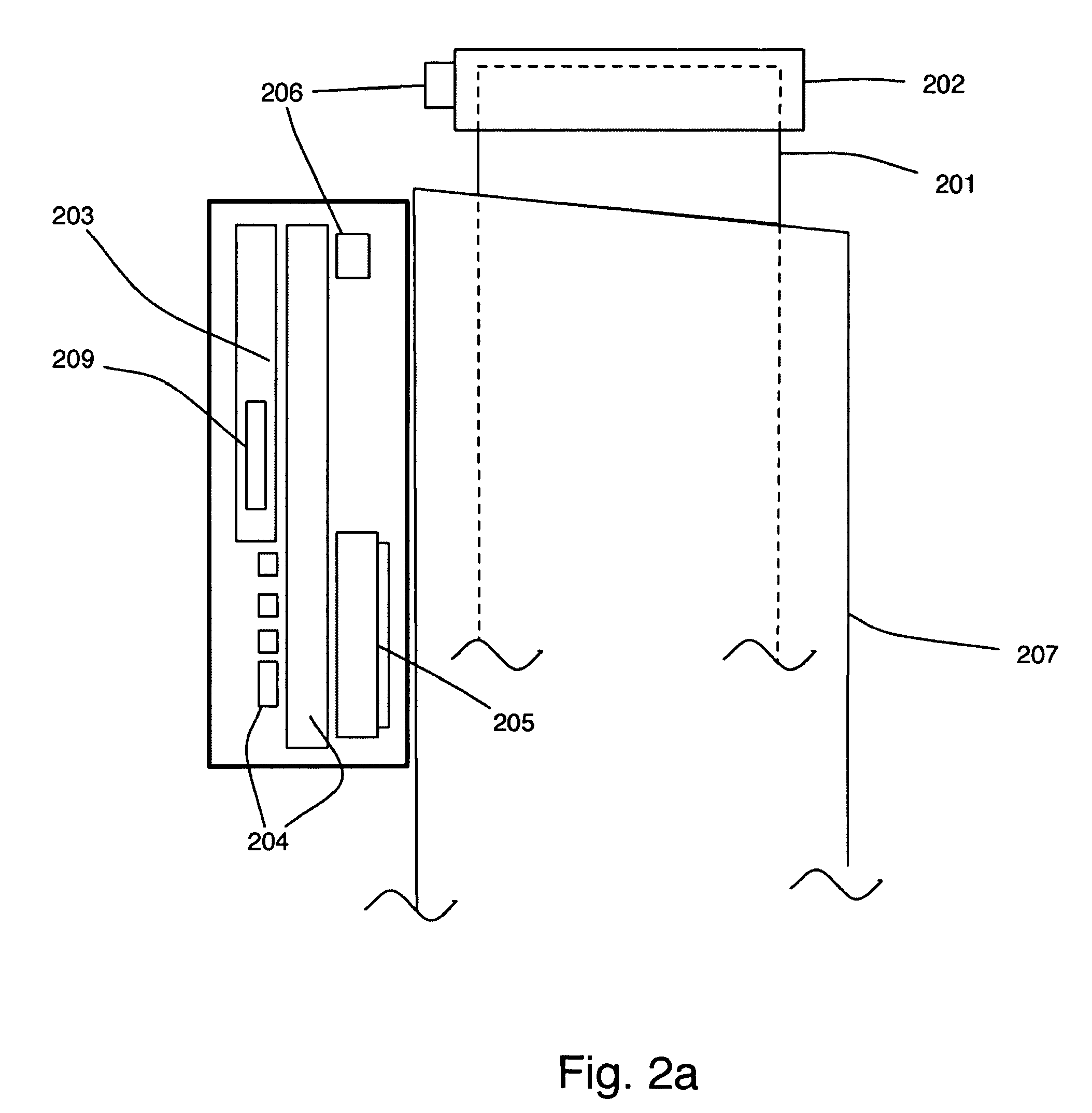
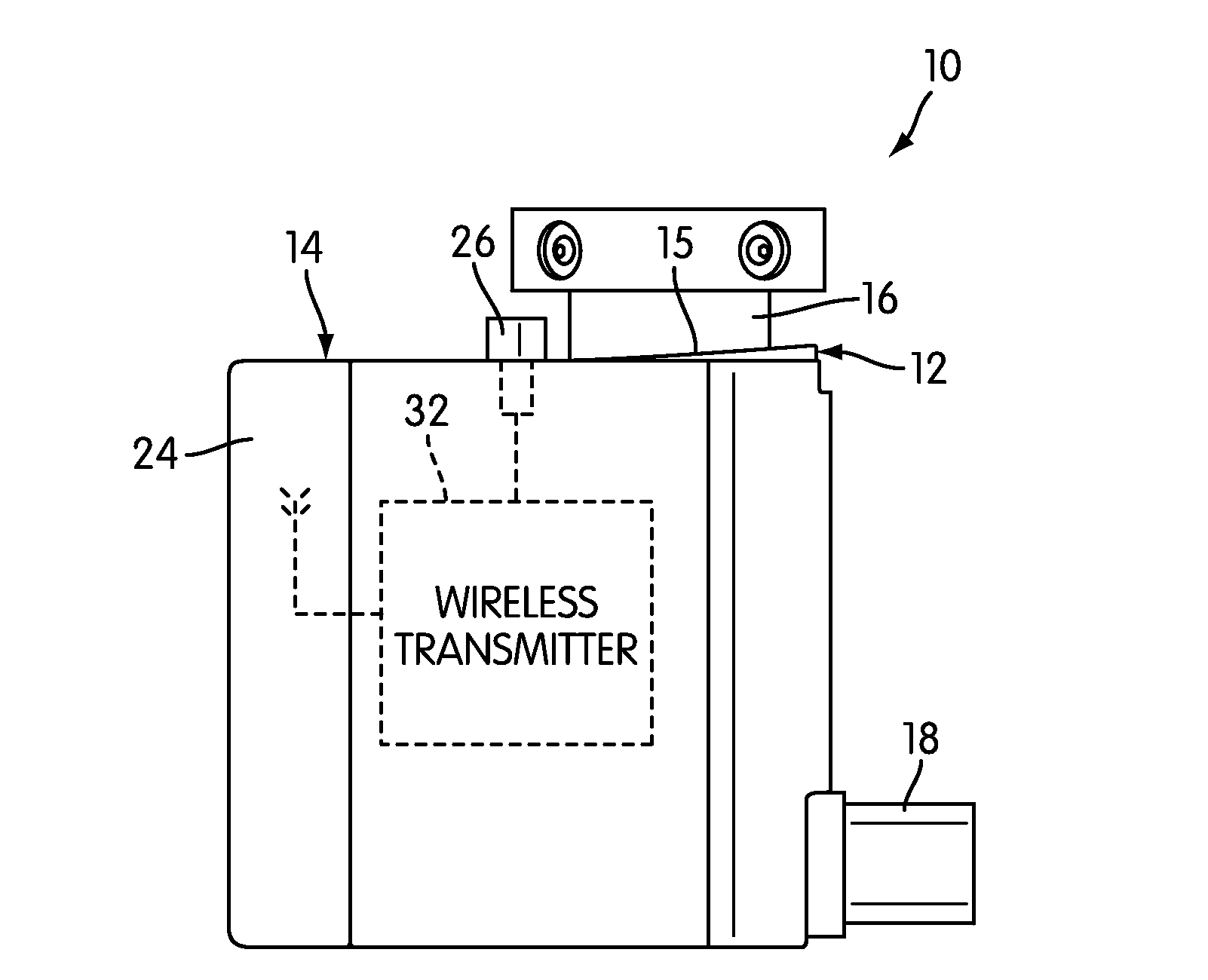
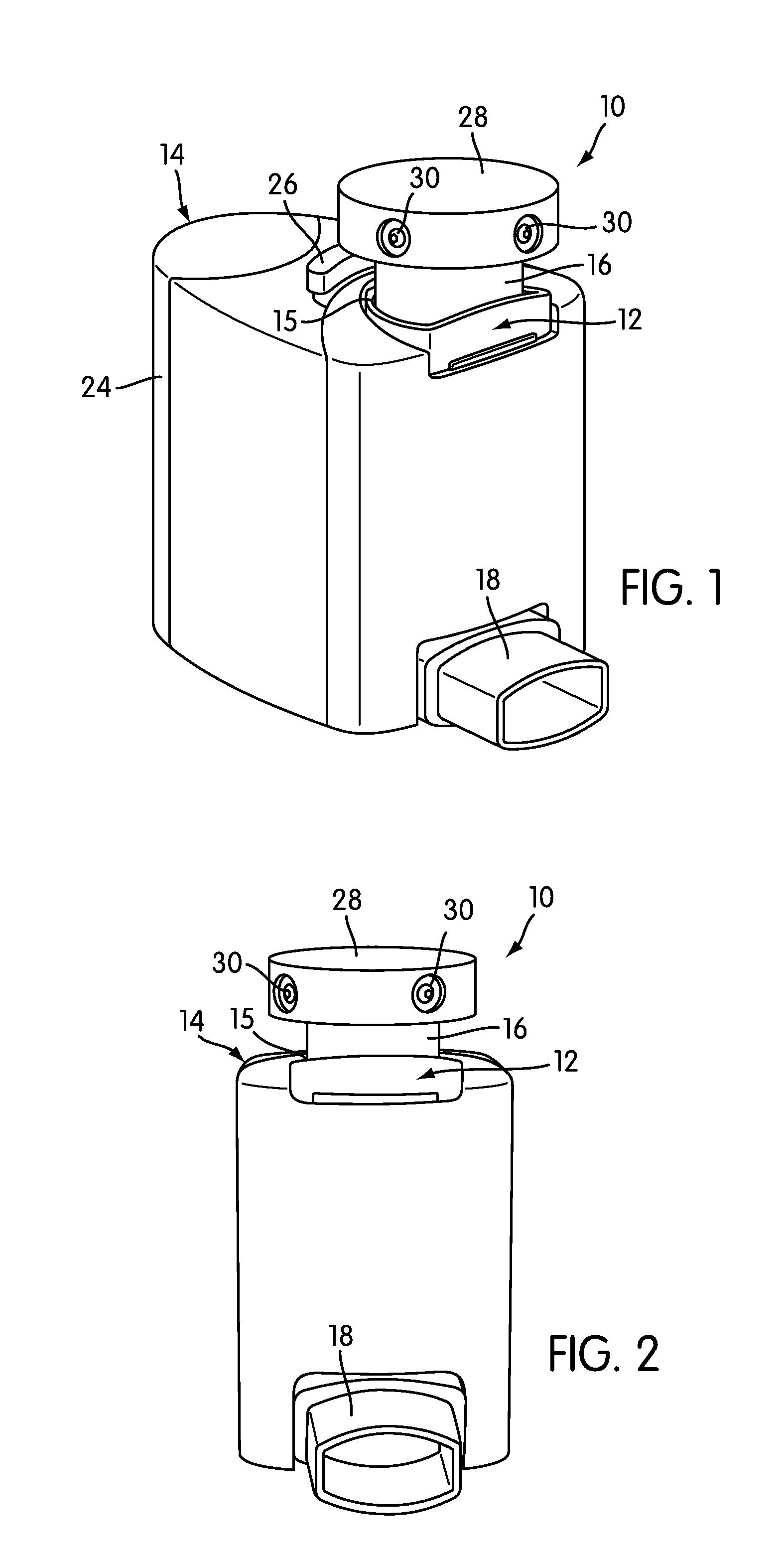
Instrumented metered-dose inhaler and methods for predicting disease exacerbations
The present invention is directed to devices, systems, and methods for monitoring inhaled drug usage to predict when an acute attack or exacerbation of a disease, such as a respiratory disease, is imminent. Instrumented inhalers that use modular designs with standard components are disclosed, as are systems for monitoring the instrumented inhalers. Also disclosed are methods for determining whether or not a patient's inhaled drug usage pattern indicates that an acute attack or disease exacerbation is imminent, and notifying appropriate medical personnel of any usage patterns indicative of an attack or disease exacerbation. If such an attack or exacerbation is imminent, additional therapeutic agents may be dispensed to the patient or other interventions made.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Instrumented Metered-Dose Inhaler and Methods for Predicting Disease Exacerbations
ActiveUS20100094099A1Easy to addEnhanced couplingRespiratorsDrug and medicationsInhaled drugRespiratory disease
The present invention is directed to devices, systems, and methods for monitoring inhaled drug usage to predict when an acute attack or exacerbation of a disease, such as a respiratory disease, is imminent. Instrumented inhalers that use modular designs with standard components are disclosed, as are systems for monitoring the instrumented inhalers. Also disclosed are methods for determining whether or not a patient's inhaled drug usage pattern indicates that an acute attack or disease exacerbation is imminent, and notifying appropriate medical personnel of any usage patterns indicative of an attack or disease exacerbation. If such an attack or exacerbation is imminent, additional therapeutic agents may be dispensed to the patient or other interventions made.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Salmeterol superfine formulation
The present invention relates to a pharmaceutical formulation for use in the administration of a long-acting β2-agonist by inhalation. In particular this invention relates to a chemically stable, highly efficient salmeterol HFA solution formulation to be administered by pressurised metered dose inhalers (pMDIs) characterized by a deep lung penetration. The invention also relates to methods for the preparation of said formulation and to its use in respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD).
Owner:CHIESI FARM SPA
Pressurised metered dose inhalers (MDI)
InactiveUS20050142071A1Eliminate side effectsEfficient inhalationBiocideDispersion deliveryMeter dose inhalerAnodic Aluminum Oxide
Owner:CHIESI FARM SPA
Insulated canister for metered dose inhalers
An insulated cartridge for use in a metered dose system, such as a metered dose inhaler or a topical sprayer, for a pressurized or non-pressurized system, characterized by comprising an inner container surrounded by an outer container, and the inner container and the outer container The space between the walls defines a gap. The gap can be filled with vacuum, air or a material with low thermal conductivity.
Owner:NOVARTIS AG
Inhalation therapy assembly and method
InactiveUS20020069869A1Conveniently and easily allowBreathing masksRespiratory masksInhalationIntensive care medicine
The inhalation therapy assembly and method of use described herein increases the efficiency of metered dose inhalers by allowing delivery of the doses to a collapsible reservoir which can be manually pumped, ensuring that medicants contained therein are properly and completely delivered to the patient. Terminal and proximal valves of the one-way diaphragm type allow flow of the aerosol medicants while preventing improper expulsion. An exhalation valve is adjustable to ensure the patient expires suitably to permit proper medicant absorption.
Owner:FARMER MEDICAL
Particulate materials
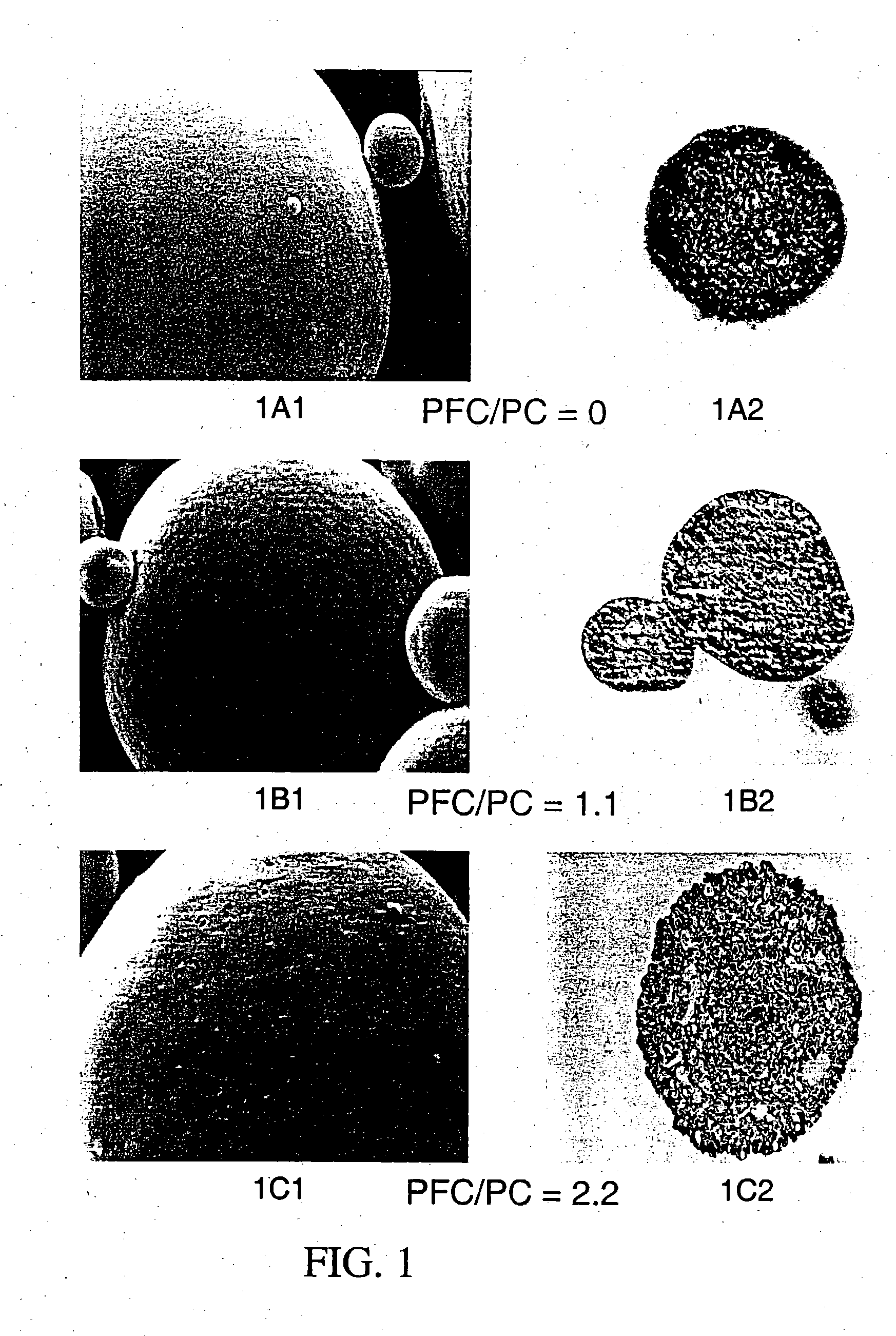
The present invention relates to active substances in particulate form, to methods for preparing them, to formulations containing them and to uses of such substances and formulations. A preferred embodiment is directed to particulate suspensions having improved flocculation behaviour in a suspension vehicle, such as a hydrofluoroalkane propellant used in metered dose inhalers.
Owner:NEKTAR THERAPEUTICS INC
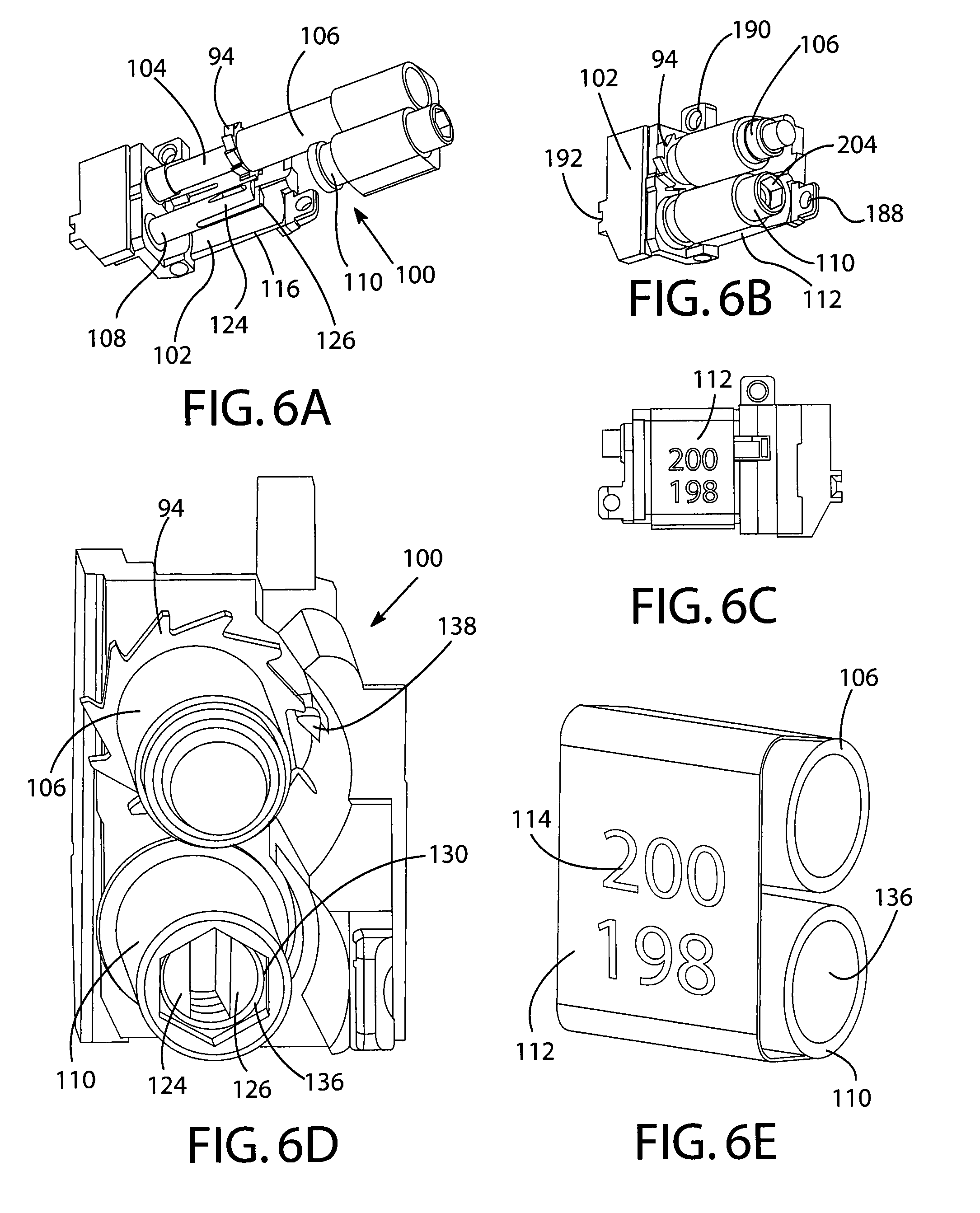
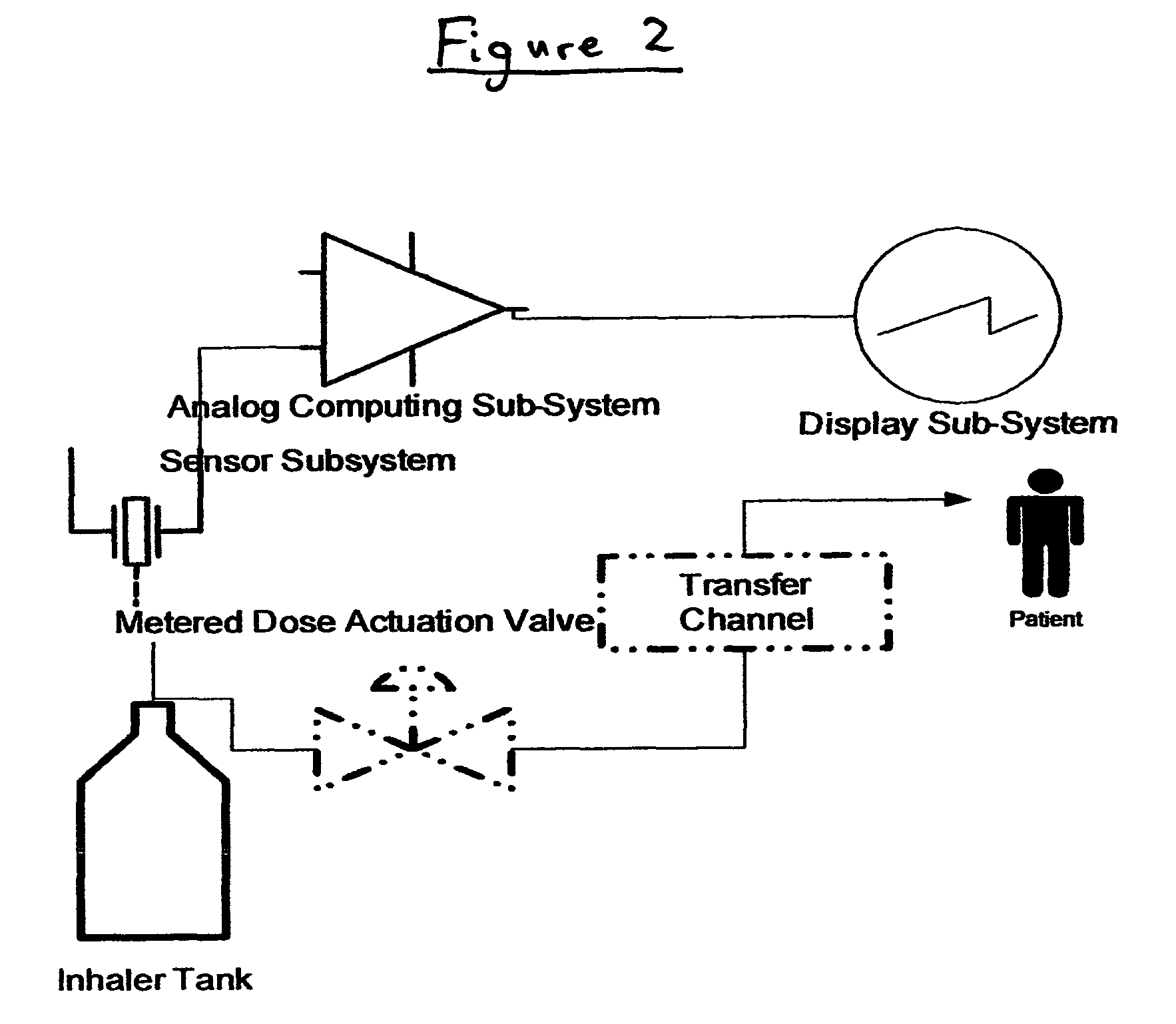
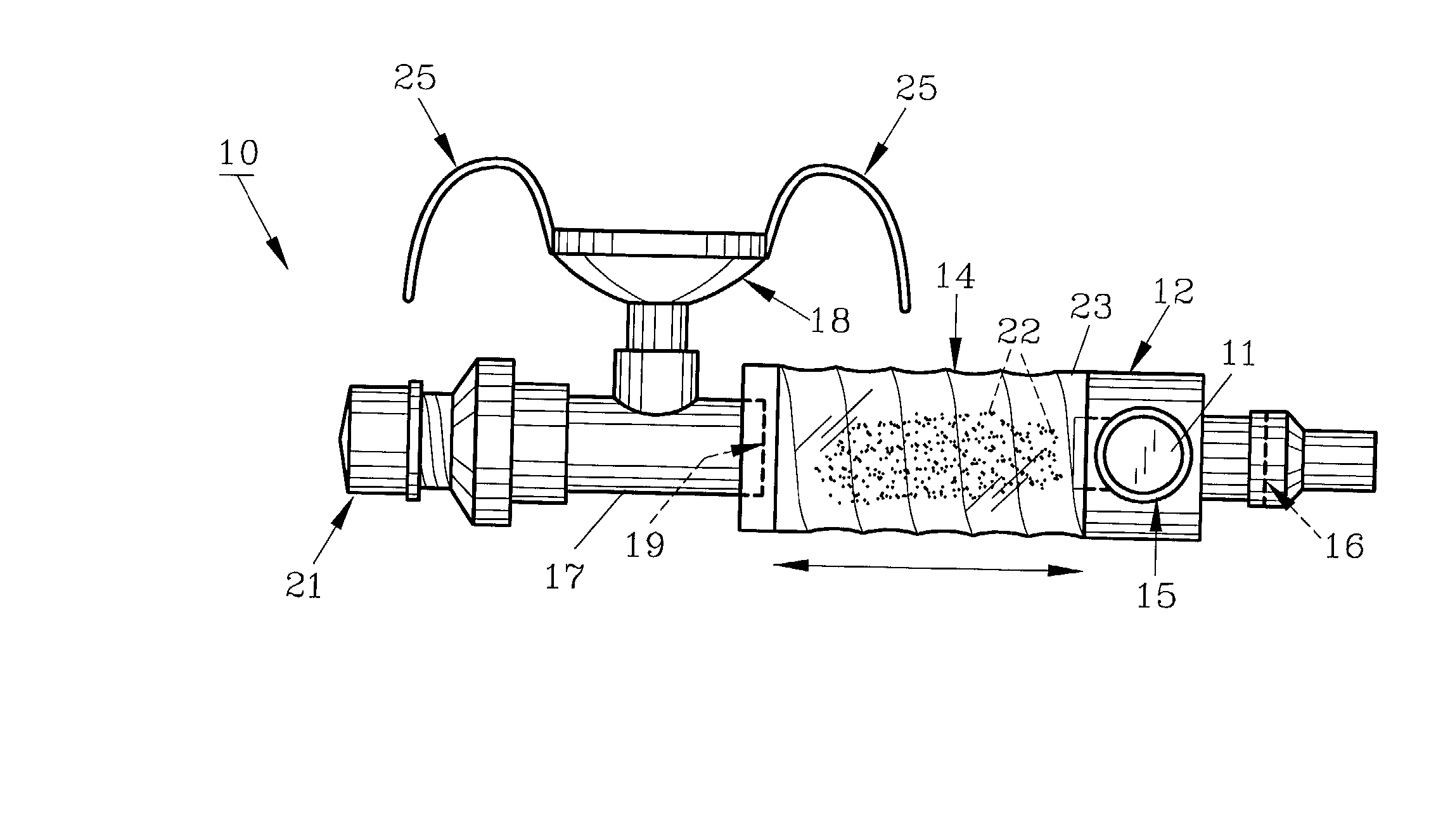
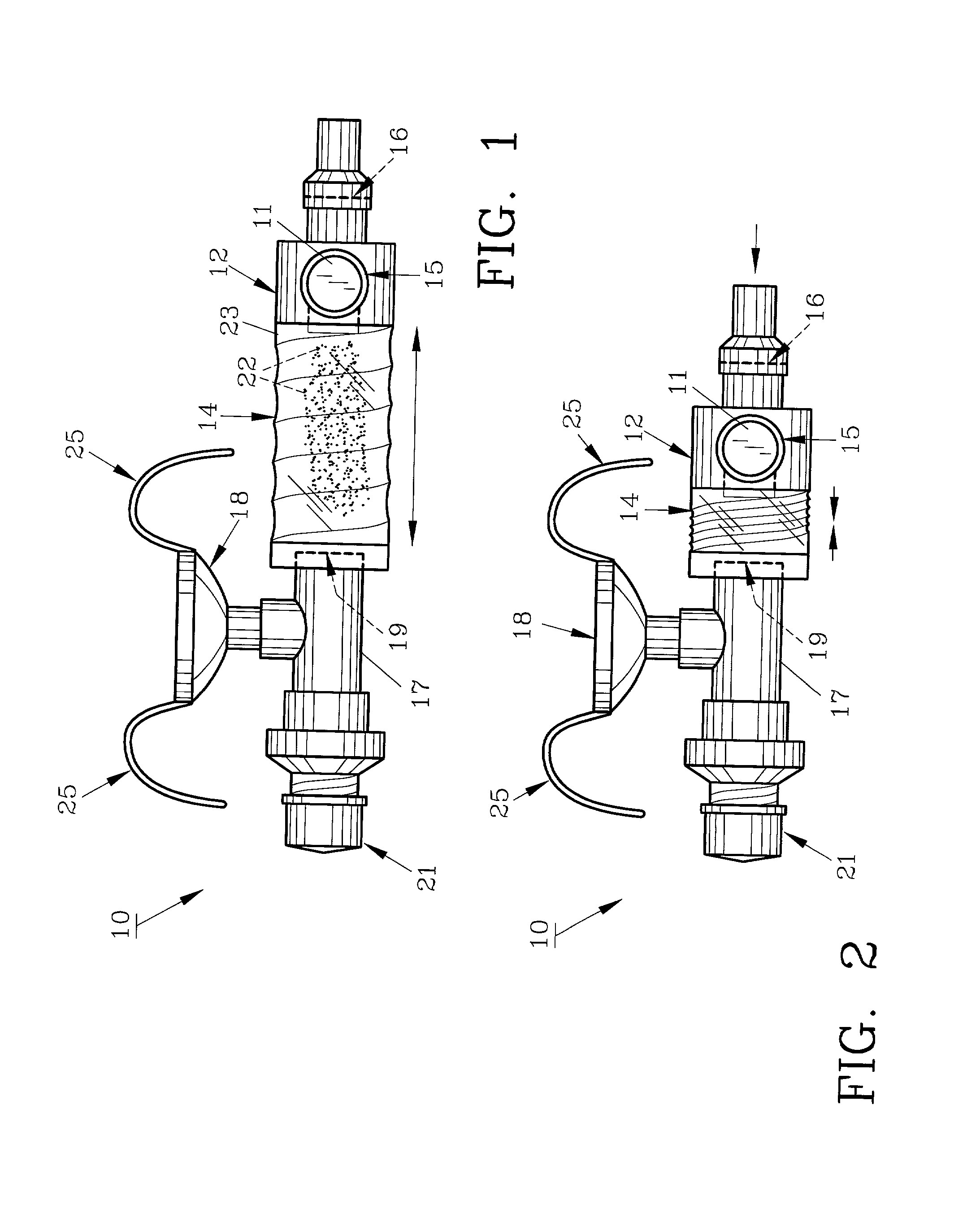
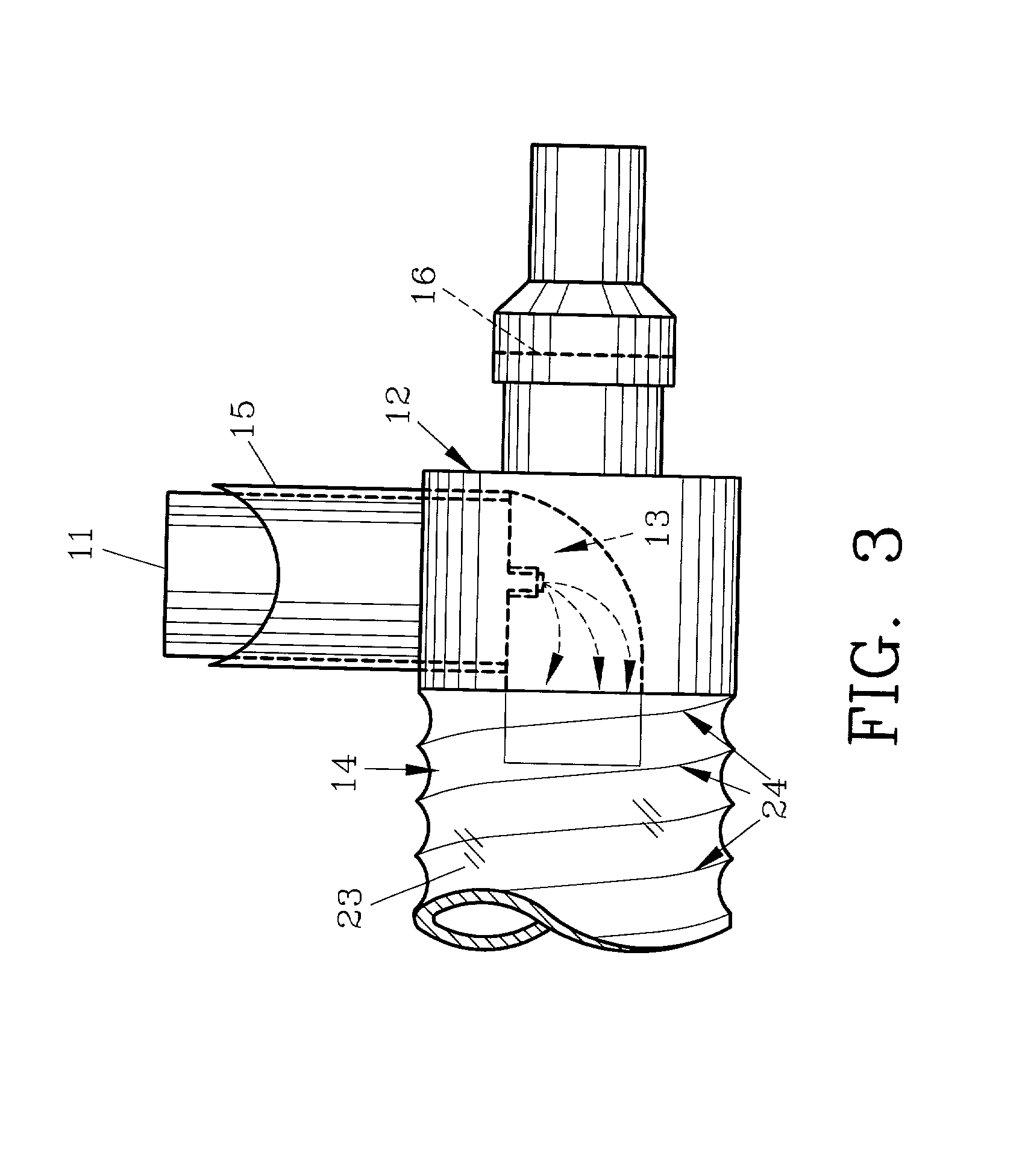
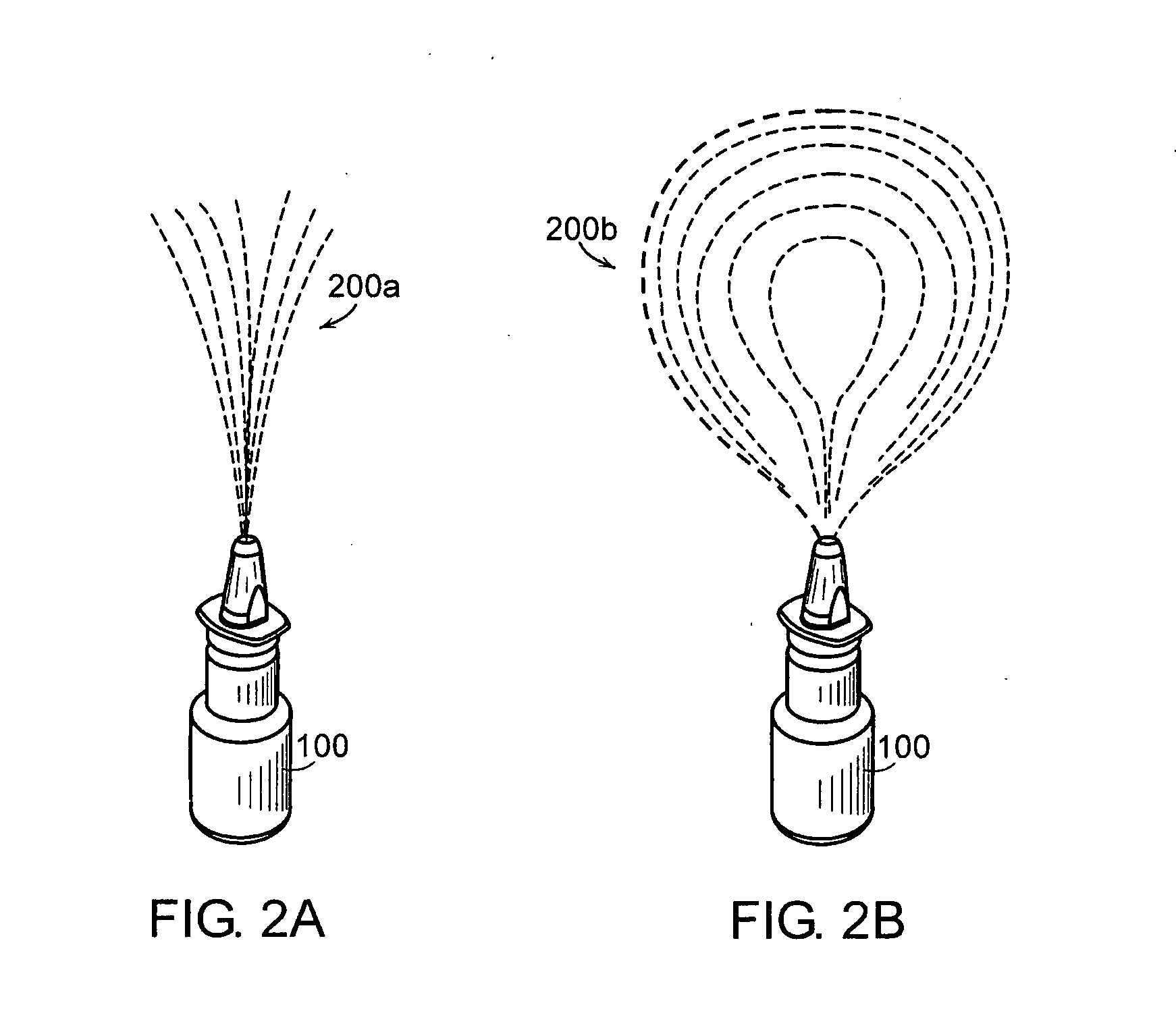
Method and apparatus for measuring manual actuation of spray devices
ActiveUS20050001054A1Low variabilityHigh sensitivitySelf-acting watering devicesWatering devicesTransducerEngineering
An assembly that provides operational information about operation of a spray device, such as a nasal spray pump or metered dose inhaler. A linkage adapted to extend between a mounting assembly, connected to a stationary part of the spray device, and an adapter assembly, connected to a movable part of the spray device, is in operational relationship with a transducer to enable the transducer to indicate a mechanical relationship between the movable and stationary parts of the spray device corresponding to the operation of the spray device. A data collection and processing system may be connected to the transducer for determining information that may be used to program an automated actuation system.
Owner:PROVERIS SCI CORP
Pressurised metered dose inhalers (MDI)
InactiveUS7223381B2Eliminate side effectsEfficient inhalationBiocidePowder deliveryMeter dose inhalerAnodic Aluminum Oxide
Owner:CHIESI FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com