Patents
Literature
43 results about "Salmeterol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
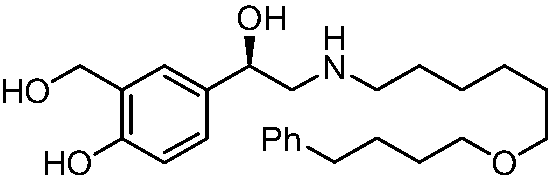
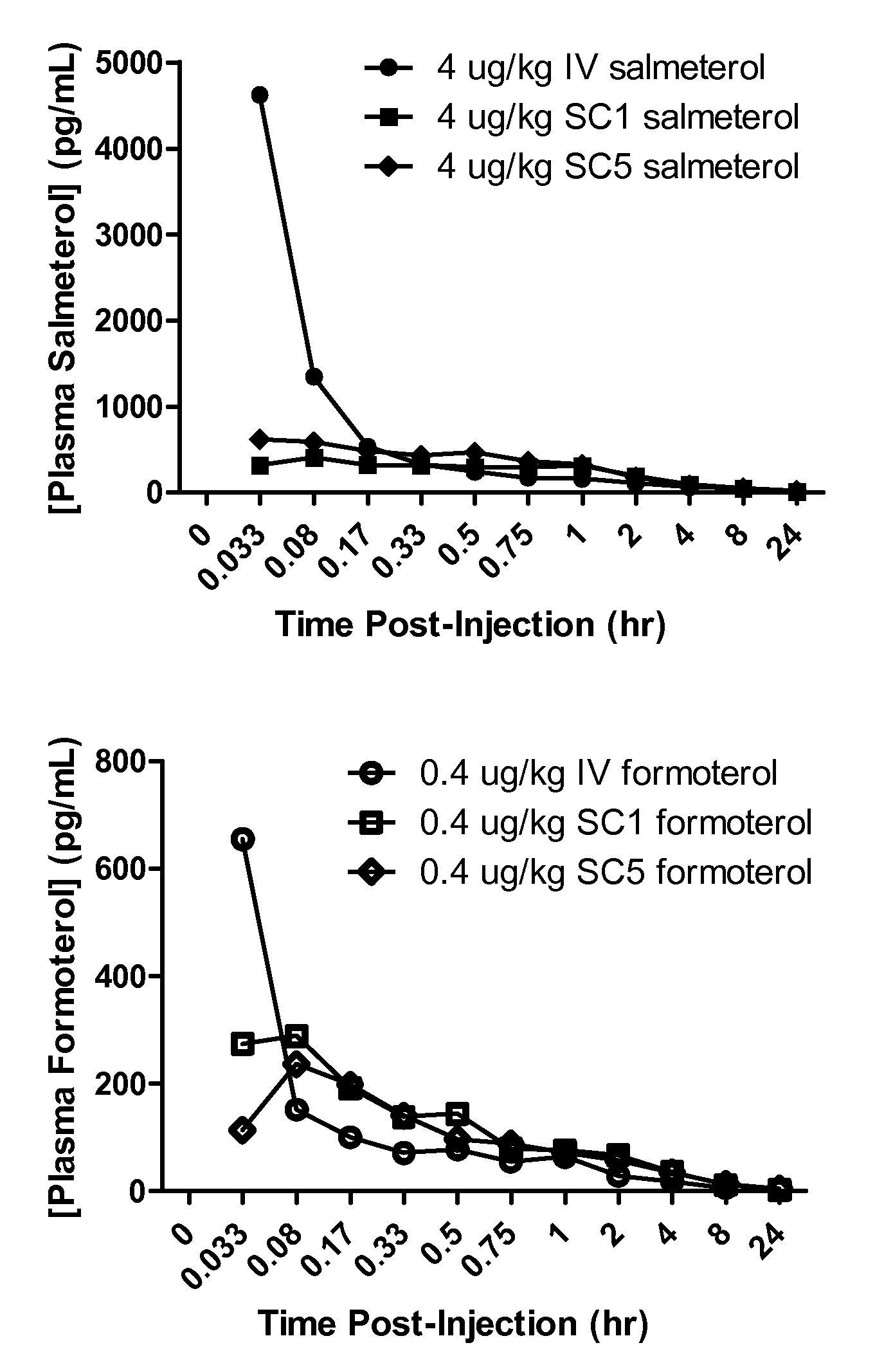
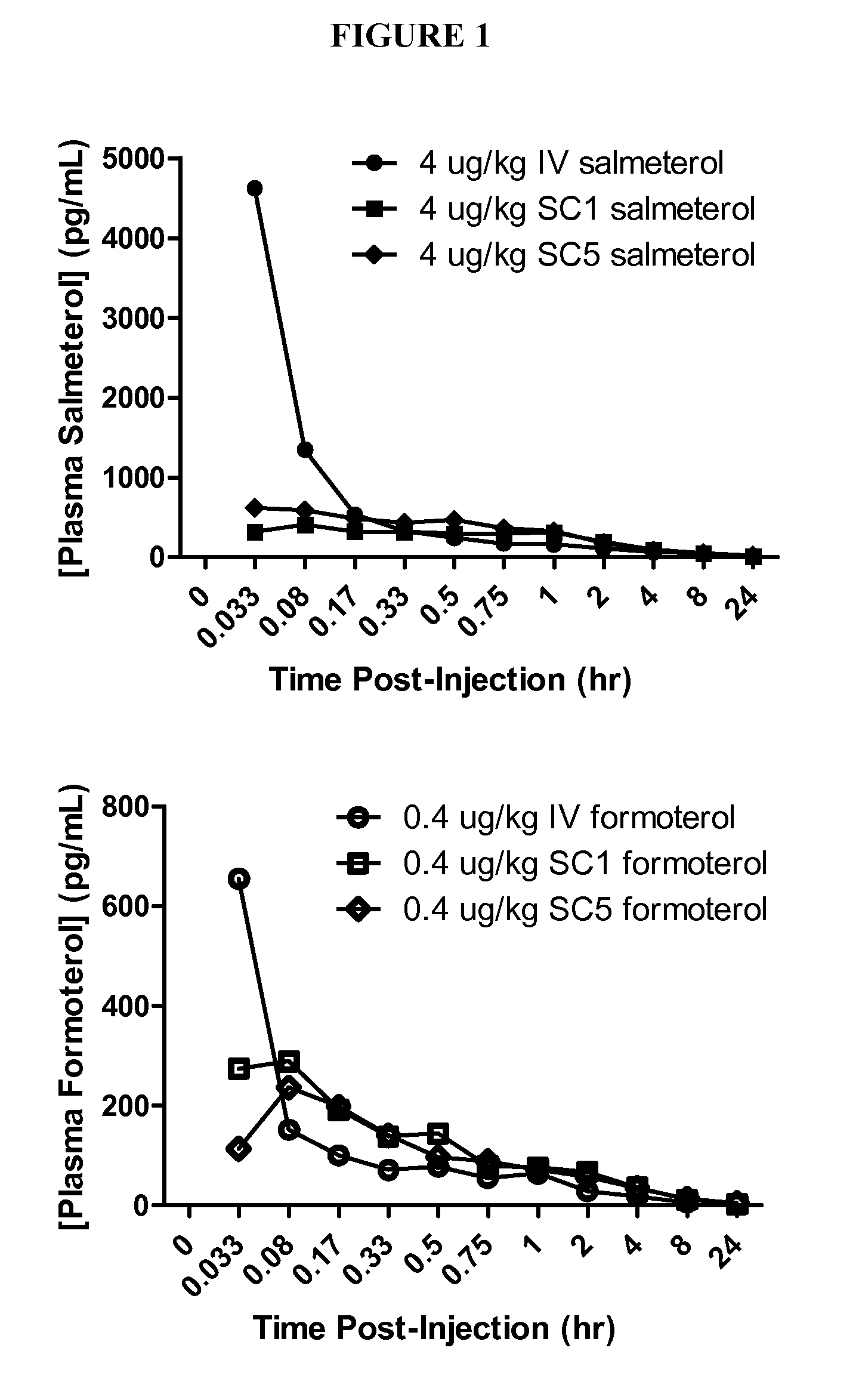
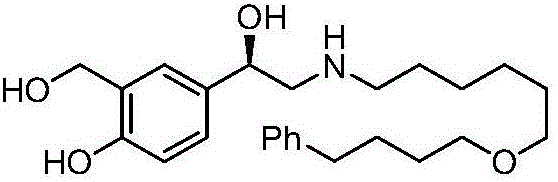
Salmeterol is used as a long-term (maintenance) treatment to prevent or decrease wheezing and trouble breathing caused by asthma or ongoing lung disease (chronic obstructive pulmonary disease-COPD, which includes chronic bronchitis and emphysema). It should only be used long-term if your asthma symptoms are not controlled by your other asthma medications (such as inhaled corticosteroids). Salmeterol must not be used alone to treat asthma. (See also Warning section.) It is also used to prevent asthma brought on by exercise (bronchospasm).
Salmeterol superfine formulation
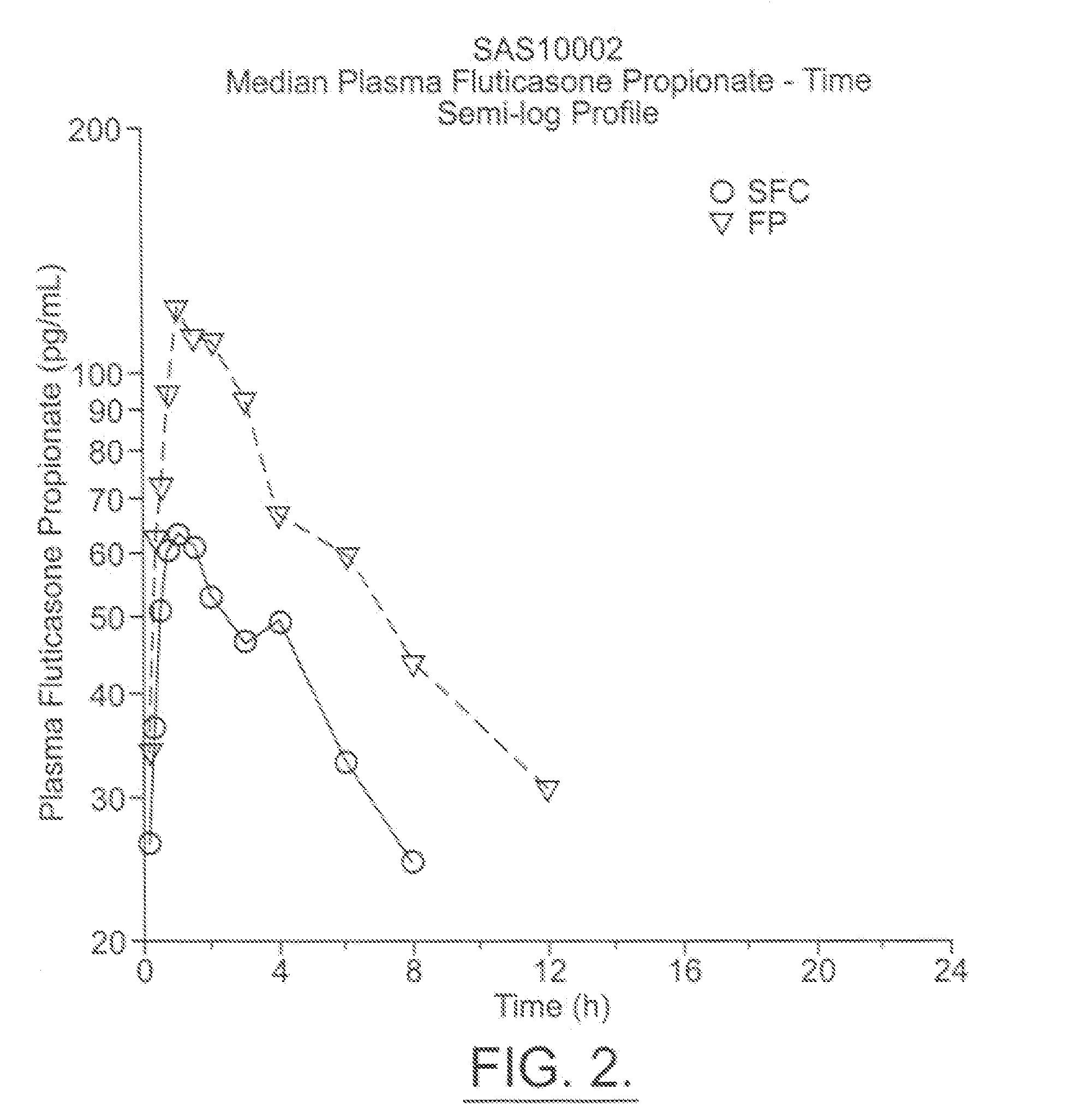
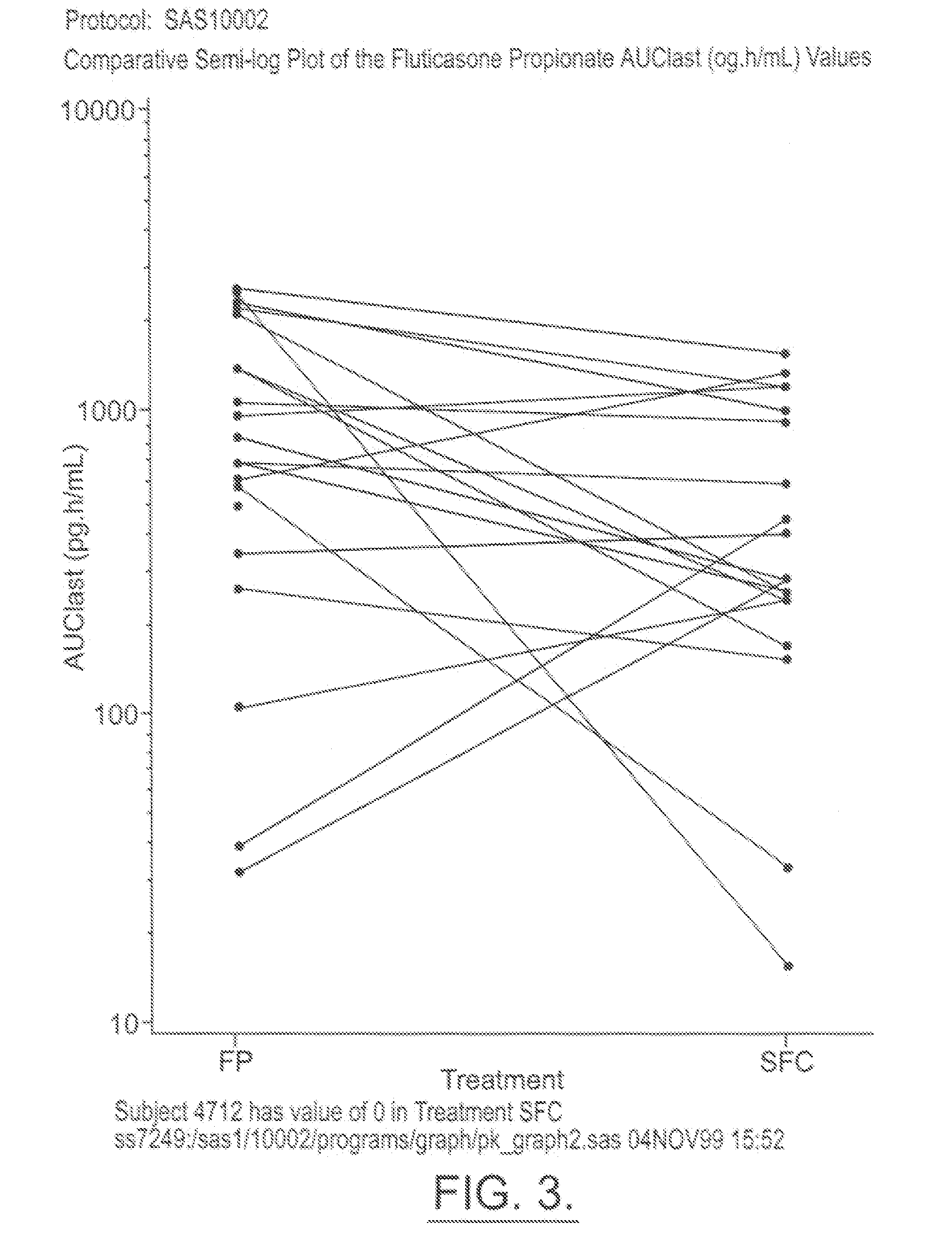
The present invention relates to a pharmaceutical formulation for use in the administration of a long-acting β2-agonist by inhalation. In particular this invention relates to a chemically stable, highly efficient salmeterol HFA solution formulation to be administered by pressurised metered dose inhalers (pMDIs) characterized by a deep lung penetration. The invention also relates to methods for the preparation of said formulation and to its use in respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD).
Owner:CHIESI FARM SPA
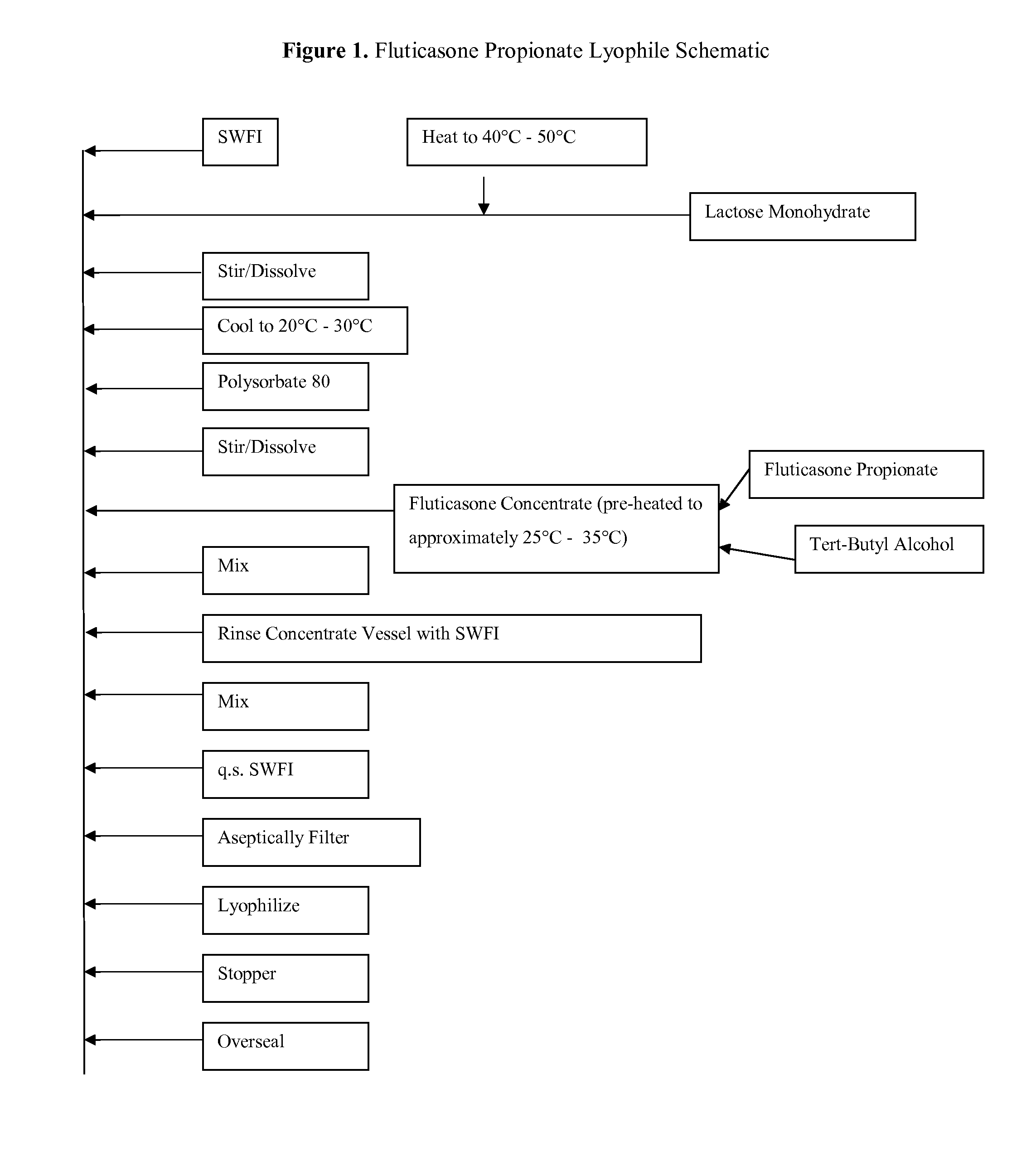
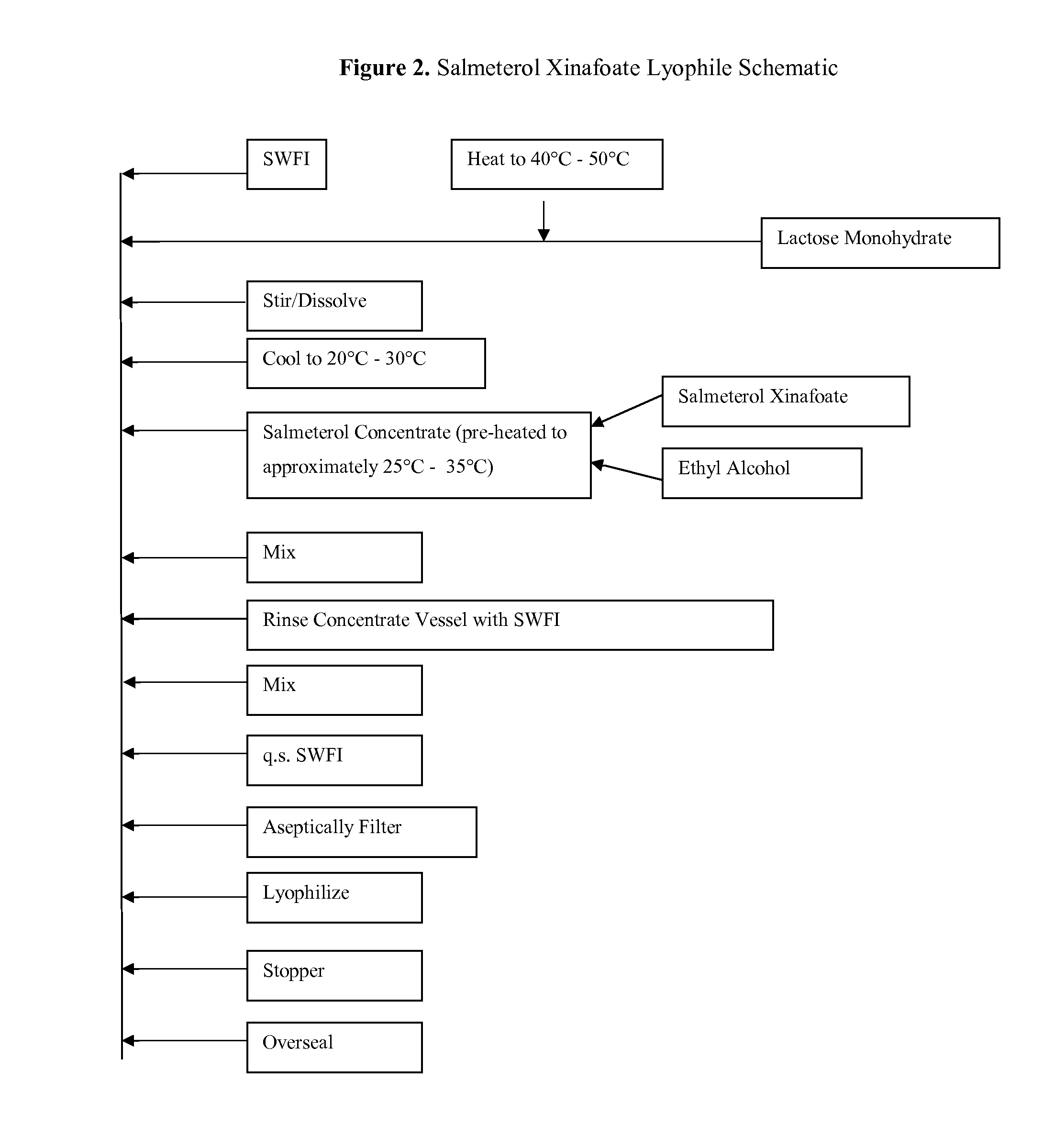
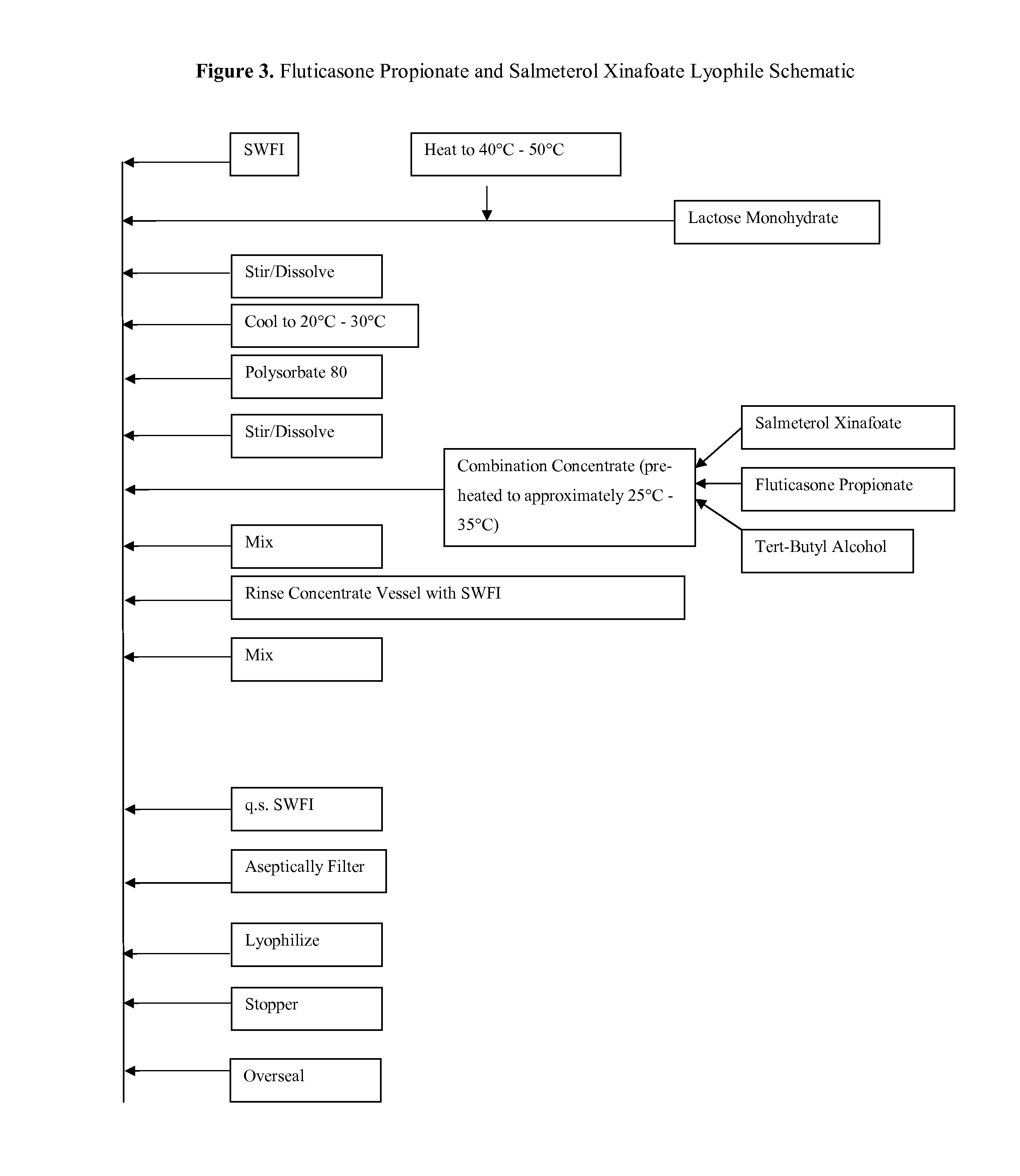
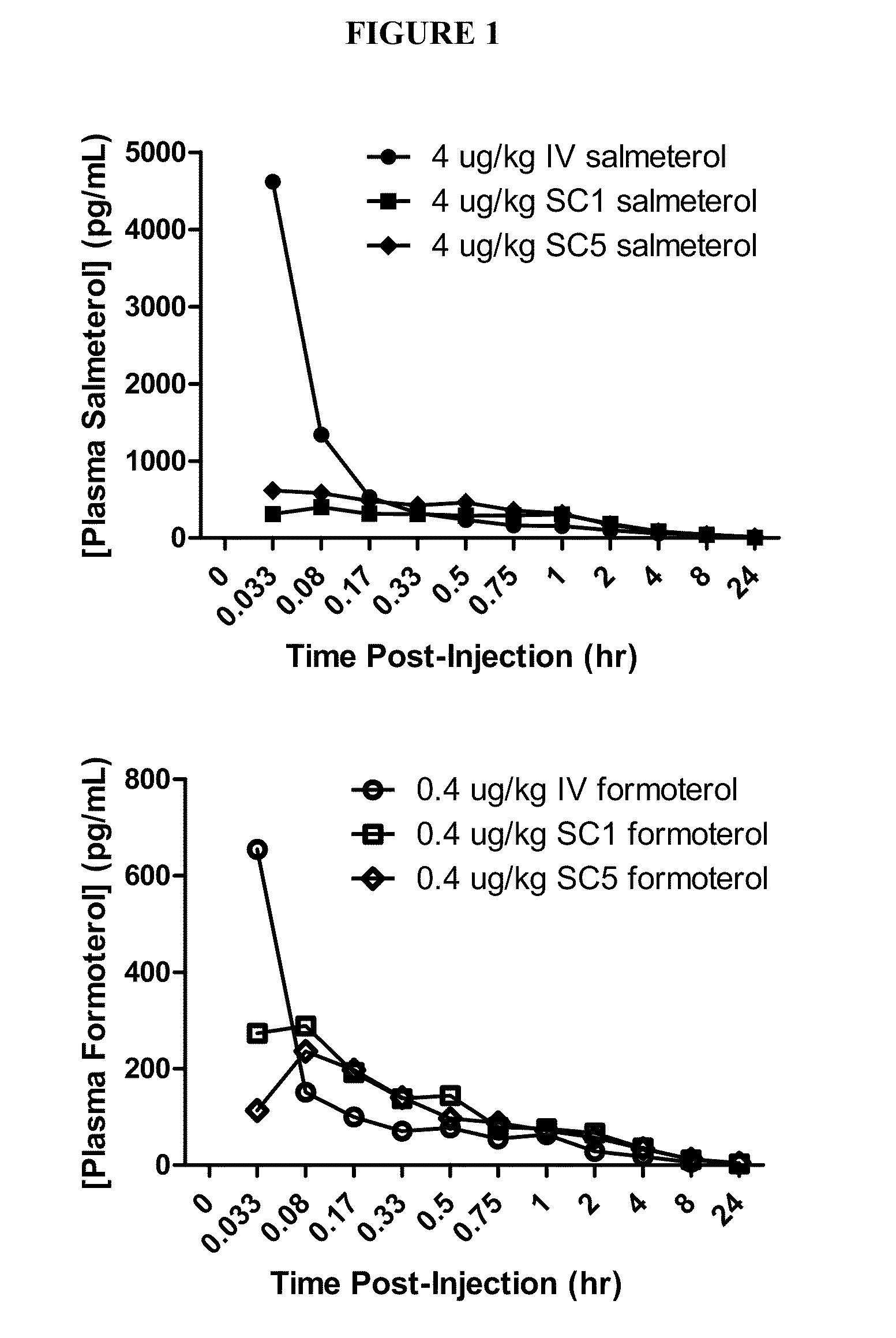
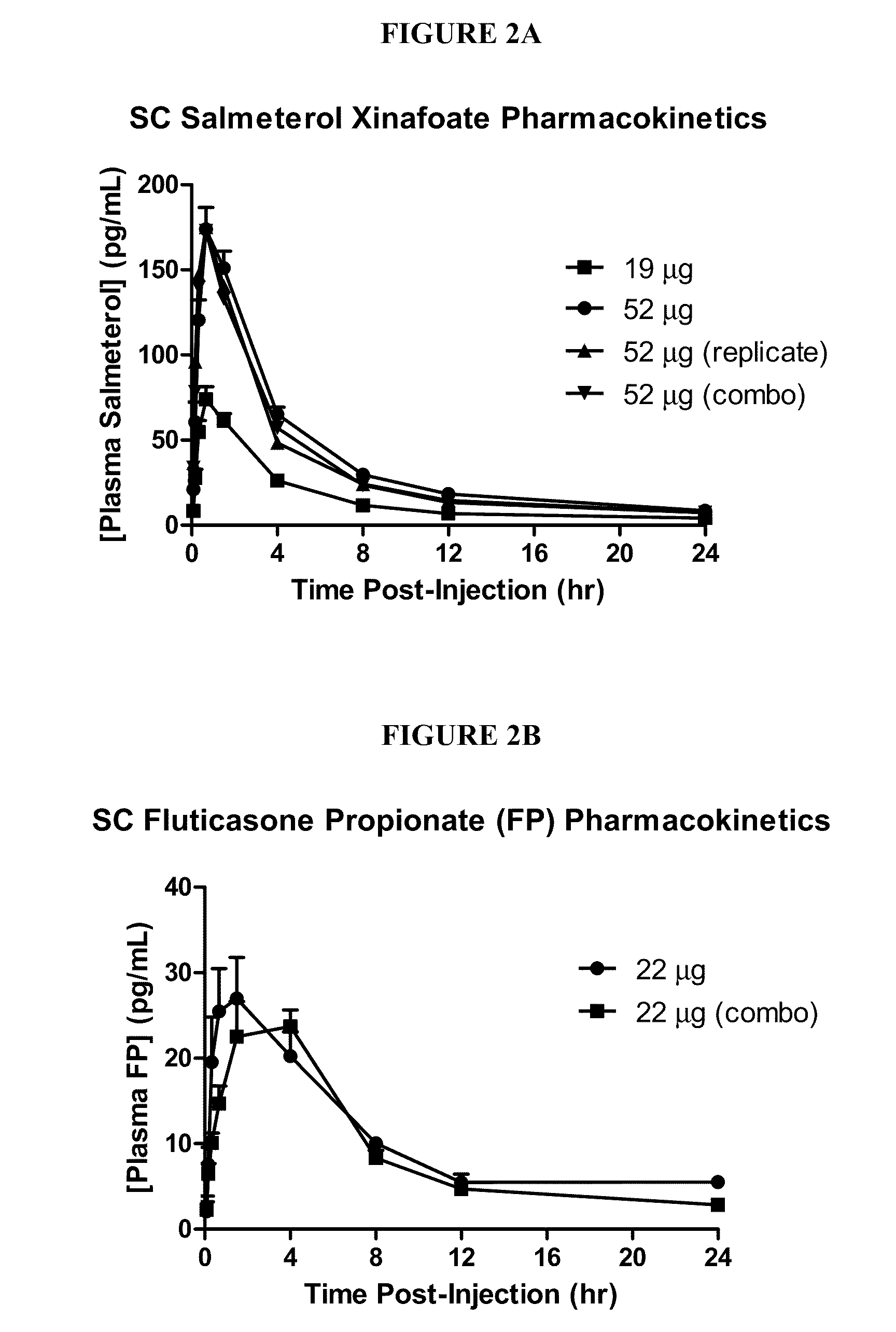
Lyophilized Cake Formulations
Provided herein are lyophilized cake forms of fluticasone, salmeterol, or a pharmaceutically acceptable salt or a combination thereof which provides room temperature stability for an extended period of time. Upon reconstitution with an acceptable solvent (e.g., a carrier or diluent), the reconstituted pharmaceutical or cosmetic formulation provides a sterile, non-suspension form suitable for parenteral injectable administration, including subcutaneous injection.
Owner:LITHERA
Tiotropium aerosol inhalant and its preparation method
InactiveCN1557308APromote absorptionImprove complianceOrganic active ingredientsAerosol deliveryIrritationSodium cromoglicate
Owner:SHANGHAI PUKANG PHARMA +2
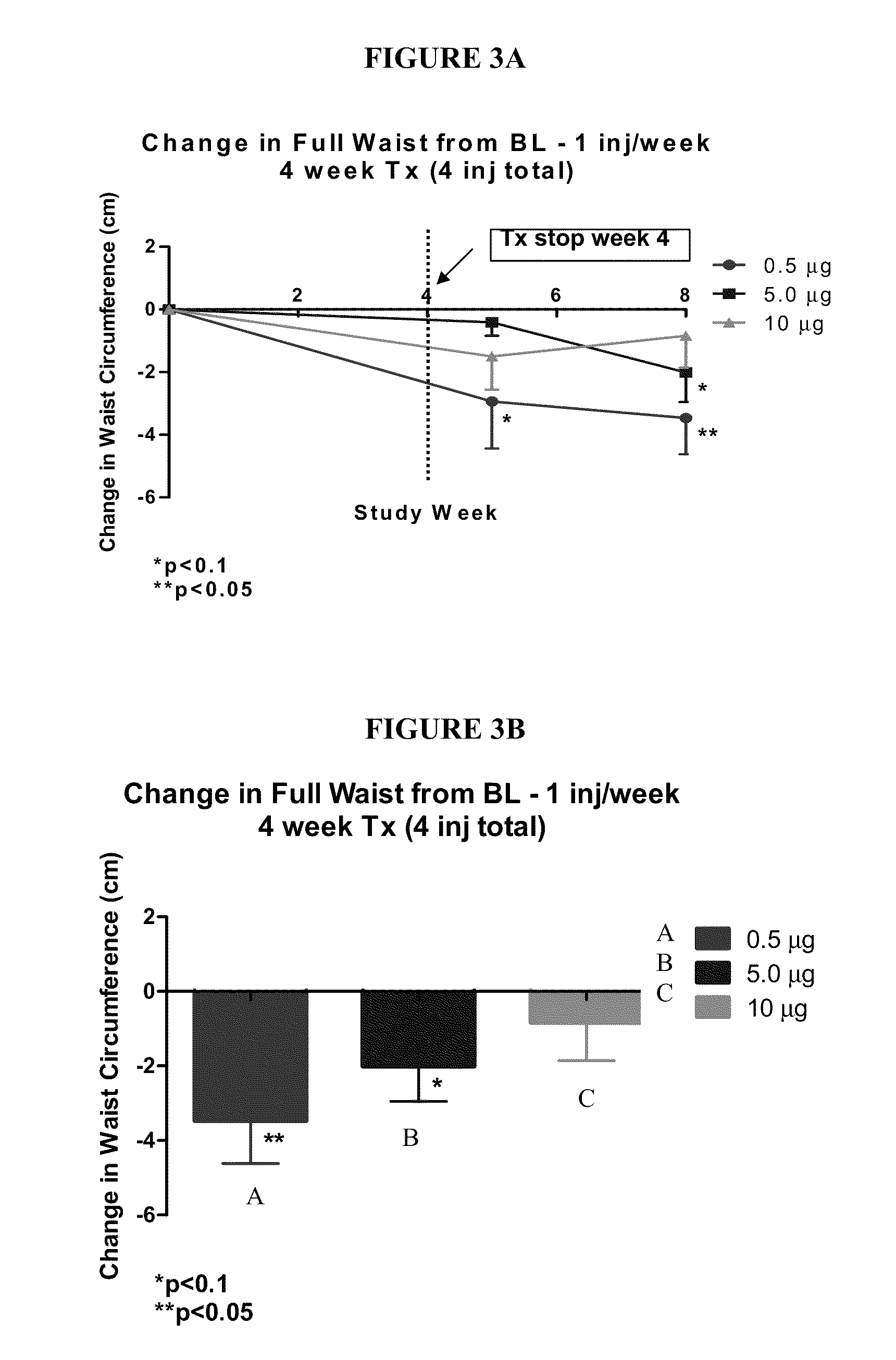
Methods for administration and formulations for the treatment of regional adipose tissue
InactiveUS20110130373A1Reducing a regional fat depositReducing desensitizationPowder deliveryCosmetic preparationsAdditive ingredientAdrenergic receptor agonists
Provided herein are pharmaceutical formulations, methods, and systems for treating regional fat deposits and fat-related conditions and indications. Methods comprise administering a pharmaceutical formulation consisting essentially of a long-acting beta-2 adrenergic receptor agonist, for example, salmeterol, suitable for subcutaneous administration. Methods further comprise administering a pharmaceutical formulation that is suitable for subcutaneous injection comprising: (a) a lipophilic long-acting selective beta-2 adrenergic receptor agonist and / or glucocorticosteroid, or a salt, optical isomer, racemate, solvate, or polymorph thereof; and (b) at least one subcutaneously acceptable inactive ingredient.
Owner:LITHERA
Methods for Administration and Formulations for the Treatment of Regional Adipose Tissue
InactiveUS20120046257A1Reducing a regional fat depositReducing desensitizationPowder deliveryCosmetic preparationsAdditive ingredientAdrenergic receptor agonists
Provided herein are pharmaceutical formulations, methods, and systems for treating regional fat deposits and fat-related conditions and indications. Methods comprise administering a pharmaceutical formulation consisting essentially of a long-acting beta-2 adrenergic receptor agonist, for example, salmeterol, suitable for subcutaneous administration. Methods further comprise administering a pharmaceutical formulation that is suitable for subcutaneous injection comprising: (a) a lipophilic long-acting selective beta-2 adrenergic receptor agonist and / or glucocorticosteroid, or a salt, optical isomer, racemate, solvate, or polymorph thereof; and (b) at least one subcutaneously acceptable inactive ingredient.
Owner:LITHERA
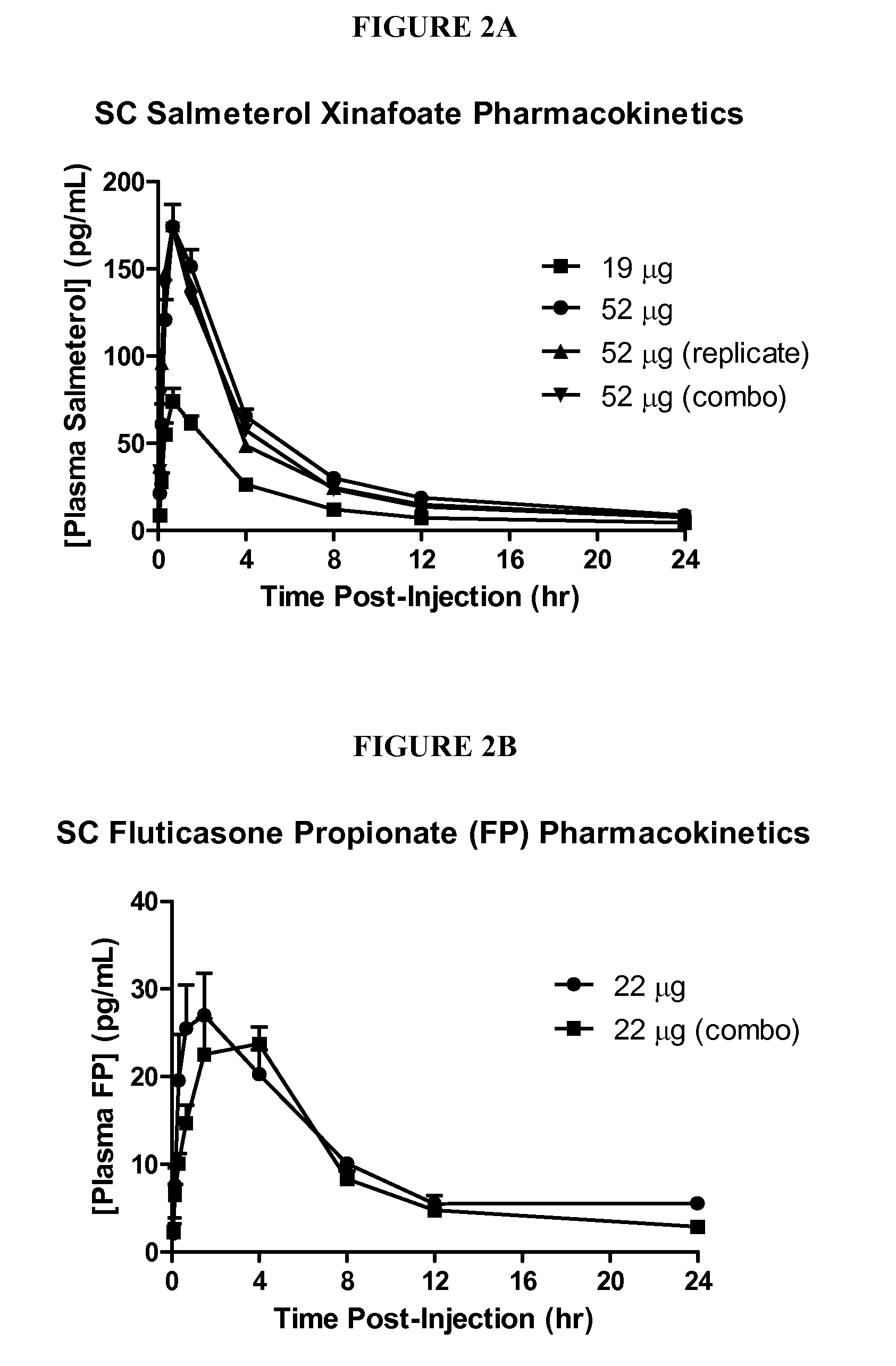
Inhalation Drug Combinations
InactiveUS20070122351A1Less systemic exposureEliminate side effectsPowder deliveryBiocideDiseaseFluticasone propionate
A method for treating respiratory disorders by administrating by inhalation an effective amount of a β2-receptor agonist, an acceptable amount of a corticosteriod, and HFA 134a, to a patient in need thereof, is disclosed. Preferably, the β2-receptor agonist is salmeterol or a physiologically acceptable salt thereof, and the corticosteriod is fluticasone propionate or a solvate thereof. The combination of salmeterol, fluticasone proprionate, and HFA 134a may lower the risk of cardiac arrhythmias, sudden death, or hypercorticism that are sometimes associated with the simultaneous administration of a β2-receptor agonist and an anti-inflammatory corticosteroid.
Owner:GLAXO GROUP LTD
Composition for curing branchial asthma
InactiveCN1425378AWide Range of Weight RatioGood effectOrganic active ingredientsRespiratory disorderAntioxidantActive component
The present invention relates to a kind of medicine composite for treating branchial asthma. The medicine composite consists of active component including salmeterol or its medicinal salt and budesonide in the molar ratio of 1 to 0.38-20, as well as non-active components including solvent, cosolvent, propellant, antioxidant, dilutent and lubricant. The present invention has large range of medicine dosage, long lasting period and high comprehensive effect, and may be prepared into various preparation forms.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing anti-asthmatic medicament of salmeterol
InactiveCN101712622AHigh yieldEasy to operateOrganic compound preparationAmino-hyroxy compound preparationOrganic layerEthyl acetate
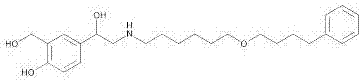
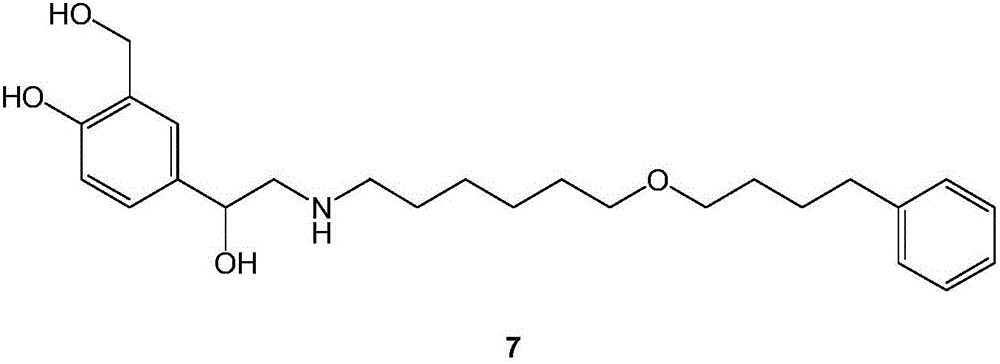
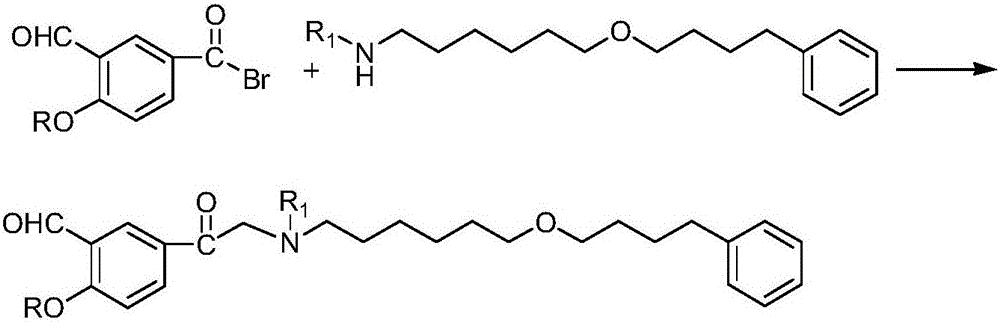
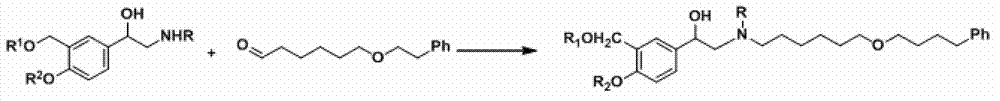
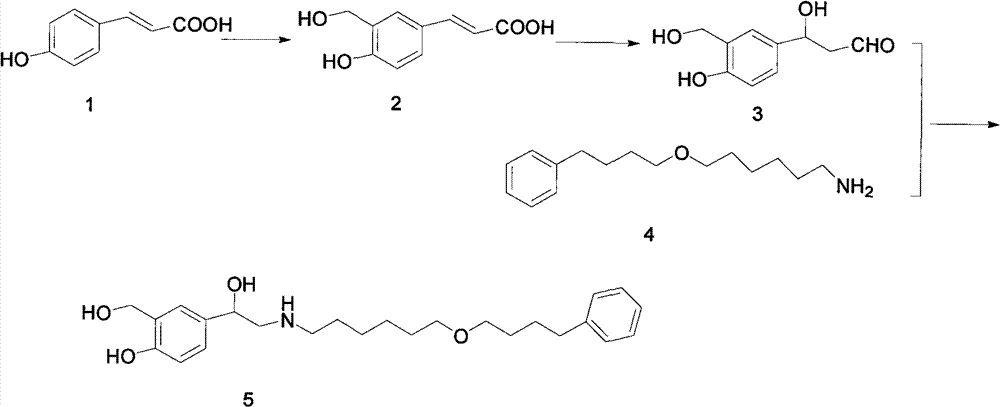
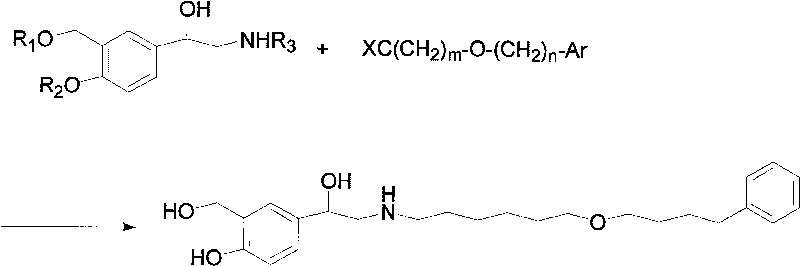
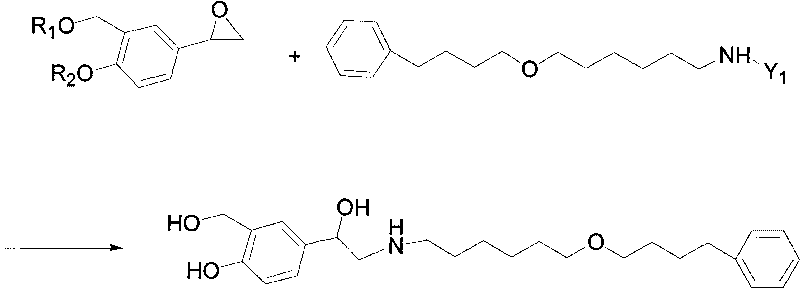
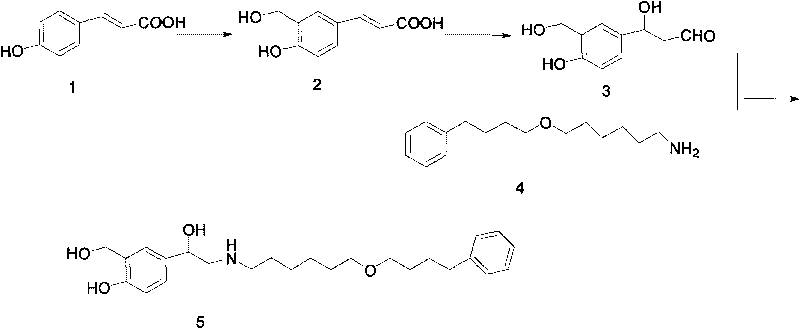
The invention discloses a method for preparing an anti-asthmatic medicament of salmeterol. The method comprises the following steps of: adding paraxylene, parahydroxyphenylacrylic acid, formaldehyde and acid medium into a reactor, heating, cooling, filtering, washing and drying to obtain 3-hydroxymethyl-4-hydroxyl-phenylacrylic acid; adding the 3-hydroxymethyl-4-hydroxyl-phenylacrylic acid into the reactor, then adding oxyful, lipase and ethyl acetate to finally obtain 3-hydroxymethyl-4-hydroxyl-(beta-hydroxy)-phenylpropyl aldehyde, adding the 3-hydroxymethyl-4-hydroxyl-(beta-hydroxy)-phenylpropyl aldehyde and 6-(4-phenylbutoxy)hexylamine into the reactor, heating for reflowing, cooling, filtering, adding sodium borohydride in batches into filtrate, stirring at normal temperature, then evaporating to dryness, extracting, combining organic layers, drying, filtering and evaporating to dryness to obtain a solid, and separating by using the column chromatography to obtain the final product. The invention has novel and short synthetic route, convenient experiment operation, higher yield of the target product and lower production cost.
Owner:ZHEJIANG WANLI UNIV
Method for synthesizing (R)-salmeterol
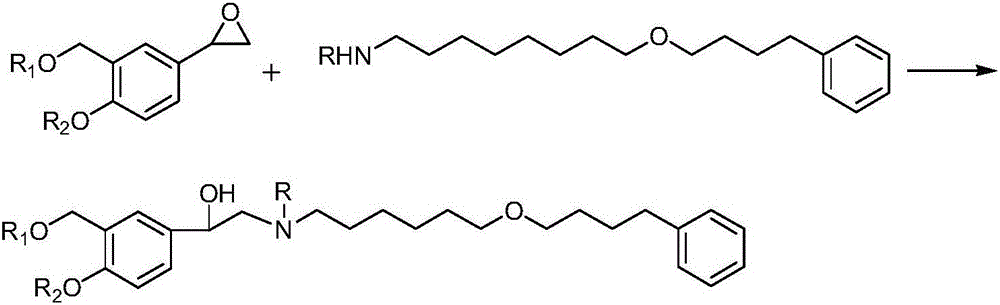
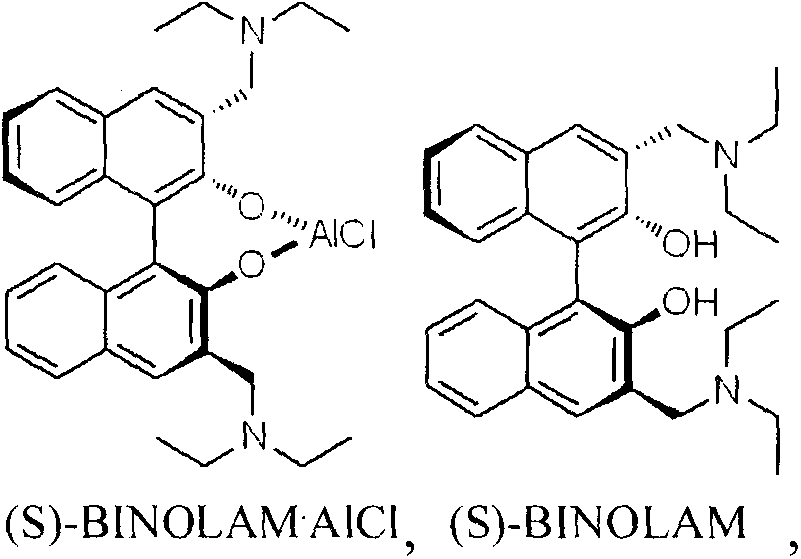
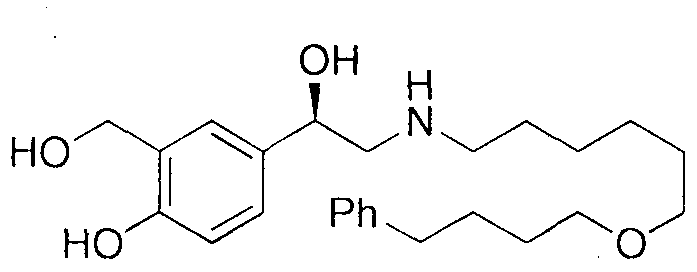
The invention discloses a method for synthesizing (R)-salmeterol. The method is implemented by taking p-hydroxy benzaldehyde as a raw material through the steps of carrying out a methylolation reaction on the p-hydroxy benzaldehyde so as to obtain prochiral aldehyde for protecting double hydroxide radicals in an acetal form; then, through taking (S)-BINOLAM.AlCl as a chiral catalyst, carrying out an asymmetric nucleophilic addition reaction so as to obtain a chiral cyanohydrin intermediate; and reducing the chiral cyanohydrin intermediate so as to obtain primary amine, reacting the primary amine with mesylate [6-(4 phenylbutoxy]hexane, and carrying out hydrolysis on the obtain product so as to remove protecting groups, thereby obtaining a final product (R)-salmeterol. In the invention, a chiral catalyst precursor (S)-BINOLAM can be reused; and the method has relatively high yield and relatively good enantioselectivity, and is short in synthetic route, simple and convenient in operation and low in cost.
Owner:NANJING UNIV OF TECH
Inhalation Drug Combinations
InactiveUS20070122352A1Good curative effectReduce systemic side effectsHalogenated hydrocarbon active ingredientsBiocideDiseaseFluticasone propionate
A method for treating respiratory disorders by administrating by inhalation an effective amount of a β2-receptor agonist, an acceptable amount of a corticosteroid, and HFA 134a, to a patient in need thereof, is disclosed. Preferably, the β2-receptor agonist is salmeterol or a physiologically acceptable salt thereof, and the corticosteroid is fluticasone propionate or a solvate thereor. The combination of salmeterol, fluticasone proprionate, and HFA 134a may lower the risk of cardiac arrhythmias, sudden death, or hypercorticism that are sometimes associated with the simultaneous administration of a β2-receptor agonist and an anti-inflammatory corticosteroid.
Owner:GLAXO GROUP LTD
Process for the manufacture of 4-(6-bromohexyloxy)-butylbenzene
InactiveUS6835857B2Increased space-time yieldHigh purityOrganic compound preparationEther preparation by ester reactionsDiluent4-phenylbutanol
The present invention relates to an improved process for preparing 4-(6-bromohexyloxy)-butylbenzene by reacting 4-phenylbutanol with 1,6-dibromohexane in the presence of a base and a phase transfer catalyst, wherein 4-phenylbutanol in a diluent is metered into a mixture consisting of 1,6-dibromohexane, a base, a phase transfer catalyst and a diluent, and the use of the 4-(6-bromohexyloxy)-butylbenzene thus prepared for producing salmeterol in a method known per se.
Owner:BOEHRINGER INGELHEIM PHARMA KG
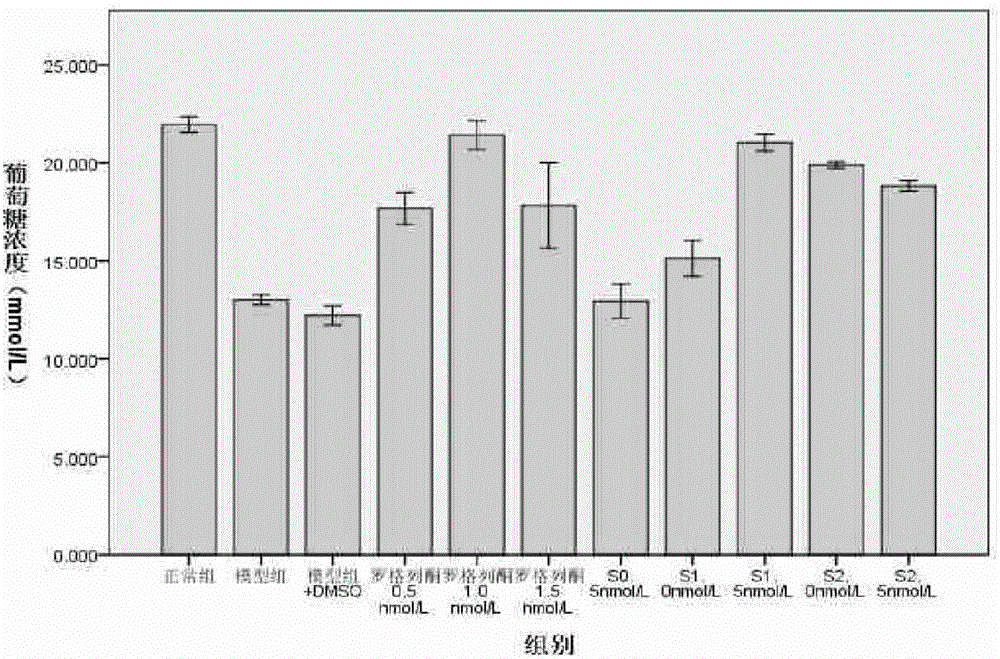
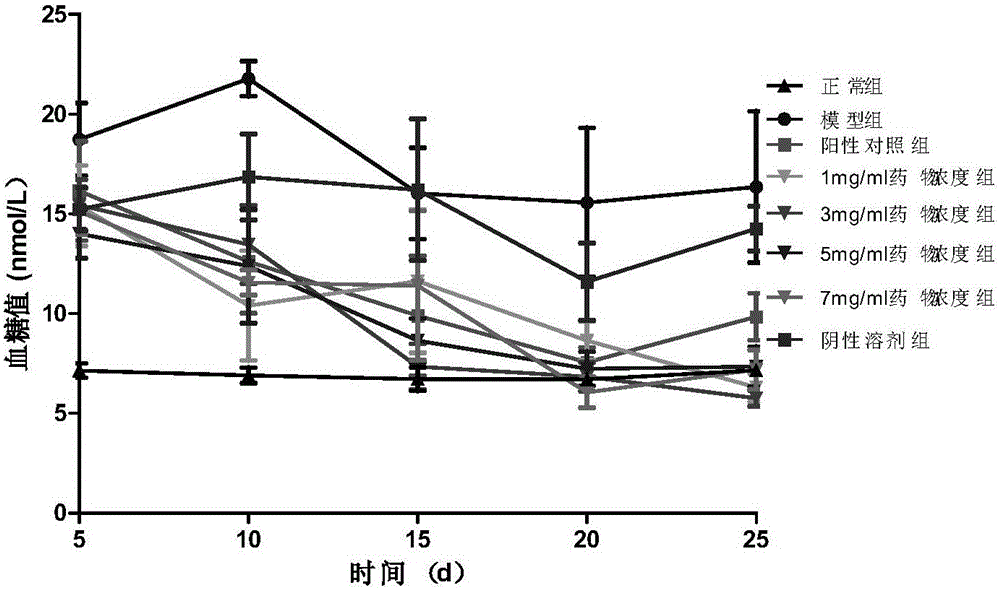
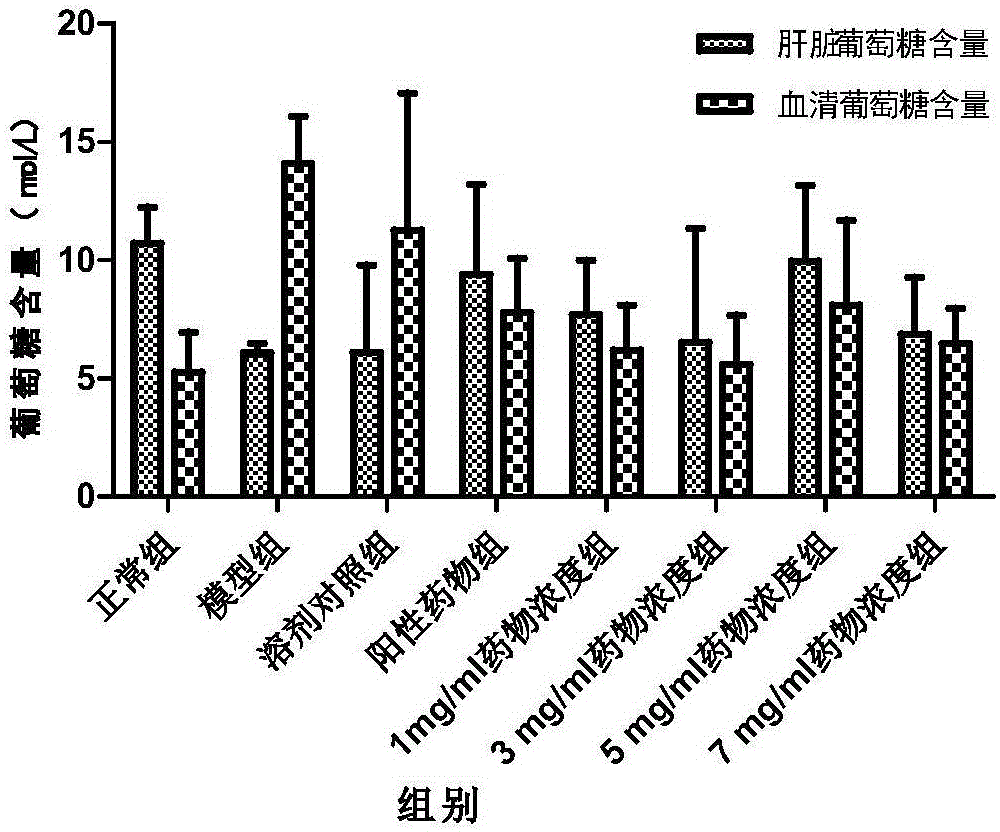
Application of salmeterol in medicine for treating type 2 diabetes and insulin resistance
The invention provides application of salmeterol in medicine for treating type 2 diabetes and insulin resistance. The salmeterol has effects of lowering blood sugar, resisting diabetes and increasing the cell glucose consumption of a 3T3-L1 insulin resistance model. Measuring of PPAR gamma protein expression quantity through an ELISA method shows that the salmeterol can increase the PPAR gamma protein expression quantity. The salmeterol has evident curative effect on the type 2 diabetes.
Owner:CHONGQING UNIV OF TECH
Salmeterol superfine formulation
Owner:CHIESI FARM SPA
Synthesis method of R-salmeterol
ActiveCN105884625AEasy to routeMild reaction conditionsOrganic compound preparationOrganic chemistry methodsSynthesis methodsStructural formula
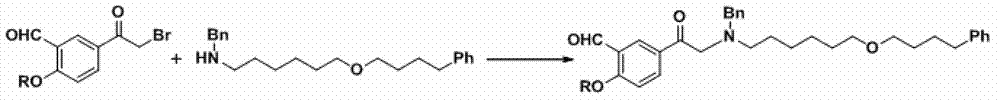
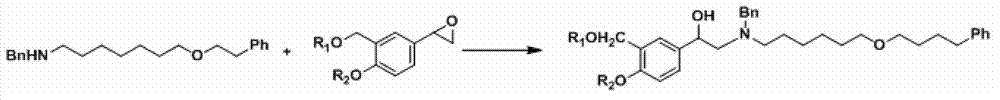
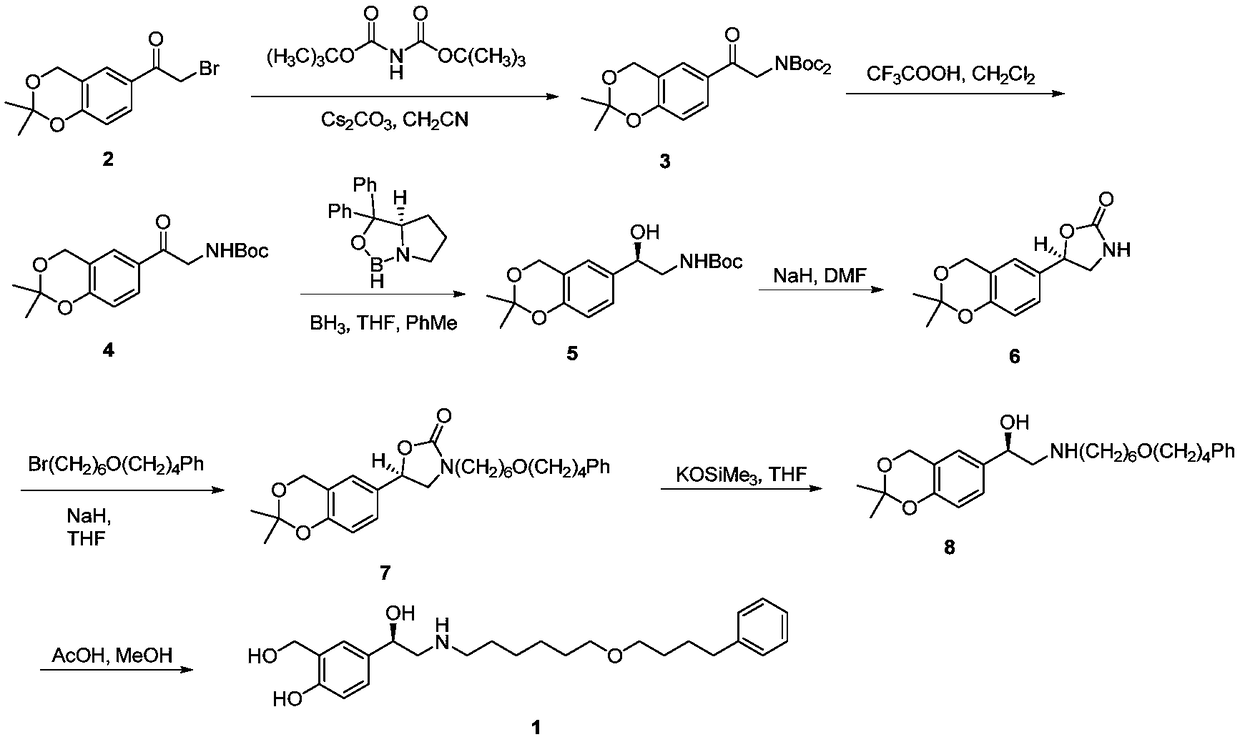
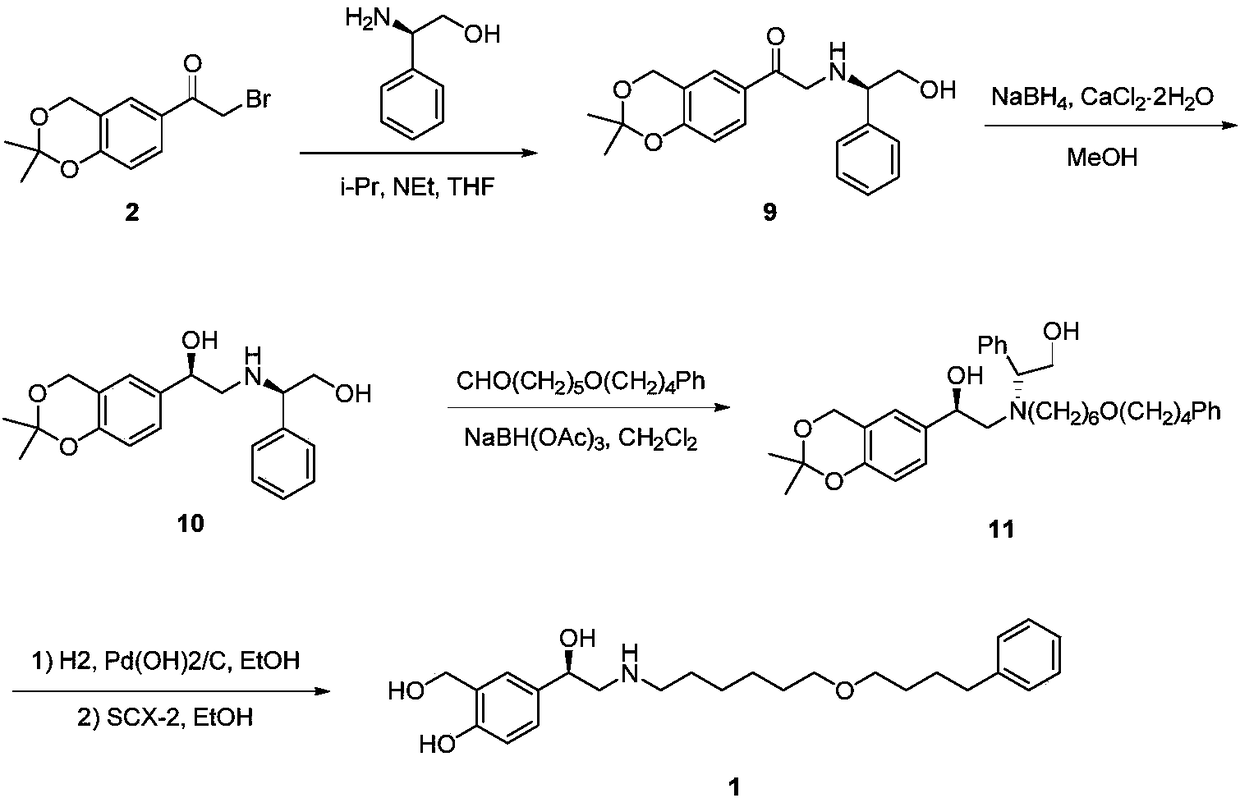
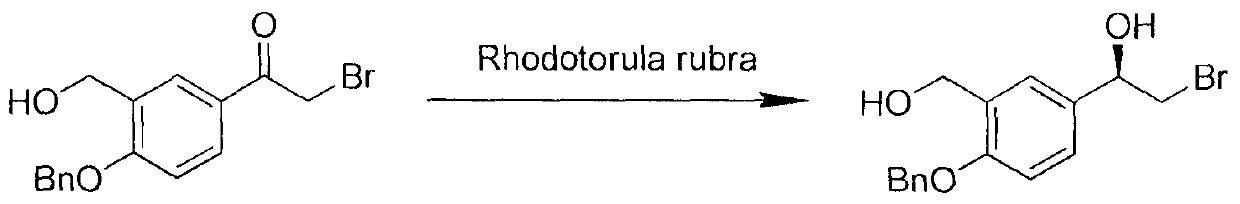
The invention provides a synthesis method of R-salmeterol (V). The synthesis method comprises the following steps that a compound (I) and N-benzyl-6-(4-phenyl butoxy)-1-hexylamine take a reaction to obtain a compound (II); the compound (II) is hydrolyzed under the acidic condition to obtain a compound (III); the compound (III) is subjected to asymmetric reduction to obtain a compound (IV); the compound (IV) is subjected to deprotection to obtain the R-salmeterol (V). The route is simple; the target product of R-salmeterol (V) is obtained by using 2-acetoxyl group-5-(2-bromoacetyl) phenmethyl acetic ester and the N-benzyl-6-(4-phenyl butoxy)-1-hexylamine as raw materials through total four steps of reaction. The synthesis method has the advantages that the reaction conditions are mild; the operation is simple and convenient; the yield is high; the stereoselectivity is good; the production cost is low; the synthesis method is suitable for industrial production; great practical application values and social economical benefits are realized. The structural formula is shown as the accompanying drawing.
Owner:JOINCARE HAIBIN PHARM CO LTD
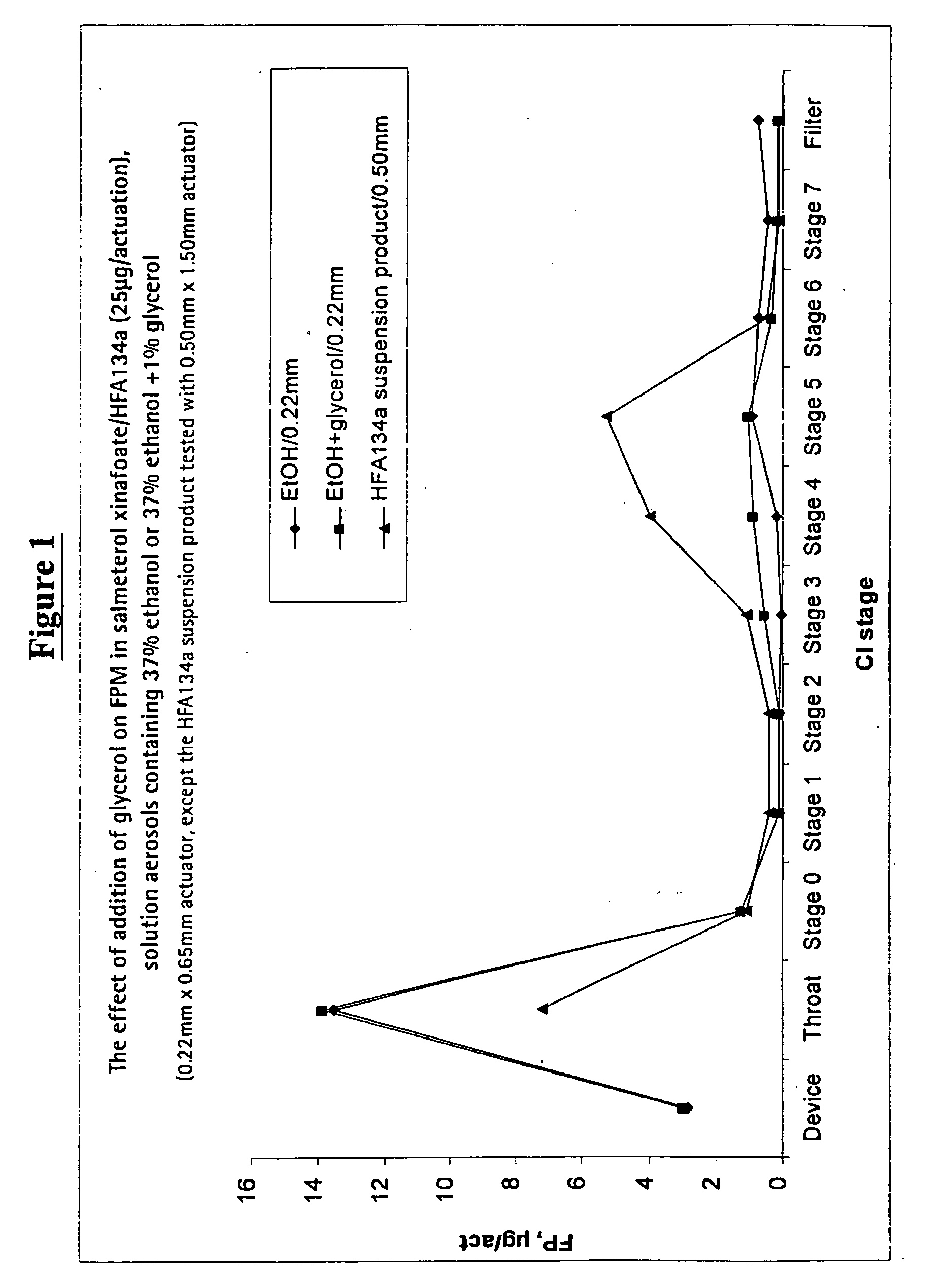
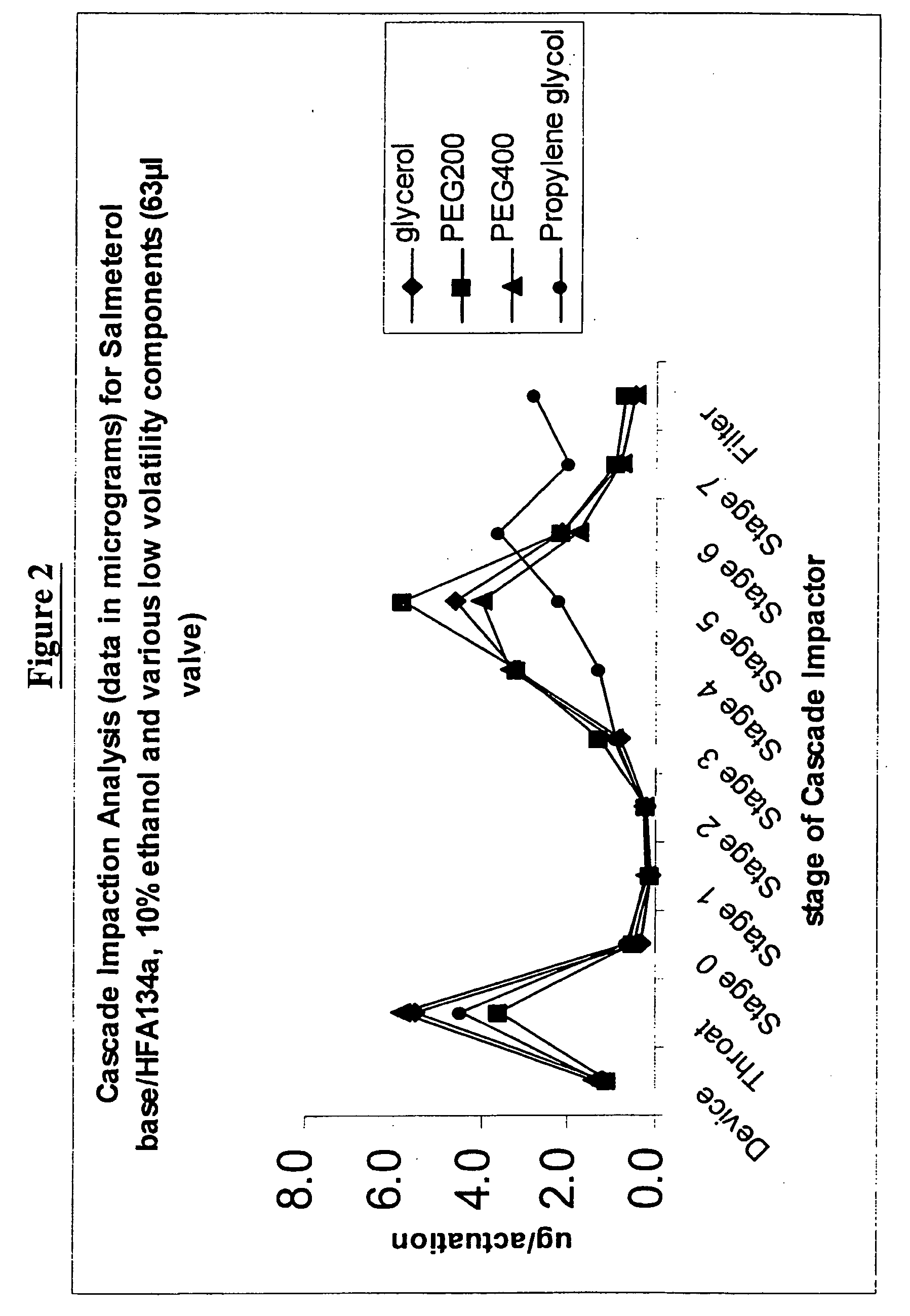
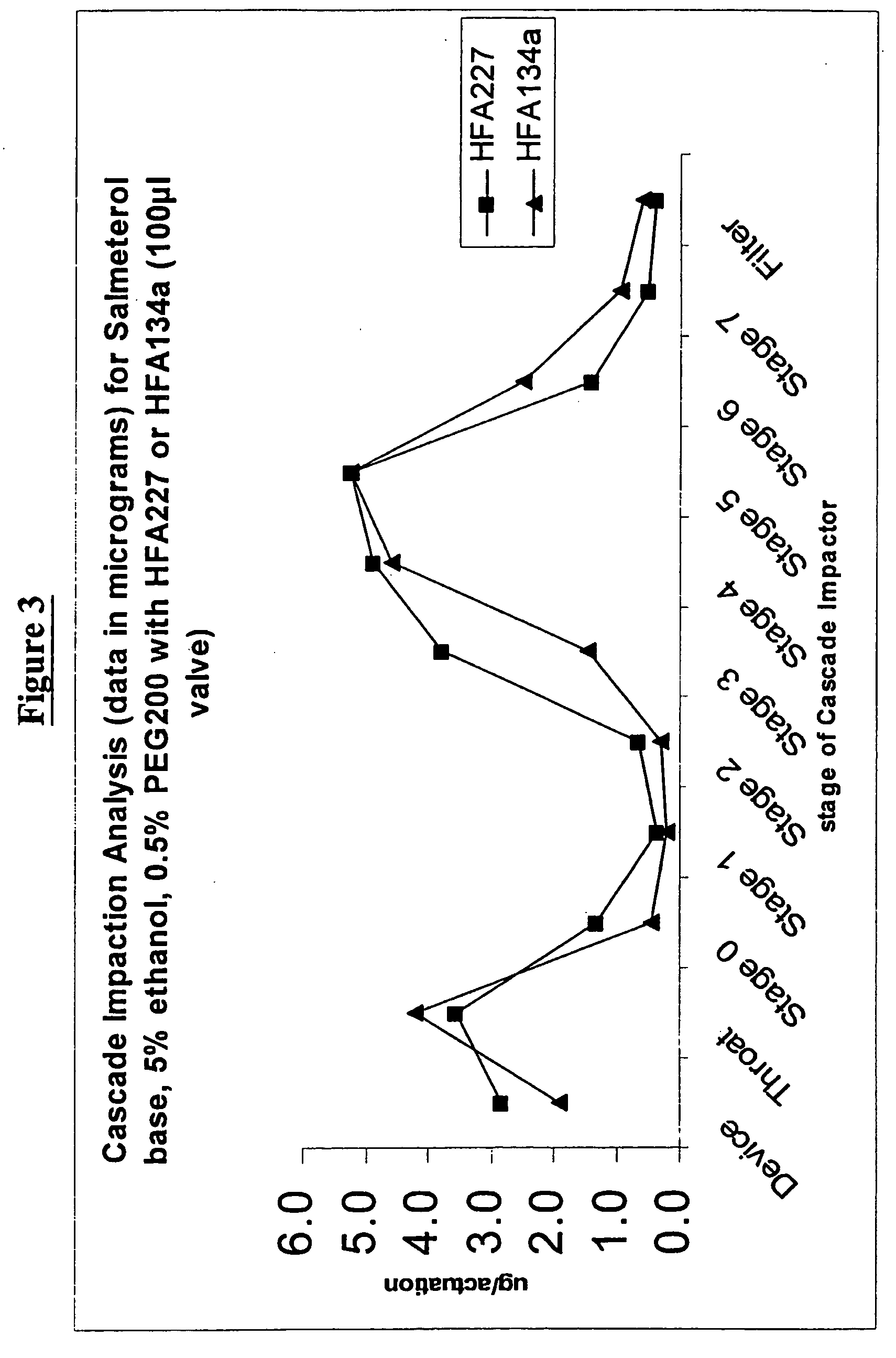
Pharmaceutical formulations of salmeterol
InactiveUS20050048001A1Better dosing uniformityHigh FPMOrganic active ingredientsDispersion deliveryDrug aerosolMedicine
There is provided according to the invention a pharmaceutical aerosol formulation which comprises: (i) salmeterol or a pharmaceutically acceptable salt thereof and (ii) a hydrofluoroalkane (HFA) propellant, characterised in that the salmeterol or pharmaceutically acceptable salt thereof is completely dissolved in the formulation.
Owner:CRIPPS ALAN LESLIE +1
Formula and preparation method of salmeterol/fluticasone
InactiveCN111067869ADisruption of regularityWide range of parcelsOrganic active ingredientsPharmaceutical non-active ingredientsFluticasone propionatePropanoic acid
The invention discloses a formula of salmeterol / fluticasone. The formula of the salmeterol / fluticasone comprises liposomes, as well as ingredients encapsulated in the liposomes. The ingredients encapsulated in the liposomes are as follows: 10-20 parts of salmeterol, 20-40 parts of fluticasone propionate, 1-5 parts of acesulfame, and 0.1-0.5 parts of an emulsifier; and parts by mass of the liposomes are 10-30 times of the sum of the parts by mass of the ingredients. In the complex prepared by encapsulating the ingredients with the liposomes, the two ingredients, namely the salmeterol and the fluticasone propionate, are mixed so as to achieve good uniformity, so that the phenomenon of component separation does not occur; and moreover, the liposomes are soft to touch, so that the problem of insufficient fluffy feeling due to direct mixing of solid particles is solved.
Owner:长沙而道新能源科技有限公司
Preparation and application of bis [-6-oxo-(3-deoxgmonoester citrate-4)]-beta-cyclodextrin HPLC column material
ActiveCN106984291AAchieve separationOther chemical processesOrganic compound preparationCefuroximeEnantiomer
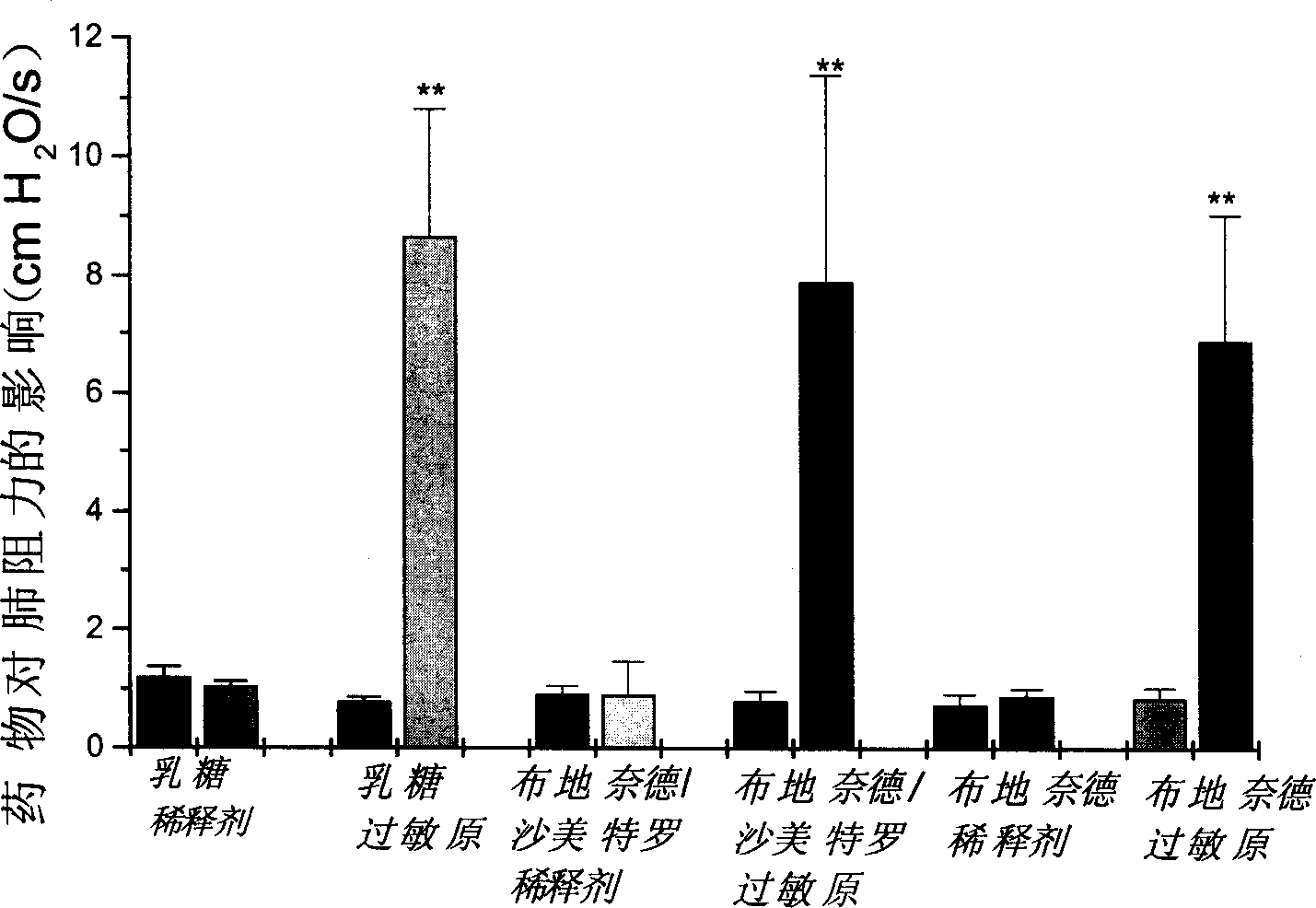
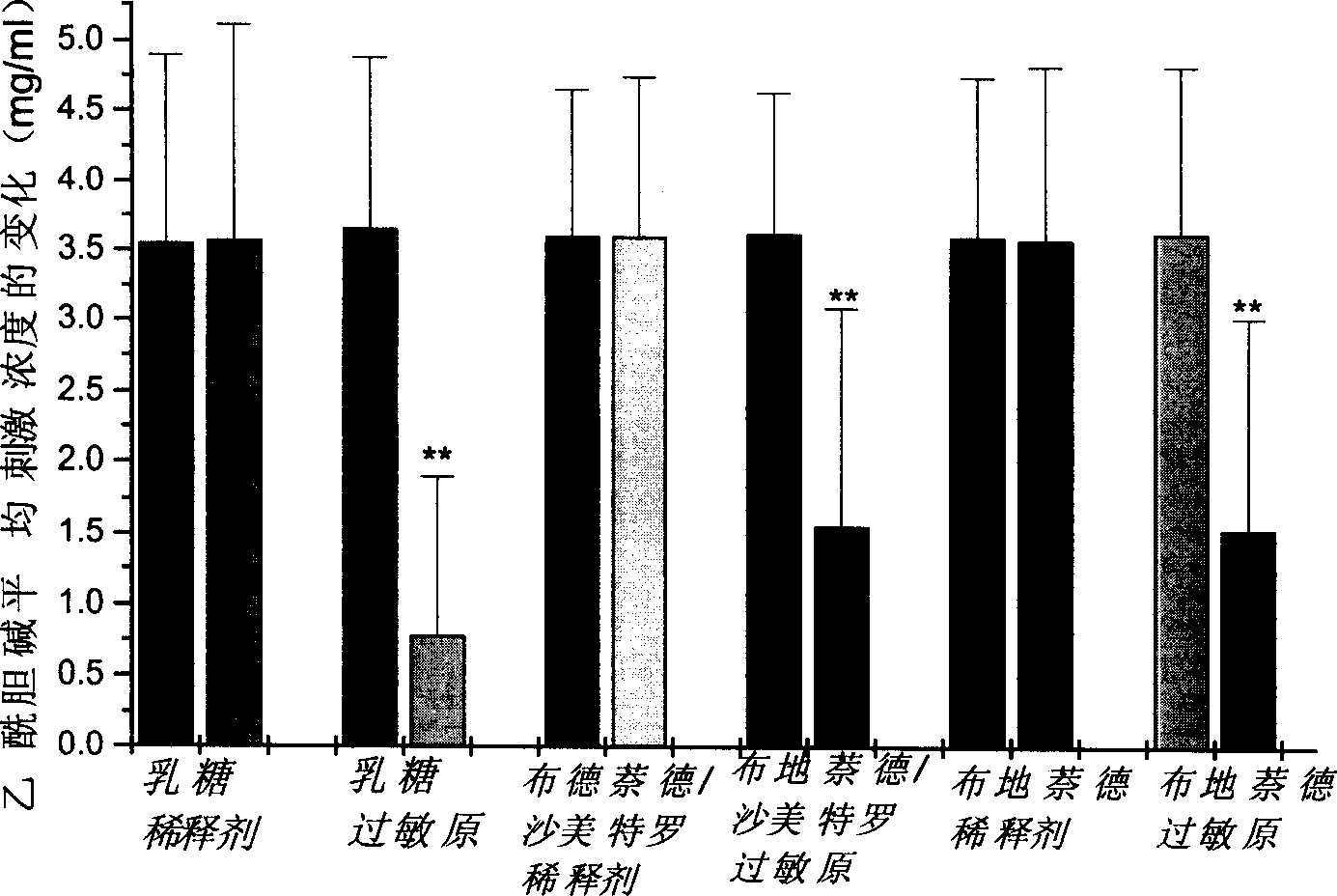
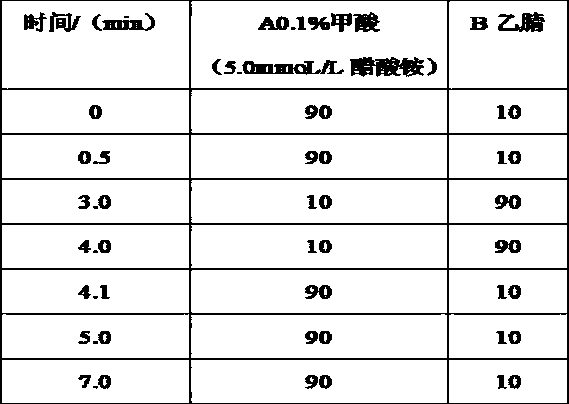
The invention relates to the technical field of preparation and application of chiral high-performance liquid chromatography column materials, and discloses preparation and application of beta-CD-D2 high-performance liquid chromatography chiral column material. A high-performance liquid chromatography chiral column is prepared from beta-CD-D2 and is used for splitting chiral medicine enantiomers. The preparation and the application of the beta-CD-D2 high-performance liquid chromatography chiral column material comprise the following steps: 1, constructing a chiral environment by using beta-CD-D2, bonding the chiral environment with high-performance liquid chromatography silica beads into a fixed phase filler, then preparing the fixed phase filler into a chiral column, and applying the chiral column into high-performance liquid chromatography; 2, finding out an optional formula and an optional condition by using a proper characterization means, and optimizing the method and the condition; 3, establishing a preparation method of a salmeterol single enantiomer by using the prepared beta-CD-D2 chiral column, and splitting a chiral compound by using a cyclodextrins derivative for the first time; and 4, splitting drugs by using the prepared beta-CD-D2 chiral column, and establishing a method for splitting and qualitatively and quantitatively analyzing seven chiral drugs including salmeterol, terbutaline, procaterol, cetirizine, lamivudine, cefuroxime and ceftriaxone.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Method for determining salmeterol, indacaterol and olodaterol in pork
InactiveCN108982712ASolve detection gapsHigh ion responseComponent separationIndacaterolSpectrometer
The invention provides a method for determining salmeterol, indacaterol and olodaterol in pork. The method includes the following steps: step 1, preparing samples; step 2, extracting to-be-detected samples; step 3, determining detection conditions of a liquid chromatography-tandem mass spectrometer and performing detection; step 4, drawing standard working curves; step 5, according to liquid chromatograms of to-be-detected solutions in the S2, calculating the contents of the olodaterol, the indacaterol and the salmeterol in the pork. The method has the advantages that the salmeterol, the indacaterol and the olodaterol in the pork are simultaneously determined for the first time, and the blank in current detection of the salmeterol, the indacaterol and the olodaterol in the pork is filled up; ion response values are high, sensitivity is high, detection limits are low, qualitativeness, quantitativeness, accuracy, rapidness, high efficiency and sensitivity are truly realized, and the method can serve as a reliable measure to detect the three drugs in the pork.
Owner:食药环检验研究院(山东)集团有限公司
Preparing method of salmeterol and fluticasone super fine particles
InactiveCN106474136ASmall particlesImprove uniformityOrganic active ingredientsGranular deliveryFluticasone propionateSolvent
The invention relates to a preparing method of salmeterol and fluticasone super fine particles. The preparing method comprises the steps of dissolving the raw materials for preparing the super fine particles into a solvent to prepare a solution, and then adding the solution into a spray dryer to be subjected to spray drying, so that the salmeterol and fluticasone super fine particles are prepared, wherein the raw materials comprise salmeterol, fluticasone propionate and auxiliaries, the volume added quantity of the solution is 10%-30% of the total capacity of the spray dryer, and the mass concentration of the solution is 5%-10%. According to the preparing method of the salmeterol and fluticasone super fine particles, the salmeterol and fluticasone dry particles are finer, uniformity is better, and distribution of the two medicines is more reasonable.
Owner:ZHANGJIAGANG HUACHANG PHARMA
Preparation and application of bis[-6-oxo-(3-deoxycitrate monoester-4)]-β-cyclodextrin hplc column material
ActiveCN106984291BAchieve separationOrganic compound preparationOther chemical processesFormularyCefuroxime
The invention relates to the technical field of preparation and application of chiral high-performance liquid chromatography column materials, and discloses preparation and application of beta-CD-D2 high-performance liquid chromatography chiral column material. A high-performance liquid chromatography chiral column is prepared from beta-CD-D2 and is used for splitting chiral medicine enantiomers. The preparation and the application of the beta-CD-D2 high-performance liquid chromatography chiral column material comprise the following steps: 1, constructing a chiral environment by using beta-CD-D2, bonding the chiral environment with high-performance liquid chromatography silica beads into a fixed phase filler, then preparing the fixed phase filler into a chiral column, and applying the chiral column into high-performance liquid chromatography; 2, finding out an optional formula and an optional condition by using a proper characterization means, and optimizing the method and the condition; 3, establishing a preparation method of a salmeterol single enantiomer by using the prepared beta-CD-D2 chiral column, and splitting a chiral compound by using a cyclodextrins derivative for the first time; and 4, splitting drugs by using the prepared beta-CD-D2 chiral column, and establishing a method for splitting and qualitatively and quantitatively analyzing seven chiral drugs including salmeterol, terbutaline, procaterol, cetirizine, lamivudine, cefuroxime and ceftriaxone.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Salmeterol lipid complex and preparation method thereof
The invention discloses a salmeterol lipid complex and a preparation method thereof. The complex comprises salmeterol, a lipid material, a buffer solution and the like. The lipid complex can compriseone or more lipids. In addition, the invention further discloses the ratio of the high-activity compound salmeterol to the lipids in the complex. The encapsulation efficiency of the salmeterol lipid complex can be increased, and in combination with a pharmaceutically acceptable vector or an optional medicament formula, the complex can prevent or treat related diseases, and has the characteristicsof good stability, high targeting property, low toxic and side effects and the like. The preparation method involved in the invention is simple, and is high in operability, and industrialized production is easy to implement.
Owner:ENKANG PHARMA GUANGZHOU LTD +1
Method for preparing salmeterol transdermal patch
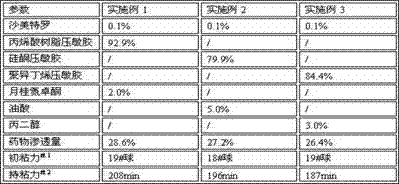
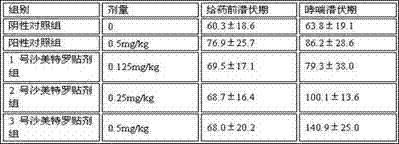
InactiveCN107875142AObvious antiasthmatic effectIncreased antiasthmatic effectOrganic active ingredientsOrganic non-active ingredientsTransdermal patchSolvent
The invention discloses a salmeterol transdermal patch, which contains salmeterol and its salts, a pressure-sensitive adhesive, a penetration enhancer, a solvent, and contains 0.05% to 0.1% of salmeterol and its salts, Sensitive glue 74.9%~98.5%, penetration enhancer 0.5~10%, solvent 1%~15%. It has excellent properties in transdermal absorption rate, mechanical properties and preparation process, and is used for the prevention and treatment of asthma.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Medicinal composition product containing salmeterol and roflumilast
The invention relates to a combination consisting of inhalation / oral roflumilast or a pharmaceutically acceptable salt thereof and inhalation salmeterol or a pharmaceutically acceptable salt thereof. The medicinal composition product is administrated synchronously, sequentially or separately, and is applied to treatment or preventive treatment of respiratory diseases or symptoms thereof, particularly treatment of diseases accompanied with obstruction or inflammation such as chronic obstructive pulmonary disease (COPD) or asthma.
Owner:QINGDAO CITY CHENGYANG DISTRICT PEOPLES HOSPITAL
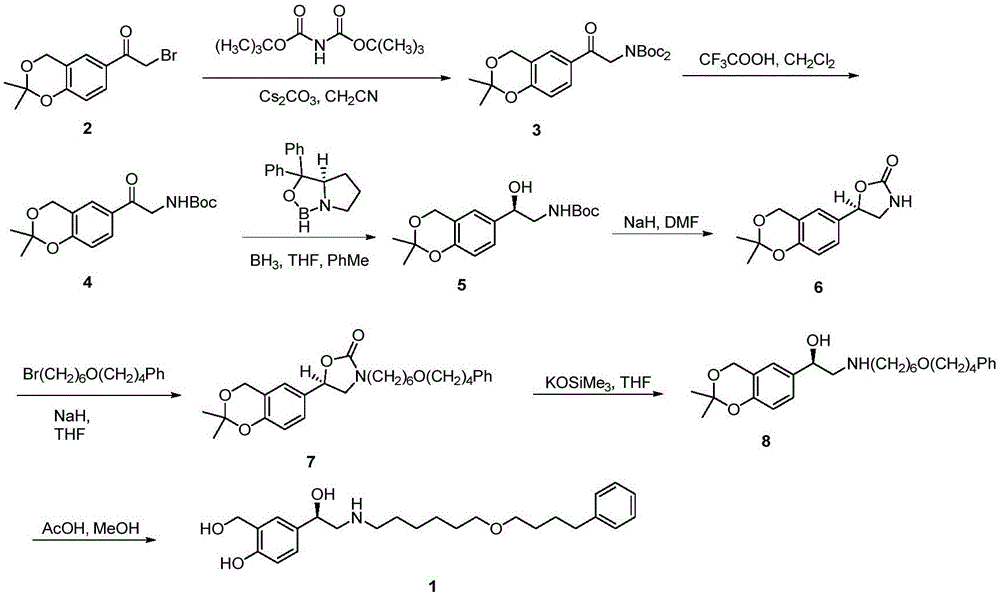
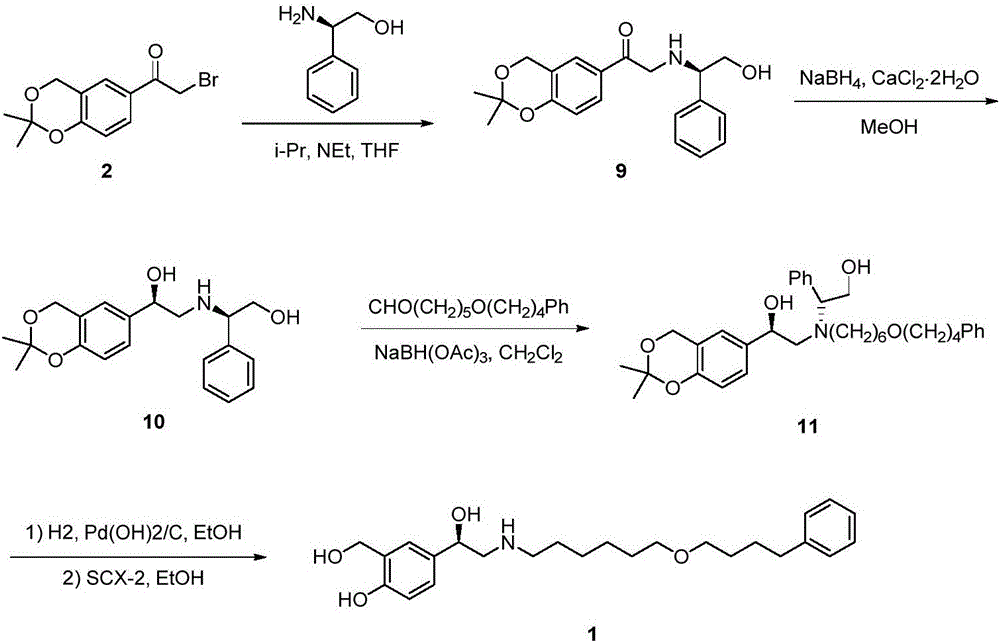
Preparation method of salmeterol base
InactiveCN106397228AHigh yieldHigh purityOrganic compound preparationAmino-hyroxy compound preparationPalladium on carbonHydrolysis
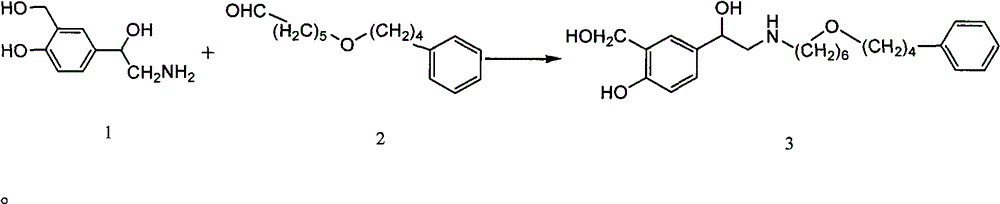
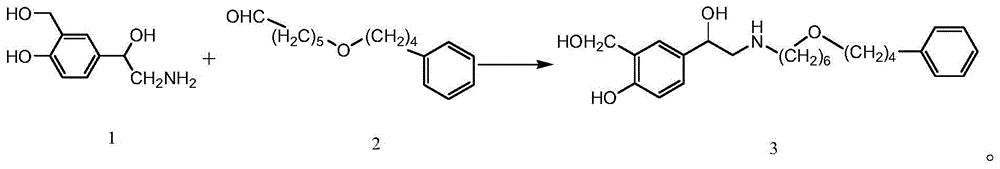
The invention discloses a preparation method of salmeterol. The method includes the following steps: carrying out a condensation reaction on a compound 2 and a compound 3 under an alkaline condition to obtain an intermediate 4; carrying out acidic hydrolysis to obtain an intermediate 5, and reducing the intermediate 5 to obtain an intermediate 6; and removing a benzyl group from the intermediate 6 by using palladium on carbon (Pd / C) to obtain a salmeterol base. The preparation method has the advantages of mild reaction conditions, simple post-treatment, low cost, high yield high product purity, and easiness in realization of industrialization.
Owner:LUNAN PHARMA GROUP CORPORATION
Salmeterol preparation method
ActiveCN103420856BLow costReduce usageOrganic compound preparationHydroxy compound preparationMalonic acidBromine
The invention discloses a salmeterol preparation method which aims to overcome the defects in the prior art. According to the preparation method, 2-bromine ethylbenzene, which is easier to obtain, is taken as raw material, and the key intermediate 4-phenylbutanol is obtained through condensation, decarboxylation and reduction of 2-bromine ethylbenzene and malonic acid ester; N-benzyl-6-(4-phenyl butyl)-hexylamine is obtained through two-step condensation of 4-phenylbutanol; the key intermediate 5-[2-(benzyl (6-(4-phenyl butyl) hexyl) amino) acetyl] salicylaldehyde is obtained through condensation of N-benzyl-6-(4-phenyl butyl)-hexylamine and 5-chloracetyl-salicylaldehyde; finally, the salmeterol is obtained through simple steps of reduction, deprotection, salifying and the like. According to the preparation method, the technology is stable, the reaction condition is moderate, the selectivity is excellent, the aftertreatment is simple to operate, the intermediates are easily to separate, and the product has high purity and high yield, so that a novel thinking and a novel method for large-scale production of salmeterol are provided.
Owner:ASYMCHEM LAB TIANJIN +4
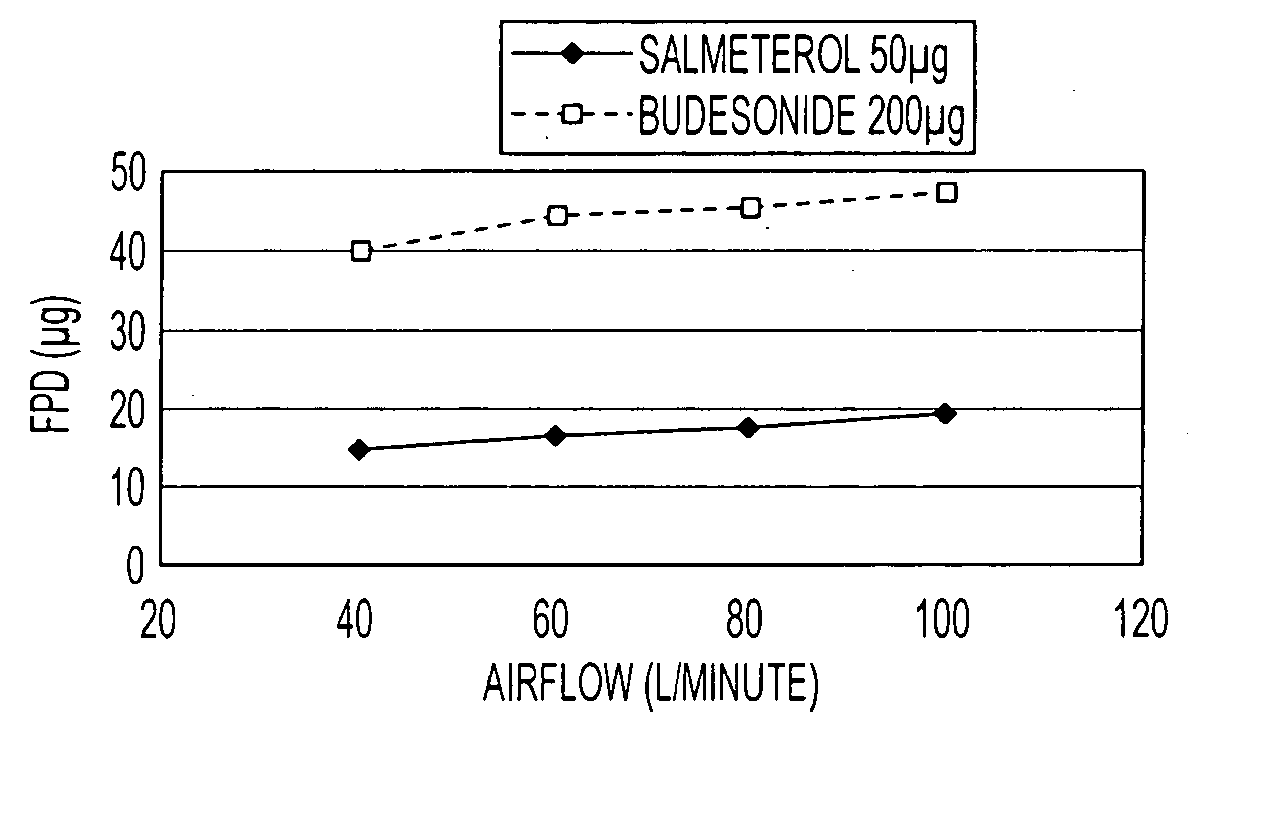
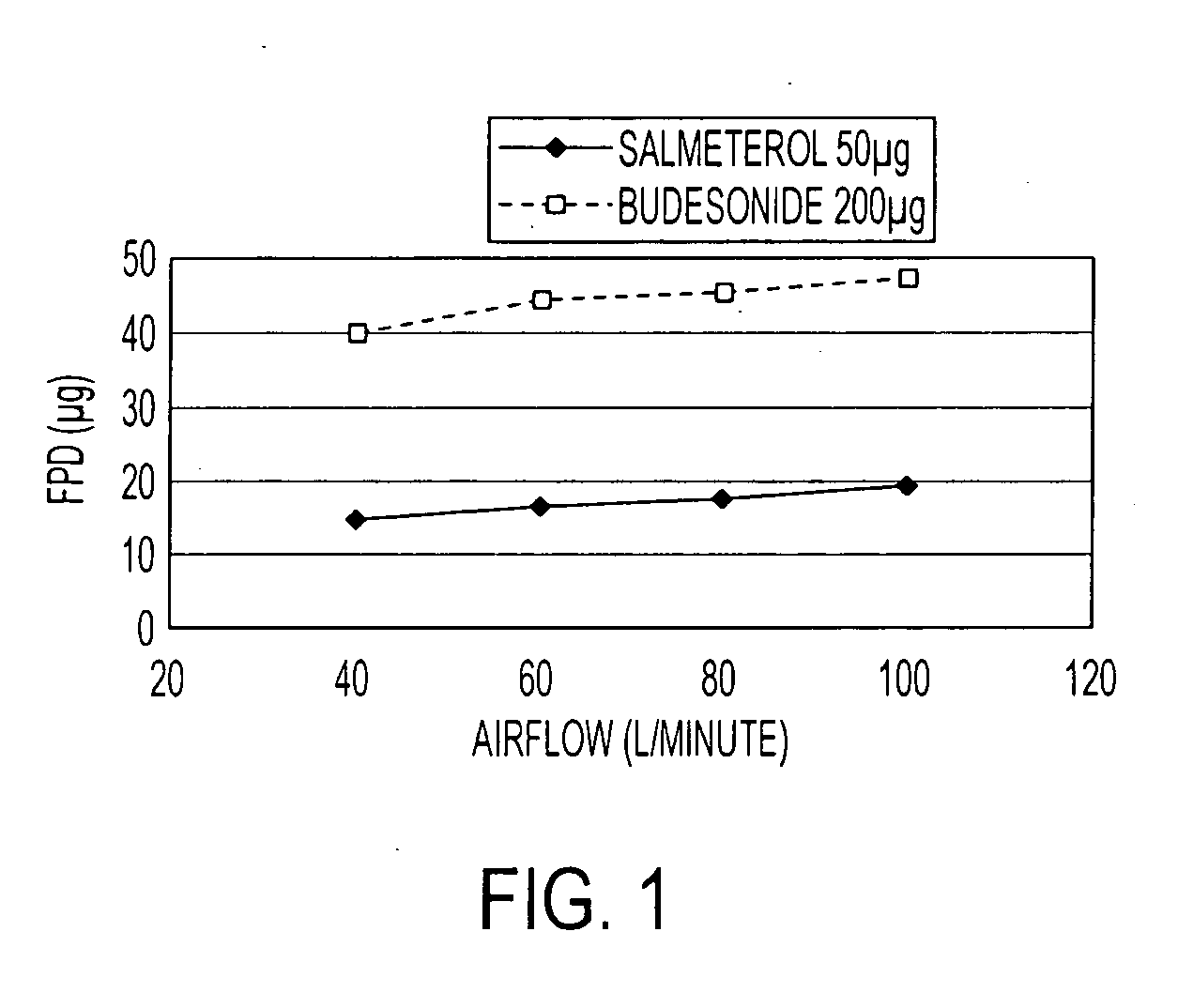
Pharmaceutical composition comprising salmeterol and budesonide for the treatment of respiratory disorders
InactiveUS20080107740A1Great efficacy and durationQuick effectBiocideOrganic active ingredientsDiseaseMedicine
Pharmaceutical composition for inhalation, containing as active ingredient effective amounts of salmeterol or a physiologically salt of salmeterol or a solvate thereof, and budesonide or a therapeutically salt of budesonide or a solvate thereof, wherein the molecular ratio of salmeterol component to budesonide component is in the range 1:2 to 1:50, together with a pharmaceutically acceptable carrier.
Owner:GALEPHAR M F
Salmeteroxinamic acid ester particles for inhalation and preparation method of salmeteroxinamic acid ester particles
PendingCN112933064AAvoid cloggingIncrease the feeding speedPowder deliveryOrganic active ingredientsInhalationDrugs preparations
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a salmeteroxinamic acid ester microparticle for inhalation and a preparation method thereof, and the salmeteroxinamic acid ester microparticle for inhalation comprises the following components: salmeteroxinamic acid ester and a co-crushing agent in a mass ratio of 1: (10-70). The preparation method comprises the following steps: firstly, according to the mass ratio, mixing salmeteroxamic acid ester with the co-crushing agent for 0.5-1 hour; smashing the mixture obtained in the step 1) in a self-airflow smashing mode, wherein the smashing pressure is 0.6 Mpa, the feeding speed is 0.5 g / min, and obtaining the salmeteroxinamic acid ester particles for inhalation. Compared with independent crushing, the method provided by the invention has the advantages of higher feeding speed and higher product yield, powder blockage in the crushing cavity and the airflow channel is avoided, and the obtained product has smaller particle size.
Owner:SHENZHEN HAIBIN PHARMA
A kind of synthetic method of r-salmeterol
ActiveCN105884625BEasy to routeMild reaction conditionsOrganic compound preparationOrganic chemistry methodsSynthesis methodsStructural formula
The invention provides a synthesis method of R-salmeterol (V). The synthesis method comprises the following steps that a compound (I) and N-benzyl-6-(4-phenyl butoxy)-1-hexylamine take a reaction to obtain a compound (II); the compound (II) is hydrolyzed under the acidic condition to obtain a compound (III); the compound (III) is subjected to asymmetric reduction to obtain a compound (IV); the compound (IV) is subjected to deprotection to obtain the R-salmeterol (V). The route is simple; the target product of R-salmeterol (V) is obtained by using 2-acetoxyl group-5-(2-bromoacetyl) phenmethyl acetic ester and the N-benzyl-6-(4-phenyl butoxy)-1-hexylamine as raw materials through total four steps of reaction. The synthesis method has the advantages that the reaction conditions are mild; the operation is simple and convenient; the yield is high; the stereoselectivity is good; the production cost is low; the synthesis method is suitable for industrial production; great practical application values and social economical benefits are realized. The structural formula is shown as the accompanying drawing.
Owner:JOINCARE HAIBIN PHARM CO LTD
Method for preparing anti-asthmatic medicament of salmeterol
InactiveCN101712622BHigh yieldEasy to operateOrganic compound preparationAmino-hyroxy compound preparationChromatographic separationOrganic layer
The invention discloses a method for preparing an anti-asthmatic medicament of salmeterol. The method comprises the following steps of: adding paraxylene, parahydroxyphenylacrylic acid, formaldehyde and acid medium into a reactor, heating, cooling, filtering, washing and drying to obtain 3-hydroxymethyl-4-hydroxyl-phenylacrylic acid; adding the 3-hydroxymethyl-4-hydroxyl-phenylacrylic acid into the reactor, then adding oxyful, lipase and ethyl acetate to finally obtain 3-hydroxymethyl-4-hydroxyl-(beta-hydroxy)-phenylpropyl aldehyde, adding the 3-hydroxymethyl-4-hydroxyl-(beta-hydroxy)-phenylpropyl aldehyde and 6-(4-phenylbutoxy)hexylamine into the reactor, heating for reflowing, cooling, filtering, adding sodium borohydride in batches into filtrate, stirring at normal temperature, then evaporating to dryness, extracting, combining organic layers, drying, filtering and evaporating to dryness to obtain a solid, and separating by using the column chromatography to obtain the final product. The invention has novel and short synthetic route, convenient experiment operation, higher yield of the target product and lower production cost.
Owner:ZHEJIANG WANLI UNIV
Preparation method for salmeterol
InactiveCN102584609BHigh yieldMild reaction conditionsOrganic compound preparationAmino-hyroxy compound preparationHydrogenSolvent
The invention discloses a preparation method for salmeterol expressed by formula 3. The preparation method comprises the following steps: in a solvent, under the catalysis of palladium carbon, causing the compound 1, compound 2 and hydrogen to be subjected to reduction ammoniation reaction, thereby obtaining the salmeterol. The preparation method provided by the invention has the advantages of mild reaction conditions, simple post-processing, lower cost, higher yield and easiness in realization of industrialization.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
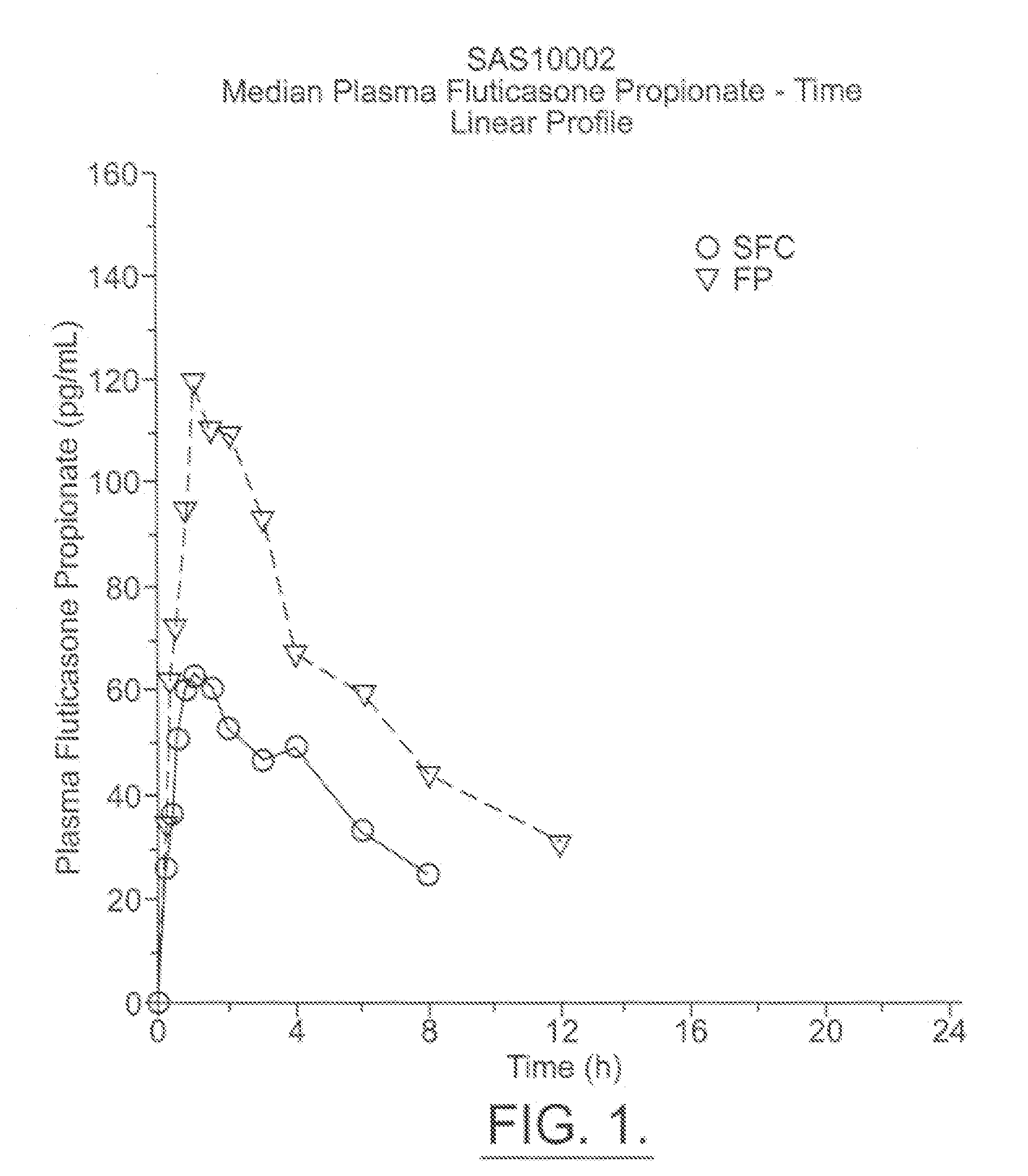



![Preparation and application of bis [-6-oxo-(3-deoxgmonoester citrate-4)]-beta-cyclodextrin HPLC column material Preparation and application of bis [-6-oxo-(3-deoxgmonoester citrate-4)]-beta-cyclodextrin HPLC column material](https://images-eureka.patsnap.com/patent_img/e365b304-e735-424a-95fb-085a209962e0/HDA0001260942960000011.png)
![Preparation and application of bis [-6-oxo-(3-deoxgmonoester citrate-4)]-beta-cyclodextrin HPLC column material Preparation and application of bis [-6-oxo-(3-deoxgmonoester citrate-4)]-beta-cyclodextrin HPLC column material](https://images-eureka.patsnap.com/patent_img/e365b304-e735-424a-95fb-085a209962e0/HDA0001260942960000012.png)
![Preparation and application of bis [-6-oxo-(3-deoxgmonoester citrate-4)]-beta-cyclodextrin HPLC column material Preparation and application of bis [-6-oxo-(3-deoxgmonoester citrate-4)]-beta-cyclodextrin HPLC column material](https://images-eureka.patsnap.com/patent_img/e365b304-e735-424a-95fb-085a209962e0/HDA0001260942960000021.png)
![Preparation and application of bis[-6-oxo-(3-deoxycitrate monoester-4)]-β-cyclodextrin hplc column material Preparation and application of bis[-6-oxo-(3-deoxycitrate monoester-4)]-β-cyclodextrin hplc column material](https://images-eureka.patsnap.com/patent_img/a029a9f3-afd0-4749-bc09-f815bfd14bab/HDA0001260942960000011.png)
![Preparation and application of bis[-6-oxo-(3-deoxycitrate monoester-4)]-β-cyclodextrin hplc column material Preparation and application of bis[-6-oxo-(3-deoxycitrate monoester-4)]-β-cyclodextrin hplc column material](https://images-eureka.patsnap.com/patent_img/a029a9f3-afd0-4749-bc09-f815bfd14bab/HDA0001260942960000012.png)
![Preparation and application of bis[-6-oxo-(3-deoxycitrate monoester-4)]-β-cyclodextrin hplc column material Preparation and application of bis[-6-oxo-(3-deoxycitrate monoester-4)]-β-cyclodextrin hplc column material](https://images-eureka.patsnap.com/patent_img/a029a9f3-afd0-4749-bc09-f815bfd14bab/HDA0001260942960000021.png)